How Camest Thou in This Pickle?
JERRY M. MELILLO
Addressing the problem of increasing concentrations of carbon dioxide in the atmosphere brings to mind a simple question from Shakespeare’s The Tempest, “How camest thou in this pickle?” The answer is anything but simple. The developed world has so far been primarily responsible for disrupting the global carbon cycle. However, in the future parts of the developing world are likely to become the dominant emitters of carbon dioxide (CO2) through the burning of fossil fuels. I believe developed nations should lead by example by reducing their CO2 emissions and promoting sensible programs to sequester carbon. I also believe developed countries should share carbon-efficient energy-generating technologies with developing countries as rapidly as possible. After all, a mole of CO2 contributes to the greenhouse effect regardless of its source.
In attempting to address carbon cycle problems through engineering, we must anticipate that the interventions may have negative environmental consequences. We are dealing here with complex nonlinear ecosystems with yet-to-be-defined limits and thresholds, and we must move forward slowly. In addition to the global carbon cycle, humans have disrupted other major biogeochemical cycles, including those of nitrogen, sulfur, and phosphorus. Addressing these problems will require wise engineering combined with a deep understanding of ecological, economic, and social systems. Actions taken by and in the developing world will be crucial.
Earth’s climate is a function of complex interactions among the sun, atmosphere, oceans, land, and living things. Several gases in the atmosphere, including CO2, absorb heat radiated from the Earth’s surface and create the “greenhouse effect,” a natural feature of our climate system. Humans have been changing the
composition of the atmosphere by burning fossil fuels and clearing forests for agriculture and other uses for the past 1,000 years. But until about 100 years ago these activities had a minor effect on the global carbon cycle and the climate system (NAST, 2000). Since the late 1800s, increasing emissions associated with human actions have been responsible for a 30 percent increase in the concentration of atmospheric CO2. Many aspects of climate, including warming, have also occurred (Figure 1).
From 1950 to 1995 the developed world accounted for about three-quarters of total CO2 emissions associated with the burning of fossil fuels. In 1995, for example, 73 percent of total CO2 emissions from human activities came from developed countries (OSTP, 1995). The United States was the largest single source, accounting for 22 percent of the total, with carbon emissions per person exceeding 5 tons per year. Elsewhere in the developed world, Western Europe accounted for 17 percent, Eastern Europe and the former Soviet Union for 27 percent, and Asia for 7 percent. China was the largest single source among developing countries, accounting for 11 percent of the total, with carbon emissions per person about one-tenth those of the United States (Figure 2).
In the next few decades as much as 90 percent of the world’s population growth is expected to occur in developing countries (Figure 3), some of which will concurrently undergo rapid economic growth. Per capita energy use in developing countries, which is now only one-tenth to one-twentieth of U.S. energy use, will also rise. If present trends continue, developing countries will account for more than half of total global CO2 emissions by 2035. China—today the second-largest source of CO2 emissions—will become the largest emitter sometime between 2010 and 2015.
As scientists currently understand the global carbon cycle, natural carbon sinks in the ocean and on land eventually absorb between one-half and two-thirds of emissions from human activities. The rest of the emitted carbon remains in the atmosphere. Therefore, to reduce the rate at which CO2 accumulates in the atmosphere, we must either reduce emissions or increase carbon uptake by the land and the oceans.
Both developed and developing countries must eventually be involved in managing the emissions side of the global carbon cycle. Developed nations should lead the way because they are responsible for most of the CO2 that has accumulated in the atmosphere since the late 1800s. Developed countries must also share carbon-efficient, energy-generating technologies with the developing world as soon as possible because developing nations with rapidly growing economies, such as China, are making capital investments now in power plants with lifetimes of at least several decades. It is in everyone’s interest that these power plants be as carbon efficient as possible.
Both land and ocean ecosystems have the capacity to store or sequester carbon. On land vegetation currently stores about 550 billion metric tons of carbon and soil stores another 1,500 billion metric tons. The ocean, which is a
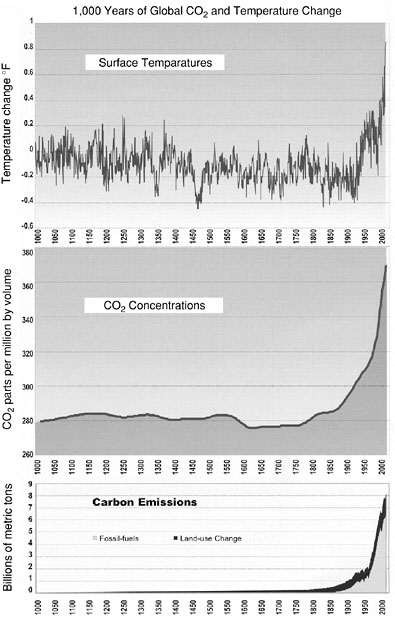
FIGURE 1 Records of surface temperatures, CO2 concentrations, and carbon emissions in the Northern Hemisphere. Surface Temperatures: Reconstruction of annual average surface air temperatures derived from historical records, tree rings, and corals (until about 1900) and direct measurements of air temperatures (after about 1900). CO2 Concentrations: Derived from measurements of CO2 concentration in air bubbles in the layered ice cores drilled in Antarctica (for period before 1957) and from atmospheric measurements (since 1957). Carbon Emissions: Reconstruction of past emissions of CO2 from land clearing and fossil fuel combustion since about 1750 (and linearly projected back to zero in 1000). Source: NAST, 2000.
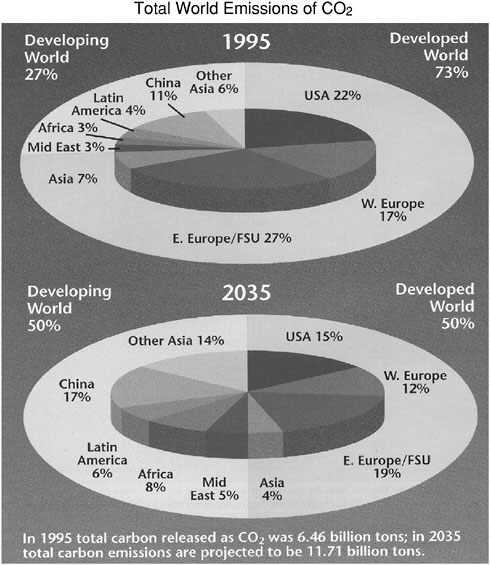
FIGURE 2 Relative distribution of total world CO2 emissions associated with the burning of fossil fuels (estimated for 1995 and projected for 2035). Source: OSTP, 1995.
much larger carbon storehouse, contains about 38,000 billion metric tons of carbon, most of it in deep waters (Schlesinger, 1997).
In the past decade scientists and engineers have been exploring ways to increase carbon storage in both land and ocean ecosystems. Schemes for sequestering more carbon on land include reforestation (Birdsey and Heath, 1993) and
the elimination of traditional tillage practices in managing agricultural soils (Lal et al., 1998). Interestingly, many of the proposed carbon sequestration schemes for land ecosystems would also have other benefits. For example, reforestation of hillsides would protect soil against erosion from heavy rains, and the buildup of organic matter in agricultural soils would increase their capacity to retain nutrients and water.
Schemes for increasing carbon storage in ocean ecosystems are not as well developed. One proposal that has received considerable attention would entail manipulating the biological component of the Southern Ocean in an attempt to store carbon in the deep ocean for centuries (Abraham et al., 2000; Boyd et al., 2000; Chisholm et al., 2000; Watson et al., 2000). This scheme is based on the observation that in the Southern Ocean a lack of available iron in sunlit surface waters limits the growth of phytoplankton—microscopic ocean plants—that form the basis of the marine food web. Using sunlight and dissolved nutrients, phytoplankton convert CO2 to organically bound carbon. Animals eat the tiny marine plants, and microorganisms, such as bacteria, then decompose both plant and animal wastes. As the organic carbon passes through the marine food web, most of it is converted back to CO2 and escapes into the atmosphere. A small amount, however, is transported to the deep ocean, where it, too, is eventually converted back to CO2 but remains for about 1,000 years. The rate at which the CO2 is “pumped” to the deep ocean is largely related to the composition of phytoplankton
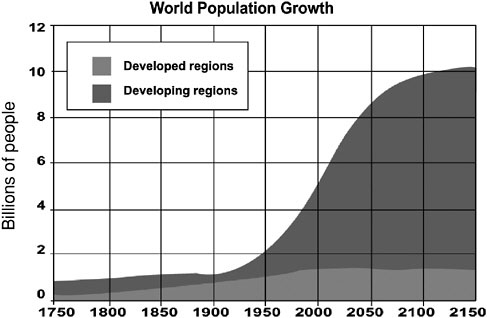
FIGURE 3 Population growth in the developed and developing world from 1750 to 2150.
species and their productivity, both of which are controlled by the availability of iron in the Southern Ocean. The idea is to add iron to this region of the ocean to increase carbon sequestration in deep waters.
This concept was tested in February 1999 when 8,663 kilograms of an iron compound were added to a circular patch of ocean 8 kilometers in diameter, located 2,000 kilometers south-southwest of Hobart, Tasmania (Boyd et al., 2000). As expected, iron fertilization led to a dramatic phytoplankton bloom and a shift in this community from small-celled to large-celled species, primarily diatoms. In a report on the results in Nature, Watson et al. (2000) conclude that “the experiment confirms that modest sequestration of atmospheric CO2 by artificial additions of iron to the Southern Ocean is in principle possible, although the period and geographical extent over which sequestration would be effective remain poorly known.”
Even if iron fertilization of the Southern Ocean resulted in a modest increase in the sequestration of atmospheric CO2, this increase could come at a high price. In a critique of this scheme, S.W. Chisholm (2000), an MIT-based marine biologist, has argued that it would threaten ocean ecosystems by changing the structure of the marine food web. She reasoned that the iron-fertilization scheme could also produce many unintended side effects, such as deoxygenation of the deep ocean and the generation of greenhouse gases that are more potent than CO2.
Other elements in the environment contribute to climate change. The increasing demand for food has led to the clearing of forests for cropland and pastures and the recent addition of large quantities of nitrogen and phosphorus as fertilizer. Today the fixation of nitrogen associated with production of food and energy is greater than natural nitrogen fixation in terrestrial ecosystems. Our growing demand for energy has also resulted in the burning of fossil fuels, wood, and other forms of biomass. These activities are major sources, not only carbon but also of sulfur and nitrogen in the atmosphere, where they affect the climate system and the chemistry of precipitation. The unprecedented mobilization of nitrogen, sulfur, and phosphorus has led to a range of environmental consequences at the local, regional, and global scales.
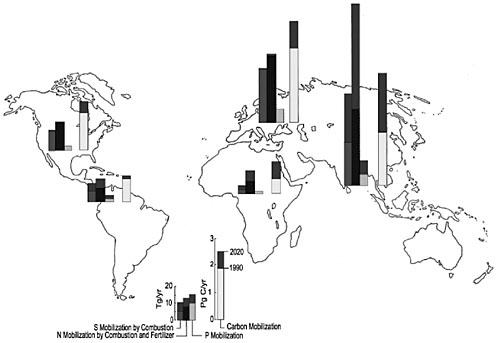
In the past century, most of the nitrogen, sulfur, and phosphorus mobilization has occurred in North America and Europe (Galloway et al., 1998). In the last few decades, however, Asia, which has more than half of the world population and many of the most rapidly growing economies, has substantially affected the global cycles of nitrogen, sulfur, and phosphorus (Figure 4). Estimates show that Asia today is affecting mobilization of these nutrients almost as much as North America and Europe combined. At the start of the new millennium, Asia accounted for 40 percent of nitrogen mobilization, 35 percent of sulfur mobilization, and 35 percent of phosphorus mobilization. Equally important is that mobilization in Asia is increasing rapidly. During the 1980s, for example, nitrogen mobilization in Asia from fertilizer alone doubled from 20 to 40 tetragrams of nitrogen per year (Tg N/year).
Mobilized carbon, nitrogen, sulfur, and phosphorus ultimately accumulate in the Earth system’s major reservoirs—the atmosphere, land, freshwaters, and oceans. The site and the magnitude of accumulation determine the environmental consequences. Nitrogen loading of the atmosphere as nitrous oxide contributes to the greenhouse effect and global climate change. Nitrogen loading can also lead to increases in tropospheric ozone and decreases in stratospheric ozone. Elevated levels of tropospheric ozone can cause human health problems and reduce crop production. Decreased levels of stratospheric ozone can mean that more ultraviolet radiation reaches the Earth’s surface, causing human health problems. Loading of land ecosystems with nitrogen can acidify ecosystems. Loading of the atmosphere with sulfur increases its turbidity and acidity, which in turn can affect the radiation balance and acidify poorly buffered land and freshwater ecosystems. Finally, loading freshwater ecosystems with phosphorus can lead to a chain of events that includes increases in aquatic plant productivity, reductions in oxygen levels in the water column, and ultimately reductions in habitat quality for aquatic animals, including fish.
The rapid increase in carbon, nitrogen, sulfur, and phosphorus mobilization observed by Asia is expected to continue. By 2020 nitrogen mobilization is likely to double—from 45 Tg/year to 100 Tg/year. Asia is likely to consume about 50 percent of the phosphorus fertilizer used worldwide, compared with 35 percent in 1990. The region will also account for about half of all sulfur emissions to the atmosphere, compared with about 35 percent in 1990.
Continued increases in the rates at which nitrogen, sulfur, and phosphorus are mobilized will make managing our planet and sustaining and enhancing the quality of life at regional and local scales even more difficult. Developed countries must share their knowledge of the causes of atmospheric change and their technologies for enhancing our quality of life and reducing the adverse effects of disruptions to life-sustaining cycles of key elements.
Managing the Earth and its life-support systems will require a partnership among engineers, scientists, and policy makers. Together, we must develop and pursue an adaptive strategy in which new knowledge based on sound science and engineering is used to benefit humankind.
REFERENCES
Abraham, E.R., C.S. Law, P.W. Boyd, S.J. Lavender, M.T. Maldonado, A.R. Bowie. 2000. Importance of stirring in the development of an iron-fertilized phytoplankton bloom. Nature 407(6805): 727–730.
Birdsey, R.A., and L.S. Heath. 1993. Carbon Sequestration Impacts of Alternative Forestry Scenarios. Washington, D.C.: U.S. Department of Agriculture.
Boyd, P.W., A.J. Watson, C.S. Law, E.R. Abraham, T. Trull, R. Murdoch, D.C.E. Bakker, A.R. Bowie, K.O. Buesseler, H. Chang, M. Charette, P. Croot, K. Downing, R. Frew, M. Gall, M. Hadfield , J. Hall, M. Harvey, G. Jameson, J. LaRoche, M. Liddicoat, R. Ling, M. T. Maldonado, R. M. McKay, S. Nodder, S. Pickmere, R. Pridmore, S. Rintoul, K. Safi, R. Strzepek, P. Sutton, K. Tanneberger, S. Turner, A. Waite, and J. Zeldis. 2000. A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature 407(6805): 695–702.
Chisholm, S.W. 2000. Oceanography: stirring times in the Southern Ocean. Nature 407(6805): 685–687.
Galloway, J.N., D.S. Ojima, and J.M. Melillo. 1998. Asian change in the context of global climate change: an overview. Pp. 1–17 in Asian Change in the Context of Global Climate Change: Impact of Natural and Anthropogenic Changes in Asia on Global Biogeochemical Cycles, J.N. Galloway and J.M. Melillo, eds. Cambridge, U.K.: Cambridge University Press.
Lal, R., J.M. Kimble, R.F. Follett, and C.V. Cole. 1998. The Potential of U.S. Cropland to Sequester Carbon and Mitigate the Greenhouse Effect. Ann Arbor: Sleeping Bear Press.
NAST (National Assessment Synthesis Team). 2000. Climate Change Impacts on the United States: The Potential Consequences of Climate Variability and Change. Washington, D.C.: U.S. Global Change Research Program.
OSTP (Office of Science and Technology Policy). 1995. Climate Change: State of Knowledge. Washington, D.C.: Executive Office of the President.
Schlesinger, W.H. 1997. Biogeochemistry: An Analysis of Global Change. New York: Academic Press.
Watson, A.J., D.C.E. Bakker, A.J. Ridgwell, P.W. Boyd, and C.S. Law. 2000. Effect of iron supply on Southern Ocean CO2 uptake and implications for glacial atmospheric CO2. Nature 407(6805): 730–733.