INTRODUCTION
At the World Health Assembly in May 1980, the World Health Organization (WHO) declared the world free of smallpox. Smallpox vaccination of civilians is now indicated only for laboratory workers directly involved with smallpox (variola virus) or closely related orthopox viruses (e.g., monkeypox, vaccinia, and others). Recent questions raised by the terrorist attacks in fall 2001 have renewed concerns about possible outbreaks of smallpox resulting from its use as a biological weapon. The risk of smallpox occurring as a result of a deliberate release by terrorists is not known, but is considered very low. Smallpox vaccine (vaccinia virus) is a highly effective immunizing agent against smallpox; however, its use is not without risk and reintroduction of a wide-scale vaccination program must be done judiciously, if at all.
The Advisory Committee on Immunization Practices (ACIP) provides advice and guidance to the Secretary and the Assistant Secretary for Health, Department of Health and Human Services (DHHS) and the Director, Centers for Disease Control and Prevention (CDC), regarding the most appropriate application of antigens and related agents (e.g., vaccines, antisera, immune globulins) for effective disease control in the civilian population. ACIP develops written recommendations for the routine administration of vaccines to the pediatric and adult populations, along with schedules regarding the appropriate periodicity, dosage, and contraindications applicable to the vaccines. Additionally, ACIP reviews and reports regularly on existing immunization practices and recommends improvements in national immunization efforts.
In 1980, ACIP developed guidelines recommending the use of vaccinia vaccine to protect laboratory workers from possible infection while working with nonvariola orthopoxviruses (e.g., vaccinia and monkeypox). In 1984, those recommendations were included in guidelines for biosafety in microbiological and biomedical laboratories. The guidelines expanded the recommendations to include persons working in animal care areas where studies with orthopoxviruses were being conducted. They further recommended that such workers have documented evidence of satisfactory smallpox vaccination within the preceding
three years. CDC has provided vaccinia vaccine for these laboratory workers since 1983.1 In 1991, ACIP further expanded smallpox vaccination recommendations to include healthcare workers involved in clinical trials using recombinant vaccinia virus vaccines and lengthened the recommendations for revaccination for persons working with vaccinia virus, recombinant vaccinia viruses, or other nonvariola orthopoxviruses to every 10 years.
In June 2001, ACIP made recommendations for use of smallpox (vaccinia) vaccine to protect persons working with orthopoxviruses, and to prepare for a possible bioterrorism attack involving smallpox. Because of the fall 2001 terrorist attacks, CDC asked ACIP to again review and update its previous recommendations for smallpox (vaccinia) vaccination. As a result of this review, ACIP issued supplemental recommendations for vaccination of 1) the general population and 2) persons designated to respond or care for a suspected or confirmed case of smallpox. In addition, the proposed policy clarified and expanded the primary strategy for control and containment of smallpox in the event of an outbreak.
To supplement this review process, CDC asked the Institute of Medicine (IOM) of the National Academy of Sciences (NAS) to convene a public conference to discuss the scientific, clinical, procedural, and administrative aspects of various immunization strategies. This report summarizes the discussions of that meeting. Held on June 15, 2002 in Washington, D.C., the meeting was expository, not deliberative, and its discussions and conclusions do not reflect the opinions of either IOM or the NAS.
OPENING REMARKS2
The threat of smallpox has not changed appreciably since ACIP last reviewed smallpox immunization policy in June 2001. It remains difficult to obtain the virus, prepare it, and distribute it. What has changed is the availability of vaccine.
Dryvax, the vaccinia (smallpox) vaccine currently licensed in the United States, is a lyophilized, live-virus preparation of infectious vaccinia virus, produced in 1975 by Wyeth Laboratories, Inc., of Marietta, Pennsylvania. Vacciniad vaccine does not contain smallpox (variola) virus. Previously, the vaccine had been prepared from calf lymph with a seed virus derived from the New York City Board of Health strain of vaccinia virus. Vaccine was administered by us
ing the multiple-puncture technique with a bifurcated needle. A reformulated vaccine, produced by using cell-culture techniques, is now being developed.
In fall 2001, there were 150,000 ampules of Dryvax, available at 100 doses per ampule, which would vaccinate 15 million people. However, because Dryvax is a dried product, once reconstituted it begins to deteriorate at a rapid rate, so there is a finite period of time in which it can be used, which can create substantial wastage. In September 2001, DHHS placed an order for 40 million doses of vaccine with Acambis, Inc. The 20-year contract would purchase a new vaccine produced in tissue cell culture, to be available in 2004. However, the September 11, 2001, attacks and the release of the anthrax organisms through the mail spurred the government to acquire more vaccine more quickly. Acambis and Baxter are currently producing 200 million doses of a stable tissue cell culture vaccine to be available by the end of 2002. Also in 2002, Aventis Pasteur located in a storage facility 85 million doses of vaccine prepared from calf lymph, produced in 1958. This vaccine has been tested and is available if needed; however, the newer vaccine produced in tissue cell culture is preferable.
Now that sufficient vaccine will be available for the entire U.S. population should it be needed, a responsible immunization strategy must be developed. Previous experience with immunization has shown that serious complications can arise in as much as 20 percent of those who come in contact with vaccinees but are not yet vaccinated and are susceptible to complications for a variety of reasons.
SCIENTIFIC BACKGROUND ON SMALLPOX AND SMALLPOX VACCINATION
Smallpox Disease3
The last case of naturally occurring smallpox occurred almost 25 years ago, and 24 years ago the last episode occurred in Birmingham, England, with the laboratory escape of variola virus. As a result of its eradication, virtually all clinicians, particularly in northern countries, are unfamiliar with this disease and research on human smallpox has practically stopped. Eradication was relatively easy to achieve because humans are the only reservoirs and vectors, the disease is clinically manifest, and there is no carrier or latent state. Moreover, one episode gives lifelong protection, transmission occurs when the disease is manifest, there is a stable vaccine, and it is relatively straightforward to trace chains of transmission.
The smallpox virus replicates in the cytoplasm. The virus enters the respiratory tract and multiplies in the oropharynx. There is a brief burst of viremia that goes into cells of the reticuloendothelial system, followed by a second viremia into the skin, after which the patients then manifest the disease, which then again spreads via respiration. The incubation period for smallpox is 10 to 12 days. The prodrome, which is a mean of two to three days, is very severe, with high fever, backache, headache, and prostration. The first few days involve a macular phase—a reddish rash that is not distinctive, followed in a couple of days by papules, then vesicles, then pustules, which can become confluent over the entire body. After about two weeks there is crusting, hypopigmentation and pitting, scarring, and eventually hyperpigmentation. The infectiousness period occurs when the lesions are heaviest.
There are five known classifications of smallpox. The ordinary form is the most common (~90 percent) with a 30 percent case fatality rate. The flat form accounts for about 5 percent of cases, and has a 97 percent case fatality rate. The hemorrhagic form accounts for less than 3 percent of cases but has a 100 percent fatality rate. The other classifications of smallpox are the modified form (occurring in less than 2 percent of cases and having less than a one percent fatality rate) and V. sine eruptione (occurring in less than 1 percent of cases with no known fatalities). There are no specific strains associated with hemorrhagic disease, thus it is believed to be a host response. Patients with hemorrhagic disease die despite post-exposure vaccination. The hemorrhagic cases do not look like smallpox and many of them will not resemble an infectious disease. It is likely that initially these cases will come into emergency rooms, perhaps diagnosed as acute leukemia or a variety of other things, in which case emergency room personnel are not likely to have taken the necessary precautions one would take if smallpox were suspected.
Conditions that resemble the maculopapular eruptions of smallpox include drug eruptions, measles, secondary syphilis, and vaccine reactions. Chickenpox, monkeypox, and generalized vaccinia can resemble the papulovesicular eruptions of smallpox. With newer molecular approaches to diagnosis, however, more rapid and precise screening, if not confirmation, of variola and chicken pox can help in diagnosis. However, cell culture is the only reliable diagnostic tool for the orthopoxes when the clinical symptoms are indistinguishable.
Smallpox is transmitted person-to-person by large airborne droplets, that is, face-to-face contact of 2 to 2.5 meters. Thus homes and hospitals are major transmission sites. However, carriers are symptomatic so investigations done with due diligence can prevent further spread. In general, it has been believed that smallpox can not be carried by the wind and travel great distances, although outbreaks in hospitals might have been due to movement of the virus through air ducts.
There are certain features of smallpox making it, in temperate areas, a winter or early spring disease, and in the tropics, a hot, dry season disease, mainly because the virus persists longer on droplets in aerosols, and the nasopharynx might be more eroded and therefore more susceptible to invasion by the virus.
The most important epidemiological index for smallpox spread or that of any infectious disease is the number of persons in an environment who, when in contact with a patient, will come down with the disease. Studies in Asia and Africa found that the attack rate in unvaccinated persons ranged from roughly 40 to 90 percent with variola major. Despite being a somewhat milder disease, the secondary attack rate for variola minor is still about 50 percent. The case fatality rate increases as years from vaccination increase, from nearly 0 percent if vaccination occurred less than 10 years prior to contact to over 10 percent when vaccination occurred more than 20 years prior to contact. Deaths from smallpox are generally due to secondary infection of lesions, pneumonia, toxemia, and hypotension. Death rates in unvaccinated patients, particularly those with the more severe form of the disease, can be as high as 50 percent.
Smallpox Control Strategies and Vaccine Availability4
Although the smallpox vaccine works well in a pre-exposure and post-exposure setting, quarantine and isolation are also valuable means by which to control spread of the disease. Estimates of vaccination efficacy originally were not based on controlled clinical trials, but rather on comparisons of secondary attack rates among vaccinated and unvaccinated family contacts of cases. Vaccination status was determined by the presence of a scar and did not account for vaccine potency, scarring secondary to skin infection rather than vaccine take, or “on-time” vaccination. Estimates of pre-exposure vaccination efficacy were conservative, yet the general medical opinion is that successful vaccination or re-vaccination within three years provided 90 to 97 percent efficacy against disease. However, even with vaccination, both flat and hemorrhagic smallpox continue to have high case fatality rates—in the 90 percent range—which might reflect a host response rather than protective immunity.
Effectiveness of post-exposure vaccination ranges from 20 to 90 percent. For those receiving primary post-exposure vaccination, the efficacy is around 70 percent—yielding either protection from disease or manifestation of modified smallpox, which has a much lower case fatality rate. In re-vaccinated individuals, efficacy protections are over 80 percent. Effectiveness is clearly present in those vaccinated less than seven days after exposure.
Experience in developing countries, in which hospitals had very high rates in terms of smallpox transmission, demonstrated that poor infection control practices were the cause of rapid spread of the virus. Airborne precautions, including discharge of air to the outside or through a HEPA filter, closed doors, and using a N-95 or better respirator would be expected to prevent this disease.
A fitted respirator can provide 90 percent protection against any type of air leakage. Contact precautions can also help control the spread of the virus, for example, use of hand washing, masks, and eye protective gear.
Several characteristics of smallpox led to its control and eventual eradication: 1) cases could be identified because smallpox is a clinically evident disease and there is no subclinical illness; 2) the disease moves relatively slowly— transmission does not occur during prodrome and maximum transmission is at the time of substantial illness; and 3) the vaccine is highly effective.
Mass vaccination was the earliest strategy used. It was not until 1968 that surveillance and containment became the strategy that finally eliminated smallpox. In this approach, cases are searched for and when clinically evident disease is found a ring of immunity is created and if possible, contacts are isolated or quarantined. Determining the size of the ring is the challenge.
Accumulating evidence suggests that surveillance and containment were more effective than mass vaccination in the eradication of smallpox. In West and Central Africa in 1968–1969, cases continued to occur in spite of mass vaccination, until surveillance and containment were initiated. Prolonged and intense exposure was the norm for person-to-person transmission of smallpox, suggesting that control of the movement of these contacts was central to containment.
Operationally, surveillance and containment begins with case detection, followed by vaccination and quarantine of contacts of cases, and delineation of functional and geographic boundaries around cases or outbreaks (e.g., wide-area vaccination), followed by communication among areas about cases.
Protocols are in place for vaccine handling, dilution, and administration in the United States. There are 162 million doses of calf-lymph-derived vaccine and there will be 362 million doses of cell-cultured-derived vaccine by January 2003. The vaccines are currently part of the national pharmaceutical stockpile, located in four regions throughout the United States. Initial shipments can be sent with a confirmed case of smallpox via Vaxicools—self-contained storage and transport units holding 300,000 doses. Any site in the United States can be reached within 12 hours. The entire stockpile could be deployed to multiple locations within a 5-day period.
In summary, vaccination provides high levels of protection, both pre- and post-exposure. Current infection control practices should prevent occupational and nosocomial acquisition of smallpox. Surveillance and ring containment is the most effective means to control this disease in populations with relatively high levels of immunity from immunization, as well as in parts of the world where there are low levels of immunity, both from immunization as well as from naturally occurring disease.
Smallpox Vaccination: Efficacy, Availability, Duration of Immunity, and Timing5
Successful primary vaccination confers full immunity to smallpox in greater than 95 percent of persons for a period of approximately 5 to 10 years. Successful re-vaccination provides protection for 10 to 20 years.
The 15.4 million doses of Dryvax that had been produced in 1982 or earlier were tested in a dilutional study (dilutions were 1:5 and 1:10), the results of which were published in the April 25, 2002, issue of The New England Journal of Medicine.6 Vaccination initially was successful in a high percentage of individuals with the 1:5 dilution—a 99.1 percent take rate—compared to the 97.2 percent take rate of undiluted doses. The 1:10 dilution had a 97.1 percent take rate, not statistically different from the 1:5 dilution or the undiluted sample. Thus, the diluted vaccine can be added to the current stockpile (the 15.4 million doses can be diluted to create 77 million doses).
The duration of smallpox immunity has not been satisfactorily measured. Studies of case-fatality rates in Liverpool, England, in the early 1900s showed that when decades separated vaccination from the time of a smallpox outbreak, non-vaccinated individuals had a much higher case fatality rate than vaccinated individuals.
A review by Thomas Mack of the introduction of smallpox in Europe from 1950 to 1971 looked at case fatality rate vis-a-vis vaccination status.7 The case fatality rate among 680 cases of variola major was 52 percent for those never vaccinated and as high as 11 percent for those vaccinated more than 20 years before exposure. The data for those vaccinated between 1 and 20 years before exposure suggest a duration of immunity.
Immunity is defined by surrogates of immunity—which can be neutralizing antibody, cellular immunity, and skin reactions. A 1990 study looked at the persistence of neutralizing antibody after re-vaccination against smallpox.8 The titer is significantly decreased after the first 3 years after re-vaccination but remains stable at a low level for at least 30 years thereafter. Whether that low level is protective is not clear but clinical observations from other studies suggest that it is.
Cellular immunity is more problematic in its measurement and relevance. A study was conducted of 26 healthy male military recruits who were vaccinated 15 to 18 years earlier.9 Blood samples collected before re-vaccination to study antigen-specific proliferative response—an indicator of cellular immunity—indicated that there was virtually no existing specificity of responses of lymphocyte proliferation prior to vaccination. However, a more recent study found that T-cell vaccinia-specific immunity can actually persist up to several decades following immunization.10
Skin reaction to vaccinia in people who previously had smallpox vaccine provides an additional source of projections about the state of immunity. In a study published in 1968, immunity to smallpox of 425 people in Afghanistan who previously not only were vaccinated but also actually had smallpox showed that 9 to 11 years after their disease more than 50 percent actually had takes, suggesting that they had lost immunity to pox viruses.11
An NIAID protocol is studying 80 individuals from 32 to 60 years old who have been previously vaccinated at least once, but not more recently than 1971. Neutralizing antibody, cell-mediated immunity will be analyzed, as well as interferon-gamma using ELISPOT assays. Baseline measurements will aim to establish the long-term persistence of immunity 30 years or longer.
As for vaccination timing, if administered within four to five days following exposure it may prevent or significantly ameliorate subsequent illness. In an outbreak in Bangladesh of over 1,300 cases, including 372 deaths, few if any individuals who were vaccinated as late as 5 days into their incubation period developed clinical disease, and vaccination performed after 5 days actually reduced the clinical attack rate by 50 percent.12
In summary, primary smallpox vaccination probably provides full immunity for at least three to five years. However, beyond that, the immunity duration is still somewhat uncertain. Post-exposure vaccination within several days may prevent or ameliorate disease. However, vaccine with vaccinia, although highly effective, is one of the least safe of all licensed human vaccines. These data must be considered in deciding whether to proceed with voluntary pre-emptive mass vaccinations without credible threat of smallpox attack, voluntary pre-emptive vaccination of “first responders” only, or the use of ring versus mass vaccination in the event of a smallpox attack.
Smallpox Vaccination Safety13
Data on the safety of vaccinia are 35 to 40 years old. There is very little in the way of controlled data and immunological knowledge at the time was primitive. Moreover, differences in administration of vaccinia produced different reactions, depending on the number of insertions and therefore the amount of virus delivered.
The first and probably most common reaction to vaccine is erythema multiforma, which occurs 7 to 14 days after vaccination. After re-vaccination, it may occur much sooner. It is sporadic and most likely an allergic or toxic reaction to components of the virus. The rash differs from a macular rash, becoming maculopapular, occasionally vesicular or even pustular, and urticarial. In rare cases, Stevens-Johnson syndrome occurs after vaccination. Diagnosis is by clinical appearance and by temporal association with the vaccine. The treatment is symptomatic, primarily benadryl. Stevens-Johnson syndrome requires more extensive measures, including systemic and topical steroids.
In the past, diseases (including tetanus, syphilis, streptococcal and staphylococcal infection) may have been transmitted from patient to patient due to methods that involved dipping the needle into the bottle prior to vaccinating. Further, the use of totally occlusive dressings in the past to prevent the spread of virus created an anaerobic environment with the potential for subsequent infectious complications. In recent studies, semi-permeable occlusive dressings have been used.
Accidental vaccination (by ingestion or injection) sometimes occurred with no serious adverse consequences, as compared to accidental inoculation, which could have quite serious consequences (such as keratitis, burns, eczema vaccinatum). About 20 percent of complications were, in fact, due to transmission of vaccinia from a vaccinee to some other person.
Traumatic and surgical wounds predisposed individuals to accidental inoculation, as did dermal infection of any type that disrupts the skin (such as eczema, which could predispose those individuals to eczema vaccinatum). Mucosal inoculation occurred via dental extraction, tonsillar extraction, and other mucosal lesions. Young infants and children tended to have more of these complications than others, for obvious reasons. The vaccination site itches, and by scratching they would transfer the virus on to their hands. Because transfer was often by hand, inflammatory eye disease predisposed some individuals to peri-orbital and corneal lesions as a result of their rubbing their eyes. Bathing can result in autoinoculation, particularly in young infants who have lesions elsewhere on their body.
Antiviral agents and vaccinia immunoglobulin (VIG)14 are useful treatments for these complications, except for use in the eye, although doses are not clearly established. The recommended dosage of the currently available VIG for treatment of complications is 0.6 ml/kg of body weight. VIG must be administered intramuscularly and should be administered as early as possible after the onset of symptoms. Future reformulations of VIG might require intravenous administration.
There remains a need for pharmaceutical therapy, either for the management of smallpox or for the management of smallpox side effects. The eventual development of such drugs would materially change the severity and, therefore, frequency and relevance of the side effects. The development of a drug could become an alternative to vaccination, particularly in some of the containment-oriented scenarios.
Generalized vaccinia is likely to be a problem should vaccination begin. Despite its appearance, it is a benign disease with multiple lesions that heal, except in rare cases of persistent recurrent lesions. However, extensive immunological studies are needed to understand why this disease occurs. Progressive vaccinia is a greater concern. It occurs in immunologically-deficient individuals, primarily in those with cell-mediated immune deficiencies. The disease involves progressive enlargement of the primary site, with viremic spread to other parts of the body, and each lesion expands as does the primary site until the lesions overcome the individual and become fatal. Children with severe combined immunodeficiency do not survive vaccinia and children with hypogammaglobulanemia can be overwhelmed by virus and die. Other populations that are vulnerable if inoculated include those with graft-versus-host disease following solid organ transplantation, cancer survivors, and HIV-infected individuals. Thus, appropriate screening for contraindications to vaccination should be implemented and should include vaccinated persons as well as their contacts. Because there are a growing number of asymptomatic and unknown HIV-positive individuals in society, vaccination strategies must consider the implications of HIV testing.
CDC-ACIP SMALLPOX VACCINATION POLICY REVIEW
CDC’s Draft Policy Options15
In June 2001, ACIP published a statement on vaccinia vaccines in the Morbidity and Mortality Weekly Report. In February 2002, CDC asked ACIP to re-visit the issue in light of the terrorist attacks in fall 2001. In response, ACIP and the National Vaccine Advisory Committee (NVAC) formed a joint working group on smallpox to review a series of questions regarding possible immunization plans. In addition, four community forums were convened in New York, San Francisco, St. Louis, and San Antonio.
Information provided to ACIP indicated that the risk for smallpox occurring as a result of a deliberate release by terrorists is considered low, and the population at risk for such an exposure cannot be determined. Therefore, pre-exposure vaccination is not recommended for any group other than laboratory or medical personnel working with non-highly attenuated orthopoxviruses.
Recommendations regarding pre-exposure vaccination should be made on the basis of a calculable risk assessment that considers the risk for disease and the benefits and risks regarding vaccination. Because the current risk for exposure is considered low, benefits of vaccination do not outweigh the risk regarding vaccine complications. If the potential for an intentional release of smallpox virus increases later, pre-exposure vaccination might become indicated for selected groups (e.g., medical and public health personnel or laboratorians) who would have an identified higher risk for exposure because of work-related contact with smallpox patients or infectious materials.
CDC asked ACIP to consider three questions and develop options under each. The results of its deliberations, presented as options, follow each question:
Question 1: With no known cases of smallpox worldwide, should there be any change in the current recommendation for not vaccinating members of the general public?
Option 1: In the absence of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP does not recommend vaccination of members of the general public (i.e., no change from the current recommendation).
Option 2: In the absence of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP does not recommend that members of the general public be vaccinated; however, members of the general public
may choose to be vaccinated. (This is a negative recommendation by ACIP, but there is choice by members of the public.)
Option 3: In the absence of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP recommendations for smallpox vaccine do not now include members of the general public; however, members of the general public may choose to be vaccinated. (ACIP is neutral, and there is choice by the public.)
Option 4: In the absence of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP recommends vaccination for those members of the general public who decide to receive the vaccination.
Question 2: In addition to laboratory workers who work with viruses related to smallpox, are there other individuals in specific occupational groups who should be vaccinated to enhance smallpox preparedness? If so, what guidelines should be used to determine which individuals should be vaccinated?
Option 1: In the absence of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP does not recommend pre-exposure vaccination for any individuals other than laboratory or medical personnel who work with non-highly attenuated orthopox viruses.
Option 2: In the absence of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP recommends smallpox vaccination of persons pre-designated by the appropriate bioterrorism and public health authorities who have responsibility for direct contact or investigation of the initial cases of smallpox.
Option 3: In the absence of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP recommends extending Option 2 above to include smallpox vaccination of “essential” medical and non-medical service personnel pre-designated by the appropriate bioterrorism and public health authorities.
Question 3: Should there be any change in the current recommendation that surveillance and containment be the primary strategy for control of smallpox in the event of a case or an attack?
Option 1: In the event of a confirmed smallpox case or a confirmed smallpox bioterrorism attack, ACIP recommends surveillance and containment (ring vaccination) be the primary strategy for the control and containment of smallpox.
Option 2: In the event of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP recommends surveillance and containment (ring vaccination) be the primary strategy for the control and containment of smallpox, and that it be supplemented by vaccination of medical, health,
law enforcement, and other personnel who would assist in responding to, managing, and investigating the outbreak or attack.
Option 3: In the event of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP recommends that surveillance and containment be the primary strategy for control and containment of smallpox, and encourages offering vaccination to those people in the affected community(ies) who would like to be vaccinated.
Option 4: In the event of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP recommends surveillance and containment and mass vaccination of members of the general public be used as concurrent strategies for the control and containment of smallpox.
The options considered by ACIP assume: that the threat level is low; that there will be sufficient VIG available should widespread vaccination occur; that appropriate pre-vaccination screening for contraindications can and will be implemented; that the current vaccine is an investigational new drug; and that vaccination programs will be conducted by federal, state, and local health agencies. In addition to vaccination, appropriate infection control and use of personal protective measures will be utilized by health care workers and others in the event of a case or an attack.
Summary of Regional Meetings16
As part of the process developed by ACIP and the NVAC Smallpox Vaccine Group, CDC convened a series of meetings over a two-week period in New York City, San Francisco, St. Louis, and San Antonio to engage the public in the deliberations.
NVAC noted in February 2002, that the development of policies and programs on bioterrorism preparedness would benefit from public dialogue involving medical and related groups, as well as the lay public. Nearly 500 people attended the 4-day-long meetings: representatives from 43 agencies and organizations and 23 members of the public, primarily from the health care professions, spoke. Written comments have been received from 25 individuals. In addition, in May 2002, 130 organizations were represented at a meeting to discuss the ACIP recommendations. Additionally, the Association of State and Territorial Health Officers has been actively engaged in discussing the policy options, which is critical given the need for state and local health officials to be involved in the decision-making process.
The public forums sought input on the ACIP options described above. A summary of the public response follows:
Most participants favored Option 1, Question 1, regarding vaccination of the public, that is, “In the absence of a confirmed smallpox case, or a confirmed smallpox bioterrorism attack, ACIP does not recommend vaccination of members of the general public (i.e., no change from the current recommendation).” The reasons for favoring this option included shortage of VIG, risks to vaccine recipients and their contacts, and distrust of government. Some persons expressed a preference for a permissive recommendation reflecting their desire to make a personal choice in consultation with their physician.
As for Question 2, providing vaccinations to selected occupational groups, most who commented supported expanding vaccination beyond the current limited group, which is only those working with orthopoxes, to either Option 2 or Option 3. Those supporting Option 2 stressed the need for state smallpox response teams to rapidly respond should a suspected or a proven case occur. Those favoring Option 3 represented health care agencies and organizations, and noted that many occupations face risks, including primary care providers, laboratory workers, home health care providers, and others. Other participants wanted vaccination of other essential groups in bioterrorism if an emergency should occur, such as firefighters, transportation workers, and law enforcement workers—those necessary for the continued functioning of society. Limited support was expressed for Option 1. A common theme was, irrespective of those to whom the vaccine would be recommended, immunization of first responders should be voluntary, with fully informed consent.
Regarding Question 3—the use of surveillance and containment as a control strategy—little support surfaced for mass vaccination once an attack occurred throughout the United States. Those who commented readily appreciated the success of the smallpox eradication campaign, but expressed considerable doubts about whether that program would be sufficient in a bioterrorism attack given today’s highly mobile society. They noted the difference between natural smallpox, that is, endemic disease and smallpox resulting from an attack, which might be in multiple places. The need for flexibility in the policy was noted.
Surveys of public opinion, such as by the Harvard School of Public Health, indicate that a substantial number of Americans, if offered the vaccine, might accept it. However, focus groups convened by CDC indicate that there are considerable gaps in knowledge and substantial misunderstandings, both on the part of the public and the medical community.
THE MODELING BASIS FOR VACCINATION POLICY OPTIONS
Three models on which vaccination policies might be based were presented, as was a report of a 1971 smallpox outbreak in a region of the former Soviet Union, which is now the city of Aralsk, Kazakhstan.
Model 117
A simple rule for deciding whether to be vaccinated states: if the risk of smallpox is greater than the risk of serious vaccine-related side effects (i.e., those requiring medical care and possibly VIG), then vaccine should be immediately available to the public.
In defining the risk of smallpox one has to consider the following: What is the probability of release? What is the likely number of people initially infected before it is verified that an outbreak has occurred? What is the probability of contacting one of those persons before it is known that a release or an outbreak has occurred? What is the probability of transmission from a person who has smallpox to an unvaccinated person? What is the effectiveness of the vaccine? Against these probabilities and projections of risk of smallpox, the actual risk of serious vaccine side effects must be weighed.
There are some assumptions in this model. The first is one of risk neutrality, that is, the negative value of smallpox is equal to the negative value of serious side effects from the vaccine. The second assumption is that the assessment relates to what is known today, before a confirmed case of smallpox occurs. Once there has been a confirmed case or an outbreak identified, the value and necessity of accepting pre-exposure smallpox vaccination must be reassessed. The third assumption is that the model is valuable to the individual, not to society.
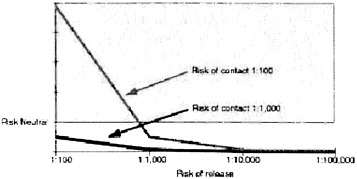
The models use probabilities based on assumptions and on earlier studies. The probability of release ranges from 1:100 to 1:100,000. The risk of contacting somebody who has smallpox before an outbreak is recognized ranges from 1:100 to 1:10,000. The probability of transmission between someone who is infectious and someone who is not vaccinated is 70 percent. Vaccine efficacy for this set of results is set at 98 percent. The probability of serious vaccine-related side effects is 1:100,000.
Using these probabilities and variables one can calculate the point at which vaccination makes sense for hospital personnel (Figure 1). If the risk of contact is greater than one in 100, and the risk of release is greater than one in 1,000, then vaccination is warranted.
In addition, one must carefully consider the risk of contact. There are approximately 100 million emergency room visits every year. There are a lot of personnel in emergency rooms who could be eligible for pre-exposure vaccination. But, these numbers make the probability of contact very low.
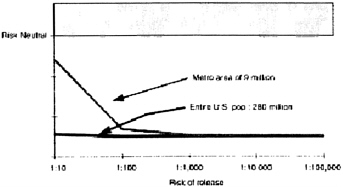
As for the general public, even if the risk of release is 1:10, in a population of approximately 280 million, pre-exposure vaccination is not warranted (Figure 2). However, not everybody in the United States is at equal risk of coming in contact with the first number of people infected with smallpox. Those living in metropolitan areas are at higher risk than those living in rural areas. Sensitivity
analysis is also critical, that is, how many cases of serious vaccine-related side effects are comparable to one case of smallpox? Even if this value is set at 40, the impetus for pre-exposure vaccination is still lacking.
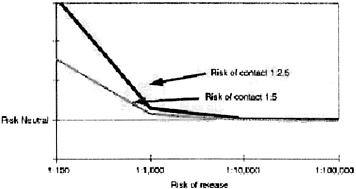
Risks for investigative teams are obviously higher (Figure 3). The risk of contact could be as high as 1:2.5, but the risk of transmission is likely to be lower, perhaps 40 percent if the investigator takes proper precautions. These individuals are candidates for pre-exposure vaccination.
Model 218
The first step in this model was to develop plausible attack and response scenarios. Four scenarios covered a range of possibilities: 1) a hoax; 2) a laboratory release in which one family is affected; 3) an aerosol attack on a large building resulting in 350 initial cases in a big city; and 4) a coordinated attack on airports with varying degrees of success.
A systematic literature review conducted in cooperation with the Southern California Evidence Based Practice Center encompassed 6,000 titles. Over 1,500 articles and books were selected and reports of 41 post-World War II smallpox outbreaks in western Europe and North America were identified and reviewed. From these reviews inferences were drawn about what smallpox spread was like before control measures were put in place. In western Europe, health workers were fairly well vaccinated, whereas the general public was not. Thus, some assumptions and probabilities could be derived from that context that could be comparable to the current environment.
Based on this model, if health care workers across the country were not vaccinated pre-exposure and there was a single building attack, ring vaccination and isolation would result in 2,117 seriously ill and 400 dead. With prior health care worker vaccination, that would drop to 1,830 ill and 245 dead. Many of the dead will not be in the city where the attack occurred, but will die as a result of infection from contacts. Another consideration is the impact that exposure might have on health care workers. They might be too ill or fearful to report to work. They will be concerned about exposing their families to infection. However, this model suggests that families of vaccinated health care workers are actually at less risk in the case of an outbreak than the general population because the individual who would bring the infection into the home is most likely using protective measures in the workplace.
If policy makers think that the chance of an attack on a single large building, for example, a federal building, is 19 percent or greater over the next five years, then vaccinating first responders and other health care workers makes
sense. However, if the mayor of a large city receives credible warning that the risk of an attack on city hall is 1:100, he or she is likely to demand pre-exposure vaccination for his or her employees.
For relatively small attack scenarios, for example, a laboratory release, ring vaccination and isolation are effective enough to achieve containment, however, 43 deaths will still occur. However, current models are relying on outdated data. There are now better barrier methods and institutional isolation is likely to be more effective than it was 50 years ago.
Model 319
To analyze smallpox response options, this model mathematically embeds the response logistics into disease transmission models and then tailors the logistics models for alternative response policies. The idea is to evaluate options in terms of deaths, disease, and duration of response to arrive at a good policy decision. In this model, there is an option to switch to a broader vaccination strategy if ring vaccination fails to contain the spread within the first two generations of cases.
A trace or ring vaccination model was contrasted with mass vaccination (i.e., 100 percent of the population as opposed to a random 60 or 70 percent). A switch from trace to mass vaccination is modeled if the former policy does not contain the outbreak following the first two generations of cases. The “race to trace” model depends on several operational variables: linking the disease progression in index cases and their contacts, availability of resources, availability of sufficient numbers of persons who actually find and vaccinate people, and the length of time it takes to do so. In this sense, the model can also be used as a staffing tool, for example, how many vaccinators would you actually need to achieve different outcomes? The disease stages and transmission progression in this model are taken from the classical smallpox literature. However, the model assumes worst case population mixing patterns—so-called free mixing.
Using the New York metropolitan area as a working example, assume there is a large attack on 1,000 persons at Pennsylvania Station. The initial transmission in this model is three new infections per initial case at the start, the so-called reproductive number. A person is infectious, on average, for three days. At the beginning of the epidemic, such a person would transmit three infections, on average. The detection delay is estimated at five days. Changing the detection delay is very similar to changing the initial attack size. If it takes longer to detect an attack, there will be more transmission before a response begins. The contact tracing accuracy in this base case is 50 percent, that is, 50 percent of all contacts can be located and vaccinated (this can vary from 10 to 100 percent). This
model also assumes vaccine efficacy at 97.5 percent, and a vaccine fatality rate of one in one million.
With these parameters, using only traced vaccination and case isolation, there would be 97,000 deaths and 324,000 cases over a response that would last for 440 days until the end of the epidemic. By contrast, post-attack mass vaccination would result in only 525 deaths, 1,720 cases, and would only take 115 days—a ratio of 180:1 of deaths per traced versus mass vaccination.
The CDC interim policy under such an example would greatly reduce the toll from trace vaccination but exceed the toll from mass vaccination, at 3,345 deaths over 160 days until the end of the epidemic. So, the cost of delay of starting with choice vaccination before switching to mass vaccination would be 2,820 lives.
When does it make sense to do trace versus mass vaccination? The two critical parameters are the initial attack size and the reproductive rate. Under the base case scenario of 50 percent tracing accuracy, mass vaccination would result in fewer deaths for any initial attack size or reproductive number. In the event of a very low initial attack size and a low reproductive number, tracing results in fewer deaths. If tracing is 100 percent accurate, one would still favor mass vaccination for any reproductive number greater than 3.6.
Under the worst-case scenario, even if only 35 persons were infected in the initial attack, for any reproductive number greater than 1.5, one would prefer mass vaccination. What this means is that increasing the initial attack size from 1 to 35 is all it takes to overcome the benefit of going to perfect tracing accuracy from 50 percent.
In a less severe scenario, suppose that all household contacts or close contacts were found in time and that those deaths were preventable by vaccination. The open question is the number of transmissions that would be extra-household or outside someone’s close circle of contacts. Even in this scenario, the qualitative result holds—a small initial attack size and small reproductive number favor trace vaccination. Larger numbers of both favor mass vaccination.
The consequences of making a mistake are asymmetric. If it turns out that, in fact, the tracing approach is optimal and one chooses mass vaccination there will be additional deaths, but the additional number will not be large. On the other hand, if it turns out that mass vaccination is actually optimal, but the choice is for trace vaccination, the number of excess deaths could be quite large, in the hundreds to thousands. What this says is that one might consider trace vaccination only if there is extreme confidence that the initial attack size and reproductive numbers are favorable. If there is a good chance that the policy will switch to mass vaccination, then in terms of minimizing the number of deaths, one should use mass vaccination from the outset.
Pre-attack vaccination reduces the reproductive rate at the time of the attack, and makes post-attack surveillance and containment much more attractive.
If tracing fails to contain the epidemic, the disease will spread widely and consequently many people will be vaccinated. If trace vaccination results in 80
percent of the population getting vaccinated, but over a much longer period of time, and mass vaccination results in a 100 percent vaccination rate, the risk to the immunosuppressed is similar. However, screening out the immunosuppressed and quarantining them for their own protection over the duration of an attack would allow for rapid mass vaccination. Under the tracing approach, it is not realistic to imagine quarantining people for such long periods of time.
In conclusion, mass vaccination allows many fewer deaths over a much wider range of scenarios.
Soviet Outbreak of Smallpox20
A preliminary report that a 1971 smallpox outbreak in the former Soviet Union was triggered by a secret bioweapon field test suggests that smallpox can be aerosolized and that the Soviets might have experimented with turning an extremely deadly smallpox strain into a weapon.
The 1971 epidemic in the city of Aralsk, Kazakhstan, on the northern shore of the Aral Sea, affected 10 people. Three of the patients, who had never been vaccinated, developed the fatal hemorrhagic form of smallpox, which in other outbreaks occurred in fewer than two percent of cases. Two of the deaths from hemorrhagic disease were in infants under the age of one year.
According to the official report, all of the others who were ill had discrete classical smallpox, or what they called varioloid or mild smallpox. All of them had been vaccinated. The strain appeared to be unusually infectious, because 3 of the 25 people who were vaccinated against smallpoxand were close to a vaccinated patient got sick, an unusually high percentage. With 10 cases, the ability to do robust statistical calculation is obviously limited but inferential statistics have been used where possible.
News about the outbreak never reached the West until a classified official account, written in the 1970s, was sent to the Monterey Institute of International Studies in California in 2001 by a Kazakh scientist. The report concluded that the first patient most likely contracted smallpox while on a two-month voyage on the Lev Berg, an ecological research ship. The report stated that she probably picked up the virus during visits to Uyaly or Komsomolsk, two cities where the boat docked during its voyage, then brought the virus to her home in Aralsk. This account is problematic, given the mismatch between smallpox’s incubation period and the onset of her symptoms and the fact that the individual reported that she never disembarked at any of the ports of call. In addition, despite very aggressive searching, according to the official report, no other cases of smallpox were identified in the area and no delegations from Afghanistan, the nearest
country that had endemic disease at the time, were in the country at the time, so they were unable to find the source from which the index case of infection came.
An alternative explanation is that she was infected when the ship passed close to Vozrozhdeniye Island, a top-secret outdoor testing site for bioweapons. The former Chief Sanitary Physician of the Soviet Union was quoted in the November 13, 2001, Moscow News saying that around the time of the outbreak a research vessel in the Aral Sea approached the island at 15 kilometers distance and that smallpox virus reached the index case. At the time, there was smallpox virus stock on the island. It is probably the case that smallpox was aerosolized, which answers the age-old question of whether or not smallpox is in fact aerosolizable and infectious in that state.
A criticism of this theory is based on speculation that ultraviolet (UV) light would quickly kill the virus in an aerosol cloud wafting over the Aral Sea. However, it is possible that aerosol tests would have been carried out at night to reduce UV exposure. One possible reason for why only one person on board the vessel became infected is that the index case was particularly vulnerable because she spent much more time on deck than other crew members.
Studying the strain or tissue samples from the 1971 outbreak, which could be stored somewhere in Russia, would answer some questions about the outbreak and the source of the isolates. Initially, the biodefense laboratory in Siberia where the Russian smallpox isolates are kept denied any knowledge of the incident, but has since agreed to search for specimens.
LESSONS LEARNED FROM RAPID MOBILIZATION FOR ANTHRAX MASS PROPHYLAXIS: IMPLICATIONS FOR SMALLPOX
Three jurisdictions in the Washington, D.C., metropolitan area were affected by the release of anthrax spores via the mail in fall 2001. Michael Richardson of the Department of Health in Washington, D.C., Susan Allan of the Arlington County, Virginia, Department of Human Services, and Georges Benjamin of the Maryland Department of Health and Mental Hygiene provided their perspectives on the anthrax response and its implications for a smallpox response strategy.
District of Columbia21
The anthrax exposures via a letter to Capitol Hill through the Brentwood Postal Facility in Washington, D.C., necessitated the rapid deployment of a mass
prophylaxis for postal workers in the Washington metropolitan area. Modification to the National Pharmaceutical Stockpile (NPS) Use Plan facilitated distribution of prophylaxis to over 15,000 individuals within a 2-week period. Incident management was conducted through the District of Columbia Department of Health using an incident command structure sited at the recently closed D.C. General Hospital.
Challenges were encountered in four main areas: 1) event management, 2) dispensing of medications, 3) resources, and 4) communications. Each area presented overlapping and confounding issues because of the uniqueness of the Washington Capitol Region.
The anthrax attack was distinctive in some ways because it was a risk population that was well defined in terms of time of exposure in the work area, although that definition was fluid and changed as the response progressed. Over a 2-week period, 17,759 individuals were seen and initially prescribed ciprofloxacin (160,160 tablets distributed), later substituted by doxycycline (497,880 capsules distributed). Individuals were given an initial 10-day supply, and then a 50-day supply. Notably, 300 Department of Health staff were involved in this prophylaxis effort.
Because there were numerous jurisdictions involved (D.C., Maryland, Virginia, the federal government) coordination was essential and a lead agency had to be defined. Resource requirements, such as staff, transportation, and telecommunications, were time limited but a valuable lesson learned was that it is best to assume the worst-case scenario and then scale down resource requirements as more information becomes available. In planning for future events, public health officials learned that they needed to determine the required staff categories in advance and model resource needs, for example, individuals trained to give vaccinations, mental health workers to provide counseling and support, and individuals trained in the informed consent process. They also realized that in distributing medications they had to identify and design the distribution plan in advance and develop protocols as well as patient education materials. Moreover, the departments had to identify and track persons and their doses, and clinical evaluations were often required in the dispensing area. These requirements were made more difficult by postal employees who were reluctant to disclose medical information. In addition, many citizens felt that there was differential treatment between congressional workers and postal employees.
One of the most important lessons learned was the need for a credible spokesperson to discuss events and responses publicly and to give recommendations. The types of information and messages that are necessary to reach diverse audiences was a challenge for the District of Columbia health officials, who faced vast ethnic, racial, and socioeconomic diversity. It was also difficult to get real-time information out to health care workers in the field, so vital staff had to be equipped with two-way pagers and establish inter-site communications. Dedicated transport vehicles were needed for each site. Timeline information was necessary, as was dissemination of information from a system that was not geared to give real-time information in a changing situation, where the parame
ters and the scale of the attack were not known. This required the establishment of an expedited approval process for release of information and the creation of a call center.
Smallpox raises additional concerns that were not present during the anthrax attacks—primarily the potential for quarantine, isolation, or civil confinement. Another challenge that will be faced in smallpox that was not as significant with anthrax is identifying the risk population so decisions can be made about vaccination or control measures. Related to this is defining the first responder category. Classically, in incident and disaster management first responders are firefighters, emergency medical personnel, and police, but in smallpox the first responders might also be private physicians and their staff.
Civil cooperation with government recommendations is another issue to be considered. Local officials found in the anthrax response that if information was communicated within a reasonable period of time, and if the spokespeople were credible, for the most part the citizens did what was asked of them. However, the past may not be the future. In planning a response, the ability to manage the safety of people and manage their movements must be evaluated, given that some people distrust the government. Educated health workers will be key in communicating with the public in a way that protects their health and promotes public health goals. D.C. public health officials learned that communicating all information to the media, especially when dealing with uncertainty, was not always productive. An appropriate response involves protecting civil rights and liberties and responsible communication, which includes the right of the public to receive accurate information.
Arlington County, Virginia22
National policy is only as good as its implementation in each and every community. Thus, constraints on implementation of policy in the community have to be considered as policy decisions are being made.
There is considerable pressure from the public to receive the smallpox vaccine. The Arlington County Department of Human Services has been receiving requests from members of the community for smallpox vaccination for three years. These have included physicians who have brought their families to open immunization programs to get the vaccine for their families. The appropriate decision about immunization in the absence of immediate crisis should be based on an assessment of health benefits and risks, an assessment that currently does not support a mass vaccination policy.
At the height of the anthrax event, physicians in the Arlington area were inappropriately dispensing ciprofloxacin in response to patient anxiety. Public
health officials need to be cognizant of how their gestures and actions say at least as much to the public as do formal education efforts. To say there is no risk, and then offer a prescription or a vaccine, is sending a mixed message, which could breed distrust in the future.
The need to pre-vaccinate health workers is not clear. Personnel in local public health departments and emergency rooms know how to use masks and other appropriate precautions in the face of unknown and possibly suspicious disease. However, it would be reassuring and even constructive to ensure that pre-vaccinated teams, regionally located, are available and quickly deployed in the first hours or days of identification and response. Beyond that core group, the number of personnel who might expect or demand pre-vaccination could become unmanageably and unjustifiably large, and could include community physicians, nurses, physical therapists, school nurses, and hospital personnel responsible for laundry and food preparation. Where should the line be drawn?
To the extent that the pre-vaccination of health professionals begins, as a policy matter the obligations to treat should be imposed, that is, ensuring that these individuals are aware of their legal and moral responsibilities to the public to remain on the job in the event of an outbreak. Physicians in the private sector do not always regard themselves as part of a public response—they feel obligated to their individual patients, but not necessarily to the general public. This attitude could be changed through an effective professional education program. On the other hand, police and firefighters, who understand their duty to the public, are very clearly telling public health departments that they want to be vaccinated and treated, and want to be provided with their own antibiotic packs should they need them.
Public health departments are familiar and comfortable with surveillance and containment; on a daily basis this strategy is used with tuberculosis and meningitis. However, the issue of magnitude is daunting in the event of a widespread outbreak or attack. At that point, a strategy is needed for rapidly mobilizing immunizations for those immediately on the forefront of investigation and response.
How to contain an immunization strategy is problematic because the public will panic and demand treatments they do not need. This was certainly the case with anthrax. Physicians could play a significant role in containing panic but they did not feel prepared during the anthrax crisis to reasonably deny their patients what they wanted. Moreover, other vaccine shortages have left physicians feeling vulnerable and ill prepared to meet patient needs.
Whatever the eventual policy becomes regarding smallpox vaccine, it cannot be considered in a vacuum. A larger system of disease preparedness and protection must be developed. If the health care community is prepared for anything, not just smallpox, then everyone is better off. That requires better education and preparation of physicians and an improved public health infrastructure.
Maryland23
In fall 2001, five letters containing anthrax spores were sent through the mail through four big regions of the country—the Washington metropolitan region (Maryland, Northern Virginia, and the District of Columbia), Florida, New York and New Jersey, and Connecticut. There were a small number of people directly affected in terms of illness, but more than 33,000 people were prescribed ciprofloxacin or another prophylaxis. In total, there were 18 confirmed cases—11 were the inhalation form and 7 cutaneous—of which there were 5 deaths.
The State of Maryland quickly learned that its knowledge base was inadequate and original assumptions were incorrect. Prior to 2001, public health officials assumed that anthrax was difficult to weaponize and deliver in mass, that it would stay put once delivered—that is, it would not aerosolize,—that only those who opened the letter would be exposed, and that at least 8,000 spores were needed to become ill. It was also assumed that inhalation anthrax was 90 percent fatal and that 60 days of antibiotic treatment was sufficient.
A poor knowledge base and false assumptions could hold true for smallpox. Public health officials cannot claim to know what the delivery mechanism will be and therefore must think broadly about disease tracking and disease surveillance strategies. If an attack occurs, one would assume that multiple regions of the United States will be involved, and that public health will not only have to be involved in the health delivery and surveillance aspects of a response, but, also, the immediate briefing and education of the public and policy makers at a variety of levels. Moreover, it is likely that hoaxes will accompany real threats.
Based on the anthrax experience, one can assume that demand for vaccination will be high, as it is every time there is a meningitis outbreak in a school. A vaccination plan will be needed that is clearly, unequivocally, and uniformly communicated to the public. There has to be national leadership and an effective spokesperson who can address multiple audiences and ensure that the message is delivered in multiple languages and formats.
A major challenge is facing public perceptions about risk and accurately communicating the risks of disease, vaccination, and treatments. People understand and process risks differently, based on their own experiences. Those differences must be anticipated and accounted for in communication plans. The first smallpox death, with all the pockmarks, is going to radically change public perceptions of risk and therefore the way risk communication should evolve. Likewise, the first death from the vaccine will change the risk perceptions and expectations of the public. Moreover, public health officials must anticipate how a smallpox vaccine death might negatively alter public attitudes about other vaccination programs.
In Maryland, less than 10 percent of routine vaccinations are given by the public health community. More trained vaccinators are needed. There must be equal access to vaccine for the poor and underserved and a means established by which the uninsured will be covered should they experience vaccine complications. Other employment-related issues to consider include lost time at work, workers’ compensation, particularly if vaccination is expanded to a broader range of health care workers, and workers who have no insurance or cannot take time off from work. For low wage earners, vaccination might not be an effective use of their time based on the risks.
In terms of vaccine delivery, medical liability issues must be resolved, as must ethics review of research protocols. Just because a protocol is approved by an Institutional Review Board at the federal level does not mean it will be approved by a local board. Vaccine wastage is a real concern for the public health community because even if it is medically warranted, the public might not be willing to accept that waste of resources. A big question is whether health care providers will be willing to give a vaccine that causes some people to die when there is no known case of the disease on the planet.
Control strategies, such as isolation and quarantine, will be controversial. Standard procedures will be needed to avoid high-risk exposure to vaccinia and create preventive measures to avoid secondary exposure. At some point, the emergency medical system might have to be redesigned to send infectious cases to a designated facility.
Finally, public health officials always will be faced with the challenge that they can only make recommendations based on the science available at the time. Nevertheless, the public must be informed about the assumptions underlying the recommended policy and how the assumptions and policy might change as new information becomes available. The anthrax response failed in that regard— public health officials did not communicate to the public that things were going to change. Although public health departments were continuing to chase the epidemic and were being responsible public health officials, there was no media strategy already developed to tell the American public that the official response might change, so be prepared.
From a scientific perspective, spokespersons can articulate where the uncertainty lies and where the problems are but from the emergency management perspective, which operates on a paramilitary structure, decisions have to be made based on the best information available at the time. Bringing these two cultures together, and coming up with a unified message, is critically important so public health does not lose its ability to make an impact in future times of emergency.
EMERGENCY RESPONDERS
ACIP is deliberating various policy options for pre-exposure vaccination of specific occupational groups at high risk for exposure to the smallpox virus.
Representatives of emergency responders, such as emergency medical technicians, firefighters, and emergency services, were asked to provide commentary on the ACIP options.
Emergency Medical Technicians24
The National Association of Emergency Medical Technicians (NAEMT) represents the professional interests of over 870,000 emergency medical service responders, including EMTs, paramedics, and EMS first responders. A NAEMT position paper published June 7, 2002, describes four protective measures recommended for smallpox vaccination in the event of bioterrorism.
The first measure calls for the active participation of EMS organizations in community threat assessment in conjunction with relevant public health authorities. Participation of EMS organizations in such assessments would ensure accurate and timely communication to EMS organizations about the nature and level of the threat—potentially shortening the implementation time frame for actions necessary to protect the safety of emergency medical personnel—and would provide a mechanism for EMS input into the process.
The second measure calls for amending the EMT and paramedic national standardized curriculum to insure that all EMTs and paramedics are adequately educated about all terrorism responses, their implications, and their impacts on the health, safety, and well being of the EMT community.
The third measure calls for the voluntary smallpox vaccination of EMTs and paramedics in the absence of a confirmed smallpox case or a confirmed smallpox bioterrorism attack. The voluntary smallpox vaccination initiative should be modeled after the past precedents established in the OSHA standard pertaining to blood-borne pathogens.
A combination of tactics and technology allows EMTs and paramedics to operate safely while providing patient care and rescue services in a threatening environment. It is not likely, at least in the early, unrecognized stages of a bioterrorism attack, that those infectious smallpox patients seen as part of an emergency medical response will be appropriately diagnosed in sufficient time to adequately protect the responding personnel. Once there has been a recognition of a bioterrorism attack, it is not likely that a vaccination program could be completed in sufficient time to prevent a significant attenuation, or perhaps a complete collapse of the emergency medical response system. Affording EMT personnel access to the smallpox vaccination before the event is the tactical application of available technology that is both prudent and necessary.
Many EMTs, paramedics, and EMS first responders return home in the same clothing and the same condition that they left work in, which could result
in transmission of infection to their families. The fourth protective measure proposes that smallpox vaccination be made available to the immediate family of an emergency medical service member without delay upon recognizing that a smallpox emergency has been identified. The foundation for this proposal is clearly delineated in the NAEMT position paper. Adopting this proposal is logical, if only in limiting the propagation of the disease by eliminating the families of rescuers as potential sources of infection.
Fire Chiefs25
There are 26,000 local fire departments in the United States with variable organizational structures. Perhaps the only unifying component of the vast majority of these systems is that fire services provide EMS first response, as well as the majority of emergency ambulance transport. The anthrax attacks highlighted the role of the fire service in providing hazardous material clean up, placing an enormous strain on local response systems.
At this time, the International Association of Fire Chiefs does not feel it is appropriate to vaccinate fire service personnel pre-exposure, in part due to the risks of the vaccine. Regarding Question 3, the use of ring vaccination as the primary control strategy in the event of a confirmed outbreak, the International Association of Fire Chiefs favors Option 2, that is, supplementing ring vaccination with nationwide vaccination of those who would assist in the containment, including fire service personnel. Any vaccination policy must be supplemented with an aggressive public and health care provider education campaign.
Emergency Services26
The California Governor’s Office of Emergency Services (OES) is one of the largest emergency management organizations in the United States. Under the authority of the California Emergency Services Act, as well as other California legislation, OES is responsible for the mitigation, planning, preparation for, and coordination of California’s emergency response to, as well as a recovery from, the effects of multi-hazard emergencies that effect lives, property, and the environment. OES works cooperatively primarily with local and regional agencies in California. California also stands ready to assist other states with the resources and expertise of the emergency management communities within California.
While OES does not represent any one class or discipline of responder, it does have extensive experience working with and coordinating the efforts of
both traditional and non-traditional emergency responders in various situations. It is not the intent of OES to second guess or judge the risk assessments that have been made by other subject matter experts on these issues.
In reaching its recommendations regarding smallpox vaccination, OES made two additional assumptions surrounding the use of the live vaccinia vaccine. The first is that respect for individual autonomy requires that any use of smallpox vaccine in any situation be voluntary. Alternatives for the protection of individuals, and also the community exist in any situation where an individual refuses smallpox vaccination for whatever reason. It follows from this assumption as a second assumption that absent the re-introduction of smallpox into the wild, individuals, including emergency response members, have a reasonable expectation to remain free from either inadvertent or unwanted exposure to vaccinia virus via transmission from recently vaccinated individuals.
California OES agrees with CDC’s risk assessment that the risks of the smallpox vaccine outweigh the benefits for the general public, and that the vaccination of the public should not be undertaken at present. In support of that assessment, OES strongly encourages the public health community to present a unified and unequivocal recommendation regarding this option (Option 1).
It has been the experience of OES that members of the general public respond in a cooperative and constructive manner when presented truthful information by subject matter experts who speak with a consistent message. Since the adoption of a position of no public vaccination will require educating the public about the risk assessment that forms the basis for that recommendation, any perceived equivocation will make successful implementation of such a policy difficult at best.
Question 2, regarding the possible expansion of pre-exposure vaccination, is perhaps the most difficult question to look at from the emergency management perspective. The specific occupational groups that make up the disciplines that are required to investigate, contain, control, and treat a reintroduction of smallpox into the community are heterogeneous, and occupy a spectrum of risk that is intermediate between the orthopox virus laboratory workers and the general public.
In constructing a rational policy regarding this question, OES believes it is useful to focus on the specific missions of the various occupational groups, and consider the time periods and the natural history of the smallpox outbreak involved. Given the existence of alternative and complementary protective measures—such as respiratory protection, isolation, and immediate post-exposure vaccination—it seems prudent to focus pre-exposure vaccination on the groups that could be reasonably expected to be at highest risk of exposure early in a smallpox outbreak. These groups appear to be primarily epidemiologists, public health responders, and others who would be investigating unusual vesicular rashes or disease outbreaks in the population. OES believes it is neither ethical nor realistic to ask these occupational groups to investigate the earliest stages of what may be a smallpox outbreak, given the existence of an effective vaccine,
without being offered voluntary pre-exposure vaccination. These higher-risk occupational groups are best identified in the context of an organized, pre-planned smallpox investigation team or smallpox investigation program that has specific tasks and individuals assigned. These teams could variously be located at either regional or state levels, and created in response to a specific state-level risk assessment.
OES supports the development of specific guidelines to assist the states in developing the appropriate response mechanisms that take into account each state’s unique risk, the resources available, and the geographic proximity to federal smallpox response resources.
OES recognizes that this pre-exposure vaccination recommendation does not include most traditional first responders such as general fire responders, law enforcement, and paramedical responders, as well as other health care workers. In addition to the lower relative risk that these groups have for an unrecognized exposure to wild smallpox virus, that others have observed, the logistical difficulties of a widespread voluntary vaccination program, the impacts of an already stressed emergency response system resulting from the decreased productivity of these key responders due to local vaccine reactions, as well as the possible logistical and financial costs associated with any restriction on the movement or occupational activity of these recently vaccinated responders, makes a general first responder vaccination program both non-effective and unwarranted at present. Any perceived needs within the first responder community should not allow inadvertent harm to occur to this population through good intentions.
Adoption of any recommendation for a limited pre-exposure responder vaccination must be accompanied by an intensive education process to address: 1) the legitimate concerns of those at lower risk, non-vaccinated first responders; 2) the efficacy of complementary smallpox vaccination strategies; and 3) the distinctions among risks assigned to various occupational groups. Most first responders understand and accept the inherent risks involved in their chosen profession and will respond appropriately to a reasoned explanation of the scientific and public health rationale for this recommendation. Emergency responders understand they cannot function in an absolute risk-free environment, and will respond as long as the risks have been minimized to the lowest practical level.
The recommendation of selected pre-exposure vaccination appears to represent the lowest practical risk level regarding smallpox. OES recognizes that knowledgeable persons may differ in the designation of the relative higher versus lower intermediate risk groups, and strongly urges that to the maximum extent possible, objective and consistent guidelines be used by the states in identification of these occupational groups. It is vital that these pre-exposure smallpox vaccination volunteers be thoroughly educated in the risks and benefits, that appropriate safeguards be in place to reduce inadvertent subjective coercion of the responders’ decision to receive a pre-exposure vaccination, and that these persons, because of their vaccinated status, be fully utilized in a smallpox response program in the event of reintroduction of smallpox virus into the population. In addition, mechanisms are needed to insure that rapid post-exposure vac
cination is available to all first responders if this recommendation is to have any credibility with the emergency response community.
With regard to Question 3, OES recommends that the fundamental biology of variola be considered in light of the collective human experience, which favors primarily ring containment. However, it is naive to suspect that there would not be a huge public demand for vaccination if a smallpox outbreak were to occur. Therefore, any vaccination plan will have to prioritize levels of community vaccination.
HEALTH CARE PROVIDERS
ACIP is deliberating various policy options for pre-exposure vaccination of specific occupational groups at high risk for exposure to the smallpox virus. Representatives of a number of health care provider groups provided their perspectives on the vaccination policy options.
Nurses27
The Omaha medical response system consists of nine subcommittees that have developed component plans in the areas of pharmacy, public health, laboratory equipment, training, medical health services, media response, communications, alternate care facilities, and hospital response. The hospital response plan includes a memorandum of understanding among all hospitals regarding sharing of resources, personnel, and equipment, as well as localizing infected individuals in one facility. A quick reference guide was prepared listing possible biological and chemical agents and guidelines for treatment. These guides will be available to EMTs and placed on handheld devices.
The Emergency Nurses Association and the American Nurses Association support a broad public awareness campaign regarding smallpox, the nature of the disease, and the risks and benefits of the vaccine. A well-designed campaign will help affirm public opinion and minimize panic. In addition, health care providers must be educated to recognize smallpox and universal systems for surveillance of symptoms are needed in emergency departments, offices, and clinics. The key to control by ring vaccination will be, first and foremost, identification of the illness.
It is likely that too many unimmunized health care providers will be exposed and develop the disease long before a single case is identified and isolated. Education of health care providers regarding recognition, isolation procedures, protection, and vaccination are likely to assist in early identification and reduce exposure. Currently, nurses are not educated in smallpox vaccination
procedures, and are not taught the techniques. Competencies will have to be established either before an outbreak, or very quickly following. At minimum, each community should have protected and competent public health officials who can begin vaccination procedures and care for infected patients.
In the event of a smallpox outbreak, emergency department staff should be considered first responders. Research shows that as many as 70 percent of all victims from major known disasters will use means other than public transportation to get to hospital emergency departments. Emergency department staff, along with law enforcement, physicians, pre-hospital providers, public health workers, and laboratory personnel, should be considered at high risk for disease following exposure, and should be included in immediate vaccination plans.
Recall of staff to provide care to patients following confirmed cases of smallpox may be difficult if staff is not immunized. Most staff will report to duty following a disaster when there is minimal or no risk. However, the response rate is likely to be lower if staff are required to care for patients with a highly contagious and deadly disease. The unknown factor of recall of staff, combined with the nursing shortage, will place care delivery at risk.
Although everyone recognizes the need to prepare for the possibility of smallpox coming into their hospitals, no one wants to be pre-designated a contagious hospital, even if that means getting the vaccine to their staff over others. Should one hospital be identified first as a contaminated site, cases must be localized to that site and staff immunized.
The nurse’s organizations agree with CDC’s recommendation that if there are no known outbreaks of smallpox, the general public need not be vaccinated. In the absence of a confirmed case or confirmed bioterrorism attack, persons, pre-designated by public health authorities who will have direct contact with or will investigate the initial cases of smallpox, and those considered essential medical personnel should be vaccinated. Those who should be considered essential medical personnel are first responders; emergency department personnel; law enforcement; ambulance service providers; those who will be called to assist in transportation, fire, and rescue units; and all hospital personnel who assist in the internal investigation and care of patients. These teams could then safely respond and provide early care in an incident. Designated state, local, and national public health individuals who would have the authority to investigate exposures should be vaccinated.
In the event of a confirmed smallpox case, or confirmed bioterrorism event involving smallpox, surveillance and containment will be needed to identify, control, and contain the disease. Public health officials should determine the exposure risk, and direct vaccinations be given to those at risk. Federal, state, and local public health officials should determine the national mass immunization response plan. Because of the mobility of the world’s population, CDC should work with the World Health Organization for a global response plan to smallpox, or a confirmed smallpox outbreak. The strategy for ring containment around each known case of smallpox will be difficult given the possibility that a single case exposure could expose individuals on different continents.
The fear created by an outbreak of smallpox may prompt the public to demand vaccination. If the United States has sufficient vaccine, CDC should consider universal vaccination in the cases where they deem it necessary, depending on risk. Open and public dialogue regarding the advantages and disadvantages of vaccination is needed now.
Emergency Room Physicians28
Emergency physicians are obligated to treat all patients, regardless of their illness, so they must be prepared for anything. The experience with anthrax taught the public health community that it was not prepared, and conflicting, delayed, and sometimes inaccurate reports from public health officials exacerbated the inefficiency of the response and its logistics. Strategies had to be constantly revised as new information became available. Lessons were learned about the difficulties of communicating uncertain information through the media.
The anthrax experience also taught the health professions that even when the risks are low, public concern is contagious, including among hospital personnel. This is something that emergency personnel in an urban area like New York City must be concerned about, that is, the difficulty of responding to a few cases of smallpox exposure when there might be multiple dispersion in a public and densely populated area. The effectiveness of a ring containment strategy is not clear.
Our current response requires that more senior physicians see patients coming in with unexplained rashes, because these individuals are more likely to have been vaccinated and retained some residual immunity. If a case is suspected, immediate isolation will be imposed. Unless the risk of smallpox is zero, the proper response is to be prepared through a voluntary vaccination process for a qualified group of health care providers, including emergency physicians.
Family Physicians29
Two assumptions about smallpox must be considered. First, that the risk is low; there is no longer a naturally occurring case of smallpox, and there is no evidence to suggest that that the risk of an attack is anything but low. Second, planners accept the defined risk of the vaccine. In fact, the risk calculations about the vaccine from 1968 may be low, considering today’s population. The
mobility of our population and the interactions that individuals have with health care workers and responders who may be at high risk is significant.
Given those two assumptions, however, the American Academy of Family Physicians recommends no change in the current CDC guideline vaccination policies for the general population. With regard to other workers who may be vaccinated, the Academy supports the vaccination of investigative teams at the state and local levels.
If there is a known smallpox event, the Academy supports CDC’s surveillance and containment strategies, but would also support the flexibility of allowing other measures to be implemented as needed, based on that event. For example, if there was a laboratory accident, ring containment might be the only strategy needed. In contrast, if there was a mass aerosolized event, in which a significant portion of the United States was exposed, other strategies would have to be considered. In any case, there must be continued enhancement of communications between the public health and private sectors. Clearly, the anthrax incidents and recent vaccine shortages have taught us that lesson.
Pediatricians30
If there were mass vaccination in this country, children and their families would suffer the adverse effects to a significantly greater degree than the healthy adult population for many reasons. First, children are more likely to self-inoculate, as they are not able to control scratching of lesions. Moreover, their parents are likely to be inoculated by the same process. Second, eczema is a common problem in small children, and vaccine would have to be withheld from affected individuals or they might develop eczema vaccinatum. When children experience these side effects, because of the difficulty of managing them at home, they are more likely to be hospitalized.
Day care attendance must also be considered. Should children in day care be immunized and if so, should they be withheld from day care for a certain period of time? If so, their parents would have to care for them during that time, which has a cascade of effects.
Mass immunization of children is an important component of preventive health care in this country. When universal immunization was in place, children under one year of age were not immunized, primarily because at that age congenital immunodeficiencies were not yet identifiable. In addition, infants are more likely to have uncontrolled atopic dermatitis than older children, which might be a risk factor for progressive vaccinia disease.
It is important to consider smallpox vaccination in the context of other immunization campaigns. Recent vaccine shortages have created new pressures on universal immunization strategies that might be far more important to address
than universal immunization for smallpox. With limited resources, society may very well be substituting protection against disease that does exist in this country—pneumococcus, influenza, varicella—for protection against a disease that does not exist. Forcing health care providers who care for children to make that choice is simply not something that society should do.
Any communication campaign concerning smallpox must include children and the people who care for them—schools, churches, temples, and community organizations that work with children. Messages have to be crafted that are understandable to children in a way that helps them deal with the issue rather than alarm them. If the risk is small then that message must be conveyed and the public reassured that the risk will be reassessed periodically.
Hospital Workers31
The Service Employees International Union represents 1.5 million workers in the United States, Canada, and Puerto Rico, including nurses, doctors, EMTs, laboratory workers, and other hospital support staff workers, mostly interacting with patients and working directly in patient care areas.
Public education is particularly important for smallpox given that the general public, based on its experience with other vaccines, will assume that the vaccine is entirely safe. An education and communication strategy must clearly outline the risks of smallpox and the vaccine, an area in which CDC does not have a great track record following its handling of the anthrax attacks. Many members of the public will remember the disparate handling of employees in the Hart Senate Office Building—mostly upper-middle class, white congressional staff—and employees of the Brentwood postal facility—a predominantly black, blue collar population. In addition, members of the Service Employees International Union are already asking why CDC is vaccinating its own employees and not offering the same protection to hospital personnel. Until public confidence can be bolstered and public education implemented, a mass smallpox vaccination program should be delayed. In the absence of public education, an announcement by DHHS limiting the vaccine to select groups of first responders is likely to be greeted skeptically and followed by legislation making the vaccine available to all members of the public based on constituent demands.
The final policy must include an explanation as to why certain groups are to be vaccinated and others are not. Once it is decided which frontline health care workers should be vaccinated, it is clear that such vaccinations must be voluntary. Workers who refuse to be vaccinated should not be discriminated against in any way or reassigned to different jobs. In addition, as vaccinated individuals may pose a risk to their co-workers or patients for a period of two weeks, plan
ners need to consider paying vaccinated workers during this period of time rather than requiring that they use sick leave or vacation time.
Hospital Systems32
HealthPartners is a health care system that includes financing and health care delivery, employing approximately 550 physicians. Syndromic surveillance for the respiratory syndromes associated with bioterrorism events is conducted daily. HealthPartners also has a major teaching hospital affiliated with the University of Minnesota. The hospital has 64,000 emergency room visits per year; it has a level-one trauma center and a burn center serving the upper five Midwestern states.
Planning for smallpox should be done in consideration of risks. If a bioterrorism event unfolds in ways analogous to previous smallpox epidemics—a few cases here and there, perhaps building over time—then vaccination policy should be more conservative. However, if one thinks about September 11—the unthinkable and unimaginable—it is not difficult to envision a bioterrorism event in which there is a release of an agent by aerosol, reaching hundreds or thousands of people simultaneously. In this case, the strategy should be quite different. Thus, which underlying assumptions about the risk of such an event should be used in planning a vaccination strategy? Regardless, under no scenario should mass immunization of the public be conducted.
Question 2 is where the differences arise, depending on the scenario. If there are just a few localized cases, immunizing first responders is sufficient. However, in the mass casualty event it would be far better to think about broader voluntary immunization of personnel in the health care and emergency response systems. It is not logical to assume that early cases of this would necessarily selectively show up in emergency rooms. The realistic planning assumption is that ambulatory care offices, both of primary care physicians and urgent care facilities, would see these cases. Thus, there are two logical breaks in terms of the planning: one is the specialized response team, and the second is the primary first contact.
Question 3 is also sensitive to the scenario. In a localized situation, surveillance and ring immunization would be an appropriate strategy. However, the models presented by earlier speakers suggest that a mass immunization campaign would be the response for a more massive outbreak. The real concerns for health systems are issues such as liability and indemnification, availability of clear and explicit protocols and guidelines for the administration of vaccines and dealing with the complications, and programs for education of health professionals, not only in terms of the illnesses and the complications but also in the techniques and methods of immunization.
Policies must be in place at the state and local levels to allow rapid licensure of health professionals imported from other jurisdictions and states to help one jurisdiction deal with a serious crisis. Triage and quarantine issues, as well as surge capacity in our institutions, must be planned for in advance. And most importantly, there must be a clear plan for dealing with immunization capacity between the public health and private sectors.
Special Populations33
The HIV-positive population is representative of populations with cell-mediated immunity defects in general, for example, patients with organ transplants, or individuals treated with chronic corticosteroids or cancer chemotherapy. There are approximately 900,000 people living with HIV infection in the United States, although one-third to one-half are unaware of their serostatus, which means that any strategy based on mass vaccination is going to have some surprises unless everyone is screened for HIV status—an unrealistic prospect. Therefore, several issues relevant to this population must be considered when planning a smallpox immunization strategy.
First, the immune response in this population is blunted; anyone with a CD4 count of less than 200 probably counts for at least a third of the 900,000 infected individuals. These individuals do not respond to any of the current vaccines, and it might be problematic getting a response to vaccinia.
Second, the risk of progressive vaccinia to this population is real and it is not clear whether VIG will be effective. One case report from the military involved a patient unknown to have HIV infection until developing progressive vaccinia and dying. The cause of death was unclear, although there was rapid progression of HIV infection.
Third, the potential for secondary transmission to household members, spouses, and so forth, would pose a risk to this group, even if they are excluded from immunization programs.
Fourth, what is the risk of smallpox in a person with HIV infection? The mortality rate is 30 percent but it might be 100 percent in a person with advanced HIV infection.
Thus, anyone with defects in cell-mediated immunity should be excluded from mass vaccination or targeted vaccination in the absence of disease. With face-to-face exposure, however, the risk-benefit ratio changes and the inclination would be to vaccinate. That decision could be fine-tuned depending on the CD4 count of the individual in question.
The HIV population and some other special populations have a well-established and focused group of care providers. If there is a policy to vaccinate
these populations, implementation will depend on the acceptance of those physicians. About 3,000 physicians write 80 percent of the prescriptions for HIV drugs. Although it is not a big group, it is a critical group to reach.
ETHICAL, LEGAL, AND SOCIAL ISSUES
Ethical, legal, and social issues must be considered in addition to scientific and medical issues in designing a public health policy for smallpox prevention and response. Speakers were asked to provide commentary on ethical issues, indemnification for adverse events, communication strategies, and risk communication.
Ethical Considerations34
Public health involves the abilities of the state, under certain circumstances, to sometimes require individuals to act in ways that they might not otherwise act. Public health officials need to think about how to address situations in which individuals choose to do the opposite of what ACIP recommends.
For example, what if ACIP recommends against vaccination and an individual patient insists on being vaccinated, perhaps because of an unconfirmed bioterrorism attack? Or, as another example, what if someone not on the restricted list of personnel claims that due to the essential nature of his or her job, he or she should be vaccinated? In ordinary clinical care, health care providers aim to respect patient autonomy. The patient gets to judge the risks and benefits, in conjunction with the physician, and even if the physician is trying to dissuade a patient from trying a drug that might be risky, ultimately it is the patient’s choice. In the public health arena, society is not necessarily always going to respect the same level of patient autonomy.
Fairness, justice, and equity are additional principles to consider in developing any type of plan. Although the anthrax situation brought to the forefront issues concerning race, class, and access to power, it is important to remember that the science of epidemiology is often shaded with and confounded by sociodemographic characteristics. For example, those at highest risk for HIV infection come from segments of populations that are already disadvantaged in many other ways. When there is a negative confluence of morbidity and social factors, there is often a lot of controversy.
These factors have to be considered in making recommendations so that policy makers can anticipate the circumstances in which ACIP makes a recommendation about immunization and an individual refuses to comply. Perhaps
they refuse because of existing medical risks (e.g., impaired cell-mediated immunity), religious objections, or fear of side effects. But many might refuse because of mistrust of government, in particular its ability to present fair and considered public health messages. Are some of those reasons more compelling? The classic public health dilemma is always when is state coercion warranted, and how can such intervention be conducted in a way that respects civil liberties?
Ethical analyses in public health differ in some respects from clinical medicine. Informed consent is still important, but it is more difficult in the case of smallpox because the data are uncertain, incomplete, and changing, and there are serious time constraints in trying to get consent before vaccination. The determination of risks and benefits to some extent is going to be made by the government and if the new cell-based vaccine is under an IND, then the vaccine cannot be prescribed in the same way that approved vaccines currently are prescribed, which restricts patient choice and possibly access. Fairness is a particular concern when society requires people to do things they do not want to do, or forbids or discourages them from doing things they want to do. If access to the vaccine is restricted, then there must be a plan for how that access will be determined at the local level to ensure fairness. If people are being treated differently in one city with one outbreak versus another city with a different outbreak, there are going to be concerns about fairness and equity.
This conference has focused primarily on the ACIP recommendations, however, the decision-making process by which the recommendations are made, and the process by which they will be modified as new information becomes available, are equally important. The public must believe that policy makers considered all of the options, weighed all the risks and benefits, thought hard about it, and made the best decision under the circumstances, acknowledging that there was uncertainty. In public health, perceptions may be as important or even more important than reality. No matter how sound the guidelines, unless they are presented to the public in ways that they will understand and find acceptable, they will not work.
Indemnification for Adverse Events35
Vaccines generally are susceptible to litigation and liability for several reasons. Typically they are given to large numbers of healthy people. Thus, if someone experiences an adverse event, it can be relatively easy to persuade a jury that the adverse event was caused by the vaccine. If vaccines are distributed in the interest of public health rather than in the interest of profit, it by no means mitigates or reduces the possibility of liability litigation. This is especially true if
it turns out after a vaccine is distributed that the threat does not materialize, which occurred in 1976 with the swine flu vaccine. In addition, although it is typical for vaccines to be accompanied by warnings, such warnings, no matter how carefully constructed, often fail to hold up in the litigation process.
There are several reasons why smallpox vaccine could be far more susceptible to litigation than the typical vaccine. First, FDA review will to some extent be attenuated and could be incomplete at the time the vaccine is actually distributed. Second, the side effects are far more serious and common than those experienced with vaccines that people have become accustomed to over the past few decades. In addition, many of the individuals experiencing side effects will be those who were not actually vaccinated and thus they will be seen by juries as completely innocent, and possibly uninformed, victims.
In general, the public is not aware of the risks of smallpox vaccine. If the vaccine is distributed, there is a good chance it will be distributed quickly. It may not be provided by physicians. All of these factors make the smallpox vaccine especially susceptible to tort liability litigation, which could add up to billions of dollars if the vaccine is used on a mass scale. It is obvious that this kind of liability could threaten bankruptcy for a small vaccine firm, even for a medium-sized vaccine firm. Thus, policies are needed regarding liability exposure for smallpox vaccine. Tightly targeted tort reform could limit punitive damages, a measure unlikely to be acceptable.
Another option is indemnification after liability has occurred, possibly through Executive Order 10789. The problem with this form of indemnification is that it is discretionary—there does not have to be indemnification if liability occurs. If ex post, it would apply after a firm has gone through the litigation process. It covers “reasonable” liability expenses, which makes for a great deal of uncertainty about what kind of indemnification would actually occur. Perhaps most limiting of all, it only applies to vaccines that are given to consumers and patients directly by the federal government.
A third alternative would be indemnification through specific legislation, which also raises problems. Again, it would be ex post, after the firm has gone through the litigation process, and would be subject to political considerations.
A fourth alternative is government assumption of risk for the smallpox vaccine, an alternative which was implemented for the swine flu vaccine and which is likely to be the most realistic alternative.
Communication36
Vaccine programs, like most public health efforts, require public cooperation and participation to be effective. Indeed, public health programs have, with carefully developed communication strategies, usually succeeded in garnering public trust, cooperation, and participation. Mostly, Americans cooperate with
public health officials and programs, although there have been exceptions to this rule, including the reluctance of soldiers to accept anthrax vaccine.
These are difficult times because the needed public trust is low. The public health community did not perform well in response to the anthrax releases. If there is any doubt, ask the postal workers. Public trust is the key ingredient for success, and constitutes one broad measure of the effectiveness of our communications. Trust depends on the public understanding what and how actions will protect people. In the case of smallpox, the public will need to understand the important distinction between protecting individuals with vaccine versus using vaccine in the population to stop the spread of disease.
Public health actions should not be in conflict with public understanding, or lead to a suspicion that there are unrelated political motives or goals in play. Perhaps most importantly, to be effective, public health officials must not adopt reassurance as an objective. Elected officials often demand that public health officials reassure the public. In truth, the public is comforted only by knowing that public health officials are more concerned about and alert to threats to the public health than are individual citizens. Public health officials are never trusted if they are perceived as offering reassurance rather than vigilance and protection.
As a corollary, secrecy is counterproductive and destroys trust. The public should know what the public health officials know. Many citizens may choose to trust their word, and follow their advice, but others will want more information. Experience teaches that information should not be withheld and simplification carries risks. Clear and understandable explanations are indispensable. It helps to be able to explain complex and difficult ideas, but simplification must not even appear to be a way to withhold information. With every useful simplification, communicators must be able to demonstrate a willingness to expand and explain in greater depth and complexity. A knowledgeable person can master understandable simplification as well as the complexity.
Finally, politics will prevail over science in the international context. If smallpox poses a threat or a serious risk to Americans, it poses similar risks to everyone on earth. If the nation is caught up in war imagery, the other six billion people on this planet may see a focus on protecting Americans in a very different light. A vaccination strategy focused entirely on Americans seems likely to trigger international mistrust about our motives and could promote fears of genocide.
Risk Communication37
What is going to be the best risk communication strategy once the final smallpox vaccination policy is announced? Based on the complex scientific and technical information regarding smallpox vaccine, how are public health officials going to transform this information into a message, and communicate it to the right audiences at the right time with the intended effect? Dr. Ed Baker, CDC, has said, “As we move into the 21st century, risk communication may well become the central science of public health practice.”
Since September 11, the Consortium for Risk and Crisis Communication has been conducting tracking studies on the public’s attitude, awareness, and beliefs about post-September 11 events, in particular smallpox, smallpox vaccine, anthrax, and anthrax vaccine. Since September 11, there have been 572 media mentions of the smallpox vaccine (particularly as it relates to national security) in the top 20 national daily newspapers. Of interest, public health is now viewed by the public as a national security issue, which provides a strong communications leverage point for any communications campaign.
Although there has been much good coverage of the issues, the public does not understand the information, suggesting that it has not been put into a publicly understandable form. A large majority of the American public lacks basic and correct information, and physicians are poorly informed, not only about the disease, but also about the adverse effects of the vaccine.
A public information campaign will have to be designed to correct misperceptions, identify missing facts and concepts, fill data and information gaps, reinforce correct beliefs, emphasize peripheral ones, dispel myths and rumors, overcome resistance, and anticipate and minimize controversy. The campaign, which could begin today, requires trust in the source of information and trust in the messenger.
The message should be tested for public opinion and the new policy, once decided, should be positioned within the context of public health and national security. Moreover, a common message that outlines both risks and benefits must be sent across all organizations. A “step risk” communication approach is needed, recognizing that people are at varying stages in their awareness and knowledge.
The individual chosen to communicate this message must have numerous attributes. He or she must: convey calmness and resolve; recognize the enormity of events; identify the nature and source of harm; acknowledge uncertainty; be highly visible; take charge of the situation; explain why and how risk information might change; elaborate concrete steps to minimize harm and risk; keep the public informed about any new developments; expose bad news; express personal and honest emotions; deliver candid and complete answers; present clear,
strong, and empathic messages; and anticipate the psychological impact of the communications.
PUBLIC HEALTH RISKS AND BENEFITS
Four speakers provided commentary on the public health risks and benefits that need to be considered in planning for a smallpox outbreak or attack.
Perspective 138
When focusing on public health risks and public health benefits, it is important to remember that people “do what they do,” that not everyone will respond to the same information in the same way, which creates a challenge for public health officials. The most dangerous thing in public health is having different people doing different things during a public health crisis. This was obvious in the aftermath of the anthrax release when diverse groups of people offered the anthrax vaccine made different choices.
During the anthrax crisis, public health officials told everybody to go to D.C. General Hospital, get in line, get educated, and get ciprofloxacin. They were able to communicate fairly effectively that people cannot transmit anthrax to each other. It will be more difficult communicating transmission risks for smallpox or Ebola when public health departments desperately are going to need people to do what they are asked.
The traditional public health approach of prevention, education, and outreach will have to be replaced with outreach first, followed by engagement, then education and prevention. Communication does not occur if one is not engaging his or her audience. Unfortunately, the anthrax crisis was the first chance the public health community had to deliver a clear public health message across the United States and engage the American public, and they did not do it very well. Unsurprisingly, those who trust government did what they were asked and those who do not did something else. This first failure will follow us into the next public health crisis.
Further, the public health community needs to consider the issues involved in communicating public health risks and engendering trust and not panic. Public health officials need to stop talking about percentages and start talking about individuals. We need to reach out to people from diverse communities in diverse ways so in the middle of a crisis, all those diverse people get up and go in one
direction, because it is essential and they must believe it is essential. Trust is something that comes through consistent behavior.
Perspective 239
Among members of the American Society for Microbiology those working in clinical microbiology laboratories have expressed concern about possible smallpox infection. Many of these workers are young enough to have never been vaccinated, and they are likely to be on the front line of a smallpox response. Although it is easy to consider in theory that good microbiology practice would be sufficient to avoid infection of these workers, they are not confident that this would necessarily be the case. Thus, it is important that this critical group of health care personnel feel that due consideration has been given to their protection and that they have a say in the determination of that policy.
The same is true for other hospital and laboratory personnel. Not only are there all the usual considerations about the danger of the vaccination to those being immunized, but also in the hospital setting is the greater problem of potential exposure to large numbers of immunodepressed or -suppressed patients. If, in fact, the probability of a smallpox attack is low, then it might not be warranted to immunize all these hospital and laboratory personnel. On the other hand, if the probability of an attack is higher than has been suggested, an altogether different public discussion should occur.
Perspective 340
If, in fact, a smallpox case arises, it is by definition going to be a bioterrorist attack and there likely will be multiple introductions at multiple sites at multiple times. Although education and consensus are important, once these events occur the media and many of the scientists who agreed at one point on the response policy will assert that they had a very different policy in mind.
If there is an introduction of smallpox, the public health community will not be able to prevent the second wave and thus will encounter many vaccination complications. In public health, however, perspective is important. There were 30,000 deaths during the 1968 flu outbreak in comparison to a speculated 2 cases of smallpox or 500 cases of progressive vaccinia. On the other hand, the political reaction will be pronounced: once there is one case of smallpox everyone is going to want access to the vaccine. Consequently, mass vaccination is going to take place whether or not ring vaccination is the official policy.
The priority has to be to protect those people who are initially at highest risk, the first responders. The major reason to employ ring vaccination, in the presence of what will surely rapidly become mass vaccination, is to ensure near 100 percent coverage, which will never occur with a mass vaccination campaign.
Pre-exposure vaccination has very little purpose, because there is no end to the list of first responders. There are good data suggesting that post-exposure vaccination within the first four or five days is essentially 100 percent effective. An epidemic was stopped cold in Bangladesh by posting vaccinators around the clock at the doorway to the hospital. Anybody who entered the smallpox hospital, where there were hundreds of cases, got vaccinated, and none of those people ended up getting smallpox.
Successful response depends on our success in deploying core public health disciplines. It requires meticulous local planning and preparation, which includes sufficient local vaccine stocks—not vaccine stocks that can be pushed out in 12 hours—but stocks located in every major metropolitan area and municipality, with needles to go with them, trained vaccinators, and surge capacity. If the anthrax events proved anything, it is that the public health community cannot plan for a specific event. Public health capacity must be expanded to respond to the unexpected.
Perspective 441
Public health crises inevitably involve changing facts and situations, which contribute to the uncertainty and complexity of risk communication. For example, in the context of smallpox in 2002, versus 30 years ago, there are now more people who are on chemotherapy, recipients of organ transplants, living with HIV, and in day care, and the nation is witnessing increases in childhood asthma and eczema. Social changes have occurred as well. There is greater distrust of government. The United States population has reached 280 million people, with more and more living in urbanized environments. The fraction of the population that is immigrant with English as a second language has grown, and many of them are undocumented with no access to health care. The mobility of the population is stunning. As a result of all these changes, planners must be wary of their assumptions about “who we are, who we are trying to protect, and what we are trying to do.” And they must now deal with the concept of malignant intent, which was not factored in decades ago.
Experience with swine flu vaccine taught the public health community that efforts like developing consent forms and mobilizing enough people to vaccinate enough people are incredibly difficult to mount and can be halted for political
reasons. Moreover, public health plans can never achieve 100 percent immunization when people do not have insurance or access to health care because city emergency rooms are closing down for cost containment reasons.
Jonathan Mann used to emphasize that the infrastructure to fight AIDS had to be built to the capacity necessary for everything else that needed doing, because otherwise it would either not work at all or disappear as soon as there was a departure of personnel, money, or a particular program. There is a lot of work to be done in building the public health infrastructure and there are a lot of biological agents to worry about besides smallpox. Whatever the public health community does, it needs to build the infrastructure that society has been negligent about in terms of infectious disease in general.
PUBLIC COMMENTS AND DISCUSSION
Several additional issues emerged in the general discussion and are briefly summarized below.
Use of Scarce Public Health Resources and Insuring Dual Use Approaches
An immunization program will entail costs, thus decisions must be made about trade-offs. Spending money on a program that would potentially be very effective at addressing a single bioweapons disease might come at the expense of other public health measures that might provide more protection against a broader range of bioagents. However, developing a plan for smallpox response also readies the public health system for other infectious diseases, whether naturally occurring or deliberately spread.
Vaccine Development and Approval
New vaccines will be subject to an IND, which will require the usual protections for those volunteering to be research subjects, including IRB review, informed consent, and data and safety monitoring. By recruiting health care professionals into those trials it might be possible to not only test the vaccine but also potentially immunize a larger ring of individuals who by occupation are not at the highest risk (first responders) but are at greater risk of exposure than the general population. In addition, there should be provisions in the usual IND granting process—if the response is going to be in an emergent situation—to suspend some of the usual procedures and guidelines, or the vaccine will not be delivered.
In testing new vaccines, it will be important to fine tune the dosage and correlate it with the take and reaction. Public response to the clinical reactions experienced by those receiving experimental doses will be important to consider and measure, anticipating that a more risk averse reaction is likely to occur if only the high-dose, more radical reactions are publicized.
For new vaccine development, a vaccine compensation injury fund and indemnification must be available or manufacturers will continue to be wary of conducting R&D in this area.
International Considerations
In developing a smallpox plan for the United States, the ability of the rest of the world to also respond must be considered, especially those countries that have neither the resources nor infrastructure to conduct surveillance and containment or that might face complex cultural challenges in communicating the need for immunization, isolation, or quarantine. In setting domestic vaccine policies, public health officials must also consider what, how, and when the United States makes recommendations to other countries.
Uncertainties
One area of uncertainty in the many public discussions about the vaccination policy options is the risk-benefit ratio. Government officials have said that the risk of an attack is very low, but not zero. The uncertainty of the threat level, the characteristics of the virus that would be used in an attack, and the mechanisms of dispersal make it difficult to evaluate the benefit of the vaccine. Another area of uncertainty is that much of the clinical and epidemiological experience with smallpox infection and the vaccine derive from an era with very different population characteristics than today. Two key differences are the large number of Americans who have no prior immunity to smallpox and the number of people who are immune-compromised.
Communication and Education
The lack of an effective communication strategy can be a significant hindrance to an effective public health response, even with a fairly benign vaccine that is well accepted and without risk. Right now, the assumption is that once the nation sees crucial people getting vaccinated, everyone is going to want to be vaccinated. An alternative possibility is that when people learn about this vaccine through effective public education efforts, they will understand that the vaccine carries risks but they will also understand that if they are exposed, the vaccination is very effective within a few days and there will be enough vaccine
for those who need it. A plan should be made for advance notification of the public about the risks and the benefits of the vaccine. Citizens should be encouraged to consider in advance whether vaccination is something they would want to pursue in the event of an outbreak or attack.
An important lesson from the anthrax investigation was the importance of a strong, credible spokesperson. Once the vaccination policy is announced, it is important that it be explained thoroughly to the public, particularly to those who are at risk for adverse effects of vaccination. Depending on the policy decided upon, it will be critical that it be explained not only to those who will receive the vaccine, but also—if the plan does not involve mass vaccination—to those who are not given access.
EPILOGUE
A summary of the June 15, 2002 IOM meeting was presented at the June 19–20, 2002 meeting of the Advisory Committee on Immunization Practices. The ACIP made its recommendations (below) on June 20th. These recommendations are currently under consideration by CDC and the Department of Health and Human Services.
Draft Supplemental Recommendations of the ACIP Use of Smallpox (Vaccinia) Vaccine, June 2002
Draft approved by ACIP on June 20, 2002 (SOURCE: http://www.cdc.gov/nip/smallpox/supp_recs.htm [accessed August 2002])
Pre-Release Vaccination of the General Population
Under current circumstances, with no confirmed smallpox, and the risk of an attack assessed as low, vaccination of the general population is not recommended, as the potential benefits of vaccination do not outweigh the risks of vaccine complications.
Recommendations regarding pre-outbreak smallpox vaccination are being made on the basis of an assessment that considers the risks of disease and the benefits and risks of vaccination. The live smallpox (vaccinia) vaccine virus can be transmitted from person to person. In addition to sometimes causing adverse reactions in vaccinated persons, the vaccine virus can cause adverse reactions in the contacts of vaccinated
persons. It is assumed that the risk of serious adverse events with currently available vaccines would be similar to those previously observed and could be higher today due to the increased prevalence of persons with altered immune systems.
Pre-Release Vaccination of Selected Groups to Enhance Smallpox Response Readiness
Smallpox Response Teams
Smallpox vaccination is recommended for persons pre-designated by the appropriate bioterrorism and public health authorities to conduct investigation and follow-up of initial smallpox cases that would necessitate direct patient contact.
To enhance public health preparedness and response for smallpox control, specific teams at the federal, state and local level should be established to investigate and facilitate the diagnostic work-up of the initial suspect case(s) of smallpox and initiate control measures. These Smallpox Response Teams might include persons designated as medical team leader, public health advisor, medical epidemiologists, disease investigators, diagnostic laboratory scientist, nurses, personnel who would administer smallpox vaccines, and security/law enforcement personnel. Such teams may also include medical personnel who would assist in the evaluation of suspected smallpox cases.
The ACIP recommends that each state and territory establish and maintain at least one Smallpox Response Team. Considerations for additional teams should take into account population and geographic considerations and should be developed in accordance with federal, state, and local bioterrorism plans.
Designated Smallpox Healthcare Personnel at Designated Hospitals
Smallpox vaccination is recommended for selected personnel in facilities pre-designated to serve as referral centers to provide care for the initial cases of smallpox. These facilities would be pre-designated by the appropriate bioterrorism and
public health authorities, and personnel within these facilities would be designated by the hospital.
As outlined in the CDC Interim Smallpox Response Plan and Guidelines state bioterrorism response plans should designate initial smallpox isolation and care facilities (e.g., type C facilities). In turn, these facilities should pre-designate individuals who would care for the initial smallpox cases. To staff augmented medical response capabilities, additional personnel should be identified and trained to care for smallpox patients.
Implementation of Recommendations
The ACIP recognizes that the implementation of the supplemental recommendations presented in this document requires addressing a number of issues, and that this will take time. The issues include provider and public education, health care provider training, availability of vaccine and VIG, developing the appropriate investigational new drug protocols, screening, strategies to minimize vaccine wastage, vaccine adverse event surveillance, and other logistical and administrative issues.