4
Control Principles and Programs
CONTROL PRINCIPLES
Although there are large gaps in the understanding of Mycobacterium avium subsp. paratuberculosis (Map) transmission, enough detail is known that the essential control program components for dairy herds were proposed almost a half-century ago (Organisation for European Economic Co-operation, 1956) and reiterated more recently (Moyle, 1975). They differ surprisingly little from current proposals for the control of Johne’s disease (JD) (Collins, 1994; Rossiter and Burhans, 1996).
For dairy herds, the recommendations include:
-
Taking precautions against introducing the disease through purchased animals
-
Isolating and slaughtering clinically infected animals
-
Culling recent offspring of clinical cases as soon as possible
-
Removing calves from dams immediately upon birth (before suckling)
-
Isolating calves in separate calf-rearing area
-
Harvesting colostrum from cows with cleaned and sanitized udders
-
Feeding colostrum to calves by bucket, and thereafter feeding only milk replacer or pasteurized milk
-
Preventing contamination of calf feedstuffs, water, or bedding by effluent from the adult herd
-
Applying manure from the adult herd only to cropland or to pastures grazed by adult stock
Few empirical studies of those control program components have been done, and their justification is based on biologic plausibility, limited observation, and anecdotal evidence.
Implementation of herd or flock level control programs, establishment of test-negative or low-risk herds and flocks, and reduction of environment and food contamination with Map are attainable current goals. The possibility of eradication of JD in the United States should be evaluated after significant progress in control programs is attained.
Environmental Factors in Infection Control Practices
In most, if not all, affected species, Map is believed to be transmitted primarily in a fecal-oral cycle shed in the feces of infected animals and then ingested by susceptible animals. Such a cycle is also the primary means by which most other communicable enteric infectious agents are transmitted. For Map transmission by indirect contact, factors include the number of organisms shed in the feces and the organism’s survival characteristics in the environment. The relationship between Map and the environment is complex, involving factors such as the physical characteristics of the substrate material (feces, water, milk, manure slurry, dust, environmental surface, dirt), temperature, pH, water activity or content, and competing microorganisms. The relationships are not well defined for the many combinations encountered in the farm environment, but decisions must still be made for control programs. More importantly, current information relates to the duration of environmental survival of a large inoculation of laboratory-origin Map, which could respond differently from Map originating directly from an infected host (Mitscherlich and Marth, 1984). More information on the environmental survival characteristics of Map is needed to determine how long the organism remains infectious once the area or material (water, grass, forage, or other feedstuff) becomes contaminated, leading to an estimate of dose and response over time. All of this is critical information for determining how to manage livestock flow through housing facilities or paddocks and for how to otherwise minimize disease transmission. Some recommendations do not provide risk estimates of cost relative to benefit. For example, there is a recommendation that livestock producers keep young animals from grazing pasture that has been fertilized with Map-contaminated manure for at least one year after birth. But because producers could regard that practice as infeasible and the risk relatively insignificant, they choose to ignore it. In fact, these recommendations may well
conflict with recommendations being made or required of the operation for other reasons, such as comprehensive nutrient management plans.
There is not enough information available about the environmental survival of Map to support adequate risk assessments of many farm practices. Map survival information also is needed to address emerging off-farm issues, such as concerns about the persistence of Map in waterways that receive farm effluent or the survival of Map in other farm products (such as forages) potentially contaminated with Map through normal irrigation or manure spreading.
Producer Awareness and Adoption of Biosecurity Practices and Control Measures
Infectious disease biosecurity (preventing the introduction of disease to a farm) has two major components: The first is to reduce the likelihood of introduction of an infectious agent into a group (external biosecurity). The second is to reduce the likelihood of transmission once a disease is present (internal biosecurity or biocontainment). Unfortunately, many dairy producers have not adopted long-advocated practices for either, and many are largely unaware of JD and its associated preventive measures (National Animal Health Monitoring System [NAHMS], 1996b, 1997a). According to NAHMS, an astounding 10 percent of dairy producers admitted never having heard of JD, 35 percent knew of it by name only, 37 percent knew some basics, and only 18 percent considered themselves knowledgeable about the disease. Many of those operations were at significant risk: 64 percent had milk cows born off the operation, 44 percent had purchased cattle during the previous year, and only 9 percent of those who had purchased cattle during the previous year normally required testing of additions to the herd. The most important prepurchase information—the infection status of the herd of origin—was probably unidentified in most of the cases, although the survey did not address this issue (NAHMS, 1996b, 1997a).
In addition to low awareness, there has been poor adoption of general biosecurity practices by producers. Of the dairy operations in the NAHMS (1996b) survey that brought mature cattle onto the operation, 38 percent had required no previous vaccinations, and fewer than half required vaccinations for bovine virus diarrhea (BVD), infectious bovine rhinotracheitis (IBR), or leptospirosis. Sixty-six percent of the producers required no testing of purchased cattle for JD. Only 26 percent of dairy producers who purchased cows required an individual-cow somatic-cell count for mastitis, a disease Wells and colleagues (1998) ranked of highest concern because it causes the largest direct losses to the producer. Purchasing such animals is most likely the way many infectious agents are introduced into a herd. MacNaughton (2001) stated that “the trigger that pushes many herds from elevated-level test results to penalty-level test results is the introduction of new animals into the milking herd without taking biosecurity precautions.”
Low awareness and poor adoption of biosecurity practices are not unique to the dairy industry. A NAHMS study (1997b) of 2713 randomly selected producers of U.S. beef cow-calves reported that understanding of bovine JD was even lower among that group than it was among dairy producers: seventy percent had never heard of the disease, 22 percent recognized its name only, 5 percent knew some basics, and 2 percent classified themselves as “fairly knowledgeable” (Dargatz et al., 1999). The frequency of possible JD exposure via purchased animals in the beef cow-calf herds surveyed was similar to that in the dairy herd survey (NAHMS, 1996a), with 39 percent of operations purchasing cattle during the survey year and 22 percent of the calved-cow inventory consisting of purchased animals.
As with external biosecurity practices, producers are slow to adopt well-established internal biosecurity measures. The NAHMS study (1996b, 1997b) of 1219 randomly selected dairy herds across the United States and reported that 55 percent used a common hospital and calving area, 47 percent left newborn calves with their dams more than six hours, 32 percent did not clean udders before harvesting colostrum, 23 percent used the same equipment to handle both young stock feed and herd manure, and 12 percent allowed contact between calves younger than six months and adult cows. This lack of prevention is not unique to U.S. dairy producers. A recent study of 534 Australian dairy producers (Wraight et al., 2000) reported that 48 percent had adopted none of six long-recommended control measures. This failure is even more surprising in light of the facts that these producers ranked JD second only to scours as the calfhood infection of greatest concern in Australia, and the proportion adopting no control measures was not significantly different between herds with and without Map infection recognized on their premises.
Failure to adopt internal biosecurity measures by dairy producers is not limited to Map control. Mastitis control practices are also not adopted by a significant proportion of producers, which is even more surprising given that mastitis is more prevalent and it causes the largest farm losses attributable to disease (Wells et al., 1998). The efficacy of these control program components has been reported in an extensive literature from experimental studies and field trials beginning in the mid-1960s (Neave et al., 1969).
There are likely any number of reasons for low producer awareness of JD and poor adoption of biosecurity practices, but one factor could be a general failure on the part of livestock veterinarians to educate their clients. Dairy producers reported that the most important source of health information was a veterinarian—at 78 percent, far outranking any other source (trade journals, university extension services, other professionals). A lack of contact with veterinarians also does not appear to be the problem: veterinarians made between 13 and 24 visits to the surveyed farms during the year prior to the survey (NAHMS, 1996b, 1997a). Beef producers also ranked veterinarians as their most important source of health information, outranking any other source almost threefold (NAHMS, 1996a).
Education to increase producer awareness is an essential component of JD control, but it will be insufficient by itself to generate widespread
compliance. Additional incentives could be required. In a Canadian mastitis program initiated in 1989 based on bulk-tank somatic-cell counts in milk, penalties for violating standards and premiums for exceeding standards resulted in adoption of control practices that reduced somatic cell counts. Premiums appeared to generate the greatest response: premiums of approximately one-tenth the magnitude of the penalties resulted in similar compliance (Geyer, 1990). This response to market price signals is interesting in light of the fact that information on the production losses associated with subclinical mastitis was readily available from studies carried out in the mid-1970s (Hortet and Seegers, 1998). It appears that many dairy producers who do not respond to information about unobservable losses will respond if there is direct market feedback. Thus, providing additional information to dairy producers that includes the economics of subclinical Map infection is likely to have far less influence on their adoption of Map control practices than will a market price signal—even when the unobserved losses are greater than the drop in market price.
At least in the cattle industries, a major weakness of current biosecurity programs is the failure of producers to adopt well-established control practices, not a lack of scientific support for such programs. Clearly, research into factors that affect producer adoption of control practices, particularly in association with their veterinarians, is a major need. In fairness to the producers, rapid changes in the industry structure—the growth in average herd size, the emergence of off-site rearing, and the intensification of management and housing systems over recent decades—has contributed to disease risk. But those same structural changes also increase the producers’ need for specialized knowledge. Little published work addresses the veterinary profession’s role in this area (Brown et al., 1988). To its credit, the National Johne’s Working Group (NJWG) recognized the lack of producer understanding as an impediment to Map control and proposed an educational plan that includes marketing and follow-up effectiveness evaluation (Hansen, 1997). In a subsequent five-year review (Whitlock et al., 2000a) in which “educational issues for producers” was ranked as the highest current concern, it was not clear whether the proposed plan was fully funded or fully adopted. The USDA NAHMS 2002 Dairy Survey, which is currently under way, could provide some evidence about the effectiveness of the education program. Furthermore, given both the lack of understanding about the disease and the lack of control program adoption among cattle producers, similar studies of owners of the other ruminant livestock species are probably needed. No evidence on the adoption of control practices or on the knowledge of producers of the other ruminant species affected by Map disease is currently available.
Control Programs with a Single-Agent Focus
With the possible exception of the New York State Cattle Health Assurance Program (NYSCHAP, 2002), all current on-farm Map control programs, as well as those for most other agents, are focused on controlling a single infectious agent without considering other agents transmitted by the same
mechanisms. Those programs also ignore the associated broader fundamental interests of the stakeholders. This rather myopic focus on a single agent reflects two outdated paradigms: first, that an infectious agent by itself is the necessary and sufficient cause of disease, and second, that infectious disease is a linear process that starts with the entry of the agent into a susceptible host, continues with a series of pathologic events, and culminates in clinical disease and sometimes death (Morris, 1995; Schwabe, 1982, 1993). The modern paradigm places infectious disease in a causal web (Thrusfield, 1995), so infection is not simply a matter of the agents being present or absent (the focal point of historical control programs [Morris, 1995; Schwabe, 1982, 1993]). The occurrence of disease is the result of many factors that act as a set of sufficient causes (Rothman, 1976). The infectious agent, the host, and the environment interact in complex, dynamic ways over time. An infectious agent most often acts as an opportunist, rather than as a primary pathogen. As a consequence, individual animals in a herd manifest the effects of this causal web in different ways. Suboptimal production of milk is the most common manifestation, and thus the most important economically. Subclinical infection is the next-most-prevalent and economically important manifestation, followed by clinical disease, which is least common and economically least important.
In this modern paradigm, the excessive incidence or prevalence of a controllable infectious disease is more correctly viewed as a sign that one or several major risk factors are at work, and that the risk factors work in concert to present an opportunity for other infectious agents to flourish, as well. The fact that a significant number, if not a majority, of similar herds are not infected by a particular agent, or that they do not exhibit an excessive amount of a very common infectious agent, is primary evidence that control interventions are possible. The sets of risk factors against which interventions can be made differ in the strength of their influence on infection and disease risk for different farms and management systems.
Some have suggested that, with this shift in the way infectious diseases are viewed, livestock veterinarians need to shift focus from traditional hands-on diagnosis and treatment of individual animals (after disease has already occurred), to a more comprehensive approach that includes disease prevention, herd health, and client education (Leman, 1988; Radostits, 2001; Schwabe, 1982, 1993). Although not an explicit component of its current program, the NJWG has adopted this concept for cattle herds and is promoting it through its education materials that were distributed to states throughout the country, as well as to all members of the American Association of Bovine Practioners. A glance at the tables of contents of the recent editions of major texts used in agricultural animal disease courses, however, reveals content that is still mostly organized by specific disease, suggesting that the veterinary curriculum retains its traditional focus on the individual animal. Morris (1995) suggested that teaching veterinary students in this new paradigm will be difficult because their lack of experience with complex problems limits their ability to learn the methods necessary to solve them.
From the producer perspective, the most important and fundamental goals are, first, the short-term, and then the long-term economic survival of their own farming enterprises. Many chronic, difficult-to-detect infectious agents (including Map) reduce herd profitability through decreased production, increased morbidity and mortality, and decreased product value, which in turn jeopardize a herd’s economic viability. Many agents are difficult to control once they become established and, for most of those agents, the most important risk factor for acquiring the infection is the purchase of infected animals. Both the ease of acquiring the infection and the subsequent difficulty with control are consequences of the silent—and thus difficult to detect—carrier state of infected animals.
From the consumer perspective, the fundamental objectives are wholesome, high-quality foods produced without unwarranted environmental impact. Wholesomeness includes minimal risk from any zoonotic agent in livestock-origin foodstuffs, not just the mitigation of risk attributable to a specific infectious agent in a specific product. Expending stakeholders’ resources on the control of one agent, such as in an indemnity program targeted at a single agent, while ignoring the other agents of concern to the stakeholders is less than optimal. For example, some of the individual-agent control measures typically recommended can either directly or indirectly increase the risk of a herd’s acquiring other infectious agents. Those in turn can have direct and indirect farm-level economic effects or adverse consequences for human health. For example, application of a test-and-cull program increases the need for replacement animals, which often are purchased rather than reared on the farm. Although it is commonly recommended that purchased replacements be tested for Map, little consideration appears to have been given to preventing the acquisition of other economically and zoonotically important infectious agents. Because the largest risk factor for acquiring most communicable infectious agents is the purchase of a subclinically infected animal, and given the demonstrated lack of knowledge on the part of producers about disease biosecurity, such recommendations must be placed within an integrated disease biosecurity program that considers other infectious agents of concern.
Another Map control recommendation that can increase the risk of acquiring other infectious agents is contract rearing of heifers off-site (Groenendaal and Galligan, 1999)—18 percent of dairy producers with more than 200 lactating cows in a herd follow this practice (NAHMS, 1996b). In many contract rearing operations, neonatal calves originating from different farms initially are housed in close quarters and commingled after weaning. This creates an opportunity for the exchange of infectious agents between calves from different herds. Although many disease agents can establish long-term carrier states (Map, bovine leukemia virus, IBR virus, Mycoplasma bovis, Staphlococcus aureus, Salmonella enterica var Dublin, Leptospira borgpetersenii serovar hardjo-type Bovis), the individual health consequences and subsequent biosecurity risks associated with return of such calves to the herd of origin are currently unknown. If pregnant heifers are commingled at midgestation, BVD virus also must be added to the list because of the potential
for fetal infections that result in persistent infections after birth. Both the prevalence of contract rearing and the plausibility of its attendant disease risk are sufficient to warrant further assessment and risk analysis, for Map and other infectious agents.
Single-agent control programs also commonly overlook the on- and off-farm economies of integrating multiple control programs. For example, the fecal and serum samples taken during a Map control program also could be used for surveillance for other infectious agents. To do so reduces the on-farm expense associated with the sampling for Map, because the costs are apportioned across other agents as well, reducing the unit cost of this aspect of controlling Map. Similarly, diagnostic laboratory testing based on integrated technology— microarrays, multiplex primers in PCR (polymerase chain reaction), and different antibody-signal conjugates in ELISA (enzyme-linked immunosorbent assay)—could be used to reduce the supply and labor costs per agent because they would be apportioned across the agents.
Finally, the approach of starting with the infectious agent and then packaging information on how to control associated risk factors is a convenient focus and a convenient way to organize information for researchers, administrators, and veterinary practitioners, but it does not meet the immediate needs of the producer. On a dairy farm, the producer is focused on managing an intensive, complex animal husbandry system, which requires the day-to-day balancing of many competing risks and expenditures of scarce resources. Time is the farm manager’s most precious resource, and acquiring new information takes time. Information is needed on everything from current commodity prices to where to find new milkers. As a result, information on disease control must be delivered concisely and efficiently. Most researchers and practitioners consider themselves information seekers (looking for any and all information on a topic with little regard to its current applicability), but producers are information satisfiers, who seek only the information they need to make a specific decision. Information relevant to a current problem—where to purchase heifers to fill the new free-stall barn—is of considerably higher value than information related to a potential future problem, such as the possibility of JD developing in five years in one of those purchased heifers. A control practice could be completely feasible and biologically correct in the long term, but if implementing it results in cash-flow problems that lead to bankruptcy in the short-term, it is a failure.
Producers are best served by “pull” information systems, such as the Johne’s Information Center at http://johnes.org/ (Collins and Manning, 2002a), which allow them to find and use information as the need arises, rather than “push” information systems, such as newsletters or journal articles. The occurrence of a particular infectious agent in a herd is a consequence of one or more risk factors being out of control. If the focus is on controlling the risk factors, which could be common to several infectious agents, rather than on the agent itself, the producer will be more likely to adopt the control practice because there is a greater return on the investment. In contrast, if the focus and information packaging follow the traditional approach of controlling
cryptosporidiosis, salmonellosis, Map, leptospirosis, rota and corona viruses individually, the common principles are lost. In the end, the producer concludes that control of any of these agents is impractical, or of low priority, and moves on to more immediate, higher priority issues. If the starting point is, for example, how to manage the control points of a set of common risk factors for enteric infectious disease rather than understanding the control details of the infectious agents themselves, producer compliance is likely to be higher.
Ultimately, the control program must motivate most producers to change their behavior when such a change is needed. Even complete understanding without behavioral change is a failure. Some with long experience on control programs are not optimistic (Franks, 2001). At least for dairy producers, the Milk and Dairy Beef Quality Assurance Program appears to have been a failure: it has been adopted by only a small proportion of producers (Gibbons-Burgener et al., 1999, 2000). What is needed is an integrated approach to on-farm disease control that meets the needs of the livestock producer and motivates behavioral change. This is not a novel idea; several groups have proposed a more integrated approach to disease control by using a hazard analysis critical control point (HACCP) strategy (which targets control at the most critical points in the causal web) and establishing good farming practices (Noordhuizen and Welpelo, 1996; Weber and Verhoeff, 2001; Wells and Ott, 1998). Some are beginning to appear on the Internet, including those presented at http://www.gov.mb.ca/agriculture/foodsafety/gpp/ (Manitoba Agriculture and Food, 2001), which lists a manual of practices and offers’ measurements for self-assessment. General outlines for a given type of management and husbandry situation will be the same but must be sufficiently flexible to be easily adaptable to the specific circumstances of each farm. Finally, the information must be packaged and delivered in a manner that is in harmony with the information management style of producers and that motivates them to change their practices. Motivation can require feedback signals in the form of market access or market price differentials established through testing by the downstream purchaser of the farm product.
Vaccination
As a component of biosecurity control programs, vaccination is problematic for several reasons. First, producers adopting vaccination may be less likely to employ other management practices that, although requires more work, might be more effective. A recent NAHMS report stated: “When considering disease control programs, many producers and veterinarians think primarily about vaccinating. Yet there are many other management practices that can be used to minimize both disease occurrence and the risk of introducing new diseases onto operations” (NAHMS, 1996a). Second, the proportion of producers voluntarily adopting vaccination may be low. A study of beef-cow calf producer biosecurity practices based on NAHMS Beef 1997 data found that although the producers importing purchased cattle into their herd were approximately twice as likely to vaccinate for two common viral infectious agents of cattle, IBR and BVD, as those who did not, only 25 percent did so
(Sanderson et al., 2000). Third, evidence suggests that a significant proportion of producers often do not use existing vaccinations appropriately. In a study of BVD vaccination and biosecurity practices in 387 randomly selected Pennsylvania dairy herds after a regional outbreak of clinical BVD, Rauff et al. (1996) found that, although 82 percent of producers indicated that they routinely vaccinated for BVD, the authors regarded only 27 percent of the herds as adequately vaccinated. Because of these vaccination errors and weaknesses in associated biosecurity practices, the authors concluded that 30 percent of the herds were at high risk of a clinical BVD outbreak. Finally, some producer skepticism against vaccination may be warranted as the evidence of field efficacy for many of the currently available commercial enteric and respiratory disease vaccines is limited at best (Perino and Hunsaker, 1997; Radostits, 1991) and anecdotal reports of vaccine failures are common. Federal vaccine licensing standards do not require demonstration of field efficacy under circumstances in which producers could reasonably expect the vaccine to be an important component of disease control. Adoption of vaccination and other biosecurity practices may be limited if the producer perceives the risk for that specific disease to be low for their herd and is willing to take the risk or that the expense of implementing the biosecurity step will be higher than the actual cost of an outbreak of the disease (Sanderson et al., 2000).
Herd-Level Control
A herd plan provides the basis for organizing control strategies on a farm. Prerequisites are knowledge of herd goals and resources for voluntary programs and agreements between herd owners and their advisors on the intensity necessary to achieve herd goals with respect to control or eradication of Map (Rossiter and Burhans, 1996). Incorporating Best Management Practices concepts will facilitate control of other economically important diseases and could result in greater producer compliance overall.
Many states have adopted control programs for Map test-positive herds and status programs for test-negative herds. Control programs should be tailored to the individual herd, but working within state program standards will allow recognition of control progress or test status and could be necessary for access to state laboratory and technical resources.
Dairy Herds
Whitlock (2001) divides control principles into two categories. First is management practices that prevent highly susceptible newborn and young animals from ingesting manure from infected animals. Second is reducing farm contamination with Map by culling infected animals. Rossiter and Hansen (2000) list three management principles for control of Map: reducing infections by manure management, reducing infections by colostrum and milk management, and reducing infections by management of infected animals. Test-and-cull strategies are not likely, by themselves, to be effective in herd Map
control. Better hygiene and management are more effective control tools (Groenendaal and Galligan, 1999).
Veterinarians and other consultants who work with dairy farmers to implement Map control programs should promote management recommendations that adapt recognized control principles to specific situations. Most control measures fall into one of three generally accepted categories of Map control (Rossiter and Hansen, 2000; Rossiter et al., 1998; Sockett, 1994):
-
Protect young stock from older animals and from feces-contaminated feed and water:
-
Clean and disinfect maternity and calf pens after each use.
-
Calve cows in clean, dry, dedicated maternity pens.
-
Remove calves immediately after birth to clean, dry calf pens, stalls, or hutches.
-
Feed colostrum only from test-negative cows.
-
After colostrum feeding, use pasteurized milk or use milk replacer.
-
Raise calves separate from the adult herd for at least the first year of life.
-
Do not allow shared feed or water between adults and young stock; do not offer feed refusals from adult cattle to young stock.
-
Avoid vehicular and human traffic from adult animal areas to young stock areas.
-
-
Prevent manure contamination of feed and water sources:
-
Use separate equipment for handling feed and manure.
-
Design and maintain feedbunks and waterers to minimize risk of contamination with manure.
-
Do not spread manure on grazing land.
-
-
Reduce total farm exposure to the organism:
-
Immediately cull all animals with clinical signs of JD.
-
Cull culture-positive animals as soon as possible; for cows with low or moderate fecal culture colony counts, removal at the end of lactation may be acceptable.
-
Test adult cattle at least annually by serum or fecal tests; positive serum test results should be confirmed by fecal culture.
-
Purchase replacement animals from test-negative herds; if this is not possible, assess the status of the herd of origin through owner or veterinarian statements, by negative serum ELISA tests of at least thirty adult animals, or both.
-
Beef Herds
Control plans for beef-cow calf herds follow the same principles as those for dairy herds, but must adapt the procedures to meet calf management
needs. In addition to the management procedures suggested for dairy herds, some specific control measures for beef herds (adapted from Hansen and Rossiter, 2000) include the following:
-
Avoid manure build-up in pastures and corrals where late-gestation cows are kept.
-
Provide a clean calving area, with low cow density.
-
Move cow-calf pairs to clean pasture as soon as bonding occurs.
-
Move feedbunks, waterers, and creep-feed areas frequently to avoid exposing calves to manure build-up.
-
Do not put weaned calves on pastures used by cows.
-
Blood or fecal test the entire breeding herd annually; avoid calving-out and raising offspring from test-positive animals.
-
If possible, calve first-calf heifers in an area separate from older cows.
Sheep Flocks
Sheep flock JD control or eradication programs have been more widely implemented in Australia than in other sheep-raising countries. The Australian program is based on negative serologic testing (ELISA or AGID [agar gel immunodiffusion]) of a sample of the adult flock (2 years or older) and management that includes boundary fencing and introducing flock additions only from flocks of similar Map status. The flock-sampling program, which is designed to detect 2 percent or greater infection prevalence with 95 percent confidence, requires testing 400–500 sheep from each flock. Fecal culture of samples from sheep with histologically confirmed JD has been unrewarding because of low test sensitivity. Recent use of Middlebrook agar or a modified BACTEC radiometric medium have greatly improved the sensitivity of detection and allowed the use of pooled (up to 50 animals per pool) fecal samples in control programs (Whittington et al., 1999).
Goat Herds
There are few organized programs for control of Map in goat herds. Elements to consider in developing a strategy for elimination of Map from infected herds include identification and removal of infected animals, reduction in the rate of new infections in susceptible animals through improved sanitation and kid-rearing techniques, and vaccination to increase host resistance to new infections (Smith and Sherman, 1994).
Zoos and Game Farms
A recent workshop addressed control programs for zoos accredited by the American Zoo and Aquarium Association (Proceedings of the Workshop on Diagnosis, Prevention and Control of Johne’s Disease in Non-Domestic Hoofstock, 1998). Plans suited to the unique needs and management of game farms have not been developed, but they could be guided by relevant aspects of similar control plans for domesticated and zoo animals.
CURRENT CONTROL PROGRAMS
National Programs
In the past decade, there have been several attempts to establish nationwide management programs to eliminate JD from animal herds. In 1993, a task force of the Johne’s disease committee of USAHA (United States Animal Health Association) drafted a model herd certification program to provide states with an example from which to develop or standardize programs. Some states modified their programs to conform, but relatively few herd owners have elected to pursue herd certification. The primary deterrents cited were the amount of testing required and the associated costs. In 1997, the newly formed NJWG appointed a committee to design a scientifically sound and more affordable herd certification program. The program was evaluated by veterinarians, veterinary associations, cattle breed associations, and interested industry groups, and a revised version was adopted by USAHA in October 1998 as the U.S. Voluntary Johne’s Disease Herd Status Program for Cattle (Whitlock et al., 2000a). The standards have been adopted in whole or in part by at least 20 states. In 1999, additional guidelines were developed to address JD-affected herds: the Minimum Recommendations for Administering and Instituting State Voluntary Johne’s Disease Programs for Cattle. Although the proposed program standards are not required by the federal government, resources for national coordination of JD programs have been appropriated by Congress.
Two additional programs have been proposed. In 2001, the USDA Animal and Plant Health Inspection Service (APHIS) released for comment draft program standards for a voluntary control program. The National Milk Producers Federation has developed a federally funded program to provide reimbursement for producers with JD-related expenses.
Control programs have in general relied on management techniques to identify infected herds and then clear those herds of the disease, because effective treatment and vaccination strategies do not exist. In addition, because of the long subclinical phase and the limited sensitivity of diagnostics, eradication programs require a long-term commitment.
NJWG and Control Plans
The United States Animal Health Association has long had an interest in and concern about JD in domesticated animals. Its work is conducted primarily by committees, and until the formation of a separate JD Committee in 1987, the Tuberculosis Committee was the focal point for consideration of JD issues through the Johne’s Subcommittee. By 1990, the Johne’s Committee had created several subcommittees to address specific tasks.
In 1994, the USAHA’s JD Committee recommended formation of NJWG, which was to be broadly representative of the many public- and private-sector groups with interest in control of JD. At its first meeting, NJWG adopted a mission statement:
The NJWG will serve as a resource for animal agriculture in assessing any association between Johne’s and human health. Recognizing that JD has major economic implications for producers, the NJWG will develop and coordinate implementation of a National Johne’s program. The program will be designed to protect the public and animal health, reduce the economic burden upon producers and bring about a uniform approach for control, herd certification, and eventual eradication of this insidious and costly disease in the United States, (approved 4/9/95, Colorado Springs, Colorado; USAHA, 2002).
NJWG set out the following initial objectives:
-
Evaluate information about Map as a zoonotic pathogen and assess the likelihood that animals serve as a reservoir of infection.
-
Evaluate the potential for Map to contaminate foods of animal origin.
-
Identify and encourage research needed to develop a strategy for control and herd certification.
-
Evaluate domestic and international economic consequences and suggest good management practices for prevention of entry and spread of Map.
-
Develop policy objectives and goals for disease control and herd certification.
Subcommittees study economics, state control programs, research status and priorities, laboratory diagnostic issues, education, small ruminants, certification, strategic planning, serology quality control, validation of check tests, and certification of veterinary inspection. Through its subcommittees and the entire working group, NJWG has made significant progress toward meeting the initial objectives. In September 2000, a survey of NJWG members and others with interest in JD identified issues for additional committee concern and effort. The top three priorities were producer education, economic incentives for testing and adding value for “status” herds, and issues of serologic tests (quality control and false positives).
The National Johne’s Working Group has emerged as a prime mover both in setting the agenda and in developing the tools needed to address issues of JD control. NJWG has set short-term, intermediate, and long-term goals. Because the group includes representatives of most of the major organizations with animal-agriculture interests, decisions made by NJWG are likely to be supported and promoted by those organizations.
NJWG 1998 Program
The 1998 NJWG program (U.S. Animal Health Association, 1998; see Appendix B) is designed as a model for improving the equivalency of state control programs, as a framework for the establishment of new state programs,
and to assist state veterinarians and state JD advisory committees as they considered implementation of herd certification programs. The model is a voluntary herd status program, which identifies minimum requirements for operation of a scientifically sound approach to identification of low-risk herds. It provides for confidentiality of testing results at the discretion of the producer (within state limits), but it also encourages producers who have entered the program to reveal test results as a way to promote the value added by JD-free herd certification.
The program recommends an initial risk evaluation, to inform producers of existing on-farm factors associated with the spread of JD and to introduce to them the Best Management Practices that have been identified to prevent the introduction and spread of JD. Important practices that must be adopted before entry into the program are the prevention of commingling, the prevention of exposure to the manure or raw milk of susceptible species over a period of 12 months, and the permanent identification of individual cattle.
There are two paths through the program: the standard and the fast track. If the producer can certify that the herd has been free of JD for five years, that the property has been JD free for one year, and that no cattle have been introduced from JD-infected herds for five years, then the herd can be entered into the certification program through the fast track. This allows entry directly into Level 2, by-passing the first of four levels of certification. The fast track permits producers to proceed more quickly than the standard track does, but it requires a greater financial investment at program entry. The fast track will allow herds to reach Level 4 in two years with three tests; the standard track requires at least three years and four tests to reach Level 4. However, the standard track allows entry to the program with a minimal investment of funds and gradually increases the producer’s investment in the program.
The four levels differ primarily by the number of herd animals tested and the types of diagnostic tests performed (Tables 4–1, 4–2). Previously infected herds and JD-vaccinated herds can enter the program after infected animals are removed or vaccination is discontinued and the number of nonvaccinated animals meets the testing criteria. There is also a provision for an appeal process, for example, if results from an ELISA or fecal-culture test are disputed.
On-farm biosecurity measures are required to prevent the spread of JD from animals of unknown status. Exposure to pooled milk (for calves) or to manure from untested cattle should be eliminated, and cattle should not be grazed or have other contact with other JD-susceptible species. Cattle being transported should be hauled in cleaned and disinfected trailers, and commingling should be avoided.
Table 4–1. Standard-Track Certification from the U.S. Voluntary Johne’s Disease Herd Status Program for Cattle
|
Level |
Criteria |
|
1 |
Program entry requirements have been met, and negative ELISA tests have been performed on 30 second- or higher-lactation animals. A sample size of 30 was selected to optimize herd sensitivity and herd specificity and to maintain a fixed cost for all herds entering the program. |
|
2 |
The herd has met the requirements for Level 1, and negative ELISA on a statistical subset of second- or higher-lactation animals has been performed. The Level 2 testing must be completed between 10–14 months after any Level 1 testing. |
|
3 |
The herd has met requirements for Level 2 and has negative fecal-culture-test results on a statistical subset of second- and higher-lactation animals. Bulls 2 years of age and older must be included in this testing. The fecal culture must be collected between 10–14 months after any Level 2 testing. |
|
4 |
The herd has met the requirements for Level 3 and has a negative ELISA on a statistical subset of second- or higher-lactation animals. Level 4 testing must be completed between 10–14 months after any Level 3 testing. Level 4 status is maintained by achieving negative ELISA results on 30 second- or higher-lactation animals every 10–14 months. |
|
SOURCE: USAHA, 1998 |
|
Table 4–2. Fast-Track Certification from the U.S. Voluntary Johne’s Disease Herd Status Program for Cattle
|
Level |
Criteria |
|
2 |
Fast-track program entry requirements have been met, and negative ELISA results have been obtained on a statistical subset test of second- or higher-lactation animals. |
|
3 |
The herd has met requirements for Level 2 fast-track and has negative fecal-culture test results on 30 second- or higher-lactation animals. (A history of JD freedom for 5 years before program entry adds sufficient confidence to allow fast-track herds to test 30 animals rather than the statistical subset used in the standard tack to obtain Level 3 status.) Level 3 testing must be completed 10–14 months after any Level 2 testing. |
|
4 |
The herd has met the requirements for fast-track Level 3 and has negative ELISA results on a statistical subset test of second- or higher-lactation animals. Level 4 testing must be completed 10–14 months after any Level 3 testing. Level 4 status is maintained by achieving negative ELISA results on 30 second- or higher-lactation animals every 10–14 months. |
|
SOURCE: USAHA, 1998 |
|
A key feature of the program is the status of herd additions. Young cattle are required to come from program herds of similar status. Older animals have more stringent testing and biosecurity requirements, if purchased from nonprogram herds. Replacement cattle must come from herds of equivalent status. Semen and embryos may be used from any herd.
USDA 2002 Program
The USDA’s recently approved “Uniform Program Standards for the Voluntary Bovine Johne’s Disease Control Program” (USDA, 2001a) for beef and dairy cattle was developed with input from NJWG and USAHA (USDA, 2001a; see Appendix C). It is intended to be a federal-state-industry cooperative program, in which all levels have important roles. States will have primary administrative responsibilities through specified positions and groups, and will be supported by federal agencies and industry. The program is voluntary, so producer incentive for participation relies on the potential for the added market value associated with products from known-status herds.
As with the NJWG control program, the USDA draft program is intended to provide minimum national standards, which local authorities may exceed as warranted. The program has administrative, technical, and testing components.
Administratively, the USDA program mandates the appointment of a qualified designated state JD coordinator who assists producers, health officials, and veterinarians in combating JD through training, interpreting results, and helping to review and develop herd management plans. The coordinator also advises and informs other federal and state animal health officials, specifically USDA’s JD program officials and a mandated state JD group, about JD activities and issues. The coordinator may also decide to incorporate the services of private veterinary practitioners in the development of technical program elements, such as herd management plans, and should then implement a JD certification program for those veterinarians to ensure their qualifications.
The program stipulates that the coordinator should serve as a member of the mandated JD advisory group and that a producer should be its chair. It is recommended that the membership should consist of dairy producers, beef producers, university or extension faculty, diagnostic-laboratory personnel, regulatory veterinary medical officers, and beef and dairy veterinary practitioners, and that they meet at least once a year.
There are three primary technical program elements: education of producers, development of good management strategies, and herd testing and classification. The education element of the program is the producer’s entry point to the program. At this stage, the producer should receive information about the disease; about good management practices for the control, elimination, and prevention of JD; and about the JD control program itself.
The intermediate element involves establishing approved but voluntary management plans and practices that are producer- or market-driven. The first step is to conduct an on-farm risk evaluation, which can be used as the basis for a management plan. The JD risk assessment instrument assesses “maximum risk” values for each management group of animals on a farm. The numerical values constitute a consensus opinion of JD control experts; the numerical values attempt to weight the greatest risk to the youngest groups of animals in recognition of available information on transmission risks. The management plan should focus on biosecurity measures to minimize exposure to and spread of JD through infected animals and a contaminated environment. Minimum
management practices, such as the prevention of commingling of infected and uninfected animals, the use of single-source colostrum, individual housing, and the permanent and individual identification of all cattle, should be put in place. Annual renewal of participation in the program is based on repeating the risk assessment and updating the herd management plan.
Herd testing and classification is the third element of the USDA draft control program. An initial screening test on a random sampling of a herd determines whether the herd will enter the test-positive or the test-negative track of this third element. There are different designated sampling schemes for testing, which vary by the number, age, and sex of the cattle selected for testing, and which are similar to the NJWG sampling scheme. Vaccinated herds are eligible to enter the program without additional testing or culling, as long as an organism detection method is used. Appeal of a positive test result for an animal is allowed.
Test-Positive Component
The USDA program has instituted a test-positive component to gather information about the prevalence of JD-infected herds. Test-positive herds must be enrolled in the program and abide by the education, herd management, identification, renewal, and appeal procedures as laid out in the first two elements of the program. They also must conform to guidelines for herd additions and testing, following a protocol similar to the random-sampling protocol laid out in the NJWG program.
Additionally, the USDA program has a four-level test-positive-classification scheme, ranging from Level A to Level D (Table 4–3). Herds can advance through the levels to enter the test-negative classification ladder. Level A indicates a negative herd-screening result, and producers whose herds qualify for Level A are encouraged to apply to enter the test-negative program component. Level D indicates greater than 15 percent positive herd prevalence.
Test-Negative Component
Entry into the test-negative component requires the same education, herd management, identification, renewal, and appeal procedures as in the test-positive track. The test-negative component has more stringent guidelines for herd additions and testing, similar to those of the NJWG program. Testing should be performed by or under the supervision of a recognized health official, and the tests must be done by an approved laboratory. As in the test-positive track, advancement is possible, and there are standard and fast tracks that are similar to those of the NJWG program (Tables 4–4, 4–5).
Table 4–3. Test-Positive Requirements from the Uniform Program Standards for the Voluntary Bovine Johne’s Disease Control Program
|
Level |
Criteria |
|
A |
An annual herd test reveals no screening or official Johne’s-test-positive animals. Can be maintained by achieving negative screening test results on 30 second- or higher-lactation animals every 10–14 months. Herds achieving Level A should be encouraged to enter the test-negative program at Level 1 . The following qualifications must be met: • Negative test results on at least 30 randomly selected second- or higher-lactation animals (3 years old or older), or • Negative test results on the whole herd and on bulls over 2 years of age. |
|
B |
An annual whole-herd test and bulls over 2 years of age reveals less than 5% of animals positive to a screening or official JD test. |
|
C |
An annual whole-herd test and bulls over 2 years of age reveals at least 5%, but not more than 15%, of animals positive to a screening or official JD test. |
|
D |
Herds should be classified as Level D if either of the following apply: • A test on at least 30 randomly selected second- or higher-lactation animals (3 years or older) reveals one or more test-positive animals, or • A whole-herd test with the addition of bulls over 2 years of age reveals more than 15% of the animals positive to a screening or official JD test. |
|
SOURCE: USDA, 2001a |
|
Table 4–4. Standard-Track Test-Negative Components from the Uniform Program Standards for the Voluntary Bovine Johne’s Disease Control Program
|
Level |
Criteria |
|
1 |
The herd owner has developed a herd management plan and agreed to abide by the requirements of the test-negative component. The herd has had negative screening-test results on 30 second- or higher-lactation animals. |
|
2 |
Herds have met the Level 1 requirements and have had negative screening-test results on a statistical subset of second- or higher-lactation animals. Level 2 testing must be completed within 10–14 months of any Level 1 testing. |
|
3 |
Herds have met the Level 2 requirements and have had negative fecal-culture-test results on a statistical subset of second- and higher-lactation herd members. Bulls 2 years and older must be included in this testing. The fecal culture must be collected within 10–14 months of any Level 2 testing. |
|
4 |
Herds have met the Level 3 requirements and have had negative screening-test results on a statistical subset of second- or higher-lactation animals. Level 4 testing must be completed within 10–14 months of any Level 3 testing. |
|
SOURCE: USDA, 2001a |
|
Table 4–5. Fast-Track Test-Negative Components from the Uniform Program Standards for the Voluntary Bovine Johne’s Disease Control Program
|
Level |
Criteria |
|
1 |
Skip this level if the owner signs a declaration that no cows were seen or diagnosed with JD in the past five years and has an approved herd plan in place. The State may require the declaration to be cosigned by the herd veterinarian. The signed declaration must include the following statements: • I am fully aware of the management and disease history of the herd during the past five years. • JD is not known or suspected to have existed in the herd during the past five years or on the property during the past 12 months. • No cattle have been introduced from known infected herds during the past five years. |
|
2 |
Herds have met the Level 1 requirements and have had negative screening-test results on a statistical subset of second- or higher-lactation animals. Level 2 testing must be completed within 10–14 months of any Level 1 testing. |
|
3 |
Herds have met the Level 2 requirements and have had negative fecal-culture-test results on 30 second- or higher-lactation cows and all bulls 2 years or older. The fecal culture must be collected within 10–14 months of any Level 2 testing. |
|
4 |
Herds have met the Level 3 requirements and have had negative screening-test results on a statistical subset of second- or higher-lactation animals. Level 4 testing must be completed within 10–14 months of any Level 3 testing. |
|
SOURCE: USDA, 2001a |
|
Laboratory Approval
Laboratories that are being used for JD testing must have official approval specifically for JD testing. Private, university, state, and federal laboratories can participate, but state animal health officials must be able to audit them. Whether they perform analytical or screening tests, laboratories must meet performance validation standards, as described in Boxes 4–1 and 4–2. Currently approved diagnostic tests and laboratories are listed in Tables 4–6 and 4–7.
|
BOX 4–1 Approval Process for Laboratories Performing Official Johne’s Disease Tests
|
|
BOX 4–2 Approval Process for Laboratories Performing Johne’s Disease Screening Tests (Serology Tests)
|
Table 4–6. National Veterinary Services Laboratory Approved Johne’s Disease Tests
|
Test |
Description |
|
Fecal, tissue culturea |
Culture is the standard for organism-based tests, although culture methods are not currently standardized. Protocols for recommended methods are available from NVSL upon request. Sensitivity of fecal- and tissue-culture tests is estimated at 40%; specificity is 99% for tests that are done correctly. |
|
Direct DNA probe (without PCR) |
DNA probes can detect Map without culture. DNA tests are relatively rapid (3 days) but more expensive than culture methods. They can miss low shedders. Sensitivity is estimated at 40%; specificity is about 99.9%. |
|
Radiometric culture |
Radiometric-based methods have been adapted from those used to isolate the bacterium that causes human tuberculosis. Radiometric culture can detect small amounts of bacteria, and it is faster than standard fecal-culture methods (7 weeks vs. 16 weeks). It is more expensive than other methods, it requires technicians to handle radioactive materials, and it requires specialized instrumentation to read the culture vials. Sensitivity is estimated at 40%; specificity is about 99%. |
|
Histology of tissue |
No check test is currently available. Microscopic identification of characteristic changes and of Map in tissue is a definitive test for JD. Tissue changes and bacteria can be seen in the intestinal lining and in nearby ileum, mesenteric, and ileocecal lymph nodes of infected animals. Sensitivity, which depends on disease stage and the number and type of specimens examined, typically exceeds that of other methods. Specificity is considered to be 100%, based on defined criteria (granuloma with acid-fast bacteria observed by board-certified pathologist). |
|
USDA-licensed ELISA |
Animals identified as positive by ELISA should be considered infected until disease is ruled out by an official JD test. ELISA is used for screening and to inform management decision making. Sensitivity is estimated at 25% for nonclinical cases, 85% for clinical cases. Specificity is estimated at 98–99%. |
|
aSOURCE: http://www.usaha.org/njwg/jdtests.html (8/1/02). |
|
Table 4–7. Canadian and U.S. National Veterinary Services Laboratory Approved Laboratories for Johne’s Disease (Nov. 1, 2001)
|
State/Province |
City |
Laboratory |
Fecal |
PCR |
Serology |
|
Canada |
|||||
|
Alberta |
Edmonton |
Alberta Agriculture, Food & Rural Development Food Safety Division |
Yes |
No |
No |
|
Veterinary Pathology Laboratory Alberta Ltd. |
No |
No |
Yes |
||
|
Virology-Serology Laboratory AHLB-Alberta Agriculture |
No |
No |
Yes |
||
|
Lothbridge |
Palliser Animal Health Laboratories |
No |
No |
Yes |
|
|
State/Province |
City |
Laboratory |
Fecal |
PCR |
Serology |
|
Canada |
|||||
|
British Columbia |
Abbotsford |
Animal Health Monitoring Laboratory |
Yes |
Yes |
Yes |
|
Manitoba |
Winnipeg |
Veterinary Services Laboratory |
No |
No |
Yes |
|
Saskatchewan |
Regina |
Prairie Diagnostic Services |
Yes |
No |
Yes |
|
Saskatoon |
Prairie Diagnostic Services Bacteriology/Mycology |
Yes |
No |
Yes |
|
|
United States |
|||||
|
Alabama |
Auburn |
Charles S.Roberts Veterinary Diagnostic Laboratory Alabama Department of Agriculture |
No |
No |
Yes |
|
Arkansas |
Little Rock |
Arkansas Livestock & Poultry Arkansas State University |
No |
No |
Yes |
|
Arizona |
Tucson |
Arizona Veterinary Diagnostic Laboratory |
No |
No |
Yes |
|
California |
Davis |
California Animal Health & Food Safety Laboratory University of CA, Davis |
Yes |
No |
Yes |
|
Fresno |
California Animal Health & Food Safety Laboratory University of CA |
Yes |
No |
Yes |
|
|
San Bernadino |
California Animal Health & Food Safety Laboratory University of CA |
Yes |
No |
Yes |
|
|
Colorado |
Denver |
Rocky Mountain Regional Animal Health Laboratory CO Department of Agriculture |
Yes |
Yes |
Yes |
|
Fort Collins |
Veterinary Diagnostic Laboratory CO State University |
Yes |
No |
Yes |
|
|
Rocky Ford |
Colorado State University Veterinary Diagnostic Laboratory Rocky Ford Branch |
No |
No |
Yes |
|
|
Delaware |
Dover |
Delaware Department of Agriculture |
No |
No |
Yes |
|
Florida |
Kissimmee |
Animal Diagnostic Laboratory FL Department of Agriculture |
No |
No |
Yes |
|
Live Oak |
Live Oak Diagnostic Laboratory |
No |
No |
Yes |
|
|
Georgia |
Tifton |
Veterinary Diagnostic and Investigations Laboratory University of GA |
No |
No |
Yes |
|
Iowa |
Ames |
Veterinary Diagnostic Laboratory IA State University |
Yes |
No |
Yes |
|
Diagnostic Bacteriology Laboratory, NVSL USDA/APHIS/VS |
No |
No |
Yes |
||
|
National Animal Disease Center USDA-ARS |
Yes |
No |
No |
||
|
Diagnostic Bacteriology Laboratory, NVSL USDA/APHIS/VS |
Yes |
No |
No |
||
|
State/Province |
City |
Laboratory |
Fecal |
PCR |
Serology |
|
United States |
|||||
|
Idaho |
Boise |
Idaho Bureau of Animal Health Laboratories |
No |
No |
Yes |
|
Caldwell |
Caine Veterinary Teaching Center University of Idaho |
No |
No |
Yes |
|
|
Illinois |
Centralia |
Animal Disease Laboratory Illinois Department of Agriculture |
Yes |
No |
Yes |
|
Galesburg |
Animal Disease Laboratory Illinois Department of Agriculture |
Yes |
No |
No |
|
|
Urbana |
Labs of Veterinary Diagnostic Medicine College of Veterinary Medicine |
Yes |
No |
Yes |
|
|
Indiana |
Dubois |
Animal Disease Diagnostic Laboratory |
Yes |
No |
No |
|
West Lafayette |
Animal Disease Diagnostic Laboratory Purdue University |
Yes |
Yes |
Yes |
|
|
Kentucky |
Hopkinsville |
Breathitt Veterinary Center Murray State University |
Yes |
No |
Yes |
|
Lexington |
Livestock Disease Diagnostic Center University of Kentucky |
Yes |
No |
Yes |
|
|
Maryland |
College Park |
Animal Health Diagnostic Laboratory Maryland Department of Agriculture |
No |
No |
Yes |
|
Frederick |
Animal Health Diagnostic Laboratory |
Yes |
No |
No |
|
|
Maine |
Westbrook |
IDEXX Laboratories, Inc. |
No |
Yes |
Yes |
|
Michigan |
East Lansing |
Animal Health Diagnostic Laboratory Michigan State University |
Yes |
Yes |
Yes |
|
Laboratory Division Michigan Department of Agriculture |
Yes |
No |
Yes |
||
|
Lansing |
Biostar Research, Inc. (Antell Biosystems) |
Yes |
Yes |
Yes |
|
|
Minnesota |
St. Paul |
Minnesota Veterinary Diagnostic Laboratory |
Yes |
No |
Yes |
|
Missouri |
Cameron |
NW Missouri Veterinary Diagnostic Laboratory Missouri Department of Agriculture |
Yes |
No |
Yes |
|
Columbia |
Veterinary Medical Diagnostic Laboratory University of Missouri |
Yes |
No |
Yes |
|
|
Fayette |
Allied Monitor, Inc. |
Yes |
No |
Yes |
|
|
Gray Summit |
Veterinary Services Laboratory Purina Research Center |
No |
No |
Yes |
|
|
State/Province |
City |
Laboratory |
Fecal |
PCR |
Serology |
|
United States |
|||||
|
Jefferson City |
Animal Health Laboratory Missouri Department of Agriculture |
Yes |
No |
Yes |
|
|
Springfield |
Missouri Veterinary Diagnostic Laboratory Missouri Department of Agriculture |
No |
No |
Yes |
|
|
Mississippi |
Jackson |
Mississippi Veterinary Diagnostic Laboratory |
No |
No |
Yes |
|
Montana |
Bozeman |
Veterinary Diagnostic Laboratory Montana Department of Livestock |
No |
No |
Yes |
|
North Carolina |
Raleigh |
Rollins Animal Disease Diagnostic Laboratory |
No |
No |
Yes |
|
North Dakota |
Fargo |
North Dakota State Veterinary Diagnostic Laboratory North Dakota State University |
Yes |
No |
Yes |
|
Nebraska |
Lincoln |
Veterinary Diagnostic Center University of Nebraska, Lincoln |
No |
No |
Yes |
|
New Jersey |
Trenton |
Division of Animal Health Laboratory New Jersey Department of Agriculture |
Yes |
No |
Yes |
|
New York |
Ithaca |
Veterinary Diagnostic Laboratories New York State College of Veterinary Medicine |
Yes |
No |
Yes |
|
Ohio |
Reynoldsburg |
Animal Disease Diagnostic Laboratory Ohio Department of Agriculture |
Yes |
No |
Yes |
|
Oklahoma |
Stillwater |
Oklahoma Animal Disease Diagnostic Laboratory Oklahoma State University |
Yes |
No |
Yes |
|
Oregon |
Salem |
Animal Health Laboratory Oregon Department of Agriculture |
Yes |
No |
Yes |
|
Pennsylvania |
Harrisburg |
Pennsylvania Veterinary Laboratory Department of Agriculture |
Yes |
Yes |
Yes |
|
Kennett Square |
Johnes Research Laboratory University of Pennsylvania |
Yes |
No |
Yes |
|
|
Quakertown |
Quakertown Veterinary Clinic |
No |
No |
Yes |
|
|
South Carolina |
Columbia |
Clemson Veterinary Diagnostic Center Clemson University |
No |
No |
Yes |
|
South Dakota |
Brookings |
Animal Disease Research & Diagnostic Laboratory South Dakota University |
Yes |
Yes |
Yes |
|
Tennessee |
Nashville |
C.E.Kord Animal Disease Laboratory |
Yes |
No |
Yes |
|
Texas |
Amarillo |
Texas Veterinary Medical Diagnostic Laboratory Texas A&M University |
Yes |
No |
Yes |
|
State/Province |
City |
Laboratory |
Fecal |
PCR |
Serology |
|
United States |
|||||
|
College Station |
Texas Veterinary Medical Diagnostic Laboratory Texas A&M University |
Yes |
Yes |
Yes |
|
|
Washington |
Olympia |
Washington State Department of Agriculture Lab Services |
Yes |
No |
Yes |
|
Pullman |
Washington Animal Disease Diagnostic Laboratory Washington State University |
Yes |
No |
Yes |
|
|
Wisconsin |
Barron |
Wisconsin Veterinary Diagnostic Laboratory |
Yes |
No |
No |
|
Madison |
Wisconsin Animal Health Laboratory Wisconsin Department of Agriculture |
Yes |
No |
Yes |
|
|
Madison |
Johnes Testing Center University of Wisconsin, Madison |
Yes |
No |
Yes |
|
|
West Virginia |
Charleston |
Animal Health Division West Virginia Department of Agriculture |
No |
No |
Yes |
|
SOURCE: USDA, 2002 |
|||||
NMPF 2002 Proposal
The National Milk Producers Federation (NMPF) has recommended plans for JD management and testing. NMPF along with other farm organizations, represents many of the milk-processing cooperatives in the United States. Although the NMPF plan has evolved over time and iterations have varied in programmatic cost and scope, the March 20, 2002, version of the NMPF proposal is representative of this group’s current goals.
The proposal seeks federal funding to support state JD program activities. About $49 million in federal support is requested to fund JD testing by ELISA and fecal culture and to fund state infrastructure and pay laboratory costs. Funding would be earmarked for states with programs administered through state-approved JD advisory committees. Funding to support testing would be available only to those herds with Johne’s herd management plans approved by the chief livestock veterinarian in the state and based on herd risk assessment conducted under the direction of a USDA-approved Johne’s certified veterinarian. Fecal-culture-positive animals would be ear-punch identified and removed from the herd before drying-off (end of lactation). Culture-positive animals would be removed to slaughter only, except heavily infected animals (TNTC on fecal culture), which would be rendered.
The goal of the NMPF initiative is to encourage voluntary testing of herds and the establishment of herd plans that incorporate Best Management Practices for JD control and eradication. An earlier program initiative proposed
additional funding for indemnity support to producers who remove fecal-culture-positive animals from their herds (NMPF, 2002, 2003).
State Programs
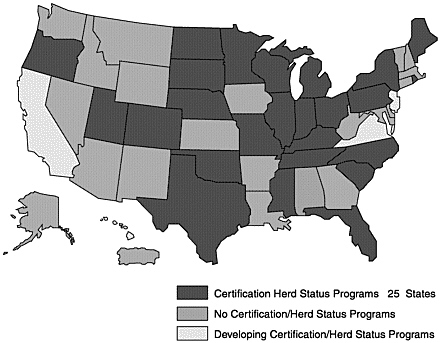
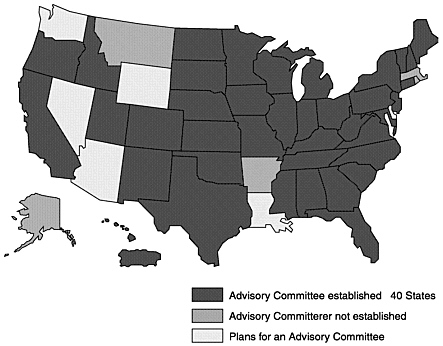
Many states have established, or are moving toward, JD programs that are compatible with national standards (Figures 4–1, 4–2, and 4–3; Table 4–8). A few states have JD programs that predate the national effort. Each program has some unique approaches to JD control.
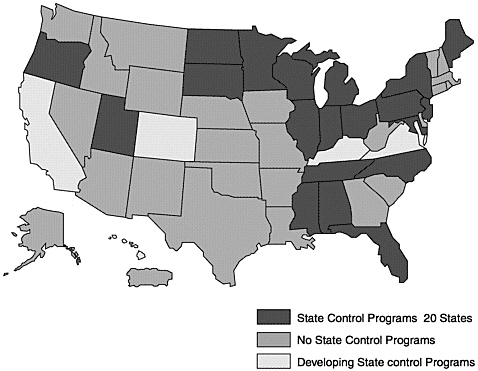
Figure 4–1. Johne’s Disease State Control Programs, January 2002
SOURCE: USDA, 2003a
New York
JD control programs are an integral part of the larger NYSCHAP, which is administered by the state’s Department of Agriculture and Markets and by the New York State Animal Diagnostic Laboratory. NYSCHAP enrolls herds in three categories of participation:
-
Participating herd A farm risk assessment is conducted and a herd plan outlines management procedures to reduce the risk of introducing Map to the farm or to prevent spreading of the organism to susceptible animals on the farm.
-
Control herd In addition to risk assessment and management procedures, producers of these herds include testing in the control plan.
-
Status herd Producers of herds that are negative on serum or fecal testing are encouraged to enter a herd status program that is similar to the national voluntary JD program. Special features of the New York program are the use of a kinetics-based enzyme-linked immunosorbent assay (KELA) that allows characterization of individual animal test results by level of risk for Map infection.
In 2001, the New York Animal Diagnostic Laboratory adopted a liquid-medium Trek system for fecal culture of Map, enabling a much reduced turn-around time (NYSCHAP, 2001). More than 400 New York herds are currently participating in some form of control program (Dr. John Huntley, New York State Department of Agriculture and Markets, personal communication, October 2001).
Pennsylvania
Pennsylvania has had JD control programs for more than 20 years. Early efforts were based on fecal-culture identification of infected animals with permanent quarantine or slaughter-with-indemnity of culture-positive animals. The effort in Pennsylvania includes three complementary programs:
-
Thirty Free Tests Program Any dairy or beef producer may request serum ELISA on 30 animals. Testing is done within the state laboratory system at no charge to the producer. Producers are encouraged to sample 30 randomly selected second-lactation or older animals, but they may choose any 30 animals. Test results are informational for the producer only, but herd owners are encouraged to enter their herds in one of the other state programs, based on the results of the 30 tests.
-
Voluntary Johne’s Disease Control Program A voluntary JD control program is available for herds with at least one animal with JD-positive ELISA or other test results. The program is consistent with USAHA-recommended program standards, and it includes a participation stage without testing and a control stage with testing.
-
Voluntary Johne’s Disease Status Program Pennsylvania has adopted the proposed national voluntary JD status program for test-negative herds (Pennsylvania Bureau of Animal Health and Diagnostic Services, 2000).
More than 30,000 animals from 1,100 herds have been tested through the Thirty Free Tests Program. About 500 herds are enrolled in management, control, or status programs. No indemnity is offered currently (Philip DeBok, Pennsylvania Department of Agriculture, personal communication, 2001).
Wisconsin
The current JD program in Wisconsin has been in place since July 2000. It is a voluntary program that encourages testing to establish a herd classification. Any cattle or goats offered for sale as breeding animals include an implied warranty that the animals are free of JD unless the seller offers, in writing, a Wisconsin Johne’s Disease Management classification. Herd classification is established through testing of “test eligible” animals (bulls 24 months of age or older, cows 36 months of age or older, goats 18 months of age or older). The following classifications have been established:
-
Entire eligible herd tested or test 30 eligible animals or 10 percent of the eligible animals, whichever is greater; no test positives
-
Entire eligible herd tested; fewer than 5 percent test positive
-
Entire eligible herd tested; 5–15 percent test positive
-
Entire eligible herd tested; more than 15 percent positive or 30 eligible animals or 10 percent of the herd tested; one or more positives
-
No testing done; maximum risk
Herd owners may elect one of three testing options:
-
Random herd test Thirty eligible animals or 10 percent of eligible animals, whichever is greater
-
Entire herd test All eligible animals tested at one time
-
Split herd test With a state Department of Agriculture approved plan, test all eligible animals over a period not to exceed one year
Testing may be by fecal culture or by serum ELISA in cattle, or by fecal culture in goats (Wisconsin Department of Agriculture, Trade and Consumer Protection, 2000).
Although considerable program experience has been gained through the New York, Pennsylvania, and Wisconsin programs, these and other programs have not been objectively evaluated and reported. Each of these programs has changed significantly over time, making it difficult to evaluate program effectiveness and impact. Collection and publication of this information would be extremely valuable in the formulation of a successful control strategy.
Table 4–8. Components of State Johne’s Disease Herd Status/Control Programs
Non-U.S. Programs
Few regional or national JD programs have been established outside the United States because of legal, political, and economic issues (Table 4–9). Long-term success among national programs is limited. Iceland has effectively controlled JD in sheep through a national vaccination program (Fridriksdottir et al., 2000). Australia has implemented market assurance programs that involve certification of assessed herds and flocks of cattle, sheep, goats, and alpaca (Allworth and Kennedy, 1999; Kennedy and Allworth, 1999). Specific areas of Australia have been regionalized since 1999, when Western Australia was declared disease-free for ovine and bovine JD (Kennedy and Benedictus, 2001). The Australian program is a partnership between government and seven affected industries that began modestly as an effort to assess the status of herds and flocks to identify test-negative sources. The program includes identification of test-positive herds and flocks, and there are mandatory restrictions on the sale or movement of animals from affected premises. Depopulation and restocking is being pushed at different rates by various Australian states.
Table 4–9. Components of Nationwide Johne’s Disease Herd Status/Control Programs
PRINCIPLES OF CONTROL IMPLEMENTATION
National-government-funded animal health programs typically are directed to exotic-disease exclusion and to the control or eradication of specific diseases of widely recognized economic or public-health importance. However, the emergence of the global food system and the expansion of trade in animals and animal products has increased national attention to diseases such as JD, that can affect national economic development by restricting international movement.
Despite the increased attention, the control and eradication of JD from beef and dairy cattle, sheep, and goats face formidable challenges, in part because of low awareness among producers, but also because of the insidious nature of infectious diseases. Despite the availability of adequate diagnostic tests and the increasing availability of disease-free replacement stock, there is still room for significant improvement in both areas. The economic pressure for increasing herd and flock sizes in U.S. commercial animal agriculture contributes to the spread of disease, as infected replacement animals are added to existing herds.
Regional or national coordination of prevention and control programs is critical for effectively addressing these challenges. The responsibility for initiating, coordinating, and funding regional or national animal health programs can rest on government or industry, or on a partnership between the two. Existing programs rely on varying combinations of entities, and they have emphasized the importance of a coordinated response, regardless of which agency has primary responsibility for the program. There is consistent recognition that the involvement of producers is critical to program success. An integral education component that leads to on-farm risk assessment and development of herd management plan is essential for success. Some programs rely on market-based enrollment incentives and disincentives, but other ways to engage producers also have been developed—subsidies for testing and indemnification, for example. Another possibility for promoting producer involvement is through the incorporation of Best Management Practices for a wider range of fecal pathogens, but this concept has not been incorporated into current programs. Additional research on JD and control principles and practices, and the promotion of scientifically sound practices—such as the use of likelihood ratios for interpreting ELISA results—also are important elements that are not considered in some current programs.
Regulatory elements—restricting animal movement from test-positive herds, requiring laboratory proficiency testing, and program audits with review and correction mechanisms—have been introduced to bolster the effectiveness of JD control and eradication programs. Clearly, another effective step toward eradication will be to gradually make compliance mandatory. Although there is no current mandatory national JD control or eradication program, federal authorities have one additional but limited tool to control the spread of JD: there are regulations that control interstate transport of clinically affected animals. Historically, the federal government has mandated that animals and animal products that are transported from one state to another must be accompanied by a certificate of veterinary inspection identifying the animals, attesting to animal health, and documenting relevant diagnostic test results. The federal government establishes minimum animal health requirements for interstate movement, including the requirement that animals be certified by a federally recognized veterinarian as free of any visible signs of infectious or communicable disease. This is of little benefit in controlling the spread of JD however, because most Map-infected animals are subclinically affected. Cachectic animals or animals that exhibit clinical diarrheal disease would not be certifiable for interstate movement, except directly to slaughter. Individual states require additional tests or certification regarding specific diseases. In 2000, the USDA Animal and Plant Health Inspection Service Veterinary Service promulgated rules that prohibit the interstate movement of animals known to be test positive to an officially recognized JD test, unless the animals are moving directly to slaughter, however, testing of those animals prior to movement is not required.