A National Perspective on the Immunization System
At the Los Angeles workshop, opening presentations from David Smith, president of Texas Tech University Health Sciences Center and chair of the Institute of Medicine (IOM) committee organizing the workshop, and Walter Orenstein, director of the National Immunization Program at the Centers for Disease Control and Prevention (CDC), provided an overview of immunization issues facing the country, the findings and recommendations on immunization finance from the IOM study, and CDC’s response to those recommendations.2
THE NATIONAL IMMUNIZATION PICTURE
Immunization Coverage
Over the course of the 20th century, the introduction of new vaccines and efforts to ensure that children are appropriately immunized produced substantial reductions in illness from many serious diseases (see Table 1). Dr. Orenstein reported that it even appears that measles may no longer be endemic in the United States. Nevertheless, many children and
|
2 |
An audiocast of each presentation and the speaker’s visual aids are available in electronic form at the workshop website: www.iom.edu/iom/iomhome.nsf/pages/hcs+immunization+finance+dissemination. |
TABLE 1 Change in Annual Morbidity from Vaccine-Preventable Diseases: Prevaccine Baseline and 2001
|
Disease |
Prevaccine Baseline Date |
Average Annual Baseline Cases |
2001 Cases |
% Decrease |
|
Diphtheria |
1920–1922 |
175,885 |
2 |
100.00 |
|
Haemophilus influenzae, type b and unknown (< 5 years) |
1985 |
20,000 |
183 |
99.20 |
|
Measles |
1958–1962 |
503,282 |
108* |
100.00 |
|
Mumps |
1968 |
152,209 |
231 |
99.80 |
|
Pertussis |
1922–1925 |
147,271 |
5,396 |
94.70 |
|
Poliomyelitis |
1951–1954 |
16,316 |
0 |
100.00 |
|
Rubella |
1966–1968 |
47,745 |
19 |
99.60 |
|
Tetanus |
1922–1926 |
1,314 |
27 |
97.30 |
|
*Provisional data SOURCE: Adapted from CDC (2002a). |
||||
adults have not received recommended vaccines, and disease outbreaks remain a threat if immunization coverage is below optimal levels. Dr. Smith pointed to Texas, where a pertussis outbreak has resulted in seven deaths.
Despite improvements in immunization coverage during the 1990s, national immunization rates for 2-year-olds and for adults aged 65 years and older have not yet reached the current public health objective of 90 percent coverage. For 2000, the National Immunization Survey (NIS) found that 76 percent of children ages 19 to 35 months had received all the vaccine doses necessary to complete the 4:3:1:3 immunization series3 (see www.cdc.gov/nip/coverage/NIS/00-01/toc-00-01.htm). Although coverage rates for many individual vaccines approach and even exceed 90 percent, the various missed doses lower the rates for a complete immunization series. The decline in the rate for the 4:3:1:3 series from 79 percent in 1998 is a source of concern, as are the persistent disparities in rates across states (see Figure 1).
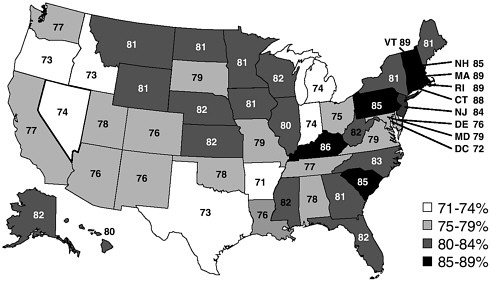
FIGURE 1 Estimated vaccination coverage with the 4:3:1:3 series, by coverage level and state. Source: Calling the Shots. Institute of Medicine (2000).
Among older adults (ages 65 and over) living in the community (i.e., not in nursing homes or other institutional settings), 67 percent reported in 1999 that they had received an influenza vaccination in the previous year, and 54 percent had ever received a pneumococcal vaccination (CDC, 2001). Immunization rates for high-risk adults ages 18 to 64 are particularly low, with data for 2000 showing that only 32 percent of this group had received an influenza vaccination in the previous year (CDC, 2002b). Low influenza immunization rates among “high- risk” children and adults younger than age 65—those with asthma or other chronic health conditions—are of particular concern.
Immunization levels are also persistently lower among low-income children and adults. Although the annual NIS and other state-based immunization surveys provide estimates of general population immunization coverage levels, they frequently rely on telephone household surveys that do not include estimates for pockets of high-risk, underimmunized populations represented by groups such as public housing districts or neighborhoods characterized by deep poverty (U.S. General Accounting Office, 1996). For example, one survey of coverage rates in 1997 for inner-city, African-American children in Los Angeles found that only half (50.6 percent) of the control group (which did not participate in a case management intervention) were up to date at 1 year of age, a marked contrast with the NIS estimates of 83 percent for the nation and 86 percent for Los Angeles County (Wood et al., 1998). Similarly, a 1994 Chicago
area study found coverage rates for the 4:3:1 series of 29 percent among African-American children living in public housing in contrast to 47 percent citywide coverage rates (Kenyon et al., 1998). Nationally, the disparity in immunization rates between children in households with incomes below the poverty level and children in higher income households is about 9 percentage points.
Federal Financing for Immunization Program Activities and Vaccine Purchase
Federal financial support for the immunization system is provided principally through the Section 317 program and Vaccines for Children (VFC). Under the Section 317 program, each state and territory and five large urban areas receive annual grants for the purchase of vaccine and for the operation of immunization program activities (infrastructure funding). The Section 317 awards are the major source of federal support for essential immunization system activities such as surveillance of vaccine coverage and efforts to improve coverage. Dr. Orenstein reported that about $182 million is available for program operation grants for 2002.
VFC, a federal entitlement program, funds the purchase of vaccine for participating health care providers to administer to eligible children. Roughly $795.5 million is available for VFC for 2002 (see www.cdc.gov/fmo/fmofybudget.htm). CDC estimates that more than 40,000 private providers are participating in VFC. In addition, federal contracts with vaccine manufacturers make it possible for states to use either federal or state funds to obtain additional vaccines at discounted prices. More than half of all vaccine is purchased under these federal contracts, with 36 percent purchased through VFC and 15 percent purchased through Section 317 (IOM, 2002b).
CHALLENGES FOR THE IMMUNIZATION SYSTEM
Dr. Smith and Dr. Orenstein noted that the nation’s immunization system is facing challenges that could undermine past achievements and hinder the effort still needed to reach targeted levels of immunization coverage. Some of these demands include sustaining current rates of coverage with the addition of new and more expensive vaccines to the immunization schedule, responding to concerns about the safety of vaccines, serving an increased number of people as a result of recommendations for expanded adolescent and adult vaccination, and adapting to changes in the health care delivery system that can affect the availability and affordability of vaccines in the private sector.
The continuing addition of vaccines and vaccine doses to the recom-
mended childhood immunization schedule is increasing the complexity and the cost of meeting immunization requirements. Between 1975 and 2000, 25 changes were made to the pediatric immunization schedule, and more changes and additions are anticipated. For example, a recommendation for influenza vaccination for all children is being considered. With children now needing up to 20 vaccine doses by age 2, the demand for immunization services is placing a growing burden on the health care delivery system. Combination vaccine products help reduce the number of separate injections required, but the use of products with different combinations of vaccines can make it more difficult to manage the immunization requirements of children who receive immunizations from multiple providers.
The high cost of newer vaccines, reflecting in part the increased costs of production and distribution, is having a marked impact on federal, state, and local budgets for vaccine purchase. The cost of these vaccines is also creating financial burdens for families, private providers, and insurers. The pneumococcal conjugate vaccine, added to the pediatric immunization schedule in 2000, costs roughly $46 per dose under the CDC purchase contract and nearly $59 per dose at regular prices (www.cdc.gov/nip/vfc/cdc_vac_price_list.htm). By comparison, comparable perdose costs for the polio vaccine are approximately $8 and $15, respectively.
A problem that has emerged in the past couple of years is persistent shortages of vaccines. Dr. Orenstein reported that vaccines against 8 of the 11 vaccine-preventable diseases among children were in short supply at the time of the workshop, a situation he described as unprecedented. In addition, supplies of the influenza vaccine have been limited or delayed in the past 2 years. The shortages appear to reflect a mix of vaccine production problems and a decline in production capacity resulting from the decisions of some manufacturers to stop making certain vaccines. CDC is advising states and health care provider organizations on modifications to the immunization schedule to accommodate the shortages, but it appears that many providers are not keeping up with the changing recommendations. The vaccine shortages and reduced number of vaccine producers are contributing to upward pressures on vaccine prices. Dr. Orenstein noted that the National Vaccine Advisory Committee would be examining issues of vaccine supply at a meeting scheduled for February 2002.
Vaccine safety is another concern. CDC routinely monitors reports of adverse events following immunization for signals of unacceptable health problems. In response to a question about the tensions between CDC’s roles in promoting immunization and in monitoring vaccine safety, Dr. Orenstein pointed out that a separate agency, the Food and Drug Admin-
istration, is responsible for licensing or recalling vaccines. He also noted that responses to vaccine safety concerns over the past few years have included modification of some vaccines by manufacturers and removal of the rotavirus vaccine from the immunization schedule. In addition, CDC supports vaccine safety studies, including the work of an IOM committee that is reviewing several safety topics over a 3-year period and the Vaccine Safety Datalink project.
To respond to other needs, CDC is also focusing attention on adult immunization, on the improvement of the surveillance and measurement tools used to monitor immunization, and on the implementation of evidence-based activities aimed at improving immunization rates. In response to questions from workshop participants about immunization registries, Dr. Orenstein noted that CDC is involved in their development through its funding to states as well as through participation in efforts to develop technical standards. In collaboration with the National Vaccine Advisory Committee, CDC has developed guidance on ways to address the confidentiality and privacy concerns associated with registries. CDC is also working with the American Academy of Pediatrics on how to improve participation in immunization registries by private physicians.
IOM CONCLUSIONS AND RECOMMENDATIONS
Dr. Smith reviewed the key conclusions and recommendations from Calling the Shots. The report calls for a renewal and strengthening of the federal–state partnership that is a fundamental element of the national immunization system. The report also recommends strategic investments in immunization efforts and closer collaboration between public and private health care systems to coordinate immunization roles and responsibilities in the wake of health care reforms.
The IOM study committee identified six fundamental roles for the nation’s immunization system:
-
Control and prevent infectious disease.
-
Assure the purchase of recommended vaccines for the total population of U.S. children and adults, with particular emphasis on the protection of vulnerable groups.
-
Assure access to such vaccines within the public sector when private health care services are not adequate to meet local needs.
-
Sustain and improve immunization coverage levels within child and adult populations, especially in vulnerable communities.
-
Conduct populationwide surveillance of immunization coverage levels, including the identification of significant disparities, gaps, and vaccine safety concerns.
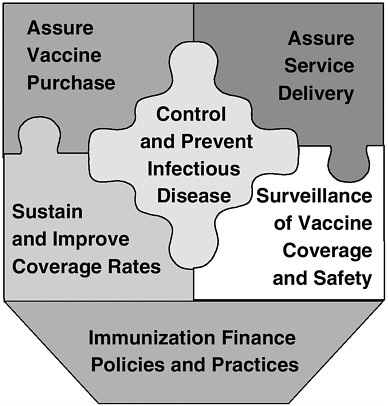
FIGURE 2 Six roles of the national immunization system.
-
Use primary care and public health resources efficiently in achieving national immunization goals.
The committee used this framework to guide its finance recommendations. The report concluded that adequate, stable, and predictable funding was necessary for the development of effective state immunization programs and that the fluctuations in Section 317 infrastructure funding during the 1990s made it difficult for states to achieve program goals. Furthermore, with only a 1-year grant period, many state immunization programs could not invest in multiyear programs to support long-term strategic planning or data collection efforts.
The committee also concluded that immunization policy should be national in scope, but flexible enough to accommodate important political, socioeconomic, and structural differences among states and communities. Furthermore, federal and state governments share responsibility for supporting vaccine purchase and the infrastructure essential for achieving and sustaining national immunization goals. Data reviewed
for the study showed that some states appeared to provide little or no state funding for immunization while others invested substantially more than they received from federal sources. Finally, the private sector, through health plans and individual health care providers, has the capacity to do more to ensure the delivery of appropriate immunization services to their members and patients, but such efforts do not replace the need for a public health infrastructure capable of assuring that the immunization needs of the whole population are addressed.
The study recommendations addressed federal and state funding levels, grant mechanisms for immunization programs, and the need for better measurement of immunization coverage. The committee concluded that annual budgets for the purchase of vaccine for children have been adequate in the past; however, this finding was made prior to the addition of the pneumococcal conjugate vaccine to the recommended schedule for children. The committee recommended increases in both federal and state budgets to provide for the purchase of additional vaccine for those high-risk adolescents and adults under age 65 who do not qualify for other federal assistance. The committee also recommended increases in financial and administrative support from federal and state governments for immunization infrastructure programs.
In addition to budgetary increases, Dr. Smith noted that the IOM report proposed new operational and reporting requirements for the federal grants linked to the six fundamental roles of the national immunization system. The committee recommended that CDC should use a formula grant mechanism to distribute Section 317 awards to states. The formula should reflect essential minimum funding levels and state need, capacity, and performance. In addition, a requirement for a state funding match should be added, and the federal grants should have a 2-year budget cycle to give states greater flexibility to plan and implement multiyear efforts. Finally, the IOM report recommended that federal and state agencies develop a set of consistent and comparable measures for use in monitoring the immunization status of children and adults enrolled in private and public health plans as well as the immunization status of populations in defined geographic areas.
CDC RESPONSES TO IOM RECOMMENDATIONS
Dr. Orenstein outlined CDC’s responses to the IOM report and recommendations. He first emphasized the value of articulating the six broad roles of the national immunization system. This framework helps explain the importance of a range of activities that includes not only vaccine purchase and direct delivery of immunization services, but also surveillance and coverage assessments.
IOM recommended that CDC provide Congress with estimates of the cost of vaccines added to the childhood immunization schedule so that those estimates can be factored into the Section 317 budget. Dr. Orenstein commented that although Congress has not requested such estimates, CDC is seeking better information about factors affecting vaccine pricing and the demand for publicly purchased vaccine. A new IOM study will examine vaccine financing strategies that reflect the roles and responsibilities of the public and private sectors in the purchase of vaccine and associated administrative costs. The study will also consider the effect of vaccine pricing on incentives for further vaccine research and production and will assess prospects for future vaccine prices.
Little progress has been made at the federal level in increasing funding for the purchase of vaccine for adolescents and adults. CDC is, however, trying to address this issue in other ways such as urging public and private health plans to include or improve coverage for adolescent and adult immunizations. CDC is also encouraging states to enroll health care providers who treat adolescents in VFC so that they can obtain free VFC vaccine for eligible patients. The enrollment of more adolescents in State Child Health Insurance Programs is also seen as an opportunity to improve access to lower cost immunization services for this population. In addition, standards for adult immunization practices, comparable to the standards developed in the 1990s for young children, were recently issued, and work is beginning on standards for immunization of adolescents. The Task Force for Community Preventive Services will be developing evidence-based guidelines for immunization interventions aimed at certain target populations.
Dr. Orenstein reported welcome increases in funding for Section 317 immunization infrastructure grants in 2001 and 2002. Even so, the funding level remains about $18 million to $19 million short of the $200 million that IOM recommended. CDC is exploring whether to extend the grant period to 2 years, but Congress reauthorized the Section 317 program before such a proposal could be submitted. CDC is working with grantees to reduce the reporting burden associated with these grants and to improve the documentation of state financial and in-kind contributions to immunization activities.
The formula grant mechanism that IOM recommended for the distribution of Section 317 infrastructure funds offers the advantages of greater equity and transparency in funding decisions, but it poses a challenge in that some states will end up receiving less funding. The recent budget increases will help ease the impact of the reallocation of funding, and CDC has agreed that a state’s funding will decrease by no more than 5 percent per year. CDC is working with the states, through the Association of State and Territorial Health Officials and the Association of Immu-
nization Managers, to develop criteria and weights for a funding formula. The proposal currently under consideration gives a weight of 12 percent to a funding base to cover minimum program needs, 78 percent to need as reflected in factors such as population size and immunization coverage rates, 5 percent to performance, and 5 percent to discretionary funding needs (e.g., responding to disease outbreaks).
CDC’s other major effort in response to the IOM recommendations is to improve the measurement of immunization coverage. Toward this end, CDC is working with the National Committee for Quality Assurance to modify immunization measures in the Health Plan Employer Data and Information Set (HEDIS) to better reflect immunization recommendations. New vaccines are to be incorporated into HEDIS measures within 3 years of their addition to the immunization schedule. A new HEDIS measure will reflect the recommendation for influenza immunization for adults ages 50 to 64. In addition, changes to the NIS will allow for greater comparability with HEDIS data and for the measurement of immunization coverage at more ages.
Dr. Orenstein ended his remarks by noting the conclusions in Calling the Shots regarding the federal and state roles in the immunization system. With states as the ultimate stewards of public health, state legislatures and state governments should be expected to sustain an immunization infrastructure. The federal role is to supplement and support state efforts. Thus, Dr. Orenstein hoped that the workshop would aid CDC in finding ways to help states fulfill their roles in the immunization system.










