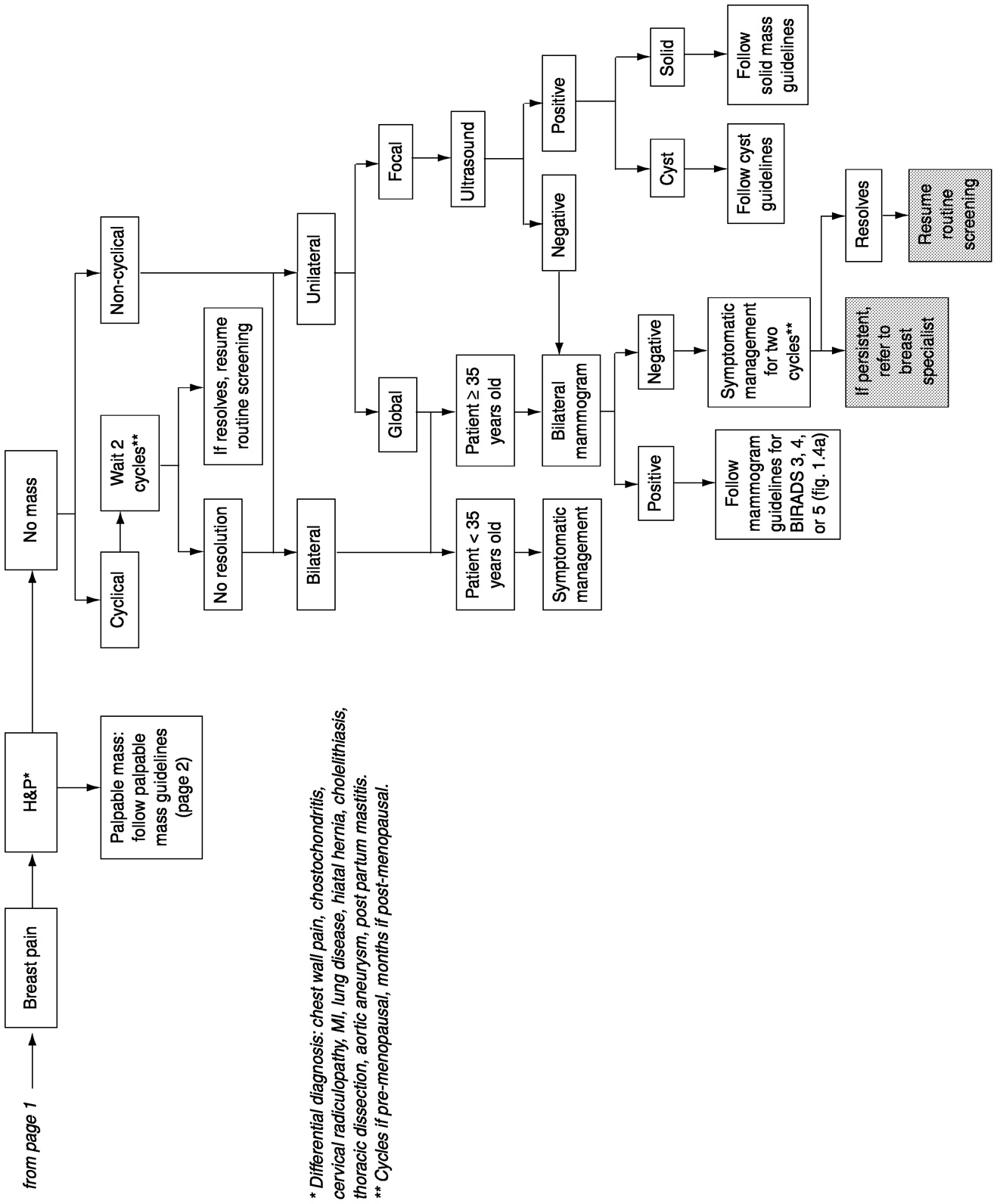
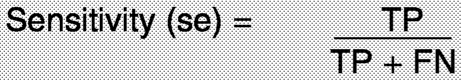Page 15
1
Introduction
Breast cancer is the most common non-skin-related malignancy and the second leading cause of cancer death among women in the United States1. Each year, more than 180,000 new cases of invasive breast cancer are diagnosed and more than 40,000 women die from the disease. Until research uncovers a way to prevent breast cancer or to cure all women regardless of when their tumor is found, early detection will be looked upon as the best hope for reducing the heavy toll of this disease. The early detection of cervical cancer by screening with the Papanicolaou smear (the Pap smear) dramatically reduced mortality from that cancer, and the rationale for the early detection of breast cancer is similar.
Fifty years ago, there was no established method for the detection of breast cancer at an early stage or for screening of the general population, but advances in technology, policy recommendations by various organizations, and legal mandates have thoroughly changed that situation (Figure 1-1). Although the use of X-ray imaging for the detection of breast cancer was first suggested in the early 1900s, mammography did not begin to emerge as an accepted technology until the 1960s, after a number of technical innovations that produced higher-quality images that were more reproducible and easier to interpret were introduced. Subsequently, some physicians began ordering mammograms to help with the diagnosis of complicated cases, and the technology was also tested as a screening
Page 16
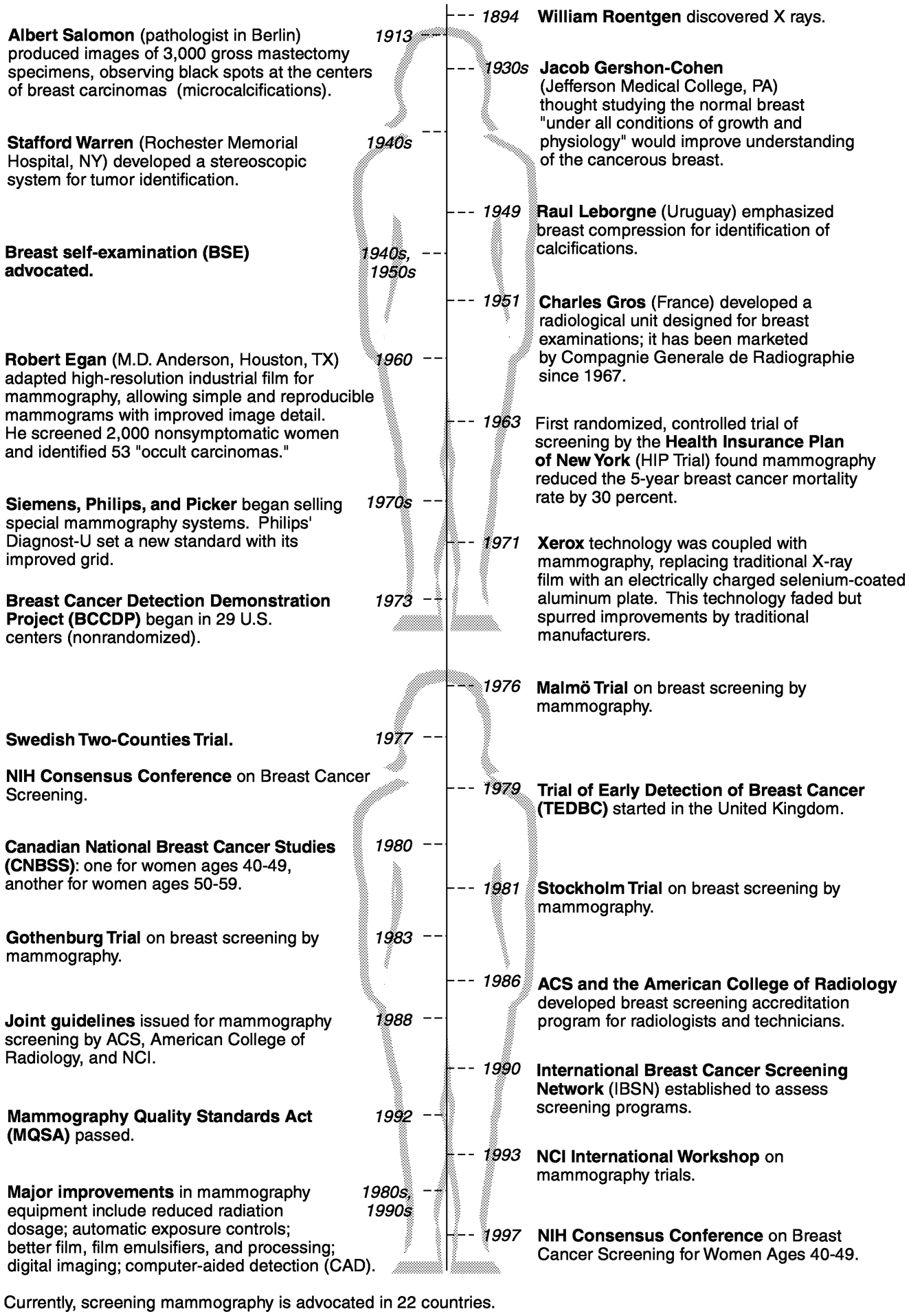
~ enlarge ~
FIGURE 1-1 A History of Breast Cancer Screening.
SOURCES: Gold et al. (1990), Kevles (1997); Jatoi (1999), Moss (1999), and Lerner (2001).
Page 17
tool (reviewed by Lerner, 2001). X-ray film mammography and physical examination of the breast are now the mainstays for early detection of breast cancer. Screening for early cancer detection has been credited for part of the recent reduction in breast cancer mortality, which had been stagnant for 40 years (Blanks et al., 2000; Hakama et al., 1997; Mettlin, 1999; Peto et al., 2000) (Figure 1-2). (Adjuvant therapy is also credited with reducing breast cancer mortality). New or improved technologies are also rapidly emerging and providing new hope of early detection.
Over the past decade, the investment in breast cancer research, including early detection, has increased substantially. Research has intensified with federal funding, and private firms have turned more attention to breast cancer detection. Programs within the U.S. Department of Health and Human Services and the U.S. Department of Defense support large numbers of investigators working on breast cancer, and recently, the National Aeronautics and Space Administration and several intelligence services have agreed to apply their imaging expertise to mammography ( Table 1-1). Biotechnology and device companies have proliferated, with many developing technologies that might improve the ability to detect breast cancer early, and established firms have also turned their attention to breast cancer, in part as the result of findings derived from the rising federal research investment.
Advances in imaging (see Chapter 2) include reducing the dose of X rays needed, enhancement of digital images, computer-assisted analysis of images, and use of alternatives to X rays such as ultrasound, magnetic resonance imaging (MRI), and optical imaging. Techniques for high-resolution imaging and image processing, many of which were developed for other applications such as space science, are now being applied to breast imaging, with hopes of improved accuracy, speed, ease of use, and perhaps lower cost. Advances in genetics and increased knowledge of the basic biology and etiology of breast cancer may also lead to novel, biologically based early detection and diagnostic methods. Use of molecular markers (see Chapter 3) may increase the accuracy of diagnostic techniques and offer new opportunities for the characterization of early disease as well as for the refinement and improvement of treatments.
However, early detection depends on more than just the development of technologies and the advance of new science. Technological advances must be thoroughly evaluated before they can become widely used by women. This evaluation takes place in many stages, including Food and Drug Administration (FDA) approval (when it is a device), adoption by health plans and providers, approval of payment for screening and detection, acceptance by women, and marketing by private firms. A wide range of factors must be considered at the various stages, including safety, accuracy, cost-effectiveness, and negative side effects. The level of evidence needed to establish efficacy, how effectiveness should be es-
Page 18
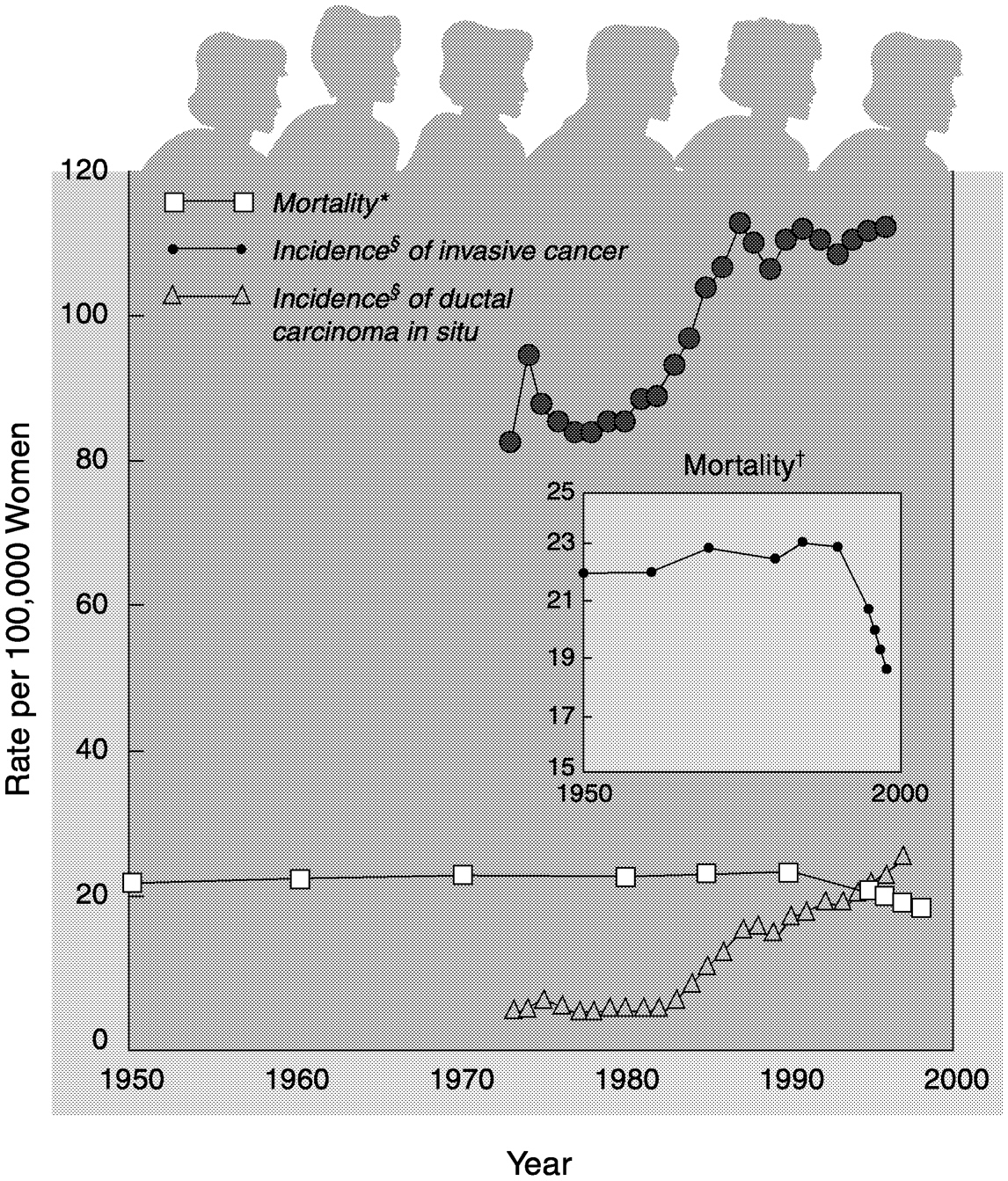
~ enlarge ~
FIGURE 1.2 Breast cancer incidence and mortality rates in the United States, 1950-1998.
*Age-adjusted to the 1940 U.S. standard population. SOURCE: Health, United States 2000, National Center for Health Statistics, National Vital Statistics System, Centers for Disease Control and Prevention.
§Age-adjusted to the 1970 U.S. standard population. SOURCE: SEER Cancer Statistics Review, 1973-1996 (Ries et al., 1999). Numbers are calculated using cancer incidence rates from the regions of the U.S. included in the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program and population data collected by the U.S. Bureau of the Census.
†Inset: Mortality data on a compressed scale to demonstrate the recent decrease. Between 1990 and 1998, mortality decreased by ∼2%/year on average.
Page 19
tablished, how costs should be measured, and how much evidence is needed before decisions about coverage are made—including offering and paying for screening—are all critical questions that need to be addressed. According to Edward Golub at the Pacific Center for Ethics and Applied Biology: “We are on the verge of an era of technological change in which the ability to do tests can determine whether we do them, which ones we do, and on whom they are done; it is crucial to understand the value and limits of [screening] tests, and how they fit into the goals of medicine. We are in danger of behaving as if all technological change is progress, and of confusing being swept along on the wave of changes with responsible exercise of the authority that society has given to the clinician in that which matters most to people, their health” (Golub, 1999, p. 14).
The purpose of the study presented in this report was to review breast cancer detection technologies in development and to examine the many steps in medical technology development as they specifically apply to methods for the early detection of breast cancer. The study committee was charged with ‘surveying existing technologies and identifying promising new technologies for early detection, assessing the technical and scientific opportunities.' The committee was further charged with examining the ‘policies that influence the development, adoption, and use of technologies.'
THEORY AND PRINCIPLES OF CANCER SCREENING
The practice of screening for cancer is based on the premise that early detection will have a positive effect on the outcome of the disease through medical intervention. The goal of screening is to reduce disease-specific morbidity and mortality through early treatment by identifying clinically occult (asymptomatic) tumors that are likely to become lethal. It is widely believed that early detection of such tumors will allow surgical removal of the cancer before the cells have begun to metastasize.
Policy decisions regarding the use of screening in large populations depends on data from clinical studies (R. Harris, 1999). In 1968, the World Health Organization proposed guidelines for clinical trials of tests used to screen for diseases (Wilson and Jungren, 1968):
1. The disorder screened for should be an important cause of morbidity, disability, or mortality.
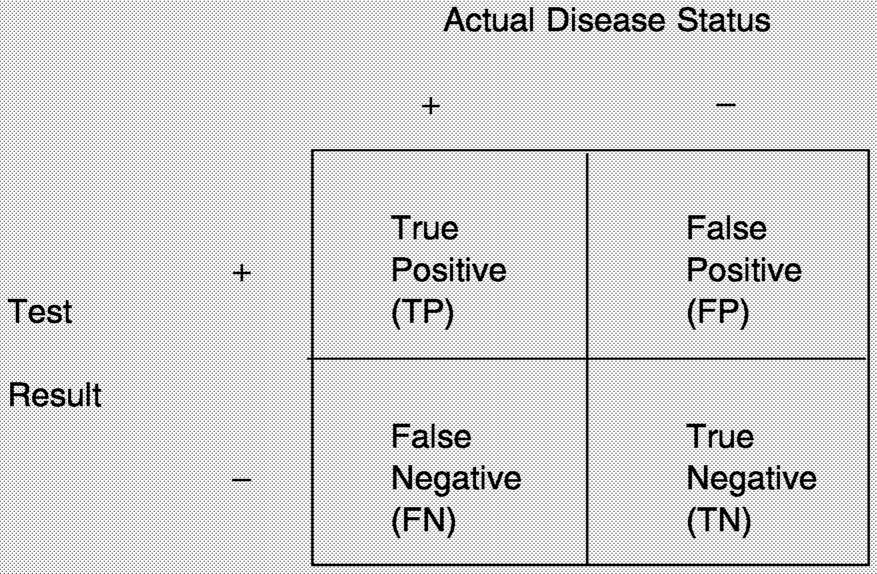
2. The tests must have acceptable performance characteristics (for example, high levels of sensitivity and specificity, see Box 1-1 for definitions of screening test performance).
3. The tests must be available and acceptable to the target population and that population's physicians.
Page 20
|
Funder or Program |
Amount ($) |
Type of Projects Supported |
|
Government Funders |
||
|
Early Detection Research Network, NCI, NIH; established 1999 (edrn.nci.nih.gov) |
$8 million awarded 10/99 to fund 18 biomarker development laboratories. $18 million awarded 5/00 to fund nine clinical/epidemiological centers, three biomarker validation labs, and a data center. Additional $2 million for Steering Committee. |
Development of biomarkers for earlier detection of cancer and risk assessment. Four components of the multi-institutional consortium: biomarker developmental laboratories, biomarker validation laboratories, Clinical/ epidemiological centers, data management, and a coordination center. Breast cancer is one focus activity. |
|
Unconventional Innovations Program, NCI, NIH, OTIR; announced 1999 (amb.nci.nih.gov/UIP.HTM) |
Initial estimate of $48 million over next 5 years. |
Development of noninvasive methods of detecting defined signatures of cancerous and precancerous cell types and developing directed interventions toward detected cells. Promotes multidisciplinary collaboration among researchers with expertise in diverse scientific fields. |
|
Phased Innovation Award NCI, NIH, OTIR; announced 1999 (otir.nci.nih.gov/funding/phasein.html) |
6 months, $100,000. |
Fosters the translation of emerging technologies from pilot research to research development, speeding the adoption of near-term technological opportunities. Example of an award includes Innovative Technologies for the Molecular Analysis of Cancer, encompassing methods and tools that enable research, such as instrumentation, techniques, devices, and analysis tools. |
Page 21
|
Phased Technology Application Award, NCI, NIH, OTIR announced 1999 (otir.nci.nih.gov/funding/phasetech.html) |
2 years, $750,000. |
Developed to complement the Phased Innovation Award. Fosters the translation of emerging technologies from evaluation to pilot application, speeding the adoption of near-term technological opportunities. |
|
Cooperative Trials in Diagnostic Imaging (ACRIN), NCI, NIH; March 1999 (www.acrin.org) |
$3 million in FY 1999, $23 million through FY 2003. |
Network established to facilitate multiinstitutional clinical trials in diagnostic imaging related to cancer, with breast cancer as one focus area. |
|
Director's Challenge: Toward a Molecular Classification of Tumors, NCI, NIH; announced 1999 (grants.nih.gov/grants/guide/rfa-files/RFA-CA-98-027.html) |
Ten 5-year grants awarded, totaling $4.1 million for first 6 months. Anticipated total cost of $50 million for 5 years. |
Discovery of molecular profiles toward goal of precise molecular diagnosis of cancer. Breast cancer is one of eight cancers targeted. |
|
Development and Testing of Digital Mammography Displays and Workstations (PA), NCI, NIH; announced April 1999 |
Open application; no set-aside. |
Development of displays and workstation design for improved image interpretation. |
|
Early Clinical Advanced Technology Program, NIST, U.S. Department of Commerce; began in 1990 (www.atp.nist.gov) |
$22 million over 5 years, starting in FY 2001, to support a total of 56 trials. |
Will support phase I and II clinical trials to rapidly evaluate new technologies in molecular level targeted imaging such as imaging probes, ligands, radiopharmaceuticals, and contrast agents. |
|
Trials of Imaging Agents, NCI, NIH; announced March 2000 |
Page 22
|
Funder or Program |
Amount ($) |
Type of Projects Supported |
|
Advanced Technology Program, NIST U.S. Department of Commerce; Began in 1990 (www.atp.nist.gov) |
NA (breast cancer portion unknown) |
Encourages innovation by bridging the gap between research laboratory and marketplace through partnerships with the private sector. Accelerates development of promising novel technologies through early-stage investment. |
|
Breast Cancer Research Program (BCRP), U.S. Department of Defense began in 1992 (cdmrp.army.mil/) |
$135 million in FY 1999. |
Supports the spectrum of breast cancer research, from basic research to clinical science. Examples of award mechanisms include special mammography awards and computer-aided diagnosis support. Has also funded research in telemammography, ultrasound, MRI, PET, and molecular targets. |
|
U.S. Department of Energy (www.doe.gov) |
$72 million in FY1999 for the Life Sciences Division (breast cancer portion unknown) |
Life Sciences Division supports research in the molecular basis of breast cancer, as well as technologies for detection (Center for Functional Imaging). |
|
National Science Foundation (www.nsf.gov) |
In 1999, more than $2.8 billion for research (unknown how much to breast cancer research) |
Research grants include projects related to early detection of breast cancer (e.g., digital mammography). Photonics Partnership Initiative will investigate possibilities for early breast cancer detection. |
Page 23
|
Private Foundations |
||
|
California Breast Cancer Research Program; established in 1993 (www.ucop.edu/srphome/bcrp) |
$75 million since 1994.; $16 million in 1999. |
Earlier detection is one priority area of the program and encompasses the development of new technologies, biomarkers, and improved access for all women. |
|
Susan G. Komen Breast Cancer Foundation; established in 1982 (www.komen.org) |
In 1998, $14.1 million distributed in research grants. Current funding for imaging technology projects has maximum amount of $250,000 for 2-year period. |
Grant program supports research in imaging technology to improve screening and diagnosis, in addition to basic, clinical, and translational research in breast cancer. Program in imaging technology dedicated to research and development for early detection and diagnosis of breast cancer. |
|
Breast Cancer Research Foundation; established in 1993 (www.bcrfcure.org) |
In 1999, gave out $6 million in grants. Minimum annual grant of $100,000 per institution. |
Funds clinical and genetic research in the causes and treatments of breast cancer, including technologies for early detection. |
|
Breast Cancer Fund; established in 1992 (breastcancerfund.org) |
Grants range from $15,000 to $50,000. |
One objective of the Innovative Research Grant category is to replace mammography with a safer, more accurate screening method. |
|
Whitaker Foundation; established in 1975 (www.whitaker.org) |
In 1998, $52 million for awards and programs. Maximum award is $240,000 over 3 years. |
Supports the field of biomedical research. Past grants have been awarded to research in technologies for breast cancer detection, including digital mammography, microwave imaging, optical tomography, etc. |
Page 24
|
Funder or Program |
Amount ($) |
Type of Projects Supported |
|
Friends...You Can Count On; established in 1995 (www.earlier.org) |
Small Grants in Cancer Detection/ Screening; awards up to $40,000 offered, may be used over 3-year period. |
Funds research on new methods to improve detection of early breast cancer, especially in the areas of biological or immunological methods for the detection of early-stage breast cancer. |
|
New Initiatives and Collaborations |
||
|
NASA/NCI collaboration: Workshop on Sensors for Bio-Molecular Signatures, June 1999 |
Development of sensory and imaging systems that will help NASA detect life on other planets and NCI to detect cancer cells in humans. These technologies would have sensitivities and detection capabilities at the molecular level and the ability to transmit information to external systems. |
|
|
NCI-Industry Forum & Workshop on Biomedical Imaging in Oncology, NCI, industry, HCFA, FDA September 1999 |
Discussed importance of coordination between various agencies and industries to improve the present system of technology development. |
|
NOTE: NIH, National Institutes of Health; OTIR, Office of Technology and Industrial Relations; FY, fiscal year; NIST, National Institute of Standards and Technology; PET, positron emission tomography; NASA, National Aeronautics and Space Administration; HCFA, Health Care Financing Administration.
Page 25
BOX 1-1
|
|
Measurement |
Question Answered |
|
How often does the test correctly identify women with breast cancer? |
|
|
How often does the test correctly identify women without the disease? |
|
|
Among women with an abnormal test result, what proportion actually have the disease? |
The positive predictive value (PPV) is a function of sensitivity, specificity, and disease prevalence (P), with the following mathematical relationship:
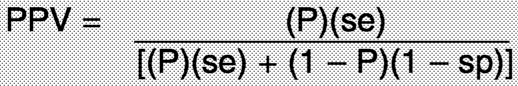
~ enlarge ~
Page 26
4. Appropriate follow-up of individuals with positive findings must be ensured.
5. Screening should provide a net benefit to the target population, and the resources required to administer the tests under screening conditions must be justified in terms of the net benefits.
In other words, the natural history of the disease should be sufficiently well known, treatments should be sufficiently effective, risks resulting from screening should be acceptably low, and efficacy in reducing disease-specific morbidity and mortality should be high enough to conclude that early detection of the disease will be beneficial. Clearly, these standards are subject to value judgments in determining the relative importance of each condition and whether the conditions are adequately met. However, it is quite useful to refer to these recommendations when evaluating potential screening technologies.
The methods used to assess the efficacy of a screening method are quite different from the approach used to assess new treatments. For instance, treatment outcome is often measured by using short-term surrogate end points that have previously been correlated with long-term outcome, but such surrogate end points generally do not exist for screening methods.
There are many difficulties in accurately determining the real benefit of any cancer screening technology or program. Two inherent biases2 must be taken in account: lead-time bias and length bias ( Figure 1-3). These biases can be minimized (but not completely eliminated) only by evaluating a screening modality through a randomized, controlled trial in which mortality is the end point. Lead-time bias is due to the assumption that the identification and treatment of tumors at an earlier point in the progression of the disease will necessarily alter the rate of progression. Thus, a woman who survives 4 years after the diagnosis of a cancer identified during screening may be thought to have an increased survival time compared with that for an unscreened woman who finds a lump and dies 2 years after the diagnosis. However, once a cancer is identified by screening and is treated, it is impossible to know how long the woman would have survived if the cancer would have gone undetected until it became palpable. Likewise, it is impossible to know whether earlier detection and treatment of the woman with a 2-year postdiagnosis survival time would have resulted in a longer life for the woman if her cancer had been detected and treated sooner.
Page 27
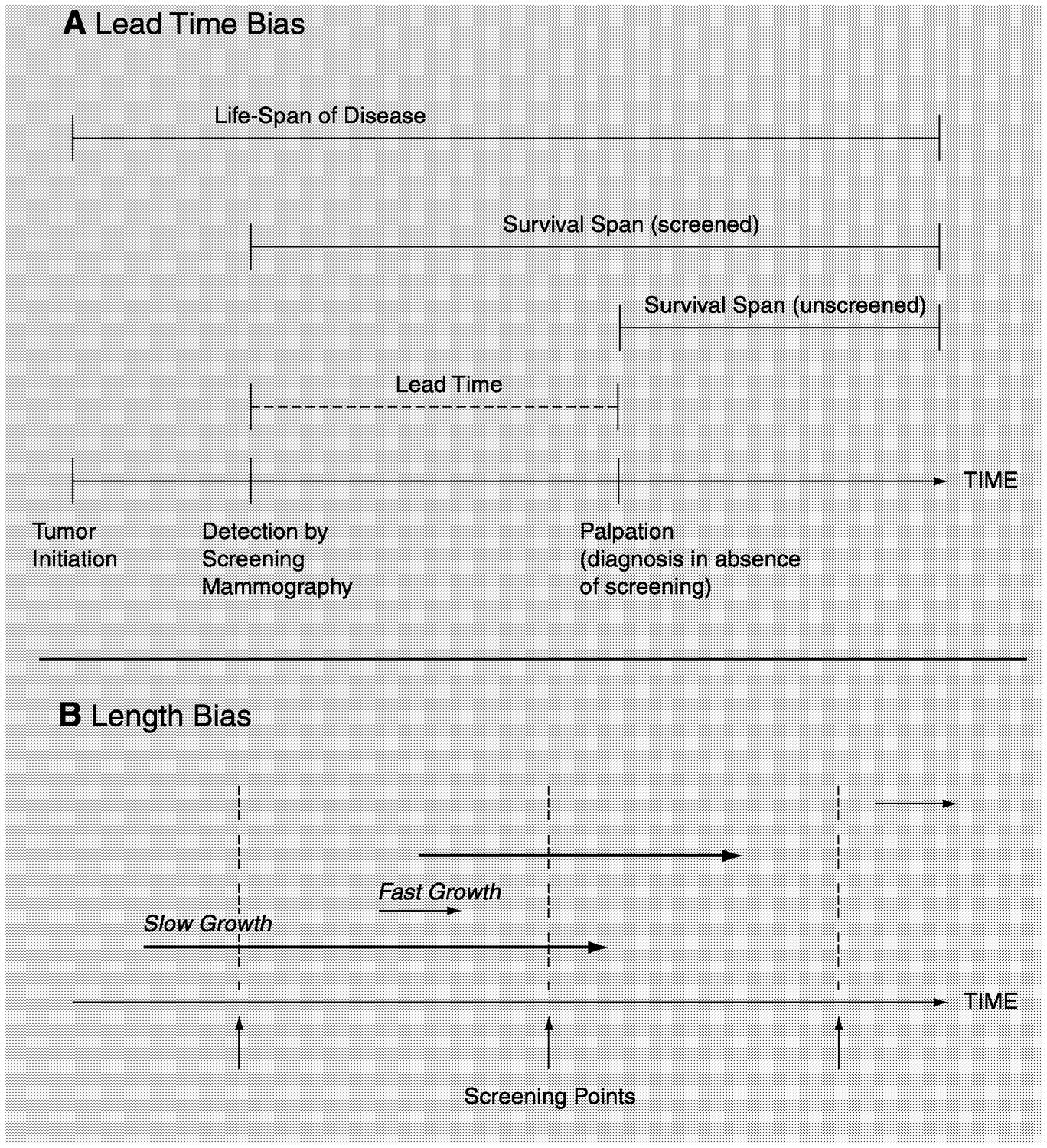
~ enlarge ~
FIGURE 1-3 Lead-time (A) and length (B) biases. In panel B, the length of the arrows represents the time required for the tumor to reach a palpable size. For a more detailed description of lead-time and length biases, see the accompanying text in Chapter 1.
Length bias reflects the fact that screening tests detect a disproportionate number of women with slowly progressing tumors. A cancer that takes several years to reach a palpable size will be detected as a smaller tumor by regular screening than one that grows to the same size in a much shorter time period. If an aggressive, fast-growing tumor is more likely to become life-threatening than a slow-growing tumor, then many women whose tumors were identified through a screening program will inherently have a more favorable outcome following treatment.
Page 28
BOX 1.2
|
Additional difficulties encountered in the assessment of screening programs include selection bias and overdiagnosis. Selection bias assumes that women who are at higher risk (see Box 1-2 for a definition of “risk”) for breast cancer will be more likely to participate in screening trials and will be more compliant with the recommended guidelines for screening mammography. Since cancer screening may be more beneficial and cost-effective for high-risk populations than for the general popula-
Page 29
tion, a selection bias may result in overestimation of the value of implementing a screening program for the general population.
“Overdiagnosis” is the result of labeling small lesions as cancer or precancer when in fact the lesions may never have progressed to a lifethreatening disease if left undetected and untreated. In such cases, some of the “cures” following early detection may not be real. This issue will be revisited in the next sections.
CURRENT PRACTICE OF BREAST CANCER DETECTION
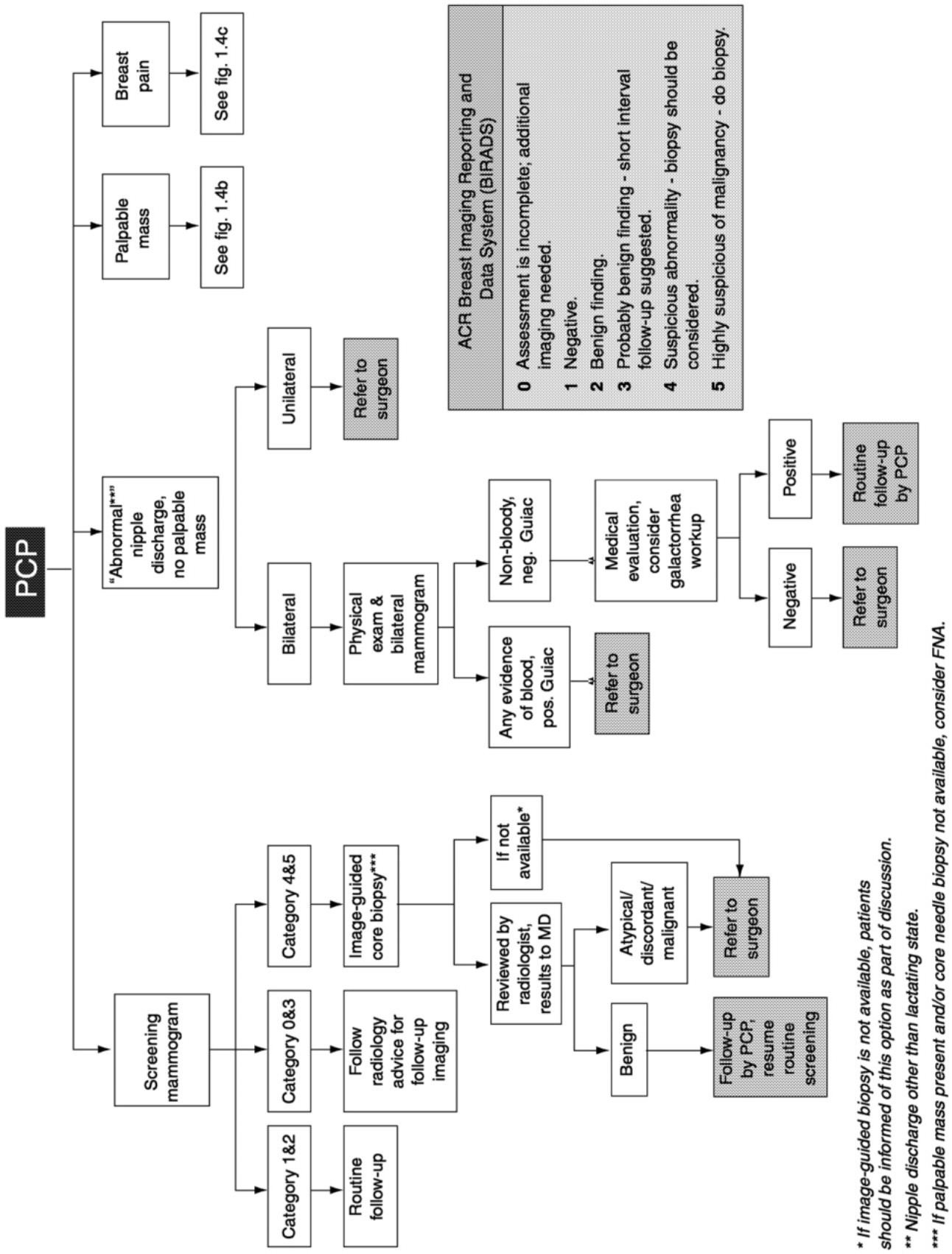
Breast cancer detection currently entails three distinct stages. The first stage is identification of an abnormality in the breast tissue either by physical examination or by an imaging technique (most commonly, mammography). Once identified, the abnormality must be diagnosed as benign or malignant by using additional imaging modalities or by biopsy and microscopic examination of the tissue morphology ( Figure 1-4). In the third stage, abnormalities labeled as malignant must be further characterized biochemically and staged according to tumor size and extent of invasion and metastasis to determine a prognosis and an appropriate course of treatment.
Monthly breast self-examination (BSE) is a common method of identifying lumps and other abnormalities in the breast. BSE in conjunction with screening mammography is currently advocated by many organizations,3 but it is also recommended for younger women who are not yet being screened by mammography. BSE was first advocated in the 1940s and 1950s, before the advent of screening mammography. Breast surgeons saw many patients whose tumors were too large for surgical removal, and they believed that regular self-examination of the breasts would result in earlier detection when surgery was still an option. Although the goal of finding smaller tumors at an earlier stage may be attained by BSE (Coleman, 1991), to date the evidence is not definitive that BSE improves the survival rate for women with breast cancer. Furthermore, BSE can lead to an increase in unnecessary biopsies, especially in younger women (Semiglazov et al., 1992, 1999; Thomas et al., 1997). Two large, randomized trials are ongoing in Russia and China4 and may help to answer some of these questions more definitively.
Page 31
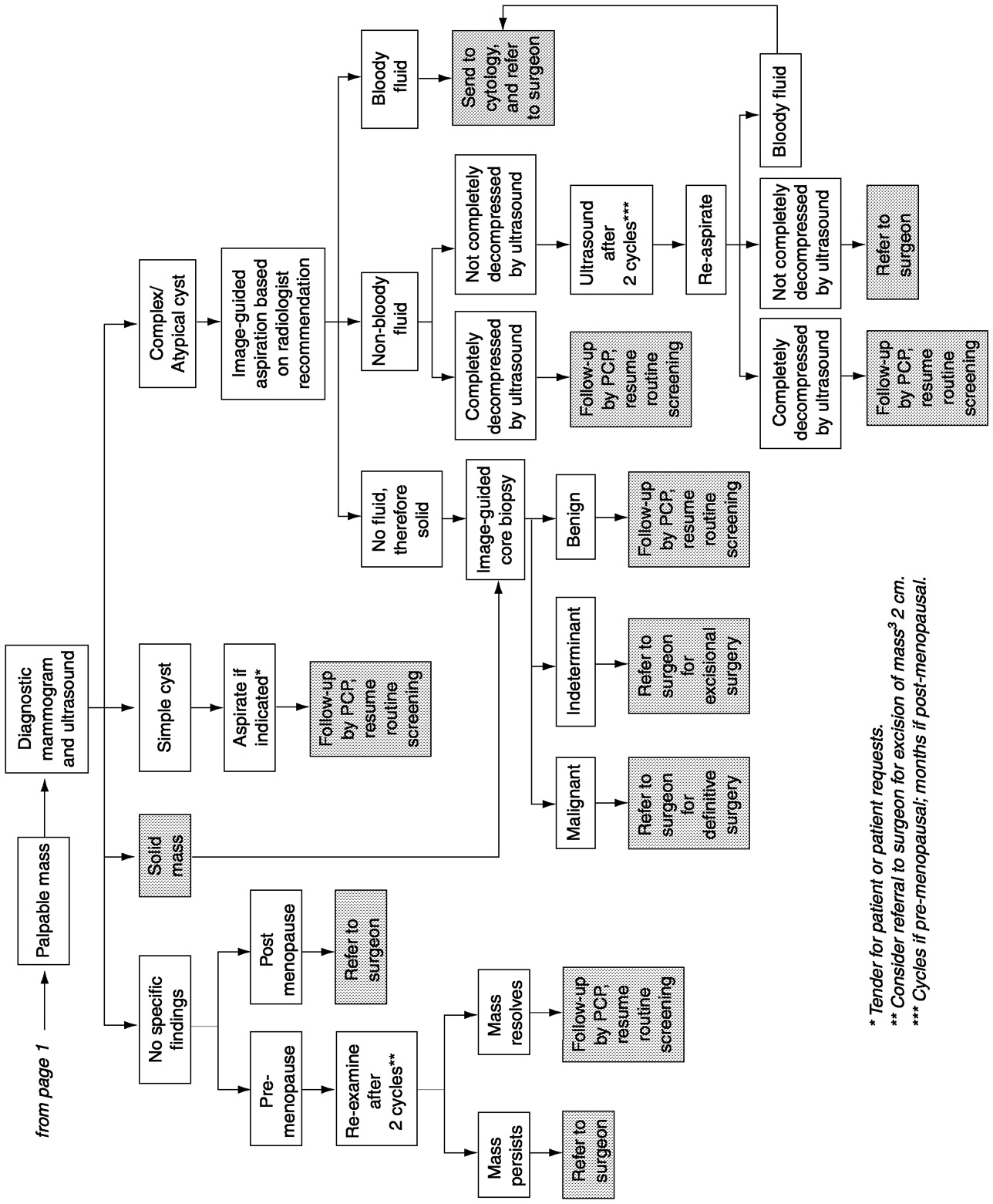
~ enlarge ~
FIGURE 1-4 Breast cancer management algorithm. SOURCE: Risk Management Foundation, Boston Massachusetts (May 23, 2000).
Page 33
Breast physical examination by physicians is also widely practiced and advocated for women of all ages. Although clinical breast examination (CBE) has been studied in conjunction with mammography and one study has shown it to be beneficial in that context (the Health Insurance Plan of New York [HIP] trial), its use as a stand-alone screening tool has not yet been fully assessed. A large trial is being undertaken in India to determine the impact of screening by physical examination alone on breast cancer mortality (Jatoi, 1999). In Canada, a large randomized clinical trial has directly compared CBE alone with screening mammography plus CBE.5 The results showed no significant difference in breast cancer mortality rates between the two study arms at 7 and 13 years after the initiation of screening (Miller et al., 1993, 2000)6, although mammography identified smaller tumors than physical examination did. The study does not question the assertion that mammography lowers breast cancer mortality, but the results suggest that a very careful standardized physical examination (lasting an average of 10 minutes) can achieve the same reduction in mortality as screening mammography. The investigators in that study did not address the question of whether mammography led to reduced morbidity as a result of less aggressive treatment for the smaller tumors found by that screening approach. This is the only study to date to directly compare CBE to screening mammography, so there are no data to confirm or refute the findings, although the undertaking of such a confirmatory study has recently been suggested (Mittra et al., 2000). However, even if the data are sound, it might be more difficult in practice to recapitulate the benefits of CBE observed in this clinical trial (that is, more difficult than it would be for mammography) because mammography is highly standardized and regulated in the United States (see the discussion of the Mammography Quality Standards Act below), whereas CBE is not. A number of recommendations for improving the practice and standardization of CBE have been made based on a review of the literature (Barton et al., 1999).
Nevertheless, physical examination will always be important, but it is not sensitive enough to identify very small tumors. Although a pad to help women perform BSE was recently approved by the FDA7 and some electronic palpation devices are under development (see Chapter 2), pros-
Page 34
pects for improving physical detection methods may depend more on increasing the number of people who do it carefully and thoroughly after training and education than on technological advances.
Screening mammography is promoted as the key to the continued reduction in breast cancer mortality through early detection. A number of organizations, including the National Cancer Institute (NCI), the American Cancer Society (ACS), and the American College of Radiology , currently recommend routine screening every 1 to 2 years for women over age 40 (U.S. Preventive Services Task Force, 1996). Several randomized controlled studies have been undertaken in four countries to assess the value of screening mammography (reviewed by Moss [1999] and Jatoi [1999]) ( Table 1-2). Most of them demonstrated a substantial reduction in rates of death from breast cancer (about 25 to 30 percent) among women screened by mammography, and meta-analysis has confirmed a clear benefit of screening mammography for women over age 50 (Kerlikowske et al., 1995; Kerlikowske, 1997). (A recent review published in The Lancet criticized six of the eight trials for methodological inadequacies in randomization procedures that led to baseline imbalances and for determining the cause of death without blinding [Gotzsche and Olsen, 2000]. The authors concluded that because the two studies without these problems showed no reduction in mortality rates for the screened groups, screening for breast cancer by mammography is unjustified. An accompanying editorial and several letters to the editor rebutted the review, pointing out that the baseline differences were very small, that many other criteria are important for the assessment of screening trials, and that one trial did not assess screening but, rather, compared two different methods of screening.) The reported reduction in breast cancer mortality among women aged 40-49 appears to be less than that of older women, with a longer time period between initiation of routine screening and observation of reduced mortality. As a result, the value of screening women younger than age 50 is still controversial, as will be discussed later in this chapter.
There are now a variety of venues for mammography breast cancer screening, including doctors' offices, private radiology practices, hospital radiology departments, imaging centers, breast clinics, and mobile mammography vans. Because the effectiveness of screening mammography is dependent on the quality of the facilities and personnel, a federal law requires all mammography facilities to be certified by FDA. The intent of the Mammography Quality Standards Act (MQSA)8 was to ensure that
Page 35
|
Trial |
Entry Years |
Age at Entry(years) |
Number of Women |
Follow-up Period (years) |
RR (95% CI) |
|
HIP Triala |
1963-69 |
40-64 |
60,696 |
18 |
0.77(0.61-0.97) |
|
Malmö |
1976-86 |
45-69 |
41,478 |
12 |
0.81 (0.62-1.07) |
|
Two-County |
1977-85 |
40-74 |
133,065 |
11 |
0.70 (0.58-0.85) |
|
Stockholm |
1981-85 |
40-64 |
59,176 |
7.4 |
0.71 (0.4-1.2) |
|
Gothenburg |
1982-88 |
40-59 |
49,553 |
10 |
0.86 (0.54- 1.37) |
|
Edinburgh |
1978-85 |
45-64 |
54,671 |
10 |
0.82 (0.61- 1.11) |
|
Canada NBSS Ia |
1980-87 |
40-49 |
50,430 |
10 |
1.10 (0.78- 1.54) |
|
Canada NBSS IIb |
1980-87 |
50-59 |
39,405 |
8.3 |
0.97 (0.62- 1.52) |
NOTE: RR, relative risk (a value less than 1 indicates fewer breast cancer deaths among screened women); CI, confidence interval, NBSS, national breast screening study.
a Compared mammography plus CBE with no screening.
b Compared mammography plus CBE with CBE alone (all others compared mammography with no screening).
SOURCES: adapted from Jatoi (1999), Kerlikowske et al. (1995), and Moss (1999).
Page 36
|
BIRADS Category |
Assessment |
Recommendations |
|
0 |
Incomplete |
Other mammographic views and techniques or ultrasound needed |
|
1 |
Negative, no findings |
Routine screening |
|
2 |
Benign finding |
Routine screening |
|
3 |
Probably benign |
Short-term follow-up to establish stability |
|
4 |
Suspicious abnormality |
Biopsy should be considered |
|
5 |
Highly suggestive of malignancy |
Appropriate action should be taken |
SOURCE: American College of Radiology: Breast Imaging Reporting and Data System (BIRADS). Reston, Virginia, American College of Radiology, 1998.
all facilities meet federal standards for equipment, personnel, and practices.9 Since its inception, the quality of mammograms has improved (Suleiman et al., 1999; U.S. General Accounting Office, 1998a,b; Wagner, 1999). However, mammography is the only medical examination that is federally regulated in this way, and the regulations substantially increase the cost of performing mammography (Inman, 1998; Wagner, 1999).
A screening mammogram actually consists of two X-ray films, taken from the side (referred to as the “mediolateral oblique view”) and from above (referred to as the “craniocaudal view”), for each breast. A diagnostic mammogram, which may include additional views or magnifications, is usually performed following a suspicious finding on a screening mammogram or when a woman has a new symptom such as a breast lump. To create a uniform system of assessing mammography results, the American College of Radiology developed the Breast Imaging Reporting and Data System (BIRADS)10 (Table 1-3). This system includes five categories
Page 37
of assessment with increasing suspicion of malignancy, along with standard follow-up recommendations for each category.
Ultimately, mammograms do not detect cancer per se. Rather, they provide a means for the identification of tissue abnormalities, which are subject to interpretation by human observers who introduce variability into the process on the basis of their prior experience, training, perceptual capabilities, vigilance, and so on. Radiologists look for microcalcifications (tiny calcium deposits), architectural distortions, asymmetrical densities, masses, and densities that have developed since the previous mammogram. In some cases, breast ultrasound may be ordered as a follow-up to mammography to rule out cysts (fluid-filled lesions) or to better characterize the lesion and its solid components. For women with a BIRADS score of 4 or 5, biopsy and histological examination are generally necessary to determine whether the abnormality identified by imaging methods is benign and harmless or malignant and life threatening.
Traditionally, a diagnostic biopsy entails an open surgical incision to remove a lump or tissue sample. In the case of nonpalpable lesions, the surgeon may be guided to the position of the abnormal tissue by “wire localization,” in which a fine wire is inserted into the suspicious area and placement of the tip is confirmed by mammography. More recently, two minimally invasive biopsy methods have been developed for breast cancer diagnosis: fine-needle aspiration (FNA) for cytology and core-needle biopsy (CNB) for pathological assessment of breast tissue (Fajardo and DeAngelis, 1997; Morrow, 1995; Scott and Morrow, 1999; Sneige, 1991). A very thin needle and syringe can be used to remove either fluid from a cyst (standard FNA) or clusters of cells from a palpable mass (fine-needle aspiration biopsy [FNAB]). It is most frequently used to confirm a cyst by removing the cyst fluid. If the mass proves to be solid or only partially filled with fluid, the needle is quickly pushed back and forth through the solid tissue while suction is applied to free some cells, which are aspirated and analyzed by a cytopathologist. The sensitivity of FNAB cytology depends on the quality of the sample obtained and the experience of the cytologist, but it generally falls in the range of 65 to 98 percent (Scott and Morrow, 1999). Unfortunately, the ability to obtain samples of sufficient size from nonpalpable lesions is limited, and the retrieval of such samples can be done only with imaging guidance (ultrasound or mammography). Thus, FNA is not as useful in such instances (Pisano et al., 1998b). Other limitations of this procedure are that it cannot distinguish between invasive cancer and in situ lesions, and it may produce inconclusive findings that require an additional core or open biopsy procedure.
CNB, although more traumatic for the patient, affords a higher rate of sensitivity. CNB uses a larger needle with a special cutting edge. The needle is inserted, under local anesthesia, through a tiny incision in the skin, and 5 to 10 small cores of tissue are removed. Tissue cores obtained
Page 38
by CNB are usually sufficiently large to allow pathologists to distinguish between invasive and noninvasive types of breast cancer. For nonpalpable lesions, CNB must be combined with an imaging modality (X ray, ultrasound, or less frequently, MRI) to target the suspicious tissue. The choice of guidance method depends on the experience of the radiologist or surgeon, the equipment available, and the type of lesion.
Novel CNB systems are also being developed with the goal of improving accuracy (Velanovich et al., 1999; Wong et al., 2000). One example is a vacuum-assisted biopsy instrument, also known as a Mammotome, in which suction is applied to the tissue while CNB is performed. This results in more tissue being drawn into the needle. In one study that compared standard stereotactic CNB with vacuum-assisted biopsy in patients with microcalcifications, CNB missed calcifications in nearly 10 percent of the patients, but vacuum-assisted biopsy did not miss calcifications in any of the 106 patients (Meyer et al., 1997). The Advanced Breast Biopsy Instrument (ABBI) is an example of a new system for removal of a larger core biopsy specimen than is possible with a Mammotome or by traditional CNB. The ABBI method uses a rotating circular knife and a thin wire heated with an electrical current to remove a large cylinder of tissue containing the abnormality.
LIMITATIONS OF CURRENT TECHNOLOGIES
Despite its demonstrated ability to detect breast cancer early and reduce disease-specific mortality rates to some degree, mammography has inherent limitations and risks like any cancer screening technology (including physical examination). Because the sensitivity and specificity are not 100 percent, screening programs will necessarily produce some false findings, both false-positive and false-negative findings, which can have detrimental effects on the screened population. Furthermore, identification of breast cancer by screening mammography does not guarantee that a woman will not die of breast cancer. Even small tumors may develop the ability to metastasize, and in these cases, early detection and treatment will not necessarily produce a cure. Some cancers will also rapidly develop during the period between screenings (termed interval cancers), and such aggressive tumors may not be amenable to treatment.
False-Positive Results
One limitation with the current technology for mammography is the high rate of false-positive results, which are abnormal findings in patients who are subsequently found to be free of breast cancer. To avoid large numbers of false-positive results, the specificity of a test must reach 99
Page 39
percent or more, but most screening tests for cancer have much lower specificities. For published reports on mammography, specificities generally fall in the range of 90 to 98 percent (Mushlin et al., 1998). The risk of having a false-positive result by mammography during routine yearly screening may be as high as 10 percent (Brown et al., 1995). One study suggests that among women who receive annual mammograms for 10 years, half will have at least one false-positive result that leads to additional tests such as diagnostic mammography, ultrasound, or biopsy (Christiansen et al., 2000; Elmore et al., 1998a). Currently, as many as three-fourths of all biopsy specimens turn out to be benign lesions. As acceptance and use of mammographic screening become more widespread, the increasing number of false-positive results becomes a cause for concern.
Short-term studies have shown that abnormal mammograms negatively affect a woman's psychological and emotional state (Lowe et al., 1999). Even when further evaluation rules out cancer, some women report impaired moods and daily functioning for up to 3 months after a suspicious finding on a mammogram (Lerman et al., 1991). A study in Norway examined perceptions of quality of life 18 months following a false-positive mammogram. Most women in that study regard their experience with a false-positive mammogram as one of the many minor stressful life incidences, with only a temporary decrease in quality of life (Gram et al., 1990). However, women with false-positive mammograms also experience heightened levels of concern about breast cancer (Lowe et al., 1999; Gram et al., 1990). Previously, it was commonly believed that fear could prevent women from returning for a second screening following a false-positive result. However, a recent study showed that women with false-positive mammograms, especially those who had no previous mammograms, were actually more likely to come in for their next scheduled visit (Burman et al., 1999; Pisano et al., 1998a).
The medical procedures that are necessary after a suspicious mammogram have additional consequences, both physical and financial. Follow-up work to an initial screening test can include diagnostic mammograms, ultrasound examinations, and needle or surgical biopsies. Pain and reduced sexual sensitivity due to surgical biopsy are possible side effects (Gram et al., 1990). Lost productivity as a result of time off for surgery and recuperation is an additional cost. Retrospective studies show that the additional costs of evaluating false-positive results can add up to one-third of the total cost of screening for all women (Elmore et al., 1998a; Lidbrink et al., 1996). Furthermore, scarring of the tissue following surgical biopsy may result in cosmetic concerns and could potentially interfere with subsequent cancer detection. However, improvements in biopsy techniques have led to smaller and less invasive procedures and have thus reduced some of these concerns.
Page 40
False-Negative Results
No screening or diagnostic technology is perfect, and thus, some false-negative results are inevitable. A normal mammogram does not guarantee that a woman is free of breast cancer because some tumors are not detected by mammography. The sensitivity of screening mammography (ability to detect occult cancer) ranges from 83 to 95 percent in published studies (Mushlin et al., 1998). Failure to detect breast cancer can generally be attributed to one of four main reasons: inherent limitations of mammography, inadequate radiographic technique, subtle or unusual lesion characteristics, and errors of interpretation. A number of studies have shown that a significant portion of breast cancers detected at follow-up mammography are visible in retrospect on the previous mammogram that was interpreted as normal (Harvey et al., 1993; van Dijck et al., 1993; Warren Burhenne et al., 2000). Regardless of the cause, a false-negative finding on a mammogram can be quite harmful to the woman whose cancer has been missed. Normal findings on a mammogram may produce a false sense of security that could prevent women from seeking appropriate medical attention, even for symptomatic lesions. A delay in diagnosis will delay treatment, perhaps in some cases to the point where treatment will no longer be effective because the tumor has had sufficient time to progress and metastasize. Because of these potential dire consequences associated with false-negative findings, the number of medical malpractice lawsuits stemming from missed cancer diagnoses has increased considerably since screening programs were widely introduced. In fact, a recent report suggests that lawsuits alleging a missed or delayed breast cancer diagnosis are now the most prevalent of all medical malpractice suits filed against radiologists and physicians in general (Berlin, 1999; Physicians Insurers Association of America, 1995, 1997) (see Chapter 6).
Lack of Data for Older Women
The risk of breast cancer increases with age throughout a woman's lifetime, and the sensitivity and positive predictive value of screening mammography also improve as women age (Kerlikowske et al., 1993; Mushlin et al., 1998) (Table 1-4). However, few data are available on the benefits of screening mammography in women age 70 and older, and thus, uniform recommendations do not exist for women in this age group. The efficacy of mammography in older women is unknown because only two randomized controlled trials included women over age 65, and the numbers were too small too provide meaningful results (Table 1-2). Furthermore, screening of some older women may be less beneficial and cost-effective because of their shortened life expectancies compared with those
Page 41
|
Age |
Probability |
|
by age 30 |
1 out of 2,525 |
|
by age 40 |
1 out of 217 |
|
by age 50 |
1 out of 50 |
|
by age 60 |
1 out of 24 |
|
by age 70 |
1 out of 14 |
|
by age 80 |
1 out of 10 |
SOURCE: National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program, Publication 97-3536, 1997.
for women in younger age groups. When a woman has serious comorbid conditions that are life limiting and would deter intervention if a tumor were discovered, screening mammography would not be helpful and is generally not recommended. However, because of the lack of data, it is difficult to determine who is likely to benefit from screening, and thus, the decision is often made on an individual basis. Recently, a retrospective cohort study among women ages 66 to 79 years suggested that some women in this age group might benefit from the continuation of screening mammography. Results indicated an increased probability for detecting localized breast cancer in conjunction with a significantly reduced risk for detecting metastatic breast cancer among screened women (Smith-Bindman et al., 2000). A case-control study from Holland found that regular screening mammography for women ages 65 to 75 was associated with a 55 percent reduction in mortality from breast cancer, although there was no reduction in breast cancer mortality associated with screening of women over age 75 (Van Dijck et al., 1996). Another recent study suggests that for women over age 70, screening mammography may be most beneficial and cost-effective for individuals with higher bone mineral density, a characteristic associated with a higher risk for breast cancer (Kerlikowske et al., 1999).
Challenges in Younger Women
Breast cancer screening for women under age 50 remains controversial (Lerner, 2001). Some studies have reported a survival benefit of mammography, and many organizations advocate regular screening in this age group, but questions have been raised as to whether the benefits outweigh the risks and costs of screening younger women (Table 1-5). As a result of the controversy, some effort has been made to develop guidelines based on risk to help women make individual decisions about when
Page 42
|
Trial |
Start Date |
Age at Entry (years) |
Follow-up Period (years) |
RR (95% CI) |
|
HIP |
1963 |
40-49 |
10 |
0.77 (0.50-1.16) |
|
Malmö I |
1976 |
45-49 |
14 |
0.67 (0.35-1.27) |
|
Malmö II |
1978 |
45-48 |
12 |
0.69 (0.44-1.09) |
|
Two-County: |
||||
|
Östergötland |
1977 |
40-49 |
13 |
1.02 (0.52-1.99) |
|
Kopparberg |
1977 |
40-49 |
13 |
0.73 (0.37-1.41) |
|
Stockholm |
1981 |
40-49 |
11.4 |
1.08 (0.54-2.17) |
|
Gothenburg |
1983 |
39-49 |
12 |
0.56 (0.32-0.98) |
|
Edinburgh |
1978-82 a |
45-49 |
10-14 |
0.73 (0.43-1.25) |
|
Canada NBSS I |
1980 |
40-49 |
10.5 |
1.14 (0.83-1.56) |
NOTE: RR, relative risk; CI, confidence interval.
aInitial randomization was 1978; additional women ages 45-49 years were randomized starting in 1982.
SOURCE: adapted from Kerlikowske (1997).
Page 43
to begin screening (Gail and Rimer, 1998). It was beyond the charge of the present committee to revisit the issue of whether women under age 50 should undergo routine screening mammography, so the discussion in this report will simply review some of the issues behind the controversy, with an emphasis on how technology development may overcome some of the difficulties associated with the screening of women in this age group.
Statistical analysis of pooled data from seven randomized clinical trials indicated that screening mammography reduced breast cancer mortality by about 16 to 18 percent in women under age 50 (Berry, 1998; Kerlikowske 1997; National Institutes of Health, 1997). However, the lag time between initiation of screening and clear demonstration of a reduced mortality was more than 10 years, whereas it was about 5 years for women over age 50. The lower absolute risk of cancer among women under age 50 implies that even if relative mortality benefits were equal for women under and over age 50, absolute risk reduction would remain considerably lower for younger women. This disparity would not be corrected by improved screening technology or adjustment of screening intervals (Sirovich and Sox, 1999). Furthermore, because breast cancer is less common among women under age 50 than among older women, more individuals need to be screened to identify a case of occult breast cancer or to prevent a death from breast cancer.
One of the difficulties with screening women in their 40s is that most such women are premenopausal and are therefore likely to have greater breast density than postmenopausal women, whose breast tissue often (but by no means always) contains a higher percentage of fatty tissue. (However, postmenopausal women on estrogen replacement therapy may have similar difficulties [Laya et al., 1996], and the number of women on such therapy has been increasing.) This tissue density can make mammograms more difficult to interpret and can thus lead to missed diagnoses, as well as increased rates of false-positive findings (resulting in unnecessary biopsies). One study found that the accuracy of screening mammography in premenopausal women varies with the phase of a woman's cycle at the time of screening (White et al., 1998), suggesting that accuracy might be improved by scheduling the mammogram during a particular phase (the follicular phase during the first 2 weeks of the cycle), but this is not standard practice at present. Physical examination (CBE and BSE) may also be impeded by dense breast tissue (Heimann et al., 1998).
Other screening modalities that are not affected by breast density might be helpful for the screening of women with dense tissue at any age, especially since there may actually be a correlation between breast density and cancer risk (Byng et al., 1998). A number of studies have been undertaken to test other technologies in this population, as discussed in more detail in Chapter 2.
Page 44
Younger women also tend to have a faster average cell growth rate, meaning that interval cancers may be more common and, thus, that screening may need to be conducted more frequently (e.g., annually or, among high-risk women, perhaps even semiannually) to be effective for women in this age group (Kerlikowske et al., 1996; Tabar et al., 1999).
These concerns associated with the screening of younger women are especially relevant to women at high risk. For example, women with inherited mutations in breast cancer susceptibility genes such as BRCA1 and BRCA2 are faced with the decision of choosing between prophylactic bilateral mastectomy or screening, often beginning at an earlier age than the general population (Burke et al., 1997). Women may also opt to participate in chemoprevention trials. Thus far, there are no definitive data to guide the decision-making process. Because of the limitations of mammography, especially for younger women, improved screening methods are seriously needed for this high-risk group. Several institutions are now studying whether alternate screening modalities, such as MRI or ultra-sound, may be more effective and cost-effective for this relatively small, specific group.
Radiation Sensitivity and Breast Cancer Screening
Since mammography was first introduced, some concerns have been raised about the potential risks associated with repeated exposure of the breast to ionizing radiation (i.e., X rays). There is no direct evidence of carcinogenic risk from mammography, but there is a hypothetical risk from screening because higher than normal rates of breast cancer have been noted in women with high-level radiation exposures to the breast that occurred from the 1930s to the 1950s as a result of exposure to atomic bomb radiation, multiple chest X rays, and radiation therapy treatments for benign disease or Hodgkin's lymphoma (Clemons et al., 2000; Feig and Hendrick, 1997). However, extrapolation of cancer risk from these very high radiation doses, which are unlike any dose a woman might receive from mammography, is difficult, if not impossible (Land, 1980), and most experts agree that the potential benefits of mammography outweigh the risks from radiation (Feig and Hendrick, 1997). Furthermore, technical improvements to mammographic methods over the years have greatly reduced the dose of radiation necessary to obtain quality mammograms.
Nonetheless, the risk of cancer following radiation exposure may not be uniform among all women. For example, a number of rare hereditary syndromes, usually diagnosed in children, are associated with cancer predisposition as well as sensitivity to ionizing radiation. Among these is ataxia telangiectasia (AT), a rare autosomal recessive disorder. It was observed by Swift and coworkers that mothers of children with AT devel-
Page 45
|
Study |
No. of Women |
Type of Breast cancer |
No. (%) of Women with AT mutations |
|
FitzGerald et al., 1997 |
202 |
Early (<40) onset |
2 (1) |
|
Broeks et al., 2000 |
82 |
Early (<45) onset |
7 (8.5)a |
|
Izatt et al., 1999 |
100 |
Early (<40) onset no FH |
0 |
|
Shafman et al., 2000 |
57 |
Contralateral |
0 |
|
Chen et al., 1998 |
88 |
FH |
3(3.4) |
|
Chen et al., 1998 |
100 |
FH |
1 (1) |
NOTE: FH, family history; AT, ataxia telangiectasia gene.
aSignificantly increased from population frequency.
oped breast cancer more frequently than predicted for the general population and that the breast cancers in these individuals were often associated with exposure to diagnostic radiation (Swift et al., 1991). Because 1 percent of the general population was predicted to be carriers of mutations of the AT gene, there was a concern that a large subset of women would be more susceptible to diagnostic radiographic procedures. Since the identification and cloning of the AT gene, a number of studies have been designed to address this important public health question. Of five studies conducted to date, only one reveals an increased risk for breast cancer in AT heterozygotes (Table 1-6).
In addition to these studies of early-onset or contralateral breast cancers occurring after radiation therapy, other study designs have thus far failed to reveal a significant role of AT heterozygosity as a risk factor for radiation-associated breast cancer. One of these studies included 52 patients with a second malignancy after receiving therapeutic radiation for Hodgkin's disease (Nichols et al., 1999). In addition, the adverse effects of radiation were not associated with AT mutations in 57 patients in two studies (Appelby et al., 1997; Shayeghi et al., 1998). Thus, the current literature does not support the theory that mutation of the AT gene is a major risk factor for radiation-induced breast cancer, although additional studies are needed.
Recent studies have also raised concerns regarding the radiation sensitivity of carriers of BRCA mutations. Initial reports showed that mice lacking the protein products of the BRCA1 and BRCA2 genes were extremely sensitive to ionizing radiation (Connor et al., 1997; Sharan et al., 1997). Recently, human tumor cell lines containing one normal copy and one mutated copy of the BRCA1 gene also showed many classical signs of radiation sensitivity (Foray et al., 1999). These results raise the possibility
Page 46
that BRCA mutations in humans may result in deleterious effects (due to the accumulation of radiation-induced mutations) in women exposed to ionizing radiation. However, the doses of gamma radiation used in these cell line experiments (in the range of 1 to 2 grays [Gy]) were far in excess of the doses that normal tissues receive during diagnostic irradiation (in the range of 1 rad, or 0.01 Gy). Further study is needed to address this issue.
Overtreatment of Early Lesions
New technologies do not merely detect breast cancer earlier, but they can also complicate the screening and treatment processes. Improvements in the sensitivity of breast imaging techniques have led to an increase in the identification of small abnormalities whose biology is not well understood. For example, many consider ductal carcinoma in situ (DCIS) to be a premalignancy because it appears cancerous but has not invaded surrounding tissues or metastasized (Figure 1-5).
The number of women diagnosed with DCIS has greatly increased since screening mammography was widely adopted. Between 1973 and 1983, DCIS accounted for only 0.3 to 5.2 percent of all cases of breast cancer diagnosed, depending on the age group. In contrast, between 1983 and 1992, DCIS constituted 12 to 18 percent of all newly diagnosed cases of breast cancer and may account for as much as 30 percent of breast cancer cases identified by screening mammography (Ernster et al., 1996). Among women ages 40 to 49, as many as 40 percent of all cases of breast cancer detected by mammography are DCIS (Ernster and Barclay, 1997). Although some DCIS lesions will develop into invasive cancers, there is no method for determination of whether a particular DCIS will develop into a life-threatening metastatic cancer. In fact, one study reported finding occult DCIS at autopsy in 40 percent of women between 40 and 50 years of age who died from other causes (Nielsen et al., 1987), although the incidence in other similar studies (6 to 18 percent) has not been quite so high (see Welch and Black [1997] and references therein).
Initially, DCIS was often treated by mastectomy, but more recently, lumpectomy (removal of tissue surrounding the lesion) followed by radiation has become more common, as it has with invasive breast cancer. However, the pattern of treatment varies greatly, and because the rate of DCIS detection has increased, thousands of mastectomies for DCIS are still performed each year (Ernster et al., 1996). In some cases, the treatment decision may be due to a patient's inaccessibility to facilities that provide radiation therapy (e.g., because of where a woman lives or because she lacks medical insurance).
Another type of noninvasive high-risk breast lesion, referred to as “lobular carcinoma in situ” (LCIS), is as perplexing or even more so than
Page 47
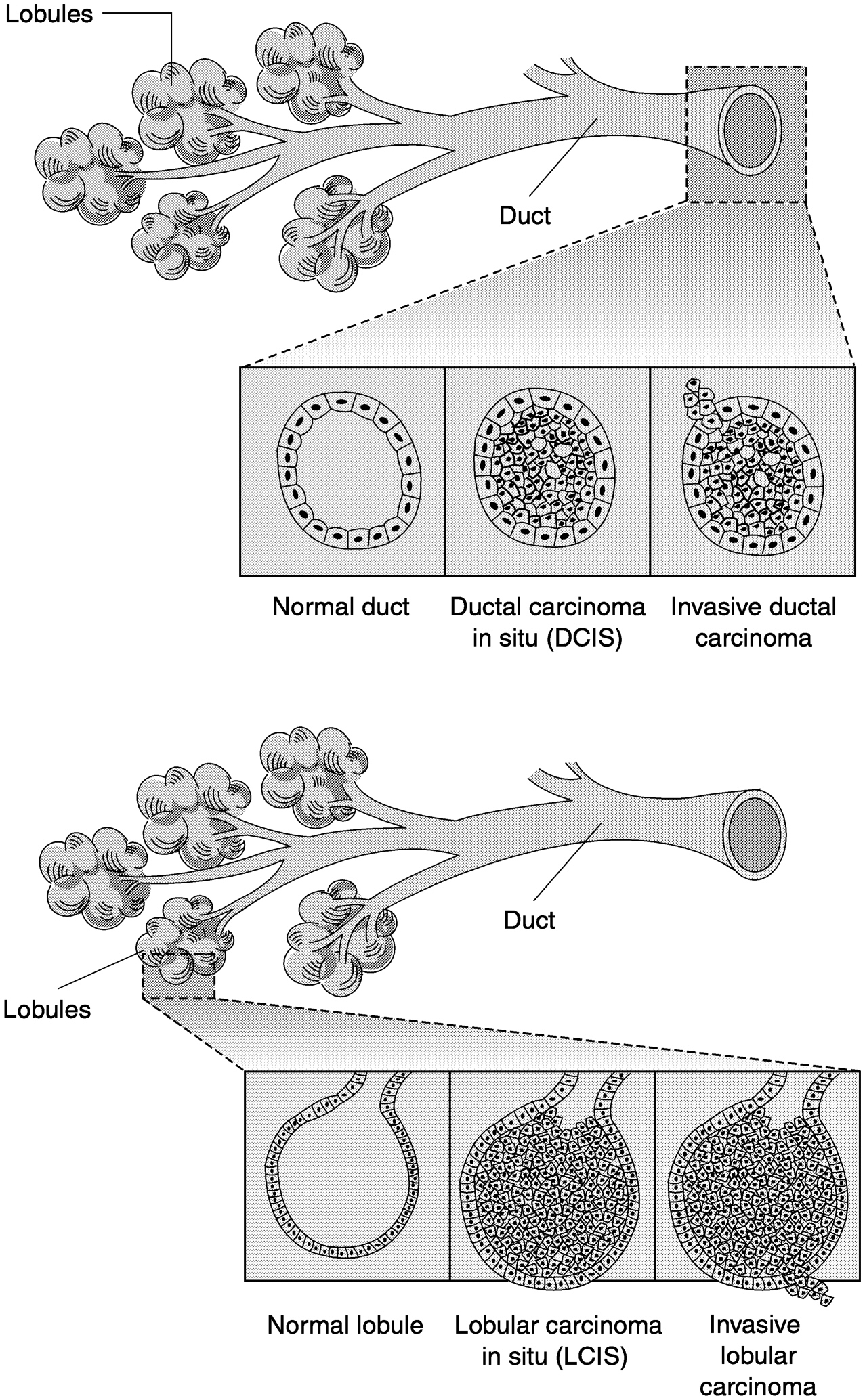
~ enlarge ~
FIGURE 1-5 Schematic representation of ductal (a) and lobular (b) carcinoma of the breast. SOURCE: adapted from Love (1995, p. 220).
Page 48
DCIS in terms of biological understanding and clinical management. Epidemiological studies have shown that patients with a history of LCIS are as likely as those diagnosed with DCIS to eventually develop invasive breast cancer (relative risk, ∼10 times that for age-matched controls) (Rosen et al., 1980; Rosen, 1981). Unlike DCIS, the risk associated with LCIS is bilateral, suggesting that LCIS may be a marker for rather than a precursor of invasive breast cancer. Furthermore, LCIS is often multifocal and bilateral, suggesting that it may arise in response to a carcinogenic “field defect.” Recent studies have unequivocally demonstrated that LCIS shares identical genetic defects with invasive cancer in the same breast (Lu et al., 1998; Nayar et al., 1997), consistent with the notion that LCIS may be both a marker for and a direct precursor to invasive tumors. LCIS is present in about 5 percent of breast biopsy specimens. It is almost always clinically occult and is encountered as an incidental finding in breasts biopsied for some other reason. Surgery is not considered an option for patients with LCIS because of its multifocal nature, and there is no universally agreed upon approach to the management of LCIS because it is not a true malignancy. In the recent chemoprevention trial conducted by the National Surgical Adjuvant Breast and Bowel Project, a history of LCIS was one of the enrollment criteria. There was a 50 percent reduction in the incidence of invasive breast cancer in the LCIS group receiving tamoxifen compared with the incidence in the group receiving a placebo, suggesting that tamoxifen may be reasonable therapy for patients with LCIS (Fisher et al., 1998a).
Increasing the ability to identify DCIS and LCIS raises important questions in regard to breast cancer screening. What are clinicians looking for, and what should they do when they find it? The biology of these small lesions and how to treat them are not as well studied, and research has been possible only since it became possible to detect them, so clarity about optimal treatment will take many years to develop.
DILEMMA OF “EARLIER” DETECTION
The efforts to develop technologies capable of pushing back the detection timeline to a stage that is even earlier than what is currently possible could provide new opportunities for early intervention, but such opportunities could also increase the difficulties associated with “over-diagnosis.” The underlying assumption in promoting early detection held by many investigators, most physicians, and almost all members of the lay population is that early detection provides an opportunity to reverse the malignancy process more effectively and perhaps with less toxicity than if the condition were identified later. The notion that earlier detection is better requires the following three assumptions: (1) that development of carcinoma of the breast proceeds through a relatively orderly
Page 49
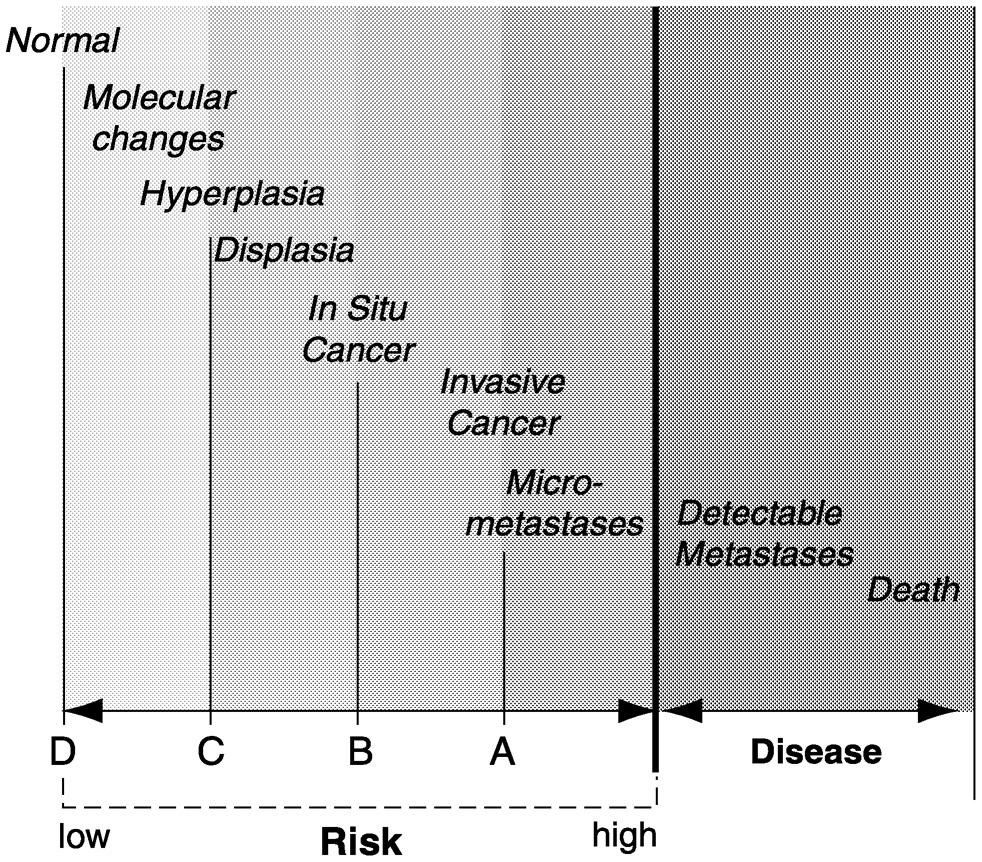
~ enlarge ~
FIGURE 1-6 Hypothetical illustration of breast cancer initiation and progression. Although most patients and physicians would consider a histological diagnosis of breast cancer, even when it is totally in situ, a “disease,” a more precise definition of disease is “a condition that causes morbidity and mortality.” In this sense, breast cancer is only a disease when one of these two conditions exists. Morbidity and mortality occur almost exclusively in the setting of clinically detectable metastases. Thus, all other stages of breast abnormalities are shown here as having variable levels of risk for the development of morbidity and mortality (i.e., disease). For more detail, refer to the section Dilemma of “Earlier” Detection in the text.
series of steps (Figure 1-6); (2) that this process includes an irreversible checkpoint (which may vary for different patients) and that once an individual is past this checkpoint the process steadily progresses so that only some sort of external intervention can prevent the ultimate inevitability of morbidity and mortality; and (3) that detection of this process before establishment of metastatic deposits will permit external intervention to prevent disease-specific morbidity and mortality more efficiently than after metastases are established. These points of detection are illustrated as A, B, C, and D, respectively by the vertical lines on Figure 1-6.
The fundamental support for these assumptions comes from the randomized clinical trials described earlier in this chapter, which showed that mammography screening of asymptomatic women results in early treatment of breast cancers that are detected and reduces disease-specific mortality by approximately 20 to 30 percent compared with that for women who have not been screened.11 Why is the decrease in mortality
Page 50
not greater? There are several possible answers to this question. Simply put, screening mammography is insufficiently sensitive in two ways: (1) it does not detect all cancers at point B, and (2) it does not detect any cancers at point C. One might argue that improvements in imaging technologies might allow clinicians to increase the number of tumors detected at point B or even move detection to point C. However, a second reason that reductions in breast cancer mortality rates due to screening mammography are limited might be embedded in the biology of the cancer itself. Some cancers may metastasize at a time well before point B on the breast cancer continuum, whereas others may do so much later. Thus, the question of whether detection is early enough to allow effective intervention may vary from one woman to the next.
The assumptions presented above raise several considerations. A distinction should be made between screening for an asymptomatic but precise condition that is considered undesirable and establishing that the individual is at risk for such a condition but does not yet and may never have it. Indeed, taken to its extreme, this distinction is blurred and perhaps even abolished in the definition of “disease.” For example, most patients and physicians would consider a histological diagnosis of breast cancer, even when it is totally in situ, a “disease.” However, a more precise definition of disease is “a condition that causes morbidity and mortality.” In this sense, breast cancer is only a disease when one of these two conditions exists. Morbidity and mortality occur almost exclusively in the setting of clinically detectable metastases.
Before the increased awareness of breast cancer and adoption of screening mammography, almost all patients who presented with breast cancer did so because the condition was at a stage at which it was truly a disease: it caused symptoms or even death. However, in more modern times, most patients are diagnosed with asymptomatic breast cancer. In this setting, almost all treatment for breast cancer (surgical removal of the primary tumor, local-regional radiation to sterilize the area, and adjuvant systemic therapy) could be considered prophylactic or preventive. Such treatments are applied to reduce the chances that the patient will develop morbidity or mortality (or “disease”). A substantial body of literature describing the results of randomized clinical trials demonstrates that each of these strategies does, in fact, reduce to some extent the individual's chances of developing morbidity or mortality from breast cancer.
Why are these distinctions important? No single condition, other than actually having symptomatic disease, carries a 100 percent chance (or risk) of developing what is designated, in this discussion, disease. How-
Page 51
ever, as one moves from left to right on the theoretical breast cancer continuum illustrated in Figure 1-6, an individual's chances of ultimately developing morbidity and mortality increase. Estimating this risk is quite difficult because, at least in the last century, few patients were left untreated once they had a diagnosis of “breast cancer,” and therefore, it is impossible to determine what percentage of patients, if any, might never have progressed to experience morbidity or mortality from breast cancer if they were left untreated.
However, one might extrapolate from certain experiences to suggest that among women at a given point in the continuum, not all will progress to the next or subsequent steps. For example, a number of pathological features such as the presence of axillary lymph nodal micrometastases are known to identify women more likely to develop morbidity and mortality after local-regional therapy. However, 20 to 30 percent of node-negative patients subsequently develop symptomatic, incurable metastases, and up to 25 percent of node-positive patients do not (Cocconi, 1995; Weidner, 1995). More recently, clinical investigators have reported that detection of distant micrometastatic breast cancer cells in circulation or even bone marrow is associated with a poorer prognosis (Braun et al., 2000; Pantel et al., 1999). However, a substantial fraction of these “positive” patients also survive without systemic therapy. Other molecular features, such as hormone receptor content, measures of proliferation, and HER-2 gene amplification, may help to fine-tune prognostic estimates, but they are far from absolute in dividing a population into those who are destined to develop “disease” and those who are not.
DCIS presents an even greater dilemma than early invasive cancers. DCIS hypothetically lies to the left of invasive cancer on the theoretical continuum illustrated in Figure 1-6. Since DCIS does not contain cells that have invaded the surrounding tissue layer, it should not be associated with metastatic cells or a very high, if any, risk of developing morbidity and mortality. Therefore, in theory, DCIS would be the preferred lesion to detect by screening (rather than invasive cancer). However, it is not known what percentage of breast cancers pass through the DCIS phase. In other words, do some breast cancers progress from left to right in Figure 1-6 with only a brief sojourn as DCIS, or do some perhaps skip it completely? Moreover, few patients who have been diagnosed with this condition have ever been studied or followed without excising of the lesion. Therefore, it is not known whether some or many in situ breast cancers would never progress beyond this phase. Indeed, it is likely that a certain proportion of these lesions might remain as in situ cancer or even regress to a less worrisome histologic state. Nonetheless, recent randomized clinical trials have suggested that women with excised DCIS who do not receive further local (surgery, radiation) or systemic (hormonal) therapy are more likely to suffer a recurrence of cancer in the breast with
Page 52
either the same diagnosis or a more advanced diagnosis (further to the right on the continuum illustrated in Figure 1-6), such as invasive breast cancer (Fisher et al., 1998; Hetelekidis et al., 1999; Silverstein and Lagios, 1997).
In summary, current definitions of breast cancer, as determined by histopathology of biopsy specimens taken from the breast, are only imperfect surrogates of whether patients will develop true disease, as defined here. A diagnosis of anything other than “normal” or “benign” breast tissue places the patient into a higher risk category, whereas treatment with surgery or with radiation or systemic therapy places the patient in a lower risk category. Treatment in this setting is always applied inefficiently because there is no effective way of determining who will benefit from it. Thus, many women receive “preventive” therapy, even though they would never develop metastatic disease, in order to reduce the risk of morbidity and mortality for those who would. Preventive therapy is less efficient the further to the left on the breast cancer continuum in Figure 1-6 one goes. As a woman's condition moves farther to the right on the continuum, women are more willing to accept previously unacceptable preventive therapies because they perceive their risk of death from breast cancer to be higher.
SUMMARY
X-ray mammography is now the mainstay for the detection, diagnosis, and localization of breast cancers. It is currently the only medical imaging procedure used as a screening tool. Screening mammography has definitively been shown to reduce, but not eliminate mortality from breast cancer when it is performed at regular intervals and followed by appropriate interventions. In randomized clinical trials, screening mammography reduced breast cancer mortality by about 25 to 30 percent among women ages 50 to 70 and by about 18 percent among women between the ages of 40 and 50. Although the incidence of breast cancer increases with age, data on the benefits of screening for older women are lacking because most randomized trials excluded women over age 70. Recent observational studies suggest that mammography is also beneficial for women over age 70, but further documentation of benefit is important. There is also some indirect evidence that screening mammography has been effective in reducing the number of deaths from breast cancer in the general population. Mortality from breast cancer in the United States, as well as some European countries, has been decreasing in recent years, and some of this reduction is consistent with the effect of screening.
However, there is clearly room for improvement in the screening and diagnosis of breast cancer because of both the technical and the biological
Page 53
limitations of the current methods. Although the clinical practice of mammography is federally regulated for quality assurance (the only clinical procedure regulated in this way), it is still technically difficult to consistently produce mammograms of high quality, and interpretation is subjective and can be variable among radiologists. Furthermore, mammography does not detect all breast cancers, including some that are palpable, and as many as three-quarters of all breast lesions biopsied because of a suspicious finding on a mammogram turn out to be benign. Mammograms are particularly difficult to interpret for women with dense breast tissue, who are at increased risk of breast cancer. The dense tissue interferes with the identification of abnormalities associated with tumors. This leads to higher rates of false-negative and false-positive findings in these women. In addition, optimal screening intervals are poorly defined because some tumors develop too quickly to be identified at the current screening intervals, especially in younger women.
The current limitations of mammography and other existing technologies (as described in this chapter) have been driving forces behind the efforts to improve mammography and other diagnostic techniques and to develop additional novel methods for the early detection of breast cancer. The purpose of the study presented in this report is to examine some of the many technologies under development (Chapters 2 and 3) and to identify potential impediments to the development of new breast cancer screening and diagnostic procedures (Chapters 4, 5, and 6). Many factors can influence the development, adoption, and use of medical technologies, including the availability of research funds, the regulatory approval process, coverage and reimbursement decisions, acceptability to the target population, and difficulties in ensuring broad access. Although many technologies described in this report are at a relatively early stage of development and it is difficult to predict their ultimate value or use, they all must clear many hurdles if they are to become part of the standard of care for women and thus play a role in reducing the toll of breast cancer.
In assessing new technologies, three distinct goals regarding early detection, listed below, need to be considered and addressed. These are revisited frequently throughout the remaining chapters.
1. Identification of a higher percentage of women with an early stage of breast cancer. This aim could be achieved by improving the accuracy (sensitivity and specificity) and accessibility of mammography and also by developing other technologies that can identify cancers that are often missed by mammography. This is the goal of many of the technologies described in Chapter 2.
2. Development of technologies that can detect early changes before the appearance of a true malignancy that increase a woman's risk of de-
Page 54
veloping invasive or metastatic breast cancer. A number of potential technologies could accomplish this aim, as described in Chapters 2 and 3. However, as these develop, the specificity (that is, the magnitude to which a woman's risk is increased as a result of having a positive test) must be considered. Women may or may not consider a given magnitude sufficient to accept a given preventive strategy. Thus, it is important that investigators and physicians not assume that if a new technology identifies a breast abnormality, even one that appears, histologically, to represent cancer, it must move a patient to the right on the cancer continuum (as illustrated in Figure 1-6). In the absence of data, such an assumption would unfairly lead a patient to accept therapies that she previously believed were unacceptable.
3. Discover more acceptable and effective means of risk reduction for women at early points along the continuum of breast cancer initiation and progression. Research on the biological processes that increase a woman's risk may also lead to new intervention strategies directed toward those processes.12 An underlying context of this discussion of early detection is that new, more acceptable preventive strategies could be applied more widely and efficiently. That is, a therapy with little or no toxicity or adverse consequences would be much more acceptable to women with only a low or a moderate risk.
In the meantime, enthusiasm for new technologies should be tempered by consideration of the ultimate goal: to reduce the morbidity and mortality from breast cancer among women. It is important to keep in mind that the ability to move toward detection at an earlier point in the continuum of abnormalities does not necessarily mean that further progress toward decreasing disease-specific morbidity and mortality will occur. It is also essential to understand what is being detected and how to appropriately intervene. Decisions about the use of new technologies should be firmly grounded in scientific evidence if investigators are to optimize the benefits and minimize the risks of early breast cancer detection.