Executive Summary
|
ABSTRACT In response to a request from Congress, the Institute of Medicine (IOM) convened a committee to conduct an analysis of the federal government’s quality enhancement processes in six government programs—Medicare, Medicaid, the State Children’s Health Insurance Program, the Department of Defense TRICARE and TRICARE for Life programs, the Veterans Health Administration program, and the Indian Health Services program. About one-third of Americans are beneficiaries of and the majority of health care providers participate in one or more of these programs. The IOM committee encourages the federal government to take full advantage of its influential position to set the quality standard for the health care sector. Specifically, regulatory processes should be used to establish clinical data reporting requirements; purchasing strategies should provide rewards to providers who achieve higher levels of quality; health care delivery systems operated by public programs should serve as laboratories for the development of 21st-century care delivery models; and applied health services research should be expanded to accelerate the development of knowledge and tools in support of quality enhancement. A strong quality infrastructure consisting of three components should be built. First, the Quality Interagency Coordinating Task Force, working with the private sector, should establish standardized performance measures to be applied in each of the government programs. Second, Congress and each of the six government programs should provide financial support and other incentives to providers to facilitate the development of information technology infrastructures. Finally, each government program should make quality reports available in the public domain for use by consumers, health care professionals, accreditation and certification bodies, and other stakeholders. |
The U.S. health care sector faces serious safety, quality, coverage, and cost challenges. The United States spends much more per capita ($4,637 in 2000) on health care than any other country (Reinhardt et al., 2002), yet Americans cannot count on receiving care that is safe and effective (Institute of Medicine, 1999; Leatherman and McCarthy, 2002). While health care represents 13 percent of the U.S. gross domestic product—about $1.3 trillion annually (Levit et al., 2002)—one in seven Americans do not even have health insurance, and there are disturbing disparities in care for certain racial and ethnic subgroups (Institute of Medicine, 2002b, 2002c).
A major redesign of the health care sector is needed (Institute of Medicine, 2001). This redesign can occur only in an environment that fosters and rewards improvement. The Institute of Medicine’s (IOM) 2001 report Crossing the Quality Chasm: A New Health System for the 21st Century calls for a “new environment for care” with payment incentives to encourage and reward innovation, precise streams of accountability and measurement reflecting quality achievements, and information and support to help engage consumers in understanding and interpreting information on quality and safety.
In this context, Congress asked the IOM to examine the federal government’s quality enhancement processes (the Healthcare Research and Quality Act of 1999, Public Law 106-129) in six government programs—Medicare, Medicaid, the State Children’s Health Insurance Program (SCHIP), the Department of Defense TRICARE and TRICARE for Life programs (DOD TRICARE), the Veterans Health Administration (VHA) program, and the Indian Health Service (IHS) program (see Table ES-1).
OVERVIEW OF CURRENT QUALITY ENHANCEMENT PROCESSES
Each of the six programs reviewed for this study has both minimum participatory standards for providers and ongoing performance assessment activities.
Minimum participatory standards for institutional providers and clinicians are intended to ensure that program participants possess minimal levels of competence and comply with health and safety requirements. For institutions, the standards include physical safety and sanitation requirements and organizational requirements that enable specific activities such as governance, credentialing of medical staff, and quality improvement processes. For clinicians, the standards generally require compliance with the licensing laws of at least one state. Minimum participation standards reflect a good deal of consistency among programs.
Across all six government programs, there has been a proliferation of
TABLE ES-1 Government Health Care Programs in Fiscal Year 2001
|
Program |
Beneficiaries |
Expenditures |
|
Medicare |
40 million aged and disabled beneficiaries |
$242.4 billion |
|
Medicaid |
42.3 million low-income persons; mostly children, pregnant women, disabled, and aged |
$227.9 billion (joint federal and state) |
|
SCHIP |
4.6 million low-income children |
$4.6 billion (joint federal and state) |
|
VHA |
4.0 million veterans |
$20.9 billion |
|
DOD TRICARE |
8.4 million active-duty military personnel and their families and military retirees |
$14.2 billion |
|
IHS |
1.4 million American Indians and Alaska Natives |
$2.6 billion |
|
TOTAL |
About 100 million peoplea |
$512.6 billion |
|
aThis estimate does not adjust for those beneficiaries who are eligible for more than one government program. SOURCES: Department of Health and Human Services, 2002; Paisano, 2002; Veterans Administration, 2001; Williams, 2002. |
||
performance assessment activities focused on the measurement of specific aspects of care processes and patient outcomes. The Medicare program relies mainly on external reviews of provider performance by quality improvement organizations (QIOs). In recent years, the Centers for Medicare and Medicaid Services (CMS) has required certain providers participating in Medicare—including Medicare+Choice plans, End Stage Renal Disease Networks, and, most recently, nursing homes—to comply with standardized quality reporting requirements. Federal law pertaining to the Medicaid program requires that states establish a plan for reviewing the appropriateness and quality of care, and most states contract with QIOs to carry out these reviews (Verdier and Dodge, 2002). The VHA, DOD TRICARE, and IHS programs all have incorporated a wealth of performance measurement activities into their health care delivery processes; in some instances, these programs also have contracts with external review organizations to review selected aspects of quality.
OPPORTUNITIES FOR IMPROVEMENT
A critical first step in addressing the nation’s serious health care safety and quality concerns is the establishment of valid and reliable measurement systems that can be used to assess the degree to which care processes are consistent with the clinical knowledge base and patients are achieving desired outcomes. Clinical quality measurement provides the essential foundation for both quality improvement and accountability.
Although the quality enhancement processes of the major government programs are moving in a reasonably consistent and appropriate direction, the current set of activities has not closed the quality gap and is unlikely to do so in the future unless changes are made. This is the case for a number of reasons:
-
A lack of consistency in performance measurement requirements both across and within individual government programs. In Medicare and Medicaid performance measurement requirements are quite extensive for managed care plans and to a lesser degree for hospitals. On the other hand, performance measurement requirements are minimal or nonexistent for noninstitutional providers under fee-for-service arrangements, which still account for the majority of health care services. States have considerable latitude in the way they choose to define, implement, and enforce quality review in Medicaid and SCHIP programs; not surprisingly, the level and degree of external review activity vary widely among and within state programs.
-
The absence of standardized performance measures, resulting in an unnecessary burden on providers and diminished usefulness of quality information. Although some government programs have undertaken efforts to adopt standardized measures, these represent isolated success stories. The majority of performance measurement activities being carried out by the major government health care programs are neither standardized nor evenly applied across the programs. For private-sector providers, who typically participate in more than one government health care program, such variability in measures results in an excessive administrative burden.
-
The lack of a conceptual framework to guide the selection of performance measures, resulting in a patchwork of measurement projects. What generally appears to be missing is a clear conceptual framework with criteria that can guide the selection of individual measures to help maximize the health of the population being served.
-
A lack of computerized clinical data. VHA and DOD have made noteworthy strides in establishing a clinical information infrastructure, and the ability of their programs to measure and improve quality through continuous feedback and the application of computerized decision sup-
-
port systems is superior to what is typically found in the private sector. On the other hand, Medicare, Medicaid, SCHIP, and DOD TRICARE contracted health services must rely to a great extent on 20th-century measurement technology (e.g., abstraction of samples of medical records and culling of information from administrative datasets).
-
The lack of a strong commitment to transparency and the availability of comparative quality data in the public domain. Key stakeholders in each of the government programs—beneficiaries, providers, accrediting and certifying entities, regulators, and purchasers—have little useful information that can guide efforts to address the serious safety and quality shortcomings of the health care sector.
-
The absence of a systematic approach for assessing the impact of quality enhancement activities. Most minimum-participatory standards have been in place for a very long time, with little effort having been made to evaluate their effectiveness and the costs of compliance.
THE NEED FOR FEDERAL LEADERSHIP NOW
The federal government has a fiduciary responsibility to taxpayers and beneficiaries to ensure that the more than $500 billion invested annually in the six government programs is used wisely. Given the current deficiencies in health care safety and quality, it is clear that the federal government should be doing much more.
No other stakeholder has the federal government’s ability to produce fundamental change throughout the health care sector. Absent strong federal leadership in addressing safety and quality concerns, progress will continue to be slow.
RECOMMENDATION 1: The federal government should assume a strong leadership position in driving the health care sector to improve the safety and quality of health care services provided to the approximately 100 million beneficiaries of the six major government health care programs. Given the leverage of the federal government, this leadership will result in improvements in the safety and quality of health care provided to all Americans.
This does not mean that the federal government should act alone. Indeed, its efforts will be far more effective if carried out in close collaboration with health care leadership from the private sector.
Each of the six government programs is already redesigning its quality enhancement processes. Unless there is more deliberate coordination, opportunities to achieve substantial gains in quality will be lost. The IOM committee that conducted this study encourages the leadership of the vari-
ous government health care programs to ensure that their quality enhancement processes adhere to the following guiding principles:
-
Government health care programs should establish consistent quality expectations and requirements and apply them fairly and equitably to all financing and delivery options within a program.
-
Government health care programs should promote and encourage providers to strive for excellence by providing financial and other rewards and public recognition to providers who achieve superior levels of quality.
-
Government health care programs should actively collaborate with each other and private-sector quality enhancement organizations with regard to all aspects of quality enhancement—including use of standardized measures and sharing of data—where doing so will likely result in greater gains in quality or reduced provider burden.
-
Government health care programs should encourage and enable active consumer participation in efforts to enhance quality through such means as the following:
-
Raising consumer awareness of the magnitude of quality and safety shortcomings and the means of addressing these problems
-
Seeking consumer input into the design and evaluation of quality enhancement processes
-
Including patient assessments of quality and service in the portfolio of performance measures
-
Providing patients with health information necessary to evaluate treatment options and participate in care management
-
Providing consumers with comparative performance data on providers and health plans
-
-
Government health care programs, in collaboration with the Agency for Healthcare Research and Quality (AHRQ), should pursue a rich agenda of applied research and demonstrations focusing on tools, techniques, and approaches to quality enhancement.
THE FEDERAL GOVERNMENT’S UNIQUE POSITION
In providing leadership to effect the needed changes in health care, the federal government should take full advantage of its unique position as a regulator; purchaser; health care provider; and sponsor of research, education, and training. As regulator, the federal government sets the standards for minimally acceptable performance. As the largest purchaser of health care services, the federal government institutes payment policies that determine the financial rewards or penalties that either spur or stifle innovations aimed at improving safety and quality. As a direct provider of health care services to veterans, military personnel and their families, and
Native Americans, the federal government can serve as a model for all aspects of health care organization and delivery. The federal government also provides support for applied health services research, much of which directly enhances the government’s ability to carry out effectively its roles as regulator, purchaser, and health care provider.
In the government health care programs that provide care through the private sector—Medicare, Medicaid, SCHIP, and to some degree DOD TRICARE—the federal government has relied primarily on regulatory approaches to promote quality. Regulatory approaches are best suited to establishing a “floor” that protects beneficiaries from providers lacking basic competencies. When it comes to encouraging providers to pursue higher standards of excellence, the regulatory approach is a blunt tool that generally fails to differentiate among grades of quality.
Purchasing strategies are aimed at raising the quality of care offered by the majority of providers. Such strategies include the provision of financial and other rewards (e.g., higher fees, Diagnosis Related Group [DRG] payments, or bonuses) to providers and health plans achieving high levels of quality. The disclosure of comparative performance data on providers and health plans draws attention to best practices in the hope of driving patient volume to the higher-quality performers, and spurring action on the part of poor and average performers to enhance their knowledge and skills or limit their scope of practice. The public disclosure of quality and safety information may also encourage professional societies, board certifying and accrediting entities, and other leadership organizations to take action to achieve broader adherence to defined standards of care.
In its provider role, the federal government assumes all the responsibilities of ownership of health care institutions, employment of the health care workforce, and management and operation of comprehensive delivery systems. In this capacity, it has an opportunity to serve as a laboratory for the testing of new financing, delivery, and information dissemination models while experimenting with various quality measurement and improvement strategies. The three government programs that provide services directly—the VHA, DOD TRICARE, and IHS programs—have led the way in building clinical information systems to support care delivery, quality improvement, surveillance and monitoring, and many other applications. Since taxpayer dollars have financed the development of these systems, more should be done to facilitate their application in other parts of the health care system.
As a major sponsor of applied health services research, the federal government provides support for the development of knowledge and the creation of tools needed to carry out more effectively the roles of regulator, purchaser, and health care provider. Through AHRQ and other ap-
plied research programs sponsored by VHA, the National Institutes of Health, and the Centers for Disease Control and Prevention, the federal government can and has assisted in the development of quality measures, survey instruments, and public reporting tools. The federal government also supports applied health services research that addresses many of the broader health care financing and delivery issues whose resolution is important to creating an environment that supports quality.
RECOMMENDATION 2: The federal government should take maximal advantage of its unique position as regulator, health care purchaser, health care provider, and sponsor of applied health services research to set quality standards for the health care sector. Specifically:
-
Regulatory processes should be used to establish clinical data reporting requirements applicable to all six major government health care programs.
-
All six major government health care programs should vigorously pursue purchasing strategies that encourage the adoption of best practices through the release of public-domain comparative quality data and the provision of financial and other rewards to providers that achieve high levels of quality.
-
Not only should health care delivery systems operated by the public programs continue to serve as laboratories for the development of innovative 21st-century care delivery models, but much greater emphasis should be placed on the dissemination of findings and, in the case of information technology, the creation of public-domain products.
-
Applied health services research should be expanded and should emphasize the development of knowledge, tools, and strategies that can support quality enhancement in a wide variety of settings.
Congress should provide the appropriate direction, enabling authority, and resources to the government health care programs for carrying out this mandate.
THE TRANSFORMATION OF QUALITY ENHANCEMENT
At present, the federal government is seriously hampered in performing its purchasing, regulatory, and provider functions by a lack of information on clinical quality—the degree to which the care received by beneficiaries is consistent with the science base (i.e., effective) and provided in a technically competent fashion (i.e., safe).
Variability in performance measures and activities across and within government programs limits the ability to draw comparisons; imposes an unnecessary burden on providers that participate in multiple programs; and makes it difficult to obtain a complete picture of the quality of care for beneficiaries, especially dual eligibles. The uneven application of performance measurement requirements across various delivery sites and beneficiary populations on the part of some government programs fails to provide equitable protections to all program beneficiaries. Moreover, providers do not receive strong, consistent signals as to where quality improvement is needed. There is also a paucity of comparative quality data available in the public domain for use by other stakeholders, including consumers, providers, professional associations, purchasers, and private accrediting and certifying entities.
RECOMMENDATION 3: Congress should direct the Secretaries of the Department of Health and Human Services, Department of Defense, and Department of Veterans Affairs to work together to establish standardized performance measures across the government programs, as well as public reporting requirements for clinicians, institutional providers, and health plans in each program. These requirements should be implemented for all six major government health care programs and should be applied fairly and equitably across various financing and delivery options within those programs. The standardized measurement and reporting activities should replace the many performance measurement activities currently under way in the various government programs.
The proposed changes in quality enhancement processes will necessitate substantial reorientation and operational change within all three government branches, so leadership and support will be necessary from the highest levels of the Department of Health and Human Services (DHHS), DOD, and VHA. At the same time, there should be a focal point for coordination and accountability. Congress should consider directing the Secretary of Health and Human Services to assume a lead role in producing an annual progress report detailing the collaborative and individual efforts of the various government programs to redesign their quality enhancement processes.
To achieve the objective of this recommendation, a stronger quality infrastructure consisting of three major components—standardized performance measures, computerized clinical information, and comparative quality reporting in the public domain—must be developed.
Standardized Performance Measures
In developing a menu of standardized performance measures for health care quality, the federal government should not reinvent the wheel. Rather, it should build on work already under way in both the private and public sectors to establish a common conceptual framework for performance measurement and reporting. In 2003, DHHS will be releasing the first National Healthcare Quality Report, which will include measures relevant to six national quality aims recommended by the IOM in an earlier report—safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity (Institute of Medicine, 2001). The National Healthcare Quality Report will also focus on priority health areas—common chronic conditions and health care needs of the population (Institute of Medicine, 2002a). Use of a common conceptual framework, common terminology, and, whenever possible, standardized measures for reporting at all levels—national, regional, and provider-specific—will facilitate understanding and action on the part of all stakeholders and reduce the burden of compliance.
The Quality Interagency Coordination Task Force (QuIC) (or some similar interdepartmental structure) should play a pivotal role in the establishment of standardized performance measures. The QuIC was created in 1998 to provide coordination across federal agencies involved in regulating, purchasing, providing, and studying health care services. Its membership already includes representatives of CMS, AHRQ, VHA, DOD, IHS, and other federal programs, and could be expanded to include representatives from state Medicaid and SCHIP programs and consumers. The QuIC should collaborate with such private-sector groups as the National Quality Forum (NQF), the Leapfrog Group, the National Committee for Quality Assurance (NCQA), the Joint Commission on Accreditation of Healthcare Organizations (JCAHO), and the Foundation for Accountability (FAACT) in the development of performance measures to avoid duplication of effort and conflict with the activities of these groups. NQF, in particular, was established in 1999 to convene public- and private-sector stakeholders to seek consensus around standardized measures (Miller and Leatherman, 1999), and CMS has worked collaboratively with NQF to develop standardized measures in particular areas (National Quality Forum, 2000).
RECOMMENDATION 4: The QuIC should promulgate standardized sets of performance measures for 5 common health conditions in fiscal year (FY) 2003 and another 10 sets in FY 2004.
-
Each government health care program should pilot test the first 5 sets of measures between FY 2003 and FY 2005 in a limited num-
-
ber of sites. These pilot tests should include the collection of patient-level data and the public release of comparative performance reports.
-
All six government programs should prepare for full implementation of the 15-set performance measurement and reporting system by FY 2008. The government health care programs that provide services through the private sector (i.e., Medicare, Medicaid, SCHIP, and portions of DOD TRICARE) should inform participating providers that submission of the audited patient-level data necessary for performance measurement will be required for continued participation in FY 2007. The government health care programs that provide services directly (i.e., VHA, the remainder of DOD TRICARE, and IHS) should begin work immediately to ensure that they have the information technology capabilities to produce the necessary data.
Although there should be a common menu of standardized performance measures, not all measures would have to be implemented in all government programs. Each program would select the subset of measures that corresponded to its beneficiaries’ clinical needs.
Computerized Clinical Data
Although it may be feasible in the short run for providers to produce the patient-level data needed for performance measurement through record abstraction or special data collection instruments, it will not always be possible to rely upon such approaches as the menu of measures expands—nor should it be. Computerized clinical data and decision support systems are a prerequisite for the safe provision of quality care (Institute of Medicine, 2001). The potential benefits of computerized clinical data and decision support have long been recognized (Institute of Medicine, 1997), but only recently has the evidence base emerged to substantiate these expectations (Balas et al., 2000; Bates et al., 1999; Classen et al., 1997; Leapfrog Group, 2000; Raymond and Dold, 2002; Shea et al., 1996).
In general, the health care delivery sector has lagged behind other industries in adopting and making innovative use of information technology. This is especially true for the private sector, and less so for the public-sector delivery systems of VHA and DOD.
The federal government has an important role to play in offering financial incentives to health care providers. It was beyond the scope of this study to develop estimates of the resources required to build the necessary information technology infrastructure, but the need for such support should not be underestimated. In the absence of adequate assistance, it
will not be possible for providers to adhere to the ambitious timetable for quality enhancement proposed in this report.
RECOMMENDATION 5: The federal government should take steps immediately to encourage and facilitate the development of the information technology infrastructure that is critical to health care quality and safety enhancement, as well as to many of the nation’s other priorities, such as bioterrorism surveillance, public health, and research. Specifically:
-
Congress should consider potential options to facilitate rapid development of a national health information infrastructure, including tax credits, subsidized loans, and grants.
-
Government health care programs that deliver services through the private sector (Medicare, Medicaid, SCHIP, and a portion of DOD TRICARE) should adopt both market-based and regulatory options to encourage investment in information technology. Such options might include enhanced or more rapid payments to providers capable of submitting computerized clinical data, a requirement for certain information technology capabilities as a condition of participation, and direct grants.
-
VHA, DOD, and IHS should continue implementing clinical and administrative information systems that enable the retrieval of clinical information across their programs and can communicate directly with each other. Whenever possible, the software and intellectual property developed by these three government programs should rely on Web-based language and architecture and be made available in the public domain.
In addition to offering financial incentives to providers, the federal government should play a stronger role in the establishment of national standards for the collection, coding, and classification of clinical and other health care data (National Committee on Vital and Health Statistics, 2001). Some degree of technical assistance may also be required, especially for safety net providers.
Comparative Quality Reporting
There are many potential uses of comparative quality data. First, providers and care systems that are working to achieve continuous improvement might use the data for benchmarking purposes and to inform decisions regarding referral of patients to specialists and hospitals. Second, patients and group purchasers might access the data when choosing health plans or providers. Third, professional groups, including board
certification entities such as the American Board of Medical Specialties and its member boards, might be able to use the data to identify best practices and to assist in making credentialing decisions. Fourth, private accreditation organizations, such as NCQA and JCAHO, and public regulatory programs might use the data in their efforts to assess provider compliance with requirements and to provide information to the public. Fifth, the data will likely be useful to states, communities, and public health groups as a tool for identifying gaps in quality and monitoring the impact of community-wide efforts to close these gaps.
RECOMMENDATION 6: Starting in FY 2008, each government health care program should make comparative quality reports and data available in the public domain. The programs should provide for access to these reports and data in ways that meet the needs of various users, provided that patient privacy is protected.
Many private-sector stakeholders, such as accreditors and purchasers, already impose quality reporting requirements on providers. The committee encourages the government programs to work with these groups on the design, pilot testing, and rollout of the above reports. Doing so will increase the likelihood that these stakeholders will incorporate the standardized measures and reports into their processes, thus further reducing administrative burden.
A mechanism should be established for pooling data from each of the government health care programs. Pooled data would facilitate benchmarking across a wide variety of financing and delivery arrangements, population subgroups, and geographic areas. The availability of such data would also allow for the analysis of more complete care patterns for beneficiaries receiving services under more than one government program.
RECOMMENDATION 7: The government health care programs, working with AHRQ, should establish a mechanism for pooling performance measurement data across programs in a data repository. Contributions of data from private-sector insurance programs should be encouraged provided such data meet certain standards for validity and reliability. Consumers, health care professionals, planners, purchasers, regulators, public health officials, researchers, and others should be afforded access to the repository, provided that patient privacy is protected.
The desirability of providing broad access to the repository by many stakeholders must be balanced by the need to both protect patient privacy and minimize harmful, unintended consequences of public disclosure. Patient-level data included in the repository should be deidentified to pre-
vent individual patient identification, and any users violating data access policies should be subject to severe penalties.
NEED FOR APPLIED HEALTH SERVICES RESEARCH
Steps should be taken to ensure that the health services research agendas developed by the various government programs are complementary, address the needs of the populations served, and advance the capabilities of quality enhancement processes to promote excellence. Given its mission to coordinate the implementation of quality enhancement processes among the six government programs and its representative membership, the QuIC is well positioned to serve as the coordinating entity through which programs would provide input on the research and development agenda. AHRQ should staff the QuIC and provide the organizational locus of QuIC research activity.
RECOMMENDATION 8: The six government health care programs should work together to develop a comprehensive health services research agenda that will support the quality enhancement processes of all programs. The QuIC (or some similar interdepartmental structure with representation from each of the government health care programs and AHRQ) should be provided the authority and resources needed to carry out this responsibility. The agenda for FY 2003–2005 should support the following:
-
Establishment of core sets of standardized performance measures
-
Ongoing evaluation of the impact of the use of standardized performance measurement and reporting by the six major government health care programs
-
Development and evaluation of specific strategies that can be used to improve the federal government’s capability to leverage its purchaser, regulator, and provider roles to enhance quality
-
Monitoring of national progress in meeting the six national quality aims (safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity)
Formulation of the federal health services research agenda should address the immediate need of the QuIC to establish a core set of standardized performance measures. Efforts should be made to address methodological issues, especially those related to the assessment of quality at the small group or individual clinician level. Attention should also be focused on the design and pilot testing of alternative reporting formats tailored to the needs of various users.
Evaluation of the impact of standardized performance measurement and reporting efforts should include assessment of the associated reduction in burden, as well as identification of opportunities for further eliminating redundancy and ineffective regulatory requirements. As discussed above, current quality enhancement processes fall into two categories: minimum participatory requirements and performance assessment. The specific performance measurement activities in each of the government programs that have been superceded by standardized measurement and reporting activities should be documented. Once a robust quality infrastructure has been established, an assessment should be conducted to determine whether some minimum participatory standards might be eliminated.
GETTING FROM HERE TO THERE
The committee has formulated a very rigorous implementation strategy that calls for the release of an initial set of comparative performance reports for a limited set of standardized measures in 3 years and a fully operational process in 6 years (see Figure ES-1). Specifically, the QuIC would identify standardized performance measure sets for 5 priority areas in FY 2003 and for 10 more in FY 2004.
Pilot testing of the first 5 sets of measures would begin immediately, with the objective of each government program’s release of comparative performance reports (probably for a few selected geographic areas) for this limited number of measures in FY 2005. Starting in FY 2007, providers participating in the government programs that offer services through the private sector would be required to submit performance data as a condition of participation. Installation of compatible information technology systems across VHA, DOD, and IHS should be completed in 2006 to enable better evaluation of quality of care in government-operated health programs. In FY 2008, each government health care program should publicly release a comprehensive set of comparative quality reports for all 15 priority areas and all provider types. A vigorous applied research and demonstration capability will be necessary throughout this period, starting in FY 2003 with the development of an agenda to address key measurement and methodological issues, design of the pilot test, conduct of periodic evaluations, and preparation of a final evaluation upon completion of the 6-year implementation period.
The committee realizes this is an ambitious agenda. It does not, however, represent a radical departure from the status quo, but rather a rapid scaling up of the most promising, cutting-edge quality enhancement projects currently under way. It is important not to underestimate the challenges of progressing from what are essentially promising pilot
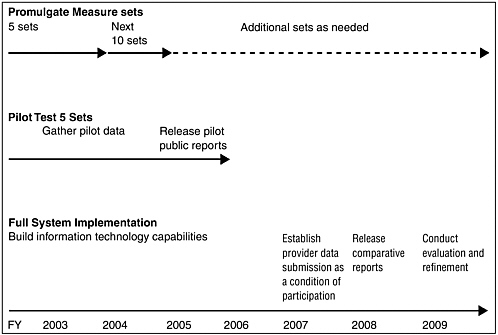
FIGURE ES-1 Implementation timeline.
projects to widespread adoption of a new strategic direction and major redesign of the quality enhancement processes of the six government programs. The committee has identified four factors that will be critical to the success of this endeavor.
First, to avoid major delays in moving forward, it would be wise to identify potential legal and regulatory barriers early on and determine how best to address these barriers expeditiously. For example, does the emphasis on public reporting of deidentified data raise any concerns with regard to the confidentiality and security provisions of the Health Insurance Portability and Accountability Act? Will additional enabling authority be required for CMS to move forward with demonstration projects that provide financial rewards to providers who achieve exemplary performance?
Second, conscious and careful redesign of the quality enhancement processes of each of the major government programs will be necessary. The standardized quality measurement and reporting activities proposed in this report are not intended to represent another layer of government oversight, but rather to replace the patchwork of quality measurement activities and projects currently under way. Nowhere will these changes be more significant than in the Medicare, Medicaid, and SCHIP programs, which rely on a mix of public- and private-sector external review entities,
and in some cases on a blend of federal and state requirements and initiatives.
The backbone of Medicare’s external quality review processes is the QIOs. The QIOs are engaged in some projects that employ common measures and methodologies across most or all states, and this approach is increasingly becoming the norm. Much of the quality-related data used by QIOs is abstracted from samples of paper medical records or culled from claims data—methods that will likely become obsolete as computerized patient-level data become more readily available. QIOs have limited experience in public reporting, although sizable resources are earmarked for this function in their Seventh Scope of Work. Contractual, cultural, organizational, and programmatic modifications will be required for the QIO program to continue playing a central role in the quality enhancement processes of Medicare and other government health care programs.
The challenges for state Medicaid and SCHIP programs will also be significant. Under the proposed restructuring, states will be asked to relinquish some flexibility and to work in partnership with each other and federal government representatives from the six programs to agree upon standardized performance measurement sets and to apply these standardized measures across their Medicaid and SCHIP programs. States have already worked with CMS, each other, and NCQA on the development of the Health Plan Employer Data and Information Set (HEDIS) standardized measures adapted for state Medicaid programs, and these measures are being used for health plans with a good deal of uniformity in adoption and application. What is being sought here is a much stronger commitment to standardized measurement and reporting.
Third, strong support from the appropriate authorization and appropriations committees of Congress will be critical to success. Federal financial assistance to providers will be essential to establishing the necessary information technology infrastructure. AHRQ will require additional funds to provide adequate support to the QuIC for the establishment and maintenance of the menu of standardized measures, the design and pilot testing of reporting formats, the establishment and operation of the repository of pooled data, and the conduct of periodic evaluations of the impact of the new quality enhancement strategies. Lastly, some initial support for each of the government programs will also be needed so they can redesign their current oversight processes and establish the capacities required to receive and process the necessary clinical data and produce reports.
Fourth, the federal and state governments should immediately begin working in partnership with health care leaders, including representatives of consumer groups, the health professions, and health care organizations. It will be challenging to transform the current quality enhance-
ment processes of the government programs into standardized quality reporting structures that are transparent and contribute to an environment rich in comparative quality information. This transformation will be much smoother if the QuIC and CMS, in particular, establish strong partnerships with stakeholders. Consumers, participating providers, and health plans should receive complete and timely information—there should be no surprises. Special efforts should be made early on to secure the support of leaders from the medical, nursing, and other health professions, and to build on the basic tenets of professionalism (American Board of Internal Medicine et al., 2002). The government health care programs should move expeditiously to address all legitimate questions and concerns about their quality enhancement processes, including the reliability and validity of information, measures, and data. All parties should recognize that problems will be encountered along the way with data, measures, and reports, necessitating continuous improvement and refinement. There must also be rewards and benefits for providers taking part in these efforts in the form of reduced regulatory burden, feedback of information useful for quality improvement, and public recognition and financial rewards for exemplary performance.
REFERENCES
American Board of Internal Medicine, ACP-ASIM Foundation, and European Federation of Internal Medicine. 2002. Medical professionalism in the new millennium: a physician charter. Ann Intern Med 136 (3).
Balas, E. A., S. Weingarten, C. T. Garb, D. Blumenthal, S. A. Boren, and G. D. Brown. 2000. Improving preventive care by prompting physicians. Arch Intern Med 160 (3):301-8.
Bates, D. W., J. M. Teich, J. Lee, D. Seger, G. J. Kuperman, N. Ma’Luf, D. Boyle, and L. Leape. 1999. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc 6 (4):313-21.
Classen, D. C., S. L. Pestrotnik, R. S. Evans, et al. 1997. Adverse drug events in hospitalized patients. JAMA 277 (4):301-6.
Department of Health and Human Services. 2002. 2002 CMS Statistics. CMS Publication No. 03437. Baltimore MD: U.S. Department of Health and Human Services.
Institute of Medicine. 1997. The Computer-Based Patient Record: An Essential Technology for Health Care. Washington DC: National Academy Press.
———. 1999. To Err Is Human: Building a Safer Health System. Washington DC: National Academy Press.
———. 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academy Press.
———. 2002a. Priority Areas for National Action: Transforming Health Care Quality. Washington DC: National Academy Press.
———. 2002b. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. B. Smedley, A. Stith, and A. Nelson, eds. Washington DC: National Academy Press.
———. 2002c. Care Without Coverage. Washington DC: National Academy Press.
Leapfrog Group. 2000. “Fact Sheet: Computer Physician Order Entry (CPOE).” Online. Available at http://www.leapfroggroup.org/FactSheets/CPOE_FactSheet.pdf [accessed May 16, 2002].
Leatherman, S., and D. McCarthy. 2002. Quality of Health in the United States: A Chartbook. New York NY: The Commonwealth Fund.
Levit, K., C. Smith, C. Cowan, et al. 2002. Inflation spurs health spending in 2000. Health Aff (Millwood) 21 (1):172-81.
Miller, T., and S. Leatherman. 1999. The National Quality Forum: a “me-too” or a breakthrough in quality measurement and reporting? Health Aff (Millwood) 18 (6):233-7.
National Committee on Vital and Health Statistics. 2001. “Information for Health: A Strategy for Building the National Health Information Infrastructure.” Online. Available at http://ncvhs.hhs.gov/nhiilayo.pdf [accessed May 14, 2002].
National Quality Forum. 2000. NQF project brief: Measuring serious, avoidable adverse events in hospital care. National Forum for Health Care Quality Measurement and Reporting.
Paisano, E. (IHS). 1 July 2002. IHS total expenditure data. Personal communication to Barbara Smith.
Raymond, B., and C. Dold. 2002. Clinical Information Systems: Achieving the Vision. Oakland CA: Kaiser Permanente for Health Policy.
Reinhardt, U. E., P. S. Hussey, and G. F. Anderson. 2002. Cross-national comparisons of health systems using OECD data, 1999. Health Aff (Millwood) 21 (3):169-81.
Shea, S., W. DuMouchel, and L. Bahamonde. 1996. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inform Assoc 3 (6): 399-409.
Verdier, J., and R. Dodge. 2002. Other Data Sources and Uses, Working Paper in the Informed Purchasing Series. Lawrenceville NJ: Center for Health Care Strategies.
Veterans Administration. 2001. “VA Budget Overview 2002.” Online. Available at http://www.va.gov/pressrel/bdgtovrw_files/bdgtovrw.htm [accessed June 28, 2002].
Williams, T. (TMA) 2 July 2002. TRICARE total expenditure data. Personal communication to Barbara Smith.




















