Page 184
5
Tissue Mechanobiology
Basic biology studies, such as those that examine microscopic or biochemical changes in tissues, are a source of our understanding of details of injury mechanisms at the tissue or cellular level. These studies are primarily performed using cadaver samples, animal models, and tissues or cells grown in culture. Direct study of the tissues of concern in live humans (e.g., nerve, tendon, disc, muscle) is limited due to ethical and methodological considerations. The purpose of this chapter is to systematically review basic biology studies in order to determine to what degree they support an association between loading and tissue injury, especially at load levels well below those that cause tissue disruption. Biological plausibility, one of the Bradford Hill criteria for causality (see Chapter 3), refers in this case to the likelihood that associations between loading and tissue damage are compatible with existing knowledge of basic biological mechanisms. These basic biology studies may also address other Bradford Hill criteria, such as specificity, temporality (tissue injury occurs after loading is initiated), and dose-response associations.
In reference to our overall model of the person in the workplace (Figure 1.2), this chapter explores the “Internal Tolerances” box, that is, the tolerance of tissues to loading. The focus is primarily on the mechanical and biological responses of tissues to repeated or continuous loading, not on damage due to a single, sudden load. However, cyclical loads are frequently compared to the single load that causes tissue to rupture or grossly fail. It is well recognized that loading is required to maintain tissue integrity. Lack of loads or disuse leads to tissue atrophy and impaired function (e.g., osteoporosis, muscle atrophy). The implications are that there may be an optimal range of loading below which atrophy occurs and above which tissue injury may occur. This chapter does not
Page 185
focus on the effects of disuse. We do, however, investigate a number of questions: Is there evidence that tissue damage occurs at levels below the strength of the tissue? Is there evidence for microtrauma or damage accumulation? If so, what is the dose-response relationship? What are the mechanisms of injury and repair? How are the responses of tissues to load modified by intrinsic factors (e.g., age, gender)? Do existing studies support or refute an association between repeated loading and injury?
For the purposes of this chapter, several terms are defined. Elastic materials are those that regain their original shape after a load is removed. For such materials, the change in shape (e.g., strain) is proportional to the applied load (within certain limits). The constant of proportionality is called the stiffness. The force (e.g., stress) necessary to cause rupture or fracture is called the strength. In some cases, cyclically applied forces that are below the tissue strength may cause rupture or fracture via damage accumulation. This is called fatigue. Tissue fatigue can also lead to changes in other mechanical properties, such as reduced tissue stiffness.
Since pain is a common and important endpoint for humans with musculoskeletal disorders, this chapter begins with a review of the pain pathways from musculoskeletal tissues to the brain. This is followed by reviews of the biological responses of six tissues—vertebral bone, spinal disc, tendon and ligament, muscle, peripheral nerve, and spinal nerve root—to loading. These reviews are based on systematic evaluations of the scientific literature. The chapter summary integrates the findings across all tissues and draws conclusions about current knowledge of injury mechanisms. We conclude with suggestions for future research directions.
PAIN PATHWAYS FROM PERIPHERAL TISSUES
Evolution has provided our bodies with many senses by which to interact with the environment. Each sense (smell, vision, hearing, taste, and somatic sensibilities) has a highly specialized neural pathway. Pain is one of the somatic sensibilities (others are touch, temperature sensation, and proprioception) and itself has its own highly specialized set of neural pathways.
This specialization begins in the peripheral tissues. “Nociceptor” is the term given to the specialized receptors that serve as injury (or noxious stimuli) detectors. Activation of nociceptors evokes pain. Pain arouses us to protect the injured or threatened body part and hence plays a crucial role in survival. Nociceptors innervate a variety of tissues in ways that are appropriate from a teleological perspective. Lightly touching the cornea can injure the eye, and so the nociceptors that serve the cornea are quite sensitive to mechanical stimuli. The skin is a more resilient tissue, and
Page 186
nociceptors that serve the skin are sensitive to higher intensities of stimuli. Not all tissues have nociceptors (e.g., fat tissues are relatively insensitive to noxious stimuli). However, muscle, periosteum, and especially the interface between ligaments or tendons and bone are richly innervated by nociceptors. Correspondingly, surgical manipulation of fat is relatively painless, whereas manipulation of muscle or bone at tendon insertion sites is painful.
Nociceptors can also assist in healing and may even be involved in neuroimmune mechanisms. When nociceptor innervation to the skin is blocked, there is delay in wound healing, and the thickness of the epidermis is reduced. Neurogenic inflammation is another function of nociceptors; activation of nociceptors prompts a release of potent vasoactive peptides that leads to redness and increased permeability of the vessels.
Signals from nociceptors are transmitted by the peripheral nerve to cells in the spinal cord. Damage to the peripheral nerve (e.g., carpal tunnel syndrome, spinal root compression) may lead to unusual sensations, the sensation of pain, or the loss of sensation (e.g., numbness) in the part of the extremity served by the nerve. The spinal cord is an important processing center for noxious information. Nociceptive inputs have connections to motor neurons in the dorsal horn; this accounts for pain-induced muscle contractions (muscle spasms). Specialized cells in the spinal cord also transmit information from nociceptors to higher brain centers. These inputs to higher centers arouse descending pathways back down the spinal column, which in turn regulate the sensitivity of the nociceptive neurons. Other inputs from peripheral pathways (e.g., touch systems) may interact with the nociceptive inputs to regulate the sensitivity of the cells in the spinal cord. Thus, the sensitivity of the pain-signaling pathways is highly plastic.
Nociceptors that serve different deep tissues have convergent inputs to the spinal cord; these lead to the phenomenon of referred pain. Thus, a person with a heart attack may feel pain in the left arm; a person with a herniated cervical disc feels muscle tenderness in the trapezius muscle, and a person with carpal tunnel syndrome may feel pain in the elbow and upper arm.
Injury may induce changes in pain sensibility. Tissues may become hyperalgesic; that is, the same stimulus produces a greater sensation of pain. Lightly touching the skin may be associated with pain (allodynia). Hyperalgesia results from two forms of sensitization: peripheral and central. Nociceptors (peripheral) themselves become more sensitive to heat and mechanical stimuli, and the spinal cord cells (central) become sensitized as well. As part of this central sensitization, the nerve fibers concerned with touch sensation acquire the capacity to activate the spinal cord cells that serve pain. This accounts for the phenomenon of allodynia,
Page 187
in which touch stimuli evoke pain in patients with inflammation of the skin and in patients with nerve injury.
LITERATURE REVIEW
The panel reviewed the scientific literature to evaluate the state of knowledge of the effects of loading on vertebral bone, spinal disc, tendon and ligament, muscle, peripheral nerve, and spinal nerve root. Online databases (e.g., Embase, MEDLINE, Pre-Medline) were searched at least back to 1980 for articles with relevant keywords (e.g., tissue type, damage, pathology, fatigue, tension, compression, repetitive, loading). Appropriate articles were considered for review only if they were published in English-language, peer-reviewed scientific journals. For each tissue type, this process identified between 28 and 190 articles for consideration. The reviews that follow summarize, for each tissue, the function and structure of the tissue; the effects of loads on microstructure, mechanical characteristics, and biological function; and the influence of heterogeneity, aging, and other factors on the response of the tissue to load. The types of load considered, along with the biological and mechanical responses, are appropriate to the tissue.
VERTEBRAL BONE AND SPINAL DISC
Structural and Functional Properties
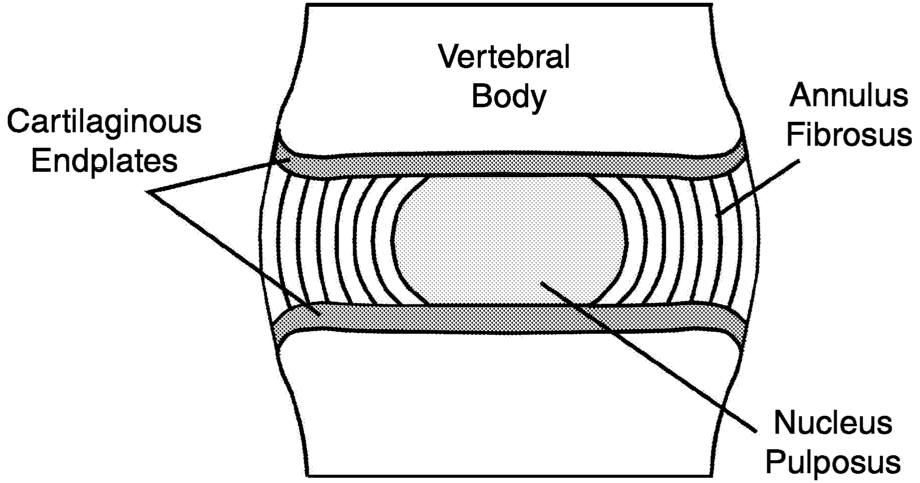
The intervertebral disc is a complex structure consisting of four distinct tissues: the nucleus pulposus, the annulus fibrosus, the cartilaginous endplates, and the adjacent vertebral bodies (Figure 5.1). The nucleus
~ enlarge ~
FIGURE 5.1 Schematic representation of the intervertebral disc (from Bass, 1999:2). Reprinted with permission from the author.
Page 188
pulposus is a viscous, mucoprotein gel that is approximately centrally located within the disc. It consists of abundant sulfated glycosaminoglycans in a loose network of type II collagen, with a water content that is highest at birth (approximately 80 percent) and decreases with age. The annulus fibrosus is a ligamentous tissue that becomes differentiated from the periphery of the nucleus and forms the outer boundary of the disc. The transition between the nucleus and the annulus is progressively more indefinite with age. The annulus is made up of coarse type I collagen fibers arranged in layers, running obliquely between the adjacent vertebral bodies. The fibers run in the same direction within a given layer, but opposite to those in an adjacent layer. The cartilaginous endplates cover the end surfaces of the opposed vertebral bodies and serve as the upper and lower surfaces of the intervertebral disc; they are composed predominantly of hyaline cartilage. The vertebral bodies consist of a trabecular (porous) bone core (centrum) surrounded by a thin shell of cortical (dense) bone. The facet joints are part of the posterior vertebral arch and serve as additional points of articulation between adjacent vertebra. They guide vertebral motion by constraining rotation and supporting some axial load.
In the adult, the cells residing within the endplate, nucleus, and inner annulus resemble chondrocytes (cartilage cells), while the cells populating the middle and outer annulus are fibroblastic (fibrous tissue cells). Because the disc is avascular, these cells receive nutrition via diffusion from adjacent vascularized tissues and convective fluid flow (Maroudas, 1988).
Normal Disc Mechanics
The disc derives its structural properties largely through its ability to attract and retain water. The proteoglycans (biochemicals that help resist compressive loading) of the nucleus osmotically pull in water, exerting a “swelling pressure” that enables it to support spinal compressive loads. The pressurized nucleus also creates tensile stress within the collagen fibers of the annulus and ligamentous structures surrounding the disc. In other words, although the disc principally supports compression, the fibers of the annulus experience significant tension. This annular and ligamentous prestress, in turn, functions synergistically with the facet joints to guide normal spinal motion (Adams et al., 1987).
Under long duration loading, in which the spinal stress exceeds the nuclear swelling pressure, water is slowly forced from the disc, principally through the semipermeable cartilaginous endplates, resulting in a creep response (continual change in height from a constant applied force). As a result of this mechanism, a significant disc water loss can occur over the course of hours due to activities of daily living (Tyrell et al., 1985).
Page 189
Diurnal loss of disc height can approach 2 mm, leading to increased spinal instability via decreased tissue prestress (Adams et al., 1987). Water loss and stability can be recovered during periods of bed rest (LeBlanc et al., 1994).
Effects of Age and Degeneration
After skeletal maturity, the intervertebral disc undergoes numerous alterations with age. These include a progressive loss of cellularity, disorganization of the extracellular matrix, and, as a result, morphological changes and alterations in biomechanical properties (Buckwalter, 1995). These age-related changes represent a form of degeneration, which may be accelerated by a number of factors and have been implicated in increasing the risk of discogenic back pain. Discogenic pain refers to pain originating from the intervertebral disc and is distinguished from back pain of other origins, such as facet joints, spinal ligaments, and muscles.
The most consistent chemical modification observed with aging is loss of proteoglycans and concomitant loss of water (Pearce et al., 1987). Secondary changes in the annulus include fibrocartilage production with disorganization of the annular architecture and increases in type II collagen (Rufai et al., 1995). These alterations precede the morphological reorganization usually attributed to degeneration: loss of disc height, disc bulge, sometimes called protrusion, and disc herniation, sometimes referred to as prolapse (Pearce et al., 1987). Disc bulge can occur when loss of water causes the disc to flatten, bulge beyond its normal margins, and may place pressure on a nerve exiting from or traversing along the spinal column. Disc bulge can also occur when fibrocartilage proliferates within the substance of the annulus fibrosus (Yasuma, 1990). Disc herniation occurs when disc material escapes through a fissure in the annulus fibrosus, which, like a bulge, can place pressure on nerve roots or the spinal cord.
Nociceptive nerve fibers are sparsely present in the outer annulus and vertebral body and extensively present in the facet joint capsule and posterior longitudinal ligament (Cavanaugh et al., 1997; Antonacci et al., 1998; Palmgren et al., 1999). With increasing degeneration, nerves can penetrate to deeper layers within the tissue (Coppes et al., 1997; Freemont et al., 1997), including the vertebral endplate (Brown et al., 1997). Innervation is thought to advance deeper into the disc in concert with vascular granulation tissue (Yoshizawa et al., 1980). These nerves can be stimulated both mechanically and chemically (Yamashita et al., 1993).
There are several mechanisms that purportedly link disc degeneration and low back pain. First, degeneration leads to tissue dehydration (Pearce et al., 1987). Breakdown of the nuclear polymeric structure results
Page 190
in reduction of its osmotic properties— the disc loses its ability to attract and retain water (Urban and McMullin, 1985). Tissue volume loss from dehydration, in turn, leads to a decrease in disc height and an increase in disc bulging (Adams et al., 1987). Both of these geometric changes can adversely affect patients by accelerating facet joint arthritis and by causing mechanical impingement on the adjacent spinal cord or nerve roots. In these cases, alterations in nerve function secondary to chronic compression are thought to be the primary mediators of back pain (Devor, 1995).
Dehydration is also correlated with decreases in disc cellularity, disorganization of the annular layers, and alterations in the density and architecture of adjacent vertebra (Vernon-Roberts, 1988). These changes can begin early in life and have significant consequences for the disc's biomechanical behavior.
Disc degeneration may cause pain indirectly via chemicals secreted by disc cells. These inflammatory factors can diffuse to and sensitize surrounding innervated tissues (McCarron et al., 1987; Kawakami et al., 1996, 1997; Kayama et al., 1998).
As mentioned previously, degenerated discs may be considered a normal consequence of aging. Indeed, a large percentage of the adult population has degenerated discs, with a significant percentage of these being asymptomatic (Wiesel et al., 1984; Powell et al., 1986). For instance, in an MRI study of symptomless adults, greater than 50 percent had disc bulges, protrusions, or vertebral endplate abnormalities (M. C. Jensen et al., 1994). While these data suggest that the presence of a degenerated disc is not diagnostic of back pain, the severity of spinal degeneration (extent and number of levels affected) does correlate with increased risk for symptoms (Luoma et al., 2000).
Influences of Loading on the Disc Via Mechanical and Biologic Pathways
The disc behaves as a composite structure when loaded: forces exerted on it are distributed among the tissues from which it is constructed (the annulus, nucleus, cartilage endplate, and adjacent vertebra). This tissue stress distribution is dependent on the type of loading (e.g., compression, flexion, lateral bending, or torsion) and duration of loading (creep response).
Tissue stress induced by spinal loading affects the disc through both mechanical and biological pathways. These pathways are usually coupled: that is, the mechanical response influences the biology, and the biological response influences the mechanics. This load-induced response can be either beneficial or detrimental.
Page 191
An important detrimental mechanical response is overload injury (material failure). This occurs when the tissue stress exceeds the tissue strength. The human tolerance to overload injury has been investigated largely in cadaveric models. These in vitro experiments demonstrate that failure will occur within the tissue that is stressed most severely. The tissue at risk, and therefore the mode of injury, is in part dependent on the type of loading (compression, flexion, lateral bending, or torsion). For instance, under pure compression the disc fails by vertebral body fracture, whereas excessive bending injures the ligaments of the neural arch (Table 5.1). Vertebral body compressive strength is strongly correlated with its cross-sectional size and bone density (Brinckmann, Biggeman, and Hilweg, 1989a, 1989b), making it feasible to predict noninvasively in humans.
Disc tissues can also be injured through a process of fatigue, where subfailure loads are applied repetitively for sufficient cycles to ultimately cause tissue failure via damage accumulation (such as during exposures
|
Loading Mode |
Injury Mode |
Average Strength |
Notes |
|
Compression |
Vertebral endplate fracture |
5.2 (± 1.8) kNa 6.1 (± 1.8) kN (male, 20-50 yrs)a 10.2 (± 1.7) kN* (male, 22-46 yrs)b |
Dependent on vertebral cross-sectional area and bone density |
|
Shear |
Neural arch, facet joint fracture |
1.0 kNc |
Uncertain |
|
Flexion |
Posterior ligaments |
73 (± 18) Nm |
measured with 0.5 - 1.0 kN compressive preload |
|
Extension |
Neural arch |
26 (± 9) Nmd |
Anterior annulus may be damaged |
|
Torsion |
Neural arch /facets |
25 - 88 Nme |
|
|
Compression plus flexion |
Posterior annulus, vertebral body |
5.4 (± 2.4) kNf |
Disc can prolapse under hyperflexion |
aBrinckmann, Biggemann, and Hilweg, 1989a, 1989b
bHutton and Adams, 1982
cMiller et al., 1986; Adams et al., 1994
dAdams et al., 1988
eFarfan et al., 1970; Adams and Hutton, 1981
fAdams and Hutton, 1982
Page 192
to extremes of whole-body vibration). Cadaveric experiments demonstrate that cyclic loading in compression, bending, torsion, shear, or combinations thereof damage vertebra (including facet joints) prior to damaging the annulus fibrosus. These studies suggest that vertebral fatigue may result from physiological loading regimens that include between 1,000 and 10,000 compressive cycles (5,000 cycles may easily accumulate in vivo during 2 weeks of industrial exposure). Cyclic compressive stress as low as 50 percent of the vertebral failure strength may result in fracture after 1,000 cycles (Hansson, Keller, and Spengler, 1987). Brinckmann, Biggemann, and Hilweg (1988) developed a probability model to predict the fatigue strength given vertebral size, density, and load magnitude. Based on this model, a “fatigue limit” of 30 percent of ultimate compressive strength has been hypothesized for living vertebrae; in other words, cyclic loading of less than 30 percent of vertebral compressive strength would never cause fatigue failure (Table 5.2). Vertebral microdamage cannot be identified utilizing clinical radiographs, bone scans, or MRIs (Hansson et al., 1980; Mosekilde and Mosekilde, 1986). The clinical significance of these pathological changes remains uncertain. Some evidence suggests that the annulus may also be injured via fatigue and damage accumulation (Gordon et al., 1991; Buckwalter, 1995; Walsh et al., 2000)
Tissue stress developed during spinal loading can influence disc biology. Within vertebra, stress can stimulate cells to produce more bone in areas of high stress or remove bone in areas of low stress. This process,
|
Relative Load |
Number of loading cycles |
||||
|
% |
10 |
100 |
500 |
1000 |
5000 |
|
60-70 |
10%a |
55% |
80% |
95% |
100% |
|
50-60 |
0% |
40% |
65% |
80% |
90% |
|
40-50 |
0% |
25% |
45% |
60% |
70% |
|
30-40 |
0% |
0% |
10% |
20% |
25% |
|
20-30 |
0% |
0% |
0% |
0% |
10% |
aValues indicate the probability of compressive failure if a motion segment is loaded for the specified number of cycles at the specified relative load. Relative load is the actual compressive load expressed as a percentage of the load required for compressive failure from single loading cycle. Data from Brinckmann, Biggemann, and Hilweg (1988).
Page 193
called remodeling, is the body's mechanism to optimize the density and shape of bones for a particular mechanical exposure (e.g., tennis players have denser bone in their dominant arms) (Cowin et al., 1985). These bone cells are also responsible for healing fractures, including microdamage resulting from fatigue (as described above). It is inferred from known bone healing times, amounting to several weeks or months (Martin et al., 1998), that minimal repair of bone microfractures would be expected in a time interval of approximately 2 weeks. This observation suggests that vertebral fatigue damage may accumulate in vivo, not be offset by healing, and lead to fractures. However, while the presence of endplate microfractures appears to increase with age (Roberts et al., 1997), whether these are responsible for patient symptoms is uncertain (Braithwaite et al., 1998).
Within the nucleus and annulus, spinal loading can alter tissue water content (via creep, as discussed above) and tissue shape, leading to altered cell metabolism. In particular, changes in water content, in addition to concomitant modifications of tissue permeability, fixed charge density, oxygen tension, and cell shape, can have adverse biologic consequences (Ohshima et al., 1989; Ohshima and Urban, 1992; Ishihara et al., 1996; Handa et al., 1997; Ishihara and Urban, 1999). For instance, Urban and coworkers utilized an in vitro model to demonstrate that disc cell function is harmed by extremes of water content (either too high or too low) induced by fluctuations of disc compression (Ohshima et al., 1995). The detrimental effect was thought to be due to alterations in the disc cells' pericellular environment. In vivo loading in animals demonstrates that altered disc cell metabolism and death may be related to spinal loading via a quantifiable dose-response relationship (Hutton et al., 1998; Lotz et al., 1998; Lotz and Chin, 2000). These studies and others demonstrate that certain regimens of spinal loading can be harmful to the disc. Implied, though not demonstrated directly, is that other regimens, involving lower compressive loads, may be beneficial.
Summary and Conclusions
The intervertebral disc manifests a complex, time-dependent response to spinal loading. Loading, in turn, alters the joints' biomechanical behavior and the tissues' biological activity. Overload injury and fatigue may cause vertebral body failure, while coupling between tissue stress and cell activity may accelerate annular and nuclear degeneration through more subtle, biological pathways.
Spinal discs degenerate with age. The independent contribution of physical force to degeneration is currently unknown due to inherent physiological variability among individuals, and because aging, by definition, signifies lengthened exposure to cumulative trauma. Furthermore,
Page 194
due to a lack of specificity of disc degeneration for back pain, the patho-physiological mechanisms linking spinal load and pain in humans are still uncertain.
However, significant data exist by which to quantify the failure and fatigue strength of vertebral bodies in humans (related to bone density and bone size). The biological response to spinal stress and its contribution to damage accumulation have been demonstrated in animal and laboratory models, yet the extent by which this pathway affects humans still needs to be established.
TENDONS AND LIGAMENTS
Properties of Tendon and Ligament and Injury Endpoints
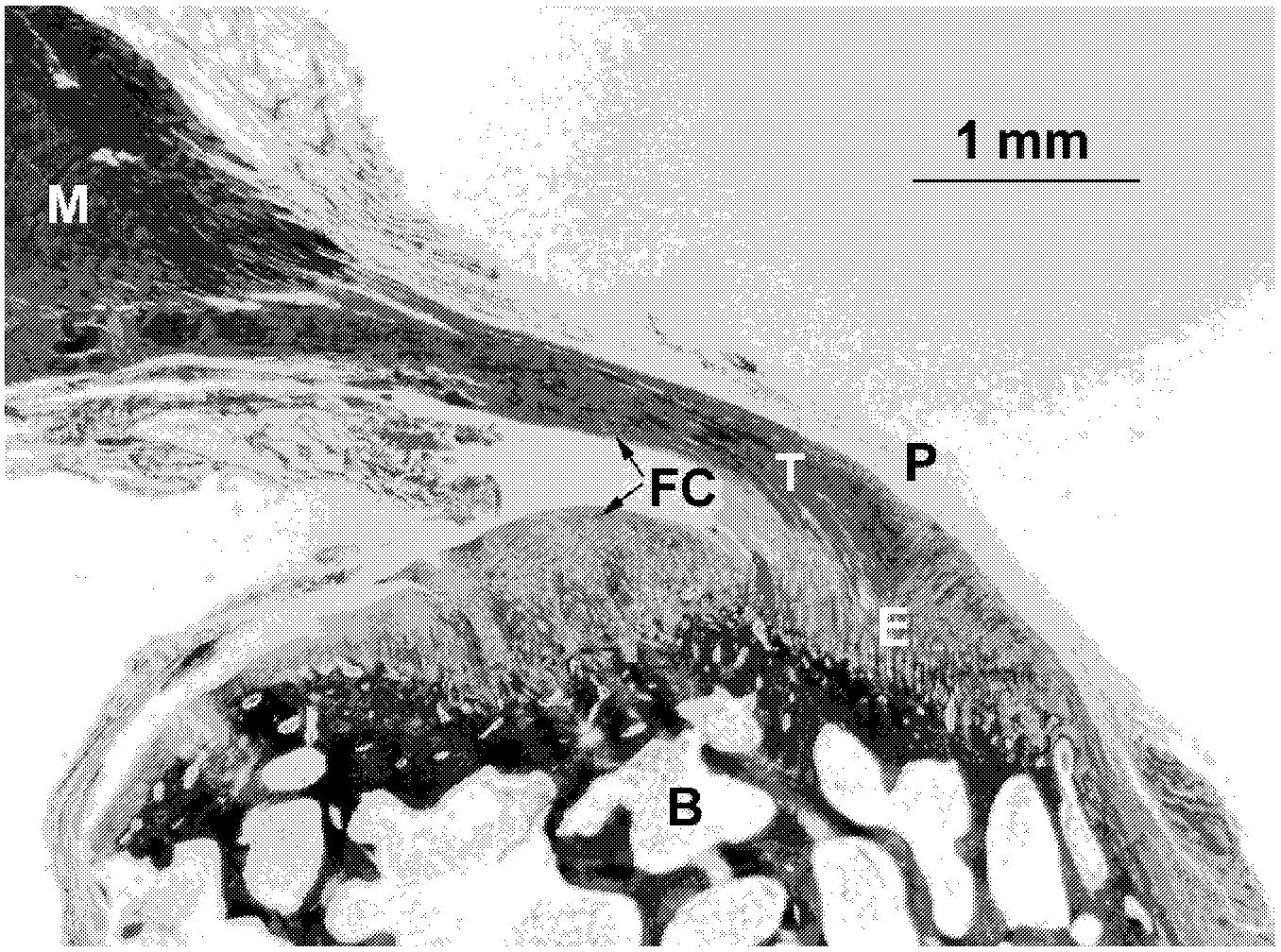
Tendon and ligament are composed of dense connective tissue. The collagen fibrils visible with the electron microscope are grouped into fibers and fascicles that are visible with a light microscope. The fibers and fascicles are enclosed in a thin film of loose connective tissue called endotendon or endoligament. The whole tendon is wrapped in a connective tissue called the epitenon, which in turn is surrounded by the paratenon, a loose, areolar connective tissue (Figure 5.2). In some areas of the body, for example at the wrist, the paratenon forms a double layer sheath lined with synovial cells. This tendon sheath or tenosynovium facilitates smooth gliding of the tendon.
Tendons connect muscle to bone, while ligaments connect bone to bone. Tendons and ligaments primarily transmit tension forces but can also experience shear and compressive loads (Luo et al., 1998). Compression occurs when the tendon path is altered, for example around a bony structure or pulley system, or if there is impingement between the bony structures. Cellular remodeling and adaptation of these tissues occurs in response to different types of loading. When a tendon experiences compressive loading in addition to tension, the tendon in this region is gradually transformed from linear bands of collagen fascicles into irregular patterned fibrocartilage. The transformation is accompanied by changes in proteoglycans (Malaviya et al., 2000).
During tendon gliding, the amount of friction against the surrounding sheath and tissue depends on the amount of tension in the tendon, the friction coefficient, and the arc of contact (Uchiyama et al., 1997). Friction force can generate heat and cause thermal effects indirectly and can stimulate cellular reaction directly (Birch, Wilson, and Goodship, 1997). Joint movement determines the amount of tendon excursion; therefore, the specific joint posture, as well as tendon tension, are important determinants of compressive and shear load.
Page 195
~ enlarge ~
FIGURE 5.2 Control tendon. Tendon (T) with adjacent tissues from a rabbit epicondyle. Peratenon (P), enthesis (E), fibrocartilage (FC), muscle (M), and bone (B). Trichrome stain. (Reproduced with permission from Karen King and David Rempel, 2001.)
Mechanical Properties
Tendon and ligament have characteristic mechanical properties characterized by stiffness, failure strength, and viscoelasticity; these properties have been extensively studied. For example, the strength of human finger flexors is approximately 1,500 N, which corresponds to a strain (length change) of approximately 13 percent (Pring, Amis, and Coombs, 1985). In general, the tension experienced in the tendon during activities of daily living, occupational tasks, and even sport activities is likely to be low compared with the failure strength. For example, a pinch force of 4 kg is likely to require between 70 and 230 N of tensile force in the flexor digitorum superficialis tendon (Dennerlein et al., 1998).
The mechanical fatigue properties of human tendon have been evaluated by subjecting tendon to cyclical loading (Schechtman and Bader, 1997; Hubbard and Chun, 1988). Extensor tendons from the foot, subjected to a cyclic square tension-tension stress waveform at physiological frequencies, failed according to a log-linear model: S = 101.3 − 14.8 log (N). The number of cycles to failure (N) was inversely related to the stress (S),
Page 196
even at the lowest stress levels (10 percent of failure strength, 70 hours of loading), suggesting the absence of an endurance limit.
Pathology of Human Tendons
The taxonomy for disorders of the tendon and adjacent structures may be confusing. Tendinitis is the common term used to describe pain at \the site of a tendon and may be accompanied by swelling, warmth, anderythema. However, some tendon disorders, for example, lateral epicondylitis (“tennis elbow”), have no acute inflammatory cells on histologic examination. Instead, at surgery there are tears in the tendon with disorganized collagen, vascular hyperplasia, and fibroblast proliferation near cleavage planes in the tendon (Kraushaar and Nirschl, 1999; Coonrad and Hooper, 1973). It has been proposed that these disorders characterized by degenerative changes be called tendinosis (Kraushaar and Nirschl, 1999). Furthermore, tendon disorders can be classified based on the anatomy of the tendon and its surrounding tissues (Viikari-Juntura, 1984; Clancy, 1990):
(a) Tenosynovitis refers to inflammation of the tendon sheath or paratenon. This can occur where the finger flexors pass through the carpal tunnel (e.g., flexor tenosynovitis) or on the back of the wrist (e.g., fourth extensor compartment tenosynovitis). Histologically these are characterized by edema with inflammatory cells and vascularization of the paratenon.
(b) Stenosing tenosynovitis (tenovaginitis) occurs when tendon gliding is restricted due to thickening of the tendon or sheath (e.g., de Quervain's disease or trigger finger). Histologically, the tendon sheath and tendon nodule demonstrate fibrocartilage metaplasia with increased chondrocytes and gylcosaminoglycan matrix (Sampson et al., 1991).
(c) Peritendinitis refers to inflammation of only the paratenon in areas in which there is no tendon sheath. This can occur at the back of the wrist, at the second extensor compartment (e.g., intersection syndrome), where the extensor carpi radialis tendons pass below the muscle bellies of the abductor pollicis longus and the extensor pollicis brevis, which may or may not be lined by the synovium. Histologically, the paratenon areolar tissue is infiltrated with edema, thickening, hypervascularity, and inflammatory cells and the process may extend to the adjacent muscle.
(d) Tendinosis occurs when there are degenerative alterations within the tendon without the evidence of inflammatory cells. Histologic find-
Page 197
ings are partial ruptures, collagen fiber disorientation, fibroblast hyperplasia, neovascularization, local necrosis, and glycosaminoglycans laid down between tendon fibrils. Tendinosis is observed at the time of surgical treatment of rotator cuff “tendinitis” and lateral epicondylitis (Chard, 1994; Kraushaar, 1999). Whether an early, inflammatory response precedes these changes is unknown. Histologic changes consistent with tendinosis are present in the rotator cuff of 40 percent of cadavers over the age of 50 (Chard, 1994).
Mechanisms of Injury
The mechanism of injury for the various tendon disorders may vary depending on local anatomy and the forces experienced by the tendon and adjacent tissues. A limited number of well-designed animal models have been developed to investigate mechanisms of injury by studying the effects of repetitive motion and loading on the adaptation and pathological changes of soft tissue; Archambault and colleagues (Archambault, Wiley, and Bray, 1995) have recently reviewed these models. The long-term effect of exercise on tendons may be positive, by increasing tendon cross-sectional area and strength, if conditioning duration and repetition rates are controlled (Woo et al., 1980). Remodeling of the tendon can occur with development of fibrocartilaginous tissue along the tendon. At points where the tendon wraps around bone or a pulley and is subjected to transverse compressive loading in addition to tension, tendon remodeling occurs, with the development of fibrocartilaginous tissue with elevated glycosaminoglycan content (Malaviya et al., 2000; Perez-Castro and Vogel, 1999). This tissue diminishes when the compressive loading is removed. These fibrocartilaginous changes are congruent with the pathology observed in the tendon and tendon sheath of humans with stenosing tenosynovitis. The factor that induces the fibrocartilagenous change (e.g., ischemia, compressive force, frictional heat) is unknown.
Repetitive stimulation of the rabbit ankle flexor has been used to investigate the pathogenesis of peritendinitis and tendinosis of the Achilles tendon (Rais, 1961; Backman et al., 1990). In the Backman et al. study (1990), rabbits were exercised in a kicking machine, producing passive flexions and extensions of the ankle joint combined with active contractions of the ankle flexors. The animals were exercised for 5 to 6 weeks, 3 days per week, for 2 hours, at a rate of 150 flexions and extensions per minute. The peak load was estimated at 15 percent of maximal muscle force. Although the rate of loading is high, the number of hours per week is low relative to what might be experienced by humans. Light microscopic examination showed degenerative changes of the tendon and increased number of capillaries, infiltrates of inflammatory cells, edema,
Page 198
and fibrosis in the paratenon. This animal model demonstrates tissue changes due to repetitive loading that are congruent with the pathology of peritendinitis and tendinosis in humans.
Recent studies have suggested that peritendinitis due to repeated loading may be mediated by an early inflammatory response. Using microdialysis techniques, it has been observed that metabolism is accelerated and is accompanied by an elevation of prostaglandin E2 and thromboxane B2 in the peritendinous region of the human tendon with dynamic loading (Langberg et al., 1999). In animals trained on a treadmill for 3 to 5 days, the IGF-I immunoreactivity throughout the cytoplasm of the tendon and paratenon fibroblasts was increased (Hansson et al., 1988).
Based on human pathology findings, it has been suggested that tendinosis associated with repeated loading is due to microtears in the tendon, such as side-to-side dehiscence of the fascicles or longitudinal disruption of the fibers (Kraushaar, 1999). Not only are partial tears observed in pathological specimens but repair activity (e.g., fibroblast proliferation, angiogenesis, matrix production) is found near cleavage planes in the tendon. Morphologic changes consistent with microtrauma are elevated in the flexor tendons of horses after galloping exercises (Patterson-Kane et al., 1997, 1998b). Elevated temperature in the core of the tendon associated with repeated strain and compromised blood flow and hypoxia have also been postulated as mechanisms leading ultimately to degenerative changes in the central core of the tendon. However, the evidence supporting either pathway is limited.
An animal model for rotator cuff tendinosis was developed in the rat with treadmill running (Carpenter et al., 1998). Overuse led to an increase in cellularity and collagen disorganization in the tendon compared with controls. This was accompanied by biomechanical changes of an increase in tendon cross-sectional area and a decrease in tissue stiffness. Tendons with a surgical injury plus overuse exhibited a worse histologic grade than those with overuse alone. The study demonstrated that damage to the supraspinatus tendon can be caused by overuse and intrinsic injury, overuse and extrinsic compression, and overuse alone. The changes were congruent with the pathological changes observed in human rotator cuff tendinosis. The differences in anatomy between the rat and human shoulder may be considered a limitation; however, a detailed review of 33 species of animals revealed that the rat was the most appropriate based on acromion anatomy and function.
Effects of Age and Other Factors
In the rat, Achilles tendon strength decreases with age (Simonsen, Klitgaard, and Bojsen-Moller, 1995). Certain kinds of exercise can prevent
Page 199
some of this age-related loss of strength. In the rat, the aging process was not prevented by strength training, but it was compensated to some degree by swim training (Nielsen, Skalicky, and Viidik, 1998). Whether aging increases the risk of injury associated with cyclical loading is unknown.
Summary
Basic science studies support the conclusion that repetitive motion or overuse loading can cause chronic injury to tendon tissues. The external loading exposures are related to the internal stress in the tissue and to interaction between tissues. The resultant physiological and cellular responses can lead to either biological adaptation or chronic pathology. The injury pattern includes inflammatory changes with fibrosis in the paratenon, with evidence of degenerative changes in the tendon, specifically edema, collagen disorganization, and fibrosis. The damage may be initially mediated by inflammatory activity and microtrauma.
Some elements of the pathophysiology pathway are still uncertain. For example, the very early ultrastructural, cellular, and biochemical responses to repetitive tissue loading have not been well explored. The response of tendons at different sites of the body to repeated loading may not be homogeneous. For example, the findings observed in the Achilles tendon may differ from those that might be observed in the extensor carpi radialis tendon insertion into the epicondyle. Stenosing tenosynovitis (e.g., trigger finger) may be associated with repeated loading, but there are no animal models for this condition.
In addition, a better understanding of the specific biomechanical factors that cause injury would be extremely useful for prevention efforts. It is unclear whether the problem with repeated loading has more to do with the rate of loading, the peak loads, cumulative tendon travel, or simply the duration of loading. These issues are complex, and the questions are likely to be resolved only with animal models.
SKELETAL MUSCLE
Skeletal Muscle Body Function Related to Work Performance
Skeletal muscle is unique as the body machine that powers external human work. Skeletal muscle is an elongated, contractile tissue that generates force and shortens when activated to contract by stimuli from alpha (α) motor neurons that originate in the spinal cord. Central nervous system stimuli and spinal reflexes activate the α motor neurons and skeletal muscles to bring about coordinated and efficient movement of limbs, maintenance of posture, and withdrawal from painful stimuli. Because
Page 200
skeletal muscle generates the force for body movement and external work, it also is a source of physical load to other tissues, such as tendons, joints, and nerves.
Although skeletal muscle is a working machine, it has inherent self-repair and adaptation mechanisms that allow it to maintain its structure and remodel over time. In the absence of excessive external forces, muscle does not usually damage itself from overuse because it fatigues, or fails to contract, before the point of irreversible contractile or cellular damage. Skeletal muscle cells, or fibers, recover from fatigue within minutes to hours. Damage or injury to skeletal muscle invariably occurs as a result of external forces that exceed the tolerance limits of the muscle's passive (e.g., connective tissue) and active contractile structures; the nature of the damage is directly related to skeletal muscle structure and the molecular mechanism of force generation.
Skeletal Muscle Structure and Contractile Mechanism
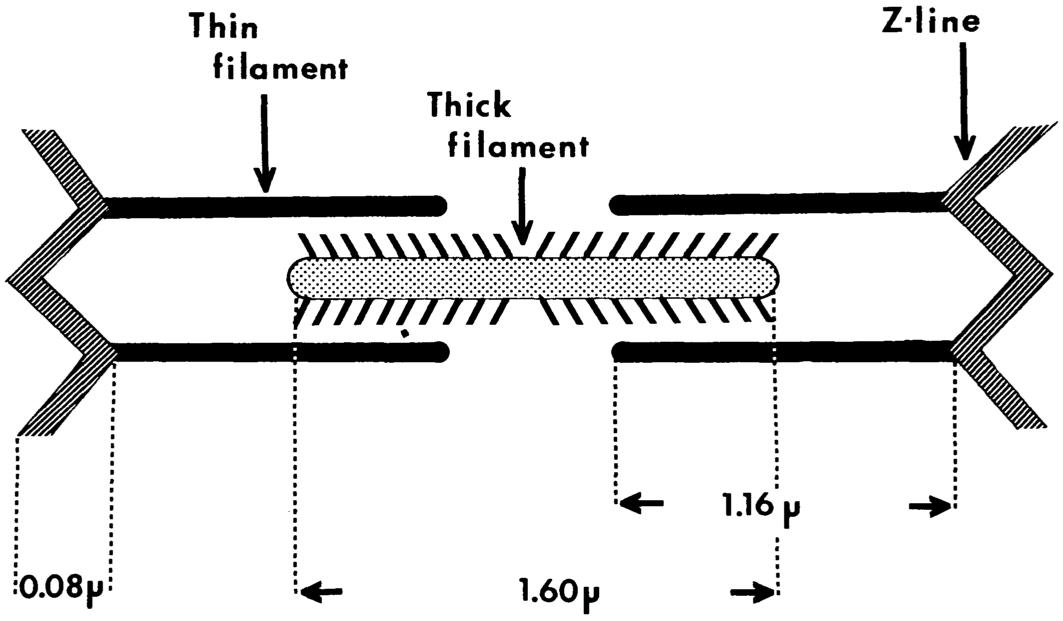
Skeletal muscle has a highly structured architecture. Within each skeletal muscle fiber, or cell, interdigitating thick and thin protein filaments are organized longitudinally into repeating sarcomeres and cross-sectionally into lattices that form myofibrillar bundles (see Figure 5.3a and Figure 5.3b). Thick filaments of the protein myosin form a lattice in the center of each sarcomere, and thin filaments of the protein actin insert into the thick
~ enlarge ~
FIGURE 5.3a Schematic of a skeletal muscle sarcomere, showing interdigitating actin protein thin filaments and myosin protein thick filaments. The sarcomere is the smallest structural unit of skeletal muscle. (From Kaldor and DiBattista, 1978:7. Reprinted with permission.)
Page 201
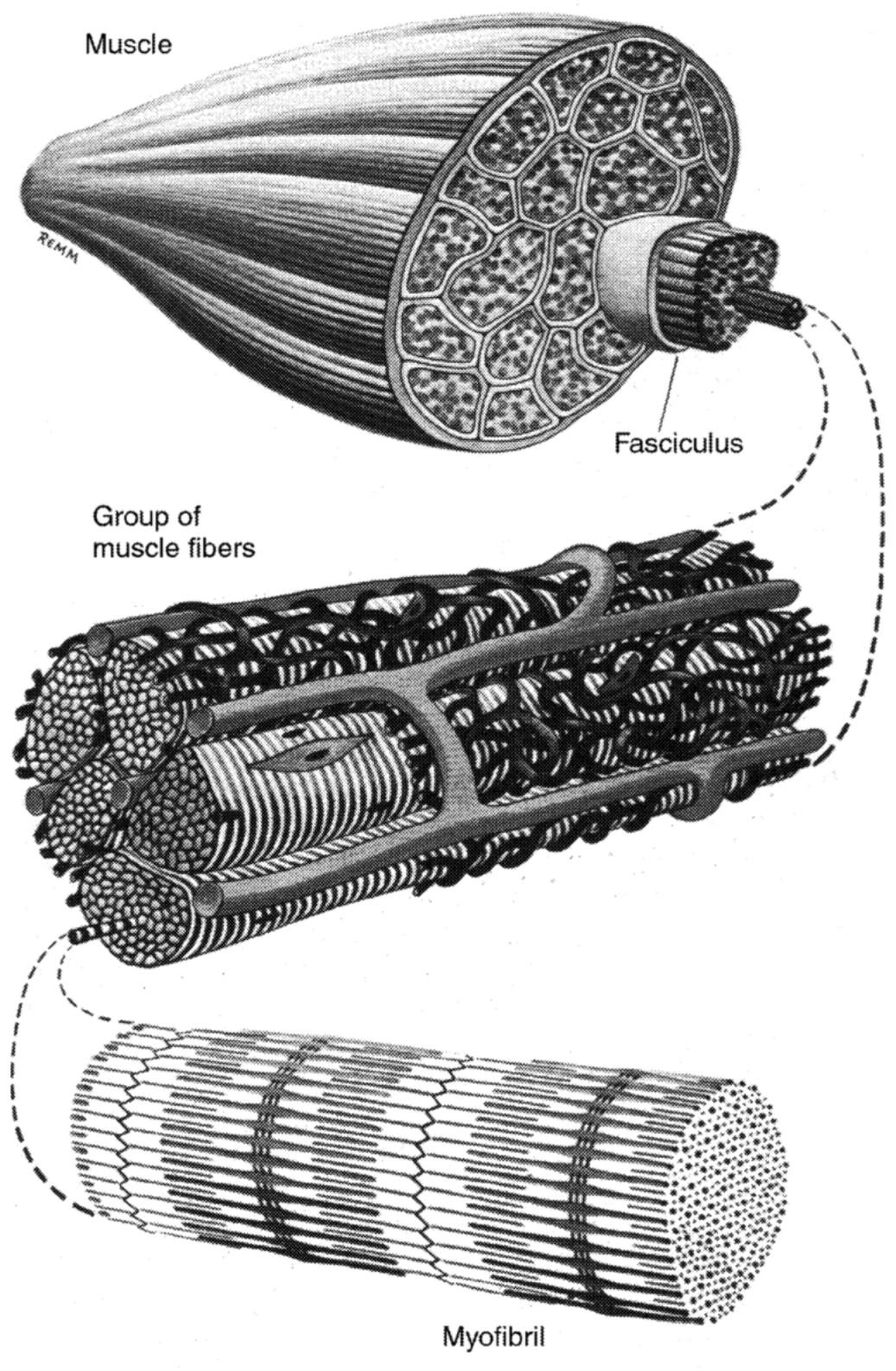
~ enlarge ~
FIGURE 5.3b Schematic of skeletal muscle structure showing the muscle, a group of fibers (i.e., muscle cells) surrounded by blood vessels, and a single myofibril of many that constitute the single fiber. (Adapted from Warwick and Williams, 1973:481, Figure 5.8. Reprinted with permission.)
filament lattice from both directions toward the center of each sarcomere. The thick filament myosin heads are the molecular force generators that form cross-bridge attachments to the thin filaments and pull the thin filaments toward the center of the sarcomere, thereby generating force and shortening. The fuel for cross-bridge movement is the energy stored
Page 202
in the chemical adenosine triphosphate (ATP); myosin heads hydrolyze ATP as a part of the cross-bridge cycle and convert the chemical energy released to force generation and heat. Each muscle fiber has a metabolic enzyme system, glycolytic and oxidative for replenishment of cellular ATP.
The smallest intact unit of a skeletal muscle that contracts in response to nerve stimulation is the single cell, or muscle fiber. Each muscle cell, or fiber, is made up of multiple myofibrils, creating the cross-sectional area that determines the fiber's maximal force generation. The skeletal muscle's maximal force generation is determined by the combined cross-sectional area of all of its fibers. The fiber's length is that of the entire muscle, with 2.0-2.2 μm long resting sarcomeres joined end to end at Z protein discs and repeating longitudinally tendon to tendon. The summed shortening of all of the sarcomeres in a fiber determines the total shortening of the fiber, and thus the muscle. Individual muscle fibers are bound together in parallel by connective tissue sheaths to form the muscle. The connective tissue of skeletal muscle serves as passive resistance to stretching of the sarcomeres by external forces and varies in content among skeletal muscles of an individual and among individuals.
Activation of Skeletal Muscle Contraction
The hard wiring of the nervous system determines the pattern of activation, or recruitment, of muscle fibers within a given muscle for either voluntary or reflex contraction. Each muscle fiber is served by only one α motor neuron that drives its contraction; but a single α motor neuron may branch and serve many skeletal muscle fibers. All of the fibers fed by a single α motor neuron are stimulated together and behave as a motor unit. Small motor units have relatively few fibers and are used for fine, delicate movements; large motor units have hundreds to thousands of fibers and are recruited for large forces and gross movement.
Recruitment order of motor units is from small to large for voluntary skeletal muscle contractions. As a consequence, the smaller motor units contract more frequently. Studies of muscle electrical activity have confirmed the preferential activation of small motor units during repetitive, low-intensity, stereotypical movement (Sjøgaard and McComas, 1995; Jensen, Pilegaard, and Sjøgaard, 2000). Because the skeletal muscle fibers of these low-threshold small motor units carry a disproportionate burden, they are referred to as “Cinderella” fibers (Hägg, 1991). Cinderella fibers are the ones at greatest risk of focal injury during low-intensity, repetitive work.
The fibers in a given motor unit are all activated together; therefore, they share the same adaptive, or conditioned, state, and they fatigue and recover as a unit. As a result of variations in use, motor unit fibers are
Page 203
transformed into one of two basic types: fast twitch, fatigable, or slow-twitch, fatigue resistant. Within the fast-twitch category, the primary metabolic enzymes vary from primarily glycolytic to glycolytic/oxidative; the diameter of the glycolytic fibers is larger than that of glycolytic/ oxidative fast-twitch fibers. In general, fast-twitch fibers usually have greater diameters than slow-twitch ones. Slow-twitch fibers have primarily oxidative metabolic enzymes, with relatively more mitochondria, and are the fibers with lowest cross-sectional area and maximal force generation. Cinderella fibers are predominantly slow twitch; they are the motor units recruited first, then fast-twitch oxidative/glycolytic, and, finally fast-twitch glycolytic in maximal voluntary contraction (Hennemen and Olson, 1965; Hennemen, Somjen, and Carpenter, 1965a, 1965b; Hennemen et al., 1974).
The average person has a mixture of slow-twitch and fast-twitch motor units in every skeletal muscle. There is little evidence that fibers in a given motor unit convert from one type to the other as a result of exercise or training. This innate fiber type distribution may determine the capacity of an individual for various types of physical work as well as for forms of athletic performance; slow-twitch fibers enhance endurance, and fast-twitch enhance sprint-type performance.
Aside from a motor unit increasing in size through reinnervation of denervated muscle fibers, motor units do not increase in size after birth, because new muscle fibers are not created postnatally. As a consequence of aging, fast-twitch fibers appear vulnerable to dennervation and reinnervation by new branches of α motor neurons of slow-twitch motor units, as shown by Kadhiresan and coworkers (Kadhiresan, Hassert, and Faulkner, 1996) in a study of rats. Thus, aging leads to larger slow-twitch and smaller fast-twitch motor units.
Evidence of Skeletal Muscle Damage and Mechanisms of Injury
Decrements in skeletal muscle force generation in the face of repetitive use without nerve damage is evidence of either fatigue of motor units or skeletal fiber damage. The difference between the two is that force loss from fatigue is recovered within minutes to hours of rest, but fiber damage is repaired more slowly and may be irreversible. Fatigue of skeletal muscle fibers is failure of the fiber to contract in response to continuing α motor neuron stimulation (Bigland-Ritchie, Furbush, and Woods, 1986). While the fatigued fibers are not themselves permanently damaged, they can put other motor units at risk of structural damage due to inappropriate recruitment or excessive strain from external loads. In contrast to fatigue, recovery from fiber structural damage takes weeks to months, depending on rest and reuse, and the damage may result in complete loss
Page 204
of the damaged fibers with persistent reduction of maximal force generation. The only way to increase the maximal force generation capacity of a muscle after fiber loss is through hypertrophy of the remaining skeletal muscle fibers.
Fiber structural damage is also accompanied by products of cell inflammatory and necrotic processes, edema, microscopic evidence of contractile structure disruption, and leaking of intrafiber proteins and enzymes through disrupted cell membranes (Sjøgaard, 1986; Jensen, Jorgensen, and Sjøgaard, 1994; Skjeldal et al., 1993; Hagberg, Michaelson, and Ortelius, 1982). All of these indicators of fiber structural damage should be accompanied by a decrement in force generation if skeletal muscle damage has occurred. However, for repetitive contraction of skeletal muscle fibers under conditions leading to damage, loss of force may be accompanied only by clinical symptoms and pain as precursors to measurable structural damage (Sjøgaard and Jensen, 1997). It is hypothesized that loss of force generation is the result of molecular damage to the myosin heads, or cross-bridges; the methods for testing this hypothesis in intact muscle have not been developed.
Muscle injury may also be mediated by other mechanisms unrelated to contractile structure. When muscle contractions result in an intramuscular pressure exceeding capillary closing pressure (about 30 mmHg) muscle ischemia may result (Sjøgaard and Sjøgaard, 1998; Sadamoto, Bonde-Petersen, and Suzuki, 1983; Jarvholm et al., 1988a, 1988b; Shepherd et al., 1981). Data indicate that intramuscular pressure is inhomogeneous during contractions (Sejersted et al., 1984; Sejersted and Hargens, 1985; Laughlin, Mohrman, and Armstrong, 1984; Frank et al., 1999; Sexton and Poole, 1995) and that damage by this mechanism is most likely to affect small muscles (Sjøgaard and Jensen, 1997) and slow-twitch fibers (Bai et al., 1998) during prolonged static contractions. Intramuscular pressure ≥ 30 mmHg over an 8-hour period can cause muscle fiber atrophy, splitting, necrosis, and other derangements (Hargens et al., 1981; Pedowitz et al., 1990). The interaction between the increased metabolic demand of active muscles and the relative ischemia via increased intramuscular pressure has been hypothesized to contribute to derangements in intracellular pH/lactic acid, calcium, and potassium homeostasis (Sjøgaard, 1990, 1988; Sjøgaard, Savard, and Juel, 1988; Wilkie, 1986; Hermansen, 1981; Saltin et al., 1981; Sjøgaard et al., 1986; Chase and Kushmerick, 1988; Donaldson, Hermansen, and Bolles, 1978; Sjøgaard and Jensen, 1997). However, these mechanisms may relate mainly to the onset of muscle fatigue and not to fiber damage. There is evidence that reperfusion after ischemia leads to microvascular and cellular dysfunction, initiating longer-term symptoms and functional change in skeletal muscle (Seyama, 1993; Skjeldal et al., 1993; Jerome, Kong, and Korthuis, 1994).
Page 205
In general, skeletal muscle behavior can be viewed from the perspective of the material fatigue model for considering musculoskeletal disorders. Laboratory studies of skeletal muscle show that there is a correlation between the number of contraction cycles and the extent of injury; these data are in agreement with clinical findings of repetitive use of affected muscles (Ranney, Wells, and Moore, 1995; Dennett and Fry, 1988). Stereotypical, repetitive motion is a major risk factor for development of musculoskeletal disorders (Fredriksson et al., 1999; Ohlsson et al., 1995; Ekberg et al., 1995). The muscles of each person have their own endurance limits, creating variations in each person's injury threshold for repetitive motion work. Also, multiple skeletal muscle contractions can be performed at low force before an actual injury, that is, before the person's endurance limit of force times the number of contraction cycles is reached. Working muscles initially tolerate the stress but require rest periods for recovery in order to avert damage.
People do experience symptoms of fatigue, such as discomfort and inability to work as efficiently, as they approach their endurance limit. The small, slow-twitch skeletal muscle fibers are more resistant to physiological fatigue than fast-twitch fibers. Since slow-twitch fibers are also characteristic of the small motor units recruited for low-force, repetitive, endurance work, they are the most vulnerable for being contracted to the point of muscle damage. Small muscles used for low-force, repetitive work are at risk for this type of damage (Lindman et al., 1991; Dennett, 1998; Larsson et al., 1988), as are small, slow-twitch motor units of larger muscles such as the trapezius. Larsson and colleagues (1990) observed a correlation between pain, reduction in muscle blood flow, and mitochondrial changes in slow-twitch fibers of the trapezius muscle for patients with a work history of performance of repetitive, static contractions; the pain and reduced blood flow to the trapezius muscle persisted long after elimination of the work.
The existing measures for damage of muscle appear inadequate for detecting endurance limits and early structural damage at the molecular level. Consequently, the correlation of the symptoms experienced by the worker to beginning structural damage have not been determined in scientific studies.
Types of Contractions and Associated Injury
Muscles always generate force due to cross-bridge cycling and internal sarcomere shortening during contraction, but the type of contraction varies based on what happens to the overall length of the muscle. During an active contraction, the overall length of the muscle may remain con-
Page 206
stant, yielding an isometric contraction; decrease, yielding a concentric contraction; or increase (i.e., stretch to a longer length), yielding an eccentric contraction. The first two types of active contraction, isometric and concentric, do not normally result in structural damage (McCully and Faulkner, 1985; Armstrong, Ogilvie, and Schwane, 1983; Newham, Jones, and Edwards, 1983; Newham et al., 1983; Balnave, Davey, and Allen, 1997; Lieber and Fridén, 1988; Lieber, Woodburn, and Fridén, 1991; Faulkner, Jones, and Round, 1989). The exception would be repetitive isometric (static) or concentric (kinetic) contractions of small motor units with slow-twitch muscle fibers under conditions in which fatigue does not protect them from overuse, as discussed above (Dennett and Fry, 1988; Larsson et al., 1988; Guidotti, 1992; Ranney, Wells, and Moore, 1995; Larsson, Oberg, and Larsson, 1999).
Due to the cross-bridge mechanism of skeletal muscle force generation and sarcomere structure, there is an optimal length for overlap of thick and thin filaments and cross-bridge formation, reflected as optimal length muscles for maximal force production. Contracting skeletal muscle outside this optimal length range creates a greater risk of structural damage, in addition to reducing force generation. In these less than optimal length ranges, sarcomeres in a fiber may have nonuniformity of force generation, causing hypercontraction of some and overstretching of others along the length of the fiber. Eventually, chronically stretched muscles will lengthen and chronically shortened muscles will shorten, by addition and deletion of sarcomeres at the ends of their fibers, respectively. This remodeling takes days to weeks; in the interim, the muscle is working inefficiently, and the muscles performing at longer lengths are at particular risk of damage to sarcomere structure.
The third type of muscle contraction, eccentric, offers the greatest risk for structural damage to fibers; this risk cannot be reduced by fiber remodeling or exercise training. External loads or work that cause sarcomeres to lengthen during active cross-bridge attachment are very likely to result in structural damage. Evidence of muscle damage may include loss of active force generation capacity, inflammation, necrosis, hemorrhage, and connective tissue tearing. Loss of force generating capacity may precede the other signs of eccentric contraction damage and is hypothesized to be due to shearing of, or damage to, myosin cross-bridges (Jones et al., 1986; Ogilvie et al., 1988; Fridén, Sjøstrom, and Ekblom, 1983; Newham et al., 1983; Newham, Jones, and Edwards, 1986; McCully and Faulkner, 1986; McComas, 1996; Macpherson, Dennis, and Faulkner, 1997; McCully and Faulkner, 1985; Lieber and Fridén, 1993; Brooks, Zerba, and Faulkner, 1995).
Regardless of the etiology, muscle injury results in an inflammatory response (Cannon et al., 1990; Kokot et al., 1988; Smith et al., 1989). Evi-
Page 207
dence indicates that oxygen free-radical mediated injury, edema, impaired perfusion, and other elements associated with acute inflammation contribute to both the progressive injury noted after injurious eccentric contraction and the so-called reperfusion injury seen after prolonged muscle ischemia (Korthuis et al., 1985; Walker et al., 1987; Granger, 1988; Messmer et al., 1988; Rubin et al., 1990; Seyama, 1993; Skjeldal et al., 1993; Jerome, Kong, and Korthuis, 1994).
Average force, strain, and work done to stretch the muscle (i.e., average force × strain) are the main physical factors in the initiation of muscle fiber injury during eccentric contraction (Brooks and Faulkner, 1996; Brooks, Zerba, and Faulkner, 1995; Hunter and Faulkner, 1997; Lynch and Faulkner, 1998). For repetitive eccentric contractions, available data indicate that an exponential relationship exists between stress and the number of cycles to failure, so that greater stress requires fewer cycles to failure. On the basis of the materials fatigue model, there should exist an endurance limit, or stress threshold, below which any number of contraction cycles could be applied without leading to injury (Armstrong, Warren, and Warren, 1991; Warren et al., 1993). However, the injurious effect of duty cycle and total duration of eccentric contraction have been incompletely characterized; therefore, a damage threshold for human work has not been identified.
Passive Stretch Injury of Skeletal Muscle
Passive stretch is the lengthening of a skeletal muscle while it is relaxed and not actively generating force. Passive stretch has been shown in a variety of studies to be a cause of skeletal muscle damage. The specific conditions requisite for passive stretch injury are not fully elucidated, in part because of different models used to apply passive stretch in scientific studies. However, certain results are common across studies.
The velocity, excursion, duty cycle, and total duration of passive stretch determine the total energy imparted to the muscle (Nikolaou et al., 1987). The combined effect of these variables must exceed a threshold for injury. An amplitude threshold was determined in a study by Noonan and others (1994) of rabbit skeletal leg muscles. Muscle stretch at 20 percent of load to failure showed no decrement in maximal contractile force. In contrast, stretch at 30 percent of load to failure showed significant loss of maximal contractile force and hemorrhage with focal areas of muscle fiber rupture. The relationship of amplitude and duty cycle of passive stretch in injury appears more complex. Cycling of passive stretch has been shown to cause a decrease in maximal force generation without abnormal microscopic or ultrastructural changes (Lieber and Fridén, 1988; Lieber, Woodburn, and Fridén, 1991). A plausible biological
Page 208
explanation for this is that the cycles of passive stretch damage the cross-bridges that attach and detach in relaxed muscle at a rate of 32 s−1 at 37° C (Eisenberg, Hill, and Chen, 1980). Such damage to cross-bridges would not be visible using a conventional microscope.
The mechanism of muscle damage during passive stretch has not yet been fully elucidated. Similarly, the effects of cyclical, passive loading and the role of duty cycle and total duration have not been systematically studied.
Vibration Injury of Skeletal Muscle
Working with hand-held vibrating tools has been linked to neurologic, vascular, and musculoskeletal disorders (Pelmear and Taylor, 1992; Armstrong et al., 1987; Stromberg et al., 1997; Färkkilä et al., 1979). In terms of skeletal motor unit function, vibration exposure impairment during intermittent and sustained maximal voluntary contractions has been shown in humans to include reduced electromyogram (EMG) firing rate, decreased motor unit firing rate, and decreased skeletal muscle force generation (Bongiovanni, Hagbarth, and Stjernberg, 1990). The effects on skeletal muscle per se are not so well documented, and the decrease in skeletal muscle maximal force generation is due at least in part to reduced firing of α motor neurons (Färkkilä, 1978; Färkkilä et al., 1980). Relocation of nuclei to the center of muscle fibers, although not structural damage per se, is used as a marker of injury in vibration-exposed muscles because this nuclear change is observed to be a common feature of neuromuscular disorders. In studies, using centralized nuclei as a marker for injury, only the skeletal muscles most directly exposed to the vibration were found to be affected by it (Necking et al., 1992, 1996b; Dubowitz, 1985).
The results of studies of rats by Necking and coworkers (Necking et al., 1996a, 1996b) show that frequency displacement and duration of the vibration interacted as determinants of changes in fiber nuclei location in the contracting skeletal muscle. The most direct indication of skeletal muscle damage from exposure to vibration during active contraction is that plasma levels of intracellular muscle enzymes increase, suggesting disruption of the skeletal muscle fiber cell membrane (Miyashita et al., 1983; Okada, 1986).
Muscular weakness is a common complaint among vibration-exposed workers (Färkkilä, 1978; Färkkilä et al., 1980; Pyykko et al., 1986), and reduced hand grip strength, corrected for aging, may persist for years after exposure (Färkkilä et al., 1986). However, the evidence of skeletal muscle damage per se has not been thoroughly studied for conditions leading to vibration-induced loss of force generation.
Page 209
Age-Related Skeletal Muscle Injury and Risk
Aging is associated with skeletal muscle decrements in force, power, endurance, and recovery from injury. There are also age-related changes in motor unit innervation and in muscle morphology and metabolism (Kirkendall and Garrett, 1998; Bemben, 1998; Faulkner, Brooks, and Zerba, 1990; Shephard, 1999).
In humans, the decrease in muscle strength begins around age 40 and is more dramatic in humans after age 65; most of this decline is associated with inactivity (Faulkner, Brooks, and Zerba, 1990; Kirkendall and Garrett, 1998; Brooks and Faulkner, 1990, 1994; Shephard, 1999). However, approximately 20 percent of the age-related skeletal muscle weakness cannot be explained by the decrease in muscle mass or cross-sectional area associated with inactivity (Brooks and Faulkner, 1988; Bruce, Newton, and Woledge, 1989; Phillips et al., 1992; Brooks and Faulkner, 1994; Degens, Hoofd, and Binkhorst, 1995; Brown and Hasser, 1996; Jubrias et al., 1997). As evidence for this, training does not completely protect against the changes due to aging (Faulkner and Brooks, 1995).
Aged skeletal muscle is also more vulnerable to injury. In studies of rats subjected to eccentric contractions, researchers have demonstrated that aged skeletal muscle fibers are more easily injured by single and multiple eccentric contractions, muscle fibers regenerate less, and structural and functional recovery is not complete (Zerba, Komorowski, and Faulkner, 1990; Brooks and Faulkner, 1990, 1996; Carlson and Faulkner, 1989). However, extensive studies of the effects of eccentric contraction on aged human muscle have not been published.
Summary
The scientific studies reviewed support the conclusion that repetitive mechanical strain exceeding tolerance limits, imposed in a variety of ways, results in chronic skeletal muscle injury. This conclusion must be tempered by the limitations of the animal studies, which examined only a limited number of independent variables for short time periods that do not match the time frame for chronic work-related exposures. A major void in this area is concrete animal data that links repetitive use to injury after chronic exposure at levels of use that do not cause short-term injury. The conclusions related to repetitive mechanical strain and chronic skeletal muscle injury are dependent on extrapolation of data from short-term animal experiments. However, human studies support the same conclusions, even though the measures of dependent and independent variables are less definitive and the experimental conditions are less controlled. More importantly, the earliest molecular contractile changes in skeletal
Page 210
muscle structural injury have not been identified, and measures to detect them are not available for animals or humans.
Standardizing work will not necessarily guarantee safety or similar risk of injury for all workers. Constant, or standardized, external loads and strains, encountered in the performance of work, will have a different impact on each person, because of individual variations in skeletal muscle (mass, type, condition, structure) and for a given person over time, because of effects of aging and adaptation. For example, younger conditioned persons with the largest contractile mass, or skeletal muscle cross-sectional area, will have the greatest contraction force generation to oppose external load. Similarly, people with longer skeletal muscles have the capacity to withstand the larger length changes, and a person's relative proportion of slow- versus fast-twitch fibers will determine their tolerance for low-intensity endurance versus high-intensity burst-type work. This creates person-based variations in the risk and degree of injury for fixed work and external loads. Better noninvasive measures of skeletal muscle injury threshold are needed, since the match of work to task will be imprecise.
PERIPHERAL NERVE
Structure and Function
Peripheral nerves carry electrical impulses from peripheral tissues (e.g., skin, tendon, muscle) to the spinal columns and from the spinal column to the periphery (e.g., vessels, muscle). A nerve is composed of hundreds or thousands of axons, which are each an extension of a nerve cell body located in the spinal cord. The axon is surrounded by Schwann cells to form myelinated nerve fibers ( Figure 5.4). Myelinated and non-myelinated nerve fibers are grouped together in bundles, called fascicles, and surrounded by a perineurial membrane. The amount of connective tissue in and surrounding the nerve varies by level. For example, nerves located superficially in the limb or parts of the peripheral nerve that cross a joint contain an increased quantity of connective tissue, possibly as a response to repeated loading (Sunderland, 1978).
The energy needs of impulse propagation and nutritional transport (axonal transport) are provided by a unique microvascular system. The small vessels supplying the nerve from the surrounding tissue have a coiled appearance that permits the normal gliding of the nerve during movement. When the vessels reach the nerve, they divide into branches running longitudinally in various layers of the nerve. In the endoneurium the environment is protected by a blood-nerve barrier. There are no lymphatic vessels to drain the endoneurial space; therefore, when edema
Page 211
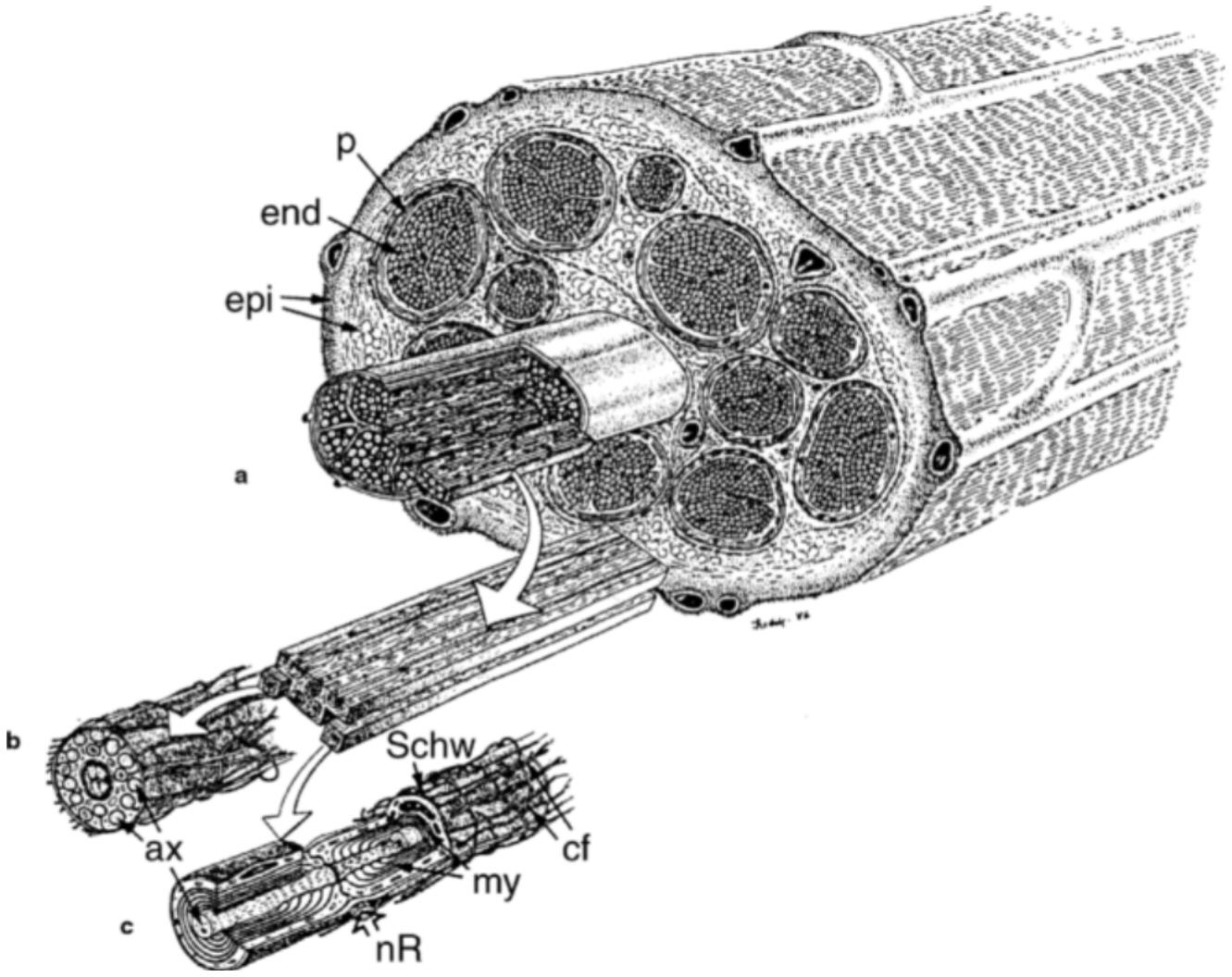
~ enlarge ~
FIGURE 5.4 Drawing of a peripheral nerve with bundles of nerve fibers surrounded by perineurium (p) forming 12 fascicles. The fascicles are embedded in a loose connective tissue called the epineurium (epi). Myelinated (c) and nonmyelinated (b) fibers are shown with Schwann cells (Schw), myelin sheath(my), axons (ax), and nodes of Ranvier (nR). (Reproduced, with modification, from Lundborg, 1988:186. Reprinted with permission.)
forms in this space, the pressure in the fascicle may increase and rapidly interfere with the endoneurial microcirculation (Lundborg and Dahlin, 1996).
Short-Term Effects of Compression
The effects of loading on the peripheral nerve have recently been reviewed (Rempel et al., 1999). The primary mechanism of mechanical injury to the nerve is by regional compression or nerve stretching. Extraneural compression pressures as low as 20 mmHg can decrease intraneural microvascular flow, and pressures of 30 mmHg can impair axonal transport. By increasing vascular permeability, a brief low-pressure (30 mmHg) compression of the nerve can lead to endoneurial edema formation, which persists for at least 24 hours after the compression is
Page 212
removed. In turn, the resultant edema reduces blood flow in the nerve. Both increasing duration of compression and higher pressure lead to greater edema formation.
The effect of fluctuating extraneural pressure on nerve function was investigated in a rat tibial nerve model, wherein a sinusoidal pressure pattern was applied at 1 Hz for 20,000 cycles (Szabo and Sharkey, 1993). The study indicated that when extraneural pressure fluctuates rapidly, the effect on nerve function is associated with the mean value of the pressure waveform, rather than the minimal or peak value.
Long-Term Effects of Nerve Compression
The long-term biological effects of brief, graded nerve compression have been studied in several animal models using small inflatable cuffs (Powell et al., 1986; Dyck et al., 1990). Pressures of 0, 10, 30, and 80 mmHg were applied for 2 hours to a nerve; then at intervals up to 28 days the nerves were examined for evidence of injury. Within 4 hours endoneurial edema formed within all compressed nerves and persisted for the entire time of the study. Inflammation and fibrin deposits occurred within hours of compression, followed by a proliferation of endoneurial fibroblasts and capillary endothelial cells. Within days, vigorous proliferation of fibrous tissue was noted, with marked fibrosis at day 28 and sheets of fibrous tissue extending to adjacent structures. Demyelination and axonal degeneration were first observed a week after compression. The degree of axonal degeneration and demyelination were correlated with the initial pressure.
To model chronic nerve compression, other investigators have placed short silicon tubes of varying internal diameters or loose ligatures around the rat sciatic or sural nerve (Mackinnon et al., 1994; Sommer et al., 1993). These are very effective models for studying pain-related behavior (Mosconi and Kruger, 1996). The biological response of the nerve is similar to that found in the cuff experiments, with early perineural edema followed by a short-term inflammatory response, fibrosis, demyelination, and, finally, nerve fiber degeneration. It is not possible to precisely control the compression level with these chronic models.
Vibration Exposure
Work with handheld vibrating tools can lead to a complex of symptoms known as the hand-arm vibration syndrome, in which sensorineural disturbances are prominent (Strömberg et al., 1996). Biopsies of the posterior interosseus nerve 5 cm proximal to the wrist, from men exposed to hand vibration at work, revealed such pathological changes as break-
Page 213
down of myelin and the presence of interstitial and perineurial fibrosis in comparison to controls (Strömberg, 1997). The histology results suggest that demyelination may be a primary lesion in the neuropathy, which is followed by fibrosis associated with incomplete regeneration or with organization of an edema. Similar pathological changes are seen in the small nerves at the fingertips from patients exposed to vibrating handheld tools (Takeuchi et al., 1986). Animal models exposing peripheral nerves to vibration demonstrate an initial edema formation followed by demyelination and later a loss of axons (Lundborg et al., 1990, 1987; Ho and Yu, 1989; Chang, Ho, and Yu, 1994).
Summary
Several animal models demonstrate that low magnitude, short- or long-term compression of a peripheral nerve leads to a biological response of endoneurial edema, demyelination, inflammation, axon degeneration, and fibrosis. The degree of axonal degeneration is dependent on the applied pressure in a dose-response pattern. The critical pressure or threshold causing acute changes in nerve function is known, but the critical pressure-duration threshold for chronic nerve compression is unknown.
Exposure to vibrating hand tools at work can lead to permanent peripheral nerve injury. Animal models of vibration exposure confirm a pathophysiological process of edema formation followed by demyelination and axonal degradation. No animal model has been developed to evaluate the effects of repetitive hand-finger loading on nerve structure and function.
SPINAL NERVE ROOTS
Structure and Function of Spinal Nerve Roots
The nerve roots are located along the axis of the spine and serve as routes of communication between the central and peripheral nervous systems. Enclosed by the vertebral bones, the spinal nerve roots are relatively well protected from external trauma. However, spinal canal pathology that compromises the neural space, such as disc herniation or protrusion, spinal stenosis, and degenerative disorders, can create high risk of injury, even under what might be considered moderate physical exposures. Furthermore, nerve roots do not possess so much protective connective tissue as do the peripheral nerves, which makes them particularly sensitive to mechanical and chemical irritation.
Structurally, the axons of the nerve root are located in the endoneural space, which is similar to that of the peripheral nerve but with five times less collagen. The root sheath separates the nerve root from the cerbro-
Page 214
spinal fluid, which is surrounded by the spinal dura mater. The vascular supply is complex and may be involved in the pathophysiology of injury. The vessels from the periphery and from the spinal cord meet in the proximal one-third of the nerve root; it has been suggested that this region is particularly vulnerable to injury from ischemia. The blood-nerve barrier in the nerve root is not so well developed as in peripheral nerves; this creates a higher risk of edema.
Acute Nerve Root Compression
The most common mode of nerve root injury is mechanical compression. Recent experiments that precisely control nerve root compression reveal that capillary blood flow can be disrupted by venular occlusion with pressures as low as 5 to 10 mmHg. Such a compression-induced impairment of the vasculature will impede nerve root nutrition and lead to nerve root dysfunction. There is no significant secondary route of nutrition via diffusion from the cerebrospinal fluid.
Low-pressure compression of a nerve root will lead to an increase in the vascular permeability and intraneural edema formation (Olmarker, Rydevik, and Holm, 1989), a response well documented for peripheral nerves (Rydevik and Lundborg, 1977). In peripheral nerves, such edema may increase the endoneurial fluid pressure (Low and Dyck, 1977; Lundborg, Myers, and Powell, 1983; Rydevik, Myers, and Powell, 1989), which in turn may impair the endoneurial capillary blood flow and jeopardize the nerve root nutrition (Myers et al., 1982; Low, Dyck, and Schmelzer, 1982; Low et al., 1985). Edema may negatively affect the nerve root for a longer period than the compression itself, since the edema usually persists for some time after the removal of a compressive agent. The presence of an intraneural edema is also related to subsequent formation of intraneural fibrosis (Rydevik, Lundborg, and Nordborg, 1976), which may delay recovery in some patients with nerve compression disorders. Experimental compression studies have demonstrated that the sensory fibers are more susceptible to compression than the motor fibers (Pedowitz et al., 1992; Rydevik et al., 1991).
Chronic Experimental Nerve Root Compression
Compression that evolves gradually may allow time for the remodeling and adaptation of axons and vasculature. In this case, the clinical consequences of compression may be less severe than if the compression was applied acutely. Despite this, a very gradual increase in compression, over two weeks, still results in structural and functional changes consistent with constriction (Delamarter et al., 1990; Cornefjord et al., 1997).
Page 215
Following compression of nerve root and dorsal root ganglion, there can be an increase in a chemical factor called substance P, which is a neurotransmitter related to pain transmission (Cornefjord et al., 1995). This finding suggests that compression may lead to pain via both mechanical and chemical pathways.
Mechanical Deformation, Biochemical Factors, and Pain
Mechanical deformation of nerve roots may induce impulses that are perceived by the individual as pain. For instance, mechanical stimulation of nerve roots or peripheral nerves results in nerve impulses of short duration; these impulses are prolonged if the nerve tissue had been exposed to mechanical irritation by a chronic gut ligature (Howe, Loeser, and Calvin, 1977; Cavanaugh, Özaktay, and Vaidyanathan, 1994). Severe mechanical deformation, such as ligation of the nerve root, is generally not painful (Chatani et al., 1995; Kawakami et al., 1994a, 1994b).
An increase in the level of neurotransmitters related to pain transmission has been found in the dorsal root ganglion in response to whole-body vibration of rabbits (Weinstein, 1986; Weinstein et al., 1987). A similar increase has also been seen in the dorsal root ganglion and nerve root after local constriction of the same nerve root (Cornefjord et al., 1995).
Recent data suggest that chemical factors in nucleus pulposus can sensitize a nerve root, making it more susceptible to mechanical perturbations. Applied individually, nucleus pulposus or slight mechanical movement will not produce pain behavior in a rat model, whereas the combination of the two factors produces pain (Olmarker and Myers, 1998; Olmarker et al., 1998). This sensitization is thought to be orchestrated by the cytokine TNF-alpha (Olmarker and Larsson, 1998).
Summary
Although experimental models of low-grade compression have not been so extensively applied to the spinal root as they have to the peripheral nerve, they reveal similar initial damage mechanisms. Compression or direct mechanical stimulation may release cytokines and neurotransmitters (e.g., substance P, TNF-alpha) that stimulate pain transmission. However, due to experimental difficulties associated with chronic pain models, the entire pathway leading to chronic pain remains uncertain. The pathway is likely to involve a combination of compression of the dorsal root combined with the release of biochemical mediators of pain.
Page 216
SUMMARY
The structural tissues—bone, disc, tendon, and muscle—demonstrate fatigue failure well below their strength. For example, tendons subjected to repeated stretching at 10 percent of strength will ultimately fail. For tissues studied in detail, the relationship between load and number of cycles to failure follows a log-linear dose-response model. The demonstration of this relationship provides evidence for damage accumulation. The ultrastructural correlates to damage accumulation have been observed for some tissues. For example, vertebral bone subjected to cyclical loading develops microfractures. In other tissues, such as the disc, the ultrastructural correlates have yet to be documented.
Although a threshold for fatigue damage below which no damage accumulates has been postulated, such thresholds have yet to be proven. For example, 30 percent compression strength has been postulated as the fatigue threshold for the disc. The duration of fatigue testing at low stress levels (e.g., 10 percent) has been short relative to the time frame for repair and remodeling. Tissue repair and remodeling takes place over weeks and months, but the fatigue tests are conducted for hours or days. The documentation of thresholds for fatigue damage would be a valuable adjunct to the current data.
In vivo animal models are necessary to investigate the whole organism's response to tissue loading. To some degree, the ultrastructural damage due to cyclical loading may be repaired as long as the time frame for the repair and remodeling is not long relative to the rate of damage and as long as the remodeling mechanism is not overwhelmed. With a repair system in place, one would expect a load-duration or a load-repetition threshold below which there is no damage accumulation and a disorder would never manifest. In addition, some injury mechanisms, for example, those mediated by inflammation or ischemia, can only be thoroughly investigated with in vivo models.
In vivo animal models have been developed for the disc, tendon, muscle, and nerve that can support the investigation of the effects of cyclical loading on cellular, biochemical, and mechanical endpoints of tissues in the intact organism. Generally, these studies demonstrate specific damage endpoints, with sustained or repeated loading, that are similar to the pathology observed in humans. For example, the rabbit tendinitis model developed by Backman et al. (1990) demonstrated edema, increased capillary network, and inflammatory cells in the paratenon and degenerative changes in the tendon, findings also observed in histopathology studies of human tenosynovitis and epicondylitis. Most of the in vivo findings have been observed in more than one laboratory. These studies, in addition to demonstrating the specificity of endpoints, also
Page 217
document the temporal relationship between exposure and effect. Due to the different levels of development of these models and the methods used to evaluate tissue damage, the depth of knowledge of the mechanism of injury varies from tissue to tissue.
In vertebral bone, strength diminishes and damage is irreversible with each trabecular fracture. However, the role of the smaller microcracks in the pathophysiology of bone damage is uncertain; they may be a harbinger of fractures, or they may be repaired. In humans, vertebral microfractures are not visible using current imaging methods (e.g., X-ray, CT, MRI), which contributes to the known poor correlation between images of the spine and low back pain. Both disc and vertebra demonstrate fatigue failure, but vertebral endplates fail before the disc in response to repeated loading. Cumulative spinal loading affects disc pressure and water content, and these factors, in turn, influence cell viability and function. In vivo loading of sufficient magnitude can cause altered cell metabolism and cell death following a dose-response relationship.
In vivo animal models that expose tendons to repeated loading demonstrate an inflammatory response with fibrosis in the peritenon. The process may be mediated by an initial release of inflammatory mediators and microtrauma. In the central tendon, degenerative changes are observed with edema, collagen disorganization, and fibrosis. Although the initial steps may include microtrauma, they are not well characterized.
In muscle, prolonged fatigue damage can occur with single or cyclical loads. Eccentric loading is more damaging than concentric loading. Repeated eccentric loading can produce a persistent force decrement with structural damage to the sarcomeres (Z-line streaming, fiber necrosis, and inflammation). A threshold or endurance limit for injury has not been identified. The mechanical fatigue injury mechanism may be complemented by physiological mechanisms (e.g., intramuscular pressure, Cinderella hypothesis).
In the peripheral nerve, compression causes edema accumulation, elevated endoneural pressure, vascular disruption, fibrosis, demyelination, and axon injury. The steps linking the initial effects to demyelination and permanent nerve damage are uncertain. Compression of the nerve for a sufficient duration also leads to chronic pain. Exposure to vibration causes a similar process of edema formation followed by demyelination and axon degradation. Although the relationship between nerve injury and compression follows a dose-response model, the critical pressure and duration relationships for chronic nerve compression have not been determined. For the spinal nerve roots, adjacent tissue compression may release cytokines that stimulate pain transmission.
Age can influence the mechanical and biological properties of bone, disc, muscle, and nerve. Increasing age leads to increased degenerative
Page 218
changes in vertebrae and discs, increased accumulation of extracellular matrix in the peripheral nerve, and reduced muscle strength. However, the independent role of age in modifying the negative effects of cyclical loading on these tissues is not determined. The role of gender as a covariate in the response of tissues to cyclical loading has not been investigated.



































