1
Nerve Agents GA, GB, GD, GF, and VX1
Acute Exposure Guideline Levels
SUMMARY
The nerve agents for which AEGL analyses have been performed include the G-series agents (GA [tabun], GB [sarin], GD [soman], and GF) and nerve agent VX. These agents are all toxic ester derivatives of phosphonic acid containing either a cyanide, fluoride, or sulfur substituent group; they are commonly termed “nerve” agents as a consequence of their anticholin-
esterase properties. These compounds were developed as chemical warfare agents, and one (agent GB, or sarin) was used by terrorists in the 1995 exposure incident that took place in the Tokyo subway system. The chemical names of these five agents are as follow: agent GA, dimethylamidocyanoethylphosphate (CAS Registry No. 77–81–6); agent GB, isopropyl methylphosphonofluoridate (CAS Registry No. 107–44–8); agent GD, pinacolyl methylphosphonofluoridate (CAS Registry No. 96–64–0); agent GF, O-cyclohexylmethyl-fluorophosphonate (CAS Registry No. 329–99–7); and agent VX, O-ethyl-S-(diisopropylaminoethyl) methyl phosphonothiolate (CAS Registry No. 50782–69–9).
The G agents are all viscous liquids of varying volatility (vapor density relative to air between 4.86 and 6.33) with faint odors (“faintly fruit,” or “spicy,” odor of camphor). Toxic effects may occur at vapor concentrations below those of odor detection. Agent VX is a amber-colored liquid with a vapor density of 9.2 (air=1) and is considered odorless. As a consequence, agent VX vapor possesses no olfactory warning properties.
The vapor pressures and acute toxicity of these agents are sufficiently high for the vapors to be rapidly lethal. Within the G-series, GB is considered a greater vapor hazard than agent GD. Agent GA represents a smaller vapor hazard and is expected to present a relevant contact hazard. The vapor density of agent GF is intermediate between that of agents GA and GD. Agent VX, which has a vapor density (9.2) greater that of any G agent under consideration, was deliberately formulated to possess a low volatility; VX is approximately 2,000 times less volatile than nerve agent GB (DA 1990). As a consequence, agent VX is a persistent, “terrain denial” military compound with the potential to off-gas toxic vapor for days following surface application.
Exposure to acutely toxic concentrations of nerve agents can result in excessive bronchial, salivary, ocular, and intestinal secretions and sweating, miosis, bronchospasm, intestinal hypermotility, bradycardia, muscle fasciculations, twitching, weakness, paralysis, loss of consciousness, convulsions, depression of the central respiratory drive, and death. Minimal effects observed at low vapor concentrations include miosis (contraction of the pupils of the eye, with subsequent decrease in pupil area), tightness of the chest, rhinorrhea, and dyspnea (Dunn and Sidell 1989).
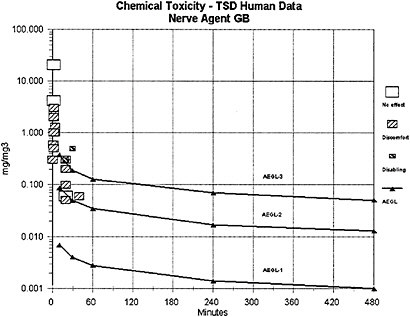
The results of agent GB vapor exposure studies conducted with human volunteers indicate that the threshold for miosis and other minimal toxic effects falls in the range of 0.05–0.5 mg/m3 for 10–30 minute (min) exposures. The findings are based on the results of low-concentration nerve agent exposures of informed volunteers who were under clinical supervi-
sion during the periods of exposure as well as for postexposure periods of several months.
A concern associated with symptomatic exposures to anticholinesterase compounds such as the nerve agents is the possibility of chronic neurological effects. There is, at present, no evidence indicating that asymptomatic exposures to any of the nerve agents result in chronic neurological disorders. In general, the available epidemiological data indicate that most clinical signs of toxicity resolve within hours to days; severe miosis can require several months after exposure for resolution. However, several studies have shown that subclinical signs may persist for longer periods. Following the chemical terrorist attacks with nerve agent GB (sarin) that occurred in Japan in 1994 and 1995, clinical signs of agent toxicity were no longer apparent in the surviving victims 3 months (mo) after the exposures had occurred; however, several studies conducted on a small number of asymptomatic individuals 6–8 mo after the attack revealed subclinical signs of neurophysiological deficits as measured by event-related and visual evoked potentials, psychomotor performance, and increases in postural sway.
Small but measurable changes in single fibre electromyography (SFEMG) of the forearm were detectable between 4 and 15 mo following exposure to a concentration of agent GB that produced minimal clinical signs and symptoms in fully informed human subjects who were under clinical supervision in compliance with Helsinki accords (Baker and Sedgwick 1996). The SFEMG effects were not clinically significant and were not detectable after 15–30 mo. In a separate study of workers who had been occupationally exposed to agent GB (sarin), altered electroencephalograms (EEGs) were recorded 1 year (y) or more after the last exposure had occurred. Spectral analysis of the EEGs indicated significant increases in brain beta activity (12–30 Hz) in the exposed group when compared with nonexposed controls, and sleep EEGs revealed significantly increased rapid eye movement in the exposed workers; however, those observations were not clinically significant. Increases in beta activity were also observed in rhesus monkeys 1 y after being dosed with GB at 5 mg/kg. Slight, but nonsignificant, increases in beta activity, without deleterious effects on cognitive performance, were reported for marmosets injected with GB at 3.0 mg/kg and tested 15 mo later. The significance of subclinical neurological effects for the long-term health of exposed individuals has not been determined.
Animal data from vapor and oral exposure studies for the G-series nerve agents and agent VX suggest that agents GB and VX do not induce
reproductive or developmental effects in mammals. Oral exposure studies of agent GD in lab animals as well as injection exposure studies of agent GA likewise suggest a lack of reproductive or development effects for these agents. Neither agent GB nor agent VX were found to be genotoxic in a series of microbial and mammalian assays, but agent GA was reported to be weakly mutagenic. There is no evidence indicating that agents GB, GA, or VX are carcinogenic.
Derivation of G-Agent AEGL Estimates
The base of data for toxicological effects in humans is more complete for agent GB than for any of the other nerve agents under consideration in this analysis. Furthermore, agent GB is the only G agent for which sufficient human data are available to directly derive AEGL-1 and AEGL-2 estimates, and the only G agent for which sufficient laboratory animal data are available for deriving an AEGL-3 value for all five AEGL time periods.
AEGL-1 and AEGL-2 Values for G-series Agents
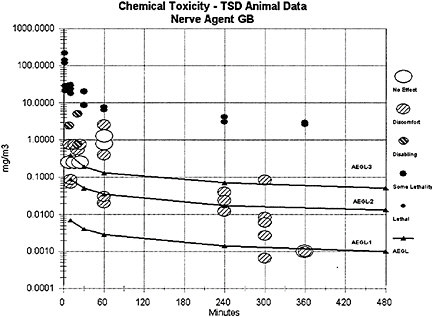
The AEGL-1 values for agent GB were derived from a well-conducted study on adult female Sprague-Dawley rats exposed whole-body in a dynamic airflow chamber to a range of GB vapor concentrations (0.01 to 0.48 mg/m3) over three time durations (10 min, 60 min, or 240 min) (total of 283 agent-exposed rats of which 142 were female and 141 were male) (Mioduszewski et al. 2002b). With the inclusion of range-finding experiments and controls (N=130), a total of 423 rats were used in this well-conducted study, which involved highly credible protocols for GB vapor generation and measurement. Analysis of rat pupil diameters assessed pre-and postexposure allowed determination of EC50 values for miosis (defined as a postexposure pupil diameter of 50% or less of the preexposure diameter in 50% of the exposed population). Blood samples collected from tail vein and heart at 60 min and 7 d postexposure indicated no significant change from preexposure baseline in monitored blood RBC-ChE, butyrylcholinesterase (BuChE) or carboxylesterase. No other clinical signs were evident throughout the duration of the study. Gender differences (females more susceptible) were statistically significant at 10 min (p= 0.014) and 240 min (p=0.023), but not at 60 min (p=0.054). This is a
well-defined animal end point in a susceptible gender, and it is transient, reversible, and nondisabling.
In terms of potential effects on humans, an EC50 for miosis is not considered an adverse effect. This degree of miosis is the first measurable change, by modern and reproducible techniques, in the continuum of response to anticholinesterase compounds. In bright daylight or under bright lighting, a 50% reduction in pupil diameter would result in greater visual acuity among some members of the affected exposed population and no marked reduction in visual acuity for the majority of the affected population. In twilight or dim light conditions, 50% reduction in pupil diameter in some persons would result in reduced visual acuity and less-than-optimal performance of tasks requiring operation of vehicular controls, monitoring or tracking on computer screens, reading of fine text, or shifts in focus between near and far fields. For individuals with central cataracts, the effects would be more pronounced at all illumination levels. During the Tokyo Subway Incident (terrorist release of GB), persons experiencing ≥50% reduction in pupil diameter were able to self-rescue and to render aid to others.
Data from GB vapor studies of nonhuman primates (marmosets, 5 h exposures to GB vapor concentrations at 0.05 to 150 µg/m3) (van Helden et al. 2001, 2002) and human volunteers (minimal and reversible effects of miosis, rhinorrhea, headache, etc., after a 20-min exposure to a GB vapor concentration at 0.05 mg/m3) (Harvey 1952; Johns 1952) are considered secondary and supportive. The human data of Harvey (1952) and Johns (1952) indicate that some adult humans exposed to concentrations within the exposure range tested by Mioduszewski et al. (2002b) would experience some discomfort (headache, eye pain, nausea, etc.) in addition to miosis corresponding to ≤50% pupil area decrement but no disability (see definition of AEGL-1 provided in NRC [2001]). Compared to the available human data, the miosis data derived from the study on rats (Mioduszewski et al. 2002b) are considered a more reliable data set because they are based on current and multiple analytical techniques for quantifying exposures and measuring miosis and because they apply an experimental protocol incorporating sufficiently large test and control populations. With the additional knowledge that the EC50 exhibited by rats in the study of Mioduszewski et al. (2002b) is transient and reversible, the determination was made that EC50 for miosis in female (susceptible gender) SD rats is an appropriate end point for estimating AEGL-1 values. Mioduszewski et al. (2002b) is considered the critical study for derivation of AEGL-1 estimates for agent GB.
The weight-of-evidence analysis indicates reasonable concordance among AEGL-1 estimates derived from the female Sprague-Dawley rat, the marmoset, and the human data sets identified above. Application of the Mioduszewski et al. (2002b) rat miosis data did not significantly change the interim values for AEGL-1 (based on the human experimental data of Harvey [1952] and Johns [1952]) but confirmed that the interim values were representative, protective, and could be retained as final AEGL-1 values.
The AEGL-2 values for agent GB were derived from a study in which miosis, dyspnea, photophobia, inhibition of red blood cell cholinesterase (RBC-ChE), and changes in single fibre electromyography (SFEMG) were observed in human volunteers following a 30-min exposure at 0.5 mg/m3 (Baker and Sedgwick 1996). The SFEMG changes noted in the study were not clinically significant and were not detectable after 15–30 mo. Baker and Sedgwick considered SFEMG changes a possible early indicator or precursor of the nondepolarising neuromuscular block associated with intermediate-syndrome paralysis in severe organophosphorous insecticide poisoning cases. They concluded that the electromyographic changes were persistent (>15 mo), but that they were reversible and subclinical.
Although not considered debilitating or permanent effects in themselves, SFEMG changes are considered an early indicator of exposures that potentially could result in more significant effects. Selection of this effect as a protective definition of an AEGL-2 level is considered appropriate given the steep dose-response toxicity curve of nerve agents (Aas et al. 1985; Mioduszewski et al. 2000, 2001, 2002a). The concept of added precaution for steep dose-response is consistent with the emergency planning guidance for nerve agents that was developed by the National Center for Environmental Health of the Centers for Disease Control and Prevention (Thacker 1994).
Animals exposed to low concentrations of the G agents exhibit the same signs of toxicity as humans, including miosis, salivation, rhinorrhea, dyspnea, and muscle fasciculations. Studies on dogs and rats indicate that exposures to GB at 0.001 mg/m3 for up to 6 h/d are unlikely to produce any signs of toxicity.
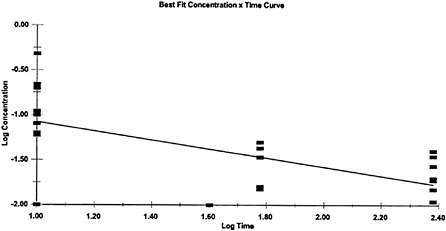
Because exposure-response data were not available for all of the AEGL-specific exposure durations, temporal extrapolation was used in the development of AEGL values for some of the AEGL-specific time periods. The concentration-exposure time relationship for many systemically acting vapors and gases may be described by Cn×t=k, where the exponent n ranges from 0.8 to 3.5. The temporal extrapolation used here is based on
a log-log linear regression of the LC01 lethality of GB in female Sprague-Dawley rats (Mioduszewski et al. 2000, 2001, 2002a) and a log-log linear regression of female SD rat miosis data following GB vapor exposure for durations of 10–240 min (Mioduszewski et al. 2002b). Regression analysis of the LC01 values yields an n value of 1.93 with an r2 of 0.9948, and regression analysis of the miosis data yields an n value of 2.00 with an r2 of 0.4335 (24 data points; see Appendix B). Given that all mammalian toxicity end points observed in the data set for all nerve agents represent different points on the response continuum for anticholinesterase exposure, and that the mechanism of acute mammalian toxicity (cholinesterase inhibition) is the same for all nerve agents, the experimentally derived n=2 from the Mioduszewski et al. (2000, 2001, 2002a,b) rat lethality and miosis data sets is used as the scaling function for all the AEGL derivations rather than a default value. An n of 1.16 (r2=0.6704) was calculated for comparison using other data (human volunteer) and other end points (e.g., GB-induced miosis in humans; see Appendix B). However, because of uncertainties associated with some of the exposure measurements in the earlier studies, the Mioduszewki et al. rat data were determined to be the best source of an estimate for n. The n value of 2 was used to extrapolate for exposure time periods for which there were no experimental data. Those included (1) the 8-h AEGL-3 value (extrapolated from experimental data for 6 h); (2) the 30-min and 8-h AEGL-1 values (extrapolated from 10-min and 4-h experimental data; and (3) all of the AEGL-2 values (extrapolated from experimental data for 30 min).
In consultation with experimental investigators at Porton Down (United Kingdom) and the TNO Prins Maurits Laboratory (Netherlands), the analysis has determined that the miotogenic response of mammalian eyes to agent GB vapor exposure is similar across species. The species evaluated include standard laboratory animals (rabbits, rats, guinea pigs), nonhuman primates (marmosets), and humans. As a consequence, the interspecies uncertainty factor (UF) for the critical AEGL-1 end point of miosis is considered equal to 1. To accommodate known variation in human cholinesterase and carboxylesterase activity that may make some individuals susceptible to the effects of cholinesterase inhibitors such as nerve agents, a factor of 10 was applied for intraspecies variability (protection of susceptible populations). A modifying factor is not applicable. Thus, the total UF for estimating AEGL-1 values for agent GB is 10.
The fact that AEGL-2 analyses for agent GB are based on data from human volunteers (Baker and Sedgwick 1996) precludes the use of an interspecies UF. As was the case in the AEGL-1 estimations, a factor of 10 was
applied for intraspecies variability (protection of susceptible populations). A modifying factor is not applicable. Thus, the total UF for estimating AEGL-2 values for agent GB is 10.
In comparison to the data set for agent GB, the data sets characterizing the toxicity of agents GA, GD, and GF are less complete. However, the database for the G agents as a group is considered reasonably complete in that there is/are (1) experimental data for multiple species, including humans; (2) documented nonlethal and lethal end points that follow an exposure-response curve; (3) a known mechanism of toxicity common to all the G agents with all end points representing a response continuum to inhibition of cholinesterase activity; and (4) no uncertainties regarding other toxic end points such as reproductive or developmental effects or carcinogenicity. Because the mechanism of action is the same for all the G agents, data uncertainty is reduced, and target organ effects are expected to be identical, but different in magnitude. Thus, it was possible to develop AEGL estimates for agents GA, GD, and GF by a comparative method of relative potency analysis from the more complete data set for agent GB. This concept has been applied before in the estimation of G-series nerve agent exposure limits, most recently by Mioduszewski et al. (1998).
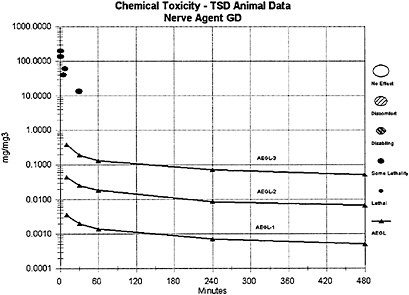
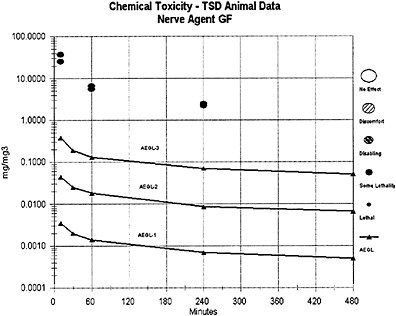
The AEGL-1 and AEGL-2 values for agents GA, GD, and GF were derived from the AEGL-1 and AEGL-2 values for GB using a relative potency approach based on the potency of the agents needed to induce LOAEL effects of miosis, rhinorrhea, and SFEMG and agent concentration in milligrams per cubic meter. Agents GA and GB were considered to have an equivalent potency for causing miosis; thus, the AEGL-1 values for agents GA and GB are equal in milligrams per cubic meter. Agents GD and GF are considered approximately 2 times as potent as agents GB or GA for these end points, and equipotent to each other for AEGL-1 and AEGL-2 effects. Thus, the AEGL-1 and AEGL-2 concentration values for agents GD and GF are equal to 0.5 times those values derived for agents GA and GB, in milligrams per cubic meter.
AEGL-3 Values for G-Series Agents
AEGL-3 values for agent GB were derived from recent inhalation studies in which the lethality of GB vapor in female Sprague-Dawley rats was evaluated for 10-, 30-, 60-, 90-, 240-, and 360-min time periods (Mioduszewski et al. 2000, 2001, 2002a). Both experimental LC01 and LC50 values were evaluated. The use of a rat data set resulted in selection
of an interspecies UF of 3; the full default value of 10 was not considered appropriate because the mechanism of toxicity in rats and humans is the same, and lethality represents one point on the response continuum for these anticholinesterase compounds. The full default value of 10 for intraspecies uncertainty was considered necessary to protect susceptible populations. Because a modifying factor is not applicable, the composite UF for AEGL-3 determination for agent GB is equal to 30.
The AEGL-3 values for agent GA were derived from the AEGL-3 values for GB using a relative potency approach based on lethality of the agents; the potency of agent GA was considered to be only one-half that of agent GB for this end point. Thus, the AEGL-3 concentration values for agent GA are equal to 2.0 times the AEGL-3 values for agent GB, in milligrams per cubic meter.
The lethal potencies of agents GD and GF are considered equivalent and equipotent to that of agent GB; thus, the AEGL-3 concentration values for agent GB, GD, and GF are equal in milligrams per cubic meter, and the same composite UF (30) was applied in the derivation of the AEGL-3 values for agents GB, GD, and GF. For comparison, AEGL-3 values for GD were alternately derived from a secondary and short-term GD inhalation study of rat lethality for exposure times ≤30 min (Aas et al. 1985). As was the case in the derivation of the GB AEGLs, an n value of 2 was used for extrapolating to different time periods; however, because of the sparse data set for GD, the full default values for interspecies (10) and intraspecies (10) uncertainty were applied to the Aas et al. (1985) data. Because a modifying factor is not applicable, a composite UF of 100 was used for the Aas et al. (1985) data, whereas in the GB AEGL derivation from the Mioduszewski et al. (2000, 2001, 2002a) rat lethality data, a composite UF of 30 was used. The resulting 10-min AEGL-3 (0.27 mg/m3) and 30-min AEGL-3 (0.15 mg/m3) estimates for agent GD from Aas et al. (1985) are very similar to those for GB (0.38 mg/m3 for 10 min and 0.19 mg/m3 for 30 min) from Mioduszewski et al. (2000, 2001, 2002a) and support the assumption of lethal equipotency for agents GB and GD.
Derivation of Agent VX AEGL Estimates
Insufficient data are available from which to directly derive AEGL values for VX from human or animal inhalation toxicity studies. The few studies available are historical and are considered nonverifiable because of flawed study design, poor sampling techniques, or suspect contamination
of sampling and detection apparatus. Nevertheless, available literature clearly indicates that inhibition of cholinesterase activity is a common mechanism of toxicity shared by the G-series nerve agents and nerve agent VX. Thus, it was possible to develop AEGL estimates for agent VX by a comparative method of relative potency analysis from the more complete data set for nerve agent GB. The concept has been applied before in the estimation of agent VX exposure limits, most recently by Reutter et al. (2000). There are a number of estimates in the literature regarding the potency of VX relative to agent GB; all estimates indicate that vapor toxicity for agent VX is greater than that for agent GB. Comparable RBC-ChE50 data from clinically supervised human volunteers (Grob and Harvey 1958; Sidell and Groff 1974), who were exposed to agents GB and VX during well-conducted studies, are available for estimation of relative potency. The human data indicate that agent VX is approximately 4 times more potent than agent GB for inducing the RBC-ChE50 end point, which is considered an early and quantitative measure of the response continuum known for those compounds. Thus, the GB:VX relative potency ratio of 4 is considered an appropriate estimate of GB:VX relative potency for all VX AEGL determinations.
All mammalian toxicity end points observed in the data set for nerve agent VX as well as the G-series agents represent different points on the response continuum for anticholinesterase effects. Further, the mechanism of mammalian toxicity (cholinesterase inhibition) is the same for all nerve agents. In consequence, the experimentally derived n=2 from the Mioduszewski et al. (2000, 2001, 2002a,b) rat miosis and lethality data sets for agent GB are used as the scaling function for the agent-VX AEGL-1, AEGL-2, and AEGL-3 derivations rather than a default value.
By applying the GB:VX relative potency concept outlined above (the relative potency of GB:VX equal to 4), the AEGL-1 analyses for agent VX are derived from miosis data for adult female SD rats exposed to GB vapor for three time durations of significance for AEGLs (10, 60, and 240 min) (Mioduszewski et al. 2002b). Data from a GB vapor study of nonhuman primates (marmosets, 5 h exposures to GB vapor concentrations at 0.05–150 µg/m3) (van Helden et al. 2001, 2002) and human volunteers (minimal and reversible effects of miosis, rhinorrhea, headache, etc., after a 20-min exposure to a GB vapor concentration at 0.05 mg/m3) (Harvey 1952; Johns 1952) are considered secondary and supportive. The same UFs and logic applied in the derivation of AEGL-1 and AEGL-2 values for agent GB (e.g., interspecies UF of 1, intraspecies UF of 10) are used here for estimat-
ing AEGL-1 and AEGL-2 values for agent VX. With application of a modifying factor of 3 for the sparse VX data set, the total UF for estimating AEGL-1 values for agent VX (from the GB data set of Mioduszewski et al. [2002b]) is 30.
By further application of the GB:VX relative potency concept outlined above, the AEGL-2 values for agent VX were derived from a GB vapor exposure study of human subjects in which miosis, dyspnea, photophobia, inhibition of red blood cell cholinesterase (RBC-ChE) to approximately 60% of individual baseline, and small but measurable changes in SFEMG of the forearm occurred following a 30-min exposure at 0.5 mg GB/m3 (Baker and Sedgwick 1996).
The fact that AEGL-2 analyses for agent VX are based on data from clinically supervised human volunteers exposed to GB vapor (Baker and Sedgwick 1996) precludes the use of an interspecies UF. With application of a factor of 10 for intraspecies variability and a modifying factor of 3 for the sparse VX data set, the total UF for estimating AEGL-2 values for agent VX (from the GB data set of Baker and Sedgwick [1996]) is 30.
By further application of the GB:VX relative potency concept outlined above, the AEGL-3 values for agent VX were derived from recent inhalation studies in which the lethality of GB to female Sprague-Dawley rats was evaluated for the 10-, 30-, 60-, 90-, 240-, and 360-min time periods (Mioduszewski et al. 2000, 2001, 2002a). Both experimental LC01 and LC50 values were evaluated. The same UFs and logic applied in the derivation of AEGL-3 values for agent GB (interspecies UF of 3 and an intraspecies UF of 10) are used here for agent VX. With the additional application of a modifying factor of 3 for the sparse VX data set, the total UF for AEGL-3 determination for agent VX is equal to 100.
Research Needs
G-Series Agents
Further data analysis and experimentation is needed to more fully understand gender differences in susceptibility to nonlethal and lethal end points among the test population of SD rats. Interspecies susceptibility could be more fully characterized by determining if similar results can be obtained for the same protocol with different test species (particularly nonhuman primates).
The scarcity of dose-response data for agents GA, GD, and GF forces
the AEGL analysis to rely on assumptions of relative potency that need experimental confirmation.
Agent VX
It is noted that additional research to more fully characterize VX is needed in the following areas:
-
The toxicity of VX vapor in whole-animal systems. It is noted that specific experimental focus should be on obtaining data that would reduce uncertainties regarding the relative potency of agents GB and VX, or the potency of agent VX, for critical effects such as miosis, rhinorrhea, and lethality. Such studies could be adequately performed on a limited test population and scale.
-
The emissions profile expected during VX release, especially the generation and yield of VX vapors versus aerosol.
-
Comparative examination of agents GB and VX with regard to noncholinergic mechanisms in an effort to correlate whole-organism toxic responses with those reported for in vitro rat hippocampal cells in culture. The primary goal would be to generate a more refined determination of GB:VX relative potency.
Final AEGL estimates for the G-series nerve agents and VX are given in the summary table below.
1. INTRODUCTION
This evaluation of the AEGL values for the nerve agents GA, GB, GD, and GF is based on studies and data that are documented in the open literature as well as some unclassified documents with limited distribution requirements. Because of the military-specific nature of these compounds, some additional reports from the United States and elsewhere with classified or restricted distribution requirements exist. However, because of the open review process established by the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances, classified and other restricted-distribution reports are not cited in this evaluation, to the best of our knowledge.
TABLE 1–1 Summary of Final AEGL Values for Nerve Agents GA, GB, GD, GF, and VXa
|
Agent |
Classification |
10-min |
30-min |
1-h |
4-h |
8-h |
End Point (Reference) |
|
GA |
AEGL-1 (Nondisabling) |
0.0010 ppm (0.0069 mg/m3) |
0.00060 ppm (0.0040 mg/m3) |
0.00042 ppm (0.0028 mg/m3) |
0.00021 ppm (0.0014 mg/m3) |
0.00015 ppm (0.0010 mg/m3) |
Based on relative potency from GBb |
|
|
AEGL-2 (Disabling) |
0.013 ppm (0.087 mg/m3) |
0.0075 ppm (0.050 mg/m3) |
0.0053 ppm (0.035 mg/m3) |
0.0026 ppm (0.017 mg/m3) |
0.0020 ppm (0.013 mg/m3) |
Based on relative potency from GBb |
|
|
AEGL-3 (Lethal) |
0.11 ppm (0.76 mg/m3) |
0.057 ppm (0.38 mg/m3) |
0.039 ppm (0.26 mg/m3) |
0.021 ppm (0.14 mg/m3) |
0.015 ppm (0.10 mg/m3) |
Based on relative potency from GBc |
|
GB |
AEGL-1 (Nondisabling) |
0.0012 ppm (0.0069 mg/m3) |
0.00068 ppm (0.0040 mg/m3) |
0.00048 ppm (0.0028 mg/m3) |
0.00024 ppm (0.0014 mg/m3) |
0.00017 ppm (0.0010 mg/m3) |
EC50 for miosis observed in adult female SD rats exposed to a range of GB vapor concentrations (0.01–0.48 mg/m3) for 10, 60, and 240 min (Mioduszewski et al. 2002b) and miosis data from secondary and supportive studies with |
|
Agent |
Classification |
10-min |
30-min |
1-h |
4-h |
8-h |
End Point (Reference) |
|
|
marmosets (van Helden et al. 2001, 2002) and humans (Harvey 1952; and Johns 1952) |
||||||
|
|
AEGL-2 (Disabling) |
0.015 ppm (0.087 mg/m3) |
0.0085 ppm (0.050 mg/m3) |
0.0060 ppm (0.035 mg/m3) |
0.0029 ppm (0.017 mg/m3) |
0.0022 ppm (0.013 mg/m3) |
Miosis, dyspnea, RBC-ChE inhibition, single fibre electromyography (SFEMG) changes in human volunteers exposed at 0.5 mg/m3 for 30 min (Baker and Sedgwick 1996) |
|
|
AEGL-3 (Lethal) |
0.064 ppm (0.38 mg/m3) |
0.032 ppm (0.19 mg/m3) |
0.022 ppm (0.13 mg/m3) |
0.012 ppm (0.070 mg/m3) |
0.0087 ppm (0.051 mg/m3) |
Based on experimental SD rat lethality data (LC01 and LC50); whole-body dynamic exposure to concentrations between 2 and 54 mg/m3 for 3, 10, 30, 60, 90, 240, and 360 min (Mioduszewski et al. 2000, 2001, 2002a) |
|
GD |
AEGL-1 (Nondisabling) |
0.00046 ppm (0.0035 mg/m3) |
0.00026 ppm (0.0020 mg/m3) |
0.00018 ppm (0.0014 mg/m3) |
0.000091 ppm (0.00070 mg/m3) |
0.000065 ppm (0.00050 mg/m3) |
Based on relative potency from GBd |
|
|
AEGL-2 (Disabling) |
0.0057 ppm (0.044 mg/m3) |
0.0033 ppm (0.025 mg/m3) |
0.0022 ppm (0.018 mg/m3) |
0.0012 ppm (0.0085 mg/m3) |
0.00085 ppm (0.0065 mg/m3) |
Based on relative potency from GBd |
|
|
AEGL-3 (Lethal) |
0.049 ppm (0.38 mg/m3) |
0.025 ppm (0.19 mg/m3) |
0.017 ppm (0.13 mg/m3) |
0.0091 ppm (0.070 mg/m3) |
0.0066 ppm (0.051 mg/m3) |
Based on relative potency from GB; supported by Wistar rat LC50; dynamic chamber exposures at 21 mg/m3 for three time periods of ≤30 min (Aas et al. 1985)e |
|
GF |
AEGL-1 (Nondisabling) |
0.00049 ppm (0.0035 mg/m3) |
0.00028 ppm (0.0020 mg/m3) |
0.00020 ppm (0.0014 mg/m3) |
0.00010 ppm (0.00070 mg/m3) |
0.000070 ppm (0.00050 mg/m3) |
Based on relative potency from GBd |
|
|
AEGL-2 (Disabling) |
0.0062 ppm (0.044 mg/m3) |
0.0035 ppm (0.025 mg/m3) |
0.0024 ppm (0.018 mg/m3) |
0.0013 ppm (0.0085 mg/m3) |
0.00091 ppm (0.0065 mg/m3) |
Based on relative potency from GBd |
|
|
AEGL-3 (Lethal) |
0.053 ppm (0.38 mg/m3) |
0.027 ppm (0.19 mg/m3) |
0.018 ppm (0.13 mg/m3) |
0.0098 ppm (0.070 mg/m3) |
0.0071 ppm (0.051 mg/m3) |
Based on relative potency from GBe |
|
Agent |
Classification |
10-min |
30-min |
1-h |
4-h |
8-h |
End Point (Reference) |
|
VXf |
AEGL-1 (Nondisabling) |
0.000052 ppm (0.00057 mg/m3) |
0.000030 ppm (0.00033 mg/m3) |
0.000016 ppm (0.00017 mg/m3) |
0.0000091 ppm (0.00010 mg/m3) |
0.0000065 ppm (0.000071 mg/m3) |
Derived by relative potency from EC50 for miosis observed in adult female SD rats exposed to a range of GB vapor concentrations (0.01–0.48 mg/m3) for 10, 60, and 240 min (Mioduszewski et al. 2002b) and miosis data from secondary and supportive studies of van Helden et al (2001, 2002), Harvey (1952), and Johns (1952) in marmosets and humans, respectivelyg |
|
|
AEGL-2 (Disabling) |
0.00065 ppm (0.0072 mg/m3) |
0.00038 ppm (0.0042 mg/m3) |
0.00027 ppm (0.0029 mg/m3) |
0.00014 ppm (0.0015 mg/m3) |
0.000095 ppm (0.0010 mg/m3) |
Derived by relative potency from study of GB vapor exposure to exercising human volunteers exposed at 0.5 mg/m3 for 30 min; miosis, dyspnea, inhibition of RBC-ChE, changes in single fibre electromyography (SFEMG) (Baker and Sedgwick 1996)h |
|
|
AEGL-3 (Lethal) |
0.0027 ppm (0.029 mg/m3) |
0.0014 ppm (0.015 mg/m3) |
0.00091 ppm (0.010 mg/m3) |
0.00048 ppm (0.0052 mg/m3) |
0.00035 ppm (0.0038 mg/m3) |
Derived by relative potency from experimental SD rat lethality data (LC01 and LC50); whole-body dynamic exposure to GB vapor concentrations between 2 and 54 mg/m3 for 3, 10, 30, 60, 90, 240, and 360 min (Mioduszewski et al. 2000, 2001, 2002a)i |
|
aThe derived AEGL values are for vapor exposures only. Percutaneous absorption of nerve agent vapors is known to be an effective route of exposure; nevertheless, percutaneous vapor concentrations needed to produce similar adverse effects are greater than inhalation vapor concentrations by several orders of magnitude. (For agent VX, the percutaneous vapor concentrations needed to produce similar adverse effects are greater than inhalation vapor concentrations by an approximate factor of 10.) Thus, the AEGL values presented are considered protective for both inhalation and percutaneous routes of exposure. bBased on relative potency equal to that of agent GB (see Section 4.3 and Mioduszewski et al. [1998]). cAgent GA is considered approximately one-half as potent as GB in lethality; thus, AEGL-3 values for GA are estimated by multiplying each time-specific AEGL-3 value for agent GB by a factor of 2 (see Section 4.3 and Mioduszewski et. al. [1998]). dAgents GD and GF are considered approximately twice as potent as agents GA and GB for causing miosis, and they are equipotent to each other. Thus, AEGL-1 and AEGL-2 values are estimated by multiplying each time-specific AEGL-1 or AEGL-2 value for agent GB by a factor of 0.5 (see Section 4.3 and Mioduszewski et al. [1998]). eBased on a relative potency for lethality of GD=GF=GB and lethality data of Aas et al. (1985) (which provides a 10-min AEGL-3 estimate of 0.27 mg/m3 and a 30-min AEGL-3 value of 0.15 mg/m3 and is thus supportive of the GD AEGL-3 estimate derived from relative potency) (see Section 4.3 and Appendix A). fBased on relative potency. Agent VX is considered approximately 4 times more potent than agent GB (see Section 4.3.4, Grob and Harvey [1958], and Sidell and Groff [1974]). |
|||||||
The chemical-warfare agents discussed here are highly toxic organophosphate ester derivatives of phosphonic acid. They are commonly termed “nerve” agents as a consequence of their anticholinesterase properties and subsequent adverse effects on smooth and skeletal muscle function as well as the central nervous system. As a group, nerve agents are divided into the G-series agents (“G” for German, identifying these agents as among those secretly developed by the German Ministry of Defense before and during World War II—they contain a fluorine or cyanide substituent group) and the V agents (which contain a sulfur substituent group) (Sidell 1997). The G agents addressed in the current analysis include GA, or tabun (dimethylamidocyanoethylphosphate; C3H11N2O2P); GB, or sarin (isopropyl methylphosphonofluoridate; C4H10FO2P); GD, or soman (pinacolyl methylphosphonofluoridate; C7H16FO2P); and GF (O-cyclohexyl-methylfluorophosphonate; C7H14FO2P). The V agent discussed in this document is VX (S-(diisopropyl aminoethyl) methyl phosphonothiolate, O-ethyl ester; C11H26NO2PS). Agent VX is a persistent, “terrain denial” compound with a deliberately formulated low volatility; it is designed to contaminate surfaces.
Organophosphate (OP) nerve agents have been specifically designed and formulated to cause death, major injuries, or incapacitation to enemy forces in wartime. They are particularly effective in a military sense because of their potency. Detailed descriptions of nerve agent toxicity can be found in reviews by NRC (1999), Mioduszewski et al. (1998), Opresko et al. (1998), Sidell (1997), Munro et al. (1994), and Watson et al. (1989), among others.
Munitions containing agents GA, GB, and VX are stored at various military installations within the continental United States as part of the domestic unitary chemical warfare agent stockpile, which is undergoing congressionally mandated destruction (Carnes and Watson 1989). “Unitary” (as opposed to binary) munitions are those in which undiluted agents have been placed for immediate release upon firing or detonation.
According to information recently released by the Army at public meetings held in June 2001 in Pueblo, Colorado, the status of the project is as follows:
-
Disposal operations at Johnston Atoll were completed on November 29, 2000. Over one million munitions, containing over 2,030 tons of agents HD, GB, and VX, were destroyed.
-
As of June 6, 2001, the Tooele, Utah, facility had destroyed over 5,100 tons of agent GB, representing 37.4% of the original inventory at
-
Tooele. The Tooele facility began operations in August 1996. Demilitarization operations there are scheduled for completion in FY04.
-
Agents GB and/or GA are under secure storage and awaiting destruction at military facilities near Anniston, Alabama; Pine Bluff, Arkansas; Richmond, Kentucky; Tooele, Utah; and Umatilla, Oregon.
-
Agent VX is under secure storage and awaiting destruction at military facilities near Anniston, Alabama; Newport, Indiana; Pine Bluff, Arkansas; Richmond, Kentucky; Tooele, Utah; and Umatilla, Oregon.
-
The remaining demilitarization facilities are in various stages of construction.
Small quantities of agent GD are held in research and development facilities in the United States. Agents GA, GB, GD, and VX are listed as materiel thought to be located at some nonstockpile sites (DA 2001; USACMDA 1993a,b) and are being dealt with during installation restoration activities. The Chemical Weapons Convention (April 1997; Convention on the Prohibition of the Development, Production, Stockpiling and Use of the Chemical Weapons and on Their Destruction) has increased the interest in, and pace of, nonstockpile installation restoration.
Agent GF is believed to have been manufactured within Iraq during the Persian Gulf War (1990–1991) when precursors of agent GB (but not GF) were embargoed. Agent GF is currently considered of little strategic interest (Sidell 1997) but is included for completeness. With the possible exception of agent GF, all of the G agents identified above are considered potential military or terrorist threats.
Public and institutional concerns exist regarding potential agent release during unitary stockpile disposal, nonstockpile installation restoration activities, and potential chemical terrorism events (e.g., IOM 1999; Carnes 1989; NRC 1999; FEMA/DA 1996; DHHS 1988). A new dimension was added to consideration of this issue when it was determined that nerve agent GB had been used by a non-state terrorist group in two attacks on civilians in Japan during 1994 and 1995 (Sidell 1997; IOM, 1999). As a consequence, current domestic community emergency planning and preparedness often includes protocols for treating and managing exposure to chemical warfare agents (particularly nerve agents).
Experimental research specifically designed to improve the state of existing data sets quantifying toxic responses of mammals to nerve agent vapor exposure is currently underway and is supported by multiple military services. The AEGL analysis developed in this technical support document makes use of the most recent research findings (Mioduszewski et al. 2000, 2001, 2002a,b; van Helden et al. 2001, 2002; Anthony et al. 2002) from the
initiative. As the effort progresses, more of the assumptions necessary for developing AEGL estimates will be clarified. As new data and results become available in the next several years, assumptions will evolve. It is acknowledged that the current estimates represent a work in progress that will be updated as necessary.
Historical military approaches to chemical warfare (CW) agent protection and treatment of young and healthy soldiers are not necessarily suitable for application to heterogeneous civilian populations, and guidelines are needed for “safe and effective evacuation, decontamination, and other protective action” in the event of CW agent release in a civilian setting (IOM 1999). The development of AEGLs is intended to help address that need.
At present, the only CW agent control limits published in the United States for use in civilian community emergency preparedness planning are those developed by the Department of Health and Human Services (DHHS 1988; Thacker 1994). For the agents GA and GB, the current time-weighted average (TWA) applied as a no-adverse-health-effect level for 24-h continuous exposure to the general population is 3×10−6 mg/m3. For the same agents, the 8-h TWA applied as a no-adverse-health-effect level for 8-h continuous workplace exposure for worker populations is 1×10−4 mg/m3 (53 Fed. Reg. 8504 [1988]; DHHS 1988). Agents GD and GF, which are not part of the unitary stockpile, were not evaluated by DHHS in 1988. For VX, the TWA applied as a no-adverse-health-effect level for 24-h continuous exposure to the general population is 3×10−6 mg/m3; the 8-h TWA applied as a no-adverse-health-effect level for 8-h continuous workplace exposure to worker populations is 1×10−5 mg/m3 (DHHS 1988).
As part of a regularly scheduled review process, the Centers for Disease Control and Prevention (CDC) is currently reevaluating the 1988 agent control limits with application of recent risk assessment models and updated scientific data (67 Fed. Reg. 895 [2002]; DHHS 2002). The review is in progress (as of September 2002), and the CDC has not yet released a final position.
Acute Threshold Effects Levels developed by the CDC (Thacker 1994) are values of cumulative exposure (Ct) (concentration in mg/m3 multiplied by time in minutes, or mg·min/m3—Ct does not express the amount retained within the organism [Sidell 1997]). These cumulative exposure values are considered by the CDC to represent “lowest-observed-effect-levels” that “could be exceeded without danger” to the public and form the basis for planning protective actions, such as emergency evacuations, in the Chemical Stockpile Emergency Preparedness Program (CSEPP) of the Federal
Emergency Management Agency and the Department of the Army. The Acute Threshold Effect Levels are described by the CDC as protective of the general population (including consideration of vulnerable subgroups, such as infants, the elderly, and debilitated or ill persons) (Thacker 1994). The value for agent GB is 0.5 mg·min/m3, a protective cumulative exposure at which miosis is not expected to occur in humans (McNamara and Leitnaker 1971). If projected GB concentrations resulting from a release result in GB Cts >0.5 mg·min/m3, then the CDC considers protective measures (such as evacuation or shelter-in-place) warranted as a means of providing maximal protection to the general public. At the time of publication, the CDC has not established similar values for other G agents. The Acute Threshold Effects Level for agent VX is 0.4 mg·min/m3.
The database for toxicological effects in humans is more complete for agent GB than for the other G agents and for agent VX. Further, agent GB is the only G agent for which sufficient human data are available for use in deriving AEGL-1 and AEGL-2 estimates and the only G-agent for which sufficient laboratory animal data are available for deriving AEGL-1 and AEGL-3 values for all five AEGL time periods. In consequence, estimates for agents GA, GD, GF, and VX are, out of necessity, based on extrapolations of potency relative to the toxicity of agent GB.
Data for the derivation of AEGL-3 values for agent GB are from recent experimental studies of lethality in Sprague-Dawley rats (Mioduszewski et al. 2000, 2001, 2002a). AEGL-3 values for agent GD are derived from relative potency comparison with agent GB and limited inhalation lethality data for experimental exposures to Wistar rats (Aas et al. 1985).
All literature published in this technical support document is unclassified (i.e., not secret at any level, not confidential), including critical studies. Classified material relevant to AEGL assessment for these agents has been reviewed by document developers and has been found to contain no significant data that are not also found in unclassified reports. The technical support document itself was determined to be unclassified following examination by the Intelligence and Security Office of the U.S. Army Soldier and Biological Chemical Command (SBCCOM) (Aberdeen Proving Ground, Maryland) in July 2000.
Given the nature of the compounds under review, military literature is a major source of the relevant toxicity data. In consequence, some of the significant sources possess “limited distribution,” which is a separate issue from “classification.” Several sources possess a restricted distribution because of treaty restrictions on data access with allies, concerns regarding distribution of engineering information characterizing agent dissemination
or vapor generation contained in other sections of the same document, and related issues. To ensure public access to pertinent toxicity data originating from “limited distribution” materials, pertinent data from those sources have been incorporated into the technical support document. The technical support document itself was “cleared and approved for public access” by the Intelligence and Security Office of the U.S. Army SBCCOM (Aberdeen Proving Ground, Maryland) in July 2000. If additional details are desired, the U.S. Army Center for Health Promotion and Preventive Medicine will assist any request on a one-to-one basis. The point of contact is Ms. Veronique Hauschild (U.S. Army Center for Health Promotion and Preventive Medicine, Environmental Health Engineering, Bldg. E-1675, Aberdeen Proving Ground, MD 21010–5403).
All human exposure studies presented in this evaluation meet the criteria for acceptance for use in the AEGL process (e.g., there is evidence that subjects provided informed consent and that the studies were performed under appropriate clinical supervision) (NRC 2001).
The G agents are all viscous liquids of varying volatility (see Tables 1– 2 through 1–5), with faint odors (“faintly fruity” or “spicy,” odor of camphor) (DA 1990a,b; Dutreau et al. 1950; McGrath et al. 1953; MODa, unpublished material; all as cited in Marrs et al. 1996). However, these agents are considered odorless in field concentrations for all practical (military) purposes (DA 1990a,b). Odor thresholds are somewhat undefined (DA 1974, 1990a,b, 1992). Agent GA has been reported to have a faintly fruity odor, although it has no odor when pure (DA 1974, 1990a,b, 1992). For agent GB, the odor threshold was reported to be less than 1.5 mg/m3 (DA 1974, 1990b, 1992; MODa, unpublished material, as cited in Marrs et al. 1996). For agent GD, the odor threshold was reported to be between approximately 1.5 mg/m3 and 7.0 mg/m3 (MODa, unpublished material, as cited in Marrs et al. 1996). Approximately 65% of adult subjects (N=34) exposed to GD at 3.3 to 7.0 mg/m3 exhibited “mild nasal and airway symptoms” (Dutreau et al. 1950); a “median detectable concentration by odor for man is 7±2.4 mg/m3.” However, Dutreau et al. (1950) warn that it is doubtful that an untrained civilian could detect agent GD in sufficient time to avoid a partially incapacitating exposure. Agent GF is reported to have a sweet or musty odor of peaches and has an odor threshold between about 10.4 mg/m3 and 14.8 mg/m3 (McGrath et al. 1953, as cited in Marrs et al. 1996; DA 1990b).
As a class, G agents are more volatile and less persistent than the V agents; the vapor pressures and acute toxicity of the G-series agents are sufficiently high for the vapors to be rapidly lethal (USACHPPM 1996).
Within the G series, GB is considered a greater vapor hazard than agent GD (USACHPPM 1996). Agent GA represents a smaller vapor hazard and is expected to present a relevant contact hazard (USACHPPM 1996). The vapor pressure of agent GF is intermediate between that of agents GA and GD.
Agent VX is an amber-colored liquid with a molecular weight of 267.38; it has a vapor density of 9.2 (air=1) and a liquid density of 1.006 g/ml at 20 °C; its water solubility is 3 g per 100 g at 25 °C and 7.5 g per 100 g at 15 °C; and it has a low volatility (10.5 mg/m3 at 25 °C) (DA 1990b). Agent VX is approximately 2,000 times less volatile than nerve agent GB (sarin) (DA 1990b). Because agent VX is considered odorless (Koon et al. 1959; DA 1990b), it possesses no olfactory warning properties.
Chemical and physical data for agents GA, GB, GD, GF, and VX are presented in Tables 1–2 through 1–6.
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
The acute lethal action of G agents and other anticholinesterase compounds results from their effects on the respiratory system at several levels: bronchoconstriction and excessive tracheobronchial secretion, paralysis of the diaphragm and other respiratory muscles, and depression of the CNS respiratory center (Mioduszewski et al. 1998).
G Agents
Based on extrapolations from historical animal data, the LCt50 for military personnel undergoing vapor exposures to GB has been estimated at 35 mg·min/m3 for 2–10 min exposures at moderate temperatures (65–75 °F) for an individual with a respiratory minute volume of 15 liters (Reutter and Wade 1994). Reutter and Wade (1994) also estimated LCt50 values for military personnel undergoing vapor exposures to agents GA, GD, and GF; the estimates are 70 mg·min/m3 for GA, 35 mg·min/m3 for GD, and 35 mg·min/m3 for GF. This Army report remains classified except for a summary table cited here that contains information on median exposure levels. The recommended LCt50 estimate for vapor exposure given in Reutter and Wade (1994) was calculated for 2-min exposure periods and then proposed
TABLE 1–2 Chemical and Physical Data for Nerve Agent GA
|
Parameter |
Value |
Reference |
|
Chemical name |
Dimethylamidocyanethylophosphate |
Clark 1989; DA 1974, 1988, 1990a,b, 1992; Britton and Grant 1988; Small 1984; Windholz et al. 1983 |
|
Synonyms |
Tabun; ethyl N,N-dimethyl phosphoro-amidocyanidate; N,N-dimethyl phosphoroamidocyanidate, ethyl ester. |
|
|
Chemical formula |
C5H11N2O2P |
|
|
Chemical structure |
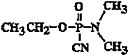 |
DA 1990b |
|
Molecular weight |
162.13 |
DA 1990b |
|
CAS Registry Number |
77–81–6 |
DA 1974, 1990a,b, 1992 |
|
Physical state |
Colorless to brown liquid |
DA 1990b |
|
Solubility in water (g/L) |
98 (25 °C); 72 (20 °C) |
DA 1990b |
|
Vapor pressure (mm Hg, 20 °C) |
0.037 |
DA 1990b |
|
Vapor density (air=1) |
5.63 |
DA 1990b |
|
Liquid density (g/mL, 25 °C) |
1.073 |
DA 1990b |
|
Melting point |
−50 °C |
DA 1974, 1992 |
|
Boiling point |
245 °C |
DA 1974, 1992 |
|
Flash point |
78 °C |
DA 1974, 1992 |
|
Conversion factors in air |
ppm=(0.15)×mg/m3 (calculated) mg/m3=(6.6)×ppm (calculated) |
Calculated from procedure outlined in ACGIH 2002 using molecular weight |
|
logKow |
1.18 |
Britton and Grant 1988 |
|
Bioconcentration factor (BCF) |
Not available |
|
|
Henrys’ law constant (atm m3/mol) |
1.52×10−7 |
Opresko et al. 1998 |
TABLE 1–3 Chemical and Physical Data for Nerve Agent GB
|
Parameter |
Value |
Reference |
|
Chemical Name |
Isopropyl methylphosphonofluoridate |
Clark 1989; DA 1974, 1988, 1990a,b, 1992; Britton and Grant 1988; Small 1984; Windholz et al. 1983 |
|
Synonyms |
Sarin; methyl phosphonofluoridate, isopropyl ester |
|
|
Chemical formula |
C4H10FO2P |
|
|
Chemical structure |
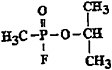 |
DA 1990b |
|
Molecular weight |
140.10 |
DA 1990b |
|
CAS Registry Number |
107–44–8 |
DA 1974, 1990a,b, 1992 |
|
Physical state |
Colorless liquid |
DA 1990b |
|
Solubility in water (g/L) |
Miscible with water |
DA 1990b |
|
Vapor pressure (mm Hg at 20 °C) |
2.10 |
DA 1990b |
|
Vapor density (air=1) |
4.86 |
DA 1990b |
|
Liquid density (g/mL, 20 °C) |
1.102 |
DA 1990b |
|
Melting point |
−56 °C |
Clark 1989; DA 1974, 1988, 1990a,b, 1992; Britton and Grant 1988; Small 1984; Windholz et al. 1983 |
|
Boiling point |
158 °C |
|
|
Flash point |
>138 °C |
|
|
Conversion factors in air |
ppm=(0.17)×mg/m3 (calculated) mg/m3=(5.7)×ppm (calculated) |
Calculated from procedure outlined in ACGIH 2002 using molecular weight |
|
logKow |
0.15 |
Britton and Grant 1988 |
|
Bioconcentration factor (BCF) |
Not available |
|
|
Henrys’ law constant (atm m3/mol) |
5.34×10−7 |
Clark 1989; DA 1974, 1988, 1990a,b, 1992; Britton and Grant 1988; Small 1984; Windholz et al. 1983 |
TABLE 1–4 Chemical and Physical Data for Nerve Agent GD
|
Parameter |
Value |
Reference |
|
Chemical name |
Pinacolyl methylphosphonofluoridate |
Sidell 1997; Clark 1989; DA 1974, 1988, 1990a,b, 1992; Britton and Grant 1988; Small 1984; Windholz et al. 1983 |
|
Synonyms |
Soman; phosphonofluoridic acid, methyl-1,2,2-trimethylpropyl ester |
|
|
Chemical formula |
C7H16FO2P |
USACHPPM 1996 |
|
Chemical structure |
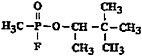 |
DA 1990b |
|
Molecular weight |
182.178 |
DA 1990b |
|
CAS Registry No. |
96–64–0 |
USACHPPM 1996 |
|
Physical state |
Colorless liquid |
DA 1974 |
|
Solubility in water (g/L) |
21 (20 °C) |
DA 1990b |
|
Vapor pressure (mm Hg at 25 °C) |
0.40 |
Clark 1989; DA 1974, 1988, 1990a,b, 1992; Britton and Grant 1988; Small 1984; Windholz et al. 1983 |
|
Vapor density (air=1) |
6.33 |
DA 1990b |
|
Liquid density (g/mL, 25° C) |
1.0222 |
DA 1990b |
|
Melting point |
−42 °C |
DA 1990b |
|
Boiling point |
198 °C |
DA 1990b |
|
Flash point |
121 °C |
DA 1990b |
|
Conversion factors in air |
ppm=(0.13)×mg/m3 (calculated) mg/m3=(7.5)×ppm (calculated) |
Calculated from procedure outlined in ACGIH 2002 using molecular weight |
|
logKow |
1.02 |
Britton and Grant 1988 |
|
Bioconcentration factor (BCF) |
Not available |
|
|
Henrys’ law constant (atm m3/mol) |
4.56×10−6 |
Opresko et al. 1998 |
TABLE 1–5 Chemical and Physical Data for Nerve Agent GF
|
Parameter |
Value |
Reference |
|
Chemical name |
O-cyclohexyl-methylfluorophosphonate |
DA 1990b |
|
Synonyms |
Cyclohexyl methylphosphonofluoridate (CMPF) |
|
|
Chemical formula |
C7H14FO2P |
DA 1990b |
|
Chemical structure |
 |
DA 1990b |
|
Molecular weight |
180.2 |
DA 1990b |
|
CAS Registry Number |
329–99–7 |
DA 1990b |
|
Physical state |
Liquid |
DA 1990b |
|
Solubility in water |
0.37% (20 °C); almost entirely insoluble in water |
DA 1990b |
|
Vapor pressure (mm Hg, 25 °C) |
0.044 |
DA 1990b |
|
Vapor density (air =1) |
6.2 |
DA 1990b |
|
Liquid Density (g/mL, 20° C) |
1.1327 |
DA 1990b |
|
Melting point |
−30 °C |
DA 1990b |
|
Boiling point |
239 °C |
DA 1990b |
|
Flash point |
94 °C |
DA 1990b |
|
Conversion factors in air |
ppm=(0.14)×mg/m3 (calculated) mg/m3=(7.4)×ppm (calculated) |
Calculated from procedure outlined in ACGIH 2002, using molecular weight |
|
logKow |
Not available |
|
|
Bioconcentration factor (BCF) |
Not available |
|
|
Henrys’ law constant (atm m3/mol) |
Not available |
|
as a 2–10 min exposure estimate in the summary table. Thus, the LCt50 of 35 mg·min/m3 assumes only short-term exposures of 2–10 min.
TABLE 1–6 Chemical and Physical Data for Nerve Agent VX
|
Parameter |
Value |
Reference |
|
Chemical name |
O-ethyl-S-(diisopropylaminoethyl)methyl phosphonothiolate |
Munro et al. 1999; DA 1990b |
|
Synonyms |
Agent VX; S-(2-diisopropylaminoethyl) O-ethyl methyl phosphonothiolate; ethyl-S-dimethylaminoethyl methylphosphonothiolate |
|
|
Chemical formula |
C11H25NO2PS |
|
|
Chemical structure |
 |
DA 1990b |
|
Molecular weight |
267.38 |
DA 1990b |
|
CAS Registry Number |
50782–69–9 |
DA 1990b |
|
Physical state |
Oily, amber-colored liquid |
DA 1990b |
|
Solubility in water (g/L) |
3 g per 100 g at 25 °C 7.5 g per 100 g at 15 °C |
DA 1974 |
|
Vapor pressure (mm Hg, 20° C) |
0.0007 mm Hg at 20 °C |
DA 1990b |
|
Vapor density (air=1) |
9.2 |
DA 1990b |
|
Liquid density |
1.006 g/cc at 20°C |
DA 1990b |
|
Melting point |
−39 °C (calculated) |
DA 1990b |
|
Boiling point |
298 °C |
DA 1990b |
|
Flash point |
159 °C |
DA 1990b |
|
Conversion factors in air |
mg/m3=(10.936)×ppm ppm=(0.0914)×mg/m3 |
Calculated from procedure outlined in ACGIH 2002 using molecular weight |
|
logKow |
Not available |
|
|
Bioconcentration factor (BCF) |
Not available |
|
A subcommittee of the National Research Council’s Committee on Toxicology (COT) has examined the Reutter and Wade (1994) analysis and recommends that the proposed LCt50 estimates for agents GA, GB, GD, and GF for estimating vapor inhalation and percutaneous exposure effects in exposed military populations “should be lowered” in light of the need for additional data characterizing vapor inhalation and percutaneous vapor toxicity. Furthermore, the subcommittee considered the estimates of Reutter and Wade (1994) inappropriate for civilian applications (NRC 1997).
Agent VX
From animal data, Reutter and Wade (1994) estimated a LCt50 for military personnel of 15 mg·min/m3 for 2–10 min vapor exposures at moderate temperatures (65–75 °F) for an individual with a respiratory minute volume of 15 L. As in the case for agent GB, this LCt50 estimate was calculated for 2-min exposure periods and then proposed as a 2–10 min exposure estimate. Thus, the LCt50 for VX at 15 mg·min/m3 assumes only short-term exposures of 2–10 min.
The subcommittee recommends that the Reutter and Wade (1994) proposed LCt50 estimate of 15 mg·min/m3 for military personnel “should be lowered” because of the low to moderate degree of confidence in the estimation, which considered effects from vapor inhalation and percutaneous vapor exposures. Further, the subcommittee considered the estimates of Reutter and Wade (1994) inappropriate for civilian applications (NRC 1997).
Bide and Risk (2000) estimated the human 10-min LCt50 value for a VX aerosol based on lethality data for several animal species (see Section 3.1). The human LCt50 value was an estimated 7 mg·min/m3 for a 70 kg man breathing 15 L/min for 10 min.
2.1.1. Case Reports
Agent GB
In 1994 and 1995, two incidents of chemical terrorism involving nerve agent GB (sarin) occurred in Japan; in both incidents, civilian populations were deliberately exposed to lethal concentrations by followers of a cult
originally local to Japan (Lillibridge 1995; Morita et al. 1995; Okumura et al. 1996; Sidell 1996). Because of the state of emergency at the time of release and the initial unknown nature of the source, exposures and dose-response could not be quantified.
The first incident occurred in June of 1994 in the central highland city of Matsumoto, Japan, where seven people died shortly after exposure to an unknown vapor later determined to be agent GB (Morita et al. 1995) released into a residential area during the night. The Matsumoto incident resulted in 56 hospital admissions as well as 253 cases in which the affected individuals sought medical consultation. Reports of “mild symptoms” were presented by eight out of 53 rescue personnel and one attending physician (Morita et al. 1995). Prompt deaths (N=3) and those who died before arriving at the hospital (N=4) appear to have been the result of respiratory insufficiency. At the time of the Morita et al. (1995) report, one patient remained “in a vegetative state because of anoxic encephalopathy”; a report on the outcome of that case has not yet been found.
The second occurrence, widely known as the Tokyo Subway Incident, took place on March 20, 1995. The same terrorist group responsible for the Matsumoto incident employed sources of passive, evaporative release of nerve agent GB in five individual subway cars serving three separate subway lines during morning commuter rush hours (Lillibridge 1995; Okumura et al. 1996; Sidell 1996). Of the 5,510 persons known to have been given medical attention, there were eight prompt deaths; four more died later (hours to days). The “later” group included individuals who had initially presented with “critical” respiratory effects requiring mechanical ventilation and intensive care (Lillibridge 1995). The 12 fatalities included commuters and subway transport employees, and death appeared to be the result of respiratory insufficiency. On hospital day 28, an additional death occurred as a consequence of “severe hypoxic brain damage” sustained during the release incident (Okumura et al. 1996). This delayed fatality was a previously healthy woman, 21 years of age, who presented without heartbeat or spontaneous respiration at the hospital but was revived with CPR and treated with agent antidotes. Plasma and RBC cholinesterase returned to normal within a period of days, but the patient eventually succumbed to hypoxic brain damage (Okumura et al. 1996).
Neuropathological examination of one individual who died 15 mo after being severely exposed to agent GB during the Tokyo subway terrorist attack indicated that the victim suffered marked nerve-fiber decrease in the sural nerve and moderate nerve-fiber loss in the sciatic nerve, with no changes in the dorsal root ganglion, dorsal roots, or posterior column of the
spinal cord (Himuro et al. 1998). The victim’s CNS showed severe hypoxic-ischemic changes, which made it difficult to assess the specific effects of agent GB. Himuro et al. (1998) concluded that the observations were consistent with the “dying back” of the peripheral nervous system and might have been indicative of delayed neuropathy associated with inhibition of neuropathy target esterase (NTE). Himuro et al. (1998) cite as additional evidence of sarin-induced distal axonopathy an earlier study (Ishiyama 1996) in which degeneration of intramuscular nerve fascicles with preservation of the anterior horn cells was observed in a patient who died 1 mo after the subway attack.
2.2. Nonlethal Toxicity
Exposure to acutely toxic concentrations of nerve agents can result in excessive bronchial, salivary, ocular, and intestinal secretion, sweating, miosis, bronchospasm, intestinal hypermotility, bradycardia, muscle fasciculations, twitching, weakness, paralysis, loss of consciousness, convulsions, and depression of the central respiratory drive (Dunn and Sidell 1989). Minimal effects seen at very low exposure levels include miosis and rhinorrhea. The effects of exposures to very low concentrations of the nerve agents are evaluated in the literature, which includes clinical case reports as well as several studies using human volunteers. Key to acceptance of human subject data for use in the AEGL process is evidence that subjects provided informed consent and that the studies were performed under appropriate clinical supervision (NRC 2001). These criteria were met by the nonlethal studies summarized in Section 2.2.2.
A number of investigators consider both miosis and rhinorrhea to be early signs of exposure to cholinesterase inhibitors (Sidell 1997; Mioduszewski et al. 2002b; H.van Helden, Pulmonary and CNS Pharmacology Lab, TNO, the Netherlands, personal communtication; S.Tattersall, Biomedical Sciences Division, Porton Down, United Kingdom, personal communication). The presence of rhinorrhea can be indicative of inhalation exposure and/or development of systemic effects, while miosis in the absence of other signs or symptoms is a local effect to the pupillary muscles of the eye. In consequence, the presence of miosis is considered an appropriately sensitive indicator of direct vapor exposure and has the additional advantage of being readily recognized and quantifiable.
Recent nerve agent releases by terrorist groups have exposed civilian populations. Survivors of the incidents have been examined, and the resulting evaluations are summarized in Section 2.2.1.
2.2.1. Case Reports
Agent GB
Clinical case reports exist for the survivor population of the 1994 agent GB (sarin) release in Matsumoto, Japan, and the 1995 sarin release in Tokyo; no estimates of exposure concentrations could be found in the literature for either of these incidents. In the Matsumoto incident detailed above (see Section 2.1.1), Morita and his colleagues (Morita et al. 1995) published the clinical and laboratory findings of 264 people who sought treatment and the results of health examinations performed on 155 Matsumoto residents at 3 weeks (wk) postexposure. During initial treatment, severely poisoned individuals exhibited severe miosis, tachycardia followed by bradycardia, salivation, rhinorrhea, muscle fasciculations, and abnormal epileptiform EEGs. Other reported acute exposure signs and symptoms included headache, vision disturbances, fatigue, dizziness, nausea, dyspnea, ocular pain, and dysesthesia of the extremities. Clinical findings for the same group at the time of examination included decreases in serum cholinesterase, erythrocyte acetylcholinesterase, and serum triglycerides as well as serum potassium and chloride and increases in serum creatine kinase, leucocytes, and ketones in urine. For a period of up to 30 d following the incident, some of the severely exposed population exhibited slight continuous fever and some epileptiform EEG abnormalities (N=2 out of nine “severely affected people”). Nevertheless, follow-up examination revealed no persistent abnormal physical findings in any individual; acetylcholinesterase activity in erythrocytes and serum cholinesterase returned to normal within 3 mo in the examined population. Among some severe or moderately affected persons, subclinical miosis and some neuropathy were present 30 d after exposure. Morita et al. (1995) state that, in most people, “almost all symptoms of sarin exposure disappeared rapidly and left no sequelae.”
In the hours following the Tokyo subway release of agent GB, the emergency department of St. Luke’s International Hospital (located near
the affected subway stations) received 640 patients (Okumura et al. 1996). Additional details of the incident are provided above (see Section 2.1.1). Of the 640 admissions, 528 (82.5%) were diagnosed by Okumura and his colleagues (1996) as “mild” and exhibited “only eye signs or symptoms” such as miosis, eye pain, dim vision, and decreased visual acuity. Of the remaining 112 patients, one died in the emergency department, 107 were admitted as “moderate” cases (exhibiting “systemic signs and symptoms” such as weakness, fasciculations, convulsions, difficult breathing), and four were admitted as “severe” cases “requiring emergency respiratory support” such as intubation. Of the four severe cases, two patients experienced cardiac arrest but were revived, treated with agent antidotes and anticonvulsants, and eventually recovered fully (discharged on hospital day 3 and 5). Of the remaining two, both of whom required cardiopulmonary resuscitation, one recovered after vigorous treatment and was discharged on day 6. The remaining severely affected patient originally presented with no pulse and died on hospital day 28. For the three severe cases discharged, RBC-cholinesterase remained below normal activity levels for 51–72 d.
In the early 1970s, three men (ages 27, 50, and 52 y) working at Edgewood Arsenal (now Aberdeen Proving Ground in Edgewood, Maryland) in a chemical agent area containing stored containers of agent GB (sarin) were brought to an emergency room after sudden onset of rhinorrhea and respiratory discomfort approximately 20 min prior to arrival at the emergency room (Sidell 1974). It was determined later that one of the agent GB (sarin) containers in the work area had developed a leak and that the three individuals exhibiting signs had been working in the general area of the room where the leaking container was located. Examination indicated the presence of “mild respiratory distress, marked miosis with slight eye pain, rhinorrhea, a moderate increase in salivation, and scattered wheezes and rhonchi throughout all lung fields” (Sidell 1974). The men received no therapy but were observed for 6 h after emergency room arrival and were asymptomatic upon discharge except for eye irritation and “decreased vision in dim light.” Blood cholinesterases were monitored and pupil diameter was recorded photographically for a period of 4 mo following exposure. Although 60–70% recovery of the ability to dark-adapt occurred within 2 wk, complete recovery of the ability to dark-adapt required 2 mo. Sidell (1974) did not report any estimates of the GB agent concentrations the men were exposed to.
Also in the early 1970s, a 52-y-old man in full protective gear employed in cleaning an agent GB-contaminated area at Edgewood Arsenal
(now Aberdeen Proving Ground in Edgewood, Maryland) experienced breathing difficulty and increased oral and nasal secretions (Sidell 1974). It was later determined that there was a crack in the man’s voicemitter diaphragm through which exposure most likely had occurred. Upon arrival at the emergency room 5–10 min after the first symptom, he was convulsing and cyanotic. Other evident signs included labored breathing, muscular fasciculations, miosis, salivation, and rhinorrhea. He was treated aggressively with agent antidotes and provided assisted ventilation, and he recovered sufficiently to be able to walk through the ward by 9 h postadmission. Red blood cell cholinesterase (RBC-ChE) was monitored, as were EKGs. “While ChE activity in his blood was undetectable,” the individual was conscious and alert (Sidell 1997). By 18 h postadmission, miosis was still evident. On day 4 and thereafter, the patient was asymptomatic; upon discharge 4 wk postexposure, he was “fully ambulatory and doing well.” A 4-mo-postexposure EKG “was entirely within normal limit” (Sidell 1974). Sidell (1974) did not report any estimate of GB agent concentrations to which this individual was exposed.
In another incident of accidental exposure to GB vapors (0.09 mg/m3 for an undefined duration resulting from a faulty ventilation hood), two men (ages 46 and 53 y) exhibited significantly lowered RBC-ChE for 80–90 d (one showed depression to 19% of baseline activity, the other to 84% of baseline activity) and extreme miosis that persisted for 30–45 d (Rengstorff 1985). These men exhibited no other signs or symptoms of nerve agent poisoning and required no treatment with antidotes.
2.2.2. Acute Studies
Agent GB
Vapor Exposures
Fairley and Mumford (1948) exposed 16 male volunteers to GB at 0.3 mg/m3 for 0.5 min. Nine of the test subjects reported that they could detect the agent by smell; seven reported tightness of the chest, and 16 reported rhinorrhea.
McKee and Woolcott (1949) evaluated the effects of low concentrations of agent GB on 14 male volunteers. A single exposure to GB at 0.6 mg/m3 for 1 min or at 0.06 mg/m3 for 40 min resulted in miosis and slight tightness of the chest (4/4 subjects exhibited those signs and symptoms in
both the 1-min and 40-min tests; within 24 h, signs and symptoms resolved in subjects receiving 1-min exposures, although more than 48 h were required for resolution in subjects receiving 40-min exposures). Exposure of five individuals to GB at 0.06 mg/m3 for 20 min/d resulted in miosis, but only after the fourth day of exposure. When the subjects were exposed to GB at 0.06 mg/m3 for 40 min/d, miosis occurred on the first or second day and additional symptoms (headache, blurred vision, eye pain) appeared on the second, third, and fourth day of exposure.
In summarizing the toxicity studies conducted at Porton Down, United Kingdom, Mumford (1950) concluded that the threshold for ocular effects is 1.5–5.0 mg·min/m3 (exposure times of 5–6 min) and that exposures to GB at 6–12 mg·min /m3 (exposure times of 5–8 min) would result in moderate to severe discomfort due to miosis and frontal headaches.
In a study reported by Johns (1952) and Harvey (1952), 128 adult males volunteered to be exposed to GB concentrations ranging from 0.05 mg/m3 to 3.0 mg/m3 for 2–20 min in a chamber. The corresponding Cts ranged from 1.0 mg·min/m3 to 6.0 mg·min/m3. The analytical methods used to measure the chamber concentrations of GB were not reported. Regression analysis of 150 observations, including 55 controls, indicated that the point at which a 50% decrease in pupil diameter would be attained was approximately 4.1 mg·min/m3, with 90% confidence limits of about 2.7 and 5.7 mg·min/m3 (Johns 1952). At the lowest test exposure level (0.05 mg/m3 for 20 min), there were mean maximum decreases in pupil diameter of 0.82 mm (right eye) and 1.00 mm (left eye) (total of eight observations) compared with 0.36 mm (right eye) and 0.33 mm (left eye) in controls (55 observations). Johns (1952) defines “mild miosis” as a “decrease of 1 to 2 mm” in pupil diameter that usually disappears within 24 h. Although mild miosis, as defined by the author, was observed in some subjects at the lowest Ct tested (Ct=1.0 mg·min/m3), other subjects exhibited mean maximal pupil decreases of <1 mm. This indicates that a likely response threshold was attained at this level of cumulative exposure. The results of the Johns (1952) study are presented in Table 1–7. It should be noted that untreated controls exhibited a pupil diameter decrease of ≥0.33 mm. Johns (1952) attributes this difference to observer bias and points out that there is still a relative difference between the control group and the exposure groups.
From the same overall study, Harvey (1952) reported signs and symptoms resulting from the GB exposures; those results are presented in Table 1–8.
TABLE 1–7 Decrease in Pupil Diameter (mm) Following GB Vapor Exposures
|
|
Exposure Duration |
||||||||||
|
|
20 min |
4 min |
2 min |
||||||||
|
Concentration (mg/m3) |
0 |
0.05 |
0.2 |
0.3 |
1.0 |
1.3 |
0.5 |
1.0 |
2.0 |
2.3 |
3.0 |
|
Number of observations |
55 |
8 |
11 |
11 |
12 |
4 |
15 |
9 |
8 |
7 |
10 |
|
Right eye; mean maximal decrease in pupil diameter (mm) |
0.36 |
0.82 |
2.18 |
2.91 |
2.75 |
2.00 |
0.51 |
1.72 |
2.50 |
2.36 |
3.00 |
|
Left eye; mean maximal decrease in pupil diameter (mm) |
0.33 |
1.00 |
2.18 |
3.00 |
2.59 |
2.22 |
0.60 |
1.67 |
2.92 |
2.07 |
3.00 |
|
Source: Johns 1952. |
|||||||||||
TABLE 1–8 Number of Test Subjects Showing Effects from GB Vapor Exposures
|
|
Exposure Duration |
|||||||||
|
|
20 min |
2 min |
||||||||
|
Concentration (mg/m3) |
0 |
0.05 |
0.1 |
0.2 |
0.3 |
0 |
0.5 |
1.0 |
2.0 |
3.0 |
|
Number of test subjects |
4 |
14 |
34 |
11 |
12 |
4 |
15 |
9 |
15 |
10 |
|
Headache |
1 |
2 |
1 |
1 |
8 |
|
4 |
1 |
|
4 |
|
Eye pain |
|
2 |
|
|
6 |
1 |
3 |
|
|
6 |
|
Dimness of vision |
|
|
|
|
7 |
|
|
|
4 |
7 |
|
Twitching of lids |
|
|
|
|
2 |
|
|
2 |
2 |
|
|
Rhinorrhea |
|
3 |
20 |
11 |
12 |
|
2 |
9 |
15 |
10 |
|
Salivation |
|
|
|
|
2 |
|
|
|
|
|
|
Throat irritation |
|
|
|
|
5 |
|
1 |
|
3 |
|
|
Tightness in chest |
|
1 |
12 |
2 |
9 |
|
|
6 |
11 |
4 |
|
Sweating |
|
|
|
|
4 |
|
|
|
|
|
|
Cramps |
|
1 |
|
|
6 |
|
|
|
1 |
2 |
|
Nausea |
|
1 |
|
|
3 |
1 |
|
|
1 |
|
|
Vomiting |
|
|
|
|
1 |
|
|
|
1 |
|
|
Giddiness |
|
|
|
|
5 |
|
|
|
|
|
|
Concentration difficulty |
|
|
|
|
8 |
|
|
|
|
|
|
Malaise (“Grippe”) |
|
2 |
|
|
6 |
|
1 |
|
7 |
7 |
|
Source: Harvey 1952. |
||||||||||
In tests on human volunteers, Sim (1956) found that pupil constriction occurs more slowly and is less severe following exposure to GB at 5 mg/m3 than at 10 or 15 mg/m3 (1–3 min exposures). Some of the test subjects (number not given) reported restricted vision and eye pain.
Rubin et al. (1957) evaluated the effects of agent GB on the visual threshold of three adult volunteers. The test subjects were exposed to GB at 2 mg/m3 for 2 min with the eyes protected or unprotected. With the eyes unprotected, the exposure resulted in moderate miosis and no other obvious signs of cholinesterase inhibition, but there was a significant elevation of the absolute visual threshold in the dark-adapted eye.
Oberst et al. (1968) conducted inhalation studies in which 125 volunteers were exposed to low concentrations of GB vapors in order to measure levels of GB retention and changes in RBC-ChE activity. In one series of tests in which resting subjects (N=90; minute volumes 5.6–8.4 L) were exposed to GB (concentrations in the supply chamber were 16.2 to 22.7 mg/m3, average 20.7 mg/m3) for 2 min, the calculated retained dose was 3.4–3.8 µg/kg and the percent inhibition of RBC-ChE activity was 39–63% (average 49%). In a second series of tests, in which exercising men (N= 35; minute volumes 41.5–64.9 L) were exposed to GB (supply chamber concentrations were 3.9 to 4.53 mg/m3, average 4.19 mg/m3) for 2 min, the calculated retained dose was 3.2–4.0 µg/kg and the percent inhibition of RBC-ChE activity was 29–58% (average 47%). The reported 2-min ChE50 dose for all 125 subjects (grouped data) was 3.95 µg/kg. From these data, the 2-min EC50 for cholinesterase inhibition can be estimated as approximately 21 mg/m3 for resting men breathing about 7 L/min and about 4 mg/m3 for exercising men breathing about 50 L/min. In these studies the subjects inhaled GB through a nosepiece or a mouthpiece; therefore, the potential effects of the agent on the eyes (i.e., miosis) could not be determined.
McNamara and Leitnaker (1971) applied mathematical and conceptual models to human and animal data and estimated that the threshold for neuromuscular effects and the ECt50 of GB for miosis in humans would be 4.0 mg·min/m3 (0.2 mg/m3 for 20 min). They further suggested that miosis would not occur at a Ct of 0.5 mg·min/m3 (0.016 mg/m3 for 30 min). McNamara and Leitnaker (1971) also estimated the Ct at which 50% inhibition of blood cholinesterase would occur; it was reported to be 20 mg·min/m3 (0.67 mg/m3 for 30 min). Blood cholinesterase activity was not expected to be affected at a Ct of 0.5 mg·min/m3 (0.016 mg/m3 for 30 min).
Callaway and Dirnhuber (1971) evaluated the “miotogenic potency” of GB vapor in humans exposed to GB “under goggles” (62 miosis responses
in 26 human volunteers). The “goggle” experiments were designed to deliver GB vapor directly to the air volume around the eye and enclose the vapor as a means of controlling the exposure (no inhalation or percutaneous exposure) and delivering the vapor directly to the surface of the eye (thereby reducing variability). An airstream of GB vapor (flow rate 0.1 L/min) was delivered to the space enclosed by each goggle. The unexposed pupil area of each eye was the baseline for pupil area decrement determinations for each eye. Exposure time periods ranged from 10 min to 5 h. Callaway and Dirnhuber (1971) reported a 50% loss of pupil area in the human dark-adapted eye at a Ct of 3.13 mg·min/m3 (95% confidence interval [CI]=2.15–4.57 mg·min/m3). A 90% loss of pupil area occurred at a Ct of 13.85 mg·min/m3 (95% CI=6.00–32.02 mg·min/m3).
Baker and Sedgwick (1996) exposed eight human volunteers to GB at 0.5 mg/m3 for 30 min in an exposure chamber. During the exposure, test subjects walked at a rate of 96 paces per minute and breathed normally. It was reported that the test Ct of 15 mg·min/m3 caused an inhibition of RBC-AChE activity to approximately 60% of individual baseline (reduction of 40%) at both 3 h and 3 d postexposure. Subjects exhibited miosis and, in some cases, photophobia and mild dyspnea following exposure. Respiratory symptoms resolved within minutes, and ocular effects resolved within 48 h. There were no clinical neuromuscular signs or symptoms; however, small changes in single fibre electromyography (SFEMG) of the forearm were measured at 3 h and 3 d postexposure and were still detectable at the first follow-up examination 4 to 15 mo postexposure. These changes were not detectable at the second follow-up examination 15 to 30 mo after exposure. Baker and Sedgwick (1996) suggested that these electrophysiological changes “may indicate subclinical onset of a non-depolarising type of neuromuscular block” that is fully reversible and has no clinical significance.
Oral Exposures
In clinical studies conducted by Grob and Harvey (1958), GB was administered orally in aqueous solution to eight normal subjects. Doses of 0.002 to 0.022 mg/kg resulted in 15–75% reduction in plasma and RBC-ChE activity. Grob and Harvey (1958) reported that the oral dose producing 50% depression of RBC-ChE was 0.01 mg/kg.
Intra-arterial Exposures
In clinical studies conducted by Grob and Harvey (1958), GB was administered by intra-arterial injection to eight normal subjects. Grob and Harvey (1958) reported that the intra-arterial dose of GB producing 50% depression of RBC-ChE was 0.003 mg/kg.
Agent GD
Fairley and Mumford (1948) exposed 15 male volunteers to GD at 0.3 mg/m3 for 0.5 min. Fourteen men reported that they could detect the agent by smell, seven reported tightness in the chest, and 11 reported rhinorrhea.
Agent GA
Uhde and Moore (1945, as cited in Mioduszewski et al. 1998) reported that four men exposed to T2104 (agent GA) at a concentration of 0.35 mg/m3 for 2 min were able to detect the agent by smell, and all reported slight, transient tightness of the chest, but none exhibited miosis. Ten men exposed to GA at 1.6 mg/m3 for 2 min were able to detect the agent by smell, reported tightness of the chest, and exhibited miosis.
Agent VX
Local effects occurring at points of contact in the eyes and respiratory tract following exposure to low concentrations of VX vapor include miosis, rhinorrhea, and slight bronchoconstriction (Sidell 1992). These effects may occur without a significant decrease in activity of blood cholinesterases and without any signs of systemic toxicity (Sidell 1992). The ECt50 for mild effects (ocular effects, accompanied perhaps by chest tightness and rhinorrhea) resulting from vapor exposures has been estimated at 0.09 mg·min/m3 for 2–10 min exposures at moderate temperatures (65–75 °F) for a respiratory minute volume of 15 L (Reutter and Wade 1994). Exposures sufficiently high to result in systemic uptake can result in muscular weakness, tremors, difficulty breathing, convulsions, paralysis, and death. The
ECt50 for severe effects resulting from vapor exposures has been estimated at 10 mg·min/m3 for 2–10 min exposures at moderate temperatures (65–75 °F) for a respiratory minute volume of 15 L (Reutter and Wade 1994).
According to an unclassified NRC report (NRC 1997), the Reutter and Wade (1994) estimated ECt50 of 0.09 mg·min/m3 for mild effects (ocular effects and rhinorrhea in humans) is based on the study by Bramwell et al. (1963) (percutaneous and direct ocular exposure to humans). The Bramwell et al. (1963) study is not considered credible for reasons that are discussed below under “Inhalation Exposures.” This conclusion also is supported by the evaluation of a U.S. Surgeon General’s review panel in an August 2000 public hearing (67 Fed. Reg. 894 [2002]; DHHS 2002).
Because agent VX is considered odorless, it possesses no olfactory warning properties.
Vapor Exposures
Sixteen volunteers participated in an odor detection study of stabilized and unstabilized VX (Koon et al. 1959). The agent was inhaled through an osmoscope attached to a chamber containing freshly generated agent vapor. The osmoscope permitted dilutions of the agent vapor with room air to yield concentrations down to one-sixty-fourth that in the chamber (0.05– 3.34 mg/m3). Each subject sniffed the agent in the morning and in the afternoon on two successive days (presumably only one sniff at each time point). The estimated total doses for the four exposures ranged from 0.01 to 0.13 µg/kg. No significant changes in RBC- or plasma-ChE activity were demonstrated. Three subjects reported headaches the evening of the last test, and three other subjects reported slight chest tightness, dryness of the mouth, and nasal irritation for 30 min following the test. There was no agreement as to description of the odor. The median detectable concentration for VX vapor was estimated to be 3.6 mg/m3 (95% CI=0.8–16.4 mg/m3).
One of the few experimental attempts to evaluate human exposure to VX vapor for time durations greater than a few minutes is the historically important study of Bramwell et al. (1963) in which eight individuals were exposed for time periods ranging from 2.25 seconds (s) to 24 min to VX vapor concentrations ranging from 0.23 mg/m3 to 5 mg/m3 (Cts=0.7 to 25.6 mg·min/m3). The Bramwell et al. (1963) study is not considered credible because of its seriously flawed exposure protocol but is presented here for completeness and context. The test subjects were exposed while stand-
TABLE 1–9 ChE Inhibition in Humans Following Exposure to VX Vapors
|
|
|
Exposure Conditions |
Max ChE Inhibition (% depression) |
||
|
Trial |
Subject |
Time (min) |
Concentration (mg/m3) |
Ct (mg·min/m3) |
|
|
R1 |
SHE |
3 |
0.2 |
0.6 |
20 |
|
R2 |
BIS |
3 |
0.35 |
0.9 |
18 |
|
R3 |
LAD |
3 |
0.31 |
0.9 |
22 |
|
R4 |
BUR |
3 |
0.37 |
1.1 |
17 |
|
R5 |
BRA |
3 |
0.4 |
1.2 |
14 |
|
R6 |
HOP |
3 |
0.48 |
1.4 |
10 |
|
R7 |
CRO |
3 |
0.57 |
1.7 |
12 |
|
R8 |
SHE |
1.5 |
1.6 |
2.4 |
26 |
|
R9 |
BRA |
1.5 |
1.73 |
2.6 |
25 |
|
R10 |
BUR |
1.5 |
1.73 |
2.6 |
21 |
|
R11 |
BIS |
1.5 |
1.93 |
2.8 |
28 |
|
R12 |
LAD |
1.5 |
2.0 |
3.0 |
41 |
|
R13 |
HOP |
1.5 |
2.07 |
3.1 |
18 |
|
R14 |
HOL |
1.5 |
2.07 |
3.1 |
28 |
|
R15 |
CRO |
1.5 |
2.4 |
3.6 |
20 |
|
R16 |
CRO |
6 |
0.8 |
4.8 |
44 |
|
R17 |
LAD |
7 |
0.79 |
5.5 |
70 |
ing or seated at the mouth of a tunnel from which VX vapor was flowing in an airstream at 1 m/min at a temperature of 32 °C. Only the head and neck of the test subjects were exposed. A total of 19 exposures were conducted without respiratory protection (see Table 1–9). All but one of the tests were conducted with eyes closed without the use of eye protection in the form of goggles or face mask. The only symptoms noted during the exposures were slight tightness in the throat and upper respiratory tract; these symptoms were not reported by all subjects. In the individual exposed with eyes open (0.31 mg/m3 for 3 min), miosis developed suddenly 20 to 30 min postexposure and was maximal at 1.5 h postexposure. In the individuals exposed with eyes closed, some miosis usually developed 1 to 3 h postexposure. The degree of miosis was quite variable among the individuals and appeared to be concentration-dependent. The miosis was often accompanied by a fluttering or twitching of the eyelids. Although the muscle effects were clearly reported by the subjects, they were not always obvious to the observers. Rhinorrhea occurred within 30 min of exposure in 14 of 19 trials. In four other trials, it developed more slowly; in one, it did not develop at all. Excessive salivation, lasting for about an hour, was reported in one subject after a 6-min exposure to a concentration of 1.06 mg/m3. Two hours postexposure, one individual experienced some nausea and sweating; RBC-ChE activity was 60% depressed at that time. These effects abated somewhat and then recurred later when ChE inhibition had reached 70%. Several individuals also experienced malaise and lethargy. Based on all 19 trials, the inhaled dose estimated to produce inhibition of 50% of the RBC-ChE activity (ChE50) was 13 µg/kg. However, the authors thought that apprehension had increased the subjects’ minute volume during initial exposures. That would have effectively increased the dose to which the individuals were exposed and was thought to account for a relatively shallow probit slope. When those data were excluded, the estimated ChE50 was about 8 µg/kg, which was thought to compare favorably with intravenous data.
The Bramwell et al. (1963) study is not considered credible for use in deriving AEGLs for agent VX. Reutter et al. (2000) examined the Bramwell et al. study as a potential critical study for the estimation of worker population levels (WPLs) and general population levels (GPLs) for chronic exposure to VX vapor (8-h time-weighted average for WPL; 24-h continuous exposure for GPL). Reutter et al. (2000) rejected the Bramwell et al. study because of multiple deficiencies; the concentration of VX to which the subjects were exposed could not be determined (subjects were seated in front of a “tunnel” down which generated VX vapor flowed in an
airstream of known velocity), both C and t were varied (resulting in no replicate cumulative exposures), and the organic sol vent benzene was used to help disperse the agent in the airstream to which subjects were exposed (Bramwell et al. did not address the potential effect of the carrier solvent on agent absorption by the subject). The majority of a U.S. Surgeon General’s review panel concurred with the appraisal of Bramwell et al. (1963) at a public hearing convened by the CDC to examine the Reutter et al. (2000) report (67 Fed. Reg. 894 [2002]; DHHS 2002).
Oral Exposures
In clinical studies conducted by Sidell and Groff (1974), single oral doses of VX at 2–4.5 µg/kg (stock solution in absolute ethanol diluted in a solution of saline and dextrose and swallowed by each subject under supervision) produced gastrointestinal symptoms in 5 of 32 test subjects (more specific dose-response data not reported). Regression analysis of the dose-response data indicated that the RBC-ChE50 was 2.3 µg/kg. Sidell and Groff (1974) reported that the oral dose of VX needed to produce 70% ChE inhibition (4 µg/kg) was 3 times greater than that needed to produce the same effect after intravenous administration.
Sim et al. (1964) reported no signs of toxicity in seven human volunteers receiving VX at 1.43 µg/kg/d for 7 d (in four daily doses of 500 mL drinking water); however, average RBC-ChE activity was reduced 60% (to 40% of baseline values). The Sim et al. (1964) study resulted in a lower RBC-ChE50 value than the Sidell and Groff (1974) oral study, probably because of the cumulative effects of VX given over the 7 d in the Sim et al. study. The total dose in the Sim et al. (1964) study was about twice that used in the Sidell and Groff (1974) oral study.
Intravenous Exposures
Several studies have been conducted in which human volunteers were injected intravenously with VX. The experiment of Kimura et al. (1960) was performed with the informed consent of the participants, under full clinical supervision and in a hospital setting considered suitable at the time (resuscitation team at bedside “to administer atropine, oximes, oxygen, artificial resuscitation, and tracheotomy if indicated”). Kimura et al. (1960) reported that a 30-s intravenous injection of 0.04 µg/kg in one adult test
subject caused frontal and retrobulbar headaches starting 45 min after the injection. The subject reported being tired and appeared irritable to observers, but no change in RBC or whole blood cholinesterase activity was observed. A subsequent 30-s intravenous injection of 0.08 µg/kg 3.5 h later resulted in a 2-fold increase in airway resistance, a 25–30% decrease in respiratory rate, and a 15% drop in pulse rate 15 min after the exposure, but no change in RBC-ChE. Headaches began 20 min postexposure, and minute volume increased from 15 L to 32 L 30–45 min postexposure. Peak effects (increased sweating, lightheadedness, and abdominal cramping) appeared about 45 min after the dose was administered. A single 30-s intravenous dose of 0.225 µg/kg in one test subject resulted in a 27% decrease in baseline RBC-ChE activity within 15 min as well as retrobulbar headaches. Many of these observed effects are for the single subject participating in the dose-response range-finding study—Dr. Van Sim, MD, a principal investigator of the reported study. Six additional subjects (volunteers identified by subject code) received VX at 1 µg/kg by intravenous infusion over 1.75 to 4 h periods and exhibited 50–60% depression in cholinesterase activity but no signs of toxicity (except for one 84-kg individual who reported headaches).
The Kimura et al. (1960) study meets the criteria for acceptance of human subject data for use by the AEGL process (e.g., evidence that subjects provided informed consent and that the studies were performed under appropriate clinical supervision).
In clinical studies conducted by Sidell and Groff (1974), 34 test subjects were given VX by intravenous injection. The administered dose ranged from 1.2 to 1.7 µg/kg. An intrvenous dose of 1.5 µg/kg administered to 18 test subjects resulted in dizziness, nausea, and vomiting in 11, 4, and 6 individuals, respectively; RBC-ChE was depressed 55–90% from baseline values (average about 75%). The test subjects exhibited a significant decrement in performance on a number facility test within 1 h after treatment. Regression analysis of the dose response data indicated that the RBC-ChE50 was 1.1 µg/kg (three individuals tested at 1.2 µg/kg, 1.3 µg/kg, 1.4 µg/kg, and 1.7 µg/kg; four at 1.6 µg/kg; and 18 at 1.5 µg/kg; estimated from graphic presentation of the data) (Sidell and Groff 1974).
Percutaneous Exposures
Dermal vapor absorption is a low priority for this compound, although there are certain release events that generate a dermal vapor threat. It is
generally acknowledged that a specific toxicological end point for vapor exposure to nerve agent VX would be achieved at a lower concentration exposure for the inhalation route than for other routes (e.g., the estimated human LCt50 for percutaneous vapor exposure to agent VX is 150 mg·min/m3, while the estimated human LCt50 for inhalation vapor exposure to agent VX is <15 mg·min/m3) (NRC 1997). Thus, AEGL estimates based on inhalation exposures are considered protective for both inhalation and dermal routes.
In studies conducted by Bramwell et al. (1963, as cited in Reutter et al. 2000) eight individuals were exposed for time periods ranging from 2.25 s to 24 min to VX vapor concentrations ranging from 0.23 mg/m3 to 5 mg/m3 (Cts=0.7 to 25.6 mg·min/m3). The test subjects were exposed while standing or seated at the mouth of a tunnel from which VX vapor was flowing in an airstream at 1 m/min at a temperature of 32 °C. Only the head and neck of the test subjects were exposed. Thirty-five of the exposures were performed with eyes closed (but without the use of eye protection in the form of goggles or face mask) and with respiratory protection (a nose clip was used and the subjects were breathing through a spirometer connected to a respirator canister). ChE inhibition was measurable within an hour of exposure and was greatest at 8–12 h postexposure. No signs or symptoms were noted during the exposure periods; however, 30 min or more after the initial exposure, miosis appeared in nearly all subjects and became maximal several hours later. It was usually accompanied or followed by fluttering and twitching of the eyelids and was more pronounced at the higher concentrations. Flushing of the skin of the head and neck was observed in five of the eight subjects, and all eight individuals reported local sweating in one or more tests. Although some subjects had the perception that they were experiencing “tunnel vision” postexposure, visual perimetry studies following three of the exposures were not confirmatory. Nor were there any changes in visual acuity or color vision. Five hours postexposure, one subject developed flatulence and abdominal discomfort. An hour later he did not feel well and was experiencing waves of nausea. Eight hours postexposure, he deteriorated rapidly and experienced severe nausea and vomiting. At that time, his RBC-ChE activity was only 30% of baseline; no further inhibition occurred. Bouts of vomiting and malaise continued, and he experienced cold sweating, pallor, and a feeling of motion sickness—minus the vertigo. At 12 h postexposure, he was able to sleep, but experienced a nightmare shortly after falling asleep. By the next morning no signs or symptoms remained. The Bramwell et al. (1963) study is not considered credible for deriving AEGL values.
Lubash and Clark (1960) reported that percutaneous doses of undiluted VX (20 µg/kg or 35 µg/kg) applied to the volar forearm of male volunteers resulted in significant decreases in blood ChE as well as signs and symptoms of toxicity (lightheadedness, nausea, vomiting, diarrhea, hyperactive bowel sounds, epigastric discomfort, insomnia, and nightmares) in two of four subjects dosed with 20 µg/kg and in two of four subjects dosed with 35 µg/kg (eight total subjects).
Sim (1962) reported that head and neck areas were the most sensitive to percutaneously applied VX. A dose of VX at 5 µg/kg applied to these areas resulted in signs and symptoms of systemic toxicity (nausea, vomiting, and weakness) in 54% (28 of 40) of the tested individuals. Whole blood ChE was 50% of normal in 5.8 h and 33.5% of normal in 8.5 h. It was estimated that a VX dose of 5.1 µg/kg would be necessary to result in RBC-ChE30 (this end point was chosen because median ChE depression of 30% was associated with the onset of gastrointestinal signs and symptoms of nausea and vomiting).
Cresthull et al. (1963) studied the effects of percutaneous absorption of VX vapors on whole blood ChE activity in 29 male volunteers. Exposures were to the arm or forearm. The VX concentrations ranged from 1.2 to 12.2 mg/m3 and the exposure times were from 2 to 75 min (Cts ranged from 6 to 765 mg·min/m3). Two men were exposed at 1.2–1.5 mg/m3 for 5 min (500 cm2 surface area exposed); six to 2.5–4.9 mg/m3 for 5–10 min (500 cm2 surface area exposed); four to 4.8–7.3 mg/m3 for 12–20 min (500 cm2 surface area exposed); ten to 4.5–8.0 mg/m3 for 20–60 min (1,000 cm2 surface area exposed); and seven to 8.5–12.2 mg/m3 for 60–75 min (1,000 cm2 surface area exposed). The median decrease in whole blood ChE in those groups was 5%, 3%, 8%, 18%, and 43%, respectively. Although whole blood ChE was inhibited as much as 76% at 20 h after exposure, none of the test subjects exhibited any toxic signs. Cresthull et al. (1963) estimated that the whole blood ChE50 vapor concentration for percutaneous exposures would be 141 mg·min/m3. The value was reported to be not statistically meaningful because of the wide confidence limits (lower 95% CI=35 mg/m3); however, by comparison with data for exposures to VX aerosols, Cresthull et al. concluded that the estimated ChE50 of 141 mg·min/m3 was acceptable. Cresthull et al. (1963) also estimated that 1 to 1.25 h whole-body percutaneous exposure to a Ct of 38 mg·min/m3 (0.51 mg/m3 for 75 min) would not cause any signs of toxicity other than “partial” lowering of whole blood ChE (activity inhibition between 0% and 31% from baseline).
Bowers et al. (1964) evaluated behavioral changes in 93 volunteers who were exposed percutaneously to small amounts of liquid EA-1701 (agent VX). The actual amounts of VX applied were not reported. The test subjects were divided into three postexposure groups depending on the level of reduction in their whole blood ChE (there was no control group). Of 32 individuals whose whole blood ChE was 81–100% of control values (not explicitly stated but presumed to be individual preexposure values) following exposure, 6% showed symptomatology of intellectual impairment (impairment of ability to perform simple arithmetic tasks, inability to perform serial sevens, impairment of performance in reading or standard games of concentration, and other subjective symptoms such as “impairment in orientation”), and 3% reported unusual dreams. Of the 24 whose whole blood ChE was 40–80% of control values, 4% showed symptomatology of intellectual impairment (by the measures reported above), 33% reported unusual dreams, 8% exhibited anxiety (determined by the appearance of palpitations coupled with other, subjective symptoms such as “restlessness”), and 4% exhibited psychomotor depression (determined by the appearance of reply latency, slowed speech, and evidence of fatigue in addition to other, subjective symptoms such as reported feelings of being “slowed down”). Of the 37 whose ChE was 10–40% of control values, 57% showed symptomatology of intellectual impairment (by the measures reported above), 38% reported unusual dreams, 30% exhibited anxiety, and 57% exhibited psychomotor depression. The more severely affected cases exhibited mood alterations as determined by Clyde mood card sort before and after exposure, and some developed nausea and vomiting. Miosis, bronchoconstriction, hypermotility of the lower bowel, and muscle fasciculations were not observed in any of the test subjects. Bowers et al. (1964) concluded that, with the exception of excessive dreaming, psychological symptomatology did not develop in the exposed individuals unless whole blood ChE fell to 40% or less of control values. Very few of the test subjects whose blood ChE was 80% or more of control values exhibited any signs.
Data compiled by Sidell (1992) revealed that, for individuals exposed to VX percutaneously, gastrointestinal signs (vomiting) occurred in 0.6% (1/166) when RBC-ChE activity was at 50% of control values and in 8% (2/24) when RBC-ChE levels were 40–49% of controls. Thirty-three percent exhibited such signs when RBC-ChE levels were 30–39% of controls, and 45% (19/42) exhibited signs when RBC-ChE levels were 20–29% of
controls. Sixty-seven percent (16/24) exhibited effects when RBC-ChE levels fell to less than 20% of control values.
2.2.3. Epidemiologic Studies
There are no human epidemiologic studies with dose-response data suitable for deriving AEGL estimates for the G agents.
Occupational exposures to agent GB have been associated with altered electroencephalograms (EEGs) (Duffy et al. 1979; Burchfiel and Duffy 1982). Burchfiel and Duffy (1982) evaluated the wake and sleep EEGs of 77 industrial workers who had been exposed at least once to agent GB (sarin); however, no exposures had occurred in the year preceding the study. Spectral analysis of the EEGs indicated significant increases in brain beta activity (12–30 Hz) in the exposed group compared with nonexposed controls, and sleep EEGs indicated significantly increased rapid eye movement in the exposed workers. Combinations of EEG components were subjected to computer analysis in an attempt to identify an exposed individual by EEG characteristics; however, the results were inconclusive. Burchfiel and Duffy (1982) concluded that there might be a threshold for this type of effect. In evaluating the data of Burchfiel and Duffy (1982), DHHS (1988) considered the EEG changes to be “of questionable significance—given the difficulty of demonstrating such changes and the absence of clinically significant effects even when EEG changes are present.”
A retrospective analysis of possible chronic or delayed adverse health effects among servicemen who participated in chemical agent effects and therapy testing at Edgewood Arsenal during the years 1955–1975 was conducted by the Committee on Toxicology of the National Research Council (NRC 1985). The primary source of information was provided by participant response to a questionnaire, but there were no exposure data from which to derive a dose-response relationship. The chronic health effects of concern were “excess cancer risk, and adverse mental, neurologic, hepatic and reproductive effects.”
Evaluation of questionnaire response indicated that data provided by subjects historically tested with anticholinesterase compounds did not significantly differ from that of control subjects or those tested with other compounds when self-evaluations of current health status were compared. The report candidly pointed out that the experimental design and comparison groups available were such that “only large effects were likely to be
uncovered” because of the resulting large standard errors, self-reporting, and the potential for more than one exposure to eventually result in development of the same biological end point (NRC 1985).
A number of studies have been conducted on individuals exposed to agent GB as a result of terrorist attacks in Japan. Morita et al. (1995) reported clinical findings for several hundred people who were exposed to agent GB in the city of Matsumoto in 1994. Subclinical miosis and neuropathy were still present in some individuals 30 d after exposure; however, most individuals exhibited no clinical signs of toxicity 6 mo after the exposure.
Several follow-up studies have examined the health of victims of the Tokyo subway terrorist attack that occurred in March of 1995. No clinical abnormalities were detected in 640 patients examined 3 mo after the incident (Okumura et al. 1996). Kato and Hamanaka (1996) examined 96 victims for ocular effects. The primary ocular signs and symptoms included miosis, conjunctival injection, and ocular pain. Some individuals had temporary blurring of vision, 36 patients complained of subjective accommodation impairment, and in 30 patients there were indications that agent GB (sarin) had caused a reduction in intraocular pressure (intraocular pressure was 11.6±1.9 mm Hg within 2 h of exposure but increased to 14.6± 1.8 mm Hg when the pupil diameter returned to normal). These signs and symptoms spontaneously resolved within 3–21 d after exposure in most cases. Kato and Hamanaka (1996) note that none of the victims developed corneal injury, glaucoma attack, or retinal detachment, and although the ocular condition of the patients returned to normal, they suggest that exposure to agent GB may increase the risk of angle-closure glaucoma caused by anterior shift of the lens, retinal detachment, and vitreous hemorrhage caused by extensive contraction of the ciliary muscles. Murata et al. (1997) evaluated neurophysiological deficits in 18 victims of the subway attack who had exhibited signs and symptoms of agent GB poisoning (i.e., headache, miosis, increased lacrimation, dyspnea, nausea, diarrhea, paraesthesia, and decreased serum ChE activity). It was reported that 6 mo after the exposure, the exposed but no longer symptomatic individuals exhibited significantly prolonged latencies in event-evoked potentials and visual evoked potentials suggestive of persistent cognitive and visual dysfunction.
In another study, Yokoyama et al. (1998a,b) evaluated chronic neurobehavioral effects in nine male and nine female patients 6–8 mo after the incident. Although this study is for a very small number of those affected
(only 18 out of approximately 5,500 people) and suffers from low recruitment, the results will be presented here for completeness. The neurobehavioral tests included (1) digit symbol (psychomotor performance), (2) picture completion (visual perception), (3) digit span (attention and memory), (4) finger tapping (psychomotor performance), (5) reaction time (psychomotor performance), (6) continuous performance test (sustained visual attention), (7) paired-associate learning (learning and memory), (8) General Health Questionnaire (GHQ) (psychiatric symptoms), and (9) Profile of Mood States (POMS) (mood). Fifteen controls were used in the tests. Analysis of covariance of the test results suggested to the investigators that “perhaps a chronic effect on psychomotor performance [digit symbol test only] was caused directly by acute agent GB (sarin) poisoning; on the other hand, the effects of psychiatric symptoms (GHQ) and fatigue (POMS) appeared to result from post-traumatic stress disorder induced by exposure to sarin.” Yokoyama et al. (1998c) have also reported vestibulocerebellar effects (increased postural sway) in 18 patients tested 6–8 mo after the incident. Postural sway was significantly greater than controls in exposed females but not in males. In both genders postural sway was correlated with the plasma cholinesterase activity measured immediately after the exposure.
The U.S. Department of Defense reported in 1997 that military personnel might have been exposed to nerve agents as a result of the demolition of Iraqi munition storage sites. Retrospective studies have evaluated the post-war health of soldiers who may have been exposed to nerve agents. Landrigan (1997) reviewed the principal epidemiologic studies published before 1997. Kang and Bullman (1996) reported a 0.8% higher death rate among Gulf War veterans (10.4%) compared with other veterans of the same time period (9.6%); this difference was largely due to accidents, and no excess deaths from suicides or specific diseases were observed. Gray et al. (1996, 1999) reported no consistent pattern for increased occurrence of any specific disease or hospitalization among Gulf War veterans. Further, Gray et al. (1999) indicate that “this data analysis does not support the hypothesis that Gulf War veterans are suffering postwar morbidity from subclinical nerve agent exposure.”
Other epidemiologic studies have reported an increase in neurologic disorders among selected groups of Gulf War veterans but have not linked any reported signs, symptoms, or clinical effects with potential nerve agent exposure (Goldstein et al. 1996; Kotler-Cope et al. 1996; Haley et al. 1997a,b; Haley and Kurt 1997; Hom et al. 1997; Schwartz et al. 1997; Vasterling et al. 1998).
Epidemiologic studies regarding human exposure to agent VX were not found in the available literature.
2.3. Neurotoxicity
The G agents (GA [tabun], GB [sarin], GD [soman], and GF) and agent VX are toxic organophosphate ester derivatives of phosphonic acid. They are commonly termed “nerve” agents as a consequence of their potent anticholinesterase properties and subsequent adverse effects on both smooth and skeletal muscle function as well as the central and peripheral nervous systems. These neurotoxic properties were discussed in detail in Sections 2.1 and 2.2.
Although the inhibition of cholinesterases within neuroeffector junctions or the effector itself is thought to be responsible for the major toxic effects of nerve agents, these compounds can affect nerve impulse transmission by more direct processes as well (e.g., direct effects on neurotransmitter receptors) (see Section 4.2).
2.4. Developmental and Reproductive Toxicity
The retrospective study of agent-exposed servicemen discussed in preceding sections (NRC 1985) requested self-reported information on fertility. Two comparison groups of men were used. One was a “no chemical test” (NCT) group who met the requirements for military service but did not meet the more rigorous requirements (physical and mental screening exams for contraindications) necessary for chemical exposure tests. Those individuals were exposed to placebos, equipment only, or “FDA approved drugs” not otherwise identified. A second comparison group comprised men tested with compounds other than those being evaluated in a particular test, the “other chemical test” (OCT) group. These individuals also met the requirements for military service. They were exposed to test chemicals other than the chemicals of interest. The OCT compounds appear to include cannabinoids, “approved drugs,” and “innocuous chemicals and controls” not otherwise identified (NRC 1985). When the collected data were adjusted for volunteer age when the last test was performed (to accommodate national trends toward smaller and delayed families), there “was no difference between the observed fertility pattern of men exposed to anticholinergic chemicals and that expected on the basis of men who were
exposed to other chemicals” (NRC 1985). Nevertheless, these data are not useful for application to the derivation of an AEGL given that no exposure data were collected.
Iranian soldiers and civilians were exposed to multiple chemical warfare agents during the Iran/Iraq conflict. Exposures may have been to the nerve agents GA and/or GB as well as to the vesicant sulfur mustard. Follow-up studies have been conducted on some of the individuals. It has been reported that the offspring of these chemical warfare victims born after the Iran/Iraq conflict were more likely to have birth defects than those born before the war (Pour-Jafari 1994a). It was also reported that the off-spring had an altered gender ratio (Pour-Jafari 1994b). Because of the possibility that exposures to multiple chemicals had occurred, it is impossible to determine if, or to what extent, exposure to any of the G agents contributed to the reported effects.
There have been several reports of potential increased incidence of birth defects among the offspring of military personnel who served in the Persian Gulf War. Araneta et al. (1997) reported a slight increase (relative risk 3.03, with 95% CI=0.63–20.57) in Goldenhar syndrome among infants born in military hospitals to Gulf War veterans. Goldenhar syndrome is a craniofacial anomaly of unclear etiology. According to Araneta et al. (1997), suggested associations with its occurrence have included chromosomal abnormalities, a genetic pattern of inheritance, maternal diabetes, or prenatal exposure to several controlled or therapeutic drugs (e.g., cocaine, tamoxifen); the role of male-mediated effects is undefined. At least one mother of one case infant exhibited mild facial asymmetry upon examination, and the family of another case infant had a history of birth defects. In all five cases of confirmed Goldenhar syndrome among the 34,069 infants born to veterans of the Gulf War and included in this retrospective study, only the paternal parent served in the military. Among nondeployed veterans (two cases of a total 41,345 births examined), only the paternal parent served in the military.
Araneta and colleagues (1997) point out that differences in prevalence rates of Goldenhar syndrome among the offspring of Gulf War veterans (14.7, 95% CI=5.4–36.4) are not significantly different from those of nondeployed veterans (4.8,95% CI=0.8–19.5) because of the small sample sizes and wide confidence intervals. Araneta et al. (1997) determined that, with the sample size maintained as a constant, “the risk would have had to be at least 5.75 times higher among the Gulf War veterans’ infants in order to be statistically significant.”
As a consequence of this finding of no significance, the Goldenhar syndrome issue was not further pursued in the AEGL analysis for chemical warfare agents. Its utility is further compromised by the fact that there is no confirmed report of veteran exposure to chemical warfare agents, and the Gulf War fathers in the Araneta et al. (1997) study all served in different units and were deployed in theater at different times.
No data are available regarding the potential reproductive and developmental toxicity of agent VX in humans.
2.5. Genotoxicity
There is no information available to evaluate the genotoxicity of G agents or agent VX in humans.
2.6. Carcinogenicity
There are no human data to suggest that G agents or agent VX are carcinogenic.
2.7. Summary
G-series Agents
Available information on the acute inhalation toxicity of agent GB (sarin) to humans is summarized in Table 1–10. Minimal effects observed at low concentrations include miosis, tightness of the chest, rhinorrhea, and dyspnea. The threshold for minimal effects appears to fall in the range of 0.05 to 0.5 mg/m3 for 10–30 min exposures. The results from different studies are not consistent in identifying the threshold, and that may be due to differences in individual sensitivities, breathing rates of the test subjects, experimental protocols, or analytical methods.
A number of investigators consider both miosis and rhinorrhea to be early signs of exposure to cholinesterase inhibitors (Sidell 1997; Mioduszewski et al. 2002b; H. van Helden, Pulmonary and CNS Pharmacology Lab, TNO, the Netherlands, personal communication; S.Tattersall, Biomedical Sciences Division, Porton Down, United Kingdom, personal
TABLE 1–10 Human Experimental Data For GB Vapor (Single Exposures)
|
Study |
GB Concentration (mg/m3) |
Duration |
Ct (mg·min/m3) |
Signs and Symptoms |
|
Harvey 1952 |
0.05 |
20 min |
1 |
Headache, eye pain, rhinorrhea, tightness in chest, cramps, nausea, malaise (N=14) |
|
Johns 1952 |
0.05 |
20 min |
1 |
Mild miosis (mean maximum decrease in pupil diameter 1–2 mm) in some of the test subjects (150 observations) |
|
McKee and Woolcott 1949 |
0.06 |
20 min |
1.2 |
No reported effects (N=5) |
|
McKee and Woolcott 1949 |
0.06 |
40 min |
2.0 |
“Threshold” for miosis; no other signs or symptoms (N=4) |
|
Fairley and Mumford 1948 |
0.3 |
0.5 min |
0.15 |
Rhinorrhea in 16 and tightness in chest in 7 (N=16) |
|
Baker and Sedgwick 1996 |
0.5 |
30 min |
1.5 |
Miosis, dyspnea, photophobia, 40% inhibition of RBC-ChE, changes in SFEMG (N=8) |
|
McKee and Woolcott 1949 |
0.6 |
1 min |
0.6 |
Miosis and slight tightness in chest (N=4) |
|
Rubin et al. 1957 |
2 |
2 min |
4 |
Miosis; no other signs of ChE inhibition (N=3) |
|
Callaway and Dirnhuber 1971 |
|
10 min to 5 h |
3.13a |
50% pupil area decrement |
|
Study |
GB Concentration (mg/m3) |
Duration |
Ct (mg·min/m3) |
Signs and Symptoms |
|
Callaway and Dirnhuber 1971 |
|
10 min to 5 h |
13.85b |
90% pupil area decrement |
|
Oberst et al. 1968 |
4.19 |
2 min |
8.38 |
47% inhibition of RBC-ChE; no other effects; eyes not exposed (breathing rate 5.6–8.4 L/min) |
|
Oberst et al. 1968 |
20.7 |
2 min |
41.4 |
49% inhibition of RBC-ChE; no other effects; eyes not exposed (breathing rate 47–65 L/min) |
|
Note: Entries are from primary sources and known experimental data a95% confidence limits 2.15–4.57 mg·min/m3. b95% confidence limits 6.00–32.02 mg·min/m3. |
||||
communication). The presence of rhinorrhea can be indicative of inhalation exposure and/or development of systemic effects, while miosis alone in the absence of other signs or symptoms is a local effect to the pupillary muscles of the eye. As a consequence, the presence of miosis is considered an appropriately sensitive indicator of direct vapor exposure and has the additional advantage of being readily recognized and quantifiable.
There is no evidence that exposure to any of the G agents results in developmental or reproductive toxicity, nor are there any data available to evaluate potential genotoxicity in humans. The G agents have not been identified as human carcinogens.
Agent VX
Experimental data on the effects of acute VX exposures to humans are summarized in Tables 1–11 and 1–12; very few studies have been conducted using exposures to VX vapor, and available data are not of sufficient quality to be used directly in the development of AEGL estimates.
A comparison of the results of the intravenous studies, in terms of the estimated absorbed dose, allows for an evaluation of the dose-response relationship (Table 1–11).
Studies indicate that an intravenous dose of about 1 µg/kg can result in 50% ChE depression and some symptoms of toxicity (headaches); an intravenous dose of about 0.1 µg/kg is unlikely to affect RBC-ChE, but may cause mild effects (headache, chest tightness, dyspnea); and an intravenous dose of 0.01 µg/kg may be below the effects threshold. The estimated equivalent air concentrations for these dose levels, using standard default values for body weight (70 kg) and breathing rate (0.0138 m3/min), are also listed in Table 1–11. They are highly derivative values and are only presented for comparative purposes.
Experimental data from the Bramwell et al. (1963) study are summarized in Table 1–12. Although the Bramwell et al. (1963) data are considered suspect, they provide a means of comparison with the equivalent concentrations estimated from the intravenous data. Both sets of data suggest that a 10-min exposure to VX at 0.5 mg/m3 or higher may produce substantial depression of RBC-ChE activity and some clinical signs of toxicity.
Available data summarized suggest that a 10-min exposure to VX at 0.5 mg/m3 or higher may produce depression of RBC-AChE activity and some clinical signs of toxicity.
TABLE 1–11 Human Experimental Data for VX
|
Dose (µg/kg) |
Exposure Route |
Estimated Equivalent Concentration (mg/m3)a |
End Point |
Reference |
|
0.01–0.13 (estimated) |
Inhalation (sniff test) |
0.05–3.34 |
No ChE change; headache, chest tightness, dryness of the mouth |
Koon et al. 1959 |
|
0.04 |
Intravenous (30 s injection) |
0.41 (0.02 for 10 min)b |
No ChE change; headache, tiredness, irritability |
Kimura et al. 1960 |
|
0.12 |
Intravenous (2 doses over 3.5 h) |
0.003 (0.06 for 10 min)b |
No ChE change; headache, light headedness, abdominal cramps, decrease in respiration and pulse rates, increase in airway resistance and minute volume |
Kimura et al. 1960 |
|
1.0 |
Intravenous (over 1.75–4 h) |
0.021(0.5 for 10 min)b |
50–60% depression in ChE activity; headaches in 1/6 individuals |
Kimura et al. 1960 |
|
1.0 |
Intravenous (1 dose) |
10 (0.51 for 10 min)b |
50% inhibition of RBC-ChE |
Sidell and Groff 1974 |
|
1.5 |
Intravenous (1 dose) |
15 (0.76 for 10 min)b |
75% depression in ChE: dizziness (11/18), nausea (4/18), vomiting (6/18) |
Sidell and Groff 1974 |
|
1.43 |
Oral (1 dose/d for 7 d) |
NA |
60% inhibition of RBC-ChE; no signs or symptoms of toxicity |
Sim et al. 1964 |
|
2.3 |
Oral |
NA |
50% inhibition of RBC-ChE |
Sidell and Groff 1974 |
TABLE 1–12 Human Experimental Data For VX Vapor from Bramwell et al. (1963)a
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
Acute inhalation lethality data for agents GB, GA, GD, GF, and VX for several laboratory species are summarized in Tables 1–13 through 1–17. Additional lethality information is presented in the following subsections.
3.1.1. Nonhuman Primates
Agent GB
The National Defense Research Committee (NDRC) (1946) reported an LCt50 value of 150 mg·min/m3 for 10-min exposure in five monkeys. Cresthull et al. (1959) reported that inhalation doses of GB at 19.2–48.4 µg/kg (0.55-min exposures) resulted in 33–100% mortality in monkeys.
Johnson et al. (1988) conducted a series of lethality studies in which nonhuman primates (baboons) were exposed to GB by the inhalation pathway. Baboons were considered to be a more appropriate test species than rodents or dogs because of the similarities between baboon and human lungs in both biochemical and functional characteristics. Young male baboons were exposed to GB vapor at 1.25–1.3 LD50 (N=6) or GD vapor at 2 LD50 (N=5). As a result of these tests, Johnson et al. (1988) (see also Anzueto et al. [1990]) reported that inhalation exposures were in close agreement with the reported intravenous LD50 values (approximately 13 µg/kg). Woodard et al. (1994) reported an intravenous LD50 of 14.7 µg/kg for GB in rhesus monkeys.
Vapor inhalation studies were conducted by Oberst (1961) on monkeys (species not identified) fitted with masks through which GB concentrations were administered. The eyes were protected by eyepieces and were not exposed. The resulting 2-min LCt50 value of 42 mg·min/m3 is reported in Table 1–9.
Agent GA
DA (1974) (secondary source) reported LCt50 values of 187 and 135 mg·min/m3 for monkeys.
TABLE 1–13 Acute Inhalation Lethality Values for Agent GB in Animals (Toxicity Value, LCt50)
|
Species |
Duration (min) |
Ct (mg·min/m3) |
Reference |
|
Monkey |
10 |
74 |
DA 1974 |
|
Monkey |
2 |
42 |
DA 1974 |
|
Monkey |
0.167 |
27 |
DA 1974 |
|
Monkey |
10 |
150 |
NDRC 1946a |
|
Monkey |
2 |
42 |
Oberst 1961a |
|
Dog |
10 |
60 |
DA 1974 |
|
Dog |
2 |
56 |
Oberst 1961a |
|
Rabbit |
10 |
120 |
DA 1974 |
|
Guinea pig |
1 |
140 |
Oberst 1961a |
|
Guinea pig |
10 |
180 |
DA 1974 |
|
Rat |
5 |
164 (f) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
10 |
181 (f) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
30 |
255 (f) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
60 |
383 (f) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
90 |
401 (f) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
240 |
727 (f) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
360 |
947 (f) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
5 |
230 (m) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
10 |
226 (m) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
30 |
265 (m) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
60 |
453 (m) |
Mioduszewski et al. 2000, 2001a |
|
Species |
Duration (min) |
Ct (mg·min/m3) |
Reference |
|
Rat |
90 |
433 (m) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
240 |
982 (m) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
360 |
1040 (m) |
Mioduszewski et al. 2000, 2001a |
|
Rat |
10 |
220 |
DA 1974 |
|
Rat |
10 |
300 |
Cohen et al. 1954a |
|
Rat |
1 |
220 (m), 118 (f) |
Callaway and Blackburn 1954a |
|
Rat |
5.0–6.7 |
191 (f) |
Schoene et al. 1985a |
|
Mouse |
30 |
501 |
Bide et al. 1999 |
|
Mouse |
30 |
600 |
Husain et al. 1993a |
|
Mouse (active) |
10 |
240 |
DA 1974 |
|
Mouse (resting) 10 |
310 |
DA |
1974 |
|
aItalic entries are from primary sources and known experimental data. Abbreviations: f, female; m, male. |
|||
Agent GD
In tests conducted on baboons, Johnson et al. (1988) found that the effects of inhalation exposures were in close agreement with the reported intravenous LD50 values of 6.6 µg/kg. Adams et al. (1975) reported a 15-d intramuscular LD50 of 6.57 µg/kg.
3.1.2. Dogs
Agent GB
NDRC reported LCt50 values of 100–150 mg·min/m3 for 10-min exposures. Cresthull et al. (1959) reported that inhalation doses of GB at 25.1
TABLE 1–14 Acute Inhalation Lethality Values for Agent GA in Animals (Toxicity Value, LCt50)
|
Species |
Duration (min) |
Ct (mg·min/m3) |
Reference |
|
Monkey |
10 |
187 |
DA 1974a |
|
Monkey |
10 |
135 |
DA 1974a |
|
Dog |
2 |
320 |
DA 1974a |
|
Rabbit |
10 |
960 |
DA 1974a |
|
Rat |
10 |
450 |
DA 1974a |
|
aDA (1974) is a secondary source. |
|||
and 26.0 µg/kg (0.6- and 2.23-min exposures) were not lethal to dogs, but inhalation doses of GB at 32.5 µg/kg or higher caused 40% or more mortality. Bide et al. (1999) and Yee et al. (1999) developed a three dimensional probit model to calculate lethality values (LC05, LC50 LC95) from historic laboratory data and to estimate equivalent human values. Using the species-specific constants for inhalation rates, body mass, et cetera, provided by these authors, the 30-min LC50 value for dogs was calculated to be 4.3 mg/m3.
Inhalation studies were conducted by Oberst (1961) on dogs (breed not identified) fitted with masks through which GB vapor flowed. The eyes were protected by eyepieces and were not exposed. The resulting 2-min LCt50 value of 56 mg·min/m3 is reported in Table 1–9.
Agent GA
DA (1974) (secondary source) reported an LCt50 value of 320 mg·min/m3 for dogs.
Agent VX
Bide and Risk (2000) cite several earlier studies in which the LCt50 values for VX aerosols were reported to be 15 mg·min/m3 (whole body) (Krackow 1956) and 15.1 mg·min/m3 (Punte and Atkinson 1960).
TABLE 1–15 Acute Inhalation Lethality Values for Agent GD in Animals (Toxicity Value, LCt50)
|
Species |
Duration (min) |
Ct (mg·min/m3) |
Reference |
|
Rabbit |
10 |
160 |
DA 1974 |
|
Rat |
1 |
196 (m) 135 (f) |
Callaway and Blackburn 1954a |
|
Rat |
5.3–8.5 |
211 (f) |
Schoene et al. 1985a |
|
Rat |
10 |
279 |
DA 1974 |
|
Rat |
10 |
230 |
DA 1974 |
|
Rat |
<30 (threshold at 16) |
400 (threshold at 335) |
Aas et al. 1985a |
|
Guinea pig |
8 |
480 |
Langenberg et al. 1998a |
|
aEntries are from primary sources and known experimental data. Abbreviations: f, female; m, male. |
|||
3.1.3. Rats
Agent GB
NDRC (1946) reported LCt50 values of 150–300 mg·min/m3 for 10-min exposures. Bide et al. (1999) and Yee et al. (1999) developed a three dimensional probit model to calculate lethality values (LC05, LC50, LC95) from historic laboratory data and to estimate equivalent human values. Using the species-specific constants provided, the 30-min LC50 value for rats was calculated to be 8.2 mg/m3.
In studies conducted by Mioduszewski et al. (2000, 2001, 2002a), the acute lethal toxicity of GB to male and female Sprague-Dawley rats was evaluated for time periods of 10, 30, 60, 90, 240 and 360 min in a whole-body dynamic chamber. The final report of this study (Mioduszewski et al. 2001, 2002a) is further documentation of the findings presented below. Ten males and 10 females were used for each concentration-time (Ct) combination, and 50 males and 50 females were used for each time point. GB concentrations ranged from about 2 mg/m3 to 56 mg/m3. Agent concentrations were confirmed in the exposure chamber by three procedures (“Edgewood” bubblers, solid sorbent tubes, and a phosphorous monitor) to allow
TABLE 1–16 Acute Inhalation Lethality Values for Agent GF in Animals (Toxicity Value, LCt50)a
point and continuous determinations (Mioduszewski et al. 2000, 2001, 2002a). Lethality was assessed at 24 h and at 14 d. Female rats were reported to be more sensitive to GB vapor toxicity than males over the range of exposure concentrations and durations studied (Mioduszewski et al. 2000, 2001, 2002a). Gender differences were reported to be significant at p<0.01. Probit analysis (MINITAB, version 13) presented in Mioduszewski et al. (2000) gave the following 14-d LC50 values for female rats exposed to GB vapor: 18.1 mg/m3 for 10 min; 8.51 mg/m3 for 30 min; 6.39 mg/m3 for 60 min; 3.03 mg/m3 for 4 h; and 2.63 mg/m3 for 6 h.
Probit analysis presented in Mioduszewski et al. (2000) gave the following 14-d LC50 values for male rats: 22.6 mg/m3 for 10 min; 8.84 mg/m3 for 30 min; 7.55 mg/m3 for 60 min; 4.09 mg/m3 for 240 min; 2.89 mg/m3 for 360 min.
Based on a probit analysis of the data (Mioduszewski et al. 2000), the estimated 14-d LC01 values for the females are as follows: 11.54 mg/m3 for 10 min; 5.84 mg/m3 for 30 min; 4.01 mg/m3 for 60 min; 2.09 mg/m3 for 4 h; 1.76 mg/m3 for 6 h.
This is the critical study and data set (females) for determination of AEGL-3 values.
GB vapor exposure significantly inhibited rat blood cholinesterase activity in the Mioduszewski et al. (2000, 2001, 2002a) study. However, no correlation between severity of clinical signs and cholinesterase inhibition was reported.
Cohen et al. (1954) reported that exposure of rats to GB at 30 mg/m3 for 10 min (Ct=300 mg·min/m3) resulted in mortality rates of close to 50%
TABLE 1–17 Lethality in Laboratory Species Following Inhalation Exposure to Agent VX Vapor (End Point, LCt50)
|
Species |
Duration (min) |
Ct (mg·min/m3) |
Reference |
|
Mouse (head only) |
10 min |
13.6 |
Koon et al. 1960, as cited in NRC 1997 |
|
Mouse (whole body) |
10 min |
4.0 |
Koon et al. 1960, as cited in NRC 1997 |
|
Mouse (nose only) |
– |
71 |
Carroll et al. 1957 |
|
Mouse (whole body) |
– |
16.1 |
Carroll et al. 1957 |
|
Goat |
10 min |
9.2 |
Koon et al. 1960, as cited in NRC 1997 |
|
Mouse |
6 h/d, 5 d/wk, 2 wk |
0.9 |
Crook et al. 1983 (noncredible data) |
|
Rat |
6 h/d, 5 d/wk, 2 wk |
24.9 |
Crook et al. 1983 (noncredible data) |
|
Guinea pig |
6 h/d, 5 d/wk, 2 wk |
238.6 |
Crook et al. 1983 (noncredible data) |
|
Rabbit |
6 h/d, 5 d/wk, 2 wk |
No deaths |
Crook et al. 1983 (noncredible data) |
and a decrease in brain cholinesterase activity levels to less than 5% of normal.
Schoene et al. (1985) reported an LCt50 of 191 mg·min/m3 (95% CI= 178–204 mg·min/m3) for exposure times of 5.0–6.7 min for female Wistar rats. The corresponding 5-min LC50 is 38 mg/m3. Callaway and Blackburn (1954) reported LCt50 values of 220 mg·min/m3 for male albino rats and 118 mg·min/m3 for female albino rats (1-min exposures).
Agent GA
DA (1974) (secondary source) reported an LCt50 value of 450 mg·min/m3 for the rat.
Agent GD
In a study designed to secondarily examine agent GD toxicity, Aas et al. (1985) reported that the LCt50 for GD in rats (six animals tested at each of three exposure levels for periods of time <30 min) was 400 mg·min/m3. Aas et al. (1985) graphically present their data as an LCt-versus-mortality curve. The lethality threshold estimated from the curve is about 335 mg·min/m3. Because the reported GD concentration was fixed at 21 mg/m3 for the duration of the study, the exposure time corresponding to the lethal threshold is 16 min (see Table 1–11).
The principal objective of the Aas et al. (1985) study was to test an experimental dynamic flow system that would allow study of highly toxic vapors. In consequence, it was necessary to continually generate small amounts of the toxic material in question. Agent GD (soman) was the compound selected to best test the system. Secondary objectives of the study were to determine the (short-term) inhalation toxicity of agent GD (soman) and to study inhibition of acetylcholinesterase, cholinesterase, and carboxylesterase activity in the respiratory tract (relative to other tissues).
Schoene et al. (1985) reported an LCt50 of 211 mg·min/m3 (95% CI= 195–229 mg·min/m3) for exposure times of 5.3–8.5 min for female Wistar rats. Callaway and Blackburn (1954) reported LCt50 values of 196 mg·min/m3 for male albino rats and 135 mg·min/m3 for female albino rats (1-min exposures) (Table 1–11).
Agent GF
Callaway and Blackburn (1954) reported LCt50 values of 181 mg·min/m3 for male albino rats and 110 mg·min/m3 for female albino rats (1 -min exposures). Kassa and Cabal (1999) reported an intramuscular LD50 of 80 µg/kg for rats.
A recent study of GF vapor inhalation toxicity in male and female SD rats reported 24-h postexposure LC50 and LCt50 values for three exposure periods (10, 60, and 240 min) (Anthony et al. 2002). Young adult rats were exposed whole-body in a dynamic 750-L chamber under protocols similar to those previously published by Mioduszewski et al. (2001, 2002a) but with additional accommodation for the lesser volatility of agent GF. For female rats, Anthony et al. (2002) report 24-h LC50 values as follows: 10 min, 25.3 mg/m3; 60 min, 5.56 mg/m3; 240 min, 2.22 mg/m3. For male rats,
24-h LC50 values are as follows: 10 min, 36.8 mg/m3; 60 min, 6.60 mg/m3; 240 min, 2.48 mg/m3. These results are summarized as LCt50 values in Table 1–16.
Agent VX
Crook et al. (1983) reported an LCt50 of 24.9 mg·min/m3 for animals exposed 6 h/d, 5 d/wk, for 2 wk; however, the results of the study are not considered credible (see Section 3.2).
Bide and Risk (2000) exposed outbred male CD1(SD)BR rats to NaCl aerosols containing entrained VX. The animals (five per test group) were tested with a nose-only inhalation system and for an exposure time of 12 min. Test concentrations were not reported. The observed LCt50 was 67 mg·min/m3.
In studies conducted by Maxwell (1992) on Sprague-Dawley rats, the subcutaneous LD50 for VX was reported to be 0.027 µmol/kg. DA (1974) (secondary source) reports intragastric LD50 values of 0.1 mg/kg and 0.077–0.1280 mg/kg for rats.
3.1.4. Mice
Agent GB
NDRC (1946) reported LCt50 values of 150–300 mg·min/m3 for 10-min exposures, 360 mg·min/m3 for a 20-min exposure, and 420 mg·min/m3 for a 30-min exposure (14 mg/m3 for 30 min). Clement (1992) reported a subcutaneous LD50 value of 170 µg/kg for male CD-1 mice with body weight ranging from 30–40 g (five animals per test group). A subcutaneous LD50 of 0.212 mg/kg was reported for male and female Shanghai mice (18–22 g body weight, eight mice per group, five exposure groups) (Luo and Liang 1997). Bide et al. (1999) and Yee et al. (1999) developed a three dimensional probit model to calculate lethality values (LC05, LC50, LC95) from recently conducted laboratory experiments on mice and historic toxicity data for other laboratory species and to estimate equivalent human values. Using the species-specific constants provided by Bide et al. (1999), the 30-min LC50 value for the mouse was calculated to be 16.7 mg/m3.
The LCt50 for agent GB in female Swiss albino mice has been reported
to be 600 mg·min/m3 (Husain et al. 1993), equivalent to a 30-min exposure at 20 mg/m3. Lohs (1960) reported a 30-min inhalation lethality value of value of 5 mg/m3.
Agent GD
Lohs (1960) reported a 30-min inhalation lethality value of 1 mg/m3.
Agent GF
Inhalation lethality data for agent GF were not found in the available literature. Luo and Liang (1997) reported an LD50 of 0.346 mg/kg in mice injected with the agent subcutaneously. Clement (1992) reported a subcutaneous LD50 value of 243 µg/kg for male CD-1 mice with body weights ranging from 30–40 g (five animals per test group).
Agent VX
Ten-minute LCt50 values of 4.0 mg·min/m3 (whole body) and 13.6 mg·min/m3 (head only) have been reported for mice exposed to VX vapors (Table 1–6) (Koon et al. 1960, as cited in NRC 1997). LCt50 values of 71 mg·min/m3 for female mice for nose-only exposures and 16.1 mg·min/m3 for whole-body exposures were reported by Carroll et al. (1957) for female mice; however, in this study it was reported that the concentration of the agent in the exposure chamber was not measured directly but was estimated from the mortality level, which was correlated with the LD50 for an intravenous injection (estimated to be 17 µg/kg).
Crook et al. (1983) reported an LCt50 of 0.9 mg·min/m3 for animals exposed 6 h/d, 5 d/wk, for 2 wk to VX vapors; however, the results of this study are not considered credible.
Koplovitz et al. (1992) exposed Swiss ICR mice intramuscularly to GB and VX. The resulting acute (24-h) LD50 are as follows: LD50 for GB of 204.81 µg/kg, LD50 of VX of 13.07 µg/kg.
Bide and Risk (2000) exposed outbred male CD1(ICR)BR mice to NaCl aerosols containing entrained VX. The animals (five per test group) were tested with a nose-only inhalation system and for a exposure time of
12 min. Test concentrations were not reported. The observed LCt50 was 72 mg·min/m3. Bide and Risk (2000) also cite several earlier studies in which the LCt50 values for VX aerosols were reported to be 7 mg·min/m3 (Krackow 1956) and 6.1 mg·min/m3 (Punte and Atkinson 1960).
3.1.5. Guinea pigs
Agent GB
NDRC (1946) reported LCt50 values of 150–250 mg·min/m3 for 10-min exposures. Bide et al. (1999) and Yee et al. (1999) developed a three dimensional probit model to calculate lethality values (LC05, LC50, LC95) from historic laboratory data and to estimate equivalent human values. Using the species-specific constants provided, the 30-min LC50 value for guinea pigs was calculated to be 7.5 mg/m3.
Oberst (1961) conducted inhalation studies on guinea pigs administered GB vapor by means of face masks “which filled the face without leakage.” The author does not mention eye protection for guinea pigs. The resulting 1-min LCt50 of 140 mg·min/m3 is reported in Table 1–9.
Atchison et al. (2001) reported that subcutaneous injections of 0.6 LD50 GB once per day, 5 d/wk, in young male Hartley guinea pigs (600 g) resulted in 50% mortality (two of four) after 2 wk of exposure and 100% mortality after 3 wk. The subcutaneous LD50 for guinea pigs was reported to be 42 µg/kg.
Agent GD
Allon et al. (1998) reported an inhalation LD50 of 101 µg/kg for guinea pigs, considerably higher than the reported intravenous LD50 values of 22 µg/kg (Sterri et al. 1982) and 3.5 µg/kg (Due et al. 1993). For guinea pigs weighing 0.84 kg and breathing 0.4 m3/d (EPA defaults), a dose of 101 µg/kg would be equivalent to an exposure to GD at 0.009 mg/m3 for 24 h, 0.22 mg/m3 for 1 h, or 0.44 mg/m3 for 30 min. Langenberg et al. (1998) reported an 8-min LCt50 of 480 mg·min/m3 for guinea pigs. This value is equivalent to an LC50 of 60 mg/m3 for 8 min or 16 mg/m3 for 30 min (assuming linear scaling).
Atchison et al. (2001) reported that subcutaneous injections of 0.6 LD50
GD once per day, 5 d/wk, in young male Hartley guinea pigs (600 g) resulted in 50% mortality (two of four) after 2 wk of exposure and 100% mortality after 9 wk. The subcutaneous LD50 for guinea pigs was reported to be 28 µg/kg.
Agent VX
Crook et al. (1983) reported an LCt50 of 238 mg·min/m3 for animals exposed 6 h/d, 5 d/wk, for 2 wk; however, the results of this study are not considered credible.
Koplovitz et al. (1992) exposed Hartley albino guinea pigs (subcutaneous) to GB and VX. The resulting acute (24-h) LD50 are as follows: LD50 for GB of 41.26 µg/kg, LD50 for VX of 6.89 µg/kg.
Bide and Risk (2000) exposed outbred male (HA)BR guinea pigs to NaCl aerosols containing entrained VX. The animals (five per test group) were tested with a nose-only inhalation system and for an exposure time of 12 min. Test concentrations were not reported. The observed LCt50 was 30 mg·min/m3. Bide and Risk (2000) also cite several earlier studies in which the LCt50 values for VX aerosols were reported to be 30 mg·min/m3 (whole body) (Krackow 1956) and 29.5 mg·min/m3 (Punte and Atkinson 1960).
Atchison et al. (2001) reported that subcutaneous injections of 0.6 LD50 VX once per day, 5 d/wk, in young male Hartley guinea pigs (600 g) resulted in 33% mortality (two of six) after 10 wk of exposure and 83% mortality (five of six) after 9 wk. One animal survived the full 13 wk of treatment. The subcutaneous LD50 for guinea pigs was reported to be 9 µg/kg.
3.1.6. Rabbits
Agent GB
NDRC (1946) reported LCt50 values of 120–250 mg·min/m3 for 10-min exposures. Bide et al. (1999) and Yee et al. (1999) developed a three dimensional probit model to calculate lethality values (LC05, LC50, LC95) from historic laboratory data and to estimate equivalent human values. Using the species-specific constants provided, the 30-min LC50 value for rabbits was calculated to be 5.6 mg/m3.
Agent GA
DA (1974) (secondary source) reported an LCt50 of 960 mg·min/m3 for a 10-min exposure.
Agent VX
Bide and Risk (2000) cite an earlier study in which the LCt50 values for a VX aerosol was reported to be 22.1 mg·min/m3 (Punte and Atkinson 1960).
3.1.7. Cats
NDRC (1946) reported an LCt50 value of 100 mg·min/m3 for a 10-min exposure. Bide et al. (1999) and Yee et al. (1999) developed a three dimensional probit model to calculate lethality values (LC05, LC50, LC95) from historic laboratory data and to estimate equivalent human values. Using the species-specific constants provided by Bide et al. (1999), the 30-min LC50 value for cats was calculated to be 3.9 mg/m3.
3.1.8. Goats
Agent VX
A single 10-min LCt50 of 9.2 mg·min/m3 has been reported for goats (Table 1–6) (Koon et al. 1960, as cited in NRC 1997).
3.1.9. Hamsters
Agent VX
Bide and Risk (2000) cite several earlier studies in which the LCt50 values for VX aerosols were reported to be 17 mg·min/m3 (whole body) (Krackow 1956) and 14.7 mg·min/m3 (Punte and Atkinson 1960).
3.1.10. Summary of Acute Lethality Data in Animals
Acute inhalation lethality data for agents GB, GA, GD, GF, and VX for several laboratory species are summarized in Tables 1–13 through 1–17.
In addition to the data presented in Sections 3.1 through 3.9, Tables 1– 13 through 1–17 contain data obtained from several historical reference handbooks (NDRC 1946; DA 1974). The DA (1974) reference source also contains LCt50 values for exposure times less than 10 min (i.e., from 2 s to 2 min). Christensen et al. (1958) used data sets for agent GB to develop LCt50-exposure time curves for each species. The original sources of the lethality data are cited by Christensen et al. (1958). A similar data set (exposures from 2 s to 12 min) was also used by Yee (1996) (see also Yee et al. [1999] and Bide et al. [1999]) who evaluated the relationship between lethal concentrations and exposure times.
3.2. Nonlethal Toxicity
3.2.1. Nonhuman Primates
Agent GB
Increases in high frequency beta activity were observed by Burchfiel and Duffy (1982) in the EEGs of rhesus monkeys who had been injected with agent GB (sarin) 1 y earlier (with either a single 5 µg/kg intravenous dose or with a series of intramuscular injections of 1 µg/kg, given once per week for 10 wk). Control animals did not show any changes in EEG. Neurobehavioral tests were not conducted on the exposed animals. In a similar series of tests in which marmosets were injected intramuscularly with 3 µg/kg, a slight but nonsignificant increase in beta activity was observed 15 mo after the exposure (Pearce et al. 1999). Behavioral tests indicated no deleterious effects on cognitive performance. RBC-ChE activity was reduced by 51.3% in the dosed animals.
Christensen et al. (1958) cite several earlier studies (Cresthull et al. 1957; Callaway and Crichton 1954) in which the incapacitation Ct50 for GB for monkeys was reported to be 67–75% of the LCt50 value. The ICt50 value is estimated to be 47 mg·min/m3 for a 2-min exposure (derived from the graphic presentation of the data given by Christensen et al. [1958]). Incapacitation was defined as convulsions, collapse, or death. Anzueto et al. (1990) reported that inhalation of 30 µg/kg (2 times the LD50) by four
baboons resulted in cardiac arrhythmias, apnea, and a significant decrease in mean blood pressure. Single intramuscular injections of 6 µg/kg to marmosets resulted in adverse behavioral effects when the animals were tested for hand-eye coordination, but no adverse effects were seen in a visual discrimination test (Wolthuis 1992). A dose of 3 µg/kg had no adverse effects on behavior, and hand-eye coordination was improved (versus each individual animal’s baseline performance prior to exposure) in three of six animals (Wolthuis 1992).
Ashwick and deCandole (1961) reported that subcutaneous GB doses of greater than 0.04 mg/kg would result in convulsions in monkeys.
van Helden et al. (2001, 2002) exposed nearly equal numbers of male and female marmosets (Callithrix jacchus, Harlan, United Kingdom) (whole-body) to GB vapor concentrations at 0.05 to 150 µg/m3 for 5 h. The lowest cumulative exposure at which the internal dose became measurable (based on fluoride-regenerated GB from blood BuChE) was 0.04±0.01 mg·min/m3 (N=5). The LOAELs for miosis, EEG effects, and visual evoked response (VER) were determined by testing one animal at each of the following concentrations: 7.5, 15, 25, 50, and 150 µg/m3. Controls (N =5) were exposed to air for 5 h. For miosis, the LOAEL (10% decrease in pupil size compared with controls; estimated at approximately 20% decrement in pupil area; p<0.05) was reported to be 2.5±0.8 mg·min/m3. The LOAEL (p<0.05) for changes in EEG parameters was 0.2 mg·min/m3 (indicative value), and the LOAEL for VER was 25 mg·min/m3 (indicative value). The blood AChE activity for marmosets was significantly (p< 0.05) inhibited at all GB vapor exposure concentrations and exhibited dose dependence. Although van Helden et al. (2001, 2002) reported that the EEG signal appeared to be more sensitive to GB than the eye, they noted that the EEG effects are more complex and concluded that “the miotic response, showing a clear dose-relationship, might therefore be considered at this moment as the most reliable biomarker of exposure to low levels of GB.”
Agent GD
Anzueto et al. (1990) reported that inhalation of GD at 13.14 µg/kg (2 times the LD50) by five baboons resulted in cardiac arrhythmias, apnea, and a significant decrease in mean blood pressure. Lipp and Dola (1980) reported that intramuscular injections of 30–75 µg/kg would result in seizure activity and convulsions in female rhesus monkey.
3.2.2. Dogs
Agent GB
Harris et al. (1953) exposed four mongrel dogs in a chamber to an average Ct of 10.5 mg·min/m3 for an exposure duration of 20 min/d (equivalent to an average concentration of 0.53 mg/m3), 5 d/wk for 2 mo. The only reported clinical sign was miosis, which appeared with each exposure but disappeared prior to the next exposure. However, when each daily exposure was increased to 15 mg·min/m3, toxic signs (body tremors, dyspnea, loss of muscle control, convulsions) occurred within 7–10 d and several dogs died. When the Ct was again reduced to 10 mg·min/m3, all signs but miosis disappeared and RBC-AChE stabilized at a level between zero and 20% of normal in the surviving dogs. Sixty-one percent of the total blood ChE activity in dogs is found in the RBC (Osweiler et al. 1985).
Fogleman et al. (1954) exposed three beagle dogs (average body weight 11.4 kg) to agent GB (sarin) vapors (face-only) for three successive exposure periods (4, 6, and 6 wk) with intervening time periods to allow for complete recovery of RBC-AChE (recovery times not reported). During each test period, the animals were exposed for time periods ranging from 8–24 min/d for 5 d/wk. In the first exposure period (series I), a concentration of 0.24–0.26 mg/m3 for 8, 16, or 24 min/d for a total of 17–20 exposures produced only mild salivation and rhinorrhea. These effects were thought to be due to the type of mask used on the animals (the Snell dog mask). RBC-AChE activity was not recorded. In series II, in which the Saunders-Fogleman dog mask was used, 39 exposures over 6 wk at a concentration of 0.73–0.75 µg/L (0.73–0.75 mg/m3) for 8, 16, or 24 min/d produced dyspnea when the daily total amount of agent GB (sarin) retained exceeded 2 µg/kg. One of the three dogs in series II exhibited gluteal muscle fasciculations when the total retained dose reached 64.5 µg/kg (after about 23 exposures). In series III (exposures at 2.38–2.43 mg/m3 for 8, 12, or 16 min/d and a total of 30 exposures over 6 wk), dyspnea and fasciculations in the region of the gluteal muscle occurred when the daily retained dose exceeded 2 µg/kg. RBC-AChE activity of all three dogs dropped to approximately 65% of normal (percent of preexposure value) after the first exposure. After the fourth exposure, RBC-AChE activity in the dog exposed for 16 min/d dropped to zero while the RBC-AChE activity in the other two dogs was about 35% of normal (range of 32–38% for the 12-min and 8-min exposure animals, respectively). Analysis of expired air allowed
for the estimation of the total amount of agent GB (sarin) retained in the body; average measured retention rates ranged from 78.9% to 84.3% in the series III tests.
Jacobson et al. (1959) exposed male beagle dogs (three per group) at 0.04 mg/m3, 4 h/d, 5 or 7 d/wk for 6 mo or at 0.50 mg/m3, 20 min/d, 5 or 7 d/wk for 6 mo. The lowest test concentration of 0.04 mg/m3 for 4 h/d resulted in decreased RBC-AChE activity (to less than 30% of the baseline values), miosis, dyspnea, increased salivation, and rhinorrhea. The effects were more severe in animals exposed 7 d/wk rather than 5 d/wk and in animals exposed to the higher concentration for 20 min/d rather than the lower concentration for 4 h/d. Miosis persisted throughout the entire 6-mo test period. Jacobson et al. (1959) autopsied two dogs in each exposure group at the end of the 6-mo period and found “some thickening of the musculature of the bronchioles and alveolar duct…dilation of the mucous glands in the bronchial trees and some flattening of the epithelium…(and) …some emphysematous areas and interstitial pneumonitis.” Histopathology of other organs was not reported. Brain ChE activity (measured in one dog per exposure group and in one control at autopsy, by the manometric method of Ammon [1933] as modified by Cohen et al. [1954]) was not significantly affected by the GB exposure except possibly in the dog exposed at 0.5 mg/m3, 20 min/d, 7 d/wk. In the latter case, brain ChE activity was 45% of the control value. Weekly electrocardiograms (EKGs) did not show any changes in the exposed animals except those that might be associated with hypoxia. Hematological counts showed no significant changes; clinical chemistry was not reported.
Weimer et al. (1979) exposed purebred beagle dogs at 0, 0.0001, or 0.001 mg/m3 for 6 h/d, 5 d/wk, for up to 52 wk. Four male and eight female beagles were exposed to each test concentration; however, only two females per exposure group were exposed for the full 52-wk period. In the exposed animals, statistically significant changes in RBC-AChE activity occurred occasionally (blood samples drawn after 1 and 2 wk of exposure, and thereafter on a monthly basis). However, these changes did not follow a clear dose-response or duration-response pattern. Two of 12 dogs exposed at 0.0001 mg/m3 and three of 12 exposed at 0.001 mg/m3 exhibited abnormal EKGs at the time of sacrifice (one each at 4, 12, and 52 wk and two at 24 wk); elevated P waves were suggestive of right atrial hypertrophy. However, there was no evidence of enlargement or physical abnormalities of the heart. Weimer et al. (1979) noted that the anomalies could have been preexisting conditions. Baseline EKGs, which were available only for
four dogs exposed for 2 mo and for four dogs exposed for 36 wk (and surgically modified to allow for periodic physiological measurements), did not reveal any evidence of EKG abnormalities. The absence of baseline data for the other test animals precludes identifying the reported EKG changes as being caused by the GB exposure, and statistical analysis of the data is not possible because of the small number of test animals (only two animals per exposure group were tested for each exposure duration).
3.2.3. Rats
Agent GB
Kassa et al. (2001) exposed male albino Wistar rats for 60 min in an inhalation chamber once or repeatedly to GB concentrations at 0.8, 1.25, or 2.5 mg/m3. The lowest concentration (level 1) was reported to be asymptomatic based on clinical and laboratory measurements. The second concentration (level 2) was reported to be asymptomatic based on clinical signs but produced a significant inhibition of RBC-AChE (by 30%). The level 2 concentration was tested using a single exposure or three exposures during 1 wk. The highest test concentration (level 3) was reported to be a nonconvulsive symptomatic exposure. Controls were exposed to pure air only. Three months following the exposure, the control and exposed animals (10 per test group) were evaluated for GB-induced effects using biochemical, hematological, neurophysiological, behavioral, and immunotoxicological methods. None of the exposed animals showed any clinical signs of intoxication 3 mo after exposure; their body weights did not differ significantly from control values, and there were no changes in hematological or biochemical parameters, including blood and brain cholinesterase. Test animals exposed at 0.8 mg/m3 (level 1) for 60 min showed no alterations in immune function, as measured by in vitro spontaneous or lipopolysaccharides-stimulated proliferation of spleen cells, or by in vitro evaluation of the production of reactive nitrogen intermediates (N-oxides), indicative of bactericidal efficacy of peritoneal macrophages. Level 1 test animals also showed no neurotoxic effects after 3 mo when monitored using a functional observatory battery (FOB) and a test of excitability of the CNS on the basis of the observation of convulsive activity after intraperitoneal administration of pentamethylenetetrazol. The only significant effect (p<0.05) observed in rats exposed once to 1.25 mg/m3
was an increase in stereotyped behavior. Effects observed in rats exposed three times to 1.25 mg/m3 included a significant increase (p<0.05) in the excitability of the CNS, significant alterations of mobility score (p<0.01) and gait disorder (p<0.001) characterized by ataxia, and a significant increase in stereotyped behavior (p<0.001). Animals exposed once to 2.5 mg/m3 exhibited significant changes in some immune functions (p<0.05), mobility score (p<0.01), activity (p<0.01), gait score (p<0.01), gait disorder (p<0.001), and stereotyped behavior (p<0.01).
Henderson et al. (2000, 2001, 2002), Conn et al. (2002), and Kalra et al. (2002) exposed male F344 rats at 0.0, 0.2, or 0.4 mg/m3 (nose-only) for 1 h/d for 1 d, 5 d, or 10 d, with sacrifices at 1 d after exposure and at 1 mo after exposure. Tests were conducted under normal temperatures (25 °C) and under heat stress (32 °C) conditions (core body temperature raised 1 °C). Study parameters included overt symptoms of toxicity, body temperature and activity, body weight, breathing patterns, cytokine levels in brain, Con-A-stimulated mitogenesis in splenic lymphocytes, number of cholinergic receptor sites in brain, and apoptosis in brain cells. It is reported that no overt symptoms of neurotoxicity (tremors) occurred at either exposure level after a single, 1-d exposure. Single exposures were associated with little inhibition of RBC cholinesterase activity (inhibition of “7 and 11% for the low- and high-exposure groups, respectively”) (Henderson et al. 2002); after the 10-d exposure, RBC-ChE activity was reduced 60% for the high-exposure group. Inhibition of plasma cholinesterase activity in heat-stressed animals was greater (approximately 20%) following a single exposure than after repeated exposures (no significant activity changes after 10 d; p≤0.05) (Henderson et al. 2002). Cholinesterase changes measured after single exposures were not associated with clinical signs. Repeated exposures induced some signs of suppression of the immune system in terms of reduced ability to maintain body temperature, and a dose-dependent reduction in the response of splenic lymphocytes to mitogens was recorded. In addition, dose-dependent induction of cytokine expression (IL-1β, IL-6, and TNF-α) was observed in the brain. No signs of increased apoptosis were seen in any of the rats exposed for 1, 5, or 10 d. Further, heat stress in combination with sarin exposure led to an increase in the number of M3 receptor sites in olfactory and adjacent areas of the rat brain. Repetitive heat stress alone reduced body weight gain; sarin exposure did not affect body weights (Henderson et al. 2002).
To better characterize the relationship between miosis and GB vapor exposure concentration and duration, Mioduszewski et al. (2002b) exposed
young adult (8–10 wk) male and female Sprague-Dawley rats to GB vapor at a range of concentrations (0.01–0.48 mg/m3) and three time durations (10, 60, and 240 min). A total of 283 rats (142 female, 141 male) were exposed whole-body “to GB vapor in a 750-L dynamic airflow inhalation chamber” following published protocols (Mioduszewski et al. 2001, 2002a). Including range-finding experiments and controls (N=130), a total of 423 rats were employed in this well-conducted study. Approximately 30 min postexposure, rat pupil diameters were assessed by means of individual examination with a simple microscope fitted with a reticule eyepiece. Blood samples were also collected (24 h preexposure, 60 min postexposure, and 7 d postexposure at sacrifice) from tail vein and heart (postmortem only) for RBC and plasma carboxylesterase (CaE) and cholinesterase activity determinations using a modified Ellman method (Ellman et al. 1961). Animals were also observed for development of clinical signs during 7 d postexposure. The miosis data were used to generate EC50 and ECt50 values for both genders for each of the three exposure durations (female EC50 was 0.068 mg/m3 for 10 min, 0.020 mg/m3 for 60 min, and 0.012 for 240 min or 0.68 mg·min/m3 for 10 min, 1.20 mg·min/m3 for 60 min, and 2.88 mg·min/m3 for 240 min) (male EC50 was 0.087 mg/m3 for 10 min, 0.030 mg/m3 for 60 min, and 0.024 mg/m3 at 240 min or 0.87 mg·min/m3 for 10 min, 1.80 mg·min/m3 for 60 min, and 5.76 mg·min/m3 at 240 min). The Mioduszewski et al. (2002b) study defined the EC50 and ECt50 points as the statistical concentration (or cumulative exposure [Ct]) required for postexposure pupil diameters of 50% or less of the pre-exposure pupil diameter in 50% of the exposed population. Gender differences (females more susceptible) were statistically significant at 10 min (p=0.014) and 240 min (p=0.023), but not at 60 min (p=0.054). Whole-body exposure to GB vapor did not result in significant activity inhibition for any blood enzyme monitored—RBC-AChE, plasma BuChE, or CaE for any GB vapor concentrations and duration tested. The authors conclude that “observable clinical signs associated with whole-body GB vapor exposure can be limited to miosis, even in the absence of significant changes in AChE, BuChE, or CaE activity” (Mioduszewski et al. 2002b, p. 21).
This is the critical study and data set (female Sprague-Dawley rats) for determination of AEGL-1 values for agent GB.
In tests conducted by Cohen et al. (1954), hypertonicity and hyperactivity of the musculature, increased response to stimuli, rigidity, and convulsions were seen in some test animals exposed at 50 mg·min/m3 (1 mg/m3 for 50 min, daily). Brain cholinesterase activity became depressed only after
erythrocyte cholinesterase activity had dropped to about 30% of the normal levels (after approximately 58 d exposure at a Ct of 75 mg·min/m3). All rats exposed to an LCt50 at 300 mg·min/m3 for 10 min had brain ChE values below 5% of normal.
Noninhalation studies have demonstrated that single exposures to GB can result in neurobehavioral changes. An intraperitoneal dose of 50 µg/kg resulted in decreases in rearing and grooming behavior and locomotive activity in male Wistar rats (Nieminen et al. 1990). A subcutaneous injection of 61 µg/kg increased spontaneous motor activity in male Sprague-Dawley rats; a dose of 71 µg/kg produced conditioned flavor aversions; 84 and 115 µg/kg caused significant decreases in spontaneous locomotive activity; and doses of 98 and 115 µg/kg resulted in significant decrements in rotorod performance. At exposures ≤84 µg/kg, no significant effects in rotorod performance were observed (Landauer and Romano 1984). Male Sprague-Dawley rats exposed to a single 100 µg/kg intramuscular dose of GB (LD50) showed significant inhibition of cholinesterase in brain and blood plasma and an increase in choline acetyl transferase activity in cortex and brain stem, but not in the mid-brain (Khan et al. 2000a,b). Olson et al. (2000) reported that subcutaneous doses of GB (once per day for 4 d) sufficient to lower whole blood cholinesterase by 20–30% caused no neurobehavioral or neuropathologic effects in rats. That finding is consistent with what Cohen et al. (1954) reported above.
Young et al. (2001) evaluated the correlation of blood cholinesterase levels with sarin-induced toxicity in female, nonpregnant CD rats (Crl:COBS CD [SD BR Rat Outbred]) treated by gavage with type I sarin at 380 µg/kg once per day for 10 d. Based on the results of previous studies, a dose of 380 µg/kg was expected to result in 30% mortality. Baseline blood cholinesterase values were determined before treatment. After the first dose, there was a drop in plasma cholinesterase which remained low throughout the 10-d test period. A statistically significant correlation (p< 0.0001) was found between body weight loss and plasma cholinesterase levels during the period of dosing. However, RBC-cholinesterase levels were not different between control and treated animals. Neither plasma nor RBC-AChE baseline cholinesterase activity levels nor the relative or absolute decline in cholinesterase values could be used as predictors of mortality in the treated animals.
Abu-Qare and Abou-Donia (2001) examined the ability of a single intramuscular dose of agent GB (80 µg/kg) alone, or in combination with a single oral dose of pyridostigmine bromide, to induce markers of oxida-
tive stress. Urine samples of treated and control adult SD rats were collected at various times post-treatment (16–96 h). No increase in the concentrations of stress markers 3-nitrotyrosine and 8-hydroxy-2'-deoxyguanosine was detected following a single dose of sarin.
Jones et al. (2000) investigated potential subchronic neurotoxic effects of GB concentrations administered in fractions of the LD50 dose (intramuscular at 0.01, 0.1, 0.5, and 1×LD50) to male Sprague-Dawley rats, after which the rats were maintained for 90 d. Potential changes in blood-brain barrier (BBB) permeability were monitored in the cortex, brainstem, midbrain, and cerebellum; other parameters monitored included plasma butyrylcholinesterase activity as well as m2-selective muscarinic acetylcholine receptor (m2-mAChR) and nicotinic acetylcholine receptor (nAChR) ligand binding. Plasma butyrylcholinesterase activity recovers rapidly and cannot, therefore, serve as a reliable biomarker for potential long-term toxicity of sarin exposure. Ninety days after single sarin exposure, changes in brain regional binding densities of the two receptors were noted; the clinical significance of those changes was not reported.
In a subchronic inhalation study conducted on Fischer 344 rats, no signs of toxicity were observed in animals exposed to GB at 0.0001 or 0.001 mg/m3 6 h/d, 5 d/wk (excluding holidays), for up to 24 wk (Weimer et al. 1979). In a continuation of these studies, Sprague-Dawley/Wistar (colony) and Fischer 344 rats were exposed at 0, 0.0001, or 0.001 mg/m3 6 h/d, 5 d/wk, for up to 52 wk (Weimer et al. 1979). Fifty animals of each gender of each strain were exposed to each test concentration, and blood samples were drawn for RBC-ChE determination at the time of sacrifice. During a 3-wk period, the test animals exhibited heat stress due to loss of chamber temperature control (temperatures exceeded 90 °F) and many of the rats died (16 in the low exposure group and 12 in the high exposure group). Fluctuations in blood chemistries (including RBC-AChE) for the exposed animals were no greater than controls, and although statistically significant changes in RBC-AChE occurred occasionally, the changes did not follow a clear dose-response or duration-response pattern. Atrophy of the seminiferous tubules was observed in Fischer 344 rats exposed to GB; however, Weimer et al. (1979) noted that this inbred strain of rat is susceptible to numerous genetically based defects that may appear under experimental conditions of stress. The tests were repeated using the same experimental protocol for 12 and 24 wk, and none of the exposed rats in the second assay exhibited testicular atrophy. A high incidence of tracheitis oc-
curred in both the Fischer rats and in the colony rats exposed to GB. The most severe cases reportedly occurred in the high-exposure group. The incidence of tracheitis in colony rats is summarized in Table 1–18; the results were analyzed statistically using the Fisher Exact Test (statistical analysis was not provided by Weimer et al. [1979]). Although there were statistically significant differences between exposed and control groups after 4, 8, and 12 wk of exposure, the differences were not significant for longer exposure durations. A similar response was seen in Fischer rats (i.e., a few cases of tracheitis early in the study, but none in animals exposed for 52 wk).
Tracheitis was often common in animal colonies during the time of the Weimer et al. (1979) study and is now considered reflective of incomplete infectious-disease control in the colony. This evidence of disease, coupled with the loss of chamber temperature control and subsequent heat-stress deaths of test animals, compromise the results and disqualify Weimer et al. (1979) from use as a critical study for AEGL estimation.
Agent GD
Walday et al. (1991) exposed male Wistar rats to GD at 0.05 or 0.2 mg/m3 for a single 40-h period. No clinical signs of toxicity were seen during the exposures. Acetylcholinesterase and butyrylcholinesterase were significantly inhibited in all tissues except the brain.
Agent GF
A recent study of lethal GF vapor exposure toxicity in male and female SD rats also reported sublethal clinical signs of tremors, convulsions, salivation, and miosis following whole-body dynamic chamber exposures (Anthony et al. 2002). Blood samples were also drawn for BuChE activity determinations. A range of near-lethal vapor concentrations were employed for three exposure durations (10, 60, and 240 min). Because the experimental protocol was designed for LC50 lethality-effects determination, effective concentration determinations (EC50, ECt50) for nonlethal effects were not estimated by Anthony et al. (2002). Miosis was observed in all exposed rats during the first hour postexposure; the effect was reversible
TABLE 1–18 Incidence of Tracheitis in Colony Rats Exposed to Agent GBa
|
|
Exposure Group |
||
|
Exposure Period |
Control |
0.0001 mg/m3 |
0.001 mg/m3 |
|
4 wk |
0/10 |
5/10b |
0/10 |
|
8 wk |
0/10 |
4/10b |
9/10c |
|
12 wk |
0/10 |
5/8c |
5/7c |
|
16 wk |
0/9 |
0/10 |
1/10 |
|
20 wk |
0/10 |
0/5 |
2/6 |
|
24 wk |
1/10 |
1/5 |
0/6 |
|
36 wk |
0/9 |
2/5 |
2/7 |
|
52 wk |
2/10 |
1/10 |
6/10 |
|
6 mo |
0/19 |
7/19c |
9/28c |
|
aStatistical analysis using the Fisher Exact Test. bSignificantly different from control, p<0.05. cSignificantly different from control, p<0.01; the postexposure population was made up of groups of each rodent strain held for 6-mo observation after the experimental exposure period ended. Source: Weimer et al. 1979. |
|||
and pupil sizes were normal at 14 d postexposure. Preliminary analysis of BuChE activity indicates statistically significant depression “immediately after exposure” and statistically significant elevations at 14 d postexposure to near-lethal vapor concentrations; at neither time period is the BuChE delta correlated with cumulative exposure (Ct) (Anthony et al. 2002).
Agent VX
Crook et al. (1983) conducted VX vapor exposure studies in male and female Sprague Dawley rats. Crook and his colleagues consider their results to be nonverifiable and suspect for the reasons outlined earlier. These data are thus considered too unreliable for any application to development of AEGL estimates for agent VX.
3.2.4. Mice
Agent GB
Signs suggestive of delayed neuropathy have been observed in female Swiss albino mice (N=6) exposed to GB at 5 mg/m3 for 20 min daily for 10 d (Husain et al. 1993). Muscular weakness of the limbs and slight ataxia occurred on the day 14 after the start of the exposures (the number of animals showing these effects was not specified). Significant (p<0.001) inhibition of NTE activity in the brain (59.2%), spinal cord (47.4%), and platelets (55.4%) was observed in the test animals (N=6). Histological examination of the spinal cord revealed focal axonal degeneration that was reported to be moderate in two animals and light in four. The same exposure inhibited blood AChE by 27.3% and brain AChE by 19.2% but was not associated with any anti-AChE symptoms. The LCt50 for this strain of mice was reported to be 600 mg·min/m3 (Husain et al. 1993), presumably for a 1-min exposure.
Agent VX
Crook et al. (1983) conducted VX vapor exposure studies in male and female ICR mice. Crook and his colleagues consider their results to be nonverifiable and suspect for the reasons outlined earlier. The data are thus considered too unreliable for any application to development of AEGL estimates for agent VX.
3.2.5. Guinea pigs
Agent GB
Van Helden et al. (2001, 2002) exposed male Dunkin-Hartley albino (HSD-Harlan [Harlan]) guinea pigs (whole-body) to GB vapor concentrations at 0.05 to 150 µg/m3 for 5 h. The lowest cumulative exposure at which the internal dose became measurable (based on fluoride-regenerated GB from blood BuChE) was 0.010±0.002 mg·min/m3 (N=12). The LOAELs for miosis, EEG effects, and visual evoked response (VER) were
evaluated at 7.5, 15, 25, 50, and 150 µg/m3 (N=2 per group). Controls (N =6) were exposed to air for 5 h. For miosis, the LOAEL (5% decrement in pupil size compared with controls; estimated to be equivalent to approximately 10% decrement in pupil area; p<0.05) was reported to be 1.8±0.3 mg·min/m3. The LOAEL (p<0.05) for changes in EEG parameters and VER was 0.8 mg·min/m3 (indicative value). There was no significant decrease in blood AChE activity at any GB vapor exposure concentration tested.
Atchison et al. (2001) reported that subcutaneous injections of 0.4 LD50 GB once per day, 5 d/wk, for 13 wk in young male Hartley guinea pigs (600 g) resulted in no clinical signs of acute toxicity and no changes in body weight, body temperature, complete blood counts, or blood chemistry; however, RBC-ChE activity was decreased by about 90%. The subcutaneous LD50 for guinea pigs was reported to be 42 µg/kg.
Agent GD
Benschop et al. (1998) evaluated the toxicokinetics and effects of single inhalation exposures of the four stereoisomers of soman to guinea pigs. The test animals (male albino outbred guinea pigs of the Dunkin-Hartley type, 450–620 g body weight) were anesthetized and atropinized and then exposed, nose-only, for 5 h to each of the four stereoisomers, at a concentration of 20 ppb (160±16 µg/m3). During the exposure there was a gradual increase in the inhibition of RBC-AChE, which correlated well with the increase in the concentration of the toxic stereoisomers (C(±)P(−) soman) in the blood. Inhibition of AChE in the brain and diaphragm was not significant at the end of the exposure period.
Atchison et al. (2001) reported that subcutaneous injections of 0.4 LD50 GD once per day, 5 d/wk, for 13k w in young male Hartley guinea pigs (600 g) resulted in no clinical signs of acute toxicity, no agent related pathology, and no change in blood chemistry other than a 91% inhibition of RBC-ChE. The subcutaneous LD50 for guinea pigs was reported to be 28 µg/kg.
Agent VX
Atchison et al. (2001) reported that subcutaneous injections of 0.2 LD50 VX once per day, 5 d/wk, for 13 wk in young male Hartley guinea pigs
(600 g) resulted in no clinical signs of acute toxicity and no changes in body weight, blood count, blood chemistry of gross, or histopathology; however, RBC-ChE activity was inhibited about 90%. The subcutaneous LD50 for guinea pigs was reported to be 9 µg/kg.
Crook et al. (1983) conducted VX vapor exposure studies in male and female Hartley guinea pigs. Crook and his colleagues consider their results to be nonverifiable and suspect for the reasons outlined earlier. These data are thus considered too unreliable for application to development of AEGL estimates for agent VX.
3.2.6. Rabbits
Callaway and Dirnhuber (1971) performed a study in which pupil area decrement was measured from electronic flash photographs of dark-adapted eyes for which baseline pupil area had been previously determined. The nominal cumulative exposure (Cts) necessary to produce 50% and 90% decrement in total pupil area were determined and compared for GB vapor (46 eye measurements from 14 rabbits), GD vapor (153 eye measurements from 48 rabbits), and T-2715 (GF analog) vapor (85 measurements on 19 rabbits). The cumulative exposure needed to produce miosis sufficient to generate 90% pupil area decrement was 2.71 mg·min/m3 (95% CI=1.84– 4.00 mg·min/m3) for agent GB, 2.19 mg·min/m3 (95% CI=1.45–3.29 mg·min/m3) for agent GD, and 1.79 mg·min/m3 (95% CI=1.40–2.29 mg·min/m3) for agent GF. The cumulative exposure needed to produce miosis sufficient to generate 50% pupil area decrement was 1.32 mg·min/m3 (95% CI=1.05–1.67 mg·min/m3) for agent GB, 0.59 mg·min/m3 (95% CI=0.49–0.70 mg·min/m3) for agent GD, and 0.75 mg·min/m3 (95% CI=0.65–0.87 mg·min/m3) for agent GF.
Callaway and Dirnhuber (1971) also evaluated the “miotogenic potency” of GB vapor in rabbits exposed to GB “under goggles” (43 miosis responses in 10 albino rabbits). The “goggle” experiments were designed to deliver GB vapor directly to the air volume around the eye and enclose the vapor as a means of controlling the exposure (no inhalation or percutaneous exposure) and delivering the vapor directly to the surface of the eye (thereby reducing variability). An airstream of GB vapor was delivered to the space enclosed by each goggle. The unexposed pupil area of each eye was considered to be the baseline for pupil area decrement determinations for each eye. Exposure periods ranged from 10 min to 5 h. Callaway and Dirnhuber (1971) reported a 50% decrement of pupil area in
the rabbit dark-adapted eye (goggles) at a Ct of 2.33 mg·min/m3 (95% CI =1.65–3.31 mg·min/m3). A 90% decrement of pupil area occurred at a Ct of 7.68 mg·min/m3 (95% CI=4.90–19.50 mg·min/m3).
Agent VX
Crook et al. (1983) conducted VX vapor exposure studies in male and female New Zealand white rabbits. Crook and his colleagues consider their results to be nonverifiable and suspect for the reasons outlined earlier. These data are thus considered too unreliable for any application to development of AEGL estimates for agent VX.
In tests conducted by Goldman et al. (1988), blood cholinesterase levels were monitored in female rabbits (three per dose group) injected subcutaneously with VX at 0, 0.25, 1.0, 4.0, or 8.0 µg/kg once per day for 7 d. The 8.0 µg/kg dose was severely toxic (1/3 died, 2/3 ataxic). RBC-ChE activity was inhibited to 0.71 of the control value in the 0.25-µg/kg group, to 0.36 of the control value in the 1-µg/kg group, and to 0.24 of the control value in the 4.0-µg/kg group.
In a study of miotogenic potency, Callaway and Dirnhuber (1971) exposed the eyes of male and female “albino” rabbits (N=45; no strain identified; 94 observations) to concentrations of VX agent vapor ranging from approximately 0.5 µg/m3 to 25 µg/m3 for varying time periods (approximately 2–400 min). Pupil diameters were recorded only after attaining maximal decrease, and decrease in pupil area per Ct was expressed as a percentage of the original area of the same eye. Maximal pupil diameter decrease usually occurred at times >30 min postexposure. The “percentage decrease” data underwent probit transformation to derive Cts necessary to produce 50% and 90% decrease in pupil area in the dark-adapted eye. For comparison, experimental exposures to nerve agents GB and GD under a similar protocol were also performed by the authors (Callaway and Dirnhuber 1971). Their results are reported in Table 1–19 below.
3.2.7. Summary of Nonlethal Toxicity in Animals
The summary of animal toxicity data has focused on short-term, subchronic, or chronic exposures to agent GB (Table 1–20). Results of inhalation exposure studies are emphasized; however, some pertinent data for other exposure pathways are included.
TABLE 1–19 Miosis in Rabbits Following Vapor Exposure to Agents VX, GB, and GD
|
Agent |
50% Pupil Area Decrease (mg·min/m3) |
95% CI |
90% Pupil Area Decrease (mg·min/m3) |
95% CI |
Slope (b) |
|
VX |
0.04 |
0.03–0.05 |
0.23 |
0.12–0.45 |
1.70 |
|
GB |
1.32 |
1.05–1.67 |
2.71 |
1.84–4.00 |
4.11 |
|
GD |
0.59 |
0.49–0.70 |
2.19 |
1.45–3.29 |
2.24 |
|
Source: Callaway and Dirnhuber 1971. |
|||||
The miotogenesis studies of GB vapor exposure recently published by van Helden et al. (2001, 2002) (male and female marmosets and male guinea pigs) and Mioduszewski et al. (2002b) (male and female SD rats) were well conducted, employed modern protocols, and examined a range of exposure durations significant to the AEGL process. Mioduszewski et al. (2002b) is the critical study for deriving AEGL-1 values for agent GB; van Helden et al. (2001, 2002) is a secondary and supportive study.
3.3. Neurotoxicity
The G agents (GA [tabun], GB [sarin], GD [soman], and GF) and agent VX are toxic organophosphate ester derivatives of phosphonic acid. They are commonly termed “nerve” agents as a consequence of their potent anticholinesterase properties and subsequent adverse effects on both smooth and skeletal muscle function as well as the central and peripheral nervous system. Although the inhibition of cholinesterases within neuroeffector junctions or the effector itself is thought to be responsible for the major toxic effects of nerve agents, these compounds can apparently affect nerve impulse transmission by more direct processes as well (for example, direct effects on muscarinic receptors) (see Section 4.2).
As described in Section 3.2.3, Kassa et al. (2001) evaluated the neurotoxic effects of agent GB in male albino Wistar rats exposed for 60 min, once or repeatedly, to concentrations at 0.8, 1.25, or 2.5 mg/m3. The lowest concentration was determined asymptomatic based on clinical and laboratory measurements. The second concentration was determined asymptomatic based on clinical signs, but produced a significant inhibition of RBC-AChE (30%). The highest test concentration was a nonconvulsive
TABLE 1–20 Nonlethal Toxicity of Agent GB Vapor to Animalsa
|
Species |
Exposure |
Duration |
End Point |
Comments |
Reference |
|
Dog |
10.5 mg·min/m3 20 min/d |
2 mo |
LOAEL |
Miosis |
Harris et al. 1953 |
|
Dog |
15 mg·min/m3 20 min/d |
7–10 db |
LOAEL |
Body tremors, dyspnea, loss of muscle control, convulsions |
Harris et al. 1953 |
|
Dog |
0.24–0.26 mg/m3; 8, 16, 24 min/d |
17–21 times over 4 wk |
NOAEL |
Nose only exposures; no reported toxic signs; ChE was not monitored |
Fogleman et al. 1954 |
|
Dog |
0.73–0.75 mg/m3; 8, 16, 24 min/d |
30 times over 6 wk |
LOAEL |
Dyspnea, gluteal muscle fasciculations in one of three test animals |
Fogleman et al. 1954 |
|
Dog |
2.38–2.43 mg/m3; 8, 12, 16 min/d |
30 times over 6 wk |
LOAEL |
Dyspnea; gluteal muscle fasciculations; RBC-ChE levels 0, 35%, and 35% of normal after 4 d |
Fogleman et al. 1954 |
|
Dog |
0.04 mg/m3 4 h/d, 5 d/wk |
6 mo |
LOAEL |
Decreased RBC-ACHE; dyspnea, salivation, rhinorrhea, miosis |
Jacobson et al. 1959 |
|
Dog |
0.001 mg/m3 6 h/d, 5 d/wk |
52 wk |
NOAEL |
Abnormal EKGs in some dogs; however, baseline measurements were not available for all the test animals |
Weimer et al. 1979 |
|
Rabbit |
1.32 mg·min/m3 |
10 min to 5 h |
ECt50 |
50% miosis |
Callaway and Dirnhuber 1971 |
|
Rabbit |
2.71 mg·min/m3 |
10 min to 5 h |
ECt50 |
90% miosis |
Callaway and Dirnhuber 1971 |
|
Guinea pig |
0.8 mg·min/m3 |
5 h |
LOAEL |
EEG changes and visual evoked response |
van Helden et al. 2001, 2002 |
|
Guinea pig |
1.8 mg·min/m3 |
5 h |
LOAEL |
Miosis |
van Helden et al. 2001 |
|
Marmoset |
0.2 mg·min/m3 |
5 h |
LOAEL |
EEG changes |
van Helden et al. 2001, 2002 |
|
Marmoset |
2.5 mg·min/m3 |
5 h |
LOAEL |
Miosis |
van Helden et al. 2001, 2002 |
|
Marmoset |
25 mg·min/m3 |
5 h |
LOAEL |
Visual evoked responses |
van Helden et al. 2001, 2002 |
|
Mouse |
5 mg/m3, 20 min/d |
10 d |
LOAEL |
Muscular weakness of the limbs and slight ataxia; inhibition (p< 0.001) of NTE activity in the brain (59.2%), spinal cord (47.4%), and platelets (55.4%); focal axonal degeneration of spinal cord; blood AChE inhibited by 27.3% and brain AChE by 19.2% |
Husain et al. 1993 |
|
Rat |
0.068 mg/m3 (female) |
10 min |
EC50 |
Miosis |
Mioduszewski et al. 2002b |
|
Species |
Exposure |
Duration |
End Point |
Comments |
Reference |
|
Rat |
0.020 mg/m3 (female) |
60 min |
EC50 |
Miosis |
Mioduszewski et al. 2002b |
|
Rat |
0.012 mg/m3 (female) |
240 min |
EC50 |
Miosis |
Mioduszewski et al. 2002b |
|
Rat |
0.087 mg/m3 (male) |
10 min |
EC50 |
Miosis |
Mioduszewski et al. 2002b |
|
Rat |
0.030 mg/m3 (male) |
60 min |
EC50 |
Miosis |
Mioduszewski et al. 2002b |
|
Rat |
0.024 mg/m3 (male) |
240 min |
EC50 |
Miosis |
Mioduszewski et al. 2002b |
|
Rat |
0.8 mg/m3 |
60 min |
NOAEL |
Asymptomatic |
Kassa et al. 2001 |
|
Rat |
1.25 mg/m3 |
60 min |
NOAEL |
Asymptomatic but with significant inhibition of RBC-ChE |
Kassa et al. 2001 |
|
Rat |
2.5 mg/m3 |
60 min |
LOAEL |
Changes in immune system and neurobehavioral effects |
Kassa et al. 2001 |
|
Rat |
0.4 mg/m3 1 h/d |
1 d |
NOEL |
No overt neurotoxicity (tremors) observed |
Henderson et al. 2000, 2001, 2002 |
|
Rat |
0.001 mg/m3 6 h/d, 5 d/wk |
24 wk |
NOAEL |
No observed inhibition of blood ChE |
Weimer et al. 1979 |
symptomatic exposure. Controls were exposed to pure air only. Three months following the exposure, the control and exposed animals (10 per test group) were evaluated for GB-induced effects using biochemical, hematological, neurophysiological, behavioral, and immunotoxicological methods. None of the exposed animals showed any clinical signs of intoxication 3 mo after exposure. Test animals exposed to GB at 0.8 mg/m3 exhibited no neurotoxic effects after 3 mo, when monitored using a functional observatory battery (FOB) and a test of excitability of the CNS, on the basis of observation of convulsive activity after intraperitoneal administration of pentamethylenetetrazol. The only significant effect (p<0.05) observed in rats exposed once to GB at 1.25 mg/m3 was an increase in stereotyped behavior. Effects observed in rats exposed three times at 1.25 mg/m3 included a significant increase (p<0.05) in the excitability of the CNS, significant alterations of mobility score (p<0.01), gait disorder (p< 0.001) characterized by ataxia, and a significant increase in stereotyped behavior (p<0.001). Animals exposed once at 2.5 mg/m3 exhibited significant changes in mobility score (p<0.01), activity (p<0.01), gait score (p <0.01), gait disorder (p<0.001), and stereotyped behavior (p<0.01).
3.4. Developmental and Reproductive Effects
Due to the limited database for evaluating developmental or reproductive effects of nerve agent vapor inhalation exposure, other exposure routes were also examined.
3.4.1. Rats
Agent GB
The reproductive and developmental toxicity of GB was evaluated in a pilot study in which Sprague-Dawley rats were exposed to GB vapors (Denk 1975). In one series of inhalation tests, male rats were exposed to GB at 0.1 or 1 µg/m3 for 6 h/d, 5 d/wk, for 1, 2, 8, or 12 wk or 6, 9, or 12 mo and then mated to unexposed females. Nineteen days after mating, the females were sacrificed and examined for number of corpora lutea, deciduomata, number of fetal deaths, and number of live fetuses. Mated pairs of rats were also exposed to the same GB concentrations for 1, 2, or
3 wk or until the pups were whelped. The incidence of intrauterine deaths was recorded and all fetuses were examined for abnormalities. In a third series of tests, males and females were exposed to agent GB (sarin) for 10 mo and then mated. The F1 generation was mated at 12 wk of age, as was the F2 generation. The number and gender of offspring, number of preweaning deaths, number weaned, and pup weights at various ages were recorded. Denk (1975) reported reduced rates of whelping in the F0 generation, but reduced whelping rates were also seen in the controls, and this effect was thought to be due to the age of the animals at mating (12 mo old). No other adverse effects with respect to dominant lethal mutations, reproductive performance, fetal toxicity, and teratogenesis were observed.
Oral exposure studies in laboratory animals indicate that developmental or reproductive effects are not likely, even at dose levels that are maternally toxic. LaBorde and Bates (1986) (see also LaBorde et al. [1996]) conducted developmental toxicity studies on agent GB type I and GB type II using CD rats. The test animals were dosed with 0, 100, 240, or 380 µg/kg orally on days 6–15 of gestation. Females were weighed on gestational day 0, gestational days 6–16, and before death on gestational day 20. The test animals were observed for clinical signs of toxicity. At sacrifice, gravid uteri were weighed and examined for number and status of implants (alive, resorbed, or dead). Individual fetal body weight and internal or external malformations were recorded. Maternal toxicity (evidenced by excessive salivation, ataxia, lacrimation) and mortality (8/29 for GB type I and 13/29 for GB type II) occurred in the high-dose group. There were no significant differences among treatment groups in the incidence of resorptions or in the average body weight of live fetuses per litter. The only fetal morphological anomaly was fetal hydroureter, which occurred at a rate of 5.2%, 1.9%, 5.3%, and 2.1% with GB type I; and 4%, 5%, 3.2%, and 0.5% with GB type II in the 0-, 100-, 240-, and 300-µg/kg dose groups, respectively. The observed effect was not dose related and was therefore considered a spontaneous variant. Skeletal and cartilage variants occurred between dose groups, but they were not statistically significant.
Agent GA
There are intraperitoneal and subcutaneous exposure studies of agent GA in which developmental and reproductive toxicity were studied in maternal CD rats (Bucci et al. 1993). In both studies, the LOAEL for ma-
ternal toxicity (salivation, lacrimation, nasal discharge, diarrhea) was attained in the absence of fetal malformations or adverse effects on fetal implantations or fetal weight.
Agent GD
Developmental studies in maternal rats orally exposed to agent GD (soman) were reported by Bates et al. (1990); the protocol was the same as that employed in the GB oral exposure studies of LaBorde and Bates (1986) and LaBorde et al. (1996) reported earlier. At doses that produced significant maternal toxicity and mortality, there was no evidence of fetal toxicity or prenatal mortality as evidenced by postimplantation loss, average body weight of live fetuses per litter, or malformations (Bates et al. 1990).
Agent VX
In studies conducted by Schreider et al. (1984), pregnant rats were dosed with VX at 0.25, 1.0, or 4.0 µg/kg by subcutaneous injection on days 6–15 of gestation (doses higher than 4.0 µg/kg were expected to cause excessive deaths). The animals were sacrificed on day 20 of gestation. The examined fetuses showed no evidence of malformations. Fetal body weight, litter size, and gender ratio were within normal limits.
Goldman et al. (1988) administered VX by subcutaneous injection to Sprague-Dawley rats on days 6–15 of gestation. The administered doses were 0, 0.25, 1.0, or 4.0 µg/kg/d. Body weight, frequency of visceral and skeletal abnormalities, litter size, and gender ratios were evaluated. There was no statistical evidence that VX affected any of the parameters studied. Blood cholinesterase levels were not monitored.
In a modified dominant lethal study, Goldman et al. (1988) administered VX by subcutaneous injection to male and/or female Sprague-Dawley rats and observed the effects on various parameters including terminal body weight, testes weight, testicular histopathology, maternal weight, implantation sites, resorptions, and total corpora lutea. The test animals were dosed with VX at 0 (saline control), 0.25, 1.0, or 4 µg/kg/d for 10 wk. Triethylenemelamine was used as a positive control. Exposure to VX produced no significant changes in body or organ weights. VX had no adverse effects on pre-implantation losses as evaluated by number of im-
plants, live fetuses, dead fetuses, and resorptions. Microscopic examination of the testes did not reveal any abnormalities that could be attributed to VX exposure.
In a three-generation study, male and female Sprague-Dawley rats were dosed by subcutaneous injection with VX at 0 (saline controls), 0.25, 1.0, or 4.0 µg/kg/d, 5 d/wk (Goldman et al. 1988). The F0 generation (11– 12 males and 24 females per dose group) was dosed for about 105 d after which they were mated, and the dosing continued through gestation and weaning (total duration of dosing 21–25 wk). Dosing of the F1 generation began after weaning and continued for approximately 126 d after which they were mated, and dosing continued through gestation and weaning (total duration 24–27 wk). Five males and five females of each dose group of the F2 generation were sacrificed at weaning. The study included analysis of pup mortality in each of the generations, body and organ weight changes and hematological parameters in the F0 generation, and histopathological examination of tissues (including nervous system, reproductive system, gastrointestinal tract, lung, liver, and kidney) of the F1 parental males and females, the F1 weanlings, and the F2 weanlings. Blood cholinesterase activity levels were not monitored during the study. VX exposure had no adverse effect on the number of pups born in the F1 or F2 generation. Perinatal mortality (i.e., percent of pups born dead or dying within 24 h of birth) was not significantly different among dose levels for both generations; however, perinatal mortality in the high-dose group (5.7%) was considerably higher than that in the lower-dose groups (1.2%). Pup mortality from birth to weaning was significantly related (p<0.01) to VX exposure, primarily for the F1 generation pups in the 4.0-µg/kg/d dose group. Goldman et al. (1988) attributed this increase to the effect of VX on the dams, which resulted in an increased cannibalism of the pups by the dams. The investigators concluded that under the conditions of the test, there was no evidence of direct VX reproductive toxicity. The hematological studies conducted on dosed males of the F0 generation revealed no significant VX-associated effects. In females dosed with VX at 4.0 µg/kg/d, statistically significant decreases occurred in hemoglobin, hematocrit, mean corpuscular volume, and mean corpuscular hemoglobin. Body and organ weight analysis and histopathological examination revealed three effects that may have been dose-related changes in brain weight, incidence of eosinophilic gastritis, and incidence of pituitary cysts; however, Goldman et al. (1988) attributed the first two effects to statistical chance and considered the third not biologically significant. The overall conclu-
sion of the investigators was that there were no organ-weight or microscopic changes that could be attributed specifically to the action of VX.
3.4.2. Guinea Pigs
Pregnant guinea pigs were administered GD orally (7 µg/kg/d) on gestation days 42, 43, and 44 (Mehl et al. 1994). It had been determined prior to the study that a dose of 13 µg/kg, while tolerated by nonpregnant females, was “highly toxic” to pregnant animals. The administered dose of GD caused no significant change in brain weight of neonates, the end point of concern, or in total body weight.
3.4.3. Rabbits
Agent GB
LaBorde and Bates (1986) (see also LaBorde et al. [1996]) conducted developmental toxicity studies on agent GB type I and GB type II using New Zealand rabbits. The same protocol as previously outlined for the rat oral studies by these same investigators (see Section 3.4.1) was employed in the rabbit study. The test animals were dosed with GB at 0, 5, 10, or 15 µg/kg orally on days 6–19 of gestation. No fetal toxicity or teratogenicity was observed. The only observed fetal anomaly was retinal folding, which occurred at a rate of 6.8%, 3.9 %, 4.3 %, and 7.4% for GB type I and 17%, 18%, 25%, and 19% for GB type II in the 0-, 5-, 10-, and 15-µg/kg dose groups, respectively. The frequency of the anomaly was not dose-related and was, therefore, considered to be a spontaneously occurring malformation. Maternal toxicity, evidenced by excessive salivation, ataxia, and lacrimation, occurred at the highest dose.
Agent GA
The developmental and reproductive toxicity of GA was studied in maternal New Zealand white rabbits dosed intraperitoneally or subcutaneously (Bucci et al. 1993). In both studies, the LOAEL for maternal toxicity
(salivation, lacrimation, nasal discharge, diarrhea) was attained in the absence of fetal malformations or adverse effects on fetal implantations or fetal weight.
Agent GD
Developmental studies in maternal rabbits orally exposed to agent GD (soman) were reported by Bates et al. (1990); the protocol was the same as that employed in the GB oral exposure studies of LaBorde and Bates (1986) and LaBorde et al. (1996) reported earlier. At doses that produced significant maternal toxicity and mortality, there was no evidence of fetal toxicity or prenatal mortality as evidenced by post-implantation loss, average body weight of live fetuses per litter, or malformations (Bates et al. 1990).
Agent VX
Goldman et al. (1988) administered subcutaneous doses of VX at 0, 0.25, 1.0, and 4.0 µg/kg/d to New Zealand white rabbits on days 6–19 of gestation. Animals were also observed daily for signs of toxicity. The does were sacrificed on day 29 of gestation. Body weight, fetal weights, fetal deaths, frequency of visceral and skeletal abnormalities, litter size, and gender ratios were evaluated. There was no statistical evidence that VX affected any of the parameters studied. Blood cholinesterase levels were monitored in a 7-d pilot study, which also included a dose of 8 µg/kg. The 8-µg/kg dose was severely toxic to the rabbits (1/3 died, 2/3 ataxic). The dose of 0.25 µg/kg resulted in a level of RBC-AChE inhibition equal to 0.71 of the control value, but produced no signs of toxicity.
3.4.4. Sheep
Agent VX
The effects of VX on the development and reproduction of sheep were evaluated by Van Kampen et al. (1970) following an accidental release of VX in Skull Valley, Utah. Of some 6,300 affected animals, about 4,500
died or were killed (Van Kampen et al. 1970). Seventy-nine surviving animals pregnant at the time of exposure, and their lambs, were evaluated for changes in RBC-AChE activity and for signs of toxicity over a 6-mo postexposure period. RBC-AChE activity in the ewes remained significantly depressed for about 4 mo and then returned to normal. Ewes that were sacrificed at 2-wk intervals had no gross or microscopic evidence of damage to the central nervous system. Torticollis (wryneck) developed in one ewe 1 wk following exposure and persisted for 9 mo. (Van Kampen et al. [1970] reported that a similar effect was seen in one of 38 ewes dosed in the laboratory with an undisclosed amount of VX.) Of the lambs born 2–3 mo after the exposure of the ewes, only one (total number examined not reported) exhibited a deformity (extra oral opening below the right ear), but Van Kampen et al. believed the anomaly originated developmentally and before the poisoning episode. None of the lambs displayed neurotoxic signs or symptoms, and their whole blood cholinesterase activity was not reduced even when suckling from exposed and affected ewes. Five months after exposure, the ewes exposed in the field as well as ewes dosed with an undisclosed amount of VX 4 mo prior were mated to unexposed males. Examination 4 mo later indicated that fetal growth and development were normal except for one fetus that appeared stunted (total number examined not reported). The investigators concluded that VX had little or no effect on fetal growth or development.
3.4.5. Summary
Animal data from vapor and oral exposure studies for agent GB suggest that agent GB does not induce reproductive or developmental effects in mammals. Oral exposure studies of agents GA and GD in laboratory animals as well as injection exposure studies of agent GA suggest the lack of reproductive or developmental effects for these agents. Available data indicate that agent VX does not cause reproductive or developmental effects.
3.5. Genotoxicity
Agent GB
In bioassays using bacteria and mammalian cell cultures, agent GB was
not genotoxic or mutagenic when tested with or without metabolic activation (Goldman et al. 1987). GB did not induce biologically significant increases in mutations (e.g., highest concentration tested failed to exceed a doubling of the spontaneous rate) when tested in the Ames Salmonella assay using five revertant strains (TA135, TA100, TA98, TA1537, and TA1538) (Goldman et al. 1987). GB type I and GB type II did not induce significant increases in forward mutations when tested on mouse L5178Y lymphoma cells at concentrations of 50, 100, or 200 µg/mL (Goldman et al. 1987). An increase in sister chromatid exchanges (SCE) was not observed in Chinese hamster ovary cells exposed in vitro to GB at 200 µg/mL (Goldman et al. 1987). Mice treated in vivo with a maximally tolerated intraperitoneal dose of GB at 360 µg/kg did not exhibit a significant increase in SCE in splenic lymphocytes (Goldman et al. 1987). Exposure of rat hepatocytes to GB concentrations as high as 2.4×10−3 M resulted in a decrease in DNA repair synthesis, leading Goldman et al. (1987) to conclude that GB probably did not damage DNA directly but that it might inhibit DNA synthesis after non-agent-induced DNA damage had occurred.
Agent GA
Genotoxicity and mutagenicity data for agent GA are available from microbial assays and in vitro and in vivo tests on laboratory animals (Wilson et al. 1994). GA was found to be weakly mutagenic in eight of 11 Ames Salmonella assays using the revertant strains TA98, TA100, TA1535, and TA1538 and S-9 activation. GA also induced dose-related increases in mutation rates when tested on mouse L5178Y lymphoma cells without metabolic activation; the increase observed at a test concentration of 100 µg/mL was nearly 3 times that of the control. An increase in sister chromatid exchanges (SCE) was observed in Chinese hamster ovary cells exposed in vitro to GA concentrations at 25–200 µg/mL. Dose-responses were linear and highly statistically significant; however, the number of SCEs did not exceed twice the control value at any of the concentrations tested. C57B1/6 mice treated in vivo with a maximally tolerated intraperitoneal dose of GA at 700 µg/kg did not exhibit a significant increase in SCE in splenic lymphocytes. Exposure of rat hepatocytes to GA concentrations as high as 200 µg/mL resulted in inhibition of unscheduled DNA synthesis. From the results of these studies (i.e., three positive responses in five assays), Wilson et al. (1994) concluded that GA was a weakly acting mutagen.
Agent VX
In tests on microorganisms and mammalian cell cultures, VX was not found to be mutagenic or was only weakly mutagenic (Crook et al. 1983; Goldman et al. 1988). Crook et al. (1983) reported that VX gave negative results when tested in the mouse micronucleus assay (exposures for 6 h/d for 9 d to VX at 0.002 mg/m3) and when tested in the Ames assay with five strains of Salmonella typhimurium (compared with positive controls; no other data reported). VX did not induce biologically significant increases in mutations when tested in the Ames Salmonella assay using five revertant strains (TA135, TA100, TA98, TA1537, and TA1538) with and without metabolic activation (Goldman et al. 1988). In tests using the yeast Saccharomyces cerevisiae, VX did not induce recombinants following exposures to concentrations as high as 100 µg/mL (Goldman et al. 1988). VX also failed to induce forward mutations when tested on mouse L5178Y lymphoma cells at concentrations less than 50 µg/mL (Goldman et al. 1988). Although doses of VX at 50 and 100 µg/mL resulted in increased numbers of mutations; these were not more than 1.5 times the control level. (A 2-fold increase was considered the minimum required to establish a positive result.)
Crook et al. (1983) reported that VX gave negative results for mutagenicity when tested in the sex-linked recessive lethal assay using Drosophila melanogaster.
Summary
Agents GB and VX were not found to be genotoxic in a series of microbial, cellular and mammalian assays. Agent GA was reported to be weakly mutagenic in some microbial assays.
3.6. Carcinogenicity
Agent GB
As part of the chronic inhalation studies conducted by Weimer et al. (1979), the tissues of animals exposed to GB for up to 1 y were examined for microscopic lesions including tumors. The test species included ICR
Swiss mice, strain-A mice, Sprague-Dawley/Wistar rats, Fischer 344 rats, and purebred beagle dogs. The exposures were to GB at 0.0001 or 0.001 mg/m3 6 h/d, 5 d/wk. Weimer et al. (1979) reported that agent-related tumors did not occur in any of the exposed species. Pulmonary tumors did occur in strain-A mice; after 52 wk of exposure, pulmonary adenomas were present in 3/19 animals exposed to GB at 0.0001 mg/m3, in 3/20 animals exposed to GB at 0.001 mg/m3, and in 0/20 controls. For animals maintained for 6 mo postexposure, the incidence rates for pulmonary adenocarcinomas were 5/19, 6/18, and 9/29, respectively. However, these lesions were not considered to be agent-related. Strain-A mice have a high natural propensity to form pulmonary tumors; the incidence of spontaneous pulmonary tumors being about 53% in animals 12 mo of age and 90% in animals 18 mo of age (Heston 1942). Overall, the studies of Weimer et al. (1979) indicate that agent GB is not carcinogenic.
Agent GA
No long-term animal carcinogenicity studies have been carried out on GA. Neoplastic lesions were not observed in male and female CD rats injected intraperitoneally with GA at up to 28.13, 56.25, or 112.5 µg/kg/d for 90 d (Bucci et al. 1992); however, this subchronic study was of insufficient duration to fully evaluate tumor incidence rates. No other animal data are available to assess the potential carcinogenicity of GA.
Agent VX
Standard long-term carcinogenicity studies have not been conducted on laboratory animals exposed to agent VX. Neoplastic lesions were not observed in male and female CD rats injected subcutaneously with 0.25, 1.0, or 4.0 µg/kg/d for 90 d (Goldman et al. 1988). No other animal data are available to assess the potential carcinogenicity of VX.
Summary
There is no evidence that agents GB, GA, or VX are carcinogenic. It is noted that a 90-d study, such as that performed by Bucci et al. (1992) for agent GA, is of insufficient duration to fully evaluate tumor incidence rates.
3.7. Summary
G Agents
Acute lethality data for inhalation exposures to the G agents are available in the form of LCt50 values for exposure times of 10 min or less. In only one published study was information presented from which a lethality threshold could be estimated for agent GD (Aas et al. 1985). Acute inhalation studies on rats exposed to agent GB vapor for the time periods of 10, 30, 90, 240, and 360 min have been conducted by the U.S. Army’s Edgewood Chemical Biological Center (ECBC) at Aberdeen Proving Ground, Maryland (Mioduszewski et al. 2000, 2001, 2002a). Mioduszewski et al. (2000, 2001, 2002a) is the critical study for deriving AEGL-3 values for agent GB. Nonlethal toxicity studies conducted primarily on dogs indicate that low concentrations of the G agents may cause miosis, salivation, rhinorrhea, dyspnea, and muscle fasciculations. Studies on dogs and rats indicate that exposures to GB at 0.001 mg/m3 for up to 6 h/d are unlikely to produce any signs of toxicity.
Animal data from vapor and oral exposure studies suggest that agent GB does not induce reproductive or developmental effects in mammals. Oral exposure studies of agents GB and GD in lab animals as well as injection exposure studies of agent GA suggest the lack of reproductive or development effects for these agents. Agent GB was not found to be genotoxic in a series of microbial and mammalian assays, but agent GA was reported to be weakly mutagenic. There is no evidence that agents GB and GA are carcinogenic.
Agent VX
Credible acute lethality data for vapor inhalation exposure to agent VX vapors are available for only two species (mice and goats) (Koon et al. 1960, as cited in NRC 1997). LCt50 values are 13.6 mg·min/m3 for mice and 9.2 mg·min/m3 for goats. In a short-term inhalation study, no signs of toxicity except miosis were seen in rats, mice, guinea pigs, or rabbits exposed to VX vapor concentrations at 0.0002 mg/m3 or less (6 h/d, 5 d/wk, for 2 wk) (Crook et al. 1983).
The available data indicate that VX does not cause reproductive or developmental toxicity. There is no evidence suggesting that VX is genotoxic or carcinogenic.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism, Toxicokinetics, and Disposition
4.1.1. Absorption
Although nerve agents may be absorbed through any body surface, the route through which absorption is most rapid and complete is the respiratory tract. It has been reported that as much as 70% of an inhaled dose of agent GB is retained in guinea pigs, dogs, and monkeys (Oberst 1961). In studies conducted on human volunteers, Oberst et al. (1968) found that mean percent retention of an inhaled dose of agent GB ranged from about 80% to 90%. Resting men (minute volume 6.9–7.9 L/min) retained a similar percent of the inhaled dose regardless of whether they were breathing exclusively through the mouth or nose; however, exercising men (minute volume 42.5 L/min) retained a significantly lower percentage (80%). Toxicity studies on nonhuman primates indicate that the intravenous and inhalation dose levels producing a similar level of effect are about the same, also suggesting that absorption through the respiratory tract may be close to 100% of the inhaled dose (Johnson et al. 1988; Anzueto et al. 1990). However, in species such as rodents that are nasal breathers, a proportionally greater amount of toxicant may be removed before reaching the lungs (the mechanisms of removal are thought to be hydrolysis or a reaction with epithelial tissues). In guinea pigs, Allon et al. (1998) found that approximately 29% of an inhaled dose of a racemic mixture of agent GD (soman) reached the blood.
4.1.2. Toxicokinetics
Spruit et al. (2000) conducted toxicokinetic studies of (±) sarin in anesthetized, atropinized, restrained guinea pigs. The test animals were exposed (nose-only) to doses corresponding to 0.4 and 0.8 LCt50 for 8-min exposure times. Toxicokinetics was also studied after an intravenous bolus corresponding to 0.8 LD50. The LC50 for sarin was calculated by probit analysis to be 47 mg/m3 (95% CL=44–50 mg/m3), and the LCt50 for an 8-min exposure was estimated to be 376 mg·min/m3. In both the intravenous and inhalation studies the concentration of the nontoxic (+) isomer in the blood was below detection limits (<5 pg/mL blood). In the intravenous test, the toxicokinetics of the toxic (−) isomer followed a bi-exponential
equation. In the inhalation tests, the blood AChE activity decreased to about 70% of control values at 0.4 LCt50 and to about 15% of control values at 0.8 LCt50; however, there were no effects on respiratory parameters (respiratory minute volume or respiratory frequency). The toxic (−) isomer appeared to be rapidly absorbed and the toxicokinetics followed a discontinuous process with a mono-exponential equation for the exposure period and a bi-exponential equation for the postexposure period.
Benschop et al. (2000) (see also Benschop [1999]) studied the toxicokinetics of several VX stereoisomers [(±)−] in hairless guinea pigs (intravenous and percutaneous exposures) and marmosets (intravenous exposures only). Following an intravenous dose of 28 µg/kg (marmosets) or 56 µg/kg (guinea pigs), VX was found in the blood at toxicologically relevant levels even after 6 h. Detoxification proceeded at a slower rate in marmosets than in guinea pigs. The VX metabolite, O-ethyl methyphosphonic acid (EMPA), was found in the blood of the exposed animals; however, the metabolite contributed only 5% to the recovery of the phosphonyl moieties related to the VX dose. Metabolites of VX were also evaluated in in vitro studies by treating liver homogenates and plasma from hairless guinea pigs, marmosets, and humans with the radio-labeled compounds, 35S-VX and [14CH3-P]-VX. The potential toxic metabolite VX-N-oxide was not found. Desethyl-VX was found after incubation of VX in plasma of all three species; however, because of its slow rate of formation, Benschop et al. (2000) concluded that it would be unlikely that this compound would be present at toxicologically relevant levels after administration of VX in vivo. In vitro studies with 35S-VX revealed that a significant part of the thiol-containing leaving group (S-2-(N, N-diisopropylamino)ethane thiol, DPAT) was bound to proteins such as albumin. It was found that the sulfur-containing leaving group was also transformed into a variety of oxidation products.
4.1.3. Disposition and Metabolism
There are a number of enzyme systems in mammalian blood and tissues capable of the binding with and/or metabolically detoxifying organophosphate nerve agents. A primary disposition pathway is the binding of the compounds with blood cholinesterases and carboxylesterases. Of the cholinesterases present in blood (RBC- and plasma-ChE), VX preferentially inhibits RBC-ChE (Sidell and Groff 1974). Plasma cholinesterase may likely serve as a buffer to offset the binding of nerve agents (and pref-
erential binding of agent VX) to RBC-AChE. It has been reported that pretreatment with human plasma cholinesterase protected laboratory rats (Ashani et al. 1993) and monkeys (Raveh et al. 1997) from lethal and other acute toxic effects of VX exposure.
The G-agents have a strong affinity for carboxylesterases (Jokanović 1989), in contrast to agent VX, which has a quaternary ammonium group that prevents it from being a substrate for carboxylesterases. In tests on male SD rats, Maxwell (1992) experimentally confirmed that endogenous carboxylesterases provide “significant protection” against in vivo toxicity of the organophosphorous (OP) agents GA, GB, and GD, but not VX. Maxwell (1992) goes on to conclude that “CaE [carboxylesterase] detoxification does not appear to be important” against exposures to lethal concentrations of agent VX in the laboratory rat.
Because of the lack of other reactive esterases, agent VX induces a toxic response at lower concentrations than the G agents.
While carboxylesterases were once widely considered to be absent from the blood plasma of humans, carboxylesterases are, indeed, present in human erythrocytes and monocytes as well as in human liver, kidney, lung, skin, and nasal tissue (Cashman et al. 1996). Additional literature documents the presence of carboxylesterases in many human tissues and fluids, including brain, milk, mammary gland, pancreas, small intestine, colon, stomach, placenta, and plasma and serum (Chanda et al. 2002; Kaliste-Korhonen et al. 1996). The lung carboxylesterases are associated with alveolar macrophages (Munger et al. 1991). Further, carboxylesterases are present in human tissues and organs where exposure to nerve agent vapors would likely first occur (nasal tissues and the lung), would be distributed (erythrocytes, monocytes, plasma), and would generate effects (brain, stomach, colon, etc.). Carboxylesterase is also present in human serum. Chanda et al. (2002) indicate that full characterization of the OP-protective capabilities of carboxylesterases requires assessment not only of the amount but also of the affinity exhibited by carboxylesterases for the inhibitor as well as the total carboxylesterase activity unlikely to be inhibited (inhibitor resistant esterase activity [IRE]). The detoxification potential of carboxylesterases is multifaceted and is an area requiring further experimental characterization.
It is acknowledged that the CaE profile in humans is not well known and that there are few data from which to characterize the contributions that CaE may make to human protection from anticholinesterase poisoning. Chanda et al. (2002) consider that full characterization of CaE amount, affinity, and IRE in human tissues will be necessary before accurate predic-
tions can be made regarding CaE detoxification potential following anticholinesterase exposures to humans.
Phosphorylphosphatases associated with the hydrolysis of GD (somanase), GA (tabunase), and GB (sarinase) have been reported. Sterri et al. (1980) reported that the liver of rats was capable of hydrolyzing GD at a rate of 743 µmol/g of liver per hour. At low substrate concentrations some phosphorylphosphatases have been shown to be stereospecific in their activity. Sarinase from the plasma of rats preferentially catalyzes the hydrolysis of the less toxic isomer of agent GB (Christen and van den Muysenberg 1965); however, tabunase targets the toxic stereoisomer of agent GA (reviewed by Gupta et al. [1987]). Somanase from rat liver (Wahllander and Szinicz 1990) or swine kidney (Nordgren et al. 1984; Benschop et al. 1981) preferentially inhibits the less toxic isomers of agent GD; however, another hepatic enzyme in rat liver has been reported to be capable of hydrolyzing all four isomers of GD (Little et al. 1989). The same hepatic enzyme also catalyzed the hydrolysis of agents GA and GB (Little et al. 1989). In studies conducted on rats dosed subcutaneously with agent GB, GD, or GF at 75 µg/kg, Shih et al. (1994) found that the major metabolite formed by a nonsaturable mechanism and excreted in the urine was an alkylmethyl phosphonic acid. Little et al. (1986) identified 3H-labeled GB metabolites in the tissues of mice following intravenous administration of a sublethal dose (80 µg/kg). Most of the label was associated with free isopropyl methylphosphonic acid (IMPA), the hydrolytic metabolite of GB. In individuals allegedly exposed to GB, Noort et al. (1998) found O-isopropyl methylphosphonic acid in serum samples, and Nakajima et al. (1998) reported that methylphosphonic acid and isopropyl methylphosphonic acid were detected as urinary metabolites of GB. Distribution of the low-sarinase allele appears to be somewhat ethnically related. The Japanese population has a higher frequency of the low-sarinase isoform (allele frequency of 0.66) than other ethnic groups (0.24 to 0.31) (Yamasaki et al. 1997).
A-esterases (paraoxonase/arylesterase) present in the blood and liver are also capable of hydrolyzing phosphate esters (Cashman et al. 1996). Paraoxonase is one A-esterase from humans known to hydrolyze the phosphorus-fluorine bond of the nerve agents GB and GD (Davies et al. 1996). A-somanase isolated from human liver (Wang et al. 1998) is capable of hydrolyzing agent GD as well as agent GA with P-F or P-CN bonding, but cannot hydrolyze paraoxon or nerve agent VX with P-O or P-S bonding. Agent GB was not tested in the studies of Wang et al. (1998). A-
esterases are considered to provide protection against the adverse effects of some OP compounds (Pond et al. 1995).
Paraoxonase is polymorphic in human populations, and individual differences are wide (LaDu et al. 1986, as cited in Davies et al. 1996; Furlong et al. 1988, 1989). In one population tested, differences in paraoxonase activity among three genotypes was approximately 6-fold (Kujiraoka et al. 2000). Among a “caucasoid” population sampled in Seattle, Washington, a 40-fold variation in human serum paraoxonase activity was observed (Furlong et al. 1989) and was associated with three phenotypes: homozygotes for the low-activity allele, heterozygotes, and homozygotes for the high-activity allele.
Individuals expressing certain isomeric forms of the enzyme with low hydrolyzing activity are considered to be more susceptible to organophosphate anticholinesterase poisoning (Yamasaki et al. 1997). The polymorphic paraoxonase gene (PON1) has an important role in the detoxifying metabolism of nerve agents and OP insecticides. The PON1R192 paraoxonase isoform hydrolyzes agents sarin (GB) and soman (GD) slowly when compared with the PON1Q192 isoform (Furlong et al. 2002; Davies et al. 1996). The human population can be organized into three PON1*192 genotypes: PON1Q192 homozygotes; heterozygotes; and PON1R192 homozygotes (Furlong et al. 2002; Allebrandt et al. 2002). Frequency distributions of the PON1*192 variants have been examined in ethnically diverse populations (Allebrandt et al. 2002). The allele expressing low activity for agent GB and agent GD hydrolysis (PON1R192) is significantly more frequent in African Americans (sampled in Brazil and North America) and Asians (sampled in China, Japan, and Canada) than in individuals of Indo-European descent (sampled in East India, Turkey, Canada, Russia, Germany, North America, England, France, the Netherlands, and Brazil). Nevertheless, Furlong et al. (2002) point out that “genotyping alone provides no information about PON1 levels, which can vary up to 13-fold between individuals” (homozygous for the low-activity allele) (see also Furlong et al. [1989] and Davies et al. [1996]).
The serum paraoxonase activity ranges observed by Furlong et al. (1989) and discussed in Davies et al. (1996) illustrate the presence of human genetic variability in one of several metabolic detoxification systems that can denature certain G agents. It is understood, however, that mere determination of serum paraoxonase activity alone is not sufficient to characterize whole-organism susceptibility to anticholinesterase exposure. There are many other metabolic detoxification mechanisms that are also
simultaneously active (e.g., RBC-ChE, tissue carboxylesterases). Further experimentation will be necessary before 13-time or 40-time differences in human serum paraoxonase activity can be translated into quantitative differences in whole-organism susceptibility to anticholinesterase compounds.
Some investigators have previously considered that low levels of paraoxonase in newborns may contribute to the observed sensitivity of newborn rats to organophosphate insecticides (Benke and Murphy 1975; Burnett and Chambers 1994, as cited in Davies et al. 1996). A recent investigation (Chanda et al. 2002) presents in vitro and in vivo evidence that carboxylesterases “are critical for explaining age-related sensitivity” of rat pups to the OP insecticide chlorpyrifos. The presence of low carboxylesterase activity, however, does not sufficiently characterize the greater susceptibility of rat pups to neurotoxic effects of some OP insecticides (Chanda et al. 2002).
A novel mouse-liver enzyme, unrelated to the paraoxonases, has been found to hydrolyze agents GB and GD (Billecke et al. 1999) in an in vitro assay of soluble fraction extracts from commercially available frozen mouse livers.
4.1.4. Distribution and Excretion
Several studies have examined the tissue distribution and excretion of G agents and their metabolites following parenteral administration to rodents. In studies conducted on rats dosed subcutaneously with agent GB (sarin), agent GD (soman), or GF at 75 µg/kg, Shih et al. (1994) found that the major route of elimination for all three agents was urinary excretion. For GD, the lung was the major organ of accumulation. McPhail and Adie (1960) dosed rabbits with radio-labeled (32P) GB and found the highest levels of radioactivity in the lungs and kidney. Kadar et al. (1985) injected mice intravenously with a LD50 dose of 3H-labeled agent GD. High levels of radioactivity were found in the lung and skin at 5 min to 24 h after the injection, with very small amounts in the CNS. Considerable accumulation of the label occurred in the urine, gall bladder, and intestinal lumen, suggesting that these were the main pathways of excretion. Little et al. (1986) measured the distribution of 3H-labeled agent GB (sarin) and sarin metabolites in the tissues of mice following intravenous administration of a sublethal dose (80 µg/kg). Within 1 min all tissues contained large amounts
of the label, of which less than 10% represented agent GB (sarin). High concentrations of the metabolite were found in the kidneys and lungs, and only trace amounts of 3H-labeled agent GB (sarin) were found in the brain within 15 min. In a continuation of these studies, Little et al. (1988) evaluated the distribution of 3H-labeled agent GD (soman) and agent GB (sarin) in the brain of mice following sublethal intravenous doses (25 µg/kg and 80 µg/kg). The nerve agents were distributed evenly throughout the brain tissue with the exception of a 2- to 5-fold greater concentration in the hypothalamus.
4.2. Mechanism of Toxicity
The acute toxicity of the nerve agents is considered to be initiated by inhibition of acetylcholinesterase (AChE), an enzyme responsible for deactivating the neurotransmitter acetylcholine at neuronal synapses and myoneural junctions. Nerve agents phosphorylate the enzyme, thereby preventing deactivation of acetylcholine. Although the inhibited cholinesterase can be reactivated by the process of dephosphorylation, that is not possible once the nerve agent-cholinesterase complex undergoes “aging,” which is thought to happen because of a loss of an alkyl or alkoxy group. Agent GD ages very rapidly, with a t1/2 (time required for 50% of the enzyme to become resistant to reactivation) of 1.3 min (Harris et al. 1978). The aging half-time for agent GA is 46 h, as calculated from a rate constant of 2.5× 10−4 per minute (de Jong and Wolring 1978), and the t1/2 for agent GB has been reported to be 5 h (Sidell and Groff 1974). In the latter case, approximately 5% of the GB-enzyme complex reactivated spontaneously. In contrast to the results of these latter studies, Grob and Harvey (1958) had earlier reported that both GA and GB combined with ChE almost irreversibly within 1 h when tested in vitro. The complex formed between ChE and agent VX does not age significantly (half-life about 48 h), and the rate of spontaneous reactivation in humans has been reported to be as fast as 1% per hour (Sidell and Groff 1974).
Although nerve agents exert toxic effects on the central and peripheral nervous system indirectly through AChE inhibition (Koelle 1976, 1981), nerve agents may also affect nerve impulse transmission by additional mechanisms at neuromuscular junctions (Somani et al. 1992) and at neurotransmitter receptor sites in the CNS. Rao et al. (1987) reported that VX caused an increase in acetylcholine release at neuromuscular junctions in
the frog by an interaction with the nicotinic acetylcholine receptor-ion channel complex. Aas et al. (1987) reported alterations in muscarinic receptors in rat bronchi and lung tissue after subacute inhalation exposures to agent GD. In the CNS, nerve agents may act directly on muscarinic, nicotinic, and glutamate receptors. Bakry et al. (1988) reported that nanomolar concentrations of agent GD affected muscarinic ACh receptors that have a high affinity for [3H]-cis-methyldioxalane binding. Rocha et al. (1998, 1999) reported that, in cultured rat hippocampal neurons, VX at 0.01 nM reduced the evoked release of the neurotransmitters γ-aminobutyric acid (GABA) and reduced the amplitude of evoked GABAergic postsynaptic currents. VX concentrations >1 nM decreased the amplitude of evoked glutamatergic currents. In the presence of a Na+ channel blocker, VX increased the frequency of GABA- and glutamate-mediated miniature postsynaptic currents, a Ca+ dependent effect reported to be unrelated to cholinesterase inhibition (Rocha et al. 1999). Chebabo et al. (1999) reported that 0.3–1 nM of agent GB reduced the amplitude of GABA-mediated postsynaptic currents but had no effect on the amplitude of glutamatergic-mediated postsynaptic currents. The observed effect was thought to be due to the direct interaction of GB with muscarinic acetylcholine receptors present on presynaptic GABAergic neurons. Chebabo et al. (1999) suggest that the selective reduction in the action-potential-dependent release of GABA in the hippocampus might account for GB-induced seizures. Lallement et al. (1991a,b) had earlier suggested that GD-induced overstimulation of glutamatergic receptors contributed to maintenance of seizures. Although these data indicate that nerve agents may have direct effects on the nervous system unrelated to AChE inhibition, the in vitro data do not provide a means of relating electrophysiological alterations in rat hippocampal neurons or determining a dose conversion to the integrative end point of whole-body lethality. Neither do they allow qualitative/quantitative comparisons directly relevant to lethality. The results were obtained largely from single cells in isolation from whole organisms and systems, and extrapolation from observations on individual cells is not presently possible. At present, nM-induced amplitude changes in postsynaptic currents in rat hippocampal neurons in vitro cannot be correlated to dose levels resulting in multisystem failure and death such as are needed for AEGL estimation.
It should be further noted that the effects of nerve agents on GABAergic transmission in the CNS may have profound implications for behavioral effects in laboratory animals and humans and may also contrib-
ute to the induction of convulsions at higher doses (Bakshi et al. 2000). Nevertheless, given the present undefined application of noncholinergic data to AEGL estimation, reliance on the primary assumption of anticholinesterase action is consistent with recognized opinion (Bakshi et al. 2000).
Recent studies with cholinesterase inhibitors such as galantamine, which affect neuronal nicotinic AChE receptors in a similar manner to that reported for VX, have shown that such compounds have therapeutic benefits for patients with mild to moderately severe Alzheimer’s disease (Maelicke et al. 2001). As such, these compounds might be helpful in stabilizing behavior in such patients by improving memory and cognitive and daily function.
As pentavalent phosphorous-containing compounds, the G agents may also indirectly generate neurotoxic effects through a noncholinergic mechanism involving the kinase-mediated protein Ca 2+/calmodulin kinase II (Ca 2+/CaM kinase II) (de Wolff et al. 2002; Abou-Donia and Lapadula 1990). The Ca 2+/CaM kinase II protein becomes activated by OP-induced phosphorylation and reacts with the cytoskeletal proteins found in neurofilaments to produce axonal degeneration in the large-diameter tracts of the spinal cord.
It is also understood that OP compounds interact with detoxification enzymes such as the carboxylesterases and A-esterases and that the degree of such interaction may alter the magnitude and extent of the toxic cascade following AChE inhibition (Pope and Liu 2002). Recent studies indicate that full characterization of the OP-protective capabilities of carboxylesterases requires assessment not only of the amount but also of the affinity exhibited by carboxylesterases for the inhibitor as well as the total carboxylesterase activity unlikely to be inhibited (inhibitor resistant esterase activity [IRE]) (Chanda et al. 2002). The detoxification potential of carboxylesterases is multifaceted and is an area requiring further experimental characterization.
4.3. Relative Toxic Potency
Because of the sparse animal and human toxicity data for agents GA, GD, GF, and VX, AEGLs for those agents will necessarily be derived from the AEGLs for agent GB by a relative potency method. The database for the nerve agents as a group is considered reasonably complete in that there
exist (1) experimental data for multiple species, including humans; (2) documented nonlethal and lethal end points that follow an exposure-response curve; (3) a known mechanism of toxicity common to all the nerve agents with the all end points representing a response continuum to inhibition of cholinesterase activity; and (4) no uncertainties regarding other toxic end points such as reproductive or developmental effects or carcinogenicity.
Because the mechanism of action is the same for all the nerve agents, data uncertainty is reduced and target organ effects are expected to be identical and to differ only in magnitude. Thus, a comparative method of relative potency analysis from the more complete data set for agent GB is appropriate. This approach has been applied before, in the estimation of nerve agent exposure limits (Watson et al. 1992; Mioduszewski et al. 1998). The relative toxic potency of cholinesterase inhibitors can be expressed in several ways, based on in vitro or in vivo data.
4.3.1. In Vitro Potency
The in vitro potency can be measured by either the bimolecular rate constant (ki, in M/min) for the reaction of the agent compound with the enzyme or by the molar concentration causing 50% inhibition of the enzyme (I50) in vitro. The relationship between I50 and ki for a fixed time (t) of incubation is expressed by the following equation (Eto 1974):
As summarized by A.D. Little, Inc. (1985), ki values for GB are in the range of 1×106 to 2×107 M−1/min−1 for acetylcholinesterase in rat brain tissue, and 1×107 M−1/min−1 for butyrylcholinesterase in human serum. Reported ki values for agent GD are 3.7×107 M−1/min−1 for acetylcholinesterase in rat brain tissue, and 1×107 M−1/min−1 for butyrylcholinesterase in human serum (A.D. Little, Inc. 1985). More recently, Maxwell (1992) reported ki values of 4.5 (±0.7)×106 M−1/min−1 for agent GA, 1.2 (±0.3) ×107 M−1/min−1 for agent GB, and 3.6 (±0.5)×107 M−1/min−1 for agent GD in in vitro tests conducted on rat brain AChE.
I50 data for several G agents have been tabulated by Dacre (1984). The pI50 (negative log of the molar concentration causing 50% inhibition of
cholinesterase) was reported to be 8.4–8.6 for GA and 9.2 for GD (Dacre 1984; Holmstedt 1959). Grob and Harvey (1958) reported that the in vitro potency of GB (I50=0.3×10−8 mol/L) was 5 times that for GA (I50=1.5× 10−8 mol/L).
The ki values for agent VX have been reported to be 1.4±0.3×108 M/min, respectively, for in vitro tests conducted on rat brain AChE (Maxwell 1992). In comparison, Maxwell (1992) reported a ki value of 1.2 ±0.3×107 M/min for agent GB. The corresponding I50 values are 5.8×10−8 M for agent GB and 5.0×10−9 M for agent VX. The GB:VX ratio for the I50 values is 11.7, indicating that VX is nearly 12 times more potent than GB in inhibiting rat brain acetylcholinesterase in vitro. This comparison is one way to express the relative potency of agent VX.
4.3.2. In Vivo Potency
Relative potency of nerve agents can also be expressed in terms of the in vivo dose necessary to produce the same toxic effect by a specific exposure route.
G Agents
A summary of the estimated inhalation and visual effects values for the G agents is given in Tables 1–21 and 1–22. The information presented on animal toxicity values is derived from Callaway and Dirnhuber (1971) and Mioduszewski et al. (2002b) for nonlethal visual effects; and Oberst (1961), Callaway and Blackburn (1954), Mioduszewski et al. (2001, 2002a), and Anthony et al. (2002) for lethality. Another source is the largely unpublished experimental data summarized by the NDRC in 1946.
Estimates of lethality and severe effect levels in humans are based on extrapolations from animal data and on modeling studies. Several of the estimates are presented in Table 1–21, together with the limited human data for miosis. For the end point of miosis, ratios for ECt50, ECt90, and threshold effects are summarized in Table 1–21 for both experimentally derived and estimated toxicity values. For miosis as a critical effect, comparison of effective doses to achieve 50% pupil area decrement in the eye of the albino rabbit (Callaway and Dirnhuber 1971) indicates that agents GD and GF are more miotogenic than GB at approximately 50% of the GB Ct (GB/ GD of 2.24; GB/GF of 1.76; relative potency to agent GB of approximately
TABLE 1–21 Comparison of Visual Effects Values for G Agents
|
|
Toxicity value (ECt [mg·min/m3]) |
Ratios |
|||||||
|
Species |
GB |
GA |
GD |
GF |
GB:GA |
GB:GD |
GB:GF |
GD:GF |
References |
|
Human (10 min to 5 h) (ECt90, miosis) |
13.85 |
|
Callaway and Dirnhuber 1971 |
||||||
|
Human (20 min) (ECt50, miosis) |
4 |
|
Johns 1952 |
||||||
|
Human (ECt50, incapacitation) |
2.5 |
7.5 |
0.4 |
|
0.33 |
6.25 |
|
Wells et al. 1993a |
|
|
Human (10 min to 5 h) (ECt50, miosis) |
2.33 |
|
Callaway and Dirnhuber 1971 |
||||||
|
Human (20 min) (No effect, miosis) |
1.2 |
|
McKee and Woolcott 1949 |
||||||
|
Human (2 min) (ECt50, mild effects) |
0.5 |
0.5 |
0.2 |
0.2 |
1.0 |
2.5 |
2.5 |
1.0 |
Reutter and Wade 1994a (unclassified summary table) |
|
Human (2 min) (ECt50, mild effects) |
0.5 |
0.5 |
0.25 |
0.25 |
1.0 |
2.0 |
2.0 |
1.0 |
Mioduszewski et al. 1998a |
|
Human (2–10 min) (ECt50, mild effects) |
<2 |
|
NRC 1997a |
||||||
|
Human (1 min) (<ECt01, miosis) |
0.5 |
|
McNamara and Leitnaker 1971a |
||||||
|
Rat (f, m) (10 min) (ECt50 miosis) |
0.68, 0.87 |
|
Mioduszewski et al. 2002b |
||||
|
Rat (f, m) (60 min) (ECt50, miosis) |
1.20, 1.80 |
|
Mioduszewski et al. 2002b |
||||
|
Rat (f, m) (240 min) (ECt50, miosis) |
2.88, 5.76 |
|
Mioduszewski et al. 2002b |
||||
|
Guinea pig (5 h) (LOAEL, miosis) |
1.8 |
|
van Helden et al. 2001, 2002 |
||||
|
Marmoset (5 h) (LOAEL, miosis) |
2.5 |
|
van Helden et al. 2001, 2002 |
||||
|
Rabbit (10 min to 5 h) (ECt, 50% miosis) |
1.32 |
0.59 |
0.75b |
2.24 |
1.76 |
0.79 |
Callaway and Dirnhuber 1971 |
|
Rabbit (10 min to 5 h) (ECt, 90% miosis) |
2.71 |
2.19 |
1.79b |
1.23 |
1.51 |
1.22 |
Callaway and Dirnhuber 1971 |
|
aSecondary sources. bData for agent T2715, (2-methylcyclohexyl methylphosphonfluoridate), analog for agent GF. |
|||||||
TABLE 1–22 Acute Lethal Inhalation Toxicity Values for G-Agents
|
Species (Exposure Time) |
Toxicity Value (LCt50 [mg·min/m3]) |
Ratios |
|||||||
|
GB |
GA |
GD |
GF |
GB:GA |
GB:GD |
GB:GF |
GD:GF |
Reference |
|
|
Monkey |
74 |
187 |
|
0.40 |
|
DA 1974a |
|||
|
Monkey (2 min) |
42 |
135 |
|
0.31 |
|
Oberst 1961; DA 1974 |
|||
|
Monkey (10 min) |
150 |
250 180 |
|
0.71b |
|
||||
|
Geometric Mean (monkey data) |
0.44 |
|
|||||||
|
Rat (female) (1-min) |
118 |
|
135 |
110 |
|
0.87 |
1.07 |
1.23 |
Callaway and Blackburn 1954 |
|
Rat (male) (1-min) |
220 |
|
196 |
181 |
|
1.12 |
1.22 |
1.08 |
Callaway and Blackburn 1954 |
|
Rat (10-min) |
220 |
450 |
230 279 |
|
0.49 |
0.87d |
|
DA 1974a |
|
|
Rat (female) (10-min; 24-h lethality) |
184 |
|
253 |
|
0.73 |
|
Mioduszewski et al. 2001; Anthony et al. 2002 |
||
|
Rat (female) (60-min; 24-h lethality) |
387 |
|
334 |
|
1.16 |
|
Mioduszewski et al. 2001; Anthony et al. 2002 |
||
2) and that the GD:GF ratio approximates 1.0 (equal to 0.79). For 90% pupil area decrement in the rabbit, agents GD and GF are again more effective than agent GB for inducing this end point (GB/GD of 1.23, GB/GF of 1.51; relative potency range to agent GB of approximately 1.2 to 1.5) with a GD:GF ratio of 1.22. A protective determination of relative potency to agent GB is 2.0. Thus, agents GD and GF are considered equipotent and approximately twice as potent as agent GB for inducing miosis. For more severe effects, such as lethality, resulting from vapor exposures, the relative potency estimates presented in Table 1–22 indicate that agents GB, GD, and GF are equally potent and are twice as potent as agent GA.
At a public hearing in 2000 convened by the Chemical Demilitarization Branch of the Centers for Disease Control and Prevention, a U.S. Surgeon General’s review panel concluded that because (1) the data base for GB is relatively robust, and (2) the data for the other G agents are limited, it is appropriate to utilize a relative potency approach for comparing G agents (67 Fed. Reg. 895 [2002]; DHHS 2002).
Agent VX
The in vivo doses of agents VX and GB required to produce the same level of blood cholinesterase inhibition in the same species by a specific exposure route are shown in Table 1–23. In humans, the experimentally determined RBC-AChE50 for VX is 0.0023 mg/kg for an oral dose (Sidell and Groff 1974). In contrast, for agent GB, an oral dose of 0.010 mg/kg is required to produce about the same level of effect (Grob and Harvey 1958). The GB:VX ratio for this effect is approximately 4.3. In studies conducted by Gupta et al. (1991) in which rats were injected subcutaneously, VX was found to be 10 times more toxic than GB for ChE inhibition and myonecrosis end points. The relative potency of agents VX and GB are shown in Table 1–23.
In studies conducted by Maxwell (1992) on Sprague-Dawley rats, subcutaneous LD50 values for GB and VX were 0.51 and 0.027 µmol/kg, respectively, indicating that VX is about 19 times more toxic than GB in rats for subcutaneous lethality, on a molar basis (if the micromoles of each compound are converted to grams using 140 as the molecular weight of GB and×10−5 g/kg for GB and 8.022×10−5 g/kg for VX, resulting in a GB:VX ratio of 9.9). Analysis of parenteral data for Hartley albino guinea pigs (subcutaneous) and Swiss ICR mice (intramuscular) in studies by Koplovitz
TABLE 1–23 Relative Potency Estimates for Agents GB and VX Experimental Data
|
Species |
Toxicity End Point |
Units |
GB |
VX |
GB:VX Ratio |
|
Human |
Inhalation Ct ChE50a |
mg-min/m3 |
42 |
~6.5 |
~6.5 |
|
Human |
Oral RBC-ChE50b |
µg/kg |
10 |
2.4 |
4.3 |
|
Human |
Intra-arterial/intravenous RBC-ChE50b |
µg/kg |
3 |
1.1 |
2.7 |
|
Monkeyl |
Intravenous LD50c |
µg/kg |
20 |
6–11.9 |
1.8–3.3 |
|
Ratl |
µg/kg |
45–63 |
6.9–10 |
4.5–9.1 |
|
|
Mouse |
Intramuscular LD50e |
µg/kg |
204.81 |
13.07 |
15.7 |
|
Mousel |
Intravenous LD50c |
µg/kg |
100 |
12–15 |
6.7–8.3 |
|
Mousel |
mg·min/m3 |
240–310 |
4–13 |
18.5–77.5 |
|
|
Mousel |
µg/kg |
1000 |
36–59 |
17–28 |
|
|
Guinea pig |
Subcutaneous LD50e |
µg/kg |
41.26 |
6.89 |
5.99 |
|
Rat |
Subcutaneous LD50g |
µmol/kg |
0.57 |
0.03 |
19 (9.9)k |
|
Ratl |
Oral LD50c |
µg/kg |
870–1,060 |
77–128 |
6.8–13.8 |
|
Rabbitl |
mg·min/m3 |
2,000 |
8.3–28 |
71–241 |
|
|
Rabbit |
Vapor exposure; 50% pupil area decrementi |
mg·min/m3 |
1.32 |
0.04 |
33 |
|
Rabbit |
Vapor exposure; 90% pupil area decrementi |
mg·min/m3 |
2.71 |
0.23 |
11.8 |
|
aGB, Oberst et al. (1968); VX, Bramwell et al. (1963) (estimated from tabulated data; not verifiable). |
|||||
et al. (1992) resulted in acute (24-h) LD50 estimates as follows: in the guinea pig, LD50 for GB of 41.26 µg/kg, LD50 for VX of 6.89 µg/kg; and in the mouse, LD50 for GB of 204.81 µg/kg, LD50 for VX of 13.07 µg/kg. Inhalation lethality data for mice include LCt50 values of GB at 240 mg·min/m3 (forced activity); GB at 310 mg·min/m3 (resting animals); VX at 4 mg·min/m3 (total animal exposures); and VX at 13.6 mg·min/m3 (head-only exposures) (DA 1974; Koon et al. 1960, as cited in NRC 1997).
The Cts necessary to generate 50% and 90% decrease in pupil area in the albino rabbit eye (Callaway and Dirnhuber 1971) were summarized in Table 1–19. The calculated Cts for 50% decrease are 1.32 mg·min/m3 for GB and 0.04 mg·min/m3 for VX (a GB:VX ratio of 33), while the Cts for 90% decrease are 2.71 mg·min/m3 for GB and 0.23 mg·min/m3 for VX (a GB:VX ratio of 11.8) (see Table 1–23). Callaway and Dirnhuber (1971) consider the 90% decrement to be a more definite end point; furthermore, this degree of pupil area decrease has operational significance. However, because Callaway and Dirnhuber (1971) do not document incidence data, neither an EC50 nor an EC90 for a given percentage miosis, as defined by current experimental protocols, can be reliably determined for their exposed rabbit population.
Primary experimental data for GB:VX comparisons for the same end point are available for five mammalian species (human, rat, mouse, guinea pig, rabbit; see Table 1–23). In all cases, agent VX is more potent than agent GB (range of 2.7 to 33).
Human Estimates ofGB and VX Toxicity
Estimates of lethality and severe effect levels in humans are based on extrapolations from animal data and on modeling studies. Several of these estimates are presented in Table 1–24, together with the limited human experimental data (vapor inhalation, oral, intra-arterial, and intravenous exposures) for ChE50 levels. The GB:VX ratios for these experimentally derived end points fall in the range of 2.7 to 6.5. For the end point of miosis, ECt50 estimates range from 0.06 mg·min/m3 to 0.09 mg·min/m3 for VX and 0.5 mg·min/m3 to 1.5 mg·min/m3 for GB, resulting in overall GB:VX ratios of 5.6 to 25 (secondary sources and nonverifiable data).
Human inhalation exposures (Oberst et al. 1968; Bramwell et al. 1963), human oral exposures (Grob and Harvey 1958; Sidell and Groff 1974), and human intra-arterial and intravenous exposures (Grob and Harvey 1958;
TABLE 1–24 Human Toxicity Estimates for Agents GB and VX
|
Toxicity End Point (Exposure Time) |
GB (mg·min/m3) |
VX (mg·min/m3) |
GB:VX Ratio |
|
Inhalation ChE50a |
42b |
~6.5c |
6.5 |
|
Oral RBC-ChE50a |
10 µg/kgd |
2.3 µg/kge |
4.3 |
|
Intra-arterial/intravenous RBC-ChE50a |
3 µg/kgd |
1.1 µg/kge |
2.7 |
|
LCt50 (2–10 min) |
35f |
15f |
2.3 |
|
ECt50 (2–10 min) |
25f |
10f |
2.5 |
|
LCt05 |
20g |
6g |
3.3 |
|
ECt05 (severe) |
1g |
5g |
3 |
|
LCt01 |
10d |
− |
− |
|
ECt05 (mild) |
8g |
3g |
2.7 |
|
No deaths |
6h |
− |
− |
|
ECt05 (ocular, miosis) |
1.5g |
0.06g |
25 |
|
Ocular threshold |
1.0i |
0.04i |
25 |
|
ECt50 (ocular, miosis; 2–10 min) |
0.5f |
0.09f |
5.6 |
|
ECt50 (ocular, miosis) |
>0.5k |
0.09k |
>5.6 |
|
aExperimental data with human subjects; all other estimates are extrapolations based on animal data. bOberst et al. (1968); resting men, breathing 7 L/min. cBramwell et al. (1963); estimated from tabulated data—not verifiable. dGrob and Harvey (1958). eSidell and Groff (1974). |
|||
Sidell and Groff 1974) are included in the experimental database summarized in Tables 1–23 and 1–24; reported end points for each human study were ChE50. The Bramwell et al. (1963) study of VX inhalation toxicity is considered a flawed and nonverifiable source because the human subjects were not exposed to a rigorously controlled atmosphere (breathing zone concentrations could not be determined and potential effects of the carrier solvent [benzene] on agent absorption by subject was not evaluated, etc.). In consequence, the GB:VX ratio for inhalation ChE50 (which includes the VX Ct from Bramwell et al. [1963]) is not as credible as the comparable ratio derived from the well-conducted human oral exposure studies of Grob and Harvey (1958) and Sidell and Groff (1974).
4.3.3. Comparison of Exposure Standards
G-Series Agents
The current occupational exposure limits for the G-series nerve agents, as published by the CDC (DHHS 1988) are 0.0001 mg/m3 for GB and GA to be applied as a no-adverse-health-effect level for 8-h continuous workplace exposure. The resulting GB:GA ratio is 1.0. The current general population exposure limits for the G-series nerve agents, as published by the CDC (DHHS 1988) are 0.000003 mg/m3 for GB and GA, to be applied as a no-adverse-health-effect level for 24-h continuous exposure (provides a GB:GA ratio of 1.0). Agents GD and GF are not part of the unitary stockpile and were not evaluated by the CDC in 1988.
The U.S. Department of the Army has prepared a health criteria document for the G-series agents (Mioduszewski et al. 1998) in which exposure limits for the G agents were derived using the relative potency approach and the currently accepted exposure limits for agent GB. As part of a regularly scheduled review process, the CDC is currently reevaluating the 1988 agent control limits with application of recent risk assessment models and updated scientific data (67 Fed. Reg. 895 [2002]; DHHS 2002). The review is currently (September 2002) in progress, and the CDC has not yet released a final position.
Agent VX
The current occupational exposure limit for VX, as published by the
CDC (DHHS 1988), is 0.00001 mg/m3. Compared with the CDC recommended value of 0.0001 mg/m3 for agent GB, the resulting GB:VX ratio is 10. The current general population exposure limits for VX and GB, as published by the CDC (DHHS 1988), are 0.000003 mg/m3 for GB and 0.000003 mg/m3 for VX, resulting in a GB:VX ratio of 1.
The U.S. Department of the Army has prepared a health criteria document for VX (Reutter et al. 2000) in which exposure limits for VX are derived using the relative potency approach and the currently accepted exposure limits for agent GB (see Mioduszewski et al. [1998] and DHHS [1988], as detailed above). The exposure limits developed by Reutter et al. (2000) were based on minimal effect levels, and miosis was considered to be the most appropriate end point to use for comparison. Reutter et al. (2000) consider a ratio of 10 to be a protective estimate of the relative potency of miosis for agents GB and VX.
Embedded within the Army’s logic (USACHPPM 1998; Reutter et al. 2000) regarding the choice of 10 for the relative potency of VX:GB are two elements: downward adjustment to allow for the greater effect of VX on the eye, and upward adjustment to allow for the more rapid recovery (reversibility) of eye effects from VX exposure compared with recovery following GB exposure. These adjustments are based on the human intravenous studies of Kimura et al. (1960) and a calculational model based on ChE activity recovery (McNamara et al. 1973). Ocular exposure to VX vapor is estimated to cause eye effects at approximately one-twenty-fifth of the GB Ct required to attain the same effect. VX “ages” (irreversibly binds to cholinesterase) very slowly (t1/2 of 48 h) when compared with agent GB (t1/2 of 5 h) (Sidell and Groff 1974), and some spontaneous enzyme recovery occurs even in the absence of antidote. In general, recovery from the effects of VX vapor exposure is 4 times greater than that for agent GB (McNamara et al. 1973). Thus, an effective concentration of VX relative to GB is four-twenty-fifths, or 0.16, or approximately one-sixth. The ratio of 1:10 used by the Army in deriving exposure criteria for VX (Reutter et al. 2000) was to allow for a greater margin of safety.
4.3.4. Selection of Nerve Agent Potency Values for Use in Deriving AEGLs
Recent publication of science policy by the EPA Office of Pesticides to guide the use and application of data on cholinesterase inhibition (EPA 2000) recommends a weight-of-evidence approach for evaluating toxicity
end points for anticholinesterase compounds. This approach is consistent with that of Storm et al. (2000), who consider that the most defensible means of deriving (occupational) inhalation exposure limits for organophosphates should be based on weight-of-evidence. In the weight-of-evidence approach, first priority is given to clinical signs and physiological or behavioral effects in humans and animals followed by
-
Symptoms in humans.
-
Cental nervous system acetylcholinesterase inhibition.
-
Peripheral nervous system acetylcholinesterase inhibition.
-
Red blood cell acetylcholinesterase inhibition.
-
Plasma cholinesterase inhibition in humans and animals.
In general, the guidelines consider blood ChE inhibition to be an imperfect measure because of the need for individual baseline measurements for comparison and the fact that there is no fixed percentage of blood ChE activity change that can distinguish adverse from nonadverse effects (EPA 2000; Storm et al. 2000). A number of nerve agent exposure investigations have noted the poor association between blood (RBC and plasma) cholinesterase activity and anticholinesterase intoxication (Koelle 1994; Sidell 1992; Rubin and Goldberg 1957; Mioduszewski et al. 2002b). Circulating ChE activity does not parallel tissue ChE activity, and minimal blood ChE activity has been observed in association with normal tissue function (Sidell 1992). In the recent GB vapor exposure study of Mioduszewski et al. (2002b), “miosis was not correlated with, or even accompanied by, significant reduction of circulating AChE, BuChE, or CaE” as a consequence of GB vapor whole-body exposure to SD rats. These results further document the fact that miosis alone, and in the absence of signs such as ChE or CaE activity inhibition, is a local effect and reflects an exposure much less than that required to produce a systemic clinical effect. Thus, selection of the local effect of miosis as a critical AEGL end point allows a greater margin of protection against the potential for exposures that would generate systemic effects.
The findings of Mioduszewski et al. (2002b) are consistent with those for human volunteers exposed to GB vapor in the study of Rubin and Goldberg (1957).
Although RBC-ChE inhibition in the blood is an acceptable surrogate for central nervous system inhibition, plasma ChE is more labile and is considered a less reliable reflection of enzyme activity change at neuro-
effector sites (EPA 2000; Young et al. 1999; California Environmental Protection Agency 1998). In consequence, plasma-ChE activity inhibition is considered a biomarker of effect and is here rejected as a critical end point from which to develop a reliable estimate of relative potency. Relative RBC-AChE inhibition or an observable sign (i.e., miosis) in a test species are considered more appropriate end points for deriving relative potency estimates.
G Agents
Experimental determination of miosis (90% decrease in pupil area) in the eyes of albino rabbits directly exposed to a range of GB, GD, and GF vapor concentrations for periods of time ranging from 2–10 min to 5–6 hours (Callaway and Dirnhuber 1971) is a suitable study for estimating relative potency between the G-series nerve agents.
Although there are acknowledged analytical weaknesses in the protocol and data of Callaway and Dirnhuber (1971), this experiment is the only study found in the literature for which the same end point is measured in the same species following exposure to each of several G-series agents. There are no comparable human experimental data. The resulting potency ratios, estimated from cumulative exposure (Ct) values in the literature, are presented in Table 1–21.
For AEGL-1 and AEGL-2 effects, GB and GA are considered equipotent, and GD and GF are each considered equipotent to each other, and more potent than GB by a factor of 2.0 for miosis (see Table 1–22 and the review by Mioduszewski et al. [1998]). Thus, for an equivalent effective concentration (EC) for miosis
EC of GA (mg/m3)=EC of GB (mg/m3);
EC of GD (mg/m3)=EC of GB (mg/m3)÷2; and
EC of GF (mg/m3)=EC of GB (mg/m3)÷2.
For AEGL-3 effects, GB, GD, and GF are considered equipotent, while GA is considered less potent than agent GB by a factor of 2 (see Table 1–22 and the review by Mioduszewski et al. [1998]). As previously discussed in Section 3.1.3, a secondary and short-term GD vapor inhalation study of rat lethality was performed for GD dynamic chamber exposure times of ≤30 min (Aas et al. 1985). In addition, a recent study of GF vapor inhalation
lethality in male and female SD rats reported 24-h LC50 and LCt50 values for 3 durations of exposure (10, 60, and 240 min) (Anthony et al. 2002). The assumptions for agent GD and GF lethal potency relative to agent GB is generally supported by analysis of the Aas et al. (1985) and Anthony et al. (2002) rat lethality data. Thus, for lethal concentrations (LC) of the G agents
LC of GB (mg/m3)=LC of GD (mg/m3)=LC of GF (mg/m3); and
LC of GA (mg/m3)=LC of GB (mg/m3)×2.
Agent VX
The AEGL standing operating procedures (NRC 2001) state the following: “It is important to emphasize that only toxicity data obtained directly from a primary reference source is used as the basis for ‘key’ toxicity studies from which the AEGL values are derived. Additionally, all supporting data and information important to the derivations of an AEGL value is obtained solely from the primary references.” In the studies listed in Tables 1–23 and 1–24, the verifiable experimental data for humans, rats, and rabbits provide a range of VX:GB relative potencies (RPs) of 2.7 to 33.
Of the various animal data available for developing a GB:VX relative potency factor, the rabbit miosis study of Callaway and Dirnhuber (1971) offers advantages in that VX and GB vapor were tested by the same investigators using the same protocols and test species (see Table 1–23). Nevertheless, it is understood that agent measurements collected during the study were hampered by the limited capabilities and techniques for determining agent vapor concentrations in the early 1970s. Furthermore, when compared with current low-light digital methods, the protocols employed to measure rabbit miosis in Callaway and Dirnhuber (1971) are today considered semisubjective. In addition, the study documentation does not fully report miosis incidence in the agent-exposed rabbit population.
When making cross-compound comparisons for use in developing human exposure guidelines, there is a preference for human data sets. Three exposure routes have been examined in the analysis presented in Tables 1–23 and 1–24. Remarkable concordance (RP range of 2.7 to 6.5) is noted.
The flawed and nonverifiable study of Bramwell et al. (1963) was described in previous sections. The GB:VX ratio for inhalation ChE50
(which includes the VX Ct from Bramwell et al. [1963]) is not as credible as the comparable ratio derived from the well-conducted human oral and intra-arterial and intravenous exposure studies of Grob and Harvey (1958) and Sidell and Groff (1974). In addition, the oral exposure studies evaluate the effects of known agent doses (µg/kg). The GB:VX ratio resulting from the oral exposure studies is considered more protective (RP=4.3) than that derived from the direct systemic intra-arterial and intravenous studies (RP =2.7). Of the values derived from available human data, the GB:VX ratio calculated from oral dose exposures needed to achieve RBC-ChE50 is the most appropriate for the present application.
With no adjustments for differences in recovery or reversibility (aging), direct application of experimental data from human subjects for the ChE50 end point supports a GB:VX RP estimate approximating 4.3. With rounding, the GB:VX RP equals 4.0. Because the ChE50 end point is part of the continuum of response for these anticholinesterase compounds, it is consistent to apply the same RP for estimating AEGL-1, AEGL-2, and AEGL-3 values for agent VX.
Until additional data from well-conducted experimental studies are available, the current relative potency approach (RP=4) is reasonable, is supported by existing human experimental data, and meets the requirements of the standing operating procedures for developing AEGLs (NRC 2001).
4.4. Structure-Activity Relationships
Mager (1981) conducted a quantitative structure-activity analysis of organophosphorus compounds having anticholinesterase properties. The toxicity end point used in the analysis was the intraperitoneal LD50 value for the mouse. The calculated values were similar to the observed values. The observed values (−log LD50) were 0.70, 0.25,0.22, −0.01, and 2.30 for GB, GD, GA, GF, and VX respectively; the calculated values (−log LD50) were 0.43, 0.19, 0.11, 0.01, and 2.41 for GB, GD, GA, GF, and VX, respectively. In this analysis, agent GB was determined to be 3–4 times more toxic than GD and GA; however, it was noted by Mager (1981) that only the L-enantiomorph of GB was tested and that that isomer is 10–20 times more toxic than the D-isomer. The relative potency of the L-isomer is not necessarily reflective of the relative potency for different mixtures of GB isomers. The optical stability of the isomers can be maintained in the laboratory only under special storage conditions involving solvent solutions and
temperature control (Boter et al. 1966). Those same conditions are not maintained in munition storage or the field.
The toxicokinetics of VX stereoisomers [(±)−] have been examined and preliminary results documented in recent reports from the TNO Prins Maurits Laboratory (Benschop 1999; Benschop et al. 2000). Benschop and his colleagues studied the toxicokinetics of several VX stereoisomers [(±)−] in hairless guinea pigs (intravenous and percutaneous exposures) and marmosets (intravenous exposures only). Following an intravenous dose of 28 µg/kg (marmosets) or 56 µg/kg (guinea pigs), VX was found in the blood at toxicologically relevant levels after 6 h. Detoxification proceeded at a slower rate in marmosets than in guinea pigs. Desethyl-VX was found after incubation of VX in plasma of all species tested; however, because of its slow rate of formation, Benschop et al. (2000) concluded that it would be unlikely that this compound would be present at toxicologically relevant levels after administration of VX in vivo.
4.5. Other Relevant Information
4.5.1. Breathing Rates and Toxicity
For chemicals that are as acutely toxic as the nerve agents, and for which the concentration-response curves are expected to be very steep, a critical factor associated with the estimation of the potential inhalation toxicity is the breathing rate of individuals who might be exposed. In the case of the nerve agents, the vapor concentration producing a similar level of effect can be considerably different depending on the inhalation rate. In studies conducted on 125 human volunteers exposed to nerve agent GB, Oberst et al. (1968) demonstrated that the same end point (50% of RBC-ChE depression) could be attained with 2-min exposures to GB concentrations as high as 16.2–22.7 mg/m3 (average 20.7 mg/m3) in men breathing 5.6–8.4 L of air per minute; however, concentrations of only 3.9–4.53 mg GB/m3 (average 4.19 mg/m3) were needed to produce the same effect in exercising men breathing 41.5–64.9 L of air per minute. Oberst et al. (1968) reported that the retained dose (mg/kg) in both test groups was very similar.
Minute volumes up to about 25 L/min should cover most situations involving civilian populations; however, breathing rates may be higher under stressful evacuation conditions. Dosimetric adjustments based on
breathing rate are not normally considered by the AEGL protocol (NRC 2001, 57–62). In the case of the nerve agents, such a dosimetric adjustment would not be necessary for the AEGL-1 (and, to some extent, the AEGL-2) values, which are based on a local effects to the eye (miosis) as the most sensitive indicator of direct vapor exposure toxicity (see also Section 2.7 of this document). Changes in breathing rate would not affect this end point.
As is true for AEGL-3 determinations for agent GB, the composite UF applied in the determination of an AEGL-3 for agent VX does not include any adjustment for interspecies differences in dosimetry due to species differences in breathing rates, minute volumes, and body weight. For systemic poisons that are 100% absorbed, the minute volume-body weight normalization method results in a human equivalent concentration approximately 3.5 times greater than that for rats for the same end point (NRC 2001). However, for high exposure levels, such as those at the AEGL-3 level, absorption may be less than 100% and the estimated human equivalent exposure may be excessively high, resulting in an underestimation of the toxicity of the compound (NRC 2001). Another possible dosimetric adjustment is one using the inhaled dose against the body weight raised to the three-fourth power (EPA 1992). This approach is supported by the results of chronic toxicity studies but may not be relevant for acute lethality end points (NRC 2001). When applied to breathing rates, the adjustment predicts that rats would receive a dose about 4 times greater than humans. When this adjustment for breathing rate is combined with the adjustment for toxicity (EPA 1992), the two cancel each other out, and the conclusion is reached that equivalent exposures result in equivalent results in both rats and humans (NRC 2001). Use of the EPA RfC dosimetric method for systemically acting Category 2 gases (gases that are moderately water soluble and intermediate in reactivity and would therefore be distributed throughout the respiratory tract and readily absorbed into the blood stream) results in the prediction that humans would receive a dose ranging from 6,000 to 50,000 times greater than a rodent (depending on the species) for an equivalent exposure (NRC 2001). These numbers are not considered biologically reasonable or scientifically credible by the NRC (2001).
Given the uncertainties surrounding the issue of dosimetric adjustment across species, and the fact that no dosimetric correction would be the most conservative public-health approach, the NAC/AEGL committee decided that it would not use dosimetry corrections across species unless there were sufficient data on a specific chemical to support their use. Dosimetric
adjustments for nerve agents are complicated by the fact that species response to cholinesterase inhibitors are affected to an extent by levels of endogenous enzymes that bind with the inhibitors. Some of these detoxification pathways are present in rodents but not in humans (see Section 4.5.3). Therefore, a dosimetric adjustment alone may be insufficient to account for interspecies differences in response to nerve agents. In consequence, no dosimetric adjustment is required for these compounds, including nerve agents.
4.5.2. Delayed Neuropathy
Exposure to some organophosphate ChE inhibitors results in delayed neurotoxic effects (distal neuropathy, ataxia, and paralysis, which has been referred to as organophosphate-induced delayed neuropathy [OPIDN]) several days to several weeks after exposure. These effects, characterized by axon and myelin degeneration, are not associated with the inhibition of AChE and had been thought to be a consequence of the inhibition (and subsequent aging) of an enzyme known as neuropathy target esterase (NTE) (Abou-Donia 1993; Ehrich and Jortner 2002). As pentavalent phosphorous-containing compounds, the G agents and agent VX may also indirectly generate neurotoxic effects through a noncholinergic mechanism involving the kinase-mediated protein Ca 2+/calmodulin kinase II (Ca 2+/CaM kinase II) (de Wolff et al. 2002; Abou-Donia and Lapadula 1990). The Ca 2+/CaM kinase II protein becomes activated by OP-induced phosphorylation, and reacts (proteoloysis) with the cytoskeletal proteins found in neurofilaments to produce axonal swelling and degeneration in the large-diameter tracts of the spinal cord. The proteolysis and axonal degeneration are accompanied by accumulation of myelin debris, perturbed ionic gradients, and cellular edema (de Wolff et al. 2002).
For some OP compounds, delayed neuropathy can be induced in experimental animals at relatively low exposure levels, whereas for others the effect only is seen following exposure to supralethal doses, when the animal is protected by antidotes from acute cholinergic effects caused by ChE inhibition. In either case, there is evidence that a threshold exists below which delayed neuropathy does not occur. Studies reviewed by Somani et al. (1992) indicate that, in chickens (a species particularly susceptible to delayed neuropathic effects), a 70% decrease in brain NTE activity 24–48 h after exposure is related empirically to the subsequent development of
delayed neuropathy. According to Husain et al. (1995), a minimum of 45% NTE inhibition is associated with delayed neuropathy after multiple exposures.
G Agents
It has been shown that agents GB, GA, and GD inhibit NTE in vitro (Vranken et al. 1982). Supralethal doses of all three G agents produced delayed neuropathy in antidote-protected chickens in vivo (Gordon et al. 1983; Willems et al. 1984). Doses of 120×LD50 for agent GA resulted in mild neuropathic signs, and delayed neuropathy was observed at 120–150 ×LD50 for GD in a single surviving hen, but not at GD doses of 38×LD50. Delayed neuropathy was also observed in chickens administered agent GB at 30–60×LD50. In all of these challenge tests, nerve agents were administered to adult chickens previously protected from lethality by large antidote doses (Gordon et al. 1983; Willems et al. 1984). Because chickens are considered a sensitive species for this effect, it would appear that the potential for delayed neuropathy would be a concern only for those human individuals surviving a single exposure to concentrations greater than 30× LD50 for the G agents. There are also some delayed neuropathy data for animals receiving serial exposures.
Although not comparable to the single, one-time exposure assumption basic to AEGL determinations, the serial exposure data are useful to illustrate the high-concentrations of G agents necessary to induce delayed neuropathy. Signs indicative of delayed neuropathy have been observed in chickens receiving serial subcutaneous injections of one-tenth LD50 of agent GB on each of 10 successive days (a total of 1×LD50) (Husain et al. 1995) and in mice exposed to GB vapors at 5 mg/m3 (one-sixth LD50) for 20 min/d on each of 10 successive days (a total of 1.66×LD50; Husain et al. 1993). Rats receiving daily gavage doses of GB for 90 d at the maximum tolerated (nonlethal) dose (MTD) did not exhibit neuropathy (Bucci et al. 1991; Bucci and Parker 1992). Of the four G agents evaluated in this report, agent GB has the greatest potential for inducing delayed neuropathy after single, large exposures in excess of those necessary to cause death.
Another type of delayed neuropathy that has been associated with exposures to some organophosphate anticholinesterase agents is referred to as an “intermediate syndrome” (Senanayake and Karalliedde 1987; Brown and Brix 1998). Recovery of muscle function after a well-defined cholin-
ergic phase has been followed by reappearance of paralysis between 24 and 96 h postexposure (Baker and Sedgwick 1996; Senanayake and Karalliedde 1987). This delayed response has involved respiratory and proximal limb muscles, neck flexors, and motor cranial nerves (Senanayake and Karalliedde 1987). Paralytic symptoms have been documented to persist as long as 18 d, and some cases require ventilatory support (Senanayake and Karalliedde 1987). Intermediate syndrome is considered to be a reversible neuromuscular effect resulting from a nondepolarizing neuromuscular block. For the purposes of AEGL estimation, single fibre electromyographic changes observed in humans following agent GB vapor exposures are considered a subclinical and protective indication of syndrome onset (Baker and Sedgwick 1996).
Agent VX
No clinical or experimental evidence is available to indicate that VX causes delayed neuropathy in humans (see Munro et al. [1994] for review). Delayed neuropathy was not observed in three strains of antidote-protected chickens given a single subcutaneous dose of VX as large as 0.15 mg/kg (estimated to be 5–10 times the lethal level). Repeated intramuscular injections of VX (0.04 mg/kg/d and equivalent to 1.3×LD50 for this species per day, 3 d/wk for 30 d or 5 d/wk for 90 d) also did not produce any signs of OPIDN (Goldman et al. 1988; Wilson et al. 1988). For comparison, the LD50 value for an intramuscular injection of VX in chickens is about 0.03 mg/kg (Goldman et al. 1988).
In 90-d subchronic studies conducted on Sprague-Dawley rats, Goldman et al. (1988) found no incidence of tissue degeneration in brain, spinal cord, or peripheral nerves that could be associated with daily subcutaneous injections of VX at up to 4 µg/kg for 5 d/wk. However, in tests conducted on rats, Lenz et al. (1996) found that continuous subcutaneous exposure (via an osmotic pump) to 57 µg/kg/d (1.3 times the subcutaneous LD50 of 45 µg/kg) for 14 d resulted in 75–90% reduction in NTE in the brainstem, midbrain, and soleus muscle. Myopathy was seen in the soleus muscle of the test animals.
There is no clinical or experimental evidence that agent VX induces a delayed neuropathy of the “intermediate syndrome” type.
In summary, delayed neuropathy was not observed in three strains of antidote-protected chickens given a single subcutaneous dose of VX equiv-
alent to 5–10 times the lethal dose. Further, repeated supralethal intramuscular injections of VX (each injection being equivalent to 1.3 times the LD50) for either 3 d/wk over 30 d or 5 d/wk over 90 d produced no signs of organophosphate-induced delayed neuropathy (Goldman et al. 1988; Wilson et al. 1988). It is true that, in rats, continuous subcutaneous exposure via osmotic pump to a daily supralethal dose equivalent to 1.3 times the subcutaneous LD50 for 14 d is reported to generate myopathy in the soleus muscle (Lenz et al. 1996). Nevertheless, application of the Lenz et al. (1996) results seems appropriate only for individuals who survive exposures to lethal concentrations (which are well above final AEGL-3 values).
4.5.3. Intra- and Interspecies Variability in Esterase Activity and Response to Nerve Agents
Intraspecies Variability
Differences between individuals in blood cholinesterase activity may affect their susceptibility to the toxic effects of nerve agents. It has been shown that a small subpopulation of men and women possess genetically determined variants in their plasma ChE resulting in very low activity levels (Harris and Whittaker 1962; Lehmann and Liddell 1969) (see also Jokanović and Maksimović [1997] for review). Studies reviewed by Bonderman and Bonderman (1971) indicate that homozygous individuals have plasma-ChE activity reduced to less than 25% of the normal value. For heterozygous individuals, mean plasma-ChE activity is 64% of normal (range 28–114%) (Lehmann and Liddell 1969). Morgan (1989) reported that about 3% of individuals may have genetically determined low levels of plasma cholinesterase and may therefore be unusually sensitive to some anticholinesterase compounds. The frequency of the atypical homozygous phenotype is estimated at 0.025% (Hayes 1982).
Several studies indicate that plasma- and RBC-ChE activity is significantly lower in women than in men (Rider et al. 1957; Reinhold et al. 1953; Augustinsson 1955; Kaufman 1954; all as cited in Hayes 1982 and Wills 1972). Gender differences of 10% in plasma- or RBC-ChE activity have been reported (Wills 1972). Plasma-ChE activity may also be depressed in pregnant women and in individuals with liver disease, heart disease, allergic conditions, and neoplasms (Wills 1972). Such individuals may also be
at a greater risk from exposure to OP compounds. Although some investigators consider gender differences in plasma ChE activity to be confined to young persons (Shanor et al. 1961), data are available suggesting that adult females may be more susceptible to nerve agents than males. Yokoyama et al. (1998c) reported vestibulocerebellar effects (increased postural sway) in a small population of patients tested 6–8 mo after being exposed to agent GB (sarin) during the Tokyo subway terrorist attack. Both female and male patients (nine of each gender) had similar levels of plasma cholinesterase inhibition following the attack, and in both genders, postural sway was correlated with plasma-ChE activity; however, only in females was the increase in sway significantly greater than controls.
Females are here considered to be part of the susceptible subpopulation. In the Mioduszewski et al. (2000, 2001, 2002a,b) studies on rats, females were statistically more sensitive than males for the lethality end point. For agent GF, LCt50 values were generally lower in adult female rats than in adult male rats (Anthony et al. 2002). The observed increased susceptibility of females is taken into account by the intraspecies uncertainty factor (UF) for susceptible subpopulations in AEGL estimation. Additional gender comparisons found in the literature are included in Table 1–25.
While the biological role of plasma cholinesterase is at present unknown, it is acknowledged that plasma cholinesterase may likely serve as a buffer to offset the binding of nerve agents (and preferential binding of agent VX) to RBC-AChE. For example, pretreatment with human plasma cholinesterase has protected laboratory rats (Ashani et al. 1993) and monkeys (Raveh et al. 1997) from lethal and other acute toxic effects of VX exposure. Thus, variability in plasma cholinesterase activity is a parameter of concern for characterization of population susceptibility to nerve agent exposure.
As discussed in Section 4.1, A-esterases (paraoxonase/arylesterase) present in the blood and liver are also capable of hydrolyzing phosphate esters (Cashman et al. 1996; Davies et al. 1996; Wang et al. 1998; and Pond et al. 1995). Further, paraoxonase is known to be polymorphic in human populations, and individuals express widely different enzyme levels (see Section 4.1) (LaDu et al. 1986, as cited in Davies et al. 1996; Furlong et al. 1988, 1989; Kujiraoka et al. 2000).
Individuals expressing certain isomeric forms of the enzyme with low hydrolyzing activity are considered to be more susceptible to organophosphate anticholinesterase poisoning (Yamasaki et al. 1997). The polymor-
TABLE 1–25 Comparison of Acute (1–10 min) Lethal Inhalation Toxicity Values for G Agents for Male and Female Rats
|
|
Toxicity Value LCt50 (mg·min/m3) |
||||||||
|
|
Agent GB |
Agent GD |
Agent GF |
||||||
|
Species |
Females |
Males |
Ratio F:M |
Females |
Males |
Ratio F:M |
Females |
Males |
Ratio F:M |
|
Rat (5-min)a |
164 |
230 |
0.71 |
|
|||||
|
Rat (10-min)a |
181 |
226 |
0.80 |
|
|||||
|
Rat (1-min)b |
118 |
220 |
0.54 |
135 |
196 |
0.69 |
110 |
181 |
0.61 |
|
Rat (10-min)c |
184 |
231 |
0.80 |
|
253 |
368 |
0.69 |
||
|
Geometric Mean |
|
0.70 |
|
0.69 |
|
0.64 |
|||
|
Note: Entries from primary sources and known experimental data. aMioduszewski et al. (2000, 2001, 2002a). bCallaway and Blackburn (1954). cMioduszewski et al. (2001) for agent GB; Anthony et al. (2002) for agent GF; 24-h lethality. |
|||||||||
phic paraoxonase gene (PON1) has an important role in the detoxifying metabolism of nerve agents and OP insecticides. The PON1R192 paraoxonase isoform hydrolyzes agents sarin (GB) and soman (GD) slowly compared to the PON1Q192 isoform (Furlong et al. 2002; Davies et al. 1996). The human population can be organized into three PON1*192 genotypes: PON1Q192 homozygotes; heterozygotes; and PON1R192 homozygotes (Furlong et al. 2002; Allebrandt et al. 2002). Frequency distributions of the PON1*192 variants have been examined in ethnically diverse populations (Allebrandt et al. 2002). The allele expressing low activity for agent GB and agent GD hydrolysis (PON1R192) is significantly more frequent in African-Americans (sampled in Brazil and North America) and Asians (sampled in China, Japan, and Canada) than in individuals of Indo-European origin (sampled in East India, Turkey, Canada, Russia, Germany, North America, England, France, the Netherlands, Brazil). Nevertheless, Furlong et al. (2002) point out that “genotyping alone provides no information about PON1 levels, which can vary up to 13-fold between individuals” (homozygous for the low-activity allele) (see also Furlong et al. [1989] and Davies et al. [1996]).
Some investigators have previously considered that low levels of paraoxonase in newborns may contribute to the observed sensitivity of newborn rats to organophosphate insecticides (Benke and Murphy 1975; Burnett and Chambers 1994, as cited in Davies et al. 1996). A recent investigation (Chanda et al. 2002) presents in vitro and in vivo evidence that carboxylesterases “are critical for explaining age-related sensitivity” of rat pups to the OP insecticide chlorpyrifos. Further, the presence of low carboxylesterase activity, although important, does not sufficiently characterize the greater susceptibility of rat pups to neurotoxic effects of certain OP insecticides (Chanda et al. 2002).
Distribution of the low sarin-hydrolysis allele (PON1R192) appears to be somewhat ethnically related. The Japanese population has a higher frequency of the low sarin-hydrolysis isoform (allele frequency of 0.66) (Yamasaki et al. 1997) than Caucasian groups documented in the literature (0.24 to 0.31) (Serrato and Marian 1995; Ruiz et al. 1995; Antikainen et al. 1996).
Carboxylesterases are another enzyme group capable of binding with certain OP compounds and are present in human erythrocytes and monocytes as well as in human liver, kidney, lung, skin, and nasal tissue (Cashman et al. 1996; Chanda et al. 2002; Kaliste-Korhonen et al. 1996; Munger et al. 1991). As detailed in Section 4.1, the detoxification potential
of carboxylesterases is multifaceted and is an area requiring further experimental characterization.
Interspecies Variability
Differences exist among animal species in the types of esterases found in the blood as well as in their relative activity, and those differences may affect a species’ susceptibility to specific OP compounds. Baseline RBC-AChE activity in humans is slightly higher than that in monkeys but much higher than levels measured in sheep, rats, and other species (see Table 1– 26) (Ellin 1981). Species differences also exist in plasma cholinesterase levels. In humans, about 50% of the total blood ChE consists of plasma ChE (Osweiler et al. 1985). Plasma-ChE activity constitutes about 40% of the total blood ChE in dogs, about 30% in rats, and 20% in monkeys, but only 10% in sheep, horses, and cows (Wills 1972). Cohen et al. (1971) reported that plasma ChE activity in humans was 2 times greater than that in mice and 4 times greater than that in rats. Because of its more rapid turnover time when compared with RBC-AChE, plasma ChE may function as a repository and primary detoxification pathway for many OP compounds. This logic also applies to the carboxylesterases, discussed more fully in the earlier section on intraspecies variability.
It is acknowledged that the CaE profile in humans is not well known and that there are few data from which to characterize the contributions that CaE may make to human protection from anticholinesterase poisoning. Chanda et al. (2002) consider that full characterization of CaE amount, affinity, and IRE in human tissues will be necessary before accurate predictions can be made regarding CaE detoxification potential following anticholinesterase exposures to humans. Interspecies variation in response to some nerve agents may be accounted for largely by carboxylesterase binding (Somani et al. 1992). The G agents readily bind with carboxylesterases (Fonnum and Sterri 1981; Jokanović 1989; Clement 1994; Maxwell et al. 1987; Jokanović et al. 1996), and Maxwell (1992) demonstrated that endogeneous carboxylesterase activity provided rats with protection against the lethal effects of agents GA, GB, and GD, but not VX. In rodents, detoxification of G agents might be accounted for largely by carboxylesterases binding, and in the case of GD, binding appears to occur specifically with the most toxic stereoisomer of the agent (Cashman et al. 1996). Inhibition of carboxylesterase activity significantly increased the
TABLE 1–26 Baseline RBC-ChE Activity in Different Speciesa
acute toxicity of GD, GB, and GA to laboratory animals (Clement 1984; Jokanović 1989; Maxwell et al. 1987), and induction of carboxylesterase activity by pretreatment with phenobarbital substantially reduced the acute toxicity of GD and GA, but not GB (Clement 1983, 1984; Jokanović 1989). In contrast, selective inhibition of acetylcholinesterase or butyrylcholinesterase had no effect on the acute toxicity of GD to mice (Clement 1984). Because rodents have high levels of plasma carboxylesterases, they may be less susceptible to the G agents than humans.
Interspecies variability in response to nerve agents can be evaluated in terms of lethal and nonlethal end points.
G-series Agents
Available experimental agent GB LCt50 data for the monkey, dog, and rat are presented in Table 1–13. Data for rats (Table 1–25) show that females of these species are more susceptible than males. Comparisons of female rat LCt50 values with those of dogs and monkeys indicate that, in
terms of lethality, adult female SD rats are less susceptible to agent GB than adult dogs or monkeys by approximate factors of 2.0 to 4.0. Because rats are a CaE-rich species and dogs and monkeys were once thought to possess no plasma carboxylesterase (Augustinsson 1959), these differences in susceptibility may be due, in part, to species differences in CaE.
In the case of human lethality estimates, Bide et al. (1999) estimated GB inhalation toxicity values for humans by application of allometric model extrapolation from extensive experimental animal data. The calculated 2-min adult human LCt50 was approximately 31 mg·min/m3, equivalent to a 2-min LC50 of 15.5 mg/m3. In contrast, the 2-min LC50 for female SD rats is 52 mg/m3 (derived from a 2-min LCt50 of 104 mg/m3 as reported by Mioduszewski et al. [2000, 2001, 2002a]). Therefore, the ratio of the 2-min LC50 values for female rats and humans is approximately 3.4 (52/15.5). This comparison indicates that, when challenged with a lethal concentration of GB vapor, adult female SD rats are likely to be more resistant than adult humans by a factor between 3.0 and 3.5.
Few comparative studies have been conducted for nonlethal end points. However, some information is available on the miotogenic potency of agent GB in several species, including humans. In a study conducted by Johns (1952), 128 adult male volunteers were exposed to agent GB concentrations ranging from 0.05–3.0 mg/m3 for 2–20 min in an exposure chamber. Regression analysis of 150 observations, including 55 controls, indicated that the point at which a 50% decrease in pupil diameter would be attained was approximately 4.1 mg·min/m3, with 90% confidence limits of about 2.7 and 5.7 mg·min/m3. At the lowest test exposure level (0.05 mg/m3 for 20 min, equal to a Ct of 1 mg·min/m3) there was a mean maximum decrease in pupil diameter of 0.82 mm in the right eye and 1.00 mm in the left eye (total of eight observations) compared with 0.36 mm in the right eye and 0.33 mm in the left eye in controls (55 observations). Although mild miosis (defined by the author as a decrease of 1 to 2 mm in pupil diameter) was observed in some subjects at a Ct of 1.0 mg·min/m3, other subjects exposed to the same Ct exhibited mean maximal pupil decreases of <1 mm.
Callaway and Dirnhuber (1971) evaluated the “miotogenic potency” of GB vapor in humans and rabbits exposed to GB “under goggles” (62 miosis responses in 26 human volunteers and 43 miosis responses in 10 albino rabbits). Nevertheless, it is understood that agent measurements collected during this study were hampered by the limited capabilities and techniques for determining agent vapor concentrations in the early 1970s. Further, when compared with current low-light digital methods, the protocols em-
ployed to measure rabbit miosis in Callaway and Dirnhuber (1971) are considered semisubjective. In addition, the study documentation does not fully report miosis incidence in the agent-exposed rabbit population. An airstream of GB vapor (flow rate 0.1 L/min) was delivered to the space enclosed by each goggle. The unexposed pupil area of each eye was the baseline for pupil area decrement determinations for each eye. Exposure time periods ranged from 10 min to 5 h. Callaway and Dirnhuber (1971) reported a 50% decrement in pupil area in humans at a Ct of 3.13 mg·min/ m3 (with 95% confidence limits of 2.15–4.57 mg·min/m3) and in rabbits at a Ct of 2.33 mg·min/m3 (with 95% confidence limits of 1.65–3.31 mg·min/m3). A 90% decrement in pupil area occurred in humans at a Ct of 13.85 mg·min/m3 (with 95% confidence limits of 6.00–32.02 mg·min/m3) and in rabbits at a Ct of 7.68 mg·min/m3 (with 95% confidence limits of 4.90–19.50 mg-min/m3). Callaway and Dirnhuber (1971) reported that comparison of the values for 90% area decrement suggests that the human eye “may be somewhat less sensitive to GB than the rabbit eye in that it appears to be more difficult to produce a maximal miosis with low concentrations of GB vapor in humans than in rabbits, but this has not been validated statistically.”
Van Helden et al. (2001, 2002) exposed marmosets and guinea pigs (whole-body) to GB vapor concentrations at 0.05 to 150 µg/m3 for 5 h. In guinea pigs, the LOAEL for miosis (5% decrement in pupil size compared with controls; estimated to be equivalent to approximately 10% decrement in pupil area; p<0.05) was reported to be 1.8±0.3 mg·min/m3. In marmosets, the LOAEL for miosis (10% decrease in pupil size compared with controls; estimated at approximately 20% decrement in pupil area; p< 0.05) was reported to be 2.5±0.8 mg·min/m3. Van Helden et al. (2001, 2002) reported that the guinea pig and marmoset LOAEL values were not significantly different.
Mioduszewski et al. (2002b) exposed young adult male and female SD rats (whole-body) to a range of GB vapor concentrations (0.01–0.48 mg/m3) for three time durations (10, 60, and 240 min). The results (ECt50 for miosis) are summarized in Table 1–15.
The results of the Callaway and Dirnhuber (1971), van Helden et al. (2001, 2002) and Mioduszewski et al. (2002b) studies suggest that, in terms of miosis, the response of mammalian eyes appears to be quantitatively very similar across species (including humans).
Agent VX
Interspecies differences in susceptibility to VX have also been reported. In subacute inhalation studies conducted on rats, mice, guinea pigs, and rabbits (exposures were 6 h/d, 5 d/wk, for 2 wk), Crook et al. (1983) determined from calculated LCt50 values that mice (LCt50=0.9 mg·min/m3) were more sensitive to VX than rats (LCt50=24.9 mg·min/m3), and rats were more sensitive than guinea pigs (LCt50=238.6 mg·min/m3). Rabbits were more resistant than guinea pigs.
The detoxification potential of endogenous carboxylesterase to protect against the lethal effects of nerve agent exposure was tested by Maxwell (1992) in (male) SD rats. Nerve agents GA, GB, GD, or VX in isotonic saline were administered by subcutaneous injection. The degree of in vivo CaE inhibition was measured in the plasma, lung, and liver of exposed rats. In vivo protection provided by endogenous CaE was estimated by comparing differences in LD50 following nerve agent exposures to rats with inhibited CaE activity (following administration of the probe, 2-(O-cresyl)-4H-1,3,2-benzodioxaphosphorin-2-oxide) versus nerve agent exposures to rats without inhibited CaE activity. Maxwell determined that endogenous CaE in the rat provided no significant protection against in vivo lethal exposures to nerve agent VX under the experimental protocol employed; furthermore, Maxwell concluded that “CaE detoxification does not appear to be important” against exposures to lethal concentrations of agent VX.
In conclusion, the SD rat in vivo experimental results of Maxwell (1992) indicate that endogenous CaE in this species confers no protection against lethal exposures of nerve agent VX. Thus, rats exposed to VX should not be considered more robust than other species possessing a different CaE profile (e.g., humans).
4.5.4. Unique Physicochemical Properties
As discussed by Somani et al. (1992), organophosphate nerve agents consist of stereoisomers resulting from the presence of a chiral phosphorus atom in the molecule. Limited data (mainly from studies with agents GD and GB) indicate that the stereoisomers may differ considerably in their toxic potency. In general, most toxicity studies have utilized racemic mixtures of these agents.
The volatility of agent VX is 10.5 mg/m3 at 25 °C (DA 1990). The Department of the Army considers agent VX to be “about 2,000 times less volatile than [nerve agent] GB” (DA 1990). A volatility of 3.0±0.5 mg/m3 was reported for a temperature of 25 °C in tests in which the vapor phase was in equilibrium with the aerosol phase (Frostling 1974).
4.5.5. Concurrent Exposure Issues
Two issues might be of concern: (1) simultaneous exposure to multiple nerve agents or related organophosphate compounds, and (2) simultaneous exposure through multiple exposure pathways.
Multiple Exposures to Similar Chemicals
Because of their similarity in mechanism of action, it can be expected that the toxic effects of the nerve agents would be additive. Clement (1994) and Luo and Liang (1997) reported that the toxicity of agents GB and GF were basically additive when administered together by subcutaneous injection to mice. Nevertheless, the various nerve agents are deliberately stored in separate locations and will undergo demilitarization and destruction at separate times. Furthermore, the agents are deliberately stored and secured separately prior to destruction. Thus, the chance for the release of more than one agent while under storage or during the disposal process is minimal.
The acute toxicity for numerous organophosphate insecticides in current use is identical to that of the nerve agents (i.e., initiated by cholinesterase inhibition). The vapor concentrations of insecticides causing acute toxic effects are considerably higher than nerve agent vapor concentrations producing the same end points. Information on lethality levels for some organophosphate insecticides, listed on the Registry of Toxic Effects of Chemical Substances (RTECS) (NIOSH 1999), are shown in Table 1–27. The most acutely toxic of the insecticides listed in Table 1–27 is methyl parathion, for which a 4-h LC50 value of 34 mg/m3 has been reported for rats. In comparison, the 10-min LC50 values for rats for agents GA, GB, and GD are 45 mg/m3, 22 mg/m3, and 23 mg/m3, respectively. Using a direct linear extrapolation, the corresponding 4-h LC50 values can be estimated to be 1.9 mg/m3 for GA, 0.92 mg/m3 for GB, and 0.95 mg/m3 for GD,
TABLE 1–27 Inhalation Lethality Values for Organophosphate Pesticides
|
Chemical |
Species |
Exposure Time (h) |
LC50 (mg/m3) |
Reference |
|
Tetraethyl dithiopyrophosphate |
Rat (f) Rat (m) |
4 4 |
38 59 |
Kimmerle and Klimmer 1974 |
|
Methyl parathion |
Rat |
4 |
34 |
EGESAQ 1980 |
|
Parathion |
Rat |
4 |
84 |
AMRL 1977 |
|
Phosmet |
Rat |
4 |
54 |
Izmerov et al. 1982 |
|
Pirimiphos-methyl |
Rat |
4 |
>150 |
Kagan et al. 1983 |
|
Methamidophos |
Rat |
4 |
162 |
Hartley and Kidd 1983–1986 |
|
Disulfoton |
Rat |
NA |
200 |
Klimmer 1971 |
|
Ethion |
Rat |
NA |
864 |
FCH 1991 |
|
Naled |
Mouse |
6 |
>1,500 |
Hartley and Kidd 1983–1986 |
|
Fonophos |
Rat |
1 |
1,900 |
Hartley and Kidd 1983–1986 |
|
Acephate |
Mouse |
5 |
>2,200 |
Berteau and Chiles 1978 |
|
Abbreviation: NA, not available. |
||||
or approximately 15- to 30-fold more toxic than methyl parathion. The toxicity of VX is considerably greater; the 10-min LCt50 value in mice is only 4 mg·min/m3. Thus, the organophosphate insecticides are considerably less potent than nerve agents.
Comparison of the large differences in LC50 values between the G agents, agent VX, and commercial insecticides illustrates that the effects of concurrent exposure would be dominated by the more potent nerve agents. In consequence, concurrent exposure is of far less significance than exposure to each nerve agent alone.
Multiple Exposure Through Different Exposure Pathways
Nerve agents can be absorbed through the skin as well as through the respiratory tract. The extent of skin absorption of a vapor depends on the physicochemical characteristics of the agent and the presence of moisture on the skin. A comparison of the relative toxicity of the nerve agent vapors through inhalation and skin absorption can be made by evaluating the reported LCt values for each pathway.
In studies on human subjects, Freeman et al. (1954) reported that doses up to 400 mg of liquid agent GA applied to the skin of the forearm (5 mg/kg) and allowed to evaporate to dryness caused no clinical signs but resulted in a 30% decrease in RBC-ChE activity. The degree of liquid versus vapor absorption through the skin was not measured in the Freeman et al. (1954) study.
Although the human exposure study of Bramwell et al. (1963) might have provided potential percutaneous ECt50 values for severe or threshold effects in humans, the study is flawed by a defective protocol (no reliable estimate of agent exposure to the subjects; see discussion in Section 2.2.2).
Information on the percutaneous toxicity of the G series nerve agents and agent VX was reviewed by a subcommittee of the National Research Council Committee on Toxicology in Review of Acute Human-Toxicity Estimates for Selected Chemical-Warfare Agents (NRC 1997). Following evaluation of relevant human and animal studies, the NRC summarized human toxicity estimates. Differences between ECt50 values for mild effects resulting from vapor inhalation exposures (GA and GB, 0.5 mg·min/m3; GD and GF, 0.2 mg·min/m3) and the ECt50 values for threshold effects resulting from percutaneous vapor exposures (GA, 2,000 mg·min/m3; GB, 1,200 mg·min/m3; GD and GF, 300 mg·min/m3) are all in excess of 102. The NRC
(1997) considered the GD and GF percutaneous vapor values to be in need of further research and the inhalation vapor estimates to be “low.” Nevertheless, the NRC recommendations suggest that, for mild effects, the vapor inhalation pathway is several orders of magnitude (approximately 103) more effective than the percutaneous vapor pathway. There are similar order-of-magnitude differences for severe effects (NRC 1997).
In Chapter 6 of NRC (1997), “Review of Acute Human-Toxicity Estimates for VX,” relevant human and animal studies are summarized. The NRC reported percutaneous vapor VX LCt50s of 11.5 mg·min/m3 for mice and 100–150 mg·min/m3 for clipped goats (body-only) (Koon et al. 1960). In comparison, a whole-body (inhalation and percutaneous) vapor LCt50 of 9.2 mg·min/m3 was reported for goats; the comparable value for mice was 4.0 mg·min/m3 (Koon et al. 1960). It appears that VX vapor exposures involving inhalation are more effective in causing lethality than percutaneous vapor exposures alone; the difference in effectiveness for the lethality end point is approximately 3 for mice and between 11 and approximately 16 for goats.
Human toxicity estimates listed by NRC (1997) include a VX ECt50 value of 0.09 mg·min/m3 for mild effects resulting from vapor inhalation exposures and an ECt50 value of 10 mg·min/m3 for threshold effects resulting from percutaneous vapor exposures. The latter value was considered by NRC to have an associated low degree of confidence, and further research was recommended. However, these recommendations suggest that for mild effects, the vapor inhalation pathway is several orders of magnitude more effective than the percutaneous vapor pathway. For severe effects, the NRC (1997) presented an ECt50 value of VX at 10 mg·min/m3 for vapor inhalation exposures and an ECt50 value of 25 mg·min/m3 for percutaneous vapor exposures as interim values (low confidence, further research recommended), indicating that for this end point, the inhalation pathway is 2.5 times as effective as the percutaneous pathway for the severe effects ECt50.
The issue of differential toxicity associated with physical states of the same compound has been illustrated in the case of certain industrial compounds; an example is n-butyl acetate (OXO Process Panel 1995). n-butyl acetate has commercial use in fine furniture manufacture as a vehicle in spray finish application. In studies of rats exposed to n-butyl acetate atmospheres generated by either evaporation (vapor exposure) or “atomization” (submicron aerosol exposure), lethality was profoundly different and almost entirely dependent on the physical state of n-butyl acetate to which
rats were exposed. Irritation and hypoactivity were noted in animals exposed to n-butyl acetate as the vapor (6,800 ppm for 4 ho), but the animals recovered within 1 d and went on to gain weight during the 14-d recovery period. Exposure to comparatively low concentrations (approximately 150 ppm v/v) of n-butyl acetate as the aerosol resulted in severe lung damage and mortality. During industrial spray application of finishes in wood furniture manufacture, aerosol particles of n-butyl acetate are extremely short-lived, and measurement of worker breathing zone exposure found only the vapor (OXO Process Panel 1995). In consequence, it was determined that toxicity information on the vapor form of n-butyl acetate was more appropriate than information on the aerosol form in establishing n-butyl acetate occupational exposure limits (ACGIH 1996).
The above examples support the need for research characterizing the emissions profile expected during VX release. Parameters essential to accurate quantification by modern methods and protocols include the following: generation and yield of vapors versus aerosols; rate of aerosol conversion to the vapor; atmospheric degradation half-times; deposition rates; and rates of degradation as influenced by humidity, temperature, and ultraviolet light. Until these parameters are more fully characterized in determinations of differential toxicity of VX vapor and aerosols, AEGL determinations will necessarily be based on the assumption of exposure to VX as the vapor.
It is acknowledged that droplets and/or aerosols may be present during certain release events. Nevertheless, the community emergency preparedness need for guidelines is presently focused on vapor exposure. There is interest and potential for developing a comparable guideline for exposures to nerve agent aerosols at some future time.
4.5.6. Critical Effect End Point
Blood cholinesterase levels are too variable to use as critical effect end points in deriving AEGLs for the nerve agents. Although there are some estimates of enzyme inhibition levels that are associated with acute effects, individual response will vary not only with baseline ChE levels but also with certain characteristics of physiological status (e.g., anemia, liver dysfunction or infection, pregnancy, etc.) which are transient and thus result in dynamic individual susceptibility through time (Lessenger and Reese 1999; Bakerman 1984; Rider et al. 1957; Ciliberto and Marx 1998; Haboubi and Thurnham 1986; Phillips 1995). Local effects on the eyes
(miosis) and upper respiratory tract (rhinorrhea) are more sensitive and consistent indicators of exposure. A number of investigators consider both miosis and rhinorrhea to be early signs of exposure to cholinesterase inhibitors. The presence of rhinorrhea can be indicative of inhalation exposure and/or development of systemic effects, although miosis alone in the absence of other signs or symptoms is a local effect to the pupillary muscles of the eye. In consequence, the presence of miosis is considered an appropriately sensitive indicator of direct vapor exposure and has the added advantage of being readily recognized and quantifiable.
The logic of not using ChE depression as a critical effect is consistent with the science policy of EPA’s Office of Pesticide Programs (EPA 2000). According to EPA, there is no predetermined percentage of enzyme activity inhibition that separates adverse from nonadverse effects. The weight-of-evidence analysis advocated by this science policy document for selection of critical effects considers first the “clinical signs and other physiological and behavioral effects in humans and animals,” after which “symptoms in humans” are considered, and then changes in blood cholinesterase. The recommended sequence is as follows:
-
Clinical signs and other physiological and behavioral effects in humans and animals.
-
Symptoms in humans.
-
Cental nervous system acetylcholinesterase inhibition.
-
Peripheral nervous system acetylcholinesterase inhibition.
-
Red blood cell acetylcholinesterase inhibition.
-
Plasma cholinesterase inhibition in humans and animals.
Miosis can be observed before significant ChE depression can be measured; setting AEGL values on the basis of miosis (a local effect) will protect against significant ChE activity depression (a systemic effect).
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
G-Series Agents
Candidate human data from which to develop AEGL-1 values for the G agents are available in the studies of Harvey (1952) and Johns (1952),
the study of McKee and Woolcott (1949), and the study of Baker and Sedgwick (1996). In the study described by Harvey (1952) and Johns (1952), several male volunteers who were exposed to GB at 0.05 mg/m3 for 20 min experienced mild effects including miosis, rhinorrhea, and tightness in the chest (see Tables 1–7 and 1–8). Miosis and rhinorrhea were clinically observed. Harvey (1952) and Johns (1952) quantified miosis as the maximal decrease in pupil diameter measured with a modified fixed focus prism telescope in a clinical setting. In the study of McKee and Woolcott (1949), five male subjects were exposed to GB at 0.062 mg/m3 for 20 min/d without any signs of clinical effects until day 4, when miosis was observed. A single exposure to GB at 0.6 mg/m3 for 1 min or 0.06 mg/m3 for 40 min resulted in miosis and slight tightness of the chest. In the Baker and Sedgwick (1996) study, eight healthy male servicemen who were exposed to GB at 0.5 mg/m3 for 30 min developed miosis and several also exhibited photophobia and dyspnea. In addition, RBC-ChE activity was inhibited to approximately 60% of individual baseline at 3 h and 3 d postexposure, and small but measurable changes occurred in single fibre electromyography (SFEMG) of the forearm. The latter, which were detectable in the lab between 4 and 15 mo postexposure, were not considered clinically significant by Baker and Sedgwick. The SFEMG changes were not detectable after 15–30 mo.
In tests on 125 volunteers, Oberst et al. (1968) observed no signs or symptoms of toxicity in resting men (breathing rate about 7 L/min) following 2-min exposures to an average GB concentration of 20.7 mg/m3 or in exercising men (breathing rate 50 L/min) following 2-min exposures to an average GB concentration of 4.19 mg/m3. Linear extrapolation of the lower concentration results in a 30-min exposure to GB at 0.27 mg/m3, less than the exposure used in the Baker and Sedgwick (1996) study.
The above studies do not agree in identifying the concentration of GB at which effects first appear, and there are even inconsistencies within some of the studies. One possibility for the conflicting results is human variability, since few subjects were used in each study. Another possibility is that the analytical measurements were not accurate. There are no details on analytical procedures in the study of Harvey (1952) or Johns (1952), where it is stated that “known concentrations” were used. The Baker and Sedgwick (1996) study provides a description of the analytical method and indicates that exposure concentrations were verified before and after exposure.
Agent VX
Clearly defined human concentration-response data for low-level inhalation exposures to agent VX are not available. The human toxicity studies that have been conducted with VX are not considered adequate for deriving exposure limits. The study conducted by Bramwell et al. (1963) suggests that an inhalation dose of about 8 µg/kg causes a 50% ChE depression as well as signs of toxicity including miosis, rhinorrhea, and nausea; however, this suspect study involved multiple exposures to the same individuals, short exposure durations (maximum of 7 min), and an experimental protocol (open tunnel rather than exposure chamber) in which the individual exposures to VX may have varied. The Bramwell et al. (1963) study is therefore not considered credible because of its seriously flawed exposure protocol.
Other experimental data indicate that inhalation exposures equivalent to internal doses in the range of 0.01–0.13 µg/kg result in mild signs of toxicity and no change in ChE (Koon et al. 1959). As extrapolated from historical animal data, the human ECt50 for miosis has been estimated at 0.09 mg·min/m3 (Reutter and Wade 1994).
5.2. Summary of Animal Data Relevant to AEGL-1
G-Series Agents
Acute inhalation toxicity data are available for agents GA and GB for several animal species. In most cases, the studies were designed to estimate LCt50 values, and they are not directly suitable for application to an AEGL-1 estimation. Several studies, however, have identified minimal effect levels. Van Helden et al. (2001, 2002) reported LOAELs for miosis of 2.5 ±0.8 mg·min/m3 for marmosets and 1.8±0.3 mg·min/m3 for guinea pigs exposed to agent GB for 5 h. The LOAEL values for miosis in the two species were not statistically different (van Helden et al. 2001, 2002). Mioduszewski et al. (2002b) reported ECt50 values for miosis in male and female SD rats exposed (whole-body) to GB vapor for time durations of 10, 60, and 240 min. Miosis was defined by the authors as “post-exposure pupil diameter 50% or less of the pre-exposure pupil diameter.” The ECt50 determinations for both genders are summarized in Table 1–21.
In studies conducted by Harris et al. (1953), dogs were able to tolerate daily exposures to GB at an average Ct of 10.5 mg·min/m3 (equivalent to an average concentration of 0.53 mg/m3 for 20 min), 5 d/wk, for 2 mo. The only reported clinical sign was miosis, which appeared with each exposure but disappeared before the next exposure. However, when each daily exposure was increased to 15 mg·min/m3, toxic signs (body tremors, dyspnea, loss of muscle control, convulsions) occurred within 7–10 d and several dogs died. Henderson et al. (2000, 2001, 2002), Conn et al. (2002), and Kalra et al. (2002) exposed male F344 rats to GB at 0.2 mg/m3 or 0.4 mg/m3 (nose-only) for 1 h/d for 1 d, 5 d, or 10 d, with sacrifices at 1 d after exposure and at 1 mo after exposure. Henderson et al., Conn et al., and Kalra et al. reported that there were no overt signs or symptoms of neurotoxicity (tremors) under non-heat stress conditions at either GB exposure concentration and that single GB exposures “did not alter body weight, breathing patterns, routine brain histopathology, or apoptosis in brain cells.”
Agent VX
There are no single exposure studies available for deriving AEGL-1 values for VX. In a nonverifiable study, Crook et al. (1983) reported no signs of toxicity except miosis in rats, mice, guinea pigs, or rabbits exposed to VX vapor concentrations up to 0.0002 mg/m3 for 6 h/d, 5 d/wk, for 2 wk. A test concentration of 0.004 mg/m3 resulted in rat and mice mortality. The available animal data indicate that VX does not cause reproductive or developmental toxicity, and there is no evidence suggesting that VX is genotoxic or carcinogenic.
In an examination of miotogenic potency, Callaway and Dirnhuber (1971) consider that agent VX is an order of magnitude more effective than agents GB or GD at producing miosis in the eyes of male and female albino rabbits.
5.3. Derivation of AEGL-1 for Agent GB
The estimation of interim AEGL-1 values relied on the Harvey (1952) study (66 Fed. Reg. 21940 [2001]). Of 14 individuals exposed to the lowest concentration for the longest exposure time (0.05 mg/m3 for 20 min), the
following signs and symptoms were reported: two headaches, two eye pain, three rhinorrhea, one tightness in the chest, one cramps, one nausea, and two malaise. Of human studies available, this analysis gave the lowest LOAEL of 0.05 mg/m3 for a 20-min exposure and was chosen as the basis for deriving the interim AEGL-1 values. The miosis effects data of Johns (1952) were considered as supportive. The subjects were male “normal human volunteer” service personnel between the ages of 22 and 59 and under clinical supervision during the periods of exposure as well as for postexposure periods of several months. Derivation of the interim values is detailed in Appendix A.
The final analysis relies on the Mioduszewski et al. (2002b) study of miosis induction to young adult SD rats as the basis for AEGL-1 estimation, with retention of van Helden et al. (2001, 2002; marmosets), Harvey (1952) (humans) and Johns (1952) (humans) as secondary and supportive studies.
The selection of miosis induction as the basis for deriving final AEGL-1 values is supported by the evaluation of a U.S. Surgeon General’s review panel on agent exposure limits convened by the Chemical Demilitarization Branch of the National Center for Environmental Health of the Centers for Disease Control and Prevention (CDC) (67 Fed. Reg. 894 [2002]; DHHS 2002). Although the CDC has not yet finalized its position, the review panel generally concluded that cholinesterase activity depression is too variable for application as a critical effect in the estimation of nerve agent exposure limits and that miosis is an appropriate and readily quantified critical effect.
The AEGL-1 values for agent GB were derived from a well-conducted study on adult female Sprague-Dawley rats exposed whole-body in a dynamic airflow chamber to a range of GB vapor concentrations (0.01–0.48 mg/m3) over three time durations (10 min, 60 min, or 240 min) (total of 283 agent-exposed rats, of which 142 were female and 141 were male) (Mioduszewski et al. 2002b). With the inclusion of range-finding experiments and controls (N=130), a total of 423 rats were employed in this well-conducted study documenting highly credible protocols for GB vapor generation and measurement. A sufficient number of individual animals were exposed at each interval (10 min, 52 female SD rats; 60 min, 35 female SD rats; 240 min, 55 female SD rats). Analysis of rat pupil diameters assessed pre- and postexposure allowed generation of EC50 determinations for miosis (defined as a postexposure pupil diameter of 50% or less of the preexposure diameter in 50% of the exposed population). Blood samples
collected from tail vein and heart at 60 min and 7 d postexposure indicated no change from preexposure baseline in monitored blood RBC-ChE, butyrylcholinesterase (BuChE) or carboxylesterase. No other clinical signs were evident throughout the duration of the study. Gender differences (females more susceptible) were statistically significant at 10 min (p= 0.014) and 240 min (p=0.023) but not at 60 min (p=0.054). As the female rat appears to be more susceptible than the male for at least two of the AEGL exposure durations of interest, the AEGL-1 estimations are calculated from the female data set. This data set selection for the most susceptible gender will provide a more protective estimation of AEGL-1. This is a well-defined animal end point in a susceptible gender and is transient, reversible, and nondisabling. (Further details of this study are provided in Section 3.2.3.)
Data from the GB vapor study of nonhuman primates (marmosets; 5 h exposures to GB vapor concentrations of 0.05 to 150 µg/m3) (van Helden et al. 2001, 2002) and human volunteers (minimal and reversible effects of miosis, rhinorrhea, headache, etc., after a 20-min exposure to a GB vapor concentration of 0.05 mg/m3) (Harvey 1952; Johns 1952) are considered secondary and supportive. The human data of Harvey (1952) and Johns (1952) indicate that some adult humans exposed to concentrations within the exposure range tested by Mioduszewski et al. (2002b) would experience some discomfort (headache, eye pain, nausea, etc.) in addition to miosis corresponding to ≤50% pupil area decrement, but no disability (see definition of AEGL-1 provided in NRC [2001]). The studies of Harvey (1952) and Johns (1952) also show that miosis is transient and reversible, with reversibility occurring within hours to days (depending on degree of miosis). This is consistent with other human data documenting miosis after nerve agent vapor exposures. In consequence, with the knowledge that the EC50 exhibited by rats in the study of Mioduszewski et al. (2002b) is also transient and reversible, the determination is made that EC50 for miosis in female SD rats is an appropriate end point for estimating AEGL-1 values (Mioduszewski et al. 2002b).
Because exposure-response data were unavailable for all of the AEGL-specific exposure durations, temporal extrapolation was used in the development of AEGL values for the AEGL-specific time periods. The concentration-exposure time relationship for many systemically acting vapors and gases may be described by Cn×t=k, where the exponent n ranges from 0.8 to 3.5. The temporal extrapolation used here is based on a log-log linear regression of the LC01 lethality of GB to female Sprague-
Dawley rats (Mioduszewski et al. 2000, 2001, 2002a) and a log-log linear regression of female SD rat miosis data following GB vapor exposure for time durations of 10 min to 240 min (Mioduszewski et al. 2002b). Regression analysis of the LC01 values yields an n value of 1.93 with an r2 of 0.9948, while regression analysis of the miosis data yields an n value of 2.00 with an r2 of 0.4335 (24 data points; see Appendix B). Given that all mammalian toxicity end points observed in the data set for all nerve agents represent different points on the response continuum for anticholinesterase exposure, and that the mechanism of acute mammalian toxicity (cholinesterase inhibition) is the same for all nerve agents, the experimentally derived n=2 from the rat lethality and miosis data sets is used as the scaling function for all the AEGL derivations rather than a default value. An n of 1.16 (r2=0.6704) was calculated for comparison using other data (human volunteer) and other end points (e.g., GB-induced miosis in humans; see Appendix B). However, due to uncertainties associated with some of the exposure measurements in these earlier studies, the Mioduszewki et al. rat data were determined to be the best source of an estimate for n.
Derivation of AEGL-1 Values Using Animal Data
AEGL-1 values can be derived from the data set presented by van Helden et al. (2001, 2002) for GB-induced miosis in marmosets exposed to agent GB vapor for 5 h, as well as the data set presented by Mioduszewski et al. (2002b) for rats exposed to GB vapor for 10, 60, and 240 min.
Van Helden et al. (2001, 2002) reported a LOAEL for threshold miosis of 2.5±0.8 mg·min/m3, and considered miosis to be significantly different (p<0.05) from controls when a 10% decrease in marmoset pupil size was observed (estimated to be equivalent to an approximate 20% decrement in pupil area). Van Helden et al. (2001, 2002) also reported that there was no significant difference between the LOAEL for miosis in marmosets and in guinea pigs. The EPA IRIS database and the NLM Hazardous Substances Databank were searched for additional information on the miotogenic response of marmosets to cholinesterase inhibitors, but no relevant data were found.
The recent miosis and lethality data of Mioduszewski et al. (2000, 2001, 2002a,b) in rats have been subjected to regression analysis (see Appendix B). In consequence, the nonlethality (miosis) and lethality data of Mioduszewski and his colleagues are determined to be the best source of
an estimate for the n value for GB response. The Mioduszewki et al. (2000, 2001, 2002a,b) data sets are robust and compound-specific for the most completely characterized G-series nerve agent, agent GB. As outlined earlier, the mechanism of mammalian toxicity for nerve agents is known, and all end points observed in human and animal studies represent a response continuum to anticholinesterase exposure. Accordingly, it is valid to apply an n value derived from compound-specific miosis and lethality data to time scaling for nonlethal as well as lethal effects. This position is consistent with that of the recently published science policy of the EPA Office of Pesticide Programs (EPA 2000). Furthermore, this approach is preferable to the use of default values.
For AEGL-1 derivation, an interspecies uncertainty factor (UF) of 1 and an intraspecies UF of 10 were used, resulting in a composite UF of 10. To estimate an interspecies UF, miosis data for a number of species were compared. Van Helden et al. (2001, 2002) exposed marmosets and guinea pigs (whole-body) to GB vapor to estimate a LOAEL for miosis in both species. They determined that there was no significant difference between guinea pigs and marmosets at the 5% level. Contact with leading investigators in the field (H.van Helden, Pulmonary and CNS Pharmacology Lab, TNO, the Netherlands, personal communication; S.Tattersall, Biomedical Sciences Division at Porton Down, United Kingdom, personal communication) was performed to determine availability of experimental data characterizing miosis following nerve agent vapor exposure to mammals. Dr. Tattersall pointed out that Porton Down has not performed systematic measurements of miosis in recent years, and that the only other extant report of relevant data was Callaway and Dirnhuber (1971), cited in this document. These investigators have independently concluded that the miotogenic response of mammalian eyes to agent GB vapor exposure is quantitatively similar across species, including standard laboratory animals (rabbits and guinea pigs), nonhuman primates (marmosets), and humans (please see Table 1–21). In consequence, the interspecies UF for the AEGL-1 end point of miosis in young adult female SD rats is set equal to 1.
The intraspecies UF of 10 used in the derivation of the AEGL-1 is based on the known polymorphic variation in human cholinesterase and carboxylesterase activity that may make some individuals susceptible to the effects of cholinesterase inhibitors such as nerve agents. A factor of 10 was applied for protection of susceptible populations.
The database for agent GB is reasonably complete. Strong arguments for not incorporating an additional modifying factor include the following:
-
Data are available for multiple species.
-
Data characterizing both lethal and nonlethal end points have been used in the analysis; the end points possess exposure-response data.
-
The mechanism of toxicity is known.
-
The n value is derived from experimental data and is not the default.
-
There are no uncertainties regarding reproductive and developmental effects or issues of carcinogenicity.
In consequence, no modifying factor was used in the estimation of AEGL-1 values.
For comparison, from the marmoset data of van Helden et al. (2001, 2002), k was derived using a composite UF of 10.
([0.0083 mg/m3]/10)2×(5 h)=k;
k=3.4×10−6 mg/m3×h.
From the experimental data of Mioduszewski et al. (2002b), k was derived as follows for the 10-min to 30-min extrapolation:
([0.068 mg/m3]/10)2×(10/60) h=k;
k=7.7×10−6 mg/m3×h.
For the 4-h to 8-h extrapolation, k was derived as
([0.012 mg/m3]/10)2×4 h=k;
k=5.8×10−6 mg/m3×h.
The Interim AEGL-1 estimates and the estimates from the van Helden et al. (2001, 2002) (marmoset; 5-h exposure) and Mioduszewski et al. (2002b) (female SD rat; 10-min, 60-min, and 240-min exposures) data sets are summarized for comparison in Table 1–28 below. The interim values (66 Fed. Reg. 21940 [2001]) are bolded.
Comparison of AEGL estimates from this rich database for GB vapor-induced miosis in the eyes of mammals exhibits remarkable concordance and corroboration across species. There is little to no change between the interim estimates derived from historical human data (Harvey 1952; Johns 1952; 66 Fed. Reg. 21940 [2001]) and that derived from the female rat miosis data published in 2002 (Mioduszewski et al. 2002b). Any differences are usually a single digit in the fourth decimal place. Estimates based
TABLE 1–28 Alternate AEGL-1 Estimates for Nerve Agent GB
|
Time Period |
Interim Value (66 Fed. Reg. 21940 [2001])a (mg/m3) |
Alternate 1b (mg/m3) |
Alternate 2c (mg/m3) |
|
10 min |
0.0069 |
0.0045 |
0.0068 |
|
30 min |
0.0040 |
0.0026 |
0.0039 |
|
1 h |
0.0028 |
0.0019 |
0.0020 |
|
4 h |
0.0014 |
0.00092 |
0.0012 |
|
8 h |
0.0010 |
0.00065 |
0.0010 |
|
Note: n=2; interspecies UF=1 (research staff of TNO and Porton Down consider miosis response in all mammal eyes exposed to nerve agent vapors to be similar across species); intraspecies UF=10 (adjustment for possible susceptible individuals); total UF=10. aDetermined using human data from Harvey (1952) and Johns (1952) (20-min exposure). See Appendix A for details of derivation. bDetermined using marmoset miosis data from van Helden et al. (2002) (5-h exposures). cDetermined using female SD rat miosis data from Mioduszewski et al. (2002b) (10-min, 60-min, and 240-min exposures). |
|||
on marmoset data (a single exposure period of 5 h) differ from the interim values by an approximate factor of 1.5. Given that variation of this magnitude in the AEGL estimates does not reflect response differentiation with any precision, the GB interim values for AEGL-1 are considered adequately representative and protective against miosis resulting from GB vapor exposure to the public. The female rat miosis experiment of Mioduszewski et al. (2002b) is the critical study for final AEGL-1 determination.
The recommended AEGL-1 values are summarized in Table 1–29. The calculations of exposure concentrations for female SD rats and humans scaled to AEGL-1 time points are shown in Appendix A.
5.4. Derivation of AEGL-1 Values for Agents GA, GD, and GF
The relative potency approach was used to estimate AEGL-1 values for agents GA, GD, and GF. A discussion of the relative toxic potencies for these agents is given in Section 4.3. It was determined that for the end point of miosis, the effect usually observed at the lowest exposure concen-
TABLE 1–29 AEGL-1 Values for Agent GB (mg/m3 [ppm])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.0069 mg/m3 (0.0012 ppm) |
0.0040 mg/m3 (0.00068 ppm) |
0.0028 mg/m3 (0.00048 ppm) |
0.0014 mg/m3 (0.00024 ppm) |
0.0010 mg/m3 (0.00017 ppm) |
trations, the potency of GA is identical to that of GB. Agents GD and GF are each considered approximately twice as potent as agents GB or GA for miosis, and equipotent to each other for AEGL-1 effects. Thus, the AEGL-1 concentration values for agents GD and GF are equal to 0.5 times the values derived for agents GA and GB (Table 1–30).
5.5. Derivation of AEGL-1 for Agent VX
Because of inadequacies in the human and animal toxicologic database for agent VX, the present analysis recommends that the AEGL-1 for agent VX be derived from the critical study (Mioduszewski et al. 2002b) for the agent-GB AEGL-1 using a relative potency approach. The experimental protocol for the Mioduszewski et al. (2002b) study is described fully in Section 3.2.1.
A relative potency (RP) of 4 is used to derive the AEGLs for VX. The well-conducted (and clinically supervised) human exposure studies of Grob and Harvey (1958) and Sidell and Groff (1974) report RBC-ChE50 values following single oral or intra-arterial/intravenous exposures to GB and VX (see analysis presented in Tables 1–23 and 1–24). Of the values derived from available human data, the GB:VX ratio (RP=4.3, rounded to 4.0) calculated from oral dose exposures needed to achieve RBC-ChE50 is the most appropriate for the present application. Details of this logic are provided in Section 4.3. The comparative miosis study of Callaway and Dirnhuber (1971) is considered secondary and supportive of the concept that agent VX is more potent than GB for the miosis end point.
By applying an RP factor of 4 to the miosis data set of Mioduszewski et al. (2002b), the comparative concentrations for VX were estimated to be one-fourth that of GB, or 0.017 mg/m3, for a 10-min exposure, 0.005 mg/m3 for a 60-min exposure, and 0.003 mg/m3 for a 240-min exposure. The VX concentrations were further adjusted by a composite UF of 30; 1 for
TABLE 1–30 AEGL-1 Values for Agents GA, GD, and GF (mg/m3 [ppm])
|
Agent |
10 min |
30 min |
1 h |
4 h |
8 h |
|
GA |
0.0069 mg/m3 (0.0010 ppm) |
0.0040 mg/m3 (0.00060 ppm) |
0.0028 mg/m3 (0.00042 ppm) |
0.0014 mg/m3 (0.00021 ppm) |
0.0010 mg/m3 (0.00015 ppm) |
|
GD |
0.0035 mg/m3 (0.00046 ppm) |
0.0020 mg/m3 (0.00026 ppm) |
0.0014 mg/m3 (0.00018 ppm) |
0.00070 mg/m3 (0.000091 ppm) |
0.00050 mg/m3 (0.000065 ppm) |
|
GF |
0.0035 mg/m3 (0.00049 ppm) |
0.0020 mg/m3 (0.00028 ppm) |
0.0014 mg/m3 (0.00020 ppm) |
0.00070 mg/m3 (0.00010 ppm) |
0.00050 mg/m3 (0.000070 ppm) |
interspecies uncertainty (miosis response is similar across species), 10 for intraspecies variability to accommodate known human variation in ChE and carboxylesterase activity (protection of susceptible populations), and a modifying factor of 3 for the sparse VX data set. To derive AEGL-1 values for different time periods (10 min to 30 min and 4 h to 8 h), the data were scaled using the relationship Cn×t=k (ten Berge et al. 1986). An n value has not been determined experimentally for VX; however, because the primary mechanism of action (cholinesterase inhibition) is the same as that for agent GB, the n value of 2 used in the derivation of the AEGL values for GB is also appropriate for deriving all AEGL values for VX. In consequence, the experimentally derived n=2 from the Mioduszewski et al. (2000, 2001, 2002a,b) rat miosis and lethality data sets for agent GB is here used as the scaling function for the agent-VX AEGL-1 values, rather than a default value. Until additional data from well-conducted experimental studies are available, the current value of n is reasonable, is supported by existing data, and meets requirements of the standing operating procedures for estimating AEGL values (NRC 2001).
The 10-min to 30-min extrapolation was
C2×t=k;
([0.017 mg/m3]/30])2×(10/60) h=k;
k=5.0×10−8 mg/m3×h.
The 4-h to 8-h extrapolation was
C2×t=k;
([0.003 mg/m3]/30)2×4 h=k;
k=4.0×10−8 mg/m3×h.
The resulting AEGL-1 values for VX are summarized in Table 1–31. The calculations of exposure concentrations for humans scaled for all AEGL-1 time points are shown in Appendix A.
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
Human data to derive an AEGL-2 for the G agents are provided in the studies of Harvey (1952), Johns (1952), and Baker and Sedgwick (1996). In the Harvey (1952) study an array of signs and symptoms, including headache, eye pain, dimness of vision, twitching of eyelids, rhinorrhea, salivation, throat irritation, tightness in the chest, cramps, nausea, vomiting, giddiness, difficulty in concentrating, and malaise were reported in individuals exposed to GB at 0.3 mg/m3 for 20 min. Twelve subjects were exposed at this GB concentration—all experienced rhinorrhea, eight suffered from headaches, and seven reported dimness of vision. In the Baker and Sedgwick (1996) study, eight healthy male servicemen who were exposed to GB at 0.5 mg/m3 for 30 min developed miosis, and several also exhibited photophobia and dyspnea. In addition, RBC-ChE activity was inhibited to approximately 60% of individual baseline at 3 h and 3 d postexposure, and small but measurable changes occurred in single fibre electromyography (SFEMG) of the forearm. The latter effects, which were detectable in the lab between 4 and 15 mo postexposure, were “not significantly different from the control value,” with “control” defined as preexposure baseline readings for each individual subject (Baker and Sedgwick 1996). The SFEMG changes were not detectable after 15–30 mo.
6.2. Summary of Animal Data Relevant to AEGL-2
Animal inhalation data are insufficient to derive AEGL-2 values.
TABLE 1–31 AEGL-1 Valuesa for Agent VX (mg/m3 [ppm])
6.3. Derivation of AEGL-2 for Agent GB
The present analysis applies the Baker and Sedgwick (1996) study as the basis of the AEGL-2 values. Of the human studies conducted on GB that were available for evaluation, the Baker and Sedgwick study is recent, was conducted following a rigorous experimental protocol, and used modern analytical methods for determining the exposure concentrations (GB at 0.5 mg/m3 for 30 min). Furthermore, this study was performed under Helsinki accords and clinical supervision and was conducted with the cooperation of fully informed human subjects (N=8, “fit male servicemen”). The observed effects included miosis in eight of eight subjects, dyspnea and photophobia in some individuals (number not given), inhibition of RBC-ChE to approximately 60% of individual baseline at 3 h and 3 d postexposure in (eight of eight subjects), and small but measurable changes in single fibre electromyography (SFEMG) of the forearm (in five of eight subjects). Nevertheless, the fact that the SFEMG abnormalities were detectable in the lab between 4 and 15 mo postexposure makes these effects long-lasting, and they are therefore included under the definition of AEGL-2.
Respiratory effects resolved within minutes, and visual effects resolved within 48 h. The SFEMG changes noted in the study were not clinically significant, and were not detectable after 15–30 mo. Baker and Sedgwick considered SFEMG changes to be a possible early indicator or precursor of the nondepolarising neuromuscular block found associated with intermediate syndrome paralysis in severe organophosphorous insecticide poisoning cases (Senanayake and Karalliedde 1987). The study concluded that these electromyographic changes were persistent (>15 mo), but that they were reversible and subclinical. Subclinical and reversible effects are not normally included within the definition of AEGL-2 effects. However, because SFEMG changes may be a precursor of intermediate syndrome (see Section
4.5.2), and because of the steepness of the dose-response curve for nerve agents, the use of this end point for establishing AEGL-2 values is considered a protective approach. This concept of added precaution for steep dose-response is consistent with emergency planning guidance for nerve agents previously developed by the National Center for Environmental Health of the Centers for Disease Control and Prevention (Thacker 1994).
As previously described in the development of AEGL-1 values for the G agents (Sections 5.3 and 5.4), an n value of 2 derived from a linear regression of both miosis and lethality data for GB vapor exposure to female SD rats (Mioduszewski et al. 2000, 2001, 2002a,b) is appropriate for use as a scaling function for all nerve agents. AEGL-2 values for exposure times different from the experimental time of 30 min were thus scaled using an n of 2.
A composite UF of 10 was used in the calculation. To accommodate known variation in human cholinesterase and carboxylesterase activity that may make some individuals susceptible to the effects of cholinesterase inhibitors such as nerve agents, a factor of 10 was applied for intraspecies variability (protection of susceptible populations). Because human data were used, an interspecies UF was not required. The database for agent GB is reasonably complete. As was true for the AEGL-1 estimations, there are strong arguments for not incorporating an additional modifying factor. In consequence, no modifying factor was used in the estimation of AEGL-2 values.
From the experimental data, k was derived as
([0.5 mg/m3]/10)2×(0.5 h)=k;
k=0.0013 mg/m3×h.
The resulting estimates of AEGL-2 are summarized in Table 1–32.
6.4. Derivation of AEGL-2 Values for Agents GA, GD, and GF
The relative potency approach is used to estimate AEGL-2 values for agents GA, GD, and GF. A discussion of the relative toxic potencies for these agents is given in Section 4.3. It was determined that for the end point of miosis, the effect usually observed at the lowest exposure concentrations, the potency of GA is identical to that of GB. Agents GD and GF are each considered approximately twice as potent as agents GB or GA for
TABLE 1–32 AEGL-2 Values for Agent GB (mg/m3 [ppm])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.087 mg/m3 |
0.050 mg/m3 |
0.035 mg/m3 |
0.017 mg/m3 |
0.013 mg/m3 |
|
(0.015 ppm) |
(0.0085 ppm) |
(0.0060 ppm) |
(0.0029 ppm) |
(0.0022 ppm) |
miosis, and equipotent to each other for AEGL-2 effects. Thus, the AEGL-2 concentration values for agents GD and GF are equal to 0.5 times those values derived for agents GA and GB (Table 1–33).
6.5. Derivation of AEGL-2 Values for Agent VX
Acute inhalation toxicity studies on animals have identified median lethal concentrations; however, these studies are inadequate for deriving AEGL-2 values because of the lack of dose-response data for the appropriate time periods. Some information for agent VX is available from a repeat exposure study in which a VX concentration of 0.004 mg/m3 for 6 h/d, 5 d/wk, for 2 wk resulted in severe signs of toxicity (tremors, convulsions, salivation, and bloody tears) and 100% mortality of mice, 35% mortality in rats, and 3% mortality in guinea pigs (Crook et al. 1983). Exposure to 0.0002 mg/m3 under the same experimental protocol resulted in no toxic signs but miosis and ChE depression. The Crook data set is considered nonverifiable. An AEGL-2 effect for a single 6-h exposure would most likely fall within the range of 0.0002 and 0.004 mg/m3.
There are no definitive data identifying the minimal exposure level at which severe, irreversible, or escape-impairing effects of acute exposure to agent VX would occur. Because of the inadequacy of the human and animal toxicologic database for agent VX, the AEGL-2 for agent VX is derived from the AEGL-2 for agent GB using a relative potency approach.
The Baker and Sedgwick (1996) study of GB vapor exposure in human volunteers is used as the basis of the AEGL-2 values for agent VX, as described in Section 6.3.
By applying a relative potency of 4, the comparable VX exposure is one-fourth that of GB, or 0.125 mg/m3, for a 30-min exposure. The VX concentration was adjusted by a composite UF of 30; 1 for interspecies uncertainty (human data), 10 for intraspecies variability to accommodate known human variation in ChE activity (protection of susceptible populations), and a modifying factor of 3 for the sparse VX data set. To derive AEGL-2 values for different time periods, the data were scaled using the
TABLE 1–33 AEGL-2 Values for Agents GA, GD, and GF (mg/m3 [ppm])
|
Agent |
10-min |
30-min |
1-h |
4-h |
8-h |
|
GA |
0.087 mg/m3 (0.013 ppm) |
0.050 mg/m3 (0.0075 ppm) |
0.035 mg/m3 (0.0053 ppm) |
0.017 mg/m3 (0.0026 ppm) |
0.013 mg/m3 (0.0020 ppm) |
|
GD |
0.044 mg/m3 (0.0057 ppm) |
0.025 mg/m3 (0.0033 ppm) |
0.018 mg/m3 (0.0022 ppm) |
0.0085 mg/m3 (0.0012 ppm) |
0.0065 mg/m3 (0.00085 ppm) |
|
GF |
0.044 mg/m3 (0.0062 ppm) |
0.025 mg/m3 (0.0035 ppm) |
0.018 mg/m3 (0.0024 ppm) |
0.0085 mg/m3 (0.0013 ppm) |
0.0065 mg/m3 (0.00091 ppm) |
relationship Cn×t=k (ten Berge et al. 1986). An n value has not been determined experimentally for VX. However, because the mechanism of action (cholinesterase inhibition) is the same as that for agent GB, the n value of 2, as used in the derivation of the AEGL values for GB, is also appropriate for deriving AEGL values for VX. In consequence, the experimentally derived n=2 from the Mioduszewski et al. (2000, 2001, 2002a,b) rat lethality data set for agent GB is here used as the scaling function for the agent VX AEGL-2 values, rather than a default value; therefore
C2×t=k;
([0.125 mg/m3]/30)2×0.5 h=k;
k=8.7×10−6 mg/m3×h.
The resulting AEGL-2 values are summarized in Table 1–34. The calculations of exposure concentrations for humans scaled for all AEGL-2 time points are shown in Appendix A.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
Human lethality data resulting from exposure to any of the G agents were not available for deriving an AEGL-3.
TABLE 1–34 AEGL-2 Valuesa for Agent VX (mg/m3 [ppm])
7.2. Summary of Animal Data Relevant to AEGL-3
Agent GB
Data on the lethality of GB are available for several laboratory species (see Table 1–9). Mioduszewski et al. (2000, 2001, 2002a) reported LCt50 and LC50 values for rats for exposure time periods of 10, 30, 60, 240, and 360 min. Bide et al. (1999) (see also Yee et al. [1999]), determined LC50 values for mice for time periods of 1 s to 30 min and estimated LC50 values for five other laboratory species and humans using a three-dimensional probit model.
Agent GD
In an experimental exposure study designed to secondarily examine agent GD toxicity, Aas et al. (1985) reported that the LCt50 for GD in rats (six animals tested at each of three exposure levels for periods of time <30 min) was 400 mg·min/m3. Aas et al. (1985) graphically present their data as an LCt-versus-mortality curve. As estimated from this curve, the lethality threshold for rats exposed to GD is about 335 mg·min/m3. Because the reported GD air concentration was fixed at 21 mg/m3, the exposure time corresponding to the threshold was back-calculated to equal 16 min.
Note that the principal objective of the Aas et al. (1985) study was to test an experimental dynamic flow system that would allow study of highly toxic vapors. Secondary objectives of the study were to determine the (short-term) inhalation toxicity of agent GD (soman) and to study inhibition of acetylcholinesterase, cholinesterase, and carboxylesterase activity in the respiratory tract (relative to other tissues).
Agent GF
A recent study of GF vapor inhalation toxicity in male and female SD rats reported 24-h postexposure LC50 values for three exposure periods (10, 60, and 240 min) (Anthony et al. 2002). Young adult rats were exposed whole-body in a dynamic 750-L chamber under protocols similar to those previously published by Mioduszewski et al. (2001, 2002a) but with additional accommodations for the lesser volatility of agent GF. For female rats, Anthony et al. (2002) report 24-h postexposure LC50 values as follows: 10 min, 25.3 mg/m3; 60 min, 5.56 mg/m3; 240 min, 2.22 mg/m3. For male rats, 24-h postexposure LC50 values are as follows: 10 min, 36.8 mg/m3; 60 min, 6.60 mg/m3; 240 min, 2.48 mg/m3. These results are summarized as LCt50 values in Table 1–16. The preliminary data of Anthony et al. (2002) document 24-h lethality and LC50 only (Table 1–16). In consequence, these data are not comparable to the 14-d postexposure rat LC01 information available from the Mioduszewski et al. studies for GB vapor inhalation lethality. Furthermore, the preliminary nature of the Anthony et al. (2002) documentation precludes LC01 determination by benchmark dose analysis at this time.
7.3. Derivation of AEGL-3 for Agents GB and GD
Agent GB
The most complete lethality data set for the relevant time periods is that presented by Mioduszewski et al. (2000, 2001, 2002a). The final report of this study (Mioduszewski et al. 2001, 2002a) is further documentation of the findings presented below. The acute lethal toxicity of GB to male and female Sprague-Dawley rats was evaluated for time periods of 10, 30, 60, 90, 240, and 360 min in a whole-body dynamic chamber. Ten males and 10 females were used for each concentration-time (Ct) combination, and 50 males and 50 females were used for each time point. GB concentrations ranged from about 2 mg/m3 to 54 mg/m3. Agent concentrations were confirmed in the exposure chamber by three procedures to allow point and continuous determinations (Mioduszewski et al. 2000, 2001, 2002a). Lethality was assessed at 24 h and at 14 d postexposure. Female rats were reported to be more sensitive to GB vapor toxicity than males over the range of exposure concentrations and durations studied. Please note that
comparison of LCt50 values for male and female rats exposed to vapor concentrations of GB from Mioduszewski et al. (2000, 2001, 2002a) and Callaway and Blackburn (1954) reports indicates that the range of ratios (F:M) is 0.54 to 0.80, with a geometric mean of 0.67 (see Table 1–25). Gender differences for lethality are reported by Mioduszewski et al. (2000, 2001, 2002a) to be statistically significant at p<0.01.
Probit analysis (MINITAB, version 13) presented in Mioduszewski et al. (2000) gave the following 14-d LC50 values for female rats exposed to agent GB vapor: 18.1 mg/m3 for 10 min, 8.51 mg/m3 for 30 min, 6.39 mg/m3 for 60 min, 3.03 mg/m3 for 4 h, and 2.63 mg/m3 for 6 h. Based on a probit analysis of the data (Mioduszewski et al. 2000), the estimated LC01 values for the females are as follows: 11.537 mg/m3 for 10 min, 5.836 mg/m3 for 30 min, 4.006 mg/m3 for 60 min, 2.087 mg/m3 for 4 h, and 1.761 mg/m3 for 6 h. Mioduszewski et al. (2000) note that these estimates of LC01 are associated with large error bars.
The AEGL-3 for agent GB was derived from the lethality data for female Sprague-Dawley rats Mioduszewski et al. (2000, 2001, 2002a).
Regarding selection of the species to be used in neurotoxicity tests, the EPA Health Effects Test Guidelines (OPPTS 870.6200, Neurotoxicity Screening Battery) published by the Office of Prevention, Pesticides and Toxic Substances (EPA 1998) state that “in general, the laboratory rat should be used.” The experimental protocol of Mioduszewski et al. (2000, 2001, 2002a) followed the OPPTS guidelines concerning the species, age, gender, and number of animals per dose and control group. Furthermore, in their recent review of organophosphates insecticide toxicity data, Storm et al. (2000) consider rat organophosphate inhalation data to be a defensible basis for developing (occupational) exposure limits, especially in the absence of human exposure data.
As previously discussed in the text on AEGL-1 and AEGL-2 values, the recent miosis and lethality data of Mioduszewski et al. (2000, 2001, 2002a,b) are determined to be the best source of an estimate for the n value for GB response (see Appendix B). Therefore, n=2 is used as the scaling function for AEGL-3 derivations in the equation (Cn×t=k) according to the methods often Berge et al. (1986) to derive an 8-h AEGL-3 from the 6-h LC01. All the other time-specific AEGL-3 values were derived directly from the LC01 values for female SD rats in the Mioduszewski et al. (2000) study.
Given that the AEGL-3 estimation for the G-series nerve agents is derived from a lethal inhalation toxicity study of adult female SD rats (Mioduszewski et al. 2000, 2001 2002a), it is reasonable to consider the
whole-organism response of lethality as an appropriate end point by which to compare data for rats (a CaE-rich species) with data for monkeys and dogs, two experimental species considered in earlier studies to possess no plasma carboxylesterase (Augustinsson 1959). Available experimental LCt50 data for the monkey, dog, and rat are presented in Table 1–13. As shown in Table 1–13, LCt50 values for 10-min exposures to GB are 310 mg·min/m3 in mice, 181–226 mg·min/m3 in rats, 60 mg·min/m3 in dogs, and 74 mg·min/m3 in monkeys. There is a 2- to 3-fold difference between rats and monkeys, and a 3- to 4-fold difference between rats and dogs. These comparisons indicate that, when challenged with a lethal concentration of GB vapor, adult female SD rats are more resistant than adult dogs or monkeys by approximate factors of 2 to 4. Species differences in carboxylesterase concentrations may account, in part, for these observed differences. Please see Section 4.5.2 for a more detailed discussion of carboxylesterases as detoxification enzymes for nerve agent exposures.
In the case of human lethality estimates, Bide et al. (1999) estimate GB inhalation toxicity values for humans by application of allometric model extrapolation from extensive experimental animal data. Their study estimates that a 2-min adult human LCt50 approximates 31 mg·min/m3 (a 2-min LC50 of 15.5 mg/m3). The resulting 2-min LC50 ratio with the female SD rat from Mioduszewski et al. (2000, 2001, 2002a) (2-min LCt50 of 104 mg/m3 or 2-min LC50 at 52 mg/m3) is
female SD rat:human (estimated)=52/15.5=3.4.
This comparison indicates that, when challenged with a lethal concentration of GB vapor, adult female SD rats (Mioduszewski et al. 2000, 2001) are likely to be more resistant than adult humans by a factor between 3.0 and 3.5.
The following summarizes the above analysis of interspecies UF for AEGL-3 estimates:
-
The literature regarding carboxylesterase (CaE) in lab animals and humans indicates that CaE is present in human plasma as well as numerous other human tissues and organs (including those where exposure and distribution leading to death by G agent vapor toxicity would likely occur).
-
Interspecies data for comparison of the whole-organism response of lethality indicates that, when challenged with a lethal concentration of GB vapor, adult female SD rats are more resistant than adult dogs or monkeys by approximate factors of 2 to 4. Species differences in carboxyl-
-
esterase concentrations may account for these differences. Model predictions of human LCt50 indicate a rat:human ratio of between 3.0 and 3.5.
-
The known detoxification potential of carboxylesterases is multifaceted and encompasses consideration of CaE amount, affinity, and inhibitor resistant esterase activity. The present state of incomplete characterization for human CaE precludes accurate prediction regarding CaE detoxification potential in a population of humans exposed to anticholinesterase compounds.
In conclusion, recent literature indicates that CaE detoxification potential exists in numerous human organs and tissue, including blood plasma. It is acknowledged that further experimental characterization of CaE detoxification potential in humans will be necessary before accurate prediction of the contributions CaE may make to human protection from anticholinesterase poisoning. Interspecies comparisons of lethality data for rats and monkeys (as well as estimated human LCt50 values) has been performed. The results indicate that an interspecies UF (rat-to-human) of approximately 3 for AEGL-3 determination is a reasonable characterization of the present state of knowledge for this parameter.
To accommodate known variation in human cholinesterase and carboxylesterase activity that may make some individuals susceptible to the effects of cholinesterase inhibitors such as nerve agents, a factor of 10 was applied for intraspecies variability (protection of susceptible populations). Because a modifying factor is not applicable for reasons previously outlined for AEGL-1 and AEGL-2, the composite UF for AEGL-3 determination for agent GB is equal to 30. From the experimental data, k was derived from the 6-h LC01 as
([1.761 mg/m3]/30)2×6.0 h=k;
k=0.021 mg/m3×h.
The resulting AEGL-3 estimates for agent GB are summarized in Table 1– 35.
Benchmark Exposure Analyses
A benchmark exposure calculation has been performed on the female rat 14-d vapor lethality data presented in the Mioduszewski et al. (2001)
TABLE 1–35 AEGL-3 Values for Agent GB (mg/m3 [ppm])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.38 mg/m3 (0.064 ppm) |
0.19 mg/m3 (0.032 ppm) |
0.13 mg/m3 (0.022 ppm) |
0.070 mg/m3 (0.012 ppm) |
0.051 mg/m3 (0.0087 ppm) |
report in accordance with guidance provided in the NRC standing operating procedures (NRC 2001, 45). For comparison, a NumberCruncher Statistical System analysis has also been completed.
There appears to be some degree of controversy around using the BMD approach for acute lethality data. The SOP workgroup will address this issue in the future.
There are eight models that accept dichotomous data in the Benchmark Dose software package available on the EPA Web site (http://cfpub.epa.gov/ncea/cfm/bmds.cfm): gamma, logistic, log-log multistage, probit, log-probit, quantal-linear, quantal-quadratic, and Weibull. Evaluations were performed with all eight (multiplied by five time points, times the 5% response for the 95% Lower Confidence Limit (LCL) and the 1% Maximum Likelihood Estimate (MLE), as per the SOP). The Weibull and gamma programs would not run with the input data; contact with the EPA Webmaster eventually revealed, through systems testing, that these two models require entry of a zero-concentration effect value in order to converge. The Mioduszewski et al. (2001) data set does not contain any zero-concentration effects data, and its addition would be an artificial alteration of the data set. It was concluded that the content of the data set is not compatible with requirements of the Weibull and gamma models; thus no analyses of the vapor lethality data were performed with these two models.
Tables summarizing the statistical results of the Benchmark Exposure Concentration analysis are included in Appendix C. Table C-1 is a summary of LC01 values obtained from all the Benchmark Dose software routines; the ones on the lower tier of the table (logistic, multistage, quantal-linear, quantal-quadratic) are poor fits and are rejected from any further consideration. The first column following the exposure times is the set of MLE LC01 values used to develop the AEGL-3 estimates published in the Federal Register notice of May 2, 2001 (66 Fed. Reg. 21940 [2001]). The LC01 values in the second column following the exposure times are those published by Mioduszewski et al. (2001). All remaining values presented in Table C-1 were based on the raw experimental data presented in Mioduszewski et al. (2001).
The MINITAB log probit seems to be a reasonable fit with the lethality data, and the experimental results on which this analysis is based are published in Mioduszewski et al. (2001, 2002a).
Because the statistical routines used to evaluate the data in Mioduszewski et al. (2000) differ slightly from those used in Mioduszewski et al. (2001), the LC01 values employed in developing interim AEGL-3 determinations also differ slightly. The resulting LC01 and AEGL-3 estimates developed with the same calculational approach—with the UF and n values applied in the AEGL-3 determinations presented earlier (see Appendix A)—are summarized in Tables C-1 and C-2 of Appendix C. The Federal Register interim values for AEGL-3 (see Table C-2) are consistently lower or equal to the Mioduszewski et al. (2001) log probit derived estimates, with the single exception of the 4-h value. In the case of the 4-h value, the NAC interim AEGL-3 (0.070 mg/m3) is somewhat greater than the 4-h AEGL-3 estimate derived from the Mioduszewski et al. (2001) log probit derivation (0.059 mg/m3; see Table C-2), by 0.011 mg/m3. The variation is slight.
The LC01 values presented in Mioduszewski et al. (2001), although slightly different from the preliminary results considered (Mioduszewski et al. 2000), represent a better documented and more widely accessible data set. These differences are acknowledged.
Agent GD
A relative potency approach is used to estimate AEGL-3 values for agent GD, and a discussion of the relative potency of agent GD and GB is provided in Section 4.3. The lethal potency of agent GD is considered equivalent to that of agent GB (see Table 1–22).
A secondary and short-term GD inhalation study of rat lethality for exposure times ≤30 min (Aas et al. 1985) lends support to the assumption of lethal equipotency for agents GB and GD when used as a secondary study for derivation of 10-min and 30-min AEGL-3 values and as a comparison with the values derived by the relative potency method. Aas et al. (1985) calculated an LCt50 of 400 mg·min/m3 for GB and graphically presented their data as an LCt-versus-mortality curve. As estimated from this curve, the lethality threshold for rats exposed to agent GD (six animals tested at each of three exposure levels for periods of time <30 min) is about 335 mg·min/m3. Because the GD air concentration was fixed at 21 mg/m3,
the exposure time corresponding to the threshold could be back-calculated, and was found to be 16 min. This lethality threshold was used to derive a comparative estimate of the AEGL-3.
Regression analysis of the data of Aas et al. (1985) was not possible from the information provided. Because the principal mode of action (cholinesterase inhibition) for the G agents is identical, n=2 was used for deriving comparative AEGL-3 values from the GD data of Aas and his colleagues. Because of the sparse data set for GD, the full default values for interspecies (10) and intraspecies (10) uncertainty were applied. Because a modifying factor is not applicable, a composite UF of 100 was used in deriving comparative 10-min AEGL-3 and 30-min AEGL-3 estimates for agent GD from the data provided by Aas et al. (1985).
AEGL-3 values for exposure times different from the experimental times were scaled using an n of 2. From the experimental data, k was derived as
([21 mg/m3]/100)2×(16/60) h=k;
k=0.012 mg/m3×h.
The resulting comparative AEGL-3 estimates include a 10-min AEGL-3 estimate of 0.27 mg/m3 and a 30-min AEGL-3 estimate of 0.15 mg/m3. Details of the comparative derivation are provided in Appendix A.
The values derived from the Aas et. al (1985) study are in good agreement with those derived by means of relative potency comparison with agent GB for the same time periods.
The recommended AEGL-3 value estimates are those derived by relative potency comparison with agent GB (with agent GD being considered equipotent to agent GB for lethal effects), and are summarized in Table 1– 36 below.
7.4. Derivation of AEGL-3 Values for Agents GA and GF
A relative potency approach is used to estimate AEGL-3 values for agents GA and GF. A discussion of the relative potency of these agents to cause lethality is given in Section 4.3 and summarized in Table 1–22. The lethal potency of GA is considered to be one-half that of GB, and agent GF is considered to be equipotent to GB for lethality (Table 1–37). The preliminary GF lethality report of Anthony et al. (2002) is not sufficiently com-
TABLE 1–36 AEGL-3 Values for Agent GD (mg/m3 [ppm])
|
10-min |
30-min |
1-h |
4-h |
8-h |
|
0.38 mg/m3 (0.049 ppm) |
0.19 mg/m3 (0.025 ppm) |
0.13 mg/m3 (0.017 ppm) |
0.070 mg/m3 (0.0091 ppm) |
0.051 mg/m3 (0.0066 ppm) |
plete (no documentation of threshold lethality) to support an independent AEGL-3 estimation for agent GF (see Section 7.2).
7.5. Derivation of AEGL-3 Values for Agent VX
Credible data on the acute vapor exposure lethality of agent VX are available for only two laboratory species, mice and goats. The LCt50 values are 4.0 mg·min/m3 for mice and 9.2 mg·min/m3 for goats for 10-min exposures (Koon et al. 1960). However, LC01 estimates cannot be derived from the Koon et al. study, and the analytical methods employed in measurement of experimental VX concentrations are not considered acceptable by modern standards.
Because of inadequacies in the human and animal toxicological database for agent VX, the AEGL-3 for agent VX is derived from the AEGL-3 for agent GB using a relative potency approach. The AEGL-3 for agent GB was derived from the lethality data for female Sprague-Dawley rats (Mioduszewski et al. 2000, 2001, 2002a), as discussed more fully in Section 7.3.
Probit analysis (MINITAB, version 13) presented in Mioduszewski et al. (2000) gave the following 14-day LC50 values for female rats exposed to agent GB vapor: 18.1 mg/m3 for 10 min, 8.51 mg/m3 for 30 min, 6.39 mg/m3 for 60 min, 3.03 mg/m3 for 4 h, and 2.63 mg/m3 for 6 h.
Based on a probit analysis of the data (Mioduszewski et al. 2000), the estimated 14-day LC01 values for the females are as follows: 11.54 mg/m3 for 10 min, 5.84 mg/m3 for 30 min, 4.01 mg/m3 for 60 min, 2.09 mg/m3 for 4 h, and 1.76 mg/m3 for 6 h.
By applying the relative potency of 4 as described earlier, the estimated 14-day LC01 for female SD rats exposed to VX vapor are as follows: 2.89 mg/m3 for 10 min, 1.46 mg/m3 for 30 min, 1.00 mg/m3 for 60 min, 0.52 mg/m3 for 4 h, 0.44 mg/m3 for 6 h.
TABLE 1–37 AEGL-3 Values for Agents GA and GF (mg/m3 [ppm])
|
Agent |
10 min |
30 min |
1 h |
4 h |
8 h |
|
GA |
0.76 mg/m3 (0.11 ppm) |
0.38 mg/m3 (0.057 ppm) |
0.26 mg/m3 (0.039 ppm) |
0.14 mg/m3 (0.021 ppm) |
0.10 mg/m3 (0.015 ppm) |
|
GF |
0.38 mg/m3 (0.053 ppm) |
0.19 mg/m3 (0.027 ppm) |
0.13 mg/m3 (0.018 ppm) |
0.070 mg/m3 (0.0098 ppm) |
0.051 mg/m3 (0.0071 ppm) |
The derived LC01 values above were adjusted by a total UF of 100. The use of a rat data set resulted in selection of an interspecies UF of 3; the full default value of 10 was not considered appropriate for the interspecies UF because the mechanism of toxicity in both laboratory rodents and humans is cholinesterase inhibition, and the experimental results of Maxwell (1992) indicate that endogenous carboxylesterases in rats confer no protection against lethal exposures of nerve agent VX. To accommodate known variation in human cholinesterase activity, the full default value of 10 for intraspecies uncertainty was considered necessary to protect susceptible populations. With the additional application of a modifying factor of 3 for the sparse VX data set, the total UF for AEGL-3 determination for agent VX is equal to 100.
To derive an AEGL-3 for a time periods not included with the experimental protocol of Mioduszewski et al. (2000, 2001, 2002a) (i.e., the 8-h AEGL), the 6-h data were scaled using the relationship Cn×t=k (ten Berge et al. 1986). An n value has not been determined experimentally for VX. However, because the mechanism of action (cholinesterase inhibition) is the same as that for agent GB, the n value of 2 used in the derivation of the AEGL values for GB is appropriate for deriving AEGL values for VX. The experimentally derived n=2 from the Mioduszewski et al. (2000, 2001, 2002a,b) rat miosis and lethality data sets for agent GB are used here as the scaling function for the agent-VX 8-h AEGL-3 values, rather than a default value. Therefore, using the 6-h estimated LC01,
C2×6 h=k;
([0.44 mg/m3]/100)2×6 h=k;
k=1.16×10−4 mg/m3×h.
The resulting AEGL-3 values are summarized in Table 1–38. The calculations of exposure concentrations for humans scaled for all AEGL-3 time points are shown in Appendix A.
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity End Points
A summary of the AEGLs for agents GA, GB, GD, GF and VX is shown in Table 1–39.
G-Series Agents
In consultation with experimental investigators at Porton Down (United Kingdom) and the TNO Prins Maurits Laboratory (Netherlands), the current analysis has determined that the miotogenic response of mammalian eyes to agent GB vapor exposure is similar across species. The species evaluated include standard laboratory animals (rabbits, rats, guinea pigs), nonhuman primates (marmosets), and humans. In consequence, the interspecies UF for the critical AEGL-1 end point of miosis is considered equal to 1. To accommodate known variation in human cholinesterase and carboxylesterase activity that may make some individuals susceptible to the effects of cholinesterase inhibitors such as nerve agents, a factor of 10 was applied for intraspecies variability (protection of susceptible populations). A modifying factor is not applicable. Thus, the total UF for estimating AEGL-1 values for agent GB is 10.
For the development of AEGL-2 values, the database for toxicological effects in humans is more complete for agent GB than for any of the other G agents. Sufficient human data are available to directly derive AEGL-2 values for agent GB. The toxicity end points used to derive the value were considered to be appropriate, and the lowest of the available exposure concentrations was used. The data were limited, however, by the maximum time of exposure of 30 min (Baker and Sedgwick 1996). The AEGL-1 and AEGL-2 values for agents GA, GD, and GF were derived from the AEGL-1 and AEGL-2 values for GB using the relative potency approach based on the potency of the agents to induce miosis. Agents GA and GB were considered to have an equivalent potency for causing miosis. Agents GD and
TABLE 1–38 AEGL-3 Valuesa for Agent VX (mg/m3 [ppm])
GF were considered equipotent for AEGL-1 and AEGL-2 effects, and twice as potent as agent GB. The use of these relative potency measures for the derivation of AEGL-2 values for GA, GD, and GF was a conservative approach compared with an estimate based on a fraction (one-third) of the AEGL-3 concentration level (NRC 2001), which would have resulted in higher AEGL-2 values for GA, GD, and GF.
Lethality data for SD rats were available for the derivation of an AEGL-3 for agent GB. The AEGL-3 values for agents GA, GD, and GF were derived from the AEGL-3 values for GB using a relative potency approach. Agents GB, GD, and GF were considered to have an equivalent lethal potency. The lethal potency of agent GA was considered to be only one-half that of agents GB, GD, and GF.
A secondary derivation of 10-min and 30-min AEGL-3 values for agent GD relied on data from an inhalation study of male Wistar rats exposed to GD for periods up to 30 min (Aas et. al. 1985). The values derived from this secondary study (0.27 mg/m3 for 10 min and 0.15 mg/m3 for 30 min) are in good agreement with those derived by means of relative potency comparison with agent GB (0.38 mg/m3 for 10 min and 0.19 mg/m3 for 30 min) for the same time periods.
Cross-comparison of the AEGL estimates for agent GB with the available human toxicity data (Figure 1–1; see also Tables 1–7, 1–8, and 1–9) reveals that the AEGL-1 values for agent GB are substantially below any reported effect levels, and the AEGL-2 values are below any of the exposure levels that might be considered disabling. Although available human data are clustered in time periods <60 min, it is clear that AEGL-3 estimates are below exposures considered capable of inducing reversible AEGL-2 effects (miosis, dyspnea, changes in SFEMG, etc.) (Baker and Sedgewick 1996) and overlap those where reversible discomfort is observed. Comparisons with animal toxicity data (Figure 1–2) also indicate that the estimated AEGL values for GB are below most reported effect
TABLE 1–39 Relational Comparison of AEGLs for Nerve Agentsa GA, GB, GD, GF, and VX
|
Agent |
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
GA |
AEGL-1 (Nondisabling) |
0.0010 ppm (0.0069 mg/m3) |
0.00060 ppm (0.0040 mg/m3) |
0.00042 ppm (0.0028 mg/m3) |
0.00021 ppm (0.0014 mg/m3) |
0.00015 ppm (0.0010 mg/m3) |
Based on relative potency from GBb |
|
AEGL-2 (Disabling) |
0.013 ppm (0.087 mg/m3) |
0.0075 ppm (0.050 mg/m3) |
0.0053 ppm (0.035 mg/m3) |
0.0026 ppm (0.017 mg/m3) |
0.0020 ppm (0.013 mg/m3) |
Based on relative potency from GBb |
|
|
AEGL-3 (Lethal) |
0.11 ppm (0.76 mg/m3) |
0.057 ppm (0.38 mg/m3) |
0.039 ppm (0.26 mg/m3) |
0.021 ppm (0.14 mg/m3) |
0.015 ppm (0.10 mg/m3) |
Based on relative potency from GBc |
|
|
GB |
AEGL-1 (Nondisabling) |
0.0012 ppm (0.0069 mg/m3) |
0.00068 ppm (0.0040 mg/m3) |
0.00048 ppm (0.0028 mg/m3) |
0.00024 ppm (0.0014 mg/m3) |
0.00017 ppm (0.0010 mg/m3) |
EC50 for miosis observed in adult female SD rats exposed to a range of GB vapor concentrations (0.01 to 0.48 mg/m3) for 10, 60 and 240 min (Mioduszewski et al. 2002b) AND miosis data from secondary and supportive studies with |
|
|
marmosets (van Helden et al. 2001, 2002), and humans (Harvey 1952; and Johns 1952). |
||||||
|
|
AEGL-2 (Disabling) |
0.015 ppm |
0.0085 ppm |
0.0060 ppm |
0.0029 ppm |
0.0022 ppm |
Miosis, dyspnea, RBC-ChE inhibition, single fibre electromyography (SFEMG) changes in human volunteers exposed to 0.5 mg/m3 for 30 min (Baker and Sedgwick 1996) |
|
AEGL-3 (Lethal) |
(0.087 mg/m3) 0.064 ppm (0.38 mg/m3) |
(0.050 mg/m3) 0.032 ppm (0.19 mg/m3) |
(0.035 mg/m3) 0.022 ppm (0.13 mg/m3) |
(0.017 mg/m3) 0.012 ppm (0.070 mg/m3) |
(0.013 mg/m3) 0.0087 ppm (0.051 mg/m3) |
Based on experimental SD rat lethality data (LC01 and LC50); whole-body dynamic exposure to concentrations between 2–54 mg/m3 for 3, 10, 30, 60, 90, 240, and 360 min |
|
|
Agent |
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
|
(Mioduszewski et al. 2000; 2001, 2002a) |
||||||
|
GD |
AEGL-1 (Nondisabling) |
0.00046 ppm (0.0035 mg/m3) |
0.00026 ppm (0.0020 mg/m3) |
0.00018 ppm (0.0014 mg/m3) |
0.000091 ppm (0.00070 mg/m3) |
0.000065 ppm (0.00050 mg/m3) |
Based on relative potency from GBd |
|
AEGL-2 (Disabling) |
0.0057 ppm (0.044 mg/m3) |
0.0033 ppm (0.025 mg/m3) |
0.0022 ppm (0.018 mg/m3) |
0.0012 ppm (0.0085 mg/m3) |
0.00085 ppm (0.0065 mg/m3) |
Based on relative potency from GBd |
|
|
AEGL-3 (Lethal) |
0.049 ppm (0.38 mg/m3) |
0.025 ppm (0.19 mg/m3) |
0.017 ppm (0.13 mg/m3) |
0.0091 ppm (0.070 mg/m3) |
0.0066 ppm (0.051 mg/m3) |
Based on relative potency from GB. Supported by Wistar rat LC50; dynamic chamber exposures at 21 mg/m3 for 3 time periods of ≤30 min duration (Aas et al. 1985)e |
|
|
GF |
AEGL-1 (Nondisabling) |
0.00049 ppm (0.0035 mg/m3) |
0.00028 ppm (0.0020 mg/m3) |
0.00020 ppm (0.0014 mg/m3) |
0.00010 ppm (0.00070 mg/m3) |
0.000070 ppm (0.00050 |
Based on relative potency from GBd |
|
|
mg/m3) |
||||||
|
|
AEGL-2 (Disabling) |
0.0062 ppm (0.044 mg/m3) |
0.0035 ppm (0.025 mg/m3) |
0.0024 ppm (0.018 mg/m3) |
0.0013 ppm (0.0085 mg/m3) |
0.00091 ppm (0.0065 mg/m3) |
Based on relative potency from GBd |
|
AEGL-3 (Lethal) |
0.053 ppm (0.38 mg/m3) |
0.027 ppm (0.19 mg/m3) |
0.018 ppm (0.13 mg/m3) |
0.0098 ppm (0.070 mg/m3) |
0.0071 ppm (0.051 mg/m3) |
Based on relative potency from GBe |
|
|
VXf |
AEGL-1 (Non-disabling) |
0.000052 ppm (0.00057 mg/m3) |
0.000030 ppm (0.00033 mg/m3) |
0.000016 ppm (0.00017 mg/m3) |
0.0000091 ppm (0.00010 mg/m3) |
0.0000065 ppm (0.000071 mg/m3) |
Derived by relative potency from EC50 for miosis observed in adult female SD rats exposed to a range of GB vapor concentrations (0.01 to 0.48 mg/m3) for 10, 60 and 240 min (Mioduszewski et al. 2002b) AND miosis data from secondary and supportive studies of van Helden et al (2001, |
|
Agent |
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
|
2002), Harvey (1952) and Johns (1952) in marmosets and humans, respectivelyg |
||||||
|
|
AEGL-2 (Disabling) |
0.00065 ppm (0.0072 mg/m3) |
0.00038 ppm (0.0042 mg/m3) |
0.00027 ppm (0.0029 mg/m3) |
0.00014 ppm (0.0015 mg/m3) |
0.000095 ppm (0.0010 mg/m3) |
Derived by relative potency from study ofGB vapor exposure to exercising human volunteers exposed to 0.5 mg/m3 for 30 min; miosis, dyspnea, inhibition of RBC-ChE changes in single fibre electromyography (SFEMG) (Baker and Sedgwick 1996)h |
|
AEGL-3 (Lethal) |
0.0027 ppm (0.029 |
0.0014 ppm (0.015 |
0.00091 ppm (0.010 |
0.00048 ppm (0.0052 |
0.00035 ppm (0.0038 |
Derived by relative potency from |
|
|
|
|
mg/m3) |
mg/m3) |
mg/m3) |
mg/m3) |
mg/m3) |
experimental SD rat lethality data (LC01)i and LC50); whole-body dynamic exposure to GB vapor concentrations between 2–54 mg/m3 for 3, 10, 30, 60, 90, 240, and 360 min (Mioduszewski et al. 2000; 2001; 2002a |
|
aThe derived AEGL values are for vapor exposures only. Percutaneous absorption of nerve agent vapors is known to be an effective route of exposure; nevertheless, percutaneous vapor concentrations needed to produce similar adverse effects are greater than inhalation vapor concentrations by several orders of magnitude (for agent VX, the percutaneous vapor concentrations needed to produce similar adverse effects are greater than inhalation vapor concentrations by an approximate factor of 10). Thus, the AEGL values presented are considered protective for both inhalation and percutaneous routes of exposure. bBased on relative potency equal to that of agent GB (see Section 4.3 and Mioduszewski et al. [1998]). cAgent GA is considered approximately one-half as potent as GB in causing lethality; thus, AEGL-3 values for GA are estimated by multiplying each time-specific AEGL-3 value for agent GB by a factor of 2 (see Section 4.3 and Mioduszewski et al. [1998]). dAgents GD and GF are considered approximately twice as potent as agents GA and GB for causing miosis, and equipotent to each other. Thus, AEGL-1 and AEGL-2 values are estimated by multiplying each time-specific AEGL-1 or AEGL-2 value for agent GB by a factor of 0.5 (see Section 4.3 and Mioduszewski et al. [1998]). eBased on a relative potency for lethality of GD=GF=GB and lethality data of Aas et al. (1985) (which provides a 10-min |
|||||||
|
AEGL-3 estimate of 0.27 mg/m3 and a 30-min AEGL-3 value of 0.15mg/m3 and is thus supportive of the GD AEGL-3 estimates derived from relative potency) (see Section 4.3 and Appendix A). fBased on relative potency. Agent VX is considered approximately 4 times more potent than agent GB. (See Section 4.3.4, Grob and Harvey [1958], and Sidell and Groff [1974].) gDerived from miosis effects noted in young adult female SD rats exposed to agent GB vapor at concentrations (0.010 to 0.48 mg/m3) for 10, 60 and 240 min (Mioduszewski et al. 2002b). VX concentration to achieve same end point estimated by relative potency adjustment presented in footnote f above. hDerived from transient effects noted in exercising human volunteers exposed to agent GB vapor at 0.5 mg·min/m3 for 30 min (Baker and Sedgwick 1996). VX concentration to achieve same end point estimated by relative potency adjustment presented in footnote f above. iDerived from LC01 values for female Sprague-Dawley rats exposed to GB vapor in dynamic exposure chamber (Mioduszewski et al. 2000, 2001, 2002a). VX concentrations to achieve same end point estimated by relative potency adjustment presented in footnote f above. |
levels. Estimates of AEGL-3 for GB for time periods <60 min overlap those considered to cause no effect or discomfort. Therefore, the AEGL estimates for agent GB are considered protective. Although comparative data for the other G agents are quite limited, the animal toxicity data that are available for agent GD and GF (Figures 1–3 and 1–4) further support the conclusion that the current AEGL estimates are protective for all levels of effect.
Agent VX
For the development of AEGL values for Agent VX, toxicity end points specific for each of the three AEGL levels were not available. Therefore, each of the AEGL values was derived from the corresponding critical AEGL study for agent GB, using the relative potency approach and the assumption that the potency of VX was 4 times greater than that of GB. After relative potency adjustment, the 8-h AEGL-3 for VX was scaled from the 6-h experimental data point for GB (see Appendix A).
Cross-comparison of the AEGL estimates for agent VX with available human toxicity data (Figure 1–5) reveals that the AEGL-3 estimates for agent VX are well below reported human exposure levels (median of 3.6 mg/m3) that result in mild, reversible discomfort (headache, chest tightness, and dryness of the mouth) (Koon et al. 1959). Lethal exposures in animals (Figure 1–6) occur 1 to 2 orders of magnitude above AEGL-3 estimates.
These comparisons with available experimental data indicate that the final AEGL estimates for agent VX are protective.
8.2. Comparison with other Standards and Criteria
G Agents
Exposure guidelines for the nerve agents GA, GB, and GD have been established by several organizations. All currently available guidelines are shown in Tables 1–40, 1–41, and 1–42. No comparable values have been established for exposure to agent GF. Values were not found for the following standards and guidelines: ERPGs, PELs, RELs, TLVs, and MAKs.
TABLE 1–40 Extant Standards and Guidelines for Nerve Agent GA (mg/m3)a
|
Guideline |
10 min |
30 min |
1 h |
4 h |
8 h |
24 h |
|
AEGL-1b |
0.0069 |
0.0040 |
0.0028 |
0.0014 |
0.0010 |
|
|
AEGL-2b |
0.087 |
0.050 |
0.035 |
0.017 |
0.013 |
|
|
AEGL-3b |
0.76 |
0.38 |
0.26 |
0.14 |
0.10 |
|
|
Army IDLHc |
|
0.2 |
|
|
|
|
|
DHHS TWAd |
|
|
|
|
0.0001 |
0.000003 |
|
aExisting exposure guidelines for nerve agents are published in mg/m3; the guidelines are presented here as they exist in the literature and have not been converted to parts per million. bPercutaneous absorption of G agent vapor is known to be an effective route of exposure; nevertheless, percutaneous vapor concentrations needed to produce similar adverse effects are greater than inhalation vapor concentrations by several orders of magnitude. Thus, the AEGL values presented are considered protective for both routes of exposure. cDA 1997. dDHHS 1988; 8-h value is TWA for occupational exposure; 24-h value is TWA for the general population. |
||||||
At present, the only chemical warfare exposure limits published in the United States for use in civilian community emergency preparedness planning are those developed by the Department of Health and Human Services (DHHS 1988; Thacker 1994). For the agents GA and GB, the current time-weighted average (TWA) to be applied as a no-adverse-health-effect level for 24-h continuous exposure to the general population is 3×10−6 mg/m3. For these same agents, the 8-h TWA to be applied as a no-adverse-health-effect level for 8-h continuous workplace exposure for worker populations is 1×10−4 mg/m3 (DHHS 1988). Agents GD and GF, which are not part of the unitary stockpile, were not evaluated by DHHS in 1988. As part of a regularly scheduled review process, the Centers for Disease Control and Prevention (CDC) is currently reevaluating the 1988 agent control limits with application of recent risk assessment models and updated scientific data (67 Fed. Reg. 895 [2002]; DHHS 2002). This review is currently (September 2002) in progress, and the CDC has not yet released a final position.
TABLE 1–41 Extant Standards and Guidelines for Nerve Agent GBa
|
Guideline |
No Time Period (Ct) (mg·min/m3) |
10-min (mg/m3) |
30-min (mg/m3) |
1-h (mg/m3) |
4-h (mg/m3) |
8-h (mg/m3) |
24-h (mg/m3) |
|
AEGL-1b |
|
0.0069 |
0.0040 |
0.0028 |
0.0014 |
0.0010 |
|
|
AEGL-2b |
|
0.087 |
0.050 |
0.035 |
0.017 |
0.013 |
|
|
AEGL-3b |
|
0.38 |
0.19 |
0.13 |
0.070 |
0.051 |
|
|
Army IDLHc |
|
|
0.2 |
|
|
|
|
|
DHHS TWAd |
|
|
|
|
|
0.0001 |
0.000003 |
|
DHHS ATELe |
0.5 |
|
|
|
|
|
|
|
aExisting exposure guidelines for nerve agents are published in mg/m3; the guidelines are presented here as they exist in the literature and have not been converted to parts per million. bPercutaneous absorption of G agent vapor is known to be an effective route of exposure; nevertheless, percutaneous vapor concentrations needed to produce similar adverse effects are greater than inhalation vapor concentrations by several orders of magnitude. Thus, the AEGL values presented are considered protective for both routes of exposure. cDA 1997. dDHHS 1988; 8-h value is TWA for occupational exposure; 24-h value is TWA for the general population eAcute Threshold Effects level (ATEL); Thacker 1994; based on linear extrapolation equivalent to 10 min to 0.05 mg/m3, 30 min to 0.017 mg/m3, 1 h to 0.0083 mg/m3, 4 h to 0.0021 mg/m3, or 8 h to 0.0010 mg/m3. |
|||||||
TABLE 1–42 Extant Standards and Guidelines for Nerve Agent GD (mg/m3)a
|
Guideline |
10 min |
30 min |
1 h |
4 h |
8 h |
24 h |
|
AEGL-1b |
0.0035 |
0.0020 |
0.0014 |
0.00070 |
0.00050 |
|
|
AEGL-2b |
0.044 |
0.025 |
0.018 |
0.0085 |
0.0065 |
|
|
AEGL-3b |
0.38 |
0.19 |
0.13 |
0.070 |
0.051 |
|
|
Army IDLHc |
|
0.06 |
|
|
|
|
|
Army TWAc |
|
|
|
|
0.00003 |
0.000003 |
|
aExisting exposure guidelines for nerve agents are published in mg/m3; the guidelines are presented here as they exist in the literature and have not been converted to parts per million. bPercutaneous absorption of G agent vapor is known to be an effective route of exposure; nevertheless, percutaneous vapor concentrations needed to produce similar adverse effects are greater than inhalation vapor concentrations by several orders of magnitude. Thus, the AEGL values presented are considered protective for both routes of exposure. cDA 1997. |
||||||
The CDC has also established an acute threshold effects level (ATEL) for agent GB (Thacker 1994). An ATEL is a cumulative exposure (Ct) (concentration in mg/m3 multiplied by time in minutes [mg·min/m3]; Ct does not express the amount retained within the organism [Sidell 1997]). These ATELs are considered by CDC to represent “lowest-observed-effect-levels” that “could be exceeded without danger” to the public and form the basis for planning protective actions, such as emergency evacuations, in the Chemical Stockpile Emergency Preparedness Program (CSEPP) of the Federal Emergency Management Agency and the Department of the Army. The Acute Threshold Effect Levels are described by CDC as protective of the general population (including consideration of vulnerable subgroups such as infants, the elderly, and debilitated or ill persons) (Thacker 1994). The ATEL for agent GB is 0.5 mg·min/m3, a protective cumulative exposure at which miosis is not expected to occur in humans (McNamara and Leitnaker 1971). If projected GB concentrations resulting from a release event were to result in Cts >0.5 mg·min/m3, then the CDC would consider
protective measures (such as evacuation or shelter-in-place) warranted as a means of providing maximal protection to safeguard the general public. The CDC did not designate the exposure time periods applicable to the ATEL.
For comparison to the AEGLs for the G agents, the CDC ATEL for GB can be converted (by direct linear extrapolation) to the following time-specific agent concentrations: 10-min, 0.05 mg/m3; 30-min, 0.017 mg/m3; 1-h, 0.0083 mg/m3; 4-h, 0.0021 mg/m3; or 8-h, 0.0010 mg/m3. These estimated concentrations are all greater than the agent-GB AEGL-1 values (with the exception of the 8-h interval, for which the ATEL and the AEGL-1 values are nominally equal) and less than the agent-GB AEGL-2 values. The estimated AEGL-1 and AEGL-2 values are thus in keeping with the CDC ATEL for agent GB (Thacker 1994).
Acute threshold effects levels have not been established for agents GA, GD, and GF.
Agent VX
Exposure guidelines for the nerve agent VX have been established by several organizations. All currently available guidelines are shown in Table 1–43.
At present, the only chemical warfare agent exposure limits published in the United States for use in civilian community emergency preparedness planning are those developed by the Department of Health and Human Services (DHHS 1988; Thacker 1994). For agent VX, the current time-weighted average (TWA) to be applied as a no-adverse-health-effect level for 24-h continuous exposure to the general population is 3×10−6 mg/m3. The 8-h TWA to be applied as a no-adverse-health-effect level for 8-h continuous workplace exposure for worker populations is 1×10−5 mg/m3 (DHHS 1988). As stated earlier, this review is currently (September 2002) in progress, and the CDC has not yet released a final position.
ATELs have also been developed by the CDC for agent VX (Thacker 1994). The ATEL value for agent VX is 0.4 mg·min/m3; the CDC did not designate specific exposure time periods applicable to the ATEL. If projected VX concentrations resulting from a release event resulted in VX Cts >0.4 mg·min/m3, the CDC would consider protective measures (such as evacuation or shelter-in-place) warranted as a means of providing maximal protection to safeguard the general public.
TABLE 1–43 Extant Standards and Guidelines for Nerve Agent VXa
|
Guideline |
No Time Period (Ct) (mg·min/m3) |
10 min (mg/m3) |
30 min (mg/m3) |
1 h (mg/m3) |
4 h (mg/m3) |
8 h (mg/m3) |
24 h (mg/m3) |
|
AEGL-1b |
|
0.00057 |
0.00033 |
0.00017 |
0.00010 |
0.000071 |
|
|
AEGL-2b |
|
0.0072 |
0.0042 |
0.0029 |
0.0015 |
0.0010 |
|
|
AEGL-3b |
|
0.029 |
0.015 |
0.010 |
0.0052 |
0.0038 |
|
|
Army IDLHc |
|
|
0.02 |
|
|
|
|
|
DHHS TWAd |
|
|
|
|
|
0.00001 |
0.000003 |
|
DHHS ATELe |
0.4 |
|
|
|
|
|
|
|
aExisting exposure guidelines for nerve agents are published in mg/m3; the guidelines are presented here as they exist in the literature, and have not been converted to parts per million. bThese final values are for vapor exposures only. cDA 1997. dDHHS 1988; 8-h value is TWA for occupational exposure, 5 d/wk; 24-h value is TWA for the general population, 7 d/wk. eThacker 1994. |
|||||||
For comparison to AEGLs for VX, the CDC ATEL for VX can be converted by extrapolation to the following time-specific agent concentrations: 10-min, 0.04 mg/m3; 30-min, 0.013 mg/m3; 1-h, 0.0067 mg/m3; 4-h, 0.0017 mg/m3; or 8-h, 0.00083 mg/m3. These values are in excess of AEGL-2 estimates for VX exposure durations ≤1 h. They are approximately equivalent to AEGL-2 estimates for VX exposure durations ≥4 h.
Because the VX AEGLs are derived in this report by comparison to the AEGL for agent GB, it should be noted that the Acute Threshold Effects Level for agent GB is 0.5 mg·min/m3.
8.3. Data Adequacy and Research Needs
G Agents
Confidence in the AEGL-2 values is limited by the sparse human experimental data for all AEGL-specific time frames and G-series agents. The human data from which the AEGL-2 values were derived were limited by short exposure times (30 min). The AEGL-1 values for agent GB derived from rat data (Mioduszewski et al. 2002b) are supported by 5-h exposure studies conducted on marmosets (van Helden et al. 2001, 2002) and 20-min exposure studies conducted with human volunteers (Harvey 1952; Johns 1952).
Observed differences among studies in identifying the toxicity threshold for low-dose exposures may be due to differences in individual sensitivities and breathing rates among the test populations, the steepness of the dose-response curve, and/or differences in experimental protocols or analytical methods. These variables need further characterization.
Further data analysis and experimentation are needed to more fully understand the degree of susceptibility to lethal exposure exhibited by female populations of test animals. Interspecies susceptibility could be more fully characterized by determining if similar results can be obtained under the same protocol with different test species (particularly nonhuman primates).
The scarcity of dose-response data for agents GA, GD, and GF forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL values for these three compounds are largely derivative (with the exception of 10-min and 30-min AEGL-3 values for agent GD, which are confirmed by short-term experimental lethality data for Wistar rats provided by Aas et. al. [1985]).
The relative potency assumptions for estimating AEGL-1, AEGL-2, and AEGL-3 values for agents GA, GD, and GF from the available database for agent GB need experimental confirmation.
Additional research is needed to compare and contrast effects of all nerve agents on noncholinergic neurotransmitters and neurotransmitter receptor sites for correlating reported effects observed in rat hippocampal cells in vitro (see Section 4.2) with whole-organism responses to acutely toxic dose levels. Extrapolation of findings from such neurophysiological research to whole organisms may allow a more refined quantitative analysis of the nerve agent relative potency.
Agent VX
The available experimental human acute toxicity data for agent VX are limited by the short exposure times (7 min or less), uncontrolled atmospheres for inhalation studies, inadequate exposure protocols, or inappropriate exposure routes. Longer-term inhalation exposure data would be useful for deriving more reliable AEGL-1 values. The Bramwell et al. (1963) study is a flawed and suspect source and could not be applied in deriving exposure estimates.
Only a single nonlethal animal inhalation toxicity study has been conducted on agent VX (Crook et al. 1983). The results of this animal study are considered nonverifiable and are thus not adequate for deriving an AEGL-1 or an AEGL-2 for VX.
Credible acute animal lethality data for inhalation vapor exposures to agent VX are available only in the form of LCt50 values for exposure times of 10 min or less for two species, mice and goats (Koon et al. 1960, as cited in NRC 1997). Available animal lethality data for VX do not cover the time periods relevant for deriving AEGL-3 values. A comprehensive animal lethality data set is needed for directly deriving AEGL-3 estimates.
Confidence in the AEGL values derived for agent VX is limited by sparse human inhalation data for the AEGL-specific time frames and a definitive assessment of the exposure-response relationship for humans exposed to VX vapor. Derivation of AEGL-1, AEGL-2, and AEGL-3 values using the relative potency method and comparison with agent GB is limited by uncertainties associated with the derivation of the values for agent GB as well as uncertainties associated with estimating the potency of VX relative to that of GB.
Further experimentation is needed to more fully understand the degree of sensitivity to lethal exposure exhibited by female populations of test animals. Interspecies sensitivity could be more fully characterized by determining if similar results can be obtained with different test species (particularly nonhuman primates).
It is noted that specific experimental focus should be put on obtaining data that would reduce uncertainties regarding the relative potency of agents GB and VX, or the potency of agent VX, for critical effects such as miosis, rhinorrhea, and lethality; such studies could be adequately performed on a limited test population and scale. Tests should be performed with a single species (preferably laboratory rat), both genders, and should involve vapor exposures over the range of 10 min to 8 h (minimally, for 10 min, 1 h, and 6 h). It is further recommended that dosages selected begin slightly below the recommended AEGL-1 estimate and increase in 10-fold increments to include the anticipated LCt50. Clinical effects should be observed, and histopathology and necropsy performed on a sample from each Ct group.
In addition, research characterizing the emission profile of vapor and aerosols expected during VX release events is needed for estimation of potential differential exposure and toxicity. Agent VX parameters needing quantification by modern methods and protocols include: generation and yield of vapors versus aerosols; particle size ranges; atmospheric half-times; deposition rates; and rates of degradation in air under various states of humidity, temperature, UV, et cetera. Until these parameters can be more fully characterized to inform the discussion of differential toxicity between VX vapors and aerosols, AEGL determinations will be necessarily based on the assumption of exposure to VX vapor.
9. REFERENCES
Aas, P., T.A.Veiteberg, and F.Fonnum, F. 1987. Acute and sub-acute inhalation of an organophosphate induced alteration of cholinergic muscarinic receptors. Biochem. Pharmacol. 36:1261–1266.
Aas, P., S.H.Sterri, H.P.Hjermstad, and F.Fonnum. 1985. A method for generating toxic vapors of soman: Toxicity of soman by inhalation in rats. Toxicol. Appl. Pharmacol. 80:437–445.
Abou-Donia, M.B. 1993. The cytoskeleton as a target for organophosphorous ester-induced delayed neurotoxicity (OPIDN). Chem.-Biol. Interactions 87: 383–393.
Abou-Donia, M.B., and D.M.Lapadula. 1990. Mechanisms of organophosphorous ester-induced delayed neurotoxicity: Type I and type II. Ann. Rev. Pharmacol. Toxicol. 30:405–440.
Abu-Qare, A.W., and M.B.Abou-Donia. 2001. Combined exposure to sarin and pyridostigmine bromide increased levels of rat urinary 3-nitrotyrosine and 8-hydroxy-2'-deoxyguanosine, biomarkers of oxidative stress. Toxicol. Lett. 123:51–58.
ACGIH (American Conference of Governmental Industrial Hygienists). 1996. Supplements to the Sixth Edition; Documentation of the Threshold Limit Values and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH. (pp. 1–7 for n-butyl acetate).
ACGIH (American Conference of Governmental Industrial Hygienists). 2002. 2002 TLVs and BEIs; Threshold Limit Values and Biological Exposure Indices for chemical substances and physical agents. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
A.D. Little, Inc. 1985. Pharmacokinetic parameters of selected organophosphate compounds with anticholinesterase activity. AAMRL-TR-85–040 (DTIC AD-A157 923). Prepared by Arthur D. Little, Inc., Cambridge, MA, for Air Force Aerospace Medical Research Laboratory, Aerospace Medical Division, Air Force Systems Command, Wright-Patterson Air Force Base, Ohio 45433.
Adams, N.L., J.von Bredow, and H.V.deVera. 1975. Intramuscular Lethality of GD (Soman) in the Rhesus monkey. EB-TR-76039. Biomedical Laboratory, Edgewood Arsenal, MD.
Allebrandt, K.V., R.L.R.Souza, and E.A.Chautard-Freire-Maia. 2002. Variability of the paraoxonase gene (PON1) in Euro- and Afro-Brazilians. Toxicol. Appl. Pharm. 180:151–156.
Allon, N., L.Raveh, E.Gilat, E.Cohen, J.Grunwald, and Y.Ashani. 1998. Prophylaxis against soman inhalation toxicity in guinea pigs by pretreatment alone with human serum butyrylcholinesterase. Toxicol. Sci. 43:121–128.
AMRL (Aerospace Medical Research Laboratory). 1977. Proceedings of the Annual Conference on Environmental Toxicology (7th). Report No. AMRL-TR-76–125. Aerospace Medical Research Laboratory, Wright-Patterson, Air Force Base, OH, Oct. 13–15, 1976.
Ammon, R. 1933. The enzymatic hydrolysis of acetylcholine. Arch. Ges. Physiol. 233:486.
Anthony, J.S., M.V.Haley, J.H.Manthei, R.A.Way, D.C.Burnett, B.P.Gaviola, D.R.Sommerville, R.B.Crosier, R.J.Mioduszewski, E.M.Jakubowski, J.L. Montgomery, and S.A.Thomson, S.A. 2002. Inhalation toxicity of GF vapor in rats as a function of exposure concentration and duration and its potency comparison to GB. Late-breaking poster presented at 41st Annual Meeting of the Society of Toxicology, Nashville, TN (21 Mar 2002).
Antikainen, M., S.Murtomaki, M.Syvanne, R.Pahlman, E.Tahvanainen, M. Jauhiainen, M.H.Frick, and C.Ehnholm. 1996. The Gln-Arg 191 polymor
phism of the human paraoxonase gene (HUMPONA) is not associated with the risk of coronary artery disease in Finns. J. Clin. Invest. 98:883–885.
Anzueto, A., R.A.DeLemos, J.Seidenfeld, G.Moore, H.Hamil, D.Johnson, and S.G.Jenkinson. 1990. Acute inhalation toxicity of soman and sarin in baboons. Fund. Appl. Toxicol. 14:676–687.
Araneta, M.R., C.A.Moore, R.S.Olney, L.D.Edmonds, J.A.Karcher, C. McDonough, K.M.Hiliopoulos, K.M.Schlangen, and G.C.Gray. 1997. Goldenhar syndrome among infants born in military hospitals to Gulf War veterans. Teratology 56:244–251.
Ashani, Y., J.Grunwald, L.Raveh, et al. 1993. Cholinesterase prophylaxis against organophosphorous poisoning. AD-A277096. U.S. Army Medical Research, Development, Acquisition and Logistics Command, Ft. Derrick, MD.
Ashwick, W.E., and C.A.deCandole. 1961. A Further Trial of Atropine, Metaraminol, and P2S in the Treatment of GB Poisoning in the Monkey. Suffield Technical Paper No. 208. Defence Research Board, Department of National Defence, Canada.
Atchison, C.R., C.Holmes, S.Akers, et al. 2001. Guinea pig model for low dose chronic exposure to nerve agent. Proceedings of the Scientific Conference on Chemical and Biological Defense Research. March 6–8, 2001, Hunt Valley, MD.
Augustinsson, K.-B. 1955. The normal variation of human blood cholinesterase activity. Acta Physiol. Scand. 35:40–52. (As cited in Hayes 1982.)
Augustinsson, K.-B. 1959. Electrophoresis studies on blood plasma esterases. I. Mammalian plasmata. Acta. Chem. Scand. 13:571–592.
Baker, D.J., and E.M.Sedgwick. 1996. Single fibre electromyographic changes in man after organophosphate exposure. Hum. Exp. Toxicol. 15:369–375.
Bakerman, S. 1984. ABC’s of Interpretive Laboratory Data, 2nd Ed. Interpretative Laboratory Data, P.O. Box 7066, Greenville, NC (p. 127).
Bakry, N.M., A.H.El-Rashidy, A.T.Eldefawi, and M.E.Eldefawi. 1988. Direct action of organophosphate anticholinesterases on nicotinic and muscarinic acetylcholine receptors. J. Biochem. Toxicol. 3:235–259.
Bakshi, K.S., S.N.J.Pang, and R.Snyder, eds. 2000. Review of the U.S. Army’s Health Risk Assessments for oral exposure to six chemical-warfare agents. J. Toxicol. Environ. Health (Part A):59(5–6):281–526.
Bates, H.K., J.B.LaBorde, J.C, Dacre, and J.F.Young. 1990. Developmental toxicity of soman in rats and rabbits. Teratology 42:15–23.
Benke, G.M., and S.D.Murphy. 1975. The influence of age on the toxicity and metabolism of methyl parathion and parathion in male and female rats. Tox. Appl. Pharmac. 31:254–269.
Benschop, H.P. 1999. Toxicokinetics of O-Ethyl S-(2-Diisopropylaminoethyl) Methylphosphonothioate [(±)−VX] in Hairless Guinea Pigs and Marmosets-Identification of Metabolic Pathways. Annual Report (Project: DAMD17–97– 2–7001). Annual Report (Project: DAMD17–97–2–7001). Medical Research and Materiel Command, Fort Derrick, MD.
Benschop, H.P., C.A.G.Konings, and L.P.A.de Jong. 1981. Gas chromatographic separation and identification of the four stereoisomers of 1,2,2-trimethylpropyl methylphosphonofluoridate (soman). Stereospecificity of in vitro “detoxification” reactions. J. Am. Chem. Soc. 103:4260–4262.
Benschop, H.P., H.C.Trap, H.E.T.Spruit, et al. 1998. Low-level nose-only exposure to the nerve agent soman: toxicokinetics of soman stereoisomers and cholinesterase inhibition in atropinized guinea pigs. Toxicol. Appl. Pharmacol. 153:179–185.
Benschop, H.P., M.J.van der Schans, and J.P.Langenberg. 2000. Toxicokinetics of O-Ethyl S-(2-Diisopropylaminoethyl)Methylphosphonothioate [(±)−VX] in Rats, Hairless Guinea Pigs and Marmosets—Identification of Metabolic Pathways. Final Report (Project: DAMD17–97–2–7001). Prepared for U.S. Army Medical Research and Materiel Command, Fort Detrick, MD.
Berteau, P.E., and R.E.Chiles. 1978. Studies on the inhalation toxicity of two phosphoramidothiate insecticides to rodents and quail. Toxicol. Appl. Pharmacol. 45:232.
Bide, R., J.Armour, and E.Yee. 1999. An approach to obtain estimates of human toxicity. Part III: A reasonable, defendable procedure to obtain human inhalation toxicity estimates (LCt05, LCt50 and LCt95) directly from animal toxicity data. Presented to the NATO Challenge Subgroup, 12–14 May, 1999, San Antonio, TX.
Bide, R.W., and D.J.Risk. 2000. Inhalation Toxicity of Aerosolized Nerve Agents. 1. VX Revisited. Technical Report DRES TR 2000–063. Defence Research Establishment Suffield, Alberta, Canada.
Bide, R.W., and D.J.Risk. 2001. GB toxicity in mice exposed for 20 min to 720 min (U). DRES-TR-2000–173. Defence Research Establishment Suffield, Ralston, Alberta, Canada (in review).
Billecke, S.S., S.L.Primo-Parmo, C.S.Dunlop, J.A.Doorn, B.N.LaDu, and C.A. Broomfield. 1999. Characterization of a soluble mouse liver enzyme capable of hydrolyzing diisopropyl phosphoroflouridate. Chemico-Biological Interactions 119–120:251–256.
Bonderman, R.P., and D.P.Bonderman. 1971. Atypical and inhibited human serum pseudocholinesterase: A titrimetric method for differentiation. Arch. Environ. Health 22:578–581.
Boter, H.L., A.J.J.Ooms, G.R.Van DenBerg, and C.Van Dijk. 1966. The synthesis of optically active isopropyl methylphosphonofluoridate (sarin). Recueil 85:147–150.
Bowers, M.B., E.Goodman, and V.M.Sim. 1964. Some behavioral changes in man following anticholinesterase administration. J. Nerv. Mental Dis. 138:383–389.
Bramwell, E.C.B., W.S.S.Ladell, and R.J.Shephard. 1963. Human exposure to VX vapour. Porton Technical Paper No. 830. Porton Down, Salisbury, Wiltshire, UK.
Britton, K.B., and C.L.Grant. 1988. Prediction of octanol-water partition coeffi
cients of organophosphates. Evaluation of structure-function relationships. Special Report 88–11, U.S. Department of the Army, Corps of Engineers Cold Regions Research and Engineering Laboratory, Hanover, NF.
Brown, M.A., and K.A.Brix. 1998. Review of health consequences from high-, intermediate- and low-level exposure to organophosphorus nerve agents. J. Appl. Toxicol. 18:393–408.
Bucci, T.J. and R.M.Parker. 1992. Toxicity studies on agents GB and GD (phase II): 90-day subchronic study of GB (sarin type II) in CD rats. Final report (DTIC: AD-A248618). E515 and E516. National Center for Toxicological Research. Prepared for the U.S. Department of the Army, Biomedical Research and Development Laboratory, Ft. Detrick, MD.
Bucci, T.J., R.M.Parker, J.A.Crowell, J.D.Thurman and P.A.Gosnell. 1991. Toxicity studies on agents GB and GD (phase II): 90-day subchronic study of GB (sarin type I) in CD rats. FDA 224–85–0007 (DTIC: AD-A248617). Prepared for the U.S. Department of the Army, Biomedical Research and Development Laboratory, Ft. Detrick, MD.
Bucci, T.J., R.M.Parker, J.A.Crowell, J.D.Thurman, and P.A.Gosnell. 1992. Toxicity studies on agent GA (phase II): 90-day subchronic study of GA (tabun) in CD rats. Final report (DTIC: AD-A258020). Prepared for the U.S. Department of the Army, Biomedical Research and Development Laboratory, Ft. Detrick, MD.
Bucci, T.J., J.D.Fikes, R.M.Parker, K.H.Denny, and J.C.Dacre. 1993. Developmental toxicity study (segment II: teratology) of tabun in CD rats and New Zealand white rabbits. Prepared for the U.S. Department of the Army, Medical Research and Development Command, Ft. Detrick, MD.
Burchfiel, J.L., and F.H.Duffy. 1982. Organophosphate neurotoxicity: Chronic effects of sarin on the electroencephalogram of monkey and man. Neurobehav. Toxicol. Teratol. 4:767–778.
Burnett, W.T., and J.E.Chambers. 1994. Age-related toxicity of the insecticides parathion and chlorpyrifos. Toxicologist 14:390. (As cited in Davies 1996.)
California Environmental Protection Agency. 1998. Medical Supervision. Pesticide and Safety Information, Series A, No. HS-8. Worker Health and Safety Branch, California Environmental Protection Agency, Department of Pesticide Regulation, Sacramento, CA (Sept 20, 1998).
Callaway, S., and J.W.Blackburn. 1954. A comparative assessment of the vapour toxicities of GB, GD, GF, T-2132, T-2137, and T-2146 to male and female rats. Porton Technical Paper No. 404. Chemical Defence Establishment, Porton Down, England.
Callaway, S., and D.Crichton. 1954. The production of casualties in monkeys with GB vapor. Porton Technical Paper No. 424. Chemical Defence Establishment, Porton Down, England.
Callaway S., and P.Dirnhuber. 1971. Estimation of the concentration of nerve agent vapour required to produce measured degrees of miosis in rabbit and
human eyes. Technical Paper No. 64. Chemical Defence Establishment, Porton Down, Salisbury, Wiltshire, UK.
Carnes, S.A. 1989. Disposing of the chemical weapons: A desired end in search of an acceptable means. Environ. Prof. 11(4):279–290.
Carnes, S.A., J.A.Boyette, F.C.Kornegay, et al. 1989. Preliminary Assessment of the health and environmental impacts of incinerating M55 rockets stored at Pine Bluff Arsenal, Lexington-Blue Grass Depot Activity, and/or Anniston Army Depot and Pine Bluff Arsenal, ORNL 6197, Oak Ridge National Laboratory, Oak Ridge TN.
Carnes, S.A., and A.P.Watson. 1989. Disposing of the U.S. chemical weapons stockpile: An approaching reality. JAMA 262:653–659.
Carroll, N.C., F.C.G.Hoskin, M.K.McPhail, and D.K.Myers. 1957. Vapour toxicity of VE and VX to female mice. STP 121. Suffield Experimental Station, Canada.
Cashman, J.R., B.Y.Perotti, C.E.Berkman, and J.Lin. 1996. Pharmacokinetics and molecular detoxication. Environ. Health Perspect. 104:23–40.
Chanda, S.M., T.L.Lassiter, V.C.Moser, S.Barone, Jr., and S.Padilla. 2002. Tissue carboxylesterases and chlorpyrifos toxicity in the developing rat. HERA 8:75–90.
Chebabo, S.R., M.D.Santos, and E.X.Albuquerque. 1999. The organophosphate sarin, at low concentrations, inhibits the evoked release of GABA in rat hippocampal slices. Neurotoxicol. 20(6):871–882.
Chen, S.-M., and M.-G.Chi. 1986. Direct effect of VX and soman on nicotinic receptors. Acta Pharmacol. Sinica 7:401–406.
Christen, P.J., and J.A.C.M.van den Muysenberg. 1965. The enzymatic isolation and fluoride catalyzed racemisation of optically active sarin. Biochim. Biophys. Acta. 110:217–220.
Christensen, M.K., P.Cresthull, and F.W.Oberst. 1958. ICt99 and LCt1–99 estimates of GB Vapor for man at various exposure times. Chemical Warfare Laboratories Report 2266, Army Chemical Center, MD.
Ciliberto, C.F., and G.F.Marx. 1998. Physiological changes associated with pregnancy. Physiology 9(2) [Online]. Available at www.nda.ox.ac.uk/wfsa/html/u09/u09_005.htm [accessed on January 28, 2002].
Clark, D.N. 1989. Review of reactions of chemical agents in water. DTIC: AD-A213 287. Defense Technical Information Center, Alexandria, VA.
Clement, J.G. 1983. Effect of pretreatment with sodium phenobarbitol on the toxicity of soman in mice. Biochem. Pharmacol. 32:1411–1415.
Clement, J.G. 1984. Role of aliesterase in organophosphate poisoning. Fund. Appl. Toxicol. 4:S96–S105.
Clement, J.G. 1992. Efficacy of various oximes against GF (Cyclohexylmethylphosphonofluoridate) poisoning in mice. Arch. Toxicol. 66:143–144.
Clement, J.G. 1994. Toxicity of the combined nerve agents GB/GF in mice: Efficacy of atropine and various oximes as antidotes. Arch. Toxicol. 68:64–66.
Cohen, B.M., P.J.Christen, and E.Mobach. 1971. The inactivation by oximes of sarin and soman in plasma from various species. I. The influence of diacetylmonoxime on the hydrolysis of sarin. J.A.Cohen Memorial Issue, Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen (Proceedings of the Royal Netherlands Academy of Sciences and Letters). North-Holland, Amsterdam, pp. 113–131.
Cohen, B.S., F.W.Oberst, J.W.Crook, and C.Harris. 1954. Effect of repeated exposures of GB vapor on erythrocyte and brain cholinesterase in rats. Medical Laboratories Research Report No. 297. Army Chemical Center, MD.
Conn, C.A., K.Dokladny, M.G.Ménache, E.B.Barr, W.Kozak, A.Kozak, M. Wachulec, K.Rudolph, M.J.Kluger, and R.F.Henderson. 2002. Effects of sarin on temperature and activity of rats as a model for Gulf War Syndrome neuroregulatory functions. Toxicol. Appl. Pharm. 184:77–81.
Cresthull, P., W.S.Koon, F.P.McGrath, and F.W.Oberst. 1957. Inhalation effects, incapacitation and mortality for monkeys exposed to GA, GB, and GF Vapors. Chemical Warfare Laboratory Report No. 2179. Army Chemical Center, MD.
Cresthull, P., W.S.Koon, and N.P.Musselman. 1963. Percutaneous exposure of the arm or forearm of man to VX vapor. U.S. Army Chemical Research and Developmental Laboratories, Technical Report No. 3176. Edgewood Arsenal, MD.
Cresthull, P., J.W.Crook, M.K.Christensen, et al. 1959. Relationship between the LD50 and ChE50 values for monkeys and dogs exposed to GB by the inhalation and intravenous routes. Chemical Warfare Laboratory Report No. 2280. Army Chemical Center, MD.
Crook, J.W., P.Hott, E.J.Owens, et al. 1983. The effects of subacute exposures of the mouse, rat, guinea pig, and rabbit, to low-level VX concentrations. U.S. Army Armament Research and Development Command, Chemical Systems Laboratory, Technical Report ARCSL-TR-82038, Aberdeen Proving Ground, MD.
DA (Department of the Army). 1974. Chemical agent data sheets, vol. 1. Edgewood Arsenal Special Report, EO-SR-74001. U.S. Department of the Army, Edgewood Arsenal, Aberdeen Proving Ground, MD.
DA (Department of the Army). 1987. Safety regulations for chemical agents H, HD, HT, GB and VX. AMC-R385–131. U.S. Department of the Army, Material Command, Alexandria, VA.
DA (Department of the Army). 1988. Final programmatic environmental impact statement for the chemical stockpile disposal program. U.S. Department of the Army, Office of the Program Executive Officer, Program Manager for Chemical Demilitarization, Aberdeen Proving Ground, MD.
DA (Department of the Army). 1990a. Occupational health guidelines for evaluation and control of occupational exposure to nerve agents GA, GB, GD and VX. DA Pam 40–8. U.S. Department of the Army, Headquarters. Washington, DC.
DA (Department of the Army). 1990b. Potential Military Chemical/Biological Agents and Compounds. Field Manual FM 3–9 (NAVFAC P-467, AFR 355– 7), Headquarters, Department of the Army, Department of the Navy, Department of the Air Force, Washington, DC (12 December 1990).
DA (Department of the Army). 1991. Occupational health guidelines for evaluation and control of occupational exposure to nerve agents GA, GB, GD and VX. DA Pam 40–8. U.S. Department of the Army, Headquarters, Washington, DC (Interim change I01, Revision, 15 May 91).
DA (Department of the Army). 1992. Material safety data sheets: Lethal nerve agents GA, GB, VX. U.S. Department of the Army, Edgewood Research, Development and Engineering Center, Aberdeen Proving Ground, MD.
DA (Department of the Army). 1997. The Army Chemical Agent Safety Program. Army Regulation 385–61 (AR 385–61). Headquarters, Department of the Army, Washington, DC. (26 Feb 1997).
DA (Department of the Army). 2001. Transportable treatment systems for nonstockpile chemical warfare materiel, Programmatic Environmental Impact Statement, Vol. I and II. U.S. Department of the Army, Project Manager for Non-Stockpile Chemical Materiel, Aberdeen Proving Ground, MD (February 2001).
Dacre, J.C. 1984. Toxicology of some anticholinesterases used as chemical warfare agents—a review. Pp. 415–426. in: Cholinesterases: Fundamental and Applied Aspects, M.Brzin, E.A.Barnard, and D.Sket, eds. New York: Walter de Gruyter.
Davies, H.G., R.J.Richter, M.Keifer, C.A.Broomfield, J.Sowalla, and C.E.Furlong. 1996. The effect of human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat. Genet. 14:334–336.
de Jong, L.P.A., and G.Z.Wolring. 1978. Effect of 1-(AR)alkyl-2-hydroxyiminomethyl-pyridinium salts on reactivation and aging of acetylcholinesterase inhibited by ethyl dimethylphosphor- amidocyanidate (tabun). Biochem. Pharmacol. 27:2229–2235.
de Wolff, F.A., N.Treijtel, and M.Vermeulen. 2002. Mechanisms of peripheral neurotoxicity. Chapter. 15, pp. 282–303, in Site-Selective Neurotoxicity, D.S. Lester, W.Slikker, and P.Lazarovici, eds. New York: Taylor and Francis Publishers.
Denk, J.R., 1975. Effects of GB on mammalian germ cells and reproductive performance. EB-TR-74087, U.S. Department of the Army, Edgewood Arsenal, Aberdeen Proving Ground, MD.
DHHS (U.S. Department of Health and Human Services, Centers for Disease Control and Prevention). 1988. Final recommendations for protecting the health and safety against potential adverse effects of long-term exposure to low doses of agents GA, GB, VX, Mustard Agent (H, HD, T), and Lewisite (L). Fed. Reg. 53:8504–8507, 1988.
DHHS (Department of Health and Human Services). 2002. Airborne exposure
limits for chemical warfare agents GA (Tabun), GB (Sarin), and VX. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Fed. Reg. 67(5):894–901 (8 January 2002).
Due, H.A., H.C.Trap, H.J.Van der Wiel, and H.P.Benschop. 1993. Effect of pretreatment with CBDP on the toxicokinetics of soman stereoisomers in rats and guinea pigs. Arch. Toxicol. 67:760–711.
Duffy, F.H., J.L.Burchfiel, P.H.Bartels, M.Gaon, and V.M.Sim. 1979. Long-term effects of an organophosphate upon the human electroencephalogram. Toxicol. Appl. Pharmacol. 47:161–176.
Dunn, M.A., and F.R.Sidell. 1989. Progress in the medical defense against nerve agents. J. Amer. Med. Assoc. 262:649–652.
Dutreau, C.W., F.P.McGrath, and E.H.Bray, Jr. 1950. Toxicity studies on GD. 1. Median lethal concentration by inhalation in pigeons, rabbits, rats, and mice. 2. Median detectable concentration by odor for man. MDRR 8, CMLEM-52. Chemical Corps, Medical Division, Army Chemical Center, MD (ADE 470029 058).
EGESAQ (Egezsegtudomany). 1980. 24:173 (Kultura, POB 149, H-1389, Budapest, Hungary).
Ehrich, M., and B.S.Jortner. 2002. Organophosphorous-induced delayed neuropathy. Chapter 2, pp. 17–25 in Handbook of Neurotoxicity, Vol. 1, E.J.Massaro, ed. Totowa, NJ: Humana Press.
Ellin, R.I. 1981. Anomalies in theories and therapy of intoxication by potent organophosphorus anticholinesterase compounds. U.S. Army Medical Research and Development Command, Biomedical Laboratory, Report No. USABML-SP-81–003. Aberdeen Proving Ground, MD. DTIC, ADA101364.
Ellman, G.L., K.D.Courney, V.Andres, Jr., and R.M.Featherstone. 1961. A new rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 7:88–95.
EPA (U.S. Environmental Protection Agency). 1992. Draft report: A cross-species scaling factor for carcinogen risk assessment based on equivalence of mg/kg3/4/day. Fed. Reg. 57(109):24152–24173.
EPA (U.S. Environmental Protection Agency). 1998. Health Effects Test Guidelines. OPPTS 870.6200 Neurotoxicity Screening Battery. Office of Prevention, Pesticides and Toxic Substances. EPA 712-C-98–238, U.S. Environmental Protection Agency, Washington, DC (August 1998).
EPA (U.S. Environmental Protection Agency). 2000. Office of Pesticide Programs science policy on the use of data on cholinesterase inhibition for risk assessment of organophosphorus and carbamate pesticides. Office of Pesticide Programs, U.S. Environmental Protection Agency, Washington, DC (August 18, 2000 ).
Eto, M. 1974. Organophosphorus Pesticides: Organic and Biological Chemistry. Cleveland, OH: CRC Press. Pp. 123–231.
Fairley, A., and S.A.Mumford. 1948. Detection of the G gases by smell. PTP 74. Porton Down, England.
FCH (Farm Chemicals Handbook). 1991. Farm Chemicals Handbook, Meister Publishing, Willoughy, OH.
FEMA/DA (Federal Emergency Management Agency/U.S. Department of the Army). 1996. Planning Guidance for the Chemical Stockpile Emergency Preparedness Program. Federal Emergency Management Agency and U.S. Department of the Army, Washington, DC.
Fogleman, R.W., J.P.Saunders, and R.T.Goring. 1954. The respiratory effects of inhaled toxic vapors dispersed in air. II. The subacute effects on dogs of low inhaled dosages of GB administered by the dosimetric technique. Medical Laboratory Research Report, MLRR 287. Army Chemical Center, MD.
Fonnum, F., and S.H.Sterri. 1981. Factors modifying the toxicity of organophosphorous compounds, including soman and sarin. Fund. Appl. Toxicol. 1:143–147.
Freeman, G., F.N.Marzulli, A.B.Craig, et al. 1954. The toxicity of liquid GA applied to the skin of man. Chemical Corps Medical Laboratory Research Report No. 250 (abstract only available).
Frostling, H. 1974. The vapour pressure of O-ethyl, S-3 (N,N-diisopropylamino)-ethylmethyl phosphonothiolate. Acta Chemica Scand. A28:83–85.
Furlong, C.E., R.J.Richter, S.L.Seidel, L.G.Costa, and A.G.Motulsky. 1988. Role of genetic polymorphism of human plasma paraoxonase/arylesterase in hydrolysis of the insecticide metabolites chlorpyrifos oxon and paraoxon. Am. J. Hum. Genet. 43:230–238.
Furlong, C.E., R.J.Richter, S.L.Seidel, L.G.Costa, and A.G.Motulsky. 1989. Spectrophotometric assays for the enzymatic hydrolysis of the active metabolites of chlorpyrifos and parathion by plasma paraoxonase/arylesterase. Analyt. Biochem. 180:242–247.
Furlong, C.E., W-F.Li, D.M.Shih, A.J.Lusis, R.J.Richter, and L.G.Costa. 2002. Genetic factors in susceptibility: Serum PON1 variation between individuals and species. HERA 8:31–43.
Goldman, M., L.S.Rosenblatt, B.W.Wilson, et al. 1988. Toxicity studies on agent VX: Final report. Laboratory for Energy-Related Health Research, University of California, Davis, CA.
Goldman, M., A.K.Klein, T.G.Kawakami, and L.S.Rosenblatt. 1987. Toxicity studies on agents GB and GD. DTIC: ADA187841. Laboratory for Energy-Related Health Research, Prepared for the U.S. Department of the Army, Medical Research and Development Command, Fort Derrick, MD.
Goldstein, G., S.R.Beers, and L.A.Morrow. 1996. A preliminary neuropsychological study of Persian Gulf veterans. J. Int. Neuropsychol. Soc. 2:368–371.
Gordon, J.J., R.H.Inns, M.K.Johnson, L.Leadbeater, M.P.Maidment, D.G.Upshall, G.H.Cooper, and R.L.Rickard. 1983. The delayed neuropathic effects of nerve agents and some other organophosphorous compounds. Arch. Toxicol. 52:71–82.
Grant, W.M. Toxicology of the Eye, Third Edition. 1986. Springfield, Illinois: Charles C.Thomas, Publisher.
Gray, G.C., B.D.Coate, C.M.Anderson, et al. 1996. The postwar hospitalization experience of U.S. veterans of the Persian Gulf War. New England J. Med. 335:1505–1513.
Gray, G.C., T.C.Smith, J.D.Knoke, and J.Heller. 1999. The postwar hospitalization experience of Gulf War veterans possibly exposed to chemical munitions destruction at Khamisiyah, Iraq. Amer. J. Epidemiol. 150:532–540.
Grob, D., and J.C.Harvey. 1958. Effects in man of the anticholinesterase compound Sarin (isopropyl methyl phosphonofluoridate). J. Clin. Invest. 37:350–368.
Gupta, R.C., G.T.Patterson, and W.-D.Dettbarn. 1987. Acute tabun toxicity: Biochemical and histochemical consequences in brain and skeletal muscles of rat. Toxicol. 46:329–341.
Gupta, R.C., G.T.Patterson, and W.-D.Dettbarn. 1991. Comparison of cholinergic and neuromuscular toxicity following acute exposure to sarin and VX in rat. Fund. Appl. Toxicol. 16:449–458.
Haboubi, N.A., and D.E.Thurnham. 1986. Effect of ethanol on erythrocyte acetylcholinesterase activity. Ann. Clin. Biochem. 23:458–462.
Haley, R.W. 1998. Counterpoint: Haley replies. Amer. J. Epidemiol. 148:334– 338.
Haley, R.W., and T.L.Kurt. 1997. Self-reported exposure to neurotoxic chemical combinations in the Gulf War: A cross-sectional epidemiologic study. J. Amer. Med. Assoc. 277:231–237.
Haley, R.W., T.L.Kurt, and J.Hom. 1997a. Is there a Gulf War Syndrome? Searching for syndromes by factor analysis of symptoms. J. Amer. Med. Assoc. 277:215–222.
Haley, R.W., J.Hom, and P.S.Roland. 1997b. Evaluation of neurologic function in Gulf War veterans: A blinded case-control study. J. Amer. Med. Assoc. 277:223–230.
Harris, H., and M.Whittaker. 1962. The serum cholinesterase variants: Study of twenty-two families selected via the “intermediate” phenotype. Ann. Hum. Genet. 26:59–72.
Harris, C., W.Koon, J.W.Christensen, and F.W.Oberst. 1953. Do pathological lesions develop in dogs repeatedly exposed to low concentrations of GB vapor? Medical Laboratory Research Report, MLRR 155. Army Chemical Center, MD.
Harris, L.W., W.C.Heyl, D.L.Stitcher, and C.A.Broomfield. 1978. Effects of 1,1-oxydimethylene-bis-(4-tert-butyl pyridinium chloride) (SAD-128) and decamethonium on reactivation of soman- and sarin-inhibited cholinesterase by oximes. Biochem. Pharmacol. 27:757–761.
Hartley, D., and H.Kidd, eds. 1983–1986. Agrochemicals Handbook. Nottingham, UK: Royal Soc. Chem.
Harvey, J.C. 1952. Clinical observations on volunteers exposed to concentrations of GB. Medical Laboratories Research Report No. 114, Publication Control No. 5030–114, MLCR 114 (CMLRE-ML-52). Army Chemical Center, MD.
Hayes, W.J. 1982. Pesticides Studied in Man. William and Wilkins, Baltimore, MD.
Henderson, R.F., E.B.Barr, W.B.Blackwell, C.R.Clark, C.A.Conn, R.Kalra, T.H.March, M.L.Sopori, Y.Tesfaigzi, M.G.Ménache, and D.C.Mash. 2002. Response of rats to low levels of sarin. Toxicol. Appl. Pharm. 184:67– 76.
Henderson, R.F., C.A.Conn, E.B.Barr, T.H.March, J.R.Krone, M.L.Sopori, Y. Tesfaigzi, M.Wachulec, and D.C.Mash. 2000. Effect of low level sarin exposure on physiological parameters in rats. Presented at the 39th Annual Meeting of the Society of Toxicity, March, 2000, Philadelphia, PA . Toxicologist 54(1):266 (#1248).
Henderson, R.F., E.B.Barr, C.R.Clark, M.L.Sopori, S.P.Singh, C.A.Conn, Y. Tesfaigzi, T.H.March, and D.C.Mash. 2001. Effects of inhalation exposure to low levels of sarin in Fischer 344 rats. Presented at the 40th Annual Meeting of the Society of Toxicity, March, 2001, San Francisco, CA (#1142).
Heston, W.E. 1942. Inheritance of susceptibility to spontaneous pulmonary tumors in mice. J. Natl. Cancer Inst. 3:79–82.
Himuro, K., S.Murayama, K.Nishiyama, et al. 1998. Distal axonopathy after sarin intoxication. Neurobiology 51:1195–1197.
Holmstedt, B. 1959. Pharmacology of organophosphorous cholinesterase inhibitors. Pharmacol. Rev. 11:567–688.
Hom, J., R.W.Haley, and T.L.Kurt. 1997. Neuropsychological correlates of Gulf War Syndrome. Arch. Clin. Neuropsychol. 12:531–544.
Husain, K., R.Vijayaraghavan, S.C.Pant, S.K.Raza, and K.S.Pandey. 1993. Delayed neurotoxic effect of Sarin in mice after repeated inhalation exposure. J. Appl. Toxicol. 13:1435.
Husain, K., S.C.Pant, S.K.Raza, R.Singh, and S.Das Gupta. 1995. A comparative study of delayed neuropathy in hens following repeated administration of organophosphorous compounds. Indian J. Physiol. Pharmacol. 39:47–50.
IOM (Institute of Medicine). 1999. Chemical and Biological Terrorism: Research and Development to Improve Civilian Medical Response. Committee on Research and Development Needs for Improving Civilian Medical Response to Chemical and Biological Terrorism Incidents, Institute of Medicine, Washington, DC: National Academy Press.
Ishiyama, I. 1996. Problems on the possibility in forensic diagnosis. Nippon Hoigaku Zasshi 50:220 (abstract).
Izmerov, N.F., I.V.Sanotsky, and K.K.Sidorov. 1982. Toxicometric Parameters of Industrial Toxic Chemicals under Single Exposure. USSR Commission for UNEP (United Nations Environmental Programme), Centre of International Projects, GKNT, Moscow.
Jacobson, K., M.K.Christensen, I.Dearmon, Jr., and F.W.Oberst. 1959. Studies on chronic exposures of dogs to GB vapor (isopropyl methylphosphonofluoridate). Arch. Ind. Health 19:5–10.
Johns, R.J. 1952. The effect of low concentrations of GB on the human eye.
Chemical Corps Medical Laboratories Research Report No. 100, Publication Control No. 5030–100 (CMLRE-ML-52). Army Chemical Center, MD.
Johnson, D.E., A.Anzueto, H.F.Hamil, J.H.Brewer, G.T.Moore, J.J.Siedenfeld, and R.A.Delemos. 1988. Studies of the effects of organophosphorus agent exposures on the lung. Southwest Research Institute, San Antonio, TX. Report prepared for the U.S. Army Medical Research and Development Command, Fort Detrick, MD. Defense Technical Information Center Report No. AD-A203861.
Jokanović, M. 1989. Role of carboxylesterase in soman, sarin and tabun poisoning in rats. Pharmacol. Toxicol. 65:181–184.
Jokanović, M., M.Kosanović, and M.Maksimović. 1996. Interaction of organophosphorus compounds with carboxylesterases in the rat. Arch. Toxicol. 70:444–450.
Jokanović, M., and M.Maksimović. 1997. Abnormal cholinesterase activity: Understanding and interpretation. Eur. J. Clin. Chem. Clin. Biochem. 35:11– 16 .
Jones, K.H., A.M.Dechkovskaia, E.A.Herrick, A.A.Abdel-Rahman, W.A.Khan, and M.B.Abou-Donia. 2000. Subchronic effects following a single sarin exposure on blood-brain and blood-testes barrier permeability, acetylcholinesterase, and acetylcholine receptors in the central nervous system of rat: A dose-response study. J. Toxicol. Environ. Health A 61:695–707.
Kadar, T., L.Raveh, and G.Cohen. 1985. Distribution of 3H-Soman in mice. Arch. Toxicol. 58:45–49.
Kagan, I.U.S., G.A.Voitenko, T.N.Pan’shina, et al. 1983. Combined toxicological and hygienic study of the organophosphate insecto-acaricide actellic [in Russian]. Gig. Sanit. 1983(6):32–35.
Kaliste-Korhonen, E., K.Tuovinen., and O.Hanninen 1996. Interspecies differences in enzymes reacting with organophosphates and their inhibition by paraoxon in vitro. Hum. Exp. Toxicol. 15:972–980.
Kalra, R., S.P.Singh, S.Razani-Boroujerdi, R.J.Langley, W.B.Blackwell, R.F. Henderson, and M.L.Sopori. 2002. Subclinical doses of the nerve gas sarin impair T-cell responses through the autonomic nervous system. Toxicol. Appl. Pharm. 184:82–87.
Kang, H.K., and T.A.Bullman. 1996. Mortality among U.S. veterans of the Persian Gulf War. New England J. Med. 335:1498–1504.
Kassa, J., M.Pecka, M.Tich , J.Bajgar, M.Koupilová, J.Herink, and Z. Kročová. 2001. Toxic effects of sarin in rats at three months following single or repeated low-level inhalation exposure. Pharmacol. Toxicol. 88:209–212.
Kassa, J., and J.Cabal. 1999. A comparison of the efficacy of acetylcholinesterase reactivates against cyclohexyl methylphosphonofluoridate (GF Agent) by in vitro and in vivo methods. Pharmacol. Toxicol. 84:41–45.
Kato, T., and T.Hamanaka. 1996. Ocular signs and symptoms caused by exposure to sarin gas. Amer. J. Opthalmol. 121:209–210.
Kaufman, K. 1954. Serum cholinesterase activity in the normal individual and in people with liver disease. Ann. Intern. Med. 41:533–545.
Khan, W.A., A.Dechkovskaia, K.H.Jones, et al. 2000a. Acute sarin exposure leads to cholinergic dysregulation in central nervous system of rats. Presented at the 39th Annual Meeting of the Society of Toxicity, March, 2000, Philadelphia, PA. Toxicologist 54(1):265 (#1245).
Khan, W.A., A.M.Dechkovskaia, E.A.Herrick, K.H.Jones, and M.B.Abou-Donia. 2000b. Acute sarin exposure causes differential regulation of choline acetyltransferase, acetylcholinesterase, and acetylcholine receptors in the central nervous system of the rat. Toxicol. Sci. 57(1):112–120.
Kimmerle, G., and O.R.Klimmer. 1974. Acute and subchronic toxicity of sulfotep. Arch. Toxicol. 33:1–16.
Kimura, K.K., B.P.McNamara, and V.M.Sim. 1960. Intravenous administration of VX in man. U.S. Army Chemical Research and Development Laboratory, Technical Report No. 3017. Army Chemical Center, MD.
Klimmer, O.R., ed. 1971. Pflanzenschutz-und Schaedlingsbekaempfungsmittel: Abriss einer Toxikologie und Therapie von Vergiftungen, 2nd Ed. Hattingen, Fed. Rep. Germany.
Koelle, G.B. 1976. Cholinesterases and anticholinesterase agents. Berlin: Springer-Verlag. Pp. 123–154.
Koelle, G.B. 1981. Organophosphate poisoning—an overview. Fundam. Appl. Toxicol. 1:129–134.
Koelle, G.B. 1994. Pharmacology of organophosphates. J. Appl. Toxicol. 14: 105–109.
Koon, W.S., P.Cresthull, J.W.Crook, et al. 1959. Odor detection of VX vapor with and without a stabilizer. Chemical Warfare Laboratory Report, No. 2292. Aberdeen Proving Ground, MD.
Koon, W.S., J.W.Crook, and F.W.Oberst. 1960. Progress Report on the Toxicity of VX Vapor to Mice and Goats. Army Chemical Warfare Laboratories Report, No. TM 24–37. Army Chemical Center, MD.
Koplovitz, I., W.Harris, D.R.Anderson, W.J.Lennox, and J.R.Stewart. 1992. Reduction by pryidostigmine pretreatment of the efficacy if atropine and 2-PAM treatment of sarin and VX poisoning in rodents. Fund. Appl. Toxicol. 18:102–106.
Kotler-Cope, S., J.B.Milby, et al. 1996. Neuropsychological deficits in Persian Gulf War veterans: A preliminary report. Presented at the annual meeting of the International Neuropsychological Society, Chicago, IL.
Krackow, E.H. 1956. Toxicology of the V agents. CWLR 2065. Chemical Corps. Research and Development Command, Chemical Warfare Laboratories, Army Chemical Center , Aberdeen Proving Ground, MD.
Kujiraoka, T., T.Oka, M.Ishihara, T.Egashira, T.Fujioka, E.Saito, S.Saito, N. Miller, and H.Hattori. 2000. A sandwich enzyme-linked immunosorbent assay for human serum paraoxonase concentration. J. Lipid Research 41:1358–1363.
Laborde J.B., and H.K.Bates. 1986. Developmental toxicity study of agent GB-DCSM Types I and II in CD rats and NZW rabbits. Final Report. AD-A168 331. National Center for Toxicological Research, Jefferson, AR.
LaBorde, J.B., J.K.Bates, J.C.Dacre, and J.F.Young. 1996. Developmental toxicity of sarin in rats and rabbits. J. Toxicol. Environ. Health 47:249–265.
LaDu, B.N., J.I.Piko, H.W.Erickson, M.Vincent-Viry, and G.Seist. 1986. An improved method for phenotyping individuals for the human serum paraoxonase arylesterase polymorphism. Ann Biol. Clin. 44:369–372.
Lallement, G., P.Carpenteir, I.Pernot-Marino, D.Baubichon, A.Collet, and G. Blanchet. 1991 a. Involvement of the different rat hippocampal glutamatergic receptors in development of seizures induced by soman. Neurotoxicology 4:655–664.
Lallement, G., P.Carpenteir, A.Collet, I.Pernot-Marino, D.Baubichon, and G. Blanchet. 1991b. Effects of soman-induced seizures on different extracellular amino acid levels and on glutamate uptake in rat hippocampus. Brain Res. 563:234–340.
Landauer, M.R., and J.A.Romano. 1984. Acute behavioral toxicity of the organophosphate sarin in rats. Neurobehav. Toxicol. Teratol. 6:239–243.
Landrigan, P.J. 1997. Illness in Gulf war veterans, causes and consequences. JAMA 277:259–261.
Langenberg, J.P., E.T.Spruit, H.van der Wiel, et al. 1998. Inhalation toxicokinetics of soman stereoisomers in the atropinized guinea pig with nose-only exposure to soman vapor. Toxicol. Appl. Pharmacol. 151:79–87.
Lehmann, H., and J.Liddell. 1969. Human cholinesterase genetic variants and their recognition. Br. J. Anaesth. 41:235–244.
Lenz, D.E., D.M.Maxwell, and L.W.Austin. 1996. Development of a rat model for subacute exposure to the toxic organophosphate VX. J. Amer. College Toxicol. 15(suppl. 2):S69–S77.
Lessenger, J.E, and B.E.Reese. 1999. Rational use of cholinesterase activity testing in pesticide poisoning. J. Am. Board Fam. Pract. 12:307–314.
Lillibridge, S. 1995. Trip Report: United States Medical Delegation to Japan. From S.R.Lillibridge, Acting Chief, Disaster Assessment and Epidemiology Section, to R.J.Jackson, Director, National Center for Environmental Health, Centers for Disease Control and Prevention, Department of Health and Human Services, Atlanta, GA (25 April 1995).
Lipp, J., and T.Dola. 1980. Comparison of the efficacy of HS-6 versus HI-6 when combined with atropine, pyridostigmine and clonazepam for soman poisoning in the monkey. Arch. Int. Pharmacodyn. 246:138–148.
Little, J.S., C.A.Broomfield, M.K.Fox-Talbot, L.J.Boucher, B.MacIver, and D.E. Lenz. 1989. Partial characterization of an enzyme that hydrolyzes sarin, soman, tabun and diisopropyl phosphonofluoridate (DFP). Biochem. Pharmacol. 38:23–29.
Little, P.J., M.L.Reynolds, E.R.Bowman, and B.R.Martin. 1986. Tissue disposi
tion of 3H sarin and its metabolites in mice. Toxicol. Appl. Pharmacol. 83:412– 419.
Little, P.J., J.A.Scimeca, and B.R.Martin. 1988. Distribution of [3H] diisopropylfluorophosphate, [3H] soman, [3H] sarin and their metabolites in mouse brain. Drug Metab. Disposition 16:515–520.
Lohs, K, von. 1960. Zur Toxikologie und Pharmakologie organischer Phosphorsäureester. Dtsch Gesundheitsweren 15:2133–2179.
Lubash, G.D., and B.J.Clark. 1960. Some metabolic studies in humans following percutaneous exposure to VX. U.S. Army Chemical Research and Developmental Laboratories, Technical Report No. 3003. Edgewood Arsenal, MD.
Luo, C., and J.Liang. 1997. Evaluation of the combined toxic effects of GB/GF and efficacy of Jielin injection against combined poisoning in mice. Toxicol. Letters 92:195–200.
Maelicke, A., M.Samochocki, R.Jostock, A.Fehrenbacher, J.Ludwig, E.X.Albuquerque, and M.Zerlin. 2001. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol. Psychiatry 49:279–288.
Mager, P.P. 1981. Grouped observations in quantitative structure-toxicity relationships of organophosphorus poisons. Pharmazie 36:513–514.
Marrs, T.C., R.L.Maynard, and F.R.Sidell. 1996. Chemical Warfare Agents; Toxicology and Treatment. Chichester, NY: John Wiley and Sons.
Maxwell, D.M. 1992. The specificity of carboxylesterase protection against the toxicity of organophosphorus compounds. Toxicol. Appl. Pharmacol. 114:306–312.
Maxwell, D.M., K.M.Brecht, and B.M.O’Neill. 1987. The effect of carboxylesterase inhibition on interspecies differences in soman toxicity. Toxicol. Lett. 39:35–42.
McGrath, F.P., V.J.Von Berg, and F.W.Oberst. 1953. Toxicity and perception of GF vapor. Medical Laboratory Research Report, MLRR 185. Army Chemical Center, MD.
McKee, W.H.E., and B.Woolcott. 1949. Report on exposures of unprotected men and rabbits to low concentrations of nerve gas vapour. PRP-143. Chemical Defence Establishment, Porton Down, UK.
McNamara, B.P., and F.Leitnaker. 1971. Toxicological basis for controlling emission of GB into the environment. Edgewood Arsenal Special Publication 100–98 . Department of the Army, Edgewood Arsenal, Medical Research Laboratory, Edgewood Arsenal, MD.
McNamara, B.P., F.J.Vocci, and F.C.Leitnaker. 1973. Proposed limits for human exposure to VX vapor in nonmilitary operations, Edgewood Arsenal Special Publication, EASP 1100–1 (R-1). Aberdeen Proving Ground, MD.
McPhail, M.K., and P.A.Adie. 1960. The distribution of radioactive phosphorus in the blood and tissues of rabbits treated with tagged isopropyl methylphosphonofluoridate (Sarin) Can J. Biochem. Physiol. 38:945–951.
Mehl, A., T.M.Schenke, B.J.Johnsen, and F.Fonnum. 1994. The effects of tri
chlorfon and other organophosphates on prenatal brain development in the guinea pig. Neurochem. Res. 19:569–574.
Mioduszewski, R.J., S.A.Reutter, L.L.Miller, E.J.Olajos, and S.A.Thomson. 1998. Evaluation of airborne exposure limits for G-agents: Occupational and general population exposure criteria. ERDEC-TR-489, Edgewood Research Development and Engineering Center, Aberdeen Proving Ground, MD.
Mioduszewski, R.J., J.Manthei, R.Way, D.Burnett, B.Gaviola, W.Muse, S. Thomson, D.Sommerville, and R.Crosier. 2000. Estimating the probability of sarin vapor toxicity in rats as a function of exposure concentration and duration. Proceedings of the International Chemical Weapons Demilitarization Conference (CWD-2000), The Hague, NL. May 21–24, 2000.
Mioduszewski, R.J., J.Manthei, R.Way, D.Burnett, B.Gaviola, W.Muse, J. Anthony, D.Durst, D.Sommerville, R.Crosier, S.Thomson, and C.Crouse. 2001. ECBC Low Level Operational Toxicology Program: Phase I-Inhalation toxicity of sarin vapor in rats as a function of exposure concentration and duration. ECBC-TR-183, Edgewood Research Development and Engineering Center, Aberdeen Proving Ground, MD (August 2001).
Mioduszewski, R.J., J.Manthei, R.Way, D.Burnett, B.Gaviola, W.Muse, S. Thomson, D.Sommerville, and R.Crosier. 2002a. Interaction of exposure concentration and duration in determining acute toxic effects of sarin vapor in rats. Toxicol. Sci. 66:176–184.
Mioduszewski, R.J., J.Manthei, R.Way, D.Burnett, B.Gaviola, W.Muse, S. Thomson , D.Sommerville, R.Crosier, J.Scotto, D.McCaskey, C.Crous, and K.Matson. 2002b. Low-level sarin vapor exposure in rats: Effect of exposure concentration and duration on pupil size. ECBC-TR-235. Edgewood Chemical Biological Center, U.S. Army Soldier and Biological Chemical Command, Aberdeen Proving Ground, MD (May 2002).
MODa (Ministry of Defense). Unpublished report. Detection of G gases by smell. United Kingdom, Ministry of Defense. (As cited in Marrs et al. 1996.)
Morgan, D.P. 1989. Recognition and Management of Pesticide Poisonings. 4th Ed. EPA-540/9–88–001. U.S. Environmental Protection Agency, Washington, D.C.
Morita, H., N.Yanagisawa, T.Nakajima, M.Shimizu, H.Hirabayashi, H.Okudera, M.Nohara, Y.Midorikawa, and S.Mimura. 1995. Sarin poisoning in Matsumoto, Japan. The Lancet 346:290–293.
Mumford, S.A. 1950. A physiological assessment of the nerve gases. Porton Memorandum No. 39.
Munger, J.S., G.-P.Shi, E.A.Mark, D.T.Chin, C.Gerard, and H.A.Chapman. 1991. A serine esterase released by human alveolar macrophages is closely related to liver microsomal carboxylesterases. J. Biol. Chem. 266:18832– 18838.
Munro, N.B., A.P.Watson, K.R.Ambrose, and G.D.Griffin. 1990. Treating exposure to chemical warfare agents: Implications for health care providers and community emergency planning. Environ. Health Perspect. 89:205–215.
Munro, N.B., K.R.Ambrose, and A.P.Watson. 1994. Toxicity of the organophosphate chemical warfare agents GA, GB, and VX: Implications for public protection. Environ. Health Perspect. 102:18–38.
Munro, N.B., S.S.Talmage, S.G.D.Griffin, L.C.Waters, A.P.Watson, J.F.King, and V.Hauschild. 1999. The sources, fate, and toxicity of chemical warfare agent degradation products. Environ. Health Perspect. 107:933–974.
Murata, K., S.Araki, K.Yokoyama, T.Okumura, S.Ishimatsu, N.Takasu, and R.A.White. 1997. Asymptomatic sequelae to acute sarin poisoning in the central and autonomic nervous system 6 months after the Tokyo subway attack . J. Neurol. 244:601–606.
Nakajima, T., K.Sasaki, H.Ozawa, Y.Sekijima, H.Morita, Y.Fukushima, and N. Yanagisawa. 1998. Urinary metabolites of sarin in a patient of the Matsumoto sarin incident. Arch. Toxicol. 72:601–603.
NDRC (National Defense Research Committee). 1946. Chemical Warfare Agents and Related Chemical Problems. Part I–II, Vol. 1. National Defense Research Committee, Washington, DC.
Nieminen, S.A., A.Lecklin, O.Heikkinen, and P.Ylitalo. 1990. Acute behavioural effects of the organophosphates sarin and soman in rats. Pharmacol. Toxicol. 67:36–40.
NIOSH (National Institute for Occupational Safety and Health). 1999. Registry of Toxic Effects of Chemical Substances (RTECS) online file, retrieved November 1999. National Institute for Occupational Safety and Health, Cincinnati, OH. MEDLARS online information retrieval system, National Library of Medicine, Washington, DC.
Noort, D., A.G.Hulst, D.Platenburg, M.Polhuijs, and H.P.Benschop. 1998. Quantitative analysis of O-isopropyl methylphosphonic acid in serum samples of Japanese citizens allegedly exposed to sarin: Estimation of internal dose. Arch. Toxicol. 72:671–675.
Nordgren, I., G.Lundgren, G.Puu, and B.Holmstedt. 1984. Stereoselectivity of enzymes involved in toxicity and detoxification of soman. Arch. Toxicol. 55: 70–75.
NRC (National Research Council). 1985. Possible long-term health effects of short-term exposure to chemical agents. Vol. 3, Final Report: Current health status of test subjects. Committee on Toxicology, Board on Toxicology and Environmental Health Hazards, Commission on Life Sciences, National Research Council. Washington, DC: National Academy Press.
NRC (National Research Council). 1997. Review of the Acute Human-toxicity Estimates for Selected Chemical Warfare Agents. Committee on Toxicology, Subcommittee on Toxicity Values for Selected Nerve Agents and Vesicant Agents. Washington, DC: National Academy Press.
NRC (National Research Council). 1999. Review of the U.S. Army’s Health Risk Assessment for oral exposure to six chemical warfare agents. Committee on Toxicology, Subcommittee on Chronic Reference Dose for Chemical Warfare Agents. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Committee on Toxicology, Subcommittee on Acute Exposure Guideline Levels. Washington, DC: National Academy Press.
Oberst, F.W. 1961. Factors affecting inhalation and retention of toxic vapors. Pp. 249–266 in Inhaled Particles and Vapours, C.N.Davis, ed. London: Pergamon Press.
Oberst, F.W., W.S.Koon, M.K.Christensen, et al. 1968. Retention of inhaled sarin vapor and its effect on red blood cell cholinesterase activity in man. Clin. Pharmacol. Ther. 9:421–427.
Okumura, T., N.Takasu, S.Ishimatu, S.Miyanoki, A.Mitsuhashi, K.Kumada, K. Tanaka, and S.Hinohara. 1996. Report on 640 victims of the Tokyo subway sarin attack. Ann. Emerg. Med. 28:129–135.
Olson, C.T., J.A.Blank, and R.G.Menton. 1998. Neuromuscular effects of low level exposures to sarin, pyridostigmine, DEET, and chlorpyrifos. Drug Chem. Toxicol. 21(Suppl. 1):149–169.
Olson, C.T., J.A.Blank, P.H.Kinney, A.W.Singer, G.B.Freeman, and R.A.Lordo. 2000. Neurologic assessment of rats following low doses of sarin, pyridostigmine, chlorpyrifos, and DEET. Presented at the 39th Annual Meeting of the Society of Toxicity, March, 2000, Philadelphia, PA. Toxicologist 54:265 (#1244).
Opresko, D.M., R.A.Young, R.A.Faust, S.S.Talmage, A.P.Watson, R.H.Ross, K.A.Davidson, and J.King. 1998. Chemical warfare agents: Estimating oral reference doses. Rev. Environ. Contam. Toxicol. 156:1–183.
Osweiler, G.D., T.L.Carson, W.B.Buck, and G.A.Van Gelder. 1985. Clinical and Diagnostic Veterinary Toxicology, 3rd Ed. Dubuque, IA: Kendall/Hunt.
OXO Process Panel. 1995. Letter report: Comments of the CMA OXO Process Panel on the Proposal to Reduce the TLV of N-Butyl Acetate. Written statement presented by the Chemical Manufacturers’ Association OXO Process Panel to the ACGIH TLV Subcommittee. (Signed cover by G.D.Strickland, CMA Vice President, Technical Services, 25 Feb 1995, CMA, 2501 M. Street, NW, Washington, DC.)
Pearce, P.C., H.S.Crofts, N.G.Muggleton, D.Ridout, and E.A.M.Scott. 1999. The effects of acutely administered low dose sarin on cognitive behavior and the electroencephalogram in the common marmoset. J. Psychopharm. 13:128– 135.
Phillips, A. 1995. Clinical Toxicology Review, Massachusetts Poison Control Center. 17(7) [Online]. Available at http://www.maripoisoncenter.com/ctr/9504cholinesterase.html
Pond, A.L., H.W.Chambers, and J.E.Chambers. 1995. Organophosphate detoxication potential of various rat tissues via A-esterase and aliesterase activities. Toxicol. Letters 78:245–252.
Pope, C., and J.Liu. 2002. Nonesterase actions of anticholinesterase insecticides.
Chapter 3, pp. 29–43 in Handbook of Neurotoxicology, Vol. 1, E.J.Massaro, ed. Totowa, NJ: Human Press.
Pour-Jafari, H. 1994a. Congenital malformations in the progenies of Iranian chemical victims. Vet. Hum. Toxicol. 36:562–563.
Pour-Jafari, H. 1994b. Secondary sex ratios in progenies of Iranian chemical victims. Vet. Hum. Toxicol. 36:475–476.
Punte, C.L., and J.C.Atkinson. 1960. Inhalation toxicity and speed of action of VX with suggested estimates for man based on animal data. CWL Special Publication 2–30. U.S. Army Chemical Warfare Laboratories, Army Chemical Center, MD.
Rao, K.S., Y.Aracava, D.L.Rickett, and E.X.Albuquerque. 1987. Noncompetitive blockade of the nicotinic acetylcholine receptor-ion channel complex by an irreversible cholinesterase inhibitor. J. Pharmacol. Exp. Ther. 240:337–344.
Raveh, L., E.Grauer, J.Grunwald, E.Cohen, and Y.Ashani. 1997. The stoichiometry of protection against soman and VX toxicity in monkeys pretreated with human butyrylcholinesterase. Toxicol. Appl. Pharmacol. 145:43–53.
Reinhold, J.G., L.G.Tourigny, and V.L.Yonan. 1953. Measurement of serum cholinesterase activity by a photometric indicator method, together with a study of the influence of sex and race. Am. J. Clin. Pathol. 23:645–653.
Rengstorff, R.H. 1985. Accidental exposure to Sarin: Vision effects. Arch. Toxicol. 56:201–203.
Reutter, S.A., and J.V.Wade. 1994. “Table 1; Summary of Existing and Recommended Estimates (U),” unclassified summary table, Review of existing toxicity data and human estimates for selected chemical agents and recommended human toxicity estimates appropriate for defending the soldier. ERDEC-SP-018. U.S. Department of the Army, Edgewood Research Development and Engineering Center, Aberdeen Proving Ground, MD (secret report).
Reutter, S.A., R.J.Mioduszewski, and S.A.Thomson, S.A. 2000. Evaluation of airborne exposure limits for VX: Worker and general population exposure criteria. ECBC-TR-074. Edgewood Chemical Biological Center, U.S. Army Soldier and Biological Chemical Command, Aberdeen Proving Ground, MD.
Rider, J.A., J.L.Hodges, J.Swader, and A.D.Wiggins. 1957. Plasma and red cell cholinesterase in 800 “healthy” blood donors. J. Lab. Clin. Med. 50:376–383.
Rocha, E.S., S.R.Chebabo, M.D.Santos, Y.Aracava and E.X.Albuquerque. 1998. An analysis of low level doses of cholinesterase inhibitors in cultured neurons and hippocampal slices of rats. Drug Chem. Toxicol. 21(Suppl. 1):191–200.
Rocha, E.S., M.D.Santos, S.R.Chebabo, Y.Aracava and E.X.Albuquerque. 1999. Low concentrations of the organophosphate VX affect spontaneous and evoked transmitter release from hippocampal neurons: Toxicological relevance of cholinesterase-dependent actions. Toxicol. Appl. Pharmacol. 159:31–40.
Rubin, L.S., S.Krop, and M.N.Goldberg,. 1957. Effect of sarin on dark adaptation in man: Mechanism of action. J. Appl. Physiol. 11:445–449.
Rubin, L.S., and M.N.Goldberg. 1957. Effect of sarin on dark adaptation in man: Threshold changes. J. Appl. Physiol. 11:439–444.
Ruiz, J., H.Blanche, R.W.James, M.C.Garin, C.Vaisse, G.Charpentier, N.Cohen, A.Morabia, P.Passa, and P.Froguel. 1995. Gln-Arg 192 polymorphism of paraoxonase and coronary artery disease in type 2 diabetes. Lancet 346: 869–872.
Schoene, K., D.Hochrainer, H.Oldiges, M.Krügel, N.Franzes, and H.J.Bruckert. 1985. The protective effect of oxime pretreatment upon the inhalative toxicity of sarin and soman in rats. Fund. Appl. Toxicol. 5:S84–S88.
Schreider, J.P., J.R.Rowland, L.S.Rosenblatt, and A.G.Hendrick. 1984. The teratology effects of VX in rats. Draft Final Report. Laboratory for Energy-Related Health Effects Research. University of California, Davis, CA. Prepared for U.S. Army Medical Bioengineering Research and Development Laboratory, Fort Derrick, MD.
Schwartz, D.A., B.N.Doebbelin, and J.A.Merchant. 1997. Self-reported illness and health status among Gulf War veterans. JAMA 277:238–245.
Senanayake, N., and L.Karalliedde. 1987. Neurotoxic effects of organophosphate insecticides: An Intermediate Syndrome. N. Engl. J. Med. 316:761–763.
Serrato, M., and M.J.Marian. 1995. A variant of human paraoxonase/arylesterase (HUMPONA) gene is a risk factor for coronary artery disease. J. Clin. Invest. 96:3005–3008.
Shanor, S.P., G.R.van Hees, N.Baart, E.E.Erdos, and F.F.Foldes. 1961. The influence of age and sex on human plasma and red cell cholinesterase. Am. J. Med. Sci. 242:357–361.
Shih, M.L., J.D.McMonagle, and T.W.Dolzine. 1994. Metabolite pharmacokinetics of soman, sarin and GF in rats and biological monitoring of exposure to toxic organophosphorus agents. J. Appl. Toxicol. 14:195–199.
Sidell, F.R. 1974. Soman and Sarin: Clinical manifestations and treatment of accidental poisoning by organophosphates. Clin. Toxicol. 7:1–17.
Sidell, F.R. 1990. What to do in case of an unthinkable: Chemical warfare attack or incident. Postgraduate Medicine 88:70–83.
Sidell, F.R. 1992. Clinical considerations in nerve agent intoxication. Pp. 155–194 in: Chemical Warfare Agents, S.M.Somani, ed. New York: Academic Press, Inc.
Sidell, F.R. 1996. Chemical agent terrorism. Ann. Emerg. Med. 28:223–224.
Sidell, F.R. 1997. Nerve agents. Pp. 129–179 in Medical Aspects of Chemical and Biological Warfare, F.R.Sidell, E.T.Takafuji, and D.R.Franz, eds. Published by the Office of the Surgeon General, at TBMM Publications, Borden Institute, Walter Reed Army Medical Center, Washington, DC.
Sidell, F.R. and W.A.Groff. 1974. The reactivatibility of cholinesterase inhibited by VX and sarin in man. Toxicol. Appl. Pharmacol. 27:241–252.
Sim,V.M. 1956. Effect on pupil size of exposure to GB vapour. Porton Technical Paper 531. Chemical Defence Experimental Establishment, Directorate of Chemical Defence Research and Development, Porton Down, England.
Sim, V.M. 1962. Variability of different intact human-skin sites to the penetration of VX. CRDLR 3122, Chemical Research and Development Laboratories, Aberdeen Proving Ground, MD.
Sim, V.M., C.McClure, Jr., F.J.Vocci, et al. 1964. Tolerance of man to VX-contaminated water. Technical Report CRDLR 3231. U.S. Department of the Army , Chemical Research and Development Laboratories, Edgewood Arsenal, MD.
Small, M.J. 1984. Compounds formed from the chemical decontamination of HD, GB, and VX and their environmental fate. Technical Report 8304 (DTIC: AD A149515). U.S. Department of the Army, Medical Bioengineering Research and Development Laboratory, Fort Derrick, MD.
Somani, S.M., R.P.Solana, and S.N.Dube. 1992. Toxicodynamics of nerve agents. Pp 67–123 in Chemical Warfare Agents, S.M.Somani, ed. New York: Academic Press, Inc.
Spruit, H.E., J.B.Langenberg, H.C.Trap, H.J.van der Wiel, R.B.Helmich, H.P. van Helden, and H.P.Benschop. 2000. Intravenous and inhalation toxicokinetics of sarin stereoisomers in atropinized guinea pigs. Toxicol. Appl. Pharmacol. 169(3):249–254.
Sterri, S.H., S.Lyngaas, and F.Fonnum. 1980. Toxicity of soman after repetitive injection of sublethal doses in rat. Acta Pharmacol. Toxicol. 46:1–7.
Sterri, S.H., O.Kloster, and G.Valdal. 1982. Reduction of blood cholinesterase activities following administration of soman by different routes in guinea pigs. Acta Pharmacol. Toxicol. 50:326–331.
Storm, J.E., K.K.Rozman, and J.Doull. 2000. Occupational exposure limits for 30 organophosphate pesticides based on inhibition of red blood cell cholinesterase. Toxicology 150:1–29.
ten Berge, W.F., A.Zwart, and L.M.Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Materials 13:301–309.
Thacker, S.B., Assistant Surgeon General, Acting Director, National Center for Environmental Health, Center for Disease Control and Protection, U.S. Department of Health and Human Services. 1994. Letter establishing “Recommended Acute Threshold Effects Levels for Determining Emergency Evacuation Distances in the CSEPP Program,” to Col. J.M.Coverstone, Deputy for Chemical Demilitarization, Office of the Assistant Secretary (I, L &E), The Pentagon, Washington, DC (24 June 1994).
Uhde, G.I., and R.F.Moore,. 1945. Eye effects of T2104. Porton Technical Report 2693. CDE Porton Down, England.
USACHPPM (U.S. Department of the Army, Center for Health Promotion and Preventive Medicine). 1996. Detailed and general facts about chemical agents. Technical Guide 218 (TG-218). U.S. Army Center for Health Promotion and Preventive Medicine, 5158 Blackhawk Road, Aberdeen Proving Ground, MD.
USACHPPM (U.S. Army Center for Health Promotion and Preventive Medicine). 1998. Memorandum for Record (12 November 1998) prepared by V. Hauschild, Environmental Health Risk Assessment and Risk Communication Program. In Progress Review: Meeting Regarding Evaluation of Airborne Exposure Limits for VX, held Oct. 14, 1998, Aberdeen Proving Ground, MD.
USACMDA (U.S. Department of the Army, Chemical Materiel Destruction Agency). 1993a. Interim survey and analysis report. Program Manager for Non-Stockpile Chemical Materiel, Non-Stockpile Chemical Materiel Program, U.S. Department of the Army, Chemical Materiel Destruction Agency, Aberdeen Proving Ground, MD.
USACMDA (U.S. Department of the Army, Chemical Materiel Destruction Agency). 1993b. Survey and analysis report. Program Manager for Non-Stockpile Chemical Materiel Program, U.S. Department of the Army, Chemical Materiel Destruction Agency, Aberdeen Proving Ground, MD.
van Helden, H.P.M., J.P.Langenberg, and H.P.Benschop. 2001. Low Level Exposure to GB Vapor in Air: Diagnosis/Dosimetry, Lowest Observable Effect Levels, and Performance Incapacitation. Award No. DAMD17–97–1–7360. TNO Prins Maurits Laboratory, Final Report to the U.S. Army Medical Research and Materiel Command, Fort Derrick, MD (April 2001).
van Helden, H.P.M. H.C.Trap, W.C.Kuijpers, B.Groen, J.P.Oostdijk, R.A.P. Vanwersch, I.H.C.Philippens, J.P.Langenberg, and H.P.Benschop. 2002. Low Level Exposure to GB Vapor in Air: Diagnosis/Dosimetry, Lowest Observable Effect Level, and Performance-Incapacitation. Research and Technology Organisation (RTO) Meeting Proceedings 75, Operational Medical Issues in Chemical and Biological Defense [RTO-MP-075, AC/323 (HFM-060) TP/37] held in Estoril, Portugal, 14–17 May 2001. North Atlantic Treaty Organisation, Research and Technology Organisation, BP 25, 7 Rue Ancelle, F-92201 Neuilly-sur-Seine CEDEX , France.
Van Kampen, K.R., Shupe, J.L., Johnson, A.E., et al. 1970. Effects of nerve gas poisoning in sheep in Skull Valley, Utah. J. Amer. Vet. Med. Assoc. 156: 1032–1035.
Vasterling, J.J., K.Brailey, J.I.Constans, and P.B.Sutker. 1998. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology 12:125–133.
Vranken, M.A., H.C.DeBisschop, and J.L.Willems. 1982. In vitro inhibition of neurotoxic esterase by organophosphorous nerve agents. Arch. Int. Pharmacodyn. Ther. 260:316–318.
Wahllander, A., and L.Szinicz. 1990. Detoxification of soman in the perfused rat liver: Quantitative uptake and stereoisomer metabolism. Arch. Toxicol. 64: 586–589.
Walday, P., P.Aas, and F.Fonnum. 1991. Inhibition of serine esterases in different rat tissues following inhalation of soman. Biochem. Pharmacol. 41(1):151– 153.
Wang, Q., M.Sun, H.Zhang, and C.Huang. 1998. Purification and properties of soman-hydrolyzing enzyme from human liver. J. Biochem. Molecular Toxicol. 12(4):213–217.
Watson, A.P., K.R.Ambrose, G.D.Griffin, S.S.Leffingwell, N.B.Munro, and L.C. Waters,. 1989. Health effects of warfare agent exposure: Implications for stockpile disposal. Environ. Prof. 11:335–353.
Watson, A.P., T.D.Jones, and J.D.Adams. 1992. Relative potency estimates of acceptable residues and reentry intervals after nerve agent release. Ecotoxicology and Environ. Safety 23:328–342.
Weimer, J.T., B.P.McNamara, E.J.Owens, J.G.Cooper, and A.van de Wal. 1979. Proposed revision of limits for human exposure to GB vapor in nonmilitary operations based on one-year exposures of laboratory animals to low airborne concentrations. ARCSL-TR-78056. U.S. Army Armament Research and Development Command, Chemical Systems Laboratory, Aberdeen Proving Ground, MD.
Wells, C.H., M.T.Collins, D.E.Vanderveer, and W.A.Wells. 1993. Standards for chemical and biological warfare hazard modeling, Volume I. Toxicity of nerve agents and mustard in humans. Abridged edition (unclassified). AL-TR-1993– 0026, Jaycor, Beavercreek, OH. Report to the U.S. Air Force, Armstrong Laboratory, Ergonomics Analysis Branch, Wright-Patterson Air Force Base, OH .
Willems, J.L., M.Narcaise, and H.C.DeBisschop. 1984. Delayed neuropathy by the organophosphorous nerve agents soman and tabun. Arch. Toxicol. 55:76– 77.
Wills, J.H. 1972. The measurement and significance of changes in the cholinesterase activities of erythrocytes and plasma in man and animals. CRC Crit. Rev. Toxicol. 1:153–202.
Wilson, B.W., J.D.Henderson, T.P.Kellner, M.Goldman, R.J.Higgins, and J.C. Dacre. 1988. Toxicity of repeated doses of organophosphorous esters in the chicken. J. Toxicol. Environ. Health 23:115–126.
Wilson, B.W., T.G.Kawakami, N.Cone, J.D.Henderson, L.S.Rosenblatt, and M. Goldman. 1994. Genotoxicity of the phosphoramidate agent tabun (GA). Toxicology 86:1–12.
Windholz, M., S.Budavari, R.F.Blumetti, and E.S.Otterbein, eds 1983. The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals. 10th Ed. Rahway, NJ: Merck and Co.
Wolthuis, O.L. 1992. Behavioral effects of low doses of cholinesterase inhibitors in robot-tested marmosets. DTIC: AD A262776. Prepared for the U.S. Department of the Army, Medical Research and Development Command, Fort Detrick, MD. TNO Medical Biological Laboratory, Rijswijk, Netherlands.
Woodard, C.L., C.A.Calamaio, A.Kaminskis, D.A.Anderson, L.W.Harris, and D.G.Martin. 1994. Erythrocyte and plasma cholinesterase activity in male and female rhesus monkeys before and after exposure to sarin. Fund. Appl. Toxicol. 23:342–347.
Yamasaki, Y., K.Sakamoto, H.Watada, Y.Kajimoto, and M.Hori. 1997. The Arg 192 isoform of paraoxonase with low sarin-hyrolyzing activity is dominant in the Japanese. Japan. Hum. Genet. 10:67–68.
Yee, E., J.Armour, and R.Bide. 1999. An approach to obtain estimates of human toxicity. Part II: A three dimensional probit based, nonlinear dose response model for calculation of the mortality-concentration-time response surface. Presented to the NATO Challenge Subgroup, 12–14 May, 1999, San Antonio, TX.
Yee, E. 1996. A Non-linear Dose-Response Model with an Application to the Reconstruction of the Human Mortality Response Surface from Acute Inhalation Toxicity with Sarin. DRES-SM-1476. Defence Research Establishment Suffield, Ralston, Alberta, Canada.
Yokoyama, K., S.Araki, K.Murata, M.Nishikitani, T.Okumura, S.Ishimatsu, and N.Takasu. 1998a. Chronic neurobehavioral effects of Tokyo subway Sarin poisoning in relation to posttraumatic stress disorder. Arch. Environ. Health 53:249–256.
Yokoyama, K., S.Araki, K.Murata, M.Nishikitani, T.Okumura, S.Ishimatsu, N. Takasu, and R.A.White. 1998b. Chronic neurobehavioral and central and autonomic nervous system effects of Tokyo subway Sarin poisoning. J. Physiol. (Paris) 92:317–323.
Yokoyama, K., S.Araki, K.Murata, M.Nishikitani, T.Okumura, S.Ishimatsu, and N.Takasu. 1998c. A preliminary study of vestibulocerebellar effects of Tokyo subway sarin poisoning in relation to gender differences: Frequency analysis of postural sway. J. Occup. Environ. Med. 40:17–21.
Young, R.A., D.M.Opresko, A.P.Watson, R.H.Ross, J.King, and H.Choudhury. 1999. Deriving toxicity values for organophosphate nerve agents: A position paper in support of the procedures and rationale for deriving oral RfDs for chemical warfare nerve agents. Human Ecol. Risk Assess. 5:589–634.
Young, J.F., B.J.Gough, R.L.Suber, and D.W.Gaylor. 2001. Correlation of blood cholinesterase levels with toxicity of sarin in rats. J. Toxicol. Environ. Health 62:161–174.
Zhao, D.-L., Z.-X. Wang, S.-Q. Pei, and C.-H.Liu. 1983. Effects of soman, sarin, and VX on the specific binding of 3H-QNB in rat cerebral cortex homogenates. Acta. Pharmacol. Sinica 4:225–228.
APPENDIX A
DERIVATION OF AEGL VALUES
Derivation of AEGL-1
|
Key study: |
Mioduszewski et al. (2002b). Miosis measured in adult female SD rats exposed to a range of GB vapor concentrations for 10, 60, and 240 min. At 10 min, N =52 females; at 60 min, N=35 females; at 240 min, N=55 females. |
|
Toxicity end point: |
EC50 for miosis (defined as “a postexposure pupil diameter 50% or less of the preexposure pupil diameter”) observed in adult female SD rats |
|
EC50 for GB: |
10-min EC50=0.068 mg GB/m3 60 min EC50=0.020 mg GB/m3 240 min EC50=0.012 mg GB/m3 |
|
Scaling: |
Cn×t=k (ten Berge et al. 1986). Current analyses based on a linear regression of the lethality and miosis data for female SD rats. The LC01 of GB to female Sprague-Dawley rats (Mioduszewski et al. 2000, 2001, 2002a) yields an n value of 1.93 with an r2 of 0.9948, while miosis (female Sprague-Dawley rats; Mioduszewski et. al. 2002b) yields an n value of 2.00 with an r2 of 0.4335 (see Appendix B). The anticholinesterase mechanism of mammalian toxicity for nerve agents is known, and all endpoints observed in human and animal studies represent a response continuum to anticholinesterase exposure. Accordingly, it is valid to apply an n value derived from compound-specific lethality data to time scaling for non-lethal effects, and an n value derived for miosis, to consideration of other toxic effects resulting from anticholinesterase exposure. This position is consistent with that of the recently published Science |
|
|
Policy of the EPA Office of Pesticide Programs (EPA 2000). Therefore, n=2 is used as the scaling function for all AEGL time point derivations. |
|
Uncertainty factors: |
Interspecies: 1 (miosis response to GB vapor is similar across mammal species) Intraspecies: 10 (adjustment for possible susceptible individuals) |
|
Calculations: |
(C/uncertainty factors)2×t=k 10-min to 30-min extrapolation ([0.068 mg/m3]/10)2×(10/60) h=k 7.7×10−6 mg/m3 h=k 4-h to 8-h extrapolation ([0.012 mg/m3]/10)2×4 h=k 5.8×10−6 mg/m3 h=k |
|
10-min AEGL-1: |
C=0.068 mg/m3 10-min AEGL-1=(0.068 mg/m3)/10=0.0068 mg/m3 |
|
30-min AEGL-1: |
C2×(0.5 h)=(7.7×10−6 mg/m3)×h 30-min AEGL-1=0.0039 mg/m3 |
|
1-hAEGL-1: |
C=0.020 mg/m3 1-h AEGL-1=(0.020 mg/m3)/10=0.0020 mg/m3 |
|
4-h AEGL-1: |
C=0.012 mg/m3 4-h AEGL-1=(0.012 mg/m3)/10=0.0012 mg/m3 |
|
8-h AEGL-1: |
C2×(8 h)=(5.8×10−6 mg/m3)×h 8-h AEGL-1=0.00085 mg/m3=0.001 mg/m3 |
Derivation of AEGL-1 for Agent GB (Sarin) from Human Data Set
|
Key study: |
Harvey (1952) (see also Johns [1952]) |
|
Toxicity end point: |
Rhinorrhea, headache, tightness in chest, cramps, nausea, and miosis (mean maximal decrease in pupil diameter) observed in human volunteers exposed to GB at 0.05 mg/m3 for 20 min. Multiple observations at GB vapor Cts ranging from 0.0 to 6.0 mg·min/m3. |
|
LOAEL for GB: |
0.05 mg/m3 for 20 min |
|
Scaling: |
Cn×t=k (ten Berge et al. 1986). Current analyses based on same logic as for AEGL-1 derivation from female SD rat miosis data of Mioduszewski et al (2002b). |
|
Uncertainty factors: |
Interspecies: 1 (human data were used) Intraspecies: 10 (adjustment for possible susceptible individuals) |
|
Calculations: |
(C/uncertainty factors)2×t=k |
|
|
([0.05 mg/m3]/10)2×(20/60) h=k 8.25×10−6 mg/m3×h=k |
|
10-min AEGL-1: |
C2×(0.167 h)=(8.25×10−6 mg/m3)×h C=0.0069 mg/m3 |
|
30-min AEGL-1: |
C2×(0.5 h)=(8.25×10−6 mg/m3)×h C=0.0040 mg/m3 |
|
1-h AEGL-1: |
C2×(1 h)=(8.25×10−6 mg/m3)×h C=0.0028 mg/m3 |
|
4-h AEGL-1: |
C2×(4 h)=(8.25×10−6 mg/m3)×h C=0.0014 mg/m3 |
|
8-h AEGL-1: |
C2×(8 h)=(8.25×10−6 mg/m3)×h C=0.0010 mg/m3 |
Derivation of AEGL-2 for Agent GB (Sarin)
|
Key study: |
Baker and Sedgwick (1996). Eight healthy male servicemen exposed to GB at 0.5 mg/m3 for 30 min in an exposure chamber. During the exposure, test subjects walked at a rate of 96 paces per minute and breathed normally. |
|
Toxicity end point: |
Observed effects include miosis in eight of eight subjects, dyspnea and photophobia in some individuals (number not given), inhibition of RBC-ChE to approximately 60% (range of 54–66%) of individual baseline at 3 h and 3 d postexposure in eight of eight subjects, and small but measurable changes in single fibre electromyography (SFEMG) of the forearm that were detectable in the lab between 4 and 15 mo postexposure (five of eight subjects). Respiratory effects resolved within minutes; ocular effects resolved within 48 h. Electromyographic changes considered subclinical by study authors. No clinical effects. |
|
GB concentration: |
0.5 mg/m3 for 30 min |
|
Scaling: |
Cn×t=k (ten Berge et al. 1986); n=2, see earlier discussion of AEGL-1 scaling |
|
Uncertainty factors: |
Interspecies: 1 (human data were used) Intraspecies: 10 (for possible susceptible individuals) |
|
Calculations: |
(C/uncertainty factors)2×t=k |
|
|
([0.5mg/m3]/10)2×0.5 h=k 0.0013 mg/m3×h=k |
|
10-min AEGL-2: |
C2×(0.167 h)=(0.0013 mg/m3)×h C=0.087 mg/m3 |
|
30-min AEGL-2: |
C2×(0.5 h)=(0.0013 mg/m3)×h |
|
30-min AEGL-2: |
C2×(0.5 h)=(0.0013 mg/m3)×h C=0.050 mg/m3 |
|
1-h AEGL-2: |
C2×(1 h)=(0.0013 mg/m3)×h C=0.035 mg/m3 |
|
4-h AEGL-2: |
C2×(4 h)=(0.0013 mg/m3)×h C=0.017 mg/m3 |
|
8-h AEGL-2: |
C2×(8 h)=(0.0013 mg/m3)×h C=0.0125 mg/m3 |
Derivation of AEGL-3 for Agent GB (Sarin)
|
Key study: |
Mioduszewski et al. (2000, 2001, 2002a). Fourteen-day acute lethal toxicity of GB to female Sprague-Dawley rats was evaluated for time periods of 10, 30, 60, 90, 240, and 360 min in a whole-body dynamic chamber. Ten females were used for each concentration-time (Ct) combination, and 50 females were used for each time point. GB concentrations ranged from about 2 to 54 mg/m3. |
|
Toxicity end point: |
Fourteen-day acute lethal toxicity of GB to female Sprague-Dawley rats. Female rats were reported to be more sensitive to GB vapor toxicity than males over the range of exposure concentrations and durations studied. Gender differences for lethality are reported by Mioduszewski et al. (2000, 2001, 2002a) to be statistically significant at p<0.01. Probit analysis (MINITAB, version 13) presented in Mioduszewski et al. (2000) gave the following 14-d LC50 and LC01 values for female rats: LC50 18.1 mg/m3 for 10 min in female rats 8.51 mg/m3 for 30 min in female rats |
|
|
6.39 mg/m3 for 60 min in female rats 3.03 mg/m3 for 4 h in female rats 2.63 mg/m3 for 6 h in female rats LC01 11.54 mg/m3 for 10 min in female rats 5.84 mg/m3 for 30 min in female rats 4.01 mg/m3 for 60 min in female rats 2.09 mg/m3 for 4 h in female rats 1.76 mg/m3 for 6 h in female rats |
|
Scaling: |
Cn×t=k (ten Berge et al. 1986); n=2, see earlier discussion of AEGL-1 scaling; to extrapolate from 6-h value to an 8-h estimate. Scaling to derive 8-h LC01. |
|
Uncertainty factors: |
Interspecies: 3 (female rat data); full default value of 10 not considered appropriate since the mechanism of toxicity in rats and humans is the same, cholinesterase inhibition. Intraspecies: 10 (for possible susceptible individuals) |
|
Calculations: |
(C/uncertainty factors)2×t=k |
|
|
([1.76mg/m3]/30)2×6.0 h=k 0.021mg/m3×h=k |
|
10-min AEGL-3: |
C=11.54 mg/m3 10-min AEGL-3=(11.54 mg/m3)/30=0.38 mg/m3 |
|
30-min AEGL-3: |
C=5.84 mg/m3 30-min AEGL-3=(5.84 mg/m3)/30=0.194 mg/m3 |
|
1-h AEGL-3: |
C=4.01 mg/m3 1-h AEGL-3=(4.01 mg/m3)/30=0.133 mg/m3 |
|
4-h AEGL-3: |
C=2.09 mg/m3 4-h AEGL-3=(2.09 mg/m3)/30=0.069=0.070 mg/m3 |
|
8-h AEGL-3: |
C2×(8 h)=(0.021 mg/m3)×h 8-h AEGL-3=0.051 mg/m3 |
Derivation of AEGL-3 for Agent GD (Alternate Estimate)
|
Key study: |
Aas et al. (1985) |
|
Toxicity end point: |
Threshold for mortality in rats estimated to be 335 mg·min/m3 for an exposure period of 16 min. The lethal threshold is equivalent to an exposure to a GD concentration of 21 mg/m3 for 16 min. |
|
Scaling: |
Data insufficient to derive an n value from the Aas et al. (1985) study. The anticholinesterase mechanism of mammalian toxicity for nerve agents is known, and all end points observed in human and animal studies represent a response continuum to anticholinesterase exposure. Accordingly, it is valid to apply an n value derived from the compound-specific lethality and miosis data for nerve agent GB (Mioduszewski et al. 2000, 2002, 2002a,b) to time scaling for all AEGL effects. This position is consistent with that of the recently published science policy of the EPA Office of Pesticide Programs (EPA 2000). Therefore, n=2 is used as the scaling function for all AEGL time point derivations for the G-series nerve agents. |
|
Uncertainty factors: |
Interspecies: 10 (sparse data set for agent GD) Intraspecies: 10 (for possible susceptible individuals; sparse data set for agent GD) |
|
Calculations: |
(C/uncertainty factors)2×t=k ([21 mg/m3]/100)2×(16/60) h=0.012 mg/m3×h |
|
10-min AEGL-3: |
C2×(0.167)=(0.012 mg/m3)×h |
|
30-min AEGL-3: |
10-min AEGL-3=0.27 mg/m3 C2×(0.5 h)=(0.012 mg/m3)×h 30-min AEGL-3=0.15 mg/m3 |
Derivation of AEGL-1 for Agent VX Vapor from Agent GB Toxicity Data
|
Key study: |
Mioduszewski et al. (2002b). Miosis measured in adult female SD rats exposed to a range of GB vapor concentrations for 10, 60, and 240 min. At 10 min, N =52 females; at 60 min, N=35 females; at 240 min, N=55 females. |
|
Toxicity end point: |
EC50 for miosis (defined as “a postexposure pupil diameter 50% or less of the preexposure pupil diameter”) observed in adult female SD rats |
|
EC50 for GB: |
10-min EC50=0.068 mg/m3 60-min EC50=0.020 mg/m3 240-min EC50=0.012 mg/m3 |
|
Estimated EC50 for VX: |
10-min EC50=0.017 mg/m3 (based on relative potency of 4 as described in Section 4.3) 60-min EC50=0.005 mg/m3 (based on relative potency of 4 as described in Section 4.3) 240-min EC50=0.003 mg/m3 (based on relative potency of 4 as described in Section 4.3) |
|
Scaling: |
Cn×t=k (ten Berge et al. 1986). Current analyses based on a linear regression of the lethality and miosis data for female SD rats. The LC01 of GB to female Sprague-Dawley rats (Mioduszewski et al. 2000, 2001, 2002a) yields an n value of 1.93 with an r2 of 0.9948, while miosis (female Sprague-Dawley rats) (Mioduszewski et. al. 2002b) yields an n value of 2.00 with an r2 of 0.4335. The anticholinesterase |
|
|
mechanism of mammalian toxicity for nerve agents is known, and all end points observed in human and animal studies represent a response continuum to anticholinesterase exposure. Accordingly, it is valid to apply an n value derived from compound-specific lethality data to time scaling for nonlethal effects, and an n value derived for miosis to consideration of other toxic effects resulting from anticholinesterase exposure. This position is consistent with that of the recently published science policy of the EPA Office of Pesticide Programs (EPA 2000). Therefore, n=2 is used as the scaling function for all AEGL time point derivations. |
|
Uncertainty factors: |
Interspecies: 1 (miosis response to GB vapor similar across mammal species) Intraspecies: 10 (adjustment for possible susceptible individuals) |
|
Modifying factor: |
3 (for sparse VX data base) |
|
Calculations: |
(C/uncertainty factors)2×t=k |
|
|
10-min to 30-min extrapolation ([0.017 mg/m3]/30)2×(10/60) h=k 5.0×10−8 mg/m3×h=k 4-h to 8-h extrapolation ([0.003 mg/m3]/30)2×4 h=k 4.0×10−8 mg/m3×h=k |
|
10-min AEGL-1: |
C=0.017 mg/m3 10-min AEGL-1=(0.017 mg/m3)/30=0.00057 mg/m3 |
|
30-min AEGL-1: |
C2×(0.5 h)=(5.0×10−8 mg/m3)×h 30-min AEGL-1=0.00033 mg/m3 |
|
1-h AEGL-1: |
C=0.0050 mg/m3 |
|
4-hAEGL-1: |
C=0.003 mg/m3 4-h AEGL-1=(0.003 mg/m3)/30=0.00010 mg/m3 |
|
8-hAEGL-1: |
C2×(8 h)=(4.0×10−8 mg/m3)×h 8-h AEGL-1=0.000071 mg/m3 |
Derivation of AEGL-2 for Agent VX Vapor from Agent GB Toxicity Data
|
Key Study: |
Baker and Sedgwick (1996) |
|
Toxicity end point: |
Miosis in all subjects, dyspnea and photophobia in some individuals, inhibition of RBC-ChE to approximately 60% of individual baseline at 3 h and 3 d postexposure, and small but measurable changes in single fibre electromyography (SFEMG) of the forearm that were detectable in the lab between 4 and 15 mo postexposure. Baker and Sedgwick (1996) considered these SFEMG changes to be subclinical and reversible. Current analysis considered SFEMG changes to be a protective interpretation of the AEGL-2 definition. |
|
GB concentration: |
0.5 mg/m3 for 30 min |
|
Estimated VX concentration: |
0.125 mg/m3 for 30 min (based on relative potency of 4 for miosis and mild effects as described in Section 4.3) |
|
Scaling: |
Cn×t=k (ten Berge et al. 1986). The n value of 2 used for agent GB is also used for agent VX (see text) |
|
Uncertainty factors: |
Interspecies: 1 (human data were used) Intraspecies: 10 (adjustment for possible susceptible individuals) |
|
|
individuals) |
|
Modifying factor (MF): |
3 (for sparse VX data base) |
|
Calculations: |
(C/[uncertainty factors×MF])2×t=k |
|
|
([0.125 mg/m3]/[10×3])2×0.5 h= 8.7×10−6 mg/m3 ×h |
|
10-min AEGL-2: |
([8.7×10−6 mg/m3]/0.1667 h)1/2=0.0072 mg/m3 |
|
30-min AEGL-2: |
([8.7×10−6 mg/m3]/0.5 h)1/2=0.0042 mg/m3 |
|
1-h AEGL-2: |
([8.7×10−6 mg/m3]/1 h)1/2=0.0029 mg/m3 |
|
4-h AEGL-2: |
([8.7×10−6 mg/m3]/4 h)1/2=0.0015 mg/m3 |
|
8-h AEGL-2: |
([8.7×10−6 mg/m3]/8 h)1/2=0.00104 mg/m3 |
Derivation of AEGL-3 for Agent VX Vapor from Agent GB Toxicity Data
|
Key study: |
Mioduszewski et al. (2000, 2001, 2002a) |
|
Toxicity end point: |
GB-induced lethality in female Sprague-Dawley rats |
|
GB LC50: |
18.1 mg/m3 for 10 min 8.51 mg/m3 for 30 min 6.39 mg/m3 for 60 min 3.03 mg/m3 for 4 h 2.63 mg/m3 for 6 h |
|
GB LC01: |
11.54 mg/m3 for 10 min 5.84 mg/m3 for 30 min 4.01 mg/m3 for 60 min 2.09 mg/m3 for 4 h 1.76 mg/m3 for 6 h |
|
VX estimated LC01: |
2.89 mg/m3 for 10 min 1.46 mg/m3 for 30 min 1.00 mg/m3 for 1 h 0.52 mg/m3 for 4 h 0.44 mg/m3 for 6 h (relative potency of 4 when compared with GB; see Section 4.3) |
|
Scaling: |
Cn×t=k (ten Berge et al. 1986). The n value of 2 used for agent GB is also used for agent VX (see text) to derive the 8-h AEGL-3 from the 6-h LC01. |
|
Uncertainty factors: |
Interspecies: 3 (rat data) Intraspecies: 10 (adjustment for possible susceptible individuals) |
|
Modifying factor (MF): |
3 (for sparse VX data base) |
|
Calculations: |
(C/[uncertainty factors×MF])2×t=k |
|
|
([0.44 mg/m3]/[10×3×3])2×6 h= 1.16×10−4 mg/m3×h |
|
10-min AEGL-3: |
(2.89 mg/m3)/100=0.029 mg/m3 |
|
30-min AEGL-3: |
(1.46 mg/m3)/100=0.015 mg/m3 |
|
1-h AEGL-3: |
(1.00 mg/m3)/100=0.010 mg/m3 |
|
4-h AEGL-3: |
(0.52 mg/m3)/100=0.0052 mg/m3 |
|
8-h AEGL-3: |
([1.16×10−4 mg/m3]/8 h)1/2=0.0038 mg/m3 |
APPENDIX B
Concentration-Time Curve for Sarin-Induced Miosis in Humans and Lethality in SD Rats
Data from Johns (1952), McKee and Woolcott (1949), and Baker and Sedgwick (1996) were used to assess the concentration-time relationship for agent GB-induced miosis
|
Time |
Conc. |
Log Time |
Log Conc. |
Regression Output: |
|
|
20 |
0.05 |
1.3010 |
−1.3010 |
Intercept |
0.2371 |
|
2 |
0.5 |
0.3010 |
−0.3010 |
Slope |
−0.8629 |
|
2 |
1 |
0.3010 |
0.0000 |
R Squared |
0.6704 |
|
20 |
0.2 |
1.3010 |
−0.6990 |
Correlation |
−0.8188 |
|
2 |
2 |
0.3010 |
0.3010 |
Observations |
16 |
|
4 |
1 |
0.6021 |
0.0000 |
|
|
|
2 |
2.3 |
0.3010 |
0.3617 |
||
|
4 |
1.3 |
0.6021 |
0.1139 |
||
|
20 |
0.3 |
1.3010 |
−0.5229 |
||
|
2 |
3 |
0.3010 |
0.4771 |
||
|
1 |
0.6 |
0.0000 |
−0.2218 |
||
|
40 |
0.06 |
1.6021 |
−1.2218 |
||
|
20 |
0.05 |
1.3010 |
−1.3010 |
||
|
2 |
0.5 |
0.3010 |
−0.3010 |
||
|
1 |
0.6 |
0.0000 |
−0.2218 |
||
|
40 |
0.06 |
1.6021 |
−1.2218 |
||
|
n= |
1.16 |
|
|||
|
k= |
1.88 |
|
|||
Data from Mioduszewski et al. (2000) were used to assess the concentration-time relationship for lethality (LC50) in female Sprague-Dawley rats exposed to GB vapor
|
Time |
Conc. |
Log Time |
Log Conc. |
Regression Output: |
|
|
10 |
18.1 |
1.0000 |
1.2577 |
Intercept |
1.7577 |
|
30 |
8.51 |
1.4771 |
0.9299 |
Slope |
−0.5325 |
|
60 |
6.39 |
1.7782 |
0.8055 |
R Squared |
0.9927 |
|
240 |
3.03 |
2.3802 |
0.4814 |
Correlation |
−0.9964 |
|
360 |
2.63 |
2.5563 |
0.4200 |
Observations |
5 |
|
n= |
1.88 |
|
|||
|
k= |
2000.81 |
|
|||
Data from Mioduszewski et al. (2000) were used to assess the concentration-time relationship for lethality (LC01) in female Sprague-Dawley rats exposed to GB vapor
|
Time |
Conc. |
Log Time |
Log Conc. |
Regression Output: |
|
|
10 |
11.54 |
1.0000 |
1.0622 |
Intercept |
1.5532 |
|
30 |
5.84 |
1.4771 |
0.7664 |
Slope |
−0.5188 |
|
60 |
4.01 |
1.7782 |
0.6031 |
R Squared |
0.9948 |
|
240 |
2.09 |
2.3802 |
0.3201 |
Correlation |
−0.9974 |
|
360 |
1.76 |
2.5563 |
0.2455 |
Observations |
5 |
|
n= |
1.93 |
|
|||
|
k= |
986.04 |
|
|||
|
Chemical: |
GB Vapor |
|
Study: |
Mioduszewski et al., 2002b |
|
Species: |
SD Rat |
|
Gender: |
Female |
|
Time |
Concentration |
Log Time |
Log Concentration |
Regression Output |
|
|
10 |
0.060 |
1.0000 |
−1.2218 |
Slope −0.5010 |
|
|
10 |
0.063 |
1.0000 |
−1.2007 |
R2 0.4335 |
|
|
10 |
0.080 |
1.0000 |
−1.0969 |
Correlation −0.6584 |
|
|
10 |
0.100 |
1.0000 |
−1.0000 |
Observations 24 |
|
|
10 |
0.110 |
1.0000 |
−0.9586 |
|
|
|
10 |
0.200 |
1.0000 |
−0.6990 |
||
|
10 |
0.220 |
1.0000 |
−0.6576 |
||
|
10 |
0.480 |
1.0000 |
−0.3188 |
||
|
40 |
0.010 |
1.6021 |
−2.0000 |
||
|
60 |
0.015 |
1.7782 |
−1.8239 |
||
|
60 |
0.016 |
1.7782 |
−1.7959 |
||
|
60 |
0.034 |
1.7782 |
−1.4685 |
||
|
60 |
0.043 |
1.7782 |
−1.3665 |
||
|
60 |
0.050 |
1.7782 |
−1.3010 |
||
|
240 |
0.011 |
2.3802 |
−1.9586 |
||
|
240 |
0.011 |
2.3802 |
−1.9586 |
||
|
240 |
0.015 |
2.3802 |
−1.8239 |
||
|
240 |
0.015 |
2.3802 |
−1.8239 |
||
|
240 |
0.019 |
2.3802 |
−1.7212 |
||
|
240 |
0.020 |
2.3802 |
−1.6990 |
||
|
240 |
0.027 |
2.3802 |
−1.5622 |
||
|
240 |
0.035 |
2.3802 |
−1.4559 |
||
|
240 |
0.040 |
2.3802 |
−1.3979 |
||
|
n= |
2.00 |
|
|||
|
k= |
0.07 |
||||
APPENDIX C
Benchmark Exposure Analysis of GB Vapor Lethality Data for Female SD Rats
In August 2001, Mioduszewski et al. (2001) published their final report describing the experimental results of lethality studies in male and female adult SD rats exposed to varying Cts of GB vapor in a whole-body dynamic exposure chamber. Preliminary results of the experiments performed by Mioduszewski and his colleagues (documented in Mioduszewski et al. [2000]) were the basis for the AEGL-3 estimates submitted to the National Advisory Committee in 2000 that attained interim status.
A benchmark exposure calculation has been performed on the female rat 14-d vapor lethality data presented in the Mioduszewski et al. (2001) report, in accordance with guidance provided in the standing operating procedures (NRC 2001; Section 2.2.2.3.3). For comparison, a NumberCruncher Statistical System analysis has also been completed.
There appears to be some degree of controversy around using the benchmark dose approach for acute lethality data. The SOP workgroup will address this issue in the future.
There are eight models that accept dichotomous data in the benchmark dose (BMD) software package available on the EPA Web site(http://cfpub.epa.gov/ncea/cfm/bmds.cfm); gamma, logistic, log-log multistage, probit, log-probit, quantal-linear, quantal-quadratic, and Weibull. Evaluations were performed with all eight (multiplied by five time points, times the 5% response for the 95% lower confidence limit (LCL) and the 1% maximum likelihood estimate (MLE), as per the SOP). The Weibull and gamma programs would not run with the input data; contact with the EPA site Webmaster eventually revealed, through systems testing, that these two models require entry of a zero-concentration effect value in order to converge. The Mioduszewski et al. (2001) data set does not contain any zero-concentration effects data, and its addition would be an artificial alteration of the data set. It was concluded that the content of the Mioduszewski et al. (2001) data set is not compatible with requirements of the Weibull and gamma models; thus no analyses of the vapor lethality data were performed with these two models.
Tables C-1a,b and C-2 summarize the statistical results of this benchmark exposure analysis. Table C-1a,b is a summary of LC01 values obtained from all the BMD software routines; the ones on the low-er tier of the table (logistic, multistage, quantal-linear, quantal quadratic) are poor
TABLE C-1a Comparison of MINITAB Estimates of LC01 for Female Rat GB Vapor Inhalation Lethality with NumberCruncher Probit and BMD Analysis (14-d Lethality, Values in mg/m3)
|
Exposure Time |
Basis of Interim AEGL (Mioduszewski et al. 2000) |
MINITAB (Mioduszewski et al. 2001) (95% CI) |
Number Cruncher Probit (SE) |
BMD Probit (BMDL) |
BMD Log Probit (no zero-concentration control) (BMDL) |
BMD Log Probit (zero-concentration control) (BMDL) |
|
10 min |
11.54 |
11.56 (6.71–13.71 |
9.28 (all doses) (1.57) 10.48 (no low dose) (1.65) |
10.77 (6.53) |
11.56 (8.67) |
11.56 (8.67) |
|
30 min |
5.84 |
5.62 (3.71–6.54) |
5.27 (0.67) |
4.74 (2.42) |
5.62 (4.37) |
5.62 (4.37) |
|
60 min |
4.01 |
4.23 (1.32–5.10) |
4.05 (0.78) |
3.76 (3.85; imprecise) |
6.25 (5.51) |
4.24 (2.24) |
|
240 min (4 h) |
2.09 |
1.77 (1.07–2.16) |
1.59 (0.27) |
1.33 (0.44) |
1.77 (1.27) |
1.77 (1.27) |
|
360 min (6 h) |
1.76 |
1.83 (1.04–2.12) |
1.83 (0.21) |
1.71 (1.54; imprecise) |
2.41 (1.54) |
1.83 (1.27) |
TABLE C-1b Comparison of MINITAB Estimates of LC01 for Female Rat GB Vapor Inhalation Lethality with NumberCruncher Probit and BMD Analysis (14-d Lethality, Values in mg/m3)
|
Exposure Time |
BMD Logistic (BMDL) |
BMD Mulitstage (BMDL) |
BMD Quantal Linear (BMDL) |
BMD Quantal Quadratic (BMDL) |
|
10 min |
10.78 (7.53) |
7.92 (1.51) |
0.38 (0.26) |
2.43 (1.99) |
|
30 min |
5.53 (4.07) |
2.99 (0.43) |
0.12 (0.09) |
1.01 (0.86) |
|
60 min |
5.48 (3.54) |
2.74 (0.12) |
0.08 (0.06) |
0.74 (0.63) |
|
240 min (4 h) |
1.61 (1.07) |
1.07 (0.11) |
0.03 (0.03) |
0.33 (0.29) |
|
360 min (6 h) |
2.15 (1.30) |
1.13 (0.07) |
0.04 (0.03) |
0.31 (0.27) |
TABLE C-2 Comparison of AEGL-3 Values Calculated From Estimates of LC01 for Female Rat GB Vapor Inhalation Lethality (14-d Lethality, Values in mg/m3)a
|
Exposure Time |
NAC Interim AEGLb |
MINITAB Log Probitc |
NumberCruncher Probitb |
BMD Probit |
BMD Log Probite |
|
10 min |
0.38 |
0.39 |
0.31 |
0.36 |
0.39 |
|
30 min |
0.19 |
0.19 |
0.18 |
0.16 |
0.19 |
|
60 min |
0.13 |
0.14 |
0.13 |
0.13 |
0.21 |
|
240 min (4 h) |
0.07 |
0.059 |
0.053 |
0.044 |
0.059 |
|
480 min (8 h) |
0.051 |
0.053 |
0.053 |
0.049 |
0.070 |
|
aInterim values compared with those derived from Mioduszewski et al. (2001), MINITAB, NumberCruncher Probit, BMD Probit, and BMD Log Probit. bPublished in 66 Fed. Reg. 21947 (2001) and approved as interim values at NAC-21 on June 10–13, 2001. Derived from estimates of LC01 provided by Mioduszewski et al. from ongoing analysis of female rat lethality data documented in Mioduszewski et al. (2000). cDerived from LC01 values provided in “Appendix A: Probit Analysis” of ECBC-TR-183, ECBC Low-level Operational Toxicology Program; Phase 1—Inhalation Toxicity of Sarin Vapor in Rats as a Function of Exposure Concentration and Duration, by Mioduszewski et al. (2001). These estimates of LC01 were generated by the probit analysis routine provided in Version 13 of MINITAB, a commercial statistical package offered by Minitab, Inc., of State College, Pennsylvania. The probit analysis in the reliability/survival section of the MINITAB package provides a log-probit analysis of the entered data. This log-probit analysis was duplicated during September 2001 in the Life Sciences Division of ORNL by entry of the female rat lethality data (14-d) from Table 3 (page 38) from ECBC-TR-183 into Release 13 (February 2000) of MINITAB. Performance of the BMD Log Probit analysis, with (forced) entry of a zero-concentration control, generated LC01 values equal to those generated by the log-probit MINITAB analysis (e.g., 10 min of 11.56 mg/m3; 30 min of 5.62 mg/m3 ; 60 min of 4.24 mg/m3; 4 h of 1.77 mg/m3; 6 h of 1.83 mg/m3). dNumberCruncher Statistical System Survival Analysis, Version 5.5 (copyright 1991, Dr. Jerry L Hintz, Kaysville, UT). eNo zero-concentration control. Abbreviations: BMD, Benchmark Dose. |
|||||
fits and are rejected from any further consideration. The first column following the exposure times is the set of MLE LC01 values used to develop the AEGL-3 estimates published in the Federal Register (66 Fed. Reg. 21940 [2001]) notice of May 2, 2001. The LC01 values in the second column following the exposure times are those published by Mioduszewski et al. (2001). All the remaining values presented in Table C-1a,b were based on the raw experimental data presented in Mioduszewski et al. (2001). The MINITAB log probit seems to be a reasonable fit with the lethality data. The experimental results on which this analysis is based are published in Mioduszewski et al. (2001, 2002a).
Because the statistical routines used to evaluate the data in Mioduszewski et al. (2000) differ slightly from those used in Mioduszewski et al. (2001), the LC01 values employed in developing interim AEGL-3 determinations also differ slightly. The resulting LC01 and AEGL-3 estimates developed with the same calculational approach, UFs, and n values as were applied in the AEGL-3 determinations presented earlier (see Appendix A), are summarized in tables C-1a,b and C-2. The Federal Register interim values for AEGL-3 (see Table C-2) are consistently lower or equal to the Mioduszewski et al. (2001) log-probit-derived estimates, with the single exception of the 4-h value. In the case of the 4-h value, the NAC interim AEGL-3 (0.070 mg/m3) is somewhat greater than the 4-h AEGL-3 estimate derived from the Mioduszewski et al. (2001) log-probit derivation (0.059 mg/m3; see Table C-2), a difference of 0.011 mg/m3. The variation is slight.
The LC01 values presented in Mioduszewski et al. (2001), although slightly different from the preliminary results considered (Mioduszewski et al. 2000), represent a better documented and more widely accessible data set. These differences are acknowledged.
APPENDIX D
DERIVATION SUMMARY FOR ACUTE EXPOSURE GUIDELINE LEVELS FOR NERVE AGENTS
Derivation Summary For Agent GA (CAS No. 77–81–6) (Tabun; Dimethylamidocyanophosphate)
|
AEGL-1 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.0010 ppm (0.0069 mg/m3) |
0.00060 ppm (0.0040 mg/m3) |
0.00042 ppm (0.0028 mg/m3) |
0.00021 ppm (0.0014 mg/m3) |
0.00015 ppm (0.0010 mg/m3) |
|
Key reference: |
Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D., Crosier, R., Scotto, J., McCaskey, D., Crouse, C., and Matson, K. 2002b. Low-level sarin vapor exposure in rats: Effect of exposure concentration and duration on pupil size. ECBC-TR-235. Edgewood Chemical Biological Center, U.S. Army Soldier and Biological Chemical Command, Aberdeen Proving Ground, MD. (May 2002). |
|||
|
Secondary reference: |
(1) Van Helden, H.P.M.Trap, H.C, Kuijpers, W.C., Groen, B., Oostdijk, J.P., Vanwersch, R.A.P., Philippens, I.H.C., Langenberg, J.P. and Benschop, H.P. 2002. Low Level Exposure to GB Vapor in Air: Diagnosis/Dosimetry, Lowest Observable Effect Level, and Performance-Incapacitation. Research and Technology Organisation (RTO) Meeting Proceedings 75, Operational Medical Issues in Chemical and Biological Defense [RTO-MP-075, AC/323 (HFM-060) TP/37] held in Estoril, Portugal, 14–17 May 2001. North Atlantic Treaty Organisation, Research and Technology Organisation, BP 25, 7 Rue Ancelle, F-92201 Neuilly-sur-Seine CEDEX, France. (2) Harvey, J.C. 1952. Clinical observations on volunteers exposed to concentrations of GB. Medical Laboratories Research Report No. 114, Publication Control No. 5030–114, MLCR 114 (CMLRE-ML-52). Army Chemical Center, MD. (3) Johns, R.J. 1952. The effect of low concentrations of GB on the human eye. Chemical Corps Medical Laboratories Research Report No. 100, Publication Control |
|||
|
|
No. 5030–100 (CMLRE-ML-52), Army Chemical Center, MD. |
|||
|
Test species/strain/gender/number: Based on analysis from Section 4.3 of this document and Mioduszewski et al. (1998) that potency of GA is equal to that of agent GB for AEGL-1 effects (please see derivation for GB AEGL-1 derived from female Sprague-Dawley rat data). |
||||
|
Exposure route/concentrations/durations: Based on analysis from Section 4.3 and Mioduszewski et al. (1998) that potency of GA is equal to that of agent GB (please see derivation for GB AEGL-1) for AEGL-1 effects, and derived from GB vapor inhalation exposures for 10, 60, and 240 min in female SD rats. |
||||
|
Effects: Derivation of AEGL-1 values based on relative potency from Section 4.3 and Mioduszewski et al. (1998) study showingthat the relative potency of GA is equal to that of agent GB (please see derivation for GB AEGL-1) and from GB vapor exposure study of EC50 for miosis observed in adult female SD rats exposed to a range of GB vapor concentrations (0.01–0.48 mg/m3) for 10 min (52 females), 60 min (35 females) and 240 min (55 females) (Mioduszewski et al. 2002b). |
||||
|
End point/concentration/rationale: Derivation of AEGL-1 values based on relative potency from Section 4.3 and Mioduszewski et al. (1998) study showing that the relative potency of GA is equal to that of agent GB (please see derivation for GB AEGL-1) for AEGL-1 effects and EC50 for miosis determination (a reversible, local, nondisabling, and transient effect) in the Mioduszewski et al. (2002b) study of young female SD rats exposed to agent GB. EC50 concentrations for miosis in female SD rats (the susceptible gender) are used as points of departure for AEGL-1 estimation. The miosis effects data of van Helden et al. (2001, 2002) (nonhuman primates), Harvey (1952) (human volunteers), and Johns (1952) (human volunteers) are supportive. The EC50 for miosis (postexposure pupil diameter 50% or less of the preexposure pupil diameter in 50% of exposed population) (Mioduszewski et al. 2002b) is not considered an adverse effect for humans. |
||||
|
Uncertainty factors/rationale: Based on relative potency from Section 4.3 and Mioduszewski et al. (1998) study showing that the relative potency GA is equal to that of agent GB (please see derivation for GB AEGL-1). Total uncertainty factor: 10 Interspecies: 1—miosis response to GB vapor exposure is similar across multiple mammalian species. Intraspecies: 10—for susceptible human subpopulations. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the |
||||
|
effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3.). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for agent GB). |
||||
|
Animal to human dosimetric adjustment: None applied. |
||||
|
Time scaling: Based on relative potency from Section 4.3 and Mioduszewski et al. (1998) study showing that potency of GA is equal to that of agent GB (please see derivation for GB AEGL-1) and Cn×t=k where n=2 and k= 7.7×10−6 mg/m3×h for 10- to 30-min extrapolation, and k=5.8×10−6 mg/m3 ×h for 4-h to 8-h extrapolation for agent GB. |
||||
|
Data adequacy: Based on relative potency equal to that of agent GB (please see derivation for GB AEGL-1). The scarcity of dose-response data for agent GA forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL values for agent GA are derivative. The relative potency assumptions for estimating AEGL-1 values for agent GA from the available database for agent GB need experimental confirmation. |
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.013 ppm (0.087 mg/m3) |
0.0075 ppm (0.050 mg/m3) |
0.0053 ppm (0.035 mg/m3) |
0.0026 ppm (0.017 mg/m3) |
0.0020 ppm (0.013 mg/m3) |
|
Key Reference: Baker, D.J., Sedgwick, E.M. 1996. Single fibre electromyographic changes in man after organophosphate exposure. Hum. Exp. Toxicol. 15:369–375. |
||||
|
Test species/strain/gender/number: Based on relative potency estimate from Section 4.3 of this document and Mioduszewski et al. (1998)—potency of GA is equal to that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2 derived from human volunteer data). |
||||
|
Exposure route/concentrations/durations: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—potency of GA is equal to that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2) and derived from GB vapor inhalation to humans in exposure chamber (GB at 0.5 mg/m3 for 30 min while walking at a rate of 96 paces per min and breathing normally [Baker and Sedgwick 1996]). |
||||
|
Effects: Derivation of AEGL-2 values based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that potency of GA is equal to that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2) and from GB vapor exposure study (Baker and Sedgwick 1996) in which observed effects included miosis in eight of e subjects, dyspnea and photophobia in some individuals, inhibition of RBC-ChE to approximately 60% (range of 54–66.1%) of individual baseline at 3 h and 3 d postexposure in eight of eight subjects, and small but measurable changes in single fibre electromyography (SFEMG) of the forearm that were detectable in the lab between 4 and 15 mo postexposure (but not detectable at 15–30 mo postexposure) in five of eight human volunteers exposed to GB at 0.5 mg/m3 for 30 min. SFEMG changes considered subclinical. No permanent effects. |
||||
|
End point/concentration/rationale: Derivation of AEGL-2 values based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that potency of GA is equal to that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2). Presence of miosis in all subjects exposed to GB vapor (Baker and Sedgwick 1996), in addition to the other observed signs in portions of the exposed population, indicates a greater level of effect than the EC50 considered for AEGL-1 determination; thus, there exists a heightened potential for reduced visual acuity following a 30-min exposure to GB at 0.5 mg/m3. SFEMG effects are documented as long-lasting, but are subclinical and fully reversible. Such effects are not usually included as a basis for AEGL-2 estimation. However, due to the known steep |
||||
|
dose response for nerve agent vapor exposure, incorporation of the long-lasting SFEMG endpoint is here considered a protective interpretation of the AEGL-2 definition. The point of departure for AEGL-2 estimation is 0.5 mg GB/m3 for 30 min. |
||||
|
Uncertainty factors/rationale: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that potency of GA is equal to that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2). Total uncertainty factor: 10 Interspecies: 1—human data Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3.). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for agent GB). |
||||
|
Animal to human dosimetric adjustment: None applied (human data) |
||||
|
Time scaling: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—potency of GA is equal to that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2) and Cn×t=k where n=2 and k=0.0013 mg/m3×h |
||||
|
Data adequacy: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—potency of GA is equal to that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2). The scarcity of dose-response data for agent GA forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL values for agent GA are derivative. The relative potency assumptions for estimating AEGL-2 values for agent GA from the available database for agent GB need experimental confirmation. |
|
AEGL-3 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.11 ppm (0.76 mg/m3) |
0.057 ppm (0.38 mg/m3) |
0.039 ppm (0.26 mg/m3) |
0.021 ppm (0.14 mg/m3) |
0.015 ppm (0.10 mg/m3) |
|
Key reference: |
(1) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D. and Crosier, R. 2000. Estimating the probability of sarin vapor toxicity in rats as a function of exposure concentration and duration. Proceedings of the International Chemical Weapons Demilitarization Conference (CWD-2000), The Hague, NL. May 21–24, 2000. (2) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B., Muse, W., Anthony, J., Durst, D., Sommerville, D., Crosier, R., Thomson, S., and Crouse, C. 2001. ECBC Low Level Operational Toxicology Program: Phase I—Inhalation toxicity of sarin vapor in rats as a function of exposure concentration and duration. ECBC-TR-183, Edgewood Research Development and Engineering Center, Aberdeen Proving Ground, MD. (August 2001). (3) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D., and Crosier, R. 2002a. Interaction of exposure concentration and duration in determining acute toxic effects of sarin vapor in rats. Toxicol. Sci. 66:176–184. |
|||
|
Test species/strain/gender/number: Based on relative potency estimate from Section 4.3 of this document and Mioduszewski et al. (1998)—potency of GA is approximately one-half that of agent GB for lethality (please see derivation for GB AEGL-3 derived from female Sprague-Dawley rat data). |
||||
|
Exposure route/concentrations/durations: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—potency of GA is approximately one-half that of agent GB for lethality (please see derivation for GB AEGL-3) and study of rat inhalation toxicity in dynamic mode exposure chamber (Mioduszewski et al. 2000, 2001, 2002a). |
||||
|
Effects: Derivation of AEGL-3 values based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that potency of GA is approximately one-half that of agent GB for lethality (please see derivation for GB AEGL-3) and from GB vapor exposure study (Mioduszewski et al. 2000, 2001, 2002a) in which lethality and sublethal clinical signs monitored during and after exposure. Only lethality data reported at this time. |
||||
|
End point/concentration/rationale: Derivation of AEGL-3 values based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that potency of GA is approximately one-half that of agent GB for lethality (please see derivation for GB AEGL-3) and from GB vapor exposure study (Mioduszewski et al. 2000, 2001, 2002a) which derived 14-d lethality estimates for female Sprague-Dawley rats (female rats were reported to be overall more sensitive to GB vapor toxicity than male rats over the range of exposure concentrations and durations studied). Gender differences in sensitivity are reported to be statistically significant at p<0.01 (Mioduszewski et al. 2000, 2001, 2002a). Probit analysis (MINITAB, version 13) presented in Mioduszewski et al. (2000) study provided 14-d LC50 and LC01 values for female rats. End point concentrations for LC01 reported for female SD rats (the susceptible gender) are used as points of departure from which to derive AEGL-3 estimates. |
||||
|
Uncertainty factors/rationale: Based on relative potency estimate from Mioduszewski et al. (1998) study showing that potency of GA is approximately one-half that of agent GB for lethality (please see derivation for GB AEGL-3) Total uncertainty factor: 30 Interspecies: 3—The full default value of 10 was not considered necessary because the mechanism of toxicity in both rats and humans is the same, cholinesterase inhibition. Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase and carboxlyesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for agent GB). |
||||
|
Animal to Human Dosimetric Adjustment: None applied, insufficient data |
||||
|
Time scaling: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—potency of GA is approximately one-half that of agent GB for lethality (please see derivation for GB AEGL-3) and Cn×t=k where n=2 and k=0.021 mg/m3×h to extrapolate from 6-h value to 8-h estimate of LC01. |
||||
|
Data adequacy: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) that potency of GA is approximately one-half that of agent GB for lethality (please see derivation for GB AEGL-3). The scarcity of dose-response data for agent GA forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL values for agent GA are derivative. The relative potency assumptions for estimating AEGL-3 values |
Derivation Summary for Agent GB (CAS No. 107–44–8) (Sarin; Isopropylmethylphosphonofluoridate)
|
AEGL-1 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.0012 ppm (0.0069 mg/m3) |
0.00068 ppm (0.0040 mg/m3) |
0.00048 ppm (0.0028 mg/m3) |
0.00024 ppm (0.0014 mg/m3) |
0.00017 ppm (0.0010 mg/m3) |
|
Key reference: |
Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D., Crosier, R., Scotto, J., McCaskey, D., Crouse, C., and Matson, K. 2002b. Low-level sarin vapor exposure in rats: Effect of exposure concentration and duration on pupil size. ECBC-TR-235. Edgewood Chemical Biological Center, U.S. Army Soldier and Biological Chemical Command, Aberdeen Proving Ground, MD. (May 2002). |
|||
|
Secondary reference: |
(1) van Helden, H.P.M.Trap, H.C, Kuijpers, W.C., Groen, B., Oostdijk, J.P., Vanwersch, R.A.P., Philippens, I.H.C., Langenberg, J.P. and Benschop, H.P. 2002. Low Level Exposure to GB Vapor in Air: Diagnosis/Dosimetry, Lowest Observable Effect Level, and Performance-Incapacitation. Research and Technology Organisation (RTO) Meeting Proceedings 75, Operational Medical Issues in Chemical and Biological Defense [RTO-MP-075, AC/323 (HFM-060) TP/37] held in Estoril, Portugal, 14–17 May 2001. North Atlantic Treaty Organisation, Research and Technology Organisation, BP 25, 7 Rue Ancelle, F-92201 Neuilly-sur-Seine CEDEX, France. (2) Harvey, J.C. 1952. Clinical observations on volunteers exposed to concentrations of GB. Medical Laboratories Research Report No. 114, Publication Control No. 5030–114, MLCR 114 (CMLRE-ML-52). Army Chemical Center, MD. (3) Johns, R.J. 1952. The effect of low concentrations of GB on the human eye. Chemical Corps Medical Laboratories Research Report No. 100, Publication Control No. 5030–100 (CMLRE-ML-52), Army Chemical Center, MD. |
|||
|
Test Species/Strain/Sex/Number: Rat, young adult (8–10 wk) female Sprague Dawley. Numbers of exposed individuals per time duration as follows: 10 |
||||
|
min (52 females), 60 min (35 females) and 240 min (55 females) (Mioduszewski et al 2002b). |
||||
|
Exposure route/concentrations/durations: Whole body vapor exposure in 750-L dynamic airflow inhalation chamber to a range of GB vapor concentrations (0.01–0.48 mg/m3) for 10 min, 60 min, and 240 min (Mioduszewski et al. 2002b). |
||||
|
Effects: Mioduszewski et al. (2002b) document EC50 for miosis observed in adult female SD rats exposed to a range of GB vapor concentrations (0.01– 0.48 mg/m3) for durations of 10 min (52 females), 60 min (35 females), and 240 min (55 females). EC50 for miosis defined as a postexposure pupil diameter 50% or less of the preexposure pupil diameter in 50% of the exposed rat population (Mioduszewski et al. 2002b). EC50 for miosis is a reversible, local, and transient effect. No significant changes from baseline noted in blood RBC-ChE, BuChE, or carboxylesterase. No other clinical signs observed in the exposed rats. |
||||
|
End point/concentration/rationale: Mioduszewski et al. (2002b) document EC50 for miosis from their study of young female SD rats as a well-defined animal endpoint that is reversible, local, transient, and nondisabling. The EC50 concentrations for miosis in female SD rats (the susceptible gender) are used as points of departure for AEGL-1 estimation; the resulting AEGL-1 estimates are thus protective. The miosis effects data of van Helden et al. (2001, 2002) (nonhuman primates), Harvey (1952) (human volunteers), and Johns (1952) (human volunteers) are supportive. The EC50 for miosis effect (postexposure pupil diameter 50% or less of the preexposure pupil diameter in 50% of exposed population) (Mioduszewski et al. 2002b) is not considered an adverse effect for humans. |
||||
|
Uncertainty factors/rationale: Total uncertainty factor: 10 Interspecies: 1—miosis response to GB vapor exposure is similar across multiple mammalian species. Intraspecies: 10—for susceptible human subpopulations. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None. Strong arguments for not incorporating an additional modifying factor include the following: |
|
1. Data are available for multiple species. 2. Data characterizing both lethal and nonlethal endpoints have been used in the analysis; these end points possess exposure-response data. 3. the mechanism of toxicity is known 4. the n value is derived from experimental data and is not the default 5. there are no uncertainties regarding reproductive and developmental effects or issues of carcinogenicity. In consequence, no modifying factor was used in the estimation of AEGL-1 values. |
||||
|
Animal to human dosimetric adjustment: None applied. |
||||
|
Time scaling: Cn×t=k where n=2 and k=7.7×10−6 mg/m3×h for 10- to 30-min extrapolation, and k=5.8×10−6 mg/m3×h for 4-h to 8-h extrapolation. Current analyses based on a linear regression of the lethality and miosis data for female SD rats. The LC01 of GB to female Sprague-Dawley rats (Mioduszewski et al. 2000a,b, 2001, 2002a) yields an n value of 1.93 with an r2 of 0.9948, while miosis (EC50 of GB to female Sprague-Dawley rats) (Mioduszewski et al 2002b) yields an n value of 2.00 with an r2 of 0.4335 (see Appendix B). The anticholinesterase mechanism of mammalian toxicity for nerve agents is known, and all end points observed in human and animal studies represent a response continuum to anticholinesterase exposure. Accordingly, it is valid to apply an n value derived from compound-specific lethality data to time scaling for nonlethal effects, and an n value derived for miosis, to consideration of other toxic effects resulting from anticholinesterase exposure. This position is consistent with that of the recently published science policy of the EPA Office of Pesticide Programs (EPA 2000). Furthermore, this approach is preferable to use of default values. Therefore, n=2 is used as the scaling function for all AEGL time point derivations for agent GB. |
||||
|
Data adequacy: Confidence in the AEGL-1 values is high due to the robust data set. A total of 423 rats were used in this well-conducted study, of which 130 were controls. Three vapor exposure durations, each of which is an AEGL exposure interval (10, 60, and 240 min) were incorporated into the study design, and a sufficient number of individuals were exposed at each interval: 10 min (52 females), 60 min (35 females), and 240 min (55 females) (Mioduszewski et al. 2002b). |
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.015 ppm (0.087 mg/m3) |
0.0085 ppm (0.050 mg/m3) |
0.0060 ppm (0.035 mg/m3) |
0.0029 ppm (0.017 mg/m3) |
0.0022 ppm (0.013 mg/m3) |
|
Key reference: Baker, D.J., Sedgwick , E.M. 1996. Single fibre electromyographic changes in man after organophosphate exposure. Hum. Exp. Toxicol. 15:369–375. |
||||
|
Test species/strain/gender/number: human volunteers (healthy male servicemen), N=8 adults |
||||
|
Exposure route/concentrations/durations: Inhalation in exposure chamber; 0.5 mg/m3 for 30 min while walking at a rate of 96 paces per minute and breathing normally. |
||||
|
Effects: Baker and Sedgwick (1996) document effects observed in human volunteers (0.5 mg/m3 for 30 min while subjects in an exposure chamber walked at a rate of 96 paces per minute and breathed normally), including miosis in eight of eight subjects, dyspnea and photophobia in some individuals, inhibition of RBC-ChE to approximately 60% (range of 54– 66.1%) of individual baseline at 3 h and 3 d postexposure in eight of eight subjects, and small but measurable changes in single fibre electromyography (SFEMG) of the forearm that were detectable in the lab between 4 and 15 mo postexposure (in five of eight human volunteers), but not detectable at 15–30 mo postexposure. Respiratory effects resolved within minutes; ocular effects resolved within 48 h. SFEMG changes considered subclinical. No permanent effects. |
||||
|
End point/concentration/rationale: Presence of miosis in all subjects, in addition to other observed signs in portions of exposed population in the key study, indicates a greater level of effect than the EC50 considered for AEGL-1 determination; thus, there exists a heightened potential for reduced visual acuity following a 30 min exposure at 0.5 mg/m3. SFEMG abnormalities detectable in the lab between 4 and 15 mo after a single experimental exposure documents these effects as long-lasting. Such subclinical and fully reversible effects are not usually included as a basis for AEGL-2 estimation. However, due to the known steep dose response for nerve agent vapor exposure, incorporation of the long-lasting SFEMG effect end point is here considered a protective interpretation of the AEGL-2 definition. The point of departure for AEGL-2 estimation is 0.5 mg/m3 for 30 min. |
||||
|
Uncertainty factors/rationale: Total uncertainty factor: 10 Interspecies: 1—human data |
||||
|
Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for AEGL-1). |
||||
|
Animal to human dosimetric adjustment: None applied (human data) |
||||
|
Time scaling: Cn×t=k where n=2 and k=0.0013 mg/m3×h. The recent data of Mioduszewki et al. (2000, 2001, 2002a,b) are determined to be the best source of an estimate for the n value for GB response (see also Appendix B and derivation for AEGL-1). The Mioduszewski et al. (2000, 2001, 2002a,b) data set is robust and compound-specific for the most completely characterized G-series nerve agent, agent GB. As outlined earlier, the mechanism of mammalian toxicity for nerve agents is known, and all end points observed in human and animal studies represent a response continuum to anticholinesterase exposure. Accordingly, it is valid to apply an n value derived from compound-specific miosis and lethality data to time scaling for all AEGL effects. This position is consistent with that of the recently published science policy of the EPA Office of Pesticide Programs (EPA 2000). Furthermore, this approach is preferable to use of default values. The current analysis is based on a linear regression of the lethality of GB to female Sprague-Dawley rats (Mioduszewski et al. 2000b), which yields an n value of 2.00 (see Appendix B). Therefore, n=2 is used as the scaling function for the AEGL-2 derivations. |
||||
|
Data adequacy: Confidence in the AEGL-2 values is limited by the lack of human experimental data for all AEGL-specific time frames and the lack of a clearly defined exposure-response relationship. The human data from which the AEGL-2 values were derived were limited by the short exposure time (30 min). Observed differences among human studies in identifying the toxicity threshold for low-dose exposures may be due to differences in individual sensitivities and breathing rates among the test populations, the steepness of the dose-response curve, and/or differences in experimental protocols or analytical methods. |
|
AEGL-3 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.064 ppm (0.38 mg/m3) |
0.032 ppm (0.19 mg/m3) |
0.022 ppm (0.13 mg/m3) |
0.012 ppm (0.070 mg/m3) |
0.0087 ppm (0.051 mg/m3) |
|
Key reference: |
(1) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D. and Crosier, R. 2000. Estimating the probability of sarin vapor toxicity in rats as a function of exposure concentration and duration. Proceedings of the International Chemical Weapons Demilitarization Conference (CWD-2000), The Hague, NL. May 21–24, 2000. (2) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B., Muse, W., Anthony, J., Durst, D., Sommerville, D., Crosier, R., Thomson, S, and Crouse, C. 2001. ECBC Low Level Operational Toxicology Program: Phase I—Inhalation toxicity of sarin vapor in rats as a function of exposure concentration and duration. ECBC-TR-183, Edgewood Research Development and Engineering Center, Aberdeen Proving Ground, MD. (August 2001). (3) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D., and Crosier, R. 2002a. Interaction of exposure concentration and duration in determining acute toxic effects of sarin vapor in rats. Toxicol. Sci. 66:176–184. |
|||
|
Test species/strain/gender/number: Sprague-Dawley rats (8 to 10 wk old, from Charles River Laboratories), 10 females per concentration-time (Ct) combination, 50 females per time point, |
||||
|
Exposure route/concentrations/durations: Inhalation in dynamic mode exposure chamber; individuals exposed whole-body to one of five concentrations (2–56 mg/m3) for one of seven exposure times (3, 10, 30, 60, 90, 240, or 360 min). |
||||
|
Effects: Mioduszewski et al. (2000, 2001, 2002a) document lethality and sublethal clinical signs monitored during and after experimental exposure. Only lethality data reported at this time. |
||||
|
End point/concentration/rationale: Mioduszewski et al. (2000, 2001, 2002a) document 14-d acute lethal toxicity of GB to female Sprague-Dawley rats. Female rats were reported to be more sensitive to GB vapor toxicity than males over the range of exposure concentrations and durations studied. Gender differences for lethality are reported by Mioduszewski et al. (2000, 2001, 2002a) to be statistically significant at p<0.01; thus, selection of |
||||
|
end point concentrations for LC01 reported for female SD rats (the susceptible gender) as points of departure from which to derive AEGL-3 estimates is protective. Probit analysis (MINITAB, version 13) presented in Mioduszewski et al. (2000) study gave the following 14-d LC50 and LC01 values for female rats: |
||||
|
LC50 18.1 mg/m3 for 10 min in female rats 8.51 mg/m3 for 30 min in female rats. 6.39 mg/m3 for 60 min in female rats 3.03 mg/m3 for 4 h in female rats 2.63 mg/m3 for 6 h in female rats |
||||
|
LC01 11.54 mg/m3 for 10 min in female rats 5.84 mg/m3 for 30 min in female rats. 4.01 mg/m3 for 60 min in female rats 2.09 mg/m3 for 4 h in female rats 1.76 mg/m3 for 6 h in female rats |
||||
|
Uncertainty factors/rationale: Total uncertainty factor: 30 Interspecies: 3 (female rat data). The full default value of 10 was not considered necessary because the mechanism of toxicity in both rats and humans is the same, cholinesterase inhibition. Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for AEGL-1). |
||||
|
Animal to human dosimetric adjustment: None applied (insufficient data) |
||||
|
Time scaling: Cn×t=k where n=2 and k=0.021 mg/m3×h. The recent data of Mioduszewski et al. (2000, 2001, 2002a,b) are determined to be the best source of an estimate for the n value for GB response (see also Appendix B and derivation for AEGL-1). The Mioduszewski et al. (2000, 2001, 2002a,b) data set is robust and compound-specific for the most completely characterized G-series nerve agent, agent GB. As outlined earlier, the mechanism of mammalian toxicity for nerve agents is known, and all endpoints observed in human and animal studies represent a response continuum to anticholinesterase exposure. Accordingly, it is valid to apply an |
|
n value derived from compound-specific miosis and lethality data to time scaling for all AEGL effects. This position is consistent with that of the recently published science policy of the EPA Office of Pesticide Programs (EPA 2000). Furthermore, this approach is preferable to use of default values. The current analysis is based on a linear regression of the lethality of GB to female Sprague-Dawley rats (Mioduszewski et al. 2000), which yields an n value of 2.00 (see Appendix B). Therefore, n=2 is used as the scaling function for the AEGL-3 derivations. Time scaling was used to extrapolate from 6-h value provided by Mioduszewski et al. (2000) data to an 8-h estimate; scaling to derive 8-h LC01. All other time-specific AEGL-3 values were derived directly from the LC01 values for female SD rats in the Mioduszewski et al. (2000) study. |
||||
|
Data adequacy: Further data analysis and experimentation is needed to more fully understand the degree of sensitivity to lethal exposure concentrations exhibited by female populations of test animals. Interspecies sensitivity could be more fully characterized by determining if similar results can be obtained under the same protocol with different test species (particularly nonhuman primates). |
Derivation Summary for Agent GD (CAS No. 96–64–0) (Soman; Pinacolyl Methylphosphonofluoridate)
|
AEGL-1 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.00046 ppm (0.0035 mg/m3) |
0.00026 ppm (0.0020 mg/m3) |
0.00018 ppm (0.0014 mg/m3) |
0.000091 ppm (0.00070 mg/m3) |
0.000065 ppm (0.00050 mg/m3) |
|
Key reference: |
Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D., Crosier, R., Scotto, J., McCaskey, D., Crouse, C., and Matson, K. 2002b. Low-level sarin vapor exposure in rats: Effect of exposure concentration and duration on pupil size. ECBC-TR-235. Edgewood Chemical Biological Center, U.S. Army Soldier and Biological Chemical Command, Aberdeen Proving Ground, MD. (May 2002). |
|||
|
Secondary reference: |
(1) van Helden, H.P.M. Trap, H.C, Kuijpers, W.C., Groen, B., Oostdijk, J.P., Vanwersch, R.A.P., Philippens, I.H.C., Langenberg, J.P. and Benschop, H.P. 2002. Low Level Exposure to GB Vapor in Air: Diagnosis/Dosimetry, Lowest Observable Effect Level, and Performance-Incapacitation. Research and Technology Organisation (RTO) Meeting Proceedings 75, Operational Medical Issues in Chemical and Biological Defense [RTO-MP-075, AC/323 (HFM-060) TP/37] held in Estoril, Portugal, 14–17 May 2001. North Atlantic Treaty Organisation, Research and Technology Organisation, BP 25, 7 Rue Ancelle, F-92201 Neuilly-sur-Seine CEDEX, France. (2) Harvey, J.C. 1952. Clinical observations on volunteers exposed to concentrations of GB. Medical Laboratories Research Report No. 114, Publication Control No. 5030– 114, MLCR 114 (CMLRE-ML-52). Army Chemical Center, MD. (3) Johns, R.J. 1952. The effect of low concentrations of GB on the human eye. Chemical Corps Medical Laboratories Research Report No. 100, Publication Control No. 5030–100 (CMLRE-ML-52), Army Chemical Center, MD. |
|||
|
Test species/strain/gender/number: Based on relative potency from Section 4.3 of technical support document and Mioduszewski et al. (1998)—agent |
||||
|
GD is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1 derived from rat data). |
||||
|
Exposure route/concentrations/durations: Based on relative potency from Section 4.3 and Mioduszewski et al. (1998)—agent GD is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1) and derived from GB vapor inhalation exposures for 10, 60, and 240 min in female SD rats. |
||||
|
Effects: Derivation of AEGL-1 values based on relative potency from Section 4.3 and Mioduszewski et al. (1998) study showing that potency of GD is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1) and from GB vapor exposure study of EC50 for miosis observed in adult female SD rats exposed to a range of GB vapor concentrations (0.01–0.48 mg/m3) for 10 min (52 females), 60 min (35 females), and 240 min (55 females) (Mioduszewski et al. 2002b). |
||||
|
End point/concentration/rationale: Derivation of AEGL-1 values based on relative potency from Section 4.3 and Mioduszewski et al. (1998) study showing that potency of GD is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1) and EC50 for miosis determination (a reversible, local, nondisabling and transient effect) in the Mioduszewski et al. (2002b) study of young female SD rats exposed to agent GB. The EC50 concentrations for miosis in female SD rats (the susceptible gender) are used as points of departure for AEGL-1 estimation. The miosis effects data of van Helden et al. (2001, 2002) (nonhuman primates), Harvey (1952) (human volunteers), and Johns (1952) (human volunteers) are supportive. The EC50 for miosis (postexposure pupil diameter 50% or less of the preexposure pupil diameter in 50% of exposed population) (Mioduszewski et al. 2002b) is not considered an adverse effect for humans. |
||||
|
Uncertainty factors/rationale: Based on relative potency from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GD is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1). Total uncertainty factor: 10 Interspecies: 1—miosis response to GB vapor exposure is similar across multiple mammalian species. Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for agent GB). |
||||
|
Animal to human dosimetric adjustment: None applied |
||||
|
Time scaling: Based on relative potency from Section 4.3 and Mioduszewski et al. (1998)—agent GD is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1) and Cn×t=k where n=2 and k=7.7×10−6 mg/m3×h for 10-min to 30-min extrapolation and k=5.8×10−6 mg/m3×h for 4-h to 8-h extrapolation for agent GB. |
||||
|
Data adequacy: Based on relative potency determination from Section 4.3 and Mioduszewski et al. (1998)—agent GD is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1). The scarcity of dose-response data for agent GD forces the AEGL-1 analysis to rely on assumptions of relative potency. Thus, AEGL-1 values for agent GD are derivative. The relative potency assumptions for estimating AEGL-1 values for agent GD from the available database for agent GB need experimental confirmation. |
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.0057 ppm (0.044 mg/m3) |
0.0033 ppm (0.025 mg/m3) |
0.0022 ppm (0.018 mg/m3) |
0.0012 ppm (0.0085 mg/m3) |
0.00085 ppm (0.0065 mg/m3) |
|
Key reference: |
Baker, D.J., Sedgwick , E.M. 1996. Single fibre electromyographic changes in man after organophosphate exposure. Hum. Exp. Toxicol. 15:369–375. |
|||
|
Test species/strain/gender/number: Based on relative potency estimate from Section 4.3 of this document and Mioduszewski et al. (1998)—agent GD is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2 derived from human volunteer data). |
||||
|
Exposure route/concentrations/durations: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—agent GD is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2) and derived from GB vapor inhalation to humans in exposure chamber; 0.5 mg/m3 for 30 min while walking at a rate of 96 paces per minute and breathing normally (Baker and Sedgwick 1996). |
||||
|
Effects: Derivation of AEGL-2 values based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GD is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2) and from GB vapor exposure study (Baker and Sedgwick 1996) in which observed effects included miosis in eight of eight subjects, dyspnea and photophobia in some individuals, inhibition of RBC-ChE to approximately 60% (range of 54–66.1%) of individual baseline at 3 h and 3 d postexposure in eight of eight subjects, and small but measurable changes in single fibre electromyography (SFEMG) of the forearm that were detectable in the lab between 4 and 15 mo postexposure (but not detectable at 15–30 mo postexposure) in five of eight human volunteers exposed to GB at 0.5 mg/m3 for 30 min. SFEMG changes considered subclinical. No permanent effects. |
||||
|
End point/concentration/rationale: Derivation of AEGL-2 values based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GD is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2). Presence of miosis in all subjects exposed to GB vapor (Baker and Sedgwick 1996), in addition to other observed signs in portions of exposed population, indicates a greater level of effect than the EC50 considered for AEGL-1 determination; thus, there exists a heightened potential for reduced visual acuity following a 30-min exposure to 0.5 mg GB/m3. SFEMG effects are documented as long-lasting, but are subclinical and fully reversible. Such |
||||
|
effects are not usually included as a basis for AEGL-2 estimation. However, due to the known steep dose response for nerve agent vapor exposure, incorporation of the long-lasting SFEMG end point is here considered a protective interpretation of the AEGL-2 definition. The point of departure for AEGL-2 estimation is 0.5 mg/m3 for 30 min. |
||||
|
Uncertainty factors/rationale: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GD is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2). Total uncertainty factor: 10 Interspecies: 1 (human data) Intraspecies: 10—for susceptible human subpopulations. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for agent GB). |
||||
|
Animal to human dosimetric adjustment: None applied (human data) |
||||
|
Time scaling: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—agent GD is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2) and Cn×t=k where n=2 and k=0.0013 mg/m3×h for agent GB. |
||||
|
Data adequacy: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—agent GD is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2). The scarcity of dose-response data for agent GD forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL values for agent GD are derivative. The relative potency assumptions for estimating AEGL-2 values for agent GD from the available database for agent GB need experimental confirmation. |
|
AEGL-3 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.049 ppm (0.38 mg/m3) |
0.025 ppm (0.19 mg/m3) |
0.017 ppm (0.13 mg/m3) |
0.0091 ppm (0.070 mg/m3) |
0.0066 ppm (0.051 mg/m3) |
|
Key reference: |
(1) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D. and Crosier, R. 2000. Estimating the probability of sarin vapor toxicity in rats as a function of exposure concentration and duration. Proceedings of the International Chemical Weapons Demilitarization Conference (CWD-2000), The Hague, NL. May 21–24, 2000. (2) Mioduszewski, R.J., Manthei, J., Way R., et al. 2001. ECBC Low Level Operational Toxicology Program: Phase I—Inhalation toxicity of sarin vapor in rats as a function of exposure concentration and duration. ECBC-TR-183, Edgewood Research Development and Engineering Center, Aberdeen Proving Ground, MD. (August 2001). (3) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D., and Crosier, R. 2002a. Interaction of exposure concentration and duration in determining acute toxic effects of sarin vapor in rats. Toxicol. Sci. 66:176–184. (4) Aas, P., Sterri, S.H., Hjermstad, H.P., Fonnum, F. 1985. A method for generating toxic vapors of soman: toxicity of soman by inhalation in rats. Toxicol. Appl. Pharmacol. 80:437–445. (Secondary study.) |
|||
|
Test species/strain/gender/number: Based on relative potency estimate from Section 4.3 of this document and Mioduszewski et al. (1998)—agent GD is equipotent to agent GB for lethality (please see derivation for GB AEGL-3 derived from female Sprague-Dawley rat data). |
||||
|
Exposure route/concentrations/durations: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—agent GD is equipotent to agent GB for lethality (please see derivation for GB AEGL-3) and study of rat inhalation toxicity in dynamic mode exposure chamber (Mioduszewski et al. 2000, 2001, 2002a). |
||||
|
Effects: Derivation of AEGL-3 values based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GD is equipotent to agent GB for lethality (please see derivation for GB AEGL-3) and from GB vapor exposure study (Mioduszewski et al. 2000, 2001, 2002a) in which lethality and sublethal clinical signs monitored during and after exposure. Only lethality data reported at this time. |
||||
|
End point/concentration/rationale: Derivation of AEGL-3 values based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GD is equipotent to agent GB for lethality (please see derivation for GB AEGL-3) and from GB vapor exposure study (Mioduszewski et al. 2000, 2001, 2002a) which derived 14-d lethality estimates for female Sprague-Dawley rats (female rats were reported to be overall more sensitive to GB vapor toxicity than male rats over the range of exposure concentrations and durations studied). Gender differences in sensitivity are reported to be statistically significant at p<0.01 (Mioduszewski et al. 2000, 2001, 2002a). Probit analysis (MINITAB, version 13) presented in Mioduszewski et al. (2000) study provided 14-d LC50 and LC01 values for female rats. End point concentrations for LC01 reported for female SD rats (the susceptible gender) are used as points of departure from which to derive AEGL-3 estimates. |
||||
|
Uncertainty factors/rationale: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GD is equipotent to agent GB for lethality (please see derivation for GB AEGL-3). Total uncertainty factor: 30 Interspecies: 3 (female rat data). The full default value of 10 was not considered necessary because the mechanism of toxicity in both rats and humans is the same, cholinesterase inhibition. Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially suscep tible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3). Therefore, a factor of 10 was retained. From the secondary study of Aas et al. (1985) Interspecies: 10 (rat data). Sparse data set for agent GD. Intraspecies: 10—for susceptible human subpopulations, and sparse data set for agent GD. Some individuals possess abnormally low levels of blood cholinesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3. of technical support document). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for agent GB). |
||||
|
Animal to human dosimetric adjustment: None applied (insufficient data). |
||||
|
Time scaling: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) that agent GF is equipotent to agent GB for lethality (please see derivation for GB AEGL-3) and Cn×t=k where n=2 and k=0.021 mg/m3×h to extrapolate from 6-h value to 8-h estimate of LC01. |
|
Data adequacy: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—agent GD is equipotent to agent GB for lethality (please see derivation for GB AEGL-3). The relative potency assumptions for estimating AEGL-3 values for agent GD from the available database for agent GB need experimental confirmation. The scarcity of dose-response data for agent GD forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL-3 values for agent GD are derivative, with the exception of 10-min and 30-min values, which are confirmed by short-term experimental lethality data for Wistar rats as provided in a secondary study by Aas et al. (1985). |
||||
|
In the secondary study (Aas et al., 1985) of male Wistar rats (200–250 g), six animals tested at each of three exposure levels for periods of time <30 min by means of a dynamic inhalation chamber system; constant air concentration of GD at 21 mg/m3 for undefined exposure periods (each less than 30 min). Aas et al. (1985) reported lethality and enzyme activity changes. Time scaling includes an n=2 and k=0.012 mg/m3×h to derive 10-min and 30-min AEGL-3 estimates for agent GD. The Aas et al. (1985) data allow an estimate of the threshold for mortality in rats under this study protocol as equal to 335 mg·min/m3 for an exposure period of 16 min. This lethal threshold is equivalent to an exposure to a GD concentration of 21 mg/m3 for 16 min. Resulting AEGL-3 estimates (a 10-min AEGL-3 estimate of 0.27 mg/m3 and a 30-min AEGL-3 estimate of 0.15 mg/m3) are in good agreement with those derived by means of relative potency comparison with agent GB for the same time periods (see Appendix A for details of derivation). |
Derivation Summary for Agent GF (CAS No. 329–99–7) (O-cyclohexyl-methlyfluorophosphonate)
|
AEGL-1 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.00049 ppm (0.0035 mg/m3) |
0.00028 ppm (0.0020 mg/m3) |
0.00020 ppm (0.0014 mg/m3) |
0.00010 ppm (0.00070 mg/m3) |
0.000070 ppm (0.00050 mg/m3) |
|
Key reference: |
Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D., Crosier, R., Scotto, J., McCaskey, D., Crouse, C., and Matson, K. 2002b. Low-level sarin vapor exposure in rats: Effect of exposure concentration and duration on pupil size. ECBC-TR-235. Edgewood Chemical Biological Center, U.S. Army Soldier and Biological Chemical Command, Aberdeen Proving Ground, MD. (May 2002). |
|||
|
Secondary reference: |
(1) van Helden, H.P.M. Trap, H.C, Kuijpers, W.C., Groen, B., Oostdijk, J.P., Vanwersch, R.A.P., Philippens, I.H.C., Langenberg, J.P. and Benschop, H.P. 2002. Low Level Exposure to GB Vapor in Air: Diagnosis/Dosimetry, Lowest Observable Effect Level, and Performance-Incapacitation. Research and Technology Organisation (RTO) Meeting Proceedings 75, Operational Medical Issues in Chemical and Biological Defense [RTO-MP-075, AC/323 (HFM-060) TP/37] held in Estoril, Portugal, 14–17 May 2001. North Atlantic Treaty Organisation, Research and Technology Organisation, BP 25, 7 Rue Ancelle, F-92201 Neuilly-sur-Seine CEDEX, France. (2) Harvey, J.C. 1952. Clinical observations on volunteers exposed to concentrations of GB. Medical Laboratories Research Report No. 114, Publication Control No. 5030–114, MLCR 114 (CMLRE-ML-52). Army Chemical Center, MD. (3) Johns, R.J. 1952. The effect of low concentrations of GB on the human eye. Chemical Corps Medical Laboratories Research Report No. 100, Publication Control No. 5030–100 (CMLRE-ML-52), Army Chemical Center, MD. |
|||
|
Test species/strain/gender/number: Based on relative potency from Section 4.3 of this document and Mioduszewski et al. (1998)—agent GF is approxi |
||||
|
mately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1 derived from rat data). |
||||
|
Exposure route/concentrations/durations: Based on relative potency from Section 4.3 and Mioduszewski et al. (1998)—agent GF is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1) and derived from GB vapor inhalation exposures for 10, 60, and 240 min in female SD rats. |
||||
|
Effects: Derivation of AEGL-1 values based on relative potency from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GF is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1) and from GB vapor exposure study of EC50 for miosis observed in adult female SD rats exposed to a range of GB vapor concentrations (0.01–0.48 mg/m3) for 10 min (52 females), 60 min (35 females), and 240 min (55 females) (Mioduszewski et al. 2002b). |
||||
|
End point/concentration/rationale: Derivation of AEGL-1 values based on relative potency from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GF is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1) and EC50 for miosis determination (a reversible, local, nondisabling and transient effect) in the Mioduszewski et al. (2002b) study of young female SD rats exposed to agent GB. EC50 concentrations for miosis in female SD rats (the susceptible gender) are used as points of departure for AEGL-1 estimation. The miosis effects data of van Helden et al. (2001, 2002) (nonhuman primates), Harvey (1952) (human volunteers), and Johns (1952) (human volunteers) are supportive. The EC50 for miosis (postexposure pupil diameter 50% or less of the preexposure pupil diameter in 50% of exposed population) (Mioduszewski et al. 2002b) is not considered an adverse effect for humans. |
||||
|
Uncertainty factors/rationale: Based on relative potency from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GF is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1). Total uncertainty factor: 10 Interspecies: 1—miosis response to GB vapor exposure is similar across multiple mammalian species. Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for agent GB). |
|
Animal to human dosimetric adjustment: None applied (human data). |
||||
|
Time scaling: Based on relative potency from Section 4.3 and Mioduszewski et al. (1998)—agent GF is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1) and Cn×t=k where n=2 and k=7.7×10−6 mg/m3×h for 10- to 30-min extrapolation, and k=5.8×10−6 mg/m3×h for 4-h to 8-h extrapolation for agent GB. |
||||
|
Data adequacy: Based on relative potency determination from Section 4.3 and Mioduszewski et al. (1998)—agent GF is approximately twice as potent as agents GB and GA for AEGL-1 effects (please see derivation for GB AEGL-1). The scarcity of dose-response data for agent GF forces the AEGL-1 analysis to rely on assumptions of relative potency. Thus, AEGL-1 values for agent GF are derivative. The relative potency assumptions for estimating AEGL-1 values for agent GF from the available database for agent GB need experimental confirmation. |
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.0062 ppm (0.044 mg/m3) |
0.0035 ppm (0.025 mg/m3) |
0.0024 ppm (0.018 mg/m3) |
0.0013 ppm (0.0085 mg/m3) |
0.00091 ppm (0.0065 mg/m3) |
|
Key reference: Baker, D.J., Sedgwick , E.M. 1996. Single fibre electromyographic changes in man after organophosphate exposure. Hum. Exp. Toxicol. 15:369–375. |
||||
|
Test species/strain/gender/number: Based on relative potency estimate from Section 4.3 of this document and Mioduszewski et al. (1998)—agent GF is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2 derived from human volunteer data). |
||||
|
Exposure route/concentrations/durations: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—agent GF is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2) and derived from GB vapor inhalation to humans in exposure chamber; GB at 0.5 mg/m3 for 30 min while walking at a rate of 96 paces per minute and breathing normally (Baker and Sedgwick 1996). |
||||
|
Effects: Derivation of AEGL-2 values based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GF is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2) and from GB vapor exposure study (Baker and Sedgwick 1996) in which observed effects included miosis in eight of eight subjects, dyspnea and photophobia in some individuals, inhibition of RBC-ChE to approximately 60% (range of 54–66.1%) of individual baseline at 3 h and 3 d postexposure in eight of eight subjects, and small but measurable changes in single fibre electromyography (SFEMG) of the forearm that were detectable in the lab between 4 and 15 mo postexposure (but not detectable at 15–30 mo postexposure) in five of eight human volunteers exposed to GB at 0.5 mg/m3 for 30 min. SFEMG changes considered subclinical. No permanent effects. |
||||
|
End point/concentration/rationale: Derivation of AEGL-2 values based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GF is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2). Presence of miosis in all subjects exposed to GB vapor (Baker and Sedgwick 1996), in addition to other observed signs in portions of exposed population, indicates a greater level of effect than the EC50 considered for AEGL-1 determination; thus there exists a heightened potential for reduced visual acuity following a 30-min exposure to GB at 0.5 mg/m3. SFEMG effects are documented as long-lasting, but are subclinical and fully reversible. Such effects are not |
||||
|
usually included as a basis for AEGL-2 estimation. However, due to the known steep dose response for nerve agent vapor exposure, incorporation of the long-lasting SFEMG end point is here considered a protective interpretation of the AEGL-2 definition. The point of departure for AEGL- 2 estimation is 0.5 mg/m3 for 30 min. |
||||
|
Uncertainty Factors/Rationale: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998) study showing that agent GF is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2). Total uncertainty factor: 10 Interspecies: 1 (human data). Intraspecies: 10—for susceptible human subpopulations. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for agent GB). |
||||
|
Animal to human dosimetric adjustment: None applied (human data). |
||||
|
Time scaling: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—agent GF is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2) and Cn×t=k where n=2 and k=0.0013 mg/m3×h for agent GB. |
||||
|
Data adequacy: Based on relative potency estimate from Section 4.3 and Mioduszewski et al. (1998)—agent GF is approximately twice as potent as agents GB and GA for AEGL-2 effects (please see derivation for GB AEGL-2). The scarcity of dose-response data for agent GF forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL values for agent GF are derivative. The relative potency assumptions for estimating AEGL-2 values for agent GF from the available database for agent GB need experimental confirmation. |
|
AEGL-3 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.053 ppm (0.38 mg/m3) |
0.027 ppm (0.19 mg/m3) |
0.018 ppm (0.13 mg/m3) |
0.0098 ppm (0.070 mg/m3) |
0.0071 ppm (0.051 mg/m3) |
|
Key Reference: |
(1) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D. and Crosier, R. 2000. Estimating the probability of sarin vapor toxicity in rats as a function of exposure concentration and duration. Proceedings of the International Chemical Weapons Demilitarization Conference (CWD-2000), The Hague, NL. May 21–24, 2000. (2) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B., Muse, W., Anthony, J., Durst, D., Sommerville, D., Crosier, R., Thomson, S, and Crouse, C. 2001. ECBC Low Level Operational Toxicology Program: Phase I—Inhalation toxicity of sarin vapor in rats as a function of exposure concentration and duration. ECBC-TR-183, Edgewood Research Development and Engineering Center, Aberdeen Proving Ground, MD. (August 2001). (3) Mioduszewski, R.J., Manthei, J., Way, R., Burnett, D., Gaviola, B.Muse, W., Thomson, S., Sommerville, D., and Crosier, R. 2002a. Interaction of exposure concentration and duration in determining acute toxic effects of sarin vapor in rats. Toxicol. Sci. 66:176–184. |
|||
|
Secondary reference: |
Anthony, J.S., Haley, M.V., Manthei, J.H., Way, R.A., Burnett, D.C., Gaviola, B.P., Sommerville, D.R., Crosier, R.B., Mioduszewski, R.J., Jakubouwski, E.M., Montgomery, J.L., Thomson, S.A. 2002. Inhalation toxicity of GF vapor in rats as a function of exposure concentration and duration and its potency comparison to GB. Late-breaking Poster presented at 41st Annual meeting of the Society of Toxicology, Nashville, TN (21 Mar 2002). |
|||
|
Test species/strain/gender/number: Based on relative potency estimate from Section 4.3 of this document and Mioduszewski et al. (1998)—agent GF is equipotent to agent GB for lethality (please see derivation for GB AEGL-3 derived from female Sprague-Dawley rat data). This assumption is supported by the recent study of Anthony et al. (2002). |
||||
|
Exposure route/concentrations/durations: Based on relative potency estimate from Section 4.3, Mioduszewski et al. (1998), and Anthony et al. (2002)— |
||||
|
agent GF is equipotent to agent GB for lethality (please see derivation for GB AEGL-3) and study of rat inhalation toxicity in dynamic mode exposure chamber (Mioduszewski et al. 2000, 2001, 2002a). |
||||
|
Effects: Derivation of AEGL-3 values based on relative potency estimate from Section 4.3 and the Mioduszewski et al. (1998) and Anthony et al (2002) studies showing that agent GF is equipotent to agent GB for lethality (please see derivation for GB AEGL-3) and from GB vapor exposure study (Mioduszewski, et al. 2000, 2001, 2002a) in which lethality and sublethal clinical signs monitored during and after exposure. Only lethality data reported at this time. |
||||
|
End point/concentration/rationale: Derivation of AEGL-3 values based on relative potency estimate from Section 4.3 and the Mioduszewski et al. (1998) and Anthony et al. (2002) studies showing that agent GF is equipotent to agent GB for lethality (please see derivation for GB AEGL-3) and from GB vapor exposure study (Mioduszewski et al. 2000, 2001, 2002a) which derived 14-d lethality estimates for female Sprague-Dawley rats (female rats were reported to be overall more sensitive to GB vapor toxicity than male rats over the range of exposure concentrations and durations studied). Gender differences in sensitivity are reported to be statistically significant at p<0.01 (Mioduszewski et al. 2000, 2001, 2002a). End point concentrations for LC01 reported for female SD rats (the susceptible gender) are used as points of departure from which to derive AEGL-3 estimates. Probit analysis (MINITAB, version 13) presented in the Mioduszewski et al. (2000) study provided 14-d LC50 and LC01 values for female rats. |
||||
|
Uncertainty factors/rationale: Based on relative potency estimate from Section 4.3 and the Mioduszewski et al. (1998), and Anthony et al. (2002) studies showing that agent GF is equipotent to agent GB for lethality (please see derivation for GB AEGL-3). Total uncertainty factor: 30 Interspecies: 3 (female rat data). The full default value of 10 was not considered necessary because the mechanism of toxicity in both rats and humans is the same, cholinesterase inhibition. Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (see Section 4.5.3.). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: None (see derivation for agent GB). |
||||
|
Time scaling: Based on relative potency estimate from Section 4.3 of this document, Mioduszewski et al. (1998), and Anthony et al. (2002)—agent GF |
|
is equipotent to agent GB for lethality (please see derivation for GB AEGL-3) and Cn×t=k where n=2 and k=0.021 mg/m3×h to extrapolate from 6-h value to 8-h estimate of LC01. |
||||
|
Data adequacy: Based on relative potency estimate from Section 4.3, Mioduszewski et al. (1998), and Anthony et al. (2002)—agent GF is equipotent to agent GB for lethality (please see derivation for GB AEGL-3). The scarcity of dose-response data for agent GF forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL-3 values for agent GF are derivative. The relative potency assumptions for estimating AEGL-3 values for agent GF from the available database for agent GB need experimental confirmation. |
Derivation Summary for Agent VX Vapor (CAS No. 50782–69–9) (O-Ethyl-S-(Isopropyl-Aminoethyl)Methyl Phosphonothiolate)
|
AEGL-1 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.000052 ppm (0.00057 mg/m3) |
0.000030 ppm (0.00033 mg/m3) |
0.000016 ppm (0.00017 mg/m3) |
0.0000091 ppm (0.00010 mg/m3) |
0.0000065 ppm (0.000071 mg/m3) |
|
Key reference: |
Mioduszewski et al. (2002b). Low-level sarin vapor exposure in rats: Effect of exposure concentration and duration on pupil size (ECBC-TR-235). Edgewood Chemical Biological Center, U.S. Army Soldier and Biological Chemical Command, Aberdeen Proving Ground, MD. |
|||
|
Secondary references: |
(1) Grob, D., and Harvey, J.C. 1958. Effects in man of the anticholinesterase compound Sarin (isopropyl methyl phosphonofluoridate). J. Clin. Invest. 37:350–368 (2) Sidell, F.R., and Groff, W.A. 1974. The reactivatibility of cholinesterase inhibited by VX and sarin in man. Toxicol. Appl. Pharmacol. 27:241–252. |
|||
|
Test species/strain/gender/number: Based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974)—potency of agent VX is approximately 4 times that of agent GB for AEGL-1 effects (please see derivation for GB AEGL-1, derived from female Sprague-Dawley rat data for EC50 miosis). |
||||
|
Exposure route/concentrations/durations: Based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974)—potency of agent VX is approximately 4 times that of agent GB for AEGL-1 effects (please see derivation for GB AEGL-1) and derived from GB vapor exposures to female Sprague-Dawley rats for EC50 miosis at 10, 60, and 240 min |
||||
|
Effects: Derivation of AEGL-1 values based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974) studies showing that potency of agent VX is approximately 4 times that of agent GB for AEGL-1 effects (please see derivation for GB AEGL-1) and VX estimates derived from data in GB vapor exposure study (Miodiszewski et al. 2002b) in which EC50 for miosis (a postexposure pupil diameter 50% or less of the preexposure pupil diameter in 50% of the exposed rat population) in female SD rats was determined for 10, 60, and 240 min. Estimated concentrations of VX |
||||
|
expected to produce similar effects are 0.017 mg/m3 for 10 min, 0.005 mg/m3 for 60 min, and 0.003 mg/m3 for 240 min. |
||||
|
End point/concentration/rationale: Derivation of AEGL-1 values based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974) studies showing that potency of agent VX is approximately 4 times that of agent GB (please see derivation for GB AEGL-1) and determination that the EC50 for miosis is based on a well-defined animal end point in a susceptible gender and is reversible, local, nondisabling, and transient (Mioduszewski et al. [2002b] study of young female SD rats exposed to GB). Estimated concentrations of VX expected to produce similar effects are 0.017 mg/m3 for 10 min, 0.005 mg/m3 for 60 min, and 0.003 mg/m3 for 240 min; selection of these estimated EC50 concentrations for female SD rats (the susceptible gender) as the points of departure for agent VX AEGL-1 estimation is thus protective. The miosis effects data of van Helden et al. (2001, 2002) (nonhuman primates), Harvey (1952) (human volunteers), and Johns (1952) (human volunteers) are supportive. The EC50 for miosis (a postexposure pupil diameter 50% or less of the preexposure pupil diameter in 50% of the exposed rat population) (Mioduszewski et al. 2002b) is not considered an adverse effect for humans. The AEGL values are estimates for VX vapor exposures only. |
||||
|
Uncertainty factors/rationale: Based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974) studies showing that potency of agent VX is approximately 4 times that of agent GB (please see derivation for GB AEGL-1). Total uncertainty factor: 30 Interspecies: 1—miosis response to nerve agent vapor exposure is similar across mammalian species (as for agent GB AEGL-1 estima tion). Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (Morgan 1989; Wills 1972; Opresko et al. 1998). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: 3 (for sparse VX database). |
||||
|
Animal to human dosimetric adjustment: None applied (human data). |
||||
|
Time scaling: Based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974)—potency of agent VX is approximately 4 times that of agent GB (please see derivation for GB AEGL-1) and Cn×t=k where n= 2, and k=5.0×10−8 mg/m3×h for 10 to 30 min extrapolation, and k=4.0× 10−8 mg/m3×h for 4-h to 8-h extrapolation. |
|
Data adequacy: Based on relative potency—potency of agent VX is approximately 4 times that of agent GB (please see derivation for GB AEGL-1). The scarcity of dose-response data for agent VX forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL values for agent VX are derivative. The relative potency assumptions for estimating AEGL-1 values for agent VX from the available database for agent GB need experimental confirmation. |
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.00065 ppm (0.0072 mg/m3) |
0.00038 ppm (0.0042 mg/m3) |
0.00027 ppm (0.0029 mg/m3) |
0.00014 ppm (0.0015 mg/m3) |
0.000095 ppm (0.0010 mg/m3) |
|
Key reference: |
Baker, D.J. and Sedgwick, E.M., 1996. Single-fibre electromyographic changes in man after organophosphate exposure. Hum. Exp. Toxicol. 15:369–375. |
|||
|
Secondary references: |
Grob, D., and Harvey, J.C. 1958. Effects in man of the anticholinesterase compound Sarin (isopropyl methyl phosphonofluoridate). J. Clin. Invest. 37:350–368; Sidell, F.R., and Groff, W.A. 1974. The reactivatibility of cholinesterase inhibited by VX and sarin in man. Toxicol. Appl. Pharmacol. 27:241–252. |
|||
|
Test species/strain/gender/number: Based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974)—potency of agent VX is approximately 4 times that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2 derived from human volunteer data). |
||||
|
Exposure route/concentrations/durations: Based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974)—potency of agent VX is approximately 4 times that of agent GB (please see derivation for GB AEGL-2) and derived from GB vapor inhalation to humans in exposure chamber; 0.5 mg/m3 for 30 min while walking at a rate of 96 paces per minute and breathing normally (Baker and Sedgwick 1996). |
||||
|
Effects: Derivation of AEGL-2 values based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974) studies showing that potency of agent VX is approximately 4 times that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2). VX estimates derived from GB vapor exposure study (Baker and Sedgwick 1996) in which observed effects included miosis in eight of eight subjects, dyspnea and photophobia in some individuals, inhibition of RBC-ChE to approximately 60% of individual baseline at 3 h and 3 d postexposure in eight of eight subjects, and small but measurable changes in single fibre electromyography (SFEMG) of the forearm that were detectable in the lab between 4 and 15 mo postexposure (but not detectable at 15–30 mo postexposure) in five of eight human volunteers exposed to GB at 0.5 mg/m3 for 30 min. SFEMG changes considered subclinical. No permanent effects. |
||||
|
End point/concentration/rationale: Derivation of AEGL-2 values based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974) |
||||
|
studies showing that potency of agent VX is approximately 4 times that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2) and determination that a 30-min exposure to GB at 0.5 mg/m3 results in a greater level of effect than the EC50 considered for AEGL-1 determination (see Baker and Sedgwick [1996]); thus, there exists a heightened potential for reduced visual acuity following experimental exposures. Estimated concentration of VX expected to produce similar effects is 0.125 mg/m3 for 30 min; this concentration is the point of departure for agent VX AEGL- 2 estimations. Inclusion of the long-lasting but subclinical and fully reversible SFEMG effect is here considered a protective interpretation of the AEGL-2 definition, given the known steep dose response for nerve agent vapor exposure. The AEGL values are estimates for VX vapor exposures only. |
||||
|
Uncertainty Factors/Rationale: Based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974) studies showing that potency of agent VX is approximately 4 times that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2). Total uncertainty factor: 30 Interspecies: 1 (human data). Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (Morgan 1989; Wills 1972; Opresko et al., 1998). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: 3 (for sparse VX database). |
||||
|
Animal to human dosimetric adjustment: None applied (human data) |
||||
|
Time scaling: Based on relative potency from Grob and Harvey (1958) and Sidell and Groff (1974)—potency of agent VX is approximately 4 times that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2) and Cn×t=k where n=2 and k=8.6×10−6 mg/m3×h. |
||||
|
Data adequacy: Based on relative potency estimate from Grob and Harvey (1958) and Sidell and Groff (1974)—potency of agent VX is approximately 4 times that of agent GB for AEGL-2 effects (please see derivation for GB AEGL-2). The scarcity of dose-response data for agent VX forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL values for agent VX are derivative. The relative potency assumptions for estimating AEGL-2 values for agent VX from the available database for agent GB need experimental confirmation. |
|
AEGL-3 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.0027 ppm (0.029 mg/m3) |
0.0014 ppm (0.015 mg/m3) |
0.00091 ppm (0.010 mg/m3) |
0.00048 ppm (0.0052 mg/m3) |
0.00035 ppm (0.0038 mg/m3) |
|
Key reference: |
Mioduszewski et al. 2002a. Interaction of exposure concentration and duration in determining acute toxic effects of sarin vapor in rats. Toxicol. Sci. 66:176–184. |
|||
|
Secondary references: |
(1) Grob, D., and Harvey, J.C. 1958. Effects in man of the anticholinesterase compound Sarin (isopropyl methyl phosphonofluoridate). J. Clin. Invest. 37:350–368. (2) Sidell, F.R., and Groff, W.A. 1974. The reactivatibility of cholinesterase inhibited by VX and sarin in man. Toxicol. Appl. Pharmacol. 27:241–252. |
|||
|
Test species/strain/gender/number: Based on assumptions previously stated from Grob and Harvey (1958) and Sidell and Groff (1974)—potency of agent VX is approximately 4 times that of agent GB (please see derivation for GB AEGL-3 derived from female Sprague-Dawley rat data) and RBC-ChE50 is part of an anticholinesterase response continuum. |
||||
|
Exposure route/concentrations/durations: Based on assumption previously stated from Grob and Harvey (1958) and Sidell and Groff (1974)—that the potency of agent VX is approximately 4 times that of agent GB (please see derivation for GB AEGL-3) and the study of rat inhalation toxicity in dynamic mode exposure chamber (Mioduszewski et al. 2000, 2001, 2002a). |
||||
|
Effects: Derivation of AEGL-3 values based on assumption previously stated from Grob and Harvey (1958) and Sidell and Groff (1974) studies showing that potency of agent VX is approximately 4 times that of agent GB (please see derivation for GB AEGL-3) and from GB vapor exposure study (Mioduszewski et al. 2000, 2001, 2002a) in which lethality and sublethal clinical signs were monitored during and after exposure. Only lethality data reported at this time. |
||||
|
End point/concentration/rationale: Derivation of AEGL-3 values based on assumption previously stated from Grob and Harvey (1958) and Sidell and Groff (1974) studies showing that potency of agent VX is approximately 4 times that of agent GB (please see derivation for GB AEGL-3) and from GB vapor exposure study (Mioduszewski et al. 2000, 2001, 2002a) of female SD rats exposed to GB vapor. Gender differences for lethality are significant. The LC01 values for GB exposure were multiplied by a factor of 0.25 to estimate the LC01 value for agent VX. These estimated concentrations for VX |
||||
|
LC01 in female SD rats (the susceptible gender) are the points of departure for agent VX AEGL-3 estimations, which are thus protective. The AEGL values are estimates for VX vapor exposures only. |
||||
|
Uncertainty factors/rationale: Based on assumption previously stated from Grob and Harvey (1958) and Sidell and Groff (1974) studies showing that potency of agent VX is approximately 4 times that of agent GB (please see derivation for GB AEGL-3). Total uncertainty factor: 100 Interspecies: 3—female rat data. The full default value of 10 is not considered appropriate because the mechanism of toxicity in lab rodents and humans is ChE inhibition. Intraspecies: 10—for possible susceptible human individuals. Some individuals possess abnormally low levels of blood cholinesterase and carboxylesterase activity that may make them especially susceptible to the effects of cholinesterase inhibitors such as nerve agents (Morgan 1989; Wills 1972; Opresko et al. 1998). Therefore, a factor of 10 was retained. |
||||
|
Modifying factor: 3 (for sparse VX database). |
||||
|
Animal to human dosimetric adjustment: None applied (insufficient data) |
||||
|
Time scaling: Cn×t=k where n=2 and k=1.16×10−4 mg/m3×h, based on the assumption that the scaling function for agent VX is similar to that derived for agent GB from the experimental rat data of Mioduszewski et al. (2000, 2001, 2002a,b). Extrapolation from 6-h experimental value to 8-h AEGL-3. |
||||
|
Data adequacy: The scarcity of dose-response data for agent VX forces the AEGL analysis to rely on assumptions of relative potency. Thus, AEGL values for agent VX are derivative. The relative potency assumptions for estimating AEGL-3 values for agent VX from the available database for agent GB need experimental confirmation. |