Immunization Safety Review: Hepatitis B Vaccine and Demyelinating Neurological Disorders
Immunization to protect infants and children from many infectious diseases is one of the greatest achievements of public health. Immunization is not without risks, however. It is well established, for example, that the oral polio vaccine can on rare occasion cause paralytic polio, that some influenza vaccines have been associated with a risk of Guillain-Barré syndrome, and that vaccines sometimes produce anaphylactic shock. Given the widespread use of vaccines, state mandates requiring vaccination of children for entry into school or day care, and the importance of ensuring that trust in immunization programs is justified, it is essential that safety concerns receive assiduous attention.
The Immunization Safety Review Committee was established by the Institute of Medicine (IOM) to evaluate the evidence on possible causal associations between immunizations and certain adverse outcomes, and to then present conclusions and recommendations. The committee’s mandate also includes assessing the broader significance for society of these immunization safety issues.
In this fourth report in a series, the committee examines the hypothesis that the hepatitis B vaccine increases the risk for demyelinating disorders of the central or peripheral nervous systems, including multiple sclerosis (MS) and Guillain-Barré syndrome (GBS).
THE CHARGE TO THE COMMITTEE
Since the mid-1990s, challenges to the safety of immunizations seem to have gained prominence in public and scientific debate. Given these persistent and growing concerns about immunization safety, the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH) recognized the need for an independent, expert group to address immunization safety in a timely and objective manner. The IOM has been involved in such issues since the 1970s. (A brief chronology can be found in Appendix C.) In 1999, as a
result of IOM’s previous work and its access to independent scientific experts, CDC and NIH began a year of discussions with IOM to develop the Immunization Safety Review project to address vaccine safety issues both existing and emerging.
The Immunization Safety Review Committee is responsible for examining a broad variety of immunization safety concerns. Committee members have expertise in pediatrics, neurology, immunology, internal medicine, infectious diseases, genetics, epidemiology, biostatistics, risk perception and communication, decision analysis, public health, nursing, and ethics. While all the committee members share the view that immunization is generally beneficial, none of them has a vested interest in the specific immunization safety issues that come before the group. Additional discussion of the committee composition can be found in the Foreword written by Dr. Kenneth Shine, President of the IOM.
The committee is charged with examining three immunization safety hypotheses each year during the three-year study period (2001–2003). These hypotheses are selected by the Interagency Vaccine Group, whose members represent several units of the Department of Health and Human Services (DHHS)— the National Vaccine Program Office, the National Immunization Program, and the National Center for Infectious Diseases at the CDC, the National Institute for Allergy and Infectious Diseases at the NIH, the Food and Drug Administration (FDA), the National Vaccine Injury Compensation Program at the Health Resources and Services Administration (HRSA), and the Centers for Medicare and Medicaid Services (CMS, formerly the Health Care Financing Administration)—and the Department of Defense and the Agency for International Development. For each topic, the Immunization Safety Review Committee reviews relevant literature and submissions by interested parties, holds an open scientific meeting, and directly follows the open meeting with a one- to two-day closed meeting to formulate its conclusions and recommendations. The committee’s findings are released to the public in a brief consensus report 60–90 days after its meeting.
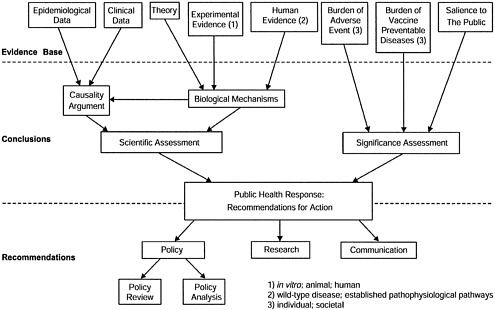
For each hypothesis to be examined, the committee assesses both the scientific evidence and the significance of the issue for society.
-
The scientific assessment has two components: an examination of the epidemiological and clinical evidence regarding a possible causal relationship between the vaccine and the adverse event, and an examination of theory and experimental evidence from human or animal studies regarding biological mechanisms that might be relevant to the hypothesis.
-
The significance assessment addresses such considerations as the burden of the health risks associated with the vaccine-preventable disease and with the adverse event. Other considerations may include the perceived intensity of public or professional concern, or the feasibility of additional research to help resolve scientific uncertainty regarding causal associations.
The findings of the scientific and significance assessments provide the basis for the committee’s recommendations regarding the public health response on the issue. In particular, the committee addresses needs for immunization policy review, current and future research, and effective communication strategies. See Figure 1 for a schematic representation of the committee’s charge.
THE STUDY PROCESS
The committee held an initial organizational meeting in January 2001. CDC and NIH presented the committee’s charge at the meeting, and the committee conducted a general review of immunization safety concerns and determined its methodology for assessing causality. This approach would be used for the hypotheses to be considered at subsequent meetings. A website (www.iom.edu/imsafety) and a listserv were created to provide public access to information about the committee’s work and to facilitate communication with the committee. The conclusions and recommendations of the committee’s first three reports—Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism (IOM, 2001a), Immunization Safety Review: Thimerosal-Containing Vaccines and Neurodevelopmental Disorders (IOM, 2001b), and Immunization Safety Review: Multiple Immunizations and Immune Dysfunction (IOM, 2002)— are summarized in Appendix A.
For its evaluation of the hypothesis on hepatitis B vaccine and demyelinating neurological disorders, the committee first commissioned a background paper; its purposes were to review current understanding of the biological mechanisms thought to be involved in MS and other demyelinating disorders, and to analyze the relationship of those mechanisms to the putative role of hepatitis B vaccine in such disorders. The committee also held an open scientific meeting in March 2002 (see Appendix B) for presentations on issues germane to the topic. The commissioned paper and all information presented to the committee at the open meeting can be viewed on the project website (www.iom.edu/imsafety). In addition, the committee reviewed an extensive collection of material from the published, peer-reviewed, scientific and medical literature. A reference list of materials reviewed by the committee, even if not cited in this report, can be found on its website.
THE FRAMEWORK FOR SCIENTIFIC ASSESSMENT
Causality
The Immunization Safety Review Committee has adopted the framework for assessing causality developed by its predecessors (convened by the IOM in 1991 and 1994 under the congressional mandate of P.L. 99–660) to address questions of immunization safety. The categories of causal conclusions used by the committee are as follows:
-
No evidence
-
Evidence is inadequate to accept or reject a causal relationship
-
Evidence favors rejection of a causal relationship
-
Evidence favors acceptance of a causal relationship
-
Evidence establishes a causal relationship.
Assessments begin from a position of neutrality regarding the specific vaccine safety hypothesis under review. That is, there is no presumption that a specific vaccine (or vaccine component) does or does not cause the adverse event in question. The weight of the available clinical and epidemiological evidence determines whether it is possible to shift from that neutral position to a finding for causality (“the evidence favors acceptance of a causal relationship”) or away from causality (“the evidence favors rejection of a causal relationship”). The committee does not conclude that the vaccine does not cause the adverse event merely if the evidence is inadequate to support causality. Instead, it maintains a neutral position, concluding that the “evidence is inadequate to accept or reject a causal relationship.”
Although no firm rules establish the amount of evidence or the quality of the evidence required to support a specific category of causality conclusion, the committee uses standard epidemiological criteria to guide its decisions. The most definitive category is “establishes causality,” which is reserved for those relationships where the causal link is unequivocal, as with the oral polio vaccine and vaccine-associated paralytic polio or with anaphylactic reactions to vaccine administration. The next category, “favors acceptance” of a causal relationship, reflects evidence that is strong and generally convincing, although not firm enough to be described as unequivocal or established. “Favors rejection” is the strongest category in the negative direction. The category of “establishes no causal relationship” is not used because it is virtually impossible to prove the absence of a relationship with the same surety that is possible in establishing its presence.
If the evidence is not reasonably convincing either in support of or against causality, the category “inadequate to accept or reject a causal relationship” is used. Evidence that is sparse, conflicting, of weak quality, or just suggestive either toward or away from causality falls into this category. Some authors of similar assessments use phrases such as “the evidence does not presently support a causal association.” The committee believes, however, that such language does not make the important distinction between evidence indicating that a relationship does not exist (category 3) and evidence that is indeterminate with regard to causality (category 2). The category of “no evidence” is reserved for those cases in which there is a complete absence of clinical or epidemiological evidence.
The sources of evidence considered by the committee in its scientific assessment of causality include epidemiological and clinical studies directly addressing the question at hand. That is, the data relate to the effects of the vaccine(s) under review and the specific adverse health outcome(s) under review— in the case of this report, the effects of hepatitis B immunization on the risk for demyelinating neurological disorders.
Epidemiological studies carry the most weight in a causality assessment; these studies measure health-related exposures and outcomes in a defined sample of subjects and make inferences about the nature and strength of associations between exposures and outcomes in the overall population from which the study sample was drawn. Epidemiological studies can be categorized as observational or experimental (clinical trial), and as uncontrolled (descriptive) or controlled (analytic). Among these various study designs, experimental studies generally have the advantage of random assignment to exposures and are therefore the most influential in assessing causality. Uncontrolled observational studies are important but are generally considered less definitive than controlled studies. In uncontrolled observational studies where observations are made over time, confounding from factors such as changing case definitions or improving case detection may affect the apparent incidence and prevalence of the adverse outcomes studied.
Case reports and case series are generally inadequate by themselves to establish causality. Despite the limitations of case reports, the causality argument for at least one vaccine-related adverse event (the relationship between vaccines containing tetanus toxoid and Guillain-Barré syndrome) was strengthened most by a single, well-documented case report on recurrence of the adverse event following re-administration of the vaccine, a situation referred to as a “rechallenge” (IOM, 1994).
Biological Mechanisms
Terminology
Evidence considered in the scientific assessment of biological mechanisms includes human, animal, and in vitro studies related to biological or pathophysiological processes by which immunizations could cause an adverse event. This kind of review has been referred to in previous reports of this committee (IOM, 2001a, 2001b) and others (IOM, 1991, 1994) as an assessment of the “biological plausibility” of a causal relationship. Although evidence regarding biological plausibility can never prove causality, the committee had previously described a spectrum of biological plausibility, ranging from not plausible to established. An agreed upon hierarchy of evidence required for assessments of biological plausibility does not exist, nor does an associated terminology (Weed and Hursting, 1998).
The committee noted in its last report (IOM, 2002) that the term biological plausibility is a source of confusion on at least two fronts. First, it is associated with guidelines (sometimes referred to as the Bradford Hill criteria) for causal inference from epidemiological evidence (Hill, 1965). In that context, an assessment of the biological plausibility of an association demonstrated by epidemiological analysis is meant to ensure that such an association is consistent with current biological knowledge. It is also meant to guard against attributions of causality to biologically implausible statistical associations that might result from studies that have not adequately accounted for important variables. A review of the biological plausibility of an association might add reassurance that the epidemiological findings point toward or reflect causality. On occasion, however, an epidemiological observation has been attributed to an apparently reasonable biological mechanism that, on further investigation, appeared not to be relevant for the pathophysiology involved.
This committee, however, is often faced with a set of circumstances in which the epidemiological evidence is judged inadequate to accept or reject a causal association between a vaccine exposure and an adverse event of concern. It is then left with the task of examining proposed or conceivable biological mechanisms that might be operating if an epidemiologically sound association could be shown between vaccine exposure and an adverse event. Even without epidemiological evidence, the identification of sound biological mechanisms could influence the development of an appropriate research agenda and give support for policymakers, who frequently must make decisions in situations of incomplete information regarding causality. In addition, there is often value in investigating and understanding possible biological mechanisms even if the available epidemiological evidence suggests the absence of a causal association. A review of biological data could give support to the negative causality assess-
ment, for example, or it could prompt a reconsideration or further investigation of the epidemiological findings. If new epidemiological studies were to question the existing causality assessment, the biological data could gain prominence in the new assessments.
Second, the committee understands that some readers of its reports are confused by what are perceived as contradictions between the findings on causality and on biological plausibility. Although the committee has previously stated that biological plausibility can range across a spectrum, readers sometimes regard the term with a degree of certainty or precision the committee never intended. When other evidence of causality is available, data on biological mechanisms add supportive evidence. However, in the absence of other evidence pointing to a causal relationship, use of the term biological plausibility, as ingrained in the language of causal inference, seems to add confusion.
Thus, the committee found that for the purpose of its reports, the lack of clarity in the phrase “biological plausibility” warranted the adoption of new terminology and a new approach to its discussion of biological data. As it began doing in the report that immediately preceded this one (IOM, 2002), the committee will review evidence regarding “biological mechanisms” that might be consistent with the proposed relationship between a vaccine exposure and given adverse events. This assessment of the biological data is presented in a section of the report that is distinct from any argument regarding the causality of such relationships.
This approach is not meant to imply that the current understanding of biological processes does not shape or guide the committee’s assessments of causality. In fact, the current thinking on a possible biological explanation for a relationship between immunization and an adverse event will influence the design of a good epidemiological analysis. The essential consideration of “confounders” in epidemiological studies depends on an understanding of the biological phenomena that could underlie or explain the observed statistical relationship. Only when important confounders are considered can a statistical observation be considered as evidence of causality. However, absent evidence of a statistical association, or convincing clinical evidence, biological mechanisms cannot be invoked as proof of causality.
Categories of Evidence
The committee has established three general categories of evidence on biological mechanisms:
-
Theory only: A reasonable mechanism can be hypothesized that is commensurate with scientific knowledge and that does not contradict known physical and biological principles, but it has not been demonstrated in whole or in part in humans or in animal models. Postulated mechanisms by which a vac-
-
cine might cause a specific adverse event but for which no coherent theory exists would not meet the criteria for this category. Thus, “theoretical only” is not a default category, but one that requires thoughtful and biologically meaningful suppositions.
-
Experimental evidence that the mechanism operates in animals, in vitro systems, or humans: Experimental evidence often describes effects on just one or a few of the steps in the pathological process required for expression of disease. Showing that multiple components of the theoretical pathways operate in reasonable experimental models increases confidence that the mechanisms could possibly result in disease in humans. The evidence can be derived under highly contrived conditions. For example, achieving the results of interest may require extensive manipulation of the genetics of an animal system, or in vivo or in vitro exposures to vaccine antigen that are extreme in terms of dose, route, or duration. Other experimental evidence is derived under less contrived conditions. For example, a compelling animal or in vitro model exists whereby administration of a vaccine antigen under conditions similar to human use results in a pathological process analogous to a human disease pathology. Mechanistic evidence also could come from studies in humans, but this is distinct from the evidence (about incidence of adverse events following immunization) that derives from randomized controlled trials or other population-based epidemiological studies, which contribute to the causality assessment.
-
Evidence that the mechanism results in known disease in humans: For example, a wild-type infection causes the adverse health outcome, or another vaccine has been demonstrated to cause the same adverse outcome by the same or a similar mechanism. Data from population-based studies of the effects of the vaccine administration on the occurrence of the adverse outcomes under review are considered not as evidence regarding the biological mechanisms but as evidence regarding causality.
If the committee identifies evidence of biological mechanisms that could be operational, it will offer a summary judgment of that body of evidence as weak, moderate, or strong. Although the committee tends to judge biological evidence in humans as “stronger” than biological evidence from highly contrived animal models or in vitro systems, the summary strength of the evidence also depends on both the quantity (e.g., number of studies or number of subjects in a study) and quality (e.g., the nature of the experimental system or study design) of the evidence. Obviously, the conclusions drawn from this review depend on both the specific data and on scientific judgment. To ensure that its own summary judgment is defensible, the committee intends to be as explicit as possible regarding the strengths and limitations of the biological data.
Published and Unpublished Data
Published reports that have been subjected to a rigorous peer review process carry the most weight in the committee’s assessment. In general, the committee cannot rely heavily on unpublished data in making its scientific assessments (regarding either causality or biological mechanisms) because they have not undergone a formal review and must therefore be interpreted with caution. Immunization safety studies and other data reviewed by the committee are funded by a variety of sources—NIH, CDC, vaccine manufacturers, research advocacy organizations, or foundations. The committee relies on editorial and peer review procedures to ensure the disclosure of potential conflicts of interest that might be related to the source of funding for the research study. The committee does not investigate the source of funding of the published research reports it reviews, nor does the funding source influence the committee’s interpretation of the evidence.
Unpublished data and other reports that have not undergone peer review do have value, however, and they are often considered by the committee; they might be used, for example, in support of a body of published literature with similar findings. If the committee concluded that the unpublished data were well described, had been obtained using sound methodology, and presented very clear results, the committee could report, with sufficient caveats in the discussion, how those data fit with the entire body of published literature. Only in extraordinary circumstances, however, could an unpublished study refute a body of published literature.
The committee acknowledges that its approach differs from the state of the art for evidence-based reviews of clinical practices in medicine, which does not include consideration of unpublished or non-peer-reviewed information or of studies with flawed experimental designs (U.S. Preventive Services Task Force, 1996). However, the Immunization Safety Review Committee was convened specifically to assess topics that are often of immediate and intense concern. In some cases, the committee’s review will take place as data are only beginning to emerge. Thus, given the unique nature of this project, the committee decided that it was important to review and consider as much information as possible, including unpublished information. The committee does not perform primary or secondary analyses of unpublished data, however. In reviewing unpublished material, the committee applies generally accepted standards for assessing the quality of scientific evidence, as described above. (All unpublished data reviewed by the committee and cited in this report are available—in the form reviewed by the committee—through the public access files of the National Academies. Information about the public access files is available at 202–334–3543 or www.national-academies.org/publicaccess.)
UNDER REVIEW: HEPATITIS B VACCINE AND DEMYELINATING NEUROLOGICAL DISORDERS
The Interagency Vaccine Group asked the Immunization Safety Review Committee to address the concern that the hepatitis B vaccine causes demyelinating neurological disorders. A plasma-derived vaccine was first marketed in the United States in the early 1980s, and it was supplanted later in the decade by vaccines produced with the aid of recombinant technology.
Concern about the safety of the hepatitis B vaccine emerged with an analysis of the first three years of post-marketing surveillance reports on various demyelinating neurological disorders following administration of the plasma-derived vaccine. Those reports suggested a possible association with Guillain-Barré syndrome (GBS) (Shaw et al., 1988). In the early 1990s, a previous IOM committee concluded that the evidence was inadequate to accept or reject a causal relationship between hepatitis B vaccine and either GBS or a general category of central nervous system demyelinating diseases (IOM, 1994). Concern about the vaccine and neurological disorders has persisted, focusing most prominently on the possibility of a causal link with MS, a central nervous system demyelinating disease. Concerns were very salient in France recently and led to immunization policy change (as discussed in a subsequent section of the report).
For this review, the committee addressed the relationship between hepatitis B vaccine and the following neurological diseases: the central nervous system (CNS) demyelinating diseases of MS (onset or relapse), acute disseminated encephalomyelitis (ADEM), optic neuritis, and transverse myelitis and the peripheral nervous system (PNS) demyelinating diseases of GBS and brachial neuritis. The committee chose to focus on these specific conditions because they are serious neurological disorders and known clinical entities. Published epidemiological studies and case reports investigating their association with hepatitis B vaccine are available, and a substantial body of literature exists on the pathophysiology of several of these conditions (e.g., MS, ADEM, and GBS). Key features of these diseases, and of hepatitis B infection and the hepatitis B vaccine, are described below.
The committee recognizes that this report addresses only a portion of the full range of concerns about the hepatitis B vaccine. In particular, some members of the public believe that the hepatitis B vaccine, which is first administered to many infants within hours of birth, is associated with infant death. The death of any child—whether following immunization or a vaccine-preventable disease—is tragic, and the committee agrees that such deaths are of intense concern. However, in the context of the current review, it is not clear that the putative association in such infant deaths is with an immune-mediated neurological assault. The committee notes that the Interagency Group on Vaccines has dis-
cussed the possibility of asking for a review, at a later time, of the question of newborn and infant death following vaccination.
The committee also is not assessing the possible role of hepatitis B vaccine in relation to undefined conditions with a neurological component, given the lack of information on which to base a causality assessment. The committee is aware of the case reports of disabling health effects experienced by some recipients of hepatitis B vaccine, but those reports do not offer adequate information for a meaningful review of either the health outcomes or the mechanisms by which the vaccine might produce those outcomes. The committee does not dismiss or undervalue the disability experienced by these people. Their suffering is real and information on the pathophysiology of these cases is needed. A detailed review of these individual cases would be required to begin gaining a meaningful understanding of the putative link between receipt of the hepatitis B vaccine and the subsequent onset of neurological dysfunction. The Immunization Safety Review Committee, however, is neither equipped nor charged to conduct such a review.
Adverse Neurological Events
The adverse events considered in this report are all diseases involving demyelination of nerve cell axons in either the central or peripheral nervous systems. Myelin, a principal component of what is referred to as the white matter of the nervous system, normally surrounds the axons of many nerve cells, providing protection and contributing to the transmission of signals through the nervous system. In the CNS (the brain, spinal cord, and optic nerves), myelin is synthesized by oligodendrocytes; in the PNS, it is synthesized by Schwann cells. Oligodendrocytes typically ensheath several axonal processes, and the expression of myelin genes by the oligodendrocytes appears to depend on the presence of astrocytes. In contrast, the external cell membrane of each Schwann cell surrounds a single axon, and the expression of myelin genes is regulated by contact between the axon and the myelinating Schwann cell.
Demyelination occurs when the sheaths around nerve cell axons are damaged by inflammatory or other injurious processes. The focus of this report is on autoimmune mechanisms of demyelination. This exposes the axon to the risk of injury and interferes with the transmission of nerve impulses. Remyelination can occur and is more effective in the PNS than in the CNS. This is probably related to the respective tissue environments, with peripheral nerves exposed to growth factors and other mediators that are not present in or are less accessible to the CNS (Waubant and Stuve, 2002).
Multiple Sclerosis
MS is the most common chronic inflammatory demyelinating disease of the CNS in humans. In the United States, approximately 300,000 individuals, about 0.1 percent of the population, have been diagnosed with the disease (Noseworthy et al, 2000). Women are affected approximately twice as often as men. The incidence of the disease is highest in persons between the ages of 20 and 40 years, but it is also diagnosed in children as young as 2 years and in older individuals. MS is more frequent in populations of Northern European origin than in other ethnic groups (IOM, 2001c). The prevalence of the disease is between 50 and 250 cases per 100,000 population in high-risk areas such as the Scandinavian countries or the northern United States, whereas it is less than 5 cases per 100,000 in Africa and Japan (Waubant and Stuve, 2002).
Clinically, MS is characterized by a variety of neurological signs and symptoms, reflecting the occurrence of inflammatory demyelinating lesions throughout the CNS. Common presenting symptoms include focal sensory deficits, focal weakness, a loss of vision, double vision, imbalance, and fatigue. Sexual impairment as well as urinary and bowel dysfunction may occur. Approximately 50 percent of patients with MS may display some degree of cognitive impairment and psychiatric symptoms. The severity of the disease can range from subclinical forms that are diagnosed only after death (from other causes) to hyperacute forms that lead to death within the first few months after disease onset. About 20 percent of patients have a “benign” form of the disease that is characterized by little accumulation of disability even after 10 or more years from the onset of the disease, but 50 percent of MS patients develop a significant limitation in their ability to walk and require assistance within 15 years (Noseworthy et al., 2000).
Four principal disease patterns have been identified (Lublin and Reingold, 1996). More than 80 percent of patients with MS initially experience a relapsing-remitting course, with clinical exacerbations of neurological symptoms that are followed by complete or partial recovery. Exacerbations can last from one day to several weeks. Incomplete recovery from relapses can result in accumulation of disability. Approximately 50 percent of patients with the relapsing-remitting form of MS will experience a more progressive course of the disease after 10 years. Patients with this secondary progressive course of MS experience a gradual worsening of their disability, with or without superimposed exacerbations. Another 10 to 15 percent of patients have primary progressive MS, a form associated with the gradual progression of symptoms from onset without exacerbation or remission. A very small proportion of patients (1–5 percent) experience a course called progressive relapsing MS, which is progressive from onset and includes a few superimposed exacerbations during the course of the disease.
The clinical diagnosis of MS requires evidence of recurrent episodes of clinical exacerbations (dissemination in time) that represent dysfunctions in dif-
ferent anatomic locations within the CNS (dissemination in space). The diagnosis is often established only after the second attack, with the clinical onset of the disease defined retrospectively as the first clinical attack. Biological changes detectable in neuroimaging studies can precede the first appearance of clinical symptoms, but the timing of the onset of those changes is difficult to establish. Relapses are defined by the clinical onset of new, recurrent, or worsening neurological symptoms related to CNS dysfunction that last for 24 or more hours in the absence of fever or infection.
Certain neuroimaging and laboratory tests can be used to support a diagnosis of MS, but none of the tests is specific for MS. Magnetic resonance imaging (MRI) is the most sensitive of these paraclinical diagnostic tests, detecting lesions in 95 percent of patients (Waubant and Stuve, 2002). Neuroimaging studies may show disease activity that is not reflected in clinical symptoms and disability; only lesions of sufficient size give rise to neurological symptoms. Examination of cerebrospinal fluid (CSF) can provide information about inflammation and immunological disturbances (McDonald et al., 2001). Two or more oligoclonal IgG bands are detected by electrophoresis in more than 80 percent of MS patients (Waubant and Stuve, 2002). Abnormal visual evoked potentials can also provide independent evidence of neurological lesions.
Diagnostic criteria have been established by clinical neurologists to facilitate the reproducible diagnosis of MS (Poser et al., 1983). A new set of diagnostic criteria account for the increasing contribution of MRI findings (McDonald et al., 2001). (See Box 1.)
The cause of MS remains elusive, but disease susceptibility appears to involve both genetic and environmental factors. Genetic factors are reflected in an increased risk of developing MS among family members of MS patients. For first-degree relatives, the risk of developing MS is 10–20 times higher than in the general population. Even so, their absolute risk remains small, only a 1–3 percent lifetime risk of developing the disease. The concordance rate among dizygotic (fraternal) twins and other siblings is 2–5 percent and it is 30–35 percent in monozygotic (identical) twins (Waubant and Stuve, 2002). Genetic studies of families with more than one MS-affected member indicate that the major histocompatibility complex (MHC) class II region on chromosome 6p21 is the most consistently identified susceptibility locus. The strongest association is with HLA-DR2 (DRB*1501, DQB*0602) (Oksenberg et al., 2001), but as many as 15 to 20 other loci may contribute to MS susceptibility. Some genes may affect susceptibility to developing the disease and others may modify the clinical expression of the disease or the response to disease-modifying treatments. Regional differences in the incidence of MS and in clinical disease patterns may reflect differences in the distribution of genetic risk factors.
|
BOX 1 McDonald Diagnostic Criteria for Multiple Sclerosis
|
|
||||||||
The possible role of environmental factors is indicated by several epidemiological studies that have suggested that individuals who migrate after age 15 from regions with a high prevalence of MS to regions with a low prevalence of the disease, or vice versa, carry their native risk for contracting MS (Alter et al., 1966; Kurtzke et al., 1970). This suggests that exposure to an environmental factor, possibly an infectious agent, during childhood is critical for the development of MS. Reports of localized “clusters” (defined areas of unexpected high prevalence) of MS also suggest that a transmissible agent may contribute to this
illness (Waubant and Stuve, 2002). But other analyses point to confounding factors that leave the role of environmental exposures uncertain (Noseworthy et al., 2000).
Acute Disseminated Encephalomyelitis
ADEM, an inflammatory demyelinating disease of the CNS that can result in permanent and severe neurological disability, occurs most commonly in children and adolescents. In contrast to MS, which involves recurring or progressive neurological consequences of demyelination, ADEM is normally defined as a monophasic disorder (that is, relapses do not occur) that results from a discrete episode of inflammatory demyelination. ADEM most often occurs following an infection, and it is associated with several viral pathogens, including the measles, rubella, and varicella zoster viruses. It is less commonly associated with the influenza, mumps, coxsackie B, Epstein-Barr, herpes simplex, human immunodeficiency, and human herpes-6 viruses. ADEM has also been reported following bacterial infections with Mycoplasma pneumoniae and Legionella cincinnatiensis. The incidence of ADEM after measles is approximately 1 case per 1,000 infections; the incidence after varicella and rubella is 1 case per 10,000 infections and 1 case per 20,000 infections, respectively (Stuve and Zamvil, 1999).
ADEM is also reported after vaccinations, for example, with receipt of the measles, mumps, and rubella vaccines, although causality is not certain (Stuve and Zamvil, 1999). The incidence of ADEM following receipt of live-virus measles vaccine is 1–2 per million, substantially lower than that following measles infection (Stuve and Zamvil, 1999). There are also case reports of confirmed or possible ADEM in recipients of the Semple rabies vaccine (Murthy, 1998), Japanese encephalitis vaccine (Plesner et al., 1998), and hepatitis B vaccine (Hynson et al., 2001, Tourbah et al., 1999). Post-immunization ADEM was originally associated with early rabies vaccines, which may have been contaminated with the animal CNS tissue then used to produce the vaccine antigen (Stuve and Zamvil, 1999). Exposure to the animal CNS tissue could have initiated the human equivalent of experimental autoimmune encephalomyelitis (EAE) (Waubant and Stuve, 2002).
ADEM usually affects infants and young children but has also been reported in middle-aged and elderly individuals. Neurological symptoms can begin during or shortly after an acute viral illness. Following a vaccination, clinical symptoms have been reported to occur after 1 to 3 weeks (Waubant and Stuve, 2002). Three recent case series suggest that the clinical presentation of the disease may differ with the age of onset (Dale et al., 2000; Hynson et al., 2001; Schwarz et al., 2001). Typically, patients initially develop fever and nonspecific respiratory illness. Common clinical features of ADEM include meningismus, ataxia, weakness, and spasticity. The most frequent symptoms in pediatric pa-
tients include headaches, seizures, and impaired conscious state and sometimes coma associated with respiratory distress; whereas adult patients often display milder neurological symptoms and, less often, fever and infectious symptoms. Transverse myelitis may represent a variant of ADEM restricted to the spinal cord. After a period of stabilization, patients frequently improve.
A diagnosis of ADEM is supported by findings of a high white blood cell count, an elevated sedimentation rate, and an indication of extensive subcortical white matter disease from MRI scans of the brain. The cerebrospinal fluid (CSF) often shows a mild lymphocytic pleocytosis and elevated levels of proteins. Elevated levels of IgG and the presence of oligoclonal bands on electrophoresis are frequently detected in the CSF of adult patients suspected of having ADEM, but are found less often in the CSF of pediatric patients. As in other conditions causing acute injury to CNS myelin, increased cellular immune responses to myelin basic protein have also been demonstrated.
Although ADEM is by definition a monophasic disease, case reports and case series note that some children and young adults experience neurological relapses after a period of complete or partial remission (Dale et al., 2000; Hynson et al., 2001; Stuve and Zamvil, 1999). This disease entity has been referred to as relapsing disseminated encephalomyelitis (Stuve and Zamvil, 1999) or multiphasic disseminated encephalitis (Dale et al., 2000). Relapses may be identical to or distinct from the presenting symptoms, and those that occur within 2 months of the original onset of ADEM should be considered as a continuation of the initial symptoms. Recurrences that occur years after the initial symptoms or years after discontinuation of steroids prescribed for treatment would meet criteria for relapsing-remitting MS (Hynson et al., 2001). Studies with conventional MRI and examination of CSF have failed to establish specific criteria to differentiate ADEM, MS, and other disseminated CNS white matter diseases, and there is no consensus on differentiating ADEM recurrences from MS relapses.
Optic Neuritis
Optic neuritis is caused by an inflammation of the optic nerve, with lesions occurring behind the orbit but anterior to the optic chiasm (IOM, 1994). Diagnosis of optic neuritis can be aided through MRI scans. Symptoms include rapid vision loss, pain associated with eye movement, dimmed vision, abnormal color vision, altered depth perception, and Uhthoff’s phenomenon—in which visual loss is associated with an increase in body temperature (IOM, 2001c). Symptoms generally worsen during the first three to seven days before improving (IOM, 2001c). The majority of cases resolve within a few weeks to months of onset.
Optic neuritis can occur as an isolated monophasic disease, or it may be a symptom of other demyelinating diseases such as ADEM or MS. Optic neuritis is frequently, though not always, followed by a diagnosis of MS. The risk for
developing MS within 15 years of an episode of optic neuritis is estimated to range from 45 to 80 percent (Purvin, 1998).
Transverse Myelitis
Transverse myelitis, which typically occurs after viral or bacterial infections may represent a variant of ADEM restricted to the spinal cord. Transverse myelitis is inflammation across the width of the spinal cord. The inflammation can cause myelin damage or demyelination, which leads to problems in nerve conduction from the spine to the rest of the body. Symptoms may begin as pain, weakness, or tingling, but then progress within weeks to paralysis, urinary retention, or loss of bowel control. Specific symptoms are dependent on the location of the myelitis. For example, if the thoracic region of the spinal cord is affected, symptoms will be present in the torso and parts of the arms. In addition, symptoms occur in areas controlled by parts of the spinal cord that fall below the area of inflammation. Using myelitis in the thoracic region again as an example, symptoms will also be present in the legs and bowel, and urinary functions, which are controlled by segments of the spinal cord below the thoracic region.
The exact cause of transverse myelitis is unknown, though viral infections, vaccination for rabies, autoimmune mechanisms, or insufficient blood flow through the spinal cord are some of the factors that may contribute to its development. Because no known cure exists for transverse myelitis, treatment is primarily devoted to symptom management and alleviation—through steroid treatment to control inflammation and through physical therapy—to maintain muscle strength and mobility.
Guillain-Barré Syndrome
GBS is the most common acquired peripheral demyelinating disease in humans (Waubant and Stuve, 2002). Its incidence is estimated at 1 to 2 cases per 100,000 population per year both in children and adults (IOM, 1994). GBS often occurs several days or weeks after an infectious event, commonly a diarrheal illness or a viral upperrespiratory infection. From 10 to 30 percent of all cases are associated with Campylobacter jejuni infections. Viral pathogens associated with GBS include human immunodeficiency virus (HIV), Epstein-Barr virus, and cytomegalovirus. An increased risk for GBS has also been linked to exposure to certain vaccines, most notably the 1976 influenza vaccine (the swine flu vaccine), the 1992–1993 and 1993–1994 influenza vaccine (Lasky et al., 1998), and rabies vaccines produced from nervous tissue of infected animals (used outside the United States). A previous IOM committee concluded that the evidence favored acceptance of a causal relation with receipt of vaccines containing tetanus toxoid and with receipt of the oral polio vaccine (IOM, 1994). A study (Rantala et al., 1994) published after the release of that IOM report suggests to some
that the relationship with the oral polio vaccine is not, in fact, causal (Sutter et al., 1999). Other risk factors appear to include surgery and malignant disorders, especially lymphomas (IOM, 1994).
The characteristic clinical feature of GBS is an acute, rapidly progressive, ascending, and symmetric weakness, with loss of deep tendon reflexes and possible tingling in the feet and hands, and muscle aches (myalgia). Facial, oculomotor, oropharyngeal, and respiratory muscles may also be involved, and some patients may require respiratory support. The severity of clinical deficits typically peaks within the first 2 weeks of onset, but some deficits may continue to progress for 3 to 4 weeks. Most patients will improve and return to normal functioning within 6 to 9 months, but some patients experience relapses or a prolonged disease course with residual neurological deficits.
Though there is no known cure for GBS, treatment with plasmapheresis (also called plasma exchange) is an effective means for relieving symptoms and aiding recovery (Raphael, 2001). During plasmapheresis, the plasma, which contains antibodies thought to aggravate GBS, is removed from a patient’s blood and temporarily replaced by albumin. Additional treatments in use are intravenous immunoglobulin (IVIG) and corticosteroid administration. IVIG has been demonstrated to aid recovery, though not faster than plasmapheresis (Hughes et al., 2001). Previously, corticosteroid treatment was thought to benefit GBS patients, though a recent review did not demonstrate significant improvements in disability (Hughes and van der Meche, 2000).
Peripheral Neuritis
Peripheral neuritis or neuropathy refers to a collection of disorders that damage the peripheral nerves. The damage may occur to a specific nerve or nerve group, as well as multiple nerve groups, and can lead to nerve destruction or demyelination. Guillain-Barré syndrome (GBS) and brachial neuritis are two examples of such conditions. GBS is described above. Brachial neuritis is characterized by a deep, severe pain in the shoulder and upper arm. The pain generally subsides within days or weeks, but weakness and muscle atrophy in the affected arm are common side-effects. Although brachial neuritis usually occurs on one side of the body, it may be bilateral. Brachial neuritis can be treated with analgesics and physical therapy (McCarty et al., 1999; Miller et al., 2000).
Hepatitis B Virus Infection and Hepatitis B Vaccines
The hepatitis B virus (HBV) can produce an acute or chronic3 infection causing inflammation of the liver. Symptoms of acute HBV infection include
jaundice, fatigue, joint pain, abdominal pain, loss of appetite, nausea, and diarrhea. About 30 percent of adult infections are asymptomatic (Coleman et al., 1998), but they can become chronic. Among infants infected at birth, the risk of chronic infection is 90 percent; among persons infected as adults, about 6 percent develop chronic infections (CDC, 2001a). Chronic carriers of HBV are at increased risk for cirrhosis and hepatocellular carcinoma (HCC) several decades after infection4, and 15–25 percent of them die from liver disease (Lee, 1997). Cirrhosis predisposes an individual to HCC, and hepatitis-induced5 cirrhosis poses a greater risk than does alcohol-induced cirrhosis (El-Serag, 2001). Worldwide, chronic HBV infection is the most common cause of HCC, and chronic carriers have a five- to fifteen-times greater risk of developing HCC than the general population (El-Serag, 2001).
Rates of hepatitis B infection vary widely throughout the world. The highest prevalence is in some regions of Southeast Asia, China, and Africa, where over half of the population will contract acute hepatitis B infection. Of these infections, approximately 8 percent become chronic (Lee, 1997). Parts of Southern and Eastern Europe, the Middle East, Japan, Western Asia, and areas of Central and South America have an intermediate prevalence (2–7 percent) of chronic HBV infection (Maddrey, 2000). The prevalence of chronic infection in North America, Western Europe, and Australia is less than 2 percent (Maddrey, 2000), but rates of infection are higher among foreign-born populations who have come from high-prevalence countries.
HBV is transmitted through bodily fluids, with the highest viral concentrations found in blood, serum, and wound exudates (Halsey, 2002). Semen, vaginal fluid, and saliva have moderate viral concentrations. HBV can remain viable outside the body for more than 7 days (Mast, 2002) and its relative infectivity is 100 times greater than that of HIV (Hilleman, 2001). Newborns can be infected by vertical transmission of the virus from an infected mother (also called perinatal transmission). Children can also be infected by horizontal transmission of the virus through contact with an infected family member or contaminated articles in the household. Other routes of horizontal transmission include sexual contact, intravenous drug use and needle sharing, and occupational exposure to bodily fluids of infected persons.
A vaccine against HBV first became available in the United States in 1982. This vaccine used inactivated alum-adsorbed hepatitis B virus surface antigen (HBsAg) particles purified from human plasma from persons with chronic HBV infections. Plasma-derived hepatitis B vaccine is no longer produced in the
United States, but such vaccines are still being produced in other countries. The recombinant hepatitis B vaccines in use in the United States since the late 1980s are produced using Saccharomyces cerevisiae (baker’s yeast), into which a plasmid containing the gene for HBsAg has been inserted (CDC, 1990). The resulting vaccine consists of HBsAg protein adsorbed to aluminum hydroxide. Some recombinant vaccines used in other countries are produced using other methods. Three doses of hepatitis B vaccine are required for full immunization. In the United States, current recommendations call for universal immunization of children, with doses administered at birth, at 1 to 2 months, and at 6 to 18 months. For children born to mothers who are HBsAg-negative, the first two doses can be given at 1 to 2 months and 4 months, respectively.6 Immunization is also recommended for all unimmunized adolescents and for adults at high risk of exposure to HBV. High-risk adults include, but are not limited to, health care workers and public-safety workers who have exposure toblood in the workplace, hemodialysis patients, household contacts and sex partners of HBV carriers, and adoptees from countries where HBV is endemic (CDC, 1991).
SCIENTIFIC ASSESSMENT
Causality
As has been specified, the committee’s review focused on six possible adverse outcomes: multiple sclerosis, acute disseminated encephalomyelitis, optic neuritis, transverse myelitis, Guillain-Barré syndrome, and brachial neuritis.
Most of the epidemiological evidence identified by the committee examines the relationship between exposure to hepatitis B vaccine and the incidence of newly diagnosed cases (incident cases) of MS or of a first episode of a CNS demyelinating disorder (which can be consistent with MS but not yet meet criteria for a diagnosis of MS). One study examined the risk for relapse in patients with diagnosed MS. Some of the studies on MS also examined the risk for other adverse outcomes, but overall, evidence regarding these outcomes is limited. In the one study regarding GBS, the vaccine exposure was to the plasma-derived hepatitis B vaccine, which is no longer produced in the United States. The evidence regarding ADEM, transverse myelitis, and brachial neuritis was primarily case reports.
The case reports are discussed first, followed by a more detailed review of the epidemiological studies available for each outcome. Because the committee viewed MS as the condition of greatest concern, that evidence is reviewed first, followed by the evidence for the more general category of CNS demyelinating disorders and then the other specific outcomes. A table summarizing the controlled epidemiological studies and the committee’s conclusions appears at the end of each outcome (see Tables 1, 2, 3).
Case Reports
Case reports are useful for describing the domain of concerns, but the data are usually uncorroborated clinical descriptions that are insufficient to permit meaningful comment or to contribute to a causality argument. Case reports can, however, lead to hypothesis generation, and the committee values their potential utility in this regard. Case reports of “challenge-rechallenge” regarding a person who received a vaccine (or drug or other challenge) more than once and reacted adversely with the same disorder each time can contribute to the causality assessment. For a challenge-rechallenge case to weigh heavily in a causality assessment, there must be certainty that the diagnosis is correct and that alternative etiological factors have been excluded.
The committee identified two published case reports of neurological disease following challenge and rechallenge with hepatitis B vaccine. The first pertains to a woman diagnosed with leukoencephalitis7 following each of her second and third shots of recombinant hepatitis B vaccine (Konstantinou et al., 2001). In general, the documentation is inadequate for this case report to provide evidence of causality. In particular, laboratory data suggestive of an immune reaction is not supplied. The second case report pertains to sensorineural deafness (Biacabe et al., 1997). Although the patient appears to have suffered hearing loss twice after vaccination, the time frame is too short to be suggestive of an immunemediated reaction. Documentation for this case report was insufficient to understand if there is an immune mechanism operating in the disease process. Finally, a tabulation prepared by CDC of case reports submitted to the Vaccine Adverse Events Reporting System (VAERS) between 1990 and 2001 lists eight cases stating evidence of rechallenge adverse events. These included a variety of clinical descriptions, including neuropathy, MS, and brachial neuritis. None of the challenge-rechallenge cases provides the level of laboratory or diagnostic detail to be contributory to a causality argument.
VAERS received several reports of demyelinating disease following hepatitis B vaccination from November 1, 1990 through December 31, 2001 (CDC, 2002). There were 408 unique reports (foreign reports were excluded). The re
ports were classified according to seven outcome categories based on indexing terms (COSTART) found in the reports, not diagnostic or medical coding terms. Reports included 125 cases of MS, 15 cases of brachial neuritis, 83 cases of optic neuritis, 46 cases of peripheral neuritis, 91 cases of GBS, 30 cases of ADEM or demyelinating disease not otherwise specified, and 109 cases of myelitis. Most of these reports were included in only one outcome category, but some (73) were classified in more than one.
The published medical literature provides case reports of several demyelinating diseases following hepatitis B vaccination. These range, for example, from optic neuritis in a 28-year-old man (Albitar et al., 1997), to GBS in a 45-year-old woman (Creange et al., 1999), and transverse myelitis in a 40-year-old health care worker (Tartaglino et al., 1995).
Multiple Sclerosis
Uncontrolled Observational Study: Incident MS
British Columbia, Canada. In a letter, Sadovnick and Scheifele (2000) reported on an ecological study to examine the incidence of MS in adolescents 11–17 years old in the periods immediately before and after the 1992 implementation of a hepatitis B vaccination program for students in grade 6 (11- and 12-year-olds) in British Columbia, Canada. The prevaccination study population consisted of an estimated 288,647 students who attended grade 6 between January 1986 and September 1992, representing 1.14 million person-years of observation. The postvaccination observation period ran from October 1992 to September 1998. The British Columbia Centre for Disease Control determined that 92.3 percent of the 289,651 students who attended grade 6 during that time completed the vaccination series, providing 966,000 person-years of observation. Data on MS cases were obtained from the medical records of the only pediatric hospital in the province, the database of the provincial MS clinic, and pediatric neurologists in the province.
A total of nine MS cases with adolescent onset occurred in the prevaccination period, and five cases occurred in the postvaccination period. The authors reported that a χ2 test showed that the difference was not statistically significant. They concluded that these data provided no evidence associating hepatitis B vaccination with an increased risk for onset of MS during adolescence.
Controlled Observational Studies: Incident MS
United States-Nurses’ Health Study. Ascherio and colleagues (2001) studied the relationship between hepatitis B vaccination and the risk of MS in women participating in the Nurses’ Health Study and the Nurses’ Health Study II, using a nested case-control design. Potential cases were initially identified on the basis of self-reports of having received a diagnosis of MS from a physician. The treating physician (neurologist or internist) confirmed the certainty of the
diagnosis (definite, probable, possible, or not MS), and provided the information on the patient’s clinical history and laboratory results. The Poser criteria (Poser et al., 1983) were applied to validate the physicians’ diagnoses. Women with a diagnosis made after April 1, 1998, were excluded from the study. Controls were randomly selected. Each woman with MS had five healthy controls (no history of MS or breast cancer) and one control with breast cancer (to test for recall bias among women with a serious disease). Controls were matched according to birth year, study cohort, and date of diagnosis (for controls with breast cancer). The date of the first neurological symptoms was used as the date of onset of MS, which served as the index date for both cases and their matched controls.
Information on exposure to hepatitis B vaccine was obtained from cases and controls through a mailed questionnaire. The response rate was 95 percent for women with MS and 88 percent for the controls. Women who reported never having been vaccinated were considered not exposed. For women who reported vaccination against hepatitis B, employer records were used to verify vaccine receipt and the date(s) of vaccination. Women who reported exposure to hepatitis B vaccine but whose vaccination records could not be obtained (35 percent of cases and 35–37 percent of controls) were excluded from the study. Subjects who were left unmatched were also excluded. This left a study population of 192 women with incident MS (definite or probable), 534 healthy controls, and 111 controls with breast cancer.
Conditional logistic regression was used to calculate the relative risk of MS for women who had been vaccinated compared with those who had not. Two risk intervals were analyzed: receipt of the first dose of hepatitis B vaccine within two years of the index date and receipt of at least one dose of the vaccine at any time before the index date. The analyses with the healthy controls showed a relative risk of MS of 0.7 (95% CI 0.3–1.7) for vaccination within two years of the index date and 0.9 (95% CI 0.5–1.6) for vaccination any time before the index date. The results of multivariate analyses (adjustment for ancestry, place of birth, smoking history, and history of certain infections) were similar. The analyses with controls with breast cancer produced a relative risk of MS of 1.0 (95% CI 0.3–4.2) with vaccination within two years of the index date and 1.2 (95% CI 0.5–2.9) with vaccination any time before the index date. As with the healthy controls, the results of the multivariate analyses for the two risk intervals were similar.
To examine the effect of the introduction of the recombinant hepatitis B vaccine in 1987, the analysis was limited to women with onset of MS after 1986. The relative risk of MS was 0.6 (95% CI, 0.2–1.5) for the women who were vaccinated within two years of the index date and 0.7 (95% CI, 0.4–1.3) for those who were vaccinated any time before the index date. Similarly, no elevation of risk for MS was found when the analysis was limited to the women in the Nurses’ Health Study II cohort, who are younger and more likely to have re-
ceived the hepatitis B vaccine, or when self-reported vaccination dates were used to include women with missing vaccination records in the analysis.
The authors concluded that the data do not support an association between hepatitis B vaccination and risk of multiple sclerosis. The authors note that selection bias was minimized by the nature of nested case-control studies and the high response rates, which were similar both for cases and controls. Recall bias was minimized by the inclusion of only those women who had vaccination records. The wide two-year exposure interval helped minimize the effect of potential inaccuracies in the estimation of the date of onset of MS. A limitation noted by Dr. Ascherio at the committee’s March 2002 meeting was the lack of power to detect an increase in the risk of MS within two months after vaccination. He commented, however, that even if demyelination occurred within two months, it might take several months or years for clinical symptoms to become apparent.
France. In 1998, Fourrier and colleagues (1999) conducted a multicenter case-control study to examine the association between hepatitis B vaccination and MS. An unpublished update of the study, by Bégaud and colleagues, is currently in press and was presented to the committee at its March 2002 meeting (Fourrier and Bégaud, 2002). Seventeen French neurology departments participated in the study. Eligible subjects were identified through neurologists’ review of the medical records of all inpatients and outpatients seen at the participating neurology departments between January 1994 and December 1995.
Cases were defined as patients who had their first CNS demyelinating episode within six months before their examination. The definition of a demyelinating episode was similar to that of an episode of MS. Controls (two per case) were matched by center, age, sex, and date of first examination at center and included patients with conditions such as migraine, noninflammatory rheumatological diseases, vascular diseases, or other neurological diseases that were not likely to affect the probability of vaccination. The records of possible cases and controls were reviewed and verified by independent experts. There were 236 cases and 355 controls eligible for analysis; 193 cases (matched to 297 controls) had a definite or probable diagnosis of MS. The mean age was 33.6 years for cases and 34.2 years for controls.
Information about vaccine exposure was obtained through telephone interviews. Included in the vaccination history was exposure to one or more doses of hepatitis B vaccine as well as to hepatitis A, tetanus, poliomyelitis, diphtheria, yellow fever, influenza, meningitis, tuberculosis, and cholera vaccines. A total of 127 definite or probable MS cases and 217 controls provided vaccination certificates to document their vaccine exposure.
Conditional logistic regression was used to calculate adjusted odds ratios. Considering only subjects with documented hepatitis B vaccine exposure and cases with definite or probable incident MS, the adjusted odds ratio for a first CNS demyelinating episode within two months of hepatitis B vaccination was 1.6 (95% CI 0.4–5.6). (Results for all cases are discussed below in the section on
first episode of a CNS demyelinating disorder.) The authors concluded that the results demonstrate no strong association between the receipt of hepatitis B vaccine and the occurrence within two months of a first demyelinating episode in adults. However, the authors noted that because of low statistical power, the study cannot exclude the possibility of a small increase in risk.
United States-Vaccine Safety Datalink. At the committee’s March 2002 meeting, DeStefano (2002) presented unpublished findings from a case-control study examining the relationship in adults between hepatitis B vaccination and the development of central nervous system demyelinating diseases, specifically MS and optic neuritis. The study also examined the risk related to the timing of vaccination. The results for MS are discussed here and those for optic neuritis are discussed below. Data for both cases and controls were obtained from three health maintenance organizations (HMOs) that participate in CDC’s Vaccine Safety Datalink (VSD) project. Automated outpatient and hospital discharge data for 1995–1999 were screened, and cases were confirmed by review of medical records. Cases were defined as having a physician diagnosis of MS or optic neuritis (ON) on their medical records or a diagnosis by a specialist, or as meeting the International Panel criteria for MS (two demyelinating episodes separated in space and time). Up to three controls were selected for each case and matched according to year of HMO enrollment (minimum of one year enrollment), age, and sex. Patients who had a prior diagnosis of MS or ON in their medical charts were excluded. A total of 440 cases (332 with MS and 108 with optic neuritis) and 950 controls participated in the study.
Hepatitis B vaccine exposure was determined on the basis of medical chart reviews and telephone interviews for those vaccinated outside the HMO. Exposure was categorized as ever or never vaccinated before the index date (i.e., the date of onset for the matched case). The time intervals between vaccination and the index date were 0–1 year, 1–5 years, and more than 5 years. Of the cases, 7.7 percent had received a hepatitis B vaccination before the index date; of the controls, 8.1 percent had been vaccinated.
Odds ratios were calculated using a conditional logistic regression stratified by matching variables and adjusted for family history, race and ethnicity, place of birth, Scandinavian ancestry, smoking, and marital status. Using the case definition based on the presence in the medical record of a physician diagnosis of MS, the risk of MS following hepatitis B vaccination was OR=0.8 (95% CI, 0.5–1.4). Similar results were obtained using case definitions based on specialist diagnosis (OR=0.9, 95% CI, 0.5–1.5) or on the International Panel criteria (OR =0.8, 95% CI, 0.4–1.4). The adjusted odds ratios for timing of hepatitis B vaccination and risk of demyelinating disease was 0.8 (95% CI, 0.4–1.8) for less than one year before index date, 1.6 (95% CI, 0.9–3.0) for one to five years before the index date, and 0.6 (95% CI, 0.2–1.4) for more than 5 years before the index date.
The authors concluded that the results do not support the hypothesis that hepatitis B vaccination causes or triggers the development of MS. The authors cite as strengths of the study identifying cases and controls from the large HMO population covered by the VSD project, minimizing recall bias by focusing on recently diagnosed cases, using medical records to establish the timing of onset of MS and of vaccination, and having obtained consistent results with different case definitions. A limitation of the study was the need to rely on self-report to obtain information on vaccinations obtained outside the HMOs. About half of both cases and controls received such vaccinations, but excluding the self-reported data had little effect on the results.
Other Studies: Passive Surveillance Data
Cases of demyelinating disease reported to VAERS and to other passive surveillance systems are briefly mentioned in this and the following sections. Because of the limitations of passive surveillance data like VAERS (Ellenberg and Chen, 1997; Singleton et al., 1999), the committee concluded that these reports were not informative on the issue of causality.
VAERS. A total of 408 unique reports of demyelinating disease following hepatitis B vaccination were received from November 1, 1990 through December 31, 2001 (foreign reports were excluded). Of these, a total of 125 cases of MS were reported8.
Controlled Observational Study: Multiple Sclerosis Relapse
Vaccines in Multiple Sclerosis (VACCIMUS)-France, Spain, and Switzerland. Confavreux and others (2001) conducted a multicenter case-crossover study to examine whether vaccination increases the risk of relapse in MS. The study subjects were MS patients from neurology departments associated with the European Database for Multiple Sclerosis network. With the case-crossover design, patients served as their own controls. Those eligible for the study had a definite or probable diagnosis of MS according to the Poser criteria (Poser et al., 1983) and had at least one relapse between January 1993 and December 1997. The index relapse was the first during this period that was confirmed by a medical visit or hospitalization and that was preceded by a relapsefree period of 12 months. Neurologists reviewed patients’ medical records to confirm the diagnosis of MS and categorized the index relapse as either definite, probable, or possible. A total of 643 subjects were included in the study.
Vaccination histories during the period January 1992 through December 1997 were collected from study subjects by telephone interview and were confirmed with written documentation, usually a copy of the vaccination record. Vaccine exposures included hepatitis B, tetanus, influenza, hepatitis A, typhoid,
yellow fever, typhoid-paratyphoid, tetanus-poliovirus, tetanus-diphtheria, and tetanus-poliovirus-diphtheria vaccines. During the 12 months before the index relapse, 135 subjects had a confirmed vaccination of any sort, and 39 had a confirmed hepatitis B vaccination. Hepatitis B vaccination exposure was assessed in terms of a two-month risk period immediately before the index relapse and four two-month control periods during the 10 months preceding the index relapse.
A conditional regression analysis was used to calculate the relative risk of MS relapse associated with exposure to the hepatitis B vaccine or to other vaccines. For hepatitis B vaccination, the relative risk of relapse was 0.67 (95% CI, 0.20–2.17); the relative risk of relapse associated with any vaccine exposure was 0.71 (95% CI, 0.40–1.26). The authors concluded that vaccination does not increase the short-term risk of a relapse among patients with MS who had been relapse-free for at least 12 months. However, the authors noted that the study findings are inconclusive with regard to long-term risks. Limitations cited by the authors include lower power for assessing risks associated with specific vaccines, exclusion of those patients with frequent or minor relapses, and an assumption of constancy of vaccine exposure and equality of risk after each exposure. Study strengths included limited confounding by the nature of the case-crossover study design, high response rates and validation of vaccine exposures, limited recall bias through collection of exposure data without specific reference to the index relapse, and results that are unaffected by a change in length of effect periods.
Causality Argument
For risk of incident MS in adults, the committee reviewed three controlled studies, of which one was published (Ascherio et al., 2001), and the other two were unpublished (DeStefano et al., 2002; Fourrier and Bégaud, 2002) (see also Table 1). Fourrier and Bégaud’s (2002) presentation to the committee was an unpublished update of data from a published abstract by Fourrier and others (1999). Given the strengths in the study design of the published study, and the consistent finding from all the studies that there is no effect of hepatitis B vaccine on incident MS, the committee concludes that the evidence favors rejection of a causal relationship between hepatitis B vaccine administered to adults and incident multiple sclerosis. For risk of MS relapse in adults, the committee reviewed one controlled published study (Confavreux et al., 2001) which reported no effect of hepatitis B vaccine on MS relapse in adults.
On the basis of this well-designed study, the committee also concludes that the evidence favors rejection of a causal relationship between hepatitis B vaccine administered to adults and multiple sclerosis relapse.
There are no controlled epidemiological data regarding the relation of hepatitis B vaccination to the risk for MS in young children. The ecological study from British Columbia (Sadovnick and Scheifele, 2000) regarded children 11–
17 years of age, and its relevance to infants and young children is not clear. Over 200 million doses of hepatitis B vaccine have been sold and distributed to in the United States since 1990, and no reports have been submitted to the VAERS concerning MS in children under age 6 years of age (CDC, 2002). For other neurological conditions following hepatitis B vaccination among children in this age group, VAERS has received 1 report each of brachial neuritis and optic neuritis, 8 reports of GBS, 6 reports of myelitis, and none of peripheral neuritis. Although underreporting to VAERS is substantial and MS occurs so rarely in infants and children, the lack of VAERS reports of MS in infants and children might indicate that MS is not occurring subsequent to hepatitis B vaccination in infants and children. However, the committee cannot extend the causality conclusion for MS, which is based on epidemiological studies in adults, to include the risk to infants and young children.
First Episode of a Central Nervous System Demyelinating Disorder
Controlled Observational Studies
France-Single Center Study. Touzé and others (2000) conducted a case-control study to examine the relationship between the hepatitis B vaccination and the onset of a first episode of central nervous system demyelination. Cases and controls were identified by a review of medical records for hospitalization or consultation at the Neurology Federation of the Hôpital de la Pitié-Salpêtriére in Paris, France. Cases were seen between January 1, 1994, and December 31, 1995, and had a first episode of CNS demyelination between July 1, 1993, and December 31, 1995. Controls were selected from patients who had been seen between January 1, 1995, and December 31, 1995, for conditions unrelated to vaccination and that were not a contraindication for vaccination. Controls were matched to cases by age, sex, and date of consultation or hospitalization. A total of 121 cases and matched controls were included in the analysis.
The case definition for a first CNS demyelinating episode was as follows: (1) neurological symptoms, reported to the physician, showing evidence of an attack on the CNS and compatible with an attack on the white matter; (2) symptom duration of 24 hours or longer; (3) possibly combined with abnormalities on various complementary tests (e.g., MRI, examination of CSF); or (4) absence of other explanation. The index date for each case-control pair was the date of onset of the case’s CNS demyelinating episode.
Information on vaccination history between January 1993 and December 1995 was collected using a mailed questionnaire and confirmed with a copy of the subject’s vaccination certificate or by phone interview. The vaccine exposures recorded in addition to hepatitis B included hepatitis A, tetanus, poliomyelitis, diphtheria, typhoid, yellow fever, meningococcus, influenza, and BCG. The risk associated with exposure to one or more doses of hepatitis B vaccine was tested for two time intervals: 0–60 days and 61–180 days before the index date.
Conditional logistic regression was used to calculate the odds ratio for the risk for onset of a first episode of CNS demyelination following hepatitis B vaccination, adjusted for age, marital status, country of birth, and living environment. For the postvaccination time interval of 0–60 days, the adjusted odds ratio was 1.7 (95% CI 0.5–6.30); for the interval of 61–180 days, the adjusted odds ratio was 1.5 (95% CI 0.5–5.3). The authors concluded that the results do not demonstrate an association between hepatitis B vaccination and the onset of the first episode of CNS demyelination. The low statistical power of the study (35% power to detect a relative risk of 2), however, made it difficult to exclude the possibility of such an association. The authors also noted that selection and recall biases may have been present in the study, but the direction or size of their effects cannot be determined. Although selection of cases and controls was independent of vaccination history, cases were more likely to live in rural areas where hepatitis B vaccination is less common. The authors note that controls from the general population might have been more suitable but that the likelihood of lower response rates could have introduced other selection biases.
France-Multicenter Study. The multicenter case-control study reported by Fourrier and others (1999) and Fourrier and Bégaud (2002) is discussed in more detail above. Described here are the data and findings related to the risk of a first episode of a CNS demyelinating disorder. Such episodes were defined as neurological symptoms compatible with CNS white matter lesions, lasting at least 24 hours, and leading to a medical consultation (Fourrier and Bégaud, 2002). A total of 236 cases and 355 matched controls were identified through a neurologist’s review of the medical records of all inpatients and outpatients seen at the participating neurology departments between January 1994 and December 1995. Cases were defined as patients who had their first CNS demyelinating episode within six months before their examination. Information on vaccine exposure was obtained through telephone interviews and confirmed with vaccination certificates for 152 cases and 253 controls.
A conditional logistic regression was used to calculate adjusted odds ratios for risk of a first episode of CNS demyelinating disorder following hepatitis B vaccination. For subjects with a vaccination certificate, the odds ratio for the period 0–2 months following vaccination was 1.4 (95% CI 0.4–4.5), and for 2– 12 months postvaccination, it was 1.0 (0.6–1.9). The authors concluded that the results demonstrate that there is no strong association between receipt of the hepatitis B vaccine and occurrence of a first demyelinating episode in adults, but because of low statistical power the study cannot exclude the possibility of a small increase in risk.
United States-Vaccine Safety Datalink. As described above, at the committee’s March 2002 meeting DeStefano (2002) presented unpublished findings from a case-control study using the VSD to examine the relationship in
TABLE 1 Evidence Table: Controlled Observational Studies—Hepatitis B Vaccine Exposure and Incident MS and MS Relapse
|
Citation |
Design |
Population |
Vaccine Assessment |
Outcome |
Results |
Comment |
Contribution to Causality Argument |
|
Ascherio et al. (2001) Published Paper |
Nested casecontrol |
192 cases 645 matched (birth year, study cohort, and date of diagnosis) controls (534 healthy controls and 111 controls with breast cancer). (Nurses’ Health Study I and II, United States). |
Receipt of at least 1 dose of Hep B. Self-reported by mailed questionnaire. Verified vaccination date by reviewing employer records. |
Definite or probable MS diagnosis. Self-reported MS. Confirmation by treating neurologist. Incident MS diagnosed before 4/98. Certainty of diagnosis based on neurologists’ judgment and validated by applying Poser criteria to clinical and lab results. |
RR (95% CI), Healthy Controls Hep B w/in 2 yrs: 0.7 (0.3–1.7) any time before index date: 0.9 (0.5–1.6) Multivariate Hep B w/in 2 yrs: 0.7 (0.3–1.7) any time before index date: 0.8 (0.5–1.5) Controls w/breast cancer Hep B w/in 2 yrs: 1.0 (0.3–4.2) any time before index date: 1.2 (0.5–2.9) Multivariate Hep B w/in 2 yrs: 1.3 (0.3–6.1) any time before index date: 1.3 (0.5–3.7) Women with onset of MS after 1986 (healthy controls) Hep B w/in 2 yrs: 0.6 (0.2–1.5) |
Authors note that selection and recall bias minimized by study design, similar response rates in cases and controls, and inclusion of women who had vaccination records. Wide 2-year exposure minimized effect of inaccuracies in estimating date of onset of MS. |
The study suggests no association between Hep B and incident MS in women |
|
Citation |
Design |
Population |
Vaccine Assessment |
Outcome |
Results |
Comment |
Contribution to Causality Argument |
|
|
any time before index date: 0.8 (0.4–1.4) Multivariate Hep B w/in 2 yrs: 0.5 (0.2–1.5) any time before index date: 0.7 (0.4–1.3) |
|
|||||
|
Fourrier and Bégaud, (2002) Unpublished Update/ Presentation to the Committee |
Case-control |
127 Cases 217 Controls. CASES: First episode of CNS DD w/in 6 months prior to examination. CONTROLS: (Two per case) Matched by center, age, sex, and date of first examination at center and had conditions that were not likely to affect probability of vaccination. (Multi-center study, France) |
Receipt of 1 or more doses of Hep B. From telephone interviews verified upon review of certificate provided by subjects. Exposure to other vaccines also assessed. |
Definite or probable MS dx. Patients seen from 1/1/94– 12/31/95 in 17 French neurology departments or first episode of CNS DD w/in 6 months prior to examination. Cases and controls identified by neurologists from review of medical records and verified by independent experts. |
Adjusted OR (95% CI) First CNS DD w/in 2 months of Hep B 1.6 (0.4–5.6) Other vaccines: ORs were close to one and not significant |
Authors noted that because of low statistical power, the study cannot exclude the possibility of a small in crease in risk. The presentation is an updated analysis of data from a published abstract by Fourrier et al. (1999). The manuscript version of this update is in press (Begaud et al., in press). |
The study does not suggest an association between Hep B and incident MS in adults but its unpublished status limits its contribution to the causality argument. |
|
Citation |
Design |
Population |
Vaccine Assessment |
Outcome |
Results |
Comment |
Contribution to Causality Argument |
|
DeStefano (2002) Unpublished Presentation to the Committee |
CaseControl |
332 Cases (N/A) Matched Controls. CASES: Physician diagnosis of MS on medical records or diagnosis by specialist, or as meeting International Panel criteria for MS. CONTROLS: Up three per case and matched according to year of HMO enrollment, age, and sex. (Vaccine Safety Datalink, U.S.) |
Receipt of one or more doses of Hep B before index date. Medical chart review and telephone interview for non-HMO vaccinated CASES: 7.7% had Hep B before index date. CONTROLS: 8.1% vaccinated. |
Physician dx of MS in medical record. Potential cases found from screening outpatient and discharge data from three HMOs for 1995–99. 556 confirmed by chart review, 440 cases (MS or ON) contacted and participated in telephone interview. |
Adjusted OR (95%CI) Ever vs. Never vaccination MD Dx: 0.8 (0.5– 1.4) Specialist Dx: 0.9 (0.5–1.5) Int’l Panel Criteria: 0.8 (0.4–1.4) |
Authors cite study’s strengths as including study sample from large HMO population, minimized recall bias by using recently diagnosed cases, use of medical records to establish timing and onset of MS and of vaccination, and consistent results using different case definitions. Limitations include reliance on selfreported information from subjects who were vaccinated outside HMO. Exclusion of self-reported date had little effect on results. |
The study suggests no association between Hep B and incident MS in adults but its unpublished status limits its contribution to the causality argument. |
|
Confavreux et al. (2001) Published Paper |
Casecrossover |
643 subjects with MS (definite or probable) and at least one index relapse. CASES: Patients |
Vaccination history collected from study subjects by telephone interview and confirmed with written medical |
Relapse in patients with diagnosed MS between 1/93– 12/97. Neurologist cate |
RR (95% CI): Received vaccine in 2 months prior to relapse Hep B=0.67 (0.20– 2.17) |
Authors note that findings were inconclusive with regard to long-term risks. Study limitations include lower power for assessing risks associated with specific vaccines, |
The study suggests no association between Hep B and MS relapse in adults. |
|
Citation |
Design |
Population |
Vaccine Assessment |
Outcome |
Results |
Comment |
Contribution to Causality Argument |
|
|
who experienced an index relapse between 1/93– 12/97 with 12 mos. prior, being relapse-free. CONTROLS: Same patients but at 8t months prior to risk period where no relapse occurred. (European Database for Multiple Sclerosis Network; France, Spain, Switzerland). |
documentation. Vaccines included HepB, tetanus, influenza, Hep A, typhoid, yellow fever, typhoid-paratyphoid, tetanus-poliovirus, tetanusdiphtheria, or tetanus-poliovirus-diphtheria |
gorized relapse as either definite, probable, or possible. Confirmed by neurologist through review of medical files. |
|
exclusion of patients with frequent or minor relapses, and assumptions of constancy of vaccine exposure and equality of risk after each exposure. Study strengths include limited confounding by nature of study design, high response rates and validation of vaccine exposures, limited recall bias, and results unaffected by change in length of effect periods. |
|
|
|
CI: confidence interval CNS DD: central nervous system demyelinating disorder Dx: diagnosis Hep A: hepatitis A vaccine Hep B: hepatitis B vaccine |
MS: multiple sclerosis N/A: number not available ON: optic neuritis OR: odds ratio RR: relative risk |
||||||
adults between hepatitis B vaccination and the development of CNS demyelinating diseases, specifically MS and optic neuritis. The study also examined risks related to the timing of vaccination. The results for MS and optic neuritis alone are discussed elsewhere; discussed here are the results of analyses for the two outcomes combined.
Automated HMO outpatient and hospital discharge data for 1995–1999 were screened, and cases were confirmed by review of medical records. Cases were defined as having a physician diagnosis of MS or optic neuritis on their medical records or a diagnosis by a specialist, or as meeting the International Panel criteria for MS (two demyelinating episodes separated in space and time). Up to three controls were selected for each case. A total of 440 cases (332 with MS and 108 with optic neuritis) and 950 controls participated in the study. Hepatitis B vaccine exposure was determined on the basis of medical chart reviews and telephone interviews for those vaccinated outside the HMO.
Odds ratios for the risk of incident demyelinating disease following hepatitis B vaccination were calculated using a conditional logistic regression stratified by matching variables and adjusted for family history, race and ethnicity, place of birth, Scandinavian ancestry, smoking, and marital status. Using the case definitions based on either a specialist’s diagnosis or the presence in the medical record of a physician diagnosis of MS or optic neuritis, the odds ratio for onset of either of these conditions following hepatitis B vaccination was 0.9 (95% CI, 0.6–1.5). When the timing of hepatitis B vaccination was considered, the adjusted odds ratios for demyelinating disease were 0.8 (95% CI, 0.4–1.8) for vaccination less than 1 year before index date, 1.6 (95% CI, 0.9–3.0) for 1 to 5 years before the index date, and 0.6 (95% CI, 0.2–1.4) for more than 5 years before the index date.
As for MS, the authors concluded that the results do not support the hypothesis that hepatitis B vaccination causes or triggers the development of CNS demyelinating diseases.
United Kingdom-General Practitioners Research Database. A case-control study reported in an abstract by Sturkenboom and colleagues (1999) examined the possible association between hepatitis B vaccination and the occurrence of demyelinating diseases. The automated, population-based General Practitioners Research Database (GPRD) in the United Kingdom was used to select cases and controls. Cases had a specialist’s diagnosis of demyelination, MS, optic neuritis, or transverse myelitis. Up to six controls were matched to each case on the basis of age, sex, and physician practice. The index date for cases and their matched controls was the date of the case’s first symptoms consistent with the demyelinating disease. Vaccination history was obtained from automated medical, prescription, and prevention records.
The cases identified included 360 individuals with MS and 140 individuals with diagnoses of demyelination. Receipt of hepatitis B vaccine within 12 months of the index date was associated with a 1.6-fold increase in the risk of
demyelination or MS (CI 0.6–4.0). Given the low statistical power of this study, the authors were unable to conclude whether this finding signals the presence or absence of increased risk for demyelination or multiple sclerosis associated with receipt of hepatitis B vaccine.
United States-Claims Data. A letter from Zipp and others (1999) reported on a retrospective cohort study to assess the risk of demyelinating diseases after hepatitis B vaccination. Data for the study were obtained from records for 1988–1995 in a U.S. health care database. The database, for six pharmaceutical services affiliated with HMO plans, contains information on enrollment and all physician, hospitals, and pharmacy claims for members of those HMOs. A total of 134,698 persons with at least one year of enrollment and no previous record of demyelinating disease were included in the study.
The rate of incident CNS demyelinating episodes in persons who were vaccinated against hepatitis B was compared in the three years after vaccination with the rate in age- and sex-matched controls (three or four per vaccinated subject) who were not vaccinated. The demyelinating disease diagnoses included were optic neuritis, myelitis, and optic neuritis, demyelinating disease of the CNS, ADEM, and MS. Diagnoses and ICD-9 (International Classification of Diseases, Ninth Revision), classifications were made by neurologists or ophthalmologists.
Relative risks for incidence of demyelinating disease were calculated for several periods of follow-up after vaccination and for three age groups. At six months the relative risk was 1.3 (95% CI 0.4–4.8); at 1 year, 1.0 (95% CI 0.3– 3.0); at two years, 1.0 (95% CI 0.4–2.4); and at three years, 0.9 (95% CI 0.4– 2.1). With a 3-year follow-up, the relative risks by age were as follows: 0–14 years, 0.40 (95% CI 0.1–3.1); 15–44 years, 1.2 (95% CI 0.3–4.5); and >44 years, 1.09 (95% CI 0.2–5.3). The authors concluded that there was no evidence that hepatitis B immunization causes demyelinating disorders.
Other Studies: Passive Surveillance Data
The committee reviewed two studies that compared the estimated and observed cases of MS after hepatitis B vaccination. Because data reported in these studies are based on a passive surveillance system with several assumptions and estimates based on data with an unclear denominator, there is an undefined and unexplained level of uncertainty in their results. While the estimates provide some information about the potential magnitude of incident cases, they contribute little to understanding causality. The articles are summarized below.
France. In a letter, Fourrier and others (2001) compared the number of observed cases of CNS demyelinating diseases with the number of cases expected under the hypothesis that no association exists between receipt of hepatitis B vaccine and risk of demyelinating disorder. An unpublished update was presented at the committee’s March 2002 meeting (Fourrier and Bégaud, 2002).
Observed cases were first episodes of CNS demyelinating disorders reported to the French National Pharmacovigilance System that involved persons ages 20–44 years who received hepatitis B vaccine between January 1994 and December 1996, and who experienced the demyelination episode within two months after the vaccination. Two independent neurologists validated the case reports. The number of expected cases was calculated based on an estimated annual incidence rate of 42.9 per million demyelinating disorders for persons aged 20–44 years. In their updated analysis, the authors found 83 eligible cases among 280 reports and estimated that 102.6 cases would have been expected. Using data on the number of vaccine doses distributed in 1994–1996 for use by persons aged 20–44 years, they also estimated that 7.18 million persons in that age group had been vaccinated.
A comparison between the observed and expected case shows no statistically significant difference, based on Poisson cumulative probabilities. The authors note, however, that the difference would be significant (p<0.05) with only 121 reported cases, a 1.5-fold increase over the actual number of eligible reported cases. Given the potential for under-reporting in a passive surveillance system, the authors do not believe that the available data can rule out a possible association between hepatitis B vaccination and a first episode of a CNS demyelinating disorder.
In another report from France, Soubeyrand and others (2000) provided a description of cases of CNS demyelinating disorders reported after receipt of GenHevac B, a recombinant hepatitis B vaccine used in France. The reports span the period from May 1989, when GenHevac B was first marketed, through December 1998. These spontaneous reports were submitted directly to Aventis Pasteur or its drug safety monitoring departments or through regional drug safety monitoring centers. Some reports were actively collected by the Hepatitis B vaccine network.
Using data on the number of vaccine doses distributed, the authors estimate that 10.8 million persons were vaccinated with GenHevac B from 1989 through 1998. A total of 187 cases of CNS demyelinating disorder were reported (0.54 per 100,000 doses distributed). Of these cases, 142 were classified as MS, 34 as optic neuritis, and 11 as myelitis. The average interval from vaccination to onset was 60 days, with a range from one day through five years. Most of the reports (102 of 187) were submitted in 1998 but related to demyelinating events that occurred in previous years. The authors attribute this increase in reporting to publicity about concerns regarding hepatitis B vaccine.
Based on an annual average MS incidence of two cases per 100,000 individuals, 917 cases of MS would have been expected among the vaccinated population during the 1989–1998 period. Noting that this number is almost five times greater than the number of reports actually received, the authors conclude that these findings provide no support for the hypothesis that receipt of GenHevac B vaccine increases the risk for onset of CNS demyelinating disorders.
The risk estimates from passive surveillance data reported in the studies by Fourrier and colleagues (2001) and by Soubeyrand et al. (2000) provided some information about the potential magnitude of incident cases but still included a level of uncertainty. Given the limitations of these two studies, they contributed little to the causality argument.
Causality Argument
For risk of a first episode of a central nervous system demyelinating disorder, the committee reviewed five controlled studies (Touzé et al., 2000; Fourrier and Bégaud, 2002; DeStefano, 2002; Sturkenboom et al., 1999; Zipp et al., 1999), of which only one was a published paper with full analysis (Touzé et al., 2000) (see also Table 2). Fourrier and Bégaud’s (2002) presentation to the committee was an unpublished update of data from a published abstract by Fourrier and others (1999). The outcome examined in each of these studies was a first episode of CNS DD (which can be consistent with MS but not yet meet the criteria for MS). The results of the studies were inconsistent; all of the studies— including the one published study (Touzé et al., 2000)—had limitations of either power or of study design, and none had the methodological strengths of the published studies (Ascherio et al., 2001; Confavreux et al., 2001) that provided support for the committee’s causality conclusions regarding incident and relapse MS. Therefore, the committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and the first episode of a central nervous system demyelinating disorder.
Acute Disseminated Encephalomyelitis
Uncontrolled Observational Study
British Columbia, Canada. As described above, Sadovnick and Scheifele (2000) reported in a letter on an ecological study of the incidence of MS in adolescents aged 11–17 years in the periods immediately before and after the 1992 implementation of a hepatitis B vaccination program for students in grade 6 (11- and 12-year-olds) in British Columbia, Canada. In this study they also compared the incidence of postinfectious encephalomyelitis in the two periods. For the same populations of students, they obtained data on cases of postinfectious encephalomyelitis from medical records of the province’s one children’s hospital. For the prevaccination period, they identified four cases among adolescents aged 11–17 years (and 25 cases in younger children); for the postvaccination period, they found three cases in adolescents, none of which occurred when those children were in grade 6 (when the vaccine would have been administered). (Twenty-eight cases occurred in younger children during the postvaccination period.) The authors reported no statistical analysis of these
data, but they concluded that the data provided no evidence for a link between hepatitis B vaccination and postinfectious encephalomyelitis.
Other Studies: Passive Surveillance Data
VAERS. VAERS received a total of 30 cases of ADEM or demyelinating disease not otherwise specified9 following hepatitis B vaccination from November 1, 1990 through December 31, 2001.
Causality Argument
Based on the evidence, the committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and ADEM.
Optic Neuritis
Controlled Observational Study
United States-Vaccine Safety Datalink. As described above, DeStefano (2002) presented to the committee, at its March 2002 meeting, unpublished findings from a case-control study examining the relationship in adults between hepatitis B vaccination and the development of central nervous system demyelinating diseases, specifically MS and optic neuritis. The study also examined the risk related to the timing of vaccination. The results for optic neuritis are discussed here; the results for MS are discussed above.
Automated HMO outpatient and hospital discharge data for 1995–1999 were screened to select cases and controls, and cases were confirmed by review of medical records. Cases were defined as having a physician diagnosis of MS or optic neuritis on their medical records or a diagnosis by a specialist, or as meeting the International Panel criteria for MS (two demyelinating episodes separated in space and time). Up to three controls were selected for each case. A total of 440 cases (108 with optic neuritis) and 950 controls participated in the study. Hepatitis B vaccine exposure was determined on the basis of medical chart reviews and telephone interviews for those vaccinated outside the HMO. Odd ratios were calculated using conditional logistic regression stratified by matching variables and adjusted for family history, race and ethnicity, place of birth, Scandinavian ancestry, smoking, and marital status. With a case definition based on the presence in the medical record of a physician diagnosis of optic neuritis, the odds ratio for optic neuritis following hepatitis B vaccination was 1.2 (95% CI 0.5–3.1). The results were similar using a case definition based on specialist diagnosis (OR=1.1, 95% CI, 0.4–2.7). The authors conclude that the results do not support the hypothesis that hepatitis B causes the development of optic neuritis. They cite as strengths of the study that it is a large population-based study, that
recall bias is minimized by inclusion of recently diagnosed cases and use of medical records, and that the results are consistent with different case definitions.
Other Studies: Passive Surveillance Data
VAERS. VAERS received a total of 83 cases of optic neuritis10 following hepatitis B vaccination from November 1, 1990 through December 31, 2001.
United States. Shaw and others (1988) published results from a passive post-marketing surveillance between June 1, 1982, and May 31, 1985, of neurological disorders reported following receipt of the plasma-derived hepatitis B vaccine, which is no longer used in the United States. The authors estimated that almost 850,000 persons received one or more doses of the vaccine during the surveillance period. The 41 reports of neurological disease included 5 cases of convulsions, 10 of Bell’s Palsy, 9 of GBS, 5 of lumbar radiculopathy, 3 of brachial plexus neuropathy, 5 of optic neuritis, and 4 of transverse myelitis. The five cases of optic neuritis were in adults and occurred 1–6 weeks after receipt of doses one to three of plasma-derived vaccine. Information about cases with transverse myelitis and GBS are reported below.
Causality Argument
Only one controlled, unpublished study was available (DeStefano, 2002) (see also Table 3). Thus, the committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and optic neuritis.
Transverse Myelitis
Other Studies: Passive Surveillance Data
VAERS. VAERS received 109 cases of myelitis11 following hepatitis B vaccination from November 1, 1990 through December 31, 2001.
TABLE 2 Evidence Table: Controlled Observational Studies—Hepatitis B Vaccine Exposure and a First Episode of Central Nervous System Demyelinating Disorder
|
Citation |
Design |
Population |
Vaccine Assessment |
Outcome |
Results |
Comment |
Contribution to Causality Argument |
|
Touzé et al. (2000) Published Paper |
Case-control (Pilot) |
121 cases 121 matched controls. From review of medical records CASES: a first hospitalization or consultation between 1/1/94 to 12/31/95. CONTROLS: a first (?) hospitalization or consultation between 1/1/95–12/31/95. Conditions unrelated to vaccination and were not a contraindication for vaccination. Matched by age, sex, date of consultation/ hospitalization. (Federation de Neurologie, France) |
One or more doses of Hepatitis B. Collected using mailed questionnaire and confirmed by phone interview or receipt of certificate. Exposure to other vaccines also obtained (hepatitis A, tetanus, poliomyelitis, diphtheria, typhoid, yellow fever, meningococcus, influenza, BCG). |
First episode of CNS demyelination disorder occurred between 6/93–12/95. CNS DD definition: Neuro symptoms which required consultation with a physician, showing evidence of an attack on the CNS and compatible w/ attack on white matter, duration of 24 hrs or longer, possibly combined w/ abnormalities on various complementary tests, absence of other explanation. |
Adjusted OR (95% CI) 0–60 days after vaccination: 1.7 (0.5–6.3) 61–180 days after vaccination: 1.5 (0.5–5.3) |
Low statistical power (35% power to detect a relative risk of 2). Difficult to exclude possibility of an association between HepB and onset of first episode of CNS demyelination. Authors note selection and recall biases may be present in the study but their effects cannot be determined. Cases more likely to live in rural areas where HepB is less common. |
The study does not suggest an association between HepB and first demyelinating episode in adults. |
|
Citation |
Design |
Population |
Vaccine Assessment |
Outcome |
Results |
Comment |
Contribution to Causality Argument |
|
Fourrier and Bégaud, (2002) Unpublished Update/ Presentation to the Committee |
Case-control |
152 Cases 253 Matched Controls. CASES: First episode of CNS DD w/in 6 months prior to examination. CONTROLS: (two per case) Matched by center, age, sex, and date of first examination at center and had conditions that were not likely to affect probability of vaccination. (Multi-center study, France) |
Receipt of one or more doses of Hepatitis B vaccine. From telephone interviews; verified upon review of certificate provided by subjects. Exposure to other vaccines also assessed. |
Patients seen from 1/1/94– 12/31/95 in 17 French neurology departments or first episode of CNS DD w/in 6 months prior to examination. Cases and controls identified by neurologists from review of medical records and verified by independent experts. CNS DD Definition: neuro symptoms compatible with CNS white matter lesions lasting at least 24 hours, leading to a medical consultation. |
Adjusted OR (95% CI) 0–2 months following HepB: 1.4 (0.4–4.5) 2–12 months following HepB 1.0 (0.6–1.9) |
The authors note that because of low statistical power the study cannot exclude the possibility of a small increase in risk. The presentation was an updated analysis of data from a published abstract by Fourrier et al. (1999). The manuscript version of this update is in press (Bégaud et al., in press). |
The study does not suggest an association between HepB and first demyelinating episode in adults but its unpublished status limits its contribution to the causality argument. |
|
DeStefano (2002) Unpublished |
Case-Control |
440 Cases 950 Matched Controls. CASES: Physician diagnosis of MS (332) or ON |
Medical chart review and telephone interview for non-HMO vaccination. |
Potential cases found from screening outpatient and discharge data from 3 HMOs for |
Adjusted OR (95%CI) Ever vs. Never vaccinated MD Dx: 0.9 (0.6– 1.5) |
Authors cite study’s strengths as including study sample from large HMO population, minimized recall bias by using recently diagnosed |
The study suggests no association between HepB and first demyelinating episode in adults |
|
Citation |
Design |
Population |
Vaccine Assessment |
Outcome |
Results |
Comment |
Contribution to Causality Argument |
|
Presentation to the Committee |
|
(108) on medical records or diagnosis by specialist, or as meeting International Panel criteria for MS. CONTROLS: Up to three per case and matched according to year of HMO enrollment, age, and sex. (Vaccine Safety Datalink, United States). |
|
1995–99. 556 confirmed by chart review, 440 cases (MS or ON) contacted and participated in telephone interview. |
Timing of HepB and risk of DD: <1 yr before index date: 0.8 (0.4–1.8) 1–5 yrs before index date 1.6 (0.9–3.0) >5 yrs before index date 0.6 (0.2–1.4) |
cases, use of medical records to establish timing and onset of MS or ON and of vaccination, and consistent results using different case definitions. Limitations include reliance on self-reported information from subjects who were vaccinated outside HMO. Exclusion of self-reported date had little effect on results. |
but its unpublished status limits its contribution to the causality argument. |
|
Sturkenboom (1999) Published Abstract |
Case-control |
Cases: 360 w/ MS and 140 w/ demyelination. CASES: Had specialist’s diagnosis of demyelination, MS, ON, or transverse myelitis. |
From automated medical, prescription, and prevention records. |
Specialist’s diagnosis of demyelination, MS, ON, or transverse neuritis. |
Exposure to Hep B within 12 months prior to index date was associated with a 1.6-fold increase (0.6–4.0) of risk of demyelination or MS compared with non-use |
Authors report low statistical power. Authors unable to conclude whether finding indicates presence or absence of increased risk of demyelination or MS associated with receipt of HepB. |
The study does not suggest an association between HepB and first demyelinating episode in adults but its status as an abstract without publication of final results limits its contribution to |
|
Citation |
Design |
Population |
Vaccine Assessment |
Outcome |
Results |
Comment |
Contribution to Causality Argument |
|
|
CONTROLS: Up to six controls (matched on age, gender, and practice). (General Practitioners Research Database, United Kingdom) |
|
the causality argument. |
||||
|
Zipp et al. (1999) Published Letter |
Cohort |
134,698 enrolled in U.S. health care database from 1988— 1995. EXPOSED: 27,229 UNEXPOSED: 107,469 (3–4 age- and sex-matched controls for vaccinated persons). (United States) |
1 or more Hep B doses identified from claims data. |
Demyelinating disease diagnoses included ON, myelitis and ON, demyelinating disease of the CNS, ADEM, or MS. Diagnosis or ICD-9 classifications made by either neurologist or ophthalmologist. |
RR (95% CI) demyelinating disease, by follow-up interval 6 mos: 1.3 (0.4–4.8) l yr: 1.0 (0.3–3.0) 2 yrs: 1.0 (0.4–2.4) 3 yrs: 0.9 (0.4–2.1) RR (95% CI) 3 yr. follow-up, by age (years) 0–14:0.40 (0.1–3.1) 15–44:1.20 (0.3–4.5) >44:1.09 (0.2–5.3) |
Study had 80% power to detect a RR of 2.5–3.0 or more for demyelinating diseases 1–3 years after vaccination. |
The study does not suggest an association between HepB and first demyelinating episode in adults but its status as a letter limit its contribution to the causality argument. |
|
ADEM: acute disseminated encephalomyelitis CI: confidence interval CNS: central nervous system CNS DD: central nervous system demyelinating disorder HepB: hepatitis B vaccin |
MS: multiple sclerosis ON: optic neuritis OR: odds ratio RR: relative risk |
||||||
Causality Argument
The committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and GBS.
Brachial Neuritis
Other Studies: Passive Surveillance Data
VAERS. VAERS received a total of 15 cases of brachial neuritis12 following hepatitis B vaccination from November 1, 1990 through December 31, 2001.
Causality Argument
Only case reports were available. Thus, the committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and brachial neuritis.
Biological Mechanisms
Although biological data do not provide an independent basis for evaluating causality, they can help validate epidemiologically based conclusions for or against causal associations. Such data can also guide further investigation when epidemiological evidence is inconclusive.
Demyelinating disorders of the central and peripheral nervous systems appear to involve inflammatory, immune-mediated processes that some worry might be triggered or promoted by the activation of the immune system in response to hepatitis B vaccination. In trying to assess whether and how the vaccine might have such effects, the committee reviewed biological evidence, summarized below, regarding several points. Described first are basic immunemediated processes in demyelinating disorders and mechanisms by which immune responses to infection or vaccination might play a role. Discussed next is evidence from animal models and studies in humans as to whether the hepatitis B vaccine might be expected to induce relevant immune-system responses. Because the vaccine antigen is a protein produced by the hepatitis B virus, evidence regarding HBV infection and demyelinating disorders is also reviewed.
Immune-Mediated Processes in MS and Other Demyelinating White-Matter Diseases
There is considerable evidence that demyelination in the CNS or PNS with diseases like MS, ADEM, and GBS is the result of inflammatory, immune
mediated processes (Noseworthy et al., 2000; Stuve and Zamvil, 1999; Waubant and Stuve, 2002). The principal difference in the pathophysiological mechanisms of central and peripheral demyelinating diseases is that the CNS diseases involve a process that can penetrate the blood-brain barrier. In MS, the demyelinated plaques or lesions center on venules, which suggests that systemic immune cells diffuse out of the vasculature and contribute to the pathology. In the early stages of the development of these plaques, an inflammatory response is evident and dominated by T lymphocytes, specifically CD+4 and CD+8 cells, and scattered B and plasma cells. Also present are various cytokines, including interleukins (IL-2, 3, 4, 6, 10, and 12), beta interferon, tumor necrosis factor, and transforming growth factor.
The initial event in the formation of MS plaques appears to be activation of autoreactive T cells. There are several autoantigens within the brain that could trigger the activation of T cells, but none has yet been proven as the causative antigen. Candidates include myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG), and proteolipoprotein (PLP). Activated CD+4 T cells that are specific for CNS antigens are increased in MS patients, but it is unclear if they are ravelin-specific. MS is associated with a Th1-based immune response and an imbalance between increased IL-12 production and decreased IL-10 production locally and systemically during disease activity.
Theoretical Mechanisms for Infection-Induced Immune-Mediated Injury
Infection can induce immune-mediated tissue injury. In most cases, this injury is short-lived and resolves as the immune system eliminates active infection. The injury is a consequence of the immune response to the foreign invader, and when the invader is eliminated, the damaging immune process ceases. In some diseases, however, infection appears to induce an injurious immune response directed, at least in part, against self antigens. True autoimmune injury must be distinguished from immune-mediated injury that results from persistent but undetected infection. If the infectious agent was not detected, ongoing immune-mediated responses to that agent and the resulting injury of host tissues could be interpreted as autoimmunity, when in fact the immune response was directed against the foreign microbe and not against self.
Major mechanisms proposed to account for the activation of self-reactive T and B cells and the induction of autoimmunity by infection include molecular mimicry, bystander activation, and non-specific or polyclonal activation of self-reactive T or B cells (Albeit and Inman, 1999; Bach and Chatenoud, 2001; Benoist and Mathis, 2001; Davidson and Diamond, 2001; Marrack et al., 2001; Regner and Lambert, 2001; Rose, 2001; Singh, 2000; Wucherpfennig, 2001; Zinkernagel, 2001). These mechanisms, and the evidence regarding the possibility that they actually contribute to autoimmune-mediated neurological diseases, are discussed in a later section. For more in- depth discussion of these mechanisms, see a previous report of this committee (IOM, 2002).
Molecular mimicry. An antigenic epitope from a microbe that is structurally similar to (mimics) an epitope of a self-molecule has the potential to trigger the activation of self-reactive, naïve T or B lymphocytes. Once activated, self-reactive T cells could expand in number and mature into effector (memory) T cells that have a lower threshold for activation by self antigens. These cells would also gain the ability to migrate to specific tissues, produce additional mediators/cytokines, and mediate injury on contact with cross-reacting self antigens. In addition, they would gain the potential to help B cells that are responding either to the same antigen as the T cells or to other self-antigens that are physically linked to it.
Bystander activation. Bystander activation results when an infection creates environmental conditions that allow the activation of self-reactive T and B cells that are normally held in check. It does not require that antigens of the infectious agent be structurally similar to self-antigens. Bystander activation may be mediated in part by infection-induced death of host cells, which results in the release of greater amounts of self peptides or in the generation of novel self peptides (i.e., novel or cryptic epitopes not normally found in the absence of the infection). As part of this process, molecules derived from the microbes (and perhaps also from the necrotic host cells—e.g., heat-shock proteins) would stimulate other components of the immune system. Tissue damage, resulting from an ongoing, heterologous, infectious, or inflammatory process can lead to the liberation or exposure of host antigens in a context that allows for presentation to, activation of, and expansion of self-reactive lymphocytes.
Nonspecific or polyclonal T cell activation. It is also possible that infections could activate a variety of T or B lymphocytes that would otherwise respond only to certain antigens. This non-antigen-specific response is referred to as a polyclonal or oligoclonal response. For example, toxins produced by Streptococcus pyogenes and Staphylococcus aureus can act as superantigens, binding both to T cell receptors and to MHC molecules and activating a substantial fraction (greater than 5 percent) of the total T cells of an individual. This overactivation of the immune system leads to the acute “toxic-shock” syndromes associated with the toxin-producing strains of these bacteria. Another example is polyclonal B cell activation following Epstein-Barr virus (EBV) infection, that is capable of enhancing autoimmune reactions. These nonspecific immune responses are usually self-limited, however, and resolve as the infection is cleared.
If self-reactive T cells are activated by a nonspecific immune response, they could induce autoimmunity. Superantigen-induced activation normally ends in the programmed death (apoptosis) of the activated cells, terminating the response. However, other microbial products, like endotoxin, that trigger the innate immune response can enhance the survival of superantigen-activated T cells (Vella et al., 1995, 1997), and could—in the context of genetic differences in mechanisms controlling the death of activated T cells—prolong the survival of self-reactive T cells, in theory allowing them to mediate self-injury.
TABLE 3 Evidence Table: Controlled Observational Studies—Hepatitis B Vaccine Exposure and First Episode of Optic Neuritis
|
Citation |
Design |
Population |
Vaccine Assessment |
Outcome Measures |
Results |
Comment |
Contribution to Causality Argument |
|
DeStefano (2002) Unpublished Presentation to the Committee |
Case-Control |
108 Cases (N/A) Matched Controls. CASES: Physician diagnosis of ON in medical records or diagnosis by specialist. CONTROLS: Up to 3 per case and matched according to year of HMO enrollment, age, and sex. (Vaccine Safety Datalink, United States). |
Receipt of one or more doses of Hep B before index date. Medical-chart review and telephone interview for non-HMO vaccination. |
Physician diagnosis of ON in medical record. Potential cases found from screening outpatient and discharge data from three HMOs for 1995– 99. 556 confirmed by chart review, 440 cases (MS or ON) contacted and participated in telephone interview. |
Adjusted OR (95%CI) Ever vs. Never vaccinated MD Dx: 1.2 (0.5–3.1) specialist’s dx: 1.1 (0.4–2.7) |
Authors cite study’s strengths as including study sample from large HMO population, minimized recall bias by using recently diagnosed cases, use of medical records to establish timing and onset of ON and of vaccination, and consistent results using different case definitions. |
The study suggests no association between Hep B on a first episode of ON in adults but its unpublished status limits its contribution to the causality argument. |
|
ADEM: acute disseminated encephalomyelitis CI: confidence interval CNS: central nervous system CNS DD: central nervous system demyelinating disorder Hep B: hepatitis B vaccine |
MS: multiple sclerosis N/A: number not available ON: optic neuritis OR: odds ratio RR: relative risk |
||||||
Theoretical Mechanisms for Vaccine-Induced Demyelinating Diseases
These mechanisms—molecular mimicry, bystander activation, and superantigen stimulation—could lead to the development of central and peripheral demyelinating disease in response to immunization. Molecular mimicry refers to the process by which a microbial antigenic determinant cross-reacts with a self-protein. If the self-protein is a myelin-related protein, the subsequent immunological response leads to autoimmune demyelination. Bystander activation refers to a process by which a microbial infection (or other stimulus) leads to the release of a large quantity of normally sequestered host proteins and the subsequent destruction of host tissue, which could include central or peripheral myelin. Superantigens are proteins that are produced by viruses and bacteria that can activate T (or B) cells in ways that are independent of the antigen specificity of the responding lymphocyte, allowing for the activation of lymphocytes that are autoreactive. It is conceivable that antigenic stimulation from vaccines could trigger any of these potentially damaging mechanisms. There is no reason in theory why hepatitis B surface antigen in the vaccine could not function in this way. Thus, there is a theoretical basis for a hepatitis B vaccine-induced immune response that could possibly lead to demyelination. As is discussed in the subsequent section, the evidence in support of this theory, however, is scant and indirect.
Evidence from Animal Models for a Possible Role for Hepatitis B Vaccine in Demyelination
The most well-established animal model for MS and ADEM is experimental autoimmune (often, but erroneously, referred to as allergic) encephalomyelitis (EAE)—a syndrome induced in susceptible strains of mice and rats. The inducing agent is usually immunization with myelin antigens or the transfer of T lymphocytes reactive against myelin proteins. There are many similarities between EAE models in animals and MS and ADEM in humans, including genetic susceptibility, greater female susceptibility, the clinical presentation, and the pathology. However, the effects of therapeutic agents are not always consistent between EAE and MS or ADEM. For example, administration of beta-interferon leads to improvement both in MS and EAE, whereas use of systemic gamma-interferon worsens MS but cures EAE. Nevertheless, the similarities of EAE to the human demyelinating diseases of MS and ADEM provide a strong indication that immunization with certain antigens can trigger autoimmune processes that produce demyelinating injuries.
There is, however, no evidence that exposure to hepatitis B vaccine (which contains only one protein, HBsAg) leads to EAE. A study published in 1985 demonstrated inflammatory infiltrates in the CNS of rabbits immunized with the
HBV polymerase, but these animals did not develop EAE (Fujinami and Oldstone, 1985). The HBV polymerase shares six consecutive amino acids with an encephalitogenic site of rabbit myelin basic protein. Although this experiment has been long presented as evidence that the hepatitis B vaccine could cause demyelination, its relevance to the current debate is negligible. First, the animals did not develop EAE. Second, the precipitating antigen in the experiment is not similar to the hepatitis B surface antigen used in the current vaccine.
Another important animal model for MS is demyelination induced by Theiler’s murine encephalomyelitis virus (TMEV). One strain of TMEV produces inflammatory demyelination of the spinal cord in mice. The effect appears to be mediated in part by T cells directed against viral antigens. Other viruses are also associated with demyelination in animal models, including several strains of mouse hepatitis virus. Despite the usefulness of these animal virus models, the ability to extrapolate from them to human MS is limited.
Animal models for peripheral demyelinating disease also exist but are less well investigated than the models for CNS disease. The most studied model is experimental allergic neuritis (EAN), which has been best described in Lewis rats. EAN can be induced by active immunization with a homogenate of whole peripheral nerve tissue or with peripheral myelin extracts, combined with complete Freund’s adjuvant (Hahn, 1996). The Lewis rats develop progressive limb paralysis about 10 days after immunization with myelin constituents. A related model, experimental neuritis, is similar but requires that a triggering agent (such as various viruses or antigens) be administered concomitantly with the myelin tissue (Hjorth et al., 1984). In this model, the swine influenza vaccine was shown to trigger autoimmune responses and peripheral demyelination.
Evidence from Humans
Clinical or in vitro studies with human cells. There is inconclusive evidence that molecular mimicry between hepatitis B vaccine proteins and CNS antigens plays a role, or even exists, in the pathogenesis of MS. In fact, there is no significant homology between the amino acid sequences of HBsAg, the main component of the hepatitis B vaccine, and the myelin proteins MOG, MBP, and PLP. This makes it unlikely that a T cell-mediated immune response against these CNS autoantigens would be triggered by the hepatitis B vaccine on the basis of molecular mimicry. However, in a recent report that describes the onset of clinically definite MS after hepatitis B vaccination, hepatitis B surface antigen-specific CD4+ T cell clones (obtained from the cerebrospinal fluid of this one vaccine recipient by limiting dilution cloning) proliferated in response to various immunodominant peptides derived from all the aforementioned CNS autoantigens (Gran et al., 2000). The significance of these very preliminary findings needs to be confirmed.
A humoral autoimmune response may still be conceivable, as antibodies recognize both conformational and linear epitopes. Although several studies reported the specificity of intrathecal oligoclonal bands against specific pathogens in other CNS diseases, no antibody against HB virus—or, more specifically, against HBsAg—has been identified in MS.
Evidence of autoimmunity in humans following hepatitis B vaccination. There is some evidence that the hepatitis B vaccine is associated with alopecia (hair loss) (Wise et al., 1997). At the time that this article was written, VAERS had received 46 cases of alopecia following hepatitis B vaccination, and 4 of those cases involved positive rechallenge. At least one form of alopecia (alopecia areata) is hypothesized to be autoimmune-mediated. However, the pathophysiological similarities between alopecia and demyelinating conditions are not strong, and the relationship between hepatitis B vaccine and alopecia is not established as causal. Therefore, the findings on alopecia provide only weak evidence of biological mechanisms relevant to the immune-mediated neurological outcomes under review.
Effects of hepatitis B virus infection in humans. When an infectious agent has been associated with a particular adverse health outcome, the possibility exists that a vaccine against that agent could have a similar effect. The primary effect of HBV infection is hepatic, but occasional extrahepatic manifestations occur, such as rash, arthritis, and arthralgias. Central or peripheral neurological manifestations are not a prominent feature of HBV infection, but anecdotal reports in review articles mention an association between acute infection and MS. This association has not been well documented, and a cause-and-effect relationship is far from certain. Although several viruses (e.g., Epstein-Barr virus) are implicated as risk factors for both incident cases of MS and relapses, the hepatitis B virus is not prominent in the discussions of viral triggers.
Known Effects of Other Vaccines
Several vaccines are considered causally associated with demyelinating diseases of either the central or peripheral nervous system. Both rabies and measles vaccines are rarely followed by ADEM, a demyelinating disease of the CNS, but the relationship has not been shown to be causal. The swine influenza vaccine used in 1976–1977 in the United States (CDC, 2001b), and the 1992–1993 and 1993–1994 influenza vaccines (Lasky et al., 1998) were associated with an increased risk of developing GBS, a peripheral demyelinating disease. Also, a previous IOM committee concluded that the oral polio vaccine was associated with GBS (IOM, 1994), but data (Kinnunen et al., 1998; Rantala et al., 1994) published after that report was issued suggested to some that the association was
not causal (Sutter et al., 1999). In addition, that same IOM committee found that the evidence favors acceptance of a causal relationship between tetanus-toxoid-containing vaccines and brachial neuritis, a peripheral nerve disorder possibly linked to immune-mediated reactions (IOM, 1994).
Conclusions Regarding Biological Mechanisms
In summary, there is a theoretical basis for mechanisms by which a vaccine could cause a central demyelinating disease, like MS or ADEM, or a peripheral demyelinating disease like GBS. There is no reason, in theory, why the hepatitis B vaccine could not operate in this way.
The following indirect biological evidence relates to the theory that hepatitis B vaccine could be associated with demyelinating disease:
-
Animal models show that under contrived experimental conditions immunization leads to a demyelinating disease similar in many respects to MS, ADEM, or GBS
-
A causal relationship has been found between several vaccines (other than hepatitis B vaccine) and ADEM, GBS, and contralateral brachial neuritis.
It should be noted, however, that animal models have not provided evidence that HBsAg serves as a trigger for the onset of demyelinating disease; the in vitro evidence from human T-cell lines is very preliminary, infection with HBV is not proven to cause MS or GBS, and no vaccine has been causally associated with MS. The biological evidence is scant and not clearly and directly relevant to the hepatitis B surface antigen in the vaccine. There is no evidence that HBsAg is capable of bystander activation of a Th1-type response, is a superantigen, is a molecular mimic with a myelin-related antigen, or otherwise induces nonspecific polyclonal activation. Thus, the committee concludes that there is weak evidence for biological mechanisms by which hepatitis B vaccination could possibly influence an individual’s risk of the central or peripheral nervous system disorders of MS, first episode of CDD, ADEM, optic neuritis, transverse myelitis, GBS, or brachial neuritis.
SIGNIFICANCE ASSESSMENT
The Immunization Safety Review Committee’s charge for the present series of studies includes consideration of the public health response to the immunization safety concerns they examine. Most previous IOM studies on immunization safety, by contrast, were limited to conclusions from causality assessments and to recommendations for future research. The public health response to an immu-
nization safety concern potentially encompasses a broad range of activities, including policy reviews, new research directions, and changes in communication to the public and to health care providers about issues of immunization safety. In formulating the breadth and direction of the recommended public health response, the committee considers not only its conclusions regarding causality and biological mechanisms, but also the significance of the immunization safety issues for society—the context in which policy decisions must be made.
Public concerns about immunization safety must be examined carefully because most vaccines are given to healthy children not only for their direct protection but also to help protect others in the population. In fact, to achieve this broader level of protection, certain vaccines are mandatory in all 50 states for school and daycare entry. Exemptions on medical grounds (contraindications) are allowed, although they are considered too limited by some (Fisher, 2001). Exemptions are also allowed on religious grounds in 48 states and on philosophic grounds in 17 (Sheedy, 2002). Use of such exemptions in most communities is rare, however, and it is argued that these public health mandates, because they are imposed on healthy children, place a special responsibility on the government for rigorous attention to safety issues, even for rare adverse outcomes.
In the present case, the committee considered the possibility that the exposure of adults and infants to the hepatitis B vaccine might increase the risk for demyelinating neurological disorders such as MS, GBS, and peripheral neuritis. This issue initially gained attention because of reports collected in the three years after the introduction of the plasma-derived vaccine, of demyelinating disease, particularly GBS, occurring after vaccination (Shaw et al., 1988). More recently, and particularly in France, concern has arisen that the vaccine might be associated with the onset or exacerbation of MS. In addition, there are concerns about the need for universal immunization of infants and young children against a disease that many people, including some pediatricians, believe is not a major or immediate threat to the health of most infants.
Reviewed here, as part of the committee’s assessment of the significance of this issue, are the disease burden (e.g., seriousness, treatment, complications) associated with MS—a chronic disease and the most common of the neurological conditions covered in the report—and with hepatitis B virus infection. Also discussed are indications of public concern that infants and children receive the vaccine at all.
Disease Burden Associated with Multiple Sclerosis
MS is a serious and usually progressively debilitating disease that is diagnosed most often in young adults, who may live with its effects for decades. The demyelinating injuries that occur to the central nervous system have a farreaching impact on physical functioning, with consequences for psychosocial
functioning. Some of the most prominent symptoms reported by MS patients13 are cognitive impairment, depression, spasticity and weakness, ataxia and tremor, bladder and bowel dysfunction, visual disturbances, fatigue, sexual dysfunction, and pain (IOM, 2001c). New therapeutic tools can modify the course of the disease for some patients, but there is no cure for MS.
The cost of care for people with MS is high. The new therapeutic medications have been estimated to cost $8,000–10,000 per year and often are not fully reimbursed (Rudick, 1999). Health insurance may cover only some of the costs of care and may exclude, or offer only partial coverage for, services intended to improve functioning or devices to improve mobility (IOM, 2001c). A 1990 survey of families with a member who had MS found that insurance covered approximately 75 percent of medical bills, but 28 percent of the respondents reported that the coverage was inadequate to cover their costs (Catanzaro and Weinert, 1992). The survey also found that 70 percent of low-income families, 43 percent of medium-income families, and 24 percent of high-income families had difficulty meeting health care needs and/or basic living needs (Catanzaro and Weinert, 1992).
The economic impact of MS extends to adverse effects on employment, which can mean loss of income and access to health insurance. MS is often diagnosed at an age when many people are beginning to firmly establish their careers. A diagnosis of MS often requires adjustments to work schedules, which often may lead to decreased income. Symptoms of MS such as fatigue, spasticity, incontinence, and restricted mobility make it difficult to perform jobs in traditional settings. Barriers to employment for people with MS have been classified as personal (an individual’s specific disease symptoms, education, and employment history); societal (inaccessible job location, and public attitudes and prejudices); and programmatic (vocational rehabilitation and policies determined by programs such as Social Security or Medicaid) (IOM, 2001c). The impairments that affect opportunities for employment can also affect access to a variety of services and resources, including some kinds of basic health care.
Hepatitis B Vaccine Safety Concerns in France
The possibility that the hepatitis B vaccine may contribute to the development of MS and other autoimmune disorders provoked concerning France in the early 1990s after the publication of two case reports on CNS demyelination following hepatitis B vaccination (Herroelen et al., 1991). The reports received strong media attention, and fears about the safety of the vaccine quickly spread throughout France and then to Europe and North America. Throughout the 1990s, reports linking the hepatitis B vaccine to MS continued to appear.
In 1998, the French health authorities held a meeting of experts to evaluate the evidence for a connection. Participants concluded there was no causal evidence of a link between hepatitis B vaccination and MS (Dittman, 2000). However, concern surrounding the issue remained high, and in October 1998, the French Minister of Health authorized the suspension of school-based hepatitis B vaccination for 11- and 12-year-olds. Two primary reasons were cited: a preference that hepatitis B vaccination be given through an adolescent’s physician, which would allow for a more thorough discussion of informed consent, and the invocation of the precautionary principle.14 French health officials made no changes to the policies for infant and adult immunization against HBV, and continued to stress the importance of universal HBV immunization (Monteyne and Andre, 2000).
Additional evidence of the safety concerns about the hepatitis B vaccine in France is evident in the legal system. In April 1997 and June 1998, a court found in favor of three individuals who filed claims against the vaccine manufacturer for illnesses the plaintiffs attributed to receipt of the hepatitis B vaccine. The rulings were appealed in April 1999, and two of the three cases were overturned by a higher court (Monteyne and Andre, 2000). In another lawsuit, compensation was awarded in May 2000, to three individuals who developed MS after receipt of hepatitis B vaccine (Jefferson and Heijbel, 2001). Additional cases are currently being pursued, including a claim against the French government for understating the risks of hepatitis B vaccine and overstating the benefits of the vaccine for the average person (Marshall, 1998).
Hepatitis B Virus Infection
CDC estimates that 1.25 million people in the United States have chronic HBV infections and that 20–30 percent of these infections were acquired in childhood (CDC, 2001a). There are also 4,000 to 5,000 deaths each year from chronic liver disease related to HBV infection (Mast, 2002). The number of new HBV infections has fallen from an average of 450,000 per year in the 1980s to about 80,000 in 1999 (CDC, 2001a). Much of the decline has occurred among children and adolescents and is attributed to routine immunization. Acute HBV infection can result in serious illness and some deaths, but chronic infection is a greater concern because of the increased risk of cirrhosis and hepatocellular carcinoma (HCC) in the infected person and the continuing risk for transmission of the virus to others.
Perinatal infections of infants born to mothers who test positive for the presence of HBsAg can be treated successfully when infants receive hepatitis B
immune globulin (HBIG) and hepatitis B vaccine soon after birth (CDC, 1991). HBIG can also be used at older ages soon after suspected exposure to HBV. Once chronic infections occur, they are treated with two principal therapies: immune modulators such as interferon-alpha (IFN-α) and antiviral agents such as lamivudine. Interferon-alpha therapy has limitations in terms of the population that best responds to the treatment, as well as unfavorable side-effects (Maddrey, 2001). Treatment with lamivudine appears to be better tolerated and available to patients for whom IFN-α is not recommended (Maddrey, 2001).
The incidence of HCC has increased in the United States from an average of 1.4 cases per 100,000 per year for 1976–1980 to 2.4 cases per 100,000 per year for 1991–1995 (El-Serag, 2001). Incidence is highest in persons aged 65 and older but is shifting toward younger ages (El-Serag, 2001). In countries where early childhood HBV infection is widespread, HCC occurs in childhood. HCC is rapidly fatal, with a median survival time of only 0.6 years (El-Serag, 2001).
People who develop HCC have few treatment options. Surgical removal of a tumor is sometimes considered but may not be possible because of the prior damage to the liver from cirrhosis (Schafer and Sorrell, 1999). Liver transplants often have a poor outcome because HBV infects the transplanted organ (Walsh and Alexander, 2001). Chemotherapy does not improve survival (Schafer and Sorrell, 1999). In addition, complications of HCC can include ascites, encephalopathy, and bleeding varices, which may confound hospital treatment.
Prevention Strategies
In the United States and many other countries, the current strategy for preventing HBV infection emphasizes universal immunization of infants, with a first vaccine dose given soon after birth. When hepatitis B vaccines first became available in the 1980s, the prevention strategy in the United States focused on immunizing populations, pregnant women in particular, considered at high risk for HBV infection. The strategy was later expanded to include all pregnant women (CDC, 1990). These approaches proved unsuccessful in reducing hepatitis B infections because vaccination rates in many high-risk populations remained low. Moreover, in the 1980s, up to 30 percent of HBV cases in adults could not be linked to known risk factors (Alter et al., 1990).
Infant immunization was also recommended because HBV infections occurred in children of mothers who were HBsAg-negative (CDC, 1991). CDC has estimated that in 1991, prior to the recommendation for universal infant immunization, approximately 16,000 children between the ages of 0 to 9 years acquired nonperinatal HBV infections (Armstrong et al., 2001). Slightly more than half of them were children of foreign-born mothers (See Table 4). Although rates of HBV infection in the United States are highest in Asian populations, the CDC estimates suggest that non-perinatal infections occurred in 8,700 non-Asian children, about 54 percent of the total.
TABLE 4 Estimated Number of Non-perinatal HBV Infections in Non- Asian and Asian Children, Ages 0–10 Years in 1991
CDC used these figures to calculate the impact of infant immunization in the first 10 years of life for the 1998 birth cohort, which includes a greater proportion of children of foreign-born HBsAg-positive mothers than in 1991 (Armstrong et al., 2001). Those estimates indicate that without immunization approximately 6,800 perinatal infections and 18,700 nonperinatal infections of children 0 to 9 years old would have occurred. About 12,10015 of these children would develop chronic infections, and 3,00016 of them would eventually die from cirrhosis or HCC. With 90 percent of children now receiving three doses of hepatitis B vaccine by age 2, it is estimated that as many as 2,700 deaths from cirrhosis or HCC will be averted in the 1998 birth cohort (See Table 5 for calculations).
TABLE 5 Estimated Number of Chronic Hepatitis B Infections and Related Deaths in 1998 Birth Cohort with No Immunization Program
|
15 |
Chronic infection could be expected to occur in approximately 60% of the children infected before age 2 and in 25% of the children infected between ages 2 and 9 years (Armstrong et al., 2001 citing McMahon et al., 1985 and Edmunds et al., 1993). Of those chronically infected, approximately 25% would die several decades (and in some cases only within years) after infection from cirrhosis or HCC (See Table 5). |
|
16 |
In contrast to other vaccine- preventable diseases, 400–500 deaths were attributed to measles in the United States between 1960 and 1962 before universal childhood measles immunization, and 800–1000 deaths were attributed to H. influenzae type b between 1986–1988 before conjugate vaccines were available for use in infants (Halsey, 1993). |
In addition, universal infant immunization was demonstrated to be cost-saving. Margolis and colleagues (1995) provided an economic analysis of various vaccination strategies, including routine hepatitis B immunization of infants plus prevention of perinatal infection, in the 1991 birth cohort. Overall, the cost of preventing perinatal infection and having routine infant vaccination would be less than the medical and work-loss costs associated with the estimated 5 percent lifetime risk of infection. A perinatal prevention and infant immunization program would reduce the estimated lifetime risk of infection by at least 68 percent.
Nevertheless, gaps continue to exist in the prevention of perinatal infection. CDC estimates that in 1997 about 16,000 children were born to HBsAg-positive mothers (Mast, 2002). Data from the period 1991–2001 show that in various states 3 percent to 16 percent of mothers were not screened for HBsAg status (Mast, 2002). Earlier data suggested that mothers who were not screened were more likely to be HBsAg-positive (Mast, 2002 citing Silverman et al., 1991). Data from the mid-1990s also showed that children born to mothers with an unknown HBsAg status might be less likely to be vaccinated at birth than children born to women known to be HBsAg-negative. Thus, the infant immunization recommendation is expected to reduce significant morbidity and mortality that would not be prevented through a targeted immunization program only.
Acceptance of the Hepatitis B Vaccine in the United States
After the first hepatitis B vaccine was introduced in 1982, health care providers and others (including those for whom it was recommended) expressed concerns about adverse effects from the vaccine, particularly concerns about contracting HIV/AIDS (Samaranayake et al., 1987; Spence and Dash, 1990). This concern arose because the original vaccine was produced using blood plasma from persons who were chronic hepatitis B virus carriers who theoretically carried a risk of transmitting other viral infections, including HIV. Although testing demonstrated that the vaccine manufacturing process inactivated potential bloodborne pathogens (Francis et al., 1986), fears lingered. The shift in the late 1980s to the current hepatitis B vaccine, a recombinant yeast-derived product that contains no human blood or bloodproducts, has helped eliminate this original concern about the safety of the vaccine.
In 1991 the Advisory Committee on Immunization Practices (ACIP) recommended that all infants receive a first dose of hepatitis B vaccine at birth (CDC, 1991), and in 1996, ACIP recommended vaccination of all 11- and 12-year-olds who had not previously received hepatitis B vaccine (CDC, 1996). Several studies in the mid-1990s examined the perceptions and acceptance of the new policies by health care providers, parents, and adolescents. Some of the studies reported a reluctance among pediatricians and family physicians to begin administering the hepatitis B vaccine at birth (Bertolino, 1996; Freed et al., 1994). This reluctance did not appear to be based on safety concerns, but on
many practitioners’ beliefs that hepatitis B was not a disease risk in their practices; also some practitioners had concerns about reimbursement. One study reported that the indication of the hepatitis B vaccine’s safety was a positive influence on practitioners’ willingness to administer the first dose at birth (Loewenson et al., 1994). However, a recent survey of pediatricians found that 11 percent of the respondents remained concerned about the safety of administering a vaccine to neonates (Cooper et al., 2001).
Nevertheless, the committee found little overall indication that safety concerns are a major barrier to acceptance of hepatitis B vaccination in the United States. Its widespread acceptance is reflected in the increases since the mid-1990s in coverage rates among young children. In 1995, 68 percent of children aged 19–35 months had received three doses of hepatitis B vaccine. For 2000, the National Immunization Survey data show a 90.3 percent coverage among children in that age group (CDC, 2001c). Although coverage rates are high, a 1999 congressional hearing (HGR Committee, Serial No. 106–97)—which addressed the risks and benefits, adverse effects reported to VAERS, and the process of approving and recommending the hepatitis B vaccine—provides evidence of concern by some parents and federal policymakers.
Information on the Safety of the Hepatitis B Vaccine
In previous reports (IOM, 2001a,b), the committee has noted that parents and advocacy groups have had difficulty obtaining unbiased and relevant information to address their vaccine-related concerns. In a brief experiment of its own, the committee experienced similar difficulties. The committee has recommended that CDC and FDA, in particular, evaluate their prominent forms of communication to learn how people with a need and desire to know—especially parents of young children—receive, understand, and respond to vaccine riskbenefit information. The committee also recommended that CDC, FDA, NIH, AAP, and similar organizations consider developing more effective ways of obtaining input from the concerned public in the design of their communication approaches and materials. To implement this organizational shift, new strategies and research efforts focused on vaccine risk-benefit perception and related decisionmaking will be required (IOM, 2002). At the committee’s March 2002 public meeting, CDC reported that such research has been initiated in order to understand the views of first-time parents. The committee considers this action a positive first step and encourages more comprehensive research of this type in the future.
Members of the public or advocacy groups have voiced their concerns about each topic the committee has studied, and they have told us of the difficulty in finding information that they trust and that meets their decisionmaking needs. Given the increasing number of people who search the Internet for health information (Taylor and Leitman, 2002), for this report the committee chose to per-
form a limited analysis of web-based information. The purpose of this effort was to explore how readily information could (or could not) be found in response to a specific question: “Does the hepatitis B vaccine cause multiple sclerosis?” The committee attempted to simulate the types of searches that concerned parents and stakeholders might undertake in pursuit of answers, and as part of the simulation we assumed that they would focus primarily on the top 10 items retrieved by each search. The committee analyzed its results by the search engine or site studied, keywords used, the numbers of “hits” for each search, and the nature of the top 10 items found. The focus was on locating items that would provide minimally technical information, based on the belief that those items would be the most desirable as the starting point for information seekers lacking technical backgrounds. The committee did not attempt to analyze the content within items for their literacy level, or design, nor did the it evaluate the websites for design or potential impacts on users’ perceptions. For each item, the committee attempted to assess the intended audience based on the readability and whether the information was pragmatic (Purcell et al., 2002).
For the first set of searches, the committee assumed that the concerned public would turn to standard search engines to locate appropriate information. Thus, the committee conducted keyword searches using Netscape, Lycos, Google, and Yahoo, (see Table 6).
TABLE 6 Search Engine Results from Using Selected Keywords,* Accessed on April 17, 2002
The numbers of hits varied considerably within a search engine and were related to the exact terms used. For example, using “hep B” always brought up fewer links than “hepatitis B.” The use of “vaccine” among the keywords greatly narrowed the number of sites found, but did not always result in sites more appropriate to the question. In the top 10 links of each search, many of the sites related personal experiences with the vaccine or MS, or were advocacy sites. A few of the searches produced a government agency or medical organization link in the top 10, while some produced no relevant information at all. Us-
ing such search engines, the concerned public is most likely to find anecdotal rather than scientifically based information. It would take a considerable amount of time for a parent to find and assess balanced and relevant information in this way.
For the second set of searches, the committee examined websites of five organizations, both within and outside government, to which parents and the concerned public would likely turn for answers to their vaccine-related questions, (see Table 7).
TABLE 7 Specific Website Results from Using Selected Keywords,* Accessed on May 13, 2002
|
Organization |
Number of Keyword Combinations |
Range of the Number of Hits |
Maximum Number of Top 10 Items Designed for Parents or the Public |
Content of Top 10 Items |
|
CDC** |
10 |
17–984 |
4 (first item in 2 of the 11 searches) |
Most items were designed for public health professionals or researchers. A few were intended for clinicians. |
|
FDA** |
5 |
41–89 |
0 |
Most items were either technical research reports or voluntary recalls. One item was for clinicians. |
|
NIH |
5 |
0–974 |
0 |
All items were technical. |
|
WebMD |
11 |
0–12 |
2 |
Some items were for hepatitis C ornon-MS outcomes. No item addressed the hep B vaccine-MS question in great detail. |
|
AAP*** |
4 |
0–12 |
1 |
Most items were designed for mass media, advocacy or policy purposes. None found on the “families” page. |
|
NOTE: CDC=Centers for Disease Control and Prevention, FDA=Food and Drug Administration, NIH=National Institutes for Health, AAP=American Academy of Pediatrics. *Keywords used were Hep B or Hepatitis B, Hep B vaccine or Hepatitis B vaccine, MS or multiple sclerosis. At least two terms (one for “exposure” and one for the “outcome”) were used for any search. **This website allowed for both “basic” (or simple) and “advanced” searches. The range includes results for both types. ***This site permitted the use of “near” as a connector between keywords. The use of this term produced the most items, but none were designed for parents. |
||||
Within organizations’ sites, there did not appear to be any apparent logic or “lessons” that could be readily consulted to determine which keywords or search approach would bring up links most relevant to the test question. The results of the committee’s searches were highly variable and dependent on the selection and combination of specific keywords and a connecting word (“and” or “near”). The numbers of hits within a site were not clearly related to the use of a “basic” or “advanced” search method, but varied by one to two orders of magnitude, depending on particular combination of key and connector words. On the CDC’s site, the combination <hepatitis B vaccine AND multiple sclerosis> in the “basic” search function located all four documents. On the FDA site, a similar combination of words yielded only one item focused on the committee’s question. However, the relevant information was near the middle of a 21-page document, making it time-consuming for a reader to find.
The AAP site searches brought up few links that appeared to be designed for the concerned public, and no relevant information was provided on AAP’s page for families. The information retrieved was mostly media and advocacy materials, so the site may not appear to parents to be for them, or to present a balanced view of the vaccine. The WebMD search found two documents with information about the hepatitis B vaccine in which the concern about MS is mentioned. The searches done of the NIH and FDA websites retrieved no documents that appeared to be designed for the concerned public.
Another issue is that the titles of many links make it very difficult to know whether the site had any relevance to the question. For example, some of the CDC searches brought up links with complex link names or even had unrelated terms such as “ebola” or “all terrain vehicles” in the brief text under the title.
Although the committee only searched for web-based materials and only in a limited exercise, its modest experiment demonstrated some of the barriers that face concerned parents and others when they attempt to locate information appropriate to their issues. There are potentially many technical reasons for the wide variations in the search results, even within one organization’s site, but these reasons are not apparent to the site user. It is clear that the concerned public may spend a great deal of time seeking meaningful information, often without success even if they are experienced Internet users (Eysenbach and Kohler, 2002).
Conclusions
The committee’s assessment of the significance of concerns about possible neurological disorders as a result of hepatitis B vaccination took several factors into account: the burden of hepatitis B infection in infants, in adults, and in the population; the burden of neurological conditions, particularly MS; and indica-
tions of the extent of concern about immunizing infants and children against hepatitis B.
Although concern about neurological disorders following immunization is justified on the basis of their total burden to individuals and to society and the known relationship of some vaccines to some neurological conditions, the benefits of hepatitis B vaccine in preventing liver disease are undeniable. However, because the benefits of hepatitis B vaccine are realized long after immunization, and because the population at highest risk of hepatitis B infection is in adolescents, young adults, and members of high-risk occupations, the universal newborn and infant immunization recommendation is difficult for some parents to understand. The theoretical risks of the vaccine are salient for them, but the known benefits are not. The committee concludes that concerns about the hepatitis B vaccine remain significant in the minds of some parents and workers who are required to take the vaccine because of occupational risk.
RECOMMENDATIONS FOR PUBLIC HEALTH RESPONSE
The scientific and policy issues considered by the committee lead to recommendations for targeted public health attention. The committee has found that although there is no evidence of increased risk of MS in adults following hepatitis B vaccination, the evidence is inconclusive in regard to risk for MS in infants and children. The evidence is also inconclusive for the other neurological diseases considered. However, because the hepatitis B vaccine is recommended by federal and national advisory bodies for use in infants and required by states for school entry and for employment in high-risk occupations (such as the health care field) and because the basis for the recommendation for universal immunization for infants and children is not fully understood by some parents and some health care providers, public health attention in the form of further research and improved communication is required.
Policy Review
The committee does not recommend a policy review of the hepatitis B vaccine by any of the national and federal vaccine advisory bodies, on the basis of concerns about demyelinating neurological disorders.
Research
Although the committee concluded that the epidemiological evidence is inadequate to accept or reject a causal relationship between the hepatitis B vaccine and most of the demyelinating disorders it reviewed, the committee found a
theoretical basis for the hypothesis when it considered biological mechanisms. The committee identified only indirect and scant evidence that relevant biological mechanisms could be operational. Because none of the evidence is specific to the hepatitis B vaccine, the summary judgment was that the evidence is weak. Given this, and the fact that the committee identified very little information about the possible effects of the vaccine on demyelinating disorders in infants and children, the committee recommends further research.
Surveillance
The committee emphasizes the need for continuing surveillance of vaccine recipients and possible adverse events. MS rarely appears in childhood but begins to appear in early adulthood (Noseworthy et al., 2000; Waubant and Stuve, 2002). The evidence that the onset of MS may appear a decade or more after exposure to a risk factor (environmental or microbial) raises the possibility that long-term follow-up might be needed to determine an effect on the rate of MS. Because the hepatitis B vaccine has been routinely administered to newborns and infants since 1991, surveillance of this exposed and aging group provides an opportunity to study its incidence of MS. The incidence of other central and peripheral nervous system disorders, such as optic neuritis, ADEM, GBS, brachial neuritis, and transverse myelitis should also be examined in this group. In addition, there should be continued surveillance of health workers who have received the vaccine. The committee recommends surveillance of MS and other central and peripheral nervous system demyelinating disorders, specifically in health care workers and those born since 1991.
Surveillance of these outcomes would be strengthened by having standard diagnostic approaches and case definitions to permit epidemiologic investigations. The lack of standardized case definition for adverse events following vaccination is a recurring concern for the committee and for all who study immunization safety. Specifically, the committee recommends the development of case definitions and guidance for diagnostic evaluation of the demyelinating disorders it has reviewed for purposes of improved vaccine adverse event surveillance and, when appropriate, causality assessment. The committee notes and encourages the work recently begun by the Brighton Collaboration to develop, through an international consensus process, a set of standard definitions for adverse events (brightoncollaboration.org), as well as the newly established Clinical Immunization Safety Assessment centers (www.cdc.gov/programs/immun8.htm). The committee has not reviewed these efforts in sufficient detail to recommend whether or not the entities behind them can or should assume the responsibility for this recommendation, or whether a distinct effort is needed.
Infection with the hepatitis B virus increases the risk of developing cirrhosis and hepatocellular carcinoma (El-Serag, 2001). Surveillance of these secondary outcomes of hepatitis B infection may provide a clearer understanding of the impact of the hepatitis B vaccination program. Continued surveillance of acute infections also remains important for the same reason. Furthermore, because hepatitis B infections can be asymptomatic, supplemental surveillance, such as the serological testing that has been conducted as part of the National Health and Nutrition Examination Survey, is needed for more accurate estimates of incidence and prevalence. Therefore, the committee recommends continued surveillance of hepatitis B disease and increased surveillance of secondary diseases, such as cirrhosis and hepatocellular carcinoma.
Basic and Clinical Science
The committee has not recommended large-scale epidemiological studies at this time to address concerns about the demyelinating conditions it has reviewed. But in recognition of its inability to reject causality for most of these conditions, and of the limited evidence regarding biological mechanisms, the committee recommends continued research in animal and in vitro models, as well as in humans, on the mechanisms of immune-mediated neurological disease possibly associated with exposure to vaccines.
Communication
The committee has continuing concerns that the public’s need for relevant information is not being effectively met. The committee again recommends that government agencies and professional organizations responsible for immunizations critically evaluate their communication services with increased understanding of and input from the intended users. It is important to ensure that the content and format of communication methods and tools are appropriate, readily accessible, and relevant to the public.
SUMMARY
Immunization advisory bodies recommend that all infants, adolescents, and high-risk adults receive the hepatitis B vaccine for protection from serious liver disease, including cirrhosis and hepatocellular carcinoma. These recommendations have been viewed skeptically by some because of concerns about the safety of the vaccine and because of a perception that hepatitis B infection is not a serious risk to the general population. The Immunization Safety Review Committee reviewed the evidence regarding the hypothesis that the hepatitis B
vaccine causes demyelinating neurological disorders, such as multiple sclerosis and Guillain-Barré syndrome.
There is a theoretical basis for the hypothesis that vaccines, including the hepatitis B vaccine, could cause demyelinating disorders. A review of the scant and indirect evidence that relevant biological mechanisms could operate in humans in response to the hepatitis B vaccine to produce disease provides weak support for this theory. However, the committee found that the epidemiological evidence (i.e., from studies of vaccine-exposed populations and their control groups or of patients with these diseases and their control groups) favors rejection of a causal relationship between the hepatitis B vaccine in adults and multiple sclerosis. The evidence was inadequate to accept or reject a causal relationship between the hepatitis B vaccine and all other demyelinating conditions.
Demyelinating disorders are often quite devastating, as are the conditions resulting from chronic hepatitis B infection. The committee found evidence that some parents and health care workers are skeptical about the vaccine more due to a perception that the vaccine is unnecessary, rather than due to a large concern about the safety of the vaccine. The committee is aware, however, that there are some people who very much object to the vaccine both on the basis of the perception that not all infants and children are at risk for hepatitis B infection and on the basis of concerns about its safety. Because of the lack of epidemiological data on conditions other than MS in adults, the committee recommends further attention in the form of research and communication.
However, the committee does not recommend that national and federal vaccine advisory bodies review the hepatitis B vaccine on the basis of concerns about demyelinating disorders. See Box 2 for a summary of all conclusions and recommendations.
|
BOX 2 Committee Conclusions and Recommendations SCIENTIFIC ASSESSMENT Causality Conclusions The committee concludes that the evidence favors rejection of a causal relationship between hepatitis B vaccine administered to adults and incident multiple sclerosis. The committee also concludes that the evidence favors rejection of a causal relationship between hepatitis B vaccine administered to adults and multiple sclerosis relapse. The committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and the first episode of a central nervous system demyelinating disorder. The committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and ADEM. |
|
The committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and optic neuritis. The committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and transverse myelitis. The committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and GBS. The committee concludes that the evidence is inadequate to accept or reject a causal relationship between hepatitis B vaccine and brachial neuritis. SIGNIFICANCE ASSESSMENT The committee concludes that concerns about the hepatitis B vaccine remain significant in the minds of some parents and workers who are required to take the vaccine because of occupational risk. PUBLIC HEALTH RESPONSE RECOMMENDATIONS Policy Review The committee does not recommend a policy review of the hepatitis B vaccine by any of the national and federal vaccine advisory bodies on the basis of concerns about demyelinating neurological disorders. The committee recommends continued surveillance of hepatitis B disease and increased surveillance of secondary diseases such as cirrhosis and hepatocellular carcinoma. Basic and Clinical Science The committee recommends continued research in animal and in vitro models, as well as in humans, on the mechanisms of immune-mediated neurological disease possibly associated with exposure to vaccines. Communication The committee again recommends that government agencies and professional organizations responsible for immunizations critically evaluate their communication services with increased understanding of, and input from, the intended users |
REFERENCES
Albert LJ, Inman RD. 1999. Molecular mimicry and autoimmunity. N Engl J Med 341(27):2068–74.
Albitar S, Bourgeon B, Genin R, Fen-Chong M, N’Guyen P, Serveaux MO, Atchia H, Schohn D. 1997. Bilateral retrobulbar optic neuritis with hepatitis B vaccination. Nephrol Dial Transplant 12(10):2169–70.
Alter M, Leibowitz U, Speer J. 1966. Risk of multiple sclerosis related to age at immigration to Israel. Arch Neurol 15(3):234–7.
Alter MJ, Hadler SC, Margolis HS, Alexander WJ, Hu PY, Judson FN, Mares A, Miller JK, Moyer LA. 1990. The changing epidemiology of hepatitis B in the United States. Need for alternative vaccination strategies. JAMA 263(9):1218–22.
Armstrong GL, Mast EE, Wojczynski M, Margolis H. 2001. Childhood hepatitis B virus infections in the United States before hepatitis B immunization. Pediatrics 108(5):1123–1128.
Ascherio A, Zhang SM, Hernan MA, Olek MJ, Coplan PM, Brodovicz K, Walker AM. 2001. Hepatitis B vaccination and the risk of multiple sclerosis. N Engl J Med 344(5):327–332.
Bach JF, Chatenoud L. 2001. Tolerance to islet autoantigens in type 1 diabetes. Annu Rev Immunol 19:131–61.
Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, Comi G, Ader HJ, Losseff N, Valk J. 1997. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain; 120 (Pt 11):2059–69.
Benoist C, Mathis D. 2001. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol 2(9):797–801.
Bertolino JG. 1996. Newborn hepatitis B immunization rates in primary care practices. Arch Pediatr Adolesc Med 150(11):1173–6.
Biacabe B, Erminy M, Bonfils P. 1997. A case report of fluctuant sensorineural hearing loss after hepatitis B vaccination. Auris Nasus Larynx 24(4):357–60.
Catanzaro M, Weinert C. 1992. Economic status of families living with multiple sclerosis. Int J Rehabil Res 15(3):209–18.
CDC (Centers for Disease Control and Prevention). 1990. Protection against viral hepatitis: recommendations of the Immunization Practices Advisory Committee (ACIP). Morb Mortal Wkly Rep 39(RR-2):1–26.
CDC. 1991. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: Recommendations of the Immunization Practices Advisory Committee (ACIP). Morb Mortal Wkly Rep 40(RR-13):1–19 .
CDC. 1996. Recommended childhood immunization schedule—United States, January-June 1996. MMWR Morb Mortal Wkly Rep 44(51–52):940–3.
CDC. 2001a. Viral hepatitis B: Fact sheet.
CDC. 2001b. Influenza vaccine 2001–2002. Vaccine Information Sheet.
CDC. 2001c. National, state, and urban area vaccination coverage levels among children aged 19–35 months—United States, 2000. Morb Mortal Wkly Rep 50(30):637–41.
CDC. 2002. Descriptive summary of VAERS reports indicating possible demyelinating disease following hepatitis-B vaccination, all ages, 1999–2001. Sent to Immunization Safety Review Committee by the Centers for Disease Control and Prevention.
Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. 1997. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med 336(26):1855–9.
Coleman PJ, McQuillan GM, Moyer LA, Lambert SB, Margolis HS. 1998. Incidence of hepatitis B virus infection in the United States, 1976–1994: estimates from the National Health and Nutrition Examination Surveys. J Infect Dis 178(4):954–9.
Confavreux C, Suissa S, Saddier P, Bourdes V, Vukusic S, for the Vaccines in Multiple Sclerosis Study Group. 2001. Vaccinations and the risk of relapse in multiple sclerosis. N Engl J Med 344(5):319–326.
Cooper A, Yusuf H, Rodewald L, Malik T, Pollard R, Pickering L. 2001. Attitudes, practices, and preferences of pediatricians regarding initiation of hepatitis B immunization at birth. Pediatrics 108(6):E98.
Creange A, Temam G, Lefaucheur JP. 1999. Lumbosacral acute demyelinating polyneuropathy following hepatitis B vaccination. Autoimmunity 30(3):143–6.
Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. 2000. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain 123(Pt 12):2407–22.
Davidson A, Diamond B. 2001. Autoimmune diseases. N Engl J Med 345(5):340–50.
DeStefano F. 2002. Risk of Demyelinating Disease after Hepatitis B Vaccination: The Vaccine Safety Datalink. Presentation to Immunization Safety Review Committee.
Dittmann S. 2000. Special address: Safety of hepatitis B vaccination. Vaccine 18 (Suppl 1):S10–1.
Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. 1993. The influence of age on the development of the hepatitis B carrier state. Proc R Soc Lond B Biol Sci 253(1337):197–201.
El-Serag HB. 2001. Epidemiology of hepatocellular carcinoma. Clin Liver Dis 5(1):87–107, vi.
Ellenberg S, Chen R. 1997. The complicated task of monitoring vaccine safety. Public Health Reports 112:10–20.
Eysenbach G, Kohler C. 2002. How do consumers search for and appraise health information on the world wide web? Qualitative study using focus groups, usability tests, and in-depth interviews. BMJ 324(7337):573–7.
Fisher BL. Comments made at January 11, 2001 Meeting. Institute of Medicine. Washington, DC.
Fourrier A, Bégaud B. 2002. Pharmacovigilance and Case-Control Study of Hepatitis B Vaccine and Multiple Sclerosis. Presentation to Immunization Safety Review Committee.
Fourrier A, Bégaud B, Alperovitch A, Verdier-Taillefer M, Touzé E, Decker N, Imbs J. 2001. Hepatitis B vaccine and first episodes of central nervous system demyelinating disorders: A comparison between reported and expected number of cases. Br J Clin Pharmacol 51(489–490).
Fourrier A, Touzé E, Alperovitch A, Bégaud B. 1999. Association between hepatitis B vaccine and multiple sclerosis: a case-control study. Pharmacoepidemiol Drug Safety; 8:S140-S141.
Francis DP, Feorino PM, McDougal S, Warfield D, Getchell J, Cabradilla C, Tong M, Miller WJ, Schultz LD, Bailey FJ, et al. 1986. The safety of the hepatitis B vaccine. Inactivation of the AIDS virus during routine vaccine manufacture. JAMA 256(7):869–72.
Freed GL, Bordley WC, Clark SJ, Konrad TR. 1994. Universal hepatitis B immunization of infants: reactions of pediatricians and family physicians over time. Pediatrics 93(5):747–51.
Fujinami RS, Oldstone MB. 1985. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science 230(4729):1043–5.
Gran B, Bielekova B, McFarland HF, Martin R. 2000. Development of multiple sclerosis after hepatitis B vaccination: An immunologic case report. Neurology 54(Suppl 3):A164.
Hahn AF. 1996. Experimental allergic neuritis (EAN) as a model for the immune-mediated demyelinating neuropathies. RevNeurol (Paris) 152(5):328–32.
Halsey NA. 1993. Discussion of Immunization Practices Advisory Committee/American Academy of Pediatrics recommendations for universal infant hepatitis B vaccination. Pediatr Infect Dis J 12(5):446–9.
Halsey NA. 2002. Recommendations for Newborn and Infant Hepatitis B Vaccination: Historical Perspective. Presentation to Immunization Safety Review Committee.
Herroelen L, de Keyser J, Ebinger G. 1991. Central-nervous-system demyelination after immunisation with recombinant hepatitis B vaccine. Lancet 338(8776):1174–5.
Hill AB. 1965. The environment and disease: Association or causation? Proc R Soc Med 58:295– 300.
Hilleman MR. 2001. Overview of the pathogenesis, prophylaxis and therapeusis of viral hepatitis B, with focus on reduction to practical applications. Vaccine 19(15–16):1837–48.
Hjorth RN, Bonde GM, Piner E, Hartzell RW, Rorke LB, Rubin BA. 1984. Experimental neuritis induced by a mixture of neural antigens and influenza vaccines. A possible model for Guillain-Barre syndrome. J Neuroimmunol 6(1):1–8.
Hughes RA, Raphael JC, Swan AV, van Doorn PA. 2001. Intravenous immunoglobulin for Guillain-Barre syndrome. Cochrane Database Syst Rev (2):CD002063.
Hughes RA, van der Meche FG. 2000. Corticosteroids for treating Guillain-Barre syndrome. Cochrane Database Syst Rev (2):CD001446.
Hynson JL, Kornberg AJ, Coleman LT, Shield L, Harvey AS, Kean MJ. 2001. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology 56(10):1308– 12.
IOM (Institute of Medicine). 1991. Adverse Events Following Pertussis and Rubella Vaccines. Washington, DC: National Academy Press.
IOM. 1994. Adverse Events Associated -with Childhood Vaccines: Evidence Bearing on Causality. Washington, DC: National Academy Press.
IOM. 2001a. Immunization Safety Review: Measles-Mumps-Rubella Vaccine and Autism. Washington, DC: National Academy Press.
IOM. 2001b. Immunization Safety Review: Thimerosal-Containing Vaccines and Neurodevelopmental Disorders. Washington, DC: National Academy Press.
IOM. 2001c. Multiple Sclerosis: Current Status and Strategies for the Future. Washington, DC: National Academy Press.
IOM. 2002. Immunization Safety Review: Multiple Immunizations and Immune Dysfunction. Washington DC: National Academy Press.
Jefferson T, Heijbel H. 2001. Demyelinating disease and hepatitis B vaccination: Is there a link? Drug Saf 24(4):249–54.
Kinnunen E, Junttila O, Haukka J, Hovi T. 1998. Nationwide oral poliovirus vaccination campaign and the incidence of Guillain-Barré Syndrome. Am J Epidemiol 147(1):69–73.
Konstantinou D, Paschalis C, Maraziotis T, Dimopoulos P, Bassaris H, Skoutelis A. 2001. Two episodes of leukoencephalitis associated with recombinant hepatitis B vaccination in a single patient. Clin Infect Dis 33(10):1772–3.
Kriebel D, Tickner J. 2001. Reenergizing public health through precaution. Am J Public Health 91(9):1351–5.
Kurtzke JF, Beebe GW, Nagler B, Nefzger MD, Auth TL, Kurland LT. 1970. Studies on the natural history of multiple sclerosis. V. Long-term survival in young men. Arch Neurol 22(3):215–25.
Lasky T, Terracciano GJ, Magder L, Koski CL, Ballesteros M, Nash D, Clark S, Haber P, Stolley PD, Schonberger LB, Chen RT. 1998. The Guillain-Barre syndrome and the 1992–1993 and 1993–1994 influenza vaccines. N Engl J Med 339(25):1797–802.
Lee WM. 1997. Hepatitis B virus infection. N Engl J Med 337(24):1733–5.
Loewenson PR, White KE, Osterholm MT, MacDonald KL. 1994. Physician attitudes and practices regarding universal infant vaccination against hepatitis B infection in Minnesota: Implications for public health policy. Pediatr Infect Dis J 13(5):373–8.
Lublin FD, Reingold SC. 1996. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 46(4):907–11.
Maddrey WC. 2000. Hepatitis B: An important public health issue. J Med Virol 61(3):362–6.
Maddrey WC. 2001. Hepatitis B—an important public health issue. Clin Lab 47(1–2):51–5.
Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. 1995. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. JAMA 274(15):1201–8.
Marrack P, Kappler J, Kotzin BL. 2001. Autoimmune disease: Why and where it occurs. Nat Med 7(8):899–905.
Marshall E. 1998. A shadow falls on hepatitis B vaccination effort. Science 281(5377):630–1.
Mast E. 2002. Recent Analyses of Hepatitis B Viral Infections in Children. Presentation to Immunization Safety Review Committee.
McCarty EC, Tsairis P, Warren RF. 1999. Brachial neuritis. Clin Orthop (368):37–43.
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS. 2001. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 50(1):121–7.
McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, Maynard JE. 1985. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 151(4):599–603.
Miller JD, Pruitt S, McDonald TJ. 2000. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician 62(9):2067–72.
Monteyne P, Andre FE. 2000. Is there a causal link between hepatitis B vaccination and multiple sclerosis? Vaccine 18(19):1994–2001.
Murthy JM. 1998. MRI in acute disseminated encephalomyelitis following Semple antirabies vaccine. Neuroradiology 40(7):420–3.
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. 2000. Multiple sclerosis. N Engl J Med 343(13):938–52.
Oksenberg JR, Baranzini SE, Barcellos LF, Hauser SL. 2001. Multiple sclerosis: Genomic rewards. J Neuroimmunol 113(2):171–84.
Plesner AM, Arlien-Soborg P, Herning M. 1998. Neurological complications to vaccination against Japanese encephalitis. Eur J Neurol 5(5):479–485.
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW. 1983. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13(3):227–31.
Purcell GP, Wilson P, Delamothe T. 2002. The quality of health information on the Internet. BMJ 324(7337):557–8.
Purvin V. 1998. Optic neuritis. Curr Opin Ophthalmol 9(6):3–9.
Rantala H, Cherry JD, Shields WD, Uhari M. 1994. Epidemiology of Guillain-Barré syndrome in children: Relationship of oral polio vaccine administration to occurrence. J Pediatr 124(2):220–3.
Raphael JC, Chevret S, Hughes RA, Annane D. 2001. Plasma exchange for Guillain-Barre syndrome. Cochrane Database Syst Rev (2):CD001798.
Regner M, Lambert PH. 2001. Autoimmunity through infection or immunization? Nat Immunol 2(3):185–8.
Rose NR. 2001. Infection, mimics, and autoimmune disease. J Clin Invest 107(8):943–4.
Rothwell PM, McDowell Z, Wong CK, Dorman PJ. 1997. Doctors and patients don’t agree: cross sectional study of patients’ and doctors’ perceptions and assessments of disability in multiple sclerosis. BMJ 314(7094):1580–3.
Rudick RA. 1999. Disease-modifying drugs for relapsing-remitting multiple sclerosis and future directions for multiple sclerosis therapeutics. Arch Neurol 56(9):1079–84.
Sadovnick AD, Scheifele DW. 2000. School-based hepatitis B vaccination programme and adolescent multiple sclerosis. Lancet 355(9203):549–50.
Samaranayake LP, Lamey PJ, MacFarlane TW, Glass GW. 1987. Attitudes of general dental practitioners towards the hepatitis B vaccine. Community Dent Oral Epidemiol 15(3):125–7.
Schafer DF, Sorrell MF. 1999. Hepatocellular carcinoma. Lancet 353(9160):1253–7.
Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. 2001. Acute disseminated encephalomyelitis: A follow-up study of 40 adult patients. Neurology 56(10):1313–8.
Shaw FE Jr, Graham DJ, Guess HA, Milstien JB, Johnson JM, Schatz GC, Hadler SC, Kuritsky JN, Hiner EE, Bregman DJ, Maynard JE. 1988. Postmarketing surveillance for neurologic adverse events reported after hepatitis B vaccination. Experience of the first three years. Am J Epidemiol 127(2):337–52.
Sheedy K on behalf of CDC. Personal communication regarding vaccine exemptions. 2002.
Silverman NS, Darby MJ, Ronkin SL, Wapner RJ. 1991. Hepatitis B prevalence in an unregistered prenatal population. Implications for neonatal therapy. JAMA 266(20):2852–5.
Singh B. 2000. Stimulation of the developing immune system can prevent autoimmunity. J Autoimmun 14(1):15–22.
Singleton JA, Lloyd JC, Mootrey GT, Salive ME, Chen RT. 1999. An overview of the vaccine adverse event reporting system (VAERS) as a surveillance system. Vaccine 17:2908–2917.
Soubeyrand B, Boisnard F, Bruel M, Debois H, Delattre D, Gauthier A, Soum S, Thebault C. 2000. Translated from [Central nervous system demyelinating disease following hepatitis B vaccination with GenHevac B. Review often years of spontaneous notifications (1989–1998)]. Presse Med 29(14):775–80.
Spence MR, Dash GP. 1990. Hepatitis B: Perceptions, knowledge and vaccine acceptance among registered nurses in high-risk occupations in a university hospital . Infect Control Hosp Epidemiol 11(3):129–33.
Sturkenboom MCJM, Abenhaim L, Wolfson C, Roulet E, Heinzelf O, Gout O. 1999. Vaccinations, demyelination, and multiple sclerosis study (VDAMS). Pharmacoepidemiol Drug Safety; 8:S170–S171.
Stuve O, Zamvil SS. 1999. Pathogenesis, diagnosis, and treatment of acute disseminated encephalomyelitis. Curr Opin Neurol 12(4):395–401.
Sutter RW, Cochi SL, Melnick JL. 1999. Live attenuated poliovirus vaccines. In Plotkin S, Orenstein W. Vaccines. 3rd ed. New York: W.B. Saunders Company. Pp. 364–408.
Tartaglino LM, Heiman-Patterson T, Friedman DP, Flanders AE. 1995. MR imaging in a case of postvaccination myelitis. Am J Neuroradiol 16(3):581–2.
Taylor H, Leitman R. 2002. Cyberchondriacs continue to grow in America. Health Care News 2(9):1–4.
Tintore M, Rovira A, Martinez MJ, Rio J, Diaz-Villoslada P, Brieva L, Borras C, Grive E, Capellades J, Montalban X. 2000. Isolated demyelinating syndromes: comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. R Am J Neuroradiol 21(4):702–6.
Tourbah A, Gout O, Liblau R, Lyon-Caen O, Bougniot C, Iba-Zizen MT, Cabanis EA. 1999. Encephalitis after hepatitis B vaccination: Recurrent disseminated encephalitis or MS? Neurology 53(2):396–401.
Touzé E, Gout O, Verdier-Taillefer MH, Lyon-Caen O, Alperovitch A. 2000. Translated from [The first episode of central nervous system demyelinization and hepatitis B virus vaccination]. Rev Neurol (Paris) 156(3):242–6.
U.S. Preventive Sevices Task Force. 1996. Guide to Clinical Preventive Services. 2nd ed. Baltimore: Williams and Wilkins.
Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. 1995. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity 2(3):261–70.
Vella AT, Mitchell T, Groth B, Linsley PS, Green JM, Thompson CB, Kappler JW, Marrack P. 1997. CD28 engagement and proinflammatory cytokines contribute to T cell expansion and long-term survival in vivo. J Immunol 158(10):4714–20.
Walsh K, Alexander GJ. 2001. Update on chronic viral hepatitis. Postgrad Med J 77(910):498–505.
Waubant E, Stuve O. 2002. Suspected mechanisms involved in multiple sclerosis and putative role of hepatitis B vaccine in multiple sclerosis. Commissioned Background Paper for IOM Immunization Safety Review Committee.
Weed DL, Hursting SD. 1998. Biologic plausibility in causal inference: Current method and practice. Am J Epidemiol 147(5):415–25.
Wise RP, Kiminyo KP, Salive ME. 1997. Hair loss after routine immunizations. JAMA 278(14):1176–8.
Wucherpfennig KW. 2001. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest 108(8):1097–104.
Yusuf HR, Daniels D, Smith P, Coronado V, Rodewald L. 2000. Association between administration of hepatitis B vaccine at birth and completion of the hepatitis B and 4:3:1:3 vaccine series. JAMA 284(8):978–83.
Zinkemagel RM. 2001. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med 345(18):1331–5.
Zipp F, Weil JG, Einhaupl KM. 1999. No increase in demyelinating diseases after hepatitis B vaccination. Nat Med 5(9):964–5.