2
Resources, Responsibilities, and Dynamics in the Military’s Vaccine Mission
The process of acquiring and maintaining the availability of vaccines for use by the U.S. military is supported by an intricate, multitiered, and continually changing Department of Defense (DoD) organizational structure that encompasses military and civilian elements and that operates within the respective branches of the armed forces. The U.S. Congress has designated the U.S. Army as the lead agent for DoD infectious diseases research.1 The steps leading to the availability of a vaccine that protects military personnel against an infectious disease include identification of a need, research, development, testing, production, evaluation, regulatory compliance, and procurement. In this chapter, the committee describes these steps and associated DoD organizational components to the extent that they are relevant to its charge.
Over the course of this study, this committee has come to appreciate, though not completely comprehend, the complex and convoluted nature of the system by which DoD acquires vaccines. The complexity of this system is, perhaps, best depicted in Figure 2-1.
A more detailed description of this process—as it is understood by the committee—follows.
VACCINE MISSION OF THE U.S. ARMY MEDICAL RESEARCH AND MATERIEL COMMAND
The U.S. Army Medical Research and Materiel Command (USAMRMC), a subordinate command of the U.S. Army Medical Command (MEDCOM)
(Figure 2-2), is charged with solving medical problems and providing the armed forces with solutions to these problems in the form of medical products; among these solutions are vaccines. USAMRMC’s primary goal is to protect and sustain the health of the warfighter (USAMRMC, 2001a). To accomplish this goal, USAMRMC is “responsible for medical research, product development, technology assessment and rapid prototyping, medical logistics management, health facility planning, and medical information management and technology” (USAMRMC, 2001a).
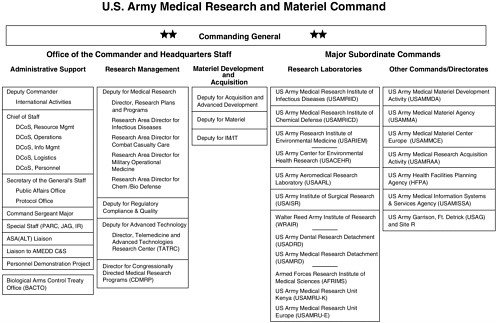
With activities throughout the United States and overseas, USAMRMC comprises its headquarters, six research laboratory commands, and six2 administrative commands or directorates. These laboratory and administrative commands are named as USAMRMC’s major subordinate commands in Figure 2-3. Approximately 4,600 military and civilian personnel are assigned to headquarters and the 12 subordinate units (USAMRMC, 2001a).
As the army’s medical materiel developer and logistician, USAMRMC has specified five major core capabilities (USAMRMC, 2001c):
-
Medical research and development
-
Logistics and acquisition
-
Information management/information technology
-
Advanced technologies
-
Congressional programs
As part of its medical research and development charge, USAMRMC has the responsibility for managing research as well as product development related to, among other things, vaccines and therapeutic agents aimed at preventing and controlling naturally occurring infectious diseases that are perceived to threaten the operational effectiveness of the armed forces. However, USAMRMC does not manage the advanced development of vaccines against biological agents that may be weaponized; DoD assigns that mission to the Joint Vaccine Acquisition Program (JVAP) (see also footnote 13).
Despite its role in vaccine acquisition, USAMRMC is not formally involved in determining DoD policy for vaccine use. The Office of the Assistant Secretary of Defense for Health Affairs (ASD[HA]) is charged with establishing and implementing policies relating to health care services for members of the armed forces. The civilian expert members of the Armed Forces Epidemiological Board (AFEB)—a standing scientific advisory committee under the executive agency of the army established by P.L. 92-463 (AFEB, 2001)—serve as scientific advisers to DoD and address such issues as disease control and health maintenance and disease prevention, including the use of vaccines.
|
2 |
The U.S. Army Garrison at Ft. Detrick is not included in this total, although it is included in Figure 2-3. |
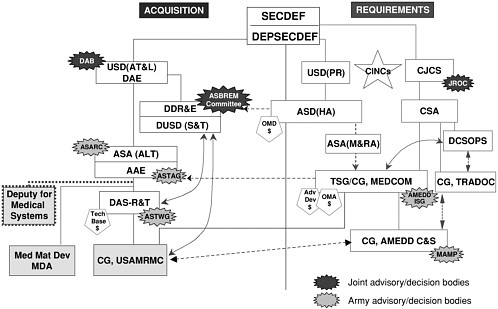
FIGURE 2-1 Military infectious disease-related research, development, and acquisition activities: USAMRMC interfaces with army and Office of the Secretary of Defense organizations. Abbreviations: AAE, Army Acquisition Executive; Adv Dev, advanced development; AMEDD C&S, Army Medical Department Center and School; AMEDD ISG, Army Medical Department Integration Steering Group; ASA(ALT), Assistant Secretary of the Army for Acquisition, Logistics, and Technology; ASA(M&RA), Assistant Secretary of the Army for Manpower and Reserve Affairs; ASBREM, Armed Services Biomedical Research, Evaluation and Management (Committee); ASD(HA), Assistant Secretary of Defense for Health Affairs ASARC, Army Systems Acquisition Review Council; ASTAG, Army Science and Technology Advisory Group; ASTWG, Army Science and Technology Working Group; CG, Commanding General; CINCs, Commanders in Chief; CJCS, Chairman, Joint Chiefs of Staff; CSA, Chief of Staff, U.S. Army; DAB, Defense Acquisition Board; DAE, Defense Acquisition Executive; DAS-R&T, Deputy Assistant Secretary of the Army for Research and Technology; DCSOPS, Deputy Chief of Staff for Operations and Plans, U.S. Army; DDR&E, Director of Defense Research and Engineering; DEPSECDEF, Deputy Secretary of Defense; DUSD(S&T), Deputy Under Secretary of Defense for Science and Technology; JROC, Joint Requirements Oversight Council; MAMP, Mission Area Materiel Plan; MEDCOM, Medical Command, U.S. Army; Med Mat Dev, medical materiel developer; OMA, Operations and Maintenance, U.S.Army; OMD, Operations and Maintenance, Department of Defense; SECDEF, Secretary of Defense; Tech Base, technology base; TRADOC, Training and Doctrine Command, U.S. Army; TSG, The Surgeon General USD(AT&L), Under Secretary of Defense for Acquisition, Technology, and Logistics; USAMRMC, U.S. Army Medical Research and Materiel Command; USD(PR), Under Secretary of Defense for Personnel and Readiness. SOURCE: Glenn (2000).
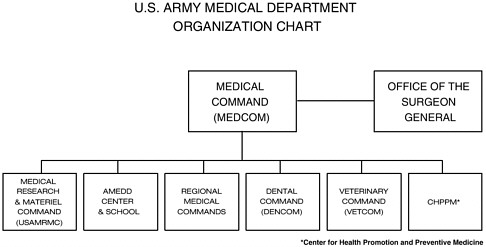
FIGURE 2-2 U.S. Army Medical Department (AMEDD) organizational chart. SOURCE: USAMRMC (2002b).
Basic Research Resources
Medical research and development activities within USAMRMC are conducted at six major laboratories, three laboratory detachments, and three overseas laboratories (USAMRMC, 2001b):
-
U.S. Army Aeromedical Research Laboratory
-
U.S. Army Institute of Surgical Research
-
U.S. Army Medical Research Institute of Chemical Defense
-
U.S. Army Medical Research Institute of Infectious Diseases
-
U.S. Army Research Institute of Environmental Medicine
-
U.S. Army Center for Environmental Health Research
-
-
Walter Reed Army Institute of Research
-
U.S. Army Dental Research Detachment
-
U.S. Army Medical Research Detachment
-
Armed Forces Research Institute of Medical Sciences—Thailand
-
U.S. Army Medical Research Unit—Europe
-
U.S. Army Medical Research Unit—Kenya
-
Infectious disease-related research activities are carried out within several of these laboratories, specifically, the Walter Reed Army Institute of Research (WRAIR) in Silver Spring, Maryland; WRAIR’s affiliated overseas laboratories
(listed above); and the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) at Fort Detrick, Maryland. USAMRIID maintains Biological Safety Level 4 research facilities. This capability permits research to be conducted with lethal pathogens (e.g., viruses that cause hemorrhagic fevers). The WRAIR complex includes a facility for the production of pilot lots of vaccines, which allows scientists to move prototype vaccines rapidly into production under good manufacturing practices (GMP)3 “that assure [the] purity, quality & consistency” of the product (Goldenthal, 2000).
The military also carries out infectious disease-related research at laboratories operated by the Navy Bureau of Medicine, including those at the Naval Medical Research Center, now colocated with WRAIR, and its affiliated overseas laboratories—the Navy Medical Research Unit 3 in Egypt, the Navy Medical Research Unit 2 (NAMRU-2) in Indonesia, and the Naval Medical Research Center Detachment in Peru.
Military Infectious Diseases Research Program
USAMRMC’s core medical research and development program is divided into four research area directorates (RADs) (USAMRMC, 2001a):
-
RAD1: Military Infectious Diseases Research Program (MIDRP)
-
RAD2: Combat Casualty Care Research Program
-
RAD3: Military Operational Medicine Research Program
-
RAD4: Medical Chemical and Biological Defense Program
RAD1 is the research directorate that manages MIDRP, which is charged with development of products to protect deployed warfighters against naturally occurring infectious diseases. MIDRP management (represented as Research Area Director for Infectious Diseases in Figure 2-2) coordinates the diverse and diffuse infectious disease-related research and development activities of USAMRMC and, on the basis of congressional direction,4 coordinates the infectious disease-related research activities of DoD research laboratories worldwide, including laboratories that are not within USAMRMC’s direct command, such as the Navy laboratories.
MIDRP’s mission is “to conduct, for the Department of Defense, a focused and responsive world class infectious diseases research and development program leading to fielding of effective and improved means of protection and treatment
to maintain maximal global operational capability with minimal morbidity” (Hoke, 2000a, p. 5). USAMRMC cites as support for this program a September 1999 executive order that refers specifically to “diseases endemic to an area of operations” and states that “it is the Policy of the United States Government to provide our military personnel with safe and effective vaccines, antidotes, and treatments that will negate or minimize the effects of these health threats” (Clinton, 1999).
Because it represents the naturally occurring infectious disease-related research interests of USAMRMC, MIDRP’s scope could extend to any naturally occurring infectious disease, endemic or newly emerging, that is judged to be capable of influencing the outcome of military operations by producing excessive morbidity, mortality, or disturbances to morale or whose occurrence could result in the excessive consumption of resources. MIDRP operates as a source of information and proponency for vaccine-related research and makes recommendations to the commanding general of USAMRMC regarding the allocation of funds to the organizations that will conduct that research. Approximately 1,000 people—including uniformed and civilian scientists—are available to support the infectious disease-related research mission of USAMRMC (Hoke, 2000a).
A summary of fiscal year (FY) 2002 USAMRMC infectious disease-related research funding is shown in Table 2-1.
TABLE 2-1 USAMRMC Infectious Disease-Related Research Funding,a FY 2002 (in millions)
|
Budget Activityb |
Vaccine |
Other |
Total |
|
6.1 Basic research |
2.8 |
6.5 |
9.3 |
|
6.2 Exploratory development |
20.7 |
11.6 |
32.3 |
|
6.3 Advanced development |
7.9 |
6.3 |
14.2 |
|
MIDRP funding total |
31.4 |
24.4 |
55.8 |
|
6.4 Demonstration and validation |
3.8 |
0.2 |
4.0 |
|
6.5 Engineering and manufacturing development |
2.1 |
1.2 |
3.3 |
|
Advanced development funding total |
5.9 |
1.4 |
7.3 |
|
aThe MIDRP director explained that figures given include relevant funding for the Science and Technology Evaluation Program and Science and Technology Objectives. WRAIR overhead is a large item that is distributed in proportion to the size of the two types of support. The figures in this table do not include human immunodeficiency virus-related research activities, which are included on a separate funding line. bDecisions related to the allocation of 6.1 to 6.3 funds rest with MIDRP; decisions related to the allocation of 6.4 and 6.5 funds do not. SOURCE: Hoke (2002). |
|||
Advanced Development and Logistics Resources
Intersecting with the basic laboratory-based research and development activities that MIDRP coordinates are USAMRMC’s advanced development and logistics management functions (Major Subordinate Commands listed in Figure 2-3), which include the following (USAMRMC, 2001a):
-
Advanced development:
U.S. Army Medical Materiel Development Agency (USAMMDA)
-
Contracting:
U.S. Army Medical Research Acquisition Activity (USAMRAA)
-
Medical logistics:
U.S. Army Medical Materiel Activity (USAMMA) and
U.S. Army Medical Materiel Center Europe
-
Health facilities planning:
Health Facilities Planning Agency
Three of the four major subordinate commands of USAMRMC play significant roles in the vaccine acquisition process: USAMMDA, USAMRAA, and USAMMA. The USAMRMC website describes the responsibilities of these subordinate commands as follows (USAMRMC, 2001a, p. 23):
The U.S. Army Medical Materiel Development Activity (USAMMDA), Fort Detrick, Maryland, develops and fields medical products for U.S. Armed Forces, in conjunction with the Army Medical Department Center and School (the medical combat developer) and the U.S. Army Medical Materiel Activity (the medical logistician). Concepts/products developed in the USAMRMC laboratories are transitioned to USAMMDA for advanced development. USAMMDA plans, manages, and directs execution of medical materiel development to achieve U.S. Army and Joint Service materiel system objectives to meet cost, schedule, and performance. The USAMMDA also manages clinical data and coordinates with the Food and Drug Administration for approval of medical materiel for human use. The USAMMDA’s vision is to provide world-class medical solutions for U.S. warfighters.
The U.S. Army Medical Research Acquisition Activity (USAMRAA), Fort Detrick, Maryland, provides contracting support to the USAMRMC and its worldwide network of laboratories, to the Fort Detrick Army Garrison, military tenant activities, Army-wide projects sponsored by The Surgeon General, and congressionally mandated programs. The USAMRAA vision is to be a leader in innovation and the premier federal organization committed to acquisition excellence. The USAMRAA staff has leaders in innovation who are committed to acquisition excellence. They provide expert advice on procurement and assistance issues.
The U.S. Army Medical Materiel Agency (USAMMA), Fort Detrick, Maryland, serves as the Army Surgeon General’s central focal point and executive agent for all strategic medical logistics. Its mission is to deliver and sustain responsive medical logistics support for all worldwide military health care operations. The USAMMA serves as the AMEDD’s [Army Medical Department] fielding command for all new medical materiel, and centrally manages a variety of strategic logistics programs such as war reserve and critical item asset management, deployment of materiel handoff teams, and operational oversight of medical materiel acquisition vehicles. Core skills and technologies center on conducting
life-cycle management for commercial and nondevelopmental items, sustaining and modernizing the medical force, supporting exercises and contingency operations, and promoting medical logistics information and knowledge. USAMMA personnel develop and implement innovative logistics concepts and technologies, manage strategic war reserve and critical items (e.g., anthrax vaccine), and manage the acquisition life cycle for medical materiel.
RESEARCH, DEVELOPMENT, AND ACQUISITION IN CONTEXT
To make the journey from a recognized military medical problem to a licensed and procured or available vaccine, an idea must pass through a complex series of priority-setting and budgeting processes and through the hands of various USAMRMC managers, as well as numerous DoD stakeholders outside USAMRMC. Research priorities evolve through multiple channels. Officially, a military product begins life as a perceived need—a problem that needs a solution. Needs are first formalized as Future Operational Capabilities (FOCs). FOCs are worded very generally and allow consideration of solutions based on doctrine,5 training, leader development, organization, materiel (products), or the soldier (DA, 1999). Preference is given to the quickest, least expensive solutions (often those that involve doctrine) over the slowest, most expensive solutions (often those that involve materiel) (DA, 1999).
MIDRP, with input from the service requirements offices, drafts product-related objectives for review and modification by the Joint Technology Coordinating Group-2,6 recommends draft objectives to the USAMRMC commanding general, and develops research plans that reflect the goals outlined in FOCs.
Regarding materiel solutions for infectious diseases, FOCs allow, for instance, consideration of vaccines, drugs, immunotherapies, immunoprophylactic preparations, vector control products, and diagnostic tests. As part of its threat identification and prioritization duties, the Army Medical Department (AMEDD) Center and School7 reviews the products that are being sought through MIDRP and offers an assessment of their importance, providing feedback to MIDRP about its priorities. Informal dialogue between MIDRP and the AMEDD Center and School is ongoing, and inputs on infectious diseases threats are obtained from a number
of sources.8 The AMEDD Center and School also has the responsibility to produce a list of infectious disease threats (Scott, 2000). However, the most recent threat list produced by AMEDD Center and School (approved by U.S. Army Training and Doctrine Command [TRADOC]) was produced in 1986, with modifications in 1987 and 1988 (Hoke, 2002; TRADOC, 1986).
The Assistant Secretary of the Army for Acquisition, Logistics, and Technology (ASA[ALT]) provides funds to support technology base-related research efforts (6.1 through 6.3 research). MIDRP provides guidance regarding research priority setting and manages the distribution of funds for that research (Table2-1). Each year, army research and development laboratories—both medical and non-medical—submit to ASA(ALT) nominations of products to be selected as Army Science and Technology Objectives (STOs). STOs are identified, refined, reviewed, and prioritized annually through a process that involves input from a large number of interested parties, including USAMRMC,9 leading to final approval of the STO program by TRADOC.10 Approved STOs receive priority funding (TRADOC, 1999). Multiyear funding for research is not available without a STO. Within USAMRMC, the promise to provide specified STO funding is considered firm. At present there are about 200 STOs throughout the army. Of those, approximately 30 STOs are medical, and 8 of those11 are within the purview of MIDRP.
New product development efforts may begin without formal documentation of a specific need. In addition to formal STO efforts, USAMRMC maintains other basic research activities under its Science and Technology Evaluation Program (STEP). When enough is known to allow formulation of a specific product plan, USAMRMC can then propose it as a STO.
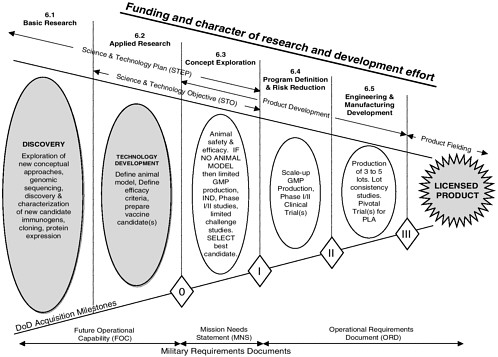
In FY 2001, infectious disease vaccine-related research12 STEPs included work on malaria vaccines, means for the prevention of diarrheal diseases, flavivirus vaccines, the malaria genome project, hepatitis virus vaccines, meningococcal vaccines, vaccine delivery, protection from viruses that cause hemorrhagic fevers and other highly lethal viruses, rickettsial diseases, and the prevention of human immunodeficiency virus (HIV) infections in military personnel (Hoke, 2000a). Figure 2-4 outlines the research and development path for vaccines.
At the end of the 6.3 program phase, projects are reviewed to determine their suitability for advanced development. If a successful candidate (in the context of this report, a candidate vaccine against a naturally occurring infectious disease) emerges from the research and development technology base—the domain of MIDRP—it is transitioned to the advanced development stage, at which time the product leaves MIDRP management and becomes the charge of USAMMDA,13 another part of USAMRMC. The transition to advanced development requires formal documentation—in the form of an Operational Requirements Document (ORD)—of a specific need for the product. An ORD specifies performance and other operational parameters for the product, including estimates of the funds that will be required, personnel requirements, and measurable capabilities and characteristics of the proposed system (DoD, 2001e). Typically, 5 to 10 years might pass after the start of work on a product before an ORD is written.
Once MIDRP recommends a product for transition to advanced development, a USAMMDA product manager works with a research coordinator to collect information and prepare a development plan. The vaccine product is then presented to representatives of other DoD organizational elements involved in the acquisition and procurement of medical materiel for approval. The core team members are the combat developer (AMEDD Center and School), the materiel developer (USAMMDA), and the logistician (USAMMA). Whether the potential product makes the transition to advanced development depends on an assessment
of technical feasibility, need, and the availability of funds. At this point in the process, products are categorized on the basis of the estimated total program cost (DoD, 2001e). Acquisition categories (ACATs) determine the level of DoD review, decision authority, and the procedures applicable for a given acquisition project (DoD, 2001e). At present, each vaccine product is managed as a distinct acquisition system. Vaccine products are managed as ACAT III (less than major) systems, the lowest priority of the three ACAT levels (i.e., ACAT I, ACAT II, and ACAT III) (DoD, 2002; Personal communication, W. Howell, Department of Defense, February 28, 2002).14
Consideration has been (and continues to be) given to packaging vaccines together in project groups to increase their visibility within DoD and, subsequently, to increase their opportunities for funding. Also, a planned reorganization of the advanced development is to be implemented in July 2002.15 This reorganization is intended to refine the management of acquisition activities within USAMRMC. Cumulatively, these changes are to bring USAMRMC acquisition practices more in line with DoD norms. These changes will not, however, directly affect basic research and development (vis-à-vis MIDRP).
The vaccine product competes with other products for funding for advanced development and other resources, such as staff expertise. The funding path for advanced development research (6.4 and 6.5) originates with the Deputy Chief of Staff for Operations and Plans, U.S. Army (DCSOPS) and differs from the funding path for technology base research (6.1 through 6.3). Funding for advanced development of vaccines against infectious diseases is substantially less (approximately $7.3 million total in FY 2002) than funding for technology base research funding ($55.8 million total in FY 2002; see also Table 2-1). At present approximately seven16 (Hoke, 2002) vaccine products are in advanced development.
Progress to advanced development can stall even though a technically feasible product candidate may have been worked on for years. For instance, limited
funds may be directed to projects that DoD considers—according to official priority ranking or by decision-maker discretion—to be relatively more important. Diseases may be sufficiently localized or so rare that populations appropriate for efficacy testing are not available; or projects may face financial, ethical, or regulatory constraints. Also, in the absence of partners in advanced development of a product, further basic scientific development may be impractical.
Successful advanced development efforts proceed from advanced clinical trials and upscaling of manufacturing for Phase III efficacy trials through to the submission of a Biologics License Application to the Food and Drug Administration.
PROCUREMENT, STORAGE, AND DISTRIBUTION IN CONTEXT
Within DoD, funds for the purchase of medical products and the maintenance of medical products that have been acquired (including vaccines) are separate from funds for research and development. Program 6 funds are used to fund 6.1 through 6.5 research and development, and Program 8 funds are used to fund operations and maintenance.
Once a product is licensed, AFEB and other organizations consider recommendations for use of the product by military personnel. Each service is responsible for procuring its own required vaccines in coordination with USAMMA, the designated lead agent for vaccine supply (AFEB, 1999). Medical care facilities purchase vaccine products recommended for routine use for the protection of the health of the members of the armed forces (e.g., adenovirus vaccine), usually directly from the vendor. Vaccine products recommended for use for the protection of new recruits or for general use among all members of the armed services are procured with funds for medical care (Defense Health Care). The USAMRMC commanding general has no authority in this process. Some vaccines recommended for use in specific deployments do, however, fall within the nominal authority of USAMRMC through USAMMA.
Vaccines that are DoD-wide requirements may be purchased from stocks held by the Defense Logistics Agency and its inventory control point, the Defense Supply Center, Philadelphia (DSCP). At the time of this writing, DSCP reports that it stocks influenza and yellow fever vaccines (Hoke, 2002). Vaccines of importance to the military that DSCP does not stock include those that are no longer available (those for the prevention of adenovirus infection, cholera, Lyme disease, and plague) as well as those otherwise available to prevent Haemophilus influenzae type B infection; hepatitis A; hepatitis B; Japanese encephalitis; measles, mumps, and rubella; meningococcal disease; pneumococcal disease; polio (the inactivated vaccine); rabies; tetanus; diphtheria; typhoid; and varicella (DSCP, 2002). DSCP manages the procurement and distribution only of those vaccines that are licensed by the Food and Drug Administration (AFEB, 1999). Available vaccines that DSCP does not stock are usually obtained directly from the manufacturer on an as-needed basis. Some purchase agreements (prime vendor
agreements) include clauses that obligate a supplier to meet military needs during surges in demand (DMM, 2002). However, shortages and other supply issues can affect timely access to many of the vaccines listed above that are otherwise considered putatively available (DSCP, 2002).
EXTERNAL INTERACTIONS
A number of cooperative agreements facilitate DoD’s research efforts, including Collaborative Research and Development Agreements (CRADAs), Small Business Innovation Research awards, Dual-Use Science and Technology (DUST) agreements, and other mechanisms. DoD uses these agreements to secure relationships with companies, academic institutions, the governments of other nations, and U.S. government agencies other than DoD.17 USAMRAA processes and monitors internal and external agreements.
The CRADA is a common mechanism used to make external vaccine-related research and development agreements and one of the few means by which DoD can accept resources from external sources. In late 2001, as many as 78 infectious disease-related CRADAs involving as many as 69 partners were active (Hoke, 2002). Laboratory commanders or USAMRMC negotiate CRADAs. Partners use CRADAs to form collaborative relationships, often for the development of specific products. CRADAs allow DoD’s partners to supply DoD with people and in-kind resources. Laboratory commanders use CRADAs to acquire additional resources to work on products that are related to the objectives of MIDRP, extending their work into research areas where limited resources do not permit full government funding (Booz Allen and Hamilton, 1999, p. 17). As a result DoD laboratories may incur obligations to partner companies. Despite its charge to oversee research related to infectious diseases, the MIDRP management office (RAD1) reports that it is neither involved in CRADA development or approval nor routinely informed of the terms of such arrangements.
The DUST program supports initiatives that may have some use in the civilian sector as well as utility to the military. The program is funded by DoD dollars drawn from research and development funds. A complex formula governs the amounts provided by companies and by the government. Scientists initiate DUST agreements to take advantage of the available funds. In contrast to the approval process for CRADAs, the MIDRP management office reports that it is heavily involved in the review of proposed DUST agreements, providing input regarding feasibility and program relevance.
Examples of current partnerships are shown in Table 2-2.
TABLE 2-2 Selected Current Vaccine-Related Agreements
|
Award Type |
Partner Organization |
Project Title |
|
DUST agreement |
|
|
|
|
Acambis, Inc. |
Development of a live attenuated vaccine for the prevention of diarrhea caused by enterotoxigenic Escherichia coli (ETEC) |
|
|
Acambis, Inc. |
Development of a subunit vaccine for the prevention of Campylobacter infection |
|
Small Business Innovation Research |
|
|
|
|
3rd Millennium Inc. |
Development of World Wide Web-driven bioinformatic platform of DNA microarrays |
|
|
Eikos LLC |
Platforms for rapid DNA microarray prototyping |
|
Small Business Technology Transfer |
|
|
|
|
Antex Biologics Inc. |
Development of a prototype multivalent oral vaccine for travelers |
|
CRADA |
|
|
|
|
Multiple partners include: • U.S. government agencies • Non-U.S. government agencies • Pharmaceutical companies • Biotechnology companies • U.S. academic institutions • Non-U.S. academic institutions |
Examples of projects include: • Research of candidate vaccines for the prevention of HIV infection and AIDS • Development of a cholera vaccine for military personnel • Research and development of vaccine products against ETEC • Malaria vaccine development • Shigella vaccine development |
|
SOURCE: Adapted from Hoke (2002). |
||
Government Agencies and Nongovernment Organizations
Many international organizations and U.S. agencies other than DoD share USAMRMC’s vaccine development mission to various degrees, although their resources, specific areas of focus, and underlying purposes and the populations that they serve may differ. From MIDRP’s perspective, the National Institutes of Health (NIH) is a critical contributor to basic infectious disease-related research.
NIH recently opened the Vaccine Research Center. NIH has devoted substantial funds to globally important infectious disease-related research, and some of this research is also of interest to USAMRMC (such as research on HIV—$2.8 billion/year [NIH, 2002b]—and malaria—$71 million/year [Personal communication, W. Crum, NIAID Office of Financial Management, June 14, 2002]). A dramatic increase in NIH funding for research on biological agents that may be weaponized, totaling $1.5 billion for the National Institute of Allergy and Infectious Diseases alone, is requested in the President’s proposed FY 2003 budget (NIH, 2002a).
The Centers for Disease Control and Prevention (CDC) conducts outbreak investigations and disease control efforts and has been charged with, for example, the creation of a new national stockpile of smallpox vaccine (Gordon, 2001). CDC also maintains stockpiles of vaccines—mostly mandated pediatric vaccines such as the measles-mumps-rubella vaccine, tetanus-diphtheria toxoid, and inactivated polio vaccine—through the companies that manufacture the vaccines to ensure continued access to these vaccines for public health. CDC is considering whether to stock new vaccines, such as Wyeth’s pneumococcal conjugate vaccine, Prevnar, and Merck’s varicella vaccine, Varivax (Vaccine Stockpile Strategy, 2002). Although the Food and Drug Administration’s role is mostly regulatory, the agency also maintains a research capability in biologics evaluation and research and contributes to DoD’s research programs through the CRADA mechanism.
International organizations such as the World Health Organization and its regional affiliates (such as the Pan American Health Organization) also undertake vaccine-related research and development and facilitate the development of programs to control vaccine-preventable diseases. Many DoD medical research laboratories serve as reference laboratories for CDC (e.g., USAMRIID) (USAMRMC, 2001a) and the World Health Organization (e.g., NAMRU-2) (NAMRU-2, undated).
Interactions among these organizations and between these organizations and DoD vary in their levels of formality, extent, and effectiveness. At present, some program-level coordination of research efforts between DoD and other federal agencies and international organizations exists. DoD representatives participate in the National Vaccine Program Office Interagency Group, and DoD sends liaisons to the National Vaccine Advisory Committee (NVPO, 2001). The DoD research program in retrovirology is in frequent contact with HIV vaccine development offices at NIH. DoD, NIH, and CDC voluntarily coordinate their malaria vaccine programs through the Federal Malaria Vaccine Coordinating Committee (FMVCC, 2001).
Academia
DoD also maintains relationships with academic researchers and institutions for vaccine-related research and development. These relationships exist primarily
at the research laboratory level. For example, NIH provides funds to the University of Massachusetts at Worcester to study the pathogenesis of dengue hemorrhagic fever. The bulk of the field research funded through this grant is carried out at the Armed Forces Research Institute of Medical Sciences, a subsidiary of WRAIR, and at other institutions in Thailand. MIDRP also provides funding for these projects. Although this collaboration is not specifically vaccine related, MIDRP considers it to be productive because it lays substantial groundwork that will be needed to field-test an anticipated vaccine against dengue virus.
MIDRP seeks input from academia through its peer review program for proposed research. Since 1999, all internal research funded by MIDRP at army and navy laboratories has been subject to review by external scientists.
Industry
DoD’s relationships with industry are complex. USAMRMC research laboratories interact with industry at the vaccine research and development stage (see Table 2-2 for examples) and at the vaccine procurement stage. Successful partnerships have been developed for the procurement of vaccines against influenza virus, Japanese encephalitis virus, and hepatitis A virus. Difficulties with procurement and maintenance have, however, halted or threatened the continued production of vaccines that are needed, such as vaccines against adenovirus, plague, and tetanus (Hoke, 2002). Over the years DoD has developed vaccines against diseases including Rift Valley fever, Argentine hemorrhagic fever, eastern equine encephalitis, western equine encephalitis, and Venezuelan equine encephalitis for which no commercial manufacturers have been identified.
Vaccines developed or marketed by foreign manufacturers for locally endemic diseases may be of use to DoD from time to time (e.g., the vaccine against Japanese encephalitis virus). Other vaccine products (e.g., the vaccine against tick-borne encephalitis) have followed or are following similar development and marketing paths but have not yet been licensed.
Also of note are instances in which a vaccine developed by the Army might have international use that is greater than its direct use to the DoD (e.g., Rift Valley fever). A 1990 analysis suggests that nearly 80 percent of the difference in disease burden between the poorest and richest 20 percent of the world’s population, in terms of death and disability-adjusted years, was attributable to communicable disease (Widdus, 2001). Many of the vaccines developed to protect deployed U.S. forces may also be of benefit to the world’s poorest populations, perhaps compelling DoD interest in a wider range of vaccine development efforts than might be dictated by market forces alone. The committee observes that, overall, the availability of a vaccine for military use is subject to many complex and changeable interests within—and external to—DoD.