4
Recommendations with Accompanying Analysis of Limitations Imposed by Current Department of Defense Structure for Managing Acquisition of Vaccines Against Infectious Diseases
Substantial shifts have occurred in the geopolitical, budgetary, and psychological framework within which the Institute of Medicine (IOM) committee that has prepared this report began its work 2 years ago. The September 11, 2001, terrorist attacks heightened the nation’s sense of vulnerability, and contamination of the U.S. mail with anthrax spores focused the public’s attention on bioterrorism and infectious disease threats. To the Department of Defense (DoD), however, the reality of infectious disease threats predated this recent national interest. DoD’s longstanding interest in the use of vaccines to protect military personnel against infectious disease threats is reflected in this committee’s charge as well as in DoD’s separate request to an expert panel led by Franklin Top, Jr., (DoD, 2001d) for advice on its vaccine production capability. These two reports and the recent statement by the IOM Council (IOM, 2001) encouraging the creation of a National Vaccine Authority share a common sense of urgency in suggesting that changes are needed in the processes by which the government acquires vaccines. At the same time, the President’s fiscal year (FY) 2003 budget proposal, the heightened public perception of infectious disease threats, and the attention now focused on biodefense provide unparalleled opportunities for change and set the stage for DoD to act.
Thus far in this report, the committee has presented mostly factual, descriptive information about the need for vaccines, their use in the U.S. military, and the organizational procedures through which DoD advances a vaccine from the point of recognizing the need for a vaccine to making it available for use by military personnel. Here, the committee presents its discussion of those organizational, procedural, and scientific components and provides its analysis of how the pieces might be made to fit better and how the overall process of vaccine acqui-
sition might be improved. Wherever possible, the committee cites specific evidence to support its conclusions. However, in a number of instances no such data were available and the committee was forced to rely on the perceptions of those interviewed by the committee or on indirect evidence, often in combination with the past experiences of committee members in their interactions with both military and civilian vaccine acquisition systems. In such cases, the committee has made every effort to note the lack of hard evidence supporting its contention.
Protecting the health of military personnel is essential to national security. The committee presented in Chapter 1 historic evidence that infectious diseases have posed significant threats to the health of the nation’s armed forces. Chapter 3 describes those vaccines that are available to the military for the prevention of infectious diseases. A review of the data presented in this report (e.g., Chapter 3) makes it clear that no vaccine is available for many of the infections that have previously posed problems for U.S. forces on overseas deployments (e.g., dengue, diarrhea, and tick-borne encephalitis, to name a few of those listed in Table 1-2). Thus, it is clear that infectious diseases remain a major concern even as the twwenty-first century unfolds. The considerable number of overseas deployments of U.S. forces on warfighting and peacekeeping missions in recent years suggests that the risk of exposure of military personnel to both naturally acquired and intentionally released infectious agents remains real and present.
Vaccines are often the most cost-effective way to protect individuals from infectious diseases, but their value is easily overlooked both within the civilian public health sector and within the military community. For example, a successful antiballistic missile defense system may provide dramatic evidence for its utility when it destroys an incoming warhead, but a safe and effective vaccine leaves no trace of its success when the immune response that it has engendered in the immunized soldier thwarts the early stages of a potentially lethal infection and prevents an incapacitating illness or death. On the basis of its review of the circumstances surrounding the loss of the adenovirus vaccines and the lack of an available licensed plague vaccine (Table 3-3) and (until very recently) an anthrax vaccine, as outlined below, the committee believes that DoD must assign a higher priority to vaccine acquisition than it has in the past.
For the purposes of this discussion, the committee defines acquisition as the process by which DoD ensures that appropriate vaccines are available for the protection of its forces. This process represents a continuum extending from the first recognition of need, to the setting of priorities, to the maintenance of a technology base permitting internally conducted or externally contracted product-oriented research, advanced product development, and clinical studies leading to licensure (whether or not DoD is in partnership with an industrial entity), as well as the establishment and maintenance of effective manufacturing facilities and, ultimately, the procurement (purchase) and stockpiling of vaccine for use by DoD for protection of members of the U.S. armed forces.
The committee’s main conclusion is that DoD’s current vaccine acquisition procedures, coupled with its complex annual budgeting process, significantly hamper its vaccine acquisition activities and thwart effective coordination with vaccine manufacturers. The evidence that led the committee to this conclusion is laid out in the pages that follow. These limitations result in an inability to develop the vaccines that are needed (as evidenced by the large number of vaccines listed in Table 3-5 that are no longer being actively developed for protection of the armed forces), instability in essential vaccine-related research programs (which is reflected in wide fluctuations in budget authority, as described below), and the failure to have available for immediate use those vaccines that are critical for the protection of military personnel, as cited above. The ultimate cost of this inefficient acquisition process is that military readiness is placed at risk. Some militarily important vaccines are not available, in whole or in part, because of poorly aligned acquisition processes and an inadequate commitment of financial resources rather than because of unmet scientific or technological hurdles. This is particularly true for the vaccines listed in Table 3-5, including, for example, the attenuated Junin virus (Argentine hemorrhagic fever) vaccine, for which evidence supporting substantial clinical efficacy has been amassed in a trial carried out among civilian populations in South America (Maiztegui et al., 1998).
DoD’s current approach to vaccines originates with the best intentions, involves skilled individuals, millions (but not sufficient millions) of dollars, and intricate planning. Nevertheless, the committee’s assessment after hearing from many of those involved in the acquisition process, as well as several executives from the companies that manufacture vaccines, is that the current vaccine acquisition process has limitations that make the path from basic research to procurement and use of vaccines both inefficient financially and cumbersome. These limitations result in occasional outright failure (as in the case of the loss of the adenovirus vaccines) and unacceptable delays (in the case of the anthrax vaccine) in vaccine acquisition. The lack of vaccines when and where they are needed risks the success of future military operations and the health of personnel and potentially places national security in jeopardy.
The committee’s recommendations cover four broad aspects of the acquisition process:
-
Organization, authority, and responsibility
-
Program and budget
-
Manufacturing
-
Regulatory status of special-use vaccines
After first presenting its nine recommendations in Box 4-1, the committee provides a discussion, building its case with examples and presenting the reasoning that has resulted in each recommendation.
|
BOX 4-1 Committee Recommendations Organization, Authority, and Responsibility The committee recommends that the Department of Defense:
Program and Budget The committee recommends that the Department of Defense:
Manufacturing The committee recommends that the Department of Defense:
Regulatory Status of Special-Use Vaccines The committee recommends that the Department of Defense:
|
ORGANIZATION, AUTHORITY, AND RESPONSIBILITY
Early in the committee’s deliberations, one DoD representative attempted to clarify the DoD process for setting vaccine research and development priorities with an illustrative slide, presented here as Figure 4-1 (and earlier as Figure 2-1). It clearly conveys the complex gauntlet awaiting the potential acquisition of a new vaccine from the time of the first conception of its need through the late stages of development. Figure 4-1 also vividly demonstrates the absence of a single organizational locus of authority and responsibility for that process. Not only is no individual in charge, but too many individuals and entities are responsible for other, unrelated activities in addition to their responsibilities for vaccines and the development of effective countermeasures against infectious disease threats. The committee believes that DoD’s vaccine acquisition program does not—and cannot—work effectively with its management structured in this fashion.
Perhaps the best example of how such diffuse management arrangements thwart effective vaccine acquisition is the loss of the adenovirus type 4 and 7 vaccines that the U.S. military used very effectively for many years to prevent acute respiratory disease among trainees. The committee heard from representatives of both DoD and the vaccine manufacturer (Wyeth) concerning the events that led up to the decision by the latter to cease manufacture of the vaccine because of its inability to make changes to its manufacturing facility required by the Food and Drug Administration (FDA) under the terms of its existing contract with DoD. What the committee heard was the inability of the manufacturer to identify any single point of authority within DoD that was sufficiently knowledgeable about the issues and sufficiently empowered to make changes in the contract with the manufacturer necessary to maintain vaccine production. No single entity in DoD had sufficient breadth of authority or responsibility to approve further research and development or to authorize modifications to the manufacturing facility once the vaccine had become licensed, even though this meant that production of the vaccine would cease and that future procurement would not be possible. The end result was the recurrence of serious adenovirus respiratory infections among basic trainees, a problem that continues to the present.
This particular issue was the subject of an interim report (IOM, 2000a; also provided as Appendix A to this report) released by the IOM committee that has prepared this report. Although one cannot be certain that a consolidation of all responsibility for vaccine acquisition within a single authority in DoD would have prevented the loss of these vaccines, the committee is convinced that the disjointed authority for advanced vaccine development and vaccine procurement that exists within DoD contributed significantly to the lack of the additional investment required for continued production of this vaccine.
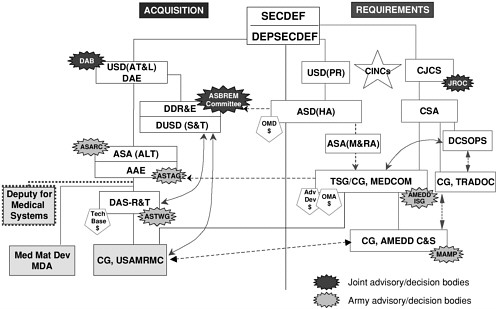
FIGURE 4-1 Military infectious disease-related research, development, and acquisition activities: USAMRMC interfaces with army and Office of the Secretary of Defense organizations. The acronyms and abbreviations included in this figure are identified in the caption to Figure 2-1 of this report. SOURCE: Glenn (2000).
Another expert committee commissioned by DoD recently reached a similar conclusion (DoD, 2001d). Soon after IOM constituted the committee that has authored this report at the request of the U.S. Army Medical Research and Materiel Command (USAMRMC), the Deputy Secretary of Defense directed the Acting Assistant Secretary of Defense for Health Affairs (ASD[HA]) and the Director of Defense Research and Engineering to form a group, chaired by Franklin Top, Jr., and charged it with a task—based on the requirements outlined in P.L. 106-398—that significantly overlapped that of the IOM committee. Working independently and with different emphases, the two committees identified similar systemic problems and arrived at similar recommendations to address them, including the need for centralized and coordinated management and strengthened, supportive expert advice.
These committees are not the first to note organizational and procedural problems within the DoD’s acquisition processes. The DoD Reorganization Act of 1986 called on DoD to “reduce and streamline the defense bureaucracy” (Republican Policy Committee, 1986). DoD, itself, has recognized the need to reform its acquisition system—agency wide. In 1994, the Secretary of Defense released a report entitled Acquisition Reform: Mandate for Change outlining the need to change the acquisition system. It noted, “The problem is that the DoD acquisition system is a complex web of laws, regulations, and policies. . . While each rule individually has (or had) a purpose for its adoption, and may be important to the process as a whole, it often adds no value to the product itself, and when combined, contributes to an overloaded system that is often paralyzed and ineffectual, and at best cumbersome and complex” (DoD, 1994, pp. 5, 6). In 2001, DoD again addressed the inefficiency of the acquisition system in its Quadrennial Defense Review Report, which notes that “two major institutional processes—the planning, programming and budgeting system and the acquisition process—create a significant amount of the self-imposed institutional work in the Department. Simplifying these processes will support a streamlining of the entire organization [the Department of Defense]” (DoD, 2001c, p. 52). The General Accounting Office (GAO), in testimony before Congress on February 27, 2002, notes that despite DoD’s heavy dependence on acquisition—“close to $100 billion annually to research, develop, and acquire weapon systems and tens of billions more services and information technology” (GAO, 2002, p. 1)—its acquisition system is inefficiently managed. GAO studies found that responsibility for acquiring services is diffuse and “with little visibility or control at the DoD- or military department level” (GAO, 2002, p. 3). The report notes that DoD “is seeking to adapt the same revolutionary business and management practices that helped the commercial sector gain a competitive edge” (GAO, 2002, p. 3).
The GAO outlines, in its testimony, several suggested changes that may improve the efficiency of the DoD acquisitions system, including restructuring programs so that requirements and needs are better matched, making sure that decision makers are open to funding the lifecycle of a product, and assuring that
those making decisions—in terms of time and money spent on a product—are sufficiently knowledgeable about the product and are persons vested with the authority “to make informed tradeoff decisions” (GAO, 2002).
Diffuse Management Responsibility
As detailed above, no identifiable decision maker within DoD has the responsibility and authority for vaccine acquisition. No single organizational agent within DoD drives the vaccine acquisition system or acts as a galvanizing motivator. No single organizational unit within DoD has the authority to address problems arising with licensed products to maintain product availability.
Because no single authority within DoD oversees the vaccine acquisition effort, the DoD decision-making structure for vaccine acquisition is fragmented at each step of the process, including research, development, production, licensure, and the purchase and stockpiling of vaccines. The fragmentation of these processes hinders the creation of priorities and the acquisition of vaccines that the military needs. It leads to misalignment of resources, creates disparities between vaccine research efforts and relevant military medical operations, and leaves large gaps within the research and development process. It prevents any long-term stability across the many years during which a new vaccine is conceptualized, moves through the preclinical and clinical research stages, and finally, is licensed. Furthermore, just as budgetary authority is disjointed, so is program authority. Even the various research and development components—technology base and advanced development—do not share an effective prioritization mechanism. The committee was unable to identify a single list of priorities for vaccine acquisition that each of these separate DoD entities involved in the vaccine acquisition continuum uses. This disconnect can result in the misdirection of resources.
Consolidating responsibility and authority for the acquisition of vaccines within a single organizational entity or vaccine authority would provide a seamless process by which DoD could acquire vaccines to provide the protection that its forces require. Vaccine acquisition would be enhanced by developing and imposing a common means of prioritization at all levels of the vaccine acquisition effort, by eliminating unnecessary bureaucracy and overlapping, redundant programs, by improving communication among those responsible for different aspects of the vaccine acquisition continuum, by eliminating the waste of program resources, and by managing vaccine acquisition as part of a higher-priority DoD acquisition category (e.g., acquisition category I).
Having expended considerable time in attempting to understand the complexities of the current acquisition process, the committee concludes that DoD should create a single vaccine authority by concentrating responsibility and authority for the entire vaccine life cycle—up to, but not including, policy and clinical decisions concerning the use of vaccines. This entity should be the con-
trolling authority for the acquisition of vaccines and related biological countermeasures and not simply a coordinating body. It should report to the highest levels within DoD. To succeed, this vaccine authority must have the following:
-
sufficient authority to influence vaccine development, including adequate budgetary authority with assured funding for operations (such as for the procurement of vaccine products after the research period) and control over any government-owned manufacturing facility, such as the government-owned, contractor-operated (GOCO) facility now being considered by DoD;
-
adequate staffing to manage and accomplish all phases of the acquisition process, from priority setting to vaccine research and development, product development, manufacture, and stockpiling;
-
personnel with the financial, regulatory, and legal expertise required for all aspects of the vaccine acquisition process integrated within a single office;
-
clearly defined relationships with the ASD(HA), the DoD and army offices involved with providing funding for science and technology-related activities and program direction, and the commanding general of USAMRMC;
-
a placement in the DoD organizational hierarchy that would allow it to control decisions throughout the vaccine acquisition process and to coordinate decisions related to policies for vaccine use; and
-
a stable, adequate, and well-defined budget.
The committee does not have a specific recommendation about where within DoD the operational elements of a single vaccine authority should be placed. It did consider, however, the qualifications and characteristics that a single vaccine authority would possess and how it would work. The committee believes that placement of the vaccine authority at a high level in DoD—at the Pentagon, with the individual in charge of the authority reporting to the highest levels of DoD— is necessary to achieve the task. That organizational placement would not preclude USAMRMC’s holding the operational lead for vaccine-related activities.
A November 2001 statement from the IOM Council proposed the development of a somewhat similar authority, the National Vaccine Authority, to confront the problems that the public health sector faces in acquiring limited-use vaccines. The problems that the IOM Council sought to address have much in common with those that are part of the scope of this committee’s charge. The IOM Council’s statement argues that the creation of a single National Vaccine Authority would help to ensure the availability of vaccines that have limited commercial potentials but that are critically needed for the civilian sector.
Although the committee recommends the creation of a single vaccine acquisition authority within DoD, it recognizes that a vaccine is more than a product that can be built simply to predetermined specifications, purchased on bid from the manufacturing sector, and stockpiled for future use. A vaccine is part of a complex and continuously evolving biological system that is intended to protect
the warfighter against an infectious disease. As with any complex system, a vaccine requires constant, well-integrated, and coordinated attention to each facet of its development and maintenance, including disease surveillance, prioritization, research and development, and product refinement in a continuously changing regulatory environment. The committee cites DoD’s recent loss of the adenovirus type 4 and 7 vaccines as prima facie evidence of the need for DoD to adopt a systems approach to vaccine acquisition that spans all steps in the acquisition process.
Recommendation 1.
Combine all DoD vaccine acquisition responsibilities under a single authority within DoD that attends to the entire spectrum of responsibility—from definition of a potential threat against which a vaccine may be needed through research and development, advanced product development, clinical trials, licensure, manufacture, procurement, and continued maintenance of manufacturing practice standards and regulatory compliance.
Fragmented Acquisition Programs for Vaccines and Related Biological Countermeasures for Weaponized and Naturally Occurring Infectious Disease Threats
The health of warfighters is at risk both from natural infectious disease threats and from weaponized forms of infectious disease agents that might be intentionally deployed against U.S. forces in combat settings or against civilian populations as agents of terror. Whether natural or weaponized, these two forms of infectious disease threats share much in common. A number of specific pathogens such as those causing plague or hemorrhagic fevers are real and present threats in both contexts. Vaccines have been shown to be capable of providing protection against both natural and weaponized infectious disease threats, drawing in each case on what is a common science and technology base.
The maintenance of separate acquisition programs for threats to military operations from naturally occurring infectious diseases and threats from the intentional and hostile use of biological materials inhibit DoD’s ability to make rational decisions related to vaccine acquisition. This complex arrangement arose from DoD’s response to congressional direction to consolidate activities related to the acquisition of chemical and biological warfare defense measures. Thus, DoD created the Joint Vaccine Acquisition Program (JVAP) to manage the advanced development of vaccines to protect warfighters against weaponized infectious disease agents. Although well intended, the creation of JVAP has led to new problems. Separate management prevents unified thinking on the acquisition of vaccines such as those against the plague bacterium and the Rift Valley fever virus, each of which could be a natural and a weaponized threat to military
personnel. Limited expertise and equally limited budgetary resources are divided in the present scheme, in which DoD has split the responsibility and the authority for the procurement of vaccines against naturally occurring and potentially weaponized infectious disease threats and has established no unifying prioritizing mechanism with which it can manage its limited vaccine development resources. JVAP was intended to streamline acquisition procedures and raise visibility of the need for biodefense products, but these potential benefits have not yet been realized in the acquisition of new vaccine products.
The committee could identify no justification for the separation in the acquisition processes for vaccines against naturally occurring and potentially weaponized infectious disease threats. There is substantial overlap in the agents, technical approaches, and hurdles to be overcome in developing vaccines against the infectious agents that comprise both types of threats. The problem here is not simply that JVAP and USAMRMC’s infectious disease program are duplicative. That would be true if both sets of programs were functioning adequately. The reality is that the loss of previously available vaccines and the failure to produce new products indicate that neither program is operating effectively—in part because they are separate. The costs and risks are therefore even higher.
In its second recommendation, the committee seeks to fuse the positive characteristics of JVAP—providing a single point of contact and the authority to use a higher DoD acquisition category—and the medical research expertise and experience of the various components of USAMRMC.
Recommendation 2.
Consolidate the infrastructure, funding, and personnel for DoD programs for the acquisition of vaccines against weaponized biological agents and naturally occurring infectious diseases.
Lack of Sufficient Advisory Structure
The committee recognizes the need for and strongly recommends the creation of an ongoing, senior advisory structure to guide high-level decision making related to the acquisition of vaccines and other medical countermeasures against infectious disease threats. The proliferation of prestigious panels now looking at vaccine acquisition and availability is a potent indication of the lack of a center of strong advocacy and advice at present.
Previously, the Armed Forces Epidemiological Board (AFEB), which reports to the surgeons general of the various services, played a major positive role in military vaccine development. DoD now supports AFEB under the authority and budget of ASD(HA) and also calls upon AFEB for advice concerning a broad range of health care and environmental issues. The committee notes that its present scope is much broader than infectious diseases and that AFEB, as it is
constituted at present, has neither a sufficient breadth of expertise in infectious diseases nor enough understanding of the vaccine acquisition process (as outlined in the following paragraph) to fill the specialized advisory role that the committee envisions. With the proposed large increase in FY 2003 funding for biodefense, the need to provide effective advice to the government on how to spend the additional funds for military vaccine needs will, if anything, become more acute. The committee considered two routes that might bolster this function.
The first possible approach would be to reconfigure AFEB so that it includes additional individuals with specialized expertise in tropical and geographic medicine and persons with direct experience in vaccine acquisition, including vaccine research, development, manufacture, and procurement. Although this approach might be favored given the long and prestigious history of AFEB, the addition of these responsibilities might diminish the board’s effectiveness in meeting or carrying out its non-infectious disease-related responsibilities. Furthermore, the need for expert external advice concerning DoD’s vaccine acquisition activities may be too important to relegate to a subcommittee of AFEB.
Second, as indicated above, AFEB operates under the authority of and reports to ASD(HA). To adequately fulfill the advisory role envisioned for the single vaccine authority by the committee, its advisory body must report to the same level of DoD as the vaccine authority itself, that is, at the highest levels of the department. These factors thus argue in favor of the creation of a new advisory structure, one that the committee believes must be able to function effectively independently of DoD’s vaccine acquisition authority and with sufficient scope and authority of its own to ensure the protection of the group’s ability to provide unbiased advice and the perception that it is providing such advice. AFEB’s role within DoD, its multiple other responsibilities, and its organizational position within the department therefore pose significant challenges.
As a third alternative, DoD could seek an independent (non-DoD) expert body to create and maintain a standing advisory committee under contract.
Any of these options—a restructured and reenergized AFEB, a new advisory committee within DoD, or a newly created, ongoing, independent advisory group outside of DoD—would provide DoD with a group of senior advisers who could evaluate the priorities and operations of a consolidated DoD vaccine authority and who would have the potential to become strong proponents for the work that DoD does regarding vaccines against infectious diseases of military importance. A respected and well-connected champion could help articulate the needs so that the upper echelons of DoD could better understand them and, therefore, within their own fiscal and political constraints and opinions, act to support these important efforts.
Recommendation 3.
Ensure that there is an effective, ongoing senior advisory group—one providing perspectives from both within and outside of the DoD—to assess
program priorities and accomplishments, to act as a proponent for vaccines and other infectious disease countermeasures, and to maintain active relationships with current science and technology leaders in the academic, government, and corporate sectors.
FUNDS AND PROGRAM MANAGEMENT
Funding and program management streams are maintained separately in DoD. Although many of the same organizational units are involved in both processes, the processes themselves are largely distinct. Figure 4-1 illustrates the interactions among the different units of DoD involved in the establishment of requirements and the processes for infectious diseases-related research, development, and acquisition. The complexities of the DoD acquisition system for vaccines and related biological countermeasures against infectious diseases give rise to budgeting and programming difficulties.
These budgeting and programming difficulties are not newly recognized. In 1981 DoD created the Armed Services Biomedical Research Evaluation and Management Committee to “facilitate management coordination, improve information exchange, and accomplish medical research, development, testing and evaluation activities” (DoD, 1996, p. 1–6). In 2001, a panel of experts convened by the Deputy Secretary of Defense found that these problems still linger and should be addressed (DoD, 2001d). The IOM committee concurs.
Complicated Funding Process, Inadequate Funds
Budget decisions are made at many levels of DoD and the Department of the Army and are heavily influenced by the competing priorities of line commanders and various staff components of the armed services and DoD. Segmentation of the military research and development budget makes the process even more complex. The research requirements and budget decisions for the development of components of the technology base follow a pathway very different from that for advanced product development. The budgeting process is further complicated by a split between activities related to naturally occurring infectious diseases and those related to potentially weaponized biological agents that results in research redundancies and fragmented funding, as discussed above. Furthermore, once a vaccine product has been developed and licensed, its procurement and stockpiling for future use are supported by yet other sources of funds. For example, if a vaccine procurement problem that required additional research for its solution were identified but research funding was no longer available, efforts to acquire the vaccine or maintain its availability might languish.
Procurement and maintenance funds, which are provided to the Defense Health Program for ongoing support of health care operations within the military,
are not available to support changes in vaccine manufacturing processes or facilities that the supplier may request in response to new regulatory requirements imposed by FDA after licensure of a vaccine. At the same time, funds designated for the technology base and advanced product development may not be deemed suitable for making improvements to a licensed product. This schism in the funding stream is matched by a similar schism in the recognition of responsibility for maintaining effective and acceptable manufacturing processes and facilities by various components of DoD after the licensure of a vaccine. In the case of the adenovirus type 4 and 7 vaccines, for example, this disjointed budgetary process for vaccine acquisition appears to have led directly to the loss of these vaccines by the military. The current process fails to recognize that a vaccine represents a complex biological defense system, not a static product.
It is also noteworthy that the current system produces a budget that is inadequate to effectively support the full spectrum of vaccine acquisition activities that are needed. Although the committee was unable to obtain specific information concerning budgets before 1993, in part because of the difficulties of comparing shifting organizational components over time, it has the strong impression that substantial declines have occurred in terms of the real funding available to support vaccine acquisition activities over the last three decades. The declining budget has resulted in a reduction in the breadth of infectious disease-related research in USAMRMC, which affects both basic and applied research, as well as the product development activities supported by USAMRMC contracts. The number of infectious disease agents that are now actively and credibly studied within DoD laboratories has been reduced over time, as USAMRMC has repeatedly restructured its research programs in an effort to retain adequate funding for what it has considered a core set of priorities. Expertise related to rickettsial and parasitic diseases, for example, has been eroded, and the robust basic and applied infectious disease research programs that spearheaded the development of meningococcal, adenovirus, and hepatitis A vaccines in the 1970s and 1980s have not been replaced by similar, cutting-edge, industry-attracting research and development activities in the 1990s and beyond.
A tangible example of the effect of budget reductions is that USAMRMC is no longer capable of effectively meeting FDA’s requirements for maintaining the ongoing investigational new drug (IND) status of a number of encephalitis and hemorrhagic fever virus vaccines, such as the attenuated vaccine developed for protection against Junin virus infections. The very real impact of this lapse in IND status is that DoD will not be able to offer protection even to those research laboratory personnel working with these dangerous agents through the Special Immunizations Program (SIP) managed by U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), let alone offer protection to troops who could be exposed to these threats in the field. Budget limitations and vaccine availability concerns force USAMRIID to maintain a cap on the number of individuals with access to vaccines administered by SIP (Boudreau and Kortepeter,
2002). The current enrollment cap has effectively made these vaccines unavailable to nonmilitary, academic researchers. Table 3-5 lists eight vaccines that were previously developed and championed by DoD but that are no longer being produced and that, as a result, are available in very limited quantities at present. This list highlights just one facet of the long-term consequences of what the committee senses has been a contraction in the breadth of DoD’s vaccine development programs.
Table 4-1 shows the somewhat erratic nature of the funding that has supported the DoD infectious disease science and technology base since 1993. From FY 1993 to FY 2000 there were no sustained increases and the budget clearly failed to keep pace with inflation. A substantial increase in funding in FY 2001 was matched by a decline in funding in FY 2002, demonstrating a lack of the reliable levels of support required to sustain stable, long-term research and development projects such as those required for vaccine acquisition. The record indicates a stagnant investment in funding for vaccines, one that has actually decreased in terms of inflation-adjusted dollars, despite real increases in development costs and regulatory burdens.
The commanding general of USAMRMC spoke to the IOM committee in April 2000 about these budgetary reductions and described a $320 million shortfall in unfunded requirements within USAMRMC over the next 5 years. This included $30 million of army “must-be-funded” items.
As a result of budget constraints that DoD has placed on the science and technology base, it has become increasingly difficult to support the broad technical base needed for the diagnosis, treatment, and prevention of infections that are uncommon among U.S. residents but prevalent elsewhere in the world and that therefore present potential threats to military personnel deployed outside the United States. Erosion of the technology base and the professional expertise available for vaccine development within the armed forces have led to a greater dependence by DoD on the commercial sector to accomplish its vaccine-related aims. This is evidenced by the relatively small number of vaccines being developed by DoD (Table 3-6) and the prominent roles that commercial vaccine manufacturers play in the development of many of these vaccines (e.g., the primary role of GlaxoSmithKline in the development of the hepatitis E vaccine).
The record shows that DoD has no long-term, stable budget to attain and sustain what it needs in terms of vaccine development and production capacity. In addition, discussions between the committee and military decision makers and leaders in the vaccine manufacturing industry make it clear that the uncertain nature of the appropriation process of the federal government makes it difficult to maintain continuous scientific and financial commitment from either within or outside of DoD. As a result, vaccines whose development is technically possible and within the country’s grasp scientifically, such as the adenovirus vaccines, or vaccines for which administrative hurdles overshadowed technological obstacles, such as the anthrax vaccine (Zoon, 2000) and a vaccine against plague (FDA,
TABLE 4-1 History of Funding for Science and Technology Base Through the USAMRMC Research Area Directorate for Infectious Diseases, FYs 1994 to 2002 (thousands of dollars)
|
|
2002 |
2001 |
2000 |
1999 |
1998 |
1997 |
1996 |
1995 |
1994 |
|
Total for infectious diseases |
52,507 |
55,823 |
41,054 |
39,282 |
51,525 |
45,182 |
42,717 |
42,040 |
41,715 |
|
Total for human immunodeficiency virus |
16,421 |
26,838 |
29,638 |
19,684 |
38,367 |
20,485 |
24,992 |
32,028 |
36,778 |
|
Vaccines |
|
||||||||
|
Diarrheal |
6,315 |
8,017 |
1,382 |
1,262 |
1,342 |
1,608 |
1,536 |
1,354 |
1,547 |
|
Malaria |
7,318 |
8,688 |
5,774 |
4,856 |
4,938 |
5,360 |
5,343 |
5,201 |
4,875 |
|
Meningitis |
644 |
903 |
554 |
544 |
493 |
470 |
375 |
451 |
400 |
|
Hepatitis |
0 |
723 |
906 |
569 |
474 |
910 |
904 |
975 |
1,122 |
|
Dengue |
3,754 |
3,791 |
2,886 |
2,316 |
2,349 |
2,404 |
3,204 |
2,350 |
2,284 |
|
Hantavirus |
849 |
1,144 |
736 |
761 |
1,407 |
1,398 |
1,480 |
1,510 |
1,315 |
|
Total for vaccines |
18,880 |
23,266 |
12,238 |
10,308 |
11,003 |
12,150 |
12,842 |
11,841 |
11,543 |
|
SOURCE: Provided by Hoke (2002). |
|||||||||
1997c; Greer Laboratories, 2001), have not been available to the military as they are needed.
Budget constraints also limit the ability of USAMRMC to successfully move potential vaccine candidates forward into Phase I and II clinical trials. Budget problems often become more severe at the end of development, when industrial development costs for vaccines generally escalate because of the scale-up of the manufacturing process and the need for clinical trials. Yet, the USAMRMC budget has a severely limited advanced development component. As noted in Chapter 2, advanced development funding for vaccines (excluding the human immunodeficiency virus [HIV] vaccine program) was approximately $5.9 million total in FY 2002 (Hoke, 2002), representing only a very small fraction of the resources that the U.S. military needs to acquire licensed vaccines against a large number of potential infectious disease threats. As a point of reference, the committee notes that the current budget provided for development and acquisition of new smallpox and anthrax vaccines within the Department of Health and Human Services (DHHS), generated in response to the nation’s call for greater civilian biodefense activities, totals several $100 million.
As indicated above, in arriving at a budget for the procurement of established vaccines, DoD does not include funds for the resources needed to improve or maintain a vaccine—or, for that matter, the funds needed to revamp a vaccine production system to meet current manufacturing practice standards, which change over time. DoD provides no funds to support changes to the production system needed to respond to new regulatory specifications from FDA. The expense of modernizing the production facilities has not previously been accounted for in the procurement process. The loss of the manufacturing facilities for the adenovirus type 4 and 7 vaccines serves as a specific example of this problem and has led to significant outbreaks of adenovirus disease at training installations and one or more deaths among military recruits.
Obtaining resources sufficient for the purchase of a vaccine—even one that has been developed through the DoD—requires independent funds and decision making from parts of DoD (e.g., through the Defense Health Program) that are not tightly linked to DoD’s upstream research and development activities. This provides further evidence of the fragmentation of priority setting and management of the vaccine acquisition process discussed above. To develop a budget, DoD must consider the costs of the entire acquisition process, including costs for the sustained manufacture of a vaccine. To do that, the decision maker must understand the process, where the money is going, and what the expenditure is achieving.
At present, the budget available for the acquisition of vaccines is insufficient for the task. Although the committee recognizes the extreme competition for resources that exists among the many important programs within DoD, it believes that DoD, like the civilian sector, has not invested sufficiently in the acquisition of new vaccines. Explanations may rest, in part, on the great successes achieved
in controlling such militarily important diseases as tetanus, meningococcal meningitis, and hepatitis A and hepatitis B and in the almost minimal numbers of casualties from infectious diseases in recent conflicts. This may have led to a sense of complacency concerning the risks posed by naturally occurring infectious diseases. Any complacency about infectious disease threats disappeared, however, in the wake of the anthrax attacks against civilian targets in the fall of 2001.
As the committee drafted this report, the President highlighted the need for a large increase in funding for biodefense-related research and product acquisition in his proposed FY 2003 budget. The committee notes that the growth in funding for the research activities of the National Institutes of Health (NIH) anticipated by the President’s proposed budget will likely lead to the discovery of novel immunization strategies and better ways to positively manipulate the human immune system. These advances—coupled with enhancements in relevant areas of the nation’s research infrastructure—are likely to provide significant spin-offs for DoD as it attempts to address militarily important naturally occurring infectious diseases.
However, in this atmosphere of increased resources fueled by a heightened awareness of the public’s vulnerability to bioterrorist actions, the committee cautions that the United States must sustain its investment in vaccine development activities over many years if it is to successfully develop useful vaccines. There are concerns that the infusion of new funds may be short-lived and thus may fail to meet long-term needs for investment in the critical infrastructure required for vaccine acquisition. The current budget is not adequate to support DoD’s acquisition of even a few of the many vaccines needed to protect U.S. forces.
Recommendation 4.
Provide budget resources commensurate with the task.
Fragmented Prioritization and Program Management System
The fragmentation apparent in the budgeting process is also evident in DoD’s management system for determining infectious disease-related research priorities. The programming process, for example, suffers because it falters at an important first step: the setting of priorities. The specific infectious disease threats to the armed forces include a broad spectrum of microbial and parasitic organisms. As the global demography and the global ecology change and new infectious diseases emerge, the civilian population of the United States and the U.S. military will continue to need a broad-based research program that is capable of coping with these changes. Setting priorities is an important part of the process of creating program goals.
Resources are not sufficient to develop effective vaccines or biological and medical countermeasures to protect warfighters against all potential infectious disease threats. Given this reality, the need for an effective prioritization mechanism is paramount. At present, USAMRMC does not use a defined process to prioritize the research goals on which it is expending its limited resources. The fact that resources are inadequate to meet all requirements only strengthens the need for a well-defined and validated process that ensures appropriate input from intelligence sources and formal periodic review of priorities in light of the changing international and political landscapes and scientific advances and failures.
The manner in which USAMRMC ranks disease threats, research goals, and specific research projects remains unclear to the IOM committee, despite the many hours that it spent in deliberation and hearing briefings from more than a dozen people. As evidence for the failure of the present system, one could cite the absence of a list comparable to the Category A list1 of the Centers for Disease Control and Prevention (CDC) to guide the activities of USAMRMC. The task of generating a priority list of infectious disease threats to warfighters rests with the Army Medical Department Center and School. However, the committee found that no such list is available to the Medical Infectious Diseases Research Program. The committee acknowledges that it cannot be certain that having a weighted, prioritized list of disease threats would alter research budget allocation decisions in the short term or the health of troops in the long term. Nevertheless, the committee strongly recommends the development and use of a well-defined and validated priority-setting mechanism. Such a mechanism could be developed by using as tools, for example, weighted, prioritized lists to reduce the chance for misunderstanding.
The U.S. government has sought external guidance in prioritization methodology in the past. The Institute of Medicine itself has issued numerous reports. In the mid-1980s, at the request of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the IOM released a two-volume report—covering domestic and international needs—that presented a quantitative methodology for choosing which vaccines to place on accelerated development paths (IOM, 1985, 1986). Estimates of expected health benefits (based on morbidity and mortality) and expected costs (including costs averted by vaccination and the costs of a vaccination program) were compared for a set of candidate vaccines. The authoring committee noted the method’s value as a decision tool
rather than a decision maker. The report illustrated how altering the assumptions and viewpoints quantified in the model would alter the priority rankings.
More than a decade later—and around the time IOM formed the committee issuing this report—the IOM report Vaccines for the 21st Century: A Tool for Decisionmaking described a different model for guiding vaccine research direction (IOM, 2000b). The model assigned candidate vaccines to one of four levels based on cost (of research and development, vaccine use, health care, vaccine efficacy and utilization, among others) and quality-adjusted life years (based on, for example, severity of illness and time spent with illness). The report, consistent with its predecessors, emphasized that the cost-effectiveness model “can provide an estimate of the cost of achieving the anticipated health benefit for each of the vaccines studied, but it cannot determine whether that health benefit is worth the cost” (IOM, 2000b, p. 57). The value of the cost-effectiveness model relates to its ability to summarize and compare different kinds of costs and benefits; to clarify assumptions; and to test, using multiple sensitivity analyses, the effect of those and alternative assumptions on the result. Although the report used quality-adjusted life years as its measure of benefit, the analytic technique could be adapted for DoD by examining outcomes such as days unavailable for military duty or other measures of unit combat effectiveness.
A CDC expert panel, in February 2002, published a matrix of “reviewable, reproducible means for standardized evaluations” of civilian effects from potential biological threat agents. The report included a Category A list of select agents that were generated by this methodology (Rotz et al., 2002). The model assigned points based on specific characteristics of a potential agent, such as whether hospitalization would be required for an infected person; what mortality rates would be expected for untreated persons with the infection; whether there would be potential for person-to-person transmission and continued dissemination of the infection (based on various assumptions regarding the route of infection); and the degree of potential public fear or panic as predicted by measures of media-registered public awareness.
A somewhat different approach to priority setting is offered by scenario-planning exercises. Scenario planning promotes the construction of different sets of priorities depending on various possible scenarios that are envisioned for the future. It includes the use of milestones to indicate potential changes in or revalidation of present priorities as advancing time and changing circumstances dictate the greater or lesser likelihood of one future scenario over another. A formal process for scenario planning would be useful in prioritizing threats based on estimated risk exposures and anticipated outcomes in the event of infection and would provide an effective interface between intelligence agencies and the DoD decision makers who manage the vaccine acquisition process. Scenarios are cited by private industry advisors as more than predictive and decision-making tools, providing participants “within the organization . . . a common vocabulary and an effective basis for communicating complex—sometimes paradoxical—conditions
and options” (GBN, 2002). Formal scenario-planning exercises compel a group of individuals “to question their broadest assumptions about the way the world works so they can foresee decisions that might be missed or denied” (GBN, 2002). Such planning can provide “a specific point at which the required value judgments are described and incorporated . . . [as] one means of isolating these differences of opinion (which are often incorporated into decision making in an ill-defined way) and determining if they affect the ultimate priorities” (IOM, 1986, p. 2). The end result of scenario planning would be a prioritized list or database of disease threats weighted by potential importance to military operations and subject to periodic review and modification as the geopolitical landscape evolves over time.
An additional level of prioritization might involve determination of scientific opportunities and constraints. DoD should take the weighted, prioritized lists generated by scenario-planning exercises and match these with the scientific opportunities for vaccine development, as well as the anticipated costs and resources required to get a particular product on the shelf. DoD is one of many players in vaccine research and development. Given the magnitude of related research activities in the civilian sector, DoD should use the prioritization process to help refine its research agenda so that it uses its finite intellectual and other resources to its best advantage. For example, DoD might decide that investment in development of a particular vaccine, although strongly indicated by scenario planning because of an anticipated need to protect troops in deployments, would be redundant given ongoing investments in the same research area by industry, NIH, or private foundations. Although the parallel pursuit of different strategies for developing an effective vaccine against a single pathogen by different federal agencies could be justifiable, DoD should examine its entire portfolio of requirements against the spectrum of research conducted outside of DoD when determining where to invest its precious research and development dollars. The committee is not persuaded that DoD has engaged sufficiently in such considerations, which would optimize the management of research and development resources in ways expected to maximize the returns (over both the short term and the long term) on investments.
Whether DoD generates a weighted, prioritized list of disease threats, a weighted list of research priorities, or a formal scenario-planning exercise, the process used in its planning efforts should involve experts from academia, DoD laboratory commanders, DoD preventive medicine officers, and the intelligence community. Furthermore, a prioritization scheme should consider not only vaccines but also other medical countermeasures, including prophylactic drugs. Whatever procedures are followed, the committee recommends that DoD consolidate its prioritization efforts within the framework of the total acquisition process defined above. The product of the prioritizing exercise should be reviewed by the reorganized AFEB or whatever ongoing group of senior advisers is convened in response to Recommendation 3, given above. In addition, the priority-
setting process should be iterative and should be performed at least annually within the context of a single DoD vaccine authority. Each year, the output of this prioritization effort would inform decisions on the allocation of the budget among the proposed vaccine research and acquisition items.
Recommendation 5.
Actively encourage the development, distribution, and use of a well-defined and validated research priority-setting mechanism. Such a mechanism could involve the use of prioritized, weighted lists of infectious disease threats and formal scenario-planning exercises and would require the use and synthesis of infectious diseases surveillance and epidemiologic information.
A Declining DoD Technology Base Limits Vaccine Acquisition
Budget competition within DoD pits efforts to build and maintain the military’s technology base against projects focused on specific products. Although such a process may produce a list of credible products, it runs the risk of eliminating research in areas low on the list, resulting in a continuing narrowing of the research abilities and scientific horizons of the laboratories. Predicting future infectious disease threats to members of the armed forces is an imperfect science, with emerging disease threats and unpredictable global politics adding to the uncertainty. DoD must have ready access to the pool of knowledge and skills needed to maintain the basic scientific research that is essential for the U.S. military to launch nimble and effective responses to shifting infectious disease threats. The committee thus believes strongly that the maintenance of a broad technology base and an infrastructure for research related to the epidemiology of infectious diseases (e.g., DoD overseas medical research laboratories) is an absolutely necessary adjunct to research and development directed at specific vaccine products.
Although the breadth of the technology base is tied to the magnitude of funding made available for infectious disease-related science and technology, as discussed in Chapter 2, the committee emphasizes that it is also dependent on program priorities. Past successes with effective hepatitis A, Japanese encephalitis, and adenovirus vaccines were built on what appears to have been a more substantial infectious disease technology base within the military than exists now. With the stronger base, DoD did not have to envision these specific successful end products at the inception of the related research programs.
The impact of the elimination of the military draft on the infectious disease technology base over the past several decades may be easy to overlook. Although the committee was unable to obtain specific data supporting the contention that the elimination of the military draft has had an impact on the infectious disease technology base, it holds a strong impression that the shift to all-volunteer mili-
tary forces in the early 1970s led to a significant reduction in the numbers of young investigators with medical and doctoral degrees entering into and passing through DoD’s infectious diseases research laboratories. Historically, those individuals who remained in service formed the core of DoD’s professional technology base. In recent years, as many of these individuals have qualified for retirement and have left the service, the scientific cadre within DoD does not appear to have been replenished in a manner that would preserve earlier capabilities. DoD could consider the implementation of loan forgiveness programs to attract highly trained researchers. An example of such a program is NIH’s Loan Repayment Program for Clinical Researchers, which repays the education loans of individuals who agree to engage in clinical research at NIH for a minimum of 2 years (AAMC, 2002). A potential unplumbed pool of future infectious diseases vaccine researchers is graduates of the Uniformed Services University of the Health Sciences (USUHS). DoD might consider adding research incentives to its established recruitment programs for clinicians, in addition to creating an M.D./Ph.D. dual degree curriculum at USUHS to create a cadre of military physician scientists with interests in infectious disease control.
An effective system must be dynamic and able to respond to new threats, to maximize the potential of biotechnology, and to use individuals with a diversity of skills and from a diversity of disciplines at all steps in the vaccine acquisition process. Vaccines are complex biological systems. Therefore, an effective process for the acquisition of vaccines must be multidisciplinary in nature, resting on a broadly constituted, diverse technology base extending from disease surveillance and risk assessment technologies to the intricacies of molecular and structural biology, vaccine design and manufacture, and, ultimately, the clinical trials and regulatory science that underlie the licensure and deployment of the final product.
Recommendation 6.
Include programming goals that ensure greater strength and continuity in the science and technology base for the full spectrum of infectious disease threats, including research related to the epidemiology of infectious diseases, the nature of protective immunity, and both early and advanced vaccine product development.
Lack of Integration with Other Public-Sector and with Private-Sector Vaccine Development Efforts
As it is structured at present, the DoD vaccine acquisition program is not well integrated with vaccine-related programs maintained by other public-sector agencies. In fact, the U.S. government has charged no less than five federal agencies in three separate departments with various aspects of the response to infectious diseases. In addition to DoD, DHHS (through CDC, FDA, and NIH),
the Department of Veterans Affairs, and, to a lesser extent, other federal agencies carry out activities related to prevention, treatment, and research regarding the control of infectious diseases. Other than broad areas of responsibility, there is no clear articulation of the roles played by each agency in the development of specific vaccines. In addition, with a few exceptions, there is no effective mechanism for the systematic coordination of all such activities across agencies. These exceptions include the Federal Malaria Vaccine Coordinating Committee and the ad hoc creation of formal contracts such as those that exist between DoD investigators and investigators at FDA’s Center for Biologics Evaluation and Research on the development of flavivirus vaccines.2 To the present IOM committee, the lack of an effective mechanism for the systematic coordination of activities results in an uncoordinated response in the development of specific vaccines.
Although the National Vaccine Program Office and the National Vaccine Advisory Committee have statutory responsibilities for the coordination of vaccine acquisition-related activities among federal agencies and despite the extensive work of their members in smoothing the flow of information, they have not assumed the authoritative stature necessary to act effectively. The IOM committee believes that the ineffectiveness is guaranteed by the absence of budgetary authority. This observation strongly informs the committee’s own recommendations that the proposed single vaccine authority in DoD have controlling authority for vaccine acquisition, including budgetary authority and adequate funding resources. Such authority is needed to carry a vaccine through the process from an idea to a product that is licensed and continually available.
The parallel efforts under way to create a larger smallpox vaccine stockpile provide a perfect example of how coordination could be improved. A Phase I clinical trial of a cell culture-derived vaccine, developed through the DoD’s JVAP, began in April 2002 (Gay, 2002). Meanwhile, DHHS, through CDC, has a contract with a different manufacturer to make a cell culture vaccine to supplement the current stockpile of the previously manufactured smallpox vaccine. The two projects use similar development techniques and are creating essentially the same vaccine. Although DoD is “in continued discussion with the Department of Health and Human Services about collapsing the two individual programs into one nationwide program” (Johnson-Winegar, 2001), the two programs remain distinct. Having multiple manufacturers does provide security for the civilian market (such as for the diphtheria–tetanus product). For special-use vaccines, however, when there are needed vaccines not being developed because of lack of funding, the nation cannot afford that kind of redundancy.
Similar circumstances will occur again and again as it becomes increasingly evident that DoD interests overlap and intersect with civilian interests in estab-
lishing effective biodefense measures. For example, the infectious disease agents on the CDC Category A list of select agents (CDC, 2000a; Rotz et al., 2002), which NIH is using to guide it in setting research priorities (NIAID, 2002b) as it prepares to receive the $1.8 billion that the President’s proposed budget directs for defenses against bioterrorism, overlap extensively with the agents covered by DoD’s research programs on biodefense and naturally occurring infectious diseases. In addition, as noted in the Preface to this report the nation’s perception of the risks that infectious agents pose as instruments of terror is evolving rapidly, and the committee has written its recommendations against the backdrop of these evolving changes. These exigencies have also figured prominently in the recent decision by the IOM Council to publish a statement regarding the creation of a National Vaccine Authority, as discussed above.
DoD stands to benefit greatly from the infusion of funds to support civilian biodefense activities. DHHS has recognized the need for DoD to play an important role in creating for civilian populations effective vaccine defenses against agents on the Category A list. Because of the more diverse nature of the civilian population in terms of age and underlying health status (NIAID, 2002b), it will be significantly more complex and difficult to meet safety and efficacy requirements for vaccines to protect the civilian population than for vaccines that protect warfighters.
Having presented its reasons for giving a single authority within DoD responsibility for efforts related to the acquisition of vaccines against potential weaponized and naturally occurring infectious disease agents, the committee is aware that separation of the DoD and NIH vaccine acquisition and development programs may also appear arbitrary. A major issue to be addressed as NIH invests its financial resources is the limited numbers of informed infectious disease investigators and vaccinologists who are available to respond to the call for the development of new vaccines. To spread this finite human capital over two or three entities of the federal government could diminish the possibility of success of each. Thus, if the creation of a National Vaccine Authority, as urged by the IOM Council, becomes a reality, the committee encourages those involved to devise a means whereby DoD may contribute to and benefit from that authority’s responsibility, management, and budget, while preserving a level of operational independence deemed critical by the committee for DoD to meet its unique requirements.
The committee noted above that there is strong overlap in the infectious disease agents of interest to DoD, either as natural threats or in weaponized formulations, and those of national security concern as potential weapons of terror. Agents on both lists include those responsible for smallpox, anthrax, plague, tularemia, and the viral hemorrhagic fevers. In addition, the science and technologies underlying the development and production of vaccines to protect civilian populations are identical to those underlying the development and production of vaccines to protect military forces. Also, as mentioned above, on a
national scale, only limited numbers of infectious disease researchers, virologists, and microbiologists have the experience and technical competence required to work with these agents and others on the Category A list of select agents.
There is a clear need for the close coordination of the vaccine development and production efforts of the civilian sector and those of DoD. All of these efforts are ultimately supported by the same budget and have similar and overlapping, although distinct, goals. DoD interaction with public–private partnerships, such as the International AIDS Vaccine Initiative and the Malaria Vaccine Initiative of the Program for Appropriate Technology in Health, could be better coordinated to everyone’s benefit. The challenge will be to develop a mechanism by which DoD maintains an effective voice for its unique needs within the structure of a unified national effort.
Recommendation 7.
Leverage DoD research efforts by building greater interactions and an effective formalized coordinating structure that links DoD research activities to vaccine development activities carried out by DHHS and other public and private groups.
MANUFACTURING
Vaccines succeed only when they are administered to people at risk. No matter how successful the research or skillful the efforts at vaccine development, unless manufacturers produce the vaccines, they cannot prevent disease. Two critical factors in industry’s decision to manufacture a vaccine are whether the price that it can charge will outweigh the cost of production and whether the required commitment of corporate resources will mean the loss of significant opportunities for the production of other, potentially more profitable products. Unfortunately, vaccine manufacturing costs are high. DoD has not always succeeded in carefully setting priorities (and, therefore, committing its funds prudently) to ensure that critical vaccines are available when and where they are needed. There are several contributory reasons, as outlined in the following sections.
Government–Industry Relationships and the Economics of Vaccines
One of the reasons underlying past failures in DoD’s vaccine acquisition efforts is that DoD lacks an ongoing and coordinated relationship with the small number of remaining large vaccine manufacturers3 that collectively possess decades of experience in vaccine development, delivery, and other logistics
crucial to bringing a vaccine to market quickly and cost efficiently. Meanwhile, the U.S. military has no independent, large-scale manufacturing capability. DoD thus needs what these companies can offer. The fact that some important vaccines that have been developed in the past and for which the science and technology base is well understood—such as the adenovirus and anthrax vaccines—have not been available to the military because the manufacturers ceased production or FDA halted distribution makes clear that this is not a theoretical problem but a real one. DoD needs to create stable incentives and contractual obligations for manufacturers to remain motivated and capable of producing vaccines over the long term. The recent shortages of even those vaccines that are used routinely in the civilian population emphasizes the fragility of the vaccine supply in the United States. Some would consider it a national crisis.4
Although the life span of a vaccine may be very long, regulatory science is an evolving field, and changes in manufacturing processes and facilities may be mandated by additional requirements that FDA imposes as it seeks to incorporate new discoveries into its regulatory efforts or to take steps to enhance the safety and efficacy of the products that it regulates. Thus, the costs of product development do not necessarily end with the licensure of a vaccine but potentially continue as long at the vaccine is in use or stored for potential use.
DoD could forge more successful relationships with manufacturers if it better understood the need for long-term commitments as well as the basis on which the vaccine industry works. The vaccine industry is highly regulated by FDA and is dominated by a few large corporations. Those corporations use limited resources to take very large risks when they set out to develop and market a new vaccine. Industry and consulting reports estimate the cost of developing a new vaccine, including facility construction, at $300 to $500 million (DoD, 2001d; IOM, 1993). The costs could be somewhat less if use of a special-use vaccine is to be restricted to military populations, if a single facility could be used to manufacture multiple products, and if smaller Phase II and Phase III safety and efficacy trials were deemed reasonable by FDA given the anticipated scope of use of the vaccine. However, because none of these points can be ensured, the costs of developing a special-use vaccine are still very high. Such expenditures make sense to vaccine manufacturers only when the prospects that they will receive a return on the investment are good—a direct benefit to the shareholder that can justify the investment made by the boards of these publicly held corporations.
One former industry executive estimated that a finished product bringing in less than $100 million a year would not be considered worth the investment required to produce it because its development would tie up the limited technical resources and the expertise of the personnel available to the company for vaccine
or drug discovery. Such opportunity costs are not acceptable to a vaccine manufacturer who is operating in a competitive external market. Even though the investment may ultimately be profitable, a decision within the company to commit the resources to develop such a vaccine will compete with other opportunities to invest those resources, including opportunities offering potentially much greater returns to shareholders. The industry also shies away from short-term, small projects because they are unstable and disrupt larger operations.
Vaccine development is as complicated for special-use vaccines as it is for those with wider commercial potentials, and thus, with the possible exceptions described above, special-use vaccines are potentially as expensive. An acceptable level of safety must be demonstrated to FDA’s satisfaction before licensure. However, the potential financial benefit to the manufacturer from the development of a special-use vaccine—if calculated based on market forces alone—is much less than the potential financial benefit expected from the development of a vaccine with large market potential. In support of these arguments, committee members recalled the absence of a response from even a single major vaccine manufacturer when DoD recently urged the development of a plague vaccine.
The government may pursue several available routes if it chooses to ensure the availability of the special-use vaccines that DoD needs (1) to strengthen and expand its partnerships with individual manufacturers to produce vaccines, (2) to encourage the development of a consortium of major vaccine manufacturers to address this need, and (3) to build its own manufacturing capacity. These approaches are not mutually exclusive, and each is costly and potentially difficult, particularly for products that may have limited markets, if any, within the U.S. civilian population.
Partnerships
Research partnerships between the U.S. military and industrial organizations have resulted in impressive successes—for example, the hepatitis A and Japanese encephalitis vaccines—and experts see promise in other current vaccine efforts supported by industrial partners. The great expansion of Cooperative Research and Development Agreements (CRADAs) signals that more DoD research laboratories are depending on industry partners for support.
The CRADA process successfully creates partnerships with industry, but only for specific projects and only for those that the industrial partner deems to be in its best interest overall. CRADAs may be attractive to industry when they are tied to the development of a vaccine with large commercial potential, such as the hepatitis A vaccine; but they are much less attractive in situations in which the civilian market is limited or absent, as in the case of the plague vaccine. In addition, CRADAs cannot support long-term commitments and, thus, they provide an approach that yields no more than piecemeal results. That dynamic can— and, indeed, does—greatly influence the structure of the vaccine program. How-
ever, CRADAs do not fundamentally alter the concerns raised above about the need for the chief executive officer of a large vaccine manufacturer to invest the company’s research and development resources in ways that maximize financial returns to the company and the shareholder. In addition, since DoD does not allocate enough advanced development funding to develop all the products that it needs, there is a substantial risk that acquisition efforts will falter in areas where no CRADAs exist.
DoD faces an industrywide lack of interest in the vaccines that it so urgently needs to protect its forces against many infectious disease threats. The committee asked current and past vaccine industry executives to describe the factors that lead manufacturers to avoid the development of vaccines with a limited target population. The views expressed to the committee are summarized here.
-
Lower profits. The costs of a vaccine targeted to a limited population are similar to those of a universally recommended vaccine, but the revenues are lower; thus, the profit margin is much lower.
-
Higher risk. Even the low potential profit is at risk because the government does not guarantee purchase of the vaccine supply that it has requested.
-
Limited resources. All projects (including projects focused on the discovery of potentially profitable new drugs for civilian markets) are in competition with one another for corporate resources. Even a large company does not have sufficient funds or personnel to pursue all products in which it may be interested, and special-use vaccines fare badly when choices are made among competing projects. The diversion of uniquely qualified people to a short-lived project hurts potentially more profitable projects by depriving the latter projects of their expertise and thus represents an unacceptable opportunity cost to corporate entities.
-
Safety. Some biological agents that must be cultivated in the process of producing vaccines for the military require expensive biological containment laboratories and present potential risks for workers, despite the use of such facilities as a precaution.
-
Regulatory and statutory requirements. Regulatory requirements have become substantially more complex and demanding in recent years. The recent deaths of two individuals during university-sponsored clinical research activities (which were not related to the evaluation of vaccines) have heightened the public’s awareness of the risks of such research and have resulted in a substantially intensified degree of oversight and more complex documentation and paperwork requirements for investigators engaging in research with human subjects. These requirements have contributed to the increase in the cost of producing vaccines by current good manufacturing practices and the escalation of expenses involved in shepherding new vaccines through to licensure. These changes in the regulatory burden include tendencies on the part of FDA to ask for larger population sizes in studies examining the risks of adverse events associated with the use of
-
vaccines, factors that run the risk of driving up substantially the costs of the Phase II and III clinical studies required for licensure. In addition, research involving agents on the Category A list of select agents now involves increasingly complex regulatory and legal oversight because of the USA Patriot Act of 2001,5 which imposes significant responsibilities on the employer with respect to those employees that it uses in such research, including the exclusion of persons from certain countries, those who have sustained legal difficulties, or those who have received other than honorable discharges from military service. There is the risk that additional rules now under consideration may substantially enhance the burdens associated with research on these agents and thus drive both industrial and academic research laboratories toward less regulated areas of research and vaccine development.
-
Indemnification issues. Even when all available precautions are taken, there is still an intrinsic risk that a company may be subjected to litigation as a result of an unexpected adverse reaction to a vaccine. Except for the vaccines covered by the National Vaccine Injury Compensation Program, no federal compensation is available. Without a large revenue stream as insurance, product liability becomes a potentially unacceptable risk. One former industry executive observed that GlaxoSmithKline may not have stopped production of the Lyme disease vaccine, despite substantial liability issues, if the market had been better (Associated Press, 2002).
Liability is further complicated for work with military populations, sometimes characterized as a captive pool. Even when working under strict rules and complying with all FDA requirements, industry would want the federal government to indemnify or provide product liability insurance. Industry cites the compensation claims that followed the mass use of the swine flu vaccine in 1976 as an indication that indemnification is necessary. Insiders cite as a more recent example the government’s refusal to indemnify Wyeth for the risk of claims related to the anthrax vaccine as a critical factor in the company’s decision not to produce the vaccine during the Gulf War.
In summary, the normal conduct of business for industry involves tendering and cost bidding. However, the process does not operate in this fashion when industry is called on to work with the U.S. military. The DoD makes no continuing, long-term funding commitment to industry, it pays no attention to investments for infrastructure or the need for an acceptable profit margin to support the infrastructure, and makes no commitment to purchase a stable or predictable volume of vaccine over time. Single-phase contracts are unsatisfactory for the planning of construction or for the allocation of scarce human resources by industry, especially when the next phase might go to another entity.
Industry representatives also voiced expectations that military managers may attempt to influence management decisions that they believe are more properly made by industry and that the budgets proffered by the military will often be insufficient to meet the task requested of industry. The overall impression held by industry, which the committee cannot refute, is that DoD has never been willing to commit sufficient resources to justify the investment that a company would need to make with its own limited resources to produce the products requested. DoD has not been able to adequately fund the infrastructure needed, maintain facilities, or ensure adequate volumes of future purchases. It fails to appreciate the needs for the large industrial vaccine manufacturer to have a stable market for its products and to collect a sufficient margin on its sales to ensure its future growth and survival within a very competitive international marketplace.
The perspectives described above make clear why established, large manufacturers have little interest in developing vaccines that would be used only by the military to protect its forces against infectious diseases and for which a profitable commercial market would not be found in the civilian sector. Meanwhile, although small newcomers to the vaccine industry may be willing to bid on projects of limited scope, such untested partners cannot reliably provide the vaccines that the military requires. This was demonstrated in the recent failure of a small company to provide the plague vaccine.
The committee believes that DoD could better attract the interest of industry by working to mitigate the concerns of industry outlined above. An initial step would be to create a centralized, empowered authority within DoD that would manage all interactions and negotiations with the industry, from early partnerships in research and development to the final procurement and stockpiling of licensed products. This would require a change in military organization such as the establishment of a single vaccine authority, as discussed above in Recommendation 1. The concentrated responsibility would permit the companies to deal with DoD representatives who are both (1) knowledgeable about vaccines and public health and (2) given the authority to commit the necessary resources.
The single vaccine authority could negotiate with active and potential industry partners for multiyear, multiphase contracts (or another suitable financial-legal arrangement) with clear milestones for both parties. These would allow the construction of additional facilities and the maintenance of a cadre of personnel who would produce the requested vaccines for clinical trials and who, after licensure, would manufacture vaccine lots for continued use. The presence of a single vaccine authority within DoD would allow informed consideration of industry requests such as the consideration of cost-plus terms for the research and development phase; fixed-cost contracts for the later development, manufacture, and distribution phases; or, after successful licensing, sale of facilities to industry. The single vaccine authority could also negotiate the financing and future ownership of fixed assets; pricing policy, including price ceilings; incentives—
including, for example, the available tax advantages (for research and development, production, and distribution); and staffing arrangements.
DoD might consider how to make better use of legislation to stimulate production of special-use vaccines—those for which a profitable market base is limited—that are particularly needed and unavailable for military use. U.S. orphan drug legislation has stimulated a notable increase in the introduction of drugs to treat rare diseases (FDA, 2002d; Lichtenberg, 2001), but vaccines—although within the purview of the Orphan Drug Act—have worldwide sales that are relatively small compared with drug sales (AEI, 1997) and have realized smaller gains following introduction of orphan product legislation. For instance, as of 1997 only 8 of 152 approved orphan products were vaccines (Lang and Wood, 1999). The financial incentives offered by the Act, such as tax credits, are not helpful to a tax-exempt government developer but it is unclear to this committee why this legislation has not generated more interest from manufacturers for vaccines for military use. DoD and FDA might explore alternative applications of or changes to current law to encourage private industry (as partners or contractors) and DoD itself to produce special-use vaccines.
DoD must assure potential manufacturers that the costs and benefits are reasonably predictable and somewhat guaranteed. An element in any such a calculation would be a consideration of the opportunity costs, that is, what it would cost industry to develop a product wanted by the military in terms of a reduction in its ability to pursue other, potentially more profitable products. The need for long-term financial support for the maintenance of the availability of critical vaccines cannot be overemphasized. Funds must be committed to maintain production facilities so that current good manufacturing practice requirements are met as necessary. A predictable market would involve the generation and maintenance of a vaccine stockpile, the purchase of guaranteed volumes in the future, and reasonable assumptions regarding pricing.
The lack of a policy that is acceptable to industry regarding indemnification against nonnegligent, adverse reactions is a major obstacle to DoD’s ability to attract industry participation in the vaccine acquisition process. DoD should examine the indemnification approaches that the federal government uses for childhood vaccines and civilian employees of the army to see if they might be adapted to use for vaccines for the protection of forces.
DoD and its potential partners in industry must delineate a set of mutually acceptable ground rules for the division of responsibility for the early steps in research, particularly those that lead to proof of principle, and the appropriate handling of the intellectual property that may emerge from joint research endeavors. The burgeoning success of technology management offices within many universities provides strong evidence that the fruits of research can financially benefit laboratories outside the industrial sector. Such benefits are appropriate and should not slow the pace of industrial partnership; rather, they should serve as an impetus for further collaboration between DoD laboratories and industrial vaccine manu-
facturers. Companies will be attracted to DoD partnerships if the companies are allowed to retain intellectual property rights for use in civilian markets. DoD needs to ensure that the ownership of its intellectual property is being properly and adequately exploited to leverage its research activities. Greater attention to the potential value of intellectual property may be of benefit to the overall DoD research effort.
A Government-Organized Consortium of Major Vaccine Manufacturers
The committee considered the possibility that government–industry partnerships might be managed through an industry consortium that would be formed to deal with the military’s requests for special-use vaccines. Such a consortium could be a single industrial vaccine authority working in partnership with the single DoD authority envisioned as described above to distribute the real and intangible costs of military vaccine development among multiple corporate entities in the industry. Such a consortium could field individual requests from DoD and work to locate an interested and qualified manufacturer that would enter into specific discussions with DoD. The mechanism could effectively distribute among the companies that are members of the consortium the opportunity costs involved in investing in the development of vaccines whose manufacture is requested by the federal government. Participation in the consortium could be recognized as a moral imperative, possibly facilitating the acceptance by shareholders of less than optimal business investments made by company boards in the interest of national and international security.
The consortium idea has its proponents and its detractors. Successful examples of different commercial entities with competing interests that have worked together for the benefit of all participants include SEMATECH’s experience with semiconductors (SEMATECH, 2002) and Airbus Industrie’s experience with the aviation industry (Airbus, 2002). An advantage particularly apt to vaccine development is the ability of a consortium to maximize the use of limited technical and professional expertise and other vaccine research and development resources. Although one can easily envision the problems that would need to be overcome to get competing industrial entities to work together in such a fashion, it seems plausible that the development of special-use vaccines by those with the greatest expertise would be particularly cost-effective. A wariness of the need to share proprietary information is a concern, however.
The idea of a consortium of major vaccine manufacturers has been proposed previously, but the reception to the proposal has been tepid. Several industry executives expressed the opinion, however, that it may now be a reasonable goal given the events of September 11, 2001, and the subsequent anthrax attacks. Any such consortium arrangement would require coordination with the Department of Justice, however, to ensure that consortium members would not be subject to collusion or antitrust investigations for these activities. Presumably, industry’s
concerns over potential antitrust action by the government would be minimized if the government itself took an active step to organize and promote the consortium.
Government-Owned Contractor-Operated Vaccine Production Facilities
Even with improved relations and successful partnerships between DoD and industry, it is unlikely that the private sector will produce all of the vaccines that the U.S. military needs. For that reason, DoD has explored the development of its own production facility. The Salk Institute operated its Government Services Division (TSI-GSD) at Swiftwater, Pennsylvania, and produced vaccines for DoD until 1995.6 DoD has not had a manufacturing resource similar to TSI-GSD since then, however. The committee notes that as part of an accelerated program of medical biodefense measures the Defense Authorization Act for FY 20027 authorizes DoD to design, construct, and operate a vaccine production facility and to contract for the private production of vaccines there.
Evidence supporting the need for a dedicated manufacturing resource can be found in Table 3-5. Table 3-5 lists eight vaccines that DoD had developed and that TSI-GSD manufactured under contract with the federal government. None of these vaccines are currently in production and thus their availability is severely restricted. None of these vaccines, which were or still are administered as INDs, have commercial markets that have interested or are likely to interest an industrial manufacturer of vaccines to invest in their further development. In these circumstances, if DoD is to make needed vaccines available to its forces, it must have access to a production facility outside of the commercial market.
All the items listed in Table 3-4 and some of those listed in Table 3-5 are likely candidates for production in a government facility. However, the committee emphasizes that regulatory requirements would prevent a single government-owned facility from producing all the vaccines needed. To produce more than one product, DoD would have to build the vaccine manufacturing plants with a modular design that would allow separate manufacturing suites to be used for different types of vaccines.
Government-funded manufacturing facilities could be operated through various models. First, DoD could operate its own facility. Second, if it developed these facilities as government-owned contractor-operated (GOCO) entities, a capable contractor could provide dedicated staff and the facility could operate with a flexibility not allowed by government personnel and budget rules. Third, such facilities could be operated by an industrial consortium, one that could mobilize expertise as required from the ranks of those working within its separate component corporations.
It is important to acknowledge that use of a GOCO facility would be expensive for at least three reasons. First, manufacturers would exert strong pressure by competing for employees who are highly experienced in the manufacture of vaccines. This means that salaries would need to be competitive with the best in the industry. Despite this, the employment of a highly skilled and experienced private-sector workforce could make up for the higher nominal cost of salaries in terms of efficiency and flexibility and could provide a greater ultimate return on the government’s investment. On the other hand, staffing of the GOCO facility by industry would create more competition for scarce expertise.
Second, the use of a GOCO facility would require the federal government to take on the full capital cost of building a production facility. This would be expensive because the facilities would require the use of high-containment technology not only for production but also for the testing of products. Although this approach could drive up the initial investment costs, it could provide corporate entities with an incentive to develop products that might not have sufficient market potential to be profitable otherwise. However, if the federal government makes a decision to invest capital for the construction of vaccine production facilities, an alternative to the GOCO concept would be to work out arrangements with specific companies, in which the federal government would subsidize construction of vaccine production facilities that the manufacturer could use to produce other vaccines when the facility was not in use for the production of vaccines for the U.S. military. This is the mirror image of the suggestion made by some that a GOCO facility might manufacture vaccines for the civilian sector when it was not in use making vaccines for the military, an idea that is anathema to the industry. Because the former situation would shift operations and management costs to the manufacturer, some within the industry are of the opinion that this would be a more efficient and less costly approach than the GOCO approach.
Third, and finally, as with any vaccine manufacturing arrangement, a GOCO facility would receive the strong FDA oversight that is essential to maintaining the efficacy and safety of vaccines. Such oversight is especially vital during the rush to respond to emergencies. The steps that need to be taken to comply with all regulatory requirements are costly, however. In addition, bacterial and viral vaccines provide unique challenges in terms of production and quality and safety control compared with the challenges for other types of pharmaceuticals.
After weighing the available evidence, the committee agreed that DoD should consider establishing a GOCO vaccine manufacturing facility, although it did not believe that the development of such a facility would in any way mitigate the need for DoD to revamp its system for managing the overall process by which it acquires vaccines, including integrating the upstream research and development activities and the downstream production and procurement activities, as outlined above. DoD’s fragmented and complex organization for vaccine acquisition must become more efficient and cost-effective, regardless of who operates the actual manufacturing process.
Recommendation 8.
Work toward improving manufacturing arrangements to better ensure consistent vaccine availability by addressing issues related to long-term commitments, predictable volume and price, indemnification, and intellectual property issues. These arrangements should include consideration of the development of vaccine-specific partnerships between the federal government and individual private manufacturers, a consortium of private vaccine manufacturers, and government-owned, contractor-operated (GOCO) vaccine production facilities.
REGULATORY STATUS OF SPECIAL-USE VACCINES
At present, the USAMRMC Special Immunizations Program (SIP) manages the use of 14 vaccines whose future availability is highly endangered. By and large, these vaccines are required for the protection of laboratory workers and other individuals who are at exceptional risk of exposure to the pathogens against which these vaccines are directed. Nine of these vaccines remain unlicensed and are available only under IND status, despite use over the past 30 years in varying numbers of recipients.
Long-term use of these products under continued IND status is problematic. FDA uses IND status to provide a basis for clinical investigations that ultimately lead to the demonstration of the safety and efficacy of a vaccine for use by humans; IND status is thus a step on the pathway toward licensure. The use of IND status to make a vaccine available for a specifically circumscribed but ongoing use without any formal intent of advancing the product through the regulatory pipeline toward the goal of licensure by FDA ignores the intent of an IND classification. The committee believes such practices should end. If DoD needs these vaccines, it needs to establish active development programs for eachone.
The committee realizes, however, that there are several substantial obstacles to moving these products from IND status to full licensure by FDA. Demonstration of efficacy may be difficult for these vaccines, because most of the vaccines that SIP manages are designed to prevent rare infections whose natural occurrences are unpredictable. That greatly limits the possibility of completing conventional clinical efficacy trials with these vaccines. The ability to conduct experimental challenge tests with these vaccines is severely limited or absolutely prohibited by well-accepted ethical rules guiding experimentation involving human subjects.
Although FDA recently finalized a rule that allows greater acceptance of efficacy data stemming from animal challenge experiments in making determinations for licensure (FDA, 2002c), the facilities and resources capable of conducting such animal challenge experiments are severely limited. The U.S. govern-
ment operates only two functional Biological Safety Level 4 (BSL-4) laboratories. Furthermore, studies with animals to demonstrate the efficacy of these vaccines would require more rhesus monkeys than are now available. Increasing those laboratory capabilities would entail considerable expense, although the committee notes that the President’s proposed FY 2003 budget may provide the funds to increase the number of BSL-4 facilities. Importantly, however, the costs of completing more extensive efficacy studies with animals with each of the vaccines whose development has been arrested at the IND level could exceed the financial benefit that would be provided if the vaccines were available as fully licensed products. Although the committee recognizes that direct financial benefit is not the only incentive to move from IND status to fully licensed status, it recognizes that cost-effectiveness analyses will be applied to any future programs that are proposed with this intent.
Demonstrating to FDA’s satisfaction that these vaccines are sufficiently safe to warrant licensure also poses special problems. For licensure of a product, FDA requires, rightfully, that safety testing involve sufficient numbers of subjects to detect vaccine-related adverse events that might occur at relatively low frequencies. This typically entails the enrollment of 10,000 or more subjects in Phase III clinical trials. These numbers may be justified for prelicensure studies of vaccines that are to be used universally or in large numbers of recipients, but studies of that size are tremendously difficult to conduct for vaccines whose use by DoD is intended to be restricted to small numbers of individuals (such as potentially exposed military personnel) no matter how critical the need.
The committee is aware that if a special-use vaccine were to be licensed by FDA, it could be used outside of the SIP framework, and could be prescribed by a physician for civilian travelers to areas in which the target disease is endemic or in response to an outbreak or a terrorist action. Committee discussion of how to help DoD acquire and maintain special-use vaccines was, therefore, couched in a speculative framework. One approach considered was to base licensure of these special-use products on safety standards that are established with a level of confidence appropriate for the number of intended recipients. For example, in the case of a vaccine with very limited intended use, it might be reasonable to base licensure on the results of safety trials involving much smaller numbers of subjects. A lack of serious adverse events among 300 subjects receiving the vaccine in a clinical study would predict that the vaccine would not produce a serious side effect more than 1 percent of the time. This level of risk might be acceptable if only small numbers of persons were intended to receive the licensed product, particularly if the risk of exposure was substantial and the consequences of infection in a nonimmunized person were severe. Such information concerning the limitations on safety data could be provided as part of package insert and could be used to guide decisions concerning the risks versus the benefits of immunization with that special-use vaccine under specific circumstances. In contrast, when considering the licensure of a vaccine for use in the civilian sector, FDA has
consistently sought evidence demonstrating a significantly lower risk of serious adverse events.
Basing safety evaluations on such statistical considerations would not be intended to short-circuit the requirement that licensed products be demonstrated to be safe as well as effective but would aim to establish parameters for safety that would recognize the expected use of the vaccine and the numbers of military personnel who might use it. Limitations on the database supporting safety would therefore need to be considered as part of the policies guiding the use of these vaccines, and these should be considered along with the magnitude and severity of the risk in the absence of immunization. If such a system were put into operation, postlicensure surveillance for adverse events would assume a new level of importance. Importantly, such surveillance is likely to be more feasible, under the conditions of ongoing military operations, than the usual data collection practices involved in the conduct of Phase III studies of a product with IND status. In effect, when standard, large-scale clinical trials of safety cannot be done, rather than prohibit the use of a vaccine that would protect warfighters against highly dangerous pathogens, a better overall strategy would be to use the vaccine according to a new FDA licensure arrangement and conduct active postlicensure surveillance for adverse events.
Yet additional problems face licensure of these vaccines with IND status because the basic research on some of these products was carried out long ago. Some data regarding the process used to manufacture the remaining stocks are not available for FDA review. For other products, it may be necessary to repeat earlier work with newer and more costly technologies.
Despite these financial implications, there are other costs, such as the substantial political costs and the crisis of trust that DoD incurred when veterans objected to the use of INDs during the Gulf War. The concern—which continues today—may have been fueled in part by people equating “investigational” with “unsafe.” To avoid such understandable concerns, DoD could work with FDA to define a new category, one that might be suitably placed between IND status and full licensure or that might be a subset of licensure. The committee does not intend to imply that products being used only as an IND and manufactured years ago are either safe or effective (although they may well be). Neither is it dismissive of the critical need to demonstrate the safety and efficacy of vaccines as an important factor in the future protection of warfighters against infectious disease threats. It also recognizes that some IND products may never be suitable for licensure. What it does seek is a pragmatic solution to an impossible set of circumstances that threaten to limit access to useful preventive measures during military operations that entail demonstrable risks of infectious diseases.
The committee suggests that DoD work with FDA to identify options related to the status of vaccines that have not been licensed. Ideas to be considered for a new FDA-sanctioned status extend from a status that is no more than a change in terminology to a status that reflects a wholesale new approach to licensure that
recognizes alternative mechanisms to the assessment of the safety and efficacy of vaccines in humans that could be applied to vaccines that are to have only limited routine use or that would be used only under unusual circumstances when the risks of infection are perceived to be exceptionally high. The Defense Authorization Act of 20028 mandates a study to examine how the government might accelerate the approval process for biodefense vaccines. Although the committee would have included vaccines against naturally occurring infectious diseases in that scope explicitly, it believes that methods devised to overcome the obstacles to the acquisition of biodefense vaccines will be applicable to vaccines designed to address both kinds of exposures. Achieving success in whichever direction DoD chooses to go will require that scientists effectively communicate a sense of urgency through the program and budget hierarchies.
Recommendation 9.
Vigorously seek a new paradigm for the regulation of special-use vaccines that remain in IND application status with the FDA and that have no reasonable prospects for licensure under current rules. The new paradigm should take into account the circumstances of the vaccine’s anticipated use in setting requirements for the demonstration of safety and efficacy.
CONCLUSION
Military scientists have a notable record of accomplishment when it comes to vaccines, including primary or significant roles in the development of vaccines against meningococcal meningitis, hepatitis A, Japanese encephalitis, and other dangerous infectious diseases. Partly because of the success of the DoD research programs, the public and even DoD nonmedical research personnel know little about them or the threats that their products have ameliorated. For example, the prior availability and the benefits of the adenovirus type 4 and 7 vaccines went unnoticed by most training center commanders until the vaccines were lost from the armamentarium and adenovirus disease reemerged at military training installations. By creating a single vaccine authority and a credible advisory board and responsibility over the entire life cycle of a vaccine, from priority setting to stockpiling of licensed products, DoD would enhance not only the effectiveness but also the visibility of its vaccine program, improving the chance of its being provided with a budget of sufficient magnitude to allow it to accomplish its mission. It is a mission of enormous importance. Immunization is often the most effective means of preventing infectious diseases, either in civilian or military populations, and whether caused by naturally encountered infectious agents or purposeful exposures related to bioterrorism or biological warfare.
The committee urges DoD to work more aggressively with decision makers in the U.S. Congress and in the executive branch to recognize that infectious disease agents—whether they occur naturally or are weaponized as agents of biological warfare or terror—threaten military operations and, therefore and implicitly, the welfare of the nation. Decision makers must recognize (1) the past, imminent, and possible future successes of vaccines in minimizing those threats; (2) the strong track records and reputations of military research programs in developing vaccines used by the U.S. military as well as in civilian settings; (3)the contributions that DoD’s medical research efforts make to foreign policy and national security; (4) the threats to continued vaccine development and the ultimate use of vaccines posed by organizational and fiscal limits; and, consequently, (5) the need for adequate, stable funding and strong management authority. Such changes would allow DoD to optimally advance and exploit the technology available for vaccine development, and to provide the best possible protection of the nation’s armed forces against infectious diseases.
In summary, DoD’s vaccine acquisition system, despite its distinguished history, diffuses responsibility and is inadequately funded; therefore, it cannot produce the effort required to respond to the magnitude of its task.








































