4
Diborane1
Acute Exposure Guideline Levels
SUMMARY
Diborane (CAS Registry No. 19287–45–7) is a highly unstable gas, and it is combustible upon exposure to moist air or high heat. The presence of some contaminants may lower the ignition temperature to at or below room temperature. Because of its strong reducing character, it has many industrial uses; it can be used as a rubber vulcanizer, as a catalyst for olefin polymerization, as an intermediate in the production of other boron hy-
drides, and as a doping gas in the semiconductor industry. Diborane was also investigated in the 1950s as a potential rocket fuel.
Data on acute exposures of humans to diborane were limited to case reports of accidental work-related exposures. Signs and symptoms of exposure included chest tightness, shortness of breath and dyspnea, wheezing, nonproductive cough, and precordial pain. Workers exposed to diborane generally experienced a complete recovery within a short period following cessation of exposure. No quantitative information was given regarding the exposure terms of these individuals, and the data were therefore unsuitable for derivation of AEGLs. No reports of human fatalities after diborane exposure were found in the literature. Reported odor thresholds range from 1.8 parts per million (ppm) to 3.6 ppm.
Data on lethal and nonlethal consequences of diborane exposure were available for several animal species, including dogs, rats, mice, hamsters, rabbits, and guinea pigs. Fifteen-minute LC50 values in rats ranged from 159 ppm to 182 ppm, and 4-hour (h) LC50 values ranged from 40 ppm to 80 ppm in rats and 29 ppm to 31.5 ppm in mice. Animals exposed to lethal and nonlethal concentrations developed pulmonary hemorrhage, congestion, and edema, and death was related to these severe pulmonary changes. Recent studies in rats and mice have also uncovered the development of multifocal and/or diffuse inflammatory epithelial degeneration in the bronchioles following exposure to diborane. These pulmonary changes produced by exposure to nonlethal concentrations were completely reversible in rats by 2 weeks (wk) after an acute exposure and were being repaired in the mouse by 2 wk postexposure. The signs of toxicity and repair of pulmonary lesions following acute exposure to nonlethal concentrations in animals were similar to the human case reports. It is likely that the mechanism of toxicity is due to direct interaction of diborane with cellular components, especially because diborane is such a potent reducer. There appears to be a similar mechanism of toxicity among species, because the cause of death from diborane exposure has always been from pulmonary damage, including edema, hemorrhage, and congestion. Mice appeared to be the more sensitive species, and the mice data were therefore used for the derivations of AEGLs.
An AEGL-1 value was not recommended because the AEGL-2 value is below the odor threshold of diborane and no other data pertaining to end points relevant to AEGL-1 definition were available. Absence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects.
The AEGL-2 values were based on reversible histological changes in the lungs in male ICR mice following a 2-h acute inhalation exposure to diborane at 5 ppm. No effects were observed in mice exposed at 5 ppm for 1 h, and exposure at 5 ppm for 2 h resulted in 4/10 mice developing multifocal and/or diffuse inflammatory epithelial degeneration in the bronchioles (Nomiyama et al. 1995). Studies have demonstrated that these lesions are reversible. There were no other treatment-related changes, such as changes in behavior, appearance, body or organ weight, or hematological or clinical chemistry indices. A total uncertainty factor (UF) of 10 was applied to the AEGL-2 value. An interspecies UF of 3 was applied because the most sensitive species, the mouse, was used and the end point of toxicity, reversible histological changes in the lungs, was the most sensitive end point. Further support for the UF of 3 is that signs of toxicity and repair of pulmonary lesions following acute exposure to nonlethal concentrations of diborane in animals were consistent with the human response reported by case reports. There appears to be a similar mechanism of toxicity among species because the cause of death from diborane exposure is due to acute pulmonary damage, including edema, hemorrhage, and congestion. An intraspecies UF of 3 was applied because using the default UF of 10 generates AEGL values that are inconsistent with existing empirical data. For example, the derived 1-h AEGL-2 value is 1.0 ppm with a total UF of 10. Mice exposed at 1 ppm for up to 8 h exhibited no effects of diborane exposure (Nomiyama et al. 1995). In addition, mice exposed at 0.7 ppm for 6 h/day (d), 5 d/wk for up to 4 wk developed only slight pulmonary infiltration of polymorphous neutrophils (Nomiyama et al. 1995) and rats exposed at 0.96 ppm for 6 h/d, 5 d/wk for 8 wk developed changes in bronchoalveolar lavage fluid that were not accompanied by histopathological changes (Nomiyama et al. 1996). The use of a higher UF would result in AEGL values that would be below concentrations causing effects in any species for an end point that is supposed to be disabling or cause irreversible effects in a human population.
The AEGL-3 values were based on the estimate of a 4-h LC01 of 9.2 ppm obtained by log-probit analysis of data from a 4-h LC50 study in male ICR mice (Uemura et al. 1995). A total UF of 10 was applied to the AEGL-3 value. An interspecies UF of 3 was applied because there did not appear to be much variation between species in sensitivity to lethal concentrations of diborane. The 4-h LC50 values determined by different authors for mice and rats were within a factor of 2.8 (4-h LC50 values ranged from
29 ppm to 31.5 ppm in mice and from 40 ppm to 80 ppm in rats). The lung was the target organ in all species tested, and the biological response remained the same, becoming more severe with increasing concentrations until death occurred from anoxia as a consequence of severe pulmonary changes. An intraspecies UF of 3 was applied because using the default UF of 10 generates AEGL values that are inconsistent with existing empirical data. For example, the derived 1-h AEGL-3 value is 3.7 ppm with a total UF of 10. Mice exposed at 5 ppm for up to 4 h developed only inflammatory epithelial degeneration in the bronchioles, with exposure for 8 h resulting in increased lung weights (Nomiyama et al. 1995). Mice exposed at 15 ppm for 4 h developed pulmonary changes, including edema, congestion, and inflammatory epithelial degeneration, that were generally resolved or in the process of being resolved within 14 d postexposure (Uemura 1996). The use of a higher UF would result in AEGL values that would be below concentrations causing effects in any species for an end point which is supposed to be life-threatening in a human population.
The derived AEGL values were scaled to 10-minute (min), 30-min, 1 -h, 4-h, and 8-h exposures using Cn×t=k. To calculate n for diborane, a regression plot of the EC50 values was derived from the studies by Nomiyama et al. (1995) and Uemura et al. (1995) investigating 1-, 2-, and 4-h exposures at 1, 5, or 15 ppm, with multifocal and/or diffuse inflammatory epithelial degeneration in the bronchioles as the end point of toxicity. Although n values have generally been derived using lethality data, it was considered appropriate in this case to use the nonlethal pulmonary changes. Toxicity studies demonstrated that the lung remained the target organ at all concentrations of exposure, and the biological response remained the same, becoming more severe with increasing concentration until death occurred from anoxia as a consequence of severe pulmonary changes. From the regression analysis, the derived value of n=1 was used in the temporal scaling of all the AEGL values (C1×t=k; Haber’s law). The 10-min AEGL-3 value was set equal to the 30-min value of 7.3 ppm because the NAC considers it inappropriate to extrapolate from the exposure duration of 4 h to 10 min. Although it is considered appropriate to extrapolate from a 2-h exposure to a 10-min exposure duration in the AEGL-2 derivation, the 10-min value of 6.0 ppm would approach that of the 10-min AEGL-3 value of 7.3 ppm. Therefore, the 10-min AEGL-2 value was set equal to the 30-min value. The AEGL values are listed in the table below.
TABLE 4–1 Summary of AEGL Values for Diborane
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1 (Nondisabling) |
NRa |
NR |
NR |
NR |
NR |
Not recommended because the AEGL-2 value is below the odor threshold, and no other data pertaining to end points relevant to the AEGL-1 definition were available |
|
AEGL-2 (Disabling) |
2.0 ppm (2.2 mg/m3) |
2.0 ppm (2.2 mg/m3) |
1.0 ppm (1.1 mg/m3) |
0.25 ppm (0.28 mg/m3) |
0.13 ppm (0.14 mg/m3) |
LOAEL for pulmonary changes in male ICR mice; 5 ppm for 2 h (Nomiyama et al. 1995) |
|
AEGL-3 (Lethality) |
7.3 ppm (8.0 mg/m3) |
7.3 ppm (8.0 mg/m3) |
3.7 ppm (4.1 mg/m3) |
0.92 ppm (1.0 mg/m3) |
0.46 ppm (0.51 mg/m3) |
4-h LC01 of 9.2 ppm estimated from a 4-h LC50 in male ICR mice (Uemura et al. 1995) |
|
aAbsence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects. Abbreviation: NR, not recommended. |
||||||
1. INTRODUCTION
Although the boron hydrides were first described in 1879, their possible uses were not investigated until the military became interested in their potential for use as rocket fuels in the 1950s (Rozendaal 1951; Stumpe 1960). The three most studied boron hydrides were pentaborane, a liquid, decaborane, a solid, and diborane, a gas. Diborane is highly unstable and can spontaneously combust at temperatures of 40–50 °C. The presence of contaminants may lower the ignition temperature to at or below room temperature (Budavari et al. 1996). It rapidly hydrolyzes in water to produce boric acid, hydrogen, and heat. Because of its strong reducing character, diborane has many industrial uses; it is used as a rubber vulcanizer, a catalyst for olefin polymerization, an intermediate in preparation of other boron hydrides, and a doping gas in the semiconductor industry (Budavari et al. 1996). “Certain base adducts of borane, BH3, such as (C2H5)3N.BH3 [1722– 26–5], (CH3)2S.BH3 [13292–87–0], tetrahydrofuranborane [14044–65–6], and C4H8O.BH3 are more easily and safely handled than B2H6 and are commercially available. They find wide use as reducing agents and in hydroboration reactions” (Rudolph 1978). Currently, diborane is one of the most used speciality gases in the semiconductor industry in Japan (655 kg consumed in 1993), and its increasing usage has prompted more refined toxicity studies than were previously available in the literature (Nomiyama et al. 1996). Information on diborane production and use data in the United States is limited. Two companies in the United States are listed as producing diborane: one having the capacity to produce 45 metric tons per year, and the other producing diborane on demand. Dopants in general, including boron trifluoride, diborane, arsine, and phosphine, were predicted to have a 9% average annual growth rate between 1994 and 1999 (Chemical Economics Handbook 1996). The physicochemical data of diborane are presented in Table 4–2.
The odor of diborane is described as repulsive and sickly sweet (Budavari et al. 1996). The median detectable odor concentration of diborane was determined to be 2–4 mg/m3 (1.8–3.6 ppm) (Krackow 1953) and 2.5 ppm (Amoore and Hautala 1983), which is above the occupational exposure limits set for this compound (ACGIH 1991, 1996). Toxicity data in humans were limited to case reports. Studies addressing lethal, nonlethal, and reproductive toxicity of diborane in experimental animals were available.
TABLE 4–2 Chemical And Physical Data
|
Parameter |
Value |
Reference |
|
Synonyms |
Boroethane, diboron hexahydride, boron hydride |
Budavari et al. 1996; ACGIH 1991 |
|
Molecular formula |
B2H6 |
Budavari et al. 1996 |
|
Molecular weight |
27.67 |
Budavari et al. 1996 |
|
CAS Registry Number |
19287–45–7 |
ACGIH 1991 |
|
Physical state |
Gas |
Budavari et al. 1996 |
|
Color |
Colorless |
Budavari et al. 1996 |
|
Solubility |
Hydrolyzes in water |
Budavari et al. 1996 |
|
Vapor pressure |
>1 atm at 20 °C 27,460 mm Hg (15 °C) |
ACGIH 1991; Lockheed Martin Energy Systems, Inc. 1988 |
|
Specific gravity (water=1) |
0.210 (15 °C) |
Budavari et al. 1996 |
|
Density (air=1) |
0.965 |
Braker and Mossman 1980 |
|
Melting point |
−165 °C |
Budavari et al. 1996 |
|
Boiling point |
−92 5 °C |
Budavari et al. 1996 |
|
Flammability limits |
Spontaneous ignition in air at 40– 50 °C |
Budavari et al. 1996 |
|
Conversion factors |
1 ppm=1.1 mg/m3 1 mg/m3 =0.91 ppm |
ACGIH 1996 |
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
LC50 values in humans have been reported to be 159 ppm for 15 min, and 30–90 mg/m3 (27–82 ppm) for 4 h (Braker and Mossman 1980;
Lockheed Martin Energy Systems, Inc. 1988). However, no references were cited and no information was given regarding the derivation of these values. These values are therefore inappropriate for use in derivations of AEGLs.
2.2. Nonlethal Toxicity
2.2.1. Case Reports
There are no studies in humans reporting the effects following exposures to known concentrations of diborane. The case reports in the literature concerning accidental workplace exposures provide some characterization of signs and symptoms associated with diborane poisoning. It is doubtful that workplace exposures were limited to diborane alone, but most likely included exposures to other chemicals such as the similar boron hydrides decaborane and pentaborane.
In 1957, Rozendaal summarized case reports of workers exposed to boron hydrides. One worker developed fatigue, shortness of breath, chills, and fever a few hours after diborane exposure. He was later diagnosed with “pneumonia” and treated with penicillin and made a complete recovery in 3 d. In this paper, Rozendaal compared the symptoms that developed following diborane exposure with those of “metal fume fever.”
Lowe and Freeman (1957) conducted a survey of dispensary records and laboratory data from 83 people who were potentially exposed to boron hydrides during a 3 y period. They noted that 2 out of 38 people exposed to diborane were hospitalized. Commonly reported symptoms of exposure included tightness, heaviness, and burning sensations of the chest, shortness of breath, a nonproductive cough, and precordial pain. Chest X-rays from the two hospitalized patients showed nonspecific infiltration, which cleared up in 1–2 d. Chronic exposures to low levels of diborane were associated with central nervous system-type symptoms, including lightheadedness, dizziness, vertigo, chills, and fever. Muscular weakness and fatigue were often noted but were generally gone by the next day. Tremors, which seldom occurred, were localized and of short duration.
Rousch (1959) described similar symptoms in workers exposed to diborane and commented that if exposed men were asked to describe their symptoms most referred to cough, chest tightness, and headache, adding that only a “few whiffs” of the gas were needed to develop the symptoms.
The symptoms tended to develop within a few minutes and generally lasted a few hours.
Cordasco et al. (1962) described the effects of boron hydride exposures recorded between 1956 and 1960. Of the 26 cases of acute diborane exposures, 18 exhibited respiratory problems, including chest tightness and pain, dyspnea, nonproductive cough, and wheezing, generally lasting from 3 to 5 d. Ten percent of the cases experienced nausea, anorexia, and hyper-salivation. There were 33 reported cases of subacute exposures, with 8 cases of respiratory involvement. Symptoms associated with exposure to low concentrations for longer periods included chest tightness, nonproductive cough, lightheadedness, headache, fatigue, and drowsiness. Inspiratory and expiratory rhonchi were the most prominent clinical findings during chest examinations of patients exposed both on an acute and subacute basis. Cordasco also recounted two case reports of acute exposures to diborane. One exposed worker developed breathing difficulty, severe tightness in the upper chest, weakness, and slight twitching of the hands, all of which continued for 2 h. Dyspnea and cough continued over the next 3 d, and rales were heard in both lungs. A chest X-ray showed infiltration in both lungs. Five days after exposure, the patient’s cough and dyspnea were gone, and his lungs were clear. Thirteen days later, the worker was again exposed to diborane, and he experienced severe shortness of breath and diffuse chest tightness. Examination indicated medium dry rales in the posterior bases of the lungs, and a chest X-ray showed “pneumonitis.” The patient was treated with penicillin and chloramphenicol, and he was asymptomatic 7 d later. A chest X-ray 3 wk after exposure showed a disappearance of the lesions. The second patient exposed to diborane immediately developed shortness of breath, vertigo, and dry cough. He was given oxygen for 20 min, after which he felt fine. He was again exposed to diborane 6 d later and developed a dry cough. He had moist rales at both bases of the lungs 7 d later, and an X-ray taken 9 d after the second exposure revealed pneumonitis in both bases. The patient received treatment with penicillin and isoproterenol and returned to normal shortly thereafter.
2.2.2. Epidemiology Studies
Epidemiologic studies regarding human exposure to diborane were not found in the available literature.
2.2.3. Other
Reported odor thresholds for diborane are 2–4 mg/m3 (1.8–3.6 ppm) (Krackow 1953) and 2.5 ppm (Amoore and Hautala 1983).
2.3. Developmental and Reproductive Effects
No human developmental and reproductive toxicity data concerning diborane were found in the available literature.
2.4. Genotoxicity
No human genotoxicity data on diborane were found in the available literature.
2.5. Carcinogenicity
No data were found in the available literature regarding the carcinogenic potential of diborane.
2.6. Summary
While there were several case reports describing the effects of diborane exposure in humans, exposure durations and concentrations were missing, and it was doubtful that workplace exposures were limited to just diborane. Commonly reported signs and symptoms associated with acute diborane exposure included chest tightness, nonproductive cough, dyspnea, precordial pain, fatigue, and wheezing. The symptoms developed shortly after exposure, and generally disappeared within a week. Three patients showed signs of apparent “pneumonitis” or “pneumonia” and experienced a complete recovery. Repeated and chronic exposures produced signs and symptoms such as headache, lightheadedness, fatigue, dizziness, chest tightness, and cough. No carcinogenicity, reproductive, or developmental toxicity data in humans were available.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Dogs
A dog anesthetized by intravenous administration of pentobarbital sodium was exposed to diborane at 350 ppm for 15 min by intratracheal cannulation (Kunkel et al. 1956). The concentration of diborane was calculated from the measured rates of air and diborane flow into a gas chamber, and the mixture was then delivered from the chamber to the animal by a polyethylene tube. The exposed dog’s blood pressure began to drop and its respiration and thoracic movements were increased within 5 min of gassing. The animal died 4 min after exposure ceased, by which time edema fluid was noted to be flowing from the tracheal cannula. A terminal ECG showed sinus bradycardia and increased T-wave voltage. Signs of pulmonary congestion, hemorrhage, and edema were found during necropsy. In addition, the liver was congested and casts were found in the renal tubules.
Using the same method of exposure, three more anesthetized dogs were exposed at 40–125 ppm for 2–2.5 h (Kunkel et al. 1956). Exposure to diborane increased intestinal peristalsis in all dogs and produced hyperactivity of the EEG in two of the three dogs. One of these dog’s EEG returned to normal, while the other dog exhibited depressed cortical activity followed by bradycardia and finally ventricular fibrillation leading to death. Pulmonary edema was found in the dog during necropsy. A second dog had died by the end of the experiment, showing gross evidence of pulmonary edema. The dogs used by Kunkel et al. were of mixed breed and gender.
Comstock et al. (1954) exposed male beagle dogs to diborane at 6 or 0.8–1.7 mg/m3 (5 or 0.7–1.5 ppm) in a gassing chamber for 6 h/d, 5 d/wk, for up to 6 months (mo). By the twenty-fifth exposure, there was 100% mortality (2/2) in the dogs exposed at 6 mg/m3 (5 ppm). The dogs developed respiratory distress as soon as the first exposure, and exhibited signs of respiratory infection by the ninth exposure. Pathological examination of one of the dogs revealed acute and chronic nasopharyngitis, acute tracheitis, chronic bronchitis and bronchopneumonia, and liver and kidney congestion. At the lower concentrations, death occurred in one of two dogs after 130 exposures. The dog that died exhibited hyperpnea and anorexia,
and necropsy of the other dog after 6 mo of exposure found no changes attributable to treatment.
3.1.2. Rats
Krackow (1953) reported the results of a 4-h LC50 study conducted in rats. Rats were exposed to diborane concentrations ranging from 45 ppm to 100 ppm for 4 h. The 4-h LC50 was determined to be approximately 50 ppm. Affected rats showed pulmonary edema and hemorrhage upon necropsy. The author did not give many details about how the study was conducted, such as the number and gender of the animals used, the methods of exposure, if the concentrations were measured or nominal, and the study duration.
Krackow (1953) also reported the results of a 15-min LC50 study in rats. The 15-min LC50 was approximately 175–200 mg/m3 (159–182 ppm), with death typically occurring within 2 h. Krackow then described the overall purpose of this experiment, which was to test the effectiveness of several therapeutic drugs on decreasing mortality following diborane exposure. The description of the study matches the study design and results of a paper by Kunkel et al. (1956), and is most likely the same study. Groups of 10 albino rats were exposed at approximately 175 ppm in a gas chamber for 15 min and then observed up to 10 d following exposure. Although some therapeutic drugs produced a slight decrease in mortality rate, all animals exposed to diborane had evidence of pulmonary edema and hemorrhage upon postmortem examination. In addition, casts were noted in the renal tubules. Death typically occurred within a few hours after exposure.
Comstock et al. (1954) investigated acute and chronic diborane exposures in male albino rats. To assess acute toxicity, groups of six male rats were exposed to diborane at 52–492 mg/m3 (47–446 ppm) in a glass jar for periods of time ranging from 60 to 240 min. No controls were used. The mortalities resulting from acute exposures are as follows: 2/6 rats exposed at 47 ppm for 240 min; 46/54 rats exposed at 60–140 ppm for 240 min; 22/24 rats exposed at 158–446 ppm for 60 min; 3/6 exposed at 159 ppm for 120 min; and 6/6 exposed at 228 ppm for 120 min. Exposed animals exhibited respiratory distress, and the animals exposed to the two highest concentrations of diborane (287 and 446 ppm) died within 2 h of exposure, experiencing difficulty in breathing and gasping before death. The remaining mortalities generally occurred within 5 d after exposure. Necropsy
revealed pulmonary congestion and edema with focal areas of hemorrhage. To assess chronic toxicity, 18 rats were exposed at 6 mg/m3 (5 ppm), and 20 rats were exposed at 0.8–1.7 mg/m3 (1–2 ppm) for 6 h/day, 5 d/wk, for up to 6 mo. Groups of 10 rats were used as controls. At the highest concentration (5 ppm), 17/18 exposed rats died. At the lower concentrations, death occurred in 5/10 rats exposed 21 times and in 5/10 rats exposed 60 times. Comstock reports that the deaths of the rats were distributed between the seventh and one hundred and thirteenth exposure. The only sign of toxicity in rats was mild rhinitis, and limited pathological examinations of the animals did not indicate any abnormalities. The Comstock et al. study has been criticized for its lack of control data (for the acute study), limited pathological data (especially the lack of changes in both rats and guinea pigs), and lack of evidence of the cause of mortality in the rats (EPA 1988).
Jacobson and Lawson (1962) investigated the effects of age and strain on the 4-h LC50 value in male rats following exposure to diborane in a gassing chamber. The two strains of rats investigated were a derived Wistar strain (CRDL) and rats from Edgewood Breeding Farms (EBF). The authors noted that the rats were affected by chronic murine pneumonia, which was a common affliction in laboratory rodents at that time. The first experiment determined that the 4-h LC50 values in 2-mo-old EBF rats and 5-mo-old CRDL rats were 40 and 80 ppm, respectively. The second experiment examined 2- and 5-mo-old EBF rats and 5-mo-old CRDL rats. These animals were exposed at 53 ppm for 4 h, and the LC50 values were derived using a slope function calculated from previous experiments. Although the authors felt this slope function was appropriate because of the identical slope functions observed in previous experiments, it is inappropriate to calculate an LC50 from a single exposure because there is too much uncertainty. The LC50 values were estimated to be 42, 65, and 74 ppm for 2- and 5-mo-old EBF rats and 5-mo-old CRDL rats, respectively. The LC50 values for the 2-mo-old EBF and 5-mo-old CRDL rats were comparable to those determined in the previous experiment. The authors concluded there was no significant difference in the sensitivity between the two strains of rats investigated, but the age of the animals did appear to have a substantial effect on susceptibility. The authors were not sure if this difference was an effect of age or weight. The authors also recorded signs of toxicity in exposed rats, which included labored breathing and froth from the nose. Necropsy showed edematous lungs in rats which were killed the day of exposure, but lungs from rats killed later appeared normal, suggesting that
the lesions were repaired. When edema was present, it was observed both macroscopically and microscopically, and was sometimes confined to peribronchial and perivascular lymphatic spaces.
3.1.3. Mice
Female mice from Carworth Farms (CF), approximately 2.5 to 3 mo old, were exposed to various concentrations of diborane in a dynamic exposure chamber for 4 h (actual exposure concentrations not provided) (Jacobson and Lawson 1962). They were observed for 14 d postexposure, and the total mortality rate over this period was used to calculate the 4-h LC50 of 29 ppm. The authors recorded signs of toxicity in the exposed mice, which included labored breathing and froth from the nose.
Groups of 10 4-wk-old male ICR mice were exposed to air containing measured concentrations of diborane at 0, 11.3, 22.1, 35.1, 37.7, or 44.8 ppm in a dynamic exposure chamber for 4 h (Uemura et al. 1995). A toxic gas monitor was used to measure the diborane chamber concentrations at 1-min intervals. The animals were observed for 2 wk postexposure. There was a concentration-response relationship between exposure levels and body-weight suppression (absolute values not provided). All treatment groups had a severe decrease in body weight for 3 d following exposure, returning to normal 4 or 5 d after exposure. The only exception was the high-concentration group, which rebounded 8 d after exposure. Mortality typically occurred within 24 h of exposure. The mortality rate in the study was 0/10, 3/10, 3/10, 9/10, and 9/10 at each concentration (11.3, 22.1, 35.1, 37.7, or 44.8 ppm, respectively), and the 4-h LC50 was calculated to be 31.5 ppm.
In the same paper, Uemura et al. (1995) investigated the effects of acute exposure in male ICR mice following exposure to diborane at 15 ppm for 1, 2, 4, or 8 h (10 mice per group). The mice were observed for 3 d and then sacrificed. One mouse from the 8-h exposure group died before the end of the study period. The histopathological examination showed findings consistent with pulmonary irritation, including mucous exudate, degeneration and necrosis of the epithelial lining, and inflammatory cellular infiltration into the nasal cavity.
3.1.4. Rabbits
Kunkel et al. (1956) exposed anesthetized rabbits (mixed breed and gender) by head exposure to an unknown concentration of diborane gas until the animals died. These rabbits were anesthetized by intravenous administration of approximately 30 mg pentobarbital sodium per kilogram body weight. The rabbits exhibited voluntary apnea upon first contact with the gas, eventually returning to normal respiration. There was a sharp decrease in blood pressure and bradycardia before death, leading to ventricular fibrillation or cessation of ventricular activity. Death was caused by pulmonary edema.
3.1.5. Hamsters
Separate groups of male and female golden hamsters were exposed to diborane at 50, 75, 100, 150, 200, 300, 400, 500, 600, 800, or 1,000 ppm in a dynamic exposure chamber, and control animals were exposed to filtered air (Stumpe 1960). The diborane was first mixed with nitrogen and then diluted with compressed air and fed into the exposure chamber. Diborane concentration was calculated using the measured flow rates of diborane and the diluents. The exposures were terminated when all of the animals in the respective group had died. The lungs were the target organ of diborane toxicity, and death was from pulmonary edema. The mean exposure time to death decreased with increasing diborane concentrations from 50 ppm to 600 ppm (from 497 min to 33 min, respectively). From 600 ppm to 1,000 ppm, the mean exposure time to death did not change, suggesting that the minimum time required for irreversible pulmonary changes leading to death was approximately 30 min. There was an earlier onset of toxicity with increasing diborane concentrations. At the beginning of the exposures, the animals huddled together and activity subsided. Soon after, the rate and depth of respiration in the animals were observed to increase, and the animals became restless and pawed at their faces. As the exposures progressed, the animals became increasingly active and would at times fall onto their sides or backs, but were able to right themselves until near death. When near death, the animals took deep, prolonged, and gasping breaths and had periods of apnea. A pinkish froth coming from the nares and mouths was noted in animals in the 500- to 1,000-ppm treatment groups.
Necropsy showed that changes were confined to the lungs. The color of the lungs was a bright red in exposed groups, progressing to a reddish brown in the higher exposed groups. No gross liver or kidney changes were noted. Microscopic examination of the lungs revealed capillary dilation and vascular congestion, edema, focal areas of atelectasis and peripheral emphysema, and sloughing of the mucosal epithelium of the bronchioles and bronchi with degeneration of mucosal cells. Pulmonary changes were more severe in the 500- to 1,000-ppm treatment groups. Vascular congestion, particularly of the glomerular capillaries, was found in the kidneys. The gender or weight of the animals did not appear to influence the sensitivity of the animal to diborane poisoning.
3.2. Nonlethal Toxicity
3.2.1. Dogs
Kunkel et al. (1956) exposed dogs (mixed breed and gender) anesthetized with pentobarbital sodium to various concentrations of diborane by intratracheal cannulation. The concentration of diborane was calculated from the measured rates of air and diborane flow into a gas chamber, and the mixture was then delivered from the chamber to the animal by a polyethylene tube. In one experiment, three dogs were exposed to diborane at 6–14 ppm for 45 min to 4 h. The results of testing during this exposure period showed normal electrocardiograms, no effects on blood pressure, and only slight increases in respiration; however, postmortem examination showed severe pulmonary hemorrhages and slight pulmonary edema. In another experiment, three dogs were exposed at 53–63 ppm for 15 min. The animals exhibited increased rate and depth of respiration. The dog exposed at 63 ppm showed a drop in blood pressure and bradycardia, whereas the other two dogs had normal electrocardiograms. Pulmonary congestion, hemorrhage, and some edema were found on necropsy. Other organs showed some congestion but otherwise appeared normal. Another dog was exposed at 5 ppm diborane for 4 h on one day and 3 h the next day. Near the end of the second exposure, the rate and depth of respiration increased and blood pressure decreased. It was noted that the dog appeared to be in shock after the second exposure, and the authors speculated that the prolonged period of anesthesia might have been the cause. Necropsy revealed pulmonary congestion and petechial hemorrhage in the medulla of the
kidneys. Lastly, one dog exposed at 125 ppm for 30 min showed increased peristalsis of the small intestine within minutes, and the EEG activity increased after 30 min of exposure. However, all activity had returned to normal within 1 h after exposure. The dog was killed 5 h after exposure, at which time there were no signs of pulmonary edema or cyanosis.
3.2.2. Rats
Nomiyama (1995) investigated the effects of acute inhalation exposure of diborane on bronchoalveolar lavage fluid (BALF) and blood parameters in male Wistar rats. Rats were placed in a dynamic exposure chamber, and diborane concentrations were measured at 1-min intervals with a toxic gas monitor. In the first phase of the study, groups of 10 rats (8-wk-old) were exposed at 20 ppm for 4 h and control rats were exposed to filtered room air. The rats were killed immediately, 1 d, 3 d, or 14 d after exposure. Eight rats per group were used to analyze changes in BALF and blood, and two rats per group were examined for pulmonary histopathological changes. The organs of all rats were weighed and examined grossly. No differences in behavior, external appearance, or body weight were observed during the study. There were only sporadic changes in the hematology and clinical chemistry of the animals, and these changes did not indicate any time-related trends. BALF analysis revealed that the proportion of neutrophils, activities of α1-antitrypsin and superoxide dismutase, and levels of total phospholipids were initially increased, but all returned to normal by 2 wk with the exception of α1-antitrypsin activity, which had decreased but was still statically elevated compared with controls. Histopathological examination showed infiltration of polymorphonuclear neutrophils into the bronchus the day of exposure, becoming milder 1 and 3 d after exposure. Multifocal and/or diffuse inflammatory epithelial degeneration in the respiratory bronchioles was observed 3 d after exposure, but these lesions were not detected 2 wk after exposure.
In the second phase of this study, Nomiyama (1995) investigated similar biological end points 3 d after a 4-h exposure to diborane at 0, 0.9, or 9.2 ppm (control rats were exposed to filtered room air). Each group contained 12 male Wistar rats (13-wk-old): 10 per group were used for BALF analysis and 2 per group were used for histopathological evaluation. The organs of all animals were weighed and examined grossly. The liver-to-body weight ratio was significantly decreased in the high-concentration
group (absolute value not provided). BALF analysis showed a number of changes, including concentration-related increases in total protein, α1-antitrypsin activity, and total phospholipids, increases in lactate dehydrogenase (LDH) and in the proportion of neutrophils and lymphocytes, and a concentration-dependent decrease in the proportion of macrophages. The activity of serum α1-antitrypsin was increased in the 9.2-ppm exposure group. Histopathological examination revealed inflammatory epithelial degeneration in the bronchioles in the high-concentration group.
Groups of 12 14-wk-old male Wistar rats were exposed at 0.11 or 0.96 ppm (control rats were exposed to filtered room air) for 8 wk (6 h/d, 5 d/wk) in dynamic exposure chambers (Nomiyama et al. 1996). Diborane concentration was measured at 1-min intervals using a toxic gas monitor. At the conclusion of the exposures, two rats per group were examined for histopathological lesions, while the other 10 per group were assigned to BALF analysis. The organs of all rats were weighed and examined grossly. The liver-to-body weight ratio was significantly decreased in the high-concentration group (absolute value not provided). BALF analysis indicated that the proportion of neutrophils and the levels of alkaline phosphatase increased with concentration, while the percentage of macrophages decreased in the 0.96-ppm group only. The high-concentration group also had a significant increase in the total and individual number of phospholipids and lactate dehydrogenase (LDH) activity. Analysis of the serum indicated increases in α1-antitrypsin and superoxide dismutase activities in both exposure groups. The changes measured in BALF and serum, which signified inflammation and cell damage, exhibited a concentration-dependent effect in the lungs. Despite changes in biochemical markers, however, there were no histopathological, behavioral, or external changes noted in any of the treatment groups, suggesting that the observed changes should be reversible following cessation of exposure to diborane.
Krackow (1953) summarized preliminary results of an experiment assessing chronic toxicity in rats (gender and strain not given) exposed at 1 to 4 mg/m3 (0.9–3.6 ppm) for 6 h/d, 5 d/wk. At that time, the experiment was in its fourth month. Pathological pulmonary changes, varying from congestion to pneumonia, were observed 1 to 4 wk after exposures started. Microscopic evaluation of pulmonary tissue showed round cell infiltration of the tracheal mucosa. Other organs examined sometimes showed congestion but were otherwise normal. No mortality had occurred at that time point. The paper discussing the final results of this study was not found in the available literature. Krackow also referred to repeated dose studies in
which rats (strain and gender not given) exposed at 6 mg/m3 (5.4 ppm) for 6 h/d, 5 d/wk, showed evidence of pulmonary damage in 2–3 wk.
3.2.3. Mice
Uemura et al. (1995) investigated acute effects of diborane on 5-wk-old male ICR mice. Ten mice per group were exposed in a dynamic exposure chamber to diborane at 15 ppm for 1, 2, 4, or 8 h or to filtered room air for 8 h. Diborane concentrations were measured at 1-min intervals with a toxic gas monitor. Actual exposure concentrations at the various time periods were 12.3, 13.1, 13.6, and 14.4 ppm, respectively. Mice were sacrificed 3 d after exposure. During the exposure periods, the mice in the treatment groups exhibited signs such as face washing movements and restlessness, and some mice in the 4- and 8-h groups had ruffled fur and systemic tremors. Upon termination of the experiment, mice from the 2-, 4-, and 8-h exposure groups had statistically decreased body weights (93%, 84%, and 86% of controls, respectively) and increased lung and trachea weights (125%, 129%, and 142% of controls, respectively). In addition, mice from the 4- and 8-h exposure groups had statistically decreased weights of the liver (83% and 79% of controls, respectively) and kidney (90% of controls). There were no hematological or clinical chemistry changes related to treatment. Histopathological examination demonstrated a time-response relationship for multifocal and/or diffuse inflammatory epithelial degeneration in the bronchioles. Clara cells with mitotic figures were frequently observed in the inflamed epithelium. The longer exposed mice had more frequent and severe cellular infiltration in the respiratory bronchioles, congestion, edema, and bleeding (see Table 4–3). The increasing severity of these lesions with time was probably due to direct contact of diborane with the epithelium. The one dead mouse from the 8-h exposure group had findings consistent with pulmonary irritation, including mucous exudate, degeneration and necrosis of the epithelial lining, and inflammatory cellular infiltration in the nasal cavity.
Groups of 10 5-wk-old male ICR mice were exposed to diborane at 15 ppm for 4 h and sacrificed immediately, 1 d, 3 d, or 2 wk after exposure, and controls were exposed to filtered room air for 4 h and sacrificed immediately or 2 wk after exposure (Uemura 1996). Exposures were conducted in a dynamic exposure chamber, and diborane concentrations were mea-
TABLE 4–3 Prevalence of Microscopic Lesions in Lungs of Mice Exposed at 15 ppm Diboranea
sured at 1-min intervals with a toxic gas monitor. Face-washing and restlessness were observed soon after the exposure started, and ruffled fur and hypoactivity were noted in some mice in the 3-d and 14-d postexposure groups. Immediately after diborane exposure, infiltration of polymorphonuclear neutrophils at the bronchiolus and some infiltration of macrophages into the alveoli were observed. Macrophage infiltration into the alveoli became more prominent 1 d and 3 d postexposure. Inflammatory epithelial degeneration, edema, and congestion were present in the lungs of all of the mice by 1 d postexposure, becoming more severe 3 d postexposure. Lung weight increased with the severity of the pulmonary lesions (see Table 4–4). Electron microscope analysis showed deposition of fine, fibrillar materials in the alveoli and bronchiolus immediately, with evidence of phagocytosis by macrophages 3 d after exposure. The nature of the fibrillar materials was unclear; boron atoms were not detected by disperse X-ray analysis. By 2 wk postexposure, the edema and congestion had almost completely subsided; however, lung weights were still significantly increased. Peribronchiolar thickening and infiltration of inflammatory cells into the bronchiolar walls were observed where inflammatory epithelial degeneration had previously been detected. Infiltration of lymphocytes into the subepi-
TABLE 4–4 Summary of Lung Weights and Prevalence of Microscopic Lesions in Lungs of Mice Exposed at 15 ppm Diborane for 4 Hoursa
|
|
Time After Exposure |
||||
|
End Point |
Control |
Immediate |
1 d |
3 d |
14 d |
|
Lung/body weight ratio |
0.57b 0.54c |
0.74e (130)d |
0.83e (146) |
0.8e (156) |
0.63e (111) |
|
Microscopic Finding |
|||||
|
PMN neutrophils at bronchiolus |
0 |
10 |
5 |
0 |
0 |
|
Macrophages in alveolus |
0 |
2 |
9 |
9 |
0 |
|
Inflammatory epithelial degeneration of the bronchioles |
0 |
0 |
10 |
10 |
0 |
|
Edema and congestion |
0 |
0 |
10 |
10 |
0 |
|
Peribronchiolar thickening |
0 |
0 |
0 |
0 |
10 |
|
Subepithelial inflammatory cellular infiltration |
0 |
0 |
0 |
0 |
10 |
|
aTotal of 10 animals per group. bControls sacrificed immediately after exposure. cControls sacrificed after 14 days. dNumber is parenthesis is the percentage of controls. eStatistically different from controls: p<0.01. Source: Data taken from Uemura 1996. |
|||||
thelial space of the bronchioles was observed 14 d postexposure. A summary of microscopic lesions observed in the lungs of exposed mice in presented in Table 4–4. There were no observed exposure-related changes in clinical chemistry indices, in the nasal cavity, or in the major bronchi.
Nomiyama et al. (1995) investigated the effects of acute and repeated diborane exposures in 5-wk-old male ICR mice. Exposures were conducted in an exposure chamber, and diborane concentrations were measured at 1-min intervals with a toxic gas monitor. For the acute study, groups of 10 mice were exposed at 1 or 5 ppm for 1, 2, 4, or 8 h, and control mice were exposed to filtered room air. Mice were sacrificed 3 d after exposure. There were no mortalities, behavioral or neurological signs, or changes in external appearance observed during the study, and no changes in hematol-
ogy or clinical chemistry were noted at termination. Absolute lung weight and lung weight relative to body weight was significantly increased in the group of mice exposed at 5 ppm for 8 h (119% and 117% of controls, respectively). Histopathological evaluation indicated a time-response relationship in the inflammatory epithelial degeneration in the bronchioles observed in the 5-ppm groups exposed for 2, 4, or 8 h (4/10, 9/10, and 10/10 animals affected, respectively, versus 0/10 for control and 1-h exposure group). No significant histopathological changes were noted in the groups of mice exposed at 1 ppm.
In a repeated-concentration inhalation exposure study by Nomiyama et al. (1995), groups of 10 mice were exposed to diborane at 0.2 or 0.7 ppm or to filtered room air for 2 or 4 wk (6 h/d, 5 d/wk) and were sacrificed the day after the last exposure. No differences in behavior or external appearance were observed during the study. There were no concentration-related changes in body weight gain or in clinical chemistry or hematology end points. An apparent concentration-response relationship was observed in the slight infiltration of polymorphous neutrophils noted in the 0.2- and 0.7-ppm exposure groups after a 2-wk and 4-wk exposure period. This infiltration was mainly found in the peribronchiolar region.
Uemura et al. (1995) investigated the effects of repeated inhalation exposures of diborane in 5-wk-old male ICR mice. Ten mice per group were exposed in a dynamic exposure chamber at 5 ppm for 6 h/d, 5 d/wk, for 2 or 4 wk, and control mice were exposed to filtered air for the same time periods. Diborane concentrations were measured at 1-min intervals with a toxic gas monitor. No deaths were observed during the study. The mice in the treatment groups exhibited similar but less frequent signs that were observed in an acute exposure study (face washing movements, restlessness, ruffled fur, and systemic tremor). The 4-wk treatment groups exhibited a slight decrease in body weight 2 and 4 d after exposure (94% and 95% of controls, respectively), and both treatment groups had significant increases in lung weight (164% and 204% of controls, respectively). There were no clinical chemistry changes related to treatment, and hematology changes were minimal. The respiratory histopathological lesions included mucous exudate and inflammatory cells in the nasal cavity, macrophage and plasma cells in the alveolus, lymphoid hyperplasia in the perivascular and peribronchial regions, and pulmonary congestion and edema. The observed hyperplasia and desquamation of Clara cells were more severe in the 4-wk exposure groups than in the 2-wk exposed groups.
3.2.4. Guinea pigs
Comstock et al. (1954) exposed 10 guinea pigs to diborane at 0.8–1.7 mg/m3 (0.7–1.5 ppm) for 6 h/d, 5 d/wk, for up to 6 mo. No control animals were used. All animals survived 95 exposures, and pathological examinations of the animals did not reveal any abnormalities. The Comstock et al. study has been criticized for its lack of control data, limited pathological data (especially the lack of changes in the rats and guinea pigs), and lack of evidence of the cause of mortality in the rats (EPA 1988).
3.3. Developmental and Reproductive Effects
Groups of 12 14-wk-old male Wistar rats were exposed at 0.11 or 0.96 ppm for 8 wk (6 h/d, 5 d/wk) in exposure chambers (Nomiyama et al. 1996). Diborane concentrations were measured at 1-min intervals using a toxic gas monitor. The animals were killed the day after the last exposure. Both testes from each animal were examined, and sperm from the head plus body and tail of the right epididymis were examined and counted. There were no significant findings in the testes from animals in any of the treatment groups (Nomiyama et al. 1996). A study by Shen et al. (1994, as cited in Uemura [1996] and Nomiyama et al. [1996]) found that mice exposed at 0.7 ppm for 4-wk developed sperm abnormalities and testicular toxicity.
3.4. Genotoxicity
The mutagenic potential of diborane was assessed on S. typhimurium TA98, TA100, TA1535, and TA1537 and E. coli WP2 uvrA following a 2 h exposure at 25 °C using a gas sampling bag. Diborane was diluted with helium in one gas sampling bag, and the appropriate bacterial plate was place in another gas sampling bag. To perform the exposure, the gas sampling bag containing the bacterial plate was filled with the appropriate concentration of the diluted gas. Diborane was mutagenic at concentrations of 0.2–0.5% (v/v; 2,000–5,000 ppm) to TA98 and TA100 with or without metabolic activation and to WP2 uvrA at a concentration of 0.5% (5,000 ppm) with metabolic activation (Araki et al. 1994).
3.5. Carcinogenicity
No data were found in the literature concerning the carcinogenic potential of diborane in animals.
3.6. Summary
Inhalation exposure lethality data were available for dogs, rats, mice, rabbits, and hamsters, and nonlethal toxicity data were available for dogs, rats, and mice (see tables 4–5 and 4–6, respectively). The 4-h LC50 values ranged from 40 ppm to 80 ppm in rats and from 29 ppm to 31.5 ppm in mice. Younger rats were more sensitive to lethality, having LC50 values of approximately 41 ppm compared with older rats’ range of 50–80 ppm. The lung was the target organ of diborane toxicity, and death was generally from pulmonary edema. Pathological changes resulting from acute diborane exposures included pulmonary edema, congestion, and hemorrhage. Recent studies in rats and mice have also uncovered the development of multifocal and/or diffuse inflammatory epithelial degeneration in the bronchioles following exposure to diborane. Pulmonary changes produced by exposure to nonlethal concentrations were completely reversible in rats by 2 wk after an acute exposure, and these lesions were being repaired in the mouse by 2 wk postexposure. These signs of toxicity and repair of pulmonary lesions following acute exposure to nonlethal concentrations in animals were similar to the human case reports. In addition to pulmonary changes, some laboratory animals also showed effects in the kidneys and liver.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Diborane quickly hydrolyzes in water to produce boric acid, hydrogen, and heat and should undergo the same reaction in the lungs. No studies were found in the literature specifically addressing the metabolism and disposition of diborane in humans or animals.
TABLE 4–5 Summary of Acute Lethal Inhalation Data in Laboratory Animals
|
Species |
Concentration (ppm) |
Exposure |
Time |
Effect Reference |
|
Dog |
350 |
15 min |
Death—pulmonary edema |
Kunkel et al. 1956 |
|
Dog |
40–125 |
2–2.5 min |
Death—pulmonary edema (2/3) |
Kunkel et al. 1956 |
|
Rat |
159–182 |
15 min |
Calculated LC50 |
Krackow 1953 |
|
Rat |
50 |
4 h |
Calculated LC50 |
Krackow 1953 |
|
Rata |
40, 42 |
4 h |
Calculated LC50 |
Jacobson and Lawson 1962 |
|
Ratb |
65 |
4 h |
Calculated LC50 |
Jacobson and Lawson 1962 |
|
Ratc |
80, 74 |
4 h |
Calculated LC50 |
Jacobson and Lawson 1962 |
|
Rat |
158–446 |
1 h |
Death (22/24) |
Comstock et al. 1954 |
|
Rat |
159 |
2 h |
Death (3/6) |
Comstock et al. 1954 |
|
Rat |
228 |
2 h |
Death (6/6) |
Comstock et al. 1954 |
|
Rat |
47 |
4 h |
Death (2/6) |
Comstock et al. 1954 |
|
Rat |
60–140 |
4 h |
Death (46/54) |
Comstock et al. 1954 |
|
Mouse |
29 |
4 h |
Calculated LC50 |
Jacobson and Lawson 1962 |
|
Mouse |
31.5 |
4 h |
Calculated LC50 |
Uemura et al. 1995 |
|
Mouse |
15 |
8 h |
Death—pulmonary damage (1/10) |
Uemura et al. 1995 |
|
Rabbit |
Unknown |
Until death |
Death—pulmonary edema |
Kunkel et al. 1956 |
|
Hamster |
50–1,000 |
Until death |
Death—pulmonary edema |
Stumpe 1960 |
|
aTwo month EBF rat. bFive month EBF rat. cFive month Wistar (CRDL) rat. |
||||
TABLE 4–6 Summary of Acute Nonlethal Inhalation Data in Laboratory Animals
|
Species |
Concentration (ppm) |
Duration |
Effectsa |
References |
|
Dogb |
6–14 |
45 min to 4 h |
No blood pressure effects, normal electrocardiograms, slightly increased respiration Postmortem: severe pulmonary hemorrhage and slight edema |
Kunkel et al. 1956 |
|
Dogb |
53–63 |
15 min |
Increased rate and depth of respiration, drop in blood pressure, bradycardia Postmortem: Pulmonary congestion, hemorrhage, edema; other organs showed congestion |
Kunkel et al. 1956 |
|
Dogb |
125 |
30 min |
Increased small intestine peristalsis and EEG; all activity returned to normal within 1 h. |
Kunkel et al. 1956 |
|
Rat |
0.9 9.2 |
4 h |
3 d postexposure BALF: Concentration-dependent increase in total protein, α1-antitrypsin activity, and total phospholipids; increases in LDH, neutrophils, and lymphocytes; concentration-dependent decrease in macrophages Serum: α1-antitrypsin activity increased in the 9.2 ppm group Histopathology: Inflammatory epithelial degeneration in the bronchioles in the 9.2 ppm group |
Nomiyama 1995 |
|
Species |
Concentration (ppm) |
Duration |
Effectsa |
References |
|
Rat |
20 |
4 h |
Immediately after, 1 d, 3 d, or 14 d postexposure: BALF: Neutrophils, total phospholipids, activities of α1-antitrypsin and superoxide dismutase initially increased; all returned to normal by 14 days postexposure except a significant decrease in α1-antitrypsin by 14 days Histopathology: Bronchial polymorphonuclear neutrophil infiltration immediately after exposure, becoming milder by 1 and 3 d postexposure; inflammatory epithelial degeneration in the bronchioles 3 d postexposure; returning to normal by 14 d postexposure |
Nomiyama 1995 |
|
Mouse |
1 |
1, 2, 4, 8 h |
3 d postexposure: No observable effects |
Nomiyama et al. 1995 |
|
Mouse |
5 |
1, 2, 4, 8 h |
3 d postexposure: Lung weight: Increased in 8-h group Histopathology: Time-response relationship for inflammatory epithelial degeneration (0/10, 4/10, 9/10, 10/10, respectively) |
Nomiyama et al. 1995 |
|
Mouse |
15 |
1, 2, 4, 8 h |
Observations: all groups—face washing, restlessness; 4-and 8-h groups—ruffled fur, systemic tremors 3 d postexposure: Weights: Liver, kidney, spleen, thymus, body weight |
Uemura et al. 1995 |
4.2. Mechanism of Toxicity
Although originally postulated that the production of boric acid from the hydrolysis of diborane was responsible for toxicity, lethal concentrations of diborane would produce only nonlethal levels of boric acid (Stumpe 1960). The half-life of diborane in a condition of saturated humidity at room temperature was reported to be 0.8–1.5 h (Nippon Sanso Co. 1986, as cited in Nomiyama et al. [1995]). It has been postulated that the heat produced by the highly exothermic reaction of diborane hydrolysis would produce local damage in the lungs (Nomiyama et al. 1995), although this has not yet been proven. It is likely that the mechanism of toxicity is due to direct interaction of diborane with cellular components, especially since diborane is such a potent reducer. There appears to be a similar mechanism of toxicity between species because the respiratory tract has consistently been the target organ, and the cause of death from diborane exposure has always been from pulmonary damage, including edema, hemorrhage, and congestion.
4.3. Structure-Activity Relationships
The use of structure-activity relationships was not necessary for derivation of inhalation exposure guidelines for diborane.
4.4. Other Relevant Information
4.4.1. Species Variability
There did not appear to be much variation between species in sensitivity to lethal concentrations of diborane, and the cause of death from these exposures was a consequence of pulmonary damage including congestion, hemorrhage, and edema. The 4-h LC50 values determined by different authors for mice and rats were all within a factor of 2.8 (ranging from 29 ppm to 80 ppm). Mice were more sensitive than rats in lethal and nonlethal studies.
4.4.2. Confounding Factors
Many acute inhalation experiments in animals were conducted in the 1950s and 1960s. A problem that was frequently encountered when using rodents as a test species during this time period was the prevalence of chronic murine pneumonia. This posed a problem when investigating the response following inhalation exposure to toxic compounds, because one could not be certain that the observed response was truly reflective of exposure to the toxicant. Indeed, Jacobson and Lawson (1962) pointed out that lack of subtle pulmonary changes in their study was probably a consequence of the infection. Fortunately, more recent studies have been conducted investigating the toxicity of diborane, thereby increasing the confidence in the data. Despite the presence of pneumonia in mice used in the study reported by Jacobson and Lawson (1962), the 4-h LC50 value for mice was the same as that of the study conducted by Uemura et al. (1995). When considering all of the 4-h LC50 values derived for mice and rats by the various groups over 42 y, the numbers vary only by a factor of 2.8.
4.4.3. Concentration-Exposure Duration Relationship
The experimentally derived exposure values are scaled to AEGL time frames using the concentration-time relationship given by the equation Cn×t=k, where C=concentration, t=time, and k is a constant. The values of the exponent n generally are in the range of 1–3.5 and “should always be derived empirically from acute inhalation toxicity experiments, in which both the concentration and exposure period are variables” (ten Berge 1986). To calculate n for diborane, a regression plot of the EC50 values was derived from the studies by Nomiyama et al. (1995) and Uemura et al. (1995) investigating 1-, 2-, and 4-h exposures at 1, 5, or 15 ppm, with multifocal and/or diffuse inflammatory epithelial degeneration in the bronchioles as the end point of toxicity (see Appendix B). Although n values have generally been derived using lethality data, it was considered appropriate in this case to use the nonlethal pulmonary changes. Toxicity studies demonstrated that the lung remained the target organ at all concentrations of exposure, and the biological response remained the same, becoming more severe with increasing concentration until death occurred from anoxia
as a consequence of severe pulmonary changes. From the regression analysis, the derived value of n=1 was used in the temporal scaling of all the AEGL values (C1×t=k; Haber’s law).
5. DATA ANALYSIS FOR AEGL-1
5.1. Human Data Relevant to AEGL-1
The only quantifiable human data available was the reported odor threshold range of 2–4 mg/m3 (1.8–3.6 ppm). Case reports of accidental workplace exposures did not contain information about exposure concentration and duration.
5.2. Animal Data Relevant to AEGL-1
There were no animal data relevant for the derivation of an AEGL-1.
5.3. Derivation of AEGL-1
An AEGL-1 value was not recommended because the AEGL-2 value is below the odor threshold of diborane and no other data pertaining to end points relevant to AEGL-1 definition were available. Absence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects.
6. DATA ANALYSIS FOR AEGL-2
6.1. Human Data Relevant to AEGL-2
No human data relevant to the derivation of the AEGL-2 were found in the available literature.
6.2. Animal Data Relevant to AEGL-2
Data relevant to the AEGL-2 were available based on several end
points of toxicity following acute exposure to diborane in animals. Nomiyama et al. (1995) and Uemura et al. (1995) found severe inflammatory epithelial degeneration in the bronchioles in mice 3 d following exposures to diborane at 5 ppm for 2, 4, or 8 h (4/10, 9/10, 10/10) and at 15 ppm for 1, 2, 4, or 8 h (8/10, 10/10, 10/10, and 10/10). Many of the mice exposed at 15 ppm also had other respiratory changes, including pulmonary bleeding, congestion, edema, and increased lung and tracheal weights (Uemura et al. 1995). In another study, mice exposed at 15 ppm for 4 h also developed inflammatory epithelial degeneration in the bronchioles by 3 d postexposure. These lesions were replaced by peribronchiolar thickening by 14 d postexposure, and infiltration of lymphocytes into the subepithelial space of the bronchioles was noted (Uemura 1995). Data on rats following acute inhalation exposure to diborane were also available; however, those data indicate that the mouse is a more sensitive model.
6.3. Derivation of AEGL-2
The AEGL-2 values were based on reversible histological changes in the lungs in male ICR mice following a 2-h acute inhalation exposure to diborane at 5 ppm. No effects were observed in mice exposed at 5 ppm for 1 h, while exposure at 5 ppm for 2 h resulted in 4/10 mice developing multifocal and/or diffuse inflammatory epithelial degeneration in the bronchioles (Nomiyama et al. 1995). Studies have demonstrated that these lesions are reversible. There were no other treatment-related changes, such as changes in behavior or appearance, body or organ weight, or in hematological or clinical chemistry indices. A total uncertainty factor (UF) of 10 was applied to the AEGL-2 value. An interspecies UF of 3 was applied because the most sensitive species, the mouse, was used, and the end point of toxicity, reversible histological changes in the lungs, was the most sensitive end point. Further support of a value of 3 is that signs of toxicity and repair of pulmonary lesions following acute exposure to nonlethal concentrations in animals were consistent with the human response reported by case reports. There appears to be a similar mechanism of toxicity between species because the cause of death from diborane exposure has always been from pulmonary damage, including edema, hemorrhage, and congestion. An intraspecies UF of 3 was applied because using the default UF of 10 generates AEGL values that are inconsistent with existing empirical data. For example, the derived 1-h AEGL-2 value is 1.0 ppm with a total UF of 10. Mice exposed at 1 ppm for up to 8 h exhibited no effects of diborane
exposure (Nomiyama et al. 1995). Mice exposed at 0.7 ppm for 6 h/d, 5 d/wk for up to 4 wk developed only slight pulmonary infiltration of polymorphous neutrophils (Nomiyama et al. 1995) and rats exposed at 0.96 ppm for 6 h/d, 5 d/wk for 8 wk developed changes in bronchoalveolar lavage fluid that were not accompanied by histopathological changes (Nomiyama et al. 1996). The use of a higher UF would result in AEGL values that would be below concentrations causing effects in any species for an end point that is supposed to be disabling or cause irreversible effects in a human population.
The experimentally derived exposure value was then scaled to AEGL time frames using the concentration-time relationship given by the equation Cn×t=k, where C=concentration, t=time, and k is a constant (ten Berge 1986). The value of n=1 was used for the temporal scaling based on the derivation presented in Section 4.4.3. and Appendix B.
Although it is considered appropriate to extrapolate from a 2-h exposure to a 10-min exposure duration, the 10-min value of 6.0 ppm approaches that of the 10-min AEGL-3 value of 7.3 ppm. Therefore, the 10-min value was set equal to the 30-min value. AEGL-2 values are presented in Table 4–7.
These values are supported by another study in which the next highest LOAEL for inflammatory epithelial degeneration in the bronchioles in mice was identified as 15 ppm for a 1-h exposure (Uemura et al. 1995).
7. DATA ANALYSIS FOR AEGL-3
7.1. Human Data Relevant to AEGL-3
There were no human data appropriate for derivation of an AEGL-3 found in the available literature. It should be noted that no reports of human mortality following diborane exposure were found in the available literature.
7.2. Animal Data Relevant to AEGL-3
Lethality data were available for several species including dog, rat, mouse, rabbit, and hamster. The 4-h LC50 values for the rat ranged from 40
TABLE 4–7 AEGL-2 Values For Diborane (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
2.0 (2.2) |
2.0 (2.2) |
1.0 (1.1) |
0.25 (0.28) |
0.13 (0.14) |
ppm to 80 ppm, and 15-min LC50 values for the rat ranged from 159 ppm to 182 ppm. The mouse was slightly more sensitive to lethality than the rat, with 4-h LC50 values of 29 and 31.5 ppm.
7.3. Derivation of AEGL-3
A 4-h LC50 study in mice was calculated by Uemura et al. (1995). This was a well-defined study in which the mortality ratios at each dose were given. An LC01 value could then be estimated by a log-probit analysis of these data. The estimated LC01 value of 9.2 ppm was used in the derivation of the AEGL-3. A total uncertainty factor (UF) of 10 was applied to the AEGL-3 value. Because there was little observed variation between species in sensitivity to lethal concentrations of diborane, an interspecies UF of 3 was applied. The 4-h LC50 values determined by different authors for mice and rats were within a factor of 2.8 (4-h LC50 values ranged from 29 ppm to 31.5 ppm in mice and from 40 ppm to 80 ppm in rats). The lung was the target organ in all species tested, and the biological response remained the same, becoming more severe with increasing concentrations until death occurred from anoxia as a consequence of severe pulmonary changes. An intraspecies UF of 3 was applied because using the default UF of 10 generates AEGL values that are inconsistent with existing empirical data. For example, the derived 1-h AEGL-3 value is 3.7 ppm with a total UF of 10. Mice exposed at 5 ppm for up to 4 h developed only inflammatory epithelial degeneration in the bronchioles, with exposure for 8 h resulting in increased lung weights (Nomiyama et al. 1995). Mice exposed at 15 ppm for 4 h developed pulmonary changes including edema, congestion, and inflammatory epithelial degeneration that were generally resolved or in the process of being resolved within 14 d postexposure (Uemura 1996). The use of a higher UF would result in AEGL values that would be below concentrations causing effects in any species for an end point that is supposed to be life-threatening in a human population.
The experimentally derived exposure value was then scaled to AEGL
TABLE 4–8 AEGL-3 Values For Diborane (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
7.3 (8.0) |
7.3 (8.0) |
3.7 (4.1) |
0.92 (1.0) |
0.46 (0.51) |
time frames using the concentration-time relationship given by the equation Cn×t=k, where C=concentration, t=time, and k is a constant (ten Berge 1986). The value of n=1 was used for the temporal scaling based on the derivation presented in Section 4.4.3. and Appendix B. The 10-min AEGL-3 value was set equal to the 30-min value of 7.3 ppm because the NAC considers it inappropriate to extrapolate from the exposure duration of 4 h to 10 min. AEGL-3 values are presented in Table 4–8.
8. SUMMARY OF AEGLs
8.1. AEGL Values and Toxicity End Points
A summary of the AEGL values for diborane is provided in Table 4–9. Derivation of an AEGL-1 was not recommended. Absence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects. The AEGL-2 was based on the lowest diborane concentration at which histopathological changes become evident in male ICR mice. These lesions consisted of multifocal and/or diffuse severe inflammatory epithelial degeneration in the respiratory bronchioles. The AEGL-3 value was based on a 4-h LC50 study in male ICR mice. Using the data from this study, an LC01 value was calculated, and this LC01 value was used to derive the AEGL-3.
A useful way to evaluate the AEGL values in context of existing empirical data is presented in Figure 4–1. For this plot, the toxic response was placed into severity categories. The severity categories fit into definitions of the AEGL health effects: no effects, discomfort, disabling, some lethality (an experimental concentration at which some of the animals died), and lethal (100% mortality). The effects that place an experimental result into a particular category vary according to the spectrum of data available on a specific chemical and the effects from exposure to that chemical. The doses often span a number of orders of magnitude, especially when human data exist. Therefore, the concentration is placed on a log scale. The graph
TABLE 4–9 Summary of AEGL Values (ppm [mg/m3])
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1a (Nondisabling) |
NR |
NR |
NR |
NR |
NR |
|
AEGL-2 (Disabling) |
2.0 (2.2) |
2.0 (2.2) |
1.0 (1.1) |
0.25 (0.28) |
0.13 (0.14) |
|
AEGL-3 (Lethal) |
7.3 (8.1) |
7.3 (8.1) |
3.7 (4.1) |
0.92 (1.0) |
0.46 (0.51) |
|
aAbsence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects. Abbreviation: NR, not recommended. |
|||||
in Figure 4–1 plots the diborane AEGL values along with the existing acute animal toxicity data for diborane in terms of the categories assigned to them. From this plot, one sees that the AEGL values are below any exposure concentration in animals resulting in any effects and should therefore be protective of human health.
8.2. Comparisons with Other Standards and Guidelines
Standards and guidance levels for workplace and community exposures are listed in Table 4–10. The 1-h AEGL-2 value of 1.0 ppm is the same as the ERPG-2 value, and the 1-h AEGL-3 value of 3.7 ppm is similar to the ERPG-3 value of 3 ppm. The 30-min AEGL-3 value of 7.3 ppm is below the IDLH value of 15 ppm. The IDLH was established in 1994 and is based upon the rat 15-min LC50 value reported in the Krackow (1953) study. The AEGL-3 is based upon a 4-h rat LC01 calculated using the Uemura et al. (1995) study.
8.3. Data Adequacy and Research Needs
Data were not available for derivation of an AEGL-1. Although the odor threshold could be considered relevant to an AEGL-1 because the odor is considered repulsive, the AEGL-2 value is below the odor threshold and this end point is therefore not appropriate. No other human or animal
TABLE 4–10 Extant Standards and Guidelines for Diborane
|
|
Exposure Duration |
||||
|
Guideline |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 |
NR |
NR |
NR |
NR |
NR |
|
AEGL-2 |
2.0 ppm |
2.0 ppm |
1.0 ppm |
0.25 ppm |
0.13 ppm |
|
AEGL-3 |
7.3 ppm |
7.3 ppm |
3.7 ppm |
0.92 ppm |
0.46 ppm |
|
ERPG-1 (AIHA)a |
|
|
NA |
|
|
|
ERPG-2 (AIHA) |
|
|
1 ppm |
|
|
|
ERPG-3 (AIHA) |
|
|
3 ppm |
|
|
|
PEL-TWA (OSHA)b |
|
|
|
|
0.1 ppm |
|
IDLH (NIOSH)c |
|
15 ppm |
|
|
|
|
REL-TWA (NIOSH)d |
|
|
|
|
0.1 ppm |
|
TLV-TWA (ACGIH)e |
|
|
|
|
0.1 ppm |
|
MAK (Germany)f |
|
|
|
|
Not established at the present |
|
MAC (the Netherlands)g |
|
|
|
|
0.1 mg/m3 0.1 ppm |
|
aERPG (emergency response planning guidelines) (AIHA 1996) The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. An ERPG-1 for diborane was not appropriate based on the fact that the odor is detectable at a concentration recommended for the ERPG-2. The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protection action. The ERPG-2 for diborane is based on the dog exposure data (Kunkel et al. 1956) that suggest that no effects other than minor irritation should be expected from exposure at 1 ppm for 1 h. Exposure at a nominal concentration |
|||||
data pertaining to end points relevant to the AEGL-1 definition were available.
Human case reports of accidental workplace exposure to diborane report reversible signs and symptoms of exposure including chest tightness, shortness of breath and dyspnea, wheezing, nonproductive cough, and precordial pain. However, nothing is known about the actual exposure concentrations. Data in animals have shown concentration-dependent respiratory effects including reversible histological respiratory lesions and pulmonary edema, hemorrhage, and/or congestion leading to death. These
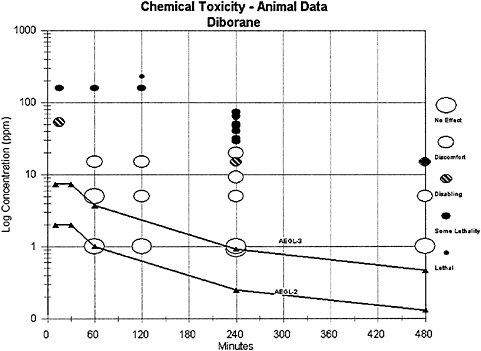
FIGURE 4–1 Category plot of animal toxicity data compared to AEGL values.
signs of toxicity and repair of pulmonary lesions following acute exposure to nonlethal concentrations in animals were consistent with the human response reported by case reports. Therefore, the animal data are considered appropriate for development of an AEGL-2. Uncertainties remain about interindividual variabilities in the toxic response to diborane, but the category plot (Figure 4–1) demonstrates that the AEGL values should be protective.
Information about the lethality of diborane in humans was not available. Lethality data from animals were considered appropriate for development of an AEGL-3 because the lung was the target organ in all species tested, and the biological response remained the same, becoming more severe with increasing concentrations until death occurred from anoxia as a consequence of severe pulmonary changes. Uncertainties remain about interindividual variabilities in the toxic response to diborane, but the category plot (Figure 4–1) demonstrates that the AEGL values should be protective.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1991. Documentation of the Threshold Limit Values and Biological Exposure Indices: (Diborane), 6th Ed. ACGIH, Cincinnati, OH.
ACGIH (American Conference of Governmental Industrial Hygienists). 2000. Threshold Limit Values and Biological Exposure Indices: (Diborane). ACGIH, Cincinnati, OH.
AIHA (American Industrial Hygiene Association). 1996. The AIHA 1996 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guidelines Handbook. AIHA, Fairfax VA.
Amoore, J.E., and E.Hautala. 1983. Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3(6):272–290.
Araki, A., T.Noguchi, F.Kato, and T.Matsushima. 1994. Improved method for mutagenicity testing of gaseous compounds by using a gas sampling bag. Mutation Res. 307:335–344.
Braker, W., and A.L.Mossman. 1980. Diborane. Pp. 219–222 in: Matheson Gas Data Book, Sixth Edition. Secaucus, NJ: Matheson Gas Products.
Budavari, S., M.J.O’Neil, A.Smith, P.E.Heckelman and J.F.Kinneary, eds. 1996. The Merck Index, 12th Ed. Rahway, NJ: Merck & Co., Inc.
Chemical Economics Handbook. 1996. Boron minerals and chemicals. SRI Interactional.
Comstock, C.C., L.Feinsilver, L.H.Lawson, and F.W.Oberst. 1954. Inhalation toxicity of diborane in dogs, rats, and guinea pigs. (Chem. Corp. Med. Lab. Research Report No. 258.) Army Chemical Center, MD: Chemical Corps Medical Laboratories.
Cordasco, E.M., R.W.Cooper, J.V.Murphy, and C.Anderson. 1962. Pulmonary aspects of some toxic experimental space fuels. Dis. Chest. 41:68–74.
EPA (U.S. Environmental Protection Agency). 1988. Research and Development: Reportable Quantity Document for Diborane.
German Research Association (Deutsche Forschungsgemeinschaft). 1999. List of MAK and BAT Values, 1999. Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area, Report No. 35. Federal Republic of Germany: Wiley-VCH.
Jacobson, K.H., and L.H.Lawson. 1962. The effect of age or weight on the toxicity of diborane. Toxicol. Appl. Pharmacol. 4:215–219.
Krackow, E.H. 1953. Toxicity and health hazards of boron hydrides. A.M.A. Arch. Induct. Hyg. Occup. Med. 8:335–339.
Kunkel, A.M., E.F.Murtha, A.H.Oikemus, D.E.Stabile, J.P.Saunders, and J.H. Wills. 1956. Some pharmacologic effects of diborane. A.M.A. Arch. Indust. Health. 13:346–351.
Lockheed Martin Energy Systems, Inc. 1988. MSDS for Diborane. Air Liquide America Corporation, Houston, TX.
Lowe, H.J., and G.Freeman. 1957. Boron hydride (borane) intoxication in man. A.M.A. Arch. Indust. Health. 16:5233–533.
Ministry of Social Affairs and Employment (SDU Uitgevers). 2000. Nationale MAC (Maximum Allowable Concentration) List, 2000. The Hague, The Netherlands.
Nippon Sanso Co. 1986. The resolution analysis of diborane. Technical Report, Nippon Sanso Co.
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHS). National Institute for Occupational Safety and Health, Cincinnati, OH. PB94195047, National Technical Information Service, Springfield, VA.
NIOSH (National Institute for Occupational Safety and Health). 1999. NIOSH Pocket Guide to Chemical Hazards. NIOSH Publication 94–116, U.S. Department of Health and Human Services; U.S. Government Printing Office, Washington, DC.
Nomiyama, T. 1995. Inhalation toxicity of diborane in rats assessed by bronchoalveolar lavage examination. Arch. Toxicol. 70:43–50.
Nomiyama, T., K.Omae, C.Ishizuka, K.Hosoda, Y.Yamano, H.Nakashima, T. Uemura, and H.Sakurai. 1996. Evaluation of the subacute pulmonary and testicular inhalation toxicity of diborane in rats. Toxicol. Appl. Pharmacol. 138:77–83.
Nomiyama, T., K.Omae, T.Uemura, H.Nakashima, T.Takebayashi, C.Ishizuka, K.Yamazaki, and H.Sakurai. 1995. No-observed-effect level of diborane on the respiratory organs of male mice in acute and subacute inhalation experiments. J. Occup. Health. 37:157–160.
OSHA. 1996. Limits for Air Contaminants. CFR, Title 29, Part 1910.1000, Table Z-1, p. 16.
Rousch, G. 1959. The toxicology of the boranes. J. Occup. Med. 1:46–52.
Rozendaal, H.M. 1951. Clinical observations on the toxicology of boron hydrides. A.M.A. Arch. Ind. Hyg. Occup. Med. 4:257–260.
Rudolph, R.W. 1978. Boron hydrides, heteroboranes, and their metallo derivatives. In: Kirk-Othmer Encyclopedia of Chemical Technology, 3rd Ed. New York, NY: John Wiley and Sons, Inc.
Shen, M.S., K.Omae, T.Uemura, T.Nomiyama, C.Ishizuka, K.Mori, and H. Sakurai. 1994. Effects of diborane on sperm in mice [in Japanese]. Jpn. J. Ind. Health. 36:28–29.
Stumpe, A.R. 1960. Toxicity of diborane in high concentrations. A.M.A. Arch. Ind. Health. 21:519–524.
ten Berge, W.F. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mat. 13:301– 309.
Uemura, T. 1996. Development of pulmonary lesions following acute exposure to diborane in male ICR mice. J. Occup. Health. 38:71–79.
APPENDIX A
Derivation of AEGL Values
Derivation of AEGL-1
An AEGL-1 value was not derived because it was not appropriate. Absence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects. The AEGL-2 value is below the odor threshold of diborane and no other data pertaining to end points relevant to AEGL-1 definition were available.
Derivation of AEGL-2
|
Key study: |
Nomiyama et al. (1995) |
|
Toxicity end point: |
LOAEL for inflammatory epithelial degeneration in the bronchioles in male ICR mice (4/10) was 5 ppm for 2 h. |
|
Scaling: |
C1×t=k (based on concentration and exposure relationships in Nomiyama et al. [1995] and Uemura et al. [1995]). |
|
Uncertainty factors: |
Total uncertainty factor: 10 Interspecies: 3 Intraspecies: 3 |
|
Calculations: |
(C/uncertainty factors)n×t=k |
|
|
([5 ppm]/10)1×2 h=1 ppm·h |
|
10-min AEGL-2: |
Although it is considered appropriate to extrapolate from a 2-h exposure to a 10-min exposure duration, the 10-min value of 6.0 ppm approaches that of the 10- |
|
|
min AEGL-3 value of 7.3 ppm. Therefore, the 10-min value was set equal to the 30-min value. |
|
|
10-min AEGL-2=(20 ppm)/10=2 ppm |
|
30-min AEGL-2: |
C1×0.5 h=1 ppm·h C1=2 ppm C=2 ppm |
|
1-h AEGL-2: |
C1×1 h=1 ppm·h C1=1 ppm C=1 ppm |
|
4-h AEGL-2: |
C1×4 h=1 ppm·h C1=0.25 ppm C=0.25 ppm |
|
8-h AEGL-1: |
C1×8 h=1 ppm·h C1=0.125 ppm C=0.13 ppm |
Derivation of AEGL-3
|
Key study: |
Uemura et al. (1995) |
|
Toxicity end point: |
The data (mortality ratios versus concentration) used to generate a 4-h LC01 in male ICR mice were given in the study. Based on those data, an LC01 value in mice was calculated to be approximately 9.2 ppm. |
|
Scaling: |
C1×t=k (based on concentration and exposure relationships in Nomiyama et al. [1995] and Uemura et al. [1995]). |
|
Uncertainity factors |
Total uncertainty factor: 10 Interspecies: 3 |
|
|
Intraspecies: 3 |
|
Calculations: |
(C/uncertainty factors)n×t=k |
|
|
([9.17 ppm]/10)1×4 h=3.668 ppm·h |
|
10-min AEGL-3: |
Inappropriate to scale from 4 h to 10 min; the 10-min value was set equal to the 30-min value. |
|
|
10-min AEGL-3=7.3 ppm |
|
30-min AEGL-3: |
C1×0.5 h=3.668 ppm·h C1=7.336 ppm C=7.3 ppm |
|
1-h AEGL-3: |
C1×1 h=3.668 ppm·h C1=3.668 ppm C=3.7 ppm |
|
4-h AEGL-3: |
C1×4 h=3.668 ppm·h C1=0.917 ppm C=0.92 ppm |
|
8-h AEGL-3: |
C1×8 h=3.668 ppm·h C1=0.4585 ppm C=0.46 ppm |
APPENDIX B
Time Scaling Calculations
The relationship between dose and time for any given chemical is a function of the physical and chemical properties of the substance and the unique toxicological and pharmacological properties of the individual substance. Historically, the relationship according to Haber (1924), commonly called Haber’s law (NRC 1993a) or Haber’s rule (i.e., C×t=k, where C=exposure concentration, t=exposure duration, and k=a constant) has been used to relate exposure concentration and duration to effect (Rinehart and Hatch 1964). This concept states that exposure concentration and exposure duration may be reciprocally adjusted to maintain a cumulative exposure constant (k) and that this cumulative exposure constant will always reflect a specific quantitative and qualitative response. This inverse relationship of concentration and time may be valid when the toxic response to a chemical is equally dependent upon the concentration and the exposure duration. However, an assessment by ten Berge et al. (1986) of LC50 data for certain chemicals revealed chemical-specific relationships between exposure concentration and exposure duration that were often exponential. This relationship can be expressed by the equation Cn×t= k, where n represents a chemical specific, and even a toxic-end-point specific, exponent. The relationship described by this equation is basically the form of a linear regression analysis of the log-log transformation of a plot of C versus t. Ten Berge et al. (1986) examined the airborne concentration (C) and short-term exposure duration (t) relationship relative to death for approximately 20 chemicals and found that the empirically derived value of n ranged from 0.8 to 3.5 among this group of chemicals. Hence, these workers showed that the value of the exponent n in the equation Cn×t= k quantitatively defines the relationship between exposure concentration and exposure duration for a given chemical and for a specific health effect end point. Habers rule is the special case where n=1. As the value of n increases, the plot of concentration versus time yields a progressive decrease in the slope of the curve.
To calculate n for diborane, a regression plot of the EC50 values was derived from the studies by Nomiyama et al. (1995) and Uemura et al. (1995) investigating 1-, 2-, and 4-h exposures at 1, 5, or 15 ppm, with multifocal and/or diffuse inflammatory epithelial degeneration in the bronchioles as the end point of toxicity. Although n values have generally been
derived using lethality data, it was considered appropriate in this case to use the nonlethal pulmonary changes. Toxicity studies demonstrated that the lung remained the target organ at all concentrations of exposure, and the biological response remained the same, becoming more severe with increasing concentration until death occurred from anoxia as a consequence of severe pulmonary changes. EC50 values were derived by probit analysis of the data, and were then analyzed using a linear regression analysis of the log-log transformation of a plot of C versus t to derived a value of n for diborane.
The derived EC50 for the 1-, 2-, and 4-h exposures were 9.68, 5.07, and 2.72 ppm, respectively.
Linear regression analysis of plot of log-log transformation of plot of C versus t:
|
Time (min) |
Concentration |
Log Time |
Log Concentration |
|
240 |
2.72 |
2.3802 |
0.4346 |
|
120 |
5.07 |
2.0792 |
0.7050 |
|
60 |
9.68 |
1.7782 |
0.9859 |
|
n=1.09 |
|||
Regression plot of EC50 values—concentration versus time.
APPENDIX C
DERIVATION SUMMARY FOR ACUTE EXPOSURE GUIDELINE LEVELS FOR DIBORANE (CAS No. 19287–45–7)
|
AEGL-1 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
NR |
NR |
NR |
NR |
NR |
|
Data adequacy: Although the odor threshold could be considered relevant to an AEGL-1 because the odor is considered repulsive, the AEGL-2 value is below the odor threshold and this end point is therefore not appropriate. No other human or animal data pertaining to end points relevant to the AEGL-1 definition were available. Absence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects. |
||||
|
Abbreviation: NR, not recommended. |
||||
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
2.0 ppm |
2.0 ppm |
1.0 ppm |
0.25 ppm |
0.13 ppm |
|
Key reference: |
Nomiyama, T., Omae, K., Uemura, T., Nakashima, H., Takebayashi, T., Ishizuka, C., Yamazaki, K., and Sakurai, H. 1995. No-observed-effect level of diborane on the respiratory organs of male mice in acute and subacute inhalation experiments. J. Occup. Health. 37:157–160. |
|||
|
Test species/strain/gender/number: Young male ICR mice, 10 per exposure group. |
||||
|
Exposure route/concentrations/durations: Inhalation; 5 ppm for 1, 2, 4, or 8 h. |
||||
|
Effects: 5 ppm: 1 h, no effects; 2 h, inflammatory epithelial degeneration in the bronchioles (4/10); 4 h, inflammatory epithelial degeneration in the bronchioles (9/10); 8 h, inflammatory epithelial degeneration in the bronchioles (10/10). |
||||
|
End point/concentration/rationale: 5 ppm for 2 h resulted in reversible inflammatory epithelial degeneration in the bronchioles. |
||||
|
Uncertainty factors/rationale: Total uncertainty factor: 10 Interspecies: 3—An interspecies UF of 3 was applied because the most sensitive species, the mouse, was used, and the end point of |
||||
|
toxicity, reversible histological changes in the lungs, was the most sensitive end point. Further support of a value of 3 is that signs of toxicity and repair of pulmonary lesions following acute exposure to nonlethal concentrations in animals were consistent with the human response reported by case reports. There appears to be a similar mechanism of toxicity between species because the cause of death from diborane exposure has always been from pulmonary damage, including edema, hemorrhage, and congestion. Intraspecies: 3—An intraspecies uncertainty factor of 3 was applied because using the default uncertainty factor of 10 generates AEGL values that are inconsistent with existing empirical data. For example, the derived 1-h AEGL-2 value is 1.0 ppm with a total uncertainty factor of 10. Mice exposed at 1 ppm for up to 8 h exhibited no effects of diborane exposure (Nomiyama et al 1995). Mice exposed at 0.7 ppm for 6 h/d, 5 d/wk for up to 4 wk developed only slight pulmonary infiltration of polymorphous neutrophils (Nomiyama et al. 1995) and rats exposed at 0.96 ppm for 6 h/d, 5 d/wk for 8 wk developed changes in bronchoalveolar lavage fluid that were not accompanied by histopathological changes (Nomiyama et al. 1996). The use of a higher uncertainty factor would result in AEGL values that would be below concentrations causing effects in any species for an end point that is supposed to be disabling or cause irreversible effects in a human population. |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Not applicable |
||||
|
Time acaling: Cn×t=k where n=1; based on a regression plot of the EC50 values derived from the studies by Nomiyama et al. (1995) and Uemura et al. (1995) investigating 1-, 2-, and 4-h exposures at 1, 5, or 15 ppm, with multifocal and/or diffuse inflammatory epithelial degeneration in the bronchioles as the end point of toxicity. Although it is considered appropriate to extrapolate from a 2-h exposure to a 10-min exposure duration, the 10-min value of 6.0 ppm approaches that of the 10-min AEGL-3 value of 7.3 ppm. Therefore, the 10-min value was set equal to the 30-min value. |
||||
|
Data adequacy: Human case reports of accidental workplace exposure to diborane report reversible signs and symptoms of exposure including chest tightness, shortness of breath and dyspnea, wheezing, nonproductive cough, and precordial pain. However, nothing is known about the actual exposure concentrations. Data in animals have shown concentration and time dependent respiratory effects including reversible histological respiratory lesions and pulmonary edema, hemorrhage, and/or congestion leading to death. These signs of toxicity and repair of pulmonary lesions following acute |
|
exposure to nonlethal concentrations in animals were consistent with the human response reported by case reports. Therefore, the animal data are considered appropriate for development of an AEGL-2. Uncertainties remain about interindividual variabilities in the toxic response to diborane, but the category plot (Figure 4–1) demonstrates that the AEGL values should be protective. |
|
AEGL-3 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
7.3 ppm |
7.3 ppm |
3.7 ppm |
0.92 ppm |
0.46 ppm |
|
Key reference: |
Uemura, T., Omae, K., Nakashima, H., Sakurai, H., Yamazaki, K., Shibata, T., Mori, K., Kudo, M., Kanoh, H., and Tati, M. 1995. Acute and subacute inhalation toxicity of diborane in male ICR mice. Arch. Toxicol. 69:397–404. |
|||
|
Test species/strain/gender/number: Young male ICR mice, 10 per exposure group. |
||||
|
Exposure route/concentrations/durations: Inhalation: 0, 11.3, 22.1, 35.1, 37.7, 44.8 ppm for 4 h. |
||||
|
Effects: |
Concentration |
Mortality |
|
|
|
|
11.3 ppm |
0/10 |
|
|
|
|
22.1 ppm |
3/10 |
|
|
|
|
35.1 ppm |
3/10 |
|
|
|
|
37.7 ppm |
9/10 |
|
|
|
|
44.8 ppm |
9/10 |
|
|
|
LC50: 31.5 ppm (provided in reference) LC01: 9.17 ppm (calculated by log-probit analysis) |
||||
|
End point/concentration/rationale: 9.17 ppm for 4 h was the calculated LC01, which is the threshold for lethality, a defined end point for the AEGL-3. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3—An interspecies uncertainty factor of 3 was applied because there did not appear to be much variation between species in sensitivity to lethal concentrations of diborane. The 4-h LC50 values determined by different authors for mice and rats were within a factor of 2.8 (4-h LC50 values ranged from 29 ppm to 31.5 ppm in mice and from 40 ppm to 80 ppm in rats). The lung was the target organ in all species tested, and the biological response remained the same, becoming more severe with increasing concentrations until death occurred from anoxia as a consequence of severe pulmonary changes. Intraspecies: 3—An intraspecies uncertainty factor of 3 was applied because using the default uncertainty factor of 10 generates AEGL values that are inconsistent with existing empirical data. For example, the derived 1-h AEGL-3 value is 3.7 ppm with a total uncertainty factor of 10. Mice exposed at 5 ppm for up to 4 h developed only inflammatory epithelial degeneration in the bronchioles, with exposure for 8 h additionally resulting in increased lung weights (Nomiyama et al. 1995). Mice exposed at 15 ppm for 4 h developed pulmonary changes including |
||||
|
edema, congestion, and inflammatory epithelial degeneration that were generally resolved or in the process of being resolved within 14 d postexposure (Uemura 1996). The use of a higher uncertainty factor would result in AEGL values that would be below concentrations causing effects in any species for an end point which is supposed to be life-threatening in a human population. |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Not applicable |
||||
|
Time Scaling: Cn×t=k where n=1; based on a regression plot of the EC50 values derived from the studies by Nomiyama et al. (1995) and Uemura et al. (1995) investigating 1-, 2-, and 4-h exposures at 1, 5, or 15 ppm, with multifocal and/or diffuse inflammatory epithelial degeneration in the bronchioles as the end point of toxicity. The 10-min AEGL-3 value was set equal to the 30-min value of 7.3 ppm because the NAC considers it inappropriate to extrapolate from the exposure duration of 4 h to 10 min. |
||||
|
Data adequacy: Information about the lethality of diborane in humans was not available. Lethality data from animals were considered appropriate for development of an AEGL-3 because the lung was the target organ in all species tested, and the biological response remained the same, becoming more severe with increasing concentrations until death occurred from anoxia as a consequence of severe pulmonary changes. Uncertainties remain about interindividual variabilities in the toxic response to diborane, but the category plot (Figure 4–1) demonstrates that the AEGL values should be protective. |






















































