Page 9
3 Human Caused Forcings
Are concentrations of greenhouse gases and other emissions that contribute to climate change increasing at an accelerating rate, and are different greenhouse gases and other emissions increasing at different rates?
Is human activity the cause of increased concentrations of greenhouse gases and other emissions that contribute to climate change?
What other emissions are contributing factors to climate change (e.g., aerosols, CO, black carbon soot), and what is their relative contribution to climate change?
How long does it take to reduce the buildup of greenhouse gases and other emissions that contribute to climate change?
Do different greenhouse gases and other emissions have different draw down periods?
Are greenhouse gases causing climate change?
GREENHOUSE GASES
The most important greenhouse gases in Earth's atmosphere include carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), water vapor (H2O), ozone (O3), and the chlorofluorocarbons (CFCs including CFC-12 (CCl2F2) and CFC-11 (CCl3F)). In addition to reflecting sunlight, clouds are also a major greenhouse substance. Water vapor and cloud droplets are in fact the dominant atmospheric absorbers, and how these substances respond to climate forcings is a principal determinant of climate sensitivity, as discussed in Section 1. The CO2, CH4, N2O and H2O are both produced and utilized in many biological processes, although the major source of gaseous water is evaporation from the oceans. Ozone is created in the atmosphere by reactions initiated by sunlight. The CFCs are synthetic compounds developed and released into the atmosphere by humankind. In addition, sulfur hexafluoride (SF6) and perfluorocarbon gases such as carbon tetrafluoride (CF4) are very potent and nearly inert greenhouse gases with atmospheric lifetimes much longer than 1000 years.
The natural atmosphere contained many greenhouse gases whose atmospheric concentrations were determined by the sum of the ongoing geophysical, biological, and chemical reactions that produce and destroy them. The specific effects of humankind's activities before the industrial era were immersed in all of the natural dynamics and became noticeable only in the immediate vicinity, as with the smoke from small fires. The theoretical realization that human activities could have a global discernible effect on the atmosphere came during the 19th century, and the first conclusive measurements of atmospheric change were made during the last half of the 20th century. The first greenhouse gas demonstrated to be increasing in atmospheric concentration was carbon dioxide, formed as a major end product in the extraction of energy from the burning of the fossil fuels—coal, oil, and natural gas—as well as in the burning of biomass.
The common characteristics of greenhouse gases are (1) an ability to absorb terrestrial infrared radiation and (2) a presence in Earth's atmosphere. The most important greenhouse gases listed above all contain three or more atoms per molecule. Literally thousands of gases have been identified as being present in the atmosphere at some place and at some time, and all but a few have the ability to absorb terrestrial infrared radiation. However, the great majority of these
Page 10
chemical compounds, both natural 1 and anthropogenic, are removed in hours, days, or weeks, and do not accumulate in significant concentrations. Some can have an indirect greenhouse effect, as with carbon monoxide (CO). 2 If the average survival time for a gas in the atmosphere is a year or longer, then the winds have time to spread it throughout the lower atmosphere, and its absorption of terrestrial infrared radiation occurs at all latitudes and longitudes. All the listed greenhouse gases except ozone are released to the atmosphere at Earth's surface and are spread globally throughout the lower atmosphere.
The lifetime of CH4 in the atmosphere is 10–12 years. Nitrous oxide and the CFCs have century-long lifetimes before they are destroyed in the stratosphere. Atmospheric CO2 is not destroyed chemically, and its removal from the atmosphere takes place through multiple processes that transiently store the carbon in the land and ocean reservoirs, and ultimately as mineral deposits. A major removal process depends on the transfer of the carbon content of near-surface waters to the deep ocean, which has a century time scale, but final removal stretches out over hundreds of thousands of years. Reductions in the atmospheric concentrations of these gases following possible lowered emission rates in the future will stretch out over decades for methane, and centuries and longer for carbon dioxide and nitrous oxide.
Methane, nitrous oxide, and ozone all have natural sources, but they can also be introduced into the atmosphere by the activities of humankind. These supplementary sources have contributed to the increasing concentrations of these gases during the 20th century.
Carbon Dioxide
While all of the major greenhouse gases have both natural and anthropogenic atmospheric sources, the nature of these processes varies widely among them. Carbon dioxide is naturally absorbed and released by the terrestrial biosphere as well as by the oceans. Carbon dioxide is also formed by the burning of wood, coal, oil, and natural gas, and these activities have increased steadily during the last two centuries since the Industrial Revolution. That the burning of fossil fuels is a major cause of the CO2 increase is evidenced by the concomitant decreases in the relative abundance of both the stable and radioactive carbon isotopes 3 and the decrease in atmospheric oxygen. Continuous high-precision measurements have been made of its atmospheric concentrations only since 1958, and by the year 2000 the concentrations had increased 17% from 315 parts per million by volume (ppmv) to 370 ppmv. While the year-to-year increase varies, the average annual increase of 1.5 ppmv/year over the past two decades is slightly greater than during the 1960s and 1970s. A marked seasonal oscillation of carbon dioxide concentration exists, especially in the northern hemisphere because of the extensive draw down of carbon dioxide every spring and summer as the green plants convert carbon dioxide into plant material, and the return in the rest of the year as decomposition exceeds photosynthesis. The seasonal effects are quite different north and south of the equator, with the variation much greater in the northern hemisphere where most of Earth's land surface and its vegetation and soils are found.
The atmospheric CO2 increase over the past few decades is less than the input from human activities because a fraction of the added CO2 is removed by oceanic and terrestrial processes. Until recently, the partitioning of the carbon sink between the land and sea has been highly uncertain, but recent high-precision measurements of the atmospheric oxygen:nitrogen (O2:N2) ratio have provided a crucial constraint: fossil fuel burning and terrestrial uptake processes have different O2:CO2 ratios, whereas the ocean CO2 sink has no significant impact on atmospheric O2. The atmospheric CO2 increase for the 1990s was about half the CO2 emission from fossil fuel combustion, with the oceans and land both serving as important repositories of the excess carbon, i.e., as carbon sinks.
Land gains and loses carbon by various processes: some natural-like photosynthesis and decomposition, some connected to land use and land management practices, and some responding to the increases of carbon dioxide or other nutrients necessary for plant growth. These gains or losses dominate the net land exchange of carbon dioxide with the atmosphere, but some riverine loss to oceans is also significant. Most quantifiable, as by forest and soil inventories, are the above- and below-ground carbon losses from land clearing and the gains in storage in trees from forest recovery and management. Changes in the frequency of forest fires, such as from fire suppression policies, and agricultural practices for soil conservation may modify the carbon stored by land. Climate variations, through their effects on plant growth and decomposition of soil detritus, also have large effects on terrestrial carbon fluxes and storage on a year-to-year basis. Land modifications, mainly in the middle latitudes of the northern hemisphere, may have been a net source of carbon dioxide to the atmosphere over much of the last century. However, quantitative estimates have only been possible over the last two decades, when forest clearing had shifted to the tropics. In the 1980s land became a small net sink for
1While the activities of mankind are part of the natural world, the convention exists in most discussions of the atmosphere that “natural processes” are those that would still exist without the presence of human beings; those processes that are significantly influenced by humans are called “anthropogenic”.
2Both carbon monoxide and methane are removed from the atmosphere by chemical reaction with hydroxyl (OH). An increase in the carbon monoxide uses up hydroxyl, slowing methane removal and allowing its concentration and greenhouse effect to increase.
3Fossil fuels are of biological origin and are depleted in both the stable isotope 13C and the radioactive isotope 14C, which has a half-life of 5600 years.
Page 11
carbon, that is, the various processes storing carbon globally exceeded the loss due to tropical deforestation, which by itself was estimated to add 10–40% as much carbon dioxide to the atmosphere as burning of fossil fuels. In the 1990s the net storage on land became much larger, nearly as large as the ocean uptake. How land contributes, by location and processes, to exchanges of carbon with the atmosphere is still highly uncertain, as is the possibility that the substantial net removal will continue to occur very far into the future. 4
Methane
Methane is the major component of natural gas and it is also formed and released to the atmosphere by many biologic processes in low oxygen environments, such as those occurring in swamps, near the roots of rice plants, and the stomachs of cows. Such human activities as rice growing, the raising of cattle, coal mining, use of land-fills, and naturalgas handling have increased over the last 50 years, and direct and inadvertent emissions from these activities have been partially responsible for the increase in atmospheric methane. Its atmospheric concentration has been measured globally and continuously for only two decades, and the majority of the methane molecules are of recent biologic origin. The concentrations of methane increased rather smoothly from 1.52 ppmv in 1978 by about 1% per year until about 1990. The rate of increase slowed down to less than that rate during the 1990s, and also became more erratic; current values are around 1.77 ppmv. About two-thirds of the current emissions of methane are released by human activities. There is no definitive scientific basis for choosing among several possible explanations for these variations in the rates of change of global methane concentrations, making it very difficult to predict its future atmospheric concentrations.
Both carbon dioxide and methane were trapped long ago in air bubbles preserved in Greenland and Antarctic ice sheets. These ice sheets are surviving relics of the series of ice ages that Earth experienced over the past 400,000 years. Concentrations of carbon dioxide extracted from ice cores have typically ranged between 190 ppmv during the ice ages to near 280 ppmv during the warmer “interglacial” periods like the present one that began around 10,000 years ago. Concentrations did not rise much above 280 ppmv until the Industrial Revolution. The methane concentrations have also varied during this 400,000 year period, with lowest values of 0.30 ppmv in the coldest times of the ice ages and 0.70 ppmv in the warmest, until a steady rise began about 200 years ago toward the present concentrations. Both carbon dioxide and methane are more abundant in Earth's atmosphere now than at any time during the past 400,000 years.
Other Greenhouse Gases
Nitrous oxide is formed by many microbial reactions in soils and waters, including those processes acting on the increasing amounts of nitrogen-containing fertilizers. Some synthetic chemical processes that release nitrous oxide have also been identified. Its concentration remained about 0.27 ppmv for at least 1,000 years until two centuries ago, when the rise to the current 0.31 ppmv began.
Ozone is created mainly by the action of solar ultraviolet radiation on molecular oxygen in the upper atmosphere, and most of it remains in the stratosphere. However, a fraction of such ozone descends naturally into the lower atmosphere where additional chemical processes can both form and destroy it. This “tropospheric ozone” has been supplemented during the 20th century by additional ozone—an important component of photochemical smog—created by the action of sunlight upon pollutant molecules containing carbon and nitrogen. The most important of the latter include compounds such as ethylene (C2H4), carbon monoxide (CO), and nitric oxide released in the exhaust of fossil-fuel-powered motor vehicles and power plants and during combustion of biomass. The lifetime of ozone is short enough that the molecules do not mix throughout the lower atmosphere, but instead are found in broad plumes downwind from the cities of origin, which merge into regional effects, and into a latitude band of relatively high ozone extending from 30°N to 50°N that encircles Earth during Northern Hemisphere spring and summer. The presence of shorter-lived molecules, such as ozone, in the troposphere depends upon a steady supply of newly formed molecules, such as those created daily by traffic in the large cities of the world. The widespread practice of clearing forests and agricultural wastes (“biomass burning”), especially noticeable in the tropics and the Southern Hemisphere, contributes to tropospheric ozone.
The chlorofluorocarbons (CFCs) are different from the gases considered above in that they have no significant natural source but were synthesized for their technological utility. Essentially all of the major uses of the CFCs—as refrigerants, aerosol propellants, plastic foaming agents, cleaning solvents, and so on—result in their release, chemically unaltered, into the atmosphere. The atmospheric concentrations of the CFCs rose, slowly at first, from zero before first synthesis in 1928, and then more rapidly in the 1960s and 1970s with the development of a widening range of technological applications. The concentrations were rising in the 1980s at a rate of about 18 parts per trillion by volume (pptv) per year for CFC-12, 9 pptv/year for CFC-11, and 6 pptv/year for CFC-113 (CCl2FCClF2). Because these molecules were
4The variations and uncertainties in the land carbon balance are important not only in the contemporary carbon budget. While the terrestrial carbon reservoirs are small compared to the oceans, the possibility of destabilizing land ecosystems and releasing the stored carbon, e.g. from the tundra soils, has been hypothesized.
Page 12
identified as agents causing the destruction of stratospheric ozone, 5 their production was banned in the industrial countries as of January 1996 under the terms of the 1992 revision of the Montreal Protocol, and further emissions have almost stopped. The atmospheric concentrations of CFC-11 and CFC-113 are now slowly decreasing, and that of CFC-12 has been essentially level for the past several years. However, because of the century-long lifetimes of these CFC molecules, appreciable atmospheric concentrations of each will survive well into the 22nd century.
Many other fluorinated compounds (such as carbon tetrafluoride, CF4, and sulfur hexafluoride, SF6), also have technological utility, and significant greenhouse gas capabilities. Their very long atmospheric lifetimes are a source of concern even though their atmospheric concentrations have not yet produced large radiative forcings. Members of the class of compounds called hydrofluorocarbons (HFCs) also have a greenhouse effect from the fluorine, but the hydrogen in the molecule allows reaction in the troposphere, reducing both its atmospheric lifetime and the possible greenhouse effect. The atmospheric concentrations of all these gases, which to date are only very minor greenhouse contributors, need to be continuously monitored to ensure that no major sources have developed. The sensitivity and generality of modern analytic systems make it unlikely that any additional greenhouse gas will be discovered that is already a significant contributor to the current total greenhouse effect.
AEROSOLS
Sulfate and carbon-bearing compounds associated with particles (i.e., carbonaceous aerosols) are two classes of aerosols that impact radiative balances, and therefore influence climate.
Black Carbon (soot)
The study of the role of black carbon in the atmosphere is relatively new. As a result it is characterized poorly as to its composition, emission source strengths, and influence on radiation. Black carbon is an end product of the incomplete combustion of fossil fuels and biomass, the latter resulting from both natural and human-influenced processes. Most of the black carbon is associated with fine particles (radius <0.2 µm) that have global residence times of about one week. These lifetimes are considerably shorter than those of most greenhouse gases, and thus the spatial distribution of black carbon aerosol is highly variable, with the greatest concentrations near the production regions. Because of the scientific uncertainties associated with the sources and composition of carbonaceous aerosols, projections of future impacts on climate are difficult. However, the increased burning of fossil fuels and the increased burning of biomass for land clearing may result in increased black carbon concentration globally.
Sulfate
The precursor to sulfate is sulfur dioxide gas, which has two primary natural sources: emissions from marine biota and volcanic emissions. During periods of low volcanic activity, the primary source of sulfur dioxide in regions downwind from continents is the combustion of sulfur-rich coals; less is contributed by other fossil fuels. In oceanic regions far removed from continental regions, the biologic source should dominate. However, model analyses, accounting for the ubiquitous presence of ships, indicate that even in these remote regions combustion is a major source of the sulfur dioxide. Some of the sulfur dioxide attaches to sea-salt aerosol where it is oxidized to sulfate. The sea salt has a residence time in the atmosphere on the order of hours to days, and it is transported in the lower troposphere. Most sulfate aerosol is associated with small aerosols (radius <1µm) and is transported in the upper troposphere with an atmospheric lifetime on the order of one week. Recent “clean coal technologies” and the use of low sulfur fossil fuels have resulted in decreasing sulfate concentrations, especially in North America and regions downwind. Future atmospheric concentrations of sulfate aerosols will be determined by the extent of non-clean coal burning techniques, especially in developing nations.
CLIMATE FORCINGS IN THE INDUSTRIAL ERA
Figure 1 summarizes climate forcings that have been introduced during the period of industrial development, between 1750 and 2000, as estimated by the IPCC. Some of these forcings, mainly greenhouse gases, are known quite accurately, while others are poorly measured. A range of uncertainty has been estimated for each forcing, represented by an uncertainty bar or “whisker.” However, these estimates are partly subjective, and it is possible that the true forcing falls outside the indicated range in some cases.
Greenhouse Gases
Carbon dioxide (CO2) is probably the most important climate forcing agent today, causing an increased forcing of about 1.4W/m2. CO2 climate forcing is likely to become more dominant in the future as fossil fuel use continues. If fossil fuels continue to be used at the current rate, the added
5Eighty-five percent of the mass of the atmosphere lies in the troposphere, the region between the surface and an altitude of about 10 miles. About 90% of Earth's ozone is found in the stratosphere, and the rest is in the troposphere.
Page 13
FIGURE 1 The global mean radiative forcing of the climate system for the year 2000, relative to 1750, and the associated confidence levels with which they are known. (From IPCC, 2001; reprinted with permission of the Intergovernmental Panel on Climate Change.) 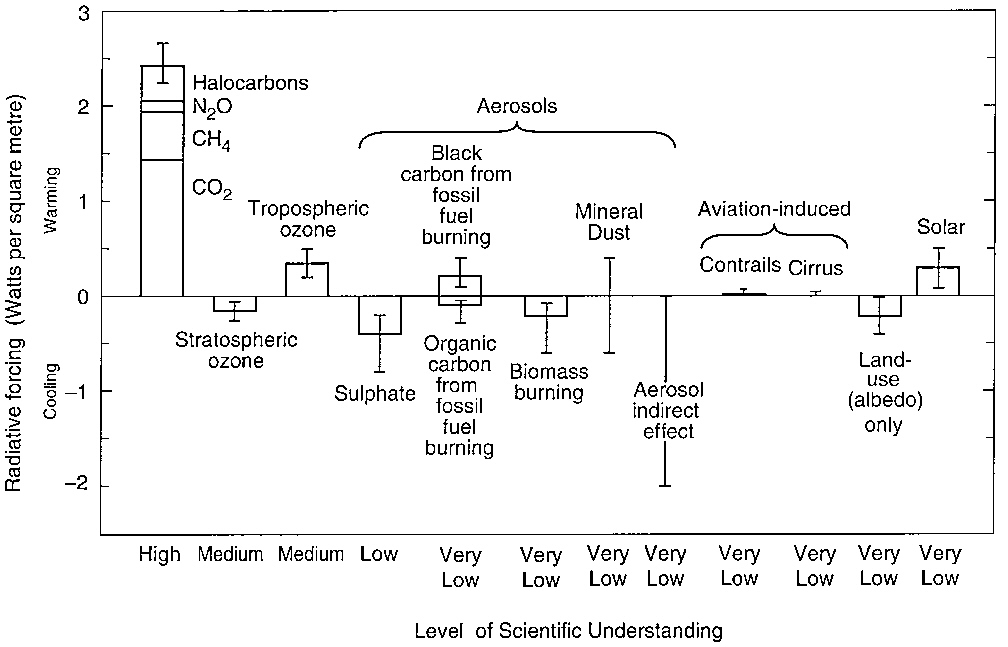
~ enlarge ~
CO2 forcing in 50 years will be about 1W/m2. If fossil fuel use increases by 1–1.5% per year for 50 years, the added CO2 forcing instead will be about 2W/m2. These estimates account for the non-linearity caused by partial saturation in some greenhouse gas infrared absorption bands, yet they are only approximate because of uncertainty about how efficiently the ocean and terrestrial biosphere will sequester atmospheric CO2. The estimates also presume that during the next 50 years humans will not, on a large scale, capture and sequester the CO2 released during fossil-fuel burning.
Other greenhouse gases together cause a climate forcing approximately equal to that of CO2. Any increase in CH4 also indirectly causes further climate forcing by increasing stratospheric H2O (about 7% of the CH4 is oxidized in the upper atmosphere), as well as by increasing tropospheric O3 through reactions involving OH and nitrogen oxides. The total climate forcing by CH4 is at least a third as large as the CO2 forcing, and it could be half as large as the CO2 forcing when the indirect effects are included.
Methane is an example of a forcing whose growth could be slowed or even stopped entirely or reversed. The common scenarios for future climate change assume that methane will continue to increase. If instead its amount were to remain constant or decrease, the net climate forcing could be significantly reduced. The growth rate of atmospheric methane has slowed by more than half in the past two decades for reasons that are not well understood. With a better understanding of the sources and sinks of methane, it may be possible to encourage practices (for example, reduced leakage during fossil-fuel mining and transport, capture of land-fill emissions, and more efficient agricultural practices) that lead to a decrease in atmospheric methane and significantly reduce future climate change. The atmospheric lifetime of methane is of the order of a decade, therefore, unlike CO2, emission changes will be reflected in changed forcing rather quickly.
Tropospheric ozone (ozone in the lower 5–10 miles of the atmosphere) has been estimated to cause a climate forcing of about 0.4W/m2. Some of this is linked to methane increases as discussed above, and attribution of the ozone forcing between chemical factors such as methane, carbon monoxide, and other factors is a challenging problem. One recent study, based in part on limited observations of ozone in the late 1800s, suggested that human-made ozone forcing could be as large as about 0.7–0.8W/m2. Surface level ozone is a major ingredient in air pollution with substantial impacts on human health and agricultural productivity. The potential human and economic gains from reduced ozone pollution and its importance as a climate forcing make it an attractive target for further study as well as possible actions that could lead to reduced ozone amounts or at least a halt in its further growth.
Aerosols
Climate forcing by anthropogenic aerosols is a large source of uncertainty about future climate change. On the basis of estimates of past climate forcings, it seems likely that aerosols, on a global average, have caused a negative climate forcing (cooling) that has tended to offset much of the positive forcing by greenhouse gases. Even though aerosol distributions tend to be regional in scale, the forced climate response is expected to occur on larger, even hemispheric and global, scales. The monitoring of aerosol properties has not been adequate to yield accurate knowledge of the aerosol climate influence.
Estimates of the current forcing by sulfates fall mainly in the range –0.3 to –1W/m2. However, the smaller values do not fully account for the fact that sulfate aerosols swell in size substantially in regions of high humidity. Thus, the sulfate forcing probably falls in the range –0.6 to –1W/m2. Further growth of sulfate aerosols is likely to be limited by concerns about their detrimental effects, especially acid rain, and it is possible that control of sulfur emissions from combustion will even cause the sulfate amount to decrease.
Black carbon (soot) aerosols absorb sunlight and, even though this can cause a local cooling of the surface in regions of heavy aerosol concentration, it warms the atmosphere and, for plausible atmospheric loadings, soot is expected to cause a global surface warming. IPCC reports have provided a best estimate for the soot forcing of 0.1–0.2W/ m2, but with large uncertainty. One recent study that accounts for the larger absorption that soot can cause when it is mixed internally with other aerosols suggests that its direct forcing
Page 14
is at least 0.4W/m2. It also has been suggested that the indirect effects of black carbon—which include reducing low-level cloud cover (by heating of the layer), making clouds slightly “dirty” (darker), and lowering of the albedo of snow and sea ice—might double this forcing to 0.8W/m2. The conclusion is that the black carbon aerosol forcing is uncertain but may be substantial. Thus there is the possibility that decreasing black carbon emissions in the future could have a cooling effect that would at least partially compensate for the warming that might be caused by a decrease in sulfates.
Other aerosols are also significant. Organic carbon aerosols are produced naturally by vegetation and anthropogenically in the burning of fossil fuels and biomass. Organic carbon aerosols thus accompany and tend to be absorbed by soot aerosols, and they are believed to increase the toxicity of the aerosol mixture. It is expected that efforts to reduce emissions of black carbon would also reduce organic carbon emissions. Ammonium nitrate (not included in Figure 1) recently has been estimated to cause a forcing of –0.2W/m2.
Mineral dust, along with sea salt, sulfates, and organic aerosols, contributes a large fraction of the global aerosol mass. It is likely that human land-use activities have influenced the amount of mineral dust in the air, but trends are not well measured. Except for iron-rich soil, most mineral dust probably has a cooling effect, but this has not been determined well.
The greatest uncertainty about the aerosol climate forcing—indeed, the largest of all the uncertainties about global climate forcings—is probably the indirect effect of aerosols on clouds. Aerosols serve as condensation nuclei for cloud droplets. Thus, anthropogenic aerosols are believed to have two major effects on cloud properties: the increased number of nuclei results in a larger number of smaller cloud droplets, thus increasing the cloud brightness (the Twomey effect), and the smaller droplets tends to inhibit rainfall, thus increasing cloud lifetime and the average cloud cover on Earth. Both effects reduce the amount of sunlight absorbed by Earth and thus tend to cause global cooling. The existence of these effects has been verified in field studies, but it is extremely difficult to determine their global significance. Climate models that incorporate the aerosol-cloud physics suggest that these effects may produce a negative global forcing on the order of 1 W/m2 or larger. The great uncertainty about this indirect aerosol climate forcing presents a severe handicap both for the interpretation of past climate change and for future assessments of climate changes.
Other Forcings
Other potentially important climate forcings include volcanic aerosols, anthropogenic land use, and solar variability. Stratospheric aerosols produced by large volcanoes that eject gas and dust to altitudes of 12 miles or higher can cause a climate forcing as large as several watts per square meter on global average. However, the aerosols fall out after a year or two, so unless there is an unusual series of eruptions, they do not contribute to long-term climate change.
Land-use changes, especially the removal or growth of vegetation, can cause substantial regional climate forcing. One effect that has been evaluated in global climate models is the influence of deforestation. Because forests are dark and tend to mask underlying snow, the replacement of forests by crops or grass yields a higher albedo surface and thus a cooling effect. This effect has been estimated to yield a global cooling tendency in the industrial era equivalent to a forcing of –0.2W/m2. Land use changes have been an important contributor to past changes of atmospheric carbon dioxide. However, the impacts of such changes on climate may be much more significant on regional scales than globally, and largely act through changes of the hydrologic cycle. Such impacts are currently poorly characterized because they depend on complex modeling details that are still actively being improved.
Solar irradiance, the amount of solar energy striking Earth, has been monitored accurately only since the late 1970s. However, indirect measures of solar activity suggest that there has been a positive trend of solar irradiance over the industrial era, providing a forcing estimated at about 0.3 W/m2. Numerous possible indirect forcings associated with solar variability have been suggested. However, only one of these, ozone changes induced by solar ultraviolet irradiance variations, has convincing observational support. Some studies have estimated this indirect effect to enhance the direct solar forcing by 0.1 W/m2, but this value remains highly uncertain. Although the net solar forcing appears small in comparison with the sum of all greenhouse gases, it is perhaps more appropriate to compare the solar forcing with the net anthropogenic forcing. Solar forcing is very uncertain, but almost certainly much smaller than the greenhouse gas forcing. It is not implausible that solar irradiance has been a significant driver of climate during part of the industrial era, as suggested by several modeling studies. However, solar forcing has been measured to be very small since 1980, and greenhouse gas forcing has certainly been much larger in the past two decades. In any case, future changes in solar irradiance and greenhouse gases require careful monitoring to evaluate their future balance. In the future, if greenhouse gases continue to increase rapidly while aerosol forcing moderates, solar forcing may be relatively less important. Even in that case, however, the difference between an increasing and decreasing irradiance could be significant and affect interpretation of climate change, so it is important that solar variations be accurately monitored.






