6
Analyzing Risks
The primary objective for managing PCB-contaminated sediments is the reduction of risk. A critical part of this evaluation is the characterization of existing and potential risks to affected parties. In analyzing risks from PCB-contaminated sediments, the primary focus has been on human health and ecological effects from exposure, the emphasis being on the bioaccumulation of PCBs through the aquatic food web and the human health effects associated with the consumption of contaminated seafood. Risks associated with water consumption and inhalation near contaminated sites are also considered. In addition, PCB contamination might result in economic, social, or cultural impacts to affected parties. These impacts might include loss of a commercial or recreational fishery, decline in property values, reduced commercial opportunities, increased health risks associated with changes from a fish-based to a possibly less healthy diet, and loss of cultural traditions (e.g., the passage of fishing and hunting rites from one generation to the next).
Analyzing all the risks associated with PCB-contaminated sediments is a complicated, multifaceted task and is best addressed within a prescribed, methodical framework of an environmental risk assessment (ERA). Although a number of frameworks are available for conducting ERAs (as described in Chapter 3), the committee endorses the application of the general framework developed by the Presidential/Congressional Commission on Risk Assessment and Risk Management (1997). The U.S. Environmental Protection Agency
(EPA) guidance for human health and ecological risk assessment (EPA 1997b, 1999) is generally consistent with the commission’s framework and is commonly used in conducting ERAs at PCB-contaminated-sediment sites. This guidance is limited to human health and ecological risk assessment and needs to be extended to explicitly include social, cultural, and economic impacts.
This chapter provides an overview of the ERA process and discusses the use and limitations of scientific information in each of the steps of a risk assessment of PCB-contaminated sediments. The steps that are described include exposure assessment to PCBs; ecological effects and human health effects from PCB exposure; PCB risk characterization; social, cultural, and economic impacts of PCB contamination; and comparative risk assessment. A summary of findings and specific recommendations for conducting ERAs at sites with PCB-contaminated sediments are given at the end of this chapter.
ENVIRONMENTAL RISK ASSESSMENT
ERA provides a process to evaluate the probability that adverse effects are occurring or might occur in the future because of the presence of contamination (see Box 6-1). The framework for this assessment is designed to follow a flexible, tiered approach beginning with a screening-level assessment followed by more detailed evaluations of the site. In this approach, initial or screening-level assessments are used to identify the issues and possibly rebut the presumption of risk. This assessment is typically based on minimal data and very protective assumptions. Three outcomes are possible from this screening-level assessment. First, the screening assessment might indicate that the degree or extent of contamination is sufficiently small to pose no significant risk. Second, the risk might be predicted to be relatively great, but the extent of contamination is sufficiently small to make effective management technically feasible and relatively cost-effective. In such cases, the decision to initiate a particular risk-management strategy or not may be taken without further refinement of the risk assessment. Third, if potential risks cannot be rebutted and the extent of contamination is such that a rapid and effective risk-management strategy cannot be easily identified and applied, a more refined ERA should be conducted. A refined ERA should begin with a baseline assessment to quantify the existing and potential risks associated with PCB contamination as described in this chapter. The baseline risk assessment should be followed by an examination of potential risk-management options (Chapter 7), the development of a risk-management strategy (Chapter 8), the implementation of the risk-management strategy (Chapter 9), and a short- and
|
BOX 6–1 Issues in Environment Risk Assessment The analysis of risk for PCB-contaminated sediments is best addressed within the systematic structure of an ERA. The discussions of risk assessment throughout this chapter largely focus on the following questions:
|
long-term evaluation of the risk-management strategy to determine if the management goals have been achieved (Chapter 10).
The ERA process consists of three steps (problem formulation, analysis, and risk characterization). Problem formulation involves defining the specific contaminants of concern, delineating the areas of concern, and identifying populations potentially at risk and their size. The analysis phase for human health and ecological risk assessments includes an identification of exposure pathways, a characterization of exposures, and an assessment of the relationship between exposures and effects. Finally, the risk-characterization phase involves quantifying overall risks to humans and wildlife. Each of these steps is discussed in further detail below.
In this discussion, problem formulation, analysis, and risk characterization are presented sequentially, but the committee emphasizes that the ERA should be considered an iterative process. Information obtained during the analysis or risk characterization can lead to a reevaluation of the problem formulation, new data collection or analysis, or even a reevaluation of the initial problem
definition and risk-management goals. Similarly, selection of a management option or the evaluation of risk-management results can lead to a reformulation of the problem and might require additional data collection and analysis, such as congener-specific measurements of PCBs in key receptors or media. The extent to which additional site-specific information is collected should be balanced with the costs of conducting the ERA and the costs of risk management. The committee stresses that references to cost refer not only to monetary costs or risks but also to social and political costs of actions or lack of actions in a timely manner.
PROBLEM FORMULATION
The problem-formulation stage for PCB-contaminated-sediment sites involves discussions among the various affected parties to identify the specific geographic areas of concern, all possible risks to humans and wildlife from immediate and long-term exposure to PCBs and from remedial activities, the identification and size of the populations potentially at risk, and the possible presence of co-contaminants at the site. This information is used to identify clearly the assessment endpoints, select measurement endpoints, and develop a conceptual model for the site. At most sites with PCB-contaminated sediments, human health assessment endpoints include both carcinogenic and noncarcinogenic effects (e.g., children born with learning dysfunctions). Special consideration should be given to certain sensitive subpopulations, such as women of child-bearing age, pregnant women, and young children. Other populations who eat fish from contaminated water ecosystems on a regular basis might also be at increased risk. Such populations include many American Indian tribes, immigrants from fishing cultures, such as Southeast Asia, and subsistence fishers who rely upon fish as a major source of protein. For ecological assessment endpoints, reproductive success and population sustainability of resident fish, piscivorous and other predatory birds, and marine mammals are often considered.
Assessment endpoints are used to select measurement endpoints, for which indirect effects, sensitivity and response time, diagnostic ability, and practicality issues are considered. Measurement endpoints are responses (e.g., litter size in mink) that can be measured more easily than assessment endpoints (e.g., reproductive success in mink) but are related quantitatively or qualitatively to the assessment endpoints. Whenever practical, multiple measurement endpoints should be chosen to provide additional lines of evidence for each assessment endpoint. For example, for humans, it might be possible to measure PCB concentrations in food and in human tissues. For
predatory fish, birds, and mammals, it might be possible to measure concentrations of PCBs in prey and in predator tissue. Additional measurement endpoints should be selected to assess effects from other chemicals, from nonchemical stressors (e.g., habitat alterations), and from the proposed remedial actions. If feasible, measurement endpoints should be compared with a reference site that has many of the same characteristics as the study area.
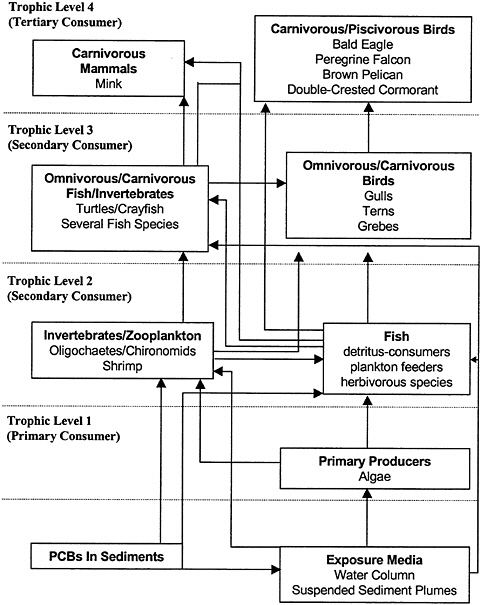
Wildlife and humans can be exposed to PCBs either directly from abiotic media, such as sediments, water, or air, or indirectly through diet. As an example, PCBs can enter the food chain by accumulating in benthic invertebrates that are in close contact to the sediments. These invertebrates then can be eaten by other wildlife and thus PCBs accumulate up the food chain. One of the most important aspects of the determination of the potential risk of PCBs to biota is the “food web” or “pathway analysis.” Although exposure to important receptors can be postulated, the best method of assessing the potential for exposure is to measure the concentrations of PCBs in key dietary items. The relative merits of measuring and modeling exposures will be discussed later in this chapter.
The primary issue in the exposure assessment is the determination of the biologically available fraction of PCBs that are buried in sediments. PCBs might be buried deep enough in sediments to be below the biologically available zone. Furthermore, PCBs bound to sediments that are in the biologically available zone might be bound in such a manner that they are not biologically available. Specifically, some congeners might be less available because of the nature of their binding to sediment particles (Froese et al. 1998). The movement of PCBs out of sediments is a slow process. Otherwise, the concentrations of PCBs in contaminated sediments would dissipate to a point where they would no longer represent a toxicological risk to wildlife or humans. The slow movement of PCBs from the buried sediments also indicates that the available fraction is relatively small. The goal of the exposure assessment is to discern the fraction of PCBs that are available and the rate of release or movement into the food chain or the transport away from the source. In general, the goal of the exposure assessment is to determine the concentration of each congener that will be accumulated into various levels of a food chain of wildlife species in the vicinity of a location containing PCBs in the sediments.
The first step in the exposure assessment is to determine the species most likely to be exposed. As part of the problem-formulation phase, a conceptual model of exposure pathways is developed (see Box 6-2). This conceptual model can then be used to conduct a pathways analysis to determine the level of exposure expected for each trophic level or individual receptor (e.g., see Figure 6–1). These estimates of exposure can be either measured or predicted. In either case, the concentration of PCBs must be measured or predicted in
|
BOX 6–2 Conceptual Model Considerations In preparing a conceptual model of the site, consideration should be given to the following (modified from EPA 1998):
|
either critical tissues of receptors or their diets. In general, to minimize the uncertainties in predictions, it is suggested to minimize the length of pathways along which predictions are to be made. Ultimately, it should be possible to link concentrations to top predators to concentrations in the sediments. That link is necessary to derive a proposed threshold concentration in sediments. The threshold concentration would be the cleanup criterion for a particular site. Uncertainties in the exposure assessment can be minimized by collecting measured values for certain key parts of the exposure pathways. For instance, measuring concentrations of PCBs in fish can serve as an integrated measure of the biologically available fraction of PCBs in sediments. The concentrations in fish can be used directly by comparing them to dietary toxicity reference values (TRV) or by using them to predict exposures to higher trophic levels. Similarly, the concentrations can be linked to concentrations in sediments with just a few links. The use of measured concentrations of PCBs in fish is suggested as the most relevant means of measuring exposure of receptors to PCBs in contaminated sediments.
contaminant concentrations and effects. Both activities involve the evaluation of available scientific data and an assessment of the relevance of the data to assessment endpoints and to exposure pathways. Exposure characterization describes sources of PCBs and other contaminants, their distribution in the environment, and their exposure to ecological and human populations. Characterization of the potential effects on humans and the environment involves the evaluation of PCB dose-response and other contaminant-response relationships or evidence that exposure to PCBs and other contaminants cause an observed response. Quantitative uncertainty analysis is typically performed in the analysis phase. The products of this phase are summary profiles that describe exposure and contaminant-response relationships.
For PCBs, the analysis of chemical exposure and contaminant-response relationships is complicated by the fact that PCBs are not a single compound, but rather a complex mixture of congeners whose composition in the environment can be drastically different from the original Aroclor mixtures. The changes in PCB-congener composition result from environmental processes, including differential volatilization, solubility, sorption, anaerobic dechlorination, and metabolism, and are referred to as “environmental weathering.” Environmental weathering of PCBs is an important consideration in determining the fate and effects of PCBs, as presented in Boxes 6-3 and 6-4. Further details of the analysis phase, such as PCB exposure assessment, and human health and ecological effects are presented below.
PCB Exposure Assessment
The purpose of an exposure assessment is to determine the concentrations of PCBs in various environmental compartments, including sediment, water, benthic invertebrates, and fish, and to evaluate dietary exposures to PCBs of higher trophic level organisms, such as birds, aquatic mammals, and humans. The receptors of interest and the conceptual model for the site serve as the basis for the exposure-assessment studies.
The questions to be addressed in the exposure studies are as follows:
-
What are the existing exposure levels of PCBs in the sediments?
-
What are the expected exposure levels of PCBs for each potential risk-management option?
An assessment of present exposure is best addressed through direct measurement of PCBs in specific organisms or in their diet. Measurements of PCB effects in organisms may also be used (e.g., fish production or survival).
|
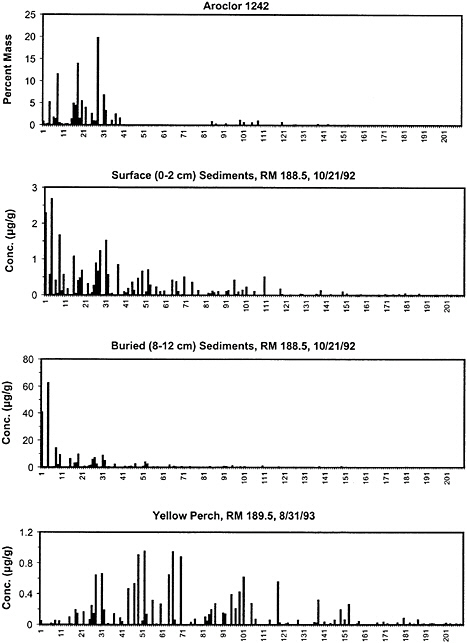
BOX 6–3 PCB Weathering in the Upper Hudson River General Electric used PCB oils in the manufacture of electrical capacitors at plants in Hudson Falls and Fort Edwards, New York, from the late 1940s through 1977. Over this time, Aroclor 1242 was the primary PCB oil used in the plant sites. (Late in the production period, General Electric switched from Aroclor 1242 to Aroclor 1016, which is a reformulated version of Aroclor 1242 with a similar congener distribution.) The congener distribution for Aroclor 1242 is shown in panel A of Figure 6–2 and is largely composed of dichlorobiphenyls (BZ 4–15) and trichlorobiphenyls (BZ 16–39) (see Appendix H for list of BZ numbers). During and subsequent to the production period, PCBs were released into the Hudson River, contaminating 200 miles of river from Hudson Falls to New York City. Sediment and fish samples collected in Thompson Island Pool (the first impoundment on the upper Hudson River, a few miles downstream of the General Electric plant sites) are shown in Figure 6–2 and provide a dramatic example of PCB weathering in the environment. The congener distribution for a surface- (0–2 cm) sediment sample in Thompson Island Pool (panel B) shows an enrichment of tetrachlorobipenyls (BZ 40–81) and pentachlorobiphenyls (BZ 82–127) compared with the original Aroclor 1242 mixture. This enrichment of the more-chlorinated-PCB congeners in surface sediments is attributed to the preferential binding of more-chlorinated PCB congeners to sediments. The surface-sediment congener distribution also shows an enrichment in a few of the less-chlorinated congeners, such as BZ 1, 4, and 19, which are known dechlorination endproducts. This enrichment might be due to dechlorination in the surface sediments or particle mixing and diffusion of these congeners from deeper sediments where dechlorination is more pronounced (see panel C). Although the extent of dechlorination is extensive at this location in Thompson Island Pool, it does not appear to be as significant as that at other locations in the Hudson River and at other PCB sites with lower contamination levels. The congener distribution for yellow perch from Thompson Island Pool is shown in panel D. These data indicate that as PCBs from the sediments or from continuing discharges from the plant sites are transferred through the food web, there is a clear shift in the distribution to more-chlorinated congeners. As discussed throughout this chapter, the changes in the congener distributions, which are collectively referred to as environmental weathering, have a profound effect on the transport, fate, bioaccumulation, and toxicity of PCBs and must be explicitly considered in the evaluation of risk. |
|
BOX 6–4 Pattern Recognition Principal components analysis was performed on the PCBtotal-normalized concentrations of individual congeners (Figure 6–3) (Froese et al. 1998). A variance of 47% was explained by principal components 1 and 2 (PC1 and PC2). The results of the principal components analysis support the hypothesis that the pattern of relative concentrations of PCB congeners in sediments was different from that in tissues of organisms, including the benthic invertebrates in the sediments. Furthermore, the patterns of PCB congeners were significantly different in the tree swallows than in the benthic invertebrates. This difference indicates that the pattern of relative concentrations of PCBs changes because of such processes as weathering, bioaccumulation, and metabolic processes as the individual congeners move from one trophic level to the next. Environmental weathering changes the relative concentrations of PCB congeners because of differential solubilities, volatilities, and sorption coefficients (Mackay et al. 1983). In addition, metabolism by microorganisms (Mavoungou et al. 1991) and animals (MacFarland and Clarke 1989) can cause relative proportions of some congeners to increase and others to decrease (Boon and Eijgenraam 1988; Borlakoglu and Walker 1989). Mean ratios of lipid-normalized mono- and non-ortho-substituted congeners to total concentrations of PCBs were not significantly different among trophic levels. Concentrations of PCB congeners 110, 81, and 77 were less in bird eggs than in invertebrates. That difference might be due to differential metabolism between birds and invertebrates (Boon et al. 1997). Alternatively, congeners 126, 157, and 156, perhaps due to their more fully occupied meta-positions, did not appear to be metabolized significantly in different biota (Boon et al. 1989). |
Evaluation of future exposures under natural attenuation or other risk-management options are typically performed using simulation models. This approach would have to include other chemicals if significant co-contaminants are present at the site. Field monitoring and PCB exposure models are discussed below.
Field Monitoring
Present exposure levels of PCBs (and co-contaminants) are determined by measuring concentrations in relevant environmental media, such as sediments, water, benthic organisms, and fish, and determining dietary exposure rates,
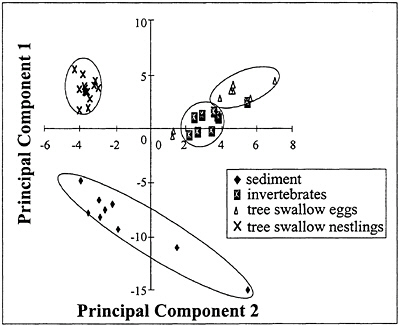
FIGURE 6–3 Principal components analysis of PCBtotal-normalized concentrations of individual congeners. Source: Adapted from Froese et al. (1998).
particularly for higher trophic level organisms, such as birds, aquatic mammals, and humans. For this assessment, sample collection for sediments may include surface-sediment grab samples (representing the top 2–10 centimeters (cm) of sediment) or sliced-sediment core samples (representing the top 1–2 meters (m) of sediment with cores sliced in 2–20 cm intervals, depending on the specific sediment site). Water-column samples are collected and analyzed as either whole-water or filtered-water samples. Benthic organisms are analyzed as composite whole-organism samples. Fish are analyzed as individual or composite whole-fish samples for smaller fish and as fish fillets for larger, edible fish. Dietary exposure rates are determined from PCB concentrations in food items (e.g., fish) times food consumption rates. For birds and aquatic mammals, food consumption rates are estimated from observed feeding behavior, gut content studies, or bioenergetic calculations, which relate food consumption to organism respiration and growth. For humans, food consumption rates can be determined from individual diet surveys or food-market sale records. In calculating dietary exposure, PCB concentrations in contaminated prey are typically defined on a whole-body basis for birds and aquatic mammals, whereas PCB concentrations in fish fillet are used in
calculating dietary exposure rates for humans. There might be specific situations in which calculations should take into account consumption of a specific portion of a harvested organism, such as the tomale of the lobster, which is considered to be a delicacy by some consumers, and can have PCB concentrations much higher than the claw or tail-muscle tissue (Farrington et al. 1986).
Because PCBs are a group of compounds and the absolute and relative concentrations of PCBs in sediments are changing as a function of space, time, and trophic level, the method used to quantify PCBs can have a great impact on the risk-assessment process. Therefore, a discussion of the various methods available is provided here, and a more detailed discussion is provided in Appendix F. There is a great deal of variation in the quality and quantity of information obtained by different methods, as well as in the costs of the analyses. Thus, there are tradeoffs between the type of information collected and the number of samples that can be studied. No single correct allocation of resources is appropriate for every site. Rather, a decision on allocation should be made in the problem-formulation stage of an assessment.
Analyses of PCBs in sediment, water, and fish samples are performed by gas chromatography. This requires extraction of PCBs from the sample and usually some cleanup of the PCB extract by one or more methods before analysis. In the analysis, a gas chromatograph (GC) is used to separate individual PCB congeners or combinations of congeners on the basis of physical and chemical properties, such as volatility or polarity. Over the past 20 years, open tubular capillary GC columns have replaced older packed GC columns for routine laboratory work. The use of open tubular capillary columns, which offer improved resolution, better selectivity, and increased sensitivity, is typically referred to as high-resolution gas chromatography (HRGC). Within the GC system, PCBs are detected by an electron capture detector (ECD), an electrolytic conductivity detector (ELCD), or mass spectrometry (MS). The chromatographic output of the GC allows identification of individual PCB congeners or combinations of congeners on the basis of the resolution of the capillary column and the detector.
Quantification of GC output is performed using Aroclor or congener-based methods. The Aroclor methods rely on comparison of select chromatographic peaks in the sample with the pattern of peaks in a series of pure Aroclor standards to estimate Aroclor concentrations. The method has been applied to chromatographs using both older packed GC columns and open tubular capillary columns and provides a useful comparison of present analyses to historical measurements. Comparison of chromatographic peaks in environmental samples with those found in commercial Aroclors, however, is of little value due to the alteration of PCB-congener distributions in the environment by physical-chemical weathering processes (e.g., differential
volatilization, solubility, and sorption) and biochemical weathering reactions (e.g., anaerobic dechlorination and metabolism). Because congeners degrade at different rates depending on the environment, commercial Aroclor products are difficult to identify and difficult to quantify in the environment. The weathered multicomponent mixtures might have significant differences in peak patterns compared with Aroclor standards. The degree and position of chlorine substitution influences not only physical and chemical properties but also toxic effects. Thus, it is important to consider not only the total PCB concentration in a sample but also to characterize the distribution of individual PCB congeners in a sample. The changes in the absolute and relative concentrations of PCBs in the environment are one of the primary uncertainties in the risk assessment and one of the potential areas for disagreement among scientists and risk managers.
Congener-based methods provide a more accurate (albeit more costly) approach in quantifying total PCB concentrations in environmental samples. Analytical methods more commonly used at PCB-contaminated sites also provide direct quantification of 13 to 108 individual or coeluting congeners (see Appendix F) that can be used in more detailed exposure and toxicity evaluations.
Final measurement of PCB concentrations are reported in terms of Aroclor, total PCB, or concentrations of individual PCB congeners for sediment (as micrograms of PCB per gram of dry sediment), water (as nanograms of PCB per liter of water), and benthic organisms and fish (as micrograms of PCB per gram wet weight of organism). Organic carbon normalization of sediment data and lipid normalization of concentrations in benthic organisms and fish are often used to recognize the preferential sorption of PCBs into these phases. Measured PCB concentrations may be used directly to assess toxicity. For example, toxicity in fish and lower trophic organisms have been reported in terms of exposed water concentrations, exposed sediment concentrations, or tissue residue concentrations. For organisms at higher trophic levels, PCB concentrations in contaminated food are used to calculate dietary exposure rates that, for example, may be compared with allowable dosage rates (e.g., reference dose (RfD)), maximum allowable toxicant concentration (MATC), or toxicant reference value (TRV)).
In addition to assessing present exposure levels, nonchemical data should be collected at the site for use in developing PCB exposure models, assessing nonchemical impacts, and evaluating the applicability of various risk-management options (e.g., natural attenuation, source control, dredging, stabilization, ex situ treatment). Information may include access to the site, hydrodynamics and hydrology, climatology, time-series suspended material concentrations, and sediment bed properties (e.g., horizontal and vertical mapping of sediment
grain size, mineralogy, water content and credibility, presence of boulders and/or debris, and depth to bedrock or impermeable hardpan, i.e., hard-packed sediment). In addition, sampling can be performed to determine external solids loading and continuing PCB sources from upstream waters, contaminated industrial sites, wastewater treatment plants, combined sewer overflows, storm drains, and the atmosphere.
Accumulation of PCBs in Sediments
Data collected at a number of sites provide a general picture of PCB-contaminant behavior in sediments, benthic organisms, and fish.
At PCB-contaminated sites, sediments, particularly fine-grained, organicrich sediments, serve as a long-term repository for PCBs. The horizontal and vertical distributions of PCBs in sediments are the result of temporal and spatial deposition patterns of fine- and coarse-grained sediment and the time history of PCB loadings to the system. The distribution of coarse- and fine-grained bottom sediments might exhibit a large degree of spatial variability; coarse-grained sediments are likely to be present in high-velocity regions and deposition of fine-grained sediment occur in more low-energy areas. The presence of cobbles, boulders, underlying bedrock or hard pan are also important considerations, particularly in evaluating dredging alternatives.
Since maximum production and use of PCBs in the United States occurred in the late 1960s and 1970s, the greatest concentrations of PCBs are often found at depth in sediment depositional areas. In addition, continuing sources of PCBs from industrial sites, wastewater discharges, storm-water discharges, and the atmosphere are still occurring (albeit at reduced rates) and might be adding to the inventory of PCBs in sediments. PCB input from contaminated ground water might add to PCB inventories in surface waters and sediments. At some sites, migration of PCBs from contaminated sediments may also be a concern. Although localized “hot spots” (i.e., areas with relatively high concentrations of PCBs) have been documented, the extent of PCB contamination is affected by water and sediment-transport processes and extends over larger areas of sediments.
As discussed previously, PCB-congener distributions in sediments are different from distributions of parent Aroclors because of physical, chemical, and biochemical weathering processes. These processes favor the preferential retention of more-chlorinated congeners in sediments and the transformation of certain more-chlorinated congeners to less-chlorinated congeners by dechlorination in anaerobic sediment layers. With the possible exception of a thin surficial layer, sediments that are likely to contain significant quantities
of PCB contaminants (i.e., fine-grained, organic-laden sediments) are usually anaerobic. Under these conditions, reductive dechlorination, which results in the selective removal of chlorines from the PCB molecules, can occur.
Although PCB dechlorination does not have a dramatic effect on total mass of PCB concentrations, dechlorination might result in a significant reduction in some types of toxicity. For example, PCB congener 126 (3,3′,4,4′,5-pentachlorobiphenyl; IUPAC Number 126), which is one of the most potent PCB congeners exhibiting dioxin-like toxicity, has been reported to decrease by as much as 10- to 100-fold as a result of reductive dechlorination (Quensen et al. 1998).
Accumulation of PCBs in Benthic Organisms and Fish
PCB-contaminated sediments might serve as long-term sources of PCBs to sediment-dwelling organisms and to fish through various exposure pathways. These exposures are largely affected by PCBs in the top 5–10 cm of sediments and not by buried PCBs. This top layer, which is continuously reworked by sediment-dwelling organisms and remains in direct contact with the overlying water, is typically referred to as the “biologically active zone.”
In some cases, desorption of PCBs from sediments in the biologically active zone can be kinetically inhibited and limit the bioavailability of PCBs to organisms. The degree to which PCB are bioavailable is a function of sediment properties, the time history of contamination, and PCB-congener properties. The resulting accumulation of PCBs in bottom-dwelling organisms is usually related to local contamination conditions. To account for preferential binding of PCBs to organic carbon in sediments and lipid content in the organisms, PCB concentrations in bottom-dwelling organisms and sediments are generally related using lipid-normalized accumulations of PCBs in organisms and organic carbon-normalized PCB concentrations in sediments. Because fish typically have a larger home range than bottom-dwelling organisms, aerial-weighted-average concentrations of PCBs in surface sediments, and not hot spot concentrations, are often considered to be a better indicator of potential PCB concentrations in fish.
PCB-congener distributions in water and fish differ from distributions in sediments because of the preferential release of less-chlorinated PCBs to the water, the preferential accumulation of more-chlorinated PCBs through the food web, and the potential metabolism of some PCB congeners. Transfer of PCBs through food webs typically results in biomagnification. Other contaminants, which might also be present in PCB-contaminated sediments, can accumulate in aquatic food webs. As discussed later in this chapter, most of
the toxicity of PCBs in fish and higher trophic organisms is attributed to dioxin-like congeners and not total PCB concentrations. Thus, in most situations, the congeners causing the arylhydrocarbon receptor (AhR)-mediated effects are the critical contaminants for risk assessments and determine both the potential for risk of a particular mixture of PCB congeners and the allowable total concentration of PCBs.
Finally, PCBs buried in sediments below the biologically active zone should not be dismissed when evaluating PCB accumulation in bottom-dwelling organisms and fish. Erosion and reworking of sediments by large storms (e.g., the 100-year flood) or other catastrophic events (e.g., dam failure) have in some cases resulted in reintroduction of buried PCBs into the biologically active zone. The potential for such events should be characterized as part of the ERA.
PCB Exposure Models
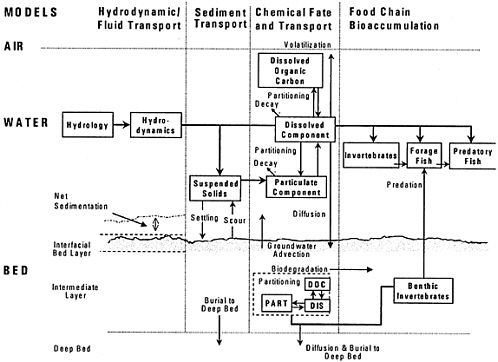
Mechanistic mass-balance models have been used in conjunction with field-monitoring data at a number of sites (1) to quantify the relationships among external sources and PCB exposure concentrations in sediments, water, and biota, and (2) to project future contamination responses under various scenarios of natural recovery or other management options. The overall modeling approach used at PCB-contaminated sites is typically presented in terms of several components (or submodels) as outlined in Figure 6–4. The overall approach considers hydrodynamic and fluid transport, sediment transport, chemical fate and transport, and bioaccumulation models. Since PCBs have a strong affinity for organic carbon, organic carbon distributions have also been modeled explicitly for a few sites. Further details regarding the modeling components—hydrodynamic transport, sediment transport, organic carbon, PCB fate and transport, and bioaccumulation—are given below.
Hydrodynamic Transport
Hydrodynamic monitoring and model calculations serve as the basis for the overall assessment and are used to define the downstream transport of dissolved and particulate PCBs.
Hydrodynamic models have evolved over the past few decades from simple hydrodynamic calculations (e.g., for the James River Estuary (Farley et al. 1983)) to complex multidimensional hydrodynamic computer simulations (e.g., for the Hudson River (Blumberg et al. 1999; QEA 1999b; TAMS Consultants et al. 2000)). These models require site-specific data for calibration of bottom-roughness parameters. Adjustments in the geometry of numerical grid elements have also been used in model calibration. Such adjustments have been used to account for fine-scale features in the river channel that cannot be properly captured in the resolution of the numerical grid. In these evaluations, particular emphasis is usually given to high-flow and storm events, where the energy associated with higher water velocities is considered to have an important role in the reworking and remobilization of contaminated sediments and in transporting particle-bound PCBs further downstream.
Sediment Transport
Sediment transport plays a critical role in the water-column transport and ultimate bioavailability of PCBs. Sediment-transport models have been applied to rivers and estuaries with reasonable success, for example, in the Fox River (Gailani et al. 1991). Because of the site-specific nature of the sediment-transport process, these models typically require collection of large amounts of data for model parameterization, calibration, and verification. Required information includes flow-dependent loading rates for cohesive and noncohesive solids from upstream waters, tributaries, wastewater treatment plants, combined sewer overflows, and other sources of solids. Information is also necessary on channel bathymetry; stream flows and tidal elevations; and sediment maps of fine-grained material, coarse-grained material, and hard bottom along a channel.
For model calculations, settling velocities of cohesive sediments are typically defined by empirical correlation to particle size and concentration and water-column turbulence (Ziegler and Nisbet 1995). Sediment erosion potentials are also required and are determined empirically as a function of flow-dependent boundary shear stress (McNeil et al. 1996). Bottom shear stresses, which are a function of water velocities and are obtained directly from hydrodynamic modeling results, are used to drive the sediment-transport calculation. Calibration parameters include particle-size composition of the solids loading, median particle sizes, and active layer depth of the noncohesive sediments (Ziegler 1999a,b). In the calibration, model results are compared with observed suspended solids and spatial patterns of sediment accumulation (as determined from changes in channel bathymetry, from radionuclide dating
of sediment cores, or from records of maintenance dredging for harbors). Model calibration is usually limited, however, by the availability of good quality data for high-energy storm events (e.g., the 10-year, 50-year, and 100-year storms). In many cases, available data are not sufficient to ensure a unique set of model calibration parameters. Additional emphasis should therefore be given to supplemental data-collection efforts and model-sensitivity studies to determine the best representation of sediment transport at the site.
Although there are inherent uncertainties associated with sediment-transport modeling, model results have greatly aided our understanding of sediment-transport dynamics at many PCB-contaminated sites. For example, in riverine systems, sediment erosion during low and moderate flows is negligible, and a large fraction of the total sediment transport typically occurs during a relatively small number of high-flow events. In this regard, sediment-transport modeling results provide critical information in assessing the depth of sediment scour during high-flow events and in identifying mixing of buried PCBs with the biologically active sediment layer, water column, or both.
Organic Carbon
Since PCBs have a strong affinity for particulate (POC) and dissolved organic carbon (DOC), organic carbon concentrations are needed to compute the partitioning of PCBs among freely dissolved, DOC-bound, and particulate phases. In many cases, measured POC and DOC concentrations are used directly in PCB partitioning calculations. Spatial and temporal variations in POC and DOC concentrations are observed at many sites and are associated with watershed runoff events, sewage-discharges locations, and seasonal phytoplankton productivity. In cases in which phytoplankton productivity has a dominant role in controlling spatial and temporal distributions of POC and DOC (e.g., Green Bay), POC and DOC concentrations may be specified from results of a dynamic eutrophication model calculation.
PCB Transport and Fate
Following evaluations for hydrodynamics, organic carbon, and sediment transport, chemical-fate calculations are performed using mass-balance numerical models to examine the transport and ultimate fate of PCBs in sediment and water-column segments. Because of large differences in physical-chemical behavior, biochemical reactions, and toxicity of PCBs (which will be discussed later), congener-specific or homologue-specific
model calculations are considered to provide a better representation of PCB transport and fate than that provided by total PCB models. It is also important to note that model comparisons to a more detailed congener-specific or homologue-specific data set provide a more rigorous test of model calibration and hence a reduction in model uncertainty.
The partitioning of PCBs between the freely dissolved, DOC-bound, and particulate phases is essential in describing PCB transport, transfer, and transformation behavior. In modeling studies, partitioning reactions are usually assumed to be fast in comparison to other environmental processes and are typically modeled as instantaneous (or equilibrium) reactions. Partitioning between freely dissolved and particulate phases are either derived from field measurements or assumed to be a direct function of the octanol-water partitioning coefficient and the fraction of organic carbon on the suspended or sediment solids. To account for differences in PCB partitioning to lower-molecular-weight DOC compounds and octanol, partitioning of PCBs between the freely dissolved and the DOC-bound phases is typically assumed to be a fraction of the octanol-water partition coefficient.
PCBs bound to particles are subject to settling, resuspension, burial, and water-column suspended-solids transport processes, as defined by hydrodynamic and sediment-transport evaluations (Figure 6–4). In addition to sediment-transport processes, exchange of freely dissolved and DOC-bound PCBs can occur across the sediment-water interface and between the surface and the deeper sediments. Exchange between the sediment pore water and the overlying water column is dependent on the detailed hydrodynamic structure at the water-sediment interface (e.g., through hydrodynamic pumping of water through sediment bed forms) and can be greatly enhanced by biological activity. In the biologically active sediment layer, which can extend down to depths of approximately 5–10 cm, rates of diffusion and particle mixing are greatly enhanced by bioturbation. In the deeper sediment, particle mixing is virtually nonexistent, and exchange of freely dissolved and DOC-bound PCBs is largely a function of molecular diffusion and is generally considered to be slow.
Freely dissolved PCBs in the water column are also subject to volatilization across the air-water interface. The process preferentially affects lower-chlorinated PCB congeners because of the decreased hydrophobicity and increased likelihood that these congeners will be present in the water column as dissolved chemical. The overall loss of PCBs by volatilization can be substantial in water bodies with long hydraulic residence times.
Transformations of organic molecules can also occur by hydrolysis, photolysis, biodegradation, and reductive dechlorination reactions. Although PCBs were originally thought to be refractory, a number of studies have shown that certain PCB congeners can be degraded under aerobic conditions or microbially dechlorinated under anaerobic conditions in aquatic environ-
ments (Abramowicz 1990). For more details on aerobic and anaerobic transformation see Appendix E. Aerobic degradation of PCBs is therefore limited to the overlying water and possibly the surficial layer of oxic sediments, whereas anaerobic dechlorination of PCBs can occur deeper in the sediment column.
The major conclusion from aerobic degradation studies (Bedard et al. 1986; 1987a,b) is that biodegradation of PCBs can occur by the attack of a dioxygenase enzyme at vicinally unchlorinated (2,3 or 5,6) carbon atoms or at an unchlorinated (3,4 or 4,5) site. These attacks result in cleavage of the biphenyl ring and can be carried out by a variety of naturally occurring bacteria. Congeners with chlorines at both ortho (2,6) positions on either ring are generally not degraded as readily as congeners lacking this characteristic.
Under anaerobic conditions, organisms leave the biphenyl ring intact while removing chlorines from the ring, thereby producing less-chlorinated congeners. Although details of the dechlorination process are not fully understood, reductive dechlorination has been shown to proceed primarily through the selective removal of meta (3,5) and para (4) chlorines (Quensen et al. 1988; Abramowicz 1990; Abramowicz et al. 1993; Rhee et al. 1993a,b,c). Anaerobic dechlorination has been observed at a number of locations, including the upper Hudson River (see Figure 6–2). Anaerobic dechlorination is not thought to be important at sites with low concentrations of PCBs, possibly due to PCB concentrations being below a dechlorination threshold value.
Mathematical expressions for the various processes affecting the fate and transport of PCBs are fairly well established, and appropriate ranges for modeling coefficients have been determined from laboratory and field studies and previous modeling applications. A time sequence of PCB concentrations in the sediments and overlying water is typically used for model calibration. Because several modeling coefficients can be adjusted in the calibration procedure, a good comparison between PCB model results and field data alone does not guarantee the proper selection of all modeling coefficients. This difficulty in calibration is likely to be reduced but not eliminated in congener-specific models. Professional judgment therefore plays an important role in model calibration for PCB fate and transport. Uncertainties in model calibration usually stem from a lack of detailed knowledge of water-sediment exchange rates, sediment mixing rates, and depths of the biologically active sediment layer.
PCB Bioaccumulation
Accumulation of PCBs in organisms is typically viewed as a dynamic process that depends on direct uptake from water, dietary exposure, depura-
tion (from back diffusion, excretion, and egestion), and metabolic transformation of PCBs within the organism. For phytoplankton and other plant species, direct uptake from water is described by diffusion of PCBs through cell membranes. For benthic invertebrates, direct uptake from water and ingestion of contaminated sediments might have important roles. For fish and higher trophic level organisms, PCB exposure from ingestion of contaminated prey will often dominate, and biomagnification of PCBs from one trophic level to the next is likely to occur (see Box 6-5).
Models of PCB bioaccumulation in organisms are available with several levels of detail, ranging from simple empirical models to complex food-web models. Simple empirical formulations, which use partition coefficients such as bioaccumulation factors (BAFs), biomagnification factors (BMFs) (Starodub et al. 1996), and biota-sediment accumulation factors (BSAFs),1 describe distributions of PCBs in various environmental compartments (see Chapter 2 for definitions of BAFs and BMFs). BAF, BSAF, and BMF values are typically determined from field or laboratory data, and their application in bioaccumulation calculations is based on the assumption that partitioning of PCBs among water, sediment, and organisms will remain invariant over time.
Empirical BSAFs, which are not based on fugacity theory, are used to predict the accumulation of contaminants from sediments into higher trophic levels (Ankley et al. 1992a,b; Cook et al. 1993). These values implicitly consider the disequilibrium that exists, or can exist, between the sediment and pelagic species (Cook et al. 1993). When applied to aquatic species such as benthic invertebrates, BSAFs are defined as ratios of lipid-normalized concentrations of compounds to organic carbon-normalized concentrations of the same compounds in sediments (Ankley et al. 1992a). When predicting higher-order accumulations, for example, in birds that eat aquatic organisms, similar BMF ratios are used. Although the BSAF-BMF method can be empirical in nature (Cook et al. 1993), it also could be based on fugacity theory (Clark et al. 1988; Mackay and Paterson 1991; Ling et al. 1993) through the use of several assumptions, including that the system is at steady state. A further assumption under both theories is that the accumulation ratios (BSAFs and/or BMFs) are constants that can be applied from one location to another (Neely and Mackay 1982; Velleux and Endicott 1994). These descriptors are useful for static or slowly varying systems but are of little use in dynamic systems where the time responses for PCBs in organisms might be very different from PCB responses in water and sediments.
|
BOX 6–5 Biota-Sediment Accumulation Factor (BSAF)/Biomagnification Factor (BMF) of PCBs Among Trophic Levels A study was conducted on the changes in the absolute and relative concentrations and relative patterns of individual PCB congeners, as well as total PCB concentrations, among trophic compartments. It was determined whether toxic potentials of PCB mixtures change as a function of trophic level when accumulated from the sediments of Saginaw Bay. PCB concentrations were measured in sediments, emergent aquatic insects (primarily chironomidae), and eggs and nestlings of tree swallows (Tachycineta bicolor) from sites within the Saginaw River watershed (Nichols et al. 1995). In addition, the BSAF/BMF method was used to calculate sediment PCB concentrations theoretically protective of tree swallows on the basis of estimates of the toxicity of PCBs or 2,3,7,8-tetrachlorodibenzo-p-dioxin equivalents (TEQs) to other bird species (Giesy et al. 1994a; Ludwig et al. 1996). Average lipid-normalized PCBtotal concentrations were not different among the invertebrates, eggs, or nestlings. The average organic carbon-normalized PCBtotal in sediments was about an order of magnitude less than tissue values. The fact that concentrations of PCBs were not different suggests that there is no net biomagnification of PCBs at these trophic levels. That is consistent with fugacity theory (Mackay et al. 1992) and suggests that BSAF-BMF methodologies might be appropriate for predicting the total mass of PCBs that would be accumulated at higher levels of the food chain in the Saginaw River. Furthermore, this observation indicates that the changes in relative concentrations of individual PCB congeners do not have a great influence on the total mass of PCBs predicted to occur in tissues of higher trophic levels. In addition, these results suggest that the concentrations of total PCBs in the tissues of the tree swallow eggs and nestlings were near steady state. |
More detailed, dynamic models of PCB transfers through food webs have been developed over the past 10–15 years (Thomann and Connolly 1984, Thomann et al. 1992a,b; Gobas 1993, Gobas et al 1993). In this approach, accumulation of PCBs by individual organisms is described by uptake of PCBs from water, sediments, contaminated prey, depuration, and metabolism. PCB uptake by higher trophic species is explicitly linked to accumulations in
lower trophic levels by the food-web feeding structure. Overall, the food-web bioaccumulation models are similar in their construct and reflect a cross-fertilization of ideas among investigators (see comparison of Thomann and Gobas models in Burkhard 1998). For slowly varying systems (such as large lakes), steady-state bioaccumulation model calculations are often sufficient. For dynamic systems (such as contaminated rivers or estuaries and bays with migratory fish), time-variable model calculations are usually required.
The formulation of dynamic bioaccumulation models is based on fish bioenergetic relationships (e.g., the relationship of fish weight to growth, respiration, food consumption, and gill transfer rates) and laboratory-derived kinetic relationships based on information for PCB uptake and depuration processes. The models require site-specific information on food-web feeding structure, whole-body lipid content, and in certain cases, fish migration patterns. A time sequence of measured PCB tissue residue concentrations in exposed organisms is used in calibrating PCB-uptake efficiencies and depuration rate coefficients. Model calibration, however, is often limited by available data. The greatest uncertainties in model calibration usually stem from a lack of information on food-web feeding preferences and inadequate data sets for PCB tissue residues. The latter limitation is exacerbated by variations in PCB tissue residues that are often observed for a given species (i.e., intraspecies variability). Multiple samples of fish are usually required to address this issue of intraspecies variability and to properly define the statistics of exposed populations; this data need is particularly problematic at sites where there are endangered or threatened species such as the Pacific salmon in Puget Sound. Such analyses are also confounded by the need to correlate tissue levels to site of exposure, and such correlations might be difficult to determine for migrating species of fish, birds, and mammals. Following model calibration, simulations are performed to determine future PCB tissue residue concentrations in food-web species on the basis of projected exposure concentrations in sediment and overlying water that are determined from model simulations for PCB transport and fate. For example, projected responses for PCB concentrations in fish from Thompson Island Pool on the upper Hudson River are discussed in Box 6-6. The relative importance of downstream transport, volatilization, burial, dechlorination, and food-chain transfer can also be examined using model simulation results.
Summary of Exposure Modeling Results
Modeling the fate and bioaccumulation of PCBs at contaminated-sediment sites requires a clear understanding of hydrodynamics, sediment transport, and organic carbon behavior.
|
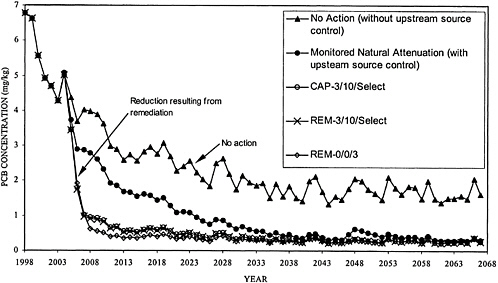
BOX 6–6 The Use of Models in Evaluating Management Options As part of its Hudson River PCB Reassessment, EPA Region II has developed mathematical models describing the transport, fate, and bioaccumulation of PCBs in the upper Hudson River (TAMS 2000) and has applied the models to examine the effectiveness of various management options. Model projections for PCB concentrations in Thompson Island Pool fish are shown in Figure 6–5 for five possible remediation scenarios:
Similar modeling studies were performed by the General Electric Company (QEA 1999b). Model projections for PCB concentrations in largemouth bass in Thompson Island Pool are shown in Figure 6–6. Both the EPA and General Electric models gave comparable results for Thompson Island Pool. Differences in the model projections in Figures 6–5 and 6–6 appeared to be largely attributed to different assumptions used in describing the remedial actions (i.e., implementation and effectiveness of source controls, dredging volumes and areas, commencement and duration of dredging, and capping of dredged areas). EPA and General Electric model projections for sections of the river below Thompson Island Dam show less agreement. This example demonstrates how models can be used to project the relative effectiveness of various management options and how models developed by independent investigators can be used to test the consistency of model formulation and calibration of transport, fate, and bioaccumulation of PCBs at specific sites. |
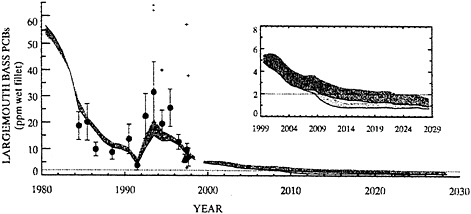
FIGURE 6–6 Predicted PCB3+ concentrations in Thompson Island Pool largemouth bass over time period from 1980 to 2029 for both natural recovery (upper line represents the uncertainty in the projection) and dredging of Reacher 8, 7 and cohesive sediments between 2005 and 2016 (lower line) to a residual concentration of zero. Dotted line at 2 ppm represents Food and Drug Administration tolerance limit; circles represents New York State Department of Environmental Conservation; triangles represents exponent; diamonds represents General Electric; crosses indicate values excluded from the annual averages. Source: Adapted from QEA (1999b).
Hydrodynamic models are rather advanced and can be used to determine a description of temporal and spatial distributions in flow. Sediment-transport models are still in the development stage and require a large collection of data for model calibration. The greatest uncertainty in sediment-transport model predictions is associated with high-flow events (e.g., 10-year, 50-year, or 100-year flooding events) when the largest amount of sediment is expected to be mobilized. Organic carbon distributions in the sediment and overlying water are usually determined by field sampling and are assumed to be constant from year to year. For certain studies, field measurements have been complemented by eutrophication model results.
Formulations of models to predict PCB fate and bioaccumulation are well-developed. In applying these models to PCB-contaminated-sediment sites, care should be taken to include reliable projections of external PCB loads, and to critically examine the model calibration procedures. Once pathways are established and models are calibrated and validated, they can be used to predict future trends and the results of various remedial actions. However, because several modeling coefficients can be adjusted in the calibration procedure, a good comparison of PCB model results to field data alone does not guarantee the proper selection of sources, pathways, and coefficients. This
difficulty in calibration is likely to be reduced but not eliminated in congener-specific models. Professional judgment, therefore, is important in the calibration of models for both fate and bioaccumulation. The most significant sources of uncertainty in PCB models of the transport and fate of PCBs are specification of water-sediment exchange rates, of sediment mixing rates, and of the depth of the biologically active sediment layer. Sources of uncertainty in PCB bioaccumulation models stem from a lack of information on food-web feeding preferences and inadequate data sets to define the statistical and temporal variations in PCB tissue residues. Because those factors are critical in determining future exposures to PCBs, it is often beneficial to have model calibrations performed by more than one group and to conduct detailed model comparisons. Review of model results by expert panels is also recommended. Publication of model results in peer-review literature, although desirable, should not be considered a sufficient criterion for model acceptance. Even with these uncertainties in calibration, PCB exposure models have provided and continue to provide important information for the decision-making process.
One of the major issues in conducting exposure assessments is predicting the movement of PCB congeners from the sediments to biological receptors. Due to the fact that the absolute and relative concentrations of PCB congeners can change over time and that different congeners with different chemical-physical characteristics result in different partitioning characteristics, the exposure profile can change over time and depending on trophic levels.
PCB Effects Assessment
The purpose of effects assessment is to determine PCB dose-response and other stressor-response relationships and show evidence that exposure to PCBs or other stressors causes an observed human or ecological response. In this context, the emphasis in the assessment of the human response is on incremental effects in the individual, and the assessment of the ecological response typically measures an adverse effect in a given population or component of the food web (e.g., the effects of PCBs on survival or reproduction of a given species). Commercial PCB mixtures are known to elicit a broad spectrum of toxic responses that are dependent on several factors, including chlorine content, purity, dose, species and strain, age and sex of animal, and route and duration of exposure (Giesy and Kannan 1998). Metabolism must be considered when assessing toxicity, because the persistence of a congener can affect its toxicity. Immunotoxicity, carcinogenicity, and developmental toxicity as well as biochemical effects of commercial PCB mixtures have been extensively investigated in various laboratory animals, fish, and wildlife species.
In this section, the congener-specific approaches for assessing exposure and effects—TCDD-toxicity equivalents (TEQs) and BSAFs—are first described, followed by a discussion of ecological and human health effects associated with PCBs.
Congener-Specific Exposure and Effects
In the risk-characterization process, estimates of exposure and hazard are required as discussed earlier in this chapter. When assessing the potential for PCBs in sediments to result in exposure and or effects in biota, simple thermodynamic relationships are often applied. These relationships, sometimes referred to as transfer coefficients—BCF, BAF, and BSAF (discussed in the section PCB Bioaccumulation earlier in this chapter)—are simple ratios of the concentrations of PCBs in various environmental compartments. Exposure and effects estimates are complicated by the fact that PCBs are a mixture, and the relative concentrations of individual congeners in the mixture are subject to change. For this reason, the simple ratios do not apply to the total concentrations of PCBs but rather to individual congeners and are a function of their chemical properties and those of the environment in which they occur. Thus, it is generally inappropriate to apply such simple relationships to total PCB concentrations. To account for the changes in the relative concentrations and thus toxic potency, the TEQ approach has been developed. Because the concentration of TEQs in an environmental or biological sample is a function of both the concentrations of individual congeners and their relative toxic potencies, it is inappropriate to apply simple thermodynamic ratios to concentrations of TEQs. Since both TEQ and BSAF techniques are applied in risk assessments of PCB-contaminated sediments, both of these concepts and their utility are discussed below.
Toxicity Equivalent Values
When considering exposure to and toxicity of PCBs, it is important to take into account the concentrations of individual PCB congeners, in addition to the total PCB concentrations, because the various congeners elicit different types and magnitudes of effects. Coplanar PCB congeners can bind to and cause pleiotrophic effects through the AhR. The toxic effects of noncoplanar PCBs are not mediated through the AhR. Some of the neurotoxic effects of PCBs appear to result from the noncoplanar PCBs. (See Appendix G for a further discussion of the neurotoxic effects of PCBs.) For the effects mediated by the AhR, the critical toxicants to which wildlife are exposed are not total PCBs but
rather concentrations of the congeners that are structurally similar to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and bind to the AhR (Ludwig et al. 1996; Giesy et al. 1994a,b). Concentrations of TEQs represent the total potential of the dioxin-like PCB congeners to cause TCDD-like toxicity. The values represent a weighted average of the concentrations of individual non-ortho and mono-ortho-substituted PCB congeners corrected for their relative potencies to cause AhR-mediated effects. However, it is important to note that the TEQ approach does not account for all effects, such as some neurotoxicity, that can be mediated by the noncoplanar PCBs through mechanisms not involving the AhR.
The ratio between the TEQs in sediments and that in invertebrates is a function of bioavailability. The TEQ concentration, based on organic carbon-normalized and lipid-normalized concentrations, decreased by a factor of about 3.3 from the sediments to the invertebrates (see Table 6–1). This factor probably represents the measure of bioavailability of the non- and mono-ortho-substituted congeners due to factors not accounted for by organic carbon normalization. The organic carbon in sediments is not exactly the same as invertebrate lipids (Karickhoff et al. 1979), and it has been suggested that the correction factor from sediment organic carbon to tissue lipids is approximately 1.7, with lipids dissolving more solute than sediments (Sabljic et al. 1995). If the ratio of normalized concentrations from sediment to invertebrate is corrected for this factor, a ratio of 1.9 is calculated. Theoretically, based on fugacity theory, if the organic carbon in the sediments is equivalent to that in biota, this value should be 1.0. Thus, only about half of the mass of PCB in
TABLE 6–1 Average TEQs Calculated from the Average Sum of Non- and Mono-Substituted PCBs and the Resulting TEQs Ratios Across Trophic Levels
|
Sample Matrix |
Sum Non- and Mono-PCB |
TEQs |
TEQs Trophic Ratios |
||
|
ng/g Lipid |
SD |
ng/g Lipid |
SD |
||
|
Sediment1 |
2.2×103 |
4.2×102 |
1.1×10−1 |
2.2×10−2 |
— |
|
Invertebrate |
8.3×102 |
6.8×102 |
3.0×10−2 |
2.0×10−2 |
Invertebrate/ sediment, 0.3 |
|
Egg |
1.4×103 |
7.8×102 |
8.8×10−2 |
4.1×10−2 |
Egg/invertebrate, 3.0 |
|
Nestling |
2.4×103 |
3.5×103 |
1.1×10−1 |
1.6×10−1 |
Nestling/egg, 1.3 |
|
1Pediment values are ng/g organic carbon. Abbreviations: ng/g, nanograms per gram; SD, standard deviation. Source: Adapted from Froese et al. (1998). |
|||||
the sediment, normalized to organic carbon, is available for equilibrium partitioning—that is, the biologically available fraction is approximately 0.5. The estimate of bioavailability is complicated somewhat by the fact that invertebrates might have sediment in their guts (Ingersoll et al. 1995). Thus, the ratio between the two compartments may be biased either up or down, depending on the effect that the gut sediment has on the fugacity of the system.
Comparison of calculated TEQ values (Table 6–2) with PCBtotal indicates that the average lipid-normalized PCBtotal concentrations did not change across trophic levels, but the distributions of the congeners changed significantly. Potency is defined as the ratio of TEQs normalized to a PCB congener, PCB 153, that contributes a significant proportion of the total PCB mass and does not selectively weather or degrade (Jones et al. 1993). The potency ratio used in the study of Froese et al. (1998) was defined as the quotient of TEQ concentrations of adjacent trophic levels. For the samples analyzed by Froese et al., most of the calculated concentration of TEQ (87%; SD=3%) was contributed by PCB 126.
No change in the relative potential from the egg to the nestling (potency ratio (nestling/egg)=1.0) was observed by Froese et al. (1998) (Table 6–2). However, the potency changed by nearly a factor of 3 from the sediment-dwelling benthic invertebrates to the tree swallow eggs (potency ratio (egg/invertebrate)=2.6). A potency ratio of 1.0 would be expected for the nestling/egg ratio, because there was little opportunity for significant metabolism or accumulation of PCBs from the egg to the nestling. For the initial trophic transfer, the adult bird accumulates, metabolizes, and transfers the organochlorines into the developing egg. The nestlings retain the egg burden of compounds, which are diluted by growth, but also begin further accumulation through feeding (Jones et al. 1994). There was a sharp decrease in the toxic potential from the sediment to the invertebrates (ratio=0.01). As discussed above, this decrease is probably due in part to the differences in bioavailability of the lipophilic compounds in the organic carbon on the sediment versus in the lipid of the invertebrates. The relative proportion of those congeners that contribute the most to the toxic potential have been shown to increase with trophic level from sediments to birds (Jones et al. 1993, 1994). The relative potency factors observed here are similar to those that can be calculated for tree swallow eggs and nestlings from Green Bay, Wisconsin (Ankley et al. 1993). Using those data, the relative potencies were calculated based on total PCBs of 1.6×10−5, 1.8×10−5 and 1.1×10−5 for tree swallow eggs, newly hatched chicks, and 16-day old nestlings, respectively. The average of these potencies (1.5×10−5) is 2.2 times greater than that of the Saginaw Bay tree swallow eggs and nestlings (6.6×10−6).
PCB 153 is often used for normalizing PCB data, because it is a major component of several technical PCB mixtures (Clark et al. 1988). PCB 153
TABLE 6–2 Relative Concentration Ratios of Congeners 153+132 to PCBtotal, Relative Potencies of TEQ Normalized to Either PCB 153 or PCBtotal, and the Corresponding Potency Ratios
|
Sample Matrix |
PCB 153/ PCBtotal |
Potency Based on PCB 153 |
Potency Based on PCBtotal |
Potency Based on PCBcoplanar |
Enrichmenta |
|||
|
Potency |
Potency Ratioa |
Potency |
Potency Ratio |
Potency |
Potency Ration |
|||
|
Sediment |
0.014 |
4.8×10–3 |
|
6.6×10–5 |
|
5.1×10–5 |
|
120 |
|
Invertebrate |
0.032 |
4.8×10−5 |
0.01 |
1.5×10−5 |
0.02 |
3.6×10−5 |
0.7 |
1.5 |
|
Tree swallow egg |
0.048 |
1.3×10−4 |
2.6 |
7.1×10–6 |
3.9 |
4.8×10−5 |
1.7 |
8.0 |
|
Tree swallow nestling |
0.055 |
1.3×10−4 |
1.0 |
6×10−6 |
1.2 |
6.2×10−5 |
0.8 |
4.6 |
|
aPotency Ratios and Enrichment are calculated as described below in Equations 1 and 2, respectively. The potency ratio is always the potency of the higher trophic level matrix divided by the potency of the matrix that is one trophic level lower. Source: Adapted from Froese et al. (1998). |
||||||||
is a di-ortho-substituted hexachlorinated congener that does not have vicinal unsubstituted carbon atoms and thus is not significantly metabolized by most organisms. On a DB-5 chromatographic column, PCB 153 co-elutes with PCB 132. The relative ratios of these two congeners are variable and depend on a number of factors, including metabolic processes across trophic levels or specific species (Niimi et al. 1996). PCB 132 can be metabolized, whereas PCB 153 cannot. Additionally, other PCB congeners are metabolized, ultimately resulting in the relative ratio of PCB 153 increasing with trophic level (Table 6–2). The effect of this increase is that the use of the PCB 153+132 peak for normalization might underestimate the potency, particularly if there are significant metabolism differences between tr1 to tr2 (Equation 1).
(1)
where PCB 153tr is the concentration of PCB congener 153 in trophic level; TEQtr is the equivalent concentration of 2,3,7,8-TCDD in trophic level; and tr1 and tr2 is the trophic levels 1 and 2, respectively.
However, the error caused by this effect is small, because the ratio of the PCB 153+132 peak to PCBtotal does not change drastically among trophic levels (Table 6–2). When the relative potencies normalized to either the mass of 153+132 or that of PCBtotal congeners, the potential bias of each normalization method must be considered, particularly when comparing data from different sources. Ratios of the relative potencies among trophic levels demonstrated a decrease in the relative toxic potency from sediment to invertebrates but an increase in the relative toxic potency between invertebrates and tree swallow eggs (Table 6–2).
The relative enrichment of TEQs with respect to the original Aroclor mixture in the same trophic level can also be calculated (Table 6–2). Enrichment was defined as the PCB 153-normalized ratios of TEQ concentrations in samples to TEQ concentrations of the Aroclor. This ratio provides a measure of the extent of change of the relative toxic potency of the PCB mixture in the environment, measured as TEQ increases from the original combination of technical Aroclor mixtures that were released into the environment (Equation 2).
(2)
where TEQsample is 2,3,7,8-TCDD TEQs contributed by PCB in the sample; TEQAroclors is 2,3,7,8-TCDD TEQs from Comstar-weighted original Aroclor
contributions; PCB 153sample is the concentration of PCB 153 in the sample; and PCB 153Aroclors is the concentration of PCB 153 from Comstar-weighted original Aroclor contributions.
Enrichment of TEQs for invertebrates (Table 6–2) indicates that little change occurred in the PCB composition due to weathering or metabolic processes. However, enrichment occurred for tree swallow eggs and nestlings. That was likely due to metabolic processes that selectively dechlorinate and/or excrete some congeners, while allowing others, primarily the more AhR-active non- and mono-ortho-substituted congeners, to accumulate (Boon and Eijgenraam 1988; Tanabe et al. 1987). The relative enrichment of TEQs for sediments is 120, which is 15 to 80 times greater than the apparent relative enrichment for biota in the trophic levels above the sediment. It is unclear why a relatively greater enrichment of TEQs was observed in sediment.
Biota-Sediment-Accumulation-Factor Values
The BSAF has been proposed as an empirical relationship to predict the lipid-normalized concentrations of residues from the total organic carbon (TOC)-normalized concentration of the same residue in sediments (Ankley et al. 1992a,b; Tracey and Hansen 1996). The BSAF approach has been proposed for use as a regulatory tool in risk-assessment methodologies involving contaminated sediments (Parkerton et al. 1993).
BSAF values measured by Froese et al. (1998) varied, depending on whether they were calculated on the basis of the PCBtotal, the sum of non- and mono-ortho-substituted PCBs, or TEQs (see Table 6–3). BSAF calculations based on total PCBs were between 8 and 11, and those based on non- and
TABLE 6–3 BSAF Valuesa for Each Matrix Based on Total PCBs, the Sum of the Non- and Mono-Ortho-Substituted PCB Congeners, and TEQs
mono-ortho-substituted congeners ranged from 0.4 to 1.1. The BSAF is operationally defined and can be site- and species-specific (Lake et al. 1990). The actual BSAF values measured are dependent on the chemical-physical properties of both the residue and sediments. Although TOC may be similar among sediments, there are qualitative, in addition to quantitative, differences that are not accounted for in the BSAF. In addition, the relative importance of TOC, inorganic properties, and size of sediment particles can have an influence on the BSAF values. Also, duration of the residence in the sediment and exposure of biota to the sediments can influence the BSAF value particularly for superhydrophobic compounds that take a long time to reach steady-state with the sediment and biota (Hawker and Connell 1985). Finally, composite samples of emergent invertebrates integrate the overall exposure of organisms to an area in which actual concentrations of residues (such as PCBs), type of sediment, and TOC content might be quite patchy. Thus, in calculating a BSAF, relatively great variations in values can be observed if a small number of samples of TOC-normalized sediment-residue concentrations are used to estimate the denominator of the BSAF. That was the case in the study by Froese et al. (1998) in which the average TOC-normalized total PCB concentration in sediments was 1.7 ng of PCB/g of TOC (standard deviation=2.6 μg of PCB/g of TOC), a range of more than 34-fold between the least and greatest values. If the greatest value was used instead of the average value, the BSAF would be approximately 1.0. Thus, the anomalously high value of the BSAF for total PCBs is probably due to such variation. Based on this type of sensitivity analysis, a range of as much as 35-fold would be expected in BSAF values calculated in this manner. The use of BSAF values in risk assessments assumes that these values do not vary among locations or that an overall average value can be calculated for a region. Although that is not necessarily an invalid assumption, the range of BSAF values should be considered in addition to the average values in interpreting the results of risk assessments.
Fugacity theory predicts that if the organic carbon in the sediment and the lipid in the animal tissues is equivalent as a solvent for the contaminant of interest, the BSAF should be 1.0 in systems at steady state (Hoke et al. 1994). That theoretical value is generally not observed in data collected from the field, because the octanol-equivalent fat fraction for sediment dry-weight organic matter is about 0.3 (Karickhoff et al. 1979; Sabljic et al. 1995). Thus, the BSAF is approximately 1.7 if it is calculated from organic carbon-normalized concentrations in the sediment and lipid-normalized concentrations in organisms.
BSAF values for total PCBs are often greater than would be expected if based on fugacity theory. The high values might be related to the contribution of PCBs in sediments within the guts of the invertebrates. Similarly, anoma-
lously large BSAF values have been observed for accumulation of other compounds from sediments by invertebrates (Eadie et al. 1985; Landrum 1989; Landrum et al. 1992). However, BSAF values of 1–2 have also been reported for PCBtotal (Ankley et al. 1992a,b). When BSAF values were based on the sum of non- and mono-ortho-substituted PCB congeners or TEQ, BSAF values were less than or equal to 1.0 and increased with increasing trophic level (Table 6–3).
BSAF values have been reported to range from 0.1 to 10 for benthic invertebrates (DiToro et al. 1991). The BSAF values observed for the distribution of total PCBs between sediments and infaunal invertebrates can range by up to 2 orders of magnitude, but a global average value of 1.7 has been suggested for use in risk assessments when BSAF values have not been determined for a particular site (Landrum and Poore 1988). For instance, BSAFs for accumulation of PCBs from marine sediments by infaunal organisms, such as mollusks (Mercinaria mercinaria) and polychaetes (Neghtys incisa), ranged from 1.7 to 4.6, depending on the PCB congener (Lake et al. 1990), and BSAFs for accumulation of PCB 153 by the mayfly (Hexagenia limbata) ranged from 4.5 to 15.5 (Drouillard et al. 1996). Other BSAF values for mayfly accumulation of PCB congeners from sediments have been reported to range from 4.8 to 6.5 (Boese et al. 1995). BSAFs for accumulation of individual PCB congeners by the mussel (Malacoma nasta) ranged from 0.19 for PCB 209 to 4.74 for PCB 118 (Landrum and Poore 1988). Those authors found a mean BSAF of 2.84 for all of the PCB congeners that they examined. In a compilation of reported values (Tracey and Hansen 1996), BSAFs ranged from 0.80 for the mayfly and 0.93 for the blue mussel (Mytilus edulis) to 5.11 for the bivalve mollusc (Yoldia limatula) and 5.30 for the midge (Chironimid spp.). The mean of median BSAF values for various species was 2.1 (Tracey and Hansen 1996).
The results of the assessment of the patterns of relative concentrations of PCB congeners as a function of trophic level indicate that when BSAF values are based on the sum of non- and mono-ortho-substituted PCB congeners or TEQs, the observed BSAF values are similar to fugacity theory predictions. Thus, the concentrations of TEQs measured in tissues of tree swallows might be used to predict an integrated measure of concentrations of TEQ in sediments, and vice versa, by the application of a fairly simple partitioning model.
Effects of PCBs on Wildlife
In the sections below, the committee summarizes the information on adverse effects associated with exposure to PCBs on wildlife and humans. The types and thresholds for effects of PCBs is presented for lower trophic organisms—that is, plankton and invertebrates, fish, birds, and nonhuman mammals.
(More detailed information on the toxic effects of PCBs on organisms may also be found in Appendix G.)
Generally, PCBs and other dioxin-like chemicals are not particularly toxic to lower trophic-level biota, including algae, zooplankton, and invertebrates, because these species lack the AhR that mediates the critical mechanism of toxicity. Numerous studies have been conducted with both individual congeners and Aroclor mixtures to assess the toxicity of PCBs to invertebrates. The EPA ECOTOX: Ecotoxicology Database contains 1,687 individual toxicity data citations for PCBs in aquatic organisms, including algae, zooplankton, invertebrates, and fish.
Because PCBs usually are associated with, or bound to, sediments, attempts have been made to relate PCB concentrations in sediment to effects concentrations. These effects concentrations, as well as the national ambient water quality criteria for total PCBs, are presented in Appendix G. It should be noted, however, that these so-called sediment effects concentrations have several limitations. Specifically, there is a lack of causal association due to the presence of co-contaminants and a poor correlation to observed effects. In addition, the bioavailability and toxicity of PCBs in sediments can vary, depending on the sediment organic carbon concentration. In general, the primary uses of invertebrate PCB data for ecological risk assessments are to provide information on site-specific bioavailability and for input to dietary models for biota that consume invertebrates. Because of the poor correlation between concentrations of PCBs and effects in benthic invertebrates and the inherent limitations of the apparent-effects-threshold (AET)-type approaches, such as effects range medium (ERM), due to the co-occurrence of other contaminants, the values developed by these approaches are underestimates of the actual threshold concentration for adverse effects. Therefore, the use of these methods is not recommended.
A more appropriate approach would be to apply the equilibrium partitioning (EqP) theory to calculate sediment quality criteria (Giesy and Hoke 1990). In this method, the site-specific organic carbon content of the sediment is used in conjunction with the organic carbon partitioning coefficient for the compound (Koc) to predict the concentration of PCBs in sediment pore water. This predicted concentration is then compared with the water-quality criterion for fresh or saltwater, as appropriate to calculate a hazard quotient (HQ). By assuming a HQ value of 1.0, the critical concentration in bulk sediments can be calculated. There are Aroclor data available for the calculation, but as noted above, they appear to be of limited value because of the relatively high concentrations required to elicit effects. Furthermore, coplanar PCBs are not expected to be substantially more toxic to invertebrates than other PCBs because of the absence of the cellular receptor required to mediate toxicity.
Assessments of ecological risks for receptors of concern (such as fish,
birds, and mammals) are made by comparing observed or projected chemical concentrations (or dosage rates) to toxicity reference values (TRVs). In this approach, TRVs represent concentrations of chemicals in water, food, or the tissue of the receptor (or dietary dosage rates) that will not cause toxicological effects. Ideally, TRVs are derived from chronic toxicity studies in which an ecologically relevant endpoint was assessed in the species of concern, or a closely related species. Although TRVs can be based on no-observed-adverse-effects levels (NOAELs), they also can be calculated from the lowest-observed-adverse-effects levels (LOAELs). With robust data sets, TRVs calculated from LOAELs might incorporate less uncertainty than TRVs derived from NOAELs. Alternatively, TRVs can be expressed as the geometric mean of the NOAEL and LOAEL to provide a conservative estimate of a threshold of effect (Tillitt et al. 1996).
The development of TRVs for site assessment is based on information on chronic toxicity effects in laboratory and field-based studies of target, sentinel, or surrogate species. Toxic effects of PCBs include developmental effects, reproductive failure, liver damage, cancer, wasting syndrome, and death (Metcalfe and Haffner 1995). These effects are primarily attributed to the non-ortho and mono-ortho-substituted or coplanar PCBs (Safe 1990; Giesy et al. 1994a; Metcalfe and Haffner 1995). The toxicity of the coplanar PCBs, TCDD, and other polychlorinated dibenzohydrocarbons (PCDHs) is expressed through their ability to bind to and activate the AhR (Poland and Knutson 1982; Gasiewicz 1997; Blankenship et al. 2000). Although most of the toxicity of PCBs is attributed to dioxin-like congeners acting through an AhR-mediated mechanism of action (Blankenship et al. 2000), PCB congeners with more than two ortho-substituted chlorine atoms have also been reported to cause chronic (primarily neurobehavioral) toxicity through non-AhR-mediated effects (Giesy and Kannan 1998). These effects, which have been observed, only occur at much greater concentrations than AhR-mediated effects. Although PCB congeners with more than two ortho-substituted chlorine atoms constitute the greatest mass of total PCBs in the environment, toxicity associated with the coplanar, dioxin-like congeners will almost invariably be dominant in higher organisms (i.e., fish, birds, and mammals) that possess the AhR.
Causal links between chronic toxicity effects and Aroclor or total PCB concentrations have been reported in a number of laboratory studies. These results, however, might not be directly applicable to field conditions, because they were largely performed using parent Aroclor mixtures and do not account for possible reduction or enrichment in toxic potency by environmental weathering (Williams et al. 1992; Corsolini et al. 1995a,b; Quensen et al. 1998). For example, microbially mediated anaerobic reductive dechlorination is common in sediments and can eliminate the most toxic coplanar congeners (Zwiernik
et al. 1998). In contrast, certain aquatic animals selectively bioaccumulate the coplanar dioxin-like congeners. Such accumulation can result in a greater relative proportion of these toxic congeners in tissues than in Aroclor mixtures (Williams et al. 1992; Corsolini et al. 1995a,b).
Based on the dominance of AhR-mediated effects, an alternative approach is to correlate observed toxicity to the concentrations (or equivalent concentrations) of dioxin-like congeners. This approach has been shown to work quite well for birds and mammals (Giesy et al. 1994a; Leonards et al. 1995). In determining equivalent dioxin-like concentrations, each PCB, dioxin, and other PCDH congener is considered to bind with different affinity to AhR and therefore to exhibit a different potency for eliciting biological effects (Safe 1990; Ahlborg et al. 1994; Van den Berg et al. 1998). Although the toxic potency of each of these chemicals varies, the potencies can be normalized to that of TCDD, the most potent of the PCDHs, using a toxicity equivalency factor (TEF) (Van den Berg et al. 1998). The concentration of TCDD toxicity equivalents (TEQs) is then determined by multiplying the concentration of a chemical and its TEF as follows (Equation 3):
(3)
where PCDHi represents the concentration of a specific PCDH, and n is the total number of PCDH congeners contributing to dioxin-like toxicity.
Risk assessments are meant to be accurate representations of the potential for risk but are designed to be protective rather than predictive. Even though PCBs are a mixture of compounds with different relative potencies, the concentration of the entire mixture to which an organism is exposed is determined by the congeners or class of congeners with the maximum toxic potency. Because the potential for adverse effects is a function of the exposure concentration and sensitivity of the organism of interest, the best way to determine the critical toxicant is by comparing hazard quotients (Equation 4).
(4)
The compound or compounds with the greatest HQ values are the critical toxicant—that is, the toxicants that would have the greatest overlap between exposure and TRV values would determine the maximum allowable exposure to the entire complex mixture.
When this analysis has been conducted for a variety of species at a variety of locations, it was found that when the allowable concentration of PCB mixtures is based on the HQ for TEQs for dioxin-like effects, concentrations of non-AhR-active congeners are less than the threshold for effects caused by the nondioxin-like congeners. In other words, if the risk assessments are based on TEQs, wildlife will be protected from the effects of nondioxin-like compounds as well.
A large number of laboratory and field toxicity studies have been conducted over the years using Aroclor, total PCB, individual PCB congeners, TCDD, and TEQ concentrations as a measure of PCB exposure. Results for lower trophic aquatic organisms, fish, birds, and mammals are summarized below. However, because organisms are exposed to environmentally weathered mixtures that contain not only PCB congeners but also polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), polychlorinated naphthalenes (PCNs), and possibly other AhR-active compounds; the potential effects of PCBs should be interpreted in the context of these other compounds (Giesy et al. 1994b). However, at most PCB-contaminated sites, PCB coplanar congeners will likely be the primary contributor to AhR-mediated toxicity.
Short discussions of the thresholds for toxic effects for lower trophic level organisms, fish, birds, and nonhuman mammals are given in the following sections. (A detailed analysis of mechanisms of analysis and TRVs is given in Appendix G.)
Toxicity Results for Lower Trophic Organisms
PCBs and other dioxin-like chemicals are not particularly toxic to lower trophic organisms, such as phytoplankton, zooplankton, and invertebrates, because these species lack the cellular AhR that is required to mediate dioxinlike toxicity. PCBs do exhibit a minimum level of toxicity due to nonspecific or “narcotic” effects in these organisms. This level of toxicity is related to the critical body concentration (McCarty and Secord 1999).
Toxicity data indicate that waterborne or tissue PCB concentrations that might cause adverse effects for lower trophic level organisms are much greater than PCB concentrations reported for contaminated environments (Niimi et al. 1996). For example, Niimi et al. (1996) reported adverse effects in phytoplankton and zooplankton at PCB concentrations in water greater than 0.5–1.0 μg/L, which is roughly 100–1,000 times greater than PCB concentrations reported for contaminated ecosystems. Effects thresholds for PCB-contaminated sediments have been suggested (e.g., effects range low (ERL) and ef-
fects range median (ERM) values of 0.0227 and 0.18 mg of PCB/kg of dry weight) (Long and Morgan 1991). These threshold values, however, are suspect because of the lack of a causal relationship between PCB-sediment concentrations and effects. Confounding factors affecting the relationship between sediment concentrations and effects can include differences in PCB bioavailability as a function of sediment organic carbon and the presence of co-contaminants. Therefore, ERM and ERL values are deemed to be unreliable and should not be used for ERAs.
Toxicity Results for Fish
Adult fish are exposed to PCBs and related compounds via water, sediment, and food. Eggs and embryos can accumulate these lipophilic chemicals from the female during vitellogenesis. Bioaccumulation of PCBs by fish is dependent on the physical and chemical characteristics of individual congeners and on the biotransformation and elimination rates of congeners by fish. Early-life stages are generally the most sensitive to chemical contaminants. Thus, accumulation of these persistent chemicals during this life stage is critical to the characterization of risks from PCB exposures.
There are considerable field and laboratory data (from water, dietary, intraperitoneal-injection, and egg-injection exposure) available in which concentrations of PCB congeners, TCDD, and TEQs in eggs or other fish tissue have been measured and related to particular adverse effects. Exposure to TEQs results in effects, such as mortality during early development and thyroid hyperplasia in adult fish (Walker and Peterson 1991). Other toxic effects and histopathological lesions seen during early development are characterized by cardiovascular and circulatory changes, edema, hemorrhages, and mortality (Walker and Peterson 1991). Results of risk assessments demonstrate that maximum allowable tissue concentrations in fish tissue are more dependent on the potential for effects in piscivorous wildlife than on direct effects in the fish themselves.
The majority of fish toxicity data for PCBs is from laboratory experiments. For example, 96-hour LC50 values (the dose that is lethal to 50% of a test population) for fathead minnows are reported to be in the range of 8–15 μg/L for different PCB mixtures (Nebecker et al. 1974). The major route of exposure to fish in the wild, however, is not likely to be through the water but rather via the food chain. However, tissue-based effects thresholds are independent of exposure pathway, because both effects and measured concentrations in the eggs of rainbow trout were the same whether exposure occurred by maternal transfer, water uptake, or injection (Walker et al. 1996). Concen-
trations of PCBs in tissue, therefore, provide a better means of assessing toxicity, because they bypass the issue of water-versus-food-web exposure.
Life stages of marine fish species were evaluated for PCB concentrations in tissues and for critical effects (Jarvinen and Ankley 1999). Niimi et al. (1996) presented a similar table with greater tissue residue-based effects threshold values. Effects are observed in the high microgram per kilogram to low milligram per kilogram range.
If tissue residue concentrations are not available, residue concentrations can be estimated from measured water or sediment concentrations by using equilibrium partitioning relationships, such as the bioconcentration factors (BCF), bioaccumulation factors (BAF), BSAFs, or more-complex trophic-transfer models.
Toxicity Results for Birds
There are sufficient toxicological data available for birds to evaluate both dietary and tissue residue-based effects levels for PCBs. However, data are not available for all of the pertinent species at particular locations. Thus, uncertainty factors are still applied to determine TRVs. As with other organisms, birds demonstrate considerable differences in species sensitivities to PCBs and related dioxin-like compounds. In particular, chickens, which are the most frequently used species for PCB exposures, are among the most sensitive of avian species. Therefore, it is important to use species-specific toxicity data whenever possible.
Toxic effects of PCBs and related compounds in birds have been studied by in vivo exposure, in ovo exposure by injection, and in vitro exposure with cultured avian hepatocytes. Due to better control of exposure, dose, and timing, egg-injection studies are often of more use in deriving TRVs. In most cases, embryotoxic and teratogenic effects of PCBs seem to be the most sensitive and ecologically relevant endpoints in birds (Hoffman et al. 1998). Several in ovo studies of dioxin-like compounds are adequate for determining tissue-based-toxicity LOAEL, NOAEL, and EC50 (the concentration that causes an effect in 50% of a test population) values that can be used in developing TRVs for certain PCB congeners (Powell et al. 1996a,b; Henshel et al. 1997; Hoffman et al. 1998).
Toxicological studies (Ankley et al. 1993; Murk et al. 1994; Tillitt et al. 1996; Boon et al. 1997) have been found that correlate effects from PCB dietary exposure to Aroclors and that correlate tissue residue effects levels in eggs to Aroclors, total PCBs, PCB congeners, and TCDD and TCDD-equivalents (see Box 6-7). These studies show good consistency. Aroclor results
|
BOX 6–7 Special Consideration of PCB 77 PCB 77, one of the more potent non-ortho PCB congeners, contributes one of the greatest mole fractions to the TEQ content of most technical PCB mixtures. Due to its greater susceptibility to metabolism and degradation, it requires special consideration. Although originally considered resistant to degradation, and readily biomagnified because of its lack of ortho substitution (Tanabe et al. 1987), PCB 77 behaves differently from other non-ortho PCBs and differently from other members of the tetrachlorinated homologue group (Willman et al. 1997; Froese et al. 1998). Therefore, it is not appropriate to assume that PCB 77 or other individual congeners have similar properties within a homologue group. Based on biomagnification factors derived from field studies, it is unlikely that PCB 77 would biomagnify from fish to bird eggs to the same extent as congeners 126 and 169. In a study of bird eggs and hatchlings from the lower Fox River and Green Bay, Wisconsin, it was observed that PCB 77 decreased when hatchlings reached an age of approximately 25 days old, whereas all other congeners continued to accumulate (Ankley et al. 1993). Those authors suggested that the decrease was due to metabolism of PCB 77. This observation is in agreement with other studies that have observed metabolic degradation and depletion of 77 in bird microsomes (Murk et al. 1994), marine mammals (Boon et al. 1997), and mink (Tillitt et al. 1996) relative to other congeners. Similarly, Bright et al. (1995) reported that PCB 77 was diminished rather than enriched with increasing trophic status (going from sediment to sea urchins to four-horn sculpins). In a study of Green Bay, Willman et al. (1997) found that PCB 77 decreased in relative abundance both within total PCBs and within its homologue group with increasing trophic level. Due to the predominance of PCB 77’s contribution to total TEQs (using avian TEFs) in PCB-contaminated sediments, PCB 77 is a critical congener to model when characterizing risk to avian species. Thus, modeling of congener 77 must take the above factors into account. |
are of limited use, because the studies were conducted using unweathered Aroclor mixtures, and very few of the studies were conducted on wildlife species. Total PCB results, particularly results for the last 10 years, have
largely been performed using “weathered” PCBs. For risk assessments, PCB concentrations in bird eggs can be estimated using biomagnification factors.
Toxicity Results for Nonhuman Mammals
Evaluations on the effects of environmental contamination on the health of aquatic mammals have presented a considerable challenge. Although the results of semi-field studies have been confounded by the occurrence of cocontaminants in the diet and by the lack of dose-response relationships, studies in such animals as seals have suggested that PCBs were the main cause of the observed toxic effects (de Swart et al. 1994; Ross et al. 1995). Furthermore, the exposures mimicked observed field situations, and, therefore, these values were considered suitable for deriving a toxicity benchmark to assess risks of PCBs to aquatic mammals.
A few studies are available for assessing toxicity in seals (de Swart et al. 1994; Ross et al. 1995), otter (Leonards et al. 1995), and mink (Tillitt et al. 1996). However, logistic considerations make the sampling of a large number of wild animals difficult, and ethical concerns discourage in vivo studies on captive animals. Thus, much of the mammalian toxicology is based on study with rodents. However, mink are among the most sensitive animals that have been studied to date. Risk assessments based on mink often result in the least allowable concentrations in sediments, and if mink are used as a surrogate in risk assessment, most other species, including humans, should be protected.
Lipid-normalized NOAEL values of 11 microgram per gram (μg/g) in seal blood and of 9 μg/g in otter liver were reported by Kannan et al. (2000) for PCBs. A lipid-normalized LOAEL value of 210 picogram/gram (pg/g) for TEQs in seal blubber was reported by Ross et al. (1995). That value is less than the reported range of 400–2,000 pg/g for otters and mink, implying that seals are more sensitive to TEQs.
Summary of Effects in Wildlife
PCBs have been demonstrated to cause a wide range of effects in animal models. Because of the co-occurrence of other stressors and accessory factors, it is more difficult to demonstrate PCB-specific effects in wildlife and almost impossible to demonstrate in human populations. The best evidence of the effects in populations outside the laboratory are those effects in wildlife, especially birds. Effects in mammals and fish are less obvious, in part because it is more difficult to follow individual organisms throughout their develop-
ment. In PCB-contaminated environments, there is good evidence of their effects in some wild populations of birds. The effects attributed to exposure to PBS include alteration of enzyme activities, suppression of the immune system, embryo-lethality, and developmental abnormalities. The effects observed in the field have been substantiated in the same wildlife species and surrogate animal models under laboratory conditions. Depending on the degree of contamination, the effects in birds can be minor to severe. In some locations, the weight of evidence is strong that PCBs have caused adverse effects in individuals and that this exposure has resulted in population-level effects. In other situations, the potential for effects must be inferred.
Human Health Effects from PCBs
Much of the information on the human health effects of PCBs have been gleaned from three major types of studies: occupational studies (Bertazzi et al. 1987; Brown 1987; Sinks et al. 1992 Kimbrough et al. 1999); retrospective and prospective studies of populations exposed to relatively high concentrations of PCBs through accidental exposure (e.g., Yusho and Yucheng incidents; Kuratsune et al. 1972; Masuda 1985; Rogan et al. 1988); and longitudinal prospective cohort studies of selected populations, such as the Michigan PCB study (Humphrey 1983), the Michigan aging sport fishermen study (Schantz 1996), and the Michigan angler study (Courval et al. 1999). In addition to those studies of populations exposed postnatally, scientists are studying several cohorts of children exposed to low-to-moderate (Rogan et al. 1988; Huisman et al. 1995; Patandin et al. 1999) or moderate-to-high (Lonky et al. 1996; Mergler et al. 1998) levels of PCBs in utero. In the studies on developmental effects, the more highly exposed cohorts had parents who ate PCB-contaminated fish (generally caught from the North American Great Lakes waters), used contaminated cooking oil (e.g., Yusho and Yucheng incidences), or for the Michigan cohort, were exposed to PCBs in food (mostly meat and dairy) from PCB-treated silos on farms. The more moderately exposed infants were exposed to PCBs from their parents diet, which presumably reflects the diet of the “typical” Dutch or American citizen, since the mothers were recruited from the general population.
These developmental cohort studies have produced similar results, demonstrating that the more highly exposed infants exhibited neurodevelopmental deficits or changes in behavior. For example, the infants in the 90th percentile exposure group in the study by Rogan et al. (1986) exhibited hypotonicity and hyporeflexia based on the Brazelton Neonatal Behavioral Assessment (Rogan et al. 1986). Interestingly, most of the neurobehavioral delays or deficits
observed have been correlated more with measures of prenatal exposure to PCBs than with measures of lactational exposure to PCBs, even when the neurobehavioral observations (including learning) were made years later (up to 11 years later for the study by Jacobson and Jacobson 1996) (Jacobson et al. 1985, 1990; Gladen et al. 1988). Similarly, alterations in immunological parameters were most strongly correlated with prenatal rather than postnatal measures of PCB exposure, although the effects from postnatal exposure disappear more quickly (primarily within 2–3 years following exposures) (Weisglas-Kuperus et al. 1995).
These observations in humans are supported by longitudinal studies of rhesus monkeys exposed to PCBs in utero (while the mother was being fed PCBs) or in breast milk (as long as 3 years after maternal exposure to PCBs). In one study, mothers were fed Aroclor 1016 at 0, 0.007, or 0.028 milligram per kilogram (mg/kg) of body weight per day throughout the gestational and lactational periods. Hyperpigmentation in the infants was observed in both dose groups. However, decreased birth weight and impaired performances of spatial discrimination, spatial learning, and memory were observed only in the high-dose group (Levin et al 1988; Schantz et al. 1991). An additional set of studies followed the offspring of females dosed with Aroclor 1248 at 0, 0.5, 1, and 2.5 parts per million (ppm), 3 days per week throughout the prenatal and the 4-month lactational period. Offspring born up to 3 years after exposure to the mothers receiving the highest dose were also followed and tested. The infants of the mothers in the highest dose group had reduced body weight and deficits in discrimination-reversal learning, hyperactivity when young, and hypoactivity as adolescents (Bowman et al. 1978, 1981). Adolescent and young adult monkeys, born up to 3 years after the mothers were no longer fed the PCBs, still exhibited neurobehavioral deficits on a spatial alteration task.
Because of the relatively great sensitivity of the developing fetus and the correspondence between human and animal data, many risk assessors now choose to use the developmental neurotoxicity data as the basis for setting reference doses (RfDs) for human health risk assessment. A review of RfDs derived by various agencies in the first half of the 1990s (Rice 1995) shows that even the highest RfD with the least protective assumptions (0.1 μg/kg/day for the less-chlorinated PCBs) (Aroclors 1016 and 1242), and 0.05 μg/kg/day for the more-chlorinated PCBs) results in an acceptable level in fish tissue of approximately 0.05 mg/kg. However, this value used only the RfD for the more-chlorinated PCBs, because bioaccumulation and biotic metabolic processes favor accumulation of the more-chlorinated congener over the more readily (but still slowly) metabolized, less-chlorinated PCB congeners.
Therefore, even though potentially higher levels of exposure might result from postnatal exposure in breast milk, the more biologically sensitive and
significant exposure period is prenatal. To address that, most states have set advisories for fish consumption at lower PCB concentrations for women of child-bearing age than for men.
Cancer
Cancer, including stomach, squamous-cell carcinoma (Svensson et al. 1995), and non-Hodgekin’s lymphoma (Rothman et al. 1997), has been linked to PCB exposure. However, the link between exposures to PCBs and cancer incidence has been somewhat weaker for some cancers (Sinks et al. 1992; Brown 1987; Bertazzi et al. 1997). Unlike the effects of prenatal and perinatal exposure to radiation, onset of cancer does not appear to be an effect of developmental exposure to PCBs. Rather, PCBs have been shown to be promoters of tumorigenesis after initiation by a genotoxic agent that, unlike PCBs, can form DNA adducts with subsequent DNA damage. Thus, it is unlikely that PCBs are complete carcinogens (Delzell et al. 1994).
Limitations of Human Health Risk Assessments for PCBs
The classic problem with determining relative risk due to mixtures of compounds, like PCBs, is posed by the following question, “How do we account for the toxicity of the individual congeners when potencies differ between congeners and between endpoints (effects) for a given congener?” One limitation of the epidemiological studies of the effects of PCBs is the problem of measuring rare effects induced in plastic organ systems or for endpoints where there is a strong cellular repair mechanism. Cancer is a rare effect, and the correlation between exposure to a given chemical, such as PCBs, and the increased incidence of cancer is difficult, if not impossible, to make. First, the manifestation of the effect (cancer or a tumor) is delayed, and estimates of prior exposure, which could have been in utero or several decades earlier, are almost impossible to accurately assess retrospectively. All of the epidemiological studies of occupational exposures are weak in this respect (Brown 1987; Sinks 1992; Kimbrough et al. 1999). Longitudinal prospective studies can reduce this limitation, but it is difficult to follow a large enough cohort over the time period required to assess the association between exposure and future cancer incidence.
When assessing biomarkers that might be linked to later incidents (and incidence) of disease, changes in these biomarkers, such as induced P4501A activity, are also linked to chemicals that are often co-contaminants of PCBs.
In addition, the animal body is so plastic, and has so many compensatory and repair mechanisms that even when biomarkers, such as indicators of immune function (e.g., numbers of T or B lymphocytes, antibody titers, or evaluation of neutrophil function or T-cell activation), are compromised and exposure is known (e.g., the Dutch infants prospective study by Weisglas-Kuperus et al. 1995), it is difficult, if not impossible, to observe a statistically significant increased incidence of infection (Tryphonas 1998).
An additional limitation of the assessment of potential effects of PCBs in humans relates to multiple sources. In laboratory studies, all exposures can be theoretically controlled. Thus, the experimenter can choose the route and absolute exposure level. For wildlife studies, exposure can be assessed by taking tissue biopsies throughout the exposure period or by sacrificing animals or eggs to assess population or subpopulation exposure levels. For human studies, biopsies are a possibility but are not always taken and are rarely taken multiple times throughout the exposure period. Biopsies assess net exposure from all sources, but it can be prohibitively expensive to run full congener analyses on all biopsies taken. Few studies, even those for which almost full congener analyses are available, have analyzed the correlation between each individual congener and the endpoint of interest. Therefore, some congeners, albeit present in relatively high concentrations in some environmental samples (e.g., PCBs 47, 48, or 52), are routinely ignored. To address that, data sets for which there are more complete data, should be fully evaluated to determine the relative congener potencies for the less-studied congeners and their endpoints.
RISK CHARACTERIZATION
Risk characterization provides a formalized method for combining information from exposure to PCBs with dose-response relationships to determine the likelihood that adverse effects are occurring or will occur. Because information on exposure and effects is incomplete, this characterization should include an evaluation of the inherent uncertainties underlying the risk calculations. Risk-management decisions might result in competing risks; therefore, it is not appropriate to select extreme safety factors that result in gross overestimation of human and ecological risks from PCB-contaminated sediments. If no alternative risks exist, it might be more appropriate to take a very conservative approach in estimating the risks of PCBs in sediments. However, that is not the case, and the most accurate estimate of risk is required for rational decision-making. It is possible to overestimate the potential risks by 100- to 1,000-fold with the use of safety factors. Ecological and human risk characterizations are described below, followed by a discussion of uncertainty. This
discussion is largely based on standard approaches that are used by EPA to characterize risk at PCB-contaminated sites.
Ecological Risk from Chemical Exposure
The potential risks of PCB exposure to ecological receptors are evaluated by comparing the results of exposure assessment (e.g., measured or modeled concentrations of chemicals in receptors of concern) with TRVs. In this approach, a hazard quotient (HQ) is calculated as in Equation 2. HQ values exceeding a value of 1 are typically considered to indicate potential risk to the average individual within the local population (e.g., reduced or impaired reproduction). If an HQ indicates that risks are present for the average individual, then risks are typically considered to be present for the local population. If an HQ suggests that effects are not expected to occur for the average individual, then they are considered likely to be insignificant for the local population. Recent advancements in modeling and geographic information systems have made it more feasible to consider ranges of variability in both measured and predicted exposures and responses. Application of probabilistic approaches, when appropriate, is encouraged. EPA has developed guidelines for the application of such approaches such as those of the EPA Workgroup on Probabilistic Risk Assessment for Pesticides (ECOFRAM) (EPA 1999).
Human Risk from Chemical Exposure
Risk characterizations for human health effects are developed on an individual, and not population-wide, basis. As a consequence, risk characterizations need to be performed for various subgroups within the population to account for differences in sensitivity and exposure rates (e.g., subsistence fishers). For each identified subgroup, chemical dose, or chronic daily intake (GDI), is determined from concentrations of PCBs in food and water consumption or air inhaled. For example, the GDI for ingestion of PCB-contaminated fish is given (Equation 5):
(5)
where C is the exposure concentration (e.g., mg of PCB/kg of fish); CR is the contact rate (e.g., g of fish ingested/day); EF is the exposure frequency; ED is
the exposure duration that, for example, may be determined from U.S. Census data based on population mobility; BW is the average body weight of the individual over the exposure period; and AT is the time over which exposure is averaged.
Variations in exposure are expected within an identified subpopulation because of individual differences in fish consumption rates, body weight, and PCB concentrations in consumed fish. Therefore, EPA guidance calls for an evaluation of both a central estimate of risk and an estimate of risk to an individual for a reasonable maximum exposure (RME). According to EPA guidance (1998a), central-tendency estimates are intended to reflect central estimates of exposure or dose, and RME estimates are intended to reflect persons at the upper end (i.e., above about the 90th percentile) of the distribution. Since the RME estimate is typically based on estimates of likely high-end exposure factors, the actual percentile associated with the RME is difficult to determine.
A preferred approach for estimating the RME uses probabilistic methods, such as a Monte Carlo analysis (EPA 1997a). The RME is expressed as probabilities of overlap between the distribution of the exposure and the effects (Solomon et al. 2000). The advantage of the Monte Carlo method is that an explicit estimate of the likelihood, or probability, of exposure can be obtained given sufficient data on parameter distributions (e.g., a population’s fish ingestion rate, body weight, exposure period, and PCB concentrations in fish). In the Monte Carlo method, the range and relative likelihood of exposure is calculated by randomly selecting parameter values from their probability distributions and calculating a CDI. CDI calculations are repeated many times using this approach to obtain a distribution of chemical exposures. A set of selected CDI percentiles (e.g., the 90th, 95th and 98th percentiles) are then determined for subsequent risk calculations. Risk calculations for human health are performed separately for hazards associated with noncancer effects, such as reproductive impairment, developmental disorders, disruption of specific organ functions, learning problems, and for incremental risk of developing cancer due to PCB exposure.
For noncancer hazards, risk is expressed by the ratio of the CDI to an RfD using the HQ in Equation 6:
(6)
HQs are then summed over all chemicals of potential concern and all applicable exposure routes to determine the total hazard index (HI). If an HI is greater than 1, unacceptable exposures might be occurring, and there might be a
concern for potential noncancer effects. The relative value of the HI, however, should not be translated into an estimate of the severity of the hazard or a prediction for a specific disease. This index is simply an index to guide decision-making and further analysis.
For cancer, risk is expressed as a probability and is based on the cancer potency of the chemical, known as the cancer slope factor (CSF, Equation 7). The CSF is the plausible upper-bound estimate of carcinogenic potency used to calculate risk from exposure to carcinogens by relating estimates of lifetime CDI to the incremental risk of an individual developing a cancer over a lifetime. This calculation is based on total concentrations of PCBs, and the CSF is based on technical Aroclor mixtures.
Cancer Risk=CDI×CSF.
(7)
To account for certain PCS congeners exhibiting dioxin-like toxicity, supplemental calculations for dioxin cancer risks should be performed using measured, modeled, or estimated concentrations of TCDD-toxicity equivalents (TEQs) in estimating CDI values. The primary, although not exclusive, source of information for CSFs and RfDs used in Superfund risk assessments is the EPA Integrated Risk Information System (IRIS) database, which is updated periodically to incorporate new data. Although the committee notes that EPA’s cancer classification for dioxins remains controversial as of publication of this report, the values are used as examples. For example, acceptable upper-bound risks for cancer are in the range of 1×10−4 to 1×10−6 (or an increased probability of developing cancer of up to 1 in 10,000 to 1 in 1,000,000).
Uncertainty
When potential risks posed by PCBs have been assessed for cancer, reproductive impairment, and neurobehavioral effects, the allowable daily consumption of PCBs in the diet (primarily from fish) were similar (Williams et al. 1992). Due to the conservative nature of the linear extrapolation applied in developing the RfD based on cancer risk, the allowable exposure for cancer risk would be approximately 2-fold less than that for noncancer endpoints. This level of uncertainty is relatively small, compared with the other uncertainties encountered in risk assessments. Furthermore, when risk assessments have been based on AhR-mediated effects (dioxin-like effects on reproduction) and non-AhR-mediated effects (nondioxin-like effects of di-ortho-substituted PCBs on neurobehavioral endpoints), the allowable exposures were similar (Giesy and Kannan 1998), AhR-mediated endpoints resulting in approximately 2-fold greater HQ values. Thus, basing risk assessments on AhR-
mediated effects should be protective of non-AhR-mediated effects. However, multiple lines of evidence based on both mechanisms of action should be considered to correct for site-specific differences in PCB congener patterns.
At each step of the risk-assessment process, there are sources of uncertainty that can ultimately affect the decision-making process. These include the following:
-
Sampling error and representativeness: Chemical exposure estimates may include systematic error and random error associated with sample location (e.g., river mile), spatial heterogenities (e.g., coarse-grained versus fine-grained sediment, local vegetation), intraspecies variability, and temporal variation (e.g., seasonal variations associated with hydrological events and biological cycles).
-
Sample analysis and quantification: Chemical measurements might not represent true concentrations in the environment because of possible inaccuracies in extraction and laboratory analysis of PCBs and co-contaminants. Measurement error might also be associated with dry weight and organic carbon content of sediments and wet weight and lipid content of biota. Measurement of PCB congeners at or near the detection limit is also a possible source of error for the dioxin-like congeners (e.g., PCB 126, concentrations of which are generally small but can be an important contributor to concentrations of TEQs).
-
Conceptual model: Conceptual models are constructed to provide a realistic yet tractable description of chemical exposure pathways. If a certain contaminant pathway of either in-place or external sources of PCBs is not included or not properly characterized in the conceptual model, errors will be propagated throughout the analysis.
-
Natural variation and parameter error: Natural variations in measurement of PCB concentrations, lipid content, age, body weight, feeding rate, and duration of exposure for an endpoint species might result in errors in specifying parameters for exposure assessment.
-
Model error: Inappropriate selection or aggregation of variables (e.g., use of total PCBs), incorrect functional forms, and incorrect boundary conditions can lead to errors in exposure estimates.
-
Toxicological uncertainties: A large number of toxicological studies were conducted in the laboratory using unweathered Aroclor mixtures. Uncertainties in toxicological endpoints may therefore be associated with laboratory stress of organisms and differences in laboratory and field exposures (e.g., weathering, presence of sediments, effects of dissolved organic carbon, effects of co-contaminants, and bioaccumulation effects). Intraspecies variability, interspecies variability, less-than-lifetime exposures, and acute-versus-chronic
-
toxicity testing are additional sources of uncertainty in defining dose-response relationships.
The exposure potential for PCBs via inhalation is based on the chemical-physical properties of the congeners. Natural-fate processes, such as anaerobic dechlorination of PCBs in sediments, result in the transformation of more-chlorinated, less-volatile PCBs (such as those found in commercial PCB mixtures) to less-chlorinated, more-volatile PCBs. As a result, the inhalation exposure potential from excavated sediments might be greater than the potential expected based on volatility data for commercially available Aroclors (Chiarenzelli et al. 1998). Furthermore, increased ambient-air PCB concentrations can occur during remediation operations because of increased physical disturbance of the sediments. These increased concentrations have been reported during wet excavation at several PCB-contaminated sites (e.g., Bloomington, Indiana) (Bloomington Herald Times 2000).
Summary of Risk Characterization
Currently applied methods for characterizing ecological and human health risks appear appropriate for PCB-contaminated sediments. Risk and the associated uncertainties of risk should be determined on a site-specific basis. For the overall analysis of risk from chemical exposure, the general sense is that there is typically a moderate degree of uncertainty associated with exposure assessment; an order of magnitude of uncertainty associated with ecological dose-response; and greater than an order of magnitude of uncertainty associated with the quantification of human health effects. As a result, the continued use of uncertainty factors in risk calculations is recommended to ensure to the extent possible that risk estimates are protective. At the same time, if this application of safety factors is not minimized and used in a transparent manner, the potential risks associated with PCBs in sediment will be overstated. Overstated risks can unduly alarm the public and force an active management solution that will not reduce risk and thus will be inappropriate. Furthermore, if some risks are overstated while others are not considered, such as those associated with active remediation, a biased decision will be reached, and the environment might be damaged with a reduction of risk, or in some cases, risks might be increased due to inappropriate or unnecessary remedial actions.
ECONOMIC, SOCIAL, AND CULTURAL IMPACTS
In addition to the potential effects of PCBs on human and ecological health, PCB contamination might result in various economic, social, and cul-
tural impacts on the affected parties. For the purposes of assessment, these impacts fall into two general classes: direct (and indirect) human use effects and non-use values (Marine Policy Center 2000). For the first class, human uses that are curtailed or terminated because of PCB contamination and that may result in economic damages are typically considered. These uses include the following:
-
Lost benefits from commercial fishing: Direct economic impacts are associated with losses in sale of the catch and increased unemployment of fishers in the region.
-
Lost benefits to recreational fishers (recreational experience): Economic impacts are felt directly through a decrease in tourism and marina activity. Reopening of river reaches as a “catch-and-release” fishery or with consumption advisories still results in lost benefits from personal consumption of recreationally harvested fish, which is an important component of the fishing “experience.”
-
Reduction in property values: Highly desirable waterfront property might be reduced in value because of the presence of PCB-contaminated sediments, particularly near PCB hot spots. This loss might occur even if there is no apparent danger to human health.
-
Increased costs of port, harbor, and navigation channel dredged-material disposal: PCB contamination in dredging zones might be felt directly through the increased costs in disposal of contaminated dredged material or indirectly through curtailment in navigational dredging and associated loss in economic benefits from port activities and marina operations.
-
Increased health effects due to changes in diet from a fish-based diet to a less healthy diet: For example, Ken Jock, of the St. Regis Mohawk Tribe (NRC Public Session, Albany, New York, November 8–9, 1999), cited increases in diabetes and thyroid diseases that are believed to be associated with changing from a fish-based diet.
-
Loss of benefits to recreational swimmers and boaters: Although scientific information suggests that human health risk from incidental contact with river water and sediments is low, some individuals might avoid swimming and boating in contaminated areas because of a fear of being exposed to PCBs.
-
Increased costs of drinking water: Although dissolved PCB concentrations in waters overlying PCB-contaminated sediments are typically below the maximum contaminant level goal for PCBs in drinking water (0.5 μg/L), individuals might incur costs, such as the purchase of bottled water because of uncertainty about PCB contamination.
-
Miscellaneous costs. For example, Bill Acker, a concerned citizen (NRC Public Session, Green Bay, Wisconsin, September 27–29, 1999), cited
-
a personal hardship associated with unexpected costs of disposing of PCB-contaminated zebra mussels harvested from his beachfront on Green Bay.
The second general class of impacts are the so-called non-use (or passive) values that are intangible and arise from the satisfaction that individuals experience from a particular environment, ecosystem, or river “culture,” in the absence of any physical use. Many of these impacts are difficult to quantify but ultimately are related to one’s willingness to sacrifice other benefits for the preservation of the environment for present and future generations. Examples of non-use include the following:
-
Protected species and their habitats: Protecting the viability and promoting the recovery of species in a watershed might be an environmental good that has value to humans even if they do not use the species directly (e.g., the reemergence of bald eagles on the Hudson River).
-
Ecosystem services: Non-use values might arise from the knowledge that the ecosystem, or even critical components of the ecosystem, is functioning properly.
-
Human culture: The existence of a distinct community or culture that is strongly linked to the ecosystem might be threatened by contamination. For example, Ken Jock, of the St. Regis Mohawk Tribe (NRC Public Session, Albany, New York, November 8–9, 1999), cited how the loss of fishing and hunting grounds along the St. Lawrence River has caused many of the men to leave the reservation to find work, resulting in a breakdown of family and a loss of tribal traditions.
COMPARATIVE RISK ASSESSMENT
Determining an overall assessment of risk from PCB-contaminated sediments is a challenging task that requires sound scientific information and consensus-building among affected parties. Acceptable risk levels for human health, ecological, economic, social, and cultural impacts should therefore be established at the onset of the process. The relative importance of various risk factors should be discussed continually by the affected parties throughout the risk-assessment process. If the nature and extent of all the risks are not considered by the affected parties, a risk that affects only a small population or one that affects a large population might be given the same emphasis. In comparing one risk against the same type of risk, establishing the extent of each risk is extremely important. Approaches for comparing risk are discussed in Chapter 8.
Because the notion of what is an acceptable risk can vary greatly among
the affected parties, rectifying differences among the parties can be an extremely difficult task. This problem is only exacerbated by the large spatial extent of many PCB-contaminated sites. For example, civic group leaders from areas along the upper Hudson (i.e., above the Federal Dam at Troy, New York) were very accepting of fishing bans and fish consumption advisories as a long-term solution for PCB contamination (see Box 6-8). However, civic group leaders from areas below the Federal Dam placed greater emphasis on human health effects and a safe Hudson River fishery (NRC Public Session, Albany, New York, November 8–9, 1999). These perceptions stem from the fact that the downstream communities see only benefits from active remediation. Upstream communities, however, which will be disrupted by dredging operations and will be exposed to greater risks during the implementation of any management options, recognize that there are conflicting risks and a balance is needed between the risks and the benefits of such activities. The challenge in resolving such a disagreement is balancing the risk to both affected parties, which will be a negotiated settlement. However, this need not always be the case as is evidenced by the experiences of communities near the Duwamish Waterway in Washington (see Box 6-9).
Examples of questions that need to be considered in comparative risk assessment include the following:
-
Should human health be the primary driver in assessing overall risk (even though there might be a great deal of uncertainty associated with human health risk estimates)?
-
How should economic, social, and cultural impacts be factored into risk-based decisions? For example, should jobs and the local economy take precedence over ecological concerns?
-
How should short-term and long-term risks be compared? For example, should short-term increases in PCB exposure levels during remedial activity be acceptable in achieving long-term reduction of risk?
To foster an open discussion of risks, summary information should be tabulated and periodically disseminated to all affected parties. For example, a summary table, such as Table 6–4, can be constructed to provide a concise comparison of the primary risks that are driving the decision process. For each risk, information should be provided on the populations or subgroups affected, a quantitative or qualitative measure of impact, an estimate of uncertainty (or in the case of disagreement, the range of conflicting risk estimates), and appropriate references for each risk estimate. Information in the table can be expanded or revised throughout the decision process (e.g., to account for additional concerns of affected parties or new estimates of existing or potential risks).
|
BOX 6–8 Risk Perception Along the Hudson River Research into what influences the public’s perception of risk (Hance et al. 1988) has separated risk into the more easily measured “hazard” and the more subjective and emotion-laden “outrage.” Some of the factors that affect “outrage” were garnered by the committee during their visit to the Hudson River, and discussion with affected parties include the following:
To some people on the upper Hudson River, the process of dredging is perceived as “coercion” and is seen as being controlled by “others.” Thus, to these people, it is the process of dredging itself that is perceived as the risk, a risk to their enjoyment of the river and a risk to their economic livelihoods if they have marinas along the river. Clearly, people’s perceptions of the most appropriate course of action relative to PCBs in the sediments of the Hudson River are divergent and, in some cases, diametrically opposed. These opinions are based on many factors, including perceptions of who gains and who loses, that are beyond the scope of a scientific review of the risks involved in the management of PCB-contaminated sediments. |
|
BOX 6–9 Responsive Process versus Unresponsive Process—Examples from the Duwamish Waterway and the Hudson River In Puget Sound, PCBs contaminated the Duwamish Estuary and Commencement Bay, among other places. The regional EPA personnel and local regulatory officials coordinating the remediation planning and completion processes involved many members of the community from the beginning of the process. Community members helped choose remediation solutions and participated in many of the less hazardous remediation and restoration activities. In this example, the remediation activities were, in part, individually (or community) controlled and were perceived of as “voluntary” (chosen by the community). In addition, the community perceived the remediation and restoration process to be responsive to their desires. By contrast, the communities along the Hudson River are not a part of any active decision-making processes, despite an elaborate community involvement structure that has been established (see www.epa.gov/hudson/public-participation.htm). By all accounts, the community is allowed to provide input or feedback to the planning processes at selective times; times chosen by the EPA project managers, not the community. The Hudson River community involvement process used by EPA does not appear to allow community involvement in any decision-making or even in problem-formulation phases and does not appear to be responsive to community needs and frustrations. This lack of community involvement has contributed to the impass relative to the appropriate course of action to be taken on the Hudson River. |
CONCLUSIONS AND RECOMMENDATIONS
Risk management of PCB-contaminated-sediment sites should comprehensively evaluate the broad range of risks posed by PCB-contaminated sediments and associated management actions. These risks should include societal, cultural, and economic impacts as well as human health and ecological risks.
Risk assessments and risk-management decisions should be conducted on a site-specific basis and should incorporate all available scientific information.
Current management options can reduce risks but cannot completely eliminate PCBs and PCB exposure from contaminated-sediment sites. Because
TABLE 6–4 Summary of Comparative Risks
|
Category |
Examples of Types of Risk |
Populations or Subgroups Affected |
Quantitative or Qualitative Measure of Impacts |
Estimates of Uncertainty |
Reference |
|
Human health effects |
Cancer and noncancer risks associated with PCB exposure |
|
|||
|
Ecological effects |
PCB toxicity to organisms, loss of habitat during and after risk-management activities |
|
|||
|
Societal impacts |
Changes in a community social structure due to loss of traditional work patterns; reduced maternal-offspring contact due to avoidance of breast-feeding |
|
|||
|
Cultural impacts |
Loss of American Indian fishing rites |
|
|||
|
Economic impacts |
Loss of commercial and recreational fisheries, decline in property values, increased costs of dredged material disposal |
|
|||
all options will leave some residual PCBs, the short- and long-term risks they pose should be considered when evaluating management strategies.
Research should focus on an improved understanding of the risks associated with exposures to PCB-contaminated sediments. Because the composition of PCB mixtures in the environment changes over time, risk characterizations should be based on the specific congeners present at a site. New studies on the toxicity and fate of PCBs in the environment are being conducted, and the results from these studies should be used to inform risk assessments at contaminated sites.
PCBs are often found at a site in conjunction with other chemicals of concern (e.g., pesticides, polycyclic aromatic hydrocarbons, dioxins, furans, and metals). Research is needed to determine the interactions among these chemicals and the impact that these interactions can have on site-specific risk assessments and subsequent risk-management efforts.
Models used to describe all relevant PCB exposure pathways—from the contaminated sediments, through the aquatic food web, and to specific receptors—must consider exposures to sensitive populations, including but not limited to the elderly, pregnant women, infants, and children; culturally or economically unique populations; and sensitive and endangered wildlife and their habitats. These models, which have inherent uncertainty, require calibration that should be conducted by multiple researchers. It is important that the model results are peer reviewed.
REFERENCES
Abramowicz, D.A. 1990. Aerobic and anaerobic biodegradation of PCBs: a review. Crit. Rev. Biotechnol. 10(3):241–251.
Abramowicz, D.A., M.J.Brennan, H.M.van Dort, and E.L.Gallagher. 1993. Factors influencing the rate of polychlorinated biphenyl dechlorination in Hudson River sediments. Environ. Sci. Technol. 27(6):1125–1131.
Ahlborg, U.G., G.C.Becking, L.S.Birnbaum, A.Brouwer, H.J.G.M.Derks, M. Feeley, G.Golor, A.Hanberg, J.C.Larsen, A.K.D.Liem, S.H.Safe, C.Schlatter, F.Wærn, M.Younes, and E.Yrjänheikki. 1994. Toxic equivalency factors for dioxin-like PCBs. Chemosphere 28(6):1049–1067.
Ankley, G.T., P.M.Cook, A.R.Carlson, D.J.Call, J.A.Swenson, H.F.Corcoran, and R.A.Hoke. 1992a. Bioaccumulation of PCBs from sediments by oligochaetes and fishes: comparison of laboratory and field studies. Can. J. Fish. Aquat. Sci. 49(10):2080–2085.
Ankley, G.T., K.Lodge, D.J.Call, M.D Balcer, L.T.Brooke, P.M.Cook, R.G.Kreis Jr., A.R.Carlson, R.D.Johnson, G.J.Niemi, R.A.Hoke, C.W.West, J.P.Giesy, P.D.Jones, and Z.C.Fuyin. 1992b. Integrated assessment of contaminated sediments in the lower Fox River and Green Bay, Wisconsin. Ecotoxicol. Environ. Saf. 23(1):46–63.
Ankley, G.T., G.T.Niemi, K.B.Lodge, H.J.Harris, D.L.Beaver, D.E.Tillitt, T.R. Schwartz, J.P.Giesy, P.D.Jones, and C.Hagley. 1993. Uptake of planar polychlorinated biphenyls and 2,3,7,8-substituted polychlorinated dibenzofurans and dibenzo-p-dioxins by birds nesting in the Lower Fox River/Green Bay, Wisconsin. Arch. Environ. Contam. Toxicol. 24(3):332–344.
Bedard, D.L., M.L.Haberl, R.J.May, and M.J.Brennan. 1987a. Evidence for novel mechanisms of polychlorinated biphenyl metabolism in Alcaligenes eutrophus H850. Appl. Environ. Microbiol. 53(5):1103–1112.
Bedard, D.L., R.Unterman, L.H.Bopp, M.J.Brennan, M.L.Haberl, and C.Johnson.
1986. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl. Environ. Microbiol. 51(4):761–768.
Bedard, D.L., R.E.Wagner, M.J.Brennan, M.L.Haberl, and J.F.Brown, Jr. 1987b. Extensive degradation of Aroclors and environmentally transformed polychlorinated biphenyls by Alcaligenes eutrophus H850. Appl. Environ. Microbiol. 53(5):1094–1102.
Bertazzi, P.A., L.Riboldi, A.Pesatori, L.Radice, and C.Zocchetti. 1987. Cancer mortality of capacitor manufacturing workers. Am. J. Ind. Med. 11(2):165–176.
Bertazzi, P.A., C.Zocchetti, S.Guercilena, D.Consonni, A.Tironi, M.T.Landi, and A.C.Pesatori. 1997. Dioxin exposure and cancer risk: a 15-year mortality study after the “Seveso accident”. Epidemiology 8(6):646–652.
Blankenship, A.L., K.Kannan, S.A.Villalobos, J.Falandysz, and J.P.Giesy. 2000. Relative potencies of individual polychlorinated naphthalenes and halowax mixtures to induce Ah receptor-mediated responses. Environ. Sci. Tehnol. 34(15):3153–3158.
Bloomington Herald Times. 2000. EPA offers to relocate some neighbors of site: PCB levels in air high enough to delay cleanup at Lemon Lane Landfill. Pp. A1. July 21, 2000.
Blumberg, A.F., L.A.Khan, and J.P. St. John. 1999. Three-dimensional hydrodynamic model of New York Harbor Region. J. Hydraul. Eng. 125(8):799–816.
Boese, B.L., M.Winsor, H.Lee, S.Echols, J.Pelletier, and R.Randall. 1995. PCB congeners and hexachlorobenzene biota sediment accumulation factors for Macoma nasuta exposed to sediments with different total organic carbon contents. Environ. Toxicol. Chem. 14(2):303–310.
Boon, J.P., and F.Eijgenraam. 1988. The possible role of metabolism in determining patterns of PCB congeners in species from the Dutch Wadden Sea. Mar. Environ. Res. 24(1–4):3–8.
Boon, J.P., F.Eijgenraam, J.M.Everaarts, and J.Duinker. 1989. A structure-activity relationship (SAR) approach towards metabolism of PCBs in marine animals from different trophic levels. Mar. Environ. Res. 27(3–4):159–176.
Boon, J.P., J.van der Meer, C.R.Allchin, R.J.Law, J.Klungsoyr, P.E.G.Leonards, H.Spliid, E.Storr-Hansen, C.Mckenzie, and D.E.Wells. 1997. Concentration, dependent changes of PCB patterns in fish-eating mammals: structure evidence for induction of cytochrome P450. Arch. Environ. Contam. Toxicol. 33(3):298–311.
Borlakoglu, J.T., and C.H.Walker. 1989. Comparative aspects of congener specific PCB metabolism. Eur. J. Drug Metab. Pharmacokinet. 14(2):127–131.
Bowman, R.E., M.P.Heironimus, and J.R.Allen. 1978. Correlation of PCB body burden with behavioral toxicology in monkeys. Pharmacol. Biochem. Behav. 9(1):49–56.
Bowman, R.E., M.P.Heironimus, and D.A.Barsotti. 1981. Locomotor hyperactivity in PCB-exposed rhesus monkeys. Neurotoxicology 2(2):251–268.
Bright, D.A., W.T.Dushenko, S.L.Grundy, and K.J.Reimer. 1995. Effects of local and distant contaminant sources: polychlorinated biphenyls and other organochlorines in bottom-dwelling animals from an Arctic estuary. Sci. Total. Environ. 160–161:265–283.
Brown, D.P. 1987. Mortality of workers exposed to polychlorinated biphenyls—An update. Arch. Environ. Health. 42(6):333–339.
Burkhard, L.P. 1998. Comparison of two models for predicting bioaccumulation of hydrophobic organic chemicals in a Great Lakes food web. Environ. Toxicol. Chem. 17(3):383–393.
Chiarenzelli, J., R.Scrudato, B.Bush, D.Carpenter, and S.Bushart. 1998. Do large-scale remedial and dredging events have the potential to release significant amounts of semivolatile compounds to the atmosphere? Environ Health Perspect 106(2):47–49.
Clark, T., K.Clark, S.Paterson, D.Mackay, and R.J.Norstrom. 1988. Wildlife monitoring modeling and fugacity. Environ. Sci. Technol. 22(2):120–127.
Cook, P.M., R.J.Erickson, R.L.Spehar, S.P.Bradbury, and G.T.Ankley. 1993. Interim Report on Data and Methods for Assessment of 2,3,7,8-Tetrachlorodibenzo-p-dioxin Risk to Aquatic Life and Associated Wildlife. U.S. Environmental Protection Agency, Washington, D.C. NTIS/PB93–202828.
Corsolini, S., S.Focardi, K.Kannan, S.Tanabe, A.Borrell, and R.Tatsukawa. 1995a. Congener profile and toxicity assessment of polychlorinated biphenyls in dolphins, sharks and tuna collected from Italian coastal waters. Mar. Environ. Res. 40(1):33–54.
Corsolini, S., S.Focardi, K.Kannan, S.Tanabe, and R.Tatsukawa. 1995b. Isomer-specific analysis of polychlorinated biphenyls and 2,3,7,8-tetrachlorodibenzo-p-dioxin equivalents (TEQs) in red fox and human adipose tissue from central Italy. Arch. Environ. Contam. Toxicol. 29(1):61–68.
Courval, J.M., J.V.DeHoog, A.D.Stein, E.M.Tay, J.He, H.E.Humphrey, and N. Paneth. 1999. Sport-caught fish consumption and conception delay in licensed Michigan anglers. Environ. Res. 80(2 Pt 2):S183–S188.
Delzell, E., J.Doull, J.P.Giesy, D.Mackay, I.C.Monro, and G.M.Williams. 1994. Interpretive review of the potential adverse effects of chlorinated organic chemicals on human health and the environment: polychlorinated PCBs. Regul. Toxicol. Pharmacol. 20(1 Part 2):S1–S1056.
de Swart, R.L., P.S.Ross, L.J.Vedder, H.H.Timmerman, S.Heisterkamp, H.Van Loveren, J.Vos, P.J.H.Reijnders, and A.D.M.E.Osterhaus. 1994. Impairment of immune function in harbor seals (Phoca vitulina) feeding on fish from polluted waters. Ambio 23(2):155–159.
DiToro, D.M., C.S.Zarba, D.J.Hansen, W.J.Berry, R.C.Swartz, C.E.Cowan, S.P. Pavlou, H.E.Allen, N.A.Thomas, and P.R.Paquin. 1991. Technical basis for establishing sediment quality criteria for nonionic organic chemicals using equilibrium partitioning. Environ. Toxicol. Chem. 10:1541–1583.
Drouillard, K.G., J.J.H.Ciborowski, R.Lazar, and D.D.Haffner. 1996. Estimation of the uptake of organochlorines by the mayfly Hexagenia limbata (Ephemeroptera; Ephemeridae). J. Great Lakes Res. 22(1):26–35.
Eadie, B.J., W.R.Faust, N.R.Morehead, and P.F.Landrum. 1985. Factors affecting bioconcentration of PAH (polynuclear aromatic hydrocarbon) by the dominant benthic organisms of the Great Lakes. Pp. 363–377 in Polynuclear Aromatic Hydrocarbons: Mechanisms, Methods and Metabolism, M.Cooke and A.J.Den-nis, eds. Columbus, OH: Battelle Press.
EPA (U.S. Environmental Protection Agency). 1997a. Uncertainty Factor Protocol for Ecological Risk Assessment. Toxicological Extrapolations to Wildlife Receptors. Ecosystems Protection and Remediation Division. U.S. Environmental Protection Agency, Region VIII, Denver, CO. February. [Online]. Available: http://www.epa.gov/Region8/superfund/risksf/trv-ucfs.pdf.
EPA (U.S. Environmental Protection Agency). 1997b. Ecological Risk Assessment Guidance for Superfund: Process for Designing and Conducting Ecological Risk Assessments. EPA 540-R-97–006. Office of Emergency and Remedial Response, U.S. Environmental Protection Agency, Washington, DC. June. NTIS PB97–963211.
EPA (U.S. Environmental Protection Agency). 1998. Risk Assessment Guidance for Superfund: Volume 1. Human Health Evaluation Manual (Part D, Standardized Planning Reporting and Review of Superfund Risk Assessments). Interim. EPA/540/R-97/033. OSWER-9285.7–01D. Office of Emergency and Remedial Response, U.S. Environmental Protection Agency, Washington, DC. NTIS PB97–963305.
EPA (U.S. Environmental Protection Agency). 1998a. Exposure Factors Handbook. Vol. I, II, III. National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency. [Online]. Available: http://www.epa.gov/ncea/exposfac.htm. [September 01, 2000].
EPA (U.S. Environmental Protection Agency). 1999. ECOFRAM Update: Probabilistic Risk Assessment Tools for Pesticides. Office of Pesticide Programs, U.S. Environmental Protection Agency. 2pp. October 21, 1999. [Online]. Available: http://www.epa.gov/oppefed1/ecorisk/update.html.
Farley, K.J., J.A.Mueller, and D.J.O’Connor. 1983. Distribution of kepone in the James River Estuary. J. Environ. Engr. 109(2):396–413.
Farrington, J.W., A.C.Davis, B.J.Brownawell, B.W.Tripp, C.H.Clifford, and J.B. Livramento. 1986. The biogeochemistry of polychlorinated biphenyl in the Acushnet River Estuary, Massachusetts. Pp. 174–197 in Organic Marine Geochemistry, M.L.Sohn, ed. ACS Symposium Series 305. Washington, DC: American Chemical Society.
Froese, K.L., G.T.Ankley, D.A.Verbrugge, G.J.Niemi, C.P.Larsen, and J.P.Giesy. 1998. Bioaccumulation of polychlorinated biphenyls from sediments to aquatic insects and tree swallow eggs and nestlings in Saginaw Bay, Michigan, USA. Environ. Toxicol. Chem. 17(3):484–492.
Gailani, J., C.K.Ziegler, and W.Lick. 1991. Transport of suspended solids in the lower Fox River. J. Great Lakes Res. 17(4):479–494.
Gasiewicz, T.A. 1997. Dioxins and the Ah receptor: probes to uncover processes in neuroendocrine development. Neurotoxicology 18(2):393–413.
Giesy, J.P., and R.A.Hoke. 1990. Freshwater sediment quality criteria: toxicity bioassessment. Pp. 265–348 in Sediments: Chemistry and Toxicology of In-Place Pollutants, R.Baudo, J.P.Giesy, and H.Muntau, eds. Ann Arbor: Lewis.
Giesy, J.P., and K.Kannan. 1998. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment. Crit. Rev. Toxicol. 28(6):511–569.
Giesy, J.P., J.P.Ludwig, and D.E.Tillitt. 1994a. Dioxins, dibenzofurans, PCBs, and
colonial fish-eating water birds. Pp. 249–307 in Dioxins and Health, A.Schecter, ed.. New York: Plenum Press.
Giesy, J.P., J.P.Ludwig, and D.E.Tillitt. 1994b. Deformities in birds of the Great Lakes region: assigning causality. Environ. Sci. Technol. 28(3):128A–135A.
Gladen, B.C., W.J.Rogan, P.Hardy, J.Thullen, J.Tingelstad, and M.Tully. 1988. Development after exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene transplacentally and through human milk. J. Pediatr. 113(6):991–995.
Gobas, F.A.P.C. 1993. A model for predicting the bioaccumulation of hydrophobic organic chemicals in aquatic food webs: application to Lake Ontario. Ecol. Model. 69:1–17.
Gobas, F.A.P.C., J.R.McCorquodale, and G.D.Haffner. 1993. Intestinal absorption and biomagnification of organochlorines. Environ. Toxicol. Chem. 12(3):567–576.
Hance, B.J., C.Chess, and P.M.Sandman. 1988. Improving Dialogue with Communities: A Risk Communication Manual for Government, Environmental Communication Research Program, New Jersey Agricultural Experimental Station. Trenton, NJ: Division of Science and Research.
Hawker, D.W., and D.W.Connell. 1985. Relationships between partition coefficient, uptake rate constant, clearance rate constant and time to equilibrium for bioaccumulation. Chemosphere 14(9):1205–1219.
Henshel, D.S., B.Hehn, R.Wagey, M.Vo, and J.D.Steeves. 1997. The relative sensitivity of chicken embryos to yolk- or aircell-injected 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ. Toxicol Chem. 16(4):725–732.
Hoffman, D.J., M.J.Melancon, P.N.Klein, J.D.Eisemann, and J.W.Spann. 1998. Comparative developmental toxicity of planar polychlorinated biphenyl congeners in chicken, american kestrels, and common terms. Environ. Toxicol. Chem. 17(4):747–757.
Hoke, R.A, G.T.Ankley, A.M.Cotter, T.Goldenstein, P.A.Kosian, G.L.Phipps, and F.M.VanderMeiden. 1994. Evaluation of equilibrium partitioning theory for predicting acute toxicity of field-collected sediments contaminated with DDT, DDE and DDD to the amphipod Hyalella azteca. Environ. Toxicol. Chem. 13(1):157–166.
Huisman, M., C.Koopman-Esseboom, V.Fidler, M.Hadders-Algra, C.G.van der Paauw, L.G.Tuinstra, N.Weisglas-Kuperus, P.J.Sauer, B.C.Touwen, and E.R. Boersma. 1995. Perinatal exposure to polychlorinated biphenyls and dioxins and its effect on neonatal neurological development. Early Hum. Dev. 41 (2):111–127.
Humphrey, H.E.B. 1983. Population studies of PCBs in Michigan residents. Pp. 299–310 in: PCBs: Human and Environmental Hazards, F.M.D’Itri, and M.A. Kamrin, eds. Boston, MA: Butterworth.
Ingersoll, C.G., G.T.Ankley, D.A.Benoit, E.L.Brunson, G.A.Burton, F.J.Dwyer, R.A.Hoke, P.F.Landrum, T.J.Norberg-King, and P.A.Winger. 1995. Toxicity and bioaccumulation of sediment-associated contaminants using freshwater invertebrates: a review of methods and applications. Environ. Toxicol. Chem. 14(11):1885–1894.
Jacobson, J.L., and S.W.Jacobson. 1996. Intellectual impairment in children ex-
posed to polychlorinated biphenyls in utero. N. Engl. J. Med. 335(11):783–789.
Jacobson, S.W., G.G.Fein, J.L.Jacobson, P.M.Schwartz, and J.K.Dowler. 1985. The effect of intrauterine PCB exposure on visual recognition memory. Child. Dev. 56(4):853–860.
Jacobson, J.L., S.W.Jacobson, and H.E.Humphrey. 1990. Effects of exposure to PCBs and related compounds on growth and activity in children. Neurotoxicol. Teratol. 12(4):319–326.
Jarvinen, A.W., and G.T.Ankley. 1999. Linkage of Effects to Tissue Residues: Development of a Comprehensive Database for Aquatic Organisms Exposed to Inorganic and Organic Chemicals. Pensacola, FL: Society of Environmental Toxicology and Chemistry (SETAC). 364 pp.
Jones, P.D., J.P.Giesy, J.L.Newsted, D.A.Verbrugge, D.L.Beaver, G.T.Ankley, D.E. Tillitt, and K.B.Lodge. 1993. 2,3,7,8-tetrachlorodibenzo-p-dioxin equivalents in tissues of birds at Green Bay, Wisconsin, USA. Arch. Environ. Contam. Toxicol. 24(3):345–354.
Jones, P.D., J.P.Giesy, J.L.Newsted, D.A.Verbrugge, J.P.Ludwig, M.J.Ludwig, H. Auman, T.J.Kubiak, and D.Best. 1994. Accumulation of 2,3,7,8-tetrachlorodibenzo-p-dioxin equivalents by double crested (Phalacrocorax auritus, Pelicaniformes) chicks in the North American Great Lakes. Ecotoxicol. Environ. Saf. 27(2):192–209.
Kannan, K., A.L.Blankenship, P.D.Jones, and J.P.Giesy. 2000. Toxicity reference values for the toxic effects of polychlorinated biphenyls to aquatic mammals. Hum. Ecol. Risk Assess. 6(1):181–201.
Karickhoff, S.W., D.S.Brown, and T.A.Scott. 1979. Sorption of hydrophobic pollutants on natural sediments. Water Res. 13(3):241–248.
Kimbrough, R.D., M.L.Doemland, and M.E.LeVois. 1999. Mortality of male and female capacitor workers exposed to polychlorinated biphenyls. J. Occup. Environ. Med. 41(3):161–171.
Kuratsune, M., T.Yoshimura, J.Matsuzaka, and A.Yamaguchi. 1972. Epidemiological study on Yusho, a poisoning caused by ingestion of rice oil contaminated with a commercial brand of polychlorinated biphenyls. Environ. Health Perspect. 1:119–128.
Lake, J.L., N.I.Rubinstein, H.I.I.Lee, C.A.Lake, J.Heltshe, and S.Pavignano. 1990. Equilibrium partitioning and bioaccumulation of sediment-associated contaminants by infaunal organisms. Environ. Toxicol. Chem. 9(8):1095–1106.
Landrum, P.F. 1989. Bioavailability and toxicokinetics of polycyclic aromatic hydrocarbons sorbed to sediments for the amphipod Pontoporeia hoyi. Environ. Sci. Toxicol. 23(5):588–595.
Landrum, P.F., and R.Poore. 1988. Toxicokinetics of selected xenobiotics in Hexagenia limbata. J. Great Lakes Res. 14(4):427–437.
Landrum, P.F., W.A.Frez, and M.S.Simmons. 1992. The effect of food consumption of the toxicokinetics of benzo(a)pyrene and 2,2',4,4',5,5'-hexachlorobiphenyl in Mysis relicta. Chemosphere 25(3):397–415.
Leonards, P.E.G., T.H.de Vries, W.Minnaard, S.Stuijfzand, P.de Voogt, W.P. Cofino, N.M.van Straalen, and B.van Hattum. 1995. Assessment of experimental data on PCB-induced reproduction inhibition in mink, based on an isomer- and
congener-specific approach using 2,3,7,8-tetrachlorodibenzo-p-dioxin toxic equivalency. Environ. Toxicol. Chem. 14(4):639–652.
Levin, E.D., S.L.Schantz, and R.E.Bowman. 1988. Delayed spatial alternation deficits resulting from perinatal PCB exposure in monkeys. Arch. Toxicol. 62(4):267–273.
Ling, H., M.Diamond, and D.Mackay. 1993. Application of the QWASI fugacity/ equivalence model to assessing sources and fate of contaminants in Hamilton Harbor. J. Great Lakes Res. 19(3):582–602.
Long, E.R., and L.G.Morgan. 1991. The Potential for Biological Effects of Sediment Sorbed Contaminants Tested in the National Status and Trends Program. Office of Oceanography and Marine Assessment, National Ocean Service, Rock ville, MD. GRA&I 14. NTIS PB91–172288.
Lonky, E., J.Reihman, T.Darvill, J.Mather Sr., and H.Daly. 1996. Neonatal behavioral assessment scale performance in humans influenced by maternal consumption of environmentally contaminated Lake Ontario fish. J. Great Lakes Res. 22(2):198–212.
Ludwig, J.P., H.Kurita-Matsuba, H.J.Auman, M.E.Ludwig, C.L.Summer, J.P. Giesy, D.E.Tillitt, and P.D.Jones. 1996. Deformities, PCBs and TCDD-equivalents in double-crested cormorants (Phalacrocorax auritus) and Caspian terns (Hydroprogne caspia) of the upper Great Lakes 1986–1991: testing the hypotheses of cause-effect relationships. J. Great. Lakes Res. 22(2):172–197.
MacFarland, V.A., and J.U.Clarke. 1989. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environ. Health Perspect. 81:225–239.
Mackay, D., and S.Paterson. 1991. Evaluating the multimedia fate of organic chemicals: a level III fugacity model. Environ. Sci. Technol. 25(3):427–436.
Mackay, D., W.Y.Shiu, J.Billington, and G.L.Huang. 1983. Physical chemical properties of polychlorinated biphenyls. Pp. 59–70 in: Physical Behavior of PCBs in the Great Lakes, D.Mackay, S.Patterson, S.J.Eisenreich, and M.S. Simmons. eds. Ann Arbor, MI: Ann Arbor Science.
Mackay, D., S.Paterson, and W.Y.Shiu. 1992. Generic models for evaluating the regional fate of chemicals. Chemosphere 24(6):695–717.
Marine Policy Center. 2000. Cost-Effective Reduction of PCB-contamination in the Hudson River and Estuary. Final Report to the Hudson River Foundation for Science and Environmental Research, New York, NY. Grant No. 008/991. Research Project R/B-147-PD. Marine Policy Center, Woods Hole Oceanographic Institution, Woods Hole, MA.
Masuda, Y. 1985. Health status of Japanese and Taiwanese after exposure to contaminated rice oil. Environ Health Perspect. 60:321–325.
Mavoungou, R., R.Masse, and E.Sylvestre. 1991. Microbial dehalogenation of 4 4' dichlorobiphenyl under anaerobic conditions. Sci. Total. Environ. 101(3):263–268.
McCarty, J.P., and A.L.Secord. 1999. Nest-building behavior in PCB-contaminated tree swallows. AUK 116(1):55–63.
McNeil, J., C.Taylor, and W.Lick. 1996. Measurements of erosion of undisturbed bottom sediments with depth. J. Hydraul. Eng. 122(6):316–324.
Mergler, D., S.Belanger, F.Larribe, M.Panisset, R.Bowler, M.Baldwin, J.Lebel, and K.Hudnell. 1998. Preliminary evidence of neurotoxicity associated with eating fish from the Upper St. Lawrence River Lakes. Neurotoxicity 19(4–5):691–702.
Metcalfe, C.D., and G.D.Haffner. 1995. The ecotoxicology of coplanar polychlorinated biphenyls. Environ. Rev. 3(2):171–190.
Murk, A., D.Morse, J.Boon, and A.Brouwer. 1994. In vitro metabolism of 3,3',4,4'-tetrachlorobiphenyl in relation to ethoxyresorufin-o-deethylase activity in liver microsomes of some wildlife species and rat. Eur. J. Pharmacol. 3(2–3):253–61.
Nebecker, A.V., F.A.Puglisi, and D.L.Defoe. 1974. Effect of polychlorinated biphenyl compounds on survival and reproduction of the fathead minnow and flagfish. Trans. Am. Fish. Soc. 103(3):562–568.
Neely, W.B., and D.Mackay. 1982. Evaluative model for estimating environmental fate. Pp. 127–143 in: Modeling the Fate of Chemicals in the Aquatic Environment, K.L.Dickson, A.W.Maki, and J.Cairns Jr., eds. Ann Arbor, MI: Ann Arbor Science.
Nichols, J.W., C.P.Larsen, M.E.McDonald, G.J.Niemi and G.T.Ankley. 1995. Bioenergetics-based model for accumulation of PCBs by nesting tree swallows, Tachycineta bicolor. Environ. Sci. Technol. 29(3):604–612.
Niimi, A.J., H.B.Lee, and D.C.G.Muir. 1996. Environmental assessment and ecotoxicological implications of the co-elution of PCB congeners 132 and 153. Chemosphere 32(4):627–638.
Parkerton, T.F., J.P.Connolly, R.V.Thoman, and C.G.Uchrin. 1993. Do aquatic effects of human health end points govern the development of sediment-quality criteria for nonionic organic chemicals? Environ. Toxicol. Chem. 12(3):507–523.
Patandin, S., C.I.Langting, P.G.Mulder, E.R.Boersma, P.J.Sauer, and N.Weisglas-Kuperus. 1999. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J. Pediatr. 134(1):33–41.
Poland, A., and J.C.Knutson. 1982. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Ann. Rev. Pharmacol. Toxicol. 22:517–554.
Powell, D.C., R.J.Aulerich, J.C.Meadows, D.E.Tillitt, J.P.Giesy, K.L.Stromberg, and S.J.Bursian. 1996a. Effects of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) injected into the yolks of chicken (Gallus domesticus) eggs prior to incubation. Arch. Environ. Contam. Toxicol 31(3):404–409.
Powell, D.C., R.J.Aulerich, K.L.Stromborg, and S.J.Bursian. 1996b. Effects of 3,3',4,4'-tetrachlorobiphenyl, 2,3,3',4,4'- pentachlorobiphenyl, and 3,3',4,4',5-pentachlorobiphenyl on the developing chicken embryo when injected prior to incubation. J. Toxicol. Environ. Health 49(3):319–338.
PCCRARM (Presidential/Congressional Commission on Risk Assessment and Risk Management). 1997. Framework for Environmental Health Risk Management: Final Report. Washington, DC: The Commission.
QEA (Quantitative Environmental Analysis). 1999a. PCBs in the Upper Hudson
River. Vol. 2. A Model of PCB Fate, Transport, Bioaccumulation. Prepared for General Electric, Albany, New York. May.
QEA (Quantitative Environmental Analysis). 1999b. PCBs in the Upper Hudson River. Errata. Prepared for General Electric, Albany, New York. July.
Quensen, J.F., J.M.Tiedje, and S.A.Boyd. 1988. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science 242(4879):752–754.
Quensen, J.F, 3rd., M.A.Mousa, S.A.Boyd, J.T.Sanderson, K.L.Froese, and J.P. Giesy. 1998. Reduction of aryl hydrocarbon receptor-mediated activity of polychlorinated biphenyl mixtures due to anaerobic microbial dechlorination. Environ. Toxicol. Chem. 17(5):806–813.
Rhee, G.-Y., B.Bush, C.M.Bethoney, A.DeNucci, H.-M.Oh, and R.C.Sokol. 1993a. Anaerobic dechlorination of aroclor 1242 as affected by some environmental conditions. Environ. Toxicol. Chem. 12(6):1033–1039.
Rhee, G.-Y., B.Bush, C.M.Bethoney, A.DeNucci, H.-M.Oh, and R.C.Sokol. 1993b. Reductive dechlorination of aroclor 1242 in anaerobic sediments: pattern, rate and concentration dependence. Environ. Toxicol Chem. 12(6):1025–1032.
Rhee, G.-Y., R.C.Sokol, B.Bush, and C.M.Bethoney. 1993c. Long-term study of the anaerobic dechlorination of aroclor 1254 and without biphenyl enrichment. Environ. Sci. Technol. 27(4):714–719.
Rice, D.C. 1995. Neurotoxicity of lead, methylmercury, and PCBs in relation to the Great Lakes. Environ. Health Perspect. 103(suppl. 9):71–87.
Rogan, W.J., B.C.Gladen, K.L.Hung, S.L.Koong, L.Y.Shih, J.S.Taylor, Y.C.Wu, D.Yang, N.B.Ragan, and C.C.Hsu. 1988. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science 241(4863):334–336.
Rogan, W.J., B.C.Gladen, J.D.McKinney, N.Carreras, P.Hardy, J.Thullen, J. Tinglestad, and M.Tully. 1986. Neonatal effects of transplacental exposure to PCBs and DDE. J. Pediatr. 109(2):335–341.
Ross, P.S., R.L.de Swart, P.J.H.Reijnders, H.van Loveren, J.G.Vos, and A.D.M.E. Osterhaus. 1995. Contaminant-related suppression of delayed-type hypersensitivity and antibody responses in harbor seals fed herring from the Baltic Sea. Environ. Health Perspect. 103(2):162–167.
Rothman, N., K.P.Cantor, A.Blair, D.Bush, J.W.Brock, K.Helzlsouer, S.H.Zahm, L.L.Needham, G.R.Pearson, R.N.Hoover, G.W.Comstock, and P.T.Strickland. 1997. A nested case-control study of non-Hodgkin lymphoma and serum organochlorine residues. Lancet 350(9073):240–244.
Sabljic, A., H.Gutsen, H.Verhaar, and J.Hermens. 1995. QSAR modeling of soil sorption: improvements and systematics of log Koc vs. log Kow corrections. Chemosphere 31(11–12):4489–4514.
Safe, S. 1990. Polychlorinated biphenyls(PCBs), dibenzo-p-dioxins(PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit. Rev. Toxicol. 21(1):51–88.
Schantz, S.L. 1996. Developmental neurotoxicity of PCBs in humans: what do we know and where do we go from here? Neurotoxicol. Teratol. 18(3):217–227.
Schantz, S.L., E.D.Levin, and R.E.Bowman. 1991. Long-term neurobehavioral effects of perinatal polychlorinated biphenyl (PCB) exposure in monkeys. Environ. Toxicol. Chem. 10(6):747–756.
Sinks, T., G.Steele, A.B.Smith, K.Watkins, and R.A.Shults. 1992. Mortality among workers exposed to polychlorinated biphenyls. Am. J. Epidemiol. 136(4):389–398.
Solomon, K., J.Giesy, and P.Jones. 2000. Probabilistic risk assessment of agrochemicals in the environment. Crop Protect. 19(8–10):649–655.
Starodub, M.E., P.A.Miller, G.M.Ferguson, J.P.Giesy, and R.F.Willis. 1996. A protocol to develop acceptable concentrations of bioaccumulative organic chemicals in sediments for the protection of piscivorous wildlife. Toxicol. Environ. Chem. 54(1–4):243–259.
Svensson, B.G., Z.Mikoczy, U.Stromberg, and L.Hagmar. 1995. Mortality and cancer incidence among Swedish fishermen with a high dietary intake of persistent organochlorine compounds. Scand. J. Work Environ. Health 21(2):106–115.
TAMS Consultants, Inc., Limno-Tech, Inc., Menzie-Cura & Associates, Inc., and Tetra Tech, Inc. 2000. Phase 2 Report-Review Copy, Further Site Characterization and Analysis, Vol. 2D—Revised Baseline Modeling Report Hudson River PCBs Reassessment RI/FS. Prepared for U.S. Environmental Protection Agency, Region 2 and U.S. Army Corps of Engineers, Kansas City District. January. [Online]. Available: http://www.epa.gov/hudson/rbmr-bk3&4.pdf [December 19, 2000]
Tanabe, S., N.Kannan, A.Subramanian, S.Watanabe, and R.Tatsukawa. 1987. Highly toxic coplanar PCBs occurrence, source, persistency and toxic implications to wildlife and humans. Environ. Pollut. 47(2):147–163.
Thomann, R.V., and J.P.Connolly. 1984. Model of PCB in the Lake Michigan trout food chain. Environ. Sci. Technol. 18(2):65–71.
Thomann, R.V., J.P.Connolly, and T.F.Parkerton. 1992a. An equilibrium model of organic chemical accumulation in aquatic food webs with sediment interaction. Environ. Toxicol. Chem. 11(5):615–629.
Thomann, R.V., J.P.Connolly, and T.F.Parkerton. 1992b. Modeling accumulation of organic chemicals in aquatic food webs. Pp. 153–186 in: Chemical Dynamics in Fresh Water Ecosystems, F.A.P.C.Gobas and J.A.McCorquodale, eds. Chelsea, MI: Lewis.
Tillitt, D.E., R.W.Gale, J.C.Meadows, J.L.Zajicek, P.H.Peterman, S.N.Heaton, P.D.Jones, S.J.Bursian, T.J.Kubiak, J.P.Giesy, and R.J.Aulerich. 1996. Dietary exposure of mink to carp from Saginaw Bay. 3. Characterization of dietary exposure to planar halogenated hydrocarbons, dioxin equivalents, and biomagnification, Environ. Sci. Technol. 30(1):283–291.
Tracey, G.A., and D.J.Hansen. 1996. Use of biota-sediment accumulation factors to assess similarity of nonionic organic chemical exposure to benthically-coupled organisms of differing trophic mode. Arch. Environ. Contam. Toxicol. 30(4):467–475.
Tryphonas, H. 1998. The impact of PCBs and dioxins on children’s health: immunological considerations. Can. J. Public Health 89(Suppl.1):49–52, 54–57.
Van den Berg, M., L.Birnbaum, A.T.C.Bosveld, B.Brunström, P.Cook, M.Feeley, J.P.Giesy, A.Hanberg, R.Hasegawa, S.W.Kennedy, S.W., T.Kubiak, J.C. Larsen, F.X.van Leeuwen, A.K.Liem, C.Nolt, R.E.Peterson, L.Poellinger, S. Safe, D.Schrenk, D.Tillitt, M.Tysklind, M.Younes, F.Waern, and T. Zacharewski. 1998. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 106(12):775–792.
Velleux, M., and D.Endicott. 1994. Development of a mass balance model for estimating PCB export from the Lower Fox River to Green Bay. J. Great Lakes Res. 20(2):416–434.
Walker, M.K., and R.E.Peterson. 1991. Potencies of polychlorinated dibenzo-p-dioxin, dibenzofuran and biphenyl congeners, relative to 2,3,7,8-tetrachlorodibenzo-p-dioxin for producing early life stage mortality in rainbow trout (Oncorhynchus mykiss). Aquatic Toxicol. 21:219–238.
Walker, M.K. P.M.Cook, B.C.Butterworth, E.W.Zabel, and R.E.Peterson. 1996. Potency of a complex mixture of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners compared to 2,3,7,8,-tetrachlorodibenzo-p-dioxin in causing early life stage mortality. Fundam. Appl. Toxicol. 30(2):178–186.
Weisglas-Kuperus, N., T.C.Sas, C.Koopman-Esseboom, C.W.van der Zwan, M.A. De Ridder, A.Beishuizen, H.Hooijkaas, and P.J.Sauer. 1995. Immunologic effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants. Pediatr. Res. 38(3):404–410.
Williams, L.L., J.P.Giesy, N.DeGalan, D.A.Verbrugge, D.E.Tillitt, G.T.Ankley, and R.A.Welch. 1992. Prediction of concentrations of 2,3,7,8-TCDD equivalents (TCDD-EQ) from total concentrations of PCBs in fish fillets. Environ. Sci. Technol. 26(6):1151–1159.
Willman, E.J., I.B.Manchester-Neesvig, and D.E.Armstrong. 1997. Influence of ortho-substitution on patterns of PCB accumulation in sediment, plankton and fish in a freshwater estuary. Environ. Sci. Technol. 31(12):3712–3718.
Ziegler, C.K. 1999a. Sediment Stability at contaminated sediment sites. Pp. 26–27 in: Contaminated Sediment Management Technical Papers, Sediment Management Work Group, Detroit, MI. Fall 1999. [Online]. Available: http://www.smwg.org/ [January 02, 20001].
Ziegler, C.K. 1999b. Effective decision-making models for evaluating sediment management options. Pp. 9–11 in: Contaminated Sediment Management Technical Papers, Sediment Management Work Group, Detroit, MI. Fall 1999. [Online]. Available: http://www.smwg.org/ [January 02, 20001].
Ziegler, C.K., and B.S.Nisbet. 1995. Long-term simulation of fine-grained sediment transport in large reservoir. J. Hydraul. Eng. 121(11):773–781.
Zwiernik, M.J., J.F.Quenson, 3rd, and S.A.Boyd. 1998. FeSO4 amendments stimulate extensive anaerobic PCB dechlorination. Environ. Sci. Technol. 32(21): 3360–3365.