Executive Summary
ABSTRACT
The protection of individuals who volunteer to participate in research is essential to the ethical conduct of human research. In response to mounting concerns about the well-being of research participants and the ability of current approaches to ensure their protection, the Department of Health and Human Services commissioned the Institute of Medicine to perform a comprehensive assessment of the national system for providing research participant protection. The resulting analysis emphasizes the responsibilities and functions of human research participant protection programs (HRPPPs), providing substantive descriptions of the activities intrinsic to a robust protection program. In its work, the committee suggests a systems approach to providing protection, offers several broad recommendations for reform, and proposes practical suggestions to improve the oversight of human research at the institutional level.
In the committee’s framework, the HRPPP is a system composed of interdependent elements that come together to implement policies and practices that ensure appropriate protection of research participants. The exact structure of an HRPPP will vary among research organizations and protocols according to the protection needs intrinsic to a particular study. Despite this flexibility, however, there are basic protection functions necessary to ensure the safety of participants and it is essential that all be met. These functions include: comprehensive review
of protocols (including scientific, financial conflict of interest, and ethical reviews); ethically sound participant-investigator interactions; on-going and risk-appropriate safety monitoring; and quality improvement and compliance activities. Furthermore, to be effective, HRPPPs should operate within environments that emphasize accountability for the provision of participant protection, assure adequate resources for robust protection activities, provide ethics education programs to those conducting and those overseeing research with humans, and seek open communication and interaction with all stakeholders in the research enterprise.
A series of recommendations focuses on improving ethics review of protocols, reforming the informed consent process, improving access to information by participants and those responsible for review and monitoring of protocols, enhancing safety monitoring, compensating those who are harmed as a result of their participation in research, and developing a standard of quality improvement in HRPPPs.
Recommendations focused at the national level include: extending federal requirements for protection to include every research project involving human participants, regardless of funding source or research setting; collecting, assessing, and disseminating data about the overall system; and establishing an independent, nonpartisan advisory body that includes the perspectives of participants, scientists, ethicists, and research administrators to ensure that the national protection system receives objective and ongoing assessment.
In response to mounting concerns about the well-being of research participants1 and the capability of current procedures to ensure participant protection, the Department of Health and Human Services (DHHS) commissioned the Institute of Medicine (IOM) to perform a comprehensive assessment of the national system for providing participant protection. Specifically, the IOM was asked to make recommendations regarding mechanisms to improve the structure and function of protection activities, as well as ways to continually evaluate performance of these activities. This in-depth analysis was intended to emphasize the responsibilities and functions of the individual human participant protection program (not restricted to Institutional Review Boards [IRBs]) and was to include the prospect of accreditation as a useful tool to achieve the desired performance improvements. This task was broken into two phases.
The first phase of work by the Committee on Assessing the System for Protecting Human Research Participants (“the committee”) focused almost exclusively on accreditation. While examining the issues relevant to this subject, the committee introduced the concept of the Human Research Participant Protection Program (HRPPP) as the appropriate functional unit to implement and oversee protection functions. The committee’s first report, Preserving Public Trust: Accreditation and Human Research Participant Protection Programs, provided a foundation for establishing an HRPPP, but was unable to provide much more than a sketch of the intended program (IOM, 2001a). The current report represents the culmination of the committee’s deliberations and provides substantive descriptions of the functions and responsibilities intrinsic to a robust participant protection program.
In phase two, the committee was charged with the following tasks:
-
Review the ethical foundations for protecting human participants in research.
-
Assess and describe the current system for protecting human participants and make recommendations for potential enhancements and improvements to
-
ensure informed consent,
-
monitor ongoing research,
-
accommodate private IRBs, multicenter research, and non-medical research,
-
ensure continuous improvement in the system, and
-
educate researchers, participants, and others involved in research with human participants.
-
-
Assess the potential impact of recommended changes on resource needs and how to address them.
-
Consider the effects of accreditation on improving human participant protection activities.
-
Determine the need and develop potential mechanisms for on-going independent review of the national system.
Many of the issues and policies pertinent to the committee’s task have been in flux, with a number of commissions and organizations, including research institutions, professional associations, and the federal government, all working to find solutions to previously identified problems. In addition to countless news stories highlighting and influencing public discussion,2
reports have been issued,3 accreditation programs have been launched,4 privacy regulations have been promulgated and revised,5 Congressional hearings have been held,6 and legislation has been drafted.7 This committee has endeavored, however, to formulate recommendations that reflect the current state of policy development, the present regulatory framework, and the efforts undertaken by others.
In contrast to other reports on these issues, this committee focused on the roles and responsibilities of the individual HRPPP, with the majority of recommendations directed toward improving the protection of the individual research participant through HRPPP policy and procedural enhancements.
STATEMENT OF THE PROBLEM
As recently detailed by the National Bioethics Advisory Commission (NBAC), many highly regarded groups have assessed the strengths and weaknesses of the national system for ensuring the ethical protection of volunteer research participants.8 Proposals for reform have been presented to the public, the Executive Branch of the federal government, and Congress. However, a fact that has repeatedly confounded this committee’s deliberations is the lack of data regarding the scope and scale of current protection activities. This absence of information seriously handicaps an objective assessment of protection program performance and needs and the development of useful policy directions. Nonetheless, the evidence is abundant regarding the significant strains and weaknesses of the current system, and this committee has reached the conclusion that major reforms are in order.
First, significant doubt exists regarding the capacity of the current system to meet its core objectives. Although all stakeholders agree that participant protection must be of paramount concern in every aspect of the research process, a variety of faults and problems in the present system have been noted. The common finding is that dissatisfaction with the current system is widespread.
Second, it has been shown that IRBs are “under strain” and “in need of reform” (AAU, 2001; GAO, 1996; Levine, 2001a; NBAC, 2001b; OIG 1998a, 2000a). The complexity of the issues, the variability in the research settings, the limitations of funding options, the demands of investigators and participants for access to research, and the accountability for institutional compliance have magnified and complicated IRBs’ responsibilities. This heavy burden has made it difficult both to recruit knowledgeable IRB members and to allow them sufficient time for the necessary ethical reflection.
Third, the existing regulatory framework (i.e., the Common Rule9 and the IRB system it created) cannot adequately respond to the complex and ever-changing research environment, with weaknesses related to gaps in authority, structure, and resources. Some of the problems can be addressed through interpretive guidance and clarification issued by the pertinent federal agencies and offices and through collaborations between federal authorities and private entities. However, in instances relating to deficiencies in authority, Congressional action is needed.
MAJOR RECOMMENDATIONS
The major recommendations of this report aim to ensure the protection of every research participant. The committee envisions a three-part strategy to achieve this goal, including refocusing the mission of the IRB on the thorough ethical review and oversight of research protocols; recognizing research participants’ contributions and integrating them into the system; and maintaining high standards for and continuing review of HRPPP performance. Specific recommendations are organized around these themes in the following summary. By contrast, the report itself has been sequenced according to the natural progression of the research process. Additionally, two tables at the conclusion of this summary categorize the committee recommendations into those that will require direct government action and those that will not (Tables ES.1 and ES.2). The tables also indicate which agencies, organizations, offices, or individuals have the primary responsibility for the implementation of each recommendation.
Protect Every Research Participant
The protection of research participants is fundamental and should remain paramount to any research endeavor. In today’s complex research environment, appropriate protection can most effectively be provided through a program of systematic and complementary protection functions within which roles and accountability are clearly articulated. HRPPPs, henceforth referred to as “protection programs” or simply “programs,” should be viewed as a system of interdependent elements that involve the research organization, the IRB, the investigators, the sponsors, and most importantly, the volunteer participants. Redundancies in the system that do not add value should be eliminated. To this end, each research entity should develop clear, efficient, and effective processes and procedures. In addition, open and defined communication among those involved in participant protection should be established and maintained.
The diligent application of HRPPP policies and practices will ensure that participants in any research project are protected against undue risk, that informed consent to participate in the research is provided, and that all efforts are made to ensure that participants’ rights, privileges, and privacy are protected throughout the entire research process. Therefore, protection requirements should be extended to include every research project that involves human participants, regardless of funding source or research setting.
The specific structure of a protection program is secondary to its performance of several essential functions. These functions include:
-
comprehensive review of protocols (including scientific, financial conflict of interest, and ethical reviews),
-
ethically sound participant-investigator interactions,
-
ongoing (and risk-appropriate) safety monitoring throughout the conduct of the study, and
-
quality improvement (QI) and compliance activities.
Recommendation: Adequate protection of participants requires that all human research be subject to a responsible Human Research Participant Protection Program (HRPPP) under federal oversight. Federal law should require every organization sponsoring or conducting research with humans to assure that all of the necessary functions of an HRPPP are carried out and should also require every individual conducting research with humans to be acting under the authority of an established HRPPP. (Recommendation 2.1)
Establish Accountability Within an Ethical Research Culture
Ultimate responsibility for the adequacy of an HRPPP resides at the highest level of an organization. An effective protection program requires the unequivocal support of the leaders of the relevant research organizations and the research sponsors. These leaders should engender an institutional culture that facilitates and improves the ethical and scientific quality of research within their purview. Four specific conditions should undergird the establishment of such a culture:
-
accountability—to assure the quality and performance of the protection program,
-
adequate resources—to assure that sufficiently robust protection activities are in place,
-
ethics education programs—to provide research personnel and oversight committees with the knowledge necessary to carry out their obligation to conduct or oversee ethically sound research, and
-
transparency—to ensure open communication and interaction with the local community, research participants, investigators, and other stakeholders in the research enterprise.
Each organization should tailor these pre-requisite conditions to its mission, the breadth and substance of its program, and the context of its community. When multiple organizations are involved in a research project, at least one of them should assume responsibility for obtaining appropriate and documented assurances from the other participating organizations that a robust protection program is in place at each site.
Recommendation: The authority and responsibility for research participant protections should reside within the highest level of the research organization. Leaders of public and private research organizations should establish a culture of research excellence that is pervasive and that includes clear lines of authority and responsibility for participant protection. (Recommendation 2.2)
Establishing the appropriate research culture will require ongoing efforts to educate researchers, research administrators, IRB members, and participants about research ethics and participant protection issues, as well as continuous QI activities. The Office for Human Research Protections (OHRP), with input from a variety of scholars in science and ethics, should coordinate the development and dissemination of core education elements and practices for human research ethics for those conducting and those overseeing such research. The individual research organization is responsible for ensuring that its personnel are educated about their responsibilities
and expected conduct (see Recommendation 2.4). The sponsor also shares some responsibility for ensuring that the research organization it engages employs only qualified personnel and has the resources to conduct the study. The stimulation of a high-quality research culture is one area in which the committee believes that developing accreditation programs may offer a significant contribution by focusing an organization’s attention on QI and specific resource needs.10 The committee suggests in Chapter 6 that adding a standard within accreditation programs directed at establishing and identifying accountability for specific protection functions would facilitate performance improvement within accredited protection programs (see Recommendation 6.3).
Provide Sufficient Resources
Protection programs should have the dedicated financial and nonfinancial resources needed to implement and sustain a sufficiently robust system of protection, including adequate space, equipment, and personnel, in addition to an appropriate annual budget. Research organizations, sponsors, and investigators agree that funds should be allocated for investigators and staff and to cover the out-of-pocket costs of research, but no satisfactory agreement has been reached regarding how to fund specific protection activities.
Unfortunately, few published data quantify the costs of ethics review and other protection activities (such as safety monitoring); thus, it is difficult to determine reasonable funding levels for offices or the individuals involved in the process.
Adequate resources are essential for the protection of research participants and are a real part of the cost of doing research. Therefore, to assure successful protection programs, public and private research sponsors and research organizations should partner to develop benchmark guidelines for critical functions and to provide the necessary funding sources (see Recommendation 2.3).
Refocus Institutional Review Board Mission on Ethical Review of Protocols
As the demands on the research oversight system have grown, so has the reliance upon IRBs to accomplish all protection tasks. This is a disser-
|
10 |
Accreditation efforts have been undertaken by AAHRPP and NCQA. The progress and potential contributions of accreditation programs are discussed in Chapter 6. |
vice to research participants, because IRBs, which are intended to focus on the ethical review and oversight of proposals, find it exceedingly difficult to both manage the increasing volume of protocol actions and ensure the safety of research volunteers, particularly when these boards are often under-resourced.
In this committee’s refocused paradigm, the responsibilities for managing institutional risk, ensuring institutional compliance with all relevant research rules and regulations, and assessing potential conflicts of interest related to proposed research should be assigned to other units within the research program or organization and not the IRB (see Chapter 6). Often, such units already exist and may be retooled to add the relevant participant protection focus to their responsibilities.
To reflect this refocused role, the committee recommends moving away from the term “Institutional Review Board,” which conflates institutional interests with those of participants, and suggests adopting a more functionally appropriate term.
Recommendation: The Institutional Review Board (IRB), as the principal representative of the interests of potential research participants, should focus its full committee deliberations and oversight primarily on the ethical aspects of protection issues. To reflect this role, IRBs should be appropriately renamed within research organizations’ internal documents that define institutional structure and policies. The committee suggests the name “Research Ethics Review Board” (Research ERB). (Recommendation 3.1)
From this point forward in this report, the term “Research ERB” will be used in the context of the committee’s envisioned HRPPP, and the term “IRB” will be reserved for comments regarding the existing protection framework.
All members of the Research ERB should have a core body of knowledge, and a critical mass of the membership,11 either scientist or nonscientist, should possess a specialized knowledge of human research ethics. The research organization’s goal should be to create or associate with a Research ERB in which unaffiliated members, nonscientists, and those who represent the local community and/or the participant perspective comprise at least 25 percent of the membership (see Recommendation 3.5). Although the committee recognizes that identifying this increased proportion of willing and able unaffiliated and nonscientist individuals will be difficult and that they will require additional training, the proportional shift is important to the integration of the participant or community and could help
insulate Research ERBs from potential conflicts of interest at the organizational level.
Further, as modern IRBs have tended to become larger and to reflect a broader range of scientific expertise, some IRB deliberations have tended to be dominated by the scientific perspective, increasing the potential to marginalize the perspectives of nonscientist members and those who focus on ethics-based concerns (Cho and Billings, 1997; Peckman, 2001). Therefore, the refocused Research ERB’s deliberative objective should aim for consensus rather than majority control (see Recommendation 3.6). No protocol should be approved without three-quarters of the voting members concurring. Just as a vote of unanimity would effectively give a veto to a single dissenting committee member, allowing a simple majority to approve a protocol in the face of substantial minority opinion can too easily suppress responsible ethical opinions.
Distinguish Scientific, Conflict of Interest, and Ethics Review Mechanisms
The scientific and ethical review of protocols should be equally rigorous. Therefore, each review requires distinct, although overlapping, expertise. Research ERBs that are constituted to emphasize the ethical dimensions of protocol review should not be expected to have a primary membership with the range of knowledge and skills needed to adequately assess the scientific and technical merits of every protocol under their purview. Although the in-depth scientific evaluation of proposals is fundamental to the comprehensive ethics review of any protocol, the Research ERB need not conduct the initial scientific review. Instead, summaries of the scientific review should be submitted to the Research ERB as a component of its ethics-focused deliberations.
Furthermore, there is a need to ensure that no financial or other interests on the part of the investigator, research organization, or the Research ERB (as a body or as individual members) will distort the conduct of research with human participants.12 While there are nonfinancial self-interests intrinsic to the pursuit of research questions, the frequency and complexity of potential financial conflicts of interest in research are expanding, and the federal government and relevant professional and industry groups should continue to consider their potential ramifications and pursue rigorous policies for handling them (see Chapter 6). A process for scrutinizing potential financial conflicts of interest in any protocol is vital to the subse-
quent evaluation of participant risks and benefits by the Research ERB (see Chapter 3).
Despite the need for review from three distinct perspectives (scientific, ethical, and financial conflict of interest), the interrelated nature of these perspectives requires that a single body be vested with the authority to make final protocol determinations and be accountable for those determinations. This body is and should remain the Research ERB. The focused reviews of scientific merit and potential financial conflicts of interest should inform the ethics review process for each protocol (see Figure ES.1).
Recommendation: Research organizations and research sponsors should ensure that Human Research Participant Protection Programs utilize distinct mechanisms for the initial, focused reviews of scientific and financial conflicts of interest. These reviews should precede and inform the comprehensive ethical review of research studies by the Research Ethics Review Board (Research ERB) through summaries of the relevant findings submitted to the Research ERB for full board consideration. (Recommendation 3.2)
Emphasize Risk-Appropriate Protection
The degree of scrutiny, the extent of continuing oversight, and the safety monitoring procedures for research proposals should be calibrated to a study’s degree of risk. Minimal risk studies should be handled diligently, but expeditiously, while studies involving high risk should receive the extra time and attention they require. Although federal regulations provide several mechanisms for expeditiously reviewing certain kinds of research involving no more than minimal risk,13 classifications of studies by risk level currently lack refinement and consistency. The development of such a stratification schema would be extremely useful to the research oversight community in their efforts to provide uniform and appropriate protection to research participants. This committee, as well as the panel constituted by the Committee on National Statistics and the Board on Behavioral, Cognitive, and Sensory Sciences and Education to examine issues pertinent to social and behavioral science,14 believes that federal intervention and guidance nuanced to match the different risk strata are warranted (see Recommendations 3.3 and 5.1, and Appendix B).
|
13 |
45 CFR 46.100, 21 CFR 56.104. |
|
14 |
The Panel on IRBs, Surveys, and Social Science Research is focusing on issues of human research participant protections in social, behavioral, and economic research. Its initial conclusions and recommendations are in Appendix B; its final report will be released in early 2003. |
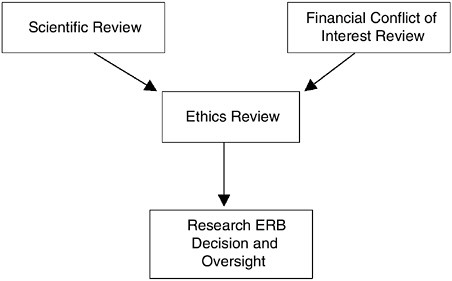
FIGURE ES.1 The Confluence of the Research Review Process
Increase Program Productivity
The effective oversight and management of the rapidly expanding number of multisite studies, particularly in the high-risk clinical domain, is an area of substantial concern (NBAC, 2001b; OIG, 1998a); full-scale IRB review of protocols by all participating organizations does not necessarily increase participant protection. Therefore, the committee encourages the streamlining of multisite trial review, recommending one primary scientific review committee and one primary Research ERB assume the lead review functions, subject to acceptance by the local committees and boards at participating sites (Recommendation 3.7).
The extreme variability in the approval decisions and regulatory interpretations among IRBs is one of the weaknesses in the current protection system. To better clarify regulatory intent and appropriate ethical practices, OHRP and the National Human Research Protections Advisory Committee (NHRPAC)15 should convene conferences and establish working groups to develop and disseminate best practices, case presentations, and conference proceedings for local HRPPPs, their Research ERBs, and research investigators (Recommendation 3.8).
Recognize and Integrate Participant Contributions
As stated in this committee’s first report, participants and their representatives should be meaningfully included in the review and oversight of research to ensure that pertinent concerns are heard and that researchers conduct studies that meet participant needs (IOM, 2001a). The public should also be educated generally about the nature of the research process and the need for well-designed research studies.
Revitalize Informed Consent
Informed consent should be an ongoing process that focuses not on a written form or a static disclosure event, but rather on a series of dynamic conversations between the participant and the research staff that should begin before enrollment and be reinforced during each encounter or intervention (see Box 4.3). Multidisciplinary approaches should be tailored to individual differences in participant education and learning capabilities.
Recommendation: The informed consent process should be an ongoing, interactive dialogue between research staff and research participants involving the disclosure and exchange of relevant information, discussion of that information, and assessment of the individual’s understanding of the discussion. (Recommendation 4.1)
The informed consent conversation(s), as well as the written consent document, should not be obscured by language designed mainly to insulate the institution from liability.16 Rather, the process should ensure that participants clearly understand the nature of the proposed research and its potential risks and benefits to them and society.
Recommendation: Forms signed by individuals to provide their legally valid consent to participate in research should be called “consent forms” rather than “informed consent forms.” Research Ethics Review Boards should ensure that the focus of the informed consent process and the consent form is on informing and protecting participants, NOT on protecting institutions. (Recommendation 3.4)
Increase System Accessibility
The system of protections established by any protection program should be transparent and open to the public if research institutions, federal agen-
cies, and private companies are to maintain the community’s trust and ask individuals to participate in research. Transparency is best achieved by providing graded levels of information and guidance to interested parties (see Recommendation 2.5).
Open communication should also occur among all relevant stakeholders to achieve this transparency and to ensure that individuals can question how research protocols were developed, reviewed, and implemented. Furthermore, those who stand to benefit or be harmed by the research should have an opportunity to comment on the research design and operation, to participate in the research, and to have access to study findings. They should also expect the research will not involve unnecessary duplication of previous studies.
In 2000, the National Library of Medicine established a clinical trials registry,17 which has expanded to serve as the Food and Drug Administration (FDA)-required site for submissions about clinical trials subject to the FDA databank requirement18 and to include information from several other trial registries (see Chapter 7). Although the development of such registries is an important first step toward providing high-quality clinical trial information to the public, currently no centralized system exists for disseminating information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies.
To ensure that information about all clinical trials is available, the committee proposes the creation of a comprehensive and soundly structured clinical trials registry for use by the public. Material submitted to Research ERBs could serve as the backbone of this registry.19 The committee believes that although the challenges and resource requirements involved in such an undertaking are significant, clinical trials are of such public concern that the effort should be pursued (see Recommendation 7.2).
Compensate Participants for Research-Related Injury
Despite decades of discussion on the ethical obligation to compensate participants for research-related injury, little information is available regarding the number of such injuries and the cost of providing compensation for them (ACHRE, 1995; DHEW, 1977; NBAC, 2001a,b; President’s Commission, 1982a). In the face of real potential for diminished public trust in
the research community,20 providing reasonable compensation for legitimate instances of research harm is critical to restoring credibility.
Although some activities in this area are ongoing, and international experience is available for guidance, determining the portion of participant illness and injury that is attributable to the research itself is a key area that will require de novo research. To guide public policy and accreditation standard development in this area and to help establish the potential magnitude of such claims, DHHS should assemble data on the incidence of research injuries and conduct economic analyses of their costs (see Recommendation 6.7).
It is the committee’s impression that many research organizations conducting clinical trials agree to provide at least short-term medical care for those who suffer research-related injuries (DoD, 2002; IOM, 1994a; NIH CC, 2000; 38 CFR 17.85), but that few research organizations cover other relevant costs. These observations refer only to harm resulting from research properly conducted in accordance with the protocol; harm due to negligence or malfeasance in science can and does end up in the tort system. However, a no-fault system could allow injured parties speedier claims resolution while permitting (as now) the pursuit of tort remedies for product defects or for negligent design or execution of studies.
The responsibility for no-fault compensation programs should fall initially on the institution or organization accountable for conducting the research, and its terms should be specified in the documentation accompanying the participant’s agreement to participate. The committee supports the findings of the many reports addressing this topic—that a comprehensive research participant protection system should include a compensation mechanism for medical and rehabilitative costs (ACHRE, 1995; DHEW, 1977; NBAC, 2001a,b; President’s Commission, 1982a). The committee further believes that the next step in this process should be to pilot test mechanisms to provide remuneration for lost work time.
Recommendation: Organizations conducting research should compensate any research participant who is injured as a direct result of participating in research, without regard to fault. Compensation should include at least the costs of medical care and rehabilitation, and accrediting bodies should include such compensation as a requirement of accreditation. (Recommendation 6.8)
Maintain Vigilance
Collect National Level Data About the System
The absence of sufficient data regarding human research activities significantly impedes the thorough examination of system performance. The value of data to support both problem definition at the national level and QI at the program level cannot be overemphasized (see Chapter 6). Collecting such data would be a considerable and lengthy undertaking, and the committee recognizes that some information needs may be better met through targeted studies. Scientific surveys involving representative samples rather than a full census would serve policy-setting priorities cost effectively (Recommendation 6.1).
Enhance Safety Monitoring
The safety of research volunteers must be guaranteed from the inception of a protocol, through its execution, to final completion and reporting of results. Continual review and monitoring of risk-prone studies is needed to ensure that emerging information has not altered the original risk-benefit analysis. Therefore, risk-appropriate mechanisms are needed to track protocols and study personnel; provide assurances that data are valid and collected according to applicable practices (e.g., Good Clinical Practice); and ensure that participants’ safety, privacy, and confidentiality are protected throughout a study. Protection measures should be monitored by various means at all levels to ensure that consent has been properly given and that all adverse events have been identified and appropriately reported by the investigator to the relevant institutional body, sponsor, and federal agency(ies).
Recommendation: Research organizations and Research Ethics Review Boards should have written policies and procedures in place that detail internal oversight and auditing processes. Plans and resources for data and safety monitoring within an individual study should be commensurate with the level of risk anticipated for that particular research protocol. (Recommendation 5.1)
An area of intense concern regarding the ongoing safety monitoring of research protocols, particularly high-risk clinical trials, is the ability of protection programs and their Research ERBs to appropriately collect, interpret, and report adverse event information (see Chapter 5). Federal oversight agencies, therefore, should harmonize safety monitoring guidance, develop standardized practices for defining and reporting adverse events, and monitor all federally regulated studies that pose substantial risks to participants with equal rigor and scrutiny (see Recommendations 5.3 and 5.5).
Continuously Improve Quality
To maximize the strength and efficiency of participant protection functions, the effectiveness and value of program policies and practices should be continuously assessed and improved. Protection programs can use systematic QI analysis tools to determine the underlying causes of shortfalls and develop procedures to eliminate them and improve work processes. The committee, in its first phase of deliberations regarding accreditation, emphasized the need to incorporate QI mechanisms into program performance assessment (IOM, 2001a).
The lack of empiric data on the performance of protection programs, the absence of defined measurable outcomes or other criteria for their ongoing evaluation, and the scant knowledge of approaches and methods by which programs have been improved have hindered efforts to initiate QI measures. Research sponsors should initiate programs and locate funding to develop criteria for evaluating program performance and enhancing QI practices. In doing so, specialists from many disciplines could contribute to a new empiric knowledge base that would inform both the leadership of individual HRPPPs and policy makers.
Recommendation: Research sponsors should initiate research programs and funding support for innovative research that would develop criteria for evaluating program performance and enhancing the practice of quality improvement. (Recommendation 6.2)
As observed in this committee’s first report, accreditation programs represent one promising approach to assessing the protection functions of research organizations in a uniform and independent manner, and may serve as a useful stimulus for QI programs (IOM, 2001a). The committee reiterates its support for pilot testing voluntary accreditation as an approach to strengthening participant protections, but repeats its recommendation that DHHS should arrange for a substantive, independent review and evaluation of HRPPP accreditation before determining its ultimate role in the participant protection system (see Recommendation 6.4).
Manage Potential Conflicts of Interest
Confidence about the current system of participant protection is undermined by the perception that harm to research participants may result from conflicts of interest involving the researcher, the research organization, and/ or the research sponsor. This concern is particularly acute regarding financial conflicts of interest, as the relationships between the academic and private research enterprises continue to evolve. Therefore, mechanisms for identifying, disclosing, and resolving conflicts of interest should be strengthened, especially those involving financial relationships (see Chapter 6).
Strong organizational leadership and the promotion of an ethically based research culture (possibly complemented through appropriate accreditation standards) may help avoid the need for management policies regarding potential self-interests; however, a dedicated conflict of interest review process will remain essential. Guidelines for acceptable levels of conflict and policies for managing conflict should continue to be developed so that common professional standards can be implemented and refined.
In the committee’s view, because the Research ERB lacks the necessary resources or authority to ensure the appropriate management of potential conflicts of interest, the responsibility for assessing and managing financial conflicts of individuals (investigators, research staff, and Research ERB members) should lie with the research organization (see Recommendation 6.5). Likewise, organizations should ensure that an independent, external mechanism is in place for the evaluation of potential institutional conflicts (see Recommendation 6.6). In both instances, conflict of interest information should be communicated in a timely and effective manner to the Research ERB, which should make the final assessment with regard to ensuring participant protections.
The impact of institutional conflicts of interest as well as nonfinancial conflicts of interest at all levels of the research enterprise have not been explored sufficiently and are issues that, like the development of professional norms for individual conflicts of interest, should be rigorously pursued by federal agencies and appropriate interest groups.
Periodically Assess the National System
Complexity, opacity, and contradiction abound in interpretations of the rules and regulations that apply to human research, often confounding clear communication between agencies and institutions. Although the language of the Common Rule deserves a careful and comprehensive reassessment for clarity and relevancy after more than 20 years of use, its revision would be time-consuming and difficult, as each signatory agency must agree to the changes. Eventually, Congress will need to take the necessary steps to broaden and strengthen the federal oversight system and to make appropriate Common Rule modifications as needed.
One mechanism through which continuing and periodic review of the national participant protection system could be provided is NHRPAC.21 This committee was created by the Secretary of DHHS in concert with the creation of OHRP in June 2000 to provide expert advice and recommenda-
|
21 |
The recent dissolution of NHRPAC underscores the need for Congressional direction in the establishment of a nonpartisan, independent advisory committee focused on the policy issues relevant to ensuring the protection of research participants, as discussed in Chapter 7. |
tions to the DHHS Secretary, the Director of OHRP, and other departmental officials on a broad range of topics pertaining to or associated with the protection of human research participants.
If NHRPAC, or any advisory committee, is to successfully guide federal policy and preserve the public trust, there must be no appearance or existence of conflict in its membership or organization, and the committee should be perceived as an independent entity.However, creating the necessary balance in a federal advisory committee presents a significant challenge, considering the breadth of federal agencies with oversight responsibility and the profound stake the public has in human research activities.
The committee therefore proposes the establishment of a nonpartisan, independent body of experts to ensure that the national protection system receives objective public advice (Recommendation 7.1). Inherent within the multidisciplinary concept for this advisory committee’s membership is balanced representation of the perspectives of participants, a range of scientific disciplines, bioethics, and IRB experts.
Such a committee could provide ongoing advice and guidance on the scientific, technological, and ethical issues related to participant protection in clinical and social/behavioral research and could provide the capacity and mechanism for examining national system performance changes over time to provide policy makers and the research community with options for ensuring continuous improvement.
CONCLUDING REMARKS
Policy makers and the scientific community should ensure that the interests and dignity of every research participant are diligently protected throughout the research process. The complexity and multifaceted nature of research requires that many offices and individuals interact and coordinate activities to form a systemic HRPPP. Tables ES.1 and ES.2 organize the committee recommendations according to their need for government action, and also serve to highlight those parties within protection programs and the federal government that possess primary responsibility for the implementation of each recommendation. The recommendations offered within this report are intended to guide HRPPPs and policy makers as they work to guarantee that research participants’ safety and rights are protected throughout their involvement in any research study and that the national research enterprise is worthy of the public’s trust and continued support.
TABLE ES.1 Recommendations That Require Direct Government Action
|
|
The Department of Health and Human Services |
|
|||||
|
Recommendation |
Office of the Secretary |
Congress |
NHRPAC |
OHRP |
FDA |
NIH |
Relevant Regulationsa |
|
2.1 Require federal protections to be in place for all research with human participants, regardless of the source of research funding.b |
X |
X |
|
45 CFR 46.101 |
|||
|
2.4 Coordinate the development and dissemination of ethics education practices. |
|
X |
|
45 CFR 46.107(a) and 46.111(a)(1) |
|||
|
3.3 Coordinate the development of guidance for risk classification. |
|
X |
|
45 CFR 46.111(a)(b) 21 CFR 56.111(a)(b) |
|||
|
3.8 Convene conferences and/or establish working groups to develop and disseminate best practices, case presentations, and conference proceedings for use by local protection programs. |
|
X |
X |
|
|||
|
5.2 Ensure that research organizations are notified of deficiency warnings and all related communications issued by regulatory agencies. |
|
X |
X |
|
|||
|
5.3 Harmonize safety monitoring guidance for research organizations, including standard practices for defining and reporting adverse events.c |
|
X |
X |
X |
45 CFR 46.103(b)(5) 21 CFR |
||
|
5.4 Issue a yearly report summarizing the results of research monitoring activities in the United States, including OHRP and FDA findings from inspections conducted the previous year. |
|
X |
|
X |
X |
|
56.108(b) 21 CFR 312.32 |
|
5.5 When potential risks to participants warrant high levels of scrutiny, NIH-funded studies should be monitored with the same rigor and scrutiny as trials carried out under an IND. |
|
X |
21 CFR 312.23 21 CFR 312.53 |
||||
|
6.1 Commission studies to gather baseline data on the current national system of protections for research participants. |
|
X |
|
||||
|
6.4 Pilot test and evaluate voluntary accreditation as an approach to strengthening human research participant protections.d |
|
X |
|
||||
|
6.7 Assemble data on the incidence of research injuries and conduct economic analyses of their costs to establish the potential magnitude of claims that would arise under a no-fault compensation system for such injuries. |
|
X |
|
||||
|
7.1 Authorize and appropriate support for a standing independent, multidisciplinary, nonpartisan committee on human research participant protections. |
X |
|
|||||
|
|
The Department of Health and Human Services |
|
|||||
|
Recommendation |
Congress |
Office of the Secretary |
NHRPAC |
OHRP |
FDA |
NIH |
Relevant Regulationsa |
|
7.2 Facilitate the establishment of a comprehensive clinical trials registry. |
|
X |
|
X |
X |
X |
|
|
7.3 Groups addressing bioterrorism response mechanisms and research should pay special attention to the protection of research participants. |
|
X |
|
X |
X |
X |
|
|
NOTE: Within the table, entities with primary responsibility for the implementation of committee recommendations are indicated by an X. In many instances, additional agencies may be involved in the implementation of recommendations (e.g., FDA should participate in the development of guidance for risk classification [Recommendation 3.3]); however, only the lead responsibilities are indicated within the table. a The relevant regulations are provided for reference. Unless otherwise noted, the committee recommendations can be implemented without further promulgation or revision of regulations. In several instances, the development of guidance documents directed at specific issues may be useful to facilitate the recommended action. b Implementation would necessitate modification to current regulatory language. c In addition to the agencies within the Department of Health and Human Services (DHHS), all federal agencies sponsoring or overseeing human research should be consulted to ensure that reporting requirements for advers events involving nonphysical harms are similarly harmonized. d DHHS is responsible only for ensuring that an independent evaluation of accreditation is undertaken. |
|||||||
TABLE ES.2 Recommendations That Can Be Implemented in the Absence of Direct Government Action
|
Recommendation |
Research Organization |
Sponsors |
Research ERBsa |
Investigators |
Research Participants |
Accreditation Programs |
Relevant Regulationsb |
|
2.2 Establish a pervasive culture of research excellence that includes clear lines of authority and responsibility for participant protection. |
X |
X |
|
||||
|
2.3 Provide the necessary financial support to meet the obligation to ensure HRPPPs have adequate resources. |
X |
X |
|
45 CFR 46.103(a)(b) |
|||
|
2.4 Ensure that investigators, IRB members, and other individuals substantively involved in human participant research are adequately educated to perform their respective duties. |
X |
X |
|
X |
45 CFR 46.107(a) and 46.111(a)(1) |
||
|
2.5 Foster communication with the general public and research participants to assure that the protection process is open and accessible to all interested parties. |
X |
X |
|
||||
|
Recommendation |
Research Organization |
Sponsors |
Research ERBsa |
Investigators |
Research Participants |
Accreditation Programs |
Relevant Regulationsb |
|
3.1 Rename the Institutional Review Board to reflect its role as the principal representative of the interest of potential research participants. |
X |
|
45 CFR 46.102(g) 21 CFR 56.102(g) 21 CFR 50.3(i) |
||||
|
3.2 Ensure that focused scientific and financial conflicts of interest reviews precede and inform the comprehensive ethical review of research studies by the Research ERB. |
X |
X |
|
45 CFR 46.107(e)(f), 46.115(a)(1), and 46.116 |
|||
|
3.4 Ensure that the focus of the informed consent process and consent forms is on informing participants, NOT protecting institutions. |
X |
X |
X |
X |
|
45 CFR 46.116 21 CFR 50 |
|
|
3.5 Reserve 25 percent of Research ERB membership for individuals with unaffiliated and non-scientific perspectives. |
X |
|
X |
|
45 CFR 46.107 21 CFR 56.107 |
||
|
3.6 Conduct Research ERB deliberations by consensus and, in the absence of consensus, require 3/4 majority for approval. |
|
X |
|
45 CFR 46.108(b) 21 CFR 56.108(c) |
|||
|
3.7 Streamline the reviews of multisite trials by assigning lead review committees |
X |
X |
X |
|
45 CFR 46.114 21 CFR 56.114 |
||
|
4.1 Conduct the informed consent process as an on-going, interactive exchange between research staff and research participants. |
|
X |
X |
X |
|
45 CFR 46.116 21 CFR 50 |
|
|
4.2 Research participants should understand their potential role in the study, the rationale of the study, and what is required of them to prevent unanticipated harm to themselves and to maintain the scientific integrity of the study. |
|
X |
X |
|
45 CFR 46.116 and 21 CFR 50 |
||
|
5.1 Create written policies and procedures that detail internal monitoring and auditing processes. |
X |
X |
X |
|
45 CFR 46.103(b) 21 CFR 56.108(a) |
||
|
Recommendation |
Research Organization |
Sponsors |
Research ERBsa |
Investigators |
Research Participants |
Accreditation Programs |
Relevant Regulationsb |
|
5.2 Ensure that Research ERBs are notified of deficiency warnings and all related communications issued by regulatory agencies and of any serious violations identified through sponsor-requested monitoring reports. |
X |
X |
|
||||
|
5.5 When potential risks to participants warrant high levels of scrutiny, NIH-funded studies should be monitored with the same rigor and scrutiny as trials carried out under an IND. |
X |
X |
|
21 CFR 312.23 21 CFR 312.53 |
|||
|
5.6 Ensure an independent Data and Safety Monitoring Board/ Data Monitoring Committee is assigned to high-risk studies or those involving participants with life-threatening illnesses. |
X |
X |
X |
|
45 CFR 46.111(a)(6) 21 CFR 56.11(a)(6) |
||
|
6.2 Initiate research programs and funding support for innovative research that would develop criteria for evaluating program performance and enhance the practice of quality improvement. |
X |
|
|||||
|
6.3 Include an accreditation standard directed at establishing and identifying accountability for specific protection functions. |
|
X |
|
||||
|
6.4 Pilot test and evaluate voluntary accreditation as an approach to strengthening human research participant protections. |
|
X |
|
||||
|
6.5 Ensure prospective, in-depth review of potential individual financial conflicts of interest. |
X |
|
42 CFR 50 21 CFR 54 45 CFR 46.116 |
||||
|
6.6 Establish an external mechanism to review potential institutional conflicts of interest. |
X |
|
|||||
|
6.8 Compensate any research participant who is injured as a direct result of participating in research, without regard to fault. Include compensation for research-related injury as a requirement of accreditation.c |
X |
X |
|
X |
45 CFR 46.116(a)(6) |
||
|
Recommendation |
Research Organization |
Sponsors |
Research ERBsa |
Investigators |
Research Participants |
Accreditation Programs |
Relevant Regulationsb |
|
7.3 Groups addressing bioterrorism response mechanisms and research should pay special attention to the protection of research participants. |
X |
X |
X |
X |
|
||
|
NOTE: Within the table, entities with a primary responsibility for the implementation of committee recommendations are indicated by an X. In several instances, other elements within an HRPPP may possess a secondary role in the systemic implementation of the recommendation (e.g., the Research ERB); however, to preserve the clarity of the table, only primary relationships are indicated. Likewise, while the pursuit of a recommended action may be facilitated by a government organization, this indirect role is not represented within the table.Some recommendations are contained within both tables due to their multistep nature or substantial overlapping responsibility. a In accordance with Recommendation 3.1, Research Ethics Review Board (Research ERB) is used to designate the traditional IRB panel. b The relevant regulations are provided for reference. Unless otherwise noted, the committe recommendations can be implemented without further promulgation or revision of regulations. In some instances, the development of guidance documents directed at specific issues may be useful to facilitate the recommended action. c If it is determined through the evaluation of the suggested demonstration programs covering lost income due to temporary or permanent disability resulting from research harm that a federal insurance program is the appropriate solution to broad implementation of this responsibility, Congressional intervention could be required (see Chapter 6). |
|||||||




























