Executive Summary
ABSTRACT
Anthrax Vaccine Adsorbed (AVA) was licensed in 1970 to provide protection against infection with Bacillus anthracis. AVA was initially administered on a limited basis, primarily to protect veterinarians and workers processing animal products such as hair or hides that could be contaminated with anthrax spores. In the 1990s, with growing concerns about the possible use of anthrax as a biological weapon, use of the vaccine was substantially expanded. The Department of Defense (DoD) vaccinated some of the military personnel deployed for the Gulf War in 1991 and in 1998 initiated the Anthrax Vaccine Immunization Program, calling for mandatory vaccination of all U.S. service members. By late 2001, roughly 2.1 million doses of AVA had been administered. Production of AVA was suspended in 1998 when the facility manufacturing the vaccine was closed for renovations, which were undertaken to meet regulatory requirements of the Food and Drug Administration (FDA).
Concerns about the efficacy and safety of AVA, and about vaccine production, led Congress to direct the DoD to support an independent examination of AVA by the Institute of Medicine. In October 2000, the Institute of Medicine convened the Committee to Assess the Safety and Efficacy of the Anthrax Vaccine. The committee reviewed all available data, both published and unpub-
lished, and heard from representatives of DoD, FDA, and other federal agencies; from the vaccine manufacturer BioPort; from researchers studying the efficacy and safety of the vaccine; and from service members and others with concerns about the safety or efficacy of the vaccine. After the bioterrorism of fall 2001, the committee accelerated its original timetable for its review.
As indicated by evidence from studies in both humans and animals, the committee concluded that AVA, as licensed, is an effective vaccine to protect humans against anthrax, including inhalational anthrax. Moreover, because the vaccine exerts its protection via an antigen crucial to the action of the bacterium’s toxins, AVA should be effective against anthrax toxicity from all known strains of B. anthracis, as well as from any potential bioengineered strains.
After examining data from numerous case reports and especially epidemiologic studies, the committee also concluded that AVA is reasonably safe. Within hours or days following vaccination, it is fairly common for recipients to experience some local events (e.g., redness, itching, swelling, or tenderness at the injection site), while a smaller number of vaccine recipients experience some systemic events (e.g., fever and malaise). But these immediate reactions, and the rates at which they occur, are comparable to those observed with other vaccines regularly administered to adults. The committee found no evidence that vaccine recipients face an increased risk of experiencing life-threatening or permanently disabling adverse events immediately after receiving AVA, when compared with the general population. Nor did it find any convincing evidence that vaccine recipients face elevated risk of developing adverse health effects over the longer term, although data are limited in this regard (as they are for all vaccines).
Regarding manufacture of AVA, the committee reviewed and evaluated the steps taken by BioPort to win FDA approval of its production process. With the newly validated manufacturing process being used in a renovated facility, AVA will be produced under strict controls according to current FDA requirements. The newly produced vaccine is expected to have greater assurance of consistency than the vaccine produced at the time of its original licensure.
It remains important to continue and improve monitoring efforts to detect any adverse health effects caused by AVA and other vaccines. Also needed are studies to establish a quantitative correlation of protective levels of antibodies in animals with antibody levels in humans after full immunization. Direct tests of the efficacy of AVA are neither feasible nor ethical in humans. However, corre-
lates of protection in animal models can be used to test the efficacy of AVA, as well as new vaccines against anthrax. The production, testing, and licensure of a new vaccine requiring fewer doses and producing fewer local reactions are needed.
Anthrax Vaccine Adsorbed1 (AVA) was licensed in 1970. More than 2 million doses have been administered, and most of those doses have been given since 1998 to U.S. military personnel to protect them against possible exposure to anthrax spores used as biological weapons. The terrorist attacks of September 11, 2001, and the subsequent distribution through the U.S. mail of potent doses of anthrax spores drew new attention to the risks of anthrax exposure and to questions about the anthrax vaccine.
Until the 1990s, AVA had primarily been used by a small population with a risk of occupational exposure to anthrax (e.g., textile mill workers and veterinarians). In 1990, concerns that Iraq had biological weapons containing anthrax spores motivated the U.S. military to administer AVA to an estimated 150,000 service members deployed for the Gulf War. The existence of an Iraqi biological weapons program was confirmed in the mid-1990s (Henderson, 1999; Zilinskas, 1997), and in 1997 the Department of Defense (DoD) announced a plan to vaccinate all U.S. service members with the licensed anthrax vaccine. DoD’s Anthrax Vaccine Immunization Program (AVIP) began in March 1998 with personnel scheduled for deployment to higher-risk areas (e.g., Korea and Southwest Asia). In 2000 a limited vaccine supply, the result of delays in federal approval for release of newly manufactured vaccine lots, began slowing plans to vaccinate all military personnel. As more service members were vaccinated under the mandatory AVIP, some raised concerns about the safety or the efficacy of AVA, and more than 400 personnel refused vaccination (Weiss, 2001). Some had also suggested a link between vaccination with AVA and illnesses in Gulf War veterans.
STUDY PROCESS AND INFORMATION SOURCES
Responding to the concerns about the anthrax vaccine and AVIP, the U.S. Congress directed DoD to enter into a contract with the National Research Council for a study of the vaccine’s efficacy and safety.2 In October 2000 the Institute of Medicine (IOM) convened the Committee to
Assess the Safety and Efficacy of the Anthrax Vaccine to carry out that study. Committee members were selected for their expertise in microbiology; vaccine research, development, manufacture, and evaluation; post-marketing surveillance of adverse events; regulatory and licensing procedures; epidemiology; biostatistics; immunology; and health surveillance.
The charge to the committee included consideration of the types and severity of adverse reactions, sex differences in adverse reactions, long-term health implications, the efficacy of AVA against inhalational exposure to all known anthrax strains, and the correlation of the safety and efficacy of the vaccine in animal models to its safety and efficacy in humans. The study was also to address the issue of validation of the manufacturing process, with consideration of discrepancies identified by the Food and Drug Administration (FDA) in February 1998, the definition of vaccine components, and identification of gaps in existing research. (See Appendix A for the Statement of Task.) The charge did not include evaluation of the DoD policy to vaccinate all service members, so the committee did not include an evaluation of the threat from biological warfare agents in its purview. Similarly, the committee was not asked to address the challenges in bio-weapons vaccine development and procurement generally, which have recently been discussed in a statement from the Council of the Institute of Medicine (http://www.iom.edu/IOM/IOMHome.nsf/Pages/Vaccine+Development) and in reports by the Gilmore Commission (http://www.rand.org/nsrd/terrpanel/) and DoD (http://www.defenselink.mil/pubs/ReportonBiologicalWarfareDefenseVaccineRDPrgras-July2001.pdf ).
Since the terrorist attacks of September 11, 2001, and subsequent mail distribution of anthrax spores, interest in AVA has greatly increased. Consideration of the full range of topics concerning civilian use of the anthrax vaccine was beyond the purview of this report. However, some of the issues that the committee did address should also be of interest for civilians.
The committee held eight deliberative meetings plus four public work-shops. At those workshops, the committee heard from representatives of DoD, FDA, and other federal agencies; from the manufacturer of AVA, BioPort; from researchers studying the efficacy and safety of the vaccine; and from service members and others with concerns about the safety or efficacy of the vaccine. The committee also commissioned a review of the available literature on adverse events associated with other vaccines routinely administered to adults.
The committee examined both published and unpublished data from studies of the safety and efficacy of AVA. The investigators involved in many of those studies presented their data and discussed their findings at committee workshops. In addition, several analyses of existing data were carried out at the committee’s request.
ANTHRAX AND ANTHRAX VACCINE
Anthrax is caused by infection with Bacillus anthracis, a gram-positive, nonmotile, spore-forming organism (Brachman and Friedlander, 1999; Dixon et al., 1999). It is primarily a disease of wild and domestic animals. Historically, humans have contracted the disease through contact with infected animals or animal products, such as hair or hides, contaminated with anthrax spores. Depending on the site of infection, anthrax can occur in a cutaneous, gastrointestinal, or inhalational form. The disease had become extremely uncommon in any form in the United States until the bioterrorist incidents of the autumn of 2001 caused an outbreak of both cutaneous and inhalational cases of the disease. As of November 28, 2001, there had been 11 cases of inhalational anthrax, 5 of which were fatal, and 7 confirmed and 5 suspected cutaneous anthrax infections (CDC, 2001b). More than 30,000 people may have been exposed to anthrax spores (CDC, 2001a,b).
The virulence of B. anthracis derives from the production of a capsule and three toxin proteins: protective antigen (PA), edema factor (EF), and lethal factor (LF). To produce active toxins, PA must bind to cellular receptors and then to either EF or LF. AVA, the vaccine currently licensed for human use in the United States, is a cell-free filtrate containing PA as the principal immunogen. It is administered in six subcutaneous injections of 0.5 milliliters each. The first three doses are given 2 weeks apart, and the following doses are given 6, 12, and 18 months after administration of the first dose. Annual booster doses are required.
ANTHRAX VACCINE EFFICACY
The committee’s observations and findings addressed the efficacy of immunization with the licensed vaccine, AVA, against inhalational anthrax and all known anthrax strains (see Chapter 3). Of particular concern is exposure to anthrax spores processed for use in biological weapons. The committee also examined what is known and what must still be established regarding the correlation of protection in animal models with immunity in humans.
It is important to note that efficacy is relative, not absolute. The degree of protection provided by a vaccine is determined by a variety of factors, which can include the size of the inoculum of exposure, the strain of the pathogen, and the host response. Even a vaccine considered highly effective may fail to protect some individuals under some circumstances.
Evaluating Efficacy of AVA
The efficacy of a PA-containing anthrax vaccine similar to AVA against anthrax infection was established by a randomized controlled field study of
textile mill workers (Brachman et al., 1962). Subsequent data from the Centers for Disease Control and Prevention (CDC) support the results of that study (FDA, 1985). The small number of inhalational cases in those studies provides insufficient information to establish the vaccine’s efficacy against inhalational infection, but the data suggest that the vaccine has a protective effect.
Animal studies are essential for further investigation of the efficacy of AVA and other anthrax vaccines against inhalational disease because studies with humans are neither feasible nor ethical. Cases of inhalational anthrax are very rare, even where anthrax occurs naturally in the environment or as an occupational hazard. Moreover, human research subjects cannot be deliberately exposed to potentially lethal agents, such as anthrax spores, for no therapeutic reason and without the availability of a proven treatment.
Finding: Because additional clinical trials to test the efficacy of AVA in humans are not feasible and challenge trials with volunteers are unethical, by necessity animal models represent the only sources of the supplementary data needed to evaluate AVA’s efficacy.
Animal models with pathological and immunological characteristics similar to those of humans could be considered the most appropriate ones for the evaluation of vaccine efficacy. The pathophysiology of anthrax in nonhuman primates, such as the macaque, most closely resembles the patho-physiology of anthrax in humans. Among the smaller and more available laboratory animals, rabbits most closely resemble nonhuman primates in terms of the pathology of anthrax and their response to the anthrax vaccine.
Finding: The macaque and the rabbit are adequate animal models for evaluation of the efficacy of AVA for the prevention of inhalational anthrax.
Efficacy of AVA Against All Known B. anthracis Strains
Several different B. anthracis strains are found in nature worldwide (Fellows et al., 2001; Keim et al., 2000), and analysis of tissue samples from victims of the release of anthrax spores from the Soviet biological weapons facility at Sverdlovsk in 1979 indicated the presence of several B. anthracis strains (Grinberg et al., 2001; Jackson et al., 1998). It is important to establish whether AVA can afford protection against the full range of naturally occurring or engineered B. anthracis strains.
Studies have shown that the protection that AVA affords guinea pigs differs by bacterial strain (Auerbach and Wright, 1955; Fellows et al., 2001; Ivins et al., 1994; Little and Knudson, 1986; Turnbull et al., 1986),
but AVA and a predecessor vaccine protected rabbits and monkeys against the numerous strains tested (Auerbach and Wright, 1955), including those that defeated the vaccine in guinea pigs (Fellows et al., 2001). No AVA-resistant strains have been demonstrated in nonhuman primates. Observational data from studies with humans also support the efficacy of AVA against a variety of strains, though exposure strains were not evaluated in the studies (Brachman et al., 1962; CDC, 1967–1971).
PA is the principal immunogen in AVA, and the efficacy of AVA against a broad spectrum of B. anthracis strains is consistent with the critical role of PA in the pathogenesis of anthrax (Bhatnagar and Batra, 2001; Cataldi et al., 1990; Smith and Keppie, 1954). As shown in Figure ES-1, PA must be competent to carry out multiple complicated tasks: it must bind to its receptor, form a heptamer, and bring EF and LF into the cell.
There is concern that natural mutations or bioengineered alterations of the PA component of anthrax could result in vaccine-resistant strains. Studies (Sellman et al., 2001; see also Mogridge et al., 2001) have shown, however, that a PA heptamer is deactivated by the presence of even a few mutant subunits. A deactivated heptamer is unlikely to be able to deliver EF and LF to the cytosol. The committee considers it improbable that a mutant PA that retains its function yet escapes the vaccine-elicited protective antibodies directed to the wild-type PA could be constructed at this time.
The likely difficulty of successfully altering PA is supported by evidence that the B. anthracis genome is highly conserved among strains isolated across a wide geographical area (Jackson, 2001; Keim et al., 1997) and that PA is also highly conserved (Jackson, 2001; Price et al., 1999). Because PA is critical to virulence and because its structure is so highly conserved, it appears likely that changing its structure would alter and thus eliminate its toxic action.
Finding: It is unlikely that either naturally occurring or anthrax strains with bioengineered protective antigen could both evade AVA and cause the toxicity associated with anthrax.
Establishing Animal Model Correlates of Anthrax Vaccine Efficacy
Several recent studies have used passive protection to demonstrate a relationship between levels of circulating anti-PA antibody and protection from challenge with anthrax spores (Barnard and Friedlander, 1999; Beedham et al., 2001; Little et al., 1997; McBride et al., 1998; Pitt et al., 2001; Reuveny et al., 2001).
Finding: The available data indicate that immunity to anthrax is associated with the presence of antibody to protective antigen.
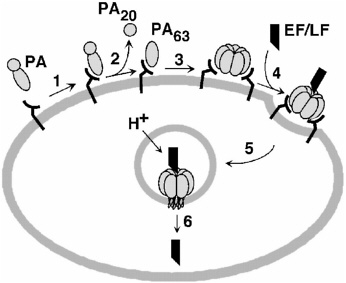
FIGURE ES-1 Model of anthrax toxin action. (1) PA binds to cellular receptor. (2–3) The protein is cleaved and activated to form a heptameric prepore. (4) LF, EF, or both bind to the heptamer, and the resulting complex is taken into an acidic compartment in the cell through endocytosis. (5–6) The acidic pH initiates the heptamer to pierce the membrane of the cell and translocate LF, EF, or both into the cytosol, where the toxins lead to damage. [Reprinted, with permission, from Biochemistry 38:10432–10441 (1999). Copyright 1999 by American Chemical Society.]
The information reviewed by the committee demonstrates that both humans and certain laboratory animals manifest the same disease after infection with the same anthrax organism and that both are protected by immunization with AVA, which elicits the production of antibodies to PA. This information establishes a qualitative correlation between protection in animal models and protection in humans. To move forward with research on the current anthrax vaccine or any new vaccines, however, a quantitative correlation of the protective levels of antibodies in animals with the antibody titers obtained after full immunization in humans is needed. Those correlates in animal models can then be used to test new vaccines for efficacy with confidence that the data from studies with animals will be predictive of the clinical results for immunized humans. The data from animal studies already developed suggest that serological correlates of human immunity can be developed in appropriate animal models. The committee commends this work and encourages its further development.
Recommendation: Additional passive protection studies with rabbits
and monkeys including the transfer of animal and human sera are urgently needed to quantify the protective levels of antibody in vivo against different challenge doses of anthrax spores.
Recommendation: Additional active protection studies should be conducted or supported to develop data that describe the relationship between immunity and both specific and functional quantitative antibody levels, including studies of
-
the relationship between the vaccine dose and the resulting level of antibody in the blood of test animals that protects the animals from challenge;
-
the relationship between the level of antibody that protects animals from challenge and the level of antibody present in humans vaccinated by the regimen currently recommended for the licensed product; and
-
the vaccine dose that results in a level of antibody in the blood of human volunteers similar to that in the blood of protected animals.
Postexposure Use of Anthrax Vaccine
As a result of the inhalational exposure to anthrax spores from letters mailed in the autumn of 2001, questions about the postexposure efficacy of AVA have arisen. No data from studies with humans are available, but two papers provide information from studies with rhesus monkeys.
These limited data suggest that use of the vaccine in combination with an appropriate antibiotic for 30 days could provide excellent postexposure protection against inhalational anthrax. Although the additional benefit from receiving the vaccine after a prolonged period of antibiotic use is not proven, reliance on the vaccine alone after exposure is clearly insufficient, as some protection is needed during the time required for an immune response to develop. Additional studies on the postexposure use of AVA with antibiotics are needed.
Recommendation: DoD should pursue or support additional research with laboratory animals on the efficacy of AVA in combination with antibiotics administered following inhalational exposure to anthrax spores. Studies should focus on establishment of an appropriate duration for antibiotic prophylaxis after vaccine administration.
Conclusions Regarding Efficacy
A vaccine similar to AVA was shown to be effective against cutaneous anthrax in humans in the field trial supporting the original application for
licensure of AVA (Brachman et al., 1962). Although that study had too few cases to evaluate the vaccine’s efficacy for the prevention of inhalational disease, the five inhalational cases observed during the trial occurred only among nonvaccinated or placebo recipients. Data from CDC on cases reported between 1962 and 1974 also indicated that the vaccine offered protection against the cutaneous form of the disease (FDA, 1985). Further-more, laboratory experiments indicate that AVA provides effective protection against inhalational challenge in rabbits and macaques, the animal models in which the disease is most reflective of the disease in humans (Fellows et al., 2001; Ivins et al., 1996, 1998; Pitt et al., 2001). Because PA is critical to the virulence of B. anthracis and because PA’s structure is so highly conserved, it appears likely that changing its structure would alter and thus eliminate its toxic action. Data from studies with animals suggest that AVA will offer protection against strains with PA-based toxicity. Finally, the available data indicate that immunity to anthrax is associated with the presence of antibodies to PA, such as those stimulated by the anthrax vaccine.
Finding: The committee finds that the available evidence from studies with humans and animals, coupled with reasonable assumptions of analogy, shows that AVA as licensed is an effective vaccine for the protection of humans against anthrax, including inhalational anthrax, caused by any known or plausible engineered strains of B. anthracis.
ANTHRAX VACCINE SAFETY
As with any pharmaceutical product or medical procedure, the use of vaccines carries a risk of adverse health effects that must be weighed against the expected health benefit. Expectations for the safety3 of vaccines are especially high because, in contrast to therapeutic agents, which are given when a disease is known to be present (or at least suspected), vaccines are usually given to people who are healthy to protect them against a disease that they may not be exposed to in the future.
The committee evaluated case reports and epidemiologic studies providing information about the safety of the anthrax vaccine. Case reports can help to generate hypotheses about possible associations but are rarely sufficient by themselves to confirm such associations. Formal epidemiologic studies are usually needed to determine whether those adverse events iden-
tified in case reports occur in exposed populations at a rate that exceeds the background rate in unexposed populations.
The case reports relating to AVA come primarily from the Vaccine Adverse Event Reporting System (VAERS), a passive surveillance system that collects reports on adverse events following the use of any vaccine licensed in the United States (see Chapter 5). A subset of the committee reviewed each of 120 VAERS reports on serious adverse events associated with AVA. The committee also heard testimony regarding adverse events following vaccination with AVA. These statements, some of which concerned cases reported to VAERS, added valuable insight into the conditions that some military personnel are experiencing.
In evaluating the epidemiologic studies of adverse events following receipt of AVA (see Chapter 6), the committee gave additional weight to those that (1) used active surveillance rather than self-reports of post-immunization events; (2) included sufficiently large numbers of subjects; (3) had clearly specified, objective criteria for the definition of adverse events; and (4) had sufficiently long postimmunization follow-up intervals to allow identification of later-onset events. Those studies that included a suitable unimmunized comparison group or in which evaluators were blinded to vaccination status were especially useful to the committee.
Conclusions Regarding AVA Vaccination and Adverse Events
Substantial data are now available from VAERS, epidemiologic studies with data from the Defense Medical Surveillance System (DMSS), and other epidemiologic studies for assessments of the health outcomes following vaccination with AVA. Immediate-onset health events are observable within hours or days following vaccination; later-onset events would be observable only months or years following vaccination.
Epidemiologic studies that have used either active surveillance (Brachman et al., 1962; Pittman, 2001b,c; Pittman et al., 1997, 2002, in press) or passive surveillance (Hoffman et al., submitted for publication; Pittman, 2001a; Pittman et al., 2001a,b; Wasserman, 2001) have consistently found local injection-site reactions, including redness, induration, edema, itching, or tenderness (see Table 6-1 for details). Systemic events, such as fever, malaise, and myalgia, are also associated with vaccination with AVA but are generally less common than injection-site reactions. The types of local and systemic reactions associated with AVA and the rates at which they were observed are comparable to those observed with other vaccines regularly administered to adults, such as diphtheria and tetanus toxoids and influenza vaccines (Treanor, 2001). Although these immediate-onset health effects can result in brief limitation of activities or the loss of time from work (Hoffman et al., submitted for publication; Wasserman,
2001), they are self-limited and result in no serious, permanent health impairments (AMSA, 2001a,b,c; Grabenstein, 2000; Lange et al., 2001a,b; Rehme, 2001; Rehme et al., 2002; Mason et al., 2001, submitted for publication; Sato, 2001a,b; Sato et al., 2001).
Finding: The data available from VAERS, DMSS, and epidemiologic studies indicate the following regarding immediate-onset health events following receipt of AVA:
-
Local events, especially redness, swelling, or nodules at the injection site, are associated with receipt of AVA, are similar to the events observed following receipt of other vaccines currently in use by adults, and are fairly common.
-
Systemic events, such as fever, malaise, and myalgia, are associated with receipt of AVA, are similar to the events observed following receipt of other vaccines currently in use by adults but are much less common than local events.
-
Immediate-onset health effects can be severe enough in some individuals to result in brief functional impairment, but these effects are self-limited and result in no permanent health impairments.
-
There is no evidence that life-threatening or permanently disabling immediate-onset adverse events occur at higher rates in individuals who have received AVA than in the general population.
Sex differences are seen in local injection-site reactions. Women are more likely than men to experience and report erythema, local tenderness, subcutaneous nodules, itching, and edema (Hoffman et al., submitted for publication; Pittman, 2001a,b; Pittman et al., 2001a,b, 2002; Wasserman, 2001). In addition, some systemic effects, including fever, headache, malaise, and chills, were sometimes more often reported by women than by men (Hoffman et al., submitted for publication; Pittman, 2001a; Pittman et al., 2001a,b), but rates of clinically observed systemic reactions generally did not differ substantially between men and women (Pittman, 2001b; Pittman et al., 2002). For female service members, reactions following vaccination against anthrax may be more likely to have an adverse effect on their ability to perform their duties (Hoffman et al., submitted for publication; Wasserman, 2001). Studies of other vaccines have also generally found higher rates of local reactions among women but similar rates of systemic reactions between men and women (Treanor, 2001). The factors that account for these sex differences are not known, but they could be a function of differences in muscle mass, differences in the doses per unit of body mass, physiologic factors, or differences in care-seeking behavior. Future studies of vaccination against anthrax should continue to analyze data for men and women separately.
Finding: The available data from both active and passive surveillance indicate that there are sex differences in local reactions following vaccination with AVA, as there are following administration of other vaccines. For female service members, reactions following vaccination with AVA can have a transient adverse impact on their ability to perform their duties. The factors that account for these sex differences are not known.
Recommendation: Future monitoring and study of health events following vaccination(s) with AVA (and other vaccines) should continue to include separate analyses of data for men and women.
Some of the data reviewed by the committee showed lot-to-lot differences in the reactogenicities of AVA doses (CDC, 1967–1971; Pittman, 2001a; Pittman et al., 2001a,b).
Unlike most vaccines, AVA is licensed for subcutaneous rather than intramuscular administration. The limited evidence from a small study that tested changes in the AVA dosing schedule and route of administration (Pittman, 2001b; Pittman et al., 2002) suggests that subcutaneous administration contributes to the local reactions but not systemic reactions associated with AVA. With other vaccines, subcutaneous administration is also associated with higher rates of local erythema or induration (Treanor, 2001), reactions commonly reported following the administration of AVA.
Finding: The currently licensed subcutaneous route of administration of AVA and the six-dose vaccination schedule appear to be associated with a higher incidence of immediate-onset, local effects than is intramuscular administration or a vaccination schedule with fewer doses of AVA. The frequencies of immediate-onset, systemic events were low and were not affected by the route of administration.
Recommendation: DoD should continue to support the efforts of CDC to study the reactogenicity and immunogenicity of an alternative route of AVA administration and of a reduced number of vaccine doses.
Some have expressed concerns about potential later-onset and chronic health effects resulting from AVA use. The available information regarding later-onset health effects is limited, as for all vaccines, but provides no convincing evidence of elevated risks of later-onset health events (AMSA, 2001a,b,c; Grabenstein, 2000; Lange et al., 2001a,b; Mason et al., 2001, submitted for publication; Peeler et al., 1958, 1965; Rehme, 2001; Rehme et al., 2002; Sato, 2001a,b; Sato et al., 2001; White et al., 1974). DMSS, which provides the best source of data for studying later-onset health effects, provides data on service personnel who have documented histories of vaccination with AVA and who have been observed for up to 3 years.
Although AVA has been administered to military personnel for more than 3 years, unreliable documentation of vaccinations before 1998 limits analysis of DMSS data for observation of potential vaccine-related health effects over longer periods.
Finding: The available data are limited but show no convincing evidence at this time that personnel who have received AVA have elevated risks of later-onset health events.
Recommendation: DoD should develop systems to enhance the capacity to monitor the occurrence of later-onset health conditions that might be associated with the receipt of any vaccine; the data reviewed by the committee do not suggest the need for special efforts of this sort for AVA.
The studies reviewed did not examine the use of AVA in children, the elderly, or individuals with chronic illnesses. In addition, information regarding the outcomes of pregnancy following use of the vaccine is limited. These limitations should be taken into account if AVA is considered for use in the general population.
ANTHRAX VACCINE MANUFACTURE
The committee was charged with addressing “validation of the manufacturing process focusing on, but not limited to, discrepancies identified by the Food and Drug Administration in February 1998.” The committee could not directly validate the manufacturing process and did not wish to second-guess FDA’s inspection and determination of validity. It was possible, however, to review and evaluate the steps by which BioPort worked to validate the AVA manufacturing process (see Chapter 7).
Documents that BioPort provided to the committee gave detailed information about findings from FDA inspections conducted since 1998, the company’s responses to those findings, and FDA’s evaluation of BioPort’s progress. The committee paid special attention to materials on product characterization and process validation. It also considered the recent and increasing investments by BioPort and DoD in facility renovations and improvements in documentation of the manufacturing process, as well as the transfer, with approval from FDA’s Center for Biologics Research (CBER), of filling operations to a contractor meeting Good Manufacturing Practices standards. The committee noted BioPort’s access to technical support and assistance from CBER and DoD research and development resources. The results of these efforts were reflected in BioPort’s reports of progress in correcting deficiencies previously noted by FDA, as reported at the committee’s July 2001 meeting. This progress was confirmed by FDA.
On January 31, 2002, FDA approved BioPort’s supplements to its Biologics License Application covering facility renovations, changes to the package label, and the contracted filling operations.
In evaluating BioPort’s efforts to meet the manufacturing requirements for AVA, the committee noted FDA’s changes and modernizations and improvements in the regulation of biologics, as well as the continuing effort at constructive criticism and response between the agency and the manufacturer. The committee also considered the history of the AVA manufacturer—in particular, the switch from a state-owned to a privately owned and operated interstate commercial venture—and the coincident changes in FDA oversight and validation requirements. Finally, the committee was mindful of the scientific and technical advances in vaccine manufacture and characterization that have occurred since the original licensure of the AVA product.
Finding: FDA’s process of plant inspection and FDA’s validation of the vaccine manufacturing process have changed and have become more stringent with time.
Finding: With high-priority efforts by the manufacturer and FDA, the manufacturing process for AVA has been validated so that vaccine manufactured postrenovation has been approved for release and distribution.
BioPort has responded to numerous specific citations from FDA regarding the manufacturing process and equipment and has now received FDA approval of its license supplements. In the committee’s judgment, the cumulative effects of the changes in materials, equipment, and processes in response to FDA citations, as well as the changes in the regulatory climate and in scientific knowledge, are likely to result in greater assurance of consistency in the final AVA product.
Finding: AVA will now be produced by a newly validated manufacturing process under strict controls, according to current FDA requirements. As a result, the postrenovation product has greater assurance of consistency than that produced at the time of original licensure.
FUTURE NEEDS
Despite recent FDA approval of the license supplement for AVA manufacturing renovations, package insert, and contract filler, the committee is convinced that relying on AVA and the current specifications for its use is far from satisfactory. There is a need for research toward the development of a different and better anthrax vaccine, as well as a need for improvements in monitoring the safety of the current vaccine.
Future Use of AVA
Finding: Current events in both the military and civilian arenas highlight and confirm the importance of ensuring both the availability and the quality of the nation’s anthrax vaccine.
With the deployment of U.S. troops to Afghanistan and surrounding areas and domestic bioterrorism incidents involving exposure to B. anthracis spores, vaccination against anthrax is likely to resume and possibly expand. This means that AVA is likely to be given to a much larger population than was anticipated at the time that the vaccine was licensed.
Meanwhile, the current supply of AVA is limited because of manufacturing difficulties, which have now been overcome. On the basis of information provided by BioPort and FDA, the committee notes that the AVA manufacturing process has been modified to incorporate more modern technology and procedures. These changes are expected to increase assurance of the consistency of the final product, which remains a relatively crude vaccine by current standards.
Although greater assurance of product consistency will occur, the levels of immunogenicity, safety, and stability of the postrenovation AVA product must be characterized. The committee emphasizes that the surveillance methods recommended below are the same as those that would be expected for any widely used vaccine and are not unique to AVA.
Finding: The AVA product produced in a renovated facility by a newly validated manufacturing process could differ from the prerenovation product in terms of its reactogenicity, immunogenicity, and stability. The information available to the committee suggests that AVA lots manufactured postrenovation may show less variation in reactogenicity because of greater consistency in the production process, and there is no a priori basis to believe that the postrenovation product will be more reactogenic or less immunogenic than the older vaccine.
Recommendation: As with all vaccines, AVA lots produced post-renovation should be monitored for immunogenicity and stability, and individuals receiving these lots should be monitored for possible acute or chronic adverse events of immediate or later onset.
Surveillance for Adverse Events
DoD has supported an independent civilian advisory panel called the Anthrax Vaccine Expert Committee (AVEC) to review each VAERS report associated with AVA.
The Future and AVEC
The committee found AVEC’s expert scrutiny of VAERS reports for signals that might require further action to be an important component of surveillance for the safety of AVA. However, the value of such a review process may not be limited to AVA. Furthermore, the IOM committee is generally skeptical about attribution of causality, such as those that AVEC makes, from reports to a surveillance system like VAERS, especially given the potential for misclassification of reported events when considering them as possibly related or unrelated to vaccination. The committee emphasizes that a review of case reports to VAERS is appropriate only for the generation of hypotheses. More emphasis should therefore be placed on the use of AVEC-derived hypotheses to trigger additional analyses, such as those that can be performed with data from DMSS. Toward that end, AVEC and the Army Medical Surveillance Activity (the office responsible for DMSS) should maintain regular and frequent communication, with signals from the former leading to analyses by the latter. “Signals” are the earliest indication of a possible causal relationship between an exposure and a health event. Such signals can come from the anecdotal experiences of patients with an adverse event after the exposure or from preliminary analyses of data. A signal does not mean that a causal relationship exists, as there may be other explanations for the apparent association. Instead, a signal is merely an indication that further investigation is needed.
Although AVA appears to be associated with certain undesirable but self-limited or easily treated adverse events, the committee saw no indication from the currently available data of a need to continue special monitoring programs for AVA. Nevertheless, monitoring of vaccine safety in general and the safety of vaccines for use by members of the military in particular must be a priority. The committee observed several areas in which surveillance for the safety of vaccines in general and AVA in particular might be improved.
Finding: Given the concerns raised by some service members about the safety of the anthrax vaccine, the creation of AVEC was an appropriate complement to other resources in FDA, CDC, and DoD for the monitoring of vaccine safety concerns. The results of the extra monitoring did not indicate the existence of any sentinel events that were not detected in the existing FDA and CDC reviews. The committee finds no scientific reason for the continued operation of AVEC in its present form.
The IOM committee’s observations about AVEC reflect no fault with the members of AVEC or its performance as that committee is constituted; rather, the IOM committee observes that AVEC was designed to pay extra
attention to safety concerns regarding the safety of AVA and that the data do not warrant the continuation of such exceptional attention. The resources supporting AVEC activities related to AVA alone could be more wisely invested in improved monitoring of the safety of vaccines in general.
Recommendation: DoD should disband AVEC in its current form and instead assist FDA and CDC in establishing an independent advisory committee charged with overseeing the entire process of evaluating vaccine safety. The proposed advisory committee can also assist on an ad hoc basis in the interpretation of potential signals detected in VAERS or other sources regarding the safety of any vaccine. The newly established FDA drug safety committee might be an appropriate model.
Should DoD choose to continue AVEC, the committee urges DoD to recommend a shift in AVEC’s focus from making attributions of causality in individual cases to seeking any patterns or rate thresholds that have been crossed in terms of the serious adverse events reported to VAERS. AVEC could then develop criteria for signals from VAERS data for any vaccine that warrants additional follow-up and could in general further systematize its processes by developing standard operating procedures and a regular schedule for examination of aggregate VAERS data. Background rates of illnesses as well as the biological plausibility of hypothesized effects must be taken into consideration as part of the method used to identify signals of possible safety concerns.
Recommendation: If DoD chooses to continue AVEC, DoD should consider redefining the panel’s role so that it serves as an independent advisory committee that responds on an ad hoc basis to specific requests to assist in the interpretation of potential signals detected by others (e.g., CDC and FDA) and reported to VAERS or other sources regarding the safety of all vaccines administered to service personnel rather than continuing the panel’s current role of rereviewing each VAERS report related to AVA.
Additional Sources of Data on Adverse Events
Ensuring the best use and interpretation of VAERS reports requires complementary information from other sources that can be used to help analyze the signals that may be suggested by VAERS reports. One such resource is DMSS. DMSS can be used both to generate and test hypotheses. If VAERS raises a hypothesis, it can be further evaluated in DMSS. DMSS data can also be used to generate hypotheses (as in its quarterly screening reports); these then need to be evaluated in more detail within DMSS, including more detailed data analyses and efforts that might involve review
of medical records, for example. Formal testing of these hypotheses would require additional studies, however, in separate data sets.
Finding: DMSS is a unique and promising population-based resource for monitoring the emergence of both immediate-onset and later-onset (perhaps up to 5 years) health concerns among military personnel and for testing hypothesized associations between such health concerns and exposures resulting from military service, including vaccines.
Because DMSS is designed to record all medical encounters without depending on the decision of a patient or a physician to report a particular encounter, DMSS data may be cross-checked with the more open-ended but much less complete case reports collected through VAERS.
Recommendation: DoD should develop a capability for the effective use of DMSS to regularly test hypotheses that emerge from VAERS and other sources regarding vaccine-related adverse events.
Finding: DoD personnel have used DMSS to conduct valuable analyses in response to concerns about health effects that might be associated with vaccination with AVA. Yet DoD personnel working with DMSS data are necessarily limited in time and focus. DMSS data could therefore yield valuable insights in the hands of civilian researchers.
Recommendation: DoD should actively support and advance the development of DMSS data resources and the staffing of units that will allow the continuing rapid and careful analysis of these data, including but not limited to the proposed collaboration between CDC and the Army Medical Surveillance Activity.
Recommendation: DoD should investigate mechanisms that can be used to make DMSS data available to civilian researchers, as is done by civilian agencies, with appropriate controls and protections for privacy.
As discussed in Chapter 6, data on the later-onset adverse effects of vaccines are available for few, if any, vaccines. Although the committee found no data indicating that vaccination with AVA is associated with any later-onset adverse events or with any severe or lasting adverse events, some service members have had serious concerns about possible links between AVA and such adverse events. To make it possible to conduct studies of later-onset health concerns, DoD could take steps to improve access to data on the chronic or later-onset effects, if any, of vaccines in general.
Recommendation: DoD should carefully evaluate options for longer-term follow-up of the possible health effects of vaccination against
anthrax (and other service-related exposures). The committee recommends consideration of the following specific steps:
-
Encourage participation in the Millennium Cohort Study4 as part of a program to ensure adequate monitoring for any possible later-onset health effects that might be associated with vaccination with AVA or other service-related exposures.
-
Collaborate with the Department of Veterans Affairs (VA) to monitor service members who receive medical care through VA facilities after separation from military service. Linking of data from DMSS to data from VA is a possible tool. Even though those who receive their medical care through VA may be an unrepresentative minority of all former military personnel, valid comparisons may be possible between those within that population who received a vaccine or other exposure and those who did not.
-
Collaborate with VA to obtain fact-of-death information from the Beneficiary and Records Locator System and with the Social Security Administration to obtain death files. Data on the cause of death should be obtained from the National Death Index as needed.
-
Ensure the long-term maintenance of DMSS and other relevant paper and electronic records so that retrospective studies will be feasible if health concerns are identified in the future.
New Anthrax Vaccine Development
Although AVA appears to be sufficiently safe and effective for use, it is far from optimal.
Finding: The current anthrax vaccine is difficult to standardize, is incompletely characterized, and is relatively reactogenic (probably even more so because it is administered subcutaneously), and the dose schedule is long and challenging. An anthrax vaccine free of these draw-backs is needed, and such improvements are feasible.
Initially, the committee urges that improvements to the currently licensed vaccine, AVA, be made as quickly as possible. The committee welcomes anticipated improvements in the assurance of lot-to-lot consis-
|
4 |
The Millennium Cohort Study is a survey recommended by the U.S. Congress and sponsored by DoD. The study will monitor a total of 140,000 U.S. military personnel during and after their military service for up to 21 years to evaluate the health risks of military deployment, military occupations, and general military service (see http://www.millenniumcohort.org/about.html). |
tency in the postrenovation vaccine. The committee also believes that it is likely that the rates of adverse events and the general acceptability of AVA will improve with a change in the route of administration (from the subcutaneous to the intramuscular route) and with a reduction in the total number of injections required and that such improvements would be desirable. Research to assess the effects of those changes in vaccine administration was under way as this report was being written.
The committee concluded, however, that a new vaccine, developed according to more modern principles of vaccinology, is urgently needed. The committee did not comment on any particular new vaccine development program, and a review of research related to the development of a new vaccine was beyond its charge. The committee recognizes that research on new vaccines against anthrax is under way at DoD, the National Institutes of Health, and various university laboratories and strongly encourages continued and further support of work on promising new vaccines. Further research with AVA on topics such as correlates of immunity in animals, the components necessary to stimulate protective immunity, and the best way to administer the vaccine should aid in the development of new and improved vaccine products for protection against anthrax.
Recommendation: DoD should continue and further expedite its research efforts pertaining to anthrax disease, the B. anthracis organism, and vaccines against anthrax. Research related to anthrax should include, in particular, efforts such as the following:
-
DoD should pursue and encourage research to develop an anthrax vaccine product that can be produced more consistently and that is less reactogenic than AVA;
-
DoD should pursue and encourage research regarding the B. anthracis capsule;
-
DoD should pursue and encourage research on the mechanisms of action of the anthrax toxins; such research could lead to the development of small-molecule inhibitors;
-
DoD should pursue and encourage research to map the epitopes of the protective antigen that correlate with specific functional activities;
-
DoD should pursue and encourage research to test the therapeutic potential of antitoxin proteins or antibodies; and
-
DoD should pursue and encourage research into additional potential virulence factors in B. anthracis, and into other possible vaccine candidates.
|
BOX ES-1 Chapter 3 Findings and Recommendations Findings
Recommendations
|
|
BOX ES-2 Chapter 5 Findings and Recommendations Findings
Recommendations
|
|
BOX ES-3 Chapter 6 Findings and Recommendations Findings
|
|
Recommendations
|
|
BOX ES-4 Chapter 7 Findings
|
|
BOX ES-5 Chapter 8 Findings
|
|
BOX ES-6 Chapter 8 Recommendations
|
|
sible between those within that population who received a vaccine or other exposure and those who did not.
|
REFERENCES
AMSA (Army Medical Surveillance Activity). 2001a. Quarterly Report—January 2001. Surveillance of Adverse Effects of Anthrax Vaccine Adsorbed. Washington, D.C.: Army Medical Surveillance Activity, U.S. Army Center for Health Promotion and Preventive Medicine.
AMSA. 2001b. Quarterly Report—April 2001. Surveillance of Adverse Effects of Anthrax Vaccine Adsorbed. Washington, D.C.: Army Medical Surveillance Activity, U.S. Army Center for Health Promotion and Preventive Medicine.
AMSA. 2001c. Surveillance of Adverse Effects of Anthrax Vaccine Adsorbed: Results of Analyses Requested by the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine. Washington, D.C.: Army Medical Surveillance Activity, U.S. Army Center for Health Promotion and Preventive Medicine.
Auerbach BA, Wright GG. 1955. Studies on immunity in anthrax. VI. Immunizing activity of protective antigen against various strains of Bacillus anthracis. Journal of Immunology 75:129–133.
Barnard JP, Friedlander AM. 1999. Vaccination against anthrax with attenuated recombinant strains of Bacillus anthracis that produce protective antigen. Infection and Immunity 67(2):562–567.
Beedham RJ, Turnbull PC, Williamson ED. 2001. Passive transfer of protection against Bacillus anthracis infection in a murine model. Vaccine 19(31):4409–4416.
Bhatnagar R, Batra S. 2001. Anthrax toxin. Critical Reviews in Microbiology 27(3):167–200.
Brachman PS, Friedlander AM. 1999. Anthrax. In: Plotkin SA, Orenstein WA, eds. Vaccines, 3rd ed. Philadelphia, Pa.: W. B. Saunders Co. Pp. 629–637.
Brachman PS, Gold H, Plotkin S, Fekety FR, Werrin M, Ingraham NR. 1962. Field evaluation of a human anthrax vaccine. American Journal of Public Health 52:632–645.
Cataldi A, Labruyere E, Mock M. 1990. Construction and characterization of a protective antigen-deficient Bacillus anthracis strain. Molecular Microbiology 4(7):1111–1117.
CDC (Centers for Disease Control and Prevention). 1967–1971. Application and Report on Manufacture of Anthrax Protective Antigen, Aluminum Hydroxide Adsorbed (DBS-IND 180). Observational Study. Atlanta, Ga.: Centers for Disease Control and Prevention.
CDC. 2001a. Update: investigation of bioterrorism-related anthrax and adverse events from antimicrobial prophylaxis. MMWR (Morbidity and Mortality Weekly Report) 50(44): 973–976.
CDC. 2001b. Update: investigation of bioterrorism-related inhalational anthrax—Connecticut, 2001. MMWR (Morbidity and Mortality Weekly Report) 50(47):1049–1051.
Dixon TC, Meselson M, Guillemin J, Hanna PC. 1999. Anthrax. New England Journal of Medicine 341(11):815–826.
FDA (Food and Drug Administration). 1985. Biological products: bacterial vaccines and toxoids: implementation of efficacy review. Proposed rule. Federal Register 50(240): 51002–51117.
Fellows PF, Linscott MK, Ivins BE, Pitt ML, Rossi CA, Gibbs PH, Friedlander AM. 2001. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine 19(23– 24):3241–3247.
Grabenstein JD. 2000. The AVIP: status and future. Presentation to the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Meeting I, Washington, D.C.
Grinberg LM, Abramova FA, Yampolskaya OV, Walker DH, Smith JH. 2001. Quantitative pathology of inhalational anthrax. I. Quantitative microscopic findings. Modern Pathology 14(5):482–495.
Henderson DA. 1999. The looming threat of bioterrorism. Science 283(5406):1279–1282.
Hoffman K, Costello C, Engler RJM, Grabenstein J. Submitted for publication. Using a patient-centered structured medical note for aggregate analysis: determining the side-effect profile of anthrax vaccine at a mass immunization site.
Ivins BE, Fellows PF, Nelson GO. 1994. Efficacy of a standard human anthrax vaccine against Bacillus anthracis spore challenge in guinea pigs. Vaccine 12(10):872–874.
Ivins BE, Fellows PF, Pitt MLM, Estep JE, Welkos SL, Worsham PL, Friedlander AM. 1996. Efficacy of a standard human anthrax vaccine against Bacillus anthracis aerosol spore challenge in rhesus monkeys. Salisbury Medical Bulletin 87(suppl):125–126.
Ivins BE, Pitt ML, Fellows PF, Farchaus JW, Benner GE, Waag DM, Little SF, Anderson GW Jr, Gibbs PH, Friedlander AM. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16(11–12): 1141–1148.
Jackson PJ. 2001. Genetic diversity in B. anthracis. Presentation to the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Meeting III, Washington, D.C.
Jackson PJ, Hugh-Jones ME, Adair DM, Green G, Hill KK, Kuske CR, Grinberg LM, Abramova FA, Keim P. 1998. PCR analysis of tissue samples from the 1979 Sverdlovsk anthrax victims: the presence of multiple Bacillus anthracis strains in different victims. Proceedings of the National Academy of Sciences USA 95(3):1224–1229.
Keim P, Kalif A, Schupp J, Hill K, Travis SE, Richmond K, Adair DM, Hugh-Jones M, Kuske CR, Jackson P. 1997. Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. Journal of Bacteriology 179(3): 818–824.
Keim P, Price LB, Klevytska AM, Smith KL, Schupp JM, Okinaka R, Jackson PJ, Hugh-Jones ME. 2000. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. Journal of Bacteriology 182(10):2928–2936.
Lange JL, Lesikar SE, Brundage JF, Rubertone MV. 2001a. Screening for adverse events following anthrax immunization using the Defense Medical Surveillance System. Presentation to the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Meeting II, Washington, D.C.
Lange JL, Lesikar SE, Brundage JF, Rubertone MV. 2001b. Update: surveillance of adverse events of Anthrax Vaccine Adsorbed. Presentation to the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Meeting IV, Washington, D.C.
Little SF, Knudson GB. 1986. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infection and Immunity 52(2):509–512.
Little SF, Ivins BE, Fellows PF, Friedlander AM. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infection and Immunity 65(12):5171–5175.
Mason KT, Grabenstein JD, McCracken LR. 2001. Hearing loss after anthrax vaccination among US Army aircrew members. Unpublished manuscript.
Mason KT, Grabenstein JD, McCracken LR. Submitted for publication. US Army Aviation Epidemiology Data Register: physical findings after anthrax vaccination among US Army aircrew members, a prospective matched-pair, case-control study.
McBride BW, Mogg A, Telfer JL, Lever MS, Miller J, Turnbull PC, Baillie L. 1998. Protective efficacy of a recombinant protective antigen against Bacillus anthracis challenge and assessment of immunological markers. Vaccine 16(8):810–817.
Mogridge J, Mourez M, Lacy B, Collier RJ. 2001. Role of protective antigen oligomerization in anthrax toxin action. In: Program and Abstracts of the Fourth Annual Conference on Anthrax. Washington, D.C.: American Society for Microbiology.
Peeler RN, Cluff LE, Trever RW. 1958. Hyper-immunization of man. Bulletin of the Johns Hopkins Hospital 103:183–198.
Peeler RN, Kadull PJ, Cluff LE. 1965. Intensive immunization of man: evaluation of possible adverse consequences. Annals of Internal Medicine 63(1):44–57.
Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, Gibbs P, Dertzbaugh M, Friedlander AM. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19(32):4768–4773.
Pittman PR. 2001a. Anthrax vaccine: an analysis of short-term adverse events in an occupational setting—the Special Immunization Program experience over 30 years. Presentation to the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Meeting II, Washington, D.C.
Pittman PR. 2001b. Anthrax vaccine: dose reduction/route change pilot study. Presentation to the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Meeting II, Washington, D.C.
Pittman PR. 2001c. Anthrax vaccine: the Fort Bragg Booster Study. Presentation to the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Meeting II, Washington, D.C.
Pittman PR, Sjogren MH, Hack D, Franz D, Makuch RS, Arthur JS. 1997. Serologic Response to Anthrax and Botulinum Vaccines. Protocol No. FY92-5, M109, Log No. A-5747. Final report to the Food and Drug Administration. Fort Detrick, Md.: U.S. Army Medical Research Institute of Infectious Diseases.
Pittman PR, Gibbs PH, Cannon TL, Friedlander AM. 2001a. Anthrax vaccine: short-term safety experience in humans. Manuscript.
Pittman PR, Gibbs PH, Cannon TL, Friedlander AM. 2001b. Anthrax vaccine: short-term safety experience in humans. Vaccine 20(5–6):972–978.
Pittman PR, Kim-Ahn G, Pifat DY, Coon K, Gibbs P, Little S, Pace-Templeton J, Myers R, Parker GW, Friedlander AM. 2002. Anthrax vaccine: safety and immunogenicity of a dose-reduction, route comparison study in humans. Vaccine 20(9–10):1412–1420.
Pittman PR, Hack D, Mangiafico J, Gibbs P, McKee KT Jr., Friedlander AM, Sjogren MH. In press. Antibody response to a delayed booster dose of anthrax vaccine and botulinum toxoid . Vaccine.
Price LB, Hugh-Jones M, Jackson PJ, Keim P. 1999. Genetic diversity in the protective antigen gene of Bacillus anthracis. Journal of Bacteriology 181(8):2358–2362.
Rehme P. 2001. Ambulatory medical visits among anthrax-vaccinated and unvaccinated personnel after return from Southwest Asia. Presentation to the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Meeting IV, Washington, D.C.
Rehme PA, Williams R, Grabenstein JD. 2002. Ambulatory medical visits among anthrax-vaccinated and unvaccinated personnel after return from Southwest Asia. Military Medicine 167(3):205–210.
Reuveny S, White MD, Adar YY, Kafri Y, Altboum Z, Gozes Y, Kobiler D, Shafferman A, Velan B. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infection and Immunity 69(5):2888–2893.
Sato PA. 2001a. DoD-wide surveillance of hospitalizations for long-term adverse events potentially associated with anthrax immunization. Presentation to the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Meeting IV, Washington, D.C.
Sato PA. 2001b. Questions on updated data. E-mail to Joellenbeck L, Institute of Medicine, Washington, D.C., November 21 .
Sato PA, Reed RJ, Smith TC, Wang LZ. 2001. DoD-wide medical surveillance for potential long-term adverse events associated with anthrax immunization: hospitalizations. Provisional report for IOM of data from January 1998 to March 2000. San Diego, Calif.: U.S. Department of Defense Center for Deployment Health Research at the Naval Health Research Center.
Sellman BR, Mourez M, Collier RJ. 2001. Dominant-negative mutants of a toxin subunit: an approach to therapy of anthrax. Science 292(5517):695–697.
Smith H, Keppie J. 1954. Observations on experimental anthrax: demonstration of a specific lethal factor produced in vivo by Bacillus anthracis. Nature 173:869–870.
Treanor JJ. 2001. Adverse reactions following vaccination of adults. Commissioned paper. Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Institute of Medicine, Washington, D.C.
Turnbull PC, Broster MG, Carman JA, Manchee RJ, Melling J. 1986. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infection and Immunity 52(2):356–363.
Wasserman GM. 2001. Analysis of adverse events after anthrax vaccination in U.S. Army medical personnel. Presentation to the Institute of Medicine Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Meeting IV, Washington, D.C.
Weiss R. 2001, September 29. Demand growing for anthrax vaccine: fear of bioterrorism attack spurs requests for controversial shot. Washington Post. p. A16.
White CS, Adler WH, McGann VG. 1974. Repeated immunization: possible adverse effects. Annals of Internal Medicine 81(5):594–600.
Zilinskas RA. 1997. Iraq’s biological weapons: the past as future? JAMA 278(5):418–424.
































