6
Steller Sea Lion Decline: Environmental Context and Compendium of Evidence
Evaluation of the main hypotheses proposed for the causes of decline and failure to recover of the western Steller sea lion population depends on understanding how food web linkages affect sea lions. Every species in an ecosystem, including humans, contributes to and is affected by the linkages represented in a food web. Food webs are described by identifying who eats whom, based on direct observation, stomach and scat analyses, or prey item chemical signatures based on stable isotope and fatty acid analyses. Understanding how linkages influence population and ecosystem dynamics is a far greater challenge because the complexity of interactions precludes analysis through static observation. Only through perturbation of one or more populations is it possible to evaluate the dynamic nature of food webs. Though much is known about the descriptive structure of food webs, the dynamic properties are less well understood. Humans are part of the food web; in the current case, they may change food web dynamics through direct takes of sea lions, removal of sea lions’ preferred prey, removal of alternate prey items of sea lion predators, or some combination of the above. For example, humans depleted populations of several whale species, possibly inducing killer whales to increase their predation on sea lions, seals, and otters.
This chapter describes four concepts that provide a context for analyzing the role of food web interactions in Steller sea lion population dynamics and then applies these concepts to evaluate the many hypotheses proposed to explain the Steller sea lion population decline.
FOOD WEB CONCEPTS
Bottom-Up and Top-Down Control
Food web linkages connect species across different trophic levels (Paine, 1980). The functional significance of any linkage can be viewed from the perspective of either the consumer or the prey. When a population’s size is limited by the availability of prey, it is described as bottom-up control; when the size of a population is determined by predation, it is described as under top-down control. Bottom-up control characterizes populations that decline or fail to expand because there is insufficient food for growth, reproduction, or survival. Top-down control characterizes populations whose size is regulated by the abundance and feeding habitats of the species that prey on them.
Direct and Indirect Food Web Linkages
Food web linkages can connect two species directly or indirectly if there are one or more intermediate species. Although hypotheses based on direct effects could be sufficient to explain the decline of Steller sea lion populations, indirect effects may influence the pattern of decline and thereby complicate analysis of direct impacts (see Box 6.1). How the number of intermediate species affects the strength of the interaction is generally unknown, but the number of potential indirect linkages is far greater than the number of direct linkages. Therefore, it is insufficient to consider only the availability of specific prey items or the foraging patterns of generalist predators because changes among other members of the food web may indirectly affect sea lion survival.
Humans have exploited many large and small predators in marine ecosystems, acting as an agent for top-down control of marine populations. If top-down forcing is important, the depletion of apex predators should have strong effects on food webs through increased abundance of species at lower trophic levels; removal of predators disrupts the trophic cascade (Paine, 1980; Carpenter and Kitchell, 1993). The consequences of these shifts in marine food webs are complex, difficult to predict, and often unrecognized.
Scale and Connectivity
Most analyses of food web dynamics have focused on the linkages among species in a common ecosystem. There is growing evidence for the importance of linkages across ecosystems. Linkages of this sort occur in a variety of forms and connect otherwise functionally distinct ecosystems
|
BOX 6.1 Some Plausible Examples of Indirect Effects The Bowen et al. (2001) report presented a number of hypotheses concerning the history of Steller sea lion population trends since 1960 to identify the most informative parameters for guiding future research programs. These hypotheses address direct effects that may explain the decline in Steller sea lions. For example, the discussion of trophic consequences of fishing has focused on reduced food availability. A broader classification of “fishing effects” would also include indirect influences of diverse trophic interactions (Wootton, 1994), estimated to be approximately 50% of all ecological interactions (Schoener, 1993; Menge, 1995). One example, captured in the following quotation from Loughlin and York (2000), is the implication that factory trawlers, simply by their presence and activity, can aggregate sea lions and killer whales. “Predation [by killer whales] is often focused in small areas, i.e., where sea lions are localized near large fish processing vessels, resulting in exacerbation of local declines” (p.43). As a second plausible example, bycatch (nontarget animals caught, killed, or injured during fishing operations) could provide a new source of food for bottom-dwelling organisms, including various flat fish. Arrowtooth flounder had several strong recruitment years in the 1980s; biomass peaked in 1995 (five-fold increase over 1980 biomass) and by the 2001 estimate had declined about 20% due to lower recruitment levels in the 1990s (Wilderbuer and Sample, 2001). The years of high recruitment occurred when the pollock fishery used bottom trawling gear that causes incidental mortality of benthic organisms (National Research Council, 2002). The high bycatch of that fishery may have contributed to the resurgence of arrowtooth flounder. These fish may pose a competitive threat to sea lions because they also prey on young pollock, other demersal fish, and invertebrates. In a third indirect effect scenario, the 10-fold increase in jellyfish over the past decade could also deplete the sea lions preferred prey (Brodeur et al., 1999). Plausible interaction pathways could include direct competition with age 0 pollock for zooplankton or predation on the smaller pollock. This massive increase in jellyfish might constrain the recruitment of many commercially and ecologically significant fish stocks. In the Black Sea, studies have documented the top-down control of commercially valuable fish stocks by a comb jelly (Shiganova, 1998). Further sampling and analysis of these food web linkages in the Bering Sea would be needed to fully evaluate the impacts of this increase in jellyfish on recruitment of important sea lion prey species. Indirect effects of fishing activity—especially if concentrated seasonally and spatially—could account for some of the unexplained mortality in the western population of Steller sea lions. In any event, despite the difficult challenge of unraveling their impacts, indirect effects should not be dismissed as either biologically unusual or dynamically trivial. |
over a wide range of spatial and temporal scales. Many marine species have dispersive life stages that can be carried great distances by ocean currents. Also, large animal movements can link disparate ecosystems in important ways. The altered foraging behavior of killer whales (to include sea otters in their diet) provides a link between the kelp forest ecosystems of the Aleutian archipelago and the food web of the open ocean (Estes et al., 1998).
Previous shifts in the abundance of key species continue to affect the dynamics of present-day food webs. For instance, the progressive removal of herbivorous fishes and invertebrates by historical fisheries from Caribbean coral reefs in conjunction with a mass die-off of sea urchins was likely responsible for declines in coral abundance due to overgrowth by algae (Jackson et al., 2001). Thus, it is possible that the Steller sea lion population decline and failure to recover is in part influenced by events distant in either time or space.
Alternative Stable States
Because food webs have complex multiple linkages, the response of these systems to disturbance is often nonlinear (Ruesink, 1998). For instance, if a major predator is removed, the increased availability of prey resources may allow expansion of other predatory species to a new, relatively stable equilibrium. This alternative stable state, dominated by a different assemblage of species, may inhibit the return of the food web to its previous status (Lewontin, 1969; Holling, 1973; May, 1977; Sheffer et al., 2001). This concept has important implications for management because of the possibility that disturbed ecosystems may not return to their previous state of equilibrium. Hence, even if the causes of the Steller sea lion decline are identified and addressed, the western sea lion population still may fail to reach its former abundance.
MULTIPLE WORKING HYPOTHESES
At least eight hypotheses have been proposed to explain the rapid decline of the western stock of Steller sea lions. As pointed out in Box 1.1 (Chapter 1), these various hypotheses cannot be accepted or rejected through the method of strong inference. The data necessary to conduct determinative analyses were simply not collected during the years of the rapid decline. However, this is not to say that relevant data are entirely lacking, particularly with regard to current trends in the population. Numerous types of information on Steller sea lions and their environment have been obtained over the years, some fortuitously and some for the specific purpose of trying to better understand sea lion ecology and popu-
lation biology. Although none of this information is sufficient to prove or eliminate hypotheses, much of it can be rated according to its consistency with any given hypothesis. When all of the information is assessed in aggregate, a weight of evidence argument emerges that allows ranking of the hypotheses according to conformity with available information.
The main hypotheses that have been proposed to explain the Steller sea lion decline are described in Table 6.1. Each hypothesis is presented separately for the sake of clarity, but this should not be taken to imply that the hypotheses necessarily act independently of each other nor does it preclude the possibility that the recent decline results from a combination of the hypothesized causes.
FOOD LIMITATION—BOTTOM-UP HYPOTHESES
Under the bottom-up scenario, the Steller sea lion decline is attributed to a deficiency in food resources. This deficiency could be manifested as depletion of prey, reduced abundance of preferred prey species, or reduced accessibility to prey due to local depletion or disturbance of fish stocks. Nutritional limitation caused by either a climate regime shift and/or a fisheries effect requires that either the quantity or quality of food is insufficient for the recovery or maintenance of the Steller sea lion population. This could come about from starvation conditions, nutritional impacts on reproductive success, or increasing susceptibility of animals to disease.
During the period of rapid decline in the 1980s, the demographics of the western stock gave some indications that Steller sea lions were nutritionally stressed. In 1985, sea lions were on average smaller, were slower to reach reproductive maturity, and had a lower birth rate than in the 1970s (Calkins and Goodwin, 1988; York, 1994). There was also evidence of higher rates of abortion and lower juvenile survival (Pitcher et al., 1998). Nutritional stress may have been a contributing factor in causing the rapid decline of the western population of sea lions, but models indicate that reduced prey availability alone is unlikely to account for the dramatic decline in the size of the population (see Chapter 3).
In 1991 the Alaska Sea Grant College Program (1993) sponsored an international conference entitled “Is It Food?” to ascertain what kind of physiological or biochemical changes would be expected in a chronically or acutely food-stressed pinniped. Because of the difficulty in handling Steller sea lions, the first field studies to address these questions were conducted during the summer on newborn pups and adult females on rookeries. They utilized an east versus west comparative approach with the hypothesis that the declining western population would be stressed relative to the stable eastern population. The studies looked at pup growth
TABLE 6.1 Eight Major Hypotheses Proposed to Explain the Steller Sea Lion Population Decline. Each hypothesis is characterized by purported demographic mechanism(s) of population change, food web forcing directions, and the acronyms used later in Table 6.2. Although the cause of the sea lion decline likely falls within this breadth of hypotheses, more than one of the listed hypotheses may have contributed to the decline, additively, interactively, or in various degrees of relative importance in different places or at different times.
|
Hypothesis |
Mechanism of Population Limitation |
Forcing Direction |
Acronym |
|
1. Fisheries removal |
Starvation and/or reproductive failure because of nutritional limitation |
Bottom-up |
FR |
|
2. Climate change/regime shift |
Starvation and/or reproductive failure because of nutritional limitation |
Bottom-up |
CE |
|
3. Predation |
Elevated mortality from attack by predators |
Top-down |
PRED |
|
4. Direct take |
Elevated mortality from shooting or other purposeful killing |
Top-down |
DT |
|
5. Subsistence harvest |
Elevated mortality from shooting for food or other subsistence uses of sea lions |
Top-down |
SH |
|
6. Incidental take/entanglement |
Elevated mortality from entanglement in fishing gear due to injury or drowning |
Top-down |
IT/ENT |
|
7. Disease |
Elevated mortality or reproductive failure caused by parasites, viruses, or bacteria |
Top-down |
D |
|
8. Pollution/biotoxins |
Elevated mortality or reproductive failure from poisonous or toxic substances, either natural or human produced |
Top-down or Bottom-up |
PO |
rates, maternal attendance patterns, blood chemistry profiles, milk quality, at-sea metabolic rate estimates, thermoregulatory measurements, and several other variables that had been outlined in the “Is It Food?” conference. These studies in the mid-1990s found that animals in the western population were at least as healthy as in the southeastern Alaskan populations
based on several measurements of body condition such as birth size, pup growth, and adult size. This research was summarized in the “Is It Food? II” conference in 2001 (see DeMaster and Atkinson, 2002).
Conclusions based on these results are limited because of sample size (less than 20 adult females and less than 100 pups), seasonality (they were only conducted in the summer on rookeries), and insensitivity to subtle differences between populations. Despite these limitations, the studies suggest that it is unlikely that newborn pup survival has been compromised by acute or chronic malnutrition over the past decade. These studies have now been expanded to juveniles on a year-round basis because new capture methods allow large numbers of juveniles to be handled. All preliminary evidence shows similar results: sea lions in the western population show no indication of being nutritionally stressed relative to sea lions in the eastern population (Richmond and Rea, 2001). The consensus statement drafted from the “Is It Food? II” conference (Alaska Sea Grant College Program, 1993) states that nutritional limitation is probably not a major contributor to the population decline over the past 10 years. Additional studies on animals from a variety of locations would be necessary to establish whether these results apply generally to Steller sea lions throughout the western range.
The following sections describe the two mechanisms proposed to cause a decrease in the availability or quality of the food supply for Steller sea lions throughout the history of the decline. These two mechanisms, climate regime shifts and fishery removals, may have had a combined effect that limited the availability of common Steller sea lion prey items during the earlier phases of the decline.
Climate Regime Shift
The regime shift hypothesis links climate-forced environmental changes to changes in the welfare of Steller sea lions through indirect trophic interactions in the marine food web. Several different mechanisms have been proposed that link climatic regime shifts to declines in Steller sea lions in the 1970s and 1980s. A reduction in the abundance of herring, capelin, and sand lance and a concomitant increase in large piscivorous fish associated with the 1977 climatic regime shift (see Chapter 2) may have adversely affected Steller sea lions by reducing the proportion of high-calorie fish in their diet (see discussion of the junk food hypothesis below). Merrick et al. (1997) showed that declines in sea lion populations correlate with a decrease in sea lion dietary diversity, which may be indicative of a change in the availability of prey species (Anderson and Piatt, 1999).
The regime shift hypothesis largely rests on statistical inference, wherein correlation analyses identify statistically significant associations between many 20th-century climate, fishery, and ecosystem survey records across the broad geography of the North Pacific and Bering Sea (e.g., see Anderson and Piatt, 1999; Hare and Mantua, 2000). Potential mechanisms linking climate changes to ecosystem changes in the North Pacific and Bering Sea are reviewed in Chapter 2. Testing the regime shift hypothesis is essentially limited to a “wait and see” approach that cannot distinguish between the impacts of natural environmental changes and other perturbations. Based on a recent shift to cooler upper-ocean temperatures and a weakening of the wintertime Aleutian Low beginning in 1998, the climate may have shifted to a “cool phase” Pacific Decadal Oscillation (PDO) state similar to what existed before the steep decline in Steller sea lion populations (Hare and Mantua, 2000; Schwing and Moore, 2000; Peterson and Mackas, 2001). If PDO regime shifts exert a significant and reversible forcing on sea lion abundance, the western population should begin to recover in response to the 1998-2002 climate trends.
Fishery Removals
The spatial and temporal scales of commercial fisheries provide important insights for evaluating the possible effects of fishery removals on the nutritional status of Steller sea lions. Fisheries and their potential interactions with Steller sea lions were discussed in detail in Chapter 5. This section first evaluates whether fisheries have depleted prey resources at a regional scale on an interannual basis to the extent that there is insufficient fish biomass to sustain the extant number of sea lions. The second part of this section considers the potential for fisheries to deplete sea lion prey at a local scale.
Evidence for Broad-Scale Depletion
Fishery and stock assessments indicate that walleye pollock (except the Donut Hole stock), Pacific cod, and Atka mackerel stocks are not overfished (North Pacific Fishery Management Council, 2001a, 2001b). Periodic strong year classes drive much of the change in fish stock abundance. Although parental abundance affects recruitment at low stock levels, some if not most of the recruitment variability in groundfish stocks in the North Pacific Ocean appears to be associated with variability in environmental conditions. Regime shifts toward winters with a deepened Aleutian Low Pressure System tend to be associated with higher frequencies of strong year classes among groundfish stocks (Hollowed and Wooster, 1992, 1995).
Are there enough fish to support a healthy population of Steller sea lions? This is a difficult question to answer without making many assumptions, but it addresses only the simplest consequence of the fishery. The more comprehensive question is whether the appropriate species, sizes, and densities of prey are available at spatial and temporal scales necessary for foraging sea lions. As will be shown, this is also a difficult question to answer given the poor state of knowledge about sea lion foraging ecology, fine-scale distributions of fishes, and effects of fishing on fish school dynamics.
In the 2000 Biological Opinion (BiOp #3), the National Marine Fisheries Service attempted to determine whether there was sufficient groundfish prey for Steller sea lions by calculating the amount of food consumed by sea lions relative to the biomass of the groundfish in the Gulf of Alaska, Aleutian Islands, and Bering Sea in 1999. This yielded a ratio of biomass consumption to availability of 1:54. Similarly, the agency deduced that a historical high number of 184,000 Steller sea lions would consume about 1.7 million metric tons (mt) annually, for a ratio of consumption to availability of 1:21. This comparison indicated that the current availability of fish biomass is higher for the 1999 population than for the prefishery population of sea lions.
Another approach for examining simple food availability is to compare trends in pollock, Pacific cod, and Atka mackerel biomass with Steller sea lion counts. Figure 6.1 shows estimates of exploitable fish biomass. Steller sea lion counts correspond to index sites in the Gulf of Alaska and Aleutian Islands, as there are no index rookeries in the Bering Sea. Also, analyses of sea lion counts were restricted to years in which at least 24 or 35 index rookeries were observed in the Gulf of Alaska and Aleutian Islands, respectively; counts from years with fewer sites tended to be inconsistent with counts from adjacent years.
In the Gulf of Alaska, sea lion numbers declined from the 1950s through the 1970s, a period during which pollock abundance was increasing (Figure 6.1a). The most rapid decline of sea lions occurred from 1977 to 1985, when pollock landings peaked in the Gulf of Alaska (Figure 5.2) and there was a large increase in the percentage of groundfish taken in Steller sea lion critical habitats (Figures 5.10 and 5.11). Trawl surveys in 1984-1996 did not show any decrease in high-density pollock abundance. However, many sea lions were taken as bycatch in the fishery. During this same time period in the 1980s, Pacific cod abundance increased (Figure 6.1c). Abundance of all three species declined from the late 1980s to the present. However, regardless of whether one considers pollock biomass alone or combined pollock and cod biomass, there have been more of these fish species available per Steller sea lion since the mid-1980s than prior to 1980 (see Figure 6.2a).
In the Bering Sea and Aleutian Islands, Steller sea lion counts were high from the mid-1960s to the late 1970s, at a time when eastern Bering Sea pollock abundance appears to have been low to moderate (Figure 6.1b). Steller sea lions declined sharply in the 1980s at a time when pollock, cod, and Atka mackerel abundances were increasing (Figure 6.1b, d, e). Interestingly, pollock biomass apparently declined during 1989-1992, whereas the Steller sea lion decline abated during 1990-1992 in the Aleutian Islands. As with the Gulf of Alaska, the biomass of pollock and the combined biomass of pollock, cod, and Atka mackerel per sea lion were higher after 1985 than prior to 1980 (Figure 6.2b).
As mentioned earlier in Chapter 5, these comparisons are fraught with assumptions and therefore should be interpreted with caution. Several factors tend to lead to overestimation of the fish biomass available to Steller sea lions. For example, in this analysis the committee chose index sites because this subset of rookeries provides the best measure of trends over time. However, by definition, these indices underestimate the total abundance of Steller sea lions because they do not include counted animals on nonindex sites and uncounted animals at sea during the surveys. Additionally, only index sites in the Gulf of Alaska within the range of the western stock of Steller sea lions were considered. Increases in the abundance of Steller sea lions in southeastern Alaska (eastern stock) were not included, although fish biomass estimates are gulf-wide values. Moreover, pollock in the northwestern portion of the eastern Bering Sea may not be available to sea lions, but the values represent total exploitable pollock biomass over the entire continental shelf.
Other assumptions of this analysis contribute to underestimation of prey biomass available to Steller sea lions. For instance, biomass estimates of Aleutian Islands pollock were not included because of questions about the discreteness of this stock. In 2000 a bottom-trawl survey estimated 105,500 mt of pollock in the Aleutian Islands (Ianelli et al., 2001). Survey trends indicate that pollock abundance in this area peaked in 1983, declined until 1994, and increased since then. Likewise, pollock biomass in the Aleutian Basin and Bogoslof Island areas were not considered in our analysis. It also should be noted that Steller sea lions target juvenile pollock (Merrick and Calkins, 1996), yet young pollock are not fully sampled by the survey gear and tend to be underestimated in stock assessments. Finally, our estimates do not include other components of the sea lion diet, including fish (e.g., salmon, herring, flatfishes, rockfishes, sand lance, capelin) and invertebrates (e.g., octopus, squid). Given these caveats, this analysis does not provide support for the hypothesis that the recent decline in the Steller sea lion population is due to depletion of sea lion prey by the groundfish fisheries.
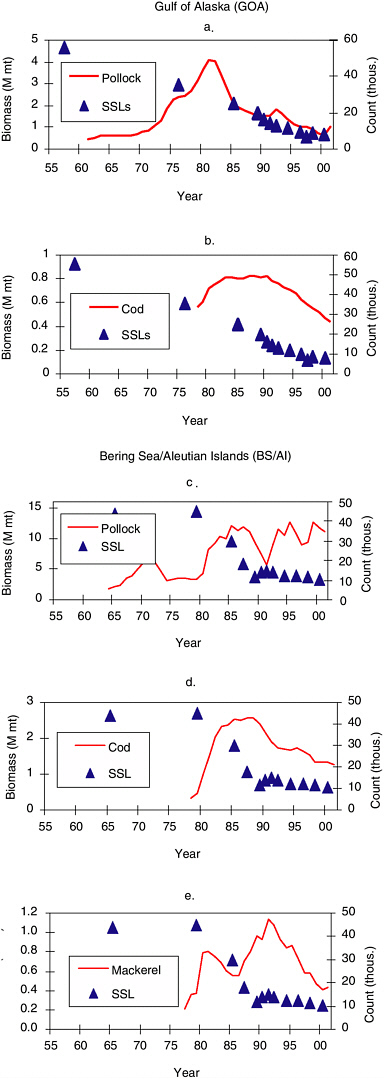
FIGURE 6.1 Trends of Steller sea lion (SSL) index counts in the Gulf of Alaska (GOA) versus exploitable biomass of (a) GOA pollock and (b) GOA Pacific cod. Trends of SSL index counts in the Aleutian Islands versus exploitable biomass of (c) Bering Sea/Aleutian Islands (BS/AI) pollock, (d) BS/AI Pacific cod, and (e) BS/AI Atka mackerel. Index counts are for those years in which a minimum of 24 rookeries in the Gulf of Alaska or 35 rookeries in the Aleutian Islands were observed.
SOURCE: Data from National Marine Fisheries Service, National Marine Mammal Laboratory, Seattle, available at www.afsc.noaa.gov/.
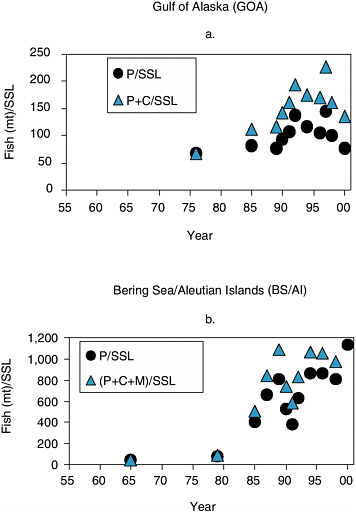
FIGURE 6.2 Relative availability of fish biomass of pollock (P), pollock and cod (P+C), or pollock, cod, and mackerel (P+C+M) per Steller sea lion (SSL) index count for (a) Gulf of Alaska and (b) Bering Sea/Aleutian Islands. Fish biomass estimates are based on stock assessments conducted for 2001.
SOURCES: Data from Dorn et al. (2001); Ianelli et al. (2001); Lowe et al. (2001); Thompson and Dorn (2001); Thompson et al. (2001).
Evidence for Localized Depletion
Although the North Pacific groundfish are managed conservatively based on single-species harvest strategies, concerns remain over other ecological effects, such as localized depletion in the vicinity of sea lion rookeries, have remained (National Marine Fisheries Service, 2000). As
described in Chapter 5, few studies have been conducted on localized depletion of sea lion prey by commercial fisheries. There is evidence of localized depletion of Atka mackerel in some areas in the early to mid-1990s (Fritz, 1999). Atka mackerel tend to stay in a given area, rendering this species particularly susceptible to local depletion. The recovery of biomass to prefishery levels by the start of the subsequent fishing season indicates that depletion is temporary, but effects of this seasonal depletion on foraging Steller sea lions are unknown.
Preliminary results from an ongoing study of pollock suggest that trawl fisheries in the Barnabas trough off Kodiak Island do not cause localized depletion (Wilson et al., 2002). The biomass and distribution of pollock were relatively stable over periods of days to weeks. Also, there was no discernable effect on the vertical distribution or school fractal dimensions. These preliminary results, and reports of high-density pollock areas in the Gulf of Alaska during 1984-1996 (Shima et al., 2002), indicate that localized depletion of pollock is unlikely. Additional research is needed on other species and in other areas before concerns can be fully resolved about localized depletion by fishery removals.
Summary of Evidence for Fishery Removal Hypothesis
Under the fishery removal hypothesis, fisheries may have sufficiently depleted the availability of prey for Steller sea lions, causing nutritional stress with adverse ramifications on reproduction and survival. Owing to the lack of studies specifically designed to test this hypothesis, it cannot be accepted or rejected. Data derived from fish stock assessments and commercial fisheries indicate that the overall levels of fish biomass in the federal fishery management areas are large relative to the current population of Steller sea lions. However, additional research on seasonal fish movements and migrations, effects of fishing on fish school size and distribution, and sea lion foraging ecology are needed to more fully resolve the issue of localized depletion.
FOOD QUALITY
The junk food hypothesis posits that Steller sea lions are consuming a lower-quality diet (i.e., eating fewer fatty fish such as capelin and herring and more lean fish such as pollock) because of an ecosystem-level change in their environment. The most recent publication on this matter (Thomas and Thorne, 2001) suggested that sea lions cannot find enough herring in their home range and therefore eat pollock instead. The diet diversity hypothesis (Merrick et al., 1997) is a corollary of this idea; it postulates that sea lions require a more diverse diet to thrive and correlates low
|
BOX 6.2 Diet Diversity Analyses of Steller sea lion scats revealed a strong inverse relationship between dietary diversity and the estimated depth of population decline (Merrick et al., 1997). Those populations that had declined the most also had the least diverse diets, and in keeping with this pattern, dietary diversity was greatest for the increasing sea lion population in southeastern Alaska. In some areas the less diverse diet consisted mostly of pollock, apparently supporting the junk food hypothesis that pollock are of insufficient nutritional quality to maintain a healthy sea lion. Similar patterns were observed in the central and western Aleutians, although there the dominant prey type was Atka mackerel. However, the inverse relationship between dietary diversity and the degree to which food resources are limiting to populations of consumers could indicate that dietary diversity in Steller sea lions decreases when prey resources are not limiting. A positive relationship between dietary diversity and the degree of food resource limitation is more consistent with foraging theory (Emlen and Emlen, 1975; Krebs, 1978). Many foraging models predict that when prey resources are abundant, consumers will specialize on the most beneficial of these. As consumer populations increase and the most beneficial or valuable prey are depleted, increasingly less valuable prey are added to the diet, thus increasing dietary diversity. |
dietary diversity with declining populations (see Box 6.2). On a less optimal diet, it is assumed that sea lions would be unable to eat a sufficient amount of food to meet their metabolic demands.
Development of a metabolic cost analysis for Steller sea lions requires knowing a suite of factors that can generally be divided into the costs of thermoregulation, swimming and diving (i.e., hydrodynamics), basal metabolic demands (i.e., digestion, growth), and reproduction (pregnancy and lactation for females, harem maintenance for males). Although all of these values can be modeled or estimated based on body size or other mammal species, unless they are actually measured on Steller sea lions, it will be not be possible to know if Steller sea lions balance the overall equation of demand and supply similarly to other species. Given the potentially subtle or chronic nature of the nutritional stress, modeling efforts may not be able to provide the refinement of data necessary to determine if sea lions are food limited.
Because the diets of animals cannot be controlled in the wild, there is no direct way to test the junk food hypothesis in the field. Therefore, several laboratory-based studies have examined how Steller sea lions
digest and process a variety of different fishes. Studies of digestive efficiency, gut passage rates, otolith recovery conditions, metabolic rate control, and swimming energetics are being conducted on captive animals. The first study, based on short-term feeding trials, showed that juvenile Steller sea lions could not maintain their body mass on a diet of pollock relative to herring (Rosen and Trites, 2000). More recent analysis of these data suggests that seasonal effects may have influenced the results and may only apply to juveniles, not adults (Trites, 2001).
Ongoing research programs at the Alaska SeaLife Center (ASLC) in Seward have been conducting long-term feeding trials (3 years) with young adult Steller sea lions. Preliminary results indicate that sea lions show seasonal and gender-specific responses to dietary change and maintain body condition under different dietary regimes (Castellini et al., 2001). In similar long-term studies with harbor seals in Alaska, body condition could not be predicted based on a diet of herring or pollock (Castellini et al., 2000). Current studies examine prey items in order to measure and assess their nutritional quality (calories, vitamins, lipid levels, protein levels, iron content, etc.) by both region and season. For example, sea lions in the feeding trials at the ASLC are being fed diets representative of predecline, postdecline, and southeastern Alaska conditions. Ongoing studies will provide a nutritional assessment of these diets. As of 2002, ongoing scat studies suggest regional and seasonal differences in the prey items found in sea lion scats (Sinclair and Zeppelin, 2002) and shifts in dietary diversity correlate with Steller sea lion population trends. However, this correlation may be coincidental rather than an indication of a causal link between diet and population decline.
Bowen et al. (2001) challenge the diet diversity theory because a link between diversity of the Steller sea lion diet and their lack of recovery has not been established. The Alaska Steller Sea Lion Recovery Team (Kruse et al., 2001) looked at the same evidence from Merrick as reported in BiOp #3 and recommended further consideration of the potential effect of diet diversity on Steller sea lion population trends.
In summary, the field-based physiology and feeding ecology studies have focused on defining how animals in different regions or at different ages compare with predicted responses of a nutritionally stressed population, while the laboratory-based programs have tried to quantify the metabolic pathways and control between prey quality and Steller sea lion condition. To date, evidence from these studies does not support the hypothesis that food limitation is currently reducing the condition of adult females, or impairing their ability to raise a pup, or compromising the health of animals up to 2 years of age.
PREDATION AND TAKES BY HUMANS— TOP-DOWN HYPOTHESES
Population growth rates decline fastest when adult survival is decreased (see Chapter 3). Therefore, factors that increase adult mortality are expected to have the greatest proportional impact on the population. Both natural (predation by killer whales or sharks, infectious disease, or toxins) and anthropogenic (deliberate kills and incidental takes) sources of adult mortality could have dramatic impacts on the demographics of the Steller sea lion population.
Previous estimates of these various sources of adult mortality were too low to explain the rapid, range-wide decline in the western population (Chapter 3). However, predation by killer whales may have been higher than previously assumed, and there is substantial uncertainty in the estimates of deliberate and incidental takes by humans. Relatively small increases in either the natural or anthropogenic mortality parameters in the Ecosim model described in Chapter 3 could improve the fit of the model to the observed pattern of sea lion decline. Additionally, these sources of mortality could readily explain the continuing, albeit slower, decline of the sea lion population. These top-down mechanisms are also consistent with recent studies that have found no evidence of reduced health or fitness in comparisons of declining and increasing populations of sea lions.
Predation on Steller Sea Lions (Killer Whales and Sharks)
Ecology has been slow to consider the role of predation in population regulation and food web dynamics, despite early examples (Elton, 1927; Brooks and Dodson, 1965; Paine, 1966) and a well-known conceptual framework (Hairston et al., 1960). But there is growing evidence from diverse ecosystems for the importance of predation and top-down forcing (Estes et al., 2001). The earliest and most compelling evidence comes from aquatic systems: lakes (Carpenter and Kitchell, 1993), streams (Power, 1990), and coastal environs (Paine, 1966; Estes and Palmisano, 1974). However, there are few known examples from oceanic ecosystems, despite the remarkable diversity and abundance of large predators in the sea. Studies of pattern and process in the ocean have been dominated by a nearly exclusive focus on bottom-up forcing mechanisms.
Evidence for predation can be evaluated based on the following three questions: Who are the potential predators? Are they sufficiently abundant and wide ranging to explain the decline based on what is known of their diets and nutritional requirements? Do current or historical records provide evidence for predator-based shifts in the sea lion population?
There are currently three contenders for the predators: salmon sharks (Lamna ditropis), Pacific sleeper sharks (Somniosus pacificus), and killer whales (Orcinus orca). Both shark species are abundant in the western Gulf of Alaska and their population numbers appear to have increased considerably in the 1990s (Lee Hulbert, National Marine Fisheries Service, Auke Bay Laboratory, personal communication, 2002, available at http://fakr.noaa.gov/oil/sharks.htm). Salmon sharks are large, fast-swimming, aggressive predators. Although related shark species attack and consume pinnipeds, there are no such records for salmon sharks, which appear to be largely or exclusively piscivorous. Sleeper shark stomachs sometimes contain remains of harbor seals and small cetaceans (Bigelow and Schroeder, 1948; Bright, 1959; Lee Hulbert, National Marine Fisheries Service, Auke Bay Laboratory, personal communication, 2002), but as yet there is no evidence they attack Steller sea lions. Also, there are no reports of shark attacks or shark wounds on sea lions. Finally, because large sharks are rarely if ever observed, it is doubtful that they could occur in sufficient abundance to account for declining Steller sea lion populations in the Aleutian Islands and southeastern Bering Sea.
The third candidate predator, the killer whale, attacks and consumes a diverse array of marine mammals and fish, including many species of large and small cetaceans, pinnipeds, and sea otters (Matkin et al., 1999). Considerable dietary variation exists among groups or individual killer whales (Ford et al., 1998). Some killer whales appear to feed largely or exclusively on marine mammals, described by some researchers as transients (Ford and Ellis, 1999). Killer whale attacks on Steller sea lions have been reported, and sea lion remains were found in the stomach contents of two of eight killer whale carcasses recovered in Alaska (Barret-Lennard et al., 1995). One of these two killer whales, a beach-cast female recovered in Prince William Sound in 1992, contained flipper tags from 14 Steller sea lions tagged as pups on Marmot Island in 1987-1988 (Saulitis et al., 2000). Killer whales have been implicated in the collapse of sea otter populations in coastal waters across the Aleutian archipelago and northern Gulf of Alaska (Estes et al., 1998). Theoretically, a switch of fewer than four killer whales to feeding exclusively on sea otters can account for the additional annual mortality in the central Aleutian Islands during the rapid decline of the sea otter population. Like the sea otter, sea lion declines could be explained by remarkably small changes in killer whale foraging behavior based on the energetic requirements of killer whales. Scientific surveys of killer whale abundance have only recently been conducted, so the historical size of the killer whale population in Alaska is unknown. However, the killer whale population in the southeastern Bering Sea has recently been estimated at 391 (95% confidence interval = 171-894), and this area
represents only a fraction of the range of the western population of Steller sea lions (Waite et al., 2002).
If killer whale predation on Steller sea lions increased, what might have triggered this change? Possible explanations include an increased abundance of predators, a change in feeding behavior, or a decrease in the availability of other marine mammal prey species. Existing information is insufficient to answer this fundamental question. The North Pacific and Bering Sea ecosystems have experienced a number of significant perturbations, including the depletion of great whales between the late 1940s and early 1970s, the establishment of large commercial fisheries, and changes in the oceanic food web caused by a climate regime shift in the late 1970s. These or possibly other factors may have influenced the population dynamics and foraging behavior of the ecosystem’s apex predators.
Because killer whales must consume considerable amounts of prey (in terms of weight) to meet their metabolic requirements, a relatively small change in the rate of killer whale predation on Steller sea lions could account for the historical decline. Although Branson (1971) reported that attacks by killer whales on pinnipeds were unknown prior to about the 1970s, there is no direct evidence that increased predation since then was the primary cause of Steller sea lion decline in the 1980s. Hence, the role of killer whale predation in the historical decline is indeterminate, but further research on killer whales should indicate whether predation is preventing the recovery of the remaining sea lion population.
Subsistence Harvests
Alaska natives have hunted Steller sea lions since prehistoric times. Archeological sites show that indigenous peoples have harvested sea lions for the past 3,000 to 4,000 years (Laughlin, 1980). Sea lion remains have been confirmed from archeological sites throughout the range of the western stock, including Prince William Sound, Kenai Peninsula, Kodiak Archipelago, and the Aleutian Islands. Steller sea lions comprised up to 70% of the estimated biomass of animal remains at some sites (Haynes and Mishler, 1994). Traditional uses of sea lions include meat for food, hides to cover kayaks, skin of flippers for soles of boots, stomachs for the leg part of boots, intestines for raincoats, and bladders as floats for fishing nets and lines and as sacks to store oil and other liquids (Haynes and Mishler, 1994).
Various traditional hunting methods have been reported, including harpooning on rocks by kayakers in the Kodiak Archipelago (Haynes and Mishler, 1994), gun and spear in the Aleutian Islands (Nelson, 1887), and clubbing on land at Nunivak Island in the Bering Sea (Haynes and Mishler, 1994). Elliott (1887), as cited in Haynes and Mishler (1994), described
annual drives that were conducted from September to November on the Pribilof Islands. Hunting was conducted at night when animals were driven into corrals. After a total of 200 to 300 animals were corralled over several nights, sea lions were then herded over 11 miles for 5 days to 3 weeks to the village where they would be slaughtered. Russian orthodox missionaries documented that an average of 2,000 animals were harvested annually on St. George Island in the 1830s (Haynes and Mishler, 1994). By the 1870s, harvests of Steller sea lions from the Pribilof Islands declined to several hundred per year (Nelson, 1887; reproduced in Appendix D).
Reports by subsistence hunters indicate that Steller sea lions declined in abundance in the Aleutian Islands and Bering Sea during the 1800s. In the late 1870s, Pribilof Islanders reported to Nelson (1887) that 70 years earlier sea lions occupied most of the shore of St. George Island and numbered several hundred thousand animals. They further recounted: “By direction of the Russians they were driven off repeatedly until they left the place, and the shore was then occupied by fur seals.” By the late 1870s Nelson noted that, while Steller sea lions used to be abundant along the Aleutian Islands, “they are now so scarce among these islands, and the ones that are found there frequent places so difficult to access, that the Aleuts secure very few of them each year.” Also, on the Pribilof Islands (referred to at the time as the Fur Seal Islands), Nelson noted that the drive was much more difficult than in previous years, and it was “almost or quite impossible to collect the full number.” At the same time, Nelson noted that “they are still rather common at a few points along the north shore of Unimak Island and the peninsula of Alaska” and “eastward and southward they occur all along the coast to California, where their range overlaps that of the southern species [California sea lion].”
During the 20th century, subsistence harvests of Steller sea lions by Alaska natives were documented intermittently. Haynes and Mishler (1994) reported on harvests in selected areas during 1981-1989. The largest documented harvest from one area was 178 animals from six communities on Kodiak Island in 1983. The sporadic coverage of different areas in different years and lack of data from some harvest areas makes it difficult to estimate total statewide harvests by year. The number of Steller sea lions killed by subsistence hunters could be significantly underestimated because not all hunters and communities have been interviewed and there is substantial uncertainty in the estimates of how many animals were lethally shot but not recovered.
The Alaska Department of Fish & Game conducted systematic interviews of approximately 2,100 households from 60 coastal communities to estimate the subsistence harvest of Steller sea lions in Alaska during 1992-1998. Details were reported in annual reports by the department, summa-
rized by Angliss et al. (2001). About 99% of the harvest was taken from the western stock. In 1992 an estimated 549 animals were taken (370 were harvested, 179 were struck and lost). On average, 414 animals were taken (330 harvested, 84 struck and lost) annually during 1993-1995. The reported age composition was 64% males, 19% females, and 17% of unknown sex, and the reported age class composition was 31% adults, 62% juveniles, 3% pups, and 4% unknown. Since 1995 the reported annual takes have declined to as low as 171 animals killed, of which 128 were harvested and 43 were lost.
DELIBERATE AND INCIDENTAL MORTALITY FROM HUMAN ACTIVITIES
Shooting of Steller Sea Lions
In the first half of the 20th century, many fox farms existed along the coast of Alaska. Not uncommonly, Steller sea lions were harvested as a cheap source of protein for fox food. During the early to mid-1940s, anecdotal reports revealed incidents of sea lion slaughters by American war-planes stationed in Alaska. In Kodiak “the big PBY’s . . . they’d come through the narrows . . . a hundred feet off the water and they’d open up those big fifty caliber guns shooting at the sea lions on Triplet Islands. I was sitting there on the beach . . . and here are these tracers hitting the sea lions and the bluffs with great big sparks flying. The sea lions were dropping off the top of the islands. And man, you talk about slaughter” (Ed Opheim, former cod and salmon fisherman, Kodiak, personal communication, September, 2001).
Shooting of Steller sea lions was legal prior to the Marine Mammal Protection Act of 1972. However, after passage of this act, fishermen were allowed to continue to shoot sea lions that were destroying their gear or causing a threat to human safety. It was not until 1990 when the species was listed as threatened under the Endangered Species Act that the discharge of firearms near Steller sea lions was fully prohibited. Recent court cases and anecdotal information indicate that some illegal shooting of Steller sea lions continues despite these laws.
Predator Control Programs
In 1951 the Alaska Department of Fisheries instituted a targeted predator control program, mainly for harbor seals but also including sea lions. The rationale was to reduce the impacts of seals and sea lions on the salmon gillnet fisheries. Complete tallies of the number of sea lions killed are unavailable, but unpublished accounts suggest that while a small
fraction of the total population was taken, kills on some rookeries were substantial. For example, agency hunters killed virtually all the pups on Amatuli Island in the central Gulf of Alaska during a season once in the 1950s and once in the 1960s.
Experimental Harvests of Steller Sea Lions
Experimental harvests were conducted from 1959 until 1972 as a more cost-effective alternative to earlier bounty programs for controlling sea lion populations (Thorsteinson et al., 1961). The harvested animals were to be used as food for humans, feed for fox farms in Alaska, and feed for mink ranches in the Pacific Northwest. A total of 45,178 Steller sea lion pups were harvested between 1963 and 1972 from islands in the Kodiak Archipelago to the eastern Aleutian Islands (Merrick et al., 1987). In addition to the experimental harvest, 3,000 to 6,000 pups were taken annually during the 1960s for the fur market (Vania, 1972).
Prior to 1990, shooting was the primary collection method for research on the reproductive biology, physiology, and diet of Steller sea lions. Although a complete count has not been compiled, in several studies biologists reported takes in the range of 80-114 animals (Mathisen, 1959; Calkins et al., 1998).
Shooting by Fishermen
There is a long history of fishermen shooting sea lions in Alaska (for a review, see Hoover, 1988). In part, shooting was motivated by the belief that declines in salmon runs during the 1930s through the 1950s was partly due to predation by sea lions (Mathisen, 1959). More importantly, fishermen shot many animals in attempts to reduce lost catches and gear damage by sea lions. Sea lions were reported to take fish from a variety of fishing gears, including salmon gillnets, troll and purse seines, herring purse seines, halibut and sablefish longlines, and groundfish trawls (Thompson et al., 1955; Mathisen, 1959; Thorsteinson et al., 1961; Thorsteinson and Lensink, 1962; Hoover, 1988). As early as the 1880s, it was reported that seals and sea lions “prey upon cod, frequently taking them from the line” around Kodiak (Bean, 1887). Sea lions puncture crab pot floats and rip holes in purse seines and gillnets, resulting in lost catch and lost and damaged gear.
At least through the 1970s, lost catch and damaged gear by Steller sea lions were considered to be a serious problem, and there were several attempts to quantify the losses. Based on a poll of halibut fishermen, the International Pacific Halibut Commission estimated that 1.3 million pounds of halibut worth $270,000 were damaged or destroyed by sea
lions in 1958 between Cape Saint Elias and the Trinity Islands (Thorsteinson et al., 1961). Matkin (1977) estimated that 8.3% of the salmon catch was damaged during the first 4.5 weeks of the Copper River gillnet fishery in 1977. Three-fourths of the loss was attributed to Steller sea lions, and one-fourth was associated with harbor seals (Phoca vitulina). Associated damage to gillnets, nearly all of which was attributed to sea lions, was estimated at 2.5% of the gross value of the catch. During the 1940s to 1970s, there were several published accounts of significant loss of sablefish and halibut to sea lions as longline gear was retrieved (Hoover, 1988).
There is little documentation of the number of Steller sea lions killed by fishermen. A partial survey of salmon trap operators in the Kodiak and Alaska Peninsula areas indicated that 816 animals were killed in spring 1954 (Thompson et al., 1955). Matkin and Fay (1980), as cited by Hoover (1988), estimated that 305 sea lions were shot and killed in association with the drift gillnet fishery at the Copper River delta in 1978. Anecdotal information and public testimony suggest that shooting of animals by fisherman was quite substantial, at least until the 1980s. In the early to mid-20th century, gillnet and other fishermen regularly shot animals that approached their gear, and many people would go to rookeries and haulouts to shoot animals in the Kodiak area (Ed Opheim, former cod and salmon fisherman, Kodiak, personal communication, September, 2001). These shootings appear to have been prevalent throughout coastal Alaska. Later, during the king crab fisheries in the 1960s and 1970s, sea lions were shot for crab bait and crab fishermen shot animals in frustration over punctured floats and lost crab pots. Fishermen active in the joint-venture fishery in Shelikof Strait shot many Steller sea lions to prevent them from taking fish and damaging trawl nets that were towed near the surface for delivery to the motherships. Anecdotal reports by participating fishermen indicate that the number of animals shot was close to the number caught in trawls during those years. Lack of systematic reporting of sea lion kills by fishermen makes it impossible to provide reliable estimates of the impact of shooting on the population (see Box 6.3).
Incidental Takes and Entanglement in Fishing Gear
Incidental mortality of Steller sea lions in lost (ghost) fishing gear is largely unknown. Most reported entanglements involve fishing net fragments and closed plastic packing bands, such as those used to hold together boxes of bait (Calkins, 1985). Two- to three-year-old animals seem to be most vulnerable. Young sea lions may become entangled in salmon and herring gillnets, but larger individuals are able to break free (Loughlin et al., 1983). Loughlin et al. (1986) examined approximately
|
BOX 6.3 Anecdotal Mortality There is a long history in Alaska of shooting sea lions for their meat and pelts, for sport, or to reduce sea lion interference with fishing operations. Prior to 1990, a prevalent attitude among many fishermen was that sea lions were a nuisance and a cause of damaged gear and lost catches. Currently, the presence of observers on factory trawlers makes it unlikely that these fisheries illegally shoot sea lions. The number of sea lions killed in other fisheries is unknown, but even small numbers of shootings could contribute to the decline of the small remaining populations. Except for subsistence harvest, shooting of sea lions has been illegal since 1990. Still, the committee heard many anecdotal accounts of unreported “takes” of Steller sea lions, suggesting that the tradition may continue at a higher incidence than the recent estimates (Loughlin and York, 2000). The following examples are from a variety of unconventional sources. Shooting, both legal and illegal, will have a particularly severe impact on the dwindling population, and better estimates of this mortality are urgently required.
|
16,000 adults and 14,000 pups at 17 rookery and 15 haulout sites in the central and eastern Aleutian Islands in summer 1985. Only 11 animals showed evidence of entanglement; most involved nets or twine. However, these were individuals that survived the encounter, and mortality due to entanglement is unknown. The amount of netting and packing straps varies with beach location and exposure on Kodiak Island (Calkins, 1985), and entanglement rate might be expected to vary with type and amount of fishing gears, which vary seasonally by area. Entanglements of Steller sea lions on longline hooks (Hoover, 1988) and salmon troll gear (Angliss et al., 2001) are occasionally reported.
Steller sea lions may become captured during commercial and sport fishing operations. Most accounts of sea lion deaths were reported during the foreign trawl fisheries, which undoubtedly have caught Steller sea lions since their inception (Perez and Loughlin, 1991). Unfortunately, recorded observational data are limited to onboard observer programs restricted to certain fisheries, vessel size classes, and years of operation. Hence, recorded takes underestimate the total number of sea lions killed in the commercial fisheries. Observers aboard foreign and joint-venture trawl vessels recorded 3,661 marine mammals (90% were Steller sea lions) as incidental takes during 1973-1988 (Perez and Loughlin, 1991). The highest catches of sea lions were taken in a joint-venture pollock trawl fishery in Shelikof Strait in 1982-1984 and foreign fisheries near Kodiak and in the Aleutian Islands in earlier years. Also, sea lions were commonly taken in foreign trawl fisheries in the southeastern Bering Sea in 1978-1981 (Loughlin et al., 1983). In Bering Sea fisheries, most incidentally caught Steller sea lions were males, whereas in the Gulf of Alaska and probably in the Aleutian Islands most takes were females and subadult males (Loughlin and Nelson, 1986; Perez and Loughlin, 1991).
To estimate total incidental mortality, observed sea lion takes must be adjusted for sea lion survival rate, fraction of observer days spent recording sea lion takes, and fraction of the fleet with onboard observers. This assumes that sea lion takes were the same on unobserved vessels, an assumption that could underestimate the number of takes if fishing practices are different in the absence of an observer. In 1979, 34% of incidentally caught sea lions were alive when brought onboard foreign trawlers and escaped into the water when released from the net (Loughlin and Nelson, 1986). In contrast, sea lion mortality on U.S. trawlers participating in the joint-venture fishery was nearly 100% because net codends were tied off after retrieval and remained in the water a long time before transfer to the foreign processing vessel.
Annually, about 800 marine mammal mortalities (predominantly sea lions) were estimated for foreign fisheries in Alaska during 1972-1976. This most likely underestimates mortality because there were a limited
number of observers during this time period and reporting protocols were informal and variable (Loughlin et al., 1983). During 1978-1981, only 10% of foreign fishing vessels (mean = 251 vessels per year) carried observers. The estimate of 800 annual mortalities does not include animals taken by U.S. domestic fishermen, including those who participated in the joint-venture fisheries. During the 1980s, observer coverage rates approached 100% for foreign trawl and longline fishing vessels and foreign processing vessels participating in joint-venture fisheries with U.S. catcher boats.
In 1980 a joint-venture fishery developed to harvest large schools of spawning pollock in Shelikof Strait (Loughlin and DeLong, 1983; Loughlin and Nelson, 1986). In this fishery alone, total mortalities of sea lions were estimated to be 958 to 1,436 in 1982, 216 to 324 in 1983, and 237 to 355 in 1984 (Loughlin and Nelson, 1986). During these years, a single tow was observed with more than 20 sea lions, but occasionally fishermen reported takes of 50 to 100 sea lions in a single tow.
Particular aspects of the joint-venture pollock fishery contributed to high incidental mortalities of Steller sea lions in Shelikof Strait in the early 1980s. Roe was the most valuable seafood product, and large volumes of fish were discarded, perhaps attracting sea lions to the area (Loughlin and DeLong, 1983). Nets were retrieved, sometimes with many sea lions in the vicinity, and the method of transfer of codends from catcher to processing vessels led to the drowning of ensnared sea lions.
A number of factors contribute to the variability in rates of incidental takes of sea lions by the joint-venture trawl fishery for pollock in Shelikof Strait in the 1980s (Loughlin and Nelson, 1986). Fishing locations varied substantially; compared to 1983 and 1984, the fishery in 1982 occurred farther east and closer to shore, where presumably more sea lions were encountered (see Figure 6.3). Also, more sea lions were observed in Shelikof Strait in mid- to late April than in January to March. During 1982-1984, 80% of caught sea lions were taken in April. Presumably, sea lions move into the area in greater numbers in April in advance of the pupping and breeding season, which begins in May. The highest catch rates of sea lions in 1982 may be partly explained by the persistence of this fishery in April, compared to the termination of the fishery in early April in 1983 and 1984.
Time of day is another important factor affecting the bycatch of Steller sea lions. In the joint-venture fishery, trawl tows were made at all hours of the day, but only 18% of the catch of Steller sea lions occurred during daylight (see Figure 6.4). Most sea lions were caught during a 4-hour period between 11 p.m. and 3 a.m.
During the 1990s, incidental takes of Steller sea lions were monitored for domestic groundfish trawl, pot, and longline fisheries conducted in the Gulf of Alaska, Aleutian Islands, and Bering Sea (Angliss et al., 2001).
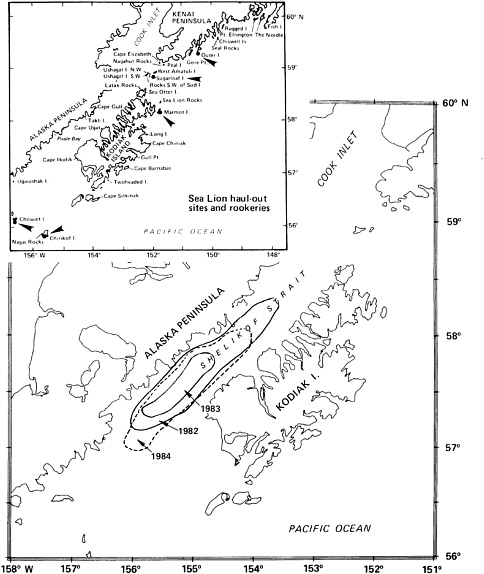
FIGURE 6.3 Generalized areas of incidental catch of Steller sea lions during the pollock joint-venture trawl fishery in Shelikof Strait in 1982-1984. Locations on the inset depict sea lion rookeries (arrows) and haulouts.
SOURCE: Loughlin and Nelson (1986); reprinted with permission from the Society of Marine Mammalogy.
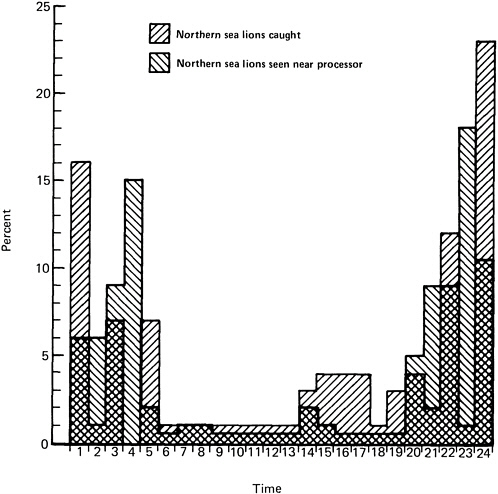
FIGURE 6.4 Percent of incidental catches of Steller (Northern) sea lions by time of day (hour) compared to percent of Steller sea lions seen near processing vessels, as reported by observers in the joint-venture pollock fishery in Shelikof Strait in 1983-1984.
SOURCE: Loughlin and Nelson (1986); reprinted with permission from the Society of Marine Mammalogy.
No mortality has been observed in the pot fisheries since 1990 and in the longline fisheries since 1995. The one observed mortality in the longline fishery was expanded by the fraction of the observed fleet to estimate a total mortality of four animals. The highest mortality from any gear/ region combination was from the Bering Sea trawl fishery, where it was estimated that seven sea lions were killed annually during the 1990s.
A salmon drift gillnet fishery with 4 to 5% observer coverage in 19901991 recorded two sea lion deaths. Approximately 2 to 5% of the salmon setnet and gillnet fisheries were observed in Cook Inlet in 1999 with no reported mortalities of sea lions.
In summary, low rates of incidental fishing mortality of Steller sea lions have been reported for the 1990s. However, observers do not cover all fisheries, and the only information on unobserved fisheries comes from self-reporting of marine mammal takes or beach standings of entangled animals. All such reports are voluntary.
Summary of Effects of Kills by Humans
It is not clear whether apparent declines in Steller sea lions in the Aleutian and Pribilof Islands between the 1830s and 1870s were related to subsistence harvests or other factors. For the next 100 years, good data on subsistence harvest are lacking. Contemporary data on subsistence harvests are available only for the 1990s, and these harvests represent less than 1% of the abundance of the western stock of Steller sea lions. However, total mortality from subsistence harvest could be higher if the number of unrecovered animals is higher than the current estimates. At this time, the subsistence harvest is not considered to be a major contributing factor to the current decline (National Marine Fisheries Service, 2000).
It is difficult to fully assess the effects of incidental mortality from fisheries because there were low rates of observer coverage during the 1970s and early 1980s. Using all available observer data, Perez and Loughlin (1991) estimated that annual incidental mortality of Steller sea lions might have declined from approximately 1,000 to 2,000 in the early 1970s and 1982 to fewer than 100 animals in 1988. If these estimates are representative of total mortality from all fisheries, then fishery-induced mortality explains only a minor portion of the decline of the western Steller sea lion population. However, extrapolations of total kill rates depend heavily on assumptions about unobserved foreign and domestic vessels and fisheries during these years. The true number of kills is unknown but could be considerably higher than official estimates. Also, although poorly documented, it appears indisputable that historical shooting of sea lions by fishermen and others was commonplace and likely represented a very large number of animals. Because it has been illegal to
discharge firearms within 100 yards of Steller sea lions since 1990, the amount of shooting in recent years is even less well known, but some anecdotal evidence suggests that the practice persists. Hence, the “smoking gun” in the mystery of the Steller sea lion population decline could, at least in part, be the unreported and illegal takes.
INFECTIOUS DISEASE
Theoretical studies suggest infectious diseases may regulate host abundance by exerting density-dependent effects on host reproduction or survival (Anderson, 1979). However, few empirical studies on free-living animals have determined whether effects of disease on populations are density dependent, as most disease investigations on wildlife have focused on determining the proximate causes of large die-offs. Such die-offs are most often the result of epidemics of disease in host populations that have not previously been exposed to the disease or that have become more susceptible to an introduced agent due to changes in immune status. Although not generally considered regulators of host population density, severe epidemics may reduce host population density to such an extent that stochastic events or previously unimportant ecological factors may further reduce the host population size (Harwood and Hall, 1990).
The importance of infectious disease epidemics in causing declines of marine mammal populations is unclear because few die-offs have been investigated sufficiently to determine their cause, and it is often difficult to accurately determine host population numbers. Recent epidemics in marine mammals have caused dramatic mortality, but the effects on host population numbers vary. For example, approximately 17,000 harbor seals (70% of the population) died in the phocine distemper (PDV) epidemic in Europe in 1988, but 10 years later the population had recovered to pre-epidemic numbers (Reijnders et al., 1997). The source of infection for this morbillivirus outbreak in marine mammals is unclear. The 1988 PDV epidemic was believed to have resulted from the introduction of a virus into a naïve population, due to the large numbers of animals without antibodies prior to the outbreak. Based on this assumption, a mathematical model investigating the infection dynamics of this disease in 1992 predicted that reintroduction of the virus resulting in large-scale mortality would not occur for at least 10 years (Grenfell et al., 1992). A new outbreak of PDV is currently occurring in the North Sea (Jensen et al., 2002; cwss.www.de/news/news/Seals/01-seal-news.html). In 1988 it appeared the mortality was lower in seal populations that had experienced previous mortality events (Grenfell et al., 1992).
Endemic diseases have more subtle effects and may be more important in regulating marine mammal populations than previously thought.
Infectious diseases such as brucellosis are known to cause population declines in terrestrial mammals due to spontaneous abortion and reproductive failure. Although these types of disease organisms have only recently been isolated from marine mammals, they may be prevalent in free-ranging populations (Dunn et al., 2001). Food limitation may increase the impact of macroparasites, resulting in population crashes, as observed in Soay sheep populations in Scotland (Gulland, 1992). Even if only a few individuals show signs of infection, it is possible that parasitic nematodes have a significant influence on the host population size (Hudson and Dobson, 1995). There are a wide variety of parasitic nematodes in marine mammals, but their effects on host population dynamics are unknown (Dailey, 2001).
A number of infectious disease agents known to occur in marine mammals of the Arctic and Pacific could cause epidemics, endemic mortality, or decreased reproduction in Steller sea lions. These include morbilliviruses, influenza virus, phocine herpesviruses, caliciviruses, Leptospira spp., Brucella spp., Chlamydia psittaci, Toxoplasma spp., and various species of nematodes (Dierauf and Gulland, 2001). If an epidemic hit a population of Steller sea lions, it would be expected to spread from one area, cause mortality in animals of all ages, and leave survivors with antibodies to the causative agent (Heesterbeek and Roberts, 1995). Although the rapid decline of sea lions is consistent with the first two of these conditions, to date, no antibodies have been detected to morbilliviruses or influenza virus—the two viruses most likely to cause such an epidemic (Burek et al., 2001). Antibodies to these viruses are also absent from sea otters (Enhydra lutris) and northern fur seals (Callorhinus ursinus) that share waters around the Aleutians with Steller sea lions and are susceptible to these diseases (Hanni et al., in press; Terry Spraker, Colorado State Veterinary School, Fort Collins, personal communication, 2000). Thus, it is unlikely, but not impossible, that a viral epidemic caused the rapid decline of Steller sea lions during the 1980s. If morbillivirius did cause an undetected epidemic, however, experience from the European harbor seal epidemics suggests that it would not continue to affect population size in the absence of other factors and that the population should show signs of recovering to pre-epidemic numbers. To definitively eliminate the hypothesis that a viral epidemic caused the rapid decline of Steller sea lions would require more comprehensive tests of banked serum samples and molecular tests for the presence of disease agents in preserved tissue samples.
Endemic diseases could inhibit recovery of the Steller sea lion population. Antibodies to phocid herpesvirus, caliciviruses, and leptospires have been detected in Steller sea lions (Barlough et al., 1987; Calkins and Goodwin, 1988; Zarnke et al., 1997; Burek et al., 2001). All of these agents
cause mortality or reproductive failure in other marine mammal species, but their effects on Steller sea lions have not been documented because neither sick nor dead animals have been available for examination. In other marine mammals, these endemic diseases cause reproductive failure through abortions or poor pup survival. Higher levels of haptoglobin, a nonspecific indicator of inflammation, have been detected in Steller sea lions in declining populations (Zenteno-Savin et al., 1997). Inflammation is often a consequence of infectious disease; therefore, higher haptoglobin levels could be an indication that declining populations of sea lions have higher rates of infection. The prevalence of these disease organisms in the Steller sea lion population is unknown. Although antibodies to some disease agents have been found in sera, few studies have confirmed the presence of the disease-causing agent in tissue samples. Chlamydia spp. was observed in tissues of an aborted fetus (Spraker and Bradley, 1996), and antibodies to this organism are widespread in adult Steller sea lions, especially females (Burek et al., 2001). Further studies should be directed at determining the prevalence of infection in Steller sea lion populations and the effect of infection on reproduction and survival.
Macroparasites such as nematodes, flukes, and tapeworms are common in pinnipeds and may cause mortality in malnourished animals. California sea lions (Zalophus californianus) that suffer food deprivation or feed on unusual prey species during El Niños have heavier parasite burdens than animals in other years and can die from parasitic ulcers (Fletcher et al., 1998). These macroparasites have been found in Steller sea lions, possibly compounding the effects of malnutrition and increasing juvenile mortality. Little is known about the species of parasites or their prevalence and intensity of infection in Steller sea lions. Future studies should identify macroparasites, determine the prevalence and intensity of infection, and determine whether infection intensity correlates with nutritional status.
In conclusion, little is known about the prevalence of infectious diseases in Steller sea lions or their morbidity. Both eastern and western populations of Steller sea lions have antibodies to agents that could decrease survival and reproduction. The prevalence and intensity of infections need to be assessed to determine whether they play a role in the decline of Steller sea lion populations. Although a viral disease could have occurred in the 1980s, to date there is no direct evidence of an epidemic.
TOXINS
Biotoxins produced by harmful algal blooms have caused episodic mortality in a number of marine mammal populations around the United States, from manatees (Trichecus manatus) off Florida to California sea
lions off California (Bossart et al., 1998; Scholin et al., 2000). A bloom of saxitoxin-producing algae is believed to have caused a die-off of about 50% of the Mediterranean monk seal population, although controversy still surrounds this event due to the lack of fresh carcasses for examination (Hernández et al., 1998). Harmful algal blooms producing toxins such as domoic acid occur in Steller sea lion foraging habitats, but their effect on these animals is unknown because no carcasses have been found or examined. It is unlikely that a large mortality event occurred, however, because these toxins cause dramatic clinical signs that would have been readily detected. In addition, mortality of other species (including fish and birds) usually occurs, and these were not reported in areas of sea lion declines. Furthermore, to account for the pattern of sea lion decline, the bloom of toxic algae would have to spread from the central Gulf of Alaska to the western Aleutians but not to southeastern Alaska. It is unlikely that such an event would have gone unnoticed. However, retrospective analysis of stored tissues for biotoxins would be necessary to completely rule out the possibility that algal toxins contributed to the rapid decline of Steller sea lions.
There is an extensive literature on the effects of toxic contaminants on mammalian reproduction (reviewed by O’Hara and O’Shea, 2001). There are also data on the levels of a number of elements in marine mammal tissues (e.g., cesium, cadmium, mercury, selenium) and persistent organic compounds such as polychlorinated biphenyls (PCBs) and dichloro-diphenyl-trichloro-ethane (DDT), but few data exist on dose-response effects even for well-known contaminants in marine mammals. It is thus not possible to determine whether the levels of contaminants measured in tissue samples affect the survival of Steller sea lions. The levels of some xenobiotic compounds have been determined in a limited number of Steller sea lion tissues. PCB and DDT levels in blubber of sea lions sampled between 1976 and 1981 in the Bering Sea were lower than in sea lions from the Gulf of Alaska (Lee et al., 1996). Levels were lower in females than males, as occurs in most marine mammal species, due to the lactational transfer of lipophilic toxins. Levels of PCBs and DDTs were higher in Steller sea lions than in ringed and harp seals from Arctic waters but were comparable to levels in gray seals from the east coast of Canada and lower than in California sea lions with normal gestation periods. Both gray seals and California sea lion populations are currently increasing. It is thus unlikely that the contaminant levels in Steller sea lions are causing direct mortality in this species, although more subtle effects on physiology could occur. There may be species-specific effects, and combinations of contaminants may have more deleterious effects than single compounds. Thus, it is not possible to eliminate the possibility that contaminants affect
the physiology of Steller sea lions by measuring a few compounds in blubber at any one time during development.
Positive associations between organochlorine burdens and reduced immune function have been observed in harbor seals, but the overall effect of the health of the population is still unclear (deSwart et al., 1994). If contaminants were causing immunosuppression in Steller sea lions, an increase in prevalence and susceptibility to infectious disease should be observed in declining populations, but these epidemiological observations are lacking. Differences in contaminant burden have been inferred from fecal levels of PCBs in Steller sea lions that could result from regional differences in the prey population (Beckmen et al., 2001). Because there has been considerable military activity in the Aleutians, it is possible that certain sites have localized contamination with unidentified compounds. Estimates of vital rates of Steller sea lions in different locations may uncover differences in local mortality and reproduction that are indicative of toxic contamination. Further epidemiological studies focusing on associations between contaminant levels in tissues of individuals and life history parameters, coupled with determination of the significance of reproductive failure and infectious disease in the dynamics of Steller sea lions are needed to determine whether contaminants could play a role in limiting sea lion recovery.
WEIGHT OF EVIDENCE
Steller sea lion behavior, physiology, life history, and environment can be analyzed with regard to how they would be expected to change under each of the eight hypotheses described above using a simple positive or negative response variable (Bowen et al., 2001). In the analysis presented below, the disease category is divided into epidemics and endemics because these two processes are expected to produce different responses in sea lions. Biotoxins are included in the epidemic disease category because the response variables should change in the same direction.
As discussed in the Bowen et al. review, the directional changes represent the most likely responses based on current information but do not represent predictions. Use of this analysis should be tempered by consideration of the following:
-
For some response variables the direction of change under a given hypothesis is debatable. Change may depend on whether the effect is size selective, is local or regional, or reduces performance rather than increases mortality (e.g., disease, pollution).
-
The magnitude of change may vary with the intensity of the effect. Hence, it is possible to have a false correlation if an effect appears to be consistent but is too minor to affect population size.
-
Data used to determine the direction of each response variable may be biased due to sampling errors such as age or size-specific effects, local effects generalized to the whole population range, or time-frame dependence (what is observed in one year becomes generalized over a decade).
In analyzing the weight of evidence for each hypothesis, the committee assumes that each variable changes as the result of direct interaction. The various hypotheses listed in Table 6.1 can be characterized as either bottom-up or top-down forcing mechanisms (e.g., Hunter and Price, 1992). Bottom-up forcing hypotheses are defined by their impact on sea lion food availability and include biomass removed by fisheries, climate change, and regime shifts. Top-down forcing hypotheses are defined by their impact on survival (assuming food is not limiting) and include disease, predation, and human killing of all kinds. Pollution has impacts that could reflect either top-down or bottom-up effects. Grouping hypotheses simplifies the task of pattern assessment because the response variables are predicted to change in a consistent manner depending on whether the direction of forcing is bottom up or top down. For example, foraging time should increase when per capita food availability decreases because prey has been depleted by fishery removals or productivity has changed due to a climate regime shift. Foraging time should decrease as per capita food availability increases if predation or human takes reduce the size of the sea lion population relative to the prey base.
The committee extended this analysis by including the available observational evidence for comparison with the expected direction of change (Table 6.2). The directionality of the observed response is determined by comparison of sea lions in the western population with sea lions in the eastern population. For this purpose, characteristics of sea lions in the eastern population are assumed to be representative of the western population prior to the start of the decline. The rationales for the expected response and data sources for the observed response are described in Box 6.4
Table 6.2 lists relevant behavioral, physiological, and life history metrics of Steller sea lions, and features of the associated ecosystem for which data are available. The expected directions of change were derived from observations or ecological models as first described in the “Is It Food?” workshop (Alaska Sea Grant College Program, 1993). Eberhardt (1977) used similar reasoning to provide a general framework for assess-
TABLE 6.2. Matrix of Expected/Observed Directional Changes in the Response Variables Under Hypotheses Proposed to Explain the Decline of the Western Steller Sea Lion Stock. The response variable changes are given as either higher (H) or lower (L), for example under a bottom-up forcing, the predicted impact of fishery removals is lower (L) birth mass. However, higher (H) birth masses have been observed. Therefore the entry in the table is L/H. Matches between observed and expected (excluding unknowns) are in bold.
|
BOX 6.4 A Brief Rationale for the Directions of Population Change inTable 6.2 Birth mass—Reduced food availability is expected to limit a female’s ability to expend energy on her offspring. Bottom-up forcing mechanisms should thus lead to a reduced birth mass, while top-down forcing mechanisms, if per capita food availability is not limiting, should lead to increased birth mass. The directional effects of disease and environmental pollutants are indeterminate: if these factors increase morbidity, the outcome (fewer sea lions and less competition) would resemble a top-down mechanism, but if they reduce fitness, the outcome (less energy available during gestation), would resemble a bottom-up mechanism. Comparisons of pup birth mass in declining rookeries with birth weights in stable or increasing rookeries in southeastern Alaska during the 1990s have shown that birth masses are similar (Brandon and Davis, 1999). Pup growth rate—An argument similar to that made for expected changes in birth mass. Pup growth rates (Brandon and Davis, 1999) and masses at 1 month (Merrick et al., 1995; Rea et al., 1998) were higher in the western population. Body condition—An argument similar to that made for expected changes in birth mass, except that disease and environmental contaminants should lead to reduced body condition. Adult females in the west have been observed to have greater mass and more body fat (Davis et al, 1996; Adams, 2000; Michael Castellini, University of Alaska, Fairbanks, personal communication, 2002). Foraging trip duration—As per capita food availability declines, the time needed to obtain nutritional resources necessary for maintenance and reproduction should increase. Therefore, bottom-up forcing scenarios suggest increased foraging trip duration, while top-down forcing mechanisms, by increasing per capita food availability, suggest reduced foraging trip duration. Foraging trip length for females with pups was lower at western rookeries in the mid-1990s (Brandon and Davis, 1999; Andrews et al., 2002). Dive depth—Diving is costly in terms of time and energy expenditure and, in the case of deep dives, is the less efficient metabolic pathway associated with anaerobic diving. Thus, sea lions should dive no deeper than necessary to obtain the nutritional resources needed for maintenance and reproduction. Increased dive depths are consistent with bottom-up forcing scenarios, and reduced dive depths are consistent with top-down forcing mechanisms, both through effects on per capita food availability. Foraging range—An argument similar to that made for expected changes in foraging trip duration and dive depth. Measurements of foraging range in 1997 at a rookery in the central Aleutians and one in southeastern Alaska indicate that foraging range is lower in the west (Andrews et al., 2002). |
|
Field metabolic rate—As per capita food availability declines, the search and pursuit times required to obtain nutritional resources needed for maintenance and reproduction should increase—hence, an argument similar to that made for expected changes in foraging behavior. Andrews et al. (2002) measured metabolic rate at the same rookeries as described above and found a lower rate in the Aleutians. Beach strandings—Expected changes in the number of stranded or moribund sea lions vary less consistently across categories of forcing mechanisms and in some cases are contingent on a variety of important details. Most forms of food limitation should lead to increased numbers of weakened and emaciated animals onshore. Predation by killer whales (assuming that attacks are rarely unsuccessful and that all or most of the carcasses are consumed) produce no or minimal visual evidence. In contrast, shark predation is associated with diagnostic scars on pinnipeds in areas where shark attacks have been observed. If shooting occurs on- or near shore, elevated numbers of stranded carcasses would be expected, even though sea lions normally sink after being shot and killed. Shooting farther from shore might result in the carcasses sinking or decomposing before they drift ashore. These same arguments apply to subsistence harvest and incidental losses in fishing gear. Eventual mortality from wounds inflicted during a subsistence hunt might lead to elevated numbers of beached carcasses, whereas animals dying from entanglement in fishing gear would likely be too far from shore for this to happen. Other piscivores(surface feeding and diving piscivorous seabirds)—Since piscivorous seabirds feed on earlier life stages of many of the same prey as Steller sea lions, their populations also would be expected to decline under a bottom-up forcing scenario. Most top-down forcing scenarios, in contrast, predict no change in seabird populations. Killer whales or sharks in the northern hemisphere do not consume seabirds, at least so far as is known. A subsistence harvest for pinnipeds should have little effect on seabird populations. The effects of gear entanglement and incidental takes in fisheries is less certain due to the substantial differences in body size between seabirds and marine mammals. Nonetheless, the most likely direct impacts of significant incidental mortality on seabirds in fishing gear are reduced populations. Disease in Steller sea lions would be expected to leave seabird populations unchanged. The expected effects of environmental contaminants are less certain—direct effects on sea lions should leave seabird populations unchanged, whereas effects on the prey base might cause both to decline. Food availability—Prey abundance should decline under the bottom-up forcing scenarios and increase under the top-down scenarios. The effect of environmental contaminants on food availability is unknown. |
ing marine mammal population status. Box 6.4 provides a brief rationale for the directions of change given in Table 6.2.
SYNOPSIS
If the rationale for the expected patterns of decline (Box 6.4) is generally correct, the weight of evidence for causality of the Steller sea lion decline since 1990 is most consistent with a top-down forcing scenario. Predation and human-induced mortality provide a good fit to the available data. Currently available assay data for disease and contaminants do not indicate additional mortality from these factors. The virtual absence of beach strandings at first appears inconsistent with unreported shooting. However, it is known that most Steller sea lions sink after being shot in the water. In their study of sea lion stomach contents, Imler and Sarber (1947) shot at least 20 animals in the water and only one (<5%) floated. Likewise, in another feeding study, Fiscus and Baines (1966) killed 34 animals in the water and 23 (68%) sank before they could be recovered. It is possible that the continuing decline in the population is caused by a combination of mortalities from killer whale predation, illegal shooting, incidental takes in fishing gear, and subsistence harvest.
Evidence gathered in the 1990s is generally incompatible with a bottom-up forcing scenario. Indicators of sea lion health and foraging behavior suggest that the western population is not food limited when compared to the stable, slowly increasing population in southeastern Alaska. Indirect effects of fishing or other ecosystem changes may influence sea lion population trends (Box 6.1), but support for these potential mechanisms would require a more in-depth understanding of food web interactions in the region.
During the rapid decline of the sea lion population in the 1980s, some studies suggested a decrease in sea lion fitness consistent with a bottom-up forcing scenario. Although groundfish fishery yields were high during this period, the biomass of these species was also high. Hence there was no global reduction in the amount of groundfish available to sea lions. The possibility remains that local depletion of some fish stocks, such as Atka mackerel, may have occurred in Steller sea lion habitat, but there is less support for local depletion of pollock stocks. Changes in the abundance of forage fish species related to the regime shift in the late 1970s may have affected sea lion fitness, but these effects do not appear sufficient to account for the large mortality of sea lions in the 1980s. Although a disease epidemic could explain a rapid drop in population, to date there is no indication of such an event based on immunological analysis of serum samples. Similarly, available data do not support widespread mortality from biotoxins or contaminants. Top-down sources of mortality
also apply to the earlier phase of the decline, and in the case of human takes, the levels are known to be higher before 1990 than in the most recent decade. Shooting of sea lions was legal to protect fishing gear, and high levels of bycatch mortality were reported in the joint-venture fisheries during the 1980s. Also, it is possible that predation by killer whales was higher than previously estimated. Therefore, even during the rapid decline of the western population, it is likely that a combination of top-down and bottom-up forcing mechanisms were responsible for the high mortality of Steller sea lions.








































