3
Animals Engineered for Human Health Purposes
INTRODUCTION
Since genetic engineering has the potential to alter the uses to which domestic animals are put, it also can lead to fundamental changes in the relationship between (1) individuals of the same species or population, (2) different species, (3) engineered animals and their products, and (4) the products and humans. There currently are major research efforts underway to develop the use of genetically engineered animals as sources for production of nontraditional materials for human use. Such uses can be divided into three major categories: biopharmaceuticals for animal or human use; live cells, tissues, and organs for xenotransplantation; and raw materials for processing into other useful end products (the latter use is discussed in Chapter 4). Several possible concerns that might in practice arise from the first two uses are discussed in the following sections.
BIOPHARMACEUTICAL PRODUCTION
A large number of genes encoding useful protein products—hormones, blood proteins, and others—has been introduced into domestic animals, leading to their expression in milk, eggs, or blood (Dove, 2000; Table 3.1). So far, none of these animals has been used for commercial production. However, a recent report suggests that the same technology might be extended to the large-scale
production of vaccines (Stowers et al., 2001). Such “biopharming” applications have the potential to use well-established agricultural methods to produce large amounts of valuable products at relatively low expense as compared to fermentation. Although the end products of these applications will be novel, by and large, the process of production and the potential concerns are not likely to differ greatly from those seen in current practice, such as the use of animals or animal cell cultures to prepare live vaccines (Brown et al., 2001), hormones, or traditional products such as meat, milk, or leather. These standard products are subject to specific regulatory procedures, and essentially the same regulatory framework is expected to apply for products of both biopharming and standard technology as regards common issues such as purity of the final product, microbial contamination, levels of adventitious DNA, and the like. Nevertheless, a few more specialized concerns arise.
Contamination or Spread of Novel Pathogens
As discussed in Chapter 2, there is a theoretical potential for microorganisms to acquire—by recombination or transduction—genes from the vector constructs used to insert the transgene. Although there is no example yet of acquisition of any gene, including drug resistance markers, by bacterial flora living in a transgenic animal, the spread of introduced genes remains a possibility, albeit remote.
Of greater concern is the possibility for generation of potentially pathogenic viruses by recombination between sequences of the vector used to introduce a transgene and related, but nonpathogenic, viruses that might be present in the same animal. These concerns are particularly acute for retroviral vectors. Retroviruses appear to be efficient vehicles for inserting transgenes into many species, including chickens (Crittenden and Salter, 1990; Briskin et al., 1991), mice (Jahner et al., 1985), cattle (Chan et al., 1998), fish, and shellfish (Sarmasik et al., 2001), and might prove more successful than pronuclear injection of DNA in the generation of transgenic offspring. In many species, including chickens and pigs, there are endogenous proviruses (including the porcine endogenous viruses, PERVs, discussed below) that are competent for low-level replication in the host animal, but have no apparent pathogenic consequences (Boeke and Stoye, 1997). Endogenous proviruses are DNA sequences that were derived from infection of germline cells with a retrovirus and that are transmitted from parent to progeny like any normal gene. Their attenuation relative to their exogenous, pathogenic counterparts often is due to differences in transcriptional regulatory sequences in long terminal repeats (LTR’s; Rosenberg and Jolicoeur, 1997). Since many vectors, such as the widely-used ones derived from murine leukemia virus (MLV), have LTR sequences derived from pathogenic viruses, the presence of both vector and
endogenous provirus in all cells of a transgenic animal provides the potential for generating pathogenic recombinant viruses by straightforward and well-understood mechanisms. Such concerns are particularly acute in chickens and pigs, where infectious proviruses very similar in sequence to those used for vectors are known to be present (Boeke and Stoye, 1997). In mice, there is a well-studied model in which recombination between benign endogenous proviruses or endogenous proviruses, and infecting viruses early in the life of the animal, can cause a high incidence of lymphoma (nearly 100 percent in some mouse strains) 6 months later (Stoye et al., 1991; Rosenberg and Jolicoer, 1997). Given this example, it is reasonable to expect that viruses of much greater pathogenicity are likely to arise in an animal when there is a possibility of recombination between vector and endogenous viral sequences.
Similar concerns arise with the use of vectors based on lentiviruses for the introduction of genes (see Chapter 2). Recombination of lentiviruses in circulation in domestic animal populations, such as Feline Immunodeficiency Virus (FIV) in cats and Bovine Immunodeficiency Virus (BIV) in cattle, with vectors based on Human Immunodeficiency Virus (HIV) is improbable due to the large genetic distance between them. However, vectors based on FIV and BIV are being developed (Curran et al., 2000; Berkowitz et al., 2001), and their use to introduce transgenes into the corresponding species would significantly increase the probability of generating more pathogenic recombinants.
TABLE 3.1 Potential uses of transgenic animals for pharmaceutical production.
|
Species |
Theoretical Yield (g/yr of Raw Protein) |
Examples of Products Under Development |
|
Chicken |
250 |
Monoclonal antibodies Lysozyme Growth hormone Insulin Human serum albumin |
|
Rabbit |
20 |
Calcitonin Superoxide dismutase Erythropoietin Growth hormone IL-2 α-glucosidase |
|
Goat |
4,000 |
Antithrombin III Tissue plasminogen activator Monoclonal antibodies α-1-Antitrypsin Growth hormone |
|
Sheep |
2,500 |
α-1-Antitrypsin |
|
|
|
Factor VIII Factor IX Fibrinogen |
|
Cow |
80,000 |
Human serum albumin Lactoferrin α-Lactalbumin |
|
Source: Modified from Dove, 2000. |
||
Ensuring Confinement of Unwanted Animals
Although biopharm animals are not intended for consumption by humans or other animals, there are grounds for concern that adequate controls be in place to ensure that this does not happen without appropriate approval (see Chapter 4). As long as they do not contain the product of the introduced gene, there might be no strong reason to believe that eating or using products from transgene-containing animals would pose a threat to human health; the possibility of such a threat combined with the lack of regulatory oversight for such uses argues strongly for confinement measures.
Although it has been stated that such animals will be too valuable to the owners to allow their misappropriation (Wall, 2001), the fact that the products of interest usually are produced only by lactating females means that half the transgene-containing animals essentially will be valueless, as will the females at the end of their period of useful production. “No takes,” or animals generated from manipulated embryos, but culled because of lack appropriate expression of the transgene product (or lacking the transgene itself) also are inevitably generated in significant numbers during the production of transgenics. Thus, companies using biopharm animals are likely to seek approval for marketing food or rendered products from surplus animals, and the regulatory agencies will need to be ready to deal with such requests. Of greater concern is the possibility that surplus animals (and their carcasses) might, through inadvertence or theft, find their way into the food or rendering chain, or be used for breeding, thus allowing uncontrolled spread of the transgene into the general population. This would create a regulatory problem of dealing with unapproved transgenes after their release into the food chain—a problem analogous to that posed by the appearance in food products of Starlink, a transgenic maize unapproved at the time for human consumption (Fox, 2001).
XENOTRANSPLANTATION
Xenotransplantation differs from other uses of genetically engineered animals in that it has the potential to create something entirely new—permanent
human–animal chimeras—in which cells of distantly-related species survive and function for long periods of time in the most intimate contact possible. Given its potential for alleviating human diseases due to irreversible tissue or organ failure (Table 3.2), and given the acute shortage of human organs for transplant, there are very active research programs underway, in both commercial and academic laboratories, to overcome the significant immunologic and physiologic barriers, and thereby to bring xenotransplantation into standard medical practice. This topic and associated concerns about infection have been reviewed in great detail elsewhere (Boneva et al., 2001), and only an overview is given here.
At present, the only animal under serious consideration as a xenotransplant donor is the pig. For regulatory purposes, human cells cultured ex vivo with the cells of any other animal, such as mouse cell lines, also are considered to be xenotransplants (DHHS, 2001); co-cultivation with mouse cell lines has been used in the preparation of some cultured skin grafts as well as human stem cell lines; Thomson et al., 1998). While nonhuman primates, such as the baboon, would seem to have physiologic and immunogenetic advantages such as the lack of a hyperacute immune response, their scarcity as well as the difficulty of clearing them of adventitious infectious agents (as well as ethical concerns) render them impractical for further consideration.
The field of xenotransplantation covers a great many procedures, ranging from implantation of single cells to treat Parkinson’s disease and tissues, such as pancreatic islets, to treat diabetes; extracorporeal use of intact organs, such as perfusion of patient blood through pig livers to provide short-term support in cases of liver failure; to transplantation of whole organs—heart, kidney, liver, and so on. While whole-organ xenotransplantation remains far in the future, development of the simpler modalities is underway, and hundreds of human
TABLE 3.2 Applications of xenotransplantation.
subjects have received porcine cells or tissues as part of clinical trials in the United States, Russia, Israel, and many European countries (Paradis et al., 1999). Given the nature of infectious disease issues, regulatory concerns are not limited to the United States alone, but extend to the international health community as well.
The development of xenotransplantation as a part of clinical practice promises great benefits in terms of making possible essentially infinite supplies of replacement tissues and organs where severe shortages exist today. This development naturally will entail both great potential benefit as well as considerable risk to the study participant, but such risk is not qualitatively different from that entailed in the development of any other new medical procedure and will not be considered further. The principal concern is that the uniquely close relationship created between recipient and host will allow novel opportunities for transmission of infectious disease, and possible creation of new disease agents in the process. While the history of close contact between humans and pigs is a very long one, and one would imagine that all possible transmission of infectious agents between the two species already would have been seen and thoroughly studied, it is possible that the “co-culture” environment of a transplant would be qualitatively different in ways that would allow different outcomes. Two different types of agents are discussed separately.
Exogenous Infectious Agents
In general, bacteria and parasites that might cause problems readily can be excluded from source flocks, leaving viruses as the principal concern (Onions et al., 2000). As can be seen in Table 3.3, the number of viral agents that are of potential concern is very large. Not all of the viruses are on the list because of their potential to cause human disease; some would cause serious disease among the donor animals and others are sensitive indicators of breaks in biosecurity, and so forth. In principle, since all of theses agents are horizontally (one animal to another) or vertically (mother to offspring) transmitted, they can be eliminated by proper management—proper containment, vaccination, close monitoring, culling, birth by Caesarian section, etc. In practice, elimination is going to prove a very difficult task, since the numbers of agents are very large and there is a lack of reliable assays for detecting many of them. Nevertheless, problems resulting from transmission of exogenous infectious agents are not qualitatively different from the present situation with human donors (allotransplantation), where infection with agents transmitted with the transplanted organ (such as Epstein–Barr virus and cytomegalovirus) is a major problem. In fact, it is anticipated that reduction in the risk of acute morbidity
TABLE 3.3 Exogenous pig viruses of concern in xenotransplantation.
|
Family |
Species |
Category |
|
Picornaviridae |
Foot and mouth disease |
|
|
|
Enterovirus 1 Talfan/Teschen |
2, 5 |
|
|
Enterovirus (other serogroups) |
5 |
|
|
Enterovirus swine vesicular disease |
5 |
|
|
Human enteroviruses |
1 |
|
|
Encephalomyocarditis |
|
|
|
Rhinovirus |
5 |
|
Caliciviridae |
Enteric calicivirus |
1 |
|
|
Swine hepatitis E |
1 |
|
Astroviridae |
Porcine astrovirus |
5 |
|
Togaviridae |
Western encephalitis |
1 |
|
|
Eastern encephalitis |
1 |
|
|
Venezuelan encephalitis |
1 |
|
|
Getah |
1 |
|
|
Chikungunya |
1 |
|
Flaviviridae |
Japanese B encephalitis |
1 |
|
|
Louping Ill/TBE complex |
1 |
|
|
Wesslebron disease |
1 |
|
|
Apoi |
2 |
|
|
Dengue fever |
1 |
|
|
West Nile fever |
1 |
|
|
Classical swine fever (hog cholera) |
5 |
|
|
Bovine viral diarrhoea |
5 |
|
|
Border disease |
5 |
|
Coronaviridae |
Transmissible gastroenteritis |
4, 5 |
|
|
Porcine respiratory coronavirus |
4, 5 |
|
|
Epidemic diarrhea |
4, 5 |
|
|
Haemagglutinating encephalomyelitis |
4, 5 |
|
|
Porcine reproductive & respiratory disease syndrome |
4, 5 |
|
|
Porcine torovirus |
5 |
|
Paramyxoviridae |
Murine parainfluenza virus type 1 (Sendai) |
2 |
|
|
Parainfluenza 2 |
2* |
|
|
Parainfluenza 3 |
2 |
|
|
Blue eye disease |
5 |
|
|
Menangle |
1 |
|
|
Nipah |
1 |
|
Rhabdoviridae |
Vesicular stomatitis |
1 |
|
|
Rabies |
1 |
|
Bornaviridae |
Bornavirus |
2, 5 |
|
Orthomyxoviridae |
Influenza A |
1 |
|
|
Influenza C |
5 |
|
Bunyaviridae |
Akabane |
1, 5 |
|
|
Batai |
1, 5 |
|
|
Hantavirus |
1, 5 |
|
Arenaviridae |
Lymphocytic choriomeningitis |
1, 5 |
|
Reoviridae |
Ibaraki |
5 |
|
|
Reovirus 1 to 3 |
2 |
|
|
Rotavirus A, B, C, E. |
2 |
|
Birnaviridae |
Porcine picobirnavirus |
5 |
|
Retroviridae |
Porcine endogenous |
2 |
|
Hepadnaviridae |
Hepatitis B |
|
|
Circoviridae |
Porcine circovirus |
5 |
|
Parvoviridae |
Porcine parvovirus |
4, 5 |
|
Papovaviridae |
Porcine polyomavirus |
3 |
|
|
Porcine genital papillomavirus |
3, 5 |
|
Adenoviridae |
Porcine adenovirus serotypes 1 to 4 |
3 |
|
Herpesviridae |
Pseudorabies |
2 |
|
|
Porcine cytomeglovirus |
5 |
|
|
Porcine lymphotropic herpesvirus type 1 |
3 |
|
|
Porcine lymphotropic herpesvirus type 2 |
3 |
|
Poxviridae |
Swinepox |
5 |
|
|
Vaccinia |
2 |
|
|
Cowpox |
1, 5* |
|
|
Orf/pseudocowpox |
1, 5* |
|
Desoxyviridae |
African swine fever |
5 |
|
NOTE: 1=Zoonotic. 2=Replicates in human cells or weak evidence for zoonotic potential. 3=Might undergo abortive replication and possibly oncogenic replication. 4=Belongs to a family with evidence of frequent changes in host range or pathogenicity. 5=Undesirable as indicates a breakdown in biosecurity and/or might compromise health of the pigs. *=Although the virus has not been detected in pigs, it has been included for reasons such as its wide host range. Source: Onions et al., 2000. Courtesy of D. Onions. |
||
and mortality resulting from the transmission of infectious agents with transplanted organs will be a significant benefit of xenotransplantation.
Porcine Endogenous Retroviruses
PERVs present quite a different situation and level of concern since they are inherited as part of the host genome and, therefore, cannot be removed easily from donor animals. All pigs contain multiple (around 50) PERV proviruses in their genome, at least several of which encode infectious virus. PERVs are gammaretroviruses, closely related to MLV, that can be classified into three subtypes, A, B, and C, based on their envelope gene sequences (Takeuchi et al., 1998). Subtypes A and B can infect many types of human cells in culture. Subtype C is much less infectious for humans. Most breeds of pig carry proviruses capable of yielding infectious virus of all three subtypes. Although most pigs carry about the same number of proviruses in their DNA, there is considerable diversity in location, implying that their insertion into the genome must have occurred rather recently (on an evolutionary time scale). Based on extensive experience with related endogenous proviruses of mice, it is highly likely that the majority of proviruses contain some sort of genetic defect, and that only a small number are responsible for release of infectious virus. Taken together with the polymorphism in the presence or absence of specific proviruses, it might well be possible to breed animals lacking infectious proviruses for use as xenotransplant donors.
PERVs have not yet been shown to cause disease (or even viremia) in pigs or any other species in which they have been detected. Nor has their presence been detected (by polymerase chain reaction, PCR, or serology) in more than 150 human recipients of pig cells or tissues (Paradis et al., 1999), although a low level of infection of recipient cells can be observed in immunodeficient mice transplanted with porcine islets of Langerhans (Van der Laan et al., 2000). Nevertheless, given the release of viruses infectious to human cells by many types of pig cells; the close similarity of these viruses to viruses known to cause cancer, immunodeficiency, and other diseases in mice and cats; the well-known adaptability and variability of retroviruses; and the example of the rapid worldwide spread of HIV and AIDS, there is serious concern that the novel association between pig and human tissues might create novel evolutionary opportunities for the virus, leading to the appearance of a new pathogen. Although such a pathogen could have serious long-term adverse consequences for the transplant recipient, this issue is not an area of concern since it is far outweighed by the potential benefits of the transplant. The real issue of concern is that the xenotransplant setting might provide the opportunity for the virus to evolve into a pathogen that also could be transmitted from one individual to another efficiently enough to create a new epidemic disease.
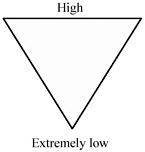
Such an evolutionary pathway would require a series of events, each increasingly improbable, as indicated by the scale shown in Table 3.4 (J. P. Stoye, 2001). As implied by the table, it is virtually certain that many cells in the transplant would express infectious PERV following transplantation, and it
is likely that some local infection of host cells would occur. The subsequent events necessary for generation of pathogenic, transmissible viruses increasingly are unlikely, but on some unknown, arbitrary scale. Although the probability of inadvertent creation of a new epidemic generally is judged to be extremely small (particularly given the long history of intimate association between humans and pigs), it cannot be ignored altogether. Current FDA policy is to permit xenotransplantation trials to proceed, but to require close monitoring of recipients, and (insofar as possible) of their contacts (DHHS, 2001). Attempts also are being made to identify specific proviruses responsible for production of infectious virus and then to selectively breed them out of lines of animals to be used as transplant donors (Herring et al., 2001).
TABLE 3.4 Theoretical scale of risks associated with PERV transmission from xenotransplants.
|
Event |
Cumulative Probability |
|
Expression of infectious virus |
 |
|
Localized infection of host cells |
|
|
Spreading infection in the host |
|
|
Persistent viremia |
|
|
Disease (e.g., lymphoma, “AIDS”) |
|
|
Transmission to close contacts |
|
|
Spreading, epidemic transmission |










