3
Carbon Monoxide
This chapter reviews physical and chemical properties and toxicokinetic, toxicologic, and epidemiologic data on carbon monoxide. The Subcommittee on Submarine Escape Action Levels used this information to assess the health risk to Navy personnel aboard a disabled submarine from exposure to carbon monoxide and to evaluate the Navy’s proposed submarine escape action levels (SEALs), proposed to avert serious health effects and substantial degradation in crew performance from short-term exposures (up to 10 d). The subcommittee also identifies data gaps and recommends research relevant for determining the health risk attributable to carbon monoxide exposure.
BACKGROUND INFORMATION
Carbon monoxide is a colorless, odorless, tasteless gas (Budavari 1989). It is very flammable and burns in air with a bright blue flame. It is highly poisonous to humans. The chemical and physical properties of carbon monoxide are summarized in Table 3–1.
Trace quantities of carbon monoxide in the environment are considered normal (WHO 1999). Plants can both metabolize and produce this gas. Carbon monoxide is also produced by incomplete combustion of carbon-containing materials (ACGIH 1996), and automobile exhaust is a major source of carbon
TABLE 3–1 Summary of Physical and Chemical Properties for Carbon Monoxide
|
Characteristic |
Value |
|
Formula |
CO |
|
CAS number |
630–08–0 |
|
Molecular weight |
28.01 |
|
Boiling point |
–191.5°C |
|
Melting point |
–205°C |
|
Vapor density |
0.968 |
|
Conversion factors at 25°C, 1 atm |
1 ppm=1.14 mg/m3 1 mg/m3=0.87 ppm |
|
Abbreviations: atm, atmosphere; CAS, Chemical Abstract Service; mg/m3, milligrams per cubic meter; ppm, parts per million. Source: Budavari (1989); Lide (1991); NRC (1994). |
|
monoxide in the environment (NRC 1994). Another common environmental source is cigarette smoke. Indoor sources include gas stoves, furnaces, and fires. Carbon monoxide is produced inside the body by hemoglobin metabolism (NRC 1994).
TOXICOKINETIC CONSIDERATIONS
Carbon monoxide is absorbed through the lungs at an approximate rate of 25.8±8 mL/min-mm Hg (Jones et al. 1982). It is then absorbed into the bloodstream from the lungs. There is competitive binding between carbon monoxide and oxygen to hemoglobin in the red blood cell, forming carboxyhemoglobin (COHb) and oxyhemoglobin, respectively (WHO 1999). The rate of formation of COHb can be predicted by a model described by Coburn et al. (1965). The affinity of hemoglobin for carbon monoxide is approximately 200–250 times its affinity for oxygen (NRC 1985). The amount of COHb depends mainly on the concentration and duration of carbon monoxide exposure, and the barometric pressure. To a lesser extent, it is also dependent on minute volume, blood volume in lung capillaries, body temperature, rate of endogenous carbon monoxide production, average partial pressure of oxygen in the lung capillaries, and the exact ratio of the affinity of blood for carbon monoxide and oxygen. Figure 3–1 shows the relationship of carbon monoxide concentration in ambient air and the COHb concentration formed at various exposure times in human volunteers
(Spencer and Schaumburg 2000). Although the primary reaction of carbon monoxide is with hemoglobin, it also interacts with myoglobin, cytochromes, and metalloenzymes (e.g., cytochrome c oxidase and cytochrome P450) (WHO 1999). The health importance of these secondary reactions is not well understood.
COHb cannot carry oxygen, and the effect of binding is a reduction of tissue partial pressure of oxygen (pO2) (NRC 1985). When carbon monoxide binds to hemoglobin, the affinity of the remaining hemoglobin for oxygen is increased. Therefore, the presence of COHb in the blood shifts the oxyhemoglobin dissociation curve to the left, and tissue pO2 must decrease to much lower values for the oxyhemoglobin to release its oxygen. The combination of the decrease in oxygen-carrying capacity of the blood and the impaired release of oxygen to the tissues results in a greater tissue oxygen, deficiency than is produced by an equivalent reduction in ambient pO2 (as at high altitude) or by an equivalent reduction in hemoglobin (as in anemia).
An additional hypothesis about the mechanism of carbon monoxide-mediated toxicity invokes reoxygenation injury subsequent to the hypoxic episode. Hyperoxygenation facilitates the production of reactive oxygen species, which, in turn, lead to the formation of oxidized proteins and nucleic acids (Zhang and Piantadosi 1992). Carbon monoxide exposure also has been shown to result in lipid peroxygenation (Thom 1990). Reoxygenation after exposure to carbon monoxide is also associated with oxidative stress and the ensuing production of oxygen radicals as a result of the conversion of xanthine dehydrogenase to xanthine oxidase (reperfusion injury; Thom 1992).
The lung eliminates carbon monoxide. More than 99% of this gas is eliminated unchanged, and less than 1% is converted to carbon dioxide (Stewart 1975). The time required for absorption or elimination of half the final volume of COHb in healthy, sedentary adults at sea level is 4 h (breathing air) or 1.5 h (breathing oxygen) (Stender et al. 1977).
HUMAN TOXICITY DATA
Carbon monoxide causes toxicity by binding reversibly to the hemoglobin molecule, forming COHb, which decreases the oxygen-carrying capacity of the blood. This capacity is further reduced because the presence of COHb alters the dissociation of oxyhemoglobin in the tissues.
Carbon monoxide toxicity has been studied well in humans. If sufficiently prolonged, exposure at concentrations of 200–1,200 ppm can result in a progression of such hypoxic symptoms as headache, decreased night vision, abnormal visual evoked response, nausea, abnormal fine manual dexterity, vomiting,
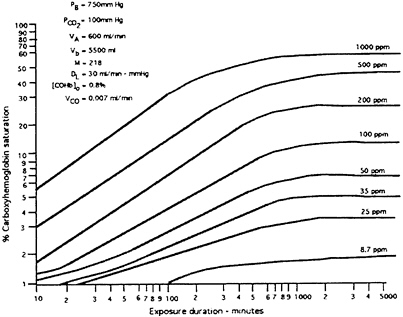
FIGURE 3–1 Carbon monoxide concentrations reached in blood (% saturation at various durations of exposure) in a normal human subject as a function of inspired CO. Abbreviations: PB, barometric pressure; PCO2, average partial pressure of carbon dioxide in lung capillaries; VA, alveolar ventilation rate; Vb, blood volume; M, equilibrium constant; DL, diffusing capacity of the lungs; [COHb]o, control value prior to carbon monoxide exposure; VCO, rate of endogenous carbon monoxide production. Source: Peterson and Stewart (1970). Reprinted with permission from Experimental and Clinical Neurotoxicology, 2nd Edition; copyright 2000, Oxford University Press.
convulsions, and if no treatment is administered, death (NRC 1985). At 35% COHb, manual dexterity is impaired; at 40%, mental confusion is observed. Death can occur at 67% COHb. After treatment to increase pO2, a complete recovery can be expected if tissue anoxia has not been too severe. The primary target organs of carbon monoxide are the heart and the brain, both of which have a critical need for oxygen (NRC 1994). The remainder of this section reviews the available human toxicity data on cardiovascular and central nervous system (CNS) effects after exposure to carbon monoxide; those data are summarized in Table 3–2.
TABLE 3–2 Human Toxicity Data, Inhalation Exposure to Carbon Monoxide
|
COHb (%) a/v |
Concentration, ppm |
Exposure Duration |
Effect |
Reference |
|
2 |
50 |
1 h and 20 min |
Impaired vigilance. No effects on response latency, short-term memory, and ability to subtract numbers mentally. |
Beard and Grandstaff 1975 |
|
2.3 |
26 |
2 h and 15 min |
No effect on vigilance, heart rate, minute volume. |
Horvath et al. 1971 |
|
2.3–3.1 |
50 |
80–125 min |
Gradual decrease in vigilance performance. |
Fodor and Winneke 1972 |
|
2.4 |
12 |
24 h/d, 8 d |
3 of 16 subjects showed changes in P waves. 1 had marked S-T or T changes (subject had localized heart myopathy). |
Davies and Smith 1980 |
|
2.5 |
50 |
25 min |
Increased minute volume, reductions in duration (21–20 min) subjects could exercise maximally at 35°C. |
Drinkwater et al. 1974 |
|
2.7 |
50 |
25 min |
No effects on maximal oxygen uptake, minute volume, and heart rate. |
Raven et al. 1974 |
|
3.4 |
NR |
NR |
Deficit in driving skills. |
Wright et al. 1973 |
|
3–7.7 |
35, 76 |
4 h |
No effect on visual task performance at 3%. Impairment of vigilance tasks at 4–7.7%. |
Putz et al. 1976, 1979 |
|
COHb (%) a/v |
Concentration, ppm |
Exposure Duration |
Effect |
Reference |
|
4.3–5 |
50 |
4 h |
During exercise, increase in heart rates, decrease in VO2MAX, decreased time to exhaustion. |
Horvath et al. 1975 |
|
4.5 |
NR |
NR |
Decrements in visual sensitivity. |
McFarland et al. 1944, as cited in NRC 1985 |
|
5 |
100 |
2.5 h |
No decrease in motor performance. |
Mihevic et al. 1983 |
|
5–10 |
70 |
8 h/d, 13 yr |
Holland Tunnel workers exposed for approximately 8 h/d for 13 yr. No adverse health effects observed. |
Sievers et al. 1942 |
|
5.7 |
NR |
NR |
No decrement in vigilance. |
Benignus et al. 1977; Davies et al. 1981 |
|
6.6 |
111 |
2 h and 15 min |
Impaired vigilance. No effect on heart rate, minute volume. |
Horvath et al. 1971 |
|
6.6 |
125 |
15 min-3 h |
No effect on tracking performance, ability to estimate time lapse. |
Mikulka et al. 1970 |
|
6.9 |
111 |
2 h and 15 min |
No effect on vigilance, heart rate, minute volume. |
O’Hanlon 1975 |
|
7.0 |
100 |
2.5 h |
Impaired visual vigilance (no effect on auditory vigilance). Impaired performance in the Amthauer’s Intelligence Structure Test. |
Bender et al. 1972; Bunnell and Horvath 1989 |
|
COHb (%) a/v |
Concentration, ppm |
Exposure Duration |
Effect |
Reference |
|
7.1 |
50 |
24 h/d, 8 d |
Feeling of fatigue. Increased minute volume, heart rate. Changes in P-waves. |
Davies and Smith 1980; Davies et al. 1981 |
|
7.3 (nonsmokers) –9.3 (smokers) |
NR |
NR |
No change in subjective symptoms, pulmonary function, oxygen metabolism, blood characteristics in healthy subjects performing moderate aerobic exercise at 50% of VO2MAX |
DeLucia et al. 1983, as cited in NRC 1985 |
|
7.5 |
250 |
15 min-1 h and 20 min |
No effect on vigilance, response latency, short-term memory, ability to do subtraction. 23% reduction in the duration the subjects could exercise maximally. |
Beard and Grandstaff 1975; Ekblom and Huot 1972 |
|
8–9 |
50–200 |
2.5–24 h |
No effect on mental capacity, manual dexterity, hand steadiness, reaction time, estimation of time lapse, visual function. |
Stewart et al. 1970; Ettema et al. 1975; Benignus et al. 1987; Luria and McKay 1979 |
|
8.5 |
650 |
45 min |
Increased reaction time to visual stimuli. |
Ramsey 1973 |
|
10 |
90 |
4–6 h |
Increased response time, decreased precision in maintaining separation distance in driving. |
Ray and Rockwell 1970 |
|
COHb (%) a/v |
Concentration, ppm |
Exposure Duration |
Effect |
Reference |
|
10.4 |
200 |
3 h |
No effect on time perception, tracking performance. |
O=Donnell et al. 1971 |
|
10.7–13.2 |
NR |
NR |
Minimal changes observed in heart rate, other physiologic responses in smokers and nonsmokers (aged 22–55) after long periods of activity. |
Gliner et al. 1975, as cited in NRC 1985 |
|
11 |
75 |
24 h/d, 7 d |
Changes in P-wave, S-T segment, or T-wave (6 of 9 subjects), supraventricular extrasystoles (1 of 9 subjects). |
Davies and Smith 1980 |
|
11 |
700 |
NR |
Gradual decrease in driving performance. |
McFarland 1973 |
|
12 |
100 |
8 h |
No effect on manual dexterity, hand steadiness, reaction time, estimation of time lapse. |
Stewart et al. 1970 |
|
12 |
950 |
45 min |
Increased reaction time. No effect on depth perception; light detection sensitivity. |
Ramsey 1973 |
|
12.6 |
200 |
2 h and 40 min |
No effect on vigilance performance. |
Benignus et al. 1977 |
|
13–16 |
200–500 |
1–4 h |
Mild headache. |
Stewart et al. 1970 |
|
17 |
11,600 for 4–5 min, then 141.6 for remainder of experiment |
2 h and 15 min |
No effect on threshold to visually detect motion, pattern, contrast. No effect on luminance threshold. |
Hudnell and Benignus 1989 |
|
COHb (%) a/v |
Concentration, ppm |
Exposure Duration |
Effect |
Reference |
|
18 |
200 |
5 h |
No impairment on ability to estimate time lapse. |
Stewart et al. 1973 |
|
15–20 |
|
No change in oxygen uptake in tissues during submaximal exercise for 5–60 min. |
Chevalier et al. 1966; Ekblom and Huot 1972; Pirnay et al. 1971; Vogel and Gleser 1972; Vogel et al. 1972 |
|
|
20 |
100 |
NR |
Headache in 1 of 49 subjects. No other symptoms. No changes in spinal and cranial nerve reflexes, heart rate, systolic and diastolic blood pressure, respiratory rate, muscle persistence time. No effect on static steadiness or response time in making simple choices of letter and color. |
Schulte 1963 |
|
23 |
NR |
NR |
No changes in plasma volume, hematocrit, total protein concentration. |
Parving 1972 |
|
37 |
860 |
NR |
Severe headache, dizziness, difficulty in concentrating, polycythemia. |
DiMarco 1988 |
|
COHb (%) a/v |
Concentration, ppm |
Exposure Duration |
Effect |
Reference |
|
30–40 |
NR |
NR |
Severe headache, nausea and vomiting, syncope. |
Stewart 1975, as cited in NRC 1985 |
|
40–45 |
NR |
NR |
Subjects unable to perform tasks requiring only slight physical exertion. |
Chiodi et al. 1941, as cited in NRC 1985 |
|
50–60 |
NR |
NR |
Coma and convulsions. |
Stewart 1975, as cited in NRC 1985 |
|
67–70 |
NR |
NR |
Lethal if not treated. |
Stewart 1975, as cited in NRC 1985 |
|
Abbreviations: NR, not reported; VO2MAX, maximum oxygen uptake. |
||||
Cardiovascular Effects
The myocardium is more sensitive than any other muscle tissue to the decrease in available blood oxygen caused by formation of COHb. Cardiac dysfunction attributable to the effects of carbon monoxide binding to cardiac myoglobin also could contribute to decreased perfusion of the heart and the CNS (Cobb and Etzel 1991). As COHb concentrations increase, there is a gradient of symptoms that reflects increasing cardiovascular dysfunction (Table 3–2). Some factors have been shown to modulate the clinical effects of carbon monoxide exposure. Greater oxygen demand in actively exercising (versus sleeping) patients enhances susceptibility to carbon monoxide intoxication (Meredith and Vale 1988). The degree and duration of hypotension, the presence of cardiac or pulmonary disease or anemia, as well as cardiac dysfunction (arrhythmias or other conditions) induced by carbon monoxide exposure also influence clinical outcome (Ehrich et al. 1944; Stewart 1976).
Cardiac function in healthy persons could be affected by low to moderate carbon monoxide exposures (Davies and Smith 1980). Six matched groups of young healthy subjects lived in a closed environmental chamber for 18 d and were exposed continuously to carbon monoxide at concentrations of 15 or 50 ppm in air during the middle 8 d. Unequivocal P-wave electrocardiogram (ECG) changes were observed during exposure in 3 of the 15 subjects exposed at 15 ppm (2.4% COHb) and in 6 of 15 subjects exposed at 50 ppm (7.1% COHb), compared with none of the 14 exposed at 0 ppm (0.5% COHb).
The ability to perform physical work is drastically reduced when COHb concentrations reach 40%. Chiodi et al. (1941) observed that subjects with 40– 45% COHb were unable to perform tasks requiring only slight physical exertion. COHb concentrations of 15–20% do not appear to affect the ability to do physical work (submaximal exercise for short durations) (Chevalier et al. 1966; Ekblom and Huot 1972; Pirnay et al. 1971; Vogel and Gleser 1972; Vogel et al. 1972). In those studies, a slight increase in heart rate was observed. Gliner et al. (1975) exposed smokers and nonsmokers aged 22–55 to carbon monoxide (13.2 and 10.7% COHb, respectively) during long periods of activity (3.5 h within a 4-h period) at 35% of maximal aerobic capacity. The subjects showed only minimal changes in physiologic response.
The critical concentration at which COHb reduces maximum oxygen uptake (VO2MAX) is 4.3%, according to Horvath et al. (1975). The same investigators also reported decreases of 4.9% and 7% in work time to exhaustion at COHb of 3.3% and 4.3%, respectively. Those findings were confirmed in a double-blind study of 6 healthy male firefighters exposed to carbon monoxide (5.0–5.5% COHb for 4 h) (Klein et al. 1980). The firefighters showed a decrease in time to exhaustion.
Sievers et al. (1942) studied Holland Tunnel workers exposed for approximately 13 years at an average concentration of 70 ppm for multiple periods lasting 2 h each during an 8 h shift. No adverse health effects were observed in those workers. COHb concentrations were 5–10%. In a retrospective study of bridge and tunnel officers exposed to carbon monoxide at an average COHb concentration of less than 5%, Stern et al. (1988) reported a significant increase in mortality from arteriosclerotic heart disease. Given that continuous COHb burdens of 5% might carry a significant cardiovascular risk, it is possible that the cardiovascular effects of carbon monoxide in submariners, particularly those who smoke cigarettes, could be amplified by the imposition of a 1.5–5% COHb produced by ambient carbon monoxide in a submarine (Bondi et al. 1978; Davies 1973; Goldsmith and Landaw 1968; McIlvaine et al. 1969).
Central Nervous System Effects
Numerous studies have assessed the effects of carbon monoxide on such CNS functions as vigilance, sensory effects, coordination and tracking, driving ability, and cognitive behavior. Despite extensive investigations, the COHb concentrations that trigger an effect on human perceptual function and cognition have not been quantified. COHb concentrations as low as 4.5% perturb ability to discriminate small differences in light intensity (McFarland et al. 1944). Transient alterations of visual thresholds are associated with 5% COHb (McFarland et al. 1944). A significant performance decrement in the ability to compare the duration of tones was reported after exposure to 50 and 100 ppm carbon monoxide for 90 and 50 min, respectively (Beard and Grandstaff 1975). Other studies have not corroborated effects on perceptual function and cognition at comparably low COHb concentrations (Table 3–2). Differences in experimental design-in particular, in task duration–have been suggested to be, at least partially responsible for the discrepant results (Beard and Grandstaff 1975; Crystal and Ginsberg 2000; Stewart 1976).
Vigilance assessment measures how well a person performs at detecting small environmental changes that take place at unpredictable intervals and, therefore, demand continuous attention (NRC 1985). Decreases in vigilance due to carbon monoxide exposure have been reported by several investigators, but the data do not show conclusively which concentration of COHb will trigger impaired vigilance. Beard and Grandstaff (1975) reported impaired vigilance in subjects with 2% COHb. Horvath et al. (1971) and O’Donnell et al. (1971) reported a decrement in performance in subjects with 6.6% COHb. However, vigilance was not affected in subjects with 0.9% and 2.3% COHb. Winneke et al. (1978) did not observe any decrements in vigilance at COHb up to 11%. Putz et al. (1976, 1979) exposed subjects to carbon monoxide and found that visual-task performance was decreased at 5% COHb. This effect was not observed at 1% or 3% COHb. Using a vigilance paradigm different from that used by Putz et al. (1976, 1979), Benignus et al. (1977) and Davies et al. (1981) found no decrement in vigilance in subjects with up to 5.7% COHb.
Several studies have tested visual function after exposure to carbon monoxide. To minimize variability, McFarland et al. (1944) studied brightness discrimination in a small group of well-trained subjects. The subjects reported decreases in visual sensitivity at approximately 4.5% COHb. Another study reported no adverse effects on visual discrimination or depth perception in subjects with 8% or 12% COHb (Ramsey 1973). Luria and McKay (1979) reported no decrement in night vision with COHb of 9%. Davies et al. (1981) reported that at 7% COHb there was no effect on visual function. The studies described above used various visual paradigms, which could account for the differences in results.
Two studies that used subjects with COHb concentrations of up to 15% reported no changes in the ability to do tasks involving coordination (Stewart et al. 1970; Wright et al. 1973). Stewart et al. (1970) used several tests of manual dexterity. In one, subjects had to pick up small pins, place them in small holes, and then put collars over the pins. No effects attributable to carbon monoxide exposure were found at COHb up to 15%. Wright et al. (1973) also observed no effects on a number of coordination tasks at COHb of 5% and 7%. One study did report a decrement in a hand-eye coordination task (5% COHb) (Putz et al. 1976).
Several investigators have assessed the effects of carbon monoxide exposure on driving performance. McFarland (1973) studied subjects with 17% COHb who drove instrumented automobiles under highway conditions. Carbon monoxide did not cause a serious decrease in driving ability, but there was a statistically significant increase in roadway viewing time. No differences were reported in steering-wheel reversals. At 10% COHb and higher, Ray and Rockwell (1970) reported a significant difference in time required to respond to a velocity change in a lead car.
Bender et al. (1972) investigated the effects of exposure that resulted in 7% COHb on the ability to learn 10 nonsense syllables. The exposure caused a decrease in ability to recite the syllables and a decrease in ability to recite a series of digits in reverse order. The exposed subjects showed no change in ability to perform other tasks involving calculation problems, analogies, shape selection, dot counting, and letter recognition.
In approximately 3% of patients recovering from acute carbon-monoxide-induced coma, a severe, sometimes fatal, neurologic condition develops (Choi 1983; Min 1986). No clinical or laboratory results predict which patients are at risk for the delayed neuropsychiatric syndrome. Age appears to be a risk factor (Ernst and Zibrak 1998). Although the syndrome is widely held as a characteristic of carbon monoxide poisoning, a similar illness also can develop upon hypoglycemia, heroin overdose, strangulation, and anesthetic accident (Plum et al. 1962). The delayed syndrome is characterized as a postanoxic encephalopathy syndrome. It commonly develops 1–4 wk after an acute episode, with carbon-monoxide-induced coma and a period of recovery and lucid state. Thereafter, irritability, confusion, or manic symptoms appear, followed by a spastic or parkinsonian gait. Masked faces and rigidity are common, and most patients become somnolent and unable to walk On occasion, patients become mute or comatose, and some die. Notably, the progress of the disease can be halted at any stage, and some patients can make partial or full recovery. The most striking change associated with the delayed syndrome is diffuse demyelination in the cerebral hemispheres. Unlike injury patterns that occur in the acute carbon mon-
oxide syndrome, the corpus callosum, fornix, and anterior commissure are commonly spared.
EXPERIMENTAL ANIMAL TOXICITY DATA
Numerous studies have been conducted to test carbon monoxide toxicity in experimental animals. Several are reviewed in this section and summarized in Table 3–3.
Acute Exposure
There is evidence that acute exposure (20 min to 6 h) to carbon monoxide causes cardiovascular and CNS effects in experimental animals. Dogs exposed at 100 ppm (6.5% COHb) for 2 h (Aronow et al. 1979) and monkeys exposed at 100 ppm (9.3% COHb) for 6 hours showed a decrease in ventricular fibrillation threshold (DeBias et al. 1976). No effects on unconditioned reflex and conditioned avoidance tests were observed in rats exposed to 400 ppm (27% COHb) for 4 h (Mullin and Krivanek 1982). Failure of unconditioned reflex and a decrement in conditioned avoidance were observed in rats exposed to 800 ppm (34% COHb) for 4 h (Mullin and Krivanek 1982), and increased time to traverse a maze was observed in rats exposed to 2,000 ppm (75% COHb) for 30 min (Annau 1987). Monkeys exposed to 900 ppm (25–30% COHb) for 20–30 min showed a decrement in the ability to perform behavioral tasks (Purser and Berrill 1983). After 10–15 min exposure, the monkeys also had a decrease in carbon dioxide output.
Repeated Exposure
Repeated (subchronic and chronic) exposure of experimental animals to carbon monoxide increases hemoglobin concentration and hematocrit in several animal species. These effects were observed in exposures at 50 ppm for 6 mo (7.3% COHb) in dogs (Musselman et al. 1959), at 96 ppm for 90 d (7.5% COHb) in rats (Jones et al. 1971), and at 200 ppm for 90 d (16–20% COHb) in rats and monkeys (Jones et al. 1971). The effects were not observed at lower COHb concentrations, for example, in exposures at 20 ppm for 2 yr (3.4% COHb) in monkeys (Eckardt et al. 1972), at 51 ppm for 90 d (5% COHb) in monkeys and rats (Jones et al. 1971), and at 66 ppm for 2 yr (7.4% COHb) in monkeys (Eckardt et al. 1972).
TABLE 3–3 Experimental Animal Toxicity Data, Exposure with Carbon Monoxide
|
Species |
Concentration, ppm |
Duration |
COHb (%) |
Effect |
Reference |
|
ACUTE EXPOSURE |
|||||
|
Dog |
100 |
2 h |
6.5 |
Decreased ventricular fibrillation threshold. |
Aronow et al. 1979 |
|
Monkey |
100 |
6 h |
9.3% |
Decreased ventricular fibrillation threshold. |
DeBias et al. 1976 |
|
Rat |
400 |
4 h |
27 |
No effect on unconditioned reflex, conditioned avoidance tests. |
Mullin and Krivanek 1982 |
|
Monkey |
900 |
20–30 min |
25–30 |
Effect on ability to perform behavioral tasks. Metabolism affected (decreased CO2 output) after 10–15 min exposure. |
Purser and Berrill 1983 |
|
Rat |
800 |
4 h |
34 |
Failure of unconditional reflex, decrement in conditioned avoidance. |
Mullin and Krivanek 1982 |
|
Rat |
2000 |
30 min |
75 |
Increased time to traverse a maze. |
Annau 1987 |
|
REPEATED EXPOSURE |
|||||
|
Monkey |
20 |
22 h/d, 7 d/wk, 2 yr |
3.4 |
No change in hematocrit, hemoglobin concentration, red blood cell count. No histopathology in heart, brain, lung. |
Eckardt et al. 1972 |
|
Monkey, Rat |
51 |
24 h/d, 90 d |
5 at 48 h |
No toxic signs and histopathology. No changes in hematocrit, hemoglobin concentration. |
Jones et al. 1971 |
|
Species |
Concentration, ppm |
Duration |
COHb (%) |
Effect |
Reference |
|
Dog |
50 |
24 h/d, 6 mo |
7.3 |
12% increase in hemoglobin concentration. No change on EKG, histology. |
Musselman et al. 1959 |
|
Monkey |
66 |
22 h/d, 7 d/wk, 2 yr |
7.4 |
No change in hematocrit, hemoglobin concentration, red blood cell count. No histopathology in heart, brain, lung. |
Eckardt et al. 1972 |
|
Rat |
96 |
24 h/d, 90 d |
7.5 at 48 h |
No toxic signs and histopathology. Increased hematocrit and hemoglobin concentrations. |
Jones et al. 1971 |
|
Monkey |
96 |
24 h/d, 90 d |
10 at 48 h |
No toxic signs, histopathology. No effects on hematocrit, hemoglobin concentrations. |
Jones et al. 1971 |
|
Monkey Rat |
200 |
24 h/d, 90 d |
16–20 |
No toxic signs, histopathology. Increased hematocrit, hemoglobin concentrations. |
Jones et al. 1971 |
|
Monkey |
400 |
0.5 h on/0.5 h off, 10 h/d for 12 mo |
18–23 |
Did not cause atherosclerosis. |
Bing et al. 1980 |
|
Dog |
100 |
5 3/4 h/d, 6 d/wk for 11 wk |
20 |
Increases in red blood cell count after 8 wk; returned to normal after 11 wk |
Brieger 1944 |
|
Dog |
100 |
5 3/4 h/d, 6 d/wk for 11 wk |
20 |
T-wave changes, myocardial degeneration. |
Ehrich et al. 1944 |
|
Dog |
100 |
5 3/4 h/d, 6 d/wk for 11 wk |
20 |
Disturbance of postural and position reflexes and of gait. Histologic changes in cortex, white matter of the cerebral hemispheres, globus pallibus, brain stem 3 mo after exposure. |
Lewey and Drabkin 1944 |
|
Monkey |
380 |
24 h/d, 99 d |
31 |
No decrement on operant behavior: visual and auditory response times, learned pressing of a lever. |
Theodore et al. 1971 |
|
Monkey, Dog, Rat, Mouse |
400 for 71 d, then 500 for 97 d |
24 h/d, 71 and 97 d |
32–33 |
40% increase in hemaglobin concentration, 30% increases in hematocrit, blood volume. No effect on plasma volume, body weight, survival. No histopathology in brain, heart. In rats, heart and spleen increased in weight. |
Theodore et al. 1971 |
|
Rat |
500 |
21 h/d, 62 d |
42 |
Enhanced development of NaCl-induced hypertension, cardiomegaly, splenomegaly, elevated hemoglobin concentration, hematocrit. |
Shiotsuka et al. 1984 |
|
Rat |
25 50 |
24 h/d, 2 mo |
NR |
At 25 ppm, no effects on serum corticosterone and thyroxine, hypothalamic norepinephrine concentration, adrenal catecholamines concentration, organ weights, body weight. At 50 ppm, reduced serum thyroxine concentration, increased adrenal catecholamine concentration. No effect on organ weight. |
Vyskocil et al. 1986 |
|
Species |
Concentration, ppm |
Duration |
COHb (%) |
Effect |
Reference |
|
|
100 |
|
At 100 ppm, reductions in serum thyroxine and hypothalmic norepinephrine concentrations, increases in adrenal catecholamines and serum cortocosterone, no effect on body organ weight except for a slight decrease in liver weight. |
|
|
|
Abbreviation: NR, not reported. |
|||||
Several studies have examined the morphologic effects of carbon monoxide exposure in experimental animals. Dogs exposed at 100 ppm for 5.75 h/d, 6 d/wk for 11 wk (20% COHb) exhibited histopathologic changes in the cortex, white matter of the cerebral hemispheres, globus pallidus, and brain stem 3 mo after exposure (Lewey and Drabkin 1944). These animals also showed disturbance of postural and position reflexes and of gait. No histopathologic changes were observed in exposures at 50 ppm for 6 mo (7.3% COHb) in dogs (Musselman et al. 1959), at 200 ppm for 90 d (16% COHb) in rats (Jones et at. 1971), at 66 ppm for 2 yr (7.4% COHb) in monkeys (Eckardt et al. 1972), at 200 ppm for 90 d (20% COHb) in monkeys (Jones et al. 1971), and at 400 ppm for 71 d (32% COHb) followed by 500 ppm for 97 d (33% COHb) in monkeys, dogs, rats, and mice (Theodore et al. 1971).
NAVY’S RECOMMENDED SEALS
The Navy has proposed a SEAL 1 set at 75 ppm and a SEAL 2 set at 85 ppm. These values are based on a model developed by Coburn et al. (1965) for estimating COHb concentrations from environmental exposures. COHb at these values would range from 8.3% to 12.4%.
ADDITIONAL RECOMMENDATIONS FROM THE NRC AND OTHER ORGANIZATIONS
Recommended exposure guidance levels for carbon monoxide from the NRC and other organizations are summarized in Table 3–4. The 24-h emergency exposure guidance level (EEGLs) (NRC 1985) is the most relevant guidance level to compare to the SEALs. EEGLs were developed for healthy military personnel in emergency situations. An important difference between EEGLs and SEALs is that EEGLs allow mild, reversible health effects, whereas SEALs allow moderate, reversible health effects. Therefore, the SEAL values are higher than the corresponding EEGL values.
SUBCOMMITTEE ANALYSIS AND RECOMMENDATIONS
Submarine Escape Action Level 1
On the basis of its review of human and experimental animal health-effects and related data, the subcommittee concludes that the Navy’s proposed SEAL 1 of 75 ppm for carbon monoxide is too conservative. The subcommittee rec
TABLE 3–4 Recommendations from Other Organizations for Carbon Monoxide
|
Organization |
Type of Exposure Level |
Recommended Exposure Level |
Reference |
|
ACGIH |
TLV-TWA |
25 ppm |
ACGIH 1998 |
|
AIHA |
ERPG-1 |
200 ppm |
AIHA 2001 |
|
|
ERPG-2 |
350 ppm |
|
|
|
ERPG-3 |
500 ppm |
|
|
DFG |
MAK (8 h/d, 40 h/wk) |
30 ppm |
DFG 1997 |
|
|
Peak Limit (30 min maximum duration, 4 times per 8 h) |
60 ppm |
|
|
IPCS |
Derived guidelines values for carbon monoxide concentration in ambient air |
|
WHO 1999 |
|
|
15 min |
87 ppm |
|
|
|
30 min |
52 ppm |
|
|
|
1 h |
26 ppm |
|
|
|
8 h |
9 ppm |
|
|
NAC |
Proposed 8-h AEGL-1 |
NR |
Federal Register: May 2, 2001.66(85):21940– 21964. |
|
|
Proposed 8-h AEGL-2 |
27 |
|
|
|
Proposed 8-h AEGL-3 |
130 |
|
|
NASA |
SMAC: |
|
NRC 1994 |
|
|
1 h |
55 ppm |
|
|
|
24 h |
20 ppm |
|
|
|
7d |
10 ppm |
|
|
|
30 d |
10 ppm |
|
|
|
180 d |
10 ppm |
|
|
NIOSH |
REL |
35 ppm (Time Weighted Average) |
|
|
|
200 ppm (Ceiling Concentration) |
|
|
|
NIOSH |
IDLH |
1,200 ppm |
NIOSH 2000 |
|
NRC |
EEGL: |
|
NRC 1985 |
|
|
10 min |
1,500 ppm |
|
|
|
30 min |
750 ppm |
|
|
|
60 min |
400 ppm |
|
|
|
24 h |
50 ppm |
|
|
Abbreviations: ACGIH, American Conference of Governmental Industrial Hygienists; AIHA, American Industrial Hygiene Association; DFG, Deutsche Forschungsgemeinschaft; IPCS, International Programme on Chemical Safety; NAC, National Advisory Committee; NASA, National Aeronautics and Space Administration; NIOSH, National Institute for Occupational Safety and Health; NRC, National Research Council; OSHA, Occupational Safety and Health Administration; WHO, World Health Organization; AEGL, acute exposure guideline level; EEGL, emergency exposure guidance level; ERPG, emergency response planning guidelines; IDLH, immediately dangerous to life and health; MAK, maximum concentration values in the workplace; NR, not recommended; PEL, permissible exposure limit; REL, recommended exposure limit; SMAC, spacecraft maximum allowable concentration; TLV-TWA, Threshold Limit Value—time weighted average. |
|||
ommends a SEAL 1 of 125 ppm; exposure at this concentration would not result in blood COHb concentration above 15%. Studies in humans show that COHb concentrations of approximately 15% do not result in significant effects on perceptual function or cognition in healthy individuals (Stewart et al. 1973; Hudnell and Benignus 1989).
It should be noted that the SEAL 1 was recommended within the context of inspired air at sea level, where the oxygen concentration is at 20.95%. The oxygen concentration in a disabled submarine may be lower. Oxygen and carbon monoxide compete for binding sites on hemoglobin. When the oxygen pressure is low in the lung capillaries, as might be found in crew members on a disabled submarine, there is more unoxygenated hemoglobin for carbon monoxide to bind to. Therefore, carbon monoxide exposure becomes more dangerous to crew members in a submarine with lower oxygen concentration than one in which the oxygen concentration is 20.95%. The partial pressure of carbon monoxide (Pco) should be proportional to the partial pressure of alveolar oxygen (PAo2). Therefore, in a disabled submarine with 16% oxygen concentration instead of 20.95%, the inspired Po2 would be 114 mm Hg, PAo2=64 mm Hg (PAo2=(PB-47)-50 mm Hg; where PB is barometric pressure). Because Pco/Po2 =1/220×Po2, in equilibrium the ratio would reduce the allowable carbon monoxide from the proposed 125 ppm carbon monoxide to 80 ppm (64/100×125).
Submarine Escape Action Level 2
On the basis of its review of human and experimental animal health-effects and related data, the subcommittee concludes that the Navy’s proposed SEAL 2 of 85 ppm for carbon monoxide is too conservative. The subcommittee recommends a SEAL 2 of 150 ppm, which would not result in blood COHb concentration above 20%. Human data suggest that at a COHb concentration of approximately 20%, some submariners could experience mild headache and some decrement in cognitive function (Schulte 1963; Parving 1972; Stewart et al. 1973). Such effects would not impair the ability of a crew to escape from a disabled submarine. The recommended SEAL 2 is also supported by Theodore et al. (1971) where monkeys were exposed to a carbon monoxide concentration of 380 ppm for 90 d (COHb=31%) and there were no adverse health effects.
As pointed out in the discussion on SEAL 1, the recommended value for SEAL 2 is set for oxygen concentration at sea level (20.95%). Given that the oxygen concentration in the disabled submarine will likely be lower, it is necessary to correct the SEAL 2 for this change. Applying the same assumptions and calculations, one derives a SEAL 2 of 96 ppm (64/100×150) for a submarine in which the oxygen concentration is at 16%.
DATA GAPS AND RESEARCH NEEDS
The subcommittee recommends that the Navy consider conducting the following studies:
-
Studies to determine whether the rates of formation and elimination of COHb will increase under hyperbaric conditions. Such studies should be conducted because carbon monoxide exerts its toxic effects by reduction of the oxygen-carrying capacity of the blood. Thus, the ratio of the pressure of carbon monoxide and the pressure of oxygen will determine the toxicity of carbon monoxide. This ratio will not be affected by absolute pressure.
-
Studies on the additive effects of carbon monoxide and hydrogen cyanide, at concentrations likely to be produced in disabled submarines.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1996. Carbon Monoxide. Pp. 1–4. Supplements to the 6th Edition Documentation of the Threshold Limit Values and Biological Exposure Indices. Cincinnati, OH: ACGIH
ACGIH (American Conference of Governmental Industrial Hygienists). 1998. Pp. 81– 83 in TLVs and BEIs Threshold Limit Values for Chemical Substances and Physical Agents. Cincinnati, OH: ACGIH
AIHA (American Industrial Hygiene Association). 2001. The AIHA 2001 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides Handbook Fairfax, VA: American Industrial Hygiene Association.
Annau, Z. 1987. Complex maze performance during carbon monoxide exposure in rats. Neurotoxicol. Teratol. 9(2):151–155.
Aronow, W.S., E.A.Stemmer, and S.Zweig. 1979. Carbon monoxide and ventricular fibrillation threshold in normal dogs. Arch. Environ. Health 34(3):184–186.
Beard, R.R., and N.W.Grandstaff. 1975. Carbon monoxide and human functions. Pp. 1–27 in Environmental Science Research, Vol. 5. Behavioral Toxicology, B.Weiss and V.G.Laties, eds. New York: Plenum Press.
Bender, W., M.Goethert, and G.Malorny. 1972. Effect of low carbon monoxide concentrations on psychological functions. Staub-Reinhalt Luft. 32(4):54–60.
Benignus, V.A., K.E.Muller, C.N.Barton, and J.D.Prah. 1987. Effect of low level carbon monoxide on compensatory tracking and event monitoring. Neurotoxicol. Teratol. 9(3):227–234.
Benignus, V.A., D.A.Otto, J.D.Prah, and G.Benignus. 1977. Lack of effects of carbon monoxide on human vigilance. Percept. Mot. Skills 45(3 Pt 1):1007–1014.
Bing, R.J., J.S.Sarma, R.Weishaar, A.Rackl, and G.Pawlik. 1980. Biochemical and histological effects of intermittent carbon monoxide exposure in cynomolgus monkeys (Macaca fascicularis) in relation to atherosclerosis. J. Clin. Pharmacol. 20(8– 9):487–499.
Bondi, K.R., K.R.Very, and K.E.Schaefer. 1978. Carboxyhemoglobin levels during a submarine patrol. Aviat. Space Environ. Med. 49(7):851–854.
Brieger, H. 1944. Carbon monoxide polycythemia. J. Ind. Hyg. Toxicol. 26(10):321–327.
Budavari, S., ed. 1989. Carbon monoxide. Pp. 275 in The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th Ed. Rahway, NJ: Merck.
Bunnell, D.E., and S.M.Horvath. 1989. Interactive effects of heat, physical work and CO exposure on metabolism and cognitive task performance. Aviat. Space Environ. Med. 60(5):428–432.
Chevalier, R.B., R.A.Krumholz, and J.C.Ross. 1966. Reaction of nonsmokers to carbon monoxide inhalation: cardiopulmonary responses at rest and during exercise. JAMA 198(10):1061–1064.
Chiodi, H., D.B.Dill, F.Consolazio, and S.M.Horvath. 1941. Respiratory and circulatory responses to acute carbon monoxide poisoning. Am. J. Physiol. 134:683–693.
Choi, I.S. 1983. Delayed neurologic sequelae in carbon monoxide intoxication. Arch. Neurol. 40(7):433–435.
Cobb, N., and R.A.Etzel. 1991. Unintentional carbon monoxide-related deaths in the United States, 1979 through 1988. JAMA 266(5):659–663.
Coburn, R.F., R.E.Forster, and P.B.Kane. 1965. Considerations of the physiological variables that determine the blood carboxyhemoglobin concentration in man. J. Clin. Invest. 44(11):1899–1910.
Crystal, H.A., and M.D.Ginsberg. 2000. Carbon Monoxide. Pp. 318–329 in Experimental and Clinical Neurotoxicology, Second Ed., P.S.Spencer, H.H.Schaumburg, and A.C.Ludolph, eds. New York, NY: Oxford University Press.
Davies, D.M. 1973. Sixty days in a submarine: the pathophysiological and metabolic cost. J.R.Coll. Physicians Lond. 7(2):132–144.
Davies, D.M., and D.J.Smith. 1980. Electrocardiographic changes in healthy men during continuous low-level carbon monoxide exposure. Environ. Res. 21(1):197– 206.
Davies, D.M., E.J.Jolly, R.J.Pethybridge, and W.P.Colquhoun. 1981. The effects of continuous exposure to carbon monoxide on auditory vigilance in man. Int. Arch. Occup. Environ. Health 48(1):25–34.
DeBias, D.A., C.M.Banerjee, N.C.Birkhead, C.H.Green, S.D.Scott, and W.V.Harrer. 1976. Effects of carbon monoxide inhalation on ventricular fibrillation. Arch. Environ. Health 31(1):42–46.
DeLucia, A.J., J.H.Whitaker, and L.R.Bryant. 1983. Effects of combined exposure to ozone and carbon monoxide (CO) in humans. Pp. 145–159 in Advances in Modern Environmental Toxicology, Vol. 5, S.D.Lee, M.G.Mustafa, and M.A.Mehlman, eds. Princeton, NJ: Princeton.
DFG (Deutsche Forschungsgemeinschaft). 1997. List of MAK and BAT Values 1997. Maximum Concentrations and Biological Tolerance Values at the Workplace, 1st Ed. Report No. 33. Weinheim: Wiley-VCH.
DiMarco, A. 1988. Carbon monoxide poisoning presenting as polycythemia. N. Engl. J. Med. 319(13):874.
Drinkwater, B.L., P.B.Raven, S.M.Horvath, J.A.Gliner, R.O.Ruhling, N.W.Bolduan, and S.Tagucki. 1974. Air pollution, exercise, and heat stress. Arch. Environ. Health 28(4):177–181.
Eckardt, R.E., N.H.McFarland, Y.C.Alarie, and W.M.Busey. 1972. The biologic effect from long-term exposure of primates to carbon monoxide. Arch. Environ. Health 25(6):381–387.
Ehrich, W.E., S.Bellet, and F.H.Lewey. 1944. Cardiac changes from CO poisoning. Am. J. Med. Sci. 208:511–523.
Ekblom, B., and R.Huot. 1972. Response to submaximal and maximal exercise at different levels of carboxyhemoglobin. Acta. Physiol. Scand. 86(4):474–482.
Ernst, A., and J.D.Zibrak. 1998. Carbon monoxide poisoning. N. Engl. J. Med. 339(22):1603–1608.
Ettema, J.H., R.L.Zielhuis, E.Burer, H.A.Meier, L.Kleerekoper, and M.A.de Graaf. 1975. Effects of alcohol, carbon monoxide and trichloroethylene exposure on mental capacity. Int. Arch. Occup. Environ. Health 35(2):117–132.
Fodor, C.G., and G.Winneke. 1972. Effect of low CO concentrations on resistance to monotony and on psychomotor capacity. Staub-Reinhalt Luft 32(4):46–54.
Gliner, J.A., P.B.Raven, S.M.Horvath, B.L.Drinkwater, and J.C.Sutton. 1975. Man’s physiologic response to long-term work during thermal and pollutant stress. J. Appl. Physiol. 39(4):628–632.
Goldsmith, J.R., and S.A.Landaw. 1968. Carbon monoxide and human health. Science 162(860):1352–1359.
Horvath, S.M., T.E.Dahms, and J.F.O=Hanlon. 1971. Carbon monoxide and human vigilance: a deleterious effect of present urban concentrations. Arch. Environ. Health 23(5):343–347.
Horvath, S.M., P.B.Raven, T.E.Dahms, and D.J.Gray. 1975. Maximal aerobic capacity at different levels of carboxyhemoglobin. J. Appl. Physiol. 38(2):300–303.
Hudnell, H.K., and V.A.Benignus. 1989. Carbon monoxide exposure and human visual detection thresholds. Neurotoxicol. Teratol. 11(4):363–371.
Jones, R.A., J.A.Strickland, J.A.Stunkard, and J.Siegel. 1971. Effects on experimental animals of long-term inhalation exposure to carbon monoxide. Toxicol. Appl. Pharmacol. 19(1):46–53.
Jones, H.A., J.C.Clark, E.E.Davies, R.E.Forster, and J.M.Hughes. 1982. Rate of uptake of carbon monoxide at different inspired concentrations in humans. J. Appl. Physiol. 52(1):109–113.
Klein, J.P., H.V.Foster, R.D.Stewart, and A.Wu. 1980. Hemoglobin affinity for oxygen during short-term exhaustive exercise. J. Appl. Physiol. 48(2):236–242.
Lewey, F.H., and D.L.Drabkin. 1944. Experimental chronic carbon monoxide poisoning of dogs. Am. J. Med. Sci. 208:502–511.
Lide, D.R. 1991. CRC Handbook of Chemistry and Physics, 72nd Ed. Boca Raton: CRC Press.
Luria, S.M., and C.L.McKay. 1979. Effects of low levels of carbon monoxide on visions of smokers and nonsmokers. Arch. Environ. Health 34(1):38–44.
McFarland, R. 1973. Low-level exposure to carbon monoxide and driving performance. Arch. Environ. Health 27(6):355–359.
McFarland, R.A., F.J.W.Roughton, M.H.Halperin, and J.I.Niven. 1944. The effects of carbon monoxide and altitude on visual thresholds. J. Aviat. Med. 15:381–394.
McIlvaine, P.M., W.C.Nelson, and D.Bartlett Jr. 1969. Temporal variation of carboxyhemoglobin concentrations. Arch. Environ. Health 19(1):83–91.
Meredith, T., and A.Vale. 1988. Carbon monoxide poisoning. Br. Med. J. 296(6615):77–79.
Mihevic, P.M., J.A.Gliner. and S.M.Horvath. 1983. Carbon monoxide exposure and information processing during perceptual-motor performance. Int. Arch. Occup. Environ. Health 51(4):355–363.
Mikulka, P.R.O’Donnell, P.Heinig, and J.Theodore. 1970. The effect of carbon monoxide on human performance. Ann. N.Y. Acad. Sci. 174(1):409–420.
Min, S.K. 1986. A brain syndrome associated with delayed neuropsychiatric sequelae following acute carbon monoxide poisoning. Acta Psychiat. Scand. 73(1):80–86.
Mullin, L.S., and N.D.Krivanek 1982. Comparison of unconditioned reflex and conditioned avoidance tests in rats exposed by inhalation to carbon monoxide, 1,1,1-trichloroethane, toluene or ethanol. Neurotoxicology 3 (1):126–137.
Musselman, N.P., W.A.Groff, P.P.Yevich, F.T.Wilinski, M.H.Weeks, and F.W.Oberst. 1959. Continuous exposure of laboratory animals to low concentration of carbon monoxide. Aerosp. Med. 30:524–529.
NIOSH (National Institute for Occupational Safety and Health). 2000. Immediately Dangerous to Life and Health Concentration (IDLHs). [Online]. Available: http://www.cdc.gov/niosh/idlh/630080.html. [Updated October 28, 2000].
NRC (National Research Council). 1985. Carbon Monoxide. Pp.17–38 in Emergency and Continuous Exposure Guidance Levels for Selected Airborne Contaminants, Vol. 4. Washington, DC: National Academy Press.
NRC (National Research Council). 1994. Carbon Monoxide. Pp. 61–90 in Spacecraft Maximum Allowable Concentrations for Selected Airborne Contaminants, Vol. 1. Washington, DC: National Academy Press.
O’Donnell, R.D., P.Mikulka, P.Heinig, and J.Theodore. 1971. Low level carbon monoxide exposure and human psychomotor performance. Toxicol. Appl. Pharmacol. 18(3):583–589.
O’Hanlon, J.F. 1975. Preliminary studies of the effects of carbon monoxide on vigilance in man. Pp. 61–75 in Behavioral Toxicology, B.Weiss and G.Laties, eds. New York: Plenum Press.
Parving, H-H. 1972. The effect of hypoxia and carbon monoxide exposure on plasma volume and capillary permeability to albumin. Scand. J. Clin. Lab. Invest. 30(1):49– 56.
Peterson, J.E., and R.D.Stewart. 1970. Absorption and elimination of carbon monoxide by inactive men. Arch. Environ. Health 21(2):165–171.
Pirnay, F., J.Dujardin, R.Deroanne, and J.M.Petit. 1971. Muscular exercise during intoxication by carbon monoxide . J. Appl. Physiol. 31(4):573–575.
Plum, F., J.B.Posner, and R.F.Hain. 1962. Delayed neurological deterioration after anoxia. Arch. Intern. Med. 110:18–25.
Purser, D.A., and K.R.Berrill. 1983. Effects of carbon monoxide on behavior in monkeys in relation to human fire hazard. Arch. Environ. Health 38(5):308–315.
Putz, V.R., B.L.Johnson, and J.V.Setzer. 1976. Effects of CO on Vigilance Performance. Effects of Low-Level Carbon Monoxide on Divided Attention, Pitch Discrimination, and the Auditory Evoked Potential. DHEW (NIOSH) 77–124. Cincinnati, OH: U.S. Department of Health, Education, and Welfare, National Institute of Occupational Safety and Health.
Putz, V.R., B.L.Johnson, and J.V.Setzer. 1979. A comparative study of the effects of carbon monoxide and methylene chloride on human performance. J. Environ. Pathol. Toxicol. 2(5):97–112.
Ramsey, J.M. 1973. Effects of single exposures of carbon monoxide on sensory and psychomotor response. Am. Ind. Hyg. Assoc. J. 34(5):212–216.
Raven, P.B., B.L.Drinkwater, R.D.Ruhling, N.Bolduan, S.Taguchi, J.Gliner, and S.M. Horvath. 1974. Effect of carbon monoxide and peroxyacetyl nitrate on man’s maximal aerobic capacity. J. Appl. Physiol. 36(3):288–293.
Ray, A.M., and T.H.Rockwell. 1970. An exploratory study of automobile driving performance under the influence of low levels of carboxyhemoglobin. Ann. N.Y. Acad. Sci. 174(1):396–408.
Schulte, J.H. 1963. Effects of mild carbon monoxide intoxication. Arch. Environ. Health 7:524–530.
Shiotsuka, R.N., R.T.Drew, and R.W.Wehner. 1984. Carbon monoxide enhances development of hypertension in Dahl rats. Toxicol. Appl. Pharmacol. 76(2):225– 233.
Sievers, R.F., T.I.Edwards, and A.L.Murray. 1942. A Medical Study of Men Exposed to Measured Amounts of Carbon Monoxide in the Holland Tunnel for 13 Years. Public Health Bulletin No. 278. Washington, DC: U.S. Government Printing Office.
Spencer, P.S., and H.H.Schaumburg, eds. 2000. Experimental and Clinical Neurotoxicology, 2nd Ed. New York: Oxford University Press.
Stender, S., P.Astrup, and K.Kjeldsen. 1977. The effect of carbon monoxide on cholesterol in the aortic wall of rabbits. Atherosclerosis 28(4):357–367.
Stern, F.B., W.E.Halperin, R.W.Hornung, V.L.Ringenburg, and C.S.McCammon. 1988. Heart disease mortality among bridge and tunnel officers exposed to carbon monoxide. Am. J. Epidemiol. 128(6):1276–1288.
Stewart, R.D. 1975. The effect of carbon monoxide on humans. Annu. Rev. Pharmacol. 15:409–423.
Stewart, R.D. 1976. Proceedings: the effect of carbon monoxide on humans. J. Occup. Med. 18(5):304–309.
Stewart, R.D., P.E.Newton, M.J.Hosko, and J.E.Peterson. 1973. Effect of carbon monoxide on time perception. Arch. Environ. Health 27(3):155–160.
Stewart, R.D., J.E.Peterson, E.D.Baretta, R.T.Bachand, M.J.Hosko, and A.A. Herrmann. 1970. Experimental human exposure to carbon monoxide. Arch. Environ. Health. 21(2):154–164.
Theodore, J., R.D.O’Donnell, and K.C.Back. 1971. Toxicological evaluation of carbon monoxide in humans and other mammalian species. J. Occup. Med. 13:242–255.
Thom, S.R. 1990. Carbon monoxide-mediated brain lipid peroxidation in the rat. J. Appl. Physiol. 68(3):997–1003.
Thom, S.R. 1992. Dehydrogenase conversion to oxidase and lipid peroxidation in brain after carbon monoxide poisoning. J. Appl. Physiol. 73(4):1584–1589.
Vogel, J.A., and M.A.Gleser. 1972. Effect of carbon monoxide on oxygen transport during exercise. J. Appl. Physiol. 32(2):234–239.
Vogel, J.A., M.A.Gleser, R.C.Wheeler, and B.K.Whitten. 1972. Carbon monoxide and physical work capacity. Arch. Environ. Health 24(3):198–203.
Vyskocil, A., M.Tusl, and K.Zaydlar. 1986. The effect of carbon monoxide on hormone levels and organ weights in rats. J. Appl. Toxicol. 6(6):443–446.
WHO (World Health Organization). 1999. Pp. 1–18 in Carbon Monoxide, Environment al Health Criteria 213, 2nd Ed. Geneva: WHO.
Winneke, G., G.Fodor, and H.Schlipkoter. 1978. Carbon monoxide, trichloroethylene, and alcohol: reliability and validity of neurobehavioral effects. Pp. 461–469 in Multidisciplinary Perspectives in Even-Related brain Potential Research, Proceedings of the Fourth International Congress on Event-Related Slow Potentials of the Brain (EPICIV), Univ. of NC and U.S. EPA, Hendersonville, NC, April 4–10, 1976, D.P.Otto, ed. EPA-600/9–77–043. Research Triangle Park, NC: U.S. Environmental Protection Agency.
Wright, G., P.Randell, and R.J.Shephard. 1973. Carbon monoxide and driving skills. Arch. Environ. Health 27(6):349–354.
Zhang, J., and C.A.Piantadosi. 1992. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J. Clin. Invest. 90(4):1193–1199.




























