APPENDIX E Functional and Organic Hybrid Materials
ORGANIC BUILDING BLOCKS FOR CHROMOPHORE STRUCTURE AND PHOTOREFRACTIVE COMPOSITE MATERIALS
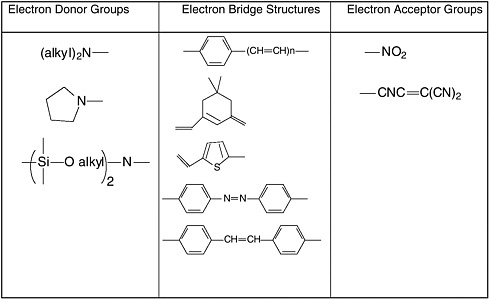
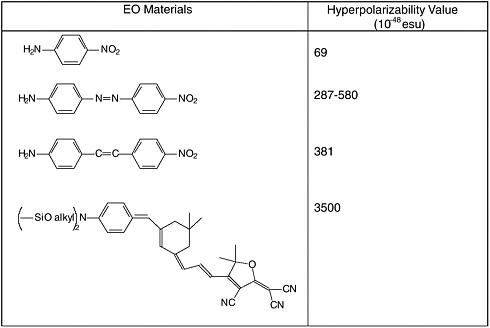
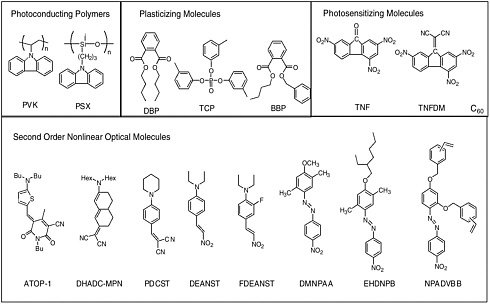
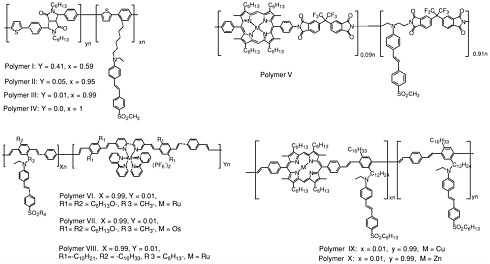
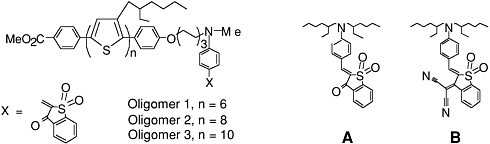
Common electron donor, π electron bridge, and electron acceptor functional groups are shown in Figure E-1, and a very small sample of typical electro-optic (EO) chromophore structures and their mb values are given in Figure E-2. Figure E-3 shows polymers and various molecular components used in preparing photorefractive composite materials.
SCHEMES FOR PHOTOREFRACTIVE POLYMERS AND ORGANIC MATERIALS
Photorefractive (PR) polymers and organic materials can be produced through several different approaches, as discussed in Chapter 6. Examples of two current approaches are shown in the next two figures. Figure E-4 shows several functional polymers reported to exhibit PR effects including functional polyurethanes, functional conjugated polymers, functional polyimides, and conjugated polymers containing transition metal complexes. Figure E-5 shows several amorphous molecular materials exhibiting high PR performances.
USING MOLECULES AS SWITCHES
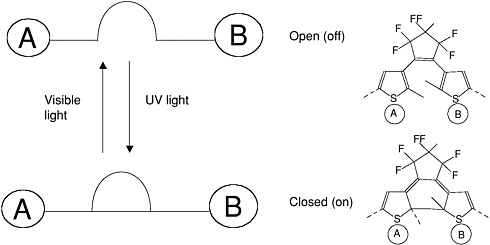
Examples of simple molecular switches are shown in the next two figures. The photochromic molecule in Figure E-6 can undergo a photo-induced intramolecular change in its molecular
orbital structure that favors or reduces electron transfer depending upon its final molecular configuration (Fraysse et al., 2000).
The supermolecular structure rotaxane, shown in Figure E-7, is reported to have mechanical switching capabilities. In a rotaxane structure a macromolecular ring (analogous to a bead) encircles a linear molecular chain (thread) that is terminated by bulky stopper groups that prevent the ring from disengaging. The linear molecular chain also contains polar/ nonpolar regions or docking stations that control the transport process. In the example shown in Figure E-7, the macromolecular ring consists of two positively charged bipyridinium groups separated by p-xylyl groups. The linear chain consists of flexible polyether groups segmented between benzidene and biphenol electron donating groups. Migration (switching) of the ring between the benzidine or biphenol stations depends upon temperature and oxidation reduction (Bissell et al., 1994).
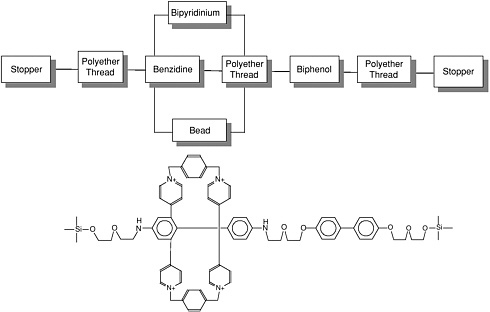
FIGURE E-7
Bead-on-a-thread molecular switch. SOURCE: Reprinted with permission from Bissell et al. (1994). Copyright 1994 by Macmillan Magazines Limited.
MULTICOMPONENT ORGANIC MATERIALS FOR ENHANCED CARRIER MOBILITY
Multicomponent organic materials can enhance carrier mobility by providing multiple absorption pathways and high interfacial areas between electron and hole transport regions, providing a mechanism for exciton dissociation. One approach is shown schematically in Figure E-8.
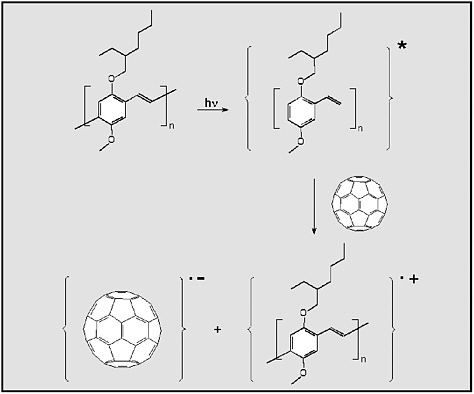
FIGURE E-8
The photo-induced electron transfer from a conjugated polymer (MEH-PPV) to C60. First, the conjugated polymer absorbs a photon of light and becomes electronically excited; second, this excited molecule transfers an electron to a nearby C60 molecule within 3 × 10–13 seconds. The result is a radical-cation/radical-anion pair. SOURCE: Reprinted with permission from Yu et al. (1995). Copyright 1995 by the American Association for the Advancement of Science.
REFERENCES
Bissell, R.A., E. Cordova, A.E. Kaifer, and J.F. Stoddart. 1994. Chemically and electrochemically switchable molecular shuttle. Nature 369:133-137 [see also Borman, S. 1994. Reversible molecular switch synthesized. Chem. Eng. News, May 16, pp. 8-9, and Borman, S. 1999. Key step made toward molecular computing. Chem. Eng. News 77(29):11-12].
Fraysse, S., C. Coudret, and J.-P. Launay. 2000. Synthesis and properties of dinuclear complexes with a photochromic bridge: An intervalence electron transfer switching “on and off.”Eur. J. Inorg. Chem. 7:1581-1590.
Yu, G., J. Gao, J.C. Hummelen, F. Wudl, and A.J. Heeger. 1995. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 270:1789.