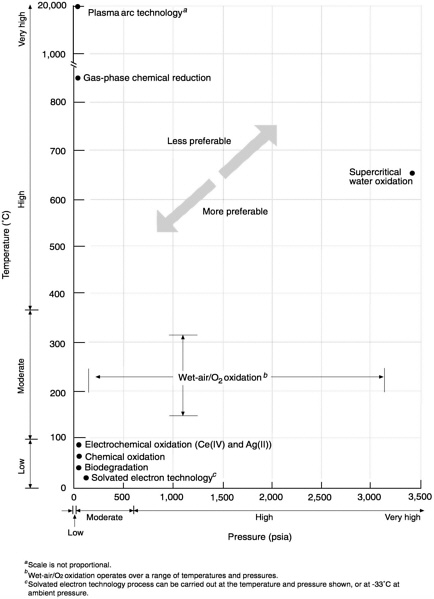
4
Descriptions and Evaluations of Technologies
The committee selected eight candidate technologies to evaluate: chemical oxidation, WAO, biodegradation, electrochemical oxidation [Ag(II) and Ce(IV)], SCWO, SET, plasma-arc technology, and GPCR. In this chapter, these technologies are described briefly (roughly in order of increasing operating temperature), evaluated, and ranked according to the criteria described in Chapter 3. None of these technologies has been tested on neutralents, and their effectiveness can only be estimated based on their use in similar applications. Experimental studies, including measurements of their destruction effectiveness on actual neutralents, will be necessary.
Each technology description is followed by tables representing the qualitative assessment of individual committee members assigned to investigate that technology based on their expertise. The committee took these qualitative assessments into account in its overall ranking of technologies.
CHEMICAL OXIDATION
On balance, chemical oxidation is a promising technology for mineralizing (i.e., converting organic compounds to inorganic salts, water, and carbon dioxide) RRS and MMD neutralents and for converting other components to less toxic materials. Experimental studies will be necessary to verify its effectiveness.
Description
Hydrogen peroxide, potassium permanganate, OxoneTM, 1 peroxydisulfate, and ultraviolet (UV)-activated hydrogen peroxide or ozone oxidation are all viable oxidants for the treatment of nonstockpile neutralents. 2 Under appropriate operating conditions and with sufficient reagent, the organic compounds present in neutralents can be expected to be mineralized with any of these oxidants. 3
For chemical oxidation not activated by UV light, conventional process equipment and procedures are used. The reactions are carried out at 80°C to 100°C at atmospheric pressure in aqueous solutions. When an organic phase is present, vigorous agitation is necessary to suspend and disperse the organic materials in the aqueous phase.
Processes employing UV activation 4 require special equipment. The solution containing the material to be oxidized must be pumped past a quartz tube containing a UV lamp to expose it to UV radiation (the solution must be transparent to UV radiation). If ozone is used, it must be generated in an ozone generator. The oxidation system is usually operated semicontinuously (i.e., a large batch of feed is prepared in a feed tank, pumped past the UV source, and returned to the feed tank). This operation is continued until the desired degree of oxidation is obtained. The contents of the feed tank are then discharged.
Evaluation
Chemical oxidation is a simple, well established industrial process that uses standard equipment under relatively mild conditions. The only gas evolved is carbon dioxide, and the aqueous reaction products can be evaporated to leave inorganic salts that can be stabilized and sent to a landfill. Because all reagents would be in aqueous solutions at
|
1 |
Oxone, a registered trademark of DuPont Specialty Chemicals, is a triple salt (2KHSO5.KHSO4.K2SO4). The active component is KHSO5, the potassium salt of monoperoxysulfuric acid (Cooper et al., 1999; DuPont Specialty Chemicals, 1992; Mikolajczyk, 1996). |
|
2 |
Palladium and other catalysts can also facilitate chemical oxidation. |
|
3 |
Oxidation with hypochlorite has been studied but does not appear to be as effective (Soilleaux, 1998). |
|
4 |
UV radiation is capable of decomposing ozone or hydrogen peroxide to form hydroxyl radicals that can oxidize most organic compounds (Holm, 1998). The hydroxyl radical has a high oxidation potential exceeded only by fluorine. |
ambient pressure and below 100°C, dioxins and furans are not formed.
The cost of reagent is expected to be relatively high. Several pounds of reagent may be necessary to mineralize one pound of neutralent. However, because the total volume of neutralent will not be large, the cost of reagent is not expected to be an important consideration.
Because UV light cannot penetrate an opaque solution, the opacity of the feed will have to be considered for processes that include UV activation. Fouling and periodic cleaning of optical surfaces are design and operating considerations.
The biggest potential disadvantage of chemical oxidation is that it may not fully mineralize all of the compounds in the neutralents or that it may not mineralize them rapidly enough to be practical. This question can only be resolved through further research. The committee was not aware of any direct experience with the mineralization of neutralent by chemical oxidants. However, the oxidation or mineralization of closely related materials, including mustard, nerve agents, and their hydrolysates, has been documented. Laboratory-scale studies at the Army's Edgewood Research, Development and Engineering Center on the reaction of VX, GB, GD (soman), and
TABLE 4-1a Chemical Oxidation: Top Priority Criteria
|
Criterion |
Rating |
|
Technical Effectiveness |
|
|
Integral part of a coherent system |
Good. All or parts of this technology could easily be integrated with existing nonstockpile treatment technologies. |
|
Destruction efficiency |
Potentially good, but must be verified by experiment. Complete mineralization if large quantities of chemical oxidant are used under correct conditions. |
|
Inherent Safety |
|
|
Minimal storage and transportation of hazardous material |
Good. Oxidizing agents must be transported and stored. Storage and transportation are routine. |
|
Minimal toxicity and flammability (process materials) |
Good. No highly toxic or flammable materials used. |
|
Containable temperature and pressures |
Fair. For hydrogen peroxide, conditions that minimize opportunities for decomposition must be used. The stability of hydrogen peroxide-containing reaction liquors depends on the concentration of the hydrogen peroxide, the temperature, and the materials present. Experimental studies will be necessary to determine the highest concentrations of hydrogen peroxide that can be safely employed. |
|
Pollution Prevention |
|
|
Minimum toxicity of effluents |
Good. Organic compounds can be effectively and rapidly destroyed. Under adequate conditions, nontoxic products, up to and including products of mineralization, can be produced. For hydrogen peroxide, this may require up to 3.5 pounds of peroxide per pound of organic compound. Chloroform can be mineralized. |
|
Minimal use of processing materials |
Poor. Large volumes of reagent may be necessary for oxidation, and large volumes of cement may be necessary for stabilization of residues. |
|
Solids, liquids, and gaseous wastes |
Fair. Carbon dioxide will be produced if mineralization is accomplished. A liquid waste stream will be produced, which can be converted to a solid via stabilization. No data have been generated on this topic, and treatability studies will be necessary. |
|
Minimal number of processing steps |
Good. May be a one-step or two-step process. Oxidation with or without stabilization is part of the process. |
|
Temperatures, pressures, corrosion, plugging, and other operating difficulties minimized to prevent unprogrammed shutdowns |
Good. Temperature and pressure are moderate. For hydrogen peroxide, conditions must be moderate. |
|
Permit Status |
|
|
Allowed by regulations and capable of meeting schedules imposed by records of decision and treaties |
Good. Permitting should be relatively easy. |
mustard with hydrogen peroxide and peroxydisulfate had very favorable results (Hovanek et al., 1993; Yang, 1995, 1999). Using peroxydisulfate,VX was mineralized to carbon dioxide, nitrate, sulfate, and phosphate.
The use of hydrogen peroxide or Fenton's reagent was a key feature of the technology developed by ARCTECH and tested on hydrolysates for the ACWA Program. The procedures were shown to be effective at the bench scale for hydrolysates of VX, GB, and mustard. However, the ARCTECH technology was judged not to meet the demonstration selection criteria of the ACWA Program (see NRC, 1999b).
A chemical destruction process that uses base hydrolysis and oxidation with peroxydisulfate salts to mineralize chlorinated and other organic compounds has been developed at the Lawrence Livermore National Laboratory (Lawrence Livermore National Laboratory, 2000). Peroxidisulfate oxidation of MMD neutralents was proposed by Teledyne-Commodore in response to the Commerce Business Daily announcement promulgated by Stone and Webster. This process is likely to require a large excess of peroxydisulfate, leading to the formation of large quantifies of sulfate in the waste stream (Yang, 1995).
UV/hydrogen peroxide oxidation (followed by biodegradation) was a feature of the Parsons/AlliedSignal technology for nerve agents demonstrated (unsuccessfully) for the ACWA Program (NRC, 1999b, 2000a). The combination of biological and UV/hydrogen peroxide treatment was able to achieve only 40 to 60 percent destruction of Schedule 2 compounds from GB hydrolysate, somewhat more for VX hydrolysate. The poor performance of the UV/hydrogen peroxide was attributed to the black color of the waste stream. The Gas Research Institute has conducted extensive studies on UV-enhanced ozone-based or hydrogen peroxidebased chemical oxidation of organic compounds associated with former gas plant sites (GRI, 2000).
Overall, chemical oxidation is a good candidate for treating neutralents because of its technical effectiveness, its good pollution-prevention qualities, its robustness, and its low cost. The technology can be easily integrated into existing nonstockpile treatment systems and is commercially used to treat other waste streams. Organic compounds can be destroyed at low temperature and pressure, and toxic emissions are minimal because no large gas streams, such as those encountered in combustion processes or in GPCR, are involved. Formation of chlorodibenzodioxins and chloro-dibenzofurans is precluded because of the low temperatures. Based on commercial experience with chemical oxidation technology, capital and operating costs are expected to be moderate.
The ratings for chemical oxidation are summarized in Table 4-1a and Table 4-1b .
TABLE 4-1b Chemical Oxidation: Important Criteria
|
Criterion |
Rating |
|
Robustness |
|
|
Stability and continuity of operation |
Good, although chemical oxidation remains to be demonstrated. Stabilization of solids is not expected to cause problems. |
|
Cost |
|
|
Minimal total costs |
Good. Capital and operating costs are expected to be moderate. |
|
Practical Operability |
|
|
Minimal training time |
Good. Operations are conventional, and training should be similar to training for workers in chemical plants. |
|
Continuity |
|
|
Vendor likely to remain in operation and raw materials likely to remain available |
Unknown. No specific vendor. |
|
Space Efficiency |
|
|
Minimal weight, area, and volume of operating equipment |
Fair. Equipment, although conventional, may be large. |
|
Materials Efficiency |
|
|
Use of internally recyclable materials |
Unknown. |
|
Beneficial reuse of wastes |
Fair. Metals could be recycled, but not other residues. |
|
Use of externally recyclable materials |
Not applicable. |
ELECTROCHEMICAL OXIDATION
The committee considered two forms of electrochemical oxidation, the silver Ag(II) process and the cerium Ce(IV) process (the “CerOx ” process). The Ag(II) process has been advanced as a candidate for treating assembled chemical weapons but is probably not suitable for treatment of RRS neutralents because of their high chlorine content. Although Ag(II) and Ce(IV) are more potent oxidizers than the chemical oxidants discussed above, electrochemical processes are less desirable for treating neutralent wastes because they generate large quantities of hazardous effluents and because corrosive effluents could cause operating problems.
Ag(II) Process
Description
This process has been patented for oxidizing organic wastes using Ag(II), an unstable form of silver and one of the strongest oxidizing agents known. Any carbon in the waste stream is completely oxidized to carbon dioxide with traces of carbon monoxide. Other elements end up as salts (e.g., fluorines to fluorides, sulfur to sulfates). Chlorine precipitates out with the silver as silver chloride. The process is operated at 90°C and at atmospheric pressure.
A solution of silver nitrate in 8-molar nitric acid is electrolyzed to produce the Ag(II) cations at the anode of a commercially available electrochemical cell. A semi-permeable membrane separates the anode and the cathode compartments of the cell to prevent mixing of the anolyte and catholyte solutions but allowing the passage of cations and water across the membrane.
The anolyte and catholyte solutions form two separate recirculating loops. The anolyte solution is circulated through the reaction vessel into which the organic wastes are introduced. Solids formed in the anolyte loop are removed by a hydrocyclone. In the cathode loop, the nitric acid is reduced to nitrous acid and water. This solution is passed through a nitrogen oxide reformer to regenerate nitric acid. Off-gases are passed through a scrubber. If no chlorine is present, the silver ions are recovered and recycled to the anolyte loop.
Evaluation
Ag(II) is expected to be an effective oxidizing agent for destruction of MMD neutralent. However, the large quantities of chloroform present in the RRS neutralent would result in the formation of large quantities of silver chloride, which would probably plug up the electrochemical cells.
The Ag(II) process also has several disadvantages. First, large quantities of concentrated nitric acid, which is extremely corrosive and a strong oxidizer, are required. Second, significant amounts of silver nitrate must be added (although, in principle, the silver is recovered in a recycling step), and silver is an expensive and regulated metal. Third, the large quantities of nitrogen oxides generated at the cathode must be reformed back to nitric acid, and waste gases must be scrubbed.
The Ag(II) process has been evaluated by the ACWA Program as an alternative technology for the disposal of assembled chemical weapons. The process was demonstrated with nerve agents, but not with mustard, at Porton Down in Great Britain. Two units have been constructed for the ACWA Demo II tests that were completed in September 2000—a small unit was tested with the chemical agent, and a large unit was tested with energetic materials. The results of these tests should be of interest to the NSCMP because either unit would be large enough to treat all of the neutralent generated from the MMD. The results of the tests were not available at the time this report was written.
The Ag(II) process as evaluated by the committee's criteria has both advantages and disadvantages. It has the technical capability to treat neutralent from the MMD but may be ineffective in treating neutralent from the RRS because of the large quantities of chloroform. The Ag(II) process has good space efficiency and stable continuous operation with reasonable controls, and the silver can be recycled. The major disadvantage for RRS neutralent is that large quantities of silver salts and chlorides are generated, which could lead to problems with corrosion and precipitation. Another disadvantage is that large quantities of silver (a toxic heavy metal) and nitric acid (a corrosive) are required for the operation of this technology, which could increase toxic emissions and effluents.
The ratings for electrochemical oxidation Ag (II) are summarized in Table 4-2a and Table 4-2b .
TABLE 4-2a Electrochemical Oxidation Ag(II): Top Priority Criteria
|
Criterion |
Rating |
|
Technical Effectiveness |
|
|
Integral part of a coherent system |
Fair. This process has not been interfaced with the neutralent process and has not been used to treat neutralent. The process is more attractive for treating MMD wastes than RRS wastes because of the high chlorine content in the latter. |
|
Destruction efficiency |
Good. Ag(II) has one of the highest known oxidation potentials. |
|
Inherent Safety |
|
|
Minimal storage and transportation of hazardous material |
Fair. Nitric acid is a hazardous material that is transported routinely. Large quantities of silver nitrate may be required. |
|
Minimal toxicity and flammability (process materials) |
Poor. Silver is a toxic heavy metal; nitric acid is highly corrosive. |
|
Containable temperature and pressures |
Excellent. Moderate temperatures (90°C), and pressures allow reliable containment. |
|
Pollution Prevention |
|
|
Minimum toxicity of effluents |
Good. Organic compounds can be effectively and rapidly destroyed. Under adequate conditions, nontoxic products, up to and including products of mineralization, can be produced. Chloroform can be mineralized. |
|
Minimal use of processing materials |
Fair. Large quantities of nitric acid and AgII are required. |
|
Solids, liquids, and gaseous wastes |
Poor. Large quantities of silver nitrate and nitric acid are required. |
|
Minimal number of processing steps |
Good. Small number of steps. |
|
Temperatures, pressures, corrosion, plugging, and other operating difficulties minimized to prevent unprogrammed shutdowns |
Fair. The system must be glass lined. Joints will have leakage and corrosion problems. Corrosion and precipitation could be serious problems, leading to plugging of the electrochemical cells. |
|
Permit Status |
|
|
Allowed by regulations and capable of meeting schedules imposed by records of decision and treaties |
Not known. Large quantities of silver salts, including silver chloride, will be produced, although the vendor intends to recycle all of the silver. Silver II has been operated at pilot scale at Dounreay and Porton Down, United Kingdom. There is no information as to whether or not it has been permitted as a full-scale facility. |
TABLE 4-2b Electrochemical Oxidation Ag(II): Important Criteria
|
Criterion |
Rating |
|
Robustness |
|
|
Stability and continuity of operation |
Fair. |
|
Cost |
|
|
Minimal total costs |
Fair. Requires an inventory of silver in the form of silver nitrate. The silver can be reconstituted by an outside firm. |
|
Practical Operability |
|
|
Minimal training time |
Fair. Training time is likely to be substantial. |
|
Continuity |
|
|
Vendor likely to remain in operation and raw materials likely to remain available |
Good. |
|
Space Efficiency |
|
|
Minimal weight, area, and volume of operating equipment |
Fair. Equipment, although conventional, may be large. |
|
Materials Efficiency |
|
|
Use of internally recyclable materials |
Good. Modular system was demonstrated at Aberdeen in spring 2000. Data are not yet available. |
|
Beneficial reuse of wastes |
Good. Silver will be recycled. |
|
Use of extemally recyclable materials |
Not applicable. |
CerOx Process
Description
The CerOx process is similar to the Ag(II) process except that it uses 0.8M Ce(IV) solution in 3-molar nitric acid at 100°C to oxidize and destroy organic compounds. Unlike Ag(II), Ce(IV) is stable. The Ce(IV) is produced and regenerated by the electrolysis of Ce(III) in a bipolar electro-chemical cell, which the vendor calls a “T-cell. ”
The system has two circulating loops, one for the anolyte solution and one for the catholyte solution. In the anolyte loops, Ce(III) is oxidized to Ce (IV) in the T-cell and passed through the reaction chamber where the organic wastes are introduced gradually. Carbon is converted to carbon dioxide; chlorine compounds are converted to elemental chlorine, which is scrubbed and converted to hypochlorite; sulfur and other elements are converted to salts, such as sulfates. These salts remain in anolyte solution, which must be periodically replaced as the concentration of the salts increases.
The catholyte loop provides the second electrode for the electrolysis. The nitric acid in this loop is reduced to nitrous acid and then reformed back to nitric acid and nitric oxide. Water is produced in the process, but much of it is removed by evaporation because the operating temperature is very close to the boiling point (100 °C).
The CerOx process uses very few reactants, principally nitrate (which is recycled), nitric acid, and sodium hydroxide scrubbers to treat off-gases. The biggest cost is for electrical power to operate the electrolysis T-cells.
Evaluation
The CerOx process avoids some, but not all, of the deficiencies of the Ag(II) process. Cerium is much cheaper than silver and much less toxic, and its release to the environment is not as strictly regulated. Unlike the Ag(II) process, the CerOx process could potentially be used to treat both RRS and MMD neutralents, although the high concentration of chlorine in RRS neutralent would result in the formation of large amounts of toxic chlorine gas that would have to be scrubbed. Like the Ag(II) process, CerOx uses large quantities of corrosive nitric acid and generates large quantities of nitrogen oxides at the cathode, which must be reformed and the waste gases scrubbed. Finally, CerOx is not as mature a technology as Ag(II) and would require a larger investment for further development.
The CerOx process was initially developed by the Lawrence Livermore National Laboratory and patented by
the Battelle Pacific Northwest Laboratory. Recently, it has been licensed to CerOx Corporation, which has constructed and is operating a small unit at the University of Nevada in Reno for the destruction of laboratory organic wastes. The unit is designed to treat one 35-gallon barrel of waste at a time.
During a visit to the Reno facility, the committee noted that no provisions were being made for the separation of inorganic salts that accumulate in the anolyte solution. When the salts become too concentrated, the solution must be replaced so they do not plug up the T-cell.
Although the process has been demonstrated with a variety of different wastes, the ownership of the unit has still not been transferred to the university; thus the process remains under development and ownership of the vendor. No doubt Ce(IV) can destroy many common organic wastes, such as methylene chloride. However, the process has never been tested with any of the neutralents. Based on the process chemistry of the unit and discussions with the operator, the committee believes the unit at Reno would be adequate in size to treat all of the neutralents from the MMD and RRS.
In summary, the Ce(IV) process has the potential to treat RRS and MMD neutralents with fewer disadvantages than the Ag(II) process. The Ce(IV) process uses a less toxic, cheaper, and not strictly regulated substance (cerium) and operates at low temperature and pressure. The process has some disadvantages. Large amounts of nitric acid (a corrosive) are used, and large amounts of chlorine gas are generated from the process. Therefore, in terms of the pollution-prevention criteria, the Ce(IV) process was rated poor. However, chlorine gases could be scrubbed using pollution-control equipment. The most serious disadvantage is that the technology is not mature enough for immediate use.
The ratings for electrochemical oxidation Ce(IV) are summarized in Table 4-3a and Table 4-3b .
TABLE 4-3a Electrochemical Oxidation Ce(IV): Top Priority Criteria
|
Criterion |
Rating |
|
Technical Effectiveness |
|
|
Integral part of a coherent system |
Fair. Process has not been interfaced with the neutralent process and has not been used to treat neutralent. |
|
Destruction efficiency |
Good. |
|
Inherent Safety |
|
|
Minimal storage and transportation of hazardous material |
Good. Nitric acid is a hazardous material that is transported routinely. |
|
Minimal toxicity and flammability (process materials) |
Good. Cerium is much less toxic than silver. |
|
Containable temperature and pressures |
Excellent. Moderate temperatures (90°C) and pressures allow reliable containment. |
|
Pollution Prevention |
|
|
Minimum toxicity of effluents |
Probably good. Carbon is oxidized to carbon dioxide, hydrogen to water, and nitrogen to either nitrogen oxide (scrubbed) or elemental nitrogen. Sulfur is converted to sodium sulfate. Chlorine is converted to Cl2, which is scrubbed. |
|
Minimal use of processing materials |
Fair. Large quantities of nitric acid and Ce(IV) are required. |
|
Solids, liquids, and gaseous wastes |
Poor. Large volumes of gaseous effluents will require scrubbing. Large quantities of water remain. |
|
Minimal number of processing steps |
Good. Small number of steps. |
|
Temperatures, pressures, corrosion, plugging, and other operating difficulties minimized to prevent unprogrammed shutdowns |
Fair. The system must be glass lined. Joints will have leakage and corrosion problems. Corrosion could lead to plugging of the electrochemical cells. The design of the cell is not known at this time. |
|
Permit Status |
|
|
Allowed by regulations and capable of meeting schedules imposed by records of decision and treaties |
Not known. Probably OK because release of cerium to the environment is not regulated. |
Table 4-3b Electrochemical Oxidation Ce(IV) Process: Important Criteria
|
Criterion |
Rating |
|
Robustness |
|
|
Stability and continuity of operation |
Good. |
|
Cost |
|
|
Minimal total costs |
Good. Cerium is much cheaper than silver. |
|
Practical Operability |
|
|
Minimal training time |
Fair. Training time likely to be substantial. |
|
Continuity |
|
|
Vendor likely to remain in operation and raw materials likely to remain available |
Fair. Technology provider (CerOx Corporation) is a small company that licenses the process. |
|
Space Efficiency |
|
|
Minimal weight, area, and volume of operating equipment |
Good. System for demonstration, which is modular, was demonstrated at Aberdeen in spring 2000. Data are not yet available. |
|
Materials Efficiency |
|
|
Use of internally recyclable materials |
Good. Nitric acid is recycled. |
|
Beneficial reuse of wastes |
Not applicable. |
|
Use of externally recyclable materials |
Not applicable. |
BIODEGRADATION
Biodegradation is not a feasible treatment method for RRS neutralents because chloroform, which is present in high concentrations, is highly resistant to oxidation by this method. As a treatment method for MMD neutralents, biodegradation is also doubtful for a number of reasons, as discussed below.
Description
Biotreatment processes use microorganisms to destroy certain organic compounds in dilute aqueous solutions. Aerobic processes result in the partial or total oxidation of neutralent compounds, although the structures of some compounds render them highly resistant. Oxygen is supplied, usually as air that is sparged into the reactor. Nutrients, such as nitrogen in the form of an ammonium salt, and a carbon source, such as dextrose, are often added.
Evaluation
Biodegradation is generally perceived to be natural and, therefore, a publicly acceptable approach to the destruction of wastes. The operating temperature is near ambient, precluding the formation of chlorinated dioxins and furans. Biodegradation is used to treat sewage in many communities, and the safety and reliability of this technology are taken for granted. The U.S. Army is currently testing chemical hydrolysis as a method of destroying chemical agents (HD mustard) at Aberdeen, Maryland, and VX nerve agent at Newport, Indiana. (The results of the tests were not available at the time this report was written.) The hydrolysate resulting from the treatment of HD mustard agent, which contains thiodiglycol and sodium chloride as the primary reaction products, will be treated at Aberdeen using biodegradation technology. The VX hydrolysate, which contains thiol amine and methyl phosphonic acid as the primary reaction products, is not readily amenable to treatment by biodegradation (NRC, 2000b).
A recent evaluation by industry experts indicates that the biodegradability of several compounds present in RRS and MMD neutralents, such as chloroform and hydantoins (major components of RRS waste streams), are extremely resistant to biotreatment (Dekleva and Gannon, 2000). Other compounds that are present at lower concentrations, such as 1,1,2-trichloroethane and 1,1,1,2-tetrachloroethane, are also
very resistant to biotreatment. Because the process also requires that microbes be acclimatized for each composition in the waste streams, the quantities of microbes may not be sufficient to accomplish this task. Thus, biotreatment is not a promising treatment for RRS waste streams.
MMD waste streams may be more amenable to biotreatment although they would have to be diluted at least 100-fold with water, and the carbon-nitrogen-phosphorus ratios would have to be adjusted. However, these streams also contain low levels of chloroform, hexachlorobenzene, and hexachlorobutadiene, all of which are known or expected to be resistant to biotreatment. Thus, the treatment of MMD neutralent by biodegradation is not likely.
The ratings for biodegredation are summarized in Table 4-4a and Table 4-4b .
TABLE 4-4a Biodegradation: Top Priority Criteria
|
Criterion |
Rating |
|
Technical Effectiveness |
|
|
Integral part of a coherent system |
Poor. The biological treatment system cannot be easily transported. |
|
Destruction efficiency |
Poor. Not usable for RRS neutralent; destruction efficiency rarely exceeds 90 percent. |
|
Inherent Safety |
|
|
Minimal storage and transportation of hazardous material |
Good. Nutrients and other raw materials for the process are not expected to be hazardous. If the solid products are hazardous, they will be rendered nonhazardous before release from the site. |
|
Minimal toxicity and flammability (process materials) |
Good. No highly toxic or flammable materials used. |
|
Containable temperature and pressures |
Excellent. Operating temperatures are near ambient, and the pressure is near atmospheric. |
|
Pollution Prevention |
|
|
Minimal toxicity of effluents |
Fair. A catalytic oxidizer (catox) may be necessary to destroy organic compounds in the large air stream that passes through the bioreactor. Past studies have shown that measurable amounts of chlorodibenzodioxins and chlorodibenzofurans are formed in the catox, if employed for off-gas treatment. |
|
Minimal use of processing materials |
Poor. Very large processing material streams, such as carbon source for bioreactor, are necessary. |
|
Solids, liquids, and gaseous wastes |
Poor. Large volumes of gases and large volumes of sludge and salts are necessary. |
|
Minimal number of processing steps |
Poor. Numerous unit operations are required to remove reaction products from the bioreactor effluent and recycle the water. |
|
Temperatures, pressures, corrosion, plugging, and other operating difficulties minimized to prevent unprogrammed shutdowns |
Good. Mild conditions should lead to minimal unprogrammed shutdowns. |
|
Permit Status |
|
|
Allowed by regulations and capable of meeting schedules imposed by records of decision and treaties |
Fair. If catox system is employed for treatment of off-gas. Excellent otherwise. |
TABLE 4-4b Biodegradation: Important Criteria
|
Criterion |
Rating |
|
Robustness |
|
|
Stability and continuity of operation |
Fair. Many unresolved issues for biotreatment process. |
|
Cost |
|
|
Minimal total costs |
Fair. Capital and operating costs are expected to be high. Transportation and set-up costs will also be high. |
|
Practical Operability |
|
|
Minimal training time |
Good. Operations are conventional, and training should be similar to training for typical chemical plants. |
|
Continuity |
|
|
Vendor likely to remain in operation and raw materials likely to remain available |
Good. The company that offers the immobilized cell bioreactor technology (the biological technology of most interest) operates the technology commercially and is large and stable. The anticipated nutrients and other raw materials are commodities and are expected to be commonly available. |
|
Space Efficiency |
|
|
Minimum weight, area, and volume of operating equipment |
Poor. Equipment, although conventional, may be very large. The weight and volume will be high, and the footprint will be large. |
|
Materials Efficiency |
|
|
Use of internally recyclable materials |
Fair. Some reaction liquor can be internally recycled. |
|
Beneficial reuse of wastes |
Poor. Metals, if any, could be recycled, but not other residues. |
|
Use of externally recyclable materials |
Not applicable. |
SOLVATED-ELECTRON TECHNOLOGY
SET would be an inappropriate technology for the treatment of MMD neutralents, which have a high water content. The sodium reagent used for the operation of SET would react with water causing a release of hydrogen gas and requiring excessive quantities of reagent for further treatment. SET could potentially be used to treat RRS neutralents with very little, if any, water.
Description
SET involves the reaction of organic compounds with solutions of metallic sodium in anhydrous liquid ammonia. When sodium is dissolved in liquid ammonia, it forms sodium cations. The electrons released in the process are solvated by ammonia and are highly mobile in the solution. Teledyne-Commodore has proposed using SET for the treatment of assembled chemical weapons and also as a treatment technology for RRS neutralents in response to Stone & Webster's Commerce Business Daily announcement.
Whereas most of the technologies discussed in this report are oxidation processes, SET is a chemical reduction process (the only other reduction process is GPCR, described below). The SET process can be carried out at −33°C, the boiling point of liquid ammonia, and at atmospheric pressure, or at ambient temperature and slightly elevated pressures (125 psia to 182 psia).
In general, solvated electrons are attracted to the covalent bond between carbon and a more electronegative species, such as chlorine, fluorine, phosphorus, sulfur, or oxygen. The result is a rupture of molecular bonds and some molecular reorganization leading to a complex mixture of chemical species.
After the reduction process has gone to completion, as indicated by persistence of the intense bright blue color of the SET solution, the reaction products are hydrolyzed. Hydrolysis destroys the excess sodium with the release of hydrogen and brings about other reactions that are not yet fully understood.
Evaluation
SET has the advantage of operating at low temperatures and low to moderate pressures, although the engineering advantages may be outweighed by the difficulty of handling anhydrous ammonia and sodium metal, both of which are
TABLE 4-5a Solvated-Electron Technology: Top Priority Criteria
|
Criterion |
Rating |
|
Technical Effectiveness |
|
|
Integral part of a coherent system |
Fair. Not suitable for MMD neutralent and not tested for RRS neutralent. |
|
Destruction efficiency |
Fair. Not usable for aqueous waste stream of the MMD. |
|
Inherent Safety |
|
|
Minimal storage and transportation of hazardous material |
Fair. Uses liquid ammonia and metallic sodium. |
|
Minimal toxicity and flammability (process materials) |
Poor. Ammonia is toxic; both ammonia and metallic sodium have caused fires. |
|
Containable temperature and pressures |
Good. Operates at ambient temperature and slightly elevated pressures that are easy to control. |
|
Pollution Prevention |
|
|
Minimum toxicity effluents |
Fair. Gases released from the SET process and subsequent hydrolysis are mainly hydrogen, ethane, and ethylene. The liquid product contains complex organics, which must be further treated by oxidation. The final oxidation products are suitable for landfill. |
|
Minimal use of processing materials |
Fair. Requires metallic sodium and anhydrous liquid ammonia; both present handling challenges. |
|
Solids, liquids, and gaseous wastes |
Good. Hold-test-release systems are used for effluent gases, and the likelihood of explosion is very low. Aqueous effluents can be treated with caustic or hypochlorite. Solid wastes can be stabilized in cement and disposed of in a landfill. |
|
Minimal number of processing steps |
Fair. Processing steps include SET, hydrolysis, oxidation, solidification/stabilization, and ammonia recovery. |
|
Temperatures, pressures, corrosion, plugging, and other operating difficulties minimized to prevent unprogrammed shutdowns |
Poor. Optimum conditions not well defined; final oxidation step not well understood. |
|
Permit Status |
|
|
Allowed by regulations and capable of meeting schedules imposed by records of decision and treaties |
Fair. Would require a Subtitle X permit and a great deal more work. |
toxic and have been known to cause fires. Based on tests conducted by Teledyne-Commodore, SET is very effective with highly halogenated materials and, therefore, may be able to mineralize the main constituents of RRS neutralents. However, the agent breakdown products are likely to be transformed into residual organic compounds that will require additional treatment (Mitretek, 1999). In addition, SET process efficiency is poor when treating process aqueous waste streams, such as MMD neutralents.
SET is also less mature than some of the other treatment technologies considered by the committee. The optimum operating conditions have not been defined, and the final hydrolysis step is not fully understood. The treatment steps for the vapor streams have not been defined. SET also requires a Subtitle X permit,5 which would require that uncertainties about its operation be resolved.
The ratings for electron technology are summarized in Table 4-5a and Table 4-5b .
|
5 |
Both liquid/gaseous ammonia and metallic sodium are widely used in industry, but not for treatment of hazardous wastes. A Subtitle X permit is required under RCRA for processes that treat wastes regulated as hazardous in nonstandard ways. (Standard treatment processes, such as incineration and tank treatment, are permitted under other RCRA subtitles.) |
TABLE 4-5b Solvated-Electron Technology: Important Criteria
|
Criterion |
Rating |
|
Robustness |
|
|
Stability and continuity of operation |
Poor. Many unresolved issues with respect to the final oxidation step. |
|
Cost |
|
|
Minimal total costs |
Fair. Capital and operating costs are likely to be high. Materials cost are relatively low. |
|
Practical Operability |
|
|
Minimal training time |
Poor. The process is unusual and, therefore, will require specialized training of personnel. |
|
Continuity |
|
|
Vendor likely to remain in operation and raw materials likely to remain available |
Excellent. Teledyne Brown Engineering has been in business since 1953 and is a division of Teledyne Technologies, an $800-million publicly traded company. Commodore Applied Technologies is also publicly traded but is much smaller and less financially stable. Supplies, mainly sodium and ammonia, are readily available commodities. |
|
Space Efficiency |
|
|
Minimal weight, area, and volume of operating equipment |
Poor. While the equipment may be transportable, not enough is known to characterize the system as space efficient. |
|
Materials Efficiency |
|
|
Use of internally recyclable materials |
Good. Ammonia is recovered. |
|
Beneficial reuse of wastes |
Fair. Possibility of recovery of energy from off-gases. |
|
Use of externally recyclable materials |
Fair. The ammonia is recovered for recycling. |
WET-AIR/O2 OXIDATION
WAO is a promising treatment for both RRS and MMD neutralents. WAO operates under slightly more aggressive temperature and pressure conditions than chemical oxidation processes. The process is used commercially and has an established track record with compounds similar to those found in neutralents.
Description
The WAO process oxidizes and hydrolyzes organic contaminants in water at temperatures of 150°C to 315°C and pressures of 150 psia to 3150 psia, below the critical temperature of water and pressure (374°C and 3,204 psia). If pure oxygen is used instead of air as the oxidizing agent, the gas volumes that must be managed are greatly reduced.
Organic compounds containing carbon, hydrogen, and oxygen are converted to carbon dioxide, water, and short-chain, biodegradable compounds, such as acetic acid and formaldehyde. Depending on reaction conditions, further biotreatment of residues may be necessary. Toxic heavy metals in the neutralent would have to be precipitated and filtered out prior to biotreatment. Sulfur-containing organics are mineralized to sulfate ions in solution; phosphorus-containing organics are converted to phosphate ions; chlorine-containing organics are converted to chloride ions; nitrogen-containing organics are converted to ammonium ions, nitrate ions, nitrogen gas, or nitrous oxide gas, depending on the organic nitrogen compound; cyanides are converted to carbon dioxide and ammonium ions.
Two U.S.-based vendors, Battelle and Zimpro (now part of U.S. Filter), have demonstrated successful WAO equipment. Battelle's assisted hydrothermal oxidation process uses WAO or SCWO to destroy halogenated and other wastes under conditions that avoid the formation of acid gases. Oxygen or other oxidants are often added. Battelle claims the process thoroughly destroys (mineralizes) organic wastes, including chemical warfare agents, at substantially faster oxidation rates than SCWO at comparable operating temperatures. Reaction times for WAO range from one to two hours. Gases are treated with a thermal oxidizer prior to release, and no provision is made for gas containment. Battelle has used the technology on a small pilot scale in a continuous mode.
Zimpro has installed more than 300 WAO systems world-wide. The process has been used commercially to treat spent
TABLE 4-6a Wet-Air/O2 Oxidation: Top Priority Criteria
|
Criterion |
Rating |
|
Technical Effectiveness |
|
|
Integral part of a coherent system |
Good. The RRS and MMD neutralents might have to be diluted with water to a chemical oxygen demand (COD) of 120,000 mg/L or less, but the system can be field-erected or prefabricated in modules. |
|
Destruction efficiency |
Fair. Short-chain biodegradable organics may remain in the effluent and require further treatment. Methyl phoshonic acid is resistant and would have to be studied further. Arsenic would be converted to arsenate, which would have to be precipitated and filtered out prior to final polishing with biodegradation. |
|
Inherent Safety |
|
|
Minimal storage and transportation of hazardous material |
Excellent. |
|
Minimal toxicity and flammability (process materials) |
Excellent. No reagents required other than water and air or oxygen. |
|
Containable temperature and pressures |
Excellent. High destruction efficiency can be achieved at temperatures under 300°C and pressure under 2,075 psia. |
|
Pollution Prevention |
|
|
Minimal toxicity of effluents |
Good. Gas phase contains carbon dioxide, oxygen, and nitrogen and no dioxins. Sulfur, phosphorous, and chlorine remain in the liquid phase as dissolved salts, and oxygen probably does as well. The liquid phase also contains biodegradable organics. Solids produced can be stabilized, placed in drums, and disposed of in a permitted landfill. |
|
Minimal use of processing materials |
Excellent. Needs water and air or oxygen. |
|
Solids, liquids, and gaseous wastes |
Fair. Most wastes are aqueous liquids but are potentially suitable for discharge to a POTW. |
|
Minimal number of processing steps |
Good. Process uses pumps, compressors, heat exchangers, and a reactor. Final effluent requires biological treatment. |
|
Temperatures, pressures, corrosion, plugging, and other operating difficulties minimized to prevent unprogrammed shutdowns |
Good. Technology provider (Zimpro) has not experienced corrosion, plugging, or other operating difficulties in 300 commercial installations. |
|
Permit Status |
|
|
Allowed by regulations and capable of meeting schedules imposed by records of decision and treaties |
Excellent. Process has been designated best available data by the EPA for many land-banned hazardous wastewaters. |
caustic liquors, high-strength petrochemical wastewater streams, coke oven gas liquors, and municipal sludge from ethylene facilities and petroleum refineries.
Evaluation
WAO is a strong candidate for the treatment of both RRS and MMD neutralents because the process requires only the addition of water and air or oxygen, and no dioxins are formed (in fact, Battelle claims dioxins are destroyed). WAO is most effective on dilute aqueous solutions (e.g., chloroform must be diluted to less than 20,000 mg/L), and RRS and MMD neutralents might have to be diluted with water to reduce their chemical oxygen demand. 6 When a titanium liner is used, no evidence of corrosion has been observed in experimental studies conducted with the feedstocks, temperatures, and pressures described below. This is a major advantage over SCWO technologies.
WAO is currently used in more than 300 commercial installations, and the Environmental Protection Agency (EPA) has specified WAO as a best-demonstrated available technology for the treatment of hazardous wastewater containing
|
6 |
For RRS and MMD neutralents, a dilution of 4 to 14 times may be necessary for maximum effectiveness. |
TABLE 4-6b Wet-Air/O2 Oxidation: Important Criteria
|
Criterion |
Rating |
|
Robustness |
|
|
Stability and continuity of operation |
Good. Tests would be necessary on RRS and MMD neutralents specifically, but process has a long commercial history of treating other refractory organics. |
|
Cost |
|
|
Minimal total costs |
Good. Costs are reported as less than incineration, but the effluent does need to be treated biologically to achieve complete mineralization. |
|
Practical Operability |
|
|
Minimal training time |
Good. Temperatures and pressures are moderate, and the process is similar to others used in the chemical industry. |
|
Continuity |
|
|
Vendor likely to remain in operation and raw materials likely to remain available |
Excellent. Zimpro, the principal vendor is part of U.S. Filter, which in turn is owned by Vivendi, the largest environmental firm in the world. |
|
Space Efficiency |
|
|
Minimum weight, area, and volume of operating equipment |
Good. Skid mounted units are available. |
|
Materials Efficiency |
|
|
Use of internally recyclable materials |
Poor. None of the materials used are recyclable. |
|
Beneficial reuse of wastes |
Poor. Waste is mainly wastewater which could potentially be treated for reuse but currently is not. |
|
Use of externally recyclable materials |
Poor. Raw materials are air or oxygen and water, neither of which is available in recycled form. |
a variety of wastes classified under P and U in 40 CFR 261. U.S. Filter has tested the destruction of many types of wastes in WAO testing laboratories and pilot-plant facilities. In tests on pesticides, reported destruction efficiencies were greater than 99 percent for malathion at 200°C, dyfonate at 260°C, parathion at 260°C, and glyphosate at 260°C to 280°C. For example, in a test on chloroform reported in 1985, concentration was reduced from 4,500 mg/L to 3 mg/L in 60 minutes at 275°C (Dietrich et al., 1985). The Illinois Waste Management and Research Center at the University of Illinois in Champaign-Urbana also has several reactors designed to carry out WAO studies.
WAO has achieved excellent destruction efficiencies to biodegradable compounds with inorganic and organic cyanides, chlorinated aliphatics, halogenated aromatics containing nonhalogen functional groups, and amines. Halogenated aromatics without other functional groups (e.g., PCBs and chlorobenzene), and alkyl phoshonic acids are relatively resistant. Zimpro has done bench-scale tests on 30 organic compounds, including acenaphthene, acrylonitrile, 2-chlorophenol, 2,4-dinitrotoluene, 1,2-diphenyl hydrazine, pentachlorophenol, chloroform, carbon tetrachloride, 1,2-dichloroethane, hexachlorcyclopentadiene, chlorobenzene, 2,4-dichloroaniline, 2,4,6-trichloroaniline, 1-chloronaphthalene, malathion, Kepone, Arochlor 1254, and 1,2-dichlorobenzene. They achieved greater than 99 percent destruction efficiencies in bench-scale tests of WAO on 2-chlorophenol, chloroform, carbon tetrachloride, 1,2-dichloroethane, 2,4-dichloroanaline, 2,4,6-trichloroanaline, and 1-chloronaphthalene (Dietrich et al., 1985). Excellent destruction efficiencies were obtained for biodegradable compounds with inorganic and organic cyanides, chlorinated aliphatics, halogenated aromatics containing nonhalogen functional groups, and amines. Zimpro successfully treated these organic compounds by conducting these tests at temperatures between 240°C and 280°C and at pressures of 750 psia to 2,075 psia. The committee expects these ranges to be comparable for processing of neutralents in both the MMD and RRS.
WAO operates at somewhat elevated temperatures and pressures (150 °C to 315°C and 150 psia to 3,150 psia). WAO may be effective in treating neutralents from RRS and MMD, but some organics that require further treatment may remain in the effluents. Effects of high salt concentration on rates of destruction remain to be tested.
The ratings for WAO are summarized in Table 4-6a and Table 4-6b .
SUPERCRITICAL WATER OXIDATION
SCWO has the potential to treat both RRS and MMD neutralent waste streams, producing a relatively clean effluent containing mainly inorganic salts and water. However, high operating temperatures and pressures, as well as corrosion and potential plugging caused by salts, have made it difficult to operate SCWO processes on a routine basis.
Description
SCWO is a hydrothermal process for the oxidative destruction of organic wastes. The system involves introducing air or oxygen in the presence of high concentrations of water heated above the critical temperature and pressure of pure water (374°C, 3,204 psia). 7 The reaction mechanisms for the destruction of organic compounds generally involve free-radical chain reactions with oxidative radicals. Thermal bond cleavage and polar or ionic reactions, including hydrolysis, also occur.
In the SCWO process, the feed stream (aqueous waste) to the reactor is heated, pressurized, mixed with oxidant, and pumped through a flow reactor at supercritical conditions designed to provide the required residence time (typically, several seconds to a minute). Heat produced by the reaction can be recovered (or must be removed) based on the heating value of the feed stream. If the heating value is too low to heat the reactor, supplemental fuel can be added. Downstream of the reactor, the system is depressurized, either before or after cooling. Solids produced from oxidation reactions can be recovered prior to or following pressure let-down. The effluent is then passed through gas/liquid separators, and the gas stream and aqueous streams can be treated further.
SCWO process effluents include vent gases, liquid effluents (neutralized with NaOH), and crystallized salts, all of which must comply with regulatory requirements prior to disposal. The gases primarily consist of oxygen, nitrogen, and carbon dioxide but may also contain trace quantities (7 to 28 ppb in testing to date) of volatile organic compounds (VOCs). Any remaining VOCs in the effluent gases are filtered out by passing them through activated carbon filters.
Evaluation
In the committee's judgment, SCWO would effectively mineralize agent neutralents. RRS neutralents, however, contain considerable quantities (more than 50 percent) of chloroform and other chlorinated compounds that may be highly corrosive to the SCWO liner. Experience in processing nonstockpile neutralents in a SCWO unit will be necessary to quantify the nature, locations, and rates of corrosion.
Problems associated with the stability of SCWO (e.g., maintenance of temperature and pressure for at least 20 hours, control of salt accumulation) appear to have been resolved for a test of SCWO processing of NaOH-based VX neutralents. In testing on materials of construction with this neutralent, salts accumulated at a rate of about one pound per hour, limiting runs with neutralent to 20 hours (runs with surrogate continued for about 40 hours) (Dekleva and Gannon, 2000). However, issues related to the mechanisms and locations of salt buildup, the chemical composition of the salts produced, and the effectiveness of flushing away salts are still unresolved. Pressure containment is another issue that must be addressed. 8
A removable titanium or platinum liner for processing nonstockpile neutralents has been suggested by the SCWO technology proponent because it could withstand reactions with acidic chloride compounds found in RRS neutralents. Although the corrosion of reactor liners and the erosion of metal parts may be mitigated by periodic replacement of the liner and frequent maintenance, the associated frequency, cost, and down time will have to be determined.
The ratings for SCWO are summarized in Table 4-7a and Table 4-7b .
|
7 |
See NRC, 1998b, Table 3-1, for comparisons of operating SCWO systems. |
|
8 |
“Failure of the pressure containment system (piping, SCWO reactor, post-reactor air cooler, or pressure let-down system) could result in rapid depressurization and dispersal of hot fluids and debris at high velocities. Similarly, failure of the pressure let-down system could result in a large pressure surge that could rupture equipment downstream. .. The pressure let-down system may be the weak link in the full-scale SCWO process chain” (NRC, 1998b). |
TABLE 4-7a Supercritical Water Oxidation: Top Priority Criteria
|
Criterion |
Rating |
|
Technical Effectiveness |
|
|
Integral part of a coherent system |
Fair. Oxidation of neutralents from the RRS and MMD can be the final step following neutralization of nonstockpile chemical agent fill. |
|
Destruction efficiency |
Good. Neutralization of agent and RRS or MMD must reduce agent concentration in neutralent to under 50 ppm as per RCRA permit. SCWO should reduce any remaining agent to below detection limits. |
|
Inherent Safety |
|
|
Minimal storage and transportation of hazardous material |
Fair. Neutralents are under engineering controls. Liquid effluent is evaporated, and salts are crystalized, placed into drums, and sent to a hazardous waste landfill following TCLP testing. |
|
Minimal toxicity and flammability (process materials) |
Good. Some kerosene is used to start the process. |
|
Containable temperature and pressures |
Poor. Operates at high temperature and pressure, 650°C and 3,400 psia. Pressure containment failure can result in ejection of liquid, but volume is limited and pressure relief valves prevent over-pressurization. Pressure containment failures, resulting from overtemperature (reducing the strength of the containment material) has occurred. Water pump failure is a typical problem. |
|
Pollution Prevention |
|
|
Minimal toxicity of effluents |
Good. Liquid effluent is evaporated, and salts are crystallized, put into drums, and sent to a hazardous waste landfill following TCLP testing. Gases (oxygen, nitrogen, and carbon dioxide) are filtered and released. Brines and salts are tested and put into drums and can be sent to hazardous waste landfill. Fate of arsenic compounds is an issue. |
|
Minimal use of processing materials |
Good. Requires start-up fuel, oxidant (air), and NaOH to reduce acidity of brine. |
|
Solids, liquids, and gaseous wastes |
Fair. Most wastes are liquids that can be evaporated to obtain leachable salts, which can be disposed of in a Subtitle C landfill. |
|
Minimal number of processing steps |
Good. Uses a reactor, compressors, pressure let-down, evaporators, effluent air coolers, pumps, and gas/liquid separator. |
|
Temperatures, pressures, corrosion, plugging, and other operating difficulties minimized to prevent unprogrammed shutdowns |
Poor. Corrosion and plugging have been serious problems, but pilot SCWO at Dugway Proving Ground has operated for several 20-hour runs using VX hydrolysate without plugging and with stable temperature and pressure. Extent of unprogrammed shutdowns is not known because of lack of operating experience with nonstockpile neutralents for representative times. |
|
Permit Status |
|
|
Allowed by regulations and capable of meeting schedules imposed by records of decision and treaties |
Fair. The contractor expects RCRA Part B permit for SCWO at Newport Chemical Agent Disposal Facility from the state regulatory agency. |
TABLE 4-7b Supercritical Water Oxidation: Important Criteria
|
Criterion |
Rating |
|
Robustness |
|
|
Stability and continuity of operation |
Fair. Using NaOH-based VX hydrolysates, 20-hour runs at stable temperatures and pressures have been achieved. No operating experience with nonstockpile neutralents. |
|
Cost |
|
|
Minimal total costs |
Good. Capital and operating costs are expected to be moderate. |
|
Practical Operability |
|
|
Minimal training time |
Fair. Need more information on training time and necessary skill levels. Maintenance time requirements may be high if frequent shutdowns for salt removal are necessary. Process monitoring and control strategies should to be tailored to SCWO. |
|
Continuity |
|
|
Vendor likely to remain in operation and raw materials likely to remain available |
Good. Technology provider has operating experience and will be operating a unit processing VX hydrolysates at a chemical stockpile disposal facility in Newport, Indiana. Vendor also operates a unit in Utah. |
|
Space Efficiency |
|
|
Minimum weight, area, and volume of operating equipment |
Fair. The reactor at Dugway Proving Ground, which has a volume of 1.51 ft3 and, at its targeted flow rate of 17.7 lbs/hr, can process 141.6 lbs of material per 8-hour day. This is less than half of the reactor 's design flow rate, and, thus, the Dugway reactor should be able to process about 300 lbs per 8-hour day. Four or five flatbed trucks are required to transport the unit and its ancillary equipment. |
|
Materials Efficiency |
|
|
Use of internally recyclable materials |
Excellent. Evaporator condensate is recycled. Otherwise, there is nothing to recycle. |
|
Beneficial reuse of wastes |
Poor. Solid wastes are put into drums and sent to a hazardous waste landfill. No beneficial uses for this material. |
|
Use of externally recyclable materials |
Not applicable. |
GAS-PHASE CHEMICAL REDUCTION
Although GPCR appears to be capable of destroying nonstockpile neutralents from both the RRS and MMD, the process generates large volumes of effluent gases that require a complex treatment system.
Description
The GPCR process uses hydrogen and steam at temperatures of approximately 850°C at atmospheric pressure to convert organic wastes into substances that are either less toxic or convertible to less toxic materials. The overall process requires a high-temperature reaction vessel followed by a gas-scrubbing train to remove inorganic by-products. Residence time in the reactor is only a few seconds.
In the GPCR reactor, which contains a hydrogen-rich atmosphere, organic chemicals are reduced to methane, water, carbon soot, and other by-products, including acid gases, such as HCL from chloroform in RRS neutralent, hydrogen sulfide, phosphorus-containing products from VX neutralent, and arsenic-containing products from lewisite neutralent. These products, as well as the carbon soot, must be treated or scrubbed, adding to the complexity of the process. Catalytic steam reformers supply hydrogen gas to the GPCR reactor by steam reforming of natural gas. Vertical radiant tube heaters with internal electric heating elements are used to heat the inside of the reactor. When the gases leave the reactor, they pass through primary and secondary caustic scrubbers to remove acid gases, water, and fine particulates. The gas stream exiting the secondary scrubber is a mixture of hydrogen, methane, carbon monoxide, carbon dioxide, nitrogen, and trace quantities of light hydrocarbons. This gas is stored in tanks, sampled, and tested. If permitted, the gas can be used as an auxiliary fuel for the steam boiler.
Evaluation
GPCR, a well established thermal treatment technology operating in a pyrolytic mode, is capable of very high destruction efficiency. GPCR is the only one of the eight processes considered that involves the chemical reduction of neutralent with hydrogen and steam. The process generates large volumes of effluent gases compared to low-temperature
oxidation processes. GPCR is a complex process that requires the management of hot hydrogen gas in the reactor, separate scrubbing of effluent acid gases, the recovery of phosphorus and arsenic, and the control of carbon soot buildup in the reactor.
The technology provider has stated that GPCR reactors have been operating reliably in Canada and Australia for several years at commercial scales (several tons per day) to treat a variety of feedstocks, including polychlorinated biphenyls (PCBs), polychlorinated aromatic hydrocarbons, dichlorodiphenyltrichloroethane, chlorobenzenes, and toluene. According to the process developer, the reactor can operate effectively over a temperature range of 750°C to 950°C.
Testing with HD, VX, and VX hydrolysates has been conducted in laboratory and bench-scale tests at the Edgewood Research, Development and Engineering Center. In 1996, 1,440 grams of VX and 2,450 grams of HD were destroyed with a destruction efficiency of 99.999999 percent (eight 9's) in a portable pilot reactor. Tests with nonstockpile neutralents, however, have not been conducted.
To the best of the committee's knowledge, no commercial scale GPCR reactor has been permitted to operate in the United States. As a result, processing nonstockpile neutralents by GPCR may be delayed because no regulatory experience with either the process or with destruction of the feedstock for this process (MMD and RRS neutralents) is available. In addition, the proposed use of the GPCR off-gas as a boiler fuel poses unique permitting challenges (NRC, 1999b). A demonstration of the GPCR system was completed in September 2000 as part of the second set of ACWA demonstrations (Demo II). Results were not available at the time this report was written.
The ratings for GPCR are summarized in Table 4-8a and Table 4-8b .
TABLE 4-8a Gas-Phase Chemical Reduction: Top Priority Criteria
|
Criterion |
Rating |
|
Technical Effectiveness |
|
|
Integral part of a coherent system |
Good. Part of technology package to dispose of stockpile chemical munitions that can be a stand-alone process for nonstockpile CWM; can process either neat agent or neutralent. |
|
Destruction efficiency |
Good. High efficiencies have been achieved with similar compounds. |
|
Inherent Safety |
|
|
Minimal storage and transportation of hazardous material |
Good. Hydrogen is transported and stored routinely. |
|
Minimal toxicity and flammability (process materials) |
Fair. Prevention of leaks important to contain hydrogen. |
|
Containable temperature and pressures |
Fair. Reactor operates at near ambient pressure but at 850°C. |
|
Pollution Prevention |
|
|
Minimal toxicity of effluents |
Good. Scrubbed gases containing hydrogen, methane, CO, and CO2. Solids containing phosphorous, arsenic, and possibly sulphur must be disposed of. |
|
Minimal use of processing materials |
Excellent. Process requires only fuel, NaOH for caustic scrubbing, and water. |
|
Solids, liquids, and gaseous wastes |
Poor. Primary emissions are gases, some of which are recycled as boiler fuel. Carbon soot is also produced and must be disposed of. |
|
Minimal number of processing steps |
Fair. Process is moderately complex, especially for scrubbing reactor effluent gases. Process involves feed to the reactor, the reactor itself, scrubbing of gases, steam reforming, and handling of materials and storage equipment. Because process is integrated, it must be controlled to allow excess materials to be recirculated. |
|
Temperatures, pressures, corrosion, plugging, and |
Fair. Potential for plugging as a result of carbon (soot) buildup. Reactor must be cleaned other operating difficulties minimized to prevent unprogrammed shutdowns periodically to prevent this. Operating experience with neutralents containing chlorine, phosphorous, and other nonstockpile constituents will show if plugging and corrosion are problems. |
|
Permit Status |
|
|
Allowed by regulations and capable of meeting schedules imposed by records of decision and treaties |
Undetermined. Full-scale reactor has not been permitted in the United States (but was tested as part of ACWA Program). Data is not yet available. Trial burns to obtain RCRA and other permits may be required. Permit for using reactor off-gas as a boiler fuel will be required. |
TABLE 4-8b Gas-Phase Chemical Reduction: Important Criteria
|
Criterion |
|
|
Robustness |
|
|
Stability and continuity of operation |
Excellent. A reactor operating since 1995 in Australia is capable of processing liquid feedstocks, including PCBs, polyaromatic hydrocarbons, chlorobenzenes, and dichlorophenyltrichloroethane. Start-up and shutdown have been demonstrated. More information necessary about scale-down of reactor to meet nonstockpile needs and about operation with neutralents. |
|
Cost |
|
|
Minimal total costs |
Good. Appears to be competitive, but no cost data are available for processing small quantities of neutralents. |
|
Practical Operability |
|
|
Minimal training time |
Good. Appears to be well established for materials being processed but not for operation with neutralents. Additional scrubbers, permitting concerns, materials handling requirements (e.g., arsenic, phosphorous, carbon) may reduce practical operability. |
|
Continuity |
|
|
Vendor likely to remain in operation and raw materials likely to remain available |
Excellent. Vendor operates facilities in Canada and Australia and will be part of ACWA Demo II. Expected to remain in business. No unique raw materials required. |
|
Space Efficiency |
|
|
Minimal weight, area, and volume of operating equipment |
Good. Appears to be scalable. Not all size requirements for equipment, including scrubbers and steam reformers, are known. |
|
Materials Efficiency |
|
|
Use of internally recyclable materials |
Good. Some of the reactor effluent gas is recyclable as boiler fuel. |
|
Beneficial reuse of wastes |
No. Liquid and solid wastes are not recyclable but must be disposed of. |
|
Use of externally recyclable materials |
Not applicable. Facilities (e.g., landfills) that will accept these wastes have not been identified. |
PLASMA-ARC TECHNOLOGY
Plasma-arc technology is a very high-temperature process that probably would effectively destroy both RRS and MMD neutralents although the large quantities of water present in MMD neutralents could present problems. Plasma-arc generates large volumes of high-temperature vapor streams that require containment and high-quality treatment.
Description
Plasma-arc technologies utilize electrical discharges in various gases to produce a field of intense radiant energy and high-temperature ions and electrons that cause dissociation of chemical compounds in a containment chamber. The process, operating at temperatures as high as 20,000°C, occurs in a closed hearth reactor. The reaction chamber is maintained at slightly less than atmospheric pressure to prevent the release of hazardous effluents. Material exposed to the plasma environment is transformed into atoms, ions, and electrons. However, small molecules form as the gases leave the reaction zone.
Three types of waste streams are produced: plasma off-gas that is first treated to completely destroy VOCs and then cleaned by a two-stage scrubber, followed by a sophisticated filtration system prior to release; wastewater from the water treatment system used in the purification of the off-gases; and vitrified inorganic products that fall to the bottom of the containment vessel.
Evaluation
Plasma-arc systems can achieve high destruction efficiencies, reportedly higher than 99.99999 percent (seven 9's). They are most efficient when used to treat low-volume, highly concentrated feed streams. Consequently, they would be less efficient when used with neutralents from the RRS and MMD, the latter of which would require that the system process large amounts of water. The committee is concerned
TABLE 4-9a Plasma-Arc Technology: Top Priority Criteria
|
Criterion |
Rating |
|
Technical Effectiveness |
|
|
Integral part of a coherent system |
Excellent. Could be integrated with existing technologies. System available in either a fixed or mobile configuration. |
|
Destruction efficiency |
Excellent. Has achieved destruction efficiencies of seven 9's or better with similar chemical compounds in Germany and Switzerland. |
|
Inherent Safety |
|
|
Minimal storage and transportation of hazardous material |
Excellent. Requires no storage of hazardous materials. |
|
Minimal toxicity and flammability (process materials) |
Excellent. Requires no storage of toxic or flammable materials. |
|
Containable temperature and pressures |
Poor. Temperatures can run as high as 20,000°C. Subatmospheric pressures permit reliable containment. |
|
Pollution Prevention |
|
|
Minimum toxicity of effluents |
Good. Exhaust gases are filtered and scrubbed. Arsenic is removed from the scrubber, recycled, and eventually forms a slag. |
|
Minimal use of processing materials |
Fair. Helium or another suitable gas used in starting the process is replaced by nitrogen or air. Water is used as a coolant. |
|
Solids, liquids, and gaseous wastes |
Poor. Converts the neutralent primarily into an off-gas that requires extensive treatment. Solids are formed into a slag. |
|
Minimal number of processing steps |
Good. Requires a pollution control system, but there are not many steps. |
|
Temperatures, pressures, corrosion, plugging, and other operating difficulties minimized to prevent unprogrammed shutdowns |
Good. System requires only weekly preventive maintenance based on an 80-hour- per-week operating schedule. Torch electrode is replaced after 20 hours. |
|
Permit Status |
|
|
Allowed by regulations and capable of meeting schedules imposed by records of decision and treaties |
Poor. System has not been permitted to operate in the United States. May be considered by regulators as incineration. |
about the very high operating temperatures of this system and the need for extensive off-gas treatment.
A plasma-arc process was tested for the destruction of assembled chemical weapons for the Army's ACWA Program. The test configuration included a 300-kW unit that used nitrogen as the plasma gas. Tests conducted on dimethyl methyl phosphonate and hydrolysates of HD and VX achieved high destruction efficiencies but generated products of environmental concern, including C2N2, hydrogen cyanide, and metal cyanides. The by-products with a different plasma gas would be different.
The Committee on Review and Evaluation of Alternative Technologies for Demilitarization of Assembled Chemical Weapons concluded, in concurrence with the Army and the Dialogue (a citizen group), that the plasma torch apparatus demonstrated for the ACWA Program did not qualify for further consideration for the demilitarization of assembled chemical weapons. That committee noted, however, that “the variety of equipment problems encountered in the demonstration were due to the immaturity of the particular demonstration equipment and not due to a fundamental inability of plasma-based technologies to achieve acceptable results” (NRC, 1999b).
A patented plasma-arc process, PLASMOX®, is currently operational in Europe in both fixed and mobile configurations. The process combines features of pure plasma and incineration operations. The first step in the process is a plasma treatment unit, which is started on helium but is maintained during processing by using either nitrogen or air as the plasma gas. Operation with nitrogen would represent a pure plasma mode, while operation on air would represent a plasma-augmented incineration mode. Off-gases from the plasma unit are fed to a secondary combustion chamber employing air or oxygen at 1,000°C to 1,200°C. This operation is a main feature of incinerators. The off-gas handling system includes a gas cooler and quencher, a two-stage
TABLE 4-9b Plasma-Arc Technology: Important Criteria
|
Criterion |
Rating |
|
Robustness |
|
|
Stability and continuity of operation |
Excellent. |
|
Cost |
|
|
Minimal total costs |
Good. Capital and operating costs are expected to be average. Costs of transportation and setup will be low. |
|
Practical Operability |
|
|
Minimal training time |
Good. Operations are simple, training in the handling of toxic chemicals should be similar to training for typical chemical plants. |
|
Continuity |
|
|
Vendor likely to remain in operation and raw materials likely to remain available |
Good. Large, stable engineering company. The equipment is comprised mostly of off-the-shelf items. |
|
Space Efficiency |
|
|
Minimum weight, area, and volume of operating equipment |
Good. Mobile system can be moved on three standard trailers. |
|
Materials Efficiency |
|
|
Use of internally recyclable materials |
Good. Majority of waste products recycled internally. |
|
Beneficial reuse of wastes |
Fair. Metals can be recycled, but not other residues. |
|
Use of externally recyclable materials |
Not applicable |
scrubber, a dust filter, a high-efficiency particulate air filter, and an activated carbon filter. When treating chlorinated materials, the process generates small quantities of dioxins, which are removed in the carbon filter. A mature technology that has been operated for extended periods of time in Europe, this technology has been used to destroy lewisite, mustard, adamsite, and phosgene.
The ratings for plasma-arc technology are summarized in Table 4-9a and Table 4-9b .
OVERALL RANKINGS
The committee's qualitative demarcation of the axes in Figure 4-1 into low, moderate, high, and very high temperature and pressure ranges corresponds roughly with the extent of engineering controls required for safe operation. Biodegradation, chemical oxidation, and electrochemical oxidation fall into the low-temperature, low-pressure range; SET, when operated at room temperature, operates at slightly higher pressures. WAO is operated over a range of moderate temperatures and moderate to very high pressures. GPCR and plasma-arc processes (as well as the Army's baseline incineration technology) operate in high and very high-temperature, low- to moderate-pressure regimes; SCWO requires high temperatures and very high pressures.
Other things being equal, the lower the operating temperature and pressure required for a technology, assuming that it can achieve acceptable performance, the more likely it is to be simpler, cheaper, and less risky to health and the environment. As the operating temperature and pressure increase, several undesirable changes may occur: the sophistication (and cost) of the required engineering controls increases; undesirable by-products may be formed (e.g., tar soot, polyaromatic hydrocarbons, chlorodibenzodioxins); and containment and the treatment of high-temperature vapor streams become issues of concern. SCWO might be an exception to the general rule that undesirable by-products may be formed in high-temperature processes. The committee began with a bias toward technologies in the low-temperature, low-pressure category. Nevertheless, after much study and debate, two of these (electrochemical oxidation and SET) were given lower rankings because of other concerns (e.g., toxicity and the corrosiveness of process chemicals). Biodegradation was the only technology that was judged to be unacceptable.
The technologies are described below in order of their ranking, from highest to lowest.
Low-Temperature Chemical Oxidation in Water
Chemical oxidation in water at less than 100°C appears to be the most attractive technology. This technology has a reasonable chance of destroying MMD and RRS neutralent successfully and does not require exotic or significantly toxic
chemicals. Other than carbon dioxide from the oxidation of carbon-containing molecules, the process should not produce a vapor stream. Control of acidity by adjusting the pH will be necessary when handling chlorinated materials. Problem metals, such as arsenic and mercury, would be captured by demonstrated technologies, such as ion-exchange, precipitation, or activated carbon, and disposed of through recycling or in a RCRA landfill. Teledyne-Commodore, an existing vendor, has proposed using peroxidisulfate (oxidant species) to treat MMD neutralents.
Wet-Air/O2Oxidation
WAO has almost all of the advantages of chemical oxidation but two significant disadvantages: (1) the process usually uses an air stream as the source of oxygen; and (2) WAO operates at higher temperature and pressure than chemical oxidation. The air stream could contain oxides of nitrogen, which must be treated before discharge, although one vendor (Zimpro) has not found detectable levels of nitrogen oxides in the effluent from any of the tests conducted with air. Use of pure oxygen instead of air does create safety problems. Therefore, the concentrations of organics in the effluent gas must not exceed the lower explosive limits of oxygen. Substituting oxygen for air would significantly reduce the amount of vapor that would have to be contained and cleaned. A process using oxygen instead of air would be a higher temperature analog of the chemical oxidation technologies described above. Zimpro is already set up to demonstrate this technology and has already demonstrated it using oxygen in the treatment of other organic compounds.
Oxidation in Water by Cerium IV
Although oxidation in water by Ce(IV) is a low-temperature process, it may produce a significant vapor stream because of the formation of chlorine. The process also uses nitric acid, which must be regenerated. Oxidation by Ce(IV) should be pursued only if low-temperature chemical oxidation and WAO cannot break down the neutralents to the point that the resultant materials could be discharged to a POTW or equivalent facility. However, because there is an existing vendor for this technology, and if actual or synthetic neutralent can be made readily available, it may be possible to test this technology with a minimum of time and effort. If so, this should be done as a potential backup technology, although low-temperature chemical oxidation should remain the technology of choice.
Oxidation in Water by Silver II
The only advantage of oxidation in water by Ag(II) over oxidation by Ce(IV) is that Ag(II) may have greater oxidizing power. Otherwise, this technology has several disadvantages. Silver salts are far more toxic and expensive than cerium salts. In addition, silver forms a highly insoluble, toxic salt with chloride, which could be present in large amounts and would require extensive handling, collection, and recycling. Ag(II) technology should be considered a low priority for development funding. However, because an existing vendor for this technology is already conducting tests under the ACWA Program, if actual or synthetic neutralent can be made readily available, it may be possible to test this technology with a minimum of time and effort. If so, that should be done as a potential backup, although low-temperature chemical oxidation should remain the technology of choice.
Supercritical Water Oxidation
SCWO is a potentially simpler process than any of the technologies discussed so far except for low-temperature chemical oxidation, the primary choice. However, developers have been trying unsuccessfully to solve the corrosion and plugging problems associated with SCWO for almost two decades. The Army is attempting to demonstrate an improved SCWO process for the Chemical Stockpile Disposal Program and for the ACWA Program. Unless the Army demonstration is very successful, however, SCWO should only be considered for treatment of RRS and MMD neutralents if there is reasonable assurance that further investments will not be prohibitive. However, if actual or synthetic neutralent can be made readily available, it may be possible to test this technology with a minimum of time and effort in combination with the Army's tests of SCWO for the Chemical Stockpile Disposal Program. If so, that should be done. SCWO could be a potential backup technology but should not compromise the preference for low-temperature chemical oxidation.
Solvated-Electron Technology
SET uses metallic sodium in anhydrous liquid ammonia, both of which require careful and complicated handling. In addition, liquid ammonia requires refrigeration or operation at moderate pressure. This technology has no obvious advantages over the preferred technologies for destruction of RRS neutralents and is not suitable for treatment of MMD neutralents. SET should, therefore, be ranked lower than low-temperature, low-pressure, less-complex process technologies.
Gas-Phase Chemical Reduction
GPCR technology would generate a voluminous, high-temperature vapor stream that would require significant efforts to contain, scrub, and otherwise treat. The technology has apparently been demonstrated by vendors outside the United States to the satisfaction of the regulators and the community. Except for stronger destruction capability,
GPCR has no obvious advantages over the preferred technologies for destruction of RRS and MMD neutralents and should be ranked lower than low-temperature, low-pressure, less-complex process technologies.
Plasma-Arc Technology
Like GPCR, plasma-arc technology would generate a large, very high temperature vapor stream that would require significant efforts to contain, scrub, and treat. This technology has apparently been demonstrated by vendors outside the United States to the satisfaction of the regulators and the community of the hosting country. Other than stronger destruction capability, plasma-arc technology has no obvious advantages over the preferred technologies and should be ranked lower than low-temperature, low-pressure, less-complex process systems.
Biodegradation
The committee could not find any record of tests of stand-alone biological processing for treating any form of RRS or MMD neutralent. (However, an immobilized-cell biological reactor was tested by Parsons/AlliedSignal and was found to be effective for treating mustard hydrolysate, 9 a different type of waste stream). Aeration of the bioponds might generate significant secondary air emissions that would require treating large volumes of off-gas. Preliminary modeling suggests that the chloroform in the RRS neutralent would be particularly difficult to destroy by biodegradation. Small amounts of metals and biologically recalcitrant organic compounds in both the RRS and the MMD neutralents may also pose major problems. Thus, stand-alone biological processing would probably be applicable only to MMD neutralent, if at all. In addition, a test of using biodegradation to treat GB and VX hydrolysate was unsuccessful, which raises questions about the effectiveness of using biodegradation to treat GB and VX neutralent. Overall the committee felt that no investment in biodegradation was warranted for destruction of neutralents.
|
9 |
The Army refers to the destruction of chemical agent via hydrolysis as chemical neutralization. The term is derived from the military definition of “neutralize,” to render something unusable or nonfunctional. Hydrolysis is a reaction of a target compound with water, often catalyzed by an acid or a base, in which a chemical bond is broken in the target, and the components of water, OH− and H+, are inserted at the site of the bond cleavage. Hydrolysate is the product resulting from hydrolysis. The technical definition of neutralization is a chemical reaction between an acid and a base to form a salt and water. Neutralent is the product generated from neutralization. |