6
Proposed Studies on the Acceptability of the Anthrax Vaccine
While the issue of acceptability is not explicit in the congressional mandate to the Centers for Disease Control and Prevention (CDC) for research on the anthrax vaccine, it is an important consideration. When people are reluctant or unwilling to accept a vaccine, it clearly poses important obstacles to achieving the protection that the vaccine might afford. Concerns on the part of some service members and members of the public have affected the acceptability of Anthrax Vaccine Adsorbed (AVA) since the Department of Defense (DoD) implemented the Anthrax Vaccine Immunization Program (AVIP). These concerns are reviewed briefly in Chapter 2 and in greater detail in another report from the Institute of Medicine (IOM, 2002).
A study in Air National Guard and Air Force Reserve forces indicated that the level of concern about AVA was high (GAO, 2000). Similarly, a CDC pilot study of vaccine providers and health care administrators at an Air Force base in August 2000 found that 20 percent of the respondents were concerned about the safety of the vaccine and that only 15 percent believed they were likely to be exposed to anthrax spores (CDC, 2002a). These studies were carried out in the context of a service-wide mandatory AVA vaccination program at a time when the public had only a limited awareness of the potential threat of exposure to anthrax spores.
Circumstances have changed, however. Bioterrorist events in the fall of 2001 resulted in the exposure of large numbers of civilians to anthrax spores and five deaths from inhalational anthrax. In addition, new lots of AVA have been released by FDA following approval of renovations of the manufacturing facility (Masiello, 2002). An IOM review of the available evidence about the vaccine has found the vaccine’s safety to be comparable to that of other adult vaccines (IOM, 2002). Finally, the current vaccination program is limited to those military personnel “at higher risk whose performance is essential for certain mission critical capabilities” (Wolfowitz, 2002). The committee reviewed CDC’s proposed studies on acceptability in light of the current circumstances.
OBJECTIVES AND CRITICAL RESEARCH QUESTIONS FOR CDC RESEARCH ON THE ACCEPTABILITY OF THE ANTHRAX VACCINE
CDC’s stated objectives for the acceptability component of its anthrax vaccine research program are displayed in Box 6-1. At the request of the committee, CDC also identified a set of critical research questions, shown in Box 6-2.
CDC intends to investigate the acceptability of the anthrax vaccine by identifying concerns about it among military vaccine recipients through large surveys on knowledge, attitudes, and beliefs (KABs) powered to yield estimates by service and by subgroups within each service; a patient satisfaction survey;
|
BOX 6-1 CDC Objectives for Research on the Acceptability of the Anthrax Vaccine
SOURCE: CDC, 2002b, p. 14. |
|
BOX 6-2 Critical Research Questions Regarding the Acceptability of the Anthrax Vaccine, as Identified by CDC
SOURCE: CDC, 2002c. |
and other assessment tools. Detailed documentation of the acceptability of the vaccine within the military population is the major thrust of the research program as planned. However, the committee believes that a different prioritization is appropriate. Rather than emphasizing detailed measurements of the level of concern about the anthrax vaccine in surveys with large numbers of participants, it would be more appropriate to focus on learning how educational interventions can improve acceptability. While the development
of strategies and training materials that are aimed at increasing the acceptability of the vaccine is listed among the CDC research objectives, it is given little attention in the protocols provided to the committee.
Similarly, the committee disagrees with the relative priorities that have been assigned to the critical research questions. The committee considers the questions related to the development of interventions to be as or more important than the questions currently emphasized by CDC regarding determining the percentage of military personnel with concerns regarding AVA. While documenting the concerns of military service members about the vaccine is important, this information is most useful in the context of learning how these concerns might be met through interventions. Thus, the committee views developing and testing interventions as a more appropriate focus for CDC’s efforts than producing exhaustive information on the percentages of service members with concerns. The need for such a change in emphasis is discussed further in conjunction with the proposals for individual studies.
SURVEY OF KNOWLEDGE, ATTITUDES, AND BELIEFS REGARDING THE ANTHRAX VACCINE AMONG MILITARY PERSONNEL
The stated goal for this portion of CDC’s research on the acceptability of the anthrax vaccine is to assess the KABs of military service and health care personnel regarding AVA (CDC, 2002a,d). Representative surveys of the military population are planned for two different time points to provide an understanding of the factors influencing perceptions of the safety and efficacy of the anthrax vaccine and to direct the development of appropriate educational materials. CDC contracted with Research Triangle Institute (RTI) to devise a study design to gather information from a representative sample of the U.S. military’s active and reserve populations. The specific aims of the study are shown in Box 6-3.
|
BOX 6-3 Specific Aims of the Survey of Knowledge, Attitudes, and Beliefs Regarding the Anthrax Vaccine Among Military Personnel, as Identified by CDC Primary specific aims:
Secondary aims:
SOURCE: CDC, 2002a, p. 1. |
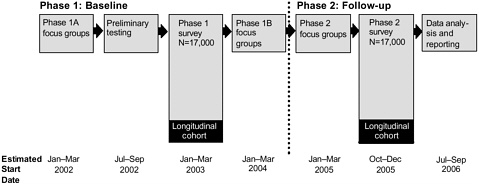
FIGURE 6-1 Proposed components and estimated timeline for the KAB survey of military personnel regarding the anthrax vaccine
Study Design
RTI’s plan for this study involves two phases that rely on complementary focus groups and surveys. Figure 6-1 offers a schematic illustration of the timing of the focus groups and surveys, as described in the draft proposal.
Focus Groups
Two phases of focus group meetings are planned to aid in the development of the survey and to test the educational materials that CDC develops on the anthrax vaccine. RTI will recruit focus group partic ipants from geographically diverse military installations. To ensure that the qualitative information gathered will provide representative viewpoints of the U.S. military’s active and reserve populations, plans call for approximately one-third of the total participants to be non-Caucasians. Twenty-six focus groups are planned for the first phase to make it possible to segment the groups by gender, experience with AVA, medical training, military branch or component, and rank. Focus groups in phase 1 of the study design are further subdivided into phase 1A and phase 1B.
Phase 1A focus groups will be used to gather information that can be applied to the development of the survey for phase 1 of the study, as well as to explore the KABs of military personnel regarding their sources of information about AVA and the perceived credibility of these sources. In phase 1A, the focus groups will also be used to determine which forms of communication participants prefer for receiving educational information about AVA.
Educational materials designed on the basis of the phase 1A focus group discussions will be tested 12 to 15 months later in phase 1B. Phase 1B focus groups will be exposed to an educational message about AVA followed by a series of semi-structured, open-ended questions about how they received the message. Focus group discussions that result from the questions will be used to explore ways to revise and refine potential educational messages. A structured questionnaire will also be administered to participants to gauge their knowledge about AVA.
Phase 2 will use eight focus groups that are scheduled to begin meeting shortly after the completion of phase 1B focus groups. The groups will be designed to distinguish key changes from earlier focus groups in the KABs of participants regarding AVA. Information collected in the phase 2 focus groups
will be used to develop questions for the complementary phase 2 survey, scheduled to commence shortly after completion of the focus groups.
RTI will provide CDC with brief summaries of the individual focus groups, including participants’ sociodemographic characteristics, and will compile the findings from the focus groups in both phases 1 and 2 on the educational needs of military personnel related to AVA and AVA vaccination. On the basis of those findings, RTI will recommend how CDC should address the unmet educational needs of military personnel, propose methods to increase the acceptability of AVA, and suggest the preferred information and media sources of military personnel.
Surveys
Using information gathered from the focus groups, RTI will develop two surveys (repeated crosssectional surveys) that will be administered approximately 2 years apart. As with the focus groups, survey participants will be a randomly selected representative sample of U.S. active and reserve military personnel. Each survey will be administered to approximately 17,000 military personnel who will complete the survey form in classroom settings on military bases. The sample determination, based on stratified random sampling with proportional allocation, was designed to assure representation of all service and regional components of the military population. The phase 1 survey will be administered after the phase 1A focus groups and after the resumption of the AVIP. The phase 2 survey will be administered after the phase 2 focus groups.
The survey in phase 1 will be used as a baseline to gather key information from the participants regarding their KABs toward AVA and other vaccines and their exposure to information about vaccines. The phase 1 survey will also determine if respondents’ KABs toward AVA and other vaccines are influenced by prior experience with vaccines. A measurement of KABs regarding military vaccines other than AVA will be included to allow for comparison and contrast to AVA. In addition, the baseline survey will be used to estimate participants’ perceptions of the frequency and severity of AVA-related complications and to determine the need for CDC-developed AVA educational interventions. The survey will be stratified by gender, anthrax vaccination history, and enlistment and duty status.
The follow-up survey (phase 2 survey) will include a longitudinal comparison to assess temporal changes in KABs regarding the anthrax vaccine. For the longitudinal comparison, selected individuals will participate in both the baseline and follow-up surveys. Their responses will be evaluated to determine whether the observed temporal changes in KABs are associated with interventions alone or with other baseline or societal factors. The analysis will control for anthrax vaccination status.
Before beginning the surveys, RTI plans preliminary tests to ensure the validity of the survey instrument. The testing to validate the survey instrument will include preliminary cognitive testing interviews; a pilot study for each of the active services, a National Guard unit, and a reserve unit; and a psychometric analysis test. Six questions from the Balanced Inventory of Desirable Responding (BIDR)—a 40-question survey that measures self-deceptive enhancement and impression management—will be incorporated into the phase 1 and 2 surveys as a covariate to control for social desirability bias in survey responses. The preliminary testing will also validate the BIDR questions included in the survey.
Committee Comments
As designed and described in the summary and protocol provided by CDC, this study will provide a thorough assessment of military KABs regarding the anthrax vaccine. The design phase of the study has been expanded to include cognitive and psychometric tests and a pilot survey, as recommended by this committee in its interim report (IOM, 2001). The summary and protocol raise questions, however, about the overall objectives or motivation for the study and the need for the very large sample size proposed. Although the committee found in its interim report that the rationale for investigating the KABs of service personnel was appropriate, the additional information reviewed by the committee in preparing the present
report does not justify the need for the level of detail that is driving the use of a study population of 17,000 persons.
It will certainly be helpful to know more about the concerns of military personnel regarding the anthrax vaccine, vaccines in general, and some of the influences on and sources of this concern, but this information should not be an end in itself. Since scientific experts have not found evidence to link AVA with adverse events other than immediate-onset reactions typical of those observed with other vaccines administered to adults (IOM, 2002) and because intelligence assessments indicate that U.S. forces face a real threat of exposure to biological weapons (Wolfowitz, 2002), this licensed vaccine is likely to continue to be used by the military and for selected civilian populations.
Thus information about knowledge, attitudes, and beliefs about AVA is valuable to the extent that it can facilitate or further some constructive action to increase the acceptability of the vaccine. The CDC protocol, however, does not indicate that the results of the phase 1 survey will be used to guide the formulation of different intervention strategies. Furthermore, such a large study could create a burden on the many respondents and on military units, which would have to aid in scheduling times and locations for participants to complete the survey. The available resources might be better applied to the development of intervention materials.
The protocol notes that the assessment of CDC educational materials is a secondary aim for the study. If the ultimate motivation for the study is the development or refinement of such materials, there appears to be a lack of planning toward this end in the study design. Specific materials could be drafted at the start, and tested and refined with focus groups rather than after an exhaustive survey. Materials would be targeted for military personnel and their family members, as well as vaccine providers and other health care providers likely to care for those with concerns about the vaccine. A smaller sample size for the survey, on the order of 3,000, would seem adequate to provide data regarding KABs for this purpose. The survey could also be enhanced by including a question about potential new anthrax vaccines, in addition to the planned questions about military vaccines other than AVA. The focus groups and survey might also aid in the development of effective educational materials by gathering information on how the respondents prefer to hear health messages and on how they view alternative approaches to achieving acceptance.
Concerns have been expressed (Hubbell, 2002) that the survey will lack credibility because military personnel will doubt the confidentiality of their responses and therefore will not feel free to answer the survey questions honestly. The committee agrees that the study is vulnerable to such concerns and recognizes that the survey designers can do only a limited amount to address them. While assurances can be given to study participants that their answers will remain individually anonymous, results may be reported at group or unit levels. Participants may feel constrained to provide responses that will not discredit their group or unit. RTI plans to use questions from the BIDR to control for potential social desirability bias in responses, but this cannot address the overall problem of concerns about confidentiality.
The committee was also concerned about the study’s timeline. The phase 1 survey is anticipated in 2003, with the report on the entire study to be completed by 2006. Since the next 4 years may bring changes in the way the current anthrax vaccine is administered or even see approval of a new anthrax vaccine, it is important that the study gather information to facilitate new materials or interventions relevant to a potential new anthrax vaccine or military vaccines more generally.
Finding: With its large sample size, the current design of the study of knowledge, attitudes, and beliefs regarding AVA primarily addresses the acceptability of the vaccine among military personnel. Further documentation of the prevalence of attitudes and beliefs regarding the vaccine is unlikely to significantly advance the acceptability of the vaccine, which should be the major goal. Instead, qualitative research techniques such as focus groups and smaller-scale surveys can be used to determine the breadth, depth, and underlying reasons for the attitudes and beliefs regarding AVA. This information can serve as the basis for targeted interventions, the impact of which can be assessed with subsequent surveys.
Recommendation: In view of the study timeline and research needs, CDC should modify the design of the KAB study of military personnel to focus on more timely development of educational interventions and the evaluation of their impact on the acceptability of AVA and a broader range of vaccines, including a new anthrax vaccine.
The draft protocol provided to the committee indicates that the focus groups used to gather information to design the survey will be assembled so that enlisted personnel, enlisted women, Reserve and National Guard units, health care personnel, and officers are each represented by a separate group (CDC, 2002a). Participants will be drawn from each main branch of the military. The study design appropriately takes gender into account in the plans for the focus groups. The committee notes that minority racial or ethnic groups may also have different opinions about AVA that they might not feel free to express in a heterogeneous group. Therefore, the committee recommends a focus group design that takes different racial and ethnic groups into account.
Finding: Potential differences between racial and ethnic groups in knowledge, attitudes, and beliefs about AVA and military vaccines generally may be important.
Recommendation: CDC should design the focus groups and preliminary survey to take into account different racial and ethnic groups.
SURVEY OF CIVILIAN AND MILITARY HEALTH CARE PROVIDERS REGARDING THE ANTHRAX VACCINE AND THE REPORTING OF POSSIBLE VACCINE-ASSOCIATED ADVERSE EVENTS
This study is planned to obtain representative data on the knowledge, awareness, attitudes, and practices of both military and civilian health care providers regarding the reporting of adverse events following immunization to the Vaccine Adverse Event Reporting System (VAERS) (CDC, 2002e). The study is also intended to obtain information on providers’ general knowledge of and attitudes towards anthrax vaccination. Information obtained from the study will be applied to the development of appropriate vaccine benefit and risk communication materials, including educational and promotional materials targeted to providers regarding anthrax vaccine safety and reporting of adverse events. CDC also anticipates gathering information from the participants that might be used to improve VAERS from the reporter’s perspective.
Study Design
Limited detail about this study was available in the information provided to the committee. According to the study summary, the survey of health care providers is to be carried out in two phases through a contract with RTI. In the first phase, RTI will recruit military and civilian health care providers from selected sites in eight geographically diverse areas to ensure multiple viewpoints. The focus groups will be used to collect qualitative data from health care providers about reporting of adverse events to VAERS following immunization. Preselected focus groups of military health care personnel participating in the KAB survey on AVA (discussed above) will be asked additional questions about adverse event reporting.
The second phase of the study will consist of a mail-out survey of military and civilian physicians. Three study populations will be targeted: active-duty military physicians who are likely to provide anthrax vaccine and other vaccines; civilian physicians in solo or two-physician practices who are likely to provide vaccines; and civilian physicians in a group practice who are likely to provide vaccines. Military physicians will be selected proportionately by the region of the country in which they are stationed and by their branch of service. Civilian physicians selected to participate in the survey will be in office-based practices in the same cities as major military facilities, under 65 years of age, and have a primary specialty that would make them likely to administer vaccines.
The survey instrument will be a self-administered questionnaire that will require 15 to 20 minutes to complete and that can be processed using a scanning optical-mark reader. Results from the focus groups of phase 1 will be used to add to or modify existing survey instruments to develop the questionnaire. The mail-out survey will integrate questions about office practices and technology, vaccine-related adverse event reporting, and knowledge and attitudes regarding the anthrax vaccine and other vaccines. In addition, each questionnaire will be used to assess the sociodemographic characteristics of the respondent.
To provide sufficient sample size to detect differences of interest between the groups, RTI will mail the surveys to 538 participants from each of the three health provider categories. According to CDC, the survey response rate is expected to be 65 percent following multiple waves of mailings and phone calls.
Committee Comments
CDC has responded to the committee’s previous recommendation (IOM, 2001) to broaden the survey beyond a narrow focus on KABs regarding VAERS to include KABs about the anthrax vaccine. The committee endorses this modification and notes that broadening the survey still further to provide information about health care provider KABs regarding vaccination in general would provide additional improvement. In the committee’s view, the survey should include not only providers who administer vaccines, but also those who deliver care and advice when a service member has a concern or adverse event following vaccination.
Given the effort and time that will go into the survey, the committee believes that it should be designed to produce results that will be applicable to the current anthrax vaccine and to potential new anthrax vaccines; to vaccines more generally; and to mandatory vaccination, such as that required by the military. This information could be very useful for the development and targeting of educational and promotional materials for health care providers on anthrax vaccine safety and on the reporting of adverse events, as described in the study summary.
It was not clear from the draft protocol why the civilian health care providers were stratified by size of practice. The committee cautions that this may unnecessarily handicap the analysis by limiting the size of the groups.
Finding: As proposed, the survey of civilian and military health care providers has a focus on knowledge, attitudes, and beliefs concerning VAERS and vaccination with AVA. Additional questions oriented toward the development of educational materials concerning AVA and other vaccines, immunization, and adverse events could broaden its usefulness. In addition, further articulation of links between the study and development of educational materials is needed.
Recommendation: In addition to gathering information on KABs about VAERS and the current anthrax vaccine, CDC should modify the survey of health care providers to study KABs about a new anthrax vaccine, other military vaccines, and vaccines in general, with a focus on information useful for timely development and testing of appropriate educational materials. The study population should include health care providers who may treat service members with adverse events following vaccination as well as those who administer vaccines.
REFERENCES
CDC (Centers for Disease Control and Prevention). 2002a. Protocol 5: national survey of knowledge, attitudes, and beliefs regarding the anthrax vaccine among military personnel (draft). Anthrax Vaccine Safety and Efficacy Plan. Atlanta: Centers for Disease Control and Prevention.
CDC. 2002b. Section 1: anthrax vaccine safety and efficacy plan. Anthrax Vaccine Safety and Efficacy Plan. Atlanta: Centers for Disease Control and Prevention.
CDC. 2002c. Section 3: critical research questions table. Anthrax Vaccine Safety and Efficacy Plan.Atlanta: Centers for Disease Control and Prevention.
CDC. 2002d. Section 9: study summary: national survey of knowledge, attitudes, and beliefs regarding the anthrax vaccine among military personnel. Anthrax Vaccine Safety and Efficacy Plan. Atlanta: Centers for Disease Control and Prevention.
CDC. 2002e. Section 10: study summary: survey of civilian and military healthcare providers regarding the anthrax vaccine and the reporting of possible vaccine-associated adverse events. Anthrax Vaccine Safety and Efficacy Plan. Atlanta: Centers for Disease Control and Prevention.
GAO (General Accounting Office). 2000. Anthrax Vaccine: Preliminary Results of GAO’s Survey of Guard/Reserve Pilots and Aircrew Members. GAO-01-92T. Washington, D.C.: GAO.
Hubbell K. 2002. Letter to the CDC. E-mail to Clay B, Committee on Government Reform, U.S. House of Representatives, January 9.
IOM (Institute of Medicine). 2001. CDC Anthrax Vaccine Safety and Efficacy Research Program. Interim Report. Washington, D.C.: National Academy Press.
IOM. 2002. Joellenbeck LM, Zwanziger LL, Durch JS, Strom BL, eds. The Anthrax Vaccine: Is It Safe? Does It Work? Washington, D.C.: National Academy Press.
Masiello SA. 2002. Your request to supplement your biologics license application to include Hollister-Stier Laboratories LLC, Spokane, Washington, as a contract manufacturer for the filling of Anthrax Vaccine Adsorbed (AVA) has been approved. Letter to: Giri, L, BioPort Corporation, Lansing, Mich.
Wolfowitz P. 2002. Reintroduction of the Anthrax Vaccine Immunization Program (AVIP). Memorandum for Secretaries of the Military Departments; Chairman of the Joint Chiefs of Staff; Undersecretaries of Defense; Assistant Secretaries of Defense; General Counsel, Department of Defense; Inspector General, Department of Defense; Directors of Defense Agencies; Commandant of the U.S. Coast Guard. Department of Defense, Washington, D.C.









