DAVID RITTENBERG
November 11, 1906–January 24, 1970
BY DAVID SHEMIN AND RONALD BENTLEY
DAVID RITTENBERG WAS a leader in the development of the isotopic tracer technique for the study of biochemical reactions in intermediary metabolism. In a brief but historic paper published in Science in 1935, Rittenberg and Rudolph Schoenheimer described work at the Department of Biochemistry at Columbia University’s College of Physicians and Surgeons. Their pioneering experiments used deuterium, 2H, the heavy, stable isotope of hydrogen, to trace the fate of various compounds in the animal body. The metabolites containing 2H had properties essentially indistinguishable from their natural analogs by the methods commonly used. Nevertheless, the presence of the isotope made it possible to trace their metabolic fate. Thus, if a 2H-containing compound, B, was isolated after feeding the 2H-labeled compound, A, to an animal, the metabolic conversion A → B was established. Prophetically, these authors noted that “the number of possible applications of this method appears to be almost unlimited.” Subsequent developments have shown that they were true prophets.
In the mid-1930s little was known about the chemical reactions used by living systems to synthesize and degrade cellular components. One difficulty was that methods for the isolation and purification of carbohydrates, lipids, and
proteins were primitive and methods for the detailed study of enzymes were lacking. Moreover, the important role played by comparatively simple molecules with low relative molecular mass in the biosynthesis of more complex molecules was not yet appreciated. In the first of 15 papers published in the Journal of Biological Chemistry with the general title “Deuterium as an Indicator in the Study of Intermediary Metabolism” (“the deuterium series”), Schoenheimer and Rittenberg elaborated as follows on other problems inherent in metabolic studies (1935).
The study of the metabolism of substances which occur in nature in large amounts and are continually synthesized and destroyed in the animal body presents almost insuperable difficulties. If substances such as natural fatty acids, amino acids, etc., are administered to an animal, we lose track of them the moment they enter the body, since they are mixed with the same substances already present. Furthermore, if a substance A is given to an animal and an excess of a substance B is afterwards discovered in the body or in the excretions, we can never be sure that the substance A has been converted into B, for a stimulation of the formation of B from some other source may equally well have occurred. The difficulty in following physiological substances in the course of their transportation in the body, and their conversion into other substances, accounts for our ignorance with respect to many of the most fundamental questions concerning intermediate metabolism. The solution of these problems will be possible only when direct methods for tracing such substances are available.
The isotope work at the College of Physicians and Surgeons (P&S) provided a realistic experimental method that overcame many of the difficulties and did provide for direct tracing of metabolites.
While there had been previous uses of tracers in biology, both chemical and isotopic, the P&S results reported initially in 1935 were the first in which an isotope was systematically introduced into an organic compound so that a defined reaction could be studied. For example, cholest4-en-3-one was reduced with 2H to provide [4,5-2H2]-5ß-
cholestan-3-one. The latter metabolite was converted to 2H-containing coprosterol (5ß-cholestan-3ß-ol) in both a dog and a human subject, thus indicating that the biological reduction of the ketone function in cholestan-3-one was possible.
Although humans had ingested 2H2O previously, this historic experiment was the first in which a substrate, specifically labeled with 2H, was ingested by a human. The experimental detail deserves to be noted, if only for the ingenious use of another biological indicator system.
A healthy man, 24 years of age, took 1 g of the ketone at 5 o’clock in the afternoon on two successive days. The substance was dissolved in butter and taken with bread. 5 Hours before the first, and five hours after the last coprostanone meal he ate grapes, the seeds of which served as an indicator in the stools…. The isolation of the sterols was performed on the portion of stool excreted between the two grape seed markers, in which the greatest concentration of the newly formed coprosterol was to be expected.
The brave volunteer, not identified in 1935, was actually Hans Hirschmann; the conversion was presumably accomplished by his intestinal bacterial flora. In any case, he survived the experiment and later obtained a Ph.D. degree with O.Wintersteiner at P&S. Hirschmann had a distinguished subsequent career at Case Western Reserve University, retiring as emeritus professor in 1978.
Rittenberg had found himself at P&S through the convergence of several unusual circumstances. At Columbia University’s main campus Harold Urey had identified and isolated deuterium in 1932. Rittenberg, one of Urey’s graduate students, obtained a Ph.D. degree in 1934 for his 30-page thesis, “Some Equilibria Involving Isotopes of Hydrogen.” Urey was anxious to promote the study of both chemical and biological properties of deuterium, and received funding from the Rockefeller Foundation. In 1934 Rittenberg had no prospects of a position as a physical chemist. He
was instead assigned to H.T.Clarke, chair of the Department of Biochemistry at P&S to promote biological uses of 2H. At that time one interest of Clarke’s was the possibility of demonstrating optical activity for a compound, Cab1H2H. Clarke also suggested to Rittenberg a “roving commission” to talk with other members of the department about possible uses of 2H. These developments have been reviewed comprehensively by the historian Robert E.Kohler (Historical Studies in Physical Sciences, vol. 8, pp. 257–98, R. McCormmach and L.Pyerson, eds., Johns Hopkins University Press, Baltimore, London, 1977).
A second circumstance was that Schoenheimer, forced from his position as head of the Institute of Pathology at the University of Freiburg by the policies of the Third Reich, had accepted a position in Clarke’s department in 1933. There he continued a long-standing interest in sterol metabolism. When Rittenberg presented to him the prospect of the use of 2H Schoenheimer quickly appreciated the possibilities. Schoenheimer had, in fact, some prior experience, having worked with Hevesy on the partition of radioactive lead between normal and tumor tissue. Clearly, the meeting of these two individuals was propitious. While Schoenheimer could formulate problems in metabolism, Rittenberg was perhaps the only individual at that time with the necessary background and training to tackle the difficult experimental details required for biochemical work with 2H. This was still the era of “string and sealing wax,” when experimenters used glass blowing and workshop skills to make a necessary apparatus. To appreciate Rittenberg’s skill in that art one has only to consult the methods articles that he wrote with elegant figures of complex glassware. In particular, the second part of the deuterium series contains methods for the generation of 2H as gas; the combustion of samples to water, followed by purification by distillation; and the use of the
submerged-float technique to determine density and hence 2H content. In 1937 measurement of the refractive index of water samples and the falling-drop technique were added to the methods.
Significant results with the new technique were obtained very quickly after Rittenberg’s approach to Schoenheimer sometime in 1934. Before the brief announcement of the use of deuterium as an indicator in the August 16, 1935, issue of Science, the first four papers of the deuterium series had already been submitted to the Journal of Biological Chemistry (June 26, 1935) and they appeared in volume 111 (1935) of that journal. The deuterium series continued until 1938. Over that period, results obtained in connection with fatty acid metabolism were more interesting than those concerned with sterol metabolism. The degradation and synthesis of saturated fatty acids was shown to proceed two carbon atoms at a time, and saturated fatty acids could be converted to monounsaturated fatty acids and vice versa. A particularly significant observation was that when mice were fed 2H-labeled fatty acids, most of the 2H was recovered in the fat tissues rather than being immediately utilized. Of the 15 papers in the deuterium series only the last was not coauthored by Rittenberg, and 8 of them were authored only by Rittenberg and Schoenheimer.
By 1937, again thanks to Urey, the stable, heavy isotope of nitrogen, 15N, became available and opened up the investigation of nitrogen metabolism. Rittenberg was now confronted with a new challenge. At that time the only practicable method for 15N assay required a mass spectrometer, an instrument not then commercially available. Rittenberg, with the help of I.Sucher, A.Keston, and F.Rosebury, constructed at P&S a 180° mass spectrometer of the Bleakney type similar to that used by Urey. For assay a sample of dinitrogen gas was bled into the high-vacuum system of the
mass spectrometer through a glass capillary leak. The size of the leak was important. The international standard was eventually defined as the diameter of a hair from Rittenberg’s head. Inside the spectrometer the intensities of ions due to 14N14N (m/z ratio=28) and 14N15N (m/z ratio=29) were determined.
It was necessary to devise means whereby nitrogen of a metabolite (e.g., an amino acid) could be converted to dinitrogen. A two-step process was used. Ammonia from a Kjeldahl digestion was trapped in a dilute solution of H2SO4. The ammonium salt was then oxidized by sodium hypobromite:
2 NH4+ + 2 OH- + 3 NaOBr → N2 + 3 NaBr + 5 H2O
The reaction had to be carried out in a vacuum system. To mix the reagents a rather cumbersome rotating flask was used initially. Finally, Rittenberg devised a two-legged, Y-shaped tube (see Figure 1). After freezing the contents of each leg and evacuation on a separate vacuum line the two solutions were thawed and mixed; following a further freezing the tube was attached to the mass-spectrometer vacuum system. While the tubes were generally known as Rittenberg tubes, their characteristic shape gave rise to the name “Rittenberg trousers” in Israel. With his excellent glass-blowing capabilities Rittenberg could always locate and repair any pinhole leaks in a vacuum system with the aid of a Tesla coil. Drawings of the Kjeldahl apparatus and of the rotating flask set up were provided by Rittenberg in 1946.
The first 15N work at P&S was a study of hippuric acid metabolism in 1937. By this time the isotope group had expanded and there were five authors on the paper (Schoenheimer, Rittenberg, M.Fox, A.S.Keston, S.Ratner). Ammonia was found to be converted to both glycine and
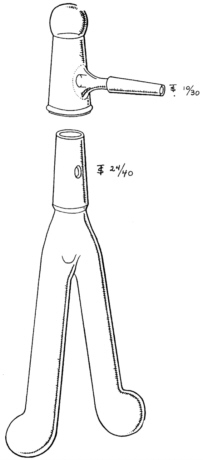
FIGURE 1 This elegant drawing of a Rittenberg tube was provided in the article on gas sample preparation published by Sprinson and Rittenberg (1948).
hippuric acid in animals. The hippuric acid was absorbed from the intestinal tract without hydrolysis and glycine was used directly for hippurate formation. In a final sentence it was confidently asserted that “the nitrogen isotope should prove to be as useful in the study of the intermediary metabolism of nitrogen compounds as deuterium is in the study of fat and sterols.” Descriptions of the use of isotopes in metabolic studies and specifically of the use of 15N were provided for a general scientific audience in Science in 1938 and 1939. A series of papers with the general title
“Studies in Protein Metabolism” was published between 1937 and 1941 and, like the deuterium studies, also ran to 15 papers.
In September 1941 Schoenheimer took his own life. By that time the 2H work on lipids and the 15N experiments on proteins had led to a new biochemical generalization: the dynamic state of body constituents. The large, complex macromolecules were constantly involved in rapid chemical reactions with their smaller component units, a continuing and constant process of degradation and resynthesis. The generalization overthrew the prevailing opinion that the dietary constituents were used only for repair and for energetic purposes. Schoenheimer was to have delivered three Edward K.Dunham lectures on this topic at Harvard in October 1941. His lecture drafts were revised by Clarke, Ratner, and Rittenberg and the lectures were presented by Clarke. The book form of these lectures ran to two editions and is a classic in biochemical history.
In 1941 Rittenberg was appointed director of the isotope laboratory. The research group included many individuals who later became well known, such as K.Bloch, S.Ratner, D.Shemin, and D.Sprinson. The areas of investigation widened and other stable isotopes, 13C and 18O, became available. Rittenberg and H.Waelsch did the first 13C experiment at P&S in 1940: a study of the conversion of NaH13CO3 to urea. Not until 1949 was work with 18O reported by Bentley (but carried out with the constant support and enthusiasm of Rittenberg): a study of the mechanism of hydrolysis of acetyl phosphate. There was a decline in the use of stable isotopes beginning about 1950 as radioactive isotopes also became readily available following the end of World War II.
In 1937 during the early exciting days of work with 2H, Rittenberg and Schoenheimer made an observation that
had bountiful repercussions. When 2H2O was administered to mice, it was found that 50 percent of the hydrogen atoms of cholesterol derived from the hydrogen atoms of the water. This high level of 2H incorporation eliminated large molecules (e.g., sterols) as cholesterol precursors. With considerable foresight Rittenberg and Schoenheimer stated that cholesterol “is formed by the coupling of smaller molecules, possibly those which have been postulated to be intermediates in the fat and carbohydrate metabolism.” In another laboratory, yeast grown in the presence of [2-2H] acetate contained a high 2H level in unidentified sterols. Following this observation, Bloch (a Ph.D. student with Schoenheimer) and Rittenberg proved in 1942 that [2-2H] acetate was actually a cholesterol precursor in mice and rats. A little later cholesterol biosynthesis was demonstrated by Bloch, Borek, and Rittenberg in surviving liver slices from both [2-2H] acetate and [2-2H, 1-13C] acetate. In subsequent work at the University of Chicago and at Harvard, Bloch uncovered many details of the biosynthetic pathway from acetate to cholesterol and received a Nobel Prize in 1964 (jointly with F.Lynen) for his work. The beginnings of this work clearly owed much to Rittenberg. Moreover, Rittenberg and Bloch demonstrated for the first time a role for acetate in the biosynthesis of fatty acids (1944).
Work reported by Shemin and Rittenberg in 1945 on the utilization of glycine also led to important findings. Shemin himself, with much courage, ingested over a three-day period 66 g of [15N]glycine. At stated intervals blood was withdrawn and the 15N concentration of blood proteins was determined. The data were consistent with the concept of the dynamic state of body constituents. However, when the 15N content of the heme component of hemoglobin was examined, it appeared to reach a maximum value about 20 days after the start of the experiment. Thereafter, it
remained approximately constant for 80 days and then declined. It was clear that hemoglobin was not in a dynamic state. From the data the life span of the human red blood cell could be calculated; it was found to be 127 days, much higher than the then quoted figure of 30 days. There was also an important bonus; it could be concluded that glycine was the nitrogenous precursor of heme and other porphyrins.
There were two elaborations as a result of this work. With an accurate and physiological method for determining the life span of the red blood cell, Shemin and Rittenberg organized a collaborative program with I.M.London and R.West (Department of Medicine, P&S) to study the life span of red blood cells in patients with blood abnormalities. The dynamics of red cell survival in patients with polycythemia vera, sickle cell anemia, and pernicious anemia were described in several papers. Rittenberg was also an author on a paper dealing with porphyrin formation and hemoglobin metabolism in congenital porphyria.
The second elaboration was that Rittenberg, Shemin, and London began to study the synthesis of protoporphyrin in vitro using immature non-nucleated mammalian erythrocytes and the red blood cells of the duck. Particularly with the latter system, Shemin and his colleagues elucidated the pathway for porphyrin biosynthesis in the red cell. N.Radin and Shemin, in collaboration with Rittenberg, studied the roles of both glycine and acetate in heme biosynthesis.
A further achievement of the isotope work was a method for assay of amino acids in protein hydrolysates with a very low error. Since the usual laboratory methods do not result in isotope separation, a measurement of the dilution of a labeled compound added to an unlabeled mixture provided the amount of substance originally present. The method depended only on the isotope content of added and iso-
lated material, and the weight of added isotopic material. Hence the amount of isolated substance did not need to be determined and large losses during isolation could be tolerated. The method was first described by Rittenberg and G.L.Foster in 1940. Although originally used for amino acid and fatty acid analysis, the method has general application.
After 1950 Rittenberg’s research was scaled back on account of administrative responsibilities (see below). Even so, he used glucose labeled with 18O and 13C to determine the contribution of the oxidative and nonoxidative pathways for pentose formation. His analysis of the enzyme hydrogenase in Proteus vulgaris and other microorganisms no doubt was influenced by his work as a graduate student. It was demonstrated in work with A.Krasna that the enzymatic cleavage of hydrogen is a heterolytic process, whereas the same cleavage catalyzed by platinum is homolytic.
This brief and incomplete account of the isotope work at P&S has focused on the many key contributions made by Rittenberg and on published papers that he coauthored. Many other workers in the Department of Biochemistry benefited from his advice. For instance, in several papers DeWitt Stetten acknowledged help and cooperation from Rittenberg. David Sprinson worked with Rittenberg on ammonia utilization for protein synthesis and the rate of reaction of dietary amino acids with tissue proteins before going on to the major, independent study of the pathway for shikimic acid biosynthesis, for which he received much recognition.
Historian Robert Kohler has pointed out that the isotope work at P&S “entailed a special social organization: the interdisciplinary group. An organic chemist was needed to synthesize labeled compounds; a physical chemist to build and operate the complex instruments for measuring isotope ratios; a physicist to provide concentrated heavy isotopes
(or radioactive isotopes from a cyclotron); and a biochemist to work with metabolism in animals.” In fact the group at P&S set the trend to interdisciplinary research efforts. There was a definite progression from the early experiments by Schoenheimer and Rittenberg working by themselves, and then to the large, interdisciplinary group. In all of this Rittenberg can be fairly described as the keystone. Eventually, with the development of commercial instruments and supply sources, a biochemist alone could hope to perform these specialized tasks.
The man who became the leader of the isotope group at P&S and made many contributions to biochemical knowledge was born in New York City on November 11, 1906. He was educated in public schools and received a B.S. degree from the College of the City of New York in 1929. That year marked the beginning of the Great Depression, and he must have encountered financial difficulties. He once claimed that he had made money as a consultant to a bootlegger making bathtub gin. He worked as a refractories chemist for two years at the Nonmetallic Minerals Experiment Station, U.S. Bureau of Mines, at Rutgers University. This work led to his first scientific publication with P.S.Roller: a method for the production of refractory crucibles of magnesium oxide that were recrystallized to translucence and were impervious to air under pressure at room temperature. Following this experience, he became a graduate student with Harold Urey as already noted.
Rittenberg’s contributions to the isotope tracer technique were recognized in 1941 by the Eli Lilly Award in Biological Chemistry from the American Chemical Society, Division of Biological Chemistry. A further recognition was his election to the National Academy of Sciences in 1953. Rittenberg survived Schoenheimer by almost three decades and he served as a faculty member at P&S for 36 years. He was appointed
chair of the Department of Biochemistry in 1956. He had met Chaim Weizmann, founder of the Weizmann Institute of Science in Rehovoth, Israel, in 1944 and was invited to become a member of the Planning Board of that institute. Later he became a member of the Board of Governors and was made an honorary fellow of the institute in 1967. His interest in the development of science in Israel led him to accept an invitation to join the Advisory Board of the Hadassah Medical School. He made important contributions especially in the early development of their science and in ferreting out very capable young scientists.
David Rittenberg had a well-developed sense of humor and a fund of stories. At a 1948 Cold Spring Harbor symposium “Biological Applications of Tracer Elements” he noted that modesty on the part of George Hevesy had prevented him from evaluating his own contributions. He said that “Hevesy, to employ terms suitable to a biological science, was not only one of the fathers of the isotope technique but also the attending gynecologist.” Modesty on Rittenberg’s part did not allow him to describe himself as one of the founding fathers. It has been recorded, however, that he liked to be introduced as “the most refractory chemist remaining in the field of biochemistry.”
No doubt as a young member of the Department of Biochemistry he may have overplayed his hand by being overconfident, thus creating some tension with other department members. To some the introduction of techniques by a brash physical chemist was seen as a threat and several biochemists were suspicious of the isotope technique, especially when it began to require what were for those days large sums of money. It was even hinted that 2H-labeled compounds might be toxic although Hans Hirschmann’s survival should have put that idea to rest. Moreover, there was a feeling that, at least initially, Rittenberg did not know
any biochemistry. In the late 1930s it is said that many believed the papers titled “Studies of Intermediary Metabolism with the Aid of Isotopes” should have been titled “Studies of Isotopes with the Aid of Intermediary Metabolism.” Perhaps it was inevitable that as his responsibilities increased, more tensions were created. Many may have felt that Rittenberg’s success owed much to Schoenheimer’s imagination, without realizing that Schoenheimer owed equally much to Rittenberg. Sarah Ratner has noted that toward the end of the relationship, some unpleasantness developed between Rittenberg and Schoenheimer.
David Rittenberg—born, raised, and educated in New York City—was a quintessential New Yorker. Almost all of his working life was spent in that city and once at P&S he never moved to a different institution. An anecdote illustrates his love-hate relationship with New York. When a visitor from the U.K. twice failed a New York driving test, Rittenberg offered the following advice for attempt number three: “Arrive late for the test and apologize that you were detained at Mass. Hand the examiner a cigar wrapped in a ten dollar bill. You will have no further trouble.” Needless to say, the visitor did not feel able to accept the advice. David Rittenberg and his wife, Sara, were unfailingly generous in extending hospitality to visitors to the department. Many individuals remember superb meals prepared by Sara. As a youth Rittenberg had contracted rheumatic fever and his death on January 24, 1970, was from heart disease. In his personal life, David Rittenberg was a very proud father. His son, Stephen, a 1963 graduate of P&S, is now in practice as a psychoanalyst. Moreover, two gradchildren have also become physicians.
David Rittenberg was uniquely qualified for a challenging task at a particular time and place. He fulfilled his role with skill and honor. In 1935 isotopes were strange entities,
exciting considerable interest and even generating suspicion. Almost seven decades later they are widely accepted and require no special comment. They are defined in non-specialist dictionaries and are often referred to in the press. Isotopes find extensive applications not only in biological sciences but also in such fields as chemistry, environmental studies, medicine (both in diagnosis and treatment), and physics. The darker side is that some radioactive isotopes have very adverse effects in humans. Rittenberg had unbounded optimism and enthusiasm and clearly anticipated great future developments for his isotopes. He could probably not have foreseen how extensive and important the developments would be. For example, when he went to P&S in 1934, one possible research project was to determine if the 1H → 2H substitution would give rise to measurable optical activity in the chiral substance, Cab1H2H. While this possibility has now been long established, it has also become possible to investigate stereochemical problems in compounds containing all three hydrogen isotopes, Ca1H2H3H. Work with “chiral methyl groups” is now well known and similar work with the three oxygen isotopes has concerned “chiral phosphate groups” in compounds such as RO-P16O17O18O. David Rittenberg would have been pleased and excited by such possibilities and by the ever expanding role of isotopes in the modern world.
SELECTED BIBLIOGRAPHY
(Not including abstracts, David Rittenberg and his colleagues published more than 135 research papers. A more complete listing may be obtained by contacting R.Bentley by e-mail at rbentley@pitt.edu).
1933 With H.C.Urey. Some thermodynamic properties of the H1H2, H2H2 molecules and compounds containing the H2 atom. J. Chem. Phys. 1:137–43.
1935 With R.Schoenheimer. Deuterium as an indicator in the study of intermediary metabolism. I. J. Biol. Chem. 111:163–68.
1937 With R.Schoenheimer. Deuterium as an indicator in the study of intermediary metabolism. IX. The conversion of stearic acid into palmitic acid in the organism. J. Biol. Chem. 120:155–65.
With R.Schoenheimer. Deuterium as an indicator in the study of intermediary metabolism. XI. Further studies on the biological uptake of deuterium into organic substances, with special reference to fat and cholesterol formation. J. Biol. Chem. 121:235–53.
With R.Schoenheimer et al. The nitrogen isotope (N15) as a tool in the study of the intermediary metabolism of nitrogenous compounds. J. Am. Chem. Soc. 59:1768.
1938 With R.Schoenheimer. The application of isotopes to the study of intermediary metabolism. Science 87:221–26.
1939 With R.Schoenheimer and S.Ratner. The process of continuous deamination and reamination of amino acids in the proteins of normal animals. Science 89:272–73.
With R.Schoenheimer. Studies in protein metabolism. I. General considerations in the application of isotopes to the study of protein metabolism. The normal abundance of nitrogen isotopes in amino acids. J. Biol. Chem. 127:285–90.
1942 With K.Bloch. The utilization of acetic acid for cholesterol formation. J. Biol. Chem. 145:625–36.
With R.Schoenheimer et al. The interaction of the blood proteins of the rat with dietary nitrogen. J. Biol. Chem. 144:541–44.
1944 With K.Bloch. Sources of acetic acid in the animal body. J. Biol. Chem. 155:243–54.
With D.Shemin. Some interrelationships in general nitrogen metabolism. J. Biol. Chem. 153:401–21.
1945 With D.Shemin. The utilization of glycine for the synthesis of a porphyrin. J. Biol. Chem. 159:567–68.
With K.Bloch. The utilization of acetic acid for the synthesis of fatty acids. J. Biol. Chem. 160:417–24.
1946 With D.Shemin. The metabolism of proteins and amino acids. Annu. Rev. Biochem. 15:247–72.
With D.Shemin. The life span of the human red blood cell. J. Biol. Chem. 166:627–36.
1948 Dynamic aspects of the metabolism of amino acids. Harv. Lect. 44:200–219.
With I.M.London and D.Shemin. The in vitro synthesis of heme from glycine by the nucleated red blood cell. J. Biol. Chem. 173:799–800.
1950 With D.Shemin and I.M.London. The synthesis of protoporphyrin in vitro by red blood cells of the duck. J. Biol. Chem. 183:757–65.
With N.S.Radin and D.Shemin. The role of acetic acid in the biosynthesis of heme. J. Biol. Chem. 184:755–67.
1953 With A.San Pietro. A study of the rate of protein synthesis in hu
mans. II. Measure of the metabolic pool and the rate of protein synthesis. J. Biol. Chem. 201:457–73.
1956 With A.I.Krasna. A comparison of the hydrogenase activities of different microorganisms. Proc. Natl. Acad. Sci. U.S.A. 42:180– 85.
With L.Ponticorvo. A method for the determination of the O18 concentration of the oxygen of organic compounds. Int. J. Appl. Radiat, Isot. 1:208–14.
1962 With L.Ponticorvo. On the quantitative significance of the pentose pathway in Escherichia coli. J. Biol. Chem. 237:PC2709–10.
1963 With J.C.Sadana. Some observations of the enzyme hydrogenase of Desulfovibrio desulfuricans. Proc. Natl. Acad. Sci. U.S.A. 50:900– 904.
1969 With R.Caprioli. Pentose synthesis in Escherichia coli. Biochemistry 8:3375–84.





















