5
Moving Forward with Bioavailability in Decision-Making
Soils and sediments are the ultimate sink for many persistent organic and inorganic contaminants and have the potential to impact human and environmental health for a long time. Remediation and management of contaminated soils and sediments is often technically difficult and can be very expensive when there are large volumes of contaminated material. To more rationally allocate limited environmental management and remediation resources, there is a need to improve risk assessment by including more explicit consideration of bioavailability processes.
Inadequate scientific understanding has hampered the widespread consideration of bioavailability processes in remedial decision making to date. Uncertainty in the relationship between total contaminant concentrations in soils and sediments and risk has often resulted in a conservative approach to exposure assessment in which the total contaminant present in a particular material is assumed to be available for uptake by possible receptors. Other assumptions (of relative bioavailability being less than 100 percent or about relevant exposure pathways for ecological receptors) may have led to situations where risk was underestimated. All assumptions have important implications with respect to the amount of material that must be treated and to the selection of a technology capable of reaching treatment goals. Explicitly incorporating bioavailability routinely and rigorously into the risk assessment process would offer the possibility of demonstrating in some cases that only a fraction of a contaminant’s total mass contained in a soil or sediment actually has the potential to enter potential receptors. In other cases, better understanding of bioavailability processes can lead to
more protective risk estimates, for example by refining a default relative bioavailability factor or identifying an important exposure pathway that was overlooked.
Consideration of bioavailability processes could also be used to improve evaluation of remediation technologies. For example, dredging is a common remediation technology applied to contaminated sediments. In certain cases, natural burial processes have isolated the contamination to the extent that contact between sensitive species and the contaminated matrix is not possible (a situation that can be evaluated through the use of coring studies). Dredging may promote the release of contaminants to the water column, possibly resulting in an increase in mobility and hence bioavailability. In such cases, decision-makers need to consider whether an increase in bioavailability is consistent with the goals of site remediation.
This chapter examines the developments needed in both science and decision-making approaches to promote better consideration of bioavailability processes in remediation and management of contaminated soil and sediment. The chapter examines limitations in our current understanding of bioavailability processes and their implications and what can be done to overcome these limitations. Scenarios in which consideration of bioavailability processes has the greatest potential to impact decision-making are identified, with the hope of focusing science and technology development efforts on these situations. The chapter concludes by recommending specific steps that can be taken to move forward with consideration of bioavailability processes at individual sites, in regulation and decision-making, and in scientific research.
CURRENT LIMITS OF KNOWLEDGE
As demonstrated in Chapter 3, bioavailability of contaminants in soils and sediments to human and ecological receptors is governed by a wide range of physical, chemical, and biological processes. Qualitative and quantitative understanding of some of these processes is substantial, but for other processes there is much to be learned. For example, there is much about contaminant–solid interactions that is only weakly understood. While conceptual models exist for many kinds of contaminant–solid interactions, tools to test these models are often inadequate or nonexistent. As a result, there is significant uncertainty in the models used to describe contaminant–solid interactions and in the parameter values employed in these models. As some description of contaminant–solid interaction will usually be needed for assessment of risk associated with contaminated soils and sediments, the model and parameter uncertainty will transfer directly to the exposure assessment in a risk analysis.
All models and parameters used in exposure assessment have a certain degree of uncertainty associated with them, including those used in bioavailability process considerations. In screening-level assessments for contaminated soils and sediments, this uncertainty is often recognized and dealt with by assuming
that all the contaminant mass is readily available. In practical terms this means that no special adjustments are made to account for bioavailability processes when exposures are estimated. If explicit consideration of bioavailability processes is to become more frequent, the uncertainties inherent in their measurements must be addressed and reduced, if possible.
Some general sources of uncertainty associated with bioavailability processes include:
-
a lack of knowledge about how physical, chemical, and biological processes acting at the level of soil and sediment particles influence the binding and release of chemicals;
-
variations in soil and sediment characteristics at various spatial scales;
-
a lack of knowledge about how biota modify bioavailability of chemicals in soils and sediments that come into contact with external membranes (e.g., skin) or that are taken into the body (e.g., digestive systems), and whether information obtained for one species is representative of another;
-
variations in chemical form or properties (e.g., redox state of metals or diffusive rates for organics);
-
physical, chemical, or biological changes that might, at some point in the future, change the bioavailability of a chemical.
Given these multiple sources of uncertainty, regulatory agencies have been cautious about moving away from default assumptions concerning bioavailability processes in risk estimates. It is not clear whether there is too much uncertainty associated with bioavailability tools for regulatory agencies to feel comfortable about more explicitly incorporating their results into exposure estimates. Input received by the committee indicates that there is disagreement over this issue. An individual who has a strong precautionary stance might argue against replacing certain default assumptions (e.g., of 100 percent availability) to account for site-specific bioavailability processes. On the other hand, someone who sees large trade-offs among alternatives that hinge on bioavailability considerations would likely support their inclusion in specific situations. Risk assessment practitioners well versed in uncertainty and probabilistic analyses might argue that the uncertainties could be identified and taken into account, thereby providing more complete information to the risk manager.
Explicit incorporation of information on bioavailability processes has occurred in ecological and human health risk assessments for particular types of problems and chemicals where the uncertainty has been relatively low due to extensive testing of certain contaminants and processes. Examples include exposure of humans to lead in soils (oral), and to polychlorinated biphenyls (PCBs) in soils (dermal); leaching of soil contaminants to groundwater; exposure of benthic invertebrates to non-polar organic chemicals (e.g., polyaromatic hydrocarbons or PAHs) in sediments; and site-specific determinations of bioavailability via up-
take studies from soils or sediments to benthic invertebrates, sediment invertebrates, plants, and wildlife (see Table 2-3). Clearly, the inclusion of site-specific bioavailability information has been judged to be important in a number of cases, and uncertainties were addressed at a level appropriate to risk-based decision making.
There have been many other cases, however, in which the level of uncertainty has been judged to be too high for bioavailability measurements to replace default assumptions. A prominent example is the case of the Times Beach, Missouri, Superfund site, where large amounts of dioxin-contaminated soil were excavated and incinerated (see Box 5-1). There was a limited, generic consideration of bioavailability processes in determining the dioxin action levels for soil to be excavated and treated. However, site-specific assessments of bioavailability processes were not used to guide remediation decision-making, at least in part due to uncertainty in the bioavailability process measurements.
WHY THESE LIMITATIONS AND UNCERTAINTIES MATTER
The limitations in our understanding of bioavailability processes and the large uncertainties associated with their measurement have important ramifications for site management. The most obvious is that a lack of knowledge may inadvertently support poor decisions regarding exposure assessment, which has implications for how much contamination should be cleaned up and at what cost. For example, site managers working with incomplete information may be inclined to excavate a contaminated site even if the contaminants are not bioavailable. This could present myriad problems, including increasing the bioavailability of the material and potentially the risk to other receptors, such as wildlife, that were not originally the receptors of concern.
Our lack of understanding of bioavailability processes also has important implications for the remediation of hazardous waste in situ. With regard to remedy selection, a large number of treatment and containment technologies rely on biological processes that are partially controlled by bioavailability, such as the transformation reactions of microorganisms. Without a better understanding of bioavailability processes, it is difficult to choose among technologies or to know if they are effective. (Although many might agree with the conceptual model of bioavailability processes outlined in Figure 1-1, there is little consensus on how to identify and quantify the dominant processes relevant for a specific situation.) This is aggravated by the plethora of different bioavailability tools and measurements used, many of which do not actually test a relevant endpoint. Additionally, site managers may not be cognizant of when treatment technologies unintentionally affect bioavailability. Especially for technologies that have yet to be fully tested, like phytoremediation, there may be unanticipated “side effects” that result in undesirable changes in bioavailability to certain receptors. Finally, in the last several years, approaches using simple tests to assess bioavailability at hazardous
|
BOX 5-1 Times Beach Superfund Site: How Uncertainty Influenced Decision-Making about Bioavailability The remediation of Times Beach, Missouri, has been one of the largest Superfund projects in the nation after hazardous levels of dioxin were found throughout eight square miles of the small agricultural and residential town in 1982. Waste oil used to spray the roads for dust control in 1972 and 1973 contained dioxin (2,3,7,8 TCDD). After the waste oil application to the roads, animal mortality and human illness were observed. Almost immediately a toxic chemical in the oil treatment was suspected. The U.S. Environmental Protection Agency (EPA) tested soil samples from the town’s unpaved roads and right of ways, revealing dioxin levels ranging from 1 ppb to 127 ppb. The entire Times Beach site is situated within the floodplain of the Meramec River. Shortly after the discovery of dioxin, the Meramec River flooded the city, which spread the contamination. Times Beach was evacuated in February of 1983, and the federal government used $33 million from Superfund to buy the dioxin site and relocate the residents. The Centers for Disease Control and Prevention (CDC) evaluated the health implications of dioxins in the soil at the site (Kimbrough et al., 1984)—one of the earliest examples of explicitly including bioavailability information in an assessment. CDC investigators noted that “regarding dermal absorption, there is some evidence that TCDD binds to soil and would not be as easily available for absorption.” They considered three routes of exposure: dermal contact, incidental ingestion, and inhalation. In their estimates of exposure, Kimbrough et al. (1984) used the available literature values for relative bioavailability—1 percent to estimate dermal uptake and 30 percent to estimate absorption in the digestive system. Bioavailability was not included in the estimate of inhaled dose. Interestingly, in discussing the implications of their assessment for management of the soils at Times Beach, Kimbrough et al. (1984) state: “The precise bioavailability of TCDD from soil is not known. Such bioavailability may vary with the soil type. It has been recently established that TCDD-contaminated soil from Missouri is toxic to guinea pigs and rats, if given orally. It was estimated that the [relative] bioavailability was |
waste sites have become popular. Some of these approaches do not seek to better understand underlying bioavailability processes such that their widespread application may become problematic.
Technologies Developed with the Intent to Decrease Bioavailability
A number of treatment technologies have been reported that “decrease bioavailability”—that is, treatment that impedes transfer of a contaminant from the soil or sediment matrix to a living organism. Although institutional controls and containment remedies would theoretically be encompassed by this definition, this discussion focuses on in situ treatments that aim to either (1) remove the labile fraction of contaminants (e.g., by microbial or plant mineralization), (2) convert
|
30–50 percent or more [compared to ingestion of TCDD in corn oil] (McConnell et al., 1984.)” As a result of the Kimbrough et al. (1984) study, CDC recommended a 1 ppb TCDD action level for residential areas and 20 ppb level for industrial areas. Site-specific assessments of relative bioavailability performed later (Umbreit et al., 1986a, b, 1987, 1988a, b; Shu et al., 1988), which would probably have changed the cleanup goals by a factor of about 2, were not used to guide remedial actions because:
Roads and affected areas at Times Beach containing dioxin levels over 1 ppb were excavated to a depth of four feet of contaminated soil and stored. A 50,000 cubic yard concrete tank with a flood-proof covering was used as a storage facility for the excavated soil, which was subsequently treated via incineration. Contaminated soil from 26 other dioxin sites was also brought to Times Beach to be incinerated—a fifteen-month process resulting in 265,000 tons of waste material. The incinerators ceased operation in June 1997, and the site was declared fully recovered. |
the labile fraction to a stable fraction (e.g., by the precipitation of metals), or (3) increase the mass transfer resistance of pollutants (e.g., by modifying the physical structure of the geosorbent). Examples of such technologies include biostabilization (the use of bioremediation to reduce contaminant mobility and toxicity of contaminated soils and sediments); sediment capping (reducing the ability of a bottom dwelling organism to get to the contaminant, and increasing mass transfer distance); vitrification or solidification (decreasing contaminant mobility by vastly increasing mass transfer resistance out of the solid matrix); and chemical alteration (e.g., converting a compound to a low solubility redox state via an amendment).
Biostabilization relies on the microbial degradation of contaminants serving as carbon or energy sources or as electron acceptors. It consists of an initial active
and often engineered bioremediation phase (that may last months) to remove or transform those compounds that are more bioavailable, followed by a passive bioremediation phase (lasting years) to ensure that there is no chemical migration away from the actively treated material. The concept of the second phase is that intrinsic biodegradation rates equal or exceed the rate at which low solubility compounds become available. Box 5-2 discusses the characteristic desorption curves for PAH-contaminated solids, which are a frequent target of biostabilization efforts.
One limitation of biostabilization is that the organic compounds may not meet threshold concentrations needed to drive microbial metabolism. Threshold concentrations of compounds are thought to play a role in energy maintenance and microbial enzyme induction (e.g., Schmidt et al., 1985) and they are experimentally manifested as residual concentrations of pollutants in various biodegradation tests (Bosma et al., 1996; Tros et al., 1996a, b). For a given contaminant, the value of the threshold concentration is determined by the efficiency of microbial metabolism (e.g., the relative values of specific uptake rates versus maintenance coefficients). Thus, thresholds can be affected by external mass transfer limitations, which often occur with aged pollutants in soils and sediments (Bosma et al., 1997). The existence of threshold values may be irrelevant when these values are far below concentrations that present risk. However, when these microbial threshold concentrations are above values deemed to represent a risk, biostabilization may not be a suitable remedial technology.
Other remediation approaches use isolation to reduce bioavailability by employing capping or burial to remove access of a contaminant to the biosphere. In a physically active waterbody, however, capping will not permanently remove contaminants from the bioaccessible or bioavailable location if the sedimentary environment is erosional. To evaluate the potential success of isolation techniques, it is important to take sediment cores and evaluate their sedimentation regimes.
Several technologies to reduce bioavailability of metals in soil, sediment, or other contaminated matrices rely on amending the solid phase to alter the redox or acid–base status of metals or sulfur species (NRC, 1997a). Certain metals (e.g., chromium or uranium) may have highly unavailable (low solubility) species depending on redox conditions, which can be imposed by specific technologies. This has been demonstrated at the Department of Energy (DOE) Hanford Site in Washington, where groundwater hexavalent chromium levels have been reduced from 0.060 mg/L to below detection limits (0.008 mg/L). The zone of reduction was created by injecting reagents that reduce iron naturally present in the aquifer sediments from Fe(III) to surface-bound and structural Fe(II) species, which concomitantly reduces the hexavalent chromium. Other metals may not have such speciation, but they can be precipitated as phosphates or sulfides, and hence the reduction of oxidized sulfur species can reduce their bioavailability (Benner et al., 1999). This strategy is exemplified by the case study presented in Box 5-3,
|
BOX 5-2 Biostabilization of PAH-laden Soils or Sediments Biostabilization generally refers to the situation where biological processes alone—intrinsic or stimulated—are deemed sufficient to reduce the risk associated with contaminants in soils and sediments. Although an awkward term, stabilization alludes to the fact that the labile fractions of the total contaminant are being reduced in size. This remedy has been suggested extensively for soils and sediments contaminated with PAHs, many of which have been documented to undergo microbial mineralization under various redox conditions (Kanaly and Harayama, 2000). Documenting the success of biostabilization typically requires demonstrating not only a decrease in total contaminant mass but also a decrease in the labile fraction of the contaminant pool between the onset and the end of the examined stabilization period. Popular tests to make these measurements examine the “rate of release” of contaminants using infinite sorption sinks or different extraction solvents (e.g., Cornelissen et al., 1998; Hawthorne and Grabanski, 2000), or they use toxicological endpoints (Loehr and Webster, 1997). Typical results for desorption data are shown in Figure 5-1 for two compounds in contaminated sediment. Detailed studies that directly inspect the soil and sediment phase to determine the stabilization mechanism are rare, and the actual contribution of microbial metabolic activity is only sporadically demonstrated (Ringelberg et al., 2001). 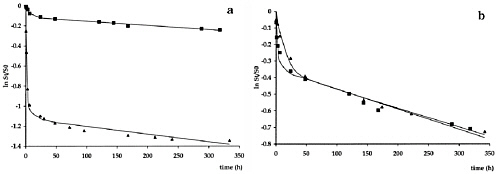
FIGURE 5-1 (A) Desorption of fluoranthene, a compound amenable to microbial degradation, before (triangles) and after (squares) bioremediation. Total fluoranthene concentration dropped from approximately 170 mg/kg to 20 mg/kg over four months of active bioremediation. The shape of the desorption curves are very different before and after bioremediation. The rapidly desorbing fraction (obtained from curve fits shown in figure) dropped from 67 percent ± 3 to 10 percent ± 4 after bioremediation. This drop in rapidly desorbing fraction was observed for all the compounds that were biodegraded, suggesting a decrease in their labile fraction, and hence biostabilization. (B) Desorption results for the non-degraded compound benzo(ghi)perylene indicating very similar shapes of the desorption curves before and after the bioremediation. SOURCE: Reprinted, with permission, from Cornelissen et al. (1998). © (1998) American Chemical Society. |
|
BOX 5-3 Soil Amendments to Reduce Lead Bioavailability at the Joplin Superfund Site Joplin, Missouri, was included on the National Priorities List because of soil contamination from the smelting of locally mined lead (Pb) and zinc (Zn) ores. As part of the remedial action undertaken for the site, 2,600 homes in Joplin have had their soil replaced with clean material. In conjunction with this cleanup, a field site was established to test the ability of different in situ soil amendments to reduce the bioavailability of soil Pb to children. This project was undertaken by the Inplace Inactivation and Natural Ecosystem Restoration Team (IINERT) of EPA’s Remedial Technology Development Forum, whose stated mission was to identify in situ technologies that could chemically and physically inactivate hazardous metals in soils by reducing the metal’s solubility and bioavailability. This box focuses on human health because of the urban focus of the risk assessment. However, there are adjacent lots at the site where soil amendments are also being tested and where ecological receptors (including plants, herbivores, and insectivores) are the primary receptors of concern. Background Several lines of evidence suggested that soil amendments, including different sources of phosphorus, high iron materials, and biosolids compost, might be successful in reducing Pb availability in situ. The solubilities of different Pb species are known to vary in relation to the mineral form (Nriagu, 1984). In the presence of phosphorus, lead can form chloropyromorphite, which has a very low Ksp (10–84.4), such that the compound is likely to be stable under most soil and gastric systems. Thus, amendments that would promote formation of this mineral became the focus of research. Controlled environment studies demonstrated that it was possible to alter the mineral form of Pb in both pure and soil systems (Ma et al., 1993, 1994a, b). Field validation of these technologies was determined to be the appropriate next phase of research, for which hypotheses were developed. The initial phase of research focused on defining an appropriate animal surrogate to measure changes in bioavailability and on determining what extractions or in vitro tests can potentially substitute for animal feeding studies. Identifying the mechanisms that are responsible for the observed reduction in bioavailability and the appropriate tools to measure changes in speciation was also a goal. Animal surrogates and in vitro testing Initial results from the field site showed that additions of both H3PO4 and biosolids compost in situ are capable of reducing Pb bioavailability in juvenile swine, and in weanling and adult rats (Casteel et al., 2001; Maddaloni et al., 2001). However, although animal feeding studies have consistently shown reduced lead bioavailability as a function of treatment, the reductions are not consistent across groups or over time after treatment (see Casteel et al., 2001 for details). A second goal of the field study was to determine whether an in vitro extraction test could substitute for in vivo trials to assess reduction in Pb bioavailability. For the Joplin site soils, the in vitro test results at an extraction pH of 2.3 were comparable to the results from the swine studies (Ruby et al., 2001). Mineral Form The final goal of the field trial was to determine the mechanisms responsible for the observed reduction in bioavailability. Using X-ray adsorption spectroscopy (XAS) and |
|
comparing field samples to known mineral forms, the formation of chloropyromorphite in the treatments that included phosphorus addition has been confirmed (Scheckel and Yang, 2001). In addition, the portion of total Pb present in this mineral phase increased over time. Mineral forms in the unamended soils have remained constant over time. There also seems to be a relationship between the observed decrease in bioavailability and the presence of pyromorphite [although the correlation is weak—r2 = 0.5546 (Ryan and Berti, 2001)]. For other treatments, results are even less clear. Although compost addition resulted in reduced bioavailability as measured by in vitro and in vivo (weanling rats) studies, XAS was not able to quantify the formation of a new mineral phase. A shift was observed from carbonate- and S-associated Pb in the control soils to what was identified as adsorbed Pb in the compost amended soils. Clearly, more information will be required before this shift can be accepted as the cause of the observed decrease in bioavailability. Conclusions On many levels, the preliminary research at the Joplin field site has been a success. It should be noted that this is the first time that feeding studies on animals have used treated soils. Thus, the methods are clearly a work in progress. All in vivo (human, pig, and rat) and in vitro studies (data unpublished) have shown that soil amendments are able to reduce the portion of total soil Pb that is bioavailable. In addition, it has been demonstrated that when P is added to the soil, the mineral form of Pb shifts, at least in part, to pyromorphite. This mineral shift appears to weakly correlate with the observed decrease in bioavailability. The stability of this mineral phase also suggests that the observed decrease in bioavailability will persist over time. During the limited sampling time since treatment addition, increasing pyromorphite concentrations have been observed for select treatment. However, this field site also illustrates some of the complexities involved in the measurement of bioavailability to assess risks posed by Pb in soil. Although all indices used in this study show decreases in bioavailability, they also show considerable variability. At this time, it is not clear if a single, appropriate index can be identified. The initial results from this field site indicate that, while it is possible to reduce the bioavailability of Pb in situ, it is not clear how to interpret or utilize these observed reductions in the regulatory arena. 
Plots at the Joplin Superfund Site being subjected to soil amendment in order to reduce metal bioavailability to residents. |
in which different soil amendments were tested for their ability to reduce the bioavailability of lead in soil to children. Both bioassays (feeding studies) and physicochemical tests (x-ray spectroscopy) were conducted to determine the effectiveness of the soil amendments.
Environmentally Acceptable Endpoints. Of specific relevance in this discussion (particularly for biostabilization) is the increasing popularity of environmentally acceptable endpoints (EAE). The EAE concept is based on the observation that many organic contaminants become less “available” as they age within soil or as the soils undergo treatment, due to changes in the way soils and sediments encapsulate chemicals over time (Alexander, 1995). It has been proposed that this reduced availability should have an impact on cleanup levels and remediation goals and should be incorporated in site-specific risk assessment (Stroo et al., 2000). In some cases, this may involve modifying the default assumptions to reflect bioavailability limitations.
This reduced availability, which has been described for organic contaminants by such mechanisms as sequestration and entrapment, has largely been inferred from the behavior of persistent hydrophobic compounds (mainly PAHs) in the field. After an initially rapid rate of chemical degradation, a period follows with little or no change in chemical concentrations. In the case where the considered chemicals are known to be biodegradable, the lack of continued decline—all other things remaining favorable for microbial activity—suggests that the chemicals themselves have largely become the limiting factor to microbial biodegradation, probably because of reduced availability. It is postulated, but rarely confirmed, that reduced availability to microorganisms relates to reduction in risk posed by the contaminants. Concomitant reductions in toxicity to other more relevant receptors has only occasionally been demonstrated (Salanitro et al., 1997; Olivera et al., 1998).
Although plausible, the lack of availability of contaminants in soils or sediments to resident microorganisms does not suffice to characterize the suite of possible bioavailability processes. As an analogy, consider the fact that exchange of metals from sediments to pore water declines as the metal–sediment association ages (Schlekat et al., 2002). While the risk to water column species may decline with contaminant aging in sediments, there will not necessarily be a change in the risk to species whose food web is connected to ingestion of the sediments themselves. Hence, the evidence on which environmentally acceptable endpoints are based (microbial availability) may be insufficient, unless multiple exposure pathways and multiple receptors are considered. The challenge to all bioavailability assessment is to quantify the relevant bioavailability processes at work in a given situation, which requires an understanding of the importance of all exposure routes and receptors.
Variability in the Tools Used. One of the difficulties inherent with implementing all of these types of remedies is that there is no consensus on the tools or methods that should be employed to measure “bioavailability reduction” in the course of remedial technology selection or on how results from those tests should be incorporated into risk assessment. As a result, the state-of-the-practice consists in applying a battery of assays to the soil or sediment under investigation that all have some relationship (however ill-defined) to contaminant bioavailability. Further, measurements that may approximate only certain bioavailability processes, such as chemical mobility measurements or the water-soluble fraction of a compound, have been employed to infer satisfactory treatment. Again using biostabilization as an example, a recent review of remedies for hydrocarbon-contaminated soils from petroleum refining, wood treating, petrochemical manufacture, and gas and electric utility sites demonstrates the wide variety of surrogate measures of bioavailability utilized. Technical report 25 in Loehr and Webster (1997) measured a reduction in total chemical concentrations as well as in toxicity (via Microtox EC50 assays) to assess reductions in bioavailability for petroleum-contaminated soils subject to soil pan and biopile treatability. In a study on bioremediation of soils artificially contaminated with a mixture of chlorophenolic compounds, reduction in the water soluble fraction as well as Microtox-inferred toxicity were used as “bioavailability reduction measures” (Dassapa and Loehr, 1991). In another recent study on soils artificially contaminated with pentachlorophenol (PCP), increased toxicity was measured using the soil bacterium Bacillus megaterium as test species, although the aqueous PCP concentration had dropped (McGrath and Singleton, 2000). This finding indicates that transformation products potentially can cause increased biological effects (compared to the parent compound), and that toxicity reduction may be an ambiguous tool for understanding “reduced bioavailability.” In yet another set of field-scale bioremediation efforts of unsaturated-zone wood treating site soils, a reduction in Toxicity Characteristic Leaching Procedure evaluations or water-soluble fraction determinations were employed to infer bioavailability reduction with time (technical reports 16, 17, and 22 in Loehr and Webster, 1997).
A recent comprehensive study on a PAH-impacted site applied seven assays to assess the degree of biostabilization and reduction in contaminant mobility that had occurred after various natural and engineered processes (Stroo et al., 2000). These assays were dermal uptake through human cadaver skin over 96 hours, absorption efficiency via 10-day oral uptake in mice, accumulation via 28-day earthworm tests, 14-day exposure earthworm toxicity, Microtox toxicity of soil slurries and aqueous extracts, Synthetic Precipitation Leaching Procedure, and a 119-day desorption test in infinite dilution matrix. Although qualitative consistency among some of the tests was found, quantitatively the results were very different. As recognized by the authors, each of the applied tests had limitations with respect to relevance to real endpoints. Further, all tests reflect a single time point analysis, and the effect of time-varying ecological and geochemical factors
is not typically addressed. The authors concluded that using any specific set of tests to adjust risk-based cleanup criteria would be subjective (although they also suggested development and adoption of a few short-term tests that could be employed in a tiered testing scheme).
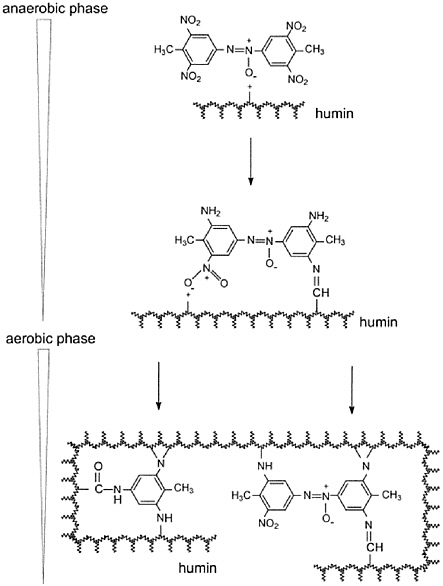
More recently, physicochemical-based assays have been applied to infer bioavailability reduction during biostabilization. For example, in PAH-contaminated sediments subject to four months of active bioremediation or two years of land farming, the degree of biostabilization was inferred by comparing the rapidly desorbing PAH fractions, before and after treatment, using the infinite dilution Tenax TA desorption technique (Cornelissen et al., 1998). A similar trend between “extractability or bioavailability” and extent of bioremediation for manufactured gas plant soils was observed when supercritical fluid extraction was used to measure the various fractions of soil-bound PAH (Hawthorne and Grabanski, 2000; Hawthorne et al., 2001). Box 5-4 discusses how multiple complementary tools might be used to address the effectiveness of biostabilization, in this case the humification of trinitrotoluene (TNT), and gain more confidence in the proposed remedial selection.
***
There is a general consensus that biostabilization and certain other treatment technologies and natural aging processes might reduce the risk associated with soil and sediment contaminants. However, this has not been conclusively demonstrated in the examples cited above. The types of correlative assays frequently used may aid in short-term decision making for site management. But in the absence of better capabilities to measure bioavailability processes, they must be applied with caution to ensure that appropriate site management decisions are made. In addition, the permanency of treatment technologies that aim to reduce bioavailability has not been addressed, in part because tools to assess bioavailability processes over long time scales and over a range of soil and sediment conditions are not yet developed. Hence, the concept of using EAE-based rather than default cleanup values may have merit, but full acceptance of this concept will be contingent on better understanding and measurement of the constituent bioavailability processes on which it integrally is based.
Technologies Developed with the Intent to Increase Bioavailability
An alternate strategy is one that recognizes that the continued presence of pollutants in soil or sediment will always invoke potential risk. Thus, some technologies attempt to increase pollutant removal or destruction by facilitating bioavailability processes. These technologies increase mass transfer from the sorbed phase via physical means (grinding or mixing to decrease diffusional paths, increasing temperature to increase mass transfer rates) or chemical means
(surfactants, co-solvents, or chelating agents to increase mass transfer by increasing the apparent aqueous solubility of hydrophobic organic compounds, or mediating changes in geosorbent matrix structure). Clearly, such technologies need to be paired with technologies that can capture or destroy the increased flux of pollutant thus generated.
The use of additives to soils or sediments to enhance the extent or rate of desorption has been examined for both inorganic and organic contaminants. Surfactants, of both chemical and microbiological origin, have been applied with varying degrees of success to enhance solubility of hydrophobic organic chemicals (particularly nonaqueous phase liquids). They typically function by micellar solubilization and mobilization of the trapped liquids by lowering the liquid– water interfacial tension (Harwell et al., 1999), leading to an increase in apparent water solubility and solubilization of sorbed contaminants (Kim et al., 2000). The surfactant generally must be present in amounts above its critical micelle concentration. Unfortunately, sorption of the surfactant itself to solids can impede the success of this approach (Dwarakanath et al., 1999; Deshpande et al., 2000). The effectiveness of surfactant use has been widely disparate, with studies demonstrating negative effects, zero effects (Löser et al., 1999), or positive effects on enhancing pollutant availability and subsequent biotransformation (Liu et al., 1995; Tiehm et al., 1997).
For inorganic contaminants, many additives have been used to increase their solubility. For example, chelating agents have been used specifically to enhance the solubility of multivalent cationic species. Technologies based on citrate addition to enhance removal of transition metals and actinides from the solid phase have been developed that rely on the formation of complexes with citric acids (Francis and Dodge, 1998). Recently, it has also been observed that chelating agents may enhance the bioavailability of hydrophobic organic pollutants, presumably by altering the geosorbent matrix, although the exact mechanism has not yet been elucidated (Yang et al., 2001). For example, White and Kottler (2002) found that citrate addition enhanced the plant uptake of weathered 2,2-bis(p-chlorophenyl) 1,1–dichloroethylene (p,p′-DDE) from soil. Nonetheless, without a complete understanding of the bioavailability process and appropriate tools to measure the constituent steps, it is difficult to ascertain with certainty the impact of these bioavailability enhancement techniques on the long-term fate of the contaminants.
Chelating agents have also been used intentionally to promote the uptake of metals and radionuclides into plants from contaminated soils. In particular, EDTA and citric acid can trigger hyperaccumulation in plants (specifically Brassicaceae) (Blaylock et al., 1997; Huang et al., 1998; Bricker et al., 2001; Chen and Cutright, 2001). This may be due to the chelator’s ability to promote desorption of metals and radionuclides from the solid phase to soil solution. Although the ensuing hyperaccumulation response is very rapid (within 24 hours) (Huang et al., 1998), and several chelating agents are readily biodegradable, this application needs to
|
BOX 5-4 Humification of TNT via Sequential Anaerobic-Aerobic Soil Slurry Treatment A technology proposed to reduce the bioavailable fraction of trinitrotoluene (TNT) in contaminated soils relies on the cometabolic reduction of the nitro substituents on the compound. Microbial reduction of TNT occurs readily, leading to nitroso, hydroxylamino, and finally amino derivatives of TNT. The rate and extent to which individual nitro substituents are reduced and the number of nitro substituents reduced per TNT molecule depend partly on the redox status of the environment (Preuß and Rieger 1995; Riefler and Smets, 2000). The nitroso and hydroxylamino functional groups formed as intermediate products during reduction have a high chemical reactivity towards solid-phase constituents. Thus, it is thought that microbial TNT reduction in the presence of the functional sites on soil might lead to biostabilization of the reduced compounds. Lenke et al. (1998) treated contaminated soil from a former munitions site (176 mg/ kg TNT, 45.6 mg/kg ADNT, 2.4 mg/kg 2,4-DANT) as a soil slurry (850 g soil/850 ml mineral medium) subject to an anaerobic fermentative step followed by an aerobic polishing step. No hydroxylaminodinitrotoluenes (HADNT) or triaminotoluenes (TAT) were detected in the slurry supernatants, and no residual methanol extractable compounds were detected after the combined anaerobic–aerobic phase (after approximately 672 hrs). (Methanol extractions are used to release rapidly desorbable fractions.) Also at a technical scale, a sequential anaerobic–aerobic incubation of a TNT- and a DNT (dinitrotoluene)-contaminated soil gave only TNT and DNT as residual extractable compounds at 1.86 and 3.45 mg/kg, respectively, from initial concentrations of 189 and 49.1 mg/kg. None of the reduction products was detected in either aqueous supernatant fractions or in alkaline, base, or methanol extracts of soil, strongly suggesting the formation of irreversibly soil-bound fractions. Further, no toxicity was detected in aqueous soil eluates after the combined anaerobic–aerobic treatment, according to tests employing a bacterium, Vibrio fisheri, an aquatic invertebrate, Daphnia magna, or the photosynthetic alga Scenedesmus subspicatus. Further, terrestrial tests indicated no earthworm mortality or plant toxicity and acceptable microbial respiratory activities of the soil after treatment. Although these results suggested some type of humification, complementary experiments were necessary to confirm these observations. To examine stability of the immobilized TNT derivatives and to differentiate between sequestration and covalent binding, samples from the lab-scale experiment that used radioactive TNT were subject to vigorous extraction–derivatization procedures (Achtnich et al., 2000). Very small amounts (1.3 percent to 2.5 percent) of initial radioactivity were extracted after the combined anaerobic–aerobic treatment with methanol. Only with 5.0 M HCl was a significant fraction extracted (8.9 percent). However, chromatographic analysis of the HCl extract showed that all radioactivity remained associated with the humic acid fraction. Silylation, which breaks open the 3-dimensional structure of soil, was able to release 73.1 percent of the initial radioactivity, but chromatographic analysis again indicated that all activity was associated with soil organic matter, and no free TNT metabolites were detected. These speciation analyses clearly supported the notion that TNT derivatives were covalently bound to soil after the two-stage process. |
|
To further understand the observed immobilization of TNT derivatives to the soil, Daun et al. (1998) examined the cometabolic reduction of TNT (0.4 mM) by a glucose fermenting enrichment culture in the presence of individual model soil components: montmorrilonite (3.3 or 10.3% w/v) and humic acid (1% w/v). They observed a very rapid decrease in aqueous phase TNT reduction metabolites, with complete absence of aqueous products after prolonged incubation (340 hrs, 220 hrs) and suggested that HADNT and TAT had undergone strong reactions with the solid phase. Separate experiments confirmed that neither acid nor base hydrolysis could release HADNT and TAT sorbed on montmorillonite and humic acids, suggesting formation of irreversible sorptive interactions. In a final study (Achtnich et al., 1999), stable isotopes of TNT were employed. 15[N3]TNT and [14C] TNT were spiked (4 g/kg) into the same TNT-contaminated soil samples (350 mg/kg) as studied by Lenke et al. (1998), and the sequential anaerobic– aerobic soil-slurry treatment was repeated at the laboratory level. Soil samples, taken at various times throughout the treatment period, were subject to both methanol extractions as well as subsequent fractionations of the soil organic matter (fulvic, humic, humin fractions) to characterize the bound fractions. 14C-based mass balances revealed a vast reduction of the methanol-extractable fraction (from 102 percent at day 1 to 1.1 percent after 83 days), with a gradual increase in the humin-bound fraction (up to 71 percent, after 83 days). Of the humin-bound fraction, only 3.4 percent was HCl-extractable, 44.4 percent could be solubilized in dimethylsulfoxide after silylation (due to humin solubilization), and 23.4 percent remained soil-bound. Importantly, NMR inspections of the humic acid-bound fraction revealed a gradual reduction in the aromatic nitro groups, intermediary accumulation of azoxy functional groups, and accumulation of aromatic amines, tertiary amines, or amides with time, while the NMR spectra of the humin-bound fraction suggested formation of azoxy compounds and imine linkages. Further, the broad NMR line widths of the metabolite spectra provided convincing evidence of strong (covalent) interactions between metabolites and humic acids or humins. Hence, convincing spectroscopic evidence of true soil immobilization of TNT metabolites during reductive transformation of TNT was presented. An illustration of the humification of the TNT derivatives is shown below in Figure 5-2. In summary, the observations of TNT disappearance (from aqueous phase) during anaerobic cometabolic reductive treatment of TNT-laden soils was confirmed to be in part due to immobilization of TNT reduction products on soil constituents (humification) via the following complementary lines of inspection: (1) aqueous phase monitoring of TNT and all its presumed transformation products, (2) extraction of solid phases with various rigorous extraction procedures, (3) ecotoxicological endpoints, (4) sorption experiments with individual TNT transformation products showing irreversibility, (5) TNT reduction experiments in the presence of model solid components, (6) mass balances employing spiked [UL14C]-TNT, and (7) NMR spectroscopic investigations employing spiked [15N]-TNT. |
be designed and timed carefully to avoid negative effects on soil microbiota and the unintentional release of contaminants to the underlying groundwater (Grcman et al., 2001; Romkens et al., 2002).
Box 5-5 discusses the second major category of treatment technologies designed to increase contaminant bioavailability—the use of physical mixing and changes in temperature to enhance the biodegradation of hexachlorocyclohexane in soil.
Remediation Strategies with Unintentional Effects on Bioavailability
A number of technologies used for the remediation of contaminated soils or sediments operate through principles of increasing the mobility—and consequently the bioavailability—of contaminants. In some cases, however, technologies that function around principles other than enhancing mobility are also capable of increasing bioavailability, often unintentionally. Although this unintentional effect has been recognized in some cases, it is likely that, in an absence of complete understanding of a technology, such effects might be more common than anticipated.
An example of an unintentional increase in contaminant bioavailability can occur during the dredging of contaminated sediments. In dredging operations there is considerable concern regarding the short- and long-term potential to increase contact between receptors and contaminants after dredging as compared to the levels of exposure that would occur if sediments were not disturbed (NRC, 1997b, 2001a). The objective of the dredging process is to remove sediments from the bed, capture the sediment particles, and then transport the contaminated materials to confined disposal or ex-situ remediation processes. The unintentional increase in bioavailability that results may be the outcome of one or more specific processes that occur during or after the dredging is complete. For example, mobilized sediment particles that are subject to transport in the water column may not be adequately captured and have the potential to come into contact with receptors. Certainly efforts to retain a high fraction of the sediment particles are a component of dredging practices, but the small fraction of sediment that escapes is often significant in the analysis of risk at contaminated sites.
Adding to the short-term risk of dredging is the release of contaminants to the water column as bed sediments are brought into contact with overlaying waters. Similar to the concern with sediment transport, any dissolved-phase contaminants are free to move with the flow of water and come into contact with receptors. This mechanism of release may take place only for short periods but can result in the release of contaminants into the aqueous phase at levels considerably higher than was occurring prior to dredging (via diffusing from the sediment bed).
An example of long-term concerns of sediment dredging results from the storage of the materials in confined disposal facilities where redox conditions are
|
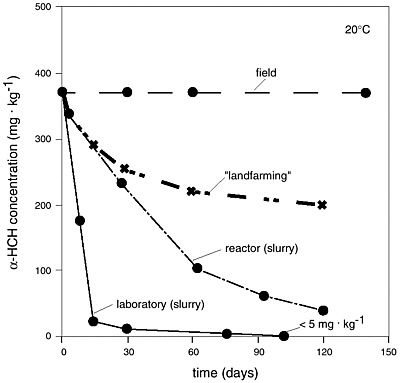
BOX 5-5 Mixing to Enhance Bioavailability as Measured by Biodegradation Rates It has often been observed that some contaminants are recalcitrant to microbial attack after a certain time, despite favorable environmental conditions (Erickson et al., 1993)—an observation on which biostabilization is premised. In situations where further microbial degradation is desired, it may be possible to manipulate other factors such as physicochemical phenomena and supply of electron donors and acceptors to restart the microbial degradation process (Ramaswami and Luthy, 1997). The kinetics of mass transfer can control the overall biotransformation rates only if the mass transfer of the substrate or other critical reactant, such as the electron acceptor, is slower than the potential biodegradation rate. The ratio between these two rates is referred to as a Damköhler number; if this value is much greater than unity then physicochemical processes such as desorption, dissolution, or diffusion occur much more slowly than biodegradation, limiting the overall biotransformation rate. If the biodegradation rate is limited by external mass transfer of electron donor or acceptor, then mechanical mixing may enhance the overall rate by increasing contact and the surface area per unit volume. This is illustrated in Figure 5-3 where the biodegradation rate for α-hexaclorocyclohexane [α-HCH] in unmixed soil in the field is practically zero. The rate increases significantly with tilling, and even more so with mixing in a slurry reactor or mixing in a laboratory apparatus. The implication from the data in Figure 5-3 and related desorption tests (Rijnaarts et al., 1990) is that the biodegradation of α-HCH is mass transfer (diffusion or desorption) limited. Thus, activities that can increase mass transfer by reducing the particle size, such as mixing, can enhance biodegradation rates. Temperature has a similar influence in that increasing temperature generally increases mass transfer rates for volatile and semivolatile compounds and thus affects contaminant bioavailability. Indeed, this partially forms the basis of thermal treatment technologies for subsurface contamination. |
often different than those in bed sediments. This change in redox conditions has the potential to perturb the partitioning behavior of contaminants associated with the dredge spoils. In particular, those heavy metals that are prone to precipitation under reducing conditions (often present in bed-sediments), but are soluble in aerobic, oxidizing environments (perhaps found in the water column or confined disposal facilities) may become more available and more mobile in a confined disposal facility. The leaching of contaminants from confined disposal represents a long-term concern in sediment management, and may result in greater impacts on the waters near the disposal site than would have occurred in the region of initial contamination without dredging. None of these potential outcomes is desirable or intentional, but all must be considered in the dredging of sediments.
Similar concerns are considered in the excavation of contaminated soils, where particulate matter is prone to atmospheric transport, and volatile contaminants may be lost to the gas phase. In certain cases, the potential for such releases
results in the decision not to excavate or dredge, as the resulting exposure to receptors would be greater than that which would occur without intervention.
Examples of how treatment and containment technologies impact bioavailability processes (intentionally or unintentionally) are summarized in Table 5-1.
WHEN WILL CONSIDERATION OF BIOAVAILABILITY PROCESSES MAKE A DIFFERENCE?
Explicit consideration of bioavailability processes in site-specific risk assessment can be technically difficult, time consuming, and costly. As the preceding discussion indicates, uncertainties associated with data and models pertaining to bioavailability processes must be confronted and dealt with. Experience shows that decisions to consider bioavailability processes occur on case- or topic-specific bases. An important dimension for the risk manager to consider is the value
TABLE 5-1 The Effects of Remedial Technologies on Bioavailability
|
Technology |
Primary Effect on Bioavailability |
Other Considerations |
|
Bioremediation/ Biostabilization |
Mineralizes labile forms or otherwise transforms contaminants into a chemical form that is more resistant to uptake. |
May not be effective for contaminants that resist rapid transfer to the aqueous phase; resistant fraction may need to be assessed for possible entry into other life forms. |
|
Phytoremediation |
Promotes uptake and transformation of contaminants in plants. |
Phytoremediation processes often result in the translocation of inorganic contaminants to tissues that have the potential for consumption by herbivores and direct entry into terrestrial food chains. Hence they might enhance bioavailability at an ecosystem level scale. |
|
Sediment capping |
Produces a barrier of “clean” materials to prevent transport of contaminated material to bottom dwelling macrofauna. |
May alter the flux of materials into and out of the sediment bed resulting in changes of the biogeochemistry of the contaminated media and subsequently the physicochemical state of the contaminants. |
|
Stabilization/ vitrification |
Modifies the soil or sediment matrix to produce a material where the contamination is less prone to transport or biodegradation. |
No destruction of contaminant mass occurs such that long-term stability of the solid matrix must be considered. |
|
Redox manipulation |
Changes the chemical form of a contaminant to decrease solubility, mobility, and bioavailability. |
No destruction of contaminant mass occurs such that long-term stability of the chemical form must be considered. Also, in certain microniches, organisms may be capable of changing redox conditions or chelation processes, reversing the intent of the process. |
|
Surfactant/Co- solvent/Chelatant flushing |
Increases the apparent solubility of contaminants and may also increase bioavailability. |
Chelates, surfactants, or co-solvents may change the biogeochemistry of the site, as these compounds may be biodegradable or toxic to indigenous organisms. |
|
Physical treatment (heat, particle size reduction via mixing) |
Increases the rate or release of contamination from the solid phase, thereby increasing the potential bioavailability. |
Physical treatment may have unintended negative effects on indigenous biota and other natural processes. |
of the bioavailability information. If inclusion of bioavailability information makes little difference to the decision, a risk manager would not find this extra level of analysis helpful and, in fact, such information could detract from rather than support decision-making.
There are a number of factors that determine whether or not consideration of bioavailability processes in risk assessment will make a difference for a particular situation. These factors may be grouped into three general categories: chemical, site (setting), and regulatory. Consideration of these factors will help risk managers and assessors judge the value of assessing bioavailability processes in detail at a particular site. The five basic factors that determine whether or not consideration of bioavailability processes in risk assessment will make a difference for a particular situation are:
-
when the contaminant is a risk driver for the site;
-
when default assumptions about bioavailability processes or parameters are not appropriate for the site;
-
when a significant difference in the remediation goal is possible if bioavailability processes are considered;
-
when future conditions at the site are not likely to change and can be estimated with confidence; and
-
when there is potential for regulatory and public acceptance of consideration of bioavailability processes.
Chemical is a Risk Driver
Consideration of bioavailability processes will be most important for chemicals that pose or will pose the greatest risk to human health or the environment, or both, at a particular site—the “risk drivers.” Such chemicals are frequently persistent, bioaccumulative, or toxic (and usually some combination of these characteristics is required). However, the most important factor in determining whether a chemical is a risk driver depends on the degree of overlap between the exposure at the site and the chemical’s threshold for effect.
Chemicals that persist in the environment (i.e., those with long half-lives) are particularly important from a bioavailability perspective. Persistent chemicals have the potential to become widely distributed, which can result in prolonged exposure, greater likelihood for transfer across environmental media, and greater accumulation in organisms, resulting in greater risk. Table 5-2 gives the persistent chemicals of current greatest concern as identified by the United Nations Environment Programme and EPA. Clearly not all persistent chemicals will be important for the purposes of assessing bioavailability. For example, potassium is an element that is ubiquitous in the environment and highly persistent. However, under most situations it does not pose a risk because concentrations are less than those required to cause adverse effects. Not surprisingly, there are no docu-
TABLE 5-2 Persistent Chemicals for which Production Controls are Established or are being Sought in the United States and by the United Nations
|
Priority Persistent Bioaccumulative and Toxic Pollutants—EPA |
Persistent Organic Pollutants United Nations Environment Programme |
|
Aldrin/Dieldrin |
Aldrin |
|
Benzo(a)pyrene |
Furans |
|
Chlordane |
Chlordane |
|
DDT, DDP, DDE |
DDT |
|
Hexachlorobenzene |
Hexachlorobenzene |
|
Alkyl lead |
Heptachlor |
|
Mercury and its compounds |
Endrin |
|
Mirex |
Mirex |
|
Octachlorostyrene |
Dieldrin |
|
PCBs |
PCBs |
|
Dioxins and furans |
Dioxins |
|
Toxaphene |
Toxaphene |
mented cases of conducting bioavailability assessments for potassium. However, since it is a required element that is often limiting, plants and animals have mechanisms to bioconcentrate potassium, such that long-term continuous exposure could potentially result in adverse effects (as have been found in some clams). As always, it is the degree of exposure relative to the onset of adverse effects in the organism that will determine whether a persistent chemical is a risk driver in a given situation.
Chemicals that bioaccumulate in organisms warrant special bioavailability consideration because of the potential to cause great harm via food web amplification. This is especially important when the target organism is a threatened or endangered species. Compounds like PCBs, certain pesticides, selenium, and mercury are known to biomagnify as they pass up the food chain. Organic chemicals with very large values of octanol–water partition coefficients (Kow) will tend to bioconcentrate in the tissue of aquatic organisms. Often the root source of persistent chemicals that are amplified up the food chain is contaminated sediment or soil. Thus, decisions about the bioavailability of such chemicals in sediment or soil have important implications for bioaccumulation and food web transfer.
As the concern at sites with contaminated soil or sediment is usually risk from long-term exposure, the chronic toxicity of chemicals is usually the focus of assessment. Chemicals that exhibit the greatest potency with respect to chronic human health effects (e.g., cancer) or ecosystem effects (e.g., species reproduction) are usually risk drivers if present in sufficient abundance (i.e., sufficiently near the threshold for effect).
Finally, bioavailability process evaluations will be most useful when soil or sediment concentrations of the risk-driving chemical are driving remedial decision-making. In many cases, however, regulatory agencies rely upon a wide range of other criteria for managing contaminated soils and sediments, including the presence and thickness of free product and hot spots (which may be highly mobile and thus available) and aesthetic criteria. In situations where these factors will drive cleanup decisions (often manifested in immediate removal actions like excavation), the value of refining risk estimates to include bioavailability process information may be limited.
Default Assumptions are Inappropriate
As discussed in Chapter 2, risk assessment incorporates numerous assumptions that may be inappropriate or incorrect for a given site. For example, state soil standards have often been developed by assuming a direct pathway from the soil to the human or other receptor. Modification of contaminant concentration via fate and transport processes is neglected or considered only minimally. Sediments and surficial soils provide obvious opportunities for transport, exposure, and entry of a contaminant into an organism, while buried or encapsulated material clearly will have impeded transport to humans and biota for many exposure scenarios. If the physical setting appears to cut off the pathway, or present a pathway that provides for substantially impeded release and transport, then consideration of the relevant bioavailability processes will be warranted. Bioavailability process considerations may be of greatest value in guiding decision-making where the threat of transport and exposure is low.
Another common conservative assumption is that total chemical concentration in the solid phase correlates with negative effects in receptors. However, for some chemicals there is clear evidence that the total chemical concentration correlates poorly with receptor response (for example, elemental mercury—EPA, 1996a). In cases where such chemicals are perceived as potential risk drivers at early stages of assessment, consideration of bioavailability processes will usually be warranted. If limited bioavailability can be established early in the process, there may be no need to evaluate exposure further (although it also should be established that the conditions limiting bioavailability will not change with time). Deviating from a conservative default assumption in this way must be done cautiously, and must include tests that determine the form of the chemical present, because there may be strong or weak correlation of total chemical concentration with receptor response depending on biogeochemical conditions. A good example of such a chemical is chromium, a redox active element whose toxicity depends on the biogeochemical conditions in soils and sediments. The reduced form, chromium(III), has low toxicity due to poor membrane permeability and noncorrosivity, while chromium(VI) is highly toxic due to strong oxidation characteristics and ready membrane permeability. Chromium(III) and chromium(VI)
also have different chemical reactivities and thus different fate and transport characteristics. Biogeochemical processes can mediate chromium(III)– chromium(VI) transformation, thus greatly affecting bioavailability and toxicity. Thus, tests to determine which form of the metal is present are critical for determining cleanup goals and an appropriate remedy.
There are many other default assumptions made during human health and ecological risk assessment (see Chapter 2) that might be replaced by site-specific information about bioavailability processes. Fortunately, most states allow for site-specific risk assessment in cases where the appropriateness of default cleanup goals is challenged. With the flexibility to perform a site-specific risk assessment, the key regulatory issue then becomes the type of bioavailability process assessment allowed and the level of scientific rigor that must be associated with results for the assessments to be potentially acceptable.
A Significant Difference in Remediation Goals is Possible
Consideration of bioavailability processes in a risk assessment for a particular chemical is usually worthwhile only if there is potential for the revised exposure assessment to change the estimated risk (and thus the cleanup goal) to an extent greater than its uncertainty bounds. Conditions that increase the likelihood of a sufficiently large change in exposure and remediation goals are as follows.
First, chemical concentrations must be of the same order of magnitude as proposed action levels. As discussed in Chapter 2, bioavailability considerations for soils and sediments, in the few cases where data are available, have tended to adjust cleanup goals (acceptable contaminant concentrations) by factors of two to three. Thus, experience suggests that if contaminant concentrations are of the same order of magnitude as proposed action levels, the adjustment of cleanup goals by factors of two or three may be sufficient to keep exposure in the acceptable range. (Also, this experience indicates that adjustments by factors of two to three have potential to be accepted.) If contaminant concentrations are many times higher than the initial cleanup goals, then it is possible that no reasonable amount of bioavailability information will have a meaningful impact on environmental decision making.
Second, a revised exposure assessment may significantly change the estimated risk for those chemical–receptor combinations where small differences in concentration correspond to large differences in toxicity—that is, where the response versus dose plots are steep. Generally such plots indicate intense receptor sensitivity to the chemical. Where small differences in bioavailability that lead to small differences in exposed dose will translate into large differences in risk, refining the exposure assessment with bioavailability considerations may be worthwhile. (It should be noted that for this type of chemical there is also the need for extremely high precision in bioavailability estimates,
because small errors in determining exposure will result in large errors in the estimated risk.)
The overall risk at a site and costs for soil or sediment remedial actions often depend on the total amount of contaminant mass present. Bioavailability considerations can have a major impact on a site comprised of large amounts of material at low concentrations. This is because small adjustments in acceptable concentrations translate to large differences in the overall amount of material treated and remediation costs. This may include broadly contaminated areas such as estuaries.
Finally, in some cases consideration of bioavailability processes can expand options for remediation. This may be of particular interest to regulatory authorities when use of conventional conservative assumptions and default values necessitate solutions that have environmental or public use costs that negate some of the environmental or public health benefit. For example, if conventional remedial decision-making procedures point to a solution involving soil or sediment removal and treatment that will result in damaging a valuable resource such as a wetland or other habitat, or a boating area, regulators may be interested in finding a less destructive option. This is exemplified by the remedy chosen at the Gary, Indiana, Lagoons Superfund site, where soil is contaminated with PCBs, BTEX1, and PAHs. In this case, soil excavation was significantly scaled back in order to not disturb an adjacent wetland ecosystem after it was determined (during site-specific investigations) that the PCBs adjacent to the wetlands posed less of a risk than would be imposed by excavation.
Future Conditions are Not Likely to Change
Consideration of bioavailability processes will make the greatest difference in decision making if the estimated risk can be projected into the future with certainty. This will be possible to do with confidence when the pathway of concern, site conditions, and key bioavailability processes are not likely to change with time. Obviously, there are many factors that may change the bioavailability or toxicity of a compound in the future. This may in fact be beneficial, as in the case of some organic compounds in soil for which aging is shown to decrease the compound release rate and extent. In this case, bioavailability decreases with time. Alternatively, changing the future conditions may lead to an increase in bioavailability via the modification of the geochemical setting, changes in the exposure pathway of concern, and the introduction of different receptors. Likewise, organisms can change the form of a chemical (e.g., when a chemical is eaten it may become more bioavailable to the predator). Examples of these changes are given below in Table 5-3. If an assessment of the potential future changes introduces a large degree of uncertainty, it is unlikely that evaluation of
TABLE 5-3 Examples of Factors That May Affect the Availability of Soil and Sediment Contaminants over Time
|
Factor |
Causes and Possible Effect on Contaminant Availability |
|
Physical disturbance |
This can result from human activities (e.g., land use change and associated soil excavation) or natural phenomena (e.g., earthquakes, volcanoes, floods, wave action). Depending on the degree of physical disturbance, contaminants that were previously unavailable may become more available. |
|
Changes in pH or ionic strength |
This may occur as a result of natural changes (plant growth) or human activities (disposal of waste materials) in the vicinity of contamination. Changes in pH can affect the speciation and consequently the availability of many metals as well as the binding of organic compounds to solids. |
|
Aging or weathering |
Aging refers to physical and chemical changes in the bonds between contaminants and solids as their contact time increases (see Chapter 3). These processes generally reduce the bioavailability of contaminants from soils and sediment over time. |
|
Moisture |
Natural and anthropogenic changes in the hydrologic regime (droughts and floods) near a contaminated site can change the moisture content of soils. Increasing moisture content may favor transfer of chemical contaminants from soil to bioreceptors. |
|
Temperature |
Temperature change can be induced by certain remediation strategies such as thermal treatment. In general higher temperatures increase the desorption of volatile chemical contaminants from solids. In some cases, higher temperatures may change reaction conditions resulting in a transformation that influences bioavailability. |
|
Biota |
Chapter 3 discusses various processes by which organisms help release contaminants from solid phases (bioturbation, excavation, siderophore action) or transform contaminants in solution (e.g., methylation of mercury). These processes often affect bioavailability of chemicals by changing the redox environment in which the chemical resides. |
|
SOURCE: Adapted from Menzie et al. (2000). |
|
bioavailability processes will yield results with sufficient certainty to impact decision-making.
Regulatory and Public Acceptance is Possible
The potential for results from bioavailability process analyses performed in risk assessment to support remediation decision-making depends on the regulatory domain and public acceptance. Before undertaking a bioavailability process assessment, the likelihood of acceptance of the results by regulators and the
public needs to be evaluated. Conditions for which regulatory and public acceptance of bioavailability information is most likely are described below.
If site conditions, the contaminant of interest, and the default cleanup objectives are similar to those at other sites where remedial action is needed or underway, investment in an assessment of bioavailability processes may be warranted because regulators and the public will have familiarity with the problem. Acquisition of process data and knowledge and application of new measurement tools for bioavailability assessment may help with formation of more cost-effective solutions. An example is the swine test to assess bioavailability of lead in soil, which was applied by EPA to test soils at the Palmerton Zinc Pile Superfund Site (see Box 2-5). The results of the swine testing did not affect the remediation decision at the Palmerton site, as they pointed to acceptable lead soil concentrations in the range estimated by the default assumptions. However, the experience gained at the Palmerton Site and elsewhere led to subsequent applications of the swine testing at approximately 20 other lead-contaminated sites, including several high-volume waste sites. Remediation decisions were influenced by the swine test results at some of these sites (Weis, 2000).
A bioavailability assessment is difficult to justify if a relevant regulatory body has a policy stance against explicit consideration of particular bioavailability processes. Some state environmental agencies and EPA regions have included in guidance to their remediation project managers and risk assessors recommendations or policy directives to refrain from consideration of certain bioavailability processes in estimating exposure (see Table 2-8).
In contrast, some state environmental agencies and EPA regions have developed guidance for consideration of bioavailability processes in risk assessment. EPA Region 10, for example, developed guidance for bioavailability considerations in human health risk assessments for arsenic contaminated soil (see Chapter 2). Washington state has very recently amended its Model Toxics Control Act to allow for incorporation of new scientific information which could be used to modify the “gastrointestinal absorption fraction” and other bioavailability default assumptions (G. McCormack, Washington Department of Ecology, personal communication, 2003). While this has only been done in a few states as of this writing, and for a limited range of bioavailability processes and contaminants, the existence of guidance signifies openness to bioavailability process evaluation.
NEXT STEPS
The preceding chapters have shown that there is a variety of physical, chemical, and biological processes that determine the availability of contaminants in soils and sediments to ecological receptors; that consideration of these bioavailability processes is inherently part of the risk assessment process; that validated measurement techniques and models exist for some bioavailability processes, but not for many others; and that uncertainty about how to measure and
describe some key bioavailability processes has led to limited use and regulatory acceptance of comprehensive bioavailability process evaluation in risk assessment. This chapter has identified soil and sediment contamination scenarios in which consideration of bioavailability processes can have a significant impact on remediation planning and decision-making. Clearly, limitations in measurement tools, models, and understanding serve as impediments to comprehensive assessment of bioavailability processes for many contaminated soil and sediment sites. Yet, just as clearly, there are substantial opportunities for consideration of bioavailability processes to advance risk-based remediation.
Various actions are needed to make progress in using bioavailability processes in risk assessment and decision-making at individual sites, in acknowledging bioavailability processes in regulations and creating appropriate guidance for management of contaminated soils and sediments, and in better understanding bioavailability processes on a mechanistic level.
In Risk Assessment and Decision-making at Individual Sites
In order for bioavailability processes to be considered more explicitly in risk-based management at individual sites, key issues that represent obstacles need to be addressed aggressively. These include (1) selecting appropriate bioavailability process measurement and modeling tools; (2) assessing and (when possible) reducing uncertainty in understanding, models, and parameters for particular bioavailability processes; (3) developing coordinated long-term monitoring of bioavailability processes critical to the risk-based remedial plan implemented; and (4) involving community groups in remediation planning at early stages.
Tools Selection
Chapter 4 described numerous existing and emerging measurement and modeling tools important for bioavailability processes, and it gave the criteria on which the merits of individual tools should be judged and validated. Bioavailability analyses are necessarily site-specific, and it is important that tools appropriate for the particular site and context be selected for assessment of bioavailability processes. Because development of tools relevant to bioavailability is a rapidly growing field with new techniques becoming available on a regular basis, there can be considerable confusion regarding which tools and how many of them to choose in order for the results to be useful in decision making.
In the last five years, scientists, risk assessors, and EPA have advocated relying on a weight-of-evidence approach as a way of making decisions in the face of limited information and imperfect tools. Although the term “weight-of-evidence” is used in different ways by different groups, two concepts associated with the term have important ramifications for choosing bioavailability tests.
First, “weight-of-evidence” can be used to refer to selecting individual bioavailability measurement tools based on the strength of the information that they produce, as well as on how that information will be used for risk-based decision making (Menzie et al., 2000). In this regard, four principles have been outlined that would give a specific tool greater weight: (1) soil–chemical relevance, (2) receptor relevance, (3) pathway relevance, and (4) acceptance or validation of the tool. These factors align closely with discussion earlier in this chapter (pages 377–383) and the criteria outlined for tool validation in Chapter 4. As discussed in Menzie et al. (2000), the degree to which these four factors are satisfied increases user confidence in the tool. Early attempts to explicitly consider bioavailability processes in risk assessment have frequently used inappropriate tools (that usually were not relevant and/or validated), which has contributed to concern about inclusion of bioavailability in risk-based decision-making for contaminated soils and sediments.
On a broader scale, the term “weight-of-evidence” is used to refer to how one uses the combined results of multiple tests. Here the term is synonymous with providing “multiple lines of evidence” about bioavailability processes at a site. For example, this approach might combine empirical measures with measures of bioaccumulation, toxicity, and others parameters. EPA has recently provided guidance on how to use this approach to better identify stressors in aquatic ecosystems (EPA, 2000a) and how to collect sediments (EPA, 2001a), suggesting that the agency would be amenable to using this approach for bioavailability assessments. It is highly consistent with the Chapter 4 notion that each method has unique advantages and limitations and that an integrated suite of tools (see Box 5-4 for an example) is preferable to a single tool. Several recent publications discuss the tenets of this approach for use during human health and ecological risk assessment (Menzie et al., 1996; Burton et al., 2002a,b; Chapman et al., 2002).
The “multiple lines of evidence” approach provides an opportunity to make near-term progress at sites and to overcome some of the pessimism felt by the regulatory community regarding bioavailability because of the lack of mechanistic tools currently available. Its use is an implicit recognition that although our empirical techniques are not able to unambiguously predict bioavailability, they represent progress over the assumption that receptors are exposed to the total contaminant mass bound to soils or sediments. Nonetheless, because of the limitations of empirical tools in their ability to make predictions or be applicable to other sites, the multiple lines of evidence approach should be accompanied by substantial efforts to promote the development of more precise tools. This means employing measurements and models that relate directly to bioavailability process mechanisms to the maximum extent possible. Mechanistic knowledge and insight enables clearer explanation of existing site conditions and how they will respond to a particular remediation or management strategy as well as more confident long-term projections of reliability and durability of remediation and management solutions. For example, mechanistic models based on kinetics will allow
understanding of potential future contaminant release from the solid phase. Empirical knowledge based on measurements that aggregate processes has substantial limitations in this regard. When it is possible to choose tools that will provide better mechanistic understanding, this opportunity should be exploited and not bypassed in favor of conventional empirical assessment approaches.
Given the complexities of bioavailability processes identified in this report, it is likely that mechanistic tools and predictive model development will be a multi-decade effort. Adopting a multiple lines of evidence approach today would utilize and build on the currently existing battery of empirical tests, many of which may have future application for site-specific validation of mechanistic models of exposure and effects. As more robust methods evolve, the need for a multiple lines of evidence approach should diminish concomitant with our increasing ability to predict impacts, leading to greater acceptance of risk assessment that includes explicit consideration of bioavailability processes.
Assessment and Reduction of Uncertainty
At the present time, many bioavailability processes are hidden within default assumptions that are highly simplified and likely to be uncertain (although this uncertainty is generally not reported). More explicit, site-specific consideration of bioavailability processes in risk assessment can reduce this uncertainty. However, if there is (even perceived) substantial uncertainty associated with a bioavailability process that controls the ultimate estimated risk, there may be a tendency to not measure that process explicitly and instead to use conservative assumptions. For example, if the rate of contaminant desorption from a sediment is suggested for consideration in an ecological risk assessment, a slow rate of desorption may decrease significantly the concentration of contaminant predicted to occur in fish. Consideration of this bioavailability process will only be acceptable, however, if the desorption rate for current and projected site conditions can be measured with a fair degree of certainty. If the desorption rate and how it will change as site conditions evolve is not well understood, conservative assumptions such as release of all contaminant to the aqueous phase or equilibrium partitioning may be invoked.
For these reasons, it is important to recognize the uncertainty and variability in each bioavailability process descriptor and the potential for propagation of error in risk assessment. More substantive efforts to manage or reduce the uncertainties, especially for key bioavailability processes, have the potential to greatly reduce the degree of uncertainty in the overall risk assessment. The influence of bioavailability process uncertainty and variability on the overall risk can be assessed qualitatively, quantitatively through sensitivity analysis (deterministic risk evaluation), or through stochastic risk assessment. These approaches are discussed in greater detail in Box 5-6.
|
BOX 5-6 Methods for Assessing Uncertainty in Risk Estimates Most risk assessments are performed in a deterministic manner, that is, with single values for the various parameters in exposure and toxicity models (Burmaster and Wilson, 1998). This results in a single value estimate of risk. All of the exposure and toxicity model parameters have some associated uncertainty and variability, however. Attempts to examine the effects of uncertainty and variability in critical parameter values for a particular risk assessment usually involve performance of sensitivity studies in which the value of a critical parameter is systematically varied while holding all other parameter values constant. This can be done for any number of parameters and is often done for several. The manner in which differences in model results are treated defines various kinds of sensitivity analyses (Cullen and Frey, 1999). Important changes in risk predictions that may result from simultaneous changes in two or more uncertain parameters can be missed with sensitivity analysis, however. The uncertainty and variability in exposure and toxicity model parameter values can be taken into account more rigorously by describing some or all key model parameters with a probability distribution (Morgan and Henrion, 1990; Burmaster and Wilson, 1998; Cullen and Frey, 1999). The risk model is then run many times with different combinations of parameter values sampled from each of the distributions using any of a number of stochastic sampling strategies (e.g., Monte Carlo, Latin Hypercube). Resulting risk model outputs are compiled and used to construct a probabilistic distribution of risk. This process is known as stochastic risk assessment. It is used mostly in the research community at present, though EPA (1997a, 1997b; 2001b) and other organizations are encouraging its use in practice. Simultaneous consideration of distributions for critical parameters in stochastic risk assessment provides a rigorous estimate of the uncertainty in the risk estimate, provided that the parameter distributions are reasonably well defined. This also provides other useful information and insights. For example, a study of the uncertainty in a site-specific risk assessment resulting from variable site physicochemical properties revealed greater uncertainty in risk for more mobile and less degradable compounds present as soil contaminants (Labieniec et al., 1997). The uncertainty in the site properties affecting transport and hence exposure had more importance for these compounds, and that was reflected in the predicted risk distribution. A primary limitation to use of stochastic risk assessment is the lack of sufficient data to define the probabilistic distributions for exposure and toxicity parameters. Site physicochemical data are often quite limited, necessitating the assumption of distributions if a stochastic approach is to be employed. Similarly, toxicity data are often too limited or too dependent on specific test conditions for meaningful assignment of distributions to parameters such as reference doses, carcinogenic slope factors, or absorption fractions. In addition, there is the uncertainty associated with the selected exposure or toxicity model (including the extrapolation of animal results to humans). Nevertheless, there is much work under way to develop accurate distributions for exposure and toxicity parameters, such that stochastic risk assessment should become increasingly useful and routine as part of bioavailability assessment. |
Long-Term Monitoring
A more rigorous evaluation of bioavailability processes during risk assessment will likely alter both the prioritization of remediation efforts at contaminated sites and decisions pertaining to the remedial technology(s) chosen at individual sites. These impacts on decision-making are profound and have the potential to change the current landscape of contaminated soils and sediments management. Whether these decisions provide long-term protection to humans and the environment will depend, in part, on how much is known about bioavailability processes over time. Thus, the consideration of bioavailability processes in risk assessment must include evaluation of future system states via coordinated and process-based long-term monitoring, including the potential for events that might reintroduce unacceptable exposure conditions. Events that could alter contaminant bioavailability at sites where contamination has been left in place include changes in land use, fluctuations in site geochemistry, or the introduction of a new sensitive receptor in the area. Most current bioavailability information is derived from studies shorter in duration than the time frames of interest in site management. These studies are conducted under more consistent physical, chemical, and biological conditions than would be expected at any particular contaminated site. Thus, our understanding of temporal changes in underlying bioavailability mechanisms is limited.
The need for long-term monitoring to enable confident assessment of system behavior over time is a recognized component of most remediation strategies and is generally not complicated in a conceptual sense. Long-term monitoring is also a statutory requirement for those sites regulated under Superfund where contamination is left on-site at levels above those necessary to allow unrestricted use of the land. In the case of bioavailability processes, there is almost no guidance on approaches for long-term monitoring that specifically target the stability of the contaminant “form” instead of total contaminant concentration. Furthermore, monitoring may need to shift from classical site monitoring of total contaminant levels to include the activities of receptors, changes in site-specific processes (e.g., geochemistry), plans for future land use, and other factors.
Depending on the certainty of the bioavailability assessment conducted, a range of monitoring efforts may be appropriate. No further action may be required in some cases where certainty is relatively high, extensive long-term monitoring may be needed at some sites, and a range of possibilities can occur in between. In addition, the need for long-term monitoring may decrease over time if reliable data indicate a high potential for future system stability and other statutory requirements are met. Box 5-7 discusses the development of site-specific monitoring tools for assessing bioavailability over the long term at a hazard-ous waste site. While there are often legal and practical constraints involved with the design of long-term monitoring programs, from a scientific perspective the stronger the commitment to monitoring the greater the payoff in terms of confidence in describing system performance.
As discussed in Chapter 4, a number of methods have been or are being developed to assay the physical state of contaminants in soils and sediments. It is likely that a subset of these approaches will find routine use as monitoring tools. Also, it is likely that new methods will continue to be developed and incorporated into long-term monitoring strategies, such as the deployment of absorbents in the water column to measure potential bioavailability of contaminants over time. As methods are adopted and changed in the future, it is possible that the results of testing will alter the analysis of risk from what might be derived today. To that end, the introduction of bioavailability processes into risk assessment extends not only the time frame of monitoring, but also the time frame for decision-making. Regardless of the origin of change, it may be necessary to rethink risk assessments into the future and be prepared to respond accordingly to avoid unwanted exposure, or to stop ongoing activities that are no longer needed to reduce risk.
Community Concerns and Risk Communication
Experience has demonstrated that communities often have concerns about consideration of bioavailability processes in risk assessments for decision-making at hazardous waste sites. Perhaps most importantly, bioavailability assessments may be viewed as a “do-nothing” or “do-less” approach. Given that incorporation of bioavailability adjustments into risk assessments may raise acceptable contaminant concentrations in soil or sediment, it may be viewed as simply a justification for leaving more contamination in place. Second, in some cases evidence is often insufficient to justify the use of bioavailability process information. Because bioavailability process studies may not be conducted for the ultimate receptor of concern, or may yield results with considerable uncertainty, a community may not be confident that the scientific evidence is adequate to apply the results within their community. This can be exacerbated by the fact that standardized methods for evaluating some key bioavailability processes are lacking. Third, the long-term effectiveness of leaving “unavailable” contaminants in soils and sediments is unknown. Because the bioavailability of contaminants from soil or sediment may increase or decrease over time, or if site conditions change, exposure to the contaminants may increase or decrease in the future. Finally, monitoring requirements may be perceived as insufficient. The previous section discussed the need for monitoring of bioavailability processes over time to ensure that contaminant availability to receptors remains within an acceptable range. Given the potential cost of long-term monitoring, a community may not be confident that it will be conducted adequately, or for a sufficient period.
To date, bioavailability process evaluations at hazardous waste sites have been applied primarily for human health risks from metals in soils, and at a limited number of mining and smelting sites. Because only a small number of communities have had to grapple with bioavailability issues, it is uncertain which community concerns will predominate. Of the limited cases to date where com-
|
BOX 5-7 Monitoring Tools for Assessing Long-term Bioavailability in Leadville, Colorado A Superfund site in Leadville, Colorado is characterized by high metal, pyritic alluvial tailings deposits along the Upper Arkansas River. The remedy at the site will involve amending the tailings with chemicals in situ to reduce the bioavailability of the metals as opposed to a more conventional soil removal and replacement. The threat posed by the tailings is primarily to the surrounding ecosystem because of the inability of the tailings to support a vegetative cover. The bare tailings along the banks of the river tend to erode into the river, resulting in damage to river biota. The in situ amendment selected to reduce the bioavailability of the tailings includes application of municipal biosolids (224 Mt ha–1) and limestone (224 Mt ha–1). Surface application of this type of amendment has been shown to reduce surface as well as subsoil acidity, thereby reducing the solubility of the metals in the system (Brown et al., 1997). Biosolids also provide both inorganic and organic specific adsorption sites for metals (Zhenbin et al., 2001). In addition, the improvements in soil nutrient and physical properties associated with biosolids application will permit establishment of a vegetative cover on the tailings, and thereby reduce the potential for re-entrainment (Sopper, 1993). By selecting an in situ amendment to reduce the bioavailability of the contaminants, project costs were reduced, allowing additional acreage to be treated. According to the project manager, a local repository for excavated soil was unavailable, and the costs to excavate and transport to a front range disposal facility were prohibitive. To date, about $1.25 million has been spent to treat 35 acres or about 42,000 cubic yards using the in situ remedy. Remedial Assessment EPA’s Environmental Response Team, a division of Superfund, has been in charge of developing an appropriate monitoring scheme for the site. Addition of amendments to metals-contaminated soils to reduce the bioavailability of metals in situ is considered an “emerging” technology by EPA, and a standard array of tests has not been developed 
Alluvial tailings deposits along the Upper Arkansas River outside Leadville. Surface salt consists of metal sulfates with zinc concentrations as high as 9 percent. |
|
for performance evaluation. Scientists are attempting to develop appropriate tests and criteria to evaluate the effect of the amendment on the functioning of the ecosystem, focusing on the worst-case exposure pathways in order to be conservative. The monitoring approach centers on soil function and biological activity in the remediated area as compared to both control uncontaminated and control contaminated areas. Increased soil function and biological activity is taken to be indicative of decreased bioavailability of the contaminants. The emphasis is on determining if the amendment has restored functionality to the system. Importantly, there has not been a corresponding effort to assess the speciation or fate of the metals. Ecosystem function is addressed in increasing orders of complexity—first soil functionality, then plant health, and finally the diversity and health of larger communities. The evaluation is being developed to answer the following questions. The tests being used to answer each question are listed below the question itself.
A functioning ecosystem will be viewed as effective proof that the bioavailability of the contaminants has been reduced as a result of the in situ amendment. |
munities have been presented with bioavailability information (see Box 5-8), the responses have ranged from strong support (Oak Ridge, Tennessee) to acceptance (Bartlesville, Oklahoma) to strong objection (Aspen, Colorado).
Nonetheless, consideration of bioavailability process information for contaminated soils and sediments is inherently part of the risk assessment process, whether for protection of ecological receptors or human health. As discussed in Chapter 2, all risk assessments for soil and sediments contain implicit assumptions about bioavailability (a common default assumption being that the contaminant is equally bioavailable from soil or sediment as it was in the original laboratory toxicity study for the chemical). Thus, bioavailability does not present a risk communication problem unique from the risk assessment processes. The public should be introduced to the concept of bioavailability, and the consideration of bioavailability processes, as being a fundamental component of risk assessment no different from other exposure parameters or toxicity values used in risk assessment, and around which there may be considerable uncertainty.
Whether default assumptions about bioavailability processes are replaced with site-specific measurements will depend on whether such measurements are technically justifiable, and whether a good job of public outreach and communication is performed at a specific site. The quality of the risk communication (as outlined in Box 5-9) will determine whether the public is likely to evaluate the use of bioavailability processes on their scientific merits.
The technical components that should be included in any public communication program regarding application of bioavailability adjustments should include the following:
-
factors that affect bioavailability from soils or sediments;
-
the concepts of absolute bioavailability and relative bioavailability;
-
the technical basis for the established toxicity values, and how bioavailability was handled in the derivation of those values;
-
selection of a model for bioavailability studies and why it was chosen;
-
how uncertainty was handled (e.g., different bioavailabilities in different animals in the study, uncertainty in overall study); and
-
how the bioavailability information is incorporated into the risk assessment.
Specific interests or concerns of the community may dictate detail, or additional areas that need to be addressed.
The potential community concerns discussed above should be dealt with in a direct and honest manner by providing the public with timely information about any bioavailability studies that are proposed for a specific site, and their outcome and implications for the site. With respect to the concern that consideration of bioavailability processes is simply used to justify a “do-less” approach, it should be conceded that there is an element of truth in this. The reality is that bioavailability process studies are rarely undertaken simply to improve the accuracy
of a risk assessment. Rather they are generally performed to justify site cleanup goals that are more financially or technically feasible, and that involve leaving appreciable amounts of contaminant mass in place, while still being protective of public health and the environment. On the other hand, there can be a strong scientific basis for the incorporation of site-specific bioavailability process information into the risk assessment process; such technical information should be provided to the public. Bioavailability assessment may receive greater scrutiny, given its relative newness, than other site-specific studies that are performed at contaminated sites (e.g., soil ingestion studies in humans and wildlife, soil-to-house dust transfer studies, environmental exposure studies, or toxicity studies). The important fact to emphasize in communicating results is that all bioavailability process studies are aimed at exposure assessment, and are performed to reduce uncertainty regarding the magnitude of site risks and thereby support a more efficient cleanup strategy, or to support choices among different remedial alternatives.
Into the Regulatory Arena
In relatively few cases has the replacement of default assumptions about bioavailability processes with site-specific measurements been incorporated as a matter of practice into protocols that govern risk-based decision-making in the regulatory arena for contaminated soils and sediments. Consideration of physical transport processes is sometimes permitted, if these processes are adequately characterized. Use of a dilution-attenuation factor for contaminant concentration mitigation along the pathway from source to receptor is also sometimes allowed. More often than not, however, the total contaminant mass in the source area is assumed to be available to the receptors of interest, and potential attenuation of exposure via fate and transport processes is neglected. This approach is conservative with respect to protection of public health and the environment, and it deals simplistically with the issue of uncertainty. In this regulatory environment, when even basic transport processes are not routinely considered, it is difficult to introduce new kinds of data and information pertaining to processes that affect exposure.
There is no question that risk assessment methods and models will evolve to encompass our improved scientific understanding of bioavailability processes. It will always be the case, however, that process-based methods for exposure analysis will only be applicable and useful in situations for which site characterization data are adequate. Thus, the existence of thoroughly validated measurement techniques and models for particular bioavailability processes will still not guarantee the ability to perform comprehensive, process-based exposure assessments in all cases. Regulatory requirements and constraints on risk-based management of soil and sediment sites necessarily must account for this reality.
|
BOX 5-8 Case Studies of Community Concerns Regarding Bioavailability Oak Ridge, Tennessee At the Oak Ridge site, the community was in favor of applying a mercury bioavailability adjustment to soils and sediments of the East Fork of Poplar Creek. In general, the community viewed extensive remediation of soils and sediments as disruptive and of questionable benefit. The situation at this site was somewhat unique in that the community was both highly informed and highly educated (due to the presence of Oak Ridge National Labs) and actively participated in evaluating the data and science used to assess risk and develop cleanup goals. The residents of Oak Ridge readily accepted the mercury bioavailability adjustment for soils and sediments, which was applied in the risk assessment and ultimately increased the cleanup level. (Sources: M. O. Barnett, Oak Ridge National Laboratory, Environmental Sciences Division, personal communication, 2000; NEPI, 2000.) Bartlesville, Oklahoma At the National Zinc site in Bartlesville, Oklahoma, oral bioavailability studies were conducted for lead, cadmium, and arsenic in soil, and the resultant data were used in the human health risk assessments for these elements (see Box 2-4). No concerns were voiced by the community, either at public meetings or as written comments, regarding the development and application of bioavailability adjustments. A number of factors were likely involved in the community acceptance of this issue, including (1) proactive engagement of the community by the Oklahoma Department of Environmental Quality, coupled with a concerted community awareness and risk communication program, (2) existence of an active Citizens Advisory Group that represented the community throughout the entire remedial investigation/feasibility study process, and (3) |
It remains to be seen whether regulatory agencies will embrace the bioavailability concept in the short term. The resistance in some regulatory domains to allowing site-specific measurements of some bioavailability processes in risk assessment stems from many factors, including uncertain methodologies and lack of validation, public anxiety and suspicion about motives, and lack of precedent. These factors are not unique to the issue of bioavailability of contaminants in soil or sediment. Similar concerns arise in other contexts with proposals for new approaches for the protection of human health and the environment. Some examples are the application of innovative remediation technologies for site cleanup, or adoption of new methods for treating drinking water, or engineering manipulation of river or groundwater resources for ecosystem restoration. In each of these cases there is reluctance to make a substantial commitment to a new approach until more is known.
A viable way to move around these obstacles and achieve more widespread consideration of bioavailability processes in risk-based management of contami-
|
representation of the community by an expert (Dr. Frederick Oehme from Kansas State University) who reviewed and commented on the bioavailability study protocols and data interpretation. For both of the sites, project managers commented that consideration of bioavailability processes in risk assessment posed no special risk communication problem relative to the overall challenge of communicating the role and results of risk assessment in project decision-making. Aspen, Colorado The Smuggler Mountain Superfund site in Aspen was placed on the National Priority List in 1986 due to elevated levels of metals, particularly lead, in soil in the vicinity of residences. The EPA’s proposed remedial options, which included the removal of substantial amounts of soil and the deposit of funds in escrow accounts for future environmental cleanup, were opposed by the affected community, partly because the remedy for the site involved hauling tons of dirt. In addition, a blood-lead survey found that lead concentrations in the children living near the site were below that for the general population, leading to the claim that the lead at the site is not bioavailable and thus not harmful. Lead bioavailability studies in young swine that were conducted by EPA Region 8 on Aspen soils met with considerable opposition (Bernstein, 1991). However, this response was symptomatic of the already strained relations between EPA and the Aspen community at the time that the bioavailability studies were conducted, and may not have reflected public discord with the bioavailability studies themselves. |
nated soils and sediments is to invoke an adaptive management approach. This paradigm embraces two ideas. The first is that there should be various pilot studies to experiment with different techniques to see if they work or not. The second is that agencies should use the results from such efforts to develop a common systematic approach to determine how and when to incorporate bioavailability concepts into regulations in a consistent manner.
Adaptive management applies findings from carefully monitored experiments to the adjustment of future management and policy decisions in light of changing conditions and new knowledge. This approach moves away from rigid requirements that require the selection of fixed goals and the means to achieve them. Adaptive management is receiving increasing attention and application to problems of regional ecosystem management (Gunderson et al., 1995; Lee, 1993; Walters, 1997). It is being promoted for wider use in water management programs such as in the Florida Everglades and in Glen Canyon on the Colorado River (NRC, 1999, 2001b) and has been tried in some forest and fisheries sectors
|
BOX 5-9 Tenets of Good Risk Communication As consideration of a wider range of bioavailability processes becomes more common in risk assessments, there will be an associated responsibility for risk assessors and project managers to educate potential stakeholders regarding the key bioavailability processes and related measurements, their incorporation into site-specific risk assessments, and their ultimate effect on cleanup goals. This may require that regulatory agencies institute more comprehensive risk communication programs that emphasize both the learning and explaining activities of communication, while training risk managers and others engaged in communicating risk. Communicating scientific issues regarding public health risks has been an active field of study and practice since the early-1980s (Sandman, 1986). Risk communication has come to mean communication that supplies lay people with the information they need to make informed independent judgments about risks to health, safety, and the environment (Morgan et al., 1992). The basics tenets of risk communication include (Elder, 1997; NACCHO, 1995; Sandman, 1996):
These principles of public communication hold equally well for the communication of bioavailability information as for any other type of scientific information. One of the fundamental features of public communication is that to be successful it should be treated as a process, not as a single event or mechanism. Successful examples of public communication documented by Ashford and Rest (1999) had in common that each was a process designed to improve communication with the community, educate community members and build their technical skills, and facilitate specific participation by the community in the decision-making process. In a 1996 evaluation by the Agency for Toxic Substances and Disease Registry (ATSDR) of its community involvement efforts, it was concluded that in order to build an effective working relationship, community involvement should be viewed as a dynamic and developing relationship between community members and the ATSDR. This approach to public communication is particularly important when addressing issues of bioavailability, because these issues require a considerable amount of technical information to be transmitted, and may require some time for the studies to be designed, conducted, and interpreted. This provides an opportunity to work with interested individuals or organizations within a community (or technical experts who may represent the community) to reach consensus on the design and application of such studies. |
(NRC, 1996; Taylor et al., 1997). It has also been proposed for management and remediation of PCB-contaminated sediments in rivers (NRC, 2001a) and for cleanup of hazardous waste sites (NRC, 2003). Adaptive management arose from concerns that conventional resource management approaches inadequately considered system dynamics and uncertainties, and that some problems in large-scale ecosystem and resource management can only be understood through experiments. In principle, the concept is not new. It is akin to the scientific method and engineering problem solving, as in “learning by doing.” But it is not simply trial and error. The outcomes must be based on integrated scientific experimentation with attention to uncertainties and hypothesis testing to reduce these uncertainties. The adaptive management paradigm allows a way around the stakeholder, regulatory, and policy gridlock that characterizes cleanup at many contaminated soil and sediment sites.
The strengths and limitations of the adaptive management approach (Lee, 1999; Walters, 1997) could apply to progressively incorporating bioavailability concepts into regulations as well as they do to managing forests or fisheries. To explain how such an approach might be used, it is instructive to think through a hypothetical example. AVS/SEM (see Chapter 2) is an approach that regulatory agencies around the world have considered incorporating into sediment quality guidelines for metals, although opinions differ widely as to the suitability of the method for use as a regulatory tool (EPA, 2000b). An adaptive way of incorporating the tool in regulations might involve the following:
-
Make the management decision that AVS/SEM methodology will be used to evaluate site cleanup at, for example, a large Superfund site or in a single region (like San Francisco Bay) for a finite period of time (e.g., five years).
-
Establish a conceptual model of how the method would be applied and specific methodologies for each step of the application. For example, a contaminated site might be dredged when sediments marginally exceed total metal guidelines, with the spoils being deposited in an area where AVS/SEM > 1.
-
Design hypotheses about the outcomes of the application. For example, one could hypothesize that exposure of resident organisms to metals in the disposal area (where AVS/SEM > 1) should not increase as a result of disposal of the marginally contaminated dredge spoils. Also, benthic communities in such an area should recover after spoil disposal similarly to if the dredged spoils were not contaminated.
-
Set up a formal experimental design, including marginally contaminated pilot sites, uncontaminated pilot sites, and non-dredge sites.
-
Monitor outcomes in the different experimental treatments, for example by assessing metal concentration and form, exposures in resident organisms, benthic community changes, or predator useage. Part of the goal is to better define the relationships between chemical concentrations and biological responses.
-
Study changes in the regulatory and stakeholder responses that occur as a result of the experiment over the five-year period.
-
Feed back the results of the experiment into the AVS normalization model to improve its predictive capabilities, and then make decisions about how and whether to implement such a strategy on a larger scale.
This would constitute an intermediate step between (1) removing and treating sediment with contaminant concentrations above a fixed acceptable concentration, determined via an ecological risk assessment with minimal consideration of bioavailability processes, and (2) not doing anything.
Another example is the adaptive management approach recommended for determining the efficacy of dredging and how much PCB-contaminated sediment to dredge from the Hudson River. The EPA has formulated a cleanup plan that involves a series of performance standards by which the cleanup will be evaluated regularly (EPA, 2001c). The plan attempts to accommodate concerns about increased bioavailability of PCBs during dredging. Performance indicators will include PCB concentrations in sediment, in the water column, and in fish, and the amount of dredged material that becomes suspended in the water column. Risks will be reevaluated, and cleanup plans and objectives will be adjusted as the performance monitoring information is acquired and interpreted.
The adaptive management paradigm, embracing various well-designed pilot studies, is a viable approach to moving new bioavailability process considerations into the field and the regulatory arena. The experiments could progress from small-scale to larger-scale, from short time frame to long time frame, and from narrow perspective to broad perspective. Assessment of risk can be performed simultaneously, and the influence on risk of the bioavailability process information developed can be elucidated.
Into the Scientific Arena
Expansion of bioavailability process considerations into risk assessment and remediation decision-making for contaminated soil and sediment sites requires improved scientific understanding and models for a number of key bioavailability processes. Also required are additional federal sources of funding for bioavailability research. Some specific research needs in these broad areas are outlined below.
Mechanistic Studies and Tool and Predictive Model Development
Much research on soil and sediment contamination has been driven by regulatory agendas, with associated emphasis on the need for simple measurements and models. The result is a knowledge base limited by substantial dependence on empirical measurements and models. Models for many bioavailability processes
have weak predictive capability, a serious limitation for their use in risk assessment. In the case of human health risk assessments, for example, much information on bioavailability of contaminants transported to human receptors comes from industry-funded studies at specific sites. Those are usually, and understandably, not conducted in a way that advances understanding of fundamental underlying processes.
Greater mechanistic understanding and predictive models of bioavailability processes are needed to improve the accuracy of risk assessments for contaminated soil and sediment sites. Investment in mechanistic understanding and models will prove more profitable in the long-term than reliance on empirical knowledge because models have greater predictive power for a broader range of situations. As part of this research effort it will be important to draw ties between mechanistic understanding and more operational tests for bioavailability. For example, there have been feeding studies with different lead minerals that revealed different relative bioavailabilities, and there have been measured differences in blood lead levels in humans from mining (primarily PbS) versus urban (PbCO3 or PbO) sites (Steele et al., 1990; Cotter-Howells and Thornton, 1991; Davis et al., 1992, Freeman et al., 1992, Ruby et al., 1992). But there are almost no studies that quantitatively examine both the mineralogical form of the contaminant (using X-ray absorption spectroscopy) and biological uptake (using plants or small mammal bioassays). Chapter 3 discusses other areas in need of attention, including contaminant–solid interactions, the nature and effects of aging on contaminant release rates, the role of colloids, and the feeding ecology of animals. Research areas suggested by the present chapter include better understanding of whether and when associations between contaminants and soils and sediments can be made permanent. As a corollary, describing and measuring the “activity” of solid-phase-associated contaminants should be a future research goal, including understanding how naturally occurring chemical and biochemical reactions already mediate changes in the activity of solid-phase-associated pollutants. The results from such research are needed before bioavailability explanations can be used with confidence to determine the amounts of soil and sediment remediated.
Many of the tools discussed in Chapter 4 are still in development and require future research, including some with tremendous potential for better understanding bioavailability processes. In addition to developing new tools, existing tools require research to expand their applicability to more sophisticated processes and greater numbers and types of contaminated sites. Most tools have not undergone the type of validation outlined in Chapter 4 as necessary for ensuring their accuracy and usefulness, nor can their results be generalized to multiple sites. Finally, research is greatly needed to develop a systematic approach to identifying an appropriate suite of complementary tools for use at a particular site. Such an approach should assess the state of validation for particular tools and their performance in different experimental matrices and at different sites. This would help
ensure that all important bioavailability processes relevant to a particular site are studied. At the present time, individual bioavailability tools are frequently applied, producing information that is difficult to interpret in isolation, that is extrapolated to the field without adequate scientific justification, or that is not relevant to the key bioavailability processes at a site.
Chapter 3 stressed the need for better understanding bioavailability processes at the field-scale. As a corollary, field tests are critical to determining whether proposed measurement techniques and models can accurately describe and predict bioavailability process performance at relevant scales. There has been limited investment in well-designed field experiments in which the complexity of environmental conditions can be accurately represented. Because these studies are expensive, priority should be given to selected important, recurring soil and sediment contamination problems. To provide more regulatory confidence, these studies could be conducted strictly in a pilot context before adopting the techniques widely. In addition to providing the most rigorous scientific test platform for a bioavailability measurement or modeling tool, field testing also enables realistic assessment of implementation costs and regulatory and public acceptance of the results obtained.
Funding for Bioavailability Research
Significant advances in understanding of bioavailability processes will have to come from new research. There are several potential avenues for funding of this research by federal agencies with research missions and responsibilities for managing environmental contamination. These agencies include the National Science Foundation (NSF), the National Institutes of Health, EPA, DOE, and the Department of Defense (DoD). NSF has funded a variety of studies of bioavailability processes, principally those related to interactions between environmental contaminants and media and the movement of chemicals in the environment. The National Institutes of Health, through the Superfund Basic Research Program administered by the National Institute for Environmental Health Sciences, funds a few bioavailability process studies, as does DoD, principally through the Strategic Environmental Research and Development Program. DOE is conducting research on methods for assessment of bioavailability processes as they affect remediation.
Among federal agencies, the greatest commitment to bioavailability research has been made by EPA. Over the last decade, EPA has supported nearly 100 studies on bioavailability processes through its National Center for Environmental Research. The vast majority of these research projects have involved mobility of chemicals in the environment, uptake relevant to assessing ecological risks, and bioavailability processes that might affect bioremediation. Despite this research investment, progress in understanding these bioavailability processes is quite limited. For example, the number of bioavailability field trials or mechanis-
tic studies from EPA’s Superfund program is surprising low. Bioavailability studies at complex hazardous waste sites could be instrumental in designing improved risk management at those sites.
Recently, EPA has evaluated research needs and prioritized research topics (EPA, 1999); bioavailability in human health risk assessment emerged as a high priority. For example, for soils the topic with the highest research priority was “Estimating Human Exposure and Delivered Dose.” This topic included focus points such as “evaluating the bioavailability of contaminants in various soil matrices,” “deriving dermal absorption factors for common soil contaminants,” and “developing biotransfer and bioaccumulation factors for contaminants to facilitate estimates of exposure via the food chain.” Despite this high priority, however, very little in the way of sponsored research on this topic is being funded by the agency. In fact, most of what is known about the potential oral bioavailability of contaminants from soil matrices, for example, comes not from agency-sponsored research projects, but rather from studies conducted by EPA Regions, states, and responsible parties on bioavailability of lead and arsenic from contaminated sites (e.g., EPA, 1996b; Casteel et al., 1997, 2001; Freeman et al., 1992, 1993, 1995; Roberts et al., 2002). These studies offer valuable observations regarding the absorption of contaminants from soils in specific situations, and some inferences on general behavior of absorption from soils might be gained from looking at these studies collectively. However, they are not an effective substitute for directed research because they have a different objective. The purpose of these studies was to obtain empirical measurements of relative bioavailability to support a human health risk assessment. For understandable reasons, this objective does not include an exploration of factors that might influence bioavailability processes, and therefore it is difficult to determine the extent to which these observations can be generalized or used to predict the results that might be obtained at different sites or under different conditions. Unless a greater commitment is made to fund bioavailability process studies from more of a research perspective, progress in developing information that can be utilized to advance human health risk assessments will be slow.
OVERARCHING CONCLUSIONS AND RECOMMENDATIONS
Bioavailability process considerations are not uniformly or widely embraced by scientists, regulators, or the public because of a lack of scientific and technical understanding. Explicit consideration of bioavailability processes and modeling in risk assessment would help to adjust cleanup goals by more accurately identifying that fraction of contaminant total mass that has the potential to enter receptors. Also, bioavailability process understanding would help guide the selection of appropriate remediation technologies. It is clear that more numerous validated tools and models are needed and that there should be reliance on an integrated suite of tools that lead to mechanistic understanding rather than on a single tool or
wholly empirical approaches. Ultimately, bioavailability process considerations are likely to make a difference where less than an order of magnitude adjustments in chemical concentrations are sought compared to proposed action levels, and where investments in assessment of bioavailability processes lead, over time, to familiarity with specific issues. Where site-specific consideration of bioavailability processes leads to more contaminated material remaining on site, long-term monitoring is needed to assess treatment performance, validate models, and demonstrate that contaminant bioavailability is not increasing over time. The following overarching conclusions and recommendations summarize our current understanding of processes that affect whether chemical contaminants in soils and sediments are bioavailable to humans, animals, microorganisms, and plants.
Bioavailability processes are defined as the individual physical, chemical, and biological interactions that determine the exposure of plants and animals to chemicals associated with soils and sediments. First, in the broadest sense, bioavailability processes describe a chemical’s ability to interact with the biological world. Second, bioavailability processes are quantifiable through the use of multiple tools. Third, bioavailability processes incorporate a number of steps not all of which are applicable for all contaminants or all settings. Fourth, there are barriers that change exposure at each step. Thus, bioavailability processes modify the amount of chemical in soil or sediment that is actually absorbed and available to cause a biological response.
Bioavailability processes are embedded within human health and ecological risk frameworks. The goal of bioavailability analysis is to reduce uncertainty in exposure estimates and thus improve the accuracy of the risk assessment. However, today “bioavailability” is commonly thought of in relation to one process only—absorption efficiency—such that a single “bioavailability” factor is used as an adjustment to applied dose. Most of the other bioavailability processes are hidden within the risk assessment process, and assumptions made about these processes are not clear. The knowledge base underlying many default assumptions about bioavailability processes is weak.
Mechanistic understanding of bioavailability processes is ultimately needed to improve the scientific basis of risk assessment. Thus, tools for measuring bioavailability processes that further mechanistic understanding and promote predictive model development are preferred over conventional empirical approaches. In the short term, empirical approaches are useful in generating site-specific information—provided that their results are analyzed using a weight-of-evidence approach and with an understanding that they will be replaced with more mechanistic tools as they are developed. At any given site, a suite of tools will be necessary to describe bioavailability processes in soils or sediments.
The potential for the consideration of bioavailability processes to influence risk-based decision-making is greatest when certain chemical, environmental, and regulatory factors align. Consideration of bioavailability processes is most likely to impact decision-making when the contaminant is, and is likely to remain, the risk driver; when the default assumptions made for a particular site are inappropriate; when significant change to remedial goals is likely (e.g., because large amounts of contaminated soil or sediment are involved); when conditions present at the site are unlikely to change substantially over time; and where regulatory and public acceptance is high. These factors should be evaluated before committing the resources needed for a detailed consideration of bioavailability processes.
Moving bioavailability concepts further into the hazardous waste arena will require specific actions at individual sites, further scientific research on critical bioavailability processes, and large-scale, coordinated testing of bioavailability tools and techniques at pilot sites. At individual sites, assessment of bioavailability processes must be accompanied by uncertainty analysis, process-based long-term monitoring to ensure that present assessments of bioavailability remain accurate and acceptable, and community involvement beginning at the early stages of remediation planning. Although bioavailability is not a unique risk communication problem, experience has demonstrated that communities often have concerns about consideration of bioavailability processes during risk assessments. In order to demonstrate the utility of explicitly considering bioavailability processes and to test new models and tools, adaptive management should be applied to select pilot bioavailability test sites. Adaptive management applies findings from carefully monitored experiments to the adjustment of future management and policy decisions in light of changing conditions and new knowledge.
REFERENCES
Achtnich, C., E. Fernandes, J. M. Bollag, H. J. Knackmuss, and H. Lenke. 1999. Covalent binding of reduced metabolites of [N-15(3)]TNT to soil organic matter during a bioremediation process analyzed by 15N NMR spectroscopy. Environ. Sci. Technol. 33(24):4448-4456.
Achtnich, C., H. Lenke, U. Klaus, M. Spiteller, and H. J. Knackmuss. 2000. Stability of immobilized TNT derivatives in soil as a function of nitro group reduction. Environ. Sci. Technol. 34(17):3698-3704.
Alexander, M. 1995. How toxic are toxic chemicals in soil? Environ. Sci. Technol. 29:2713-2716.
Ashford, N. A., and K. M. Rest. 1999. Public participation in contaminated communities. Cambridge, MA: Center for Technology, Policy, and Industrial Development, Massachusetts Institute of Technology.
Benner, S. G., D. W. Blowes, W. D. Gould, R. B. Herbert, and C. J. Ptacek. 1999. Geochemistry of a permeable reactive barrier for metals and acid mine drainage. Environ. Sci. Technol. 33:2793-2799.
Bernstein, J. 1991. Report from Aspen. The New Yorker. November 25. Pp. 121-136.
Blaylock, M. J., D. E. Salt, S. Dushenkov, O. Zakharova, C. Gussman, Y. Kapulnik, B. D. Ensley, and I. Raskin. 1997. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 31:860-865.
Bosma, T. N. P., E. M. W. Ballemans, N. K. Hoekstra, R. A. G. Welscher, J. Smeenk, G. Schraa, and A. J. B. Zehnder. 1996. Biotransformation of organics in soil columns and an infiltration area. Ground Water 34:49-56.
Bosma, T. N. P., P. J. M. Middeldorp, G. Schraa, and A. J. B. Zehnder. 1997. Mass transfer limitation of biotransformation: quantifying bioavailability. Environ. Sci. Technol. 31:248-252.
Bricker, T. J., J. Pichtel, H. J. Brown, and M. Simmons. 2001. Phytoextraction of Pb and Cd from a Superfund soil: effects of amendments and croppings. Journal of Environmental Science and Health, Part A—Toxic/Hazardous Substances & Environmental Engineering 36:1597-1610.
Brown, S., R. Chaney, and J. S. Angle. 1997. Subsurface liming and metal movement in soils amended with lime-stabilized biosolids. J. Environ. Qual.26:724-732.
Burmaster, D. E., and J. C. Wilson. 1998. Risk assessment for chemicals in the environment. In: The encyclopedia of biostatistics. New York: Wiley.
Burton, G. A., P. M. Chapman, and E. P. Smith. 2002a. Weight-of-evidence approaches for assessing ecosystem impairment. Human and Ecological Risk Assessment 8:1657-1674.
Burton, G. A., G. E. Batley, P. M. Chapman, V. E. Forbes, E. P. Smith, T. Reynoldson, C. E. Schlekat, P. J. den Besten, A. J. Bailer, A. S. Green, and R. L. Dwyer. 2002b. A weight of evidence framework for assessing sediment (or other) contamination: improving certainty in the decision-making process. Human and Ecological Risk Assessment 8(7):1675-1696.
Casteel, S. W., R. P. Cowart, C. P. Weis, et al. 1997. Bioavailability of lead to juvenile swine dosed with soil from the Smuggler Mountain NPL site of Aspen, Colorado. Fund. Appl. Toxicol. 36:177–187.
Casteel, S., T. J. Evans, J. Yang, and D. Moseby. 2001. Effects of treatments on soil-lead bioavailability in swine. Agronomy abstracts 2001. Annual meeting October 21-25, Charlotte, NC.
Chapman, P. M., B. G. McDonald, and G. S. Lawrence. 2002. Weight of evidence issues and frameworks for sediment quality (and other) assessments. Human and Ecological Risk Assessment 8: 1489-1516.
Chen, H., and T. Cutright. 2001. EDTA and HEDTA effects on Cd, Cr, and Ni uptake by Helianthus annuus. Chemosphere 45:21-28.
Cornelissen, G., H. Rigterink, M. M. A. Ferdinandy, and P. C. M. VanNoort. 1998. Rapidly desorbing fractions of PAHs in contaminated sediments as a predictor of the extent of bioremediation. Environ. Sci. Technol. 32:966-970.
Cotter-Howells, J., and I. Thornton. 1991. Sources and pathways of environmental lead to children in a Derbyshire mining village. Environ. Geochem. Health 13:127-135.
Cullen, A. C., and H. C. Frey. 1999. Probabilistic techniques in exposure assessment. New York: Plenum Press.
Dasappa, S. M., and R. C. Loehr. 1991. Toxicity reduction in contaminated soil bioremediation processes. Water Research 25:1121-1130.
Daun, G., H. Lenke, M. Reuss, and H.-J. Knackmuss. 1998. Biological treatment of TNT-contaminated soil. 1. Anaerobic cometabolic reduction and interaction of TNT and metabolites with soil components. Environ. Sci. Technol. 32:1956-1963.
Davis, A., M. V. Ruby, and P. D. Bergstrom. 1992. Bioavailability of arsenic and lead in soils from the Butte, Montana, mining district. Environ. Sci. Technol. 26:461-468.
Deshpande, S., L. Wesson, D. Wade, D. A. Sabatini, and J. H. Harwell. 2000. DOWFAX surfactant components for enhancing contaminant solubilization. Water Research 34:1030-1036.
Dwarakanath, V., K. Kostarelos, G. A. Pope, D. Shotts, and W. H. Wade. 1999. Anionic surfactant remediation of soil columns contaminated by nonaqueous phase liquids. Journal of Contaminant Hydrology 38:465-488.
Elder, M. 1997. The process of community involvement—a case study: the Bartlesville, Oklahoma, Lead Project. Toxicol. Ind. Health 13(2/3):395–400.
Environmental Protection Agency (EPA). 1996a. Mercury study report to Congress. EPA 600-P-94-002Ab. External Review Draft. Washington, DC: EPA Office of Research and Development.
EPA. 1996b. Bioavailability of arsenic and lead in environmental substrates: results of an oral dosing study of immature swine. EPA 910/R-96-002. Seattle, WA: EPA Region 10.
EPA. 1997a. Policy for use of probabilistic analysis in risk assessment at the U.S. Environmental Protection Agency. Available at http://www.epa.gov/ncea/mcpolicy.htm.
EPA. 1997b. Guiding principles for Monte Carlo Analysis. EPA/630/R-97/001. Washington, DC: EPA.
EPA. 1999. Waste research strategy. EPA/600/R-98/154. Washington, DC: EPA Office of Research and Development.
EPA. 2000a. Stressor identification guidance document. EPA/822/B-00/025. Washington, DC: EPA Office of Research and Development.
EPA. 2000b. Review of an integrated approach to metals assessment in surface waters and sediments. EPA-SAB-EPEC-00-005. Washington, DC: EPA Science Advisory Board Ecological Processes and Effects Committee.
EPA. 2001a. Methods for the collection, storage, and manipulation of sediments for chemical and toxicological analysis: technical manual. EPA-823-B-01-002. Washington, DC: EPA Office of Water.
EPA. 2001b. Risk assessment guidance for Superfund. Volume 3 Part A: process for conducting probabilistic risk assessment. EPA-540-R-02-002. Washington, DC: EPA OSWER.
EPA. 2001c. Whitman decides to dredge Hudson River. Press Release, U.S. Environmental Protection Agency, Washington, DC, August 1.
Erickson, D. C., R. C. Loehr, and E. F. Neuhauser. 1993. PAH loss during bioremediation of manufactured gas plant site soils. Water Research 27:911-919.
Francis, A. J., and C. J. Dodge. 1998. Remediation of soils and wastes contaminated with uranium and toxic metals. Environ. Sci. Technol. 32:3993-3998.
Freeman, G. B., J. D. Johnson, J. M. Killinger, S. C. Liao, P. I. Feder, A. O. Davis, M. V. Ruby, R. L. Chaney, S. C. Lovre, and P. D. Bergstrom. 1992. Relative bioavailability of lead from mining waste soil in rats. Fund. Appl. Toxicol. 19:388-398.
Freeman, G. B., J. D. Johnson, J. M. Killinger, S. C. Liao, A. O. Davis, M. V. Ruby, R. L. Chaney, S. C. Lovre, and P. D. Bergstrom. 1993. Bioavailability of arsenic in soil impacted by smelter activities following oral administration in rabbits. Fund. Appl. Toxicol. 21:83-88.
Freeman, G. B., R. A. Schoof, M. V. Ruby, A. O. Davis, J. A. Dill, S. C. Liao, C. A. Lapin, and P. D. Bergstrom. 1995. Bioavailability of arsenic in soil and house dust impacted by smelter activities following oral administration in cynomolgus monkeys. Fund. Appl. Toxicol. 28:215-222.
Grcman, H., S. Velikonja-Bolta, D. Vodnik, B. Kos, and D. Lestan. 2001. EDTA enhanced heavy metal phytoextraction: metal accumulation, leaching and toxicity. Plant and Soil 235:105-114.
Gunderson, L. H., C. S. Holling, and S. S. Light (eds.). 1995. Barriers and bridges to the renewal of ecosystems and institutions. New York: Columbia University Press. 245pp.
Harwell, J. H., D. A. Sabatini, and R. C. Knox. 1999. Surfactants for ground water remediation. Colloids and Surfaces: Physicochemical and Engineering Aspects 151:255-268.
Hawthorne, S. B., D. G. Poppendieck, C. B. Grabanski, and R. C. Loehr. 2001. PAH release during water desorption, supercritical carbon dioxide extraction, and field bioremediation. Environ. Sci. Technol. 35:4577-4583.
Hawthorne, S. B., and C. B. Grabanski. 2000. Correlating selective supercritical fluid extraction with bioremediation behavior of PAHs in a field treatment plot. Environ. Sci. Technol. 34:4103-4110.
Huang, J. W. W., M. J. Blaylock, Y. Kapulnik, and B. D. Ensley. 1998. Phytoremediation of uranium contaminated soils: role of organic acids in triggering uranium hyperaccumulation in plants. Environ. Sci. Technol. 32:2004-2008.
Kanaly, R. A., and S. Harayama. 2000. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J. Bacteriol. 182:2059-67.
Kim, J. Y., C. Cohen, and M. L. Shuler. 2000. Use of amphiphilic polymer particles for in situ extraction of sorbed phenanthrene from a contaminated aquifer material. Environ. Sci. Technol. 34:4133-4139.
Kimbrough, R. D., H. Falk, and P. Stehr. 1984. Health implications of 2,3,7,8-tetrachloro-dibenzodioxin (TCDD) contamination of residential soil. J. Toxicol. Environ. Health 14:47-93.
Kociba, R. J., D. G. Keyes, J. E. Beyer, R. M. Carreon, C. E. Wade, D. A. Dittenber, R. P. Kalnins, L. E. Frauson, C. N. Park, S. D. Barnard, R. A. Hummel, and C. G. Humiston. 1978. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol. Appl. Pharmacol. 46:279-303.
Labieniec, P. A., D. A. Dzombak, and R. L. Siegrist. 1997. Evaluation of uncertainty in a site-specific risk assessment. J. Environ. Eng. 123:234-243.
Lee, K. 1993. Compass and gyroscope: integrating science and politics for the environment. Covelo, CA: Island Press.
Lee, K. N. 1999. Appraising adaptive management. Conservation Ecology 3(2):3.
Lenke, H., J. Warrelmann, G. Daun, K. Hund, U. Sieglen, U. Walter, and H.-J. Knackmuss. 1998. Biological treatment of TNT-contaminated soil. 2. Biologically induced immobilization of the contaminants and full-scale application. Environ. Sci. Technol. 32:1964-1971.
Liu, Z., A. M. Jacobson, and R. G. Luthy. 1995. Biodegradation of naphthalene in aqueous nonionic surfactant systems. Appl. Environ. Microbiol. 61(1):145-151.
Loehr, R., and M. T. Webster. 1997. Effect of treatment on contaminant availability, mobility, and toxicity. Pp. 138-386 In: Environmentally acceptable endpoints in soil: risk-based approach to contaminated site management based on availability of chemicals in soil . D. G. Linz et al. (eds.). Annapolis, MD: American Academy of Environmental Engineers.
Löser, C., H. Seidel, P. Hoffmann, and A. Zehnsdorf. 1999. Bioavailability of hydrocarbons during microbial remediation of a sandy soil. Applied Microbiology and Biotechnology 51:105-111.
Ma, Q. Y., S. J. Traina, T. J. Logan, and J. A Ryan. 1993. In situ lead immobilization by apatite. Environ. Sci. Technol. 27(9):1803-1810.
Ma, Q. Y., S. J. Traina, T. J. Logan, and J. A Ryan. 1994a. Effects of NO3–, Cl–, F–, SO42–, and CO32– on Pb2+ immobilization by hydroxyapatite. Environ. Sci. Technol. 28(3):408-418.
Ma, Q. Y., S. J. Traina, T. J. Logan, and J. A Ryan. 1994b. Effects of aqueous Al, Cd, Cu, Fe(II), Ni, and Zn on Pb immobilization by hydroxyapatite. Environ. Sci. Technol. 28(7):1219-1228.
Maddaloni, M. A., N. Loiacono, C. Blum, S. Chilirud, and J. Graziano. 2001. Effects of treatments on soil-lead bioavailability (human studies). Agronomy abstracts 2001. Annual meeting October 21-25, Charlotte, NC.
McConnell, E. E., G. W. Lucier, R. C. Rumbaugh, P. W. Albro, D. J. Harvan, J. R. Hass, and M. W. Harris. 1984. Dioxin in soil: bioavailability after ingestion by rats and guinea pigs. Science 223:1077–1079.
McGrath, R., and I. Singleton. 2000. Pentachlorophenol transformation in soil: a toxicological assessment. Soil Biology and Biochemistry 32:1311-1314.
Menzie, C. A., M. H. Henning, J. Cura, K. Finkelstein, J. Gentile, J. Maughan, D. Mitchell, S. Petron, B. Potocki, S. Svirsky, and P. Tyler. 1996. Special report of the Massachusetts weight-of-evidence workgroup: a weight-of-evidence approach for evaluating ecological risks. Human and Ecological Risk Assessment 2(2):277-304.
Menzie, C. A., A. M. Burke, D. Grasso, M. Harnois, B. Magee, D. McDonald, C. Montgomery, A. Nichols, J. Pignatello, B. Price, R. Price, J. Rose, J. Shatkin, B. Smets, J. Smith, and S. Svirsky. 2000. An approach for incorporating information on chemical availability in soils into risk assessment and risk-based decision making. Human and Ecological Risk Assessment 6(3):479-510.
Morgan, M. G., and M. Henrion. 1990. Uncertainty: a guide to dealing with uncertainty in quantitative policy analysis. Cambridge, UK: Cambridge University Press.
Morgan, M. G., B. Fischhoff, A. Bostrom, L. Lave, and C. J. Atman. 1992. Communicating risk to the public. Environ. Sci. Technol. 26(11):2049–2056.
NACCHO. 1995. Don’t hazard a guess: addressing community health concerns at hazardous waste sites. Washington, DC: National Association of County & City Health Officials.
National Research Council (NRC). 1996. Upstream: salmon and society in the Pacific Northwest. Washington, DC: National Academy Press.
NRC. 1997a. Innovations in soil and ground water cleanup: from concept to commercialization. Washington, DC: National Academy Press.
NRC. 1997b. Contaminated sediments in ports and waterways: cleanup strategies and technologies. Washington, DC: National Academy Press.
NRC. 1999. Downstream: adaptive management of Glen Canyon Dam and the Colorado River ecosystem. Washington, DC: National Academy Press.
NRC. 2001a. A risk management strategy for PCB-contaminated sediments. Washington, DC: National Academy Press.
NRC. 2001b. Aquifer storage and recovery in the comprehensive Everglades restoration plan: a critique of the pilot projects and related plans for ASR in the Lake Okeechobee and Western Hillsboro areas. Washington, DC: National Academy Press.
NRC. 2003. Environmental cleanup at Navy facilities: adaptive site management. Washington, DC: National Academy Press.
NEPI. 2000. Assessing the bioavailability of metals in soil for use in human health risk assessment. Washington, DC: National Environmental Policy Institute.
Nriagu, J. O. 1984. Phosphate minerals. J. O. Nriagu and P. Moore (eds.). New York: Springer Verlag.
Olivera, F. L., R. C. Loehr, B. C. Coplin, H. Eby, and M. T. Webster. 1998. Prepared bed land treatment of soils containing diesel and crude oil hydrocarbons. J. Soil Cont. 7:657-674.
Preuß, A., and P.-G. Rieger. 1995. Anaerobic transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. In: Biodegradation of nitroaromatic compounds. J. C. Spain (ed.). New York and London: Plenum Press.
Ramaswami, A., and R. G. Luthy. 1997. Measuring and modeling physicochemical limitations to bioavailability and biodegradation. Pp. 721-729 In: Manual of environmental microbiology. C. J. Hurst (ed.). Washington, DC: American Society for Microbiology.
Riefler, R. G., and B. F. Smets. 2000. Enzymatic reduction kinetics of 2,4,6-trinitrotoluene and related nitroarenes: kinetics linked to one-electron redox potentials. Environ. Sci. Technol. 34 (18):3900-3906.
Rijnaarts, H. H. M., A. Bachman, J. C. Jumelet, and A. J. B. Zehnder. 1990. Effect of desorption and intraparticle mass transfer on the aerobic mineralization of α-hexaclorocyclohexane in contaminated calcereous soil. Environ. Sci. Technol. 24:1349-1354.
Ringelberg, D. B., J. W. Talley, E. J. Perkins, S. G. Tucker, R. G. Luthy, E. J. Bouwer, and H. L. Fredrickson. 2001. Succession of phenotypic, genotypic, and metabolic community characteristics during in vitro bioslurry treatment of polycyclic aromatic hydrocarbon-contaminated sediments. Appl. Environ. Microbiol. 67:1542-50.
Roberts, S. M., W. R. Weimar, J. T. R. Vinson, J. W. Munson, and R. J. Bergeron. 2002. Measurement of arsenic bioavailability in soil using a primate model. Toxicological Sciences 67:303-310.
Romkens, P., L. Bouwman, J. Japenga, and C. Draaisma. 2002. Potentials and drawbacks of chelate-enhanced phytoremediation of soils. Environ. Pollut. 116:109-121.
Ruby, M. V., A. Davis, J. H. Kempton, J. W. Drexler, and P. D. Bergstrom. 1992. Lead bioavailability—dissolution kinetics under simulated gastric conditions. Environ. Sci. Technol. 26:1242-1248.
Ruby, M. V., S. L. Brown, K. G. Scheckel, and D. Allen. 2001. Effects of treatments on soil-lead bioavailability: implications of in vitro extraction testing. Agronomy abstracts 2001. Annual meeting October 21-25, Charlotte, NC.
Ryan, J. A., and W. R. Berti. 2001. Field evaluation of in situ treatment to reduce soil-lead bioavailability. Agronomy abstracts 2001. Annual meeting October 21-25, Charlotte, NC.
Salanitro, J. P, P. B. Dorn, M. H. Huesemann, K. O. Moore, I. A. Rhodes, L. M. Rice-Jackson, T. E. Vipond, M. M. Western, and H. L. Wisniewski. 1997. Crude oil hydrocarbon bioremediation and soil ecotoxicity assessment. Environ. Sci. Technol. 31:1769-1776.
Sandman, P. 1986. Explaining environmental risk. Washington, DC: EPA.
Sandman, P. 1996. Responding to community outrage: strategies for effective risk communication. Fairfax, VA: American Industrial Hygiene Association.
Scheckel, K. G., and J. Yang. 2001. Effect of phosphorus treatment on lead mineralogy. Agronomy abstracts 2001. Annual meeting October 21-25, Charlotte, NC.
Schlekat, C. E., B.-G. Lee, and S. N. Luoma. 2002. Dietary metals exposure and toxicity to aquatic organisms: implications for ecological risk assessment. Pp. 151-188 In: Coastal and estuarine risk assessment. M. C. Newman, M. H. Roberts Jr., and R. C. Hale (eds.). Boca Raton, FL: Lewis Publishers.
Schmidt, S. K., M. Alexander, and M. L. Shuler. 1985. Predicting threshold concentrations of organic substrates for bacterial growth. J. Theor. Biol. 114:1-8.
Shu, H., D. Paustenbach, F. J. Murray, et al. 1988. Bioavailability of soil-bound TCDD: oral bioavailability in the rat. Fund. Appl. Toxicol. 10:648–654.
Sopper, W. E. 1993. Municipal sludge use in land restoration. Boca Raton, FL: Lewis Publishers.
Steele, M. J., B. D. Beck, B. L. Murphy, and H. S. Strauss. 1990. Assessing the contribution from lead in mining wastes to blood. Reg. Toxicol. Pharm. 11:158-190.
Stroo, H. F., R. Jensen, R. C. Loehr, D. V. Nakles, A. Fairbrother, and C. B. Liban. 2000. Environmentally acceptable endpoints for PAHs at a manufactured gas plant site. Environ. Sci. Technol. 34(18):3831-3836.
Taylor, B., L. Kremsater, and R. Ellis. 1997. Adaptive management of forests in British Columbia. Victoria, BC: British Columbia Ministry of Forests, Forest Practices Branch. 93 pp.
Tiehm, A., M. Stieber, P. Werner, and F. H. Frimmel. 1997. Surfactant-enhanced mobilization and biodegradation of polycyclic aromatic hydrocarbons in manufactured gas plant soil. Environ. Sci. Technol. 31:2570-2576.
Tros, M., G. Schraa, and A. Zehnder. 1996a. Transformation of low concentrations of 3-chlorobenzoate by Pseudomonas sp. Strain B13: kinetics and residual concentrations. Appl. Environ. Microbiol. 62:437-442.
Tros, M. E., T. N. Bosma, G. Schraa, and A. J. Zehnder. 1996b. Measurement of minimum substrate concentration (Smin) in a recycling fermentor and its prediction from the kinetic parameters of Pseudomonas strain B13 from batch and chemostat cultures. Appl. Environ. Microbiol. 62:3655-61.
Umbreit, T. H., E. J. Hesse and M. A. Gallo. 1986a. Bioavailability of dioxin in soil form a 2,4,5-T manufacturing site. Science 232:497-499.
Umbreit, T. H., E. J. Hesse and M. A. Gallo. 1986b. Comparative toxicity of TCDD contaminated soil from Times Beach, Missouri, and Newark, New Jersey. Chemosphere 15:2121-2124.
Umbreit, T. H., E. J. Hesse and M. A. Gallo. 1987. Differential bioavailability of 2,3,7,8-tetrachlorodibenzo-p-dioxin from contaminated soils. ACS Symposium Series 10(338):131-139.
Umbreit, T. H., E. J. Hesse and M. A. Gallo. 1988a. Reproductive studies of C57B/6 male mice treated with TCDD-contaminated soils from 2,4,5-trichlorophenoxyacetic acid manufacturing site. Arch. Environ. Contam. Toxicol. 17:145-150.
Umbreit, T. H., E. J. Hesse and M. A. Gallo. 1988b. Bioavailability and cytochrome p-450 induction from 2,3,7,8-tetrachlorodibenzo-p-dioxin contaminated soils from Times Beach, Missouri, and Newark, New Jersey. Drug Chem. Toxicol. 11:405-418.
Walters, C. 1997. Challenges in adaptive management of riparian and coastal ecosystems. Conservation Ecology 1(2):1.
Weis, C. 2000. Presentation to the NRC Committee on Bioavailability of Contaminants in Soils and Sediments. September 13, 2000.
White, J. C., and B. D. Kottler. 2002. Citrate-mediated increase in the uptake of weathered 2,2-bis(p-chlorophenyl)1,1-dichloroethylene residues by plants. Environ. Toxicol. Chem. 21:550-556.
Yang, Y., D. Ratté, B. F. Smets, J. J. Pignatello, and D. Grasso. 2001. Mobilization of soil organic matter by metal ion complexing agents and implications for polycyclic aromatic hydrocarbon desorption. Chemosphere 43:1013-1021.
Zehnder, A. J. B. 1991. Mikrobiologische Reinigung eines kontaminierten Bodens, Beitrag des 9. Dechema-Fachgespräches Umweltschutz, Frankfurt/Main. Pages 68-72.
Zhenbin, L., J. A. Ryan, J. L. Chen, and S. R. Al-Abed. 2001. Adsorption of cadmium on biosolidsamended soils. J. Environ. Qual. 30:903-911.