3
The CDC Anthrax Vaccine Research Plan
In appropriations legislation for fiscal year 2000, Congress requested that the Centers for Disease Control and Prevention (CDC) implement a collaborative effort to study the safety and efficacy of the anthrax vaccine (AVA). The aim of this research was to (1) examine the risk factors for adverse events, including differences in rates of adverse events between men and women; (2) determine the immunologic correlates of protection and document vaccine efficacy; and (3) optimize the vaccination schedule and administration to assure efficacy while minimizing the number of doses required and the occurrence of adverse events. Congress also specified that CDC, the National Institutes of Health, and the Department of Defense (DoD) would fully cooperate in this effort.
The research program proposed and begun by CDC to respond to the mandate from Congress consists of an array of studies. These studies were described to the committee in written materials and oral presentations over the course of five committee meetings. Changes over time in these presentations and materials reflected the evolution of the plans for and implementation of the research program. Understanding that the research program would continue to develop, the committee requested a definitive description of the plan as of February 2002 to serve as a basis for its evaluation. CDC responded by providing the document “Anthrax Vaccine Safety and Efficacy Plan,” dated February 22, 2002, which described or listed 11 studies (CDC, 2002c). This document is reproduced in Appendix C and is referred to in this chapter as the CDC Plan. It was accompanied by protocols or draft protocols for seven of the studies and less detailed descriptions of plans for the other four studies. These materials are the primary source of information used by the committee to evaluate the research program, supplemented by information gathered and discussed in the committee meetings. The committee’s report and evaluation do not reflect changes that CDC has made in the program since February 2002.
The CDC Plan states that the studies and activities it describes are intended “to evaluate vaccine immunogenicity and correlates of protection; assess alternate vaccination schedules and routes of administration to enhance vaccine safety; and enhance reporting of adverse events after vaccination. In addition to evaluating the efficacy and short- and long-term safety of AVA, CDC and its partners will use a variety of approaches to improve the acceptance of AVA amongst military personnel”(CDC, 2002c, p. 3). The document notes that the implementation of the research plan will also provide scientific benefits for researchers in several disciplines and in development and validation of a new generation of technologically advanced vaccines.
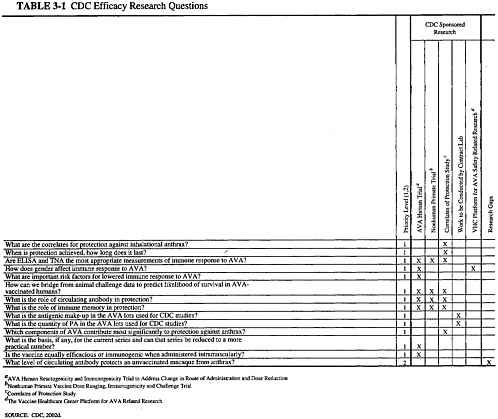
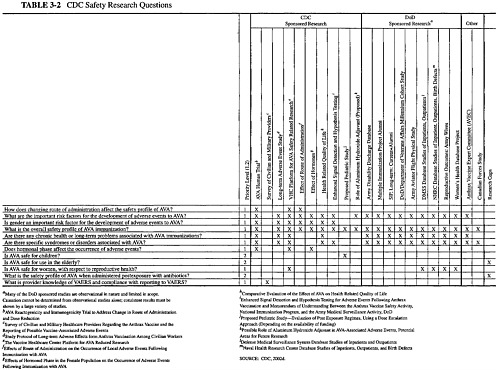
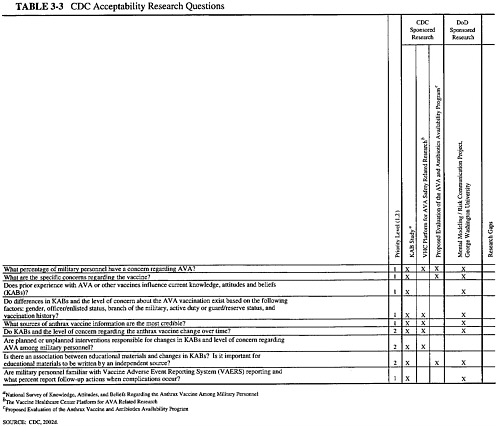
The document provided to the committee by CDC listed objectives for three research categories: efficacy, safety, and acceptability. In response to a request from the committee, CDC also listed the critical research questions in these areas, with an indication of which of the proposed studies was designed to address each question and whether other organizations were addressing the question. This information, as
well as the prioritization CDC assigned to each study, is presented in three matrices (Tables 3-1, 3-2, 3-3). This chapter summarizes the studies that CDC has planned to address each set of research objectives. More detailed descriptions of the studies and the committee’s review of each study are provided in Chapters 4, 5, and 6 of this report. The committee’s overall assessment of the research plan is presented in Chapter 7.
The research studies are being carried out or managed at CDC by two units—the National Immunization Program (NIP) and the National Center for Infectious Diseases (NCID). As its name suggests, NIP is a disease-prevention program providing for the planning, coordination, and conduct of immunization activities nationwide (CDC, 2001). Its activities include providing consultation and training to assist health departments in planning, developing, and implementing immunization programs; administering research and operational programs for prevention and control of vaccine-preventable diseases; and supporting a nationwide framework for surveillance of vaccine-preventable diseases. NIP’s support to health departments includes assistance in developing information management systems to monitor the safety and efficacy of vaccines by linking vaccine administration information with adverse event reporting and disease outbreak patterns (CDC, 2001). In keeping with these activities, the aspects of the anthrax safety and efficacy research program developed and overseen by NIP include studies of vaccine acceptability as well as of the use of data from the Vaccine Adverse Event Reporting System (VAERS) and the Defense Medical Surveillance System (DMSS) to improve information about adverse events following vaccination.
NCID is focused on the prevention of disease, disability, and death caused by infectious diseases and seeks to accomplish this goal by working with public health officials, health care professionals, and international groups (CDC, 2002a). The center’s staff conducts surveillance, epidemic investigations, epidemiologic and laboratory research, training, and public education programs to develop and promote prevention and control strategies for infectious diseases. For the anthrax vaccine research program, NCID has developed and is managing three interrelated studies of the safety and efficacy or immunogenicity of the vaccine in both humans and nonhuman primates (NHPs).
The following sections review the research objectives and critical research questions described in the CDC materials.
EFFICACY
CDC’s stated objectives for the efficacy component of its anthrax vaccine research program are displayed in Box 3-1.
The studies that have been planned to address efficacy and immunogenicity are (1) the Human Reactogenicity and Immunogenicity Trial (the Human Clinical Trial); (2) the Nonhuman Primate Vaccine Dose Ranging, Immunogenicity, and Challenge Trial (the NHP study); and (3) the Correlates of Protection Study (the ICP study). These studies are being carried out at or through NCID.
|
BOX 3-1 CDC Objectives for Research on the Efficacy of the Anthrax Vaccine
SOURCE: CDC, 2002c, p. 10. |
The Human Clinical Trial
The Human Clinical Trial is intended to compare the immunogenicity and reactogenicity of AVA when given under the currently licensed regimen (6 doses given subcutaneously over 18 months) with the immunogenicity and reactogenicity of the vaccine when given intramuscularly and with a reduced number of doses. It is anticipated to provide “the principal scientific basis for decisions regarding changes in route of vaccine administration and reduction in number of doses in the vaccination series” as well as “new understanding about anthrax pathogenesis and immunologic correlates of protection against inhalational anthrax in humans” (CDC, 2002c, p. 6). The work should also provide a scientific foundation for the development and licensing of the next generation of protective antigen (PA)-based anthrax vaccines.
The study is designed as a prospective, randomized, double-blind, placebo-controlled clinical trial to be conducted over a period of 43 months at five sites in the United States. The study population will consist of 1,560 healthy civilian adult men and women between the ages of 18 and 61 years. The study will be open to anyone meeting the eligibility criteria, but recruitment efforts will focus on groups for whom AVA vaccination for bioterrorism preparedness has been considered, including emergency first responders, federal responders, and medical practitioners. The analysis will compare men and women in terms of the reactogenicity of the vaccine and the influence of various risk factors on the occurrence of adverse events.
The study protocol received approval from the Food and Drug Administration (FDA) in fall 2001 and began enrolling participants in May 2002. CDC anticipates providing an interim analysis of the first 7 months of data to FDA in fall 2003, and presenting the final analysis to FDA in early 2007.
The Nonhuman Primate Dose-Ranging, Immunogencity, and Challenge Trial
The Nonhuman Primate Dose-Ranging, Immunogenicity, and Challenge Trial is planned to provide information from experiments with rhesus macaques about the relationship between immune responses developed from vaccination with AVA and protection from aerosol challenge with anthrax spores. Based upon the assumption that similar immune responses in humans and in nonhuman primates will be similarly protective, the study will help to provide information about the protection afforded by the vaccine (and other potential anthrax vaccines) in humans. The data will be used as evidence to support the objective of dose reduction and change in route of administration in the licensed AVA schedule for humans (CDC, 2002c). In addition, data on immune response will be collected and used in a related study (described next) to establish correlates of protection induced by AVA vaccination.
Rhesus macaques receiving a three-dose series of AVA at full dose or fixed dilutions of the full dose will be challenged with anthrax spores at different periods of time after vaccination. Vaccination with different dilutions of AVA is expected to induce different levels of immune response in the macaques. Rates of survival after lethal challenge of these animals will provide data to describe a relationship between immune response and survival. Blood sampling at intervals following vaccination and challenge will allow analysis of immune factors that may play a role in protection from challenge.
This study will be carried out at two sites. It began with vaccination of some of the animals in early 2001. Plans call for completion of the study in 2004, but data available before then could be used by FDA in making a decision concerning the potential application for a label change to permit the use of fewer doses or a different route of administration in humans.
Studies of Immune Correlates of Protection (ICP) Against Inhalational Anthrax
Studies of Immune Correlates of Protection (ICP) Against Inhalational Anthrax are planned to identify components of the rhesus macaque humoral and cell-mediated immune responses to AVA that correlate with protection against aerosol challenge by virulent B. anthracis (CDC, 2002f). The ICP studies will develop and apply a panel of immunologic assays to carry out this work.
The emphasis in the ICP studies is on the description and quantification of antibody responses to PA, lethal factor, and edema factor, using quantitative anti-PA IgG enzyme-linked immunosorbent assay and toxin neutralizing antibody assay. The same assays are being used in the human clinical trial as primary endpoints for evaluating the immunogenicity of alternative dosing schedules and routes of vaccine administration. The studies will also include more detailed analyses of the humoral response, as well as analysis of cellular immune response factors.
The studies, which are being carried out at three different sites, began in March 2001, with the first vaccinations of about half of the animals. They will follow the timeline of the closely related human clinical trial and NHP studies, with a preliminary analysis to be presented to FDA in the first quarter of 2004, and the final analysis scheduled for presentation to FDA in the first quarter of 2007.
SAFETY
CDC’s stated objectives for the safety component of its anthrax vaccine research program are displayed in Box 3-2.
The studies that address the subject of adverse events are (1) the human clinical trial, (2) a study to look for long-term adverse events, (3) cohort studies conducted in collaboration with the Vaccine Healthcare Centers (VHC) Network, (4) analysis of data from VAERS and DMSS, and (5) investigation of the possible role of the aluminum hydroxide adjuvant in adverse events following AVA vaccination.
Anthrax Vaccine Adsorbed: Human Reactogenicity and Immunogenicity Trial to Address Change in Route of Administration and Dose Reduction
The human clinical trial, described above in connection with the efficacy studies, will also provide data regarding adverse events following vaccination with AVA. CDC has proposed two study hypotheses related to reactogenicity and adverse events:
-
AVA administered by the IM route results in local reactogenicity that is decreased compared to that of SQ administration.
-
Occurrence of adverse events following AVA administration is influenced by selected risk factors. (CDC, 2002b,e)
The human clinical trial is therefore planned to provide information to permit a comparison of the rates of adverse events observed following AVA vaccination via the IM route with those observed with vaccination via the SQ route. The study will also be able to gather data on the risk factors for adverse events. Information about the study design and timeline was presented briefly above, and more detail regarding the safety aspects of the study is provided in Chapter 5.
Follow-up Study of Textile Mill Workers Vaccinated Against Anthrax
CDC has planned a retrospective cohort study to assess the possibility of chronic or later-onset adverse health effects associated with AVA vaccination. The study plans call for an examination of the mortality experience and functional status of textile mill workers who received doses of AVA 10 or more years ago. The study population is to be drawn from former workers at a textile mill that processed goat hair from the mid-1960s through the mid-1990s. CDC proposes to identify these workers through Social Security records and then to either locate survivors or obtain death certificates for those no longer alive. This process is expected to produce a study population of about 1,500 persons, based on assumptions that 15 percent of survivors will be lost to follow-up and that 70 percent of those located will participate.
Two comparison groups of unvaccinated persons are planned: one group drawn from the community in which the goat hair mill was located, and a second comparison group of persons who worked in other kinds of textile processing mills in the same region and time period as the members of the vaccinated study group.
|
BOX 3-2 CDC Objectives for Research on the Safety of the Anthrax Vaccine
SOURCE: CDC, 2002c, p. 11. |
Information is to be collected about participants’ demographic and socioeconomic characteristics and about health-related risk factors. Death certificates will be obtained to determine the date and cause of death for vaccinated workers who have died. Among survivors, if data from the self-reported medical histories reveal a statistically significant excess of certain medical conditions, the information will be verified by a review of participants’ medical records.
The study is planned to begin in 2003, with data analyzed and results reported in early 2005.
Studies Based in the Vaccine Healthcare Center Network
CDC reported plans for three studies to be conducted through the VHC Network. The VHC Network is a collaboration between DoD and CDC to address issues of safety and acceptability of vaccines within the military immunization health care system. The first VHC was established at Walter Reed Army Medical Center in Washington, D.C., in September 2001. Plans call for a total of 10 to 12 VHCs to be opened over the next 5 years.
The goals for the network are to serve as a platform for studies of vaccine-related adverse health events and to enhance the immunization-related health care of military personnel. Concerns related to AVA will be the initial focus of these activities, but the VHC Network is expected to address issues related to other vaccines as well.
The three study proposals provided to the committee replicate, using observational studies in a military population, certain components of CDC’s human clinical trial. Specifically, these studies will examine (1) the effects of the route of AVA administration on local adverse events, (2) the effect of AVA on health-related quality of life, and (3) the effect of hormonal phase on the occurrence of adverse events in women receiving AVA. The proposal notes that these studies will complement the human clinical trial by overcoming some of its limitations, in particular, the trial’s low statistical power to test some risk-factor associations and the need to wait until the completion of the study (43 months) to perform some of the analyses. Initiation of these studies depends on resumption of routine administration of AVA to military personnel scheduled for deployment to certain areas.
Enhanced Signal Detection and Hypothesis Testing for Adverse Events Following Anthrax Vaccination
CDC plans to analyze data from VAERS and DMSS to identify signals of adverse events that might be associated with receipt of AVA. These analyses are to be performed using methods of automated exploratory data analysis referred to as data mining. Signals that are identified will be investigated further using additional data from DMSS to test for evidence of a possible causal association.
VAERS is the nation’s principal system for collecting spontaneous reports of adverse events following the use of any vaccine licensed in the United States. It is jointly administered by CDC and FDA. DMSS is a system of DoD-wide databases of health-related information, including records for inpatient and outpatient care, and is coordinated by the Army Medical Surveillance Activity (AMSA). CDC is entering into a formal collaboration with AMSA that will establish an Analytic Unit based at AMSA that will conduct the analyses of DMSS data. This unit was to be established by August 1, 2002. Other collaborators include FDA and the DoD’s Anthrax Vaccine Immunization Program.
Possible Role of Aluminum Hydroxide Adjuvant
CDC identified several possible research questions that might be investigated concerning the possible role that the aluminum hydroxide adjuvant in AVA might play in adverse events following vaccination. No study proposals or protocols had been developed at the time the materials were submitted to the committee.
ACCEPTABILITY
CDC’s stated objectives for the acceptability component of its anthrax vaccine research program are displayed in Box 3-3.
Two survey-based studies are planned by CDC to address issues related to the acceptability of the anthrax vaccine and vaccines more generally.
|
BOX 3-3 CDC Objectives for Research on the Acceptability of the Anthrax Vaccine
SOURCE: CDC, 2002c, p. 14. |
Survey of Knowledge, Attitudes, and Beliefs Regarding the Anthrax Vaccine Among Military Personnel
CDC plans a large survey to assess the knowledge, attitudes, and beliefs of military personnel and military health care providers regarding AVA. Two phases of focus group meetings are planned. These will be followed by representative surveys of the military population at two different time points to provide an understanding of the factors influencing perceptions of anthrax vaccine safety and efficacy and to inform the development of appropriate educational materials. CDC has contracted with Research Triangle Institute (RTI) to design and implement the survey, which will gather information from a representative sample of the U.S. military’s active duty and reserve populations. The baseline survey is planned to take place in early 2003, with a follow-up survey anticipated in 2005. Data analysis and reporting will take place in 2006.
Survey of Civilian and Military Health Care Providers Regarding the Anthrax Vaccine and the Reporting of Possible Vaccine-Associated Adverse Events
This study is planned to obtain nationally representative data on the knowledge, awareness, attitudes, and practices of both military and civilian health care providers regarding the reporting of adverse events following immunization to VAERS. It is also intended to obtain information from providers about their general knowledge of and attitudes about anthrax vaccination. Information obtained from the study will be applied to the development of appropriate vaccine benefit and risk communication materials, including educational and promotional materials targeted to providers regarding anthrax vaccine safety and reporting of adverse events. CDC also anticipates gathering information from this study’s participants that might be used to improve VAERS from the reporter’s perspective. The study will be carried out via a mail-out survey designed and administered through a contract with RTI. The survey is planned for early 2003, with analysis and reporting of data completed later that year.
In the chapters that follow, the studies described briefly in this chapter are presented in greater detail, along with the committee’s evaluation and recommendations regarding each study. Chapter 7 provides the committee’s assessment of the research program as a whole.
REFERENCES
CDC (Centers for Disease Control and Prevention). 2001. About NIP. [Online]. Available: http://www.cdc.gov/nip/about.htm [accessed May 7, 2002].
CDC. 2002a. About the Center. [Online]. Available: http://www.cdc.gov/ncidod/about.htm [accessed May 7, 2002].
CDC. 2002b. Protocol 1: AVA human reactogenicity and immunogenicity trial to address change in route of administration and dose reduction. Anthrax Vaccine Safety and Efficacy Plan. Atlanta: Centers for Disease Control and Prevention.
CDC. 2002c. Section 1: anthrax vaccine safety and efficacy plan. Anthrax Vaccine Safety and Efficacy Plan. Atlanta: Centers for Disease Control and Prevention.
CDC. 2002d. Section 3: critical research questions table. Anthrax Vaccine Safety and Efficacy Plan.Atlanta: Centers for Disease Control and Prevention.
CDC. 2002e. Section 6: study summary: AVA human reactogenicity and immunogenicity trial to address change in route of administration and dose reduction. Anthrax Vaccine Safety and Efficacy Plan. Atlanta: Centers for Disease Control and Prevention.
CDC. 2002f. Section 8: study summary: immune correlates of protection against inhalation anthrax—part C of the anthrax vaccine research program. Anthrax Vaccine Safety and Efficacy Plan. Atlanta: Centers for Disease Control and Prevention.