3
Chemical Contaminants and Their Sources
Passengers and crew have expressed concerns regarding exposure to various chemical contaminants in the aircraft cabin and have linked adverse health effects to specific potential exposures. Whether the exposures actually occur in the cabin is a critical question. Accordingly, this chapter addresses chemical contaminants, their sources, and potential exposure to them in aircraft. Unless otherwise noted, the term contaminants in this chapter refers to chemical contaminants. Biological agents are discussed in detail in Chapter 4.
Contaminants that originate outside the aircraft are discussed first, and then contaminants that originate inside the aircraft are addressed. Finally, contaminants that can result from the environmental control system (ECS), including the main engines and the auxiliary power unit (APU), are discussed.
CONTAMINANTS WITH EXTERNAL SOURCES
Ventilation air provided to the cabin by the ECS is drawn from ambient air around the aircraft. Any pollutants in this air can be introduced into the passenger cabin. During the gate-to-gate course of a flight, an aircraft generally encounters the following types of ambient air:
-
Ground-level air at the departure or arrival airport.
-
Urban air aloft in the air basin of the departure or arrival city.
-
Tropospheric air above the mixed surface layer.
-
Air in the upper troposphere or lower stratosphere.
Therefore, the ventilation supply air can be contaminated by background urban pollution and by emissions from local airport sources when the aircraft is on the ground. Urban air pollution is also encountered shortly after takeoff and before landing; however, these periods are usually small fractions of an entire flight. Finally, when flying in the upper troposphere or lower stratosphere, an aircraft can encounter high ozone (O3) concentrations. The issues noted are explored in the following sections.
Ground-Level Pollution
Most airports are near large metropolitan areas, where pollution can exceed health-based standards. For example, in 1999, 105 million U.S. residents lived in areas that were designated “nonattainment” with respect to at least one of the criteria pollutants (EPA 2000). On a population-weighted basis, the most serious problems were posed by O3, 90 million residents; particulate matter (PM), 30 million residents; and carbon monoxide (CO), 30 million residents. In addition to urban air pollution, substantial amounts of combustion-generated pollutants are emitted on the ground at airports by, for example, aircraft jet engines and diesel-powered service vehicles. Because emissions at airports are important contributors to urban and regional air pollution, modeling and measurement programs have been established to measure the emissions and the resulting concentrations (Moss and Segal 1994). Some of the information is summarized in the following paragraphs, but it does not appear to have been used to investigate the effect of emissions at airports on air quality in passenger cabins of aircraft.
During the middle 1980s, the Emissions and Dispersion Modeling System (EDMS) was developed to assess air-quality effects of proposed airport development projects (Moss and Segal 1994). It was developed by the Federal Aviation Administration (FAA) in cooperation with the U.S. Air Force and is supported as a Windows-based simulation tool.1 Specifically, the EDMS is
|
1 |
Background information on the EDMS tool is available at http://www.aee.faa.gov/aee-100/aee-120/edms/banner.htm where it can be purchased. |
designed to emphasize the effect of emissions from aviation sources— especially aircraft, APUs, and ground support equipment—on air quality in the surrounding areas. It incorporates aircraft engine emission factors from a data bank maintained by the International Civil Aviation Organization. The effects of emissions on ambient air concentrations are predicted by means of dispersion algorithms validated by the Environmental Protection Agency. In 1998, FAA designated the EDMS as the required model to perform air-quality analyses for aviation-related air pollution.
Examples of emission data available through the EDMS are presented in Table 3–1. Emission factors for three combustion conditions are provided for six species. The emission factors express the mass of a species emitted per mass of fuel burned. The last row in the table indicates typical fuel combustion rates of a single jet engine during idle and takeoff. The product of the combustion rate and the related emission factor is the mass emission rate for the species. For example, an idling jet engine would be estimated to emit CO as follows: (fuel at 0.205 kg/s)(CO at 25 g/kg of fuel)=CO at 5.1 g/s.
The data in Table 3–1 show that emission factors for products of incomplete combustion (e.g., CO and hydrocarbons [HC]) are greatest when the jet engine is idling. However, nitrogen oxides, which are produced by high-temperature oxidation of nitrogen in combustion air, are emitted at the greatest rate during the high-power takeoff period. Sulfur oxide emissions are a consequence of sulfur in jet fuel and are independent of combustion conditions. Similarly, because carbon dioxide (CO2) and water vapor are the major products of combustion, they are emitted at rates that reflect the prevalence of carbon and hydrogen in the fuel rather than the combustion conditions.
The data in Table 3–1 do not reveal what other products of incomplete combustion and unburned fuel constituents are emitted from jet engines. Such components would generally be emitted at lower mass rates than the products listed in the table. However, some have important adverse health effects as toxic air pollutants and may be of concern even at lower rates of emission. Recent studies have begun to explore the problem of exposure of ground personnel and passengers to some pollutants (e.g., volatile organic compounds [VOCs], polycyclic aromatic hydrocarbons, and soot) at airports (Pleil et al. 2000; Childers et al. 2000). Because these studies are in their early stages, no conclusions can be drawn yet.
With respect to pollutant exposure in passenger aircraft cabins at the airport, a key factor is the duration of time on board while the aircraft is on the ground. Flight attendants have higher potential exposures because they board
TABLE 3–1 Typical Emission Factors for Selected Gaseous Species From Jet Engine Operating Regimes
|
|
Emission Factor, g/kg |
||
|
Species |
Idle |
Takeoff |
Cruise |
|
Carbon dioxide |
3,160 |
3,160 |
3,160 |
|
Water |
1,230 |
1,230 |
1,230 |
|
Carbon monoxide |
25 (10–65) |
<1 |
1–3.5 |
|
Hydrocarbons (as methane) |
4 (0–12) |
<0.5 |
0.2–1.3 |
|
Nitrogen oxides (as nitrogen dioxide)— short haul |
4.5 (3–6) |
32 (20–65) |
7.9–11.9 |
|
Nitrogen oxides (as nitrogen dioxide)— long haul |
4.5 (3–6) |
27 (10–53) |
11.1–15.4 |
|
Sulfur oxides (as sulfur dioxide) |
1.0 |
1.0 |
1.0 |
|
Fuel combustion ratea |
0.205 kg/s |
2.353 kg/s |
— |
|
aFuel combustion rate for a high-bypass GE (CF6–80) turbofan engine. Source: Data from Penner et al. (1999). |
|||
before the passengers and deplane after them. Delays on the ground after boarding because of traffic, inclement weather, or equipment malfunction would also be associated with potentially greater exposures.
Pollution During Ascent and Descent
Ambient air pollution varies markedly with altitude. Almost all air pollution of anthropogenic origin has ground-level or low-altitude sources. The pollution is mixed through the lower troposphere by meteorological processes. Specifically, wind blowing over rough ground surfaces causes turbulent mixing, and heating of the earth’s surface by the sun induces vertical motion. The well-mixed layer of the lower troposphere typically extends from a few hundred to a few thousand meters above the earth’s surface. For short-lived pollutants that are characteristic of photochemical-smog constituents, the pollutant concentrations are highest in the well-mixed layer and decline steeply above it.
Under ordinary flight circumstances, the duration of exposure of the aircraft to polluted air in the well-mixed layer is relatively brief, lasting for several
minutes after takeoff and for several minutes before landing. Sometimes, because of airport traffic, planes are placed in a holding pattern near the airport; in such cases, the duration of exposure to polluted urban air would be increased.
Ozone During Cruise
At altitudes no greater than a few thousand meters above the ground, the contribution of anthropogenic emissions to air pollution is small, at least with respect to pollutants that could affect cabin air quality. Accordingly, the primary ambient air pollutant of concern at cruise altitude is O3. In contrast with O3 formed from pollutant emissions near the earth’s surface, the O3 at altitude has natural sources. Oxygen molecules (O2) undergo photodissociation triggered by ultraviolet radiation from the sun. The oxygen atoms combine with other oxygen molecules to produce O3. O3 itself is reactive and decomposes fairly rapidly in the stratosphere either by photodissociation, by reaction with oxygen atoms, or by catalytic destruction (e.g., reactions with nitrogen oxides or chlorine oxides). The persistence and prevalence of stratospheric O3 is a consequence of the dynamic balance between rates of production and destruction (Seinfeld and Pandis 1998a).
The following subsections review various aspects of O3: atmospheric concentrations that might be encountered on flights, reactivity and possible reactions of O3 with materials present in the passenger cabin, methods of controlling O3 concentrations in passenger aircraft cabins, and studies that have measured O3 concentrations in passenger aircraft cabins.
Atmospheric Ozone Concentrations
Figure 3–1 presents a summary of annual average O3 concentrations as a function of latitude and altitude over North America. The figure shows that at cruise altitudes (9,000–12,000 m [29,500–39,400 ft]), the average O3 concentration is much higher at high latitudes (greater than approximately 60°N) than at low latitudes (approximately 30°N). For example, the O3 concentration of 35×1011 molecules per cubic centimeter at 12,000 m (39,400 ft) at a latitude of 70°N corresponds to a partial pressure of 1.0×10−7 atm (1.0×10−4 mbar) assuming a temperature of 216.7 K as in the U.S. standard atmosphere (Bolz
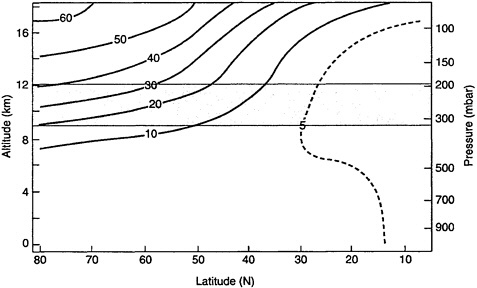
FIGURE 3–1 Annual mean vertical distribution of O3 (1011 molecules per cubic centimeter) over North America. Shaded band represents range of common cruise altitudes. Range of latitudes for continental U.S. is approximately 24°–49°N (e.g., Miami, Florida, is at a latitude of 25.77°N, and Seattle, Washington, is at 47.61 °N). Source: Adapted from Wilcox et al. (1977).
and Tuve 1973). Given a total air pressure of 0.19 atm (190 mbar) at this altitude, the O3 mole fraction would be 0.5 ppm.2 In contrast, the value of 5 ×1011 molecules per cubic centimeter at 9,000 m (29,500 ft) at a latitude of 30°N would correspond to only 0.05 ppm.
In addition to the effects of latitude and altitude, O3 varies with season and fluctuates over relatively short periods because of meteorological processes that cause air exchange between the lower stratosphere and the upper troposphere (Seinfeld and Pandis 1998b). Thus, although Figure 3–1 illustrates overall annual trends, O3 concentrations at any altitude and latitude fluctuate substantially.
In the lower troposphere, outdoor air contains only trace amounts of O3
(typically, 0.01–0.1 ppm). However, exposure to low concentrations of O3 has been associated with adverse health effects, including decreases in pulmonary function (e.g., decreases in lung capacity and increased airway resistance), inflammation of lung tissue, and increased mortality (see Chapter 5). Accordingly, various national and international government organizations have established upper limits on the concentration of O3 in the air that people breathe (see Chapter 1, Table 1–1). Specifically, the U.S. national ambient air-quality standards for O3 are 0.12 ppm for a 1-h duration and 0.08 ppm for an 8-h duration.
Ozone Chemistry
In addition to affecting human health directly, O3 can react with chemicals in aircraft to produce potentially irritating contaminants (Weschler and Shields 1997a). Products of indoor O3-alkene reactions include short-lived, highly reactive radicals, quasistable compounds (e.g., secondary ozonides), and stable aldehyde, ketones, and organic acids (see Table 3–2). The substances produced can be more irritating than their precursors (Wolkoff et al. 2000); therefore, preventing their formation and accumulation is another reason to limit O3 in the aircraft cabin.
The relative likelihood of those reactions in an aircraft depends on whether they occur in the gas phase or on surfaces. For a gas-phase (homogeneous) reaction between O3 and an indoor pollutant to have important consequences, it must occur at a rate that is at least as great as the air-exchange rate (the rate at which the cabin air is replaced with outdoor air) (Weschler and Shields 2000). Outdoor-air exchange rates tend to be much greater in aircraft than in homes or commercial buildings. Specifically, exchange rates in aircraft range from 9.7 to 27.3 exchanges per hour (Hocking 1998), and in U.S. residences and office buildings from 0.2 to 2 exchanges per hour (Murray and Burmaster 1995; Persily 1989). The greater air-exchange rate in an aircraft limits the consequences of homogeneous O3 chemistry. However, the potentially high O3 concentrations in aircraft cabins compared with ordinary buildings somewhat offset the effect of faster air exchange.
The compounds that are known to react with O3 at a rate competitive with aircraft air-exchange rates and that are most likely to be encountered in an aircraft environment include d-limonene, α-pinene, and isoprene. Sources of these compounds include solvents, cleaning fluids, and “synthetic” natural rubber materials (Budavari et al. 1989).
TABLE 3–2 Products of O3/Alkene Reactions Identified or Suspected in Indoor Settings
|
Product |
Reference |
|
Hydroxyl radical |
Nazaroff and Cass 1986; Weschler and Shields 1996; Weschler and Shields 1997b |
|
Hydroperoxy and alkylperoxy radicals |
Nazaroff and Cass 1986; Weschler and Shields 1997b |
|
Stabilized Criegee biradicals |
Finlayson-Pitts and Pitts 1999; Tobias and Ziemann 2000; Tobias et al. 2000 |
|
Unidentified radical |
Clausen and Wolkoff 1997 |
|
Hydrogen peroxide |
Li 2001 |
|
Organic hydroperoxides |
Tobias and Ziemann 2000; Tobias et al. 2000 |
|
Peroxyhemiacetals |
Tobias and Ziemann 2000; Tobias et al. 2000 |
|
Ozonides |
Tobias and Ziemann 2000; Tobias et al. 2000; Morrison 1999 |
|
Formaldehyde |
Finlayson-Pitts and Pitts 1999 |
|
Other volatile aldehydes and ketones |
Finlayson-Pitts and Pitts 1999 |
|
Fine and ultrafine particles |
Weschler and Shields 1999; Wainman et al. 2000; Long et al. 2000 |
|
Condensed-phase constituents containing carbonyl, carboxylate, and/or hydroxyl functional groups |
Yu et al. 1998; Jang and Kamens 1999; Griffin et al. 1999; Virkkula et al. 1999; Glasius et al. 2000 |
Table 3–3 compares the half-lives of d-limonene, α-pinene, and isoprene at three O3 concentrations with their half-lives in the cabin at three air-exchange rates representative of those encountered on aircraft. The table indicates that the reactions of O3 with d-limonene and α-pinene are fast enough to compete with cabin air exchange under conditions of high cabin O3.
Although the high ventilation rates on aircraft limit the time available for gas-phase (homogeneous) chemical reactions to occur, evaluating the importance of surface (heterogeneous) O3 reactions is less straightforward. Surfaces in the cabin encounter more O3 at high ventilation rates than at moderate ventilation rates. That situation promotes the O3 oxidation of chemicals associ-
TABLE 3–3 Comparison of Half-Lives of Selected Pollutants at Different O3 Concentrations and in Cabin Air at Different Air-Exchange Rates
|
Pollutant |
2nd Order Rate Constanta (ppb−1h−1) |
Pollutant Half-Life (h) at O3 Concentration (ppb) |
Pollutant Half-Life (h) in Cabin Air at Air-Exchange Rate (air changes/h) |
||||
|
100 |
200 |
300 |
7.5 |
10 |
12.5 |
||
|
d-Limonene |
1.8×10−2 |
0.38 |
0.19 |
0.13 |
0.09 |
0.07 |
0.05 |
|
α-Pinene |
7.6×10−3 |
0.92 |
0.46 |
0.31 |
0.09 |
0.07 |
0.05 |
|
Isoprene |
1.1×10−3 |
6.4 |
3.2 |
2.1 |
0.09 |
0.07 |
0.05 |
|
aData from Mallard et al. (1998) |
|||||||
ated with surfaces, especially chemicals with unsaturated carbon-carbon bonds. Such reactions generate products with a range of volatilities. Volatile products desorb from the surface and enter the gas phase, in which they are diluted with ventilation air. Therefore, although the increased ventilation rates favor the formation of reaction products, the greater production rate is offset by a greater dilution rate.
Higher ventilation rates can lead to the accumulation of semivolatile products of heterogeneous O3 chemistry on surfaces depending on their vapor pressure and rate of volatilization. In other words, the surfaces can become a reservoir for such products, which later volatilize from them. Volatilization can occur for extended periods after the initial production of the semivolatile species (Morrison and Nazaroff 1999); this means that semivolatile oxidation products can continue to be emitted from surfaces in the aircraft even when the O3 concentrations in the cabin are close to zero. Factors like a sudden change in relative humidity in the cabin could alter the rate of desorption of some compounds from surfaces. See Box 3–1 for further discussion and examples of heterogenous O3 reactions.
Methods of Controlling Ozone in Aircraft
Concern about O3 in aircraft was first expressed in the middle 1950s. In the early 1960s, Brabets and co-workers conducted a monitoring survey of O3 aboard commercial aircraft (Brabets et al. 1967). They measured real-time cabin O3 on 285 commercial jet flights. Little or no O3 was detected on flights
|
BOX 3–1 Examples of Heterogeneous O3 Reactions Consider a February flight between New York City and Chicago (about 40°N latitude). Many of the aircraft that fly this route are not equipped with O3 converters (M.Dechow, Airbus, personal communication, April 13, 2001; R. Johnson, Boeing, personal communication, April 25, 2001). Nonetheless, because of the high volume of traffic, a substantial fraction of flights on this route are assigned a cruise altitude of 10,700 m (35,100 ft). The average ambient O3 concentration at this altitude and latitude in February is approximately 260 ppb±200 ppb (Law et al. 2000). Accordingly, if one assumes that 30% of the O3 in the ventilation air is removed by indoor surfaces (see Box 3–2 for a discussion of retention ratio) and that 15% of the O3 removed by surfaces produces formaldehyde (a middle-range estimate derived from O3 interactions with recently painted latex surfaces, Reiss et al. 1995), then a central-tendency estimate of formaldehyde in cabin air as a consequence of O3-driven heterogeneous reactions could be calculated according to the following Equation: (260 ppb)(0.30)(0.15)=12 ppb. However, if one assumes that 50% of the O3 in the ventilation air is removed by indoor surfaces, on the basis of data on the 747–100 aircraft (Nastrom et al. 1980), and that 30% of the O3 removed by surfaces produces formaldehyde (an upper-bound estimate derived from O3 interactions with recently painted latex surfaces, Reiss et al. 1995), then a plausible upper-bound estimate of formaldehyde in cabin air could be calculated according to the following equation: (460 ppb)(0.50)(0.30)=69 ppb, where the ambient O3 concentration of 460 ppb is considered to be 1 standard deviation above the mean. To put these estimates into perspective, the American Conference of Governmental Industrial Hygienists has established a threshold limit value ceiling of 300 ppb for formaldehyde. This value is recommended for occupational exposure of healthy workers. The California Air Resources Board has proposed indoor air-quality guidelines for formaldehyde with 100 ppb set as the “action” value and 50 ppb set as the “target” value. These guidelines apply to the general population. Next, consider a heterogeneous process that produces a semi-volatile product, cis-2-nonenal, on carpeted and upholstered surfaces in an aircraft. The odor |
|
threshold for this compound is 2 ppt (Devos et al. 1990). At a ventilation rate of 10 air exchanges per hour, the emission rate of this compound from surfaces would have to be 20 ppt/h for its concentration to reach 2 ppt. Assume that the interior of an aircraft cabin has a volume of 200 m3 (slightly larger than an MD-80, Hocking 1998) and a surface area of 100 m2 covered with carpet and upholstery. The emission rate of cis-2-nonenal from these surfaces would have to be 0.23 μg m−2h−1 to achieve the odor threshold. As demonstrated by the studies of Morrison and Nazaroff (1999), such emission rates are easily achieved. They reported emissions of 2-nonenal of up to 200 μg m−2 h−1 from carpets exposed to O3 at 100 ppb. The examples provided show that the volatile products of heterogeneous reactions (e.g., formaldehyde) can reach meaningful, but not necessarily excessive, concentrations in cabin air. The semivolatile products of heterogeneous reactions can accumulate on surfaces, and the surface concentrations can eventually become large enough for the surface emission rates to exceed odor thresholds for some compounds. |
below the tropopause. At altitudes above the tropopause, the O3 was commonly above 0.1 ppm. The study found a maximal O3 concentration of 0.35– 0.40 ppm averaged over a 20-min period. Later, Bischof (1973) pointed out that the highest cabin O3 would be experienced during high-altitude, long-distance flights at high latitude in the spring. He measured O3 in the cabin air on 14 flights over polar areas. He reported concentrations greater than 0.1 ppm for 75% of the flight time and maximal concentrations of 0.4 ppm averaged over 4 h and 0.6 ppm averaged over 1 h.
By the middle 1970s, concern about O3 in high-altitude flights had become widespread. Studies conducted in the late 1970s confirmed that high O3 concentrations could be encountered in the passenger cabin during flight (Nastrom et al. 1980). Furthermore, symptoms that have been associated with O3 exposure were more prevalent in flight attendants on long-range, high-altitude flights than on short-haul flights (Reed et al. 1980).
The results of those studies and others precipitated regulatory action by FAA. Two regulations introduced in 1980 established O3 concentration limits for aircraft cabins (FAR 25.832 and FAR 121.578). The regulations are discussed in Chapter 1, and the regulatory language is in Appendix C. Airlines
and aircraft manufacturers comply with the regulations through a combination of air treatment, which reduces O3 in ventilation supply air, and flight planning, which reduces the likelihood of high ambient O3. Another consideration in meeting the regulations is the fact that the mole fraction of O3 in cabin air is lower than that in ambient air due to ozone’s reactivity. The retention ratio quantifies this effect (see Box 3–2 for a discussion of retention ratio and related issues).
As discussed in Chapter 2, the use of O3 converters is the only active treatment technology commonly used for reducing O3 concentrations in aircraft passenger cabins. According to SAE International (2000), a 1997 survey indicates that about 50% of the world fleet of wide-body aircraft are equipped with O3 converters. Such converters are essentially nonexistent on narrow-body commercial aircraft. More recent information from Airbus and Boeing provides greater detail on the number of aircraft equipped with O3 converters. Airbus reports that two-thirds of narrow-body aircraft (A319, A320, and A321) produced in 2000 are equipped with converters. However, Airbus was not able to estimate the fraction of its wide-body aircraft (A300, A310, and A300–600) that have O3 converters because operators may have retrofitted them with converters. All long-range aircraft (A330 and A340) are equipped with O3 converters (M.Dechow, Airbus, personal communication, April 13, 2001). Boeing reports that all 777s and 767–400 come with O3 converters as standard equipment. Converters are available as optional equipment on other Boeing models (Hunt et al. 1995), and various proportions of other aircraft models have such converters. Table 3–4 shows the approximate percentages of active Boeing aircraft with O3 converters (personal communication, Richard Johnson, Boeing, April 25, 2001). When new, converters can decompose 90– 98% of the O3 present in the air flowing through them (SAE 2000). The useful life of O3 converters is 10,000–20,000 flight hours (R.Lachelt, Engelhard, personal communication, June 12, 2001).
The introduction and use of O3 converters in some aircraft have apparently reduced the frequency and severity of high-O3 episodes in aircraft cabins, inasmuch as the widespread complaints about O3 in the 1970s have not recurred. Some concerns remain, however. Some aircraft that are not equipped with the converter may have elevated O3 concentrations. Furthermore, there is no process to ensure a high level of converter performance although FAA stipulates that the effectiveness of O3 converters should be spot-checked (Fed. Regist. 45(14):3880–3883, January 21, 1980).
|
BOX 3–2 Retention Ratio and Related Issues The retention ratio is defined as the O3 mole fraction in cabin air normalized by the O3 mole fraction in ambient air in the absence of deliberate control devices (e.g., O3 converters). The retention ratio is intended to account for inadvertent O3 decomposition in the ventilation system and in the aircraft due to reactions on surfaces. It also accounts for reactions that occur spontaneously at the high temperature of the compressor. The retention ratio is a function of the rate of reaction on these surfaces and the ventilation rate. It can be expressed as the ratio of the rate of ventilation (air-exchange rate in units of inverse time) to the sum of the rates of decomposition and ventilation. The best direct data on the O3 retention ratio are based on simultaneous measurements of cabin and ambient O3 on many flights on two aircraft—a Boeing 747–100 and a Boeing 747SP (Nastrom et al. 1980). The average for the 747–100 was 0.465, and that for the 747SP was 0.825. For demonstrating compliance with FAA regulations, airplanes can be assigned a default retention ratio of 0.7 (cited in SAE AIR910 Rev. B page 5 as Final Rule Preamble, Amdt Nos 25–56 and 121–181). The use of a lower value would require the support of direct experimental testing. Such testing would be done on the ground by introducing O3 into the supply air and measuring the resulting interior concentration. The phenomenon of O3 decomposition on surfaces has been widely studied to understand O3 in buildings; see the reviews by Nazaroff et al. (1993) and Weschler (2000). Studies have shown three important points in the present context. First, the rate of reaction of O3 on some surfaces depends on humidity, with higher rates of reaction occurring at higher humidity. That observation raises concern about the use of ground-based studies to measure O3 retention ratios on aircraft because the higher humidity on the ground may lead to lower retention ratios than would prevail during flight. Second, materials exhibit an aging effect: the rate of reaction slows after a period of consistent exposure. That observation also raises concern about the ground-based testing procedure: a short-term test on the ground at low O3 may not properly account for the aging that could occur during long-term exposure to higher O3 on a flight. Third, O3 decomposition on surfaces can produce secondary reaction products, such as aldehydes, that can have low odor and irritation thresholds (see Box 3–1). |
TABLE 3–4 Percentage of Boeing Aircraft with O3 Convertersa
|
Aircraft |
No. Delivered and Ordered Aircraft |
Percentage with O3 Catalytic Converters |
|
B717 |
60 |
0 |
|
B727–100/200 |
1,380 |
0 |
|
B737–200/300/400/500 |
2,910 |
0 |
|
B737–600/700/800/900 |
1,100 |
22 |
|
B747–100/200/300/SP |
700 |
20 |
|
B747–400 |
578 |
64 |
|
B757–200 |
1000 |
7 |
|
B757–300 |
35 |
34 |
|
B767–200 |
250 |
41 |
|
B767–300 |
600 |
42 |
|
B767–400 |
20 |
100 |
|
B777–200/300 |
335 |
100 |
|
DC-9 |
750 |
0 |
|
MD-80 |
1,175 |
0 |
|
MD-90 |
115 |
0 |
|
DC-10 |
400 |
88b |
|
MD-11 |
195 |
78 |
|
aData in table represent approximate number of active aircraft and approximate percentages with O3 catalytic converters. Table is snapshot of information as of May 2001. bPercentage with ordered retrofit kits. |
||
Maintenance procedures for O3 converters specify cleaning or replacement of the catalyst according to a schedule of flight-hours; however, the committee found no evidence that converters are subject to performance testing after cleaning and maintenance. Concern exists because catalytic converters are generally subject to poisoning by deposits of PM or chemicals (Rodriguez and Hrbek 1999; SAE 2000). Of particular concern is the potential for fouling caused by an air-quality incident. For example, any sizable exposure of the bleed-air system to engine lubricating oil, hydraulic fluid, or even jet exhaust could contribute to fouling that would degrade the performance (de-
struction efficiency) of the O3 converter. A lower destruction efficiency could lead to exceeding the regulatory limit. Specifically, a 95% O3 destruction efficiency appears to be sufficient to maintain cabin O3 below regulatory limits, but a 50% destruction efficiency would not suffice (see Box 3–3). Although the committee found no hard evidence that fouling of the O3 converter occurs, it also did not find any evidence to the contrary.
As noted above, flight planning can be used to control cabin O3 and is based on statistical summaries of atmospheric O3 as a function of altitude, latitude, and season. Illustrative data are presented in Figure 3–2. The data reinforce the point from Figure 3–1 that O3 increases with cruise altitude and tends to be higher at high latitudes than at middle latitudes. Figure 3–2 also shows that O3 varies seasonally, with higher concentrations occurring during winter and spring than during summer and fall.
By regulation (14 CFR §121.578cl; see Chapter 1 or Appendix C), flight planning to limit cabin O3 is based on the 84th percentile atmospheric O3 for a given altitude, latitude, and month. O3 is expected to exceed the planning value 16% of the time. Therefore, under the regulation, cabin O3 is permitted to exceed the numerical limits of 0.25 ppm (peak) and 0.1 ppm (time-weighted average) in a substantial fraction of flights. Depending on the relationship between the absolute maximal atmospheric O3 and the 84th percentile, permissible cabin O3 concentrations under this regulation could be considerably higher than the nominal numerical limits.
|
BOX 3–3 Effect of Decreased Destruction Efficiency of O3 Converters on Regulatory Compliance Consider a sustained encounter (greater than 3 h) of an aircraft to O3 at 0.5 ppm at an altitude of 12,000 m (39,400 ft). Assume that the cabin pressure is 0.78 atm (795 mbar), corresponding to an altitude of 2,000 m (6,560 ft). Assume a default value of 0.7 for the O3 retention ratio. In the absence of an O3 converter, the O3 mole fraction in the cabin would be 0.35 ppm, and the sea-level equivalent value would be 0.27 ppm. Addition of an O3 converter with a 50% destruction efficiency would reduce the values by 50%, which would exceed the regulatory limit of 0.1 ppm (14 CFR § 25.832 [b]; see Appendix C). However, an O3 destruction efficiency of 95% would yield a predicted sea-level equivalent cabin O3 concentration of 0.014 ppm, well below the regulatory limit. |
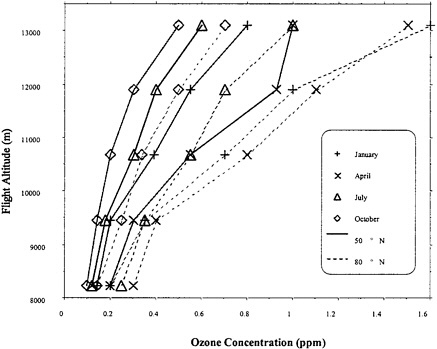
FIGURE 3–2 O3 concentrations at 84% upper-bound confidence interval, as a function of altitude, for selected months and latitudes. The 0.1-ppm concentration corresponds to 200 μg/m3, where the volume is determined at sea-level pressure, and temperature is 20°C. Source: Data from SAE (2000).
Measurements of Ozone in Aircraft
Since the introduction of FAA O3 regulations, few systematic studies of cabin air quality have included O3 measurements (Nagda et al. 1989; Nagda et al. 1992; Eatough et al. 1992; Sussell and Singal 1993; Spengler et al. 1997; Pierce et al. 1999; Waters et al. 2001). The lack of studies is due partly to limitations in the instruments available to measure O3 (see Chapter 7 for a brief discussion of O3 monitoring aboard aircraft). Only ultraviolet (UV) photometric and chemiluminescence instruments have sufficient sensitivity and accuracy to measure O3 reliably and accurately in the concentration range of interest. Furthermore, only UV photometric instruments are acceptable aboard aircraft because chemiluminescence instruments use a combustible consumable (ethyl-
ene), which precludes their use. The disadvantages of the commercially available UV photometric instruments are that they weigh more than 10 kg and require a 110-V AC power source. Lighter, smaller instruments compatible with the power systems aboard aircraft are feasible but are not commercially available. Regardless, the post-1980 studies are summarized below, along with a brief critique of their methods and findings.
The most thorough post-1980 study of cabin air quality was conducted for the Department of Transportation (DOT) by Geomet (Nagda et al. 1989; Nagda et al. 1992). It was conducted during the transition period when smoking was common on flights and when it was almost completely banned, so the emphasis was on environmental tobacco smoke and its constituents. The researchers measured many air-quality characteristics in the passenger cabins of 92 randomly selected flights between April and June 1989. Only 12 of 92 flights were longer than 5 h, and only eight were international. The proportion of aircraft equipped with O3 converters was not reported. O3 was measured with an integrated sampling technique. A pump was used to draw air at a fixed rate over glass-fiber filters that had been treated with 3-methyl-2-benzothiazolinone acetone azine (MBTH) and 2-phenylphenol according to the method of Lambert et al. (1989). Time-integrated samples were collected beginning 15 min after takeoff and concluding 30 min before scheduled arrival. The results showed relatively low O3 concentrations: averages of 0.010 ppm on smoking flights and 0.022 ppm on nonsmoking flights.3 The highest reported time-weighted average was 0.078 ppm, which is less than the regulatory limit of 0.1 ppm.
Although the study suggests that average O3 in aircraft cabins is low, the committee has several concerns regarding the study and believes that general
conclusions based on the results should be avoided. The concerns include the accuracy and precision of the sampling method, the use of integrated samplers instead of real-time measurements, and the small number of the flights evaluated. The concerns noted are discussed further in the following paragraphs.
The MBTH sampling method chosen for the DOT study (Nagda et al. 1989) was developed shortly before the study started. The primary reference (Lambert et al. 1989) is limited in scope, focusing on the colorometric change associated with the MBTH reagent and potential interferences, and does not thoroughly explore issues associated with sampling. Even with its limited scope, the study by Lambert and co-workers found that reagent response, measured by reflection absorbance, was significantly nonlinear. Furthermore, the response was not simply a function of exposure (the product of O3 concentration and time). For example, exposure at 0.2 ppm for 500 s produced a reflection absorbance 33% higher than exposure at 1.0 ppm for 100 s. Some concern exists because the aircraft samples were collected over periods of approximately 1–10 h, much longer than the sampling periods of 1–10 min used by Lambert and coworkers.
Despite considerable continuing attention to the broad issue of environmental sampling of O3, the MBTH method does not appear to have been used in any sampling studies after the DOT airliner investigation. Of the recent papers that report development of improved sampling methods, only one cites the work by Lambert and co-workers (Zhou and Smith 1997); others do not even acknowledge it (Monn and Hangartner 1990; Grosjean and Hisham 1992; Koutrakis et al. 1993; Black et al. 2000).
In addition to the concerns about the accuracy and precision of the sampling method, concerns regarding the use of integrated sampling have been raised. The evidence from real-time monitoring clearly demonstrates that O3 in aircraft is highly variable with time. Thus, integrated sampling can fail to detect important occurrences of increased cabin O3. For example, if in a 10-h flight O3 was very low for 9 h but was high (above 0.5 ppm) for 1 h, an accurate time-integrated measurement would reveal a low concentration (approximately 0.05 ppm). However, the peak (0.5 ppm) would represent a real health concern and, if it occurred above a flight altitude of 32,000 ft (9,750 m), would be above the regulatory limit (14 CFR §121.578).
The authors of the DOT study concluded, on the basis of their O3 measurements, that “all values were consistently below flight, occupational, and environmental standards by the Federal government…. This and current scientific knowledge leads to the conclusion that ozone does not pose a health hazard to cabin crew members or passengers.”
The committee notes that in at least two respects the evidence does not support the authors’ conclusion. First, high ambient O3 encountered during cruise clearly can lead to cabin O3 concentrations that exceed the DOT standard and ambient air-quality standards in the absence of effective controls. Therefore, at a minimum, the conclusion should be qualified to state “provided that control measures are functioning effectively.” Second, high ambient O3 occurs episodically, so one cannot conclude on the basis of flight-integrated measurements that the peak concentrations are below the appropriate standard.
O3 measurements on aircraft have been reported in several other post-1980 studies. However, because of methodological concerns or small sampling scope, they add little to our knowledge of O3 concentrations in the aircraft passenger cabins.
Eatough et al. (1992) measured O3 in four 4- to 5-h flights on DC-10 aircraft in which smoking was permitted. The authors used sorbent tubes, which measure time-weighted average concentrations. The flight-average values ranged from less than 0.002 to 0.020 ppm, with an overall average of 0.009 ppm. The use of integrated samplers limits the utility of the data.
In 1990, in response to a request from the Association of Flight Attendants (AFA), the National Institute for Occupational Safety and Health (NIOSH) conducted a health-hazard evaluation of cabin air quality (Sussell and Singal 1993). The investigation included real-time measurements of O3 on three Alaska Airlines short-haul flights on MD-80 aircraft during July 1990 along the West Coast of the United States. Because details of the measurement techniques were not included in the report, the accuracy of the data is difficult to judge. Nonetheless, the data indicate that episodic peaks were encountered in each flight, and peak concentrations were much larger than average O3 concentrations. The limited data reinforce the finding that the use of time-averaged samplers for O3 is inadequate to demonstrate compliance with DOT regulations.
In a study conducted for the Boeing Company, Spengler et al. (1997) made air-quality measurements in several modes of commercial transport. Measurements in aircraft passenger cabins were made on four flight segments in 1996. Integrated average O3 concentrations were “consistently below the limit of detection for the method used (i.e., 1.8 to 9.8 ppb).” Again, the use of integrated samplers limits the utility of the data.
In a research project funded by the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), O3 measurements were made on eight commercial flights of Boeing 777 aircraft (ASHRAE/CSS1999;
Pierce et al. 1999). As noted above, O3 converters are standard on ventilation systems on the Boeing 777. Four segments were domestic (1,000–1,500 miles), and four covered international routes (over 3,000 miles). Monitoring was done during July 1998. O3 was measured with a continuous, direct-reading electrochemical sensor. The reported in-flight mean O3 concentrations on domestic and international flight segments were 46 and 53 ppb, respectively.
Two technical aspects of the measurement system used by Pierce and coworkers are troubling. First, the authors reported that the instrument was calibrated with 2 ppm of NO2 in air (ASHRAE/CSS1999). It is highly unusual to calibrate a pollutant-monitoring device with a pollutant different from the one to be measured. Second, the reported accuracy of the monitoring instrument is ±0.075 ppm. Because that value is comparable with the lower concentration in the standard and is higher than the mean measured, the entire set of sampling results must be called into question. If the data were accurate, they would provide some evidence of degraded performance of the O3 converter relative to the rated efficiency of 95%. On the basis of a default retention ratio of 0.7 and converter efficiency of 95%, the inside O3 concentration should only be 3.5% of the outside concentration—0.7(1−0.95)=0.035. Performance would not appear to be as good in these flight segments. The maximal ambient O3 encountered by an aircraft is likely to be about 1.0 ppm (Nastrom et al. 1980). Therefore, with the O3 converter operating at its rated efficiency, the maximal concentration in the cabin should be no higher than about 0.04 ppm. The reported maximum of 3 times that suggests a converter efficiency of approximately 85%.
Finally, a substantial study of air quality in aircraft cabins is in its late stages. The work is being conducted by NIOSH in partnership with the FAA Civil Aeromedical Institute (Waters et al. 2001). The project includes sampling on 37 flight segments in 11 aircraft types of four airline companies. O3 is measured in real time with an electrochemical sensor. As noted earlier, such sensors have limited sensitivity and resolution. Preliminary results indicate substantially higher O3 concentrations than reported for the other post-1980 studies. On the basis of gate-to-gate measurements, the reported average O3 is 0.20 ppm, which is well in excess of the DOT standard of 0.1 ppm. The reported average peak O3 on these flights is 0.28 ppm. The reported maximal time-weighted average and peak are 0.55 ppm and 1.0 ppm, respectively. These values are high enough to trigger serious concern; however, the investigators have indicated that the results are preliminary. Because the results have not been subjected to peer review, further evaluation of the data will not be warranted until a written report has been issued.
In summary, unacceptably high O3 concentrations can occur in passenger cabins of commercial aircraft in the absence of effective controls. The 1986 National Research Council (NRC) report (NRC 1986b) on the aircraft cabin environment included this recommendation:
The Committee could find no documentation of the effectiveness of the various methods being used by the airlines to control O3. Therefore, the Committee suggests that FAA carry out a carefully designed program to ensure that cabin O3 concentrations comply with Department of Transportation regulations.
In a 1987 DOT report to Congress, a commitment was made to establish such a program:
The FAA will issue biennial action notices requiring FAA inspectors to report on the present status of all U.S. air carriers’ compliance with the existing O3 regulations. The response to the action notices will be summarized and published. Identified deficiencies will be corrected.
However, perhaps because of the low concentrations of O3 reported in the 1989 DOT study (Nagda et al. 1989; Nagda et al. 1992), no such program appears to have been implemented. For reasons discussed above, the present committee does not find that there is any basis for confidence that the DOT O3 standard is regularly met. Nor does it appear possible to demonstrate that without a continuing program that incorporates regular real-time O3 monitoring on aircraft. Therefore, the potential for high O3 in aircraft cabins remains a concern. A program of regular monitoring of cabin O3, particularly on high-altitude, high-latitude flights, is necessary to document the effectiveness of control measures. Such monitoring must use reliable and accurate measurement equipment that is capable of making real-time O3 measurements.
CONTAMINANTS WITH INTERNAL SOURCES
Sources of contaminants inside the cabin are associated with the passengers and crew in the form of bioeffluents, viruses, bacteria, allergens, and spores; these contaminants are shed from clothing or skin or expelled from oral, nasal, or rectal orifices. Structural components of the aircraft, luggage,
personal articles, food, and sanitation fluids can also be sources of vapors or particles. Furthermore, surface residues on aircraft components can be sources of cleaning compounds, pesticides, or simply accumulated debris. The sources and the mechanisms of their emission and dispersion are not specific to the aircraft cabin environment. Most public transportation conveyances will have similar sources. However, aircraft environments are somewhat different given the high surface-to-volume ratios and the relatively small volume-to-passenger ratios.
The following subsections discuss contaminants associated with passengers and their belongings, aircraft component materials, cleaning materials and dust, and pesticides. In several cases, few published data are available on the aircraft environment, and data on similar indoor environments are presented. For a discussion of similarities between building and aircraft environments, see Appendix B. As noted in the introduction to this chapter, biological agents (e.g., infectious agents and allergens) are discussed in detail in Chapter 4; they are not addressed further here.
Passengers and Belongings
Passengers and crew are sources of bioeffluents, allergens, and infectious agents. Through metabolic activity and personal sanitary habits, people can emit odors that others perceive as unpleasant. Bioeffluent emission rates are presented in Table 3–5 for several common compounds as prepared by the NRC Subcommittee on Guidelines for Developing Spacecraft Maximum Allowable Concentrations (SMACs) for Space Station Contaminants (NRC 1992).
As suggested by data in Table 3–5, the passengers and crew are the primary sources of CO2 in the cabin. Studies conducted in the aircraft environment indicate that CO2 can range from 293 to 4,238 ppm (see Table 1–2). CO2 is often used as a marker of the adequacy of ventilation, which is critical in the aircraft environment. For the building environment, Wargocki et al. (2001) found on review of the current literature that “increasing the ventilation rate improves perceived air quality, decreases the prevalence of SBS [sick-building syndrome] symptoms, improves clinical symptoms, reduces absenteeism and improves the performance of office work.”
In addition to producing bioeffluents, passengers may apply odor-producing products including nail polish, nail-polish remover, cologne, and perfume. Measurements of VOCs during cruise have identified many compounds, which are
TABLE 3–5 Human Sources of Bioeffluent Aircraft Cabin Contaminantsa
|
Substance |
Metabolic Generation Rate (mg/day per person) |
|
Alcohols |
|
|
Methanol (methyl alcohol) |
1.42 |
|
Ethanol (ethyl alcohol) |
4.00 |
|
2-Methyl-l-propanol (isobutyl alcohol) |
1.20 |
|
1-Butanol (n-butyl alcohol) |
1.33 |
|
Aldehydes |
|
|
Ethanal (acetaldehyde) |
0.08 |
|
Pentanal (valeraldehyde) |
0.83 |
|
Hydrocarbon |
|
|
Methane |
600.00 |
|
Ketone |
|
|
2-Propanone (acetone) |
0.13 |
|
Mercaptans and sulfides |
|
|
Methanethiol (methyl mercaptan) |
0.83 |
|
Ethanethiol (ethyl mercaptan) |
0.83 |
|
1-Propanethiol (n-propyl mercaptan) |
0.83 |
|
Organic acids |
|
|
2-Oxopropanoic acid (pyruvic acid) |
208.30 |
|
n-Pentanoic acid (valeric acid) |
0.83 |
|
Octanoic acid (caprylic acid) |
9.17 |
|
Organic nitrogen compounds |
|
|
1-Benzopyrrole (indole) |
25.00 |
|
3-Methylindole (skatole) |
25.00 |
|
Miscellaneous |
|
|
Hydrogen |
50.00 |
|
Ammonia |
250.00 |
|
Carbon monoxide |
33.30 |
|
Carbon dioxide |
8.8×105b |
|
aIUPAC or accepted name is provided with common name in parentheses, where relevant. bEstimate based on generation rate of 0.31 L/min, which was used by ASHRAE to determine ventilation standards (ASHRAE 1999). Source: Data from NRC (1992). |
|
listed with their concentration ranges in Table 3–6; data on other transportation modes are presented for comparison.
TABLE 3–6 Volatile Organic Compounds Frequently Detected in Passenger Compartments of Transportation Vehicles and Their Concentrations
|
|
Concentration, μg/m3a |
|||||
|
Compound |
Commercial Uses or Sources |
Aircraft (1994) |
Aircraft (1996), Boeing 777 |
Trains |
Buses |
Subways |
|
Ethanol |
Bioeffluent, distilled spirits |
280–4,300 |
290–2,600 |
170–1,700 |
50–260 |
130–300 |
|
2-Propanol |
Distilled spirits, solvent |
|
12–43 |
0–33 |
7–63 |
9–23 |
|
Acetone |
Bioeffluent, sealants, adhesives, solvent for cellulose acetate |
74–150 |
52–140 |
49–92 |
30–73 |
30–92 |
|
2-Butanone |
Solvent in resins, adhesives, nitrocellulose coatings and vinyl films, cleaning fluids; printing catalyst; in lubricating oils |
3–16 |
4–8 |
3–11 |
4–18 |
4–17 |
|
d-Limonene |
Scent in cleaners |
12–24 |
2–45 |
1–17 |
190– 490 |
1–6 |
|
Pentadiene isomer |
Combustion exhaust |
10–30 |
— |
0–30 |
0–20 |
10–20 |
|
Benzene |
Aviation fuel, gasoline, perfumes |
1–6 |
— |
2–4 |
2–6 |
4–7 |
|
Toluene |
Gasoline, solvent for paint, thinner, coatings and rubber, cosmetics |
0–29 |
9–19 |
7–54 |
15–39 |
13–27 |
|
m- & p-Xylene |
Gasoline, solvent in cosmetics |
0–8 |
2–4 |
3–9 |
6–48 |
5–50 |
|
Trichlorofluoromethane |
Aerosol sprays, blowing agent for polyurethane foam, refrigeration, fire extinguisher |
0–2 |
3–6 |
3–6 |
1–4 |
3–150 |
|
Ethyl acetate |
Whiskey fermentation |
0–4 |
0–26 |
2–20 |
2–10 |
0–4 |
|
Decane |
Gasoline, solvent |
37016 |
36926 |
36971 |
6–34 |
36966 |
Aircraft Component Materials
Many aircraft components are made of lightweight plastics. For example, the overhead luggage compartments, sidewalls, ceilings, lavatories, and bulkhead separators are made of formed plastics. Accordingly, passengers and crew can be exposed to various plasticizers, such as phthalates. Animal and human studies indicate that exposure to such plasticizers as mono(2-ethylhexyl) phthalate (MEHP), which is the primary hydrolysis product of di(2-ethylhexyl) phthalate (DEHP), may cause adverse health effects. Øie et al. (1997) suggested that phthalates play a role in asthma by mimicking prostaglandins and thromboxanes in the lungs. Doelman et al. (1990) demonstrated that MEHP induced bronchial hypersensitivity in rats, and Roth et al. (1988) concluded that DEHP from respiratory tubing used to ventilate preterm infants induced lung damage in them. More recently, Jaakkola et al. (2000) reported adjusted odds ratios for persistent wheeze of 3.42 (95% confidence interval, 1.13–10.36) and for cough of 2.41 (95% confidence interval, 1.04–5.63) in a cross-sectional study of 2,568 Finnish preschool children that evaluated the relationship between the presence of plastic wall materials in the home and respiratory health. These findings are consistent with earlier studies by Jaakkola et al. (1997). Although the health evidence linking phthalates to respiratory effects is still limited, exposures to these chemicals may be associated with several respiratory symptoms, including bronchial obstruction, asthma, and respiratory infections.
Passengers and crew may be exposed to coatings used on aircraft components. For example, because water condenses between the wrapped insulation barrier and the colder metal surface of the plane, the internal surface of the fuselage is coated with anticorrosive and antimicrobial materials. Fabric seat and floor coverings, like other commercial materials, may be treated with stainresistant and antifungal-antibacterial chemicals. Although the potential for exposure to these coating materials exists, no published data were available to determine the extent or degree of exposure of passengers and crew to these materials.
Other materials associated with the aircraft can be sources of VOCs (see Table 3–6). Some measurements of VOCs in planes suggest that foaming agents, plastic resins, and cleaning materials contribute to the contaminant burden. However, measurements of VOCs and of other organic compounds (e.g., aldehydes) are limited. Concentrations reported to date are integrated samples that do not indicate short-term concentrations exceeding odor thresholds or occupational limits. However, further investigation of VOCs in aircraft
may be warranted in that several chamber studies conducted by Mølhave (2001) and Otto et al. (1990) demonstrate that individual VOCs and mixtures of VOCs can lead to progressive eye, nose, and throat discomfort over several hours of continuous exposure.
Although the concentrations for individual compounds may be well below their individual odor thresholds, occurrence as mixtures may degrade the perceived air quality or lead to sensory irritation (Cometto-Muñiz 2001; Mølhave and Neilsen 1992; Ten Brinke et al. 1998). Cabin air quality can also be perceived as unacceptable because of interactions with temperature, humidity, and the combined effects of mixtures. Fang et al. (1998a, 1998b) conducted a series of sensory-perception tests in which subjects evaluated the quality of various air samples (clean air versus air with common indoor sources present) at different temperatures and humidities. The sources were scaled to approximate normal loadings found in office buildings. The study results indicated that as air becomes warm and moist, people cannot discriminate among emissions from different sources, and they uniformly rate the quality as low. Drier, cooler, clean air is perceived as better, and the introduction of odorants is recognized and discriminated more readily in cooler and drier air (see Figure 3–3). Although the experimental conditions used by Fang and co-workers do not extend to the low humidity range experienced on commercial aircraft, their results suggest why passengers and crew may be able to distinguish specific odors in the dry air of aircraft that cause them concern regarding air quality.
Cleaning Materials and Dust
The interiors of aircraft are cleaned between flights. One airline reported that aircraft on the ground overnight are thoroughly cleaned (e.g., floors, flight deck, and closets vacuum cleaned; tray tables, walls, and windows washed; debris collected; and galleys and lavatories wiped and sanitized), and aircraft between flights receive spot cleaning (e.g., debris collected and galleys and lavatories wiped and sanitized) (K.Vailu’u, United Airlines, personal communication, June 19, 2001). During more extensive scheduled maintenance, the interior passenger compartment components (e.g., seats, flooring, and overhead storage compartments) are removed; carpet and seat upholstery may be replaced, and portions of the air duct distribution system are removed. On the basis of committee tours of maintenance facilities, debris and soot appear to accumulate on the inside of planes.
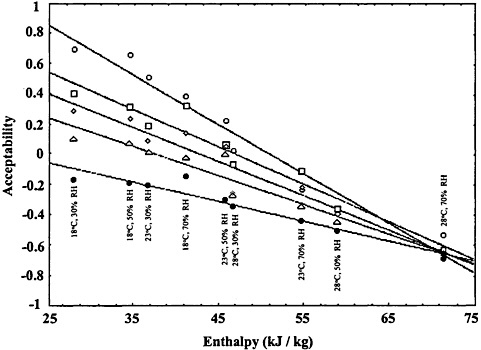
FIGURE 3–3 Acceptability of air samples with different pollutant loadings versus clean air as function of enthalpy. Enthalpy is defined as energy content of air where cool and/or dry air has low enthalpy and warm and/or moist air has high enthalpy. ○, clean air; □, wall paint; ◇, carpet; △, floor varnish; ●, sealant. Source: Adapted from Fang et al. (1998a).
As indicated above, minimal information was provided by one airline regarding cleaning practices and materials; the committee could not reach any conclusions regarding the thoroughness or adequacy of cleaning on the basis of that information. Nor could the committee determine the potential for cleaning-product residues or the debris remaining in the carpet and upholstery to be resuspended or the potential for exposure of passengers and crew. Accordingly, the following is a general discussion of cleaning practices, cleaning products, and possible effects of dust exposure; it is not specific to aircraft.
Wolkoff et al. (1998) reviewed the chemical and physical properties of cleaning agents, which are designed to facilitate the removal of debris from surfaces without damaging materials. Active components include surfactants,
corrosion inhibitors, solvents, disinfectants, complexing agents, and water softeners. Pigments, fragrances, and preservatives are also present in some products. Cleaning agents contain both volatile and nonvolatile components (see Table 3–7). When cleaning agents are applied, the emissions of volatile components peak within 2 h after application and then decay exponentially over many hours.
Denmark has required registration of washing and cleaning agents since 1986. Chemical composition data on over 2,500 agents are available. Products have been categorized into six distinct groups. Table 3–8 summarizes data on the hazards associated with those categories. The disinfectant category
TABLE 3–7 Components or Constituents of Cleaning Agents
|
Component or Constituent |
Examples |
|
Volatile Substances |
|
|
Fragrance |
Terpenes (α-pinene, limonene) |
|
Solvent |
Glycols, glycol ethers (dipropylene glycol, 2-ethoxyethanol, 2-methoxyethanol) |
|
Biocide (disinfectant) |
Formaldehyde and releasers |
|
Plasticizer (softener) |
Phthalates |
|
Residual monomer from polymer (film) |
Styrene, methacrylate |
|
Decomposed product |
Impurities in raw products |
|
Nonvolatile Substances |
|
|
Tensides |
Soap, detergent |
|
Film formers |
Wax, polish, acrylate polymer |
|
Complexing agents |
Ethylenediamine tetraacetic acid (EDTA), citrate salts |
|
Acids |
Phosphoric acid |
|
Bases |
Sodium or potassium hydroxide |
|
Fillers |
Sodium chloride or sulfate |
|
Biocides (disinfectants) |
Benzalkonium chloride, formaldehyde releasers |
|
Colors, pigments |
Different substances |
|
Source: Adapted from Wolkoff et al. (1998). |
|
TABLE 3–8 Number of Products Classified As Irritant (Xi), Harmful to Health (Xn), or Corrosive (C) and Content of CRANa Substances in Selected Danish Cleaning Agents
had the highest percentage (75%) of products classified; many were identified as irritants, corrosives, or potential causes of serious adverse, chronic health effects.
Case studies over the last 25 years have associated respiratory and eye symptoms with carpet cleaning in buildings (Persoff and Koketsu 1978, as cited in Kreiss et al. 1982; Robinson et al 1983; Schmitt 1985; Berlin et al. 1995). Excessive use or improper dilution or application was the probable cause, but these cases provide evidence that inhaled carpet-cleaning residues can cause dry cough and irritation of the throat, skin, and eyes.
More recently, workplace exposures to cleaning compounds have been clinically associated with asthma and pulmonary-function changes. McCoach et al. (1999) examined 17 cases of occupational asthma associated with floor-cleaning materials. Provocation bronchial-challenge tests of six of the workers confirmed that floor-cleaning compounds caused the asthma observed. Rosins (e.g., pine oil and tall oil) and benzalkonium chloride were the constituents
identified as bronchial irritants; they had been associated with occupational asthma in earlier studies (Innocenti 1978; Burge et al. 1986; Burge and Richardson 1994). In addition to respiratory effects, Taylor and Hindson (1982) have linked exposure to allyl phenoxyacetate in dry carpet shampoo to occupational dermatitis.
Another possible source of contaminants to which passengers and crew may be exposed is dust or PM. Vacuuming, mopping, and abrasion can resuspend particles in indoor dust. Resuspension can create personal PM exposures 50% higher than area measurements when averaged over 12–24 h (Clayton et al. 1993). However, active walking on surfaces can raise airborne dust to over 10 times preagitation conditions (Hambraeus et al. 1978). Active vacuum cleaning can also greatly increase concentrations of fine particles (Lioy et al. 1999) and cat allergens (Woodfolk et al. 1993). However, because the air-exchange rates on aircraft are high, airborne-particle exposure resulting from the activities noted would probably persist for only minutes.
Airplane dust should be similar in composition to dust in homes and offices. Dust contains minerals, metals, textile, paper and insulation fibers, combustion soot, nonvolatile organics, and various materials of biological origin (e.g., hair, skin flakes, and dander). Dust can be a vehicle for absorbed organic compounds, such as polycyclic aromatic hydrocarbons. In addition, airplane dust can include residues from cleaning agents and pesticides (see discussion below).
Little information on particle mass and number counts on airplanes exists (see Table 1–2). The previous NRC report (1986a) noted that mass concentrations of respirable particles (mass median aerodynamic diameter approximately 3.5 μm) routinely exceeded 100 μg/m3 while passengers were actively smoking in the designated areas. In the absence of smoking, mass concentrations were usually less than 50 μg/m3 and often below 25 μg/m3. More recently, measurements made on a Boeing 777 (nonsmoking flight) yielded 10-min PM2.5 concentrations of 3–10 μg/m3 during cruise and 11–90 μg/m3 during boarding (Spengler et al. 1997). Measurements reported by Nagda et al. (2001) also support findings of low concentrations of respirable particles. They reported a mean PM10 and PM2.5 concentration of less than 10 μg/m3 in the cabin during cruise. In comparison with particle mass concentrations (PM10 and PM2.5) in U.S. office buildings—indoor concentrations of 3.0–35.4 μg/m3 for PM10 and 1.3–24.8 μg/m3 for PM2.5, according to Burton et al. (2000)—airplanes would rank at the lower end of the concentration ranges during the nonsmoking cruise portion of a flight.
Ultrafine particles (aerodynamic diameters less than 0.1 μm) are formed in copious amounts in pyrolysis of oils, combustion of fossil fuels, and chemical reactions between O3 and unsaturated hydrocarbons. Episodic events of high ultrafine-particle counts would be expected when engine or ground-equipment exhaust enters the passenger cabin. In contrast with catastrophic engine oil-seal failures, which are accompanied by visible smoke in the cabin, slowly leaking oil that comes into contact with hot ECS or engine components would result in ultrafine-particle formation that might not be perceived in the cabin air. Similarly, O3 reactions known to produce ultrafine particles (see Table 3–2) could create imperceptible but large amounts of ultrafine particles. Although ultrafine particles may be introduced into the aircraft cabin, no data are available on counts.
Although no published data on health effects of particle exposure on aircraft are available, dustiness and surface soiling have been associated with complaints of comfort and irritation in schools and offices (Wallace et al. 1991; Skov et al. 1989; Norbäck and Torgen 1989; Roy et al. 1993). Skyberg et al. (1999) conducted an intervention study in which the effect of office cleaning on mucosal symptoms was investigated in 104 nonsmoking office workers. The office spaces of 49 workers (intervention group) were thoroughly cleaned. The office spaces of the other 55 workers (control group) received the usual superficial cleaning. The intervention group experienced a 27% decrease in mucosal symptoms, whereas the control group experienced an increase of 2% (p=0.02). Nasal volume increased in the intervention group by 15% but decreased in the control group by 6% (p=0.02). The indoor airborne-particle concentrations were lower by more than 20 μg/m3 in the intervention group.
Wyon et al. (2000) conducted a double-blind reverse-intervention study of enhanced filtration and air cleaning in a London office building. Occupants reported (p<0.05) less fatigue, less eye ache, clearer thinking, and improved productivity with better-filtered air. Electronically cleaned air did not have as impressive an effect on symptoms.
Mendell et al. (1999) explored enhanced submicrometer particle filtration on an office buildings without known complaints. In a double-blind study, conventional filters were periodically replaced with HEPA filters. Submicrometer-particle mass was significantly lowered, but 2-μm-particle concentrations did not change. Of 16 reported symptoms, 13 improved slightly. However, improvements reached statistical significance in only three of the symptoms (confusion, “too stuffy,” and “too humid”). This study also showed that an increase in temperature by 1°C, although well within the comfort
range, significantly increased complaints. A 1°C increase in temperature had offset the improvements in filtration by a factor of 2–5, indicating that many factors affect perceptions of occupants.
The emerging literature from well-designed double-blind studies and clinical investigations strengthens the observations reported for almost 15 years. Dust—perhaps because of the biological agents, fibers, VOCs, cleaning residues, or other components—can adversely affect the airways, skin, and eyes of occupants. Accordingly, because of the absence of published data specific to aircrafts, systematic sampling of dust on aircraft floors and seats should be conducted. Information should include dust loadings (gram per unit area) and concentration of the components (gram per gram). If problems are identified, the airlines should review their cleaning practices (e.g., cleaning frequency and materials and equipment used) and incorporate newly available techniques to assess the quality of cleaning (Kildesø and Schneider 2001).
Pesticides
The use of pesticides in aircraft for the purpose of insect control is commonly referred to as disinsection (Naumann and McLachlan 1999). The countries that require disinsection on aircraft are listed in Table 3–9 according to disinsection procedure and include Australia, New Zealand, India, and many island nations (DOT 2001). Some of the countries require disinsection only on selected flights on the basis of the origin of in-bound flights; others require disinsection on all in-bound flights. Disinsection is conducted because it is believed to protect public health, agriculture, and the native ecosystems from unwanted insect pests. However, the United States eliminated the practice of disinsection on in-bound flights in 1979 because the Centers for Disease Control and Prevention concluded that disinsection of aircraft was ineffective in preventing insect pests from entering a country and that it would pose a potential health risk to passengers and crew (Anonymous 1999).
The countries that continue to disinsect aircraft base their decisions on reported incidents in which an unwanted vector of human disease has entered the country, established itself, and caused an outbreak of disease (Naumann and McLachlan 1999; WHO 1995). Airport malaria and runway malaria have been reported. In airport malaria, a person contracts malaria from an infected mosquito transported on an aircraft from a malarious region; in runway malaria, a person contracts malaria during a stopover in a region where malaria
TABLE 3–9 Countries That Require Disinsection
|
Countries That Treat All In-Bound Aircraft |
Countries that Treat Selected In-Bound Aircraft (Origin of Aircraft Targeted for Disinsection)c |
|
|
Passengers on Boarda |
Passengers Not on Board (Method)b |
|
|
Grenada |
Australia (residual treatment) |
Czech Republic (areas of contagious diseases) |
|
India |
Barbados (residual treatment) |
Indonesia (infected areas) |
|
Kiribati |
Fiji (residual treatment) |
South Africa (areas of malaria or yellow fever) |
|
Madagascar |
Jamaica (residual treatment) |
Switzerland (intertropical Africa) |
|
Trinidad and Tobago |
New Zealand (residual treatment) |
United Kingdom (malarial countries) |
|
Uruguay |
Panama (spray treatment) |
|
|
aAerosolized spray used as treatment. bDOT (2001) also mentioned that American Samoa sprayed aircraft while passengers were not on board but did not include this country in list. cNo information provided on method of treatment or passenger presence during treatment. DOT (2001) noted that Guam requires disinsection on all flights from Commonwealth of the Northern Mariana Islands, Thailand, Philippines, Korea, Indonesia, Malaysia, the Federated States of Micronesia, Papua New Guinea, Solomon Islands, and the Republic of the Marshal Islands. Furthermore, flights from Taiwan, Korea, and Japan are disinsected during some months. Residual treatment was listed as disinsection method for Guam. Source: Information from DOT (2001). |
||
is endemic (WHO 1995). Karch and co-workers (2001) summarized cases associated with malaria vectors in European aircraft and estimated that 78 cases of airport malaria have occurred in western Europe since 1977. Of the 78, 28 cases occurred in France, primarily at the Paris Roissy Airport, which is the port of entry for aircraft from tropical Africa. The fatality rate was estimated to be about 5%. Gratz et al. (2000) reviewed cases of airport malaria and other related cases of malaria (see Table 3–10) and noted that airport malaria can be problematic if an infected person (e.g., a person who lives near an airport) has no history of travel to a malarious areas; proper diagnosis can be delayed, and the risk of death increased.
As indicated in Table 3–10, countries that continue to disinsect aircraft
TABLE 3–10 Countries in Which Confirmed or Probable Cases of Airport Malaria Have Been Reported, 1969–August 1999
|
|
Period |
|||||
|
Country |
1969–77 |
1978–86 |
1987–95 |
1996–98 |
1999 |
Total |
|
France |
9 |
3 |
11 |
3 |
— |
26 |
|
Belgium |
0 |
9 |
7 |
1 |
— |
17 |
|
Switzerland |
3 |
0 |
5 |
1 |
— |
9 |
|
United Kingdom |
4 |
3 |
0 |
7 |
— |
14 |
|
Italy |
0 |
1 |
3 |
0 |
— |
4 |
|
USA |
0 |
0 |
3 |
1 |
— |
4 |
|
Luxembourg |
— |
— |
— |
2 |
3 |
5 |
|
Germany |
0 |
0 |
2 |
1 |
1 |
4 |
|
Netherlands |
0 |
2 |
0 |
0 |
— |
2 |
|
Spain |
0 |
1 |
1 |
0 |
— |
2a |
|
Israel |
0 |
0 |
0 |
1 |
— |
1 |
|
Australia |
0 |
0 |
0 |
1 |
— |
1 |
|
Total |
|
89 |
||||
|
aOriginal table mistakenly reported zero. Source: Gratz et al. (2000). Reprinted with permission from Bulletin of the World Health Organization, copyright 2000, World Health Organization. |
||||||
justify the practice by pointing to data that show that insects are transported by aircraft (Naumann and McLachlan 1999). Data on the numbers of insects transported by aircraft appear to differ considerably and to depend on the place of origin and the time when the assessments were conducted. For example, an average of 11.4 insect per aircraft in Australia was estimated for the 1970s, while an average of 0.05 insect, including 0.04 mosquito, per aircraft in the Philippines was reported for the same period. Furthermore, an average of 0.1 mosquito per inbound flight in the United States in 1963 was estimated, while an average of 0.18 mosquito per aircraft arriving in the United Kingdom from tropical countries in 1985 was reported. The high number in the Australian studies may have been the result of accumulation from a large number of flights or the result of stopping and refueling at overseas airports at night when bright lights, which attract insects, are used to service aircraft.
Disinsection Procedures
Regardless of the controversy surrounding disinsection, some countries, as stated above, continue to disinsect aircraft. Two publications (WHO 1985; WHO 1995) form the basis of current disinsection practices that are used, or required, by various countries. For passenger cabins, five disinsection procedures appear to be used and include “blocks-away” spraying, “top-of-descent” spraying, “on-arrival” spraying, residual treatment, and pre-embarkation spraying (Naumann and McLachlan 1999).4 All procedures with the exception of pre-embarkation spraying have been approved by the World Health Organization (WHO) (WHO 1995; Naumann and McLachlan 1999). The methods are discussed below.
Blocks-away, top-of-descent, and on-arrival spraying are similar in that the passengers are on board the aircraft while the spraying is conducted (Naumann and McLachlan 1999). The procedures differ in the time of spraying. Blocks-away spraying is conducted when the aircraft is loaded, doors are closed, the aircraft is prepared for departure, and the wooden blocks are removed from the front of the aircraft tires, allowing the aircraft to taxi to the runway for takeoff. Top-of-descent spraying is conducted before descent to the port of destination. On-arrival spraying is conducted by a quarantine officer who boards the aircraft at the port of destination and sprays the cabin while the passengers are on board. The passengers must remain on board for 5 min after treatment. Top-of-descent and on-arrival spraying may result in lower total exposure of passengers and crew than blocks-away spraying because the time spent on the aircraft after the pesticide has been sprayed is shorter. WHO (1995) commented that “Member States should limit any routine requirement for disinsection of aircraft cabins and flight decks with an aerosol, while passengers are onboard, to aircraft operations originating in, or operating via, territories that they consider to pose a threat to their public health, agriculture or environment.”
Residual treatment and pre-embarkation spraying are similar in that no passengers are on board while the spraying is conducted. Because passengers are not present, these procedures tend to be preferred by the airlines (WHO 1995). Residual treatment is conducted by trained operators who certify the aircraft for a period of 8 weeks (WHO 1995); high-use surfaces require touch-
up spraying during this period. Residual treatment of an aircraft has the potential to expose passengers who fly only domestically because aircraft that are treated can also be used on domestic routes. Pre-embarkation spraying, which is being investigated as a new procedure (WHO 1995), is conducted after catering of the aircraft, before passengers board the aircraft, and within 1 h of departure.
Composition of Pesticide Sprays
The pesticide sprays are composed of a pesticide (the active ingredient), a propellant, and possibly solvents. The most commonly used pesticides are d-phenothrin and permethrin (cis:trans ratio of 25:75), which belong to a class of pesticides known as pyrethroids, and are recommended by WHO for disinsection (WHO 1995). Permethrin is considered a residual pesticide and d-phenothrin a nonresidual pesticide. No pesticides are registered in the United States for use as disinsectants in occupied aircraft (Anonymous 1999).
The disinsection procedures use sprays that vary in composition (Naumann and McLachlan 1999; WHO 1995). For blocks-away, top-of-descent, and on-arrival spraying, the aerosol sprays typically contain 2% d-phenothrin and are applied at 1 g/s and aimed to disperse 10 g of formulation per 1,000 ft3. The preflight application often used in conjunction with the procedures noted is typically a 2% permethrin formulation. For residual treatment, a 2% aqueous emulsion of permethrin is typically applied at 10 mL/m2 and aimed to deliver permethrin at a rate of 0.2 g/m2; a higher application rate may be required for carpets. For pre-embarkation spraying, an aerosol spray containing both 2% d-phenothrin and 2% permethrin is typically used with application designed to disperse 10 g of formulation per 1,000 ft3. Both pesticides are used because the d-phenothrin kills insects present in the cabin at the time of spraying, and the permethrin kills insects that enter the cabin between spraying and departure. The permethrin also has a repellant effect that discourages insects from entering the cabin.
In addition to the active ingredients, a propellant is present in the aerosol sprays (WHO 1995). Two chlorofluorocarbons (CFC 11 and CFC 12) were recommended by WHO but are no longer recommended because of their O3-depleting potential. Although partially halogenated chlorofluorocarbons have a lower O3-depleting potential, they are considered to be only transitional substitutes. Hydrofluorocarbons are considered the ideal substitutes because they have minimal O3-depleting potential. However, several of them (e.g., HFC 32,
HFC 143a, and HFC 152a) are flammable and therefore are not considered suitable for use in aircraft. HFC 134a and HFC 227ea are nonflammable and might be recommended by WHO for use in disinsection of aircraft.
In addition to the active ingredients and propellant, solvents (e.g., petroleum distillates) might be added to enhance the solubility of the active ingredients (WHO 1995). However, the amount of solvent added is typically small (0.067%). A synergist (e.g., piperonyl butoxide) might also be added to the pyrethroid formulation to increase activity of the pesticide (WHO 1995).
Pesticide Exposure
No information on quantitative measures of exposures of passengers or crew to pesticides is available. However, passengers and crew clearly receive inhalation and dermal exposures if pesticides are sprayed while they are on board the aircraft. If pesticides are sprayed before a flight (blocks-away spraying) or during a flight (i.e., top-of-descent spraying), passengers may continue to receive dermal exposures after spraying because the aerosol spray settles onto interior surfaces, which serve as a reservoir for later dermal exposures. Oral exposures can occur as a result of hand-to-mouth activity.
Passengers and crew are also exposed to pesticides through residual or pre-embarkation spraying, but these exposures are primarily oral and dermal because the cabin is treated while the passengers and crew are not on board. Inhalation exposure would not be a primary route of exposure because d-phenothrin and permethrin have low volatility.
Wipe samples, taken by a flight attendant who appeared to use the proper equipment and procedure, showed concentrations of permethrin of 0.17–0.69 μg/cm2 (J.Murawski, AFA, personal communication, March 5, 2001). The wipe samples were obtained from four surfaces after a residual spray treatment several hours before boarding of a 747–400 aircraft. The values cited may be underestimates; no data were provided on the efficiency of an alcoholswipe method to capture such a highly lipophilic substance as permethrin.
CONTAMINANTS FROM AIRCRAFT SYSTEMS
Contaminants from the aircraft systems (e.g., engines) may enter the cabin in bleed air. The stages at which air is bled from the system depend on the aircraft and the flight segment. Bleed air can also be supplied by the APU,
which is a small turbine engine usually in the tail cone of the aircraft. The APU provides bleed air to the aircraft when the main engines are not operating, or when main-engine power demands are high during specific flight segments and main-engine bleed air cannot be spared. See Chapter 2 for further details on the bleed-air system.
Problems can arise when fluids used in the operation and maintenance of the aircraft enter the bleed-air system. However, few data have been collected on contaminants that might be present in engine bleed air under normal operating or upset conditions. This section addresses the potential for lubricating oils, hydraulic fluids, deicing fluids, and their pyrolyzed products to enter the aircraft cabin.
Over the years, newspaper reports have documented incidents in which smoke, fumes, or mists have entered a passenger cabin or cockpit (Norton 1998, Acohido 2000, Arlidge and Clark 2001). Many of the incidents have been attributed to the entry of engine lubricating oils or hydraulic fluids into the cabin through the bleed-air system. The oils or fluids enter the ECS as a result of equipment failures (e.g., leaking engine-oil seals). Many estimates have been provided of the frequency of such events ranging from one in 22,000 flights (Winder 2000) to one in 1,000 flights (Hood 2001). In testimony before an Australian Senate inquiry, Jean Christophe Balouet stated that 70 “major smoke/haze events” and 500 “severe fume events” have been estimated to occur each year worldwide (Parliament of the Commonwealth of Australia 2000). The committee was not able to verify any of the estimates.
Balouet further stated that “some aircraft types, especially BAe 146, MD 80, B 737, A 300, and a limited number of companies…have been the cause of over 90% of the world wide problems identified today, whereas they represent less than 3% of world flights” (Parliament of the Commonwealth of Australia 2000). A recent study using information from several years of operation for three airlines attempted to quantify the relative frequency of air-quality incidents for aircraft (C. van Netten, University of British Columbia, personal communication, November 13, 2001). Although the results cannot necessarily be extended to other airlines, they demonstrate that the frequency of incidents can vary considerably from one aircraft to another—3.88 per 1,000 flight cycles for the BAe 146, 1.29 per 1,000 flight cycles for the A320, 1.25 per 1,000 flight cycles for the B747, 1.04 per 1,000 flight cycles for the DC-10, 1.02 per 1,000 flight cycles for the MD-80, 0.63 per 1,000 flight cycles for the B767, and 0.09 per 1,000 flight cycles for the B737. See Box 3–4 for further discussion of problems with air quality on BAe 146 and MD-80 aircraft.
The airlines do not publicize the frequency of those events. However, that
|
BOX 3–4 BAe 146 and MD-80 Aircraft Some aircraft types have been associated with air-quality problems in which smoke or fumes enter the passenger cabin or cockpit. One such aircraft is the BAe 146, which was the focus of a recent investigation by the Australian Senate (Parliament of the Commonwealth of Australia 2000). Persistent problems with this aircraft have been noted since the early 1990s. Poor cabin air quality has been attributed to excessive oil-seal leaks in the main engines or the APU. These leaks allow lubricating oil to enter the bleed-air system, where high temperatures can volatilize or pyrolyze the oil and result in smoke in the aircraft cabin or cockpit. The frequency of the leaks in Australian BAe 146 aircraft has been reported as one in 66 flights in 1992 and one in 128 flights in 1997 (Winder 2000). The outcome of the Australian Senate investigation was the issuance of an airworthiness directive (AD) by the Australian Civil Aviation Safety Authority (CASA). The AD requires investigation and reporting of any suspected cabin air contamination with engine oil (Airworthiness Directive, March 31, 2001). Furthermore, defective components must be fixed before further flight or within 10 flight hours with the provision that the source of contamination is isolated from the passenger cabin and cockpit. Another aircraft that has been associated with poor cabin air quality is the MD-80. An investigation by the AFA found that 73% of air-quality incidents in one airline were attributed to the MD-80 (Witkowski 1999). FAA, in a notice of proposed rule-making for an AD, described the problems with the MD-80 aircraft (Fed. Regist 65(11), January 18, 2000). Smoke and odor have been reported in the passenger cabins of these aircraft. An investigation indicated that the problems result from the “failure of a hydraulic pipe in the aft fuselage accessory compartment.” Failure of the component leads to leaking of hydraulic fluids in the bilge area of the tailcone and the drainage and ingestion of the fluids into the air intake of the APU, which results in the smoke and odors that have been observed in the passenger cabin and cockpit of these aircraft. The AD became effective on September 12, 2000, and requires replacement of faulty hydraulic components and installation of drain tubes and diverter assemblies in the area of the APU inlet (14 CFR Part 39). |
leaks do occur is supported by information provided in the Boeing 737 maintenance manual, which describes how air can become contaminated and how to purge contaminants from the system (Boeing 1998). Specifically, the manual indicates that “smoke or fumes” may enter “the passenger cabin or the flight compartment through the air distribution system during flight.” The problems
are attributed to leaks of oil, fuel, hydraulic fluids, or deicing fluids from the engines or APU or ingestion of the fluids into the inlet of the engines or APU.
Because no published data are available on contaminants present in bleed air (or cabin air) during an air-quality incident (e.g., leakage of engine lubricating oil or hydraulic fluid into the cabin air-supply system), data on the composition of engine lubricating oils and hydraulic fluids are useful for determining potential exposures. Components of some engine lubricating oils and hydraulic fluids are summarized in Table 3–11.
As indicated in Table 3–11, engine lubricating oils contain cresyl phosphate isomers. Specifically, these oils typically contain 3% tricresyl phosphate (TCP) with the neurotoxic isomer tri-o-cresyl phosphate (TOCP) present at 0.1% (van Netten 2000). Although the Australian material safety data sheet (MSDS) for Mobil jet oil 291 indicates “no reportable ingredients,” the U.S. MSDS for this oil indicates the presence of TCP isomers. Common ingredients in hydraulic fluids are tributyl phosphate, butyl diphenyl phosphate, and dibutyl phenyl phosphate. Although it is not indicated in the MSDSs, hydraulic fluids may contain as much as 1% TOCP (van Netten 2000).
Independent laboratory analyses confirmed the presence of the TCP isomers in Mobil jet oil 254, Mobil jet oil II, and Mobil jet oil 291 and of tributyl phosphates in Skydrol 500B-4, Skydrol LD-4, and HyJet IV-A+ (van Netten 2000; van Netten and Leung 2001). The only difference that could be identified between Mobil jet oil II and Mobil jet oil 291 was the absence of N-phenyl-l-naphthalenamine in Mobil jet oil 291. These analyses also indicated that Hyjet IV-A+ contained TCP isomers, which were not reported in the MSDS for this product.
Although information on the composition of engine lubricating oils and hydraulic fluids is useful, it does not positively identify components that would be present in bleed air as a result of equipment failures. Laboratory experiments have been conducted to determine constituents that might be generated when engine lubricating oils and hydraulic fluids are exposed to high temperatures (i.e., 525 °C) like those in some aircraft engines (van Netten 2000, van Netten and Leung 2000, van Netten and Leung 2001). The results showed that Castrol 5000, Exxon 3280, Mobil jet oil II, Mobil jet oil 254, and Mobil jet oil 291 release TCP isomers that can be trapped in air at room temperature. These compounds appear to remain airborne and are probably associated with smoke particles. Many other compounds are also generated, including substantial amounts of CO. Hydrogen cyanide (HCN) was generated in very negligible amounts under the conditions of these experiments.
TABLE 3–11 Components of Some Engine Lubricating Oils and Hydraulic Fluids, as Reported in Material Safety Data Sheets
|
Type of Oil or Fluid |
Reported Components (wt%) |
CAS No. |
|
Engine lubricating oils |
||
|
Mobil jet oil 254 |
Tricresyl phosphate (1–5%) |
1330–78–5 |
|
Mobil jet oil II |
Tricresyl phosphate (1–5%) |
1330–78–5 |
|
|
N-Phenyl-l-naphthalenamine (1–5%)a |
90–30–2 |
|
Hydraulic fluids |
||
|
Skydrol 5 |
Triisobutyl phosphate |
126–71–6 |
|
(Solutia Inc.) |
Triphenyl phosphate |
115–86–6 |
|
|
Epoxy-modified alkyl ester |
|
|
Skydrol 500B-4 |
Tributyl phosphate |
126–73–8 |
|
(Solutia Inc.) |
Dibutyl phenyl phosphate |
2528–36–1 |
|
|
Butyl diphenyl phosphate |
2752–95–6 |
|
|
Epoxy-modified alkyl ester |
|
|
Skydrol LD-4 |
Tributyl phosphate |
126–73–8 |
|
(Solutia Inc.) |
Tributyl phosphate |
126–73–8 |
|
|
Dibutyl phenyl phosphate |
2528–36–1 |
|
|
Butyl diphenyl phosphate |
2752–95–6 |
|
|
2,6-Di-tert-butyl-p-cresol |
128–37–0 |
|
|
Epoxy modified alkyl esters |
|
|
HyJet IV-A+ |
Tributyl phosphate (79%) |
126–73–8 |
|
(Chevron) |
Cyclic aliphatic epoxide (<2.9%) |
3388–03–2 |
|
|
Additives (<21%) |
|
|
aContains 1 wt% phenyl-α-naphthylamine (PAN). |
||
The hydraulic fluids Skydrol 500B-4, Skydrol LD-4, and HyJet IV-A+ appeared to generate much less CO than the engine lubricating oils under identical conditions. Specifically, the oils produced CO at 56–141 ppm, and the hydraulic fluids at 2–18 ppm. Few compounds were detected in the air when the hydraulic fluids were heated. The constituents appeared to evaporate into the air before any pyrolysis took place.
The identification of CO as a degradation product when engine lubricating oils and hydraulic fluids are subjected to high temperatures indicates a potential acute hazard to passengers and crew.
Trimethylolpropane phosphate (TMPP) was not detected in any of the experiments. This compound has been shown to form from trimethylolpropane esters and TCP isomers at high temperatures (Wyman et al. 1993; Wright 1996).
Although the experiments indicate components that might be present during an air-quality incident, it is important to know whether a constituent could be present at a hazardous concentration. Simple calculations illustrate that only very small quantities of oils need to be pyrolyzed under conditions that occur in the bleed air system to exceed commonly accepted health standards. See Box 3–5 for a sample calculation. Therefore, possible contaminant concentrations (e.g., of TCP isomers) that might result from upset conditions should be evaluated.
The foregoing discussion focused on potential contaminants in bleed air during an air-quality incident. Another issue to address is the quality of bleed air during normal operating conditions. Nagda et al. (2001) conducted a study to investigate contaminants in bleed air under normal operating conditions. They obtained measurements on three wide-body Boeing 767s (two flights each), two standard-body Boeing 737s (one flight each), and two wide-body Boeing 747s (one flight each). Contaminants in bleed air appear to have been measured only on the Boeing 767. They measured CO2, CO, PM (PM2.5 and PM10), VOCs, aldehydes, ketones, and semivolatile organic compounds (SVOCs) in bleed air.
Average CO2 concentrations in the bleed air on the Boeing 767 during boarding, ascent, cruise, and descent were 680±173 ppm, 402±5 ppm, 340 ±13 ppm, and 359±4 ppm, respectively. Average CO2 concentrations in the cabin air on the Boeing 737 and Boeing 747 aircraft during the different flight segments were 1,091–1,547 ppm. On the Boeing 767, CO2 was measured in the cabin only for a 15-min period during cruise. PM and CO concentrations were extremely low. PM2.5 and PM10 were consistently below 10 μg/m3 in bleed air and cabin air after instrument calibration. CO measurements were typically below 1 ppm (see Table 3–12).
The authors noted that VOC concentrations were generally below those measured in buildings. Maximal concentrations of VOCs detected in bleed air are summarized in Table 3–13. For comparison, maximal concentrations in cabin air are also presented, where measured. The only VOCs detected in
|
BOX 3–5 How Much Does it Take? Consider an incident in which a leaky engine seal in an aircraft results in oil pyrolysis in bleed air. Assume that one of the major pyrolysis products is formaldehyde. How much oil must be pyrolyzed during a 15-min period for the concentration of formaldehyde in the aircraft cabin to reach its threshold limit value-ceiling (TLV-C)? We begin by calculating how much formaldehyde must be emitted during a 15-min period for its concentration in the cabin to reach its TLV-C. Under quasisteady-state conditions, the concentration of a contaminant, C, emitted into the cabin of an aircraft can be calculated with the following equation: C=(E/V)λv, where E is emission rate, V is volume of the cabin, and λv is ventilation rate. Rearranging yields the following equation: E=CVλv. The TLV-C for formaldehyde is 300 ppb or 370 μg/m3 (assuming 760 mm Hg and 25°C). Assume that the volume of the cabin is 150 m3 and the ventilation rate is 23 h−1. Substituting yields E=(370 μg/m3)(150 m3)(23 h−1)=1.3×106 μg h−1=1.3 g h−1. In a 15-min period (0.25 hr), this emission rate equates to 0.3 g of formaldehyde. Pyrolysis data on one of the oils typically used in aircraft indicate that at 370°C, 0.3 g of formaldehyde is produced for each gram of oil pyrolyzed (R.Fox, Honeywell, personal communication, April 4, 2001). Therefore, 1 g of this oil, pyrolyzed over a 15-min interval, would be sufficient to produce formaldehyde in the cabin that matched the TLV-C. It should be emphasized that this is a simplified calculation. It assumes that the temperature of the bleed air is 370°C. Such a temperature would typically occur only during takeoff and climbing. It neglects the loss of formaldehyde in the airstream, especially to moist surfaces; moist surfaces may be present during takeoff and climbing. It assumes that pyrolysis occurs at a constant rate, that all the oil (1 g) is pyrolyzed and enters the ECS, and that the air in the cabin is perfectly mixed. Nonetheless, the calculation illustrates the feasibility of achieving contaminant concentrations in cabin air, during upset conditions, that exceed occupational-exposure limits. This calculation cannot be used to estimate oil seal leakage rates required to generate this concentration of formaldehyde because only a small, unknown fraction of oil leaking from a seal is likely to get into the bleed air system. |
TABLE 3–12 Carbon Monoxide in Aircraft Air
|
|
Carbon Monoxide, ppm |
||||
|
B767 Bleed |
B767 Cabin |
B737 Cabin |
B747 Cabin |
||
|
Boarding |
Average |
0.5 |
|
1.0 |
0.6 |
|
SD |
0.1 |
|
0.3 |
0.1 |
|
|
Maximum |
0.9 |
|
1.7 |
0.8 |
|
|
Ascent |
Average |
0.1 |
|
0.6 |
0.3 |
|
SD |
0.1 |
|
0.1 |
0.1 |
|
|
Maximum |
0.3 |
|
0.8 |
0.5 |
|
|
Cruise |
Average |
0.1 |
0.1 |
0.5 |
0.1 |
|
SD |
0.1 |
0.01 |
0.1 |
0.1 |
|
|
Maximum |
0.3 |
0.1 |
0.8 |
0.3 |
|
|
Descent |
Average |
0.3 |
|
0.5 |
0.2 |
|
SD |
0.1 |
|
0.01 |
0.01 |
|
|
Maximum |
0.7 |
|
0.6 |
0.3 |
|
|
Source: Adapted from Nagda et al. (2001). |
|||||
cabin air that were not detected in bleed air were vinyl acetate, 4-methyl-2-pentanone, styrene, and 1,4-dichlorobenzene with maximal concentrations of 0.94–4.9 μg/m3. The only VOC detected at a high concentration was methylene chloride in cabin air. Because bleed-air concentrations were below 10 μg/m3, the methylene chloride in cabin air was attributed to a source in the cabin.
Aldehyde and ketone concentrations were low. The authors noted that aldehyde concentrations were “lower than those encountered in ground level buildings.” For example, maximal concentrations of formaldehyde were 2.1– 3.1 μg/m3 in bleed air and 6.4–13.0 μg/m3 in cabin air. Maximal concentrations of acetaldehyde were 26.4–30.7 μg/m3 in bleed air and 20.8–70.2 μg/m3 in cabin air. Acrolein, a strong respiratory irritant, was not detected in bleed or cabin air.
SVOC concentrations were extremely low. In fact, only naphthalene was detected in bleed or cabin air. Maximal concentrations were 2.0–3.4 μg/m3 in bleed air and 0–1.6 μg/m3 in cabin air. No TCP isomers, including TOCP, were detected, and no TMPP was detected.
On the basis of data obtained in this investigation, the authors rated the quality of the bleed air as high under normal operating conditions. However,
TABLE 3–13 Maximal Volatile Organic Compound Concentrations
|
|
Maximal Concentration, μg/m3 |
|||
|
Compound |
Ascent-Bleed Air |
Cruise-Bleed Air |
Cruise-Cabin Air |
Descent-Bleed Air |
|
Chloromethane |
2.4 |
0 |
0 |
2.1 |
|
Acetone |
15 |
12 |
130 |
31 |
|
Trichlorofluoromethane |
3.3 |
2.2 |
16 |
2.3 |
|
Methylene chloride |
5.4 |
9.5 |
2,900 |
10 |
|
Trichlorotrifluoroethane |
2 |
0 |
0 |
0.8 |
|
Carbon disulfide |
0 |
2.2 |
9.2 |
0 |
|
Methyl tert-butyl ether |
2.5 |
0 |
0 |
52 |
|
2-Butanone |
1.7 |
1.7 |
9.4 |
6.1 |
|
Benzene |
0 |
0 |
0 |
7.3 |
|
Trichloroethene |
0 |
0 |
0 |
3.4 |
|
Toluene |
9.3 |
10 |
21 |
45 |
|
Tetrachloroethene |
0 |
1.1 |
12 |
2 |
|
Ethylbenzene |
1.1 |
0 |
0 |
6.8 |
|
m- and p-Xylenes |
10 |
1.5 |
24 |
28 |
|
o-Xylene |
1.6 |
0 |
0 |
7.8 |
|
Source: Data from Nagda et al. (2001). |
||||
they noted that only 10 flights were monitored, and these flights “do not provide a statistically robust sample for representing the universe of all flights.”
Other fluids that can potentially enter an aircraft ventilation system are deicing fluids. Deicing fluids are applied to aircraft on the ground before takeoff under some weather conditions (e.g., freezing rain). Deicing fluids are usually aqueous solutions of ethylene glycol and/or propylene glycol containing proprietary additives (Ritter 2001). Because ethylene glycol is considerably more toxic than propylene glycol, the latter is being used more and more. The additives may include “a surfactant, polymer thickening agent, pH buffer, corrosion inhibitor, flame retardant, or dye.” Some concern has been raised about the toxicity of several additives (e.g., urea and tolyltriazoles).
Aircraft deicing fluids are typically sprayed onto the aircraft at 150–180°F under high pressure to optimize the removal of snow or ice (Ritter 2001).
When the aircraft is scheduled to take off immediately after application, a type I deicing fluid is used; these typically contain 90% glycol and 8% water. When the aircraft is scheduled to remain on the runway for more than 15 min, it is sprayed with the deicing fluid and then with an anti-icing agent that contains 65% glycol with a polymer thickener. No published information is available on the fate of these fluids if they are taken into the engine or APU, heated to a high temperature, and released into the aircraft ventilation system. The committee notes that application does not necessarily lead to exposure.
CONCLUSIONS
-
When an aircraft is on the ground, exposure to various pollutants (e.g., O3, CO, and PM) under normal operating conditions is determined primarily by the concentrations in the ground-level air. Contaminants result from ambient air pollution and airport sources (e.g., jet engine exhaust).
-
Exposure to ambient pollutants in the departure or arrival city’s air basin is limited because of the small fraction of time spent in takeoff and landing under normal flight conditions.
-
At cruise altitude, the primary ambient air pollutant of concern is O3.
-
O3 concentrations in the aircraft cabin are controlled by active O3 destruction by O3 converters, by passive O3 destruction that occurs on the interior surfaces of the aircraft, and by flight planning. Some aircraft use O3 converters to control O3, but no formal process exists for ensuring a high level of converter performance. There is concern because O3 converters are susceptible to poisoning by deposits of PM and some chemicals.
-
The committee found no data that provided confidence that the DOT O3 standard is regularly met. Nor does it appear to be possible to demonstrate compliance without a program that incorporates regular real-time O3 monitoring on aircraft.
-
Although data on exposure to other contaminants in aircraft cabins are extremely sparse, there are some data on CO2, CO, PM, VOCs, and SVOCs under normal operating conditions. CO2 concentrations appear to be below the FAA regulatory limit, although often higher than the ASHRAE ventilation standard; CO and PM concentrations appear to be lower than health-based standards for ambient air; and VOC and SVOC concentrations appear to be similar to those in other public-transport vehicles.
-
Pesticides are used on selected international flights, but the committee
-
could not locate any quantitative data on pesticide exposure of passengers or crew members.
-
Incidents have occurred in which engine lubricating oils, hydraulic fluids, or their pyrolyzed products have entered the ECS and contaminated the cabin air. However, no available exposure data identify the contaminants present in cabin air during an air-quality incident.
-
Controlled-pyrolysis experiments in the laboratory indicate that a large number of volatile and nonvolatile agents (e.g., TCP isomers) are released from engine lubricating oils and hydraulic fluids into the ambient air, where they can be measured at room temperature. However, the components released into the passenger cabin during air-quality incidents and their possible concentrations cannot be determined from the experiments.
RECOMMENDATIONS
-
A surveillance program should be developed and conducted to monitor cabin O3 on a representative number of flights and aircraft to determine compliance with existing federal aviation regulations for O3. The program should accurately establish temporal trends in O3 concentrations and determine the effectiveness of O3 control measures. Continuing monitoring should be conducted to ensure accurate characterization of O3 concentrations as new aircraft come on line, and aircraft equipment ages and is upgraded. Monitoring should be done with reliable and accurate instrumentation that is capable of making real-time O3 measurements.
-
FAA should develop procedures for ensuring O3 converter performance. At a minimum, FAA should conduct spot checks to verify that O3 converters are operating properly, according to the Federal Register (Vol. 45, No. 14, January 21, 1980).
-
Pesticide-exposure data should be collected to determine exposures of passengers and crew.
-
Wipe samples of aircraft cabin, cockpit, and ventilation ducts should be taken and analyzed after air-quality incidents to identify the contaminants to which passengers and crew were exposed. Filters from the aircraft ventilation system should also be analyzed to identify contaminants that have collected on them.
-
Because CO is most likely produced during air-quality incidents involving leaks of engine lubricating oils or hydraulic fluids in the ECS, it should be monitored in the ducts that introduce air into the cabin or cockpit.
-
More research should be conducted to determine products that might be generated when engine lubricating oils, hydraulic fluids, and deicing fluids are exposed to high temperatures that might be encountered in the ECS.
REFERENCES
Acohido, B. 2000. Alaska airlines jet flew repeatedly with fouled air. The Seattle Times. January 21, 2000.
Anonymous. 1999. The plane truth about disinsection. Environ. Health Perspect. 107(8):A397–398.
Arlidge, J., and T.Clark. 2001. British pilots overcome by fumes. The Observer. April 22, 2001.
ASHRAE (American Society of Heating Refrigerating and Air-Conditioning Engineers). 1999. ASHRAE Standard—Ventilation for Acceptable Indoor Air Quality. ANSI/ASHRAE 62–1999. American Society of Heating Refrigerating and Air-Conditioning Engineers, Atlanta, GA.
ASHRAE/CSS (American Society of Heating Refrigerating and Air-conditioning Engineers/Consolidated Safety Services). 1999. Relate Air Quality and Other Factors to Symptoms Reported by Passengers and Crew on Commercial Transport Category Aircraft. Final Report. ASHRAE Research Project 957-RP. Results of Cooperative Research Between the American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc., and Consolidated Services, Inc. February 1999.
Berlin, K., G.Johanson, and M.Leindberg. 1995. Hypersensitivity to 2-(2-butoxyethoxy) ethanol. Contact Dermatitis 32(1):54.
Bischof, W. 1973. O3 measurements in jet airliner cabin air. Water Air Soil Pollut. 2(1):3–14.
Black, D.R., R.A.Harley, S.V.Hering, and M.R.Stolzenburg. 2000. A new portable real-time O3 monitor. Environ. Sci. Technol. 34(14):3031–3040.
Boeing. 1998. Removing smoke or fumes from the air conditioning system-trouble shooting. Pp. 101 in Boeing 737–300/400/500 Maintenance Manual. 21–00–01. Boeing Company. Nov. 15, 1998.
Bolz, R.E., and G.L.Tuve, eds. 1973. Environmental and Bioengineering. Pp. 649–705 in CRC Handbook of Tables for Applied Engineering Science, 2nd Ed. Boca Raton : CRC.
Borglum, B., and A.M.Hansen. 1994. A Survey of Washing and Cleaning Agents. AMI Repport #44. Copenhagen, (as cited in Wolkoff et al. 1998).
Brabets, R.I., C.K.Hersh, and M.J.Klein. 1967. O3 measurement survey in commercial jet aircraft. J. Aircraft 4(1):59–64.
Budavari, S., M.J.O’Neil, and A.Smith, eds. 1989. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th Ed. Rah way, NJ: Merck.
Burge, P.S., and M.N.Richardson. 1994. Occupational asthma due to indirect exposure to lauryl dimethyl benzyl ammonium chloride used in a floor cleaner. Thorax 49(8):842–843.
Burge, P.S., A.Weiland, A.S.Robertson, and D.Weir. 1986. Occupational asthma due to unheated colophony. Br. J. Ind. Hyg. 43(8):559–580.
Burton, L.E., J.G.Girman, and S.E.Womble. 2000. Airborne particulate matter within 100 randomly selected office buildings in the United States (BASE). Pp. 157–162 in Healthy Buildings 2000: Exposure, Human Responses, and Building Investigations, Proceedings, Vol. 1, O.Seppänen, and J.Säteri, eds. Helsinki, Finland: SIY Indoor Air Information.
Childers, J.W., C.L.Witherspoon, L.B.Smith, and J.D.Pleil. 2000. Real-time and integrated measurement of potential human exposure to particle-bound polycyclic aromatic hydrocarbons (PAHs) from aircraft exhaust. Environ. Health Perspect. 108(9):853–862.
Clausen, P.A., and P.Wolkoff. 1997. Degradation products of tenax TA formed during sampling and thermal desorption analysis: indicators of reactive species indoors. Atmos. Environ. 31(5):715–725.
Clayton, C.A., R.L.Perritt, E.D.Pellizzari, K.W.Thomas, R.W.Whitmore, L.A.Wallace, H.Ozkaynak, and J.D.Spengler. 1993. Particle Total Exposure Assessment Methodology (PTEAM) study: distributions of aerosol and elemental concentrations in personal, indoor, and outdoor air samples in a southern California community. J. Expo. Anal. Environ. Epidemiol. 3(2):227–250.
Cometto-Muñiz, J.E. 2001. Physicochemical basis for odor and irritation potency of VOCs. Pp. 20.1–20.21 in Indoor Air Quality Handbook, J.D.Spengler, J.M.Samet, and J.F.McCarthy, eds. New York: McGraw-Hill.
DOT (Department of Transportation). 2001. Aircraft Disinsection Requirements, Office of the Assistant Secretary for Transportation Policy, Department of Transportation. [Online]. Available: http://ostpxweb.ost.dot.gov/policy/safety/disin.htm [August 22, 2001].
Devos, M., F.Patte, J.Rouault, P.Laffort, and J.J.Gmert. 1990. Standardized Human Olfactory Thresholds. New York: Oxford University Press.
Doelman, C., P.J.Borm, and A.Bast. 1990. Plasticisers and bronchial hyperreactivity. [letter]. Lancet 335(8691):725.
Dumyahn, T.S., J.D.Spengler, H.A.Burge, and M.Muilenburg. 2000. Comparison of the Environments of Transportation Vehicles: Results of Two Surveys. Pp. 13–25 in Air Quality and Comfort in Airliner Cabins, N.L.Nagda, ed. West Conshohocken, PA: American Society for Testing and Materials.
Eatough, D.J., F.M.Caka, J.Crawford, S.Braithwaite, L.D.Hansen, and E.A.Lewis. 1992. Environmental tobacco smoke in commercial aircraft. Atmos. Environ. Part A Gen. Top. 26(12):2211–2218.
EPA(U.S. Environmental Protection Agency). 2000. National Air Quality and Emissions Trends Report, 1998. EPA454/R-00–003. Office of Air Quality Planning and Standards, Emissions Monitoring and Analysis Division, Air Quality Trends Analysis Group, U.S. Environmental Protection Agency, Research Triangle Park, NC. March 2000.
Fang, L., G.Clausen, and P.O.Fanger. 1998a. Impact of temperature and humidity on the perception of indoor air quality. Indoor Air 8(2):80–90.
Fang, L., G.Clausen, and P.O.Fanger. 1998b. Impact of temperature and humidity on perception of indoor air quality during immediate and longer whole-body exposures. Indoor Air 8(4):276–284.
Finlayson-Pitts, B.J., and J.N.Pitts, Jr. 1999. Chemistry of the Upper and Lower Atmosphere. Orlando: Academic Press.
Glasius, M., M.Lahaniati, A.Calogirou, D.Di Bella, N.R.Jensen, J.Hjorth, D.Kotzias, and B.R.Larsen. 2000. Carboxylic acids in secondary aerosols from oxidation of cyclic monoterpenes by O3. Environ. Sci. Technol 34(6):1001–1010.
Gratz, N.G., R.Steffen, and W.Cocksedge. 2000. Why aircraft disinsection? Bull. World Health Org. 78(8):995–1004.
Griffin, R.J., D.R.Cocker III, R.C.Flagan, and J.H.Seinfeld. 1999. Organic aerosol formation from the oxidation of biogenic hydrocarbons. J. Geophys. Res. D Atmos. 104(3):3555–3567.
Grosjean, D., and M.W.M.Hisham. 1992. A passive sampler for atmospheric O3. J. Air Waste Manage. Assoc. 42(2): 169–173.
Hambraeus, A., S.Bengtsson, and G.Laurell. 1978. Bacterial contamination in a modern operating suite. 3. Importance of floor contamination as a source of airborne bacteria. J. Hyg. 80(2): 169–174.
Hocking, M.B. 1998. Indoor air quality: recommendations relevant to aircraft passenger cabins. Am. Ind. Hyg. Assoc. J. 59(7):446–454.
Hood, E. 2001. OPs cause of bad trips? Environ. Health Perspect. 109(4): A156.
Hunt, E.H., D.H.Reid, D.R.Space, and F.E.Tilton. 1995. Commercial Airliner Environmental Control System, Engineering Aspects of Cabin Air Quality. Presented at the Aerospace Medical Association Annual Meeting, Anaheim, CA.
Innocenti, A. 1978. Occupational asthma due to benzalkonium chloride. [in Italian]. Med. Lav. 69(6):713–715.
Jaakkola, J.J.K., L.Øie, P.Nafstad, G.Botten, K.C.Lødrup-Carlsen, S.O.Samuelsen, and P.Magnus. 1997. Interior surface materials and development of bronchial obstruction in young children. Epidemiology 8(suppl. 4):S54.
Jaakkola, J.J.K., P.K.Verkasalo, and N.Jaakkola. 2000. Plastic interior materials and respiratory health in young children. Pp. 139–141 in Healthy Buildings 2000: Exposure, Human Responses, and Building Investigations, Proceedings, Vol. 1, O.Seppänen, and J.Säteri, eds. Helsinki, Finland: SIY Indoor Air Information.
Jang, M., and R.M.Kamens. 1999. Newly characterized products and composition of secondary aerosols from the reaction of a-pinene and O3. Atmos. Environ. 33(3):459–474.
Jenkins, R.A., M.R.Guerin, and B.A.Tomkins. 2000a. Mainstream and sidestream cigarette smoke. Pp. 49–75 in The Chemistry of Environmental Tobacco Smoke: Composition and Measurement, 2nd Ed. Boca Raton, FL: Lewis.
Jenkins, R.A., M.R.Guerin, and B.A.Tomkins. 2000b. Field studies-oxides of nitrogen. Pp. 239–252 in The Chemistry of Environmental Tobacco Smoke: Composition and Measurement, 2nd Ed. Boca Raton, FL: Lewis.
Karch, S., M.F.Dellile, P.Guillet, and J.Mouchet. 2001. African malaria vectors in European aircraft. Lancet 357(9251):235.
Kildesø, J., and T.Schneider. 2001. Prevention with cleaning. Pp. 64.1–64.18 in Indoor Air Quality Handbook, J.D.Spengler, J.M.Samet, and J.F.McCarthy, eds. New York: McGraw-Hill.
Koutrakis, P., J.M.Wolfson, A.Bunyaviroch, S.E.Froehlich, K.Hirano, and J.D.Mulik. 1993. Measurement of ambient O3 using a nitrite coated filter. Anal. Chem. 65(3):209–214.
Kreiss, K., M.G.Gonzales, K.L.Comight, and A.R.Scheere. 1982. Respiratory irritation due to carpet shampoo: two outbreaks. Environ. Int. 8:337–342.
Lambert, J.L., J.V.Paukstells, and Y.C.Chiang. 1989. 3-methyl-2-benzothiazolinone acetone azine with 2-phenylphenol as a solid passive monitoring reagent for O3. Environ. Sci. Technol. 23(2):241–243.
Law, K.S., P.H.Plantevin, V.Thouret, A.Marenco, W.A.H.Asman, M.Lawrence, P.J. Crutzen, J.F.Muller, D.A.Hauglustaine, and M.Kanakidou. 2000. Comparison between global chemistry transport model results and Measurement of O3 and Water Vapor by Airbus In-Service Aircraft (MOZAIC) data. J. Geophys. Res. 105(1):1503–1525.
Li, T-H. .2001. Generation, Characterization, Aerosol Partitioning, and Indoor Measurements of Hydrogen Peroxide for Exposure and Toxicological Assessment. Ph.D. Dissertation. Graduate School-New Brunswick of Rutgers, The State University of New Jersey and The University of Medicine and Dentistry of New Jersey, New Brunswick, NJ.
Lioy, P.J., T.Wainman, J.Zhang, and S.Goldsmith. 1999. Typical household vacuum cleaners: the collection efficiency and emissions characteristics for fine particles. J. Air Waste Manage. Assoc. 49(2):200–206.
Long, C.M., H.H.Suh, and P.Koutrakis. 2000. Characterization of indoor particle sources using continuous mass and size monitors. J. Air Waste Manage. Assoc. 50(7):1236–1250.
Mallard, W.G., F.Westley, J.T.Herron, R.F.Hampson, and D.H.Frizzell. 1998. NIST Chemical Kinetics Database: Windows Version 2Q98, NIST Standard Reference Database 17. Gaithersburg, MD: U.S. Department of Commerce.
McCoach, J.S., A.S.Robertson, and P.S.Burge. 1999. Floor cleaning materials as a cause of occupational asthma. Pp. 459–464 in Indoor Air 99, Proceedings of the 8th International Conference on Indoor Air Quality and Climate, Edinburgh, Scotland, 8–13 Aug. 1999, Vol. 5, G.Raw, C.Aizlewood, and P.Warren, eds. London: Construction Research Communications.
Mendell, M.J., W.J.Fisk, M.Petersen, M.X.Dong, C.J.Hines, D.Faulkner, J.A. Deddens, A.M.Ruder, D.Sullivan, and M.F.Boeniger. 1999. Enhanced particle filtration in a non-problem office environment: summary findings from a double-blind crossover intervention study. Pp. 974–975 in Indoor Air 99, Proceedings of the 8th International Conference on Indoor Air Quality and Climate, Edinburgh, Scotland, 8–13 Aug. 1999, Vol. 4, G.Raw, C.Aizlewood, and P.Warren, eds. London: Construction Research Communications.
Mølhave, L. 2001. Sensory irritation in humans caused by volatile organic compounds
(VOCs) as indoor air pollutants: a summary of 12 exposure experiments. Pp. 25.1– 25.28 in Indoor Air Quality Handbook, J.D.Spengler, J.M.Samet, and J.F.McCarthy, eds. New York: McGraw-Hill.
Mølhave, L., and D.N.Neilsen. 1992. Interpretation and limitations of the concept “total volatile organic compounds” (TVOC) as an indicator of human response to exposures of volatile organic compounds (VOC) in indoor air. Indoor Air 2:65–77.
Monn, C., and M.Hangartner. 1990. Passive sampling for O3. J. Air Waste Manage. Assoc. 40(3):357–358.
Morrison, G.C. 1999. O3-Surface Interactions: Investigation of Mechanisms, Kinetics, Mass Transport, and Implications for Indoor Air Quality. Ph.D. Dissertation. Department of Civil and Environmental Engineering, University of California, Berkeley.
Morrison, G.C., and W.W.Nazaroff. 1999. Emissions of odorous oxidised compounds from carpet after O3 exposure. Pp. 664–669 in Indoor Air 99, Proceedings of the 8th International Conference on Indoor Air Quality and Climate, Edinburgh, Scotland, 8–13 Aug. 1999, Vol. 4, G.Raw, C.Aizlewood, and P.Warren, eds. London: Construction Research Communications.
Moss, M.T., and H.M.Segal. 1994. The emissions and dispersion modeling system (EDMS): its development and application at airports and airbases. J. Air Waste Manage Assoc. 44(6):787–790.
Murray, D.M., and D.E.Burmaster. 1995. Residential air exchange rates in the United States: empirical and estimated parametric distributions by season and climatic region. Risk Anal. 15(4):459–465.
Nagda, N.L., M.D.Fortmann, M.D.Koontz, S.R.Baker, and M.E.Ginevan. 1989. Airliner Cabin Environment: Contaminant Measurements, Health Risks, and Mitigation Options. DOT-P-15–89–5. NTIS/PB91–159384. Prepared by GEOMET Technologies, Germantown, MD, for the U.S. Department of Transportation, Washington DC.
Nagda, N.L., M.D.Koontz, A.G.Konheim, and S.K.Hammond. 1992. Measurement of cabin air quality aboard commercial airliners. Atmos. Environ. Part A Gen. Top. 26(12):2203–2210.
Nagda, N.L., H.E.Rector, Z.Li, and E.H.Hunt. 2001. Determine Aircraft Supply Air Contaminants in the Engine Bleed Air Supply System on Commercial Aircraft. ENERGEN Report AS20151. Prepared for American Society of Heating, Refrigerating, and Air-Conditioning Engineers, Atlanta, GA, by ENERGEN Consulting, Inc., Germantown, MD. March 2001.
Nastrom, G.D., J.D.Holdeman, and P.J.Perkins. 1980. Measurements of cabin and ambient O3 on B747 airplanes. J. Aircraft 17:246–249.
Naumann, I.D., and K.McLachlan. 1999. Aircraft Disinsection. Report commissioned by the Australian Quarantine and Inspection Service. June 1999.
Nazaroff, W.W., and G.R.Cass. 1986. Mathematical modeling of chemically reactive pollutants in indoor air. Environ. Sci. Technol. 20(9):924–934.
Nazaroff, W.W., A.J.Gadgil, and C.J.Weschler. 1993. Critique of the use of deposi
tion velocity in modeling indoor air quality. Pp. 81–104 in Modeling of Indoor Air Quality and Exposure, N.L.Nagda, ed. ASTM STP 1205. Philadelphia, PA: American Society for Testing and Materials.
Norbäck, D., and M.Torgen. 1989. A longitudinal study relating carpeting with sick building syndrome. Environ. Int. 15(1–6):129–135.
Norton, D. 1998. 7 treated after hydraulic-fluid leak on plane. The Seattle Times. February 21, 1998.
NRC (National Research Council). 1986a. The physicochemical nature of sidestream smoke and environmental tobacco smoke. Pp. 25–54 in Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects. Washington, DC: National Academy Press.
NRC (National Research Council). 1986b. The Airliner Cabin Environment: Air Quality and Safety. Washington, DC: National Academy Press.
NRC (National Research Council). 1992. Guidelines for Developing Spacecraft Maximum Allowable Concentrations for Space Station Contaminants. Washington, DC: National Academy Press.
Øie, L., L.G.Hersoug, and J.Ø.Madsen. 1997. Residential exposure to plasticizers and its possible role in the pathogenesis of asthma. Environ. Health Perspect. 105(9):972–978.
Otto, D., L.Molhave, G.Rose, H.K.Hudnell, and D.House. 1990. Neurobehavioral and sensory irritant effects of controlled exposure to a complex mixture of volatile organic compounds. Neurotoxicol. Teratol. 12(6):649–652.
Parliament of the Commonwealth of Australia. 2000. Air Safety and Cabin Air Quality in the BAe 146 Aircraft. Report by the Senate Rural and Regional Affairs and Transport References Committee, Parliament House, Canberra. October 2000.
Penner, J.E., D.H.Lister, D.J.Griggs, D.J.Dokken, and M.McFarland, eds. 1999. Aviation and the Global Atmosphere. Cambridge: Cambridge University Press.
Persily, A. 1989. Ventilation rates in office buildings. Pp. 128–136 in The Human Equation: Health and Comfort , Proceedings of the ASHRAE/SOEH Conference IAQ89 San Diego, CA, April 17–20, 1989. Atlanta, GA: American Society of Heating Refrigerating, and Air Conditioning Engineers.
Persoff, R., and M.Koketsu. 1978. Respiratory irritation and carpet shampoo. Pp. 347– 349 in Proceedings of the 5th International Conference of Medichem, San Francisco, CA, Sept. 5–10, 1977, C.Hine, and D.J.Kilian, eds.
Pierce, W.M., J.N.Janczewski, B.Roethlisberger, and M.G.Janczewski. 1999. Air quality on commercial aircraft. ASHRAE J. 41(9):26–34.
Pleil, J.D., L.B.Smith, and S.D.Zelnick. 2000. Personal exposure to JP-8 jet fuel vapors and exhaust at air force bases. Environ. Health Perspect. 108(3):183–192.
Reed, D., S.Glaser, and J.Kaldor. 1980. O3 toxicity symptoms among flight attendants. Am. J. Ind. Med. 1:43–54.
Reiss, R., P.B.Ryan, P.Koutrakis, and S.J.Tibbetts. 1995. O3 reactive chemistry on interior latex paint. Environ. Sci. Technol. 29(8):1906–1912.
Ritter, S. 2001. Aircraft deicers. Chem. Eng. News 79(1):30.
Robinson, P.A., R.V.Tauxe, W.G.Winkler, and M.E.Levy. 1983. Respiratory illness in conference participants following the use of a carpet shampoo. Infect. Control 4(3):158–160.
Rodriguez, J.A., and J.Hrbek. 1999. Interaction of sulfur with well-defined metal and oxide surfaces: unraveling the mysteries behind catalyst poisoning and desulfurization. Accounts of Chemical Research 32(9):719–728.
Roth, B., P.Herkenrath, H.J.Lehmann, H.D.Ohles, J.J.Hömig, G.Benz-Bohm, J. Kreuder, and A.Younossi-Hartenstein. 1988. Di-(2-ethylhexyl)-phthalate as plasticizer in PVC respiratory tubing systems: indications of hazardous effects on pulmonary function in mechanically ventilated, preterm infants. Eur. J. Pediatr. 147(1):41–46.
Roy, S.P., G.J.Raw, and C.Whitehead. 1993. Sick building syndrome: cleanliness is next to healthiness. Pp. 261–266 in Indoor Air 93, Proceedings of the 6th International Conference on Indoor Air Quality and Climate, Vol. 6, Seppänen, R. Ilmarinen, J.J.L.Jaakkola, E.Kukkonen, J.Sateri, and H.Vuorelma, eds. Helsinki: International Society of Indoor Air Quality and Climate (ISIAQ).
SAE International (Society of Automotive Engineers International). 2000. (R) O3 in High Altitude Aircraft. Aerospace Information Report. SAE AIR910 Rev. B. Society of Automotive Engineers.
Schmitt, H.-J. 1985. Irritation of the airways following the use of a carpet shampoo [in German]. Offentl. Gesundheitswes. 47(9):458.
Seinfeld, J.H., and S.N.Pandis. 1998a. Chemistry of the stratosphere. Pp. 163–233 in Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. New York: Wiley.
Seinfeld, J.H., and S.N.Pandis. 1998b. The atmosphere. Pp. 1–48 in Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. New York: Wiley.
Seinfeld, J.H., and S.N.Pandis. 1998c. Chemistry of the troposphere. Pp. 234–336 in Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. New York: Wiley.
Skov, P., O.Valbjorn, and B.V.Pedersen. 1989. Influence of personal characteristics, job-related factors, and psychosocial factors on the sick-building syndrome. Danish Indoor Climate Study Group. Scand. J. Work Environ. Health 15(4):286– 295.
Skyberg, K., K.R.Skulberg, K.Kruse, P.O.Huser, F.Levy, and P.Djupesland. 1999. Dust reduction relieves nasal congestion: a controlled intervention study on the effect of office cleaning, using acoustic rhinometry. Pp. 153–154 in Proceedings of the 8th International Conference on Indoor Air Quality and Climate, Edinburgh, Scotland, 8–13 Aug. 1999, Vol. 4, G.Raw, C.Aizlewood, and P.Warren, eds. London: Construction Research Communications.
Spengler, J., H.Burge, T.Dumyahn, M.Muilenberg, and D.Forester. 1997. Environmental Survey on Aircraft and Ground-Based Commercial Transportation Vehicles. Prepared by Department of Environmental Health, Harvard University School of Public Health, Boston, MA, for Commercial Airplane Group, The Boeing Company, Seattle, WA. May 31, 1997.
Sussell, A., and M.Singal. 1993. Alaska Airlines, Seattle, Washington. NIOSH Health Hazard Evaluation Report. HETA 90–226–2281. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH.
Taylor, A.E., and C.Hindson. 1982. Facial dermatitis from allyl phenoxy acetate in a dry carpet shampoo. Contact Dermatitis 8(1):70.
Ten Brinke, J., S.Selvin, A.T.Hodgson, W.J.Fisk, M.J.Mendell, C.P.Koshland, and J.M.Daisey. 1998. Development of new volative organic compound (VOC) exposure metrics and their relationship to “Sick Building Syndrome” symptoms. Indoor Air 8(3):140–152.
Tobias, H.J., and P.J.Ziemann. 2000. Thermal desorption mass spectrometric analysis of organic aerosol formed from reactions of 1-tetradecene and O3 in the presence of alcohols and carboxylic acids. Environ. Sci. Technol. 34(11):2105–2115.
Tobias, H.J., K.S.Docherty, D.E.Beving, and P.J.Ziemann. 2000. Effect of relative humidity on the chemical composition of secondary organic aerosol formed from reactions of 1-tetradecene and O3. Environ. Sci. Technol. 34(11):2116–2125.
van Netten, C. 2000. Analysis of two jet engine lubricating oils and a hydraulic fluid: their pyrolytic breakdown products and their implication on aircraft air quality. Pp. 61–75 in Air Quality and Comfort in Airliner Cabins, N.L.Nagda, ed. West Conshohocken, PA: American Society for Testing and Materials.
van Netten, C., and V.Leung. 2000. Comparison of the constituents of two jet engine lubricating oils and their volatile pyrolytic degradation products. Appl. Occup. Environ. Hyg. 15(3):277–283.
van Netten, C., and V.Leung. 2001. Hydraulic fluids and jet engine oil: pyrolysis and aircraft air quality. Arch. Environ. Health 56(2):181–186.
Virkkula, A., R. van Dingenen, F.Raes, and J.Hjorth. 1999. Hygroscopic properties of aerosol formed by oxidation of limonene, alpha-pinene and beta-pinene. J. Geophys. Res. 104(3):3569–3579.
Wainman, T., J.Zhang, C.J.Weschler, and P.J.Lioy. 2000. O3 and limonene in indoor air: a source of submicron particle exposure. Environ. Health Perspect. 108(12):1139–1145.
Wallace, L.A., C.J.Nelson, and G.Dunteman. 1991. Workplace Characteristics Associated with Health and Comfort Concerns in Three Office Buildings in Washington, DC. Atmospheric Research and Exposure Assessment Lab, U.S. Environmental Protection Agency, Research Triangle Park, NC. NTIS PB91–211342. 11pp.
Wargocki, P., J.Sundell, W.Bischof, G.Brundrett, P.O.Fanger, F.Gyntelberg, S.O. Hanssen, P.Harrison, A.Pickering, O.Seppänen, and P.Wouters. 2001. Ventilation and Health in Nonindustrial Indoor environments. Report from a European multidisciplinary scientific consensus meeting. Clima 2000/Napoli 2001 World Congress, Napoli, Italy, September 15–18, 2001.
Waters, M., T.Bloom, and B.Grajewski. 2001. Cabin Air Quality Exposure Assessment. National Institute for Occupational Safety and Health, Cincinnati, OH. Federal Aviation Administration Civil Aeromedical Institute. Presented to the
NRC Committee on Air Quality in Passenger Cabins of Commercial Aircraft, January 3, 2001. National Academy of Science, Washington, DC.
Weschler, C.J. 2000. O3 in indoor environments: concentration and chemistry. Indoor Air 10(2):269–288.
Weschler, C.J., and H.C.Shields. 1996. Production of the hydroxyl radical in indoor air. Environ. Sci. Technol. 30(11):3250–3258.
Weschler, C.J., and H.C.Shields. 1997a. Potential reactions among indoor pollutants. Atmos. Environ. 31(21):3487–3495..
Weschler, C.J., and H.C.Shields. 1997b. Measurements of the hydroxyl radical in a manipulated but realistic indoor environment. Environ. Sci. Technol. 31 (12):3719– 3722.
Weschler, C.J., and H.C.Shields. 1999. Indoor O3/terpene reactions as a source of indoor particles. Atmos. Environ. 33(15):2301–2312.
Weschler, C.J., and H.C.Shields. 2000. The influence of ventilation and reactions among indoor pollutants: modeling and experimental observations. Indoor Air 10(2):92–100.
WHO (World Health Organization). 1985. Recommendations on the disinsecting of aircraft. Weekly Epidemiological Record 60(7):45–47.
WHO (World Health Organization). 1995. Report of the Informal Consultation on Aircraft Disinsection, WHO/HQ, Geneva, 6–10 November 1995, International Programme on Chemical Safety. Geneva, Switzerland: World Health Organization.
Wilcox, R.W., G.D.Nastrom, and A.D.Belmont. 1977. Period variations of total ozone and its vertical distribution. J. Appl. Meteorol. 16(3):290–298.
Winder, C. 2000. Cabin air quality in the Bae 146: the Senate Inquiry report. Hazard Alert 6:1–4. (December 1, 2000).
Witkowski, C.J. 1999. Remarks on Airliner Air Quality. Presentation at ASHRAE Conference, Chicago, IL, January 24, 1999.
Wolkoff, P., P.A.Clausen, C.K.Wilkins, and G.D.Nielsen. 2000. Formation of strong airway irritants in terpene/O3 mixtures. Indoor Air 10(2):82–91.
Wolkoff, P., T.Schneider, J.Kildesø, R.Degerth, M.Jaroszewski, and H.Schunk. 1998. Risk in cleaning: chemical and physical exposure. Sci. Total Environ. 215(1–2): 135– 156.
Woodfolk, J.A., C.M.Luczynska, F. de Blay, M.D.Chapman, and T.A.Platts-Mills. 1993. The effect of vacuum cleaners on the concentration and particle size distribution of airborne cat allergen. J. Allergy Clin. Immunol. 91(4):829–837.
Wright, R.L. 1996. Formation of the neurotoxin TMPP from TMPE-Phosphate Formulations. Tribology Transactions 39(4):827–834.
Wyman, J., E.Pitzer, F.Williams, J.Rivera, A.Durkin, J.Gehringer, P.Servé, D. von Minden, and D. Macys. 1993. Evaluation of shipboard formation of a neurotoxicant (trimethylolpropane phosphate) from thermal decomposition of synthetic aircraft engine lubricant. Am. Ind. Hyg. Assoc. J. 54(10):584–592.
Wyon, D.P., K.W.Tham, B.Croxford, A.Young, and T.Oreszczyn. 2000. The effects on health and self-estimated productivity of two experimental interventions which
reduced airborne dust levels in office premises. Pp. 641–646 in Healthy Buildings 2000: Exposure, Human Responses and Building Investigations. Proceedings, Vol. 1., O.Seppänen, and J.Säteri, eds. Helsinki, Finland: SIY Indoor Air Information.
Yu, J., R.C.Flagan, and J.H.Seinfeld. 1998. Identification of products containing— COOH, -OH, and -C=O in atmospheric oxidation of hydrocarbons. Environ. Sci. Technol. 32(16):2357–2370.
Zhou, J., and S.Smith. 1997. Measurement of O3 concentrations in ambient air: using a badge-type passive monitor. J. Air Waste Manage. Assoc. 47(6):697–703.


























































