1
Introduction
Submarines have been used by the United States and other countries as weapons platforms for a century. Worldwide, there have been 102 known cases in which submarines have become disabled and have sunk in noncombat situations, leading to a loss of approximately 2,600 lives (Commander Wayne Horn, personal commun., U.S. Navy 2000). The most recent case, which occurred in August 2000, was the disablement and sinking of the Kursk, a Russian nuclear submarine, off of the northwestern coast of Russia in the Barents Sea. The submarine was taking part in a naval exercise. One hundred and eighteen crew members perished after a possible on-board explosion occurred (Washington Post, August 30, 2000). The last accident involving a U.S. submarine occurred in May 1968. The USS Scorpion sank with 99 men on board (Somers 1972). The cause of the sinking is unknown.
The most probable cause of a submarine sinking is flooding caused by an event that breaches the outer hull. The force required would have to be substantial. Potential causes include surface collision, grounding, external explosion, and catastrophic failure of a hull valve. It is likely that such an event also would start a fire within the submarine. The immediate concern for the crew is the release of toxic gases that are produced as the combustion products of on-board fires (U.S. Navy 1998). Human exposure to these gases can lead to adverse health effects, particularly respiratory and central nervous system effects, and even
death. Other concerns include the depletion of oxygen, largely from the fire, the accumulation of carbon dioxide, and the drop in temperature (U.S. Navy 1998).
Most accidents that lead to the disablement and sinking of submarines occur at a depth of less than 300 ft. Down to 600 ft, the crew can escape from the submarine, and down to 2,000 feet, the crew can be rescued (Brown 1999). To escape, a crew member enters the escape trunk and is subjected to pressure equalization (Bond et al. 1960). He then inflates a vest, takes a deep breath, and passes through the open escape hatch. Once outside the hatch, he starts releasing air from his lungs to avoid an air embolism and is carried rapidly to the surface by buoyancy from the vest. In the future, the U.S. Navy will use British submarine escape immersion suits (SEIS) instead of Steinke hoods or inflatable vests. The SEIS suits provide more protection to the crew and contain a one-man raft that can be deployed at the surface. Two crew members can escape together, and the process can be repeated every 15 min. Therefore, it would take approximately 13 hours for a crew of 100 to escape. Numerous risks are associated with escape, including nitrogen narcosis, barotrauma (a type of high-pressure injury to the ear drum, lungs, or bowel), arterial gas embolism, decompression sickness, hypothermia, and drowning (Benton et al. 1999; Parker et al. 2000). The United States and the United Kingdom have conducted submarine escape exercises from depths of 100–600 ft. Several subjects experienced decompression sickness and barotrauma (P.K.Weathersby, Ret., U.S. Navy, personal commun.). A comparison between the health effects associated with escaping from a disabled submarine and those associated with exposure to the eight gases is presented in Table 1–1.
It is difficult to quantify the risks associated with escape from a disabled submarine, however, it is known that attempting such an escape would be extremely dangerous. Because of the substantial risks associated with escape, the Navy’s policy is that, if conditions allow, the crew of a submerged disabled submarine should wait for rescue (U.S. Navy 1998), which can be accomplished by the use of a deep submergence rescue vehicle (DSRV) or a submarine rescue chamber (SRC). The DSRV is a mini submarine that can go to a depth of 2,000 ft. It is attached to the disabled submarine, and 24 crew members are taken at a time from the submarine to a surface ship or to another submarine. The Navy currently has one DSRV, which is kept in San Diego, California. Depending on where in the world a disabled submarine is located, it can take up to 10 d for the DSRV to be transported to a site for rescue. The SRC can be used to a depth of 850 ft. It is lowered to the disabled submarine from a surface ship and can transport 6 crew members at a time to the surface.
As stated above, an event that leads to the disablement and sinking of a submarine is likely to also cause on-board fires. The toxic gases produced as combustion products could include ammonia, carbon monoxide, hydrogen
TABLE 1–1 Health Effects Associated with Escape From a Disabled Submarine versus Exposure to Toxic Gases
|
Health Effects Associated with Escapea |
Health Effects Associated with Exposure to the Gases Below the Recommended SEAL 2 Values |
|
• Arterial gas embolism • Barotrauma • Decompression sickness • Drowning • Hypothermia • Nitrogen narcosis |
• Moderate eye irritation and lacrimation from exposure to ammonia, chlorine, hydrogen chloride, hydrogen sulfide, nitrogen dioxide, and sulfur dioxide. • Moderate respiratory effects from exposure to ammonia, chlorine, hydrogen chloride, hydrogen sulfide, nitrogen dioxide, and sulfur dioxide. - Nasal irritation/lesions - Throat irritation - Chest irritation - Dyspnea - Transient pulmonary changes • Moderate central nervous system effects from exposure to carbon monoxide an hydrogen cyanide. - Headache - Abnormal vision - Decreased manual dexterity - Difficulty in concentrating - Syncope - Nausea and vomiting |
|
aMost of these health effects are likely to be fatal to a significant number of crew members during an escape from a disabled submarine, particularly if the escape is conducted from a depth greater than 300 feet. |
|
chloride, hydrogen cyanide, hydrogen sulfide, nitrogen dioxide, and sulfur dioxide. Chlorine gas also could be produced from the contact of saltwater with the submarine’s batteries. To protect crew members, scientists at the Navy Health Research Center’s Toxicology Detachment (NHRC/TD) have proposed preliminary exposure guidance levels, called submarine escape action levels (SEALs), for each of those gases.
Under current Navy policy, when a submarine fire occurs, the crew is instructed to put on emergency air breathing devices (EABs) to prevent smoke inhalation and toxic gas exposure. When a large number of crew members use EABs, expired air increases the pressure inside the submarine, which can increase the chance of decompression sickness. Minimizing the use of EABs to prevent
an increase in air pressure inside the submarine is one objective of establishing SEALs; the crew would use EABs only when SEAL 2 is reached or exceeded. The SEALs were also established to protect crew members from short-term adverse health effects—particularly to the respiratory and central nervous system—that could reduce their chances of survival during and after an escape or rescue attempt.
STATEMENT OF TASK
To protect crew members on disabled submarines from adverse health effects caused by exposure to eight toxic gases, the Chief of the Bureau of Medicine and Surgery, U.S. Navy, requested that the National Research Council (NRC) review the available toxicity data on eight gases—ammonia, carbon monoxide, chlorine, hydrogen chloride, hydrogen cyanide, hydrogen sulfide, nitrogen dioxide, and sulfur dioxide—and evaluate the scientific validity of the Navy’s proposed SEALs. NRC assigned the project to the Committee on Toxicology, and assembled the Subcommittee on Submarine Escape Action Levels. The specific task to the subcommittee was to review the toxicologic, epidemiologic, and related data to assess the validity of the Navy’s proposed SEALs. The subcommittee also was to consider the implications of exposures at hyperbaric conditions and the potential interactions for atmospheric components. Deficiencies in the database relevant to setting SEALs for the gases were to be identified and, where appropriate, recommendations for research were to be made.
DEFINITIONS OF SEALS
Two SEALs are proposed for each of the eight gases that maybe generated on a disabled submarine as a result of fires or contact of saltwater with the submarine’s batteries. SEAL 1 is defined as the maximum concentration of a gas in a disabled submarine below which healthy submariners can be exposed for up to 10 d without experiencing irreversible health effects. SEAL 2 is defined as the maximum concentration of a gas in a disabled submarine below which healthy submariners can be exposed for up to 24 h without experiencing irreversible health effects. Exposures at SEAL 1 and SEAL 2 might produce moderate, reversible effects, such as irritation of the skin, eyes, and respiratory tract, but they will not impair the functions of the respiratory system and central nervous system to the extent that impair the ability of submariners in a disabled submarine to escape or be rescued or perform specific tasks, such as shutting off a valve and using a fire extinguisher.
SEALs do not represent hard lines between safe and unsafe concentrations. If a SEAL is exceeded, some people should expect to be adversely affected.
The SEALs are based solely on scientific data relevant to health effects. Some surviving crew members in a disabled submarine are expected to perform light-to-moderate physical work and that is considered in the derivation of SEALs. It is inappropriate to use SEALs for routine exposures in a normally operating submarine.
The Navy will use the subcommittee’s SEALs as one of many parameters in its Submarine Escape and Rescue Expert System model. That model takes into account several additional parameters, such as geographical position and depth of the submarine, number and medical condition of the crew members, the ability to communicate with search and rescue forces, and compartment temperature, and is used by the senior officer to assist in making a decision on whether to escape from the disabled submarine.
The decision to escape from the submarine is a military-management decision that involves many considerations, which are beyond the charge and expertise of the subcommittee. SEALs are not standards or judgments of acceptable risk and must not be so construed. They are the subcommittee’s best judgment based on available evidence of exposure concentrations at which submariners can continue to function in an emergency situation in an environment of a disabled submarine and be unlikely to suffer irreversible effects. Like all reports of the National Research Council, this report contains only advisory information and recommendations.
THE ON-BOARD POPULATION
The U.S. Navy submariner population currently consists of an all-male, generally healthy group (personal communication, Commander Wayne Horn, U.S. Navy 2000). The average age of enlisted men is 26, and the average for officers is 31. The men are screened before assignment to a submarine for physical fitness and chronic health problems (e.g., neurologic, cardiovascular, respiratory).
Since 1938, asthma has been a disqualifying condition for submarine duty. However, a recent study reported that there is a 0.16% annual-period prevalence in the active duty enlisted Atlantic Fleet Submarine Force (Sims et al. 1999). Because asthma can develop in people during their 20s and 30s, it is possible that the condition can be diagnosed in some individuals after they are assigned to submarine duty. In most cases, submarine crew members who do have asthma exhibit only mild symptoms. It is not known whether the submarine atmosphere poses an occupational asthma risk Crew members with asthma are likely to be
more susceptible to toxic gases found in disabled submarines than would those without the condition.
THE SUBMARINE ATMOSPHERE
Today’s nuclear submarines can stay submerged for up to 90 d, although a typical patrol is approximately 60 d (Davies 1973). One reason for long periods of submergence is that the nuclear core requires no air to generate power. Another reason is that atmosphere control systems renew the air for respiration. The systems produce oxygen by electrolysis of seawater; remove carbon dioxide by chemical scrubbing; remove carbon monoxide and hydrogen by catalytic oxidation; and remove dusts, aerosols, and some toxic contaminants by active and passive filters and by electrostatic precipitation (Davies 1973). Because the concentration of oxygen in a submarine is 17–20%, the risk of fire is somewhat lower than at the surface. Air at sea level is 20.95% oxygen. Submarine air is 0.3– 0.5% carbon dioxide, compared with 0.033% in ambient air at sea level.
Submarines are equipped with mass spectrometers and nondispersive infra-red spectrophotometers that, under normal operating conditions, continuously monitor the atmosphere for compounds such as oxygen, hydrogen, carbon monoxide, carbon dioxide, water vapor, and three fluorocarbons (NRC 1988). Submarines are also equipped with colorimetric detection tubes (Dräeger tubes). Submarine atmospheres also contain trace concentrations of many volatile organic compounds, including long-chain aliphatic hydrocarbons, aromatic compounds, and halocarbons (Knight et al. 1989). It should be noted that submariners live for periods of up to several months in a totally enclosed and isolated environment beneath the sea. Any exposure to contaminants occurs 24 h/d for up to 90 d; the crew has no respite or periods of recovery away from these conditions as do workers in traditional occupational settings. Chronic exposure to these trace contaminants is not believed to be toxic to submarine crews (Davies 1973). Detailed listings of the major atmospheric contaminants found in nuclear submarines and their sources can be found in Davies (1973) and NRC (1988).
In the event of a submarine fire, crew members are likely to be exposed to higher than normal concentrations of ammonia, carbon monoxide, hydrogen chloride, hydrogen sulfide, nitrogen dioxide, and sulfur dioxide, and possibly to hydrogen cyanide gas. Exposure to chlorine gas can occur if saltwater is introduced into the battery compartment. In a disabled submarine, it is probable that the spectrophotometers used to monitor atmospheric contaminants no longer function because of power loss. Additionally, if power is lost, air would no longer circulate through the scrubbers; the air would become stagnant, leading to increased concentrations of contaminants. To monitor contaminants, the
crew is instructed to use Dräeger tubes, which can indicate a value up to 30% lower or higher than the actual gas concentration.
If a submarine becomes disabled, atmospheric pressure could rise due to increased flooding and any use of EABs. The increased pressure can lead to decompression sickness in crew members and reduce the likelihood of a successful rescue (Shake et al. 1995; Eckenhoff 1984). Development of decompression sickness symptoms, such as the bends and air embolisms, is a major risk associated with escape from a disabled submarine. The higher the internal pressure in the disabled submarine and the greater the escape depth, the greater is the risk of developing decompression sickness. Figure 1–1 illustrates the relationship between the internal pressure of a disabled submarine, its depth, and the percentage of crew members who are likely to suffer from decompression sickness.
THE NAVY’S INSTRUCTIONS FOR THE MANAGEMENT OF TOXIC GASES
The Navy’s instructions for the management of toxic gases, including the use of SEAL 1 and SEAL 2, are shown in Box 1–1. The instructions use a cumulative exposure index (CEI) approach, which considers the combined exposure to the irritant gases—ammonia, chlorine, hydrogen chloride, sulfur dioxide, and nitrogen dioxide—to result in additive and not synergistic effects. Thus, when mixtures of these gases are present, the CEI results in effectively lowering the SEAL 1 and SEAL 2 for each gas. The subcommittee believes the Navy’s approach of assuming that combined exposure to several gases resulting in additive and not synergistic effects is appropriate, and this approach is likely to protect the health of the crew. The approach is consistent with those recommended in other NRC reports (NRC 1992, 1994).
The Navy’s instructions in Box 1–1 allow for individual crew members to wear EABs if symptoms are severe. Thus, susceptible crew members can be protected without as much danger of increasing the pressure in the submarine as would happen if the entire crew were to wear EABs. Crew members wearing EABs are instructed to remove them each hour, as concentrations of some gases are likely to decrease overtime because of adsorption of some gases to submarine surfaces or because of the solubility of some gases in water.
THE SUBCOMMITTEE’S APPROACH TO ITS CHARGE
The subcommittee evaluated human data from experimental, occupational, and epidemiologic studies; data from accident reports; and experimental-animal
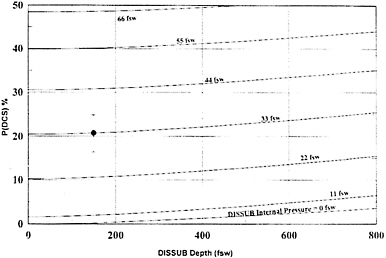
FIGURE 1–1 Relationship between the depth of the disabled submarine, the internal pressure of the submarine, and the percentage of the crew members likely to suffer from decompression sickness. Abbreviations: DCS, decompression sickness; DISSUB, disabled submarine; fsw, feet seawater. Source: Parker et al. 2000. Reprinted with permission from Aviation Space and Environmental Medicine, copyright 2000, Aerospace Medical Association, Alexandria, Virginia.
data (single and repeated exposures). The evaluations focused primarily on high-concentration inhalation exposure studies that measured respiratory and central nervous system effects. In general, the subcommittee’s approach was to recommend SEALs based directly on human data to avoid the need for incorporating uncertainty factors commonly used in the derivation of exposure guidance levels from animal data. Minor health effects (e.g., respiratory tract and eye irritation) are excluded from consideration as long as they do not become intolerable, cause irreversible effects, or impair a crew’s ability to escape.
The subcommittee believes that its recommended SEALs might produce health effects such as moderate irritation of respiratory tract, eyes, skin, or other moderate reversible effects, but would not produce any irreversible health effects in the submariners. The subcommittee did not incorporate an uncertainty factor for hypersusceptible individuals, including asthmatics, because as discussed above, the submariner population is healthy and asthma is a disqualifying condition for submarine duty. However, it is possible that some crew members may be hypersusceptible to the effects of the irritant gases or become mildly asth-
matic during the course of their employment as a submariner, and it might be necessary for those individuals to wear EABs at concentrations below the SEAL 2. Because only a small number of crew members would be expected to use EABs in that circumstance, the expired air should not significantly increase the air pressure inside the submarine.
Crew members on a disabled submarine would not be expected to be engaged in heavy physical activity (Captain K.Still, U.S. Navy, personal commun., 2001). Some crew members would need to do light work, such as the use of a fire extinguisher. However, the majority of crew members on a disabled submarine would be asked to lay down in their bunks and keep their eyes closed, which would serve to conserve oxygen, reduce carbon dioxide production, reduce the amount of toxic gases that the submariners would inhale, and reduce eye irritation.
The subcommittee believes that for the irritant gases (i.e., ammonia, chlorine, hydrogen chloride, hydrogen sulfide, nitrogen dioxide, and sulfur dioxide), the exposure concentration of the gases is more important than the exposure duration for determining toxicity, particularly for durations up to 24 h. Additionally, for several of the irritant gases reviewed in this report, an acclimation phenomenon has been well established.
Particulate matter, with significant toxicity, could be generated from onboard fires. The subcommittee believes it is possible that high-particle concentrations may interact with some of the gases and increase their toxicity. However, the subcommittee did not find data on such interactions and, therefore, was not able to consider them in recommending the SEALs.
In a disabled submarine, temperatures will likely be colder and atmospheric pressures higher than the normal atmospheric conditions used for studies from which SEALs were developed. The subcommittee emphasizes that its recommendations for SEALs are for an atmospheric pressure of 1 and a temperature of 25°C. Corrections for altered temperature and pressure will need to be made. If the pressure increases in the disabled submarine, the SEAL values should be reduced in inverse proportion to the pressure increase. The subcommittee did not find information on the effects of hyperbaric conditions on Dräeger-tube measurements. Values obtained for the gases using Dräeger tubes in a disabled submarine might need to be corrected to an atmospheric pressure of 1 and 25°C.
The Navy should be aware that the altered atmospheric conditions on a disabled submarine would affect the toxicity of the gases. For example, cold temperatures will cause crew members to shiver, which will increase the minute volume of ventilation due to an increase in metabolic rate. Lower air temperature may also result in the crew breathing unconditioned air, which is a risk factor for lower-airway disease and airway hyperactivity.
|
BOX 1–1 The Management of Toxic Gases on a Disabled Submarine
|
Carbon Monoxide (CO) Hydrogen Cyanide (HCN) Hydrogen Chloride (HCl)* Sulfur Dioxide (SO2)* Chlorine (Cl2)* Oxides of Nitrogen (NOx)* Ammonia (NH3)*
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
The subcommittee recognizes that the senior officer aboard a disabled submarine will have to consider many factors when deciding whether the crew should try to escape or await rescue. Such factors include the depth and condition of the submarine; the proximity of a rescue vehicle; the number and physical condition of survivors; the temperature of the sea water; the ambient pressure inside the submarine; the presence of airborne particles; and the concentrations of oxygen, carbon dioxide, and toxic gases. Factors specific to the toxic gases include which gases are present and how they are distributed throughout the submarine; whether the gases can be metabolized, absorbed, or neutralized by the crew; and whether the crew can acclimate to the gases at the concentrations present. Because of the lack of data on the effects of these parameters on toxicity of the gases, it is not possible to recommend SEALs for each gas for every potential scenario; therefore, the subcommittee did not consider factors such as those described above in recommending the SEALs. The senior officer on a disabled submarine should be aware of this limitation of the SEALs.
COMPARISONS BETWEEN SEALS AND EXISTING EXPOSURE GUIDANCE LEVELS
In chapters 2–9, the subcommittee presents existing exposure guidance levels for each gas, which have been recommended by various regulatory agencies and other organizations. Those levels include the NRC’s emergency exposure guidance levels (EEGLs), continuous exposure guidance levels (CEGLs), spacecraft maximum allowable concentrations (SMACs), acute exposure guidance levels
(AEGLs), the American Industrial Health Association’s emergency response planning guidelines (ERPGs), the American Conference of Governmental Industrial Hygienists’ Threshold Limit Values (TLVs), and the National Institute for Occupational Safety and Health’s immediately dangerous to life and health values (IDLHs). The AEGLs, ERPG, TLV, and IDLH values are developed for the general public or workers and take into account susceptible subpopulations, such as asthmatics and children. As described above, SEALs are developed specifically for submariners, who are healthy male adults with no history of asthma or other chronic medical conditions. Therefore, the subcommittee does not believe that it is appropriate to compare the SEALs to the AEGLs, ERPGs, TLVs, and IDLH values. Like the SEALs, the SMACs were developed for a healthy subpopulation, in this case astronauts. However, SMACs allow only mild irritation of the eyes, skin, and respiratory tract or mild central nervous system effects, whereas SEALs allow moderate reversible health effects. Additionally, SMACs take into account the changes in the astronauts’ physiological systems that they will experience from working under microgravity conditions. Therefore, EEGLs are the most relevant guidance levels to compare to the SEALs. EEGLs were developed for emergency situations involving healthy military personnel. An important difference between the EEGLs and the SEALs is that EEGLs allow mild, reversible health effects, whereas SEALs allow moderate, reversible health effects. That is, SEALs allow effects that are somewhat more intense or potent than those for the EEGLs. Therefore, the SEALs are higher than the corresponding EEGLs.
ORGANIZATION OF THE REPORT
This report has nine chapters in addition to this introduction. Chapters 2 to 9 contain the subcommittee’s reviews of the available toxicologic, epidemiologic, and other data on ammonia, carbon monoxide, chlorine, hydrogen chloride, hydrogen cyanide, hydrogen sulfide, nitrogen dioxide, and sulfur dioxide, respectively, its evaluations of the Navy’s proposed SEALs for each of the gases, and research needs for each of the gases. Chapter 10 contains the subcommittee’s conclusions and recommendations.
REFERENCES
Benton, P.J., T.J.R.Francis, and R.J.Pethybridge. 1999. Spirometric indices and the risk of pulmonary barotrauma in submarine escape training. Undersea and Hyperbaric Medicine. 26(4):213–217.
Bond, G.F., R.D.Workman, and W.F.Mazzone. 1960. Deep Submarine Escape. Report No. 346. Vol. XIX, No. 21. New London, CT: U.S. Naval Medical Research Laboratory.
Brown, D.C. 1999. Operational medicine. Submarine escape and rescue in today’s Royal Navy. J.R.Nav. Med. Serv. 85(3):145–149.
Davies, D.M. 1973. Sixty days in a submarine: The pathophysiological and metabolic cost. J.R.Coll. Physicians. Lond. 7(2):132–144.
Eckenhoff, R.G. 1984. Pressurized Submarine Rescue. NSMRL Report 1021. NTIS/AD-A143 348/1. Groton, CT: Naval Submarine Medical Research Laboratory.
Knight, D.R., D.V.Tappan, J.S.Bowman, H.J.O’Neill, and S.M.Gordon. 1989. Submarine atmospheres. Toxicol. Lett. 49(2–3):243–251.
NRC (National Research Council). 1988. Submarine Air Quality. Washington, DC: National Academy Press.
NRC (National Research Council). 1992. Guidelines for Developing Spacecraft Maximum Allowable Concentrations for Space Station Contaminants. Washington, DC: National Academy Press.
NRC (National Research Council). 1994. Science and Judgment in Risk Assessment. Washington, DC: National Academy Press.
Parker, E.C., R.Ball, P.M.Tibbles, and P.K.Weathersby. 2000. Escape from a disabled submarine: Decompression sickness risk estimation. Aviat. Space Environ. Med. 71(2):109–114.
Shake, C.L., P.K.Weathersby, B.G.Caras, and J.Parker. 1995. Helium-Nitrogen-Oxygen: Isobaric Shift and Saturation Decompression. NSMRL Report 1196. NTIS/ AD-A292 639/2. Groton, CT: Naval Submarine Medical Research Laboratory.
Sims, J.R., P.M.Tibbles, and R.P.Jackman. 1999. A descriptive analysis of asthma in the U.S. Navy Submarine Force. Aviat. Space Environ. Med. 70(12):1214–1218.
Somers, C.L. 1972. Submarine disasters in peacetime, 1900–1971. U.S. Naval Institute Proceedings, Naval Review 1972:319–329.
U.S. Navy. 1998. Memorandum from N.A.Carlson, Acting Commanding Officer, Naval Submarine Medical Research Laboratory to Officer in Charge, Naval Medical Research Institute Toxicity Detachment. Subject: The Management of Toxic Gases in a Disabled Submarine. March 2, 1998.
US. Navy. 2000. SSN688 Class Guard Book, Disabled Submarine Survival Guide, Forward Escape Trunk, Naval Sea System Command Technical Pub. S9594-APSAR-010. October 30, 2000.
Washington Post. 2000. US. theory On-board blast sank sub; analysts discount possibility of negligent Russian crew or maintenance flaws. A12, August 30, 2000.















