Background
In the United States, the purchase of vaccines and the delivery of immunization services depend on a complex mix of public and private funding and services. Although immunization coverage rates reached high levels during the past few decades, an outbreak of measles in 1989-1991 drew attention to the continuing health threat from vaccine-preventable diseases when immunization rates are low. Following the measles outbreak, the federal government substantially increased funds for immunization infrastructure grants to states through the Section 317 program administered by CDC. The Vaccines for Children (VFC) program was implemented in 1994 to fund the purchase of vaccine for qualifying children, primarily those enrolled in Medicaid or without health insurance. States also continued to receive federal funds through the Section 317 program for the purchase of vaccine, an arrangement that dates back to the early 1960s.
Despite the significant increases in federal support, many states found it difficult to expend their infrastructure awards during the one-year grant period. Large amounts of state grants were “carried over” to subsequent years. By 1996, the U.S. Congress cut back funding for Section 317 infrastructure grants, indicating that it was not certain that the states needed, or could manage, federal assistance in this area.
The 1990s also saw the addition of new, more expensive vaccines to the recommended schedule of immunizations. Health care reforms resulted in an increasing reliance on health care providers in the private sector for the delivery of immunization services, accompanied by funda-
mental changes in the larger health care delivery system resulting from the growth of managed care. In the wake of these changes, the roles of state and local public health departments became more ambiguous and more complex. The need for direct services diminished as private providers and health plans gradually acquired the capacity and resources to deliver primary care services—including immunizations—to disadvantaged groups. But a new public health role emerged, one that places greater emphasis on the need for data management and the development of community-level health indicators (IOM, 1996, 1997). This role requires multi-sector collaboration so that public agencies can assess coverage rates within small-area samples and respond to specific health care needs when the private sector is not able—or willing—to absorb the costs involved in sustaining high immunization coverage rates among hard-to-reach populations.
THE IOM STUDY
In 1998, Congress asked IOM to conduct a study of the Section 317 program and of broader questions regarding appropriate levels of effort to achieve national immunization goals. The study addressed six questions formulated by Congress and the Centers for Disease Control and Prevention. The study committee met during 1999-2000 to collect relevant information and to develop a framework to guide its deliberations.
Fact-finding for the study included several separate efforts. First, a research team directed by Gary Freed at the University of Michigan conducted a series of structured telephone interviews with immunization program officials in all 50 states regarding the effects of changes in federal policies and funding in the 1990s on the goals, priorities, and activities of state immunization programs (Freed et al., 2000). Second, IOM staff and consultants developed eight case studies of state immunization efforts, focusing on the states of Alabama, Maine, Michigan, New Jersey, North Carolina, Texas, Washington, and the counties of San Diego and Los Angeles in California (Fairbrother et al., 2000a).1 Third, members of the IOM committee, staff, and consultants also conducted four site visits in Detroit, Newark, Houston, and Los Angeles to supplement the case study materials with discussions with local providers and immunization program representatives. Fourth, the committee organized an IOM workshop on issues related to “pockets of need” in Washington, D.C., in Sep-tember 1999. Fifth, the committee commissioned background papers on
|
1 |
Each case study is available electronically via the website of the National Academy Press: www.nap.edu/html/case_studies. |
topics of adult immunization, registries, measuring immunization coverage (Fairbrother et al., 2000b), and federal immunization policy (Johnson et al., 2000) to supplement material available in the research literature. Selected materials from the case study reports and commissioned papers were published in a supplemental issue of The American Journal of Preventive Medicine in October 2000.
IOM FINDINGS AND RECOMMENDATIONS
The IOM report Calling the Shots recommends a renewal and strengthening of the federal-state partnership that is a fundamental element of the national immunization system. The report also recommends strategic investments in immunization efforts and closer collaboration between public and private health care systems to coordinate immunization roles and responsibilities in the wake of health care reforms.
In opening remarks at the Chicago workshop, David Smith highlighted the key findings and recommendations from the IOM study.2 The study committee identified six fundamental roles for the nation’s immunization system:
-
Assure the purchase of recommended vaccines for the total population of U.S. children and adults, with particular emphasis on the protection of vulnerable groups.
-
Assure access to vaccines within the public sector when private health care services are not adequate to meet local needs.
-
Control and prevent infectious disease.
-
Conduct populationwide surveillance of immunization coverage levels, including the identification of significant disparities, gaps, and vaccine safety concerns.
-
Sustain and improve immunization coverage levels within child and adult populations, especially in vulnerable communities.
-
Use primary care and public health resources efficiently in achieving national immunization goals.
The IOM study committee used this framework to guide its finance recommendations. The report concluded that adequate, stable, and pre-dictable funding was necessary for the development of effective state immunization programs and that the fluctuations in Section 317 infrastructure funding during the 1990s made it difficult for states to achieve
|
2 |
Several speakers in the workshop used visual aids in their presentations. These materials are available in electronic form at the workshop website: www.iom.edu/iom/iomhome.nsf/Pages/HCS+Immunization+Finance+dissemination. |
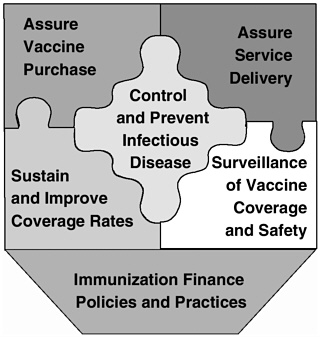
FIGURE 1 Six roles of the national immunization system.
program goals. Furthermore, with only a one-year grant period, many state immunization programs could not invest in multi-year programs with consultants or contractors to support long-term strategic planning or data collection efforts.
The committee also concluded that immunization policy should be national in scope but flexible enough to accommodate important political, socioeconomic, and structural differences among states and communities. Furthermore, federal and state governments share responsibility for supporting vaccine purchase and the infrastructure essential for achieving and sustaining national immunization goals. Data reviewed for the study showed that some states appeared to provide little or no state funding for immunization while others invested substantially more than they received from federal sources. Finally, the private sector, through health plans and individual health care providers, has the capacity to do more to ensure the delivery of appropriate immunization services to its members and patients, but such efforts do not replace the need for a more diverse public health infrastructure capable of assuring that the immunization needs of the whole population are addressed.
The study recommendations addressed federal and state funding levels, grant mechanisms for immunization programs, and the need for better measurement of immunization coverage. The committee recommended an increase in both federal and state budgets for support of immunization programs, but concluded that annual budgets for vaccine purchase for children have been adequate (this finding was made prior to the addition of the pneumococcal conjugate vaccine to the general schedule for children). The committee also recommended an increase in both federal and state budgets for the purchase of adult vaccines to provide additional vaccine for high-risk adolescents and adults under age 65 who do not qualify for other federal assistance.
In addition to budgetary increases, Dr. Smith noted that the IOM report proposed new operational and reporting requirements for federal grants to improve administrative efficiency, linking them to the six fundamental roles of the national immunization system. The committee recommended that CDC should distribute Section 317 awards to states through a formula grant mechanism, with the formula reflecting essential minimum funding levels and state need, capacity, and performance. In addition, a state match requirement should be added and the federal grants should have a two-year budget cycle to give states greater flexibility to plan and implement multi-year efforts.
Finally, the IOM report recommended that federal and state agencies should develop a set of consistent and comparable measures for use in monitoring the immunization status of children and adults enrolled in private and public health plans as well as populations in defined geo-graphic areas. For example, it would be valuable to harmonize the immunization measures of the Health Plan Employer Data and Information Set (HEDIS) and the National Immunization Survey (NIS).
Lance Rodewald and other representatives from the National Immunization Program of the Centers for Disease Control and Prevention described steps that have been taken to respond to the IOM recommendations (for further details, see www.cdc.gov/nip/news/iom-rpt-6-00.htm). CDC has revised the Section 317 grant guidance and program operations manual, giving states greater flexibility under the six broad immunization system roles outlined by the IOM study to tailor programs to local needs. In addition, discussions have begun to develop a funding formula and to improve the harmonization of HEDIS and the NIS.





