Complementary and Integrative Medical Therapies: Current Status and Future Trends
David Eisenberg
I. DEFINITIONS AND TERMINOLOGY
“Complementary,” “Alternative,” and “Integrative” Medical Approaches
Complementary and alternative medical (CAM) therapies encompass a broad spectrum of practices and beliefs (1). From an historical standpoint, they may be defined “... as practices that are not accepted as correct, proper, or appropriate or are not in conformity with the beliefs or standards of the dominant group of medical practitioners in a society” (2). From a functional standpoint, complementary (a.k.a.”alternative”) therapies may be defined as interventions neither taught widely in medical schools nor generally available in hospitals (3). Ernst et al. contend that “complementary” medical techniques “[complement] mainstream medicine by contributing to a common whole, by satisfying a demand not met by orthodoxy or by diversifying the conceptual frameworks of medicine” (4). The terminology currently in use to describe these practices remains controversial. Many commonly used labels (e.g., “alternative,” “unconventional,” “unproven”) are judgmental and may inhibit the collaborative inquiry and discourse necessary to distinguish useful from useless techniques (5). Complementary and alternative medicine (CAM) is the language currently used by the National Institute of Health (NIH) and U.S. federal agencies to describe this field of inquiry. The NIH National Center for Complementary and Alternative Medicine (NCCAM) defines CAM as “healthcare practices outside the realm of conventional medicine, which
are yet to be validated using scientific methods.” Two recent articles by Kaptchuk et al., explore the taxonomy of CAM therapies in the context of medical pluralism (6;7).
Integrative medicine refers to ongoing efforts to combine the best of conventional and evidence-based complementary therapies while emphasizing the primacy of the patient-provider relationship and the importance of patient participation in health promotion, disease prevention, and medical management. “It (integrative medicine) views patients as whole people with minds and spirits as well as bodies and includes these dimensions into diagnosis and treatment” (8). In the January 2001 British Medical Journal edition devoted entirely to integrated medicine, the Journal’s editor, Richard Smith, wrote: “It mightn’t be too pretentious (although it might) to say that such a growth (of integrative medicine) might restore the soul to medicine—the soul being that part of us that is the most important but the least easy to delineate” (9). A variety of articles and editorials have wrestled with the challenges of properly labeling and describing this field of inquiry (8;10-16).
Dietary Supplements
The Dietary Supplement Health and Education Act (DSHEA) defines dietary supplements as products (other than tobacco) intended to supplement the diet that bear or contain one or more of the following dietary ingredients: a vitamin, mineral, amino acid, herb or other botanical; or a dietary substance for use to supplement the diet by increasing the total dietary intake; or a concentrate, metabolite, constituent, extract, or combination of any ingredient described above; and intended for ingestion in the form of a capsule, powder, soft gel, or gelcap, and not represented as a conventional food or as a sole item of a meal or the diet. The DSHEA legislation stipulates that botanicals and other dietary supplements are not “drugs” and, as such, are not held to the same regulatory requirements as drugs (i.e., prerequisite evidence of both safety and efficacy). Manufacturers of dietary supplements are not allowed to make “disease claims” but are permitted to make “structure/function” claims. This has resulted in a range of interpretations and has complicated both clinical decision making and efforts to perform scientific research involving botanicals (17;18).
II. EPIDEMIOLOGY
A. Prevalence, Costs, and Patterns of Use of CAM Therapies in the United States
Findings from a 1997 follow-up national survey of complementary and alternative medicine (CAM) prevalence, costs, and patterns of use (19) include the following:
Between 1990 and 1997:
-
The prevalence of CAM use increased by 25 percent from 33.8 percent in 1990 to 42.1 percent in 1997.
-
The prevalence of herbal remedy use increased by 380 percent.
-
The prevalence of high-dose vitamin use increased by 130 percent.
-
The total number of visits to CAM providers increased by 47 percent from 427 million in 1990 to 629 million in 1997.
-
The total visits to CAM providers (629 million) exceeded total visits to all primary care physicians (386 million) in 1997.
-
In 1997, adults made an estimated 33 million office visits to professionals for advice regarding the use of herbs and high-dose vitamins.
-
Estimated expenditures for CAM professional services increased by 45 percent exclusive of inflation and in 1997 were estimated at $21.2 billion dollars.
-
Out-of-pocket expenditures for herbal products and high-dose vitamins in 1997 were estimated at $8 billion.
-
Out-of-pocket expenditures for CAM professional services in 1997 were estimated at $12.2 billion. This exceeded the out-of-pocket expenditures for all U.S. hospitalizations.
-
Total out-of-pocket expenditures relating to CAM therapies were conservatively estimated at $27.0 billion. This is comparable to the projected out-of-pocket expenditures for all U.S. physician services.
-
An estimated 15 million adults in 1997 took prescription medications concurrently with herbal remedies and/or high-dose vitamins. These individuals are therefore at risk for potential adverse drug-herb or drug-supplement interactions.
-
Current use of CAM services is likely to under-represent utilization patterns if insurance coverage for CAM therapies increases in the future.
-
Despite the dramatic increases in the use and expenditures associated with CAM services, the extent to which patients disclose their use of CAM therapies to their physicians remains low. Fewer than 40 percent of CAM therapies used were disclosed to a physician in both 1990 and 1997.
The authors concluded that CAM use and expenditures increased substantially between 1990 and 1997, attributable primarily to an increase in the proportion of the population seeking CAM therapies, rather than increased visits per patient.
Other nationally representative surveys of CAM prevalence and patterns of use have provided additional useful information. These include a study by Astin (20) which concluded that “…the majority of alternative medicine users appear to be doing so not so much as a result of being dissatisfied with conventional medicine, but largely because they find their health care alternatives to be more congruent with their own values, beliefs and philosophical orientation towards health and life.” Druss and Rosenheck’s national survey (21) found that practitioner-based CAM therapies appear to serve more as a complement than an alternative to conventional medicine; and, individuals in the top quartile of numbers of physician visits were more than twice as likely as those in the bottom quarter to have used CAM therapies during the prior year. Two recent analyses of national survey data provide additional information regarding CAM patterns of use in adults over age 65 (22) and adults with anxiety or depression (23).
A recent publication by Kessler et al. examines the long-term trends in the use of CAM in the United States (24). It found that 68 percent of adults had used at least one CAM therapy in their lifetime; and lifetime use steadily increased with age across age cohorts: approximately three in 10 respondents in the pre-baby boom cohort, five of 10 in the baby boom cohort, and seven to 10 in the post baby boom cohort reported using some type of CAM therapy by age 33. Moreover, a wide range of individual CAM therapies increased in use over time, and the growth was similar across all major sociodemographic sectors. The authors concluded, “Use of CAM therapies by a large proportion of the study sample is the result of a secular trend that began at least a half century ago. This trend suggests a continuing demand for CAM therapies that will offset health care delivery for the foreseeable future.”
A recent publication by Eisenberg et al. examined perceptions about CAM therapies relative to conventional therapies among adults who used both. The authors found that the majority of CAM therapy users: (1) perceived the combination of CAM and conventional care to be superior to either alone (79 percent); (2) typically saw a medical doctor before or concurrent with their visits to a CAM provider (70 percent); (3) had a similar level of perceived confidence in both their CAM provider and MD; and (4) they did not disclose their CAM therapy to their medical doctor (63-72 percent). Principal reasons for nondisclosure were: “It wasn’t important for the doctor to know” (61 percent); “The doctor never asked” (60 percent); “It was none of the doctor’s business” (31 percent); and “The doctor
would not understand” (20 percent). Fewer respondents (14 percent) thought their doctor would disapprove of or discourage CAM use. The authors concluded that, “Adults who use both expect to value both and that to be less concerned about their doctor’s disapproval than about their doctor’s inability to understand or incorporate CAM therapy use within the context of their medical management.” (25)
The above-mentioned surveys are all based on nationally representative random samples of adult Americans. In addition, there have been a number of convenience samples investigating CAM therapy use among individuals with a particular condition or disease. Examples include surveys involving CAM therapy use among individuals with: cancer (26-35); rheumatologic disorders (36-38); self-reported disability (39); HIV (40); inflammatory bowel disease (41); and rhinosinusitis (42); as well as surgical patients (43); and patients in an emergency department (44).
National surveys performed outside the United States suggest that CAM is popular throughout the industrialized world (45). The percentage of the population who used CAM therapies during the prior 12 months has been estimated to be 10 percent in Denmark (1987) (46), 33 percent in Finland (1982) (47), and 49 percent in Australia (1993) (48). Public opinion polls and consumers’ association surveys suggest high prevalence rates throughout Europe and the United Kingdom (49-52). The percentage of the Canadian population who saw a CAM therapy practitioner during the previous 12 months has been estimated at 15 percent (1995) (53). The wide range of utilization rates can be explained, in part, by the disparity in definitions of CAM therapy and the selection of therapies assessed.
B. Prevalence and Patterns of Use of Herbal Products, Vitamins, and Non-Herbal Dietary Supplements in the United States
A recent JAMA publication by Kaufman et al. (54) describes patterns of medication use (for both prescription and non-prescription drugs) by the ambulatory adult population of the United States. Among their findings were the observations that: (1) 40 percent of the population routinely used one or more vitamin or mineral supplements; (2) herbals and supplements were taken by 14 percent of the population over the prior week; (3) among prescription drug users, 16 percent also took an herbal or supplement.
Attitudes Toward Dietary Supplement Regulation
A recent study by Blendon et al. (55), involving Americans’ views on regulating dietary supplements, suggests that:
-
Forty-four percent of users believe MDs know “little” or “not much at all” about these products.
-
Seventy-two percent would continue use even if a government scientific study was negative.
-
Eighty-one percent would require evidence of efficacy, safety, and FDA approval prior to allowing for the sale of the product.
TABLE 1 Ten Most Commonly Used Vitamins/Minerals and Herbals/ Supplements
|
Ten Most Commonly Used Vitamins/Minerals* |
Ten Most Commonly Used Herbals/Supplements* |
||
|
Vitamin/Mineral |
% Use |
Herbal/Supplement |
% Use |
|
Multivitamin |
26 |
Ginseng |
3.3 |
|
Vitamin E |
10 |
Gingko |
2.2 |
|
Vitamin C |
9 |
Garlic |
1.9 |
|
Calcium |
9 |
Glucosamine |
1.9 |
|
Magnesium |
3 |
St. John’s Wort |
1.3 |
|
Zinc |
2 |
Echinacea |
1.3 |
|
Folic Acid |
2 |
Lecithin |
1.1 |
|
Vitamin B12 |
2 |
Chondroitin |
1.0 |
|
Vitamin D |
2 |
Creatine |
0.9 |
|
Vitamin A |
2 |
Saw Palmetto |
0.9 |
|
Any Vitamin/Mineral |
40 |
Any Use |
14 |
|
*Kaufman, et al. (54). |
|||
In light of these findings, the authors conclude that there is broad public support to increase governmental regulation to ensure that advertising claims about health benefits of dietary supplements are true.
III. EDUCATIONAL PROGRAMS
A survey of courses involving CAM at U.S. medical schools was published in the 1998 JAMA theme issue devoted to medical education (56). This article, by M. Wetzel et al., included the following results: 64 percent of the U.S. medical schools reported offering courses on CAM. Of the 123 courses reported, 68 percent were stand-alone electives and 31 percent were part of required courses. Common topics included chiropractic, acupuncture, homeopathy, herbal therapies, and mind-body techniques. The American Association of Medical Colleges has established a Special Inter-
TABLE 2
TABLE 3
|
Non-Herbal Dietary Supplement Sales |
Top Herbs, U.S. vs. Europe |
|||
|
|
$Millions |
|
United States† |
Europe‡ |
|
Glucosamine / chondroitin |
288 |
1 |
Gingko Biloba |
Gingko Biloba |
|
CoQ-10 |
41 |
2 |
St. John’s Wort |
St. John’s Wort |
|
Melatonin |
31 |
3 |
Ginseng |
Horse Chestnut |
|
Amino acids |
21 |
4 |
Garlic |
Yeast |
|
Fish oil / omega fatty acid |
14 |
5 |
Echinacea |
Hawthorn |
|
DHEA |
11 |
6 |
Saw Palmetto |
Myrtle |
|
Acidophilus |
11 |
7 |
Kava Kava |
Saw Palmetto |
|
Lecithin |
10 |
8 |
Soy |
Stinging Nettle |
|
Gelatin |
8 |
9 |
Valerian |
Ivy |
|
Glucose |
7 |
10 |
Evening Primrose |
Mistletoe |
|
Shark cartilage |
6 |
|
||
|
(Drug Store News, May 2000) |
† Drug Store News, May 2000 ‡German Commission E, 1998 |
|||
est Group devoted to CAM, and this topic continues to be discussed at the AAMC’s annual meetings and by the AAMC Council of Deans.
An article by Caspi et al. questions “whether a true integration of conventional and unconventional therapies is even possible” and addresses educational options in this regard (57).
In recent years, the NIH NCCAM has awarded multiple educational training grants to a growing number of medical schools, universities, and CAM educational institutions. These grants include the following: Fellowship Training Program Grants; Faculty Development Awards; Curriculum Development Grants; and support for CAM-related educational conferences and meetings. Ten medical schools have received curriculum development grants (R-25) and will be meeting to discuss reproducible models of CAM-related curriculum reform. (See NCCAM website: www.nccam.nih.gov for additional information; see also the Macy Foundation proceedings relating to CAM education [58].) Currently, there is no standardized curriculum involving CAM medicine educational objectives at the undergraduate, post-graduate, or continuing medical educational levels.
IV. RESEARCH: BEST EVIDENCE
In 1992, the NIH established the Office of Alternative Medicine. In November of 1998, Congress established the National Center for Complementary and Alternative Medicine (NCCAM). Its mission is: “To prevent and alleviate human suffering through research on the safety and effectiveness of CAM modalities and through research, training, and information dissemination for healthcare providers and consumers.” Currently, the NIH supports more than 200 studies involving complementary and alternative medicine therapies. (Additional information on NCCAM can be found at: http://www.nccam.nih.gov)
The NIH has also established the Office of Dietary Supplements (ODS). The scientific goals of the ODS include:
Goal 1: Evaluate the role of dietary supplements in the prevention of disease and reduction of risk factors associated with disease.
Goal 2: Evaluate the role of dietary supplements in physical and mental health and in performance.
Goal 3: Explore the biochemical and cellular effects of dietary supplements on biological systems and their physiological impact across the life cycle.
Goal 4: Improve scientific methodology as related to the study of dietary supplements.
Goal 5:Inform and educate scientists, healthcare providers, and the public about the benefits and risks of dietary supplements.
(Additional information on the ODS can be found at http://odp.od.nih.gov/ods/about/about.html)
Prior to 1990, relatively little was known about the relative safety, efficacy, cost-effectiveness, and mechanism of action of individual CAM therapies. Increasingly, however, the peer-reviewed medical literature has included consensus conferences, randomized controlled trials, systematic reviews, and meta-analyses involving CAM therapies. Noteworthy examples of recently published original trials and reviews include:
Selected Consensus Reports, Clinical Trials, and Reviews Suggesting That CAM Therapies May Be Effective and/or Warrant Further Clinical Investigation
-
Mind/Body Techniques for Pain, Insomnia (61)
-
Acupuncture for Nausea and Dental Pain (64)
-
Psychosocial Support Groups for Cancer (65)
-
Homeopathy as Distinct from Placebo (66)
-
St. John’s Wort for the Treatment of Depression (67)
-
St. John’s Wort vs. Imipramine vs. Placebo (68)
-
Gingko for the Treatment of Alzheimer’s Type Dementia (69;70)
-
Chinese Herbs for the Treatment of Irritable Bowel Syndrome (71)
-
Saw Palmetto for the Treatment of Benign Prostatic Hyperplasia (72)
-
Kava Kava for Anxiety (78)
-
Homeopathy for Vertigo (79)
-
Homeopathy for Allergic Rhinitis (80)
-
Osteopathic Manipulation for Low Back Pain (81)
-
Moxibustion for Breech Presentation (82)
-
Acupuncture for Recurrent Headaches (83)
-
Acupuncture for Post-operative Nausea (84)
-
Acupuncture for Fibromyalgia (85)
-
Distant Healing (86)
-
Intercessory Prayer (87)
-
Massage for Low-Back Pain (88)
-
Agnus Castus Extract for Premenstrual Syndrome (89)
-
Tai Chi for Balance Disorders (90)
-
Selected Herbal Therapies (e.g., Gingko, St. John’s Wort and Saw Palmetto) (91)
-
Adjunctive Non-pharmacological Analgesia for Invasive Medical Procedures (92)
Selected Clinical Trials Suggesting That CAM Therapies May Lack Efficacy
-
Acupuncture for Peripheral Neuropathy (93)
-
Hydroxycitric Acid for Obesity (94)
-
Chiropractic vs. Physical Therapy vs. Education for Low Back Pain (95)
-
Acupuncture for Tinnitus (96)
-
St. John’s Wort for Major Depression (97)
-
Homeopathy for Warts on the Hands (98)
-
Homeopathy for Muscle Soreness (99)
-
Herbal Remedies for Asthma (100)
-
Hair Analysis of Trace Minerals (101)
-
Chiropractic for Infantile Colic (102)
-
Group Psychosocial Support for Metastatic Breast Cancer (103;104)
Selected Articles Describing Significant Drug-Herb Interactions and/or Toxicity
Over the past two years, the medical literature has included several reports of clinically significant adverse events caused by the direct or indirect toxicity of herbal products. Notable examples include:
-
Case Studies Involving the Most Commonly Used Medicinal Plants (105);
-
Adverse Reactions Between St. John’s Wort and Prescription Drugs (106);
-
Open-label Study Showing St. John’s Wort Decreases Indinavir Concentrations (107);
-
Association of a Chinese Herb (Aristolochia fangchi) with Renal Failure and Urothelial Carcinoma (108);
-
Letter to Lancet Editor regarding St. John’s Wort Induced Heart Transplant Rejections (109); and
-
Summary of Ephedra’s Toxicity (110).
Selected Articles Relating to the Mechanisms of CAM Interventions and Placebo-Related Phenomena
Investigating the mechanisms of actions of CAM therapies is now a high priority for the NIH and NCCAM. Notable examples of recent publications in this area include:
-
Expectation and Dopamine Release: Mechanism of the Placebo Effect in Parkinson’s Disease (111);
-
Changes in Brain Function of Depressed Subjects During Treatment with Placebo (112);
-
Functional MRI Studies of Acupuncture in Normal Subjects—Localization of Processing (113);
-
Functional MRI Studies of Acupuncture in Normal Subjects (114);
-
Is the Placebo Powerless? (115);
-
Response Expectancies in Placebo Analgesia and Their Clinical Relevance (116); and
-
MRI Imaging of Placebo (117).
V. HOW CAM/INTEGRATIVE MEDICINE RESEARCH HAS FOLLOWED AN UNUSUAL TRAJECTORY
Conventional biomedical research typically follows a trajectory that begins with basic science and animal research, followed by Phase I, II, and III clinical (human) trials. If effective, new therapies are then evaluated for their cost-effectiveness and appropriate health care policy is ultimately developed.
This has not been the case, however, for much of complementary and integrative medicine therapies, the majority of which have not yet been formally evaluated in terms of their mechanism of action (i.e., basic science research) and clinical or cost-effectiveness (health services research). Ernst has documented the relative absence of cost-effectiveness research involving CAM Integrative Medicine interventions (118). Both basic science and health services research are emerging as high priorities for both governmental (e.g., NIH) and private sector sponsored research in this area (e.g., research sponsored by pharmaceutical companies, insurance carriers, Fortune 500 corporations).
In a recent article, Vandenbroucke and de Craen argue that CAM research provides a “mirror image” for scientific reasoning in conventional medicine. More specifically, they provide several examples in which physicians discard a theory because of new facts, or, alternatively, cling to a theory despite the facts (119).
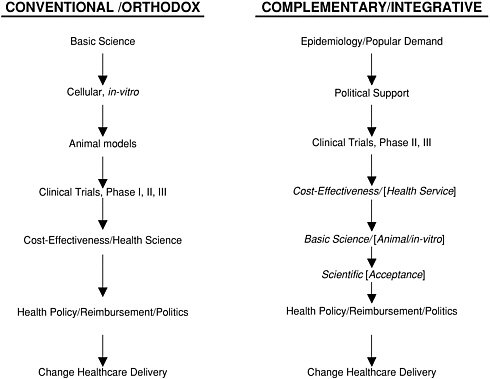
FIGURE 1
VI. CREDENTIALING AND MALPRACTICE LIABILITY CONCERNS
The oversight of educational requirements, credentialing, malpractice insurance, and scope of practice vary from state to state (120;121) (122). A tabular summary of state licensing patterns for chiropractic, acupuncture, massage therapy, homeopathy, and naturopathy is available elsewhere (120;121).
David Studdert, J.D., Ph.D., et al. examined malpractice insurance claims data from both the conventional (MD) and CAM (i.e., chiropractic, acupuncture, massage) communities (123). Their findings, published in JAMA included the observation that claims against licensed CAM practitioners occurred less frequently and typically involved injury that was less severe than claims against physicians during the same period. This article also described specific situations in which referral by a medical doctor to a licensed CAM practitioner will or will not likely be construed as negligent. The texts by Michael Cohen (124;125) also highlight many
CAM related legal concerns. An article by Cohen and Ernst addresses issues of informed consent involving CAM (126). The Annals of Internal Medicine special series on CAM has scheduled the publication of individual papers on CAM-related malpractice, credentialing and ethics in the spring of 2002. In addition, the Federation of State Medical Boards is scheduled to vote on model guidelines for the use of CAM therapies in medical practice later this year (2002).
VII. EMERGING MODELS FOR THE CLINICAL DELIVERY OF COMPLEMENTARY AND INTEGRATIVE MEDICAL THERAPIES
Increasingly, hospitals, managed care organizations, health insurers and large, self-insured corporations are developing models whereby CAM/integrative therapies are made available to members, subscribers, and employees. The spectrum of existing models, all relatively new, is broad and includes:
-
The establishment of networks of “credentialed” complementary and alternative therapy practitioners.
-
Reduced “fee-for-service” models whereby members/subscribers/ employees receive a discount on routine CAM services provided by “credentialed” networks of identified practitioners in a given geographic area. (Note: This model does not typically include reimbursement for or liability assurance regarding the delivery of CAM services.)
-
Covered benefits, which include a predetermined maximum of complementary and alternative therapy services for selected medical conditions (usually with a required referral from an MD).
-
Covered CAM benefits without a required referral from an MD.
-
“Integrated” medical services which typically include both conventional and complementary/alternative services, usually in an outpatient (ambulatory) setting. Reimbursement options vary as do referral requirements.
-
“Integrated” consultation services, i.e., the provision of complementary and alternative therapies for inpatients in hospitals.
-
The incorporation of complementary and alternative (a.k.a. “integrative”) services as part of an individual medical practice, a group medical practice, a managed care organization, a PPO, an insurance product, a community hospital, or university-affiliated teaching hospital.
-
Specialized integrative care teams consisting of conventional and complementary care providers working within a medical institution or group practice. Notable examples include integrative care teams at Beth Israel Hospital (NY), University of Maryland, Stanford University, Cedars-Sinai, and Memorial Sloan Kettering hospitals.
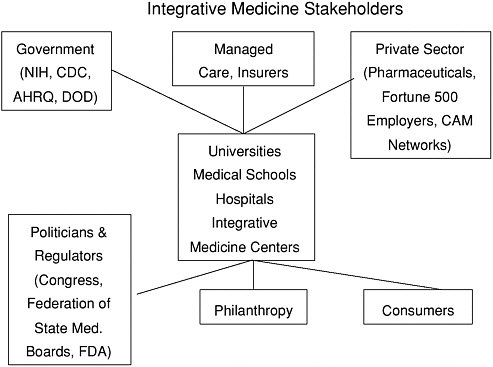
FIGURE 2
Unlike hospitalizations and physician services, complementary and alternative therapies are only infrequently included in insurance benefits. With the exception of chiropractic, CAM therapies are typically not covered by third-party reimbursement. The percentage of CAM users who paid entirely out-of-pocket for these services did not change significantly between 1990 (64 percent) and 1997 (58.3 percent) (19). Even when alternative therapies are covered, they tend to have high deductibles and copayments and tend to be subject to stringent limits on the number of visits or total dollar coverage. Because the demand for health care (and presumably alternative therapies) is sensitive to how much patients must pay out-of-pocket, current use is likely to under represent utilization patterns if (and when) insurance coverage for alternative therapies increases in the future (19). Trends involving insurance coverage for CAM therapies have recently been reviewed by Pelletier et al. (127;128). A survey by John Weeks of 27 hospital-sponsored integrative medicine clinics provides descriptive information on services, practitioners, provider mixes, and profitability issues (129).
While models of “integrative care” have recently begun to develop
nationwide, a variety of barriers to their success have become apparent. Many of these barriers were highlighted in a recent NIH request for proposals and include: 1) the need for more research; 2) the ability to translate research findings into clinical practice; 3) fiscal constraints and the absence of a financially sustainable model; 4) ignorance about CAM therapies on the part of referring physicians; 5) provider competition; 6) liability issues; 7) cultural bias and prejudice; and 8) the lack of standards pertaining to credentialing, patient triage, and third-party reimbursement. In October 2001, the NIH NCCAM issued eight awards (four RO1s and four R21s) to a spectrum of institutions and investigators to develop innovative models of integrative care.
VIII. CHALLENGES AND OPPORTUNITIES FOR STAKEHOLDERS
Further development of CAM/Integrative Medicine research will require:
-
Additional resources and an expanded commitment from both the public and private sectors to promote additional:
-
Clinical research;
-
Health services research; and
-
Basic science research.
It should be emphasized that all three are essential; moreover, basic science and health services research need to be prioritized at this time.
-
Recruitment of additional research leadership across disciplines and constituencies (e.g., more basic scientists, clinical investigators, economists, toxicologists, etc.).
-
Improved quality assurance of dietary supplements. Can botanicals be standardized for research purposes? Can the FDA, NIH, and Congress revisit current regulatory statutes in order to promote reproducible scientific inquiry as well as consumer safety?
-
A critical mass of university-affiliated CAM/Integrative Medicine programs with sufficient resources to pursue:
-
Research (clinical, basic, health services)
-
Educational reform and training
-
Clinical delivery of CAM/Integrative Medical services at university-affiliated hospitals
Note: The Consortium of Academic Health Centers for Integrative Medicine is currently being developed. This consortium currently includes medical school faculty from the Universities of Maryland, Arizona, Michigan, Minnesota, Massachusetts, Duke, Columbia, Albert Einstein, Thomas Jefferson, Georgetown, UCSF, and Harvard. The consortium is developing an agenda which relates to CAM/Integrative Medicine education, research, and clinical care.
-
A commitment to primarily pursue inter-disciplinary, inter-institutional, and, where appropriate, international collaboration wherever possible.
Note: Harvard Medical School and the UCSF School of Medicine have jointly developed an Annual International Scientific Conference on CAM/ Integrative Medicine Research. This meeting is sponsored, in part, by a grant from the NIH NCCAM. (The next research conference is scheduled for April 12-14, 2002 in Boston. For information, contact 781-245-3010.)
The successful delivery of CAM/Integrative Medical services will require:
-
More consistent standards for credentialing of CAM providers.
-
More consistent tracking of clinical and financial outcomes.
-
The establishment of appropriate guidelines regarding the use (or avoidance) of herbs, vitamins, and supplements for outpatients and inpatients.
-
Demonstration projects that provide evidence of financial and clinical offsets.
-
Demonstration projects that provide evidence of financial sustainability.
-
Demonstration projects with revenue streams that include self-pay, third-party reimbursement, philanthropy, and income from sponsored research.
-
Demonstration projects that are functionally integrated into existing medical delivery models (e.g., hospitals, clinics, group practices, MCOs, etc.).
-
Models that include access for CAM services for those with less expendable income and/or lack of medical insurance.
-
Medical-legal guidelines for conventional and CAM practitioners, institutions and third party payers so as to minimize liability exposure.
-
Partnerships and incentives involving government, the academic community, and the private sector.
Paradoxes and Policy Decisions Involving CAM and Integrative Medical Therapies
-
Is third party reimbursement for CAM/Integrative services a beneficial objective? What is the “dark side” of third party reimbursement for the CAM professions?
-
Is CAM/Integrative Medicine:
-
a valuable refinement of mainstream, conventional medical care?;
-
a “disruptive technology”?;
-
a (potentially) disruptive reconfiguration of health care delivery models?; or
-
none of the above?
-
Should academic medical centers launch model integrative care centers in the absence of scientific consensus on the efficacy, safety, and mechanism of action of each modality used? Conversely, are these model integrative care centers necessary engines of research to discern CAM efficacy and safety?
-
Can/should/will increased governmental regulation (and/or legal incentives for pharmaceutical companies) be required to address quality assurance issues regarding dietary supplements? How can the issue of intellectual property (i.e., patents) be addressed in light of existing DSHEA legislation? Should DSHEA be revisited? Amended? What would prompt Congress to do so?
-
Can reproducible models of credentialing, billing, and data tracking be devised and can existing electronic medical records systems be refined to build a national data warehouse/registry of integrative care outcomes?
-
How best to distinguish quackery/fraud/deception
from
Responsible delivery of CAM (by an individual or institution)
from
The responsible co-management of patients with a (licensed) CAM provider?
Each creates unique liability exposure and relates to specific professional sanctions.
-
How best to incorporate relevant information regarding CAM into required core curricula and training of MDs/RNs/PharmDs/dieticians, and other allied health professional at the undergraduate and postgraduate levels? Can appropriate, web-based, interactive CME programs be jointly developed across professional disciplines? Isn’t the same “core” information needed by each medical discipline?
-
How to incorporate clinically relevant CAM/Integrative Medicine examination questions into the board examinations of physicians, nurses, pharmacists, dieticians, and other health professionals, including licensed CAM providers?
-
How best to improve the quality of relevant CAM information on the Internet? For clinicians, for researchers, for patients?
-
How to pursue “integration” in the absence of co-optation of one professional discipline by another? Are there successful models of integration across (medical) disciplines? What can be learned from these?
Postscript
“Doing everything for everyone,” wrote David Grimes, “is neither tenable nor desirable. What is done should be inspired by compassion and guided by science and not merely reflect what the market will bear.” (130).
BIBLIOGRAPHY
(1) Murray RH, Rubel AJ. Physicians and healers—unwitting partners in health care [see comments]. N Engl J Med. 1992;326:61-64.
(2) Gevitz N. Other Healers: Unorthodox Medicine in America. Baltimore: Johns Hopkins University Press; 1988.
(3) Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246-52.
(4) Ernst E, Resch KL, Mills S, et al. Complementary medicine—a definition. Br J Gen Pract. 1995;45:506.
(5) Eisenberg DM, Delbanco TL, Kessler RC. Letter to the editor. N Engl J Med. 1993;329:1203.
(6) Kaptchuk TJ, Eisenberg DM. Varieties of Healing. 1: Medical Pluralism in the United States. Ann Intern Med. 2001;135:189-95.
(7) Kaptchuk TJ, Eisenberg DM. Varieties of Healing. 2: A Taxonomy of Unconventional Healing Practices. Ann Intern Med. 2001;135:196-204.
(8) Weil A, et al. Integrated medicine. BMJ. 2001;322:119-20.
(9) Smith R. Editor’s choice: Restoring the soul of medicine. BMJ. 2001;322:117.
(10) Kaptchuk TJ, Eisenberg DM. The persuasive appeal of alternative medicine. Ann Intern Med. 1998;129:1061-65.
(11) Complementary medicine: Time for critical engagement. Lancet. 2000;356:2023.
(12) Fontanarosa PB, Lundberg GD. Alternative medicine meets science. JAMA. 1998;280:1618-19.
(13) Jonas WB. Alternative medicine—learning from the past, examining the present, advancing to the future [editorial] [In Process Citation]. JAMA. 1998;280:1616-18.
(14) Angell M, Kassirer JP. Alternative medicine—the risks of untested and unregulated remedies [editorial; comment]. N Engl J Med. 1998;339:839-41.
(15) Davidoff F. Weighing the alternatives: lessons from the paradoxes of alternative medicine. Ann Intern Med. 1998;129:1068-70.
(16) Dalen JE. “Conventional” and “unconventional” medicine. Can they be integrated? Arch Intern Med. 1998;158:2179-81.
(17) Israelsen L. Botanicals and DSHEA: A health professional’s guide. Quarterly Review of Natural Medicine. 1998;Fall:251-57.
(18) Goldman P. Herbal medicines today and the roots of modern pharmacology. Ann Intern Med. 2001;135:594-600.
(19) Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, et al. Trends in alternative medicine use in the United States, 1990-1997: Results of a follow-up national survey. JAMA. 1998;280:1569-75.
(20) Astin JA. Why patients use alternative medicine: Results of a national study. JAMA. 1998;279:1548-53.
(21) Druss BG, Rosenheck RA. Association between use of unconventional therapies and conventional medical services. JAMA. 1999;282:651-56.
(22) Foster DF, Phillips RS, Hamel MB, Eisenberg DM. Alternative medicine use in older Americans. J Amer Geriatr Soc. 2000;48:1560-1565.
(23) Kessler RC, Soukup J, Davis RB, Foster DF, Wilkey SA, Van Rompay MI, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001; [In press].
(24) Kessler RC, Davis RB, Foster DA, Van Rompay MI, Walers EE, Wilkey SA, et al. Longterm trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med. 2001;135:262-68.
(25) Eisenberg DM, Kessler RC, Van Rompay MI, Kaptchuk TJ, Wilkey SA, Appel S, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: Results from a national survey. Ann Intern Med. 2001;135:344-51.
(26) Adler SR, Fosket JR. Disclosing complementary and alternative medicine use in the medical encounter: A qualitative study in women with breast cancer. J Fam Pract. 1999;48:453-58.
(27) Burstein HJ, Gelber S, Guadagnoli E, et al. Use of alternative medicine by women with early-stage breast cancer. N Engl J Med. 1999;340:1733-39.
(28) Cassileth BR, Lusk EJ, Strouse TB, Bodenheimer BJ. Contemporary unorthodox treatments in cancer medicine. A study of patients, treatments, and practitioners. Ann Intern Med. 1984;101:105-12.
(29) Goldstein J, Chao C, Valentine E, et al. Use of unproved cancer treatment by patients in a radiation oncology department: A survey. J Psychosocial Oncol. 1991;9:59-66.
(30) Lee MM, Lin SS, Wrensch MR, et al. Alternative therapies used by women with breast cancer in four ethnic populations . J Natl Cancer Instit. 2000;92:42-47.
(31) Lerner IJ, Kennedy BJ. The prevalence of questionable methods of cancer treatment in the United States. CA Cancer J Clin. 1992;42:181-91.
(32) Mooney K. Unproven cancer treatment usage in cancer patients who have received conventional therapy. Oncol Nurs Forum. 1987;Supp. 2:112.
(33) Newell SM. An investigation into the utility of the modified health belief model in predicting treatment compliance for advanced adult cancer patients in hospital outpatient clinics. Diss Abstr Int [B]. 1985;45:3201B.
(34) Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/ alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505-14.
(35) VandeCreek L, Rogers E, Lester J. Use of alternative therapies among breast cancer outpatients compared with the general population. Altern Ther Health Med. 1999;5: 71-76.
(36) Rao JK, Mihaliak K, Kroenke K, Bradley J, Tierney WM, Weinberger M. Use of complementary therapies for arthritis among patients of rheumatologists. Ann Intern Med. 1999;131:409-16.
(37) Nicassio PM, Schuman C, Kim J, Cordova A, Weisman MH. Psychosocial factors associated with complementary treatment use in fibromyalgia. J Rheumatol. 1997;24: 2008-13.
(38) Cronan TA, Kaplan RM, Posner L, Blumberg E, Kozin F. Prevalence of the use of unconventional remedies for arthritis in a metropolitan community. Arthritis Rheum. 1989;32:1604-7.
(39) Krauss HH, Godfrey C, Kirk J, Eisenberg DM. Alternative health care: Its use by individuals with physical disabilities. Arch Phys Med Rehabil. 1998;79:1440-47.
(40) Fairfield KM, Eisenberg DM, Davis RB, Libman H, Phillips RS. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Arch Intern Med. 1998;158:2257-64.
(41) Rawsthorne P, Shanahan F, Cronin NC, Anton PA, Lofberg R, Bohman L, et al. An international survey of the use and attitudes regarding alternative medicine by patients with inflammatory bowel disease. Am J Gastroenterol. 1999;94:1298-303.
(42) Krouse JH, Krouse HJ. Patient use of traditional and complementary therapies in treating rhinosinusitis before consulting an otolaryngologist. Laryngoscope. 1999;109: 1223-27.
(43) Norred CL, Zamudio S, Palmer SK. Use of complementary and alternative medicines by surgical patients. AANA Journal. 2000;68:13-18.
(44) Gulla J, Singer AJ. Use of alternative therapies among emergency department patients. Ann Emerg Med. 2000;35:226-28.
(45) Goldbeck-Wood S, Dorozynski A, Lie LG, et al. Complementary medicine is booming worldwide. BMJ. 1996;313:131-33.
(46) Rasmussen NK, Morgall JM. The use of alternative treatments in the Danish adult population. Comp Med Res. 1990;4:16-22.
(47) Vaskilampi T, Merilainen P., Sinkkonen S, et al. The use of alternative treatments in the Finnish adult population. In: Lewith GT, Aldridge D, eds. Clinical Research Methodology for Complementary Therapies. London: Hodder & Stoughton; 1993: 204-29.
(48) MacLennan AH, Wilson DH, Taylor AW. Prevalence and cost of alternative medicine in Australia. Lancet. 1996;347:569-73.
(49) Fisher P, Ward A. Complementary medicine in Europe. BMJ. 1994;309:107-11.
(50) Sermeus G. Alternative health care in Belgium. Complementary Medical Research. 1990;4:9-13.
(51) Bouchayer F. Alternative medicines: A general approach to the French situation. Comp Med Res. 1990;4:4-8.
(52) Piel E. Erfahrungen mit Naturheilmitteln-Umfrageergebnisse aus West- und Ostdeutschland. Therapeutikon. 1991;5:549-51.
(53) Millar WJ. Use of alternative health care practitioners by Canadians. Can J Public Health. 1997;88:154-58.
(54) Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States. The Slone survey. JAMA. 2002;287:337-44.
(55) Blendon RJ, et al. Americans’ views on the use and regulation of dietary supplements. Arch Intern Med. 2001;161:803-10.
(56) Wetzel MS, Eisenberg DM, Kaptchuk TJ. Courses involving complementary and alternative medicine at US medical schools. JAMA. 1998;280:784-87.
(57) Caspi O, Bell IR, Rychener D, Gaudet TW, Weil AT. Tower of Babel: Communication and medicine: An essay on medical education and complementary-alternative medicine. Arch Intern Med. 2000;160:3193-95.
(58) Josiah Macy Foundation. Education of Health Professionals in Complementary/Alternative Medicine. 2001.
(59) Bigos SJ, Bowyer OR, Braen RG, Brown K, Deyo R, Haldeman S. Acute low back problems in adults. Clinical Practice Guideline. Number 14. Rockville, Maryland: Agency for Health Care Policy and Research, US DHHS (Dept. Health and Human Services); 1994.
(60) Koes BW, Assendelft WJ, van der Heijden GJ, Bouter LM. Spinal manipulation for low back pain. An updated systematic review of randomized clinical trials. Spine. 1996;21:2860-71; discussion 2872-73.
(61) NIH Technology Assessment Statement. Integration of Behavioral and Relaxation Approaches into the Treatment of Chronic Pain and Insomnia. 1995. Bethesda, National Institutes of Health. NIH Pub #PB96113964.
(62) Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, et al. Can lifestyle changes reverse coronary heart disease? Lancet. 1990;336:129-33.
(63) Gould KL, Ornish D, Scherwitz L, Brown S, Edens RP, Hess MJ, et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA. 1995;274:894-901.
(64) NIH Consensus Conference. Acupuncture. JAMA. 1998;280:1518-24.
(65) Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer [see comments]. Lancet. 1989;2:888-91.
(66) Linde K, Clausius N, Ramirez G, Melchart D, Eitel F, Hedges LV, et al. Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo-controlled trials [see comments] [published erratum appears in Lancet 1998 Jan 17;351(9097):220]. Lancet. 1997;350:834-43.
(67) Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John’s wort for depression—an overview and meta-analysis of randomised clinical trials [see comments]. BMJ. 1996;313:253-58.
(68) Philipp M, Kohnen R, Hiller K-O. Hypericum extract versus imipramine or placebo in patients with moderate depression: Randomised multicentre study of treatment for eight weeks. BMJ. 1999;319:1534-39.
(69) Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo Biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-15.
(70) Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo Biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-32.
(71) Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: A randomized controlled trial [In Process Citation]. JAMA. 1998;280:1585-89.
(72) Wilt TJ, Ishani A, Stark G, MacDonald R, Lau J, Mulrow C. Saw palmetto extracts for treatment of benign prostatic hyperplasia: A systematic review [In Process Citation]. JAMA. 1998;280:1604-9.
(73) Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia: A meta-analysis of randomized clinical trials. Ann Intern Med. 2000;133:420-429.
(74) Silagy C, Neil A. Garlic as a lipid lowering agent—a meta-analysis. J R Coll Physicians Lond. 1994;28:39-45.
(75) Silagy CA, Neil HA. A meta-analysis of the effect of garlic on blood pressure. J Hypertens. 1994;12:463-68.
(76) McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis. A systematic quality assessment and meta-analysis. JAMA. 2000;283:1469-75.
(77) Das A, Hammad TA. Efficacy of a combination of FCHG49 glucosamine hydrochloride, TRH122 low molecular weight sodium chondroitin sulfate and manganese ascorbate in the management of knee osteoarthritis. Osteoarthritis and Cartilage. 2000;8:343-50.
(78) Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: Systematic review and meta-analysis. J Clin Psychopharmacol. 2000;20:84-89.
(79) Weiser M, Strosser W, Klein P. Homeopathic vs conventional treatment of vertigo: A randomized double-blind controlled clinical study. Arch Otolaryngol Head Neck Surg. 1998;124:879-85.
(80) Taylor MA, Reilly D, Llewellyn-Jones RH, McSharry C, Aitchison TC. Randomised controlled trial of homoeopathy versus placebo in perennial allergic rhinitis with overview of four trial series. BMJ. 2000;321:471-76.
(81) Andersson GBJ, Lucente T, Davis AM, Kappler RE, Lipton JA, Leurgans S. A comparison of osteopathic spinal manipulation with standard care for patients with low back pain. N Engl J Med. 1999;341:1426-31.
(82) Cardini F, Weixin H. Moxibustion for correction of breech presentation: a randomized controlled trial [In Process Citation]. JAMA. 1998;280:1580-1584.
(83) Melchart D, Linde K, Fischer P, White A, Allais G, Vickers A, et al. Acupuncture for recurrent headaches: A systematic review of randomized controlled trials. Cephalalgia. 1999;19:779-86.
(84) Lee A, Done ML. The use of nonpharmacologic techniques to prevent postoperative nausea and vomiting: A meta-analysis. Anesth Analg. 1999;88:1362-69.
(85) Berman BM, Ezzo J, Hadhazy V, Swyers JP. Is acupuncture effective in the treatment of fibromyalgia? J Fam Pract. 1999;48:213-18.
(86) Astin JA, Harkness E, Ernst E. The efficacy of “distant healing”: A systematic review of randomized trials. Ann Intern Med. 2000;132:903-10.
(87) Harris WS, Gowda M, Kolb JW, Strychacz CP, Vacek JL, Jones PG, et al. A randomized, controlled trial of the effects of remote, intercessory prayer on outcomes in patients admitted to the coronary care unit. Arch Intern Med. 1999;159:2273-78.
(88) Cherkin DC, Eisenberg DM, Sherman KJ, Barlow W, Kaptchuk TJ, Street J, et al. Randomized trial comparing traditional Chinese medical acupuncture, therapeutic massage, and self-care education for chronic low back pain. Arch Intern Med. 2001;161: 1081-88.
(89) Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective, randomised, placebo controlled study. BMJ. 2001;322:134-37.
(90) Hain T, Fuller L, Weil L, Kotsias J. Effects of Tai Chi on balance. Arch Otolaryngol Head Neck Surg. 1999;125:1191-95.
(91) Ernst E. Herbal medicinal products — a risk-benefit profile. Ann Intern Med. 2001;[In press].
(92) Lang E, Benotsch E, Fick L, Lutgendorf S, Berbaum M, Berbaum K, et al. Adjunctive non-pharmacological analgesia for invasive medical procedures: a randomised trial. Lancet. 2000;355:1486-90.
(93) Shlay JC, Chaloner K, Max MB, Flaws B, Reichelderfer P, Wentworth D, et al. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: A randomized controlled trial. JAMA. 1998;280:1590-1595.
(94) Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nunez C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: A randomized controlled trial [In Process Citation]. JAMA. 1998;280:1596-600.
(95) Cherkin DC, Deyo RA, Battie M, Street J, Barlow W. A comparison of physical therapy, chiropractic manipulation, and provision of an educational booklet for the treatment of patients with low back pain. N Engl J Med. 1998;339:1021-29.
(96) Park J, White AR, Ernst E. Efficacy of acupuncture as a treatment for tinnitus: A systematic review. Arch Otolaryngol Head Neck Surg. 2000;126:489-92.
(97) Shelton R, Keller M, Gelenberg A, Dunner D, Hirschfield R, Thase M, et al. Effectiveness of St. John’s wort in major depression: A randomized controlled trial. JAMA. 2001;285:1978-86.
(98) Kainz J, Kozel G, Haidvogl M, Smolle J. Homeopathic versus placebo therapy of children with warts on the hands: A randomized, double-blind clinical trial. Dermatology. 1996;193:318-20.
(99) Vickers AJ, Fisher P, Smith C, Wyllie SE, Lewith GT. Homoeopathy for delayed onset muscle soreness: a randomised double blind placebo controlled trial. Br J Sports Med. 1997;31:304-7.
(100) Huntley A, Ernst E. Herbal medicines for asthma: a sytematic review. Thorax. 2000;55: 925-29.
(101) Seidel S, Krentzer R, Smith D, McNeal S, Gilliss D. Assessment of commercial laboratories performing hair mineral analysis. JAMA. 2001;285:67-72.
(102) Olafsdottir E, Forshei S, Fluge G, Markestad T. Randomised controlled trial of infantile colic treated with chiropractic spinal manipulation. Arch Dis Child. 2001;84:138-41.
(103) Goodwin P, Leszcz M, Ennis M, Koopmans J, Vincent L, Guther H, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719-26.
(104) Spiegel D. Mind matters-group therapy and survival in breast cancer (editorial). N Engl J Med. 2001;345:1767-68.
(105) Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134-38.
(106) Ernst E. Second thoughts about safety of St. John’s Wort. Lancet. 1999;354:2014-16.
(107) Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St. John’s wort. Lancet. 2000;355:547-48.
(108) Nortier JL, Muniz Martinez M-C, Schmeiser HH, Volker MA, Bieler CA, Petein M, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342:1686-92.
(109) Ruschitzka F, Meier PJ, Turina M, Luscher TF, Noll G. Acute heart transplant rejection due to St. John’s Wort. Lancet. 2000;355:548-49.
(110) Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med. 2000;343:1833-38.
(111) de la Fuente-Fernández R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: Mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164-66.
(112) Leuchter A, Cook I, Witte E, Morgan M, Abrams M. Changes in brain function of depressed subjects during treatment with placebo. Am J Psychiatry. 2002;159:122-29.
(113) Wu M-T, Hsieh J-C. Central Nervous Pathway for acupuncture stimulation: Localization of processing with functional mr imaging of the brain-preliminary experience. Radiology. 1999;212:133-41.
(114) Hui KKS, Liu J. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: Evidence from MRI studies in normal subjects. Human Brain Mapping. 2000;9:13-25.
(115) Hróbjartsson A, Gøtzsche P. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594-602.
(116) Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77-84.
(117) Petrovic P, Kalso E, Petersson K, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Sciencexpress. 2002.
(118) White AR, Ernst E. Economic analysis of complementary medicine: a systematic review. Complement Ther Med. 2000;8:111-18.
(119) Vandenbroucke JP, de Craen AJM. Alternative medicine: A “mirror image” for scientific reasoning in conventional medicine. Ann Intern Med. 2001;135:507-13.
(120) Eisenberg DM. Advising patients who seek alternative medical therapies. Ann Intern Med. 1997;127:61-69.
(121) Sale JD. Overview of Legislative Developments Concerning Alternative Health Care in the United States. Kalamazoo,MI: John E. Fetzer Institute; 1994.
(122) Cohen MH. Complementary and Alternative Medicine: Legal Boundaries and Regulatory Perspectives. Baltimore: Johns Hopkins University Press; 1998.
(123) Studdert DM, Eisenberg DM, Miller FH, Curto DA, Kaptchuk TJ, Brennan TA. Medical malpractice implications of alternative medicine. JAMA. 1998;280:1610-1615.
(124) Cohen MH. Complementary and Alternative Medicine: Legal Boundaries and Regulatory Perspectives. Baltimore: Johns Hopkins University Press; 1998.
(125) Cohen MH. Beyond Complementary Medicine: Legal and Ethical Perspectives on Health Care and Human Evolution. Ann Arbor: University of Michigan Press; 2000.
(126) Ernst E, Cohen MH. Informed consent in complementary and alternative medicine. Arch Intern Med. 2001;161:2288-92.
(127) Pelletier KR, Marie A, Krasner M, Haskell WL. Current trends in the integration and reimbursement of complementary and alternative medicine by managed care, insurance carriers, and hospital providers. Am J Health Promot. 1997;12:112-22.
(128) Pelletier KR, Astin JA, Haskell WL. Current trends in the integration and reimbursement of complementary and alternative medicine by managed care organizations (MCOs) and insurance providers: 1998 update and cohort analysis. Am J Health Promot. 1999;14:125-33.
(129) Weeks J. Hospital-sponsored integrative clinics: an in-depth report. The Integrative Medicine Consult Quarterly Business Report. 2001.
(130) Grimes DA. Technology follies. The uncritical acceptance of medical innovation. JAMA. 1993;269:3030-33.
























