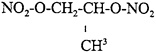SUMMARY
Otto Fuel II, a liquid propellant used exclusively by the U.S. Navy in torpedoes and other weapon systems, is a mixture of three synthetic compounds: 1,2-propylene glycol dinitrate (PGDN) (a nitrate ester explosive), dibutyl sebacate (a desensitizer), and 2-nitrodiphenylamine (a stabilizer). The
primary component and the one responsible for the toxicity of Otto Fuel II is PGDN, a volatile liquid with a disagreeable odor. Because PGDN is the primary and most toxic component of Otto Fuel II and because only PGDN is relatively volatile compared with the other components, AEGLs have been derived in terms of PGDN with the notation that the values are appropriate for Otto Fuel II.
PGDN is a systemic toxicant with effects on the cardiovascular and central nervous systems. Its vasodilatory action results in headaches during human exposures. Dizziness, loss of balance, nasal congestion, eye irritation, palpitations, and chest pains have also been reported. Methemoglobinemia has been reported at the high concentrations used in studies with animals. The air-odor threshold in healthy subjects is 0.2 parts per million (ppm), but warning properties are poor inasmuch as olfactory fatigue sets in after as little as 5 minutes (min) (Stewart et al. 1974). Within 24 hours (h) of exposure, PGDN is rapidly and completely metabolized in vivo and eliminated primarily in the urine as inorganic nitrate.
Few data were available that met the definitions of AEGL end points. One inhalation study with 20 human subjects described headaches and slight loss of balance at exposure concentrations of 0.1 to 1.5 ppm for exposure durations of up to 8 h (Stewart et al. 1974). Acute exposure of monkeys for 6 h at concentrations ranging between 70 and 100 ppm resulted in severe signs of toxicity including convulsions but no deaths (Jones et al. 1972). In the same study, exposure of rats at a higher concentration, 189 ppm for 4 h, resulted in no toxic signs. Examination of the relationship between exposure duration and concentration for both mild and severe headaches in humans over periods of 1 to 8 h determined that the relationship is C1×t=k.
The AEGL-1 values were based on concentrations at 0.5 ppm and 0.1 ppm, which were the thresholds for mild headaches in healthy individuals at exposure durations of 1 and 6 h, respectively (Stewart et al. 1974). This effect can be considered the threshold for mild discomfort (only one subject was affected at each exposure), which falls within the definition of an AEGL-1. The 0.5-ppm concentration was used to derive the 30-min and 1-h AEGL-1 values, and the 0.1-ppm concentration was used for the 4- and 8-h values. Because the time and concentration values were based on the most susceptible subject, these concentrations were adjusted by an uncertainty factor (UF) of 3 to account for potential differences in human sensitivity and scaled to the appropriate time periods using the C1×t=k relationship. A UF of 3 was considered sufficient as no susceptible populations were identified (the headache effect is the same as that experienced by patients medicated with nitro-
glycerin for angina, and the calculated concentrations of nitrite arising from inhaled PGDN are far below those inducing methemoglobinemia in infants), and the vasodilatory effects of PGDN, responsible for the headaches, are not expected to vary greatly among individuals. The UF of 3 is supported by the steep dose-response curve for induction of headaches in the key study. (The threshold concentration and the concentration that induced headaches in approximately half of the individuals differed by a factor of 2.) The 10-min AEGL-1 value was set equal to the 30-min value.
The AEGL-2 values were based on a 0.5-ppm concentration, which caused severe headaches accompanied by dizziness in one subject and slight loss of equilibrium in two subjects in one of several sensitive equilibrium tests after 6 h of exposure (Stewart et al. 1974). This concentration-exposure duration was considered the threshold for impaired inability to escape as defined by the AEGL-2. The 0.5-ppm concentration was adjusted by an intraspecies UF of 3 to protect susceptible individuals and scaled across time for the 30-min and 1-, 4-, and 8-h time periods using the C1×t=k relationship, as was done for the AEGL-1 derivation. The UF of 3 is supported by the less than 2-fold difference among individuals for the induction of narcosis by central nervous system depressants and by the steep dose-response curve for the induction of headaches in the key study: namely, a 2-fold difference in the threshold concentration and the concentration that induced headaches in the majority of tested individuals. Because of the long exposure duration of 6 h for the chosen end point, time scaling was not performed for the 10-min AEGL-2. The 10-min AEGL-2 was set equal to the 30-min value.
The AEGL-3 values were based on the 6-h exposure of squirrel monkeys at concentrations that ranged between 70 and 100 ppm. This exposure resulted in vomiting, pallor, cold extremities, semiconscousness, and clonic convulsions; these signs disappeared upon removal from the exposure chamber (Jones et al. 1972). Because a range of concentrations were encountered during the 6-h exposure, the lower concentration, 70 ppm, was selected as the basis for the AEGL-3. This value may be conservative as rats showed no effects during a 4-h exposure at 189 ppm (Jones et al. 1972). The 70-ppm concentration was adjusted by a total UF of 10. An interspecies UF of 3 was chosen because the monkey is an appropriate model for extrapolation to humans: Both the monkey and human subjects showed changes in electrical activity of the brain at similar PGDN concentrations. An intraspecies UF of 3 was considered sufficient for differences in the threshold for convulsions, which are also attributable to central nervous depression. Because the end point for the AEGL-3 values (convulsions and lethality) is different than the
end point for AEGL-1 and AEGL-2 (headache), and no data on the relationship between concentration and exposure duration are available for the end point of convulsions, the more conservative values of n=3 and n=1 were used to scale from 6 h to the shorter (30-min and 1- and 4-h) and longer time periods, respectively. The 10-min AEGL-3 was set equal to the 30-min AEGL-3. The values are supported by the results of additional studies with squirrel monkeys and dogs by Jones et al. (1972). Monkeys and dogs exposed continuously at approximately 15 ppm for 90 days (d) showed no overt clinical signs; systemic toxicity consisted of biochemical and/or non-life-threatening histological changes in the liver, spleen, and kidneys.
The values appear in Table 2–1.
1. INTRODUCTION
Otto Fuel II is a liquid propellant used exclusively by the U.S. Navy in MK-46 and MK-48 torpedoes and other weapon systems (Rivera 1974; Gaworski et al. 1985). It is a mixture of three synthetic compounds. The primary component is the explosive, 1,2-propylene glycol dinitrate (PGDN) (approximately 75%); dibutyl sebacate (23%) is added as a desensitizer, and because pure PGDN is unstable, 2-nitrodiphenylamine (2%) is added as a stabilizer (ATSDR 1995). PGDN, a nitrated ester, is a volatile liquid with a disagreeable odor. Its primary use is as a propellant in Otto Fuel II (Forman 1988). No information on production was located. Wiltshire Chemical Company in Gardena, California, was the only identified producer (ATSDR 1995).
Neither Otto Fuel II nor its components are highly acutely toxic, as indicated by oral toxicity data. The oral LD50 for Otto Fuel II in male HA/ICR mice was 1.6 mL/kg (2.24 g/kg) (Litton Bionetics 1979). For PGDN, oral LD50 values for the rat ranged from 0.25–1.19 g/kg (Clark and Litchfield 1969; Jones et al. 1972; Andersen and Mehl 1979). About 10% of topically applied PGDN is absorbed through the skin (Clark and Litchfield 1967). Dibutyl sebacate, a food flavoring agent and plasticizer, has a very low acute oral toxicity; the oral no-effect level for lethality was 16 g/kg in the rat (Bisesi 1994). The low vapor pressure of 3 mm Hg at 180°C severely limits its risk as an inhalation hazard. ATSDR (1995) reported an oral LD50 value for 2-nitrodiphenylamine in rats of 6.15 g/kg. In addition to its use in Otto Fuel II, 2-nitrodiphenylamine is an orange-colored solvent dye (Sudan yellow 1339) with a low vapor pressure of 1×10−5 mm Hg at 25°C (Baughman and Perenich 1988; ATSDR 1995).
TABLE 2–1 Summary of AEGL Values for PGDN (Otto Fuel II) (ppm [mg/m3])
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1a |
0.33 |
0.33 |
0.17 |
0.05 |
0.03 |
Mild headaches in humans (Stewart et al. 1974) |
|
(Nondisabling) |
(2.3) |
(2.3) |
(1.1) |
(0.34) |
(0.17) |
|
|
AEGL-2 |
2.0 |
2.0 |
1.0 |
0.25 |
0.13 |
Severe headaches and slight unbalance in humans (Stewart et al. 1974) |
|
(Disabling) |
(14) |
(14) |
(6.8) |
(1.7) |
(0.8) |
|
|
AEGL-3 |
16 |
16 |
13 |
8.0 |
5.3 |
Convulsions in monkeys (Jones et al. 1972) |
|
(Lethal) |
(114) |
(114) |
(93) |
(57) |
(38) |
|
|
aThe distinctive odor of PGDN will be noticeable to most individuals at the 0.33 and 0.17 ppm concentrations. |
||||||
The vapor pressures of the three components of Otto Fuel II differ considerably. During vapor generation studies with Otto Fuel II, PGDN was the only component vaporized into inhalation exposure chambers in sufficient quantity to allow direct analysis (Stewart et al. 1974; MacEwen and Vernot 1982). In light of the low toxicity of dibutyl sebacate and 2-nitrodiphenylamine and the fact that they do not vaporize to a detectable extent at test compound generation temperatures up to 45°C, the toxicity of Otto Fuel II has been evaluated in terms of PGDN. Chemical and physical data for PGDN are listed in Table 2–2.
At low concentrations, PGDN has been reported to cause cardiovascular, irritant, and central nervous system effects including headaches, nasal congestion, eye irritation, and dizziness in humans (Stewart et al. 1974; Hovath et al. 1981). In animal studies that used higher concentrations, methemo-globinemia occurred (Jones et al. 1972). The acute and subchronic effects of PGDN were studied in monkeys, dogs, rats, and guinea pigs. Several studies with humans as well as with monkeys and rats addressed neurotoxicity. The air-odor threshold in healthy subjects is 0.2 ppm, but warning properties are poor inasmuch as olfactory fatigue sets in after as little as 5 min (Stewart et al. 1974).
TABLE 2–2 Chemical and Physical Dataa
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
Although sudden deaths due to circulatory failure have been reported among workers exposed chronically to nitrated esters such as nitroglycerin and ethylene glycol dinitrate (Carmichael and Lieben 1963), no deaths attributable to cardiovascular effects were reported for U.S. Navy personnel involved in torpedo maintenance work (Horvath et al. 1981; Forman et al. 1987). The sudden deaths for workers in the explosives industry were attributed to a compensatory vasospasm that may produce coronary insufficiency upon withdrawal from nitrate ester exposure.
2.2. Nonlethal Toxicity
2.2.1. Occupational Exposures
Horvath et al. (1981) evaluated the neurophysiologic effects of acute and chronic exposure to PGDN of 87 workers employed in U.S. Navy torpedo facilities. Prior to the evaluation, the subjects reported subjective symptoms of frequent or occasional headaches (65% of respondents), nasal congestion (31%), eye irritation (26%), and dizziness (13%). Palpitations, dyspnea, chest pain, and loss of balance were reported by small percentages of workers. For the chronic exposure, evaluation of the workers included both quantitative oculomotor functions (saccades or synchronized eye tracking movements) and ataxia tests; comparison was made with a control group consisting of 21 nonexposed personnel from the same facilities. Results of the tests indicated no evidence of chronic neurotoxicity in either the study population or a subgroup of 28 workers with the longest exposure to PGDN.
In the same study (Horvath et al. 1981), acute effects were evaluated in a subgroup of 29 workers by comparing test values before and after a torpedo maintenance procedure, or “turnaround.” The maintenance procedures lasted 30–60 min. During this time, PGDN concentrations, as indicated by approximately 400 grab samples (instantaneous atmospheric samples) taken in the work area, ranged from 0.00 to 0.22 ppm (average value of 0.06 ppm; 88% were ≤0.1 ppm, 50% were ≤0.05 ppm, and only one sample was above the ACGIH TLV-Ceiling value of 0.2 ppm, which was in effect at that time).
There were no decrements in the three ataxia tests (although the mean score in one test was increased), but mean saccade velocity was statistically significantly decreased (by 37.3 degrees per second [s]), and mean saccade delay time was statistically significantly increased (by 6.4 milliseconds). There were no changes in saccade accuracy or (eye) smooth pursuit index. The changes in the saccade test parameters did not correlate with peak PGDN levels measured during the turnaround procedure. The workers involved in the turnaround did not complain of headache or nasal congestion, although one individual involved in a spill developed a headache.
Forman et al. (1987) (see also Helmkamp et al. [1984] for preliminary study) evaluated cardiac morbidity among U.S. Navy “torpedoman’s mates,” a group potentially exposed to PGDN while engaged in torpedo maintenance work. Cardiovascular events in this group were compared with both a nonexposed group of torpedomen and a nonexposed group in the job category “fire control technician”. The torpedoman’s mate group consisted of 1,352 men, with an average yearly population of 822; hospitalization records were available for 1970 through 1979. The nonexposed-torpedomen control group consisted of 14,336 individuals over the 10-y period with a yearly average of 4,906. The fire control technician control group consisted of 29,129 individuals with a yearly average of 11,198. Measured concentrations of PGDN included those of the Horvath et al. (1981) study and current surveys in which 8-h time-weighted averages were below 0.05 ppm. Cardiac incidences considered were myocardial infarction, angina pectoris, and cardiac arrhythmia. Age-adjusted incidence rates and relative risk were calculated for each group. There were higher incidences of hospitalizations for myocardial infarctions and angina pectoris but not cardiac arrhythmias in the torpedoman’s mates than in either control group. Relative risk was significant for myocardial infarction and angina pectoris when compared with the torpedoman control group (2.6 and 3.8, respectively; p<0.05) but not when compared with the fire control technicians. When incidences of myocardial infarction and angina pectoris were combined, relative risk was significant when compared with both the unexposed torpedoman and fire control technician control groups (2.6 and 2.9, respectively; p<0.05). Deaths attributable to cardiovascular events occurred in the control groups but not in the torpedoman’s mate group. The authors discuss biases in the study, including the healthy worker syndrome and the small number of actual hospitalizations. For example, only four hospitalizations for myocardial infarction and two hospitalizations for angina pectoris occurred in the torpedoman’s mates group over the 10-y period.
2.2.2. Experimental Studies
Stewart et al. (1974) exposed human volunteers to PGDN in a controlled environment chamber. “Each group underwent a training program in the chamber. The experiments were conducted in a double-blind mode. However, in those experiments in which the odor of PGDN was detectable, both the subjects and the research staff were aware that exposure to PGDN was occurring, although the magnitude of the exposure was not disclosed to them.” Exposure concentrations were 0.0 (control), 0.03, 0.1, 0.2 (range, 0.21–0.26), 0.35 (range, 0.33–0.37), 0.5, or 1.5 (1.2 and 1.5) ppm, and exposures lasted from 1 to 8 h. The exposures at 0.2 ppm were repeated on a daily basis for 5 d. Selected exposure concentrations, exposure durations, and the number of subjects tested are summarized in Table 2–3. Seventeen healthy male subjects (ages 22–25), usually in groups of three, participated in the exposures. In addition, one of the exposures (to 0.5 ppm) included two male members of the research staff, ages 45 and 51, and a 24-y-old female for a total of 20 subjects.
PGDN was generated from a sample of Otto Fuel II by blowing air across a Pyrex reservoir of the compound to the return air duct of the air conditioner. Eighty percent of the air was recirculated. The concentration of PGDN in the air was monitored continuously by an infrared spectrophotometer and by a gas chromatograph fitted with an electron capture detector. The vaporized Otto Fuel II was 99% pure PGDN as measured by infrared analysis.
Testing of the subjects consisted of both subjective evaluations and physiological and central nervous system responses observed under medical supervision. The lowest concentration at which odor was detected was 0.2 ppm (four of nine subjects), but the ability to detect the odor disappeared within 5 min. Subjective symptoms consisted of headache and eye irritation. At 0.1 ppm, two of the subjects experienced mild headache (Table 2–3). One of these subjects had developed headache during each of the control exposures and during the exposure at 0.03 ppm. The other subject developed headache after 6 h, and the headache continued for several hours postexposure.
Of the nine subjects exposed at 0.21–0.26 ppm (18 exposures; all nine subjects took part in the 8-h exposures, and three of the nine were exposed for 8 h on two separate occasions), seven developed headaches of varying intensity. The headaches were mild in intensity for two of three subjects during the 2-h exposure. During the twelve 8-h exposures, there were five incidences of mild headache and six incidences of severe headache. The number of subjects in each category of headache could not be ascertained from the data. The
TABLE 2–3 Human Responses to PGDN
|
|
0.0 ppm |
0.03 ppm |
0.1 ppm |
0.21–0.26 ppm |
0.35 ppm |
0.5 ppm |
1.5 ppm |
|||||||||||
|
|
1–8 h |
1 h |
4 h |
8 h |
1 h |
4 h |
8 h |
1 h |
2 h |
8 h |
1 h |
2 h |
8 h |
1 h |
2 h |
7.3 h |
1 h |
3.2 h |
|
Number of subjects |
2–6a |
2 |
3 |
3 |
2 |
3 |
3 |
3 |
3 |
9b |
3 |
3 |
3 |
3 |
3 |
3 |
2 |
6 |
|
Number detecting odor |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
3 |
2 |
1 |
2 |
2 |
1 |
1 |
2 |
2 |
6 |
|
Number developing mild headache |
1 |
0 |
1c |
0 |
0 |
1c |
1 |
0 |
2 |
5 |
0 |
3 |
1 |
1 |
2 |
0 |
0 |
0 |
|
Number developing severe headache |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
6 |
0 |
0 |
2 |
0 |
1 |
3 |
2 |
6 |
|
Number developing eye irritation |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
2 |
6 |
|
aGroups of eight and nine male subjects participated in a series of 4-h training sessions and all 17 male subjects, in groups of two to six, participated in a series of control exposures lasting from 1 to 8 h. bNine subjects participated in 12 exposures; numbers in column immediately below refer to incidences per 12 exposures rather than individuals. cThis individual developed a mild headache during each of the control exposures. Source: Modified from Stewart et al. (1974). |
||||||||||||||||||
visual evoked response (VER), a complex waveform representing the summed electrical activity of many neurons, was minimally altered in the majority of subjects but with no consistent pattern of response. No decrements in test performance or alterations in monitored physiological parameters occurred at this concentration. Subjects repeatedly exposed to this concentration developed tolerance to the induction of headache, but the alteration in visual evoked response morphology appeared cumulative.
At 0.35 ppm, all three subjects exposed for 2 h developed mild headaches, and one of three subjects exposed for 8 h developed a mild headache. Two of three subjects exposed for 8 h developed severe headaches. One subject also developed slight eye irritation, which persisted throughout the 2 h exposure. Four of the nine subjects detected the odor of the compound, which they described as mild at this concentration; however, the odor was not detectable after 5 min of exposure. The morphology of the visual evoked response, while variable, was altered, particularly in three subjects exposed for 8 h. The exposure produced an increase in the peak-to-peak amplitude of the 3–4–5 wave complex. The authors interpreted the VER changes as consistent with the VER changes produced by central nervous system depression.
Groups of three subjects were exposed at 0.5 ppm for time periods of 1, 2, or 7.3 h. Seven of the nine subjects developed headaches during these exposures, beginning with a mild headache after 1 h of exposure (Table 2–3). After exposure for 6.25 h, balance became impaired in two of three subjects (heel-to-toe test with eyes closed), and at 7.3 h, all three subjects had abnormal modified Romberg tests (postural stability with the eyes closed) as well as abnormal heel-to-toe tests with their eyes closed. One subject was unable to perform a normal heel-to-toe test with his eyes open. The authors compared the equilibrium disturbance with ethanol intoxication, which produced a blood alcohol concentration in the 100–150 mg/100 mL range. These three subjects also had a mean elevation of diastolic blood pressure of 12 mm Hg, which was not accompanied by alterations in pulse or cardiac rhythm. Headaches became increasingly severe and throbbing for all three subjects during exposure, and one of the three subjects reported dizziness and nausea after 6 h of exposure. Three members of the research staff, two males and one female, were exposed at this concentration for a period of 1.25 h and all developed a mild headache. These latter three exposures appear to be in addition to that of the nine subjects described above.
All eight subjects exposed at 1.5 ppm reported eye irritation (without conjunctivitis or excessive lacrimation) after 40 min of exposure. All of the subjects developed severe headaches, three after 30 min of exposure and the remaining five after 40–90 min of exposure. Headaches became so severe that
exposure was terminated after 3.2 h. Headaches persisted for 1 to 7.5 h after exposure. These subjects showed a dramatic alteration in the VER with an increased amplitude in the peak-to-peak voltage of one of the wave complexes. There was a shift to control values after 160–180 min of exposure, but VER were altered for 48 h after exposure.
None of the exposures produced changes in clinical chemistry values (blood count, blood nitrate, blood urea nitrogen, serum enzymes, and serum electrolytes or urinalysis and nitrate and nitrite urinary excretion), spontaneous electrical activity of the cortex of the brain (detected by EEG), pulse rate and sinus rhythm, or pulmonary function. Visual and auditory acuity, exercise EKG, and time estimation tests did not differ from control values for any of the exposures. Only one of several cognitive tests was affected by exposure and the change occurred only in the four subjects exposed at 1.5 ppm. The test was taken during the time the subjects were experiencing severe headaches.
2.3. Neurotoxicity
Torpedo maintenance workers exposed to PGDN at concentrations of 0– 0.22 ppm (average of approximately 0.06 ppm) for 30–60 min exhibited significantly altered responses in some oculomotor performance tests but no statistically significant decrement in balance (see Section 2.2.1 for further details) (Horvath et al. 1981). The oculomotor function changes observed in workers after acute exposures to Otto Fuel II were not observed in chronically exposed workers. Eight-hour time-weighted average (TWA) concentrations during chronic exposures Were below 0.05 ppm (Forman et al. 1987).
Human volunteers exposed to PGDN at various concentrations of also exhibited central nervous system effects (Stewart et al. 1974). At 0.35 ppm, the VER was altered, particularly at 8 h. This effect became more pronounced at 0.5 ppm and 1.5 ppm, after 45–90 min at the latter concentration. After 6.25 h of exposure at 0.5 ppm, two subjects had abnormal heel-to-toe balance tests with their eyes closed, and after 8 h, all three subjects had abnormal modified Romberg tests as well as abnormal heel-to-toe tests with eyes closed. At this time, one subject was unable to perform the heel-to-toe test with open eyes.
2.4. Developmental and Reproductive Effects
The Naval Health Research Center investigated pregnancy outcomes of
women engaged in torpedo repair work and compared their spontaneous abortion rate with three other groups: unexposed female torpedomen munitions workers, hospital corpsmen, and other uniformed U.S. Navy enlisted females (NHRC 1986). During the years of the study, 1980–1983, there were no spontaneous abortions among the five PGDN-exposed pregnant women.
2.5. Genotoxicity
No studies were located regarding genotoxic effects in humans exposed to PGDN or Otto Fuel II
2.6. Carcinogenicity
No studies were located regarding Carcinogenicity in humans exposed to PGDN or Otto Fuel II.
2.7. Summary
No deaths attributable to exposure to Otto Fuel II or its primary component, PGDN, were reported in the available literature, but relative risk for combined myocardial infarction and angina pectoris among torpedomen chronically exposed at unknown concentrations of Otto Fuel II and PGDN were significantly elevated compared with control groups (Forman et al. 1987). The number of subjects hospitalized with these cardiovascular events was small. Symptoms described during occupational exposures included headaches, nasal congestion, eye irritation, and dizziness (Horvath et al. 1981). Acute exposures to an average concentration of approximately 0.06 ppm resulted in no effects on motor coordination and only subtle changes in eye movements. There were no spontaneous abortions among five PGDN-exposed female U.S. Navy personnel. No information on genotoxicity or Carcinogenicity in humans was located.
PGDN has effects on the cardiovascular and central nervous systems. Exposure of healthy, primarily male subjects to PGDN at a concentration of 0.03 ppm for 8 h was without adverse effects. A mild headache was present in one of three subjects after exposure at 0.1 ppm for 6 h. Adverse effects became more severe at higher concentrations and shorter exposure durations: 0.2 ppm for 8 h produced severe headache in six of 12 exposures; 0.35 ppm for 8 h produced severe headache in two of three subjects and disturbance of
the VER in three of three subjects; 0.5 ppm resulted in severe headache in one of three subjects after 2 h and produced impairment of balance after 6.25 h and ataxia at 7.3 h; 1.5 ppm for 3.2 h produced throbbing and painful headaches in six of six subjects accompanied by some abnormal cognitive and coordination tests. Nonincapacitating eye irritation was also reported during the exposures at 1.5 ppm
Based on the Stewart et al. (1974) study, the threshold for odor detection in healthy adults is 0.2 ppm, and the threshold for eye irritation is 1.5 ppm, although one of three subjects developed eye irritation during a 2-h exposure at 0.35 ppm.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
No acute studies involving lethality and the exposure durations relevant to AEGL derivations were located. Jones et al. (1972) reported deaths in preliminary, range-finding studies in which single animals were exposed to PGDN. Because pure PGDN is chemically unstable, a physiologically and chemically inert stabilizer of low volatility was added. Exposure concentrations are based on the PGDN concentration of the mixture. One rabbit that was exposed to PGDN mist at a mean concentration of 240 mg/m3 (34 ppm) for 23 h/d died on day 4. The rabbit became cyanotic on day 4 and died with a methemoglobin value of 32.8%. A squirrel monkey exposed at 415 mg/m3 (approximately 60 ppm) for 23 h/d died on day 3 and had a methemoglobin level of 40.2%. In a continuous inhalation study by the same authors (Jones et al. 1972), one of nine squirrel monkeys exposed at 236 mg/m3 (33 ppm) died on day 31 of exposure. Adult filarial parasites in the abdominal cavities of a majority of the monkeys were the only gross abnormality observed at autopsy. No further details on the deaths were available. However, these and other authors (Clark and Litchfield 1969; Andersen and Mehl 1973) attribute deaths to methemoglobinemia and the resulting anoxia. Rats treated with lethal doses of PGDN (either oral or subcutaneous) were ataxic and lethargic with signs of methemoglobinemia and respiratory depression (Clark and Litchfield 1969). Death consistent with anoxia occurred up to 48 h after administration.
TABLE 2–4 Summary of Acute Sublethal Effects in Animals
|
Species |
Concentration |
Exposure Duration |
Effect |
Reference |
|
Monkey |
70–100 ppm |
6 h |
Vomiting, pallor, cold extremities, semiconscousness, clonic convulsions |
Jones et al. 1972 |
|
Rat |
189 ppm |
4 h |
No toxic signs |
Jones et al. 1972 |
3.2. Nonlethal Toxicity
Acute studies are summarized in Table 2–4. These studies and effects following longer-term exposures are described in the following text.
3.2.1. Nonhuman Primates
In a range-finding study prior to a continuous inhalation study, squirrel monkeys (number not specified) exposed at 500–700 mg/m3 (70–100 ppm) PGDN for 6 h developed vomiting, pallor, cold extremities, semiconsciousness and clonic convulsions (Jones et al. 1972). These signs disappeared within 30–45 min after removal from the exposure chambers. In a follow-up study by the same authors (Jones et al. 1972), groups of three male squirrel monkeys were exposed to PGDN at concentrations of 67, 108, or 236 mg/m3 (approximately 10, 15, or 33 ppm) continuously, 24 h/d, for 90 d. Groups of three monkeys served as control groups for each exposure. PGDN was generated from heated flasks over which pre-dried air was drawn into a dilution air stream upstream of the exposure chambers. The chamber air was sampled and monitored by a modified diphenylamine color analysis for nitrates; the concentrations were similar to nominal inputs determined gravimetrically. Hematology parameters were measured prior to and after exposure; methemoglobin was measured weekly during the exposure to 33 ppm. Although one monkey died on day 31 of exposure at 33 ppm (possibly complicated by a parasitic infection; see Section 3.1), these animals did not show signs of intoxication during the exposures, and body weight gains of surviving
animals were normal. Fatty infiltration was present in the liver of monkeys exposed at 10 ppm. Heavy iron-positive deposits consistent with mononuclear cell infiltrates and focal necrosis were present in the liver, spleen, and kidney sections of monkeys exposed at 33 ppm. Monkeys exposed at 15 and 33 ppm also had elevated serum urea nitrogen levels and decreased serum alkaline phosphatase activity. In monkeys exposed at 33 ppm, methemoglobin levels increased to 17% by day 14, declining by day 42 to approximate control levels.
3.2.2. Dogs
Groups of two male beagle dogs were exposed to PGDN at concentrations of 67, 108, or 236 mg/m3 (approximately 10, 15, or 33 ppm) continuously, 24 h/d, for 90 d (Jones et al. 1972). Six dogs (two per exposure) served as control groups. PGDN was generated as in the study with monkeys. All dogs gained weight at a normal rate, but hemoglobin and hematocrit were decreased by 63% and 37% in the two dogs in the 33-ppm exposure group. Livers showed dose-related changes, including hemosiderin deposits in the 10-ppm group, hemosiderin deposits and fatty changes in the 15-ppm group, and heavy hemosiderin deposits accompanied by focal necrosis in the 33-ppm group. The 33-ppm exposure group also had iron-positive deposits in the spleen and kidneys. The methemoglobin level reached 23% on day 14 of exposure at 33 ppm and declined thereafter but did not return to control values.
3.2.3. Rats
Six rats (strain unidentified) were exposed for 4 h to a mist of PGDN at a concentration of 1,350 mg/m3 (189 ppm) (Jones et al. 1972). No toxic signs were noted during the exposure or within the 14 d postexposure period. The mean methemoglobin level immediately postexposure was 23.5%. In repeated inhalation studies by the same authors, eight male Sprague-Dawley derived rats were exposed at a concentration of 65 mg/m3 (approximately 10 ppm) for 7 h/d, 5 d/week (wk), for a total of 30 exposures. No mortalities or clinical signs of intoxication were observed. All rats gained weight at a normal rate, hematology values remained normal, and histopathological examinations of major organs failed to reveal any effects.
Jones et al. (1972) also exposed 15 Sprague-Dawley-derived rats of both genders to PGDN at concentrations of approximately 10, 15, or 33 ppm con-
tinuously, 24 h/d, for 90 d. An additional group of 15 rats served as a control group and were treated the same as the exposed groups except for the addition of PGDN to the exposure chamber. Fatty deposits were observed in the livers of rats exposed at 10 ppm, and female rats exposed at 33 ppm showed focal necrosis of the liver and acute tubular necrosis of the kidneys. Male rats appeared normal. Methemoglobin levels of two rats exposed at 33 ppm were elevated to 12.8% by day 14 but decreased with continued exposure.
3.2.4. Guinea pigs
Groups of 15 Hartley-derived guinea pigs were exposed to PGDN at concentrations of approximately 10, 15, or 33 ppm continuously, 24 h/d, for 90 d (Jones et al. 1972). A concurrent control group of 15 animals was placed in an exposure chamber without the addition of PGDN. Fatty deposits were observed in the livers of guinea pigs exposed at 10 ppm. Guinea pigs exposed at 15 ppm consistently showed foci of pulmonary hemorrhage, and vacuolar changes occurred in the liver of all guinea pigs exposed at 33 ppm.
3.3. Neurotoxicity
Two male rhesus monkeys trained in free operant avoidance tests were exposed to PGDN at concentrations of 2–33 ppm and observed for successful completion of the avoidance test and VER (Mattsson et al. 1981). The avoidance test involved response to a red light by operating a lever within 10 s of the light cue in order to avoid an electrical shock. For the VER, the A-B-C complex, comparable to the 3–4–5 complex in the Stewart et al. (1974) study, was measured in response to flashes from a strobe light. The monkeys were tested individually, each at several concentrations, which were separated by a 1-wk interval. One monkey was exposed at 2 ppm three times and also at 7 and 20 ppm. The other monkey was exposed at 3, 10, and 33 ppm. Exposure durations were 4 h. Halothane at one-tenth of the concentration that produces anesthesia in monkeys served as a reference depressant. Free operant behavior was not affected by any PGDN concentration, but the VER was statistically significantly altered by exposure to PGDN (p<0.05). The C wave increased 20% in amplitude at 2 ppm and decreased 25% at higher concentrations; there were no changes in amplitude of the A and B waves or in the latency of the waveforms. No changes occurred in one of three trials at 2 ppm and in the trial at 10 ppm. During the course of the training, the au-
thors found that the C wave could be increased or decreased by 30% to 40% by changes in the environment or a change in the tension of the operant response lever tension, and therefore the authors suggested that the changes observed during the exposures might have been caused by the irritating or distracting properties of the vapor. Halothane produced significant increases in the A, B, and C waves and slowed the latency of the B and C waves but did not change free operant avoidance behavior.
In longer-term exposures, two rhesus monkeys were exposed to PGDN vapors for 125 d at concentrations that were increased in increments from 0.3 to 4.2 ppm (Mattsson et al. 1981). Two monkeys served as controls. Daily testing involving either the cued or free operant avoidance tests showed no effects on either type of avoidance performance, and there was no disruption of the ability to discriminate between the two avoidance schedules.
Three male squirrel monkeys previously trained to perform visual discrimination or visual acuity threshold tests were exposed continuously for 90 d to PGDN at a concentration of 262 mg/m3 (approximately 37 ppm) (Jones et al. 1972). The animals were removed from the exposure chambers for a 2-h period once a week for the respective behavior tests. A fourth trained monkey exposed to filtered room air under the same conditions served as the control. The only sign during exposure was mydriasis (excessive dilatation of the pupil of the eye), which increased from slight to moderate. There were no changes in avoidance behavior in the monkeys as determined by the visual tests.
Groups of 13–14 anesthetized male Sprague-Dawley rats that had previously been trained on the accelerod, a test of motor performance, were injected with saline (control) or 5 or 10 µL of PGDN (0.01 or 0.02 µL/kg; approximately 0.007 or 0.014 µg/kg) directly into the cisterna magna of the brain (Bogo et al. 1987). Motor performance was tested 12 min after injection, hourly for 6 h, and at 24 h in rats that had not been grossly traumatized by the injection procedure. Compared with the control group, no change in motor performance was observed in rats injected with 5 µL of PGDN. A significant decrease in motor performance was observed during the first 2 h in rats injected with 10 µL. The authors suggest that this study confirms the observations of PGDN-induced changes in human motor performance.
3.4. Developmental and Reproductive Effects
Groups of 28 or 47 (high-dose) pregnant Crl:CD BR rats were treated dermally with neat Otto Fuel II at doses of 0, 400, 2,000, or 4,000 mg/kg/d beginning on day 5 of pregnancy (Cooper et al. 1993). On day 20 of preg-
nancy the dams were euthanized and the total number of fetuses, corpora lutea, implantation sites, and resorption sites were recorded. Fetuses were examined for grossly visible abnormalities. Half of the fetuses were examined for soft tissue abnormalities, and half of the fetuses were examined for skeletal deformities. The highest dose was toxic, resulting in a maternal mortality of 53% and significantly lower body weights of surviving dams and fetuses; fetal resorptions per dam were increased and viable fetuses and fetuses per dam were significantly decreased in this group (all, p<0.05). Dams receiving 4 g/kg/d displayed a moderate erythema of the skin in the area exposed to the fuel. Body weights of dams and fetuses were also significantly reduced in the group receiving 2 g/kg/d. No other parameters were affected in the 2 g/kg/d groups, and none of the measured parameters were altered in the 400 mg/kg/d group. There was no evidence of terata erythema at any concentration.
In the same study (Cooper et al. 1993), pregnant New Zealand white rabbits were dermally treated with neat Otto Fuel II at a rate of 0, 100, 316, or 1 g/kg/d on days 6–18 of pregnancy. Although body weights of dams treated with 1 g/kg/d lagged behind the other groups on days 20 and 25 of pregnancy, no differences were present at sacrifice on day 28 of pregnancy. The number of corpora lutea, implantation sites, and resorption sites and fetal body weights did not differ significantly between treated and control groups. There was no evidence of terata. Marked erythema of the skin in the area of application was observed in the group treated with 1 g/kg/d.
Dibutyl sebacate was tested for reproductive toxicity in a dietary study with Sprague-Dawley rats: 6.25% (approximately 5.6 g/kg/d) in the diet for 10 wk prior to breeding (Smith 1953). No effect on fertility, litter size, or pup survival was found. However, pups from treated dams weighed less than pups from the control group.
3.5. Genotoxicity
The genotoxicity of Otto Fuel II was evaluated in a series of assays conducted by Litton Bionetics (1979). Otto Fuel II was not mutagenic in microbial assays involving five strains of Salmonella typhimurium or in Saccharomyces cerevisiae D4, with or without exogenous metabolic activation. The test compound was active in inducing mutations at the TK locus in L5178Y mouse lymphoma cells at concentrations that were clearly cytotoxic. Otto Fuel II failed to induce sister chromatid exchanges in the same cell line, either with or without metabolic activation. In the mouse bone marrow cytogenetic analysis, the test compound was administered acutely and
subchronically (five doses). Chromosomal aberrations were not elevated compared with the control values, but the presence of ring chromosomes suggested weak activity. Otto Fuel II was not active in the dominant lethal assay with mice.
3.6. Chronic Toxicity and Carcinogenicity
Fischer 344 rats and C57BL/6 mice were exposed to vaporized Otto Fuel II at concentrations of 0, 1.4, or 240 mg/m3 (0 and approximately 0.20 or 34 ppm) for 6 h/d, 5 d/wk for 1 y, and purebred beagle dogs were exposed at 0 or 0.20 ppm for 6 h/d, 5 d/wk for 14 months (mo) (MacEwen and Vernot 1982; Gaworski et al. 1985). For both rats and mice, groups of 100 males and 100 females were exposed at the 0- and 34-ppm concentrations and 75 animals per gender were studied at the 0.20-ppm concentration. For dogs, the exposure groups consisted of three males and three females. Separate generation systems were used for the exposures, but atmospheres were generated in a similar manner by heating the fuel and passing the vapor into the exposure chambers with a controlled air sweep. The lower concentration was monitored with an infrared analyzer; the higher concentration was monitored with a gas chromatograph. Ten male and ten female rodents from each exposure group were sacrificed at 1 y after initiation of exposures. The remaining rodents were held for 1 y postexposure. The dogs exposed at 0.20 ppm were exposed for an additional 60 d for a total of 14 mo, at which time they were necropsied. During the study, animals were weighed and monitored for hematology and clinical chemistry changes. Gross and histological examinations of all lesions and major tissues and organs were performed.
For rats, differences in the examined parameters among exposure groups were minor and generally did not reflect a dose-response relationship. Only very slight pulmonary inflammatory changes were present in the treated groups compared with the control group. Bone tumors, three osteosarcomas (male rats, one in the 0.2-ppm group and two in the 34-ppm group) and one osteoma (female rat, 0.2-ppm group) were observed in the treated rats, whereas none were observed in the concurrent control groups. These are rare tumors and could be indicative of a tumorigenic response; however, the lack of a dose-response relationship, particularly with the large differences in treatment concentrations, suggests that the tumors were not treatment related.
No remarkable changes or lesions were present in treated mice at either the end of exposure or at terminal sacrifice compared with the control groups.
In fact, lesions such as ulcerative dermatitis were generally more prevalent in the control mice than in the treated mice.
Although dogs exposed to an atmosphere containing 0.20 ppm of Otto Fuel II had slightly decreased hematocrit and hemoglobin values and increased methemoglobin levels (<5%) and liver weights relative to body weight compared with controls, there were no overt signs of toxicity and no increased incidences of tumors. As noted, these parameters were either not affected or affected to a lesser degree in mice and rats.
In a chronic toxicity study, groups of 20 male and female Sprague-Dawley rats were administered a diet containing 6.25% dibutyl sebacate (Smith 1953). The dose ranged between 2.5 and 7.2 g/kg/d over the course of the study. This concentration did not affect growth or survival. No gross or histopathological changes attributable to treatment were observed.
3.7. Summary
No data on lethality following acute exposures of ≤1 day were available. Squirrel monkeys exposed to PGDN at concentrations of 70–100 ppm for 6 h exhibited vomiting, pallor, cold extremities, semiconsciousness, and clonic convulsions. Rats exposed at 189 ppm for 4 h exhibited no overt signs of intoxication, although the mean methemoglobin level was increased to 23.5% (Jones et al. 1972).
In neurotoxicity studies with PGDN, 4-h exposures of rhesus monkeys at 2–33 ppm (Mattsson et al. 1981) and continuous exposures of squirrel monkeys at 37 ppm (Jones et al. 1972) did not change trained avoidance behavior, although the VER was significantly altered (increase in the C-wave amplitude) in the rhesus monkeys exposed at 2 ppm for 4 h. Increases in the same wave were observed in humans exposed at 0.35 to 1.5 ppm (Stewart et al. 1974). The VER changes are subclinical disruptions of the extraocular motor system and are not functionally significant. The VER changes were very minimal and were not reflected in the cognitive abilities of humans exposed at 1.5 ppm for 3 h. The cued avoidance and free operant avoidance of monkeys exposed at 2–33 ppm for 6 h were unchanged.
When applied dermally to rats and rabbits, PGDN was not teratogenic and showed no evidence as a reproductive and developmental toxicant at doses that were less than maternally toxic (Cooper et al. 1993). In rats, lower fetal weights reflected the lower body weights of surviving dams.
In a battery of mutagenicity and genotoxicity studies, PGDN tested negative except in L5178Y mouse lymphoma cells where it induced mutations at
concentrations that were cytotoxic (Litton Bionetics 1979). There were no clear treatment-related increases in tumors in rats or mice exposed to vaporized Otto Fuel II at concentrations of 0, 0.20, or 34 ppm for 1 y or in beagle dogs exposed at 0.20 ppm for 14 mo (MacEwen and Vernot 1982; Gaworski et al. 1985).
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Absorption of PGDN during inhalation occurs in humans, as evidenced by symptoms of headache and changes in blood pressure. During exposures of human subjects at 1.5 ppm for 3.2 h, the circulating concentration was less than 5 ppb (the analytical limit of detection). After 1 h of exposure at 1.5 ppm, the expired breath of the subjects contained 20–35 ppb; no PGDN was detected in the breath 15 min after exposure (Stewart et al. 1974). Dermal absorption also occurs, as evidenced by deaths of rabbits with elevated methemoglobin and urinary nitrogen levels following repeated applications of 4 g/kg (Jones et al. 1972). About 10% of topically applied PGDN is absorbed through the intact skin of rats, as indicated by blood pressure changes relative to subcutaneous injections (Clark and Litchfield 1969).
Plasma levels of PGDN of rhesus monkeys could not be detected during inhalation exposures at concentrations ≤0.8 ppm (Mattsson et al. 1981). Plasma PGDN was approximately 35 µg/mL during 20 d of exposure at 1.6 ppm and 170 µg/mL during 14 d of exposure at 4.2 ppm. Plasma PGDN was not detectable within 24 h of termination of exposure.
Metabolism of PGDN is rapid and follows first order kinetics (Kylin et al. 1964). Metabolism occurs in the liver and within the erythrocyte, resulting in mononitrates and inorganic nitrate; the latter is eliminated in the urine. Clark and Litchfield (1969) studied the metabolism of PGDN in Alderley Park rats in vitro and in vivo following a subcutaneous injection of 65 mg/kg. Blood PGDN, the mononitrates, and blood inorganic nitrite and nitrate were measured at various times after incubation or administration. In vitro metabolism took place in the erythrocytes yielding primarily propylene glycol 2-mononitrate and inorganic nitrate by 3 h; the remainder was propylene glycol 1-mononitrate and unmetabolized PGDN. Fifty percent of the 50 µg/mL dose was metabolized in the first hour and 50% of the remainder in the next hour.
Following subcutaneous injection, circulating PGDN peaked within 1 h and then declined to an undetectable level by 8–12 h postinjection. The 2-
mononitrate was the predominant isomer in blood and the time course of metabolism was similar to that of the in vitro experiment. The major metabolite in urine was inorganic nitrate, accounting for 56% of the administered dose. Parent PGDN and nitrite were almost undetectable in urine. Excretion was complete at 24 h. The following metabolic scheme was proposed: reduction of a nitrate group to yield an unstable organic nitrite-nitrate intermediate followed by hydrolysis to yield the mononitrate and inorganic nitrite. The inorganic nitrite in the blood is oxidized to inorganic nitrate, which is excreted in the urine.
4.2. Mechanisms of Toxicity
PGDN has effects on both the cardiovascular and central nervous systems. The most commonly encountered symptom of exposure to PGDN is headache due to vasodilation of cerebral blood vessels. Nitrate and nitrite esters are vasodilators, resulting in rapid lowering of systolic and, to a lesser extent, diastolic blood pressure with a compensatory tachycardia. Administration of nitrites produces dilation of meningeal blood vessels (via relaxation of vascular smooth muscle), which is the basis for the transient pulsating headache (Nickerson 1975). Headache of presumed vascular origin is a frequent complaint following therapeutic doses of the structurally similar nitrate triester nitroglycerin for the treatment of angina. Vasodilation of the dural arteries is the probable cause of headaches and nasal congestion experienced by torpedo maintenance workers in the study of Horvath et al. (1981).
Vasodilation is attributable to nitric oxide (NO), which is produced either directly from the nitroester or liberated by decomposition of NO intermediates (Feelisch and Noack 1987). Either glutathione in cells of vascular tissue or sulfhydryl groups of proteins in these tissues may be responsible for converting nitrates to NO. Nitric oxide activates guanylyl cyclase, which increases intracellular levels of cyclic guanosine 3'5'-monophosphate and thereby produces vasodilation (Kelly and Smith 1996; Robertson and Robertson 1996).
To study the effect of PGDN on cerebral blood flow, Godin et al. (1995) injected male Sprague-Dawley rats (through a jugular vein cannula) with PGDN at 0.1 to 30 mg/kg and measured cerebral blood flow with a fiberoptic laser-Doppler flow probe in contact with the brain. Following a small initial drop in cerebral perfusion that lasted 1 min, blood flow rapidly increased and reached a maximum 2 min after injection. The increase in perfusion was correlated with dose, but due to the small number of animals and individual variability, a clear dose-response relationship was not obtained.
Peripheral vasodilation can precipitate a fall in blood pressure. Intravenous injection of male Fischer 344 rats with PGDN at 0.1 to 30 mg/kg produced a dose-related fall in systolic blood pressure within 1 min (Godin et al. 1995). No drop in blood pressure in rats was observed over a 30–45 min period during exposure to an atmosphere of saturated PGDN vapor (82–90 ppm) generated from Otto Fuel II (Godin et al. 1993).
An effect on blood pressure was shown in the study by Clark and Litchfield (1969) in which subcutaneous injections of PGDN to anesthetized rats at 5, 10, 20, 40, 80, or 160 mg/kg resulted in a dose-related fall in mean arterial blood pressure (measured in the cannulized femoral artery) within 30 min with recovery over the next 12 h. The maximum drop in blood pressure correlated with the maximum concentration of PGDN in the blood. However, a drop in blood pressure did not occur in human volunteers who inhaled 0.5 ppm PGDN for 7.3 h. Rather, a mean elevation of diastolic blood pressure of 12 mm Hg was associated with severe and throbbing headaches (Stewart et al. 1974). A drop in blood pressure and decreasing stroke volume can result in brain ischemia, causing the dizziness and weakness reported by one subject after exposure at 0.5 ppm for 6 h in the Stewart et al. (1974) study as well as in occupationally exposed workers (Horvath et al. 1981).
The sudden deaths of workers in the explosives industry have been attributed to a series of cardiovascular events that occur after repeated occupational exposures (Carmichael and Lieben 1963). Acute exposures result in a depression of both the systolic and diastolic blood pressure. Continued exposure to low concentrations of nitrate esters produces a progressive rise in the diastolic blood pressure from the previously depressed level without a comparable rise in the systolic blood pressure. This narrowing of the pulse pressure combined with an increased diastolic pressure and high pulse rate, which occurs following cessation of exposure, may contribute to acute myocardial ischemia.
High doses of PGDN are associated with increased circulating methemoglobin. Methemoglobin and blood nitrate levels were not increased in human subjects exposed at concentrations up to 1.5 ppm for 3.2 h (Stewart et al. 1974). Subcutaneous injection of rats with PGDN at 0, 25, 50, 100, 200, or 400 mg/kg resulted in a dose-related methemoglobinemia with values ranging from <10% at ≤50 mg/kg to approximately 85% at 400 mg/kg (Clark and Litchfield 1969). At the LD50 (approximately 500 mg/kg) in rats, almost complete conversion of hemoglobin to methemoglobin was achieved. Maximal methemoglobin levels were reached 2–3 h after injection. Methemoglobin reached peak levels of approximately 20% during the second week of continuous inhalation exposure of dogs and monkeys to 33 ppm (Jones et al. 1972).
PGDN also acts as a central nervous system depressant in humans. The changes in the VER, disturbances in postural balance (Stewart et al. 1974), and changes in oculomotor performance (Horvath et al. 1981) are consistent with central nervous system depression. The concentrations in these studies did not greatly influence cognitive functions. Similarly, higher concentrations had little or no effect on monkeys trained in avoidance tests (Jones et al. 1971; Mattsson et al. 1981). The mechanism of central nervous system depression induced by PGDN exposure is poorly understood but may be the same as that of volatile anesthetics. The difference in susceptibility of individuals to central nervous system depressants such as volatile anesthetics varies by no more than 2-fold as indicated by the minimum alveolar concentration (MAC), the concentration that produces immobility in 50% of patients (Kennedy and Longnecker 1996; Marshall and Longnecker 1996).
4.3. Structure-Activity Relationships
As noted under Mechanisms of Toxicity (Section 4.2), nitrate and nitrite esters are vasodilators with resulting hypotension (Nickerson 1975). Therapeutic doses of nitroglycerin for relief of angina are associated with headaches of vascular origin. Both PGDN and the structurally related ethylene glycol dinitrate produce headaches in humans and methemoglobinemia and hypotension in rats (Andersen and Mehl 1979).
4.4. Other Relevant Information
4.4.1. Species Differences
The erythrocytes of several species show different susceptibilities to PGDN-induced methemoglobin formation (Wyman et al. 1985). Blood was collected from Fischer 344 rats, Hartley guinea pigs, beagle dogs, and humans, and PGDN-induced methemoglobin was determined in whole-cell preparations, hemolysates, and partially purified hemoglobin solutions. A comparison of the net rate of methemoglobin formation in erythrocytes and stroma-free hemolysates over a 4-h period revealed that dogs showed the highest rate, followed by the guinea pig; the guinea pig was greater than the rat, and the rat was greater than the human. In enzyme-free hemoglobin preparations, the rate of methemoglobin formation was essentially in the same order, with dog greater than guinea pig, guinea pig greater than rat, and rat equal to human.
Activities of the erythrocyte enzymes methemoglobin reductase, glutathione-S-transferase, catalase, superoxide dismutase, glutathione peroxidase, 6-phosphogluconate dehydrogenase, and glucose-6-phosphate dehydrogenase failed to correlate with methemoglobin formation. The primary determinant of methemoglobin formation appeared to be the structure of each species’ hemoglobin molecule.
Methemoglobin formation during chronic inhalation exposures at 33 ppm indicated that dogs and monkeys are more susceptible to PGDN-induced methemoglobinemia than rats and guinea pigs (Jones et al. 1972). Slightly higher methemoglobin levels were present in the dog than in the monkey. During a 1-y exposure of rats and dogs, similar low levels of methemoglobin were induced in dogs exposed at 0.2 ppm and rats exposed at 33 ppm (Gaworski et al. 1985).
Acute subcutaneous LD50 values in the rat, mouse, and cat were 463–524, 1,208, and 200–300 mg/kg, respectively, indicating that by this route of administration the cat is approximately twice as sensitive as the rat, which in turn is approximately twice as sensitive as the mouse (Clark and Litchfield 1969).
4.4.2. Susceptible Populations
A review of the literature on PGDN did not reveal human populations that are unusually susceptible to this chemical. The elderly, especially those with heart disease, may be susceptible to the vasodilatory effects of PGDN. However, similar nitrate esters are used to treat heart patients with angina, and those patients may have developed a tolerance to induction of headaches. Older persons with arteriosclerosis or cardiac disease may have a limited ability to constrict blood vessels or to increase cardiac output in response to the vasodilatation action of PGDN and, therefore, may have a greater degree of hypotension than other individuals (ATSDR 1995). Compensatory vasospasm following withdrawal from nitrate ester exposure has been described in chronic exposures but not following an acute exposure. Although methemoglobinemia occurred in laboratory animals at high concentrations, an in vitro study showed that the hemoglobin of humans is less susceptible to PGDN-induced methemoglobin formation than the hemoglobin of laboratory animals. The exposure levels of PGDN in the occupational and human experimental studies did not result in methemoglobinemia.
There is probably a wide variation in the susceptibility of individuals in the population to the induction of headache from various stimuli. However, in the case of individuals exposed to PGDN in the study by Stewart et al.
(1974), the difference in concentration that induced mild headache in the most susceptible individual (0.1 ppm for 8 h) and the concentration that induced headaches in approximately half of the tested individuals (0.21–0.26 ppm for 8 h) was small, indicating little individual variation with this direct-acting chemical. At the 1.5-ppm concentration, induction of severe headaches in all individuals was rapid; the range of times was between 30 and 90 min.
Infants are more susceptible to methemoglobin-generating chemicals than adults as they have reduced levels of nicotine adenine dinucleotide (NADH, the cofactor [electron donor] for methemoglobin reductase) and a high concentration of fetal hemoglobin in their erythrocytes (fetal hemoglobin is more oxidizable than adult hemoglobin) (Seger 1992). NADH lacks full activity until infants are 4 mo of age. Because infants are more susceptible than adults to methemoglobin formation from nitrites and nitrates, there may be some concern that nitrite and nitrate released from inhaled PGDN may form methemoglobin in infants. Following calculation of the AEGL values (Appendix A), the amounts of nitrogen (N) released from exposure to PGDN at the 8-h AEGL concentrations were calculated and compared with EPA’s (1999) oral reference dose (RfD) for infants (Appendix B). EPA’s RfD for nitratenitrogen (NO3−) was based on a clinical study in newborn infants that showed that ingestion of 6.4 mg/d (1.6 mg/kg/d for a 4 kg infant) of nitrate-nitrogen failed to increase circulating methemoglobin. Although nitrate to nitrite conversion occurs in the achlorhydric neonatal gut, the assumption was made that systemic bioavailability in the infant is equivalent following ingestion and inhalation. The amount of nitrate-nitrogen taken up by an infant at the 8-h AEGL-3 is 4.8 mg. The calculation shows that at the AEGL-3 the amount taken up by inhalation is below the RfD if the absorbed N were to be released as nitrate. Because there were no data for nitrite-nitrogen (NO2−), EPA applied a modifying factor of 10 to derive a RfD for nitrite-nitrogen. Metabolism studies with PGDN show that released nitrite-nitrate is rapidly converted to nitrate, and nitrite was almost undetectable in the urine. Therefore, it is unlikely that methemoglobin induced from nitrite from PGDN would approach lethal levels at AEGL-3.
4.4.3. Concentration-Exposure Duration Relationship
Data from the study by Stewart et al. (1974) suggest that the relationship between exposure concentration and exposure duration for end points of both mild and severe headaches is approximately linear (i.e., mild headaches induced by 6, 2, 2, and 1 h at exposure concentrations of 0.1, 0.2, 0.3, and 0.5
ppm, respectively, and severe headaches induced at 8, 8, 2, and 1 h at exposure concentrations of 0.2, 0.3, 0.5, and 1.5 ppm, respectively). The concentration × time product is approximately 0.5 for mild headaches and approximately 1.6 for severe headaches. The linear relationship is consistent with an n value of 1 in the relationship between concentration and time, Cn×t=k. No data were available to calculate the relationship between concentration and time for other end points.
4.4.4. Concurrent Exposures
PGDN may be absorbed percutaneously. A comparison of blood pressure changes in anesthetized rats administered PGDN by the subcutaneous and dermal routes suggests that at least 10% of a cutaneous dose penetrates the skin within 30 min (Clark and Litchfield 1969). When PGDN doses of 5 to 450 mg/kg (1.2 to 108 mg/animal) were applied to a 1-cm2 area of the shaved intact dorsal skin of anesthetized male Fischer 344 rats, the percent absorbed, as measured in the excised skin 30–45 min later, ranged from 75% at the 5 mg/kg dose to 20% at the 450 mg/kg dose (Godin et al. 1993). The relationship between the applied dose and the percent absorption was not linear over the range of applied doses.
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
Occupational exposures and the study with human volunteers indicate that exposures at low concentrations cause headaches and signs of central nervous system depression. No headaches were reported and no equilibrium disturbances were measured during occupational exposures of healthy workers to Otto Fuel II (measured as PGDN) at concentrations ≤0.22 ppm (average of approximately 0.06 ppm) for periods of 30–60 min, although subtle changes in eye movements were recorded (Horvath et al. 1981). In a study with healthy but previously unexposed male volunteers, the threshold for odor detection was 0.2 ppm (Stewart et al. 1974). Mild headaches were reported in one of three subjects after a 6-h exposure at 0.1 ppm, in two of three subjects after a 2-h exposure at 0.2 ppm, and in one of three subjects after a 1-h exposure at 0.5 ppm. Severe headaches occurred after an 8-h exposure at 0.2
(six of 12 exposures) and 0.35 ppm and after a 2-h exposure at 0.5 ppm (one of three subjects).
5.2. Summary of Animal Data Relevant to AEGL-1
Few data on acute exposures with effects that meet the definition of an AEGL-1 were located. No clinical signs of intoxication were observed in rats exposed to PGDN at a concentration of 189 ppm for 4 h when the methemoglobin level was 23.5% (Jones et al. 1972). Repeated exposures of rats at 10 ppm resulted in no toxic signs, changes in hematology parameters, or organ lesions (Jones et al. 1972).
5.3. Derivation of AEGL-1
The study by Stewart et al. (1974) with human volunteers is relevant to the derivation of AEGL-1 values. Within the definition of an AEGL-1, both healthy and susceptible individuals could experience mild discomfort. A mild headache can be considered mild discomfort and the threshold concentration-time at which one or more subjects first developed a mild headache was used to derive the AEGL-1 values. No subjects (other than the one that developed a headache during the control sessions) developed headaches during an 8-h exposure at 0.03 ppm. The highest concentrations and exposure durations that did not result in headache and the lowest concentrations and exposure durations that resulted in mild headaches are as follows:
|
No headache |
Mild headaches |
|
0.03 ppm for 8 h |
0.1 ppm after 6 h |
|
0.1 ppm for 3–4 h |
0.2 ppm (0.21–0.26) for 2 h |
|
0.2 ppm for 1 h |
0.35 ppm for ≥2 h |
|
0.35 ppm for 1 h |
0.5 ppm for 1 h |
The subjects were primarily healthy young males. Because no susceptible populations were identified (angina patients are not considered at additional risk and the absorbed dose at these concentrations is far below that inducing methemoglobinemia), an intraspecies uncertainty factor (UF) of 3 was used. The intraspecies UF of 3 is supported by the steep dose-response curve for the induction of headaches: namely, a 2-fold difference in the threshold concentra-
TABLE 2–5 AEGL-1 Values for PGDN (Otto Fuel II) (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.33 |
0.33 |
0.17 |
0.05 |
0.03 |
|
(2.3) |
(2.3) |
(1.1) |
(0.3) |
(0.17) |
tion of PGDN and the concentration that induces headache in the majority of healthy individuals.
The data from Stewart et al. (1974) provide appropriate concentrations and exposure times to derive AEGL-1 values. The starting points are 1 h at 0.5 ppm and 6 h at 0.1 ppm. Using a value of n=1 in the concentration-time scaling equation of Cn×t=k, the 30-min value was calculated from the 1-h value of 0.5 ppm (k=0.167 ppm·h) and the 4- and 8-h values were calculated from the 6-h value of 0.1 ppm (k=0.2 ppm·h). The 10-min AEGL-1 was set equal to the 30-min value. Calculations are in Appendix A, and the resulting values are listed in Table 2–5.
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
Occupational and controlled human volunteer studies indicate that exposures at low concentrations of PGDN cause headaches and central nervous system depression. No headaches were reported and no equilibrium problems were recorded during occupational exposures of healthy workers to Otto Fuel II (measured as PGDN) at concentrations ≤0.22 ppm (average of approximately 0.06 ppm) for periods of 30–60 min, although subtle changes in eye movements were recorded (Horvath et al. 1981). In a study with healthy but previously unexposed male volunteers, mild headaches were reported after a 6-h exposure at 0.1 ppm and after a 2-h exposure at 0.21–0.26 ppm (Stewart et al. 1974). Severe headaches occurred after an 8-h exposure at 0.21–0.26 and 0.35 ppm, after a 2-h exposure at 0.5 ppm, and after a 1-h exposure at 1.5 ppm. The VER was altered after 45–90 min of exposure at 0.5 ppm. Changes in VER are not considered an adverse effect in the absence of a sensory effect or motor impairment. One subject reported dizziness and nausea after 6 h of exposure at 0.5 ppm. A slight loss of equilibrium in one of several neurobehavioral tests (heel-to-toe test with eyes closed) first occurred after 6.25 h of exposure at 0.5 ppm; loss of balance increased with increasing exposure
time, becoming more severe after 8 h of exposure. There were no reported changes in cognitive tests at this concentration.
6.2. Summary of Animal Data Relevant to AEGL-2
Few data on acute exposures with effects that meet the definition of an AEGL-2 were located. No clinical signs of intoxication were observed in rats exposed to PGDN at 189 ppm for 4 h. The methemoglobin level was 23.5% (Jones et al. 1972). Exposure of monkeys to PGDN at a concentration of 33 ppm for 4 h failed to affect performance in an operant avoidance behavioral test but altered the VER (Mattsson et al. 1981).
6.3. Derivation of AEGL-2
The study by Stewart et al. (1974) with human volunteers exposed to PGDN is relevant to derivation of AEGL-2 values. Within the definition of an AEGL-2, both healthy and susceptible individuals could experience notable but nondisabling effects The alteration in VER as well as the decrease in saccade velocity observed in the occupational exposures are subclinical disruptions of the extraocular motor system and are not functionally significant. Although the slight loss of balance observed at 6.25 h of exposure at 0.5 ppm would not cause irreversible or other serious, long-lasting effects or impair the ability to escape, it could be considered a threshold for inability to escape. After exposure at 1.5 ppm, severe, throbbing headaches became incapacitating, and the exposure was terminated after 3.2 h. Eye irritation at this concentration was without conjunctivitis or excessive lacrimation.
The severe headache accompanied by slight loss of equilibrium in one of several sensitive equilibrium tests after a 6.25-h (rounded down to 6 h) exposure at 0.5 ppm was considered the threshold for inability to escape and was used to derive the AEGL-2 values. A UF of 3 was used to adjust the value as no susceptible populations were identified and the threshold for narcosis for most anesthetics does not differ among individuals by more than a factor of 2 (Kennedy and Longnecker 1996; Marshall and Longnecker 1996). The intraspecies UF of 3 is supported by the steep dose-response curve for the induction of headaches: namely, a 2-fold difference in the threshold concentration of PGDN and the concentration that induces headache in the majority of healthy individuals (Stewart et al. 1974). The 6-h 0.5-ppm concentration was
TABLE 2–6 AEGL-2 Values for PGDN (Otto Fuel II) (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
2.0 |
2.0 |
1.0 |
0.25 |
0.13 |
|
(43) |
(43) |
(6.8) |
(1.7) |
(0.8) |
adjusted by a UF of 3 and scaled to the 30-min and 1-, 4-, and 8-h time periods using Cn×t=k where n=1 (based on the concentration and time data for headaches) and k=1 ppm·h. Because of the long exposure duration of the key study, the 10-min value was set equal to the 30-min value. Calculations are in Appendix A, and the values are listed in Table 2–6.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
No human data relevant to the definition of an AEGL-3 were located.
7.2. Summary of Animal Data Relevant to AEGL-3
Few data on acute exposure with effects that meet the definition of an AEGL-3 were located. As noted for the AEGL-2 above, no overt signs of intoxication were observed in rats exposed to PGDN at a concentration of 189 ppm for 4 h. The methemoglobin level was 23.5% (Jones et al. 1972), which may be manifest as clinical cyanosis but does not produce hypoxia (Seger 1992). Exposure of an unspecified number of monkeys to PGDN at concentrations of 70–100 ppm for 6 h resulted in semiconsciousness and clonic convulsions. These signs resolved within 30–45 min after removal from exposure (Jones et al. 1972). No gross or histopathological effects were observed in the brain, spinal cord, or nerves of monkeys and dogs continuously exposed at 33 ppm for 90 d (Jones et al. 1972) or in the organs and tissues of rats and mice repeatedly exposed at 33 ppm for 1 y (MacEwen and Vernot 1982; Gaworski et al. 1985). With the exception of the death of one of nine monkeys on day 31 of exposure, this repeated daily inhalation at 33 ppm had no effect on survival. Continuous exposure of dogs and guinea pigs at 33 ppm for 90 d also had no effect on survival (Jones et al. 1972).
7.3. Derivation of AEGL-3
Two animal studies conducted with high exposure concentrations are suitable for deriving the AEGL-3 values. No deaths and no toxic signs were observed in rats exposed to a PGDN mist at 189 ppm for 4 h and no deaths occurred in monkeys exposed at ≥70 ppm (70–100 ppm) for 6 h (Jones et al. 1972). Although no deaths occurred, the severe signs during exposure of monkeys at ≥70 ppm for 6 h can be considered the threshold for lethality. These signs are consistent with central nervous system depression and/or cardiovascular effects and suggest that the monkey is more susceptible to inhaled PGDN than the rat. The study with monkeys was chosen as the basis for the AEGL-3 because the monkey is more susceptible than the rat and the respiratory tract of the monkey is more similar to the human respiratory tract than that of the rat. The magnitude of interspecies difference in susceptibility to PGDN is unknown, but the mechanism of action is similar for all mammals, and the difference between monkeys and humans would not be great (both monkeys and humans showed changes in VER at similar concentrations). Because the most susceptible test species was chosen, the 70-ppm concentration was adjusted by an interspecies UF of 3. For extreme central nervous system depression leading to convulsions, an intraspecies UF of 3 was considered sufficient (concentrations of anesthetics causing narcosis in infants and adults generally do not differ by more than a factor of 2 [Kennedy and Longnecker 1996; Marshall and Longnecker 1996]). The intraspecies UF of 3 is supported by the steep dose-response curve for the induction of headaches; namely, a 2-fold difference in the threshold concentration of PGDN and the concentration that induces headache in the majority of healthy individuals (Stewart et al. 1974). The result is adjustment by a total UF of 10. Because an n value of 1 is relevant to the end point of headaches and the end point for the AEGL-3 is convulsions, the more conservative n values of 1 and 3, with k values of 2,058 ppm·h and 42 ppm·h, were used to time-scale from the 6-h exposure duration to the longer and shorter time periods, respectively. The 10-min AEGL-3 was set equal to the 30-min value. Calculations are in Appendix A, and the resulting values are listed in Table 2–7.
These values are supported by the results of subchronic studies with squirrel monkeys and dogs (Jones et al. 1972). Monkeys and dogs exposed continuously at approximately 15 ppm for 90 d showed no overt clinical signs; systemic toxicity consisted of biochemical and/or non-life-threatening histological changes in the liver, spleen, and kidneys.
TABLE 2–7 AEGL-3 Values for PGDN (Otto Fuel II) (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
16 |
16 |
13 |
8.0 |
5.3 |
|
(114) |
(114) |
(93) |
(57) |
(38) |
8. SUMMARY OF AEGLs
8.1. AEGL Values and Toxicity End points
The AEGL values and toxicity end points are summarized in Table 2–8.
8.2. Comparisons with Other Standards and Guidelines
PGDN has limited uses and only workplace standards have been developed. Both the ACGIH TLV-TWA and NIOSH REL TWA are 0.05 ppm. The recommended ACGIH TLV-TWA is based on the study of Stewart et al. (1974) in which volunteers exposed at 0.5 ppm for 6 to 8 h showed a marked impairment of their performance on simple behavioral tests, and volunteers exposed at 0.2 ppm or greater showed a disruption of the visual evoked response and headache. The 8-h TWA value of 0.05 ppm derived by ACGIH and NIOSH for healthy workers is identical to the 4-h AEGL-1 and is slightly greater than the 8-h AEGL-1 of 0.03 ppm.
TABLE 2–8 Summary of AEGL Values (ppm [mg/m3])
|
|
Exposure Duration |
||||
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 |
0.33 |
0.33 |
0.17 |
0.05 |
0.03 |
|
(Nondisabling) |
(2.3) |
(2.3) |
(1.1) |
(0.3) |
(0.17) |
|
AEGL-2 |
2.0 |
2.0 |
1.0 |
0.25 |
0.13 |
|
(Disabling) |
(14) |
(14) |
(6.8) |
(1.7) |
(0.8) |
|
AEGL-3 |
16 |
16 |
13 |
8.0 |
5.3 |
|
(Lethal) |
(114) |
(114) |
(93) |
(57) |
(38) |
8.3. Data Adequacy and Research Needs
Data from human exposures were used to derive the AEGL-1 and AEGL-2 values. The study on which the AEGL-1 and AEGL-2 were based was well designed, conducted, and documented and used 20 volunteers. In addition, supporting data were available. Occupational data were available to support the margin of safety associated with the AEGL-1 values; developmental toxicity data were available from both occupational exposures and experimental animal studies; specific neurotoxicity tests were performed with both human and animal subjects; and a battery of genotoxicity and chronic toxicity bioassays were reported. Moreover, the mechanism of action for the headache associated with nitrate esters is well understood from medical applications.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1991. Documentation of the Threshold Limit Values and Biological Exposure Indices, Sixth ed., ACGIH, Cincinnati, OH.
Andersen, M.E., and R.G.Mehl. 1979. A comparison of the toxicology of triethylene glycol dinitrate and propylene glycol dinitrate. Am. Ind. Hyg. Assoc. J. 34:526– 532.
ATSDR (Agency for Toxic Substances and Disease Registry. 1995. Otto Fuels II. U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA.
Baughman, G.L., and T.A.Perenich. 1988. Fate of dyes in aquatic systems: I. Solubility and partitioning of some hydrophobia dyes and related compounds. Environ. Toxicol. Chem. 7:183–200.
Bisesi, M.S. 1994. Esters. In: Patty’s Industrial Hygiene and Toxicology, Vol. II, Part D, John Wiley & Sons, New York, pp. 2967–3117.
Bogo, V., T.A.Hill, and J.Nold. 1987. Motor performance effects of propylene glycol dinitrate in the rat. J. Toxicol. Environ. Health 22:17–27.
Carmichael, P., and J.Lieben. 1963. Sudden death in explosives workers. Arch. Environ. Health 7:424–439.
Clark, D.G., and M.H.Litchfield. 1969. The toxicity, metabolism, and pharmacologic properties of propylene glycol 1,2-dinitrate. Toxicol. Appl. Pharmacol. 15:175–184.
Cooper, J.R., L.H.Lee, and D.A.Macys. 1993. Developmental toxicity of Otto Fuel II in the rat and rabbit. J. Appl. Toxicol. 13:135–239.
Donovan, J.W. 1990. Nitrates, nitrites and other sources of methemo-globinemia. In: L.M.Haddad and J.F.Winchester, eds., Poisoning and Drug Overdose, 2nd
ed. WB Sanders Co., Philadelphia, PA, pp. 1419–1431.
EPA (U.S. Environmental Protection Agency). 1999. Nitrate Reference Dose. Integrated Risk Information System, Available from: http://www.epa.gov/ngispgm3/iris.
Feelisch, M., and E.A.Noack. 1987. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase . Eur. J. Pharmacol. 139:19–30.
Forman, S.A., J.C.Helmkamp, and C.M.Bone. 1987. Cardiac morbidity and mortality associated with occupational exposure to 1,2-propylene glycol dinitrate. J. Occup. Med. 29:445–450.
Forman, S.A. 1988. A review of propylene glycol dinitrate toxicology and epidemiology. Toxicol. Lett. 43:51–65.
Gaworski, C.L., H.L.Leahy, W.J.Bashe, J.D.MacEwen, E.H.Vernot, C.C.Haun, R.H.Bruner, and J.F.Wyman. 1985. A one-year inhalation toxicity study of Otto Fuel II. ADA 163 162; AAMRL-TR-85–071; NMRI-85–56, University of California, Irvine, California and Naval Medical Research Institute, Wright-Patterson Air Force Base, Ohio.
Gingell, R., R.J.Boatman, J.S.Bus, T.J.Crawley, J.B.Knaak, W.J.Krasavage, N.P.Skoulis, C.R.Stack, and T.R.Tyler. 1994. Chapter 31: Glycol ethers and other selected glycol derivatives. In: Patty’s Industrial Hygiene and Toxicology, John Wiley & Sons, New York, pp. 2943–2947.
Godin, C.R., E.C.Kimmel, J.M.Drerup, H.F.Leahy, and D.L.Pollard. 1993. Effect of exposure route on measurement of blood pressure by tail cuff in F-344 rats exposed to Otto Fuel II. Toxicol. Lett. 66:147–155.
Godin, C.R., J.He, J.M.Drerup, and J.Wyman. 1995. Effect of propylene glycol 1,2-dinitrate on cerebral blood flow in rats: a potential biomarker for vascular headache? Toxicol. Lett. 75:59–68.
Helmkamp, J.C., S.A.Forman, M.S.McNally, and C.M.Bone. 1984. Morbidity and mortality associated with exposure to Otto Fuel II in the U.S. Navy 1966–1979. AD-A148 726; Report No. 84–35, Naval Health Research Center, San Diego, California.
Horvath, E.P., R.A.Ilka, J.Boyd, and T.Markham. 1981. Evaluation of the neurophysiologic effects of 1,2-propylene glycol dinitrate by quantitative ataxia and oculomotor function tests. Am. J. Ind. Med. 2:365–378.
Jones, R.A., J.A.Strickland, and J.Siegel. 1972. Toxicity of propylene glycol 1,2dinitrate in experimental animals. Toxicol. Appl. Pharmacol. 22:128–137.
Kelly, R.A., and T.W.Smith. 1994. Pharmacological treatment of heart failure. In Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 9th Ed., J.G. Hardman et al., eds. New York: McGraw-Hill, p. 827.
Kennedy, S.K. and D.E.Longnecker. 1996. History and Principles of Anesthesiology. P. 302 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 9th Ed., J.G.Hardman et al., eds. New York: McGraw-Hill.
Kylin, B., A.Englund, H.Ehrner-Samuel, and S.Yllner. 1966. A comparative study on the toxicology of nitroglycerine, nitroglycol and propylene glycol dinitrate.
Proceedings of the 15th International Congress on Occupational Health held in Vienna, Austria, September 19–24, 1966. Vienna: Verlag der Wiener Medizinischen Akademie 3:191–195.
Litton Bionetics, Inc. 1979. Mutagenicity evaluation of Otto Fuel number 2 in the Ames Salmonella/microsome plate test. Segment report. Report by Litton Bionetics, Inc., Kensington, Maryland submitted to Department of the Navy, Office of Naval Research, Arlington, Virginia, ADA112227.
MacEwen, J.D., and E.H.Vernot. 1982. Toxic Hazards Research Unit Annual Technical Report: 1982. ADA 121 717, AFAMRL-TR-82–62, University of California, Irvine and Air Force Aerospace Medical Research Laboratory, Wright-Patterson Air Force Base, Ohio.
Marshall, B.E. and D.E.Longnecker. 1996. General Anesthetics. P. 307 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 9th Ed., J.G. Hardman et al., eds. New York: McGraw-Hill.
Mattsson, J.L., R.W.Young, C.R.Curran, C.G.Franz, M.J.Cowan, and L.J.Jenkins. 1981. Acute and chronic propylene glycol dinitrate exposure in the monkey. Aviat. Space Environ. Med. 52:340–345.
NHRC (Naval Health Research Center). 1986. Letter 3900, dated March 13, 1986. Cited in Forman, 1988.
Nickerson, M. 1975. Vasodilator drugs. In Goodman, L. and Gilman, A. (Eds.), The Pharmacological Basis of Therapeutics, MacMillan, New York, pp. 727–743.
NIOSH (National Institute for Occupational Safety and Health). 1994. NIOSH Pocket Guide to Chemical Hazards. NIOSH Publication 94–116, U.S. Department of Health and Human Services; U.S. Government Printing Office, Washington, DC.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Airborne Chemicals. Washington, DC: National Academy Press.
Rivera, J.C. 1974. Otto fuel II: Health hazards and precautions. U.S. Navy Medicine 63:7–10.
Robertson, R.M., and D.Robinson. 1996. Drugs used for the treatment of myocardial ischemia. Pp. 759–767 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 9th Ed., J.G.Hardman et al, eds. New York: McGraw-Hill.
Seger, D.L. 1992. Methemoglobin-forming chemicals. In: J.B.Sullivan and G.R. Krieger, Eds., Hazardous Materials Toxicology: Clinical Principles of Environmental Health. Baltimore, MD: Williams & Wilkins Co, pp. 800–806.
Smith, C. 1953. Toxicity of butyl stearate, dibutyl sebacate, dibutyl phthalate, and methoxyethyl oleate. AMA Arch. Ind. Hyg. Occup. Med. 7:310–318.
Stewart, R.D., J.E.Peterson, P.E.Newton, C.L.Hake, M.J.Hosko, A.J.Lebrun, and G.M.Lawton. 1974. Experimental human exposure to propylene glycol dinitrate.
APPENDIX A
DERIVATION OF AEGL VALUES
Derivation of AEGL-1
|
Key study: |
Stewart et al. 1974 |
|
Toxicity end point: |
Mild headache (threshold); 1 h at 0.5 ppm and 6 h at 0.1 ppm |
|
Scaling: |
C1×t=k based on concentrations and exposure durations for the end points of mild and severe headache in the key study. |
|
Uncertainty factor: |
3; no unusually susceptible populations were identified and the end point was a threshold effect. More severe headaches are known to occur in some patients medicated with other nitrate esters and the threshold for vasodilatation in the key study did not vary greatly among individuals. |
|
Calculations: |
C×t=k 30-min and 1-h AEGL-1: (0.5 ppm/3)×1 h= 0.167 ppm·h 4- and 8-h AEGL-1: (0.1 ppm/3)×6 h=0.2 ppm·h |
|
10-min AEGL-1: |
Set equal to the 30-min value |
|
30-min AEGL-1: |
C×t=k C×1/2 h=0.167 ppm·h C=0.33 ppm |
|
1-h AEGL-1: |
0.5 ppm/3=0.17 ppm |
|
4-h AEGL-1: |
C×t=k C×4 h=0.2 ppm·h C=0.05 ppm |
|
8-h AEGL-1: |
C×t=k C×8 h=0.2 ppm·h C=0.03 ppm |
Derivation of AEGL-2
|
Key study: |
Stewart et al. 1974 |
|
Toxicity end point: |
Severe headache and threshold for central nervous system effects after 6-h exposure at 0.5 ppm |
|
Scaling: |
C1×t=k based on concentrations and exposure durations for the end points of mild and severe headache in the key study |
|
Uncertainty factor: |
3; severe headaches are known to occur in angina patients medicated with nitroglycerin and the threshold for vasodilatation does not vary greatly among individuals. The effect was also a threshold effect for central nervous systems depression (no change in cognitive abilities; slight imbalance in one of several sensitive motor tests). Individual variation in susceptibility to central nervous system depressants such as anesthetics varies no more than 2-fold. |
|
Calculations: |
C×t=k (0.5 ppm/3)×6 h=1 ppm·h |
|
10-min AEGL-2: |
Set equal to the 30-min value |
|
30-min AEGL-2: |
C×t=k C×1/2 hour=1 ppm·h C=2 ppm |
|
1-h AEGL-2: |
C×t=k C×1 h=1 ppm·h C=1 ppm |
|
4-h AEGL-2: |
C×t=k C×4 h=1 ppm·h C=0.25 ppm |
|
8-h AEGL-2: |
C×t=k C×8 h=1 ppm·h C=0.13 ppm |
Derivation of AEGL-3
|
Key study: |
Jones et al. 1972 |
|
Toxicity end point: |
Severe effects (vomiting, pallor, cold extremities, semiconscousness, and clonic convulsions) in monkeys exposed at 70–100 ppm for 6 h; no effects in rats exposed at 189 ppm for 4 h |
|
Scaling: |
Default values of n=3 for shorter exposure durations and n=1 for longer exposure durations |
|
Uncertainty factors: |
Interspecies: 3—The monkey was more susceptible than the rat; the lowest concentration in a range was chosen (70 ppm); humans and monkeys showed changes in the visual evoked response at similar concentrations; the monkey is a good model for the human. The concentration inducing central nervous system depression does not vary greatly among mammalian species. Intraspecies: 3—Individual variation in susceptibility to central nervous system depressants such as anesthetics varies no more than 2-fold. |
|
Calculations: |
30-min and 1- and 4-h exposure durations: C3×t=k (70 ppm/10)3×6 h=2,058 ppm·h 8-h exposure duration: C×t=k |
APPENDIX B Potential Methemoglobin Formation in Infants
Calculation of N released from exposure to PGDN at the 8-h AEGL concentrations:
Assumptions:
-
a breathing rate in infants of 4.5 m3/day (U.S. EPA Exposure Factors Handbook)
-
100% of the PGDN that enters the lung is absorbed into the circulatory system
-
1 molecule of N per molecule of PGDN (M.W.=14/166) (the 2-mononitrate is the predominant metabolite in the blood)
4.5 m3×8 h/24 h×0.17 mg/m3=0.26×14/166=0.02 mg
4.5 m3×8 h/24 h×0.8 mg/m3=0.12×14/166=0.10 mg
4.5 m3×8 h/24 h×38 mg/m3=57×14/166=4.8 mg
EPA’s reference dose for nitrate-nitrogen (NO3−) is based on a clinical study in newborn infants. That study showed that ingestion of 6.4 mg/d of nitratenitrogen did not cause an increase in the circulating methemoglobin in infants. The NOEL of 6.4 mg/d for methemoglobin formation in infants is higher than the amount of nitrogen released from PGDN even assuming complete systemic bioavailability upon inhalation and complete in vivo conversion of PGDN to NO3− during exposure to the 8-h AEGL-3.
APPENDIX C
DERIVATION SUMMARY FOR ACUTE EXPOSURE GUIDELINE LEVELS PROPYLENE GLYCOL DINITRATE (CAS No. 6423–43–4)
|
AEGL-1 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.33 ppm |
0.33 ppm |
0.17 ppm |
0.05 ppm |
0.03 ppm |
|
Key reference: |
Stewart, R.D., J.E.Peterson, P.E.Newton, C.L.Hake, M.J. Hosko, A.J.Lebrun, and G.M.Lawton. 1974. Experimental human exposure to propylene glycol dinitrate. Toxicol. Appl. Pharmacol. 30:377–395. |
|||
|
Test species/Strain/Number: 20 human subjects |
||||
|
Exposure route/Concentrations/Durations: Inhalation; 0.0, 0.03, 0.1, 0.2, 0.35, 1.2, or 1.5 ppm for periods of 1 to 8 h. Subjective evaluations and physiological and central nervous system responses reported. |
||||
|
Effects: |
No headache: |
0.03 ppm for 8 h |
||
|
0.1 ppm for 3–4 h |
||||
|
0.2 ppm for 1 h |
||||
|
0.35 ppm for 1 h |
||||
|
Mild headache: |
0.1 ppm after 6 h |
|||
|
0.2 ppm (0.21–0.26 ppm) for 2 h |
||||
|
0.35 ppm for >2 h |
||||
|
0.5 ppm for 1 h |
||||
|
End point/Concentration/Rationale: Threshold for mild headache in 1 of 3 subjects after a 6-h exposure at 0.1 ppm and after a 1-h exposure at 0.5 ppm. The threshold for mild headache falls within the AEGL-1 definition of mild discomfort. |
||||
|
Uncertainty factors/Rationale: Total uncertainty factor: 3 Interspecies: Not applicable; human subjects tested. Intraspecies: 3—no unusually susceptible populations were identified. Because the time and concentration values were based on a threshold, these concentrations were adjusted by an uncertainty factor of 3 to account for differences in human sensitivity. More severe headaches are often experienced by heart patients medicated with nitroglycerin for angina and these concentrations are far below those inducing methemoglobinemia in infants. |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Not applicable; human data used. |
||||
|
Time scaling: Cn×t=k where n=1 (k=0.167 ppm·hour for the 30-min value and 0.2 ppm·h for the 4- and 8-h values). Data from the key study suggest that the relationship between exposure concentration and exposure duration for end points of both mild and severe headaches is approximately linear (i.e., mild headaches induced by 6, 2, 2, and 1 h at exposure concentrations of 0.1, 0.2, 0.3, and 0.5 ppm, respectively, and severe headaches induced at 8, 8, 2, and 1 h at exposure concentrations of 0.2, 0.3, 0.5, and 1.5 ppm, respectively). The concentration×time product is approximately 0.5 for mild headaches and approximately 1.6 for severe headaches. The linear relationship is consistent with an n value of 1 in the relationship between concentration and time, Cn×t=k. The 1-h value was used to extrapolate to the shorter duration (30 min) and the 6-h value was used to extrapolate to the longer durations (4 and 8 h). The 10-min value was set equal to the 30-min value. |
||||
|
Data adequacy: The key study was well designed, conducted, and documented; used 20 human subjects; and utilized a range of concentrations and exposure durations. Occupational exposures support the 8-h AEGL value. The mechanism of headache induction (vasodilation) is well understood and occurs following therapeutic administration of nitrate esters to humans. Animal studies utilized several mammalian species and addressed metabolism, neurotoxicity, developmental and reproductive toxicity, and potential carcinogenicity. |
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
2.0 ppm |
2.0 ppm |
1.0 ppm |
0.25 ppm |
0.13 ppm |
|
Key reference: |
Stewart, R.D., J.E.Peterson, P.E.Newton, C.L.Hake, M.J. Hosko, A.J.Lebrun, and G.M.Lawton. 1974. Experimental human exposure to propylene glycol dinitrate. Toxicol. Appl. Pharmacol. 30:377–395. |
|||
|
Test species/Strain/Sex/Number: 20 human subjects |
||||
|
Exposure route/Concentrations/Durations: Inhalation; 0.0, 0.03, 0.1, 0.2, 0.3, 0.35, 1.2, or 1.5 ppm for periods of 1 to 8 h. Subjective evaluations and physiological and central nervous system responses reported. |
||||
|
Effects: |
Severe headache: 0.21–0.26 ppm for 8 h 0.35 ppm for 8 h 0.5 ppm for 2 h 1.5 ppm for 1 h Change in visual evoked response: 0.35 ppm for 8 h Threshold for impairment of balance: 0.5 ppm for 6 h Threshold for abnormal cognitive test: 1.5 ppm for 3.2 h |
|||
|
End point/Concentration/Rationale: A 6-h exposure at 0.5 ppm which resulted in severe headache and was the threshold for loss of equilibrium falls within the AEGL-2 definition of threshold for impaired ability to escape. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 3 Interspecies: Not applicable; human subjects tested. Intraspecies: 3—no unusually susceptible populations were identified. The threshold for vasodilatation does not vary greatly among individuals. Furthermore, severe headaches are often experienced by heart patients medicated with nitroglycerin for angina and these concentrations are far below those inducing methemoglobinemia in infants. The threshold for anesthetic effects also does not differ greatly among individuals. |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Not applicable, human data used. |
||||
|
Time scaling: Cn×t=k where n=1 and k=1 ppm·h. Data from the key study suggest that the relationship between exposure concentration and exposure duration for end points of both mild and severe headaches is approximately linear (i.e., mild headaches induced by 6, 2, 2, and 1 h at exposure concentrations of 0.1, 0.2, 0.3, and 0.5 ppm, respectively, and severe headaches induced at 8, 8, 2, and 1 h at exposure concentrations of 0.2, 0.3, 0.5, and 1.5 |
||||
|
ppm, respectively). The concentration × time product is approximately 0.5 for mild headaches and approximately 1.6 for severe headaches. The linear relationship is consistent with an n value of 1 in the relationship between concentration and time, Cn×t=k. Because of the long exposure duration of the key study, the 10-min AEGL-2 was not time-scaled, but was set equal to the 30-min value. |
||||
|
Data adequacy: The key study was well designed, conducted and documented; used 20 human subjects; and utilized a range of concentrations and exposure durations. The mechanism of headache induction (vasodilation) is well understood and occurs following therapeutic administration of nitrate esters to humans. Animal studies utilized several mammalian species and addressed metabolism, neurotoxicity, developmental and reproductive toxicity, and potential carcinogenicity. |
|
AEGL-3 |
||||
|
10 min |
30 min |
1 min |
4 min |
8 min |
|
16 ppm |
16 ppm |
13 ppm |
8.0 ppm |
5.3 ppm |
|
Key reference: |
Jones, R.A., J.A.Strickland, and J.Siegel. 1972. Toxicity of propylene glycol 1,2-dinitrate in experimental animals. Toxicol. Appl. Pharmacol. 22:128–137. |
|||
|
Test species/Strain/Sex/Number: Squirrel monkeys (number and sex not stated) |
||||
|
Exposure route/Concentrations/Durations: Inhalation; 70–100 ppm for 6 h |
||||
|
Effects: |
Severe effects (vomiting, pallor, cold extremities, semiconsciousness, and clonic convulsions) |
|||
|
End point/Concentration/Rationale: The 6-h exposure at 70–100 ppm was a NOEL for lethality in monkeys |
||||
|
Uncertainty factors/Rationale: Total uncertainty factor: 10 Interspecies: 3—the monkey was more susceptible than the rat, the lowest concentration in a range was chosen, humans and monkeys showed changes in the visual evoked response at similar concentrations, and the monkey is a good model for the human. Intraspecies: 3—the threshold for central nervous system effects (narcosis) does not vary greatly among individuals. |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Not applied. |
||||
|
Time scaling: Default values of n=3 and n=1 for shorter and longer time-scaling durations, respectively, with respective k value of 2,058 ppm·h and 42 ppm·h, because no data were available for time scaling the central nervous system end points of convulsions and narcosis. Because of the long exposure duration of the key study, the 10-min value was not time scaled but was set equal to the 30-min AEGL-3. |
||||
|
Data adequacy: Although the key study lacked details of methodology, the AEGL-3 values are supported by the additional observation of no adverse effects in rats exposed at a concentration of 189 ppm for 4 h (Jones et al. 1972). The AEGL-3 values are also supported by subchronic and chronic exposures of several animal species at concentrations up to 34 ppm with no life-threatening effects. |
||||