Summary
Wastewater treatment in the United States is a major cornerstone of efforts to keep the nation’s waters clean. Sewage sludge is the solid, semisolid, or liquid residue generated during treatment of domestic sewage. Since the early 1970s, the U.S. Environmental Protection Agency (EPA) and the wastewater treatment industry have promoted recycling of sewage sludge. With the prohibition of ocean disposal of wastewater residuals in 1992, the use of sewage sludge as soil amendments (soil conditioners or fertilizers) or for land reclamation has been increased to reduce the volume of sewage sludge that must be landfilled, incinerated, or disposed of at surface sites. Approximately 5.6 million dry tons of sewage sludge are used or disposed of annually in the United States; approximately 60% of that is used for land application. Depending on the extent of treatment, sewage sludge may be applied where little exposure of the general public is expected to occur on the sites, such as on agricultural land, forests, and reclamation sites, or on public-contact sites, such as parks, golf courses, lawns, and home gardens. EPA estimates that sewage sludge is applied to approximately 0.1% of available agricultural land in the United States on an annual basis.
The regulation governing land application of sewage sludge was established by EPA in 1993 in the Code of Federal Regulations, Title 40 (Part 503), under Section 405 (d) of the Clean Water Act. The regulation is intended to protect public health and the environment. The Part 503 rule established management practices for land application of sewage sludge, concentration limits and loading rates for chemicals, and treatment and use requirements
designed to control and reduce pathogens and attraction of disease vectors (insects or other organisms that can transport pathogens). In this report, the term biosolids refers to sewage sludge treated to meet the land-application standards in the Part 503 rule or any other equivalent land-application standards.
The chemical and pathogen land-application standards in the Part 503 rule were developed differently. For chemicals, EPA conducted extensive risk assessments that involved identifying the chemical constituents in biosolids judged likely to pose the greatest hazard, characterizing the most likely exposure scenarios, and using scientific information and assumptions to calculate concentration limits and loading rates (amount of chemical that can be applied to a unit area of land). Nine inorganic chemicals in biosolids are currently regulated, and EPA is considering the addition of a class of organic chemicals (dioxins) to its regulation. Monitoring data on some of the regulated inorganic chemicals indicate a decrease in their concentrations over the past decade, due in part to the implementation of wastewater pretreatment programs. Thus, the chemical limits for biosolids can be achieved easily. In contrast to the chemical standards, the pathogen standards are not risk-based concentration limits for individual pathogens but are technologically based requirements aimed at reducing the presence of pathogens and potential exposures to them by treatment or a combination of treatment and use restrictions. Monitoring biosolids is required for indicator organisms (certain species of organisms believed to indicate the presence of a larger set of pathogens).
THE COMMITTEE’S TASK
In response to the Clean Water Act requirement to reassess periodically the scientific basis of the Part 503 rule and to address public-health concerns, EPA asked the National Research Council (NRC) to conduct an independent evaluation of the technical methods and approaches used to establish the chemical and pathogen standards for biosolids, focusing specifically on human health protection and not ecological or agricultural issues. The NRC convened the Committee on Toxicants and Pathogens in Biosolids Applied to Land, which prepared this report. The committee was asked to perform the following tasks:
-
Review the risk-assessment methods and data used to establish concentration limits for chemical pollutants in biosolids to determine whether they are the most appropriate approaches. Consider the NRC’s previous (1996) review and determine whether that report’s recommendations have
-
been appropriately addressed. Consider (a) how the relevant chemical pollutants were identified; (b) whether all relevant exposure pathways were identified; (c) whether exposure analyses, particularly from indirect exposures, are realistic; (d) whether the default assumptions used in the risk assessments are appropriate; and (e) whether the calculations used to set pollutant limits are appropriate.
-
Review the current standards for pathogen elimination in biosolids and their adequacy for protecting public health. Consider (a) whether all appropriate pathogens were considered in establishing the standards; (b) whether enough information on infectious dose and environmental persistence exists to support current control approaches for pathogens; (c) risks from exposure to pathogens found in biosolids; and (d) new approaches for assessing risks to human health from pathogens in biosolids.
-
Explore whether approaches for conducting pathogen risk assessment can be integrated with those for chemical risk assessment. If appropriate, recommend approaches for integrating pathogen and chemical risk assessments.
MAJOR FINDINGS AND RECOMMENDATIONS
The committee recognizes that land application of biosolids is a widely used, practical option for managing the large volume of sewage sludge generated at wastewater treatment plants that otherwise would largely need to be disposed of at landfills or by incineration. In responding to its charge, the committee searched for evidence on human health effects related to biosolids exposure, reviewed the risk assessments and technical data used by EPA to establish the chemical and pathogen standards, and reviewed the management practices of the Part 503 rule. The committee did not attempt to determine whether the approaches used by EPA to set the 1993 biosolids standards were appropriate at the time of their development, and the committee’s findings and recommendations should not be construed as either criticism or approval of the standards issued at that time. The committee found that EPA has not yet addressed certain recommendations of the 1996 NRC report that pertain to the scope of the present study. The committee is aware that some interested parties were anticipating that this report might make a determination of whether EPA should continue to promote land application of biosolids. However, such a determination was not part of the committee’s charge. Nor was the committee asked to judge the adequacy of the individual standards in protecting human health. The committee’s report instead is focused on identifying how current risk-assessment practices and knowledge regarding chemi-
cals and pathogens in biosolids can be used to update and strengthen the scientific basis and credibility of EPA’s biosolids regulations.
In this report, the committee documents numerous findings and a number of recommendations for addressing public-health concerns, uncertainties, and data gaps about the technical basis of the biosolids standards. To delineate issues needing the greatest attention, the committee identified the following overarching findings and recommendation based on its review and synthesis of the specific findings and recommendations of each chapter.
Overarching Findings
There is no documented scientific evidence that the Part 503 rule has failed to protect public health. However, additional scientific work is needed to reduce persistent uncertainty about the potential for adverse human health effects from exposure to biosolids. There have been anecdotal allegations of disease, and many scientific advances have occurred since the Part 503 rule was promulgated. To assure the public and to protect public health, there is a critical need to update the scientific basis of the rule to (1) ensure that the chemical and pathogen standards are supported by current scientific data and risk-assessment methods, (2) demonstrate effective enforcement of the Part 503 rule, and (3) validate the effectiveness of biosolids-management practices.
Overarching Recommendations
-
Use improved risk-assessment methods to better establish standards for chemicals and pathogens. Risk-assessment methods for chemicals and pathogens have advanced over the past decade to the extent that (1) new risk assessments should be conducted to update the scientific basis of the chemical limits, and (2) risk assessments should be used to supplement technological approaches to establishing regulatory criteria for pathogens in biosolids.
-
Conduct a new national survey of chemicals and pathogens in sewage sludge. The committee endorses the recommendation of a previous NRC committee that a new national survey of chemicals be performed. The committee further recommends a survey of pathogen occurrence in raw and treated sewage sludges. The survey should include a careful examination of management practices to ensure that risk-assessment principles are effectively translated into practice. Data from the survey should be used to provide feedback for continuous improvement in the science and technology of biosolids applied to land.
-
Establish a framework for an approach to implement human health investigations. A procedural framework should be established to implement human health investigations, including short-term investigations of unusual episodes of release, exposure, or disease and large-scale preplanned studies of exposures and their association, if any, with disease. The framework should have mechanisms to document state-of-the-art successes, both technological and administrative, in preventing or remediating exposure to pathogens and toxicants and their adverse health outcomes. Further, the framework should include a means for tracking allegations and sentinel events (compliance, management, or health based), investigations, and conclusions. Such tracking should be systematic and developed in cooperation with states.
-
Increase the resources devoted to EPA’s biosolids program. To remedy the deficiencies and to implement the recommendations described in this report, more funding and staff resources are needed for EPA’s biosolids program. EPA should support and facilitate greater delegation of authority to states to administer the federal biosolids regulation. Resources are also needed for conducting needed research and to revise the regulation as appropriate and in a timely fashion.
These recommendations are discussed in greater detail below and in the following chapters.
Health Effects
Toxic chemicals, infectious organisms, and endotoxins or cellular material may all be present in biosolids. There are anecdotal reports attributing adverse health effects to biosolids exposures, ranging from relatively mild irritant and allergic reactions to severe and chronic health outcomes. Odors are a common complaint about biosolids, and greater consideration should be given to whether odors from biosolids could have adverse health effects. However, a causal association between biosolids exposures and adverse health outcomes has not been documented. To date, epidemiological studies have not been conducted on exposed populations, such as biosolids appliers, farmers who use biosolids on their fields, and communities near land-application sites. Because of the anecdotal reports of adverse health effects, the public concerns, and the lack of epidemiological investigation, the committee concluded that EPA should conduct studies that examine exposure and potential health risks to worker and residential populations. Studies of wastewater treatment workers exposed to raw sewage sludge should not be used as substitutes for studies of populations exposed to biosolids. The types and routes of exposure to sewage sludge and biosolids constituents can be quite different, and there are major differences in the populations exposed. For example, exposures to
biosolids go beyond the wastewater treatment plant to other worker populations, such as appliers and farmers, and to the general public, such as communities living near land-application sites and consumers of crops grown on biosolids-amended soils. Exposed populations may also include sensitive subpopulations, such as children, immunocompromised individuals, and the elderly, who are unlikely to be prevalent in the workplace.
Findings: There is a lack of exposure and health information on populations exposed to biosolids. Therefore, although the land application of biosolids has occurred for many years with little, if any, systematic documented evidence of adverse effects, there is a need to gather epidemiological data and to investigate allegations of health incidents. EPA needs to study more rigorously the exposure and health risks, or the lack thereof, in worker and community populations exposed to biosolids.
Recommendations: Although routine human health surveillance of all populations exposed to biosolids is impractical, the committee recommends that EPA promote and support response investigations, targeted exposure surveillance studies, and a few well-designed epidemiological investigations of exposed populations. This recommendation is intended to provide a means of documenting whether health effects exist that can be linked to biosolids exposure. The committee recommends the following types of studies:
-
Studies in response to unusual exposures and unusual occurrences of disease. Occasionally, the occurrence of unusual events can provide information on the agents of disease. For example, an outbreak or a symptom of disease might occur following a known exposure or an unusual exposure scenario. In both instances, exposure and health outcomes should be determined.
-
Preplanned exposure-assessment studies. Such studies should characterize the exposures of workers, such as biosolids appliers and farmers, and the general public who come into contact with constituents of biosolids either directly or indirectly. The studies would require identification of microorganisms and chemicals to be measured, selection of measurement methods for field samples, and collection of adequate samples in appropriate scenarios. A possible exposure-assessment study would be to measure endotoxin exposure of workers at biosolids production and application sites and of communities nearby.
-
Complete epidemiological studies of biosolids use. These studies should be conducted to provide evidence of a causal association, or a lack thereof, between biosolids exposure and adverse human health effects. They should include an assessment of the occurrence of disease and an assessment or measurement of potential exposures. An example of a longitudinal epidemio-
-
logical study would be an evaluation of health effects in a cohort of biosolids appliers. These workers should be characterized by duration and level of exposure, and given appropriate follow-up. Because complete epidemiological studies are expensive and require extensive data analysis, priority should be given to studies that can address serious or widespread problems and help reduce uncertainty.
Chemical and Pathogen Standards
EPA’s 1993 chemical and pathogen standards for biosolids were based on the scientific and technical information available at that time and the expectation that the prescribed biosolids-management practices specified in the Part 503 rule would be effective in preventing harmful exposure to biosolids constituents. To assure the public that the standards are protective of human health, it is important that EPA demonstrate that its chemical limits and pathogen-reduction requirements are supported by current scientific data and risk-assessment methods. Management practices (e.g., 10-meter setback from water bodies) are designed to control the potential risks; therefore, it is important to verify the effectiveness of the practices. In addition, EPA must demonstrate that the Part 503 rule is being enforced.
Findings: The committee found that no substantial reassessment has been done to determine whether the chemical or pathogen standards promulgated in 1993 are supported by current scientific data and risk-assessment methods. In addition, EPA does not have an adequate program to ensure compliance with the biosolids regulations and has not documented the effectiveness of its prescribed management practices. Although there is no documented scientific evidence that the Part 503 rule has failed to protect public health, there is a need to address scientific and management questions and uncertainties that challenge EPA’s biosolids standards.
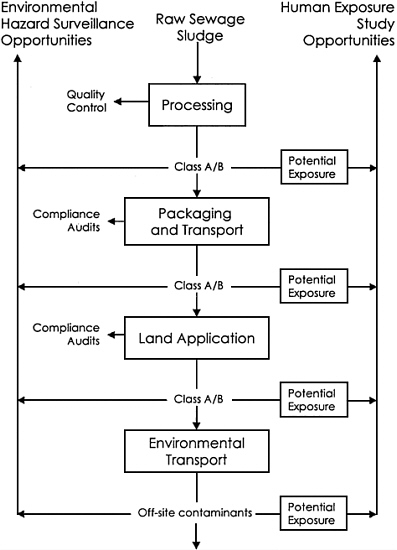
Recommendations: EPA should expand its biosolids oversight activities to include procedures for (1) assessing the reliability of the biosolids treatment processes, (2) monitoring compliance with the chemical and pathogen standards, (3) conducting environmental hazard surveillance, and (4) studying human exposure and health. The committee recommends that Figure S-1 be used by EPA as a framework for establishing such a program. The central part of the figure presents the general process by which biosolids are produced and used for land application. Depicted on the left side of the figure are opportunities for conducting environmental hazard surveillance. At these
stages, biosolids or environmental samples should be collected and analyzed to verify that (1) treatment technologies for pathogen control are effective (quality control), (2) chemical standards are met (compliance audits), and (3) unanticipated hazards are identified. An important part of this verification process is a review of the management practices required for land application, because the practices are predicated on the assumption that exposure to hazardous agents is further reduced by the implementation of such practices. Studies should be conducted to determine whether the management practices specified in the Part 503 rule achieve their intended effect. Additional risk-management practices should be considered in revising the Part 503 rule. Considerations should include setbacks to residences or businesses, setbacks to private and public water supplies, limitations on holding or storage practices, slope restrictions, soil permeability and depth to groundwater or bedrock, and greater distance to surface water.
The right side of the figure depicts the various points in the process where human exposures can occur. Field research should be conducted to assess potential exposure to biosolids constituents of concern. Results from this research could be used to identify populations that should be monitored or studied at particular times and locations for abnormal health conditions and potential biosolids exposure (see earlier recommendations for response and epidemiological studies). Studying environmental samples and reports of adverse health outcomes can provide feedback to support or improve the risk-assessment and risk-management processes.
The major aspect of the framework studied by the committee was the technical basis of the 1993 chemical and pathogen land-application standards of the Part 503 rule. Recent EPA guidance recommends that risk assessment of complex mixtures ideally be based on studies of the mixture rather than on selected individual components. Such an approach is not feasible for biosolids, however, because studies of biosolids as complex mixtures are lacking. Furthermore, although methods for conducting risk assessments of chemical mixtures are available, no work has been done on risks from pathogen mixtures, much less chemical-pathogen mixtures.
Finding: Because of data gaps and lack of risk-assessment methods for complex mixtures, it is not possible at this time to integrate pathogen risk assessment with chemical risk assessment. Thus, it remains necessary to use a component-based approach to assessing risks from chemicals and pathogens in biosolids. There have been substantial improvements in conducting risk assessments since the Part 503 rule was promulgated, and guidance for using these improved methods to update and strengthen the scientific basis of the chemical and pathogen standards is provided below.
Chemical Standards
In developing the original (1993) Part 503 rule, EPA selected 10 inorganic chemicals (arsenic, cadmium, chromium,1 copper, lead, mercury, molybdenum,2 nickel, selenium, and zinc) to regulate for land application. Risk assessments were conducted on each chemical to establish concentration limits and loading rates. However, methods for conducting risk assessments have evolved substantially since the 1993 regulations were established. One of the major developments has been a growing recognition of the need to include stakeholders in the risk-assessment process. Stakeholders are groups who are potentially affected by the risk, groups who will manage the risk, and groups who will be affected by efforts to manage the source of the risk. Stakeholders can provide information and insights into how biosolids are used in practice and the nature of potential exposures to chemicals and pathogens. Involving stakeholders throughout the risk-assessment process provides opportunities to bridge gaps in understanding, language, values, and perspectives and to address concerns of affected communities. Other important developments in risk assessment in recent years include improvements in measuring and predicting adverse health effects, advancements in measuring and predicting exposure, explicit treatment of uncertainty and variability, and improvements in describing and communicating risk.
In developing its 1993 chemical standards, EPA selected chemicals, exposure conditions, and risk-assessment assumptions that were intended to be representative and conservative enough to be applicable to all regions of the United States and to all land-application sites, including agricultural fields, forests, and reclamation sites. Thus, the standards were expected to account for possible variations in biosolids composition, geographic and environmental conditions, or application and management practices. EPA relied heavily on its 1988–1989 National Sewage Sludge Survey (NSSS) to identify chemicals to regulate, using percent detection and concentration values to exempt some
chemicals from regulation and to establish ceiling-concentration limits for others. A 1996 NRC report (Use of declaimed Water and Sludge in Food Crop Production) questioned the reliability of the results of the NSSS because of limitations in sampling analyses and data-reporting methods. Improvements in industrial wastewater pretreatment processes and changes in chemical uses have occurred over the past decade. Chemicals not included in the NSSS analyses have since been identified as potential concerns, and data gaps on toxicity and fate and transport characteristics that prevented risk assessment from being performed on some chemicals a decade ago might now be filled. In addition, the committee found no adequate justification for EPA’s decision to eliminate from regulation all chemicals detected at less than 5% frequency in the NSSS (or 10% frequency in subsequent reanalysis). It should be noted that there are still data gaps that will continue to limit risk-assessment capability on many of the chemicals, including those newly identified as potential concerns.
EPA considered 14 major exposure pathways in setting the 1993 limits for the nine regulated chemicals. Nine of the pathways resulted in exposure to humans, two to animals, two to soil organisms, and one to plants. The pathways were evaluated for agricultural and nonagricultural application scenarios. For all nine of the regulated chemicals, agricultural scenarios produced the lowest limits that were subsequently used in the regulation. EPA elected to evaluate the human exposure pathways for a theoretical, highly exposed individual (HEI) (i.e., a hypothetical individual assumed to remain for an extended period of time at or adjacent to the site where maximum exposure occurs). The degree of realism for the HEI varied among the exposure pathways, and it was not clear to the committee whether exposure estimates were comparably conservative for all pathways. Moreover, each pathway was evaluated independently, and no consideration was given to exposure from multiple pathways.
Current risk-assessment practice is to perform comprehensive, multipathway risk assessments that estimate aggregate exposures for each receptor population (i.e., groups with potential exposure to contaminated media). Such risk assessments are based on a conceptual site model that identifies the biosolids sources (e.g., biosolids tilled into soil or applied to the surface for agricultural soil), the pathways by which biosolids constituents might be released and transported, and the nature of human contacts with the constituents. General practice has changed from using the HEI as the receptor of concern, because such an individual is unlikely to exist, to using an individual with reasonable maximum exposure (RME). An RME individual is a hypothetical individual who experiences the maximum exposure that is reasonably expected to occur (i.e., an upper-bound exposure estimate). RMEs
should be based on receptor populations of concern, such as a farm family living adjacent to and downhill from a land application site.
A number of risk algorithms were used to calculate the 1993 chemical limits. The general algorithms are still valid, but some fate and transport models and exposure parameter assumptions used in the calculations have advanced since 1993, and some alternative assumptions have been supported by new studies. Chemical limits should be based on an integrated evaluation of all exposure pathways that might affect the identified receptors.
Findings: The committee found the technical basis of the 1993 chemical standards for biosolids to be outdated. EPA has not reevaluated its chemical standards since promulgation, so the data and methods used for the original regulations are well over a decade old. There have been substantial advances in risk assessment since then, and there are new concerns about some adverse health outcomes and chemicals not originally considered. Because of the diversity of exposed populations, environmental conditions, and agricultural practices in the United States, it is important that nationwide chemical regulations be based on the full range of exposure conditions that might occur. Furthermore, there is a need to investigate whether the biosolids produced today are similar in composition to those used in the original assessments.
Recommendations: Using current risk-assessment practices, EPA should reassess the standards for the regulated chemicals and conduct another chemical selection process to determine whether additional chemicals should be considered for regulation. On the basis of the revised risk assessments and chemical selection, EPA can determine whether the standards or risk-management process should be revised and whether additional chemicals should be regulated. Because the land-application standards are to be relevant nationally, it is important that the revised risk assessments reflect regional variations in climate, hydrology, and biosolids use and characteristics, and that standards are protective of populations reflecting reasonable estimates of maximum exposure. The chemical standards should be reevaluated and updated periodically to ensure that they are supported by the best available scientific data and methods. Important elements for updating the risk assessments are the following:
-
As recommended by an earlier NRC committee, a new national survey of chemicals in biosolids should be conducted. EPA should review available databases from state programs in designing a new survey. Other elements that should be included in the survey are an evaluation of the adequacy of detection methods and limits to support risk assessment; consideration of chemical categories, such as odorants and pharmaceuticals, that were not previously
-
evaluated; and assessment of the presence of multiple species of certain metals, such as mercury and arsenic, that have different toxicity end points. Data from this survey should be used to identify any additional chemicals for potential regulation.
-
Aggregate exposure assessments should be performed. A conceptual site model should be used to identify major and minor exposure pathways for various application scenarios. Special consideration should be given to identifying the application practices and environmental conditions that are likely to result in the greatest human exposure. Risks from long-term low-level exposures, as well as short-term episodic exposures, such as those that can occur with volatile chemicals, should be evaluated.
-
An RME individual, rather than an HEI, should be evaluated for each exposure pathway. Use of the RME is a more informed and reasonable estimate of exposure than the HEI because it reduces reliance on the subjective application of default assumptions and reflects improved methods of characterizing population exposure. When the RME individual is likely to be exposed by more than one pathway, exposures should be added across pathways.
-
Fate and transport models and exposure parameter assumptions used in the risk assessment should be updated to reflect the most current information on the RME individual for each exposure pathway.
-
Representatives of stakeholders should be included in the risk-assessment process to help identify exposure pathways, local conditions that could influence exposure, and possible adverse health outcomes.
Pathogen Standards
Pathogens are disease-causing microorganisms. The two land-application classifications for biosolids, Class A and Class B, are based on pathogen content. Class A biosolids have pathogen densities below specified detection limits, whereas Class B biosolids have pathogen densities above those limits. No risk assessments were conducted to establish the 1993 pathogen standards for these classes. Instead, EPA established technologically based requirements to reduce the presence of pathogens by treatment or a combination of treatment and use restrictions. To meet Class A requirements, demonstration of pathogen reduction is required by using one of several prescribed treatments. Monitoring of indicator organisms is required of Class A biosolids at the time of use, distribution, or land application to verify that treatment processes have reduced pathogen concentrations as expected (i.e., below the specified detection limits). Class B biosolids must also undergo treatment to reduce the presence of pathogens but, unlike Class A biosolids, Class B biosolids may
have detectable concentrations of pathogens. Because of that, site restrictions are required to minimize contact with the biosolids until environmental factors (e.g., heat and desiccation) have further reduced the presence of pathogens. Site restrictions include restrictions on crop harvesting, animal grazing, and public access for designated periods of time. However, there is no requirement that on-site measurements be taken at Class B application sites to confirm that the treatment and the use restrictions resulted in below-detection pathogen concentrations. Such on-site measurements would help to estimate potential risks and the efficacy of site-management requirements.
EPA considered a spectrum of bacteria, viruses, protozoa, and helminths in setting its 1993 pathogen standards. New information on some of these and other organisms are now available for updating hazard identification. Humans may be exposed to pathogens in biosolids from ingestion of contaminated food, water, or soil; dermal contact; and inhalation of bioaerosols (aerosolized biological particles). There is also the potential for humans to be exposed via secondary transmission from exposure to pathogens shed from infected individuals either by direct contact or by routes through the environment. Some exposure pathways, such as the inhalation pathway, were not adequately evaluated by EPA in the development of the 1993 Part 503 pathogen requirements. EPA also did not address sufficiently the potential for surface-water contamination by runoff, groundwater contamination, and secondary transmission of disease.
The reliability of biosolids treatment processes in reducing pathogens is essential for public-health protection. There is a need to better document the reliability of EPA’s prescribed treatment processes and to establish that management controls intended to reduce pathogens by natural attenuation are effective. An important consideration in making these determinations is ensuring that the pathogen detection methods used are accurate and precise. Substantial advances in detection and quantification of pathogens in the environment have been made since the 1993 promulgation of the Part 503 rule. For example, new molecular techniques for detecting pathogens (e.g., polymerase chain reaction) are now available. In addition, new approaches to environmental sample collection and processing are available. However, improved standardized methods for measuring pathogens in biosolids and bioaerosols need to be developed.
As with the chemical standards, EPA based its 1993 pathogen standards on selected pathogens and exposure conditions that were expected to be representative and conservative enough to be applicable to all areas of the United States and all types of land applications. This includes the recognition that pathogen survival in soils can range from hours to years, depending on the specific pathogens, biosolids application methods and rates, initial patho-
gen concentrations, soil composition, and meteorological and geological conditions. Little is know about pathogen transport and survival in bioaerosols. Quantitative microbial risk-assessment (QMRA) methods similar to those used in chemical assessments have been developed for microbial agents in drinking water and food. These methods are not as well established as those for chemicals, and there are important differences between the two. One of the major differences is that microbial risk assessment must include the possibility of secondary transmission of disease, either through person-to-person contact or from transmission of the pathogen to others through air, food, or water. The importance of secondary transmission depends in part on the level of acquired immunity to the pathogen in the community, a phenomenon that has no analog in chemical risk assessment.
Findings: Given the variety of pathogens that have the potential to be present in biosolids, the committee supports EPA’s approach to establishing pathogen reduction requirements and monitoring indicator organisms. However, the reliability of EPA’s prescribed treatment techniques should be better documented using current pathogen detection technology, and more research on environmental persistence and dose-response relationships is needed to verify that current management controls for pathogens are adequate to maintain minimal exposure concentrations over an extended period of time. QMRA methods have developed sufficiently to provide better risk information that should be used to establish or support existing regulatory criteria.
Recommendations:
-
EPA should conduct a national survey of pathogen occurrence in raw and treated sewage sludges. Important elements in conducting the survey include use of consistent sampling methods, analysis of a broad spectrum of pathogens that could be present in sewage sludge, and use of the best available (preferably validated) pathogen measurement techniques.
-
QMRAs should be developed and used to establish regulatory criteria (treatment requirements, use restrictions, and monitoring) for pathogens in biosolids. For example, EPA could stipulate an acceptable risk level for a particular pathogen. QMRA could then be used to estimate the concentration of that pathogen in biosolids either at the point of application (where there is immediate potential for exposure) or following any required holding period. EPA could then determine experimentally based relationships between the maximum acceptable pathogen concentration and the process conditions (e.g., time, temperature, pH, chemical doses, and holding times) and/or the pathogen indicator concentrations (either density or reduction through treatment). On the basis of those relationships, regulatory criteria and monitoring for land
-
application can be updated or developed to ensure consistent attainment of target pathogen concentrations. To conduct QMRAs, a conceptual site model should be used to identify all potential routes of exposure; additional input data (e.g., dose-response and pathogen-survival data) should be collected; and consideration should be given to potential secondary transmission of infectious disease. QMRAs also can be used to analyze sensitivity and to ascertain what critical information is needed to reduce uncertainty about the risks from exposure to pathogens in biosolids. The pathogen standards should be reevaluated and updated periodically to ensure that they are supported by the best available scientific data and methods and to ensure that anecdotal information is not being used for the predication of past, current, or future regulations.
-
EPA should foster development of standardized methods for measuring pathogens in biosolids and bioaerosols.
-
EPA should promote research that uses improved pathogen detection technology to better establish the reliability of its prescribed pathogen treatment processes and biosolids-use controls to achieve and maintain minimal exposure over time. In setting pathogen treatment requirements, it might be useful to establish metrics for typical (mean) treatment performance and concentrations not to be exceeded.
-
Research should be conducted to assess whether other indicator organisms, such as Clostridium perfringens, could be used in regulation of biosolids. Such indicators, along with traditional indicators and operational parameters, may be suitable for monitoring day-to-day regulatory compliance.