SUMMARY
Methyl isocyanate (MIC) is one of the most reactive of all isocyanates and is rapidly degraded in aqueous medium (Varma and Guest 1993). Because of its reactivity, MIC is used as an intermediate in the synthesis of N-methylcarbamate and N-methylurea insecticides and herbicides (Hartung 1994). During the night of December 2–3, 1984, an estimated 30 tons of
MIC were released from a chemical plant in Bhopal, India, resulting in one of the worst industrial accidents in history (Karlsson et al. 1985).
Signs of severe irritation to the respiratory tract were reported for victims of the Bhopal disaster, and autopsies revealed the cause of death to be acute pulmonary edema (Weill 1988). Long-term pulmonary and ocular sequelae have been documented in survivors. The spontaneous abortion rate (Arbuckle and Sever 1998) and the infant death rate (Varma 1987) among women who were pregnant at the time of the release were significantly increased in the months following the disaster. Numerous animal studies corroborate the epidemiological findings in humans. A compilation of case reports in industrial workers consistently noted skin and respiratory irritation in MIC-exposed workers but no definitive case of sensitization (Ketcham 1973). The mechanism of action for the pulmonary, skin, and ocular toxicity is irritation, but the mechanism of action for the systemic effects is unknown.
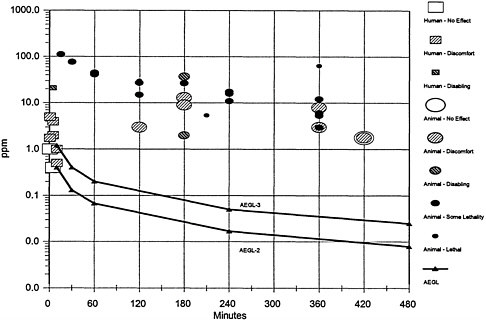
AEGL-1 values were not derived. Although human and animal data were available for irritation levels, the irritation threshold for MIC may be above the level of concern for systemic effects. Experimental studies in humans indicate that both duration of exposure and concentration of MIC contribute to the severity of irritation. However, extrapolation from the short experimental durations to the longer AEGL time points may not be predictive of adverse health effects. It is not known at what concentration the risk for systemic effects, other than pulmonary edema, becomes a concern. The concentrations causing irritation in humans after several minutes (1–4 parts per million [ppm]) are similar to, or higher than, the concentrations resulting in embryo and fetal lethality in well conducted animal studies. Therefore, the results of controlled human exposures were not used in derivation of AEGL-1. However, it should be noted that exposures to MIC at concentrations below those used to calculate AEGL-1 might be associated with systemic toxicity.
Systemic toxicity data from rats and mice were used for derivation of AEGL-2. An increase in cardiac arrhythmias occurred in rats 4 months (mo) after a 2-hour (h) exposure to 3 ppm (Tepper et al. 1987). Pregnant Swiss-Webster mice were exposed to analytically monitored concentrations of MIC at 0, 2, 6, 9, and 15 ppm for 3 h on gestation day 8 (Varma 1987). Placental weights and fetal body weights were significantly reduced at all concentrations. Exposures to concentrations at 9 ppm and 15 ppm resulted in deaths of two dams in each group, a significant increase in complete litter resorption among surviving dams, and fetuses with significant reduc-
tions in the lengths of the mandible and long bones. The single exposure concentration of 2 ppm for 3 h was an experimentally derived LOAEL for reduced fetal body weights in the absence of maternal toxicity. Values scaled for the derivation of the 10- and 30-minute (min), and 1-, 4-, and 8-h time points were calculated from the equation Cn×t=k where n=1. The value of n was empirically derived from regression analysis of lethality data for rats. Identical AEGL-2 values are derived based on the exposures of 3 ppm for 2 h and 2 ppm for 3 h. The experimental concentrations were reduced by a factor of 3 to estimate a threshold for effects on cardiac arrhythmias or fetal body weights. A total uncertainty factor (UF) of 30 was applied, including 3 for interspecies variation because similar developmental toxicity results have been obtained in both rats and mice and 10 for intraspecies variation because the mechanism of action for developmental toxicity is unknown.
The neonatal survival study with mice conducted by Schwetz et al. (1987) was used for derivation of AEGL-3 values. Pregnant mice were exposed to MIC at 0, 1, or 3 ppm for 6 h/day (d) on gestation days 14–17. Dams were allowed to litter for evaluation of neonatal survival. No maternal toxicity was observed at either exposure concentration. A concentration-related increase in the number of dead fetuses at birth was observed in both MIC exposure groups, and an increase in neonatal mortality during lactation was observed in the 3-ppm group. No differences in neonatal body-weight gain occurred during lactation between the treated and control groups. The 6-h exposure to 1 ppm was used to derive AEGL-3 values and is considered a NOEL for pup survival during lactation. Values scaled for the derivation of the 10- and 30-min, and 1-, 4-, and 8-h time points were calculated from the equation Cn×t=k where n=1. The value of n was empirically derived from regression analysis of lethality data for rats. A total UF of 30 was applied, including 3 for interspecies variation because similar developmental toxicity results have been obtained in both rats and mice and 10 for intraspecies variation because the mechanism of action for developmental toxicity is unknown. According to Section 2.7 of the standing operating procedures (NRC 2001), 10-min values are not to be scaled from an experimental exposure time of ≥4 h. However, because n was derived from exposures ranging from 7.5 min to 4 h, extrapolation from 6 h to the 10-min AEGL-3 value is valid in this instance.
The proposed values for the three AEGL classifications for the five time periods are listed in the table below.
TABLE 3–1 Summary of AEGL Values for Methyl Isocyanate
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1a (Non-disabling) |
NR |
NR |
NR |
NR |
NR |
|
|
AEGL-2 (Disabling) |
0.40 ppm (0.94 mg/m3) |
0.13 ppm (0.32 mg/m3) |
0.067 ppm (0.16 mg/m3) |
0.017 ppm (0.034 mg/m3) |
0.008 ppm (0.02 mg/m3) |
Decreased fetal body weights (Varma 1987); cardiac arrhythmias (Tepper et al. 1987) |
|
AEGL-3 (Lethal) |
1.2 ppm (2.8 mg/m3) |
0.40 ppm (0.95 mg/m3) |
0.20 ppm (0.47 mg/m3) |
0.05 ppm (0.12 mg/m3) |
0.025 ppm (0.06 mg/m3) |
Decreased pup survival during lactation (Schwetz et al. 1987) |
|
aExposure to MIC at concentrations below those used to calculate AEGL-1 may be associated with systemic toxicity. Abbreviations: NR, not recommended. |
||||||
1. INTRODUCTION
Methyl isocyanate (MIC) is one of the most reactive of all isocyanates and is rapidly degraded in aqueous medium (Varma and Guest 1993). Because of its reactivity, MIC is used as an intermediate in the synthesis of N-methylcarbamate and N-methylurea insecticides and herbicides (Hartung 1994).
During the night of December 2–3, 1984, a release occurred in a chemical plant in Bhopal, India, where MIC was used as an intermediate in the production of carbamates. An estimated 30 tons of MIC were released resulting in one of the worst industrial accidents in history (Karlsson et al. 1985). Signs of severe respiratory tract irritation were reported for victims of the Bhopal disaster and autopsies revealed the cause of death to be pulmonary edema (Varma and Guest 1993). Long-term pulmonary and ocular disease have been documented in survivors (Andersson et al. 1990).
TABLE 3–2 Chemical and Physical Data for Methyl Isocyanate
|
Parameter |
Value |
Reference |
|
Synonyms |
Isocyanatomethane; isocyanic acid methyl ester; MIC |
Budavari et al. 1996 |
|
Chemical formula |
C2H3NO |
Budavari et al. 1996 |
|
Molecular weight |
57.05 |
Budavari et al. 1996 |
|
CAS Registry Number |
624–83–9 |
|
|
Physical description |
Liquid |
Budavari et al. 1996 |
|
Vapor pressure |
400 torr at 20.6 °C |
Budavari et al. 1996 |
|
Vapor density (air=1) |
2.0 |
Hartung 1994 |
|
Melting/boiling point |
−45 °C/39.1 °C |
EPA 1986 |
|
Solubility in water |
0.067 g/mL |
Hartung 1994 |
|
Conversion factors in air |
1 ppm=2.34 mg/m3 1 mg/m3=0.43 ppm |
NIOSH 1997 |
|
Reactivity |
Exothermic reaction with water can lead to explosion |
EPA 1986 |
Numerous animal studies corroborate the epidemiological findings in humans. Unlike other isocyanates, MIC is not a sensitizer.
Selected physicochemical properties of MIC are listed in Table 3–2.
2. HUMAN TOXICITY DATA
2.1. Bhopal Disaster
On the night of December 2/3, 1984, an estimated 30 tons of MIC gas were released over Bhopal, India from a carbamate factory when water entered the storage tank. The reaction of water and MIC is exothermic and resulted in increased pressure and temperature until the tank’s safety valve ruptured. Total duration of the release was approximately 1 h. Atmospheric conditions maintained the MIC cloud close to the ground, and light winds moved it towards a heavily populated area. Dispersion calculations by Karlsson et al. (1985) estimate concentrations of MIC to have ranged from
3,000 ppm at 270 m downwind to 10 ppm at 5,500 m downwind. However, the concentrations to which the population was actually exposed are unknown.
The official death toll was 2,250 individuals, with another 50,000 incapacitated and about 100,000 treated in area hospitals. In addition, about 1,000 livestock were killed. The area with the heaviest casualties was 6–7 km2 south of the factory and severe injuries occurred in a region of about 25 km2 (Karlsson et al. 1985). In general, deaths were not instantaneous but occurred in phases over the next few days following the release. Only a few deaths were recorded within the first few hours; a second phase occurred between 8 and 12 h, and the greatest number of deaths occurred between 24 and 72 h after the MIC release (Varma 1989; Varma and Guest 1993).
Numerous accounts have been published detailing the effects of MIC on the population. The most frequently reported symptoms were burning and/or watering of the eyes, coughing, respiratory distress, pulmonary congestion, nausea, vomiting, muscle weakness, and CNS involvement secondary to hypoxia (Kamat et al. 1985; Misra et al. 1987; Lorin and Kulling 1986; Andersson et al. 1988; Weill 1988; Kamat et al. 1992). The frequency of reports of cough as an initial symptom most closely followed the distribution of deaths in the exposed populations (Andersson et al. 1988), deaths resulting from pulmonary edema (Weill 1988) or cardiac arrest following pulmonary edema (Varma and Guest 1993). Between areas in which deaths occurred and areas where only symptoms were reported, distance to the factory was a contributing factor, but the duration of exposure (up to 4 h) did not appear to vary (Andersson et al. 1988). Although most survivors improved within 2 weeks (wk), many had restrictive respiratory function with radiographic changes suggestive of interstitial deposits (Kamat et al. 1985).
Long-term health effects from the accident have been reported for populations followed for up to 3 years (y). One hundred and five days after the accident, a survey of children residing within 2 km of the factory at the time of the accident showed that 83.5% had persistent cough, 47.5% had breathlessness, 48.1% had rhonchi, and 43.2% had wheezing compared with abnormal respiratory findings in 8.5% of children living 8–10 km away; abdominal pain and anorexia were also increased by 8.0–9.8% in children living closer to the factory (Irani and Mahashur 1986). Another survey, which included adults and children, documented persistent respiratory, ophthalmological, neuromuscular, and gastrointestinal symptoms 15
wk after the accident (Naik et al. 1986). In more heavily exposed groups (defined by distance to the factory or number of symptoms), cognitive functions were impaired and consolidations were observed on chest radiographs after 1 y (Misra and Kalita 1997), with breathlessness, chest pain, and nausea/vomiting more frequent after 3 y (Andersson et al. 1990). Small airway obstruction, as measured by reductions in pulmonary function tests and/or abnormal chest radiographs, were found in victims at 1 y (Misra and Kalita 1997), 2 y (Kamat et al. 1992), and 10 y (Cullinan et al. 1997) after the accident. A study carried out 1–7 y after the accident found reductions in pulmonary function correlated with increases in inflammatory cells measured in bronchoalveolar lavage fluid and with radiographic abnormalities (Vijayan and Sankaran 1996).
Immediate and long-term ocular toxicities were also a major consequence of the MIC release in Bhopal. However, no cases of blindness were attributed to exposure (Andersson et al. 1984, 1985, 1988). Immediately after the accident, tearing, photophobia, profuse lid edema, and superficial corneal ulceration were reported (Andersson et al.,1984; Dwivedi et al. 1985). Superficial interpalpebral erosion of the cornea and conjunctiva observed initially (Andersson et al. 1988) was followed about 2 mo later by the typical whorling pattern of new growth and healing of the corneal epithelium (Andersson et al. 1984, 1985). Results showed that the incidence of cataract, conjunctivitis, corneal opacity, and hyperemia of the conjunctivae remained increased 3 mo after the accident in individuals residing within 2 km of the factory. In this survey, males were more affected than females, which the author attributed to the fact that the males ran in an attempt to find safety, and thus, increased their exposure, leaving the females behind in whatever shelter was available (Maskati 1986). From a follow-up study 3 y after the accident, Andersson et al. (1990) concluded that “Bhopal eye syndrome” may include full resolution of the initial interpalpebral superficial erosion, a subsequent increased risk of ocular infections, hyperresponsive phenomena such as irritation, watering, and phlyctens, and possibly cataracts. Eye irritation, reduction in vision, and persistent corneal opacity were also reported after 6–9 mo (Raizada and Dwivedi 1987) and after 2 y (Khurrum and Ahmad 1987).
Of interest here are reports comparing the effects of MIC with effects following exposure to related chemicals. In general, isocyanate exposures produce immunologic sensitization. However, evaluation of sera from Bhopal patients revealed low antibody titers that were transient (Karol et al. 1987). In a comparison to hydrogen cyanide, the absence of cyano-
methemoglobin was noted (Misra et al. 1987), as was the fact that MIC pulmonary lesions were not characteristic of those seen after cyanide intoxication (Varma 1989; Weill 1988).
Based on model predictions, the actual consequences in Bhopal, and limited animal and human toxicity data, Karlsson et al. (1985) estimated the effects of MIC in humans from “short exposure” durations (Table 3–3). Although the authors did not define “short” duration, their models were based on 1 h, which was the approximate duration of the release. It is interesting to note that the Karlsson et al. (1985) results, especially for irritation of mucus membranes, are in close agreement with the results of controlled human inhalation studies described below.
2.2. Nonlethal Toxicity
2.2.1. Case Reports
Case reports have been submitted to EPA under TSCA sections 8D and 8E. One letter included a compilation of case reports in which industrial workers consistently noted skin and respiratory irritation from MIC exposure. There were no definitive cases of dermal or respiratory sensitization. All MIC-exposed workers apparently recovered completely when removed from the source of exposure. No exposure concentrations or durations were given (Union Carbide 1973). Another letter (Union Carbide 1966) described possible sensitization in a worker analyzing MIC by infrared spectroscopy. This individual had severe swelling and redness of the face after handling the chemical, but was not aware of exposure at the time (i.e., no odor or irritation was reported). Details of concurrent exposures and/or confounding factors were not included.
2.2.2. Experimental Studies
The odor threshold for MIC in air is 2.1 ppm (EPA 1986; AIHA 1989).
Four subjects were each exposed for 1–5 min to MIC at 0.4, 2, 4, or 21 ppm (Kimmerle and Eben 1964). At 0.4 ppm, no odor was detected and no irritation was reported by any of the volunteers. Minor but distinct irritation of mucous membranes (particularly lacrimation) was noted without odor at 2 ppm. Ocular irritation became more pronounced at 4 ppm. Exposure to 21 ppm was intolerable for even a moment.
TABLE 3–3 Symptoms of MIC at Short Exposure Timesa
Eight volunteers were exposed to MIC at an analyzed concentration of 1.75 ppm for 1 min (Mellon Institute 1970). All individuals reported eye irritation, seven had tearing, and three reported nose and throat irritation. Complaints of these effects ceased within 10 min, except that one woman reported a sensation of “something in her eye” for 45 min. Six of the same individuals were subsequently exposed to MIC at 0.5 ppm for 10 min. All reported eye irritation, five had tearing, four had nose irritation, and two reported throat irritation. One person detected an odor after 3 min. Additional experimental details were not available.
In a slightly larger study, seven male volunteers were exposed to nominal concentrations at 0.3, 1.0, 2.5, or 5.0 ppm for 1 min or 1 ppm for 10 min (Mellon Institute 1963a). No effects were reported for 0.3 or 1.0 ppm for 1 min. Exposure at 2.5 and 5.0 ppm resulted in eye irritation in 4/7 and 7/7, nose irritation in 2/7 and 2/7, and tearing in 1/7 and 7/7, respectively. Throat irritation was also reported by one individual, and 3/7 could detect an odor during exposure at 5.0 ppm. During the 10-min exposure at 1 ppm, eye irritation and tears were reported in 7/7 individuals by 4 and 5 min, respectively, and nose and throat irritation were reported by 3/7 after 9 min of exposure; no odor was detected.
2.3. Developmental and Reproductive Toxicity
A door-to-door survey was carried out 4.5 mo after the accident in Bhopal, India, to determine the reproductive outcomes of women who were pregnant at the time of the release; the data obtained consisted of self-reported symptoms and pregnancy outcomes. The spontaneous abortion rate was 24.2% in the exposed area versus 5.6% in a control area. No specific
pattern of congenital defects was observed in term infants (reviewed in Arbuckle and Sever [1998] and Shepard [1995]). Similar results were obtained in a survey conducted approximately 9 mo after the accident (Varma 1987). Among 865 women reporting that they were pregnant at the time of the accident, 43.8% of these pregnancies did not result in a live birth; background rates of spontaneous abortion were not given. Of the 486 live births, 14.2% of the infants died within 30 d as compared with a background infant death rate of 2.6–3%. None of these surveys reported stage of pregnancy at the time of exposure, severity of maternal symptoms, or concentrations or duration of exposure.
2.4. Genotoxicity
At 1,114 d after the MIC release in Bhopal, cytogenetic studies were conducted on peripheral blood lymphocytes from exposed men and women (Ghosh et al. 1990). The frequency of chromosomal aberrations was generally greater in exposed individuals, with females showing a higher incidence than males. Nondisjunction was rare and frequencies of sister chromatid exchanges (SCE) and depression in mitotic and replicative indices could not be related to exposure.
In another study examining blood lymphocytes from MIC-exposed individuals, SCE frequencies were increased more than 3 times compared with a control population, and chromosomal breaks were observed in 10 of 14 (71.4%) MIC-exposed individuals versus 6 of 28 (21.4%) unexposed controls (Goswami 1986).
2.5. Carcinogenicity
No information was found regarding the carcinogenic potential of MIC in humans.
2.6. Summary
Although 2,250 deaths were reported following the MIC release in Bhopal, the concentration of MIC causing lethality in humans is unknown. Dispersion calculations estimated airborne concentrations to range from
3,000 ppm at 270 m downwind to 10 ppm at 5,500 m downwind. Persistent ocular and pulmonary pathology has been described for the survivors of the accident. There was an increase in self-reported spontaneous abortions and a decrease in the number of live births among women pregnant at the time. The chemical has been confirmed to be an irritant to mucous membranes in both experimental and epidemiological studies. Eye irritation, usually with lacrimation, was the most common symptom reported in controlled inhalation studies at concentrations of MIC ranging from approximately 1 ppm to 5 ppm. MIC has not definitively been shown to be a sensitizer.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
LC50 values for guinea pigs, rats, and mice are summarized in Table 3– 4. An LC50 for rabbits was not found in the available literature. Acute lethality studies, including clinical effects where available, are discussed below by species.
3.1.1. Rabbits
Two rabbits per group were exposed to MIC at 5.4 ppm for 6.75, 3.5, or 2 h or at 1.8 ppm for 7 h (Dow Chemical 1990). Although the concentrations were listed as nominal, the report stated that the analytical method (infrared absorption) was adequate for monitoring concentrations as low as 1.8 ppm. A description of the exposure chamber was not included. All animals exposed at 5.4 ppm died 1–2 wk postexposure, apparently due to respiratory infections. Animals exposed at 1.8 ppm survived. At 5.4 ppm for durations ≥3.5 h, the eyes were red and had evidence of corneal injury when observed with fluorescein. Ocular damage was slight in animals exposed at 5.4 ppm for 2 h and equivocal in animals exposed at 1.8 ppm for 7 h. No experimental details or further discussion was included.
Male albino rabbits were exposed in a flow-through chamber to a monitored concentration of MIC at 1,260 ppm for 30 min (Pant et al. 1987). Although numbers of deaths were not stated, it is unlikely most animals survived exposure at this concentration. At necropsy, lung weights were
TABLE 3–4 Summary of LC50 Values
|
Species (Gender) |
Number of Animals Per Group |
LC50 (ppm) |
Duration (h) |
Reference |
|
Guinea pigs (male and female) |
6/gender |
5.4 |
6 |
Dodd et al. 1985, 1986 |
|
Guinea pigs (not stated) |
Not stated |
10.6 |
4 |
Mellon Institute 1970 |
|
Guinea pigs (male) |
8 |
26.5 |
3 |
Ferguson and Alarie 1991 |
|
Rat (male and female) |
6/gender |
6.1 |
6 |
Dodd et al. 1985, 1986 |
|
Rat (not stated) |
6 |
17.5 |
4 |
Mellon Institute 1970 |
|
Rat (male) |
6 |
11.1 |
4 |
Fait and Dodd 1981 |
|
Rat (female) |
6 |
11.0 |
4 |
Fait and Dodd 1981 |
|
Rat (not stated) |
20 |
5 |
4 |
Kimmerle and Eben 1964 |
|
Rat (not stated) |
6 |
27.4 |
2 |
Mellon Institute 1970 |
|
Rat (not stated) |
20 |
21 |
2 |
Kimmerle and Eben 1964 |
|
Rat (not stated) |
6 |
41.3 |
1 |
Mellon Institute 1970 |
|
Rat (male) |
20 |
45.01 |
1 |
ManTech Environmental 1992 |
|
Rat (female) |
4 |
171 |
0.25 |
Dodd et al. 1987 |
|
Mouse (male and female) |
6 |
12.2 |
6 |
Dodd et al. 1985, 1986 |
|
Mouse (male) |
8–41 |
26.8 |
3 |
Varma et al. 1988 |
|
Mouse (male) |
6 |
112.4 |
0.5 |
Vijayaraghavan and Kaushik 1987 |
increased 2- to 2.5-fold, and the lungs had large hemorrhagic patches. Histologically, the epithelial lining of the bronchioles was necrotic and sloughed, and the alveoli were edematous.
3.1.2. Guinea Pigs
Groups of eight male English short-haired guinea pigs were exposed for 3 h to analytical concentrations of MIC at 6, 13, 19, 27, or 37 ppm (Ferguson and Alarie 1991). Each animal was held in a whole body plethysmograph attached to a primary chamber into which MIC was delivered. A 3-h LC50 of 26.5 ppm was calculated. Pulmonary performance, as measured by respiratory frequency, amplitude, coughing, flow-volume measurements, O2 uptake, and CO2 output, was evaluated for up to 1 y in the survivors. At 19 ppm and 37 ppm, 2/8 and 6/8 animals exposed died within 48 h, respectively; no animals survived more than 24 h following exposure at 27 ppm. Coughing was observed in animals exposed at ≥19 ppm; the frequency increased with concentration and persisted in survivors for more than 5 d postexposure. Deterioration of pulmonary performance (decreased breathing frequency and abnormal flow-volume loops) was observed in animals exposed at 6 ppm and 13 ppm, but complete recovery occurred within a few weeks. Because all measures of pulmonary performance in the 6- and 13-ppm animals returned to normal, these groups were terminated at 1 and 2 mo postexposure, respectively. Gross necropsy was unremarkable in the animals exposed at 6 ppm and 13 ppm. Histologically, the surface density of the epithelial layer of all conducting airways was decreased at 6 ppm after 1 mo but had returned to normal in the 13-ppm animals after 2 mo. In animals surviving exposure at 19 ppm or 37 ppm, impairment of pulmonary performance, indicative of chronic obstructive lung disease, was observed after 1 y. These higher exposure groups had concentration-related decreases in tidal volume and abnormal flow-volume loops in response to CO2 challenge. At necropsy 1 y postexposure, the lungs from the animals exposed at 19 ppm or 37 ppm showed large areas of scarring, and portions of the lobes appeared atelectatic. Histologically, there was an increase in fibrous connective tissue in the main bronchi, an increase in septal thickness of the alveoli, and destruction of the alveolar walls, all of which were more severe at 37 ppm than at 19 ppm. In a companion study to the one just described, no evidence of inhibition of oxygen utilization (cyanide-like effect) was reported for the 37-ppm group (Alarie et al. 1987).
Dodd et al. (1985, 1986) determined a 6-h LC50 of 5.4 ppm for male and female guinea pigs (N=6). Analytically determined mean exposure concentrations in the flow-through chamber were 0, 1.0, 2.4, 5.4, and 10.5 ppm. No adverse effects were observed at 1 ppm and 2.4 ppm. Clinical signs included lacrimation and/or perinasal wetness at ≥5.4 ppm and irregular or labored breathing at 10.5 ppm. In animals that died, red nasal discharge was observed and the lungs were grossly hyperemic. Histologically, congestion, necrosis, and rhinitis of the nasal cavity, necrosis of the larynx and trachea, and congestion, hemorrhage, edema, hyaline membrane formation, and necrosis of the lungs were observed. The bronchioles were nearly obliterated by exfoliated epithelial cells. Severity of these lesions was concentration-related, and epithelial regeneration was observed in survivors (Fowler and Dodd 1985, 1986).
Female Hartley guinea pigs (minimum of four per group) inhaled 25, 125, 225, or 675 ppm for 15 min in a static exposure chamber (Dodd et al. 1987). Chamber concentrations were analytically determined prior to exposure. The 15-min LC50 was calculated as 112 ppm, and the approximate time to death was 1–3 d postexposure. Number of deaths at each concentration was not given. Clinical signs at concentrations ≥225 ppm included lacrimation, nasal wetness, rubbing of eyes and nose with forepaws, partial to complete closure of the eyelids, salivation, mouth breathing, and short periods of hyperactivity; prior to death animals were prostrate and gasping. Histologically, necrosis of the epithelia lining the conducting airways resulted in sloughing of large sheets of epithelial cells (Fowler et al. 1987). Sheets of epithelial cells, together with fibrin and mucus, occluded the more distal airways causing atelectasis. The severity of the lesions was concentration-related.
Male Hartley guinea pigs (group sizes not stated) were exposed to target concentrations of MIC at 25, 125, or 225 ppm for 15 min in a static exposure chamber, and blood samples were taken immediately or up to 16 h postexposure (Troup et al. 1987). All animals in the 225-ppm group died prior to the 16-h sample, and only one animal in the 125-ppm group survived; time to death was not stated. No effects were observed on red blood cell cholinesterase activity. Increased hemoglobin concentration and hematocrit developed in animals exposed at ≥125 ppm. At the highest concentration, neutrophilia and increases in creatine kinase were observed. These changes were indicative of a generalized hypoxic injury with concomitant pathophysiologic alterations.
In an immunotoxicity study, five pairs of female Sprague-Dawley guinea pigs were exposed in a static exposure chamber to a target concentration at 650 ppm (644–702 ppm) until one of the pair died (11–15 min) (Kolb et al. 1987). The survivor was sacrificed, and plasma was obtained from both animals and analyzed for complement consumption. In both animals, complement activation had occurred as indicated by a reduction in CH50, C3, and C5 activity levels. Plasma protein concentrations were significantly elevated in animals that had died but not in animals that were sacrificed.
Groups of four male guinea pigs were exposed to “metered” (nominal) concentrations of MIC at 15.63, 31.25, or 62.5 ppm for 4 h followed by a 14-d observation period (Mellon Institute 1966). A description of the exposure chamber was not included. All animals died following exposure to the highest concentration, 3/4 died after exposure at 31.25 ppm, and 0/4 died after exposure at 15.63 ppm; deaths occurred within 48 h. Animals showed immediate signs of ocular and nasal irritation at the “higher” concentrations and were gasping after 10 min at 62.5 ppm. Gross necropsy revealed hemorrhage of the lungs. Further experimental details were not given. A 4-h LC50 of 10.6 ppm was reported for guinea pigs, but experimental details were not included (Mellon Institute 1970).
3.1.3. Rats
Groups of 20 male Fischer rats were exposed in whole-body, flow-through chambers for 1 h to analyzed concentrations of MIC at 22.7, 33.5, 46.5, 49.7, or 62.5 ppm (ManTech Environmental 1992). Mortalities were 0, 4, 7, 16, and 16 animals, respectively, resulting in a calculated 1-h LC50 of 45 ppm with 95% confidence limits of 38.62–52.46 ppm. Most rats in all exposure groups exhibited dyspnea, rales, salivation, lacrimation, and clear or red nasal discharge upon removal from the chamber. Clinical signs during the 14-d observation period included redness around the nose and eyes, gasping, wheezing, cold to touch, rough hair coat, diarrhea, discolored inguinal fur, and emaciation. All surviving rats lost weight during the first week postexposure, and survivors exposed at ≥33.5 ppm continued to have body-weight loss during the second week. Gross findings in rats that died included red or pale puffy lungs, gas-filled stomach, and yellow and gas-filled intestines. Puffy, pale or red lungs, tan or gray areas, and/or red foci in the lungs were observed in all animals necropsied at the end of the observation period.
Dodd et al. (1985, 1986) determined a 6-h LC50 of 6.1 ppm for male and female Fisher 344 rats combined (N=6/gender/group). Exposure was whole-body, and analytically determined exposure concentrations in the flow-through chamber were 1, 2.4, 5.4, 10.5, and 20.4 ppm. Clinical signs included lacrimation and/or perinasal wetness, irregular or labored breathing, decreased activity, and ataxia as well as body-weight loss during the observation period. Gross observations included dark red to brown material encrusted around the mouth, nares, and eyes and lung congestion. In animals that died, congestion, epithelial necrosis, and inflammation of the nasal tissues, larynx, trachea, and lungs with edema in the lungs were confirmed histologically. Squamous metaplasia in the upper airways and submucosal fibroplasia in the bronchi and bronchioles became more pronounced in animals with longer survival times (Fowler and Dodd 1985, 1986).
Four-hour LC50s of 11.1 ppm and 11.0 ppm were calculated for male and female Fisher 344 rats (N=6/gender), respectively (Fait and Dodd 1981). Exposure was whole-body and analytically determined exposure concentrations in the flow-through chamber were 5.2, 15.24, 25.6, and 36.07 ppm. Clinical signs during and postexposure included respiratory difficulty, lacrimation, nasal discharge, dark red staining of the fur in the perioral and perinasal regions, and decreased motor activity; the severity of these signs was concentration-related. Gross necropsy revealed the lungs to be dark red with mottling.
Two- and four-hour LC50s for rats (strain not specified) were calculated to be 21 ppm and 5 ppm, respectively (Kimmerle and Eben 1964). The 2-h value was based on analytical concentrations of MIC at 2, 20, and 21 ppm that resulted in deaths of 0/20, 10/20, and 10/20 animals, respectively. The 4-h value was based on analytical concentrations of 5, 9, and 23 ppm, which killed 10/20, 16/20, and 20/20 animals, respectively. All deaths occurred 1–7 d after exposure. Signs of toxicity at concentrations >2 ppm included mucous membrane irritation (not further described), labored breathing, and lung edema. In an additional experiment by the same authors, exposure at 22 ppm for 1 h resulted in the death of 7/20 in 3–8 d with similar clinical signs.
Four-hour exposures of Wistar rats to MIC at 62.5 ppm killed 6/6 animals within 2 d, but no deaths occurred following exposure at 31.2 ppm (Mellon Institute 1963b). A description of the exposure chamber was not included. This range-finding data was used as the basis for LC50 studies in which groups of six male Wistar rats were exposed to varying concentrations for durations of 7.5 min to 4 h (Mellon Institute 1970). At concentra-
tions of ≥8.9 ppm (durations not specified) the animals exhibited signs of eye, nose, and lung irritation, including gasping and labored breathing. Transient eye and nose irritation was observed during exposure at 4.47 ppm. LC50 values for 4 h, 2 h, 1 h, 30 min, 15 min, and 7.5 min were listed as 17.5, 27.4, 41.3, 76.6, 216, and 541 ppm, respectively. Although additional experimental details were not given, the report stated that the results were based on analytically verified concentrations.
Female Wistar rats (N=5 to 8) were exposed whole-body to analytical concentrations of MIC at 297, 420, 528.7, or 665.7 ppm for 30 min in a static exposure chamber (Vijayaraghavan and Kaushik 1987). The 30-min LC50 was calculated as 439.5 ppm. The cyanide antidote sodium thiosulfate, given before or after exposure, had no effect on mortality. The histological changes in the lungs were reported in another study in which animals were exposed in static chambers to approximately 1, 0.5, and 0.33 times this 30-min LC50 (Pant et al. 1987). Rats that survived had granular deposition of the lining cells of the bronchioles and epithelial necrosis leading to desquamation of the bronchiolar epithelium and congestion and edema. The severity of the lesions increased with MIC concentration.
Female Sprague-Dawley rats (minimum of four per group) were exposed whole-body at 100, 600, or 1,000 ppm (analytically determined) for 15 min in a static exposure chamber (Dodd et al. 1987). The 15-min LC50 was calculated as 171 ppm and the time to death was 1–3 d postexposure. Clinical signs at concentrations ≥600 ppm included lacrimation, nasal wetness, rubbing of eyes and nose with forepaws, partial to complete closure of the eyelids, salivation, and mouth breathing. Histologically, necrosis of the epithelia lining the conducting airways resulted in sloughing of large sheets of epithelial cells (Fowler et al. 1987). The sheets of epithelial cells, together with fibrin and mucus, plugged the more distal airways causing atelectasis. The severity of these lesions was concentration-related.
Female rats (strain not specified; N=4) were exposed to concentrations of MIC ranging from 1.8 to 230 ppm for durations of 0.1–7 h (Dow Chemical 1990). Although the concentrations were listed as nominal, the report stated that the analytical method (infrared absorption) was adequate for monitoring concentrations as low as 1.8 ppm; however, the analytical concentrations were not reported. A description of the exposure chamber was not included. Exposures at 230 ppm for ≥0.5 h, 90 ppm for ≥2 h, 35 ppm for ≥4 h, and 13.8 ppm for ≥4 h resulted in the deaths of all animals. Exposures resulting in some or no mortalities are as follows: 230 ppm for 0.2 h, 1/4; 230 ppm for 0.1 h, 0/4; 90 ppm for 1 h, 3/4; 35 ppm for 2 h, 3/4;
35 ppm for 1 h, 0/4; 13.8 ppm for ≤2 h, 0/4; 5.4 ppm for 7 h, 3/4; 5.4 ppm for ≤4 h, 0/4; 1.8 ppm for ≤7 h, 0/4. At concentrations ≥5.4 ppm, clinical signs indicated eye and nasal irritation, and necropsy revealed moderate to slight lung congestion. Liver and kidney pathology was also stated, but not defined, for most of these animals. No irritation was observed from exposures at 1.8 ppm for 4 or 7 h with questionable to slight lung pathology observed at necropsy.
Male and female F344/N rats (N=5/gender/time point) were exposed to MIC at 0, 3, 10, 15, 20 or 30 ppm for 2 h and necropsied at various time points up to 91 d postexposure (Bucher et al. 1987a). Exposure was whole-body in flow-through chambers. Chamber concentrations were monitored continuously and did not vary more than ±10% of nominal. Clinical signs during exposure included irritation and restlessness with rubbing of the eyes and ears at concentrations of 10–15 ppm; at 20 ppm, rats walked with a low carriage and the eyes were partially closed; at 30 ppm, animals lay flat with their eyes closed, had excessive lacrimation, and a frothy, reddish discharge was seen from the nose and mouth. Upon removal from the chamber, concentration-related clinical signs included weakness, ruffled fur, respiratory distress (gasping, moist rales, open mouth, abdominal breathing), and decreased body-weight gain. No animals died during exposures, but deaths were observed 15–18 h later in the 20- and 30-ppm groups, with a higher proportion of males dying during the first 3 d. These initial deaths were followed by a 5- to 7-d period in which few deaths occurred before more animals began dying. All male rats died by day 28 and only two female rats survived to 91 days following exposure at 30 ppm. Survival at 10 ppm was approximately 70% for both males and females, and at 20 ppm it was approximately 25% and 40% for males and females, respectively. Because animal survival was presented in graphic form, the exact numbers could not be discerned and control data were not included. At necropsy, lung weights were increased in the 10- and 30-ppm males and in the 30-ppm females throughout the postexposure period. Additional investigations showed no clinical chemistry or hematologic changes, no inhibition of blood or brain cholinesterase, and no effect on lethality following sodium nitrite and sodium thiosulfate administration (Bucher et al. 1987a). No gross or microscopic evidence of epithelial erosion or ulceration of the cornea or adjacent tissues was observed at any time following exposure (Gupta et al. 1987).
Tissues from the rats in the Bucher et al. (1987a) study discussed above were examined for gross and histopathological changes immediately after
exposure at 10 ppm or 30 ppm and through 91 d; a 2-h, 3-ppm group was also included (Bucher et al. 1987b). The severity of all lesions was concentration- and gender-related, and the 30-ppm males were the most affected. Grossly, reddish white encrustations were observed around the mouth, nose, and eyes of dead and moribund animals. The middle and median lobes of the lung were commonly consolidated, and petechiae and ecchymoses were observed in the lungs of the 30-ppm group. Microscopic lesions in animals exposed at 3 ppm included necrosis of the respiratory epithelium of the trachea, but the lesions were essentially resolved by day 14. At concentrations of 10 ppm and 30 ppm, necrosis of both respiratory and olfactory epithelium was followed by inflammation, epithelial regeneration, and intraluminal fibrosis. The severity of the inflammatory lesions decreased over time, but intraluminal fibrosis, mild bronchitis and bronchiolitis, and mucous plugs persisted throughout the 91-d period (Bucher et al. 1987b). Necrosis and degeneration followed by regeneration of both the respiratory and olfactory epithelia of the nasal mucosa were confirmed by ultrastructural analysis with transmission electron microscopy (Uraih et al. 1987).
Groups of six male albino rats were exposed whole-body to nominal MIC concentrations at 8, 16, 32, or 64 ppm for 6 h followed by a 15-d observation period (IRDC 1964). In addition, two animals in the 16-ppm group were sacrificed at 24 h for interim histopathological evaluation. Exposure concentrations were calculated on the basis of air flow through the chamber and the amount of material vaporized. Slight nasal irritation was noted in animals exposed at 8 ppm, and all animals survived. At ≥16 ppm, animals exhibited labored breathing and signs of respiratory distress. All animals died within 24 h of exposure to 64 ppm, and by the end of the observation period, 5/6 and 1/4 animals in the 32- and 16-ppm groups, respectively, died. Hemorrhagic areas were observed at necropsy in the lungs of all rats exposed at ≥16 ppm. After the 15-d observation period, histopathological evaluation revealed inflammatory lesions in the lungs of all exposed rats, the severity of which was concentration-related. Rats that died or were sacrificed during the first or second postexposure day had pulmonary edema, early acute bronchitis, congestion, and hemorrhage.
Male LAC:P rats (N=3/concentration/time point) were exposed whole-body to MIC for 1 h in a static exposure chamber, and lesions of the respiratory tract were assessed at various time points over 3 wk. Nominal chamber concentrations were 8.4, 42, 105, 210, or 420 ppm (Nemery et al.
1985a; Dinsdale et al. 1987). During exposure, all animals showed signs of eye and nose irritation and had a clear nasal exudate. A concentration-related decrease in respiratory rate led to respiratory acidosis and hypoxemia. Within 24 h, one rat exposed at 210 ppm died and three exposed at 420 ppm died; four others were killed moribund. Following exposure at 105 ppm, one animal died 10 d postexposure, and another died 6 wk postexposure. Animals exposed at 105 ppm were examined for ultrastructural changes in the respiratory tract. The authors stated that a “raft” of cellular debris and fibrin lined most of the airways during the first week after exposure, but repair to the underlying epithelium was well advanced within 2–3 d. The majority of airways were lined by a normal epithelium within 3 wk postexposure, but isolated foci of hyperplasia were present, and a few of the bronchioles were blocked by fibrin, although others showed signs of residual peribronchial fibrosis. The study authors stated that the initial chamber concentrations “must be reduced by a factor of about four” to obtain the time-weighted average (TWA) concentrations over 1 h. Their explanation for this statement was that an initial injection of MIC into the tank gave the reported nominal concentration, but that did not reflect the real TWA concentration over the 1-h exposure. No data were included to support this statement.
Male Lister hooded rats were exposed, in pairs, for 2 h to MIC at 11, 21, 31, or 65 ppm (Salmon et al. 1985). Exposures were whole-body in flow-through chambers. No animals died during exposure, but at 65 ppm, one rat died 45 h after exposure and the other was killed in extremis at 50 h. Necropsy of these animals revealed hemorrhagic patches on the lungs and pulmonary edema. During exposures animals exhibited slow and irregular breathing and were inactive; the severity of the clinical signs was concentration-related. When examined 20 h postexposure, erosions of the corneal epithelium were observed in all animals exposed at ≥21 ppm. The surviving animals were then followed for up to 14 mo (Gassert et al. 1986). One rat exposed at 31 ppm died at 6 mo, and one exposed at 11 ppm died at 8 mo, following sudden onset of respiratory distress. Necropsy of exposed animals at 14 mo showed a history of mild respiratory infection in all animals as evidenced by lymphoid hyperplasia adjacent to bronchiolar airways. Mild interstitial fibrosis in the peribronchiolar regions was seen in all animals. In the eyes, eosinophil and lymphoid infiltrate was most prominent in the animals exposed at 21 ppm.
Pairs of Charles Foster rats were exposed to MIC at 3.52 or 35.32 ppm for 10 min and necropsied immediately after death (Sethi et al. 1989).
During exposure, dyspnea, congestion in the eyes, bloody lacrimation, and nasal secretion were observed. Animals exposed at 35.32 ppm died within 4 min of exposure, and animals exposed at 3.52 ppm died 39 min and 293 min postexposure. Lung hemorrhages, dilated and congested trachea, pulmonary edema, and brain edema were observed at necropsy. The main histological findings were necrosis of the bronchial epithelium, lung edema, and congestion of the vessels in several visceral organs. It should be noted that for these exposure concentrations, the effects are more severe than (and are not consistent with) those reported in other similar studies. Additional details on the exposure system, generation of the test atmospheres, and monitoring of chamber concentrations were not provided.
Histological lesions in the lungs of male Lister rats were assessed up to 10 wk following a single 30-min exposure to nominal concentrations at 233, 465, or 930 ppm in static exposure chambers. The concentration of 465 ppm was stated as the LC50, but details concerning the LC50 experiment were not reported (Jeevaratnam and Sriramachari 1994; Sriramachari and Jeevaratnam 1994). During exposures, the animals displayed acute respiratory distress. At 24-h postexposure, the numbers of deaths were 0/8, 4/10, and 7/12, respectively. Initially, acute necrotizing bronchitis of the respiratory tract accompanied by congestion, hyperemia, and interstitial and alveolar edema were observed with the severity related to concentration. This was followed at 4 wk by regeneration of the bronchial epithelium, but persistent interstitial pneumonitis, thickened alveolar septa, and areas of atelectasis were prevalent. At 10 wk postexposure, the only surviving animals were from the 233-ppm group. Those animals developed diffuse interstitial pulmonary fibrosis, but restoration of the bronchial epithelium was nearly complete. In a related study (Jeevaratnam et al. 1990), biochemical changes in rats were monitored up to 24 h after exposure at 465 or 930 ppm for 30 min. None of the animals in the high concentration group survived beyond 4 h postexposure. At both exposure concentrations, rats were afflicted with hyperglycemia, lactic acidosis, elevated plasma urea, and slight inhibition of plasma cholinesterase activity; erythrocyte cholinesterase activity remained unaffected.
Male rats (six per group) were exposed under a whole-body protocol at 1,344, 1,844, 4,607, 9,219, or 18,438 ppm (analytical concentrations) for 8 min in static exposure chambers. A series of toxicological studies was conducted on the surviving low-concentration animals. All animals died within 24 h after exposures at ≥4607 ppm. Mortality at 1,344 and 1,844 ppm was approximately 10–20% and 30–40%, respectively. Pronounced
clinical signs were indicative of severe irritation, and gross necropsy showed consolidation of the small lobes of the lung and distention of the gastrointestinal tract with gas. Food and water consumption and body weights of the survivors were initially reduced after exposure, followed by a gradual recovery. Peripheral emphysema and congestion of the alveolar capillaries persisted through 14 d postexposure with some reduction in severity over time (Dutta et al. 1988). Biochemical analyses conducted 7 or 14 d after exposure at 1,344 ppm showed an increase in total serum lactate dehydrogenase (Gupta et al. 1988), increases in lung levels of aniline hydroxylase and glutathione-S-transferase activities, decreased lung glutathione levels, increases in cytochrome P-450 and cytochrome b5 content in the lung (Mishra et al. 1988), impaired alveolar and peritoneal macrophage functions and delayed type hypersensitivity reactions (Dwivedi et al. 1988; Saxena et al. 1988), and an increased susceptibility to bacterial E. coli endotoxin (Saxena et al. 1988). In a companion study, alterations in the biochemical and cytological constituents of bronchoalveolar lavage fluid (BALF) were monitored over a period of 30 d following exposure at 1,344 ppm for 8 min (Gupta et al. 1991). Total protein, sialic acid, and lactic acid contents of BALF were increased followed by a gradual return to baseline between day 3 and day 30. Lactic dehydrogenase levels and the numbers of polymorphonuclear neutrophils increased over time.
Groups of three rats were exposed under a whole body protocol to high concentrations of MIC until death. The exposure concentration and timeto-death for all animals in each group were 171,600 ppm for 7 min (flow-through), 6,619 ppm for 18 min (flow-through), 8,580 ppm for 30 min (static), 832 ppm for 110 min (static), and 343 ppm for 196 min (static). Static exposure at 51 ppm for 6 h resulted in deaths of two of three rats by 8 d postexposure. Clinical signs included lacrimation, dyspnea, nasal discharge, and gasping (Eastman Kodak 1966).
3.1.4. Mice
Male Swiss-Webster mice were exposed using a whole-body regimen to MIC at 0, 9, 18, or 40 ppm for 3 h and were observed for 14 d following exposure (Varma et al. 1988). Concentrations in the flow-through chamber were monitored throughout exposure. Deaths in the MIC-exposed groups were 0/8, 5/11, and 31/41, respectively, for a calculated 3-h LC50 of 26.8 ppm. Clinical signs of toxicity were similar for all exposure groups with
the animals scratching their noses and lacrimating. The animals ceased activity and remained listless for the duration of exposure but could be aroused by tapping on the chamber. Decreased respiratory rates were observed but not quantified. Concentration-related reductions in body weights were observed 1–2 d after exposure followed by gradual recovery. However, in the 40-ppm group, recovery of body weights was followed by a second phase of decrease from day 7 to day 8 postexposure. In separate studies, mortalities at 40 ppm were delayed in mice that had been deprived of food for 24 or 48 h and were reduced in mice injected with 2 mg dexamethasone per kilogram body weight prior to MIC exposure. Death was not prevented by administrations of sodium thiosulfate, ethanol, and atropine before or dexamethasone after MIC exposure.
A 6-h LC50 of 12.2 ppm for male and female B6C3F1 mice combined (N=6/gender) was reported by Dodd et al. (1985, 1986). Analytically determined exposure concentrations in the flow-through chamber were 1, 2.4, 5.4,10.5, and 20.4 ppm. Clinical signs at ≥5.4 ppm included lacrimation and/or perinasal wetness, irregular or labored breathing, decreased activity, ataxia, and/or hypothermia as well as body-weight loss in all treated groups during the observation period. Gross observations included mild perinasal exudate and discoloration of the lungs. In animals that died, congestion, necrosis, and inflammation of the nasal tissues, necrosis of the larynx and trachea, and edema and bronchiolar epithelial necrosis in the lungs were observed histologically. Squamous metaplasia in the upper airways and submucosal fibroplasia in the bronchi and bronchioles became more pronounced in animals with longer survival times (Fowler and Dodd 1985, 1986).
Male Swiss albino mice (N=6) inhaled analytical concentrations of MIC at 83.8, 94.0, 132.8, or 236.2 ppm for 30 min in a static exposure chamber (Vijayaraghavan and Kaushik 1987). The 30-min LC50 was calculated as 112.4 ppm. Sodium thiosulfate, given before or after exposure, failed to influence mortality.
Male and female B6C3F1 mice inhaled MIC at 0, 3, 10, or 30 ppm for 2 h (Boorman et al. 1987a,b; Bucher et al. 1987a). Exposure was conducted using whole-body in flow-through chambers. Chamber concentrations were monitored continuously and did not vary more than ±10% of nominal. Five animals per group were necropsied within 3 h after exposure and on days 1, 3, 7, 14, 49, and 91. During exposures, animals were inactive, especially at 30 ppm, and dyspnea was observed upon removal from the chamber. Deaths began 15–18 h after exposure to MIC at 30 ppm fol-
lowed by 5–7 d during which few deaths occurred. Sixteen of 80 (20%) males in the 30-ppm group died, with seven deaths occurring within the first 24 h. Two females died, one each in the 30- and 10-ppm groups. Lung weights of males exposed at 30 ppm were significantly increased beginning on day 3 postexposure (Bucher et al. 1987a). Treatment-related lesions of the respiratory system were observed in both genders, with the severity greater in males. At 30 ppm there was extensive erosion and necrosis of the epithelia in the nasal cavity, trachea, and main bronchi. In the nasal cavity, recovery was essentially complete with the exception of small areas in the olfactory epithelia where lesions persisted in the males through day 91. In the trachea and major bronchi, fibrin and cellular debris were present in the airways, which in some cases had organized and formed fibrotic projections. Fibrosis was more severe in the males and chronic alveolitis and atelectasis were evident. Similar but less severe lesions were seen at 10 ppm. Acute inflammation and erosion of the respiratory epithelium were the main effect at 3 ppm. Complete recovery occurred in mice exposed at 3 or 10 ppm (Boorman et al. 1987a,b). Degeneration followed by rapid regeneration of the respiratory and olfactory epithelia was also confirmed ultrastructurally by transmission electron microscopy for males exposed at 10 or 30 ppm (Uraih et al. 1987). In contrast to many of the victims of the accident in Bhopal, ocular lesions in mice were not found (Boorman et al. 1987a). Clinical pathology and hematology end points and blood and brain cholinesterase activities were not affected by treatment. The cyanide antidotes sodium nitrite and sodium thiosulfate were not effective against mortality (Bucher et al. 1987a).
3.2. Nonlethal Toxicity
3.2.1. Guinea Pigs
Tissue hypoxia and metabolic acidosis were observed in female Hartley guinea pigs (N=2 to 3) statically exposed at 240 or 628 ppm for 15 min (Fedde et al. 1987). Following exposure, the animals were anesthetized and artificially ventilated for measurement of blood gases. Both concentrations of MIC resulted in marked reductions in the partial pressure of oxygen and in arterial pH. The partial pressure of oxygen remained low after ventilation with 100% oxygen, indicating severe intrapulmonary blood shunting. Hemoglobin and hematocrit were not affected by this protocol. Gross ob-
servation of the lungs showed atelectasis, multifocal hemorrhages, and long strands of viscous material in the trachea and primary bronchi.
Hypoxemia and metabolic acidosis were confirmed in another study in which the oxygen binding properties of the blood were determined following whole-body exposure of female Hartley guinea pigs (number not reported) to a mean concentration at 698 ppm (range 618–804 ppm) for 15 min in a static chamber (Maginniss et al. 1987). Erythrocyte volume, methemoglobin concentration, oxygen binding capacity, and combined red cell organic phosphate concentration were not affected. However, lactic acidosis was indicated by increased circulating lactate concentrations, and oxygen equilibrium curves were significantly right-shifted (reduction in hemoglobin-oxygen affinity). In consequence, hemoglobin-oxygen saturation decreased from 66% for the controls to 42% for the MIC-exposed animals resulting in hypoxia.
The potential of inhaled MIC to cause respiratory sensitization was investigated in guinea pigs (Mellon Institute 1970). Adult male albino guinea pigs were exposed to an analytical concentration at 1 ppm for 2 h/d, 3 times per week for 3 wk. A description of the exposure chamber was not provided in the Mellon report. After a 3 wk incubation interval, groups of seven animals were exposed for 2 h at either 1 or 5 ppm. No signs suggesting tracheal edema or other evidence of respiratory allergic response were observed.
3.2.2. Rats
Lesions in the respiratory tract of male F344 rats (N=5) were examined 7 d after a single 6-h exposure to a monitored concentration of MIC at 3 ppm (Mitsumori et al. 1987). Exposures were carried out using a whole-body protocol and flow-through chambers. A marked reduction in body weight was noted during the recovery period. Inflammation was seen throughout the respiratory tract with regeneration of the respiratory epithelium in the nasal passages and trachea. Erosion of the respiratory epithelium in the bronchi was present. Severe inflammation of the alveoli correlated with atelectasis.
Groups of 50 male and 50 female F344 rats were given a single 2-h exposure at 0, 1, 3, or 10 ppm and then held for 2 y (Bucher and Uraih 1989). Survival and body-weight gains of exposed animals were similar to the controls throughout the study. At the end of the 2-y period, 42% of
males and 36% of females exposed at 10 ppm had evidence of intraluminal fibrosis of secondary bronchi. It was not stated whether these were analytical or nominal concentrations of MIC; however, in other work by these authors, concentrations were reported as analytical. Also, a description of the exposure chamber was not given.
Pulmonary function (Stevens et al. 1987) was assessed through 13 wk and cardiopulmonary function (Tepper et al. 1987) was assessed at 4 and 6 mo after a single exposure of male F344 rats at 3, 10, or 30 ppm for 2 h (number not reported). Exposure was whole-body in flow-through chambers. Chamber concentrations were monitored continuously and did not vary more than ±10% of nominal. None of the animals exposed at 30 ppm survived beyond 1 wk, but details of deaths were not reported. Body weights of rats exposed at 10 ppm were significantly decreased throughout the 6-mo study. Several measures of lung volume were increased by exposure at 10 ppm. Total lung capacity was increased to 120% of control at 4 wk and to 140% of control by 13 wk postexposure. Residual volume and end expiratory volume were markedly increased through 13 wk. Single-breath diffusion capacity to carbon monoxide was depressed at 1 and 2 wk postexposure and distensibility of the lung was depressed at 1 wk, but these end points were similar to controls thereafter (Stevens et al. 1987). Minute ventilation was significantly increased during CO2 challenge 4 mo after exposure but was similar to controls after 6 mo. Increased lung recoil was indicated by an increase in maximum expiratory flow and a decrease in expiratory time. Also at 4 mo postexposure, an increase in cardiac arrhythmias was observed in both the 3- and 10-ppm groups. At 6 mo, forced expiratory flow-volume curves indicated persistent airway obstruction. Wet and dry lung weights of rats exposed at 10 ppm were increased through 4 mo but were not reported for the 6-mo time point (Tepper et al. 1987).
Male Sprague-Dawley rats (number not reported) were exposed in a static exposure chamber to MIC at 100, 600, or 1,000 ppm for 15 min and blood samples were taken immediately or up to 16 h postexposure (Troup et al. 1987). Some animals in the 1,000-ppm group died prior to the 16-h sample, but the number and time to death were not stated. No effects were observed on red blood cell cholinesterase activity. Increases in hemoglobin concentration, hematocrit, erythrocytes, reticulocytes, mean corpuscular volume, neutrophils, and creatinine kinase occurred in animals exposed at 1,000 ppm. An increase in reticulocytes also occurred at 600 ppm. These changes were indicative of a generalized hypoxic injury with concomitant pathophysiologic alterations.
3.2.3. Mice
Sensory irritation to MIC was evaluated in male Swiss-Webster mice (N=4) exposed to analytical concentrations between 0.5 and 7.6 ppm for 90 min. Exposures were head-only in flow-through chambers. The RD50 (concentration that causes a 50% reduction in breathing rate) was 1.3 ppm (Ferguson et al. 1986). In the same study, pulmonary irritation was evaluated in tracheally cannulated (TC) mice. The RD50TC was found to be 1.9 ppm (Ferguson et al. 1986). Another study using male ICR mice reported an RD50 of 2.9 ppm (James et al. 1987).
Groups of 50 male and 50 female B6C3F1 mice were given a single 2-h exposure to MIC at 0, 1, 3, or 10 ppm and then held for 2 y (Bucher and Uraih 1989). Survival and body-weight gains of exposed animals were similar to the concurrent controls throughout the study. Permanent or chronic lung lesions were not described for mice. The publication failed to state whether the results were expressed as analytical or nominal concentrations of MIC; however, in other work by these authors (Bucher et al. 1987a), concentrations were reported as analytical. Also, a description of the exposure chamber was not given.
Female B6C3F1 mice (N=10) were exposed whole-body in flow-through chambers to analytical concentrations of MIC at 0, 1, or 3 ppm for 6 h/d for 4 consecutive days, and systemic immunity was evaluated within 3–5 d (Luster et al. 1986). Humoral immunity (antibody response to sheep erythrocytes) and natural killer cell activity were not affected, and resistance to infectious agents (Listeria monocytogenes, mouse malaria parasite, influenza virus) or to B16F10 transplantable tumor cells was not compromised by exposure.
3.3. Developmental and Reproductive Toxicity
Pregnant Sprague-Dawley rats (N=11) were exposed whole-body in flow-through chambers to MIC at 9 ppm (analytical) for 3 h on gestation day (GD) 10 (Varma et al. 1990). Dams were sacrificed on GD 20 and the uterine contents examined for live fetuses and postimplantation loss. Group mean values for maternal body weights, fetal weights, and placental weights were significantly (p≤0.05; Student’s t-test) reduced to 87%, 82%, and 85%, respectively, of the control levels. The total number of resorptions was significantly (p≤0.05) increased in the treated dams (76 versus 2 in the control group) resulting in a significant (p≤0.05) decrease
in the number of live fetuses; a total of 86 live fetuses were reported for the treated group versus 119 for the control group. Four of 11 (36%) treated dams had no live fetuses. Data for numbers of resorptions and live fetuses were only given as group totals and not on a per-litter basis. External, visceral, and skeletal examinations of the fetuses were not conducted.
Male and female Charles Foster rats (N=5) were exposed “once before mating” to MIC at 0, 0.212, 0.265, or 0.353 ppm for 30 min (Singh et al. 1994). Concentration-related decreases in maternal body weights occurred during gestation, and average fetal body weights and lengths were reduced in all treated groups. The percent resorptions was 3, 12, 20, and 32, respectively, based on group totals for numbers of implantations and resorptions; however it was not possible to discern from the data the number of resorptions per dam or the number of dams with whole litter resorption. The percent of fetuses (litter incidence not given) with limb defects and skeletal and visceral malformations was increased in the treated groups in a concentration-related manner. No information was given describing the exposure chambers, statistical procedures, test atmosphere generation, or concentration determinations. Therefore, the Singh et al. report was not utilized in weight-of-evidence considerations on MIC.
Pregnant Swiss-Webster mice (N=12 to 24) were exposed in flow-through chambers to analytically monitored concentrations of MIC at 0, 2, 6, 9, or 15 ppm for 3 h on GD 8 (Varma, 1987). The number of animals in each group was 24, 11, 12, 12, and 18, respectively. Dams were sacrificed on GD 18, the uterine contents were examined for postimplantation losses, and the live fetuses were examined for external, visceral, and skeletal abnormalities. Two dams in each of the 9- and 15-ppm groups died. Among survivors, complete litter resorption occurred in 0/24 (0%), 1/11 (9%), 1/12 (8%), 8/10 (80%), and 12/16 (75%), respectively. Maternal body weights of the 15-ppm group were significantly (p≤0.05) reduced on GD 18, mainly due to a decrease in body-weight gain within 48 h of exposure; only data for the 15-ppm group were presented graphically. Group mean placental weights and fetal body weights were significantly (p≤0.05) reduced at all concentrations to 78–93% and 73–93%, respectively, of the concurrent control. Fetuses from dams exposed at 9 ppm and 15 ppm also had significant (p≤0.05) reduction in the lengths of the mandible and long bones (group means compared with fetuses from control dams; statistical test not stated).
In a similar study (Varma et al. 1990), pregnant Swiss mice (total of 51 control and 63 MIC-exposed animals) were exposed in flow-through chambers to MIC at 9 ppm (analytical) for 3 h on GD 8. Dams (N=4–18/d)
were sacrificed on GD 10–18, and the uterine contents examined for live fetuses and postimplantation loss. Group mean values for maternal body weights, fetal weights, and placental weights were significantly (p≤0.05; Student’s t-test) reduced at all postexposure periods compared with controls, and by GD 18 these parameters were 64%, 82%, and 79%, respectively, of the control levels. Fetal deaths were observed within 2 d after maternal exposure. Complete litter resorption was observed in 70% of animals. A separate experiment was conducted to determine whether fetal toxicity of MIC was due to a decrease in maternal progesterone levels. Progesterone levels were found to be significantly higher in dams that retained pregnancy than in mice that lost their litters. However, daily administration of progesterone after exposure did not decrease fetal death.
Schwetz et al. (1987) conducted a series of developmental and reproductive toxicity studies with Swiss (CD-1) mice. Exposure was whole-body in flow-through chambers. Pregnant females (N=39–44) were exposed at 0, 1, or 3 ppm for 6 h/d on GD 14–17; dams were allowed to litter for evaluation of neonatal survival. Analytical concentrations in the exposure chamber were within 10% of nominal. Exposure to MIC had no effect on maternal survival, body weight, clinical signs, or gestation length. A significant (p<0.05) increase in the total number of dead fetuses at birth was observed in both exposure groups (3.3% and 6.4% dead, respectively, versus 0.4% of controls). An increase in pup mortality during lactation was observed in the 3-ppm group, with the most pronounced effect during lactation days 0–4 (11.3% died versus 2.0% of controls). Pup mortalities in the 3-ppm group resulted in significantly (p<0.05) fewer pups per litter on lactation days 0–4. No differences in pup body weights occurred during lactation between the treated and control groups.
Schwetz et al. (1987) also evaluated 30 male and female Swiss (CD-1) mice that inhaled 0, 1, or 3 ppm 6 h/d for 4 consecutive days. Mating trials were conducted during weeks 1, 8, and 17 postexposure. This same exposure regimen was conducted with 30 males followed by mating to untreated females for 8 wk. No significant effect on body weight, demeanor, fertility, or litter size and no evidence of a dominant lethal effect was observed.
In a dominant lethal assay, 24 male Wistar rats were exposed using a whole-body protocol to MIC at 1,344 ppm for 8 min in a static exposure chamber and sequentially mated to unexposed females for 3 wk (Agarwal and Bose 1992). Total deaths among exposed males during days 1–7, 8–14, and 15–21 were 13, 5, and 3, respectively. No evidence of dominant lethality and no effects on epididymal sperm density and morphology were
observed. A transient reduction in reproductive performance of exposed males during days 1–14 postexposure was attributed to general stress.
3.4. Genotoxicity
A small but significant increase in the frequency of sister chromatid exchange (SCE) was observed in lung cells from mice exposed to MIC at 1, 3, or 6 ppm 6 h/d for 4 d; SCEs were not induced in peripheral lymphocytes (Kligerman et al. 1987). No evidence of chromosomal effects or micronuclei formation were observed in the bone marrow of mice exposed at 3, 10, or 30 ppm for 2 h (Shelby et al. 1987) or to up to 7.20 µL evaporated in a 22 L chamber for two 10-min exposures separated by 24 h (Kar et al. 1989).
MIC failed to induce mutations in any of five strains of Salmonella or in the Drosophila sex-linked recessive lethal assay following exposure of males by inhalation, feeding, or injection (Mason et al. 1987). However, dose-related increases in the number of thymidine kinase mutants were induced in L5178Y mouse lymphoma cells, and the frequencies of SCE and chromosomal aberrations increased in Chinese hamster ovary cells (Shelby et al. 1987).
Dominant lethality studies in mice have been described in Section 3.3.
3.5. Chronic Toxicity and Carcinogenicity
Groups of male and female F344 rats and B6C3F1 mice were given a single 2-h exposure to MIC at 0, 1, 3, or 10 ppm and then held for 2 y (Bucher and Uraih 1989). The authors did not state whether these were analytical or nominal concentrations of MIC; however, in other work by these authors, concentrations were reported as analytical. No neoplastic lesions were observed in male or female mice or in female rats. In male rats the incidence of pheochromocytomas of the adrenal gland were 7/46, 14/46, 18/48, and 16/50, respectively, and the incidence of adenomas of pancreatic acinar cells were 0/50, 2/50, 0/49, and 6/50, respectively. The authors cautioned that although the incidence rates may indicate weak evidence for carcinogenicity and there was no evidence for dose-response, the adrenal tumor incidences were only slightly greater than the historical control mean of 22% and both tumor types are not uncommon in rats. Neither of these organs have been found to be targets for MIC.
3.6. Summary
Numerous studies have been conducted to determine the effects of MIC in animals following inhalation exposure. LC50 values for guinea pigs, rats, and mice (Table 3–4) show little species variability. It is interesting to note that in most studies, deaths did not occur during exposure—animals began dying within 24–48 h postexposure with another phase of deaths often occurring several days later. This pattern was similar to that in humans following the Bhopal accident in which deaths were not instantaneous but occurred after a lag period and in phases (Section 2.1).
Clinical signs of toxicity and histological lesions of the respiratory tract following exposure to MIC were consistent among all species tested. Generally, necrosis of the respiratory epithelia was followed by regeneration and intraluminal fibrosis in the bronchi. The severity of the initial lesion was concentration-dependent and the fibroplasia became more pronounced over time. In rats and guinea pigs, the histological lesions were shown to correlate with persistent decrements in pulmonary function.
Reported no-effect levels for signs of irritation included 3 ppm for 2 h in rats (Bucher and Uraih 1989; Stevens et al. 1987) and 10 ppm for 2 h in mice (Bucher and Uraih 1989). Protocols that elicited clinical signs but were experimentally derived no-effect levels for death included 8 ppm for 6 h in rats (IRDC 1964); 2.4 ppm for 6 h in rats, guinea pigs, and mice (Dodd et al. 1985, 1986); 230 ppm for 0.1 h, 35 ppm for 1 h, and 5.4 ppm for 4 h in rats (Dow Chemical 1990); and 9 ppm for 3 h in mice (Varma et al. 1988).
Developmental and reproductive toxicity has also been shown in rats and mice following inhalation exposure to MIC. Maternal toxicity was observed in both species following exposures at ≥9 ppm. Fetal body weights were decreased following a single maternal exposure at 9 ppm for 3 h in rats and at ≥2 ppm for 3 h in mice (Varma et al. 1987). Embryo-lethality was increased in rats exposed at 9 ppm for 3 h on GD 10 (Varma et al. 1990) and in mice exposed at 9 or 15 ppm for 3 h on GD 8 (Varma 1987; Varma et al. 1990). In mice, increased numbers of dead fetuses at birth occurred following maternal exposure at 1 or 3 ppm and increased pup mortality during lactation occurred following maternal exposure at 3 ppm for 6 h/da on GD 14–17 (Schwetz et al. 1987). It should be noted that the slight increases in stillbirths observed in mice following multiple inhalation exposures at 1 or 3 ppm (Schwetz et al. 1987) did not appear as increased resorptions following single exposures at 2 or 6 ppm (Varma 1987).
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Guinea pigs inhaled 14C-MIC at 0.38 to 15.2 ppm for 1–6 h and deposition in the airways was analyzed by autoradiography (Kennedy et al. 1993). Radioactivity was highest in the proximal airways; the level of radioactivity in the airway tissue was directly related to exposure concentration times duration. Radioactivity was detected throughout the entire nasal respiratory epithelial layer in a concentration-related manner. In the tracheobronchial region and in the lung, the label accumulation was at the subepithelial level extending to the terminal bronchiole but radioactivity was not detected in the alveolar region.
The uptake and distribution of 14C-MIC was measured in arterial blood of guinea pigs following exposure to concentrations at 0.5–15 ppm for periods of 1–6 h (Ferguson et al. 1988). Circulating 14C showed immediate uptake with clearance gradual over a period of 3 d. A similar profile was observed for urine and bile. Uptake in the upper respiratory tract passages and distribution was to all examined tissues. In pregnant mice similarly exposed to 14C-MIC, the label was detected in all tissues examined, including the uterus, placenta, and fetus (Ferguson et al. 1988).
4.2. Mechanism of Toxicity
Results from human and animal studies indicate that MIC is a severe irritant to mucous membranes. Ocular irritation was the most pronounced symptom reported in human experimental studies (Kimmerle and Eben 1964; Mellon Institute 1963a, 1970). The most frequently reported symptoms among the exposed population in Bhopal, India, were burning of the eyes, coughing, respiratory distress from pulmonary congestion, watering of the eyes, nausea, vomiting, muscle weakness, and CNS involvement secondary to hypoxia (Kamat et al. 1985; Misra et al. 1987; Lorin and Kulling 1986; Andersson et al. 1988; Weill 1988; Kamat et al. 1992). Human (Varma and Guest 1993) and animal (Fowler and Dodd 1986) fatalities are attributed to pulmonary edema.
Developmental toxicity was observed in rodents following controlled exposure to MIC. The mechanism of the systemic toxicity is unknown.
Cyanide does not contribute significantly to the toxicity of MIC.
Cyanomethemoglobin was not noted in Bhopal victims (Misra et al. 1987), pulmonary lesions are not characteristic of cyanide intoxication (Varma 1989; Weill 1988), and standard thiosulfate/nitrite cyanide antidotes have not been successful in preventing deaths in animal studies (Nemery et al. 1985b; Bucher et al. 1987a; Varma et al. 1988). Finally, the time-to-death in both humans and animals was not consistent with that associated with high dose cyanide intoxication (Varma and Guest 1993).
MIC has not been shown to be a sensitizer in either humans (Ketcham 1973; Union Carbide 1966) or animals (Mellon Institute 1970). The chemical is not an effective inhibitor of cholinesterases (Brown et al. 1987).
4.3. Other Relevant Information
4.3.1. Species Variability
Comparison of LC50 values for the guinea pig, rat, and mouse shows species variation no greater than 2-fold. For example, 6-h LC50 values were 6.1, 5.4, and 12.2 ppm, respectively (Dodd et al. 1985, 1986), while 4-h values were 10.6 ppm for the guinea pig (Mellon Institute 1970) and 11– 17.5 ppm for the rat (Fait and Dodd 1981; Mellon Institute 1970). Comparison of time-to-death from a 4-h exposure in the studies of Dodd et al. (1985, 1986) and Fowler and Dodd (1986), shows that at concentrations of 15.2–36.1 ppm guinea pigs died either during exposure or 1 d postexposure, whereas rats died 1–4 d postexposure. In addition, lesions of the respiratory tract of guinea pigs were more severe than lesions observed in rats and mice. Mice and rats are generally obligate nasal breathers, whereas guinea pigs are optimal mouth breathers when exposed to respiratory irritants. Therefore, a greater amount of MIC is deposited in the lung of the guinea pig, resulting in greater damage. Als,o guinea pigs can undergo involuntary bronchoconstriction and die in response to some irritants, which may be the reason that guinea pigs appear more sensitive to MIC than rats or mice.
4.3.2. Concentration-Exposure Duration Relationship
Values scaled for the derivation of the 10- and 30-min, and 1-, 4-, and 8-h time points were calculated from the equation Cn×t=k (ten Berge et
al. 1986) where n=1. Using 7.5-, 15-, 30-, 60-, 120-, and 240-min LC50 values of the Mellon Institute (1970), the least-squares linear curve fit of the graph (Appendix A), log time versus log LC50, resulted in the equation y=3.48−1.01x. The slope of the line, 1.0, was identified as the exponent n.
5. DATA ANALYSIS FOR AEGL-1
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
5.1. Summary of Human Data Relevant to AEGL-1
Experimental data on the effects of MIC on humans were not available for the derivation of AEGL-1. While no adverse health effects were reported following inhalation exposures at 0.4 ppm for 1–5 min (Kimmerle and Eben 1964) or at 1 ppm for 1 min (Mellon Institute 1963a), ocular and upper respiratory tract irritation and lacrimation were reported after exposure at 0.5 ppm for 10 min (Mellon Institute 1970) and at 1 ppm for 10 min (Mellon Institute 1963a). Eye irritation and lacrimation, in the absence of consistent odor perception, were also reported following exposures at 2 or 5 ppm for 1–5 min (Kimmerle and Eben 1964), at 1.75 ppm for 1 min, (Mellon Institute 1970), or at 2.5 or 5.0 ppm for 1 min (Mellon Institute 1963a). Nose and throat irritation were also reported by some, but not all, of the volunteers in these experiments.
These studies, taken together, indicate that both duration of exposure and concentration of MIC contribute to the severity of MIC-induced irritation. Extrapolation from the short experimental durations to the longer AEGL time points may not be predictive of potential adverse effects. Also, it is not known at what concentration the risk for systemic toxicity, including end points of developmental toxicity, becomes a concern. The concentrations causing irritation in humans after several minutes are similar to, or higher than, the concentrations resulting in embryo and fetal lethality in
well conducted animal studies. Therefore, the human experimental exposure data were not used in derivation of AEGL-1.
5.2. Summary of Animal Data Relevant to AEGL-1
Animal data relevant to derivation of AEGL-1 were not found. Exposure concentration-duration regimens that were reported as no-effect levels for signs of irritation were similar to protocols used in other studies that caused embryolethality. Many animal studies used concentrations of MIC sufficient to result in severe damage to the respiratory system.
5.3. Derivation of AEGL-1
Methyl isocyanate has notoriously poor warning properties (AIHA 1989), and AEGL-1 values were not derived. Although human and animal data were available for irritation levels, the ambient air concentrations associated with MIC sensory irritation may be greater than those associated with systemic toxicity. However, it should be noted that exposures to MIC at concentrations below those used to calculate AEGL-1 might be associated with systemic toxicity.
6. DATA ANALYSIS FOR AEGL-2
AEGL-2 is the airborne concentration of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
6.1. Summary of Human Data Relevant to AEGL-2
Human data relevant to AEGL-2 derivation are limited to studies that used very short exposure durations. Among volunteers exposed at 1 ppm for 10 min, 7/7 had eye irritation and tears by 4 and 5 min, respectively, and nose and throat irritation were reported by 3/7 after 9 min (Mellon Institute 1963a). Eye irritation and tearing were also reported for individuals ex-
posed at 2 or 4 ppm for 1–5 min (Kimmerle and Eben 1964) and at 0.5 ppm for 10 min (Mellon Institute 1970).
6.2. Summary of Animal Data Relevant to AEGL-2
Pregnant Swiss-Webster mice were exposed to analytically monitored concentrations of MIC at 0, 2, 6, 9, and 15 ppm for 3 h on GD 8 (Varma 1987). Placental weights and fetal body weights were significantly reduced at all MIC concentrations. Maternal toxicity was evident as deaths of two of the 11 treated dams in the 9- and 15-ppm groups and decreased maternal body weights at 15 ppm. Exposures at 9 or 15 ppm also resulted in a significant increase in complete litter resorption among surviving dams, and fetuses with significant reductions in the lengths of the mandible and long bones. Decreased maternal body weight and increased resorptions also occurred in rats exposed at 9 ppm for 3 h on GD 10 (Varma et al. 1990). Taken together these studies indicate that in pregnant rats and mice exposed once to MIC by inhalation, significant reductions in fetal body weight occur in the absence of maternal toxicity but embryo and fetal lethality occur at concentrations that result in overt maternal toxicity.
An increase in cardiac arrhythmias occurred in rats 4 mo after a 2-h exposure at 3 ppm (Tepper et al. 1987).
No effects on survival, body weights, or lung histopathology were noted in rats 2 y after exposure at 1 or 3 ppm for 2 h (Bucher and Uraih 1989).
6.3. Derivation of AEGL-2
Animal data were used for derivation of AEGL-2 values. The exposure of mice at 2 ppm for 3 h on GD 8 was a LOEL for lower fetal body weights in the absence of maternal toxicity (Varma 1987), and the exposure of rats at 3 ppm for 2 h was a LOEL for cardiac arrhythmias (Tepper et al. 1987). These exposure concentration and duration scenarios yield identical AEGL-2 values when used for derivation. Details of the calculations are shown in Appendix B. Values scaled for the derivation of the 10- and 30-min and 1-, 4-, and 8-h time points were calculated from the equation Cn×t=k (ten Berge et al. 1986) where n=1 as discussed in Section 4.4.2. The experimental concentrations were reduced by a factor of 3 to estimate a threshold
TABLE 3–5 AEGL-2 Values for Methyl Isocyanate (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.40 (0.94) |
0.13 (0.32) |
0.067 (0.16) |
0.017 (0.034) |
0.008 (0.02) |
for effects on fetal body weight and cardiac arrhythmias. A total uncertainty factor of 30 was applied, including 3 for interspecies variation, because similar results for developmental toxicity have been obtained in both rats and mice, and 10 for intraspecies variation, because the mechanism of action for developmental toxicity is unknown. Proposed AEGL-2 values are presented in Table 3–5.
7. DATA ANALYSIS FOR AEGL-3
AEGL-3 is the airborne concentration of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
7.1. Summary of Human Data Relevant to AEGL-3
Human data relevant to derivation of AEGL-3 were not found. Concentrations that resulted in death, spontaneous abortion, or infant death during the accidental release in Bhopal, India, are unknown.
7.2. Summary of Animal Data Relevant to AEGL-3
Schwetz et al. (1987) exposed pregnant mice at 0, 1, or 3 ppm for 6 h/d on GD 14–17. Dams were allowed to litter for evaluation of neonatal survival. No maternal toxicity was observed at either exposure concentration. A concentration-related increase in the number of dead fetuses at birth was observed in both exposure groups and an increase in pup mortality during lactation was observed in the 3-ppm group. Pup mortalities in the 3-ppm group resulted in significantly (p<0.05) fewer pups per litter on lactation days 0–4. No differences in pup body weights occurred during lactation between the treated and control groups. It should be noted that the slight
increases in stillbirths observed in mice following multiple inhalation exposures at 1 or 3 ppm (Schwetz et al. 1987) did not appear as increased resorptions following single exposures at 2 or 6 ppm (Varma 1987).
Pregnant rats (Varma et al. 1990) and mice (Varma 1987) exposed at 9 ppm for 3 h on GD 10 or 8, respectively, had increased resorptions and decreased maternal body weights, fetal body weights, and placental weights.
7.3. Derivation of AEGL-3
The neonatal survival study with mice by Schwetz et al. (1987) was used for derivation of AEGL-3 values. The study was well conducted and included sufficient concentration-response data. Although the exposures were repeated on 4 consecutive days, the exposure to the fetus is considered similar to a single exposure because the stage of development and potential susceptibility changes daily throughout gestation. In addition, the lower pup survival seen experimentally following repeated maternal exposure, is the same end point as fetal and infant death in humans observed following the Bhopal accident. Therefore, the NAC/AEGL FACA committee considered this study appropriate for derivation of AEGL-3 values.
The 6-h exposure at 1 ppm was used to derive AEGL-3 values using the equation Cn×t=k where n=1 as described in Section 4.4.2. This concentration was a NOEL for pup survival during lactation. A total uncertainty factor (UF) of 30 was applied, including 3 for interspecies variation, because similar developmental toxicity results have been obtained in both rats and mice, and 10 for intraspecies variation, because the mechanism of action for developmental toxicity is unknown. According to the standing operating procedures of the NAC/AEGL FACA committee, 10-min values are not to be scaled from an experimental exposure time of ≥4 h. However, because n was derived from exposures ranging from 7.5 to 240 min, extrapolation from 6 h to the 10-min AEGL-3 value is valid in this instance. Details of the calculations are shown in Appendix B. AEGL-3 values for MIC are listed in Table 3–6.
Exposures of 9 ppm for 3 h from the developmental toxicity studies in rats (Varma et al. 1990) and mice (Varma 1987) could also be used to derive AEGL-3 values. Derivations based on those data, using the UF of 30, result in AEGL-3 values of 5.4, 1.8, 0.9, 0.23, and 0.11 ppm. They would be less conservative AEGL-3 values that might not be protective of neonates.
TABLE 3–6 AEGL-3 Values for Methyl Isocyanate [ppm (mg/m3)]
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
1.2 (2.8) |
0.40 (0.95) |
0.20 (0.47) |
0.05 (0.12) |
0.025 (0.06) |
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity End Points
The derived AEGL values for various levels of effects and durations of exposure are summarized in Table 3–7. Data were not available for derivation of AEGL-1; the irritation threshold may be above the level of concern for systemic effects. However, it should be noted that exposures to MIC at concentrations below those used to calculate AEGL-1 might be associated with systemic toxicity. AEGL-2 is based on lower fetal body weights in mice and cardiac arrhythmias in rats. AEGL-3 is based on decreased neonatal survival in mice. Derivation summaries, including key studies and end points for each AEGL level, are given in Appendix C.
A useful way to evaluate the AEGL values in context of existing empirical data is presented in Figure D-1 of Appendix D. For this plot, the toxic response was placed into severity categories. The severity categories fit into definitions of the AEGL health effects: no effects, discomfort, disabling, partial lethality, and lethality (100% mortality). The effects that place an experimental result into a particular category vary according to the spectrum of data available on a specific chemical and the effects from exposure to that chemical. The concentrations often span a number of orders of magnitude, especially when human data exist, and are therefore plotted on a log scale. The graph in Figure D-1 plots the AEGL values for MIC along with the existing acute human and animal toxicity data for MIC in terms of the categories assigned to them. From this plot, it is apparent that the AEGL values are below any exposure concentration in animals resulting in adverse effects (including developmental toxicity) and should therefore be protective of human health.
8.2. Comparison with Other Standards and Criteria
Existing guideline exposure levels for MIC are listed in Table 3–8. All
TABLE 3–7 Summary of AEGL Values (ppm [mg/m3])
|
|
Exposure Duration |
||||
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1a (Nondisabling) |
NR |
NR |
NR |
NR |
NR |
|
AEGL-2 (Disabling) |
0.40 (0.94) |
0.13 (0.32) |
0.067 (0.16) |
0.017 (0.034) |
0.008 (0.02) |
|
AEGL-3 (Lethal) |
1.2 (2.8) |
0.40 (0.95) |
0.20 (0.47) |
0.05 (0.12) |
0.025 (0.06) |
|
aExposure to MIC at concentrations below those used to calculate AEGL-1 may be associated with systemic toxicity. Abbreviation: NR, not recommended. |
|||||
of the standards listed have a skin notation. The American Industrial Hygiene Association (AIHA 2000) derived an ERPG-1 based on the belief that nearly all individuals could be exposed for 1 h at 0.025 ppm without experiencing or developing adverse effects more serious than mild irritation or perception of a clearly objectionable odor. The 1-h AEGL-2 and AEGL-3 values are substantially less than the ERPG-2 and ERPG-3 values because of the different end points setting the levels. The ERPG-2 was based on the RD50 of 1.3 ppm, the increased spontaneous abortion in Bhopal, and the fact that 1 ppm was a NOAEL in rats exposed 6 h/d for 4 or 8 d; the AGEL-2 was based on reduced fetal body weight. The ERPG-3 was based on nonlethal acute inhalation data in mice and guinea pigs (5.4 ppm for 6 h and 10.6 ppm for 2 h, respectively) and the 1-h LC50 of 41.3 ppm in rats; in contrast, the AEGL-3 was based on fetotoxicity in rodents following maternal exposure during gestation. The AEGL-3 was of particular concern given the reports of spontaneous abortion among women pregnant during the Bhopal accident.
The NIOSH IDLH (NIOSH 1996) is greater than the 30-min AEGL-3. NIOSH based their IDLH on acute inhalation toxicity data in humans from Kimmerle and Eben (1964) and from the Mellon Institute (1963a).
The Dutch MAC (2000) of 0.02 ppm is equal to the TWA values of NIOSH, OSHA, and ACGIH, but the German MAK (2000) of 0.01 ppm is less than these. The MAK excursion peak category is I (with an excursion factor of 1) for a substance for which local irritant effects determine the MAK value.
TABLE 3–8 Extant Standards and Guidelines for Methyl Isocyanate Exposure Duration
|
|
Exposure Duration |
||||
|
Guideline |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 |
NR |
NR |
NR |
NR |
NR |
|
AEGL-2 |
0.40 ppm |
0.13 ppm |
0.067 ppm |
0.017 ppm |
0.008 ppm |
|
AEGL-3 |
1.2 ppm |
0.40 ppm |
0.20 ppm |
0.05 ppm |
0.025 ppm |
|
ERPG-1 (AIHA)a |
|
0.025 ppm |
|
||
|
ERPG-2 (AIHA) |
0.50 ppm |
||||
|
ERPG-3 (AIHA) |
5.0 ppm |
||||
|
PEL-TWA (OSHA)b |
|
0.02 ppm |
|||
|
PEL-STEL (OSHA)c |
0.02 ppm |
||||
|
IDLH (NIOSH)d |
|
3 ppm |
|
||
|
REL-TWA (NIOSH)e |
|
0.02 ppm |
|||
|
TLV-TWA (ACGIH)f |
0.02 ppm |
||||
|
MAK (Germany)g |
0.01 ppm |
||||
|
MAK Peak Limit (Germany)h |
|
Category I (excursion factor 1) |
|
||
|
MAC (The Netherlands)i |
|
0.02 ppm |
|||
|
aERPG (emergency response planning guidelines) (AIHA 1995). The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-1 for MIC is based on belief that nearly all individuals could be exposed to this concentration without experiencing or developing serious effects. The ERPG-2 is the maximum airborne concentration below which it is believed nearly |
|||||
8.3. Data Adequacy and Research Needs
Mainly qualitative data are available regarding acute exposures of
humans to MIC. Although extensive research has been published describing the immediate and long-term effects on humans following the Bhopal disaster, exact exposure conditions are lacking. Experimental studies in humans are of extremely short duration (i.e., minutes) and do not adequately define a dose-response. Animal data have shown concentration-dependent effects including irritation and histological lesions of the respiratory tract, developmental toxicity, and neonatal and adult lethality. Because the nonlethal and lethal effects in humans and animals are qualitatively similar, the animal data were considered relevant and appropriate for development of AEGL values as described in the standing operating procedures of the National Advisory Committee for AEGLs.
The most notable data deficiencies were the absence of quantitative human exposure data, the absence of a well-defined exposure-response curve for the toxic effects in animals, an understanding of the mechanism of action for systemic effects, and an understanding of individual variability in the toxic response to MIC.
Critical research needs include defining thresholds for effects and how these thresholds may vary with exposure concentration and duration. Such data would be valuable for affirming the AEGL values. In addition, the mechanism of systemic toxicity is unknown, and therefore, research into the mechanism(s) of systemic toxicity and the relationship to irritation effects would be instrumental in reducing uncertainties in quantitative health risk issues.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1991. Methyl Isocyanate. Pp. 1022–1024 in Documentation of the Threshold Limit Values and Biological Exposure Indicies, 6th Ed. ACGIH, Cincinnati, OH.
ACGIH (American Conference of Governmental Industrial Hygienists). 2001. 2001 TLVs and BEIs Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices, ACGIH, Cincinnati, OH. 41 pp.
Agarwal, D.K., and M.Bose. 1992. Inhalation toxicity of methylisocyanate: Assessment of germ cell mutagenicity and reproductive effects in rats. In. J. Exp. Biol. 30:504–508.
AIHA (American Industrial Hygiene Association). 1989. Table 5.3 Reported odor thresholds from all code sources. In: Odor Thresholds for Chemicals with Established Occupational Health Standards. AIHA, Fairfax, VA. 68 pp.
AIHA (American Industrial Hygiene Association). 1995. The AIHA 1995 Emer-
gency Response Planning Guidelines and Workplace Environmental Exposure Level Guides Handbook. AIHA, Fairfax, VA. 19 pp.
Alarie, Y., J.S.Ferguson, M.F.Stock, D.A.Weyel, and M.Schaper. 1987. Sensory and pulmonary irritation of methyl isocyanate in mice and pulmonary irritation and possible cyanidelike effects of methyl isocyanate in guinea pigs. Environ. Health Perspect. 72:159–167.
Andersson, N., M.K.Muir, and V.Mehra. 1984. Bhopal eye. Lancet 2:1481.
Andersson, N., M.K.Muir, A.G.Salmon, C.J. Wells, R.B.Brown, C.J.Purnell, P.C.Mittal, and V.Mehra. 1985. Bhopal disaster: Eye follow-up and analytical chemistry. Lancet 1:761–762.
Andersson, N., M.K.Muir, V.Mehra, and A.G.Salmon. 1988. Exposure and response to methyl isocyanate: Results of a community based survey in Bhopal. Br. J. Ind. Med. 45:469–475.
Andersson, N., M.K.Ajwani, S.Mahashabde, M.K.Tiwari, M.K.Muir, V.Mehra, K.Ashiru, and C.D.Mackenzie. 1990. Delayed eye and other consequences from exposure to methyl isocyanate: 93% follow up of exposed and unexposed cohorts in Bhopal . Br. J. Ind. Med. 47:553–558.
Arbuckle, T.E., and L.E.Sever. 1998. Pesticide exposures and fetal death: A review of the epidemiologic literature. Crit. Rev. Toxicol. 28:229–270.
Boorman, G.A., R.Brown, B.N.Gupta, L.C.Uraih, and J.R.Bucher. 1987a. Pathological changes following acute methyl isocyanate inhalation and recovery in B6C3F1 mice. Toxicol. Appl. Pharmacol. 87:446–456.
Boorman, G.A., L.C.Uraih, B.N.Gupta, and J.R.Bucher. 1987b. Two-hour methyl isocyanate inhalation and 90-day recovery study in B6C3F1 mice. Environ. Health Perspect. 72:63–69.
Brown, W.E., A.H.Green, T.E.Cedel, and J.Cairns. 1987. Biochemistry of protein-isocyanate interactions: A comparison of the effects of aryl vs. alkyl isocyanates. Environ. Health Perspect. 72:5–11.
Bucher, J.R., B.N.Gupta, B.Adkins, Jr., M.Thompson, C.W.Jameson, J.E. Thigpen, and B.A.Schwetz. 1987a. Toxicity of inhaled methyl isocyanate in F344/N rats and B6C3F1 mice. I. Acute exposure and recovery studies. Environ. Health Perspect. 72:53–61.
Bucher, J.R., G.A.Boorman, B.N.Gupta, L.C.Uraih, L.B.Hall, and S.A. Stefanski. 1987b. Two-hour methyl isocyanate inhalation exposure and 91-day recovery: A preliminary description of pathologic changes in F344 rats. Environ. Health Perspect. 72:71–75.
Bucher, J.R., and L.C.Uraih. 1989. Carcinogenicity and pulmonary pathology associated with a single 2-hour inhalation exposure of laboratory rodents to methyl isocyanate. J. Natl. Cancer Inst. 81:1586–1587.
Budavari, S., M.J.O’Neil, A.Smith, P.E.Heckelman, and J.F.Kinneary, eds. 1996. The Merck Index, 11th Ed. Rahway, NJ: Merck and Co., Inc. 1,039 pp.
Cullinan, P., S.Acquilla, and V.R.Dhara. 1997. Respiratory morbidity 10 years after the Union Carbide gas leak at Bhopal: A cross sectional survey. Br. Med. J. 314:338–343.
Dinsdale, D., B.Nemery, and S.Sparrow. 1987. Ultrastructural changes in the respiratory tract of rats following methyl isocyanate inhalation. Arch. Toxicol. 59:385–390.
Dodd, D.E., E.H.Fowler, W.M.Snellings, I.M.Pritts, and R.L.Baron. 1985. Acute inhalation studies with methyl isocyanate. Bushy Run Research Center. Sponsored by Union Carbide Corporation. EPA/OTS; Doc #88–8600039. 100 pp.
Dodd, D.E., E.H.Fowler, W.M.Snellings, I.M.Pritts, and R.L.Baron. 1986. Acute inhalation studies with methyl isocyanate vapor. I. Methodology and LC50 determinations in guinea pigs, rats, and mice. Fundam. Appl. Toxicol. 6:747–755.
Dodd, D.E., F.R.Frank, E.H.Fowler, C.M.Troup, and R.M.Milton. 1987. Biological effects of short-term, high-concentration exposure to methyl isocyanate. I. Study objectives and inhalation exposure design. Environ. Health Perspect. 72:13–19.
Dow Chemical. 1990. The Dow Chemical Company. Results of single exposure of rats and rabbits to the vapors of methyl isocyanate. Dow report identification number D004632. EPA/OTS; Doc #86–910000362S. 7 pp.
Dutta, K.K., G.S.D.Gupta, A.Mishra, A.Joshi, G.S.Tandon, and P.K.Ray. 1988. Inhalation toxicity studies of methyl isocyanate (MIC) in rats: Part I—Pulmonary pathology and genotoxicity evaluation. Ind. J. Exp. Biol. 26:177–182.
Dwivedi, P.C., J.K.Raizada, V.K.Saini, and P.C.Mittal. 1985. Ocular lesions following methyl isocyanate contamination: The Bhopal experience. Arch. Ophthalmol. 103:1627.
Dwivedi, P.D., A.Mishra, G.S.D.Gupta, K.K.Dutta, S.N.Das, and P.K.Ray. 1988. Inhalation toxicity studies of methyl isocyanate (MIC) in rats: Part IV— Immunologic response of rats one week after exposure: Effect on body and organ weights, phagocytic and DTH response. Ind. J. Exp. Biol. 26:191–194.
Eastman Kodak. 1966. Eastman Kodak Company. Toxicity and health hazard summary. Lab. No. 63–832. EPA/OTS; Doc #86–910000056. 6 pp.
EPA (U.S. Environmental Protection Agency). 1986. Health and Environmental Effects Profile for Methyl Isocyanate. Office of Health and Environmental Assessment, EPA, Cincinnati, OH. 52 pp.
Fait, D.W. and D.E.Dodd. 1981. Methyl isocyanate four-hour acute LC50 inhalation study on rats and guinea pigs. Bushy Run Research Center. Sponsored by Union Carbide Corporation. EPA/OTS; Doc #86–920000613. 6 pp.
Fedde, M.R., D.E.Dodd, C.M.Troup, and E.H.Fowler. 1987. Biological effects of short-term, high-concentration exposure to methyl isocyanate. III. Influence on gas exchange in the guinea pig lung. Environ. Health Perspect. 72:189–195.
Ferguson, J.S., and Y.Alarie. 1991. Long term pulmonary impairment following a single exposure to methyl isocyanate. Toxicol. Appl. Pharmacol. 107:253– 268.
Ferguson, J.S., M.Schaper, M.F.Stock, D.A.Weyel, and Y.Alarie. 1986. Sen-
sory and pulmonary irritation with exposure to methyl isocyanate. Toxicol. Appl. Pharmacol. 82:329–335.
Ferguson, J.S., A.L.Kennedy, M.F.Stock, W.E.Brown, and Y.Alarie. 1988. Uptake and distribution of 14C during and following exposure to [14C]methyl isocyanate. Toxicol. Appl. Pharmacol. 94:104–117.
Fowler, E.H., and D.E.Dodd. 1985. Respiratory tract changes in guinea pigs, rats, and mice following acute exposure to methyl isocyanate vapor. Bushy Run Research Center. Sponsored by Union Carbide Corporation. EPA/OTS; Doc #88–8600039. 100 pp.
Fowler, E.H., and D.E.Dodd. 1986. Acute inhalation studies with methyl isocyanate vapor. II. Respiratory tract changes in guinea pigs, rats, and mice. Fundam. Appl. Toxicol. 6:756–771.
Fowler, E.H., D.E.Dodd, and C.M.Troup. 1987. Biological effects of short-term, high-concentration exposure to methyl isocyanate. V. Morphologic evaluation of rat and guinea pig lungs. Environ. Health Perspect. 72:39–44.
Gassert, T., C.MacKenzie, M.Kerr Muir, N.Andersson, and A.G.Salmon. 1986. Long term pathology of lung, eye, and other organs following acute exposure of rats to methyl isocyanate. Lancet 2:1403.
German Research Association (Deutsche Forschungsgemeinschaft). 2000. List of MAK and BAT Values, 2000. Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area, Report No. 36. Weinheim, Federal Republic of Germany: Wiley VCH.
Ghosh, B.B., S.Sengupta, A.Roy, S.Maity, S.Ghosh, G.Talukder, and A. Sharma. 1990. Cytogenetic studies in human populations exposed to gas leak at Bhopal, India. Environ. Health Perspect. 86:323–326.
Goswami, H.K. 1986. Cytogenetic effects of methyl isocyanate exposure in Bhopal. Hum. Genet. 74:81–84.
Gupta, B.N., S.A.Stefanski, J.R.Bucher, and L.B.Hall. 1987. Effect of methyl isocyanate (MIC) gas on the eyes of Fischer 344 rats. Environ. Health Perspect. 72:105–108.
Gupta, G.S.D., F.N.J.Jaffery, K.K.Dutta, P.N.Viswanathan, A.Mishra, and P.K. Ray. 1988. Inhalation toxicity studies of methyl isocyanate (MIC) in rats: Part II—Biochemical studies of hemolysates, lactate dehydrogenase, isoenzymes and lipid peroxidation. Ind. J. Exp. Biol. 26:183–186.
Gupta, G.S.D., J.L.Kaw, S.H.N.Naqvi, R.Dixit, and P.K.Ray. 1991. Inhalation toxicity of methyl isocyanate: biochemical and cytological profile of bronchoalveolar lavage fluid in rats. J. Appl. Toxicol. 11:157–160.
Hartung, R. 1994. Cyanides and Nitriles. In: Patty’s Industrial Hygiene and Toxicology, 4th Ed. G.D.Clayton and F.E.Clayton, eds. pp. 3161–3172. New York: John Wiley & Sons, Inc.
Irani, S.F., and A.A.Mahashur. 1986. A survey of Bhopal children affected by methyl isocyanate gas. J. Postgrad. Med. 32:195–198.
IRDC (International Research and Development Corporation). 1964. Six-hour
acute inhalation toxicity study in rats. Report 203–001. Sponsored by The Carwin Co. EPA/OTS; Doc #86–870002225. 6 pp.
James, J.T., L.C.Buettner, and S.S.Hsu. 1987. Sensory irritation of methylisocyanate vapor. J. Appl. Toxicol. 7:147–148.
Jeevaratnam, K., R.Vijayaraghavan, M.P.Kaushik, and C.S.Vaidyanathan. 1990. Acute toxicity of methyl isocyanate in mammals. II. Induction of hyperglycemia, lactic acidosis, uremia, and hypothermia in rats. Arch. Environ. Contam. Toxicol. 19:314–318.
Jeevaratnam, K., and S.Sriramachari. 1994. Comparative toxicity of methyl isocyanate and its hydrolytic derivatives in rats. I. Pulmonary histopathology in the acute phase. Arch. Toxicol. 69:39–44.
Kamat, S.R., A.A.Mahashur, A.K.B.Tiwaris, V.Potdar, M.Gaur, V.P.Kolhatkar, P.Vaidya, D.Parmar, R.Rupwate, T.S.Chatterjee, K.Jain, M.D.Kelkar, and S.G.Kinare. 1985. Early observations on pulmonary changes and clinical morbidity due to the isocyanate gas leak at Bhopal. J. Postgrad. Med. 31:63– 72.
Kamat, S.R., M.H.Patel, P.V.Pradhan, S.P.Taskar, P.R.Vaidya, V.P.Kolhatkar, J.P.Gopalani, J.P.Chandarana, N.Dalal, and M.Naik. 1992. Sequential respiratory, psychologic, and immunologic studies in relation to methyl isocyanate exposure over two years with model development. Environ. Health Perspect. 97:241–253.
Kar, R.N., K.A.Khan, and N.Sethi. 1989. Genotoxicity studies on mice after short term inhalation exposure to methyl isocyanate. Cytobios 59:167–176.
Karlsson, E., N.Karlsson, G.Lindberg, B.Lindgren, and S.Winter. 1985. The Bhopal catastrophe—consequences of a liquefied gas discharge. National Defense Research Institute, Sweden. NTIS ISSN 0347–2124.
Karol, M.H., S.Taskar, S.Gangal, B.F.Rubanoff, and S.R.Kamat. 1987. The antibody response to methyl isocyanate: Experimental and clinical findings. Environ. Health Perspect. 72:169–175.
Kennedy, A.L., G.Singh, Y.Alarie, and W.E.Brown. 1993. Autoradiographic analyses of guinea pig airway tissues following inhalation exposure to 14C-labeled methyl isocyanate. Fund. Appl. Toxicol. 20:57–67.
Khurrum, M.A., and S.H.Ahmad. 1987. Long term follow up of ocular lesion of methyl-isocyanate gas disaster in Bhopal. Ind. J. Ophthalmol. 35:136–137.
Kimmerle, G., and A.Eben. 1964. On the toxicity of methyl isocyanate and its quantitative determination in air [in German]. Arch. Toxikol. 20:235–241.
Kirk-Othmer. 1994. Encyclopedia of Chemical Technology, 4th Ed., pp. 435–436. New York: John Wiley & Sons.
Kligerman, A.D., J.A.Campbell, G.L.Erexson, J.W.Allen, and M.D.Shelby. 1987. Sister chromatid exchange analysis in lung and peripheral blood lymphocytes of mice exposed to methyl isocyanate by inhalation. Environ. Mutagen. 9:29–36.
Kolb, W.P., J.R.Savary, C.M.Troup, D.E.Dodd, and J.D.Tamerius. 1987. Biological effects of short-term, high-concentration exposure to methyl isocyanate.
VI. In vitro and in vivo complement activation studies. Environ. Health Perspect. 72:189–195.
Lorin, H.G., and P.E.J.Kulling. 1986. The Bhopal tragedy—what has Swedish disaster medicine planning learned from it? J. Emerg. Med. 4:311–316.
Luster, M.I., A.N.Tucker, D.R.Germolec, M.T.Silver, P.T.Thomas, S.J.Vore, and J.R.Bucher. 1986. Immunotoxicity studies in mice exposed to methyl isocyanate. Toxicol. Appl. Pharmacol. 86:140–144.
Maginness, L.A., J.M.Szewczak, and C.M.Troup. 1987. Biological effects of short-term, high-concentration exposure to methyl isocyanate. IV. Influence on the oxygen-binding properties of guinea pig blood. Environ. Health Perspect. 72:35–38.
ManTech Environmental (ManTech Environmental Technology, Inc). 1992. Acute 1-hour inhalation toxicity study of methyl isocyanate in rats. Project No. 6030– 003. Sponsored by Rhone-Poulenc Ag Company. EPA/OTS; Doc #86– 920000962. 25 pp.
Maskati, Q.B. 1986. Ophthalmic survey of Bhopal victims 104 days after the tragedy. J. Postgrad. Med. 32:199–202.
Mason, J.M., E.Zeiger, S.Haworth, J.Ivett, and R.Valencia. 1987. Genotoxicity studies of methyl isocyanate in Salmonella, Drosophila, and cultured Chinese hamster ovary cells. Environ. Mutagen. 9:19–28.
Mellon Institute. 1963a. The feasibility of using methyl isocyanate as a warning agent in liquid carbon monoxide. Report 26–23. Sponsored by Union Carbide Chemicals, Co. EPA/OTS; Doc #86–910000271. 5 pp.
Mellon Institute. 1963b. Range finding tests on methyl isocyanate. Report 26–75. Sponsored by Union Carbide Chemicals, Co. EPA/OTS; Doc #86–910000658. 6 pp.
Mellon Institute. 1966. Miscellaneous acute toxicity data. Report 28–141. Sponsored by Union Carbide Chemicals, Co. EPA/OTS; Doc #86–910000267. 2 pp.
Mellon Institute. 1970. Acute inhalation toxicity, human response to low concentrations, guinea pig sensitization, and cross sensitization to other isocyanates. Report 33–19. Sponsored by Union Carbide Chemicals, Co. EPA/OTS; Doc #86–910000268. 8 pp.
Ministry of Social Affairs and Employment (SDU Uitgevers). 2000. Nationale MAC (Maximum Allowable Concentration) List, 2000. The Hague, The Netherlands.
Mishra, A., G.S.D.Gupta, V.S.Kumar, K.K.Dutta, and P.K.Ray. 1988. Inhalation toxicity studies of methyl isocyanate (MIC) in rats: Part III—Studies on biotransformation enzymes. Ind. J. Exp. Biol. 26:187–190.
Misra, N.P., R.Pathak, K.J.B.S.Gaur, S.C.Jain, S.S.Yesikar, P.C.Manoria, K.N. Sharma, B.M.Tripathi, B.S.Asthana, H.H.Trivedi, V.K.Sharma, Y. Malhotra, A.Verma, D.K.Bhargava, and G.Batni. 1987. Clinical profile of gas leak victims in acute phase after Bhopal episode. Indian J. Med. Res. 86:11–19.
Misra, U.K. and J.Kalita. 1997. A study of cognitive functions in methyl-isocyanate victims one year after Bhopal accident. Neurotoxicol. 18:381–386.
Mitsumori, K., G.A.Boorman, B.N.Gupta, and J.R.Bucher. 1987. Four-day repeated inhalation and recovery study of methyl isocyanate in F344 rats and B6C3F1 mice. Fund. Appl. Toxicol. 9:480–495.
Naik, S.R., V.N.Acharya, R.A.Bhalerao, S.S.Kowli, H.Nazareth, A.A.Mahashur, S.Shah, A.V.Potnis, and A.C.Mehta. 1986. Medical survey of methyl isocyanate gas affected population of Bhopal. Part 1: General medical observations 15 weeks following exposure. J. Postgrad. Med. 32:175–184.
Nemery, B., S.Dinsdale, S.Sparrow, and D.E.Ray. 1985a. Effects of methyl isocyanate on the respiratory tract of rats. Br. J. Ind. Med. 42:799–805.
Nemery, B., S.Sparrow, and D.Dinsdale. 1985b. Methyl isocyanate: Thiosulphate does not protect. Lancet 2:1245–1246.
NIOSH (National Institute for Occupational Safety and Health). 1996. Methyl Isocyanate. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs). NIOSH, Cincinnati, OH. Last updated 8/16/96.
NIOSH (National Institute for Occupational Safety and Health). 1997. NIOSH Pocket Guide to Chemical Hazards. NIOSH, Cincinnati, OH.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
OSHA (Occupational Safety and Health Administration). 1995. Table Z-1. Limits for Air Contaminants. CFR 29 Part 1910, p. 14.
Pant, S.C., R.K.Srivastava, and R.Vijayaraghavan. 1987. Histomorphologic changes induced by methyl isocyanate in lungs of rats and rabbits. Bull. Environ. Contam. Toxicol. 38:876–881.
Raizada, J.K., and P.C.Dwivedi. 1987. Chronic ocular lesions in Bhopal gas tragedy. Ind. J. Ophthalmol. 35:453–455.
Salmon, A.G., M.Kerr Muir, and N.Andersson. 1985. Acute toxicity of methyl isocyanate: A preliminary study of the dose response for eye and other effects. Br. J. Ind. Med. 42:795–798.
Saxena, A.K., K.P.Singh, K.K.Dutta, G.S.D.Gupta, S.L.Nagale, A.Mishra, S.I.A.Zaidi, and P.K.Ray. 1988. Inhalation toxicity studies of methyl isocyanate (MIC) in rats: Part V—Immunologic response of rats two weeks after exposure: Phagocytic response, endotoxin susceptibility, local lung immunity, mitogenic and DTH response. Ind. J. Exp. Biol. 26:195–200.
Schwetz, B.A., B.Adkins, Jr., M.Harris, M.Moorman, and R.Sloane. 1987. Methyl isocyanate: Reproductive and developmental toxicology studies in Swiss mice. Environ. Health Perspect. 72:149–152.
Sethi, N., R.Dayal, and R.K.Singh. 1989. Acute and subacute toxicity study of
inhaled methyl isocyanate in Charles Foster rats. Ecotoxicol. Environ. Safety 18:68–74.
Shelby, M.D., J.W.Allen, W.J.Caspary, S.Haworth, J.Ivett, A.Kligerman, C.A. Luke, J.M.Mason, B.Myhr, R.R.Tice, R.Valencia, and E.Zeiger. 1987. Results of in vitro and in vivo genetic toxicity tests on methyl isocyanate. Environ. Health Perspect. 72:183–187.
Shepard, T.H. (Ed.) 1995. Catalog of Teratogenic Agents, 8th Ed. Baltimore: The Johns Hopkins University Press. 274 pp.
Singh, R.K., R.Dayal, A.K.Srivastava, and N.Sethi. 1994. Teratological studies on methyl isocyanate in Charles Foster rats (part I). Biol. Memoirs 20:117– 123.
Sriramachari, S., and K.Jeevaratnam. 1994. Comparative toxicity of methyl isocyanate and its hydrolytic derivatives in rats. II. Pulmonary histopathology in the subacute and chronic phases. Arch. Toxicol. 69:45–51.
Stevens, M.A., S.Fitzgerald, M.G.Ménache, D.L.Costa, and J.R.Bucher. 1987. Functional evidence of persistent airway obstruction in rats following a two-hour inhalation exposure to methyl isocyanate. Environ. Health Perspect. 72:89–94.
ten Berge, W.F., A.Zwart, and L.M.Appleman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Mat. 13:301–309
Tepper, J.S., M.J.Wiester, D.L.Costa, W.P.Watkinson, and M.F.Weber. 1987. Cardiopulmonary effects in awake rats four and six months after exposure to methyl isocyanate. Environ. Health Perspect. 72:95–103.
Troup, C.M., D.E.Dodd, E.H.Fowler, and F.R.Frank. 1987. Biological effects of short-term, high-concentration exposure to methyl isocyanate. II. Blood chemistry and hematologic evaluations. Environ. Health Perspect. 72:21–28.
Union Carbide (Union Carbide Corporation). 1966. Compilation of toxicology on methyl isocyanate—TSCA informational submittal with cover letter dated 121784. EPA/OTS; Doc #88–8500718. 189 pp.
Union Carbide (Union Carbide Corporation). 1973. MIC—Sensitization characteristics with cover sheets and letter dated 012591. EPA/OTS; Doc #86– 910000666D.
Uraih, L.C., F.A.Talley, K.Mitsumori, B.N.Gupta, J.R.Bucher, and G.A. Boorman. 1987. Ultrastructural changes in the nasal mucosa of Fischer 344 rats and B6C3F1 mice following an acute exposure to methyl isocyanate. Environ. Health Perspect. 72:77–88.
Varma, D.R. 1987. Epidemiological and experimental studies on the effects of methyl isocyanate on the course of pregnancy. Environ. Health Perspect. 72:153–157.
Varma, D.R. 1989. Hydrogen cyanide and Bhopal. Lancet 2:567–568.
Varma, D.R., J.S.Ferguson, and Y.Alarie. 1988. Inhibition of methyl isocyanate toxicity in mice by starvation and dexamethasone but not by sodium thiosulfate, atropine, and ethanol. J. Toxicol. Environ. Health 24:93–101.
Varma, D.R., and I.Guest. 1993. The Bhopal accident and methyl isocyanate toxicity. J. Toxicol. Environ. Health 40:513–529.
Varma, D.R., I.Guest, S.Smith, and S.Mulay. 1990. Dissociation between maternal and fetal toxicity of methyl isocyanate in mice and rats. J. Toxicol. Environ. Health 30:1–14.
Vijayan, V.K., and K.Sankaran. 1996. Relationship between lung inflammation, changes in lung function and severity of exposure in victims of the Bhopal tragedy. Eur. Respir. J. 9:1977–1982.
Vijayaraghavan, R., and M.P.Kaushik. 1987. Acute toxicity of methyl isocyanate and ineffectiveness of sodium thiosulphate in preventing its toxicity. Ind. J. Exp. Biol. 25:531–534.
Weill, H. 1988. Disaster at Bhopal: The accident, early findings and respiratory health outlook in those injured. Physiology 23:587–590.
APPENDIX A
Time-Scaling Calculations
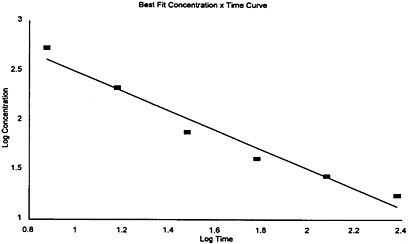
Data: LC50 Values in the Rat (Mellon Institute 1970)
|
Time (min) |
Concentration (ppm) |
Log Time |
Log Concentration |
|
7.5 |
541 |
0.8751 |
2.7332 |
|
15 |
216 |
1.1761 |
2.3345 |
|
30 |
76.6 |
1.4771 |
1.8842 |
|
60 |
41.3 |
1.7782 |
1.6160 |
|
120 |
27.4 |
2.0792 |
1.4378 |
|
240 |
17.5 |
2.3802 |
1.2430 |
|
Regression Output: |
|
|
Intercept |
3.4828 |
|
Slope |
−0.9880 |
|
R Squared |
0.9642 |
|
Correlation |
−0.9818 |
|
Observations |
6 |
|
n=1.01 |
|
|
k=3351.62 |
|
APPENDIX B
Derivation of AEGL Values
Derivation of AEGL-1
An AEGL-1 was not recommended because the irritation threshold for MIC may be above the level of concern for systemic effects such as spontaneous abortion and fetal or infant death. It is not known at what concentration the risk for these systemic effects becomes a concern. The concentrations causing irritation in humans after several minutes (1–4 ppm) are similar to, or higher than, the concentrations resulting in embryo and fetal lethality in well conducted animal studies. Therefore, it should be noted that exposures to MIC at concentrations below those used to calculate AEGL-1 might be associated with systemic toxicity. The absence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects.
Derivation of AEGL-2
|
Key Studies: |
Varma (1987) and Tepper et al. (1987) |
|
Toxicity end point: |
Decreased fetal body weights in offspring from mice exposed at 2 ppm for 3 h on GD 8 and cardiac arrhythmias in rats exposed at 3 ppm for 2 h; the concentrations were reduced by a factor of 3 to estimate a threshold for effects. |
|
Scaling: |
C1×t=k (based on regression analysis of LC50 values in the rat). |
|
Uncertainty factors: |
Total uncertainty factor: 30 Interspecies: 3. A factor of 3 was applied for interspecies variation because similar results have been obtained in both rats and mice. Intraspecies: 10. A factor of 10 was applied for intra- |
|
|
species variation because the mechanism of action for developmental toxicity is unknown. |
|
Calculations: |
(C1/uncertainty factors)×t=k |
|
|
([0.67 ppm]/30)1×3 h=0.067 ppm·h; or ([1 ppm]/30)1×2 h=0.067 ppm·h |
|
10-min AEGL-2: |
([0.067 ppm·h]/0.167 h)1=0.40 ppm |
|
30-min AEGL-2: |
([0.067 ppm·h]/0.5 h)1=0.13 ppm |
|
1-h AEGL-2: |
([0.067 ppm·h]/1 h)1=0.067 ppm |
|
4-h AEGL-2: |
([0.067 ppm·h]/4 h)1=0.017 ppm |
|
8-h AEGL-2: |
([0.067 ppm·h]/8 h)1=0.008 ppm |
Derivation of AEGL-3
|
Key Study: |
Schwetz et al. (1987) |
|
Toxicity end point: |
Experimentally derived NOEL for pup mortality during lactation in mice exposed at 1 ppm for 6 h on gestation days 14–17 |
|
Scaling: |
C1×t=k (based on regression analysis of LC50 values in the rat). |
|
Uncertainty factors: |
Total uncertainty factor: 30 Interspecies: 3. A factor of 3 was applied for interspecies variation because similar results have been obtained in both rats and mice. Intraspecies: 10. A factor of 10 was applied for intraspecies variation because the mechanism of action for developmental toxicity is unknown. |
APPENDIX C
DERIVATION SUMMARY FOR ACUTE EXPOSURE GUIDELINE LEVELS FOR METHYL ISOCYANATE (CAS No. 624–83–9)
|
AEGL-1a |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
NR |
NR |
NR |
NR |
NR |
|
aExposure to MIC at concentrations below those used to calculate AEGL-1 may be associated with systemic toxicity Abbreviation: NR, not recommended. |
||||
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.40 ppm |
0.13 ppm |
0.067 ppm |
0.017 ppm |
0.008 ppm |
|
Key references: |
(1) Varma, D.R. 1987. Epidemiological and experimental studies on the effects of methyl isocyanate on the course of pregnancy. Environ. Health Perspect. 72:153–157. (2) Tepper, J.S., M.J.Wiester, D.L.Costa, W.P.Watkinson, and M.F.Weber. 1987. Cardiopulmonary effects in awake rats four and six months after exposure to methyl isocyanate. Environ. Health Perspect. 72:95–103. |
|||
|
Test species/strain/number: Female mouse/Swiss-Webster/11–24; male rat/F344/number not stated. |
||||
|
Exposure route/concentrations/durations: Inhalation at 2, 6, 9, or 15 ppm for 3 h on GD 8 (2 ppm was determinant for AEGL-2). Inhalation at 3, 10, or 30 ppm for 2 h (3 ppm was determinant for AEGL-2). |
||||
|
Effects: 2, 6, 9, and 15 ppm, reduced fetal body weights; 9 and 15 ppm, increases in complete litter resorption and maternal mortality; 30 ppm, mortality of all animals; 10 ppm, increased minute ventilation during CO2 challenge and increased wet and dry lung weights 4 mo after exposure; 3 and 10 ppm, increase in cardiac arrhythmias 4 mo after exposure. |
||||
|
End point/concentration/rationale: Reduced fetal body weight and increased cardiac arrhythmias; experimental concentration reduced by a factor of 3 to estimate a threshold for effects. |
||||
|
Uncertainty factors/rationale: Total uncertainty factor: 30 Interspecies: 3—developmental toxicity did not vary greatly among species. Intraspecies: 10—the mechanism of developmental toxicity is unknown. |
||||
|
Modifying factor: None |
||||
|
Animal to human dosimetric adjustment: Insufficient data |
||||
|
Time scaling: C1×t=k |
||||
|
Data quality and support for the AEGL values: AEGL-2 values for MIC were based on two well conducted animal studies and are supported by human data. |
|
AEGL-3 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
1.2 ppm |
0.40 ppm |
0.20 ppm |
0.05 ppm |
0.025 ppm |
|
Key reference: |
Schwetz, B.A., Adkins, B., Jr., Harris, M., Moorman, M., and Sloane, R. 1987. Methyl isocyanate: reproductive and developmental toxicology studies in Swiss mice. Environ. Health Perspect. 72:149–152. |
|||
|
Test species/strain/number: Mouse/Swiss (CD-1)/39–44 |
||||
|
Exposure route/concentrations/durations: Inhalation at 1 or 3 ppm for 6 h/d on gestation days 14–17 (1 ppm was determinant for AEGL-3). |
||||
|
Effects: 3 ppm, decreased pup survival during lactation day 0–4; 1 ppm, no effects on pup survival. |
||||
|
End point/concentration/rationale: No effect level for pup mortality |
||||
|
Uncertainty factors/rationale: Total uncertainty factor: 30 Interspecies: 3—developmental toxicity did not vary greatly among species. Intraspecies: 10—the mechanism of developmental toxicity is unknown. |
||||
|
Modifying factor: none |
||||
|
Animal to human dosimetric adjustment: Insufficient data |
||||
|
Time Scaling: C1×t=k |
||||
|
Data quality and support for the AEGL values: AEGL-3 values for MIC were based on a well conducted animal study and supported by other animal data. |
||||