critically about the nature of these solutions to answer the subsequent questions correctly. More discrimination is required to differentiate acetic acid from NaF and from MgCl2 solution behavior.
Modification 2. If the stoppers were removed from the flasks, from which flask would the solute escape most readily? Explain the answer.
Modification 3. The contents of which flask(s) could be used as reactant(s) in an esterification reaction? Explain.
This would become a more challenging question if varying concentrations of the compounds in water were used. This change would make the question quantitative and more difficult. The quantitative nature of Question 7 would make it less appropriate as a companion for question 8. These two questions should be of like nature and degree of difficulty. To avoid the quantitative aspect of concentration calculation, it would be appropriate to ask the student to identify the type of substance for each question and to write a dissociation, ionization, hydration equation (as necessary) for these substances in water and discuss their relative activity.
These three items are examples of particulate representations linked to symbolic representations or macroscopic phenomena. Such questions are not part of the AP or IB examinations.
-
Which particulate representation corresponds to the equation?
2 SO2 + O2 → 2 SO3
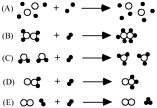
-
Which would best represent a mixture of the gases helium and chlorine?
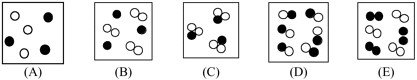
-
If the contents of these two beakers are poured into a third beaker, what will the resulting mixture look like?
-
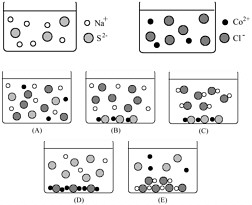
This is an example of an error analysis–type item.
-
A student titrated 10.00 mL of fruit juice with 12.84 mL of 9.580 x 10-2 M NaOH. Which step is NOT correct in this calculation of mass of citric acid in 1.00 mL of juice?
C3H5O(COOH)3 + 3 NaOH → Na3(C3H5O(COO)3 + 3 H2O
-
Moles NaOH = (12.84 mL) (9.580 x 10-2 mol/L).
-
Moles citric acid = (1.230 mol NaOH) (1 mol citric acid / 3 mol NaOH).
-
Mass citric acid in sample = (0.4100 mol citric acid) (192.12 g/mol citric acid).
-
Mass citric acid in 1 mL fruit juice = (78.77 g citric acid) / (10.00 mL fruit juice).
-
All the steps are correct.
This is an example of a laboratory-related item.
-
Consider a laboratory exercise in which you prepare approximately 200 mL of a pH = 4.35 buffer. The chemicals available for your use are: 1M solutions of acetic acid, ammonium acetate, ammonium chloride, aqueous ammonia, hydrochloric acid, sodium acetate, and sodium hydroxide (acetic acid Ka = 1.8 x 10-5 and ammonia Kb = 1.8 x 10-5).
-
Which chemicals will you use to make your buffer? Explain your choice; use chemical equations where appropriate.
-
How much of each chemical will you need? Show calculations to support your answer.
-
Outline the procedure you will follow to make your buffer. Include specific equipment and glassware you will use.
-
How will you prove that you successfully prepared the buffer?
ADDITIONAL SUGGESTIONS FOR SECTION II, PART B
The following are some other suggestions for Section II, Part B that are not specifically tied to the 1999 version of the examination.
-
A 0.2 M sulfuric acid is added to a 0.2 M solution of barium nitrate. Describe what happens and write an equation representing the reaction.
-
A small piece of solid potassium is added to 500 mL of water in a beaker. The potassium fizzes, dances about, and bursts into flame.
-
Write an equation representing the reaction.
-
A drop of phenolphthalein solution is added to the resulting solution. What would you observe?. Explain.
-
The panel also suggests that a useful addition would require students to identify reactions as examples of one or more of the following types of reactions: acid/base, oxidation/reduction, decomposition, combination, precipitation, gas evolution, double displacement, electron transfer, and proton transfer. (This list could be expanded or reduced.)
Appendix E
Research Agenda for Advanced Studies in High School Chemistry
The chemistry panel identified a number of topics on which research is needed with respect to advanced studies in high school chemistry:
-
How do students who take advanced courses in high school chemistry move through their college studies (e.g., choice of major, continued interest and enrollment in chemistry), and how well do they succeed compared with students who do not take these programs? Longitudinal data are needed to address these questions.
-
What are the actual costs for implementing, sustaining, updating, and upgrading advanced courses in the experimental sciences? Surveys of teachers are needed to determine actual costs.
-
Is there a difference in the academic success of students whose school districts spend more money on these programs (e.g., for staff development, materials, and modern equipment and facilities) compared with districts that spend less?
-
How much is information technology being integrated into advanced study courses, and what are the requirements for achieving such integration?
-
To what extent do the AP and IB courses reflect current approaches to teaching and learning in introductory college courses?
-
How much variation exists in the granting of college credit and placement in courses to students who take advanced courses in high school?
-
What are the effects of the current ordering of prerequisite and advanced courses in science?
-
Do advanced programs favor some kinds of students over others?
-
What backgrounds, credentials, and professional experience characterize teachers who are involved with these programs? Do these differences translate into how well students learn and achieve in the courses?
-
What percentage of students who take AP Chemistry take it as their first course in chemistry? How well do these students fare in the course and subsequently in college chemistry courses?
-
What proportion of students who take AP or IB Chemistry do not take the examinations? What effect does the resulting lack of information about student learning have on the quality and quality control of the AP program in individual schools and on the AP program overall?
-
Are there advantages to having schools offer clusters of advanced study courses as opposed to isolated courses? What is the impact of not doing so (for example, in small high schools that can support only one or two advanced study courses that may not be connected with each other)?
-
Are there “critical masses” in the number of teachers in a school who teach advanced study courses? In other words, do differences in opportunities for isolated teachers to communicate with colleagues translate into differences in learning and achievement of their students?
-
Are there “critical masses” in the number of students who enroll in advanced study courses? Are students who enroll in such courses either individually (e.g., through distance learning courses) or in small numbers at an advantage or disadvantage relative to students who are enrolled in very large classes?
-
What percentage of schools have prerequisites or other screening procedures for entry into advanced study courses? Do more stringent requirements for entry into such courses translate into differences in scores on the respective assessments?
-
What kinds of physical facilities, equipment and instrumentation, and support for laboratories are available to teachers and students in advanced study programs in the experimental sciences? Do differences in the level and availability of such resources have an impact on student learning and performance?
-
Are there differences in student performance on advanced study examinations in districts or states that provide incentives to students to do well as compared with students in districts or states that do not offer these kinds of incentives?
There was a problem loading page 51.
There was a problem loading page 52.
There was a problem loading page 53.
There was a problem loading page 54.









