10
Energetics, Sociality, and Human Reproduction: Life History Theory in Real Life
Carol M. Worthman
Understanding the determinants of human fertility remains a matter of urgent practical as well as scientific concern. Many fields, including demography, economics, health sciences, and policy and political science, offer theories at varying levels of explanation and predictive power. Only one theory, evolutionary theory, offers an account at the ultimate level of design.
Since its formulation, however, evolutionary theory has challenged the efforts of anthropologists and evolutionary biologists to apply the grand theory to variation within particular species and populations, or among individuals. Ideally, these efforts involve dialectic between epistemological and empirical work, between model building and model testing. Yet even outstanding empiricists in the field emphasize the remaining challenges, reporting, for instance, “the realization that no current models that adequately explain fertility variation in traditional societies have withstood empirical scrutiny” (Hill and Hurtado, 1995:396). Both theory and the models derived from it therefore require further work.
This essay attempts to develop a fresh view of human fertility behavior and family formation by considering the intersection of three approaches— life history theory, behavioral and reproductive ecology, and developmental psychobiology. On the theoretical front, life history theory aims to integrate comparative, cross-taxonomic data into a framework comprising life course attributes such as the timing, pace, and forms of reproduction and reproductive effort. On the population level, human evolutionary and reproductive ecologists have sought to probe the value of adaptationist models for understanding variations in reproductive behavior and biology.
On the individual level, developmental psychobiologists have unpacked the roles of rearing environments in temperament, sociality and parenting, and life history strategy.
The evolutionary and functional analyses presented below suggest the need to expand current demographic models of human fertility behavior to include considerations of design and human development. Each level of analysis–evolutionary, ecological, and developmental–suggests that human reproduction involves much more than fertility, and identifies critical variables for successful human reproduction that merit more attention in demographic analysis.
IMPORTANCE OF A BIOCULTURAL PERSPECTIVE
For over 25 years, adaptationist accounts of human behavior (first under the rubric of sociobiology, then behavioral ecology) have been dominated by the calculus of cost and benefits reckoned against the bottom line of limited available resources. Reproduction occupies a central place in this calculus because fitness, or differential reproductive success, is the currency of adaptation. The logic is simple: Reproductive effort will be determined by the availability of finite resources, principally energy and time, balanced against competing demands for subsistence or survival. Human evolutionary or behavioral ecology proceeds from the assumption that “humans should have evolved fertility and mortality patterns that lead to highest contribution to the future gene pool, given the constraints provided by general human morphological, physiological, and social characteristics, and the environments in which our species lives” (Hill and Hurtado, 1995:13).
This bold proposition—that human behavior has been shaped by selective pressures to optimize fitness—inspired a wave of empirical research, the best of which sought to test the validity of this claim and probe its ability to illuminate human behavior. This research builds on an older foundation of evolutionary biology to assess whether and how adaptive design may explain the inter- and intrapopulation variations in human reproductive function. The proposition that reproductive function itself reflects design constraints posed by evolutionary processes has yielded a series of novel hypotheses that have met with empirical support while also providing fresh perspectives on both adaptation and reproduction (Ellison, 1994; Wood, 1994). Yet even the best of these studies seldom cover beliefs, values, and schemas that inform behavior—that is, culture and experiential worlds (Borgerhoff Mulder, 1995). Research on impacts on fertility of workload, nutritional status, breastfeeding and supplementation, and maternal age rarely pursues the cultural dimensions woven into the biocultural dynamics (McDade and Worthman, 1998).
Inattention to culture, human experience, and cognition (in behavioral
and reproductive ecology) reflects gaps in evolutionary thinking that are only slowly being addressed in evolutionary psychology (Crawford and Krebs, 1998; Henrich, 2001; Henrich et al., 2001). But the gaps also mirror the absence of powerful dual-inheritance models that incorporate contemporary advances in developmental and behavioral biology. Such advances demonstrate that the distinction between biological and cultural modes of transmission is gratuitous, since inheritance operates through biocultural mechanisms across ontogeny (Oyama, 1985). Using this insight, evolutionary models, reformulated as biocultural inheritance models, could build in developmental psychobiology to provide a more complete picture of human reproductive behavior that incorporates social viability as a goal for offspring, an absolute prerequisite for successful human reproduction.
The analysis here begins with an examination of life history design, particularly resource allocation over the life course to growth, reproduction, and maintenance, as effected through neuroendocrine-endocrine regulators (abbreviated as neuro-endocrine). In addition to the well-studied ecological effects on reproductive biology, less studied trade-offs with social effort are identified in foundational pathways for neuro-endocrine regulation. Thus, human physiology reflects the adaptive significance of social life and the demands for social competence and participation in that life. Then, pathways by which social ecology instructs ontogeny are reviewed, which leads directly to consideration of the environments of evolutionary adaptedness. Both sets of analyses—endocrine and epigenetic—delineate components of biocultural inheritance central to human reproduction. Thus, inheritance for humans is not appropriately viewed as dual, genetic and cultural. Rather, it has a dominant biocultural coevolutionary component that is not divisible into biological (genes) and cultural (memes) units of inheritance but runs through the mechanisms and components of epigenesis. (If we wish to model this space, we would do well to commence with close readings of Baldwin [1895] and his modern counterpart, Gottlieb [1991, 1998].)
Biocultural inheritance and the redefinition of fertility outcomes to include viability of offspring imply possibilities for parallel changes in demographic thinking. Such possibilities are exemplified in the classic formulation of the “theoretical maximum” of fertility for humans (see Figure 10-1). The maximum is intended as a biological starting point from which the components that expand interbirth intervals pare away fertility potential to determine actual fertility. But this formulation, though merely theoretical, is patently absurd.
Better models of proximate determinants of effective fertility may be constructed from schedules for actual populations, such as the Hutterites and Gainj, from which we could begin to derive general patterns of the kind represented in the top three boxes of the figure. This is the classic work of
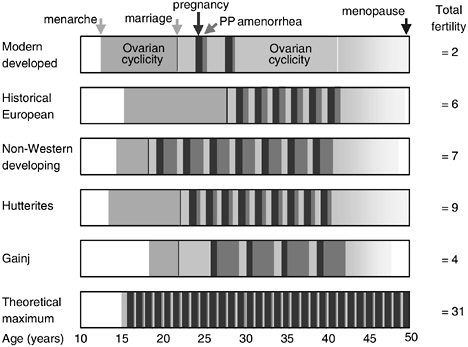
FIGURE 10-1 Scheduling in timing of reproductive events in diverse human populations (modified from Wood, 1994, which was based on Bongaarts and Potter, 1983).
demographers, which has shaped present understanding of fertility and could generate new, expanded models of determinants to help us think about fertility in creative ways. This analysis shows, however, that models of fertility behavior cannot stop with birth or lactation but must move on to identify and incorporate the key components of development that drive the production of viable offspring or the achievement of effective fertility.
LIFE HISTORY THEORY AND ENERGETICS
Life history theory concerns the evolved design constraints that shape species-specific phenotypes across the life course and that underlie the striking contrasts in life histories across the animal kingdom and even within taxa. Theorists seek design features from comparative evolutionary biology that will explain fertility and mortality schedules—classic demographic concerns. Consider the contrasts among any conceivable set of organisms, such as coelenterata, insectivora, and mammalia or sardines, whales, mice, and albatrosses (Charnov, 1993; Promislow and Harvey, 1990). Rather than
focusing on differential transgenerational genetic representation (fitness) as a basis of evolutionary analysis, life history theory concentrates on resource allocation as intrinsic to adaptive strategy. The life course is viewed as the product of an integrated suite of strategies for resource allocation, of which reproduction and its translation into fitness are a central feature.
Energy and Time
Resource allocation involves deploying energy and time across the spatiotemporal place carved out of entropy that comprises a life. The calculus is simple in principle but challenging to operationalize in detail.
First, energy. This is actually better conceived as energy (e.g., calories) plus valuable, limited material resources (e.g., micronutrients). The acquisition and the disposition of resources are both components of life history strategy, but current life history theory attends little to the input or acquisition side (a focus of behavioral ecology; Smith and Winterhalder, 1992).
The total energy captured by an organism is allocated among three domains: maintenance, growth, and reproduction. Maintenance includes (1) the metabolic cost of being alive, which covers processing and distributing food, air, and other inputs, the operation of membrane potentials, biosynthesis, resource recycling, and waste treatment; (2) materials and energy required for continuous maintenance and replacement, as in the constant turnover in bone and most other organs, cells, and cellular constituents, DNA checking and repair, and barrier maintenance through dermal and mucosal production; (3) healing from injury or unusual wear and tear; and (4) defense against, removal of, and cohabitation with micropredators (pathogens and parasites), as well as detection and evasion of macropredators (non- and conspecifics). In other words, maintenance involves all the investments that allay causes of mortality, intrinsic (e.g., aging) or extrinsic (e.g., accident and predation). The extent and quality of such investments in maintenance determine mortality schedules, or age-specific probabilities and causes of death.
Growth includes increases in body size—in length, height, weight, and overall mass. Two issues with respect to growth are the distinction between determinate and indeterminate growers and the value of size. Many species confine growth to an early period of life, ceasing to grow when adult body size is attained; others grow throughout life. The contrast in resource allocation strategies is apparent. Body size within taxa increases through evolutionary time, prompting the inference that size itself may have adaptive value (Charnov, 1993).
Reproduction comprises producing viable offspring that successfully reproduce. Life history strategy for accomplishing this may vary in many
dimensions, including timing of first reproduction; adult size and proportionate size of offspring at birth; number of offspring per reproductive event and spacing between events, types, amounts, and duration of postnatal care of offspring (parental effort); sex determination and the relative contributions of males and females to reproduction; and the slope of reproductive value with age. Evolutionary analysis has focused, virtually from its inception, on the necessary reproductive effort (Darwin 1871; Maynard Smith, 1978; Trivers, 1972; Williams, 1985), which has various costs (Borgerhoff Mulder, 1992), basically including (1) the biological costs of producing a new life (gametes, gestation, parturition) and (2) the costs of sustaining new life (parental care, including lactation, provisioning, defense, and solicitude for physical and emotional security). Costs may be expanded to cover (3) mating effort—finding, recruiting, and keeping a mate. Taxa show widely divergent mating strategies, which may comprise a significant portion of reproductive effort, particularly for males. A further dramatic expansion of the costs that can be counted as reproductive effort comes from defining inclusive fitness to incorporate reproduction by others, discounted by degree of relatedness to ego (Hamilton, 1964). This adds to the list (4) the costs of inclusive fitness, such as any form of altruism (to benefit others at one’s own expense) as well as aspects of social behavior and environmental modification. Particularly for social species, and especially when culture is employed as a major adaptive strategy, there is a final component of reproductive effort: (5) socialization cost. Socializing the young includes nurturing them (attending to emotional-cognitive needs as well as material ones), instructing them or guiding their participation in adult activities while tolerating juvenile ineptitude; providing opportunities to obtain and practice knowledge, attitudes, behaviors, and skills required for viable sociality requisite to survival and reproduction; and even actively advancing their social prospects (Blurton Jones et al., 1989, 1992).
Second, time. Like energy, time is a finite resource that must be apportioned among the same three domains as material resources and for reproduction, particularly, among the subdomains outlined above. The time invested in any reproductive event, weighed against life expectancy, determines reproductive potential or value.
The intersection of energy and time drives rates of resource demand and use, and lies at the heart of energetics. Energetic balance can be adjusted by modulating the rate of throughput. Lower throughput, due to late and infrequent reproduction, involves lower reproductive demand per unit of time than early and frequent reproduction. Lower throughput therefore relaxes the constraint of resource availability but carries the risk of dying before completing reproduction.
Trade-Offs
Such considerations demonstrate a central principle of life history organization, namely, to establish optimal trade-offs among competing demands under consistently unpredictable circumstances. Modeling trade-offs is essential for understanding the design or the biological organization of the functions and capacities of the human organism. Life history strategies can be usefully viewed, on the level of the organismic design of a species, as a set of algorithms that negotiate the trade-offs to optimize spatiotemporal allocation of limited resources.
One cardinal rule underlies the ubiquity of trade-offs: the allocation rule. Related to thermodynamic notions of energy conservation, the allocation rule states that consumable resources used for one purpose cannot be used for another. This rule will be critically examined below.
In schematic terms, maintenance may be subtracted from resource intake to determine the net resources available for growth or reproduction, which is labeled productivity. In comparison to productivity estimated from juvenile growth rates in other determinate growers, the productivity of primates appears remarkably low, only 40 percent of that of mammals in general—and the productivity of humans is only 20 percent of that of mammals, because human children grow very slowly between the ages of 5 and 10 (Kaplan et al., 2000). Slow growth affects life history strategy in several ways. It reduces the burden of energetic demands for growth (given the species has relatively low juvenile mortality). This effect may be particularly important for easing the demands on human parents, who provision their young throughout juvenility (Bogin, 1997, 1999). Slow growth may also permit greater investments in the maintenance required to reduce juvenile mortality or to meet the high metabolic costs of large brain size. Finally, low productivity may be the product of increased developmental costs not reflected in growth (e.g., play) but required for attaining adult competence.
Human Life History Strategy
Humans are large, long lived, and obligately social primates that have altricial singleton births, spaced at 2 to 5 years, which are first breastfed and then provisioned well into the second decade. Young are carried, tended, defended, instructed, and systematically exposed to experiences or adult-driven ecologies aimed not only at teaching attitudes and techniques for survival but also developing social competence and coherence as well as adult productivity. Reproductive timing is set through distinctive physiological switches that inhibit gonadal function in childhood and activate it in puberty.
Humans are determinate growers who grow slowly over a protracted period and then exhibit a growth spurt during a relatively late puberty. In puberty, adult height is achieved and energy previously used for growth becomes available for reproduction. Shifts in energy storage through changes in body composition (girls to fat, boys to muscle) attend this transition. After a phase of subfecundity, women enter a period of reproductive activity sustained over roughly two decades. Notably, they possess the unusual feature of programmed cessation of reproductive function—menopause—whereby ovarian potency declines and ceases decades before life ends. Reproductive aging leads to a remarkably ubiquitous average age at last birth of around 40 years (Bongaarts and Potter, 1983). The reproductive careers of men are not so curtailed, although they also show signs of reproductive aging.
Challenges for Life History Theory
Life history theory has excited interest because it applies formal and game theoretical models to comparative data; provides a life span, time-integrated framework; integrates across components of phenotype rather than focusing on specific features; identifies key cost-benefit trade-offs in the design of life history strategies; and thus suggests organic design criteria and evolutionary constraints. It promises to be generalizable, predictive, and hypothesis generating. Formerly, life history analysis was primarily based on species averages for the various life history parameters; variance was not included in formal analysis. However, the range of behavioral decisions used has been expanded to include state-dependent action (condition-, context-, or density-dependent) in models that can evaluate variations within and between individuals (Brommer, 2000). Notable limitations remain. These limitations do not impugn the importance and value of life history theory but should inform its application to fertility behavior.
First, variations within taxa should be distinguished from variations across taxa. Life history theory is based on formal comparative analysis across taxa, yet the goal of the present volume is to address fertility behavior within one taxon, humans. The hierarchy of life history trade-offs derived from analysis encompassing macrotaxonomic variations (between classes or phyla—e.g. fishes versus mammals) does not necessarily generalize directly to variations within species. By definition, different taxa do not share evolutionary history and thus have different design features and life history strategies, so each taxon has a different hierarchy of allocation trade-offs, as allometric analysis confirms. Analysis involving restriction of the phyletic range (within rather than across orders or families) would narrow the sweep of organic design questions and help focus on the relevant variance to partition.
Second, like evolutionary theory itself, the formal models of life history theory are highly abstract and aphysiological and do not support direct inferences about mediating mechanisms responsible for resource allocation or constraining life history design. A given life history constraint or trade-off can be met through diverse mechanisms. An additional empirical step is required to link life history to function in specific taxa. This step is abetted by identification of design patterns and constraints that do support testable hypotheses about organic design. For instance, comparative life history analysis identified adult mortality as the prime factor explaining variations among life history strategies. But a distinction between intrinsic and extrinsic sources of mortality (Charnov, 1993) proved difficult to sustain at the level of the functioning organism, though it generated a body of work that has illuminated relationships between these two sources of mortality (Ricklefs, 1998) as well as mechanisms of aging in general (Holliday, 1995; Kirkwood, 1981; Williams, 1957). A later section will deal with endocrine architecture and attempt to link this aspect of organic design and function with life history to show how trade-offs are embodied and shape fertility in humans.
A third limitation of the life history literature concerns the allocation rule and the need for more attention to the trade-offs around sociality. Perhaps the allocation rule has not received adequate critical or empirical scrutiny. The rule may actually be “bendable,” particularly with regard to the fertility behavior of highly social primates, a possibility evaluated below.
The following sections bring the strengths of life history analysis to bear on human fertility behavior while addressing limitations and expanding the scope of adaptationist thinking to substantially strengthen the analytic purchase on human reproduction.
TRANSLATION AND TRANSMISSION OF EVOLVED DESIGN
An integrated theory of reproductive ecology requires linkage of life history theory with phenotype and the mechanisms that produce life history, particularly reproductive careers. Phenotype comprises all the manifest features of an individual, including behavior. As in most species, human phenotypes adjust to environmental quality. The capacity for facultative adjustment can be gauged by the norm of reaction, or the phenotypic variation shown by a given genotype across diverse environments (Stearns and Koella, 1986). Cultural variations and the complexities associated with sociality lead to large reaction norms in human behaviors such as parenting practices or timing of marriage, but humans also exhibit substantial reaction norms in the biological bases of reproductive capacities and behavior.
Age at Menarche
One of the most thoroughly documented reaction norms for humans is age at menarche, which has been found to vary by nearly 50 percent across populations, from a low median age of just over 12 years in consistently well-nourished, healthy populations, to up to 18 years in persistently poorly nourished, less healthy ones (Eveleth and Tanner, 1990; Worthman, 1999a).
One might argue that the correlation of environmental quality with age at menarche reflects the impact of environmental insult rather than adaptive biological response, but the case of girls adopted at different ages into radically improved circumstances contradicts this interpretation. Girls from disadvantaged south Indian populations adopted at age 3 or later had an earlier age at menarche (11.1 years) than those adopted at 2 years or less (11.8 years); Proos et al., 1991). Furthermore, recent epidemiological studies of the effects of early environment on systems that drive resource allocation (Barker, 1991, 1997; Clark et al., 1996; Fall et al., 1995) have documented that fetal programming of neuro-endocrine regulation alters developmental and health outcomes across the life span (Adair, 2001; Godfrey, 1998; McDade et al., 2001; Susser and Levin, 1999; Williams and Poulton, 1999).
Secular trends and population variations in timing of reproductive maturation (indexed by menarche for girls) are related to variations in the timing of the onset of puberty in both sexes. Intensive investigation into these variations has identified maternal well-being, infant and child health, nutrition, and psychological well-being as salient ecological correlates. In teleological terms of life history theory (see Figure 10-2), maternal well-being (health, nutrition, and low stress), low juvenile mortality risk (indexed by morbidity), and sustained good nutrition reduce the resources needed for maintenance, with the net effect of increasing productivity.
Greater productivity increases the energy available for growth (in height or weight, fat or muscle) or reproduction and reduces the marginal costs of reproduction. This allows faster growth and accelerated maturation, conducive to earlier puberty. Good conditions from gestation through childhood also signal that the risk or relative cost of reproduction likely will be low (the dashed line in the figure).
At this point, limitations on the applicability of life history theory to intraspecific variations become clear, for life history predicts that adult mortality risk will be associated with earlier onset of the reproductive career (Charnov, 1993). Humans show the opposite pattern, with mortality risk associated with later puberty. As child health and survival improve in a population, child maturation accelerates, as evidenced by increased height for age and earlier age at menarche. Indeed, so close is this link that child
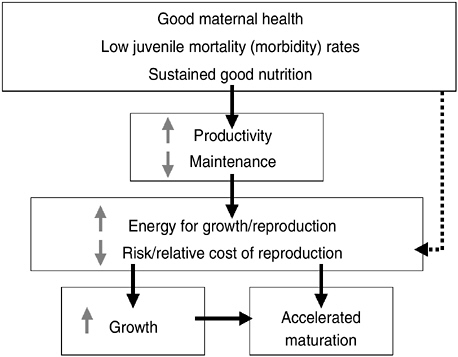
FIGURE 10-2 Secular trend in timing of puberty in terms of life history.
growth indices are used as a sensitive measure of population nutrition and health (World Health Organization, 1995).
Two Mediating Mechanisms
Phenotypic variations in reproductive behavior, such as variations in the timing of puberty, must originate somehow from evolved life history design. Two issues are involved, and lead to two distinctive but ultimately related sets of mediating mechanisms.
First is design for information use: how is life history design translated into phenotype? A case will be made here for the place of neuroendocrine-endocrine systems in the translation process. Endocrine architecture provides the concrete physiological structure for realizing the intersection of constitutional genetic endowment of the individual, fixed at conception, with environmental inputs, demands, and chance.
Second is transmission of life history design across generations. How can all the information that an organism needs to develop, survive, and succeed be communicated across generations? This is an enormous
bioinformatics challenge that cannot have a purely organic solution but must be seen as a problem of biocultural inheritance.
The following sections explore these two set of reproductive mechanisms—endocrine architecture for translating life history design into phenotype and biocultural inheritance for transmitting information to instruct translation.
Endocrine Architecture of Life History
Physiological mechanisms for producing life history phenotypes remain unspecified in life history theory, but neuroendocrine-endocrine (or neuro-endocrine) systems are obvious candidates (Finch and Rose, 1995). Hormones act like pacemakers for growth and developmental transitions. They are responsible for establishing the prolonged juvenile period in humans and govern the timing of puberty and the progression to reproductive maturity. Neuro-endocrine action thereafter orchestrates adult reproductive function, including the production of gametes, establishment and course of pregnancy, parturition, lactation, reproductive aging, and menopause.
The large array of neuro-endocrine regulatory systems handles resource partitioning among growth, reproduction, and maintenance. Interactions across this array determine productivity and negotiate resource allocation to short- and long-term life history projects, balancing the immediate demands of survival (e.g., metabolism, immune function) against long-term needs for growth or reproduction.
For instance, neuro-endocrine responses to psychosocial distress antagonize gonadal activity, for such distress arises from conditions likely to be less favorable for reproduction. Moreover, psychological distress and poor nutritional state each reduce sexual interest, and thereby dampen reproduction by both physiologic and behavioral routes. Hence, hormones mediate the interface between individual and environment, effecting triage to meet shifting demands and exigencies. They also present the means for facultative adjustment of life history parameters, mediating for instance the relationship of maternal workload to duration of postpartum amenorrhea.
Figure 10-3 presents the principal endocrine regulatory pathways that mediate energy allocation and trade-offs among growth, reproduction, and maintenance (reviewed in Worthman, 1999b, 2002). All these pathways traffic between brain and periphery, and four of them run from the hypothalamus at the floor of the brain, which regulates pituitary activity by releasing hormones or inhibitory factors (CRH, GnRH, etc.). Hypothalamic-releasing hormones stimulate or suppress pituitary output of trophic hormones (ACTH, LH/FSH, etc.) that act on target glands in the periphery (adrenals, gonads, etc.). In turn, target glands release hormones that mediate the stress response, reproductive function and behavior, growth, and metabolism.
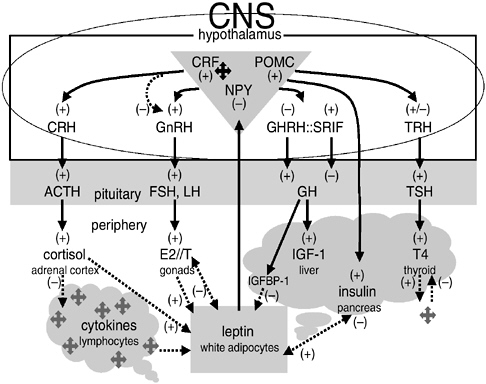
FIGURE 10-3 Representative endocrine axes, pathways, and interrelations, with particular regard to resource partitioning.
NOTE: Based on Anderson et al. (2001), Arnalich et al. (1999), Baskin et al. (1999), Bruunsgaard et al. (2000), Harris (2000), Imura et al. (1991), Mantzoros and Moschos (1998), Mantzoros et al. (2001), Owen et al. (2001, Rasmussen (2000), Straub et al. (2000), Wauters et al. (2000), and reviews in Worthman (1999a, 2002).
From the upper left to the right in Figure 10-3, the axes are (1) the hypothalamic-pituitary-adrenal (HPA) axis, which regulates arousal and stress via cortisol, a hormone that also extensively affects the other axes; (2) the hypothalamic-pituitary-gonadal (HPG) axis, which regulates gonadal function, including output of gonadal steroids (estradiol, E2; testosterone, T), which are responsible for reproductive functions and behaviors and many sex differences; (3) the somatotropic axis, which regulates growth and resource allocation via a cascade of trophic factors and binding proteins produced in the liver and locally; and (4) the thyrotropic axis, responsible for regulating metabolic rate and required for neurological development and alertness. The relationship of growth to energy availability is built into the organization of this axis.
The next two axes, at the bottom of Figure 10-3, differ from the others, involving not central coordination but distributive production and regulation, with the brain as a primary target organ. The (5) adiposal axis (bottom center) comprises white adipocytes in fat depots throughout the periphery that produce leptin, which acts in the brain and periphery with metabolic, reproductive, affective, and even hemopoeitic effects (Harris, 2000; Mantzoros, 1999; Wauters et al., 2000). This axis is significant for the present discussion because it clearly ties energetic status (not just energy stores but the balance of intake and expenditure) not only to prioritization of energy use (reproduction, metabolism) but also to mood and behavior (Baile et al., 2000; Havel, 2001; Maes, 1999; Mantzoros et al., 2001; Trayhurn and Rayner, 1996). Gonadal steroids also influence adipocyte activity and distribution (Anderson et al., 2001). Multiple neuropeptides, chiefly neuropeptide Y (NPY), proopiomelanocortin (POMC), and agouti-related peptide, mediate the actions of leptin on thermogenesis and energy metabolism and its hypothalamically mediated impact on other axes, including the gonadal. Indeed, one of the earliest observed stigmata of leptin-deficient mice was infertility (Caprio et al., 2001; Caro et al., 1996). But dense neuroendocrine pathways also tie leptin and a closely allied hormone, insulin, to motivation, affect, and behavior, which may be represented by hunger, irritability, and food intake, respectively (Mantzoros and Moschos, 1998; Schwartz et al., 1997).
The last axis, (6) the immunologic (bottom left), is stunningly complex and highly distributive. In addition to its primary place in the maintenance of physical health and integrity, the immune system strongly affects mood and behavior via cytokines released in the course of its normal pathogen-defense and repair actions (Anisman et al., 1999; Owen et al., 2001). This effect is most vividly manifested in sickness behavior—the listlessness, anorexia, social withdrawal, and cognitive blunting that accompany illness or injury (Dantzer, 2001; Hart, 1988; Yirmiya et al., 2000). Cytokine activation also suppresses sexual behavior, although animal models suggest that this effect is much more pronounced in females than males (Avitsur and Yirmiya, 1999). In addition to their potent psychobehavioral effects, cytokines trigger shunting of energy away from growth or reproduction via activation of the HPA axis and direct central effects on the HPG and somatotropic axes, suppression of thyroid activity, and peripheral actions on target organs (Rasmussen, 2000; Rivest and Rivier, 1995; von Laue and Ross, 2000).
This brief survey of endocrine architecture reveals that life history parameters have demonstrable physiological bases and furthermore raises several points about neuro-endocrine function. First, neuro-endocrine activity can be seen as clearly aimed at allocating resources among the domains of growth, reproduction, and maintenance. Neuro-endocrine path-
ways are organized to reflect the nature of life history demands, as in the contrast between the centrally regulated, clearly localized operation in reproduction versus the decentralized, distributive organization of the immune system. Potentially expensive and risky reproductive behaviors are under high selective pressure for appropriate timing, prolonged reallocation of resources, maintenance of biological states (cyclicity, pregnancy, parturition), and target orientation (e.g., placental [i.e., fetal] regulation of pregnancy, lactation dependence on infant stimuli).
Second, contrary to the classic silo model of endocrine organization reflected in the vertical pathways of Figure 10-3, each axis demonstrates systematic cross-talk. Cross-talk is understood rather differently when viewed from a life history perspective that throws into relief the necessity for mechanisms to allocate and reallocate energetic resources. Cross-talk provides the means to reallocate energy and modulate axis activity to meet ongoing relative demands and to do so in a physiologically integrated and synchronized manner.
Third, the neuro-endocrine architecture of life history not only supports biological functions comprising reproduction, growth, and maintenance but also integrally involves cognition and behavior. This involvement runs both ways: Hormones directly inform affect and behavior, as exemplified in the operation of the adiposal axis and the immune system, but behavior and cognition also influence hormones, as most directly exemplified by the HPA axis (McEwen, 2000; Seeman and McEwen, 1996). Such mechanisms coordinate the endocrine with the behavioral architectures of life history, as reflected in the functions of the brain as a primary site for experiential processing and memory, a center for physiological regulation and integration, and a target of peripheral modulation and signaling through endocrine action.
Fourth, the timing and course of life history events depend on ecological signals of environmental quality. Neuro-endocrine mechanisms transduce this information into physiological responses over the short term and long term. In the case of humans, favorable ecological circumstances include energetic conditions that are at least permissive, which are signaled by hormones like leptin, insulin, or cytokines, as well as positive social conditions, which are indexed through central processing (cognition and memory), emotion systems, and the HPA. These systems embody the individual’s knowledge of the surrounding social ecology (a formulation analogous to Kaplan’s [1995] notion of embodied capital).
Biocultural Inheritance and Fertility Behavior
The problem of intergenerational transmission for reproduction in the fullest, phenotypic, life history sense has concerned Western biologists and
philosophers for several hundred years (Gould, 1977). For much of that time, progress was hampered by an unduly materialist emphasis on an organic solution, whereas an epigenetic, biocontextual (or, for the case of humans, biocultural) inheritance model now appears more appropriate. Most recently, developmental biology and genetics have documented the significance of nongenetic factors in ontogeny. In brief, postconception environments can convey most of what the individual needs to know about the inputs and demands with which it must deal effectively (Reeve and Sherman, 1993). Accordingly, ontogeny is designed to reliably capture available information to instruct development (Changeux, 1985; Gottlieb, 1991; Oyama, 1985).
Human development therefore is integral to human reproduction, perhaps more than in any other species. Human reproduction cannot be said to have succeeded until offspring development is complete. The coevolution of biocultural mechanisms for reproduction through development is distinctively elaborated among humans. Williams (1957:400) observed that: “Any individual, of whatever age, who is caring for dependent offspring is acting in a way that promotes the survival of his own genes and is properly considered a part of the breeding population. No one is post-reproductive until his youngest child is self-sufficient.”
Recognizing the role of human development could help fill some of the gaps in life history models of fertility and bring them more in line with empirical observations. The substantial literatures on development and sociocultural processes would have to be integrated into the models of fitness determinants. Such integration sheds a different light on human reproduction and the selective pressures on it. If human development is coextensive with fertility in determining successful reproduction, the proximate determinants of fertility will require significant expansion to account for effective fertility, that is, for successful transmission of not only genotype but also biocultural patterns. Expansion of the concept of fertility to include postnatal processes requires incorporation not only of mortality risk but also components of developmental ecology required for biocultural transmission.
Individuals grow up in specific niches or proximal spatiotemporal envelopes. These may be labeled the effective environments of rearing. These environments can be diverse, given stratification and niche partitioning in human societies. Environments intersect with individual characteristics to create a wide range of developmental microniches (Bronfenbrenner, 1979; Super and Harkness, 1993), which produce diversity across individuals in embodied capital. This diversity is essential to complex human societies, so that variations in effective environments of rearing are critical to biocultural inheritance.
Diversity is possible partly because of human behavioral plasticity. Plasticity is rooted in a learning- and knowledge-oriented central nervous
system structurally and functionally organized around a life history strategy grounded in sociality allied with high-end extractiveness (Deacon, 1997; Kaplan et al., 2000). But the basis for plasticity also resides in reproductive adaptations for biocultural inheritance.
SOCIALITY AND MULTITASKING IN LIFE HISTORY
Life history theory recognizes ecological, cognitive, and behavioral dimensions but does not adequately consider sociality. Humans must belong to social groups to survive and develop and even to reproduce. Human reproduction depends on relationships, and on sharing resources with others.
The Need for Resource Sharing
The design of the species includes an unusually pronounced pattern of asynchronous energy consumption and production (Kaplan, 1997). In foraging societies, over 25 percent of lifetime energy needs is expended by children in the first 15 years, during which time they generate only 5 percent of lifetime energy production (Kaplan et al., 2000). Furthermore, forager women do not produce as much as they consume until the end of their reproductive careers, although they do show a substantial increase in productivity in the second decade. Males, by contrast, sharply escalate production over adolescence to generate a net energy excess by age 20 and produce double their own calorie consumption over most of the next three decades. Among elders, individual production falls below consumption at age 70 in women and age 60 in men (Kaplan, 1994).
In short, among foragers, provisioning of young and subvention of reproductive-age women are necessary because of asynchrony between production and consumption. Early consumption is returned in later adult production that supports children and grandchildren. The social-cognitive fabric that sustains provisioning others and concomitant lagged reciprocity is critical to human life history strategy. Yet life history models include neither social production and consumption nor social costs and benefits.
Sociodynamics, Cognition, and Learning
Sociodynamics create or influence conditions for virtually all aspects of life history—timing of weaning, growth rates and adult body size, timing of maturation and first reproduction, pace and degree of fertility, mortality risk, and so forth. Although humans are highly diverse in their beliefs, values, practices, and resultant human ecologies, they share one important similarity: social relationships universally dominate human experience and shape the material and social worlds that individuals must navigate.
Human relationships have specific properties. They persist, through time and space. They are reciprocal. They may be ascribed (kinship, group membership) or attained (friendship). They may be displaced through time and space. They are cognition intensive, mediated largely through language, the expressiveness of face and voice, and coordinated activity.
The cognitive and social impacts of language have been extensively explored. Evolution of the human face as a communicative organ and recognition marker extends a primate trend to visual orientation, freeing of the upper lip, and snout reduction and is reflected in hairlessness (incomplete in men); high integration of muscle and skin; complexity of musculature, including muscle-muscle insertions; different central neuroregulation of voluntary versus involuntary emotional expressions; and dramatically expanded representation of the face on sensory and motor cortices. Identification, reading, and tracking of faces are supported by expanded cortical regions and specific cell subsets within them for visual and emotion processing. Individually distinctive faces also aid recognition and recall of individual-specific information (social positionality, past behaviors) to guide behavior toward, treatment by, and social interactions with others.
The importance of cognition in human social relationships is attested to by high information and processing overhead. Acquisition of social knowledge is at least as important for survival and reproduction as acquisition of productive knowledge, being crucial for successful relationships. Additionally, emotional intelligence is required to navigate relationships, and all individuals must learn how to read the emotional content of situations and behaviors, communicative and otherwise, and develop the ability to regulate their own emotions and emotional expression. In this regard, emotional-cognitive bases for deferred gratification are critical to the spatiotemporally displaced reciprocity patterns humans pursue. Experiments with chimpanzees suggest that symbolic representation of “goods,” allowing abstraction and distancing, is a prerequisite (Beran et al., 1999; Boysen and Berntson, 1995). Performances of social reciprocity (e.g., conversation) are crucial to form and maintain social relationships in the short term and long term, and require integrated use of memory, signal processing, social intelligence, empathy, emotion processing, and behavior production (language, expression, gesture).
The skills necessary for such performances require extensive learning through observation, practice, and neurobehavioral honing during development. Reading emotion in others involves a formidable array of capacities: sense of self, theory of mind, cross-modality sensory mapping, integration of self and other (empathy), and expressive reciprocity to moderate ambiguity. Childhood may be extended to allow development of these emotional-cognitive skills.
Children learn to read others’ emotional expression rather slowly: of the set of internationally recognized facial expressions of emotion, only happiness is reliably recognized at ages 3 to 5, anger by age 7, fear by age 10, and surprise by age 11 (Massaro, 1998). Since both language and facial expressions of emotion suffer from signal deterioration (due to, for example, darkness, distance, poor reception, and averted face) children must learn to bridge ambiguity. They must observe, mimic, and practice not only productive skills but also social ones (Tomasello, 1993, 1999). Adult tolerance for social incompetence and support for learning (via exegesis of imitative behaviors, commands and instructions, and guided performance) create the space for social learning analogous to that created for production learning. Social skills will, if anything, be more critical to successful reproduction, and it is significant that rites of passage at adolescence emphasize implicit and explicit social instruction over or in conjunction with production.
The Adaptive Value of Social Relationships
Social relationships have high adaptive value, for they regulate access to resources. On the one hand are the tangible resources emphasized in life history analysis that constitute material capital: food, shelter, safety, labor, and mates. On the other hand are immaterial resources largely overlooked in life history theory and that comprise social capital: information, social connections and memberships, social support and opportunity, assistance and cooperation, coordination and triage, and economies of scale. Deferred reciprocities allow social capital to be translated into material capital at spatially and temporally remote situations. Conversely, material capital can be converted to social capital through sharing and obligation.
Information and opportunities to acquire information through observation, association, or assisted implementation are critical resources in human life history because they facilitate access to material and social resources. Information can be of durable or ephemeral value, but given the vicissitudes of social life and ecological circumstances, ephemeral knowledge of the immediate and transient contributes just as importantly to life history as does durable knowledge of people, places, and processes. Thus, the value of social relationships inheres also in access to information, which may be regarded as the third good in limited supply, after energy and time.
In sum, human ecology and social relationships largely define the context of individual life history and determine access to resources. Virtually all aspects of reproduction–mate acquisition, mating, fertility, parenting– are framed and mediated through social behavior and social ecology. Offspring viability is also shaped through social dynamics. The very low adult mortality on which human reproductive strategy relies depends heavily on
social variables. Human life history strategy includes cognitive and behavioral adaptations to acquire knowledge and skills to negotiate the social world and the material world it gates. Hence, social costs and benefits should be included in the energy calculus of life history theory.
The Costs of Sociality
Sociality requires energy and exacts costs that alter the calculus of resource allocation. First, social interaction places cognitive demands on the body’s most expensive organ, the brain. Such demands include attention and emotion regulation, memory and recall, and production and processing of signals (language, face and other ethological cues, symbols) according to culture- and person-specific schemas and usages. Language use greatly increases the density of information and the elliptical and inferential content in communication, increasing the need to escalate computational speed and enhance processing modes (Beran et al., 1999; Deacon, 1997).
Second, the complexity of life in a group expands exponentially with the number of members. One must track, store, and instantaneously recall each individual’s history, relationship with and interactions with oneself and with all other members of the group, inferred properties (e.g., trustworthiness), and history of social and material exchanges. The escalating computational demands imposed by group size may have contributed substantially to pressures for cortical expansion in humans. Whether or not this is true historically, humans now must meet the costly performance demands.
Third, relationships are based on spatiotemporally displaced reciprocities involving exchanges of time, material goods, and social resources. Resource sharing mitigates against acute shortfalls and helps smooth out resource availability over the life course (Kaplan, 1997). Habitual sharing comes with a cost, however. Obligations to others represent a tax on an individual, but reciprocity converts that tax into social insurance for self or offspring.
Finally, sociality brings not only support but also competition (Geary and Flinn, 2001). It is a truism of evolutionary biology and ecology that conspecifics represent the keenest competition. The costs of competition with and defense against predation by other people contribute significantly to maintenance costs. Still, the pressure of potential human competition and predation further reinforces the pressure for group membership and compliance with its costs and complex reciprocities.
Bending the Allocation Rule
Anyone who has observed and tried to code human behavior knows that multitasking is pervasive (Gosling and Petrie, 1981). Men guard goats
while mending fences, gossiping with neighbors, and watching weather patterns; women clean living areas while supervising a cooking pot, tending a fire, overseeing children, and listening to the neighbors argue. Notably, children do not stay on task and show less concurrent behavioral layering, in part because cognitive skills for multitasking are acquired gradually. The allocation rule–that resources expended for one purpose cannot be used for another–requires that resources be partible. Moderating the constraints of the rule, “bending” it even while it cannot be circumvented, should provide great adaptive advantage. Such potential advantage places a premium on strategies that increase the density of purposes achieved or constraints met in a given interval with given expenditures. Parallel processing and multitasking are therefore adaptive strategies. Multitasking is facilitated by language, bipedalism with manual dexterity and tool use, cooperative foraging, a large capacity for planning, recall, and rumination; and family and group life.
Most components of reproductive effort are amenable to multitasking. Biological processes like gamete production, pregnancy, and lactation proceed concurrently with many or all other activities. Mating effort and parenting also are layered with many other activities. Everyday tasks are integrated with social relations that in turn regulate access to mates for oneself or for offspring. Men traversing the distance to a foraging site, who on the way discuss marriageable daughters for themselves or their sons, are pursuing both food acquisition and mating or parenting advantage as well as reinforcing their social bonds and refreshing their command of the social landscape. Likewise, many aspects of child care are performed in parallel with other activities. Infants and small children are carried and tended usually as a secondary or tertiary activity. Carrying and tending exact physical and cognitive costs, but their time costs are greatly diminished by integrating them with parallel activities (as with the Hiwi and Ache, in Hurtado, 1990, 1992).
Indeed, the differential reproductive burden imposed by types of labor may relate to their compatibility with multitasking (as with the Hadza, in Hawkes et al., 1997). Among Hadza foragers, for example, the value of grandmaternal care for easing a daughter’s reproductive effort emerges as significant only in seasons when a mother’s foraging involves tasks incompatible with concurrent child care. In a radically different postindustrial setting, the costs of parenting are greatly increased for mothers working long, inflexible hours in remote offices and lacking kin support for child care. Another form of parenting effort readily or even necessarily integrated with other tasks is child socialization. Children learn essential skills, including language, subsistence activities, social negotiation, and even child care, passively and informally through observation, imitation, and guided participation rather than direct instruction (Blurton Jones and Konner, 1976;
Rogoff, 1990; Vygotsky, 1978, 1986). Finally, high productivity, generosity, and social effort by parents generate not only social capital for themselves but also future material benefits for offspring—access to scarce social and material resources (e.g., marriage opportunities or food, respectively) that may serve as a hedge against premature parental illness or death.
Implications for Life History
How, then, should sociality and multitasking be counted in life history terms? Existing theory should be pushed to cope with these realities of human reproductive behavior. Sociality raises productivity through its impact on intake and maintenance via the pathways described above. It represents a major overlooked approach to meeting other maintenance and reproductive demands. Though sociality exacts real costs, it yields tangible benefits. Social effort accordingly represents an important arena for the allocation of trade-offs. Similarly, multitasking represents a challenging phenomenon for parsing allocation in behavior analysis and adaptationist logic. The ubiquity of multitasking relating to reproduction and involving social life indicates that its omission may limit the capacity of current behavioral ecology and life history theory to contribute to the understanding of human reproductive behavior.
The importance of social relationships, their role in fertility behavior, and their value in determining long-term offspring viability are not unique to humans. The data on baboons presented by Altmann and Alberts in this volume provide compelling nuanced evidence of this. Reproductive effort comprises complex social strategizing, not just copulating and giving birth, and the insight that such strategic capacities are both significant in humans and not unique to them should broaden our view of the determinants of fertility schedules. Efforts directed at social relationships, including relationships with group members who are neither coparents nor primary kin, yield costs and benefits over time that determine mating and parenting success. Therefore, from a life history perspective, strategies to establish, maintain, and enhance social relationships are essential components of reproductive effort.
EPIGENESIS AND REARING ENVIRONMENTS
Epigenesis in Human Reproduction
As noted above, reproduction faces an information problem: how the blueprint for a fully functional adult organism can be conveyed through the union of two parent cells. It was suggested that biocultural inheritance was an aspect of the solution. This is part of the broader process of epigenesis,
the dynamic between genes and environment that informs phenotype. More formally, epigenesis comprises nonlinear developmental processes, not reducible to genes or genetic programs, that involve interactions within and among many levels of the organism and its environment (Gottlieb, 1998; Maleszka et al., 1998). Some of the most exciting work in developmental and behavioral biology over the last two decades yields insights into the nature of these interactions, and has transformed scientific views of ontogeny and adaptive design.
The problem of intergenerational transfer of information to guide ontogeny was apparently resolved by the (re)discovery of Mendelian genetics in 1900 (cited in International Human Genome Sequencing Consortium, 2001), and the blueprint analogy was reinforced by elucidation of DNA structure and function in the 1960s. But the genome simply cannot convey enough information to specify phenotype in its myriad details; indeed, initial human genome maps reported in 2001 gauge the number of protein-coding genes at 30,000 to 40,000 (International Human Genome Sequencing Consortium, 2001; Venter et al., 2001), merely double those in a fly or worm. The number of genes does not correspond to phenotypic complexity. As a crude example, the mammals mouse, whale, and human have a similar numbers of genes but possess 40, 200,000, and 85,000 million neurons, respectively (Miklos and Maleszka, 2000).
Genes provide at least the minimum information to code essential proteins and establish basic body plans. But use of the genome involves a complex metaarchitecture that is demand or context driven, and one might even say that, in ecological terms, what reproduces is the environment (Bonner, 1974). The environment is implicated in epigenesis at many levels, including replication, gene expression, splicing, posttranslational modifications, and context-dependent protein interactions (Miklos and Maleszka, 2001). Much of ontogeny–particularly that of humans, with the high premium on plasticity and longevity–is designed to capture information from the environment to “instruct” development of the organism. Environmental context can convey far more information than genes can about what the organism needs for adequate functioning.
Present knowledge of epigenesis rests on the well-studied nervous and immune systems. Epigenesis operates through (1) the proliferation of variants (e.g., neurons and neural connections, or unique cell lines) and production of transient redundancy, (2) the retention of variants that “work” best, and (3) the pruning of those that do not. Which variants work best is determined via processing of functional throughputs provided by contextual inputs or demands (e.g., visual representation or pathogen recognition; Changeux, 1985; Edelman, 1987; McDade and Worthman, 1999). The strategem of developmental Darwinism constitutes a potent means for production of phenotypic complexity honed to the circumstances in which the
organism must operate, and it does so by creating ontogenetic expectancies for functional load and contextual inputs.
Expectable Environments of Rearing
The elegant epigenetic devices for environmental programming depend on the information provided by contextual inputs and therefore can evolve only when those inputs are highly reliable. These form the expectable environments of rearing (EER), the set of environments normally encountered during ontogeny. The EER encapsulates the fitness outcomes and selective pressures to which organic design is adapted.
An overview of the human EER (see Table 10-1) integrates findings from developmental biology, developmental psychobiology, anthropology, and epidemiology to translate the epigenetic landscape into everyday human ecology. The listed features are nearly or entirely universal in human groups and have all been associated with variations in phenotypic outcomes, though of varying magnitudes.
The impact of a component of the EER on phenotype depends on several factors: (1) its predictiveness of future conditions, (2) the significance of those future conditions for fitness, (3) the timeliness of the signal for mobilization of an adaptive response, (4) the feasibility of transducing the signal into ontogenetic responses, (5) the relative fitness value versus the cost of adjusting the phenotype to one cue versus another, (6) pleiotropic effects of phenotypic adjustment on other features with adaptive value, and (7) the time horizon for realizing costs versus benefits.
This set of factors determines trade-offs around maintenance of plasticity versus reliance on context to inform ontogeny. Humans are thought to maintain an unusually high degree of plasticity in domains pertaining to information processing and behavior. Ecological circumstances, group composition, and social dynamics can range widely over the life course of an individual, so many early conditions will generalize poorly to later circumstances. Reproduction is separated from early development by over a decade or more and continues for several decades. Thus, although childhood environment strongly affects biological dimensions of life history (maturation and senescence rates), its effects on reproductive behavior (on partner relationships and infant and child care) are far more subtle than immediate ecological and cumulative sociocultural effects (Chisholm, 1993, 1996; Hill and Hurtado, 1995).
IMPLICATIONS FOR RESEARCH AND POLICY
The view of human reproduction as rooted in biocultural inheritance casts new light on current human affairs. It implies that when the fabric of
TABLE 10-1 Humans’ Expectable Environments of Rearing
|
Social ecology |
||
|
|
Child care |
|
|
|
• |
Gestation: maternal stress, nutrition, activity |
|
|
• |
Prolonged carrying (devices may be used for holding, carrying) |
|
|
• |
Infant/child signaling-caregiver response (contingency, state regulation) |
|
|
• |
Cosleeping |
|
|
• |
Breastfeeding (and weaning) |
|
|
• |
Variable caregiver competence |
|
|
• |
Provisioning into adolescence with transition to productive in(ter)dependence |
|
|
Family |
|
|
|
• |
Parents |
|
|
• |
Coresident dependent siblings |
|
|
• |
Privileged emotion communication and intersubjective regulation |
|
|
• |
Resident in family into adolescence |
|
|
Social group |
|
|
|
• |
Multiage, mixed-sex with changeable composition (mobility, mortality) |
|
|
• |
Presence of kin and nonkin |
|
|
Pervasive language use, multiple registers (e.g., information exchange, narrative) |
|
|
|
Collaboration |
|
|
|
Sharing and exchange (socially and spatiotemporally displaced reciprocities) |
|
|
|
Contexts for play, practice, and exploration (risk taking) |
|
|
|
• |
Multiage, mixed-sex play groups |
|
|
• |
Tolerance of low productivity, incompetence |
|
|
• |
Surveillance, safe spaces |
|
|
Participant observation in adult activities and competent performances |
|
|
|
• |
Feedback on imitation, provision of instruction, guided participation |
|
|
Use of analogs and symbols (visual, acoustic) |
|
|
|
Tool use |
|
|
|
Fire and thermal buffering (possibly with clothing, coverings) |
|
|
|
Sanctions (physical, verbal, social) |
|
|
Bioecology |
||
|
|
Food constituents and gut activity |
|
|
|
Energetics (resource reliability and quality, maintenance costs) and metabolic regulation |
|
|
|
Exposures to pathogens, parasites, and dirt and immune development |
|
|
|
Sensory inputs, activity patterns, and brain development |
|
|
|
Perceived safety or security and vigilance (attention and arousal regulation) |
|
human social life and culture is repatterned or rent, fertility outcomes (in terms of effective fertility) are changed.
We can expect a large measure of robustness in biocultural transmission for critical features such as language and for those under previous selection for retention of plasticity. Other areas of human developmental ecology may be more vulnerable, particularly (1) emotion and arousal regulation, particularly in affiliation, anxiety, violence and aggression, and toler-
ance of novelty and threat; (2) modes of learning; (3) capacities for behavior change; (4) risk for psychopathology, particularly depression, suicide, and substance use; and (5) health and mortality risk associated with metabolic and eating dysregulation (diabetes, obesity), bioreproductive morbidities (polycystic ovarian disease, breast cancer), and hyperarousal and vigilance.
Demographic and epidemiologic data worldwide suggest that human development, and hence human reproduction, currently face enormous pressures (not all unfavorable). These are well documented in recent surveys of world mental health (Desjarlais et al., 1995), the global burden of disease (Murray and Lopez, 1996), inequity and health (Chen and Berlinguer 2001; Dasgupta, 1993; Deaton, 2001; Gwatkin, 2000; Wilkinson, 1996), and globalization in virtually all domains (World Bank, 2001; Dollar and Collier, 2001). These pressures require attention because some may strain even the large evolved human capacities for plasticity, accommodation, and successful adaptation (Nesse and Williams, 1994; Trevathan et al., 1999). Such pressures may need to be met with thoughtful planning and action.
The issues are biocultural. To get a sense of this, consider the well-documented worldwide shift in growth and maturation rates (Eveleth and Tanner, 1990; Worthman, 1999a) and concurrent dramatic transformations in the ecology of child rearing: changing family composition and maternal employment; widespread schooling of children; urbanization and increased densities; demographic effects of emerging diseases, specifically AIDS; and escalating rates of population dislocation. These formidable challenges raise the bar for effective theory that will support new hypotheses about human behavior, stimulate research, and inform policy. Pushing past dual thinking about the human condition and operationalizing biocultural inheritance models may provide the start to understanding the human implications of these challenges.
ACKNOWLEDGMENTS
Thanks are owed to Kristen Hawkes, Daniel Lende, and Ryan Brown for close reading, critique, and significant additions to this work, and to Randy Bulatao for heroic editorial input.
REFERENCES
Adair, L.S. 2001 Size at birth predicts age at menarche. Pediatrics 107:E59.
Anderson, L.A., P.G. McTernan, A.H. Barnett, and S. Kumar 2001 The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: Influence of gender and site. Journal of Clinical Endocrinology and Metabolism 86:4951-5956.
Anisman, H., A.V. Ravindran, J. Griffiths, and Z. Merali 1999 Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Molecular Psychiatry 4:182-188.
Avitsur, R., and R. Yirmiya 1999 The immunobiology of sexual behavior: Gender differences in the suppression of sexual activity during illness. Pharmacology, Biochemistry and Behavior 64:787-796.
Baile, C.A., T.M. Della-Fera, and R.J. Martin 2000 Regulation of metabolism and body fat mass by leptin. Annual Review of Nutrition 20:105-127.
Baldwin, J.M. 1895 A new factor in evolution. American Naturalist 30:441-451, 536-553.
Barker, D.J.P. 1991 Fetal and Infant Origins of Adult Disease. London: British Medical Journal Publishing.
1997 Intra-uterine programming of the adult cardiovascular system. Current Opinion in Nephrology and Hypertension 6:106-110.
Baskin, D.G., T.M. Hahn, and M.W. Schwartz 1999 Leptin sensitive neurons in the hypothalamus. Hormone and Metabolic Research 31:345-350.
Beran, M.J., E.S. Savage-Rumbaugh, J.L. Pate, and D.M. Rumbaugh 1999 Delay of gratification in chimpanzees (Pan troglodytes). Developmental Psychobiology 34:119-127.
Blurton Jones, N., and M.J. Konner 1976 !Kung knowledge of animal behavior. In Kalahari Hunter-Gatherers. R.B. Lee and I. DeVore, eds. Cambridge, MA: Harvard University Press.
Blurton Jones, N.G., K. Hawkes, and J.F. O’Connell 1989 Studying costs of children in two foraging societies: Implications for schedules of reproduction. Pp. 365-390 in Comparative Socioecology of Mammals and Man. V. Standon and R. Foley, eds. London: Blackwell.
Blurton Jones, N., L.C. Smith, J. O’Connell, K. Hawkes, and C. Kamazura 1992 Demography of the Hadza, an increasing and high-density population of savanna foragers. American Journal of Physical Anthropology 89:159-181.
Bogin, B. 1997 Evolutionary hypotheses for human childhood. Yearbook of Physical Anthropology 40:63-89.
1999 Evolutionary perspectives on human growth. Annual Review of Anthropology 28:109-153.
Bongaarts, J., and R.G. Potter 1983 Fertility, Biology, and Behavior: An Analysis of the Proximate Determinants. New York: Academic Press.
Bonner, J.T. 1974 On Development. The Biology of Form. Cambridge, MA: Harvard University Press.
Borgerhoff Mulder, M. 1992 Reproductive decisions. Pp. 339-374 in Evolutionary Ecology and Human Behavior. E.A. Smith and B. Winterhalder, eds. New York: Aldine de Gruyter.
1995 Bridewealth and its correlates—quantifying changes over time. Current Anthropology 36:573-603.
Boysen, S.T., and G.G. Berntson 1995 Responses to quantity: Perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes). Journal of Experimental Psychology: Animal Behavior Processes 109:47-51.
Brommer, J.E. 2000 The evolution of fitness in life-history theory. Biological Reviews of the Cambridge Philosophical Society 75:377-404.
Bronfenbrenner, U. 1979 The Ecology of Human Development: Experiments by Nature and Design. Cambridge, MA: Harvard University Press.
Bruunsgaard, H., A.N. Pedersen, M. Schroll, P. Skinhøj, and B.K. Pedersen 2000 TNF-a, leptin, and lymphocyte function in human aging. Life Sciences 67:2721-2731.
Caprio, M., E. Fabbrini, A.M. Isidori, A. Aversa, and A. Fabbri 2001 Leptin in reproduction. Trends in Endocrinology and Metabolism 12:65-72.
Caro, J.F., M.K. Sinha, J.W. Kolaczynski, P.L. Zhang, and R.V. Considine 1996 Leptin: The tale of an obesity gene. Diabetes 45:1455-1462.
Changeux, J.-P. 1985 Neuronal Man. New York: Pantheon.
Charnov, E.L. 1993 Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology. Oxford: Oxford University Press.
Chen, L.C., and G. Berlinguer 2001 Health equity in a globalizing world. In Challenging Inequities in Health: From Ethics to Action. T. Evans, M. Whitehead, F. Diderichsen, A. Bhuiya, and M. Wirth, eds. Oxford: Oxford University Press.
Chisholm, J.S. 1993 Death, hope, and sex—Life history theory and the development of reproductive strategies. Current Anthropology 34:1-24.
1996 The evolutionary ecology of attachment organization. Human Nature 7:1-37.
Clark, P.M., P.C. Hindmarsh, A.W. Shiell, C.M. Law, J.W. Honour, and D.J. Barker 1996 Size at birth and adrenocortical function in childhood. Clinical Endocrinology 45(6):721-726.
Crawford, C., and D.L. Krebs, eds. 1998 Handbook of Evolutionary Psychology: Ideas, Issues, and Applications. Mahwah, NJ: Lawrence Erlbaum Associates.
Dantzer, R. 2001 Cytokine-induced sickness behavior: Where do we stand? Brain, Behavior, and Immunity 15:7-24.
Darwin, C. 1871 Descent of Man, and Selection in Relation to Sex. London: John Murray.
Dasgupta, P. 1993 An Inquiry into Well-Being and Destitution. Oxford: Clarendon Press.
Deacon, T.W. 1997 The Symbolic Species: The Co-evolution of Language and the Brain. New York: W. W. Norton.
Deaton, A. 2001 Health, Inequality, and Economic Development. Princeton, NJ: Princeton University.
Desjarlais, R., L. Eisenberg, B. Good, and A. Kleinman 1995 World Mental Health: Problems and Priorities in Low-Income Countries. New York: Oxford University Press.
Dollar, D., and P. Collier 2001 Globalization, Growth, and Poverty: Building an Inclusive World Economy. New York: Oxford University Press.
Edelman, G.M. 1987 Neural Darwinism: The Theory of Neuronal Group Selection. New York: Basic Books.
Ellison, P.T. 1994 Advances in human reproductive ecology. Annual Review of Anthropology 23:255-275.
Eveleth, P.B., and J.M. Tanner 1990 Worldwide Variation in Human Growth. New York: Cambridge University Press.
Fall, C.H., A.N. Pandit, C.M. Law, C.S. Yajnik, P.M. Clark, B. Breier, C. Osmond, A.W. Shiell, P.D. Gluckman, and D.J. Barker 1995 Size at birth and plasma insulin-like growth factor-1 concentrations. Archives of Disease in Childhood 73(4):287-293.
Finch, C.E., and M.R. Rose 1995 Hormones and the physiological architecture of life history evolution. The Quarterly Review of Biology 70(1):1-52.
Geary, D.C., and M.V. Flinn 2001 Evolution of human parental behavior and the human family. Parenting Science and Practice 1:5-61.
Godfrey, K.M. 1998 Maternal regulation of fetal development and health in adult life. European Journal of Obstetrics, Gynecology, and Reproductive Biology 78:141-150.
Gosling, L.M., and M. Petrie 1981 The economics of social organization. Pp. 315-345 in Physiological Ecology: An Evolutionary Approach to Resource Use. C.R. Townsend and P. Calow, eds. Sunderland, MA: Sinauer Associates.
Gottlieb, G. 1991 Experiential canalization of behavioral development: Theory. Developmental Psychology 27:4-13.
1998 Normally occurring environmental and behavioral influences on gene activity: From central dogma to probabilistic epigenesis. Psychological Review 105:792-902.
Gould, S.J. 1977 Ontogeny and Phylogeny. Cambridge, MA: Harvard University Press.
Gwatkin, D.R. 2000 Health inequalities and the health of the poor: What do we know? What can we do? Bulletin of the World Health Organization 78:3-18.
Hamilton, W.D. 1964 The evolution of social behavior. Journal of Theoretical Biology 12:12-45.
Harris, R.B.S. 2000 Leptin—much more than a satiety signal. Annual Review of Nutrition 20:45-75.
Hart, B.L. 1988 Biological basis of the behavior of sick animals. Neuroscience and Biobehavioral Reviews 12:123-137.
Havel, P.J. 2001 Peripheral signals conveying metabolic information to the brain: Short-term and long-term regulation of food intake and energy homeostasis. Experimental Biology and Medicine 226:963-977.
Hawkes, K., J.F. O’Connell, and N.G. Blurton Jones 1997 Hadza women’s time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Current Anthropology 36:551-577.
Henrich, J. 2001 Cultural transmission and the diffusion of innovations: Adoption dynamics indicate that biased cultural transmission is the predominate force in behavioral change. American Anthropologist 103:992-1013.
Henrich, J., R. Boyd, S. Bowles, C. Camerer, E. Fehr, H. Gintis, and R. McElreath 2001 In search of Homo economicus: Behavioral experiments in 15 small-scale societies. American Economic Review 91:73-78.
Hill, K., and A.M. Hurtado 1995 Ache Life History: The Ecology and Demography of a Foraging People. New York: Aldine de Gruyter.
Holliday, R. 1995 Understanding Aging. New York: Cambridge University Press.
Hurtado, A.M. 1990 Seasonality in a foraging society—Variation in diet, work effort, fertility, and sexual division-of-labor among the Hiwi of Venezuela. J Anthropol Res 46:293-346.
Hurtado, A.M., K. Hill , H. Kaplan, and I. Hurtado 1992 Trade-offs between female food acquisition and child care among Hiwi and Ache foragers. Human Nature 3(3):185-216.
International Human Genome Sequencing Consortium 2001 Initial sequencing and analysis of the human genome. Nature 409:860-892.
Kaplan, H. 1994 Evolutionary and wealth flows theories of fertility: Empirical tests and new models. Population Development Review 20:753-791.
1995 Does observed fertility maximize fitness among New Mexican men? A test of an optimality model and a new theory of parental investment in the embodied capital of offspring. Human Nature 6:325-360.
1997 The evolution of the human life course. Pp. 175-211 in Between Zeus and the Salmon. K.W. Wachter and C.E. Finch, eds. Washington, DC: National Academy Press.
Kaplan, H., K.R. Hill, J. Lancaster, and A.M. Hurtado 2000 A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology 9:156-185.
Kirkwood, T.B.L. 1981 Repair and its evolution: Survival versus reproduction. Pp. 165-189 in Physiological Ecology: An Evolutionary Approach to Resource Use. C.R. Townsend and P. Calow, eds. Sunderland, MA: Sinauer Associates.
Maes, M. 1999 Major depression and activation of the inflammatory response system. Advances in Experimental Medicine and Biology 461:25-46.
Maleszka, R., H.G. de Couet, and G.L.G. Miklos 1998 Data transferability from model organisms to human beings: Insights from the functional genomics of the flightless region of Drosophila. Proceedings of the National Academy of Sciences of the United States of America 95:3731-3736.
Mantzoros, C.S. 1999 The role of leptin in human obesity and disease: A review of current evidence. Annals of Internal Medicine 130:671-680.
Mantzoros, C.S., and S.J. Moschos 1998 Leptin: In search of role(s) in human physiology and pathophysiology. Clinical Endocrinology 49:551-567.
Mantzoros, C.S., M. Ozata, A.B. Negrao, M.A. Suchard, M. Ziotopoulou, S. Caglayan, R.M. Elashoff, R.J. Cogswell, P. Negro, V. Liberty, M.L. Wong, J. Veldhuis, I.C. Ozdemir, P.W. Gold, J.S. Flier, and J. Licinio 2001 Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: Evidence for possible partial TSH regulation by leptin in humans. Journal of Clinical Endocrinology and Metabolism 86:3284-3291.
Massaro, D.W. 1998 Perceiving Talking Faces: From Speech Perception to a Behavioral Principle. Cambridge, MA: MIT Press.
Maynard Smith, J. 1978 The Evolution of Sex. Cambridge: Cambridge University Press.
McDade, T.W., and C.M. Worthman 1998 The weanling’s dilemma revisited: A biocultural analysis of breastfeeding ecology. Journal of Developmental Behavioral Pediatrics, 19:286-299.
1999 Evolutionary process and the ecology of human immune function. American Journal of Human Biology 11:705-717.
McDade, T.W., M.A. Beck, C. Kuzawa, and L.S. Adair 2001 Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. American Journal of Clinical Nutrition 74(4):543-548.
McEwen, B.S. 2000 Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology 22:108-124.
Miklos, G.L.G., and R. Maleszka 2000 Deus ex genomix. Nature Neuroscience 3:424-425.
2001 Protein functions and biological contexts. Proteomics 1:169-178.
Murray, C.J.L., and A.D. Lopez 1996 The Global Burden of Disease. Cambridge, MA: Harvard University Press/World Health Organization.
Nesse, R.M., and G.C. Williams 1994 Why We Get Sick: The New Science of Darwinian Medicine. New York: Time Books.
Owen, B.M., D. Eccleston, I.N. Ferrier, and A.H. Young 2001 Raised levels of plasma interleukin-1 beta in major and postviral depression. Acta Psychiatrica Scandinavia 103:226-228.
Oyama, S. 1985 The Ontogeny of Information: Developmental Systems and Evolution. Cambridge: Cambridge University Press.
Promislow, D., and P.H. Harvey 1990 Living fast and dying young: A comparative analysis of life history variation among mammals. Journal of the Zoological Society of London 220:431-437.
Proos, L.A., Y. Hofvander, and T. Tuverno 1991 Menarcheal age and growth pattern of Indian girls adopted in Sweden. Acta Paediatrica Scandinavica 80:852-858.
Rasmussen, A.K. 2000 Cytokine actions on the thyroid gland. Danish Medical Bulletin 47:94-114.
Reeve, H.K., and P.W. Sherman 1993 Adaptation and the goals of evolutionary research. Quarterly Review of Biology 68:1-32.
Ricklefs, R.E. 1998 Evolutionary theories of aging: Confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. American Naturalist 152:24-44.
Rivest, S., and C. Rivier 1995 The role of corticotropin-releasing factor and interleukin-1 in the regulation of neurons controlling reproductive functions. Endocrine Reviews 16:177-199.
Rogoff, B. 1990 Apprenticeship in Thinking: Cognitive Development in Social Context. New York: Oxford University Press.
Schwartz, M.W., R.L. Prigeon, S.E. Kahn, M. Nicolson, J. Moore, A. Morawiecki, E.J. Boyko, and D.Porte 1997 Evidence that plasma leptin and insulin levels are associated with body adiposity via different mechanisms. Diabetes Care 20:1476-1481.
Seeman, T.E., and B.S. McEwen 1996 Impact of social environment characteristics on neuroendocrine regulation. Psychosomatic Medicine 58:459-471.
Smith, E.A., and B. Winterhalder, eds. 1992 Evolutionary Ecology and Human Behavior. New York: Aldine de Gruyter.
Stearns, S.C., and J. Koella 1986 The evolution of phenotypic plasticity in life-history traits: Predictions for norms of reaction for age- and size-at-maturity. Evolution 40:893-913.
Straub, R.H., L.E. Miller, J. Scholmerich, and B. Zietz 2000 Cytokines and hormones: Possible links between endocrinosenescence and immunosenescence. Journal of Neuroimmunology 109:10-15.
Super, C.M., and S. Harkness 1993 The developmental niche: A conceptualization at the interface of child and culture. International Journal of Behavioral Development 9:545-569.
Susser, M., and B. Levin 1999 Ordeals for the fetal programming hypothesis: The hypothesis largely survives one ordeal but not another. British Medical Journal 318:885-886.
Tomasello, M. 1993 Cultural learning. Behavioral and Brain Sciences 16:495-511.
1999 The human adaptation for culture. Annual Review of Anthropology 28:509-529.
Trayhurn, P., and D.V. Rayner 1996 Hormones and the ob gene product (leptin) in the control of energy balance. Biochemical Society Transactions 24(2):565-570.
Trevathan, W.R., E.O. Smith, and J.J. McKenna 1999 Evolutionary Medicine. New York: Oxford University Press.
Trivers, R.L. 1972 Parental investment and sexual selection. Pp. 136-179 in Sexual Selection and the Descent of Man 1871-1971. B. Campbell, ed. Chicago: Aldine.
Venter, J.C., M.D. Adams, E.W. Myers, P.W. Li, R.J. Mural, G.G. Sutton, and H.O. Smith, et al. 2001 The sequence of the human genome. Science 291:1304-1351.
von Laue, S., and R.J. Ross 2000 Inflammatory cytokines and acquired growth hormone resistance. Growth Hormone and IGF Research 10 (Suppl. B):S9-S14.
Vygotsky, L.S. 1978 Mind in Society. Cambridge, MA: Harvard University Press.
1986 Thought and Language. Cambridge, MA: MIT Press.
Wauters, M., R.V. Considine, and L.F. Van Gaal 2000 Human leptin: From an adipocyte hormone to an endocrine mediator. European Journal of Endocinology 143:293-311.
Wilkinson, R.G. 1996 Unhealthy Societies: The Afflictions of Inequality. London: Routledge.
Williams, G.C. 1957 Pleiotropy, natural selection, and the evolution of senescence. Evolution 11:398-411.
1985 Sex and Evolution. Princeton, NJ: Princeton University Press.
Williams, S., and R. Poulton 1999 Twins and maternal smoking: Ordeals for the fetal origins hypothesis? A cohort study. British Medical Journal 318:897-900.
Wood, J.W. 1994 Dynamics of Human Reproduction. New York: Aldine de Gruter.
Worthman, C.M. 1999a Evolutionary perspectives on the onset of puberty. Pp. 135-163 in Evolutionary Medicine. W. Trevathan, J. McKenna, and E. Smith, eds. New York: Oxford University Press.
1999b The epidemiology of human development. Pp. 47-104 in Hormones, Health and Behavior. C. Panter-Brick and C.M. Worthman, eds. Cambridge: Cambridge University Press.
2002 Endocrine pathways to differential well-being across the life course. In A Life Course Approach to Women’s Health, D. Kuh and R. Hardy, eds. Oxford: Oxford University Press.
Yirmiya, R., Y. Pollak, M. Morag, S.A. Reichenberg, O. Barak, R. Avitsur, H. Ovadia, J. Weidenfeld, A. Morag, M.E. Newman, and T. Pollmacher 2000 Illness, cytokines, and depression. New York Academy of Sciences 917:478-487.
World Bank 2001 Facets of Globalization. New York: Oxford University Press.
World Health Organization 1995 Physical Status: The Use and Interpretation of Anthropometry. Geneva: WHO.

































