3
Battery Technologies for Military Hybrid Vehicle Applications
INTRODUCTION
Chemical batteries have been used as electric energy storage devices for many years. With the revival of interest in electric transportation, great effort and investment have been put into the research and development of high-performance chemical batteries. Until recently, however, the battery performance has been far from meeting the requirements of the vehicle application. One of the major problems is the very limited amount of energy stored per unit weight (specific energy). Compared with conventional petroleum and internal-combustion-engine-based systems, far less energy is stored in a practical onboard battery, resulting in limited operation time. This chapter examines the current state-of-the-art of batteries for vehicle propulsion, and promising research areas that could lead to improved performance.
ENERGY DENSITY OF CHEMICAL BATTERIES
The theoretical specific energy density of selected existing batteries is shown in Table 3-1.
TABLE 3-1 Theoretical Specific Energy of Typical Existing Batteries
|
Reaction |
Voltage (V) |
Specific Energy (Wh/kg) |
|
Lead acid cell: |
|
|
|
PbO2 +2H2SO4 +Pb ↔ 2PbSO4 + 2H2O |
2.04 |
170 |
|
Edison (Ni-Fe) cell: |
|
|
|
Fe + 2NiOOH +2H2O ↔ Fe(OH)2 +2Ni(OH)2 |
1.25 |
260 |
|
Ni-Zn cell: |
|
|
|
Zn + 2NiOOH +2H2O ↔ Zn(OH)2 +2Ni(OH)2 |
1.9 |
360 |
|
Zn-chlorine cell: |
|
|
|
Zn + (Cl2+8H2O) ↔ ZnCl +8H2O |
2.1 |
410 |
|
Al-S cell: |
|
|
|
2Al +3S +3OH- ↔ 2AL(OH)3 +3 HS- |
1.3 |
910 |
|
Organic lithium: |
|
|
|
Li(y+x)C6 + Li(1-(y+x))CoC ↔ LiyC6 + Li(1-y)CoO2 |
|
320* |
|
*For maximum value of x = 0.5 and y = 0. |
||
However, practical batteries have specific energies that are much lower than their theoretical values. This is due to the need for a container, electrode support, connectors, diluted electrolyte, unreacted materials and so on. Table 3-2 shows the data for some existing batteries, and compares them with the mid-term and long-term goals of the U.S. Advanced Battery Consortium (USABC). Lithium-ion batteries have the highest current values of specific energy. These may be designed in a high-power (HP) or high-energy (HE) configuration, depending on the requirements of the load.
TABLE 3-2 Expected Practical Energy Density
|
|
Specific Energy (Wh/kg) |
|||||
|
|
Battery |
Theoretical |
Current |
Ratio |
Projected |
Ratio |
|
Existing |
Lead acid |
170 |
40 |
4.25 |
50 |
3.40 |
|
|
Adision Ni-Fe |
260 |
50 |
5.20 |
60 |
4.33 |
|
|
Ni-Zn |
260 |
50 |
5.20 |
60 |
4.33 |
|
|
Zn-Cl |
260 |
50 |
5.20 |
60 |
4.33 |
|
|
Li-ion high power |
|
85-95 |
|
||
|
|
Li-ion high energy |
|
135-150 |
|
||
|
USABC |
Mid-term |
|
80 |
|
||
|
Goal |
Long-term |
|
200 |
|
||
|
Al-based |
Al-Fe-O |
2,278 |
|
455 |
5.0 |
|
|
|
Al-Cu-O |
2,198 |
|
440 |
5.0 |
|
|
|
Al-Fe-OH |
1,903 |
|
380 |
5.0 |
|
SPECIFIC POWER CHARACTERISTICS OF CHEMICAL BATTERIES
Specific power is the maximum power per unit battery weight that the battery can deliver in a short period. Theoretically, there is no top limit for specific power. It depends mostly on the manufacturing and material processing technologies. Specific power is also important in the reduction of battery weight, especially for the high power demand applications, such as hybrid electric vehicles. The maximum power that the battery can deliver to the load is limited by the conductor resistance and the internal resistance caused by the chemical reaction. Accurate determination of battery resistance by analysis is difficult. Specific power is usually obtained by measurement.
Table 3-3 shows the status of battery systems potentially available for hybrid vehicles. Although it can be seen that specific energies are higher in advanced batteries, until recently the specific powers showed no such improvement over mature lead acid technology. Li-ion high-power and high-energy batteries with about 4000 W/kg and 600 W/kg, respectively, have been reported.1 If these results are proven in vehicle tests, they would represent a significant step forward. Work continues on the development of batteries with very high power capabilities of at
least 10 to 15 kW/kg, and tests indicate that power levels in excess of 15 kW/kg are possible. New processing techniques are expected to deliver in excess of 30 kW/kg. This will require significant advancement in processing capabilities.2 The Department of Defense (DoD) has supported and continues to support the development of higher power systems required for future needs—for example, for directed energy systems such as lasers and high-power microwaves. At the same time, the DoD requires lower power density and higher energy density batteries to satisfy the silent watch requirements and stealth operation capabilities.
TABLE 3-3 Status of Battery Systems for Hybrid Vehicles
|
System |
Specific energy (Wh/kg) |
Specific power (W/kg) |
Energy efficiency (%) |
Cycle life |
Cost (US$/kWh) |
|
Lead acid |
35-50 |
150-400 |
>80 |
500-1000 |
120-150 |
|
Nickel/cadmium |
40-60 |
80-150 |
75 |
800 |
250-350 |
|
Nickel iron |
50-60 |
80-150 |
65 |
1500-2000 |
200-400 |
|
Nickel zinc |
55-75 |
170-260 |
70 |
300 |
100-300 |
|
Nickel/metal/hydride |
70-95 |
200-300 |
70 |
750-1200+ |
200-350 |
|
Sodium/sulfur |
150-240 |
230 |
85 |
800+ |
250-450 |
|
Lithium/ion/sulfur |
100-130 |
159-250 |
80 |
1000+ |
110 |
|
Lithium-ion |
80-130 |
200-300 |
>95 |
5000+ |
200 |
|
Li-ion high power |
85-95 |
~4000 |
>95 |
— |
— |
|
Li-ion high energy |
167 |
~600 |
>98 |
— |
— |
TYPICAL MOBILITY REQUIREMENTS OF MILITARY VEHICLES FOR BATTERIES
Figure 3-1 shows the typical tractive effort and speed of military vehicles under various operational conditions. In order to evaluate the requirements to batteries, these three typical operations are selected: (1) high-speed highway operation, (2) hill-climbing operation, and (3) hard acceleration. The first operation represents the energy demand for continuous operation, and the last operation represents the power demand for intermittent operation. It is assumed that the maximum acceptable ratio of battery weight to total vehicle weight is 0.25. Table 3-4 shows the data of these three operations.
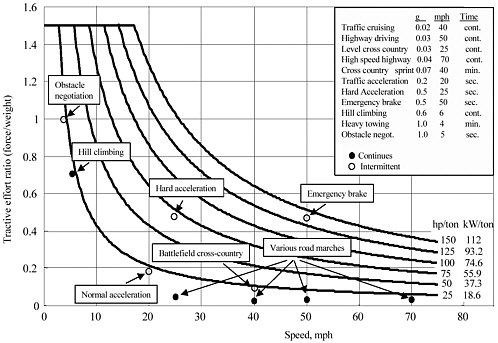
FIGURE 3-1 Power requirement of typical mobility of military vehicles.
Table 3-4. Typical Operations of Military Vehicles
|
Operation |
Speed (mph) |
Power (kW/ton) Traction |
Battery |
Time |
|
High-speed highway |
70 |
12 |
16 |
Continous |
|
Hill-climbing |
6 |
16 |
21 |
Continous |
|
Hard acceleration |
25 |
54 |
72 |
Seconds |
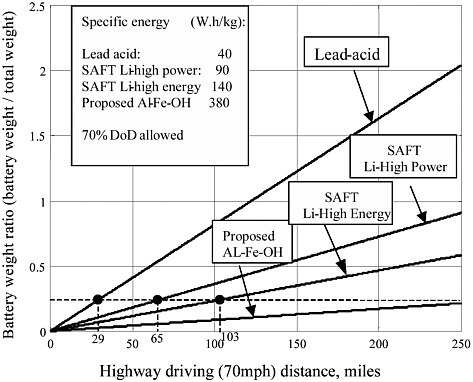
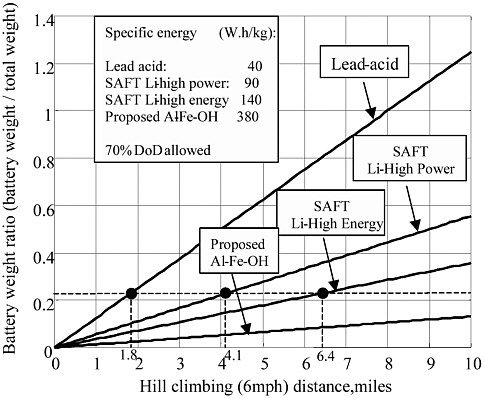
When the vehicle demands hard acceleration, sufficient battery power must be supplied. Referring to Table 3-4 and Figures 3-2 and 3-3, this power demand is about 72 kW per ton of vehicle weight. In Figures 3-2 and 3-3, the total weight is defined as the total vehicle weight minus the battery weight. The calculated ratios of battery weight to total vehicle weight for typical batteries are listed in Table 3-5. It is clear that SAFT Li-ion high power and high energy batteries can meet the power demand. Even lead acid batteries may be able to supply sufficient power for hard acceleration.
The driving range performance of the Li-ion batteries is somewhat less satisfactory, however. The maximum driving range on the highway at 70 mph is about 105 miles for the SAFT HE battery and 63 miles for the SAFT HP battery, while the maximum hill-climbing range for the SAFT HE and HP batteries is 6.4 and 4.1 miles, respectively.
TABLE 3-5 Ratio of Battery Weight to Total Vehicle Weight
|
Battery |
Specific Power (W/kg) |
Battery Weight/ Vehicle Weight |
|
Lead acid |
300 |
0.24 |
|
SAFT Li-high power |
3,000 |
0.024 |
|
SAFT Li-high energy |
500 |
0.144 |
|
Proposed Al-Fe-OH |
— |
— |
Another battery requirement for military applications is that the fully sealed and water-cooled battery packs can be submerged in 10 feet of water. These batteries should also be intelligently managed with module management and data collection systems. These systems are now being researched by companies in the United States and elsewhere.
BATTERY PERFORMANCE IMPROVEMENT TECHNIQUES
The intrinsic properties of the active electrode materials and electrolyte used determine the cell potential, capacity, and energy density, each of which has a theoretical top limitation. However, battery power capability has no theoretical top limitation. It heavily depends on manufacturing technology to reduce the battery internal resistance, which causes voltage drop on the battery terminals and consequently limits the battery power. The battery voltage drop is generally caused by reaction activity and electrolyte concentration.
The voltage drop caused by the reaction activity may be reduced by two approaches. One is to develop advanced electrode materials and electrolyte that have high chemical reaction activity on the reaction surface. The other approach is to employ electrodes with large surface areas. This will decrease the current density for a given load current and consequently reduce the voltage drop. In addition to simply increasing the geometric size, the electrode area can be dramatically increased by using active materials with high intrinsic surface area, for example, porous matrices.
The voltage drop caused by the electrolyte concentration (sometimes called a mass-transfer overpotential) can be reduced by using high concentrations of reactant species and technologies to reduce the ion transfer resistance. In addition to the voltage drop caused by the chemical reaction, there is also a voltage drop due to the electric ohmic resistance in the connectors and terminals. This can be addressed through the application of low-resistance materials.
The new technologies for improving battery power capability may also include use of nanomaterials, engineered interfaces and surfaces in materials, advanced energy storage and conversion materials, and advanced materials processing and systems manufacturing techniques.
SUMMARY
SAFT high power and high energy Li-ion batteries may be able to meet the power demands of military hybrid vehicles, though their ability to satisfy requirements for vehicle driving range on the highway or up grades appears less certain. Theoretical analysis indicates that hypothetical aluminum-based batteries potentially have high energy density, which is over two times that of the long-term goal of USABC. However, their specific powers are uncertain. Technical challenges and opportunities for improvement in battery performance are summarized in Table 3-6.
TABLE 3-6 Technical Challenges, Performance Metrics, and Research Priorities Associated with the Application of Batteries to Combat Hybrid Power Systems
|
System/Component |
Technical Challenge |
Performance Metric |
R&D Priorities |
|
Advanced battery concepts |
Validation of batteries in vehicle applications |
Specific power Specific energy |
Triple the power and energy with nanomaterials technology and new chemistries |
|
|
Safety |
|
Increased safety (eliminate flammable materials; better packing for isolation, containment, venting; thermally stable materials; diagnostics/ prognostics integrated in pack; eliminate ground fault and arcing; improved materials that reduce gassing) |
|
|
Battery management (state of health, state of charge, power availability, life prediction, temperature management, diagnostics, and prognostics) |
|
|
|
Electrode/electrolyte interface |
Voltage drop caused by limited chemical reactivity at the interface |
|
Advanced electrode/electrolyte materials with high surface reactivity |
|
|
Increased electrode surface area by increased matrix porosity or perhaps application of nanomaterials |
||
|
Electrolyte |
Voltage drop caused by mass transfer overpotential |
|
Electrolytes with high concentrations of reactant species and low ion transfer resistance |
|
Connectors and terminals |
Ohmic resistance of materials |
Minimized resistance |
Low-resistance materials |
BIBLIOGRAPHY
Crompton, T.R. 1996. Battery Reference Book, 2nd ed. Warrenton, PA: SAE International.
Kajs, John. 2001. "Combat Vehicle Mobility Requirements," seminar at Texas A&M University, Dec
Messerle, Hugo K. 1969. Energy Conversion Statics. New York: Academic Press.
Rand, D.A.J., R. Woods, and R.M. Dell. 1998. Batteries for Electric Vehicles. Somerset, England: Research Studies Press.
Severinsky, Alex J. 1994. Theoretical Limits to Application of Batteries for Automobile Propulsion (System Study). National Challenges for the Commercialization of Clean Fuel Vehicles: Conference Proceedings. Online. Available at http://www.adlabs.com/library/battery.html. Accessed December 2002.