Page 13
Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism
Immunization is widely regarded as one of the most effective and beneficial tools for protecting the public's health. In the United States, immunization programs have resulted in the eradication of smallpox, the elimination of polio, and the control and near-elimination of other once-common, often debilitating, and potentially life-threatening diseases, including measles, mumps, rubella, diphtheria, pertussis, tetanus, and Haemophilus influenzae type b.
Along with the benefits of widespread immunization, however, have come concerns about the safety of the vaccines. No vaccine is perfectly safe or effective, and vaccines may lead to serious adverse effects in some instances. Furthermore, if a serious illness is observed following vaccination, it is often unclear whether that sequence is coincidental or causal, and it can be difficult to determine the true nature of the relationship, if any, between the vaccination and the illness.
Ironically, the successes of vaccine coverage in the United States have made it more difficult for the public to weigh the benefits and risks of vaccines because the now controlled diseases and their often-serious complications, are no longer familiar. However, because vaccines are so widely used—and because state laws require that children be vaccinated before entering daycare and school, in part to protect others—it is essential that safety concerns be fully and carefully studied.
This report, the first of a series from the Immunization Safety Review Committee, presents an assessment of the evidence regarding a hypothesized causal association between the measles-mumps-rubella (MMR) vaccine and autism, the committee's conclusions and recommendations based on that assessment, and an assessment of the broader significance for society of the issues
Page 14
surrounding the MMR-autism question. Since the late 1990s, this hypothesis has received increasing attention from scientific researchers, Congress, the media, parents, advocacy organizations, public health professionals, and vaccine manufacturers (60 Minutes, 2000; U.S. House Committee on Government Reform, 2000; Wakefield et al., 1998, 2000).
ORIGINS OF THE IMMUNIZATION SAFETY REVIEW PROJECT
The federal government has responded to concerns about the safety of vaccines through several mechanisms. In 1986, Congress passed the National Childhood Vaccine Injury Act (Public Law 99-660), followed by the Vaccine Compensation Amendments of 1987 (Public Law 100-203). This legislation mandated the establishment of a National Vaccine Injury Compensation Program to handle related claims, and of the Vaccine Adverse Event Reporting System (VAERS), which is a national passive surveillance system. The legislation also provided for the development of vaccine information statements for parents of children receiving immunizations. These activities are managed by three agencies of the U.S. Department of Health and Human Services (DHHS): the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), and the Health Resources and Services Administration (HRSA). The compensation program is jointly administered by HRSA and the Department of Justice.
The legislation also called for the Institute of Medicine (IOM) to review evidence regarding possible adverse consequences of childhood immunizations. The three expert committees convened by IOM produced the reports Adverse Effects of Pertussis and Rubella Vaccines (IOM, 1991), Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality (IOM, 1994a), and DPT Vaccine and Chronic Nervous System Dysfunction: A New Analysis (IOM, 1994b). Following the completion of the third study, IOM was asked to organize the Vaccine Safety Forum to provide a framework for continued discussion of vaccine safety issues. Forum participants included representatives of government agencies, advocacy groups, and pharmaceutical companies, as well as parents, health care providers, academic researchers, and IOM staff. Forum discussions, on topics such as research strategies and risk communication, were documented in brief reports (IOM, 1996, 1997a,b) but were not intended to produce conclusions or recommendations. The final meeting of the Forum explored the early emerging data regarding the hypothesized relationship between MMR vaccine and autism. A list of research ideas from that open meeting can be found in Appendix D.
In 1995 and 1997, in response to the findings and recommendations of Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality (IOM, 1994a), the Secretary of the DHHS updated the Vaccine Injury Table, a list of post-vaccination events that must be reported to DHHS and that
Page 15
are covered by the National Vaccine Injury Compensation Program. Also in 1995, the National Vaccine Advisory Committee (NVAC) of the National Vaccine Program Office of DHHS added a Vaccine Safety Subcommittee to its efforts. In 1999, this subcommittee expanded its scope and was renamed the Vaccine Safety and Communication Subcommittee. Concern over cases of vaccine-associated paralytic poliomyelitis prompted another CDC committee—the Advisory Committee on Immunization Practices—to recommend in 1997 that the immunization schedule be changed to replace oral poliovirus vaccine with inactivated poliovirus vaccine (CDC, 2000e).
But since the mid-1990s, a number of additional challenges to the safety of vaccinations have gained attention in various settings. During 1999–2000, the Committee on Government Reform of the U.S. House of Representatives held seven hearings on vaccine-safety issues. The media have covered these issues on news programs such as 60 Minutes, 20/20, and Nightline, and the Internet is playing an increasingly important communications role. Also, many consumer and professional organizations have sponsored conferences and scientific symposia to address vaccine safety.
Given these growing concerns, CDC and the National Institutes of Health (NIH) recognized the need for an independent, expert group to address vaccine safety in a timely and objective manner. In 1999, as a result of IOM's previous work and its access to independent scientific experts, CDC and NIH began a year of discussions with IOM to develop the Immunization Safety Review project to address vaccine-safety issues both existing and emerging.
THE CHARGE TO THE COMMITTEE
The Immunization Safety Review Committee is responsible for examining a broad variety of vaccine-safety concerns. Committee members have expertise in pediatrics, neurology, immunology, internal medicine, infectious diseases, genetics, epidemiology, biostatistics, risk perception and communication, decision analysis, public health, nursing, and ethics. To preclude any real or perceived conflicts of interest, candidate members were subject to strict selection criteria that excluded anyone who had financial ties to vaccine manufacturers or their parent companies, previous service on major vaccine advisory committees, and prior expert testimony or publications on issues of vaccine safety. While all committee members share a belief in the benefits of vaccines, none of them has a vested interest in the vaccine-safety issues that will come before the group. Additional discussion of the committee composition can be found in the Foreword, written by Dr. Kenneth Shine, President of the IOM.
The committee is charged with examining three vaccine-safety hypotheses each year during the 3-year study period (2001–2003). The Interagency Vaccine Group, comprising officials from the National Vaccine Program Office at DHHS, the National Immunization Program and the National Center for Infectious Diseases at the CDC, the National Institute for Allergy and Infectious Dis-
Page 16
eases at the NIH, the Department of Defense, the FDA, the National Vaccine Injury Compensation Program at HRSA, the Health Care Financing Administration, and the Agency for International Development, selects the hypotheses to be examined by the committee. For each hypothesis examined, the committee will hold an open scientific meeting followed directly by a 1- to 2-day closed meeting for committee deliberations and formulation of conclusions and recommendations. The committee's findings will be released to the public in a brief consensus report 60-90 days after its meeting.
For each hypothesis to be examined, the committee has been asked to assess both its scientific plausibility and the significance of the issue in a broader societal context. The plausibility assessment has two components: (1) an examination of the causal relationship between the vaccine and the adverse event and (2) an examination of any pathogenic mechanism(s) in support of the hypothesis. The significance assessment addresses such considerations as the nature of the health risks associated with the vaccine-preventable disease and that of the adverse event in question. Other considerations may include the perceived intensity of public or professional concern or the feasibility of additional research to help resolve scientific uncertainty regarding causal associations.
The findings of the plausibility and significance assessments provide the basis for the committee's recommendations on public health response, immunization-policy review, current and future research, and effective communication strategies for the specific immunization-safety questions. Although the committee has been asked to make recommendations related to immunization policy, there are clear limits on this element of the charge. For example, it would exceed the authority of this committee to recommend a change in the licensure, scheduling, or administration of a vaccine. If the committee concluded that the scientific evidence or other important factors justified such action, it could recommend convening the appropriate advisory group(s) to examine the question.
THE STUDY PROCESS
The committee held an initial organizational meeting in January 2001. CDC and NIH presented the committee's charge at the meeting, and the committee conducted a general review of immunization-safety concerns and determined its methodology for assessing causality. This approach would be used for the hypotheses to be considered at subsequent meetings (see Appendix A).
To evaluate the hypothesis on MMR vaccine and autism, the committee then collected information from several sources. An extensive review was performed of the published, peer-reviewed scientific and medical literature pertinent to the hypothesis. A background paper reviewing the epidemiological studies of MMR vaccine and autism was commissioned and made available on the project's website to inform the committee and to generate discussion among committee members and other interested parties. Critiques of the paper were reviewed during the committee's deliberations. (The committee emphasizes that
Page 17
this background paper does not represent the views of the committee, only those of the authors.)
At an open scientific meeting in March 2001 ( Appendix B), academic researchers, NIH scientists and other federal officials, and representatives of vaccine safety advocacy groups gave presentations and offered comments. The formal presentations reviewed the current state of knowledge of the etiology and epidemiology of autism and current research efforts. The committee also heard presentations from researchers currently investigating the MMR vaccine-autism hypothesis. Unpublished data shared with the committee through presentations and personal communications helped inform the committee's conclusions and recommendations. A working group of the committee conferred with parents of autistic children, as well as vaccine-safety advocates and educators, to discuss their concerns regarding the MMR vaccine, autism, and the hypothesized association between the two.
THE FRAMEWORK FOR ASSESSING CAUSALITY
The Immunization Safety Review Committee has adopted the framework for assessing causality developed by the committees previously convened by the IOM (1991, 1994a) to address questions of vaccine safety. Reviews begin from a position of neutrality regarding the specific vaccine-safety hypothesis under question. That is, there is no presumption that a specific vaccine does or does not cause the adverse event in question. The weight of the available evidence determines whether it is possible to shift that position toward causality (“the evidence favors acceptance of a causal relationship”) or away from causality (“the evidence favors rejection of a causal relationship”). The committee does not conclude that the evidence favors rejecting causality merely if the evidence toward causality is inadequate. Rather, the committee requires epidemiological evidence showing no association before concluding that the evidence favors rejection of a causal relationship. Furthermore, while biological plausibility must be demonstrated in order to establish a causal relationship, demonstrated biological plausibility in the absence of adequate epidemiological evidence is not sufficient.
Standard approaches are used for evaluating evidence. Controlled epidemiological studies published in peer-reviewed journals always carry the most weight. Uncontrolled observational studies are important but generally are considered less definitive than controlled studies. Case reports and case series are reviewed, although they are generally inadequate to establish causality. Despite the limitations of case reports, the causality argument for at least one adverse event (the relationship between vaccines containing tetanus-toxoid and Guillain-Barré syndrome) was strengthened most by a single, well-documented case report on recurrence of the adverse event following re-administration of the vaccine, referred to as a “rechallenge” (IOM, 1994a).
Page 18
Unpublished or non-peer-reviewed data presented to the committee are often reviewed. Such findings could be used in support of a body of published literature with similar findings, but only in extraordinary circumstances could an unpublished study refute a body of published literature. If, however, the committee felt that the unpublished data were well described, were obtained using sound methodology, and presented very clear results, the committee could consider, with sufficient caveats in the discussion, how those data fit with the entire body of published literature.
Five categories are used to summarize the direction and strength of the evidence for causality (see Table 1). The wording of the causality categories used in the 1991 IOM report was revised in the 1994 report because the IOM had found that some people misinterpreted the 1991 language. The changes in wording are shown in Table 1 . The types and strength of evidence required to determine a specific level of causal association were the same for the two reports. The Immunization Safety Review Committee is using the wording adopted in 1994.
UNDER REVIEW: THE MMR–AUTISM HYPOTHESIS
The Immunization Safety Review Committee examined the hypothesized causal relation between MMR vaccination and autism. Autism is a complex and severe developmental disorder characterized by impairments of social interaction, impairments in verbal and nonverbal communication, and restricted or repetitive and stereotyped patterns of behaviors and interests (APA, 1994; Filipek et al., 1999). Over time, research has identified subtle differences in the onset and progression of autistic symptoms. The term “autistic spectrum disorders” (ASD), synonymous with “pervasive developmental disorders” (PDD), refers to a continuum of related cognitive and neurobehavioral disorders that reflects the heterogeneity of these symptoms. ASD includes autistic disorder, childhood disintegrative disorder, Asperger's syndrome, Rett's syndrome, and pervasive developmental disorder not otherwise specified (PDD-NOS or atypical autism). While the primary deficits are similar for all of these disorders, patients vary in the severity of their symptoms and level of cognitive impairment. Although Rett's syndrome is included in the diagnostic category of ASD, it is considered by many to be a distinct neurologic disorder and this diagnosis is not included in most research which has evaluated the association of the MMR vaccine with autism. In this report, the terms “autism,” “autistic,” and “autistic spectrum disorders” are used interchangeably to refer to this broader group of pervasive developmental disorders. The term “autistic disorder” refers to a more narrow diagnosis defined by criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (APA, 1994).
Page 19
|
Category |
IOM, 1991 |
IOM, 1994a |
Level of Evidence |
|
1 |
No evidence bearing on a causal relation |
No evidence bearing on a causal relation |
No case reports or epidemiological studies identified. |
|
2 |
Evidence insufficient to indicate a causal relation |
The evidence is inadequate to accept or reject a causal relation |
One or more case reports or epidemiological studies were located, but the evidence for the causal relation neither outweighs nor is outweighed by the evidence against a causal relation. |
|
3 |
Evidence does not indicate a causal relation |
The evidence favors rejection of a causal relation |
Only evidence from epidemiological studies can be used as a basis for possible rejection of a causal relation. Requires a rigorously performed epidemiologica study (or meta-analysis) of adequate size that did detect a significant association between the vaccine and the adverse event. |
|
4 |
Evidence is consistent with a causal relation |
The evidence favors acceptance of a causal relation |
The balance of evidence from one or more case reports or epidemiological studies provides evidence for a causal relation that outweighs the evidence agains |
|
5 |
Evidence indicates a causal relation |
The evidence establishes a causal relation |
Epidemiological studies and/or case reports provide unequivocal evidence for a causal relation. |
Most cases of ASD appear to result from prenatal or early postnatal insults (Bristol et al., 1996). Although it is clear that a vaccine given in the second year of life, as MMR is, could not cause the cases of autism originating in the prenatal or early postnatal period, the emergence of more pronounced symptoms at the time of vaccination may leave the temporal relationship with vaccine exposure uncertain. Moreover, because in some cases autistic symptoms emerge after
Page 20
a period of apparently normal development (i.e., regression), usually in the second year of life, the possibility is left open that MMR vaccination may provoke the onset of the disorder.
The MMR vaccine, which consists of three separate, attenuated viruses directed against three different diseases, has been hypothesized many times over the years to cause neurologic disorders, especially encephalitis or encephalopathy. Biologic plausibility is demonstrated for this association, because natural or wild-type measles clearly infects the central nervous system (CNS) and can lead to clinical neurologic events. In addition, rubella virus is known to produce CNS–related birth defects. Although neurologic effects are biologically plausible, the totality of biological, clinical, and epidemiological data led previous IOM committees to conclude that the evidence is inadequate to accept or reject a causal relationship between MMR vaccine and encephalopathy, subacute sclerosing panencephalitis (SSPE), or residual seizure disorder. The specific question of MMR and ASD was not addressed in the 1991 or 1994 IOM reports.
Current attention to the possible relationship between MMR and ASD stems primarily from a case series reported in 1998 (Wakefield et al., 1998). The authors investigated 12 children, consecutively referred to a London gastroenterology clinic, who exhibited regression in development (loss of previously acquired developmental milestones) and gastrointestinal symptoms. For eight of these children, according to retrospective accounts by their parents or physicians, the onset of their behavioral problems was associated with MMR vaccination. While the authors acknowledge that the study did not prove an association between MMR and the conditions seen in these children, the report generated considerable interest and concern about a possible link between MMR vaccination and ASD, and regressive autism in particular. Subsequent epidemiological studies have investigated the possible relationships among the MMR vaccine, ASD, and bowel disease. Some studies have focused on ASD with no specific relation to bowel disease; other studies have focused on the bowel disease with no particular relationship to ASD.
There are also more general concerns in the United States and the United Kingdom that the introduction and wide-scale use of the MMR vaccine coincides with an apparent increase in the incidence of autism. A report by the California Department of Developmental Services (1999) showed a significant increase between 1987 and 1998 in its caseload of children with autism, and this report is often cited as supporting an increase in ASD occurrence, although these reported increases occurred well after the licensure and introduction of MMR in the United States in 1971. The evidence from other studies of trends in ASD prevalence and incidence is unclear. While several recent reviews have found an increase in autism prevalence rates, these observed increases may reflect such factors as reporting bias, changes in diagnostic criteria for ASD, and better detection of cases (Fombonne, 1999, 2001a; Gillberg and Wing, 1999). Given these broader concerns and uncertainties about ASD, parents of autistic children who spoke to members of the IOM committee urged consideration of biologic mechanisms other than those involving bowel disorders.
Page 21
The possible association between MMR vaccine and autism has been the focus of high-level scientific research and review, both in the United Kingdom and the United States. British health authorities have issued statements that the evidence shows MMR vaccine does not cause autism and MMR vaccine should be administered in its trivalent form (U.K. DOH, 2001a). The World Health Organization (WHO) has likewise issued a statement in support of the trivalent vaccine (WHO, 2001). In the United States, the American Academy of Pediatrics (AAP), at the request of CDC, convened a workshop in June 2000 to explore the data on this relationship. The report, due to be released in May 2001, was embargoed and therefore not available to this committee for review. However, a letter from the vice-president of AAP to the AAP membership states that “The bottom line is that a considerable body of evidence does not support a causal relationship between MMR vaccine and autism or inflammatory bowel disease. No data exist to suggest that separate administration of measles-mumps-rubella vaccines would offer any potential benefit over the MMR vaccine currently used in the United States” (Cooper, 2001).
PLAUSIBILITY ASSESSMENT
The Immunization Safety Review Committee undertook to answer the following question: What is the causal relationship between the MMR vaccine and ASD? The sources of evidence considered by the committee in its plausibility assessment include biological plausibility, reports of individual cases or series of cases, and epidemiological studies. Epidemiological studies assess health-related exposures or outcomes in a defined sample of subjects and making inferences about the values of those characteristics or the associations among them in the population from which the study sample originates. Epidemiological studies can either be uncontrolled (descriptive) or controlled (analytic), observational (survey) or experimental (clinical trial). Controlled and experimental studies are given more weight in causality assessments because of their more rigorous study designs.
It is important to emphasize that the focus is on the hypothesized relationship between MMR vaccine and ASD, not the presence or absence of bowel disease in children with ASD. The committee recognizes the contribution to clinical medicine of the presentation of bowel disease in a subset of children with ASD, but the possible presence and role of measles vaccine-strain virus in the bowel of these children is not central to assessing the relationship between MMR vaccine and ASD. It does, however, suggest a potential biologic mechanism to link MMR vaccine and ASD, which is discussed below in the review of biologic plausibility. Further research on this subject might have more bearing on the possible role of measles-related virus in the etiology of bowel disease than on its role in the etiology of ASD.
Page 22
Clinical Description of Autistic Spectrum Disorders
Autism was first described by Kanner in 1943, and a serious effort by Rutter and others to define the disorder more precisely came in the 1970s (Volkmar and Lord, 1998). Efforts to develop clear definitions for each of the autistic spectrum disorders have culminated in a convergence of the diagnostic criteria in DSM-IV (APA, 1994) and the latest version of the WHO's International Classification of Diseases, ICD-10 (Filipek et al., 1999; WHO, 1993). Widespread acceptance of these diagnostic criteria is expected to produce more consistent identification and categorization of cases, which will be more conducive to research and comparative studies.
Kanner initially described “infantile autism” as exhibition of poor social and communication skills but not necessarily cognitive impairment. He described these impairments as being evident at birth or shortly thereafter and not associated with any medical conditions. Early research on autism was hampered by confusion resulting from the placement of autism in a continuum of psychotic disorders related to schizophrenia. Autism was also erroneously associated with high parental achievement, parental psychopathology, and dysfunctional parent-child interactions and care. In fact, autism occurs in families of all socioeconomic levels and ethnic backgrounds. Autism has been found to be associated with various organic abnormalities such as structural abnormalities in the brain, seizure disorders and EEG abnormalities, and mental retardation (Volkmar and Lord, 1998).
Autopsy studies of a small number of brains of individuals who had autism have shown neuroanatomic abnormalities, including decreased cell size, increased cell density, and stunting of dendritic branching bilaterally in the limbic system (Bauman and Kemper, 1997; Kemper and Bauman, 1998). The limbic system is important for learning, memory, emotion, and behavior. A decrease in Purkinje cell density and, to a lesser extent, granule cell density in the cerebellum has also been described (Bauman, 1999; Bauman and Kemper, 1997). The cerebellum is linked to control of emotion, motivation, learning, memory, and the processing and integration of sensory and motor information. The pattern of neural abnormalities in the limbic system and the lack of reactive gliosis or other evidence of an inflammatory or infectious event in the autopsied brains suggest that the etiologic insult occurred in early embryonic development (Kemper, 2001). Furthermore, the existence of Purkinje cell lesions with the preservation of related olivary neurons as described in the brains of autistic patients is consistent with a prenatal insult because cerebellar lesions after birth generally lead to regression of the olivary neurons (Bauman and Kemper, 1997).
Autism is believed to be the most genetic of all psychiatric disorders (Rutter et al., 1997). It is generally thought that the genetic mechanism is a complex interaction among multiple genes. However, interactions of other factors, including infectious, neurologic, metabolic, immunologic, and environmental insults, may also play an important role in the onset of autism. (Bristol-Power, 2001). An increased risk of autism in siblings of a child with autism and a high
Page 23
concordance rate in monozygotic twins have been found (Bailey et al., 1995; Trottier et al., 1999). In a recent study, Bailey and colleagues (1995) reevaluated the subjects of an original British twin study and also evaluated a new sample of twins. Consistent with previous studies, the study revealed a significant difference in the concordance rate of monozygotic (identical) versus dizygotic (fraternal) twins, 60% and 0%, respectively. Autism has been associated with a variety of clearly inherited (genetic) medical conditions including fragile X syndrome, tuberous sclerosis, Rett's syndrome, and phenylketonuria (Trottier et al., 1999). Furthermore, aberrations of almost all chromosomes, including the X and Y (sex-linked) chromosomes, have been described in some children diagnosed with autism (Gillberg, 1998). The frequency of the association of known medical conditions with autism has been a point of much debate, but the rate of concurrence is thought to be approximately 10% (Rutter et al., 1994).
Clinical descriptions of autism suggest several different types of presentations, including early onset and regression. In the early-onset cases, developmental abnormalities appear within the first year or few months of life, and may be apparent as early as birth. Most cases of autism appear to be early onset (Bristol et al., 1996); however, the diagnosis is characteristically not made until the second year of life, when symptoms become more prominent. In a second course suggested by the minority of cases, apparently normal development is followed by regression, or the sudden or insidious loss of previously established developmental milestones, which may exhibit a fluctuating pattern (Rapin, 1997; Tuchman et al., 1991). There is no scientifically established definition of regressive autism, and data are not available regarding the fundamental differences in course or other features between early onset and regressive autism. The distinction is drawn by the reported time-course of developmental abnormalities. Differentiation between these two courses of autism may be confounded by delayed parental recognition of developmental problems that were actually present much earlier in childhood (Mars et al., 1998; Rogers and DiLalla, 1990; Tuchman and Rapin, 1997). Furthermore, it is possible that the regressive form does not represent actual regression of development but rather a failure to progress (Volkmar, 2001). It is an important possibility that regressive autism is a manifestation of a later insult that exacerbates an earlier insult, such as those outlined above. There are conflicting views regarding the frequency and timing of regression, and these are the subject of current research efforts aimed at producing a better understanding of this course of autism. Below, the specific diagnoses classified under ASD or PDD are briefly described.
Autistic disorder occurs more often in boys than girls and is thought to have multiple etiologies that are not well described. Genetic factors are known to have a very strong influence in the etiology (Rutter et al., 1997). The standard criteria used for diagnosis, as described in DSM-IV/ICD-10 (see Table 2), include qualitative impairments of social interaction, such as lack of emotional reciprocity and failure to develop peer relationships; qualitative impairment in spoken or behavioral communication; and restrictive, repetitive and stereotyped
Page 24
|
A. A total of at least six items from (1), (2) and (3), with at least two from (1), and one each from (2) and (3):
(1) Qualitative impairment in social interaction, as manifested by at least two of the following:
(a) marked impairment in the use of multiple nonverbal behaviors such as eye-to-eye gaze, facial expression, body postures, and gestures to regulate social interaction; (b) failure to develop peer relationships appropriate to developmental level; (c) markedly impaired expression of pleasure in other people's happiness; (d) lack of social or emotional reciprocity. (2) Qualitative impairments in communication as manifested by at least one of the following:
(a) delay in or total lack of the development of spoken language (not accompanied by an attempt to compensate through alternative modes of communication such as gestures and mime); (b) in individuals with adequate speech, marked impairment in the ability to initiate or sustain conversation with others; (c) stereotyped and repetitive use of language or idiosyncratic language; (d) lack of varied spontaneous make-believe play or social imitative play appropriate to developmental level. (3) Restricted repetitive stereotyped patterns of behavior, interests and activities, as manifested by at least one of the following:
(a) encompassing preoccupation with one or more stereotyped and restricted patterns of interest that is abnormal either in intensity or focus; (b) apparently compulsive adherence to specific, nonfunctional routines or rituals; (c) stereotyped and repetitive motor mannerisms (e.g., hand or finger flapping or twisting, or complex whole body movements); (d) persistent preoccupation with parts of objects. B. Delays or abnormal functioning in at least one of the following areas, with onset prior to age three: (1) social interaction, (2) language as used in social communication, or (3) symbolic or imaginative play. C. Not better accounted for by Rett's Disorder or Childhood Disintegrative Disorder. |
Reprinted with permission from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Copyright 1994 American Psychiatric Association.
Page 25
behaviors, interests, and activities (APA, 1994; WHO, 1993). By definition these symptoms must be present prior to 36 months of age. They are usually recognized by the caretaker during the first 2 years of life and include concerns about language delays, hearing impairment, and impaired social interaction (Volkmar, 2001).
Childhood disintegrative disorder, also known as Heller's syndrome or disintegrative psychosis, is marked by a precipitous developmental deterioration of a normally progressing child, usually between 3 and 4 years of age (Filipek et al., 1999; Volkmar and Lord, 1998). The symptoms overlap those of autistic disorder but are more severe, with minimal recovery of lost language, motor, and social skills. CDD has a greater association with seizure risk than does autism (Tanguay, 2000). Because of the period of normal development followed by loss of skills in CDD, it is similar to the regressive course of autism, which as described above, is not well-defined (Filipek et al., 1999).
Asperger's syndrome, which primarily affects males, overlaps with the characteristics of autistic disorder in numerous areas and is sometimes considered a “higher-functioning” form of autistic disorder (Tanguay, 2000). Individuals with Asperger's exhibit a preservation of language skills compared to those diagnosed with autistic disorder. The DSM-IV criteria for Asperger's are the same as those for autistic disorder with respect to social impairments and abnormal patterns of behavior and interests but describe no evidence of significant language impairment and near-normal IQ. Because of the less-impaired language development, the syndrome often becomes apparent later in childhood (3-4 years of age).
Rett's syndrome, first described in 1966, is evident only in girls and only after a brief period of normal development. The syndrome is marked by development of motor impairments (apraxia and ataxia), deceleration of head growth, profound mental retardation, and breathing dysfunctions. Rett's disorder is caused by an X-linked, dominantly expressed genetic mutation that is nearly always lethal in males (Amir et al., 1999; Tanguay, 2000; Volkmar and Lord, 1998). Recent studies, however, suggest that males can also be affected by a form of Rett's Syndrome (Clayton-Smith et al., 2000; Salomao et al., 1999).
PDD-NOS, or atypical autism, is a residual category for subtypes of autism that have many of the characteristics of other pervasive developmental disorders, such as abnormal social interaction and communication skills, but that do not meet the strict DSM-IV/ICD-10 diagnostic criteria for one of the previously described disorders. Atypical autism also includes autistic characteristics that develop after 36 months of age (Volkmar and Lord, 1998).
Epidemiology of Autistic Spectrum Disorders
There is considerable uncertainty about the prevalence (the proportion of individuals in a population with a given condition) and incidence (the number of new cases) of autistic disorder and other ASD and their trends over time. A recent
Page 26
review of 23 epidemiological studies from multiple countries that were published in English between 1966 and 1998 found estimates of the prevalence of autistic disorder ranging from 0.7 per 10,000 to 21.1 per 10,000, with a median value of 5.2 per 10,000 (Fombonne, 1999). 1 In a recent unpublished update of this review, which includes 32 studies conducted between 1966 and 2001, prevalence rates of autistic disorder ranged from 0.7 per 10,000 to 72.6 per 10,000, with a median value of 8.7 per 10,000 (Fombonne, 2001b). After excluding studies with low precision and focusing on recent surveys, the best conservative estimate of the prevalence of autistic disorder is thought to be 10 per 10,000 (Fombonne, 2001b). A separate review of 18 epidemiological studies conducted outside the United States between 1966 and 1997 also concluded that the most reasonable conservative (mean) estimate of the prevalence of autistic disorder is about 10 in 10,000 children (Gillberg and Wing, 1999).
These figures do not include other categories of ASD such as Asperger's syndrome, childhood disintegrative disorder, Rett's syndrome, or atypical autism. Clearly, prevalence estimates would be higher if these categories were included. While no large-scale studies have been conducted on the prevalence of other ASD, estimates suggest that their prevalence is more frequent than the prevalence of autistic disorder (Fombonne, 1999, 2001b; Gillberg and Wing, 1999).
Most of the published literature is uninformative for gauging trends in autism rates (Fombonne, 1999, 2001b). Although recent reviews have concluded that the prevalence of autism has increased over time (Fombonne, 1999, 2001b; Gillberg and Wing, 1999), many of the studies examined varied in terms of their diagnostic criteria, case-finding methods, participation rates, precision, and the age and size of the populations studied. Thus, it is difficult to discern how much of the observed increase is real or possibly due to other factors, such as the adoption of a broader diagnostic concept of autism, improved recognition of autism, or variations in the precision of the studies (Fombonne, 1999, 2001b; Gillberg and Wing, 1999). Time trends can be evaluated only in studies in which these parameters are held constant.
Furthermore, even if the prevalence of autism has increased over time, this trend cannot be interpreted as evidence of an increase in the incidence of autism. Standardizing study methodology and focusing on incidence rather than prevalence will facilitate comparisons across populations and analysis of trends in autism over time.
Information about rates of autism in the United States and changes in incidence or prevalence is limited, reflecting a lack of epidemiological research on autism in this country. In the recent review by Gillberg and Wing (1999), only two major United States prevalence studies met the criteria for inclusion (Burd et al., 1987; Ritvo et al., 1989). These studies, both of which were published in the 1980s, were based on sufficiently large populations to avoid the risk of chance findings (Gillberg and Wing, 1999). The two studies provide similar prevalence
1 The review included only studies that surveyed autism in clearly demarcated, non-overlapping samples. (Fombonne, 1999).
Page 27
estimates of autistic disorder, 3.3 per 10,000 and 3.6 per 10,000, although these rates differ substantially from the prevalence rates found in non-U.S. studies conducted during the same period and more recently (Gillberg and Wing, 1999).
In a recent unpublished epidemiological study conducted by the CDC in Brick Township, New Jersey, the estimated prevalence of autistic disorder was 40 per 10,000 (95% CI: 28–56) while the estimated prevalence of ASD was 67 per 10,000 (95% CI: 51–87) (CDC, 2000f). These rates are higher than the rates reported in previously published studies although, as noted above, there is significant controversy about the actual rate of ASD in the United States. Factors that may have contributed to the higher rates include the intensity of case-finding methods, the small size of the target population, the heightened awareness of the issue in the community, and the use of the Autism Diagnostic Observation Schedule-G (ADOS-G) diagnostic tool, which may have led to the inclusion of children with more subtle signs of ASD. The authors comment that interpreting the rate of autism in Brick Township is difficult given the lack of comparable data on the prevalence of ASD in other large and diverse populations in the United States (CDC, 2000f). As noted in the report, the in-migration of families with children with ASD to Brick Township may have led to a clustering of cases in that town and is a possible explanation for the higher rate of autism and ASD found in this study.
The previously mentioned report from the California Department of Developmental Services (1999), which showed a large increase from 1987 to 1998 in the number of children with ASD registered in the California Developmental Services system, has been widely cited as evidence of an increase in the incidence of ASD in the United States. The report stresses that the study was not designed to measure trends in autism incidence, and the data should therefore be interpreted with caution. Several methodological limitations have been cited, including the failure to account for changes over time in the population size or composition, in diagnostic concepts, in case definitions, or in age of diagnosis (Fombonne, 2001a). The lack of epidemiological data on ASD in the United States points to the need for studies to establish reliable baseline estimates of incidence and prevalence for large and diverse populations in the United States.
Evidence Regarding Association: Biologic Plausibility
Biologic plausibility relies on the existence of a scientifically viable mechanism by which the vaccine could be associated with the adverse event in question. Evidence for this association is based on the demonstration, through clinical, animal, and in vitro studies, of the mechanism. The biological plausibility of the potential association of MMR and ASD (through discussion of immunologic mechanisms and appropriate animal models) and of MMR and autism/bowel disease (through discussion of the opioid excess hypothesis, autoimmune mechanism, and isolation of the vaccine-strain measles virus in the gut) are described below.
Page 28
Mechanisms for a Potential Causal Relationship Between MMR and ASD
Immunologic Mechanisms. An increasing concern has been voiced by some that the polyvalent MMR vaccine may cause an alteration in the immune response to one vaccine component due to the effects of one or more of the other vaccine component(s) (Wakefield and Montgomery, 1999). Vaccines are given in combination, as with MMR, to reduce the number of separate vaccinations and health-care contacts that individuals must receive to achieve full immunization coverage over a lifetime. Polyvalent vaccines are tested for their immunological efficacy and for adverse host reactions to the combination (Goldenthal et al., 1995). A concern regarding simultaneously administered vaccines is the potential alteration in immunogenicity (increase or decrease) of the component vaccines (Choo and Finn, 1999; Goldenthal et al., 1995; Insel, 1995). Some evidence from animal models suggests that prior or subsequent infection with different viruses can alter the magnitude and quality of the T cell response to these viruses, and thereby alter viral clearance and tissue injury (Selin et al., 1998, 1999). This raises the possibility that concomitant infection with multiple viruses in a combination vaccine might modify T-cell-dependent immune responses to one or more of its components. However, there is no evidence that this occurs in the context of MMR vaccine. Altered immunogenicity can be attributable to various causes, including physical or chemical interactions, interactions between live viruses, or immunological interference (Insel, 1995; Schutze et al., 1989).
There are multiple mechanisms that may be involved in an alteration of immunity as a result of the administration of a vaccine. Those that are relevant in the assessment of the causal relationship between MMR and ASD are noted here.
First, the presence of an increased number of types of viral proteins from a combination vaccine may increase the potential for peptide competition for binding to major histocompatibility complex (MHC) molecules. This can then influence the T-cell response to viral peptides in that only the dominant peptides (those with the greatest affinity to MHC molecules) will elicit a response (Griffin et al., 1994). Also, similar MHC-peptide structures formed by different peptides can induce cross-reactive T-cell responses. Such responses can result in the desirable effect of cross-protection between two different viruses or in undesirable effects, including anergy, cell death, cross-reactive response to self antigens (causing an autoimmune response), or altered function of the cross-reactive T-cell, resulting in impaired resistance (Selin et al., 1998, 1999; Welsh et al., 2000).
Second, since viral replication in the host is an important factor in the induction of a robust, protective immune response, viral interference may lead to a reduced immune response that could reduce protection. This and the other mechanisms noted above may contribute to the need to modify the concentrations or strains of the individual measles, mumps, and rubella components in combination vaccines compared to single component vaccines (Andre and Peetermans, 1986; Berger and Just, 1988; Insel, 1995). Third, T-cell-mediated immune responses, by a variety of mechanisms, might be reduced for a period of
Page 29
time following infection or immunization by vaccine-strain measles virus (Karp, 1999; Marie et al., 2001).
Finally, studies have consistently shown that pre-existing or maternally derived measles virus antibodies can interfere with the response to the vaccine for the same virus during early infancy (Galletti et al., 1995; Redd, et al., 1999). Maternal antibodies may neutralize the vaccine virus before immunity develops. For this reason, the MMR vaccine is not given prior to 12 months of age, when antibody titers are still high. It has been suggested that the maternal antibody levels are lower after receiving the measles vaccine than after a natural measles infection, which may then result in lower antibody titers in the infant and, consequently, improved ability to respond to the vaccine at an earlier age (Nates et al., 1999; Pabst et al., 1992).
While in principle these mechanisms for decreased viral immunity could impair or otherwise alter clearance of one or more of the vaccine-strain viruses in MMR, there is no biological precedent or sufficient evidence from existing research to support this scenario.
Animal Models. Animal models are useful for studying the pathogenesis of human disease. However, no adequate animal model currently exists through which to study any relationship between MMR vaccination and autism. One model that is being studied by some autism researchers is the effect of neonatal and prenatal Borna disease virus (BDV) infection on brain development in rats. BDV is a RNA virus that has also been associated with some forms of psychiatric diseases in humans. The BDV-infected rat has been proposed as an adequate model of the neuroanatomical and behavioral aspects of autism induced by a persistent viral infection with a minimal inflammatory response (Carbone et al., 1991; Pletnikov et al., 1999, 2000; Rubin et al., 1999). However, BDV is not related to measles virus, and this model has not been shown to be useful in addressing the potential link between autism and the attenuated measles virus found in MMR vaccines. In addition, BDV is not illustrative of a potential postnatal viral insult or of some symptoms of ASD that are distinctly human such as verbal communication. Investigators are continuing to develop a neurovirulence rat model for mumps vaccine strains (Rubin et al., 1998, 2000). Effective primate models of vaccine safety and immunogenicity and neurobehavioral aspects of autism have been developed (Bachevalier, 1996; Kennedy et al., 1997) but are not useful at this time to study the association between MMR vaccine and ASD onset specifically.
Potential Mechanisms Linking MMR, Bowel Disease, and Autism
Opioid Excess Hypothesis. The observation of bowel inflammation or enterocolitis in a group of children with ASD (Wakefield et al., 1998, 2000) has raised the possibility that exposure to MMR vaccine is linked to inflammation-mediated intestinal permeability that results in incomplete breakdown and excessive absorption of gut-derived peptides from certain foods (Wakefield, 2001). These peptides in turn are postulated to exert opioid effects on the central nerv-
Page 30
ous system, resulting in the dysregulation of the endogenous opioid system and disruption of normal brain development (Wakefield et al., 1998).
First proposed by Hermann and Panksepp (1978), hyperfunction of the endogenous opioid system has been implicated in symptoms associated with ASD in both animals and humans including reduced socialization, decreased crying, convulsive activity, stereotyped behavior, and reduced clinging specifically in animals (Gillberg, 1995; Sahley and Panksepp, 1987). Also, children who were exposed to opiates in utero experienced similar medical problems (Sandman et al., 1990).
Suggested causes of excess opioid levels include overproduction, reduced degradation, abnormal feedback mechanisms, and developmental delay of maturational processes that reduce opioid levels in the brain (Gillberg, 1995). This hypothesis continues to be controversial, and studies have not consistently found increased beta endorphin levels in cerebrospinal fluid in patients with childhood autism (Gillberg et al., 1985, 1990; Nagamitsu et al., 1997) or symptomatic improvement after administration of an opioid antagonist such as naltrexone (Black, 1994; Campbell et al., 1993; reviewed in Chabane et al., 2000; Leboyer et al., 1992).
Autoimmune Etiology. Immune-mediated injury can be induced by a viral infection. One mechanism for such injury can be seen in the production of a cross-reactive autoimmune response to self antigens by activated T-cells and B-cells (ter Meulen and Liebert, 1993). Measles virus is known to cause post-infectious encephalitis when T-cells directed against myelin basic protein enter the CNS through loss of integrity of brain microvascular endothelial cells (Johnson, 1987; Liebert, 1997). However, no cases of vaccine-strain measles virus have been isolated in immunocompetent individuals with encephalitis (IOM, 1994a). Induction of autoimmunity due to cross-reactive immunity to self-antigens as an explanation for MMR-induced ASD or enterocolitis is unsupported because of the absence of characteristic markers for immune injury or inflammation.
Measles Viral Presence in the Gut. Although measles virus has not been isolated by culture, it has been suggested that measles virus N protein on the surface of T- and B-cells and follicular dendritic cells from the lamina propria of the terminal ileum can be identified by immuno-histochemical procedures and flow cytometry in patients with bowel symptoms and ASD (Wakefield, 2001). There are, however, concerns regarding the significance of these findings, because it has not been possible to detect measles virus genetic material using standard RT-PCR assays. Although unpublished studies (Wakefield, 2001) suggest that the presence of measles virus genetic material can be detected using very sensitive real-time RT-PCR studies (Wakefield, 2001), conventional RT-PCR should have been sufficiently sensitive to readily detect viral genetic material in these samples, given the apparent abundance of immunologically detected viral antigens. This suggests that inadvertent sample contamination or other errors may account for the real-time RT-PCR findings. It is also possible that the
Page 31
results can be explained by the presence of nonviable vaccine-strain virus in the lymphoid tissue after degradation of the viral RNA.
A published study that has investigated persistent measles virus infection in autistic patients with bowel disease is that by Kawashima and colleagues (2000). This group reported the identification, by RT-PCR, of measles virus genetic material in the peripheral mononuclear cells (PBMC) of individuals with inflammatory bowel disease (Crohn's and ulcerative colitis) and of individuals with autism and bowel disease. They further report that the measles virus sequence from the patients with ulcerative colitis and autistic enterocolitis more closely matched that of the vaccine-strain measles virus. These findings have not been replicated by other laboratories.
Similarly, over the last several years, various investigators have reported identifying vaccine-strain measles virus in the intestinal lymphoid tissues of patients with Crohn's disease (Wakefield et al., 1993, 1999). Although an association between Crohn's disease and autism has not been suggested, these findings have bearing on the plausibility argument for persistent vaccine-strain measles virus infection. The identification of vaccine-strain measles virus in Crohn's patients remains highly controversial. Virus has not been isolated from patient samples by culture. Studies seeking to detect viral antigens by immunological approaches and viral genetic material in patient samples have given conflicting results regarding the identification of measles-virus materials in the gut of patients with inflammatory bowel disease (Afzal et al., 1998, 2000a; Chadwick et al., 1998; Lewin et al., 1995; Miyamoto et al., 1995; Wakefield, et al., 1993). The majority of studies have found no evidence for the presence of measles virus or measles virus materials (either wild-type or vaccine strain) in the gut of patients with inflammatory bowel disease (reviewed in Afzal et al., 2000). Furthermore, large epidemiological studies investigating a potential relationship between measles virus (wild-type and vaccine-strain) exposure and occurrence of inflammatory bowel disease have been inconsistent in their findings and unhelpful in establishing or refuting an association (Ekbom et al., 1996; Morris et al., 2000; Pardi et al., 1999, 2000; Thompson et al., 1995).
There is support for the biologic plausibility of persistent measles virus infection, but the only reproducible and convincing evidence for this is in the CNS. This is illustrated by the documented persistence of altered measles viruses developed from wild-type strains in brain tissues of patients with subacute sclerosing panencephalitis (SSPE) (Connolly et al., 1967; Horta-Barbosa et al., 1969; Payne et al., 1969). SSPE is a rare disorder, most commonly associated with acquisition of wild-type measles infection in early life. However, studies have shown a propensity for similar tissue tropism among wild-type and attenuated viruses (Ward and DeWals, 1997). Moreover, in the case of individuals who are immunosuppressed (e.g., by HIV infection, cancer, etc.), persistent wild-type and vaccine-strain virus in tissue and cerebral spinal fluid (CSF) has been isolated (Bitnun et al., 1999; McQuaid et al., 1998; Ohuchi et al., 1987). This raises the possibility that persistent CNS infection with vaccine-strain virus could
Page 32
occur. However, vaccine-strain measles virus has not been specifically isolated in brain tissue of immunocompetent patients with SSPE, and the evidence is inadequate to accept or reject a causal relationship between SSPE and MMR vaccination (reviewed in IOM, 1994a). In fact, both measles vaccination and MMR vaccination have been associated with a marked reduction in SSPE incidence (IOM, 1994a; Ward and DeWals, 1997). Although the gut is richly innervated, there is no evidence of persistent measles virus infection in gut neurons, and the apparent localization of measles-virus antigens and genetic material in the studies by the Wakefield group is not in intestinal neurons (Wakefield, 2001).
There are a number of other reports suggesting the presence of measles virus mRNA, as detected by RT-PCR, in blood or multiple tissues at the time of autopsy in apparently healthy individuals and in individuals with a variety of disorders (Helfrich et al., 2000; Katayama et al., 1998; Kurihara et al., 2000; Ooi et al., 2000). However, such findings are controversial and have not demonstrated the presence of vaccine-strain measles virus mRNA.
Thus, with the exception of the results from two groups (Kawashima et al., 1996, 2000; Wakefield, 2001;), there is no evidence to support persistent infection with vaccine-strain measles virus except for individuals with compromised immunity. The extant evidence is internally inconsistent, supporting the need for carefully controlled studies to explore these inconsistencies. In the absence of such studies, the evidence does not demonstrate persistent vaccine-strain measles virus infection in ASD, inflammatory bowel disease, or ASD with bowel inflammation. Furthermore, it is not possible with the available evidence to describe the direction of any relationship among vaccine-strain measles virus infection, autism, and enterocolitis—i.e., is it possible that autism creates greater susceptibility to enterocolitis following a viral insult?
Evidence Regarding Association: Case Reports, Case Series, and Uncontrolled and Controlled Epidemiological Studies
Below, we summarize the case reports and epidemiological studies related to MMR and autism. The epidemiological studies are summarized in Table 3 .
Case Reports
VAERS Reports. Over the last 10 years, the Vaccine Adverse Event Reporting System (VAERS), a national passive surveillance system, has received a total of approximately 112,000 adverse-event reports for all vaccines (CDC, 2001c). Between January 1990 and January 2001, 291 unique reports (excluding foreign reports) were identified that involved MMR or another measles-containing vaccine and any of the following adverse events: autism, speech dis-
Page 33
order, mental retardation, schizophrenic reaction, colitis, ileitis, enteritis, and/or gastrointestinal disorder.2
Overall, 40% of the 291 reports involve MMR vaccine alone; three reports are for measles-rubella (MR) vaccine or monovalent measles (M). The remainder of the reports involve a measles-containing vaccine along with some other vaccine. Of the 129 reports involving autism, 46% are for MMR vaccine alone; for the 38 reports involving gastrointestinal disorders, 55% are for MMR vaccine alone.
Among the 291 cases, 50% of the reports providing information on the timing of the adverse event indicate that symptoms developed within 9 days after vaccination. In 21% of these cases, symptoms manifested themselves the same day as the vaccination. The time interval was listed as unknown in approximately 24% of reports. The average time interval was comparable for gastrointestinal conditions but was longer for reports of autism.
About 54% of these 291 reports were for children aged 12-23 months at recognition of the adverse event. Males account for 63% all reports, but for the 129 cases with an outcome of autism they account for 88%, which is consistent with the higher proportion of males seen with many developmental disorders.
Copies of the VAERS reports were made available by CDC to the committee for review. The reports varied substantially in the amount of detail and supporting documentation provided. At the March meeting, FDA reported to the committee on plans for a follow-up study of the VAERS reports on autism to develop more detailed documentation of the timing and clinical characteristics of each case using standard assessment tools (Woo, 2001).
Nevertheless, the committee concluded that these case reports were not informative on the issue of causality. The analytic limitations of passive surveillance systems like VAERS (e.g., underreporting, lack of detail, inconsistent diagnostic criteria, inadequate denominator data) are well known (Ellenberg and Chen, 1997; Singleton et al., 1999). However, well-documented reports of similar outcomes in response to an initial exposure to a vaccine and a repeat exposure to the same vaccine, referred to as “rechallenge,” would constitute strong evidence of an association. No rechallenge cases for regressive autism in response to MMR vaccination were identified in the VAERS case reports provided to the committee. Possible rechallenge cases from another source are discussed in a subsequent section.
UK Working Party on MMR Vaccine. In the United Kingdom, the Medicines Control Agency convened a working group to review and assess several hundred case reports of children who had developed autism, Crohn's disease, or similar disorders after vaccination with MMR or the measles-rubella (MR) vaccine (MCA, 1999). It collected additional information about the possible adverse events, including sending questionnaires to parents and physicians who had cared for these children.
2 Cases identified for each outcome category are not unique. Cases may fall into multiple categories.
Page 34
|
Reference |
Type of Study |
Study Subjects |
Controls |
|
Wakefield et al., 1998 (United Kingdom) |
Case series |
12 children with a history of normal development followed by a loss of acquired skills, and gastrointestinal symptoms. In 8 of the 12 children, the onset of behavioral problems was associated by a parent or physician with MMR vaccination. |
None |
|
Peltola et al., 1998 (Finland) |
Case series |
31 vaccinees who developed gastrointestinal symptoms after MMR vaccination; drawn from adverse event reports sent to Finland's national passive surveillance system between 1982 and 1996. |
None |
|
Patja et al., 2000 (Finland) |
Case series |
169 vaccinees who reported 173 serious adverse events following MMR vaccination; drawn from adverse event reports sent to Finland's national passive surveillance system between 1982 and 1996. |
None |
|
Dales et al., 2001 (United States) |
Ecological |
Samples of children born in 1980-1994, enrolled in California (CA) kindergartens; cases of children with a diagnosis of autistic disorder reported to the CA Department of Developmental Services between 1980 and 1994. |
None |
|
Kaye et al., 2001 (United Kingdom) |
Ecological |
305 children 12 years of age and younger diagnosed with autism between 1988–99. Cases were identified through the UK general practice research database. |
None |
Page 35
|
Gillberg and Heijbel, 1998 (Sweden) |
Ecological, reanalysis of data from 1991 population study of autism (Gillberg et al.) |
55 children with autistic disorder (according to DSM-III) and 19 children with atypical autism (according to ICD-10) divided into 2 birth cohorts (as proxy for MMr exposure): individuals born 07/01/80 - 12/31/84 (post-MMr); individuals born 01/01/75 - 06/30/80 (pre-MMR) |
None |
|
Taylor et al., 1999 (United Kingdom) |
Cross-sectional prevalence study |
498 children with ASD: including 261 with typical (core) autism; 166 with atypical autism; and 71 with Asperger's syndrome. Subjects identified from special needs/disability registries and special schools in 8 health North Thames districts, UK |
Self-controlled and population controls |
|
Fombonne et al., 2001b (unpublished, under review) (United Kingdom) |
Ecological |
2400 autistic individuals born 1959–1993, divided into 4 birth year cohorts (as a proxy for changes in measles vaccine exposure): 1959–1967; 1968–1986; 1987–August 1991; September 1991-1993 |
4640 individuals with Down Syndrome |
|
Fombonne et al. 2001b (unpublished, under review) (United Kingdom) |
Cross-sectional survey |
Representative group of 97 individuals with ASD identified through a survey in Stafford (UK), who had received the MMR vaccine |
68 autistic subjects exposed to MMR and 89 subjects not-exposed to MMR |
NOTE: Studies are ordered as they appear in the text
Page 36
The Working Party reported that its detailed evaluation of 92 cases with autism/PDD and 15 cases with confirmed Crohn's disease revealed no unusual features that suggested a novel syndrome. They found that only 8 of the 92 cases with autism and 4 of the 15 cases with Crohn's disease had evidence adequate to confirm the following elements: the diagnosis, a close temporal association between administration of the vaccine and onset of symptoms, no prior history of the disorder, and absence of an alternative cause.
The Working Party concluded that it was impossible to prove or refute the suggested associations between MMR vaccine and ASD or inflammatory bowel disease due to limitations such as selection bias and lack of a control. Based on the available evidence, the Working Party found that there was no support for a causal association between MMR and autism, and there was no cause for concern about the safety of either the MMR or MR vaccine.
The IOM committee did not independently review these cases and cannot verify their usefulness in determining causality.
Case Series
United Kingdom. Wakefield and colleagues (1998) examined 12 children (11 males, 1 female; aged between 3 and 10 years) consecutively referred to the pediatric gastroenterology department of the Royal Free Hospital and School of Medicine in London, England. These children each had a history of normal development followed by a loss of acquired skills, including language, and of intestinal symptoms (diarrhea, abdominal pain, bloating, and food intolerance).
Eleven subjects were found to have chronic or acute nonspecific colitis (non-Crohn's disease or ulcerative colitis). Eight of the 12 subjects were reported to have reactive ileal lymphoid hyperplasia; three of them also had colonic lymphoid hyperplasia, and one subject had just colonic lymphoid hyperplasia. In addition, nine of the subjects were found to have lymphoid nodular hyperplasia of the terminal ileum. Urinary methylmalonic-acid excretion was significantly elevated in eight children who were tested. The authors report that there is no clear correlation between the endoscopic appearances and the histologic findings, although none of the findings from the 12 subjects were seen in a series of five ileocolonic biopsies from age-matched and site-matched controls with normal mucosa. In a later study, Wakefield and colleagues (2000) further examined the endoscopic and histopathological features of patients with developmental disorders and bowel symptoms. The cohort of 60 children includes the 12 described above.
The authors reported the following behavioral diagnoses for 10 of the children they examined: “autism” for eight subjects; “autism? disintegrative disorder?” for one subject; and “autistic spectrum disorder” for one subject. Two subjects were diagnosed with “post-vaccinial encephalitis?” or “post-viral encephalitis?”. The methods used to assess behavioral problems were not clearly stated.
Page 37
MMR vaccine was the exposure identified by parents or a doctor as linked to the onset of behavioral problems in six of the eight subjects with definitive autism and in the subject with suspected “post-vaccinial encephalitis.” The other two subjects diagnosed with autism had received MMR vaccine, but no specific exposure was linked to the onset of behavioral symptoms. Recurrent otitis media was the exposure identified for the subject with ASD; this subject had previously received MMR vaccine. Measles infection was the identified exposure in the subject with suspected “post-viral encephalitis?”; this subject also had previously received MMR vaccine. For the subject with a diagnosis of either “autism? or disintegrative disorder?”, MMR vaccine was linked to deterioration in behavior, and this subject was also reported to have shown slowed development following an earlier exposure to monovalent measles vaccine.
The time between suspected exposure and first clinical and behavioral symptoms ranged from 24 hours to 2 months, with a median of 1 week. Of the eight subjects for whom MMR had been identified as the exposure linked to the onset of behavioral problems, five had early adverse reactions (fever, rash, convulsions). Self-injury behavior was reported for three subjects; gaze avoidance (n = 2), repetitive behavior (n = 1), loss of self-help (n = 1) were also reported. One subject was reported to have recurrent viral pneumonia for 8 weeks following vaccination. For the 11 of the 12 subjects for whom age at onset was reported, the range in age at the onset of first clinical and behavioral symptoms was 12 months to 4.5 years, with a median of 15 months. The age at first bowel symptoms was reported for 6 of 12 subjects and ranged from 18 to 30 months, with a median of 19 months.
Although these findings may identify a distinctive gastrointestinal condition in a set of children diagnosed with ASD, or showing symptoms of ASD, who received MMR vaccine, they are not helpful in assessing the hypothesized causal association between MMR vaccine and autism. First, it is difficult to identify a specific time of onset of developmental and gastrointestinal problems in young children because of the overlap in timing between the typical age at which ASD symptoms are initially suspected and the schedule for MMR and other vaccinations. And second, given the relatively high vaccine coverage rates, many children with such problems will have received the MMR vaccine within months of the onset of symptoms.
Finland. In 1982 the Finnish National Board of Health and National Public Health Institute launched a long-term MMR vaccination program aimed at the elimination of measles, mumps, and rubella from Finland (Patja et al., 2000; Peltola et al., 1998). All children were to be vaccinated twice with MMR, between the ages of 14 and 18 months and at 6 years. In addition to the primary target groups, intermediate age groups were vaccinated in catch-up programs, unvaccinated adolescents were vaccinated during outbreaks, and adult groups at increase risk of exposure to these diseases (e.g., defense workers and health care workers) were also vaccinated. The live-virus MMR vaccine produced by Merck & Co., Inc. (West Point, PA) was primarily used, except in 1992-1996, when
Page 38
2,570 doses of Trivirten (Swiss Serum and Vaccine Institute, Berna, Switzerland) were administered to individuals with severe hypersensitivity. Vaccine coverage was around 95%, with almost 3 million doses distributed and approximately 1.8 million vaccines by 1996.
Following introduction of MMR, a country-wide passive surveillance system, based on reporting by health care personnel, was established to gather information about the incidence and nature of all severe adverse events following MMR vaccination. A potentially serious adverse event was defined as any event following MMR vaccination, without time limit, that met at least one of three criteria: (1) a potentially life-threatening disorder (e.g., anaphylaxis), (2) a chronic disease that possibly had been triggered by vaccination (e.g., rheumatoid arthritis, diabetes), or (3) hospitalization for reasons possibly attributable to MMR vaccine. If an event occurred, a report was filed by health care personnel. The first part of a two-part form was sent immediately, with a serum sample if possible. The second part of the form was completed 2–3 weeks later and sent with a second serum sample. Reports were evaluated, and contacts were made with the hospital or health center treating the vaccinated person if more information was needed. The authors note that passive surveillance systems may lead to under reporting and that active surveillance may more reliably detect adverse events. However, awareness of this potential problem prompted organization of an extensive campaign to encourage health care workers and the public to report serious events thoroughly.
Through 1996, a 14-year surveillance period, adverse events were reported for 437 vaccines. For 169 of these vaccines, 173 events were considered serious according to the criteria noted above. Age at the time of vaccination ranged from 13 months to 23 years. The interval from MMR vaccination to onset of symptoms ranged from a few minutes to 80 days, with peaks during the first 24 hours and at 7–10 days. These cases were grouped into several categories: death, likely allergic reactions, neurologic disorders, and miscellaneous reactions. The neurologic disorders included febrile seizures, epilepsy, undefined seizure, encephalitis, meningitis, Guillain-Barré syndrome, gait disturbance, and confusion during fever.
Patja and colleagues (2000) reviewed all 173 serious adverse events reported during this period. Of these, there was one death, 73 cases (42%) of likely allergic reactions, 77 (45%) cases of neurologic disorders, and 22 cases (13%) of miscellaneous reactions. Peltola and colleagues (1998) followed up surveillance-system reports on 31 children, aged from 1 year 2 months to 13 years at vaccination, who developed gastrointestinal symptoms, all except one after the first vaccine dose. Hospital or health records were reviewed or local public health nurses were interviewed. The interval between the reported event and follow-up ranged from 16 months to 15 years and 1 month (median of 10 years and 8 months). Neurological symptoms originally reported in these 31 children included febrile seizures (5 cases), headache (2 cases), and ataxia (1 child).
During the 14 years of MMR vaccination surveillance, no cases of ASD were reported or identified during the follow-up of the 31 children for whom
Page 39
gastrointestinal disorders were reported after vaccination. Similarly, no cases of ulcerative colitis, Crohn's disease, or any other chronic disorder affecting the gastrointestinal system were reported. The authors conclude that there is no evidence to support the hypothesis of an association between MMR vaccine and ASD or inflammatory bowel disease.
Uncontrolled, Observational (Ecological) Studies
United States. Dales, Hammer, and Smith (2001) examined trends in autism and MMR immunization coverage among young children in California to determine whether a correlation exists between the two. Data on age at first MMR immunization for children born between 1980 and 1994 who were enrolled in California kindergartens were derived from annual reviews of a sample of school records (approximately 600–1900 children per year). The California Department of Developmental Services provided data on regional service-center caseloads for children born between 1980 and 1994 and with an ICD-9 diagnosis of autistic disorder, which excludes other pervasive developmental disorders.
The authors observed a substantial increase in autism caseloads for successive birth cohorts but relatively stable immunization rates at ages 17 or 24 months. They conclude that these data do not support an association between MMR immunization and an increase in the incidence of autism.
The authors note that they were unable to link individual immunization and autism records for the same children. In addition, the data do not provide precise breakdowns of the percentage of children who received the MMR vaccine versus separate administration of monovalent or other combinations of measles, mumps, and rubella vaccines. Historical information suggests, though, that separate administration was rare in the United States during the period of study.
United Kingdom. Kaye and colleagues (2001) used population-based data from the United Kingdom general practice research database (GPRD) to conduct a time-trend analysis to estimate changes in the risk of autism, and specifically to assess the temporal relationship between MMR vaccination in the United Kingdom and the incidence of autism. The authors note that the GPRD has been used for numerous published studies and is considered to be complete with respect to vaccination records.
From GPRD records, 305 incident cases of autism in children aged 12 or younger and diagnosed between 1988 and 1999 were identified. Of these cases, 83% were male and 81% were referred to a specialist for evaluation of the diagnosis. The estimated annual incidence of diagnosed autism increased 7-fold from 0.3 per 10,000 person-years in 1988 to 2.1 per 10,000 person-years in 1999. The median age at first recorded diagnosis was 4.6 years. The authors performed further analyses to estimate the four-year risk of diagnosed autism for each annual birth cohort. These analyses were restricted to 114 boys born between 1988 and 1993 who were first diagnosed with autism between 2 and 5 years of age. The prevalence of MMR vaccination was calculated separately for
Page 40
each annual birth cohort (restricted to children who were registered with the GPRD within 60 days of birth and had at least 2 years of recorded follow up).
The 4-year risk of autism increased nearly fourfold from 8 per 10,000 person-years in 1988 to 29 per 10,000 person-years in 1993, while the prevalence of MMR vaccination remained constant at 97%. The authors hypothesized that if MMR vaccine were a major cause of the increasing incidence of autism, then the risk of autism in successive birth cohorts would be expected to stop rising within a few years of the vaccine being widely used. However, because the incidence of autism among 2- to 5–year-olds increased markedly from 1988 to 1993 while MMR vaccine coverage was over 95% for successive birth cohorts, the authors conclude that the results do not support a causal association between MMR vaccination and the risk of autism.
The authors note they did not review full clinical records for the children diagnosed with autism; such a review would be necessary to provide a more detailed characterization of these children and their diagnosis and to explore other possible explanations for the increase in the observed incidence of autism during the past decade.
Sweden. In a brief commentary, Gillberg and Heijbel (1998) reanalyzed data from a population study of autism conducted in the late 1980s in Sweden (Gillberg et al., 1991). A total of 55 children were diagnosed with autistic disorder based on DSM-III-R criteria, and an additional 19 individuals met criteria for atypical autism based on ICD-10 criteria. The MMR vaccine was introduced into Sweden for 18-month-old children in 1982, and coverage soon rose above 90%. The authors divided the subjects into two groups according to era of birth, as a proxy for exposure to the MMR vaccine: children born between January 1, 1975, and June 30, 1980 (pre-MMR), and children born between July 1, 1980, and December 31, 1984 (post-MMR). The authors hypothesized that if autism were associated with MMR vaccination, children born since July 1980, who were 18 months or younger at the time of MMR introduction, would be at increased risk of having developed autism.
The analysis indicated that 47 of the children (34 with autistic disorder, 13 with atypical autism) were born during the earlier period, and 27 (21 with autistic disorder, 6 with atypical autism) were born during the later period. Since the numbers in the later period were much less than expected had there been a strong effect at the population level of MMR on the prevalence of autism, the authors concluded that this study does not support the hypothesized association between MMR vaccine and autism. One limitation of this study is that children born from 1980 through 1984 did not have the same length of follow-up as those born earlier, with the maximum follow-up age of 4 years for those born in 1984. Although most cases of autism are diagnosed prior to this age, if there was some age dependency in the MMR effect, this study would have been unable to detect it.
Page 41
Controlled Observational Studies
United Kingdom. In a population-based study of children in eight health districts of the North East Thames region in the United Kingdom, Taylor and colleagues (1999) investigated trends in the incidence of ASD before and after the introduction of MMR vaccine in 1988 and tested for post-vaccination clustering of diagnoses or other indicators of onset. Children with ASD born since 1979 were identified in mid-1998 from computerized specialized-needs/disability registries and from records in special schools. Information was extracted on the age at which the disorder was diagnosed, the recorded age at which parents first became concerned about the child's developmental course, and the age at which regression became obvious (if it occurred). The authors identified 498 children with ASD: 261 with typical (core) autism, 166 with atypical autism, and 71 with Asperger's syndrome. When ICD-10 criteria were applied to available records, these diagnoses were confirmed for 82% of core autism cases, 31% of atypical autism cases, and 38% of Asperger's syndrome cases. While not all cases were verified according to ICD-10 criteria, most cases were documented as having been assessed by a specialist clinician. All 498 cases were included in the analyses. Data on the vaccination histories of these children were obtained from a separate regional information system. Three statistical analyses were undertaken.
First, in a time series analysis, Poisson regression was used to fit an exponential trend to the number of cases diagnosed by age 60 months for children born between 1979 and 1992. Children born later were excluded because a diagnosis might not have been made by the time of the study. The analysis of actual numbers and the fitted trend showed a significant increase in cases for core and atypical autism but produced no evidence of a sudden “step up” after MMR vaccine was introduced in the United Kingdom in either the number of cases or in the exponential trend.
The second analysis focused on children born after 1987. Among the 389 study subjects in this group, the proportion who had received MMR vaccine by their second birthday (86.4%) was reported to be similar to that for the North East Thames region in general. A further analysis was limited to 356 children who were diagnosed with core or atypical autism at age 18 months or later. Of these cases, 233 received MMR vaccine before this age, 64 never received MMR vaccine, and 59 received MMR vaccine at 18 months or later. Children with Asperger's syndrome were excluded because of small numbers and their older age at diagnosis. There were no statistically significant differences in mean age at diagnosis between those vaccinated before 18 months of age, those vaccinated afterward, and those never vaccinated. There was no temporal association between changes in the incidence of autism by birth cohort since 1987 and changes in vaccine coverage.
The final analysis focused on the timing of diagnosis, first parental concern, and regression in children who received one or more MMR vaccine doses. With one exception, the authors found no clustering of diagnosis, parental concern, or
Page 42
autistic regression in the periods following vaccination. (The authors investigated periods within 12 or 24 months after vaccination for diagnosis; periods within 6 or 12 months after vaccination for parental concern; and periods within 2, 4, or 6 months after vaccination for regression). A statistically significant clustering of parental concern was found within 6 months of diagnosis (relative incidence 1.48; 95% C.I. 1.04 – 2.12), and was attributed to a large peak in recorded parental concern at 18 months and a peak in MMR vaccination at 13 months. Since the convergence of parental reports around age 18 months may reflect recall uncertainty and age 13 months corresponds to the recommended vaccination schedule, this association was interpreted by the authors as an artifact related to the difficulty of precisely defining the onset of symptoms. The authors concluded that their analyses did not support a causal association between MMR vaccination and autism or the onset of autistic regression.
Taylor and colleagues recently updated their study by reviewing all prevalent autism cases identified by the end of 2000 in five of the eight districts in the North Thames region. Their objectives were to test whether there has been an increase in the proportion of cases with regressive features associated with MMR vaccination and whether there was an association between bowel symptoms and MMR exposure in children with and without regressive symptoms. The authors report that they found no evidence in support of these two hypotheses (Miller et al., 2001). These results have been submitted for publication and were recently presented at a conference in Cold Spring Harbor, New York (Taylor, 2001). These data have not been reviewed by the IOM committee because of the publication submission. If published in the peer-reviewed literature, these data would lend additional support to the Taylor et al. (1999) study, which found no association between MMR vaccination and autism on a population level.
United Kingdom. At the committee's public workshop on March 8, 2001, Dr. Eric Fombonne presented unpublished findings from two studies designed to address the hypothesized link between MMR vaccination and ASD. In the first study, which is currently under review, the investigators test the possible impact of change in vaccine policy in the United Kingdom on the incidence of ASD, and in particular, whether the introduction of MMR vaccine in 1988 was associated with a step-up in the incidence of ASD.
In that study, 2400 autistic subjects born between 1959 and 1993 were identified from the membership of the U.K. National Autistic Society. A group of 4,640 individuals with Down syndrome were recruited as controls. The subjects were grouped into four birth cohorts, defined on the basis of changes in measles vaccine use: (1) 1959–1967, prior to the introduction of measles-containing vaccines in the United Kingdom; (2) 1968–1986, use of only monovalent measles vaccine; (3) 1987 through August 1991, introduction of MMR
Page 43
vaccine with different vaccine products (e.g., Urabe and Jeryl-Lynn strains)3 in use; and (4) September 1991 through 1993, exclusive use of the Jeryl-Lynn strain of the mumps component in the MMR vaccine. Autism incidence rates were compared across successive periods to assess whether incidence changed as a function of vaccine use. Fombonne and colleagues found no increase in the incidence of autism from period 1 to 2 with the introduction of monovalent measles vaccine. The comparison between periods 2 and 3, when MMR was introduced, data did not show a “step-up” but rather a significant “step-down” in the incidence of autism. While this downward trend cannot be explained, the data do not support any increase in autism as a function of MMR introduction. Finally, the change to exclusive use of the Jeryl-Lynn MMR vaccine was associated with no change in the incidence rates of autism. The authors concluded from this analysis that vaccine policy changes had no effect on the incidence of autism.
The second set of research findings presented by Dr. Fombonne focused on the evidence for a new variant of ASD defined by regression and bowel symptoms. As background, he provided data and clinical descriptions predating the use of MMR vaccine demonstrating that regression has been recognized as a developmental pattern in ASD for many years and is not a new phenomenon. Three prior studies, the earliest from 1966, showed that there is wide variability in the estimated proportion of persons with ASD who manifest a regressive trajectory. Although childhood disintegrative disorder, which is characterized by normal development until age 2 or older with a rapid degeneration, is rare (Volkmar, 2001), a pattern of apparently normal development followed by fluctuating skill acquisition is well known.
Dr. Fombonne reported unpublished findings from a recent epidemiological survey in the United Kingdom that was designed to test the frequency of bowel symptoms in children with ASD and their possible association with regression using different strategies. The survey, which was conducted in Stafford, identified a representative group of 97 individuals with a diagnosis of a pervasive developmental disorder (synonymous with ASD) using the Autism Diagnostic Interview-Revised (ADI-R) instrument. Of these individuals, 96 had received the MMR vaccine. The investigator compared that group with two other groups: one comprised of 68 autistic subjects who had also been exposed to MMR and were seen in a clinic setting; the second comprised of 89 subjects, none of whom had been exposed to MMR.
Three analyses were performed. The first statistical analysis showed no differences across three samples (one pre-MMR and two post-MMR samples) in the mean age at which parents reported the first symptoms in their child, showing that in the two post-MMR samples there was no shift towards a younger age of onset as compared to the pre-MMR sample. In a second analysis, the investigator reported comparisons in the rates of regression in the epidemiological sample of 97 children, and found no evidence of an increased frequency of regression in this
3 These vaccine types refer to the mumps component of MMR. The United States uses the Jeryl-Lynn strain of the mumps component in MMR.
Page 44
post-MMR sample as compared to pre-MMR comparison group. In addition, children who regressed did not differ from those without regression in terms of age at first parental concerns or severity of autistic symptoms. A third analysis of the first epidemiological sample of 97 children, where data on gastrointestinal (GI) symptoms were also available, found no association between regression and GI symptoms in this study population. The investigator concluded that this study does not support a new variant of ASD with features of regression and bowel symptoms (Fombonne, 2001b).
Controlled Clinical Trials
No controlled clinical trials have tested the specific hypothesis of a causal association between MMR vaccine and ASD.
CAUSALITY ARGUMENT
A number of epidemiological studies (both uncontrolled and controlled) consistently provide no support for an association at the population level between MMR immunization and ASD. The study by Taylor et al. (1999) is the most extensive epidemiological study and the strongest published evidence against the hypothesis that MMR vaccine causes ASD. The case series by Peltola et al. (1998) and Patja et al. (2000) provide additional, albeit weaker, evidence of no association between MMR vaccine and ASD. In addition, four studies examined whether an ecological correlation (i.e., observations of parallel trends in the same population over time or across different populations) exists between ASD rates and MMR coverage, but found no evidence to support such an association on a population level (Dales et al., 2001; Gillberg and Heijbel, 1998; Kaye et al., 2001; Taylor et al., 1999). Although evidence of an ecological correlation would be consistent with the hypothesis that the MMR vaccine causes ASD, it would not constitute strong evidence of causality, and additional studies would be needed to establish the causal association. However, lack of an association or a negative finding does favor rejection of a causal relationship (Gellin and Schaffner, 2001; IOM, 1994a).
Findings from several unpublished studies, currently being peer-reviewed for publication, provide additional evidence of no association between MMR vaccine and ASD on a population level. The recent unpublished update (Miller et al., 2001; Taylor, 2001) of the Taylor et al. study of 1999 reportedly found no increase in the proportion of autism cases with regressive features associated with MMR vaccination and no association between bowel symptoms and MMR exposure in children with and without regressive symptoms. Similarly, the unpublished findings from a case-control study that Dr. Fombonne presented at the committee's meeting provided no evidence to support an increase in autism in the United Kingdom as a result of changes over time in measles-vaccination policy or of the introduction of MMR vaccine. In addition, in an
Page 45
epidemiological sample with assessments based on standardized diagnostic measures, he reported finding no evidence to support a new variant of ASD defined by regression and bowel symptoms or an association between bowel symptoms and regression.
Even though the collective epidemiological evidence provides no support for an association, it is important to recognize the inherent methodological limitations of such studies in establishing causality. They cannot role out the possibility of an unusual and rare response to an exposure because they do not have sufficient precision to detect very rare occurrences on a population level. A poor understanding of the risk factors and lack of a consistent case definition may also hamper the ability of epidemiological studies to detect rare adverse events. In addition, since MMR exposure is virtually universal in developed countries, elucidating any association with adverse outcomes requires imaginative use of administrative and other data sets, and complex research designs. Furthermore, because ASD is a relatively rare disorder, and because it is difficult to determine the exact onset of autistic conditions, and therefore the temporal relationship between onset and vaccination, certain epidemiological study designs (e.g., cohort studies) are impractical.
Second, the committee concludes that the original case series of children with ASD and bowel symptoms (Wakefield et al., 1998) is uninformative with respect to causality between MMR vaccine and ASD. The small number of cases, the potential selection bias (cases were referred to a gastroenterology group interested in studying the relationship between MMR vaccine and IBD), the failure to use standard methods of diagnosis, multiple diagnoses in the patients, and the lack of detail regarding the criteria for the behavioral diagnoses of the children in the series limit the utility of this study in establishing causality. Although parents made a temporal link between the onset of their children's behavioral disorders and the MMR vaccine, the authors acknowledge that their findings do not prove an association between MMR and the condition described (Wakefield et al., 1998). In addition, case reports submitted to VAERS that note a temporal association between MMR vaccination and the onset of symptoms vary substantially in their level of detail and supporting medical documentation. The committee found these reports uninformative in assessing causality.
Third, the biologic model linking MMR vaccine and ASD is incomplete and fragmentary. Potential immunologic and metabolic mechanisms have been described but have not been supported by validated and replicated controlled studies.
Finally, there is no relevant animal model. The model based on Borna disease virus infection in rats may be useful for studying the induction of symptoms of ASD by insults, especially infectious insults, to brain development during the prenatal and perinatal periods but this model is not adequate for studying the association between the MMR vaccine and the subsequent onset of ASD. Also, primate models which are effective for the study of vaccine safety and immunogenicity or the neurobehavioral aspects of ASD do not adequately represent any relationship between the MMR vaccine and ASD.
Page 46
Thus, the committee concludes that the evidence favors rejection of a causal relationship at the population level between MMR vaccine and ASD. The committee bases this conclusion on the following evidence:
-
A consistent body of epidemiological evidence shows no association at a population level between MMR vaccine and ASD.
-
The original case series of children with ASD and bowel symptoms and other available case reports are uninformative with respect to causality.
-
Biologic models linking MMR vaccine and ASD are fragmentary.
-
There is no relevant animal model linking MMR vaccine and ASD.
However, the committee notes that its conclusion does not exclude the possibility that MMR vaccine could contribute to ASD in a small number of children, because the epidemiological evidence lacks the precision to assess rare occurrences of a response to MMR vaccine leading to ASD and the proposed biological models linking MMR vaccine to ASD, although far from established, are nevertheless not disproved.
SIGNIFICANCE ASSESSMENT
In contrast to previous IOM vaccine-safety studies, which limited their conclusions to causality assessments and their recommendations to future research directions, the Immunization Safety Review Committee has been asked to make recommendations regarding a broad range of actions—including potential policy reviews, for example, and changes in communication to the public and to health care providers about issues of vaccine safety. In so doing, the committee considers the significance of the hypothesized associations between vaccines and adverse events in a broader social context—the context in which policy decisions must be made. These considerations include the burden (i.e., the seriousness, risk, and treatability) of the adverse health event in question, the burden of disease that the vaccine prevents, and the level and potential consequences of public concern about the safety of vaccine use.
Public concerns about immunization safety are particularly important to understand and to weigh, because most vaccines are given to children not only for their direct protection but also to help protect others in the population. In fact, to achieve this broader level of protection, vaccinations are mandatory in all 50 states for school and daycare entry. Exemptions on medical grounds (contraindications) are allowed though they are considered too limited by some (Fisher, 2001), and exemptions on religious and philosophic grounds are also allowed in 48 and 15 states, respectively (Evans, 1999). But such exemptions are rare, and it is argued that these public health mandates, particularly because they are imposed on healthy children, place a particular responsibility on the government for rigorous attention to safety issues, even for rare adverse outcomes.
Page 47
Basis for Concern Regarding ASD as a Possible Adverse Event
Autistic spectrum disorders are serious and incurable developmental disorders characterized by deficits in communication and behavioral, emotional, and social functioning. As the review of the literature on the epidemiology of ASD demonstrated, there are no agreed-upon estimates of the prevalence or incidence of ASD in the United States. ASD cannot be cured, but behavioral therapies are used to manage symptoms (Wing, 1997) and medication may help alleviate symptoms such as hyperactivity, anxiety, and repetitive behavior (Lainhart and Piven, 1995). Furthermore, early educational and social interventions may improve functioning and integration into society for some children with ASD (Harris and Handelman, 1997; Howlin and Goode, 1998)
ASD leads to substantial challenges for the families of affected individuals because many people with ASD remain dependent throughout their lives. Many families may spend $18,000 on special education costs per year, and $30,000 per year for more specialized programs. The annual cost of care in a residential school may be as much as $80,000–100,000 (CDC, 1999). In addition to the substantial financial strains, families of children with ASD face other demands. During its public meeting (March 2001), the committee heard about the difficulties of caring for autistic children firsthand. Parents described around-the-clock efforts to care for their child, the difficulty of finding knowledgeable and sympathetic health-care providers, the challenges in finding high-quality information, and the frustrations of seeing their child regress from being active and engaged to being aloof and nonresponsive.
Although ASD is recognized as a serious condition and many strides have been made in understanding the disease in many areas, significant gaps remain in understanding its etiology and risk factors. These gaps include uncertainty about trends in the prevalence and incidence; limited knowledge about the natural history of ASD, including early-onset and regressive forms; the lack of a strong biologic model for ASD; limited understanding of potentially associated features (e.g., immune alterations, enterocolitis); and no current basis for identifying possible subtypes of ASD with different pathogenesis related to genetic and environmental interactions. Research has been hindered by changing case definitions and the heterogeneity of study populations that may include cases linked to other known medical risk factors (e.g., Fragile X).
Basis for Concern Regarding Prevention of Measles, Mumps, and Rubella
Disease Burden
Measles is a serious infectious disease that causes significant morbidity and mortality. Complications of measles infection include otitis media (in 7–9% of cases), pneumonia (in 1–6% of cases), diarrhea (in 6% of cases), postinfectious encephalitis (in .1–.2% of cases), and subacute sclerosing panencephalitis (in .001–.018% of cases) (Redd et al., 1999; Miller et al., 1992). Most measles cases are not treated directly, but antiviral agents and other preparations are sometimes
Page 48
used in serious infections (Redd et al., 1999) and other treatments may be appropriate for certain symptoms (e.g., fever, pain) or complications (e.g., pneumonia) (Gershon, 1998). In the United States, measles is fatal in 1.0–2.0 per 1000 cases (CDC, 1998). Worldwide, approximately 1 million children die each year from measles infection (CDC, 1997c).
Mumps and rubella, though not as serious as measles, carry the risk of serious complications. As with measles, treatment is usually limited to certain symptoms and complications. The classic symptom of mumps is parotitis, which occurs in 15–20% of cases (Plotkin and Wharton, 1999). A serious complication of mumps in children is sensorineural deafness. Other complications, which occur more often in adults than children, include orchitis, pancreatitis, mumps meningoencephalitis, and mastitis (Plotkin and Wharton, 1999).
Acquired rubella, although a benign disease in itself, can cause such complications as arthralgia and arthritis in adults. Less common complications are thrombocytopenia and encephalitis (Plotkin, 1999). The greatest concern centers on rubella in pregnant women, because the infection may affect the developing fetus, causing a variety of teratological and inflammatory abnormalities, referred to as congenital rubella syndrome (CRS). It is estimated that CRS occurs in about 20–25% of infants born to women who were infected with rubella during the first 20 weeks of pregnancy (CDC, 1998), but at least one study found that 85% of infants contracted CRS when their mothers were infected with rubella at 8 weeks into the pregnancy (Peckham, 1972). The most common CRS abnormalities are sensorineural deafness, cataracts, pigmentary retinopathy, and patent ductus arteriosus (Plotkin, 1999). Rubella infections during early pregnancy can also lead to miscarriages, stillbirths, and early terminations of pregnancy (CDC, 1998).
Impact of Immunization on the Incidence of Measles, Mumps, and Rubella
The introduction of vaccines against measles, mumps, and rubella has brought dramatic reductions in the incidence of these diseases. Prior to the introduction of the measles vaccine in the United States in 1963, an average of 400,000 measles cases were reported each year (CDC, 1998) ( Figure 1). Since most children acquired measles, this number is likely to be a serious underestimate, attributable to underreporting and other factors. A more accurate estimate of measles incidence prior to 1963 is probably 3.5 million to 4 million cases per year, essentially an entire birth cohort (CDC, 1998). One analysis suggests that the 4 million cases of measles per year in the U.S. resulted in the following complications per year: 150,000 cases of respiratory complications, 100,000 cases of otitis media, 48,000 hospitalizations, 7,000 instances of seizures, and 4,000 cases of encephalitis (Bloch et al., 1985). Using the incidence rate of 4 million cases per year and the measles case fatality rate of 1.0–2.0 deaths per 1,000 cases (CDC, 1998), an estimated 4,000–8,000 deaths would have occurred annually from measles complications.
Page 49
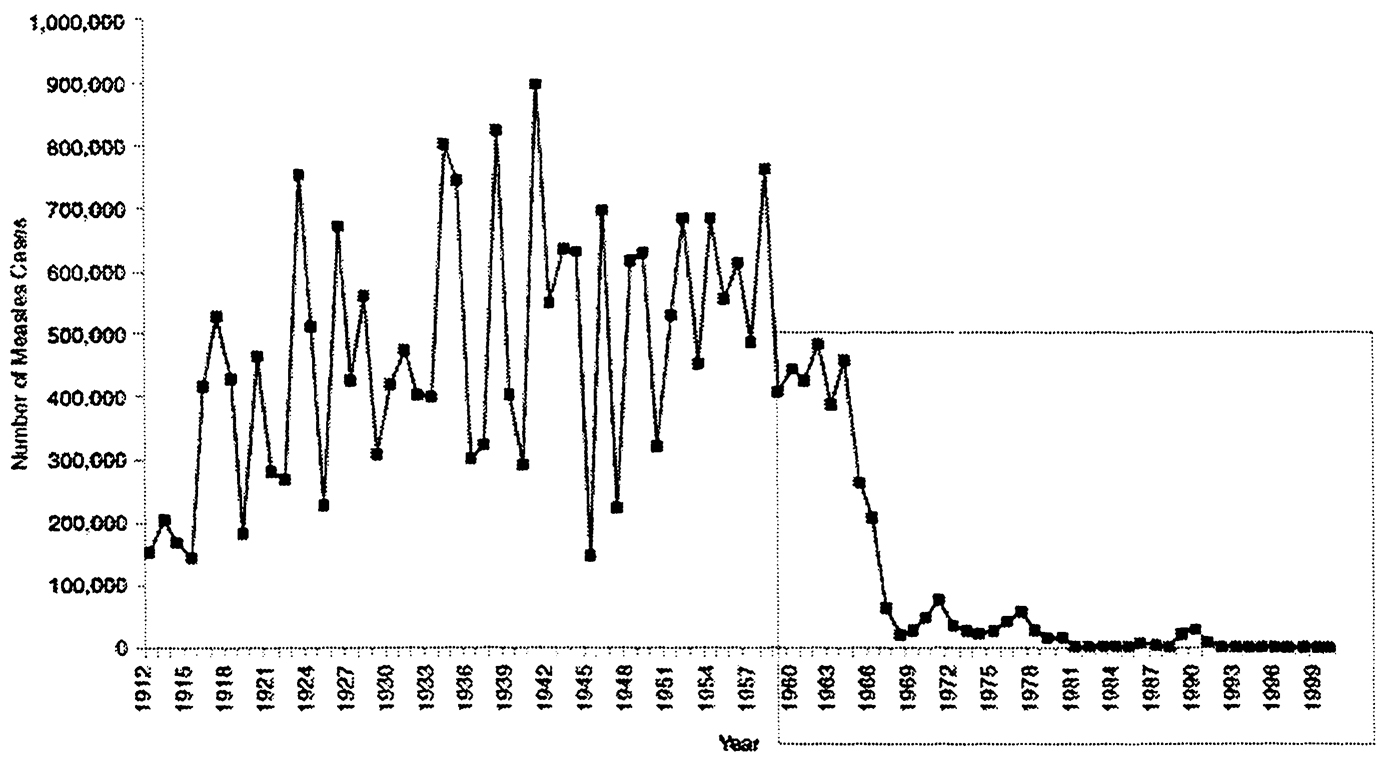
~ enlarge ~
FIGURE 1 Measles cases per year in the U.S. (1912–2000). SOURCE: CDC, 2001a.
With the measles, mumps, and rubella vaccines available, diseases prevented by these vaccines have declined ( Figure 2) and vaccine coverage rates have increased. Measles cases decreased to 22,000–75,000 per year through the late 1970s (CDC, 1998). During the period from 1981 to 1988, following the introduction of the current MMR vaccine, there were generally fewer than 5,000 cases per year, but the number rose to almost 28,000 cases in 1990 during a serious measles outbreak (Atkinson, 1992; CDC, 1998). By 1993, however, with renewed immunization efforts, transmission of indigenous measles in the United States ceased (Watson et al., 1998). In 1999, only 100 cases of measles were reported, and a majority of these were imported or import-linked cases (CDC, 2000b). By 2000, measles was no longer considered endemic in the United States (CDC, 2000b).
Similarly, there have been substantial declines in the numbers of mumps and rubella cases. Since the introduction of the mumps vaccine in 1967, cases declined from 185,691 in 1968 to 391 in 1999 (CDC, 1998, 2001e). Following U.S. licensure of the rubella vaccine in 1969 (CDC, 1998), rubella cases decreased from 57,600 in 1970 to 271 cases in 1999 (CDC, 1998, 2001e). Similarly, during the last U.S. rubella epidemic, an estimated 20,000 cases of CRS occurred in 1964–1965 before the rubella vaccine became available (CDC, 1998). In 1999, only 6 cases of CRS were reported (CDC, 2001d).
A combined MMR vaccine was originally introduced in 1971 and replaced by the current MMR vaccine in 1979. By 1998, MMR vaccination coverage had reached its highest level ever, with an estimated 92% of children aged 19–35 months vaccinated (CDC, 2000d). The coverage estimate for 1999
Page 50
is slightly lower, at 90.6% (CDC, 2000a). 4 With coverage rates at this level, it means that each year about 3.4 million children aged 12–24 months receive the MMR vaccine.4 The targeted vaccine coverage rate needed to eliminate indigenous measles in the United States is estimated to be roughly 90% or greater in each successive birth cohort at age 12 months (CDC, 1997b). However, factors such as population density across areas of the U.S. (i.e., urban versus rural areas), population movement, and immigration of individuals from countries with inadequate levels of immunization coverage may alter the vaccine coverage rates needed to eliminate indigenous cases (Fine, 1993; CDC, 2000b).
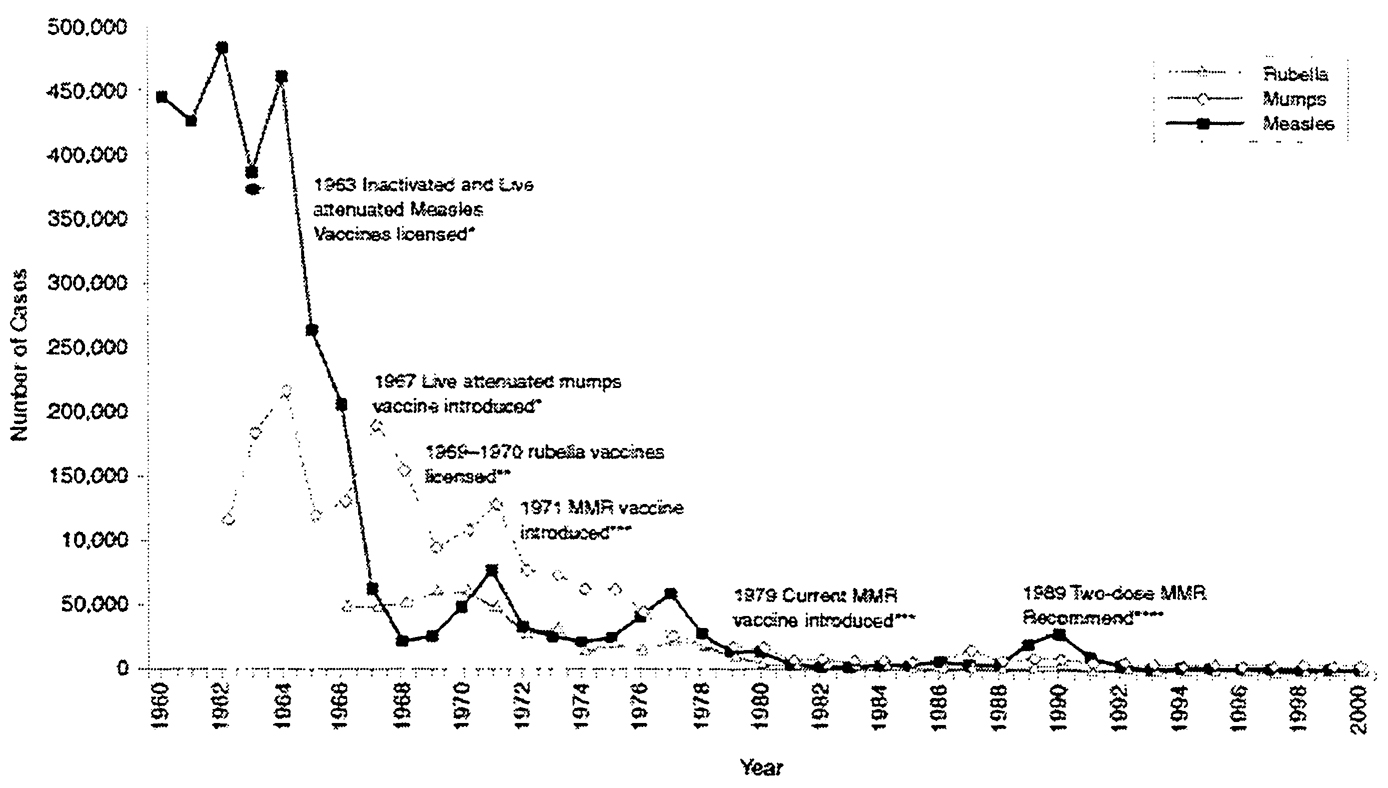
~ enlarge ~
FIGURE 2 Measles, mumps, and rubella cases per year in the U.S. (1960–2000). “Licensed” means FDA approval for use in the U.S. “Introduced” implies that the vaccine was recommended for use in the immunization program. SOURCES: CDC, 2001a. *CDC, 1998; **Plotkin, 1999; ***Redd et al., 1999; ****CDC, 1989.
Immunization Gaps and Disease Risk: U.S. Measles Outbreak in 1989–1991
Although measles cases are currently at an all time low in the United States, there is reason to be concerned that reductions in immunization coverage could still lead to disease outbreaks. As recently as 1989–1991, inadequate vaccination coverage, specifically in preschool-aged children, led to a major measles outbreak in the United States. More than 55,000 cases of measles were reported during the outbreak's 3 years, compared with about 12,000 cases during the previous 3 years (CDC, 1998). There were more than 120 deaths (CDC, 1998), with a majority occurring in preschool-aged children (55%) (Orenstein et al., 1993). Infants less than 12 months old accounted for 22% of those deaths. In addition, there were 11,000 hospitalizations, and about 20% of reported cases experienced one or more complications, including diarrhea (9%), otitis media (8%), pneumonia (6%), and encephalitis (0.1%) (Orenstein et al., 1993).
Page 51
Prior to 1989, most measles cases were reported in school-age children (5– 19 years) rather than in preschool-aged children (less than 5 years). From 1980 to 1988, the median percentage of reported cases in children less than 12 months old was 8%. During the 1989–1991 outbreak, however, the majority of cases occurred in the younger children, with up to 19% of reported cases in children less than 12 months old (Orenstein et al., 1993).
The outbreak demonstrated the vulnerability of unvaccinated younger children and led to efforts to increase immunization coverage. The current recommendation for a two-dose MMR immunization schedule aims at maximizing protection for infants and school-aged children alike. The current high rates of vaccination and historically low rates of disease support the effectiveness of this strategy. At the same time, smaller measles outbreaks continue to occur in unvaccinated populations, pointing to the continued risk of disease. In 1994, outbreaks occurred in Missouri and Illinois, where all of the cases were unvaccinated (CDC, 1994). In outbreaks in Utah and Alaska in 1996, the majority of measles infections occurred in persons who were not vaccinated or who had received only one dose of a measles-containing vaccine (CDC, 1996, 1997a). In a recent outbreak in the Netherlands from 1999 to 2000, 95% of the cases were among unvaccinated populations, the majority of whom (84%) had declined vaccination for religious reasons (CDC, 2000c).
Level and Potential Consequences of Public Concern Regarding MMR and ASD
Publication of reports hypothesizing an association between the MMR vaccine, bowel disease, and ASD (e.g., Thompson et al., 1995, Wakefield et al. 1998, Wakefield and Montgomery, 2000) has resulted in extensive and continuing attention from the public, the media, and health officials in the United States and the United Kingdom. While the media attention to this issue may be a reflection of the public's concerns about the possible link between MMR vaccine and ASD, it may also be helping to maintain and spread such concern, despite reassurances from health professionals.
UK Response to Concerns about MMR Vaccine
In the United Kingdom, MMR coverage rates for 2-year-old children declined from 92% in 1995 to 88% in 1998, where they appear to have stabilized (Communicable Disease Report, 2001). Researchers and public health authorities have suggested that publicity—in particular, coverage of the studies proposing a link between the vaccine and bowel disease and ASD—may be a factor
Page 52
in the decrease in MMR coverage (Communicable Disease Report, 2001; Pareek and Pattison, 2000; Thomas et al., 1998).
The United Kingdom's Department of Health has taken various steps to deter any further decline in MMR coverage rates. Soon after publication of the 1998 study (Wakefield et al., 1998) and after numerous media reports about the study, the Medical Research Council (MRC) held an independent scientific seminar with an ad-hoc committee of experts to review the evidence. The group reported finding no evidence to support a link with MMR and a change in MMR vaccination policy (MRC, 1998).
The findings of the Working Party on MMR Vaccine, an advisory group created by the Medicines Control Agency (MCA) in 1998, have already been discussed in the committee's review of evidence for its plausibility assessment. The group concluded in June 1999 that the available evidence did not support the suggested causal associations or give concern about the safety of MMR or MR vaccines (MCA, 1999).
Early in January 2001, two independent scientific advisory committees— the Committee on Safety of Medicines, which advises the British government on the licensing and safety of human medicines, including vaccines; and the Joint Committee on Vaccination and Immunization, which advises on vaccination/immunization policy—issued a press release reassuring the public that the MMR vaccine is safe (U.K. DOH, 2001a). However, in late January 2001, a paper questioned the safety of the MMR vaccine and suggested that the vaccine was licensed prematurely (Wakefield and Montgomery, 2000.) In response, numerous professional and medical organizations issued statements reiterating their confidence in the safety of the MMR vaccine (MCA and DOH, 2001; U.K. DOH, 2001b). Also in January 2001, the Department of Health announced a £3 million publicity campaign targeted to health professionals and parents, in order to counter publicity that called the safety of the MMR vaccine into question.
Evidence of Concern about MMR in the United States
There are several possible indicators of heightened public awareness or concern about a possible association between MMR vaccination and ASD. Reports to VAERS concerning MMR vaccine and ASD appear to have increased sharply from 29 in 1998, to 47 in 1999, and 75 in 2000. Of all such reports, 55% have been received since 1998. For VAERS reports on all vaccines, about 10,000 are received each year, and about 20% are classified as serious (Braun and Ellenberg, 1997).
The increase in VAERS reports on MMR vaccine may be due to publicity about the issue. From December 1996 to February 2001, 184 news stories on MMR vaccine and ASD appeared on television and radio; and from April 1997 to March 2001, 110 such stories appeared in print (CDC, 2001b). Although the connection between publicity and the number of VAERS reports is difficult to measure directly, there are suggestive patterns. For example, a segment on MMR vaccine and autism that appeared on CBS's 60 Minutes in November
Page 53
2000 might have contributed to a surge in reporting to VAERS; 43% of VAERS reports submitted in 2000 on measles-containing vaccine and autism, speech disorder, mental retardation, schizophrenic reaction, colitis, ileitis, enteritis, and/or gastrointestinal disorder arrived in the reporting quarter from October– December 2000.
Changes in immunization rates might also signal public concern about a vaccine. In 1998, MMR coverage in children aged 19–35 months was at its highest—92.1% (+/− 0.6, 95% CI)—but it decreased slightly to 90.6% (+/−0.6, 95% CI) in 1999 (CDC, 2000a,d). Coverage rates for other vaccines also declined slightly from 1998 to 1999 (CDC, 2000a,d).
The Internet, through which reports from a broad range of sources can be easily accessed and shared, has emerged as an important mechanism for gathering and exchanging information about vaccine-safety concerns (Gellin et al., 2000). But the quality and reliability of such information varies. For some, the Internet merely complements information obtained through published sources and through conversations with health care providers and other personal interactions. For others, however, the Internet has become a primary information source.
Various organizations and institutions have responded to the public's concern over the safety of the MMR vaccine. Since August 1999, the U.S. House of Representatives Committee on Government Reform has held seven hearings on vaccine safety. In April 2000, a hearing specifically on autism and vaccines included testimony from federal researchers, health care providers, and parents of autistic children. Parents reported on the challenges in caring for autistic children, such as the difficulties in finding medical experts, therapies, research, and appropriate education. The June 2000 American Academy of Pediatrics conference on ASD and vaccines has already been mentioned. The meeting was open to the public and included presentations from experts in epidemiology, gastroenterology, autism, virology, immunology, and neurobiology and from representatives of advocacy organizations. A report of the conference findings on the hypothesized link between MMR vaccine and ASD was released in May 2001 subsequent to the finalization of this report.
Potential Consequences: Evidence from Pertussis Outbreaks in the 1970s
There is concern that because of the seriousness of ASD and the many unknowns related to its etiology, the current focus on the possibility of a causal link between MMR and autism may alter immunization practices in ways that increase the risk of measles, mumps, or rubella infection. For example, some have suggested using monovalent vaccines in place of MMR (Wakefield and Montgomery, 2000). However, there is concern that substitution of separate monovalent vaccines will prove less effective in controlling disease. Use of three separate vaccines is likely to delay immunization against at least two of the three diseases compared with use of MMR. The added burden of additional health care visits for separate vaccinations may contribute to further delays. Such changes, or a net decline in immunization coverage for measles, mumps,
Page 54
and rubella, could cause serious public health problems. The history of wholecell pertussis vaccine use provides evidence that measurable reductions in vaccination coverage can occur in response to concerns about vaccine safety and that increases in disease can result.
Several influences contributed to a marked decline in the 1970s in pertussis-vaccine coverage in a number of countries, including Japan, Sweden, and the United Kingdom (Gangarosa et al., 1998). There was an assumption that pertussis was no longer a serious health problem, given the low number of pertussis cases. There also was publicity about risks of adverse events attributed to the vaccine, and some respected medical and public health leaders questioned the safety of the vaccine. As the public's confidence in the protective effects of the vaccine decreased, use of the vaccine was either interrupted or declined markedly. As coverage rates decreased, pertussis cases increased and several epidemics occurred. In Sweden from 1974 to 1979, DPT (diphtheria and pertussis and tetanus toxoids) vaccine coverage decreased from 90% to 12%, and in subsequent years more than 10,000 pertussis cases were reported annually. In Japan from 1974 to 1976, pertussis coverage dropped from 80% to 10%; in an epidemic in 1979, 13,000 cases were reported and 41 people died. In the United Kingdom, from the late 1960s to the late 1970s, vaccine coverage declined from 81% to 31%. From 1976 to 1988, three epidemics accounted for 300,000 cases of pertussis and at least 70 deaths (Gangarosa et al., 1998; Nicoll et al., 1998).
Conclusion
In its significance assessment, the committee considered the burden (seriousness, risk, and treatability) of the vaccine-preventable diseases (measles, mumps, and rubella), the burden of the potential adverse event (ASD), and the level of public concern surrounding this issue. Measles, mumps, and rubella are serious infectious diseases that can lead to significant morbidity and mortality. Treatment of these diseases and their associated complications is limited to symptomatic relief and physiologic support until the condition resolves. Historically, concerns about the safety of vaccines have led to declines in immunization coverage rates and outbreaks of disease, as observed during the pertussis outbreaks in United Kingdom during the 1970s. Similar disease outbreaks could easily occur, with devastating effects, were immunization rates to decline as a result of fears regarding MMR vaccine. Yet, because it is a mandatory vaccine that is administered to healthy children—in part, as a public health measure to protect the health of others—the responsibility of the government to ensure the safety of this vaccine is high, even if the hypothesized adverse outcome is rare. Thus, the seriousness of the adverse event—ASD, a group of incurable and serious behavioral disorders—requires rigorous consideration of all possible etiologies. In addition to the seriousness of both the vaccine-preventable disease and the hypothesized adverse event, the level of public concern about MMR vaccine-safety is high. The possible association between MMR vaccine and ASD
Page 55
has become the subject of parliamentary debates and congressional hearings as well as numerous media reports.
RECOMMENDATIONS
Public Health Response
Although the committee has concluded that the evidence favors rejection of the causal relationship at the population level between MMR vaccine and ASD, the committee recommends that this issue receive continued attention. It does so in recognition that its conclusion does not exclude the possibility that MMR vaccine could contribute to ASD in a small number of children and in recognition as well of the following factors discussed in this report including: the identified limitations of the evidence, the seriousness of ASD, the seriousness of the diseases prevented by the vaccine, the immense concern of parents, and the prominence of the issue in public debate.
The committee recognizes that there are conditions under which a conclusion favoring rejection of the hypothesized causal relationship would result in a recommendation that no further research, surveillance, communication, or policy attention be mounted. Those conditions include strong evidence against the relationship; an understanding that no further mechanistic or pathogenesis research could shift the balance of evidence; an adverse event of little medical or public health significance; a vaccine-preventable disease posing little danger of significant morbidity or mortality should immunization rates fall; and little public, political, or media attention to the problem. The hypothesized relationship between MMR vaccine and ASD does not meet these conditions at this time.
Specific recommendations regarding policy review, research and surveillance, and communication follow.
Policy Review
-
The committee does not recommend a policy review at this time of the licensure of MMR vaccine or of the current schedule and recommendations for administration of MMR vaccine.
Research Regarding MMR and ASD
The committee concludes that further research on the possible occurrence of ASD in a small number of children subsequent to MMR vaccination is warranted, and it has identified targeted research opportunities that could lead to firmer understanding of the relationship. The committee makes the following research recommendations, recognizing that it has no basis for judging whether the results of such research will alter the balance of evidence that led to the committee's original conclusion:
Page 56
-
Use accepted and consistent case definitions and assessment protocols for ASD in order to enhance the precision and comparability of results from surveillance, epidemiological, and biologic investigations.
Currently, a number of research studies are in progress regarding the etiology, brain structure and/or function, developmental course, and epidemiology of ASD—for example, those being conducted by the Collaborative Programs of Excellence in Autism (CPEA), a network of 10 multidisciplinary programs funded by the National Institute for Child Health and Human Development. To evaluate and compare these current and future studies, accepted and consistent case-definitions and assessment protocols are critical.
-
Explore whether exposure to MMR vaccine is a risk factor for ASD in a small number of children.
This research might be most usefully conducted once there is a marker of some kind for identifying children at risk for the “regressive” form of ASD or for key steps along a proposed pathogenic model. Currently, not enough is known about either to propose such a marker.
-
Develop targeted investigations of whether or not measles vaccine-strain virus is present in the intestines of some children with ASD.
The committee recommends this research for several reasons. The data most salient in the public debate over the hypothesized relationship between MMR vaccine and ASD is the case series reported in 1998 (Wakefield et al., 1998). It is feasible and important to resolve at least one controversy surrounding those data, the presence or absence of measles vaccine-strain virus. Positive findings would provide the basis for encouraging research to understand the role that measles vaccine-strain virus might play in the occurrence of ASD and bowel disease. Negative findings would provide the basis for directing research on pathogenic mechanisms in other directions.
Current research is attempting to answer these questions. The committee is aware that a paper has been submitted for publication that will address this issue (Wakefield, 2001). Furthermore, in conjunction with the CDC's National Immunization Program, the CPEA is beginning an autism regression and vaccination study that will assess the temporal association between MMR vaccination and autism, distinguishing between the early-onset and regressive forms; and will replicate studies of persistent measles infection in children with autism compared to control children (Bristol-Power, 2001).
-
Encourage all who submit reports to VAERS of any diagnosis of ASD thought to be related to MMR vaccine to provide as much detail and as much documentation as possible.
The limitations of passive surveillance systems such as VAERS are well recognized (Ellenberg and Chen, 1997; Singleton et al., 1999). Even though such systems have biases, better documented reports could be useful. Submis-
Page 57
sion of clinical material specifically related to the diagnosis of ASD could be helpful for further research, and documentation of the behavioral, neurologic, and cognitive status of the child prior to vaccination would be crucial.
The committee did not have the time during the preparation of this report to consider thoroughly how best to encourage improved VAERS reporting. This issue will undoubtedly re-emerge when future hypotheses are considered, and the committee hopes to address this issue more specifically in the future. Meanwhile, the committee encourages the government agencies responsible for VAERS (CDC and FDA), as well as immunization providers (physicians and nurses) and parents to use the VAERS reporting system conscientiously and thoroughly.
In particular, case reports in VAERS or elsewhere of “rechallenge” should be identified, documented, and followed up. In the context of MMR vaccine and ASD, rechallenge refers to children who appeared to have experienced some form of neurologic regression after a first dose of MMR or other measles-containing vaccine and who appeared to have experienced another regression following a second dose of MMR or other measles-containing vaccine. The committee is aware that there might be some cases of rechallenge that could be assessed (Wakefield, 2001). If well-documented and reviewed by appropriate clinicians, these reports and similar data could provide evidence in favor, but not necessarily prove causality, of the hypothesized relationship in a small number of children. It is not clear, however, that such evidence would necessarily shift the balance of evidence away from a causality determination favoring rejection of the relationship at the population level with ASD. (See IOM, 1994a, for a discussion of the contribution of rechallenge cases in causality determinations.)
-
Study the possible effects of different MMR immunization exposures.
For instance, studies might enroll children whose families have chosen not to have them receive the MMR vaccine, although the number of these children may be insufficient to draw population-level conclusions.
This recommendation should not be perceived as promoting non-vaccination or alternative schedules of vaccination. But, it is naïve to ignore the fact that some parents are selecting alternative approaches to vaccination. Children who are immunized in an alternative manner, such as different vaccine types or at different ages, should be studied, although the number of children enrolled in these studies and issues of selection bias would affect the design and interpretation of the results. However, clinical investigations could be well-designed to study these children. A careful review of the ethical implications of such a study would be essential, however, and the informed consent of the families would need to be ensured through Institutional Review Boards.
Page 58
-
Conduct further clinical and epidemiological studies of sufficient rigor to identify risk factors and biological markers of ASD in order to better understand genetic or environmental causes.
The committee further concludes that there is a need to support and continue the NIH- and CDC-funded research already under way on all aspects of ASD. The committee does so because of the burden of ASD and because some general research might one day shed additional light on the hypothesized relationship between MMR and ASD. As definitions or understanding of autism are enhanced and clarified, epidemiological studies are needed to document the prevalence and incidence of ASD, temporal trends, and the incidence and prevalence of different courses of ASD (e.g., regressive vs. early onset). As noted earlier, information about the rotes of ASD in the United States and changes in incidence or prevalence is limited, reflecting a lack of epidemiological research on ASD in this country. Further studies are also needed to identify risk factors for ASD and biological links or markers to be better able to study links with genetic or environmental causes, as well as to decrease misclassification of cases.
Furthermore, the committee would like to acknowledge the many comments, received from parents of autistic children, that part of the burden of this disease is the lack of access to knowledgeable providers and to interventions. A complete ASD research portfolio should include identifying the service needs of this population and the barriers to services; in that way, persons with ASD, and their families and caregivers, can be better served.
Communications
The committee heard repeatedly in its open sessions and in discussions with parents and advocacy groups that obtaining unbiased and relevant information on the possible relationship between MMR vaccine and ASD has been difficult. This issue is discussed generically in the next section and will be addressed more fully in the future. However, the committee specifically recommends at this time that governmental and professional organizations, CDC and FDA in particular, review some of the most prominent forms of communication regarding the hypothesized relationship between MMR vaccine and ASD—for example, information provided via the Internet. Attention should be given to how the material is perceived and used by those with the right and desire to know—the parents of children about to be immunized or those who believe their child has been adversely affected. Direct input from parents and other stakeholders would be invaluable in conducting a systematic and effective evaluation of current communication tools. The health communication section of Healthy People 2010 (U.S. DHHS, 2000) could be used to provide evaluations of Internet communications.
Page 59
General and Crosscutting Issues
In its discussion of recommendations related specifically to the MMR-autism question, the committee identified more general concerns that it could not adequately or appropriately address in this report. These concerns are briefly noted here in anticipation of more complete consideration at a later time.
First, a recurring dilemma in the minds of many concerned about vaccine safety focuses on how to align the appropriate public health attention with a possibly small vaccine safety risk. The committee had little difficulty recommending that continued attention be devoted to the hypothesized MMR and ASD link with the specific recommendation being for targeted research. However, the committee recognizes that in addressing future safety issues, a recommendation regarding the level of future public health response might be quite difficult.
The committee sees a need for a dialogue between various vaccine-safety advocates—including researchers, parents, manufacturers, immunization program implementers, and policymakers—in order to come to a common understanding of key issues that factor into this alignment. These discussions might include but are not limited to: the difficulty of proving the null hypothesis (i.e., no association between two events), identification of a level of vaccine risk that is acceptable given the benefit of the vaccine, the meaning to various stakeholders of terms such as associations and causality and the evidence required to support scientific conclusions such as these, and how to research vaccine exposure as a trigger for conditions of multifactorial etiologies. The committee regrets that it could not begin to address explicitly these issues in its first report. Some of the reactions to this report will center on our inability to do so at this time. The committee will address these issues in the future and welcomes assistance from all who are concerned about vaccine safety.
Second, the committee is concerned about the current status of research on vaccine risk and benefit communication. The committee is aware from its background reading and from discussions that some members of the public perceive serious deficiencies in the available information on the risks and benefits of vaccines. For example, some have noted that the CDC and FDA websites are difficult to navigate and that important material is “hidden” within the sites. It has also been said that the role of public input into federal advisory committees on vaccines and immunization policy is unclear and in some cases minimal. In addition, clinical-provider information about the VAERS system or about vaccine safety is reportedly lacking. Another concern is the relative lack of discussion about the ethical issues regarding provision of information on the small but predictable risks of vaccinations, even for those that are mandatory and for which exemptions on religious or philosophic grounds are uncommon.
The committee acknowledges the seriousness of these concerns but was unable to address them appropriately in this fast report. There have been many discussions in recent years about vaccine communication, but the impact of these discussions is unclear. To date, only a few papers have examined issues in vaccine risk communication (see Bostrom, 1997). More research needs to be
Page 60
done on risk communication, including a better understanding of trade-off issues and of the complexity of developing and explaining quantitative risk-benefit estimates. In the meantime, the committee urges CDC, FDA, NIH, AAP, and similar organizations to take to heart the serious concerns and earnest offers from the concerned public to help with information exchange and communication strategies.
Finally, the committee did not have time to address responsibly the appropriateness of alternative immunization schedules or practices, which might be requested in a clinical setting. This has been discussed by others, especially recently with regard to MMR vaccine, and is of great interest and concern to many.
Because the committee believes these to be issues that will emerge in many of its subsequent meetings, it will hold specific comments, conclusions, and recommendations for the future. The committee does pledge to address these matters over the next 3 years and will develop a mechanism for further input into its work in this area.
SUMMARY
The Immunization Safety Review committee concludes that the evidence favors rejection of a causal relationship at the population level between MMR vaccine and ASD. However, this conclusion does not exclude the possibility that MMR vaccine could contribute to ASD in a small number of children. Because of the limitations of the evidence, the significant public concern surrounding the issue, the risk of disease outbreaks if immunization rates fall, and the seriousness of ASD, the committee recommends that continued attention be given to this issue. This committee has provided targeted research and communication recommendations. However, the committee does not recommend a policy review at this time of the licensure of MMR vaccine or of the current schedule and recommendations regarding administration of MMR vaccine.
REFERENCES
60 Minutes. The MMR Vaccine. November 12, 2000.
, , , , , , 1998. Absence of detectable measles virus genome sequence in inflammatory bowel disease tissues and peripheral blood lymphocytes. J Med Virol 55(3): 243–249.
, , , , 2000a. Further evidence of the absence of measles virus genome sequence in full thickness intestinal specimens from patients with Crohn's disease. J Med Virol 62(3): 377–382.
, , 2000b. Clinical safety issues of measles, mumps and rubella vaccines. Bull World Health Organ 78(2): 199–204.
, , , , , 1999. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet 23(2): 185–188.
, 1986. Effect of simultaneous administration of live measles vaccine on the “take rate” of live mumps vaccine. Dev Biol Stand 65(6): 101–107.
Page 61
. 1994. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: APA.
, , , 1992. Measles surveillance— United States, 1991. Mor Mortal Wkly Rep CDC Surveill Summ 41(6): 1–12.
1996. Brief report: Medial temporal lobe and autism: A putative animal model in primates. J Autism Dev Disord 26(2): 217–220.
, , , , , , 1995. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol Med 25(1): 63–77.
1999. Autism: Clinical features and neurobiological observations. Tager-Flusberg H. Neurodevelopmental Disorders. Cambridge, MA The MIT Press. Pp.383–399.
, 1997. Neuroanatomic observations of the brain in autism. Bauman M , Kemper T , eds. The Neurobiology of Autism. Baltimore: The Johns Hopkins University Press. Pp. 119–145.
, 1988. Interference between strains in live virus vaccines. II: Combined vaccination with varicella and measles-mumps-rubella vaccine. J Biol Stand 16(4): 275–279.
, , , , , , , , , , , , 1999. Measles inclusion-body encephalitis caused by the vaccine strain of measles virus. Clin Infect Dis 29(4): 855–861.
1994. Naltrexone in infantile autism. J Autism Dev Disord 24: 236–239.
, , , , , , , 1985. Health impact of measles vaccination in the United States. Pediatrics 76(4): 524–532.
1997. Vaccine risk communication: Lessons from risk perception, decision making, and environmental risk communication research. Risk Health Safety Environ 8: 173–200.
, 1997. Descriptive epidemiology of adverse events after immunization: Reports to the Vaccine Adverse Event Reporting System (VAERS), 1991–1994. J Pediatr 131(4): 529–535.
, , , , , , , , , , , , 1996. State of the science in autism: Report to the National Institutes of Health. J Autism Dev Disord 26(2): 121–154.
2001. Presentation to Immunization Safety Review Committee. Etiology of Autism: March 8, 2001; Washington, DC .
, , 1987. A prevalence study of pervasive developmental disorders in North Dakota. J Am Acad Child Adolesc Psychiatry 26(5): 700–703.
, . 1999. Changes in the Population of Persons With Autism and Pervasive Developmental Disorders in California's Developmental Services System: 1987 Through 1998. A Report to the Legislature. California Health and Human Services Agency. State of California.
, , , , , 1993. Naltrexone in autistic children: Behavioral symptoms and attentional learning. J Am Acad Child Adolesc Psychiatry 32: 1263–1271.
, , , , 1991. Borna disease: Association with a maturation defect in the cellular immune response. J Virol 65(11): 6154–6164.
Page 62
. 1989. Measles prevention. MMWR Morb Mortal Wkly Rep 38 Suppl 9: 1–18.
1994. Outbreak of measles among Christian Science students—Missouri and Illinois, 1994. MMWR Morb Mortal Wkly Rep 43(25): 463–465.
1996. Measles outbreak among school-aged children—Juneau, Alaska, 1996. MMWR Morb Mortal Wkly Rep 45(36): 777–780.
1997a Measles outbreak—Southwestern Utah, 1996. MMWR Morb Mortal Wkly Rep 46(33): 766–769.
1997b. Measles eradication: Recommendations from a meeting cosponsored by the World Health Organization, the Pan American Health Organization, and CDC. MMWR Morb Mortal Wkly Rep 46(RR–11): 1–20.
1997c. Progress toward global measles control and elimination, 1990–1996. MMWR Morb Mortal Wkly Rep 46(38): 893–897.
1998. Measles, mumps, and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 47(RR–8): 1–57.
1999. Autism spectrum disorders among children. [Online]. Available: http://www.cdc.gov/ncbddd/fact/tasdfs.htm [accessed 2001].
2000a. Estimated Vaccination Coverage with Individual Vaccines and Selected Vaccination Series Among Children 19-35 Months of Age by State and Immunization Action Plan Area–US, National Immunization Survey, Q3/1999–Q2/2000. [Online]. Available: http://www.cdc.gov/nip/coverage/tables/99-00/antigen_iap.xls.
2000b. Measles—United States, 1999. MMWR Morb Mortal Wkly Rep 49(25): 557–560.
2000c. Measles outbreak–Netherlands, April 1999–January 2000. MMWR Morb Mortal Wkly Rep 49(14): 299–303.
2000d. National, state, and urban area vaccination coverage levels among children aged 19-35 months—United States, 1998. MMWR Morb Mortal Wkly Rep CDC Surveill Summ 49(9): 1–26.
2000e. Poliomyelitis prevention in the United States: Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 49(RR–5): 1–22.
2000f. Prevalence of Autism in Brick Township, NJ, 1998: Community Report.
2001a Measles, mumps, and rubella data. National Immunization Program.
2001b. , , . Media Coverage on MMR and autism. April 19, 2001.
2001c. . VAERS Reports. March 26, 2001.
2001d. .
2001e. Table III: Provisional cases of selected notifiable diseases preventable by vaccination, United States, weeks ending December 30, 2000, and January 1, 2000 (52nd Week). MMWR Morb Mortal Wkly Rep 49(51 and 52): 1172–1174.
, , 2000. Opiate antagonists in children and adolescents. Eur Child Adolesc Psychiatry 9 Suppl 1: 144–150.
, , , , 1998. Measles virus RNA is not detected in inflammatory bowel disease using hybrid capture and reverse transcription followed by the polymerase chain reaction. J Med Virol 55(4): 305–311.
Page 63
, 1999. Pediatric combination vaccines. Curr Opin Pediatr 11(1): 14–20.
, , , 2000. Somatic mutation in MECP2 as a non-fatal neurodevelopmental disorder in males. Lancet 356 (9232): 830–832.
. 2001. MMR vaccination coverage in the United Kingdom. Communicable Disease Report CDR Weekly 11(4). .
, , , 1967. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet 1: 542–544.
2001. MMR vaccine and autism: Interim comment from the AAP Executive Committee. AAP News.
, , 2001. Time trends in autism and in MMR immunization coverage in California. JAMA 285(9): 1183–1185.
, , , 1996. Crohn's disease after in-utero measles virus exposure. Lancet 348(9026): 515–517.
, 1997. The complicated task of monitoring vaccine safety. Public Health Reports 112: 10–20.
1999. Vaccine injury compensation programs worldwide. Vaccine 17(Suppl3): S25–S35.
, , , , , , , , , , , , , , , , , , 1999. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord 29(6): 439–484.
1993. Herd immunity: History, theory, practice. Epidemiol Rev 15(2): 265–302.
2001. Comments made at January 11, 2001, Institute of Medicine Immunization Safety Review Meeting, Washington, DC.
1999. The epidemiology of autism: A review. Psychol Med 29(4): 769–786.
2001a. Is there an epidemic of autism? Pediatrics 107(2): 411–413.
2001b. Presentation to Immunization Safety Review Committee. New Studies: March 8, 2001: Washington, DC.
, , 1995. Passively administered antibody suppresses the induction of measles virus antibodies by vaccinia-measles recombinant viruses. Vaccine 13(2): 197–201.
, , , , , , 1998. Impact of anti-vaccine movements on pertussis control: The untold story. Lancet 351(9099): 356–361.
, , 2000. Do parents understand immunizations? A national telephone survey. Pediatrics 106(5): 1097–1102.
, 2001. The risk of vaccination–the importance of “negative” studies. New Engl J Med 344: 372–373.
1998. Chapter 196 : Measles (Rubeola). Fauci AS , Braunwald E , Isselbacher KJ , Wilson JD , Martin JB , Kasper DL , Hauser SL , Longo DL . Editors. Harrison's Principles of Internal Medicine: Volume 1. 14th ed. New York: McGraw-Hill. Pp.1123.
1995. Endogenous opioids and opiate antagonists in autism: Brief review of empirical findings and implications for clinicians. Dev Med Child Neurol 37(3): 239–245.
1998. Chromosomal disorders and autism. J Autism Dev Disord 28(5): 415–425.
, 1998. MMR and autism. Autism 2: 423–424.
, , 1991. Is autism more common now than 10 years ago? Br J Psychiatry 158: 403–409.
Page 64
, , , , 1990. CSF beta-endorphins in childhood neuropsychiatric disorder. Brain Dev 12: 88–92.
, , 1985. Endorphin activity in childhood psychosis. Arch Gen Psych 42: 780–783.
, 1999. Autism: Not an extremely rare disorder. Acta Psychiatr Scand 99(6): 399–406.
, , , , 1995. Overview—combination vaccines and simultaneous administration. Past, present, and future. Ann N Y Acad Sci 754(3): xi–xv.
, , 1994. Pathogenesis of measles virus infection: A hypothesis for altered immune responses. J Infect Dis 170(Suppl 1): S24–S31.
, 1997. Helping children with autism enter the mainstream , Handbook of Autism & Pervasive Developmental Disorders. New York: John Wiley & Sons. Pp. 665–675.
, , , , , , , , , , , , 2000. A negative search for a paramyxoviral etiology of Paget's disease of bone: Molecular, immunological, and ultrastructural studies in UK patients. J Bone Miner Res 15(12): 2315–2329.
, 1998. Outcome in adult life for people with autism and Asperger's syndrome. Autism and Pervasive Developmental Disorders. Cambridge, England: Cambridge University Press. Pp. 209–241.
, 1978. Effects of morphine and naloxone on social attachment in infant guinea pigs. Pharmacology, Biochemistry, and Behaviour 9: 213–220.
, , , 1969. Subacute sclerosing panencephalitis: Isolation of measles virus from a brain autopsy. Nature 221: 974.
1995. Potential alterations in immunogenicity by combining or simultaneously administering vaccine components. Ann N Y Acad Sci 754(3): 35–47.
. 1991. Adverse Events Following Pertussis and Rubella Vaccines. Washington DC: National Academy Press.
1994a. Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality. Washington DC: National Academy Press.
1994b. DPT Vaccine and Chronic Nervous System Dysfunction: A New Analysis. Washington DC: National Academy Press.
1996. Options for Poliomyelitis Vaccination in the United States: Workshop Summary. Washington DC: National Academy Press.
1997a. Risk Communication and Vaccination: Workshop Summary. Washington DC: National Academy Press.
1997b. Vaccine Safety Forum: Summaries of Two Workshops. Washington DC: National Academy Press.
1987. The pathogenesis of acute viral encephalitis and postinfectious encephalomyelitis. J Infect Dis 155(3): 359–364.
1999. Measles: Immunosuppression, interleukin-12, and complement receptors. Immunol Rev 168: 91–101.
, , , , , 1998. Detection of measles virus mRNA from autopsied human tissues. J Clin Microbiol 36(1): 299–301.
, , , , , 2000. Detection and sequencing of measles virus from peripheral mononuclear cells from patients with inflammatory bowel disease and autism. Dig Dis Sci 45(4): 723–729.
Page 65
, , , , , 1996. Polymerase chain reaction detection of the hemagglutinin gene from an attenuated measles vaccine strain in the peripheral mononuclear cells of children with autoimmune hepatitis. Arch Virol 141(5): 877–884.
, , 2001. Mumps, measles, and rubella vaccine and the incidence of autism recorded by general practitioners: A time trend analysis. Br Med J 322(7284): 460–463.
2001. Presentation to Immunization Safety Review Committee. Neuroanatomic Observations of the Brain in Autism: March 8, 2001; Washington, DC.
, 1998. Neuropathology of infantile autism. J Neuropathol ExpNeurol 57(7): 645–652.
, , 1997. Nonhuman primate models to evaluate vaccine safety and immunogenicity. Vaccine 15(8): 903–908.
, , , , 2000. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest 105(5): 607–614.
, 1995. Diagnosis, treatment, and neurobiology of autism in children . Curr Opin Pediatr 7(4): 392–400.
, , , , , , , , 1992. Brief report: A double-blind study of naltrexone in infantile autism. J Autism Devel Disord 22: 309–319.
, , , , , 1995. Persistent measles virus infection of the intestine: Confirmation by immunogold electron microscopy. Gut 36(4): 564–569.
1997. Measles virus infections of the central nervous system. Intervirology 40(2–3): 176–184.
, , , , , , , , , , 2001. Mechanism of measles virus-induced suppression of inflammatory immune responses. Immunity 14(1): 69–79.
, , 1998. Symptoms of pervasive developmental disorders as observed in prediagnostic home videos of infants and toddlers. J Pediatr 132(3 Pt1): 500–504.
, , , , , 1998. Distribution of measles virus in the central nervous system of HIV-seropositive children. Acta Neuropathol (Berl) 96(6): 637–642.
. 1999 Working Party on MMR Vaccine. Report of the Working Party on MMR Vaccine.
. 2001. Combined measles, mumps and rubella vaccines: Response to issues raised in papers published in “Adverse Drug Reactions and Toxicological Review 19(4), 2000.
. 1998. Group Concluded No Reason for Change in MMR Vaccine Policy. News Archive 1998.
, , 1992. The epidemiology of subacute sclerosing panencephalitis in England and Wales 1970–1989. Int J Epidemiol 21(5): 998–1006.
, , 2001. IOM review on autism and MMR Vaccine. Letter.
, , , , 1995. Detection of immunoreactive antigen, with a monoclonal antibody to measles virus, in tissue from a patient with Crohn's disease. J Gastroenterol 30(1): 28–33.
Page 66
, , , , , 2000. Measles vaccination and inflammatory bowel disease: A national British Cohort Study. Am J Gastroenterol 95(12): 3507–3512.
, , , , , , , , 1997. CSF beta-endorphin levels in patients with infantile autism. J Autism Dev Disord 27(2): 155–163.
, , , , , , , 1999. Measles antibody in pregnant Argentinian women relative to vaccine-induced immunity and natural infection. Pediatr Infect Dis J 18(10): 937–939.
, , 1998. MMR vaccination and autism 1998. Br Med J 316(7133): 715–716.
, , , , 1987. Characterization of the measles virus isolated from the brain of a patient with immunosuppressive measles encephalitis. J Infect Dis 156(3): 436–441.
, , , 2000. Absence of measles virus and canine distemper virus transcripts in long-term bone marrow cultures from patients with Paget's disease of bone. Bone 27(3): 417–421.
, , , 1993. The experience with measles in the United States. Kurstak E , Editor. Measles and Poliomyelitis. Pp. 23–35.
, , , , , , 1992. Reduced measles immunity in infants in a well-vaccinated population. PediatrInfect Dis J 11(7): 525–529.
, , , , , , , 2000. Early measles virus infection is associated with the development of inflammatory bowel disease. Am J Gastroenterol 95(6): 1480–1485.
, , , , , 1999. Perinatal exposure to measles virus is not associated with the development of inflammatory bowel disease, Inflamm Bowel Dis 5(2): 104–106.
, 2000. The two-dose measles, mumps, and rubella (MMR) immunisation schedule: Factors affecting maternal intention to vaccinate. Br J Gen Pract 50(461): 969–971.
, , , , , 2000. Serious adverse events after measles-mumps-rubella vaccination during a fourteen-year prospective follow-up. Pediatr Infect Dis J 19(12): 1127–1134.
, , 1969. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med 281: 585–589.
1972. Clinical and laboratory study of children exposed in utero to maternal rubella. Arch Dis Child 47(254): 571–577.
, , , , , 1998. No evidence for measles, mumps, and rubella vaccine-associated inflammatory bowel disease or autism in a 14-year prospective study. Lancet 351(9112): 1327–1328.
, , , , 2000. Effects of neonatal rat Borna disease virus (BDV) infection on the postnatal development of the brain monoaminergic systems. Brain Res Dev Brain Res 119(2): 179–185.
, , , , 1999. Developmental brain injury associated with abnormal play behavior in neonatally Borna disease virus-infected Lewis rats: A model of autism. Behav Brain Res 100(1–2): 43–50.
Page 67
1999 Chapter 17: Rubella Vaccine. Plotkin SA , Orenstein WA , Editors. Vaccines. Third ed. Philadelphia: W.B. Saunders Co.
, 1999. Chapter 13: Mumps Vaccine. Plotkin SA , Orenstein WA , Editors. Vaccines. Third edition. Philadelphia: W.B. Saunders Co.
1997. Autism. N Engl J Med 337(2): 97–104.
, , . 1999. Chapter 12: Measles Vaccine. Plotkin SA, Orenstein WA, Editors. Vaccines. Third ed. Philadelphia: W.B. Saunders Co.
, , , , , , , , , . 1989. The UCLA-University of Utah epidemiologic survey of autism: Prevalence. Am J Psychiatry 146(2): 194–199.
, 1990. Age of symptom onset in young children with pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry 29(6): 863–872.
, , , , 1999. Viral teratogenesis: Brain developmental damage associated with maturation state at time of infection. Brain Res Dev Brain Res 112(2): 237–244.
, , 1998. Comparison of the neurovirulence of a vaccine and a wild-type mumps virus strain in the developing rat brain. J Virology 72: 8037–8042.
, , , , , , , 2000. Evaluation of a neonatal rat model for prediction of mumps virus neurovirulence in humans. J Virol 74(11): 5382–5384.
, , , 1994. Autism and known medical conditions: Myth and substance. J Child Psychol Psychiatry 35(2): 311–322.
, , , 1997. Genetic Influences and Autism. Cohen D , Volkmar F , Editors. Handbook of Autism and Pervasive Developmental Disorders. New York: John Wiley. Pp. 370–387.
, 1987. Brain opioids and autism: An updated analysis of possible linkages. J Autism Dev Disord 17(2): 201–216.
, , , , , , 1999. Rett syndrome in a boy with a 47, XXY karyotype. Am J Hum Genet 64(6): 1781–1785.
, , , , , 1990. Opioid peptides and perinatal development: Is beta-endorphin a natural teratogen? Clinical implications. Ann N Y Acad Sci 579(3): 91–108.
, , , 1989. Carrier-induced epitopic suppression is initiated through clonal dominance, J Immunol 142(8): 2635–2640.
, , , , , , , , 1999. Attrition of T cell memory: Selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11(6): 733–742.
, , , 1998. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med 188(9): 1705–1715.
, , , , 1999. An overview of the vaccine adverse event reporting system (VAERS) as a surveillance system. Vaccine 17: 2908–2917.
2000. Pervasive developmental disorders: A 10-year review. J Am Acad Child Adolesc Psychiatry 39(9): 1079–1095.
Page 68
2001. Microbiology, Immunology and Toxicology of Autism and other developmental disorders. Oral presentation at the Banbury Center Harbor Laboratory, Cold Spring Harbor.
, , , , , , 1999. Autism and measles, mumps, and rubella vaccine: No epidemiological evidence for a causal association. Lancet 353(9169): 2026–2029.
, 1993. Measles virus-induced autoimmune reactions againstbrain antigen. Intervirology 35(1–4): 86–94.
, , 1998. Rates of first measles-mumps-rubella immunisation in Wales, United Kingdom. Lancet 351(9120): 1927.
, , 1995. Perinatal and childhood risk factors for inflammatory bowel disease: A case-control study. Eur J Gastroenterol Hepatol 7(5): 385–390.
, , 1999. Etiology of infantile autism: A review of recent advances in genetic and neurobiological research. J Psychiatry Neurosci 24(2): 103–115.
, 1997. Regression in pervasive developmental disorders: Seizuresand epileptiform electroencephalogram correlates. Pediatrics 99(4): 560–566.
, , 1991. Autistic and dysphasic children. I: Clinical characteristics. Pediatrics 88(6): 1211–1218.
. 2001a. MMR vaccine given all Clear: Independent advisory bodies confirm safety of triple vaccine. Press Release #0027.
2001b. Doctors and nurses confirm support for MMR vaccination programme at health summit. Press Release #0046.
. Healthy People 2010:Understanding and Improving Health. 2nd ed. Washington, DC: U.S. Government Printing Office, November 2000.
. 2000. Autism–Present Challenges, Future Needs–Why the Increased Rates? 106th Congress.
Presentation to Immunization Safety Review Committee. Diagnosis of Autism: March 8, 2001; Washington, DC.
, 1998. Diagnosis and definition of autism and other pervasive developmental disorders. Volkmar FR , Editor. Autism and Pervasive Developmental Disorders. Cambridge: Cambridge University Press.
, 1999. Autism, viral infection and measles-mumps-rubella vaccination. Isr Med Assoc J 1(3): 183–187..
, 2000. Measles, mumps, rubella vaccine: Through a glass, darkly. Adverse Drug React Toxicol Rev 19(4): 265–83; discussion 284–292.
, , 1999. Crohn's disease: The case for measles virus, Ital J Gastroenterol Hepatol 31(3): 247–254.
, , , , , , 1993. Evidence of persistent measles virus infection in Crohn's disease. J Med Virol 39(4): 345–353.
Presentation to Immunization Safety Review Committee. March 8, 2001; Washington DC . 2001.
, , , , , , , , 2000. Enterocolitis in children with developmental disorders. Am J Gastroenterol 95(9): 2285–2295.
, , , , , , , , , , , ,
Page 69
1998. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 351(9103): 637–641.
, 1997. Association of measles infection and the occurrence of chronic inflammatory bowel disease. Can Commun Dis Rep 23(1): 1–6.
, , , 1998. The interruption of transmission of indigenous measles in the United States during 1993. Pediatr Infect Dis J 17(5): 363–6; discussion 366–367.
, , , 2000. Consequences of cross-reactiveand bystander CTL responses during viral infections. Virology 270(1): 4–8.
. The ICD-10 Classification of Mental and Behavioral Disorders: Diagnostic Criteria for Research. Geneva: WHO; 1993.
2001. Statement on the Use of the MMR Vaccine. Website: www.who.int/vaccines-diseases/safety/hottop/mmrstatement.htm [Accessed Feb. 2001] .
1997. The autistic spectrum. Lancet 350(9093): 1761–1766.
Presentation to Immunization Safety Review Committee. Autism Reports to VAERS: March 8, 2001; Washington, DC.


























































