Page 10
2
Workplace Chemical Monitoring
MONITORING CONSIDERATIONS
Chemical monitoring involves repeated analyses for chemicals that have the potential to affect the health and well-being of workers, the public, or the environment. Substances of potential concern (SOPCs) at chemical agent disposal facilities include chemical agents, agent breakdown products, other munitions-related chemicals and their decomposition products, and other substances created or released during agent processing or normal industrial repair or maintenance activities. Proper monitoring and awareness of chemical hazards are essential during all phases of operation— construction, startup and testing, agent and munitions destruction, and plant closure—and possibly even after the Army has relinquished control of the facilities.
Monitoring is generally required both for disposal processes and for maintenance activities when workers can potentially be exposed, as well as for emissions and wastes transported off site. SOPCs may be agents or nonagents; they may be found in the plant, in outdoor air, in liquid process or effluent streams, on surfaces in the plant, or in solid waste materials. Table 2-1 shows a number of examples of media that may require monitoring.
Although the nine U.S. stockpile storage/disposal sites have some common monitoring needs, each site also requires site-specific monitoring because of the differences in the types of chemical agents and munitions stored at each site and the technologies chosen for their destruction. All nine sites require monitoring of air and process waste streams for the agents being processed and related agent breakdown products of concern. All sites will also produce agent hydrolysis products because of the common practice of using decontamination solution (aqueous sodium hydroxide or sodium hypochlorite) to decontaminate equipment or to clean up after agent spills. Each site will also produce many other secondary wastes, including contaminated carbon and demilitarization protective ensemble (DPE) suits, tools, machinery, buildings (including concrete walls and floors), sumps, and soils. Secondary wastes will be similar at all sites and must be tested and ana-
|
Media Phase |
Agent or Agent Breakdown Products |
Nonagents |
|
Air |
Plant air |
Plant air |
|
Outdoor air |
Outdoor air |
|
|
Stack exhaust a |
Stack exhaust a |
|
|
Liquid |
Hydrolysate b |
SCWO effluent c |
|
Decontamination solution |
Fuels |
|
|
Brine |
Caustic solution |
|
|
Solid |
Activated carbon |
Ash a |
|
DPE d suits |
Soil |
|
|
Soil |
Concrete |
|
|
Concrete |
||
|
Equipment and tools |
a For sites with baseline incineration system.
b For Aberdeen and Newport.
c For Newport only.
d Demilitarization protective ensemble.
Page 11
lyzed to minimize worker exposure, ensure proper treatment and disposal, and meet cleanup criteria for closure. Finally, as is typical at any industrial facility, operations at each disposal site will entail a variety of maintenance and repair activities capable of generating contamination by various SOPCs. These include cleaning and degreasing with volatile solvent emissions and welding or machine operations with organic and metal emissions. Emissions from these routine industrial operations may have to be monitored on an episodic basis to validate industrial hygiene practices for controlling and minimizing worker exposures.
The sites at Aberdeen, Maryland, and Newport, Indiana, have only one agent each (HD at Aberdeen and VX at Newport) stored in bulk containers. These sites will use hot aqueous hydrolysis (hot aqueous caustic hydrolysis in the case of VX) as the first step in agent destruction. Batch analyses of liquid hydrolysates will be necessary at both sites to ensure that the defined degree of agent destruction (99.9999 percent) is met prior to secondary treatment.
Five of the stockpile sites that store (or have stored) chemical agents configured in a variety of weapons (e.g., rockets, bombs, artillery shells, mortar rounds, and mines) have used, currently use, or will use incineration as the means of disposal. Requirements for these disposal facilities include techniques for monitoring products of incomplete combustion, acid gases, and heavy metals that may elude exhaust pollution abatement systems and be emitted with exhaust gases through the common stacks.
The emphasis at the two operating baseline sites has (properly) been on gas-phase monitoring for agents because of their toxicity and the potential of airborne transport and inhalation. However, with the imminent start-up of sites using alternative liquid processing technologies and the upcoming plant closures (beginning with the closure of JACADS in 2001), monitoring for agents in liquid and solid media will become much more important.
MONITORING FOR AIRBORNE AGENT
Description
Monitoring for airborne chemical agent is a major activity at each chemical agent disposal facility. Two systems are currently being used: (1) the automatic continuous air monitoring system (ACAMS), an active system designed to provide a “near-real-time” alarm (currently ~3 to 8 minutes) if agent vapors are present; and (2) the depot area air monitoring system (DAAMS), a passive sampling system that draws air through adsorption tubes that are collected periodically for desorption and analysis in on-site laboratories. Short descriptions of these systems are provided below. More extensive descriptions can be found in the committee's report, Review of Monitoring Activities Within the Army Chemical Stockpile Disposal Program (NRC, 1994b), and the Army's Monitoring Concept Plan (U.S. Army, 1997a).
ACAMS monitors are composed of an automated air sampling system that supplies gaseous samples to a gas chromatograph that separates agent or agent-derived compounds and detects characteristic phosphorus or sulfur chemiluminescence with flame photometric detectors. ACAMS monitors can also be deployed with higher alarm levels in areas subject to operational contamination to monitor contamination levels, as well as to monitor progress during decontamination operations. ACAMS monitors are also deployed at several points in the pollution abatement system (PAS) and in the common stack for exhaust gas emissions from baseline system incinerators. Some ACAMS monitors are arranged in tandem to cut analysis cycle times in half. Alarms triggered by ACAMS monitors on the common stack automatically shut off the feed to the liquid agent incinerator to minimize potential emission of agents into the atmosphere.
DAAMS monitors contain adsorption tubes that collect chemicals from ambient air, usually over a period of several hours. These monitors are deployed in conjunction with most ACAMS monitors to provide a capability for confirming or negating an ACAMS alarm. This is important because ACAMS monitors operating at their lowest detection levels have a significant frequency of false positive alarms (NRC, 1994b, 1999a). DAAMS monitors are also deployed as perimeter monitors at disposal facility fence lines to detect any ground-level transport of agent outside the facility. Even in the absence of ACAMS alarms, DAAMS adsorption tubes are periodically collected and taken to the facility's laboratory for desorption and quantitative analysis on a research-grade gas chromatograph with flame photometric detection. A gas chromatograph with mass spectrometric detection is also available in each laboratory to help identify compounds that lead to false positive ACAMS alarms or otherwise interfere with the quantification of agents or agent derivatives.
Page 12
Systematic quality control procedures are followed to ensure the reliable operation of ACAMS and DAAMS monitors. Each ACAMS monitor is routinely challenged with dilute agent solutions to confirm that appropriate alarm levels are being maintained. DAAMS tubes spiked with known agent levels are also added to field samples undergoing analysis on a random schedule to confirm that the monitoring system can detect and quantify adsorbed agent. Electronic records of ACAMS monitor alarms and challenges and the results of analyses of DAAMS tubes are created on a daily basis and eventually archived (U.S. Army, 1997a).
Exposure Limits and Process Control Levels
The alarm levels for deployed ACAMS monitors at various facility sites are typically set at 20 percent of a specific airborne exposure limit or process control level. Thus, the absence of an ACAMS monitor alarm may be assumed to indicate that no agent concentrations of more than 20 percent of the airborne exposure limit have persisted for longer than the cycle period (~3 to 8 minutes). The Army has set exposure limits and process controls at the levels mandated (in permits) for current disposal facility operations (U.S. Army, 1997a). These are reprinted in Table 2-2 . The
|
Exposure Limit for Each Chemical Agent (mg/m3) |
||||
|
Purpose |
Applicable Level |
GB |
VX |
HD a |
|
Nonagent worker band general population level |
GPL |
3 × 10−6 |
3 × 10−6 |
(1 × 10−4) c |
|
Unmasked agent workerb,d |
TWAe,f |
1 × 10−4 |
1 × 10−5 |
3 × 10−3 |
|
Source emission limit, process control levels |
Ceiling valueg ASC GLD ECLh IDLH MPL |
1 × 10−4 3 × 10−4 NA 0.01 0.2 100 |
1 × 10−5 3 × 10−4 NA NA 0.02 NA |
3 × 10−3 3 × 10−2 0.2 NA NA 100 |
aThe presence of HT is determined by monitoring for the HD component.
bNo individual is intentionally exposed to direct skin or eye contact with any amount of neat agent or to solid materials contaminated with agent.
cThis level of detection (using a 12-hour sampling time) should be demonstrated and used at all sites where mustard is transported and destroyed.
dDevices for sampling and analyzing workplace air measure and alarm within 10 minutes when chemical agents are present in concentrations of one TWA or higher.
eThe TWA is also referred to as the worker population limit (WPL)
fTWA DAAMS monitoring may be performed using a 12-hour method.
gThe ceiling value is the maximum concentration an individual may be exposed to at any time for any duration. Practically it is the average value over the maximum time required to detect and quantify the specified concentration (U.S. Army, 1990, 1991).
hECL monitoring levels can vary depending on the monitoring application. The laboratory identifies each ECL monitoring level application in the site specific agent monitoring plan.
ASC = allowable stack concentration
ECL = engineering control level
GLD = gross level detector
GPL = general population limit (24-hr day, 7-day week)
IDLH = immediately dangerous to life and health (30 min)
MPL = maximum permissible limit (with workers in DPE suits)
NA = not applicable
TWA = time-weighted average (8-hr day, 40-hr week)
Page 13
general population limit values for VX and HD may be revised downward as a result of a review of agent standards now being done by the Army (Ruetter et al., 2000).
Assessment
ACAMS monitors, the principal agent quantification instruments throughout any disposal facility, alarm when a preset level of agent (usually 20 percent of the relevant control level for that location) has been exceeded. Signals much larger than the preset response level may saturate the signal processing algorithm, and because the duty cycle of an ACAMS monitor is less than 100 percent (i.e., samples are collected only during part of the duty cycle), confirmation that agent actually caused the signal depends on the analysis of DAAMS tubes at the same location. This analysis can give only the average agent concentration over the DAAMS tube's total exposure period, although it is reasonable to assume that most agent accumulation occurred during the period when the associated ACAMS monitor was in an alarm mode. A DAAMS tube without associated ACAMS monitoring can only indicate the average agent level between the time of deployment and the time of collection for analysis.
One potential weakness of the current airborne agent monitoring program is that ACAMS monitors are typically set to detect only the single agent currently being processed. Because individual ACAMS monitors can detect only one agent at a time, multiagent monitoring requires different ACAMS monitors for each agent. Moreover, only the agent currently being processed is usually addressed during routine DAAMS tube analysis. Thus, an accidental release of a chemical agent not being currently processed might go undetected. For instance, leaks from a mislabeled munition or a projectile filled with an unexpected or mislabeled agent in nominally agent-free areas would be missed, and contamination of the downstream processing area by the unexpected agent could go undetected. This issue was raised in the committee's 1994 monitoring report (NRC, 1994c), but the Army has judged the probability of “mislabeling” to be low enough that routine deployment of ACAMS monitors for multiagent detection is currently restricted to the plant-air carbon filtration system. Recent briefings on JACADS closure planning have indicated that multiagent monitoring will be implemented during closure operations (U.S. Army, 1999c).
Another weakness of the airborne monitoring system is the lack of real-time (< 10 seconds) agent detection. The committee has recommended that the Army develop a real-time system that uses a measurement technology independent of the gas chromatography with flame photometric detector methods used by the ACAMS and DAAMS systems (NRC, 1994b). To date, the Army's attempts to develop and demonstrate such a system have not been successful (NRC, 1999a). New interest in chemical agent detection as a key component of antiterrorism activities has spurred government and commercial activities focused on developing better airborne agent sensors (IOM, 1999). The committee has previously urged the Army to continue to monitor technological advances and to consider implementing any that are appropriate for chemical agent disposal facilities (NRC, 1999a).
The recurrent problem with the airborne monitoring system is false positives—which occur when an ACAMS alarm goes off but the presence of agent cannot be confirmed by later DAAMS tube analysis. The resulting tendency to discount alarms and to proceed as if agent were not present was graphically illustrated by an incident involving a minor release of GB at TOCDF in May 2000 (CDC, 2000).
MONITORING AGENT IN LIQUIDS AND SOLIDS
The primary processing requirement for monitoring agent in liquid media is to analyze the hydrolysates produced at Aberdeen and Newport to ensure that the mustard or VX has been thoroughly destroyed before proceeding to the secondary treatment step—biodegradation at Aberdeen and SCWO at Newport. It will also be necessary to ensure that no significant amount of agent is present in any process stream that is ready for discharge.
Because of the relatively low solubility of VX and several of its hydrolysis products in water and the salting-out effect of the high ion concentrations involved in caustic hydrolysis, there will be two liquid phases present during and after VX hydrolysis. Because the hydrolysate must be certified to be free of agent (defined as a destruction and removal efficiency [DRE] of 99.9999 percent) before it goes to the high-temperature, high-pressure SCWO reactor, both phases will have to be included in the analysis. VX, being lipophilic, is likely to partition selectively into the less dense oily phase, which constitutes about 5 percent of the hydrolysis mixture.
Page 14
Agent hydrolysis by aqueous caustic solution is also used at baseline incineration system sites where decontamination solution is used to clean contaminated tools, equipment, and structural surfaces. At these sites, spent decontamination solution is processed through the liquid agent incinerator afterburner (secondary chamber) to destroy any residual agent or toxic hydrolysis products. This option will not be available at Aberdeen or Newport, where decontamination solution may be processed in the primary hydrolysis treatment step. No agent is expected to remain in decontamination solution after several days at room temperature. However, any liquids or solids shipped to off-site disposal facilities should be analyzed before shipment if there is any possibility of agent contamination. This is also true of the brine solution (primarily sodium chloride, fluoride, sulfate, and phosphate salts) left after the scrubbing of acid gases formed by combustion in incinerators.
Solids that are known to be or suspected of being contaminated with agent include activated carbon used in the air filtration system or gas masks, DPE suits, concrete in the munitions demilitarization building (MDB) or storage igloos, and agent-exposed soil, equipment, and tools. The usual methods of analysis include (1) holding the solid(s) in an enclosed space, such as a drum, at 70ºF and analyzing the headspace vapor or (2) taking wipe samples from a solid surface and analyzing them by solvent extraction, followed by gas chromatography (U.S. Army, 1997a). The Army has also developed a scheme for classifying the degree of cleanliness of solid materials based on this headspace analysis method, designated 1X, 3X, and 5X. 1 Normally solids (e.g., shell casings) are not shipped off site unless they are at the 5X level (U.S. Army, 1997a). Although this level of decontamination may be satisfactory for steel or polymer materials, it may not be satisfactory for activated carbon, which has a high adsorptive capacity and could therefore give a very low agent vapor pressure even if a substantial loading of agent were present. If the temperature were raised, this agent could be released, posing a danger to anyone not properly prepared or equipped.
Agent in soil or concrete is not a problem during ordinary operations because gas-phase monitoring of agent suffices in areas where agent spills may occur. However, it is a potential problem during cleanup and closure operations when these materials must be certified as agent free.
MONITORING NONAGENT CHEMICALS IN AIR
Much of the public concern about incineration is based on the perception that incinerators emit chlorinated dioxins and furans, heavy metals, and other toxic substances into the atmosphere, potentially harming both the workforce and the public. The normal practice at the incineration-based disposal facilities has been to monitor only agent and carbon monoxide, carbon dioxide, nitrogen oxides (NOx), and oxygen in the stack gas to determine that the incinerator is operating properly and that combustion is nearly complete. Other nonagent stack emissions are analyzed only during trial burns required to obtain or modify operating permits. The Stockpile Committee has extensively reviewed the trial burn emissions data from both JACADS (NRC, 1994c) and TOCDF (NRC, 1999a) and determined that emissions of organic and metallic species are exceptionally low when the incinerators and their pollution abatement systems are operating as designed.
The committee has recommended that the Army consider periodically monitoring emissions for species other than agent during normal operations as a means of reassuring disposal facility workers and the public that they are not being exposed to unacceptable risk (NRC, 1994b). This issue is likely to become more important since the Environmental Protection Agency (EPA) issued a draft document indicating that one potential incinerator emission, the most potent form of dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin), is considered to be significantly more toxic than was previously thought (EPA, 2000). Regular analyses for heavy metals (e.g., Hg, Pb, etc.) should also be considered. The Army has agreed to design and assess a plan for periodic monitoring of SOPCs in stack emissions at TOCDF, but this plan has not yet been finalized or implemented.
In addition to emissions from combustion processes, other potential sources of airborne compounds from
1 The agent contamination levels 1X, 3X, and 5X are defined on Page 1 of Department of Army Pamphlet 385-61, Chemical Agent Safety, Chapter 5 ( http://www.usapa.army.mil ) (U.S. Army, 1997b). 1X indicates the item has been partially decontaminated. 3X indicates that it has been surface decontaminated by locally approved procedures and bagged or contained in an agent-tight container whose headspace analysis shows concentrations of agent higher than 0.0001 mg/m3 for GB, 0.00001 mg/m3 for VX, or 0.003 mg/m3 for mustard. 5X indicates that an item has been completely decontaminated and may be released for general use or sold to the general public in accordance with all applicable federal, state, and local regulations.
Page 15
ordinary facility activities, such as painting and welding, could be hazardous to workers. For instance, during construction of the plant at Umatilla, there was an incident in which a number of employees were treated for respiratory distress and sent to the emergency department. The chemical substance(s) responsible could not be identified, but this incident illustrates the potential for exposures to hazardous chemicals other than agent.
AGENT BREAKDOWN PRODUCTS AND CONTAMINANTS IN LIQUIDS
In addition to monitoring for mustard and VX that may remain in the hydrolysates produced at Aberdeen and Newport, respectively, monitoring must also measure the more toxic agent breakdown compounds that remain after hydrolysis. Because hydrolysis by aqueous caustic or hypochlorite solution is the method used for agent decontamination throughout the CSDP, all sites should consider this possible exposure source. Physical properties of the three most important agents and their major hydrolysis products (listed below each agent) are shown in Table 2-3 , along with CAS (Chemical Abstracts Service) registry numbers, chemical formulas, and molecular weights. As the table shows, the hydrolysis products have lower molecular weights, lower vapor pressures, and generally higher water solubilities than the agent being hydrolyzed. The decreasing lipophilicity (preference for oil over water) can be seen in the more negative values of log Kow (where Kow is the equilibrium constant for partitioning a species between octanol and water) of the hydrolysis products.
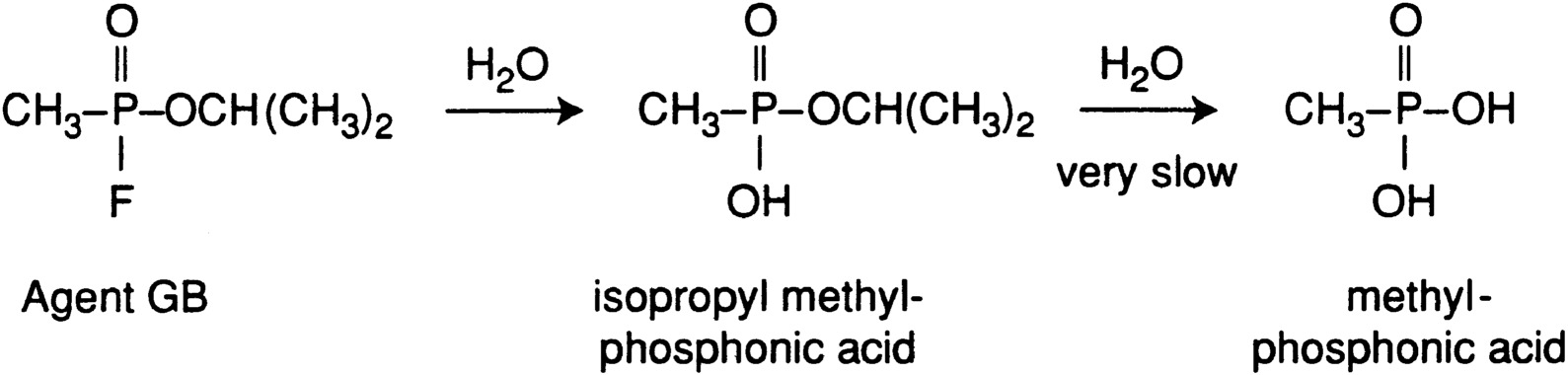
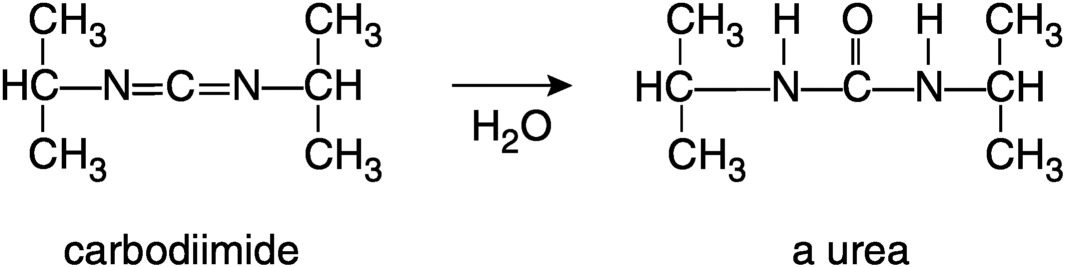
A brief review of the chemistry of agent hydrolysis is presented below based on information and figures from The Sources, Fate, and Toxicity of Chemical Warfare Agent Degradation Products (Munro et al., 1999). Figure 2-1 shows a simplified scheme for the hydrolysis of GB. The P-F bond is hydrolyzed more rapidly than the P-OR bond; the P-C bond is much more resistant to hydrolysis. The scheme is oversimplified because most nerve agents are typically only 90 to 95 percent pure; they contain stabilizers, impurities from manufacturing, and other compounds that have formed during storage. For example, because GB is sensitive to both hydrolysis and acid-catalyzed decomposition, N-N′-diisopropyl carbodiimide and tributyl amine have been added as stabilizers. The carbodiimide reacts with water even more rapidly than GB, yielding a urea, as shown in Figure 2-2.
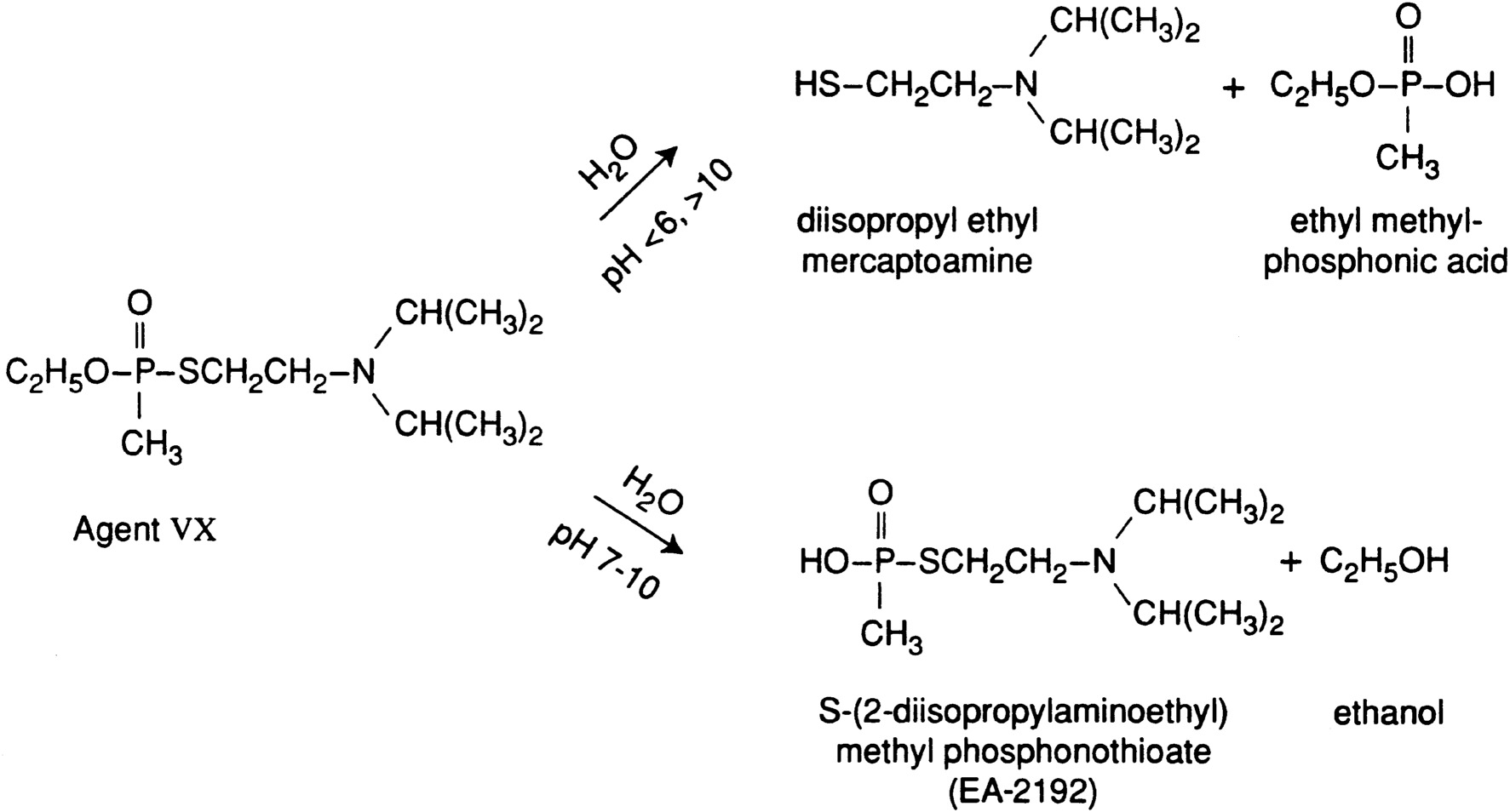
Figure 2-3 shows the simplified hydrolysis of VX. The two pathways correspond to initial hydrolysis of (1) the P-SR bond, which produces 2-diisopropyl ethyl mercaptoamine (DESH) and ethyl methylphosphonic acid (EMPA), and (2) the P-OR bond, which produces EA-2192 and ethanol. The upper path is favored at pH > 10. EA-2192 hydrolyzes more slowly than VX and is still very toxic (Munro et al., 1999). Further hydrolysis of EA-2192 produces DESH and methylphosphonic acid (MPA). The Army is currently working on analytical methods of quantifying low levels of VX in hydrolysate, but at this point an efficient, sensitive, rapid method has not been developed and demonstrated (NRC, 2000a). The Stockpile Committee has previously recommended that the Army increase its efforts to develop innovative analytical techniques with sufficient specificity, sensitivity, and speed for analyzing VX and mustard hydrolysate matrices for process monitoring under operational conditions (NRC, 2000a).
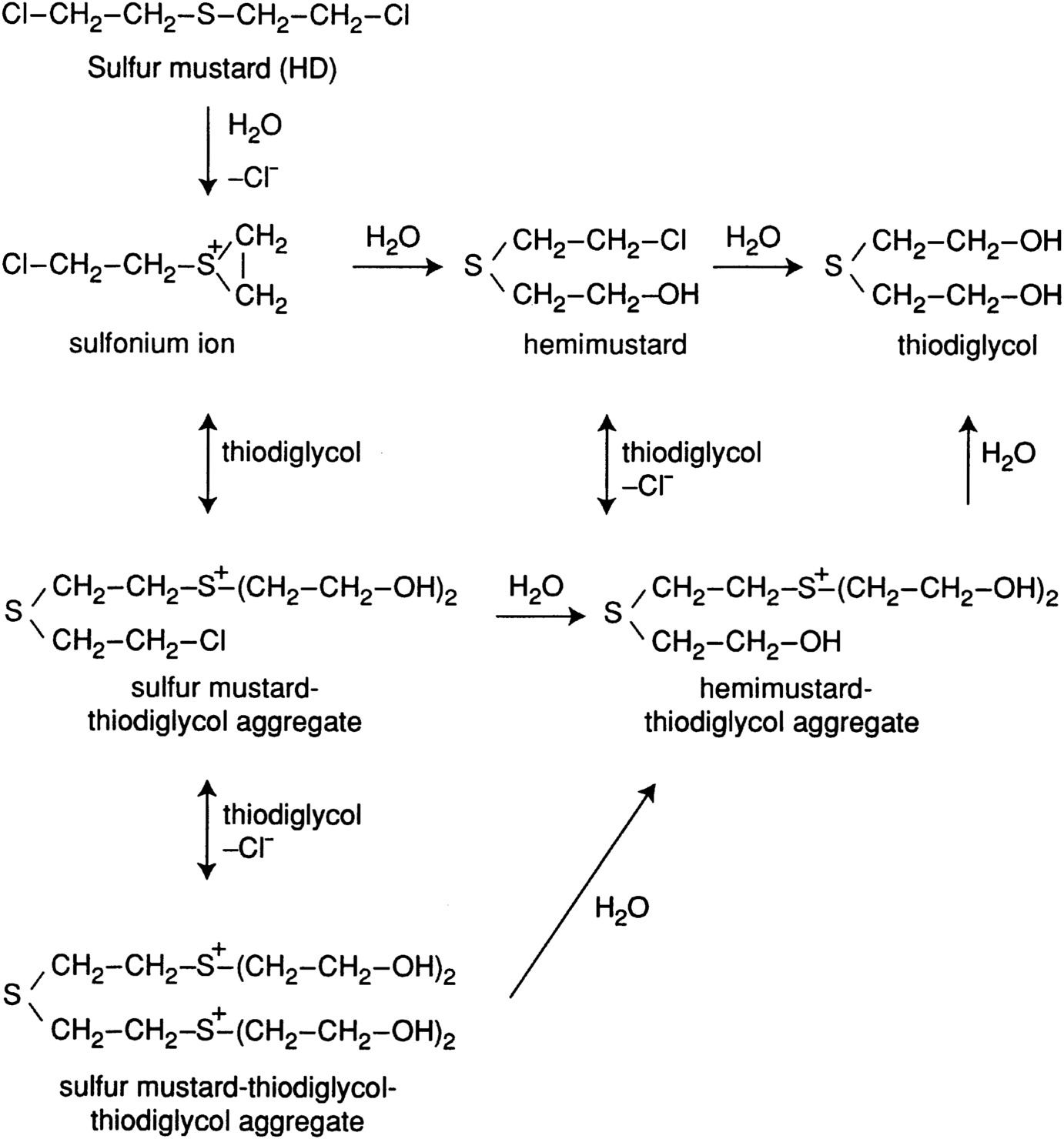
Figure 2-4 shows the major hydrolysis pathways for mustard, which are complicated by the reversible reactions of sulfonium ion and hemimustard with thiodiglycol to produce sulfur mustard thiodiglycol aggregate and hemimustard thiodiglycol aggregate. A further complication is the related formation of polymeric sludge. The low solubility of mustard in water and related high-molecular-weight compounds means that hydrolysis may be mass-transfer limited and, therefore, may require effective mixing to proceed to completion.
Currently, no monitoring program has been developed for agent degradation products. The assumption has been that breakdown products from decontamination or other activities are either less toxic or less persistent, or both. However, a recent evaluation prepared by the Army's Center for Health Promotion and Preventive Medicine and Oak Ridge National Laboratory has noted that a primary VX hydrolysis product, EA-2192, is more stable in water and is nearly as toxic as VX (Munro et al., 1999). Although EA-2192 may primarily be a concern for operations at Newport (where bulk VX will be destroyed by hydrolysis), it may also be present at other facilities if it survives normal VX decontamination operations.
Sulfur mustard is a known human carcinogen, and some of its degradation products may also be carcinogenic (IOM, 1993). Sulfur mustard acts as a vesicant or blister agent and shows acute systemic toxicity in addition to its effects on skin, eyes, and the respiratory tract.
Page 16
|
Army Name Abbreviation |
Chemical Name |
CAS Registry Number |
Formula |
Mol. Weight |
Boiling Point (ºC) |
Freezing Point (ºC) |
Vapor Pressure (mm Hg @ 25ºC) |
Volatility (mg/m3 @ 25ºC) |
Viscosity (cP)b |
Density (g/cc @ 20ºC) |
Solubility (g/L H2O @ 25ºC) |
Log Kowc |
ΔHcomb. (cal/g)b , d |
|
Sarin (GB) |
Isopropyl methylphosphonofluoridate |
107-44-8 |
C4H10FO2P |
140.1 |
158 |
−56 |
2.9b |
22,000 |
1.28 @ 25ºC |
1.102 |
Infinite |
0.299 |
5.55 |
|
IMPA |
Isopropyl methylphosphonic acid |
1832-54-8 |
C4H11PO3 |
138.1 |
0.0034 |
4.8 |
|||||||
|
MPA |
Methylphosphonic acid |
993-13-5 |
CH5PO3 |
96.0 |
Dece |
108.5 |
0.000002 |
>1000 |
−2.28 |
||||
|
VX |
O-Ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioic acid |
50782-69-9 |
C11H26NO2PS |
267.4 |
298 Dece |
−39 |
0.0007 |
10.5 |
12.3 @ 20ºC |
1.008 |
30 |
2.09 |
8.33 |
|
EA-2192 |
S-(2-Diisopropylaminoetyl)methyl- phosphonothoic acid |
73207-98-4 |
C9H22NSPO2 |
231.3 |
Infinite |
0.96 |
|||||||
|
DESH |
Diisopropyl ethyl mercaptoamine |
5842-07-9 |
C8H19NS |
161.3 |
184 f |
1.5 f |
5.4 @ 20ºf |
1.08 f |
|||||
|
EMPA |
Ethyl methylphosphonic acid |
1832-53-7 |
C3H9PO3 |
124.1 |
0.00036 |
180 |
−1.15 |
||||||
|
MPA |
Methylphosphonic acid |
993-13-15 |
CH5PO3 |
96.0 |
Dec e |
108.5 |
0.000002 |
>1000 |
−2.28 |
||||
|
Mustard (H or HD) |
Di-2-chloroethylsulfide |
505-60-2 |
C4H8C12S |
159.1 |
215-217 |
13-14 |
0.11 |
920 |
3.95 @ 20ºC |
1.27 |
0.92 |
1.37 |
4.5 |
|
TDG |
Thiodiglycol |
111-48-8 |
C4H10SO2 |
122.2 |
282f |
−16f |
0.00002 |
62.5f |
1.18 |
Infinite |
−0.77 |
aData from Munro et al.,1999, unless otherwise noted.
bData from NRC, 1994b
cKow is the partition coefficient (concentration ratio at equilibrium) for the compound in a two-phase system containing 1- octanol and water.
d ΔHcomb. is the heat of combustion (cal/g).
eDecomposes
fValues from R. Ward, Aberdeen, personal communication. Properties for DESH are estimates from ASPEN PLUS.
Page 17
~ enlarge ~
FIGURE 2-1 Simplified scheme for the hydrolysis of GB.
Source: Munro et al., 1999.
~ enlarge ~
FIGURE 2-2 Hydrolysis of stabilizer N-N′-diisopropyl carbodiimide.
~ enlarge ~
FIGURE 2-3 Simplified scheme for the hydrolysis of VX.
Source: Munro et al., 1999.
Page 18
~ enlarge ~
FIGURE 2-4 Major hydrolysis pathways for mustard.
Source: Munro et al., 1999.
Some HD degradation products retain considerable toxicity, including, in some cases, vesicant action. Examples include mustard and hemimustard-thiodiglycol aggregates, mustard sulfone, and divinyl sulfone (Munro et al., 1999).
A more complete description of the hydrolysis reactions involving these and other chemical agents and of the toxicities of the products can be found in Munro et al. (1999). Impurities found in ton containers of mustard at Aberdeen include 1,2-dichloroethane, trichloroethylene, tetrachloroethylene, 1,1,2,2-tetrachloroethane, and hexachloroethane, all of which may be subject to state and federal hazardous waste regulations (Munro et al., 1999). The Army's current plan is to
Page 19
adsorb these chlorinated hydrocarbon compounds on activated carbon and send them to an off-site contractor for disposal.
SOLIDS CONTAMINATION: SPECIAL CONSIDERATIONS RELATED TO CLOSURE
Solid secondary wastes that are known or suspected to be contaminated with agent—such as activated carbon, DPE suits, and tools—must be safely disposed of during operations and closure. Contaminated soils and concrete, particularly concrete from the MDB, will be a concern during closure. The Army's standard method of determining the level of agent contamination on solids is to put them into a closed drum or other vessel at 70ºF, wait four hours, and analyze for agent in the head space (U.S. Army, 1997a). Although this procedure may be acceptable for contaminated steel or DPE suits, it may not be satisfactory for contaminated carbon, soil, or concrete, where the strong adsorption of agent may reduce vapor pressures to values much lower than would be expected for an equilibrium between liquid and vapor. Furthermore, as currently practiced, this procedure does not detect any agent breakdown products of potential concern.
The application of ion-trap secondary ion mass spectrometry (IT-SIMS) for the analysis of VX and its breakdown products on soil and concrete and the analysis of 2-chloroethylethyl sulfide (a simulant for HD mustard) on soil have been described in recent literature (Groenewold et al., 1995, 1998, 1999, 2000). IT-SIMS has the advantages of requiring a very small sample size (only milligrams of solid) and no solvent extraction. The method can identify breakdown products, as well as agents. The development of this method or a comparable advanced surface analysis technique to screen solid samples rapidly and sensitively for agent or toxic agent breakdown products could significantly improve detection and decrease the chances of worker exposure during some routine chemical demilitarization operations and many facility closure procedures.










