3
Back to Basics: Scientific, Conflict of Interest, and Ethical Review of Research Protocols
Protecting the rights and welfare of research participants is based on respecting the relevant ethical principles that underlie such protection, including the principal of beneficence. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research states that “persons are treated in an ethical manner not only by respecting their decisions and protecting them from harm, but also by making efforts to secure their well being” (National Commission, 1979, p.2). In the context of research, individuals are sometimes placed at risk, and such risks should be carefully weighed against potential benefits, either to the individual or to society. The principle of beneficence incorporates the rules of “do no harm” and “maximize possible benefits and minimize possible harms” (National Commission, 1979, p. 6). The best means for assessing risks and benefits is through independent review of the proposed research by individuals who have no direct vested interest in its outcome.
THE NEED FOR RESEARCH ETHICS REVIEW BOARDS
As detailed in this chapter, Institutional Review Boards (IRBs) should be reshaped and reformed to serve the role for which they were originally intended—ensuring participant protection through the careful ethical review of research protocols. As such, IRBs are the cornerstone of a system in which other entities, such as research sponsors, also have obligations to protect research participants, for example, by ensuring that investigators and research staff have completed necessary education requirements. How-
ever, IRBs have come to shoulder an increasing share of these tasks in ways that were not originally intended. Because IRBs should be constituted to carry out their obligation to focus primarily on the ethical aspects of human protection issues, the committee suggests that protection programs consider changing the name of these boards to reflect this recommended fundamental shift in emphasis.
Recommendation 3.1: The Institutional Review Board (IRB), as the principal representative of the interests of potential research participants, should focus its full committee deliberations and oversight primarily on the ethical aspects of protection issues. To reflect this role, IRBs should be appropriately renamed within research organizations’ internal documents that define institutional structure and policies. The committee suggests the name “Research Ethics Review Board (Research ERB).”
Changing the name of an entity can be a useful way to signal important substantive change. In the United Kingdom and elsewhere, ethics review is carried out by what are typically called “Ethics Committees” or “Ethics Review Committees.” The International Conference on Harmonisation uses the terminology “Independent Ethics Committee.” In each case, the objective of these reviews is to ensure the ethical conduct of research and that participants’ interests are fully recognized, represented, and protected. The committee therefore recommends moving away from the term “Institutional Review Board,” which conflates institutional interests with those of participants and which may cause at least the appearance of an institutional conflict of interest.
Admittedly, the term IRB is now firmly embedded in the regulations and literature, and is likely to continue to be used despite its imperfect reflection of the function that the board is designed to serve. However, many research organizations in this country have given these bodies different names (“Committee on the Protection of the Rights of Human Subjects” or “Committee on Clinical Investigations”)1 that more accurately describe their appropriate functions, while empowering them to carry out the functions that have been assigned to IRBs by the applicable federal regulations.
This committee urges all research organizations (as well as free-standing IRBs) to signal their commitment to reform by changing the name of the bodies serving the functions of IRBs to “Research Ethics Review Board”
(“Research ERB”). The modifier “research” is intended to distinguish the body from the ethics review board commonly found in many hospitals, which is charged with providing advice and consultation on difficult issues related to health care delivery. Throughout the remainder of this report, the term “Research ERB” will be used when describing the idealized system of protections. The term “IRB” will be used when describing aspects of the current system.
The Research ERB should refer issues of institutional interest (e.g., risk management concerns, resolution of institutional or investigator conflict of interest) to the institution’s management and/or compliance office (see Chapter 6).
THREE-PRONGED REVIEW
A central tenet in the protection of research participants is the independent review of research protocols to assess their scientific merit and ethical acceptability. It is also critical to consider whether conflicts of interest on the part of the investigator, the Research ERB, or the institution place research participants at undue risk. Thus, every protocol requires an autonomous analysis of several interrelated factors (scientific merit, ethical design, and potential financial conflicts of interest)2 before it is deemed appropriate for investigators to enroll participants. Evaluation of each factor requires a specific knowledge base for sufficient assessment, both in depth and breadth. Thus, it is unrealistic to expect a single group of individuals to possess the requisite skills to competently carry out the many tasks needed to protect the rights and welfare of research participants.
Recommendation 3.2: Research organizations and research sponsors should ensure that Human Research Participant Protection Programs utilize distinct mechanisms for the initial, focused reviews of scientific and financial conflicts of interest. These reviews should precede and inform the comprehensive ethical review of research studies by the Research Ethics Review Board (Research ERB) through summaries of the relevant findings submitted to the Research ERB for full board consideration.
|
2 |
The committee acknowledges that nonfinancial conflicts of interest also pose potential threats to the integrity of study conduct. Some possible conflicts of this nature are discussed in Chapter 4, and the need for the research community to rigorously pursue policies to oversee and manage such potential conflicts is discussed in Chapter 6. In light of the need for further development of the policy discussion in this arena, the committee has largely concentrated its discussion with respect to the protocol review process on financial conflicts of interest. |
In the United States, ethics review of federally funded research and of some sponsor-funded research (specifically, clinical trials of products subject to Food and Drug Administration [FDA] regulation) is generally conducted by IRBs, as specified in federal regulations.3 The degree to which these bodies should and can explore the scientific merits of protocols has been a matter for impassioned debate since review of protocols by nonscientists was first suggested in the 1960s and 1970s (Levine, 1986; Moreno, 2001). Today, some observers assert that most IRBs as currently constituted do not routinely or sufficiently review scientific concerns, such as justification for sample size, eligibility criteria, or the qualifications of the investigator (Bohaychuk et al., 1998). Others believe that ethics and science review cannot and should not be separated because they are intrinsically tied (Freedman, 1987). In the United States, not all human research is routinely subjected to both scientific and ethical review. In some cases, particularly when no federal funds are used to sponsor the research, scientific review may not occur or may be conducted only cursorily by a group internal to the research organization. In other cases, ethics review may not occur if the research is not subject to the various federal regulations governing the conduct of research with human participants.4 As stated in Chapter 2, the committee believes that all research involving human participants, regardless of site or funding source, should be subject to an independent review and a common system of protections (Recommendation 2.1).
The protection program should ensure that each research protocol receives objective scientific review, relying on input from content experts. These experts may be found, for example, within local scientific departments, external academic institutions, pharmaceutical companies, federally organized peer-review groups (i.e., the National Institutes of Health [NIH] or National Science Foundation [NSF] study sections), or FDA Review Divisions, and they should be sufficiently insulated from the interests of the investigator or the protocol. Program procedures should clearly articulate mechanisms for documenting such insulation and for the transmittal of the findings to the relevant Research ERB. The program should be subject to external audits (by FDA, NIH, or accreditation bodies) that verify, among other things, the appropriate degree of insulation of these functions and their operations.
Although some IRBs rigorously consider the scientific merits of proposed research, the extent to which they are aware of or consider potential financial conflicts of interest is not clear. In addition to scientific and ethical considerations, it is essential to ensure that potential financial conflicts of interest involving the investigator or the institution are identified, managed,
and, if possible, eliminated. This determination is especially critical if the conflicts pose possible risks to research participants.5 In its 2001 report, the National Bioethics Advisory Commission (NBAC) suggested that IRB review of research studies is “one method for identifying and dealing with conflicts of interest that might face investigators” (2001b, p.58). However, NBAC concluded that “IRB review alone…is not sufficient to manage conflicts of interest, because the options available to IRBs to eliminate such conflicts are limited” (2001b, p.59). Indeed, given the burdens faced by IRBs in terms of work volume, expecting these boards, especially in large research organizations, to assume primary responsibility for such reviews is unrealistic (see Chapter 6).
Potential financial conflicts of interest of the investigator, Research ERB members, or the institution should be assessed by the organization’s relevant conflict of interest oversight mechanism (Recommendation 6.5) and communicated to the Research ERB. As described in Chapter 6, the conflict of interest oversight body should determine whether financial conflicts should be disclosed, managed, or are so great that they compromise the safety or integrity of the proposed research. The conflict of interest body should communicate to the Research ERB its determination of potential conflicts relevant to protecting the rights and welfare of research participants, the rationale for its determination, and any recommended conflict management plan. Such communications could be verbal or achieved by providing the Research ERB chair or administrator a copy of the conflict of interest committee’s final determination, if that conclusion suggests a conflict that poses greater than minimal risk to potential research participants. Some institutions “cross-fertilize” various review committees to maintain communication and promote awareness of the relevant issues within each committee’s purview (Dretchen, 2001). The Research ERB should use this information to determine if and how participant protection could be negatively affected, whether the recommended conflict management plan is sufficient to ensure participant protection, what information pertaining to any conflict should be disclosed to participants through the informed consent process, and whether ongoing review is required in the event that the research goes forward.
By ensuring that properly constituted bodies review protocols for scientific merit and freedom from conflicts of interest, the Research ERB should be able to focus its efforts on assessing whether the protocol meets the ethical requirements as stated in the Belmont Report (National Commission, 1979) and the federal regulations.6 Each program should ensure that
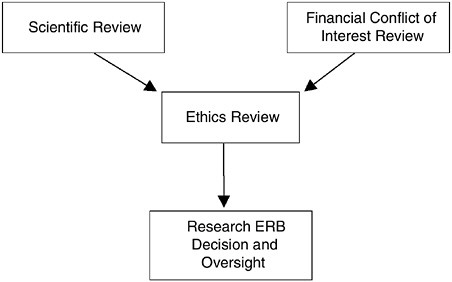
FIGURE 3.1 The Confluence of the Research Review Process
these reviews occur in accordance with established standards and are subject to a system of internal checks and balances.
Despite the need for the three distinctive reviews (science, ethics, financial conflicts of interest), their interrelated nature and their underlying considerations requires that a single body be vested with the explicit authority and accountability for the final determination regarding the ethical acceptability of research protocols. The committee believes that this body should be the newly designed Research ERB (Figure 3.1). Therefore, the focused reviews of scientific merit and the evaluation of potential financial conflicts of interest should feed into the ethics review process for each protocol, and the Research ERB should have the ultimate authority regarding participant enrollment.
ENSURING DISCRETE SCIENTIFIC REVIEW OF PROTOCOLS
Scientific and ethical reviews of research protocols are both essential because each considers different sets of questions and therefore each can yield different determinations. For example, a proposed study might be deemed scientifically sound and intellectually intriguing and yet pose significant or even intolerable risk of harm to participants. Conversely, the former Office of Protection from Research Risks (OPRR) wrote that “a proposal without scientific merit can on the surface appear to be ethically
acceptable, but the fact that it will not produce new or usable data does not justify the use of human participants regardless of the level of risk” (1993). Furthermore, a scientifically meritorious study might be ethically conducted in one cultural context but not in another (NBAC, 2001a). Unquestionably, there are areas in which the two sets of considerations intersect, for example, in determining if inclusion/exclusion criteria are wisely chosen on scientific grounds and properly justified ethically. Each review process is likely to consider aspects of the other, but in general, greater benefit can result through separate, focused reviews.
For these reasons, programs should not rely solely on one review mechanism (e.g., the Research ERB) to conduct all aspects of ethical and scientific review of protocols.7 When a Research ERB is called on to conduct the exclusive scientific review of a protocol, two primary problems can arise: 1) it can be distracted from intensive review of the ethical issues due to lack of time, or 2) it may lack the scientific expertise necessary to adequately assess the technical merit of a proposal (OIG, 1998a). An additional complication arises if the only resource available to the Research ERB to answer technical questions is the principal investigator (PI) who submitted the protocol— obviously not a disinterested party. In this case, the Research ERB would lack an appropriately independent technical resource who could address challenging scientific questions pertaining to the given protocol. The Human Research Participant Protection Program (HRPPP) should also ensure that any expert scientific panel is free of significant conflicts of interest.
Elements of Scientific Review
All protocols involving human participants should undergo an independent and rigorous scientific review to assess scientific quality, the importance of the research to increase knowledge, and the appropriateness of the study methodology to answer a precisely articulated scientific and, in some cases, clinical question. For example, the design of clinical trials should be based on sound statistical principles and methodologies, including sample size, use of controls, randomization, population stratification, stopping rules, and the feasibility of relating endpoints to objectives. Ensuring that the chosen study design minimizes bias and generates data that will answer the scientific question requires some understanding of the research process and the area under study (Spilker, 1991). These issues are pivotal to a successful study and
|
Box 3.1
|
should be evaluated by a mechanism that is distinct from the ethical review process before participants are enrolled (see Box 3.1).
This mechanism should ensure that adequate technical expertise in the evaluation of the proposal occurs, which could be accomplished by using a separate committee(s) based on expertise, by using a subcommittee of the Research ERB augmented by others in the institution with specific expertise, or by using outside experts. The result of the scientific review should include the elements shown in Box 3.1; the result should be provided to the Research ERB.
There will always be some level of overlap between scientific and ethical reviews. Research ERB review should continue to include some consideration of a protocol’s scientific merit; however, delegating the in-depth scientific review to an upstream mechanism should facilitate the Research ERB’s more focused consideration of the ethical elements of particular protocols. One advantage of ensuring a distinct scientific review mechanism is the opportunity to identify protocols that are not yet suitable for Research ERB consideration. Such protocols should be returned with suggestions for revision to the author of the proposed research. This would help ethics review meetings maximize their time to focus on a thorough deliberation of the ethical considerations of fully developed, scientifically sound protocols. In rare and controversial cases, however (e.g., proposals to conduct embryonic stem cell research or xenotransplantation research), it may be appropriate to pursue the ethical consideration of a protocol before, or in conjunction with, the evaluation of its scientific merits.
Mechanisms for Scientific Review
It is the responsibility of the research organization directly overseeing the conduct of the research to ensure an adequate process for the scientific review of protocols. As with other components within protection programs, the scientific review process should be accountable to the highest authority within the research organization, and failure to conduct an independent, nonconflicted scientific review should be met with sanctions by that authority. Therefore, a mechanism for periodic audit of the scientific review process should be established.
A variety of mechanisms can be used to ensure independent scientific review. In fact, most protocols currently undergo some level of scientific review through existing mechanisms. Measures should therefore be taken to ensure that protocols not currently subject to technical review are funneled into existing or newly created mechanisms for this purpose (Box 3.2).
Research sponsored by established industrial entities typically undergoes rigorous scientific review (FDA, 2001c; Spilker, 2001). For example, protocols designed by pharmaceutical companies often go through numerous and prolonged iterations before ultimately being approved by an internal oversight committee of physicians and scientists. In large pharmaceutical companies, scientific review committees typically comprise medical directors, clinical scientists, safety managers, and regulatory affairs professionals, and these committees report to a high-level clinical executive within the organization. In addition, they often rely on other experts in areas such as pharmacology, toxicology, and pharmaceutical development for additional support. The committees are generally part of the clinical organization of the sponsor (e.g., in the Chief Executive Officer’s office), rather than being placed outside it. This process is not entirely devoid of conflicts of interest, and care should be taken by companies to appropriately insulate their scientific review committees, perhaps by providing a charter that governs the committee’s operations and allows the committee to be audited. The charter should also indicate the qualifications required of scientific review committee members.
If protocols involve investigational drugs, devices, or biologics, they must be submitted to FDA for regulatory review, comment, and approval; they can be rejected by the agency on scientific or safety grounds. FDA reviewers are also trained scientists and physicians versed in the pertinent therapeutic area and intimately familiar with issues of inclusion/exclusion, appropriate endpoints, and safety issues. Thus, comments provided to sponsors by FDA reviewers should be made available to the Research ERB to inform the final comprehensive assessment of a protocol.
Federally funded biomedical, social, and behavioral research protocols are typically subjected to a scientific peer review process by the funding
|
Box 3.2 Academic-based research: A separate, internal committee with requisite expertise (such as departmental or GCRC [General Clinical Research Center] unit); a subcommittee of the Research ERB, perhaps augmented with others from the institution with appropriate expertise; external committees of experts (i.e., federal peer review mechanisms). Outside expert consultants should always be considered as a resource to remove the perception or the reality of conflicts of interest and to ensure a sound scientific review. Funding for scientific review should be assumed by the academic institution, which should also include auditing of the scientific review process within its overall quality assurance (QA) activities. Written assurance of the scientific review should be provided to the Research ERB. Industry-sponsored research (for FDA-regulated products): Company Protocol Review Committee independent of the author(s) of the research protocol; applicable FDA Review Division. Both should provide written assurances to the Research ERB. Privately sponsored research (not for FDA-regulated products): Protocol Review Committee (e.g., leaders in the applicable field) independent of the author of the research protocol and external to the research sponsor. The committee should provide written assurance to the Research ERB. May be funded by the research sponsor, but should operate at arm’s length and according to a charter. Federally funded research (NIH or equivalent): Protocol Review Committee independent of the author of the research protocol. Written assurance to the Research ERB should be provided (such as grant “pink sheets”). May be funded by the research sponsor, but should operate at arm’s length and according to a charter. NIH or equivalent agency should have an audit mechanism to verify adequacy of the scientific review process. Locally sponsored research (e.g., a university department using unrestricted grants): Departmental Protocol Review Committee independent of the author of the research protocol. Written assurance should be provided to the Research ERB. Should be subject to audit by institutional-level body. |
agency. The guiding principles for the initial review of research project grant applications submitted to NIH are based on the Public Health Service Scientific Peer Review Regulations, which state that peer review groups are to make recommendations concerning the scientific merit of applications. The specific criteria used to assess the merit of research project grant applications vary with types of applications reviewed. However, the review by the scientific panel is expected to reflect existing codes adopted by disciplines relevant to the research or the collective standards of the professions
represented by the membership. In addition, the evaluation is to take into consideration the investigator’s response to six points relevant to the protection of human participants, ranging from the inclusion criteria to the protection of confidentiality to the minimization of risks and to obtaining informed consent (NIH CSR, 2001, 2002). No awards are made until all expressed concerns about human participants have been resolved to the satisfaction of NIH.
At NSF, proposals are assigned to the appropriate NSF program for acknowledgment. If they meet NSF requirements, they are submitted for review. All proposals are reviewed by a scientist, engineer, or educator serving as an NSF program officer and usually by 3 to 10 other individuals outside the organization who are experts in the fields represented by the proposal. Proposers are invited to suggest names of those they believe are especially well qualified to participate in the review and/or those they would prefer not participate. These suggestions may serve as one element of the reviewer selection process, at the program officer’s discretion. Program officers may obtain comments from assembled review panels or from site visits before recommending final action. Senior NSF staff further review recommendations for awards (NSF, 2001).
Scientific review of a protocol should be particularly rigorous at the local level if the study will not be submitted for federal funding and/or will not be subjected to a peer review process similar to that of NIH or NSF. Scientific beliefs and biases, as well as competing interests by reviewers and the relationship of reviewers to investigators or chairs of departments, can affect the outcome of the review and should be considered in the selection of a scientific review mechanism. The responsibility for scientific review should not be left solely to a department chair, as he or she may lack sufficient time. The committee recognizes that it will not be practical or appropriate to subject every protocol to rigorous external peer review (e.g., a student-led research project in the social sciences). However, even in such cases, some level of internal scientific review should occur under the auspices of the departmental faculty based on a documented process that can be audited by an institution-level body.
Finally, when commercial Research ERBs are called upon to review research protocols, their standard operating procedures should provide the mechanisms to ensure that scientific review of proposed research occurs and that their primary function remains focused on the ethics review and the integration of the scientific and financial conflict of interest review elements pertinent to the research. Currently, most protocols reviewed by independent IRBs undergo intensive scrutiny by the same group for both scientific merit and ethical safeguards, with reliance on external content experts as necessary. For multisite studies, the protocol may be submitted to the IRB directly from the sponsor, often before investigator selection.
Considering the frequency of this practice, it is critical that the mechanisms for independent scientific review are defined and can be audited.
Departmental Responsibilities
Academic departments have a responsibility to establish and cultivate the highest standards in scientific conduct, including the treatment of those who participate in human studies. There are few, if any, investigators who are so senior and experienced that their proposed research cannot benefit from scientific review. In large institutions, departmental committees may already vet proposals before they are submitted to the IRB, perhaps serving as the formal scientific review mechanism. In smaller institutions, an inter-departmental review may occur. And in the case of graduate level research, the mentor or thesis committee may assume this responsibility.
Secondary gains from the use of this particular scientific review mechanism include an additional level of mentoring for new and junior investigators, continuing education of reviewers, a mechanism for monitoring and developing departmental research programs, and departmental investment in and responsibility for its research program and the consequent human participant protection needs. The organization’s standards should become an integral component of the mentoring that senior investigators provide to less experienced investigators and reviewers. Furthermore, the departmental responsibility for fostering quality research among its members is representative of the accountability for research conduct and behavior at the highest levels of a research organization (Recommendation 2.2). In this way, the local leadership provided by a department facilitates the realization of an ethically rich and robust research culture.
Communicating with the Research ERB
If the targeted scientific review mechanism, in whatever form it may take, is to be optimally utilized, a summary of the results of the scientific review must be reported to the Research ERB before the focused ethics consideration occurs, and it should be reported in a manner that is understandable to nonmedical, nonscientific members. A written, signed report might include the following items:
-
A determination that the importance of the scientific question is sufficient to merit the inclusion of human participants and the risks imposed upon them;
-
Comments on the strength of the scientific design and methodology;
-
An assessment of the practical feasibility of the research design;
-
An estimate of the probability of meeting the goals of enrollment;
-
The need for a DSMB/DMC;
-
Assurance that the relevant literature and previous studies, if available, have been taken into account by the PI and that, if necessary, experts in the field have been consulted;
-
Comments on the qualifications of the investigator to carry out the protocol and the adequacy of the facilities available to him or her.
Depending on the review mechanism utilized, communication to the Research ERB could take the form of a distinct summary specifically prepared for the Research ERB, or it might be possible to use existing forms, such as the NIH grant review summary (the “pink sheet”), if the information listed above is included. The goal is to facilitate a quick and responsive system of review capable of resolving most scientific issues with the investigators before the protocol is considered by the full Research ERB.
FINANCIAL CONFLICTS OF INTEREST AND PROTOCOL REVIEW
As stated in Chapter 2, Research ERBs should not bear the primary responsibility for identifying and managing financial conflicts of interest, as they lack the necessary resources, expertise, or authority to do so (AAMC, 2001; Glass and Lemmens, 1999; NBAC, 2001b; NHRPAC, 2001). However, the most important function in assessing potential conflicts of interest (financial or nonfinancial) in human research studies is determining whether bias or overly optimistic promises of potential benefits are clouding risk assessments. Therefore, the Research ERB should retain a central role in determining whether financial conflicts of interest have the potential to affect participant safety, and, if necessary, how participants should be informed of any resulting risk (see Chapter 6 discussion).
Investigator Conflicts of Interest
In recent years, pressure has been building to require investigators to disclose financial conflicts of interest to the IRB, so that it is aware of any potential conflicts when a protocol is reviewed (DeRenzo, 2000). However, simply disclosing conflicts to the IRB is insufficient (Cho et al., 2000; Lo et al., 2000; NBAC, 2001b). Independent conflict of interest review by another entity within the program is essential to ensure that such review is given appropriate attention, that any necessary conflict management plans are implemented, and that the relevant aspects of the review and management plan are communicated to the Research ERB for its ethics review (see Recommendation 6.5). This separate conflict of interest review should fo-
cus on whether an investigator’s or an institution’s financial interests in the proposed research are inappropriate; the results of this review need to be communicated to the Research ERB.
The role of the Research ERB with respect to financial conflicts of interest is to assess the determinations made by the research organization’s conflict of interest mechanism with specific regard to participant protection. The Research ERB is responsible for the ultimate determination of a conflict’s acceptability in terms of participant protection, and it also should determine how information about the conflict should be presented to the participant through the informed consent process (DHHS, 2001a; NBAC, 2001b). Simple means of obtaining further information also should be clearly made available to potential participants (see Chapter 2). If the investigator has a financial interest or, in the case of medical studies, is the participant’s primary caretaker, then the investigator should not be the sole person involved in the informed consent process. A number of institutions already have established financial disclosure procedures regarding the consent form. However, the effectiveness of these procedures or their protective contribution has not been determined (AAMC, 2001; DHHS, 2001a).
Although public attention and consequent reports and guidelines have focused on financial conflicts of interest, conflicts are not limited to the potential for pecuniary gain. In fact, the desire for professional advancement, fame, or the desire to make a scientific breakthrough can present very strong conflicts of interest (Angell, 2000; Kirchstein, 2000; NBAC, 2001b; NHRPAC, 2001; Spilker, 2001). These desires also have been cited as motivating factors in clinical research, and are an inherent part of the research environment (IOM and NRC, 2002). In 1978, the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research noted that “…investigators are always in positions of potential conflict by virtue of their concern with the pursuit of knowledge as well as the welfare of the human subjects of their research” (1978, p.1). The Association of American Medical Colleges addressed the issue in its 1990 report: “Such conflicts become detrimental when the potential rewards, financial or otherwise, cause deviation from absolute objectivity in the design, interpretation, and publication of research activities, or in other academic and professional decisions” (1990). In addition, subconscious biases and pre-conceptions may lead to a flawed informed consent process. These conflicts, although less quantifiable than those with financial implications and not subject to conflict of interest committee financial reviews, should be considered by the Research ERB during the review of research involving human participants. (Potential nonfinancial conflicts of interest of investigators are discussed further in Chapter 4.)
Research ERB Conflicts of Interest
Research ERBs themselves can also have conflicts of interest at multiple levels. Critics have charged that academic IRBs, by virtue of their constitution and location, are too close to the scientific community whose research they review and that their role has shifted from protecting research participants to protecting the research institution (Annas, 1991; Francis, 1996). Eventually, as the HRPPP system continues to evolve, it may be desirable for Research ERBs to become structurally independent, without any institutional links to academic centers or other research organizations. Although the process of forming independent IRBs has already begun,8 substantial restructuring of the present system undoubtedly will take several years. In the meantime, it is essential for Research ERBs within academic centers to protect and maintain their independence within organizational structures in order to reduce the risk that participant protections will be compromised by institutional interests. To signify the importance of this realignment, the committee encourages academic centers and other constituencies in the research community to begin to structure their IRBs as “independent” review boards rather than “institutional” review boards in addition to renaming the board, as suggested in Recommendation 3.1. Such a change in vocabulary will not by itself eliminate the potential for conflict when research organizations utilize internal Research ERBs within their HRPPPs. However, it may encourage the development of structural mechanisms that would insulate Research ERB operations from institutional power structures as the broader discussion concerning the appropriateness of moving to a system that utilizes completely unaffiliated and wholly independent Research ERBs develops.
Beyond the structural relationship of Research ERBs to research organizations, conflict of interest can occur at the individual member level. Members of these bodies, particularly when the board is located at an academic research institution, may have ties to the researchers whose proposals they are reviewing; they may have concern for an institution’s financial well-being and reputation; or they may have an excessive faith in science that could be harmful to human participants if potential consequences are overlooked. One possible way to address this problem is to ensure that the Research ERB has sufficient representation of members from the nonscientific and noninstitutional communities (NBAC, 2001b; Levinsky, 2002; also see Recommendation 3.5). An increase in the percent-
age of unaffiliated members serving on review boards may also help address the concerns regarding the ability of Research ERBs to make decisions without regard for the potential institutional ramifications.
However, outside representation is not enough to counter conflicts of interest—or the perception of such conflicts—that may affect the review of research. In addition to appropriate recusals at each meeting, Research ERB members should submit a disclosure statement once each year to the chair (and if it exists, to the organizational conflict of interest committee). This statement should include information about financial and nonfinancial biases including but not limited to professional relationships or competing scientific projects. If even remotely relevant, these potential conflicts should be shared with the Research ERB (as a committee or with the chair), or the board member should state that a potential conflict of interest exists and recuse her- or himself from the discussion and from voting on a particular protocol, as required in federal regulations.9
Similar problems could arise in the case of proprietary Research ERBs (i.e., established by a company to review its own research). However, in these instances, the issues are exacerbated by the explicit purpose of a company to make a profit. For the most part, these conflicts can be addressed in a manner similar to those of academically based review boards. Lemmens and Freedman argue that “this appearance of conflict [in proprietary IRBs] makes it crucial to require public scrutiny and access to information on how IRB members are protected from corporate sanction, whether they have secure positions…and whether they have any other financial interests in research undertaken by the company” (2000, p.567). These precautions should enhance the independence of the Research ERB within the company—essential to preserving the board’s judgment and the public’s trust.
Independent Research ERBs (i.e., noninstitutional or commercial boards), although avoiding the conflict of being located within an institution, have a more obvious conflict—they are paid for their review by an interested party (e.g., an investigator, a sponsor, a contract research organization [CRO], or an institution) and depend on these contracts to remain viable. But paradoxically, the financial issues that suggest the potential
conflict of interest also provide more resources than are available to academic committees, resources that, in fact, allow the independent review boards to perform robust reviews (Thacker, 2002). Individual member conflicts can be addressed as described earlier, but a further concern is involved if the independent board members, who are paid for their services, have a vested interest in approving research and facilitating friendly relations with sponsors to ensure their own income (Lemmens and Freedman, 2000; Thacker, 2002). Moreover, if a given review board does not satisfy a client, the client can end the business relationship and go “IRB shopping.”
In addressing these concerns, independent boards point out that they are paid regardless of the review outcome and that business management aspects should be, and often are, completely separated from the review function (Jacobs, 2001; Thacker, 2002). A recent FDA proposal for rulemaking aims to counter the IRB shopping concern somewhat by requiring sponsors and investigators to inform boards of any prior reviews (FDA, 2002a; Lemmens and Thompson, 2001; OIG, 1998c).10 In addition, independent Research ERBs must adhere to the federal regulations governing human research; if a board was censured by the Office for Human Research Protections (OHRP) for failing to perform its duties or was cited by FDA for compliance violations, it would lose credibility within the research community, adversely affecting its income and possibly its entire business.
ETHICS REVIEW: CLARIFYING THE ROLES AND RESPONSIBILITIES OF RESEARCH ERBS
Independent review of proposed research by Research ERBs to determine ethical acceptability should provide a comprehensive ethical assessment of protocols from the perspective of the local community and the institution. However, despite the central role of these review boards in the federal regulations and the research review process, their ability to keep pace with the enormous volume of work and the high-quality services expected of them has been in question for some time (AAU, 2001; GAO, 1996; Levine, 2001a; NBAC, 2001b; OIG 1998a, 2000a).
The Research ERB’s role is to review human research proposals that have passed scientific review to ensure that they comply with federal and institutional policies regarding the ethical treatment of research participants throughout a project. The board should focus solely on the protection of research participants and should consider itself an advocate for these volunteers. It should refer issues of organizational interest (e.g., institutional conflicts of interest, adequate reimbursement, or risk management
concerns) to organizational management or the appropriately designated office among the program elements (see Chapter 6). In addition, it should consider to what extent and in what manner conflict of interest information should be communicated to potential participants if that information might influence the investigator’s, institution’s, or participant’s assessment and judgment about the risks and potential benefits of the proposed study. In general, Research ERBs are responsible within the protection program for the functions listed in Box 3.3.
|
Box 3.3
|
Research ERBs should not be expected to perform the following tasks:
-
monitor safety and establish data and safety monitoring plans or boards;
-
comprehensively review conflict of interest;
-
investigate allegations of scientific misconduct;
-
investigate publication-related disputes and claims;
-
address indemnification and contract issues;
-
review radiation or isotope safety, biohazards, or recombinant DNA-specific issues;11
-
review material transfer agreements;
-
conduct QA or quality improvement (QI) for the program as a whole; or
-
establish ethical training programs for investigators.
Although these functions are important in the protection of research participants, they should be addressed by other program units or collaborating units within an organization (e.g., Office of Technology Transfer for Material Transfer Agreements, see Chapter 6).
In addition, training is an institutional responsibility, not that of the Research ERB (see Chapter 2). Similarly, ongoing oversight of investigator compliance with applicable professional standards, such as Good Clinical Practice, is a responsibility of the organization that employs the researcher and makes him or her available to conduct the research. The organization should establish an audit function for nonminimal risk research that flags violations and violators and reports its findings to the organization and to the Research ERB for remedy and communication with any sponsor as appropriate (see Chapters 5 and 6 for monitoring and QA discussions).
Finally, it is not the Research ERB’s responsibility to adjudicate issues regarding institutional liability, drains on institutional resources, or public relations. The committee emphasizes that the Research ERB is one element of a protection program; the organization responsible for the conduct of the research (in some cases, private sponsors) should subsume or appropriately delegate these distinct functions.
Improving Protections Through Appropriate Levels of Review
In recent years, the protocol review responsibilities of IRBs have received more focused attention at the national level (ACHRE, 1995; GAO, 1996, 2000; NBAC, 2001b; OIG 1998a,b,c,d,e; 2000a,b,c), and recent reports have highlighted the significant workload borne by the IRB system (AAU, 2001; AAUP, 2001; Bell, et al., 1998; GAO, 1996, 2000; NBAC, 2001b; OIG 1998a, 2000a). As the sheer diversity and volume of research studies have increased and IRBs have been asked to assume more and more
responsibilities, proposals have surfaced to relieve these boards of some of their more mundane and less controversial tasks. The intent of these proposals is to enable IRBs to focus on protocols that pose the greatest risks, and their recommendations often emphasize the need to establish policies that allow boards to conduct reviews in a manner commensurate with the nature and level of the risk involved and that allow senior staff to grant approval for studies that are clearly of minimal risk (NBAC, 2001b). In fact, the federal regulations provide several mechanisms by which certain kinds of research involving no more than minimal risk can be expeditiously reviewed;12 however, the fear of liability and the absence of clear federal guidance have caused some boards to hesitate to adopt such mechanisms.
Recommendation 3.3: The Office for Human Research Protections, with input from a broad spectrum of research disciplines and participant groups, should coordinate the development of guidance for risk classification.
A defined process for assigning levels of review commensurate with levels of risk is needed to help rationalize and systematize program functioning and to ensure that participants receive appropriate protection. Guidance incorporating specific examples regarding the interpretation of risk levels would be most useful to the research community.
Determining level of risk is central to ensuring that risks are minimized to the extent reasonably possible and that adequate protections are in place. It also provides the framework on which ethics review is based. The definition of “minimal risk” in the regulations13 is difficult to grasp and operationalize; examples that help to clarify this term are available in medical studies, but are seriously lacking in social and behavioral science studies (see Appendix B).
OHRP notes that some investigators fail to report studies that are more than minimal risk to IRBs (OHRP, 2000). The current culture of IRB review emphasizes local autonomy in making these decisions, but the current system, due to a lack of sufficient guidance and an environment of regulatory fear, has caused many boards to take few chances in independently determining the level of review required. In 2001, NBAC recom-
mended that “Federal policy should require research ethics review that is commensurate with the nature and level of risk involved” (2001b, p.41). However, a more refined formal stratification of studies, with guidance nuanced to match the different strata, is needed for protection programs. For example, a sample survey involving the risk of disclosure of information about a sensitive topic (e.g., sexual behavior, illicit drug use) raises different issues than would an experimental study of a medical intervention that is not life threatening (e.g., a moderate exercise program).
Research with humans can range from second-stage analysis of existing datasets, to anthropological studies of communities, to psychological research involving deception, to experiments involving potentially life-threatening interventions for serious illnesses in vulnerable populations. A major challenge facing any HRPPP is determining how to appropriately and efficiently manage this variety of activity. Although the same ethical principles apply to participant protection regardless of the methodologies employed, a “one size fits all” approach to the review and oversight of heterogeneous studies can cause inefficiency and frustration, which unacceptably diminish a program’s capacity to provide adequate protection.
Inefficiency results when excessive attention is paid to studies that involve minimal risk. Frustration results when the implementation of minor modifications to a minimal risk study is delayed because the protocol must be returned to the review board or when investigators are overruled by boards with limited understanding of the research context. The contrary result is that overwhelmed boards may not pay sufficient attention to high-risk studies that warrant careful and ongoing monitoring (NBAC, 2001b; OIG, 1998a,d).
Over the past few years, IRBs have strived valiantly to grapple with these issues, but the high volume of reports citing problems as well as discussions with the IRB community suggest to the committee that considerable inconsistency remains in how IRBs address these issues and whether they are being handled successfully.14
The committee therefore offers one possible scheme for explicitly recognizing the variations that can be expected during review, which involves dividing human research studies into three categories that might be labeled categories 0, 1, and 2:
-
Category 0 studies are exempted from regulatory oversight.
-
Category 1 studies should be submitted to Research ERB review, but are deemed eligible for expedited review.
-
Category 2 studies require full Research ERB review.
|
14 |
The committee gathered this and other information during two conference calls; one with IRB members and another with investigators. See Appendix A, “Methods,” for more information. |
Clinical trials could be stratified further according to whether or not they include a DSMB/DMC that conducts interim assessments of whether to terminate or continue a trial (see Chapter 5). Although less explicitly defined by the Common Rule,15 this distinction has clear implications regarding how research participants are protected; thus, it seems useful to divide Category 2 studies in two subcategories:
-
Category 2A studies receive full Research ERB review but no DSMB/DMC review.
-
Category 2B studies receive full Research ERB review and monitoring through a DSMB/DMC or other mechanism.
Two additional key questions pertain to the current state of stratification:
-
How effective and consistent is the current stratification of studies? That is, do investigators and Research ERBs have sufficient guidance to make the right decisions about which stratum is appropriate for any given study?
-
Is the current system of stratification adequate, or would a more refined form of staging be useful?
Systematic empirical evidence regarding these questions is lacking and needs to be developed, perhaps by using inter-rater reliability studies that compare the disposition of similar protocols across sets of review boards. Based on anecdotal evidence and testimony to the committee, it seems likely that current stratification practices vary widely and leave much to be desired (AAUP, 2001; Investigator conference call;16 NRC, 2001). Whatever the cause, the protection system is undermined by the too common occurrence of some boards exempting a study from review as allowed in the regulations,17 while other boards require review. Similarly, the granting of expedited review as allowed under the regulations18 is also practiced inconsistently. Previously, NBAC found these variations even among federal agencies that are signatories to the Common Rule (NBAC, 2001b). Review boards lack clear guidance about how to make these assessments and do not have sufficient educational materials available to assist in these determinations, which are key to ensuring adequate levels of protection.
The level of scrutiny of research proposals involving human partici-
|
15 |
45 CFR 46, Subpart A. |
|
16 |
See Appendix A for more information. |
|
17 |
45 CFR 46.101(b), 21 CFR 56.104. |
|
18 |
45 CFR 46.110, 21 CFR 56.110. |
pants needs to be calibrated with the degree of risk, to ensure that studies involving minimal risk are handled efficiently and studies involving high risk receive the careful attention they require. However, board members generally have little guidance for weighing the risks and benefits of research, as classifications of studies by level of risk currently lack refinement and consistency. Further, the fear of being found “noncompliant” by regulators has led boards (and programs) to be overly conservative in utilizing the flexibility available to them within the current mandates. While there is on-going work to devise practical methods for determining risk (Barnbaum, 2002), this issue is of such importance that this committee, as well as the Committee on National Statistics/Board on Behavioral, Cognitive, and Sensory Sciences and Education panel, believes federal intervention and guidance is warranted (see Appendix B, Recommendations 7 and 8). Therefore, the committee recommends that OHRP engage representatives of the scientific, participant, and policy communities in activities designed to develop illustrative and practical guidance for risk classification.
These activities could be used as means to move the interaction between research organizations and regulators from a focus on the punitive aspects of compliance toward a proactive conversation that emphasizes continuous improvement in the participant protection system (see Chapter 5 for further discussion).
Research Ethics Review Board Focus on Informed Consent
Recommendation 3.4: Forms signed by individuals to provide their legally valid consent to participate in research should be called “consent forms” rather than “informed consent forms.” Research Ethics Review Boards should ensure that the focus of the informed consent process and the consent form is on informing and protecting participants, NOT on protecting institutions.
The ethical foundations of research protections in the United States can be found in the three tenets identified in the Belmont Report: respect for persons, beneficence, and justice (National Commission, 1979). To assure that these principles are followed, two protections are considered fundamental to the ethical conduct of research involving human participants— informed consent and independent review by an IRB. Indeed, IRB review has traditionally included an assessment of the protocol informed consent procedure and its documentation.19 However, the committee believes it is important to distinguish between the processes in place to facilitate in-
formed decision making and the instrument for obtaining legally valid consent (the consent form). Because the facilitation of decision making is what enables potential participants to make informed and autonomous choices, the ethics review process should be reformed to emphasize this goal as paramount by ensuring that the process (as well as the content) of informed consent is based on an exchange of information and ongoing dialogue. This conclusion is based on two trends that were described anecdotally to the committee during its meetings, as well as on the experiences of committee members themselves (Levine, 2001b; Sugarman, 2001).
First, traditional informed consent often does not appropriately inform and empower the participant, because the information in the consent document increasingly serves institutional rather than participant needs. In other words, consent forms have been hijacked as “disclosure documents” for the risk management purposes of research organizations. In the event of a negative outcome and legal action, the plaintiff is generally the individual participant (or his agent), and the defendant is the research organization where the research was conducted. For this reason, heavy pressure is exerted on those organizations to build waivers into the consent form for all possible negative outcomes as a means of self-protection and/or risk management (Annas, 1991). The effect on the participant, however, is that the consent document can become of marginal relevance to his or her protection during the research study (see Chapter 4), and it may be highly technical and overly detailed. The paradox is that the mechanism for allowing participants to protect themselves from risks inherent to a specific protocol has become intermingled with the mechanism that institutions utilize to protect themselves from a different set of risks, with the interests of the institution often overwhelming those of the participant. The role of the Research ERB in this process should be as an advocate for the participant, not the institution.
The committee and others have formulated examples of methods to address this problem. One involves “layered approaches”—an initial simple and clear statement accompanied by one or more increasingly detailed explanations of core elements of consent that may be studied to the degree desired by potential participants (NAPBC, 1996; NBAC, 1999). Another approach would be to place information regarding legal and business matters, such as indemnification agreements, which represent “disclosure documents” and which are clearly distinct from the protocol-specific consent form, in attached appendixes.20
Whatever approach is taken, the goal is to encourage simplification of the consent form as part of a program’s QI efforts, to urge accreditation agencies and federal regulators to acknowledge the legitimacy of seeking such improvements, and to encourage the toleration of deliberate variation in the strategies employed to assure protection (including alternative methods for providing information within the informed consent process and for obtaining legal consent—e.g., videos or Web-based tools).
In biomedical research there is the potential for the therapeutic misconception to contribute to a participant’s failure to understand the issues presented to them through the informed consent process (Appelbaum et al., 1982; Churchill et al., 1998; King, 1995). In their 1982 paper, Appelbaum and colleagues introduced this term to focus attention on the problem that many participants do not always understand the differences between research and treatment. In fact, many participants think that by participating in a research protocol, they are receiving treatment designed by a physician with their best interests in mind, when in fact the work is driven by the demands of the research protocol.
The Research ERB should take responsibility for ensuring that participants are voluntarily participating in research and that they understand the uncertainty of potential benefits. However, Research ERBs face a dilemma when participation in a trial provides to individuals health care services that are otherwise unavailable to them, particularly in instances in which the risks are minimal.21
Research ERBs should ensure that consent forms contain study-specific information relevant to a potential participant’s decision to enroll, including information related to the nature of the study, the level and nature of its risks, reasonable expectations of benefit(s), alternatives to the research, clarification that this is research—not treatment, relevant investigator or institutional conflicts of interest, and opportunities for recourse in the event of problems during the course of the study. Research ERBs should require that disclosure language regarding liability, indemnification, and business agreements be removed from the consent form altogether, or at a minimum, be moved to an appendix in order to clearly distinguish the informed consent discussion from risk management information. Furthermore, accord-
|
21 |
As noted in Chapter 1, such situations raise complex ethical and policy questions that are beyond the scope of this committee to fully consider. However, they should be pursued by appropriate groups to provide guidance to Research ERBs. |
ing to revisions announced for the Rule implementing the Health Insurance and Portability Accountability Act of 1996 (HIPAA), Research ERBs may also utilize protocol consent forms to ensure that the appropriate provisions for protecting participant privacy and confidentiality are described to potential volunteers (see Chapter 7 for further discussion regarding the impact of HIPAA requirements). However, such additional language is contrary to the committee’s goal of simplifying consent forms.
Finally, the Research ERB should emphasize that the act of obtaining informed consent from a potential research participant should be interactive and ongoing and that obtaining written “informed consent” is tangential to the process of informed consent and merely provides a mechanism to document and record that communication with the participant regarding relevant considerations to enrollment in a protocol has taken place.
Research Ethics Review Board Membership, Qualifications, and Voting System
The effectiveness of the review process depends on the experience and commitment of board members. Reviewers should be able to make complex judgments that depend on an elaborate scientific and intellectual calculus that requires both the ability to assess the ethical appropriateness of the research design and methodology and an awareness of the important elements that affect the ability of potential participants to refuse or consent to enroll. Board members should be especially well grounded in ethics and community values, given their primary function of assessing a scientifically validated protocol in terms of its ethical soundness. In addition, board membership must be diverse, representing scientific and nonscientific, institutional and noninstitutional interests.22 Recruiting individuals who can meet all of these needs presents a major obstacle for many programs. Attempting to create the “perfect” Research ERB is a challenge that consumes much of the time of board chairs and administrators and creates a great deal of frustration (IRB conference call).23
Current federal regulations require that each “IRB have at least one member who is not otherwise affiliated with the institution and who is not part of the immediate family of a person who is affiliated with the institution.”24 The regulations also require that each IRB include “at least one member whose primary concerns are in scientific areas and at least one member whose primary concerns are in non-scientific areas.”25 However,
|
22 |
45 CFR 46.107, 21 CFR 56.107. |
|
23 |
See Appendix A, “Methods,” for more information. |
|
24 |
45 CFR 46.107(d), 21 CFR 56.107(d). |
|
25 |
45 CFR 46.107(c), 21 CFR 56.107(c). |
it is not clear that these current requirements and practices regarding board membership adequately serve the system.
Recommendation 3.5: The Research Ethics Review Board composition and the qualifications of its chair in particular should reflect its unique and preeminent responsibility to provide a thorough ethical review of proposed research studies. At least 25 percent of its membership should be reserved for unaffiliated members and those who can provide non-scientific perspectives.
While all members should have a core body of knowledge, a critical mass of the membership (i.e., a sufficient number of members to influence the tenor of the discussion), either scientific or nonscientific, should possess a specialized knowledge of human research ethics (see Figure 3.2). In addition, the goal of research organizations should be to assemble a board with at least 25 percent of its membership not affiliated with the institution, not trained as scientists, and able to represent the local community and/or the participant perspective. The committee encourages the Research ERB community to work toward developing common goals and content for Research ERB member training, so that within five years, certification programs might be available to demonstrate the initial and continuing qualifications of members who serve within participant protection programs.
Currently, the regulatory language and guidance that describe the criteria for meeting the definition of an unaffiliated member, for determining how long such members should serve and under what circumstances they may be removed, or for determining what payment should be provided for members who are otherwise not affiliated with the institution are insufficient (NBAC, 2001b). Also, nonscientific, unaffiliated members can represent either the community being studied or the community in which the research will take place. However, it is sometimes not clear whose interests such members represent and if one individual can represent more than one community (Hogg, 2001; MacQueen et al., 2001).
In order to address the lack of guidance, NBAC recommended that
Institutional Review Boards should include members who represent the perspectives of participants, members who are unaffiliated with the institution, and members whose primary concerns are in nonscientific areas. An individual can fulfill one, two, or all three of these categories. For the purposes of both overall membership and quorum determinations 1) these persons should collectively represent at least 25 percent of the Institutional Review Board membership and 2) members from all of these categories should be represented each time an Institutional Review Board meets (2001b, p.64).
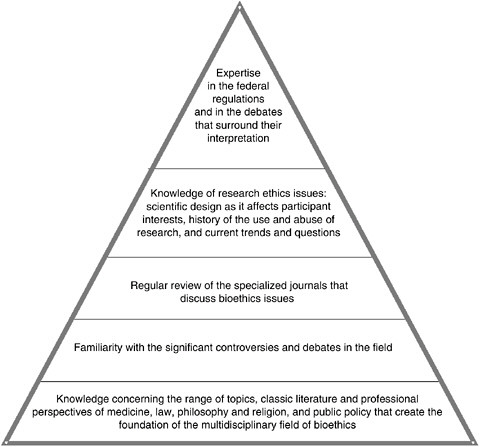
FIGURE 3.2 Content Knowledge Intrinsic to Human Research Ethics
The requisite expertise, or “specialized knowledge,” which the committee believes should be available to (or preferably found within) the membership of a Research ERB can be broken down into various levels of familiarity with the topics listed above. The depth of knowledge necessary in the particular areas correlates to the position of the issue within the pyramid, moving from the base to the top tier.
The committee agrees that the composition of the Research ERB should reflect its ultimate function as the ethical review body for human research protocols. Science and ethics expertise should be appropriately balanced with each other and with noninstitutional member perspectives. Review boards could better avoid conflicts by including a greater proportion of individuals who are unaffiliated with any of the institutions/firms involved in the research study.
Research ERB Voting System
Modern IRBs have tended to become larger as members have been recruited to reflect the broad range of scientific expertise needed to conduct informed scientific review of a diverse array of research protocols (OIG, 1998a). Consequently, some deliberations have the potential to be dominated by a scientific voice, leading to a corresponding reduction in ethics-centered reflection and the possible marginalization of the perspectives of nonscientific members (Cho and Billings, 1997; Peckman, 2001). Under the committee’s proposed refocusing of the ethics review, responsibility for the initial in-depth scientific review would lie elsewhere, enabling the Research ERB to focus on its primary functions of providing ethical review of proposed research and integrating the scientific and financial conflict of interest reviews. Consistent with this mission, the most important roles of the board chair are to constitute a proper and balanced membership, facilitate open discussion, and promote consensus. In fact, it is not required or always the case that board chairs themselves be trained physicians or scientists (Jacobs, 2001).26
Recommendation 3.6: A Research Ethics Review Board’s deliberative process should aim for consensus. If consensus cannot be achieved, approval of a protocol should require favorable votes by three-quarters of the voting members.
If the membership of the Research ERB is modified to reflect the increased number of unaffiliated and non-scientific perspectives as suggested in Recommendation 3.5, but a simple majority voting procedure remains in place, no actual mechanism or basis for change will have been established and only the appearance of change will have been created. Such a scenario would tend to divide the deliberative body, encourage power blocks and leave the less powerful perspectives in the minority. Therefore, an alternative to standard parliamentary process is needed.
Seeking consensus can be a way of acknowledging, exploring, and managing conflict and is a process that would facilitate the full expression of the minority perspective(s) and provide the basis for substantive discussion and debate. It also requires chairs who are skilled in techniques of mediation and conflict resolution. The process of facilitating consensus requires skills that include listing the players/parties who represent competing interests, characterizing their stakes and goals, identifying and maxi-
mizing the options that might be employed to accommodate competing interests, and reaching agreement on this new solution.
The committee acknowledges that sometimes mediation and the search for consensus will fail and that at some point a vote may need to be taken. The committee therefore recommends that the Research ERB adopt super-majority rules stating that no protocol will be approved unless it has the affirmative endorsement of at least three-quarters of the voting members present. A vote of unanimity would be unworkable and would effectively give a veto to a single dissenting committee member, while allowing a simple majority to approve a protocol in the face of substantial minority opinion to the contrary poses too much risk of suppressing respectable and responsible ethical opinion.
Research ERB Member Education
The need to educate board members has been discussed by every major national body charged with the review of human research protections, with each clearly stating the need for additional resources and educational programs (ACHRE, 1995; National Commission, 1978; NBAC, 2001b; President’s Commission, 1983). In addition, a 1998 survey documented strong support for increased board member education (Bell et al., 1998).
Training programs at the national, regional, or institutional level could provide education to Research ERB members about the required core body of knowledge (Box 3.4 expands this point with a biomedical focus). Although the specific content will vary based upon the portfolio of the Research ERB, the basic elements will remain constant. Currently, Public Responsibility in Medicine and Research (PRIM&R) offers a course known as “IRB 101” to train board members. PRIM&R, in cooperation with OHRP, also has produced a CD-ROM version of “IRB 101.” NIH offers a computer-based training course27 designed for NIH board members but also accessible to the public. In addition, some universities include ethics training in research design and history of science courses.
The inclusion of the perspectives of individuals not affiliated with a particular research organization in all decision-making and oversight bodies, particularly the Research ERB, is a vital component of any protection system. Specialized training for these members can help maximize their contribution to the process.
Such training could include the following:
|
27 |
Available at ohsr.od.nih.gov/irb_cbt. |
|
Box 3.4 Background: Ethical perspectives on the research enterprise, history of human research, current structure and funding of research, identifying who conducts and pays for research, setting the research agenda, methodological issues, current regulatory structure and the Common Rule. Foundation Knowledge: An introduction to clinical medicine, basic science, epidemiology, introduction to ethical principles, concepts, and issues, and skills, such as reading scientific literature and research protocols. Methodological Issues: Identifying the elements of research design, thinking about statistical power without statistical or mathematical training, the use and abuse of the placebo model, randomization before or after consent, and adequate use of animal models and competent persons before investigations with vulnerable or incapacitated persons. Difficult Design Questions: Questions of placebo arms in general, in psychiatry protocols, and as required by the FDA for review when effective treatment exists; testing of “me too” drugs when effective treatment exists and when the desire is for a more or a less expensive treatment; stratification of subjects and treatments; use of children, fetuses, or prisoners, whose involvement in research is governed by a specific sub-set of federal regulations; investigations with incapacitated persons whose use in research is subject to state law and regulation and to institutional policy in the absence of clear federal policy. Conflicts of Interest: How to think about the relationship of institutions, investigators, and participants in light of the monetary and status benefits that institutions and investigators can expect from the research, and how to manage and minimize possible conflicts. Cultural Competency: How to understand the design issues that will either encourage or discourage participation by persons of color and other vulnerable populations that have a history of being suspicious of the research enterprise. Reviewing Research Proposals—The Intellectual Calculus: Determine if a proposal presents an important question; identify who is included and excluded and why—women, children, racial minorities, prisoners, and other vulnerable populations; the risk-benefit calculus; informed consent; conflicts of interest; confidentiality; monitoring and review. Overarching Issues: International research, genetic and stem cell research, AIDS research, research with children, research in the name of national security. The Responsibilities of Research ERB Members: Ethics review, protocol assessment, research participant advocacy, accountability, community perspective. |
-
A detailed description of the process of research, the identity and roles of all who are involved, and the components of a research study;
-
A description of the process within a specific institution, including scientific review and conflict of interest review;
-
Rules of scientific ethics (and broader theories of ethics as well).
It is preferable that these training programs be designed, funded, and owned by consumer or community oriented nonprofit organizations. However, collaborations of such organizations with scientific trade associations or research organizations might also be sufficient to provide these programs.
Several programs developed by consumer groups provide intriguing models for supporting and training unaffiliated members on Research ERBs or any other research oversight committee, such as programs developed for medical specialty boards by the Citizen Advocacy Center or those targeted to the research setting by Project LEAD (Leadership, Education, and Advocacy Development) and the National Alliance for the Mentally Ill (CAC, 1994; Cowdry, 2001; Dickersin et al., 2001; Hinestrosa, 2001; NAMI, 2001; Swankin, 2001).
Certification of Research ERB Professionals
Certification for Research ERB members (and investigators) has been a problematic issue, in part because they are predominantly scientists and traditionally belong to subspecialty professional organizations. However, IRB administrators are now being certified by the Council for Certification of IRB Professionals; through the National Association of IRB Managers; and as Certified IRB Professionals, through the Applied Research Ethics National Association in conjunction with the Professional Testing Corporation. The committee supports these efforts because they encourage the development of professional staff who can facilitate the ethics review function of the Research ERB.
ORGANIZING AND INTEGRATING THE REVIEW PROCESSES
In this chapter, the committee has proposed that separate and independent scientific review and conflict of interest review mechanisms should be available to the Research ERB, and that relevant findings from those reviews should be communicated to the Research ERB to inform its comprehensive ethical assessment. It is the responsibility of the program to ensure that this process occurs. Under this new vision, Research ERBs would review the research protocol, the written and signed findings of the scientific review and financial conflict of interest review bodies, and the oral
reports given by members of these bodies as appropriate. In the final stage, the Research ERB would integrate this information and determine the acceptability of the proposed research. A critical factor in making this three-pronged review process successful is to ensure that it is not unduly extended, complicated, or confused by these improvements. Participant interests are not served if research protocols are unnecessarily delayed. Therefore, in establishing review mechanisms, it is important to remain responsive to incoming applications by aligning submission and meeting dates for the preliminary reviews with the Research ERB schedule. Furthermore, communication between investigators and the review structure should continue to originate with the Research ERB office, except in instances of identified problems at the initial review levels, in order to prevent duplication of effort and avoid confusion resulting from overlapping and possibly contradictory messages to investigators about a protocol’s status. Likewise, efforts should be made to align and consolidate other administrative procedures within the HRPPP—e.g., the creation of one master application for the submission of protocols to all appropriate review bodies.28 If the functional integration of review activities is to successfully improve protection, it must be mirrored by a similar integration of administrative activities.
Review of Multisite Studies
In addition to ensuring coordination and communication between the various review groups, special consideration should be given to protocols conducted at more than one site, and possibilities for alternative review mechanisms should be considered.
Recommendation 3.7: The review of multisite trials should be streamlined, as allowed by current regulations. One primary scientific review committee and one primary Research Ethics Review Board should assume the lead review functions, with their determinations subject to acceptance by the local committees and boards at participating sites.
The rapid growth of multisite studies, particularly in the clinical trials arena, brings a new complexity that challenges the ability of review committees to meet their responsibilities efficiently and effectively. Multisite protocol review has become a cumbersome and labor-intensive process, because in most cases each research organization’s board considers the
|
28 |
For example, Children’s Hospital Regional Medical Center in Seattle has devised a combined electronic application for the purposes of the General Clinical Research Center Advisory Committee and the IRB. In this instance, the electronic format will enable investigators to complete relevant information and avoid irrelevant questions, through a series of drop boxes. The form will be posted at www.seattlechildrens.org/research after October 2002. |
same protocol; performs the same risk assessment; examines the same, or similar, consent form; and later reviews the same, often voluminous, set of adverse event reports. In addition, multisite review can introduce considerable variability into the approvals and/or required modifications to study design or disclosure language, which actually detracts from participant protections. At best, it is an inefficient use of time and money for the review boards, investigators, and sponsors. The time involved in these reviews could be better spent attending to the participant protection needs of other studies or, in the case of the investigator, interacting with participants.
In general, the review of multisite studies by each organization participating in a study does not significantly increase the level of protection provided to research participants or enhance the scientific design of the protocol. In fact, this repetitive review can be detrimental to the protection of the other research participants within a program, as the review board is unable to provide the needed time, resources, and expertise to thoroughly evaluate other protocols.
Current federal regulations and guidance for IRBs contain provisions for the sharing of oversight responsibility with institutions in which regular collaboration takes place—including the ceding of authority for the reviews.29 In 2001, NBAC recommended that alternatives be considered for multisite review, including the use of central or lead review boards (2001b). In a multisite project, the sites could designate an independent Scientific Review Committee (SRC) and Research ERB or could designate one of the SRCs and Research ERBs as the “primary SRC and Research ERB.” The organization that has primary responsibility for obtaining the assurances should also assume the responsibility for acting decisively should violations occur, including termination of the study or the site and/or reporting violations and violators to authorities. In FDA-regulated trials, it should not be assumed that the industry sponsor has primary responsibility for the program; it would be preferable for the medical institutions involved to share that responsibility, since they are most directly and closely involved with the research participants. In addition, determinations regarding potential financial conflicts of interest should be forwarded to the lead Research ERB by the appropriate entity (i.e., the party responsible for the oversight of an investigator’s role in a project). Sponsors and federal regulators should encourage such collaboration and centralization, and local committees should reserve the right to refuse the primary review body’s determination for serious safety concerns and unique local requirements.
Under this system, the opportunity and responsibility for locally appropriate oversight would continue to be intrinsic to protocol review, and
some institutions might insist on being involved in the review process for reasonable instances of indemnification and confidentiality. The committee believes, however, that flexible approaches might provide helpful alternatives to reduce redundancy that does not enhance participant protections. Effective use of communication tools, such as the use of protocol Web sites that would enable local Research ERBs to follow the lead Research ERB’s progress, will be fundamental to successful collaboration.
Sharing Responsibilities: Alternative Review Models
Models exist in the United States for sharing program responsibilities among collaborating institutions and their Research ERBs—for example, MACRO and BRANY.30 Similarly, the United Kingdom relies on regional committees for review of multisite research, and Denmark handles multisite studies by assigning the review responsibility to a lead committee (Alberti, 2000; Holm, 2001). These approaches can reduce duplicative workload and assure that reviews take place in settings that can bring to bear the appropriate scientific and ethical expertise. For example, complex protocols may involve consulting with biostatisticians, epidemiologists, and clinical specialists who might not be available at some individual sites.
The ability to distribute costs could also place a regional program in a better position to provide the resources and infrastructure needed for various functions, such as maintaining qualified monitors for higher-risk research involving human participants. Furthermore, by ceding certain responsibilities to a regional unit, local programs could direct their efforts and resources to the remaining single-site studies for which they are responsible. This could be particularly useful to research organizations that have few resources, including small academic centers and community hospitals.
In addition, regional or centralized review could provide a cost-effective alternative to smaller institutions and study sites that cannot afford to maintain a sufficiently comprehensive program onsite. Such organizations, for example, may find it difficult to sustain Research ERBs with the associated increased costs for training, monitoring, and, should it become a standard of practice in the community, accreditation preparation and the asso-
|
30 |
MACRO is a collaboration between Baylor College of Medicine, University of Alabama at Birmingham, the University of Pennsylvania School of Medicine, Vanderbilt University, and Washington University School of Medicine. See ccs.wustl.edu/macro/aboutmacro.htm for more information. BRANY focuses on sites near New York City. The Alliance serves more than 100 sites in multiple states, from New Jersey to Hawaii. For more information, see www.brany.com. |
ciated fees. Possible examples include smaller hospitals, community-based organizations, local health agencies, and CROs.
Regional programs/Research ERBs offer a solution to another set of problems encountered by boards today—the difficulty of recruiting and maintaining active and involved members. Many recent reports have noted the problems of heavy workloads, lack of professional or personal reward for committing the time needed to do the work, problems of ascertaining and managing conflict of interest within small organizations that may have a limited choice of potential board members, and a host of similar problems (IRB teleconference;31 Levine, 2001a,b; Moreno et al., 1998; OIG 1998a). Of course, regionalized approaches would need to include an equitable fee or honorarium structure to cover the expected services of board members and of expert consultant reviews needed in specialized topic areas and would need to extend liability insurance coverage to all involved in the process (see Chapter 6).
Utilizing regional Research ERBs would also alleviate the potential conflict of interest inherent to the use of internal review boards. To encourage institutions conducting or sponsoring research to develop shared arrangements or use regional programs and to make Research ERBs more comfortable with ceding authority (but not ultimate accountability), adequate communication between local and regional programs will need to be assured. Research organizations should be kept abreast of the status and progress of studies, adverse events, media coverage, and other matters relevant to the organization, while keeping the reporting burden reasonable for the regional programs. Obviously, such a scenario could draw on the experience of the independent IRB review model.
RESOURCE NEEDS
The most important aspects of the review process depend on the experience and commitment of those who conduct the reviews. The processes of protecting human participants, which require skill, learning, wisdom, and sufficient practical experience to make complex judgments, are not tasks that can be assigned exclusively to junior faculty members or unskilled researchers. Time and focus are needed to accomplish these tasks, and if their difficult and demanding nature is to be taken seriously, the members—and not just the administrative staff—of scientific review groups and Research ERBs should be compensated for their efforts. This compensation may be monetary, may support academic promotion, or may provide release time from other duties. If the ethical review of research involving human participants is to be adequately conducted, appropriate resources
|
31 |
See Appendix A, “Methods,” for more details about the teleconference. |
should be committed to the process. These considerations apply equally to those involved in scientific review, financial conflict of interest review, and ethics review of proposed research.
Need for a Common Body of Knowledge
Recommendation 3.8: The Office for Human Research Protections and the National Human Research Protections Advisory Committee should convene conferences and/or establish working groups to develop and disseminate best practices, case presentations, and conference proceedings for use by local protection programs and their Research Ethics Review Boards.
Intellectual and educational resources are as important as financial resources in ensuring protections. The current protection system emphasizes local control and archiving of collective wisdom in the decision-making process for approving human research protocols. A more systematic literature and “case presentation” approach is needed for educational purposes and to promote consistency and high-quality decision making within Research ERBs. The lack of consistency among boards regarding, for example, the interpretation of the definition of minimal risk or investigator education requirements, can lead to contradictory—and often unproductive—directives to investigators that detract from time interacting with participants and overseeing research staff (investigator teleconference).32 Steps should be taken to improve the consistency of review board practices and regulatory interpretations through the dissemination of best practice guidelines; consensus conferences involving board chairs, investigators, ethicists and participant groups; and eventually, the building of a rich database of case dispositions analogous to case law in the legal arena. Such activities could also be useful in the exploration of complex ethical situations, such as research with children. The National Human Research Protections Advisory Committee (NHRPAC)33 has begun to address this void within the research review community through working groups focused on social and behavioral sciences and research with children.34 However, their work alone cannot be expected to meet this need.
The availability of such materials would assist Research ERBs and other review bodies in making difficult decisions by aggregating institu-
|
32 |
See Appendix A, “Methods,” for more information. |
|
33 |
As this report went to press, it was reported that DHHS had disbanded NHRPAC (Weiss, 2002). Committee discussion and recommendations directed to NHRPAC should now be directed to any future advisory body constituted to address participant protection issues. |
|
34 |
See ohrp.osophs.dhhs.gov/nhrpac/nhrpac.htm for further information about the National Human Research Protections Advisory Committee. |
tional experiences and creating precedent cases. Although it is anticipated that the continued development and implementation of accreditation programs will contribute to the dissemination of best practices, this mechanism cannot substitute for the recommended deliberations at the national level. OHRP and NHRPAC, with input from the FDA to ensure that issues particular to drug, biologic and device research are incorporated, should initiate such efforts forthwith.
SUMMARY
The adequate review of research to ensure that human research participants are protected involves the evaluation of several factors that require specific expertise. The IRB structure should be redefined and renamed the “Research ERB” to assert its mandate to conduct ethics reviews on protocols on behalf of those who will eventually be enrolled in studies. However, the newly configured Research ERB cannot be expected to carry out all of the specialized tasks required of a comprehensive protection program. Therefore, when appropriate to the research risks and context, scientific and financial conflicts of interest review should occur through distinct mechanisms that feed into and inform the Research ERB’s comprehensive ethical review of research.
In order to carry out the responsibilities that should be under the purview of the Research ERB, all members should have core knowledge regarding human research ethics. In addition, at least 25 percent of the body’s membership should have no affiliation with the institution, not be trained as scientists, and be able to represent the local community and/or participant perspectives. To ensure that no study goes forward if a substantial portion of the Research ERB objects, no protocol should be approved absent three-quarters of the voting members’ agreement.
One of the primary responsibilities of the Research ERB is the review of consent forms and the informed consent process. The board should ensure that the consent forms convey information relevant to the participant’s decision about whether to enroll and limit, or preferably delete, any language that would serve only to protect the institution.
Robust, ethical review by the Research ERB could be enhanced by better employing risk-stratification, allowing boards to deal with minimal risk studies efficiently and devote more attention to high-risk studies. The review of multisite protocols could be streamlined by designating lead scientific review and ethics review committees, whose judgments would be subject to acceptance by the local review boards (this would include the sharing of conflict of interest determinations with the lead Research ERB). A common body of knowledge and guidance should be developed at the national level to assist review boards in their deliberations and promote consistency among the research oversight community.






































