Page 159
10
Increasing Efficiencies for Hydrocarbon Activation
Harold H. Kung
Northwestern University
Hydrocarbons derived from fossil fuel are the main source of energy and raw material for petrochemicals in the industrial world. When not used in combustion to generate power and heat, fossil fuels are refined in various petrochemical transformation processes into purer and higher-valued products. This chapter continues the discussion by Leo Manzer to address opportunities for research in chemical sciences to reduce carbon (dioxide) emission. Although the large majority of carbon emission is from power generation and transportation, the discussion here focuses on hydrocarbon conversion in the chemical processing industry, with only a brief discussion of hydrocarbon conversion in fuel cell applications.
There are different ways to increase hydrocarbon conversion efficiency to minimize carbon oxides emission. In a chemical transformation process to form a desired product, carbon oxides can be emitted as a by-product in the chemical reaction, as a result of the generation of power needed to effect the desired chemical transformation, or in the generation of reactants needed for the reaction. Increased efficiencies in any of these aspects would reduce carbon emission. In this chapter, examples are presented to illustrate research opportunities that could reduce (1) carbon-containing wastes, (2) hydrogen consumption or wastes, and (3) energy needed for chemical transformation.
REDUCING CARBON-CONTAINING WASTES
In general, chemical transformation processes can be exothermic or endothermic. The examples of catalytic selective oxidation presented by Leo Manzer in Chapter 9 are exothermic reactions, in which carbon oxides are formed as by-products. Increasing selectivity for the desired product would lower carbon emission because of less COx formation in the reaction and lower energy consumption needs for downstream separation and purification. One example mentioned in Chapter 9 is the production of maleic anhydride by selective oxidation of butane (Equation 10.1). The commercial yield is only about 50%. That is, about 4 moles of COx are formed as reaction by-products for every mole of maleic anhydride produced. There is substantial room for improvement:
C4H10 + 7/2 O2 → C4H2O3 + 4 H2O. (10.1)
Page 160
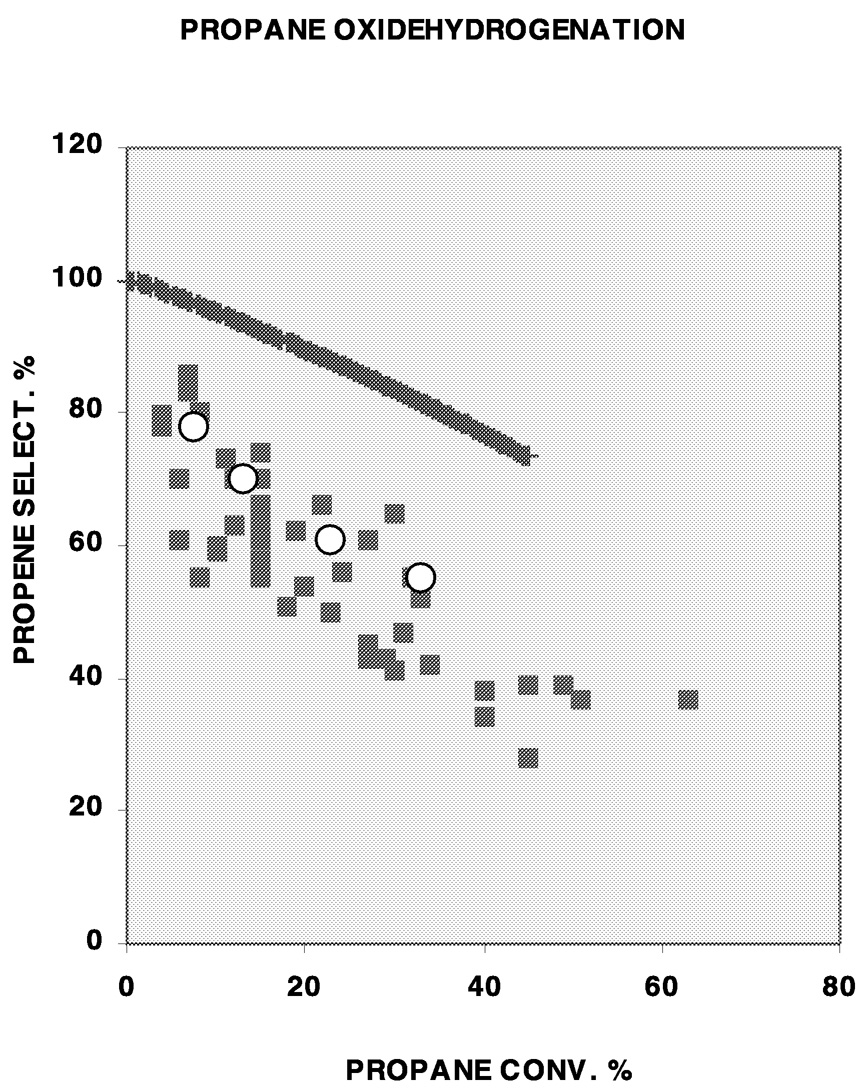
Another example is the catalytic oxidative dehydrogenation of propane to propene. This is not yet a commercial process because of the low yield of propene using known catalysts. Figure 10.1 summarizes the reported selectivity as a function of conversion of propane for the better catalysts. The data show that selectivities approaching 100% can be obtained only at low conversions, suggesting that the low selectivities at high conversions are due to the faster oxidation of propene to COx than of propane to propene. The former reaction is faster because allylic C–H bonds in propene are much weaker and more reactive than the C–H bonds in propane. Consequently, if catalytically active sites that are indifferent to C–H bond strengths can be constructed, such that the catalyst promotes the reaction between oxygen and propene as fast as that between oxygen and propane, then the selectivity-conversion relationship shown by the solid line in the Figure 10.1 could be obtained. Unfortunately, at present, there is insufficient information on the nature of the active sites in mixed oxide catalysts reported for this reaction to permit designing such an active site. One difficulty in attempts to elucidate the nature of active sites in mixed oxide catalysts for selective oxidation in general is the poorly crystalline state of the solid at the active site because of the facile motion of oxygen ions under reaction conditions. Development of more informative experimental methods and computational techniques will be very valuable in this area.
In addition to exothermic reactions, there are many chemical transformation processes that are endothermic. Examples include steam reforming of methane to generate hydrogen and cracking of hydrocarbon in the fluid catalytic cracking process. For these reactions, heat is required, which is often
~ enlarge ~
FIGURE 10.1 A summary of reported selectivity-conversion relationships in the oxidative dehydrogenation of propane to propene. Solid line is the relationship for a sequential reaction of propane to propene to COx if the first-order reaction constants for the two steps are identical. Open circles are data for homogeneous noncatalytic reaction.
Page 161
supplied by the combustion of carbon-containing fuels. In these cases, it may be possible to use unwanted by-products of the reaction as fuel, and the most efficient process (in terms of total carbon emission) would be one in which the fuel value of the waste by-products matches the endothermicity of the reaction.
REDUCING HYDROGEN CONSUMPTION AND WASTES
The hydrogen-to-carbon ratio in most petrochemicals is higher than in crude oil. Therefore, hydrogen must be added in their production. Industrial production of hydrogen is mostly by the energy-intensive steam reforming of methane or, less frequently, by partial oxidation of methane. Both processes emit carbon oxides. Thus, reducing hydrogen consumption in a process would reduce carbon emission.
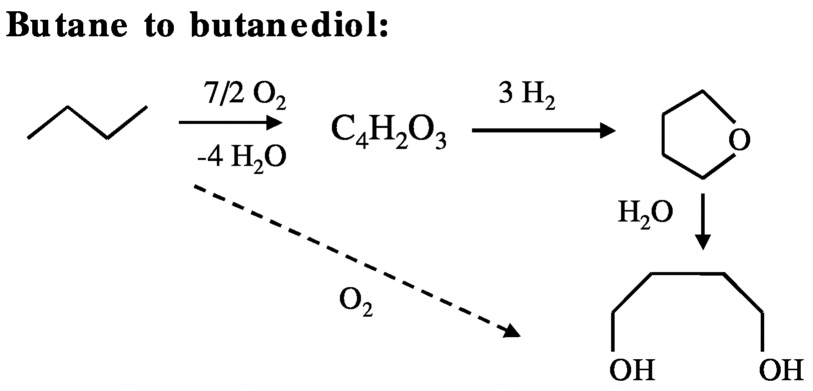
For example, the production of butanediol from butane involves three steps ( Figure 10.2 solid arrows): (1) partial oxidation of butane to maleic anhydride, (2) selective hydrogenation of maleic anhydride to tetrahydrofuran, and (3) hydration of tetrahydrofuran to butanediol. From the stoichiometry of the reactions, for every mole of butanediol produced—even if each step proceeds with 100% yield—3 moles of hydrogen are consumed.
In principle, it should be possible to produce the same product by selective oxidation of butane with oxygen (Equation 10.2 and Figure 10.2, dashed arrow).
C4H10 + O2 → HOCH2CH2CH2CH2OH. (10.2)
The feasibility of such direct oxidation of a terminal carbon, has been demonstrated recently in the selective oxidation of hexane to adipic acid (1,6-diacid) 1 by molecular oxygen catalyzed by cobalt-containing aluminum phosphate molecular sieves. Molecular modeling suggests that the hexane molecule is adsorbed in the pores of the molecular sieve in such a configuration that the two end carbons are close to the cobalt ions in the framework, enabling selective activation of the terminal C–H bonds. This parallels enzymatic action, where specific binding of the substrate with the protein leads to a favorable configuration for selective reaction with the active center.
There are other processes in which potential exists to reduce hydrogen consumption. For example, in the ammoxidation of propene to acrylonitrile (Equation 10.3), ammonia produced from hydrogen and nitrogen is used:
C3H6 + 3/2O2 + NH3 → CH2CHCN + 3H2O. (10.3)
~ enlarge ~
FIGURE 10.2 Reaction pathways for the conversion of butane to butanediol.
Page 162
~ enlarge ~
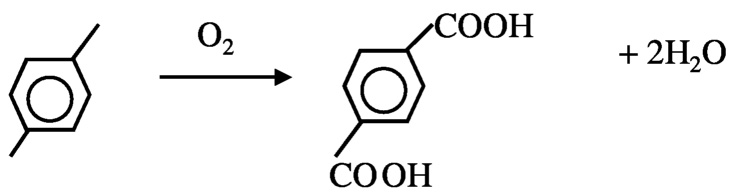
FIGURE 10.3 Current method for the formation of terephtalic acid.
If a new method can be found that uses N2 instead of NH3, there will be less carbon emission in the overall production process.
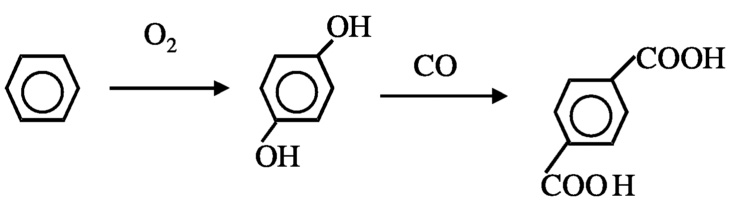
Another possibility to reduce hydrogen consumption is in the production of p-terephthalic acid, which is currently achieved by oxidation of p-xylene. Two moles of water are formed for each mole of terephthalic acid formed ( Figure 10.3). In principle, the product can be produced from benzene without loss of hydrogen ( Figure 10.4). In order to achieve this, new catalytic processes must be developed for selective hydroxylation of benzene and carbonylation of catechol. Currently, selective hydroxylation can be accomplished using a titanium silicalite catalyst and peroxide as oxidant, but a method using O2 is needed. Carbonylation of alcohol is known. The carbonylation of methanol to acetic acid using rhodium complex catalysts is a commercial process, although—as discussed next—improvement is possible.
REDUCING ENERGY NEED
In addition to the energy needed for endothermic reactions, there is substantial energy consumption in the various separation and purification steps in a chemical transformation process. Increasing selectivity in chemical reactions of the desired product, thereby decreasing the amount of by-product that has to be separated, would reduce the energy need accordingly. Additional opportunities arise when processing conditions can be modified, especially when radically new processes can be discovered. One example is methanol carbonylation.
Recently, the Cativa process for liquid-phase carbonylation to produce acetic acid has been commercialized. 2 This process uses an iridium-based catalyst instead of rhodium, produces less propionic acid and acetaldehyde, and uses a much lower water concentration in the reaction mixture. The last aspect results in a reaction product stream that contains less water, so much less energy is needed for distillation to separate water from acetic acid.
There are opportunities for further improvement. In principle, the conversion of methanol to acetic acid requires only the insertion of a CO molecule (Equation 10.4). If this could be achieved in the vapor phase, the need to separate acetic acid from water would be eliminated:
CH3OH + CO → CH3COOH. (10.4)
~ enlarge ~
FIGURE 10.4 Preferred method for the formation of terephtalic acid.
Page 163
Vapor-phase carbonylation catalyzed by copper-MOR (Cu-mordenite) has been reported. 3 However, a successful process will require a better catalyst system. The Cu-MOR catalyst suffers from deactivation, and there are substantial side reactions of hydrocarbon formation over the Brønsted acid sites.
At present, many starting materials for chemicals are light hydrocarbons obtained from petroleum fractions. As the petroleum refining process improves and the demand for chemicals increases, it might become necessary to develop specific processes to produce raw materials on demand. At that time, there would be a need to synthesize specific hydrocarbons to improve the flexibility of operation. For example, the recent report of selective dimerization of alkene could enable efficient synthesis of mediumlength hydrocarbons without the need for further separation. 4 It should be equally useful to be able to selectively cleave a longer hydrocarbon molecule into shorter ones instead of the current nonselective cracking. The potential to improve process efficiency could be further enhanced if selective isomerization of hydrocarbon could be realized. This includes both skeletal and double-bond isomerizations.
The examples mentioned above require development of new chemical transformation processes. Associated with these processes is the need to develop new catalytic systems. Improved understanding of current catalytic systems would facilitate the scientific discovery of new ones. New experimental and computational techniques are needed. Insufficient understanding of catalysis in general is demonstrated by the system of supported gold catalysts: Although these catalysts have a wide variation of reaction selectivity, there is little understanding of the nature of their active sites.
HYDROCARBON ACTIVATION FOR FUEL CELLS
Fuel cells are rather efficient energy conversion devices. Currently, the transportation industry is seriously investigating the use of fuel cells as the power source for automobiles to replace the internal combustion engine. For operational reasons, proton-exchange membrane fuel cells, combined with on-board reforming of a liquid fuel to supply hydrogen, is considered the most feasible candidate for the near future. Many technological advances, such as improvements in the fuel processing unit, are needed before fuel cell-powered vehicles are ready for large-scale market penetration.
The current fuel processing unit consists of a fuel reformer that catalytically converts the liquid fuel into a mixture of hydrogen, CO, CO2, and water. The CO concentration is then reduced using a water-gas shift unit. Much research is devoted to improve the activities of the catalysts in both the reformer and the water-gas shift unit to reduce their weight and volume. In the autothermal mode of operation of the reformer, there is first complete combustion of the fuel into water and CO2 until nearly all of the feed oxygen is consumed, followed by steam reforming of the fuel to generate hydrogen. Because steam reforming is relatively slow, the bulk of the reformer unit is occupied by catalyst for steam reforming. This provides opportunities for new approaches to fuel reforming. One possibility is to effect fuel conversion to hydrogen by selective oxidation at low temperatures. The low temperature could lead to lower CO concentration because of the more favorable equilibrium dictated by the water-gas shift reaction. If the slow steam reforming reaction is no longer needed, the size of the reformer unit could be reduced substantially.
SUMMARY
This chapter provides a few examples of the many opportunities that exist to reduce carbon emission in hydrocarbon conversion processes. Potentially substantial reduction could be achieved by new, innovative catalytic systems. In addition to improving selectivity in chemical reactions, new processes that match the number of carbon and hydrogen atoms in the reactants with those in the products should
Page 164
be developed. These processes would reduce both carbon and hydrogen wastes. The latter is important because there is carbon emission in hydrogen generation.
In order to facilitate discovery of revolutionary catalytic systems, there needs to be better understanding of the interaction of the active sites of a catalyst with various bonds in a reactant molecule and in the product. There is also a need to learn to beneficially use the interaction of the nonreacting portion of a reactant molecule with atoms in the catalyst away from the active site as a means of controlling the configuration of the reactant molecule at the active site and, thus, the reaction selectivity. These goals can be accomplished by developing new insitu characterization techniques and more powerful computational methods to understand existing catalytic systems better.
REFERENCES
1. , , and , 2000 . Angew. Chem. Int. Ed. 39: 2313.
2. , and , 2000 . Catal. Today 58: 293.
3. , , , , , and , 1996 . Proc. 11th Intern. Cong. Catal, Stud. Surf. Sci. Catal. 101; 771.
4. and , 1998 . Inorg. Chim. Acta. 270: 20.
DISCUSSION
David Bonner, Rohm and Haas Company: I think your talk and Dr. Manzer's talk together provide a beautiful synergy, and it is great that you all collaborated. If you try to boil the messages from both of the talks down to the main concepts, I would say it is clear that although there is a broad array of possibilities available to us, reinvestment economics limit some of the things that can be taken through practical development.
If reinvestment economics do shift because of carbon management considerations, through either government regulation or economics of another sort, what areas of research funding, in your opinion, should be emphasized more than they are now by policy makers in the federal government?
Harold Kung: In my summary, I suggest a number of things. We need to better understand the interactions of the entire molecule with the catalysts, not just the active sites, and how to manipulate them. Sir John Thomas's example makes use of the structure of molecules. Once we know what is required for a particular reaction, we can design a catalyst that involves how the whole molecule interacts and how the active site acts on the portion of the molecule that we want to change. I think there are a lot of opportunities there. For example, Davis and colleagues has been trying to synthesize a cavity based on what they want in their reaction.
That is just the beginning. There are a lot of research opportunities in so-called nanotechnology for catalyst design beyond the immediate active site. In my opinion, this is an area that will be very fruitful; the preceding example suggests that it can be done.
Hans Friedericy, Honeywell International: To come up with a fuel cell car that really is long-lived and will eventually compete with a diesel car, one of the keys is the membrane itself and the catalyst inside. Developing a less expensive catalyst for fuel cell systems is an area that is lacking sufficient research. You mentioned fuel cells—are you involved in that area?
Harold Kung: I mostly confined my few comments on the fuel cells to the hydrocarbon activation aspect, so I am talking about the hydrogen fuel cell. A lot of progress has been made in reducing
Page 165
platinum usage in the anode. I think James Spearot mentioned that yesterday. There is also a lot of work on increasing the temperature tolerance of the membrane.
Hans Friedericy: Has anyone been successful?
Harold Kung: There has been substantial progress, but further improvements are needed for commercialization.
Hans Friedericy: The reformers that go with fuel cell engines need a lot more work too. Honeywell has studied at least 25 different areas, and we yet have to find a membrane that can take higher temperature. Even if we have a membrane that has a better catalyst in it, we are still stuck with a fuel cell that is too expensive.
Harold Kung: Reducing the cost is definitely an issue. Many companies are working on cost issues. Fuel cell research has received a higher level of attention than ever before. It seems premature to conclude that the current problems cannot be overcome. In my presentation, I suggested a totally different way of fuel reforming. Rather than just thinking about how to come up with a better, more actively reforming catalyst in the traditional way, let's look at the process totally differently. Perhaps there can be completely new proton transfer membranes as well.
There are research opportunities in what I would call pre-competitive research that is very suitable for government funding.
Hans Friedericy: I also think you can get a lot of funding for it.
Harold Kung: Yes, fuel cells are receiving more attention these days.
Klaus Lackner, Los Alamos National Laboratory: I would like to point out that one of the largest sources of electricity is ultimately coal-burning power plants. If you really want to get the effluent clean and want to capture your CO2, ideally, in a separate stream, you end up going through a calcification process. The issue of catalysts and how to make this work is certainly on the mind of everyone working in that area. I think that if the power plant of the future is a coal plant, it is likely to be a gasification plant because I see this as the only way of collecting CO2 and all the other pollutants for that matter.
Tobin Marks, Northwestern University: This issue concerns available funding for topic A or B. Funding for academic research in the catalytic area in the United States is not very large, and the funding that is there has been shrinking. I think the program managers here would verify that. If we want to attack some of these problems, I think we have to generate more interest nationally as well as generate arguments for why this funding really is needed.







