2
Current Use of Bioavailability in the Management of Contaminated Soil and Sediment
Cleanup of contaminated soil and sediment in the United States follows a risk-based paradigm that takes into account individual exposure pathways linking sources to potential receptors. Typical pathways include contaminant leaching from soil to groundwater, contaminant release from sediments to overlying water, ingestion of contaminated sediments or soils, direct dermal contact with sediments or soils, inhalation of particulate matter or vapors containing contaminants, and ingestion of food items that have accumulated contaminants from soils or sediments. Risk management decisions for soils or sediments focus on identifying relevant pathways of exposure that pose a risk to human health or the environment and then developing appropriate remedial measures that could include treating or removing sources or cutting off pathways or both. Many of the exposure pathways discussed above are affected by the bioavailability processes shown in Figure 1-1. Thus, bioavailability processes are an integral part of risk assessment and risk-based management of contaminated soils and sediments, although their consideration is not always obvious or explicit.
Risk-based cleanup approaches typically are characterized by a tiered methodology, in which a screening-level step is used initially to assess site conditions and potential actions, followed by one or more levels of site-specific assessment. The states have set many guidance values for use at the screening-level step. For example, there are state and federal soil screening levels for the protection of human health (that often differentiate between residential and industrial land use), the protection of groundwater, and the protection of ecological receptors. Sediment guidelines for protection of ecological receptors are often used to guide
cleanup. Because they are initial screening levels, they are typically developed to be conservative (i.e., to overestimate most exposures). Although there is continued debate about whether they are conservative enough, it is undisputed that the development of such screening levels requires that assumptions be made about certain bioavailability processes. In most cases, this has involved selecting default conditions or parameters regarding the environmental fate of the chemical as well as how it might enter a human or an ecological receptor. Examples include default assumptions about the relative amount of chemical that is absorbed via dermal contact or incidental ingestion, or the manner and degree to which an organic compound in sediment is bound to organic carbon. For some screening levels (in particular empirical sediment guidelines) bioavailability processes have not been explicitly considered but probably play a role.
Understanding how bioavailability processes have been considered at a screening-level stage is an important first step for evaluating how site-specific information might be used to refine exposure and risk assessments and reduce the uncertainties inherent in their outcomes. In some cases, this might involve developing site-specific information for a particular process that can be inserted into a risk equation. As discussed below, there has been considerable work in generating site-specific information on association/dissociation and absorption (bioavailability processes A and D in Figure 1-1) for certain metals in animal models that are applicable to humans. Another type of refinement could involve making site-specific measurements of contaminant release from soils. Still other site-specific estimates—such as those encountered in ecological risk assessments— could involve measurements of available contaminant pools or tissue levels in organisms. This information can be used to both refine a risk assessment calculation and help develop models of bioavailability processes that can be used at other sites.
This chapter first describes human health risk assessment to illustrate how bioavailability processes are considered in that arena, followed by an overview of the use of bioavailability processes in ecological risk assessment. The two sections describe the current state of the practice but do not represent an endorsement by the committee. Finally, the chapter describes how “bioavailability” is considered within legal and regulatory frameworks. As will become clear, the legal and even regulatory view of what is meant by “bioavailability” is narrower than the processes illustrated in Figure 1-1, in that the primary focus has been on absorption (particularly systemic absorption for humans) and thus on direct contact with soils via the oral and dermal pathways. This underscores the significance of semantic issues discussed in Chapter 1. What should be clear from this chapter is that bioavailability processes are an integral part of risk-based management of contaminated sites. They may be considered either implicitly or explicitly, and they may be dealt with either by using default values in risk assessment equations or by using site-specific data and information.
USE OF BIOAVAILABILITY IN RISK ASSESSMENT
Because bioavailability processes influence exposure of humans and ecological receptors to chemicals in soils and sediments, and because exposure is one aspect of risk assessment, measuring or modeling bioavailability is consistent with prevailing U.S. Environmental Protection Agency (EPA) and state risk assessment paradigms. The general framework used by EPA for human health risk assessments has four major components derived from NRC (1983):
-
Hazard Identification is a systematic planning stage that identifies the major factors considered in the assessment and establishes its goals, breadth, and focus. It is essentially a scoping activity and is fundamental to the success of all subsequent components in the risk assessment. It consists of stating the objectives, developing the conceptual model, selecting and characterizing receptors, and identifying the endpoints of the assessment.
-
Exposure Assessment estimates the magnitude of actual or potential human or ecological exposure to a contaminant of concern, the frequency and duration of exposure, and the pathways of exposure. Incorporation of bioavailability information often influences estimates of exposure.
-
Dose-Response Assessment is “the process of characterizing the relation between the dose of an agent administered or received and the incidence of an adverse health effect.” This step estimates the probability that an individual will be adversely affected by a given chemical dose, relying primarily on data obtained from animal studies. Information on bioavailability processes may influence measures of toxicity and other effects.
-
Risk Characterization integrates the exposure assessment and dose-response assessment into a quantitative and qualitative expression of risk. This may include deterministic calculations, probabilistic methods, and professional judgement using various lines of evidence.
These four steps are similar in ecological risk assessment, with the following differences (EPA, 1992a; NRC, 1993). The first step is termed problem formulation, which determines the focus and scope of the assessment. Hazard identification and dose–response assessment are combined into an ecological effects assessment phase. And finally, dose–response is replaced with stressor–response to emphasize that physical changes make cause harm to ecosystems as well as chemicals (although for the purposes of this report, the focus is on chemical contaminants).
Although bioavailability processes can be considered explicitly in both human health and ecological risk assessments, there are some important differences. Unlike human health risk assessment, assessments of exposure and risk to ecological receptors consider various species ranging from invertebrates and plants to fish and wildlife. Some of these species are in intimate contact with soils

Both direct exposure via soil ingestion and indirect exposure via fish consumption are affected by contaminant bioavailability. Human health risk assessment often quantifies direct ingestion of soil (top photo), while ecological risk assessment frequently considers bioaccumulation of contaminants in animal tissues (bottom photo).
or sediments. Many are also exposed to contaminants exchanged from soils or sediments to the dissolved phase or through eating organisms that have accumulated contaminants from these media. Therefore, there are many exposure pathways and a larger number of bioavailability processes that may require simultaneous evaluation during ecological risk assessment as compared to human health risk assessment, where it is more feasible to evaluate one pathway at a time. A manifestation of this difference is that human health risk assessment often involves distinct exposure equations for the direct pathways of ingestion, dermal contact, and inhalation, within which a variable is included to account for absolute or relative bioavailability. This discrete consideration of bioavailability for individual exposure media and exposure routes is driven by the fact that human exposures can often be separated in time and space. For example, vegetables may be grown in a different section of a garden from where children play, and not all receptors have gardens. In contrast, in ecological risk assessment, at least for many receptors there is obligatory simultaneous exposure via multiple pathways and routes. Thus, ecological risk assessments include equations for some of the direct exposure pathways for wildlife (although this knowledge is not well-developed for most species) as well as many other types of measures and exposure models that differ from what is commonly employed in human health assessments. For ecological risk assessment, it is often not be possible to quantify bioavailability processes associated with each of these pathways separately, which is a primary reason for focusing on measures of bioaccumulation as an overall indicator of bioavailability.
A second important factor to consider is the acceptability of making measurements on organisms such as earthworms, plants, fish, and wildlife compared to humans. As described in Chapter 4, such measurements include toxicity tests as well as uptake or accumulation tests (determination of tissue residues of contaminants)—tests that for ethical reasons cannot be conducted in humans. Thus, there are more tools for quantifying bioavailability processes and the sum of multiple exposure routes using the actual receptor of interest during ecological risk assessment. This is not the case in human health risk assessment, where greater reliance is placed on default values and where it can be difficult to modify defaults on a site-specific basis.
Regardless of whether humans or ecological receptors are the concern at a particular site, some general criteria are useful when attempting to more explicitly consider bioavailability processes during risk assessment (Menzie et al., 2000). First, it is imperative to determine (as best as possible) the usefulness of incorporating new information on bioavailability in terms of altered outcomes at a site. Chapter 5 discusses the chemical and environmental settings for which bioavailability assessments are most likely to make a difference in site management. Second, a conceptual model of exposure for the site is critical to any bioavailability assessment. Because it is known that soils and sediments can alter contaminant bioavailability, relevant soil factors should be identified early. Fi-
nally, data on bioavailability processes should be collected using measures or models that are compatible with the risk assessment and risk management framework being used at the site.
Human Health Risk Assessment
In most situations, a quantitative assessment of risk to humans from exposure to contaminants in soils or sediments involves a comparison of the estimated magnitude of exposure with the measured toxicity of the chemical(s) in question. Bioavailability processes play a variety of important roles in these risk calculations. Although risk calculations for contaminated soils and sediments can sometimes be complex, there are three fundamental types of inputs: (1) the concentration of the chemical in soil or sediment at the point of contact with the individual, (2) variables related to the nature and extent of exposure (e.g., exposure frequency, amount of soil ingested, body weight), and (3) toxicity values for the chemical. Bioavailability processes can be reflected in all three types of inputs.
Soil concentration: Bioavailability processes A, B, and C in Figure 1-1 can influence the concentration of chemical reaching the exposed individual from its point of release or residence in the environment. Typically, these bioavailability processes are addressed either through direct measurement of soil concentration at the point of contact or through environmental fate and transport modeling.
Exposure variables: Numerical adjustments to account for bioavailability processes related to entry of soil or sediment contaminants into the body are typically included among the exposure variables. This is the usual means by which “bioavailability adjustments” are made in human health risk calculations. Clearly, the primary focus here is on bioavailability processes A and D (association/dissociation and absorption or uptake across a membrane) and to a lesser extent process E if systemic circulation is a measured endpoint.
Toxicity values: Toxic potency estimates are based on one or more critical studies which offer information on the relationship between dose of the chemical and toxic effects. Most toxicity values, in the form of cancer potency estimates or acceptable daily intake rates, are based on applied rather than absorbed doses. As a result, the toxicity value is a function, in part, of the rate and extent of absorption that occurred in the critical study. This bioavailability process—the absorption of the chemical into the body in the critical toxicity study—must be kept in mind when using toxicity values.
Human contact with contaminants in soils or sediments can occur through three direct routes of exposure: incidental ingestion, dermal contact, or inhalation of soil-derived particulates (dusts) or chemicals volatilized from soil. All three routes are usually relevant for human exposure to soils, while ingestion and dermal contact are the most likely exposure routes for sediments (see Figure 2-1).
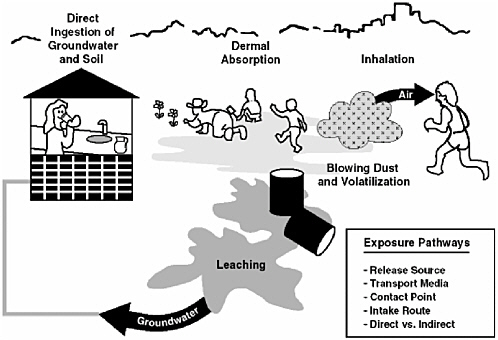
FIGURE 2-1 Major Exposure Pathways for Human Exposure to Contaminated Soils and Sediments. SOURCE: EPA Region 9 Preliminary Remediation Goals website (www.epa.gov/region09/waste/sfund/prg).
In addition to these three routes, there are other indirect pathways by which contaminants in soil and sediment can reach human receptors, notably leaching to groundwater and subsequent ingestion of well water. These routes of exposure are considered below, using contaminated soil (rather than sediment) as an example.
Incidental Ingestion
Incidental ingestion is often an important exposure route for contaminated soils in human health risk assessments. In its basic form, the intake equation for incidental ingestion of soils is:
where:
Cs = chemical concentration in the soil at the point of contact
IR = incidental ingestion rate of soil
RAF = relative absorption factor
BW = body weight
EF = exposure frequency
ED = exposure duration
AT = period over which exposure will be averaged.
The chemical concentration in soil, soil ingestion rate, and body weight are used to determine the ingestion rate for the chemical per unit body weight. The exposure frequency, exposure duration, and averaging time are used to account for periods when exposure does not occur, and to develop an average intake over time. A correction for relative bioavailability can be introduced in the form of a Relative Absorption Factor (RAF). Usually, the RAF is expressed as a ratio:
where Fs is the fraction of the dose of chemical absorbed from soil under circumstances of environmental exposure, and Fsm is the fraction of the dose absorbed from the study medium (e.g., food, water, or some liquid vehicle) used in the critical study upon which the toxicity value is based. The RAF may be an estimated or measured factor, and can be less than or greater than 1.0 (100 percent). If the absorption from soil is found or assumed to be the same as absorption in the critical study upon which the toxicity value is based, then the RAF is 1.0. Note that a RAF of 1.0 does not indicate that absorption is complete, but simply that absorption is known or estimated to be the same as that in the critical study. It is not uncommon for an ingestion intake equation to lack a RAF term. This simply means that the relative bioavailability is assumed to be 1.0.
Under some circumstances, the oral toxicity value might be expressed as an internal dose. In this situation, the RAF would be replaced by a term for absolute bioavailability from soil in order to permit an internal dose to be calculated for comparison.
Dermal Contact
A general form of the equation used to calculate the internal (absorbed) dose from dermal exposure to soil is:
where:
Cs = chemical concentration in soil on the skin
SA = skin surface area
AF = soil adherence factor (how much soil covers a unit area of skin)
ABS = absorption factor from the soil into the body
BW = body weight
EF = exposure frequency
ED = exposure duration
AT = period over which exposure will be averaged
The soil concentration, surface area, adherence factor, and body weight terms allow calculation of an amount of chemical present on the skin per unit body weight. As with exposure by ingestion, the exposure frequency, exposure duration, and averaging time terms are present to allow determination of an average exposure rate over time. Usually, the absorption factor (ABS) is intended to reflect the absolute bioavailability of the compound from soil via the dermal route (dermal bioavailability) and is used to calculate the absorbed, or internal, dose of the chemical expected to result from dermal contact. Data on dermal bioavailability from soil are extremely limited or absent for most chemicals, although default assumptions have been specified by EPA and state agencies (see later discussion).
Once the intake has been determined from the equation above, it is compared with a suitable toxicity value for dermal exposure. Unfortunately, there are very few toxicity values available specifically for dermal exposure. Instead, if the toxicity is systemic in nature (i.e., doesn’t occur through direct interaction with the skin) the applied-dose toxicity value from another route is converted to an internal-dose value in order to assess risks from dermal contact—a process known as route-to-route extrapolation. This requires knowledge or an assumption regarding the extent of absorption associated with the toxicity value. For example, an oral cancer potency value for a chemical based on a dietary study in laboratory animals could be converted to an internal dose equivalent for use in assessing risks from a chemical entering through the skin. This adjustment in the oral toxicity value would require some knowledge of the gastrointestinal absorption of the chemical in the critical study upon which the oral cancer potency estimate was derived. For cancer potency factors (such as EPA cancer slope factors), the adjustment is made by dividing the oral toxicity value by the known or inferred absolute bioavailability of the chemical from the gut in the critical cancer study. Thus, risks from dermal exposure commonly must rely on estimates of both dermal and oral absolute bioavailability of a chemical, with little supporting data for either.
An alternative approach is to compare dermal intake with an oral or inhalation toxicity value without adjustment of the toxicity value to an internal dose form. If this approach is used, the ABS term has a different meaning. Instead of representing the absolute bioavailability of the chemical through the skin, ABS is instead a relative bioavailability term, in this case quantifying the expected difference in absorption from the dermal route versus the absorption implicit in the toxicity value. If the toxicity value for comparison is based on the oral route, then the comparison point is the gastrointestinal absorption of the chemical in the
critical oral toxicity study. The example shown in Box 2-1 uses this approach. Similarly, if an inhalation toxicity value is used to assess dermal risks, then the ABS value will be based upon differences in dermal versus inhalation exposure to the chemical. Rarely are experiments conducted to generate these ABS numbers; rather they are the products of best professional judgment.
Inhalation
Calculating exposure from inhalation of contaminants from soils can be accomplished by measuring or estimating the associated concentration of the chemical in air. A simple form of inhalation intake equation is:
where:
Ca = chemical concentration in inspired air
INR = inhalation rate
BW = body weight
EF = exposure frequency
ED = exposure duration
AT = period over which exposure will be averaged
This equation calculates the average amount of chemical entering the respiratory tract per unit time and per unit body weight over a specified exposure interval. This intake value is in the form of an applied dose, and is analogous to chemicals entering the gastrointestinal tract after ingestion or coming in contact with the skin during dermal exposure. For exposure to chemicals in soils, the inhalation intake equation often uses the soil concentration and incorporates a model to calculate the corresponding air concentration of the chemical. This model can be viewed as representing the bioavailability processes that make a chemical in soil accessible to its site of entry into the body, which in this case is the lungs.
As with ingestion, risks from inhalation exposure are typically assessed through the use of estimates of applied doses resulting from exposure and of toxicity values based on applied doses. Unlike ingestion, however, both the doses and the toxicity values are often expressed in terms of concentration in air, rather than an amount of chemical per unit body weight. For example, a toxicity value for non-cancer health effects by inhalation exposure may be simply a safe concentration limit for the chemical in air. For estimating cancer risks from inhalation exposure, cancer potency can be expressed in reciprocal concentration terms, such that multiplication with the exposure concentration in air yields an excess cancer risk estimate (e.g., EPA inhalation unit risk values). In theory, if differ-
ences in pulmonary bioavailability are known to exist between the exposure situation and the critical study used to develop the inhalation toxicity value, this can be addressed through the use of a relative bioavailability or RAF term, as with exposure by ingestion. However, there are few obvious examples of situations where such an adjustment is required, and consequently it is rare in risk assessments. Instead, the implicit assumption is that the relative bioavailability associated with environmental exposure is 100 percent—that is, the pulmonary absorption of the chemical under environmental exposure conditions is equivalent to the pulmonary absorption that existed in the critical study used to derive the inhalation toxicity value.
Leaching to Groundwater
Leaching from soil to groundwater is another common pathway by which humans can be exposed to contaminants (see Figure 2-2). The calculation requires an estimate of the contaminant concentration in the infiltrating water and a determination of the dilution by mixing with underlying groundwater. Estimation of a soil concentration that will be “protective” of groundwater is achieved by working backward from the desired water concentration at the groundwater well (usually a water quality standard), via the dilution attenuation factor (DAF). The following equation for DAF is meant to account for the dilution by mixing with underlying groundwater:
where Qgw is groundwater discharge per unit aquifer thickness over the mixing depth in the aquifer (d); Ql is the leaching recharge [L3L−2T–1]. The Qgw depends upon the aquifer hydraulic conductivity (K), hydraulic gradient (i) and mixing depth (d). The Ql depends upon the area covered by the contaminated soil (L) and infiltration rate (I).
The protective soil concentration for this pathway, Cs, is estimated by assuming equilibrium partitioning between the soil- and aqueous-phase contaminant concentrations in the soil pore water using the following equation:
where Cw is the water quality standard at the receptor (such as a maximum contaminant level or MCL); Kd is the sorption distribution coefficient for the contaminant; θw and θa are the volumetric air and water contents, ρb is the soil bulk density, and H′ is the dimensionless form of the Henry’s law constant or partitioning coefficient between the air and water phases at a specified temperature. Cs is then compared to the levels of soil contamination at a specific site to determine what actions should be taken next. Unlike the previous three pathways
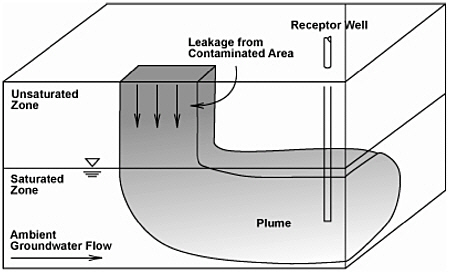
FIGURE 2-2 Conceptual View of the Leaching to Groundwater Pathway.
SOURCE: EPA (1996a).
described above, there is no explicit exposure intake equation used for the leaching to groundwater pathway. Rather, the intake equation—including dose, toxicity, and relative absorption values for ingestion of contaminated groundwater—is reflected in the water quality standard for the contaminant (Cw). For all practical purposes, the relative absorption factor for ingestion of contaminated water is assumed to be 100 percent.
Assumptions and Default Values
Direct Contact Pathways. Commonly, assessment of risks from direct contact with a soil chemical involves an evaluation of its intake from ingestion, dermal contact, and inhalation. As the preceding discussion indicates, this entails the need to make several assumptions regarding the absorption of the chemical by the various routes under different sets of conditions. Box 2-1 provides an example of these many assumptions that were made during the development of the soil cleanup criterion for the pesticide chlordane. It should be noted that assumptions also must be made about bioavailability processes A–C that lead to the chemical concentration used in the three intake equations, but these assumptions are not discussed here.
Data on the absorption of chemicals under conditions of environmental exposure are extremely limited. Also, information on absorption implicit in the toxicity values used in the calculations is required for determining absolute bioavailability. Unfortunately, the extent of absorption of a chemical that occurred as part of a critical toxicity study is almost never measured. Instead, the
|
BOX 2-1 Implicit Assumptions Regarding Bioavailability in Human Health Risk Assessments: Soil Cleanup Goals for the Pesticide Chlordane Estimation of risks to humans from direct contact with contaminated soils requires several types of bioavailability assumptions, most of which are obscure to all but those familiar with the detailed mechanics of risk calculations. To illustrate “hidden” bioavailability assumptions, derivation of a risk-based soil cleanup goal for chlordane is used as an example. The procedure used to calculate chlordane soil cleanup goals and thus Preliminary Remediation Goals (PRGs) by EPA Region 9 is considered for this example, although the formula and assumptions vary among different regulatory agencies. A PRG is a soil concentration thought to correspond to a specified risk level, given a set of default assumptions about the extent of exposure to soil. The PRG for chlordane in soil in industrial settings, based on a 10–6 excess cancer risk, is 11 mg/kg soil. Since chlordane is regarded as a carcinogen, the Region 9 PRG equation for direct exposure to carcinogens was used to develop this number. The equation for an industrial exposure scenario is: 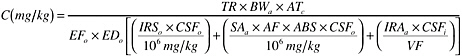 where: TR is the Target Risk (in this case, an excess cancer risk of 1 × 10–6) BWa is Body Weight for an adult worker ATc is Averaging Time, the total period over which exposure is averaged EFo is the Exposure Frequency EDo is the Exposure Duration IRSo is the incidental Soil Ingestion Rate for a worker SAa is the exposed skin Surface Area AF is the soil Adherence Factor, or the extent of soil loading on exposed skin ABS is the Absorption factor for skin, or the dermal bioavailability of the chemical VF is the Volatilization Factor, which is used to estimate the air concentration resulting from volatilization of the chemical from soil IRAa is the Inhalation Rate CSFo is the oral Cancer Slope Factor, a measure of cancer potency for oral exposure CSFi is the inhalation Cancer Slope Factor, a measure of cancer potency for inhalation exposure. This equation includes terms for intake resulting from incidental ingestion of soil, dermal contact with soil, and inhalation of chemical volatilized from soil. As the equation illustrates, development of an acceptable risk-based concentration for soil requires specific assumptions regarding several exposure parameters, including the exposure frequency, exposure duration, body weight, and incidental soil ingestion rate. With respect to bioavailability, a term for absorption of chemical through the skin, ABS, is specified; |
|
however, there are a number of other bioavailability assumptions that are implicit in the calculation. To recognize these, it is important to understand the nature of the toxicity values—in this case the cancer slope factors—and how they are used in the equation. To estimate cancer risk from chlordane, two cancer potency estimates (i.e., Cancer Slope Factors) are available from the EPA—one for oral exposure and one for inhalation exposure. The oral cancer slope factor is derived from a study in which mice fed chlordane in the diet developed liver tumors. No attempt was made to estimate the dose of chlordane absorbed by these animals, and the cancer slope factor is instead based simply on measurements of the amount of chlordane ingested daily. This is, therefore, an applied-dose toxicity value. Unless some adjustment is made, use of this cancer slope factor to estimate risks from ingestion of chlordane from soil or sediment assumes that the absorption from these media is the same as from food in the mouse cancer study. That is, the relative bioavailability is assumed to be 100 percent. If this assumption can be demonstrated to be incorrect, and the difference in absorption following ingestion from these two different sets of oral exposures can be quantified, a RAF can be introduced into the ingestion portion of the equation to correct for this. However, in this particular example, RAF is not in the equation, and it is consequently not obvious that a relative bioavailability of 100 percent is being assumed for the ingestion route of exposure. No cancer data from inhalation exposure to chlordane are available, and so EPA uses cancer potency information from oral exposure to derive an inhalation cancer potency estimate. By using the same cancer potency estimate, without adjustment, for both routes of exposure, it is assumed that bioavailability from both routes is equivalent—that the relative bioavailability for inhalation versus ingestion exposure is 100 percent. Stated more precisely, it is assumed that the absorption of volatilized chlordane from the lungs is the same as the absorption of ingested chlordane from food in the critical oral cancer study. This assumption is discussed by EPA in technical support documentation for these cancer slope factors (Toxicological Review of Chlordane [Technical], EPA, 1997a), and data are presented in support of it. However, without investigating the basis for the chlordane inhalation cancer slope factor, the bioavailability assumption associated with this toxicity value would not be evident. If the relative bioavailability were something other than 100 percent, this could again be addressed by including a RAF term in the inhalation portion of the equation, although this is seldom done. EPA does not produce toxicity values specific for the dermal route of exposure. As a consequence, toxicity values from oral or inhalation exposure must be adapted or utilized to address the contribution of dermal absorption to total risk from a chemical. In the equation above, the dermal component of the equation relies upon the oral cancer slope factor to estimate risks from dermal absorption. Here there is an explicit term for dermal bioavailability, ABS. What may not be clear to some is what this bioavailability term represents. Since it is used in conjunction with the oral cancer slope factor, which is an applied dose toxicity value, this value represents the relative bioavailability of chlordane from soil on the skin versus chlordane in the gut from food. Another approach that is commonly used in estimating risks from dermal absorption is to create a dermal cancer slope factor from either the oral or inhalation cancer slope factor. In order to do this, an |
|
internal dose version of one of these applied dose toxicity values must be derived. For chlordane, the oral cancer slope factor would be divided by its implicit absolute oral bioavailability; that is, the absolute bioavailability of chlordane from food in the gut. This internal dose version of the oral cancer slope factor could then be used with an estimate of the internal dose resulting from dermal exposure. Calculating the internal dose from dermal exposure would involve estimates of the amount of chlordane in soil on the surface of the skin and the absolute dermal bioavailability of that chlordane. Thus, as-sessment of risk can involve either an estimate of the relative bioavailability of chlor-dane by the dermal (versus oral) route, or separate estimates of both the absolute bioavailability of chlordane from soil through the skin and the absolute bioavailability of |
extent of absorption must be inferred from absorption studies that may not duplicate well the conditions of the toxicity study. Overall, the situation is usually one in which a great deal of information is needed on bioavailability processes related to absorption, but almost no data exist specific to the exposure or toxicity study conditions of interest. These limitations are overcome to a large extent by conducting relative bioavailability studies at specific sites instead of attempting to determine absolute bioavailability.
Nonetheless, the paucity of absorption data, and the expense and difficulty associated with doing site-specific studies of relative bioavailability (see Chapter 4), have led to extensive use of simplifying or default assumptions regarding chemical absorption in human health risk assessments. Regulatory agencies have not discouraged this and, as a practical matter, often specify the defaults they regard as acceptable. The most prominent default assumption imposed is this: relative bioavailability is assumed to be 100 percent unless there is compelling evidence to the contrary and a scientifically defensible adjustment factor can be derived.1 Criteria as to what constitutes an acceptable scientific basis to choose a RAF other than 1.0 have not been clearly articulated by regulatory agencies. As a result, the burden of proof required to depart from a default assumption of 100 percent relative bioavailability is poorly defined.
A default relative bioavailability assumption of 100 percent is often described as conservative. Occasionally this arises from a misconception that com-
plete absorption is being assumed. There is certainly a reason to suspect that an assumption of 100 percent relative bioavailability is conservative in many instances, simply because most toxicity tests use forms of a chemical that tend to be readily absorbed. However, this is not always the case, and treatment with the chemical in diet, for example, may represent sub-optimal conditions for absorption. Under these circumstances, it is possible that exposure to the chemical in an environmental medium may entail greater absorption than during the critical toxicity study. In this situation, an assumption of 100 percent relative bioavailability will underpredict the toxic potential of the exposure.
As discussed above, there are many situations in which information on absolute bioavailability is needed. Examples include the extent of dermal absorption of a chemical for estimating intake by the dermal route, and the extent of gastrointestinal absorption of a chemical to convert an applied-dose oral toxicity value to its corresponding internal dose form. To facilitate locating absolute bioavailability information for various chemicals, compendia are available (see EPA, 2001a; Oak Ridge National Laboratory at http://risk.lsd.ornl.gov/cgi-bin/tox/TOX_select?select=nra). In many instances, the absolute bioavailability values represent chemical-specific information derived from studies with varying degrees of similarity to the conditions of interest. For example, information on the absorption of a chemical from diet might be sought in order to develop an internal-dose form of an oral toxicity value, but the only data available may be for oral absorption of the chemical from water.
There are several other sources of uncertainty associated with this absolute bioavailability information. For example, EPA has recommended absolute bioavailability values for the dermal absorption of 92 organic and six inorganic chemicals from soil (EPA, 2001a). Each value is from a study in which dermal absorption from soil was measured, but the number of soil samples examined was limited. Often these studies used uncontaminated soils to which the chemical of
|
chlordane from the gut. In the case of chlordane, and in fact for most contaminants, hard data on these bioavailability values are absent, and professional judgement must be used to generate estimates. As shown in this example, when assessing risks to humans from contact with contaminated soils or sediments, each route of exposure requires at least one, and sometimes two or more assumptions regarding bioavailability. Most formulas for calculating risks do not include terms by which all of these assumptions are clearly shown. Even when a bioavailability term is present, the meaning is sometimes not obvious, that is, whether it is intended to represent relative or absolute bioavailability. As a result, the bioavailability assumptions incorporated into risk estimates are often obscure. |
interest was added, with or without subsequent aging. Dermal absorption of chemicals from soil could conceivably vary with soil type and with interactions between the chemical and soil. Consequently, even though the default values are based on simulated environmental exposure conditions, there is uncertainty regarding the extent to which these values are applicable to soils at contaminated sites.
For many chemicals, there is essentially no information on absolute bioavailability. For these chemicals, crude default assumptions are used based on simple chemical classifications. For example, in the absence of chemical-specific data, EPA Region 4 recommends an oral absolute bioavailability of 80 percent for volatile organic compounds, 50 percent for semi-volatile compounds, and 20 percent for inorganics. For dermal absorption of chemicals from soils, when chemical-specific data are not available, a default absolute bioavailability of 1 percent for organics and 0.1 percent for inorganics is recommended (EPA Region 4, 2000). Table 2-1 lists default absolute and relative bioavailability values for dermal and oral routes, respectively, used by EPA and the states.
The use of national default values for relative and absolute bioavailability and standardized exposure models has been most thoroughly developed for lead-contaminated sites. As mentioned in Chapter 1, mining sites were some of the first to receive attention as sites where the total amount of contaminant present may not be the best indicator of the actual human health risk. As explained in Box 2-2, EPA has developed an exposure model for lead contamination by direct contact (the Integrated Exposure Uptake Biokinetic or IEUBK Model) that focuses on the most sensitive receptor—children. It incorporates a value for the relative bioavailability of lead from soil of 60 percent (EPA, 1999a, 2001b). This value was then used to derive a national default value for absolute bioavailability of soil lead to children of 30 percent.
The IEUBK model allows for the use of more refined relative bioavailability values derived from site-specific data and information if they are available. This is actually an important feature of the model, because it has been shown that the relative bioavailability of lead in soil can vary by as much as two orders of magnitude with soil type. This variability is evident in Figure 2-3, which shows the results of 19 swine feeding studies on different soils contaminated with lead. Thus, despite having a national default value of 30 percent absolute bioavailability, there are clearly limitations with using this value in many circumstances. This underscores the limitations of default values for bioavailability processes in general. Indeed, it is because of the substantial variability with soil type observed in these studies that a significant portion of Chapter 3 is devoted to better understanding solid–contaminant interactions.
Leaching-to-Groundwater Pathway. Assumptions are also made with regard to the groundwater leaching pathway. Most important perhaps are the assumptions implicit in the MCL or water quality standard used to determine
TABLE 2-1 Examples of Default Values Used to Adjust Exposures to Account for Reduced Bioavailability of Compounds in Soil
|
Chemical |
Dermal Absorption Factor (ABS)a [source] |
Oral Relative Absorption Factor (RAF)b [source] |
|
Benzene |
0.08 [1], 0.0005 [2] |
1.0 [1] |
|
Ethylbenzene |
0.2 [1], 0.03 [2] |
1.0 [1] |
|
Toluene |
0.12 [1], 0.03 [2] |
1.0 [1] |
|
Xylenes |
0.12 [1], 0.03 [2] |
1.0 [1] |
|
Volatile organic compounds |
0.1 [5], 0.25 [6] |
1.0 [5] |
|
n-Hexane (for TPH) |
0.5 [1] |
0.91 [1] |
|
Nonane (for TPH) |
0.2 [1] |
0.91 [1] |
|
Eicosane (for TPH) |
0.1 [1] |
0.91 [1] |
|
Pyrene |
0.18 [1], 0.1 [2] |
0.91 [1] |
|
Acenaphthene |
0.2 [1], 0.1 [2] |
1.0 [1] |
|
Anthracene |
0.29 [1], 0.1 [2] |
0.91 [1] |
|
Benzo (ghi)perylene |
0.18 [1], 0.1 [2] |
0.91 [1] |
|
Flouranthene |
0.2 [1], 0.1 [2] |
1.0 [1] |
|
Fluorene |
0.2 [1], 0.1 [2] |
1.0 [1] |
|
1-Methylnaphthalene |
0.1 [1], 0.1 [2] |
1.0 [1] |
|
2-Methylnaphthalene |
0.1 [1], 0.1 [2] |
1.0 [1] |
|
Naphthalene |
0.1 [1], 0.1 [2] |
1.0 [1] |
|
Phenanthrene |
0.18 [1], 0.1 [2] |
0.91 [1] |
|
Benzo(a)anthracene |
0.18 [1], 0.1 [2] |
0.91 [1] |
|
Benzo(a)pyrene |
0.18 [1], 0.1 [2] |
0.91 [1] |
|
Benzo(b)fluoranthene |
0.18 [1], 0.1 [2] |
0.91 [1] |
|
Dibenz(a,h)anthracene |
0.08 [1], 0.1 [2] |
0.91 [1] |
|
Indeno(123,cd)pyrene |
0.2 [1], 0.1 [2] |
0.91 [1] |
|
Polycyclic Aromatic |
0.15 [3], 0.05 [4], 0.01 |
0.5 for SVOCS [5] |
|
Hydrocarbons |
[5], 0.13 [9] (0.1 for SVOCS [6, 9]) |
|
|
Lindane |
0.04 [9] |
|
|
2,4-D |
0.05 [9] |
|
|
Chlordane |
0.04 [7, 9] |
|
|
PCB Aroclors 1254 and 1242 |
0.14 [7, 9] |
0.5 [5] |
|
DDT |
0.03 [7, 9] |
0.5 [5] |
|
Pentachlorophenol |
0.25 [7, 9] |
|
|
Dioxins |
0.03 or 0.001 if OC >10% [9] |
|
|
Arsenic |
0.03 [7, 9] |
0.5 [5] |
|
Cadmium |
0.1 [7], 0.001 [9] |
0.5 [5] |
|
Leadc |
0.3 [10], 0.12 [11]c |
|
|
Inorganics |
0.01 (qualitative screen only) [8] |
0.5 [5] |
|
aABS equals the absolute bioavailability of the compound in soil via the dermal route. bRAF equals the relative bioavailability of the compound (i.e., in soil vs. in the medium used in the toxicity study). cValues for lead are absolute bioavailability. SOURCES: 1. Massachusetts Department of Environmental Protection (1992). 2. EPA Region 3 (1998). 3. California Environmental Protection Agency (1993) 4. Illinois Environmental Protection Agency (1996). 5. Michigan Department of Environmental Quality (personal communication). 6. Ohio Department of Commerce (1992). 7. Wester et al. (1990); Wester and Maibach (1996). 8. Used by U.S. Environmental Protection Agency Region 1 (EPA Region 3, 1998). 9. EPA (2001a). 10. Value used for children in the EPA IEUBK Model (EPA, 1999a, 2001b). 11. Value used for adults in the EPA adult lead model prepared by the Technical Working Group (EPA, 1996b). |
||
whether the water source poses an unacceptable risk to human health, which are similar to the assumptions discussed above regarding absorption and toxicity and thus are not discussed further here. In addition, there are numerous assumptions that go into the equations for determining the protective soil concentration, as discussed in greater detail in Box 2-3. One of the most common assumptions is that there is no dilution of the contaminant in groundwater as it travels from the source to the point of contact with humans. Partly because of this assumption, the leaching-to-groundwater pathway has been found to be the most sensitive exposure pathway for 86 of the 110 contaminants considered by EPA in setting soil screening levels (EPA, 1996a).
***
In summary, bioavailability processes are important in assessing risks to humans from both direct contact with soils and sediments and leaching of soil and sediment contaminants to water. The term “bioavailability,” when used in a human health risk assessment context, generally refers to the relative or absolute absorption of the chemical from either ingestion, dermal, or inhalation exposure. Calculating risks from direct contact with contaminated soils or sediments typi-
|
BOX 2-2 Absolute Bioavailability of Lead in Soil: The Integrated Exposure Uptake Biokinetic Model National risk assessment guidance for lead is based on information that has been developed on the behavior of this metal in the gastrointestinal system, blood, and other organs. Lead is a compound for which there is a great deal of toxicological data. The disposition of lead is fairly well understood, as are the target organs, effects, and to some extent the mechanism by which lead exerts its adverse effects. Although lead has been shown to affect every system in the body, the most sensitive target organs are the nervous system in young children, the hematopoietic system, and the cardiovascular system—with the nervous system being by far the most sensitive. For estimating child exposure to lead, EPA developed the IEUBK model, a pharmacokinetic model that takes into account multi-media exposures of young children (less than six months to six years old). This population is the most sensitive to the effects of lead, due in part to physiological conditions (e.g., efficient absorption and developing nervous system/blood brain barrier) and to behavioral conditions (e.g., hand-to-mouth contact and frequent ingestion of soils). The output of the IEUBK model is a predicted distribution of blood lead levels in children. From this distribution, the model calculates the probability that blood lead concentrations will exceed 10 mg lead per deciliter of blood (Centers for Disease Control, 1991). The specifics of the IEUBK model are given in EPA (1994a). The IEUBK model can evaluate residential exposures to lead in soil, indoor dust derived from soil, ambient air, drinking water, and food. It does not evaluate exposures via inhalation of fugitive dust derived from soil. Dust exposures in the model are via ingestion of indoor dust derived, at least in part, from soil. Since dermal absorption of lead is very low (< 0.3 percent), this pathway is typically not evaluated. The model is implemented using an EPA software program. The model includes two values for lead bioavailability in soils for incidental ingestion in children. The first is the relative bioavailability of lead from soil as compared to other exposure media (60 percent is recommended by EPA—EPA, 1999a, 2001b). This value is independent of the age of the subject. The second is the absolute bioavailability of lead in children (i.e., the amount of ingested lead that is subsequently absorbed through the gut). Because absorption is efficient in children, this value is quite high—50 percent. Combining the two factors yields an absolute bioavailability of 30 percent for lead in soil ingested by children, which is the national default value. These factors can and have been modified on a case by case basis when data from feeding studies or appropriate extraction measurements are available for site-specific soils. The approach currently used to assess exposures of lead in soils to adults is the adult model described in EPA (1996b) and referred to as the EPA Technical Review Workgroup (TRW) Model. This is a biokinetic model that estimates uptake of lead ingested incidentally with soil. Like the IEUBK model, the TRW model also includes a value for the relative bioavailability of soil lead in the digestive track of adults, which presumably could be modified based on feeding studies and extraction studies performed on site-specific soils. The relative bioavailability of lead from soil (relative to lead in water) is assumed to be 60 percent. Because the absorption of lead from water into adults is assumed to be 20 percent, this equates to an absolute bioavailability of 12 percent for lead in soil ingested by adults. |
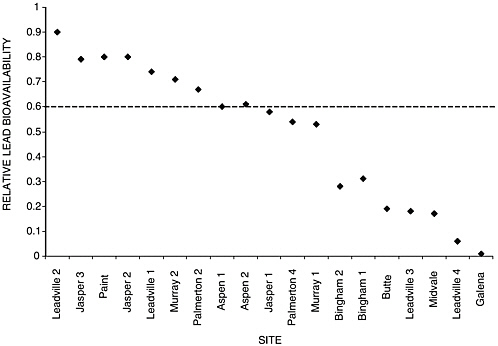
FIGURE 2-3 Swine feeding studies using 17 field soils contaminated with lead and two laboratory prepared soils (paint in soil and galena in soil). The dashed line represents the 60 percent relative bioavailability used to set the national default value for absolute bioavailability of lead in soil used by EPA. SOURCE: Reprinted, with permission, from Ruby et al. (1999). © (1999) American Chemical Society.
cally requires several bioavailability assumptions, many of which are not readily apparent. Currently, default assumptions are used extensively, although the opportunity exists to refine risk assessments by incorporating site-specific bioavailability process information using approaches described in Chapter 4.
Ecological Risk Assessment
Ecological risk assessment involves more complexity than human health risk assessment because of the types of species, physiologies, and physical/chemical processes that must be considered. Some organisms feed directly on soils and sediments and thereby access contaminants, and other species absorb dissolved chemicals across their external membranes. Still other species access contaminants that originated in soils and sediments by eating organisms exposed via the first two routes. There are also significant differences in what governs exposure between aquatic and terrestrial organisms.
Like human health risk assessment, information on bioavailability processes is generally utilized during the exposure assessment, but not always in an explicit
way. In general, the goal of the exposure assessment is to determine the concentration of each compound that will be accumulated into various levels of a food chain in the vicinity of contaminated soils or sediments—similar to determining intake in human health risk assessment. For a given exposure pathway, the most conservative approach is to assume 100 percent availability relative to the available tests of threshold toxicity. This might overestimate risk if all exposure pathways are adequately considered and toxicity tests are designed to maximize contaminant uptake. For example, compounds may be buried deep enough to be below the zone accessed by most organisms, or they may be bound to the solid phase in such a way as to be minimally available. It might underestimate risk if some important exposure pathways are missed or if toxicity tests are not conducted under conditions that maximize uptake.
Because there are many types of ecological receptors and because exposures to soils or sediments can include direct as well as indirect pathways, it is common practice to employ a conceptual model to illustrate the predominant exposure pathways. An example of a conceptual model of exposure to soil contaminants is given in Figure 2-4. There are multiple stressors and pathways—depending on the ecological receptors present as well as the spatial and vertical distribution of the contaminant—that vary in both time and space. Concentrations of individual compounds can change between compartments, including moving from water, sediment, or soils to biota, and between trophic levels. Thus, estimates of exposure can vary depending on the residue and system. Although plant and animal species use different depths within the soil system, most ecological risk assessments focus on surface soils (the upper few meters). Surface sediments, sometimes thought to be the upper 3 cm, are defined by an oxidized zone in which most animals live. However, the depth at which the animal is exposed to its microenvironment can vary from millimeters to tens of centimeters. Burrowing animals can interface with much deeper environments.
Intake equations require values for contaminant concentrations in the various compartments (solid, water, tissue), which can be either measured or predicted. To minimize uncertainties, ecological risk assessors have tried to minimize the length of pathways along which predictions are to be made. Ultimately, one would like to be able to link concentrations of contaminants in top predators to concentrations in the soils or sediments. In cases where top predators are the receptors of concern, such a linking would allow one to derive a proposed threshold concentration in soils or sediments, which would then be the cleanup criterion for a particular site.
Depending on which exposure pathways dominate, different bioavailability processes can be considered during ecological risk assessment. Table 2-2 considers where explicit bioavailability information has been typically used for four exposure pathways. To illustrate further how specific bioavailability processes are currently considered in ecological risk assessment and risk management, the following section focuses on direct contact of invertebrates with soils or sedi-
|
BOX 2-3 Assumptions Imbedded in the Leaching-to-Groundwater Exposure Pathway Soil Screening Levels (SSLs) are generic values, established by the states and EPA, that are used in screening level assessments of contaminated soil. It turns out that for a large number of chemicals, the leaching-to-groundwater pathway controls SSL values. Thus, it is important to understand the assumptions about bioavailability processes A and B that play a role in this exposure pathway—assumptions that are not apparent from simply reading the list of numeric SSLs. A better understanding of the assumptions and the default parameters selected to obtain the numeric criteria can illuminate opportunities to improve bioavailability process assumptions via more site-specific evaluation of contaminated sites. Two equations described earlier represent leaching of contaminants from the soil and subsequent mixing and dilution with underlying groundwater. Regarding the equation for the dilution attenuation factor (DAF), infiltration over the site area is presumed to be uniform and leached water is presumed to have uniform contaminant concentration. The contaminant is presumed to be uniformly distributed in the site soil, and the soils are assumed to be physically and chemically homogeneous. It is also assumed that there is no background concentration of the contaminant in the off-site groundwater. In order to generate generic SSLs, EPA established a “default” DAF of 20 to be used at all sites. This number was generated after applying the DAF equation to 300 selected groundwater sites across the country. Although the physical hydrologic properties of the subsurface soils vary from site to site, the default value is expected to be protective in most cases where the contaminants are above the water table and the site size is less than half an acre. A number of assumptions are also found in the second groundwater leaching equation, which determines the protective soil concentration of contaminant. In order to obtain numeric estimates, default physical soil property values (θw, θa, ρb) are assumed. The H′ constants are contaminant-specific properties and are tabulated in the literature |
ments and exposure to wildlife feeding on soil invertebrates and plants—selected because they frequently drive ecological risk assessment efforts.
Direct Contact of Invertebrates with Soils or Sediments
Bioavailability processes A and D in Figure 1-1 (association and dissociation of the contaminant with the solid phase and absorption through a biological membrane) play an important role in this exposure pathway and are considered during ecological risk assessment in a variety of ways. One relatively simple technique has been to develop models that predict the partitioning of metals and organics between different phases—of which there are many levels of detail— and then incorporate these into exposure assessment. In the simplest formulations, thermodynamic partition coefficients are used to describe distributions of contaminants between various environmental compartments, with the contaminant in the aqueous or organismic phase usually assumed to be available. For
|
for most compounds of concern. For hydrophobic organic pollutants, the sorption distribution coefficient (Kd) is estimated as the product of the Koc (organic carbon normalized sorption coefficient), a compound specific property that is also tabulated for many organic pollutants, and the fraction organic carbon content (foc), a soil-specific property. In order to determine default SSLs, a relatively low foc of 0.2 percent typical of a subsurface sediment is assumed for all calculations. Use of the Koc-approach assumes that sorption is controlled by linear partitioning to “normal” soil organic matter (i.e., sorption to other types of carbonaceous solids, described in Chapter 3, is assumed negligible). For a select list of inorganic pollutants (including silver, copper, nickel), the Kd values are estimated using a geochemical model (MINTEQ) or empirical data. For the generic soil screening values, the estimated Kd values are derived based on assumptions about a number of soil properties, including circumneutral pH and sorptive clay-mineral coatings. For both organic and inorganic contaminants, it is assumed that the time to reach sorption equilibrium (contaminant concentrations in the dissolved and solid phases) is rapid compared to the rate of infiltration, which may not always be true. In summary, determination of the generic soil concentrations that protect human health via the leaching-to-groundwater pathway relies upon a large number of assumptions about the soil and contaminant behavior. Some assumptions are more obvious because they are captured by “default” values. Other assumptions are less visible and underly the conceptual scenario established for the “generic” site. Clearly, collecting and applying site-specific information has the potential to reduce the uncertainty associated with using the more generic SSLs. By understanding where the default assumptions and parameters are in the leaching-to-groundwater pathway, opportunities for improving the rigor of the risk assessment via the collection of site-specific chemical and physical information are made obvious. |
metals, their distribution in soils is assumed to be controlled by both the cation exchange capacity and the organic carbon content, while in sediments, the solubility of metals complexes (both inorganic and organic) and precipitates are assumed to determine the available fraction. As described below, a simple normalization technique known as AVS/SEM has been proposed to determine the fraction of metals that are bound to sediment phases or in pore water, based on what are assumed to be the canonical factors controlling availability. For organic compounds, partitioning between solid, aqueous, and organismal phases is assumed to be dependent primarily on the organic content of soils and sediments and the organism. Another simple and empirical test, known as BSAF, has seen increasing use in determining the distribution of organic contaminants in both soil and sediment systems. In both cases, these descriptors are useful for static or slowly varying systems, but are of limited utility in dynamic systems.
Estimates of the available fraction of a contaminant pool from the exposure assessment are used in ecological risk assessments directly by comparing the
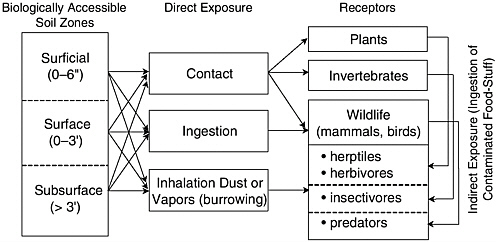
FIGURE 2-4 Hypothetical conceptual model for direct and indirect exposure of ecological receptors to soil contaminants. SOURCE: Adapted, with permission, from Menzie et al. (2000). © (2000) Journal of Human and Ecological Risk Assessment.
predicted available concentrations to threshold concentrations known to cause negative effects in invertebrates, if thresholds are known. (Such threshold levels are usually determined during simple, short-term, single media tests.) Or, as discussed in the subsequent section, estimates of the available fractions can be used to model contaminant concentrations in different phases and transfer to higher trophic levels.
Acid Volatile Sulfide Method for Metals in Sediments. A normalization technique developed for EPA to predict metal accumulation from sediment into aquatic organisms is based on redox conditions and equilibrium partitioning (EqP) theory. A redox boundary is common in aquatic sediments, although the depth of the boundary varies among sediments. Partitioning of metals between the forms typical of oxic vs. anoxic sediment is a first-order process in bulk sediments, because diffusion is the rate limiting process (Rand, 1995). For the anaerobic portion of sediments, the availability of metals is thought to be controlled, in part, by precipitation as insoluble sulfides, because the stability constants for most metal-sulfide associations are very high, and exchange from metal sulfides to water is low. Thus, it has been suggested that normalizing concentrations of metals in sediment by acid volatile sulfide (AVS) might provide a universal explanation of metal availability from sediments (DiToro et al., 1990; Hansen et al., 1996). The theory assumes that low pore water concentrations of metal translate into limited bioavailability. Because they are typically not anoxic, soils are not candidates for the AVS normalization.
TABLE 2-2 Where Bioavailability Information is Used in Ecological Risk Assessment
|
Exposure Category |
Current Use of Bioavailability Information |
|
Direct contact of invertebrates and plants with soils or sediments |
This pathway refers to exposure through feeding, exposure to pore waters within sediments, or external contact of non-predator organisms. Bioaccumulation information is the basis for many guidelines and it is the starting point for evaluating indirect exposure to fish, wildlife, and humans (see below). |
|
Release of contaminants from sediments to overlying water column |
This fate and transport process (bioavailability process A in Figure 1-1) is commonly considered for exposures to water column organisms such as fish. Releases from soils to overlying air are rarely considered for terrestrial animals and plants. |
|
Birds, mammals, and other predators feeding on plants or on soil or sediment invertebrates |
Bioavailability processes are usually considered with regard to accumulation of chemicals into animals that are food for higher organisms. Bioavailability of contaminants in soils incidentally ingested by wildlife itself is rarely considered because of the difficulty in making such measurements. |
|
Food web transfer of contaminants |
Some bioaccumulative substances such as PCBs, mercury, and selenium are transferred up the food web. For these compounds, bioavailability processes occurring at lower levels (e.g., uptake into invertebrates and plants) have a great influence on exposure of higher trophic level animals. |
Laboratory and field experiments have shown that if the ratio of AVS/SEM is greater than 1, where SEM is simultaneously extractable metal, there are likely to be no metals in solution. Most experiments were conducted with bulk sediments (e.g., Ankley et al., 1991a, b), but similar results are seen in more complex sediment typical of nature. For example, vertical redox gradients and sulfide were found to control concentrations of cadmium in lake pore water in a field setting in Quebec (Hare et al., 1994) and for cadmium, zinc, and nickel in an experimental setting (Lee et al., 2000a). There is also a body of work indicating that acute toxicity from sediments is not observed at ratios of AVS/SEM > 1, although this type of work has been mostly limited to traditional sediment bioassay approaches (i.e., dietary exposure is minimized or absent).
Despite these results, uncertainties remain about the use of AVS as the universal sediment normalizer. For example, studies to date have not defined how to determine biologically relevant AVS concentrations. Redox reactions, and thus sulfide concentrations, are heterogeneous on biologically relevant micro-scales within reduced sediments. AVS varies widely with depth in a different manner in every sediment, with time in the same sediment, and between the
outside and inside of animal burrows (see Luoma and Ho, 1993; Luoma, 1995 for reviews). So it is not clear how closely protocols for bulk sediment collection (which tends to homogenize samples) can account for the actual microenvironments to which relevant organisms are exposed (e.g., Kemble et al., 1994).
Mechanistic knowledge of sediment geochemistry suggests that factors in addition to AVS should influence the concentrations of metals in pore waters and thus metal availability from sediments. In sediments, a metal will distribute among iron oxides, manganese oxides, organic ligands, sulfides, and perhaps clay surfaces, depending upon (in simplistic terms) the balance of redox couples, the association constant with different types of binding sites, and the abundance of sites (Jenne and Luoma, 1977). In addition, most macrofauna have an obligate requirement for oxygen and therefore seek, or create, microenvironments where they can obtain oxygen. Macrofauna that burrow into sediment can irrigate their burrows with oxygenated water from above the sediment. Other macrofauna and meiofauna concentrate their activities in the oxidized zones of sediments (Rhoads and Boyer, 1983). In all these cases thermodynamics do not favor occurrence of sulfides. Samples of bulk sediment that mix microenvironments from the sediment column may misrepresent the influences of AVS and either overestimate or underestimate (more likely the former) the AVS that animals actually experience.
Finally, it is increasingly recognized that exposure to metals (and organic compounds as well) from sources other than pore water is important in many species–contaminant combinations. Indeed, a long history of study demonstrates direct uptake of metal, by some if not many species, after ingestion of the various metal forms found in sediments, including metal sulfides (Luoma and Jenne, 1977; Lee et al., 2000b). The AVS method assumes no contribution to exposure from dietary metal uptake, by ingestion of either sediments or other food sources. Lee et al. (2000b) showed that assimilation from diet was the best explanation for a disconnect between the measured cadmium, zinc, and nickel bioaccumulation by five different benthic species and the AVS/SEM predictions. While some experts promote the use of the AVS/SEM approach in risk assessment, others question its universality because of the confounding influences described above.
It should be noted that equilibrium partitioning methods similar to AVS/ SEM have been developed for predicting organic compound distribution between solid phases and pore water (DiToro et al., 1991; Nichols et al., 1995). These methods assume that organic compounds are associated with organic matter in soils and sediments, that pore water concentrations vary depending on the octanol-water partition coefficient for the compound and the amount of organic matter present, and that the pore water concentrations of these contaminants determine bioavailability to invertebrates.
BSAF Values. The biota-sediment (or soil)-accumulation factor (BSAF) is another simple empirical method used to evaluate bioavailability of contaminants to invertebrates by direct contact. Rather than considering pore water contami-
nant concentrations like the AVS/SEM method above, these factors rely on measured contaminant concentrations in tissue. Organics and sediments are used as the examples in this section because of the existence of guidance material, but similar principles apply to metals (without the normalizations) and soils.
BSAF is an empirical ratio, defined as the chemical concentration in tissue (on a lipid-normalized basis) over the chemical concentration in sediment (normalized to the organic carbon levels in the solid) (Ankley et al., 1992; Cook et al., 1993; Tracey and Hansen, 1996).
BSAF = (Ct/Fl)/(Cs/Foc)
where:
Ct = contaminant concentration in the organism
Fl = the lipid fraction in the tissue
Cs = contaminant concentration in the sediment
Foc = the organic carbon fraction in the sediment
Depending on the compound of interest and the organism, the numbers can range from much less than 1 to much greater than 1, with numbers greater than 1 indicating a compound that bioaccumulates. When predicting higher-order accumulations such as into birds that eat aquatic organisms, ratios referred to as Bio-Magnification Factors (BMFs) are used (Starodub et al., 1996; EPA, 1997b).
BSAF is a simple partitioning factor designed to account for the propensity of an organic chemical to partition into an organism vs. into the organic matter contained in sediment. Such values have the advantage of not assuming equilibrium between the sediment and benthic or pelagic species (Cook et al., 1993). BSAF is generally used to predict the potential accumulation of neutral organic compounds by benthic invertebrates from sediments, but has also been applied to accumulation by fish. For the direct ingestion pathway, BSAF is used mainly as a screening device; that is, a concentration measured in the sediment is multiplied by the BSAF to determine the amount in the organism, which is then compared to some value known to cause harm. As discussed later, BSAF values are also used as input to intake equations for wildlife exposure.
Because BSAF values are dependent on the chemical–physical properties of both the organic compound and solid as well as on the lipid content of the organism, they are site- and species-specific (Lake et al., 1990). Total organic carbon (TOC) values may be relatively constant among sediments. But other inorganic properties, the size of sediment particles, and how long the compound resides in the sediment can influence the BSAF value, especially for superhydrophobic compounds that take a long time to come to steady state with both the sediment and biota matrices (Hawker and Connell, 1985). Indeed, the actual concentrations of organic compounds, such as polychlorinated biphenyls (PCBs), and the type of sediment and TOC content may be quite heterogeneous. Thus,
there can be substantial variation in BSAF values depending on the number of samples of TOC-normalized sediment contaminant concentrations that are used to estimate the denominator of the BSAF.
As an example of the application of the BSAF technique, BSAF values measured by Froese et al. (1998) were found to vary depending on whether they were calculated based on total concentrations of PCBs, the sum of non- and mono-ortho-substituted PCBs, or TEQ (toxicity equivalence, or the PCB congeners that cause TCDD-like toxicity) (Table 2-3). BSAF values calculated based on PCBtotal normalized to TOC in sediments and to the lipid content of biota were between 8 and 11, while those based on non- and mono-ortho-substituted congeners ranged from 0.4 to 1.1. The average TOC-normalized total PCB concentration in sediments was 1.7 mg PCB/g TOC with a range of more than 34-fold between the least and greatest values, resulting in a range of as much as 35-fold for BSAF values calculated in this manner.
Although the BSAF method is empirical, it could be more mechanistically based (e.g., on fugacity theory—see Clark et al., 1988; Mackay and Paterson, 1991; Ling et al., 1993) through the use of several assumptions, including that the system is at steady state. Indeed, if the organic carbon in the sediment and the lipid in the animal tissues is equivalent as a solvent for the contaminant of interest, the BSAF should be 1.0 in systems at steady state (Hoke et al., 1994). However, this value is generally not observed in data collected from the field because the octanol-equivalent fat fraction for sediment dry weight organic matter is about 0.3 (Karickhoff et al., 1979; Sablijc et al., 1995). Thus, the BSAF is approximately 1.7 if it is calculated from organic carbon-normalized concentrations in the sediment and lipid-normalized concentrations in the tissues of the biota. Nonetheless, BSAF values for total PCBs are generally greater than would be expected based on the above assumptions. This may be related to changes in the organic matrix of the food within the guts of the invertebrates that promote further uptake. Similarly, anomalously high BSAF values have been observed for accumulation of some compounds from sediments by invertebrates (Eadie et al.,
TABLE 2-3 BSAF Valuesa for Various Matrixes Based on Total PCBs, the Sum of the Mono- and Non-ortho-substituted PCB Congeners, and TEQs
1985; Landrum et al., 1989, 1992). However, BSAF values of 1 to 2 have also been reported for PCBtotal (Ankley et al., 1992).
Numerous studies have calculated BSAF values for accumulation of PCBs from marine sediments by such organisms as mollusks (Mercinaria mercinaria) and polychaetes (Neghtys incisa) (Lake et al., 1990), the mayfly (Hexagenia limbata) (Boese et al., 1995; Drouillard et al., 1996), and the mussel (Malacoma nasta) (Landrum and Poore, 1988). Variation in BSAF is observed for individual species as well as for individual PCB congeners. In a compilation of previous studies, Tracey and Hansen (1996) reported that the mean of median BSAF values for various species is 2.10. Additional compilations of BSAFs are available for a range of ecosystems (Boese and Lee, 1992; Lee, 1992; Parkerton et al., 1993).
Interestingly, despite the variations observed, there have been calls to apply accumulation ratios (BSAFs or BMFs) from one location to another (Neely and Mackay, 1982; Velleux and Endicott, 1994). For example, for total PCBs in sediments, a global average BSAF value of 1.7 has been suggested for use in risk assessments for infaunal invertebrates where BSAF values have not been determined for a particular site (Landrum and Poore, 1988). Indeed, the BSAF approach has been proposed for use as a regulatory tool in risk assessment methodologies involving contaminated sediments (Parkerton et al., 1993), which would be useful if the values do not vary among locations or if an overall average value can be calculated for a region. However, the application of BSAF values determined at one location to other locations is limited (EPA, 2000). For example, at the Baird and McGuire Superfund site (a contaminated soil system) the upper-bound BSAF values taken from the literature were found to be three or four times higher than the site-specific measurements, which was probably explained by the high organic content of the soils (about 30 percent) that enhanced the soil binding of the pesticides (Menzie et al., 1992). Thus, it has been suggested that the method would be most useful as a first-level screening tool (Wong et al., 2001). The key concept should not be that there is a global correction, but that a site-specific correction can be made to account for certain bioavailability processes in ecological risk assessment.
***
To summarize, the commonly used paradigms to incorporate bioavailability processes into assessments of exposure by direct contact have substantial uncertainties, and, at best, may capture only crude influences. The variability in empirical predictions of bioaccumulation (BSAFs) indicates that the degree of influence that bioavailability processes have on exposure can be large. But predictions of those influences from theoretical measures either have not been validated or can differ (sometimes substantially) from the observations in nature.
Exposure of Wildlife Feeding on Invertebrates and Plants
For a variety of reasons, the pathway of wildlife feeding on invertebrates or plants often drives ecological risk assessments. Wildlife that feed on terrestrial or aquatic invertebrates and plants can be exposed to chemicals accumulated into the tissues of these organisms as well as through the incidental ingestion of soils or sediments. The simplest form of the wildlife exposure model, assuming a soil environment, is shown below:
Exposure Dose (oral, μg/g-day) = [Cfood × Ifood] + [RAF × Csoil × Soildiet × Ifood]
where:
Cfood = concentration of the contaminant of concern (COC) (μg/g) in the food (measured or estimated); this is the average concentration in the relevant exposure zone—an area determined by the size and locations of foraging areas. Estimates of Cfood can be obtained by using the BSAF described earlier multiplied by the soil or sediment concentration to yield a concentration in the animals or plant. Estimates are also provided by models or actual measurements as described in considerable detail in Chapter 4;
Ifood = amount of food ingested per day normalized to body weight (g/g-day) and usually expressed in terms of wet weight/wet weight;
RAF = relative availability factor for COCs in soil via incidental ingestion of soils;
Csoil = concentration μg/g in the relevant exposure zone; this is estimated as an average concentration in the exposure zone for chronic exposure and effects and as upper bound (e.g., maximum or hot spot concentrations) for evaluation of short-term or acute exposures;
Soildiet = fraction of soil in the diet; the product of this number and Ifood yields an estimate of the amount of soil or sediment that is incidentally ingested.
This exposure model is similar in form to the one used for humans, and the two models share similar considerations regarding bioavailability processes. The relative amounts of invertebrates, plants, and soils or sediments that are ingested are species dependent. For example, a species that feeds on earthworms or invertebrates in sediments may ingest more soil or sediment than one that feeds on invertebrates that inhabit vegetation (e.g., grasshoppers). Beyer et al. (1994) have estimated the amounts of soil and sediment ingested by various species, and these data are frequently used in ecological risk assessments for wildlife.
The first term in the equation—exposure via contaminants in food—is often the most important source of exposure for wildlife. Thus, the accumulation of compounds in lower-order organisms is a primary concern, and some of the ways in which this is evaluated were described previously. However, the spatial and
temporal scales considered for wildlife are different than those used to evaluate exposure to invertebrate and plant communities. These scales also differ among wildlife species, such that the availability of chemicals and associated exposure will vary from species to species. This is usually taken into account by explicitly considering foraging areas (see Figure 2-5) in estimating exposure concentrations. The more sophisticated wildlife exposure models take into account the foraging behavior of individual animals in the population, food and habitat quality, and the spatial distribution of habitat and contamination (Hope, 2001).
Although it is recognized that wildlife may also be exposed via incidentally ingested soils or sediments (the second term in the equation), little effort has been spent determining RAF values because of difficulties in making such a measurement. (There has been considerable effort directed at the availability of lead in sediments ingested by waterfowl species—Beyer et al., 1997, 1998a, b, 1999.) Indeed, it is much easier to estimate or measure accumulation of contaminants into food items than it is to determine the bioavailability of soil-bound chemicals in the digestive systems of various wildlife species. Regardless of the species under consideration, the RAF value for food ingestion is typically assumed to be
FIGURE 2-5 Examples of wildlife home ranges.
100 percent (with the exception of a few metals and organic chemicals and a few species of wildlife). This implies that predators absorb contaminants similarly from their food (unlikely given the very wide range of digestive physiologies), or that absorption is similar from all prey (also not likely), or both. Other than this assumption, there are few if any default values related to bioavailability that are commonly used in ecological risk assessment—unlike with human health risk assessment. Because of a lack of information, and because it is thought to be less significant than the food and soil ingestion pathways, dermal contact is rarely considered when estimating exposures of wildlife species, and therefore no default values for dermal absorption have been suggested.
***
In summary, ecological risk assessments currently use a variety of empirical measures and relatively simple models to incorporate information on bioavailability processes, particularly bioaccumulation into invertebrates. There are similarities between the wildlife exposure models and human exposure models in that both contain terms for direct ingestion of soils and sediment that may employ a relative bioavailability value. However, for wildlife this pathway is not as important a source of contamination as is food.
The methods described here represent how bioavailability is currently considered in ecological risk assessment today. There are more innovative and mechanistic models of bioavailability processes on the horizon (such as dynamic bioaccumulation models), and these are discussed, along with the specific measurement tools used for bioavailability, in Chapter 4.
Case Studies to Illustrate Use of Bioavailability in Risk Assessment
Although bioavailability processes are implicitly a part of every risk assessment, those assessments that have been specifically labeled as “dealing with bioavailability” comprise only a small subset. For those involving human health, site-specific studies have been conducted to determine relative bioavailability, which reflects the difference between uptake of solid-bound contaminant vs. contaminant in the dosing medium used for the toxicity study (Table 2-4). Relative bioavailability results from such studies have been used to adjust the default value at sites where EPA is the lead regulatory agency and at sites where a state regulatory agency has the lead (e.g., California, Michigan, New Jersey, and Oklahoma). These adjustments have been supported by in vivo animal studies, in vitro testing, environmental health studies, studies of the chemical forms of contaminants in soil, or some combination of these methods (see Chapter 4 for a discussion of methods). To date, most relative bioavailability adjustments in human health risk assessment have been made for the oral route of exposure and for inorganic contaminants (arsenic, cadmium, lead, and mercury) in soil. This reflects the
importance of the oral pathway in human exposures to contaminants in soil and the relative ease of conducting a defensible bioavailability study for inorganics as compared to organics.
Most of the examples cited in Table 2-4 illustrate decreased relative bioavailability compared to the default assumptions, and thus numerically higher cleanup standards. Box 2-4 presents one of these cases in detail—the National Zinc Site, where the site-specific bioavailability of three metals was determined. However, in some cases bioavailability studies can support the default assumption or even demonstrate higher bioavailability than is reflected in the default. The best example is provided by lead, for which there is a national default assumption of 30percent absolute bioavailability from soil to children. As described in Box 2-5, one such site is Palmerton, Pennsylvania, where the results from swine studies ended up supporting the default absolute bioavailability value for lead.
Bioavailability processes have also commonly been included in ecological risk assessments, although they have not been labeled as “bioavailability assessments or adjustments” per se. Nonetheless, there are certain pathways (e.g., sediments to invertebrates) and chemicals (persistent and bioaccumulative compounds) for which information on bioavailability processes is frequently sought and for which there has been greater regulatory acceptance (Table 2-5). Box 2-6 provides an example of where not all bioavailability processes were given equal consideration during ecological risk assessment, primarily because of the lack of acceptable measurement tools—with important implications for remediation efforts.
LEGAL AND REGULATORY FRAMEWORK
Management of contaminated soil and sediment in the United States is conducted on the basis of risk assessment, but with different levels of risk assessment employed depending on the regulatory domain and site type. As discussed previously, all risk assessments for soil and sediment contain implicit assumptions about bioavailability; the most common assumption has been that the contaminant is equally bioavailable from soil or sediment as from the medium used in the critical toxicity study. Other assumptions are also frequently made, e.g., prolonged human exposure, residential land use at a contaminated site, and direct consumption rather than dilution and attenuation during transport. Because of scientific uncertainty inherent in risk assessment and time and expense issues, the use of these generic assumptions during risk assessment has predominated over site-specific analyses. Many of these generic, default assumptions (which are often conservative) are now part of state and federal hazardous waste laws and regulations.
Research over the last ten years on hazardous waste cleanup has prompted site assessors, parties responsible for cleanup, and state and federal agencies to question the validity of the traditional generic approach in a variety of different contexts. A recent trend toward more site-specific risk assessments has led to an
TABLE 2-4 Examples of Relative Bioavailability Adjustments (RBA) in Human Health Risk Assessment
|
Site |
Contaminanta |
Test Used |
|
Anaconda, MT |
Arsenic |
In vivo—monkey |
|
|
Arsenic (in house dust) |
In vivo—monkey |
|
Butte, MT |
Lead |
In vivo—rat |
|
Carson River, NV |
Mercury |
Speciation |
|
Jasper County, MS |
Lead |
In vivo—swine |
|
Oak Ridge National Laboratory, TN |
Mercury |
In vivo, in vitro, speciation |
|
Palmerton, PA |
Lead |
In vivo—swine |
|
Rushton/North Tacoma, WA |
Arsenic |
In vivo—swine |
|
Vasquez Blvd. & I-70 Site, Denver, CO |
Arsenic |
In vivo—swine |
|
National Zinc Co. National |
Lead |
In vivo—rat, speciation |
|
Priorities List (NPL) Site, |
Cadmium |
In vivo—rat, speciation |
|
Bartlesville, OK |
Arsenic |
In vitro, speciation |
|
Crego Park, Lansing, MI |
Arsenic |
In vitro, speciation |
|
Almaden Quicksilver County Park, Los Gatos, CA |
Mercury |
In vitro, speciation |
|
Hawthorne, NJ |
Mercury |
In vitro, speciation |
|
Union Pacific RR, Sacramento, CA |
Arsenic (in slag) |
In vivo—swine |
|
Former Coal Tar Manufacturing Site, Chicago, IL |
PAHs |
In vivo—mouse |
|
Former MGP Site, Taunton, MA |
PAHs |
Literature valueg |
|
Former Koppers Wood Treating Site, Youngstown, OH |
PAHs |
Literature valueg |
|
aThe contaminant was present in soil, unless otherwise indicated. bCleanup levels at all of these sites were increased due to the site-specific bioavailability adjustment, with the exception of the Palmerton, PA, site. cAlthough studies generally determine the relative bioavailability of lead, the absolute bioavailability of lead in soil is used in the IEUBK model. The default value in this model is 30 percent absolute bioavailability. |
||
|
Rel. Bioavail. Adjustment |
Cleanup Levelb |
Regulatory Agency |
|
18.3% |
250 mg/kg |
EPA Region 8 |
|
25.8% |
|
|
|
24% (12% absolute)c |
1,200 mg/kg |
EPA Region 8 |
|
30% |
80 mg/kg |
EPA Region 9 |
|
800 mg/kg |
EPA Region 7 |
|
|
10% |
400 mg/kg |
EPA Region 4 |
|
60% (30% absolute)c |
650 mg/kg |
EPA Region 3 |
|
80% |
230 mg/kg |
EPA Region 10 |
|
42% |
100 mg/kg |
EPA Region 8 |
|
40% (20% absolute)c |
925 mg/kg |
Oklahoma DEQ |
|
33% |
100 mg/kg |
|
|
25% |
60 mg/kg |
|
|
10% |
68 mg/kg |
Michigan DEQ |
|
30% |
300–500 mg/kge |
California EPA |
|
6% |
150 mg/kg |
New Jersey DEP |
|
<0.5% |
No cleanup requiredf |
California EPA DTSC |
|
18% |
RBA used; reduced area of remediation |
EPA Region 5 |
|
29% |
No cleanup levels calculatedh |
Massachusetts DEP |
|
29% |
No cleanup levels calculatedh |
Ohio EPA and EPA Region 5 |
|
dThere are two numbers for each because more than one soil was analyzed. Both values were used in the risk assessment modeling. eCleanup goal varied in different areas of the park. fSlag containing up to 1800 mg/kg arsenic was left in place. gBased on Magee et al. (1996). hRBA accepted by regulatory agency, and used to eliminate portions of the site from remediation. |
||
|
BOX 2-4 Development and Use of Bioavailability Adjustments at the National Zinc Site The National Zinc NPL Site in Bartlesville, Oklahoma, was home to a zinc smelter that operated continuously from 1907 until the early 1990s. Most of the soil contamination around the facility resulted from the period 1907–1976, during which the facility operated as a horizontal retort smelter. Facility emissions (stack and roof emissions, and windblown concentrate) resulted in elevated concentrations of zinc, lead, cadmium, and arsenic being deposited in soils, with the greatest concentrations downwind of the facility (prevailing wind direction is northerly). Residential areas lie primarily to the north and east of the facility (Figure 2-6), and these areas were of greatest concern for human exposures to metals in soil. During the planning stages for the remedial investigation, it was concluded that site-specific studies of the oral bioavailability of lead, cadmium, and arsenic in soil would be beneficial. A detailed protocol for a study of the relative bioavailability of lead and cadmium in rats was prepared. (See Chapter 4 for a detailed discussion of whole animal uptake studies, in vitro studies, and mineralogical studies.) The rat model was selected because it had recently been published (Freeman et al., 1992) and had been used to assess oral lead bioavailability from community soils at the Butte, Montana, NPL site (EPA, 1994b). The protocol, which called for the study to be conducted in accordance with EPA’s Good Laboratory Practice regulations (40 CFR Part 792), was provided to the Oklahoma Department of Environmental Quality (DEQ) for review, which had taken over from EPA as the lead regulatory agency. The protocol was also reviewed by a toxicologist selected by the community advisory group and by an expert in the field who was independent of any of the stakeholders. Comments from all of these reviewers were considered when revising the draft protocol. In addition to the feeding study, arsenic availability was also evaluated using mineralogical and chemical extraction (i.e., in vitro) studies.  FIGURE 2-6 Site map showing the cleanup areas based on EPA goals and more site specific calculations. |
|
The rat feeding study used a surficial soil composited from five residential lots in the vicinity of the historical smelter. The in vitro study used 11 surficial soils collected from residential lots. Electron microprobe analysis was used to identify the forms of lead, cadmium, and arsenic present in these samples. The in vivo study in rats involved dosing groups of five animals with either contaminated soil or lead acetate/cadmium chloride (the positive control) mixed in feed for a period of 30 days. Four dose groups spanning a 20-fold range in doses of lead and cadmium were used for both the soil and the positive control. On day 30, the rats were sacrificed, and samples of blood, liver, kidney, and bone were collected from each animal for analysis of lead and cadmium concentration. Relative bioavailability of lead was calculated from the amount of lead in blood and bone for the soil-dosed rats relative to the amount in rats dosed with lead acetate. Relative bioavailability of cadmium was calculated in a similar manner using data from kidneys, as this is the primary site of toxic action for cadmium. Relative bioavailability values for lead and cadmium determined in this manner were 40 and 33 percent, respectively, while the in vitro study supported a relative bioavailability value of 25 percent for arsenic. These values were incorporated into the human health risk assessments for residential, occupational, and recreational exposure scenarios (Oklahoma DEQ, 1994). These bioavailability adjustments, in combination with other site-specific factors, resulted in two- to three-fold numeric increases in risk-based cleanup goals over the values initially proposed by the EPA for this site (Figure 2-7). These revised cleanup levels greatly reduced the aerial extent of soils requiring remediation (Figure 2-6), reducing remediation costs by approximately $40 million (as estimated by the responsible parties). The bioavailability studies cost less than one-hundredth of this cost saving. Critical factors in the success of the bioavailability studies at the National Zinc site included preliminary discussions of study design with the regulatory agencies, development of a detailed study protocol that was submitted for peer review to experts in the field, revision of the study protocol to address concerns raised during peer review, sharing of the study data, and detailed discussions of results and data interpretation methods.  FIGURE 2-7 Changes in soil cleanup goals at the National Zinc, NPL site from those originally set by EPA to those later determined via bioavailability tests and agreed upon by Oklahoma DEQ. |
|
BOX 2-5 Palmerton Zinc Pile Superfund Site Use of In Vivo Swine Tests to Assess Bioavailability of Lead in Soil The Palmerton Zinc Pile Superfund Site encompasses the vicinity of a former large zinc smelting operation that had two separate smelting plants. The site is located in the Lehigh Valley of eastern Pennsylvania, next to Blue Mountain. One of the smelting plants, the “East Plant,” was adjacent to Aquashicola Creek; the “West Plant” was on the Lehigh River. The smelting operations, which occurred from 1898 to 1980, resulted in long-term emissions of metals to the atmosphere and deposition of lead, cadmium, and zinc to the land near the site. The soil at many residences in the borough of Palmerton, located between the two former smelting plant sites, is sufficiently contaminated that the town itself was included as part of the Superfund site. Approximately 2,000 acres on Blue Mountain, adjacent to the smelter, were progressively defoliated, ultimately resulting in a barren mountainside that is also part of the site. Adding to the environmental impact was the disposal, over 70 years, of 33 million tons of slag at the site, creating a slag pile that extends for 2.5 miles and measures over 100 ft in height and 500 to 1000 ft in width. The name of the site derives from this pile.  In support of baseline risk assessment activities at the site, EPA used in vivo testing in juvenile swine to measure the oral bioavailability of lead in soil (Casteel et al., 1996). Soil from two locations was used, one denoted Location 2 (3,230 ppm lead) and the other Location 4 (2,150 ppm lead). The juvenile swine model, discussed in greater detail in Chapter 4, is considered by EPA to be the best method to measure the site-specific bioavailability of lead in soil (EPA, 1999a) because the gastrointestinal physiology and overall size of young swine are similar to that of young children, the population of principal concern for exposure to lead in soil. Groups of five swine were given either doses of lead-contaminated soil or oral or intravenous doses of lead acetate for 15 consecutive days. The amount of lead absorbed by each animal was determined by measuring the amount of lead in the blood (measured on nine days during the trial), and the amount of lead in liver, kidney, and |
|
bone (measured at study termination on day 15). The measured lead concentrations in blood and tissue samples from animals exposed to test soils were compared to those for animals exposed to lead acetate, and the relative bioavailability was calculated for each endpoint medium (blood, liver, kidney, bone). The relative bioavailability results for the two samples from the Palmerton site are given in Table 2-6. TABLE 2-6 Relative Bioavailability (RBA) of Lead in Juvenile Swine for Palmerton Soils
In interpreting the results, Casteel et al. (1996) recommended emphasis on the blood lead data because they are less susceptible to random errors than the tissue lead data. They defined the “plausible range” to extend from the relative bioavailability based on blood AUC to the mean of the three tissues (liver, kidney, and bone). They defined the “preferred range” to be the interval from the relative bioavailability based on blood to the mean of all four relative bioavailability values. Their “suggested point estimate” is the mid-point of the preferred range. These relative bioavailability values are presented in Table 2-7. TABLE 2-7 Aggregated Estimates of the Relative Bioavailability (RBA) of Lead in Juvenile Swine for Palmerton Soil
Because soluble forms of lead are about 50 percent absorbed (absolute bioavailability) by a child, estimates of the absolute bioavailability of lead in soil can be determined by multiplying the relative bioavailability value by 0.5. This would result in absolute bioavailability values for the two Palmerton soils (EPA’s suggested point estimate) of 0.33 (Location 2) and 0.27 (Location 4). This conversion is important because the IEUBK model (EPA, 1994a), which is used by EPA to estimate the effect of lead in soil on children’s blood lead, contains a default value of 30 percent absolute bioavailability of lead in soil to children. The results of the Palmerton bioavailability study bracketed the default values used in the IEUBK model for predicting blood lead levels (Ioven and Hubbard, 2000). Thus, the juvenile swine testing served to confirm the oral bioavailability value used in risk assessment modeling for lead in soil at the Palmerton site, and no special adjustments for bioavailability were needed or used for this site. |
|||||||||||||||||||||||||||||||||
TABLE 2-5 Examples of Including Bioavailability Processes in Ecological Risk Assessments
|
Exposure Pathways |
Chemicals |
Process and Method |
Example Sites |
|
Sediment to Invertebrates |
Lead, Cadmium, Copper, Nickel, Zinc |
These metals can be bound by sulfides, and their partitioning to pore water has been evaluated using the AVS/SEM methodology at a number of sites. |
Lake Waban, Wellesley, MA; Neponset Reservoir, Foxborough, MA; Mill River, Fairfield, CT. |
|
Sediment to Invertebrates |
PAHs, PCBs |
These organic chemicals can be bound by organic carbon in sediments, and their concentration in invertebrates has been evaluated using the equilibrium partitioning method. |
PAH-contaminated sites including many manufactured gas plant sites and locations near refineries. |
|
Soils to Invertebrates |
Pesticides, PAHs, PCBs, metals |
Bioaccumulation of these chemicals has been evaluated using various empirical or mechanistic exposure models as well as with site-specific measurements. |
Baird & McGuire, Holbrook, MA, and Oak Ridge National Laboratory, Oak Ridge, TN. |
|
Sediments to Waterfowl |
Lead |
A number of risk assessments have considered the relative bioavailability of incidentally ingested lead particles or contaminated sediments;other studies have examined lead shot. |
Chesapeake Bay, MD, and Couer d’ Alene River Basin, ID. |
|
Soils to Wildlife |
Mercury, PCBs, other chemicals |
A number of food chain models that account for bioaccumulation into invertebrates and plants have been used to evaluate exposure to higher trophic levels. |
Oak Ridge National Laboratory, Oak Ridge, TN; Baird & McGuire, Holbrook, MA; Rocky Mountain Arsenal, Denver, CO. |
|
Sediment to Fish |
Mercury, PCBs, other chemicals |
A number of fate and transport and bioaccumulation models and measurements have been used to evaluate fish exposure to contaminated sediments. |
Southern California outer continental shelf; Hudson River, NY; James River, VA; various dredged material disposal sites; San Francisco Bay. |
interest in bioavailability processes, particularly contaminant uptake or absorption. However, these processes and the bioavailability concepts they represent have not often been explicitly acknowledged in laws or regulations for hazardous waste cleanup at the federal or state level. Assuming that adequate information is obtainable, an explicit consideration of bioavailability processes should lead to more scientifically accurate and cost-effective remediation, with no greater actual risk to human health or ecological receptors than under the traditional generic approach to cleanup.
The following discussion considers the current use of bioavailability as a concept in laws and regulation governing hazardous and solid waste cleanup, and it considers whether the law allows for the implicit assumptions currently made about bioavailability to become more explicit via site-specific risk assessment. Because legal and regulatory recognition of bioavailability narrowly targets how retention of contaminants by soils and sediments alters contaminant absorption into an organism, these processes are the focus of this section. Fate and transport processes (bioavailability processes B and C in Figure 1-1) are also an established part of the risk assessment paradigm, and some limited guidance has been developed for their consideration (e.g., partitioning models for soil-water exchange). They are not the focus of further discussion in this section.
Background
Federal and state environmental regulation and directives take a variety of forms, with differing legal impacts. At the federal level, statutes passed by Congress, such as the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and the Clean Water Act (CWA), are binding nation-wide on federal and state agencies as well as on private parties. Environmental statutes ordinarily designate an agency, often EPA, to oversee compliance with the statute. As part of this responsibility, Congress may delegate rule-making authority to the federal agency to regulate in more detail with the benefit of the agency’s expertise, in accordance with the Administrative Procedure Act (APA). This process consists of proposing the rule in the Federal Register, having a comment period, and then producing the publication in final form in the Federal Register and ultimately in the Code of Federal Regulations (CFR). State agencies may administer their own complementary state environmental programs, assist in the administration of a federal environmental program, or assume responsibility for administration of a federal environmental program if the relevant federal statutory criteria are met.
As environmental issues have become increasingly complex, statutes and regulations (although voluminous) sometimes have lacked the comprehensiveness and detail necessary to put the regulatory requirements into practice. As a result, federal and state agencies have provided more detailed guidance in documents available to the public but which are not promulgated with the formality
|
BOX 2-6 Bioavailability Considerations during Ecological Risk Assessment at Clark Fork River Superfund Site Since 1864, mining and smelting of copper and zinc ore have occurred in the head-waters of the Clark Fork River drainage basin at Butte, Montana. One of the world’s largest smelters was constructed in 1900 in Anaconda, 40 km west of the mine. By the time the smelter was closed in 1980, over 1 billion megatons of ore and waste rock had been produced (Lang, 1988). The mining and smelting activities contaminated soils, sediment, groundwater, and surface water over an area one-fifth the size of Rhode Island (Moore and Luoma, 1990). The site is now the largest single Superfund site in the United States. Like many mine sites, Clark Fork is affected by a legacy of historic waste inputs. Understanding more about the bioavailability of metal contaminants in soils and sediments in abandoned mine lands is important in restoring such areas. Sediment contamination is the most unambiguous sign of mining influences on the Clark Fork River. Maximum concentrations in fine sediments are >20 μg/g Cd and >2000 μg/g Cu compared to 0.2 μg/g Cd and 20 μg/g Cu in tributaries (Axtmann and Luoma, 1991; Cain et al., 1992; Farag et al., 1995). Metal contamination in sediments and resident invertebrates decline coincidentally away from the mine (Axtmann et al., 1997) and are enriched in both sediments and invertebrates as far as 550 km downstream. Signs of stress in the region include (1) vegetation and biodiversity losses in the floodplain; (2) reduced diversity of benthic communities in the river and histopathological lesions in trout consistent with contaminant effects (Cain et al., 1992; Farag et al., 1995); (3) reduced diversity of trout to mostly one species (brown trout) in the upper ~180 km of the river and absence of native bull trout (an endangered species); (4) reduced fish standing stock to about 20 percent that of trout in rivers with similar habitats elsewhere in Montana (Hillman et al., 1995); and (5) reduced abundance of mink, which are fish predators (Szumski, 1998). An ecological risk assessment was aimed at identifying the extent to which the signs of stress in the system were the result of metal contaminants vs. other stressors like |
necessary under the APA to be considered a legally binding rule or regulation (for example, EPA’s Risk Assessment Guidance for Superfund). These guidance documents are of great practical importance and generally are assumed by regulated parties to state the methodology and criteria that must be followed to meet statutory and regulatory requirements. For example, if EPA, a regional EPA office, or a state environmental agency issues a guidance document on the use of bioavailability in risk assessment, the risk assessor generally assumes that any departure from that guidance will be closely scrutinized and questioned. Alternatively, lack of clear authorization or guidance on bioavailability would lead the risk assessor to conclude the approach is not favored or even prohibited.
Legal Recognition of “Bioavailability”
As a formal legal requirement, the term “bioavailability” currently receives little mention in any of the federal statutes and regulations governing environ-
|
nutrient runoff and dewatering of the river (Johnson and Schmidt, 1988; Hillman et al., 1995). In the river, sediment contamination was the clearest indicator of mine influences, and extensive, reliable data were available defining the levels of contamination. However the risk assessment ultimately relied more on smaller, less reliable data sets for water column metal concentrations and pore water concentrations to define risk (EPA, 1999b) and discounted the exposure pathway of direct ingestion where sediment concentrations play a large role. One major reason for this decision was that different approaches and tools reached contradictory conclusions about the direct effects from metals in sediments. For example, a comparison to the sediment quality guidelines established by NOAA for benthic invertebrates showed all sediments from the Clark Fork exceeded levels of copper and zinc likely to cause toxicity. But toxicity tests using Clark Fork sediments and an amphipod (a surrogate species found at only one place in the Clark Fork) showed no toxicity. One study (from depositional zones) found AVS in excess of SEM at all Clark Fork sites, while another using sediments from riffle sites showed SEM > AVS at many sites. The risk assessors concluded that the inability to quantify the dietary pathway of exposure (invertebrates ingesting sediment) precluded drawing any conclusions about effects from direct ingestion of food organisms or sediments. Thus, the conclusions about effects on both benthos and fish were most heavily influenced by comparisons of waterborne concentrations to toxicity test effect levels. The risk assessment concluded that the major impact on fish was pulse inputs of dissolved metal to the river, rather than chronic impacts from the sediment contamination. By discounting the sediment route of exposure, the risk assessment implicitly concluded limited bioavailability via the routes of direct contact and diet—a conclusion that will guide the approach to risk management. It is likely that management of risks from pulse inputs could be very different from management of contaminated sediment, and it may not target the true source of contamination. |
mental regulation. The only statutory reference is a brief mention of the bioavailability of restricted metals in the Clean Water Act (CWA) Section 402 permit requirements for point source discharges into navigable waters. In contrast, there are 20 or more statutory references to “bioavailability” and “bioequivalence” requirements in the pharmacological context of food and drug regulation under the Food, Drug, and Cosmetic Act.
Conducting the same word search under the comments to the federal regulations, however, leads to a dramatically different result. The term “bioavailability” appears hundreds of times in the comments to the regulations, including comments to regulations under the major statutory programs outlined below. The incorporation of “bioavailability” into the more detailed, working guidance provided by the comments to the Code of Federal Regulations suggests that the concept of bioavailability, however denominated, is recognized and available for utilization far more frequently than the formal terminology of current laws and regulations would suggest.
EPA’s only quasi-official explicit recognition of bioavailability in risk assessment is in Appendix A to the Risk Assessment Guidance for Superfund, Volume I, Human Health Evaluation Manual (Part A) (EPA, 1989a) [hereinafter “RAGS”], and there the term “bioavailability” is not even used. Instead, the Appendix refers to “adjustments for absorption efficiency.” In other words, RAGS opens the door for consideration of information that absorption of a substance at a particular site may be more or less than typically presumed under the standard risk assessment paradigm. There is no agency-wide guidance on the data necessary to substantiate such an adjustment, however, leaving that critical determination to EPA regional offices, state environmental agencies, or the judgment of the risk assessors, risk assessment reviewers, remedial project managers, and risk managers to whom RAGS is addressed.
The fact that the term “bioavailability” does not appear in the laws and regulations, but does appear in the informal guidance of regulatory comments and guidance documents necessarily leads to confusion and even conflict over the acceptability of the concept in risk assessment between regulators and risk assessors. Regulators are unable to find authoritative authorization for explicit consideration of bioavailability, while risk assessors and others involved in the scientific aspects of risk assessment find that it is acknowledged in the more informal guidance, which is of more practical importance in the actual process of risk assessment. This difference of perception is exacerbated by scientific terminology that risk assessors and scientists may recognize as referring to bioavailability processes, but that regulators and others may not. Despite this disparity in perception, there is the potential for a more explicit analysis of bioavailability processes in any federal program utilizing risk assessment to determine an acceptable level of exposure to a contaminant.
The principal federal remediation programs for soils and sediment most susceptible to a better understanding of bioavailability concepts are (1) sediment quality assessment as regulated under multiple sections of the CWA; (2) sludge disposal programs; (3) the hazardous waste remediation programs; and (4) state and federal Brownfields programs.
Bioavailability in Regulation of Soil Remediation
This section analyzes the current use of bioavailability in national and state cleanup values for soil, as well as EPA regional guidance and state approaches to using bioavailability during soil remediation. As mentioned earlier, this discussion of bioavailability focuses on how retention of contaminants by soils and sediments alters contaminant absorption into an organism.
Regulatory Programs
The regulations discussed below set up a risk-based approach to cleanup, which requires (at least) an implicit consideration of bioavailability processes.
However, there is no explicit recognition of the concept or use of the term in any of the regulations.
RCRA. The Resource Conservation and Recovery Act (RCRA) regulates the generation, transportation, treatment, storage, and disposal of waste. Both RCRA and the Safe Drinking Water Act are designed to curtail the land disposal of untreated waste and to contain releases from any remaining land disposal. CERCLA, and to a more limited extent RCRA, also are directed toward cleanup of existing contamination.
The regulatory sections of RCRA focus on prevention of contamination. Only Section 7003 of RCRA addresses the problem of remedying contamination that has already occurred. Whenever past or present handling, storage, treatment, transportation, or disposal of any solid or hazardous waste “may present an imminent and substantial endangerment to health or the environment,” the past or present owner or operator must take corrective action. RCRA and CERCLA use different “risk triggers” for specific types of response actions (Malone, 2002).
Under EPA’s regulations, any significant increase in groundwater contamination by any of a list of designated pollutants, or any hazardous waste at the site, will require cleanup. Cleanup must continue until MCLs are met, or, if impractical, until alternate concentration levels are met (Novick, 2002). Section 7003 has somewhat lessened in importance since RCRA’s regulatory expansion requiring prevention of contamination and CERCLA’s creation of a fund for cleaning up contaminated sites.
CERCLA. The purpose of CERCLA is not to prevent soil contamination but to remedy contamination after it has occurred. Whenever there is a “release” of a hazardous substance, or substantial threat of a release of a hazardous substance, EPA may respond by taking a removal action or a remedial action. Procedures for both response and removal actions are set out in a National Contingency Plan (NCP). Both actions are designed to clean up contamination, particularly when no responsible parties can be found or required to do so.
In order to finance cleanup of abandoned hazardous waste, a revolving trust fund (the “Superfund”) was established through CERCLA, funded by taxes on petrochemical feedstocks, crude oil, and general corporate income, and by general revenues. The fund may be reimbursed by “parties responsible” for the contamination; if responsible parties refuse to reimburse the fund, they can be sued by EPA. States, local governments, and private parties who conduct cleanups may also be reimbursed from the Superfund or directly by responsible parties. The hazardous waste sites in the Superfund program are on the NPL.
Over the last 15 years, EPA has produced numerous guidance documents both on risk assessment conducted under CERCLA and on remediation strategies for meeting cleanup goals. CERCLA specifies the factors to be considered in assessing treatment options and provides nine general criteria that must be considered:
-
overall protection of human health and the environment
-
compliance with the chemical-specific standards that are considered the statutorily required applicable or relevant and appropriate requirements (ARARs)
-
long-term effectiveness and permanence
-
reduction of toxicity, mobility, or volume through the use of treatment
-
short-term effectiveness
-
implementability
-
cost
-
state acceptance; and
-
community acceptance.
The preamble to the NCP makes it clear that EPA has a strong preference for treatment technologies over engineering and institutional controls, especially for “principal threat” wastes2 (EPA, 1990). EPA does not encourage solutions in which institutional controls are the sole remedy, and prefers that such controls be used in conjunction with containment strategies. As mentioned above, the only mention of bioavailability in guidance documents created for CERCLA is found in Volume A of the Risk Assessment Guidance document for Superfund.
Brownfields. Brownfields are abandoned, idled, or under-used industrial and commercial sites where expansion or redevelopment is complicated by real or perceived environmental contamination (EPA Region 5, 1996). The goal of state and EPA Brownfields programs is the restoration of Brownfields so they can once again be used as a fruitful resource.
Incorporating bioavailability assessments into state and federal Brownfields programs would do much to alleviate businesses’ concerns of unforeseen liability and lack of future profitability in property use or transfer. The purpose of any Brownfields program is to restore a site to a state of productive use. In most cases this does not mean restoration to the “highest and best” use of residential use, but rather to commercial development. Thus, an advantage of more explicitly using bioavailability tools to assess Brownfields restoration is to set the cleanup goal to the actual use and exposure levels that would occur at the site (and thereby limit cleanup costs). The more accurate the assessment of bioavailability, the more precisely tailored the future use of the property can be. Thus, granting legal recognition to bioavailability concepts as a method of improving the basis for evaluating cleanup goals has the potential to lower costs and lessen the potential liability of businesses.
EPA Guidance
At present, there is no national guidance or policy statement on the use of bioavailability in risk assessment, although a workgroup at EPA Headquarters has been preparing to issue such a statement for some time (P. Grevatt, EPA, personal communication, 2001). Nonetheless, measurements of bioavailability processes can be used to adjust and refine human health and ecological risk assessments, most readily with the authorization provided in EPA’s RAGS (EPA, 1989a). As mentioned earlier, site-specific risk assessment is infrequently conducted because many states have specified generic, conservative screening levels for contaminated soil and groundwater that are used to quickly assess contamination. However, rather than using such generic cleanup levels, states can choose to do a site-specific risk assessment that would incorporate the full range of bioavailability processes. It should be noted that EPA’s version of these generic screening levels, embodied in the Soil Screening Guidance (EPA, 1996a), provides not only initial screening levels but also a methodology for calculating risk-based, site-specific soil contaminant concentration levels in which bioavailability might be incorporated. Although the guidance covers only a subset of contamination problems (in terms of chemicals, land use, and exposure pathways), relative bioavailability adjustments could be a factor in some of the allowable scenarios (e.g., for direct ingestion of metal-contaminated soil).
In order to determine whether and how the EPA Regional Offices consider bioavailability in the hazardous waste programs they oversee, the committee heard presentations from and sent a brief questionnaire to each regional representative. The representatives were asked if the region, or the states in that region, had developed any default values for absolute or relative bioavailability, or any guidance material and policy statements regarding the use of bioavailability in environmental cleanup. Each regional office was also asked to identify any site-specific applications of bioavailability assessment for cleanup of metals or organics. The questions were phrased in terms of “bioavailability” specifically, rather than referring to the various processes (such as sequestration, mobility, leaching, etc.) that are often associated with bioavailability, in order to obtain a sense of more formal recognition of bioavailability by EPA administrators as a concept relevant to determining cleanup values. In addition, questions were directed toward human health risk assessment managers rather than ecological risk assessment managers. Table 2-8 summarizes responses to the questionnaire received from various EPA regional personnel.
The survey revealed that outright recognition, acceptance, and utilization of bioavailability factors in state and federal cleanup projects is limited at best, although the opportunity has clearly existed in the Superfund program since 1989. Several observations support this lack of acknowledgment. First, the regions are generally cautious in their recognition and utilization of bioavailability concepts. Second, there are wide variations among the regions in receptiveness to
TABLE 2-8 Formal Use of Bioavailability in Human Health Risk Assessment at the EPA Regional Level
|
Region 1 |
There is no formal regulatory guidance for the use of bioavailability, although Massachusetts and New Hampshire have policies that proscribe default values for absolute and relative bioavailability (see Table 2-1). There are several examples of site-specific calculations of absolute or relative bioavailability in cleanups in this region, indicating a willingness on the part of state agencies to consider the concept. |
|
Region 2 |
There is no region-wide regulatory guidance, and there are no state policies within the region. Site-specific relative bioavailability factors for arsenic were calculated at two sites, but these were rejected by state regulatory agencies (Maddaloni, 2000). |
|
Region 3 |
Neither the region nor any state in the region has developed guidance, although there is a list of absolute bioavailability default factors used for dermal exposure routes only. Changing the implicit assumptions about bioavailability in the baseline risk assessment is permitted only when a site-specific study has been performed (such as for lead and arsenic at the Palmerton Zinc Smelting site—see Box 2-5). |
|
Region 4 |
Though there are two high profile examples of using site-specific bioavailability factors in Region 4 (mercury site in TN, arsenic site in GA), its guidance on risk assessment states: “Bioavailability questions arise as to potential differences in uptake levels under study conditions versus environmental exposure conditions, i.e., the matrix effect. Chemical specific data is rarely sufficient to quantify this difference in bioavailability for all receptors under their varied exposure conditions. Therefore, Region 4 does not accept any adjustment in the 100 percent bioavailability default assumption in the exposure condition without extensive supporting data.” |
|
Region 5 |
Region 5 has no formal guidance on bioavailability. Nonetheless, its interest is evident in that it has conducted a study on the site-specific relative bioavailability of PAHs in soil. |
|
Region 6 |
Region 6 assumes a default relative bioavailability of 100 percent via ingestion. Other values can be used in site-specific situations if supporting scientific data are presented. |
|
Region 7 |
Region 7 has no formal guidance but normally assumes a default relative bioavailability of 100 percent via ingestion. This region has also performed lead relative bioavailability studies at a few specific sites. |
|
Region 8 |
Region 8 has no guidance other than the national default values for lead and the two national dermal values. However, the region has spearheaded many basic, site-specific studies of absolute and/or relative bioavailability primarily at large metal contaminated sites in the Rocky Mountain west. |
|
Region 9 |
Region 9 has no formal policy or default values, but it has allowed the use of relative bioavailability factors on a site-specific basis. No state in the region has prohibited the use of bioavailability factors, nor have they formulated default values. |
|
Region 10 |
There is limited guidance in Region 10 about bioavailability, including interim Region 10 guidance regarding default values for arsenic bioavailability in soil and a decision tree. State regulators rejected a proposal for assuming 40 percent relative bioavailability for arsenic, and instead choose 100 percent, noting that “it remains to be demonstrated that the results of any soil arsenic bioavailability study accurately represents bioavailability for humans or whether the results are more dependent on study conditions as opposed to actual differences in bioavailability.” The guidance has been used at Region 10 federal Superfund sites. |
the approach. Regions 2 and 7 appear to have given the concept limited consideration. Region 8, on the other hand, has been conducting extensive bioavailability studies, primarily because there are large metal-contaminated sites in that part of the country at which an explicit assessment of bioavailability could make a significant difference in cleanup costs. Regions 4 and 6 seem skeptical of explicit bioavailability assessments, although a prominent hazardous waste site in Region 4 embraced the concept for a mercury cleanup. Regions 1, 3, 5, 9, and 10 are actively exploring its use but also with varying levels of acceptability and actual utilization. These differences may be explained only partially by the regional differences in the nature, types, and costs of contaminated site cleanups.
Hesitancy to explicitly consider bioavailability processes during site-specific risk assessments, especially for human health, may reflect agency concern with costs (which can be very large for an in vivo bioavailability study), anxiety about public and community acceptance of the concept and the methods (see Chapter 5), and the absence of more formal national guidance that may lead to legal impediments or challenges. Other factors that contribute to caution on the part of regulatory agencies include lack of supporting data and concerns over available tools and study designs and their validation. Thus, despite the lack of legal impediments to its utilization, explicit bioavailability assessments are not currently a regular feature of site-specific risk assessment.

Considerations of bioavailability have been most common at large metal-contaminated sites in the West, such as where soil is affected by acid mine drainage.
State Recognition of Bioavailability
Although some information on state use of bioavailability was gleaned from the survey of regional EPA offices, there is less information about state practices. Recently, the Air Force Institute for Environment, Safety, and Occupational Health Risk Analysis surveyed state regulators to determine past use of bioavailability adjustment factors and the likelihood of utilization of bioavailability factors in the future in their state or region (unpublished data). Thirty-one (31) states responded to the survey, although three major states—California, Massachusetts, and Texas—did not. In general, the state environmental agencies do not have guidelines currently in place for the use of site-specific absolute or relative bioavailability adjustments in human health risk assessments and rely nearly exclusively on EPA risk assessment (RAGS) protocols (which as mentioned earlier are void of explicit guidance on bioavailability). Only West Virginia and Minnesota provided guidance documents addressing the use of site-specific bioavailability data (MPCA, 1999; WVDEP, 1999). New Jersey indicated plans to produce guidance that includes consideration of bioavailability. The report concludes “while there is little guidance, it appears that state regulators are willing to consider the use of bioavailability adjustments on a site-specific basis. However, it also appears that most states will follow the lead of EPA.”
Other sources indicate that at least some states (like some EPA regions) have taken a more quantitative approach to bioavailability in the form of default values other than 100 percent for the absolute and relative bioavailability of certain compounds or classes of compounds. As discussed previously, these factors are primarily used to adjust exposure via the dermal and oral ingestion pathways. Such values are particularly noteworthy in the Northeast, led by Massachusetts and New Hampshire. In Massachusetts, the default values for four classes of chemicals are to be used “as a last resort” when the risk assessor is unable to find absorption efficiency data specific to the site and the chemical of interest. Michigan has set default relative bioavailability values by compound class for both oral and dermal exposures that are lower than EPA default values or any other state and are based primarily on the best professional judgment of Michigan Department of Environmental Quality scientists.
It should be noted that once an agency has established a default value, in regulation or guidance material, there is typically widespread acceptance and application of the value to a variety of sites. Because of a desire to maintain a level of standardization between sites, there can be reluctance to consider site-specific information in lieu of using default values. Nonetheless, there is also evidence that states are increasingly allowing the use of site-specific bioavailability adjustments. In Washington State, adjustment of “soil gastrointestinal absorption fraction” and “inhalation absorption percentage” is specifically mentioned in state regulations relating to contaminated sites. These are included in a longer list of exposure parameters which “may be changed where there is ad-
equate scientific data to demonstrate that use of an alternative or additional value would be more appropriate from the conditions present at the site” (from section 173-340-708, Human Health Risk Assessment procedures, in Proposed Amendments, Washington State Register, Issue 00-16, The Model Toxics Control Act Cleanup Regulation, Chapter 173-340-WAC). These additions, newly proposed in August 2000, are now accepted.
***
In summary, although there is no legal recognition of the term “bioavailability” in soil remediation statutes, bioavailability processes can be encompassed by both the human health and ecological risk assessment paradigms used under CERCLA and RCRA. Several states and some EPA regions have specified default values (other than 100 percent) for absolute and relative bioavailability of contaminants in soil via the dermal and oral pathways that can be used during risk assessment. And in a few cases, the states and regions will allow these default values to be replaced by results from a site-specific bioavailability assessment. There are a number of sites that have successfully used site-specific bioavailability adjustments in human health risk assessments (predominantly for lead and arsenic), although state regulators appear to be waiting for more explicit approval and guidance from EPA before engaging in more widespread consideration of site-specific adjustment factors for bioavailability. Box 2-7 discusses the role that bioavailability plays in setting soil standards in a few select European countries.
Bioavailability in Regulation of Sludge Disposal
One of the most prominent and explicit uses of the bioavailability concept is its incorporation into the regulatory standards for biosolids (sludge) disposal. Biosolids are the residual material generated by municipal water treatment. They are commonly used as a fertilizer and source of organic matter in agricultural and forest soils. In addition, they are used, generally at high application rates, to restore or remediate mined soils. They contain measurable levels of trace metals, pathogens, and some trace amounts of synthetic organic compounds.
After the promulgation of the Clean Water Act, the amount of biosolids being generated increased dramatically along with concern over the potential detrimental effects of their use. As a result of this concern, EPA began to develop regulations (Part 503 Sludge Rule) that would establish standards for metals, toxic organics, and pathogens in biosolids (EPA, 1989b, 1993; Page et al., 1989). These standards were intended to assure that no adverse effects would occur as a result of land application of biosolids. In addition, because of the consideration of multiple exposure pathways in their evaluation of risk, the regulations are very comprehensive. Over time the regulations incorporated a great deal of research data, such that for all exposure pathways, other than direct human ingestion of
|
BOX 2-7 How Other Countries Use Bioavailability in Environmental Regulations for Soil Contamination Some international regulations consider bioavailability in their assessment of soil contamination and remedial options. Examples include the British Contaminated Land Act (2000), the German Soil Protection Act (1998), the Swiss Ordinance Relating to Pollutants in Soil (1986) and the Ordinance Relating to Impacts on the Soil (1998), and the Contaminated Land Policy in Flanders (1995). Bioavailability is a factor in these laws in several different ways. It is considered for determining if the site is contaminated, determining if the presence of a contaminant is cause for concern, and in determining and evaluating remedial technologies. The acts have some common characteristics. For all legislation, the presence of a contaminant is not sufficient proof that soil requires remediation. Several of the laws identify contamination by using the bioavailable rather than the total fraction of the contaminant. In Switzerland and Germany, the neutral salt extractable fraction of total soil metals has been used to determine whether a soil is contaminated and poses a threat to plants or to humans consuming the plants (VSBo, 1986; OIS, 1998). In Flanders, a microbial bioassay is used to evaluate metal bioavailability. Victor Dries of OVAM, the office in charge of implementing the Contaminated Land Policy in Flanders, notes that “for sites with a high ecological value, it is evident that [ecotoxicity] and bioavailability are the most essential parameters in deciding whether or not remediation is necessary.” The German Soil Protection Act defines “precaution” values, “trigger” values for initiation of investigations of the contamination, “action” values, and remediation requirements. The trigger, action, and precaution values take bioavailability into account both in the types of analysis that are required for determining the values as well as in the acceptable soil concentrations for different end uses. For example, trigger values for several inorganic ions for the soil-to-food plant pathways are based on the 1M NH4NO3 extractable concentration. This extract is classified as a neutral salt extract and is generally seen as reflective of the phytoavailable fraction of total metals (Hani, 1996). The elements whose trigger values are defined using this extract include cadmium, lead, copper, arsenic, and zinc. Organic matter content and particle size are also used in the German Soil Protection Act to modify these values. Use of a pathway approach to evaluate whether a contaminant has the potential to cause harm is a common thread for all of these regulations. In Britain, for example, the local authority must first identify the contaminant, then a relevant receptor, and finally a pathway by which the contaminant is either causing or has a high potential to cause harm, before a “significant pollutant linkage can be established.” The legislation in Britain, Flanders, Switzerland, and Germany lists a range of potential receptors including people, animals, plants, a group of living organisms or an ecological system. |
biosolids, the bioavailable fraction rather than the total concentration of the compounds of concern formed the basis of the rule (Chaney et al., 1982; O’Conner et al., 1990). The body of research used was more extensive for certain elements
and certain pathways. For example, over 75 data points were used to determine the uptake slopes for soil cadmium by leafy vegetables, which were a component of the diet model used to predict total cadmium in humans (EPA, 1992b). The initial molybdenum limit was based on much less data and was subsequently challenged, and additional research was carried out to define an appropriate limit (O’Connor et al., 2001). The new proposed molybdenum limit is based on a soil-to-plant uptake coefficient derived from 29 field studies.
Multiple exposure pathways, and hence bioavailability processes, were used to formulate the regulatory requirements. For each of the pathways, a different highly exposed individual was identified—either humans, animals (soil organisms, soil organism predators, and grazing livestock), or plants. Regulators considered all potential direct routes of exposure (air, water, and soil) as well as indirect exposure pathways to humans or other higher-order animals via plants and lower-order animals. So, for example, a contaminant in soil could be viewed as a direct risk to a human who ingested the soil or an indirect risk to someone who consumed livestock that ingested soil. A range of potential receptors was also taken into account for each pathway. Thus, in the example given above the potential for direct harm to the livestock grazing on biosolids-amended soil would

Biosolids being applied in Leadville, Colorado.
also be evaluated. The most limiting pathway or concentration was then used to set the regulatory limit for each individual compound (Chaney et al., 1998).
The risk assessment calculation included the following steps. First, relative metal uptake coefficients (similar to absolute bioavailability factors) for a range of food crops were calculated, defined as the geometric mean of plant metal concentrations from multi-year field studies where high rates of biosolids had been applied. Next, a diet model (the same as an intake equation), accounting for changes in food consumption patterns over a lifetime, used the uptake coefficients that had been developed from all available field studies to calculate contaminant loading. Then, increased dietary intake as a result of consuming produce grown on biosolids-amended soils was combined with background intake from other sources to determine a maximum contaminant loading in biosolids that would not result in an adverse health effect.
A range of adverse health effects was used in the regulations. The scientists developing the regulation based their human health risk assessment on the potential for a highly exposed individual to have greater than a 10–4 risk of cancer. Non-cancer endpoints were also considered. The highly exposed individual was defined as a person who consumed up to 59 percent of their produce from a home garden that had been amended with biosolids containing the highest permissible metal loading rates for 70 years. Adverse effects for plants, when they were identified as the receptor of concern, were defined as a 50 percent yield reduction in vegetative growth (EPA, 1995a). In cases where animals were the endpoint of concern (e.g., commercial grazing animals or wildlife, soil organisms, and predators of soil organisms), toxicity was defined as unacceptably high metal accumulation in target organs potentially leading to mortality. The regulations relied on target tissue levels of contaminants in organs rather than on an observable lethal or sublethal toxic effect on the organism.
***
The Part 503 regulations are unique because they attempt to protect a range of individuals from a wide number of potentially toxic agents based on their bioavailable concentration, rather than on their total concentration. The regulations strive to be protective of both chronic and acute toxicity. In developing the regulations, it was understood that in addition to the risks associated with the use of biosolids, benefits would also be derived.
Bioavailability in Regulating and Managing Sediment
The monitoring and management of contaminated sediments has recently become an area of considerable interest and activity and involves several federal agencies. EPA, the U.S. Army Corps of Engineers (USACE), the National Oceanic and Atmospheric Administration (NOAA), the U.S. Fish and Wildlife Ser-
vice, and the U.S. Geological Survey are required to do environmental monitoring and assessment of chemical contamination in sediments. For example, EPA and USACE have developed joint technical guidance for evaluating the potential for sediment contamination associated with the discharge of dredged material (1) in the ocean under the Marine Protection, Research, and Sanctuaries Act (EPA and USACE, 1991), and (2) in fresh, estuarine, and saline (near-coastal) waters under Section 404 of the CWA (EPA and USACE, 1998). In response to the Water Resources Development Act of 1992, EPA routinely conducts a national survey of sediment quality in the United States, making use of fish tissue residue data and bioaccumulation models (EPA, 1997b). Bioaccumulation testing and modeling play a role in several other CWA programs, notably Section 403 Procedural and Monitoring Guidance (EPA, 1994c, 1995b), and the Section 320 National Estuary Program (EPA, 1992c). The varying methods used in these cases are intended to link the level of a contaminant(s) in sediments to adverse effects in aquatic life or water quality, which involves an explicit consideration of bioavailability processes.
Aside from some conceptual similarities, however, the different agencies’ approaches for defining sediment quality and the links to bioavailability and

Contaminated sediments at the Wingate Road Incinerator Superfund Site.
biological effects are different. NOAA researchers have developed an empirical, statistical approach for screening sediment quality that does not explicitly address bioavailability processes at all. EPA has taken a more theoretical approach by developing criteria for protecting ecosystems from sediment toxicity using equilibrium partitioning theory to explain how certain sediment characteristics are thought to affect bioavailability. The USACE uses an experimental approach that tests the toxicity of every sediment (for disposal of dredge spoils), and thereby implicitly considers bioavailability on a sediment-by-sediment basis.
The following sections discuss three approaches for setting sediment quality criteria, which refer to recommended concentrations of contaminants in a sediment sample. All three methods take certain bioavailability processes into account, particularly association/dissociation and absorption. Sediment quality criteria are basically analogous to standards for water quality; however, sediment quality criteria are not legally enforceable. Possible exceptions are the Great Lakes sediment criteria, which are used to set enforceable water quality standards. Section 118(c)(2) of the CWA (as amended by the Great Lakes Critical Programs Act of 1990) requires EPA to publish guidance on minimum water quality standards, antidegradation policies, and implementation procedures for the Great Lakes. The resulting guidance (EPA, 1995c) incorporates bioaccumulation factors into the derivation of sediment quality criteria and values to protect human health and wildlife.
EPA Approach
EPA has formulated sediment quality criteria to be consistent with previously established standards for water quality using an approach referred to as equilibrium partitioning. Recently, these sediment quality criteria have been renamed equilibrium partitioning sediment guidelines (ESGs) by EPA (see EPA, 2001c). The approach assumes that contaminants partition between the aqueous and solid phase as a function of sediment composition and contaminant type. Sediment contamination above a concentration that results in an aqueous phase level greater than water quality standards is not acceptable and thus determines the value of the ESG. The water quality standards are based upon toxicity bioassays with benthic invertebrates, dissolved contaminants, and aqueous conditions that maximize uptake. Thus, the ESGs are described as EPA’s best estimate of the concentrations of a substance that may be present in sediment and still protect benthic organisms from direct toxicity in that sediment. EPA has conducted efforts to develop and publish ESGs for some of the 65 toxic pollutants or toxic pollutant categories (EPA, 2000, 2001c).
ESGs incorporate research that identified some of the chemical factors that influence partitioning from sediments to the dissolved phase, and thus directly address an important bioavailability process (particularly A in Figure 1-1). ESGs
can be used to address site-specific issues, and they are quantitative. Note that ESGs do not protect against synergistic or antagonistic effects of contaminants or bioaccumulative effects of contaminants, and they are not protective of wildlife or human health endpoints. ESGs are not regulations and do not impose legally binding requirements. In addition, EPA does not recommend the use of ESGs as stand-alone, pass-fail criteria for sediments. Instead, they are intended for use as screening levels.
As for other strengths and limitations, it is worth noting here that the guidelines may not be applicable where digestive uptake is a significant exposure pathway, where multiple pollutants occur, or where the bioavailability is controlled by physicochemical factors not considered in the EqP approach. The measures are more applicable for instances of acute toxicity or toxicity via direct contact to gills or surfaces of the organism as compared to chronic toxicity. Given such limitations, these measures are best used for those cases where extreme sediment contamination immediately kills fauna or flora.
NOAA Approach
In contrast to the ESGs used by EPA, numerical sediment quality guidelines (SQGs) have been suggested by researchers at NOAA (Long et al., 1995, 1998). In this approach, contaminant concentrations in sediment are correlated with large data sets of observed biological effects (usually done with toxicity tests using field-collected sediments). To date, corresponding chemical and biological effects data have been compiled from one thousand or more studies. A SQG is defined as a range between a lower and upper concentration limit. The lower limits are intended to represent concentrations below which adverse effects were not frequently expected. The upper limit values are the concentrations above which effects had a high probability of occurrence. The range between the lower and higher values can vary but is typically between factors of 2 and 10.
One limitation of this approach is that sediments often contain more than one contaminant, but the majority of studies showing biological effects were conducted by evaluating contaminants individually. Bioavailability processes are not explicitly considered in this approach at an individual site, but they have implicit influences. For example, results from sediment toxicity tests can encompass both sediment chemistry and species-specific effects. The authors state that “numerical values were not intended as regulatory criteria” (Long et al., 2000). Nevertheless, this approach is sometimes used at the local and state level, at least informally, to screen or characterize sediment contamination problems, perhaps because the guidelines are simple to apply. Because this approach is confounding with respect to bioavailability processes, it is not suggested even for screening-level assessments of sites.
USACE Approach
A third approach to sediment quality criteria is that of the USACE for managing dredge spoils. As part of its navigation dredging, the USACE disposes of contaminated sediments in confined disposal facilities for inland sites or in ocean disposal sites. The law dictating Corps activities in this regard (The Marine Protection, Research and Sanctuaries Act) has been interpreted in EPA and Corps’ guidance documents as requiring bioaccumulation testing and other bioassays for purposes of determining which materials are environmentally acceptable for ocean dumping (EPA and USACE, 1998).
Using this approach, dredged soils are first tested for bulk chemical concentrations (Tier I). If these tests indicate contamination, then the sediment elutriate is tested for concentration and toxicity via benthic bioassays (Tier II). One of the assumptions of the Tier II water column analysis is that all contaminants present in the sediment will be released to the water column during disposal and emplacement, although it is acknowledged that this assumption is highly conservative due to the tendency of many contaminants to remain associated with the sediment.
Third and fourth tier testing involve advanced site-specific toxicity and bioaccumulation experiments and bioassays with a deposit feeding bivalve. Tests are conducted during a 28-day exposure time for organisms that are selected based on their ability to metabolize the target analyte and to survive the exposure test. The first two tiers of this approach are widely used; the bioaccumulation tests in Tiers III and IV are less frequently needed because the earlier tests are pass–fail.
The approach of USACE is empirical, site-specific, and more biologically based than are the other two approaches. The tiered treatment of site-specific sediments considers different bioavailability processes at different times. However, the measures cannot be extrapolated to other circumstances, nor are the relative influences of different bioavailability processes quantified.
***
The methodology and approaches used for sediment quality criteria differ among the three agencies in fundamental ways, not the least in how certain bioavailability processes are assessed and taken into consideration. These differences could serve as a point of confusion for practitioners hoping to better quantify the risks involved in various sediment management scenarios, and they reflect the lack of consensus among environmental managers about how to deal with bioavailability processes.
CONCLUSIONS AND RECOMMENDATIONS
Considering the processes that influence bioavailability is entirely within the human health and ecological risk framework. Bioavailability processes should not be considered as “something new” that falls outside of the basic risk-based approach to hazardous waste cleanup that has been adopted in the United States. The goal of bioavailability analysis is to reduce uncertainty in exposure estimates and thus improve the accuracy of the risk assessment.
Although consideration of bioavailability processes is inherent to risk assessment, usually only some of the relevant bioavailability processes are considered explicitly, and assumptions made about the other processes are not transparent. All risk assessments contain implicit, and usually conservative, assumptions about many bioavailability processes. However, different users have chosen different processes to consider explicitly. For example, EPA has focused on the absorption aspect of bioavailability (through the use of default values for dermal and oral relative bioavailability and BSAF values) while many of the other processes have been less explicitly examined. Because of this variability, it is important to use parameters containing the word “bioavailability” (such as absolute bioavailability and relative bioavailability) only with very clear definition of the parameter and its role in the entire spectrum of bioavailability processes. The lack of mechanistic understanding and description during risk assessment precludes the development of technically sound exposure models, especially those that could incorporate temporal changes in physical, chemical, and biological factors.
Explicit consideration of bioavailability processes is more common in ecological risk assessment than in human health risk assessment. This is because it is easier and more acceptable to make measurements on ecological receptors (e.g., worms, small mammals, birds, fish) than it is on humans, and because risk managers are usually willing to manage uncertainty in ecological risk assessments (including the incorporation of bioavailability processes) differently. In addition, during ecological risk assessment there is a greater focus on how bioavailability processes influence bioaccumulation into various wildlife food items. The burden of proof is often higher for adjusting exposure estimates for human receptors than it is for ecological receptors.
There is a misconception that the default values representing bioavailability processes in risk assessment are protective and appropriate for all circumstances. The tendency to standardize regulatory risk assessment has led to the use of certain default factors (e.g., equilibrium partitioning for organic chemicals, relative bioavailability values, dilution attenuation factors) typically considered to have wide applicability across a variety of sites. Although determining these default values required explicit consideration of bioavailability processes
with adoption of a conceptual model and incorporation of quantitative assumptions, the values are sometimes based on only a few studies and may not be applicable to the site of interest. Thus, replacing default values with site-specific information should be encouraged. It should be noted that consideration of site-specific information on bioavailability processes may result in an increase or decrease compared to the “default value.”
At present there is no legal recognition of “bioavailability” in soil cleanup, although bioavailability concepts are emerging for sediment management, and they have been embraced more fully for biosolids management and disposal. The fact that the term “bioavailability” does not appear in the laws and regulations, but does appear in the informal comments to regulations and guidance documents, necessarily leads to confusion and even conflict over the acceptability of the concept. More formal recognition of “bioavailability” in state and federal regulatory contexts would eliminate at least some of the hesitancy and confusion on the part of risk assessors and managers. The lack of clear authorization or guidance on using bioavailability in site-specific risk assessments from EPA has generally led to the perception that the approach is not favored.
There is no clear regulatory guidance or scientific consensus about the level and lines of evidence needed for comprehensive bioavailability process assessment. That is, it is not clear what threshold of knowledge is sufficient to be able to replace default assumptions about bioavailability with site-specific measurements. All of the decisions made at the limited number of case histories have been unique and variable. Regulatory guidance from EPA is needed that addresses what information must be included in a bioavailability process assessment, its scientific validity, acceptable models of exposure, and other issues. This may help to guide research efforts that will further mechanistic understanding of bioavailability processes.
REFERENCES
Ankley G. T., G. L. Phipps, E. N. Leonard, D. A. Benoit, V. Mattson, P. A. Kosian, A. M. Cotter, J. R. Dierkes, D. J. Hansen, and J. D. Mahony. 1991a. Acid-volatile sulfide as a factor mediating cadmium and nickel bioavailability in contaminated sediments. Environ. Toxicol. Chem. 10:1299-1307.
Ankley G. T., M. K. Schubauer-Berigan, and J. R. Dierkes. 1991b. Predicting the toxicity of bulk sediments to aquatic organisms with aqueous test fractions: pore water vs. elutriate. Environ. Toxicol. Chem. 10:1359-1366.
Ankley, G. T., P. M. Cook, A. R. Carlson, D. J. Call, J. A. Swenson, H. F. Corcoran, and R. A. Hoke. 1992. Bioaccumulation of PCBs from sediments by oligochaetes and fishes: comparison of laboratory and field studies. Canadian Journal of Fisheries and Aquatic Sciences 49:2080-2085.
Axtmann, E. V., and S. N. Luoma. 1991. Large-scale distribution of metal contamination in the fine-grained sediments of the Clark Fork River, Montana. Applied Geochemistry 6:75-88.
Axtmann, E. V., D. J. Cain, and S. N. Luoma. 1997. Effect of tributary inflows on the distribution of trace metals in fine-grained bed sediments and benthic insects of the Clark Fork River, Montana. Environ. Sci. Technol. 3:750-758.
Beyer, W. N., D. Day, A. Morton, and Y. Pachepsky. 1998a. Relation of lead exposure to sediment ingestion in mute swans on the Chesapeake Bay, USA. Environ. Toxicol. Chem. 17(11):2298-2301.
Beyer, W. N., D. J. Audet, A. Morton, J. K. Campbell, and L. LeCaptain. 1998b. Lead exposure of waterfowl ingesting Coeur d’Alene River basin sediments. J. Environ. Qual. 27(6):1533-1538.
Beyer, W. N., E. E. Connor, and S. Gerould. 1994. Estimates of soil ingestion by wildlife. J. Wildl. Manage. 58:375-382.
Beyer, W. N., J. Spann, and D. Day. 1999. Metal and sediment ingestion by dabbling ducks. The Science of the Total Environment 231:235-239.
Beyer, W. N., L. J. Blus, C. J. Henny, and D. Audet. 1997. The role of sediment ingestion in exposing wood ducks to lead. Ecotoxicology 6:181-186.
Boese, B. L., and H. Lee II. 1992. Synthesis of methods to predict bioaccumulation of sediment pollutants. ERL-N No. N232. Narraganset, RI: EPA Environmental Research Laboratory.
Boese B. L., M. Winsor, H. Lee, S. Echols, J. Pelletier, and R. Randall. 1995. PCB congeners and hexachlorobenzene biota sediment accumulation factors for Macoma nasuta exposed to sediments with different total organic carbon contents. Environ. Toxicol. Chem. 14:303-310.
Cain, D. J., S. N. Luoma, J. L. Carter, and S. V. Fend. 1992. Aquatic insects as bioindicators of trace element contamination in cobble-bottom rivers and streams. Can. J. Fish-Aquat. Sci. 49:2141-2154.
California EPA. 1993. Documentation for the CalEPA CALTOX model.
Casteel, S. W., L. D. Brown, M. E. Dunsmore, C. P. Weis, G. M. Henningsen, W. J. Hoffman Brattin, and T. L. Hammon. 1996. Bioavailability of lead in soil samples from the New Jersey Zinc NPL Site, Palmerton, Pennsylvania. Report to EPA Region III from EPA Region VIII study team, C. P. Weis, Team Leader.
Centers for Disease Control (CDC). 1991. Preventing lead poisoning in children: statements by the Centers for Disease Control. Atlanta, GA: Centers for Disease Control and Prevention.
Chaney, R. L, S. L. Brown, and J. S. Angle. 1998. Soil-root interface: ecosystem health and human food-chain protection. Pp. 279-312 In: Soil chemistry and ecosystem health. P.M. Huang (ed.). Madison, WI: Soil Science Society of America.
Chaney, R. L., S. B. Sterett, M. C. Morella, and C. A. Lloyd. 1982. Effect of sludge quality and rate, soil pH and time on heavy metal residues in leafy vegetables. Pp. 444-458 In: Proceedings of the Annual Conference on Applied Research Practices on Municipal Industrial Waste, September 22-24, 1982. Madison, WI: Univ. of Wisconsin Exension.
Clark, T., K. Clark, S. Paterson, D. Mackay and R. J. Norstrom. 1988. Wildlife monitoring, modeling, and fugacity. Environ. Sci. Technol. 22:120-127.
Cook, P. M., R. J. Erickson, R. L. Spehar, S. P. Bradbury, and G. T. Ankley. 1993. Interim report on the assessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin risk to aquatic life and associated wildlife. Washington, DC: EPA.
DiToro, D. M., J. D. Mahony, D. J. Hansen, K. J. Scott, M. B. Hicks, S. M. Mayr, and M. S. Redmond. 1990. Toxicity of cadmium in sediments: the role of acid volatile sulfides. Environ. Toxicol. Chem. 9:1487-1502.
DiToro, D. M., C. S. Zarba, D. J. Hansen, W. J. Berry, R. C. Swartz, C. E. Cowan, S. P. Pavlou, H. E. Allen, N. A. Thomas, and P. R. Paquin. 1991. Technical basis for establishing sediment quality criteria for nonionic organic chemicals using equilibrium partitioning. Environ. Toxicol. Chem. 10:1541-1583.
Drouillard, K. G., J. J. H. Ciborowski, R. Lazar, and G. D. Haffner. 1996. Estimation of the uptake of organochlorines by the mayfly (Hexagenia limbata; Ephemeroptera; Ephemeridae). J. Great Lakes Res. 22:26-35.
Eadie, B. J., W. R. Faust, N. R. Morehead, and P. F. Landrum. 1985. Factors affecting bioconcentration of PAH (polynuclear aromatic hydrocarbon) by the dominant benthic organisms of the Great Lakes. Polynucl. Aromat. Hydrocarbons, [Pap. Int. Symp.], 8th. 363-377.
Environmental Protection Agency (EPA). 1989a. Risk assessment guidance for Superfund. Volume 1. Human Health Evaluation Manual (Part A). Interim Final. EPA/540/1-89/002. Washington, DC: EPA Office of Emergency and Remedial Response.
EPA. 1989b. Development of risk assessment methodology for land application and distribution and marketing of municipal sludge. EPA/600/6-89/001. Washington, DC: EPA.
EPA. 1990. Corrective action for solid waste management units at hazardous waste management facilities. Federal Register 55(145):30865-30867.
EPA. 1991. A guide to principal threat and low level threat wastes. OSWER 9380.3-06FS. Washington, DC: EPA.
EPA. 1992a. Ecological risk assessment guidance for Superfund: process for designing and conducting ecological risk assessments. EPA 540-R-97-006. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 1992b. Technical support document for land application of sewage sludge. NTIS PB93-110575. Springfield, VA: EPA Office of Water.
EPA. 1992c. Monitoring guidance for the National Estuary Program. EPA 842-B-92-004. Washington, DC: EPA Office of Water.
EPA. 1993. Standards for the use or disposal of sewage sludge: final rules. Federal Register 58(32):9248-9415.
EPA. 1994a. Guidance manual for the Integrated Exposure Uptake Biokinetic Model for lead in children. EPA/540/R-93/081, PB93-963510. Washington, DC: EPA.
EPA. 1994b. Baseline risk assessment for lead. Priority soils operable unit, Silver Bow Creek/Butte Area NPL Site, Butte, Montana. Prepared by CDM Federal Programs Corporation, Golden, Colorado. Denver, CO: EPA Region VIII.
EPA. 1994c. CWA Section 403: procedural and monitoring guidance. EPA 842-B-94-003. Washington, DC: EPA Office of Water.
EPA. 1995a. A guide to the biosolids risk assessments for the EPA Part 503 Rule. EPA 832-B-93-005. Washington, DC: EPA Office of Wastewater Management.
EPA. 1995b. Guidance for assessing chemical contaminant data for use in fish advisories. Volume 1, fish sampling and analysis. 2nd Ed. EPA 823-R-95-007. Washington, DC: EPA Office of Water.
EPA. 1995c. Final water quality guidance for the Great Lakes System. Federal Register 60(56):15366-15425.
EPA. 1996a. Soil screening guidance: technical background document. EPA/540/R-95/128, PB96-963502. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 1996b. Recommendations of the technical review workgroup for an interim approach to assessing risks associated with adult exposures to lead in soil. Washington, DC: EPA Technical Review Workgroup for Lead.
EPA. 1997a. Toxicological review of chlordane (technical) (CAS No. 12789-03-6) in support of summary information on the Integrated Risk Information System, December, 1997.
EPA. 1997b. The incidence and severity of sediment contamination in surface waters of the United States, Volume 1: the national sediment quality survey. EPA 823-R-97-006. Washington, DC: EPA Office of Science and Technology.
EPA. 1999a. IEUBK model bioavailability variable. EPA 540-F-00-006, OSWER 9285.7-32. Washington, DC: EPA Office of Emergency and Remedial Response.
EPA. 1999b. Clark Fork River ecological risk assessment. Denver, CO: EPA Region 8. 365pp.
EPA. 2000. Bioaccumulation testing and interpretation for the purpose of sediment quality assessment: status and needs. Washington, DC: EPA Offices of Water and Solid Waste and Emergency Response.
EPA. 2001a. Risk assessment guidance for Superfund. Volume 1: human health evaluation manual (Part E, supplemental guidance for dermal risk assessment), Interim Guidance. Washington, DC: EPA.
EPA. 2001b. User’s guide for the Integrated Exposure Uptake Biokinetic Model for lead in children (IEUBK). EPA 9285.7-42. Washington, DC: Office of Emergency and Remedial Response.
EPA. 2001c. Draft implementation framework for the use of equilibrium partitioning sediment guidelines. Guidance for using equilibrium partitioning sediment guidelines (ESGs) in water quality programs. Washington, DC: EPA Office of Water.
EPA and U.S. Army Corps of Engineers. 1991. Evaluation of dredged material proposed for ocean disposal—testing manual. EPA 503-8-91-001. Washington, DC: EPA/USACE.
EPA and U.S. Army Corps of Engineers. 1998. Evaluation of dredged material proposed for discharge in waters of the U. S.: Inland testing manual. EPA-823-B-98-004. Washington, DC: EPA/USACE.
EPA Region 3. 1998. Risk-based concentration table. Prepared by EPA Region 3 Superfund Technical Support Section. http://www.epa.gpv/reg3hwmd/risk/riskmenu.htm.
EPA Region 4. 2000. Human health risk assessment bulletins—supplement to RAGS. Washington, DC: EPA Region 4 Waste Management Division.
EPA Region 5. 1996. Basic Brownfields fact sheet. Washington, DC: EPA Office of Pub. Affairs.
Farag, A. M., M. A. Stansbury, C. Hogstrand, E. MacConnell, and H. L. Bergmann. 1995. The physiological impairment of free-ranging brown trout exposed to metals in the Clark Fork River, Montana. Can. J. Fish. Aquat. Sci. 52:2038-2050.
Freeman, G. B., J. D. Johnson, J. M. Killinger, S. C. Liao, P. I. Feder, A. O. Davis, M. V. Ruby, R. L. Chaney, S. C. Lovre, and P. D. Bergstrom. 1992. Relative bioavailability of lead from mining waste soil in rats. Fund. Appl. Tox. 19:388–398.
Froese, K. L., D. A. Verbrugge, G. T. Ankley, G. J. Niemi, C. P. Larsen, and J. P. Giesy. 1998. Bioaccumulation of polychlorinated biphenyls from sediments to aquatic insects and tree swallow eggs and nestlings in Saginaw Bay, Michigan, USA. Environ. Toxicol. Chem. 17:484-492.
Hani, H. 1996. Soil analysis as a tool to predict effects on the environment. Commun. Soil Sci. Plant. Anal. 27:289-306.
Hansen, D. J., J. D. Mahony, W. J. Berry, S. J. Benyi, J. M. Corbin, S. D. Pratt, D. M. DiToro, and M. B. Abel. 1996. Chronic effect of cadmium in sediments on colonization by benthic marine organisms: an evaluation of the role of interstitial cadmium and acid-volatile sulfide in biological availability. Environ. Toxicol. Chem. 15:2126-2137.
Hare, L., R. Carignan, and M. A. Huerta-Diaz. 1994. A field study of metal toxicity and accumulation by benthic invertebrates: implications for the acid-volatile sulfide (AVS) model. Limnol. Oceanogr. 39:1653-1668.
Hawker, D. W., and D. W. Connell. 1985. Relationships between partition coefficient, uptake rate constant, clearance rate constant and time to equilibrium for bioaccumulation. Chemosphere 14:1205-1219.
Hillman, T. W., D. W. Chapman, T. S. Hardin, S. E. Jensen, and W. S. Platts. 1995. Assessment of injury to fish populations: Clark Fork River NPL sites, Montana. Appendix G In: Aquatics resources injury assessment report, Upper Clark Fork River basin. J. Lipton, et al. (eds.). Report to the State of Montana Natural Resource Damage Program, Helena, Montana.
Hoke, R. A., G. T. Ankley, A. M. Cotter, T. Goldenstein, P. A. Kosian, G. L. Phipps, and F. M. VanderMeiden. 1994. Evaluation of equilibrium partitioning theory for predicting acute toxicity of field-collected sediments contaminated with DDT, DDE and DDD to the amphipod Hyalella azteca. Environ. Toxicol. Chem. 13:157-166.
Hope, B. 2001. A case study comparing point estimate and spatially explicit ecological exposure analysis methods. Risk Analysis 21(6):1001-1010.
Illinois EPA. 1996. Tiered approach to corrective action objectives. 35 ILL. Adm. Code 742. Springfield, IL: Illinois Pollution Control Board.
Ioven, D., and J. Hubbard. 2000. Letter from D. Ioven and J. Hubbard, EPA Region III, to L. Ehlers, National Research Council, November.
Jenne, E. A., and S. N. Luoma. 1977. Forms of trace elements in soils, sediments, and associated waters: an overview of their determination and biological availability. Pp. 110-143 In: Biological implications of metals in the environment. R. E. Wildung and H. Drucker (eds).
Johnson, H. E., and C. L. Schmidt. 1988. Clark Fork Basin Project: status report and action plan. Report to the Office of the Governor, Helena, Montana.
Karickhoff, S. W., D. S. Brown, and T. A. Scott. 1979. Sorption of hydrophobic pollutants on natural sediments. Water Res. 13:241-248.
Kemble, N. E., W. D. Brumbaugh, E. Brunson, J. D. Dwyer, C. G. Ingersoll, D. P. Monda, and D. F. Woodward. 1994. Toxicity of metal contaminated sediments from the Upper Clark Fork River, Montana, to aquatic invertebrates in laboratory exposures. Environ. Toxicol. Chem. 13(12):1985-1997.
Lake, J. L., N. I. Rublinstein, H. H. L. Lee, C. A. Lake, J. Heltshe, and S. Pavignano. 1990. Equilibrium partitioning and bioaccumulation of sediment-associated contaminants by infaunal organisms. Environ. Toxicol. Chem. 9:1095-1106.
Landrum, P. F., and R. Poore. 1988. Toxicokinetics of selected xenobiotics in Hexagenia limbata. J. Great Lakes Res. 14:427-437.
Landrum, P. F., W. R. Faust, and B. J. Eadie. 1989. Bioavailability and toxicity of a mixture of sediment-associated chlorinated hydrocarbons to the amphipod Pontoporeia Hoyl. Aquatic toxicology and hazard assessment: 12th Volume. U. M. Cowgill and L. R. Williams (eds). Philadelphia, PA: ASTM.
Landrum, P. F., H. Lee II, and M. J. Lydy. 1992. Toxicokinetics in aquatic systems: model comparisons and use in hazard assessment. Environ. Toxicol. Chem. 11:1709-1725.
Lang, W. L. 1988. Pp. 130 In: The last best place. W. Kittredge and A. Smith (eds.). Missoula, MT: Mont. Hist. Soc. Press.
Lee, J.-S., B.-G. Lee, S. N. Luoma, H.-J. Choi, C.-H. Koh, and C. L. Brown. 2000a. Influence of acid volatile sulfides and metal concentrations on metal partitioning in contaminated sediments. Environ. Sci. Technol. 34:4511-4516.
Lee, B.-G., S. B. Griscom, J.-S. Lee, H. J. Choi, C.-H. Koh, S. N. Luoma, and N. S. Fisher. 2000b. Influence of dietary uptake and reactive sulfides on metal bioavailability from aquatic sediments. Science 287:282-284.
Lee, H. 1992. Models, muddles and mud: predicting bioaccumulation of sediment-associated pollutants. Pp. 267-294 In: Sediment toxicity assessment. Chelsea, MI: Lewis Publishers.
Ling, H., M. Diamond, and D. Mackay. 1993. Application of the QWASI fugacity/equivalence model to assessing sources and fate of contaminants in Hamilton Harbor. J. Great Lakes Res. 19:582-602.
Long, E. R., D. D. Macdonald, S. L. Smith, and F. D. Calder. 1995. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Mgt. 19:81-97.
Long, E. R., L. J. Field, and D. D. MacDonald. 1998. Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environ. Toxicol. Chem. 17:714-727l.
Long, E. R., D. D. MacDonald, C. G. Severn, and C. B. Hong. 2000. Classifying probabilities of acute toxicity in marine sediments with empirically derived sediment quality guidelines. Environ. Toxicol. Chem. 19(10):2598-2601.
Luoma, S. N., and K.-T. Ho. 1993. Appropriate uses of marine and estuarine sediment bioassays. Chapter 11 In: The handbook of ecotoxicology, Volume 1. P. Calow (ed.).
Luoma, S. N., and Jenne, E. A. 1977. The availability of sediment-bound cobalt, silver, and zinc to a deposit-feeding clam. Pp. 213-230 In: Biological implications of metals in the environment. R. W. Wildung and H. Drucker (eds.).
Luoma, S. N. 1995. Prediction of metal toxicity in nature from bioassays: limitations and research needs . Pp. 609-646 In: Metal speciation and bioavailability in aquatic systems. A. Tessier and D. Turner (eds.). London: John Wiley and Sons, Ltd.
Mackay, D., and S. Paterson. 1991. Evaluating the multimedia fate of organic chemicals: a level III fugacity model. Environ. Sci. Technol. 25:427-436.
Maddaloni, M. 2000. Presentation to the NRC Committee on Bioavailability of Contaminants in Soils and Sediments. October 13, 2000. Woods Hole, MA.
Magee, B., P. Anderson, and D. Burmaster. 1996. Absorption adjustment factor (AAF) distributions for polycyclic aromatic hydrocarbons (PAHs). Human Ecol. Risk Assess. 2(4):841-873.
Malone, L. A. 2002. Environmental regulation of land use. Section 9.03(5). St. Paul, MN: West Publishing.
Massachusetts Department of Environmental Protection Office of Research and Standards. 1992. Documentation for the risk assessment short form—residential scenario. Policy #WSC/ORS-142-92. Boston, MA: Commonwealth of Massachusetts Office of the Secretary of State.
Menzie, C. A., D. E. Burmaster, J. S. Freshman, and C. A. Callahan. 1992. Assessment of methods for estimating ecological risk in the terrestrial component: a case study at the Baird & McGurie Superfund site in Holbrook, Massachusetts. Environ. Toxicol. Chem. 11:245-260.
Menzie, C. A., A. M. Burke, D. Grasso, M. Harnois, B. Magee, D. McDonald, C. Montgomery, A. Nichols, J. Pignatello, B. Price, R. Price, J. Rose, J. Shatkin, B. Smets, J. Smith, and S. Svirsky. 2000. An approach for incorporating information on chemical availability in soils into risk assessment and risk-based decision making. Human and Ecological Risk Assessment 6(3):479-510.
MPCA. 1999. Risk-based guidance for soil-human health pathway. Volume 2. Technical support document. St. Paul, MN: Minnesota Pollution Control Agency. Available at: http://hubble.pca.state.mn.us/cleanup/pubs/srv3_99.pdf.
Moore, J. N., and S. N. Luoma. 1990. Hazardous wastes from large scale metal extraction: A case study. Environ. Sci. Technol. 24:1279-1285.
Neely, W. B., and D. Mackay. 1982. Evaluative model for estimating environmental fate. Pp. 127-144 In: Modeling the fate of chemicals in the aquatic environment. K. L. Dickson, A. W. Maki, and J. Cairns Jr. (eds.). Ann Arbor, MI: Ann Arbor Science Publishers.
Nichols, J. W., C. P. Larsen, M. E. McDonald, G. J. Niemi, and G. T. Ankley. 1995. Bioenergetics-based model for accumulation of PCBs by nesting tree swallows, Tachycineta bicolor. Environ. Sci. Technol. 29:604-612.
Novick, S. 2002. The law of environmental protection. Section 13.05(3)(G). St. Paul, MN: West Publishing.
National Research Council (NRC). 1983. Risk assessment in the federal government: managing the process. Washington, DC: National Academy Press.
NRC. 1993. Issues in risk assessment. Washington, DC: National Academy Press.
O’Conner, G. A., D. Kiehl, G. A. Eiceman, and J. A. Ryan. 1990. Plant uptake of sludge-borne PCBs. J. Environ. Qual. 19:113-118.
O’Connor, G., R. M. Brobst, R. L. Chaney, R. L. Kincaid, L. R. McDowell, G. M. Pierzynski, A. Rubin, and G. G. Van Riper. 2001. A modified risk assessment to establish molybdenum standards for land application of biosolids. J. Environ. Qual. 30:1490-1507.
Ohio Department of Commerce. 1992. Risk assessment guidance document. Columbus, OH: Division of State Fire Marshall, Bureau of Underground Storage Tank Regulations.
OIS. 1998. Commentary on the ordinance of 1 July 1998 relating to impacts on the soil. Swiss Agency for the Environment, Forests and Landscape (SAEFL). Order number VU-4809-E.
Oklahoma DEQ. 1994. Technical memorandum: preliminary remediation goals for the National Zinc Site, Bartlesville, Oklahoma. Prepared by Oklahoma Department of Environmental Quality, Oklahoma City, Oklahoma.
Page, A. L., T. J. Logan, and J. A. Ryan (ed.). 1989. W-170 Peer review committee analysis of the proposed 503 Rule on sewage sludge. CSRS Technical Committee W-170, Univ. of California, Riverside.
Parkerton, T. F., J. P. Connolly, R. V. Thomann, and C. G. Uchrin. 1993. Do aquatic effects or human health end points govern the development of sediment-quality criteria for nonionic organic chemicals? Environ. Toxicol. Chem. 12:507-523.
Rand, G. M. 1995. Fundamentals of aquatic toxicology: effects, environmental fate, and risk assessment. 2nd Edition. New York: Hemisphere Publishing Corporation. 1125 pp.
Rhoads, D. C., and L. F. Boyer. 1983. The effects of marine benthos on physical properties of sediments: a successional perspective. Chapter 1 In: Animal-sediment relations: the biogenic alteration of sediments. P. L. McCall and M. J. S. Tevesz (eds.). New York: Plenum Press.
Ruby, M. V., R. Schoof, W. Brattin, M. Goldade, G. Post, M. Harnois, D. E. Mosby, S. W. Casteel, W. Berti, M. Carpenter, D. Edwards, D. Cragin, and W. Chappell. 1999. Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environ. Sci. Technol. 33(21):3697-3705.
Sablijc, A., H. Gutsen, H. Verhaar, and J. Hermens. 1995. QSAR modeling of soil sorption: improvements and systematics of log Koc vs. log Kow corrections. Chemosphere 21:4489-4514.
Starodub, M. E., P. A. Miller, G. M. Ferguson, J. P. Giesy, and R. F. Willis. 1996. The use of risk assessment techniques to develop a protocol for determining acceptable concentrations of bioaccumulative chlorinated organic chemicals in sediments for the protection of piscivorous wildlife. Toxicol. Environ. Chem. 54:243-259.
Szumski, M. J. 1998. The effects of mining related metals contamination on piscivorous mammals along the Upper Clark Fork River, MT. Ph D Thesis, Univ. Wyoming, Laramie. 148pp.
Tracey, G. A., and D. J. Hansen. 1996. Use of biota-sediment accumulation factors to assess similarity of nonionic organic chemical exposure to benthically coupled organisms of differing trophic mode. Arch. Environ. Contam. Toxicol. 30:467-475.
Velleux, M., and D. Endicott. 1994. Development of mass balance model for estimating PCB export from the lower Fox River to Green Bay. J. Great Lakes Res. 20:416-434.
VSBo. 1986. Verordnung über Schadstoffgehalt im Boden. In: Swiss ordinance on pollutants in soils No. 814. 014, Publ. Eidg. Dricksachen und Materialzentrale, Bern, Switzerland.
WVDEP. 1999. Guidance manual for the West Virginia Voluntary Remediation Program, Version 1-1. Charleston, WV: West Virginia Division of Environmental Protection.
Wester, R. C., H. I. Maibach, D. A. W. Bucks, L. Sedik, J. Melendres, C. L. Laio, and S. DeZio. 1990. Percutaneous absorption of [14C]DDT and [14C]benzo(a)pyrene from soil. Fund. Appl. Toxicol. 15:510-516.
Wester, R. C., and H. I. Maibach. 1996. Percutaneous absorption of hazardous substances from soil and water. Pp. 325-335 In: Dermatology, 5th Edition. Marzulli and Maibach (eds.).
Wong, C. S., P. D. Capel, and L. H. Nowell. 2001. National-scale, field-based evaluation of the biota-sediment accumulation factor model. Environ. Sci. Technol. 35:1709-1715.



































































