5
Hormonal Mediation of Physiological and Behavioral Processes That Influence Fertility
Judy L. Cameron
This chapter reviews the complex and diverse roles that hormones play in mediating the physiological and behavioral processes that influence human fertility. Much of the focus is on hormones of the reproductive axis, which mediate the physiological processes governing fertility and provide powerful modulation of sexual behavior. Secretion of these hormones changes over the life span. Reproductive hormones are secreted in surprisingly high levels in prenatal development and at this time help set the stage for later development of normal reproductive physiology and behavior in adulthood. There is then a period of childhood quiescence, when the reproductive axis is essentially “turned off”, followed by a cascade of hormonal changes that occur with puberty. In males, reproductive hormone secretion is rather stable in the adult years, with a slow decline in levels occurring with aging. In contrast, much greater fluxes in hormone secretion occur throughout adulthood in women, with large changes in hormone secretion occurring over the course of each menstrual cycle, followed by a period of irregular hormone secretion during the transition to menopause and ultimately a marked decline in reproductive hormone levels in the postmenopausal period. Understanding the changes in reproductive hormone secretion across the life span has implications for the design of biodemographic studies with regard to how and when reproductive function and sexual behavior are assessed and understanding the factors that influence these measures.
Many lifestyle choices and life events can modulate activity of the reproductive axis and thus impact significantly on both reproductive physiology and behavior. In the modern world, pharmacological modulation of repro-
ductive hormone levels is common. Large numbers of women take exogenous hormones in the form of contraceptives, and even greater numbers of women are given estrogen replacement therapy in the postmenopausal period. A more limited but rapidly expanding subset of individuals consume steroid hormones to regulate body strength and endurance, particularly individuals who participate in competitive sports. And with the more widespread consumption of foods that contain phytoestrogens, environmental exposure to hormones is becoming an issue of greater importance.
A variety of events that occur over the course of a normal life can also significantly influence activity of the reproductive axis. Pregnancy and lactation are associated with profound changes in the functioning of the reproductive axis, fertility, sexual behavior, and maternal behavior. Common life stresses, including metabolic stresses associated with undernutrition or the increased energy expenditure of participation in chronic vigorous exercise can suppress the activity of the reproductive axis. And psychosocial stresses provide an even more common inhibition to the reproductive axis. Even if reproductive hormone secretion is maintained, these life events can markedly alter circulating levels of reproductive hormones and thus influence fertility and sexual behavior. Biodemographic studies need to track lifestyle choices and life events to allow an accurate conceptualization of factors influencing fertility outcomes in human relationships.
Lastly, it is important to keep in mind that there are dramatic individual differences in normal circulating levels of reproductive hormones, the amount of hormone needed to maintain normal reproductive physiology and sexual behavior, and the sensitivity of individuals to the various forms of stress-induced reproductive dysfunction. We are just beginning the daunting task of elucidating the systems in the brain that underlie these individual differences. However, it is likely that the task of understanding the role that individual differences play in contributing to fertility outcomes will be even more complex.
HORMONES INFLUENCING REPRODUCTIVE FUNCTION AND BEHAVIOR
Physiological Regulation by Reproductive Hormones
This section provides an overview of the hormones that comprise the reproductive axis, how they are regulated and secreted, and their physiological actions in the body (for more detailed information see Steiner and Cameron, 1989; and Griffin and Ojeda, 2000). Particular attention is given to issues that influence the types of measurements made in the field of biodemography.
Although many people think of reproductive function as a bodily func-
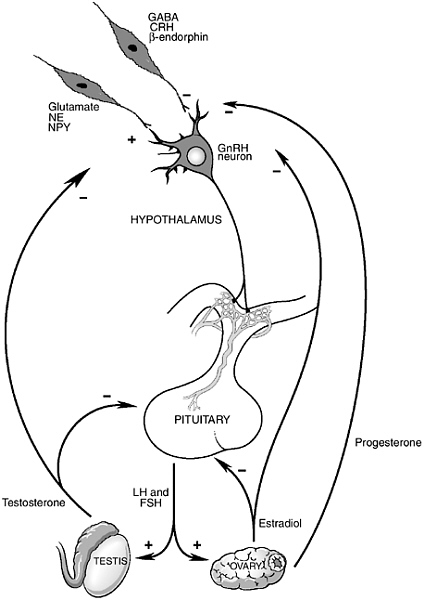
tion governed by endocrine organs of the pelvis, testes, and ovaries, specialized neurons in the brain and hormones secreted by the “master endocrine organ,” the pituitary, located just beneath the brain, play critical roles in governing reproductive function (see Figure 5-1). Not only do the brain and pituitary coordinate and provide the “central drive” to the reproductive axis throughout life, the brain is also the primary site where environmental factors that modulate reproductive function act.
The region of the brain involved in the regulation of reproductive function as well as many of the body’s other basic homeostatic functions (i.e., control of food intake, growth, response to stress, water balance, metabolic rate) is the hypothalamus. The hypothalamus sits at the base of the brain and is connected by a specialized portal blood system to the pituitary, just below. A population of specialized neurons in the hypothalamus produce the neurotransmitter, gonadotropin-releasing hormone (or GnRH, named for its ability to release the hormones in the pituitary that provide trophic support to the ovaries and testes—the gonadotropins— luteinizing hormone, LH and follicle-stimulating hormone, FSH). GnRH travels via the portal capillaries to the anterior pituitary, where it stimulates the synthesis and release of the pituitary hormones, LH and FSH.
Many neurotransmitter systems from the brainstem, limbic system, and other areas of the hypothalamus convey information to GnRH neurons (Kordon et al., 1994). These afferent systems include neurons that contain neurotransmitters that are generally stimulatory to GnRH neurons, such as norepinephrine, dopamine, serotonin, glutamate, neuropeptide Y, and galanin, as well as neurotransmitters that are generally inhibitory to GnRH neurons, such as gamma aminobutyric acid (GABA), endogenous opiate peptides, and the central hypothalamic hormone that governs the adrenal axis, corticotropin-releasing hormone (CRH). Importantly, both in normal physiological conditions and in response to environmental signals (such as changes in nutrition, exercise, and psychosocial stress) the activity of the reproductive axis is changed by modulation of the neural inputs into GnRH neurons. For example, various forms of stress can lead to a suppression of reproductive function by acting to increase inhibitory drive to GnRH neurons by increasing either ß-endorphin or CRH input into the GnRH neuronal system (Feng et al., 1991; Norman and Smith, 1992). Decreased firing of GnRH neurons leads to less GnRH stimulation of pituitary LH and FSH release and thus less stimulation of ovarian and testicular function. It is also important to understand that changes in neuronal function in a number of neurological and psychiatric diseases can be associated with alterations in both reproductive physiology and behavior. For example, changes in both reproductive function and sexual behavior are commonly reported by patients suffering from depression, anxiety disorders, and obsessive-compulsive disorders (Clayton, 2002; Shabsigh et al., 2001). The
drugs used to treat these disorders have the potential to impact reproductive function because they can affect neural input into GnRH neurons as well as treat neurotransmitter imbalances in higher cortical areas (Clayton, 2002; Montgomery et al., 2002).
GnRH is a small 10 amino acid peptide that is rapidly metabolized, so although it reaches the pituitary in adequate concentrations to stimulate the synthesis and secretion of LH and FSH, it is usually not detectable in the peripheral circulation. However, GnRH can be given systemically to stimulate activity of the reproductive axis in a number of situations where reproductive function is suppressed. In such circumstances it is important to provide GnRH in a “pulsatile” fashion (a pulse every 1 to 3 hours), in that continuous administration of GnRH will down-regulate pituitary GnRH receptors and lead to a suppression of pituitary LH and FSH release, rather than a stimulation of release (Belchetz et al., 1978). However, as one can imagine, administration of pulses of GnRH is difficult and inconvenient, usually requiring the patient to have an in-dwelling subcutaneous catheter and wear an electronic pump. Thus, restoration of normal reproductive function in individuals in which normal activity of the reproductive axis is compromised can be a fairly arduous undertaking. The converse situation, where the reproductive axis is active at an inappropriate time, such as in cases of precocious puberty, is much more easily resolved by giving a long-acting GnRH-analog (many orally active forms are available) that will provide continuous activity at the pituitary, down regulate GnRH receptors, and thus essentially shut off pituitary LH and FSH synthesis and secretion and all functions of the reproductive axis downstream from the pituitary (Moghissi, 1990).
LH and FSH are glycoprotein hormones originally named for their action at the level of the ovary in the female, but the same hormones are produced in the male and govern testicular function (Griffin and Ojeda, 2000; Steiner and Cameron, 1989). The gonadotropins are released into the peripheral bloodstream and act at cells that have specific LH and FSH receptors, primarily at the gonads. In the male, LH binds to testicular cells (Leydig cells) and stimulates the synthesis and secretion of testosterone. FSH binds to Sertoli cells in the seminiferous tubules and along with testosterone stimulates the process of spermatogenesis. In the female, FSH acts on ovarian follicles to stimulate their growth and the production of estrogen. LH acts on the fully developed follicle to stimulate ovulation and then to support the function of the transient endocrine tissue formed during the last 2 weeks of each menstrual cycle, the corpus luteum (see Figure 5-2 for an overview of hormonal changes during the female menstrual cycle). The corpus luteum secretes both estrogen and progesterone, which play a critical role in preparing the uterine endometrium for implantation of a developing embryo should fertilization occur. Not surprisingly, in that both LH
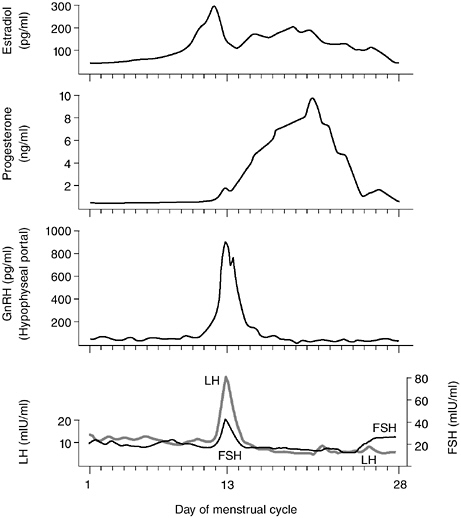
FIGURE 5-2 Diagrammatic representation of changes in plasma levels of estradiol, progesterone, LH, FSH, and portal levels of GnRH over the human menstrual cycle.
and FSH secretion are stimulated by GnRH, both hormones are released into the bloodstream in a pulsatile manner, at rates of about one pulse every 2 to 3 hours in males and at rates that vary in females from one pulse every hour to one pulse every 8-12 hours at various stages of the menstrual cycle (Soules et al., 1984; see Figure 5-3). The pulsatile nature of LH and FSH secretion can be a confound when hormone measures are collected as part of large population studies, in that a single blood sample may be collected when hormone levels are at the peak or nadir of a pulse; thus, variation
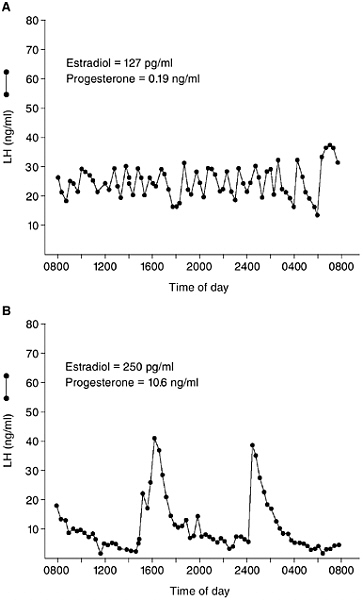
FIGURE 5-3 Examples of the pulsatile pattern of LH secretion in a woman during the late follicular phase (A) and midluteal phase (B) of the menstrual cycle. Steroid hormone levels on the day of each study are indicated on each graph. Note the dramatic slowing of pulsatile LH secretion as a result of gonadal steroid hormone negative feedback during the luteal phase. (Redrawn from Soules et al., 1984.)
within an individual can be great, making it difficult to detect group differences or changes in hormone levels in response to environmental or social conditions.
The gonadal steroid hormones are produced in a common synthetic pathway, all of them derived from the same precursor, cholesterol. Although androgens are commonly thought of as male hormones, they are produced in both the male and the female, and likewise the female hormone, estrogen, is present in the male and the female. In males, testosterone produced by the Leydig cells of the testes can act at its target tissues by binding to testosterone receptors, or first being converted to a more potent androgen, dihydrotestosterone, by the enzyme 5 α-reductase, or by being converted to estrogen by the enzyme aromatase and acting by binding to estrogen receptors. In females the pathway for estradiol production involves an intermediate step of androgen production, and thus the ovary is a source of low levels of androgens, principally androstenedione. The body produces three forms of estrogen: estradiol, which is the principal form of estrogen produced by the ovary; estrone; and estriol, which is produced predominantly by aromatization of androgens in peripheral fat tissue. Estriol production is thus related to body fat composition and is an important source of estrogen in the postmenopausal woman, once production of estradiol by the ovaries has ceased. Steroid hormones primarily travel through the bloodstream bound to proteins (>70 percent bound). In conditions where there is a change in the concentration of binding proteins in the circulation (i.e., with long-term changes in nutritional status, changes in either the level of energy availability or the ratio of protein to carbohydrate consumed; pregnancy, liver disease), the amount of steroid hormone in the circulation and its delivery to tissues are also affected. Assay procedures are generally available to measure both free and total (free + protein bound) steroid hormone concentrations in the blood, and it is important to distinguish between these.
The gonadal steroid hormones have important actions in a number of reproductive tissues. In the male, testosterone acts to stimulate development of male secondary sexual characteristics, including enlargement of the penis and testes, increased muscle mass, deepening of the voice, and stimulation of adult hair growth patterns. In the ovaries, estrogen acts to stimulate proliferation of follicular cells and maturation of the oocyte, preparing it for ovulation. At the uterus, estrogen acts during both the follicular and luteal phases of the menstrual cycle to stimulate development of the uterine lining and prepare it for implantation of a fertilized ovum. During the late follicular phase, rising levels of estrogen also act on the cervical mucosa to stimulate the elaboration of a thin, watery mucus that is amenable to sperm penetration. At the breast, estrogen stimulates development at puberty and further development during pregnancy and plays an important role in stimu-
lating milk production during lactation. Estrogen receptors are also found in many other organs, including bone, pancreas, fat, and blood vessels.
Progesterone is secreted in large quantities during the last 2 weeks of each menstrual cycle. Under the influence of progesterone, the uterine glands in the endometrium enter a secretory phase and produce large amounts of glycogen, which provides nutritional support for early development of an embryo. Withdrawal of progesterone at the end of the luteal phase leads to shedding of the uterine endometrium and menses, which marks the termination of one menstrual cycle and the initiation of the next cycle. Progesterone also acts at the cervix to thicken cervical mucus, making it hostile to sperm penetration, and at the breast in late pregnancy working in concert with estrogen to prepare for lactation. All three gonadal steroid hormones also act at receptors in the brain, as will be discussed in more detail in the section below. One of the actions of these hormones in the brain is to provide feedback regulation to the hypothalamic GnRH neurons and the pituitary gonadotropin-secreting cells.
Steroid hormone secretion is relatively stable in the adult years in males, although it must be remembered that the gonadotropins and testosterone are secreted in a pulsatile fashion. However, in the female there are marked changes in the circulating concentrations of gonadotropins and gonadal steroid hormones across the menstrual cycle (Erickson, 1978; Figure 5-2). The menstrual cycle is commonly divided into two phases, each of which is approximately 2 weeks in length. The first 2 weeks constitute the follicular phase. During this time small groups of ovarian follicles, each of which is a layer of cells surrounding an ovum, are developing and maturing, and as they do so under the trophic influence of FSH and LH, they secrete increasing concentrations of estradiol. Thus, over this 2-week time span, estradiol levels are very low during the first week and then increase exponentially in the second week. The rising secretion of estradiol by a fully developed follicle provides a positive feedback signal to the brain and pituitary, resulting in a massive release of LH and FSH at midcycle, and this “surge” of gonadotropins triggers ovulation, so that the mature follicle bursts and the ovum is released into the nearby fallopian tube and can travel to the uterus. The follicular cells that surrounded the developing ovum reorganize into a transient endocrine tissue, the corpus luteum, which produces both estradiol and progesterone in the last 2 weeks of the cycle, the luteal phase. Unless pregnancy occurs, the corpus luteum spontaneously regresses after about 2 weeks, and the withdrawal of progesterone support to the uterine lining leads to menstruation, which marks the beginning of a new cycle. Population studies that track reproductive hormone secretion must take these rather marked cyclic fluctuations in hormone levels into account in order to adequately examine how changes in hormone secretion in females
of reproductive age are linked to fertility outcomes, behavior, or environmental conditions.
Measurement of reproductive hormone levels in large field studies can be a challenge. Many of these studies are conducted at some distance from medical or laboratory facilities, where collection of blood samples, centrifugation of the samples to collect plasma, and immediate transfer to frozen storage to prevent deterioration of hormones are not possible. Fortunately, considerable advances have been made in the last decade in the development of techniques for measuring reproductive hormone levels in more easily obtainable body—fluids, saliva and urine (Campbell, 1994; Ellison, 1994; Lasley et al., 1994; Lasley and Shidleler, 1994). Improvement of the sensitivity of assay methods makes it possible to detect the low levels of hormones that are present in these fluids (Clough et al., 1992; Ellison, 1988; Stanczyk et al., 1980;). Moreover, development of collection and storage techniques that can be utilized in remote areas of the world (Lipson and Ellison, 1989; Young and Bermes, 1986) has facilitated the study of the relationship between activity of the reproductive axis and many other parameters measured in demographic population studies. Salivary samples can reflect acute changes in plasma hormone levels, while urinary measures provide an integrated assessment of steroid hormone secretion over a number of hours. Salivary samples are useful for detection of gonadal steroid hormones. Salivary steroid hormone levels reflect the levels of free hormone present in the blood (i.e., steroid that is not bound to plasma proteins). Saliva can be easily collected at frequent intervals and can be stored at room temperature for several weeks without significant deterioration of hormones. However, it is not useful for measurement of the gonadotropins, LH and FSH, and will not provide an index of changes in plasma protein levels that may be responsible for changes in free steroid hormone concentrations. Gonadotropin metabolites, as well as steroid hormones, can be measured in urine samples. And urine is particularly useful for the early detection of human chorionic gonadotropin, a placental hormone that serves as a useful indicator of early pregnancy (Canfield and O’Connor, 1991).
Behavioral Regulation by Reproductive Hormones
Sexual behavior can be divided into distinct aspects, in both males and females, which include attractiveness, sexual desire, arousal, orgasm, and reinitiation. Here we will not focus on detailed information about how each of these sexual behaviors is influenced by hormones but rather on two broad areas—sexual desire and sexual behavior. There is evidence that most aspects of sexual behavior, particularly in males, are influenced by gonadal steroid hormones. Steroid hormone receptors are abundant in the brain. Classical estrogen receptors (now called estrogen α-receptors) are
strongly concentrated in the hypothalamus but are also found in areas of the brain with strong connections to the hypothalamus (Simerly et al., 1990). More recently, a second form of estrogen receptor (estrogen ß-receptors) was identified and found to be present throughout the rostralcaudal extent of the brain, including the cerebral cortex (Shughrue et al., 1997). Specific receptors for progesterone are induced by estrogen in hypothalamic regions of the brain, and there is also some evidence for constitutive expression of progesterone receptors (Bethea et al., 1992). Androgen receptor mapping studies have shown considerable overlap in the distribution of androgen and estrogen receptors throughout the brain (Michael et al., 1995; Simerly et al., 1990). Our discussion here will focus on the effects of gonadal steroid hormones on sexual behaviors, although there is evidence that they modulate a variety of other behaviors (Cameron, 2001).
Recognition of an important link between sexual behavior and hormones arose originally from the finding that castration of adult males often results in diminished sexual activity and erectile difficulty (Luttge, 1971). In hypogonadal or castrated men, withdrawal of testosterone has been reported to result in a rapid decrease in sexual interest and activity that is reinstated with testosterone replacement (Davidson et al., 1982; Kwan et al., 1983). There are similar findings in nonhuman primate species, such that as the breeding season comes to an end and the annual cycle of testicular regression occurs, male sexual activity falls off sharply (Gordon et al., 1976). However, there is also clear evidence of tremendous variability among individuals in the rate of loss of sexual activity and the degree of diminution of sexual activity with loss of testosterone. The 1959 study by Bremer followed 244 men castrated for medical reasons and found that a third of them retained sexual interest and activity for over a year, some for up to 10 years. Similarly, in male macaques castration has been associated with a gradual reduction but not an elimination of male sexual behavior (Michael and Wilson, 1974; Phoenix et al., 1973). In normal men there is no correlation between testosterone levels and individual differences in sexual desire or behavior (Schiavi and White, 1976). This finding supports the concept that there is a threshold for testosterone actions on sexual behavior in males over which no further effects of testosterone are apparent (Meston and Frohlich, 2000). Studies in macaques suggest that other social factors interact with circulating testosterone concentrations to impact on sexual behavior. Wallen (1999) showed that suppression of testicular hormones decreased sexual activity in low-ranking male monkeys but that sexual behavior in high-ranking males was not measurably affected.
In women the factors regulating sexual desire and activity, and the role that hormones play in this regard, are even less well understood. Studies of surgical ovariectomy generally report that these women have a decrease in sexual desire from presurgical levels (Dennerstein et al., 1977; Lieblum et
al., 1983). Both estrogen therapy and androgen therapy have been shown to have some effect on the restoration of sexual behavior after surgical ovariectomy (Lieblum et al., 1983; Sherwin et al., 1985). Estrogen replacement in ovariectomized female monkeys has been shown to increase female initiation of sexual behavior (Zehr et al., 1998). At menopause, when there is a naturally occurring decrease in reproductive hormone levels (both estrogens and androgens), a decrease in sexual desire has been generally reported (McCoy and Davidson, 1985). There have been several reports of a general correlation between androgen levels and sexual interest in post-menopausal women (Lieblum et al., 1983; McCoy and Davidson, 1985), but these studies have not determined whether fluctuations in androgen levels in individuals correlate with changes in sexual interest. Estrogen treatment can also lead to an increase in sexual activity in postmenopausal women, but it is difficult to determine if this is an action at the level of the brain or is secondary to increased comfort with intercourse due to increased lubrication of the vagina (Sherwin, 1991). Studies looking for correlations in premenopausal females with circulating levels of androgens and estrogens have been somewhat confusing.
In macaques there is a clear increase in sexual behavior in midcycle (Goy, 1979; Wallen, 1990). Although this could be due to an increase in attractiveness of females to males as estrogen levels rise (Czaja et al., 1977), there is evidence that, when the male’s mobility is limited and the female can control proximity, there is a cyclic increase in the female’s approach to males (Czaja and Bielert, 1975; Pomerantz and Goy, 1983), and females will work harder on an operant task to gain access to males at midcycle (Bonsall et al., 1978). However, whether these cyclic changes in sexual interest are governed by estrogens or androgens is difficult to determine because they rise in concert, androgens being a precursor to the ovarian synthesis of estrogen. A study by Zehr et al. (1998) showing that estrogen replacement alone to ovariectomized female monkeys can stimulate female sexual initiation and earlier studies showing that in the normal menstrual cycle changes in circulating estradiol but not androgen correlate with changes in female sexual initiation (Wallen et al., 1986) support a role for estrogen in governing female sexual behavior.
Several studies in ovariectomized, estrogen-treated monkeys have suggested that adrenal androgens may play a role in modulating female sexual behavior (Baum et al., 1977; Everitt et al., 1972), but such an effect has not been seen in ovary-intact macaques (Lovejoy and Wallen, 1990). Moreover, Wallen et al. (1986) found that ovarian suppression eliminated female sexual initiation, even though the adrenals were intact and functioning. As reviewed by Wallen (2001), hormonal influences on women’s sexuality have been difficult to demonstrate and to interpret due to unwillingness of subjects to be sampled either physiologically or behaviorally and strong
influences of male partners on women’s sexual activity. However, there is evidence for changes in the level of female sexual desire in women with a peak at midcycle (Bancroft et al., 1983; Dennerstein et al., 1994; Stanislaw and Rice, 1988; Van Goozen et al., 1997). Thus, although hormones are not necessary for female sexual behavior, there is accumulating evidence that hormones modulate sexual desire (Meston and Frolich, 2000; Wallen, 2001). Further studies are needed to understand the differential roles of estrogens and androgens in this regard. As discussed for the male, there is also evidence that social factors interact with hormonal influences with regard to sexual behavior in females. Adams et al. (1997) showed that women who used less reliable contraceptives showed less pronounced midcycle increases in heterosexual initiation, but much greater midcycle increases in autosexual behavior.
HORMONAL CHANGES THROUGH THE LIFE SPAN
Early-Life Activation of the Reproductive Axis
Early in embryonic development the components of the reproductive axis are formed and become functional. GnRH neurons, which provide the central drive to the reproductive axis, are an unusual neuronal population in that they originate from outside the central nervous system, coming originally from the epithelial tissue of the nasal placode (Schwanzel-Fukuda and Pfaff, 1989; Wray et al., 1989). During embryonic development, GnRH neurons migrate across the surface of the brain into the hypothalamus. Migration is dependent on a scaffolding of neurons and glial cells along which the GnRH neurons move, with chemical signals guiding the process (Silverman et al., 1994). Failure of GnRH neurons to properly migrate leads to a clinical condition, Kallman’s syndrome, in which GnRH neuroendocrine neurons do not reach their final destination and thus do not stimulate pituitary gonadotropin secretion (Schwanzel-Fukuda et al., 1989). Patients with Kallman’s syndrome do not spontaneously enter puberty. Administration of exogenous GnRH effectively treats this form of hypothalamic hypogonadism, although, as discussed above, this requires pulsatile administration of GnRH.
Functional activity of the reproductive axis as a whole is initiated during fetal development, and surprisingly by midgestation circulating levels of LH and FSH reach values similar to those found in adulthood (Ellinwood and Resko, 1984; Kaplan et al., 1976). Later in gestational development the gonadotropin levels decline, restrained by rising levels of circulating gonadal steroids (Kaplan et al., 1976; Resko and Ellinwood, 1985). The steroids having this effect are likely placental in origin, in that following parturition there is a rise in circulating gonadotropin levels that is apparent
for the first 12 to 18 months of life (Winter et al., 1975). The decline in reproductive hormone secretion between ages one and two appears to be due to a decrease in GnRH stimulation of the reproductive axis. This decrease occurs even in agonadal individuals, and the period of elevated gonadotropin and gonadal steroid secretion can be extended by treating with administration of pulses of GnRH (Plant, 2001).
Steroid hormone secretion has effects on both primary sexual differences between males and females (i.e., differentiation of the sexual organs) and the development of secondary sexual differences (i.e., body fat distribution, muscle development, breast development, differences in hair distribution; Cooke et al., 1998; Cameron, 2001). In the case of sexual differentiation of the body, it is clear that exposure of males to various testicular secretory products, especially testosterone, during early prenatal development leads to sexual differentiation of the internal and external genitalia. Later activation of the reproductive axis at puberty, with a sustained increase in circulating testosterone then leads to the development of secondary sexual characteristics. Thus, testosterone has both “organizational” and “activational” influences on the sexual differentiation of the body. The organizational effects of gonadal hormones are conceptualized as resulting from the early influence of gonadal hormones on structural development, which do not require continued hormone exposure to maintain sexual differentiation. Activational effects are conceptualized as later stimulation of reversible influences on sexual differentiation that require continued exposure to gonadal hormones to maintain sex differences.
The concept that sex steroid hormones have important and permanent organizational effects on the developing brain was originally postulated based on experimental findings that treatment of developing mice with testosterone produced permanent effects on reproductive capacity (Barraclough and Leathem, 1954), with early treatment with testosterone blocking later activation of ovulation by estradiol. A similar coordination of early and later influences of gonadal steroid hormones on reproductive behavior also occurs in many species studied to date (Cooke et al., 1998; MacLusky and Naftolin, 1981; Phoenix et al., 1959). In general, exposure of males to testicular hormones during prenatal and early postnatal periods leads to both masculinization of some tissues and functions (i.e., masculine changes in genital structure, copulatory behavior, and other behaviors characteristic of males) and defeminization of other tissues and functions (i.e., ovulatory competence, feminine sexual behaviors like lordosis, and other behaviors characteristic of females). In rodents the critical period for steroid-hormone-mediated organization of brain regions and sexually dimorphic behaviors appears to be postnatal, with most effects occurring during the first 10 days of life. In primates, sexual differentiation of the brain
occurs prenatally, over an extended period in midgestation (Phoenix et al., 1968). There is also recent evidence in male macaques that neonatal exposure to testosterone may play a role in determining the extent of adult sexual behavior (Mann et al., 1998).
Pubertal Activation of the Reproductive Axis
Pubertal reawakening of the reproductive axis occurs in late childhood and is marked by a cascade of hormonal, physical, psychological, and behavioral changes. One of the earliest signs of puberty is an elevation of gonadotropin and gonadal steroid hormone levels specifically at night (Boyar et al., 1974), although because detection of this rise requires collecting blood samples at night it is virtually never examined in demographic studies. Investigation into the mechanisms controlling the pubertal reawakening of the GnRH pulse generator has been an area of intense investigation for the past two decades (Ojeda and Bilger, 2000; Plant, 2001). Although the mechanisms are not fully understood, significant progress has been made in identifying central changes in the hypothalamus that appear to play a role in this process. There is strong evidence of both increases in stimulatory neural input into GnRH neurons and decreases in inhibitory input. Despite an increased understanding of the neural changes occurring at puberty, the question of what signals trigger the pubertal awakening of the reproductive axis is unanswered at this time. Availability of food and nutritional status have been shown to affect the timing of puberty; however, these signals appear to be only modulators of the pubertal process in that puberty can only be moderately advanced by increasing food availability (Frisch and MacArthur, 1974). Whether there is a genetic timing mechanism that regulates puberty or whether other signals from the body are responsible for timing the reactivation of the reproductive axis awaits further research.
Changes in body habitus are the first signs of puberty detected by most individuals, although these emanate from increased levels of gonadal steroid hormones and are thus relatively late events in the reawakening of the reproductive axis. Likewise, in girls, menarche is a very late event, heralding the point where the adult cyclic interplay between the hypothalamic-pituitary-ovarian axis is initiated. As described in the section above, the increase in testosterone at puberty in males leads to development of the secondary sexual characteristics, including increased growth of facial, axillary, and pubic hair; deepening of the voice; increase in muscle mass; enlargement of the testes and penis; increased incidence of erections and ejaculations; and attainment of fertility. Sexual behavior is also dramatically increased in males at puberty, and there is a strong correlation at this
age between plasma testosterone levels and degree of sexual desire and sexual activity (Halpern et al., 1998; Udry et al., 1985).
In females the rising tide of estrogen at puberty is the primary stimulus for development of most of the secondary sexual characteristics, including breast development, widening of the hips, and increased deposition of subcutaneous body fat. The growth of axillary and pubic hair is under androgenic control and is stimulated by an increase in dehydroepiandrosterone (DHEA) from the adrenal gland. This developmental increase in DHEA is referred to as adrenarche and generally precedes puberty by several months to several years. Increases in sexual desire and sexual behavior also occur in girls at puberty. And, as in adulthood, there is controversy as to which hormones may be mediating these increases. There are correlations between androgen levels in adolescent girls and sexual interest (Udry et al., 1986) and the initiation of coitus (Halpern et al., 1997). However, the role of estrogens, which tend to covary with androgens in females, has not been adequately examined. Moreover, there is evidence that social factors can be as important as hormonal factors in determining a girl’s sexual behavior (Udry et al., 1986).
Changes with Aging
In males, testosterone levels decrease slightly with aging and there is a mean decrease in sexual behavior, although again there are large individual variations (Meston and Frohlich, 2000). Although there have been a number of reports showing an improvement in libido and erectile function in older men with testosterone and dihydrotestosterone treatment (Bain, 2001; Kunelius et al., 2002; Morley, 2001; Nolten, 2000), these have been uncontrolled open studies, with no large-scale multicenter prospective studies performed to date. Moreover, although there is fairly consistent data showing that decreases in libido and sexual activity can occur with progressive age in males (Bain, 2001; Morales and Heaton, 2001; Nolten, 2000), there is a large degree of individual variability, and within individuals who have normal testosterone levels there is no correlation between libido and testosterone levels (Rhoden et al., 2002). Most men continue to produce sperm and remain fertile well into old age. In recent years recognition that the adrenal androgen DHEA decreases with aging has led to the popular notion that DHEA treatment may increase libido and erectile function. However, several controlled double-blind studies have failed to show significant effects of DHEA supplementation on sexual function (Flynn et al., 1999; Hermann and Berger, 2001; Reiter et al., 1999).
Changes in reproductive physiology with aging are much more dramatic in females (Burger et al., 2002). The term “menopause” refers to a woman’s last menstrual cycle. During the transition to ovarian quiescence
at menopause, there is a gradual diminution of ovarian hormone production, with the earliest apparent decline occurring in ovarian inhibin production, which leads to a rise in FSH. With elevated FSH, estradiol production remains elevated in the early phases of menopause, but eventually there is a depletion of ovarian follicles and steroid hormone production falls below that necessary for stimulating the endometrium, and menopause occurs. The loss of ovarian cyclicity results from the gradual depletion of ovarian follicles, such that eventually there are no follicular cells to respond to elevated FSH levels. Thus, as menopause nears, plasma levels of estradiol are maintained initially or can actually be somewhat stimulated, but this is followed by a dramatic decline in circulating estrogen. The decline in estrogen levels leads to a number of other physiological changes, including a decrease in hormonal support of female secondary sexual characteristics, vasomotor instability known as hot flashes, increased loss of bone density, and loss of the protective effects of estrogen on the cardiovascular system. As discussed in the section above, a decrease in sexual desire in females after menopause has been generally reported, as well as a decrease in the frequency of sexual activity (McCoy and Davidson, 1985). Some studies have shown an increase in sexual interest in postmenopausal women taking estrogen (Dennerstein et al., 1980; Iatrakis et al., 1986; Sherwin, 1991), but other studies suggest that estrogen therapy alone has little effect on sexual behavior (Campbell and Whitehead, 1977; Furuhjelm et al., 1984; Sherwin et al., 1985). And, as in premenopausal women there are studies suggesting that androgen, not estrogen, levels correlate with sexual interest (Bachman et al., 1985; Bachmann and Leiblum, 1991; McCoy and Davidson, 1985).
ENVIRONMENTAL EXPOSURE TO HORMONES
Intentional consumption of steroids in the form of hormonal contraceptives, steroid hormones taken to increase muscle strength and fitness, and hormone replacement therapy in postmenopausal women are significant, particularly in Western countries. In the case of the first two, the amount of steroids consumed can be quite significant. Although not discussed in detail here, exogenous steroids have many of the same effects as endogenous steroids on target tissues (discussed in detail above). This includes both the positive effects to stimulate secondary sexual characteristics and the inhibitory effects of providing increased negative feedback support to the hypothalamus and pituitary, and thus essentially turning off or at least turning down the central drive to the reproductive axis. As a result, exogenous steroid exposure at high levels inhibits fertility. This is, in fact, the desired action in the case of contraceptives, but it is an unintended side effect of steroid hormones used to promote fitness. It is also possible for exogenous steroid hormones to influence sexual behavior; however, these
effects can be complex and are poorly understood at this time. For example, for oral contraceptives one could have concern that the increased doses of estradiol and progesterone that provide increased negative feedback to the reproductive axis and thus decrease ovarian production of androgens could potentially decrease sexual drive in women. However, studies that have examined this have generally shown no effect of oral contraceptives on female sexual behavior (Meston and Frohlich, 2000). Moreover, as discussed above, there is a report by Adams et al. (1997) suggesting that sexual behavior can be increased in women using reliable contraception, most likely due to loss of fear of becoming pregnant. This reiterates a general theme discussed throughout this chapter—that hormones and many other factors interact to determine behavioral outcomes. That is, hormone exposure modulates behavior but does not determine human reproductive behavior (Wallen, 2001).
In the past decade the concept that significant exposure to exogenous estrogen can come from environmental sources has gained increased attention (Golden et al., 1998). Concerns have arisen because of the theoretical potential for exogenous sources of estrogen to influence many aspects of reproductive biology and behavior, including altering reproductive physiology in females; altering reproductive behavior in both females and males, increasing reproductive pathologies in females, such as endometriosis, by providing additional stimulation to uterine endometrial tissue; increasing the incidence of breast, testicular, and prostate cancer by providing extra trophic support to these steroid-sensitive tissues; and increasing male fertility problems by providing negative feedback to the brain and pituitary and thus leading to a decrease in endogenous testosterone production.
The two sources of environmental estrogens that have received the greatest attention are from ingestion of PCBs (polychlorinated hydroxybiphenyls), found in plastic containers that are used more and more frequently to store beverages and from consumption of phytoestrogens found in food products such as soy. Tests of estrogenic activity of a number of PCBs have shown that the most potent is 80 times less potent than estradiol in binding to estrogen receptors, and most others are at least 100 times less potent than estradiol (Korach et al., 1988). When toxic equivalency is calculated, dietary exposure to these environmental estrogens is calculated as being no more than 0.0000025 percent of the daily intake of naturally occurring estrogenic flavonoids in the diet (Safe, 1995), and these in turn are much less potent than endogenous estradiol. Thus, it seems unlikely that exogenous estrogen exposure from PCBs in food and drink containers has any significant impact on reproductive physiology or behavior in most human populations.
In contrast, there seems little doubt that human consumption of phytoestrogens, either due to naturalistic consumption of foodstuffs high in phytoestrogens or because women intentionally consume foods with high
phytoestrogen content for their estrogenic effect, has a greater potential to have an impact on reproductive physiology. About 200 different naturally occurring phytoestrogens have been identified to date (Golden et al., 1998). These compounds vary in structure and can act as either estrogen agonists or estrogen antagonists, with their action sometimes switching as a function of dose. Of these, coumestrol, found in soy protein, has been shown to have the greatest estrogenic potency, 0.03 to 0.2 times that of estradiol. Coumestrol binds competitively to the estrogen receptor and at low doses has an estrogenic effect, but at high doses it has an antiestrogen effect in studies examining its action in cell culture. In some cultures, such as in Japan, relatively high levels of phytoestrogen metabolites of soy protein are found in the urine (Mackey and Eden, 1998). Epidemiological studies show that the Japanese have a lower incidence of such diseases as breast, endometrial, and prostate cancer, which can be aggravated by estrogen. However, whether consumption of soy phytoestrogens plays a causal role in the lower incidences of these cancers remains to be determined (Mackey and Eden, 1998). In recent years there have been several well-controlled intervention studies, in both women and men, examining the effects of soy intervention on reproductive function (Kurzer, 2002). The studies in women provide evidence for very weak effects of increased soy intake on estrogen-sensitive tissues, such as the breast, and reproductive hormone levels. The studies in men have not validated the concerns of adverse effects of phytoestrogen consumption on reproductive hormone levels or sperm production.
LIFE EVENTS THAT ALTER HORMONE LEVELS
Life events that impact the functioning of the reproductive axis are often those that produce various forms of stress. Many physical forms of stress, including energy restriction, increased energy expenditure with exercise, temperature stress, infection, pain and injury, and psychosocial stress have been associated with suppression of reproductive hormone secretion and, if sustained, a suppression of fertility (Cameron, 1997, 1998; Lachelin and Yen, 1978; Pirke et al., 1989). Stress-induced reproductive dysfunction can occur in both females and males. In adulthood, reproductive impairment in females is characterized by suppression of ovulation, a lengthening of the menstrual cycle, followed eventually by a loss of ovarian cyclicity and amenorrhea. As ovarian steroid production is decreased, there is also a decline in secondary sexual characteristics, including breast size and amount of subcutaneous fat. In males the reproductive impairment is characterized by a loss of libido, a decrease in testosterone secretion, and thus a decrease in spermatogenesis and hormonal support for secondary sexual characteristics. Chronic stress, occurring during the process of pubertal development, can impair the progression of puberty in both females and males, leading in
some cases to a very marked delay in the pubertal development of reproductive capacity and the accompanying development of secondary sexual characteristics (Carpenter, 1994).
The primary site of disruption of the reproductive axis, with all forms of stress studied in detail to date, appears to be at the level of the GnRH neurons, which provide the central neural drive to the reproductive axis. Using animal models of various stresses, it has been shown that for at least some stresses GnRH secretion is impaired (I’Anson et al., 2000). However, more typically, it is inferred that GnRH secretion is impaired in stress conditions, when a suppression of pituitary gonadotropin secretion is measured. This is further supported by the finding that, in all conditions of stress-induced reproductive dysfunction studied to date, administration of exogenous GnRH can stimulate the function of the reproductive axis, indicating that stress is not acting to directly suppress pituitary or gonadal activity (Hotchkiss and Knobil, 1994). In some forms of acute stress a fall in gonadotropin secretion can be noted within minutes to hours. With more subtle stresses, impairment of gonadotropin secretion is generally noted when the stress is present on a chronic basis.
The mechanisms by which various forms of stress impair activity of the reproductive axis appear to have some common elements, but there also appear to be mechanisms specific to each type of stress. For example, many forms of stress can activate the hypothalamic-pituitary-adrenal axis (HPA), and experimental studies have shown several mechanisms by which activation of the HPA axis can impair the central neural drive to the reproductive axis. On the other hand, certain aspects of stress, such as decreased fuel availability, only occur with some forms of stress and are likely to impair the activity of the reproductive axis via relatively specific mechanisms.
Energetic Stresses
Much of what we know about the mechanisms by which nutritional status modulates activity of the reproductive axis comes from the clinical study of patients with the psychiatric disorder anorexia nervosa (Aono et al., 1975; Marshall and Kelch, 1979; Warren and Vande Wiele, 1973). This disorder involves an obsessive fear of being fat and leads to a profound decrease in food intake, which causes extreme weight loss and can become life-threatening. Nearly all females who develop anorexia nervosa show a loss of ovarian cyclicity and amenorrhea. Amenorrhea is often apparent for great lengths of time, with normal menstrual cycles sometimes returning when patients regain weight but often lasting long after weight recovery. Measurement of circulating levels of LH and FSH show that gonadotropin secretion during the weight loss phase of anorexia nervosa is very low and often nonpulsatile in nature or pulsatile only during the nighttime period,
as it is in the early pubertal period (Boyar et al., 1974). Anorexia can often start in the teenage years and, if it does so prior to menarche, can delay menarche for many years, holding the girl in a prepubertal state long beyond the normal time of puberty (Biederman et al., 1986). This delay in activation of adult-like gonadotropin secretion and ovarian function is accompanied by minimal secretion of the ovarian steroid hormones, and thus development of secondary sexual characteristics is delayed. Such a prolongation of childhood and maintenance of a girlish body can be advantageous in certain activities, such as ballet, and the prevalence of anorexia in girls participating rigorously in such exercises is much higher than in the normal population (Warren and Stiehl, 1999). Other conditions in which there are profound changes in energy availability, such as with highly competitive exercise training, can lead to a similar suppression of the reproductive axis (Loucks et al., 1992).
While there is no doubt that severe or chronic decreases in energy availability can lead to a suppression of reproductive hormone secretion and a loss of fertility, a question that is more relevant to most demographic studies is whether mild-to-moderate changes in energy availability impact significantly on reproductive hormone secretion and fertility. Indeed, there are a number of studies with experimental animals showing that brief periods of undernutrition can lead to a suppression of reproductive hormone secretion (Cagampang et al., 1990; Cameron and Nosbisch, 1991). And brief periods of undernutrition have also been documented to decrease reproductive hormone secretion in humans (Cameron et al., 1991). However, fertility would not be expected to be compromised during brief periods of undernutrition, particularly in females who seem to be somewhat protected from the effects of mild undernutrition in the follicular phase of the menstrual cycle, the period leading up to ovulation (Berga et al., 2001; Olson et al., 1995).
The relationship between long-term mild undernutrition and activity of the reproductive axis is more controversial (Wood, 1994). There is generally little animal research addressing this question, probably because maintaining animals on diets for a prolonged period of time would be a rather inefficient method of determining the mechanisms by which energy availability modulates reproductive function, in terms of both time and money. Livestock that are maintained with suboptimal nutritional support have compromised fertility, but, again, most of the experimental work examining this subject involves rather severe undernutrition (Foster et al., 1986). In human populations, where mild-to-moderate undernutrition is relatively common, minimal impact on adult fertility has been reported (Bongaarts, 1980; Gray, 1983; Menken et al., 1981). However, there are stronger correlations between moderate nutritional compromise and later timing of puberty onset (Bhalla and Shrivatava, 1974; Chowdhuray et al., 1978;
Foster et al., 1986). Moderate levels of exercise are also associated with little impact on the reproductive axis (Rogol et al., 1992). Overall, it would appear that, although prolonged periods of mild-to-moderate undernutrition can have some impact on reproductive function and likely on fertility, the impact is relatively weak compared to the other complex of factors that modulate human reproductive ecology.
Energetic stress occurring during lactation is also an important regulator of fertility. Breast-feeding has the potential to profoundly suppress the activity of the reproductive axis (Kennedy and Visness, 1992; McNeilly, 1993). Suppression of the hypothalamic-pituitary-gonadal axis stems from both the energetic drain associated with milk production and other neuroendocrine signals that occur with lactation, including increased prolactin and oxytocin release (McNeilly et al., 1994; McNeilly, 2001). The controversy in this area has not been about whether lactation can suppress the activity of the reproductive axis but rather to what degree it suppresses reproductive processes in a wide variety of naturally occurring conditions (Lunn, 1994). It is interesting in this regard that the percentage of a mother’s daily energy intake that is required to support an infant is relatively low for humans compared to other species (Lunn, 1994). Thus, the degree of energy compromise due to lactation is less. Other important variables that impact the effects of breast-feeding on reproduction include the frequency of nursing, whether supplementation of nutritional sources for the infant is available, and the degree of food available to the mother. The role of hormones released during lactation on maternal behavior is discussed in more detail by Young (Chapter 4, this volume). It is also probable that the shift in hormonal milieu during the postpartum period of lactation has effects on sexual behavior. The relatively few studies examining sexual behavior in women in the puerperium indicate that sexual interactions are relatively lower in the first few months after the birth of a child compared to prepregnancy levels (Alder et al., 1986; al Bustan et al., 1995; Byrd et al., 1998; Kayner and Zagar, 1983). However, the reasons for the drop in frequency of intercourse are complex and include many factors other than hormonal changes, including fatigue, episiotomy discomfort, vaginal bleeding, dyspareunia, insufficient lubrication, fears of awakening the infant or not hearing the infant, and a decreased sense of attractiveness.
Psychosocial Stress
The effects of behaviorally-induced stresses—that is, psychological and social stresses—on the activity of the reproductive axis have received less attention, and their impact on the reproductive axis appears to be more variable. Moreover, in human populations it is difficult to selectively study the impact of psychosocial stress on reproductive function because this
stress rarely occurs in isolation from other stresses or in a timely fashion so that it can be easily studied. One of the best characterized forms of psychosocial-stress-induced reproductive dysfunction comes from studies of women who present to infertility clinics with functional hypothalamic amenorrhea (FHA). By definition, FHA is a state of subfertility that is not associated with substantial undernutrition or exercise, does not involve lactation, and is not associated with any organic or structural causes for decreased fertility (Berga et al., 1989; Reame et al., 1985). Studies of women with FHA show that they experience more psychological stress than other women, although they do not experience more stressful life events but rather react more profoundly to the stressful events they do experience (Giles and Berga, 1993). They also show increased activation of physiological systems that respond to stress, including increased HPA axis activity (Berga et al., 1997). Treatment of these patients with cognitive behavioral therapy or with drugs that reduce the activity of some central neural systems activated by stress can restore fertility, although not in all cases (Berga et al., 1991, 1997).
Although the majority of studies examining the effects of psychosocial stress on reproduction have documented stress-induced suppression of reproductive function, there are a handful of human studies which have reported that girls who have grown up under conditions of family stress, such as an absent father in a home, with family conflict, a girl whose parents have divorced, enter puberty at a significantly earlier age (Belsky et al., 1991; Moffitt et al., 1993; Wierson et al., 1993). However, the mechanisms by which such stress exposure would advance the onset of puberty have not been established. Moreover, there is the possibility that early stress exposure does not cause advancement of puberty but rather that the likelihood of early puberty and exposure to early life family stresses may simply be correlated because they are both influenced by a common factor(s). For example, one of the factors governing the age of menarche in a girl is her mother’s age at menarche (Graber et al., 1995). Thus, it is possible that mothers who experienced early menarche are more likely to have family conflict or divorce when their children are young as well as to have daughters who will have early menarche themselves.
A more detailed understanding of how psychosocial stress can impact reproduction comes from animal studies, with investigations in nonhuman primates having particular relevance to understanding this human condition. Nonhuman primates live in complex social groups and have higher cortical brain areas similar to humans. Moreover, the anatomical and functional organization of their reproductive axes is very similar to humans. A number of studies have shown that acute stresses, such as placing monkeys in restraint chairs (Norman and Smith, 1992), receiving aggressive attacks from other monkeys (Rose et al., 1972), and pairing with unfamiliar part-
ners (Coe et al., 1982), can rapidly suppress reproductive hormone secretion. However, not all psychological stresses have this effect. For example, exposing captive monkeys to the sight of “leather capture gloves” will lead to an increase in heart rate and cortisol, and a suppression of immune cell function, but will not suppress circulating levels of reproductive hormones (Helmreich and Cameron, 1992; Rogers et al., 1998). It seems likely that monkeys perceive the sight of capture gloves as being less threatening than a stress such as an aggressive attack by other monkeys. Thus, the perception of severity of stress is likely to be important in determining whether the stress will lead to a suppression of reproductive hormone secretion.
In nonhuman primates a number chronic social stresses have been associated with a marked and sustained suppression of reproductive hormone secretion. These include prolonged restraint stress (Goncharov et al., 1984) and troop reorganization (Sapolsky, 1983). Interestingly, in the Sapolsky study of baboon troop reorganization, several social factors appeared to modulate the response of the reproductive axis to stress in these animals. There was a high degree of correlation between aggressiveness and testosterone titers, with the more aggressive males showing higher circulating levels of testosterone. There was also an interaction between social status and the level of environmental stress, such that in periods of social stability there was no difference in plasma testosterone levels between dominant and subordinate animals, but in the period of social instability the dominant animals showed higher plasma testosterone levels than did the subordinate males. Similar findings were reported by Rose et al. (1972) in a study of male rhesus monkeys living together in a large outdoor compound, showing that plasma testosterone levels were correlated both with the number of aggressive interactions an animal experienced and the animal’s dominance rank. It has also been posited that social status (i.e., dominance rank) plays an important role in determining lifetime reproductive success in primates, with subordinate animals experiencing a greater degree of social stress and having a lesser degree of reproductive success (Cowlishaw and Dunbar, 1991; Dittus, 1979; Drickamer, 1974; Dunbar, 1980; Dunbar and Dunbar, 1977; Sade et al., 1976; Wilson et al., 1978). However, support for this hypothesis is not uniform, and this remains a controversial notion.
Some light may be shed on this issue by reports indicating a correlation between dominance rank and reproductive success in some years but not others (Cheney et al., 1988; deWaal, 1982; Duvall et al., 1976; Nishida, 1983; Witt et al., 1981). It would appear that multiple factors, including dominance rank, time of year, magnitude of stress, aggressiveness of the animal, and level of activity of the reproductive axis prior to stress exposure can all play roles in modulating the response of the reproductive axis to both acute and chronic stresses (see Figure 5-4). Thus, although it is pos-
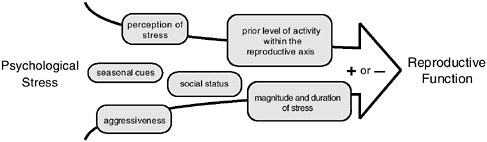
FIGURE 5-4 Schematic diagram of factors that mediate the effects of psychological stress on the activity of the hypothalamic-pituitary-gonadal axis.
sible to measure group mean responses to specific stresses and make conclusions about the effect of a stress on a species’ reproductive axis, such a mean assessment may be of little use in determining whether an individual will experience a suppression of reproductive function in response to that stress. An increased understanding of the mechanisms by which psychological and social stresses suppress the activity of the reproductive axis may well be achieved by focusing future studies on these individual differences in response to stress.
SUMMARY AND FUTURE DIRECTIONS
It is becoming clear that to fully understand the multiplicity of factors that come together to regulate and modulate reproductive physiology and reproductive behavior will require integration of demographic and biomedical approaches to these research issues. Many aspects of reproductive biology are difficult to study from the perspective of population biology approaches because of the great variation in function within an individual over a short course of time, such as with fluctuations in hormone levels over the menstrual cycle or even the fluctuation in reproductive hormone levels on an hour-to-hour basis. Biomedical approaches of studying individuals in more detail will be able to define relationships more clearly but are limited by the low power of examining relatively few individuals. What is needed is a twofold approach, first of developing studies that nest demographic and biomedical approaches together and second of using biomedical studies to inform the design of demographic studies.
The first approach would involve performance of large demographic studies to understand the relationships between variables on a global scale, with a select subset of individuals studied in much more detail to test specific hypotheses or to differentiate between possible mechanisms underlying the general relationship. The second approach simply requires further
communication between the fields of demography and biomedical sciences, such that methodologies are well understood, where possible similar measurements are made and at the least complexities understood by examining individuals are considered in the design of demographic studies. But it is also important that the information flow go in the opposite direction, so that the field of demography can play a larger role in guiding biomedical scientists toward interesting questions for detailed study.
There are a number of areas in need of further investigation to fully understand the role that hormones play in fertility outcomes. In many areas, studies with more frequent measurements are needed to more accurately assess the hormonal function that may underlie physiology and behavior. In a number of areas there is also a need for more accurate quantification of other measures. For example, in the case of assessing the actual role of undernutrition on fertility in much of the developing world, more accurate measures of the level of nutritional intake and the duration of the undernourished period would help sort out why there seems to be disagreement between demographers and biomedical scientists as to whether energetic status is an important regulator of reproductive ecology. A stronger recognition of the tremendous role that individual differences play in both reproductive physiology and behavior is needed to accurately design and interpret studies. This is true in terms of both normal functioning and reactions to various stresses and environmental conditions. Measurement of a greater number of variables in a given study is going to be essential to fully understand the interactions between hormones and other variables, such as dominance, temperament, and stress sensitivity, in determining both reproductive physiology and behavior. In the end, achieving this complex understanding will likely require a multidisciplinary approach—teams of investigators with different backgrounds working together to design studies that take into account the nuances of physiology, psychology, and population biology.
REFERENCES
Adams, D.B., A.R. Gold, and A.D. Burt 1997 Rise in female-initiated sexual activity at ovulation and its suppression by oral contraceptives. The New England Journal of Medicine 299:1145-1150.
al Bustan, M.A., N.F. el Tomi, M.F. Faiwalla, and V. Manav 1995 Maternal sexuality during pregnancy and after childbirth in Muslim Kuwaiti women. Archives of Sexual Behavior 24:207-215.
Alder, E.M., A. Cook, D. Davidson, C. West, and J. Bancroft 1986 Hormones, mood and sexuality in lactating women. British Journal of Psychiatry 148:74-79.
Aono, T., T. Kinugasa, T. Yamamoto, A. Miyake, and K. Kuracki 1975 Assessment of gonadotropin secretion in women with anorexia nervosa. Acta Endocrinologica (Copenhagen) 80:630-641.
Bachmann, G.A., and S.R. Leiblum 1991 Sexuality in sexagenarian women. Maturitas 13:43-50.
Bachmann, G.A., S.R. Leiblum, B. Sandler, W. Ainsley, R. Narcesian, R. Shelden, and H.N. Hymans 1985 Correlates of sexual desire in post-menopausal women. Maturitas 7:211-216.
Bancroft, J., D. Sanders, D. Davidson, and P. Warner 1983 Mood, sexuality, hormones and the menstrual cycle. III. Sexuality and the role of androgens. Psychosomatic Medicine 45:509-516.
Bain, J. 2001 Andropause: Testosterone replacement therapy for aging men. Canadian Family Physician 47:91-97.
Barraclough, C., and J. Leathem 1954 Infertility induced in mice by a single injection of testosterone propionate. Proceedings of the Society for Experimental Biology and Medicine 85:673-674.
Baum, M.J., B.J. Everitt, J. Herbert, and E.B. Keverne 1977 Hormonal basis of proceptivity and receptivity in female primates. Archives of Sexual Behavior 6:173-192.
Belchetz, P.E., T.M. Plant, Y. Nakai, E.J. Keogh, and E. Knobil 1978 Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202:631-633.
Belsky, J., L. Steinberg, and P. Draper 1991 Childhood experience, interpersonal development and reproductive strategy: An evolutionary theory of socialization. Child Development 62:671-675.
Berga, S.L., T.L. Daniels, and D.E. Giles 1997 Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertility and Sterility 67:1024-1030.
Berga, S.L., T.L. Loucks, and J.L. Cameron 2001 Endocrine and chronobiological effects of fasting in women. Fertility and Sterility 75:926-932.
Berga, S.L., A.B. Loucks, W.G. Rossmanith, L.M. Kettel, G.A. Laughlin, and S.S. Yen 1991 Acceleration of luteinizing hormone pulse frequency in functional hypothalamic amenorrhea by dopaminergic blockade. Journal of Clinical Endocrinology and Metabolism 72:151-156.
Berga, S.L., J.F. Mortola, L. Girton, B. Suh, G. Laughlin, P. Pham, and S.S. Yen 1989 Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. Journal of Clinical Endocrinology and Metabolism 68:301-308.
Bethea, C.L., W.H. Fahrenbach, S.A. Sprangers, and F. Freesh 1992 Immunocytochemical localization of progestin receptors in monkey hypothalamus: Effect of estrogen and progestin. Endocrinology 130:895-905.
Bhalla, M., and J.R. Shrivatava 1974 A prospective study of the age of menarche in Kampur girls. Indian Pediatrics 11:487-493.
Biederman, J., R.J. Baldessarini, J.S. Harmatz, T.M. Rivinus, G.W. Arana, D.B. Herzog, and U. Schildkrout 1986 Heterogeneity in anorexia nervosa. Biological Psychiatry 21:2113-216.
Bongaarts, J. 1980 Does malnutrition affect fecundity? A summary of evidence. Science 208:564-569.
Bonsall, R.W., D. Zumpe, and R.P. Michael 1978 Menstrual cycle influences on operant behavior of female rhesus monkeys. Journal of Comparative Physiological Psychology 92:846-855.
Boyar, R.M., J. Katz, J.W. Finkelstein, S. Kapen, H. Weiner, E.D. Weitzman, and L. Hellman 1974 Anorexia nervosa: Immaturity of the 24-hour luteinizing hormone secretory pattern. New England Journal of Medicine 291:861-865.
Boyar, R.M., R.S. Rosenfeld, S. Kapen, J.W. Finkelstein, H.P. Roffwarg, E.D. Weitzman, and L. Hellman 1974 Human puberty. Simultaneous augmented secretion of luteinizing hormone and testosterone during sleep. Journal of Clinical Investigation 54:609-618.
Bremer, J. 1959 Asexualization, A Follow-up Study of 244 Cases. New York: Macmillan.
Byrd, J.E., J.S. Hyde, J.D. DeLamater, and E.A. Plant 1998 Sexuality during pregnancy and the year postpartum. Journal of Family Practice 47:305-308.
Burger, H.G., E.C. Dudley, D.M. Robertson, and L. Dennerstein 2002 Hormonal changes in the menopause transition. Recent Progress in Hormone Research 57:257-275.
Cagampang, F.R., K. Maeda, A. Yokoyama, and K. Ota 1990 Effect of food deprivation on the pulsatile LH release in the cycling and ovariectomized female rat. Hormone and Metabolic Research 22(5):269-272.
Cameron, J.L. 1997 Stress and behaviorally-induced reproductive dysfunction in primates. Seminars in Reproductive Endocrinology 15(1):37-45.
1998 Fasting and reproduction in non-human primates. Pp. 95-109 in Pennington Center Nutrition Series: Nutrition and Reproduction. W. Hansel, G. Bray, and D.H. Ryan, eds. Baton Rouge: University of Louisiana Press.
2001 Effects of sex hormones on brain development. Pp. 59-78 in Developmental Cognitive Neuroscience. C.A. Nelson and M. Luciana, eds. Cambridge: The MIT Press.
Cameron, J.L., and C. Nosbisch 1991 Slowing of pulsatile LH and testosterone secretion during short-term fasting in adult male rhesus monkeys (Macaca mulatta). Endocrinology 128:1532-1540.
Cameron, J.L., T. Weltzin, C. McConaha, D.L. Helmreich, and W.H. Kaye 1991 Suppression of reproductive axis activity in men undergoing a 48 hour fast. Journal of Clinical Endocrinology and Metabolism 73:35-41.
Campbell, K.L. 1994 Blood, urine, saliva and dip-sticks: Experiences in Africa, New Guinea and Boston. Annals of the New York Academy of Sciences 709:312-330.
Campbell, S., and M. Whitehead 1977 Oestrogen therapy and the menopausal syndrome. Clinical Obstetrics and Gynecology 4:31-47.
Canfield, R.E., and J.F. O’Connor 1991 Biological markers of human pregnancy. Biomedical and Environmental Sciences 4:56-68.
Carpenter, S.E. 1994 Psychosocial menstrual disorders: Stress, exercise and diet’s effect on the menstrual cycle. Current Opinion in Obstetrics & Gynecology 6:536-539.
Cheney, D.L., R.M. Seyfarth, S.J. Andelman, and P.C. Lee 1988 Factors affecting reproductive success in vervet monkeys. Pp. 384-402 in Lifetime Reproductive Success. T.H. Clutton-Brock, ed. Chicago: Chicago University Press.
Clayton, A.H. 2002 Female sexual dysfunction related to depression and antidepressant medications. Current Women’s Health Reports 2:182-187.
Chowdhury, A.K.M.A., S.L. Huffman, and G.T. Curlin 1978 Malnutrition, menarche and age at marriage in Bangladesh. Social Biology 24:316-325.
Clough, K.M., F.X. Cole, S.S. Seaver, A. Vesprini, A.Y. Kuo, and B.L. Lasley 1992 Enzyme immunoassay method for total alpha gonadotropin in human urine samples. Fertility and Sterility 57:1241-1246.
Coe, C.L., D. Franklin, E.R. Smith, and S. Levine 1982 Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiological Behavior 29:1051-1057.
Cooke, B., C.D. Hegstrom, L.S. Villeneuve, and S.M. Breedlove 1998 Sexual differentiation of the vertebrate brain: Principles and mechanisms. Frontiers in Neuroendocrinology 19:323-362.
Cowlishaw, G., and R.I. M. Dunbar 1991 Dominance rank and mating success in male primates. Animal Behavior 41:1045-1056.
Czaja, J.A., and C. Bielert 1975 Female rhesus sexual behavior and distance to a male partner: Relation to stage of the menstrual cycle. Archives of Sexual Behavior 4:583-597.
Czaja, J.A., J.A. Robinson, S.G. Eisele, G. Scheffler, and R.W. Goy 1977 Relationship between sexual skin colour of female rhesus monkeys and the midcycle plasma levels of oestradiol and progesterone. Journal of Reproduction and Fertility 49:147-150.
Davidson, J.M., M. Kwan, and W.J. Greenleaf 1982 Hormonal replacement and sexuality. Clinical Endocrinology & Metabolism 11:599-623.
Dennerstein, L., G.D. Burrows, C. Wood, and G. Hyman 1980 Hormones and sexuality: Effect of estrogen and progestogen. Obstetrics and Gynecology 56:316-322.
Dennerstein, L., C. Wood, and G.D. Burrows 1977 Sexual response following hysterectomy and oophorectomy. Obstetrics and Gynecology 49:92-96.
Dennerstein, L., G. Gotts, J.B. Brown, C.A. Morse, T.M. Farley, and A. Pinol 1994 The relationship between the menstrual cycle and female sexual interest in women with PMS complaints and volunteers. Psychoneuroendocrinology 19:293-204.
deWaal, R.B.M. 1982 Chimpanzee Politics. London: Allen and Unwin.
Dittus, W.P.J. 1979 The evolution of behavior regulating density and age-specific sex rations in a primate population. Behaviour 69:265-302.
Drickamer, L.C. 1974 A ten-year summary of reproductive data for free-ranging Macaca mulatta. Folia Primatologica 21:61-80.
Dunbar, R.I.M. 1980 Determinants and evolutionary consequences of dominance among female gelada baboons. Behavioral Ecology and Sociobiology 7:253-265.
Dunbar, R.I.M., and E.P. Dunbar 1977 Dominance and reproductive success among gelada baboons. Nature 266:351-352.
Duvall, S.W., I.S. Bernstein, and T.P. Gordon 1976 Paternity and status in a rhesus monkey group. Journal of Reproduction and Fertility 47:25-31.
Ellinwood, W.E., and J.A. Resko 1984 Sex differences in biologically active and immunoreactive gonadotropins in the fetal circulation of rhesus monkeys. Endocrinology 107:902-907.
Ellison, P.T. 1988 Human salivary steroids: Methodological considerations and applications in physical anthropology. Yearbook of Physical Anthropology 31:115-142.
1994 Salivary steroids and natural variation in human ovarian function. Annals of the New York Academy of Sciences 709:287-298.
Erickson, G.F. 1978 Normal ovarian function. Clinical Obstetrics and Gynecology 21:31-52.
Everitt, B.J., J. Herbert, and J.D. Hamer 1972 Sexual receptivity of bilaterally adrenalectomised female rhesus monkeys. Physiology & Behavior 8:409-415.
Feng, Y.J., E. Shalts, L. Xia, J. Rivier, C. Rivier, W. Vale, and M. Ferin 1991 An inhibitory effect of interleukin-1 on basal gonadotropin release in the ovariectomized rhesus monkey: Reversal by a corticotropin-releasing factor antagonist. Endocrinology 128:2077-2082.
Flynn, M.A., D. Weaver-Osterholtz, K.L. Sharpe-Timms, S. Allen, and G. Krause 1999 Dehydroepiandrosterone replacement in aging humans. Journal of Clinical Endocrinology & Metabolism 84:1527-1533.
Foster, A., J. Menken, A Chowdhury, and J. Trussell 1986 Female reproductive development: A hazards model analysis. Social Biology 33:183-198.
Frisch, R., and J. MacArthur 1974 Menstrual cycles: Fatness as a determinant of minimum weight for height for their maintenance or onset. Science 185:949-951.
Furuhjelm, M., E. Karlgren, and K. Carstrom 1984 The effect of estrogen therapy on somatic and psychical symptoms in post-menopausal women. Acta Obstetricia et Gynecologica Scandinavica 63:655-661.
Giles, D.E., and S.L. Berga 1993 Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: A controlled comparison. Fertility & Sterility 60:486-492.
Golden, R.J., K.L. Noller, L. Titus-Ernstoff, R.H. Kaufman, R. Mittendorf, R. Stillman, and E.A. Reese 1998 Environmental endocrine modulators and human health: An assessment of the biological evidence. Critical Reviews in Toxicology 28:109-227.
Goncharov, N.P., D.S. Tavadyan, J.E. Powell, and V.C. Stevens 1984 Levels of adrenal and gonadal hormones in rhesus monkeys during chronic hypokinesia. Endocrinology 115:129-135.
Gordon, T.P., R.M. Rose, and I.S. Bernstein 1976 Seasonal rhythm in plasma testosterone levels in the rhesus monkey (Macaca mulatta): A three year study. Hormones and Behavior 7:229-243.
Goy, R.W. 1979 Sexual compatibility in rhesus monkeys: Predicting sexual behavior of oppositely sexed pairs of animals. Pp. 227-255 in Ciba Foundation Symposium. Sex, Hormones and Behavior. Amsterdam: Elsevier-North Holland.
Graber, J.A., J. Brooks-Gunn, and M.P. Warren 1995 The antecedents of menarcheal age: Heredity, family environment and stressful life events. Child Development 66:346-359.
Gray, R.H. 1983 The impact of health and nutrition on natural fertility. Pp. 139-162 in Determinants of Fertility in Developing Countries. R.A. Bulatao and R.D. Lee, eds. New York: Academic Press.
Griffin, J.E., and S. R. Ojeda 2000 Textbook of Endocrine Physiology, 4th ed. London: Oxford University Press.
Halpern, C.R., J.R. Udry, and C. Suchindran 1997 Testosterone predicts initiation of coitus in adolescent females. Psychosomatic Medicine 59:161-171.
1998 Monthly measures of salivary testosterone predict sexual activity in adolescent males. Archives of Sexual Behavior 27:445-465.
Helmreich, D.L., and J.L. Cameron 1992 Effects of an acute stressor on LH and cortisol secretion in rhesus monkeys: Comparison to hormone secretory responses induced by fasting. Proceedings of the 22nd annual meeting of the Society for Neuroscience, Abstract 55.14:118. Anaheim, CA (October 25-30).
Hermann, M., and P. Berger 2001 Hormonal changes in aging men: A therapeutic indication? Experimental Gerontology 36:1075-1082.
Hotchkiss, J., and E. Knobil 1994 The menstrual cycle and its neuroendocrine control. Pp. 711-749 in The Physiology of Reproduction, vol. 2. E. Knobil and J.D. Neill, eds. New York: Raven Press.
I’Anson, H., J.M. Manning, G.C. Herbosa, J. Pelt, C. R. Friedman, R.I. Wood, D.C. Bucholtz, and D.L. Foster 2000 Central inhibition of gonadotropin-releasing hormone secretion in the growth-restricted hypogonadotropic female sheep. Endocrinology 141:520-527.
Iatrakis, G., N. Haronis, G. Sakellaropoulos, A. Kourkoubas, and M. Gallos 1986 Psychosomatic symptoms of postmenopausal women with or without hormonal treatment. Psychotherapy and Psychosomatics 46:116-121.
Kaplan, S.L., M.M. Grumbach, and M.L. Aubert 1976 The ontogenesis of pituitary hormones and hypothalamic factors in the human fetus: Maturation of central nervous system regulation of anterior pituitary function. Recent Progress in Hormone Research 32:161-234.
Kayner, C.E., and J.A. Zagar 1983 Breast-feeding and sexual response. Journal of Family Practice 17:69-73.
Kennedy, K.I., and C.M. Visness 1992 Contraceptive efficacy of lactational amenorrhoea. Lancet 339:227-230.
Korach, K.S., P. Sarver, K. Chae, J.A. McLachlan, and J.D. McKinney 1988 Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: Conformationally restricted structural probes. Molecular Pharmacology 33:120-126.
Kordon, C., S.V. Drouva, G. Martinez de la Escalera, and R.I. Weiner 1994 Role of classic and peptide neuromediators in the neuronedocrine regulation of luteinizing hormone and prolactin. Pp. 1621-1681 in The Physiology of Reproduction, vol. 1. E. Knobil and J.D. Neill, eds. New York: Raven Press.
Kunelius, P., O. Lukkarinen, M.L. Hannulksela, O. Itkonen, and J.S. Tapanainen 2002 The effects of transdermal dihydrotestosterone in the aging male: A prospective, randomized, double blind study. Journal of Clinical Endocrinology & Metabolism 87:1462-1466.
Kurzer, M.S. 2002 Hormonal effects of soy in premenopausal women and men. Journal of Nutrition 132:570S-575S.
Kwan, M., W.J. Greenleaf, J. Mann, L. Crapo, and J.M. Davidson 1983 The nature of androgen action on male sexuality: A combined laboratory/self-report study on hypogonadal men. Clinical Endocrinology & Metabolism 57:557-562.
Lasley, B.L., K. Mobed, and E.B. Gold 1994 The use of urinary hormonal assessments in human studies. Annals of the New York Academy of Sciences 709:299-311.
Lasley, B.L., and S.E. Shidleler 1994 Methods for evaulating reproductive health in women. Occupational Medicine 9:423-433.
Lachelin, G.C., and S.S. Yen 1978 Hypothalamic chronic anovulation. American Journal of Obstetrics and Gynecology 130:825-831.
Lieblum, S., G. Bachmann, E. Kemmann, D. Colburn, and L. Schwartzman 1983 Vaginal atrophy in the post-menopausal woman: The importance of sexual activity and hormones. Journal of the American Medical Association 249:2195-2198.
Lipson, S.F., and P.T. Ellison 1989 Development of protocols for the application of salivary steroid analyses to field conditions. American Journal of Human Biology 1:249-255.
Loucks, A.B., J. Vaitukaitis, J.L. Cameron, A.D. Rogol, G. Skinar, and M.P. Warren 1992 The reproductive system and exercise: A state-of-the-art review. Medicine and Science in Sports and Exercise 24:S288-S293.
Lovejoy, J.C., and K. Wallen 1990 Adrenal suppression and sexual initiation in group-living female rhesus monkeys. Hormones and Behavior 24:256-269.
Lunn, P.G. 1994 Lactation and other metabolic loads affecting human reproduction. Annals of the New York Academy of Sciences 709:77-83.
Luttge, W.B. 1971 The role of gonadal hormones in the sexual behavior of the rhesus monkey and human: A literature survey. Archives of Sexual Behavior 1:61-88.
Mackey, R., and J. Eden 1998 Phytoestrogens and the menopause. Climacteric 1:302-308.
MacLusky, N.J., and F. Naftolin 1981 Sexual differentiation of the central nervous system. Science 211:1294-1303.
Mann, D.R., M.A. Akinbami, K.G. Gould, K. Paul, and K. Wallen 1998 Sexual maturation in male rhesus monkeys: Importance of neonatal testosterone exposure and social rank. Journal of Endocrinology 156:493-501.
Marshall, J.C., and R.P. Kelch 1979 Low dose pulsatile gonadotropin-releasing hormone in anorexia nervosa: A model for human pubertal development. Journal of Clinical Endocrinology & Metabolism 49:712-718.
McCoy, N.L., and J.M. Davidson 1985 A longitudinal study of the effects of menopause on sexuality. Maturitas 7:203-210.
McNeilly, A.S. 1993 Lactational amenorrhea. Endocrinology and Metabolism Clinics of North America 22:59-73.
2001 Lactational control of reproduction. Reproduction, Fertility and Development 13:583-590.
McNeilly, A.S., C.C.K. Tay, and A. Glasier 1994 Physiological mechanisms underlying lactational amenorrhea. Annals of the New York Academy of Sciences 709:145-155.
Menken, J., J. Trussell, and S. Watkins 1981 The nutrition-fertility link: An evaluation of the evidence. Journal of Interdisciplinary History 9:425-441.
Meston, C.M., and P.F. Frohlich 2000 The neurobiology of sexual function. Archives of General Psychiatry 57:1012-1030.
Michael, R.P., A.N. Clancy, and D. Zumpe. 1995 Distribution of androgen receptor-like immunoreactivity in the brains of cynomolgus monkeys. Journal of Neuroendocrinology 7:713-719.
Michael, R.P., and M. Wilson 1974 Effects of castration and hormone replacement in fully adult male rhesus monkeys (Macaca mulatta). Endocrinology. 95:150-159.
Moffitt, T.E., A. Caspi, J. Belsky, and P.A. Silva 1993 Childhood experience and the onset of menarche: A test of a sociobiological model. Child Development 63:47-58.
Moghissi, K.S. 1990 Gonadotropin releasing hormones. Clinical applications in gynecology. Journal of Reproductive Medicine 35:1097-1107.
Montgomery, S.A., D.S. Baldwin, and A. Riley 2002 Antidepressant medications: A review of the evidence for drug-induced sexual dysfunction. Journal of Affective Disorders 69:119-140.
Morales, A., and J.P. Heaton 2001 Hormonal erectile dysfunction. Evaluation and management. Urologic Clinics of North America 28:279-288.
Morley, J.E. 2001 Testosterone replacement in older men and women. Journal of Gender-Specific Medicine 4:49-53.
Nishida, T. 1983 Alpha status and agonistic alliance in wild chimpanzees (Pan troglodytes schweinfuthii). Primates 24:318-336.
Nolten, W.E. 2000 Androgen deficiency in the aging male: When to evaluate and when to treat. Current Urology Reports 1:313-319.
Norman, R.L., and C.J. Smith 1992 Restraint inhibits luteinizing hormone and testosterone secretion in intact male rhesus macaques: Effects of concurrent naloxone administration. Neuroendocrinology 55:405-415.
Olson, B.R., T. Cartledge, N. Sebring, R. Defensor, and L. Nieman 1995 Short-term fasting affects luteinizing hormone secretory dynamics but not reproductive function in normal-weight sedentary women. Journal of Clinical Endocrinology & Metabolism 65:262-267.
Ojeda, S.R., and M. Bilger 2000 Neuroendocrine regulation of puberty. Pp. 197-224 in Neuroendocrinology in Physiology and Medicine. P.M. Conn and M.E. Freeman, eds. Ottowa: Humana Press.
Phoenix, C.H., R.W. Goy, A.A. Gerall, and W.C. Young 1959 Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369-382.
Phoenix, C.H., R.W. Goy, and J.A. Resko 1968 Psychosexual differentiation as a function of androgenic stimulation. Pp. 33-49 in Perspectives in Reproduction and Sexual Behavior. M. Diamond, ed. Bloomington: Indiana University Press.
Phoenix, C.H., A.K. Slob, and R.W. Goy 1973 Effects of castration and replacement therapy on sexual behavior of adult male rhesus. Journal of Comparative Physiological Psychology 84:472-481.
Pirke, K.M., W. Wuttke, and U. Schweiger 1989 The Menstrual Cycle and Its Disorders. Berlin: Springer-Verlag.
Plant, T.M. 2001 Neurobiological bases underlying the control of the onset of puberty in the rhesus monkey: A representative higher primate. Frontiers in Neuroendocrinology 22:107-139.
Pomerantz, S.M., and R.W. Goy 1983 Proceptive behavior of female rhesus monkeys during tests with tethered males. Hormones and Behavior 17:237-248.
Reame, N.E., S.E. Sauder, G.D. Case, R.P. Kelch, and J.C. Marshall 1985 Pulsatile gonadotropin secretion in women with hypothalamic ameonorrhea: Evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. Journal of Clinical Endocrinology & Metabolism 61:851-858.
Reamy, K.J., and S.E. White 1987 Sexuality in the puerperium: A review. Archives of Sexual Behavior 16:165-186.
Reiter, W.J., A. Pycha, G. Schatzl, A. Pokorny, D.M. Gruber, J.C. Huber, and M. Marberger 1999 Dehydroepiandrosterone in the treatment of erectile dysfunction: A prospective, double-blind, randomized, placebo-controlled study. Urology 53:594-595.
Resko, J.A., and W.E. Ellinwood 1985 Negative feedback regulation of gonadotropin secretion by androgens in fetal rhesus macaques. Biology of Reproduction 33:346-352.
Rhoden, E.L., C. Teloken, P.R. Sogari, and C.A. Souto 2002 The relationship of serum testosterone to erectile function in normal aging men. Journal of Urology 167:1745-1748.
Rogol, A.D., A.Weltman, J.Y. Weltman, R.L. Seip, D.B. Snead, S. Levine, E.M. Haskvitz, D.L. Thompson, R. Schurrer, and E. Dowling 1992 Durability of the reproductive axis in eumenorrheic women during 1 year of endurance training. Journal of Applied Physiology 72:1571-1580.
Rogers, C.J., C.S. Brissette-Storkus, L.A. Hayes, J.L. Cameron, and W.H. Chambers 1998 Selective reduction in CD2 expression on CD2bright/CD8+ lymphocytes from cynomolgus monkeys (Macaca fascicularis) in response to acute stress. Journal of Neuroimmunology 86:61-72.
Rose, R.M., T.P. Gordon, and I.S. Bernstein 1972 Plasma testosterone levels in the male rhesus: Influences of sexual and social stimuli. Science 178:643-645.
Sade, D.S., K. Cushing, P. Cushing, J. Dunaif, A. Figueroa, J.R. Kaplan, C. Lauer, D. Rhodes, and J. Schneider 1976 Population dynamics in relation to social structure on Cayo Santiago. Yearbook of Physical Anthropology 20:253-262.
Safe, S.H. 1995 Environmental and dietary estrogens and human health—Is there a problem? Environmental Health Perspectives 103:346-351.
Sapolsky, R.M. 1983 Endocrine aspects of social instability in the olive baboon (Papio anubis). American Journal of Primatology 5:365-379.
Schiavi, R.C., and D. White 1976 Androgens and male sexual function: A review of human studies. Journal of Sex & Marital Therapy 2:214-228.
Schwanzel-Fukuda, M., D. Bick, and D.W. Pfaff 1989 Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Molecular Brain Research 6:311-326.
Schwanzel-Fukuda, M., and D.W. Pfaff 1989 Origin of luteinizing hormone-releasing hormone neurons. Nature 338:161-163.
Shabsigh, R., L. Zakaria, A.G. Anastasiadis, and A.S. Seidman 2001 Sexual dysfunction and depression: Etiology, prevalence, and treatment. Current Urology Reports 2:463-467.
Sherwin, B.B. 1991 The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. Journal of Clinical Endocrinology & Metabolism 72:336-343.
Sherwin, B.B., M.M. Gelfand, and W. Brender 1985 Androgen enhances sexual motivation in females: A prospective, crossover study of sex steroid administration in the surgical menopause. Psychosomatic Medicine 47:339-351.
Shughrue, P.J., M.V. Lane, and I. Merchenthaler 1997 The comparative distribution of estrogen receptor-α and β mRNA in rat central nervous system. Journal of Comparative Neurology 388:507-525.
Silverman, A.J., I. Livne, and J.W. Witkin 1994 The gonadotropin-releasing hormone (GnRH) neuronal systems: Immunocytochemistry and in situ hybridization. Pp. 1683-1709 in The Physiology of Reproduction, vol. 1. E. Knobil and J.D. Neill, eds. New York: Raven Press.
Simerly, R.B., C. Chang, M. Muramatsu, and L.V. Swanson 1990 Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. Journal of Comparative Neurology 294:76-95.
Soules, M.R., R.A. Steiner, D.K. Clifton, N.L. Cohen, S. Aksel, and W.J. Bremner 1984 Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. Journal of Clinical Endocrinology & Metabolism 58:378-383.
Stanczyk, F.Z., I. Miyakawa, and U. Goebelsmann 1980 Direct radioimmunoassay of urinary estrogen and pregnanediol glucuronides during the menstrual cycle. American Journal of Obstetrics and Gynecology 137:443-450.
Stanislaw, H., and F.J. Rice 1988 Correlation between sexual desire and menstrual cycle characteristics. Archives of Sexual Behavior 17:499-508.
Steiner, R.A., and J.L. Cameron 1989 Endocrine control of reproduction. Pp. 1289-1342 in Textbook of Physiology. H.D. Patton, A.F. Fuchs, B. Hille, A.M. Scher, and R.A. Steiner, eds. Philadelphia: W.B. Saunders.
Udry, J.R., J.O. Billy, N.M. Morris, T.R. Groff, and M.H. Raj 1985 Serum androgenic hormones motivate sexual behavior in adolescent boys. Fertility & Sterility 43:90-94.
Udry, J.R., L.M. Talbert, and N.M. Morris 1986 Biosocial foundations for adolescent female sexuality. Demography 23:217-230.
Van Goozen, S., V. Wiegant, E. Endert, F. Helmond, and N. Van de Poll 1997 Psychoendocrinological assessment of the menstrual cycle: The relationship between hormones, sexuality and mood. Archives of Sexual Behavior 26:359-382.
Wallen, K. 1990 Desire and ability: Hormones and the regulation of female sexual behavior. Neuroscience and Biobehavioral Reviews 14:233-241.
1999 Risky business: Social context and hormonal modulation of primate sexual desire. Pp. 289-323 in Reproduction in Context. K. Wallen and J. Schneider, eds. Cambridge, MA: MIT Press.
2001 Sex and context: Hormones and primate sexual motivation. Hormones and Behavior 40:339-357.
Wallen, K., D.R. Mann, M. Davis-DaSilva, S. Gaventa, J.C. Lovejoy, and D.C. Collins 1986 Chronic gonadotropin-releasing hormone agonist treatment suppresses ovulation and sexual behavior in group-living female rhesus monkeys. Physiological Behavior 36:369-375.
Warren, M.P., and A.L. Stiehl 1999 Exercise and female adolescents: Effects on the reproductive and skeletal system. Journal of the American Medical Women’s Association 54:115-120.
Warren, M.P., and R.L. Vande Wiele 1973 Clinical and metabolic features of anorexia nervosa. American Journal of Obstetrics and Gynecology 117:435-449.
Wierson, M., P.J. Long, and R.L. Forehand 1993 Toward a new understanding of early menarche: The role of environmental stress in pubertal timing. Adolescence 28:913-924.
Wilson, M.E., T.P. Gordon, and I.S. Bernstein 1978 Timing of births and reproductive success in rhesus monkey social groups. Journal of Medical Primatology 7:202-212.
Winter, J.S.D., C. Faiman, W.C. Hobson, A.V. Prasad, and F.I. Reyes 1975 Pituitary-gonadal relations in infancy. I. Patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. Journal of Clinical Endocrinology & Metabolism 40:545-551.
Witt, R., G. Schmidt, and J. Schmidt 1981 Social rank and Darwinian fitness in a multimale group of Barbary macaques (Macaca sylvanus L.). Folia Primatologica 36:201-211.
Wood J.W. 1994 Maternal nutrition and reproduction: Why demographers and physiologists disagree about a fundamental relationship. Annals of the New York Academy of Sciences 709:101-116.
Wray, S., A. Nieburgs, and S. Elkabes 1989 Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: Evidence for an embryonic origin in the olfactory placode. Developmental Brain Research 46:309-318.
Young, D.S., and E.W. Bermes, Jr. 1986 Specimen collection and processing: Sources of biological variation. Pp. 478-518 in Textbook of Clinical Chemistry. N.W. Tietz, ed. Philadelphia: W.B. Saunders.
Zehr, J.L., D. Maestripieri, and K. Wallen 1998 Estradiol increases female sexual initiation independent of male responsiveness in rhesus monkeys. Hormones and Behavior 33:95-103.