Executive Summary
Human reproductive cloning is an assisted reproductive technology that would be carried out with the goal of creating a human being. It is currently the subject of much debate around the world, involving a variety of ethical, religious, societal, scientific, and medical issues. However, this report from the National Academies addresses only the scientific and medical aspects of human reproductive cloning. Consideration of the medical aspects has required the panel to examine issues of scientific conduct and human-subjects protection. But we have not attempted to address the issue of whether producing a new individual by reproductive cloning, if it were found to be scientifically safe, would or would not be acceptable to individuals or society. Instead, the panel defers to others on the fundamental ethical, religious, and societal questions, and presents this report on the scientific and medical aspects to inform the broader debate. Our report differs in this respect from the last major report on the topic in the United States, Cloning Human Beings, a 1997 report developed by the National Bioethics Advisory Commission [1].
THE PANEL’S CONCLUSIONS AND RECOMMENDATIONS
The panel has examined and analyzed the scientific, medical, and legal literature on the issues and heard testimony at a workshop from experts in animal cloning, assisted reproductive technologies, and science, technology, and legal policy—including people who, on scientific and medical grounds, either oppose or defend human reproductive clon-
ing. After carefully considering the issues raised, we conclude that the case has not been proved that human reproductive cloning would lead to fewer negative outcomes at this time than reproductive cloning of other mammals. We therefore make the following recommendations:
Human reproductive cloning should not now be practiced. It is dangerous and likely to fail. The panel therefore unanimously supports the proposal that there should be a legally enforceable ban on the practice of human reproductive cloning. For this purpose, we define human reproductive cloning as the placement in a uterus of a human blastocyst derived by the technique that we call nuclear transplantation. In reaching this conclusion, we considered the relevant scientific and medical issues, including the record from cloning of other species, and the standard issues that are associated with evaluating all research involving human participants.
The scientific and medical considerations related to this ban should be reviewed within 5 years. The ban should be reconsidered only if at least two conditions are met: (1) a new scientific and medical review indicates that the procedures are likely to be safe and effective and (2) a broad national dialogue on the societal, religious, and ethical issues suggests that a reconsideration of the ban is warranted.
Finally, the scientific and medical considerations that justify a ban on human reproductive cloning at this time are not applicable to nuclear transplantation to produce stem cells. Because of its considerable potential for developing new medical therapies for life-threatening diseases and advancing fundamental knowledge, the panel supports the conclusion of a recent National Academies report that recommended that biomedical research using nuclear transplantation to produce stem cells be permitted. A broad national dialogue on the societal, religious, and ethical issues is encouraged on this matter.
THE FINDINGS THAT SUPPORT A BAN ON HUMAN REPRODUCTIVE CLONING
It is a serious event when any group that has potential authority over research intercedes to ban it, and the reasons must therefore be compelling. We are convinced that the scientific and medical data concerning the
likely danger to the implanted fetus or the eventual newborn if reproductive cloning of humans is attempted in the near future are compelling.
The panel has based its support for the proposed ban on human reproductive cloning on the following findings:
Finding 1: The scientific and medical criteria used to evaluate the safety of reproductive cloning must be the potential morbidity and death of the woman carrying the clone as a fetus and of the newborn and the risk to women donating the eggs.
Finding 2: Data on the reproductive cloning of animals through the use of nuclear transplantation technology demonstrate that only a small percentage of attempts are successful; that many of the clones die during gestation, even in late stages; that newborn clones are often abnormal or die; and that the procedures may carry serious risks for the mother. In addition, because of the large number of eggs needed for such experiments, many more women would be exposed to the risks inherent in egg donation for a single cloning attempt than for the reproduction of a child by the presently used in vitro fertilization (IVF) techniques. These medical and scientific findings lead us to conclude that the procedures are now unsafe for humans.
Finding 3: At least three criteria would have to be fulfilled before the safety of human reproductive cloning could be established:
-
The procedures for animal reproductive cloning would have to be improved to such an extent that the levels of observed abnormalities in cloned animals, including nonhuman primates, were no more than that seen with existing human assisted reproductive technology (ART) procedures. If that could not be achieved, researchers would have to demonstrate that humans are different from other animals with regard to cloning-related defects. Reproducible data demonstrating that a successful reprogramming of the donor nucleus and proper imprinting can be achieved in animals would be essential, as would an understanding of the mechanisms responsible for such events.
-
New methods would have to be developed to demonstrate that the human preimplantation embryos produced through the use of nuclear transplantation technology are normal with respect to imprinting and reprogramming. That would best be done by first establishing the normal state of reprogramming and imprinting in nonhuman primates and then documenting that the processes in preimplantation human embryos are substantially similar.
-
Methods would have to be developed to monitor—effectively
-
and comprehensively—preimplantation embryos and fetuses in the uterus for cloning-related defects, such as those outlined in Chapter 3; these include alterations in gene expression and imprinting.
Finding 4: The issues of responsible conduct of research raised by the prospect of cloning a person are those of medical ethics—in particular, the protection of the participants (the egg donor, the host mother, and the child produced through cloning) in any human cloning research. Participants in any human cloning research efforts require full protection as human research participants, although it should be noted that, as with fetal surgery, this protection cannot be extended fully to the cloned fetus. Human reproductive cloning has not been performed before, and its introduction, if it ever occurred, would require systematic research. That research would likely entail full review by institutional review boards and other human-subjects protections, including informed consent of donors and recipients of all biological materials.
Finding 5: If any attempts at human reproductive cloning were ever to occur, they would constitute research, not merely innovative therapy. Such research would then be subject to external technical and ethical review by review boards to ensure that the proposed experiments are both technically and ethically sound and that the rights and welfare of all research participants are protected. This institutional review process should be applied equally to both public- and private-sector research and be transparent to the public.
Finding 6: Because medical and scientific findings indicate that cloning procedures are currently not safe for humans, cloning of a human through the use of nuclear transplantation technology is not now appropriate. The panel believes that no responsible scientists or physicians are likely to undertake to clone a human. Nevertheless, no voluntary system that is established to restrict reproductive cloning is likely to be completely effective. Some organizations have already announced their intention to clone humans, and many of the reproductive technologies needed are widely accessible in private fertility clinics that are not subject to federal regulations. The panel therefore concludes that a legally enforceable ban that carries substantial penalties has a much greater potential than a voluntary system or moratorium to deter any attempt to clone a human using these techniques.
Finding 7: If no ban is imposed, it is possible that some organizations will attempt the reproductive cloning of humans. Although such attempts would most likely fail, there is a high probability they would be associ-
ated with serious risks to any possible fetus or newly born child and may harm the woman carrying the developing fetus.
Finding 8: There is concern that legislation or regulation that would ban reproductive human cloning would set a troubling precedent with respect to the restriction of innovative, experimental research and medical procedures. Modern scientific research proceeds rapidly, and its findings are unpredictable and often surprising. It is probable that at least every 5 years there will be significant new information regarding the issues of the safety and applicability of human cloning to medical practice. The above concern can be ameliorated by including in any legislation or regulation a requirement for an updated evaluation of the scientific, medical, and societal issues within 5 years. Such a requirement for periodic reviews would allow for extensive public debate regarding reproductive human cloning and the consideration of modifications to the legislation. Part of that evaluation would include a recommendation as to when the next such evaluation should be conducted.
Finding 9: Two activities will be particularly important for an updated evaluation of human reproductive cloning: a thorough scientific and medical review to evaluate whether the procedures are likely to be safe and effective and a broad national dialogue on the societal, religious, and ethical issues. As part of this process, any persons advocating the practice of human reproductive cloning would need to acknowledge the extent of the abnormalities seen in animal cloning experiments and to demonstrate that these problems—assuming that they persist—are unlikely to occur in humans.
Finding 10: Any future process designed to evaluate the scientific and medical evidence on cloning a person would likely need to involve scientists, physicians, ethicists, and the public. A public debate could be facilitated by a committee that issues regular updates on the state of the science surrounding animal cloning and reaches out to involved constituencies in a systematic manner. Such a body could derive its powers by executive order, by executive action within the Department of Health and Human Services under the Public Health Service Act, or by legislation. Among many other issues, the debate should be structured to inform the public that clones are not precise replicas, but persons with identical genetic material.
Finding 11: The science of cloning is an international one with research conducted throughout the world. Furthermore, the issue of human
reproductive cloning is the subject of worldwide debate. A number of countries and international organizations have prepared reports and issued statements on the issue. Participation by the United States in such international debates about human reproductive cloning will be beneficial to any future process to evaluate the scientific and medical evidence on this issue.
Finding 12: The limited regulation and monitoring of experimental ART procedures in the United States means that important data needed for assessing novel ART procedures are in some cases lacking, in other cases incomplete and hard to find. Because the panel was not charged to investigate ART regulation and did not solicit expert testimony thereon, we make no recommendations regarding oversight of, registration of, or required data collection from ART clinics. But we do believe that a request from Congress or the Executive Branch for a panel of experts to study the matter and report its findings and recommendations publicly would probably be useful. Having such information is likely to be beneficial to any process of evaluating future scientific and medical evidence regarding both reproductive cloning and new ART procedures.
REDUCING CONFUSION CONCERNING THE USE OF THE TERM “HUMAN CLONING”
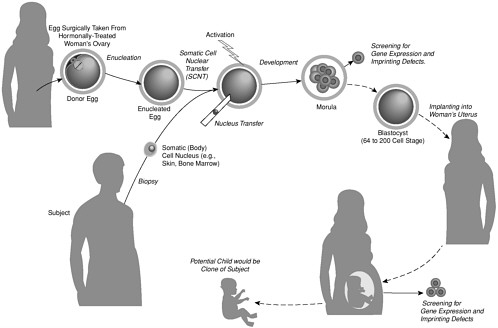
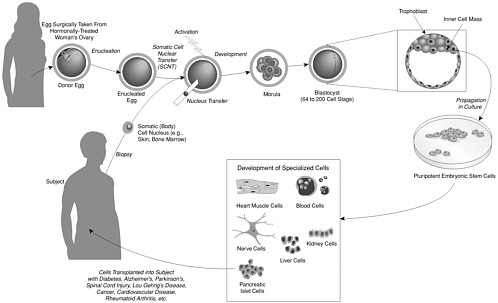
As we have just discussed, human reproductive cloning is an assisted reproductive technology that would be carried out with the goal of creating a human being (see Figure 1). There is a very different procedure, here termed nuclear transplantation to produce stem cells—but variously called nonreproductive cloning, therapeutic cloning, research cloning, or somatic cell nuclear transfer (SCNT) to produce stem cells—whose aim is the creation of embryonic stem (ES) cells for clinical and research purposes (see Figure 2).
Unlike reproductive cloning, the creation of embryonic stem cells by nuclear transplantation does not involve implantation of a preimplantation embryo, or blastocyst, in a uterus. For this reason, it cannot produce a complete, live born animal (a “clone”). Some confusion arises because in both cases researchers would use nuclear transplantation, which is an initial step in the successful procedures used to clone animals—beginning with the sheep Dolly and including several other mammals since then. In nuclear transplantation, the nucleus of an egg cell (containing its chromosomes) is removed and replaced with the nucleus of a cell taken from the body of an adult (a “somatic cell”). Thus, nuclear transplantation accurately describes the process.
For both reproductive cloning and stem cell production, a reconstructed egg cell produced by nuclear transplantation is stimulated to cause it to begin dividing. If that is successful, several sequential cell divisions can give rise to the preimplantation embryo known as a blastocyst that is composed of 64-200 cells (see Figure 2).
It is at this stage that the procedures used for reproductive cloning and for nuclear transplantation to produce stem cells become entirely different. In reproductive cloning, a blastocyst formed by the nuclear transplantation procedure is implanted in a uterus, where it begins the process of forming a fetus. Any animals produced in this way will have the same nuclear genes as the adult cells used to produce them, and when the nuclei from several somatic cells from a single animal are transferred to a series of eggs, all the animals born are said to be “clones” of the original adult animal.
Although these clones will be physically very similar, the animals will not be physically or behaviorally identical, because of various factors, including their different uterine and postnatal environments and experiences.
In nuclear transplantation to produce stem cells, cells are isolated from the blastocyst 4-5 days after the procedure, and the cells are used to make a stem cell line for further study and clinical applications. Neither the blastocyst nor the stem cells are ever placed into a uterus. Moreover, as described in Chapter 2, human stem cells do not themselves have the capacity to form a fetus or a newborn animal. Nevertheless, in the popular press and other media, the term “human cloning” has often been misleadingly applied to both this procedure and reproductive cloning whenever either is proposed to be used in a human context.
As part of our panel’s charge, we were asked, “Based on the current scientific and medical evidence, should there be a moratorium on the cloning of a person? What are the implications of doing so? Of not doing so?” This raises the question of the implications that a ban on human reproductive cloning could have for the very different process of nuclear transplantation to produce stem cells.
None of the findings summarized in the preceding section that support the panel’s conclusions regarding a ban on human reproductive cloning would support a ban on the use of the nuclear transplantation technology to produce stem cells. A recent report prepared by a different committee of the National Academies has emphasized that there is a great potential for studies on stem cells isolated through nuclear transplantation to increase the understanding and potential treatment of various diseases and debilitating disorders, as well as fundamental biomedical knowledge. The diseases and debilitating disorders include “Lou Gehrig’s disease” (amyotrophic lateral sclerosis, or ALS), Parkinson’s disease, Alz-
heimer’s disease, spinal-cord injury, cancer, cardiovascular diseases, diabetes, and rheumatoid arthritis. The necessary research would entail transfer of human somatic cell nuclei into enucleated human eggs for the purpose of deriving blastocysts and embryonic stem cells and stem cell lines; there would be no implantation in a uterus. Some have expressed concern that this research might nevertheless be misdirected to human reproductive cloning. If our recommendation for a legally enforceable ban is adopted, then any attempts at implantation that might lead to the development and birth of a newborn would be criminalized.
The committee that produced the report from the National Academies entitled Stem Cells and the Future of Regenerative Medicine considered a wide range of views on the ethical and societal issues involved in the production of human embryonic stem cells—including nuclear transplantation technology [2]. After carefully considering all sides of the issue, that committee produced the following conclusion and recommendation concerning this technology:
Conclusion: Regenerative medicine is likely to involve the implantation of new tissue in patients with damaged or diseased organs. A substantial obstacle to the success of transplantation of any cells, including stem cells and their derivatives, is the immunemediated rejection of foreign tissue by the recipient’s body. In current stem cell transplantation procedures with bone marrow and blood, success hinges on obtaining a close match between donor and recipient tissues and on the use of immunosuppressive drugs, which often have severe and potentially life-threatening side effects. To ensure that stem cell-based therapies can be broadly applicable for many conditions and people, new means of overcoming the problem of tissue rejection must be found. Although ethically controversial, the somatic cell nuclear transfer technique promises to have that advantage. Other options for this purpose include genetic manipulation of the stem cells and the development of a very large bank of ES cell lines [2].
Recommendation: In conjunction with research on stem cell biology and the development of potential stem cell therapies, research on approaches that prevent immune rejection of stem cells and stem cell-derived tissues should be actively pursued. These scientific efforts include the use of a number of techniques to manipulate the genetic makeup of stem cells, including somatic cell nuclear transfer.
Our panel includes members who participated in the workshop on stem cells held at the National Academies on June 23, 2001. This work-
shop was convened as part of the data-gathering process for the separate committee that produced the above report focused on stem cells. In our own workshop, held on August 7, 2001, we consulted with many of the world’s leaders in nuclear transplantation to produce stem cells—I. Wilmut, R. Jaenisch, R. Yanagimachi, J. Cibelli, P. Mombaerts, and A. Trounson (see Appendix C)—and we have also conducted our own extensive literature review. On the basis of this review and discussion, the panel determined that although there is a clear therapeutic potential for techniques in which stem cells are produced through nuclear transplantation (as in Figure 2), this potential is nascent and needs considerable research. The potential of this research includes developing a broader understanding of how human tissue cells develop normally and how human diseases that have a genetic component are caused at a cellular level.
The panel concludes this executive summary with a review of the scientific subjects that were covered.
ANIMAL CLONING
Since the report in 1997 of the birth of the sheep Dolly, the first successful reproductive clone of a mammal from an adult cell, reproductive cloning has been carried out with several kinds of animals. Five mammalian species have been reproductively-cloned from adult or fetal cells—sheep, mice, pigs, goats, and cattle—and similar attempts are being made, so far without success, in monkeys, dogs, and horses.
The panel reviewed the scientific literature on animal cloning and heard from animal-cloning experts at its workshop. It found that cloning efficiencies in animals remain extremely low despite several years of experimentation. This low efficiency means that any human reproductive cloning attempt would probably require large numbers of eggs. The collection of these eggs would bring with it the risk of ovarian hyperstimulation syndrome in donors, as with all in vitro fertilization (IVF). However, in the case of cloning it would probably involve either scores of women for one cloning attempt or a few women being exposed to high levels of hormones.
Furthermore, animal cloning is associated with a wide variety of abnormalities in the fetus and offspring. The abnormalities include a greater than normal size of fetus and placenta (both during gestation and after birth), poor interaction between fetal and maternal components of the placenta, greater early-gestation and late-gestation fetal morbidity and mortality, greater postnatal mortality, and various developmental defects in the immune, cardiovascular, and possibly nervous systems. In addition, it is important to note that subtle behavioral and mental defects that
could create major problems for humans may not be detectable in animal models.
The most likely reasons for the abnormalities thus far observed are failures in genetic reprogramming (the process that changes a cell nucleus from one developmental state to another) and errors in genetic imprinting (the process of establishing, maintaining, and interpreting parent-specific chemical marks on the DNA, which indicate how specific genes should function in specific cells).
On the basis of the animal data, it is also likely that human cloning will be associated with risks to the women involved. Among these risks are increased maternal morbidity and mortality and the risks inherent in the overproduction of oocytes from egg donors. The psychological burden of late-term abortions or the birth of infants with severe defects must also be considered.
HUMAN REPRODUCTIVE CLONING
Those who plan to clone humans have indicated that they will take additional precautionary steps beyond those currently undertaken in animal cloning. The steps include preimplantation testing to detect chromosome defects and errors in imprinting (methylation) at one or more DNA sites, and postimplantation testing of the imprinting (methylation) status at up to 30 DNA sites. All participants would sign an informed-consent form that would outline the risks to both the mother and the child and the low probability of success. Those who have publicly stated their intention to undertake human reproductive cloning are thus far using private funding in a nonuniversity setting, and in some cases they are operating or planning to operate outside the United States.
LESSONS FROM OTHER ASSISTED REPRODUCTIVE TECHNOLOGIES RELEVANT TO HUMAN REPRODUCTIVE CLONING
Assisted reproductive technology (ART) refers to all treatments or procedures for assisting human reproduction that include the laboratory handling of human eggs, sperm, or embryos, including in vitro fertilization (IVF). IVF involves the mixing of egg and sperm in the laboratory to generate embryos suitable for transfer to a uterus 2 or 3 days later. ART as currently practiced does not provide a basis for evaluating all the risks inherent in reproductive cloning, because reproductive cloning involves the use of adult somatic nuclei rather than the germ cell (egg and sperm) nuclei used in ART [3]. Germ-cell nuclei are preprogrammed to support early embryonic development and to respond to the egg’s regulatory
signals, whereas adult cell nuclei are not and must therefore undergo an extensive reprogramming to be successful in their new environment.
The panel compared the experiences thus far obtained in animal cloning with knowledge of current human ART procedures and found that the reproductive outcomes from cloned blastocysts observed in animals are very low compared with the efficiencies seen with current human IVF—as well as being highly variable. In addition, serious defects and deaths occur in animal cloning, often late in pregnancy and soon after birth, at rates never seen with human or most animal ART procedures.
Existing preimplantation and postimplantation testing methods are inappropriate and inadequate for the needs of human reproductive cloning. Assessing the shape and structure of embryos is of little use in determining the likelihood of successful implantation of a particular embryo, and molecular tests to detect all the possible errors in genetic imprinting and reprogramming do not yet exist. Moreover, such tests, if they become available, would be difficult to adapt to the small amount of material available for preimplantation diagnosis.
Experimental ART procedures have been minimally regulated and monitored in the United States, so there is a shortage of data pertaining to innovative ART procedures. Certification of clinics could allow greater control over any new ART procedures and collection of important information. The UK Human Fertilisation and Embryology Authority might provide a model for certifying ART clinics and clinical and research protocols and procedures, although the terms of the UK legislation would have to be adapted to the federal style of the US government.
USING NUCLEAR TRANSPLANTATION TO PRODUCE EMBRYONIC STEM CELLS
Stem cells are cells that have an extensive ability to self-renew and to differentiate (turn into specialized cells). Embryonic stem cells obtained from blastocysts (5- to 7-day-old preimplantation embryos of about 150 cells each) are particularly important because they can give rise to the widest variety of cells and are immortal. If embryonic stem cells are derived by nuclear transplantation using a nucleus from a patient as the somatic nucleus transferred into the egg, the resulting cells will be immunologically very similar to the patient’s cells. However, the nuclear DNA donor and mitochondrial DNA donor will generally be different. Only if the egg donor is the mother of the patient or the patient herself, will the stem cells be genetically identical with the patient’s cells—containing not only the same nuclear genome, but also the same mitochondrial DNA. As described in the recent report from the National Academies entitled Stem Cells and the Future of Regenerative Medicine, present research with such
cells has the goal of producing cells and tissues for therapeutic transplantation with a reduced risk of rejection [2]. However, mitochondrial gene products that differ can elicit transplant rejection (see Chapter 2).
|
Current Arguments and Counterarguments Regarding Human Reproductive Cloning Provided below is a summary of some of the current arguments and counterarguments regarding human reproductive cloning. The panel’s analysis of each is based on the scientific and medical literature and on presentations at its workshop. Argument 1: Animal-safety data do not apply, because humans are very different from the animals under study [4]. In particular, a recent study [5] indicated that an important imprinted gene in mice is not imprinted in humans; therefore, imprinting errors would not be a problem in cloned humans. Counterargument: Placental function, development, and genetic regulation are similar in humans and animal models, such as mice, so similar SCNT-related defects would be expected [6]. Numerous studies have emphasized that humans and other organisms have the same basic pathways for governing early embryonic and fetal development. Furthermore, widespread defects in all five of the mammalian species that have been reproductively cloned thus far suggest that the defects would affect basic biological functions in humans. Even if one less gene is imprinted in humans as compared to mice, humans are known to have many imprinted genes (possibly as many as 100), and any number of these are likely to cause problems in reproductively cloned humans. Argument 2: Frequent failures are seen in normal human reproduction; cloning would be no different [4]. Counterargument: Errors in normal human reproduction occur primarily early in pregnancy; many of the women in question are never aware that they are pregnant. In contrast, many of the defects in reproductively cloned animals arise late in pregnancy or after birth. Argument 3: Inappropriate culture media for the initial cells cause most cloning-related problems [7; 8]. Culture media for human assisted reproductive technologies have been better optimized [8; 4]. Synchronization between the implanted embryo and the recipient uterus has also been better in human than in animal assisted reproductive technology procedures. Counterargument: Culture effects appear to account for only some of the defects observed [9; 10]. Many defects in various organ systems are peculiar to reproductive cloning. Expertise in existing human assisted reproductive technologies is not relevant to these problems, because the defects appear to arise from biological rather than purely technical causes [9]. Argument 4: Those who have cloned animals stress the failures, but there are also many successes in animal reproductive cloning [8; 4]. |
The panel recognizes that a blastocyst derived for scientific purposes by nuclear transplantation could be implanted in a human uterus in violation of a ban on reproductive cloning. But a legally enforceable ban that
|
Counterargument: The statement is true, but does not necessarily apply to human reproductive cloning. In humans, the likelihood and benefit of success must be weighed against the probability, severity, and lifelong consequences of failure. Failures are all but certain in any human reproductive cloning attempt at this time, based on the experience with animals, and in humans, the consequences could be far more devastating. The likelihood and benefit of possible success must be weighed against the high probability and severe consequences of failure. Argument 5: Existing preimplantation and postimplantation genetic tests could be used to detect abnormalities, allowing selection of embryos to be implanted and therapeutic abortion in case of any problems. In contrast, there has been no genetic testing and weeding out of animal reproductive clones. In preimplantation testing, two cells could be removed from an eight-cell morula. One cell could be tested for correctness of the chromosome complement and the other for imprinting errors at one or more DNA sites [11]. It has been claimed that such imprinting tests have been performed with DNA from cells after somatic cell nuclear transfer (SCNT) [4], although no data have been presented. Postimplantation testing could include testing for chromosomal errors, the checking of imprinting status at up to 30 sites, and the measurement of production levels from many genes with DNA chips [12] or reverse-transcription polymerase chain reaction [11]. Counterargument: Many errors would not be detectable until late in pregnancy or after birth, when therapeutic abortion would not be an option. Many of the relevant genetic tests have not yet been developed [8; 9]; existing genetic tests appropriate for single-gene inherited disorders or gross chromosomal rearrangements are insufficient because they are not relevant to the major sources of errors expected in human cloning. Ultrasonographic tests cannot detect the small-scale defects in tissues, such as lung, that have had devastating consequences in newborn animal clones [13;14], and there is insufficient evidence regarding the possible impact of imprinting errors on brain development in humans. Argument 6: Voluntary informed consent allows potential participants to make their own decisions and elect to take the risks if they so choose. Counterargument: Our current regulatory system recognizes that when information is lacking it can be difficult or impossible to inform subjects fully. That is the case with respect to human reproductive cloning because the extent of the risks is unknown, and the greatest risk of abnormality, morbidity, and mortality is borne by the cloned fetus/child, who cannot give informed consent. In addition, there are risks borne by the woman donating the eggs and the gestational mother. When subjects cannot be fully informed, and when a procedure is clearly risky, there is a role for both regulatory agencies and professionals to limit the options available to a subject if the evidence supports such a limitation [14]. Societal concerns can also be taken into account. |
criminalizes the implantation step should be sufficient to prevent such proscribed activity. Moreover, because all nuclear transplantation experiments will require the participation of human subjects (the donor of the eggs and the donor of the somatic cell nuclei, who may be the same person or different persons), all this work would necessarily be regulated and controlled by the procedures and rules concerning human-subjects research—subjecting it to close scrutiny.
Stem cells derived directly from an adult’s own tissues are an alternative to nuclear transplantation-derived embryonic stem cells as a source of cells for therapies. Two types of adult stem cells—bone marrow and skin stem cells—currently provide the only two stem cell therapies. But, as noted in the above mentioned report, many questions remain before the potential of other adult stem cells can be accurately assessed. Few studies on adult stem cells have sufficiently defined the stem cell by starting from a single isolated cell or defined the necessary cellular environment for correct differentiation or the factors controlling the efficiency with which the cells repopulate an organ. There is a need to show that the cells derived from introduced adult stem cells are contributing directly to tissue function and to improve the ability to maintain adult stem cells in culture without having the cells differentiate. Finally, most of the studies that have garnered so much attention have used mouse rather than human adult stem cells.
The previous report also notes that unlike adult stem cells, it is well established that embryonic stem cells can form multiple tissue types and be maintained in culture for long periods of time. However, embryonic stem cells are not without their own potential problems as a source of cells for transplantation. The growth of human embryonic stem cells in culture now requires a “feeder” layer of mouse cells that may contain viruses, and when allowed to differentiate the embryonic stem cells can form a mixture of cell types at once. Human embryonic stem cells can form benign tumors when introduced into mice, although this potential seems to disappear if the cells are allowed to differentiate before introduction into a recipient.
In addition to possible uses in therapeutic transplantation, embryonic stem cells and cell lines derived by nuclear transplantation could be valuable tools for both fundamental and applied medical and biological research [2]. This research would begin with the transfer of genetically defined donor nuclei from normal and diseased tissues. The resulting cell lines could be used to study how inherited and acquired alterations of genetic components might contribute to disease processes. The properties of the cell lines could be studied directly, or the embryonic stem cells could be studied as they differentiate into other cell types. For example, the way in which cells derived by nuclear transplantation from an Alz-
heimer’s disease patient acted while differentiating into brain cells, compared with those derived from a normal patient, might yield new clues about Alzheimer’s disease. Such cell lines could also be used to ensure that research covers a more genetically diverse human population than that represented in the blastocysts stored in IVF clinics, promoting studies of the causes and consequences of genetic diseases by allowing researchers to study how embryonic stem cells with different genetic endowments differ in the way that they form cell types and tissues. Finally, studies of genetic reprogramming and genetic imprinting will be substantially enhanced through the use of stem cells derived by nuclear transplantation, compared with studies with stem cells derived from other sources.
SUMMARY
This panel was charged with assessing the scientific and medical issues surrounding human reproductive cloning. Most of the relevant data on reproductive cloning are derived from animal studies. The data reveal high rates of abnormalities in the cloned animals of multiple mammalian species and lead the panel to conclude that reproductive cloning of humans is not now safe. Our present opposition to human reproductive cloning is based on science and medicine, irrespective of broader considerations. The panel stresses, however, that a broad ethical debate must be encouraged, so that the public can be prepared to make decisions if human reproductive cloning is some day considered medically safe for mothers and offspring.
The panel’s discussion inevitably included a comparison of the methods used for reproductive cloning and for nuclear transplantation to produce stem cells. The panel is in agreement with the recent report from the National Academies entitled Stem Cells and the Future of Regenerative Medicine [2] in affirming the potential of studies on stem cells isolated through nuclear transplantation. The probable benefits include advances in fundamental biomedical knowledge, as well as the understanding and treatment of various diseases and debilitating disorders.
REFERENCES
1. NATIONAL BIOETHICS ADVISORY COMMISSION. Cloning Human Beings, Volume I: Report and Recommendations of the National Bioethics Advisory Commission. Rockville, MD. 1997 Jun. Online at: http://bioethics.gov/pubs/cloning1/cloning.pdf.
2. COMMITTEE ON STEM CELLS AND THE FUTURE OF REGENERATIVE MEDICINE, BOARD ON LIFE SCIENCES AND BOARD ON NEUROSCIENCE AND BEHAVIORAL HEALTH. Stem Cells and the Future of Regenerative Medicine. Report of the National Research Council and the Institute of Medicine. 2001 Sep.
3. KRAKAUER DC, MIRA A. Mitochondria and germ-cell death. Nature 1999 Jul 08, 400(6740):125-6.
4. BOISSELIER B, Clonaid, Bahamas. Reproductive cloning in humans. Workshop: Scientific and Medical Aspects of Human Cloning. National Academy of Sciences, Washington, D.C., 2001 Aug 7. Online at: www.nationalacademies.org/humancloning
5. KILLIAN JK, NOLAN CM, WYLIE AA, LI T, VU TH, HOFFMAN AR, JIRTLE RL. Divergent evolution in M6P/IGF2R imprinting from the Jurassic to the Quaternary. Hum Mol Genet 2001 Aug 15, 10(17):1721-1728.
6. CROSS J, University of Calgary, Alberta, Canada. Assisted reproductive technologies. Workshop: Scientific and Medical Aspects of Human Cloning. National Academy of Sciences, Washington, D.C., 2001 Aug 7. Online at: www.nationalacademies.org/humancloning
7. ANTINORI S, International Associated Research Institute, Italy. Cloning in reproductive medicine. Workshop: Scientific and Medical Aspects of Human Cloning. National Academy of Sciences, Washington, D.C., 2001 Aug 7. Online at: www.nationalacademies.org/humancloning
8. ZAVOS P, Andrology Institute of America. Human therapeutic cloning: Indications, ethics, and other considerations. Workshop: Scientific and Medical Aspects of Human Cloning. National Academy of Sciences, Washington, D.C., 2001 Aug 7. Online at: www.nationalacademies.org/humancloning
9. JAENISCH R, Massachusetts Institute of Technology/ Whitehead Institute. Scientific issues underlying cloning: Epigenetics. Workshop: Scientific and Medical Aspects of Human Cloning. National Academy of Sciences, Washington, D.C., 2001 Aug 7. Online at: www.nationalacademies.org/humancloning
10. FARIN PW, North Carolina State University. Large offspring effects in cattle. Workshop: Scientific and Medical Aspects of Human Cloning. National Academy of Sciences, Washington, D.C., 2001 Aug 7. Online at: www.nationalacademies.org/humancloning
11. ZAVOS P, Andrology Institute of America. Expert witness. Human cloning. U.S. House of Representatives, Committee on Energy and Commerce. Subcommittee on Oversight and Investigations. 2001 Mar 28. Online at: http://energycommerce.house.gov/107/hearings/03282001Hearing141/print.htm
12. BRENNER C, COHEN J. The genetic revolution in artificial reproduction: A view of the future. Hum Reprod 2000 Dec, 15 Suppl 5:111-6.
13. HILL J, Cornell University. Placental defects in nuclear transfer (cloned) animals. Workshop: Scientific and Medical Aspects of Human Cloning. 2001 Aug 7. Online at: www.nationalacademies.org/humancloning
14. WILMUT I, Roslin Institute, Scotland. Application of animal cloning data to human cloning. Workshop: Scientific and Medical Aspects of Human Cloning. National Academy of Sciences, Washington, D.C., 2001 Aug 7. Online at: www.nationalacademies.org/humancloning
15. CHARO RA, University of Wisconsin, Madison. Regulation of cloning. Workshop: Scientific and Medical Aspects of Human Cloning. National Academy of Sciences, Washington D.C., 2001 Aug 7. Online at: www.nationalacademies.org/humancloning