3
Seawater
NUTRIENTS
Measurements of the major elements composing organic matter in ocean environments are among the longest established, most fundamental, and broadly informative analyses in the marine sciences. This tradition is based on the fact that six elements (carbon, hydrogen, oxygen, nitrogen, sulfur, and phosphorus)1 make up essentially the entire mass of marine organic matter and largely govern the processes by which these elements are cycled in the ocean. One of the most widely used empirical relationships in oceanography is the Redfield-Ketchum-Richards equation (Redfield et al., 1963):
106 CO2 + 16 HNO3 + H3PO4 + 122 H2O → (CH2O)106(NH3)16(H3PO4) + 138 O2
This equation describes the ratios with which inorganic nutrients dissolved in seawater are converted by photosynthesis into the biomass of “average marine plankton” and oxygen gas O2. The opposite of this reaction is respiration, or the remineralization process by which organic matter is enzymatically oxidized back to inorganic nutrients and water. The atomic ratios (stoichiometry) of this reaction were established by
Redfield (1934), who analyzed the major elemental content of many samples of mixed plankton (phytoplankton and zooplankton) caught in nets towed through the surface ocean. They compared the carbon, nitrogen, and phosphorus composition of these collections to concentration profiles of dissolved inorganic carbon (DIC), NO3, and PO4 throughout the water column. This pioneering research demonstrated that these three elements are continually redistributed in the ocean by selective removal into plankton cells and their remains (i.e., fecal pellets), which are then efficiently respired as they sink through the marine water column.
As a result of continual nutrient removal and regeneration, the surface waters of density-stratified temperate oceans become so depleted in NO3 and PO4 that they limit the rate of photosynthesis, and hence the total amount of life that can be sustained through the food web. The extent of depletion in dissolved O2 concentrations in the deep ocean is an additional useful chemical indicator of the total amount of respiration and oxidation that has occurred since downwelling from the surface. Using the Redfield-Ketchum-Richards equation, the measured apparent oxygen utilization (AOU) can be used to estimate the amounts of CO2, NO3, and PO4 generated by heterotrophic activity within the same water parcel and, by difference, the concentrations of NO3 and PO4 that were initially present prior to downwelling. Such preformed nutrient concentrations can be useful tracers of the origins and mixing ratios of seawater that has entered the deep ocean from different surface regions (Broecker, 1974; Broecker and Peng, 1982). Measurements of the elemental composition of marine plankton thus have provided a basis for estimating the source, age, and life-sustaining potential of ocean waters for over 50 years.
In addition to the previous applications, the NO3 to PO4 ratio (N:P), for example, can be used to examine the cycling of nutrients in surface and deep waters (Wu et al., 2000). The N:P ratios at the Bermuda station (BATS) and at the Hawaii station (HOT) in surface waters are quite different (Fig. 3.1). Near Bermuda, the values of N:P range from over 30 at the surface and decrease steadily to approximately 17 below 600 m, while near Hawaii, they are nearly zero in the surface waters and increase with depth to a value of around 15 by 400 m. These results suggest that the balance between production, export, and mineralization of organic matter differs at the two sites. Furthermore, the measurement of nutrients over a 10-year period at the BATS station (Fig. 3.2) shows large variations that can be related to an extended period with a positive North Atlantic Oscillation index resulting in warmer-than-average sea surface temperatures and a reduction in the extent of convective overturn. Hence, less nitrate is mixed to the surface (Michaels et al., 2001). Future long-term studies of this type could benefit from the use of nutrient reference mate-
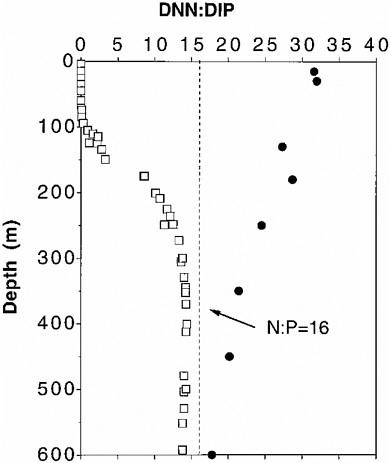
FIGURE 3.1 Vertical profiles of N:P molar ratios in the Sargasso Sea near Bermuda (31.67ºN, 64.17ºW) (•) and in the Pacific near Hawaii (HOT US JGOFS Web site at hahana.soest.hawaii.edu) (□). (Wu et al., 2000).
rials ensuring that measurements can be compared at different laboratories over long time periods.
The urgent need for nutrient standards was demonstrated during the recently completed World Ocean Circulation Experiment (WOCE) and Joint Global Ocean Flux Study (JGOFS) measurements which were made by different laboratories. The internal consistency of the nutrient data was evaluated by comparing measurements made in deep water (depth over 3500 m) at nearby stations on different cruises. If one assumes that nutrient concentrations in deep water at the same location should not
change over decadal time scales, the observed systematic nutrient differences should represent an estimate of inter-laboratory precision. Offsets were found, however, among the results of different laboratories indicating inconsistencies in the preparation of the calibration standards. Mean offsets were 0.5 to 0.7 µM for NO3, 0.05 µM for PO4, and 2 µM for Si(OH)4 (Mordy et al., 1999; Ross et al., 1999; Zhang et al., 2000). These offsets are equivalent to 1.5 to 2.0 percent of the respective deep-water nutrient concentrations. Corrections were frequently applied to the raw data to improve the internal consistency of the overall data sets, although many nutrient chemists have pointed out that such offset corrections are problematic (Gordon et al., 1999; Zhang et al., 2000). Without improvements to the accuracy and precision of nutrient measurements, it will be impossible to detect small changes in nutrient concentrations that may be important for understanding the oceanic carbon cycle.
Since certified reference materials for seawater nutrient analysis are currently unavailable, individual laboratories must prepare their own standard solutions for instrument calibration. Standard stock solutions are prepared at high concentrations (mM) so that they can be used for months without significant alterations in concentration. Working low-concentration standard solutions are unstable and need to be prepared daily by diluting stock solutions with distilled water or low-nutrient seawater. In this case, the accuracy of nutrient analysis at a given laboratory is highly dependent upon the accuracy of the daily preparation of the calibration solutions.
In the 1970s, the Sagami Chemical Research Center in Japan provided nutrient reference material for the Cooperative Study of the Kuroshio Current (the so-called CSK standards). These solutions were not prepared in seawater, which limits their general utility (see below), however they are still distributed and widely used as a common reference. French and British scientists have conducted some studies on nutrient reference material (Aminot and Keroul, 1991, 1996; Zhang et al., 1999) with limited success.
Matrix effects in the analysis of nutrients in seawater are caused by differences in background electrolyte composition and concentration (salinity) between the standard solutions and samples. This effect causes several methodological difficulties. First, the effect of ionic strength on the kinetics of colorimetric reactions results in color intensity changes with matrix composition and electrolyte concentration. In practice, analytical sensitivity depends upon the actual sample matrix. This effect is most serious in silicate analysis using the molybdenum blue method. Second, matrix differences can also cause refractive index interference in automated continuous flow analysis, the most popular technique for routine nutrient measurement. To deal with these matrix effects, seawater of
standard salinity (approximately 35) should be used as the matrix for nutrient reference materials.
TRACE METALS
Measurement of trace metal concentrations can provide fundamental insights into marine geochemical processes. Many metals are important micronutrients in seawater and can play a significant role in upper ocean biogeochemistry and carbon cycling. Under certain conditions, elevated concentrations of metals associated with human activities can have negative impacts on marine ecosystems.
Comprehensive understanding of trace metal geochemistry eluded oceanographers for decades because of the difficulty of measuring metals at very low concentrations, particularly in open ocean waters. Many of the most important metals are also highly prone to contamination. Over the last 25 years, most of these obstacles have been overcome by a relatively small number of research groups. Now, with an increasing number of investigators becoming interested in high quality trace metal measurements, there is a greater need for reference seawater with metals at realistic (i.e., low) concentrations for researchers to assess their methodologies.
In existing reference materials the concentrations of several key analytes, including iron, are too high to be useful for scientists making open ocean analysis. For instance, the concentration of iron in seawater standards provided by NRC-Canada is about 100 times greater than expected in surface ocean waters (Table 3.1).
TABLE 3.1 Comparison of Metal Concentrations in an Existing Reference Seawater (NASS-5, from NRC-Canada) and in Oceanic Seawater
|
Metal |
NASS-5 nM |
Pacific Surface Water nM |
Pacific Deep Water nM |
Reference |
|
Cadmium (Cd) |
0.2 |
0.002 |
0.8 |
Bruland, 1980 |
|
Cobalt (Co) |
0.2 |
0.01 |
0.08 |
Martin and Gordon, 1988 |
|
Copper (Cu) |
5 |
0.6 |
5 |
Bruland, 1980 |
|
Iron (Fe) |
3.8 |
0.06 |
0.7 |
Martin et al., 1989 |
|
Lead (Pb) |
0.04 |
0.04 |
0.05 |
Wu and Boyle, 1997 |
|
Zinc (Zn) |
1.6 |
0.08 |
8 |
Bruland, 1980 |
The highest priority metal that requires a reference material is iron, which limits primary production in approximately 40 percent of the world’s oceans. Iron levels are extremely low (subnanomolar) in ocean surface waters of these regions, although there is disagreement among laboratories regarding the actual concentration. Difficulties in analyzing this highly contamination-prone element, coupled with an increasing recognition that iron concentrations exhibit considerable spatial and temporal variability, make the development of low-level reference materials for iron a high priority.
There are other metals for which compelling cases can be made to produce contamination-free oceanic reference seawater. These include other bioactive metals (e.g., zinc, cobalt, cadmium, and copper), tracers of anthropogenic contamination (e.g., lead, Box 3.1), and non-bioactive metals used as tracers of geochemical and physical processes (e.g., aluminum).
The primary and immediate need is for a trace metal reference material, but a certified reference material would provide even greater benefits. A technique based on isotope dilution with detection by inductively-coupled plasma mass spectrometry (ICP-MS) (Wu and Boyle, 1998) most clearly meets the traceability criteria required for a certified reference material. Although useful for iron and several other metals, isotope dilution is not possible for monoisotopic elements like cobalt, so other techniques must also be used. Indeed, it is advisable that several techniques be used to certify a trace metal reference material.
|
Box 3.1 History of Metal Analysis in Sea Water In the 1960s much of the interest in metals centered on their role as pollutants. Considerable progress was made in characterizing the distribution of bomb-derived radionuclides, but little progress was made for stable metals. One of the first success stories was obtained by Schaule and Patterson who documented the marked effect of leaded gasoline on the distribution of lead in the upper water column (Schaule and Patterson, 1981). Wu and Boyle (1997) subsequently documented the removal of lead from the upper water column by scavenging processes, coupled with a dramatic decrease in deposition associated with the phasing out of leaded gasoline (Fig. 3.3). Such data, where decade-scale trends are superimposed over strong intra-annual variability, illustrate the importance of accuracy and precision in trace metal analysis. Widely available reference materials can help provide the analytical continuity over time and between laboratories required for such challenging trace analyses. |
Interference in Trace Metal Measurements
The major anions and cations in seawater have a significant influence on most analytical protocols used to determine trace metals at low concentrations, so production of reference materials in seawater is absolutely essential. The major ions interfere strongly with metal analysis using graphite furnace atomic absorption spectroscopy (GFAAS) and inductively coupled plasma mass spectroscopy (ICP-MS) and must be eliminated. Consequently, preconcentration techniques used to lower detection limits must also exclude these elements. Techniques based on solvent extraction of hydrophobic chelates and column preconcentration using Chelex 100 achieve these objectives and have been widely used with GFAAS.
The advent of ICP-MS has revolutionized seawater analysis for several reasons. First, while it is sensitive to interferences from sodium and chlorine, magnesium matrix effects are modest and can be corrected for. Now, many metals can be determined by co-precipitation from seawater with magnesium hydroxide (Mg(OH)2) and re-dissolution into nitric acid. This technique is simple, and avoids contaminants associated with synthetic chelates. Second, because of its high sensitivity, pre-concentration factors of 100-1000 are no longer needed, and reliable measurements can be generated from 1-10 ml of seawater. Third, because individual isotopes are measured from the mass spectra, the technique of isotope dilution can be used to determine metal concentrations. This approach, described in detail in Wu and Boyle (1997), involves spiking a sample with a stable isotope that is affected in the same way as the target analyte by matrix effects, ICP-MS sensitivity fluctuations, and variability in the recovery efficiency of the precipitate. This is a huge advantage in artifactprone matrices like seawater.
Dissolved organic matter (DOM) can complicate the analysis of metals such as iron and copper, which form strong organic complexes that render them less reactive. In oceanic waters, it is generally thought that prolonged acidification at pH 2.3 is sufficient to dissociate metals from natural organic ligands. Some workers also irradiate waters with ultraviolet light to destroy organic complexes, but a rigorous comparison of these approaches has not been carried out. It may be necessary to use the simplest procedure (acidification only) to minimize the chance of sample contamination. Dissolved organic matter also has surfactant properties that can interfere with the determination of metals by electrochemical techniques. Surfactants can also be eliminated by UV irradiation, and most electrochemists irradiate prior to determining total metal concentrations.
In coastal waters containing very high dissolved organic carbon
(DOC), acidification alone may not be sufficient to render all of a given metal reactive towards the reagents used for analysis. This may be an issue in important regions like major estuaries and waters near river deltas. Progress in this aspect of analysis may be greatly facilitated by a high-DOC reference seawater, though the lability of metals in such a sample may change with prolonged storage under acidified conditions.
Recommended Reference Materials
The principal need for trace metal reference materials is for a large volume of contamination-free seawater that can be disseminated to a large number of investigators. The highest priority for a trace metal reference material is iron. Two seawater-based reference materials are recommended: one from deep water having a relatively “high” iron concentration (approximately 0.6 nM), and one from an iron-depleted near-surface water (approximately 15 m) where the concentration is at least 10 times lower. For many analysts, the low-concentration sample would be useful for establishing blanks, and the high-concentration sample would be useful for establishing precision.
The same samples could also be used to analyze metals such as zinc, manganese, copper, molybdenum, cobalt, vanadium, lead, aluminum, cadmium, and the rare earth elements. Some of these elements have nutrient-like distributions, so surface and deep water samples would have significantly different levels. Surface water concentrations of critical elements like zinc, however, are not always low in regions where iron is low (e.g., in the Southern Ocean). For this reason, collection locations for trace element reference materials must be carefully selected. Participants at the Florida workshop in September 2001 (Appendix B), suggested the south-east tropical Pacific as a region where iron and zinc might be at low concentrations in surface waters. Manganese and aluminum concentrations may be comparable or higher in the surface sample than in the deep sample, but either sample would be valuable as a reference material for these elements.
Two additional reference materials should also be considered, one for coastal waters and one for the Great Lakes. A standard for dissolved iron and other metals in coastal water containing high concentrations of DOM would be useful in addressing the matrix effects associated with DOM described above. Interactions with organic matter can make metals more difficult to extract from seawater and cause poor recoveries (even for laboratories experienced in the analysis of seawater using clean techniques). One potential problem that will require monitoring is any change in the reactivity of dissolved iron in a very high DOM sample during prolonged storage. The Great Lakes are considered “inland seas” by the
National Science Foundation (NSF), and the Chemical Oceanography program invests substantial resources in that area. The upper water column in the center of Lake Superior might be a good place to collect water for preparation of a freshwater trace metal reference material.
A first attempt at the collection of a low-iron oceanic surface water reference material—part of a Scientific Committee for Oceanic Research (SCOR)-sponsored inter-comparison that was co-funded by the European Union—was only partially successful. The reference material had a higher iron concentration than expected after it had passed through the preparation stage, illustrating the difficulty associated with avoiding contamination of large water samples on board an iron ship. This “IRONAGES” project provided a valuable learning experience. This attempt cost approximately 100,000 Euros, for coordination of homogeneity tests, and distribution of the reference material. As of 2002, the reference material was being used in an international laboratory intercomparison.
The collection of a reference material for trace metals could complement collection of samples for other priority analytes including DOM and DOC. Seawater used for DOM analysis can also be collected in Teflon®, for example. Although samples for DOM are not normally stored in plastic bottles (as the iron reference material would be), releases of plasticizers are minimal and are not expected to interfere with the detection of individual organic compounds such as amino acids and sugars.
It is strongly recommended that sample collection be linked to inter-comparisons of field-based methodologies by different groups of investigators. This would help justify the expense of the required ship time, and satisfy a critical analytical need as real-time methodologies become more widely used by oceanographers interested in small-scale temporal and spatial variability. It would also mean that a large amount of supplemental information would be available about the collected samples.
RADIONUCLIDES
There are three classes of radionuclides occurring in seawater:
-
long-lived primary radionuclides and their daughters that have existed since their formation,
-
cosmogenic radionuclides with relatively short half-lives that are formed continually by bombardment of matter with cosmic rays, and
-
artificial radionuclides produced by human activities such as nuclear bombs and nuclear power stations.
The concentrations of these elements in seawater are extremely low. Radionuclides are primarily used as tracers to study water circulation, par-
ticle scavenging, exchange between water masses, and, in some cases, exchange between the ocean and the atmosphere or sediments.
Measurements of radionuclides in seawater have been used to study a variety of processes, including ocean mixing, cycling of materials, and carbon flux (by proxy). These measurements provide information on both process rates and mechanisms. Because of the unique and well-understood source functions of these elements, models of radionuclide behavior have often led to new understanding of the behavior of other chemically similar elements in the ocean.
Bomb-produced radionuclides in seawater have been used to determine ocean-mixing rates. The earliest measurements used cosmic ray-produced 14C (radiocarbon) to establish that the time scale for mixing of the world ocean was on the order of 1000 years. Nuclear bomb testing from 1952 to 1962 injected large amounts of 14C and 3H (tritium) into the atmosphere. The atmospheric inventory for radiocarbon essentially doubled (Levin and Hesshaimer, 2000). Although these two radionuclides are both naturally occurring, bomb-testing elevated their surface ocean activities considerably above the natural background. Tracing transient bomb-produced 14C and 3H through the ocean has provided invaluable information concerning the rate of North Atlantic deep water movement and the rate of exchange of water through the main thermocline (Ostlund and Rooth, 1990; Broecker and Peng, 1982). Transient 14C has also been used as a tracer of the transport for anthropogenic CO2 into the ocean (Broecker et al., 1980) and will be valuable in validating ocean general circulation models that will be used to predict the fate of anthropogenic CO2 in the ocean (Toggweiler et al., 1989; Guilderson et al., 2000).
Uranium—Thorium-Series Radionuclides
Naturally occurring radionuclides produced by the uranium and thorium decay series (Fig. 3.4) have been used for ocean mixing and biogeochemical studies. Sarmiento et al. (1990) used measurements of 228Ra (half-life of 5.7 years) in the Atlantic to estimate the rate of nutrient input through the thermocline and the rate of carbon mineralization. Based on 228Ra measurements in the equatorial Pacific, Ku et al. (1995) concluded that silicate limits biological production in this region. Moore et al. (1986) used 228Ra to trace horizontal mixing and advection of water parcels that had passed through the Amazon mixing zone for over 1000 km into the Atlantic.
The short-lived radium isotopes have been used in coastal and nearshore studies. Moore (2000) used 223Ra (half-life of 11 days) and 224Ra (half-life of 3.7 days) to estimate rates of cross-shelf exchange in the South Atlantic Bight. Charette et al. (2001) used this pair to estimate the age of
water in a coastal pond. These two isotopes have great potential to solve a number of problems in estuarine and coastal oceanography.
Measurements of radionuclides are also used to determine removal mechanisms and controls for carbon and metal cycling in the ocean. For example, the removal of 234Th from the euphotic zone is closely coupled to the vertical flux of particulate organic carbon. The deficiency of 234Th with respect to its parent—238U—in near-surface waters is used to estimate the export flux of particulate organic carbon (Buesseler, 1991). Measurements of 234Th and 238U in the upper water column provided the primary data relating to particulate carbon fluxes during JGOFS.
At present, there are no widely distributed certified reference materials containing all of the radionuclides in the uranium and thorium decay series. Such reference materials are needed to calibrate instruments that make radionuclide measurements and to compare analytical results from different laboratories. The most critical need is for reference materials in the 235U decay series: 231Pa, 227Ac, and 223Ra.
Because many of the radionuclide measurements in seawater require sample sizes of 20-200 liters, it is not practical to distribute true seawater reference materials containing these radionuclides. Furthermore, several of the radionuclide half-lives are only a few days to weeks. A different strategy is clearly required. The most reasonable approach is to prepare a reference material in the field by diluting solutions containing the long-lived parents of the short-lived radionuclides with a volume of seawater similar to the volume used for analyses.
Individual Standard Reference Materials containing 14C, 3H, and some naturally occurring uranium and thorium series radionuclides are available from the National Institute of Standards and Technology (NIST).
These include:
-
238U, 234U, 235U,
-
230Th,
-
226Ra, and 228Ra.
No certified reference materials are currently available for:
-
231Pa,
-
227Ac,
-
223Ra,
-
232Th, and 228Th,
-
224Ra,
-
210Pb, and
-
210Po.
There are two approaches to providing the missing certified reference materials:
-
prepare additional individual solutions of 210Pb, 210Po, 231Pa, 227Ac, 232Th, and 228Th, or,
-
prepare three mixed solutions containing:
-
238U and 235U with daughters in secular equilibrium through 226Ra and 223Ra,
-
232Th with daughters in secular equilibrium through 224Ra, and
-
210Pb with daughters in secular equilibrium through 210Po.
-
The presence of 222Rn in the 238U series makes the extension of this series through 210Pb in the same solution very difficult due to the escape of radon gas. The advantage of the second (secular equilibrium) approach is that fewer solutions need to be prepared and the activities of the short half-life nuclides in these solutions will not change with time.
The committee thus recommends that NIST continue to provide reference materials for 14C and 3H as well as 238U, 234U, 235U, 230Th, 226Ra, and 228Ra. The committee further recommends the development of three new certified reference materials containing:
-
238U and 235U with daughters in secular equilibrium through 226Ra and 223Ra,
-
232Th with daughters in secular equilibrium through 224Ra, and
-
210Pb and 210Po at secular equilibrium.
These acid solutions should contain on the order of 20 Bq/g of 238U (1 Bq/g of 235U), 20 Bq/g 232Th, and 20 Bq/g 210Pb.
CARBON ISOTOPES IN DISSOLVED INORGANIC CARBON (DIC)
The measurement of stable (12C and 13C) and radioactive (14C) isotopes in DIC in the world’s oceans provides information that can be used to study many aspects of the ocean carbon cycle. The distribution of both 13C and 14C in the ocean is governed by an interplay of biological and physical processes with carbonate chemistry. The normalization of 14C to a constant 13C as well as to a fixed point in time (1950) (Stuiver and Polach, 1977), removes biological and decay effects. This treatment allows 14C to be used as a physical tracer while 13C distributions can reveal information about biological processes. In surface waters, 13C in DIC can be used to assess the uptake of anthropogenic CO2 (Gruber and Keeling, 1999; Sonnerup et al., 1999; Quay et al., 2000). In deeper waters, 13C can be used to study oxidation of organic carbon and its impact on nutrient
concentrations (Lynch-Stieglitz et al., 1995; Mackensen et al., 1996). 14C can be used to study the aging of ocean waters and to calculate pre-bomb surface water values (Toggweiler et al., 1989a; Key and Rubin, 2002). Although thousands of these measurements have been made recently on DIC as part of the WOCE and JGOFS Global Survey programs, and there are plans to repeat measurements over the next decades (NOAA, 2001), there are no formal seawater-based reference materials available for these isotopes.
One solution to the lack of standards for the carbon isotopes in DIC is to expand the use of the existing DIC concentration standard (Box 2.3) to include 13C and possibly 14C. This would be relatively simple: preliminary tests have shown the standards to provide reproducible 13C measurements. Inclusion of 14C in any reference material will require greater planning; while the reduction in sample size required for a radiocarbon measurement afforded by accelerator mass spectrometry (AMS) has greatly increased the scope of radiocarbon studies, it has also increased the likelihood of inadvertently contaminating a sample. Many oceanographic projects use radiocarbon as a tracer in experiments at sea and in the laboratory (e.g., for the measurement of oceanic productivity), and isolated spots or equipment can be accidentally contaminated. The radio-activities typically used in tracer experiments can be several million times modern levels and very small residual amounts can contaminate samples collected for the measurement of natural levels of 14C. Table 3.2 details the concentration differences between natural levels and those typically found in productivity measurements. Because of the difficulties in methodology and the potential for cross-contamination, great care and plan-
TABLE 3.2 Abundance of 14C in Natural Samples
ning must be used when trying to prepare a seawater-based reference material for 14C in DIC.
DISSOLVED ORGANIC MATTER (DOM)
Seawater contains on average about one mg/l of dissolved organic matter (DOM), which is by far the major form of organic material in the ocean. Seawater DOM contains a mass of carbon comparable to the total amount in atmospheric CO2 and therefore is of interest as a major reservoir in the global carbon cycle. In addition, DOM is a key source of nutrition to marine organisms, with at least 50 percent of all marine primary production flowing through dissolved organic biochemicals to bacteria (the “microbial loop”). Marine DOM also complexes metals, attenuates light, promotes photochemical reactions and integrates ocean events on time scales ranging from seconds to millennia (Hansell and Carlson, 2002).
Studies of the amount, composition, and chemical reactivity of seawater DOM are challenging because of the low concentrations of the component molecules immersed in a background of 35,000 times as much dissolved salt. In addition, natural DOM mixtures are compositionally complex, with less than 10 percent of the component molecules identified to date as simple biochemicals. The only feasible method for quantifying total seawater DOM is to combust all of the component carbon to CO2, which can be precisely measured as a proxy for total organic mass— seawater DOM contains approximately 50 weight percent of carbon. Although seawater DOM has been measured in this manner for almost 100 years, it was only in the early 1990s that the oceanographic community rigorously tested available methods for DOC analysis and came to a consensus concerning appropriate protocols and reference materials for routine analysis (Box 2.2). This effort (Sharp et al., 2002) is an outstanding example of how the oceanographic community can respond to an analytical challenge once the issues are clear and support is provided for workshops and (critical) environmentally pertinent reference materials.
There are two basic approaches used to characterize seawater DOM (Benner, 2002). The first of these is to directly analyze bulk compositions (e.g., elemental or isotopic compositions) or individual compounds in the sample without concentration. This approach requires high-sensitivity methods for either broad biochemical types (e.g., total amino acids or carbohydrates) or individual compounds, often by either spectroscopic or chromatographic methods coupled to electrochemical or mass spectrometric detectors. The latter type of molecular-level analyses are now feasible for measuring individual amino acids (Lindroth and Mopper, 1979), sugars (Skoog et al., 1999), and amino sugars (Kaiser and Benner,
2000) in less than 10 ml of seawater. The great information potential of organic compounds as source and reaction indicators, coupled with continued development of more sensitive and selective measurement methods based on mass spectrometric and electrochemical detection, suggests that direct molecular measurements will continue to grow in application.
The second basic approach to characterizing seawater DOM is to concentrate a fraction of the total mixture by chemical or physical means into either dry powder or concentrated solution. The solution can be analyzed using a wide array of methods (e.g., elemental analysis, biomarker analysis, mass, or nuclear magnetic resonance spectrometry) to which these isolates are amenable.
The first practical method for concentrating milligram amounts of seawater DOM was hydrophobic adsorption from acidified solutions onto a variety of substrates including charcoal and synthetic (e.g., polystyrene) resins and subsequent elution with a dilute base (e.g., 0.1 M sodium hydroxide [NaOH]) or methanol. Although the resulting “seawater humic substances” could be isolated as low-salt powders, this method involved severe pH conditions (pH = 1 to 13) and gave relatively low (5 to 25 percent of DOC) and chemically selective recoveries. Since the early 1990s (Benner et al., 1992), the DOM isolation method of choice has been tangential-flow ultrafiltration, which separates larger organic molecules from smaller sea salts and water. Although it still recovers less than half (25 to 40 percent) of total seawater DOM, ultrafiltration does not involve large pH fluctuations and is not intrinsically selective for different chemical compound types. A major drawback of ultrafiltration is that it is necessary to process 2000-5000 L of ocean water to recover 1g of isolate. At this scale, the isolation procedure requires special equipment, considerable operator skill, and approximately a week’s time.
With the previous considerations in mind, the most practical approach to providing DOM reference materials for the oceanographic community may be to focus on providing small (1L) seawater samples for bulk chemical and molecular analysis. With some planning, it seems feasible that these reference materials could be the same as previously described for inorganic analysis. The previously discussed surface and deep seawater samples recommended for iron measurement might be particularly suitable for organic analysis. The preparation steps of filtration and acidification required for the iron samples have already been found to stabilize DOC concentrations for at least six months (Hedges et al., 1993). Although some component biochemicals may exhibit greater reactivity than total DOC in such samples, deep ocean samples contain molecules that have survived in situ for decades or more and should be relatively stable.
The stability of the organic components of surface seawater samples is more questionable, but can be tested over time for a wide variety of molecule classes.
Although iron samples must be stored in plastic (not glass) containers, some polymers such as Teflon® and high-density polyethylene are routinely used for storing DOC samples and should be suitable. Even if some plasticizer does leak from the containers, its components should not include (or interfere with the analysis of) commonly analyzed biochemicals such as amino acids and sugars. A major advantage of being able to analyze iron and DOM components of the same reference material is that the two measurements would complement each other, because the concentration and reactivity of iron is largely controlled by complexation with organic substances.
Although an available ultrafiltered DOM reference material would provide great benefit to oceanographers studying both trace organics and metals, the expense involved in obtaining sufficient material (kg) for broad distribution is disproportionately high versus that for more readily available water and sediment samples. A parallel example would be sinking particles from marine water columns, which are also of broad interest but difficult to obtain in sufficient amounts for broad distribution and use. Both ultrafiltered DOM and sinking marine particles might be candidates for a future reference material suite, or for workshop-based initiatives specific to these challenging sample types.
Existing Reference Materials for DOM
At present, soil derived humic matter and fulvic acids extracted from freshwater are available commercially and are commonly used to test techniques for DOM detection and also used as model compounds for trace metal chelation studies. The results obtained using these model compounds are frequently extrapolated to the natural environment and measurements on “real” samples provide evidence that this DOM is a good model compound. In the past, some investigators also made available organic matter isolated from marine environments using C18 resins. While these compounds come from aquatic sources, this isolation technique is chemically selective and isolates only a small percentage of oceanic DOM. Reference materials are not currently available for these compounds, which inhibits study of the role they play in a variety of oceanographic processes.
DISSOLVED GASES
Atmospheric gases can be divided into those that are relatively constant and those that vary in concentration. The most abundant (and thus relatively constant) components are nitrogen (as N2), oxygen, and argon. The variable components are primarily water vapor and gases produced at least in part by human activities. These gases include CO2, methane (CH4), and carbon monoxide (CO). Both types of gases are exchanged between the atmosphere and the surface ocean with the result that they are generally at or near solubility equilibrium. They are then distributed throughout the ocean by down-welling water masses and large-scale ocean circulation. In ocean water, some gases behave conservatively, while others, such as O2 and CO2 are influenced by biological or other processes.
Dissolved gases in seawater have been used to examine the flux of greenhouse gases (e.g., CO2, CH4, nitrous oxide [N2O]) across the air-sea interface as well as to trace and date water masses (e.g., chlorofluorocarbons [CFCs]). Biogenic gases like dimethylsulfide (DMS) are oxidized to sulfuric acid (H2SO4) in the atmosphere to produce cloud condensation nuclei. Gas-phase standards are generally available for most gases. No solution standards exist, however, for gases dissolved in seawater. This is largely due to the difficulty of preparing stable gas solution standards. At present, gas-phase standards are used to determine the reliability of measurement techniques, but these cannot be used to check the reliability of equilibration time and stripping techniques. There is a need for dissolved gas solution standards in order to put seawater measurements on a common basis. For non-greenhouse gases (noble gases) used to study ground water processes, the atmosphere can be used as a standard if reliable solubilities are available. Due to these obstacles, new gas-based reference materials are not recommended at this time.