Page 325
7
Building and Supporting the Research Enterprise
The foundations of scientific progress are laid in the building and maintenance of the research enterprise. In simplest terms, this means getting the “right” people in the “right” places, and that is the essential role of research managers. For biomedical research, this encompasses five key domains:13
1. Research funding
2. Human resources
3. Infrastructure
4. Clinical trials
5. Biotechnology and pharmaceutical firms
The drive to end the devastating effects of multiple sclerosis (MS) rests on building and sustaining these key domains of biomedical research, which are discussed in turn below. The accompanying box provides a snapshot of the National Multiple Sclerosis Society's research programs (Box 7.1).
RESEARCH FUNDING
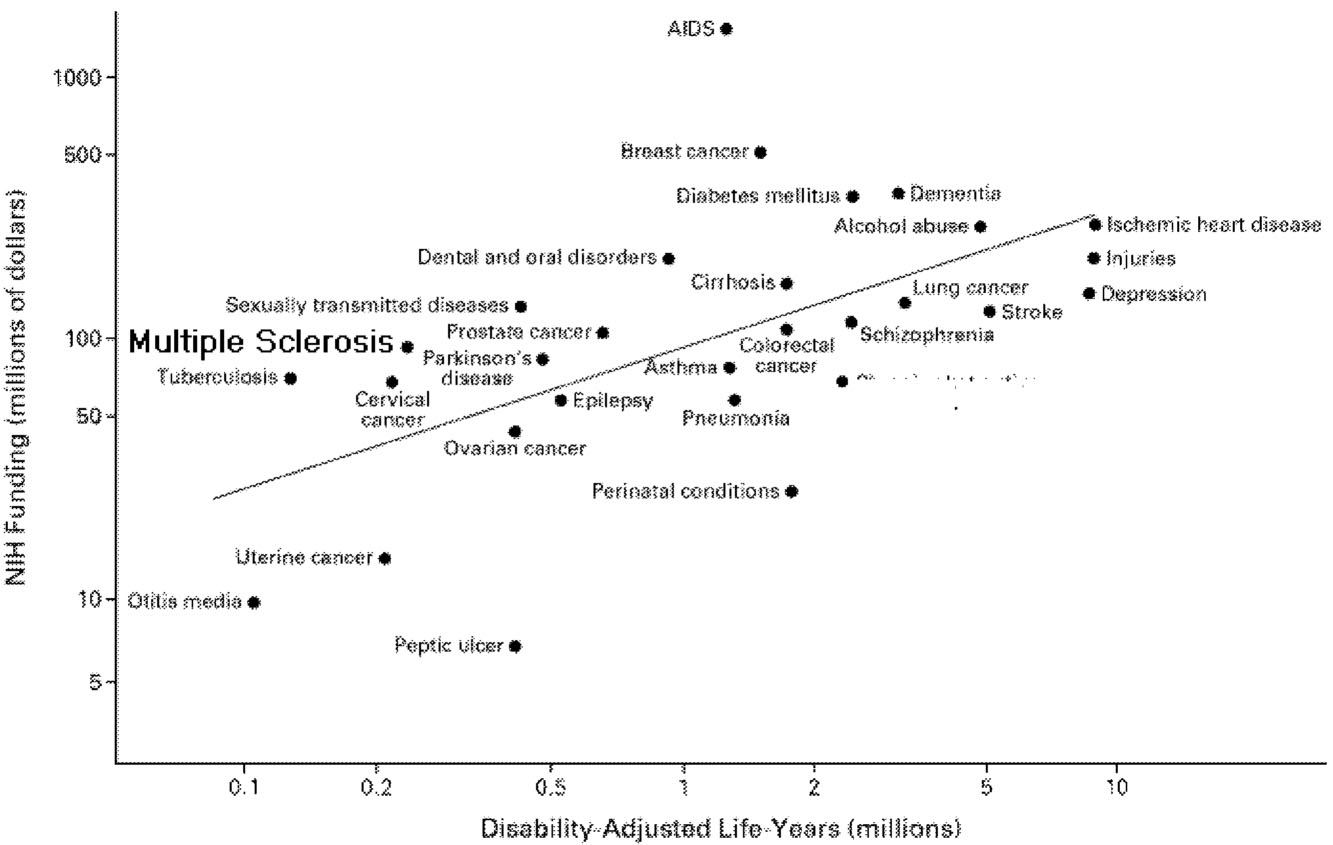
According to one analysis that compared funding levels at the National Institutes of Health (NIH) to the total burden of specific diseases, MS funding is higher than might be expected (Figure 7.1).8 In terms of funding from private health foundations that target specific diseases, MS research is also relatively well supported, at least in the United States. The research budget of the MS
Page 326
BOX 7.1National Multiple Sclerosis Society Research ProgramsThe society is the largest private sponsor of MS research in the world. Support for basic and clinical research is provided in the form of research grants and contracts, training programs, faculty awards, pilot project grants, workshop support, and other award programs including targeted, society-initiated projects. Most research program money is spent on full research grants (averaging $114,000 per year), followed by postdoctoral fellowships (averaging $32,000 per year). The top area of research emphasis is immunology, followed by glial biology (study of myelin-making cells and other support cells of the brain that do not conduct nerve signals) and infectious triggers. Some 42 percent of all newly funded research projects focus on humans or human tissues; the remaining funds support fundamental research. In the spring 1999 review cycle, annual research grant awards ranged from $70,960 to $251,855, averaging about $114,000. These awards include no more than 10 percent indirect costs. The society's research awards exceed those of other major voluntary health agencies. For example, the American Diabetes Association gives a maximum of $100,000 (including indirect costs) per year for research grants. The American Cancer Society gives a maximum stipend of $32,000 per year for postdoctoral fellowships. By contrast, National Institutes of Health research grants currently average $255,000 annually, including indirect costs, which can exceed 50 percent of total direct costs. In fiscal year 1998 (October 1, 1997-September 30, 1998), research and related expenses accounted for 18 percent of combined chapter and home office expenses and 37 percent of expenses of the home office. During FY 1999, the society supported more research than in any previous year—expending $22.5 million (unaudited) for all research programs and support activities, including some 333 new and ongoing MS research projects. Applications are reviewed and funded in two cycles per year. Research grant proposals are reviewed in the fall and spring; fellowships are reviewed once each year in the spring (for July 1 startups); pilot projects are reviewed and approved on a year-round, ad hoc basis; and health care delivery and policy (HCDP) contracts are reviewed once each year in the spring (for July 1 startups). Funding decisions are made within five months of the deadline for applications, awards are made three months later, adding up to at least eight months from submission of application to receipt of award. The comparable cycle at the National Institutes of Health is nine to ten months. Of 300 projects reviewed for all programs during FY 1999, 129 (43 percent) were found meritorious and approved for funding. Successful applicants are required to submit annual and final reports detailing progress toward their research aims and fiscal reports of their award money expenditures. When needed, the society convenes special task forces of experts in a particular field to explore new possibilities in MS research and to make recommendations for steps to stimulate research in these areas. Recent examples include the 1996 Task Force on Clinical Trial Outcome Measures, the 1997 Database Advisory Panel; and in 1998, the Task Force on Gender in MS and Autoimmunity, the |
Page 327
|
Task Force on Combination Therapy, and the Task Force on Clinico-Pathological Correlates of the MS Lesion. In 1987, the society formalized a program to attract proposals in management, care, and rehabilitation. These research grants are of one to five years' duration. In 1988, an HCDP research program was developed to fund studies on access, quality and funding of health care and quality of life for people with MS and/or family members. In FY 1996, HCDP research began to focus on large contracts addressing society-established priorities. Research to find the cause of MS and its cure is only one of several roles of the National MS Society. The society also supports professional and public education, legislative advocacy, information referral, and programs to help people with MS cope with their disease. The society counts on the voluntary services of members of six advisory panels and numerous task forces to help determine which projects are worthy of support. One panel, the Research Programs Advisory Committee, provides oversight and helps direct the society's overall research programs. This senior committee is an international panel that includes top basic and clinical MS researchers and lay leaders who are members of the society's National Board of Directors. |
~ enlarge ~
Page 329
Society is within the range of similar foundations in the United States (Table 7.1), and MS research is also relatively well funded in Great Britain.2 In sum, it appears that the overall funding levels for MS research are at least comparable to those for other chronic diseases, but it would be a mistake to conclude from total funding levels that funding for MS research is as good as or better than can be expected. The vitality of the MS research enterprise also depends on the distribution of these funds. Are they distributed in a manner that optimizes the generation of the most critical information about MS? The ideal distribution of research monies is more than a matter of simply funding the best research proposals. It also involves recruiting researchers that will generate the most innovative and productive research strategies and, likewise, establishing programs that will yield the most useful information. Such programs might range from grants awarded to individual investigators to international collaborative networks in which investigators establish common research protocols and outcome measures.
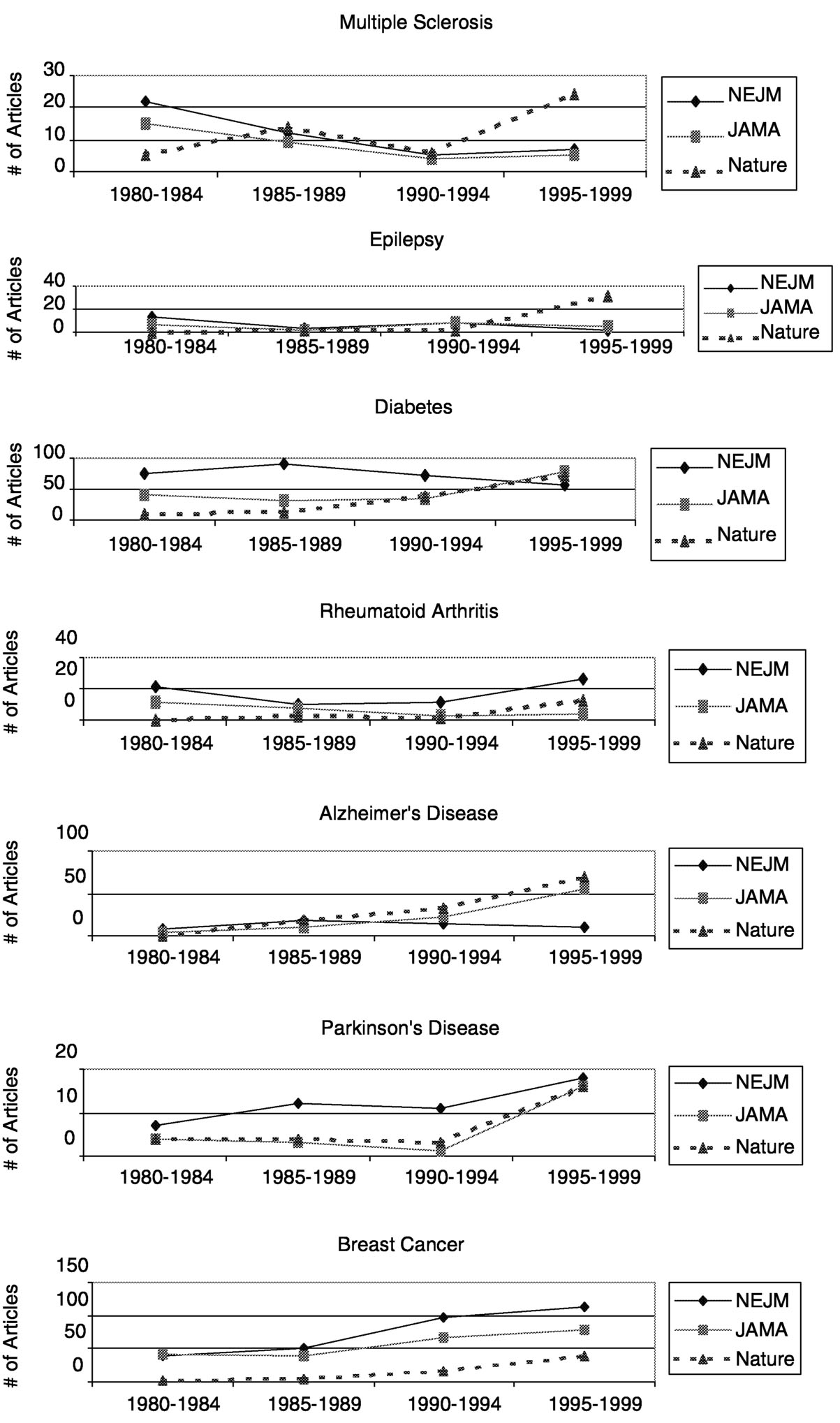
Measuring the productivity of a research discipline is anathema to many scientists, yet that is what a committee attempted to do for the field of immunology in 1999 for a joint committee of the National Academy of Sciences, National Academy of Engineering, and Institute of Medicine.13 (This request was in response to the passage of a congressional act in 1993 requiring that federal agencies develop performance measurements.) As the immunology review committee noted, although precise measurement is not possible, general conclusions can be drawn. Publication rates offer a snapshot of productivity. A review of publications from 1985 to 1999 on MS, epilepsy, diabetes, rheumatoid arthritis, Alzheimer's disease, Parkinson's disease, and breast cancer showed that MS is the only one of these that has consistently declined in terms of number of publica-
|
TABLE 7.1 Research Budgets of Selected Health Organizations in 1998 |
|||||
|
Organization |
Total Revenue |
Net Assets |
Total Expenditures |
Research Budget |
% of Budget Spent on Research |
|
Alzheimer's Association |
48.2 |
34.2 |
46.7 |
12.8 |
27 |
|
American Diabetes Association |
125.1 |
49.6 |
122.9 |
19.6 |
16 |
|
Arthritis Foundation |
114.1 |
141.9 |
103.1 |
24.2 |
23 |
|
March of Dimes |
181.3 |
43.0 |
174.7 |
35.6 |
20 |
|
Muscular Dystrophy Association |
135.0 |
113.2 |
112.5 |
24.6 |
22 |
|
National Multiple Sclerosis Society |
59.3 |
23.8 |
52.6 |
19.6 |
37 |
|
National Parkinson Foundation |
16.5 |
12.1 |
16.6 |
4.0 |
24 |
|
Huntington's Disease Society of America |
3.7 |
1.7 |
3.7 |
1.0 |
27 |
NOTE: Data in this table are based on annual reports for each organization and have been confirmed by each of the organizations listed.
Page 330
~ enlarge ~
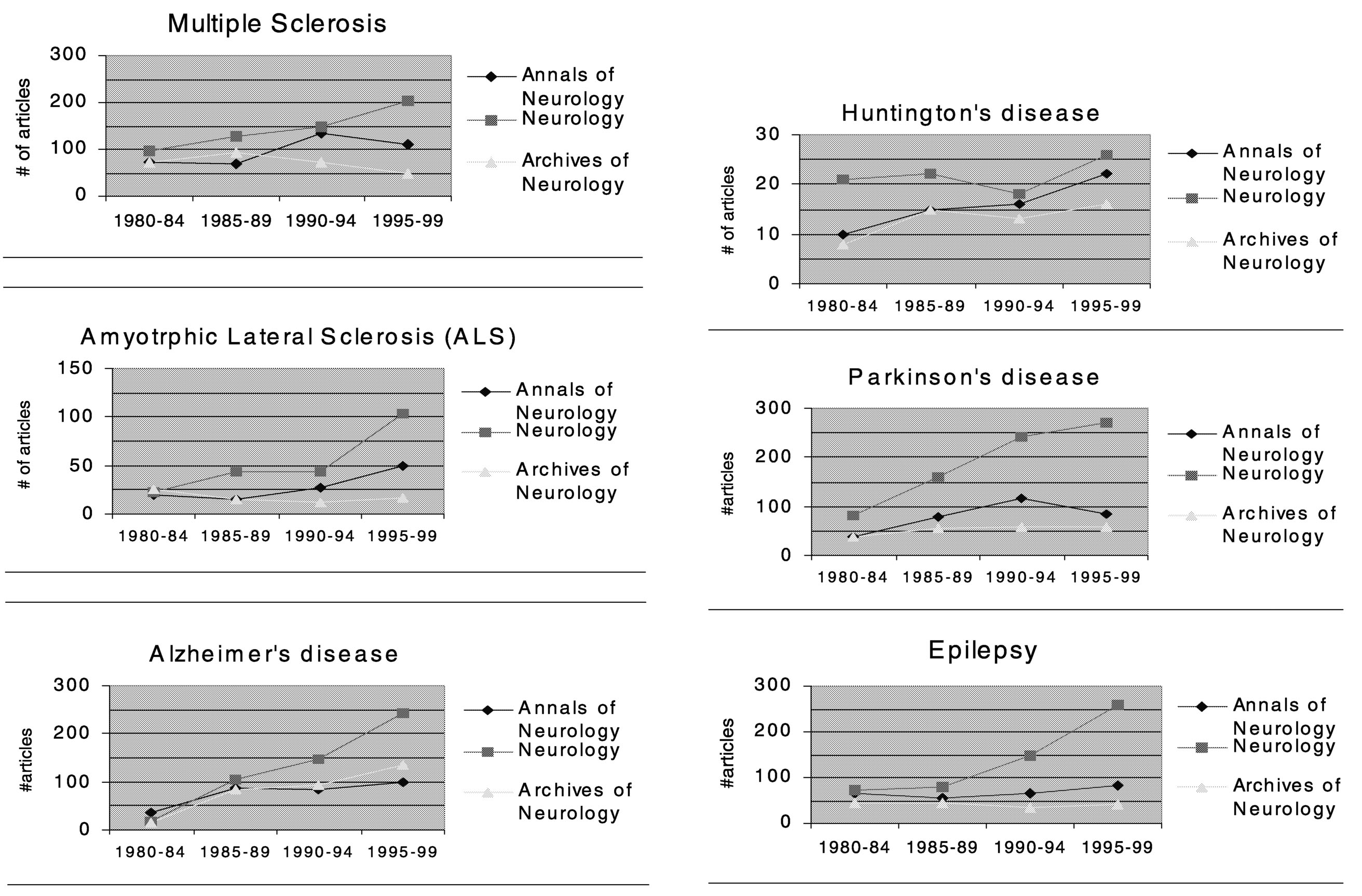
FIGURE 7.2b Summary of publications in neurology journals.
tions (Figure 7.2a). Compared to epilepsy, diabetes, rheumatoid arthritis, Alzheimer's disease, Parkinson's disease, and breast cancer, publications on MS showed the greatest downward trend in the New England Journal of Medicine and the Journal of the American Medical Association. Except for publications on rheumatoid arthritis, which declined and then rebounded, publications on the
Page 331
other diseases steadily increased or generally remained constant. The trend for MS publications was somewhat stronger in the Nature journals in the mid-1990s, after showing one of the strongest declines in the late 1980s.
As with the general medical journals, the publication rates of MS research articles in neurology journals tend to lag behind those of other neurological disorders (Figure 7.2b). Compared to articles on ALS, Alzheimer's disease, Parkinson's disease, epilepsy, and Huntington's disease, MS research articles ranked the lowest overall in terms of average percent increase in numbers of
Page 332
articles published from 1980 to 1999. Among the six neurological diseases compared, the average percent increase of MS articles ranked 5th in Neurology, 5th in Annals of Neurology, and 6th in Archives of General Neurology. (Note that these comparison are based on counting the number of articles in which a particular disease was mentioned in the title of the article, thus a few relevant articles might not have been included, but this is unlikely to cause a systematic bias in ranking of overall publication rates.)
Research Centers
There has been great interest in redirecting funds that might otherwise go to individual investigators to fund large multilaboratory centers. Indeed, the MS Society funded several centers in the 1970s and 1980s, but stopped in 1983. At the time, interest in MS research was increasing across the country, and after careful review, the Society concluded that the concentration of funds in such centers was hindering the development of MS research in other laboratories. The British MS Society also decided not to continue funding MS research centers.
Multisite research centers are ideal for certain types of research, such as clinical research requiring larger numbers of patients than can be readily enrolled in a single region, large epidemiological trials, or studies that require scarce or expensive resources such as magnetic resonance imaging (MRI) scanners or brain banks. However, centers offer less advantage for other types of research, particularly exploratory research, which is often the most innovative. For example, the early-stage, proof-of-concept clinical trials that led to the development of stem cell transplantation would not likely have been conducted in an MS research center.
The traditional model of research funding is to establish a funding program, wait for applicants, and then select the best. NIH now advertises its grant programs far more widely than in the past, but the advertising is still predominantly in a broadcast format and is not directed at specific individuals or institutions. This is also the model that has historically prevailed at many private health-research foundations, including the MS Society, but there are other models.
Public institutions are constrained by the need to provide equal access to research funds for all qualified citizens and are thus generally subjected to a variety of regulations, with their attendant administrative costs. Private foundations are not subject to such constraints and have more opportunities to adopt creative strategies in pursuit of their missions. For example, the Hereditary Disease Foundation has reaped great benefits through its departure from the “broadcast-and-wait” funding strategy. It has been very aggressive in identifying key researchers that have the appropriate techniques or outlook to contribute to the foundation's mission, and has offered them research grants with an emphasis on bringing in young people and setting them up with established scientists.
Another approach is to “superfund” a new center devoted to multidisciplinary
Page 333
research on a particular disease. Examples of this include the Davis Center for research on diabetes in Denver and the CaP CURE center for research on prostate cancer. A critical element in both of these examples is a link to an already-existing world-class research facility and a program that can attract top researchers. Such centers should emphasize inclusion of a broad range of research perspectives, training, and community-wide resource sharing and the nurturing of sustainable relationships among the organizations and investigators involved.
Funding of MS centers of excellence has been tried. The downside is the sequestering of large amounts of resources, the creation of a research community divided into “haves” and “have nots,” and the creation of conservative insider groups. Centers can also be inimical to the entrepreneurial and creative spirit.
Promoting Innovation
There are two important contributors to research innovation—ideas and programs. Innovation rarely rests on the strength of an individual. Indeed, industries or firms that are consistently innovative typically support an innovative culture, as opposed to employing a few key individuals. This suggests the importance of actively fostering innovation in MS research. In the past, the MS research community suffered from a reputation as overly insular, even stodgy. Even if this is not accurate, such perceptions can deter investigators who feel they would be unlikely to thrive in such a community.
Reflecting a deeply held belief among the research community, a 1994 report evaluating cancer research in the United States stated: “Real progress and new ideas in cancer research usually comes from unexpected and unpredictable directions.”15 The 1994 report evaluating cancer research in the United States noted that “excessive targeting of specific areas for research can be counterproductive. It can distract scientists, disrupt research programs, and divert funds from more productive lines of research.”
Innovation starts with new ideas or new perspectives on old ideas and is perhaps most likely to come from people who have been exposed to ideas and techniques from outside the field of MS research. This highlights the importance of recruitment, not only to sustain the scientific work force, but also for the infusion of new ideas.
HUMAN RESOURCES
Someone once said that if you want to ruin your reputation, go into MS research.
Jacqueline Friedman17
The Scientist
Human resources require continual renewal. A research field that fails to attract innovative, productive researchers is a stagnant field. Although data are
Page 334
not available, many people in the field of MS have the impression that too few people have entered the field in recent years. The committee agreed that recruitment of new MS researchers must remain a high priority.
The keys to building human resources are recruitment and retention, and since retention of researchers in their particular disciplines is so high, recruitment is the most important component to consider for the MS research enterprise. Researchers are recruited as they enter their research training—either in medical school or graduate school or as postdoctoral fellows. Alternatively, they are recruited in mid-career after having worked in other research areas. In terms of programs to recruit and support MS researchers, there are distinct advantages and disadvantages to recruiting new versus established researchers. New researchers are relatively inexpensive to support, but as investments they are relatively risky because they have yet to prove themselves and will have a higher attrition rate than established researchers. Established researchers, on the other hand, are more expensive to support but are already successful and provide cross-fertilization by applying knowledge and perspectives from their previous research areas to MS. Recruitment of new versus established researchers is considered separately below.
Recruitment of New Investigators
Although the committee was not in a position to analyze trends in the quality of applications for postdoctoral fellowships in MS research, it is possible to look at the interest level as reflected in the trends in numbers of applications. More applications not only indicate a higher rate of recruitment to a research field, they also provide a larger pool from which to choose the best applicants.
A 1996 survey by the MS Society of its postdoctoral fellowship program indicated that 88 percent of all former society fellows had been successful in obtaining full grant funding during their subsequent careers, and more than half reported that MS research was still their primary career activity. Whether this was a net gain or loss in terms of recruitment depends on whether these fellows were already committed to MS research and how many entered the field as opposed to left it. A moderate level of field switching is probably the ideal, because of the benefits of cross-fertilization of perspectives and techniques.
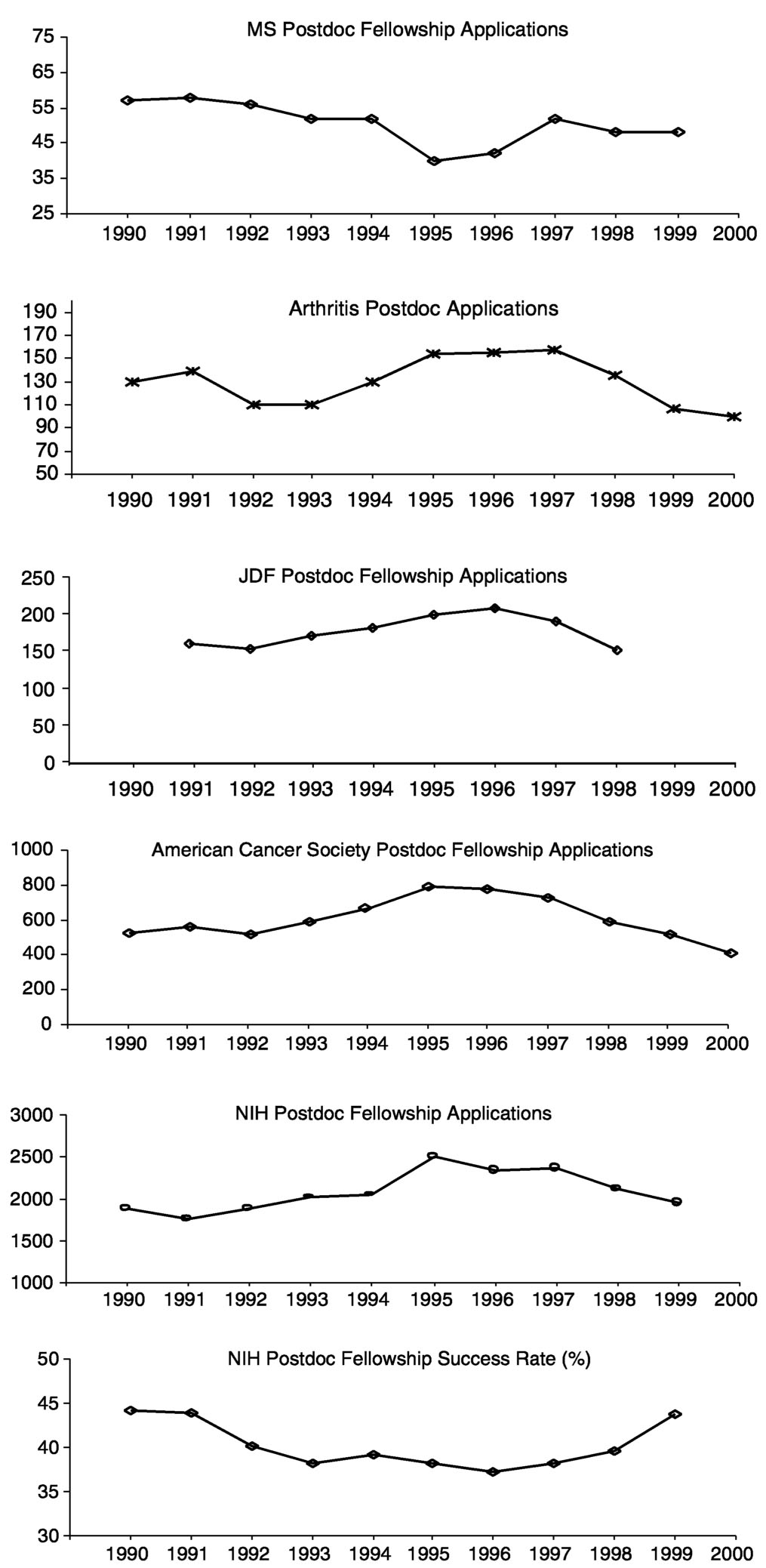
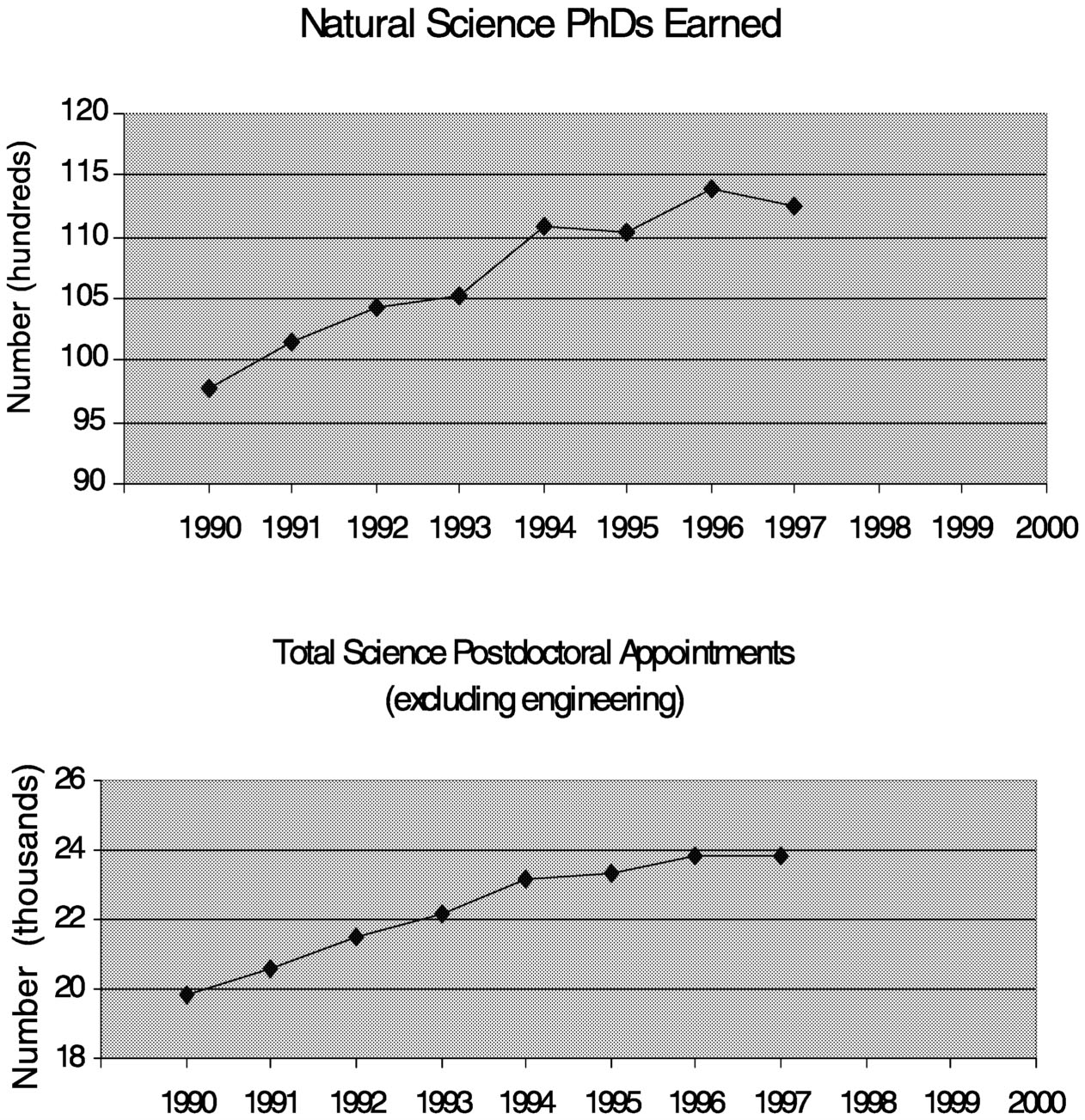
During the last decade, the number of Ph.D.s awarded in the natural sciences increased by about 20 percent, with an even greater rise in the number of postdoctoral appointments (Figure 7.3). During this same period, applications to the MS Society for postdoctoral fellowships declined by about 20 percent (Figure 7.4). At first glance, one might conclude from this that young scientists are losing interest in MS as a research area, but the situation is more complicated. For one, this trend is not unique to MS. The Arthritis Foundation, Juvenile Diabetes Foundation, and American Cancer Society all saw declines in applications after 1995, as did the NIH. The amount of the awards from these societies ($26,000 to $32,000 per year) is comparable to that of NIH fellowships. It should be noted
Page 335
~ enlarge ~
FIGURE 7.3 Ph.D. and postdoctoral fellowship trends (1997 is the last year for which these data are available). SOURCE: NSF Science and Engineering Indicators 2000.14
that applicants to the MS Society did show a different trend than those applying to the other societies in that applications declined from 1990 to 1995 when they were increasing elsewhere, indicating that at least during this period MS post-doctoral fellowships were not seen as an attractive option.
Although it has been suggested that postdoctoral application rates might have declined at private foundations because applicants' success rates were increasing at NIH, the fact that postdoctoral applications also declined at NIH suggests another explanation. One possibility is the poor job market for academic scientists that discouraged many Ph.D.s from pursuing research careers.11 In addition, the total numbers of applicants for postdoctoral fellowships at the MS
Page 336
Society each year is considerably lower than that for the other private foundations for which the committee collected data (Figure 7.4).
Finally, the recruitment of health care researchers deserves particular attention. In 2000, the Health Care Delivery and Policy Research Program Committee of the National MS Society voted to discontinue the Ph.D. fellowship program because the few applications they received were consistently too weak to merit funding. Whether this reflects too little advertising or the nature of the fellowship is unclear, but both possibilities should be considered. Alternatively, it might be more effective to recruit health care researchers at different career stages, perhaps at mid-career. One possibility might be a one-year fellowship program organized in collaboration with health care foundations that target different, but relevant, diseases. If several societies funded one to two fellows per year, those fellows could form a cohort that would, ideally, stimulate health services research for all chronic and debilitating diseases. Another alternative might be to work with the Robert Wood Johnson Clinical Scholars Program in an effort to encourage more applicants with an interest in MS research. This multidisciplinary training program is designed to allow young physicians who are committed to clinical medicine to acquire new skills and training in the nonbiological sciences important to medical care systems. Indeed, the Robert Wood Johnson Clinical Scholars Program for physicians might also offer a model for its converse—multidisciplinary programs in which survey scientists, health economists, psychologists, clinical epidemiologists, nurses, and social workers receive training to enhance their collaborations with physicians.
Recruitment Strategies
The recruitment package might be enhanced relatively inexpensively by providing an optional travel allowance to permit a fellow to spend time in the laboratory of another MS Society grantee. A strategy to stimulate recruitment into the MS field would be to encourage more junior-level applicants. Applications could be relatively simple and reviewed more frequently, or on an expedited basis, and the fellowship could offer successful applicants more options, such as greater freedom of movement between laboratories or institutions and the option of taking the fellowship with them to a beginning faculty position.
Increased recruitment might be accomplished in a variety of ways, such as by directly contacting promising Ph.D. and M.D./Ph.D. students, by advertising in journals and at meetings that students outside the MS field would attend, and by encouraging people who might not consider themselves “MS researchers” to apply.
Page 338
Declining Numbers of Physician-Researchers
For the long-term, medical schools and teaching hospitals, the NIH, health-related foundations, and the pharmaceutical industry need to examine the successful models for training health services researchers, patient oriented researchers, and clinical trials researchers and support the dissemination of those models.
American Association of Medical Colleges, 2000, p.vii1
The declining role of physicians has been a persistent concern for the last quarter of a century. In a series of reports first published in 1975 and as recently as 2000, 11 National Research Council committees have called for increased efforts to recruit physicians into research.12 (The National Research Council is the operating arm of the National Academy of Sciences.) A number of training and career development programs for physician-researchers have been established over the years, but the decline in the proportion of clinicians doing research has continued. While clearly an issue for MS research, declining numbers of physician-researchers pose problems for all areas of medical research. The committee believes that policy and program changes in medical training and research as a whole are most likely to benefit MS research.
Physicians face strong disincentives to enter research where salaries are substantially lower than in medical practice and the time available for research—at least, in the United States—is increasingly limited by the competitive health care market.12 Physicians also typically face enormous debt burden upon graduation from medical school as they consider a career in research. In 1997, the average burden of debt for U.S. medical school graduates was $64,000.12 However, debt repayment programs, although attractive, are uncommon, limited, and expensive (reviewed in 2000 by the Institute of Medicine).9 The problem of debt is probably best solved at the federal government level, rather than by individual organizations such as the MS Society. The society could, of course, contribute by encouraging the efforts of larger organizations, such as the Association of American Medical Colleges, whose mission is to serve medical education and research as a whole. The committee does not recommend that individual MS societies establish medical debt repayment programs.
Recruitment of Established Investigators: Cross-Fertilization
The strong ties of the MS Society to the research community, including the high level of integration at the international level, are among its great strengths. However, concerted efforts should be made to stimulate enduring cross-pollination among biomedical research areas.
In the past, the National Multiple Sclerosis Society has provided small amounts of funding (for example, $100,000) to researchers in established labora-
Page 339
tories, but the committee felt that this was relatively ineffective because it was too little to encourage them to pursue MS research. A number of other private health foundations, such as Huntington's, CaP CURE, and the Amyotrophic Lateral Sclerosis Association, have been successful in persuading specific investigators to join their mission by offering them attractive packages for funded research. Simply inviting individuals to apply for grants, however, is unlikely to be effective. Indeed, in the past, when the MS Society has invited specific individuals to apply for research funding, their grants generally have not passed peer review. This suggests the need for either a different recruitment strategy or a system other than peer review for quality control, at least when specific individuals have been recruited.
Another facet of cross-pollination is the collaboration between clinical and basic scientists. Although there are many researchers who combine skills in clinical and basic science, there is a conspicuous lack of basic scientists pursuing MS research, particularly in the neurosciences. This could be actively encouraged by organizing symposia in conjunction with the annual scientific meetings of other medical and basic science organizations, such as the Society for Neuroscience where MS research has received considerably less attention than other neurological diseases. Another means of stimulating more exchange between researchers and clinicians could be to provide funding for minisabbaticals in which basic scientists could work with clinicians for one- to two-month visits.
INFRASTRUCTURE
Infrastructure refers to equipment and databases, as well as administrative capabilities such as staff who can coordinate collaborations, run centers, and provide statistical guidance and analysis.
The United Kingdom MS Society played a pivotal role in the application of MRI by funding the Queen's Square facility. The MRI facility at the Canadian MS Center in Montreal, Quebec was fortuitously enabled as an unintended consequence of health coverage policy in that province. However, neuroimaging facilities are expensive. Rather than funding the purchase or development of equipment, it would be most cost-effective to fund individual investigators to spend time at sites where the development of novel technologies is under way. There they might learn to apply the technology to MS research while advising and refining to foster cutting-edge developments.
Data registries and tissue banks are other important elements of infrastructure. The Amsterdam Brain Bank and the International Bone Marrow Transplant Registry, discussed in Chapter 6, are examples of an outstanding tissue bank and patient data registry, respectively.
Page 340
CLINICAL TRIALS
Challenges to Clinical Trials: Limited Patient Pool
According to the U.S. National Cancer Institute, only 2 to 3 percent of cancer patients are ever enrolled in clinical trials.6 If enrollment rates in MS were comparable to those of cancer trials, there would be approximately 5,000 to 10,000 MS patients enrolled in clinical trials in the United States. Given a moderately sized clinical trial of 500 subjects, it could soon become difficult to find patients who had not already participated in a trial. Another complication is enrolling patients who are not already being treated with disease-modifying therapies. In the United States, approximately 110,000 MS patients are currently taking one of those therapies—close to one-third of all patients in the country (Nicholas LaRocca, personal communication).
Compared to clinical trials for drugs that are tested to treat acute conditions in the general population, MS trials are particularly challenging. Because MS is chronic, the treatments must show long-term effects, and because MS symptoms so often wax and wane, improvements in patients' condition must be shown to be statistically greater than the improvements that occur without therapeutic intervention.
In addition, MS imposes its own disincentives to participation in clinical trials. Because it is a chronic disease in which patients can remain symptom free or experience only mild symptoms for years, they might be less willing to assume the risk of a clinical trial than others, such as cancer patients, who have little reasonable hope that their health will improve without intervention. Another deterrent is that unlike cancer patients, many MS patients live for many years with severe disability and their lack of mobility is a deterrent to their participation in a clinical trial.
Participants themselves or their surrogates in patient advocacy groups have traditionally had relatively little involvement in recruiting trial participants. Yet this tradition has been successfully broken in several cases. For example, the National Breast Cancer Coalition, a patients' advocacy group, successfully intervened to improve patient accrual for a pivotal trial of Herceptin for breast cancer.* Michael Milken, the founder of CaP CURE, is another example. His appearance on Larry King's nationally televised talk-show was very successful in raising funds for prostate cancer research. A member of this committee was similarly successful after appearing on television with Montel Williams, an African-American talk-show host who was recently diagnosed with MS. Before his television appearance with Montel Williams, he had been unable to recruit enough African Americans for a genetic study of non-Caucasians, but a few days after
*Herceptin was approved by the U.S. Food and Drug Administration in 1998 and was the first biological drug for the treatment of metastatic breast cancer.
Page 341
that he found himself with hundreds of subjects. People with MS are in a unique position to help others with the disease, and their willingness to assist in the enrollment of patients in clinical trials could be an important asset.
Sponsorship of Clinical Trials
Although academic medical centers were once considered the main citadels of clinical research, most clinical studies that bring new drugs from bench to bedside are now led by pharmaceutical companies.3 In the United States, 70 percent of the money for clinical drug trials in the U.S. comes from industry rather than the federal government.5
Until recently, the pharmaceutical industry needed academic physicians to perform drug trials because the industry lacked the in-house expertise and the academic medical centers had better access to patients as subjects for trials. Pharmaceutical firms, frustrated with the slow pace of academic medical centers (in particular, with the slow reviews of industry proposals by academic research offices and institutional review boards), have increasingly turned to contract research organizations and site management organizations to design, execute, and analyze their clinical trials.3,16 In 1991, 80 percent of industry money for clinical trials went to academic medial centers. By 1998, this figure had dropped to 40 percent.7
Most investigators (and this was the sentiment among committee members) prefer that design, implementation, data analysis, and publication be controlled by academic medial centers and investigators. Many feel that clinical trials in academic centers are less likely to be compromised by conflicts between the desire to maximize scientific progress and the financial considerations of pharmaceutical firms. Yet with the rise of contract research organizations and the decline in “market share” of clinical trials in academic health centers, the control of clinical trials within academic centers is increasingly less common.
BIOTECHNOLOGY AND PHARMACEUTICAL FIRMS
As mentioned, most clinical studies that bring new drugs from the laboratory to the medicine chest are financed by pharmaceutical companies, not by government funding agencies or private foundations. Research strategies aimed at treating or curing diseases will thus fail if they do not establish informed partnerships with the sector that can deliver research results to patients. Although drug development generally begins in the realm of the research community, it bears fruit only in the realm of the marketplace. These realms are necessary partners, but they are molded by different contingencies.
Although private firms and the academic research community might share the goal of curing and treating disease, their survival depends on distinctly differ-
Page 342
ent currencies. Private firms survive by their ability to make profits. The coin of this realm is approval of new therapies. In a free-market economy, only those firms that produce profits will survive to produce medically valuable therapies. In contrast, the coin of the academic realm is publication in scientific journals. Producing profits often conflicts with full disclosure of scientific research, and it is almost axiomatic that access to data is an area of great contention in academic-industry collaborations.
From Research to Medications
Before a drug reaches the medicine chest, it must be approved for human use. In the United States, this approval comes from the Food and Drug Administration (FDA), which regulates human testing and the introduction of new drugs into the marketplace. In addition, because the U.S. market represents such a large share of the global economy, FDA regulations also influence drug development around the world. Drug approval is based on the results of clinical trials, which means that the FDA, in effect, sets the standards for clinical research. At times, these standards have been challenged as being so rigid that they impede progress and the development of critically needed medications. Although it has happened only rarely, the FDA has occasionally permitted early and expedited drug approvals where there were otherwise no existing therapeutic options. The most effective recent challenges have come from AIDS patient advocate groups who succeeded in relaxing standards for promising, but unproven medications for HIV/AIDS. In obtaining expedited drug approvals for both cancer and AIDS therapeutics, it was the affected populations that were the most effective advocates, and only those associated with long-established health organizations. Neither researchers nor research funders played a significant role.
People with MS face a somewhat similar situation, but they have not established a political organization to accelerate the development of therapeutic options as have other patient advocacy groups, such as those for breast cancer, Parkinson's disease, and AIDS. The committee neither encourages nor discourages this option but notes that patient advocates can be highly effective in stimulating the availability of therapeutic options, in terms of using their political will both to modify FDA decisions and to increase research support for particular diseases.
MS Drugs Are Orphans
One critical element in encouraging development of therapeutic options for people with MS is the existence of specific revenue-generating incentives for private firms. One such incentive is orphan drug status. The Orphan Drug Act of 1983 provides incentives to manufacturers to develop drugs to treat “orphan diseases.” An orphan disease, as defined in an amendment to the act, is one that
Page 343
affects 200,000 or fewer people in the United States.* The act's most important incentive is a period of exclusive marketing protection of seven years, during which FDA is prohibited from approving the same drug for the same indication.5,6 If a disease affects more than 200,000 people, the manufacturer sometimes subdivides the patient population into smaller units to qualify. For example, a drug for the treatment of Parkinson's disease is not likely to receive an orphan designation because its prevalence exceeds 200,000, but orphan designation has been accorded to drugs for subsets of Parkinson's patients, such as those suffering from early-morning motor dysfunction in the late stages of the disease.
The Catalytic Effect of Drug Approval
The first approval of a drug for a disease is a catalytic event in that it stimulates both academic researchers and companies to enter a field that they might have previously seen as unready for therapeutic advances. It can also create the optimism for expanded investment in research, from both the public and the private sectors. The impact of development and approval of the first disease-modifying drugs for MS might thus be much more far-reaching than their specific benefits to patients. If the history of research in other diseases is any guide, there should be a substantial surge in interest for research and development of MS therapeutics. Although early and expedited drug approval might be controversial, the stimulatory effect of encouraging follow-on research and development is worth considering.
HEALTH CARE RESEARCH
The MS Society created its Health Care Delivery and Policy Research (HCDPR) program in 1988. Since then it has grown from a small grant program to a modest contract program to a more ambitious contract program. The HCDPR program accounts for about 5 percent of the total MS Society research budget, although there are several grants funded outside this program that also include health services research.
The American Cancer Society has an extramural research budget of $171 million, of which approximately 5 percent is devoted to health services research.10 This figures compares favorably to public spending in health services research in the United States, which is proportionately much less than it is for biomedical research. The 1999 research budget for the Agency for Health Research and Quality (AHRQ), the primary funding agency that supports health services re-
*FDA can grant orphan designation to a drug intended for a condition that affects a larger population if the manufacturer's estimated expenses are unlikely to be recovered by sales in the United States. (Public Law 98-551).
Page 344
search in the United States, was $140 million, slightly less than 1 percent of the $14.8 billion NIH research budget. It is important to understand, however, that these numbers provide only rough approximations, because the lines distinguishing health services research from traditional clinical research are often indistinct. Nonetheless, it is reasonable to conclude that proportionately more health services research is supported through private foundations than by federal agencies.
There are not many applicants. A 1999 request for proposals (RFPs) on quality indicators for health care in MS and another RFP on access to health care for MS among rural populations care generated only 2 applications each. Only two applications were submitted for dissertation fellowships. It would be useful to determine whether this is because the pool of applicants is so small or because qualified applicants are either unaware of or uninterested in the opportunities.
ROLE OF VOLUNTARY HEALTH ORGANIZATIONS
The role of philanthropic funding in strengthening health research is vital in that it carries the unique capacity to invest in innovative and creative risk-taking projects.
Enriqueta Bond, president
Burroughs Wellcome Fund
Gaps and opportunities that currently exist that could be filled by foundations include training physicians and Ph.D. researchers to adapt to changing needs; support for emerging field and interdisciplinary research; support for risky research; speeding research from bench to bedside; behavioral research; public understanding of science and communication; and new partnerships.4
“The federal research grant administration process, encompassing the peer review process, has become cumbersome, inefficient, and an impediment to scientific excellence. Investigators spend as much as 30 percent of their time preparing lengthy grant applications, responding to regulations, and preparing administrative documentation. Although some time and expense are necessary, the current system siphons excessive dollars and time into efforts that do nothing to promote progress against cancer.”15 (Note that this quote was not intended as a criticism of peer review, which the report noted was a great strength of the federal research grant administration process.)
Unlike federal agencies, private research foundations are not encumbered by the obligations of a public agency, which allows them considerably more freedom to adopt flexible policies. They can also to some degree piggyback onto policies established by the federal government—for example, policies for the appropriate care and use of animals in research, trainee programs for the ethical conduct of research, and intellectual property agreements. Private foundations can more readily develop expedited grant review, and select individual investig-
Page 345
BOX 7.2CaP CURE: An Innovative Approach to Accelerating ResearchCaP CURE was founded in 1993 to develop a scientific strategy that would accelerate the development of effective therapies for prostate cancer and get help to the men who need it. It is now the world's largest private source of prostate cancer research funding, and it has provided more than $65 million in support for over 450 projects in the past six years. CaP CURE has worked together with survivors, scientists, and advocates to establish a system that encourages collaboration, reduces bureaucracy, and speeds the process of discovery. CaP CURE reaches out to private industry, the patient advocacy community, and government research institutions. These partnerships provide a model to accelerate progress in developing treatments for specific diseases. Research is funded through competitive awards, based on the peer-review model used at NIH and similar to that used by the MS Society. In addition, CaP CURE has established a fast-track grant review and award process as a central feature of its mission. Applications are limited to five pages in length, and awards are made within 90 days of the deadline for applications. The approach appears to have paid off. Applications for competitive awards have increased nearly sixfold, from 86 to 570, in the last five years. Moreover, in 1993, only 10 percent of the research awards had near-term clinical application, compared with more than 70 percent in 1997. Significant results are claimed: at least 80 CaP CURE-sponsored projects, ranging from gene therapy to therapies targeting the androgen receptor, are now making their way into clinical trials. In 1998, CaP CURE introduced young investigator awards to provide stable, four-year funding for outstanding young physician-scientists attracted to the prostate cancer field. The CaP CURE Therapy Consortium, which consists of three teams of scientists, is dedicated to rapidly incorporating discoveries. Physicians in the consortium work at 11 medical centers across the United States, testing new treatments for men with advanced prostate cancer. The consortium creates new clinical trials, recruits participants, shares results, and attracts pharmaceutical and biotechnology sponsors. CaP CURE consortium researchers face vastly fewer administrative hurdles than government-sponsored cooperative groups do, which means that they can evaluate exciting therapeutic agents with much less delay. Researchers associated with CaP CURE cite the greater involvement of patients in clinical trials and the network of clinicians engaged in the clinical trial as important factors in the recently accelerated progress of prostate cancer research, also claiming that CaP CURE has significantly shortened the time required to develop new treatments. Kenneth Pienta, M.D., of the University of Michigan Comprehensive Cancer Center notes, “In the past when I would design a new therapy, it would take me two years to test it in my own clinic. Now with the consortium, we can test these things in six months.” CaP CURE has been working with computer engineers from Oracle Corporation to develop a database that will allow scientists at each individual institution to compare and analyze findings at trials across the country. As with most clinical research, data are presently stored and tabulated at each individual institution (but see description of the IBMTR-ABMTR transplantation database in Chapter 6). CaP CURE has brought a spirit of innovation to a field that, in the past, has been criticized for discouraging new thinking. SOURCE: Stokstad, 1999,18 www.capcure.org (World Wide Web, accessed 12/1/ 2000). |
Page 346
tors for research support. Compared to a public agency, they generally have more opportunity to take proactive and flexible approaches to research support. CaP CURE, the organization founded to promote the development of therapies for prostate cancer, offers but one example of an innovative approach (Box 7.2).
REFERENCES
1. . For the Health of the Public: Ensuring the Future of Clinical Research. Report of the AAMC Task Force on Clinical Research. Washington, DC: Association of American Medical Colleges; 2000; 1.
2. . The Association of Medical Research Charities Handbook 2000. Leicestershire, UK: Chartwell Press Ltd.; 1999.
3. . 2000. Uneasy alliance—clinical investigators and the pharmaceutical industry. N Engl J Med.; 342: 1539-1544.
4. , , . The future of philanthropic support for medical/health research. How to Fund Science: the Future of Medical Research: A Workshop. February 14-16, 1999. The Aspen Institute, Wye, Maryland: American Association for the Advancement of Science. Retrieved 5/26/99 from the World Wide Web: http//www.fundingfirst.; 1999.
5. . An Industry in Evolution. Boston: Centerwatch; 1999.
6. . . A Report on the Sponsors of Cancer Treatment Clinical Trials and Their Approval and Monitoring Mechanisms. Washington, D.C.: National Academy Press; 1999.
7. . AMCs Rekindling Clinical Research Partnerships With Industry. Boston: Centerwatch; 1999.
8. , , . 1999. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med.; 340: 1881-7.
9. Institute of Medicine. Pellmar TC, Eisenberg L, editors. Bridging Disciplines in the Brain, Behavioral, and Clinical Sciences. Washington, DC: National Academy Press; 2000.
10. Institute of Medicine and National Research Council. Hewitt M, Simone JV, eds. Ensuring Quality Cancer Care. Washington, DC: National Academy Press; 1999.
11. . Trends in the Early Careers of Life Scientists. Washington, D.C.: National Academy Press; 1998.
12. . Addressing the Nation's Changing Needs for Biomedical and Behavioral Scientists. Washington, DC: National Academy Press; 2000.
13. . . Experiments in International Benchmarking of US Research Fields. Washington, DC: National Academy Press; 2000.
14. . Science and Engineering Indicators 2000. Arlington, VA: National Science Foundation; 2000.
15. NCAB Subcommittee to Evaluate the National Cancer Program. Cancer at a Crossroads: A Report to Congress for the Nation. Bethesda, MD: National Cancer Institute; 1994.
16. . The Future of Pharmaceutical Funding. How to Fund Science: the Future of Medical Research: A Workshop. February 14-16, 1999. The Aspen Institute, Wye, Maryland: American Association for the Advancement of Science. Retrieved 5/26/99 from the World Wide Web: http//www.fundingfirst.; 1999.
17. . 2000. Does multiple sclerosis have a herpesvirus connection? HHV-6 seems to play a role, but the mechanism is far from clear. The Scientist.; 14: 16.
18. . 1999. From junk bond king to cancer crusader. Science.; 283: 1100-1103.