A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites
Oswald Steward*† and Paul F.Worley‡
*Reeve-Irvine Research Center, and Departments of Anatomy/Neurobiology and Neurobiology and Behavior, College of Medicine, University of California, Irvine, CA 92697; and ‡Department of Neuroscience, The Johns Hopkins University School of Medicine, Baltimore, MD, 21205
Long-lasting forms of activity-dependent synaptic plasticity involve molecular modifications that require gene expression. Here, we describe a cellular mechanism that mediates the targeting newly synthesized gene transcripts to individual synapses where they are locally translated. The features of this mechanism have been revealed through studies of the intracellular transport and synaptic targeting of the mRNA for a recently identified immediate early gene called activity-regulated cytoskeleton-associated protein Arc. Arc is strongly induced by patterns of synaptic activity that also induce long-term potentiation, and Arc mRNA is then rapidly delivered into dendrites after episodes of neuronal activation. The newly synthesized Arc mRNA localizes selectively at synapses that recently have been activated, and the encoded protein is assembled into the synaptic junctional complex. The dynamics of trafficking of Arc mRNA reveal key features of the mechanism through which synaptic activity can both induce gene expression and target particular mRNA transcripts to the active synapses.
Information storage in the nervous system is thought to involve changes in synaptic potency that occur in response to particular patterns of activity. One candidate process is long-term potentiation (LTP), and the key features that make it an attractive candidate mechanism include: (i) LTP is long-lasting, enduring for hours and sometimes much longer, (ii) LTP is expressed selectively at synapses that have experienced particular patterns of activity (synapse specificity). (iii) LTP requires presynaptic activity in conjunction with a sufficient level of postsynaptic depolarization (the Hebb postulate), (iv) LTP can be induced by patterns of activity that central nervous system neurons actually exhibit, (v) The late stages of LTP, like the consolidation phase of memory, occur over a period of hours after the inducing event and require protein synthesis and perhaps the transcription of new gene products (for recent reviews, see refs. 1–4).
Although it is the late, protein synthesis-dependent phase of LTP that is of particular interest as a candidate mechanism of information storage, relatively little is known about the actual cellular and molecular mechanisms that bring this enduring change about. There are three general possibilities:
-
Plasticity could involve changes in the state of the existing molecules of the synapse (changes in phosphorylation state, or other posttranslational modifications). These sorts of changes are likely to account for the initial change in synaptic strength, but it is more difficult to explain how these sorts of changes could endure beyond a few hours.
-
Plasticity could involve changes in the molecular composition of existing synapses. For example, there is increasing evidence that a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors are removed from the synapse during the induction of long-term depression and inserted during the induction of LTP. There also may be changes in other synaptic constituents.
-
Plasticity could involve a structural change in synapses (increases or decreases in synapse size, formation of new synapses, or elimination of existing ones).
If enduring synaptic modifications require the selective delivery of new molecular constituents to the synapses that are to be modified, this could be accomplished in three ways: (i) The key proteins critical for modification could be synthesized in the cell body and delivered selectively to the synapses that are to be modified through some selective transport process, (ii) The key proteins could be synthesized in the cell body and be widely distributed, with synapse specificity being conferred by a selective capture mechanism (see for example ref. 5). (iii) The key proteins could be synthesized on-site as the result of translation of mRNAs that are localized at synapses. These possibilities are not mutually exclusive, and different molecules could be targeted to synapses in different ways.
The present review documents that neurons do possess a mechanism through which newly synthesized mRNA transcripts are targeted to active synapses where they mediate the local synthesis of proteins that become part of the synapse. This mechanism has been revealed through studies of the intracellular transport and synaptic targeting of the mRNA for a unique immediate early gene (IEG) called Arc (for activity-regulated cytoskeleton-associated protein). Arc, also known as Arg 3.1, is noteworthy because it is induced by neuronal activity like other IEGs, but the newly synthesized mRNA is rapidly delivered throughout dendrites. Moreover, intense synaptic activity causes the mRNA to localize selectively at synapses that had been activated. The induction of gene expression, delivery of the mRNA to dendrites, and synthesis of the protein occur during the first few hours after the inducing event—approximately the same time period in which protein synthesis-dependent synaptic modifications are occurring. Importantly, Arc protein is assembled into the matrix of the synaptic junctional complex (SJC), demonstrating that the mechanism can operate for protein constituents of the synapse. In what follows, we will summarize the key features of this mechanism and also propose a unifying hypothesis that may explain why certain synaptic proteins are locally synthesized.
Induction and Dendritic Targeting of Arc mRNA after Intense Neuronal Activity
Arc was initially discovered in screens for novel IEGs that are induced by neuronal activity in a protein synthesis-independent
|
† |
To whom reprint requests should be addressed at: Reeve-Irvine Research Center, 1105 Gillespie Neuroscience Building, College of Medicine, University of California, Irvine, CA 92697 E-mail: osteward@uci.edu. |
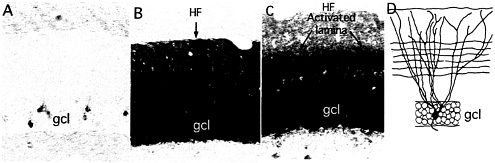
Fig. 1. Newly synthesized Arc mRNA is selectively targeted to dendritic domains that have been synaptically activated. The photomicrographs illustrate the distribution of Arc mRNA as revealed by nonisotopic in situ hybridization in nonactivated dentate gyrus (A), 2 h after a single electroconvulsive seizure (B), and after delivering high-frequency trains to the medial perforant path over a 2-h period (C). Note the uniform distribution of Arc mRNA across the dendritic laminae after an ECS and the prominent band of labeling in the middle molecular layer after high-frequency stimulation of the perforant path. (D) Schematic illustration of the dendrites of a typical dentate granule cell and the pattern of termination of medial perforant path projections. HF, hippocampal fissure; GCL, granule cell layer. (A and B) [Reproduced with permission from ref. 28 (Copyright 2001, Elsevier Science)]. (D) [Reproduced with permission from ref. 9 (Copyright 1998, Elsevier Science)].
fashion (6, 7). Arc was unique because in contrast to the mRNAs of other IEGs, Arc mRNA rapidly migrates throughout the dendritic arbor of the neuron in which it is induced. This was discovered through in situ hybridization analyses of the distribution of Arc mRNA in the dentate gyrus after a single electroconvulsive shock (ECS). For example, by 2 h after a single ECS, newly synthesized Arc mRNA is distributed throughout the molecular layer of the dentate gyrus, which contains the dendrites of dentate granule cells (Fig. 1) whereas the mRNAs for other IEGs remain tightly localized to the region of the cell body.
Because Arc is expressed as an IEG, the synthesis, intracellular trafficking, localization, and life history of Arc mRNA can be studied in a way that is not possible with mRNAs that are expressed constitutively. Evaluations of Arc mRNA distribution at different times after an ECS indicate that the mRNA reaches the most distal tips of the granule cell dendrites within 1 h after the inducing stimulus. The distance from the granule cell body layer to the distal tips of the dendrites is about 300 µm. Thus, Arc mRNA moves into dendrites at a rate of at least 300 µm per h (8).
The evaluation of Arc expression at various times after ECS also revealed that Arc mRNA was present in dendrites only transiently. Peak levels of Arc mRNA were seen 1–2 h after a single ECS; thereafter, the levels of Arc mRNA declined, returning to near control levels after about 6 h (8). Interestingly, this is approximately the same time interval during which synaptic modifications are sensitive to inhibition of protein synthesis.
Newly Synthesized Arc mRNA Is Selectively Targeted to Synapses that Have Recently Been Activated
Subsequent studies of Arc revealed another remarkable feature—that newly synthesized Arc mRNA is selectively targeted to synapses that have been strongly activated (9). This was discovered initially in studies of Arc mRNA distribution after high-frequency stimulation of the entorhinal cortical projections to the dentate gyrus using a paradigm typically used to induce LTP.
The projection from the entorhinal cortex to the dentate gyrus (the perforant path) terminates in a topographically organized fashion along the dendrites of dentate granule cells. Projections from the medial entorhinal cortex terminate selectively in the middle molecular layer of the dentate gyrus, whereas projections from the lateral entorhinal cortex terminate in the outer molecular layer. By positioning a stimulating electrode in different parts of the entorhinal cortex, it is possible to selectively activate a band of synapses that terminate on particular proximo-distal segments. High-frequency activation of the projections to middle dendritic domains (400-hz trains, eight pulses per train, delivered at a rate of 1/10 sec) strongly induces Arc expression. If high-frequency stimulation is continued as the newly synthesized mRNA migrates into dendrites, the mRNA localizes selectively in the middle molecular layer in exactly the location of the band of synapses that had been activated. This selective localization is evidenced by a prominent band of labeling for Arc mRNA in the middle molecular layer of the dentate gyrus (Fig. 1).
When the medial perforant path is activated, the levels of labeling remain quite low in the outer molecular layer, indicating that newly synthesized Arc mRNA never migrates into the distal dendrites. This finding is in contrast to the situation after an ECS, where there are high levels of labeling through-out the molecular layer (compare Fig. 1 B and C), which suggests that as the mRNA enters the dendrites, it is somehow captured in the activated dendritic segments. An analysis of the distribution of Arc mRNA after various periods of stimulation (Fig. 2) further supports this idea. After 30 min of stimulation, Arc mRNA is still confined to the cell body layer. With continued stimulation, levels of labeling in the activated dendritic lamina increase progressively, whereas there is minimal, if any, increase in labeling in the nonactivated distal dendritic segments (the outer molecular layer). Thus, newly synthesized Arc mRNA appears to be captured by active synapses, preventing the further migration of the mRNA into more distal segments.
Arc mRNA Is Targeted to Different Dendritic Domains Depending on the Population of Synapses that Are Activated
The intradendritic distribution of newly synthesized Arc mRNA is determined by the populations of synapses that are activated. For example, high-frequency stimulation of the lateral entorhinal cortex, which innervates distal dendritic segments, produces a band of labeling for Arc mRNA in the outer molecular layer. When the projections to proximal dendritic laminae are strongly activated, newly synthesized Arc mRNA localizes precisely in a band corresponding to the zone of activation. The synapses that terminate in this proximal dendritic lamina originate from large neurons in the hilus of the dentate gyrus. These project bilat-
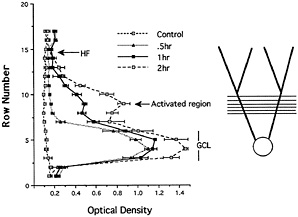
Fig. 2. Analysis of the distribution of Arc mRNA after various periods of synaptic stimulation. The graph illustrates the distribution of Arc mRNA in the molecular layer of the dentate gyrus after various periods of stimulation of the medial perforant path. After 30 min of stimulation, Arc mRNA is still confined to the cell body layer. With continued stimulation, levels of labeling increase progressively in the activated dendritic lamina, whereas there is minimal if any increase in labeling in the nonactivated distal dendritic segments (the outer molecular layer). Thus, newly synthesized Arc mRNA appears to be captured by active synapses, preventing the further migration of the mRNA into more distal segments. [Reproduced with permission from ref. 9 (Copyright 1998, Elsevier Science)].
erally, and so the projection system is called the commissural/ associational pathway. The synapses are excitatory, but stimulation of the pathway also evokes strong γ-aminobutyric acid (GABA)ergic inhibition via interneurons. Consequently, LTP can only be induced in this pathway when GABAergic inhibition is blocked (10). GABAergic inhibition can be blocked by positioning micropipettes containing bicuculline in the dentate gyrus during the period of high frequency stimulation. The diffusion of the bicuculline from the pipette blocks GABAergic inhibition locally in an area of about 1 mm diameter, thus enabling the induction of LTP (10). When the commissural pathway is activated under conditions of GABAergic blockade, Arc is strongly induced in the area surrounding the bicuculline-filled micropipette, and the newly synthesized mRNA localizes precisely in the inner molecular layer (9). Given this mechanism for targeting, it is likely, by extension, that Arc mRNA distribution also can be regulated on a finer scale, perhaps even on a synapse-by-synapse basis. The signal(s) that mediate this localization process remain to be defined.
The Selectivity of Localization Involves Targeting of mRNA to Active Domains and Migration/Depletion from Inactive Regions
A noteworthy feature of the pattern of labeling produced by stimulation of the medial entorhinal cortex is that the levels of labeling in the activated dendritic lamina are higher than in the lamina containing the more proximal dendrites of granule cells. This is true even though the mRNA would have to move through the proximal dendrites en route to the activated lamina. A possible explanation for this pattern of labeling comes from recent findings about how organelles move in dendrites. For example, mitochondria and membrane vesicles exhibit both orthograde and retrograde movements, sometimes even reversing direction (see refs. 11 and 12). The same basic bidirectional movement is exhibited by fluorescently labeled RNA granules (13). Given these patterns of movement, it seems reasonable to expect that Arc mRNA also may move bidirectionally once the mRNA enters dendrites, shuttling back and forth unless and until the mRNA docks (is captured). In this situation, the docking of the mRNA in the activated lamina in response to synaptic activation would prevent retrograde movement of the mRNA back into proximal dendritic regions.
Another possibility is that synaptic stimulation might actually cause Arc mRNA to migrate from inactive to active regions, depleting the mRNA from inactive segments. Evidence that this does occur has come from studies that use a different induction paradigm, designed to differentiate between the signals that induce Arc expression and those that mediate the localization. In this paradigm, Arc expression was induced by delivering an ECS. Then, the rat was anesthetized, and stimulation and recording electrodes were positioned so as to activate the medial perforant path on one side. The time between the ECS and the completion of the preparation for physiology was typically 30–45 min. Stimulus intensity was set so as to evoke an approximately half-maximal population spike (3- to 5-mV amplitude). Then, high-frequency trains (400-hz trains, eight pulses per train) were delivered to the perforant path beginning 1.5 h or 1.75 h after the ECS. Stimulation was delivered for 30 or 15 min, respectively, just before the animals were killed and perfused for in situ hybridization (in both cases, perfusion occurred 2 h after the ECS). The key to this experiment is that at 1.5 or 1.75 h post-ECS, Arc mRNA would have been present throughout the dendrites when the stimulation was initiated, in the pattern illustrated in Fig. 1 B.
Remarkably, as little as 15 min of synaptic stimulation was sufficient to produce a prominent band of labeling for Arc mRNA in the middle molecular layer of the dentate gyrus (Fig. 3 B). Because it occurred so quickly, the development of the band is likely to represent redistribution of the Arc mRNA that is already in the dendrite rather than transport of Arc mRNA from the cell body. After 30 min of stimulation, the band became more distinct as levels of labeling decreased in the nonactivated laminae, especially in the outer molecular layer (Fig. 3 E and F). Thus, synaptic activation caused newly synthesized Arc mRNA to rapidly redistribute to the activated zone (as is evident with 15 min of stimulation), and depleted the mRNA from nonactivated regions of the dendrites (as seen with 30 min or more of stimulation).
It is important to note that after prolonged periods of synaptic stimulation (2 h), the overall levels of labeling in the molecular layer are lower than on the side that received an ECS only (Fig. 2). This finding suggests that in addition to causing the newly synthesized mRNA to redistribute to active synaptic sites, synaptic activation may enhance mRNA degradation. This enhanced degradation could be linked to the targeting of the mRNA to the activated zone or could be caused by signals generated throughout the dendrite as a consequence of the intense depolarization. Local stabilization of mRNA is also seen in oocytes and developing embryos, where certain processes contribute to a generalized degradation of mRNA which is countered by a local stabilization in certain cytoplasmic domains (14).
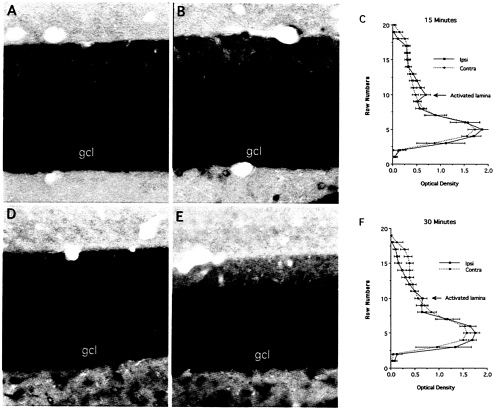
Fig. 3. Redistribution of Arc mRNA after localized synaptic activation. In these experiments, Arc expression was induced by delivering an ECS. Then, the rat was anesthetized, and stimulation and recording electrodes were positioned so as to activate the medial perforant path on one side. High frequency trains (400-hz trains, eight pulses per train) were delivered for 30 or 15 min, respectively just before the animals were killed and perfused for in situ hybridization (in both cases, perfusion occurred 2 h after the ECS). As little as 15 min of synaptic stimulation was sufficient to produce a prominent band of labeling for Arc mRNA in the middle molecular layer of the dentate gyrus (B). After 30 min of stimulation, the band became more distinct as levels of labeling decreased in the nonactivated laminae, especially in the outer molecular layer (E and F). [Reproduced with permission from ref. 28 (Copyright 2001, Elsevier Science)].
Localization of Arc mRNA in Activated Dendritic Laminae Is Associated with a Local Accumulation of Arc Protein
Immunostaining of tissue sections from stimulated animals using an Arc-specific antibody revealed a band of newly synthesized protein in the same dendritic laminae in which Arc mRNA was concentrated (Fig. 4) (9). The fact that synaptic activation leads to the selective targeting of both recently synthesized mRNA and protein suggests that the targeting of the mRNA underlies a local synthesis of the protein.
One additional important point revealed by immunocyto-chemistry is that newly synthesized Arc protein also is targeted to the nucleus. The significance of this dual targeting to active synapses and the nucleus is not yet known.
Arc Protein Is Assembled into the Postsynaptic Density (psd)/N-Methyl-D-Aspartate (NMDA) Receptor Complex (NRC)
An important clue to the function of Arc protein comes from recent evidence that the protein is concentrated in the psd and is one of a collection of proteins that are linked to the NMDA receptor. In the experiment illustrated in Fig. 5, subcellular fractions enriched in various types of cellular membranes were prepared by using established techniques (15). Band 1 contains myelin; band 2 contains nonspecialized plasma membrane; band 3 contains synaptic plasma membranes, and the pellet contains mitochondria. When band 3 is treated with detergent to remove membrane lipids and integral membrane proteins, the remaining insoluble residue represents a fraction that is highly enriched in insoluble proteins of the SJC. Western blot analyses of these subcellular fractions using antibodies against Arc and other synaptic molecules revealed that Arc protein is present at the highest relative levels in the SJC. This is the same distribution seen for other known components of the psd, like CAMKII.
A similar line of evidence comes from studies of proteins that copurify with the NMDA receptor (termed NMDAR multiprotein complex or NRC, see ref. 16). In this study, the protein constituents of the NRC were identified by mass spectroscopy combined with large-scale immunoblotting. The immunoblotting experiments revealed that Arc (going by the name Arg 3.1 in that paper) was prominently represented in the NRC: Arc was not among the proteins that were detected

Fig. 4. Arc protein accumulates in activated dendritic laminae in the same pattern as Arc mRNA. (A) Immunostaining of tissue sections from stimulated animals using an Arc-specific antibody revealed a band of newly synthesized protein in the same dendritic laminae in which Arc mRNA was concentrated. Note the sharp boundary (arrows) between the middle molecular layer (mml, the site of termination of the synapses that were activated) and the inner molecular layer. The fact that synaptic activation leads to the selective targeting of both recently synthesized mRNA and protein suggests that the targeting of the mRNA underlies a local synthesis of the protein. HF, hippocampal fissure; gcl, granule cell layer. (B) Newly synthesized Arc protein also is concentrated in the nucleus. Short arrows indicate examples of labeled nuclei.
by mass spectroscopy, however. It is interesting that a similar study, which used mass spectroscopy to identify protein constituents of the core psd, did not detect Arc (17). The starting material in that study was a psd fraction prepared by subcellular fractionation and detergent extraction, which is similar in composition to the SJC fraction. The study identified many of the same proteins that were present in the NRC, leading to the speculation that the NRC and the core psd may be different views of a subcellular structure specialized for postsynaptic signal transduction (18). The fact that Arc was not detected is probably because it is present in relatively low levels and is thus detectable by immunoblotting but not mass spectroscopy. It cannot be excluded, however, that the SJC fraction contains a slightly different complement of proteins than the psd fraction, and that Arc protein is extracted by the detergents in the preparation of the psd fraction.
The experiments above were carried out in resting animals, and so it remains to be established whether the newly synthesized Arc protein that is induced by behavioral experience or synaptic activation is also targeted to the synaptic junctional region, and if so, over what time course.
Local Protein Synthesis: A Mechanism Mediating Cotranslational Assembly of Certain Molecules into the SJC/NRC?
Since the discovery of a selective localization of polyribosomes beneath postsynaptic sites, one key question has remained unanswered: why are certain proteins synthesized locally whereas most others are synthesized in the cell body? A hypothesis is suggested by the following:
-
The psd/NRC appears to be a highly organized multimolecular structure specialized for postsynaptic signal transduction (18). It seems very likely that proper signaling requires a precise stoichiometric relationship between the different molecules making up the complex.
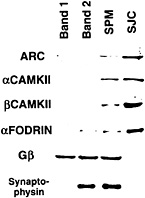
Fig. 5. Evidence from subcellular fractionation experiments that Arc protein is concentrated at the synaptic junction. Shown is a slot blot of protein samples from subcellular fractions prepared according to the procedure of ref. 15 that have been stained with various antibodies. Band 1 contains myelin; band 2 contains nonsynaptic plasma membrane; band 3 contains synaptic plasma membranes (SPM). SJC is the fraction enriched in postsynaptic densities obtained by detergent extraction of band 3. Note that Arc protein is present at the highest relative levels in the synaptic plasma membrane and SJC fractions as are a and ß isoforms of CAMKII and fodrin, which are highly enriched in psd.
-
The different protein components of the psd/NRC turn over at quite different rates. For example, Arc protein has a short half-life (a few hours). The other proteins have much longer half-lives (probably days), although the exact value is not known. The important implication of this fact is that the different molecular constituents of the psd/NRC would have to be replaced in existing psds by substitution.
-
The molecular components of the psd/NRC are almost certainly linked together through precisely controlled intermolecular interactions. Creating these links probably requires that the proteins be in particular conformations. For certain other highly organized structures, proper protein-protein interactions may require cotranslational assembly.
-
Finally, and most importantly, the mRNAs for several of the molecules that are part of the psd/NRC are present in dendrites. This is true of Arc, the a-subunit of CAMKII (19), and also shank.
Together these facts suggest the hypothesis that certain proteins are locally synthesized because they must be assembled into the psd/NRC complex by cotranslational assembly. It will be of considerable interest to take a closer look at whether any of the mRNAs encoding other constituents of the NRC complex are also present in dendrites (and conversely, whether the protein products of other dendritic mRNAs are part of the NRC).
Ribosomes at the psd?
The hypothesis for cotranslational assembly predicts that ribosomes and other components of the translational machinery would have to be closely associated with the psd as they synthesize molecules that require cotranslational assembly. Moreover, given the targeting of Arc mRNA to active synapses, one might predict an increase in ribosomes associated with the psd after high-frequency stimulation. This issue is actually difficult to address with electron microscopic techniques because the electron density of mature postsynaptic densities is nearly the same as the electron density of ribosomes. If present as singlet ribosomes, it is very likely that they would be virtually invisible with conventional electron microscopy.
In light of these ideas, it is of interest to assess the ultrastructural appearance of synapses that have experienced the intense
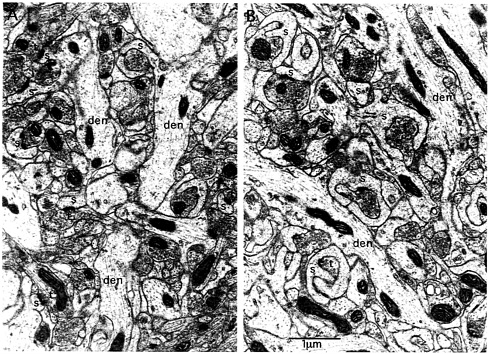
Fig. 6. Ultrastructural evidence for spine motility in synapses that have experienced intense synaptic activation. Illustrated are synapses in the middle molecular layer of the dentate gyrus on the control nonstimulated side (A) and after 2 h of high-frequency stimulation of the medial perforant path (B). Note that on the stimulated side, spines exhibit a chalise-like form that is remarkably similar to the form of highly motile spines. Animals received medial perforant path stimulation as described for 2 h and then were perfused with 2% paraformaldehyde/2% glutaraldehyde and prepared for electron microscopy. Photomicrographs then were taken in the middle molecular layer on the stimulated and control nonstimulated sides, den, dendrite; s, spine; t, terminal.
activation used herein to induce Arc expression and targeting. Electron micrographs of synapses in the middle molecular layer of the dentate gyrus after 2 h of medial perforant path stimulation reveal striking modifications of spine shape (Fig. 6 compare A, control side, with B, stimulated). In particular, the synapses in the activated zone undergo a dramatic shape change and assume a chalice-like configuration that is highly reminiscent of the shapes exhibited by highly motile spines (20, 21). Similar shape changes have been described after brief periods of stimulation in a standard LTP paradigm (22). These shapes invite the speculation that high-frequency stimulation induces a period of intense spine motility. It is noteworthy, however, that one does not find obvious examples of polyribosomes near or embedded within the psd. Indeed, polyribosomes are difficult to find in the spines that exhibit the dramatic shape changes.
These observations recall an earlier quantitative evaluation of synapse morphology after the induction of LTP that was, until now, rather curious—that fewer polyribosomes are detectable in and around synapses after inducing LTP (23). One interpretation of these observations is that strong synaptic activation triggers a translocation of ribosomes from the spine base or head to the psd, and that ribosomes that embedded in the electrondense psd become undetectable by conventional electron microscopy.
It is important to note that our hypothesis of local synthesis of psd proteins revisits an hypothesis proposed in 1981 (24). As part of a study of synaptogenesis in the cerebellar cortex, Palacios-Pru et al. (24) provided electron microscopic images of what appeared to be ribosomes in close association with immature psds on developing spines of Purkinje cells in the cerebellum. Based on these images, it was suggested that during early development, the psd was synthesized by ribosomes that were actually in immediate contact with it (see also ref. 25). If it turns out that ribosomes are embedded within mature psds and mediate cotranslational assembly of components of the NRC, what was then a controversial hypothesis will have been vindicated. Clearly it is now important to explore this issue with modern immunocyto-chemical or other techniques.
Lessons from the Study of Arc. A Candidate Mechanism for Protein Synthesis-Dependent Synaptic Modification
Studies of Arc reveal elements of a mechanism that is well-suited to mediate the sorts of molecular changes in synapses that are believed to underlie long-term synaptic plasticity.
-
The expression of the Arc gene is triggered by the patterns of synaptic activity that lead to enduring synaptic modification (LTP).
-
Arc mRNA encodes a protein that is targeted to synapses, and also to the nucleus.
-
The activity-dependent induction of Arc protein occurs during a time window that extends for a few hours after the inducing stimulus.
-
Arc mRNA and protein are targeted to synapses that have experienced particular patterns of activity.
-
Arc is induced in response to the sorts of brief behavioral experience that can lead to long-lasting synaptic modifications.
-
Finally, Arc mRNA is induced in neuron types that are thought to participate in enduring synaptic modification in response to behavioral experience (neurons in the hippocampus and cerebral cortex).
There are a number of pieces of the puzzle that are still missing, however. First, it remains to be established whether Arc protein in fact plays a role in activity-induced synaptic modification. Additional clues about the role of the protein will likely come from studies of the protein itself and its interactions with other functional molecules of the postsynaptic density. Certainly, the fact that Arc may be linked to the NMDA receptor in some way is an important clue in this regard. But even if Arc does not play a direct role, the way that Arc is handled by neurons reveals the existence of previously unknown RNA trafficking mechanisms that could be used for sorting other mRNAs that do play a key role in bringing about activity-dependent modifications.
The fascinating properties of Arc should not make us lose sight of the fact that other mRNAs are present in dendrites constitutively, including the mRNAs for molecules that have been strongly implicated in activity-dependent synaptic modification (the mRNA for the a-subunit of CAMII kinase, for example). These mRNAs that are present constitutively provide an opportunity for local regulation of the synthesis of key signaling molecules via translational regulation. Hence, gene expression at individual synapses is likely to be regulated in a complex fashion. One level of regulation would be in the mRNAs available for translation (i.e., Arc). Another level might involve regulation of translation of the mix of mRNAs that are in place, including those present constitutively (a model of which might be the translational regulation of fragile-X, refs. 26 and 27). How this is coordinated and how all of these molecules actually fit in to the molecular consolidation process remains to be established.
Thanks to Kelli Sharp and Jamie Zaffis for technical assistance. This work was supported by National Institutes of Health Grants NS12333 (O.S.) and MH 53603 (P.F.W.).
1. Bailey, C.H., Bartsch, D. & Kandel, E.R. (1996) Proc. Natl. Acad. Sci. 93, 13445–13452.
2. Mayford, M., Bach, M.E., Huang, Y.-Y., Wang, L., Hawkins, R.D. & Kandel, E.R. (1996) Science 274, 1678–1683.
3. Nguyen, P.V. & Kandel, E.R. (1996) J. Neurosci. 16, 3189–3198.
4. Jones, M.W., Errington, M.L., French, P.J., Fine, A., Bliss, T.V.P., Garel, S., Charnay, P., Bozon, B., Laroche, S. & Davis, S. (2001) Nat. Neurosci. 4, 289–296.
5. Frey, U. & Morris, R.G.M. (1997) Nature (London) 385, 533–536.
6. Link, W., Konietzko, G., Kauselmann, G., Krug, M., Schwanke, B., Frey, U. & Kuhl, K. (1995) Proc. Natl. Acad. Sci. 92, 5734–5738.
7. Lyford, G., Yamagata, K., Kaufmann, W., Barnes, C., Sanders, L., Copeland, N., Gilbert, D., Jenkins, N., Lanahan, A. & Worley, P. (1995) Neuron 14, 433–445.
8. Wallace, C.S., Lyford, G.L, Worley, P.F. & Steward, O. (1998) J. Neurosci. 18, 26–35.
9. Steward, O., Wallace, C.S., Lyford, G.L. & Worley, P.F. (1998) Neuron 21, 741–751.
10. Steward, O., Tomasulo, R. & Levy, W.B. (1990) Brain Res. 516, 292–300.
11. Ligon, L.A. & Steward, O. (2000) J. Comp. Neurol. 427, 340–350.
12. Silverman, M.A., Kaech, S., Jareb, M., Burack, M.A., Vogt, L., Sonderegger, D. & Banker, G. (2001) Proc. Natl. Acad. Sci. USA 98, 7051–7057.
13. Knowles, R.B., Sabry, J.H., Martone, M.E., Deerinck, T.J., Ellisman, M.H., Bassell, G.J. & Kosik, K.S. (1996) J. Neurosci. 16, 7812–7820.
14. Bashirullah, A., Cooperstock, R.L. & Lipshitz, H.D. (2001) Proc. Natl. Acad. Sci. USA 98, 7025–7028.
15. Cotman, C.W. & Taylor, D. (1972) J. Cell Biol. 55, 696–710.
16. Husi, H., Ward, M.A., Choudhary, J.S., Blackstock, W.P. & Grant, S.G.N. (2000) Nat. Neurosci. 3, 661–669.
17. Walikonis, R.S., Jensen, O.E., Mann, M., Provance, D.W.J., Mercer, J.A. & Kenedy, M.B. (2000) J. Neurosci. 20, 4069–4080.
18. Sheng, M. & Lee, S.H. (2000) Nat. Neurosci. 3, 633–635.
19. Burgin, K.E., Washam, M.N., Rickling, S., Westgate, S.A., Mobley, W.C. & Kelly, P.T. (1990) J. Neurosci. 10, 1788–1798.
20. Fischer, M., Kaech, S., Wagner, U., Brinkhaus, H. & Matus, A. (2000) Nat. Neurosci. 3, 887–894.
21. Kaech, S., Parmar, H., Roelandse, M., Bornmann, C. & Matus, A. (2001) Proc. Natl. Acad. Sci. USA 98, 7086–7092.
22. Desmond, N.L. & Levy, W.B. (1983) Brain Res. 265, 21–30.
23. Desmond, N.L. & Levy, W.B. (1990) Synapse 5, 139–143.
24. Palacios-Pru, E.L., Palacios, L. & Mendoza, R.V. (1981) J. Submicros. Cytol. 13, 145–167.
25. Palacios-Pru, E.L., Miranda-Contreras, L., Mendoza, R.V. & Zambrano, E. (1988) Neuroscience 24, 111–118.
26. Greenough, W.T., Klintsova, A.Y., Irwin, S.A., Galvez, R., Bates, K.E. & Weiler, I.J. (2001) Proc. Natl. Acad. Sci. USA 98, 7101–7106.
27. Weiler, I.J., Irwin, S.A., Klintsova, A.Y., Spencer, C.M., Brazelton, A.D., Miyashiro, K., Comery, T.A., Patel, B., Eberwine, J. & Greenough, W.T. (1997) Proc. Natl. Acad. Sci. 94, 5395–5400.
28. Steward, O. & Worley, P.F. (2001) Neuron, in press.







