2
Nutrient Content and Special Considerations
This chapter presents the rationale for the levels of individual nutrients recommended for the emergency food product (EFP) described in this report, and discusses additional issues to be considered.
|
The goal of an EFP is to reduce morbidity and mortality among displaced persons by providing a nutritionally complete food that will be adequate as a sole source of nutrients for as long as 15 days from the recognized time of displacement. It should provide nutrition for the period between initial displacement and establishment of a more stable food supply line. |
The EFP should be consumed with an ample quantity of water to ensure that the osmotic load provided by the EFP is diluted. This report assumes that emergency relief agencies will provide potable water supplies as a top priority. This assumption is based on assurances provided by the United States Agency for International Development.
There are five characteristics critical to the development of a successful EFP, listed in order of priority: (1) safe, (2) palatable, (3) easy to deliver, (4) easy to use, and (5) nutritionally complete. This order of priority should guide decisions about competing characteristics in developing a prototype EFP.
TABLE 2-1 The Population Distribution from Two Reports Providing Demographic Information used to Determine Nutritional Needs for Disaster Responses
|
Sub-Saharan Africaa |
The Sphere Projectb |
||
|
Age Group (yr) |
% of Population |
Age Group (yr) |
% of Population |
|
0–3 |
10 |
0–4 |
12 |
|
4–6 |
7 |
5–9 |
11 |
|
7–9 |
7 |
10–14 |
11 |
|
10–17 |
17 |
15–19 |
9 |
|
18–60 |
48 |
20–59 |
49 |
|
> 60 |
7 |
60+ |
7 |
|
|
Pregnant |
2 |
|
|
|
Lactating |
3 |
|
|
|
Male/female |
51/49 |
|
|
a Jamison and Hobbs (1994). b Sphere Project (2001). |
|||
INTRODUCTION
The nutritional advantages of a single EFP as opposed to two or more products are evident. Providing a limited selection of commodity-type foods may increase the risk of malnutrition because nutritional components that are found in only one of the foods (e.g., ascorbic acid) may be absent from the diet if that food is not selected. Under emergency conditions, diets are invariably highly monotonous, and often relief foods quickly become a medium of exchange and are commonly sold or traded for other foods, water, firewood, alcohol, and a variety of other goods and services. If a nutritionally complete food ration is divided among two or more different foods, or if foods are targeted to specific individuals such as children or pregnant women, then certain foods are more likely to be exchanged. This type of exchange can deprive the population of a portion of the profile of nutrients provided by the emergency food ration and increase the risk of malnutrition. Providing a single ration product would reduce this risk.
CHARACTERISTICS OF TARGET POPULATIONS
Characteristics of potential target populations were considered in determining the nutrient composition of the EFP. As shown in Table 2-1, some target populations may have as much as 23 percent of the population below 10 years of age and 12 to 17 percent below 5 years of age (Jamison and Hobbs, 1994; Sphere Project, 2001). Refugee groups fleeing from military conflicts may have
TABLE 2-2 Estimated Mean Per Capita Energy Requirement (EMPCER) by Body Size of Adults
|
|
Sub-Saharan Africa |
South and Southeast Asia |
United States |
|
Male height, weight |
170 cm, 63.5 kg |
165 cm, 60.1 kg |
180.4 cm, 78.1 kg |
|
Female height, weight |
155 cm, 50.0 kg |
153 cm, 49.0 kg |
163.7 cm, 55.3 kg |
|
EMPCER |
2,076 |
2,045 |
2,194 |
|
SOURCE: Institute of Medicine (IOM, 1995b). |
|||
women and children as a large proportion of the population, with only a small proportion of women pregnant or lactating.
Data from the Nutrition Collaborative Research Support Program (CRSP) in Kenya (Calloway et al., 1992; Neumann and Harrison, 1994; Neumann et al., 1991), as well as data from sub-Saharan Africa (Sphere Project, 2001) and South and Southeast Asia (James and Schofield, 1990), indicate that people from these areas have smaller body sizes than those in Western populations (Table 2-2).
While the EFP might have nonemergency uses (e.g., as a complementary food for breast-fed children 7 to 12 months of age), it has been designed as a sole food source for periods of 2 to 15 days. It is likely that the recipient population will be in poor nutritional status and may have some wasting, appetite depression, and malabsorption. The goal of this report is to provide recommendations for a product that would meet the needs of diverse populations.
General Assumptions
Given the goal outlined above, the following assumptions are made regarding the recipient population:
-
The relief food product is the only food consumed.
-
Individuals eat to meet their energy requirement.
-
Individuals in the target population are of smaller stature and body mass than similarly aged groups in the North American population (this is the same premise used in an earlier report from the Food and Nutrition Board, Estimated Mean per Capita Energy Requirements for Planning Emergency Food Aid Rations [IOM, 1995b]).
-
All individuals over the age of 6 months will consume the product.
Estimating Energy Requirements
The energy content of the EFP should be determined by the energy needs of the recipient population. However, because the EFP must be manufactured prior to knowing where it will be needed, the population’s energy needs will not be known. Recommended intakes for nutrients from recent reports in the United States and Canada are typically used as the standard for nutrient requirements and thus nutrient content (IOM, 1997a, 1998, 2000, 2001), but, as discussed earlier, energy consumption per individual may be less in the EFP target population than in the United States or Canada due to lower body weights for similar subgroups. Furthermore, because the EFP is a single food meant to support a heterogeneous population, nutrient content must be determined on an energy density basis.
Estimating Energy Requirements of the Population
A potential basis for calculating the energy requirements for a refugee population is provided in the Institute of Medicine report, Estimated Mean per Capita Energy Requirements for Planning Emergency Food Aid Rations (IOM, 1995b). The goal of this report was to establish an estimated mean per capita energy requirement (EMPCER) when little was known about the characteristics of the population. Energy requirements for 14 age and gender groups, plus pregnant and lactating women, were estimated based on body mass and assumptions about energy needs in pregnancy and lactation obtained in two refugee populations. The estimated energy requirements for adults were calculated based on an estimate of basal metabolic rate (BMR) and a physical activity level (PAL). To estimate BMR, the report used equations developed by the Food and Agriculture Organization/World Health Organization/United Nations University (FAO/ WHO/UNU, 1985). An average height of 170 cm for adult men and 155 cm for adult women was assumed (the average of adult men and women in sub-Saharan Africa; see Table 2-2). These average heights are slightly greater than those of adults in South and Southeast Asia (Table 2-2) and less than those of the U.S. population. The weights used for the estimates of BMR were the median weight for U.S. adult males of 170 cm (63.5 kg) and females of 155 cm (50 kg). The U.S. median weights (NRC, 1989) were used to provide a conservative estimate of the EMPCER for populations in most developing countries (IOM, 1995b).
For individuals under 18 years of age, values were based on data from affluent populations. Although the individuals from whom these data were derived were larger (and therefore assumed to have a greater BMR) than many children and adolescents from refugee populations, this “extra” allotment for children in developing countries was deemed appropriate on the basis that the additional food would allow some compensatory growth (IOM, 1995b). Both the adult and child values were recognized as overestimates of energy requirements, but were justified in order to establish a conservative EMPCER.
The resulting EMPCER in the report was 2,100 kcal/day (after rounding). This number is used below as the basis for the total energy content of the EFP.
Estimating Energy Requirements for Specific Life Stage and Gender Groups
The IOM (1995b) report estimated energy requirements for specific life stage and gender groups, as described above. However, it was determined that using that approach was inappropriate for determining the content of the EFP for three reasons. First, the approach could lead to underestimates of nutrient density needed because the nutrient density is based on an assumed energy intake. If energy intake is less than expected, the nutrient density will be too low to meet the micronutrient requirements. Second, the life stage and gender groups do not correspond to the current groups used in the Dietary Reference Intake (DRI) reports (IOM, 1997a, 1998, 2000, 2001). Third, the FAO/WHO/UNU (1985) equations used for infants and children under age 5 are now recognized as flawed (Butte, 1996; Torun et al., 1996).
For the above reasons, estimates of energy requirements for each life stage category were recalculated and are shown in Table 2-3. For individuals 4 years of age and older, estimated energy requirements were obtained by first calculating individual BMRs based on the age, sex, weight, and physiological status of each individual (FAO/WHO/UNU, 1985). Individual energy requirements were then calculated using the same PAL values (women: 1.56, men: 1.55) that were used by IOM (1995b).
With the exception of infants aged 7 through 12 months, the BMR and energy requirements were derived using anthropometric data from individuals in the Kenya Nutrition CRSP (Calloway et al., 1992; Neumann and Harrison, 1994; Neumann et al., 1991). Because the Kenya Nutrition CRSP did not collect anthropometry on children aged 6 through 12 months, the value for this age group was the mean weight of rural infants aged 9 months from the Mexico Nutrition CRSP (Allen et al., 1992).
The Kenya data set contains anthropometry on 1,717 individuals aged 0 to 65 years. As is common in much of the developing world, most adults and children in this population were smaller than U.S. individuals, the result of early growth stunting (Martorell and Habicht, 1986) (Figure 2-1). Additionally, the rural Kenyan population was subject to periodic food shortages and were relatively thin (Neumann and Harrison, 1994).
Estimating Energy Requirements for Infants and Children. Recent research using doubly labeled water to measure energy expenditure suggests that values derived from the FAO/WHO/UNU 1985 equations are inflated for infants and young children (Butte, 1996; Butte et al., 2000; de Bruin et al., 1998; Prentice et al., 1988). Therefore, the energy requirements for infants 9 months of age (representing the 7- through 12-month-old group) and children 2 years of age
TABLE 2-3 Median Weights, Estimated Basal Metabolic Rate (BMR), and Energy Requirements of a Rural Kenyan Populationa
|
Age |
Gender |
Weight (kg) |
BMR (kcal/d) |
Energy (kcal/d) |
Estimated Number of Emergency Food Product (EFP) Barsb per day |
|
Both |
7.0 |
371 |
578 |
1–2e |
|
|
1–3 yrd |
Both |
10.2 |
571 |
855 |
3–4 |
|
4–8 yr |
Both |
19.4 |
936 |
1,456 |
6–7 |
|
9–13 yr |
Both |
26.5 |
1,086 |
1,693 |
7–8 |
|
14–18 yr |
Boys |
42.0 |
1,378 |
2,136 |
9 |
|
|
Girls |
40.9 |
1,238 |
1,931 |
8–9 |
|
19–50 yr |
Men |
54.3 |
1,509 |
2,339 |
9–10 |
|
|
Women |
51.0 |
1,264 |
1,972 |
8–9 |
|
51+ yr |
Men |
56.1 |
1,451 |
2,249 |
9–10 |
|
|
Women |
47.0 |
1,237 |
1,929 |
8–9 |
|
a Weights from Kenya Nutrition CRSP (Calloway et al., 1992). b Each EFP bar has approximately 233 kcal; 9 bars = 2,100 kcal = one average ration per day. Each can be broken in half to yield 116 kcal. This allows distribution to young children. c Weights from Mexico Nutrition CRSP (Allen et al., 1992). d BMR estimate based on equations of Butte and coworkers (2000). e It is assumed that the EFP would be used as a complementary energy source to human milk and therefore would provide 50 percent of the estimated energy need. |
|||||
(representing the 1- through 3-year-old group) were calculated according to the formula of Butte and coworkers (2000):
Energy requirements (MJ/d) = 0.321 + 0.013 × age (mo) - 0.047 × sex + 0.139 × feeding group + 0.277 × weight,
where sex is coded as 1 for boys, and 2 for girls, and feeding group is coded as 1 for breast-feeding (nearly all children in the Kenyan and Mexican populations). Values for boys and girls were later averaged.
The Butte equations were based on breast-fed children in the United States and yielded values of similar magnitude to those derived for Mexican infants and young children: 638 kcal/day for 0- through 9-month-old infants and 843 kcal/day for 1- through 2-year-old toddlers (Butte et al., 2000). The resulting energy estimates are lower than those used in the IOM (1995b) report (800 and 1,350 kcal/day, respectively), because the IOM values are based on the energy requirements of children derived from the FAO/WHO/UNU (1985) equations and U.S. body weights.
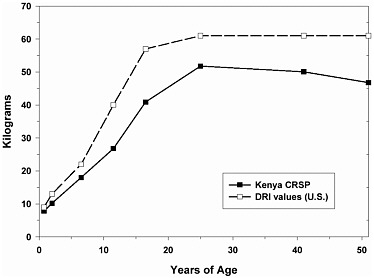
FIGURE 2-1 Reference weights of DRI life stage groups (U.S. population), and weights of rural Kenyans.
Estimating Energy Requirements for Pregnancy and Lactation. Although adequate nutrition during pregnancy and lactation are of concern in refugee populations, the EFP is designed to meet energy requirements based on the assumption that pregnant or lactating women as well as others with higher energy needs (i.e., due to physical activity or rapid growth) will consume additional food bars to meet these needs.
In 1985, FAO/WHO/UNU recommended an increased energy intake of 285 kcal/day during pregnancy. However, the actual increased energy needs during pregnancy vary widely by trimester (Prentice et al., 1996) and by population (Prentice and Goldberg, 2000). For example, the total additional energy needed during pregnancy in The Gambia has been estimated at about 7,000 kcal, or about 25 kcal/day (Prentice and Goldberg, 2000). Moreover, Prentice and colleagues (1996) have proposed that maternal energy metabolism during pregnancy may be lower as measured by change in BMR in women in developing countries versus those in affluent populations. This is believed to be due to their smaller body size. If true, then pregnant women in some emergency feeding situations may not need to consume 285 kcal beyond their nonpregnant, nonlactating energy requirement (or 1 to 2 additional food bars over the 9-bar ration). This number is near to the estimated daily increment of 229 kcal/day during the
second trimester, when pregnancy energy requirements appear to be intermediate (Prentice et al., 1996).
FAO/WHO/UNU (1985) also recommended an additional 500 kcal/day during lactation, which assumed an additional 200 kcal/day obtained from maternal fat stores. Prentice and colleagues (1996), based on an extensive review of the literature, recommended an increment of 480 kcal/day for mothers of infants 1 through 6 months of age with previous weight loss.
CHARACTERISTICS OF THE EMERGENCY RELIEF FOOD PRODUCT
Given the estimated energy requirements (Table 2-3), the proposed energy density for the EFP is 4 to 5 kcal (17 to 21 kJ)/g. To obtain this energy density, an EFP low in water (see Chapter 3) with 35 to 45 percent fat along with 10 to 15 percent protein is required (see sections below). Palatability of the EFP is a primary concern, and should dictate the final choice of ingredients (see Chapter 3). It is assumed that pregnant and lactating women will consume more than the average requirement of 2,100 kcal as needed to support pregnancy and lactation.
Nutrient Content
The methodology for determining the appropriate amount of each nutrient to be included in the EFP is summarized in Box 2-1, followed by a more detailed explanation and rationale for the approach adopted for each nutrient.
A starting premise for determining the appropriate nutrient content of the EFP is that the upper limit of an individual’s food intake is somewhat constrained by his or her total energy requirement, while the lower limit is set by many factors, including appetite, access to food, trading of food, and an individual’s ability to make his or her own food decisions. When food intake is lower than energy requirements, the nutrient density may need to be adjusted, thus highlighting a need for testing prototype EFPs developed from the specifications presented in this report.
The recommended intakes (either recommended dietary allowance [RDA] or adequate intake [AI]) as specified in the recent reports on DRIs (IOM, 1997a, 1998, 2000, 2001) were used. These reports provide recommended intakes for vitamins and minerals for 16 life stage and gender groups, plus pregnancy and lactation. It should be noted that these DRIs were established based on a selected criterion or criteria of adequacy consistent with good health, as opposed to the mere prevention of overt deficiencies. Thus the values obtained may be higher than those previously recommended by WHO. RDAs were calculated from the estimates of average requirements (EAR) using an estimate of the variability among individuals in the requirement. In most cases, the coefficient of variation of nutrient requirements was assumed to be 10 percent. The RDA is set at two
|
BOX 2-1 Summary of Methodology to Determine the Nutrient Content of the EFP
|
standard deviations above the EAR, and should meet the requirements of almost all of the U.S. and Canadian populations for which it is recommended.
For some required nutrients, it was not possible to establish an intake at which half of a life stage and gender group would be adequately nourished, while the other half would demonstrate signs of inadequacy. Thus an EAR could not be set. However, data were available that could be used to establish a level of intake that appeared adequate for most, if not all, people consuming that amount. This is called the adequate intake (AI). AIs are available for a number of the nutrients included in the EFP. Given that the data upon which an AI is
based are less certain, there is more judgment in its derivation. In some cases, the EFP may not provide the AI level due to constraints related to palatability or cost. In this case, the probability that the target population has underlying nutrient deficiencies is assumed, and what is feasible for a product to be used for 10 to 15 days as the sole source of nutrition is determined.
There are also nutrients that are deemed essential for inclusion in an EFP, but for which DRIs have not yet been determined. In this case (i.e., macronutrients and electrolytes), other recommendations for these nutrients (FAO/WHO, 2000; NRC, 1989) were considered in determining the amounts appropriate for the EFP.
Ideally, the formulation of an EFP requires information on variability in actual consumption of the relief food. Since such information is not available at this developmental stage of the product, a few cautionary flags must be raised in the use of the proposed EFP:
-
The EFP is not designed to meet all the nutrient needs for pregnancy and lactation; however, due to the energy requirements being conservatively estimated based on energy needs for smaller individuals, it should meet the requirements for most nutrients for almost all women.
-
The EFP is not appropriate for severely malnourished individuals who require medical attention. Severe malnutrition is defined in the WHO Sick Child Initiative as quoted by IOM (1995a) as the presence of any one of the following symptoms: visible severe wasting, severe pallor, clouding of the cornea, or edema of both feet.
-
The EFP is not a therapeutic nutritional supplement. (A ration distributed to the general population cannot be formulated as a therapeutic diet, as it would present too many risks of excess intake for individuals who were not severely malnourished. Severely malnourished individuals need special help, including fluid and electrolyte replacement therapy, blood transfusions for severe anemia, and medical supervision. This food product is not meant to be a substitute for this therapy, but a sustaining ration for people who have been uprooted due to war or natural disaster.)
-
The EFP is not a substitute for human milk for infants agers 0 to 6 months.
-
The EFP is not designed to meet the needs of young infants; however, it may be combined with water to produce a gruel suitable as a complementary food for older infants (7 to 12 months of age).
Determination of a Minimal Nutrient Density
At the population level, there are a number of individual minimal nutrient densities for each nutrient. If a single food must meet the nutrient requirement of most individuals in the population, this food should have a nutrient density that
meets or exceeds the minimal nutrient densities of most individuals in the population. Since food intake is limited by energy requirements, a high nutrient density is necessary to meet the nutrient requirements of an individual with low energy needs. The approach described here to establish the nutrient content for the EFP provides a complete food for individuals consuming on average as little as 855 kcal/day (1- to 3-year-old age group) to those who may require in excess of the average ration of 2,100 kcal/day (adult men); thus the EFP can be used by a diverse population.
The approach used to determine nutrient density for the EFP is as follows: for each nutrient, a minimal density value was estimated for the life stage and gender group in the population with the highest nutrient requirement relative to their energy requirement using the data on recommended nutrient intakes (Table 2-4) (IOM, 1997a, 1998, 2000, 2001; NRC, 1989; WHO, 2000), divided by the estimated average energy requirement for that life stage and gender group based on data from Kenyan refugee populations (Table 2-3). Neither pregnant nor lactating women were considered as a limiting group because for some nutrients (e.g., iodine, vitamin A) the minimal nutrient density would provide intakes that would exceed the UL (IOM, 1997a) for other groups in the population. Additional assumptions used in setting the minimal nutrient density include:
-
The relief food is the only food consumed.
-
Individual energy consumption equals energy requirement.
-
The food product should provide a nutrient density that will meet the nutrient requirements of almost all members of each life stage and gender group without exceeding the UL for any group.
These assumptions err in the direction of providing more of a nutrient than may be necessary unless energy consumption does not meet energy requirements. In most cases, the RDA values used were calculated from EARs which were originally estimated from only a few individuals with assumed variations in requirements, and then extrapolated to other age and gender groups using conservative approaches. Most of the estimates of AIs were based on mean intakes for healthy population groups that did not demonstrate any indicators of inadequacy of the nutrient, and thus could easily be overestimates of actual requirements for subgroups.
Finally, in the case of many nutrients, the minimal nutrient density was subsequently modified upward in order to ensure that possible interactions with other nutrients or storage conditions, poorer bioavailability, or assumed presence of diarrhea or disease in the recipient population were taken into account. Since increased amounts of nutrients will increase the cost and potentially may affect palatability and shelf life of the EFP, and palatability is the major factor that ensures adequate energy consumption, slight reductions in these recommended amounts may be necessary.
TABLE 2-4 Unadjusted Baseline Minimal Nutrient Density Values Using Recommended Intakes
|
Nutrient |
Limiting Group |
Baseline per 1,000 kcala |
Basis for Recommended Intake |
|
Fat |
N/A |
39–50 g |
Providing an energy density of 4–5 kcal/g |
|
Proteinb |
51+ yr, men |
34 g |
Balance studies |
|
Carbohydrate |
N/A |
100–125 g |
Seven to 12 of the 23–35 g of total carbohydrate should be from sugars for adequate palatability |
|
Sodiumc |
2–5 yr, children |
1.3 g |
Maximum level of intake |
|
Potassiumc |
2–5 yr, children |
1.7 g |
Level estimated to meet minimum requirements |
|
Chloridec |
2–5 yr, children |
2.0 g |
Level estimated to be equimolar to sodium |
|
Calcium |
9–13 yr, children |
768 mg |
Based on maximal calcium retention |
|
Phosphorus |
9–13 yr, children |
740 mg |
Based on factorial approach |
|
Magnesium |
14–18 yr, boys |
190 mg |
Amount needed to maintain magnesium balance |
|
Chromium |
— |
13.5 µg |
Based on amounts in well-balanced diets/1,000 kcal |
|
Copper |
51+ yr, women |
470 µg |
Biochemical indicators of copper status |
|
Iodine |
1–3 yr, children |
105 µg |
Balance studies |
|
Irond |
19–50 yr, women |
9 mg |
Based on iron requirement (estimated basal losses, increase in hemoglobin mass, increase in nonstorage iron, increase in storage iron) plus assumed iron absorption |
|
Manganese |
1–3 yr, children |
1.4 mg |
Average intake in healthy population |
|
Selenium |
14–18 yr, girls |
28 µg |
Maximizing plasma glutathione peroxidase activity |
|
Zinc |
14–18 yr, boys |
5.2 mg |
Level needed to match exogenous losses |
|
Vitamin A |
14–18 yr, boys |
420 µg RAE |
Level needed to maintain adequate stores |
|
Vitamin D |
51–70 yr, women |
5.2 µg |
Maintain serum 25(OH)vitamin D levels |
|
Vitamin E |
14–18 yr, girls |
7.8 mg |
Level needed to prevent hydrogen peroxide-induced hemolysis |
|
Nutrient |
Limiting Group |
Baseline per 1,000 kcala |
Basis for Recommended Intake |
|
Vitamin K |
19–50 yr, men |
~60 µg |
Average intakes in adequately nourished population groups |
|
Vitamin C |
51+ yr, men |
40 mg |
Level needed to maintain near-maximal neutrophil concentration with minimal urinary loss |
|
Thiamin |
1–3 yr, children |
0.6 mg |
Level needed for normal erythrocyte transketolase activity |
|
Riboflavin |
14–18 yr, boys |
0.6 mg |
Level needed to maintain normal erythrocyte glutathione reductase activity and urinary riboflavin excretion |
|
Niacin |
14–18 yr, boys |
7.5 mg |
NE Level needed to maintain adequate niacin metabolism as measured by excretion of metabolites |
|
Vitamin B6 |
51+ yr, women |
0.8 mg |
Level needed to replete depleted stores |
|
Folatee |
14–18 yr, girls |
207 µg |
Level needed to maintain normal homocysteine, red cell folate concentrations |
|
Vitamin B12 |
14–18 yr, girls |
1.2 µg |
Level needed to maintain normal B12 levels and hematological status in adults |
|
Pantothenic acid |
14–18 yr, girls |
2.6 mg |
Average intake in healthy population |
|
Biotin |
51+, women |
16 µg |
Average intake in healthy population |
|
Choline |
51+, men |
244 mg |
Level needed to maintain normal liver enzyme levels in young adults |
|
a Estimated energy requirements for each limiting group taken from Table 2-3. b From NRC (1989); based on reference weights from IOM (1997a) and estimated energy expenditure from Table 2-3. c Values based on estimated requirements, desirable intakes, or maximal intakes (NRC,1989). d Based on 10% iron bioavailability. e If folate is provided as synthetic folate, which is more readily absorbed, these numbers should be divided by 1.6. SOURCE: IOM (1997a, 1998, 2000, 2001). |
|||
In order to individualize and facilitate the use of the EFP to the extent possible, it is designed to be consumed in multiple subunits so that it is possible to consume from 117 kcal (one-half of a scored 233-kcal EFP bar) to 2,100 kcal (9 EFP bars, which are 1 day’s ration) or more (e.g., pregnant or lactating women or individuals with high energy expenditure) over the entire day, yet still contain adequate nutrient levels to meet the needs of smaller individuals with lower energy intakes.
Although there are conflicting data on whether individuals will consume enough of a single, biscuit-type food product to meet their energy requirements (Brown et al., 1995; Sanchez-Griñan et al., 1992), it is assumed for the purpose of this report that individuals, at least for a short period of time, will consume enough EFPs to meet their energy requirements. The nutrient content of the EFP is based on this assumption.
NUTRIENTS INCLUDED IN THE EMERGENCY RELIEF FOOD PRODUCT SPECIFICATIONS
For each nutrient or nutrient group that follows, the assumptions, including the minimal nutrient density, the limiting groups, and how the RDA, AI, or other values were utilized are discussed. Since the EFP will be used for a wide range of age groups, in those cases where maximum values were set, they were developed from the UL values included in the DRI reports (IOM 1997a, 1998, 2000, 2001).
Energy-Yielding Nutrients
Fat, protein, and carbohydrates comprise the energy nutrients. The rationale for the fat, protein, and carbohydrate levels in the EFP are discussed below.
Dietary Fat
The recommended fat content of the EFP is 35 to 45 percent of calories and takes into consideration the following:
-
the quantity of fat needed to provide a food of sufficient energy density to meet energy requirements, to be lightweight, and to be palatable;
-
the quantity of fat needed to ensure adequate absorption of fat-soluble vitamins;
-
the quality of fat needed to provide an adequate supply of essential fatty acids; and
-
the ability to protect fat from oxidation and degradation under severe storage and transport conditions.
The maximum fat content of the EFP is limited by the minimal requirements for other macronutrients, vitamins, and minerals (Jéquier, 1999; Koletzko, 1999). The principal mechanisms for increasing the energy density of a food are to either reduce water content or to increase fat content. Because fat on a weight basis is 2.25 times as energy dense as either carbohydrate or protein, a high-fat product will weigh less than lower-fat products of similar water and energy content. The reduced weight of an energy-dense food also has advantages with respect to storage and transport. Furthermore, infants and young children have comparatively high energy requirements per kilogram of body weight (Koletzko, 1999) and have limited capacities to consume food. Therefore, very-low-fat diets increase the risk of inadequate energy intakes that would result in inadequate intakes of some micronutrients in young children. FAO/WHO (1994) suggested diets of children under 2 years of age should contain 30 to 40 percent of energy from fat.
Satiation. High-fat foods are readily over-consumed, and experimental studies suggest little effect of fat per se on satiation (feeling of fullness) when energy density of the meal is held constant (Rolls, 2000; Rolls and Bell, 1999; Saltzman et al., 1997; Stubbs et al., 1996; van Stratum et al., 1978). These results suggest that an energy-dense food, regardless of fat content, is less likely to induce satiation, and therefore is likely to promote consumption of greater amounts of energy. In the case of a refugee population, in which anorexia may be common, the provision of a higher-fat, nutrient-dense food may be an important means of ensuring adequate energy intake.
Palatability. The fat content of a food can have a significant influence on its sensory properties and the quantity of the food that is consumed (Drewnowski, 1997). Fat contributes to flavor, mouth feel, moistness, and other textural properties, depending on the food and the type of fat. Relatively little research has been published concerning the influence of fat content on the palatability of products similar to the proposed EFP. Recently, Abdallah and coworkers (1998) asked 102 men to rate the pleasantness of 39 commercially available cookies and cakes. Sugar content was the best predictor of pleasantness. However, the highest ratings of pleasantness occurred with foods that were high in both sugar and fat. Moisture content bore little relationship to pleasantness after statistically controlling for the fat and sugar content of the products. Others have investigated the sensory effects of reducing the fat content of five cookies. Only a reduction of fat by 50 percent of its original recipe was associated with declines in sensory ratings (Drewnowski et al., 1998). In both studies, subjects were much more sensitive to variability in sugar content than in fat content.
Fat Intake and Absorption of Fat-Soluble Vitamins. The absorption of fat-soluble vitamins and provitamins is dependent on fat in the diet. However, the precise quantity of dietary fat needed for efficient absorption of fat-soluble
vitamins is poorly understood. A common rule of thumb is that fat energy should not fall below 10 percent of total energy (Jéquier, 1999). Thus, the fat content of the EFP is more than adequate to promote absorption of fat-soluble vitamins.
Type of Fat. As the nutritional quality of diets in developing countries improves, the availability and the percentage of energy in the diet contributed by fat increases (Tagle, 1988). The greatest concern in developing the EFP regarding type of fat is to include fats/oils that will provide the greatest stability in terms of storage of the finished product, without the inclusion of fat of animal origin. For long-term health, other aspects of dietary fat, such as the proportion of essential fatty acids or the inclusion of long chain polyunsaturated fatty acids (LC-PUFAs) is of interest as well. However, with the limited time that the EFPs will be used (15 days or less), cost and storage requirements of the finished product limit the advisability of including some of these specific fatty acids.
Polyunsaturated Fatty Acids. Polyunsaturated fatty acids are necessary for normal health in adults and normal development in the fetus and infant (Uauy et al., 1999). The essential fatty acids, α-linolenic acid (LNA, n-3) and linoleic acid (LA, n-6), present in various vegetable oils, are precursors for the other n-3 and n-6 LC-PUFAs. In animal models, synthesis of docosahexaenoic (DHA) and arachidonic acid (AA) from their essential fatty acid precursors are decreased by experimental protein and energy malnutrition (Lopez-Pedrosa et al., 1998; Marin et al., 1995) and observational studies in infants have documented associations between protein–energy malnutrition (PEM) and signs of n-6 fatty acid deficiency (Decsi et al., 1998; Holman et al., 1981; Koletzko et al., 1986; Leichsenring et al., 1995; Marin et al., 1991; Smit et al., 1997).
Studies indicate that children with sickle cell anemia and with zinc and copper deficiencies appear to have impaired ability to utilize LA and LNA (Cunnane, 1981; Enomoto et al., 1998). Research has shown considerable regional variability in the LC-PUFA content of human milk of women in developing countries, presumably due to variability in diets (Chulei et al., 1995; Koletzko et al., 1992; Laryea et al., 1995; Okolo et al., 2000; Rocquelin et al., 1998; Schmeits et al., 1999; VanderJagt et al., 2000; Xiang et al., 1999). Of relevance to some developing country populations is the fact that high LA intakes from specific vegetable oils (e.g., corn oil) may decrease the synthesis of DHA from LNA. The recommendation that a ratio of LA to LNA between 5:1 and 10:1 has been made (FAO/WHO, 1994), and seems reasonable and fairly easy to obtain from vegetable oil sources.
Although an EFP having at least 35 percent of calories from vegetable oil sources will probably not be totally devoid of such fatty acids, the constraints of manufacturing, required storage life, and the impact of oxidized unsaturated fat on flavor dictate against addition of these fatty acids.
Vitamin E, PUFA, and Oxidation. Because of their susceptibility to oxidation, very high intakes of PUFA, without a correspondingly high intake of antioxidants, can lead to vitamin E deficiency (Valk and Hornstra, 2000). Fortunately, most commonly consumed vegetable oils are good sources of vitamin E (IOM, 2000) and have relatively high vitamin E:PUFA ratios (Dupont et al., 1990). Recommendations to provide adequate vitamin E intakes in high PUFA diets have been made, and vary from 0.4 (NRC, 1989) to 0.6 mg (FAO/WHO, 1994) of α-tocopherol per gram of PUFA.
Maximum Fat Content of the EFP. The upper limit of fat for the EFP is recommended to be 45 percent of energy in order to produce a stable product that would not be unduly affected by oxidation.
Fat intakes in developing countries are often quite low and come from a small number of principal dietary sources. Average fat intakes of school-aged children ranged from 10 percent of energy (in rural Kenya where animal products are consumed in relatively small amounts) to 25 percent of energy in periurban Egypt (Beaton, 1995). In Kenya, 40 percent of the fat in the diet was polyunsaturated, much of it from corn oil (Calloway et al., 1992). In The Gambia, children’s intake of fat as a percent of energy declined from birth and stabilized at 24 months of age, when the average intake of energy from fat was 15 percent (Prentice and Paul, 2000), with most of the fat coming from groundnuts and cereals. This maximum level of fat exceeds the fat content of diets normally consumed in many developing countries, but should enhance palatability of the EFP.
In summary, recommendations regarding the fat content of the EFP are as follows:
-
Total fat should comprise 35 to 45 percent of energy.
-
Saturated fat should comprise at least 10 percent of energy.
-
Total PUFA should be 7 to 10 percent of energy.
-
The ratio of linoleic acid to α-linolenic acid should fall between 5:1 and 10:1 derived from a mixture of vegetable oils.
Protein and Amino Acid Requirements
Protein is essential for all physiological functions. Although two structural proteins, collagen and elastin, comprise about half of the proteins in the adult body, the protein associated with muscle, visceral organs, and blood is the most dynamic and most affected by poor nutritional status (Crim and Munro, 1994). Adults with good nutritional status and in protein balance turn over about 300 g of protein/day (Stein, 1995); growth during childhood and pregnancy increases this turnover. The body has no readily identifiable reserves of amino acids essential for protein synthesis. Loss of 30 to 40 percent of total body protein invariably results in death from starvation (Cahill, 1970). Rapid losses due to lack
TABLE 2-5 Recommended Amino Acid Pattern of an Emergency Relief Food Product (EFP)
|
Nutrient |
Amounta (mg/kg body weight [BW]) |
Amino Acid (mg/g Protein)c |
|
Proteinb (g/kg BW) |
1.0 |
— |
|
Isoleucine |
31 |
28 |
|
Leucine |
73 |
66 |
|
Lysine |
64 |
58 |
|
Methionine + cysteine |
27 |
25 |
|
Phenylalanine + tyrosine |
69 |
63 |
|
Threonine |
37 |
34 |
|
Tryptophan |
12.5 |
11 |
|
Valine |
38 |
35 |
|
Histidinec |
8 |
19 |
|
a The amino acid requirement for children 2 years of age was used (NRC, 1989). b Total protein based on 1 g/kg body weight, using reference body weights from the Dietary Reference Intake reports (IOM, 1997a). |
||
of food in emergency situations can thus result in serious health consequences over relatively short periods of time.
PEM may be present in populations that are likely to be recipients of the EFP (Young and Jaspars, 1995). For instance, an August 1989 survey of the Hartisheik A camp in Ethiopia indicated that 15.5 percent of reported cases of death in children less than 5 years of age were due to PEM and general malnutrition (CDC, 1990). The EFP target populations may have reduced energy intakes and low protein intakes, resulting in negative energy and nitrogen balances (Fjeld et al., 1989), reduced growth and/or lactation volume, and loss of body weight and muscle mass (Golden, 1994; Golden et al., 1977; Rice et al., 2000; Young and Jaspars, 1995). Limited muscle mass has been documented by lower body weights and mid-arm circumferences (Collins, 2000; De Onis et al., 2000; Young and Jaspars, 1995). Decreased skeletal muscle mass decreases functional capabilities (Dudley et al., 1989) and may impact the ability to perform normal life functions, as documented with PEM (Day and DeHeer, 2001; Kalra et al., 2001). Thus the EFP must provide adequate protein of appropriate quality.
Protein requirements include two components: the need for amino acids and for total protein (NRC, 1989). The EFP should meet both of these needs. The essential amino acid requirements for 2-year-old children identified by WHO (FAO/WHO/UNU, 1985), and subsequently adopted by the National Research Council (NRC, 1989), serve as the minimum amino acid pattern to use for the
EFP (34 g/1,000 kcal, or 8 g/EFP bar) along with the generally recommended amount of total protein of 1 g/kg body weight (see Table 2-5). Although the protein content may be slightly low for young children (their RDA is 1.2 g/kg body weight [NRC, 1989]), the recommendation must take into consideration that a higher protein level per kilocalorie may be too high for adults and may not be as palatable (Young et al., 1985). A maximum of 15 percent of total calories as protein is recommended to prevent renal load problems and thirst promo tion (Briend and Golden, 1993). Thus, the amount of protein recommended for the EFP is a compromise. Although the pattern of amino acids will meet the essential amino acid needs of the young child, the total protein may be limiting.
Because the EFP may be the sole food source for as long as 15 days, the protein should have a protein digestibility-corrected amino acid score (PDCAAS) of 1.0 or better (FAO/WHO, 1989). The protein and amino acids could be provided by a combination of soybean protein isolates or concentrates and grains such as wheat, and complemented with milk solids (NRC, 1989). If milk solids are used, some amount of lactose would be included, but the level should be kept below 17 g/1,000 kcal (see “Lactose,” below).
There is abundant research demonstrating the effectiveness of combinations of plant proteins such as those from soybeans and wheat flour in meeting essential amino acid needs along with total protein (Brown et al., 1982; Clegg, 1960; Dahlin and Lorenz, 1993; Friedman and Brandon, 2001; Grange et al., 1994). Wheat flour has good digestibility and provides the physico-chemical properties
for a palatable food product but is limiting in lysine content. Soy protein has lysine and is a high-quality protein, but may be limiting in methionine or sulfur amino acids for children (Friedman and Brandon, 2001). Other legume protein sources may not be sufficient. For example, the combination of wheat flour, chickpeas, and milk powder has a PDCAAS of 0.73 (FAO/WHO, 1989), which is low in lysine. Amino acids should be provided in the EFP only as intact protein and not as free amino acids. Supplementing with amino acids is not rec ommended as it will affect taste and increase cost, and can lead to problems of imbalance without adequate premixing.
Subsequent food processing should not affect protein quality. For instance, heat used in extrusion could reduce the lysine availability of the product (Clegg, 1960; Dahlin and Lorenz, 1993). Protein content in the final EFP should be within 10 percent of specifications.
Carbohydrates
Carbohydrates include monosaccharides (glucose, fructose, and galactose); disaccharides (maltose, sucrose, and lactose); oligosaccharides (maltodextrins); and polysaccharides—starch (amylose and amylopectin)—and nonstarch (cellulose, xantham, pectins, and carrageenans) (Bemiller and Whistler, 1996). Carbohydrates serve several functions as components of the EFP. They provide energy, sweetness, and desirable physical properties of the product, and are necessary for sodium absorption to maintain electrolyte status. There are also maximum levels beyond which undigested and unabsorbed carbohydrates result in gastrointestinal problems due to gas production by intestinal bacteria. Carbohydrates and fat are the two major energy sources provided by the EFP; carbo hydrate should be provided primarily as starch associated with the grains and/or legumes used as protein sources and to meet specific requirements for taste, palatability, stability, and metabolic function (FAO/WHO, 1998).
Sweetness and Physical Properties. Cookie-like products (e.g., slightly sweet biscuits) have proven to be most acceptable for a wide spectrum of cultures during various emergencies where relief food products have been used, although compressed food bars such as the Norwegian BP-5 were also acceptable (Grobler-Tanner, 2001). The only flavor found to be acceptable to widely diverse populations was sweetness (Drewnowski, 1997; Young et al., 1985). Therefore, nutrient composition recommendations for the EFP include sugars such as sucrose or corn syrup to provide sweetness and to improve the texture of the EFP. The specifications for the EFP limit total sugar levels, however, as described in the following subsections. Most of the carbohydrate in the EFP will be in the form of starch.
Glucose. A high incidence of diarrhea and malabsorption, commonly due to poor sanitation, is associated with uprooted populations (UN Subcommittee on Nutrition, 2001). Provision of potable water is the highest priority in emergency relief efforts (UNHCR, 2000), with the EFP as the primary source of electrolytes. Therefore, the emergency food product should provide glucose and sodium in quantities that will optimize intestinal absorption when consumed with ample water, yet not be so high as to be malabsorbed (Santosham et al., 1987).
Ability to absorb glucose in the small intestine and transport it with sodium remains intact during acute diarrhea (Hirschhorn, 1980). The EFP should provide 6 g of glucose for each 1 g of sodium to promote gastrointestinal uptake of sodium (Santosham et al., 1987). The sodium recommendation is 1.4 g/1,000 kcal, thus resulting in a requirement for 8.6 g of free glucose/1,000 kcal. However, the total monosaccharide level must be less than 25 percent of carbohydrates, by weight, to prevent osmotic diarrhea and elevation of the osmotic load. Use of maltodextrins to provide 8.6 g of free glucose is recommended due to the cost of free glucose compared to maltodextrins.
Lactose. Milk solids may be used in the EFP, but the level of milk sugar lactose needs to be considered. Because there may be a high incidence of adult lactase deficiency in the populations receiving the EFP, consumption of excessive lactose might be a concern if it led to abdominal discomfort, flatulence, abdominal bloating, and diarrhea (Scrimshaw and Murray, 1988). Secondary lactase deficiency also has been shown to be associated with acute gastroenteritis, malnutrition, acquired immune deficiency syndrome enteropathy, and diarrhea of infectious origin in both adults and children (Riley and Marsh, 1998; Scrimshaw and Murray, 1988). Such lactase deficiency may be transient or chronic in nature. For these reasons, use of lactose as a carbohydrate source is not recommended. Because milk solids provide high-quality protein and often are readily available for emergency feeding programs, their use as a protein source in the EFP may be desirable.
Controlled studies have shown that the majority of individuals demonstrated to be lactose maldigestors do not experience symptoms with 1 cup of milk or the equivalent amount of lactose (12 g) or more consumed at one time (Scrimshaw and Murray, 1988; Suarez et al., 1995). Many of these studies are based on results following ingestion of single test meals providing varying amounts of lactose, and tolerance to repeated intake of this amount of lactose on the same day and over a extended period of time is less clear. However, the reported milk consumption of individuals shown to be lactose maldigestors often exceeds 1 cup/day (Scrimshaw and Murray, 1988). In a controlled study by Calloway and Chenoweth (1973), four subjects shown to be lactose maldigestors were fed a diet that included 1,000 g of homogenized low-fat milk providing approximately 50 g of lactose in four divided doses for a period of 12 days. Breath hydrogen concentrations were slightly or moderately elevated in two of the subjects at this level of intake but there were few subjective complaints of discomfort due to the diet.
Although the EFP is not intended to be used in treatment of individuals with severe diarrhea or malnutrition, the use of products containing milk in feeding adults and children with these conditions demonstrates the acceptability of including milk in emergency rations. Collins and colleagues (1998) recently reported successful use of a product containing dried skim milk, vegetable oil, vitamins, and minerals as part of the diet given to adult patients with severe malnutrition in Baidoa, Somalia. Although the milk product was diluted in the first few days of treatment, the amount was gradually increased and provided 137 or 95 g of lactose/day. The latter diet was reported as being better tolerated but the investigators attributed this response to the lower protein content of the diet rather than the reduced amount of lactose.
The use of diets containing milk in treating young children with diarrhea has been studied extensively (Brown, 1991; Brown et al., 1991; Penny and Brown, 1992). A meta-analysis of clinical trials that compared the outcomes of young children treated with either lactose-containing or lactose-free diets (Brown et al., 1994) showed an overall treatment failure rate of approximately 22 percent among children treated with lactose-containing diets compared with a treatment failure rate of 12 percent among those who received lactose-free diets. On the basis of these meta-analyses the author concluded that the majority of children with acute diarrhea can safely receive undiluted, lactose-containing milks, which would contain about 12 g/240 ml, distributed over multiple feeding episodes. However, children with severe diarrhea and dehydration may have increased treatment failure rates if they receive undiluted lactose-containing milk and should be managed under close supervision. This concern, however, is not applicable to use of the EFP since it is not intended as a therapeutic treatment for individuals with severe diarrhea or malnutrition.
Based on evidence suggesting that consumption of 12 g of lactose contained in 1 cup of milk would be tolerated by populations with a high prevalence of lactose maldigestion when consumed as part of a meal, if approximately one-third of the daily ration of EFPs (and thus one-third of the lactose) is consumed during each eating episode, the maximum lactose content should be 17 g/1,000 kcal (4 g/EFP bar). Thus, children ages 1 to 3 years consuming 855 kcal/day (Table 2-3) would receive approximately 14.5 g/day or ~5 g/meal episode. This amount of lactose would allow milk solids to provide about one-third of the specified content of protein (34 g/1,000 kcal) and one-half of the calcium (768 mg/1,000 kcal) for the EFP. Lactose should only be present in the EFP due to its presence in milk solids—it should not be added.
Fiber. Generally, fiber is considered essential for human health, and the targeted population should consume fiber-containing foods if possible (NRC, 1989). However, other requirements of the EFP limit the advisability of its providing fiber. First, it is well recognized that individuals living in sub-Saharan Africa and Asia usually consume about 30 g/day of nonstarch polysaccharides, an indication of adequate fiber intake (FAO/WHO, 1998). The EFP will be used for less than 15 days and hence a lack of fiber would not result in a chronic
problem or exacerbate a condition. Furthermore, the energy density of the product needs to be high (e.g., 4.2 kcal/g is the energy density of the BP-5 [Young et al., 1988]) to meet the needs of all age groups in the population, and to facilitate ease of transport and distribution. Consequently, although the EFP will contain some fiber because of its grain and legume constituents, the level of fiber should be limited to provide maximal energy density.
Importance of Carbohydrates for Physical Activity. Individuals in need of the EFP may often be walking long distances on foot, or may be expending a large amount of energy erecting shelters, finding water, finding fuel, or meeting hygiene needs. These factors emphasize the importance of carbohydrate in the EFP in a number of ways. First, during moderate-intensity labor (e.g., less strenuous than a brisk walk, under 5.6 km/h, or at less than 40 to 50 percent VO2max), the primary metabolic fuel is fat with carbohydrate contributing about 25 percent toward total caloric expenditure (Brooks and Trimmer, 1996). However, during the course of several hours of work, muscle and liver glycogen stores can become depleted and the ability to walk or perform physical tasks declines. Adequate dietary carbohydrate intake is necessary to sustain prolonged exercise of more than 1 hour (Ivy et al., 1979) and to allay fatigue.
Second, if an insufficient amount of carbohydrate is consumed on consecutive days by individuals who exercise for prolonged periods, they likely will experience irritability, dizziness, and/or nausea in addition to fatigue (Sherman, 1983). Moreover, carbohydrate stored in muscle and liver tissue as glycogen involves water storage (i.e., 3 g of water/g of carbohydrate). This water is released when glycogen is metabolized and provides a minor, but useful, contribution to meeting fluid needs. Finally, compared to no feeding, carbohydrate intake during exercise increases endurance (Brooks et al., 2000). The EFP is convenient to eat during periods of physical activity, requires no preparation, and does not significantly divert the consumer from essential daily tasks. Individuals can thus benefit from consuming the EFP before and during periods of prolonged activity because it includes 40 to 50 percent of its calories as carbohydrate. This level of carbohydrate allows for an energy-dense ration (35 to 45 percent from fat) and for adequate protein (10 to 15 percent of energy coming from protein).
To summarize carbohydrate requirements for the EFP per 1,000 kcal/day:
-
40 to 50 percent of energy as carbohydrate, at least 50 percent of which is from starch;
-
no more than 25 percent of carbohydrates as monosaccharides;
-
at least 8.6 g of glucose from maltodextrins to allow for sodium transport;
-
no more than 17 g of lactose from milk solids (no free lactose added) per 1,000 kcal;
-
primary role for sucrose or corn syrup is to provide palatability and texture; and
-
no added fiber in order to provide an energy-dense product.
Water
In situations that require the distribution of emergency rations to distressed populations, water supplies often will be insufficient or contaminated. Since humans can live only few days without water (Brown, 1947a), this report as sumes that provision of adequate potable water is the first priority of any emergency operation. Efforts should also be made to educate indigenous group leaders regarding location of water supplies and water purification (e.g., boiling, iodination). Because of concerns over possible water shortages, the EFP is designed to contribute minimally to osmotic load, while providing essential nutrients and energy to meet the needs of most individuals in emergency situations for a short period of time.
The minimal water requirement for a fasting 70-kg adult, resting in a mild environment, is about 800 ml/day (Gamble, 1947). This is by no means consistent with good health. In the United States, for example, the average adult experiences a water turnover (all sources) of approximately 2,500 ml/day. The lowest volume of fluid required to prevent deterioration provides about 300 ml of urinary output per day. Under low-stress conditions this is equivalent to an intake of about 1,000 ml (Johnson, 1964). According to Gamble (1947) and Marriott (1950), when all water intake ceases, the minimum unavoidable water loss approximates 1,500 ml/day (or about 2 percent of body weight). In a tropical or desert climate, fluid losses may range from 300 ml/h (at rest in shade, 35º C) to 900 ml/h (walking in direct sunlight, 40º C) (Adolph, 1947); this results in total water losses of approximately 3 to 10 percent/8 h exposure for a 70-kg adult. Continuous labor in a desert environment can increase the daily water requirement to 11 L/day, primarily due to sweat losses (Brown, 1947b).
Sustained mental and physical performance are incompatible with the loss of more than 7 to 8 percent of body weight as water (Calloway, 1960). When water losses reach 15 to 25 percent of body weight, it is likely that coma, circulatory failure, and death will occur (Adolph, 1947; Leithead and Lind, 1964). The clinical conditions of heat exhaustion, heat cramps, heat syncope, and heat-stroke also are influenced or caused by perturbations of fluid–electrolyte balance (Hubbard et al., 1986).
The state of starvation involves considerable dehydration, regardless of environmental stressors. The actual body water deficit depends on the duration of starvation, water availability, body size, energy intake, dietary composition, work output, and environmental conditions. Infections (e.g., bacterial dysentery) are also common in undernourished individuals, and gastrointestinal illness, with vomiting and diarrhea, obviously increases water and electrolyte losses.
Carbohydrate Effect on Water Requirement
When water supplies are insufficient, provision of a minimum of 100 g of carbohydrate in a survival ration is needed (Johnson, 1986). Extensive studies on the composition of survival rations (Calloway, 1960; Gamble, 1947; Grande et al., 1958) have demonstrated that 100 g of carbohydrate constitutes the minimal essential ration amount. This amount of carbohydrate reduced the deficit of body water by lowering the amount of body solutes requiring excretion and by preventing ketosis, thus permitting a reduction in urine volume. The carbohydrate also was essential in maintaining the ability to perform various physical activities by preventing total depletion of glycogen stores, and provides some feeling of satiety.
Protein Effects on Water Requirement
Although muscle wasting is common in starvation, inclusion of a large amount of protein in the EFP is contraindicated because it negatively affects water balance. Assuming maximal renal concentration, the excretion of 1 g of urea nitrogen requires 40 to 60 ml of water. This means that the inclusion of 10 g of dietary nitrogen (equivalent to about 63 g of dietary protein) in a 2,100 kcal diet increases the volume of required water by 400 to 600 ml/day. Further, renal concentrating ability is severely compromised in moderate malnutrition (Golden, 2001).
Figure 2-2 depicts the effects of protein and energy content on obligatory urine volume in a multi-level study. The emergency rations tested contained four energy levels (500, 1,000, 1,500, and 2,000 kcal) and four protein levels (0, 7.5, 15, and 30 percent of total calories). In rations that contained 0 and 7.5 percent protein, increasing the caloric content of the ration from 500 to 2,000 kcal did not increase the obligatory urine volume. However, a ration that contained 30 percent protein approximately doubled the obligatory urine volume when the caloric content increased from 500 to 2,000 kcal (Calloway and Spector, 1954).
Based on these calculations and considering the renal dynamics discussed in the previous paragraph, it appears that the 2,000 kcal diet was optimal in terms of osmotic load when it contained 7.5 percent protein (approximately 40 g of protein). The osmotic load created by 15 percent protein appears to be toler able and this thus becomes the maximum allowable amount. Where water availability is of real concern, lower levels of protein should be considered maximal in developing the EFP.
Salt and Total Dissolved Solids Effects on Water Requirement
Sodium chloride (NaCl) in emergency rations requires consumption of sufficient water to dilute the added osmotic content to the level found in plasma.
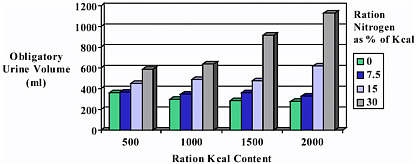
FIGURE 2-2 Influence of caloric and protein content of emergency rations on urine volume. Excretion of 1 g of urea nitrogen requires 40 to 60 ml of water.
Baker and colleagues (1963) examined the minimal water intake that is needed to dilute various amounts of dietary salt. They overloaded study participants with 11.8, 15.8, and 23.8 g of NaCl for 4 days and 32.8 g of NaCl for 10 days in a 23º C environment. Water was plentiful and was consumed ad libitum. Urinary and fecal excreta eliminated 47 percent of the total water intake and 92 percent of the salt intake. Plasma sodium levels remained constant during the course of metabolic tests, exemplifying the efficacy of renal electrolyte control. Evaluation of water balance indicated that 127 ml of water was required to dilute each gram of NaCl in a 70-kg adult leading a sedentary existence in a mild environment. Thus, the 3 g of Na supplied per 2,100 kcal from the EFP would be equivalent to 7.6 g of NaCl and require 965 ml of water.
Similarly, the human water requirement increases as the total number of osmotically active particles increases in the diet. Because underground wells, reservoirs, and streams contain dozens of minerals, the total dissolved solids (TDS) in water must be considered. Daniels and Layton (1983) have considered the concentrations of TDS in natural water sources. Although they recommend a TDS of 1,000 mg/L for field water supplies, many public drinking water sources in the United States have TDS concentrations exceeding 2,000 mg/L. This indicates that natural field water can have a significant impact on the TDS consumed each day. This should be considered when plans are formulated for the provision of water with emergency rations.
Summary of Osmotic Load and Water Requirement
There are a variety of nutrients that can increase osmotic load. Some are intracellular osmotic solutes, such as potassium, magnesium, organic phosphates, and protein; some are extracellular osmotic solutes such as sodium and its anions, chloride, and bicarbonate.
Healthy individuals have good renal control of fluid and electrolytes and maintain body equilibrium within a wide range of fluid, sodium, and potassium intakes. However, given the probable circumstances of a population in need of an EFP, body sodium, potassium, and chloride are important components that need to be monitored when a single food source is used to provide all nutrients. Given that various water sources with high levels of solids may significantly increase the osmotic load (Daniels and Layton, 1983), it is important to minimize to the extent possible that contributed by the EFP.
Electrolytes
Sodium
Sodium is essential for human health for acid–base balance, body water balance, and nerve function, and it contributes to the palatability of foods. The recommendation for sodium is based on general consumption patterns and available recommendations for maximum intakes (NRC, 1989). Although less than 1 g/day is essential for life, the chronic diarrhea that may be expected in populations requiring the EFP, along with perspiration losses due to elevated ambient temperatures and hard work, increase sodium requirements. Furthermore, additional dietary sodium enhances water retention (Shirreffs et al., 1996) and replaces sodium losses due to diarrhea. Individuals working outdoors with elevated ambient temperatures lose between 2.3 to 3.4 g Na/L of sweat (Costill et al., 1976; Dill et al., 1976). Western dietary guidelines suggest sodium intakes of no more than 2.4 g/day (NRC, 1989). Given that the EFP may be used during periods of sustained physical activity or in high ambient temperatures, the EFP should contain a minimum of 1.3 g/1,000 kcal, which is equivalent to 300 mg/EFP bar. This would provide 1 g of sodium for the 1- to 3-year age group with an average weight of 10 kg and consuming 855 kcal/day (see Tables 2-4 and 2-6). The maximum amount is 1.4 g/1,000 kcal.
Potassium
Potassium is essential for fluid balance, nerve transmission, and acid–base balance. Similar to sodium, potassium is lost in sweat, feces (diarrhea), and urine, although sweat losses are considered negligible (NRC, 1989). Golden (2001) suggests that a growing child without pre-existing deficiency may need about 1.3 g of potassium/1,000 kcal (2.7 g/2,100 kcal). The recommendation for
TABLE 2-6 Electrolyte Intake Based on Energy Needs of an Emergency Relief Food Product (EFP)a
|
Age |
Gender |
Energy Requirement (kcal/day)b |
Sodiumc (g/day) |
Potassiumc (g/day) |
Chloridec (g/day) |
|
7–12 moc |
Both |
578 |
0.83 |
0.96 |
1.3 |
|
1–3 yr |
Both |
855 |
1.0 |
1.42 |
1.9 |
|
4–8 yr |
Both |
1,456 |
2.1 |
2.43 |
3.2 |
|
9–13 yr |
Both |
1,693 |
2.4 |
2.82 |
3.7 |
|
14–18 yr |
Boys |
2,136 |
3.1 |
3.56 |
4.7 |
|
|
Girls |
1,931 |
2.8 |
3.22 |
4.2 |
|
19–50 yr |
Men |
2,339 |
3.4 |
3.90 |
5.1 |
|
|
Women |
1,972 |
2.8 |
3.29 |
4.3 |
|
51+ yr |
Men |
2,249 |
3.2 |
3.75 |
4.9 |
|
|
Women |
1,929 |
2.8 |
3.21 |
4.2 |
|
a The EFP contains 1.3 g of sodium, 1.7 g of potassium, and 2.0 g of chloride per 1,000 kcal. b Estimated energy values from Table 2-3. c Based on daily recommendations of no more than 3.0 g of sodium, desirable intake of 3.5 g of potassium, and chloride on an equimolar basis with sodium (NRC, 1989). |
|||||
the EFP is 1.7 g/1,000 kcal (396 mg/EFP bar), which is given as a desirable intake for adults (NRC, 1989) but much above the minimum requirements, and thus should provide enough to compensate for possible losses due to sweat and mild to moderate diarrhea. The maximum amount is 2.0 g/1,000 kcal (specified content + 20 percent).
Chloride
Chloride is lost in diarrhea, as well as with vomiting due to its high concentration in gastric juice. Chloride is the principal inorganic anion in extracellular fluid, and is essential for maintaining fluid and electrolyte balance (NRC, 1989). Although chloride deficiency is rarely observed, its loss mirrors sodium loss with the exception of that due to vomiting, so it is also important to ensure adequate intakes of chloride for refugee populations, particularly when consuming a single-source food product. The minimum amount contained in the EFP should be 2.0 g/1,000 kcal to match the sodium content on an equimolar basis. This provides 466 mg of chloride/EFP bar. The maximum amount is 2.2 g/1,000 kcal (specified content + 10 percent). This amount is also equimolar to the sodium level, as recommended by NRC (1989) (see Tables 2-4 and 2-6).
Summary of Electrolyte Content
Electrolyte content can influence palatability: added sodium in high amounts results in a very salty-tasting product whereas added potassium in high amounts results in a bitter-tasting product. If electrolyte losses are extensive due to chronic severe diarrhea, then therapeutic electrolyte/fluid supplements should be provided (which is beyond the scope of this report).
The nutrient density recommendations for sodium, potassium, and chloride provide additional amounts beyond the recommended intakes for healthy people (NRC, 1989). Because of its bitter taste, food sources should provide the bulk of the potassium.
Calcium, Phosphorus, and Magnesium
The nutrient content specifications for calcium, phosphorus, and magnesium were derived from the recent evaluation of requirements for these nutrients as part of the DRI process (IOM, 1997). It is assumed that growth stunting is present in the targeted populations (Neumann and Harrison, 1994). There are limited data suggesting that rapid improvement of nutritional status may improve growth, although early stunting is never fully compensated; providing bone-related nutrients early in relief efforts is potentially of benefit. The EFP specifications reflect requirements for children (IOM, 1997a).
Data presented in the DRI report (IOM, 1997a) justify the adequacy of the AI and RDA for calcium and phosphorus, respectively, during adolescence and adulthood as meeting dietary needs during pregnancy and lactation as well. Additional needs are identified during pregnancy for magnesium (IOM, 1997a); however, the individual minimal nutrient density for magnesium (Table 2-4), based on adolescent boys, is actually slightly greater than that derived for pregnancy assuming an additional 200-kcal intake. Thus, additional needs for pregnancy would be met based on the assumption that additional energy (e.g., more EFP bars) would be consumed.
Foods such as soybeans and grains should provide the primary source of these nutrients. However, poor digestibility from plant sources may require some or all of these nutrients to be added to the EFP as direct ingredients in order to provide the specified levels.
Calcium
Dietary calcium is essential for bone, neuromuscular, and cardiovascular health, as well as for many biochemical functions (IOM, 1997a). During calcium deficiency, the key calcium roles in regulatory proteins are protected at the expense of bone calcium. There is a tight regulation of serum calcium levels through exchange from and to the bone, resorption by the kidney, and absorption
from the gastrointestinal tract. Thus, the clinical sign of low calcium status is poor skeletal development, which affects growth, fracture rates, and subsequent rates of osteoporosis.
Bone growth and prevention of osteoporosis are related to chronic intakes of calcium, and there are no data suggesting that suboptimal intakes during a short emergency situation of less than 15 days would have any effect or that supraoptimal intakes during the same short time period would significantly improve bone status. Decreased bone turnover occurs in malnutrition (Branca et al., 1992), but some catch-up (or compensatory) growth is documented with children when adequate overall good nutritional status is restored (Fjeld et al., 1989; Golden, 1994). Although the EFP may provide the only source of nutrients for a very short period of time, the addition of calcium is essential to provide as nutritionally complete a diet as possible.
The minimal nutrient density for calcium is 768 mg/1,000 kcal, which is derived from the AI for children ages 9 through 13 years of 1,300 mg/day (IOM, 1997a). This assumes that these children will consume about 1,700 kcal/day, or 7 to 8 EFP bars (Table 2-3). One bar will contain about 180 mg of calcium.
The source of supplemental calcium used in the EFP should be readily absorbed (certain food sources may decrease the availability of calcium). The role of phytate, oxalic acid, and wheat bran in calcium absorption has been studied (Heaney et al., 1988, 1991; Weaver et al., 1996). Although these compounds decrease calcium absorption, overall there was no significant physiological effect on absorption when provided in a mixed diet (Heaney and Weaver, 1989; Heaney et al., 1990). It is anticipated that cereal grains and legumes will comprise the bulk of the EFP, and thus some sources of phytate will be present. Thus, an increase of 15 percent over the required amount of calcium, 180 mg/ EFP bar, is suggested to compensate. The proposed level for calcium may come from supplementation of the food sources to no more than 207 mg/EFP bar (specified content + 15 percent).
The UL for calcium should be considered since the recipient population may have low urinary volumes due to dehydration related to diarrhea and inadequate fluid intakes (Golden, 2001). Urinary loads of calcium must be considered due to calcium interactions with other nutrients that may be deficient in target populations such as iron, zinc, and possibly phosphorus (Golden, 2001). The UL for calcium for adults is 2,500 mg/day based on the adverse effect of milk alkali syndrome seen at higher intake levels (IOM, 1997a). Given the concerns related to renal solute loads discussed earlier, the maximum calcium level should not exceed 885 mg/1,000 kcal.
Phosphorous
The recommendation for phosphorus is based on its function in growth of soft and bone tissues and replacing phosphorus losses, but not on prevention of a
specific sign or symptom of a nutritional deficiency (IOM, 1997a). The phosphorus content of the EFP is set based on the minimal nutrient density of 740 mg/1,000 kcal, which is derived from the RDA of 1,250 mg for boys and girls 9 to 13 years of age based on their estimated energy needs (Table 2-3). One EFP bar of 233 kcal should contain at least 172 mg of phosphorus in an available form. The UL for phosphorus for adults is 4,000 mg based on elevated serum inorganic phosphate levels seen with very high intakes (IOM, 1997a). This level corresponds to a maximum of 1,900 mg of phosphorus/1,000 kcal.
Although potential energy and protein ingredients supply phosphorus for the EFP, the majority of phosphorus from plant foods is in the form of phytic acid, which is less bioavailable (Wyss et al., 1999; Zhou and Erdman, 1995). Furthermore, elevated levels of phytate may impair bioavailability of important trace elements such as zinc. Thus there is a concern about high levels of phytate phosphorus. Method of food processing may also affect mineral availability. Kivisto and colleagues (1986) reported that apparent absorption of magnesium and phosphorus was decreased in an extruded cereal product.
Additional phosphorus to meet the level recommended may be provided by hydrolyzed phytic acid or soluble forms of phosphorus salts such as sodium hypophosphate. The specified range for phosphorus is 740 to 880 mg/1,000 kcal, or 172 to 206 mg/EFP bar (specified content + 20 percent). It is assumed that soybean- and grain-derived ingredients will contribute most of the phosphorus.
Magnesium
Magnesium is found both in bone (about 50 percent), soft tissue, and extracellular fluid. It is a required cofactor for over 300 enzymes, many of which are involved with energy metabolism and cellular replication. Absorption of magnesium from a typical diet is approximately 50 percent, with fiber decreasing absorption (Kelsay et al., 1979), ostensibly due to its phytate content. The RDA for adults is based on balance studies; the minimal nutrient density for magnesium is based on the requirements of 14- to 18-year-old boys. The recommended amount of magnesium for this subgroup is 190 mg/1,000 kcal based on the energy requirement for this group (Table 2-3) and the RDA (410 mg/d) for magnesium (IOM, 1997a). This amount provides 45 mg of magnesium/EFP bar. A higher level in the EFP is allowed if the source is from food ingredients. The maximum content is 230 mg/1,000 kcal (specified content + 20 percent) in order to ensure that total intake of added magnesium salts is below the adult UL of 350 mg/day. The UL applies only to magnesium salts added to foods, and is a level designed to prevent diarrhea associated with magnesium supplementation. Therefore the maximum amount of added magnesium consumed per day should be below this level, with the magnesium content coming primarily from the
TABLE 2-7 Recommended Macromineral Content of an Emergency Relief Food Product (EFP)
|
Nutrient |
RDA or AIa for Nutrient Density (mg/d) |
Amount/233 kcal Food Bar (mg) |
Amount/1,000 kcal of EFP (mg) |
Amount/2,100 kcal Ration (mg) |
|
Calciumb |
1,300 |
180 |
768 |
1,620–1,865 |
|
Phosphorus |
1,250 |
172 |
740 |
1,555–1,865 |
|
Magnesium |
410 |
45 |
190 |
400c |
|
a RDA = recommended dietary allowance, AI = adequate intake. b Calcium recommended intake is an AI rather than an RDA. c The tolerable upper intake level (UL) for magnesium of 350 mg/d applies only to supplemental magnesium, not to magnesium naturally found in foods. |
||||
soybean- and grain-derived ingredients. Table 2-7 summarizes the recommended content for calcium, phosphorus, and magnesium in the EFP.
Trace Elements
Chromium
Although chromium has been shown to potentiate the action of insulin in vivo and in vitro (IOM, 2001), specific evidence of deficiency in humans has been reported in only a few isolated cases of patients receiving total parenteral nutrition and in malnourished infants who responded to oral doses of chromium chloride (Hopkins and Majaj, 1967). Because of insufficient evidence to set an EAR for chromium, AIs of 35 µg/day and 25 µg/day for men and women 19 through 50 years of age, respectively, were established based on estimated mean energy intakes. Adverse effects have not been demonstrated with excess intakes of chromium per se from food or supplements; consequently, a UL has not been established (IOM, 2001).
Early interest in chromium supplementation to improve growth and glucose utilization in malnutrition has not been applied in current practice (Carter et al., 1968; Gürson and Saner, 1973). The AI values for chromium were based on estimating average amounts of chromium in well-balanced Western diets (which were found to contain on average 13.4 µg/1,000 kcal) (IOM, 2001). Thus a ration containing a minimum of 13.4 µg/1,000 kcal could be expected to meet or exceed the chromium requirement for all healthy persons in a similar population.
This value is higher than the 1.04 µg/1,000 kcal recommended by the Sphere Project as the desirable nutrient density for refugee diets (Sphere Project, 2001). While there is a lack of evidence of deficiency or toxicity and difficulties in analyzing chromium levels in foods, it is important that a single-source food
product have some chromium present. It is suggested that the minimum chromium content of the EFP be 13 µg/1,000 kcal (3 µg/EFP bar). The maximum content is not specified in the event that higher amounts are naturally present in the EFP ingredients.
Copper
Copper deficiency is frequently observed in malnourished populations, particularly in children with protein–energy malnutrition (Ashour et al., 1999; Donma et al., 1990; Squali Houssaïni et al., 1997). Chronic and protracted diarrhea leading to copper depletion has been recognized as a particular concern in infants (Beshgetoor and Hambidge, 1998) and is also a likely risk factor for marginal copper status in adults. The high prevalence of malnutrition and diarrhea characteristic of many groups that will receive the EFP supports the need for adequate copper intake.
Factorial analysis as well as indicators such as plasma copper concentrations, serum ceruloplasmin concentration, erythrocyte superoxide dismutase activity, and platelet copper concentration, are the basis for determining recommended intakes for copper (IOM, 2001).
The minimal nutrient density value for copper was calculated (see Table 2-4) for the limiting subgroup of women 51 years of age and older. Based on the RDA of 900 g (IOM, 2001), the value required to prevent inadequate intake in almost all individuals in this group would be 470 µg of copper/1,000 kcal. Recognizing the prevalence of malnutrition and diarrhea that often afflicts populations in need of an EFP, the EFP should contain 20 percent above this amount, or 560 µg of copper/1,000 kcal (131 µg/ EFP bar).
Acute liver failure has been demonstrated in individuals consuming large amounts of copper. The UL is 1,000 µg of copper/day for children ages 1 through 3 years (IOM, 2001), more than double the proposed levels for this age group for the EFP (480 µg/855 kcal). The maximum content is 670 µg/1,000 kcal (specified content + 20 percent).
Iodine
Iodine, a component of thyroxin, is essential for thyroid function and mental development (IOM, 2001). Iodine uptake into the thyroid gland is regulated from the pituitary by thyroid stimulating hormone (TSH). Thus, iodine is part of the regulation of thyroxin production. In iodine deficiency, TSH secretion increases and this may eventually lead to goiter as well as impaired production of thyroid hormones T3 and T4, essential factors for energy regulation and postnatal brain development (Hollowell et al., 1998).
In iodine deficiency, including mild deficiency, dietary iodine supplementation has an immediate impact on thyroid function (Moulopoulos et al., 1988).
Iodine deficiency during pregnancy increases risk of poor fetal mental and physical development, including cretinism. Iodine deficiency disorder (IDD) is considered the most common cause of preventable mental retardation. Iodine deficiency is well established as a nutritional problem worldwide, regardless of refugee status (UNICEF, 2000). In 1999, WHO estimated that 740 million people per year in 130 countries were at risk of IDD, including 50 million who have some degree of IDD-related brain damage. Africa, Southeast Asia, and Asia had the highest concentration of individuals at risk (WHO, 1999b). The use of iodized salt, as well as utilization of iodine for water treatment, is common worldwide. From 1990 to 1998, two-thirds of the households living in IDD-affected countries had access to iodized salt; 20 countries had 90 percent of their households with access to iodized salt.
Iodine is stored in the thyroid gland and deficiency does not occur until that store has been depleted (Clugston and Hetzel, 1994). Aside from inadequate iodine intake, protein–calorie malnutrition also may decrease thyroid iodine levels (Ingenbleek and Malvaux, 1974). Iodine turnover is slow in individuals with adequate iodine status (Fisher and Oddie, 1969).
Iodine is rapidly absorbed in the gastrointestinal tract, and excessive iodine is excreted in the urine. One study suggests that during acute diarrhea associated with protein–calorie malnutrition, iodine may be poorly absorbed (Ingenbleek and Malvaux, 1974). Bioavailability of iodine is generally high, although there are data suggesting inhibition of iodine absorption with soy flour (Shepard et al., 1960). In some populations, linamarin found in cassava may block thyroid uptake of iodine, and there are some data indicating that other water-containing humic substances may block thyroidal iodination.
The most successful method to prevent IDD is iodination of table salt. This is the recommendation of WHO, whose major emphasis is on total prevention of IDD through this practice (WHO, 1999b). WHO recommends that in order to provide 150 µg/day of iodine via iodized salt, iodine concentration in salt at the point of production should be within the range of 20 to 40 mg of iodine (or 34 to 66 mg of potassium iodate) per kg of salt (WHO, 1996a). The EFP should contain iodine as iodized salt although the iodine could also be added as calcium iodide, potassium iodide, or potassium iodate. Dietary studies such as that by Melse-Boonstra and coworkers (2000) indicate that most individuals, regardless of economic, rural, or urban status, purchase salt for cooking.
The minimal nutrient density value for iodine is based on the subgroup of children 1 to 3 years of age and is 105 µg/1,000 kcal. Assuming that iodized table salt will be used in the EFP and provide at least 50 percent of the specified sodium content, the EFP will provide more than adequate levels of iodine to prevent IDD. In the United States, iodized salt contains 194 µg of iodine per g of sodium (Venkatesh Mannar and Dunn, 1995). Thus, if the source of all the sodium in the EFP is from iodized salt, the iodine content of the ration per 1,000 kcal would be approximately 250 µg of iodine. If one assumes that half of the
sodium in the EFP may come from nonsalt sources, then the total iodine intake would be half of this, or about 125 µg of iodine/1,000 kcal, which is above the minimum nutrient density needed.
The UL for iodine is based on observations of hypothyroidism, thyroiditis, goiter, and sensitivity reactions (Pennington, 1990). Although little research has been done on refugee populations, iodination of salt in the United States increased the incidence of excessive iodine intake (Hollowell et al., 1998; Pennington, 1990). The UL for children 1 to 3 years of age is 200 µg/day (IOM, 2001), which results in a maximum content of 230 µg/1,000 kcal (specified content + 115 percent). If most of the sodium in the EFP comes from added salt, then it is possible that a mixture of iodized and noniodized salt may be needed to keep the total iodine content below this level. However, if most of the required sodium in the EFP comes from other sources, iodine can be provided in other forms as mentioned previously.
Iron
Anemia due to iron deficiency represents a major public health problem worldwide. It has been estimated that more than 2 billion people (over 33 percent of the world’s population) are iron deficient (INACG, 1999). Young children and women of reproductive age are at greatest risk. Programs to control iron deficiency have been implemented in almost all countries; nevertheless, both anemia and iron deficiency remain endemic among many populations (de Benoist, 2001).
Subclinical and clinical consequences of iron deficiency include impaired physical work performance, developmental delays in infants, cognitive impairment, and adverse pregnancy outcomes (IOM, 2001). Although numerous confounding factors make it difficult to establish a clear relationship, iron deficiency has been reported to be associated with reversible abnormalities of immune function and increased risk of infections (Oppenheimer, 2001; Scrimshaw and SanGiovanni, 1997). A recent conference, organized to evaluate the strength of evidence that iron deficiency causes specific functional outcomes, concluded that there is a significant body of evidence to support a causal relationship among iron deficiency, deficits in work productivity, and child development; and among severe anemia, malnutrition, and increased child mortality. However, causal evidence is lacking or contradictory in support of a relationship between iron deficiency and low birth weight and infectious disease (Stoltzfus, 2001).
The potential adverse effects of excess iron intake also are recognized. High-dose iron supplements have been shown to reduce zinc absorption if both are taken without food; however, this inhibitory effect does not occur if they are consumed with food (IOM, 2001). Similarly, high-dose iron supplements often
lead to constipation and other gastrointestinal symptoms when taken without food but usually are not a problem when taken with food (IOM, 2001).
The possibility that high-dose iron supplementation may have adverse effects in individuals with severe malnutrition or infectious diseases also has received attention (Tomkins, 2000). Smith and coworkers (1989) reported an increase in mortality in children with protein–energy malnutrition who received supplements of iron and recommended that iron therapy should not be instituted during the first week of treatment. However, the increase in mortality was not statistically significant and the dosage of iron supplementation was not reported. Based on a comprehensive review of published studies on the relationship between iron and infectious diseases, Oppenheimer (2001) had the following observations of use to health planners: (1) oral iron supplementation has not been shown to cause an increased risk of infection in any age group in nonmalarious countries, (2) oral iron supplementation in malarious regions may carry up to a 50 percent increased risk of clinical malaria if given in therapeutic doses at times of malaria transmission, and (3) oral iron supplementation in therapeutic doses to older immunized children and adults in malarious regions may also carry up to a 50 percent increased risk of other infectious disease. In the studies in malarious regions showing a significant iron-associated increase in risk of nonmalarial infectious morbidity, the dosage of oral iron was 3 mg/kg/day for children 6 months to 6 years of age and 60 mg/day for anemic women.
Review of nine published and four unpublished placebo-controlled, randomized trials of iron supplementation in malarious areas by an expert panel convened by the International Nutritional Anemia Consultative Group led to a consensus statement (INACG, 1999) recommending that oral iron supplementation should continue to be recommended in malarious areas where iron-deficiency anemia is prevalent. It was recognized, however, that present evidence is insufficient to rule out the possibility of an increased risk of malarial illness in some iron-supplemented individuals.
Results of other studies, however, have shown no differences in incidence or severity of conditions such as diarrhea or respiratory infections associated with iron supplementation (Berger et al., 2000; Calder and Jackson, 2000; Oppenheimer, 2001).
It is assumed that as refugees many recipients of the EFP will be iron deficient. Although it is recognized that the deficiency cannot be reversed in 15 days, it is essential that sufficient iron is provided not only to meet basic requirements but also to support the initiation of repletion. The EFP, however, should not contain excess iron, particularly in soluble forms, because excess iron promotes oxidative changes leading to destruction of nutrients such as vitamin C, as well as to the development of rancidity.
Consideration must also be given to the form of iron used in the EFP. Use of iron-EDTA appears to have potential as a fortificant, particularly in diets of low bioavailability. It is less affected by inhibitors of iron absorption and is less
likely to cause organoleptic problems, and its efficacy has been demonstrated in several intervention studies (Bothwell, 1999). The possibility of using microencapsulated iron should also be considered (Jackson and Lee, 1991) to minimize problems such as rancidity and inhibit interaction with other nutrients.
Phytic acid, a known inhibitor of iron absorption, will influence the bioavailability of iron from food products (Reddy et al., 2000). Although various approaches are available to reduce the phytate content of the EFP, the most practical approach appears to be fortification of the product at a level of iron that would ensure a sufficient quantity of absorbable iron. Vitamin C has been shown to offset the inhibitory effect of phytate on iron absorption (Hallberg et al., 1989; Siegenberg et al., 1991), thus providing justification for a liberal content of this vitamin in the product (see later section, “Vitamin C”).
Factorial modeling was used to calculate recommended intake levels for iron for older infants, children, and adults (IOM, 2001). Using the recommended intake for adult women during their reproductive years (18 mg/day), the minimal nutrient density (Table 2-4) is 9 mg of iron/1,000 kcal. Pregnant women would need higher amounts (12.4 mg/1,000 kcal). The IOM values are based on an assumed bioavailability of 18 percent for children 1 year of age and older, pregnant women during the first trimester, and nonpregnant adults. A mixed protein diet that includes some heme iron is assumed. For children under 1 year of age, for whom the diet will contain little meat and primarily cereals and vegetables, the bioavailability is assumed to be 10 percent; for pregnant women, due to the increased rates of absorption seen during the second and third trimester, bioavailability is assumed to be 25 percent (IOM, 2001).
The provisional recommended daily iron intakes set by the Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements (FAO/WHO, 2000) for diets having 10 percent iron bioavailability are considerably higher: for girls 10 to 14 years of age, 33 mg (19.5 mg/1,000 kcal); for older adolescents, 31 mg (16 mg/1,000 kcal); and for adult women of reproductive age, 29 mg (14.7 mg/1,000 kcal). To achieve the young adolescent nutrient density, the iron content would need to be doubled to take into account the lower bioavailability.
Since a generous amount of ascorbic acid is included in the EFP, and use of an iron source that is well tolerated and more bioavailable is recommended, the EFP should contain 16.3 mg of iron/1,000 kcal (3.8 mg/EFP bar), assuming 10 percent bioavailablity. This provides the same amount of iron as 9.1 mg/1,000 kcal assuming 18 percent bioavailablility. Given the concern about excess dietary iron and possible adverse effects on immune function, as well as possible food interactions and oxidative changes, the maximum iron content of the EFP is 17.6 mg/1,000 kcal (specified content + 10 percent). A lower iron content may be necessary if this range leads to unacceptable oxidative changes in the product; however, use of encapsulated iron, as mentioned earlier, could help prevent this problem.
Manganese
Dietary manganese is essential to the action of metalloenzymes involved in the formation of bone and in the metabolism of amino acids, lipids, and carbohydrates. Manganese deficiency has been observed in animals but has only been produced experimentally in humans, thus little data is available related to deficiency in refugee populations. Early signs of inadequate manganese include hypocholsterolemia and a scaling, blistering rash on the upper torso (Friedman et al., 1987; IOM, 2001).
Recommended intakes of manganese are based on median intakes due to insufficient data to determine specific requirements and thus are AIs (IOM, 2001). The minimal nutrient density value is based on the AI for children ages 1 to 3 years (1.2 mg/day) and is 1.4 mg of manganese/1,000 kcal (see Table 2-4). This would provide 0.33 mg of manganese/EFP bar. The maximum content of the EFP is 1.7 mg/1,000 kcal (specified content + 20 percent). Risk of elevated blood manganese concentrations and neurotoxicity are the basis for UL values which range from 2.33 mg/1,000 kcal for children 1 through 3 years of age to 4.70 mg/1,000 kcal for adult males (IOM, 2001).
The level specified for the EFP is much greater than that recommended by the Sphere Project (2001) for a desirable nutrient density of 0.3 µmol/100 kcal (0.2 mg/1,000 kcal) for refugee diets, but it is within the range found in Western diets.
Selenium
The biological role of selenium is as a component of various selenoproteins. Selenoproteins include five glutathione peroxidases, two deiodinases, several thioredoxin reductases, and selenophosphate synthetase 2 (Behne and Kyriakopoulos, 2001). These proteins are important in supporting immunocompetence and defense against oxidative stress, regulation of thyroid hormone action, and regulation of the redox status of vitamin C and other molecules (IOM, 2000).
Different populations vary greatly in their dietary intake of selenium, largely because the selenium content of plant foods depends on the selenium content of the soil where the food is grown. Meat and fish may be more reliable sources of selenium; however, their content can be influenced by the selenium content in feed sources. Intakes of selenium are particularly low in New Zealand, Finland, and parts of China. Low plasma and hair concentrations of selenium and low plasma glutathione peroxidase activity are common in these countries (Thomson and Robinson, 1996; Varo et al., 1994; Xia et al., 1989). Surveys in other parts of the world suggest that marginal or deficient selenium status may be widespread (Fordyce et al., 2000; Kvicala et al., 1999; Maksimović and Djujić, 1997; Ngo et al., 1997). Low selenium intake leading to severe selenium deficiency is recognized as the major factor contributing to the development of Keshan disease, a cardiomyopathy that occurs primarily in
children living in various parts of China (Ge and Yang, 1993). The role of selenium deficiency in Kashin-Beck disease, a degenerative osteoarticular disorder that is endemic to certain low-selenium areas of Tibet, is less clear (IOM, 2000).
Because of the possibility that the selenium status of the population groups receiving the EFP may be marginal or deficient, it can be argued that the ration should provide a generous intake of selenium. Additional support for increasing selenium levels in the EFP is provided by the hypothesized role of selenium in iodine metabolism, oxidative changes associated with protein–energy malnutrition, and viral infections (Ashour et al., 1999; Beck et al., 2001; Contempre et al., 1992; Fechner et al., 2001; Golden, 1998; Levander and Beck, 1999; Ngo et al., 1997; Sive et al., 1993; Tatli et al., 2000; Vanderpas et al., 1990).
The recommended intakes for selenium are based on the criterion of maximizing plasma glutathione peroxidase activity (IOM, 2000) and on data from two intervention studies, one in China and one in New Zealand. Compared to the RDA values (55 µg/day for girls 14 to 18 years old [IOM, 2000]), intakes recommended in a preliminary FAO/WHO (2000) report are considerably lower. Based on the limiting minimal nutrient density (see Table 2-4) of 28 µg of selenium/1,000 kcal (6.5 µg/EFP bar), the minimum amount of selenium should be at this level for the EFP. The maximum content is 34 µg/1,000 kcal (specified content + 20 percent).
Attempts to identify evidence of selenium toxicity in populations living in seleniferous areas of the world have given conflicting results (Reilly, 1996). Residents of seleniferous ranches in South Dakota or Wyoming with intakes as high as 724 µg/day showed no adverse effects associated with their high intakes (Longnecker et al., 1991). In contrast, endemic selenium toxicity occurring in China led to biochemical abnormalities at selenium intakes over 750 µg/day and changes in nails and hair in susceptible individuals at intakes of at least 910 µg/day (Yang et al., 1989). Other signs of chronic toxicity included lesions of the skin, gastrointestinal tract, and nervous system (IOM, 2000).
Hair and nail brittleness and loss are the endpoints on which the UL for selenium is based (IOM, 2000). The maximum nutrient density is 103 µg/1,000 kcal based on the UL for children 4 to 8 years of age. The specified range is well under this maximum. If it is necessary to add selenium to the ration in addition to that provided by the major ingredients, it is recommended that it be in the form of selenomethionine, due to its greater bioavailability compared to selenate and selenite (IOM, 2000). Selenomethionine is the form that has been used in supplementation trials.
Zinc
Low zinc intakes and marginal or deficient zinc status are found frequently in developing countries, particularly in young children (Zinc Investigators’
Collaborative Group, 2000). However, lack of reliable laboratory biomarkers has made it difficult to accurately estimate the prevalence and severity of zinc deficiency (Hambidge, 2000). Evidence for the existence of inadequate zinc intakes has come largely from zinc supplementation trials (Hotz and Brown, 2001). Consumption of plant-based diets, especially those having a high content of phytic acid, is considered a major factor contributing to zinc deficiency (Gibson et al., 1997; Hambidge et al., 1998).
Zinc intakes recommended by FAO/WHO (2000) vary depending on the estimated bioavailability of dietary zinc. Diets are classified as having high (56 percent), moderate (35 percent), or low (15 percent) zinc bioavailability, based on the dietary content of animal and fish protein, calcium (less or greater than 1 g of calcium/day), and daily molar ratios of phytate to zinc (less than 5, 5 through 15, and greater than 15) (WHO, 1996b).
Zinc bioavailability of the traditional diets consumed by various groups of potential EFP recipients may vary considerably. Using the WHO model, estimates of zinc absorption have ranged from 15 percent for diets in Malawi, Kenya, and Guatemala to 30 percent for diets in Ghana, Guatemala, and Egypt (Gibson and Ferguson, 1998). Thus, if based on dietary zinc content alone, the zinc status of these populations might be expected to differ appreciably; however, other factors such as limited amounts of food and persistent diarrhea may lead to marginal zinc deficiency even in those populations consuming diets low in phytate, a known binder of zinc.
An increasing number of supplementation trials have demonstrated the value of increased zinc intake in promoting linear and ponderal growth in children. For example, a meta-analysis of 25 zinc supplementation trials showed that zinc supplementation had an overall positive effect on change in height (Brown et al., 1998). Subjects in these trials ranged in age from birth to 13 years, with a mean age of 3.6 years. The mean dose of zinc used for supplementation was 14 mg/day (1.5 to 50 mg/day).
A positive effect on growth, however, was not observed in all studies (Friis et al., 1997; Kikafuna et al., 1998). Inconsistent results might be attributed to inclusion of older children whose rate of growth is slower and to the presence of multiple deficiencies that would not be expected to respond to supplementation with a single nutrient (Hotz and Brown, 2001). Some evidence suggests that zinc supplementation may lead to increased activity levels in young children (Sazawal et al., 1996) and improved neuropsychological performance in schoolage children (Penland, 2000).
Because zinc deficiency is associated with diarrhea and impaired immune response (IOM, 2001), there is considerable interest in the possible therapeutic or preventive role of zinc in infectious diseases and diarrhea in children in developing countries. A recent study reported a pooled analysis of all available published and unpublished randomized controlled trials of the effect of supplemental zinc in children less than 5 years of age with acute or persistent diarrhea
(Zinc Investigators’ Collaborative Group, 2000). In the acute diarrhea trials, zinc-supplemented children had a 15 percent lower probability of continuing diarrhea on a daily basis. In the persistent diarrhea trial analysis, zinc supplementation resulted in a 24 percent lower probability of continuing diarrhea and a 42 percent lower rate of treatment failure or death.
Reports of low dietary intakes of zinc and marginal zinc nutriture in pregnant women are of concern (Fitzgerald et al., 1993; Huddle et al., 1998; Kirksey et al., 1994). Although observational studies have produced strong associations between poor maternal zinc status and various indicators of poor pregnancy outcome, supplementation trials have not produced strong or consistent results in support of dietary zinc supplementation (Caulfield et al., 1998).
The minimal nutrient density value for zinc is 5.2 mg/1,000 kcal, based on boys 14 to 18 years of age (Table 2-4). The RDA for zinc (11 mg/day for adolescent boys) is based on estimates that between 30 and 40 percent of dietary zinc would be absorbed (IOM, 2001). It is doubtful that this level of absorption would occur with the EFP given the plant-based diet with the expected level of phytate. Thus, to cover the potential for reduced absorption due to the type of food ingredients, and due to the presence of gastrointestinal problems in the potential recipient populations, the baseline value is increased by 100 percent, resulting in the specified level of zinc in the EFP of 10.4 mg/1,000 kcal (2.4 mg/EFP bar). This level seems justified on the basis of the demonstrated positive effects of zinc supplementation on growth and in the treatment and prevention of diarrhea in malnourished children
Based on estimates of the zinc content of muscle and changes that occur during malnutrition, Golden (2001) has proposed a level of 13 mg of zinc/1,000 kcal for emergency refugee rations. He concluded that this amount would allow individuals who do not have an initial deficit of zinc to gain at least 5 g/kg of body weight/day even with a diet having low zinc bioavailability. A nutrient density of 13 mg of zinc/1,000 kcal is similar to that in other emergency relief foods such as Corn Soy Blend and Unimix, and lower than that in BP-5 Compact Food or F100 (Golden, 2001). The level chosen for the EFP bar is similar to the desirable nutrient density of 9 mg/1,000 kcal recommended by the Sphere Project (Sphere Project, 2001).
The ULs for zinc, 40 mg/day for adults with lower values for children, are adjusted on a weight basis (IOM, 2001). Other reference values developed as safe upper limits of zinc intake (WHO, 1996b) are higher. The UL for zinc for children ages 1 through 3 years (IOM, 2001) is 7 mg/day, which is less than the proposed level for the EFP for this age group (which totals 8.9 mg/day, based on the estimated energy expenditure for children ages 1 through 3 years).
The UL for zinc is based on the adverse effect of excess zinc on copper status, recognizing that the studies reported were primarily in adults (IOM, 2001). Although this level of zinc in the EFP appears to be of possible concern, the expectation that less will be absorbed also holds true for the UL, and thus a
higher amount will in all likelihood not increase the risk that adverse consequences to copper status will occur, particularly when copper is also added to the EFP and the EFP will be fed over a short period of time. However, given the concern about the UL, it is important that the specifications for the EFP be tightly controlled; the maximum content is 11.4 mg/1,000 kcal (specified content + 10 percent).
Additionally, the molar phytate:zinc ratio of the EFP should be less than 10 because higher ratios are associated with suboptimal zinc status (Bindra et al., 1986; Oberleas and Harland, 1981). Consideration needs to be given to the form of zinc added to the EFP. Zinc carbonate and zinc oxide are insoluble and poorly absorbed, whereas zinc sulfate and zinc acetate appear to be better utilized (Allen, 1998) and would be the preferred ingredient.
Vitamins
Vitamin A
Vitamin A is required for normal vision, gene expression, reproduction, embryonic development, growth, and immune function (IOM, 2001). Problems associated with vitamin A deficiency reflect these functions. For example, vitamin A deficiency causes blindness as a consequence of xerophthalmia. Vitamin A deficiency is also associated with increased risk for infectious diseases (Underwood and Arthur, 1996). Conversely, infection may contribute to development of vitamin A deficiency as a result of decreased food intake as well as decreased absorption, increased utilization, and possibly increased excretion (Nalubola and Nestel, 1999).
Vitamin A deficiency is a significant public health problem in many parts of the world, especially in Africa and Southeast Asia (WHO, 2001). Clinical vitamin A deficiency affects at least 2.8 million preschool children in more than 60 countries, and it is estimated that subclinical deficiency is a problem in at least 250 million people (Stephenson et al., 2000). School-age children and pregnant women also are affected. An estimated 250,000 to 500,000 children become blind each year as a consequence of severe vitamin A deficiency (WHO, 2001). Importantly, improving the vitamin A status of deficient children, ages 6 months to 5 years, increases their chances of survival, as shown by the meta-analysis of eight studies in which the risk of mortality from diseases such as measles and diarrhea decreased by 23 percent (Beaton et al., 1994).
Many, but not all, studies have shown a beneficial effect of vitamin A supplementation in infectious disease. Meta-analyses by Fawzi and coworkers (1993) and Glasziou and Mackerras (1993) showed a significant reduction in mortality from infectious diseases in children given vitamin A. The value of vitamin A supplementation in improving morbidity, however, is less clear, and results of various studies have been equivocal. Supplementation has been shown
to reduce the severity of measles and have a beneficial effect on measles-related pneumonia. However, a beneficial effect on nonmeasles respiratory infections has not been demonstrated (Villamor and Fawzi, 2000). WHO (1999a) has recommended vitamin A supplementation in the management of uncomplicated measles in areas of known deficiency as well as in all cases of complicated measles.
Vitamin A deficiency may be an important factor contributing to poor maternal performance during pregnancy and lactation (Ladipo, 2000; Underwood and Arthur, 1996), as well as growth deficits in children (Hadi et al., 2000; West et al., 1988). Vitamin A is also important in iron metabolism. Impaired mobilization of iron stores was found in the presence of vitamin A deficiency (Lynch, 1997), and a significant increase in mean hemoglobin concentration has been shown in anemic school children given vitamin A supplements (Fishman et al., 2000; Mwanri et al., 2000). Furthermore, the results of a recent study suggest that vitamin A may enhance the absorption of iron from cereal-based meals, possibly by preventing phytic acid from binding iron in the cereals (Layrisse et al., 2000).
The adverse effects of high vitamin A intake are well recognized. Most cases of toxicity have occurred because of high-dose supplements taken over a period of months or years. The possible teratogenicity of high vitamin A levels consumed during the first trimester of pregnancy is of particular concern. However, the threshold at which risk occurs is controversial. Most of the data on birth defects associated with excess vitamin A consumption involve doses equal to or greater than 7,800 µg of preformed vitamin A/day (IOM, 2001), although Rothman and colleagues (1995) showed a significantly increased risk for malformation of cranial structures originating from neural crest cells in the infants of women who consumed more than 4,500 µg/day of preformed vitamin A from food and supplements during the first trimester of pregnancy. The ULs for women 14 through 18 and 19 through 50 years of age are 2,800 and 3,000 µg/day of preformed vitamin A, respectively (IOM, 2001).
The UL for infants is 600 µg/day, based on case reports of infants who developed bulging fontanels as a result of receiving high-dose vitamin A supplements (IOM, 2001). This value is only slightly higher than the AI of 500 µg/day for infants 7 through 12 months of age based on estimated intakes for infants receiving human milk and complementary foods (IOM, 2001). Similarly, the differences between the ULs for children 1 through 8 years of age and their corresponding RDAs are relatively small.
Recommendations for the vitamin A content of diets intended for refugee feeding vary. Beaton (1995) recommended 380 µg of retinol equivalents(RE)/1,000 kcal as a goal for fortification of the total diet for refugee feeding. This amount would be expected to meet the needs of at least 95 percent of
TABLE 2-8 Vitamin A Content Based on Energy Needs of an Emergency Relief Food Product (EFP)
|
Age |
Gender |
Energy Requirement (kcal/d)a |
Recommended Dietary Allowance for Vitamin A (µg RAE/day)b |
|
7–12 mo |
Both |
578 |
500 (AI) |
|
1–3 yr |
Both |
855 |
300 |
|
4–8 yr |
Both |
1,456 |
400 |
|
9–13 yr |
Both |
1,693 |
600 |
|
14–18 yr |
Boys |
2,136 |
900 |
|
|
Girls |
1,931 |
700 |
|
|
Girls, pregnant |
2,131 |
750 |
|
19–50 yr |
Men |
2,339 |
900 |
|
|
Women |
1,972 |
700 |
|
|
Women, pregnant |
2,172 |
770 |
|
51+ yr |
Men |
2,249 |
900 |
|
|
Women |
1,929 |
700 |
|
a Estimated energy values (from Table 2-3). b Taken from IOM, 2001. |
|||
individuals and in theory would lead to a 3-month liver reserve. The Sphere Project (2001) suggested that 500 µg RE/day can be used for planning purposes in the initial stages of an emergency. In contrast, Golden (2001) recently recommended that 2,000 µg of retinol/1,000 kcal be added to a ration intended for emergency feeding.
The vitamin A content of diets and products used for refugee relief also varies considerably. For example, the approximate vitamin A content of the BP-5 Compressed Compact Food is 1,025 µg of preformed vitamin A/1,000 kcal (Golden, 2001); Corn/Soy Blend (new), 1,850 µg; and Unimix, 1,635 µg (Beaton, 1995).
The minimal nutrient density value calculated for vitamin A and based on the RDA (900 µg RAE/day [IOM, 2001]) is for boys 14 through 18 years of age (Table 2-4). The value is 420 µg RAE/1,000 kcal. Because the vitamin A status of many of the potential recipients of the EFP may be marginal or deficient and because vitamin A is important in situations involving infectious diseases and diarrhea, the baseline value of 420 µg/1,000 kcal is likely to be too low for many
|
Tolerable Upper Intake Level for Preformed Vitamin A (µg/day) |
Intake of Vitamin A from EFPc Based on Estimated Energy Requirements (µg/day) |
|
600 |
289–578 |
|
600 |
427–855 |
|
900 |
728–1,426 |
|
1,700 |
846–1,693 |
|
2,800 |
1,068–2,136 |
|
2,800 |
966–1,931 |
|
2,800 |
1,066–2,131 |
|
3,000 |
1,170–2,339 |
|
3,000 |
1,170–2,339 |
|
3,000 |
1,086–2,172 |
|
3,000 |
1,124–2,249 |
|
3,000 |
964–1,929 |
|
c The EFP contains between 500 and 1,000 µg of preformed vitamin A/1,000 kcal (Table 2-4 and IOM, 2001). |
|
crises. It is recommended that the EFP contain a minimum of 500 µg of preformed vitamin A/1,000 kcal (117 µg/EFP bar). The maximum content is 1,000 µg of preformed vitamin A/1,000 kcal (specified content + 100 percent). Carotenoids possibly present in food ingredients do not contribute to this total due to concern for variable rates of absorption and bioconversion.
Based on assumed energy intakes, the minimum amount exceeds the U.S. and Canadian recommended intakes for all individuals (see Table 2-8). At the maximum amount, intakes of pregnant women would not exceed the UL of 3,000 µg/day of preformed vitamin A (IOM, 2001), although the intake of children 1 through 8 years of age may be 50 percent above their respective UL for the vitamin. However, the UL is designed to represent chronic intake—it is not meant to apply to malnourished individuals who are recipients of fortification or supplementation programs for the prevention and treatment of vitamin A deficiency (IOM, 2001).
If one of the initial activities in a relief program was to give children a high-dose vitamin A supplement (Golden, 2001), it could be argued that the high content of the EFP would not only be unnecessary but also might increase the risk of adverse effects. In view of the sponsors’ proposed use of this product such a
possibility is unlikely. High-dose supplements are in the range of 60,000 µg of preformed vitamin A administered as a single dose every 4 to 6 months (NRC, 1987); thus the extra amount ingested from food would represent a very small percentage of the total amount given, and consumption of the EFP is recommended for no more than 15 days. It is very important, however, that the content of vitamin A in the EFP be monitored carefully to be within the specifications given.
Vitamin D
Vitamin D (cholecalciferol) is required for calcium absorption, normal muscle function, and bone growth (IOM, 1997a). Although human requirements can be met with adequate exposure to sunlight (Holick, 1994), it is difficult to monitor and ensure adequate bioconversion. The need for dietary vitamin D for survivability or bone growth for a 15-day period for which the EFP is intended is not clear. However, based on the expectation that the target population is prone to deficiency and a dietary source will enhance the absorption and utilization of calcium, it is recommended that the EFP contain vitamin D. Although not reliably documented, vitamin D deficiency resulting from long periods of wearing full body clothing and living in environments with significant air pollution—especially from dust—is possible.
Clearly, when refugees live close to the equator, vitamin D synthesis is probably occurring when environmental and cultural conditions allow adequate skin exposure to the sun. Because of the complexity of estimating true vitamin D needs in this population, the recommendation for vitamin D content of the EFP is the AI (IOM, 1997a). Populations in need of the EFP can be expected to have a high percentage of individuals under 50 years of age. While the AI for vitamin D is 5 µg of cholecalciferol/day for the adult population 50 years of age and younger, it is 10 µg/day for those between 51 and 70 years of age, and 15 µg/day for those over 70 years of age (IOM, 1997a). The amount of vitamin D for the EFP is proposed to be 5.2 µg/1,000 kcal (1.2 µg/EFP bar), based on the needs for those over 50 years of age; those over 70 years of age were thought to be too small a group within refugee populations to be used as the basis for the vitamin D content of the EFP.
Little information is found concerning the bioavailability of vitamin D in malnourished individuals, so no adjustments were made in the recommendation. The types of ingredients likely to be used in the EFP strongly support the addition of vitamin D as cholecalciferol as there may well be no other dietary source included unless fortified milk solids are used as a protein source. Although dictated by cost, utilization of this form also reduces the potential for toxicity (as compared to 1,25(OH)2D3, the biologically active form of vitamin D). The EFP is not formulated as a therapeutic ration, thus individuals with hepatic or renal disease would need to be treated separately.
The UL for vitamin D is 50 µg of cholecalciferol/day (~24 µg/1,000 kcal), and is based on hypercalcemia at higher levels of intake on a chronic basis (IOM, 1997a). Since the recipient population is likely to have some sun exposure, this limit should be strictly adhered to, as there is a risk in displaced populations due to dehydration of compromised urinary function (Briend and Golden, 1993). The maximum content is 5.8 µg/1,000 kcal (specified content + 10 percent).
Vitamin E
Vitamin E, the primary fat-soluble antioxidant in the body, is essential for proper immune system function and for maintenance of cell membranes. Deficiencies of vitamin E have been reported in malnourished individuals (Golden, 2001). Furthermore, diarrhea and malabsorption are likely to be present in the populations served by the EFP. Absorption of vitamin E is known to be low and varies from 21 to 86 percent depending on the presence of any defects that lead to impaired absorption. Impaired absorption was taken into account in developing the recommended dietary intakes (IOM, 2000). Assuming that a smaller percentage of the dietary vitamin E in the EFP will be absorbed due to possible malabsorption, and recognizing that girls 14 to 18 years of age have the greatest nutrient density need, 20 percent is added to the minimal nutrient density value estimated for this age group (Table 2-4) of 7.8 mg of d-α-tocopherol/1,000 kcal, to provide the amount for the EFP of 9.4 mg/1,000 kcal (2.2 mg/EFP bar). The level of vitamin E should provide adequate antioxidant activity to protect against the oxidation of PUFAs after absorption. Therefore, an additional 6.6 mg of vitamin E is added to protect the maximum amount of PUFA at 10 percent of energy (which is 11 g/1,000 kcal), equivalent to 0.6 mg of vitamin E/g of PUFA.
Since it is recommended that the vitamin E be encapsulated, it is not necessary to provide additional d-α-tocopherol beyond the level specified above to serve as an antioxidant for the PUFA present in the bar. If it is not encapsulated, additional vitamin E or other antioxidants will need to be added to protect against lipid oxidation and subsequent destruction of the vitamin over the shelf life of the EFP.
The required level of d-α-tocopherol (9.4 mg/1,000 kcal) is well below the UL for all groups (1,000 mg total α-tocopherol/day [IOM, 2000]).
Vitamin K
Vitamin K functions as a cofactor for the blood clotting cascade, and a deficiency is marked by reduced levels of blood clotting factors such as prothrombin factors X, IX, VII, and protein C. Vitamin K is a cofactor for carboxylation of glutamyl residues on proteins to form γ-carboxyglutamyl proteins (Gla). Osteocalcin, a Gla protein, is essential for bone formation (IOM, 2001; Olsen, 1994).
Deficiencies of vitamin K are rare in most parts of the world, and there are no data validating vitamin K deficiencies in refugee populations. One study from India showed that breast-fed infants with diarrhea had low levels of prothrombin, suggesting that vitamin K deficiency is independent of antibiotic therapy (Kumar et al., 2001). Children with protein–energy malnutrition also have low prothrombin levels (Hassanein and Tankovsky, 1973), but this deficiency is better treated with an increase in dietary protein than with vitamin K. Besides dietary sources of vitamin K, it has been assumed that the microflora of the gastrointestinal tract synthesize menaquinone. With antibiotic therapy—or in the newborn with a sterile gastrointestinal tract—there is a decrease in vitamin K availability (Kumar et al., 2001). However, the contribution of the bacterially produced vitamin K is unknown (IOM, 2001). Thus, dietary sources are recommended, and vitamin K should be added to the EFP.
Blood clotting is important to survival, especially when there are multiple opportunities for injury due to military-type conflicts as well as the continued movement of many refugee populations. Thus, even a 15-day period of vitamin K supplementation may improve survivability by reducing blood loss due to prolonged clotting time following injury. There are data suggesting some benefit for nursing mothers to consume vitamin K to increase levels in their milk. Due to the limited data, however, the AI is the basis for the vitamin K content of the EFP.
Food composition data suggest that soybean oil (193 µg of vitamin K/100 g) could provide the vitamin K required in the EFP, thereby limiting the need for addition of vitamin K (USDA, 1994). Median and mean intakes of vitamin K have been estimated to be 80 to 120 and 60 to 210 µg/day, respectively, for adult men, the most limiting group (IOM, 2001). Levels that result in deficiency are much lower. Given little concern regarding intake above the AI, the minimum content for the EFP is set at 57 µg/1,000 kcal (14 µg/EFP bar). No maximum level is set as little evidence of adverse effects of overconsumption has been identified, except in those taking prescription anticoagulants.
Vitamin C
The function of vitamin C in protecting against oxidative stress, its necessity for wound healing, and its likely role in maintaining normal immune function (IOM, 2000) make the vitamin particularly critical for recipients of emergency rations. Vitamin C also is known to enhance the absorption of nonheme iron, which is especially important in populations where iron deficiency is a major nutritional problem, particularly among women and children (IOM, 2000).
Outbreaks of scurvy have been reported in refugee populations during the past three decades, often in populations entirely dependent on emergency food rations found to provide less than 2 mg/day of vitamin C (IOM, 1997b). It is
difficult to estimate the actual number of scurvy cases that occur, due partly to lack of adequate surveillance systems in refugee camps, but also because of the frequent existence of multiple deficiencies. Populations under siege or on the move are more likely to encounter problems obtaining fresh fruits and vegetables, the major food sources of vitamin C. Populations in some parts of Africa may have marginal intakes of vitamin C for considerable periods of time before an emergency situation occurs.
The RDA for vitamin C for adults (75 mg/day for women, 90 mg/day for men) is based on the amount needed to maintain near maximal neutrophil ascorbate concentrations with minimal urinary excretion of the vitamin (IOM, 2000). Recommended intakes for children and adolescents are derived from adult values based on body weight. The nutrient density needed to meet the needs of the most limiting group (men over age 50 years; see Table 2-4) is approximately 40 mg/1,000 kcal (IOM, 2000). This level would provide the recommended intakes of vitamin C for healthy adults and exceed those for children.
The vitamin C status of EFP recipients is assumed to be marginal given the likelihood that previous diets were low in fruits and vegetables and the occasional observation of scurvy in some refugee populations. It is also possible that storage in higher heat conditions and possible oxidation may destroy some of the vitamin C present in the EFP. Therefore, it is recommended that the vitamin C content of the EFP be 2.5 times the baseline minimal nutrient density, or 100 mg/1,000 kcal (23.3 mg/EFP bar). This level is similar to that in the BP-5 Compact Food (87 mg/1,000 kcal; Golden, 2001), and slightly lower than that in the USAID Corn/Soy Blend (106 mg/1,000 kcal [IOM, 1997b]).
Given that the ULs for children 1 through 3 and 4 through 8 years of age are 400 mg/day and 650 mg/day, respectively, it is unlikely that levels will be above the UL unless premixing problems arise. Thus, for specifications, the maximum vitamin C content of 200 mg/1,000 kcal is suggested (specified content + 100 percent).
Vitamin C is the most labile of the water-soluble vitamins, and is easily oxidized in the presence of moisture, heat, and light. It is anticipated that significant losses of vitamin C may occur during storage, but the use of an ethylcellulose-encapsulated vitamin C should provide for minimum storage losses (IOM, 1997b). With the overage recommended, adequate vitamin C will be present for the recipient.
Thiamin
Thiamin is centrally involved in carbohydrate metabolism, nucleic acid and fatty acid synthesis, and membrane and nerve conduction. Anorexia, tiredness, and weight loss are early symptoms of thiamin deficiency; more severe thiamin deficiency leads to cardiovascular and neurological symptoms, including mental changes (Brown, 1990). In adults, beriberi (severe thiamin deficiency) is
characterized by varying degrees of peripheral neuropathy and cardiovascular involvement while sustained deficiency leads to death. In young infants (2 to 3 months of age), beriberi is characterized by cardiac symptoms, cyanosis, vomiting, and dyspnea; death can occur within hours of the onset of symptoms (Tanphaichitr, 1994). In young, breast-fed infants, beriberi is due to the thiamin deficiency of the mother. Because ethanol is a thiamin antagonist, chronic and heavy alcohol consumption can lead to thiamin deficiency, manifested as Wernicke-Korsakoff syndrome (Zubaran et al., 1997). The biological half-life of thiamin is approximately 9 to 18 days (Ariaey-Nejad et al., 1970). Therefore, consumption of thiamin-poor diets rapidly leads to poor thiamin status. Thiamin deficiency (unspecified) in individuals with poor initial thiamin status has been noted in refugee situations within 2 weeks (Golden, 2001).
Losses of thiamin during cooking may be considerable due to high temperatures and discarding of cooking water (Kimura et al., 1990). Additionally, thiamin is destroyed by sulfite and chlorite, such as sodium hypochlorite, a disinfectant commonly added to water in refugee camps (Dwivedi and Arnold, 1973; Stammati et al., 1992)
Parasitic infections have been shown to be associated with poorer thiamin status in a sample of young Egyptian men (Hussein et al., 1989). Additionally, thiamin deficiency has been reported among children with severe gastroenteritis (Truswell et al., 1972).
Based on the RDA for the limiting group of children ages 1 to 3 years (0.6 mg/day [IOM, 1998]), the minimal nutrient density necessary to meet recommended intakes is 0.6 mg/1,000 kcal (Table 2-4). The thiamin content of the EFP should be the amount that conservatively meets nutritional requirements under adverse conditions. Due to possible gastrointestinal problems in the target population, and potential destruction of the vitamin due to long-term storage and temperature, the recommended thiamin content is doubled to 1.2 mg/1,000 kcal (0.28 mg/EFP bar). No UL has been set for this nutrient as there are no data on adverse effects from food or supplement intake (IOM, 1998). The maximum content is 1.4 mg/1,000 kcal (specified content + 20 percent).
Riboflavin
Riboflavin plays a central role in energy metabolism because of its role as the precursor for the coenzymes flavin mononucleotide (FMN) and flavin–adenine dinucleotide (McCormick, 1990, 1994). Both coenzymes function as catalysts for redox reactions, and are involved in numerous metabolic pathways. Riboflavin coenzymes are necessary for the functioning of the electron transport chain. Symptoms of riboflavin deficiency can include painful lesions of the lips and mouth, peripheral nerve dysfunction, and inflammation of the tongue. In general, deficiencies of riboflavin are associated with deficiencies of other nutrients (IOM, 1998).
Significant rates of riboflavin deficiency have been documented in a wide range of populations, including The Gambia (Reddy et al., 1987), northeast Thailand (Pongpaew et al., 1995), Malaysia (Shahar et al., 1999), Guatemala (King et al., 1997), and Zimbabwe (Wacker et al., 2000). In an emergency situation, prior consumption of animal products and green vegetables (major sources of riboflavin) may be limited or absent; therefore riboflavin deficiency can be assumed to be present, particularly if the crisis has been long.
In general, the bioavailability of riboflavin is quite high, although both riboflavin and FMN can form complexes with a variety of substances, including ascorbic acid, tryptophan, zinc, copper, and iron (Jusko and Levy, 1975). Riboflavin is heat stable, and therefore cooking losses are generally minimal, but the vitamin is susceptible to destruction via oxidation and exposure to light.
Diarrhea and other factors that decrease transit time can cause poor absorption (McCormick, 1994). Enhanced losses of riboflavin can occur with catabolic nitrogen losses, and protein–energy malnutrition can be associated with reduced absorption and utilization of riboflavin (McCormick, 1994). Systemic infection, even without gastrointestinal involvement, can increase the riboflavin requirement (McCormick, 1994).
Since riboflavin plays a central role in energy metabolism, the requirement should theoretically be related to energy intake and expenditure. Belko and coworkers (1983, 1984, 1985) examined the effects of dieting and moderate exercise (2.5 to 5 hr/wk) on the riboflavin status of overweight women and found that both activities increased the riboflavin requirement. In The Gambia, the seasonality of riboflavin intake may be associated with changes in energy intake and balance (Bates et al., 1994). Riboflavin requirements may also be increased by a high carbohydrate:fat ratio (Boisvert et al., 1993). Diets of this type are common in developing countries, and are frequently found in refugee situations.
The baseline minimal nutrient density value calculated for riboflavin and based on the RDA (1.3 mg/day [IOM, 1998]) was for boys 14 through 18 years of age (Table 2-4). The value is 0.6 mg/1,000 kcal. Under emergency conditions, riboflavin status may often be compromised by weight loss, heavy exercise, diarrhea, and multiple nutritional deficiencies. Therefore, the baseline value is likely to be too low for many crises. In support of this hypothesis, Bates and coworkers (1989) reported that riboflavin intakes of 1.8 to 2.5 mg/day were required to return a group of Gambian subjects to an acceptable mean erythrocyte glutathione reductase-activity concentration (EGRAC) of 1.3 to 1.4. Furthermore, Belko and coworkers (1985), studying a group of overweight women on low-calorie diets (1,200 to 1,250 kcal/day), found that riboflavin intakes of 1.0 mg/1,000 kcal were associated with elevated EGRAC levels, while a diet containing 1.2 mg/1,000 kcal provided statistically significant improvements in EGRAC values.
The importance of riboflavin in energy metabolism and its potential destruction by heat and light, and the apparent lack of adverse effects of chronic
consumption at higher than recommended levels (no UL has been established), suggest that the content of the EFP can be safely doubled to 1.2 mg/1,000 kcal (0.28 mg/EFP bar). This level should be enough to cover any additional requirements due to physical activity and/or diarrhea, and is comparable to the riboflavin content of Unimix (1.1 mg/kcal) and Corn-Soy Blend (1.3 mg/1,000 kcal), although lower than F100 (2.0 mg/1,000 kcal) (Golden 2001). The maximum content is 1.4 mg/1,000 kcal (specified content + 20 percent).
Niacin
Niacin, through its coenzymes nicotine adenine dinucleotide and nicotine adenine dinucleotide phosphate, plays a central role in energy metabolism, fatty acid and steroid synthesis, DNA repair, and calcium mobilization (Swendseid and Jacob, 1994). Severe niacin deficiency gives rise to the classic deficiency syndrome, pellagra, which is characterized by dermatitis, diarrhea, dementia, and death. Neurological symptoms include apathy, depression, and memory loss. Changes in the digestive track can lead to vomiting, diarrhea, and constipation. Early signs of mild deficiency can include ill-defined gastrointestinal problems, weakness, and lassitude (IOM, 1998). Although the prevalence of mild and marginal deficiencies has not been well documented, rates are likely to be relatively high in some maize- and sorghum-consuming populations (in which niacin deficiency is typically found due to low niacin content along with low levels of tryptophan), particularly during the “hungry season” (the weeks or months when the produce from the previous harvest is fully consumed and the next harvest is not yet ready). The body can convert the amino acid tryptophan to niacin with about 60 mg of tryptophan being needed to produce 1 mg of niacin, although this may vary by as much as 30 percent (IOM, 1998).
Niacin can be obtained either by consumption of preformed niacin or by conversion of tryptophan to niacin. Niacin bioavailability varies according to the form of niacin and the food matrix. In developing countries, many people obtain most of their niacin from grains, legumes, and green leafy vegetables, and by synthesis of niacin from tryptophan. In mature maize, and to a lesser degree in wheat and other cereals, niacin is bound to complex carbohydrates and small peptides and is biologically unavailable (WHO, 2000). Only about 30 percent of niacin in maize is bioavailable; however, heat treatment of maize under alkaline conditions, as is traditionally done in Mexico, greatly increases niacin bioavailability (Carpenter and Lewin, 1985).
Pellagra has often been observed in refugee populations. In 1989 to 1990, an outbreak of pellagra occurred among Mozambican refugees living in Malawi (Malfait et al., 1993). During an 8-month period, nearly 18,000 of 286,000 refugees (incidence = 6.3 percent) were affected (CDC, 1991). Incidence of pellagra
TABLE 2-9 Recommended Dietary Allowances (RDAs) and Tolerable Upper Intake Levels (ULs) for Niacin
|
Age (yr) |
RDA (mg of NEa/d) |
ULb (mg/d) |
Niacin Intake from the EFP Bar (mg NE/d) |
|
1–3 |
6 |
10 |
10 |
|
4–8 |
8 |
15 |
16 |
|
9–13 |
12 |
20 |
19 |
|
14–18, boys |
16 |
30 |
24 |
|
14–18, girls |
14 |
30 |
22 |
|
Adults, men |
16 |
35 |
26 |
|
Adults, women |
14 |
35 |
26 |
|
a NE = niacin equivalents. b As nicotinic acid/niacinamide added to foods or in supplements only (IOM, 1998). c Based on a maximum content of 2.9 mg NE/EFP bar (or 12.4 mg/1,000 kcal). SOURCE: IOM (1998). |
|||
was nearly eight times higher for women than men, but children under 5 years of age were relatively unlikely to be affected. This epidemic was precipitated by a disruption in local groundnut supply, a source of niacin for the population. More recently, an outbreak of pellagra in Angola affected both refugee and local populations (Baquet et al., 2000). Most cases occurred in women (83 percent), with relatively few children under 15 years of age afflicted (18 percent of cases). Other pellagra outbreaks were documented during the 1980s and 1990s in Nepal, Zimbabwe, Angola, Malawi, and Mozambique (WHO, 2000).
Because of the central role of niacin in energy metabolism, niacin requirements bear a theoretical relationship to energy. However, no research has examined the influence of energy expenditure or intake on niacin requirements (IOM, 1998). Additionally, inadequate iron, riboflavin, or vitamin B6 status reduces the efficiency of the conversion of tryptophan to niacin, although the magnitude of these effects has not been established (IOM, 1998).
The limiting subgroup for niacin is boys 14 to 18 years of age (Table 2-4), with a minimal nutrient density needed of 7.5 mg of niacin equivalents (NE)/1,000 kcal. The UL for niacin refers only to nicotinic acid or nicotinamide added to foods or taken as supplements. Thus concern about adverse effects would only arise with the amount of nicotinic acid or nicotinamide added to the EFP.
Because niacin deficiency is likely to be highly prevalent in many refugee populations, the minimal nutrient density is increased by 50 percent to 11.2 mg NE/1,000 kcal (2.6 mg NE/EFP bar). Table 2-9 provides the estimated amount of niacin intakes from the EFP based on the energy intakes estimated in Table 2-3. Given that not all the NE included in the EFP will be as an added ingredient, it is probable that the UL for nicotinic acid/niacinamide will not be exceeded at
this level of total niacin intake. However, it is important that the level contained in the EFP be carefully monitored and not be exceeded by more than 10 percent. The maximum content is 12.4 mg/1,000 kcal (specified content + 10 percent).
Golden (2001) has recommended 18 mg/1,000 kcal of niacin. The niacin content of other emergency products ranges from 10 mg/1,000 kcal (F100) to 27 mg/1,000 kcal (Oxford SK8 biscuit). The recommended level for the EFP is within the range of these other recommendations.
Vitamin B6
Vitamin B6, a group of six compounds of which the pyridoxine forms are the most prevalent in plant-based foods, is important in a wide variety of metabolic processes, including normal protein metabolism and glucose production (IOM, 1998). Adequate vitamin B6 status is also required for optimal conversion of tryptophan to niacin. Hemoglobin synthesis is dependent on adequate vitamin B6 status, and severe deficiency of vitamin B6 can lead to hypochromic, microcytic anemia. Poor vitamin B6 status is associated with compromised cell-mediated immune function. In cases of severe deficiency, infants can suffer convulsions, while symptoms in adults include depression, confusion, irritability, stomatitis, and cheilosis (IOM, 1998).
Little research has been conducted to examine the prevalence of vitamin B6 deficiency in developing countries. In periurban Egypt, 38 percent of 70 women had low vitamin B6 levels in breast milk, and low values were associated with poorer mother–infant interaction (McCullough et al., 1990). In Indonesia, approximately 40 percent of rural third-graders had plasma pyridoxal phosphate values (the most widely used vitamin B6 status index) indicative of deficiency (Setiawan et al., 2000). Vitamin B6 deficiency was observed in 26 percent of young female Chinese textile workers (Ronnenberg et al., 2000). In Europe, the SENECA study found that more than 50 percent of the elderly in some geographical areas had vitamin B6 deficiency (Haller et al., 1991). The results of these studies suggests that pre-existing deficiencies of vitamin B6 can be assumed to be present in refugee populations.
Vitamin B6 is available from a wide range of plant and animal foods. The forms of vitamin B6 in eggs, fish, and poultry are highly bioavailable. Plant pyridoxines are less bioavailable and may decrease absorption of the more bioavailable forms of the vitamin (Gregory, 1998). In developing countries, the principal sources of vitamin B6 are likely to be starchy staples and legumes. Food processing and storage adversely influence the vitamin B6 content of some foods (Leklem, 1996). Vitamin B6 in food is unstable under neutral or alkaline conditions.
Since vitamin B6 is absorbed by a nonsaturable, passive process that occurs primarily in the jejunum, the presence of parasites or diarrhea may have little effect on vitamin B6 absorption, although it has not been well explored. In order
to support the role of vitamin B6 in amino acid metabolism, some investigators have proposed an increased requirement for vitamin B6 coincident with increasing protein intake. Several studies have documented a relationship between increased protein intake and decreased vitamin B6 status. However, the precise mathematical relationship remains unclear (IOM, 1998). Several studies have examined the influence of physical activity on vitamin B6 status and metabolism, and have shown little or no relationship (Manore, 2000), although exercise is theoretically linked to increased vitamin B6 requirements.
Based on the RDA of 1.5 mg/day for the limiting subgroup of women 51 years of age and older (IOM, 1998), a minimal nutrient density value was calculated (see Table 2-4). Under the assumptions of the method, the value required to prevent inadequate intake in almost all individuals in this life stage and gender group would be 0.8 mg of vitamin B6/1,000 kcal. Given concern about losses in food processing and storage, the EFP should contain 50 percent more, or 1.2 mg/1,000 kcal (0.28 mg/EFP bar).
Large doses of oral vitamin B6 have been associated with a range of negative outcomes. The UL is 30 g/day as pyridoxine for children ages 1 through 3 years (IOM, 1998), much higher than the proposed level for the EFP. Therefore, adverse effects related to vitamin B6 should not be a problem. The maximum content is 1.4 mg/1,000 kcal (specified content + 20 percent).
Folate
Folate is a collective term for a family of compounds that are structurally and functionally related to pteroylmonoglutamic acid. Folate is involved physiologically in DNA synthesis, purine synthesis, and amino acid interconversions, including the synthesis of methionine from homocysteine (IOM, 1998). Folate deficiency leads to megaloblastic anemia, elevated homocysteine, increased risk of neural tube defects, and possibly increased risk of other congenital disorders, cancer, and vascular disease (IOM, 1998).
Major sources of folate include green vegetables and legumes. The absorption of food folate requires the conversion of polyglutamyl folates to monoglutamyl forms, which are then absorbed at physiological levels by a saturable transport process (Gregory, 2001). The bioavailability of food folate varies widely by food and may be influenced by processing of the food matrix (Castenmiller et al., 2000; Gregory, 2001). Folate absorption can be adversely effected by unidentified factors in food and by alcohol consumption. Overall, the bioavailability of food folate is estimated at about 50 percent (IOM, 1998). In contrast, synthetic folate in fortified foods is highly bioavailable ( 85 percent [IOM, 1998]).
Rates of folate deficiency in developing countries are largely unknown and may be less common than deficiencies of many other micronutrients because of the relatively low cost of legumes and greens, which are major dietary sources.
TABLE 2-10 Recommended Dietary Allowances (RDAs) and Tolerable Upper Intake Levels (ULs) for Folate
|
Age (yr) |
RDA (µg DFEa/d) |
ULb (µg synthetic folate/d) |
Synthetic Folate Intakec (µg/d) |
|
1–3 |
150 |
300 |
265 |
|
4–8 |
200 |
400 |
452 |
|
9–13 |
300 |
600 |
525 |
|
14–18, boys |
400 |
800 |
662 |
|
14–18, girls |
400 |
800 |
599 |
|
Adults, men |
400 |
1,000 |
725 |
|
Adults, women |
400 |
1,000 |
611 |
|
a As dietary folate equivalents. b As folate added to foods or in supplements only (IOM, 1998). c Based on a maximum of 80 µg DFE/emergency relief food product bar (or 340 µg/1,000 kcal). SOURCE: IOM (1998). |
|||
However, seasonality, local dietary traditions, or other health conditions may lead to observable rates of deficiency in some populations. In western Venezuela, 91 percent of individuals in a single Bari Indian community were assessed as folate deficient, whereas very little (5 percent) deficiency was observed in a second community (Diez-Ewald et al., 1997). In Malawi, 21 to 34 percent of anemic pregnant women were folate deficient (van den Broek and Letsky, 2000).
Vitamin B12 deficiency leads to functional folate deficiency because vitamin B12 acts as a cofactor in recycling folate (IOM, 1998). Early research also suggested an adverse effect of zinc deficiency on folate absorption. However, subsequent work has failed to replicate this result (Gregory, 2001).
Using the method previously outlined, the minimal nutrient density value for folate is based on the RDA of 400 µg/day (IOM, 1998) for girls 14 through 18 years of age (Table 2-4). The minimal nutrient density value is 207 µg of dietary folate equivalents (DFE)/1,000 kcal (IOM, 1998). To cover the potential for additional reduced absorption due to gastrointestinal problems, the baseline value is increased by 50 percent and the recommended level of folate for the EFP is 310 µg DFE/1,000 kcal (72 µg DFE/EFP bar).
The UL for folate is 1,000 µg/day for adults, and lower values for children are adjusted on a metabolic weight basis (IOM, 1998) (Table 2-10). The UL for folate is for folate added to foods or taken as supplements only. The UL for children ages 4 through 8 years (IOM, 1998) is 400 µg/day, which is less than the proposed level for the EFP. The UL is based on reports of adverse effects of high levels of folate masking the irreversible neurological damage seen in cases of vitamin B12 deficiency. Since some of the folate in the total amount per food
bar may be contributed by food sources, it is assumed that this level will not be exceeded. However, given a concern for inadequate mixing, the maximum content is 340 µg DFE/1,000 kcal (specified content + 10 percent). If synthetic folate is used, these values should be divided by 1.6.
Vitamin B12
Vitamin B12 is required for methyl transfer to folate; for conversion of homocysteine to methionine; and for synthesis of succinyl CoA, the Krebs cycle intermediate, from L-methylmalonyl CoA. Vitamin B12 deficiency can lead to megaloblastic anemia and neuropathy. Neuropsychiatric symptoms can include irritability, fatigue, apathy, and emotional instability (IOM, 1998). Cognitive and neuropsychiatric complications can precede anemia by a considerable period of time. As many as 90 percent of individuals with clinically observable vitamin B12 deficiency present neurological complications (IOM, 1998).
Nearly all naturally occurring vitamin B12 must be obtained by consumption of animal products, although vitamin B12 may be present in small amounts in some plant products via contamination by microorganisms (IOM, 1998). In much of the developing world, animal products are not routinely consumed due to poverty. Some individuals do not consume meat for religious and cultural reasons.
Vitamin B12 is efficiently stored in the liver, and losses are minimized in the healthy individual through enterohepatic recirculation. However, because vitamin B12 is secreted in the bile as a part of normal digestion, individuals can become deficient due to poor resorption (and absorption) of the vitamin (Stopeck, 2000). Helicobacter pylori infection of the gastrointestinal tract may be an important cause of adult vitamin B12 deficiency, and treatment of atopic gastritis with antibiotics can be an effective means of reversing B12 malabsorption (Kaptan et al., 2000; Suter et al., 1991). In the United States, an estimated 10 to 15 percent of persons aged 60 or older suffer from vitamin B12 deficiency, mostly due to poor absorption (Baik and Russell, 1999).
When initial vitamin B12 stores are abundant, deficiency due to malabsorption or a vegetarian diet can take years to manifest. However, when low stores are combined with low intake or malabsorption, deficiency occurs more rapidly. In rural Mexico, where consumption of animal products is limited, increased incidence of low levels of vitamin B12 in human milk and plasma and decreased holotranscobalamin II have been noted (Allen et al., 1995; Black et al., 1994). High rates of deficiency among children were attributed to maternal malnutrition. In an urban Mexican population, 12 percent of nonpregnant, nonlactating women had low plasma B12 values (Casanueva et al., 2000). In Guatemala, 47 percent of lactating women had low plasma B12 values, 31 percent of breast milk values were low, and 32 percent of mothers had low holotranscobalamin II
values (Casterline et al., 1997). In Malawi, 16 percent of anemic pregnant women were vitamin B12 deficient (van den Broek and Letsky, 2000). In Kenya, the vitamin B12 content of human milk was very low (Neumann and Harrison, 1994). Epidemiological research in Zimbabwe also suggests that vitamin B12 deficiency may be a public health problem in that country (Savage et al., 1994).
The significance of adequate vitamin B12 stores is indicated by research on Dutch children who were raised on a macrobiotic diet during the first 6 years of life (Louwman et al., 2000; van Dusseldorp et al., 1999). Subsequently, these children consumed lacto-ovovegetarian or omnivorous diets. However, when assessed during adolescence, vitamin B12 status remained low and cognitive function was shown to be impaired. The authors speculated that poor vitamin B12 status was the combined effect of low stores from the macrobiotic period and somewhat low subsequent intakes (van Dusseldorp et al., 1999). However, other nutrients were also deficient during the macrobiotic dietary period.
In summary, limited consumption of animal products as a result of poverty concomitant with high rates of diarrhea and gastrointestinal infection due to parasitic and other enteric diseases will likely predispose populations in developing countries to vitamin B12 deficiency, and certainly to a lack of stores of the vitamin.
Little information is available on consumption of high levels of vitamin B12 from either food or supplements and associated adverse effects; therefore data were inadequate to establish a UL for this vitamin (IOM, 1998).
The minimal nutrient density value for vitamin B12 is based on the RDA for girls 14 to 18 years of age (2.4 µg/day [IOM, 1998]), and is 1.2 µg/1,000 kcal (see Table 2-4). However, given the concerns about lack of stores and the probability of a high level of vitamin B12 deficiency, higher levels are indicated to ensure that an adequate amount of the nutrient is absorbed and that stores are replenished to the extent possible. Vitamin B12 is very stable in foods. Therefore, it is recommended that the baseline value be increased by a factor of 10 to 12 µg/1,000 kcal (2.8 µg/EFP bar). The maximum content is 14.4 µg/1,000 kcal (specified content + 20 percent).
Pantothenic Acid
Pantothenic acid is required for the synthesis of coenzyme A (CoA), which functions in a broad range of enzymatic processes, many of which involve lipid metabolism (IOM, 1998). CoA is ubiquitously distributed in cells, is required by most forms of life, and is hydrolyzed in the gut to pantothenic acid. Therefore, pantothenic acid can be obtained from a wide range of foods, and pantothenic acid deficiency is thought to be unusual.
Epidemics of deficiency, however, have occurred when food choice was severely restricted. During World War II, prisoners of war in Asia suffered symptoms that were attributed to pantothenic acid deficiency (Plesofsky-Vig, 1999).
More recently, Afghan refugees who were provided white wheat flour without other supplemental food suffered similar symptoms (Golden, 2001). Deficiency can lead to headache, irritability, fatigue, insomnia, nausea and vomiting, hypoglycemia, and paresthesia of the extremities (IOM, 1998).
Absorption of pantothenic acid occurs by active transport at low concentrations, is saturable at higher concentrations, and is passive (Fenstermacher and Rose, 1986). The effects of diarrhea on absorption of pantothenic acid are unknown.
The minimal nutrient density value for pantothenic acid is based on the AI for girls 14 to 18 years of age (5 mg/day [IOM, 1998]) and is 2.6 mg/1,000 kcal (see Table 2-4). The importance of pantothenic acid in energy metabolism, the potential for decreased absorption due to gastrointestinal symptoms or disease, and the lack of reported adverse effects of chronic consumption at higher than recommended levels (no UL has been established) suggest that the content of the EFP can safely be increased by 50 percent to 3.9 mg/1,000 kcal (0.9 mg/EFP bar). The maximum content is 4.7 mg/1,000 kcal (specified content + 20 percent).
Biotin
Biotin is a cofactor for four adenosine triphosphate-dependent carboxylases (IOM, 1998). This nutrient is necessary for normal cell growth, glucose homeostasis, and DNA synthesis. Biotin deficiency has been shown to be teratogenic in a variety of mammalian species (Mock et al., 1997).
Severe biotin deficiency is rare in the industrialized countries; it occurs due to unusual conditions such as metabolic abnormalities, heavy and sustained intake of avidin from raw egg white, and total parenteral nutrition without biotin supplementation (IOM, 1998). In developing countries, severe protein–energy malnutrition may be accompanied by poor biotin status and impaired carboxylase activity (Velázquez, 1997; Velázquez et al., 1995). Marginal biotin deficiency may be fairly common; a substantial proportion of pregnant women in Iowa exhibited evidence of biotin depletion as pregnancy progressed (Mock et al., 1997). The prevalence of biotin deficiency in both industrialized and developing countries is unknown. A study in rat models has documented an adverse effect of biotin deficiency on n-6 PUFA metabolism (Mock, 1990).
Biotin is present in a range of animal and plant foods, but for most foods the precise biotin content and its bioavailability are poorly understood (Said, 1999). Free biotin is nearly 100 percent bioavailable (Zempleni and Mock, 1999). However, much of the biotin in foods is protein-bound and bioavailability is not known (IOM, 1998).
The minimal nutrient density value for biotin is based on the estimated needs of women 51 years of age and older. Based on an AI of 30 µg/day for this group, the baseline value would be 16 µg/1,000 kcal (IOM, 1998). To cover the
potential for reduced absorption due to gastrointestinal problems, the minimal nutrient density value is increased by 50 percent to the recommended level for the EFP of 24 µg/1,000 kcal (5.6 µg/EFP bar). The maximum content is 28.8 µg/1,000 kcal (specified content + 20 percent). No UL has been established for biotin; concern about excess intake is unwarranted in this situation.
Choline
Choline is involved in the synthesis and release of acetylcholine (a neurotransmitter), and is a precursor of phospholipids and sphingomyelin (important constituents in cell membranes), and also synthesis of the methyl donor, betaine (IOM, 1998). The human body has a limited capacity for de novo choline synthesis and rates of synthesis may not be sufficient to meet the needs of at least some individuals (Zeisel, 2000).
Large amounts of choline are transferred from mother to fetus during pregnancy, and considerable amounts are delivered later to the child via human milk (IOM, 1998). Therefore, adequate maternal intakes of choline are needed to protect the mother against deficiency and to provide the infant with the choline required for normal development. Animal models have demonstrated the importance of adequate choline intake for normal brain development (Blusztajn, 1998). In the rat, choline deficiency adversely influences brain development at two times during growth: during late gestation (12 to 17 days) and postpartum (6 to 30 days) (Jones et al., 1999; Zeisel, 2000). Prenatal effects appear to be permanent (Blusztajn, 1998).
The choline content of foods is poorly characterized, and the bioavailability of many choline-containing compounds in foods is unknown (Zeisel, 2000). However, eggs contain significant amounts of choline, as do liver and peanuts (IOM, 1998).
Free choline is absorbed from the small intestine (Le Kim and Betzing, 1976). The influence of diarrhea, bacterial overgrowth, and parasitic infection on choline absorption is unknown, but it can be assumed to be adverse. No research has been done on the prevalence of choline deficiency in either industrialized or developing countries.
The minimal nutrient density value for choline is based on the AI for the subgroup of men over 50 years of age (550 mg/day [IOM, 1998]), and is 244 mg/1,000 kcal of choline (see Table 2-4). As with other water-soluble vitamins, to cover the potential for reduced absorption due to gastrointestinal problems, the minimal nutrient density value is increased by 50 percent. Therefore, the minimum content is 366 mg/1,000 kcal (85 mg/EFP bar). The maximum content is 439 mg/1,000 kcal (specified content + 20 percent).
Conclusion
The recommendations for the nutrient content and energy sources contained in this chapter meet the goal of the report: to develop a high-energy, nutrient-dense food product that would be nutritionally adequate for all people 7 months of age and older. The recommendations are designed to provide all known nutrients in quantities to satisfy the needs of the most vulnerable life stage and gender group. In addition, levels of nutrients were frequently increased above the minimal nutrient densities to compensate for poor bioavailability, processing and storage losses, and reduced absorption due to mild diarrhea, infections, or parasites. However, as was described in the beginning of this chapter, the nutritional content is not the highest priority in the design of the ration—in terms of importance, it comes after safety, palatability, ease of delivery, and ease of use.
An additional characteristic that may also be critical is cost. Since this product is intended to be an emergency stop-gap to be used no longer than 15 days while a more permanent food supply line is put in place, if cost is a consideration, it is recommended that food ingredients be analyzed for nutrient content and supplemented only as necessary. It should be assumed that the recommended amounts and sources are considered optimal, but other factors take precedence in the final formulation.
REFERENCES
Abdallah L, Chabert M, Le Roux B, Louis-Sylvestre J. 1998. Is pleasantness of biscuits and cakes related to their actual or their perceived sugar and fat contents? Appetite 30:309–324.
Adolph EF. 1947. Signs and symptoms of desert dehydration. In: Adolph EF, ed. Physiology of Man in the Desert. New York: Interscience Publishers. Pp. 226–240.
Allen LH. 1998. Zinc and micronutrient supplements for children. Am J Clin Nutr 68:495S–498S.
Allen LH, Backstrand JR, Stanek EJ 3rd, Pelto GH, Chavez A, Molina E, Castillo JB, Mata A. 1992. The interactive effects of dietary quality on the growth and attained size of young Mexican children. Am J Clin Nutr 56:353–364.
Allen LH, Rosado JL, Casterline JE, Martinez H, Lopez P, Munoz E, Black AK. 1995. Vitamin B12 deficiency and malabsorption are highly prevalent in rural Mexican communities. Am J Clin Nutr 62:1013–1019.
Ariaey-Nejad MR, Balaghi M, Baker EM, Sauberlich RE. 1970. Thiamin metabolism in man. Am J Clin Nutr 23:764–778.
Ashour MN, Salem SI, El-Gadban HM, Elwan NM, Basu TK. 1999. Antioxidant status in children with protein–energy malnutrition (PEM) living in Cairo, Egypt. Eur J Clin Nutr 53:669–673.
Baik HW, Russell RM. 1999. Vitamin B12 deficiency in the elderly. Annu Rev Nutr 19:357–377.
Baker EM, Plough IC, Allen TH. 1963. Water requirements of men as related to salt intake. Am J Clin Nutr 12:394–398.
Baquet S, Wuillaume F, Van Egmond K, Ibanez F. 2000. Pellagra outbreak in Kuito, Angola. Lancet 355:1829–1830.
Bates CJ, Powers HJ, Downes R, Brubacher D, Sutcliffe V, Thurnhill A. 1989. Riboflavin status of adolescent vs. elderly Gambian subjects before and during supplementation. Am J Clin Nutr 50:825–829.
Bates CJ, Prentice AM, Paul AA. 1994. Seasonal variations in vitamins A, C, riboflavin and folate intake and status of pregnant and lactating women in a rural Gambian community: Some possible implications. Eur J Clin Nutr 48:660–668.
Beaton GH. 1995. Fortification of foods for refugee feeding. Technical Background Report: Derivations and Analyses. Report to CIDA. Ottawa: Canadian International Development Agency.
Beaton GH, Martorell R, Aronson KA, Edmonston B, McCabe G, Ross AC, Harvey B. 1994. Vitamin A supplementation and child morbidity and mortality in developing countries. Food Nutr Bull 15:282–289.
Beck MA, Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA. 2001. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J 15:1481–1483.
Behne D, Kyriakopoulos A. 2001. Mammalian selenium-containing proteins. Annu Rev Nutr 21:453–473.
Belko AZ, Obarzanek E, Kalkwarf HJ, Rotter MA, Bogusz S, Miller D, Haas JD, Roe DA. 1983. Effects of exercise on riboflavin requirements of young women. Am J Clin Nutr 37:509–517.
Belko AZ, Obarzanek E, Roach R, Rotter M, Urban G, Weinburg S, Roe DA. 1984. Effects of aerobic exercise and weight loss on riboflavin requirements of moderately obese, marginally deficient young women. Am J Clin Nutr 40:533–561.
Belko AZ, Meredith MP, Kalkwarf HJ, Obarzanek E, Weinberg S, Roach R, McKeon G, Rose DA. 1985. Effects of exercise on riboflavin requirements: Biological validation in weight reducing women. Am J Clin Nutr 41:270–277.
Bemiller JN, Whistler RL. 1996. Carbohydrates. In: Fennema OR, ed. Food Chemistry. 3rd ed. New York: Marcel Dekker. Pp. 157–223.
Berger J, Dyck JL, Galan P, Apologan A, Schneider D, Traissac P, Hercberg S. 2000. Effect of daily iron supplementation on iron status, cell-mediated immunity, and incidence of infections in 6–36 month old Togolese children. Eur J Clin Nutr 54:29–35.
Beshgetoor D, Hambidge M. 1998. Clinical conditions altering copper metabolism in humans. Am J Clin Nutr 67:1017S–1021S.
Bindra GS, Gibson RS, Thompson LU. 1986. [Phytate][calcium]/[zinc] ratios in Asian immigrant lacto-ovo vegetarian diets and their relationship to zinc nutriture. Nutr Res 6:475–483.
Black AK, Allen LH, Pelto GH, DeMata MP, Chavez A. 1994. Iron, vitamin B12 and folate status in Mexico: Associated factors in men and women and during pregnancy and lactation. J Nutr 124:1179–1188.
Blusztajn JK. 1998. Choline, a vital amine. Science 281:794–795.
Boisvert WA, Mendoza I, Castañeda C, De Portocarrero L, Solomons NW, Gershoff SN, Russell RM. 1993. Riboflavin requirement of healthy elderly humans and its relationship to macronutrient composition of the diet. J Nutr 123:915–925.
Bothwell TH. 1999. Iron fortification with special reference to the role of iron EDTA. Arch Latinoam Nutr 49:23S–33S.
Branca F, Robins SP, Ferro-Luzzi A, Golden MHN. 1992. Bone turnover in malnourished children. Lancet 340:1493–1496.
Briend A, Golden MHN. 1993. Treatment of severe child malnutrition in refugee camps. Eur J Clin Nutr 4:750–754.
Brooks GA, Trimmer JK. 1996. Literature supports the crossover concept. J Appl Physiol 80:1073–1074.
Brooks GA, Fahey TP, White TP, Baldwin KM. 2000. Exercise Physiology. Human Bioenergetics and Its Applications. Mountain View, CA: Mayfield Publishing. Pp. 723–724.
Brown AH. 1947a. Survival without drinking water in the desert. In: Adolph EF, ed. Physiology of Man in the Desert. New York: Interscience Publishers. Pp. 271–279.
Brown AH. 1947b. Water requirements of man in the desert. In: Adolph EF, ed. Physiology of Man in the Desert. Pp. 115–135.
Brown KH. 1991. Dietary management of acute childhood diarrhea: Optimal timing of feeding and appropriate use of milks and mixed diets. J Pediatr 118:S92–S98.
Brown KH, Lopez de Romana G, Grahan GG, MacLean WC. 1982. Experience with a mixture of wheat-noodles and casein in the initial dietary therapy of infants and young children with protein energy malnutrition or acute diarrhea. Human Nutr Applied Nutr 36A:354–366.
Brown KH, Perez F, Gastanaduy AS. 1991. Clinical trial of modified whole milk, lactose-hydrolyzed whole milk, or cereal-milk mixtures for the dietary management of acute childhood diarrhea. J Pediatr Gastroenterol Nutr 12:340–350.
Brown KH, Peerson JM, Fontaine O. 1994. Use of nonhuman milks in the dietary management of young children with acute diarrhea: A meta-analysis of clinical trials. Pediatrics 93:17–27.
Brown KH, Sanchez-Griñan MI, Perez F, Peerson JM, Ganoza L, Stern JS. 1995. Effects of dietary energy density and feeding frequency on total daily
energy intakes of recovering malnourished children. Am J Clin Nutr 62:13–18.
Brown KH, Peerson JM, Allen LH. 1998. Effect of zinc supplementation on children’s growth: A meta-analysis of intervention trials. Bibl Nutr Dieta 54:76–83.
Brown ML. 1990. Thiamin. In: Brown ML, Filer LJ, Guthrie HA, Levander OA, McCormick DB, Olson RE, Steele RD, eds. Present Knowledge in Nutrition. 6th ed. Washington, DC: ILSI Press. Pp. 142–145.
Butte NF. 1996. Energy requirements of infants. Eur J Clin Nutr 50:S24–S36.
Butte NF, Wong WW, Hopkinson JM, Heinz CJ, Mehta NR, Smith EO. 2000. Energy requirements derived from total energy expenditure and energy deposition during the first 2 y of life. Am J Clin Nutr 72:1558–1569.
Cahill GH Jr. 1970. Starvation in man. N Engl J Med 282:668–675.
Calder PC, Jackson AA. 2000. Undernutrition, infection and immune function. Nutr Res Rev 13:3–29.
Calloway DH. 1960. Nutritional aspects of the all-purpose survival ration. US Armed Forces Med J 11:403–417.
Calloway DH, Chenoweth WL. 1973. Utilization of nutrients in milk- and wheat-based diets by men with adequate and reduced abilities to absorb lactose. I. Energy and nitrogen. Am J Clin Nutr 26:939–951.
Calloway DH, Spector H. 1954. Nitrogen balance as related to caloric and protein intake in active young men. Am J Clin Nutr 2:405–411.
Calloway DH, Murphy S, Balderston J, Receveur O, Lein D, Hudes M. 1992. Village Nutrition in Egypt, Kenya and Mexico: Looking Across the CRSP Projects. Final report to the U.S. Agency for International Development. Berkeley, CA: University of California, Berkeley.
Carpenter KJ, Lewin WJ. 1985. A reexamination of the composition of diets associated with Pellagra. J Nutr 115:543–552.
Carter JP, Kattab A, Abd-El-Hadi K, Davis JT, el Ghalmy A, Patwardhan VN. 1968. Chromium (III) in hypoglycemia and in impaired glucose utilization in kwashiorkor. Am J Clin Nutr 21:195–202.
Casanueva E, Drijanski A, Fernandez-Gaxiola AC, Meza C, Pfeffer F. 2000. Folate deficiency is associated with obesity and anemia in Mexican urban women. Nutr Res 20:1389–1394.
Castenmiller JJ, van de Poll CJ, West CE, Brouwer IA, Thomas CM, van Dusseldorp M. 2000. Bioavailability of folate from processed spinach in humans: Effect of food matrix and interaction with carotenoids. Ann Nutr Metab 44:163–169.
Casterline JE, Allen LH, Ruel MT. 1997. Vitamin B12 deficiency is very prevalent in lactating Guatemalan women and their infants at three months postpartum. J Nutr 127:1966–1972.
Caulfield LE, Zavaleta N, Shankar AH, Merialdi M. 1998. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am J Clin Nutr 68:499S–508S.
CDC (Centers for Disease Control and Prevention). 1990. Health and nutritional profile of refugees—Ethiopia, 1989–1990. Morb Mortal Wkly Rep 39:707–709, 715–718.
CDC. 1991. International Notes. Outbreak of pellagra among Mozambican refugees—Malawi, 1990. Morb Mortal Wkly Rep 40:209–213.
Chulei R, Xiaofang L, Hongsheng M, Xiulan M, Guizheng L, Gianhong D, DeFrancesco CA, Connor WE. 1995. Milk composition in women from five different regions of China: The great diversity of milk fatty acids. J Nutr 125:2993–2998.
Clegg KM. 1960. The availability of lysine in groundnut biscuits used in the treatment of kwashiorkor. Br J Nutr 12:325–329.
Clugston GA, Hetzel BS. 1994. Iodine. In: Shils ME, Olson JA, Shike M, eds. Modern Nutrition in Health and Disease. 8th ed. Baltimore: Lea & Febiger. Pp. 252–263.
Collins N. 2000. Assessment and treatment of involuntary weight loss and protein–calorie malnutrition. Adv Skin Wound Care 13:S4–S10.
Collins S, Myatt M, Golden B. 1998. Dietary treatment of severe malnutrition in adults. Am J Clin Nutr 68:193–199.
Contempre B, Duale NL, Dumont JE, Ngo B, Diplock AT, Vanderpas J. 1992. Effect of selenium supplementation on thyroid hormone metabolism in an iodine and selenium deficient population. Clin Endocrinol 36:579–583.
Costill DL, Coté R, Fink W. 1976. Muscle water and electrolytes following varied levels of dehydration in man. J Appl Physiol 40:6–11.
Crim MC, Munro HN. 1994. Proteins and amino acids. In: Shils ME, Olson JA, Shike M, eds. Modern Nutrition in Health and Disease. 8th ed. Baltimore: Lea & Febiger. Pp. 3–35.
Cunnane SC. 1981. Zinc and copper interact antagonistically in the regulation of linoleic acid metabolism. Prog Lipid Res 20:601–603.
Dahlin K, Lorenz K. 1993. Protein digestibility of extruded cereal grains. Food Chem 48:13–18.
Daniels JI, Layton DW. 1983. Criteria and Recommendations for Standards for Chloride in Military Field Water Supplies. Report UCRL-53481. Livermore, CA: Lawrence Livermore National Laboratory.
Day SM, DeHeer DH. 2001. Reversal of the detrimental effects of chronic protein malnutrition on long bone fracture healing. J Orthop Trauma 15:47– 53.
de Benoist B. 2001. Iron-deficiency anemia: Reexaming the nature and magnitude of the public health problem: Introduction. J Nutr 131:564S.
de Bruin NC, Degenhart HJ, Gal S, Westerterp KR, Stijnen T, Visser HK. 1998. Energy utilization and growth in breast-fed and formula-fed infants
measured prospectively during the first year of life. Am J Clin Nutr 67:885–896.
Decsi T, Molnar D, Koletzko B. 1998. The effect of under- and overnutrition on essential fatty acid metabolism in childhood. Eur J Clin Nutr 52:541–548.
De Onis M, Frongillo EA, Blossner M. 2000. Is malnutrition declining? An analysis of changes in levels of child malnutrition since 1980. Bull World Health Organ 78:1222–1233.
Diez-Ewald M, Torres-Guerra E, Layrisse M, Leets I, Vizcaino G, Arteaga-Vizcaino M. 1997. Prevalence of anemia, folic acid and vitamin B12 deficiency in two Bari Indian communities from western Venezuela. Invest Clin 38:191–201.
Dill DB, Soholt LF, Oddershede IB. 1976. Physiological adjustments of young men to five-hour desert walks. J Appl Physiol 40:236–242.
Donma O, Gunbey S, Tas MA, Donma MM. 1990. Zinc, copper, and magnesium concentrations in hair of children from southeastern Turkey. Biol Trace Elem Res 24:39–47.
Drewnowski A. 1997. Taste preferences and food intake. Annu Rev Nutr 17:237–253.
Drewnowski A, Nordensten K, Dwyer J. 1998. Replacing sugar and fat in cookies: Impact on product quality and preference. Food Qual Pref 9:13–20.
Dudley GA, Duvoison MR, Covertinos VA, Buchanan P. 1989. Alterations of the in vivo torque-velocity relationship of human skeletal muscle following 30 days exposure to simulated microgravity. Avia Space Environ Med 60:659–663.
Dupont J, White PJ, Carpenter MP, Schaefer EJ, Meydani SN, Elson CE, Woods M, Gorbach SL. 1990. Food uses and health effects of corn oil. J Am Coll Nutr 9:438-470.
Dwivedi BK, Arnold RG. 1973. Chemistry of thiamine degradation in food products and model systems: A review. J Agric Food Chem 21:54–60.
Enomoto TM, Isichei C, VanderJagt DJ, Fry DE, Glew RH. 1998. Decreased polyunsaturated fatty acids in sickle cell anaemia. J Trop Pediatr 44:28–34.
FAO/WHO (Food and Agriculture Organization/World Health Organization). 1989. Protein Quality Evaluation. FAO Food and Nutrition Paper 51. Rome: FAO.
FAO/WHO. 1994. Fats and Oils in Human Nutrition. FAO Food and Nutrition Paper 57. Rome: FAO.
FAO/WHO. 1998. Carbohydrates in Human Nutrition. FAO Food and Nutrition Paper 66. Rome: FAO.
FAO/WHO. 2000. Preliminary Report on Recommended Nutrient Intakes. Rome: FAO.
FAO/WHO/UNU (Food and Agriculture Organization/World Health Organization/United Nations University). 1985. Energy and Protein Requirements. Technical Report Series No. 724. Geneva: WHO.
Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. 1993. Vitamin A supplementation and child mortality: A meta-analysis. J Am Med Assoc 269:898–903.
Fechner A, Böhme C, Gromer S, Funk M, Schirmer R, Becker K. 2001. Antioxidant status and nitric oxide in the malnutrition syndrome kwashiorkor. Pediatr Res 49:237–243.
Fenstermacher DK, Rose RC. 1986. Absorption of pantothenic acid in rat and chick intestine. Am J Physiol 250:G155–G160.
Fisher DA, Oddie TH. 1969. Thyroid iodine content and turnover in euthyroid subjects: Validity of estimation of thyroid iodine accumulation from short-term clearance studies. J Clin Endcr 29:721–727.
Fishman SM, Christian P, West KP. 2000. The role of vitamins in the prevention and control of anaemia. Public Health Nutr 3:125–150.
Fitzgerald SL, Gibson RS, Quan de Serrano J, Portocarrero L, Vasquez A, de Zepeda E, Lopez-Palacios CY, Thompson LU, Stephen AM, Solomons NW. 1993. Trace element intakes and dietary phytate/Zn and Ca xphytate/Zn millimolar ratios of periurban Guatemalan women during the third trimester of pregnancy. Am J Clin Nutr 57:195–201.
Fjeld CAR, Schoeller DA, Brown KH. 1989. A new model for predicting energy requirements of children during catch-up growth developed using doubly labeled water. Pediatric Res 25:503–508.
Fordyce FM, Johnson CC, Navaratna UR, Appleton JD, Dissanayake CB. 2000. Selenium and iodine in soil, rice and drinking water in relation to endemic goitre in Sri Lanka. Sci Total Environ 263:127–141.
Friedman BJ, Freeland-Graves JH, Bales CW, Behmardi F, Shorey-Kutschke RL, Willis RA, Crosby JB, Trickett PC, Houston SD. 1987. Manganese balance and clinical observations in young men fed a manganese-deficient diet. J Nutr 117:133–143.
Friedman M, Brandon DL. 2001. Nutritional and health benefits of soy proteins. J Agric Food Chem 49:1069–1086.
Friis H, Ndhlovu P, Mduluza T, Kaondera K, Sandstrom B, Michaelsen KF, Vennervald BJ, Christensen NO. 1997. The impact of zinc supplementation on growth and body composition: A randomized, controlled trial among rural Zimbabwean school children. Eur J Clin Nutr 51:38–45.
Gamble JL. 1947. Physiological information gained from studies on the life raft ration. In: The Harvey Society of New York, eds. The Harvey Lectures. Lancaster, PA: The Sciences Press Printing Co. Pp. 247–273.
Ge K, Yang G. 1993. The epidemiology of selenium deficiency in the etiological study of endemic diseases in China. Am J Clin Nutr 57:259S–263S.
Gibson RS, Ferguson EL. 1998. Assessment of dietary zinc in a population. Am J Clin Nutr 68:430S–434S.
Gibson RS, Donovan UM, Heath A-LM. 1997. Dietary strategies to improve the iron and zinc nutriture of young women following a vegetarian diet. Plant Foods Hum Nutr 51:1–16.
Glasziou PP, Mackerras DE. 1993. Vitamin A supplementation in infectious diseases: A meta analysis. Br Med J 306:366–370.
Golden MH. 1994. Is complete catch-up possible for stunted malnourished children? Eur J Clin Nutr 48:S58–S71.
Golden MH. 1998. Oedematous malnutrition. Br Med Bull 54:433–444.
Golden MH. 2001. The Derivation of the Proposed Nutritional Composition of an Emergency Relief Food for Refugees and Displaced Persons. Washington, DC: U.S. Agency for International Development.
Golden MH, Waterlow JC, Picou D. 1977. Protein turnover, synthesis and breakdown before and after recovery from protein–energy malnutrition. Clin Sci Molecular Med 53:473–477.
Grande F, Taylor HL, Anderson JT, Buskirk E, Keys A. 1958. Water exchange in men on a restricted water intake and a low calorie carbohydrate diet accompanied by physical work. J Appl Physiol 12:202–210.
Grange AO, Santosham M, Ayodele AK, Lesi F, Stallings RY, Brown KH. 1994. Evaluation of a maize-cowpea-palm oil diet for the dietary management of Nigerian children with acute, watery diarrhea. Acta Peadiatr 83:825–832.
Gregory JF. 1998. Nutritional properties and significance of vitamin glycosides. Annu Rev Nutr 18:277–296.
Gregory JF. 2001. Case study: Folate bioavailability. J Nutr 131:1376S–1382S.
Grobler-Tanner C. 2001. A Study of Emergency Relief Foods for Refugees and Displaced Persons. Washington, DC: U.S. Agency for International Development.
Gürson CT, Saner G. 1973. Effects of chromium supplementation on growth in marasmic protein–calorie malnutrition. Am J Clin Nutr 26:988–991.
Hadi H, Stoltzfus RJ, Dibley MJ, Moulton LH, West KP, Kjolhede CL, Sadjimin T. 2000. Vitamin A supplementation selectively improves the linear growth of Indonesian preschool children: Results from a randomized controlled trial. Am J Clin Nutr 71:507–513.
Hallberg L, Brune M, Rossander L. 1989. Iron absorption in man: Ascorbic acid and dose-dependent inhibition by phytate. Am J Clin Nutr 49:140–144.
Haller J, Lowik MR, Ferry M, Ferro-Luzzi A. 1991. Nutritional status: Blood vitamins A, E, B6, B12, folic acid and carotene. Eur J Clin Nutr 45:63–82.
Hambidge M. 2000. Human zinc deficiency. J Nutr 130:1344S–1349S.
Hambidge KM, Krebs NF, Miller L. 1998. Evaluation of zinc metabolism with use of stable-isotope techniques: Implications for the assessment of zinc status. Am J Clin Nutr 68:410S–413S.
Hassanein EA, Tankovsky I. 1973. Disturbances of coagulation mechanism in protein–calorie malnutrition. Trop Geog Med 25:158–162.
Heaney RP, Weaver CM. 1989. Oxalate: Effect on calcium absorbability. Am J Clin Nutr 50:830–832.
Heaney RP, Weaver CM, Recker RR. 1988. Calcium absorbability from spinach. Am J Clin Nutr 47:707–709.
Heaney RP, Recker RR, Weaver CM. 1990. Absorbability of calcium sources: The limited role of solubility. Calcif Tissue Int 46:300–304.
Heaney RP, Weaver CM, Fitzsimmons ML. 1991. Soybean phytate content: Effect on calcium absorption. Am J Clin Nutr 53:745–747.
Hirschhorn N. 1980. The treatment of acute diarrhea in children: An historical and physiological perspective. Am J Clin Nutr 33:637–644.
Holick MF. 1994. McCollum Award Lecture, 1994: Vitamin D—New horizons for the 21st century. Am J Clin Nutr 60:619–630.
Hollowell JG, Staehling NW, Hannon WH, Flanders DW, Gunter ES, Maberly GF, Braverman LE, Pino S, Miller DT, Garbe PL, DeLozier DM, Jackson RJ. 1998. Iodine nutrition in the United States. Trends and public health implications: Iodine excretion data from National Health and Nutrition Examination Surveys I and III. J Clin Endocrinol Metal 83:3401–3408.
Holman RT, Johnson SB, Mercuri O, Itarte HJ, Rodrigo MA, De Tomas ME. 1981. Essential fatty acid deficiency in malnourished children. Am J Clin Nutr 34:1534–1539.
Hopkins LL Jr, Majaj AS. 1967. Improvements of impaired glucose tolerance by chromium(III) in malnourished infants. In: Kuhnau J, ed. Proceedings of the Seventh International Congress of Nutrition. Volume 5: Physiology and Biochemistry of Food Components. London: Pergamon Press. Pp. 721–723.
Hotz C, Brown KH. 2001. Identifying populations at risk of zinc deficiency: The use of supplementation trials. Nutr Rev 59:80–88.
Hubbard RW, Armstrong LE, Evans PK, De Luca JP. 1986. Long-term water and salt deficits—A military perspective. In: Predicting Decrements in Military Performance Due to Inadequate Nutrition. Washington, DC: National Academy Press. Pp. 29–53.
Huddle JM, Gibson RS, Cullinan TR. 1998. Is zinc a limiting nutrient in the diets of rural pregnant Malawian women? Br J Nutr 79:257–65.
Hussein L, Arafah A, Gaafar S. 1989. The vitamin B1 status among young Egyptian males with infection due to parasites. Int J Vitam Nutr Res 59:48– 51.
INACG (International Nutritional Anemia Consultative Group). 1999. Consensus Statemement: Safety of Iron Supplementation Programs in Malaria-Endemic Regions. Washington, DC: International Life Sciences Institute Research Foundation.
Ingenbleek Y, Malvaux P. 1974. Iodine balance studies in protein–calorie malnutrition. Arch Dis Child 49:305–309.
IOM (Institute of Medicine). 1995a. Consideration of the Nutrition Components of The Sick Child Initiative. Washington, DC: National Academy Press.
IOM. 1995b. Estimated Mean per Capita Energy Requirements for Planning Emergency Food Aid Rations. Washington, DC: National Academy Press.
IOM. 1997a. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press.
IOM. 1997b. Vitamin C Fortification of Food Aid Commodities. Washington, DC: National Academy Press.
IOM. 1998. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press.
IOM. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press.
IOM. 2001. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press.
Ivy JL, Costill DL, Fink WJ, Lower RW. 1979. Influence of caffeine and carbohydrate feeding on endurance performance. Med Sci Sports Exerc 11:6–11.
Jackson LS, Lee K. 1991. Microencapsulated iron for food fortification. J Food Sci 56:1047–1050.
Jamison E, Hobbs F. 1994. World Population Profile: 1994. Report WP/94. Washington, DC: U.S. Bureau of the Census.
James WPT, Schofield EC. 1990. Human Energy Requirements. Oxford: Oxford University Press.
Jéquier E. 1999. Response to and range of acceptable fat intake in adults. Eur J Clin Nutr 53:S84–S88.
Johnson HL. 1986. Practical military implications of fluid and nutritional imbalances for performance. In: Predicting Decrements in Military Performance Due to Inadequate Nutrition. Washington, DC: National Academy Press. Pp. 55–67.
Johnson RE. 1964. Water and osmotic economy on survival rations. J Am Diet Assoc 45:124–129.
Jones JP, Meck WH, Williams CL, Wilson WA, Swartzwelder HS. 1999. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res Dev 118:159–167.
Jusko WJ, Levy G. 1975. Absorption, protein binding, and elimination of riboflavin. In: Rivlin RS, ed. Riboflavin. New York: Plenum Press. Pp. 99–152.
Kalra V, Grover JK, Ahuja GK, Rathi S, Gulati S, Kalra N. 2001. Vitamin E administration and reversal of neurological deficits in protein–energy malnutrition. J Trop Pediatr 47:39–45.
Kaptan K, Beyan C, Ural AU, Cetin T, Avcu F, Gulsen M, Finci R, Yalcin A. 2000. Helicobacter pylori—Is it a novel causative agent in vitamin B12 deficiency? Arch Intern Med 160:1349–1353.
Kelsay JL, Behall KM, Prather ES. 1979. Effect of fiber from fruits and vegetables on metabolic responses of human subjects, II. Calcium, magnesium, iron, and silicon balances. Am J Clin Nutr 32:1876–1880.
Kikafuna JK, Walker AF, Allan EF, Tumwine JK. 1998. Effect of zinc supplementation on growth and body composition of Uganda preschool children: A randomized, controlled, intervention trial. Am J Clin Nutr 68:1261–1266.
Kimura M, Itokawa Y, Fujiwara M. 1990. Cooking losses of thiamin in food and its nutritional significance. J Nutr Sci Vitaminol (Tokyo) 36:S17–S24.
King JE, Mazariegos M, Valdez C, Castaneda C, Solomons NW. 1997. Nutritional status indicators and their interpretations in rural Guatemalan elderly: A study in San Pedro Ayampuc. Am J Clin Nutr 66:795–802.
Kirksey A, Wachs TD, Yunis F, Srinath U, Rahmanifar A, McCabe GP, Galal OM, Harrison GG, Jerome NW. 1994. Relation of maternal zinc nutriture to pregnancy outcome and infant development in an Egyptian village. Am J Clin Nutr 60:782–792.
Kivisto B, Andersson H, Cederblad G, Sandberg AS, Sandstrom B. 1986. Extrusion cooking of a high-fibre cereal product. 2. Effects on apparent absorption of zinc, iron, calcium, magnesium and phosphorus in humans. Br J Nutr 55:255–260.
Koletzko B. 1999. Response to and range of acceptable fat intakes in infants and children. Eur J Clin Nutr 53:S78–S83.
Koletzko B, Abiodun PO, Laryea MD, Bremer HJ. 1986. Fatty acid composition of plasma lipids in Nigerian children with protein–energy malnutrition. Eur J Pediatr 145:109–115.
Koletzko B, Thiel I, Abiodun PO. 1992. The fatty acid composition of human milk in Europe and Africa. J Pediatr 120:S62–S70
Kumar R, Marwaha N, Marwaha RK, Garewal G. 2001. Vitamin K deficiency in diarrhoea. Indian J Pediatr 68:235–238.
Kvicala J, Zamrazil V, Jiranek V. 1999. Characterization of selenium status of inhabitants in the region Usti nad Orlici, Czech Republic by INAA of blood serum and hair and fluorimetric analysis of urine. Biol Trace Elem Res 71:31–39.
Ladipo OA. 2000. Nutrition in pregnancy: Mineral and vitamin supplements. Am J Clin Nutr 72:280S–290S.
Laryea MD, Leichsenring M, Mrotzek M, el-Amin EO, el Kharib AO, Ahmed HM, Bremer HJ. 1995. Fatty acid composition of the milk of well-nourished Sudanese women. Int J Food Sci Nutr 46:205–214.
Layrisse M, Garcia-Casal MN, Solano L, Baron MA, Arguello F, Llovera D, Ramirez J, Leets I, Tropper E. 2000. New property of vitamin A and beta-
carotene on human iron absorption: Effect on phytate and polyphenols as inhibitors of iron absorption. Arch Latinoam Nutr 50:243–248.
Leichsenring M, Sutterlin N, Less S, Baumann K, Anninos A, Becker K. 1995. Polyunsaturated fatty acids in erythrocyte and plasma lipids of children with severe protein–energy malnutrition. Acta Paediatr 84:516–520.
Leithead CS, Lind AR. 1964. Heat Stress and Heat Disorders. Philadelphia: Davis. P. 304.
Le Kim D, Betzing H. 1976. Intestinal absorption of polyunsaturated phosphatidylcholine in the rat. Hoppe Seylers Z Physiol Chem 357:1321–1331.
Leklem JE. 1996. Vitamin B-6. In: Ziegler EE, Filer LJ, eds. Present Knowledge in Nutrition. 7th ed. Washington, DC: ILSI Press. Pp. 174–183.
Levander OA, Beck MA. 1999. Selenium and viral virulence. Br Med Bull 55:528–533.
Longnecker MP, Taylor PR, Levander OA, Howe SM, Veillon C, McAdam PA, Patterson KY, Holden JM, Stampfer MJ, Morris JS, Willett WC. 1991. Selenium in diet, blood, and toenails in relation to human health in a seleniferous area. Am J Clin Nutr 53:1288–1294.
Lopez-Pedrosa JM, Torres MI, Fernandez MI, Rios A, Gil A. 1998. Severe malnutrition alters lipid composition and fatty acid profile of small intestine in newborn piglets. J Nutr 128:224–233.
Louwman MWJ, van Dusseldorp M, van de Vijver F, Thomas CMG, Schneede J, Ueland PM, Refsum H, van Staveren WA. 2000. Signs of impaired cognitive function in adolescents with marginal cobalamin status. Am J Clin Nutr 72:762–769.
Lynch SR. 1997. Interaction of iron with other nutrients. Nutr Rev 55:102–110.
Maksimović ZJ, Djujić I. 1997. Selenium deficiency in Serbia and possible effects on health. Biomed Environ Sci 10:300–306.
Malfait P, Moren A, Dillon JC, Brodel A, Begkoyian G, Etchegorry MG, Malenga G, Hakewill P. 1993. An outbreak of pellagra related to changes in dietary niacin among Mozambique refugees in Malawi. Int J Epidemiol 22:504–511.
Manore MM. 2000. Effect of physical activity on thiamin, riboflavin, and vitamin B-6 requirements. Am J Clin Nutr 72:598S–606S.
Marin MC, De Tomas ME, Mercuri O, Fernandez A, de Serres CT. 1991. Interrelationship between protein–energy malnutrition and essential fatty acid deficiency in nursing infants. Am J Clin Nutr 53:466–468
Marin MC, De Tomas ME, Serres C, Mercuri O. 1995. Protein–energy malnutrition during gestation and lactation in rats affects growth rate, brain development and essential fatty acid metabolism. J Nutr 125:1017–1024.
Marriott HL. 1950. Water and Salt Depletion. Springfield, IL: Thomas.
Martorell R, Habicht JP. 1986. Growth in early childhood in developing countries. In: Falkner F, Tanner JM, eds. Human Growth: A Comprehensive
Treatise. Volume 3: Methodology Ecological, Genetic, and Nutritional Effects on Growth. New York: Plenum Press. Pp. 241–262.
McCormick DB. 1990. Riboflavin. In: Brown ML, Filer LJ, Guthrie HA, Levander OA, McCormick DB, Olson RE, Steele RD, eds. Present Knowledge in Nutrition. 6th ed. Washington, DC: ILSI Press. Pp 146–154.
McCormick DB. 1994. Riboflavin. In: Shils ME, Olson JA, Shike M, eds. Modern Nutrition in Health and Disease. 8th ed. Baltimore: Lea & Febiger. Pp. 366–375.
McCullough AL, Kirksey A, Wachs TD, McCabe GP, Bassily NS, Bishry Z, Galal OM, Harrison GG, Jerome NW. 1990. Vitamin B6 status of Egyptian mothers: Relation to infant behavior and maternal–infant interactions. Am J Clin Nutr 51:1067–1074.
Melse-Boonstra A, Pee S, Halati ME, Kosen SM, Muhilal S, Bloem M. 2000. The potential of various foods to serve as a carrier for micronutrient fortification, data from remote areas in Indonesia. Eur J Clin Nutr 54:822– 827.
Mock DM. 1990. Evidence for a pathogenic role of omega 6 polyunsaturated fatty acid in the cutaneous manifestations of biotin deficiency. J Pediatr Gastroenterol Nutr 10:222–229.
Mock DM, Stadler DD, Statton SL, Mock NI. 1997. Biotin status assessed longitudinally in pregnant women. J Nutr 127:710–716.
Moulopoulos DS, Mantzox JM, Souvatzoglou A, Piperingos GD, Karaiskos KS, Makriyannis D, Stontouris J, Moulopoulos SD. 1988. The relation of serum T4 and TSH with the urinary iodine excretion. J Endoc Invest 11:437–439.
Mwanri L, Worsley A, Ryan P, Masika J. 2000. Supplemental vitamin A improves anemia and growth in anemic school children in Tanzania. J Nutr 130:2691–2696.
Nalubola R, Nestel P. 1999. The Effect of Vitamin A Nutriture on Health: A Review. Washington, DC: ILSI Press. P. 10.
Neumann CG, Harrison GG. 1994. Onset and evolution of stunting in infants and children. Examples from the Human Nutrition Collaborative Research Support Program. Kenya and Egypt studies. Eur J Clin Nutr 48:S90–S102.
Neumann C, McDonald MA, Sigman M, Bwibo N, Marquardt M. 1991. Relationships between morbidity and development in mildly to moderately malnourished Kenyan toddlers. Pediatrics 88:934–942.
Ngo DB, Dikassa L, Okitolonda W, Kashala TD, Gervy C, Dumont J, Vanovervelt N, Contempre B, Diplock AT, Peach S, Vanderpas J. 1997. Selenium status in pregnant women of a rural population (Zaire) in relation to iodine deficiency. Trop Med Int Health 2:572–581.
NRC (National Research Council). 1987. Vitamin A Supplementation. Methodologies for Field Trials. Washington, DC: National Academy Press.
NRC. 1989. Recommended Dietary Allowances. 10th ed. Washington, DC: National Academy Press.
Oberleas D, Harland BF. 1981. Phytate content of foods: Effect on dietary zinc bioavailability. J Am Diet Assoc 79:433–436.
Okolo SN, VanderJagt TJ, Vu T, VanderJagt TA, VanderJagt DJ, Okonji M, Huang YS, Chuang LT, Onwuanaku C, Glew RH. 2000. The fatty acid composition of human milk in northern Nigeria. J Hum Lact 16:28–35.
Olsen RE. 1994. Vitamin K. In: Shils ME, Olson JA, Shike M, eds. Modern Nutrition in Health and Disease. 8th ed. Baltimore: Lea & Febiger. Pp. 342–358.
Oppenheimer SJ. 2001. Iron and its relation to immunity and infectious disease. J Nutr 131:616S–635S.
Penland JG. 2000. Behavioral data and methodology issues in studies of zinc nutrition in humans. J Nutr 130:361S–364S.
Pennington JA. 1990. A review of iodine toxicity reports. J Am Diet Assoc 90:1571–1581.
Penny ME, Brown KH. 1992. Lactose feeding during persistent diarrhoea. Acta Paediatr 381:133–138.
Plesofsky-Vig N. 1999. Pantothenic acid. In: Shils ME, Olson JA, Shike M, Ross CA, eds. Modern Nutrition in Health and Disease. 9th ed. Baltimore: Williams & Wilkins. Pp. 423–432.
Pongpaew P, Saowakontha S, Schelp FP, Rojsathaporn K, Phonrat B, Vudhivai N, Supawan V, Intarakhao C, Mahaweeravat U, Lumbiganon P, Sanchaisurya P, Migasena P. 1995. Vitamins B1, B2 and B6 during the course of pregnancy of rural and urban women in northeast Thailand. Int J Vitam Nutr Res 65:111–116.
Prentice AM, Goldberg GR. 2000. Energy adaptations in human pregnancy: Limits and long-term consequences. Am J Clin Nutr 71:1226S–1232S.
Prentice AM, Paul AA. 2000. Fat and energy needs of children in developing countries. Am J Clin Nutr 72:1253S–1265S.
Prentice AM, Lucas A, Vasquez-Velasquez L, Davies PS, Whitehead RG. 1988. Are current dietary guidelines for young children a prescription for overfeeding? Lancet 2:1066–1069.
Prentice AM, Spaaij CJ, Goldberg GR, Poppitt SD, van Raaij JM, Totton M, Swann D, Black AE. 1996. Energy requirements of pregnant and lactating women. Eur J Clin Nutr 50:S82–S110.
Reddy VA, Bates CJ, Goh SG, Rowland MG, Greenwood AM, Greenwood B, Paul AA. 1987. Riboflavin, folate and vitamin C status of Gambian women during pregnancy: A comparison between urban and rural communities. Tran R Soc Trop Med Hyg 81:1033–1037.
Reddy MB, Hurrell RF, Cook JD. 2000. Estimation of nonheme-iron bioavailability from meal composition. Am J Clin Nutr 71:937–943.
Reilly C. 1996. Selenium in Food and Health. London: Blackie Academic and Professional. Pp. 110–117.
Rice AL, Sacco L, Hyder A, Black RE. 2000. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull World Health Organ 78:1207–1221.
Riley SA, Marsh MN. 1998. Maldigestion and malabsorption. In: Feldman M, Sleisenger MH, Scharschmidt BF, eds. Sleisenger & Fordtran’s Gastrointestinal Disease: Pathophysiology, Diagnosis, Management. 6th ed. Philadelphia: Saunders. Pp. 1053–1054.
Rocquelin G, Tapsoba S, Dop MC, Mbemba F, Traissac P, Martin-Prevel Y. 1998. Lipid content and essential fatty acid (EFA) composition of mature Congolese breast milk are influenced by mothers’ nutritional status: Impact on infants’ EFA supply. Eur J Clin Nutr 52:164–171.
Rolls BJ. 2000. The role of energy density in the overconsumption of fat. J Nutr 130:268S–271S.
Rolls BJ, Bell EA. 1999. Intake of fat and carbohydrate: Role of energy density. Eur J Clin Nutr 53:S166–S173.
Ronnenberg AG, Goldman MB, Aitken IW, Xu X. 2000. Anemia and deficiencies of folate and vitamin B-6 are common and vary with season in Chinese women of childbearing age . J Nutr 130:2703–2710.
Rothman KJ, Moore LL, Singer MR, Nguygen UDT, Mannino S, Milunsky B. 1995. Teratogenicity of high vitamin A intake. N Engl J Med 333:1369– 1373.
Said HM. 1999. Biotin bioavailability and estimated average requirement: Why bother? Am J Clin Nutr 69:352–353.
Saltzman E, Dallal GE, Roberts SB. 1997. Effect of high-fat and low-fat diets on voluntary energy intake and substrate oxidation: Studies in identical twins consuming diets matched for energy density, fiber and palatability. Am J Clin Nutr 66:1332–1339.
Sanchez-Griñan MI, Peerson JM, Brown KH. 1992. Effect of dietary energy density on total ad-libitum energy consumption by recovering malnourished children. Eur J Clin Nutr 46:197–204.
Santosham M, Brown KH, Sack RB. 1987. Oral rehydration therapy and dietary therapy for acute childhood diarrhea. Pediatr Rev 8:273–278.
Savage D, Gangaidzo I, Lindebaum J, Kiire C, Kukiibi JM, Moyo A, Gwanzura C, Mudenge B, Bennie A, Sitima J. 1994. Vitamin B12 deficiency is the primary cause of megaloblastic anaemia in Zimbabwe. Br J Haematol 86:844–850.
Sazawal S, Bentley M, Black RE, Dhingra P, George S, Bhan MK. 1996. Effect of zinc supplementation on observed activity in low socioeconomic Indian preschool children. Pediatrics 98:1132–1137.
Schmeits BL, Okolo SN, VanderJagt DJ, Huang YS, Chuang LT, Mata JR, Tsin AA, Glew RH. 1999. Content of lipid nutrients in the milk of Fulani women. J Hum Lact 15:113–120.
Scrimshaw NS, Murray EB. 1988. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am J Clin Nutr 48:1083–1159.
Scrimshaw NS, SanGiovanni JP. 1997. Synergism of nutrition, infection, and immunity: An overview. Am J Clin Nutr 66:464S–477S.
Setiawan B, Giraud DW, Driskell JA. 2000. Vitamin B6 inadequacy is prevalent in rural and urban Indonesian children. J Nutr 130:553–558.
Shahar S, Earland J, Powers HJ, Rahman SA. 1999. Nutritional status of rural elderly Malays: Dietary and biochemical findings. Int J Vitam Nutr Res 69:277–284.
Shepard TH, Pyne GE, Kirschvink JF, McLean CM. 1960. Soybean goiter, report of three cases. N Eng J Med 262:1099–1103.
Sherman WM. 1983. Carbohydrates, muscle glycogen, and muscle glycogen supercompensation. In: Williams MH, ed. Ergogenic Aids in Sport. Champaign, IL: Human Kinetics. Pp. 3–26.
Shirreffs SM, Taylor AJ, Leiper JB, Maughan RJ. 1996. Post-exercise rehydration in man: Effects of volume consumed and drink sodium content. Med Sci Sports Exerc 28:1260–1271.
Siegenberg D, Baynes RD, Bothwell TH, Macfarlane BJ, Lamparelli RD, Car NG, MacPhail P, Schmidt U, Tal A, Mayet F. 1991. Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am J Clin Nutr 53:537–541.
Sive AA, Subotzky EF, Malan H, Dempster WS, Heese HD. 1993. Red blood cell antioxidant enzyme concentrations in kwashiorkor and marasmus. Ann Trop Paediatr 13:33–38.
Smit EN, Dijkstra JM, Schnater TA, Seerat E, Muskiet FA, Boersma ER. 1997. Effects of malnutrition on the erythrocyte fatty acid composition and plasma vitamin E levels of Pakistani children. Acta Paediatr 86:690–695.
Smith IF, Taiwo O, Golden MHN. 1989. Plant protein rehabilitation diets and iron supplementation of the protein–energy malnourished child. Eur J Clin Nutr 43:763–768.
Sphere Project. 2001. The Humanitarian Charter and Minimum Standards in Disaster Response. Herndon, VA: Stylus Publishing LLC.
Squali Houssaïni FZ, Iraqi MR, Arnaud J, Richard MJ, Favier A. 1997. Trace elements and protein–calorie malnutrition in the Fes area (Morocco). Biomed Pharmacother 51:349–351.
Stammati A, Zanetti C, Pizzoferrato L, Quattrucci E, Tranquilli GB. 1992. In vitro model for the evaluation of toxicity and antinutritional effects of sulphites. Food Addit Contam 9:551–560.
Stein TP. 1995. Protein metabolism. In: Torosian MH, ed. Hospital Based Nutrition: Science and Principles of Practice. New York: Pergamon Press. Pp. 73–87.
Stephenson LS, Latham MC, Ottesen EA. 2000. Global malnutrition. Parasitol 121:S5–S22.
Stoltzfus RJ. 2001. Iron-deficiency anemia: Reexaming the nature and magnitude of the public health problem. Summary: Implications for research and programs. J Nutr 131:697S–701S.
Stopeck A. 2000. Links between Helicobacter pylori infection, cobalamin deficiency, and pernicious anemia. Arch Intern Med 160:1229–1230.
Stubbs RJ, Habron CG, Prentice AM. 1996. Covert manipulation of the dietary fat to carbohydrate ratio of isoenergetically dense diets: Effect on food intake in feeding men ad libitum. Int J Obes 20:651–660.
Suarez FL, Savaiano DA, Levitt MD. 1995. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med 333:1–4.
Suter PM, Golner BB, Goldin BR, Morrow FD, Russell RM. 1991. Reversal of protein-bound vitamin B12 malabsorption with antibiotics in atrophic gastritis. Gastroenterol 101:1039–1045.
Swendseid ME, Jacob RA. 1994. Niacin. In: Shils ME, Olson JA, Shike M, eds. Modern Nutrition in Health and Disease. 8th ed. Baltimore: Lea & Febiger. Pp. 376–382.
Tagle MA. 1988. Changes in the patterns of food consumption in Latin America. Arch Latinoam Nutr 38:750–765.
Tanphaichitr V. 1994. Thiamin. In: Shils ME, Olson JA, Shike M, eds. Modern Nutrition in Health and Disease. 8th ed. Baltimore: Lea & Febiger. Pp. 351–365.
Tatli MM, Vural H, Koc A, Kosecik M, Atas A. 2000. Altered anti-oxidant status and increased lipid peroxidation in marasmic children. Pediatr Int 42:289–292.
Thomson CD, Robinson MF. 1996. The changing selenium status of New Zealand residents. Eur J Clin Nutr 50:107–114.
Tomkins A. 2000. Malnutrition, morbidity and mortality in children and their mothers. Proc Nutr Soc 59:135–146.
Torun B, Davies PS, Livingstone MB, Paolisso M, Sackett R, Spurr GB. 1996. Energy requirements and dietary energy recommendations for children and adolescents 1 to 18 years old. Eur J Clin Nutr 50:S37–S80.
Truswell AS, Hansen JD, Konno T. 1972. Thiamin deficiency in children with severe gastro-enteritis. S Afr Med J 46:2083–2084.
Uauy R, Mena P, Valenzuela A. 1999. Essential fatty acids as determinants of lipid requirements in infants, children and adults. Eur J Clin Nutr 53:S66–S77.
Underwood BA, Arthur P. 1996. The contribution of vitamin A to public health. FASEB J 10:1040–1048.
UNHCR (United Nations High Commissioner for Refugees). 2000. Handbook for Emergencies. 2nd ed. Geneva: UNHCR. Pp. 220–227.
UNICEF (United Nations Children’s Fund). 2000. Ending Iodine Deficiency Forever. New York: UNICEF.
UN Subcommittee on Nutrition. 2001. Report on the Nutrition Situation of Refugees and Displaced Populations. RNIS 32 & 33. Geneva: UN.
USDA (U.S. Department of Agriculture). 1994. Provisional Table on the Vitamin K Content of Foods. HNIS/PT-104. Washington, DC: U.S. Government Printing Office.
Valk EE, Hornstra G. 2000. Relationship between vitamin E requirement and polyunsaturated fatty acid intake in man: A review. Int J Vitam Nutr Res 70:31–42.
van den Broek NR, Letsky EA. 2000. Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr 72:247S–256S.
VanderJagt DJ, Arndt CD, Okolo SN, Huang YS, Chuang LT, Glew RH. 2000. Fatty acid composition of the milk lipids of Fulani women and the serum phospholipids of their exclusively breast-fed infants. Early Hum Dev 60:73–87.
Vanderpas JB, Contempre B, Duale NL, Goossens W, Bebe N, Thorpe R, Ntambue K, Dumont J, Thilly CH, Diplock AT. 1990. Iodine and selenium deficiency associated with cretinism in northern Zaire. Am J Clin Nutr 52:1087–1093.
van Dusseldorp M, Schneede J, Ueland PM, Thomas CMG, de Boer E, van Staveren WA. 1999. Risk of persistent cobalamin deficiency in adolescents fed a macrobiotic diet in early life . Am J Clin Nutr 69:664–667.
van Stratum P, Lussenberg RN, van Wezel LA, Vergroesen AJ, Cremer HD. 1978. The effect of dietary carbohydrate:fat ratio on energy intake by adult women. Am J Clin Nutr 31:206–212.
Varo P, Alfthan G, Huttunen JK, Aro A. 1994. Nationwide selenium supplementation in Finland—Effects on diet, blood and tissue levels, and health. In: Burk RF, ed. Selenium in Biology and Human Health. New York: Springer-Verlag. Pp. 199–218.
Velázquez A. 1997. Biotin deficiency in protein–energy malnutrition: Implications for nutritional homeostasis and individuality. Nutrition 13:991–992.
Velázquez A, Tercin M, Baez A, Gutierrez J, Rodriquez R. 1995. Biotin supplementation affects lymphocyte carboxylases and plasma biotin in severe protein–energy malnutrition. Am J Clin Nutr 61:385–391.
Venkatesh Mannar MG, Dunn JT. 1995. Choice and dose of iodine compound for salt iodization. In: Salt Iodization for the Elimination of Iodine Deficiency. The Netherlands: International Council for Control of Iodine Deficiency Disorders.
Villamor E, Fawzi WW. 2000. Vitamin A supplementation: Implications for morbidity and mortality in children. J Infect Dis 182:S122–S133.
Wacker J, Fruhauf J, Schulz M, Chiwora FM, Volz J, Becker K. 2000. Riboflavin deficiency and preeclampsia. Obstet Gynecol 96:38–44.
Weaver CM, Heaney RP, Teegarden D, Hinders SM. 1996. Wheat bran abolishes the inverse relationship between calcium load size and absorption fraction in women. J Nutr 126:303–307.
West KP, Djunaedi E, Pandji A, Kusdiono, Tarwotjo I, Sommer A. 1988. Vitamin A supplementation and growth: A randomized community trial. Am J Clin Nutr 48:1257–1264.
WHO (World Health Organization). 1996a. Recommended Iodine Levels in Salt and Guidelines for Monitoring Their Adequacy and Effectiveness. WHO/Nut/96.13. Geneva: WHO.
WHO. 1996b. Trace Elements in Human Health and Nutrition. Geneva: WHO. Pp. 72–104.
WHO. 1999a. Guidelines for Epidemic Preparedness and Response to Measles Outbreaks. WHO/CDC/CSR/ISR/99.1 Geneva: WHO.
WHO. 1999b. World Health Organization Sets out to Eliminate Iodine Deficiency Disorder. Press Release WHA/17. WHO, Geneva. May 25.
WHO. 2000. Pellagra and Its Prevention and Control in Major Emergencies. WHO/NHD/00.10. Geneva: WHO.
WHO. 2001. Combating Vitamin A Deficiency. Online. Available at http://www.who.int/nut/vad.htm. Accessed January 31, 2002.
Wyss M, Brugger R, Kronenberger A, Rémy R, Fimbel R, Oesterhelt G, Lehmann M, van Loon APGM. 1999. Biochemical characterization of fungal phytases (myo-inositol hexakisphosphat phosphohydrolases): Catalytic properties. Appl Environ Microbiol 65:367–373.
Xia YM, Hill KE, Burk RF. 1989. Biochemical studies of a selenium-deficient population in China: Measurement of selenium, glutathione peroxidase, and other oxidant defense indices in blood. J Nutr 199:1318–1326.
Xiang M, Lei S, Li T, Zetterstrom R. 1999. Composition of long chain polyunsaturated fatty acids in human milk and growth of young infants in rural areas of northern China. Acta Paediatr 88:126–131.
Yang G-Q, Yin S, Zhou R-H, Gu L, Yan B, Liu Y, Liu Y. 1989. Studies of safe maximal daily dietary Se-intake in a seleniferous area of China. II. Relation between Se-intake and the manifestation of clinical signs and certain biochemical alterations in blood and urine. J Trace Elem Electrolytes Health Dis 3:123–130.
Young H, Jaspars S. 1995. Nutrition, disease and death in times of famine. Disasters 19:94–109.
Young H, Fellow P, Mitchell J. 1985. Development of a high energy biscuit for use as a food supplement in disaster relief. J Food Technol 20:689–695.
Young H, Owen M, Clark J. 1988. A comparison of biscuits used in emergency relief feeding programmes in Ethiopia and eastern Sudan—1985/86. Eur J Clin Nutr 42:261–271.
Zeisel SH. 2000. Choline: An essential nutrient for humans. Nutrition 16:669– 671.
Zempleni J, Mock DM. 1999. Advanced analyses of biotin metabolites in body fluids allows a more accurate measurement of biotin bioavailability and metabolism in humans. J Nutr 129:494S–497S.
Zhou JR, Erdman JW Jr. 1995. Phytic acid in health and disease. Crit Rev Food Sci Nutr 35:495–508.
Zinc Investigators’ Collaborative Group. 2000. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: Pooled analysis of randomized controlled trials. Am J Clin Nutr 72:1516–1522.
Zubaran C, Fernandes JG, Rodnight R. 1997. Wernicke-Korsakoff syndrome. Postgrad Med J 73:27–31.
















































































