Page 55
2
Breast Imaging and Related Technologies
Medical imaging is central to breast cancer screening, diagnosis, and staging. Mammography is the most sensitive technique available for the detection of nonpalpable breast lesions, and thus, screening mammography has secured a routine place in health maintenance for women in the United States. Although it is less than perfect, screening mammography can reduce breast cancer mortality when combined with appropriate interventions (see Chapter 1).
Conventional X-ray mammography is a mature technology that provides high-quality images at low radiation doses in the majority of patients. However, conventional film-based mammography may not provide adequate diagnostic information for some women with radiodense breast tissue. It has been estimated that this technology misses about 15 percent of breast cancer lesions (Mushlin et al., 1998). In addition, studies have reported that the positive predictive value1 of conventional mammography ranges only from 15 to 40 percent (Kerlikowske et al., 1993; Kopans, 1992; Kopans et al., 1996). Consequently, 60 to 85 percent of lesions detected by mammography are benign, and thus, many biopsies could potentially be avoided. This situation creates an important incentive for the development of novel technologies to improve detection, diagnosis, and staging and monitoring of treatment for breast cancer.
Accordingly, other imaging technologies, particularly nonionizing
Page 56
modalities such as magnetic resonance imaging and ultrasound, are being tested for application to breast cancer, with promising results. At present, these methods may provide additional diagnostic specificity over X-ray mammography alone. Additional tools such as scintimammography, positron emission tomography, magnetic resonance spectroscopy, and optical imaging are under investigation as well. To date, no single imaging method appears to offer both high sensitivity and high specificity for the detection and diagnosis of breast cancer.
The previous chapter summarized the main technologies in current use for breast cancer detection, whereas this chapter looks more closely at imaging modalities under development (Tables 2-1 and 2-2). The various technologies can roughly be divided into three categories: (1) those that are currently in use, such as X-ray mammography and ultrasound, but that are being further refined; (2) those that are commonly used for medical imaging, such as magnetic resonance imaging (MRI), but that are still experimental with regard to breast cancer detection; and (3) and novel imaging modalities that may be used in the future. A 1996 report, The Mathematics and Physics of Emerging Biomedical Imaging, explains the technical background of many of these promising new technologies in greater detail than is possible here (Institute of Medicine, 1996).
The chapter describes the current state of the art as well as technological roadblocks associated with promising near-term imaging technologies. Potential longer-term solutions using alternative modalities, such as optical or microwave imaging, are also briefly addressed. In addition, this chapter describes how novel technologies may affect breast cancer detection in ways beyond image acquisition, including image processing, display, management, storage, and transmission. Common to all imaging systems is the increasing use of digital methods for signal processing, which also offers the possibility of computer-aided detection by texture analysis and pattern recognition.
FUNDAMENTALS OF IMAGING ANALYSIS
Breast imaging technologies are being developed with three distinct goals in mind: (1) to identify abnormal tissues, (2) to localize the abnormalities within the breast to facilitate further examination or treatment, and (3) to characterize the abnormalities and aid the decision-making process following identification. An ideal imaging modality would accomplish all three goals in a single use, but in reality, most current technologies cannot achieve this, so developers tend to focus on optimizing one goal at a time. In addition to these technical goals, developers hope to generate detection methods that are more practical, inexpensive, harmless, and appealing to the patient than current methods.
Many of the current medical imaging methods are used to map struc-
Page 57
| TABLE 2-1 Current Status of Imaging and Related Technologies Under Development for Breast Cancer Detection | |||
|
Current Status |
|||
|
Technology |
Screening |
Diagnosis |
FDA approved for breast imaging/detection |
|
Film-screen mammography (FSM) |
+++ |
+++ |
Yes |
|
Full-field digital mammography (FFDM) |
++ |
++ |
Yes |
|
Computer-assisted detection (CAD) |
++ |
o |
Yes |
|
Ultrasound (US) |
+ |
+++ |
Yes |
|
Novel US methods (compound, three-dimensional, Doppler, harmonic) |
o |
o |
No |
|
Elastography (MR and US) |
o |
o |
No |
|
Magnetic resonance imaging (MRI) |
+ |
++ |
Yes |
|
Magnetic resonance spectroscopy (MRS) |
−/o a |
+/o a |
No |
|
Scintimammography |
o |
+ |
Yes |
|
Positron emission tomography (PET) |
o |
o |
Yes |
|
Optical imaging |
o |
+ |
No |
|
Optical spectroscopy |
− |
o |
No |
|
Thermography |
o |
+ |
Yes |
|
Electrical potential measurements |
o |
+ |
No |
|
Electrical impedance imaging |
o |
+ |
Yes |
|
Electronic palpation |
o |
NA |
No |
|
Thermoacoustic computed tomography, microwave imaging, Hall effect imaging, magnetomammography |
NA |
NA |
No |
NOTE: This table is an attempt to classify a very diverse set of technologies in a rapidly changing field and thus is subject to change in the near future.
aEx vivo analysis of biopsy material/in vivo MRS.
Current Status Explanation of Scale
|
— |
Technology is not useful for the given application |
|
NA |
Data are not available regarding use of the technology for given application |
|
o |
Preclinical data are suggestive that the technology might be useful for breast cancer detection, but clinical data are absent or very sparse for the given application. |
|
+ |
Clinical data suggest the technology could play a role in breast cancer detection, but more study is needed to define a role in relation to existing technologies |
|
++ |
Data suggest that technology could be useful in selected situations because it adds (or is equivalent) to existing technologies, but not currently recommended for routine use |
|
+++ |
Technology is routinely used to make clinical decisions for the given application |
tural or morphological differences in tumors, such as microcalcifications, tissue masses, angiogenesis, asymmetry, and architectural distortion. Some of the more recently developed techniques can provide information about the biological or functional differences between tumors and normal tissues (Glasspool and Evans, 2000; Hoffman and Menkens, 2000). Such information is critical for making the “quantum leap” in fully achieving
Page 58
|
Technology |
Description, Mechanism |
|
Full Field Digital Mammography (FFDM) |
Detector responds to X-ray exposure, sends electronic signal to computer to be digitized and processed. Separates detector and image display. |
|
Computer-Aided Detection and Diagnosis (CAD) |
Computer programs to aid in identification of suspicious mammograms and classification as benign or malignant. Serves as a second opinion to radiologists. |
|
Ultrasound |
Use of high-frequency sound waves to generate an image. |
|
[New ultrasound technologies, in early stages of development] Compound imaging: uses several ultrasound beams that strike the tissue from different angles. Significantly reduces speckle and improves contrast and definition of small masses and microcalcifications. May cause reduction in display of some masses. Three-dimensional ultrasound imaging: permits display of a volume of tissue rather than a single slice. Examination of tumor volume and changes in tumor size over time. |
|
Page 59
|
Stage of Development |
Potential Strengths |
Current Limitations |
|
General Electric's Senographe 2000 D has FDA approval for use as both hard-copy and soft-copy displays. Studies are under way to compare FFDM with FSM. |
Ability to manipulate contrast and magnification with one exposure. Ease of image storage and retrieval. Facilitates CAD, digital tomo-synthesis, and telemammography. |
Spatial resolution and luminance of digital display are lower than those for FSM. Old film screens difficult to digitize for comparisons. Cost may be prohibitive. |
|
R2 Technology, Inc. has a program on the market. General Electric has agreement with R2 Technologies to use GE FFDM machine with R2's CAD system. |
Retrospective studies show that CAD can improve radiologists' readings and improve rate of false-negative results. |
CAD used alone has very low specificity. Sensitivity and specificity are undetermined for general screening population. |
|
Currently used as follow-up to mammography, to determine if lesion is a cyst or solid mass, or to characterize or localize a mass |
Studies suggest potential for increased use in diagnosis and perhaps even screening, especially for women with dense breasts. |
Poor ability to detect microcalcifications due to speckle. Compound imaging may help reduce speckle. |
|
Three-dimensional and power Doppler imaging: use of Doppler technology may allow assessment of tumor vascularity; it is potentially useful for predicting biological activity and predicting responses to treatment. Can be coupled with contrast agents. Ultrasound elastography: uses information from ultrasound signal to generate images showing elastic properties of tissue. Detects differences in tissue stiffness and may detect features not visible with mammography or conventional ultrasound. |
||
Page 60
|
Technology |
Description, Mechanism |
|
Magnetic Resonance Imaging (MRI) |
Image generated by signals from excitation of nuclear particles in a magnetic field. Breast tumors show increased uptake of contrast agent. |
|
[Other MRI technologies under development.] Minimally invasive prognosis and therapy monitoring: different cancer types that display distinct MRI enhancement characteristics may be important as prognostic indicators. |
|
|
MR Spectroscopy (MRS) |
Use of magnetic resonance spectra and “functional” molecular markers to measure biochemical components of cells and tissues. |
|
Scintimammography |
Image created with radioactive tracers, which concentrate more in cancer tissues than in normal tissues. Measures spatial concentration of radio-pharmaceuticals to generate planar or three-dimensional images by SPECT. |
Page 61
|
Stage of Development |
Potential Strengths |
Current Limitations |
|
National Cancer Institute trials are under way to study three-dimensional high-resolution and dynamic contrast MRI in conjunction with mammography. Completion by 2001. |
Benefits in detection: • detection of multiple malignancies • detection of invasive lobular carcinoma • screening for high-risk women with dense breasts • detection of recurrent cancers |
Lack of uniform interpretation criteria. Cannot reliably detect microcalcifications and small tumors, especially if they do not pick up the contrast agent. Overlap in uptake time course of benign and malignant tumors. |
|
“Smart” MRI contrast agents: agents “activated” by biochemical processes are then detected by MRI; can correlate cell functions with disease state, and can track cell growth and behavior. Limited by identification of appropriate markers and lack of clinical data. Pursued commercially by Metaprobe in Pasadena, CA. MR Elastography: image elastic properties of tissue. |
||
|
Studied as potential adjunct to mammography, fine needle aspirates, and assessment of lesions in vivo. |
MRS spectra of samples mayincrease accuracy of FNA analysis. Potential noninvasive method of characterizing lesions. |
High cost and low sensitivity and specificity for detection of small lesions. |
|
MIBI approved by FDA. Other radioactive compounds being studied. Used as adjunct to mammography to localize tumors, distinguish malignancies versus benign lesions, and identify metastatic cells in distal regions of the body. |
MIBI scans unaffected by dense tissue, implants, or scarring. Used when mammograms are indeterminate; can avoid the need for follow-up mammograms. High-resolution scinti-mammography uses a gamma camera and may improve resolution. Potential for SPECT monitoring of multidrug resistance. |
Radiation health risks similar to those from X rays, although small doses generally considered safe except for pregnant women. MIBI more expensive than ultrasound or mammography, but less expensive than MRI. MIBI unable to detect cancers smaller than 1 cm and less accurate for nonpalpable masses. |
Page 62
|
Technology |
Description, Mechanism |
|
Positron emission tomography (PET) |
Uses tracers such as labeled glucose to identify regions in the body with altered metabolic activity, which is common in malignant tumors. |
|
Radioactive antibodies |
Target antigens specific to breast cancer, include carcinoembryonic antigen and certain growth factor receptors. |
|
Optical imaging Elastic scattering spectroscopy (ESS), “Optical Biopsy” |
Use of fiber-optic probes to obtain spectral measurements of elastically scattered light from tissue. Generates spectral signatures that reflect architectural changes at cellular and subcellular levels. |
|
Optical tomography |
Use of light to image the breast. |
|
Infrared thermography |
Measures heat emitted by the body. Tumors can raise skin surface temperature by 2 to 3 degrees C, with heat detected by infrared cameras. Dynamic Area Telethermometry detects changes in blood flow. |
|
Electrical potential measurements |
Measurement of electrical potential at the skin surface. Proliferation of epithelial tissue disrupts normal polarization. |
Page 63
|
Stage of Development |
Potential Strengths |
Current Limitations |
|
More studies needed to determine clinical utility. |
In theory, could be useful in women with dense breasts, implants, or scars. |
Currently no technique to target biopsy specimens that are identified by PET but not visible on mammograms. PET scanners expensive and not readily available. |
|
Only small studies to date; need more clinical studies to determine role in breast cancer diagnosis. |
Some agents show promising sensitivity and specificity for breast cancer. |
Scans can be difficult to interpret. Need to identify optimal markers for imaging. |
|
Early clinical studies on transdermal needle diagnostic. |
Portable, designed for convenient clinical use. Instant diagnosis would reduce patient anxiety and allow immediate treatment. |
Currently depends on endoscopic approach, which may not be relevant to breast tissue. |
|
Systems being developed by Imaging Diagnostic Systems Inc., Dynamics Optical Breast Imaging Medical Systems, and Advanced Research Technologies, Inc. |
Low cost, speed, comfort, and noninvasiveness. Optical scans can be digitized for image manipulation and serial studies. |
Must optimize accuracy and resolution and improve target-to-background ratios. Variations in breast tissues due to age, hormone status, and genetic makeup. |
|
FDA approval in December 1999 to OmniCorder for its BioScan System, based on Dynamic Area Tele-thermometry. Computerized Thermal Imaging, Inc., is testing its system in clinical trials. |
Noninvasive, does not require compression or radiation exposure. New cameras offer improved spatial and thermal resolutions. |
Results of numerous studies have been inconsistent. Old technology, especially infrared cameras, has hindered development. |
|
Biofield Breast Exam (BBE) has received CE Mark Certification that allows the company to sell in Europe. FDA approval pending. |
BBE gives a single, numerical result that objectively determines malignancy. Inexpensive, does not require an expert reader, no discomfort, and speedy procedure. |
Two large clinical studies demonstrated specificity of 55 to 60%. |
Page 64
|
Technology |
Description, Mechanism |
|
Electrical impedance imaging |
Measures voltage at skin surface while passing small current through breast. Cytological and histological changes in cancerous tissue decrease impedance of tissue. |
|
Electronic palpation |
Quantitative palpation of breast using pressure sensors. |
|
Thermoacoustic Computed Tomography (TCT) |
Breast is irradiated with radio waves, causing different thermal expansion of tissue and generating sound waves, from which a three-dimensional image is constructed. |
|
Microwave imaging |
Transmits low-power microwaves into tissue and collects backscattered energy to create three-dimensional image. Higher water content in malignant tissues causes more scatter. |
|
Hall Effect Imaging (HEI) |
Induces vibrations by passing electric pulse through tissue while exposed to a magnetic field. |
|
Magnetomammography (MMG) |
Tags cancerous tissue with magnetic agents that are imaged with SQUID magnetometers. |
Page 65
|
Stage of Development |
Potential Strengths |
Current Limitations |
|
FDA approval in 1999 to TransScan Medical (Ramsey, NJ) for use of T-Scan 2000 device as an adjunct to mammography for women with lesions in BIRADS a 3/4. |
Potential as adjunct to mammography for women with certain indeterminate lesions. Painless, no breast compression or ionizing radiation. |
Not to be used for women with clear indications for biopsy. Currently conducting more trials to validate technology. |
|
Assurance Medical (Hopkinton, MA) is seeking FDA approval and is testing 400 women with suspicious lesions. Ultratouch (Paoli, PA) is developing robotic device (Palpagraph) and starting clinical studies for FDA approval. |
Potential to standardize performance and documentation and serially monitor physical breast exams. Preliminary studies suggest use for general screening. |
Limited sensitivity for small lesions. Clinical utility unproven. |
|
Development in early stages. To date, no large published clinical trials. Optosonics plans to initiate a study of 80 women this year. |
Does not use ionizing radiation and does not compress the breast. Retains three-dimensional structural information and images are highly consistent. |
Three-dimensional images difficult to display or analyze; more time-consuming and costly than mammography. |
|
To date, research focused on theoretical validation through computer modeling and studies with excised breast tissue. |
Does not require compression or use ionizing radiation. In theory, should produce high-contrast image, regardless of tissue density. |
Technology has been constrained by poor resolution, poor depth penetration, excessive power requirements, unsafe microwave levels, and intensive image reconstruction programs. |
|
Early stages of development; first published account of HEI in 1998. To date, HEI tested only with excised and simulated tissue. |
May be useful for a limited population of women. |
Prohibitive cost; requires an expensive, super-conducting magnet. |
|
Still untested; looking for an agent that is both magnetic and specific to cancerous tissue. |
Would not require compression or ionizing radiation. Should be equally effective with dense breasts. |
Poor spatial resolution, expensive to fabricate and operate. |
Page 66
|
Technology |
Description, Mechanism |
|
Three-dimensional interactive visualization |
Includes technologies such as virtual reality. |
the third goal of diagnostic imaging described above: tumor characterization. Again, an ideal imaging tool would provide useful data on both structure and function, but this goal is quite challenging to achieve at present.
Imaging technologies for the breast are based on physical, mechanical, electrical, chemical, and biological properties of tissue (Figure 2-1). Although the technical applications of imaging tools vary, they all have a common theme. In each case, image assembly and analysis involve identification of a signal and separation of the signal from the background. A machine or a person may do the separation step, which depends on image contrast.
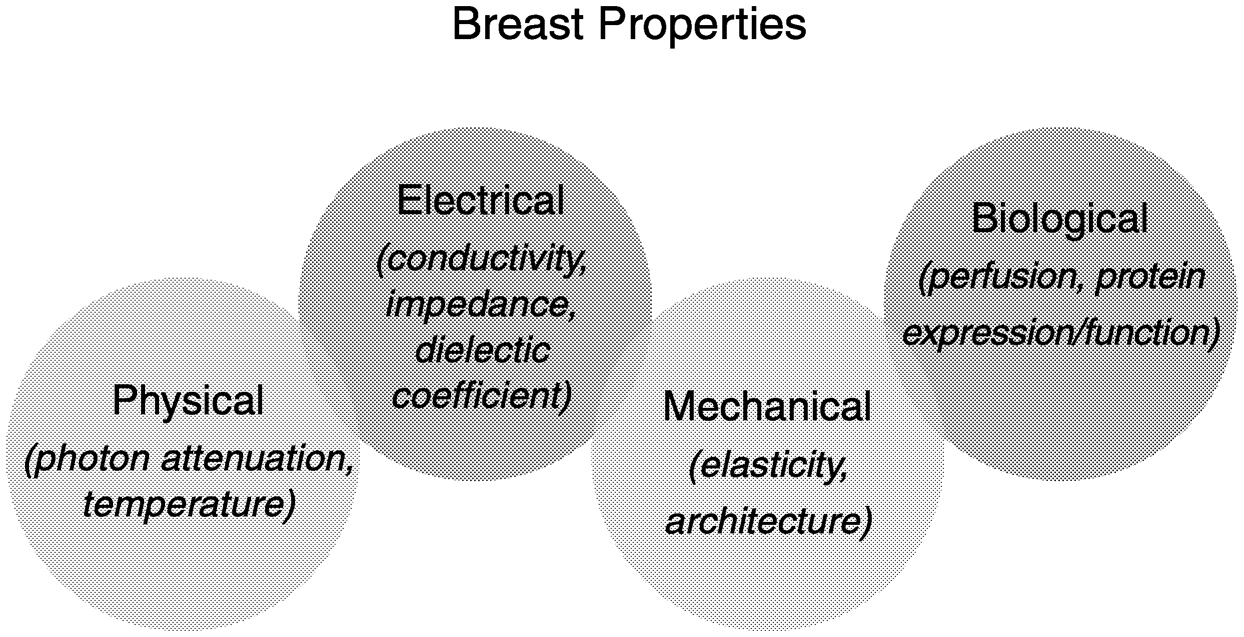
~ enlarge ~
FIGURE 2-1 Properties of breast tissue exploited by different modes of imaging. Examples of these categories are listed.
Page 67
|
Stage of Development |
Potential Strengths |
Current Limitations |
|
Primarily developed for nonmedical applications. Some early clinical research (e.g., breast MRI) |
Could potentially be used for image visualization, training, and procedure planning and support. |
Significant advances required in virtual reality technologies, including novel algorithms for breast imaging. |
DIGITAL MAMMOGRAPHY
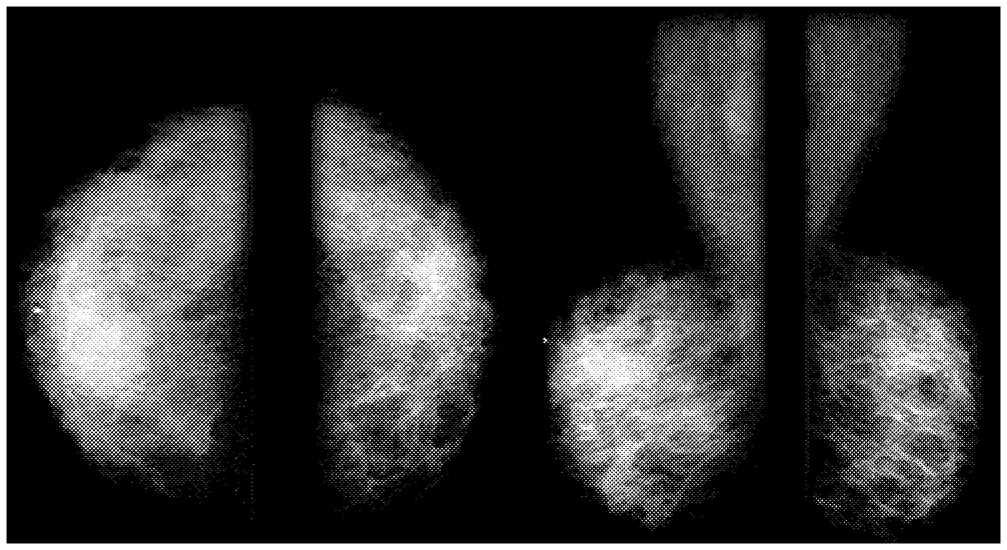
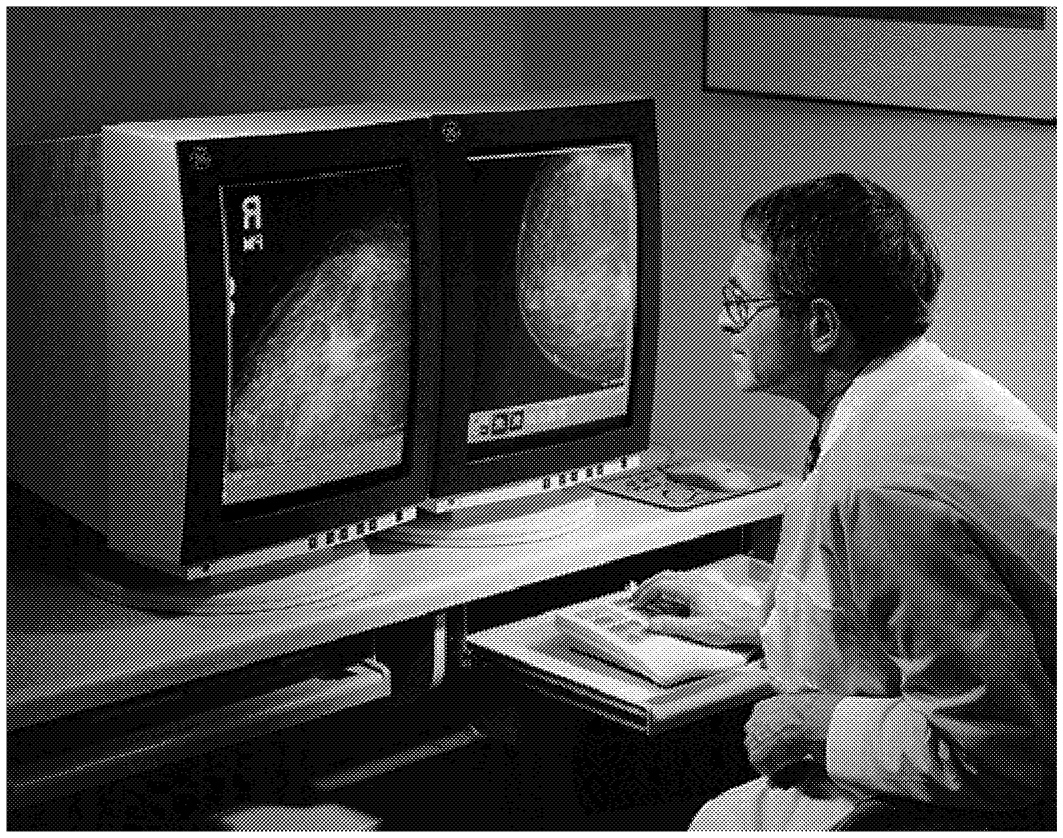
Full-field digital mammography (FFDM) systems are identical to traditional film-screen mammography (FSM) systems except for the electronic detectors that capture and facilitate display of the X-ray signals on a computer or laser-printed film (Figures 2-2 and 2-3). Proper positioning and compression of the breast are still critical for producing quality digital mammograms. The digital detector array responds to X-ray exposure and then sends an electronic signal for each detector location to a computer, where it is digitized, processed, and stored as a specific signal and location (pixel). The goal of digital mammography—to identify and localize breast abnormalities—is similar to that of traditional mammography. The primary motivation behind the development of digital X-ray mammography is the belief that it has the potential to improve image quality and therefore lesion detection (especially for dense breasts) with a lower
~ enlarge ~
FIGURE 2-2 Examples of Film Screen Mammography images of the breast. Source: Miraluma Educational CD-ROM, DuPont Radiopharmaceutical Division, The DuPont Merck Pharmaceutical Company.
Page 68
~ enlarge ~
FIGURE 2-3 Example of Full-Field Digital Mammography images of the breast, and current technology. Source: General Electric Medical Systems.
dose of radiation compared with that required for conventional film-based mammography.
Digital mammography separates image acquisition from image display, offering an infinite ability to manipulate contrast, brightness, and magnification with one exposure, a feat that is not possible with traditional FSM (Pisano et al., 2000). The ability to fine-tune the digital image can enable a more detailed examination of questionable areas without requiring a new X-ray exposure. Digital processing can also enable dynamic or real-time imaging (e.g., to assist with biopsy and localization procedures) and can enable direct use of computer-aided detection and diagnosis (CAD; see below). In addition, the technology may facilitate digital tomosynthesis—reconstruction of a three-dimensional image or hologram of the breast by combining information from different detection angles. Ease of digital image archiving, retrieval, and transmission is another advantage. For example, studies on the feasibility of satellite or long-distance transmission of digital mammograms for consultation, a process known as telemammography, are under way.
When an image is displayed on a cathode ray tube (CRT) monitor (or “soft-copy” display), digital image processing can potentially improve the lesion-to-background contrast and enhance subtle details that might be missed in a standard mammogram film. Fine-tuning of the image has not yet been proved to be beneficial for breast cancer detection, but in
Page 69
theory, image processing could improve detection of lesions in dense breast tissue, which can obscure precancerous and cancerous lesions. Manipulation of the image, however, could theoretically reduce the visibility of the lesions as well as enhance them. Thus, optimal use of digital processing may depend on image processing algorithms similar to those used with computed tomography (CT) scans.
Digital mammography currently faces some fundamental technological problems that may impede its implementation. One current limitation of digital mammography is that the spatial resolution and luminance range of images displayed on a CRT—even with the most advanced CRT technology—are significantly lower for digital mammography than for conventional FSM. Film-screen mammograms have spatial resolutions up to 20 line-pairs per millimeter (mm). The current digital display systems have, at best, 12.5 line-pairs per mm (40 micrometers [mm] per pixel) of spatial resolution. The increased contrast resolution possible in digital mammography (the ability to display subtle differences in the number of photons absorbed in adjacent areas of the breast) may or may not compensate for its lower spatial resolution. Digital mammograms can also be printed on film with a laser printer. Such a hard-copy display increases the spatial resolution and the gray-scale range so that they are comparable to those for standard FSM. However, film for use in a laser printer is costly, and often, more than one version of the mammogram must be printed to obtain optimal readability. Thus, there is a great need for the development and testing of cost-effective digital displays for high-resolution, high-contrast, large-field-of-view visualization combined with a practical rate of display and light output.
Also key to enhancing interpretation of digital mammograms is determining how to display the most important information in the image in the best (and fastest) possible way for the clinician. This requires development of computer workstations with practical user interfaces for clinical radiologists (e.g., multi-resolution, “region-of-interest” displays and “bright-light” display equivalents). Another initial limitation of FFDM is that prior films taken by standard FSM cannot be imported easily into digitized formats for serial comparisons, posing a problem for the comparison of images over time, but this will be a dilemma for any new imaging modality. Communication hardware and software also need to be developed or improved to achieve workable collaborative efforts between specialists at different locations.
Current efforts to further develop digital mammography include photostimulatable phosphors, scanning detectors, optically coupled two-dimensional arrays, large-area detectors, and new detector materials. Ideally, the detector system should be compatible with existing mammography system geometries. Specifically, the detector must image all breast tissue up to the chest wall.
Page 70
Currently, at least four manufacturers have digital mammography systems with different spatial resolutions: both the Fuji and the General Electric systems have resolutions of 100 µm, that of Fischer's system is 54 µm, and Hologic's2 digital mammography system can obtain a 41-µm resolution. (For a more detailed description of the technology associated with each of these digital detectors, see Pisano et al. [2000]). In January 2000 the Food and Drug Administration (FDA) approved the first digital mammography machine, General Electric's Senographe 2000 D digital mammography system. However, it was approved for use only with hard-copy displays, which eliminates the opportunity for enhanced soft-copy manipulation and makes computer-aided detection more difficult. In November 2000, General Electric was granted FDA approval to use the Senographe 2000 D system for soft-copy mammogram reading.3
Most clinical testing of FFDM systems has been conducted by manufacturers to obtain FDA approval, and results have not been published in many cases. However, a multicenter trial supported by the U.S. Army Breast Cancer Research and Materiel Command is comparing FFDM with FSM in a general screening population of nearly 7,000 women over age 40. Results thus far suggest that digital mammography performs no better than standard FSM in detecting malignant lesions but so far has led to fewer recalls of women for further examination than conventional mammography in a screening population (Lewin et al., 2000).
The sensitivity was 53 percent for FFDM, whereas it was 67 percent for FSM (the difference was not statistically significant) (Lewin et al., 2000). These sensitivities were lower than the typically cited values for mammography (83 to 95 percent [Mushlin et al., 1998]) because each technique detected tumors that were not detected by the other one. The use of both technologies also resulted in a higher cancer detection rate (6.4 cancers per 1,000 women screened) than would normally be expected. Among a general population of women being screened for the first time, about four to six cancers are found per 1,000 women screened. In subsequent screening rounds, about three to four cancers will be identified per 1,000 women screened.
One potential advantage of FFDM was noted in the study results (Lewin et al., 2000). The rate of calling women back for further evaluation after FFDM (11.3 percent) was lower compared with that after FSM (15 percent). This difference was statistically significant (p < 0.001). If this difference is in fact real, projection of these data to all U.S. women receiv-
2The system was originally developed by Trex Medical (Danbury Connecticut), which was recently acquired by Hologic Corp. (Bedford, Massachusetts).
3See http://www.fda.gov/cdrh/mammography/mmweb/mmweb74/rws.html.
Page 71
ing screening mammograms (about 25 million) could result in half a million fewer women being called back for follow-up procedures.
Both FSM and FFDM missed a significant number of cancers in this study (Figure 2-4). In fact, more than 800 of the first 5,000 screening examinations by FFMD and FSM had discordant interpretations (Lewin, 1999). The cause of the discrepancy in most cases was due to small differences in breast positioning and compression, even though the same technologist took the two mammograms sequentially on nearly identical machines. For the remaining one-third of the individuals with discrepant results, the difference between readings was primarily due to interpretation, which is known to vary considerably from double-reading studies (Beam et al., 1996; Thurfjell et al., 1994). Contrary to conventional wisdom, only a few of the cancers detected in individuals with discrepant results were in areas of dense tissue (Lewin, 1999).
Given the information currently available, FFDM does not appear to offer significant improvements over FSM with regard to breast cancer detection. However, the study described above is not yet complete, and the preliminary data may have insufficient statistical power to reveal important differences between FFDM and FSM. The U.S. Department of Defense will not be supporting further patient accrual to this trial, but further studies are under way. The American College of Radiology Imaging Network trial of digital mammography may be especially important in answering unresolved issues (see Chapter 4). FFDM is also at a relatively early stage of development compared with FSM and so may have more room for improvement. Furthermore, novel applications and analysis of the digital information, including tomosynthesis, telemammography, and CAD may offer additional value over FSM even if FFDM cannot detect more cancers, but the clinical utility of these applications is not yet certain.
OTHER TECHNICAL ADVANCES IN X-RAY IMAGING WITH POTENTIAL APPLICATION TO BREAST CANCER
A number of technical innovations have been suggested as ways to further improve X-ray mammography. A few examples are listed below, but relatively few data are available to assess the potential value of these techniques.
Capillary optic arrays are bundles of hollow glass capillaries that guide X rays in a manner similar to the way in which fiber optics guide light. Focused postpatient capillary optic arrays have the potential to significantly improve both contrast and resolution of mammographic images compared with those of conventional antiscatter grids (Kruger et al., 1996).
Phase-contrast X-ray imaging with coherent (or monoenergetic) X
Page 72
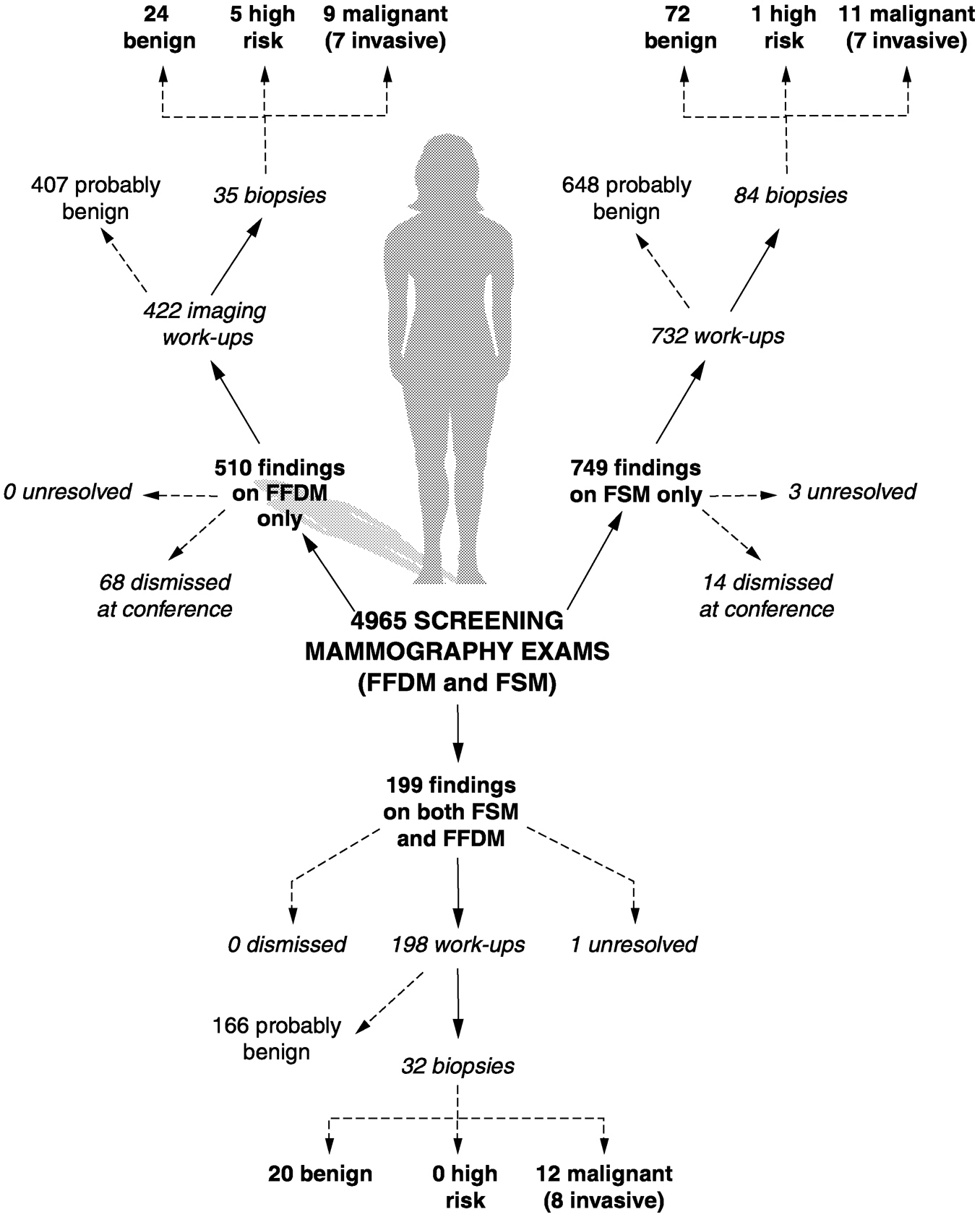
~ enlarge ~
FIGURE 2-4 Results from the Department of Defense study for the clinical evaluation of full field digital mammography for breast cancer screening. Note: updated results from this study were presented at the annual meeting of the Radiological Society of North America in November, 2000. After screening 6,768 women, 51 confirmed cancers were found; 18 were detected by both FFDM and FSM, 9 by FFDM only, and 16 by FSM only. 8 additional interval cancers were found within a year of screening. Statistically, there was still no difference between the sensitivity of the two methods. SOURCE: Lewin et al., 2001.
Page 73
rays can be a powerful technique for the detection of low-contrast details in weakly absorbing objects. Synchroton accelerators can generate nearmonoenergetic X rays as an alternative to the X rays emitted by the X-ray tubes used in conventional mammography. Another potential source of near monoenergetic X-ray radiation is the free electron laser. Phase-contrast X-ray imaging may be useful in diagnostic radiology applications such as mammography when imaging of low-contrast details within soft tissue by conventional x-ray imaging does not give satisfactory results. By using radiation doses smaller than or comparable to the doses needed for standard mammographic examinations, details that have low levels of X-ray absorption and that are invisible by conventional techniques may be detected by phase-contrast X-ray imaging (Arfelli et al., 1998a,b; 2000; Burattini et al., 1995). However, the interpretation of images through tissues with complex geometries and heterogeneous tissue types will require substantially more research.
X-ray CT has been used for more than 20 years to generate three-dimensional images of the body. X-ray computed tomographic mammography (CT/M) was first reported in 1977 to detect both benign and malignant breast disease in fatty and dense breasts. CT may also be capable of diagnosing early cancer in women who have had radiation therapy or surgery (Chang et al., 1977). CT/M imaging of the breast may facilitate diagnosis when mammography fails to detect a lesion or is unable to provide a definitive diagnosis, particularly when one is using a contrast medium (Chang et al., 1979). Although CT/M will not replace conventional mammography for routine breast examinations, it may provide an option for overcoming some limitations of mammography (Chang et al., 1980). These early development efforts resulted in a prototype product that was never brought to market, but other forms of digital CT applied to the breast are being investigated, although they have not yet been clinically evaluated in prospective trials (Nicklason et al., 1997; Pisano and Parham, 2000).
For example, tuned-aperture computed tomography (TACT) is a simpler method for tomographic viewing of individual breast tissue layers or retrieval of a true three-dimensional image. A reference system is used to reconstruct the projection geometry that produced the image. Once the projection geometry is known, it is possible to digitally reconstruct the three-dimensional image of the breast on the basis of optical aperture theory. The procedure is tailored for the breast so three-dimensional mammograms can be produced with increased patient comfort through less stringent requirements for breast compression. Multiple TACT images can be reconstructed with the same dose of radiation to the patient needed to obtain a single two-dimensional conventional digital mammogram (Webber et al., 1997).
Page 74
COMPUTER-AIDED DETECTION AND DIAGNOSIS
CAD systems consist of sophisticated computer programs that are designed to recognize patterns in images. They are intended for two different purposes: to help radiologists identify suspicious areas that may otherwise be overlooked on screening mammograms (detection schemes) and to classify breast lesions as benign or malignant (diagnosis schemes). CAD systems can be used directly on digital mammograms or on standard film-screen mammograms that have been digitized. Although CAD has a very low specificity when it is used alone without the judgment of a radiologist (Thurfjell et al., 1998), several studies now suggest that CAD can improve a radiologist's ability to detect and classify breast lesions in simulated clinical reading situations (reviewed by Nishikawa [1999]). However, further clinical studies are needed to more clearly define the value and appropriate use of the technology.
Image interpretation for screening mammograms is challenging for many reasons. Among the general screening population, about 1.5 to 6 cancers are identified for every 1,000 women screened, so radiologists must examine many films to detect a few cancers. Rapid interpretation of many images is necessary for mammography to be practical at a reasonable cost. As a result, some cancers are missed. Studies show that a significant number of cancers (as many as 30 to 65 percent) can be visualized on prior mammograms in retrospective reviews (Harvey et al., 1993; van Dijck et al., 1993; Warren-Burhenne et al., 2000). Double reading of mammograms by two radiologists can improve the cancer detection rate (by 4 to 15 percent) (Beam et al., 1996; Thurfjell et al., 1994), but such a practice is expensive and time-consuming. CAD is intended to improve detection rates in a more efficient and cost-effective manner. However, CAD use also increases the amount of time that a reader spends on each film.
Detection schemes generally use the following approach: (1) preprocessing of the image to increase the signal-to-noise ratio of the lesions being detected, (2) identification of all potential lesions, and (3) elimination of false-positive findings (using artificial neural networks and other analyses). Currently, detection schemes have a sensitivity of approximately 90 percent, with a rate of false-positive results of one to two per image (Nishikawa, 1999). It is critical to reduce the rate of false-positive results without decreasing sensitivity to increase the clinical acceptance of CAD. This is because the radiologist must scrutinize each false-positive finding which will reduce his or her productivity, decrease confidence in the computer-aided diagnosis, and, potentially, increase the number of unnecessary biopsies. Although different techniques have been developed, only two have been tested and shown to improve radiologists' performance.
A recent study of retrospective prior film review for about 500 women
Page 75
diagnosed with breast cancer found that CAD could potentially reduce the rate of false-negative results of radiologists by more than 70 percent (Warren-Burhenne et al., 2000). A common concern is that CAD might result in higher rates of callback and biopsies, only to eventually yield negative findings. However, recent data from five institutions that used conventional mammography showed no significant increase in callback rates before and after they started using CAD (∼24,000 films interpreted before CAD installation and ∼14,000 films read after CAD installation). In a concomitant study of more than 1,000 films for women previously diagnosed with breast cancer by screening mammography, CAD correctly labeled microcalcifications in 98 percent of the cases and masses in 75 percent of cases (Warren-Burhenne et al., 2000). However, the sensitivity and specificity of CAD in a general screening population have not yet been defined (although a study is under way4). Furthermore, detection of changes in a woman's mammograms over time is still technically challenging, and thus, new tools and techniques will be necessary to accomplish this goal. Comparison of serial images is confounded by variations in breast compression, patient positioning, and X-ray exposure parameters.
Further studies of CAD with digital mammography also are under way. In the United States, General Electric has an agreement with R2 Technologies, Inc. (Los Altos, California), to use R2 Technologies' detection algorithms with the FDA-approved General Electric digital mammography machine. To date, only the CAD software package produced by R2 Technologies has FDA approval and is being marketed in the United States. Several CAD detection systems are also being developed by other companies, such as Qualia Computing, Inc. (Beavercreek, Ohio), Scannis Inc. (Foster Creek, California), and CADx Medical Systems (Laval, Quebec, Canada), and these systems are being tested with populations around the world.
The commercially available CAD systems do not classify breast lesions as benign or malignant. Classification schemes work by merging features extracted from the radiograph (either automatically by the computer or manually by the radiologist) along with clinical and demographic information to give the likelihood that a lesion is cancerous. The typical techniques used are the same as those used with detection schemes. Current experimental systems used to distinguish benign and malignant lesions suggest that the positive predictive value of a radiologist's reading can be significantly increased by using CAD (Chan et al., 1999; Doi et al., 1997). The performance of CAD applied to mammography could potentially improve when direct digital image data become available.
4Qualia Computing, Inc. (Beavercreek, Ohio), is nearing completion on a study of their mammographic CAD system with 5,000 screening patients.
Page 76
Similar computer algorithms could also be developed to assess digital breast images generated by other imaging modalities. Analysis of images from multiple three-dimensional breast imaging modalities could potentially enhance diagnosis and staging by combining anatomic, physiological, and biological tumor information in a single three-dimensional image. However, such technology does not currently exist.
ULTRASOUND
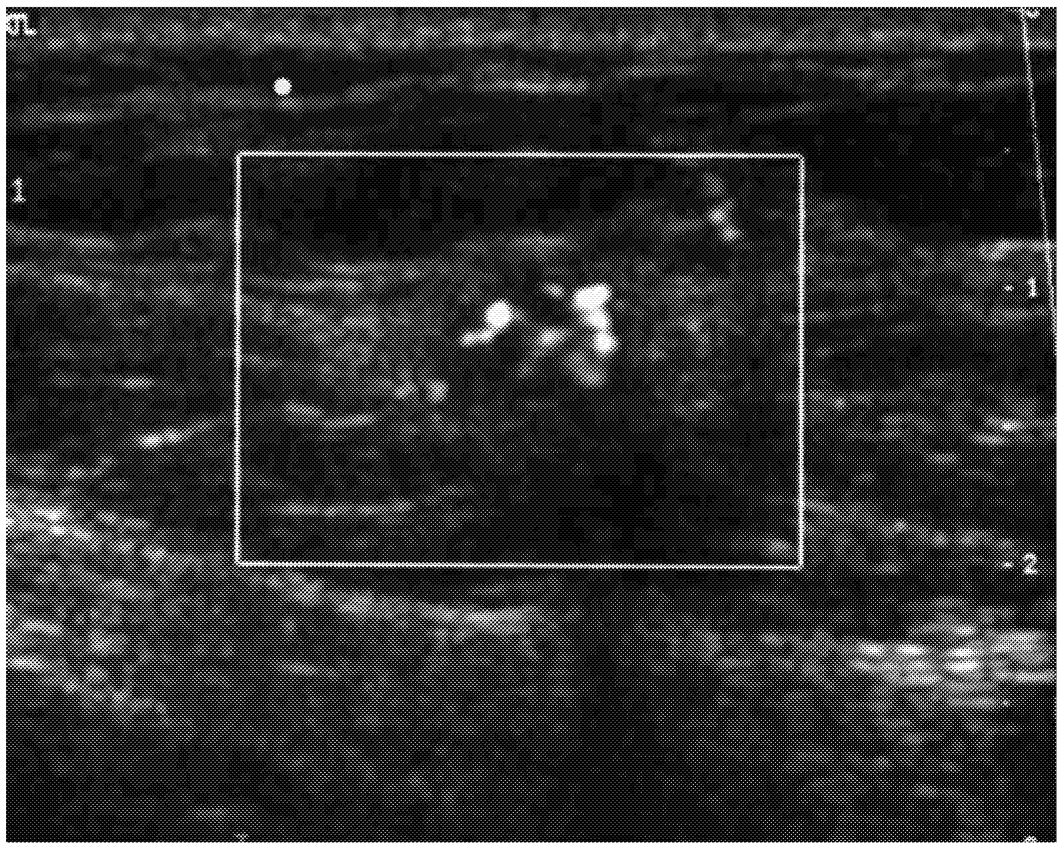
Ultrasound waves are high-frequency sound waves that reflect at boundaries between tissues with different acoustic properties. The depth of these boundaries is proportional to the time intervals of reflection arrivals. Thus, ultrasound can map an image of tissue boundaries. Traditionally used as an adjunct to mammography in the identification of cysts and in guiding aspiration and biopsy, improvements in ultrasound technology have begun to expand the role of ultrasound in the differentiation of benign and malignant breast lesions and selection of patients for biopsy (Figure 2-5).
X-ray mammograms are frequently followed up with ultrasound imaging to determine whether a lesion that appeared on a mammogram is a cyst or a solid mass. Because a fluid-filled cyst has a different “sound
~ enlarge ~
FIGURE 2-5 Example of an ultrasound image of the breast. Source: Janet Baum, Director, Breast Imaging, Beth Israel Deaconess Medical Center, Boston, MA.
Page 77
signature” than a solid mass, radiologists can reliably use ultrasound to identify cysts, which are commonly found in breasts (Feig, 1999a,c). If ultrasound cannot make a distinction between a cyst and solid mass, ultrasound imaging may also be used to guide a needle into the abnormal tissue, from which fluid or cells may be taken (Feig, 1999a,c).
Ultrasound imaging of the breast may also help radiologists evaluate some lumps that can be felt but that are difficult to see on a mammogram, especially in dense breasts. Researchers have therefore begun to evaluate ultrasound in distinguishing malignant tumors from benign lesions (Ziewacz et al., 1999). In one study of 750 breast lesions that were subsequently biopsied, ultrasound accurately diagnosed benign conditions 99.5 percent of the time. If the ultrasound findings had been used to determine who should have a biopsy and who should be monitored, more than half of the biopsies would have been avoided (Stavros, 1995). Another study of 3,000 women who primarily had palpable lesions found that when ultrasound was used with standard mammography, 92 percent of breast cancers were detected. The specificity was 98 percent. In addition, when both imaging modalities indicated the lack of a malignancy, that diagnosis was correct more than 99 percent of the time (i.e., the rate of false-negative results was 1 percent) (Duijm et al., 1997).
This combined imaging is likely to be less accurate for nonpalpable tumors, but one 1998 screening study of more than 3,500 women with dense breasts found that ultrasound could detect some early-stage, clinically occult tumors that were missed by screening mammography (Kolb et al., 1998). Thus, there may be a future role for ultrasound in the screening of younger women with dense breasts and high risk factors. However, current ultrasound technology has a field of view limited to several centimeters at maximum resolution, making full breast examination difficult and time-consuming. This is in part a result of the traditional use of ultrasound for examination of masses that are already suspected. At present, larger arrays that would increase the field of view are technologically feasible at modest extra cost.
Conventional ultrasound has been limited in its ability to detect microcalcifications, which are frequently linked to breast cancer (Merritt, 1999). This difficulty is due in part to a phenomenon called “speckle,” which arises from the interaction of the ultrasound field with the tissue. In the breast, speckle may produce small bright echoes within tissues, making them look like calcifications, so distinguishing artifacts from true calcifications can be difficult. Speckle and other noise also degrade the characterization of very small cysts and solid masses.
A new technique, called “compound imaging,” significantly reduces speckle in breast images and improves the contrast and definition of small masses and even allows visualization of microcalcifications (Merritt, 1999). Conventional ultrasound generates images by using a beam that
Page 78
strikes tissues from a single direction. New ultrasound methods use several beams that strike the tissue from different angles. This reduces speckle and other artifacts, but it may also reduce resolution.
Three-dimensional ultrasound imaging of the breast is also under investigation. Three-dimensional ultrasound displays a volume of tissue rather than a single slice. Such three-dimensional images make it possible to simultaneously view multiple planes of observations and see through and around structures without the superimposition of overlying structures. Three-dimensional images may also permit more accurate measurement of tumor volume and comparison of changes in the sizes of masses over time. In contrast to fetal and gynecological ultrasound, for which three-dimensional methods have received considerable attention, three-dimensional breast sonography is early in its development.
Ultrasound can also provide information about blood flow by mapping the amount of acoustic frequency shift as a function of blood cell motion at a particular position in tissue, the Doppler effect. The detection of increased tumor blood flow could potentially play a role in the differentiation of benign and malignant masses (Carson et al., 1998; Mehta et al., 2000), but whether this will prove to be a reliable indicator for malignancy remains to be shown in controlled clinical studies. Power Doppler is a method that shows the amount of blood cells in motion and thus in effect shows vasculature. Its sensitivity may be limited because increased vascularity may not be seen in some cancers. Ultrasound contrast agents might improve the ability of Doppler ultrasound to evaluate tumor blood supply, particularly when coupled with new signal processing methods such as harmonic and pulse inversion contrast imaging. Several contrast agents are being tested in clinical trials. Assessment of tumor vascularity could also be useful to predict the biological activities of tumors and in monitoring responses to treatment.
Elastography is another novel use of ultrasound in the breast (Ophir et al., 1999). Like palpation, elastography detects differences in tissue stiffness and other mechanical properties. Physical breast examination by inspection and palpation enables detection of breast cancer by observing differences in mechanical properties, especially stiffness, since cancerous tissue is usually much more rigid and less easily deformable than normal breast tissue. However, cysts and certain benign lesions may have mechanical properties that can mimic malignant tumors, so finding a rigid mass within the breast does not confirm malignancy.
In elastography, the mechanical properties of breast tissue are measured from point to point within the breast by ultrasound or MRI (described in the next section). These measurements are mapped into images, often called “elastograms.” There are many elastic properties of solids, including tissues, that can be determined by ultrasound or MRI measurements obtained before and after application of small deforma-
Page 79
tions or by monitoring the propagation of mechanical (infrasonic) waves. Ultrasonic and magnetic resonance elastography have the potential to distinguish breast abnormalities, such as malignant tumors, from normal tissue, benign processes, and scars. Since, in general, elastography can be done noninvasively to form images for subjective and quantitative evaluations, these methods are under active investigation. Elastic properties are not directly measured, however, and must be inferred (mathematically) by one of numerous technical strategies used to model and display the images. No clinical trials of elastography in breast cancer have yet been reported, but some feasibility demonstrations have been completed, so human clinical trials are anticipated (Muthupillai et al., 1995; Plewes et al., 2000; Sinkus et al., 2000). However, assessment of elastography could be hampered by a lack of standardization with regard to which elastic parameters should be measured and by a lack of a published characterization of normal tissue.
In summary, ultrasound imaging is well established as an adjunct to mammography for distinguishing cysts from solid lesions and as a method for localizing tumors before biopsy. Several studies suggest that it could be more widely used to characterize tumors as benign or malignant and perhaps even as a screening adjunct for specific populations. More study is needed to assess these possibilities. Ongoing technological advances in ultrasound imaging have the potential to increase the use of ultrasound in breast cancer detection even more, but their stage of development is too early to predict their ultimate utility.
MAGNETIC RESONANCE IMAGING
Magnetic resonance images are created by recording the signals generated after radiofrequency excitation of hydrogen nuclei (or other elements) in tissue exposed to a strong static magnetic field. The signals have characteristics that vary according to tissue type (e.g., fat, muscle, fibrotic tissue, and edema5). The method has minimal hazards from magnetic field effects and does not use ionizing radiation. The goal of breast MRI is similar to that of mammography: to identify structural abnormalities in the tissue. Some newer applications of MRI technology also aim to gather functional information about breast lesions. It is being developed primarily as a diagnostic tool to avoid unnecessary biopsies among women with dense breasts, but screening applications are also being studied among high-risk populations.
MRI has been used for a wide variety of medical applications since FDA approved the procedure in 1985. MRI of the human breast was first
5Women may experience some tissue edema in the first few months after surgery.
Page 80
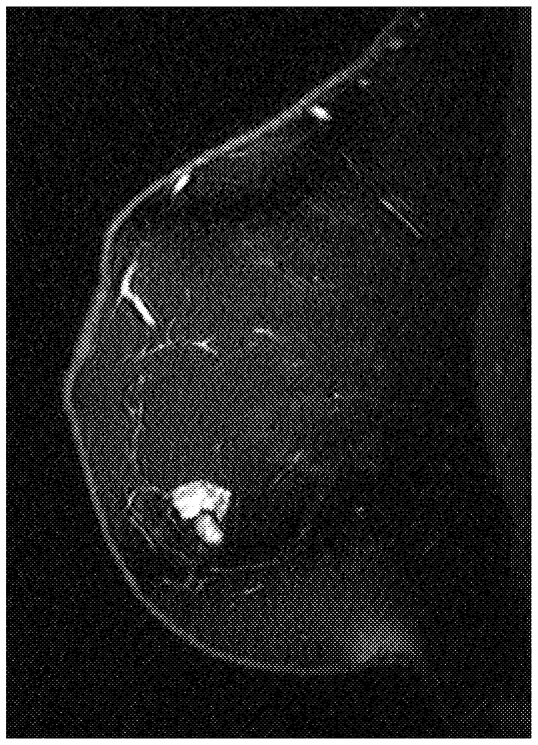
~ enlarge ~
FIGURE 2-6 Example of a magnetic resonance image of the breast. Source: Drs. D. Plewes and R Shumak of Sunnybrook and Women's College Health Centre, University of Toronto.
attempted in the 1980s, but early results were disappointing. Subsequently, intravenous contrast agents were used with a dedicated breast MRI coil, offering a clear advance. In general, malignant tumors showed intense uptake of contrast agents, whereas the surrounding normal tissue did not (Figure 2-6). Following this discovery, MRI has been studied as an emerging but as yet unproven technology for breast cancer detection. Recently, a number of investigators in this field have demonstrated the potential of breast MRI, but it is currently confined to experimental protocols.
Two different MRI techniques are being evaluated to detect breast tumors: dynamic contrast imaging and three-dimensional high-resolution imaging. Dynamic imaging aims to pinpoint tumors on the basis of how quickly they take up the contrast agent. Because malignant tumors tend to have enhanced and leaky blood vessels compared with normal tissue, they generally take up more contrast agent faster. However, studies show that there is an overlap in contrast agent uptake between benign and malignant breast tumors (Farria et al., 1999). Dynamic contrast imaging typically images only a cross section of the breast. Three-dimensional high-resolution imaging, on the other hand, generates whole-breast images, which allow radiologists to detect additional breast lesions that may be missed by dynamic contrast imaging. In the future, faster imaging technology may allow dynamic imaging information to be obtained simultaneously with three-dimensional, high-resolution, whole-breast imaging.
Studies suggest that MRI may, in some cases, be useful for the diagnosis of breast lesions identified through screening mammography or
Page 81
clinical breast examination (Tan et al., 1999; Farria et al., 1999). The sensitivity of MRI for the detection of suspicious breast lesions ranges between 88 and 100 percent (10 studies reviewed by Farria et al., 1999). One study of 225 women found the combined sensitivity of MRI and standard mammography to be 99 percent (Bone et al., 1997). The reported specificity of MRI is more variable, ranging from 28 to 100 percent (Farria et al., 1999), depending on the patient population and the interpretation technique used. The relatively low degree of specificity of MRI in some studies was mainly due to its frequent inability to distinguish between malignant tumors and benign noncystic abnormalities, such as nonmalignant solid tumors (fibroadenomas) and ductal hyperplasia. The disparity between the very high degree of sensitivity and the relatively low degree of specificity of the technology can be problematic in that “serendipitous lesions”—unexpected lesions found incidentally in the breast during the MRI workup of a lump or breast abnormality detected by mammography—are often observed (Lawrence et al., 1998). This raises the question as to whether such lesions should also be monitored or biopsied. The likelihood that these lesions are in fact cancerous seems to be low, but further study is needed to improve the decision-making process following MRI (Lawrence et al., 1998). To increase confidence in the nature of the lesions detected by MRI (e.g., benign versus malignant lesions), follow-up studies or confirmation of diagnosis by tissue biopsy may be required. Moreover, biopsy of lesions seen on MRI images but not on images obtained by other imaging methods can be difficult because MRI localization for biopsy is not a standard practice. Accessible and easy-touse guidance systems are required to perform localization or biopsy of lesions detected by MRI alone. For MRI-guided biopsy, magnet-compatible needles and other equipment using materials that do not cause image distortions in a magnetic field need further development.
MRI shows particular promise in defining the local extent (size, number, distribution) of cancer foci in women with known breast cancer who are candidates for breast-conserving therapy. Studies show that MRI may be particularly useful in defining the extent of a specific type of breast cancer, invasive lobular carcinoma. Although this type of cancer makes up only about 10 percent of all breast malignancies, it is frequently missed in mammograms and the extent of the cancer is difficult to determine by other methods. In one very small study of 20 women, MRI accurately predicted the extent of invasive lobular carcinoma in 85 percent of patients, whereas mammography accurately predicted the extent of invasive lobular carcinoma in only 31 percent of patients (Rodenko et al., 1996).
Unfortunately, MRI cannot reliably reveal microcalcifications, and MRI can miss small tumors, particularly if they do not selectively take up the contrast agent. However, despite these limitations, a negative MRI
Page 82
result could potentially rule out the presence of breast cancer in a patient whose mammogram, sonogram, and physical examination are not definitive. MRI is much more expensive than ultrasound or X-ray mammography, and MRI systems capable of imaging the human breast are not available at every institution. Nevertheless, a recent meta-analysis suggests that if the diagnostic performance of MRI is equal to or better than those reported recently, it could potentially be a cost-effective alternative to excisional biopsy in the follow-up of suspicious lesions identified by mammography (Hrung et al., 1999a).
To assess the usefulness of MRI for the diagnosis of breast cancer, the National Cancer Institute is conducting a large, multicenter study of threedimensional high-resolution MRI and dynamic contrast MRI performed in conjunction with mammography (Farria et al., 1999). One of the goals of the study, which is expected to be completed in 2001, is to establish uniform interpretation criteria for MRI of the breast. A lack of standards has hampered the clinical usefulness of MRI in the diagnosis of breast cancer. Similar studies in the United Kingdom and Europe are also under way or are being planned.
Another potential use for MRI is detection of recurrent breast cancer in breasts previously subjected to lumpectomies, because MRI scans are usually not limited by scarring and edema, unlike mammography and ultrasound, which are sometimes limited by scarring. MRI scans can also reliably detect tumors in women with breast implants or dense breasts, both of which can interfere with interpretation of X-ray mammograms. Consequently, MRI is being tested as a screening technology for high-risk women, who may begin screening at a younger age and thus are more likely to have dense breast tissue. A recent prospective trial compared MRI, ultrasound, and mammography in 192 women at high risk for breast cancer on the basis of personal or family history (Kuhl et al., 2000). The sensitivities of mammography, ultrasound, and MRI, were 33, 33 (ultrasound and mammography combined), and 100 percent, respectively. MRI identified three breast cancers that were not detected by mammography. The specificity of MRI was 95 percent based on the experience of this group in interpreting patterns of contrast enhancement and through the use of short-term follow-up MRI studies performed with 10 percent of the women. Several studies at other institutions involving more than 5,000 high-risk patients worldwide are in progress. These studies should allow a more accurate assessment of the sensitivity and specificity of MRI for high-risk populations.
Other novel applications of MRI technology are also under investigation but are generally in the early stages of development. One example is MRI elastography, which measures the mechanical properties of tissue, as described in the previous section along with ultrasound elastography.
MRI could also potentially provide a noninvasive method for assess-
Page 83
ment of prognosis, in addition to its possible role in screening and diagnosis. In this role, functional imaging of molecular markers is required. Functional MRI differs from traditional MRI by combining anatomic examination with information about biological function. For example, different histological types of breast cancer display distinct differences in MRI enhancement characteristics (Knopp et al., 1999). These differences correlate with the density and permeability of tumor vasculature, which independently predict the outcomes of breast cancer (Craft and Harris, 1994; Weidner et al., 1992).
Newer “smart” magnetic resonance contrast agents may reveal additional biochemical and physiological information, such as gene expression and other physiological processes in the form of a three-dimensional magnetic resonance image (Louie et al., 2000). The technology uses gadolinium contrast agents within a molecular shell that are activated by specific biochemical processes inside the cell and that are then detected by conventional MRI. If the gadolinium agents were activated selectively in breast cancer cells, it could be detected in the images obtained by MRI. Imaging of cell functions like gene expression that can be correlated to disease states is in the very early stages of development, but it is being pursued commercially.6 One ultimate goal of this novel imaging technique is to track cell growth and behavior in breast and other cancers (Straus, 2000), including imaging of intracellular protein communication, apoptosis (or programmed cell death), and angiogenesis (growth of new blood vessels, a hallmark of many cancers). However, so far, all research has been conducted with animals, and testing in clinical trials with humans is still likely years away. In addition, more studies must be done to identify the appropriate markers to be imaged, as discussed in more detail in the next chapter.
In addition to its potential role in screening and diagnosis, MRI may also be helpful in the development of novel minimally invasive therapies. Interactive monitoring of localized “thermotherapy” by MRI is being studied as a possible alternative to lumpectomy. The tumor cells are heated by lasers, radiofrequency ultrasound, or high-intensity focused ultrasound, and the resultant tumor cell destruction can be monitored by MRI. This method is in a very early stage of development, and its true clinical utility and potential have not been assessed in clinical trials (Farria et al., 1999). New interventions for early lesions that are simple, effective, and acceptable to women could enhance the net benefits of screening by reducing some of the problems associated with overtreatment due to screening7 (as discussed in Chapter 1).
In summary, MRI has potential as a diagnostic adjunct to mammog-
6By a company known as Metaprobe (Pasadena, California), founded by Thomas Meade.
Page 84
raphy to eliminate unnecessary biopsies. It may also have a screening role in certain high-risk populations. Ongoing studies may provide the data necessary to define the appropriate applications of the technology. Technological advances may eventually lead to broader or different uses of MRI in the future, but more study and development must occur before that can be considered.
MAGNETIC RESONANCE SPECTROSCOPY
Proton magnetic resonance spectroscopy (MRS), a method that was originally developed by physical chemists to characterize large molecules in solution, can also be used to measure biochemical components of cells and tissues (Merchant, 1994). Metabolites can increase to abnormal levels in cancer cells and these changes may be detected in tissue samples and also in vivo by MRS. The method is under active investigation as a diagnostic adjunct to mammography and other accepted imaging techniques. It is being studied as an alternate method of analysis for fine-needle aspirates (FNAs; as opposed to cytology) and also as a method for assessment of lesions in vivo to avoid unnecessary biopsies.
Because cytological analysis of FNAs is quite variable depending on the experience and skill of the individuals collecting and assessing the sample, MRS has been studied as an alternate approach to diagnosis by analysis of FNAs. The first study demonstrating the potential of MRS to distinguish benign and malignant lesions by FNA measured choline and creatine levels in 190 samples by visual reading of the spectra obtained by MRS (Mackinnon et al., 1997). More recently, the MRS spectra of more than 150 FNA samples were analyzed by a new computerized statistical classification system. Malignant lesions were distinguished from benign tissue with an accuracy of 93 percent (Mountford et al., 2000).
Studies with tumor specimens obtained by biopsy have validated the ability of the technology to measure the biochemical differences between tumor samples and normal or benign breast tissues (Gribbestad et al., 1999). Recently, several small (10 to 40 women), preliminary studies that used noninvasive MRS have found that the elevated choline content of breast tumors can be detected in vivo as well (Gribbestad et al., 1998; Kvistad et al., 1999; Roebuck et al., 1998). These results suggest that MRS spectra, which are complementary to the images obtained by MRI, could potentially be used to characterize and diagnose breast lesions in a noninvasive manner. However, the high cost and low sensitivity and specific-
7 One of the reasons that the Papanicolaou smear for screening for cervical cancer has been so successful in reducing the rate of mortality from cervical cancer is that the intervention for early lesions is simple, effective, and well tolerated and accepted by women.
Page 85
ity of the method for the detection of small lesions must be overcome before in vivo breast MRS demonstrates its clinical utility.
SCINTIMAMMOGRAPHY
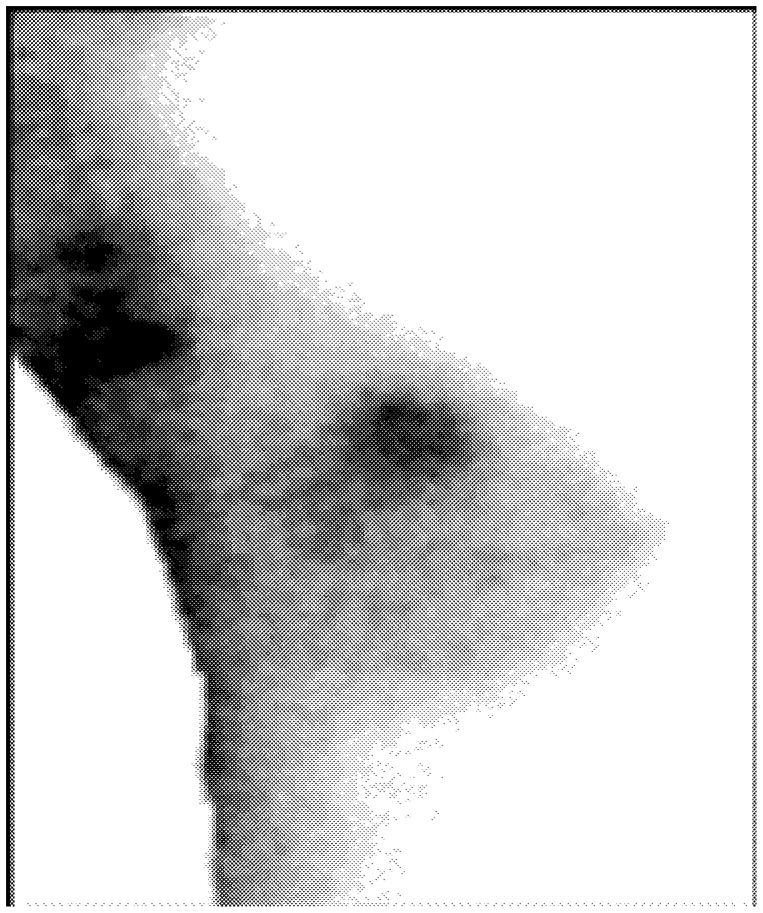
Unlike the imaging methods described thus far, in which the transmission of various forms of energy through the tissues is used to generate an image, nuclear medicine approaches rely on the emission of radioactivity from tracers that are injected into the body and that then accumulate in specific tissues. “Scintimammography” in particular uses radioactive tracers to produce an image of tumors and lesions in the breast and elsewhere ( Figure 2-7). It may be used as an adjunct to mammography to help distinguish between malignant and benign lesions. The tracers concentrate more in breast cancers than in normal breast tissues by a mechanism that is not fully understood but that may be related to the degree of cellular proliferation and vascular permeability. Several radioactive compounds are being investigated, although only one, technetium-99m sestamibi (MIBI), is approved by FDA for use in breast imaging. Scintimammography images the spatial concentration of the radiopharmaceuticals using a camera that detects gamma rays (a “gamma camera”)
~ enlarge ~
FIGURE 2-7 Example of scintimammography. Findings: Focal upake in the right breast in the area of a palpable mass. Source: Miraluma™ by duPont Merck Pharmaceutical Company.
Page 86
and may consist of traditional planar images or three-dimensional images generated by tomographic reconstruction (single-photon-emission computed tomography [SPECT]).
Because it uses radioactive compounds, scintimammography poses radiation health risks akin to those of imaging techniques that use X rays, although the small doses of radioactivity used are generally considered safe except for pregnant women and young children. MIBI imaging is generally more expensive than ultrasound imaging or diagnostic mammography but is less expensive than MRI (Allen et al., 1999).
Unlike mammograms, MIBI scans are not affected by dense breast tissue, breast implants, or scarring (Edell and Eisen, 1999). It has a limited ability to detect cancers smaller than 1 centimeter (cm), however, and MIBI imaging is less accurate for nonpalpable abnormalities than for palpable masses (Ziewacz et al., 1999). Studies indicate that the overall sensitivity of MIBI scans ranges from 75 to 94 percent and that the specificity ranges from 80 to 89 percent (reviewed by Edell and Eisen, 1999 and Stuntz et al., 1999). Based on an analysis of all published data, the Blue Cross/Blue Shield Association (BCBSA) Technology Evaluation Center (TEC) found that scintimammography did not meet its criteria (see Chapter 5) for differentiating between benign and malignant breast lesions in patients with suspicious mammograms or palpable masses (Blue Cross and Blue Shield Association Technology Evaluation Center, 1997). Its analysis of the pooled data found the sensitivity to be 94 percent for the detection of palpable masses but only 67 percent for the detection of nonpalpable lesions. Although one study of 150 women found that MIBI accurately predicted a benign lesion 97 percent of the time (negative predictive value, 97 percent) (Khalkhali et al., 1994), the pooled analysis found such predictions to be accurate for 91 percent of palpable lesions but only 69 percent of nonpalpable lesions. This BCBS TEC analysis predicted that of all the patients who would undergo a MIBI scan, 66 to 73 percent of patients could avoid a negative biopsy but 2 to 8 percent of patients would be exposed to the harms of undetected malignancy. Thus, the panel concluded that the negative predictive value of scintimammography was not sufficient to warrant its adoption as a diagnostic test.
Some clinicians also use MIBI imaging as a follow-up test for women whose mammograms indicate a mass that is “probably benign” and not suspicious enough to warrant a biopsy. Current practice is to recommend that these women have a repeat mammogram in 6 months, but since one-quarter of such women will not comply with the follow-up recommendations, MIBI can give an added level of protection against a delay in breast cancer therapy (Stuntz et al., 1999). In addition, MIBI scans can confirm a benign breast condition in women with palpable lesions whose mammograms or fine-needle aspiration results are inconclusive. MIBI scans can also detect multiple breast cancers that may be missed on a mammogram.
Page 87
Recent technical advancements may help to overcome some of the current limitations of scintimammography, such as the low resolution of MIBI scans. For example, high-resolution scintimammography (HRSM) increases resolution by using a gamma camera based on a new position-sensitive photomultiplier tube. In one study of 53 patients, it found lesions missed by standard MIBI scans and could detect a lesion as small as 7 mm (Scopinaro et al., 1999). Additional improvements in spatial resolution could further improve the clinical utility of MIBI scans for breast cancer detection. New radiopharmaceuticals may also play a role in the future use of scintimammography. Investigational compounds that show promise for breast imaging include technetium-99m, tetrofosmin and technetium-99m-MDP. These agents may be less expensive and more accurate than MIBI, although more studies are needed to determine this (Stuntz et al., 1999). Other radioactive compounds, such as thallium-201, have also been used to visualize breast tumors, but generally with less favorable imaging traits compared with those of MIBI (Ziewacz et al., 1999).
Scintimammography also has potential for use in functional imaging applications. One example of functional scintigraphic imaging uses SPECT to monitor multidrug resistance (Del Vecchio et al., 1999). Recent research suggests that MIBI can be pumped out of cells that overexpress the multidrug resistance P glycoprotein MDR1, the same mechanism that leads to resistance to chemotherapeutic agents. Rapid MIBI washout rates correlate with treatment failure. Preliminary studies suggest that patients who overexpress MDR1 and who fail breast cancer chemotherapy clear MIBI three times faster. SPECT imaging for the detection of multidrug resistance could potentially allow more individualized treatment planning by identifying those patients likely to fail chemotherapy. P-glycoprotein inhibitors, which may increase the efficacy of chemotherapy regimens in women who overexpress MDR1, are now entering phase II/III clinical trials. These would be most effective in patients who overexpress MDR1, as predicted by MIBI washout rates. Studies with many other molecular markers are also actively under way, but again, one difficulty may be in choosing the appropriate markers for use in assessment and monitoring.
In summary, scintimammography has shown diagnostic potential as an adjunct to mammography, but technical limitations such as resolution have precluded it from becoming more widely used. Although it has FDA approval, the current data do not justify its implementation on a standard basis. Technological improvements and novel radioactive compounds could potentially improve its utility, but at the moment its future is uncertain. The method also has potential for use in functional imaging applications, but further study and development are needed.
Page 88
POSITRON EMISSION TOMOGRAPHY
Positron emission tomography (PET) uses radioactive tracers such as labeled glucose to identify regions in the body with increased metabolic activity (Phelps, 2000). Because malignant tissue tends to metabolize glucose in a manner different from that of tissue with benign abnormalities, researchers have used PET to discern malignant from benign lesions in many organs and tissues, including the breast. Preliminary small studies indicate that PET scans have sensitivities between 80 and 100 percent and specificities between 75 and 85 percent, but more studies are needed to assess the clinical utility of PET scans for use in breast cancer diagnosis. In theory, scanning by PET could prove useful for the detection of breast cancers in women with dense breasts, implants, or scars. However, the inability to biopsy lesions that are identified by PET but that are not visible on a mammogram is a major impediment to accurate diagnosis (Stuntz et al., 1999). PET scanners are also quite expensive and not widely available, and the agents used are expensive to make and last only a short time. On the horizon, however, are less expensive, more commercially available PET systems and simpler radiopharmaceutical production methods, both of which could improve the usefulness of PET scans for the detection of breast malignancies (Edell and Eisen, 1999).
Researchers are also exploring the use of radioactive antibodies that target breast malignancies (Goldenberg and Nabi, 1999). These include antibodies to carcinoembryonic antigen and antibodies against other proteins that may be prevalent in breast cancer cells, such as certain growth factor receptors. Although some of these agents show promising sensitivity and specificity for the detection of breast cancer, most of the studies conducted to date have been small and the scans can be difficult to interpret. More clinical studies are needed to determine the roles of these radioactive antibodies in the diagnosis of breast cancer. A primary focus of imaging research in this area is on the development and validation of appropriate markers for breast cancer evaluation.
OPTICAL IMAGING AND SPECTROSCOPY
Investigators are developing a variety of devices and agents to aid the in vivo diagnosis of breast cancer by optical methods (Alfano et al., 1997). The use of light to image lesions in the breast was first reported by Cutler in 1929 and consisted of simple transillumination, performed by placing a light source against the breast and observing differences in the transmission of light through the tissue. During the 1980s, a digital transillumination system that used two light wavelengths (also known as diaphanography) was developed and tested, but the results were conflicting, and many systems showed low sensitivity and specificity (Moskowitz et
Page 89
al., 1989). Thus, FDA approval was not granted8 and commercialization of the technology did not go forward.
Past attempts to image tissues with light were severely restricted by the overwhelming scatter that occurs when optical radiation spreads through tissue; however, recent innovations in optical technologies have renewed interest in potential applications for breast cancer detection and characterization (Bosanko et al., 1990; Hebden and Delpy, 1997). Currently, the two main areas of interest in this field are optical spectroscopy to characterize the structure and biochemical contents of lesions and optical imaging (or tomography) to localize as well as characterize the lesions in the tissue. In each case, the procedures are being tested as an adjunct to mammography to distinguish benign and malignant lesions and thus eliminate unnecessary biopsies.
“Optical biopsy” via spectroscopy is one promising technology under investigation as a minimally invasive means of diagnosis of breast cancer (Bown et al., 2000). By exploiting the unique in vivo optical properties of normal and cancerous tissues, optical biopsy techniques may be able to discriminate between a tumor and its surrounding normal tissue in real time. For several years, researchers have been developing an optical biopsy technique known as “elastic scattering spectroscopy” (ESS) for the diagnosis of cancer. The ESS system, which is portable and which is designed for convenient clinical use, involves shining a pulse of light through an optical fiber that is placed in contact with the tissue and then performing spectral analysis on the light that is reflected back through a small volume of tissue. The resultant spectrum is influenced by light scatter due to the cellular and subcellular architectures of the tissues, as well as light absorption by chromophores in the tissue. Computer algorithms are required for the spectral analysis, and artificial neural networks are being tested for this purpose.
Tumors can be detected and cancer can be diagnosed by using spectral measurements because, in addition to significant architectural changes at the cellular and subcellular levels compared with the architecture of normal tissues, tumors may also have altered levels of natural chromophores such as hemoglobin. This approach generates spectral signatures that are relevant to the tissue parameters that pathologists address: the sizes and shapes of nuclei, the ratio of nuclear volume to cellular volume, clustering patterns, vascularity, and so on. ESS analysis is frequently mediated through endoscopes, and a few small clinical studies on the endoscopic application of ESS to bladder cancer and gastrointestinal pathologies have been published (Bohorfoush, 1996; Mourant et al., 1995). The approach is more challenging with solid organs such as the breast,
8Minutes from the FDA advisory panel meeting, 1991.
Page 90
but if ESS measurements of breast tissue were reliable, ESS could eliminate many unnecessary surgical biopsies, and the instant diagnosis could improve surgical procedures for breast cancer. Clinical studies are under way to assess the potential of the diagnostic application of ESS with a transdermal needle for the diagnosis of breast cancer. Instant diagnosis by ESS with a needle of the same size used for fine-needle aspiration cytology could reduce patient anxiety (i.e., the anxiety that occurs while waiting for a diagnosis) and, in some cases, permit immediate treatment.
Another potential application of endoscopy in breast cancer diagnosis is known as “ductoscopy.” In this case, a fiberoptic endoscope is threaded through the milk ducts of the breast via the nipple orifice. Such an approach can facilitate optical characterization of ductal lesions and could potentially be combined with microsampling methods such as tube currette cytology (Love and Barsky, 1996).
Optical imaging or tomography, which is relatively inexpensive and simple in comparison with many other imaging modalities, is also actively under investigation for a variety of cancers, including breast cancer. The technique uses light in the near-infrared range (wavelengths from 700 to 1,200 nm), which is nonionizing, to produce an image of the breast. Potential advantages of the technology include speed, comfort, and non-invasiveness. An optical scan can be taken in less than 30 seconds by simply placing an image pad over the breast without compression (Chance, 1998). Optical imaging methods offer the potential to differentiate between soft tissues that are indistinguishable by other modalities, and specific absorption by natural chromophores (such as hemoglobin) can also provide biological or functional information. Optical scanning images can also be digitized, thus allowing image manipulation, serial studies, and analysis by computer algorithms. However, hurdles that must be overcome before this technology reaches the clinic relate to accuracy and resolution, which are not yet optimized. In particular, the target-to-background ratios tend to be low. Furthermore, the physiology and thus the optical characteristics of normal and neoplastic breast tissues can be quite variable depending on the age, hormone status, and genetic background of the woman (Thomsen and Tatman, 1998).
Optical imaging systems are being commercially developed by
Imaging Diagnostic Systems Inc.9
(IMDS; Plantation, Florida), DOBI10
Medical Systems (Mahwah, New Jersey), and Advanced Research and Technology, Inc (ART; Montreal, Canada).11
The DOBI technology is based on
9Computed tomography laser mammography (CTLM;
www.imds.com/ctlm.htm).
10Dynamic optical breast imaging (DOBI;
www.dynamicsimaging.com).
11Softscan™ laser mammography was developed by ART in cooperation with the National Optics Institute of Canada, which does optical research for organizations such as the National Aeronautics and Space Administration and industrial multinational corporations.
Page 91
optical detection of angiogenesis in malignant lesions, whereas the IMDS and ART technologies use laser-based technologies to assess various optical properties of breast abnormalities. All three companies are conducting clinical trials for FDA approval for diagnostic use of their devices, but they also plan to pursue a screening approach in the future.
Optical contrast agents (Ntziachristos et al., 2000) that are selectively taken up by tumors in a fashion similar to that for the contrast agents used for MRI may improve the sensitivity and specificity of the technology, but their clinical utility is undefined. By using novel contrast agents that fluoresce after cleavage with specific enzymes, the technology also has the potential to show functional changes associated with cancer initiation and progression, but this application is at an even earlier stage of development (Mahmood et al., 1999). In addition, new types of lasers that emit rapid pulses of energy rather than a continuous wave were recently developed by physicists and may provide additional benefits to the technological advancement of this detection method.
In summary, optical imaging has long been thought to have potential as a means of breast cancer detection, but to date that potential has not yet been realized. Significant technological improvements in recent years may eventually propel this technology into the clinic, but a conclusion cannot yet be reached about its future utility. Optical biopsy methods were proposed more recently, but it is too early in the development stage to assess their clinical potential. Further studies of both applications are needed and are ongoing.
THERMOGRAPHY
Infrared thermal imaging has been used for several decades to monitor the temperature distribution over human skin. Abnormalities such as malignancies, inflammation, and infection cause localized increases in temperature that appear as hot spots or asymmetrical patterns in an infrared thermogram. Thermography, alternatively termed “thermometry” or “thermology,” was pursued for many years as a technique for breast cancer detection. Studies of thermography have focused on a range of potential uses, including for diagnosis, prognosis, and risk indication and as an adjunct to existing technologies; however, the results have been inconsistent and scientific consensus has been difficult to achieve. Thermography was largely abandoned in the 1970s, but technological advances in the intervening years have renewed interest in the technique. The use of infrared imaging is increasing in many industrial and security applications, and the transfer of military technology for medical use has prompted this reappraisal of infrared thermography in medicine. Digital infrared cameras have much-improved spatial and thermal resolutions,
Page 92
and libraries of image processing routines are available to analyze images captured both statically and dynamically.
A breast tumor can raise the temperature of the skin surface by as much as 3 degrees C compared with the temperature of the skin surface of a woman with normal tissue (Foster, 1998). Although this phenomenon is not well understood, likely mechanisms include elevated rates of tumor metabolism and elevated levels of vascularity and perfusion (Foster, 1998; Anbar, 1995). The body dissipates the heat through emitted infrared radiation, which can be detected by infrared cameras; the diagnosis of cancer is based on the difference in temperature relative to that for the contralateral breast, which serves as a built-in control. The procedure is noninvasive and does not require compression of the breast or radiation exposure.
The first published report of breast cancer detection based on temperature measurement appeared in 1956 (Lawson), and through the 1960s and 1970s, thermography was actively studied and used clinically. At one point (before passage of the 1976 Medical Device Amendment requiring FDA approval for devices) between 2,000 and 3,000 thermography clinics were actively operating in the United States (Foster, 1998). In 1977, Stephen Feig published the results of the first large clinical trial (16,000 women) to compare thermography, xeromammography (an early form of mammography), and clinical examination. The sensitivity and specificity of thermography were demonstrated to be 39 and 82 percent, respectively. By comparison, the sensitivity of xeromammography was 78 percent, with a specificity of 98 percent (Feig et al., 1977). Around the same time, the Breast Cancer Detection Demonstration Project was launched with the intent of studying mammography, clinical examination, and thermography. However, thermography was dropped early in the study because of poor results, namely, high rates of false-positive results and a low level of sensitivity (Moskowitz, 1985). Following these studies, thermography of the breast largely disappeared, and the American Medical Association12 and the Canadian Association of Radiation Oncologists (1998) do not advocate it as a technique for breast cancer detection. However, technological developments in recent years have sparked new interest in the technique.
Modern digital infrared cameras can now image the breast with significantly improved spatial and thermal resolutions (Jones, 1998). Computerized image analysis software is also being developed to analyze and compare images of one breast with those of the other. The goal is to eventually quantitate the parameters of infrared abnormalities, thus cre-
12American Medical Association, Thermography update, H-175.988, AMA Policy Finder (http://www.ama-assn.org/apps/pf_online/pf_online).
Page 93
ating an objective measurement of abnormality (Head and Elliott, 1997). A system called dynamic area telethermometry (DAT) has been developed to detect changes in neuronal control of blood flow as evidenced by small changes in heat. Research has shown that malignancy disrupts normal blood flow, and thus, these changes may be evidence of cancer (Anbar et al., 1999).
OmniCorder Technologies has integrated the DAT system with a sensor technology called quantum well infrared photodetector (Anbar et al., 1999) in developing its BioScan system. In December 1999, OmniCorder was granted FDA clearance to use BioScan as an adjunctive technology for the diagnosis of breast cancer.13 The company has just begun to manufacture systems for distribution and is also conducting trials for other uses such as management of cancer therapy. Computerized Thermal Imaging, Inc., is also developing a system that records thermal images of breast tissues to construct a three-dimensional map of the breast; the system is being tested in clinical trials for FDA approval.14
ELECTRICAL POTENTIAL MEASUREMENTS
Studies indicate that rapid proliferation of epithelial tissue in the breast disrupts the normal polarization of the epithelium. This depolarization involves both the transmembrane electrical gradient and the transepithelial electrical gradient, which are associated with the orientation of the epithelial cells with respect to their apical and basolateral surfaces. The region of depolarization can extend beyond the immediate area of the tumor to the skin surface. Thus, abnormal electrical potential measurements at the skin surface of the breast can be used as an indicator of elevated epithelial proliferation suggestive of carcinogenesis (Cuzick et al., 1998; Faupel et al., 1997; Fukuda et al., 1996). Consequently, this method has been studied for use as a tool for the diagnosis of breast cancer, with the hope of avoiding unnecessary biopsies after mammography.
Biofield Corporation (Roswell, Georgia) was the original developer of the technology that uses electrical potential measurements for the detection of cancer. As a result, the technology is often referred to as the Biofield breast exam (BBE). BBE uses an array of electrical potential sensors placed over both breasts and axillae. Reference sensors are also placed over both
13OmniCorder Technologies, Inc., The BioScan System, accessed May 2000
(http://www.omnicorer.com/dat.html).
14University of Southern California. Study examines non-invasive way to detect cancer of the breast, accessed May 2000
(http://www.usc.edu/hsc.info/pr/1vol3/329/parisky.html).
Page 94
palms. Following an equilibration period, average voltages are recorded for each of the electrical potential sensors. Differences in electrical potential can be calculated both between sensors on the symptomatic breast and between sensors on the symptomatic breast and the contralateral breast.
One unique feature of BBE is that it gives a single, numerical result that objectively determines whether the lesion is considered malignant or benign. Conversely, tests such as mammography rely on the subjective interpretation of the data by a trained reader. BBE is also relatively inexpensive because it uses very basic equipment and does not require an expert reader. It is noninvasive and not uncomfortable to women, and the procedure can be performed in less than 15 minutes (Faupel et al., 1997).
BBE has been tested primarily as a diagnostic tool for women with palpable breast lesions or nonpalpable lesions identified by mammography or ultrasonography. Two clinical studies of diagnostic BBE have been conducted. All of the women in the studies received a BBE followed by a biopsy. The electrical potential differences between sensors were retrospectively analyzed in light of the biopsy outcomes to determine which weighted sum of measurements best predicted the biopsy outcome. In the first study, which included 101 women, BBE was found to have a sensitivity of 90 percent and a specificity of 60 percent. It was also observed that for cancers measuring less than 2.5 cm, the sensitivity of BBE was 95 percent. The investigators speculated that the test's reduced sensitivity to larger tumors could be associated with the tissue necrosis seen in larger tumors. There were, however, only 19 tumors less than 2.5 cm, so this preliminary calculation of sensitivity for patients with small tumors must be validated (Fukuda et al., 1996). In a second study, which included 661 women at eight different centers, BBE was found to have a sensitivity of 90 percent and a specificity of 55 percent for women with palpable lesions (Cuzick et al., 1998).
Although Biofield has submitted a premarket approval (PMA) application, BBE has not yet been approved by the FDA and so is not used clinically in the United States. However, Biofield has received CE Mark
Certification15 for its diagnostic system, which allows the company to sell
15Since 1992, the European Parliament has enacted a series of directives intended to provide controls on product design, with the principal objective being to provide a “level playing field” for product safety requirements across the European Community. The Medical Devices Directive was enacted to provide for a harmonized regulatory environment for all medical devices sold within the European Economic Area (EEA). All products that fall within the scope of the directive must meet certain essential safety and administrative requirements and are to be marked CE to show that they comply. Such products may then be freely sold throughout the EEA without being subject to additional national regulations.
Page 95
the system in Europe. The device was certified as a diagnostic adjunct to mammography or physical examination in younger women with suspicious palpable breast lesions.
ELECTRICAL IMPEDANCE IMAGING
Transmission of a low-voltage electrical signal through the breast can be used to measure the electrical impedance of the tissues (Figure 2-8). Cytological and histological changes in cancerous tissue, including changes in the cellular and extracellular contents, electrolyte balances, and cellular membrane properties, can significantly decrease the impedance of cancerous tissue (by a factor of approximately 40 relative to that of normal tissue) (Kleiner, 1999). Electrical impedance imaging of the breast is painless, does not compress the breast, and does not use ionizing radiation. The technology also works equally well for women of all ages, including young women with dense breasts and women on estrogen replacement therapy.
TransScan Medical (Ramsey, New Jersey) has developed an electrical
~ enlarge ~
FIGURE 2.8 Example of an electrical impedance image of the breast. White Spots in the center of the displays are the nipples. The white spots in the outer sectors identified by the arrows were found to be invasive ductal carcinoma on biopsy. Source: TransScan Medical, Ramsey, NJ.
Page 96
impedance imaging device (the T-Scan 2000) as a diagnostic adjunct to X-ray mammography. The device transmits a 1-volt electrical signal through the breast via an electrode on the patient's arm. A clinician measures the electrical signal at the surface of the breast with a handheld probe containing an array of electrodes. The electrical signal is used to create a real-time, computer-displayed image of the impedance of the underlying breast tissue. Regions of low impedance suggestive of cancer are displayed as bright areas on the computer screen. The combined results of several studies conducted by TransScan Medical indicated that the T-Scan 2000, when used in conjunction with mammography with a targeted population, improved the diagnostic sensitivity by 15.6 percent and the diagnostic specificity by 20.2 percent over those of mammography alone.16 TransScan Medical has predicted, on the basis of published cancer prevalence estimates and the size of the annual screening population (25 million women in the United States), that the device could increase the number of early cancers detected by 8,000 to 9,000 and decrease the number of negative biopsies by 200,000 to 300,000. In 1999, the FDA granted premarket approval to TransScan Medical for their electrical impedance imaging device, the T-Scan 2000, for use as a diagnostic adjunct to X-ray mammography. TransScan Medical will distribute the T-Scan 2000 within the United States, and Siemens Medical Systems, Inc. (Iselin, New Jersey), has exclusive rights to distribute the T-Scan 2000 device outside of the United States. The company continues to conduct additional studies to further validate the technology.
A spectroscopic electrical impedance tomography (EITS) imaging system has also been evaluated with a very small number of women. Structural features in the EITS images have correlated with limited clinical information available on participants with benign and malignant abnormalities, cysts, and scarring from previous lumpectomies and follow-up radiation therapy (Osterman et al., 2000).
ELECTRONIC PALPATION
Electronic palpation uses pressure sensors to quantitatively measure palpable features of the breast such as the hardness and the size of
lesions17
(Oncology News, 1999). Manual physical examination of the breast currently contributes significantly to cancer detection, but it is inherently
16Center for Devices and Radiological Health, FDA, Radiological Devices Panel Meeting, August 17, 1998, accessed April 3, 2000
(http://www.fda.gov/ohrms/dockets/ac/98/transcpt/3446t1.rtf).
17The clinical assessment of electronic palpation technology: a new approach for the early detection and monitoring of breast lesions, available online
(http://www.assurancemed.com/techspec.html).
Page 97
subjective. Electronic palpation offers the potential to standardize the performance, documentation, and serial monitoring of physical breast examinations. Assurance Medical (Hopkinton, Massachusetts) and Ultratouch (Paoli, Pennsylvania) are developing electronic palpation devices.
Assurance Medical has developed a system that uses an array of pressure sensors mounted in a handheld device that is gently pressed against the breast and moved over its surface (Assurancee Medical, Inc., 1999). The resistance of the breast to the device is measured by the pressure sensors and is used to create a computer-generated image of the hardness of the underlying breast tissue. This image serves as a quantitative, objective measurement of the hardness, discreteness, and size of breast lesions for diagnosis.
The company is seeking FDA premarket approval for use of its device to measure and track the size of known, suspicious lesions. The company is testing the accuracy and reproducibility of its device with 400 women with manually palpable lesions. In that study, trained physicians or nurses first estimate the size of each lesion by manual palpation, and electronic palpation is then used to estimate the size of each lesion. The company hopes to demonstrate that there is less variability between size measurements taken by electronic palpation than by manual palpation. In cases in which the lesion is surgically removed, the electronic and manual palpation measurements are being compared with the size of the lesion as measured by a pathologist to assess the accuracy of the device.
According to the company, preliminary studies suggest that the technology might also be useful for screening. In a study with 137 women in whom 118 lesions were identified by clinical breast examination or mammography, electronic palpation successfully identified 96 of 102 palpable lesions and 12 of 16 nonpalpable lesions, for overall sensitivities of 92 percent for electronic palpation and 86 percent for clinical breast examination. Additional studies are needed to assess the specificity of electronic palpation.
A robotic device (Palpagraph™), developed by UltraTouch, has a single mechanical finger designed to mimic the action of a human finger to map relative breast density. A digital camera and other optical imaging systems create a virtual computer image of each breast consisting of cubic cells between 1 and 4 mm on a side. The robotic mechanism, guided by the virtual image, brings the mechanical finger to the center of each cell on the surface of the breast. For each surface cell, the robotic mechanism applies a series of gentle pulses to the finger, and the response is measured to fill in the underlying virtual cubic cells with density data. The finger lifts away from the breast and moves to the center of the adjacent surface cell until the entire breast, including the axillary areas, has been mapped. An average Palpagraph™ examination will take 10 to 20 min-
Page 98
utes and does not involve breast compression. An initial study, which was undertaken in Iran, tested the device in a screening setting with 850 women. The subjects, 90 percent of whom were under age 50, were screened two to three times in 6 months. The sequential palpagrams were compared to find tumors that were growing, becoming more fixed, or becoming more dense. Palpagraphy detected 22 lumps ranging from 2 to 9 mm in diameter that warranted biopsy (those in which the diameter was greater than about 4 mm). Of these lumps, eight were judged to contain malignant cancer. No consistent effort was made to detect the 22 lesions by mammography.18 The company is now preparing for clinical trials in the United States for FDA approval of the device. The device will be tested first with a population of women referred for diagnostic workup for possible breast cancer, who will be examined by mammography, palpagraphy, and clinical breast examination.
EXAMPLES OF TECHNOLOGIES IN VERY EARLY STAGES OF DEVELOPMENT
Thermoacoustic Computed Tomography
Thermoacoustic computed tomography (TCT) exposes the breast to short pulses of externally applied electromagnetic energy. Differential absorption induces differential heating of the tissue followed by rapid thermal expansion. This generates sound waves that are detected by an array of ultrasonic transducers positioned around the breast. Tissues that absorb more energy expand more and produce more sound. The timing and intensity of the acoustic waves are used to construct a three-dimensional image of the irradiated tissue (Kruger et al., 1999).
When the incident electromagnetic energy of TCT is visible light, the thermoacoustic effect is also referred to as the “photoacoustic effect.” The photoacoustic effect was first described by Alexander Graham Bell in 1861 and has been applied primarily to the spectroscopic analysis of gases, liquids, and solids (Rosencwaig, 1975). Although the thermoacoustic effect has a long scientific history, its application to medical imaging is still in the early stages of development.
TCT does not use ionizing radiation and does not compress the breast. As currently designed, the TCT ultrasonic transducers are arrayed around a hemispheric bowl that is filled with deionized water. The device is mounted beneath a table. To image the breast, the woman lays prone on the table with her breast immersed in the water through a hole in the table. The breast is scanned in approximately 1.5 minutes.
18Jeff Garwin President, UltraTouch Corporation, personal communication.
Page 99
One major limitation of traditional X-ray mammography is that it creates a two-dimensional projection of the breast that is highly dependent upon the orientation of the breast, the X-ray source, and the detector. Because TCT images retain three-dimensional structural information, unlike the images obtained by X-ray mammography, the images of a woman's breast obtained by TCT are highly consistent. Because there is less variability in the images, changes should be more apparent and easier to track longitudinally by TCT than by X-ray mammography. Three-dimensional images are, however, potentially more difficult to display and analyze, and therefore, the time and cost required for image retrieval and analysis are potentially greater for TCT than X-ray mammography.
The contrast in a TCT image is determined primarily by the electromagnetic absorption properties of the tissue being imaged (Kruger et al., 1999). Different tissues absorb electromagnetic waves of different frequencies. For radio waves in the range of 200 to 600 megahertz (MHz), there is sevenfold difference between the most and the least absorptive soft tissues. For comparison, there is only a two-fold difference between the most and the least absorptive soft tissues at X-ray frequencies (Kruger et al., 1999). In the range of 300 to 500 MHz, cancerous tissue is two to five times more absorptive than comparable noncancerous tissue, presumably because of the increased water and sodium contents of malignant cells (Chaudhary et al., 1984; Joines et al., 1980, 1994).
The electromagnetic wave pulse, the acoustic properties of the tissue, the geometry of the ultrasonic detector array, and the image reconstruction algorithm determine the spatial resolutions of TCT images (Kruger et al., 1999). To date, the leading developer of TCT, Optosonics, Inc. (Indianapolis, Indiana), has achieved in vivo imaging of the human breast with a spatial resolution of 1 mm up to a depth of 40 mm.19
The development of TCT is still in its early stages.20 To date there have been no large published clinical trials, although Optosonics is planning to conduct an exploratory study with 80 women this year in conjunction with the Indianapolis Breast Center.
Microwave Imaging
Confocal microwave imaging is a new technique that uses the differential water content of cancerous tissue versus that of noncancerous tissue to detect tumors. The technique transmits short pulses of focused, low-power microwaves into the breast tissue, collects the back-scattered
19Robert Kruger, Optosonics, Inc., personal communication, March 8, 2000.
20The original patent for the “Photoacoustic Breast Scanner” was issued in 1998 (RA Kruger, U.S. patent 5713356).
Page 100
energy via antennas positioned around the breast, and compounds these signals to produce a three-dimensional image of the breast. Normal tissue is mostly transparent to microwaves; however, the higher water content of malignant tissue and the differences in the dielectric properties of tumor tissue versus those of breast fatty tissue cause significantly more scatter of microwave energy, thus enabling detection of tumors (Meaney et al., 1999). Confocal microwave imaging has several attractive features. It does not require breast compression and does not use ionizing radiation. In theory it will produce a high-contrast three-dimensional image of the breast and should be equally effective for women with dense breasts.
Confocal microwave imaging of the breast is being developed primarily by researchers at the University of Wisconsin-Madison, Dartmouth, and Northwestern University. The work is an extension of other non-medical applications of focused microwave imaging including groundpenetrating radar for detection of land mines and detection of concealed weapons at airports (Microwave News, 2000).
Many techniques for microwave imaging of the body have been explored by other researchers, including the detection of the passive emission of microwaves by the body, microwave thermography, and active examination of the body by use of narrowband microwaves, which should provide better resolution (Larsen and Jacobi, 1986). The development of these techniques has been constrained by poor resolution, poor penetration to the required tissue depths, excessive power requirements that result in the delivery of potentially unsafe levels of microwaves to the patient, and computationally challenging techniques for image reconstruction (Bridges, 1998; Fear and Stuchly, 1999).
To date, confocal microwave imaging research has emphasized theoretical validation of the technique through computer modeling (Fear and Stuchly, 1999) and measurements of the high-frequency electrical properties of excised breast tissue. As part of the modeling, researchers have considered different antenna arrangements, tumor sizes and placements, breast sizes, and tissue compositions. The results of the modeling suggest that tumors as small as 2 mm should be detectable at a depth of 4 cm. It will probably be several years before the technique is tested with any significant number of women (Hagness et al., 1998).
Hall Effect Imaging
Hall effect imaging (HEI) is a new general-purpose imaging technique being developed on the basis of the classical Hall effect discovered in 1879 by Edwin Hall (Graham-Rowe, 1999; Wen et al., 1998; Wen, 1999). HEI induces vibrations in charged particles by passing an electric pulse through an object while it is exposed to a strong magnetic field. The vibrating particles produce sound waves that can be detected by ultra-
Page 101
sonic transducers and that can be used to create a three-dimensional image of the object.
Different materials vibrate differently according to their electrical properties. As with other emerging imaging technologies including microwave imaging and TCT, HEI is being developed to exploit the electrical properties of tissues in the body, which vary widely with tissue type and pathological state.
Although the Hall effect has been used for many years by nonmedical disciplines, it is unclear whether it will develop into a technique suitable for imaging of humans. HEI is still in its infancy: the first published account of HEI only appeared in 1998. To date HEI has been tested only with excised and simulated tissue.
Perhaps the biggest limitation to the future application of the technology is the cost. As with MRI, HEI will require an expensive, superconducting magnet to produce a sufficiently strong magnetic field. Cost alone would likely limit its usefulness as a breast cancer screening technology, but if the technique is developed, it might be useful for limited, specific populations of women, as has been the case with MRI.
Magnetomammography
Magnetic source imaging of the breast, magnetomammography (MMG), is a new technique being investigated by using extremely sensitive Superconducting Quantum Interference Device (SQUID) magnetometers.21 Researchers hope to use SQUID magnetometers to detect magnetic, tumor-specific agents introduced into the body intravenously. This is similar in principle to scintimammography, except that magnetic agents and SQUID magnetometers will replace radionuclides and gamma cameras.
SQUID magnetometers have been used clinically in a limited number of research centers for many years to detect magnetic fields produced by electrical activity in parts of the body such as the brain (magnetoencephalography) and the heart (magnetocardiography) (Clarke, 1994). MMG research is focused on developing an agent that is both magnetic and highly specific to cancerous tissue. At present, no such agent is available, and so MMG remains untested. In theory, MMG should be equally effective for women with dense breasts and would not require breast compression or ionizing radiation.
One limitation of scintimammography, which MMG will similarly have to address, has been its lack of sensitivity to some types of lesions.
21Robert Kraus, Los Alamos National Laboratory, personal communication.
Page 102
As with gamma cameras, SQUID magnetometers have poor spatial resolution. The contrast resolution may be sufficiently high to detect small tumors, provided that they have sufficient volume and provided that after injection the ratio of the concentration of the exogenous magnetic agent in the lesion to the background concentration is high. The uptake by cancerous tissue of sestamibi, one of the best agents presently available for scintimammography, is about three times that by surrounding non-cancerous tissue, but it has not yet demonstrated efficacy when it is used to detect small, nonpalpable tumors. A further difficulty is that the computational strategies needed to generate MMG images of magnetic sources are much more complex than those needed for scintimammography.
Provided a suitable magnetic agent can be developed, one of the biggest obstacles to be overcome for the implementation of MMG will be cost. SQUID magnetometers are expensive to fabricate and operate. Because they are superconductors, they must be cooled with either liquid helium or liquid nitrogen, neither of which is easily available in all areas of the country. SQUID magnetometers must also often be operated in expensive, magnetically shielded environments because the physiological signals that they are designed to measure are extremely small and easily drowned out by the earth's magnetic field and other background magnetic fields. It remains to be seen whether such special provisions will be required for MMG.
Three-Dimensional Interactive Visualization
Three-dimensional interactive visualization techniques, including virtual reality, radically alter how individuals interact with computers to understand digital data. Many components of three-dimensional interactive visualization technology have been developed for other nonmedical applications (e.g., target recognition and flight simulators) and could potentially be applied to breast imaging. Several pioneer research groups have already demonstrated improved clinical performance using three-dimensional interactive imaging, planning, and control techniques (e.g., breast MRI). Three-dimensional interactive visualization could potentially be used in breast imaging for visualization, training, procedure planning, procedure support, and prognosis. However, significant improvements in virtual reality technologies are still required, including novel algorithms for breast imaging, before this potential can be realized.
SUMMARY
At present, mammography is the only technology suitable for screening of the general population for breast cancer. It therefore serves as a “gold standard” with which new technologies will be compared. How-
Page 103
ever, this standard is imperfect, and thus, improvements in the sensitivity and specificity of mammography itself could potentially affect mortality and morbidity from breast cancer and the overall cost of screening. Many technical improvements have been made to FSM since its initial introduction, but it is not known whether these changes have led to better survival rates among screened women. Many have considered digital mammography to be a major technical improvement over traditional FSM, but studies to date have not demonstrated meaningful improvements in screening sensitivity and specificity. Although it could be argued that studies thus far have not directly tested the full potential of FFDM through the use of soft-copy image analysis, difficulties remain with regard to the limited spatial resolution and luminance range of soft-copy display. The technology could potentially facilitate novel techniques such as tomosynthesis and digital subtraction mammography with X-ray-based contrast agents, but the value of these methods has not yet been proven. Digital mammography could also potentially improve the practice of screening and diagnostic mammography in other ways, for example, by facilitating electronic storage, retrieval, and transmission of mammograms. CAD has also shown potential as a means of improving the accuracy of screening mammography, at least among less experienced readers, but again, questions remain as to how this technology will ultimately be used and whether it will have a beneficial effect on current screening practices.
Mammography is particularly limited in young women. Because breast cancer is the principal cause of death for women ages 35 to 50, efforts have been made to identify alternate or complementary screening approaches for young women at high risk. Magnetic resonance imaging and ultrasound have been studied most extensively in this regard and show considerable promise for this select population. To date, however, the data are not yet sufficient to draw sound conclusions with respect to the appropriate screening applications of these technologies. That may change in the near future, as several large studies are ongoing. Ideal detection performance may ultimately depend on multimodality imaging, as no single imaging technology can provide a high signal-to-noise ratio in all circumstances or is able to detect all significant lesions.
Most of the imaging technologies for breast cancer detection described in this chapter are being developed as diagnostic adjuncts to mammography, with the goal of avoiding unnecessary, invasive biopsy procedures. Some, such as ultrasound and MRI, may also be used in conjunction with new minimally invasive therapeutic methods that are under development. Other technologies, such as functional imaging modalities, offer additional promise as both detection modalities and prognostic aids and could potentially shift the paradigm of cancer detection, but advances in this area will require further research to identify the appropriate biological markers to be examined. If and when these developing technologies
Page 104
are adopted for such diagnostic or prognostic applications, they may also be further examined as screening modalities. However, most of the technologies are not far enough along in development to adequately assess or predict their future application or value.
Ultimately, a new technology for early breast cancer detection will be beneficial only if it can lead to a reduction in the morbidity and mortality associated with the disease. Thus, improved methods for early detection of breast cancer may bring new challenges as well as opportunities for intervention. If the information generated by new technologies cannot be acted upon appropriately to improve outcomes, then women are not likely to benefit from the technological advances. Furthermore, as imaging methods become better and better at finding very small, early lesions such as carcinoma in situ, treatment decisions can be difficult to make because so little is known about the malignant potential of these premalignant cells (Tabar et al., 2000). As a result, some women may face the diagnosis of breast cancer and the subsequent therapy for a lesion that may never have become a lethal, metastatic cancer. Research efforts into the biology and etiology of breast cancer must therefore also continue, as discussed in Chapter 3. Moreover, improvements in the understanding of breast cancer progression should lead to treatment advances, and these combined changes could eventually alter both the use and the assessment of imaging tools.


















































