III The Process of Submitting and Deciding Claims
III.A INTRODUCTION
As discussed in Section I.B.4, various public laws implemented by Title 38, Code of Federal Regulations, Part 3 (38 CFR Part 3) authorize the Department of Veterans Affairs (VA) to provide medical care and pay compensation benefits to confirmed participants or their survivors for disabilities or death related to exposure to ionizing radiation from US atmospheric nuclear testing, the occupation of Hiroshima or Nagasaki, Japan, or prisoner of war (POW) status in the vicinity of Hiroshima or Nagasaki. Veterans or their survivors can file claims covered by VA regulations (38 CFR 3.309 and 3.311) by contacting one of the 58 VA regional offices (VAROs) serving their geographic area. They can also request participation and dose information from the Defense Threat Reduction Agency (DTRA), which is the Department of Defense executive agent for the Nuclear Test Personnel Review (NTPR) program. VA regulations do not, however, require veterans or their survivors to obtain confirmation of participation or dose information from DTRA before initiating a VA claim. Veterans’ requests for medical care under VA regulations also do not require the prior filing of claims.
Veterans who believe that they have an illness related to their military service may file a claim under one of the following compensation programs:
-
VA nonpresumptive program: Several public laws, as implemented in 38 CFR 3.311, provide for VA determination of service connection and benefits for the listed diseases and others that can be documented as radiogenic. VA obtains participation and associated radiation-dose information from DTRA.
-
VA presumptive program: Several public laws, as implemented in 38 CFR 3.309, authorize VA to pay compensation for 21 types of cancer to participants in US atmospheric nuclear testing or in occupation of Hiroshima or Nagasaki. Filing a VA claim under this regulation does not require dose information from DTRA. Veterans who cannot be confirmed as participants are not eligible for VA compensation under this program.
Veterans must submit competent medical evidence that they have a medical condition covered under the VA regulations. On the basis of the information provided, VA decides under which law to file the claim. The claimant is not required to be familiar with the applicable laws and regulations.
III.B CLAIMS FILED UNDER NONPRESUMPTIVE REGULATION
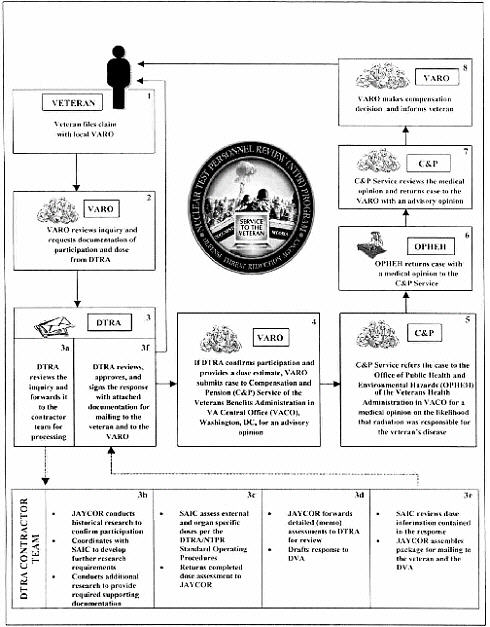
Figure III.B.1 identifies the organizations that handle a VA claim initiated through a VARO when a claim is filed under the nonpresumptive regulation (38 CFR 3.311). The chart depicts the actions and steps through which a claim inquiry passes after the VARO requests information from DTRA. DTRA has a veterans-support effort through a teamed contract with JAYCOR and Science Applications International Corporation (SAIC) to conduct historical research on veterans’ participation activities and to determine radiation doses related to those activities. DTRA reviews and approves contractor-prepared work products before submitting them to the VARO. The VARO specifies the above-cited regulation in its inquiry to DTRA for processing a claim.
To start the process, a person must file a claim for disability compensation with the local VARO. As noted above, a VA claim under this regulation does not require the claimant to obtain prior confirmation of participation or information on radiation dose from DTRA. The claimant must furnish medical evidence of a radiogenic disease listed in 38 CFR 3.311 or of any other disease plus a medical opinion that the unlisted other disease can be caused by radiation exposure. The claimed activity must involve participation in the US atmospheric nuclear testing program, occupation of Hiroshima or Nagasaki, Japan, or POW status in the vicinity of Hiroshima or Nagasaki.
The VARO asks DTRA to confirm the veteran’s participation in one of the three activities listed above. If the disease for which the claim is filed is considered a radiogenic disease, the VARO also requests an assessment of the veteran’s radiation exposure. The VA letter to DTRA should identify the veteran, describe his claimed participation, name the radiogenic disease, cite the specific controlling regulation, and include available claimant statements and supporting evidence if any.
DTRA through its contractors reviews the information provided by the VARO and conducts historical research, including interviews with the claimant for clarification if necessary, to determine the veteran’s participation status. If
participation is confirmed, DTRA provides the VARO with a report of the dose assessment. The report includes an external photon dose with upper bound and an external neutron dose if applicable, internal doses to the target organs corresponding to the radiogenic diseases identified in the VA claim, and skin dose and eye dose if applicable. Those doses account for all emissions of alpha, beta, gamma, and neutron radiation from all sources to which the veteran was exposed. A copy of the DTRA response is provided to the claimant.
The VARO sends the claim and DTRA dose assessment to the Compensation and Pension (C and P) Service of the Veterans Benefits Administration in Washington, DC, for an advisory opinion. The C and P Service asks the Office of Public Health and Environmental Hazards (OPHEH) of the Veterans Health Administration for a medical opinion of the likelihood that the veteran’s radiation exposure caused his radiogenic disease(s). For each disease, OPHEH considers the upper bound dose to the target organ, age or approximate age at exposure and at diagnosis, family and employment history, and exposure to other toxicants and carcinogens before and after military service.
Thereafter, using DTRA-reported upper-bound doses, OPHEH applies probability of causation methods, such as those based on radioepidemiological tables issued by the National Institutes of Health (NIH) (see Section III.E). Those methods are supplemented by information from other scientific or medical sources and consideration of other factors to evaluate whether it is at least as likely as not that the claimed disease(s) resulted from the reported radiation doses. OPHEH returns the case to the C and P Service with its medical opinion on the claim.
The C and P Service reviews the medical opinion from OPHEH and all the evidence of record and issues an advisory opinion to the VARO. The C and P Service notes whether, according to the regulations, the veteran’s disability from the specific diseases is the result of his military service activities.
The VARO informs the claimant about the outcome of the claim. If the claim is granted, the VARO pays compensation based on the current degree of disability resulting from the covered diseases.
Whenever a claim is denied by VA, and the veteran or other claimant believes that VA did not make a good decision because it did not review all the evidence or did not apply the law correctly, the claimant has the right to appeal a decision made by a VARO or medical center. Claimants may appeal a complete or partial denial of a claim or the amount of benefit granted.
III.C CLAIMS FILED UNDER PRESUMPTIVE REGULATION
To initiate a claim under the presumptive regulation (38 CFR 3.309), a person must file a claim for disability compensation with the VARO serving his geographic area. A VA claim under this regulation does not require the claimant to obtain prior confirmation of participation from DTRA. The claimant must furnish medical evidence of a radiogenic disease listed in 38 CFR 3.309(d). The
claimed activity must involve participation in the US atmospheric nuclear testing program, occupation of Hiroshima or Nagasaki, Japan, or POW status in the vicinity of Hiroshima or Nagasaki.
Figure III.C.1 shows that DTRA has a veterans-support effort to conduct historical research on veterans’ participation activities. As shown in the figure,
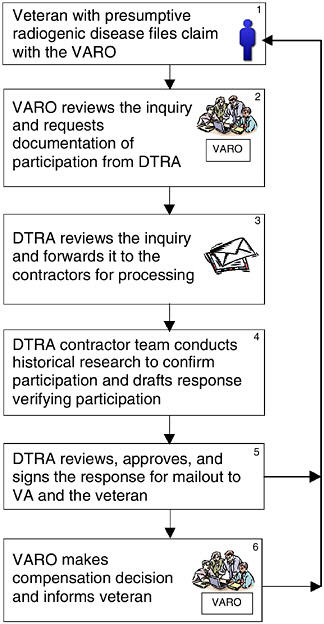
FIGURE III.C.1 How VA and DTRA process a radiation-related claim from a veteran for a presumptive disease.
only verification of participation is required for claims filed under the presumptive regulation; dose information is not required. DTRA reviews and approves contractor-prepared work products before submitting them to the VARO. The requirement for providing only participation verification to support a veteran’s claim originates in Public Law (PL) 100-321, as amended. The VARO specifies the above-cited regulation in its inquiry to DTRA for processing a claim in accordance with Figure III.C.1.
If the NTPR program verifies the veteran’s status as a participant and he has one or more of the defined presumptive diseases, his claim is granted. If the NTPR program cannot verify his participation, the claim may be refiled under the nonpresumptive regulation.
On verifying that the medical evidence meets the requirements of 38 CFR 3.309, the VARO asks DTRA to confirm the veteran’s participation in one of the three activities listed above. The VA letter to DTRA should identify the veteran, describe his claimed participation, name the radiogenic disease, cite the specific controlling regulation, and include available claimant statements and supporting evidence if any.
DTRA reviews the information provided by the VARO and conducts historical research to determine the veteran’s participation status. DTRA provides a response to the VARO and sends a copy to the claimant.
The VARO reviews the information provided by DTRA and makes a compensation decision. The VARO informs the claimant about the outcome of the claim. If the claim is granted, the VARO pays compensation based on the current degree of medical disability resulting from the covered disease.
If the veteran’s disease was diagnosed before the disease was designated as presumptive, compensation is awarded only from the time the law was enacted or modified to add the disease.
III.D COMMUNICATIONS WITH VETERANS
A veteran or his family may either communicate directly with DTRA and request a dose reconstruction or file a claim with VA. If the veteran requests a dose from DTRA and then files a claim with VA, the dose calculation may be revised in light of more specific information gathered for that veteran’s case.
In the case of a claim for a presumptive disease, the VARO requests only documentation of participation from DTRA, which then responds by letter to the VARO and sends a copy to the veteran. The VARO then informs the veteran of its compensation decision.
For a nonpresumptive disease, the veteran files a claim with the VARO, which then determines whether the disease can reasonably be considered radiogenic; if so, it requests from DTRA documentation of the veteran’s participation in the weapons testing program and an estimate of the veteran’s dose. After its research, DTRA responds to the VARO and sends a copy to the veteran. If
participation is confirmed and a dose estimate greater than zero has been provided by DTRA, the VARO submits the case to the C and P Service, which seeks an advisory opinion from OPHEH. In light of that medical opinion, the C and P Service sends its advisory opinion back to the VARO, which makes the compensation decision and informs the veteran of it.
Atomic veterans also receive other kinds of information in communications from the NTPR program. For example, veterans who file a claim for compensation or request a dose reconstruction usually receive general information, in the form of “Fact Sheets,” on the atomic testing program, the program of dose reconstruction and compensation for atomic veterans, and the magnitude and significance of doses received by the veterans. The NTPR program also communicates indirectly with atomic veterans through press releases and by submitting information for publication in the National Association of Atomic Veterans (NAAV) newsletter, especially when there are important changes in laws and regulations. Further discussion of these communications, including the committee’s comments, is given in Section VI.B.
III.E MEDICAL OPINIONS AND PROBABILITY OF CAUSATION
With the passage in 1984 of PL 98-542, compensation was made available to veterans who had radiogenic diseases, primarily cancers. The term “radiogenic” does not mean that the disease was necessarily caused by radiation, but only that credible research has established a link between exposure to radiation and an increased risk of the disease in humans. In any occurrence of cancer, there is no way for medical doctors to determine whether it was caused by radiation exposure. For a veteran to receive compensation for a nonpresumptive disease, a medical opinion is required that considers whether the radiation dose received by the veteran is at least as likely as not to have been the cause of the disease.
To assist in the determination in the case of cancer, PL 97-414 required the Public Health Service to develop radioepidemiological tables that set forth the relationships between probability of causation (PC) and radiation dose for vari-ous cancers. The purpose of the tables has been to assist in the medical determination needed for the decision about whether to award compensation. They are not the sole determining factor in the compensation decision. For example, if a radiation-exposed person has lung cancer and has been a long-time cigarette-smoker, there will be a high probability that smoking was the cause of the cancer. The probability is not 100%, and it will depend on the person’s age, how long the person has smoked, and the type of cigarettes and number smoked per day. Other factors, such as chemical exposures in the person’s occupation, can also affect the probability.
For a given exposure to radiation, scientists have developed methods for estimating the increased chance (probability) that a person will contract a particular type of cancer within a given time. Those methods have been based on exten
sive studies of the Japanese atomic-bomb survivors and medical patients who received radiation therapy. The methods assume the linear nonthreshold model for radiation effects; that is, it is assumed that there is some chance that a cancer will occur at any dose and that the probability of a cancer is proportional to the dose. In the context of a compensation decision, an important question is: Given that a person has received a particular dose of radiation and later develops a particular type of cancer, what is the likelihood that the radiation caused the cancer? The likelihood is referred to as the probability of causation, or sometimes as the attributable risk or assigned share. Although different names are used, they refer to the same calculation. The PC of a specific cancer is defined as the ratio of the estimated risk of cancer of the particular organ or tissue of concern that is due to radiation exposure to the total risk of cancer of that organ or tissue from all causes, including radiation exposure.
NIH developed the first tables for estimating PC. The tables allow one to look up, for a particular cancer diagnosis at a given age and for the radiation dose received, the estimated probability that the cancer was caused by the radiation exposure. A critical value that is often used for determining responsibility is a PC of 50%. That is, was the radiation exposure at least as likely as not the cause of a cancer? The only other risk factor that is considered in the radiation PC tables is cigarette smoking, and it is applied only to the calculations for lung cancer.
The determination of PC from radiation exposure involves a large amount of uncertainty. The uncertainty has to do with the limited epidemiological data that are available for developing the tables. In recognition of that, the Committee on Interagency Radiation Research and Policy Coordination (CIRRPC) produced additional tables (CIRRPC, 1988) based on the NIH tables. These tables give the estimated radiation dose that would be required for a given cancer type to produce the critical 50% PC value. The 50% PC values are presented in Table III.E.1. The dose associated with a PC of 50% is given for several ages at the time of radiation exposure. Because the risk of leukemia decreases after a number of years post-exposure, CIRRPC calculated different PC values for leukemia occurring within 20 years of an exposure and after 20 years. Because there is uncertainty in the dose associated with this 50% PC estimate, owing to uncertainties in the data on risks in humans, CIRRPC also gives the 5th percentile value of the corresponding uncertainty distribution. At this dose, one can be 95% confident that the dose associated with a 50% PC is not lower. The doses associated with the 95% credibility limit of a 50% PC are given in Table III.E.2. Because there is a requirement of being 95% sure that the PC value is not underestimated, the radiation dose in this case is considerably lower than in the case of estimating the dose associated with a 50% PC in Table III.E.1. CIRRPC also produced doses associated with a 99% credibility level, and these are shown in Table III.E.3.
To give the benefit of the doubt to the veteran, VA has chosen to use the doses associated with 99% credibility limits of PC rather than the 50% PC values. For example, the dose associated with a 50% PC for colon cancer in Table III.E.1
TABLE III.E.1 Organ or Tissue Doses (rad) Corresponding to a PC of 50% Based on NIH Radioepidemiological Tables (CIRRPC, 1988)a
|
|
Age at Exposure, years |
||
|
Type of Cancer |
<20 |
30 |
>40 |
|
Chronic granulocytic leukemiab |
|
||
|
within 20 years of exposure |
11.5 |
16.0 |
17.6 |
|
20 or more years post-exposure |
30.8 |
35.7 |
59.4 |
|
Acute leukemiab |
|
||
|
within 20 years of exposure |
14.7 |
22.4 |
44.5 |
|
20 or more years post-exposure |
38.7 |
44.5 |
55.6 |
|
Leukemia (excluding chronic lymphatic) |
|
||
|
within 20 years of exposure |
14.4 |
21.4 |
37.1 |
|
20 or more years post-exposure |
37.1 |
42.4 |
56.6 |
|
Colon cancer |
209.4 |
331.8 |
497.4 |
|
Esophageal cancer |
183.8 |
331.8 |
458.6 |
|
Female breast cancer |
92.3 |
157.0 |
287.3 |
|
Kidney and bladder cancer |
258.1 |
368.3 |
483.5 |
|
Liver cancer |
28.0 |
72.6 |
138.0 |
|
Lung cancer |
|
||
|
known smokersc |
258.1 |
409.0 |
546.8 |
|
othersd |
73.2 |
128.0 |
178.6 |
|
Pancreatic cancer |
112.4 |
202.2 |
297.9 |
|
Stomach cancer |
95.6 |
157.0 |
225.9 |
|
Thyroid cancer |
28.9 |
56.6 |
63.9 |
|
aDoses at ages between 20 and 30 years or between 30 and 40 years should be obtained by linear interpolation. bDose to active bone marrow. cKnown to have been a regular smoker (10 or more cigarettes per day) within 5 years of diagnosis. Doses are calculated on the basis of an assumption that the claimant is a member of the average US population that includes smokers and nonsmokers. dClaimant’s smoking habits are unknown, or claimant is known to have stopped smoking 5 years or more before diagnosis, or claimant is known to be a nonsmoker. Doses are calculated on the basis of an assumption that the claimant is a nonsmoker. |
|||
is 209.4 rad, but VA has used the lower dose of 17 rad associated with the 99% credibility limit of a PC of 50% in Table III.E.3 in evaluating whether it is at least as likely as not that radiation exposure caused a veteran’s colon cancer. To give the veterans an additional benefit of the doubt, VA also uses the upper-bound dose reported by the NTPR program as the dose to be used in determining PC. That is, the estimated upper-bound dose to a veteran is compared with the dose from the CIRRPC table giving a 99% credibility limit; if the upper bound is above the table dose, it is presumed that a PC of at least 50% is credible. To the extent that the upper bound is a reasonable representation of the uncertainty in the estimated dose, this procedure generally results in an estimate of PC that even exceeds the 99th percentile credibility limit.
TABLE III.E.2 Doses (rad) to the Affected Organ or Tissue Based on 95% Credibility Limit of PC of 50% (CIRRPC, 1988)a
|
|
Age at Exposure, years |
||
|
Type of Cancer |
<20 |
30 |
>40 |
|
Chronic granulocytic leukemiab |
|
||
|
within 20 years of exposure |
1.4 |
2.0 |
2.2 |
|
20 or more years post-exposure |
4.2 |
5.0 |
9.3 |
|
Acute leukemiab |
|
||
|
within 20 years of exposure |
1.8 |
2.9 |
6.5 |
|
20 or more years post-exposure |
5.5 |
6.5 |
8.5 |
|
Leukemia (excluding chronic lymphatic) |
|
||
|
within 20 years of exposure |
1.8 |
2.8 |
5.2 |
|
20 or more years post-exposure |
5.2 |
6.1 |
8.5 |
|
Colon cancer |
25.9 |
48.6 |
82.7 |
|
Esophageal cancer |
9.1 |
22.2 |
35.8 |
|
Female breast cancer |
26.7 |
50.9 |
104.3 |
|
Kidney and bladder cancer |
21.1 |
35.2 |
51.7 |
|
Liver cancer |
1.6 |
5.4 |
12.9 |
|
Lung cancer |
|
||
|
known smokersc |
37.8 |
69.6 |
100.6 |
|
othersd |
6.8 |
14.4 |
22.8 |
|
Pancreatic cancer |
10.3 |
23.4 |
40.0 |
|
Stomach cancer |
10.8 |
21.2 |
34.8 |
|
Thyroid cancer |
4.9 |
10.7 |
12.7 |
|
aDoses at ages between 20 and 30 years or between 30 and 40 years should be obtained by linear interpolation. A claimant with a dose less than the dose shown would have less than 5% chance of having a true PC exceeding 50%. bDose to active bone marrow. cKnown to have been a regular smoker (10 or more cigarettes per day) within 5 years of diagnosis. Doses are calculated on the basis of an assumption that the claimant is a member of the average US population that includes smokers and nonsmokers. dClaimant’s smoking habits are unknown, or claimant is known to have stopped smoking 5 years or more before diagnosis, or claimant is known to be a nonsmoker. Doses are calculated on the basis of an assumption that the claimant is a nonsmoker. |
|||
More epidemiological data on radiogenic cancers have become available in recent years, and the original NIH tables have recently been revised by the National Cancer Institute (NCI) (NCI-CDC, 2002). The National Institute for Occupational Safety and Health (NIOSH) has adopted the NCI tables and has added a few cancers to the original cancers in the NCI update. The method of calculating confidence limits of PC can be found on the NIOSH Web site at http://198.144.166.5/irep_niosh/. On the NIOSH Web site, a person enters sex, year of birth, year in which radiation exposure occurred, year in which cancer was diagnosed, type of cancer, radiation dose, and type of radiation. The Interactive RadioEpidemiological Program (IREP) method also incorporates more sophisticated ways to account for uncertainty in the process of estimating the PC value
TABLE III.E.3 Doses (rad) to the Affected Organ or Tissue Based On 99% Credibility Limit of PC of 50% (CIRRPC, 1988)a
|
|
Age at Exposure, years |
||
|
Type of Cancer |
<20 |
30 |
>40 |
|
Chronic granulocytic leukemiab |
|
||
|
within 20 years of exposure |
0.9 |
1.3 |
1.4 |
|
20 or more years post-exposure |
2.7 |
3.2 |
5.9 |
|
Acute leukemiab |
|
||
|
within 20 years of exposure |
1.1 |
1.8 |
4.1 |
|
20 or more years post-exposure |
3.5 |
4.1 |
5.5 |
|
Leukemia (excluding chronic lymphatic) |
|
||
|
within 20 years of exposure |
1.1 |
1.7 |
3.3 |
|
20 or more years post-exposure |
3.3 |
3.9 |
5.5 |
|
Colon cancer |
17.0 |
33.1 |
58.1 |
|
Esophageal cancer |
3.9 |
9.9 |
16.7 |
|
Female breast cancer |
18.8 |
37.0 |
78.6 |
|
Kidney and bladder cancer |
13.4 |
23.1 |
34.7 |
|
Liver cancer |
1.0 |
3.3 |
8.2 |
|
Lung cancer |
|
||
|
known smokersc |
25.5 |
48.8 |
72.1 |
|
othersd |
4.3 |
9.3 |
15.0 |
|
Pancreatic cancer |
5.8 |
13.7 |
24.3 |
|
Stomach cancer |
6.9 |
13.8 |
23.2 |
|
Thyroid cancer |
3.3 |
7.4 |
8.8 |
|
a Doses at ages 20 and 30 years or between 30 and 40 years should be obtained by linear interpolation. A claimant with a dose less than the dose shown would have less than 1% percent chance of having a true PC exceeding 50%. b Dose to active bone marrow. c Known to have been a regular smoker (10 or more cigarettes per day) within 5 years of diagnosis. Doses are calculated on the basis of an assumption that the claimant is a member of the average US population that includes smokers and nonsmokers. d Claimant’s smoking habits are unknown, or claimant is known to have stopped smoking 5 years or more before diagnosis, or claimant is known to be a nonsmoker. Doses are calculated on the basis of an assumption that the claimant is a nonsmoker. |
|||
for a given radiation dose. The computer code allows consideration of uncertainty in an estimated dose and uncertainties in all other parameters that enter into a calculation of PC. The code then calculates not only a central estimate of the PC value for a specified dose and its uncertainty but also the upper 97.5% and 99% credibility limits of PC, taking into account all uncertainties.
The committee has used the current NIOSH computer code (IREP) to estimate doses that can be compared with doses that were used by VA based on the CIRRPC tables as reproduced here in Tables III.E.1 through III.E.3. Table sIII.E.4 gives the comparison between the doses associated with the 50% PC values in Table III.E.1 and the doses associated with the 99% credibility limits of the 50% PC values in Table III.E.3. The values given in Table III.E.4 are only for a person
TABLE III.E.4 Comparison of CIRRPC Screening Doses (rem) with Values Based on IREP Methodologya
|
|
PC of 50% |
99% Credibility Limit |
||
|
Type of Cancer |
CIRRPCb |
IREPc |
CIRRPCb |
IREPc |
|
Colon |
209.4 |
72 |
17 |
48 |
|
Esophagus |
183.8 |
100 |
3.9 |
43 |
|
Breast (Female) |
92.3 |
|
18.8 |
55 |
|
Kidney and bladder |
258.1 |
13.4 |
57d |
|
|
Bladder |
|
NAe |
66 |
|
|
Liver |
28 |
28 |
1 |
14 |
|
Lung, smoker |
258.1 |
213 |
25.5 |
150 |
|
Lung, smoking unknown |
73.2 |
NA |
4.3 |
NA |
|
Lung, never smoked |
NA |
88 |
NA |
50 |
|
Pancreas |
112.4 |
|
5.8 |
125 |
|
Prostate |
NA |
479 |
NA |
33 |
|
Stomach |
95.6 |
143 |
6.9 |
35 |
|
Thyroid |
28.9 |
30 |
3.3 |
10 |
|
aExposure at age of 20 years, and diagnosis at age of 60 years. bCommittee on Interagency Radiation Research and Policy Coordination (1988). cInteractive RadioEpidemiological Program (IREP, 2000) developed by NCI and NIOSH. dKidney only. eNot available. NOTE: Values from IREP assume chronic exposure to photons of energy > 250 keV. |
||||
who was exposed at the age of 20 years and was diagnosed with cancer at the age of 60. The values will be very close to those for a person diagnosed with the cancer at the ages of 50 or 70, except for leukemia. What one observes is that the doses based on the 99% credibility limits of a PC of 50% are higher in the IREP calculations. The reason is that with more knowledge, the uncertainty in estimating PC has decreased, so the dose associated with a PC of 50% has increased. VA is considering using the new and improved NIOSH-NCI tables.
Leukemia is different from solid tumors, such as lung and liver cancer. The difference is that radiation-caused leukemia can occur 1–2 years after exposure, whereas it usually takes at least 10 years for most radiation-caused solid tumors to develop. Furthermore, the radiation risk of leukemia peaks at about 5–10 years after exposure, and there is no longer an observable excess risk after about 25 years; in contrast, the radiation risk of solid tumors often remains proportionally higher than the natural cancer rate throughout one’s life after the 10-year latency period. Table III.E.5 shows the doses associated with a PC of 50% and the 99% credibility limit for a PC of 50% for exposure at age 20 and various ages at diagnosis of leukemia. Although leukemia has possibly the greatest cancer risk associated with radiation exposure relative to background cancer rates, the calculations show that after a number of years, the excess risk above background is essentially minimal.
TABLE III.E.5 Doses Corresponding to Different Credibility Limits of PC for Leukemia (rem)a
|
Age at Diagnosis, year |
PC of 50% |
99% Credibility Limit |
|
30 |
10 |
5 |
|
40 |
38 |
20 |
|
50 |
140 |
58 |
|
60 |
500 |
135 |
|
aExposure at age of 20 years. |
||
Skin cancer is now also considered to be radiogenic. There are basically three major types of skin cancer. One is melanoma, which can be quite lethal, and the other two are more common nonmelanoma skin cancers, which many people develop primarily from exposure to the sun’s ultraviolet radiation. The nonmelanoma skin cancers are of two main types: squamous cell carcinoma and basal cell carcinoma. Of those three types, melanoma and basal cell carcinoma can result from exposure to ionizing radiation; squamous cell carcinoma has not been found to be radiogenic. Beta radiation can penetrate only a very short distance in human tissue. Beta radiation, however, can at times be emitted at relatively high levels by radionuclides in nuclear fallout. External beta radiation affects primarily the skin, and the NIOSH IREP program allows consideration of beta radiation in its calculations. The committee has reproduced in Table III.E.6 values of beta doses for skin cancers corresponding to different credibility limits of PC for both black and white men. Background skin cancer risks are considerably smaller in blacks, and this cancer site is the only one that requires racial specification.
Data in Tables III.E.1 through III.E.6 should provide a veteran with an idea of the radiation dose that would be required to achieve a PC of 50% at different credibility limits, including the 99% credibility limit that the 50% PC is not associated with a lower dose, as used by the VA. For a specific case, one can go
TABLE III.E.6 Doses Corresponding to Different Credibility Limits of PC for Skin Cancer (rem)a
|
|
Blacks |
Whites |
||
|
Skin Cancer Type |
PC of 50% |
99% Credibility Limit |
PC of 50% |
99% Credibility Limit |
|
Melanoma |
39 |
8 |
70 |
10 |
|
Basal cell carcinoma |
24 |
4 |
70 |
10 |
|
Squamous cell carcinoma |
NAb |
190 |
NA |
475 |
|
aChronic exposure to beta particles of energy > 15 keV. bNo value is calculated, because squamous cell carcinoma of the skin is not considered to be radiogenic. |
||||
to the NIOSH Web site and use the IREP program to determine what the 99% credibility limit of PC is for the given upper-bound radiation dose provided in a dose reconstruction and whether the 99% credibility limit of PC is 50% or higher. The values in the tables given here provide some information for the medical decision process. It must be understood that the PC values constitute only one tool used in the medical decision process, and PC is not the only factor used in deciding whether an atomic veteran with a nonpresumptive radiogenic disease receives compensation for service-connected disability.