B
Animal Reproductive Cloning Data Tables on Reproductive Cloning Efficiency and Defects
The purpose of these tables is to provide an overview of the data from animal cloning experiments done to date (August 2001). Table 1 describes the success/failure rates of reproductive cloning in animals, and Table 2 provides details of the defects or lack of observable defects in reproductively cloned animals. These data were obtained through a comprehensive review of the publications cited in the “Reference” column of each table. Only experiments that yielded live-born cloned offspring were included in the table.
Tables 1 and 2 developed by the panel are supplemented by Tables 3 and 4 developed by Lewis et al., 2001. Note that Tables 3 and 4 use the term “cytoplast” for what the panel calls “enucleated egg.”
How to read Table 1:
Example: The first line from the table can be read as following:
In the experiments described in the paper published by Campbell in 1996 (Column 12), 244 sheep embryos were created using somatic cell nuclear transplantation techniques. The donor nuclei were taken from epithelial-like cells grown from a culture of embryonic stem cells (Column 2). Of these 244 embryos, only 34, or 14%, went on to develop into the morula or blastocyst embryos that are used in the embryo transfer procedure (Column 4). All 34 of those developing embryos were transferred into the wombs of female sheep (as we can tell from Column 8, which indicates
number of embryos transferred). Of those 34 embryos, only 8 individual pregnancies resulted (Column 5). Of those 8 pregnancies, 3, or 38%, ended in miscarriage, and 5, or 63%, went on to produce live offspring (Columns 6 and 7, respectively). Of the five lambs that were born alive, only 2 (40%) survived until the time of publication. In all, 2% of the 244 embryos created resulted in live offspring (Column 9), and 12.5% of the 34 embryos transferred into recipient female sheep resulted in live offspring (Column 8).
How to read Table 2:
Any given line in Table 2 gives an overview of the clinical outcomes of each animal reproductive cloning experiment. For example, in line 1, in the sheep nuclear transplantation experiments published by Campbell in 1996 (Column 7), no information was given concerning the defects seen in miscarried fetuses (Column 3) or about the characteristics of placentas from these pregnancies (Column 6). However, Columns 4 and 5 indicate that 2/5 of the cloned lambs produced in this experiment were healthy and normal, whereas 3/5 died of unknown causes.
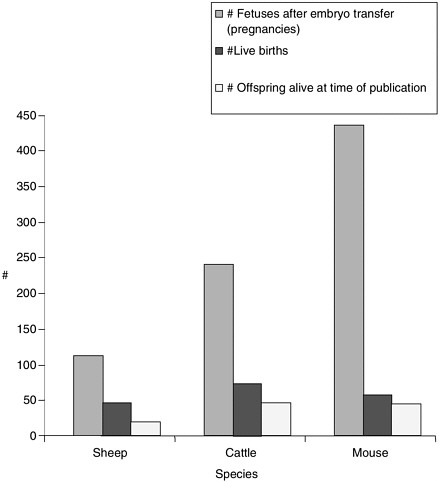
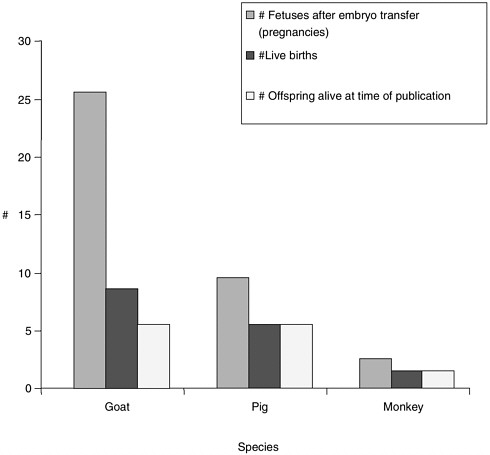
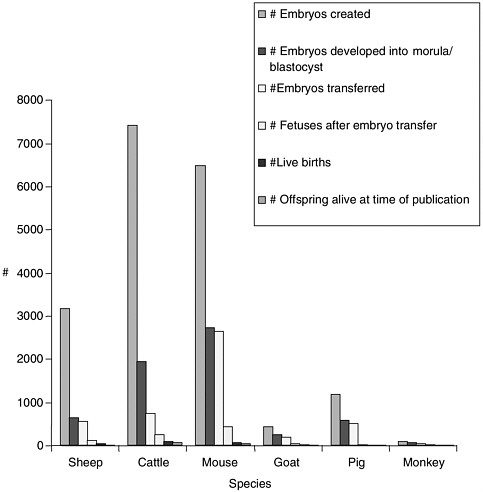
Note about Figures 1, 2, and 3
Figures 1, 2, and 3 were generated based on data presented in Table 1. Certain experiments whose results are displayed in Table 1 were omitted from the graphs due to incomplete data for all categories displayed in the graphs. Data from reproductive cloning experiments using embryonic, fetal and adult cells as nucleus donors were included in these graphs.
TABLE 1 Rates of Success/Failure of Somatic Cell Nuclear Transfer in Mammals
|
1 |
2 |
3 |
4 |
5 |
6 |
|
Speciesa |
Cell typeb |
# Embryos producedc |
# Embryos developed into morula/ blastocyst (%)d |
# Fetuses after embryo transfere |
# Fetuses miscarried (%)f |
|
Sheep |
Embryo-derived epithelial-like |
244 |
34 (14) |
8 |
3 (38) |
|
Adult mammary gland |
277 |
29 (12) |
1 |
0 (0) |
|
|
Fetal fibroblast |
172 |
47 (27) |
5 |
2 (40) |
|
|
Embryo-derived epithelial-like |
385 |
126 (33) |
15 |
11 (73) |
|
|
Fetal fibroblast |
507 |
69 (13.6) |
14 |
7 (50) |
|
|
ES cell line-derived epithelial-like |
128 |
31 (24.2) |
>9 |
>7 (~78) |
|
|
ES cell line-derived epithelial-like |
258 |
44 (17) |
>11 |
>10 (~91) |
|
|
ES cell line-derived epithelial-like |
423 |
75 (18) |
8 |
5 (63) |
|
|
ES cell line-derived fibroblast-like |
158 |
39 (31) |
10 |
7 (70) |
|
|
ES cell line-derived fibroblast-like |
187 |
51 (27) |
15 |
8 (53) |
|
|
Fetal fibroblast |
417 |
80 (19) |
20 |
6 (30) |
|
|
Cattle |
Blastomere (embryonic) |
641 |
152 (24) |
>13 |
>4 (~31) |
|
Blastomere (embryonic) |
132 |
84 (64) |
N/A |
N/A |
|
|
Embryonic stem cell |
239 |
42 (18) |
N/A |
N/A |
|
|
Fetal fibroblast |
276 |
33 (12) |
6 |
2 (33) |
|
|
Adult mural granulosa from 13 yr old cow |
621 |
259 (42) |
28 |
26 (93) |
|
7 |
8 |
9 |
10 |
11 |
12 |
|
# Live births/ Total # fetuses (%)g |
# Live births/ # Embryos transferred to uterus(%)h |
# Live births/ # Embryos produced (%)i |
# Offspring alive or healthy at time of publication/ # Live births (%)j |
Phenotypes observedk |
ReferenceL |
|
5/8 (63) |
5/34 (12.5) |
5/244 (2.0) |
2/5 (40) |
# |
Campbell 1996 |
|
1/1 (100) |
1/29 (3.4) |
1/277 (.36) |
1/1 (100) |
# |
Wilmut 1997 |
|
3/5 (60) |
3/40 (7.5) |
3/172 (1.7) |
2/3 (67) |
E# |
Wilmut 1997 |
|
4/15 (27) |
4/87 (4.6) |
4/385 (1.0) |
4/4 (100) |
# |
Wilmut 1997 |
|
7/14 (50) |
7/67 (10.4) |
7/507 (1.3) |
5/7 (71) |
BC# |
Schnieke 1997 |
|
2/>9 (<22) |
2/31 (6.5) |
2/128(1.6) |
2/2 (100) |
CE# |
Wells 1997 in vivo-matured oocytes |
|
1?>11 (<9) |
1/44 (2.3) |
1/258 (.39) |
0/1 (0) |
BEF |
Wells 1997 in vitro-matured ooctyes |
|
3/8 (38) |
3/75 (4.0) |
3/423 (.7) |
2/3 (67) |
B# |
Wells 1998n experiment 1 |
|
3/10 (30) |
3/39 (7.7) |
3/158 (1.9) |
1/3 (33) |
B# |
Wells 1998n experiment 2 |
|
7/15 (47) |
7/44 (16) |
7/187 (3.7) |
2/7 (29) |
BE# |
Wells 1998n experiment 3 |
|
14/20 (70) |
14/80 (17.5) |
14/417 (3.4) |
3/14 (21) |
E# |
McCreath 2000 |
|
N/A |
9/59 (15) |
9/641 (1.4) |
N/A |
N/A |
Chesne 1993 |
|
N/A |
19/78 (24) |
19/132 (14) |
N/A |
N/A |
Cheong 1993 |
|
N/A |
4/34 (12) |
4/239 (1.7) |
N/A |
N/A |
Sims 1994 |
|
4/6 (67) |
4/28 (14.3) |
4/276 (1.4) |
3/4 (75) |
ABCF# |
Cibelli 1998 |
|
2/28 (7.1) |
2/74 (2.7) |
2/621 (.32) |
1/2 (50) |
CD# |
Wells 1998o |
|
1 |
2 |
3 |
4 |
5 |
6 |
|
Speciesa |
Cell typeb |
# Embryos producedc |
# Embryos developed into morula/ blastocyst (%)d |
# Fetuses after embryo transfere |
# Fetuses miscarried (%)f |
|
|
Adult cumulus |
47 |
18 (38) |
5 |
0 (0) |
|
Adult oviduct epithelial |
94 |
20 (21) |
3 |
0 (0) |
|
|
Adult mural granulosa |
552 |
383 (69) |
45 |
35 (78) |
|
|
Adult mammary gland epithelium |
140 |
36 (26) |
>2 |
>1 |
|
|
Adult ear skin fibroblast |
82 |
49 (60) |
>5 |
>1 |
|
|
Fetal germ cell |
279 |
85 (30) |
>17 |
>16 |
|
|
Fetal fibroblast |
174 |
35 (20) |
>3 |
>1 |
|
|
Adult skin cell from ES cell clone |
175 |
N/A |
1 |
0 (0) |
|
|
Adult muscle |
346 |
73 (21) |
8 |
4 (50) |
|
|
Fetal fibroblast |
876 |
>110? (>13) |
>36 |
>28 (~78) |
|
|
Adult senescent fibroblast |
1896 |
87 (4.6) |
>18 |
>11 (~61), 1 inducedm |
|
|
Adult fibroblast from 17 yr old bull |
338 |
103 (30) |
12 |
6 (50) |
|
|
Many adult and fetal types |
1502 |
596 (40) |
>50 |
>26 (~52) |
|
|
Adult and fetal fibroblast |
N/A |
N/A |
>54 |
>50 (~92) |
|
|
Adult fibroblast from 21 yr old bull |
190 |
53 (28) |
6 |
1 inducedm |
|
|
Mice |
Adult cumulus |
2468 |
1385 (56) |
N/A |
N/A |
|
Embryonic stem cell |
36 |
23 (64) |
N/A |
N/A |
|
|
Mural trophectoderm |
26 |
16 (62) |
N/A |
N/A |
|
|
Adult fibroblast |
463 |
377 (81) |
N/A |
N/A |
|
|
Immature adult Sertoli cell |
1846 |
436 (24) |
235 |
219 (93) |
|
7 |
8 |
9 |
10 |
11 |
12 |
|
# Live births/ Total # fetuses (%)g |
# Live births/ # Embryos transferred to uterus(%)h |
# Live births/ # Embryos produced (%)i |
# Offspring alive or healthy at time of publication/ # Live births (%)j |
Phenotypes observedk |
ReferenceL |
|
5/5 (100) |
5/6 (83) |
5/47 (11) |
2/5 (40) |
# |
Kato 1998 |
|
3/3 (100) |
3/4 (75) |
3/94 (3) |
2/3 (67) |
# |
Kato 1998 |
|
10/45 (22) |
10/100 (10) |
10/552 (1.8) |
10/10 (100) |
ABC# |
Wells 1999 |
|
1/>2 (<50) |
1/4 (25) |
1/140 (.7) |
1/1 (1) |
# |
Zakhartchenko 1999p |
|
1/>5 (<20) |
1/16 (6.3) |
1/82 (1.2) |
0/1 (0) |
AG |
Zakhartchenko 1999p |
|
1/>17 (<6) |
1/32 (3.1) |
1/279 (.36) |
0/1 (0) |
N/A |
Zakhartchenko 1999q |
|
2/>3 (<67) |
2/7 (29) |
2/174 (1.1) |
1/2 (50) |
AB# |
Zakhartchenko 1999r |
|
1/1 (100) |
1/6 (16) |
1/175 (.57) |
0/1 (0) |
CD |
Renard 1999 |
|
4/8 (50) |
4/26 (15) |
4/346 (1.2) |
1/4 (25) |
ABG# |
Shiga 1999 |
|
8/36 (22) |
8/110 (7.2) |
8/876 (.9) |
6/8 (75) |
BCF# |
Hill 1999 |
|
6/>18 (<33) |
6/79 (7.6) |
6/1896 (.32) |
6/6 (100) |
ABD# |
Lanza 2000s |
|
6/12 (50) |
6/54 (11) |
6/338 (1.2) |
4/6 (67) |
AD# |
Kubota 2000 |
|
24/>50 (<48) |
24/172 (14) |
24/1502 (1.6) |
13/24 (54) |
ADEG# |
Kato 2000 |
|
4/>54 (<7.4) |
4/243 (1.6) |
4/? |
1/4 (25) |
BCDF# |
Hill 2000t |
|
1/6 (17) |
1/26 (3.8) |
1/190 (.52) |
1/1 (100) |
BD# |
Hill 2000u |
|
N/A |
31/1385 (2.2) |
31/2468 (1.3) |
22/31 (71) |
# |
Wakayama 1998 |
|
N/A |
2/18 (11) |
2/36 (5.6) |
N/A |
N/A |
Tsunoda 1998 |
|
N/A |
2/25 (8) |
2/26 (7.7) |
N/A |
N/A |
Tsunoda 1998 |
|
N/A |
3/274 (1.1) |
3/463 (.6) |
1/3 (33) |
BF# |
Wakayama 1999 |
|
16/235 (6.8) |
16/436 (3.7) |
16/1846 (.87) |
15/16 (94) |
GF# |
Ogura 2000v |
|
1 |
2 |
3 |
4 |
5 |
6 |
|
Speciesa |
Cell typeb |
# Embryos producedc |
# Embryos developed into morula/ blastocyst (%)d |
# Fetuses after embryo transfere |
# Fetuses miscarried (%)f |
|
|
Tail tip fibroblast |
753 |
260 (41) |
126 |
119 (94) |
|
Adult cumulus |
3920 |
N/A |
N/A |
N/A |
|
|
Fetal fibroblast |
938 |
278 (30) |
45 |
40 (89) |
|
|
Adult cumulus (from hybrid strains) |
4326 |
2583 (60) |
N/A |
N/A |
|
|
Embryonic gonadal cell |
179 |
114 (64) |
N/A |
N/A |
|
|
Embryonic stem cell (from hybrid strain) |
783 |
169 (22) |
N/A |
N/A |
|
|
Goat |
Blastomere (embryonic) |
354 |
96 |
N/A |
N/A |
|
Fetal fibroblast |
230 |
89 (39) |
20 |
17 (85) |
|
|
Fetal fibroblast |
198 |
157 (79) |
>6? |
N/A |
|
|
Pig |
Adult granulosa |
>401 |
401 (?) |
9 |
4 (44) |
|
Fetal fibroblast |
210 |
188 (90) |
N/A |
N/A |
|
|
Fetal body cell |
143 |
N/A |
N/A |
N/A |
|
|
Fetal genital ridge |
340 |
N/A |
N/A |
N/A |
|
|
Monkey |
Blastomere (embryonic) |
78 |
59 (76) |
3 |
1/3 (33) |
|
A = High birth weight B = Pulmonary problems C = Cardiovascular abnormalities D = Immune system abnormalities/infection E = Kidney and/or liver abnormalities F = Placental abnormalities G = Joint malformations or other gross deformities # = Healthy offspring produced NOTE: “N/A” indicates that no data were available in the cited publication. NOTE: ES cell = embryonic stem cell. NOTE: (~ ) indicates percentages extrapolated from data available, as shown in other columns. aThe species of animal used in the experiment. bThe cell type used as the source of the donor nucleus for the nuclear transfer. |
|||||
|
7 |
8 |
9 |
10 |
11 |
12 |
|
# Live births/ Total # fetuses (%)g |
# Live births/ # Embryos transferred to uterus(%)h |
# Live births/ # Embryos produced (%)i |
# Offspring alive or healthy at time of publication/ # Live births (%)j |
Phenotypes observedk |
ReferenceL |
|
7/126 (5.6) |
7/280 (2.5) |
7/753 (.93) |
7/7 (100) |
# |
Ogura 2000w |
|
N/A |
35/? (>.9%?) |
35/3920 (.89) |
34/35? (97?) |
# |
Wakayama 2000 |
|
5/45 (11) |
5/272 (1.8) |
5/938 (.53) |
3/5 (60) |
BGF# |
Ono 2001 |
|
N/A |
80/2573 (3.1) |
80/4326 (1.8) |
N/A |
# |
Wakayama 2001 |
|
N/A |
6/114 (5.2) |
6/179 (3.4) |
5/6 (83) |
# |
Wakayama 2001 |
|
N/A |
28/? (>16.6?) |
28/783 (.36) |
22/28 (79) |
ABF# |
Eggan 2001 |
|
45/? |
45/141 (32) |
45/354 (13) |
N/A |
# |
Yong 1998 |
|
3/20 (15) |
3/85 (3.5) |
3/230 (1.3) |
3/3 (100) |
# |
Baguisi 1999 |
|
N/A |
6/97 (6.1) |
6/198 (3.0) |
3/6 (50) |
#D |
Keefer 2001 |
|
5/9 (55) |
5/401 (1.2) |
5/>401 (<1.2) |
5/5 (100) |
# |
Polejaeva 2000 |
|
N/A |
1/110 (.9) |
1/210 (.5) |
1/1 (100) |
# |
Onishi 2000 |
|
N/A |
2/143 (1.4) |
2/143 (1.4) |
N/A |
N/A |
Betthauser 2000 |
|
N/A |
2/164 (1.2) |
2/340 (.59) |
N/A |
N/A |
Betthauser 2000 |
|
2/3 (67) |
2/29 (6.9) |
2/78 (2.6) |
2/2 (100) |
# |
Meng 1997 |
TABLE 2 Phenotypes Observed in Cloned Animals
|
1 |
2 |
3 |
4 |
|
Speciesb |
Cell typec |
Defects seen in miscarried fetusesd |
# Live birthse |
|
Sheep |
Embryo-derived epithelial-like |
N/A |
5 |
|
Adult mammary gland |
N/A |
1 |
|
|
Fetal fibroblast |
2 fetuses from one of the cell types showed abnormal liver development |
3 |
|
|
Embryo-derived epithelial-like |
N/A |
4 |
|
|
Fetal fibroblast |
1 died after delayed delivery, 2 died after sibling (2) died in utero, 2 stillborn |
7 |
|
|
ES cell line-derived epithelial-like injected into in vivo-matured oocytes |
3 late aborted fetuses underdeveloped for age; edema, hydronephrosis, testicular hypoplasia; also fetuses had variety of other defects, including cleft palate and interventricular septal defect |
2 |
|
|
ES cell line-derived epithelial-like injected into in vitro-matured oocytes |
1 late aborted fetus underdeveloped for age; edema, hydronephrosis, testicular hypoplasia |
1 |
|
|
ES cell line-derived epithelial like |
N/A |
3 |
|
|
ES cell line-derived fibroblast-like |
N/A |
3 |
|
|
ES cell line-derived fibroblast-like |
N/A |
7 |
|
|
Fetal fibroblast |
N/A |
14 |
|
5 |
6 |
7 |
|
Phenotypes of live born clonesf |
Placental defects, phenotypesg |
Referencea |
|
2/5 healthy; 2/5 died perinatally and 1/5 died at 10 days with unknown or unstated pathology |
N/A |
Campbell 1996 |
|
1/1 healthy - (Dolly) - later became overweight |
N/A |
Wilmut 1997 |
|
2/3 healthy; 1/3 died perinatally with unknown pathology |
N/A |
Wilmut 1997 |
|
4/4 healthy |
N/A |
Wilmut 1997 |
|
5/7 alive; 1/7 euthanized with heart defect; 1/7 died perinatally with meconium lodged in lung |
N/A |
Schnieke 1997 |
|
2/2 healthy |
N/A |
Wells 1997 |
|
1/1 died perinatally with respiratory failure, was underweight and had abnormal placenta that researchers hypothesize may have provided inadequate nutrition to support growth; same animal also found to have moderate bilateral hydronephrosis, although enough kidney tissue was present for normal function (as stated by researchers) |
necrosing placenta |
Wells 1997 |
|
2/3 healthy and fertile; 1/3 died perinatally with respiratory failure |
N/A |
Wells 1998h experiment 1 |
|
1/3 healthy; 1 died perinatally with respiratory failure, 1 died after being trampled by mother |
N/A |
Wells 1998h experiment 2 |
|
2/7 healthy; 5/7 died with respiratory failure, 4 of those also had kidney problem (hydronephrosis) |
N/A |
Wells 1998h experiment 3 |
|
3/14 alive, healthy; 7/14 died within 30 hrs; 4/7 died within next 12 weeks: those that died had variety of unspecified kidney, liver and brain defects |
N/A |
McCreath 2000 |
|
1 |
2 |
3 |
4 |
|
Speciesb |
Cell typec |
Defects seen in miscarried fetusesd |
# Live birthse |
|
Cattle |
Blastomere (embryonic) |
N/A |
9 |
|
Embryonic stem cell |
N/A |
4 |
|
|
Fetal fibroblast |
1 fetus aborted early, one after 249 days gestation; the late aborted fetus had abnormal placenta (hydroallantois, enlarged placentomes, edematous chorioallantois and amnion); on necropsy, fetus was oversized and had abnormal lungs and heart |
4 |
|
|
Adult mural granulosa from a 13 yr old cow |
1 case late miscarriage attributed by researchers to hydrallantois |
2 |
|
|
Adult cumulus |
N/A |
5 |
|
|
Adult oviduct epithelial |
N/A |
3 |
|
|
Adult mural granulosa |
7 miscarriages attributed by researchers to hydrallantois |
10 |
|
5 |
6 |
7 |
|
Phenotypes of live born clonesf |
Placental defects, phenotypesg |
Referencea |
|
no mention of birth condition or postnatal development of 9/9 calves, but photo shows 5/9 healthy-looking calves |
N/A |
Chesne 1993 |
|
phenotypes not described |
N/A |
Sims 1994 |
|
3/4 normal, healthy; 1/4 died perinatally with pulmonary hypertension leading to insufficient pulmonary perfusion, and exhibited heart and vessel defects |
1/4 calves born with abnormal placenta |
Cibelli 1998i |
|
2/2 calves had normal birth weight; 1/2 was initially treated for cardiac arrhythmia and is now healthy; 1/2 (the other) initially had poor suckling response and was euthanized 2 days later due to acute hemorrhagic rumenitis and abomastitis |
N/A |
Wells 1998j |
|
2/5 healthy; 3/5 died soon after birth; no abnormalities noted; environmental factors thought by researchers to have caused death |
N/A |
Kato 1998 |
|
2/3 healthy; 1/3 died soon after birth; no abnormalities noted; environmental factors thought by researchers to have caused death |
N/A |
Kato 1998 |
|
10/10 birth weights within normal range; all calves had strong suckling reflex; 1/10 required epinephrine and doxapram treatment to stimulate cardiopulmonary function at birth |
abnormalities noted in the placentas (edematous membranes, high allantoic fluid volume, enlarged umbilical vessels), although these abnormalities did not compromise fetal development according to assessment of researchers |
Wells DN 1999 |
|
1 |
2 |
3 |
4 |
|
Speciesb |
Cell typec |
Defects seen in miscarried fetusesd |
# Live birthse |
|
|
Adult mammary gland epithelium |
1 induced abortion at late gestation due to hydrallantois: fetus oversized, cysts in kidney and liver, enlarged umbilical vessels |
1 |
|
Adult ear skin fibroblast |
1 induced abortion at late gestation due to hydrallantois: fetus oversized, cysts in kidney and liver, enlarged umbilical vessels |
1 |
|
|
Fetal germ cell |
N/A |
1 |
|
|
Fetal fibroblast |
N/A |
2 |
|
|
Adult skin cell from ES cell clone |
N/A |
1 |
|
|
Adult muscle |
N/A |
4 |
|
|
Fetal fibroblast |
1/5 from miscarriage at 8 weeks; 4/5 from mothers that died late in pregnancy: 2/5 had chronic pulmonary hypertension and placental edema |
8 |
|
|
Senescent adult fibroblast |
N/A |
6 |
|
5 |
6 |
7 |
|
Phenotypes of live born clonesf |
Placental defects, phenotypesg |
Referencea |
|
1/1 healthy and normal |
N/A |
Zakhartchenko 1999k |
|
1/1 slightly oversized at birth (57 kg) and had to be euthanized at 2 days due to severe joint abnormalities, and was also noted to have liver with abnormal surface and slightly indurated |
N/A |
Zakhartchenko 1999k |
|
1/1 died, no abnormalities found; death thought by researchers to be related to pre-term delivery due to health of the mother |
N/A |
Zakhartchenko 1999L |
|
1/2 normal and healthy; 1/2 (the other) was oversized and died 3 days after birth with insufficient pulmonary function |
N/A |
Zakhartchenko 1999m |
|
1/1 had enlarged right ventricle but responded well to drug treatment, died at 7 weeks due to severe anemia - at necropsy was found to have thymus, spleen and lymph node hypoplasia |
N/A |
Renard 1999 |
|
1/4 healthy; 2/4 died w/in first 30 hrs due to inadequately inflated lungs, 1/4 (the other) could not stand after 18 days and was euthanized, 2 calves (1 that died 3 days later and the euthanized one) had astasia associated with arthrogryposis (abnormally developed joints); all cloned calves had high birth weight |
N/A |
Shiga 1999 |
|
5/8 were normal at birth, but 1/5 of these died at 6 weeks with suspected dilated cardiomyopathy; 3/8 had neonatal respiratory problems resulting in 1 death at 4 days; 2/8 were hydrallantoic pregnancies and only 1/2 of these survived; birth weights of all calves normal |
both calves that died had edematous placentas |
Hill 1999 |
|
6/6 had increased birth weight and some born with moderate polyuria/ polydypsia, several had pulmonary hypertension and respiratory distress at birth, some had fever following vaccination |
N/A |
Lanza 2000n |
|
1 |
2 |
3 |
4 |
|
Speciesb |
Cell typec |
Defects seen in miscarried fetusesd |
# Live birthse |
|
|
Adult fibroblast from 17 yr old bull |
N/A |
6 |
|
Many adult and fetal types |
N/A |
24 |
|
|
Adult and fetal fibroblast |
placental problems |
4 |
|
|
Adult fibroblast from 21 yr old bull |
N/A |
1 |
|
|
Mouse |
Blastomere (embryonic) |
N/A |
19 |
|
Blastomere (embryonic) |
N/A |
25 |
|
|
Adult cumulus |
N/A |
31 |
|
|
ES cells and mural trophectoderm |
N/A |
4 |
|
|
Adult fibroblast |
N/A |
3 |
|
5 |
6 |
7 |
|
Phenotypes of live born clonesf |
Placental defects, phenotypesg |
Referencea |
|
4/6 healthy and normal; 2/6 died perinatally: 1 with Akabane virus, 1 after labor difficulty: but no abnormalities found upon necropsy; 2 calves had above average birth weight |
N/A |
Kubota 2000 |
|
high birth weight observed in calves; 2/24 died after difficult labor; 1 died with E. coli septicemia; 8/24 died with malformations of joints thought by researchers to be caused by the Akabane virus; some calves also were observed to have kidney or gut abnormalities; 1/24 died at 3 months of unknown causes; some male clones showed aged characteristics and tissue variability in telomere length |
N/A |
Kato 2000 |
|
1/4 healthy; 2/4 died within 5 days with cardiopulmonary problems, and one of those 2 calves had a gut infection; 1/4 died at 1 month with a chronic systemic bacterial infection |
2/6 placentas examined from cloned pregnancies were normal; 4/6 were abnormal: 2/6 had flat cuboidal chronic epithelium and decreased vascularity; 2/6 had diminished cotyledonary structure |
Hill 2000o |
|
1/1 calf with lung dysmaturity and pulmonary hypertension, juvenile diabetes that responded to treatment (discontinued at 2 months), low CD45 antigen expression (required for T cell activation) |
N/A |
Hill 2000p |
|
phenotypes not described |
N/A |
Cheong 1993 |
|
6/25 (identical septuplet males) were tested for fertility and found to be fertile |
NA |
Kwon 1996 |
|
22/31 (71%) were healthy and normal; 9/31 (29%) died in first week |
N/A |
Wakayama 1998 |
|
phenotypes not described |
N/A |
Tsunoda 1998 |
|
3/3 pups grossly normal, but 2/3 died with respiratory failure |
unusually large placentas |
Wakayama 1999 |
|
1 |
2 |
3 |
4 |
|
Speciesb |
Cell typec |
Defects seen in miscarried fetusesd |
# Live birthse |
|
|
Immature adult Sertoli cells |
N/A |
16 |
|
Tail tip fibroblast |
all miscarriages were early in pregnancy |
7 |
|
|
Adult cumulus |
N/A |
35 |
|
|
Adult cumulus |
N/A |
5 tested (does not say how many were born) |
|
|
Fetal fibroblast |
N/A |
5 |
|
|
Embryonic stem cell |
N/A |
28 |
|
|
Goat |
Blastomere (embryonic) |
N/A |
45 |
|
Fetal fibroblast |
all miscarriages were early in pregnancy |
3 |
|
|
Fetal fibroblast infections |
N/A |
6 |
|
5 |
6 |
7 |
|
Phenotypes of live born clonesf |
Placental defects, phenotypesg |
Referencea |
|
15/16 pups normal; 1/16 had umbilical hernia, but was viable at birth |
unusually large but structurally normal placentas |
Ogura 2000q |
|
7/7 pups normal, healthy |
N/A |
Ogura 2000r |
|
telomeres lengthened rather than shortened in successive generations suggesting no inherited aging problem, all mice tested normal for behaviors (learning, memory, activity, agility, strength) |
N/A |
Wakayama 2000 |
|
5 of the healthy cloned mice were tested for behavioral defects - 3/10 measures of preweaning development were delayed but did appear and had no long-term effects; cloned mice were normal for learning, memory, activity and motor skills - these mice had high postnatal weight gain (not heavy at birth as in LOS) compared to controls but researchers suggest this may have been caused by the agouti gene in their background |
N/A |
Tamashiro 2000 |
|
3/5 normal and healthy; 2/5 died with umbilical hernia and respiratory deficiency |
placental hypertrophy and also placental structural abnormalities |
Ono 2001 |
|
28/28 had high birth weights, but this did not adversely affect clones in terms of survival; no respiratory or other problems |
high placental weights |
Eggan 2001 |
|
45/45 healthy |
N/A |
Yong 1998 |
|
3/3 normal, healthy |
N/A |
Baguisi 1999 |
|
3/6 healthy; 3/6 died with respiratory infections |
placentas within normal range for # of cotyledons |
Keefer 2001 |
|
1 |
2 |
3 |
4 |
|
Speciesb |
Cell typec |
Defects seen in miscarried fetusesd |
# Live birthse |
|
Pig |
Adult granulosa |
N/A |
5 |
|
Fetal fibroblast |
N/A |
1 |
|
|
Body cell and genital ridge cell |
N/A |
4 |
|
|
Monkey |
Blastomere (embryonic) |
N/A |
2 |
|
NOTE: N/A indicates that no data were available in the cited publication NOTE: ES cell = embryonic stem cell. NOTE: LOS = large offspring syndrome. aThe peer reviewed scientific article in which data for any given experiment were published. Full references can be found in the bibliography. bThe species of animal used in the experiment. cThe cell type used as the source of the donor nucleus for the nuclear transfer. dDescription of abnormalities seen in aborted cloned fetuses; in some cases, these abnormalities may be the cause of miscarriage. eThe number of live-born cloned animals. fDescription of observations of physical, physiological or genetic characteristics of live born cloned animals at time of publication of cited refernces, unless stated otherwise. gDescription of any characteristics, normal or abnormal, noted in the placentas of live born or miscarried cloned animals. hWells et al. Cloning sheep from cultured embryonic cells. Reprod. Fertil. Dev. 1998; 10:615-626. ICibelli et al. Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science 1998; 280:1256-8. jWells et al. Adult somatic cell nuclear transfer is used to preserve the last surviving cow of the Enderby Island cattle breed. Reprod. Fertil. Dev. 1998; 10:369-378. kZakhartchenko et al. Adult cloning in cattle: Potential of nuclei from a permanent cell line and from primary cultures. Mol. Reprod. Fertil. 1999; 54:264-272. LZakhartchenko et al. Potential of fetal germ cells for nuclear transfer in cattle. Mol. Reprod. Dev. 1999; 52:421-426. mZakhartchenko et al. Effects of serum starvation and re-cloning on the efficiency of nuclear transfer using bovine fetal fibroblasts. J. Reprod. Fertil. 1999; 115:325-331. nLanza et al. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science 2000 Apr 28; 288:665-669. oHill et al. Evidence for placental abnormality as the major cause of mortality in first-trimester somatic cell cloned bovine fetuses. Biol. Reprod. 2000; 63:1787-1794. pHill et al. Development rates of male bovine nuclear transfer embryos derived from adult and fetal cells. Biol. Reprod. 2000; 62:1135-1140. qOgura et al. Production of male cloned mice from fresh, cultured, and cryopreserved immature Sertoli cells. Biol. Reprod. 2000; 62:1579-1584. rOgura et al. Birth of mice after nuclear transfer by electrofusion using tail tip cells. Mol. Reprod. Dev. 2000; 57:55-59. |
|||
TABLE 3 Developmental Capacity of Cytoplasts Reconstituted with Nuclei from Embryonic Cells
|
1 |
2 |
3 |
4 |
5 |
|
Species |
Recipient cytoplast |
Donor cell type |
% Early Development: % Blastocyst (# Blastocysts/ # Cultured) |
% Term Development: % Offspring (# Live births/ # Transferred) |
|
Mouse |
Zygote |
Inner cell mass |
16% (23/142) |
19% (3/16) |
|
Trophectoderm |
1% (1/68) |
0 |
||
|
Zygote |
Pronuclear |
95% (20/21) |
no transfer |
|
|
2-cell |
13% (19/151) |
no transfer |
||
|
4-cell |
0 (0/81) |
no transfer |
||
|
8-cell |
0 (0/111) |
no transfer |
||
|
Inner cell mass |
0 (0/84) |
no transfer |
||
|
Zygote |
8-cell |
0(0/32) |
no transfer |
|
|
Inner cell mass |
0 (0/84) |
no transfer |
||
|
Zygote |
8-cell |
0(0/32) |
no transfer |
|
|
2-cell blastomere |
2-cell |
93% (40/43) |
24%a (10/41) |
|
|
Zygote |
8-cell |
51% (45/89) |
0a (0/11) |
|
|
Cumulus cell |
0 (0/91) |
no transfer |
||
|
2-cell blastomere |
4-cell |
72% (49/68) |
22% (10/46) |
|
|
8-cell |
35% (49/139) |
8% (4/48) |
||
|
Inner cell mass |
0 (0/91) |
no transfer |
||
|
Mll oocyte |
2-cell |
23% (20/88) |
15% (3/20) |
|
|
8-cell |
4% (1/26) |
0 (0/1) |
||
|
Inner cell mass |
13% (11/87) |
0 (0/11) |
||
|
2-cell |
78% (36/46) |
29% (10/34) |
||
|
4-cell |
71% (30/42) |
22% (6/27) |
||
|
8-cell |
46% (18/39) |
18% (3/17) |
||
|
4-cellc |
83% (58/70) |
43% (2/58) |
||
|
Inner cell massc |
64% (23/36) |
11% (2/18) |
||
|
Trophectodermc |
62% (16/26) |
8% (2/25) |
||
|
ES cell |
5% (47/931) |
0 (0/56) |
||
|
ES cell |
29% (312/1087) |
6% (8/132) |
||
|
Sheep |
Mll oocyte |
8-cell |
33% (8/24) |
75% (3/4) |
|
|
16-cell |
27% (13/49) |
21% (3/14) |
|
6 |
7 |
|
References |
Significant findings |
|
Illmensee 1981 |
First demonstration of developmental potential in mammals. Reproducibility of results questioned. |
|
McGrath 1984 |
Biologically impossible to achieve development with transcriptionally active nucleus. |
|
Robl 1986 |
Development more advanced with cytoplast prepared from 2-cell than zygote. |
|
Robl 1986 |
Development more advanced with cytoplast prepared from 2-cell than zygote. No development beyond 12 days gestation. |
|
Wakayama 2000b |
No development when zygotic cytoplasts were used. |
|
Robl 1987 |
Term development when 4- and 8-cell nuclei used but not more advanced. Importance of cytoplast environment. |
|
Kono 1991g |
Development to term from embryonic nuclei transferred to enucleated oocyte. |
|
Cheong 1993 |
Embryonic nuclei in G1 phase of the cell cycle can direct term development when transferred to Mll cytoplasts. |
|
Kwon 1996 |
Serial nuclear transfer of metaphase-arrested embryonic nuclei results in term development. |
|
Tsunoda 1998 |
Term development following serial nuclear transfer of inner cell mass and trophectoderm nuclei. |
|
Tsunoda 1993 |
Implantation sites but no term development. |
|
Wakayama 1999f |
Late-passage actively dividing ES cell nuclei are able to direct development to term. |
|
Willadsen 1986 |
Term development from cleavage stage blastocysts. |
|
Smith 1989 |
Transcriptionally active nuclei are able to direct development to term. |
|
1 |
2 |
3 |
4 |
5 |
|
Species |
Recipient cytoplast |
Donor cell type |
% Early Development: % Blastocyst (# Blastocysts/ # Cultured) |
% Term Development: % Offspring (# Live births/ # Transferred) |
|
|
Inner cell mass |
38% (6/16) |
11% (1/9)d |
|
|
Cultured cell line |
14% (34/244) |
14% (5/34) |
||
|
Cattle |
Pronuclear |
Pronuclear |
13% (5/38) |
100% (2/2) |
|
2- to 8-cell |
0 (0/10) |
no transfer |
||
|
Mll oocyte |
2- to 8-cell |
12% (13/111) |
0 (0/12) |
|
|
9- to 16-cell |
16% (8/50) |
28 (2/7) |
||
|
17- to 32-cell |
8% (2/24) |
no transfer |
||
|
Morula (64-cell) |
23-35% |
22%e (104/463) |
||
|
Morula (31-cell) |
24% (152/641) |
15% (9/59) |
||
|
Inner cell mass |
7% (20/304) |
13% (2/15) |
||
|
Inner cell mass |
5% (30/629) |
8% (2/26) |
||
|
Cultured inner cell mass |
27% (109/406) |
12% (4/34) |
||
|
Fetal germ cell (PGC) |
20-38% (30/149-53/140) |
5% (1/20) |
||
|
Rabbit |
Mll oocyte |
8-cell |
not assessed |
4% (6/164) |
|
8- to 16-cell |
49% (34/69) |
21% (23/110) |
||
|
32-cell |
33% (14/43) |
15% (10/67) |
||
|
Inner cell mass |
20% (17/83) |
no transfer |
||
|
Trophectoderm |
0 (0/52) |
no transfer |
||
|
Pig |
Mll oocyte |
2-cell |
9% (1/11) |
0 (0/33) |
|
4-cell |
8% (7/83) |
3% (1/34) |
||
|
8-cell |
19% (11/57) |
0 (0/21) |
||
|
Goat |
Mll oocyte |
Morulac |
31% (18/57) |
31% (45/141) |
|
Monkey |
Mll oocyte |
8-cell |
52%e (53/101) |
4% (2/53) |
|
aDevelopment assessed at 8.5 days post coitum. bWakayama et al. Nuclear transfer into mouse zygotes. Nat Genet 2000; 24:108-9. cAchieved using serial nuclear transfer. dDevelopment assessed at 42 days of pregnancy. eEmbryonic development assessed at the 2-cell stage prior to transfer. fWakayama et al. Mice cloned from embryonic stem cells. Proceedings of the National Academy of Sciences, USA 1999; 96:14984-89. |
||||
TABLE 4 Developmental Capacity of Cytoplasts Reconstituted by Nuclei from Fetal and Adult Somatic Cells
|
1 |
2 |
3 |
4 |
|
Species |
Recipient cytoplast |
Donor Cell Type |
% Early Development: % Blastocyst (# Blastocysts/ # Cultured) |
|
Mouse |
Zygote Mll oocyte |
Cumulus Cell Thymocyte |
0 (0/91) 7% (6/88) |
|
Cumulus Cell |
67% (101/151) |
||
|
Neuronal Cell |
22% (50/223) |
||
|
Sertoli cell (mature) |
40% (63/159) |
||
|
Cumulus Cell |
20% (19/93) |
||
|
Cultured follicular cell; |
34% (51/151) 3% (1/30) |
||
|
Adult Male fibroblast; |
50% (207/414) |
||
|
Cumulus cell. |
52% (206/393) |
||
|
Fibroblast cell |
23% (38/162) |
||
|
Sertoli Cell (immature) |
33% (94/284) |
||
|
Sheep |
Mll oocyte |
Fetal fibroblast |
27% (34/124) |
|
Adult Mammary (epithelial) |
12% (29/247) |
||
|
Transgenic Fetal fibroblast |
5-21% (5/82-19/89) |
||
|
Transgenic Fetal fibroblast |
6-28% (14/109, 43/154, 4/71, 19/83) |
||
|
Cattle |
Mll oocyte |
Cumulus cell |
13% (5/38) |
|
Fetal fibroblast |
12% (33/276) |
|
5 |
6 |
7 |
|
% Term Development: % Offspring / (# Live births/ # Transferred) |
References |
Significant Findings |
|
No Transfer 0 |
1. Wakayama 2000a -same footnote information as earlier Wakayama 2000 in Table 3 2. Callas, 1992 |
1. No development when zygotic cytoplasts were used. 2. Somatic nuclei are able to direct embryonic development through no term development. |
|
2% (31/1315) |
Wakayama 1998b |
Direct-injected cumulus cell nuclei direct term development; however, Sertoli and neuronal nuclei do not. |
|
2%a (1/46) |
|
Findings do not support the requirement of G0/G1 nuclei for term development. |
|
2%a (1/59) 0 (0/3) |
Kato 1999 |
Serial nuclear transfer of cultured follicular cells but not cumulus cell nuclei results in term development. nuclei can direct term development. |
|
3% (1/30); |
Wakayama 1999c; |
Male-derived adult somatic cell nuclei can direct term development; Immature, actively dividing Sertoli cell |
|
1% (2/177); |
Ogura 2000d |
|
|
1%c (2/206) |
|
|
|
0c (0/38) |
|
|
|
4% (6/134) |
|
|
|
8% (3/40) |
Wilmut 1997 |
Inducing cell to enter quiescence by serum starvation may assist in nuclear reprogramming. |
|
3% (1/29) |
|
First demonstration that nuclei from differentiated somatic fetal or adult origin can direct development to term. |
|
5/21% (1/21-1/5) |
Schnieke 1997 |
Term development of transfected somatic cell nuclei suggests an alternative method for the production of transgenic animals. One male lamb was born. |
|
0-28% (4/14, 8/43, 0/4, 2/19) |
McCreath 2000 |
Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. |
|
0 (0-19) |
Callas 1994 |
Nuclei from adult somatic cells can direct embryonic development. |
|
14% (3/28) |
Cibelli 1998e |
Cultured activity dividing fetal fibroblast nuclei can direct development to term. |
|
5 |
6 |
7 |
|
% Term Development: % Offspring / (# Live births/ # Transferred) |
References |
Significant Findings |
|
7% (2/7) |
Kubota 2000 |
Nuclei from male adult fibroblast can direct development to term. |
|
33% (2/6) |
Kato 1995 |
High rates of term development following transfer of cumulus and oviduct nuclei. |
|
50% (2/4) |
|
|
|
10% (10/100) |
Wells 1999 |
Production of calves from cultured granulosa cells. |
|
0 |
Collas and Rob, unpublished |
First production of genetically verified nuclear transfer rabbits. |
|
1.3% (5/401) |
Polejaeva 2000 |
Term development following serial nuclei transfer of cumulus cells. |
|
No Transfer 0.9% (1/110) |
Onishi 2000 |
Term development following direct injection of nuclei from fetal fibroblast cells. |
|
3% (3/112) |
Baguisi 1999 |
Production of transgenic goats from transfected fetal fibroblast nuclei. |
|
0 |
Wolf 1999 |
Donor nuclei from cell lines are capable of limited embryonic development. |
|
0 |
|
|