4
Comparison of CDC Guidelines and Proposed OSHA Rule
As described in Chapter 1, both the Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA) responded to the resurgence of tuberculosis that began in the mid-1980s and continued into the early 1990s. Beginning in 1990, CDC issued a series of tuberculosis control guidelines aimed at different settings and populations. In 1993 and 1994, OSHA issued enforcement procedures based on existing respiratory protection regulations and on statutory requirements that employers provide safe workplaces. Also, in 1994, OSHA initiated a rule-making process that led to the 1997 proposed rule. As this report was being completed in Fall 2000, OSHA had not published the final standard.
This chapter summarizes the provisions of the 1994 CDC guidelines for health care facilities and describes points of difference between the guidelines and the 1997 proposed OSHA rule. The proposed rule “incorporated the basic elements” of the 1994 guidelines with some differences (62 FR 201 at 54170 [October 17, 1997]). For example, as described later in this chapter, the CDC guidelines set forth a more extensive process for assessing the risk facing workers in a health care facility and determining which control measures apply on the basis of the level of risk. More generally, OSHA drafted the proposed rule to be enforced, and it therefore tends to be more specific and directive than the CDC guidelines. It would cover a broader range of employers and employees than the guidelines but would not extend to patients, prisoners, or visitors.
Some of the differences between the two documents reflect differences in the basic missions of CDC and OSHA. For example, consistent with its broader public health mission, the CDC guidelines for health care facilities include recommendations related to patients, family members,
and visitors as well as employees. Similarly, CDC’s discussion of tuberculosis control measures is generally more detailed, clinically oriented, and educational than the discussion of such measures in the proposed OSHA rule. Consistent with OSHA’s regulatory responsibilities, the proposed rule is often more specific and directive than the guidelines.
Both the CDC guidelines and the proposed OSHA rule were published during a period of change, and as discussed in Chapters 5, 6, and 7, circumstances continue to change. This chapter provides a descriptive overview and comparison of the guidelines as issued by CDC in 1994 and the rule as proposed by OSHA in 1997. Chapter 6 presents the committee’s assessment of the impact of the CDC guidelines, and Chapter 7 examines the likely impact of an OSHA standard. Complicating this latter assessment was the lack of a final published standard.
CDC GUIDELINES ON PREVENTING TRANSMISSION OF TUBERCULOSIS IN HEALTH CARE FACILITIES
CDC has published guidelines and recommendations for controlling tuberculosis in health care facilities (CDC, 1990b, 1994b), correctional facilities (CDC, 1996b), and facilities serving homeless people (CDC, 1992a). It has also developed guidelines focusing on special populations including migrant workers (CDC, 1992b), at-risk minority groups (CDC, 1992c), foreign-born persons (CDC, 1998b), and those with human immunodeficiency virus (HIV) infection (most recently, CDC, 1998a, 2000c).1
CDC’s 1994 guidelines for health care facilities, which replaced those issued in 1990, served as the foundation for the rule proposed by OSHA in 1997. In the fall of 2000, CDC began a reexamination of the guidelines, in particular, the recommendations on tuberculin skin testing. No new recommendations are expected before 2002.
The 1994 CDC guidelines present a three-level hierarchy of measures to prevent transmission of tuberculosis in health care facilities. As explained below, the hierarchy consists of (1) administrative controls, (2) engineering controls, and (3) personal respiratory protection.2
The guidelines for health care facilities focus primarily on hospitals, with special sections on ambulatory care settings, emergency departments, autopsy rooms, and laboratories. They also include brief discussions of emergency medical services, hospices, long-term-care facilities, correctional facilities, dental settings, home health care settings, and medical offices. The guidelines define health care workers to cover paid and unpaid workers including contract employees, students, and volunteers.
In 1996, a CDC advisory group published recommendations for the prevention of tuberculosis in correctional facilities (CDC, 1996b). These recommendations are organized around the core activities of screening, containment, and assessment rather than around the hierarchy of controls listed above. Although the recommendations reflect differences between the purposes of correctional facilities and those of health care facilities, many elements are similar to the 1994 guidelines. For example, the discussion of tuberculin skin testing and follow-up for employees is consistent with the 1994 guidelines, except that no category of prison worker is singled out for retesting more often than once a year. Prisons that have medical units are advised to follow the 1994 CDC guidelines in those units. Facility personnel are also advised to be familiar with guidelines published by American Thoracic Society (ATS) and the National Commission on Correctional Health Care (NCCHC, 1992, 1996).
CDC’s 1992 recommendations for those who work with homeless people only briefly discuss protections for homeless shelter workers (CDC, 1992a). These protections include (1) tuberculin skin testing of shelter staff upon hiring and every 6 to 12 months thereafter, (2) evaluation of those with positive tests results, and (3) provision of treatment for those with latent tuberculosis infection, as appropriate.
In contrast to government regulations, the CDC guidelines on tuberculosis are advisory, which allows more leeway for institutional interpretation and judgment. The guidelines are not enforced by a government agency responsible for monitoring compliance and proposing penalties for noncompliance. Other federal and state agencies as well as accrediting organizations may, however, require tuberculosis control measures based on or similar to those recommended by CDC.3 In some circumstances, health care
facilities might also be subject to OSHA sanctions under enforcement procedures based on the general-duty-clause of the agency’s statute (see Chapter 3 and Appendix E). In addition, health care facilities that fail to follow CDC guidance might be more vulnerable to lawsuits by patients or others who contract tuberculosis in a facility. Employees who acquire active tuberculosis (and sometimes tuberculosis infection) through occupational exposure are covered under workers’ compensation laws that preclude litigation against an employer (see Chapter 3 and Appendix E).4
Unlike some guidelines directed at health care practitioners, the 1994 CDC guidelines do not have a “sunset” provision that specifies a date after which users should not rely on the guidelines. These and similar provisions acknowledge that scientific knowledge is always advancing and that those who develop clinical practice guidelines should have a process to review and update recommendations to reflect current scientific evidence and changing circumstances (IOM, 1992; CDC, 1996a). As noted earlier, the agency recently began a reexamination of the 1994 guidelines.
PROPOSED OSHA RULE ON OCCUPATIONAL EXPOSURE TO TUBERCULOSIS
OSHA’s mission, described in Chapter 3, differs from that of CDC. In addition, its rules must meet statutory, judicial, and administrative criteria that do not apply to CDC guidelines. In developing rules, OSHA must, however, consider available guidelines, research, and other information. For example, the agency incorporated basic elements of the 1994 CDC guidelines in the 1997 proposed rule. OSHA also notes that in enforcing a final rule on tuberculosis, it would ordinarily defer to subsequently updated CDC guidelines that had provisions in conflict with the rule.
OSHA concluded that the CDC guidelines were not an enforceable alternative to the proposed rule. Nonetheless, in the commentary (preamble) on the proposed rule, the agency asks for comments on this alternative, including how “compliance and efficacy” could be determined (62 FR 201 at 54227 [October 17, 1997]).
The proposed OSHA rule also includes nonmandated guidance on certain topics. These include the writing of the required exposure control plan, the use of ultraviolet germicidal irradiation lighting systems, and
|
4 |
As described in Chapter 2, laboratory tests that can “fingerprint” strains of tuberculosis may help in evaluating whether the workplace is the source of tuberculosis in a patient or health care worker. Nonetheless, such testing in not always feasible, and it may not rule out exposure from a source in the community. As described elsewhere in this chapter, the proposed OSHA rule would provide certain financial protections for workers diagnosed with tuberculosis without requiring proof of its origin in the workplace. |
performance-monitoring procedures for high-efficiency particulate air (HEPA) filters used for contaminated air that may be recirculated into general-use areas.
The rest of this section briefly reviews differences in the settings and people covered by the CDC guidelines and the proposed OSHA rule. The remaining sections focus on differences between the exposure control measures described in the guidelines and those in the proposed rule.
Covered Settings and Employers
The proposed OSHA rule is not limited to health care workers, employers, and settings. It would cover a wide range of employers and employees including
-
Hospitals
-
Long-term-care facilities serving the elderly
-
Hospices
-
Substance abuse treatment centers
-
Home health care providers
-
Emergency medical service providers
-
Research and clinical laboratories handling tuberculosis bacteria
-
Medical examiners’ offices
-
Other facilities where certain high-hazard procedures are performed
-
Homeless shelters
-
Correctional facilities
-
Immigration detainment facilities
-
Law enforcement facilities
-
Contractors working on ventilation systems or areas that might contain airborne tuberculosis bacteria
-
Social service workers, attorneys, and teachers visiting those with suspected or confirmed tuberculosis
-
Personnel agencies or other organizations providing temporary or contract workers to covered facilities
The list of workplaces and employees provided above does not cover all those in which higher rates of tuberculosis have been documented. For example, workers in certain mining industries often have workplace-related physical conditions (e.g., silicosis) that make them more susceptible to tuberculosis. Because these workers are not anticipated to have a high risk of workplace exposure to the disease, the proposed OSHA rule does not cover them. (Also, mine safety is covered by another U.S. Department of Labor agency, the Mine Safety and Health Administration.)
Some health care settings are unexpectedly omitted from the listing in the 1997 proposed rule. For example, tuberculosis clinics are not men-
tioned, nor are HIV-AIDS clinics, although the latter serve populations at high risk of having infectious tuberculosis. Tuberculosis and HIV-AIDS clinics could, however, be affected by the proposed rule’s coverage of facilities where high-hazard procedures (e.g., cough induction or administration of aerosolized drugs) are performed. If a clinic did not perform such procedures, no provisions of the proposed rule would apparently apply. In a presentation to the committee, OSHA staff suggested that the final rule is likely to cover tuberculosis and HIV-AIDS clinics explicitly.
The proposed OSHA rule also does not cover physicians’ offices unless high-hazard procedures are performed there. Thus, physicians serving recent immigrants, AIDS patients, and other high-risk populations in high-prevalence areas would not be covered unless they performed bronchoscopies or similar procedures. The 1994 CDC guidelines state that it is likely that tuberculosis will be encountered in medical offices. They advise a risk assessment and the use of precautions consistent with that assessment. In the commentary on the proposed rule, OSHA asks whether all or some medical and dental offices should be covered.
OSHA likewise asks whether it should cover long-term care facilities other than those serving the elderly population. It specifically mentions psychiatric facilities.
Funeral homes are not covered by the proposed rule, although medical examiners’ offices would be covered. OSHA’s preliminary analysis of risk in the funeral industry was inconclusive, but more recent information provided to the agency indicates that some funeral homes use procedures that can produce airborne particles containing tuberculosis bacteria. One study of funeral home workers in Maryland found that employees involved in embalming were twice as likely as other funeral home employees to have positive tuberculin skin test results (Gershon et al., 1995b). At least one case of cadaver-to-embalmer transmission of tuberculosis has been documented (Sterling et al., 2000a).
As discussed in Chapter 3, OSHA’s statutory jurisdiction and regulations do not extend to state and local government employees unless a state has an occupational safety and health plan approved by OSHA. In states without such plans, state, county, or municipal governments would not be required to follow an OSHA rule for their hospital employees, correctional facility workers, paramedics, social workers, medical examiners, and other public employees who may be at risk of occupational exposure to tuberculosis.5 Federal government agencies such as the Veterans Health Administration are covered by OSHA standards under statute and the executive orders described in Chapter 3.
In its commentary on the proposed rule, OSHA recognizes that protective measures need to be tailored to different kinds of workplaces. To clarify the responsibilities of several kinds of employers, OSHA presents several charts outlining what the proposed rule would require. These charts cover (1) work settings where individuals with suspected or confirmed infectious tuberculosis are admitted or provided medical services; (2) work settings where early identification and transfer procedures are used for those with suspected or confirmed infectious tuberculosis; (3) employers that serve individuals who have been isolated due to suspected or confirmed tuberculosis or individuals who work in areas where air contaminated with tuberculosis likely exists; (4) home health care and home-based hospice care; (5) emergency medical services; (6) clinical and research laboratories; and (7) personnel agencies.
In facilities that rely on outside contractors to provide nursing, food service, and other kinds of workers, several different employers may share responsibilities for implementing certain protective measures. For example, a hospital may provide skin testing for its own employees while requiring each outside contractor to test contract workers. Alternatively, a contractor may be able to arrange for the hospital to provide required skin testing and other employee health services. Responsibility for some protective measures—such as the provision of isolation rooms—cannot be shared.
Covered Individuals
The specific objective of the 1997 proposed OSHA rule is to protect employees rather than patients, prisoners, visitors, or volunteers.6 The rule does not cover independent, nonemployed, nonincorporated physicians (see Chapter 3). Facilities may, however, require these physicians to certify compliance with certain measures (e.g., up-to-date skin test or successful treatment for recent infection or active disease) before they grant them privileges to see patients in the facility. Likewise, although medical, nursing, and other students are apparently not covered by the rule, the health care facilities in which they train may require that their sponsoring schools take responsibility for skin testing and certain other measures. Residents are considered employees for purposes of occupational safety and health regulation. Chapter 3 and Appendix E discuss more generally the scope of OSHA regulations.
The proposed OSHA rule would provide certain job and financial protections for employees with suspected or confirmed infectious tuberculosis that are not provided for in the CDC guidelines. These are described below in the section on administrative controls.
|
6 |
Depending on several factors, such as the receipt of significant in-kind compensation (e.g., free meals), a volunteer may sometimes be considered a worker for purposes of OSHA regulations (see Appendix E). |
COMPARISON OF GUIDELINES AND PROPOSED RULE: ADMINISTRATIVE CONTROLS
This and the next two sections of this chapter summarize and discuss the administrative, engineering, and personal respiratory protection provisions of the 1994 CDC guidelines for health care facilities. Because OSHA relied heavily on the CDC guidelines, in drafting its proposed rule, the summaries are organized around the guidelines with points of difference between the two noted in italics. The descriptions of control measures and differences in the guidelines and proposed rule were reviewed by both OSHA and CDC staff and revised as appropriate.
In its commentary on the proposal, OSHA identified some differences between the guidelines and the proposed rule. Other differences were identified in comments submitted to OSHA by various organizations including the American Hospital Association (AHA) (AHA, 1998) and the Association of Professionals in Infection Control and Epidemiology (APIC) (APIC, 1998). The committee’s own review of the CDC guidelines and the proposed OSHA rule found a few additional points of difference.
Some control measures are discussed in more detail elsewhere in this report. Tuberculin skin testing and diagnosis and treatment for latent tuberculosis infection and active disease are discussed in Chapter 3. Appendix B provides a more detailed examination of the skin test. Respiratory protections are discussed further in Appendix F.
The following discussion does not cover the 1992 CDC guidelines for those serving homeless people. The 1997 proposed OSHA rule would require homeless shelters to follow essentially the same procedures required for correctional facilities and hospitals that refer rather than treat people with suspected or confirmed tuberculosis. In 1999, acknowledging serious concerns about the practicality and cost of the requirements for homeless shelters, OSHA reopened the comment period and record on the proposed rule to solicit additional information and comments on requirements for homeless shelters. In a presentation to the committee, OSHA staff have suggested that the final rule may include fewer requirements for homeless shelters.
Administrative Controls Related to Risk Assessment, Surveillance, Worker Education, and Coordination
Table 4-1 summarizes most of the key elements of the administrative controls recommended in the 1994 CDC guidelines for health care facilities. (The elements related to patient management are summarized in the next section of this chapter.) The italicized comments in the table highlight the differences between the CDC guidelines and the proposed OSHA rule that might influence the effectiveness or the burdensomeness of a final rule. A discussion of some of the differences follows the table.
TABLE 4-1. Summary of Administrative Controls (other than diagnosis and treatment) Recommended by CDC for Health Care Facilities, with Notes (in italics) on How Proposed OSHA Rule Differs
|
Assigning Responsibility |
||
|
1. |
Assigning responsibility for the control program to qualified person(s) |
|
|
2. |
Ensuring that the program includes experts in infection control, occupational health, and engineering NOTE: Assignment of responsibility to qualified individuals is implicit in the proposed OSHA rule. |
|
|
Assessing Risk and Developing Tuberculosis Control Plans |
||
|
1. |
Analyzing tuberculosis in the community: incidence, prevalence, drug resistance NOTE: The proposed OSHA rule would require county-level information and assessment for facilities seeking exemption from certain of the rule’s provisions. |
|
|
2. |
Analyzing tuberculosis in the facility: laboratory results, discharge diagnosis including data on drug resistance, medical record review; by location(s) of treatment |
|
|
3. |
Analyzing worker tuberculin skin test conversions by work area or category NOTE: The proposed OSHA rule would not require assessment of laboratory results, data on drug resistance, medical records, or data on skin test conversions. |
|
|
4. |
Matching a facility or area within a facility to one of several risk categories based on skin test conversion data and other factors (see Figure 4-1) NOTE: The proposed OSHA rule does not explicitly rank facility risk levels and does not consider skin test conversion data in matching work area or job category characteristics to regulatory requirements |
|
|
5. |
Periodically reassessing risk based on new community data, review of patient records, observation of work practices, etc. |
|
|
6. |
Preparing and implementing written tuberculosis control plans consistent with level of risk identified in the assessment a. Writing plans for each area of a facility and each relevant worker category b. Selecting infection-control protocols for each relevant work area or job category c. Disseminating plans to relevant managers and workers d. Evaluating implementation of plans and revising it as appropriate NOTE: The proposed OSHA rule would require annual review of the written exposure control plan and updating of the plan when necessary to reflect changes in tasks, procedures, engineering controls, or job classifications. |
|
|
Establishing a Screening and Surveillance Program Consistent with the Risk Assessment |
||
|
1. |
Providing two-step baseline tuberculin skin testing for those without a documented positive test result or a documented negative test result within the past 12 months (exceptions: when results for such testing suggest that no boosting is occurring, the two-step approach can be foregone; baseline testing is optional for minimal-risk facilities) |
|
|
2. |
Providing periodic retesting at 3-, 6-, or 12-month intervals consistent with the risk assessment, area of employment, and employee characteristics NOTE: The proposed OSHA rule differs slightly on requirements for baseline skin testing and different frequencies of testing for certain workers. Unlike the CDC guidelines, the proposed rule would require skin testing within 30 days of termination of employment. |
|
|
3. |
Providing follow-up diagnostic evaluation and—when appropriate—treatment for workers with positive skin tests NOTE: The proposed OSHA rule would, in addition, require employers to provide each employee with a written medical opinion following evaluation by a physician or other licensed health care professional. |
|
4. |
Evaluating workers with symptoms or signs of tuberculosis and excluding those with infectious tuberculosis from the workplace until they are noninfectious NOTE: The medical removal provisions of the proposed OSHA rule would, in addition, require wage, benefit, and other protections for workers removed from work due to suspected or confirmed infectious tuberculosis. The proposed rule would require employers to pay for follow-up services for employees with converted skin tests or suspected or confirmed active disease. |
|
1. |
Evaluating possible workplace exposure to and transmission of tuberculosis a. Establishing procedures to identify exposure incidents, transmission of disease, and factors associated with exposure or transmission b. Investigating worker skin test conversions, diagnoses of tuberculosis in workers, and exposure incidents |
|
Educating, Training, and Counseling Workers |
|
|
1. |
Educating workers (as appropriate for their work responsibilities) about tuberculosis infection, disease, transmission, symptoms, treatment, and risks in their community and facility |
|
2. |
Training workers in tuberculosis control measures applicable to their work responsibilities NOTE: The proposed rule would also require that workers be informed about the OSHA rule. It would require annual retraining unless the employer could show that each employee had the skills and knowledge needed. The proposed rule would require that training be appropriate to employees’ level of literacy, education, and language. |
|
1. |
Counseling workers as appropriate about positive skin tests, suspected or known personal exposure to active tuberculosis, diagnosis of active disease, treatment options, etc. |
|
2. |
Offering assignments involving low risk of tuberculosis exposure to employees known to be immunocompromised NOTE: The proposed OSHA rule would not require alternative assignment for immunocompromised workers but would require that workers be educated about tuberculosis risks for individuals with these and other conditions. |
|
Coordinating with Public Health Officials |
|
|
1. |
Reporting cases of active tuberculosis |
|
2. |
Assisting in investigations of tuberculosis exposure and transmission NOTE: The proposed OSHA rule would require reporting of cases of occupational tuberculosis infection and disease to OSHA, but it does not explicitly require coordination with public health authorities. NOTE: The proposed OSHA rule includes a variety of additional recordkeeping requirements related to employee medical records, medical surveillance, employee training, engineering controls, confidentiality, record availability and transfer, and other matters. |
|
SOURCES: CDC (1994b) and 62 FR 201 (October 17, 1997). |
|
Risk Assessment and Exposure Control Plan
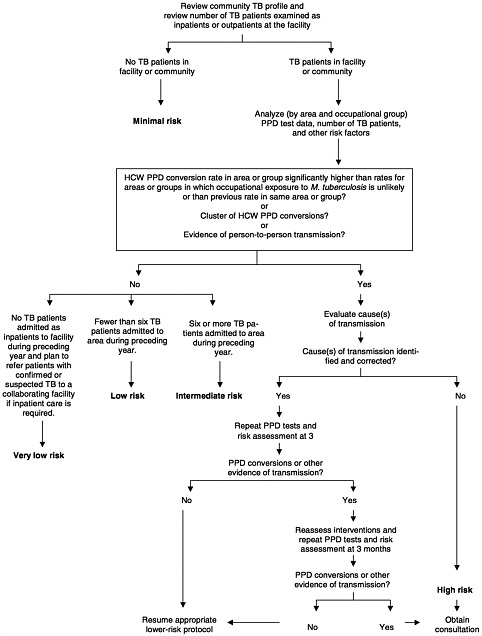
CDC Assessment Provisions The CDC guidelines recommend that all health care facilities perform a comprehensive risk assessment and then adopt tuberculosis control measures appropriate for the level of risk identified. The guidelines describe five levels of risk: minimal risk, very low risk, low risk, intermediate risk, and high risk (Figure 4–1). The minimal-risk and very-low-risk categories apply only to entire facilities; the other categories apply to areas or groups within a facility. The guidelines specify the control measures appropriate for each category. Many measures (e.g., written tuberculosis control plan, worker education, and protocols for identifying patients with active tuberculosis) are recommended for all categories.
The assignment of risk is based on an analysis of several variables including the community’s “tuberculosis profile” (including cases and rates of multidrug-resistant disease); the number and types of patients with tuberculosis seen by the facility and by areas within the facility; the facility’s policy on the treatment or referral of patients with tuberculosis; comparative levels and trends in employee tuberculin skin test conversions (by area and job category) including clusters of conversions; and evidence of person-to-person transmission of active tuberculosis within the facility. The risk assessment process also provides for review of medical records and observation of tuberculosis control practices to identify possible delays or deficiencies in identifying or treating individuals with infectious tuberculosis.
On the basis of the risk assessment, the CDC guidelines assign a facility or an area within a facility to one of five risk categories: minimal, very low, low, intermediate, and high risk. The minimal-risk category applies only to facilities with no tuberculosis patients in the facility or the community. (For this assessment, no time period—e.g., the past 2 years— is explicitly specified.) The very low risk category applies to facilities that plan to refer patients with suspected or confirmed tuberculosis, that have admitted no patients with tuberculosis during the past year, and that have not experienced comparatively high rates of tuberculin skin test conversions or conversion clusters or apparent person-to-person transmission of Mycobacterium tuberculosis. These facilities may, however, be located in communities that have had recent cases of tuberculosis.
The risk assessment recommended by CDC calls for a “profile of tuberculosis in the community served by the facility” (CDC, 1994b, p. 17). The risk assessment described in the 1997 proposed OSHA rule calls for the use of county-level tuberculosis cases without reference to a facility’s service area. County-level data are more readily and uniformly available than subcounty data. However, facilities located in large counties may draw patients from a much smaller service area; facilities may also have
service areas that include all or part of more than one county. Thus, county-level data may not provide a good picture of community risk.
Although the Table 4-1 in the 1997 CDC guidelines listing the measures applicable to different facility risk categories uses the terms “required” “optional,” and “not applicable,” the guidelines are voluntary and do not impose enforceable requirements. For minimal-risk and very-low-risk facilities, the majority of control measures still apply to both risk categories (e.g., having protocols for investigating worker skin test conversions and contacts of patients not initially diagnosed with tuberculosis, and educating workers about tuberculosis). Several tuberculosis control measures are listed not applicable (e.g., protocols for initiation of the isolation of tuberculosis patients) or optional (e.g., evaluation of ultraviolet germicidal irradiation units or other engineering controls installed in triage or waiting areas).
For the low-risk, intermediate-risk, and high-risk categories (which applies to areas within a facility), the guidelines vary only in the recommended frequency of performance for several measures including tuberculin skin testing for employees (and analysis of results), review of the medical records of patients with tuberculosis, observation of infection-control practices, evaluation of engineering controls, and reassessment of risk. For example, a low-risk facility would be expected to take these steps yearly, whereas an intermediate-risk facility would have to undertake them every 6 to 12 months—which actually involves no difference for organizations that choose the 12 month option. For the intermediate-risk category and high-risk (tuberculosis outbreak) category, the frequencies for some steps are higher than those specified in the 1997 proposed OSHA rule.
The risk assessment process recommended by CDC includes a determination of whether a facility’s conversion rate in “an area or group” is “significantly” higher than that (1) for an area or group unlikely to experience occupational exposure or (2) for the particular area or group at a previous time (CDC, 1994b, p. 10). OSHA concluded that this kind of analysis is unduly complicated and too burdensome to be required of all employers or used as a basis for determining what regulatory requirements will apply to an employer. Thus, the 1997 proposed OSHA rule does not provide for analysis of data on skin test conversions either in identifying low-risk settings or in developing or revising exposure control plans. However, in the list of issues on which it invites comments, OSHA does inquire about the benefits of requiring such analyses and the specific type of analysis that the agency might require. OSHA has not indicated whether such a requirement will appear in the final standard.
OSHA Provisions Because some facilities are very unlikely to experience occupational exposures to tuberculosis, the proposed OSHA rule
would—like the CDC guidelines—impose fewer requirements on facilities that meet certain requirements. To qualify, employers would need to document that they (or the places where their employees work):
-
neither admit nor provide medical services to individuals with suspected or confirmed tuberculosis,7
-
have had no confirmed cases of infectious tuberculosis during the previous 12 months, and
-
are located in counties that have had no confirmed cases of infectious tuberculosis during 1 of the previous 2 years and less than six cases during the other year.
These criteria do not include whether a facility has any evidence (e.g., clusters of tuberculin skin test conversions) of internal transmission of tuberculosis. No hospital in a county with one case of tuberculosis in each of the last two years could qualify for OSHA’s “low-risk” category (a label not used by OSHA), even if the hospital had admited no patients with tuberculosis.
For facilities that meet all three criteria, regulatory requirements would be limited to a written plan for controlling exposure, provision of a medical history and two-step baseline skin testing without periodic retesting, medical management and investigation after an exposure incident, job protections for workers with active tuberculosis, employee training, and record keeping. Other than requirements for baseline skin testing, the requirements are similar to those that CDC recommends for minimal-risk and very-low-risk facilities. CDC, however, advises that two-step testing can be discontinued for the minimal-risk category if experience shows little or no boosting.
Except for the facilities that meet the three criteria listed above, the proposed OSHA rule would require facilities to identify employees whose duties could be reasonably anticipated to bring them into contact with people who have suspected or confirmed infectious tuberculosis or with M. tuberculosis-contaminated air. Individual protective measures (e.g., skin testing and respirator provision and fit testing) would apply to those with such anticipated contact, which OSHA terms “occupational exposure.”
Program of Employee Tuberculin Skin Testing and Follow-up
During the course of the committee’s work, CDC’s ACET recommended that the agency examine the 1994 guidelines’ provisions on tu-
berculin skin testing. In making the recommendation, ACET members noted declines in tuberculosis cases and rates in the United States since 1994, which increases concerns about false positive test results in low-prevalence areas (see Chapter 2 and Appendix B). CDC has accepted the recommendation and has begun the process of considering revisions to the testing and other recommendations of the 1994 guidelines.
Baseline Testing The 1994 CDC guidelines describe baseline skin testing at hiring as optional for its category of minimal-risk facilities, but a footnote states that it “may be advisable so that if an unexpected exposure does occur, conversions can be distinguished from positive skin test results caused by previous exposures” (CDC, 1994b, p. 15). The 1997 proposed OSHA rule would require such testing for new employees with “occupational exposure” in all facilities, including those in its “low-risk” category. OSHA staff have indicated that the final standard may provide that employers follow CDC’s recommendations on baseline testing. Thus, if the CDC recommendations change, so would OSHA’s requirements.
Frequency of Testing The 1994 CDC guidelines establish a testing scheme that recommends 3-month, “6 to 12”-month, and 12-month testing intervals based on the risk level for a work area or occupational category. The guidelines do not call for such periodic testing for the minimal risk and very-low-risk categories. The 1997 proposed OSHA rule would require 6-month testing for some categories of workers (e.g., those performing high-hazard procedures) and 12-month testing for all others. It would not require retesting after the baseline test for facilities in its “low-risk” category. The CDC guidelines for skin testing recommend that testing frequencies be based on, among other factors, analyses of a facility’s past experience with skin test conversions. In contrast, the proposed OSHA rule reflects the agency’s focus on an employee’s type of work and its potential for creating occupational exposure.
Testing at Termination of Employment The proposed OSHA rule would also require that employers offer skin testing within 30 days of termination of employment. The CDC guidelines include no such provision. AHA reported in 1998 that less than 6 percent of hospitals undertook such testing (AHA, 1998). OSHA staff have indicated that the final rule may clarify that employers would not be required to track down employees who, for example, quit with no or little notice.
Job and Financial Protections for Workers
The proposed rule provides various job and financial protections not included in the 1994 CDC recommendations. The rule would require what
OSHA terms “medical removal protections.”8 Such protections require continuation of wages and other benefits for workers who develop suspected or confirmed infectious tuberculosis and who must be excluded from the workplace until they are confirmed to be noninfectious.9 The agency states that such protections encourage employees—especially low-income workers—who suspect that they have tuberculosis to seek early evaluation of symptoms for the good of themselves and those around them.
For workers who were unable to function adequately while wearing a properly fitted respirator, the 1997 proposed OSHA rule would require that they be assigned other tasks or have wages and other benefits continued for up to 18 months if no such tasks can be identified. Unlike the 1994 CDC guidelines, the proposed rule does not provide that immunocompromised workers be offered the voluntary opportunity of reassignment to work involving a low risk of exposure to tuberculosis.
The proposed OSHA rule would also require employers to cover the costs of skin tests, respirators, and similar services or equipment. If medical treatment for latent tuberculosis infection were advised after a skin test conversion, the employer would also have to bear the cost of such treatment. Furthermore, if an employee developed infectious tuberculosis after an exposure incident in a covered work setting, the employer would be required to cover the costs for medical evaluation and treatment.
Although the proposed rule calls for investigation of the circumstances surrounding a skin test conversion, the emphasis is on identifying possible lapses in infection control rather than on trying more definitively to determine the likelihood that the conversion resulted from occupational exposure. The CDC guidelines place more emphasis on such an investigation, in part, so that public health authorities can be informed and take steps to prevent further exposures if a likely community source of a worker’s infection is identified.
Coordinating with Public Health Authorities
The CDC guidelines—reflecting the agency’s public health orientation—highlight the importance of coordination and cooperation with public health authorities. The proposed OSHA rule is silent on such coordination. Employers would still be governed by state laws that require reporting of infectious diseases including active tuberculosis. OSHA’s
silence on requirements imposed by other federal or state laws does not alter employers’ obligations to comply with these laws.
Record Keeping
The proposed OSHA rule includes a variety of record-keeping requirements related to employee medical records, medical surveillance, employee training, engineering controls, confidentiality, record availability and transfer, and other matters. Among other purposes, these records would assist OSHA inspectors with assessing employer compliance with regulations. Many of the record-keeping requirements are consistent with standard operating procedures in larger organizations but might require new procedures for smaller organizations.
Controls Related to Patient Management
Table 4-2 summarizes the provisions of the 1994 CDC guidelines that relate specifically to the development and application of procedures for identifying, diagnosing, and treating people with tuberculosis. Again, points of difference with the 1997 proposed OSHA rule are noted in italics.
Identification of Persons with Tuberculosis
The 1994 CDC guidelines stress the critical importance of early identification, isolation, and treatment of individuals who may have infectious tuberculosis. They recommend that institutions develop protocols for such identification based on the prevalence and characteristics of tuberculosis in the populations served by the institution (CDC, 1994b). The guidelines also list common symptoms of tuberculosis but note that the “index of suspicion” will vary depending on the characteristics of the population served.
The 1997 proposed OSHA rule would require employers to develop a written tuberculosis control plan that included procedures for the prompt identification of individuals with suspected or confirmed infectious tuberculosis. OSHA’s commentary on the proposed rule notes that procedures will likely vary for different employers. It does not discuss the prevalence of tuberculosis in the population served as a factor to be considered in developing or applying these procedures.
The CDC guidelines recommend that hospitals receive laboratory analyses of sputum smears within 24 hours. The proposed OSHA rule has no parallel requirement, and the introduction to the proposed rule does not discuss the issue.
TABLE 4-2. Summary of Patient Management Recommendations by CDC, with Notes (in italics) on How Proposed OSHA Rule Differs
|
Identifying Individuals with Suspected or Confirmed Infectious Tuberculosis |
|
|
|
NOTE: In general, the proposed OSHA rule provides less detailed specification of processes for identifying, diagnosing, and treating individuals with tuberculosis. |
|
1. |
Establishing protocols to identify those with symptoms or signs of active disease a. At initial encounters in emergency department, admitting area, outpatient clinic b. Before scheduled admissions if possible |
|
2. |
Assessing suspicious symptoms or test results after admission for patients not identified earlier |
|
3. |
Initiating precautions (e.g., isolation) for suspected or confirmed cases of infectious tuberculosis NOTE: The proposed OSHA rule provides for either masking or segregation of patients before isolation or transfer to another facility. Facilities that transfer rather than admit suspected or confirmed infectious patients would be required to arrange appropriate isolation or transfer within 5 hours. |
|
4. |
Evaluating patients with suspected cases of tuberculosis unless the institution’s policy is to refer cases a. Performing appropriate laboratory tests (including tests for drug resistance) b. Providing results of smear analyses within 24 hours of collection c. Performing appropriate diagnostic radiologic procedures |
|
Treating Patients with Suspected or Diagnosed Tuberculosis (unless the policy is to refer) |
|
|
1. |
Selecting treatment regimen (including use of inpatient or outpatient care) based on patient characteristics, test results, and preferences |
|
2. |
Monitoring response and making decisions about continued treatment, isolation, discharge, etc. |
|
3. |
Performing diagnostic and treatment procedures for infectious patients in the isolation room when possible and, when not possible, scheduling procedures at times when they can be performed quickly and when waiting areas are less crowded |
|
4. |
Delaying elective surgery and elective dental procedures until patient is confirmed to be noninfectious |
|
5. |
Avoiding cough-inducing procedures on infectious patients unless absolutely necessary and performing these procedures using local exhaust ventilation devices when possible NOTE: The proposed OSHA rule refers more broadly to “high-hazard” procedures, which are defined as those that may produce aerosols that contain tuberculosis bacteria. |
|
SOURCE: CDC (1994b), and 62 FR 201 (October 17, 1997). |
|
Patient Evaluation and Management
The proposed OSHA rule would require facilities that do not treat patients with tuberculosis to either isolate those with suspected or confirmed tuberculosis or transfer them within 5 hours. The CDC guidelines do not have a specific recommendation about how quickly transfer or isolation should occur. In its discussion of the proposed rule, OSHA describes the 5-hour provision as a preliminary determination, and it asks
for comments including suggestions about alternative means of protecting employees. The agency cites one study that showed that emergency departments—once a presumptive diagnosis of tuberculosis was made— “were able to initiate isolation in an average of 5 hours from the time of patient registration” (62 FR 201 at 54252 [October 17, 1997]). OSHA also notes statements by ATS (ATS, 1992, p. 1627) describing the use of surgical masks for longer periods (not defined) as “stigmatizing, uncomfortable, and probably ineffective.” In the discussion of the proposed rule, OSHA states that if isolation or transfer cannot be accomplished within 5 hours, it must be done as soon as possible thereafter.
COMPARISON OF GUIDELINES AND PROPOSED RULE: ENGINEERING CONTROLS
The term “engineering controls” applies to an array of protective measures based on engineering principles and technologies. In the context of programs to prevent the transmission of tuberculosis, the controls apply primarily to the design, creation, and maintenance of isolation rooms or areas (e.g., booths used for cough-inducing procedures) and to ventilation or air purification procedures for general-use areas such as emergency departments and admitting areas.
Table 4-3 summarizes the engineering controls that the 1994 CDC guidelines recommend for health care institutions that serve people with tuberculosis. The italicized comments highlight differences between the CDC guidelines and the proposed OSHA rule that might affect the effectiveness or the burdensomeness of a final rule. The first category in the table—managing facility ventilation—is also an administrative control, but the topic is included here for convenience. Neither the CDC guidelines nor the proposed OSHA rule would require engineering controls for home-based services.
Warning Signs
The proposed OSHA rule describes the specific shape and text for signs outside isolation rooms. The CDC guidelines are silent on this issue.
The proposed OSHA rule also includes more extensive requirements for warning signs and labels for ultraviolet germicidal irradiation lighting systems and for ventilation systems. The proposed rule does not discuss whether signs should, under certain circumstances, provide warnings in languages in addition to English. A rule of reason would suggest that the warnings must be readable by the people whom they are intended to warn. The CDC guidelines mention the language issue in the discussion of warning signs for ultraviolet germicidal irradiation lighting systems.
TABLE 4-3. Summary of Engineering Controls Recommended by CDC for Facilities That Serve People with Tuberculosis, with Notes (in italics) on How Proposed OSHA Rule Differs
|
Managing Facility Ventilation |
|
|
1. |
Ensuring that systems are regularly checked, maintained, and overseen by staff or consultants with appropriate engineering or other expertise |
|
2. |
Ensuring that systems meet applicable federal, state, and local requirements |
|
3. |
Providing appropriate warning signs for ventilation equipment and ultraviolet lighting systems NOTE: The proposed OSHA rule, in addition, sets forth shape and text requirements for warning signs in isolation areas and includes more precise requirements for labeling of vents than are provided for in the CDC guidelines. |
|
Ventilating General-Use Areas (e.g., waiting rooms, and emergency departments) |
|
|
1. |
Designing and operating systems to move air from cleaner to less clean areas |
|
2. |
In high-prevalence communities, providing supplementary controls such as ultraviolet germicidal irradiation (UVGI) lighting or HEPA filtration system. |
|
3. |
Providing local exhaust ventilation systems (e.g., booths, tents, and laboratory hoods) for areas where high-hazard procedures (e.g., bronchoscopy, administration of aerosolized medications, and sputum induction) are performed and ventilating them to achieve 99.9 percent removal of airborne contaminants NOTE: The proposed OSHA rule does not discuss ventilation requirements for general-use areas or for local exhaust ventilation systems. The proposed rule would not require supplementary UVGI systems for general-use areas in high-prevalence communities but does provide nonmandatory guidelines for their safe use. |
|
Providing and Maintaining Isolation Rooms |
|
|
1. |
Establishing air-change rates for in-use rooms of at least 6 per hour in existing facilities and 12 per hour in new or renovated facilities NOTE: The proposed OSHA rule would not require a specific number of air changes for isolation rooms. It would require ventilation to achieve 99.9 percent removal of airborne contaminants after an isolation room is vacated by a suspected or confirmed infectious patient. |
|
2. |
Directing fresh air first to areas used by workers and then to patients (preferred strategy) |
|
3. |
Maintaining negative pressure relative to hallways and other surrounding areas |
|
4. |
Exhausting air to outside away from public areas and air intake vents (or if not possible, using HEPA filtration for exhausted or recirculated air) |
|
5. |
Daily monitoring and periodic maintenance |
|
6. |
Keeping the number of persons entering isolation room minimal NOTE: The proposed OSHA rule calls specifically for minimizing the number of employees entering isolation rooms and the time that they spend there. |
|
SOURCES: CDC (1994b), and 62 FR 201 (October 17, 1997). |
|
Ventilation Requirements
General-Use Areas
The CDC guidelines include recommendations regarding ventilation of general-use areas. The proposed OSHA rule does not include ventilation requirements for these areas.
Isolation Rooms or Areas
The CDC guidelines recommend 6 to 12 air changes per hour for an isolation room while it is in use for an infectious patient.10 The proposed OSHA rule has no specific requirement for air changes. The commentary on the rule does not explain this departure from the CDC guidelines, nor does it ask for comments.
The proposed OSHA rule does discuss air changes in the context of a requirement for ventilation following the vacating of an isolation room by an infectious patient (see below). The proposed OSHA rule would require ventilation to achieve removal of 99.9 percent of airborne contaminants. An appendix to the proposed rule includes a CDC table on local exhaust ventilation that lists the air changes and minutes that are required to achieve different removal efficiencies (90, 99, and 99.9 percent).11
The CDC guidelines do not discuss ventilation requirements when an infectious patient has vacated an isolation room, but the guidelines do include a section on ventilation of isolation tents and booths (local exhaust devices). For these spaces, the CDC guidelines recommend at least 99 percent removal efficiency.
The CDC guidelines recommend that the number of persons entering isolation rooms be “minimal,” but they do not specifically mention employees. The proposed OSHA rule would require provisions in an employer’s exposure control plan to minimize the number of employees entering isolation rooms and the time that they spend there.
COMPARISON OF GUIDELINES AND PROPOSED RULE: PERSONAL RESPIRATORY PROTECTIONS
The term “personal respirator” applies generically to a range of devices that vary in complexity from flexible masks covering the nose and mouth to units that cover the wearer’s head and have independently powered air supplies. Appendix F discusses the types and functions of personal respirators and the evidence of their effectiveness. As noted earlier, personal respiratory protection comes third in CDC’s hierarchy of tuberculosis control measures.
In the sections on respiratory protections, the 1994 CDC guidelines mention the then-applicable 1987 OSHA regulations on respiratory pro-
tection programs. Those standards were revised in 1998 (29 CFR 110.134). However, pending issuance of the standard on occupational tuberculosis, OSHA has specified provisions from earlier versions of the standard that apply pending publication of the tuberculosis standard (29 CFR 1910.139).
The National Institute for Occupational Safety and Health (NIOSH) has legal responsibility or authority for certifying personal respirators for use in a wide variety of hazardous work situations including different kinds of mining operations, construction activities, and health care services. When the 1994 CDC guidelines were issued, they specified criteria for respirators that were, at that time, met by only one type of NIOSH-certified respirator. Since then, NIOSH which is a part of CDC, has certified a new class of less expensive and simpler devices (N95 respirators) that meet the 1994 criteria in most situations.
CDC’s recommendations related to personal respiratory protections are summarized in Table 4-4, which identifies in italics differences between the guidelines and the proposed OSHA rule. Again, the first category of recommendations relates to administrative measures, which are reviewed here for convenience.
Fit Testing and Fit Checking of Respirators
The 1994 CDC guidelines recommend that workers who wear respirators should undergo an initial fit test to identify an appropriately fitting respirator and that workers be taught to check the fit of the respirator before each use. The guidelines also state that facilities should have respirator protection programs that conform to the 1987 OSHA respiratory protection standard.
The 1987 OSHA respiratory protection standard did not require annual fit testing, but the 1998 revision of the standard added such a provision (29 CFR 1910.134). Pending publication of the final tuberculosis standard, the 1998 standard did not apply to the hazard of tuberculosis. OSHA’s interim requirements for tuberculosis did not require annual fit testing (29 CFR 1910.139).
The 1997 proposed OSHA rule would require at least annual assessment of a worker’s ability to wear a respirator. Unless this assessment determined that an annual fit test was not necessary, the rule would require at least an annual fit testing of respirators for most workers. OSHA asked for comments on whether an annual evaluation of the need for fit testing was adequate.
The 1997 Federal Register notice of the proposed OSHA rule takes over five pages (in small print) to describe the required fit testing procedures. These procedures are virtually identical to those described in the 1998 general respiratory protection standard.
The 1994 CDC guidelines do not describe the fit-testing process in detail but note that all facilities in which personal respirators are used
TABLE 4-4. Summary of Personal Respiratory Protections Recommended by CDC, with Notes (in italics) on How Proposed OSHA Rule Differs
|
Managing a Facility Respiratory Protection Program |
|
|
1. |
Assigning responsibility to a person with appropriate expertise and experience to oversee worker training, device selection and maintenance, worker adherence to respirator use requirements, and other program provisions NOTE: Assignment of responsibility to qualified individuals is implicit in the proposed OSHA rule. |
|
2. |
Ensuring that respirators meet criteria relating to such matters as a. Size of particles that respirator can filter under specific conditions b. Rate of leakage where the respirator seals to the face c. Ability to fit different sizes and kinds of faces (which usually means that some range of respirator sizes must be provided) d. Ability of respirator to be tested for fit to the face of a worker |
|
3. |
Including all workers who use respirators in the respiratory protection program that facilities are required by OSHA to develop, implement, and maintain (as described in a supplement to the guidelines) |
|
Identifying Workers Needing Respirators, Primarily |
|
|
1. |
Personnel who enter patient rooms or residences to provide medical, nursing, and other services to people with suspected or confirmed infectious tuberculosis |
|
2. |
Personnel who are present during procedures such as bronchoscopy during which patients are likely to expel M. tuberculosis-bearing particles into the air and who may require higher-performing devices NOTE: The proposed OSHA rule would not make provision of higher-performing respiratory devices a requirement for workers performing high-hazard procedures. |
|
3. |
Personnel in other settings where administrative and engineering controls are not likely to be protective (e.g., personnel repairing ventilation equipment) NOTE: The proposed OSHA rule also would require either masking of patients or the use of personal respirators in two situations: (1) when personnel are transporting patients with suspected or confirmed tuberculosis and (2) when personnel are working in areas where patients with known or suspected tuberculosis are placed while awaiting transfer. |
|
Providing Respirators and Ensuring Their Proper Use |
|
|
1. |
Screening workers—at hiring and periodically thereafter (at least every 5 years) — to determine whether any medical condition precludes use of a respirator NOTE: The CDC guidelines make no explicit recommendation for periodic fit testing of personal respirators, although they briefly describe some elements of fit-testing procedures. They also note that all employers who use respiratory protection are covered by the then-applicable OSHA respiratory protection standard. The 1997 proposed OSHA rule includes explicit provisions for: (1) at least an annual assessment of worker’s ability to wear a respirator, (2) at least an annual respirator fit test unless the preceding assessment determines that a fit test is not required, (3) an assessment whenever the size or make of a respirator used by a worker changes or the worker’s facial characteristics change in ways that might affect respirator fit, and (4) use of both qualitative and quantitative fit-testing procedures. |
|
2. |
Matching workers to appropriate respirators on the basis of physical characteristics, job requirements, etc. NOTE: The proposed OSHA rule would require that employers provide alternative work for personnel who cannot function adequately while using a respirator. |
|
3. |
Training workers in a device’s appropriate use (including checking the device’s fit at each use), inspection, maintenance, and storage |
|
4. |
Cleaning, repairing, and replacing respirators as appropriate |
|
5. |
Providing respirators to those who are visiting patients with tuberculosis in isolation rooms and instructing visitors in their use NOTE: The proposed OSHA rule does not mention visitors. |
|
SOURCES: CDC (1994b), and 62 FR 201 (October 17, 1997). |
|
must have a respiratory protection program as required by OSHA. The guidelines include a supplement that discusses considerations in selecting a respirator and developing a personal respiratory protection program. In addition to referring to OSHA regulations, the 1994 guidelines also refer to a 1987 NIOSH guide. NIOSH issued a new users guide for respirators in 1998 (NIOSH, 1999; see also NIOSH, 1995, 1996).
Respirator Use Outside Isolation Rooms
The 1997 proposed OSHA rule requires respirator use when workers are either transporting unmasked individuals with suspected or confirmed infectious tuberculosis or when they are working outside isolation rooms in areas where such unmasked individuals are confined (e.g., while awaiting transport to another facility). The CDC guidelines call for masking of patients in these situations, but they also provide more generally for the use of respirators “where administrative and engineering controls are not likely to protect them” (CDC, 1994b, p. 33). The proposed rule states that OSHA cannot require masking of patients and notes that some combative individuals may not accept masking. If a known or suspected infectious person cannot be masked, then the worker transporting him or her must have personal respiratory protection.12 In the latter situation (patients not masked), protection would not be provided to others who come near the patient (e.g., including workers, visitors, and other patients who share an elevator). The proposed rule has other provisions intended to protect such individuals, for example, the requirement that exposure control plans include policies to delay the moving of patients until they are no longer infectious unless a delay would compromise care.
Reflecting its broader perspective, the CDC guidelines stress that respiratory protections used by health care workers should protect both the worker and patients. For example, workers involved in surgical proce-
dures should not use a respirator (e.g., one with an expiration valve) that might contaminate the surgical field.
Situations Requiring More Protective Respirators
The CDC guidelines note that facilities may identify certain situations (e.g., bronchoscopies on patients with diagnosed or suspected infectious tuberculosis) that warrant respiratory protections that exceed those recommended by standard criteria. The proposed OSHA rule would not require employers to identify such situations or supply more protective personal respiratory devices. OSHA, however, requested comments on whether the final rule should include such requirements.
CONCLUSION
Because OSHA relied substantially on the 1994 CDC guidelines in developing the its 1997 proposed rule, the two documents are generally similar in their basic provisions. Some differences, such as those related to record keeping, are mainly administrative. Others, particularly OSHA’s proposed financial protections for workers temporarily removed from their position while undergoing treatment for active tuberculosis, reflect differences in organizational missions and responsibilities.
The final standard is likely to differ from the 1997 proposal but specific details were not available during the course of this study. In Chapter 7, the committee’s assessment of the likely effects of a final OSHA standard examines three areas of difference that could affect its impact. These areas involve tuberculin skin testing, respiratory protections, and methods for assessing facility risk for occupational transmission of tuberculosis and requirements for control measures.